Leaching assessment as a component of environmental safety and durability analyses for NORM containing building materials
H.A. van der Sloot*; D.S. Kosson†; N. Impens‡; N. Vanhoudt‡; Talal Almahayni‡; H. Vandenhove‡; L. Sweeck‡; R. Wiegers§; J.L. Provis¶; C. Gascó**; W. Schroeyers†† * Hans van der Sloot Consultancy, Langedijk, The Netherlands
† Vanderbilt University, Nashville, TN, United States
‡ Belgian Nuclear Research Centre (SCK•CEN), Mol, Belgium
§ IBR Consult BV, Haelen, Netherlands
¶ University of Sheffield, Sheffield, United Kingdom
** CIEMAT, Unidad de Radiactividad Ambiental y Vigilancia Radiológica, Madrid, Spain
†† Hasselt University, CMK, NuTeC, Diepenbeek, Belgium
Abstract
In addition to direct exposure to radiation, inhalation of radon or inhalation of dust, other pathways of exposure need to be considered, such as release of naturally occurring radionuclides (NOR) by leaching to soil and groundwater used as sources of drinking water. Evaluation of naturally occurring radioactive materials (NORM) and NORM containing construction products by leaching has been extensively addressed. However, actual NOR measurements in eluates are very limited. The evaluation of release of dangerous substances from construction products is a topic dealt with by national legislators in Europe, in European standardization (CEN) and by US EPA, which has led to recently finalized leaching standards that are leading to the comparable results between EU and the United States. Given the physical aspects of leaching and the chemical behavior of radionuclides is the same as for their stable counterparts, it is logical to address questions about leaching of NORM and NORM containing construction products in same way. In this chapter results for a range of NORM and NORM containing products are presented, which implies that the experience gained in assessing release of stable elements is directly applicable for NORM and NORM containing construction products.
Keywords
Leaching; Leaching test methods; pH dependence; Percolation; NORM; Geochemical speciation; Reactive transport; Impact assessment
8.1 Introduction
NORMs (naturally occurring radioactive materials) may be considered for use as construction material if, in the form that they are used, they meet appropriate standards for protection of human health and the environment. When evaluating construction materials, it is important to consider the full life cycle of the material, including the use scenario as well as the potential for reuse and disposal. If demonstrated to be safe, then use of NORM offers potential sustainability benefits through reduction in energy use and reduction in mining or use of virgin natural resources for production of construction materials (e.g., aggregate, cement, concrete, paving block, tiles, filler for road works, etc.). Important pathways to be evaluated for potential human health and environmental impacts include (i) direct exposure to radiation, (ii) emission of radon, (iii) potential exposures through ingestion and inhalation, and (iv) waterborne pathways through leaching of radionuclides or other contaminants of potential concern (COPCs). Additionally, the potential benefits derived from the use of NORM require demonstration of durable products with acceptable environmental safety.
An important reason to focus on natural radioactivity is that alternative materials (by-products) used in construction as part of the new circular economy targets set by the European Commission may contain natural radioactivity in elevated levels compared with traditional materials used in construction. However, many traditional materials (e.g., granites) also may contain elevated levels of radionuclides depending on their origin and therefore, both traditional and alternative materials require consideration of radiation exposure as part of the determination of their suitability for use (as was already discussed in Chapters 3–7).
As of Jul. 2013, the Construction Products Regulation (CPR) (EU, 2011), provides the regulatory and logistic framework for management of products prepared with waste-derived materials as well as pristine materials used for construction purposes. Although the CPR, which replaced the Construction Products Directive (CPD) (EU, 1989) in 2013, extends the considerations of environment and health from only the service life to the entire lifecycle, the actual criteria to be met by construction products are still a matter for the individual Member States. Only the Netherlands and Germany have set leaching limit values for COPCs in a broad range of construction products to be used for applications with potential for impact of soil and groundwater.
Discussions are ongoing to be able to declare an end of waste (EoW) status to make easier the use of alternative materials that otherwise may be considered waste. The options to define a simple test to declare a waste as EoW (Delgado et al., 2009), have been shown to be more complicated than was initially foreseen (Saveyn et al., 2014). When a material obtains EoW status it becomes a product and is no longer regulated by waste legislation. When it is used for construction purposes, its potential impact on the environment will be regulated by the Essential Requirement 3: Health, Hygiene, and the Environment in Council Directive 89/106 (EU, 1989) and its replacement, Construction Products Regulation 305/2011 (EU, 2011). Although the test methods are being harmonized at the European level, only the Netherlands has set specific criteria on the release (leaching) of substances from construction products regardless of their origin (SQD, 2007). In Germany the main focus is on the alternative materials with potential for use in construction (German Federal Regulation—Mantelverordnunug, 2007). In most other EU Member States, a waste-derived aggregate which has obtained EoW status at EU level will not be subject to testing and compliance with environmental quality criteria. For release of radionuclides to groundwater, the criteria for radionuclides as specified in the Euratom Water Directive (EU, 2013) apply. The results from leaching tests can be applied directly for comparison with these water quality objectives for initial screening purposes. A source-path-receptor approach similar to that used for inorganic substances needs to be applied to realistically account for dilution and attenuation from the source (construction product) to the receptor (e.g., groundwater used for drinking water) when initial screening indicates leaching results to greater than criteria.
When considering end of life (EoL) for a material used in a construction application, one may expect in most circumstances that the material to be judged will be oxidized and largely carbonated (neutral to near neutral pH) due to contact with the atmosphere. For durable metallurgical slags, this relates to the surface of the particles, where the core may still be alkaline. This implies that to evaluate material performance in EoL condition, the pH dependence test (EN 14429, 2015 or EPA 1313, Garrabrants et al., 2012) is an appropriate tool, as it will indicate what changes in leaching behavior to expect starting from the materials initial pH to a final assumed pH condition in EoL status.
In the United States, beneficial use of secondary materials is governed by state regulations, with the US EPA providing guidance to the states with respect to evaluation approaches (US EPA, 2013). However, each state may choose not to follow the EPA guidance and issue their own regulations.
This chapter focuses on the waterborne pathway of potential impacts through leaching1 of radionuclides and other COPCs. Many of the historic leaching tests were based on a simulation-based approach to testing whereby the laboratory test method seeks to mimic specific field scenarios, such as attempting to mimic co-disposal with municipal solid waste as the basis for the toxicity characteristic leaching procedure (TCLP, 1992).2 Even though the percolation test (EN 14405, 2017) was used in developing the criteria in Annex 2 of the European Landfill Directive (2003), the single batch test EN 12457 (2002) or related tests (Laili et al., 2012) are used too often to address questions that are beyond their scope. Single batch test approaches do not provide a basis for comparing estimated leaching performance under different scenarios and have been shown to provide misleading results under a range of circumstance (Van Zomeren et al., 2015). More recently, there is a shift toward measurement of intrinsic leaching characteristics (i.e., characterization of leaching as a function of key release controlling parameters such as pH, liquid-to-solid ratio (L/S), or time) of a material over a range of conditions as a basis for comparison between materials, and using mass transport relationships to estimate anticipated leaching under the range of likely field conditions (Kosson et al., 2002). The implementation of characterization leaching tests has occurred through coordinated development in the United States and the European Union and is referred to by the US EPA as the Leaching Environmental Assessment Framework (LEAF; Kosson et al., 2014). The LEAF is fundamentally different from the simulation-based approach to test methods because it focuses on characterization of intrinsic material-specific leaching behaviors controlling the release of COPCs from solid materials over a broad range of test and environmental conditions, with application of the resulting leaching data to specific disposal or use conditions (Kosson et al., 2002) The LEAF approach will be used as the basis for discussion of leaching from naturally occurring radionuclides (NOR) here, based on similarities in leaching behavior between radionuclides and the corresponding stable isotopes.
Leaching of inorganic elements and naturally occurring radionuclides are controlled by the same set of chemical and physical processes and therefore can be evaluated using a common approach, including leaching test methods and descriptions of use or disposal scenarios. Leaching test results are used in conjunction with simple or detailed mass transfer models to provide a “source term” for evaluating constituent fate and transport from a source to a receptor based on the scenario to be considered. Exposure-based risk assessment or preestablished thresholds for each COPC are then used to determine if the proposed material use or disposal scenario is acceptable. For NORM, thresholds need to consider COPCs both from chemical and radiation perspectives. A tiered approach allows for simplified evaluations to be used for screening purposes, while allowing for more detailed evaluations when warranted. For all materials, including NORM, important considerations include (1) hydrogeologic setting, (2) the full lifecycle of the material (from initial production through use, reuse, and final disposition), and (3) the potential for changing material properties and local environmental conditions over time (e.g., changes in oxidation/reduction state, carbon dioxide uptake by alkaline materials, establishment of preferential flow pathways). Furthermore, the materials may change their leaching behavior in response to blending with other materials (resulting in changes in chemistry from changes in primary constituent composition) or at interfaces between dissimilar materials.
The same tools used for evaluating environmental safety associated with leaching can also play an important role in understanding the durability of many construction materials. Durability may be described in a holistic way as how a building material resists to external physical and chemical attacks, including at the interfaces with other building materials, or to internal interactions between different constituents of the building material itself. LEAF testing may be used to evaluate the susceptibility of materials to loss of primary constituents (e.g., decalcification of cements), and ingress of reactive species through contacting water (e.g., sulfate and chloride attack) or through the gas phase (e.g., carbon dioxide) (Sarkar et al., 2010; Branch et al., 2016).
Such interactions may result in changes of the principal physical properties of the building material such as its mechanical properties (e.g., elastic, flexural, tensile, compressive strength) and shape (e.g., by swelling, cracking) and of its response to subsequent physicochemical attack such as observed in freeze-thaw cycles, exposure to the atmosphere (CO2, O2), infiltration (e.g., salts from deicing), or interaction with aggressive groundwater (e.g., high sulfate concentrations leading to sulfate attack). These principal physical characteristics of building materials are described in a vast amount of literature (Hewlett, 1998; Scrivener and Young, 1995), and not repeated here. In this chapter we rather focus on the release of COPCs emphasis on naturally occurring radionuclides, recognizing that physical degradation of a material may lead to increased leaching of COPCs. However, the mechanisms underlying the responses to chemical and physical processes may predict the durability of materials, and should therefore be well understood.
Basically the same processes that affect leaching from traditional materials will influence the release of natural radionuclides from NORM. This implies that all observations on long-term leaching behavior comparing laboratory testing and field leaching (Kosson et al., 2014) apply. In addition, long-term stresses (e.g., sulfate attack, chloride attack, carbonation, oxidation) as studied in the context of the Cement Barriers Partnership (CBP) on cementitious materials used for containment of nuclear waste are also of relevance (http://www.cementbarriers.org). Studies into release behavior from masonry, geopolymers, and cement-stabilized materials are of direct relevance as well.
The leaching behavior of stable elements is not fundamentally different from that of radionuclides, which means that the leaching behavior of stable elements can be used to understand and estimate the leaching of radionuclides. Previously reported total elemental leaching (e.g., for uranium) can be used to estimate the appropriate radioactive isotopes based on naturally occurring isotopic ratios. In addition, the leaching behavior of stable isotopes can be used to estimate the behavior of radioactive isotopes present as decay products and chemical analogs can be used as a first indication of the possible behavior of specific radionuclides.
8.2 Leaching assessment
The goal of environmental leaching assessment is to provide an estimate of constituent leaching potential for materials under possible management scenarios. The approach is as accurate as practically needed, but also does not underestimate the release of COPCs, here naturally occurring radionuclides. The intended use of assessments may be to evaluate the environmental safety of specific use or disposal options for a class of materials, to evaluate effectiveness of material treatment options, or to characterize EoW conditions for materials with potential for beneficial use. The constituents identified as COPCs will be specific to the material being evaluated. With regard to naturally occurring materials, the history of the materials used will determine which radionuclides are present. This is determined by the original natural resource and the way it is processed. In particular, chemical separation processes, including thermal treatment, can result in differential separation of daughter elements from radioactive decay that behaves chemically differently. Radionuclides are therefore not necessarily in secular equilibrium, and therefore care must be taken in assuming the concentrations of daughter radionuclide relative to the initial radionuclide (Titayeva, 2000). For example, in the Bayer process, to extract aluminum from Bauxite, the process affects the secular equilibrium due to the different chemical behavior of the decay chain elements related to uranium decay (Cuccia, et al., 2011). In addition, red mud is sometimes defined as the sum of the sand and red mud residues, but in fact these are two different residues in the Bayer Process which might be mixed or used separately. Depending on the origin of the ores, the red mud may have quite different radiological composition as shown in Chapter 6.
The broad range of potential uses of environmental leaching assessment implies that there is a need for a graded or tiered approach. This approach needs to provide flexible, scenario-based assessments and allow tailoring of the needed testing and information based on the type of intended use of the assessment and the available prior or related information. Often, an initial screening assessment, using simplified testing and very conservative leaching estimates,3 can be used to eliminate specific COPCs and/or entire materials from further concern and evaluation. It is important to include such initial screening options in regulation, to limit the burden to industry without jeopardizing impact to health and environment.
Only some radionuclides from the natural decay chains may be of potential concern with respect to leaching. According to Gellerman et al. (2002), it may be stated that radionuclides like Pb-210, Po-210, and Ra-228 do not have a significant potential for groundwater contamination as long as only isolated migration is considered and hence the focus should be mainly on the long-lived Ra-226 and U isotopes, which show in practice significant migration distances in soils. On the other hand, Po-210 has similarities to the behavior of Se and thus can be mobile in the mildly alkaline pH domain. For Pb-210 and Th, the interaction with dissolved organic carbon (DOC, generated by degradation of organic matter) can lead to formation of mobile element-DOC complexes. So the environmental context needs to be considered before limiting the COPC to be considered.
Leaching of COPCs most often is strongly influenced by the general chemical state (e.g., pH, oxidation-reduction potential, and ionic strength) of the leachant in contact with the solid, the leaching of major and minor constituents, and the physical characteristics of the material that influence the degree of water contact. The extent and rate of leaching can be determined by constituent liquid-solid partitioning (including consideration of solubility, adsorption to solid phases, available content for leaching, aqueous complexation, etc.), the physical properties of the material that limit mass transport, the degree to which equilibrium is achieved, and the properties of the contacting liquid. Examples of intrinsic physicochemical characteristics are the chemical composition (including elemental speciation and molecular composition, type of mineral phases and their surface chemistry, ionic character of chemical bonds determining ion exchange capacity, type of charge-balancing ions, redox conditions, the presence of hydrophobic additives or coatings, etc.), and also the physical state of the material (crystallinity, porosity, tortuosity, and particle size). Material aging, for example, through pozzolanic reactions or ingress of reactive species (e.g., oxygen, carbon dioxide, sulfate), also may impact material chemical and physical characteristics. Thus observed leaching is the result of the chemistry and mass transfer characteristics (e.g., physical properties, water contact, diffusivity, capillarity) of the system.
Leaching chemistry is primarily controlled by the behavior of the leaching element. It may present cationic behavior (Ni, Cu, Zn, Cd, Pb, Al, Fe), oxyanionic behavior (Mo, Cr(VI), As, Se, Sb, SO4), or soluble salt behavior. The leaching behavior of different elements can be classified into groups, leading to roughly the leaching patterns as a function of pH as shown in Fig. 8.1. We restrict discussion here to the elements of concern related to NORM.
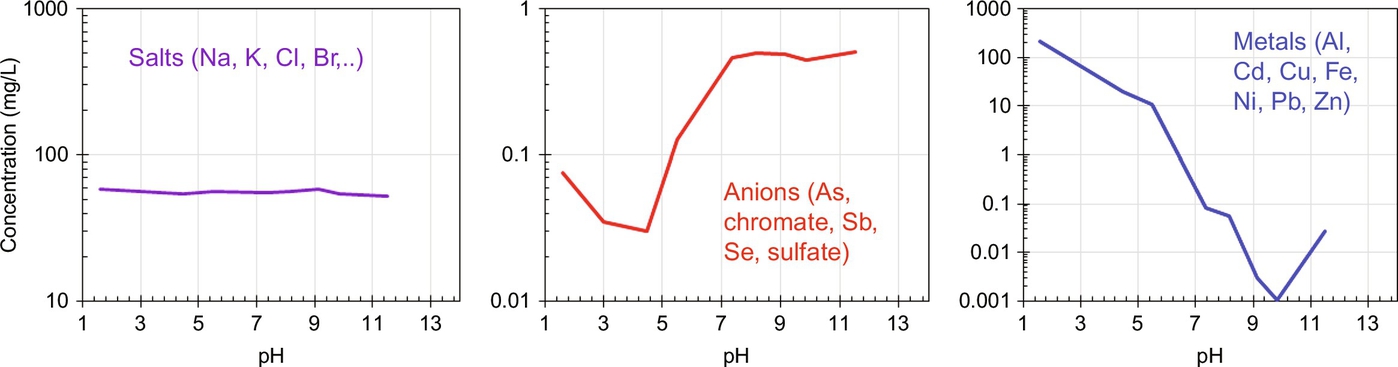
The alkali metals, group I (Na, K, and Cs), are very soluble. K-40 is an important naturally occurring radionuclide, which can leach easily in most cases, and the high predominance of the stable isotopes of these elements make them available as a charge balancing ion in aluminosilicate materials, where Al3+ is present in tetrahedral coordination. Some fraction of the Group I elements such as K+ also can be immobilized within the 3D alumino-silicate network and not present on surfaces, and thus not readily subject to ion exchange and leaching. The difference between available and nonavailable counterbalancing ions can be determined by analyzing the cation exchange capacity.
From the natural uranium and thorium decay series, the most important radionuclides with significant potential for groundwater contamination are radium and uranium if limiting the discussion to isolated migration (Gellerman et al., 2002). We therefore highlight the speciation of Ra and U that may be of concern in cementitious or alkali-activated materials.
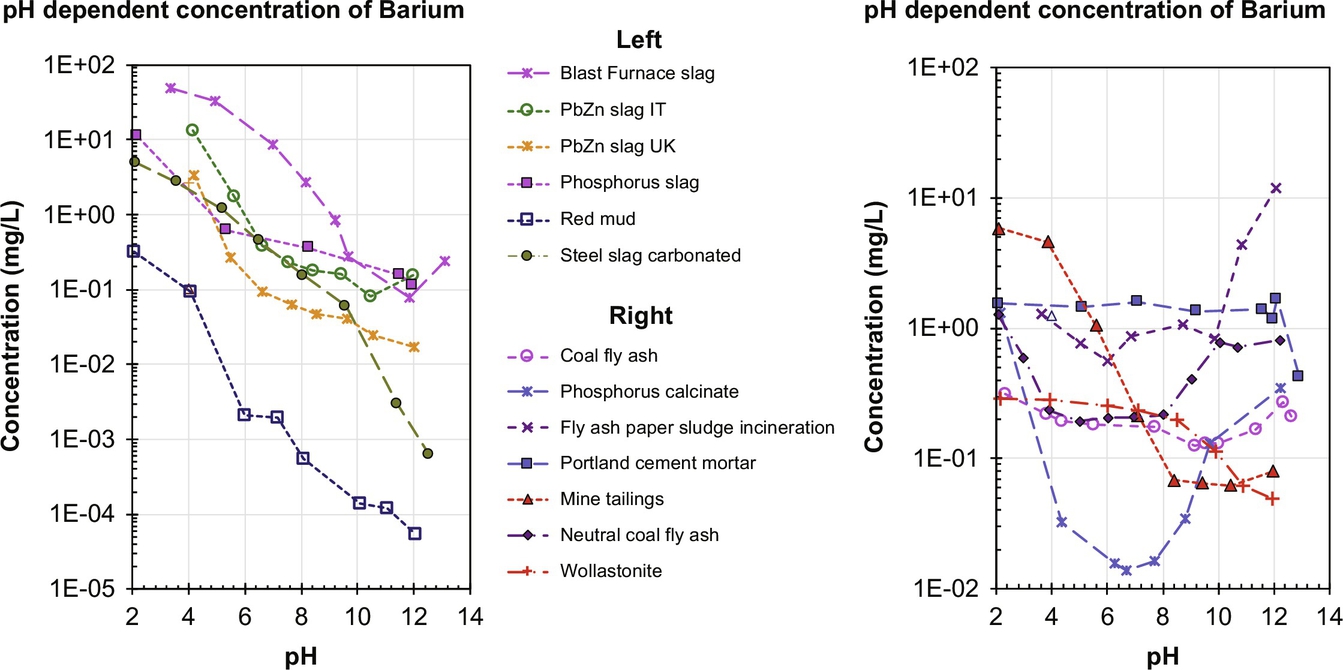
The heaviest element of the alkaline earth metals group 2 is Ra, which is of relevance to NORM as Ra-226 and Ra-228. The environmental behavior of radium is reviewed in the IAEA Technical Report Series No. 476 (2014). We highlight from this report that radium (hydrated ionic radius of 3.98 Å) behaves similar to barium (hydrated ionic radius of 4.04 Å), and therefore, when data for radium leaching are missing, barium can be used as a surrogate element for preliminary evaluation. Ra2+ forms insoluble sulfate, carbonate, and chromate salts, mostly in co-precipitation with barium, another group 2 element, as the radium concentrations rarely approach the solubility limit. However, hydroxide salts of radium are soluble. An extensive study on radium-barium co-precipitation and potential near-field impact has been provided by Grandia et al. (2008).
Uranium is a redox-sensitive element. In sufficiently oxidizing conditions to stabilize the uranyl ion (UO2 2+) uranium can migrate kilometers from its source (Merkel and Hasche-Berger, 2008). At pH≤2.5 the uranyl ion is very stable. Near pH 7, the uranyl ion forms stable complexes with phosphate and carbonate. Uranium sulfate and carbonate complexes are soluble and can migrate with ground water. Uranium may be precipitated by reduction to U(IV), or as phosphates, silicates, arsenates, vanadates, and oxyhydroxides. Tetravalent uranium forms stable hydroxides, hydrated fluorides, and phosphates of low solubility (Závodská et al., 2008).
The above explanation only takes into account the leaching element itself in combination with its complex or salt forming capacities. The way these elements are bound in the matrix may also play a crucial role in their behavior, as far as leaching or environmental stresses do not decompose the matrices themselves. Van Deventer et al. (2007) noted that in geopolymers, every s- and p-group element listed in a study of Bankowski et al. (2004) can be immobilized by the geopolymerization reaction of fly ash, while transition metals either show no immobilization or increased leachability in the metakaolin-based geopolymer, compared with the untreated fly ash. Hermann et al. (1999) demonstrated an optimized two-step technology, in which wastes from former uranium production mines in East Germany were premixed with ordinary Portland cement (PC), and successively mixed with a geopolymeric binder. Immobilizing uranium mine tailing also have been reported by Davidovits and Davidovits (1999), Gatzweiler et al. (2001), Davidovits et al. (1990), Hermann et al. (1999), and Kunze (2003).
In general, it is useful to use leaching tests to (1) characterize equilibrium partitioning between the solid and liquid phases as a function of pH and L/S and (2) determine the rate of mass transport. The materials of interest may be natural aggregates, secondary materials under consideration for beneficial use (e.g., industrial slags, flue gas desulfurization gypsum, coal fly ash, red mud), alkali-activated materials including geopolymers and construction materials containing alternative materials. The contacting water may be from percolation through porous materials, flow around porous or nonporous (or fractured) monolithic materials, or from condensation processes. The material may be water-saturated or unsaturated. The source and fate of the water (and any leached constituents) may include precipitation, runoff, groundwater, surface water, or collected leachate.
8.3 Standard leaching tests and analysis
The leaching test methods developed in EU and the United States (LEAF) are presented in Table 8.1 by type and standard reference. Both the EU and the United States provide comparable results and are designed to measure fundamental leaching parameters including:
Table 8.1
Leaching test types corresponding across different fields and jurisdictions (EU and the United States)
| CEN/TC 345 soil ISO/TC 190 soil | CEN/TC 292 waste | CEN/TC 292 WG8 | CEN/TC 351+ 60 product TC′s | |
| Matrix Test | Soil, sediments, compost, and sludge | Waste | Mining waste | Construction products |
| pH dependence test | ISO/TS21268-4 | EN14429 | EN14429 | EN14429c |
| EN14497 | EN14497 | |||
| EPA 1313a | EPA 1313 | EPA 1313 | EPA 1313 | |
| Percolation test | ISO/TS21268-3 | EN14405 | EN14405 | FprCENTS 16637-3 |
| NEN7373 | NEN7373 | |||
| EPA 1314a | EPA 1314 | EPA 1314 | EPA 1314 | |
| Monolith test | EN15863 | FprCENTS 16637-2 | ||
| NEN7375 | NEN7375 | |||
| EPA 1315a | EPA 1315 | EPA 1315 | EPA 1315 | |
| Compacted granular test | NEN7347 | FprCENTS 16637-2 | ||
| EPA 1315 | EPA 1315 | EPA 1315 | EPA 1315 | |
| Redox capacity | CEN/TS 16660b | CEN/TS 16660b | ||
| Acid rock drainage | EN15875 | |||
| Reactive surfaces | ISO 12782 Parts 1–5 | EN-ISO 12782 Parts 1–5 |
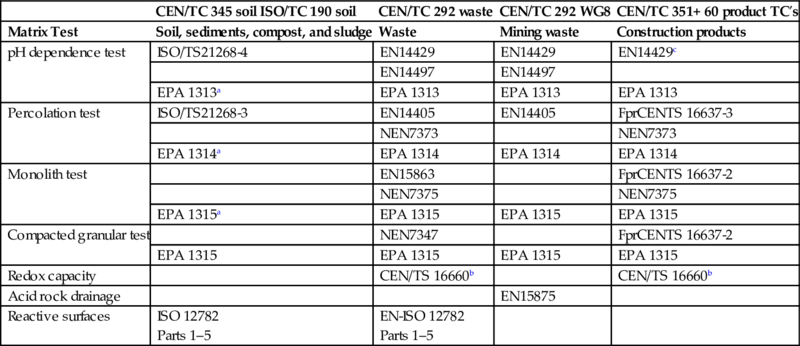
a EPA methods included in SW846.
b Based on NEN 7348.
c Not yet adopted in CEN/TC 351 (very relevant for CPR).
• Liquid-solid partitioning (LSP) as a function of eluate pH;
• LSP as a function of L/S under percolation (column flow) or batch extraction testing;
• Mass transport rates of COPCs leaching from monolithic or compacted granular materials; and
• Additional parameters for assessing special conditions, such as redox status, acid generation potential and parameters for geochemical reaction and transport modeling.
EPA Method 1313 and EPA Method 1316 are parallel batch procedures intended to characterize the LSP at conditions approaching equilibrium as a function of final extract pH and L/S, respectively. Method 1314 and Method 1315 are test methods intended to measure the rate of constituent release under percolation or diffusive/dissolution mass transport conditions, respectively. The test parameters and values specified in these methods have been described in a background information document on the LEAF leaching methods with fully validated methods available (Garrabrants et al., 2010, 2011, 2012).
8.3.1 Characterization leaching tests
Below a short description of the characterization leaching tests is given with an indication of how simplified testing can be carried out, once the release behavior of a material has been sufficiently established.
8.3.1.1 pH dependence
This test provides information about the pH sensitivity on the COPC leaching from the material (EN 14429, 2015; EN 14997, 2015; EPA Method 1313, Garrabrants et al., 2011). The listed methods lead to very comparable results (Garrabrants et al., 2011). The test consists of a number of parallel batch extractions of a material at L/S=10 mL/g (dry weight basis) during 48 h at a series of preset target pH values. The pH is adjusted at the start of the experiment with acid or base (HNO3, NaOH, or KOH) to achieve targeted endpoint pH values. After 48 h of equilibration by end-over-end rotation in polyethylene containers, the suspensions are filtered (0.45 μm) and analyzed. The test provides the response of a material to imposed pH changes and an acid-base titration to understand the response of the material to acid or base reactions under environmental scenarios (e.g., carbonation, infiltration, sulfide oxidation, soil interfaces). Results also are used to determine the available content of constituents (Kosson et al., 2014) and can be used to estimate the amount of carbonate present in cementitious materials (Branch et al., 2016).
8.3.1.2 Percolation test
The column leaching test provides information on the leaching behavior of the material as a function of the L/S (expressed in L/kg or mL/g on a dry weight basis) (EN 14405, 2017; CEN/TS16637-3, 2016; EPA Method 1314, Garrabrants et al., 2012). The listed methods lead to very comparable results (Garrabrants et al., 2012). Seven eluate fractions are collected over the L/S range 0.1–10 L/kg, with the total test duration being approx. 10 days. The eluent is demineralized water, or 1 mM CaCl2 when deflocculation of clays or organic matter is a concern. The material is tested as received, unless the particle size does not conform to the test requirements or a noncrushable material needs to be removed, and upflow (14 mL/h EN 14405; 28 mL/h CEN/TS16637-3; EPA 1314 specifies a flow rate to achieve a residence time of 0.75–1.0 day) is applied through a column with a height of about 25 cm and a diameter of 5 cm. L/S can be related to a timescale through the infiltration rate, density, and height of the application (Hjelmar, 1990; Sanchez and Kosson, 2005).
8.3.1.3 Monolith leach test
The monolith leach test provides information on the release per unit surface as a function of time and it is performed on regular shaped product samples according to standardized procedures (EN 15863, 2015; CEN/TS 16637-2, 2015; EPA Method 1315, Garrabrants et al., 2012). The listed methods lead to very comparable results (Garrabrants et al., 2012). The specimen is subjected to leaching in a closed tank. Demineralized water is used as the leaching solution at an eluent-to-product volume ratio (L/V) of approx. 5. The leaching solution is renewed after 8 h, 1, 2.25, 4, 9, 16, 36, and 64 days. The pH, electrical conductivity (EC), and, if needed, the redox potential (Eh) are measured in all eluates before filtration (0.45 μm) and chemical analysis. From this method, information about the predominant release mechanism can be obtained. The results can also be used to estimate diffusion tortuosity and observed diffusivities for specific COPCs.
8.3.1.4 Compacted granular leach test
For fine-grained materials, such as clays, that are granular, but have a very low permeability when compacted, the release is based on exposed surface area rather than percolation. The compacted granular leach test has been developed for such materials (CEN/TS16637-2 Annex, 2015; EPA Method 1315, Garrabrants et al., 2012). With the exception of the inner vessel with compacted material, the test is performed in the same manner as the monolith leach test.
8.3.1.5 Redox capacity test
The redox behavior and redox capacity test is not yet validated at the international level (CEN/TS 16660, 2015). However, it allows identification of whether a material has the potential to impose reducing properties on its leachate. Since the release behavior under reducing conditions can be very different from the materials behavior under oxidized conditions, it is important to be aware of the oxidation state of a material. In particular, industrial slags can exhibit such behavior. For example, uranium typically is more soluble under oxidizing conditions than reducing conditions.
8.3.1.6 Sorptive phase parameters
The quantities of “reactive” organic carbon in the solid phase (e.g., humic acid [HA] and fulvic acid [FA]) can be estimated by a batch procedure (van Zomeren and Comans, 2007). In short, the procedure is based on the solubility behavior of HA (flocculation at pH<1) and the adsorption of FA to a polymer resin (DAX-8). When test data are not available, an estimate can be made based on measured DOC in eluates from the pH dependence test. The amount of amorphous and crystalline iron (hydr)oxides in the waste mixture can be estimated by a dithionite extraction (Kostka and Luther III, 1994). The amount of amorphous aluminum (hydr)oxides can be estimated by an oxalate extraction (Blakemore et al., 1987). The extracted amounts of Fe and Al can be summed and used as a surrogate for hydrous ferric oxides (HFO) in geochemical speciation modeling (Meima & Comans, 1998). The methods have been standardized in ISO/TC 190 (Soil) under series ISO TS 12782, 2010 parts 1–5 (2010). An estimate of Fe and Al (hydr)oxides also can be taken from the available content as obtained from the pH dependence test (e.g., EPA Method 1313).
8.3.1.7 Chemical analysis
The eluates from laboratory tests and leachates from field scale studies are preferably analyzed for major, minor, and trace elements by ICP (Al, As, B, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Se, Si, Sn, Sr, TI, V, Zn). DOC and TIC (total inorganic carbon) can be analyzed by a Shimadzu TOC 5000a analyzer (combustion to CO2 and analysis by an infrared gas analyzer) or a similar instrument. Cl, F, phosphate, nitrate, and sulfate can be analyzed by ion-chromatography. The multielement methods are highly preferred, as the major elements dictate the chemical environment that controls release of many substances. Although the leaching methods after some modification in equipment used are suitable for organic contaminants as well, this is not further of relevance here in the context of NORM. It is important that the COPCs, analytical methods, and associated detection and quantification limits selected are consistent with applicable regulatory thresholds.
Different analytical methods are required for specific radionuclides in leaching test eluates, as generally the concentrations are too low to be determined by traditional analytical methods. Direct gamma spectrometry of eluates, although easiest to perform, often is not possible due to too low concentrations. Methods applied in the work reported below comprise the following:
• Po-210—Eluates are acidified to 0.5 N HCl. Po-209 or Po-208 is added as tracer for recovery, while stable Pb and Bi are added as nitrate salts. Ascorbic acid is added and the solution is heated to 80°C for 2 h while in contact with a small metal Ag plate. Po208 or Po-209 and Po-210 are quantified by alpha spectrometry of the Ag plate (Fleer and Bacon, 1984; van Weers and Groothuis, 1990).
• Pb-210—Acidified eluate solutions from the Po-210 measurement are evaporated to dryness and the residue subsequently dissolved in 1.5 N HCl. This solution is passed over an anion exchanger. Pb-210, Pb and Bi are eluted with demineralized water and 8 N HNO3, respectively. Pb is precipitated as PbCrO4, filtered and dried. After evaporation of the Bi containing fraction, Bi is precipitated as BiOCl and filtered, dried, and weighed. The weight is used to quantify the recovery and the self-absorption for the beta counting of Bi-210. The Pb-210 is obtained after ingrowth of Bi-210 and decay of Bi-210 in the isolated Bi fraction. Beta counting is carried out in a low background GM counter with continuous gas flow (Fleer and Bacon, 1984; van Weers and Groothuis, 1990).
• Ra-226—The concentration of Ra-226 is determined by allowing ingrowth of Rn-222 in a closed system and subsequent extraction on active carbon with He at – 60°C. At 500°C the Rn-gas is evacuated from the active carbon with He in a Lucas cell and subsequently measured by scintillation counting (Matieu et al., 1980).
8.3.2 Intercomparability of EPA and EU methods
LEAF—methods and corresponding methods from the EU (shown in Table 8.1) are similar in structure and intent. The LEAF and EU methods have only minor deviations in test structure (e.g., the number of test fractions taken) or in test parameters (e.g., specified targets or time durations). This means that the results obtained are equivalent as was demonstrated in parallel validation (Garrabrants et al., 2012). Documentation supporting the development and use of the US and EU test methods is available (Garrabrants et al., 2010; van der Sloot et al., 1997; Hjelmar et al., 2013). In Fig. 8.2 the comparability of data for Cr and Mo between the EU and the LEAF methods for pH dependence in three different materials is illustrated.

8.3.3 Uncertainty
The uncertainty associated with leaching test results is comprised of uncertainties in sample representativeness of the material for which a decision is needed, uncertainty in leaching test performance and uncertainties associated with chemical analysis of eluates. The uncertainty of testing (including analysis) is covered by the intercomparison validation carried out (Garrabrants et al., 2012). Generally, the analytical uncertainty is on the order of <10%–20% depending on laboratory specifications, provided that the measurements are not close to the method detection limit. Typical performance data for the characterization leaching tests are given in Table 8.2.
Table 8.2
Typical performance data for characterization leaching tests including analytical uncertainty specified at less than 10% (Garrabrants et al., 2012)
| Method | Repeatability (%) | Reproducibility (%) |
| pH dependence testa | 13 | 28 |
| Percolation testb | 6 | 16 |
| Monolith leach testb | 8 | 21 |
a Based on eluate concentration.
b Based on cumulative release.
Uncertainties associated with the prediction of release under field conditions are of approximately an order of magnitude, and are influenced by choices in the assumed exposure conditions (e.g., water infiltration/contact, wet/dry cycles, temperature, dimensions of the application, interaction of released substances with the near field soil system, the distance to a relevant point of compliance). A comparison of laboratory test results to field measurement of leaching is provided in Kosson et al. (2014).
8.4 Leaching test results for specific constituents and materials
8.4.1 Leaching data for U, Th, K, Po-210, Pb-210, Ra-226, and others from materials of interest
Limited data are available for the release of radionuclides from NORM with the above-mentioned characterization tests. In Fig. 8.3 results are given for Pb-210, Po-210, Ra-226, Th, and U release from granular phosphate slag by percolation (PrEN 14405, 2016). The total content, available content, relevant test conditions, and derived effective diffusion coefficients are given in Table 8.3 (Hoede et al., 1991). The eluate concentrations of Pb-201, Po-210, and Th are virtually constant, which is indicative of solubility limited release. For Ra-226 and U, the release appears sensitive to the pH change from pH 8.5 to almost 10, but still solubility limited.
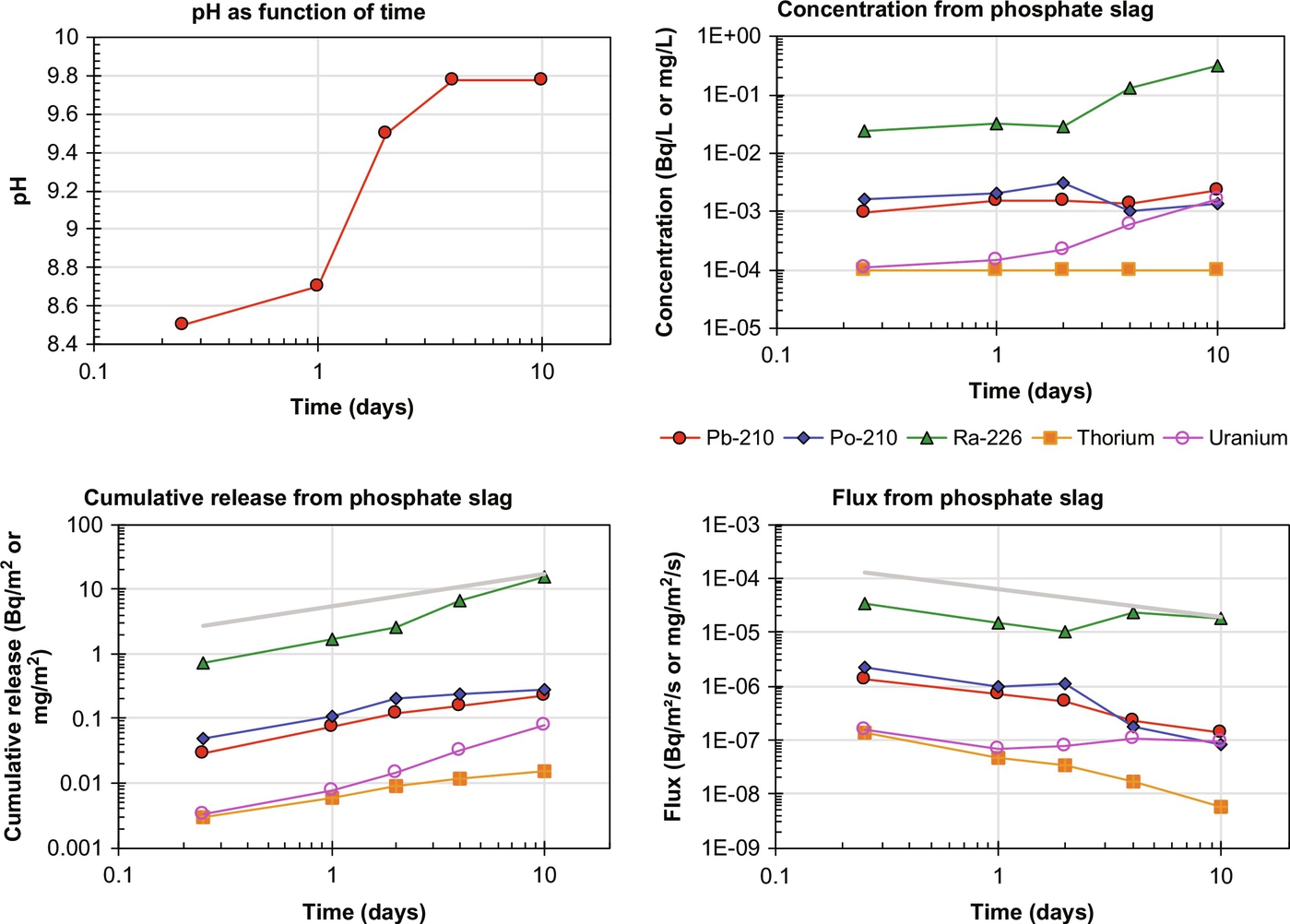
Table 8.3
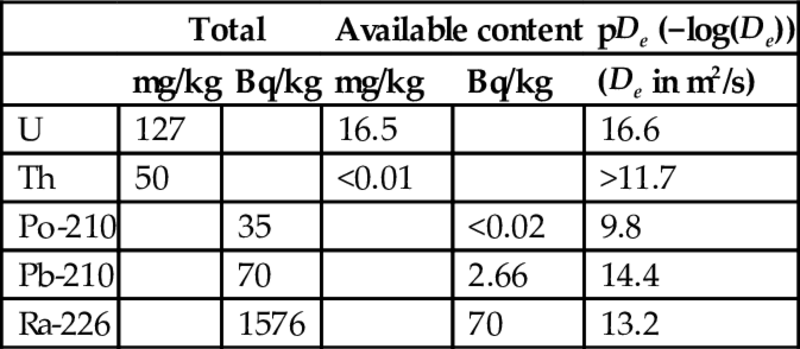
Total content, available content, test conditions, and observed diffusivities (De) for phosphorous slag (5 pieces of slag; surface area 0.2 m2; mass 5.8 kg; water volume 5 times total volume of slag)
| Total | Available content | pDe (−log(De)) | |||
| mg/kg | Bq/kg | mg/kg | Bq/kg | (De in m2/s) | |
| U | 127 | 16.5 | 16.6 | ||
| Th | 50 | <0.01 | >11.7 | ||
| Po-210 | 35 | <0.02 | 9.8 | ||
| Pb-210 | 70 | 2.66 | 14.4 | ||
| Ra-226 | 1576 | 70 | 13.2 | ||
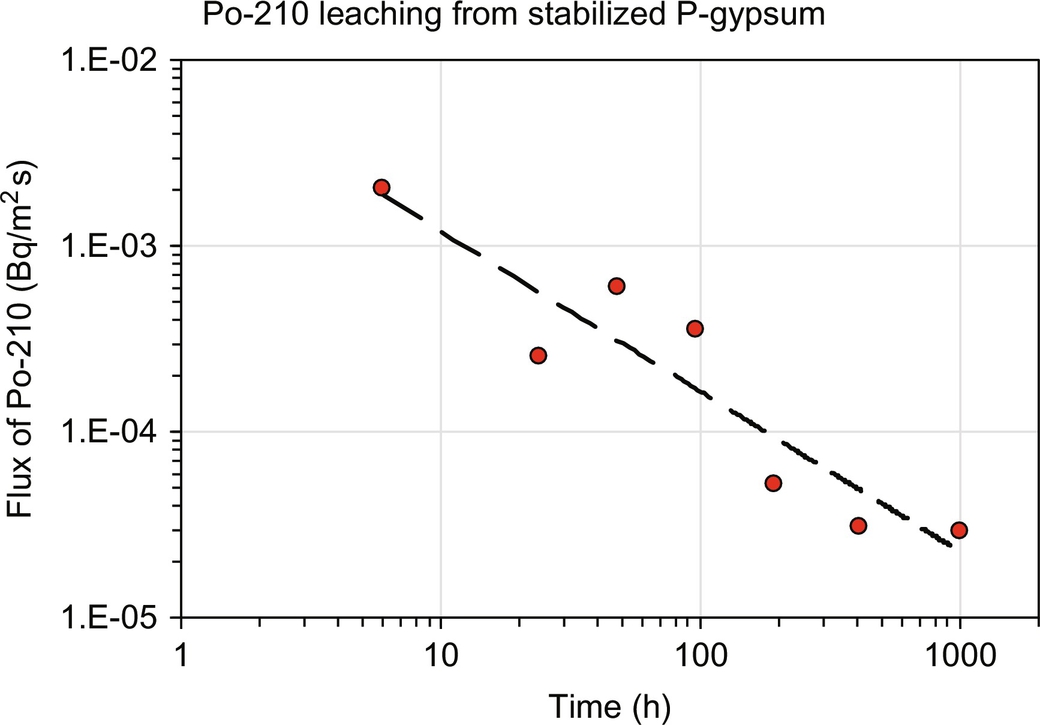
The leachability of Po-210 was measured from a monolithic cement-stabilized gypsum (van der Sloot et al., 1987) using a monolith leach test NEN 7345 (1995) similar to EN15863 and EPA Method 1315. In Fig. 8.4, the flux of Po-210 from the stabilized product is given, which indicates diffusion-controlled release (slope 0.5 in cumulative release graph and slope −0.5 in flux graph). Based on total content, the effective diffusion coefficient derived from the data is 8.2×10−15 m2/s. This seems a very low value, but when corrected based on available content the value becomes 6.0×10−12 m2/s, which is less than an order of magnitude lower than Na mobility in this matrix (3.2×10−11 m2/s). So a fraction of the Po-210 is retained in the matrix more effectively and thus the key question is whether the release remains the same when the “mobile fraction” has been depleted.
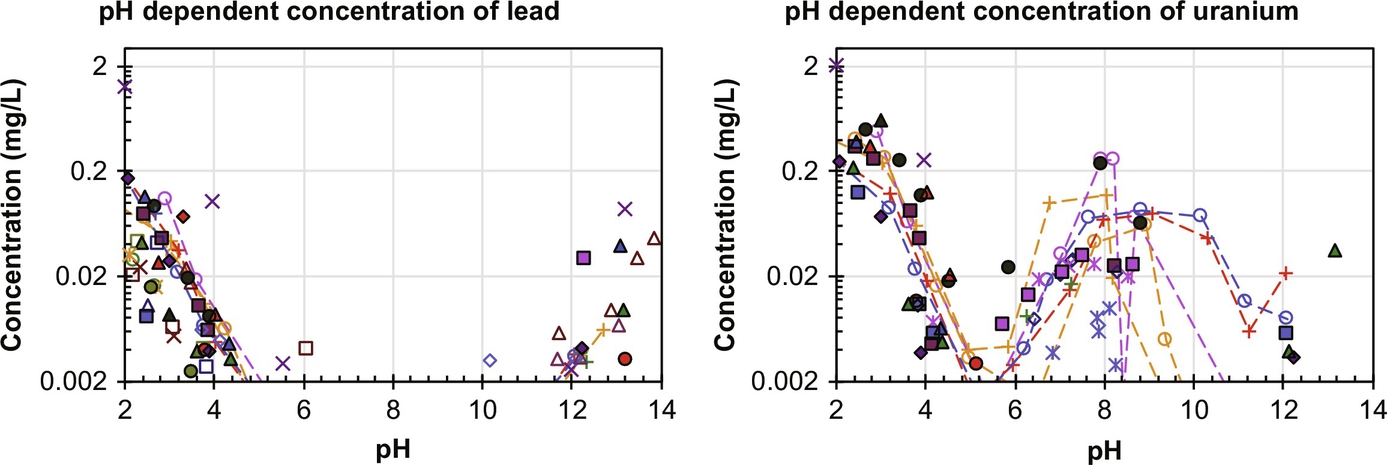
For the stable elements much more data are available for NORM as well as construction products containing NORM. In Fig. 8.5 data on U and Pb from coal fly ash (Kosson et al., 2009) are given, which illustrates for most coal fly ashes the same behavior, with a few coal ashes showing increased U leachability in the pH range 6–11, likely caused by carbonate complexation.
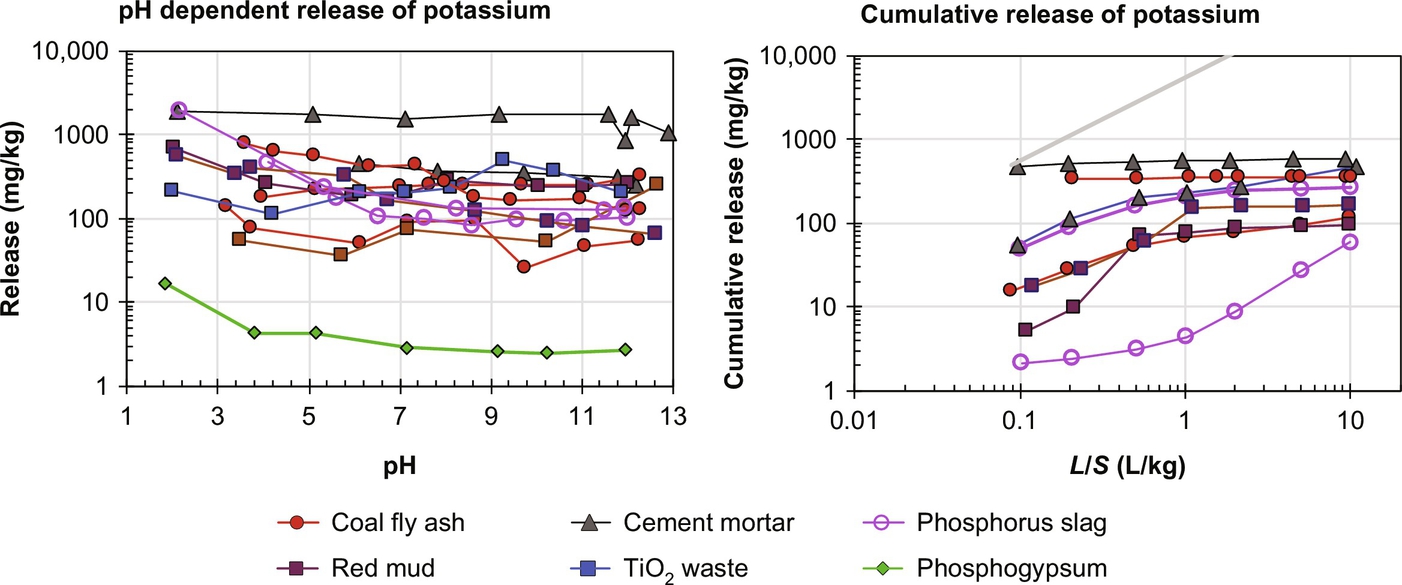
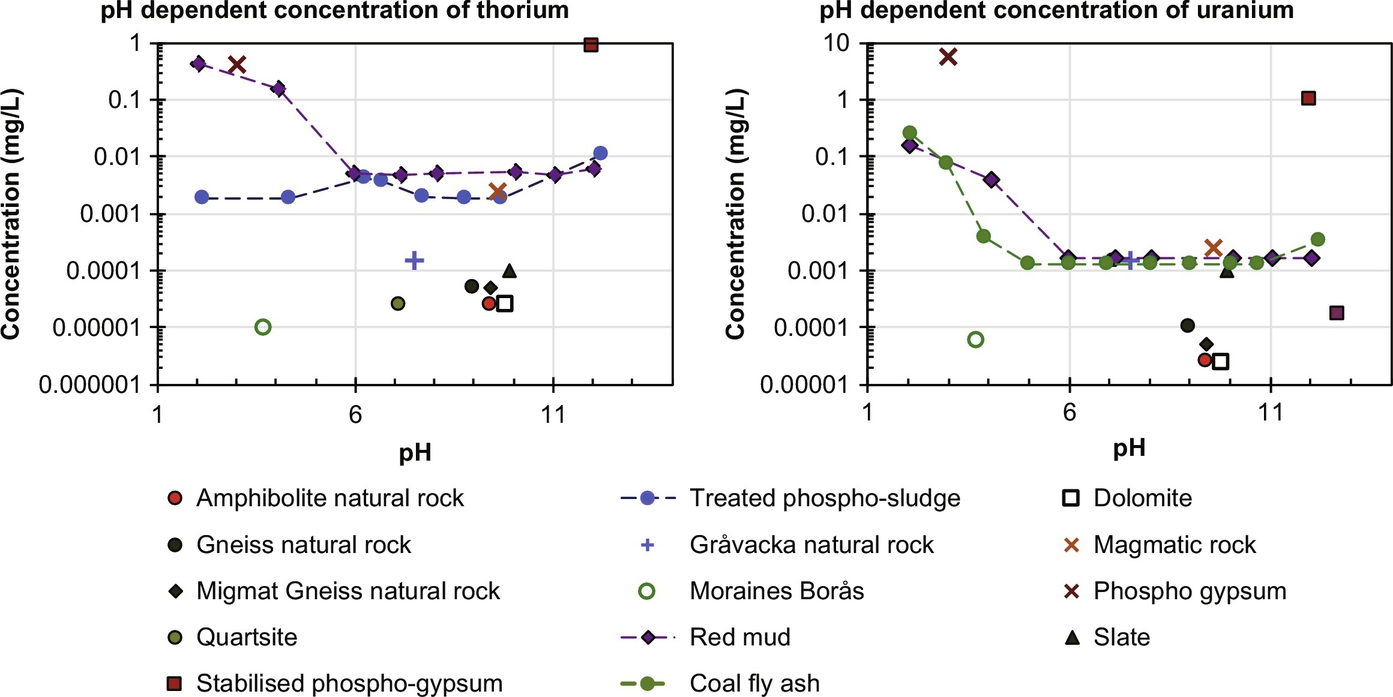
For K and hence for K-40, the release behavior from a range of NORM is mostly independent of pH and K (and K-40) are depleted from the granular material within an L/S of 1 during percolation column testing (Fig. 8.6). The activity of U-238 (30–217 Bq/kg), Th-232 (10–120 Bq/kg), and K-40 (87–303 Bq/kg) series for the same fly ashes has been reported in Roper et al. (2013). In Fig. 8.7 leaching data for U and Th are given for a selection of materials. Natural rocks generally have low leaching levels, whereas phosphogypsum and related phosphate processing wastes show elevated levels of U and Th.
In Fig. 8.8, the leaching of U from a range of monolithic NORM and monolithic products containing NORM is given for the tank leaching test (NEN 7375, similar to EN 15683 and EPA 1315). The concentration of U is more or less constant for almost all materials (except phosphate slag and steel slag armor stone), which implies diffusion-controlled release.
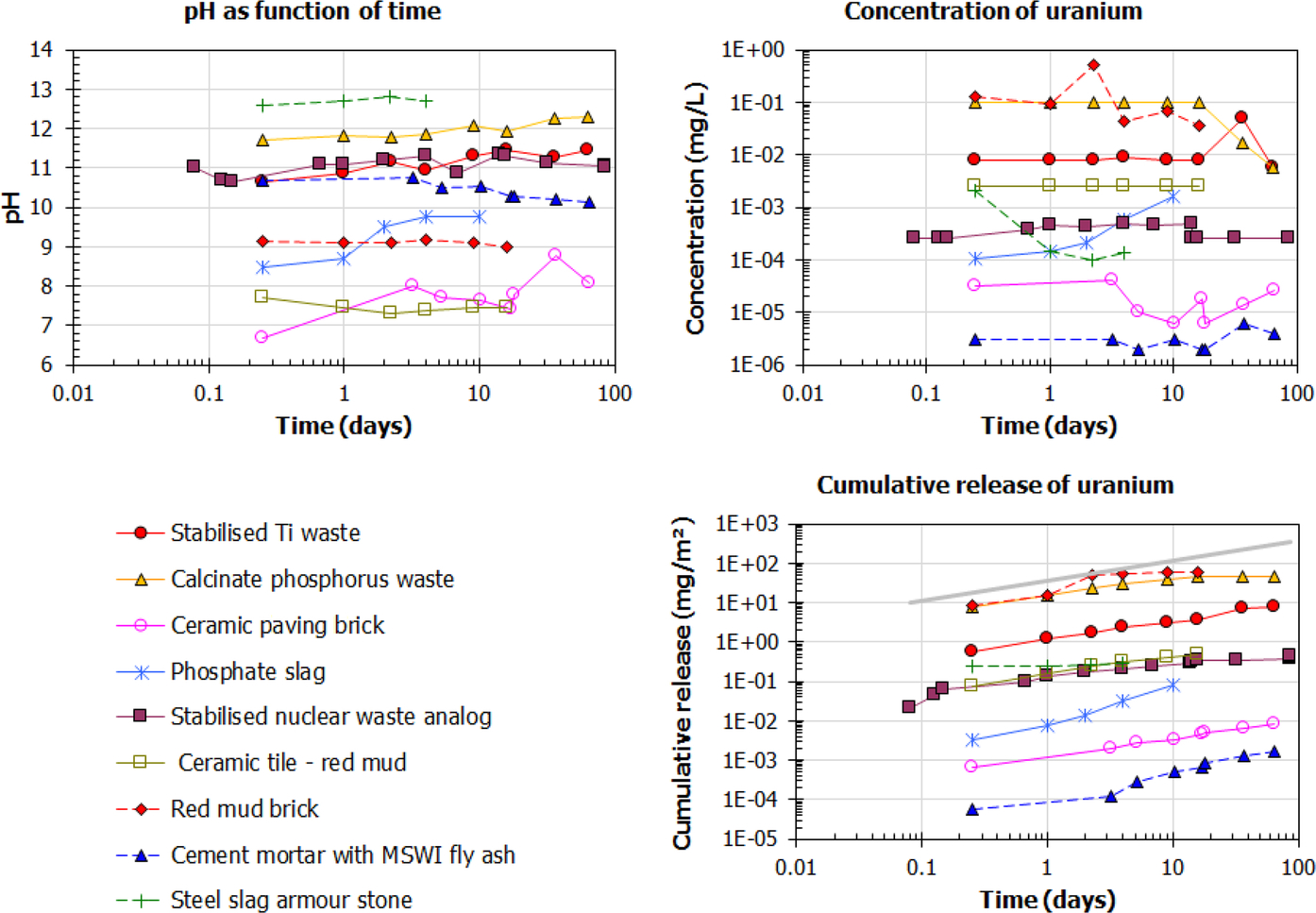
As discussed earlier, chemical analogs can be used to provide an initial understanding of the leaching behavior of some radionuclides. Ba and Ra have many similarities. Therefore the leaching behavior of Ba can give an indication of what to expect for Ra-228 and Ra-226 release. In Fig. 8.9, Ba release as a function of pH is given for a range of NORM.
8.4.2 Leaching behavior of alkali-activated cements and cements with coal fly ash
The leaching behavior of alkali-activated cements (AACs) (i.e., geopolymers) and PCs containing coal fly ash is of interest because these construction materials are made from NORM components. In addition, geopolymers have been used to reduce release of natural radioactivity (Hermann et al., 1999; Rafiza et al., 2013).
For deeper understanding of alkali-activated materials, we refer to Provis and van Deventer (2014). In addition, a comprehensive review has been written by Provis et al. (2015) on the understanding of alkali-activated materials, including comparisons between cements which are basically a calcium silicate structure, and alkali-activated materials that are aluminosilicate structures (see Chapter 7).
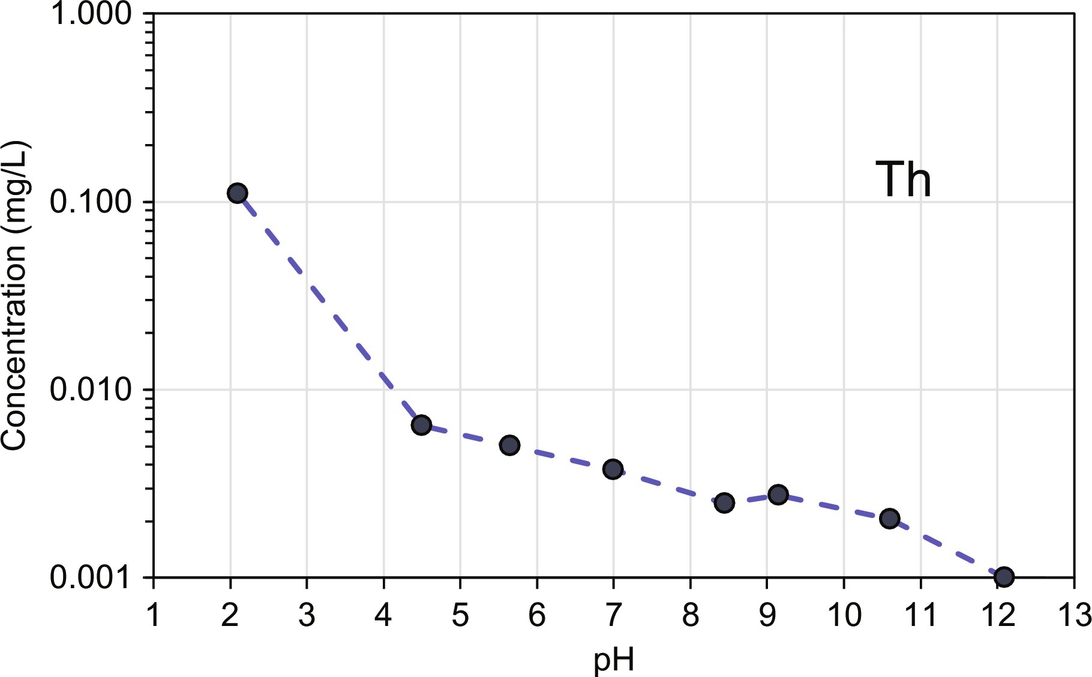
Differences in leaching between AAC and a regular PC mortar are determined by the mineral and sorptive phases controlling leachability. In AAC aluminosilicate hydrate is the primary matrix phase that is present, whereas in PC calcium silicate hydrate predominates as matrix phase. The primary identified mineral phases in the bulk matrix may be less important with respect to leaching than minor phases present on the mineral and pore surfaces within the material. On the other hand the bulk composition will mainly predict the porewater chemistry at the interface of liquid and solid within the pores. In Fig. 8.10 a comparison between AAC and PC is provided, which shows that an important difference is the lack of ettringite in AAC, where for PC ettringite is responsible for retention of several anions by substitution with sulfate at alkaline pH (see sulfate, Cr, Mo, and V). However, less Cr is leachable in AAC at alkali pH than from PC mortar because a greater proportion of Cr is present as Cr3+ than Cr6+. In PC, about 11% of the total Cr content is present as Cr6+ (van der Sloot et al., 2011). In AAC, Al concentration is increasing from pH 6 indicating different phases control Al solubility for AAC and PC. Ca solubility is lower over the entire pH range in AAC compared with PC. Also, Ba and Sr have a strong decrease in concentration with increasing pH. The behavior of these Group IIa elements in AAC, i.e., very good immobilization at alkaline pH, is in line with Van Deventer et al. (2007). In the neutral to mildly alkaline pH range (pH 5–11) silicon solubility from AAC is substantially less than for PC. Cr leaching is less in AAC and looks more like a blended cement (van der Sloot et al., 2011). Na leaching is considerably higher in AAC compared with PC because of the use of NaOH in AAC activation. Metals like Co, Cu, and Ni show increased retention for AAC than PC at pH 5–8. All other measured elements (Cd, Fe, K, Mg, Mn, P, Pb, Sb, and Zn) show very similar release behavior in level and shape indicating very similar release controlling phases. For Th no comparison data in PC are available, although the release behavior is not likely to be very different (Fig. 8.11).

Surface chemistry and interphase chemistry between different phases within the building material itself, but also at the interface with other materials in the building during its lifetime (e.g., steel reinforcement) and at EoL (e.g., in contact with other wastes, acid rain, soil, milling/erosion, freeze-thaw, or other weather conditions) may influence the response to physicochemical attacks.
Chemical and physical attack will have an effect on the release through leaching. In addition, mass transfer processes analogous (but inverse) to leaching are responsible for ingress of reactive species such as chloride, carbon dioxide. The latter lowers material pH and can lead to corrosion of reinforcement steel. Sulfate attack leads to physical deterioration. The use of leaching assessment to understand and calibrate reactive transport models to simulate these phenomena has been described elsewhere (Branch et al., 2016; Sarkar et al., 2010).
We refer to Chapter 7 for the description of the different types of alkali-activated materials and cements and the applicability of the standardized durability tests developed for PC to the different materials is critically reviewed. We selected here some examples of durability tests to highlight the important impact of durability to leaching.
For cement, standardized durability tests have been developed to determine deterioration by chloride ingress, carbonation, sulfate attack, and acid attack to assess the response of the material from external chemical stressors. In addition, intrinsic deterioration by hydration of reactive minerals (free lime) and alkali-aggregate reactions inside the material is another characteristic studied for cement and alkali-activated materials. However, durability tests which have originally been set up for PC mortar or concrete do not necessarily reflect durability if applied to alkali-activated materials (Bernall et al., 2012). For example, standard carbonation tests (ISO-1920-12, 2015 and EN 13295:2004) setup for PC-based materials, are based on elevated CO2 concentrations to increase the rate of aging. In conventional concrete carbonation may lead to reduced pH in the pore water, which can induce corrosion of the steel reinforcement (Chi et al., 2002). Application of this test to aluminosilicates would result in phase changes, which do not reflect the durability toward CO2 under realistic conditions (Bernal et al., 2010). It is expected that under natural CO2 conditions, the pore solution pH may be maintained longer by the excess of alkalis present. Understanding the impacts of aging on AAC materials, including long-term carbonation, requires further study.
A mechanistic understanding of the processes leading to leaching or chemical attack is needed to understand the macroscopic behavior of building materials and the validity of testing methods. The example of sulfuric acid attack toward a geopolymer-based product with high alkali content and a molecular ratio of Na-Si of 0.6 is used as an illustration. An extensive study was performed by Allahverdi and Skvara (2006). At a pH of 1, 2, and 3, different behavior of the geopolymer has been observed. Sulfate attack as tested with the ASTM C1012 may lead to opposite effects when applied to a geopolymer or to a PC-based concrete: whereas PC may expand under these test conditions, the geopolymer tested by Davidovits and Davidovits (1999) shrank. This difference might be related to the presence and absence of sulfate-containing AFm phases in PC and geopolymers, respectively. An extensive review on sulfate resistance in different alkali-activated materials is given in Provis and van Deventer (2014). Clearly, different responses to sulfate attack will lead to different secondary leaching behavior.
Moreover, significant differences in behavior have been observed within the different subtypes of alkali-activated materials. For instance, the type of alkali used as activator of the aluminosilicate source (metakaolin or other clay materials, Ca-rich blast-furnace slag, fly ash with high or low calcium content, red mud or other NORM residues), and the specific combination of alkali type and aluminosilicate source used, may influence the durability.
8.5 Use of geochemical speciation and reactive transport modeling
8.5.1 Modeling of radionuclide release behavior
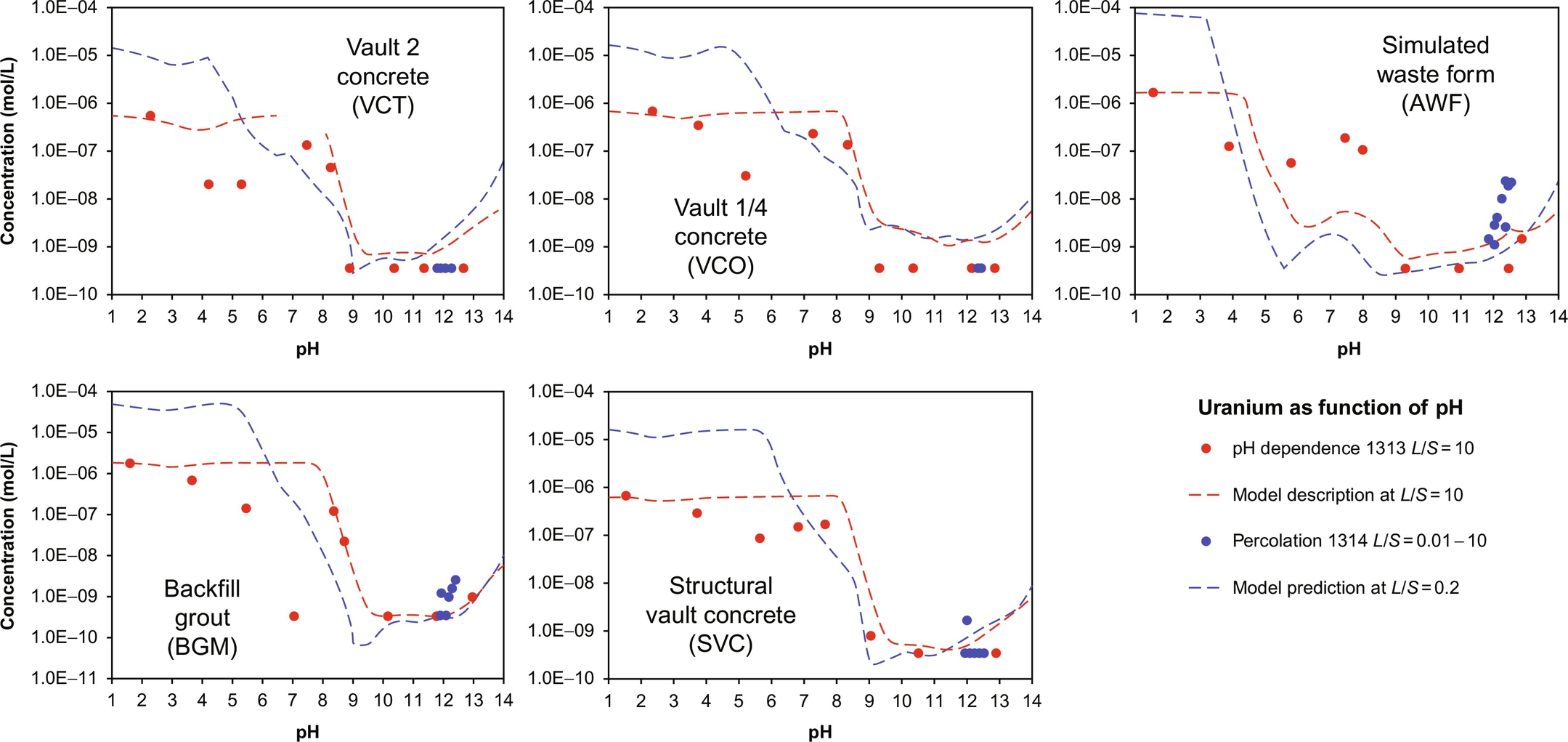
Geochemical speciation and reactive transport modeling of radionuclides is carried out with the same modeling tools as used for stable elements. Based on the test results from the pH dependence test a chemical speciation fingerprint can be derived or used from prior work (van der Sloot and van Zomeren, 2012; van der Sloot et al., 2010). For instance, when radionuclides need to be assessed in a cement mortar (hydrated cement with only fine aggregate) or concrete the chemical speciation fingerprint established for ordinary PC or blended cements can be used (Kosson et al., 2014) with the addition of the thermodynamic data for the radionuclide(s) of interest. Figs. 8.12 and 8.13 show the use of geochemical speciation modeling to simulate pH-dependent leaching test results and the mineral phases and reactions controlling the observed leaching for several cementitious materials.
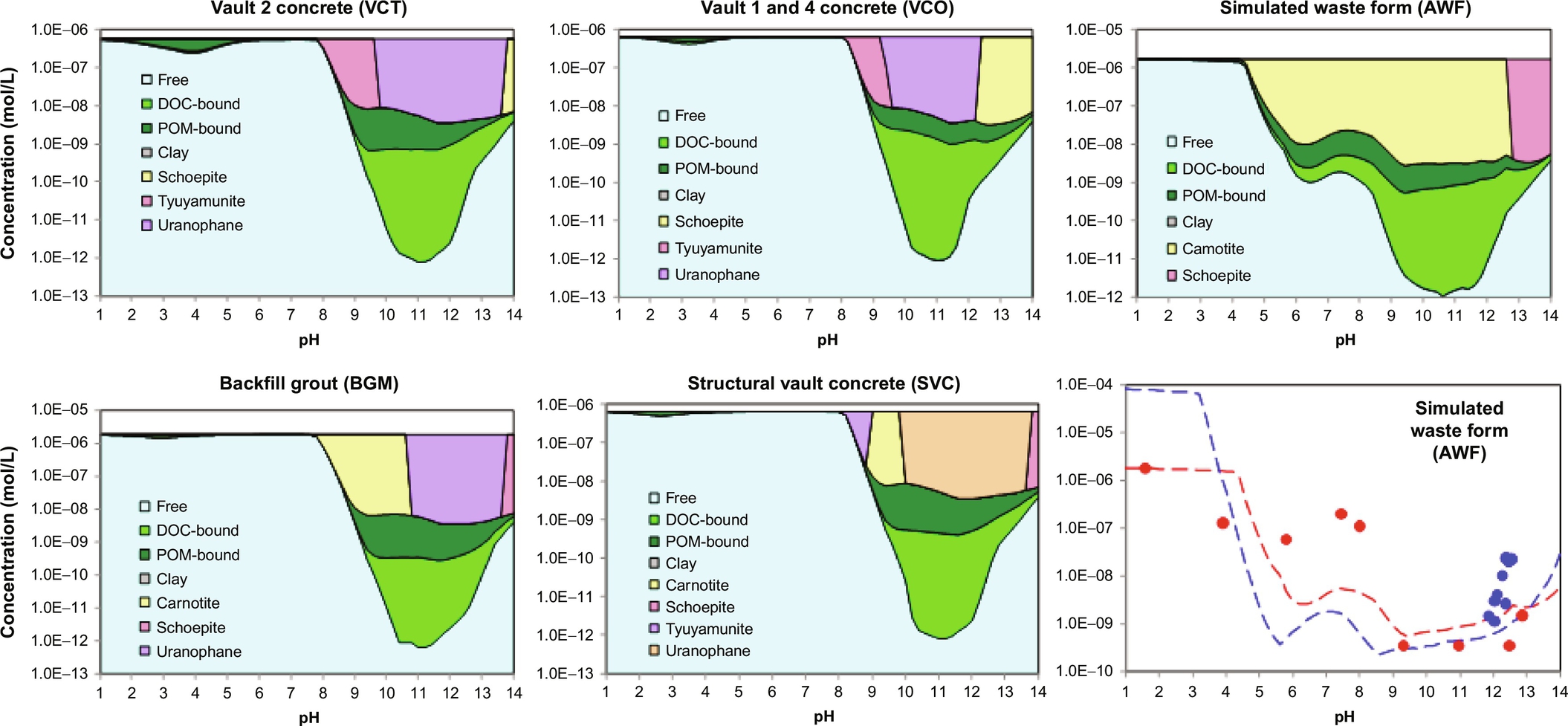
8.5.2 Influence of redox conditions and carbonation
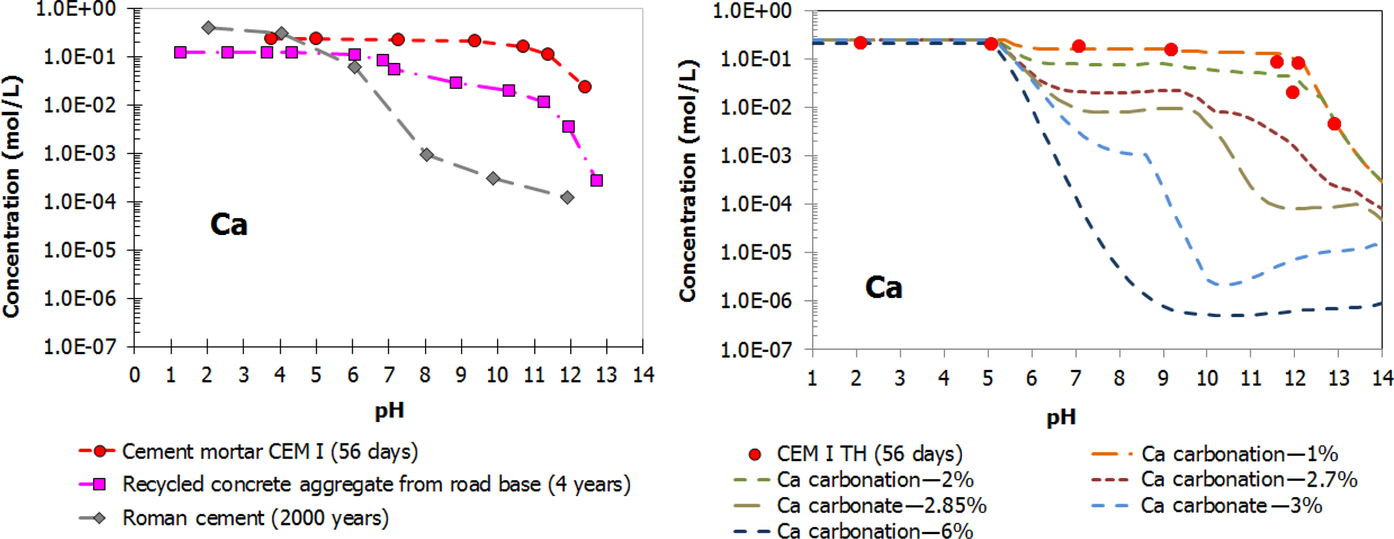
Release behavior is strongly influenced by pH, so when alkaline materials (cement-based products and many slag types) are exposed to the atmosphere, the materials will be carbonated and hence the pH decreases and the release behavior of many substances may change drastically. In Fig. 8.14 this is illustrated for Ca through measurement of field exposed material (PC concrete, recycled concrete aggregate, and Roman cement together with modeling results of increasing degree of carbonation). Ba and Sr have been shown to be affected by carbonation similarly to Ca and therefore it can be expected that Ra-226 will also be affected.
8.6 Scenario-based approach to leaching assessment
8.6.1 Overview of scenario approach
Characterization of leaching behavior using the LEAF testing approach along with scenario-specific information can be used to assemble a leaching “source term” for many environmental scenarios or levels of environmental assessment including:
• screening-level assessments at a site-specific, regional or national scale;
• detailed site-specific evaluations;
• performance comparisons between different materials or treatment processes under specific use scenarios; and
• development of chemical speciation-based models to evaluate potential material leaching behavior under field conditions that may be difficult or impossible to reproduce in the laboratory.
Assessment of the applicability and accuracy of any predictive leaching assessment approach, however, requires evaluation through the use of pilot- and full-scale field studies in which leaching predictions for a particular material based on laboratory testing may be compared to measured leachate concentrations for that material collected under field conditions. Field studies also provide information regarding the relative importance of natural processes on leaching of COPCs including water flow patterns, extent of local chemical equilibrium, and chemical changes due to aging or exposure to the environment (Fig. 8.15).
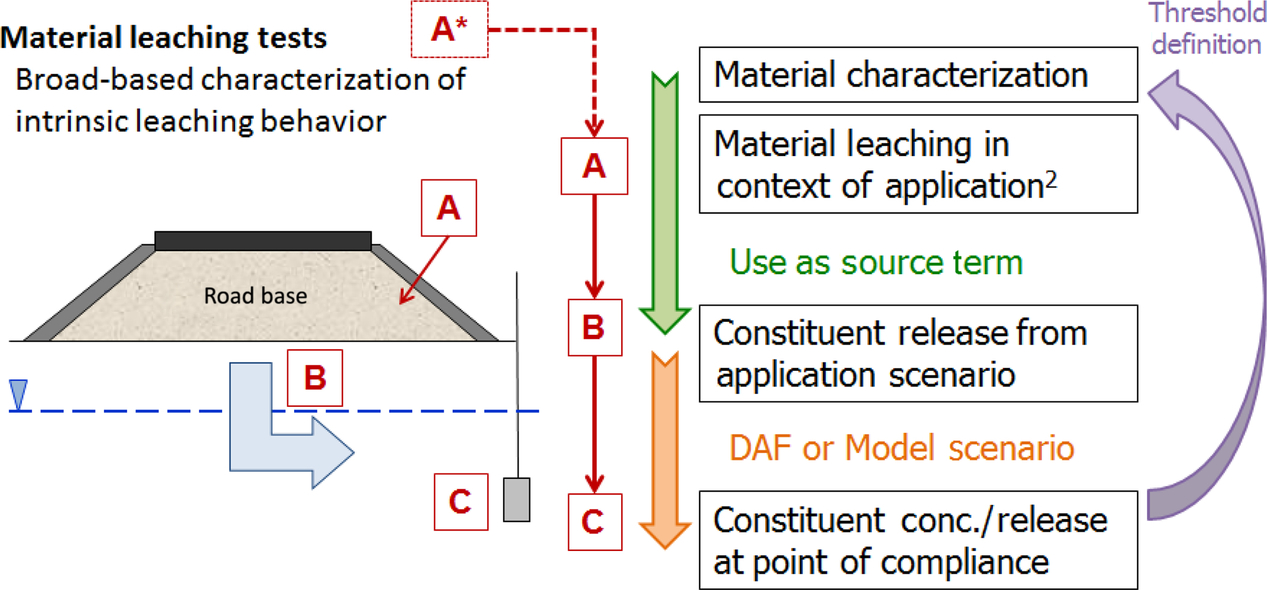
In Kosson et al. (2002), leaching assessment using a performance or “impact-based approach” was proposed, that subsequently has been referred to as LEAF. The LEAF testing methodology allows for both empirical use of testing data for specific scenarios as part of a screening assessment, and use of the leaching test data in conjunction with chemical speciation and mass transport models to provide a more realistic and refined, scenario-specific estimate of constituent leaching that can be used as a source term for risk assessment. While the screening assessment is a bounding estimate of leaching potential, consideration of waste and scenario-specific information allows many conservative assumptions to be refined with further testing data and mass transport modeling results. A tiered-approach was proposed for developing the leaching source term, considering the type of evaluation being carried out, the level of information available, and the extent of conservatism embedded in the estimate. Subsequently, the EPA published its Methodology for Evaluating Encapsulated Beneficial Uses of Coal Combustion Residuals (US EPA, 2013), which describes a tiered approach that can be applied to a more limited set of uses of two secondary materials (i.e., coal fly ash use as a cement replacement in concrete and FGD (flue gas desulfurization) gypsum use in gypsum board).
The observations and information gathered in Kosson et al. (2014) provides a basis for more detailed recommendations provided on the use of LEAF test methods, consistent with the initially proposed methodology by Kosson et al. (2002) and the EPA methodology (US EPA, 2013). These recommendations for use of leach test data only provide the approach for estimating the leaching source term (i.e., concentrations and amounts of a constituents leaching from the material under a specific scenario). Additional determinations are needed to define or account for (i) the location that serves as the basis for exposure assessment following constituent leaching release from a source scenario (e.g., point of compliance), (ii) dilution and attenuation in the vadose zone and groundwater or surface water from the point of release to the point of compliance, and (iii) appropriate exposure scenarios or reference thresholds (e.g., human health or ecological thresholds). These evaluations can be incorporated into a model of constituent fate and transport leading to possible receptor exposure (e.g., groundwater transport to a drinking water well, with water ingestion as the exposure pathway).
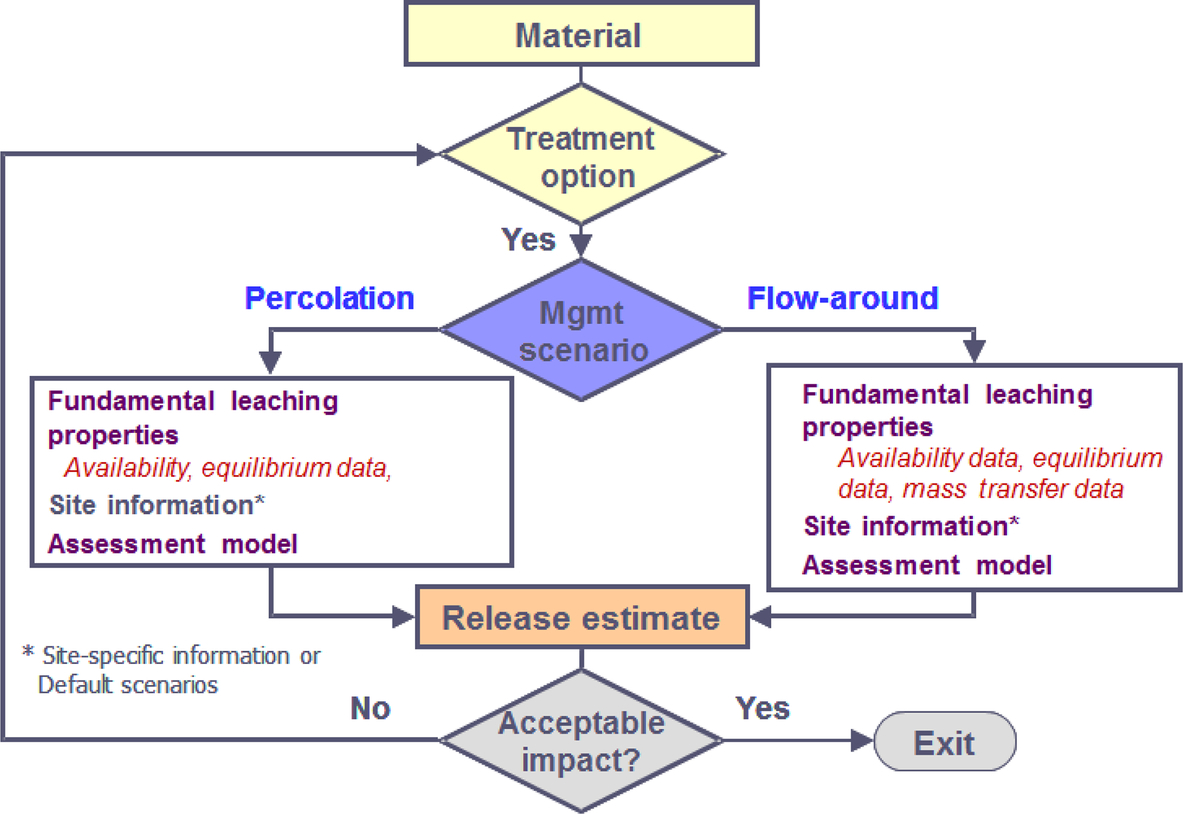
Defining the material use scenario is the first step to selecting the appropriate leaching tests and basis for interpreting the resulting data. The extent of information needed as part of the scenario definition increases as the evaluation seeks to achieve a more detailed and refined estimate of constituent leaching. The initial scenario definition should at a minimum include determination of the applicable pH domain, range of oxidation-reduction conditions, and the primary mode and amount of water contact (see Fig. 8.16).
8.6.2 Case study radiological impact assessment
The following scenario is presented in order to further illustrate the modeling approach used to estimate the radiological dose to a member of the public. A total of 2×108 kg of NORM-containing building waste is dumped on a waste heap (~20 m in height). The material is sandwiched between two layers of low permeability clay (0.5 m at the top and bottom of the material) to reduce infiltration and leaching to the local groundwater. Radionuclides from the U-238 and Th-232 decay chains are assumed to be in secular equilibrium, and their activity concentrations in the building waste material correspond to an activity concentration index for the gamma radiation emitted by building materials of 1 (EU, 2014). As such, first an activity concentration of 300 Bq/kg is considered for U-238 and its daughters, based on the Ra-226 limit in the activity concentration index. Second, an activity concentration of 200 Bq/kg is considered for Th-232 and its daughters (direct limit from the activity concentration index). The dose is estimated for the following two cases:
(1) Intact, fully functional clay (both hydrologically and chemically) and best estimate sorption (reference case)
(2) Degraded clay (i.e., clay has lost its hydraulic integrity) and decreased sorption
For the reference case, typical hydraulic properties for clay and best estimate sorption parameters (i.e., the solid-liquid distribution coefficient, Kd) are used as inputs into the calculations. For the degraded clay and reduced sorption case, the clay hydraulic conductivity is increased whereas the Kd values are decreased (by a factor of 10) to simulate increased infiltration and leaching.
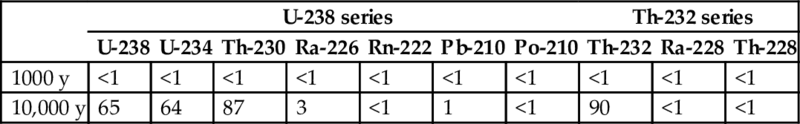
Leaching of NOR from the building waste material is simulated using the one-dimensional transport code HYDRUS-1D (Šimůnek et al., 2013). For the setup of the model, the following profile is assumed: a 20 m thick NORM layer separated from the local water table by 0.5 m of low permeable clay and 1.5 m of native sand. The water flux through the waste (i.e., the upper boundary) is set to 30 mm/year for the intact clay case and to 270 mm/year for the degraded case. The sorption parameter Kd used in the simulations is nuclide-specific to represent the different geochemical behavior of NORs in the profile materials and hence their different mobility. The simulation is performed over a time frame of 10,000 years. It should be noted, however, that during the simulation period of 10,000 years, the maximum concentration in the leachate was only reached in the worst case scenario runs (i.e., degraded clay and reduced sorption). The lower infiltration rate through the intact clay layers combined with the high sorption of the NORs considerably delayed the time to reach the maximum leachate concentration in the reference scenario. Nevertheless, the results from the worst case scenario illustrate the maximum radiological impact of the leaching NORs. The fractions of NOR leached to the aquifer in case of degraded clay and decreased sorption are presented in Table 8.4. The calculations with respect to leaching indicate that the effect of increased infiltration due to the degraded clay is secondary to the effect of decreased sorption.
Table 8.4
Percentage [%] of the total waste inventory leached for conditions of degraded clay and decreased sorption (worst case scenario)
| U-238 series | Th-232 series | |||||||||
| U-238 | U-234 | Th-230 | Ra-226 | Rn-222 | Pb-210 | Po-210 | Th-232 | Ra-228 | Th-228 | |
| 1000 y | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 10,000 y | 65 | 64 | 87 | 3 | <1 | 1 | <1 | 90 | <1 | <1 |
After leaching to the groundwater, the radionuclides are assumed to migrate to a nearby water well, 50 m downgradient, through a sandy aquifer. For radionuclide transport in the groundwater, the maximum activity concentration below the waste heap is assumed. A dilution factor is applied to the maximum concentration in the groundwater below the disposal site taking into account the area of the disposal site, the average groundwater velocity, the thickness of the aquifer, and the discharge rate.
In the next phase, a biosphere model is used to calculate the activities of the NOR in different biosphere compartments and the subsequent dose to humans. The biosphere model represents the transfer mechanisms of radionuclides in the biosphere, along with related assumptions and simplifications. Within the model, transfer is considered rapid with respect to the modeling scale and equilibrium can be assumed between the different media (e.g., soil-to-plant, uptake by animals). Radioactive decay and ingrowth are also considered in the model. To calculate the dose to man, conservative human habits are assumed. In this case study, the water in the well is used by a self-sustaining farmer. It is assumed that the farmer uses the maximum activity concentration in the well water for irrigation of food crops and pasture and livestock watering and only uses food products coming from the contaminated area. Several transfer pathways are considered in this scenario such as transfer of radionuclides from irrigated water to plants, transfer of radionuclides from irrigated water to soil, and transfer from plant to animal through watering and feed. To calculate the dose to the self-sustaining farmer, exposure pathways such as ingestion of well water, ingestion of food products (e.g., vegetables, meat, eggs, milk), external radiation from soil and inhalation of dust (ingestion), and radon when working on the field are taken into account. When considering all these transfer and exposure pathways, a maximum annual dose can be calculated, albeit this is only a very simplified scenario for illustration and there are great uncertainties with respect to the radionuclide transport assumptions. The doses a farmer could get after 10,000 years in case of the reference scenario and for the worst case scenario are presented in Table 8.5.
Table 8.5
Maximal dose rates [mSv/year] to self-sustaining farmer after 10,000 years
| Reference scenario with intact clay and best sorption | Scenario with degraded clay and reduced sorption (Kd/10) | |
| U-238 series (U-238, U-234, Th-230, Ra-226, Rn-222, Pb-210, Po-210) | 1.30×10−16 | 0.0143 |
| Th-232 series (Th-232, Ra-228, Th-228) | 1.43×10−21 | 0.0376 |
These calculations suggest that the annual dose to a member of the public following leaching of radionuclides from a waste heap with NORM containing building waste is below the maximum exposure limit of 1 mSv/year.
In addition to the dose-calculation for humans, a radiological impact assessment is also conducted for wildlife. Based on the concentration of NOR in the soil after irrigation, which is considered the peak concentration, an assessment is done using the ERICA tool for terrestrial ecosystems (Brown et al., 2008). With the ERICA tool, calculations can be performed to estimate the risks to selected animals and plants (e.g., mammal, worm, bird, grass, tree, etc.). A dose and risk quotient are calculated based on a 10 μGy h−1 no-effect-dose-rate (a risk quotient of 1 means no risk). For the worst-case scenario, in which degraded clay and increased leaching are considered, the risk quotient is ~10−6.
8.7 Conclusions and recommendations for evaluation of norm
Use of NORM residues, including use as aggregates and addition to PCs and AACs, is being considered more extensively to reduce waste management requirements and to reduce energy and natural resources usage through replacement of traditional materials. Leaching and durability assessment are important components of evaluating environmental safety and protection of human health.
The leaching of radionuclides present in NORM is controlled by the same physical-chemical processes that control leaching of stable inorganic elements. Thus leaching data from total elemental analysis of elements with radioactive isotopes (e.g., K, U, Th, Pb) can be used to gain an initial understanding of the leaching of the corresponding radioisotopes. In addition, chemical analogs can be used to provide an initial understanding of the leaching behavior of some naturally occurring radionuclides (e.g., Ba as an analog for Ra). However, there are only very limited data available on the leaching of specific radionuclides from NORM and further testing and assessments should be supported.
The leaching of specific constituents from NORM is a function of the primary material matrix, the mechanism by which each radionuclide is retained in the primary matrix (e.g., by incorporation into the matrix network structure, adsorption onto pore surfaces, co-precipitation with a distinct mineral phase, or association with solid organic matter), and the contacting water chemistry (e.g., pH, redox, carbonate concentration).
Leaching and durability of materials are closely linked for some material degradation processes. Leaching of primary components and ingress of reactive species (e.g., chloride, sulfate, carbon dioxide) through liquid or gas phase transport during aging may change the physical durability and leaching characteristics of NORM and NORM containing construction materials. Standardized tests developed for assessing aging and durability of ordinary PC materials may provide misleading results and may not be applicable for alternative cementitious binder chemistries such as AACs and mortars and concretes produced from such cements. Further evaluation of the aging processes and long-term leaching of alternative binder chemistries is needed.
The leaching assessment framework LEAF and its underlying leaching methods are applicable to a wide range of materials, field scenarios, and treatment process decisions for NORM and construction products containing NORM. CEN and US EPA leaching methods produce the same results as shown in intercomparison studies, thereby facilitating exchangeability and comparability of results. Use of these methods in characterization testing also will provide very detailed insight in controls and possible difference in release behavior of stable elements and radioisotopes and between stable elements acting as surrogates (e.g., Ba for Ra-226 and Ra-228).
For granular materials, the pH dependence test and the percolation test should be used.
For monolithic materials, the pH dependence test and monolith leach test or compacted granular leach test should be used.
For major sources and types of NORM, parallel measurements of stable and radionuclides of relevance would be highly revealing to understand release controlling phases and thereby provide better means to deal with changes occurring in the long term (carbonation, oxidation, physical degradation, etc.).
By focusing on fundamental leaching properties a single data set can be used for evaluation of multiple use and disposal options. Accumulation and comparison of data provides basis for defining material classes through ranges in release and characteristic behavior of material types, thereby reducing the need for and intensity of testing as part of quality control by the NORM residue provider. After characterization and demonstration of compatibility between stable element behavior and radio-isotopes much more simple testing will suffice for regular control, if needed. Simplified testing after characterization in a tiered approach allows for cost-effective applications.
As in EoL material is likely to become size reduced and fully oxidized due to exposure to the atmosphere, the pH dependence test provides a very useful means to assess behavior under such conditions provided a realistic pH domain is selected for the final evaluation (mostly near neutral pH as a result of carbonation or exposure to the natural environment).