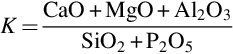From NORM by-products to building materials
J. Labrincha*,a; F. Puertas†,a; W. Schroeyers‡; K. Kovler§; Y. Pontikes¶; C. Nuccetelli**; P. Krivenko††; O. Kovalchuk††; O. Petropavlovsky††; M. Komljenovic‡‡; E. Fidanchevski§§; R. Wiegers¶¶; E. Volceanov***,†††; E. Gunay‡‡‡; M.A. Sanjuán§§§; V. Ducman¶¶¶; B. Angjusheva§§; D. Bajare****; T. Kovacs††††; G. Bator††††; S. Schreurs‡; J. Aguiar‡‡‡‡; J.L. Provis§§§§ * University of Aveiro, Aveiro, Portugal
† Eduardo Torroja Institute for Construction Sciences (IETcc-CSIC), Madrid, Spain
‡ Hasselt University, CMK, NuTeC, Diepenbeek, Belgium
§ Technion – Israel Institute of Technology, Haifa, Israel
¶ KU Leuven, Leuven, Belgium
** National Institute of Health, Rome, Italy
†† Kiev National University of Construction and Architecture, Kyiv, Ukraine
‡‡ The University of Belgrade, Belgrade, Serbia
§§ Ss Cyril and Methodius University in Skopje, Skopje, Macedonia
¶¶ IBR Consult BV, Haelen, Netherlands
*** Metallurgical Research Institute - ICEM SA, Bucharest, Romania
††† University POLITEHNICA Bucharest, Bucharest, Romania
‡‡‡ TUBITAK MRC, Kocaeli, Turkey
§§§ Spanish Institute of Cement and its Applications, Madrid, Spain
¶¶¶ Slovenian National Building and Civil Engineering Institute (ZAG), Ljubljana, Slovenia
**** Riga Technical University, Riga, Latvia
†††† University of Pannonia, Veszprém, Hungary
‡‡‡‡ University of Minho, Guimarães, Portugal
§§§§ University of Sheffield, Sheffield, United Kingdom
a Two equal first authors: coordinators of this chapter.
Abstract
The cementitious materials and ceramic industries are frequently looked as targets for the recycling and valorization of several wastes, residues, and by-products, generated from a wide variety of industries. In general, only technical (and chemical) aspects are covered on each attempt for recycling a waste in a particular product, while radiological features are rarely considered. This chapter aims to give new and more complete insights on the recycling of several industrial wastes, on four groups of construction materials: (1) construction materials based on Portland cements (both as cement itself and as concrete), (2) construction materials based on alkali-activated binders, (3) ceramics and glass-ceramics, and (4) gypsum.
For each by-product a separate section will describe (1) the technical (and chemical) aspects of the use as part of a construction material and (2) the resulting radiological properties of the designed product, when they are available.
Some ceramic industries also use radiologically active components, namely zircon and zirconia (in glazes, refractories, etc.). The radiological consequences of their production, further use/manipulation by other industrial sectors (e.g., ceramic glazes and frits production), and on the final costumers are also briefly reported.
Keywords
Cement; Concrete; Geopolymer; Ceramic; Phosphogypsum; Activity concentration index; By-product recycling
7.1 Introduction
7.1.1 Recycling of industrial by-products in building materials
The cementitious materials and ceramics industries are excellent targets for the recycling and valorization of some wastes, residues, and by-products, from a wide variety of industries.
Given the environmental challenges inherent in Portland cement manufacture (high thermal and electrical energy demand, need to quarry large quantities of limestone and clay and the emission of greenhouse gases, especially CO2), the study and development of cements based on the reuse of waste of varying origin is a priority line of research and technological innovation in the pursuit of industry sustainability. A broad range of types of waste can be used in blends with Portland cements, representing an environmentally friendly and clinker-saving way of production.
Of the 27 types of cement listed in the European Standard EN 197-1:2011 (Sanjuán and Argiz, 2011), 26 contain some manner of mineral addition which can include industrial residues such as siliceous or calcareous fly ash, blast-furnace slag, or silica fume. All of the aforementioned additions are industrial by-products and dependent on the content of natural radionuclides some of them are listed as naturally occurring radioactive materials (NORMs). The trend of industrial by-product recycling is expected to continue. The draft of the common cements standard prEN 197-1:2016 includes five new cement subtypes with higher amounts of by-products; in particular, siliceous fly ash and blast-furnace slag. In addition to the earlier, new potential cement constituents are being explored, such as ground coal bottom ash, paper sludge ash, silicon-manganese slag, copper slag, and so on (Argiz et al., 2013; Vegas et al., 2006; Sabador et al., 2007; Frias et al., 2006; García Medina et al., 2006; Siddique, 2003).
Industrial waste and by-products are used not in blends with Portland cement, but may also be added during clinkerization itself, partially or totally replacing the virgin raw materials in the raw meal (limestone in particular) or contributing as secondary fuel. Very different types of waste or by-products can be used as partial raw meal replacements, including crystallized blast-furnace slag (Puertas et al., 1988), waste from the manufacture of clay-based products (Puertas et al., 2010), aluminum recycling (Paval) (Blanco-Varela et al., 2000), etc. Efforts are also being made to use alternative fuels in OPC production: in countries such as the Netherlands, Austria, Germany, and Norway, these fuels account for over 60% of the total. The sources vary widely in nature, including shredded tires, solvents, water treatment plant sludge and used oil, among others (Pontikes and Snellings, 2014).
Another avenue for manufacturing eco-efficient cements is the development of new materials wholly different from ordinary Portland cement. Due to their mechanical and durability properties, versatility alkali-activated cements (also known as geopolymers) are among the most prominent of these new materials (Palomo et al., 2014). These cements are defined as the binders resulting from the chemical interaction between alkaline solutions and natural (clay; possibly thermally treated) or the result of human activity (industrial waste or by-products) aluminosilicates with a high- or low-Ca content, possibly having also Fe. Alkaline activation calls for two basic components: (1) a solid precursor that is prone to dissolution (most often amorphous or vitreous) and (2) an alkaline activator. The aluminosilicates may be natural products such as metakaolin or industrial by-products such as blast-furnace slag or aluminosiliceous fly ash. The alkaline solutions able to interact with aluminosilicates to generate such new binders include alkaline metal or alkaline-earth hydroxides (ROH, X(OH)2), weak acid salts (R2CO3, R2S, RF), strong acid salts (Na2SO4, CaSO4·2H2O), and R2O(n)SiO2-type siliceous salts known as waterglass (where R is an alkaline ion such as N, K, or Li). From the standpoint of end product strength and other properties, the most effective of these activators are NaOH, Na2CO3, and sodium silicate hydrate (waterglass). Industrial by-products are presently also being studied for use as possible alkaline activators. Patents have been awarded for the use of industrial waste or by-products such as ash from rice husks, silica fume, and urban and industrial vitreous waste as potential alkaline activators to replace the family of substances known as water glass (Puertas and Torres-Carrasco, 2014). Here also, the main components of these cements may be NORMs.
The foregoing is indicative of the high potential for reuse and valorization of industrial waste and by-products in the manufacturing of cement and other construction materials. To be apt for such purposes, the waste must exhibit certain chemical, physical, and microstructural characteristics that favor their reactivity and behavior. Next to the binder described so far, the aggregates to be used for mortar and concrete production can also be residues and NORM in particular. Considering that they could be used in a proportion close to 80% in concrete volume, they might have a substantial contribution in the concentration of radionuclides in the final building materials.
The main ceramics which are produced using raw materials that can contain enhanced concentrations of natural radionuclides are refractories as well as tiles in which zirconia (the main source of natural radionuclides) is mixed with other constituents. In refractories, the applications cover the production of either prefabricated units (bricks) or the use as a mortar for in situ applications, for example, in kilns. Not every zirconia can be considered as NORM and in some cases only smaller amounts of zirconia are used, hence, not every refractory has enhanced levels of natural radionuclides. This is controlled by the composition of the refractory, which depends on the required properties in terms of temperature, chemical corrosive circumstances, and whether abrasion is an issue. Refractories with enhanced levels of natural radionuclides can be found in the glass industry (kilns) and sometimes in the ceramic brick or tiles kilns. Zirconium is also a common opacifier of ceramic glazes. In general, the glazes show activity concentrations below 1 kBq/kg for the main natural radionuclides, and only their production deserves control.
Other areas where significant amounts of by-products, such as fly ash, mining tailings, etc., are incorporated are clay-based formulations, ceramic bricks for example. Despite the often notable amount of by-products employed, the concentration of natural radionuclides in such ceramic materials is, in general, similar to that of common ceramic bricks.
7.1.2 Radiological consideration for recycling of industrial by-products in building materials
As discussed in Chapter 4, in Council Directive 2013/59/Euratom a reference level of 1 mSv/year is applied to indoor external exposure to gamma radiation emitted by building materials, in addition to outdoor external exposure (EU, 2014). Therefore, Member States shall ensure that the activity concentrations of the radionuclides are determined (control the external exposure with respect to the reference level) before the materials listed below are placed on the market for use in buildings:
(b) Building materials or additives of natural igneous origin, such as:
• granitoides (such as granites, syenite, and orthogneiss);
• porphyries;
• tuff;
• pozzolana (pozzolanic ash); and
• lava.
(2) Materials incorporating residues from industries processing NORM, such as:
• phosphogypsum;
• phosphorus slag;
• tin slag;
• copper slag;
• red mud (residue from aluminum production); and
• residues from steel production.
To comply with the Council Directive 2013/59/Euratom requirements Member States shall arrange control measures with regard to their emitted gamma radiation. For screening and evaluation of building materials the Council Directive 2013/59/Euratom uses an activity concentration index (IBSS):
where CRa-226, CTh-232, and CK-40 are the measured activity concentrations (Bq/kg) for, respectively, 226Ra, 232Th, and 40K (EU, 2014).
The activity concentration index and the legislative aspects are discussed in more detail in Chapter 4. It needs to be kept in mind that the activity concentration index is only a screening parameter. In case a value of IBSS>1 is found for a given building material, then it needs to be verified that, upon use in a building, the exposure to gamma radiation is less than the reference level of 1 mSv/year (which is the real criterion for evaluation of building materials). In this chapter the activity concentration index proposed by the Council Directive is used to screen the content of natural radionuclides in several building materials.
The activity concentration index is used only for building materials (or for their constituents if the constituents are also building materials) (EU, 2014). In this chapter (and in Chapter 6) also the “activity concentration index for by-products” is considered, but this purely for the purpose of dilution calculations in order to support the discussion of building materials incorporating a given by-product. Using an “activity concentration index for by-products” would in theory mean that the by-product itself is used (for 100%) as a building material which for most by-products is an unrealistic scenario. In this way the extreme case of NORM by-product incorporation in building materials is discussed. The reported activity concentration indexes are calculated on the basis of the activity concentration for 226Ra, 232Th, and 40K from different literature references. The original values for these activity concentrations are also reported in Chapter 6.
The overall radiation hazard due to ionizing radiation from building materials includes both a gamma radiation component, which depends on their radionuclides content, and a component caused by their radon exhalation. However, most of the standards in the world, which regulate radioactivity of building materials, address the gamma radiation only, and do not require even to test the product for radon exhalation. The evaluation of the excess dose caused by building materials for the radon pathway is indeed rather complicated (Markkanen, 2011). One of the reasons is that the actual correlation between the monitored quantity and radon exhalation rate measured in laboratory and the excess indoor radon concentration on site might be rather poor. Numerous factors, such as temperature (both indoors and outdoors), air pressure and humidity fluctuations, total porosity, pore distribution and pore type (open or close), surface treating done at the building site or type of the coating material applied, influence significantly radon exhalation in dwellings. Finally, it is extremely difficult to take into account the effect of the inhabitant behavior influencing directly air exchange rate in living spaces. That is why most of the standards regulating radioactivity of building materials address the radon exhalation in a very simplified form—through the limitation of 226Ra—the precursor of 222Rn in the 238U radioactivity chain (Kovler, 2011). At present only two national standards (Austrian Standard ÖNORM S 5200 and Israeli Standard SI 5098) address radon exhalation from building products, considering 226Ra activity concentrations, radon emanation coefficient, density and thickness of the product. The detailed review of the standards regulating natural radioactivity of building materials is available in Chapter 4.
In reality, typical excess indoor radon concentration due to building materials is low: not higher than 20 Bq/m3 (Kovler, 2009), which is only 7% of the reference value introduced in the Council Directive 2013/59/Euratom (300 Bq/m3). In other words, radon cannot “compete” by its contribution with the underlying soil, which is correctly considered the most important source of indoor radon. At the same time, the building materials may also be an important source. For example, about 300,000 dwellings with walls made of lightweight concrete based on alum shale (the so-called “blue concrete”) were built between 1929 and 1975 in Sweden (Mjoenes and Aakerblom, 2001). The radon concentrations in these houses can reach 1000 Bq/m3 under low ventilation rate, while the building occupants can get an annual effective dose of 4 mSv/year—only from building material. In addition, the main part of indoor radon at the upper floors of a building originates also from building materials.
The Council Directive 2013/59/Euratom does not provide a guideline that deals separately with the radon exhalation/emanation from only the building materials. In Article 74, dealing with indoor exposure to radon, member states are expected to “promote action to identify dwellings, with radon concentrations (as an annual average) exceeding the reference level” (300 Bq/m3). In other words, radon is regulated at the level of dwellings and no distinction is made between the building materials and the soil as sources of radon. Radon exhalation/emanation is dealt with in this chapter when information is available.
7.2 Portland cement and concretes
7.2.1 Introduction
The beneficial utilization of some industrial by-products in improving the technical, environmental, and cost profiles of fresh and hardened concrete is recognized. By-products such as coal fly and bottom ash, silica fume, and ground-granulated blast-furnace slag are well-known cement constituents in blended cements and also can be added in different proportions to concrete as mineral admixtures. Some others, such as copper slag and coal bottom ash, are being used mainly as concrete aggregates.
New by-products and waste materials are being generated by various industries and the disposal of these residues raises sustainability questions. The use of some waste materials in the cement and concrete industry is a promising alternative.
7.2.2 Coal fly ash
7.2.2.1 Technical properties
The recycling of fly ash (in particular, in concrete construction) has become increasingly important in recent years due to increasing landfill costs and current interest in sustainable development. Coal fly ash is a well-known cement constituent and concrete additive (Argiz et al., 2015; Kovler, 2017). A lot of information can be found at the website of the ECOBA (European Fly Ash Association): Fly ash was successfully used in concrete around the world for the last 50 years. In the United States more than six million tons and in Europe more than nine million tons are used annually in cement and concrete.
Coal fly ash is classified into two main groups: class F and class C fly ash. When the sum of SiO2+Al2O3+Fe2O3 is higher than 70 wt% they are classed as F-type. If not, then they belong to class C (Argiz et al., 2015). In both cases, fly ash consists of fine particles that could contain some heavy metals and natural occurring radionuclides. Its management remains a major challenge all over the world. However, the utilization of fly ash is technically feasible in the cement industry. There are essentially two main applications for fly ash in cement production, (1) first as a raw material to produce Portland clinker and (2) second as a mineral or pozzolana addition. Fly ash can be added to the Portland cement clinker as a pozzolanic constituent in the production of CEM II, Portland-composite cement, CEM IV, Pozzolanic cement or CEM V, Composite cement. Table 7.1 shows the ten cement types according to the European Standard EN 197-1:2011, which are CEM II/A-V, CEM II/B-V, CEM IV/A, CEM IV/B, CEM V/A, and CEM V/B, the amount of fly ash in the cement goes from 6% to 55% (6%–20%, 21%–35%, 11%–35%, 36%–55%, 18%–30%, and 31%–49%, respectively). Incorporation of high amounts of fly ash leads to a decrease in the early strength of cement due to the early low reactivity of fly ash, which could be improved by grinding.
Table 7.1
Fly ash in common Portland cements with K, Clinker; S, Slag; D, Silica Fume; P, Natural Pozzolan; Q, Industrial Pozzolan; V, Siliceous fly ash; W, Calcareous fly ash; T, Burnt Shale; L and LL, Limestone (in LL TOC content ≤0.20 wt% and in L TOC content ≤ 0.50 wt%)
| Main types | Designation | Composition (wt%) | |||||||||||
| Main constituents | |||||||||||||
| Clinker K | S | D | Pozzolan | Fly ash | T | Limestone | Minor constituents | ||||||
| P | Q | siliceous V | calcareous W | L | LL | ||||||||
| CEM II | Portland-fly ash cement | CEM II/A-V | 80–94 | – | – | – | – | 6–20 | – | – | – | – | 0–5 |
| CEM II/B-V | 65–79 | – | – | – | – | 21–35 | – | – | – | – | 0–5 | ||
| CEM II/A-W | 80–94 | – | – | – | – | – | 6–20 | – | – | – | 0–5 | ||
| CEM II/B-W | 65–79 | – | – | – | – | – | 21–35 | – | – | – | 0–5 | ||
| Portland-composite cement | CEM II/A-M | 80–94 | <- - - - - - - - - - - - - - 6–20 - - - - - - - - - - - - - -> | 0–5 | |||||||||
| CEM II/B-M | 65–79 | <- - - - - - - - - - - -21–35 - - - - - - - - - - - - - - - -> | 0–5 | ||||||||||
| CEM IV | Pozzolanic cement | CEM IV/A | 65–89 | – | <- - - - - - - 11–35 - - - - - - - - -> | – | – | – | 0–5 | ||||
| CEM IV/B | 45–64 | – | <- - - - - - - - 36–55 - - - - - - - -> | – | – | – | 0–5 | ||||||
| CEM V | Composite cement | CEM V/A | 40–64 | 18–30 | – | <- - - - 18–30 - - - -> | – | – | – | – | 0–5 | ||
| CEM V/B | 20–38 | 31–50 | – | <- - - - 31–50 - - - -> | – | – | – | – | 0–5 | ||||

The use of coal fly ash in blended cements is increasing because it improves some properties of concrete (Argiz et al., 2015). The pozzolanic activity of coal fly ash contributes to increased strength at later ages when the concrete is kept moist. In addition, it leads in general to a lower water demand of the concrete for a given workability. This means a decrease in the water-cement ratio and capillary porosity and reduces bleeding. It also provides a low heat of hydration which is recommended in mass concrete applications to minimize cracking at early ages. Finally, coal fly ash cement in concrete means less concrete permeability as a result of producing a dense material. Given that, it provides a high-concrete resistance to sulfate ions attack, chloride ingress into the concrete, frost-thaw cycles, and alkali-silica reaction.
Typical applications of concretes made of coal fly ash cements are roller compacted concrete (RCC), which is a wide spread practice, such as in roads and dams' construction, road subbase, and soil stabilization. Also, coal fly ash has been employed as a lightweight aggregate in construction, an aggregate filler, a bituminous pavement additive, and a mineral filler for bituminous concrete (Blanco-Varela et al., 2000).
Some concrete plants produce concrete with coal fly ash as a mineral additive replacing partially Portland cement (because of pozzolanic properties of fly ash contributing in strength and durability of concrete). European Standard EN 206-1 regulates the replacement of cement with fly ash. For example, 1 kg of cement can be replaced by 2.5 kg of fly ash keeping the durability-related properties (or strength) of concrete unchanged. At the same time, the standard sets a maximum limit for such replacement, because pozzolanic reaction of fly ash occurs only, if calcium hydroxide, which is one of the products of cement hydration, is available. In other words, a presence of a minimum content of cement to trigger pozzolanic reaction of fly ash is vital. EN 206-1 allows replacing maximum 33% and 25% of cements CEM I and CEM II, respectively. If a greater amount of fly ash is used, the excess shall not be taken into account for the calculation of the replacement of cement.
Except a part of cement, fly ash can successfully replace also a part of sand, namely—its fine fraction. The partial replacement of sand in concrete becomes especially important nowadays for several countries, which suffer from the lack of high-quality quartz sand. Fly ash as a replacement of fine sand improves workability and pumpability of fresh concrete mixes. As a partial replacement of sand, fly ash can be introduced in normal-weight concrete mixes by much larger amount, than replacement of cement.
The total content of fly ash in normal-weight concrete mix at the level of 120 kg/m3 is typical, although high-volume fly ash (HVFA) concrete compositions, which were introduced in order to maximize recycling of fly ash in concrete construction, are known (ACI, 2014). LEED (the abbreviation of Leadership in Energy and Environmental Design) promotes the use of HVFA concrete, which contains up to 40% of fly ash in cement or concrete (PCA, 2005).
The following example adapted from Kovler (2011) demonstrates a typical replacement of both cement and sand by fly ash. Two concrete mixes, the reference concrete and concrete containing 120 kg/m3 of fly ash as a partial replacement of both cement and fine aggregates, are manufactured in the same concrete plant from the same raw materials—Portland cement and aggregates. Concrete compositions are shown in Table 7.2. In this example 30 kg/m3 of cement is replaced with 30×2.5=75 kg/m3 of fly ash, while the rest of fly ash (120–75=45 kg/m3) replaces a part of sand. Total content of fine materials (cement+fly ash) in this concrete mix remains constant, which guarantees the same consistency of fresh concrete at the given water content. With this replacement the main properties of concrete in both fresh and hardened states are assumed to perfectly meet the design specifications.
Table 7.2
Example of mix design for concrete with and without fly ash (kg/m3)
| Raw materials | Reference concrete | Concrete with fly ash |
| Cement | 300 | 270 |
| Coarse aggregates | 1200 | 1200 |
| Fine aggregates | 700 | 610 |
| Fly ash | – | 120 |
| Water | 150 | 150 |
| Total | 2350 | 2350 |
A clear trend is observed in the last years in construction field: the concrete grade gradually increases, because of the need to design buildings and structures for higher loads (e.g., high-rise buildings, bridges, public buildings with large span, etc.), while economizing raw materials, which results in selecting thinner cross-sections for load-bearing elements. This trend results in a gradual increase in the content of cementitious materials. These materials contain often supplementary cementitious materials, such as coal fly ash. In parallel, the uses of coal fly ash in concrete mixes as a partial replacement of either cement or sand (or both) become more and more versatile. Considering this trend, the following typical compositions seem to serve as better basis for estimating the radiological properties of modern concrete.
In Table 7.3 a typical mix design for different modern concrete compositions with and without fly ash is given. This example will be further discussed in the discussion of the radiological aspects.
Table 7.3
Example of mix design for modern concrete compositions with and without fly ash (kg/m3)
| Raw materials | Reference concrete (no FA) | Concrete containing FA as partial replacement of sand | Concrete containing FA as partial replacement of cement and sand | HVFA (high-volume fly ash) concrete |
| Cement | 400 | 360 | 320 | 160 |
| Coarse and fine aggregates | 1850 | 1800 | 1750 | 1700 |
| Fly ash as a partial replacement of cement | 0 | 0 | 50 | 40 |
| Fly ash as a partial replacement of sand | 0 | 90 | 80 | 180 |
| Water | 150 | 150 | 150 | 140 |
| Total | 2400 | 2350 | 2350 | 2220 |

7.2.2.2 Radiological properties
Coal fly ash acts as a source of gamma radiation in concrete due to the presence of the radionuclides 226Ra, 232Th, and, to a lesser extent, 40K. An overview of the activity concentrations of 226Ra, 232Th, and 40K in coal fly ashes produced in several countries is given in Chapter 6. Several authors (Kovler, 2012; Kovler et al., 2005; Chinchón-Payá et al., 2011) measured relatively higher levels of natural occurring radionuclides in coal fly ash, which is currently used in Portland cements and concretes. By contrast, the radon exhalation is controversial because of the low emanation coefficient from the coal fly ash particles, which are generated under high temperatures in coal-firing thermal plants at the process of coal combustion (Kovler et al., 2004). The coal fly ash particles have dense glassy structure, which prevents radon atoms from escaping into the surrounding cement matrix. In spite of the fact that coal fly ash participates slowly in pozzolanic reaction, and then may contribute in radon emanation, like the resulting calcium silicate hydrates, this is neutralized by the strengthening of the overall structure of cementitious matrix, which is accompanied by lowering density and reduction of radon exhalation rate of concrete with time. Drying of concrete in time is another factor reducing radon exhalation of Portland cement—fly ash concrete. These processes are described and discussed in detail by Kovler (2012).
The concentration of natural radionuclides in the resulting Portland cement will be decreased (relative to the material of origin the coal fly ash) since depended on the type of Portland cement (see Table 7.1) only a limited percentage of fly ash (up to 55 wt% of fly ash for pozzolanic cement) can be used. Therefore, recycling of coal fly ash in blended cements can have high environmental and safety advantages.
For estimating the values of I-index calculated by Eq. (7.1) of typical concrete compositions containing coal fly ash, typical activity concentrations reported by Trevisi et al. (2012) for Portland cement and European average soil (as the first approximation of the aggregates, both coarse and fine) are used. As far as coal fly ash is concerned, the minimum and maximum I-indexes (reported for coal fly ash in Chapter 6) will serve as a good assumption representing the variability of the radiological properties of this by-product.
As clearly stated in the Council Directive 2013/59/Euratom, the index should apply to the building material (concrete in our case), and not to its constituents (unless the constituents are also building materials), such as cement, aggregates, or coal fly ash. At the same time, I-index values for concrete constituents calculated by Eq. (7.2) make calculation of the overall I-index of concrete easier. Typical activity concentrations of concrete constituents for the calculation of the I-index value of modern concrete compositions containing coal fly ash are given in Table 7.4.
Table 7.4
Typical activity concentration index for concrete constituents in modern concrete compositions containing coal fly ash
| Raw materials | Typical activity concentrations [226Ra; 232Th; 40K] (Bq/kg) | I-index | Reference |
| Cement | [45; 31; 216] | 0.38 | Trevisi et al. (2012) |
| Aggregates | [36; 34; 483] | 0.45 | Trevisi et al. (2012) |
| Coal fly ash (Ireland) | [26, 11, 70] | 0.2 | Minimum value (Table 3 of Chapter 6) |
| Coal fly ash (Greece) | [469; 40; 349] | 2.2 | Maximum value (Table 3 of Chapter 6) |

The I-index calculation is based on European Commission 1999; Radiation Protection 112; Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; and Directorate-General, Environment, Nuclear Safety and Civil Protection.
Note that the I-index, as proposed by Council Directive 2013/59/Euratom, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product or cement makes the unrealistic assumption that 100% of the by-product or cement is used as a building material.
The activity concentration index of the hydrated Portland cement-based concrete is slightly lower than in the anhydrous form because of the presence of water (Puertas et al., 2015a,b).
The results of the I-index calculation of modern concrete compositions containing coal fly ash are given in Table 7.5.
Table 7.5
I-index of modern concrete compositions containing coal fly ash
| Raw materials | Reference concrete (no FA) | Concrete containing FA as partial replacement of sand | Concrete containing FA as partial replacement of cement and sand | HVFA (high-volume fly ash) concrete |
| Maximum activity of fly ash (I=2.2) | 0.41 | 0.48 | 0.51 | 0.59 |
| Minimum activity of fly ash (I=0.2) | 0.41 | 0.40 | 0.40 | 0.39 |

Taking into account that HVFA concrete is mainly used in infrastructure, but rarely applied in dwellings and other inhabited buildings, we can conclude that the I-index unlikely exceeds half of the control value (I=1). In other words, the introduction of coal fly ash into concrete mix does not lead to significant increase of gamma doses. In parallel, radon emanation of concrete, especially in the mixes containing coal fly ash as a partial replacement of sand, is usually reduced, compared with the reference concrete (Kovler, 2012, 2017). Therefore, recycling of coal fly ash in concrete construction does not represent a radiological concern.
7.2.3 Coal bottom ash
7.2.3.1 Technical properties
Coal bottom ash is generated together with fly ash in the boiler of coal-fired power plants. Therefore, its chemical composition is in many cases quite similar. However, as discussed in Chapter 6, there are important differences related to the concentrations of the incorporated trace elements, such as the naturally occurring radionuclides of concern. In addition, there are important differences in the concentrations of (partly-) volatile species, for example, alkali metals.
Most of the scientific papers published regarding studies performed on coal bottom ashes suggest its use as artificial aggregates in road bases (Churcill and Amirkhanian, 1999) and only few attempts deal with their pozzolanic properties in blended cements (Cheriaf et al., 1999; Argiz et al., 2013; Bajare et al., 2013; Bumanis et al., 2013).
7.2.3.2 Radiological properties
For the use of coal fly ash as an aggregate in road bases currently (Jan. 2017), no European directives exist. Therefore, there are no limitations from a radiological point of view in most European Member States.
In some member states, like in Sweden, a specific index for bulk incorporation in construction materials for roads and play grounds is defined (Markkanen, 1995). More information on this specific index can be found in Section 4.5.
In the unlikely case of the use of coal bottom ash in building materials then the tables with activity concentrations given in Section 6.3.1.2 can be used for the evaluation for this specific application.
7.2.4 Slags from iron and steel production
7.2.4.1 Technical properties
Slag is a by-product from the pyrometallurgical processing of various ores. The characteristics of both ferrous (steel and blast-furnace Fe) and nonferrous (Ag, Cu, Ni, Pb, Sn, and Zn) slag must be known in order to assess its possible reuse as building material. The characteristics of slag depend on the metallurgical processes that form the material and will influence its classification as waste or as a reusable product. The properties of different types of slag are discussed in detail in Chapter 6.
Ground-granulated blast-furnace slag (GGBFS) is the main by-product from iron production used in construction. Ground-granulated blast-furnace slag is also a well-known cement constituent and concrete addition. Table 7.6 shows the nine cement types according to the European Standard EN 197-1:2011.
Table 7.6
Use of blast-furnace slag in the common Portland cements
| Main types | Designation | Composition (wt%) | |||||||||||
| Main constituents | Minor constituents | ||||||||||||
| Clinker | S | D | Pozzolan | Fly ash | T | Limestone | |||||||
| K | P | Q | V | W | L | LL | |||||||
| CEM II | Portland-slag cement | CEM II/A-S | 80–94 | 6–20 | – | – | – | – | – | – | – | – | 0–5 |
| CEM II/B-S | 65–79 | 21–35 | – | – | – | – | – | – | – | – | 0–5 | ||
| Composite cement | CEM II/A-M | 80–94 | <- - - - - - - - - - - - - 6–20 - - - - - - - - - - - -> | 0–5 | |||||||||
| CEM II/B-M | 65–79 | <- - - - - - - - - - - - - 21–35 - - - - - - - - - - - -> | 0–5 | ||||||||||
| CEM III | Blast-furnace cement | CEM III/A | 35–64 | 36–65 | – | – | – | – | – | – | – | – | 0–5 |
| CEM III/B | 20–34 | 66–80 | – | – | – | – | – | – | – | – | 0–5 | ||
| CEM III/C | 5–19 | 81–95 | – | – | – | – | – | – | – | – | 0–5 | ||
| CEM V | Composite cement | CEM V/A | 40–64 | 18–30 | – | <- - - 18–30 - - -> | – | – | – | – | 0–5 | ||
| CEM V/B | 20–38 | 31–50 | – | <- - - 31–50 - - -> | – | – | – | – | 0–5 | ||||

Pelletized blast-furnace slag resulted from cooled blast-furnace slag. It has a vesicular texture and it is used as a lightweight aggregate and finely grounded as cementitious material.
Air-cooled blast-furnace slag is naturally cooled with moderate sprinkling. The crystallized slag, after crushing, sieving, and removing magnetic matter, can be used as construction aggregate, concrete bricks, road bases, and surface and Portland clinker production raw material.
Steel slag is a by-product of the steel-making process formed from the reaction of flux such as calcium oxide with the inorganic nonmetallic components present in the steel scrap. The two main types of steel slag which can be used for construction are basic oxygen furnace (BOF) steel slag and electric arc furnace (EAF) steel slag. Both types of steel slag are commonly blended with ground-granulated blast-furnace slag, coal fly ash and lime to form pavement material, skid resistant asphalt aggregate and unconfined construction fill.
BOF slags have variable compositions depending on the particularities of the metallurgical process followed and the iron ores used. In general, these slags are being used in low-end applications as their management is typically not a priority. In more detail, the main fields of application of BOF slag are aggregates for road construction (Guttm et al., 1967; Everett and Gutt, 1967) (in bound and unbound mixtures), structural fills, hydraulic engineering, fertilizers, waste water treatment, and internal use in the blast furnace.
Apart from the low-end applications, BOF slags are also used as hydraulic binders in combination with other materials. In Europe, BOF slags are mixed with GGBFS and hydraulic road binder are delivered for the stabilization of the road surfacing and upper and lower layers of the roadbed.
Studies into utilizing slag as concrete aggregates, carried out by several researchers, have shown that these slag-aggregate concretes were stronger in compressive strength than plain concrete. However, slag-aggregate concrete was found to be more vulnerable to sulfate attack in aggressive environments.
The possible utilization of slag as a building material has to be explored and the radiological impact of steel slag should be examined carefully as well during all the stages of its life cycle.
In Table 7.7 a typical mix design for different modern concrete compositions with and without blast-furnace slag is given. This example will be further discussed in the discussion of the radiological aspects.
Table 7.7
Example of mix design for modern concrete compositions with and without blast-furnace slag (kg/m3)
| Raw materials | Reference concrete (no FA) | Concrete containing slag as partial replacement of cement | Concrete containing slaga as a partial replacement of cement and concrete |
| Cement | 400 | 80 | 80 |
| Slag as partial replacement of cement | 0 | 320 | 320 |
| Coarse and fine aggregates | 1850 | 1850 | 1450 |
| Slag as partial replacement of aggregates | 0 | 0 | 400 |
| Water | 150 | 150 | 150 |
| Total | 2400 | 2400 | 2400 |

a In this assumption, for a realistic replacement, two different types of slag are to be used in order to replace both cement and aggregates.
7.2.4.2 Radiological properties
Residues from iron and steel production that are used in Portland cement and concretes can contain elevated levels of natural occurring radionuclides (Puertas et al., 2015a,b; Trevisi et al., 2012; Piedecausa et al., 2011a,b). An overview of the radiological properties of the residues is given in Chapter 6. As mentioned before ground-granulated blast-furnace slag is the main by-product from iron production that is currently used as a well-known cement constituent and concrete additive.
In Portland cement, depending of the type of cement larger percentages (up to 95% for blast-furnace cement) can be used (Table 7.6).
For concrete, containing blast-furnace slag, a possible mixing design is given in Table 7.7. For this mixing design, dilution calculations were made in order to calculate the I-index of blast-furnace slag containing concrete, based on the values of the I-index for the constituents (Table 7.8). The result of the I-index calculations is given in Table 7.9.
Table 7.8
Typical activity concentration indexes for concrete constituents in modern concrete compositions containing blast-furnace slag
| Raw materials | Typical activity concentrations [226Ra; 232Th; 40K] (Bq/kg) | I-index | Reference |
| Cement | [45; 31; 216] | 0.38 | Trevisi et al. (2012) |
| Aggregates | [36; 34; 483] | 0.45 | Trevisi et al. (2012) |
| Blast-furnace slag (Poland) | [115; 35; 192] | 0.62 | Minimum value (Table 10 of Chapter 6) |
| Blast-furnace slag (Turkey) | [178; 148; 242] | 1.41 | Maximum value (Table 10 of Chapter 6) |

The I-index calculation is based on European Commission 1999; Radiation Protection 112; Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; and Directorate-General, Environment, Nuclear Safety and Civil Protection.
Note that the I-index, as proposed by Council Directive 2013/59/Euratom, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product or cement makes the unrealistic assumption that 100% of the by-product or cement is used as a building material.
Table 7.9
I-index of modern concrete compositions containing blast-furnace slag
| Raw materials | Reference concrete (no blast-furnace slag) | Concrete containing slag as partial replacement of cement | Concrete containing slag as a partial replacement of cement and concrete |
| Maximum activity of blast furnace (I=1.41) | 0.41 | 0.55 | 0.71 |
| Minimum activity of blast-furnace slag (I=0.62) | 0.41 | 0.44 | 0.47 |

7.2.5 Copper slag
7.2.5.1 Technical properties
The physico-mechanical characteristics of copper slag suggest that it can be utilized in the cement and concrete industry (Shi et al., 2008). Granulated copper slag exhibits pozzolanic properties, and then, it could be used as a constituent for common Portland cement.
When slowly cooled and milled to be used as fine or coarse aggregate in high-strength concrete, the concrete showed comparable or even superior mechanical properties compared with conventional OPC. Depending of the composition and characteristics of copper slags, they can be used as ballast, abrasive material, fine aggregate in concrete, aggregates in hot mix asphalt pavements, cement raw material, roofing granules, glass, tiles, and so on (Al-Jabri et al., 2006, 2009; Arino-Moreno and Mobasher, 1999; Shi and Qian, 2000; Khanzadi and Behnood, 2009).
In Table 7.10 a typical mix design for different concrete compositions with and without copper slag is given. This example will be further discussed in the discussion of the radiological aspects.
Table 7.10
Example of mix design for modern concrete compositions with and without copper slag (kg/m3)
| Raw materials | Reference concrete (no FA) | Concrete containing slag as a partial replacement of concrete |
| Cement | 400 | 400 |
| Coarse and fine aggregates | 1850 | 1450 |
| Slag as partial replacement of aggregates | 0 | 400 |
| Water | 150 | 150 |
| Total | 2400 | 2400 |
7.2.5.2 Radiological properties
For concrete, containing copper slag, a possible mixing design is given in Table 7.10. For this mixing design, dilution calculations were made in order to calculate the I-index of copper slag containing concrete, based on the values of the I-index for the constituents (Table 7.11). The result of the I-index calculations is given in Table 7.12.
Table 7.11
Typical activity concentration indexes for concrete constituents in modern concrete compositions containing copper slag
| Raw materials | Typical activity concentrations [226Ra; 232Th; 40K] (Bq/kg) | I-index | Reference |
| Cement | [45; 31; 216] | 0.38 | Trevisi et al. (2012) |
| Aggregates | [36; 34; 483] | 0.45 | Trevisi et al. (2012) |
| Copper slag (Poland) | [317; 54; 886] | 1.62 | Minimum value (Table 11 of Chapter 6) |
| Copper slag (Germany) | [770; 52; 650] | 3.04 | Maximum value (Table 11 of Chapter 6) |

The I-index calculation is based on European Commission 1999; Radiation Protection 112; Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; and Directorate-General, Environment, Nuclear Safety and Civil Protection.
Note that the I-index, as proposed by Council Directive 2013/59/Euratom, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product or cement makes the unrealistic assumption that 100% of the by-product or cement is used as a building material.
7.2.6 Red mud
7.2.6.1 Technical properties
The potential uses of red mud can be classified into recovery of major or minor constituents and direct uses or incorporation into products such as concrete, tiles, and so on. Within the first group, recovery of iron, vanadium, chromium, titanium dioxide, rare earths, and aluminum oxide has been reported in the literature. With regard to applications in building materials, red mud can be used as a raw material in cement, bricks, roofing tiles, and glass-ceramics production (Thakur and Sant, 1983; Tsakiridis et al., 2004; Vangelatos et al., 2009; Singh et al., 1997; Yang and Xiao, 2008; Romero and Rincón, 2000; Vincenzo et al., 2000; Pontikes and Angelopoulos, 2013).
The use of bauxite residue in Portland cement production has been the subject of some research projects from as early as 1936 (Thakur and Sant, 1983). The iron and alumina contents of the residue are beneficial in the mix raw material to produce clinker. The residue must be pressured before its incorporation to the raw mix in a proportion below 5% (Tsakiridis et al., 2004; Vangelatos et al., 2009). Also, some special cement has been investigated in the past using of mixtures of gypsum and bauxite residue. In particular, the titanium content of the mud was found to be beneficial to cement compressive strength (Singh et al., 1997; Yang and Xiao, 2008).
Artificial aggregates made of red mud require a number of processing steps, including drying, pelletizing, and calcinations. Therefore, it is unlikely that such type of artificial aggregate could be competitive with other types due to the processing cost (Romero and Rincón, 2000).
7.2.6.2 Radiological properties
The radiological properties of red mud are discussed in detail in Chapter 6. On the basis of the activity concentration of naturally occurring radionuclides in red mud calculations can be made regarding the resulting activity concentration index of the concrete.
Especially the production of alkali-activated cement and concretes could enable the incorporation of larger percentages of red mud in concrete. This aspect is discussed in more detail in Section 7.3.5.
7.2.7 Overall discussion of radiological aspects of Portland cements and concretes
Commonly, the concentration of radionuclides, originating from residues, is decreased in the produced Portland cements and concretes due to a dilution effect. This is illustrated in Table 7.13 where the radiological properties of some investigated concretes are given.
Table 7.13
Radiological properties of investigated concrete samples (extracted from NORM4Building database)
| Country | Radionuclide concentration (Bq/kg) | I-index | Number of samples | References | ||
| 226Ra | 232Th | 40K | ||||
| China | 25.8 | 26.8 | 852 | 0.5 | 13 | Xinwei (2005) |
| Estonia | 35.1 | 11.3 | 207 | 0.2 | 1 | Lust and Realo (2012) |
| Hungary | 11 | 6 | 142 | 0.1 | 2 | Szabó et al. (2013) |
| Lithuania | 32 | 17 | 426 | 0.3 | 1 | Trevisi et al. (2012) |
| Luxembourg | 93 | 92 | 110 | 0.8 | 2 | Trevisi et al. (2012) |
| Poland | 18.5 | 16.5 | 350 | 0.3 | 2 | Zalewski et al. (2001) |
| Slovakia | 17.1 | 19.7 | 351 | 0.3 | 34 | Michael (2010), Vladar and Cabanekova (1998) |
| Spain | 23.2 | 21 | 278 | 0.3 | 9 | Chinchon-Paya et al. (2011) |
| Syria | 24.5 | 4.8 | 70 | 0.1 | 6 | Shweikani et al. (2013) |

These values are the mean values of individual entries.
The I-index calculation is based on European Commission 1999; Radiation Protection 112; Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; and Directorate-General, Environment, Nuclear Safety and Civil Protection.
Generally, the uranium series radionuclide concentration in the cement-based materials, in descending order is: Fly ash>Anhydrous calcium aluminate cement>Slags>Anhydrous Portland cement>Limestone=Silica fume (Puertas et al., 2015a,b).
Aggregates often have the greatest influence in the concrete radioactivity because they account for more than 80% of the concrete volume. Radium-rich and thorium-rich materials, for instance, granites and gneiss or others used as aggregates in concrete may enhance the indoor gamma radiation from the walls in buildings (Ackers et al., 1985; Botezatu et al., 2002). In a similar way, some industrial wastes such as blast-furnace slag, coal fly ash, and coal bottom, among others, can cause enhanced activity concentrations of concrete when they are used as aggregates (Kominek et al, 1992: Nuccetelli et al., 2015b; Skowronek and Dulewski, 2001). By contrast, natural stone of sedimentary origin such as limestone or dolomite does not enhance the radionuclide content of concrete mix. From a recent update of Trevisi et al. (2012), reporting summarized data of radioactivity concentrations of building materials in EU countries, some information can be obtained. For 226Ra, 232Th, and 40K averages (and ranges) of EU national values, expressed in Bq/kg, are 60 (14–272), 34 (8–138), and 345 (17–685), respectively (Trevisi et al., 2016). By a detailed analysis of the database it is possible to see that the highest activity concentrations are generally relevant to concretes containing NORM residues.
With regard to the radon production in concrete as a result of the presence of 226Ra, it is well-known that it depends on some characteristics of the concrete such as moisture content, porosity, tortuosity, permeability, cracks formation, and thickness of the concrete element. In general, high-moisture content in porous materials increases the radon exhalation rate (Kovler et al., 2005; Stranden et al., 1984; Yu et al., 1996). On the contrary, dehydration of concrete due to aging of materials determines a decrease of the radon exhalation rate.
7.3 Alkali-activated cement and concretes (geopolymers)
7.3.1 Introduction
In 1895 and 1908, for the first time patents demonstrated that the combination of a vitreous slag and different alkaline solutions could be used to develop a material with a performance similar to Portland cement. In the 1960s and the 1970s of the last century, relevant contributions were given by Glukhovsky (1959) at the Institute for Binders and Materials of Kyiv National University of Construction and Architecture, focusing on the alkali-carbonate activation of metallurgical slags. In early 1980s (Davidovits, 1982), in France, patented several aluminosilicate-based formulations and introduced the name “geopolymers” for these alkaline materials. Since the 1990s several research groups are working on the development of such alternative construction materials, attempting to optimize the formulations and final (mechanical, chemical, physical, and microstructural) properties. More recently, pilot-scale and industrial trials on the application of alkali-activated binders (concrete, mortars, and related materials) were conducted, and recommendations to the national and international standardization bodies were given for the practical implementations of these alternative building materials.
The alkali-activated materials (AAMs) are derived by the reaction of an alkali metal source (solid or dissolved) with a solid silicoaluminate powder (binder or precursor). This solid can be metakaolin, metallurgical slag, natural pozzolan, fly ash, or bottom ash. The alkali sources used can include alkali hydroxides, silicates, carbonates, sulfates, aluminates, or oxides (Provis and van Deventer, 2014).
According to the chemical composition of the binder, we can distinguish two main systems of AAMs:
(1) High-calcium AAMs [where the binder is mainly blast-furnace slag (BFS)].
(2) Low-calcium AAMs [where the binder is mainly fly ash (FA)].
7.3.2 Blast-furnace slag
7.3.2.1 Technical properties
In the second half of the 20th century alkali-activate slag (AAS) concretes have been successfully applied in different fields of civil engineering, particularly in Eastern Europe: Ukraine, Russia, and Poland (Shi et al., 2006). A historical study of more than 50 years old AAS concrete in Belgium was recently presented (Buchwald et al., 2015). Valuable experience for further development and applications of alkali-activated binders and concretes has been gathered from the existing applications. The lack of uniformly accepted standards is probably the major obstacle toward broader application in the construction industry. The recommendation of RILEM Technical Committee 224-AAM is that a performance-based standards regime should be implemented to provide description and regulation for alkali-activated binders and concretes (Provis and van Deventer, 2014).
Ground-granulated blast-furnace slag is the most frequently used metallurgical slag for the production of alkali-activated slag mortars, and concretes. During the alkali activation process, the vitreous phase of BFS dissolves, forming, mainly, calcium aluminosilicate hydrates (C-A-S-H) afterwards. This reaction depends on a whole series of parameters such as physical and chemical properties of BFS, properties of alkali activator (the nature, concentration, and pH of the activators), and conditions of the reaction (temperature, relative humidity, and curing time). The influence of all of these factors on the structure and properties of alkali-activated blast-furnace slag (AAS) pastes, mortars, and concretes is thoroughly presented in some other publications (Shi et al., 2006; Davidovits, 2008; Provis and van Deventer, 2014; Provis et al., 2015; Pacheco-Torgal et al., 2015).
Properties of AAS are highly affected by the activator nature (type) and concentration. The earliest binder made from BFS was lime-activated BFS. Alkali hydroxides, silicates, sulfates, and carbonates or their mixtures can successfully activate BFS.
The activator is normally used as a solution. On the other hand, one part of the binder can be produced by mixing or intergrinding solid-state activator with the BFS. However, this might cause problems due to the hygroscopic nature of the activator. Furthermore, the heat of hydration released during dissolution of solid activator may enhance the reactivity of BFS. Optimal concentration and dosage strongly depend on the nature of slag, alkali activators used, and curing conditions.
The highest strength is most commonly developed when BFS was activated with sodium silicate (water glass) as demonstrated in Fig. 7.1 (Puertas and Torres-Carrasco, 2014). However, undesirable side effects, such as fast setting and/or high-drying shrinkage, usually accompany the high strength. Such problems might be solved by extending the mixing time. The activation of BFS with NaOH or KOH results in a high-early strength, but when considering the strength at 7 days or later ages, it is usually lower in comparison to BFS activated with sodium silicate.

The selection of a suitable alkali activator most probably has the largest economic and environmental impact on the production of alkali-activated binders or concretes. The large-scale utilization of commercially produced sodium silicate as an activator will face limitations in terms of scalability, cost, practical handling issues, and the environmental cost of this product (Provis et al., 2015). New activators should be investigated in order to contribute to the fabrication of inexpensive binder systems, sustainable processes, and nonhazardous handling. Sodium sulfate, NaOH/Na2CO3 mixture, and the glass waste mixed solution or NaOH/silica fume also proved to be effective as alkali activators for BFS—see Fig. 7.1 (Puertas and Torres-Carrasco, 2014).
Curing conditions have a significant impact on the mechanical properties of AAS, as the provision of proper curing proved to be essential for high-strength development. Curing in water, the most common curing procedure for Portland cement is not recommended, as it might lead to premature leaching and unavoidable loss of strength. More appropriate options of curing at room temperature are sealed curing (in a sealed container) or curing in a humid chamber (Relative humidity>90%). Curing at elevated temperature (heat or steam curing) increases the rate of alkali activation reaction and strength development, whereby irreversible loss of water should be prevented as it might lead to the high-drying shrinkage, micro cracks formation, and strength loss (Marjanović et al., 2015). Steam and autoclave curing are significantly effective in reducing the drying shrinkage of AAS mortars. Unconventional curing by ultrasound or microwaves also has some potential (Komljenović, 2015).
The water/binder ratio plays a dominant role in strength development of AAS. Generally, a lower water/binder ratio induces higher strength. However, it depends on the activator concentration and dosage as well (Fernández-Jimenez et al., 1999). Standard water reducing admixtures, which were developed for the Portland cement systems, usually do not work properly in the alkali activation process due to high-alkaline conditions present (Palacios et al., 2009). The setting time of AAS primarily depends on the dissolution rate of the precursor material and precipitation of the reaction products. The setting time can vary significantly as a function of curing conditions and the type, concentration, and dosage of the activator used. The heat of hydration of AAS is usually lower than OPC (Križan and Živanović, 2002).
The mechanical properties of AAS concrete, such as compressive strength, flexural and splitting tensile strengths, drying shrinkage, etc., are normally assessed by using the standards of ordinary Portland cement concrete. However, some of those methods may be inappropriate for geopolymers (Provis and van Deventer, 2014).
AAS concrete has some advantages with respect to the OPC concrete, such as a low heat of hydration, a high-early strength, and an increased durability in aggressive environments. AAS concrete also shows a greater tensile strain capacity than OPC concrete due to the greater creep, the lower elastic modulus, and the higher tensile strength. Drying shrinkage and tendency to microcrack formation of AAS concrete is usually higher than OPC, particularly under dry conditions. The problem of efflorescence is also frequently present in AAS concrete, due to the high concentration and mobility of alkalis present in the pore solution, and the porosity of hardened AAS (Puertas et al., 2003). The efflorescence is rarely harmful to the product performance, but its avoiding is highly desirable due to the undesirable visual effects. The elastic properties of AAS under applied force, which are of particular importance for construction applications, can be improved by introducing fiber reinforcement such as short fibers or unidirectional long fibers into the AAS matrix. The addition of different types of fibers (polypropylene, polyvinyl alcohol, alkali-resistant glass, steel, carbon, etc.) usually increases flexural and splitting tensile strength, reducing drying shrinkage as well. Some adverse effects on workability and compressive strength were also reported (Puertas et al., 2003).
Concrete durability during long-term exploitation is of key importance for its safe and efficient functioning and it is determined by its ability to resist chemical attacks, abrasion, weathering action, or any other process of deterioration.
AAS usually contains a large amount of alkalis, which means that if AAS is being used in structural applications, an important precondition is met for the alkali-aggregate reaction (AAR) to occur. The role of calcium is known to be important in determining the rate and extent of the alkali silica reaction (ASR). AAS concrete is probably more resistant to ASR than Portland cement concrete due to the lower availability of alkalis and calcium (C-A-S-H with a lower Ca/Si ratio) (Puertas et al., 2009). However, the opposite, a lower resistant to ASR in comparison with Portland cement was also reported (Bakharev et al., 2001; Shi et al., 2015).
Carbonation is one of the most important degradation processes that can significantly affect the long-term durability of concrete infrastructures. The carbonation rate of AAS depends on the properties of BFS. For example, higher amount of MgO present in BFS reduces the carbonation rate (Bernal et al., 2014). The type and concentration of the activator used also influences the carbonation rates, as well as the water/binder ratio, the amount of BFS present in the concrete mixture, and curing conditions.
Frost resistance of AAS becomes important in cold climates when the concrete construction is exposed to freeze-thaw cycling. Deterioration of concrete can appear in two principal forms: internal cracking due to freezing and thawing cycles and surface scaling due to freezing in the presence of deicing salts (usually NaCl). However, the available literature is mainly focused on issues related to internal cracking (Cyr and Pouhet, 2015).
Different methods for testing the frost resistance of Portland cement concretes are available. However, these methods are based on different experimental conditions such as the temperatures of freezing and thawing, cycle count and duration, etc. The curing procedure prior to this or any other type of alkali-activated BFS testing is particularly important, as the proposed curing methods for Portland cement systems might not be appropriate for AAS. Generally, AAS shows very good frost resistance due to favorable characteristics of the air-void/bubble network system (Cyr and Pouhet, 2015). Sodium silicate-activated BFS concrete usually has the least porous structure, highest strength, and best frost resistance.
Chloride penetration through concrete can cause corrosion of the reinforcing steel and deterioration of reinforced concrete structures. This type of deterioration is quite common in concrete structures exposed to deicing salts or sea water. Therefore, the resistance of reinforced concrete structures to chloride penetration is quite important for designing, producing, and maintaining durable concrete structures. A great number of methods for chloride penetration testing developed for Portland cement concretes are available. The RILEM Technical Committee TC 178-TMC: “Testing and Modeling Chloride Penetration in Concrete” has tested four different groups of methods for determining chloride transport parameters in concrete: (1) natural diffusion methods, (2) migration methods, (3) resistivity methods, and (4) colorimetric methods. The RILEM Technical Committee TC 224 AAM (Provis and van Deventer, 2014) suggested that chloride ponding tests such as ASTM C1543, or rapid migration tests such as the Nord Test method NT Build 492, might be more suitable for AAMs testing. Compared with OPC, alkali-activated binders demonstrate better performance against chloride ingress, according to both accelerated (Fig. 7.2) (NordTest NT Build 492) and ponding (ASTM C1543) methods. The NordTest method is considered more reliable as an accelerated way to assess chloride durability of AAMs (Torres-Carrasco et al., 2015).

Corrosion of reinforcing steel in AAS is strongly influenced by the specific BFS chemistry (presence of sulfide). Therefore, the predictions designed for Portland cement concretes may not be applicable to the AAS concretes. Despite the fact that some reports for corrosion of reinforcing steel testing in AAS already exist, the method specifically adapted to the complex chemistry of AAS is yet to be developed.
External sulfate attack is the consequence of impact of sulfate ions present in soils, underground waters, sea water, or industrial waste waters on hardened concrete. Sulfates generally cause harmful effects on cement, depending on the type of cement used, the nature and concentration of aggressive sulfate solution, the presence of different cations and/or salts in sulfate solution, the quality of concrete, as well as concrete exposure conditions. Different methods and criteria were also used to assess the resistance of AAS mortar or concrete to external sulfate attack, most commonly based on (1) expansion, (2) flexural and/or compressive strength, and (3) the strength loss index (Komljenović et al., 2013). More detailed reports are given elsewhere (Shi et al., 2006; Provis and van Deventer, 2014; Pacheco-Torgal et al., 2015). Generally, AAS performs better in sulfate environment than Portland cement systems. However, this performance depends also on the properties of BFS, the type and concentration of the activator, concentration of sulfate solution, and cations present in the solution.
Despite the fact that most of the concrete structures are not exposed to acidic conditions, some concrete structures can be exposed to acidic aggressive environments such as specific industrial processes, acid rains, acid sulfate soils, animal husbandry, or biogenic sulfuric acid corrosion (present in sewage pipes). AAS is expected to demonstrate similar or even better acid corrosion resistance in comparison with Portland cement, due to the significant differences in the reaction products (absence of Portlandite in and low Ca/Si ratio of the BFS-based binder). According to the available literature, AAS generally shows good performance in acidic environments (Komljenovic et al., 2012; Varga et al., 2015).
7.3.2.2 Radiological properties
The radiological properties of blast-furnace slag are discussed in Chapter 6. These data can be used to assess the activity concentration of an alkali-activated cements and concrete based on blast-furnace slag.
Radiological properties of alkali-activated cements and concretes produced on the basis of blast-furnace slag and coal fly ash are discussed in Section 7.3.3.2.
7.3.3 Coal fly ash
7.3.3.1 Technical properties
Development of alkali-activated cements allows using fly ash as an aluminosilicate component (Krivenko, 1992). A specific feature of these systems is the high-initial pH value. When being appropriately used, the alkalis accelerate the first stage of destruction of the initial aluminosilicate structure, and then take an active part in the formation of compounds responsible for the strength characteristics of the material.
An important component of fly ash alkali-activated (AAFA) cement is low-calcium coal ash (up to 10% of CaO by mass, class F according to ASTM classification). In addition, also Class C ash can be used as is the case for several applications in the United States.
Curing conditions of AAFA cements also differ depending on the required properties for tailored applications: (i) special applications require autoclave curing, steam curing, and drying; (ii) common cements are cured in normal conditions (steam curing).
According to the Ukrainian Standard DSTU B V.2.7-181:2009 “Alkaline cements. Specifications” (DSTU B.V. 2.7-181, 2009), AAFA cements can be divided three classes, as shown in Table 7.14.
Table 7.14
Fly ash in the alkali-activated cements
| Class | Designation | Composition (% by mass) | |||
| Main constituents (aluminosilicates) | Alkali (Na or K) metal compounds (over 100%) | ||||
| Granulated blast-furnace slag | Clinker | Fly ash | |||
| AAC I—ASH | Alkali-activated cement with addition of fly ash | 55–90 | 0–10 | 10–35 | 1.5–12 |
| AAC III | Alkali-activated pozzolanic cement | 20–64 | 36–80 | 1.5–12 | |
| AAC V | Alkali-activated composite cement | 30–50 | 5–10 | 40–65 | 1.5–12 |

Based on DSTU B.V. 2.7-181, 2009. Alkaline Cements, Specifications. National Standard of Ukraine, Kyiv.
The main reaction products according the curing conditions are shown in Fig. 7.3 (Krivenko and Kovalchuk, 2002; Krivenko et al., 2006).

The mechanical strength of AAFA cements is similar to OPC or AAS. Their evolution with curing age is shown in Fig. 7.4. Compositions and properties of the cements are given in Table 7.15. Remarkable is the strength gain at older ages: after three years curing samples might harden over 150% of the strength obtained after 28 days.

Table 7.15
Characteristics of alkali-activated cements
| No. | Cement composition, % by mass | Properties | Flow (cone), mm (W/C) | |||||
| Clinker | Fly ash | Granulated blast-furnace slag (S) | Na2CO3 | Plasticizer | Paste of normal consistency, % | Initial setting time, min | ||
| 1 | – | 60 | 40 | 5 | 1 | 25.7 | 75 | 115/0.34 |
| 2 | 10 | 60 | 30 | 5 | 1 | 25.5 | 70 | 112/0.34 |
| 3 | 20 | 80 | – | 5 | 1 | 26.7 | 80 | 108/0.31 |
| 4 | 30 | 70 | – | 5 | 1 | 26.0 | 75 | 110/0.32 |
| 5 | OPC CEM II/A-400 (reference) | 27.8 | 85 | 112/0.38 | ||||

At the same time, AAFA concrete shows other interesting properties (Grabovchak, 2013; Krivenko et al., 2014; Krivenko et al., 2005; Kovalchuk and Grabovchak, 2013; Krivenko et al., 2010): (i) high-corrosion resistance in sea water, in Na2SO4 (10% concentration) and MgSO4 (up to 4% concentration) solutions; (ii) low shrinkage; (iii) high freeze-thaw resistance. These concretes are highly advantageous in massive structures prepared in situ; and (iv) high-temperature resistance (heat- and fire-resistant concretes). Residual strength of such materials after burning at 800°C could reach 500% comparing with the strength in normal conditions.
The durability of AAFA concretes is similar to that of previously presented AAS concretes. Sulfate resistance is outstanding and has no analogs among the traditional materials. There were obtaining concretes with strength classes C15–C40. Frost resistance, weather resistance, water impermeability, and other service properties are similar to those of traditional concretes (Kovalchuk and Grabovchak, 2013; Krivenko et al., 2010).
7.3.3.2 Radiological properties
The discussion below deals both with the radiological properties of alkali-activated cements and concretes produced on the basis of blast-furnace slag and coal fly ash since in most cases AAMs will merge several by-products that contain naturally occurring radionuclides.
The radiological properties of geopolymers or alkali-activated cement pastes have been recently studied in detail by Puertas et al. (2015a,b). Sample preparation and activation conditions used are shown in Table 7.16.
Table 7.16
Sample preparation and activation conditions of geopolymers used to study radiological features (Puertas et al., 2015a,b)
| Sample | Solution | liquid-to-solid ratio | SiO2/Na2O |
| Water glass-AA Slag | Sodium silicate | 0.4 | 0.86 |
| Glass-AASlag | NaOH/Na2CO3+glass waste | 0.4 | 0.86 |
| N/15Wg-AA fly ash | NaOH 10 M+sodium silicate | 0.3 | 0.19 |
| Glass-AA fly ash | NaOH 10 M+glass waste | 0.3 | 0.11 |

The radionuclide activity concentrations in alkaline cement pastes (Wg-AAS, Glass-AAS, N/15Wg-AAFA, and Glass-AAFA) (see Table 7.17) have been calculated considering the percentage of slag or fly ash in the anhydrous geopolymers and in the activated end products. It needs to be noted that the 40K concentration increases in such activated materials because the potassium impurities, that are often present in the NaOH activator, result in an increased 40K potassium content in the end product.
Table 7.17
Activity concentrations in raw materials (fly ash, BFS, and waste glass) and cements after alkaline activation (in Bq/kg) (uncertainty, k=2) (Puertas et al., 2015a,b)
| Series | 238U | 232Th | 40K | Indexa | |||
| Material | 234Th | 214Pb | 228Ac | 212Pb | 208Tl | ||
| Fly ash | 130±7.1 | 127.4±1.3 | 130.3±1.5 | 133.8±1.3 | 41.33±0.57 | 316.4±5.9 | 1.1815±0.0089 |
| BFS | 156.4±6.8 | 147.2±1.4 | 45.7±0.86 | 42.9±1.2 | 14.71±0.30 | 76.3±2.7 | 0.7448±0.0065 |
| Waste Glass | 11.4±1.1 | 8.73±0.19 | 5.83±0.22 | 6.28±0.12 | 1.867±0.075 | 226.8±4.4 | 0.1338±0.0020 |
| Wg-AAS | 91.5±5.6 | 48.7±1.1 | 22.84±0.71 | 23.3±0.69 | 7.7±0.39 | 77.0±5.0 | 0.3022±0.0054 |
| Waste Glass-AAS | 94.4±6.7 | 54.5±1.4 | 23.78±0.81 | 24.99±0.83 | 8.04±0.41 | 89.2±4.8 | 0.3303±0.0064 |
| N/15Wg-AAFA | 56.4±5.7 | 36.44±0.97 | 67.8±1.4 | 75.1±1.8 | 21.57±0.59 | 578±15 | 0.6531±0.0092 |
| Waste Glass-AAFA | 57.4±3.2 | 37.9±1.1 | 62.2±1.2 | 75.1±1.2 | 22.62±0.63 | 550±14 | 0.6207±0.0084 |

a Note that the I-index, as proposed by Council Directive 2013/59/Euratom, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product makes the unrealistic assumption that 100% of the by-product is used as a building material.
A paper of the radiological characterization and impact of alkali-activated concretes has been recently published (Nuccetelli et al., 2017). The publication reports results of a study on five different types of fly ash from Serbian coal burning power plants and their potential use as a binder in alkali-activated concrete (AAC), depending on their radiological and mechanical properties. Five AAC mixtures with different types of coal burning fly ash and one type of blast-furnace slag were designed. Measurements of the activity concentrations of 40K, 226Ra, and 232Th were done both on concrete constituents (fly ash, blast-furnace slag, and aggregate) (see Table 7.18) and on the five solid AAC samples (see Table 7.19). Experimental results were compared by using the activity concentration assessment tool for building materials—the activity concentration index I, as introduced by the RP 112 and EU Basic Safety Standards [RP 122, 1999;24] and in Chapter 4. All five designed alkali-activated concretes comply with EU BSS screening requirements for indoor building materials. Finally, the index I values were compared with the results of the application of a more accurate index-I (ρd), which accounts for thickness and density of building materials (Nuccetelli et al., 2015a) and the annual dose, evaluated with an accurate formula accounting for the actual density and thickness of the AAFAC concrete sample, was also calculated. Considering the actual density and thickness of each concrete sample index-I (ρd) values are lower than index-I values and the annual dose resulted negative, once the background is subtracted, in three cases and less than 2.3E-02 mSv, with uncertainties, in the other two cases. In the paper, a synthesis of main results concerning mechanical and chemical properties has also been provided.
Table 7.18
Natural radionuclide activity concentrations in Serbian fly ash and blast-furnace slag samples used in alkali-activated concretes (Nuccetelli et al., 2017)
| 232Th | 226Ra | 40K | index Ia | |
| (Bq/kg) | ||||
| Fly ash-1 | 90.9±1.7 | 123±5 | 445±7 | 1.01 |
| Fly ash-2 | 112±2 | 163±5 | 416±7 | 1.24 |
| Fly ash-3 | 42.7±0.8 | 56.2±2.1 | 199±3 | 0.47 |
| Fly ash-4 | 66.0±1.2 | 151±5 | 393±6 | 0.96 |
| Fly ash-5 | 78.3±1.5 | 152±4 | 369±6 | 1.02 |
| Blast-furnace slag | 26.5±0.5 | 108±3 | 122±2 | 0.54 |
| Fly ash from RP112 (EC, 1999) | 100 | 180 | 650 | |
| Fly ash (Nuccetelli et al, 2015b) | 80 | 207 | 546 | |
| Slag from RP112 (EC, 1999) | 70 | 270 | 240 | |
| Slagb | 63 | 147 | 246 | |

a Note that the I-index, as proposed by COUNCIL DIRECTIVE, 2013/59/EURATOM, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product makes the unrealistic assumption that 100% of the by-product is used as a building material.
b Based on new elaboration of national values given in the EU database.
Table 7.19
Natural radionuclide activity concentrations of alkali-activated concretes (AAC) samples: calculation of index I, index I (ρd), and gamma dose
| AAC samples | 232Th | 226Ra | 40K | index I | I (ρd) | D(ρd) (mSv/year) |
| (Bq/kg) | ||||||
| AAC-1 | 18.4±0.4 | 28.5±1.5 | 232±4 | 0.26±0.01 | 0.23±0.01 | 0.8E-02±1.0E-02 |
| AAC-2 | 18.6±0.4 | 28.8±1.3 | 225±4 | 0.26±0.01 | 0.24±0.01 | 1.4E-02±0.9E-02 |
| AAC-3 | 12.5±0.2 | 21.2±0.9 | 196±3 | 0.20±0.01 | 0.18±0.01 | −6.4E-02±0.6E-02 |
| AAC-4 | 14.9±0.3 | 27.7±1.7 | 218±3 | 0.24±0.01 | 0.21±0.01 | −1.6E-02±1.0E-02 |
| AAC-5 | 16.1±0.3 | 28.3±1.2 | 197±3 | 0.24±0.01 | 0.21±0.01 | −1.9E-02±0.8E-02 |

7.3.4 Steel-melting slags
7.3.4.1 Technical properties
The chemical-mineralogical composition of steel-melting slags varies within wide ranges, and this is the major drawback for recycling (Krivenko, 1986).
Alkali-activated cements made from basic steel-melting slags cured for 28 days in normal conditions show compressive strengths of 15–20 or 20–30 MPa when sodium carbonate or sodium di- and metasilicate are used as activators, respectively. Appreciable hardening is observed at later ages (Kavalerova et al., 2000): 30–42 MPa with sodium carbonate and 46–58 MPa with sodium metasilicate after 1 year; 40–50 and 60–70 MPa, respectively, for the same activators, after 4 years.
Mixtures formulated with low-basic glassy steel-melting slags tend to show slower hardening rates: only by the third day of normal curing the material acquires the resistance achieved after 24 h by the cements made from basic slags. The high-basic steel-melting slags are even less reactive and 14 days curing are required to get the strength of a basic slag formulation after 24 h.
One way to accelerate the hardening rate of such slow reactive systems is mixing with granulated blast-furnace slag. When properly combined with a high-basic crystallized steel-melting slag the obtained mixture acts as a quick-hardening cement: compressive strengths of 52–62, 66–80, and 90–110 MPa after 3, 7, and 28 days curing. The use of these slags also as aggregates in concrete mixtures can increase their consumption rates to up to 90 wt% of the final composition of the construction material (Kavalerova et al., 2000).
7.3.4.2 Radiological properties
The radiological properties for different types of steel-melting slag are reported in Chapter 6. These properties can be used for screening of these residues for use in building materials.
No publications with radiological information on alkali-activated cements or alkali-activated concretes containing steel-melting slags were found.
7.3.5 Red mud
7.3.5.1 Technical properties
The use of red mud in alkali-activated cements was studied in detail by Rostovskaya (1994). Well-succeeded attempts to incorporate high-mass percentages in the formulations (between 25% and 60% by total bindermass) were conducted, by the proper combination of red mud with glassy low-basic aluminosilicate compounds (blast-furnace slag, steel-melting slags) and high-basic Ca-containing additives (nepheline sludge, OPC), which are known to hydrate intensively in a highly alkaline environment. The alkali-activated cement containing red mud (60% by mass), ground-granulated blast-furnace slag (30% by mass), and OPC (10% by mass), using sodium silicate (Ms=2.8, ρ=1300 kg/m3) as alkaline activator, showed compressive strengths of 6.2, 30.3, and 60.0 MPa at 2, 7, and 28 days curing ages, respectively. Hydration products phase composition depends on the constituent composition but in general is constituted by a low-basic calcium hydrosilicate crystalline phase and an aluminosilicate and ferrosilicate gel phase. The concretes made using these cement formulations have high-frost resistance (200–500 cycles) and reduced shrinkage (0.16–0.20 mm/m).
There are substantial works done in incorporating bauxite reside in geopolymer composites formation (Dimas et al., 2009; He et al., 2013; Ye et al., 2014; Badanoiu et al., 2015). Different compositions and precursors (metakaolin, blast-furnace slag) were used with different activator conditions and preparations. Some authors (Ye et al., 2014) obtained mixing granulated blast-furnace slag in varying proportions with calcined red mud (800°C) specimens with 50 MPa after 28 days in a 50–50 wt% mix. In general terms, all of described inorganic polymers are characterized by a significant decrease in compressive strength with an increasing content of bauxite residue.
More chemical activity was found when the bauxite residue is thermally activated. More recently Hertel et al. (2016) treated the bauxite residue at 1100°C with carbon and silica. The resulting material was activated with K-silicate solution and was cured at 60°C for 72 h. The final material obtained with around 88.6 wt% bauxite red mud, 1.4 wt% carbon, and 10.0 wt% SiO2 reached more than 40 MPa compressive strength. A potential application of these construction materials should be pavements tiles or floor/roofing tiles.
In addition, the results (Rostovskaya, 1994) showed that red mud can also be used as an aggregate in concrete products produced by semidry pressing. This allows reaching incorporation rates of the red mud up to 75 wt%. Compressive strength of these products ranges from 20 to 40 MPa.
An example for a mix design for geopolymer concrete incorporating red mud and ggbs (compared with regular concrete) is given in Table 7.20.
Table 7.20
Example of mix design for reference concrete and an alkali-activated concrete with red mud and ggbs (kg/m3)
| Raw materials | Reference concrete | Concrete containing red mud as a partial replacement of cement and concrete |
| Portland cement | 400 | |
| Alkaline cement included ggbs—400; alkaline component —50 | 0 | 450 |
| Coarse and fine aggregates | 1850 | 0 |
| Red mud as partial replacement of aggregates | 0 | 1800 |
| Water | 150 | 150 |
| Total | 2400 | 2400 |
7.3.5.2 Radiological properties
The content of naturally occurring radionuclides in red mud is discussed in Chapter 6. This section deals with the resulting radiological properties of AAMs incorporating higher percentages of red mud.
By knowing that cumulative radiation activity of the red mud used is 599 Bq/kg, according to Ukrainian legislation the designed concrete products containing 60 wt% red muds can be used without restrictions in all fields of construction. Those formulated with 60–75 wt% waste can be used in road and airfield construction including in residential areas, while the concretes incorporating 90 wt% red muds can only be used in road, hydro engineering, and industrial constructions (Rostovskaya, 1994).
The study by Croymans et al. (2017) involves the radiological characterization of different types of alkali-activated concretes containing (up to 90 wt%) red mud from Ukraine. The authors measured activity concentrations of natural radionuclides, used different types of indexes (the index proposed by the EU-BSS and the index proposed by Markkanen (1995) for “materials used for constructing streets and playgrounds”) to evaluate the public exposure and RP122 to evaluate the occupational exposure.
Based on the mixing design in Table 7.20 and the activity concentrations given in Table 7.21, an I-index calculation for geopolymer concrete containing red mud and ggbs was made and the result is given in Table 7.22.
Table 7.21
Typical activity concentration index for concrete constituents in concrete compositions containing red mud in Europe
| Raw materials | Typical activity concentrations [226Ra, 232Th, 40K] (Bq/kg) | I-index | Reference |
| Portland cement | [45; 31; 216] | 0.38 | Trevisi et al. (2012) |
| Aggregates | [36; 34; 483] | 0.45 | Trevisi et al. (2012) |
| Blast-furnace slag (Poland) | [115.33; 34.55; 192.33] | 0.62 | Żak et al. (2008) |
| Red mud (Italy) | [97; 118; 15] | 0.9 | Minimum EU value (Table 15 of Chapter 6) |
| Red mud (Turkey) | [210; 539; 112] | 3.4 | Maximum EU value (Table 15 of Chapter 6) |
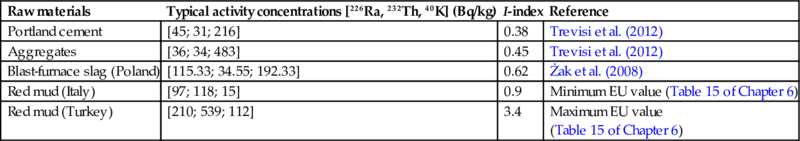
The I-index calculation is based on European Commission 1999; Radiation Protection 112; Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials; and Directorate-General, Environment, Nuclear Safety and Civil Protection.
Note that the I-index, as proposed by Council Directive 2013/59/Euratom, is only used for building materials or for their constituents if the constituents are also building materials: An I-index given for a by-product or cement makes the unrealistic assumption that 100% of the by-product or cement is used as a building material.
7.3.6 Nonferrous slag
7.3.6.1 Technical properties
In this section the technical properties of nonferrous slags (such as lead, nickel, and copper slag) that determine its use in construction materials are discussed.
Modulus of basicity (Mb=(CaO+MgO)/(Al2O3+SiO2)) of the nonferrous slags under study by Krivenko et al. (1984) varied from 0.30 to 0.60, while the crystalline phase content was 1–5 wt%. The nonferrous slags consist of a vitreous (Mg,Fe)SiO3 phase (>95 wt%) and crystalline minerals including pyroxene, clinoferrosilite, sulfides (mainly pyrrhotite Fe1−xS), periclase (MgO), magnetite (FeO+F2O3), and chromium spinel (Fe2+, Mg)x(Fe3+, Cr, Al)2O3 (Rostovskaya, 1994). Since the iron content might strongly affect the alkaline activation of such slags, a so-called quality index (K) has been introduced (Krivenko et al., 1984):
According to this quality index, nonferrous slags can be classified into three types as shown in Table 7.23 (TU 67–648-84, 1984).
Table 7.23
Characterization of nonferrous slags in terms of chemical composition and according to the activity quality index
| Characteristics | Compositional classes | ||
| I | II | III | |
| Quality index | >1.0 | 0.7–1.0 | <0.7 |
| Content of silicon oxide (SiO2), wt% | 26.0–32.0 | 33.0–52.0 | 33.0–52.0 |
| Calcium oxide (CaO) and magnesium oxide (MgO) contents, wt%, no more than | >20.0 | >17.0 | >8.0 |
| Content of iron (II) oxide (FeO), no more than | <30.0 | <30.0 | <35.0 |

Expected compressive strength values of the alkali-activated nonferrous slag cements hardened in normal conditions versus quality index and type of alkaline activator used in the process is given in Table 7.24 (Sultanov, 1985). Strength of optimal formulations can reach 100 MPa, depending on the cement type and its content, type of alkaline activator, and curing conditions. The concretes obtained by using such cements show low heat evolution, high-freeze-thaw resistance, high-abrasion resistance and can assure nuclear radiation protection.
Table 7.24
Compressive strength (MPa) of the alkali-activated nonferrous slag cements hardened in natural conditions vs. quality index and type of alkaline activator
| Alkaline activator | Values of quality index | ||
| K>1 | 0.7≤K≤1 | K<0.7 | |
| Sodium carbonate | 30–40 | 10–30 | – |
| Soda-alkali melt (waste of chemical industry) | 30–40 | 10–30 | – |
| Sodium silicate (Ms=2.8) | 30–40 | 20–30 | 10–20 |
| Sodium disilicate (Ms=2.0) | 50–80 | 30–50 | 20–30 |
| Sodium metasilicate (Ms=1.0) | 50–80 | 30–50 | 20–40 |
| Caustic alkali | 20–30 | 10–20 | – |

7.3.6.2 Radiological properties
The activity concentrations of naturally occurring radionuclides in nonferrous slag are discussed in Chapter 6. These values can be used for the evaluation of concretes depending of the percentage of nonferrous slag incorporation.
No publications with radiological information on alkali-activated cements or alkali-activated concretes containing nonferrous slags were found.
7.3.7 Granulated phosphorus slag
7.3.7.1 Technical properties
Granulated phosphorus slag is a latent cementitious material but less reactive than granulated blast-furnace slag at early age due to the lower Al2O3 content and the presence of P2O5 and F. A hydraulic index of phosphorus slag is defined as follows (Shi et al., 2006; RCT 5024-83, 1983):
Latent cementitious properties of granulated phosphorus slag can be very effectively triggered by alkaline activation.
The phase composition of cement hydration products is chiefly represented by tobermorite 1.13 nm. With addition of Portland cement, the intensity of tobermorite occurrence is higher and lines (peaks) characteristic of truscottite (Ca,Mn)14Si2 4O58(OH)8·2H2O—a mineral of the reyerite group and wenkite—Ba4Ca6(Si,Al)20 O41(OH)2(SO4)3·H2O—a mineral of the cancrinite-sodalite group appears in the X-ray diffraction patterns (Sanserbaev, 1987).
Experience obtained from the use of concrete and reinforced concrete structures from these cements in industrial, hydro engineering, and agricultural construction coincides well with assumption on the higher durability compared with those made from Portland cement concretes (Krivenko et al., 1993).
7.3.7.2 Radiological properties
No publications with radiological information on alkali-activated cements or alkali-activated concretes containing granulated phosphorus were found.
7.3.8 Overall discussion of radiological aspects of alkali-activated cements and concretes
The legislation and the methodology used for the evaluation of the radiological aspects of construction materials is discussed in, respectively, Chapters 4 and 5. The radiological properties of the by-products themselves are given in Chapter 6.
It is clear from the literature that currently (Jan. 2017) there is very little information on the radiological properties of alkali-activated cements and concretes. This is a logic situation since this type of materials is still in the research stage and there are only a limited amount of commercial applications. Alkali-activated cements and concretes can allow the incorporation of larger percentages of by-products (in comparison to Portland cements and concretes). This is one of the features that makes alkali-activated cements and concretes attractive for future applications, but also implies the requirement to control these materials from a radiological point of view. This aspect was illustrated by studies on fly ash, slag, and red mud containing alkali-activated concretes.
A good assessment regarding the activity concentration index of alkali-activated concretes can be obtained via dilution calculations if the activity concentrations of the natural occurring radionuclides in the by-products are known. Much harder is to make an assessment regarding radon exhalation/emanation from alkali-activated concretes, this will require additional measurements.
7.4 Ceramics
7.4.1 Introduction
Many industrial by-products and wastes are utilizable in the ceramic technology to substitute raw materials. The most used by-products are (i) fly ashes from power plants, incinerator of municipal wastes, and pyro metallurgical plants on the condition that they do not contain high percentages of lead or zinc; (ii) synthetic gypsum arising from sulfur decontamination of gaseous effluents; (iii) red mud from the Bayer process; (iv) foundry slags; (v) residues from the flotation process employed in the enrichment of metal containing ores; (vi) mining tails; and (vii) asbestos containing residues or asbestos fibers.
Several organic materials can usefully be employed in the ceramic technology to produce porous ceramics, while their burning is a source of energy. They mainly are (i) paper production sludges; (ii) fertilizer residues; (iii) sludge from domestic wastewater or sewage treatment; and (iv) plastics.
Literature reports several ceramic products containing wastes (Pelino, 1997; Dondi et al., 1997; Perez et al., 1996; Lee et al., 2007; VII Conference of the European Ceramic Society, 2001). They include dense and lightweight bricks, roofing tiles, paving tiles, filters, refractories, and glass-ceramics. Recycling by the ceramic technology has several advantages: (i) Possibility of substituting mining raw materials whose cost is growing due to the increasing cost of mining and land restoration; (ii) simple processes using mature technologies; (iii) fast firing cycles with high-energetic efficiency; (iv) controlled/standardized production; and (v) possibility of commercial exploitation in the large market of building materials.
The main disadvantages are related to constrains in the use of hazardous wastes and this aspect is linked to the residual porous microstructure of the ceramic material. If the microstructure is too porous, the ceramic might show low chemical resistance and enhanced leachability. On the other hand, dense products require higher temperatures and/or longer firing cycles, so the energy demand on processing is higher and final product is heavier. This goes against the sustainability principles that recommend the production/use of light materials showing less embodied energy.
The ceramic industry itself also uses zircon and zirconia (in glazes, refractories, etc.), and radiological consequences of their production, further use/manipulation by other industrial sectors (e.g., ceramic glazes and frits production), and on the final costumers also deserve a brief analysis at the end of this chapter.
7.4.2 Coal fly ash
7.4.2.1 Technical properties
The similarity in chemical (mainly SiO2 and Al2O3) and granulometric composition of fly ash and traditional raw materials makes fly ash suitable to be used in certain ceramic formulations. The marginal presence of residual carbon and heavy metals in fly ash are tolerated without problems due to the fact that the temperature employed for ceramics production is in general above 1000°C and promotes an effective incorporation of those elements in the ceramic matrix.
In the fabrication of dense ceramics, distinct fly ash samples collected at different zones of the electro filter (fly ash particle morphology in Fig. 7.5A) were mixed with clay. Fig. 7.5B shows the typical microstructure of the ceramic presenting optimal properties: porosity=2.96±0.5%, bending strength=47±2 MPa, and compressive strength=170±5 MPa. This sample was fabricated by using fly ash taken from the fourth zone of electro filter, showing particle size below 0.063 mm. The material was pressed at 133 MPa and then fired at 1100°C, using heating rate of 10°C/min. Upon sintering, diopside [Ca(Mg,Al)(SiAl)2O6] was formed and this phase is the main responsible for the properties, due to the interlocking microstructure of the crystals (Angusheva et al., 2012). Fly ash-clay blends containing distinct fly ash contents (from 10 to 90 wt%) were prepared and fired at 900–1100°C for 1 h. Fig. 7.5C exemplifies the microstructure of 40 wt% fly ash—60 wt% clay blend, pressed at 45 MPa and then fired at 1100°C for 1 h. This material shows optimal properties, i.e., density=2.09 g/cm3, water absorption=7.02%, bending strength=50.47 MPa, and E-modulus=25.35 GPa (Fidancevska et al., 2014).

Jung et al. (2001) report the fabrication of low dense (63% of theoretical density) mullite ceramics from the mixture of coal fly ash and alumina. A mixture was formulated aiming to obtain the stoichiometric mullite (71.8 wt% Al2O3 and 28.2 wt% SiO2). The shape and size of mullite particles control the relevant properties of the fired material, namely the pore structure and fracture strength. The addition of 3Y-PSZ particles (globular-shaped) inhibited grain growth of mullite particles and enhanced densification and the mechanical resistance: bending strength=395 MPa was obtained for samples sintered at 1500°C for 2 h.
Lightweight building bricks (Cicek and Cincin, 2015) obtained by mixing fly ash (88 wt%) with lime (12 wt%) were produced in a pilot-scale autoclave (cured for 6 h at 12 bars of steam pressure). Samples show water absorption=60.9%, compressive strength=7.65 MPa, and flexural strength=0.56 MPa. Their thermal conductivity was 0.225 W/mK, which is comparable to an aerated cellular concrete and much lower than of common clay bricks.
Further studies on the effect of fly ash milling on the properties of dry pressed ceramics composed of 70 wt% fly ash and 30 wt% stoneware clay are reported by Sokolar and Smetanova (2010). In the Netherlands one company is producing over decades a ceramic brick which contains ca 30 wt% of fly ash, 30 wt% of mine stone and clay (Roelofs and Wiegers, 1995). From measurements conducted in the 1990s, it was found that the level of radionuclides does not differ much from the one of common ceramic bricks produced in the Netherlands.
Another option for utilization of coal fly ash is production of glass-ceramics (Rawlings et al., 2006; Leroy et al., 2001; Lyer and Scott, 2001). According to Kim and Kim (2004) there are several ways to modify the composition of the fly ash for this application: (i) combination with other inorganic wastes such as glass cullet (composed by SiO2, CaO, and Na2O); (ii) addition of CaO and MgO as a float dolomite to promote the formation of amorphous materials; (iii) by adding TiO2 as nucleating agent, (iv) by adding CaO and Na2O as fluxing additives. Coal fly ash-containing glass-ceramics were produced by melting/quenching+thermal treatment (Deguire and Risbud, 1984). The material was melted (at 1500°C when no fluxing additives are used) and the glass was poured into graphite molds. Unusual two-stage nucleation treatment was then used: (a) 2 h at temperature between 650°C and 750°C+(b) 5–10 h at 800–950°C. Finally, the crystallization stage was done at temperature ranging from 1000°C to 1150°C. Further studies conducted by the same authors led to the conclusion that a single nucleation stage might be used instead. Some other authors (Barbieri et al., 1990) reported the development of glass-ceramics from coal fly ash, guided by the phase diagrams to anticipate the phase composition and properties of the material.
Bossert et al. (2004) reported the production of dense and porous glass-ceramics through the powder processing technology. Fly ash and waste glass (from TV monitors, windows, and flasks) were used as starting raw materials. Mixtures composed of 50 wt% fly ash and 50 wt% glass properly processed show bending strength of about 56 MPa and E-modulus near 26 GPa. Porous glass-ceramics was obtained by adding polyurethane foam (Fig. 7.6A) or C-fibers (Fig. 7.6B). E-modulus and bending strength values of the porous glass-ceramic samples were 2.7±0.5 GPa and 4.5±1 MPa when polyurethane foam is used; 7.1±1 GPa and 9.3±2 MPa for C-fibers.

Similar studies were conducted by Wu et al. (2006) but using ≤5 wt% fine SiC particles (5–25 μm) as pore creator. Wollastonite was the major crystalline phase detected while the porosity ranged from 70% to 90%. Pore size varied between 0.2 and 1.5 mm, depending on the sintering temperature.
7.4.2.2 Radiological properties
Fly ash (see Fig. 7.5A) from the thermal power plant REK Bitola, Republic of Macedonia, shows the following concentration of natural radionuclides: 266Ra: 59±6 Bq/kg; 232Th: 76±8 Bq/kg; and 40K: 376±29 Bq/kg. Depending on the wt% of incorporation and the activity concentration of the other constituents, the resulting activity concentration index for the fly ash containing ceramics can be calculated. The data from Chapter 6 can be used to calculate the activity concentration index when other types of fly ash are used.
7.4.3 Steel slag
7.4.3.1 Technical properties
Guo et al. (2011) and Khater (2002) used steel slag into the preparation of glass-ceramics. Shih et al. (2004) found that an appropriate addition of such slag could reduce the firing temperature of clay bricks. The waste was also used to produce colored paving bricks and tiles (Chen et al., 2010), and Lianyuan steel invented steel slag baking-free load-bearing tiles (Shinkai et al., 1997). The recycling of Ladle Slag (from secondary metallurgy) in the production of bricks was reported by Shinkai et al. (1997). The granulation of slag particles was introduced by Kojimori et al. (2003).
7.4.3.2 Radiological properties
In all cases, limited incorporation levels might be applied, without deleterious effects on the final properties of the products. No radiological features were reported for ceramics based on steel slag. The data from Chapter 6 on steel slag (Section 6.4.1.2) can be used to evaluate the activity concentration index for ceramics containing steel slag.
7.4.4 Aluminum-rich by-products
7.4.4.1 Technical properties
The recycling of aluminum generates slag and dross, both normally classed as hazardous wastes, can occur via ceramics products. The properties of by-product aluminum dross are discussed in Chapter 6.
Despite its potential hazardous character, alumina richness is an attractive aspect favoring its recycling. The two reutilization areas mostly explored are (Yoshimura et al., 2008): (i) refractories and (ii) composites (Al-alumina composites).
Lightweight expanded clay aggregates were produced from natural plastic clay and aluminum scrap recycling waste (ASRW), which were obtained as a result of recovering Al metal from black dross by using conventional metallurgical process (Bajare et al., 2012). ASRW contains aluminum nitride (AlN—on average 5 wt%), aluminum chloride (AlCl3—on average 3 wt%), potassium and sodium chlorides (total 5 wt%), and iron sulfite (FeSO3—on average 1 wt%). Its average chemical composition is given in Table 7.25, while the elemental analysis is shown in Table 7.26.
Table 7.25
Average chemical composition of aluminum scrap recycling waste (wt%) (Bajare et al., 2012)
| LOI, 1000°C | Al2O3 | SiO2 | CaO | SO3 | TiO2 | Na2O | K2O | MgO | Fe2O3 | Others |
| 6.21 | 63.19 | 7.92 | 2.57 | 0.36 | 0.53 | 3.84 | 3.81 | 4.43 | 4.54 | >2.6 |

Table 7.26
Elemental analysis of aluminum scrap recycling waste (wt%) (Bajare et al., 2012)
| Al | Si | Ca | Mg | Fe | Na | K | Cl | S | Cu | Pb | Zn |
| 34.4 | 4.4 | 1.32 | 2.44 | 3.60 | 1.69 | 2.31 | 4.23 | 0.07 | 0.99 | 0.14 | 0.6 |

The decomposition of volatile elements, present in the nitride, sulfite, and chlorides, will generate gases upon firing and the aluminum scrap recycling waste might act as a pore forming agent. The ceramic aggregates were produced from mixtures of carbonaceous clay and ASRW in distinct proportions (ASRW ranging from 9 to 37.5 wt%). The prepared aggregates were dried for 3 h at 105°C and then calcined for 5 min at various temperatures, ranging from 1150°C to 1270°C. Heating rate was kept constant (15°C/min). Physical and microstructural properties of sintered aggregates were then evaluated.
Apparent density of the aggregates ranged from 0.4 to 0.6 g/cm3. The pore structure is shown in Fig. 7.7, consisting of macropores with 1 mm mean diameter and micropores (size below 0.2 μm).

According to Pereira et al. (2000a), the salt slag generated from the smelting of secondary aluminum can be used in refractory bricks. Typical industrial processing conditions were followed. The incorporation of slag tends to improve the physical and mechanical characteristics of the ceramic material, due to its fluxing action. Higher incorporation levels (ca. 10 mass-%) are admissible. The same authors tested the incorporation of Al-rich salt slag in bauxitic-type refractories (Pereira et al., 2000b). It was concluded that it is possible to incorporate washed aluminum salt slags in bauxitic-type refractories. In general, the physical properties of the fired material tend to be improved with increasing slag contents (e.g., higher flexural strength). The fluxing characteristics of the slag might explain this effect. From a functional point of view, significant incorporation levels (18 wt%) are permitted.
Anodizing and powder surface coating processes are highly water consuming, not only in each consecutive chemical batch, but also to properly wash the pieces in between. As a direct consequence, a huge amount of wastewater is generated and, after proper treatments, it results in clean water and a high amount of solid waste, denominated aluminum sludge (BREF, 2006; Magalhães et al., 2005).
The clay brick ceramic industry might constitute an interesting alternative to land disposal of sludge. Marques et al. (2012) aimed to develop a thermal-resistant brick via the recycling of the aluminum sludge in bricks production. They used the production cycle of the brick plant and tried a full-scale test in brickwork, producing 10 tons of real bricks. As a conclusion anodizing sludge addition enhances the thermal behavior of bricks by 26%, without increasing the brick production cost, leading to a clear improvement of buildings' thermal comfort. The remaining physical-mechanical properties (water absorption and compression strength) of bricks still present suitable values (Marques et al., 2012).
The goal of Khezri et al. (2010) was to find an application for utilizing the sludge cake of aluminum anodizing units in order to prevent environmental pollution and finding economic profit for the factories. For this purpose, bricks with different combination of sludge, clay, and sand were made and tested with available standard. The result showed that bricks containing 40 wt% sludge have best and nearest quality standardized parameters of ordinary inner bricks. These bricks have lighter weight than the bricks in same bulk and cheaper price and also prevent sludge spreading in the environment.
Ozturk (2014) studied the utilization of anodizing sludge which is produced at high tonnages in one of the aluminum company in Turkey (Table 7.27). The research goal was the production of mullite ceramics from Al-rich sludge which contains 15–30 wt% of solid matter (90 wt% of the solid matter is boehmite (AlOOH) and the remaining is thenardite (Na2SO4) and barite (BaSO4)).
Table 7.27
Chemical composition of Al-rich anodizing sludge (wt%, XRF) (Ozturk, 2014)
| Al-rich sludge | Al2O3 | SiO2 | Fe2O3 | CaO | SO3 | Na2O | K2O | MgO | BaO |
| 70.9 | 0.78 | 0.31 | 2.06 | 20.2 | 2.95 | 0.03 | 0.97 | 1.20 |

Mullite is the stable crystalline alumino-silicate phase in the Al2O3-SiO2 system and contributes to high-performance strength, creep resistance, chemical inertness, and thermal stability in ceramic materials (Martins et al., 2004).
Ozturk (2014) applied a washing, filtering, and drying process to the anodizing sludge in order to remove sodium before the production of mullite ceramics. The sodium removal cycle was repeated until sodium was completely removed from the sludge. Then, the sodium-free powder is calcined at 1400°C for 1 h at a heating rate of 5°C/min to obtain a powder with alpha alumina (α-Al2O3) phase. The produced α-Al2O3 powder was mixed (42 wt%) with kaolin, diatomite, and clay at proportions 15%, 28%, and 15 wt%, respectively. The mixture was dry pressed and sintered at 1450–1550°C for 1–5-h (Sample code M1). The results are compared with other mixture which is prepared by using Alcoa commercial α-Al2O3 powder (Sample code M2). As a conclusion of the work it was found that if appropriately treated and mixed with natural mineral additives, the anodizing sludge can be utilized in the production of mullite-based ceramic materials (Table 7.28) (Ozturk, 2014).
Table 7.28
Physical and mechanical properties of sintered M1 and M2 samples
| Composition | Sintering conditions | Bending strength (MPa) | Density (g/cm3) | Porosity (%) | Water absorption (%) | Densification (%) |
| M1 | 1450°C—1 h | 53 | 2.02 | 26.1 | 12.88 | 63.9 |
| 1500°C—1 h | 54 | 2.27 | 13.1 | 5.76 | 71.8 | |
| 1550°C—1 h | 80 | 2.47 | 0.72 | 0.29 | 78.2 | |
| 1550°C—3 h | 81 | 2.49 | 0.71 | 0.29 | 78.8 | |
| 1550°C—5 h | 84 | 2.49 | 0.72 | 0.29 | 78.8 | |
| M2 | 1450°C—1 h | 72 | 2.15 | 0.81 | 0.81 | 70.3 |
| 1500°C—1 h | 80 | 2.13 | 1.02 | 1.02 | 68.7 | |
| 1550°C—1 h | 75 | 2.11 | 1.69 | 1.69 | 66.8 | |
| 1550°C—3 h | 72 | 2.11 | 1.75 | 1.75 | 66.8 | |
| 1550°C—5 h | 72 | 2.10 | 6.36 | 2.36 | 66.5 |

Ribeiro et al. (2004a,b, 2006), Ribeiro and Labrincha (2008) and Labrincha et al. (2006) performed detailed studies on the use of Al-anodizing sludge in the production of refractory and electrical insulating ceramics. Mullite- and cordierite-based refractory ceramic materials were produced from formulations containing 42 and 25 wt% sludge, respectively. Kaolin, ball-clay, diatomite, and talc completed the formulations. Cylindrical samples processed by uniaxial dry pressing were sintered at different temperatures. The fired properties of materials were evaluated (firing shrinkage, water absorption, bending strength, thermal expansion coefficient, refractoriness, and SEM microstructure) and demonstrated that optimal properties were obtained at 1650°C for mullite and 1350°C for cordierite bodies (Ribeiro and Labrincha, 2008). The last ones can be used up to 1300°C as refractory bricks.
Sludge-fully composed formulations were also produced and tested, revealing the formation of α-alumina and β-alumina (NaAl11O37) on samples sintered at 1450°C or above (Ribeiro et al., 2004a,b). Their electrical insulating characteristics are reported in distinct works (Labrincha et al, 2006; Ribeiro et al., 2004a,b). Mullite-based formulations (containing 42 wt% sludge) show electrical conductivity about four orders of magnitude higher than alumina-based ones (100% sludge). The last ones show insulating characteristics comparable to 90% purity alumina samples. Fig. 7.8 shows bodies processed during those works.

The same sludge was also explored in the formulation of inorganic pigments (Leite et al., 2009; Hajjaji et al., 2009), in some cases combined with other wastes (e.g., Fe-wire drawing and Cr/Ni plating sludges, marble cutting/polishing sludge/fines). Wastes-fully based formulations form stable structures at lower temperatures than commercial (chemically pure reagents) pigments, and distinct colors can be obtained, as shown in Fig. 7.9 (Hajjaji et al., 2012; Costa et al., 2007).

7.4.4.2 Radiological properties
In all these cases, no radiological studies on the considered by-products and ceramics were found. As discussed in Chapter 6 a lot of information is available on bauxite and bauxite residue but only a limited amount of radiological information is available on other aluminum-rich by-products such as aluminum dross tailings. A possible reason for this absence of information is because the activity concentration of most aluminum rich by-products, such as aluminum dross, is very low and this makes it hard to publish this type of results. The activity concentration of Egyptian aluminum dross tailings was found to be very low (Abbady and El-Arabi, 2006, see also in Section 6.5.3.2).
7.4.5 Zircon and zirconia ceramic products
7.4.5.1 Technical properties
In a wide review published in 2007 (Selby, 2007) the ceramics sector is analyzed considering its different products as glazed tiles, porcelain tiles, sanitary ware such as baths and wash basins, frits, ceramic pigments, and engineering ceramics. The main ceramics using NORM raw materials are refractories as well as tiles in which zirconia (the NORM raw material) is mixed with other constituents. In 2005, about 54 wt% of the zircon produced was consumed in ceramics production, while refractories required about 14%. In the same year, 39% of produced zirconia was used in refractories, while 33% was consumed in pigments, and 12% in advanced ceramics and catalysts (Selby, 2007).
Refractories are materials that are designed to maintain strength, dimensional stability, and chemical resistance at high temperature. They are manufactured in the form of bricks, fibers, nozzles, slide gates, valves, and grouts. One of the largest uses of zircon and zirconia in refractories is in the glass industry, where the linings of glass furnaces are made from a combination of zircon and zirconia bricks. The zircon bricks for glass furnaces typically contain 30%–40% zircon. Zirconia is commonly used for nozzles, slide gates, filters, and ceramic linings, where the zirconia content approaches 94%. Refractories are typically made from alumina, magnesia, clays, binders, and zircon or zirconia. There are two methods of fabrication: (a) mixing of the ingredients, pressing into the desired shapes, drying, and kiln firing and (b) mixing of ingredients, melting in a furnace and casting the molten mass into the desired shapes (Selby, 2007).
The main application in the ceramics field is in glazed tiles and sanitary ware. In this application the ceramic has a two-piece body—a clay-based ceramic body is covered with a silicate/borate glaze to provide waterproofing, durability, and decoration. Zircon is added to the glaze for opacification and to provide a white color. The zircon may be added in the milled form as micronized zircon or as a frit. The concentration of milled zircon in the glaze is up to 20% (Selby, 2007).
Frits are ceramic glasses containing silica and boric acid and are manufactured by melting all constituents together and then quenching in water, followed by milling. Their use allows a water-soluble constituent to be added to the glaze and converted into an insoluble form and also to control the vitrification point of the glaze. The zircon content of frits is usually 10%–20% (Selby, 2007).
In contrast to the glazed ceramics, porcelains have a one-piece ceramic body; however, they may also be glazed for decorative purposes. Porcelain ceramic tiles are more resistant to wear than the glazed variety and they are composed of clays, quartz, feldspars, and nepheline syenite together with zircon. In this application the zircon is used in the milled form at concentrations of up to 15% (Selby, 2007).
Ceramic pigments are manufactured by mixing zirconia, quartz, sodium fluoride, and an appropriate chromophore. After firing, the product is milled (Selby, 2007).
There are many “high tech” uses for zirconia in the engineering field such as coatings, grinding media, and cutting tools. Zirconia coatings are applied by plasma spraying, while grinding media are manufactured by high-pressure forming and sintering. Zirconia contents are 60%–95%. Cutting tools are made by fusion of zirconia with alumina, with a ZrO2 content of 5%–10% (Selby, 2007).
7.4.5.2 Radiological properties
Several studies were carried out about the radiological impact of ceramics industries, mainly as regard zircon and zirconia use. An overview of several studies is given in this section.
The exposure pathways in refractories are external exposure from raw materials and products, inhalation exposure from mixing and blending of components and final shaping of products, especially where this is done by grinding. Inhalation exposure can also occur from furnace dusts where enrichment in polonium and lead can occur to levels of 20–30 kBq/kg. The activity concentration of 238U in refractory products ranges from about 2.5 kBq/kg for glass refractories to about 5 kBq/kg for the more specialized zirconia products (Selby, 2007). The refractory industry could be a case for exemption from regulation, but the regulatory body would probably need to be convinced on a case-by-case basis (Selby, 2007).
Serradell et al. (2007) reported a radiological study of the ceramics industry, carried out by the Environmental Radioactivity Laboratory at the Universidad Politécnica de Valencia. The study covered three types of plant: zircon sand milling, ceramic frit production, and ceramic tile production, all of which use zircon as a raw material. These industrial activities include those that use zircon (zirconium silicate) sand as a raw material. This sand is milled for use directly by the ceramics industry or as an intermediate step for producing milled frits that are also used in the production of ceramics (see Fig. 7.10).
The milling plant studied has two lines, corresponding to dry and wet milling processes. The dry process consists of a ball mill (silex or alumina balls) and a dynamic classification system that feeds back the largest particles and produces “zircon flour.” The wet process also consists of a ball mill, followed by a dynamic size classifier and a dryer at the end of the process that produces “micronized zircon.”
Frits are also analyzed in this paper. They are intermediate materials for use in other factories producing end products. They comprise a wide variety of raw materials, of which only zircon is of radiological interest. Most of the raw material mix formulations do not contain zircon, but when they do the ZrO2 content rarely exceeds 18%. This type of plant therefore generally represents no significant radiological risk, either for the employees or for the environment (Serradell et al., 2007).
The manufacture of ceramic tiles includes a great variety of processes. The radiological study therefore focused only on those manufacturing lines in the factory that used zircon. It concluded that some values of total annual effective dose exceed the annual dose limit of 1 mSv for members of the public, mainly in the milling plants, indicating that this type of industry needs to be carefully monitored. Some areas show quite high-external dose values. Therefore, shielding walls are recommended and workers' occupancy of these areas needs to be controlled. Also, the internal dose makes an important contribution to the total dose, so it is very important to set up a highly efficient air cleaning system. Factories manufacturing frits and tiles show lower values of total effective dose—in both cases, only the zircon silo gives an effective dose exceeding 1 mSv, mainly due to external exposure (Serradell et al., 2007).
Bruzzi et al. (2000) reported on the radioactivity in raw materials and end products in the Italian ceramics industry. The natural radioactivity due to the presence of 238U, 232Th, and 40K in zirconium minerals (zircon and baddeleyite) used in the Italian ceramics industry, in tiles and in waste sludge's resulting from ceramic processes, has been measured. The average concentrations of 238U and 232Th observed in the mineral samples (>3000 and >500 Bq/kg, respectively) are higher than the concentrations found in the Earth's crust by one or two orders of magnitude. The specific activities of tiles and sludges are much lower than in zirconium minerals. The 238U and 232Th concentrations in tiles (50–79 and 52–66 Bq/kg, respectively) are not higher than in other building materials. The 238U concentration of sludges (116–193 Bq/kg) is 4–6 times higher than the mean value for the Earth's crust. In general, the data obtained confirm once more that ceramic tiles usually contain small amounts of zirconium compounds and therefore are not a cause of concern from the radioprotection point of view for members of the public; in fact, they produce negligible additional dose values. A similar conclusion was obtained by Turhan et al., from their study on radiometric analysis of raw materials and end products in the Turkish ceramics industry (Turhan et al., 2011).
7.4.6 Overall discussion of the radiological aspects of ceramics
The exposure pathways are very similar for all of the above applications in the ceramics field. The clays and zircon contribute 238U, while the feldspars and syenites contribute 40K. External exposure may arise from raw material storage and materials handling, while inhalation exposure may arise from mixing and blending, or from firing of products. For occupational exposure, the manufacture of ceramics leads to an annual effective dose of 30–200 μSv from external radiation and 10–400 μSv from inhalation, with a total annual effective dose of 10–500 μSv. Public exposure pathways occur with glazed and porcelain ceramics where the dominant pathway is external exposure. Radon in homes is also a possible pathway for these applications. The typical annual effective dose received by a member of the public from glazed ceramics amounts to 7–50 μSv from external radiation, together with an increase of 3–5 Bq/m3 in indoor radon concentration. By contrast, porcelain tiles give rise to an annual effective dose of 3–150 μSv from external radiation and an increase in radon concentration of 10–46 Bq/m3. Frits, ceramic pigments, and engineering ceramics are used only in industrial applications, so do not result in any significant public exposure pathways (Selby, 2007).
Wastes related to raw materials are recycled internally and a typical waste from a ceramic plant has an activity concentration of about 0.6 Bq/g, while waste glaze slurry has an activity concentration of less than 2 Bq/g. There are no processes in the ceramics industries for enhancing the radionuclide levels above the natural levels in the zircon or the zirconia (Selby, 2007).
On basis of doses generally found in ceramics industry, Selby proposes that this industrial sector could be a candidate for a generic exemption from regulation since the annual effective dose received by a worker is less than 1 mSv and that received by a member of the public from the use of the products is of the order of 100 μSv (Selby, 2007).
Information about tile activity concentrations in EU countries is collected in a recent update of Trevisi et al. (2012, 2016) presenting the summary of a database of building material activity concentrations in EU collected mainly by international literature. Unfortunately, information about components, in particular zircon sands, is not generally available in the papers used to build the database. In Table 7.29 a summary of tile information is reported. From analysis of the table a comment emerges: generally, values are not high, also when maximum values are considered. However, the superficial use of tiles must be taken into account to evaluate their radiological impact. For example, the application of the index I from the Council Directive 2013/59/Euratom to tiles can bring to wrong conclusions because I is appropriate to screen materials used in bulk amount, typically concrete (see Chapter 4), and not materials a few centimeters thick. For tiles a more precise screening tool accounting for thickness and density should be used, like the method reported in Section 4.7.6. Application of this type of index can show that all tiles are far from determining doses close to 1 mSv.
Table 7.29
Activity concentrations of tiles used in EU countries
| Country | No. of samples | Ra-226 (Bq/kg) | Th-232 (Bq/kg) | K-40 (Bq/kg) | ||||||
| Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | ||
| Austria | 5 | 48 | 91 | 18 | 56 | 135 | 13 | 528 | 819 | 343 |
| Bulgaria | 2 | 65 | 110 | 19 | 31 | 52 | 9 | 120 | 140 | 100 |
| Cyprus | 2 | 23 | 34 | 12 | 9 | 16 | 2 | 191 | 377 | 4 |
| Estonia | 2 | 56 | 64 | 49 | 84 | 86 | 82 | 314 | 344 | 285 |
| France | 35 | 66 | 180 | 1 | 44 | 85 | 2 | 27 | 68 | 1 |
| Germany | 7 | 71 | 88 | 50 | 62 | 70 | 55 | 433 | 560 | 310 |
| Greece | 53 | 55 | 81 | 3 | 42 | 95 | 2 | 538 | 1200 | 23 |
| Italy | 54 | 61 | 190 | 12 | 46 | 68 | 9 | 627 | 1026 | 150 |
| Luxembourg | 13 | 85 | 100 | 70 | 66 | 74 | 58 | 645 | 669 | 620 |
| The Netherlands | 15 | 51 | 61 | 43 | 55 | 66 | 43 | 553 | 600 | 480 |
| Poland | 1190 | 52 | 190 | 1 | 49 | 120 | 1 | 724 | 1410 | 60 |
| Romania | 4 | 46 | 58 | 792 | ||||||
| Slovakia | 1 | 31 | 41 | 20 | 37 | 41 | 19 | 507 | 770 | 271 |

7.5 Gypsum
7.5.1 Introduction
Gypsum (hydrous calcium sulfate) is a popular raw material for manufacturing various construction products, such as plasters, drywall (wallboard or plasterboard), ceiling tiles, partitions, and building blocks. In addition, Portland cement industry consumes up to 4%–5% of gypsum as a retarder to meet the standard requirements related to the setting times of cement. However, from the radiological perspective the uses of gypsum in plasters and finished gypsum products, which can contain up to 100% of gypsum, can be important.
The advancement of gypsum construction is due to its reduced time and cost. Gypsum and gypsum construction products are known for their excellent workability in fresh state, fast setting and hardening, excellent finish, increased fire resistance, lightweight, white color, acoustic properties for noise insulation, etc.
Gypsum raw materials are available in two forms: as a natural gypsum stone (or anhydrite—anhydrous calcium sulfate) and as a byproduct of many industrial processes, which is called chemical or synthetic gypsum (or anhydrite). The modern gypsum industry uses chemical gypsum to substitute natural gypsum whenever possible, in order to reduce the pressure on natural resources.
Flue-gas desulfurization (FGD) gypsum is a by-product of coal-firing power industry and is the most popular supplement to the supply of natural gypsum. This synthetic gypsum has a higher purity than most natural gypsum. Other types of chemical gypsum include phosphogypsum, fluorogypsum, citrogypsum, and titanogypsum, which are by-products from manufacturing phosphoric acid, hydrofluoric acid, citric acid, and titanium dioxide, respectively.
Among all kinds of gypsum, only phosphogypsum is considered as a raw material of radiological concern.
7.5.2 Phosphogypsum
7.5.2.1 Technical properties
Phosphogypsum is contaminated by chemical and radioactive materials and, therefore, in the world is mostly dumped in stockpiled in controlled areas and only about 15% is recycled; mostly as setting time retarder and in some construction elements (Tayibi et al., 2009; Yang et al., 2009).
Phosphogypsum contains, next to naturally occurring radionuclides, some trace elements such as arsenic, lead, cadmium, chromium, fluoride, zinc, antimony, copper (U.S. Environmental Protection Agency, 1990), which may be leached. Therefore, phosphogypsum is mostly dumped in stockpiled in controlled areas and only a minor part of it is recycled; mostly as setting time retarder and in some construction elements, which include the production of bricks, blocks, tiles, and artificial stone (Kumar, 2000, 2003; Weiguo et al., 2007; Tayibi et al., 2009; Yang et al., 2009). Degirmenci (2008) and some other researchers studied a possibility of the phosphogypsum utilization in combination with fly ash and lime, to produce a cementitious binder. The recent comprehensive publication of IAEA (2013) estimates that recycling rate of phosphogypsum is still very low: less than 5%.
Unfortunately, many valuable materials, which can serve future generations as raw materials in producing electrical energy, chemical, building, and other useful products, are lost in this industrial by-product. The building materials industry seems to be the largest among all the industries, which is able to reprocess the greatest amount of this industrial by-product and benefit man. However, the key problem restraining the utilization of phosphogypsum in construction is radiological effect on the human population.
Recent developments in the phosphate industry in China, India, and some other countries with rapidly developing economies have led to a major increase in the production of phosphogypsum, which in turn has stimulated interest in its use in construction and other fields. As a result, the relevant authorities have taken a greater interest in establishing the necessary conditions for the safe use of phosphogypsum (Hilton, 2008).
7.5.2.2 Radiological properties
The radium (226Ra) concentration in phosphogypsum is 200–3000 Bq/kg (U.S. Environmental Protection Agency, 1990). Building elements made of phosphogypsum, such as wallboards and similar construction elements, give yield to high values of radon emanation together with the mentioned elevated concentrations of 226Ra. Phosphogypsum can be used in Portland cement as a setting time retarder in amounts below 4–5 wt%; then, it is diluted in the concrete and normally does not influence the 226Ra activity concentration, but it increases the radon flux (Kovler et al., 2004).
Exposure levels from building materials incorporating phosphogypsum depend strongly on how the phosphogypsum is used. For example, exposure from finished building products made of phosphogypsum depends on their thickness and density, as well as on radium-226 concentration in phosphogypsum. O'Brien (1997) assumed a 226Ra activity concentration of 400 Bq/kg in phosphogypsum and calculated the annual effective dose from gamma radiation for a person continually occupying the room of dimensions up to 5 m×5 m×3 m lined from all the walls and ceiling by 10-mm wallboard, and found that it does not exceed 0.13 mSv. For the comparison, a measured annual average effective dose from gamma radiation in Australian homes is 0.9 mSv. In other words, such exposure levels are not likely to be of serious concern.
At the same time, experience suggests that the use of phosphogypsum in building materials is not being given the attention that it perhaps deserves (IAEA, 2013). In particular, relatively open microstructure and high porosity of gypsum wallboards, ceilings, masonry blocks, and other building products promotes radon exhalation. For example, Bossew (2003) and Stoulos et al. (2004) estimated radon emanation power of gypsum as 30%, while Kovler (2007) found it even higher—around 50%. Kovler (2007) explains that the reason of such high-radon emanation of gypsum is in its very special microstructure and its high-open porosity. The shape of gypsum crystals is usually longitudinal (fibroid), with well-developed surface area, while the overall density of gypsum product is low—usually 800–1200 kg m3 (Fig. 7.11). These features make radon release from gypsum relatively easy.

The final answer about the safe exposure dose can be obtained only after calculation of the total dose, not only from gamma radiation, but also from radon. O'Brien et al. (1995) estimated the contribution to the annual effective dose due to airborne contamination from phosphogypsum wallboard with enhanced radium content used as an internal lining. For ventilation rates greater than 0.5–1/h, the contribution to the total annual effective dose from inhalation of 222Rn and its progeny exhaled from the wallboard was below 1 mSv. This contribution was reduced, when the surface of the wallboard was painted or coated by cardboard, or if the very fine particles were removed from the phosphogypsum during manufacture of the wallboard (a type of phosphogypsum purification, because it is known that fine fractions are usually more contaminated). The effective doses arising from dust generation during the installation of the wallboard were also estimated to be below 1 mSv.
Unfortunately, no industrial implementation is known so far for phosphogypsum processing and utilization in construction. The central problem of recycling of phosphogypsum in construction is its slightly elevated radioactivity—mainly because of enhanced 226Ra concentration, because other chemical impurities can be extracted relatively easily, for example, by using phase transformations between different kinds of calcium sulfate hydrate and subsequent filtering of the obtained solution. Traditional technologies of purification of phosphogypsum from radium are usually not effective, because of the similarity of chemical properties of radium sulfate and calcium sulfate, when the contaminant salt is isomorphously introduced in the gypsum crystals, and therefore, cannot be washed out from the crystal surface.
In principle, there are three ways of making phosphogypsum free of radium and/or heavy metals (Weterings, 1982), viz.
• starting from clean phosphate rock, i.e., phosphate rock which is free of heavy metals and radium;
• using a clean process, i.e., a process which yields clean gypsum; and
• purifying the gypsum.
Much research has been done on methods of purifying phosphogypsum during the manufacture of phosphoric acid, to obtain grades, which are acceptable for use as plaster or as setting retarder in cement. With all the methods developed, it is mainly the phosphate content, which is decreased; the content of radium and heavy metals is hardly reduced, if at all. The purification technology is based on rearrangement of the crystal lattice during the transition to calcium sulfate hemihydrate (HH) or dihydrate (DH). There are two main variations: HH-DH processes (e.g., Nissan) and DH-HH processes (e.g., Prayon). Radium could be removed by suitable measures during the gypsum purification. Apart from the partial removal of radium, which is part of the wet gypsum purification, no procedures for the removal of radium are operative at present. In the patent literature Ra is removed by a recrystallization of the gypsum in nitric acid or in sulfuric acid with an addition of Ba-ions. Nothing is known about patent-literature concerning the removal of heavy metals from gypsum. However, none of the three alternatives, i.e., the use of clean phosphate rock, a clean phosphoric acid process, or purified gypsum, is at present practicable, and a nonstandard approach has to be used to lead to the desired result: obtaining of gypsum, which is free of 226Ra and heavy metals, provided that it is economically feasible.
Principally new technological approach has been developed recently (Kovler et al., 2015). It was found that the main contaminant of phosphogypsum is radium sulfate, the salt of extremely low solubility (2×10−4). The method is based on mixing hot phosphogypsum suspension containing special chemical reagents to extract the impurities and transition of insoluble radium salt to a soluble compound, which is successfully filtered out from the suspension. The best results demonstrated reduction of 226Ra content by an order of magnitude.
7.5.3 Overall discussion of the radiological aspects of phosphogypsum
The production cost of the environment-conscious gypsum binders can be lower than that of natural gypsum stone, because (a) the raw material is a by-product phosphogypsum, which is widely available in many countries in large quantities; (b) the following technological expenses are excluded: for mining gypsum rock, its transportation, storage, grinding, extraction of silicates, calcite, dolomite, clay, and other impurities containing in rock, with losses of a part of the ground gypsum with these impurities. At the same time, pretreatment increases the process costs.
It has to be emphasized that phosphogypsum recycling would be economically feasible, if industrial installations for the production of environmentally friendly gypsum binders and finished building products are located near phosphate plants, providing the minimum expenses for phosphogypsum transportation and for removing the small amount of the solution of the extracted impurities to the operating neutralization installations of the phosphate plant together with large amount of acidic and radioactive flows of this plant.
In spite of the fact that the problem of purification of phosphogypsum can be adequately solved on technical level, it has to be emphasized that there have been nevertheless a few attempts in different countries to produce gypsum wallboards and masonry blocks from phosphogypsum, which has been purified by simple washing and neutralized by lime (to reduce its pH and remove some acidic compounds). Such simplified treatment is certainly not sufficient to meet the environmental standards, which are getting stricter year by year.
In view of this, there is a need in parallel to develop new environmentally friendly and economically feasible technologies of purification, and also to introduce environmentally safe and economically reasonable standard regulations, which should be based on justified radiological, social, economic, and legislative concepts.
7.6 General conclusion
This chapter provided technical, chemical, and radiological information to support a safe recycling of by-products in four groups of construction materials: (1) construction materials based on Portland cements (both as cement itself and as concrete), (2) construction materials based on alkali-activated binders, (3) ceramics and glass-ceramics, and (4) gypsum.
For most of the construction materials discussed here the recycling of by-products is not a problem from a radiological perspective when taking into consideration the approach of the Council Directive 2013/59/Euratom. Several of the evaluated building materials have an I-index<1 (e.g., coal fly ash or blast-furnace slag recycling in Portland cement-based concretes) meaning that these building materials meet the gamma dose reference level set by the Council Directive 2013/59/Euratom. For only a limited amount of cases (e.g., when using 75 wt% of specific types of red mud in alkali-activated concretes) an index higher than one was found and there is a need to further verify the gamma dose reference level of 1 mSv/year. For the radiological screening of ceramics, that can be used as a layer of only a few centimeters thick and where the density can be quite different from concrete, the use of a density and thickness corrected index is recommended.
As a result of dilution, in general, the concentration of radionuclides, originating from residues, is decreased in the produced construction materials. Aggregates have the greatest influence in the concrete radioactivity because they account for the main fraction of the concrete volume. The relative fraction of by-products that can be incorporated as aggregates in construction materials is very by-product dependent and this parameter will strongly determine the resulting concentration of naturally occurring radionuclides in the construction material. This aspect was demonstrated by means of appropriate mix designs for concretes in order to make a realistic dilution calculation.
Knowing the radiological properties of by-products (Chapter 6) and resulting construction materials (this chapter) facilitates the design of new types of construction materials that are safe considering the recommendations of Council Directive 2013/59/Euratom.
For many construction materials only a limited amount, or no information at all, is available regarding the radiological aspects of these materials. New studies, many of them initiated by the COST network NORM4Building, are in preparation to tackle the gap in the knowledge.
Appendix A Toxic and radioactive waste immobilization by alkali-activated cement and concretes
Materials processed by the alkali activation technique might show strong capability to immobilize a large variety of hazardous and radioactive species (Krivenko et al., 1993; Palomo and López de la Fuente, 2003). The effectiveness level of stabilization is basically controlled by two parameters—mechanical strength and leaching resistance (Jaarsveld et al., 1998). In more detail, parameters such as the setting time (5–72 h), compressive strength (>0.35 MPa), and metal concentration in leachates (mg/L): Cd<0.5, Cr<5, Pb<5, and Zn<300 are important (Palomo and López de la Fuente, 2003; Jaarsveld et al., 1998). Metal bearing waste can have either a positive or a negative effect on the strength development. Palomo and Lopez (Krivenko et al., 1993) have concluded that boron negatively affects Portland cement hydration while it does interfere in the alkali activation process of fly ash. Boron could then be immobilized in the structure of alkali-activated fly ash-lime materials and its leaching rate can be reduced up to 100 times comparing to Portland cement- or lime-based systems.
The resistance of heavy-metal-containing AAMs to leaching in different environments strongly depends on the nature of the heavy metal and on the aggressive components of the leaching solution. Pb could be immobilized effectively by a chemical binding mechanism in AAMs, meaning that its addition in a soluble chemical form is actually preferable (Zhang et al., 2008). Heavy metals in the form of ions, such as Zn2+, Pb2+, Cd2+, and Cr6+, can be effectively stabilized in slag cements activated by NaOH, Na2CO3, and sodium silicate solution (Zhang et al., 2008; Malolepszy and Deja, 1995; Deja, 2002). The results also show that up to 2 wt% Hg2+ ions can be effectively immobilized in the alkali-activated slag cement matrix (Qian et al., 2003).
Wastes show much less interference upon the hydration process of alkali-activated cements than that of PC. However, alkali-activated cements usually exhibit higher shrinkage than PC upon hydration at room temperatures, and cracking risks of the waste-containing monolithic geopolymeric structure are higher. Some alkalis may leach out of the material structure and enter into the environment if the material is immersed (Shi and Fernández-Jiménez, 2006).