Key points
- Pre-diabetes, like diabetes itself, is defined using fasting blood glucose levels – somewhere between ‘normal’ and diabetes (6.1–6.9 mmol/l).
- Most research has concentrated on reducing the risk of people with pre-diabetes progressing to diabetes (that is blood glucose levels of 7.0 or higher).
- Pre-diabetes is more than just a glucose number, and is often associated with being overweight or obese, high blood pressure and unbalanced cholesterol levels. When it clusters like this it is called the metabolic syndrome, or the insulin resistance syndrome.
- Other medical conditions associated with the metabolic syndrome include polycystic ovary syndrome, gout, obstructive sleep apnoea and even – speculatively – Alzheimer’s disease.
- The factor linking these very different conditions is insulin itself. Although insulin is important in keeping blood glucose levels under control, it has multiple actions in several organs that are separate from its role in regulating blood glucose.
Grey areas always generate big arguments
As we saw in Chapter 2, non-diabetic blood glucose levels run between 4 and 6 mmol/l (with occasional brief excursions to 8–10 immediately after big carbohydrate-containing meals). Diabetes is diagnosed once fasting blood glucose levels rise above 7 mmol/l. So what about blood glucose levels that lie slightly higher than ‘normal’ but lower than the level used to diagnose diabetes? Can glucose levels in this grey area affect health?
Grey areas in everything are often major battle grounds, and there’s no difference here. Academics fall out with each other, while major international organisations have more measured ‘disagreements’. The difficulty is understanding where ‘normality’ ends and the grey zone starts. For example, the American Diabetes Association considers any blood glucose level of more than 5.5 mmol/l to be higher than normal, while the rest of the world uses a threshold of 6.1.
The problem is that there are so many people with fasting levels of 5.5. mmol/l or just higher that if we used this number to define even mild abnormality, then probably one-third of the world’s population would be included. Currently, in most countries, fasting glucose measurements between 6.1 and 6.9 mmol/l are considered slightly high, though not quite diabetes. But is all this numerical nit-picking of any real importance to people?
Key point: Pre-diabetes is associated with a fasting glucose level of between 6.1 and 6.9 mmol/l. It indicates an increased risk of developing diabetes and therefore its complications, but by itself it should be considered a gentle warning of future trouble, and not itself a ‘disease’ that needs ‘treatment’.
Broadly speaking, the situation in pre-diabetes is the same as the one we see in confirmed diabetes. Diabetes was originally defined as a fasting blood glucose level (7.0 mmol/l), above which people can develop complications that only occur in diabetes. The easiest of these to detect is diabetic eye disease (retinopathy; see Chapter 11). So, although we define diabetes using a blood glucose level (7.0 mmol/l or higher), that number is much less important to the individual than the risk of developing complications of diabetes. Similarly, with pre-diabetes. Discussion about whether 5.5 or 6.1 is ‘abnormal’ is much less important than whether there are any associated medical problems with any values in the grey zone. There are, as we’ll discuss shortly. First, though, let’s look at the risk of developing definite diabetes, defined as a fasting glucose level of 7.0 or higher, if you have pre-diabetes.
Pre-diabetes and the risk of developing full-blown diabetes
If you have pre-diabetic blood glucose levels, can you do anything to postpone a further rise into the diabetes range? There are a number of studies that have investigated reducing the risk of progressing from pre-diabetes to diabetes and which included large numbers of individuals studied over several years. We can now benefit from the long-term results of these trials, which started in the 1980s and continued into the early 2000s. Because pre-diabetes is not a ‘disease’ or ‘illness’ itself, most of the trials used lifestyle intervention (weight loss and exercise), though there were also a few drug-based studies.
Broadly speaking – and there’s no surprise here – the trials confirm that consistent weight loss, though not always a spectacular amount, and regular exercise do indeed delay progression to diabetic blood glucose levels. Most studies combined a portfolio of both weight loss and increased exercise, so it’s not possible to separate out whether one intervention was more effective than the other. However, in a study in Chinese people (the Da Qing study – see References for this chapter, page 211) one group was given an exercise schedule (the aim was to increase daily activity by 10–30 minutes, depending on the intensity of exercise), the other encouraged to lose weight (actual weight loss was 1.5–2.5 kg). All interventions reduced the risk of progressing to Type 2 by between 30 and 45% – diet, exercise and exercise plus diet were about equally effective. We should be cautious in extending conclusions from this population (average BMI was only slightly overweight, 26) to a more overweight population, especially when it comes to recommending increased exercise as a way of reducing progression from pre-diabetes.
Key point: Consistent weight loss and increased activity levels significantly reduce the risk of progressing from pre-diabetes to diabetes. In Chinese people increased exercise alone was effective.
Can medication postpone the onset of diabetes?
One study, the Diabetes Prevention Program in the USA (see references on page 210), found that in younger people (those aged 35–40), and those who were particularly overweight, metformin – as used in the treatment of Type 2 diabetes itself – helped, to a certain extent, to reduce the likelihood of developing diabetes. However, metformin was considerably less effective than the lifestyle portfolio (6 kg weight loss over four years and increased exercise up to 150 minutes/week), and the effect only persisted while people were taking medication. After stopping the tablets, blood glucose levels went up immediately, whereas blood glucose levels remained lower in people who had been in the lifestyle group. Although weight (and therefore blood glucose) often increases after you’ve stopped dieting, it doesn’t do so suddenly.
Key point: Medication (metformin) is less effective than lifestyle in preventing the onset of Type 2 diabetes, and its effect lasts only as long as the medication is taken.
Does pre-diabetes have health consequences?
Because pre-diabetes is diagnosed using blood glucose levels, I’ve already mentioned there’s a risk we’ll become obsessed with minute differences in numbers. If you have a blood glucose level that’s in the lower pre-diabetes range, say 6.2 mmol/l, is that ‘better’ than one that’s nearly at the diabetes level, for example 6.8? If it’s lower, then you are probably farther away from developing a diabetic blood glucose level of 7, but that may not be of great importance. People remain in the pre-diabetic range for a long time, perhaps nearly the whole of their adult lifetime. So, if we’re only going to get concerned once blood glucose levels increase from 6.7 to 7.0, then we may be missing major health concerns if pre-diabetes persists for a long time. And yes, there are significant health problems that are associated with pre-diabetes. The important thing is not to regard it just as a ‘borderline’ glucose level that needs an occasional follow-up blood test. In a world obsessed with numbers and targets, this is a risk that shows no sign of going away. We should be more concerned with health outcomes that matter to people.
The clinical triallists of the 1990s and 2000s looked in a limited way at the long-term health consequences of pre-diabetes. Because the studies initially focused on blood glucose levels, the possible broader associations of pre-diabetes, especially cardiac disease, weren’t studied, so long-term blood glucose outcomes continued to be reported. In general, people who had successfully achieved weight loss and exercise goals during the trials (which usually lasted three to five years) were at lower risk of developing Type 2 when followed up for several more years. But that’s not an earth-shattering finding, as people who had done well initially with exercise and weight loss were the most likely to continue.
However, the long-term outcomes of the Chinese Da Qing study mentioned above (and see References on page 211) were truly unexpected: 20 years after the end of the trial, those who had initially done well with exercise or weight loss had a 40% lower risk of heart disease. Of course, the risk of blood glucose climbing into the diabetes range was reduced as well – by about 50%. From other studies, though, it doesn’t look as if Europeans and Americans benefit quite as much. Nevertheless, a study in Finland found that pre-diabetic people who initially managed to maintain their lower weight and better diet continued to do so, as well as having a lower risk of crossing the blood glucose threshold into diabetes.
Key point: Although exercise and weight loss reduce the risk of developing diabetic blood glucose levels, they don’t reduce the risk of developing serious diabetes outcomes such as heart disease – except in Chinese individuals. We don’t know the reasons for these ethnic differences.
The metabolic syndrome
Let’s try and think more broadly than blood glucose. If you have pre-diabetic blood glucose levels, then you are more likely to have certain other conditions associated with it. A recognised combination of conditions or features is called a ‘syndrome’. This combination has been given various names, including ‘syndrome X’ (the original title), the ‘insulin resistance syndrome’ and, probably the most descriptive term and the one we’ll use here, the ‘metabolic syndrome’. Because they are all associated with mildly abnormal blood glucose levels, people diagnosed with Type 2 diabetes usually have features of the metabolic syndrome, but there are also many people with strictly non-diabetic glucose levels who also have the metabolic syndrome.
Figure 3.1 shows the widespread impact of the metabolic syndrome on different parts of the body. Some of these associations are not obvious (acne and gout, for example), and certainly not clearly linked with higher glucose levels.
The link is thought to lie in the many roles played by insulin – completely separate from its best-known job of reducing blood glucose levels. It’s not surprising that over millions of years insulin has developed lots of different jobs, because – after all – it is the master regulator of the body’s metabolism. We mentioned this much wider and very interesting spectrum in Chapter 1. Even when blood glucose is at levels that everyone can agree are normal, abnormal insulin action poses some major health hazards years, perhaps decades, before blood glucose levels become ‘high’ – another reason not to fixate on minute differences in nearly normal glucose values.
Key point: The health consequences of the metabolic syndrome are due to insulin acting in abnormal ways that are not linked to its effect on blood glucose.
As with diabetes, the metabolic syndrome was (and still is) formally defined using simple measurements:
- Obesity (high waist circumference – for example, more than 40 inches in men or 35 inches in women. No cheating when you’re measuring it – as if. The measurement must be taken at the level of the belly button, not the hips.
- Intermediate – pre-diabetic – glucose levels (6.1 to 6.9 mmol/l).
- High blood pressure (systolic pressure more than 140 mm).
- Relatively normal cholesterol levels, but other lipid (fat) values are abnormal – for example, low protective HDL cholesterol levels, and higher triglyceride levels.
The scientific definition requires obesity together with one of the others to be present, but in real life we need to be much more flexible, because any, all or none of these might warrant treatment in an individual. I’ll discuss some of the features of the definition itself, and then go on to discuss other and less obvious clinical associations.
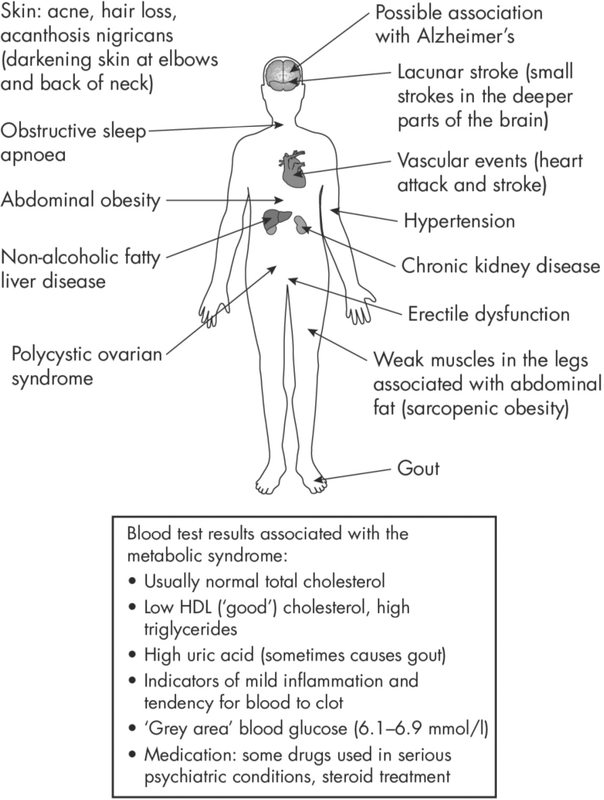
Figure 3.1: Some features of the metabolic syndrome.
Hypertension (high blood pressure)
High blood pressure is very strongly linked with both pre-diabetes and Type 2 (see Chapter 10). Embarrassingly, and in spite of decades of research, the link is mostly unexplained, but the odds are on insulin causing salt retention, one of the key features of hypertension. A good follow-on question from this would be: do hypertensive people who don’t have Type 2 have features of the metabolic syndrome? Answer: yes, many of them do. The general message is that in individuals with any features of the metabolic syndrome, we should look around for the other commonly associated features.
As discussed in more detail in Chapter 10, blood pressure is recorded using two numbers. The first (‘upper’) number, known as the ‘systolic’ blood pressure, is the only relevant one, as it reflects the peak blood pressure exerted on the major blood vessels during contraction of the heart and is therefore the measurement most strongly related to arterial damage. The definition of hypertension in the metabolic syndrome is the same as the one we use generally: systolic pressure consistently 140 mm Hg or higher.
Key point: Hypertension is a common feature of the metabolic syndrome.
Abnormal blood lipid (fat) profile
The formal metabolic syndrome definition also includes a characteristic combination, easily detected by a blood test (and often measured at the same time as total cholesterol): a low level of protective (‘good’) HDL cholesterol, which contributes to heart disease and stroke risk, and high triglyceride levels, which are more associated with food excess, especially carbohydrates. The formal definitions are triglycerides higher than 1.7 mmol/l, and HDL cholesterol lower than 1.0 mmol/l in men, and 1.2 in women. Insulin’s involvement here? Probably in the biochemical pathways in the cell where one form of fat (lipid) is converted to another. It’s worthwhile noting again that these processes have nothing to do with glucose metabolism or blood glucose levels.
Fatty liver: too much fat in the liver (and in other organs)
If you have an ultrasound scan of the abdomen for any reason, the technician or radiologist will often take at least a quick look at the liver; excess fat there is a common finding, regardless of the reason for the initial ultrasound request. ‘Fatty liver’ is the usual term, but because excess alcohol is a common cause of fat in the liver, when it isn’t a contributor (that is, alcohol intake is less than the recommended level, currently 14 units a week), it’s called ‘non-alcoholic fatty liver disease’, usually abbreviated to ‘NAFLD’. Fatty liver is rather like high blood pressure. Perhaps 30 to 40% of people with no whiff of diabetes according to glucose levels are hypertensive, but once they’ve progressed to Type 2, 80–90% have high blood pressure. The same goes for fatty liver: it’s very common in non-diabetic people, but affects about three-quarters of Type 2s.
Fatty liver/NAFLD isn’t usually considered one of the classical ‘complications’ of diabetes, which mostly affect small blood vessels and large arteries (see Chapter 11), but alarm bells about long-term (chronic) liver disease developing from fatty liver have been ringing increasingly loudly over the past few years. It starts, as we’ll see, with a metabolic abnormality – that is, insulin not doing what it should, this time in relation to fats which are manufactured in the liver. But fat that has been in liver cells for several years can set up a low level of inflammation – a non-infectious form of hepatitis. In most people the inflammation remains unchanged during their lifetime, but, for unknown reasons, in a small number the irritant effect of the inflammation stimulates the liver to try to seal off the inflammation with tough fibrous tissue. After decades, the result can be cirrhosis where all the functions of the liver – and there are lots – fail. Although only a very small proportion of people with Type 2 will progress in this way, there is concern because Type 2 is very common and large total numbers of people might be affected.
Key point: Fatty liver is detectable on ultrasound scan in many overweight people who have neither diabetic nor pre-diabetic blood glucose levels.
What causes fatty liver?
It’s worth pausing to discuss this, because it not only expands our awareness of the complications of Type 2 diabetes and pre-diabetes, but also adds to our understanding of the range of insulin’s functions that are not related to glucose levels. In fact, insulin behaves here in a similar way to how it does with glucose. You’ll remember from Chapter 1 how, in diabetic people, the liver leaks too much glucose (especially overnight) as a result of insulin not working well enough. What should be a gentle drip of the glucose tap turns into quite a big trickle. In fatty liver, though, we need to substitute a fat – triglycerides – for the glucose.
Triglycerides are synthesised from the carbohydrates we eat, and are stored (like glycogen) in the liver. Fructose, mostly now found as high-fructose corn syrup, and used as a sweetener in a huge number of manufactured products, especially non-diet soft drinks, is thought to be a particularly powerful factor in producing triglycerides. The liver itself also produces triglycerides. In people without diabetes, insulin keeps triglycerides neatly tucked away in the liver, except for very small amounts that get into the circulation from where they are used as a fuel, like glucose, to produce energy, though triglycerides do so less efficiently than glucose. In the metabolic syndrome and Type 2, insulin is less effective at preventing the liver making triglycerides, and, as in the situation with glucose, less effective at keeping it there. The liver becomes overloaded with triglycerides and related molecules, and that’s effectively the recipe for producing fatty liver.
We see this picture at its most dramatic when people with insulin-treated diabetes (usually Type 1, but also in Type 2) stop taking their insulin for whatever reason. As well as glucose leaking from the liver in large quantities, so do triglycerides. When this happens, people usually need hospital treatment for very high glucose levels, but triglycerides can be sky-high too – sometimes values of 50–100 mmol/l, compared with the usual levels of 2 mmol/l or less. It’s easy to spot: blood taken from a vein for a routine test looks creamy pink, sometimes barely red at all, because it contains so much fat. In some cases, the inflammatory effect of the high blood triglyceride level can cause pancreatitis, a severe and dangerous inflammation of the pancreas (see Figure 3.2).

Figure 3.2: Fatty liver. Triglycerides produced both from food and by the liver itself pack the liver cells. In pre-diabetes and Type 2 diabetes insulin is less effective at ensuring triglycerides do not escape from the liver into the circulation. Fatty liver and high blood triglyceride levels are common in the metabolic syndrome.
Treating fatty liver
Dramatic weight loss is the best way of reducing fat in the liver (see Chapter 4). There is optimism that bariatric surgery may be of benefit even when fatty liver disease is fairly advanced. Interestingly, high levels of physical training (for example 30–40 minutes a week for 12 weeks) reduces the amount of fat in the liver, even if there isn’t weight loss (see Chapter 8). It looks as if exercise has quite a specific effect here. There have also been several trials of drugs, many of them blood-glucose lowering drugs used in established Type 2; however, despite great hopes, none has shown improvement in the structure of the liver, which is what matters.
Key point: Exercise and weight loss are the most effective treatments for fatty liver. No drug treatment is conclusively effective.
Fat in other organs: an emerging area of interest in pre-diabetes
Although fat in the liver is easy to detect and very common, excess fat can accumulate in other organs, probably also through a combination of excess food and ineffective insulin. We’ll see in Chapter 4 that excess fat in the pancreas is now thought to be conspiring with fatty liver to account for the metabolic problems seen in Type 2 diabetes, and that substantial weight loss can dramatically reduce fat in both these key organs, with rapidly beneficial effects on blood glucose levels and the action of insulin. If fat in the liver and pancreas can have such profound metabolic effects, then can fat in other organs also have health consequences? This is a new area of research, but interest is growing. For example, excess fat around the heart can get very close to coronary arteries, and inflammation in this fat may be responsible for thickening of the coronary arteries and contribute to heart attack risk. Normal kidneys are surrounded by a substantial cushion of fat, which may not just be there to protect against trauma. Inflammation in this fat may worsen hypertension and increase protein leakage into the urine (see Chapter 11). We’ll also see in Chapter 12 that when excess fat accumulates in muscle, especially the legs, it can contribute to weakness, unsteadiness, falls and frailty in the elderly.
‘Type 3’ diabetes and a link with Alzheimer’s?
In the past few years, metabolic syndrome characteristics have been linked to a higher risk of developing Alzheimer’s disease. Research is in the very early stages and there is understandably great interest in this whole new area. There has been no shortage of dramatic headlines, to the point where ‘diabetes of the brain’ has been – prematurely – dubbed ‘Type 3’ diabetes. The brain is one of the few organs where insulin isn’t required for glucose uptake – glucose in the circulation goes straight into brain tissue so it can prioritise supplying the energy demands of billions of nerve cells, regardless of what is happening elsewhere in the body. Nevertheless, insulin is needed in the brain for a wide variety of chemical pathways, many of them involved in lipids similar to cholesterol which are needed to maintain the structure of brain cells. In other words, this is yet another example of insulin being critical to life, though definitely not through its effects on glucose. The ultimate question is whether controlling the metabolic syndrome will result in a lower risk of dementia in older age. I have no doubt that trials will be done, but the results are a good way off just yet. However, it reminds us again that the metabolic syndrome, whether or not it progresses to Type 2 diabetes diagnosed with a high glucose level, may have long-term effects on several aspects of health – some quite unexpected.
Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome is one of the conditions associated with the metabolic syndrome that is quite well understood, and abnormal insulin is clearly the culprit here. Affected women have infrequent menstrual cycles (periods) – sometimes as few as two a year – and in severe cases some degree of infertility. Cosmetically it can be very troublesome as well, as it is frequently associated with severe acne, hirsutism (excess hair on the face and body), and hair fall from the head. Many women with PCOS are overweight, but some can be very slim. Insulin action at the ovary is the problem here. It’s particularly interesting because PCOS often responds to treatment with the diabetes drug metformin, and sometimes this simple treatment restores periods and even fertility. In this instance, metformin isn’t being used to reduce blood glucose levels as it is in usual cases of Type 2, but to improve the efficiency of insulin action.
Key point: Polycystic ovary syndrome is caused by abnormal insulin action, but is frequently seen in normal-weight women who have normal glucose levels.
Finally, let’s briefly consider two conditions undoubtedly associated with the metabolic syndrome (and therefore with errant insulin action), but where the link has not yet been explained in detail: obstructive sleep apnoea and gout.
Obstructive sleep apnoea
Obstructive sleep apnoea is very common in individuals with or without diabetes. There is heavy snoring and typically breathing stops for up to 30 seconds before starting again by itself. In severe cases this cycle is repeated hundreds of times during the night. People with obstructive sleep apnoea are often drowsy during the day and are prone to dropping off to sleep – for example, during meetings, when it is embarrassing, and while driving, when the hazards are obvious. There is no doubt that obstructive sleep apnoea is associated with the metabolic syndrome, and also with severe hypertension and an increased risk of stroke; but in what way insulin and insulin resistance are involved isn’t known. Treatment with a face mask during the night, delivering air into the nose and mouth under slight pressure (continuous positive airway pressure, ‘CPAP’) can be dramatically effective in relieving symptoms and improving quality of life. It was hoped that longterm use might reduce the risk of heart attacks and strokes in sleep apnoea patients who are prone to cardiovascular disease, but a large clinical trial of people with known heart disease did not show any specific benefit after nearly seven years follow-up, possibly because CPAP treatment doesn’t consistently reduce blood pressure in these often-hypertensive people.
Gout
Gout is caused by excess uric acid in the circulation that is released near joints, especially the big toe, where it causes inflammation and, reportedly, some of the worst pain imaginable. It can affect other joints, but mostly the hands and feet. In the past it was always thought to be a particular problem of overweight old men who drank too much port, and although an attack can be brought on by drinking too much alcohol, it is very often seen in young people who don’t drink. Strangely, cherries can precipitate attacks. Because treatment is so successful, kidney failure, the worst complication of gout, and one which must have resulted in the premature death of thousands of people in the 17th and 18th centuries, is almost never seen, though kidney stones made of uric acid crystals are still very common (they can cause renal colic, another horribly agonising pain). Abnormal insulin is likely to be the culprit, which somehow increases the amount of circulating uric acid. Drug treatment is usually very effective in preventing attacks, but dietary management can help. Although gout itself is very dramatic, in most people with the metabolic syndrome, even though blood uric acid levels are often high, they are not sufficient to cause gout.
Can reducing slightly elevated uric acid levels reduce the risk of heart disease, strokes and kidney impairment? The answer isn’t known yet. Formal trials of diet, medication or combinations of these have not been done, and they’re not likely to be performed because of the long investigation period, because of the large numbers of participants needed, and because the prototype drug for reducing blood uric acid levels – allopurinol – is now over 60 years old, and so successful (and cheap) that only one additional medication has been launched. However, in people with uric acid kidney stones, careful combinations of medication, diet and specific fluids can be dramatically successful in reducing stone formation.
Other treatments for the metabolic syndrome
A few years ago, there was a flurry of interest in a fatty acid – conjugated linoleic acid – available as a health food supplement for the metabolic syndrome and to help weight loss. One short study showed no benefit, but there’s no shortage of speculative ‘fat busters’, or tablets and electrical treatments for ‘boosting metabolism’, none of which has a whiff of a proper clinical trial to support its use. Sadly, the same goes for the usual round of highly specific alternative treatments: coconut oil, cayenne pepper, grape seed extract and spinach, among others.
Summary
Formally defined, the metabolic syndrome comprises pre-diabetic blood glucose levels that are not quite normal, but are not yet at levels that define diabetes, together with elevated blood pressure, large waist size and abnormal levels of non-cholesterol lipids (HDL cholesterol and triglycerides). In people with this ‘narrow’ definition of the metabolic syndrome, some medications and stringent lifestyle interventions can help reduce the rise in blood glucose levels, and weight loss and exercise may have a legacy effect on cardiovascular events that persists for many years after clinical trials have finished. The wider scope of medically relevant conditions encompassed by the metabolic syndrome is increasing; these conditions are not usually associated with pre-diabetes, but all in some way involve abnormal actions of insulin.