Key points
Blood pressure
- High blood pressure (hypertension) affects the same organs as high blood glucose levels – so blood pressure needs just as much attention as blood glucose (but doesn’t always get it).
- Systolic (upper) blood pressure in Type 2s should be between 130 and 140 mm Hg. The lower (diastolic) measurements are no longer considered relevant to long-term outcomes.
- Good self-care in blood pressure means taking your own measurements at home using reliable equipment.
- All blood pressure medication is safe, and has been in use for at least 20 years.
- Good blood pressure control meaningfully reduces the risk of strokes, heart attacks and worsening kidney function – and the benefit is greater in Type 2s than in people without diabetes.
Cholesterol
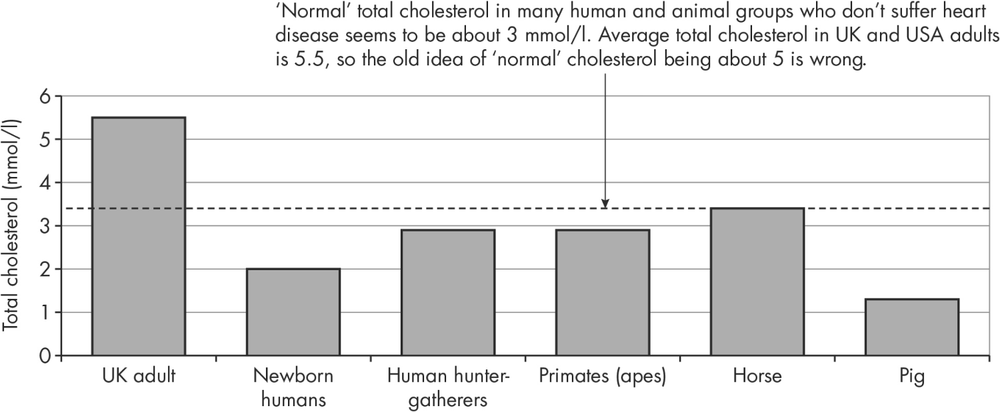
- The ‘natural’ total cholesterol level in groups who are at very low risk of heart attacks and strokes is about 3 mmol/l, compared with an average level of 5 in adults. The comparable values of LDL cholesterol – considered the most damaging fraction of cholesterol – are below 2 mmol/l.
- Diet and lifestyle can help a little, but very few people can reach the necessary low values without medication.
- Statins reduce the risk of vascular events by about 40% in people with diabetes.
- If possible, everyone who has had a heart attack or stroke should take a statin to get the LDL level to below 2 mmol/l.
- All other Type 2s should if possible also take a statin.
- Significant side-effects (especially involving the muscles) are very uncommon, but less serious side-effects are more common.
- Changing from one group of statins to another can help if you get side-effects.
- People who discontinue their statin on the basis of adverse reports in the press run a higher vascular risk. Discontinue only after a discussion with your medical team.
High blood pressure (hypertension)
Most people, even with very high blood pressure, will never experience any symptoms (everyone gets occasional headaches and dizziness), so, as with blood glucose and cholesterol, numbers are critical. Agreeing on those numbers – much like glucose and cholesterol – is another matter, but over the past few years, a general consensus has developed that people with diabetes should always have blood pressure measurements lower than 140/80 mm Hg and people who already have some complications, especially kidney, should have measurements that are nearer 130/80 mm. The ‘mm Hg’ refers to the old method of measuring blood pressure with a mercury-filled gauge, so ‘mm’ means millimetres of mercury (Hg). (Officially blood pressure numbers should be recorded as ‘mm Hg’ – Hg being the chemical symbol for mercury; but I have generally restricted it to ‘mm’ throughout the book.)
Doctors don’t help Type 2 people by often making the numbers more complicated than they need be. We still record two blood pressure numbers separated by a slash, for example 145/80. Up to about 20 years ago the number after the slash – the ‘diastolic’ value – was thought to be the most important one in determining the risk of the serious outcomes of high blood pressure, heart attacks, strokes and kidney disease (see Figure 10.1). Clinical trials of blood pressure control in the early 1990s still focused on achieving low diastolic measurements, while the first, higher number (the ‘systolic’ pressure) was mostly ignored. This has completely changed now, and every recent clinical trial has used only the systolic measurement. But doctors are terrible creatures of habit; while diastolic pressure measurements are almost irrelevant, we still always measure it, and even clinical trials that focus only on systolic blood pressure targets still measure and quote the diastolics. There are silly ‘rules’ as well, still occasionally quoted by doctors – for example, the idea that your systolic blood pressure should be lower than your age plus 100. Silly – and dangerous. For example, no person aged 60, diabetic or not, should have a systolic blood pressure of 160. Actually, it’s probably too high for a 90-year-old. Ditch this myth as well as all the others. Blood pressure is now really simple.
Key point: Systolic blood pressure should always be lower than 140 mm. Aim for around 130 if you’ve had a stroke or heart attack or if your kidneys have been stressed by diabetes. Ignore the lower diastolic number.
Measure your own blood pressure
For the remainder of this section I’m going to be radical and stop even mentioning diastolic blood pressure: from now on, I’ll only mention systolic pressures. Remember, they should always be under 140 mm.
In Type 2s not taking insulin there is still an unresolved discussion about the value of finger-prick blood glucose monitoring. The discussion will continue until we do a large clinical trial, and the wait may be a long one. Fortunately, when it comes to home-monitoring of blood pressure, the trials have been done and the answers are clear: it’s not only easy to measure your own blood pressure, but the measurements are highly reliable, and – most important of all – they’re more meaningful than those taken, often in haste, in the stressed environment of a hospital or general practice clinic, giving rise to ‘white coat hypertension’ or ‘the white coat effect’. Clinic measurements are usually significantly higher than home measurements, perhaps by as much as 10 mm.
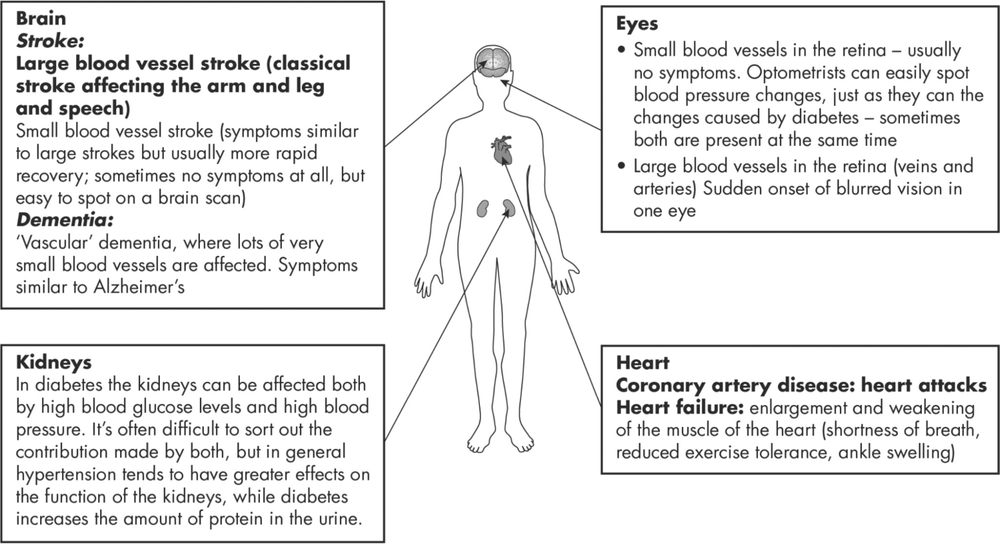
Figure 10.1: The effects of high blood pressure. These are broadly the same whether or not you have diabetes, but in Type 2 the risk of these complications is higher.
You just need the right equipment, and to take the minimum number of measurements in the correct way. The equipment is easy to deal with. For people in the UK, the British Hypertension Society keeps an updated list of validated equipment on its website (see References, page 228). It strongly recommends equipment that uses an arm cuff; devices with wrist or finger cuffs are not approved because blood pressure measurements are reliable only if they are taken at the level of the heart. Omron, Boots and Lloyds devices are the most familiar in the UK. They are usually on offer at £20 or less. Equipment that stores readings electronically is more expensive, and it’s best to get the simplest device and write down your blood pressure readings (or store them on your phone).
Key point: Home blood pressure equipment should have arm cuffs – not wrist (or even finger) cuffs, which don’t measure blood pressure reliably.
How and when to take your own blood pressure
There are lots of different recommendations. Blood pressure is highest first thing in the morning, and then settles, so take measurements later in the day – for example, late morning and evening.
- Take two measurements at about the same time of day for five to seven days before a clinic appointment or meeting your GP.
- Do a similar series of measurements about four weeks after starting a new medication or changing the dose of an existing medication.
- If your blood pressure and medication are stable, a measurement series every six months is fine.
- Don’t measure your blood pressure if you’re stressed or in pain, and before taking it sit quietly and comfortably in a chair with your feet flat on the ground (crossing your legs often increases blood pressure).
- Make sure your upper arm is bare – no tight sleeves.
- Use the same arm for each measurement. The right is usually used, but attaching the cuff requires some skill, so the left arm is fine for right-handed people. Make sure the cuff covers the bend in the elbow and that the arrow on the cuff is situated over the inner side of the elbow – nearest the body.
- Take two measurements a minute apart.
- Write down the measurements.
Key point: If you’re taking medication for high blood pressure, aim to do your blood pressure regularly – though not necessarily frequently – at home.
‘Low’ blood pressure
All doctors in diabetes clinics have heard from slightly panicked patients that they were told their blood pressure was found to be ‘low’. Is that a good or bad thing? The answer is … it depends.
I apologise for this entirely predictable ‘medical’ response, but please don’t chuck the book away in irritation; it took a long time to write. The ‘low’ blood pressure story has had a few twists and turns. First, let’s consider what is ‘normal’ blood pressure in a younger person who’s not taking blood pressure medication. Simple: it’s 120 mm or lower (120/80 or lower if you insist). There are countless young (usually slim) people, entirely fit and well who have even lower pressures – for example 90 or 100 mm. They’re in robust good health, and are likely to live to a ripe old age; their whole circulation is gentle and low pressure, but working very well, and their blood vessels won’t be stressed.
However, most of us aren’t in either of these categories: we’re no longer young, and we’re taking blood pressure medication, and that’s where ‘low’ blood pressure might not be such a good thing. In several clinical trials, Type 2s taking medication, often older people in their 60s, and who have pressures of 120 mm or lower, have a slightly increased risk of heart attacks and other vascular events, and once the pressures fall to 110 or lower, then the kidneys can be stressed as well. In addition, these low pressures can give unpleasant symptoms – for example, dizziness, especially when standing up or getting out of bed in the morning. So: aim for a blood pressure under 140 mm, but avoid 120 or lower.
Key point: The target systolic blood pressure in people with diabetes is ideally between 120 and 140 mm. It shouldn’t go below 110 mm.
Treatment of hypertension
Drug treatment
We’ve seen that hypertension is hazardous and more so in Type 2s than in non-diabetic people. Non-drug approaches to hypertension were discussed in Chapter 7. Drug treatment of high blood pressure is effective and works very quickly. It reduces the risk of stroke and heart attack, and in the longer term helps preserve kidney function. For reasons that are still not clear, but possibly related to insulin resistance and the metabolic syndrome (see Chapter 3), Type 2 diabetes and hypertension go hand in hand, so that before treatment about 80% of Type 2s have systolic blood pressure above 140 mm. It’s a very unusual Type 2 hypertensive individual who can get away with no blood pressure medication at all, and many need two, three, four or even more medications. If there are kidney problems, especially protein in the urine (see Chapter 11), medication is critical for preserving good kidney function. Specific non-drug interventions, especially the DASH diet, and reducing salt and alcohol intake, can help you reduce the number or doses of blood pressure tablets you need (see Chapter 7), but don’t expect to be able to ditch every blood pressure medication; by the time most of us develop Type 2 diabetes in our late 50s or early 60s, hypertension has often been present for as long as 30 years.
As we discussed in Chapter 7, medication for blood pressure doesn’t cause as much anxiety as blood sugar medication or drugs for cholesterol (see the statin story below), and this is largely because drugs used for hypertension are old and well established. There have been a few attempts to introduce new drugs in the past 15–20 years, but they were either withdrawn or stopped being used because of side-effects right from the start, and it’s unlikely we’ll see many more. But there’s a lot more we can do with existing (and sometimes very old and relatively unused) drugs, especially if used in logical combinations. This is particularly important in about 10% of Type 2s (amounting to a large total number of patients) whose blood pressure is difficult to control. We’ll briefly look at this phenomenon – ‘resistant’ hypertension – below. Table 10.1 lists the main categories of blood pressure medication.
Table 10.1: Major groups of blood pressure medication
The drudgery of taking blood pressure medication
Although taking two, three or more medications for anything is a chore, it may be some consolation to know that not very many years ago, blood pressure medication had to be taken twice or even three times a day. All antihypertensive medication is now once-daily. Blood pressure tends to be higher first thing in the morning, so taking your medication late at night or at bedtime may help, but morning medication is far better than forgetting to take it at night.
As with any drug treatment, some people discontinue medication; about one in five stop taking their tablets, for a variety of reasons (this seems to be particularly the case for the ACE inhibitor and ARB drugs compared with other types of medication, an unexpected finding as these groups have very few side-effects). Careful reviews with pharmacists can help increase regular tablet-taking.
People with hypertension often tell me they don’t have time to take their tablets, and always reassure me that once they retire they’ll have more time to look after themselves, and that would – of course – include absolutely regular medication-taking. Not so. A large study of people working in the Finnish public sector discovered that men and women alike were about one-third less likely to take their blood pressure medication after they retired (in men, though not women, this extended to not taking their diabetes medication either). Does this matter? Yes: in another study people admitted to hospital with a heart attack or stroke were more likely to die if they had previously taken less than 50% of their tablets (see References, page 228).
Key point: Blood pressure rises even if you miss just one day’s medication. Retired people may not be as good at taking their tablets as people in work.
Hypertension that’s difficult to control
Resistant hypertension – hypertension that is difficult to control – is defined as blood pressure of140 mm or higher in someone taking at least three medications, one of which must be a diuretic (diuretics help all other drugs to work better, especially if they’re accompanied by a low-salt diet). It’s a useful definition, because it alerts patients and doctors alike to the need for a careful medical assessment and medication review if blood pressure seems not to be well controlled. Some people turn out to have abnormal-looking adrenal glands (tiny organs perched on top of the kidneys), which can be overproducing a salt-retaining hormone called aldosterone. Blood tests, and perhaps also a CT scan of the adrenal glands, can identify an adrenal problem. But in most cases of resistant hypertension, a group of old drugs, actually diuretics themselves, has found a new lease of life, often with dramatic effects in reducing blood pressure (see Table 10.1). Their effect is greatly enhanced by intensive lifestyle measures, focusing on reducing salt intake (see Chapter 7).
Cholesterol
The cholesterol story has been running now for 70 years or more, and shows no sign of coming to an end – and certainly not a tidy end. Although there are still controversies over blood glucose and its links to the complications of Type 2, we have seen there are no street fights or headlines about blood pressure control. Why, then, has the war over cholesterol not come to a peaceful end? I think there are several reasons, all related to our poor understanding of the links between blood cholesterol, fats in food and heart attack risks. First, research, even after all this time, can’t explain why there is only a weak link between blood cholesterol levels encountered in most of the population and vascular diseases (especially heart attack and stroke), but why reducing cholesterol levels with statins has such a dramatic effect on reducing that risk.
A second unresolved problem is the relationship between the saturated fat content of food, particularly animal products, and blood cholesterol levels. Food itself contains almost no cholesterol; plants can’t manufacture cholesterol, so no vegetable contains any cholesterol. Eggs and other animal products contain small amounts, but much less than the quantities produced by the ever-versatile liver. Because food contains almost no cholesterol, it can’t be responsible for high blood cholesterol. What does, then, cause high blood cholesterol levels? Much of it is genetic, and there’s nothing we’re going to be able to do about that for a very long time. The amount of saturated fats (quite different from cholesterol) in foods derived from animals has only a weak link with blood cholesterol level, and eaten in usual quantities, meat, for example, has only a small effect on blood cholesterol values.
The final weak link in this very feeble chain is that even if you make a huge dietary effort, or lose an enormous amount of weight, there isn’t likely to be a major change in your blood cholesterol (see Chapter 7). For example, huge weight loss after bariatric surgery results in only a small reduction in total cholesterol levels. The level of harmful LDL cholesterol barely changes, and in some studies goes up. In the Look AHEAD study, in which Type 2 patients lost around 9 kg in the first year, and remained with 4–5 kg weight loss for at least four years, there were no changes in any of the cholesterol measurements.
Scientifically, all this uncertainty generates frustration and controversy, but only because we expect to be able to uncover simple links in medicine: too much ‘sugar’ in the diet ‘causes’ Type 2 diabetes (you now know that isn’t the case); too much dietary ‘fat’ causes heart disease (but not if you’re French). And so on. Nothing is that simple, so the best thing we can all do is wait for clinical science to try and sort out the whole confusing story, retain our wonder at the complexity of the way we function, and recognise the validity of highly important clinical outcome studies, such as PREDIMED, which confirmed the beneficial effect of the Mediterranean diet on something that is really important to Type 2s: reducing vascular damage. And although the Mediterranean diet is quite low in saturated fat – itself resulting in perhaps a small reduction in harm – the benefits of a high intake of polyunsaturated fats in the same diet are much greater.
Key point: We still don’t fully understand the links between dietary fat, blood cholesterol levels and the risk of heart disease.
Fatty stuff other than cholesterol
Apart from cholesterol, there is a myriad of other fatty substances, lumped together as ‘lipids’, that circulate in the blood and interact with critical organs. Recall that carbohydrates generate fats called triglycerides, which get stored wherever they can be deposited, usually in the liver, but also in the pancreas, in muscle and other organs (see Chapters 1 and 3). Other lipids, including cholesterol itself, are used to manufacture cell walls, and are therefore needed in large amounts, as we are constantly regenerating new cells. There are hundreds of these complex long-chain chemicals, all interacting with one another and being modified from one to another. But in routine practice, we measure only a few of them:
- Total cholesterol: This is the number we’re most likely to remember, though because it represents a mixture of different cholesterols it’s not the best one for estimating cardiac risk. The ‘normal’ level used to be quoted as 5 mmol/l but that’s the average level in a Western population, where heart disease is common. Laboratories have stopped quoting these arbitrary numbers, and as we’ll see below, most people with Type 2 diabetes should be aiming for levels of 3 mmol/l or lower. The accolade for the number that reflects cardiac risk most directly belongs to LDL cholesterol.
- LDL (low-density lipoprotein) cholesterol: LDL cholesterol is only one component of total cholesterol and is about 60% lower than normal. LDL cholesterol level broadly correlates with the risk of coronary heart disease and other vascular diseases – the lower the number the lower the risk – and is the measurement (see below) that has become the target for treatment in Type 2s. Just as ‘normal’ total cholesterol used to be quoted as 5 mmol/l, ‘normal’ LDL cholesterol was thought to be around 3 mmol/l. But we don’t need guesswork to establish the kind of LDL levels we should be aiming for. There are good studies of real-life LDL levels in humans and animals who never get coronary heart disease. Whether you measure it in newborn babies, primates in the wild, other wild mammals and – perhaps most relevant of all – hunter-gatherer humans, the average LDL in all these groups is about 1.6 mmol/l (equivalent to a total cholesterol of about 3 mmol/l). In Western societies only a tiny number of adults have an LDL of 3, but clearly that’s what we should be aiming for to lower the risk of heart attacks and strokes (see Figure 10.2).
- HDL (high density lipoprotein) cholesterol: As total cholesterol and LDL cholesterol levels rise, the risk of vascular complications broadly increases. But not all high levels of fats are ‘bad’; the higher the HDL cholesterol level, the lower the risk, and this is because one of the functions of HDL cholesterol is to transport cholesterol around the body and store it out of harm’s way. (Interestingly, people with Type 1 diabetes often have very high HDL levels, and those with the very highest levels tend to be protected against long-term complications.) Unfortunately Type 2s often have low HDL levels, and this is a more consistent finding than a higher total or LDL cholesterol (and is so characteristic of Type 2 and of pre-diabetes that a low HDL cholesterol is considered part of the metabolic syndrome – see Chapter 3). Like total and LDL cholesterol, diet seems to play a limited part in determining your individual HDL level, and genetics is probably the most important factor, though moderate alcohol intake and intensive exercise can increase HDL. Up to the time of the menopause, non-diabetic women have higher HDL levels than men, and this goes a long way to explaining why younger non-diabetic women have a much lower risk of heart disease than their male counterparts. Type 2 diabetes erodes this differential, so that Type 2 men and women at all ages tend to have the same, lower, HDL levels. The message is that we need to look after the hearts of all people with Type 2 whether male or female.
- Triglycerides: We encountered these diet-derived fats early on in this book. They are only a very weak predictor of heart risk, and are only very occasionally high enough to pose a cardiac risk. In Type 2, they are only moderately raised (for example, slightly higher than 1.7 mmol/l, which is considered the upper limit of ‘normal’), and this is probably more an indicator of nutritional overload and of pre-diabetes (and therefore of fatty liver) than an actual cardiac risk factor. Very high triglyceride levels (for example above 4 mmol/l) are sometimes seen in people who drink large amounts of alcohol, and there is a group of genetic disorders that can cause high levels, which in my experience in East London occurs quite often in young men of south Asian heritage. In some people, very high triglyceride levels, for unknown reasons, can attack the pancreas, resulting in painful and potentially hazardous pancreatitis, and eventually diabetes (because of the destruction of insulin-producing beta-cells in the islets).
Getting the cholesterol levels down: why bother?
The facts are simple. Reducing LDL cholesterol is more beneficial in the long term in preventing vascular diabetes complications than reducing blood glucose levels – and even than reducing blood pressure. That’s not to say LDL cholesterol is the only game in town, but we should always have our beady eyes on it.
Intensive diet (see Chapter 7) focusing on nutraceuticals known to reduce cholesterol levels may help a little. Reducing food intake in the Newcastle way will reduce triglycerides and reduce the risk of pre-diabetes and of fatty liver. But in most people these measures need support from medication – statins – in order to reduce the devastating vascular complications of Type 2, and that’s where a very long media war of attrition has been going on. But before joining the battle, let’s take a look at the evidence. First, we’ve just seen that animals and people who are at zero risk of heart disease naturally have very low levels of cholesterol and LDL. Do similar levels of cholesterol and LDL offer protection in people with Type 2?
The CARDS study (2004)
The CARDS study changed the way we thought about statin treatment in Type 2. Several studies way back in the late 1980s had already shown quite clearly that statin treatment in people who’d already had a heart attack reduced their risk of dying from another heart attack. So there was no controversy that statin treatment after a heart attack was life-saving. The medical term for treatment of this group of people who’d already had a hospital admission with a heart attack or stroke is secondary prevention – how can we prevent a second (and subsequent) event?
But the remaining question, which would apply to a much larger group of people, was whether reducing cholesterol levels in people who had not yet had a heart attack or stroke (primary prevention) could reduce the risk of either event happening in the first place. CARDS was therefore a simple study. It used the statin called atorvastatin in a low dose (10 mg daily): half the patients took the statin daily for four years while the remainder took the placebo dummy drug.
These are the blood lipid levels in the CARDS study (all values in mmol/l).
| Total cholesterol | LDL cholesterol | ||
| Value at the start of the trial | 5.4 | 3.0 | |
| At end of the trial (taking statin) | 4.0 | 1.8 | |
| Reduction | -1.4 | -1.2 |
At the start you can see the average cholesterol was not especially high – just over 5 mmol/l. Within a few months of the start of the trial, Type 2s taking atorvastatin were already having fewer vascular events (strokes and heart attacks) than those taking the placebo, but the trial had to continue until the statistics were convincing. After four years the combined risk of vascular events was reduced by 40% (see References, page 229). This was an even better result than anticipated, and from the mid-2000s nearly all Type 2s were encouraged to start long-term treatment with a statin. At the same time, the very high-risk people who’d had a heart attack or stroke were being encouraged, also on the basis of very strong evidence, that their risks would be lowered if they achieved levels similar to the ones we’ve seen in extremely low-risk groups – children and adults of native groups.
Key point: Statin treatment reduces the risks of heart attacks and strokes by nearly one-half in all people with Type 2 diabetes.
Statins, side-effects and the media battle
The only reliably effective way of getting total cholesterol levels down to 3 mmol/l and LDL to less than 2 is by regularly taking a statin. Other medications have been used, but even if they get the numbers down, they don’t do what’s really important – reduce vascular risk. Statins, like every other drug, have side-effects, but in addition to their genuine medical side-effects, the media have added to the concern about taking them. There is some evidence that when the media get into one of their periodic states over statins, they can influence people’s medication taking.
To demonstrate what effect scare stories can have, the Danes did a very clever study. Danish people, like us in the UK, are concerned about the hazards and side-effects of statins. Researchers identified negative media stories about statins, and then from their extensive national health records identified people taking statins at the time, and followed up these people to see whether they discontinued their statins – and studied any possible medical consequences.
Scarily, but not too surprisingly, heart attack rates increased by about 25%, and cardiovascular death rates by about 18% in those who had discontinued their statin. The clear message is that stopping statin treatment is a medical decision to be taken between you and your medical team, and not one that should be in the hands of a newspaper (see References, page 227). We don’t know enough individual detail about the reasons for the Danish people discontinuing statins, but it’s much more likely to be the story than actual side-effects.
However, doctors and researchers haven’t faced the problem of statin side-effects very well. They’re old drugs now, and some were introduced in the 1980s when monitoring of side-effects during and after clinical trials wasn’t as rigorous as it is now. The serious side-effects, mostly involving the muscles, became apparent very quickly, especially a very rare effect (almost literally one in a million) in which the muscles started breaking down and resulted in the kidneys shutting down. In my 35 years in the NHS I was aware of only one patient who had this serious side-effect, and he got through it without any lasting problems. Less serious muscle aches and pains are much more common, and if they are related to the statin they usually start in the first four weeks of treatment. Conversely, if they are related to the statin – we have many reasons for muscle aches and pains – then they will disappear within about two weeks of discontinuing.
The real problem isn’t the severe side-effects which are always highlighted and feared. It’s the less obvious symptoms that concern people because they continue with treatment, and since statins are lifelong treatment, even minor symptoms that go on for a long time could be cumulatively very troublesome. Because there will be no further clinical trials of statins we still don’t really know the full spectrum of mild side-effects. They include ’flu-like symptoms, stomach problems and vivid dreams; but everyone gets these from time to time, so it’s very difficult to sort out which ones are statin-related. Some people describe mild muddling of the brain (though statins definitely don’t increase the risk of Alzheimer’s – that’s been investigated in detail, and in some studies there is a lower risk of memory problems in later life in people who take statins). There’s no increased cancer risk: that’s been followed up in huge numbers of patients. But there are several non-statin drugs that may increase the risk of side-effects when taken with a statin (some blood pressure medication, for example, including the calcium channel blockers). The interaction between statins and grapefruit and grapefruit juice is well known, but occasional grapefruit is unlikely to be a problem.
Four statins are used in the UK. Simvastatin is the one that is prescribed most often, because it was used in the early major clinical trials. Atorvastatin is similar to simvastatin, but more powerful in its cholesterol-reducing effects, and many people are now started on it, rather than simvastatin, especially as its patent expired several years ago, and therefore became much less expensive. Two less widely used drugs include pravastatin (relatively weak) and rosuvastatin (powerful). They are chemically different from simvastatin and atorvastatin, metabolised in a different way and seem to be less likely to give side-effects. If you’ve developed side-effects with simvastatin or atorvastatin, it’s worthwhile changing to one of the other two.
Key point: Although all medications properly used in Type 2 diabetes help reduce long-term complications, statins are the most effective.
Other cholesterol-lowering drugs
Ezetimibe
Apart from the newly-introduced PCSK9 inhibitors (see below), ezetimibe is the only non-statin drug that both reduces LDL levels and also the risk of vascular events, but it took many years for its cardiovascular benefits to be shown in a clinical trial. It’s a fascinating drug, completely unrelated to the statins, and therefore with no statin side-effects. It works by reducing the amount of cholesterol absorbed from the gut, and is therefore similar to plant stanols/sterols in spreads and drinks (see Chapter 6). You may well ask: if there’s not very much cholesterol in most of the foods we eat, how can it have such a powerful effect? The answer is that most of the cholesterol that ends up in the gut doesn’t come from food, but is manufactured in the liver, where it passes as bile into the gall bladder which then empties into the gut. Ezetimibe works by preventing much of this own-manufacture cholesterol being absorbed. It’s less powerful than statins, reducing LDL by about 15% (compared with up to 50% reduction with statins), but in the IMPROVE-IT trial reduced heart attack and stroke rates by the amount you’d expect with this degree of cholesterol-lowering. It’s valuable in people who find it difficult to tolerate statins, and because it acts differently, it works very well in combination.
PCSK9 inhibitors
This is the most recent drug group, again completely different from statins. These drugs (examples include alirocumab and evolocumab) have to be taken by injection, much like insulin, but only every two or four weeks. (If you find these drug names impossible to pronounce, you’re no different from most doctors.) They are as effective in lowering LDL cholesterol levels as the most powerful statins at their highest doses. They are also extremely expensive, and will for the foreseeable future be restricted to people with genetically very high levels of cholesterol and a very high risk of heart disease who are unable to tolerate any statin treatment. Do they reduce the risk of heart attacks and strokes? Probably. Are they safe? Again, probably. But we’ll have to wait a good while for the ‘excitement’ that often surrounds these dramatic drugs to settle with the help of very large clinical trials, which are currently reporting.
Summary
In order to reduce the risk of vascular disease, all Type 2s need to aim for a systolic blood pressure between 130 and 140 mm, and an LDL cholesterol level under 2 mmol/l, corresponding to a total cholesterol lower than 3. Antihypertensive medication definitely reduces the risk of heart disease and stroke, so long as it’s taken regularly. For lowering cholesterol some nutraceuticals may help a little, but nearly all Type 2s need long-term statin treatment to achieve adequate cholesterol levels that will reduce the risk of vascular events, especially stroke and heart attack. Careful choice of the right statin in most cases will avoid side-effects.