THERAPEUTIC PROMISE
THERAPEUTIC PROMISE
GLOSSARY
computational modelling Use of a computer to simulate the behaviour of a biological system. Computer models can be useful to understand how systems are constructed and to test what happens when they are perturbed. The use of computational approaches in biology is called bioinformatics.
CRISPR-Cas9 Latest technology for precise genome editing. The CRISPR (‘clustered regularly interspaced short palindromic repeats’) and Cas9 system was discovered in bacteria, where it acts as a primitive immune system to protect against invading genetic material from viruses. Its ability to recognize precise DNA sequences and cut them had been exploited to engineer a powerful tool for cut-and-paste techniques on eukaryote genomes.
expressed sequence tags (EST) Short subsequence of a cloned cDNA that can be used to identify gene transcripts for quantification and for gene discovery. They are relatively short fragments that represent bits of expressed genes.
germ line Cell that gives rise to the gametes for sexual reproduction. Germ cells undergo meiosis, followed by cellular differentiation to produce mature gametes, either eggs or sperm. Gametes contain the genetic information that will be transmitted to the next generation.
induced pluripotent stem cells (iPSC) Stem cells that were generated from normal adult cells by a process of reprogramming. iPSC can be differentiated into different cell types.
lentiviral vectors Modified viruses that are used to deliver genes for gene therapy. They are RNA viruses (for example, HIV) that can be engineered to carry genes that are delivered when the virus infects the patient cells.
metagenomics Study of genetic material obtained from environmental samples. DNA sequence analysis reveals the hidden diversity of microscopic life and the microbial world. Metagenomics has expanded rapidly due to the falling price of DNA sequencing technologies.
nucleases Enzymes that cut DNA. Researchers have engineered these natural enzymes so that they can target specific DNA sequences for genome editing. For example, Zinc-finger nucleases (ZFNs) use a special protein domain that recognizes precise DNA sequences. Researchers use ZFNs, together with TALEN and CRISPR-Cas9 technologies, to cut-and-paste DNA sequences and edit genomes.
oncogenicity The capacity to induce tumours. Genes that induce cancer are called oncogenes. Genes that prevent tumour formation are called tumour suppressor genes.
oocyte Female gamete (egg) or germ cell involved in reproduction. It is produced in the ovary during female gametogenesis. Cloning experiments use ‘enucleated eggs’, which are oocytes in which the nucleus has been removed.
pluripotent Capacity of a stem cell to give rise to several different cell types. Pluripotent cells can generate all of the cell types that make up the body. Embryonic stem cells are considered to be pluripotent.
somatic cells Biological cells that form the main body of an organism. There are hundreds of different types of somatic cell types in the human body that make up the organs and tissues. Somatic cells are not transmitted to the next generation and are distinct from germ cells and gametes.
stem cells Undifferentiated cells that can differentiate to generate more specialized cell types. Embryonic stem cells can generate all the different cells in the embryo (they are pluripotent), whereas adult stem cells can normally only generate cells for specific tissues.
TALEN Enzymes that cut specific sequences of DNA. Their full name is ‘transcription-activator-like effector nucleases’ because they are made by fusing transcription proteins to nucleases. They can be engineered to cut any desired DNA sequence and have become a powerful tool for genome editing.
transgenic organism Animal or plant generated by introducing a foreign gene (a transgene) or DNA. The transgene can change the characteristics (phenotype) of the organism. Sometimes referred to as genetically modified organisms and the source of much public debate about safety issues.
virus Small infectious agent that can replicate only inside living cells. Viruses can infect all types of life, including animals and plants and bacteria. The study of viruses is called virology. Viral particles, called virions, contain genetic material (DNA or RNA) and an outer protective coat called the capsid. Most viruses are so small that they cannot be seen with a normal light microscope.
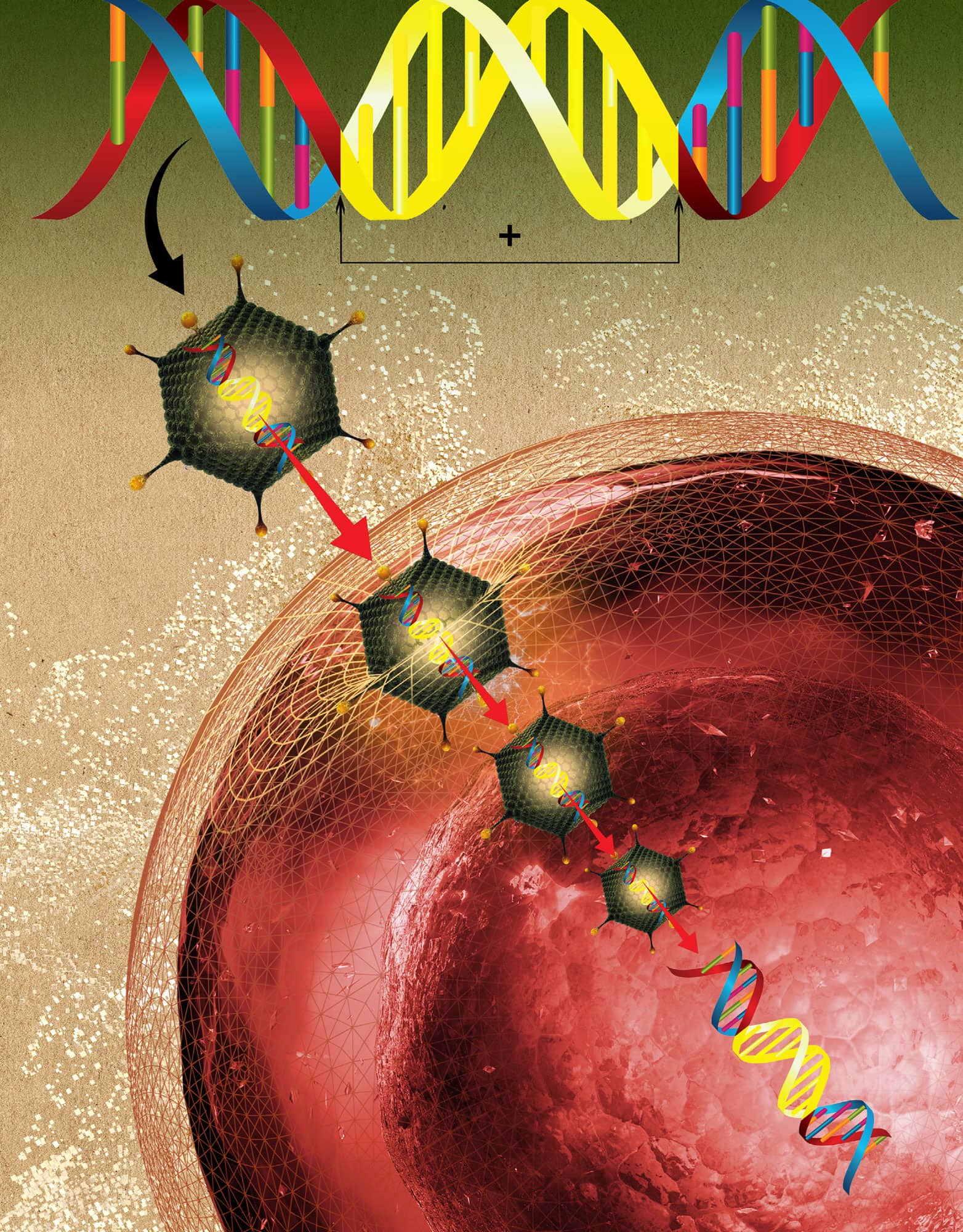
GENE THERAPY
the 30-second theory
When researchers realized that some diseases are caused by mutations in single genes, they proposed that gene therapy could be used to correct the problem gene with a normal copy. It might even be possible to add genes to change the properties of a given cell. In most cases gene therapy uses a vector (delivery agent) to transfer the therapeutic gene into the target cell. Viruses are the most effective vectors, because they persist and can often also integrate into the host genome. There are still obstacles to efficient and safe gene therapy, including the difficulty of making sure the genetic material is safely put to use and that the genes do not provoke an immune response in the body or lead to the formation of tumours. So far, gene therapy has successfully treated inherited conditions of the haematopoietic system (the organs that make blood), including severe immunodeficiencies and leukodystrophies (genetic diseases that affect the brain, spinal cord and the peripheral nerves). And progress was reported in gene therapy of haemophilia B and inherited retinal dystrophies (which causes progressive blindness). Gene therapy of the future will benefit from technological advances in vector design and production. Gene therapy is making progress to treat more complex diseases, such as cancer.
3-SECOND THRASH
Gene therapy inserts genetic material into cells to confer new characteristics, to correct genetic diseases or to strengthen defences against cancer.
3-MINUTE THOUGHT
Recently, researchers developed innovative tools (called engineered nucleases) that can cut the genome at very specific positions, promising new possibilities for precise intervention into the genome. These molecular machines make it easy to disrupt a gene or add DNA in a specific place. Such strategies could correct a genetic disorder by replacing the mutated DNA with a normal sequence, while leaving the gene in its physiological context.
RELATED TOPICS
See also
PERSONALIZED GENOMICS & MEDICINE
3-SECOND BIOGRAPHY
LUIGI NALDINI
1959–
Italian doctor who developed lentiviral vectors to be used in gene transfer
30-SECOND TEXT
Alain Fischer
Viruses invade cells and can integrate their DNA into the host cell's genome. This makes them ideal as a vector (or delivery agent) to use in DNA therapy.
PERSONALIZED GENOMICS & MEDICINE
the 30-second theory
The Human Genome Project fuelled the emergence and development of DNA sequencing technologies, which can efficiently decipher the sequence of any genome. The cost of sequencing a human genome has dropped dramatically, from billions of dollars two decades ago to just $1,000 today. This progress makes it possible to access the sequence of our own genomes. Whether we like it or not, we live in an era of personal genomics and the quantified self. A genome sequence gives access to our past, but also, to some extent, to our future. Our DNA carries genetic variations from our ancestors and tells us something about their origins. Other variations are not neutral and can have consequences for our health. ‘Reading’ the genome can provide clues about where we come from, but also about risks of developing a disease, depending on the influence of the environment and other gene variants. A practical application of personal genomics is ‘personalized medicine’. Until recently, most drugs were prescribed on the assumption that they would be effective for everyone. But by analyzing the genome sequence of an individual we can select more appropriate therapies and tailor dosages to prevent adverse side-effects.
3-SECOND THRASH
Personal genomics and personalized medicine promise to improve individual treatment because we now have access to the human genome sequence at a reasonable cost.
3-MINUTE THOUGHT
Access to individual genome sequences at an affordable cost raises numerous ethical issues. Analysis of genomic sequences is closely regulated by law in many countries to avoid any potential genetic discrimination. Having access to our own genome sequence can also be stressful. In a few clear-cut cases, a specific DNA variation is likely to impact our health. But, in most cases DNA variations only imply a potential risk.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
J. CRAIG VENTER
1946–
American biologist who played an important role in the race to sequence the human genome
FRANCIS COLLINS
1950–
American geneticist and leader of the Human Genome Project
30-SECOND TEXT
Reiner Veitia
Understanding an individual's genome can allow doctors to tailor specific treatments for a range of diseases, including cancer.

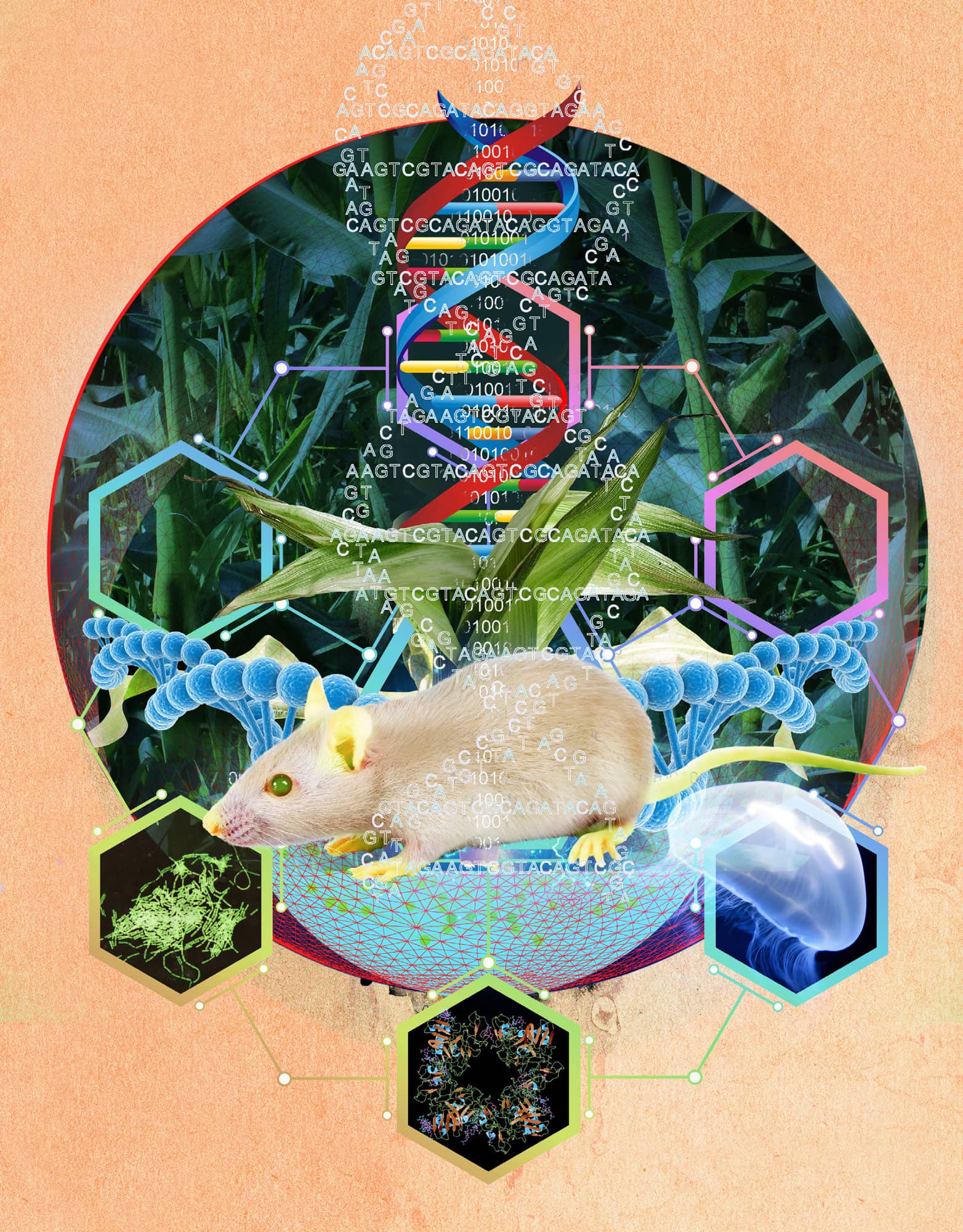
SYNTHETIC BIOLOGY
the 30-second theory
Synthetic biology is a relatively new field and scientists have many different definitions of what it is. One underlying theme is the application of engineering principles to the components of a cell, to elicit a particular action in response to an input. Advances in biotechnology and the computational modelling of biological processes enable us to manipulate existing genetic or biochemical pathways or to create artificial ones. These engineering techniques hold for molecules, cells, tissues and organisms. For example, designing an enzyme that is capable of cutting DNA at a specific sequence could be considered synthetic biology. The replacement of a DNA or protein component in a living organism by a non-natural component is also synthetic biology. Bacteria that shine light in response to a chemical in the culture medium or bacteria able to kill tumour cells are also products of synthetic biology. The common underlying theme is that the universality of the genetic code allows scientists to engineer new DNA sequences that will confer new properties to the recipient cells. This emerging discipline raises hope – because it is now possible, at least in principle, to create living organisms for many new applications.
3-SECOND THRASH
Synthetic biology involves the rational design of biological components and systems, based on knowledge of the biochemistry and functions of natural organisms.
3-MINUTE THOUGHT
The advent of synthetic biology raises ethical questions. There are concerns about potential threats to the health of living organisms or the environment if an engineered molecule or organism were to escape from a research laboratory. There are also concerns about the fairness and the (in)appropriateness of owning patents on a living organism and its components.
RELATED TOPIC
See also
GENETICALLY MODIFIED ORGANISMS
3-SECOND BIOGRAPHIES
STÉPHANE LEDUC
1853–1939
French biologist and chemist, first to use the term ‘synthetic biology’, in 1910
GEORGE CHURCH
1954–
American geneticist who has played important roles in the fields of personal genomics and synthetic biology
30-SECOND TEXT
Reiner Veitia
Synthetic biology is being used to create artificial nucleic acids, which could help scientists to answer questions about the origins of life itself.

GENETICALLY MODIFIED ORGANISMS
the 30-second theory
Imagine a mouse that gives off an eerie green glow like a jellyfish or a bacterium that makes human insulin. Although it sounds like science fiction, researchers have actually learned how to exchange genetic information to create these examples of genetically modified organisms (GMOs). Gene cloning and gene engineering techniques enable the introduction of genetic material from one species into another to create genetically modified bacteria, animals or plants. When a GMO has genetic material from a different donor species, it is called a transgenic organism. The mouse that gives off an eerie green glow is a transgenic organism: researchers cloned a gene encoding a green fluorescent protein (GFP) normally expressed only in jellyfish and created a transgenic mouse that now expresses GFP and shines green like a jellyfish. Today many economically important examples of GMOs are in the field of agriculture. These include Bt-corn and Bt-cotton, which carry a gene from the bacterium Bacillus thuringiensis. This gene encodes a toxin that kills corn borers and other insects. These Bt varieties of plants produce the toxins themselves and are resistant to many types of caterpillars and beetles.
3-SECOND THRASH
A GMO is any organism whose genetic material has been altered using genetic engineering techniques.
3-MINUTE THOUGHT
Although GMOs are rigorously tested for their safety, there has been considerable public debate about them. Some advantages of GMOs include healthier crops that require less pesticides, plants with improved nutritional value or the production of expensive drugs like human insulin in bacteria. Some disadvantages might include allergies and the threat of genes spreading to other organisms.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
HERBERT BOYER & STANLEY COHEN
1936– & 1935–
American biotechnologist and American geneticist who in 1973 made the first GMO by removing an antibiotic resistance gene from one bacterium and inserting it into another bacterium, enabling that bacterium to survive in the presence of the antibiotic
RUDOLF JAENISCH
1942–
American geneticist who created transgenic mammals by introducing foreign DNA into early mouse embryos
30-SECOND TEXT
Robert Brooker
GMOs can have many benefits, despite being a source of controversy.
CLONING
the 30-second theory
The word ‘clone’ means to make many identical copies of something. In genetics, the term ‘gene cloning’ refers to making a molecular copy of a gene. Genes can be cloned using a laboratory technique called polymerase chain reaction (PCR) (see here), in which the copying is done by an enzyme called DNA polymerase. An alternative way is to insert a gene into a plasmid (a circular DNA molecule that can copy itself independently of a cell’s chromosomal DNA) and then put the plasmid into a living host cell, such as a bacterium or yeast cell. When the host cells divide and increase in number, many copies of the cloned gene are made, too. But cloning can also be at the level of whole cells or even whole organisms. Identical twins are clones that develop from the same fertilized egg. This cloning happens by accident, when a fertilized egg divides into two cells that separate from one other, each developing into a person with the same genetic material as the other. Researchers developed ways to clone whole mammals in the laboratory. They removed the DNA from oocytes and then fused the oocyte with a cell from the individual to be cloned. This process is called reproductive cloning and the first cloned mammal was a sheep named Dolly.
3-SECOND THRASH
Cloning makes many copies of something and can be performed at the level of a gene, a single cell or a whole organism.
3-MINUTE THOUGHT
Researchers have created clones of many mammalian species, including sheep, cows, mice, goats, pigs and cats. In 2002, the first pet (a cat) was cloned. It was named CC (after the chemical symbol for carbon) and nicknamed ‘Copycat’. The cloning of mammals has many practical applications, including maintaining agriculturally valuable livestock and endangered species. However, a complete ban on human reproductive cloning was issued in many countries and research in this field is highly regulated.
RELATED TOPICS
See also
POLYMERASE CHAIN REACTION (PCR)
3-SECOND BIOGRAPHIES
JOHN GURDON
1933–
English biologist who pioneered transplanting the nucleus from a tadpole cell into an enucleated frog egg. His famous experiments in the 1960s earned him the title ‘the Godfather of cloning’ and a 2012 Nobel Prize
IAN WILMUT
1944–
English embryologist who with his colleagues at the University of Edinburgh produced clones of sheep using DNA from somatic cells
30-SECOND TEXT
Robert Brooker
Since Dolly, other mammals have been cloned, including pigs, horses and deer.

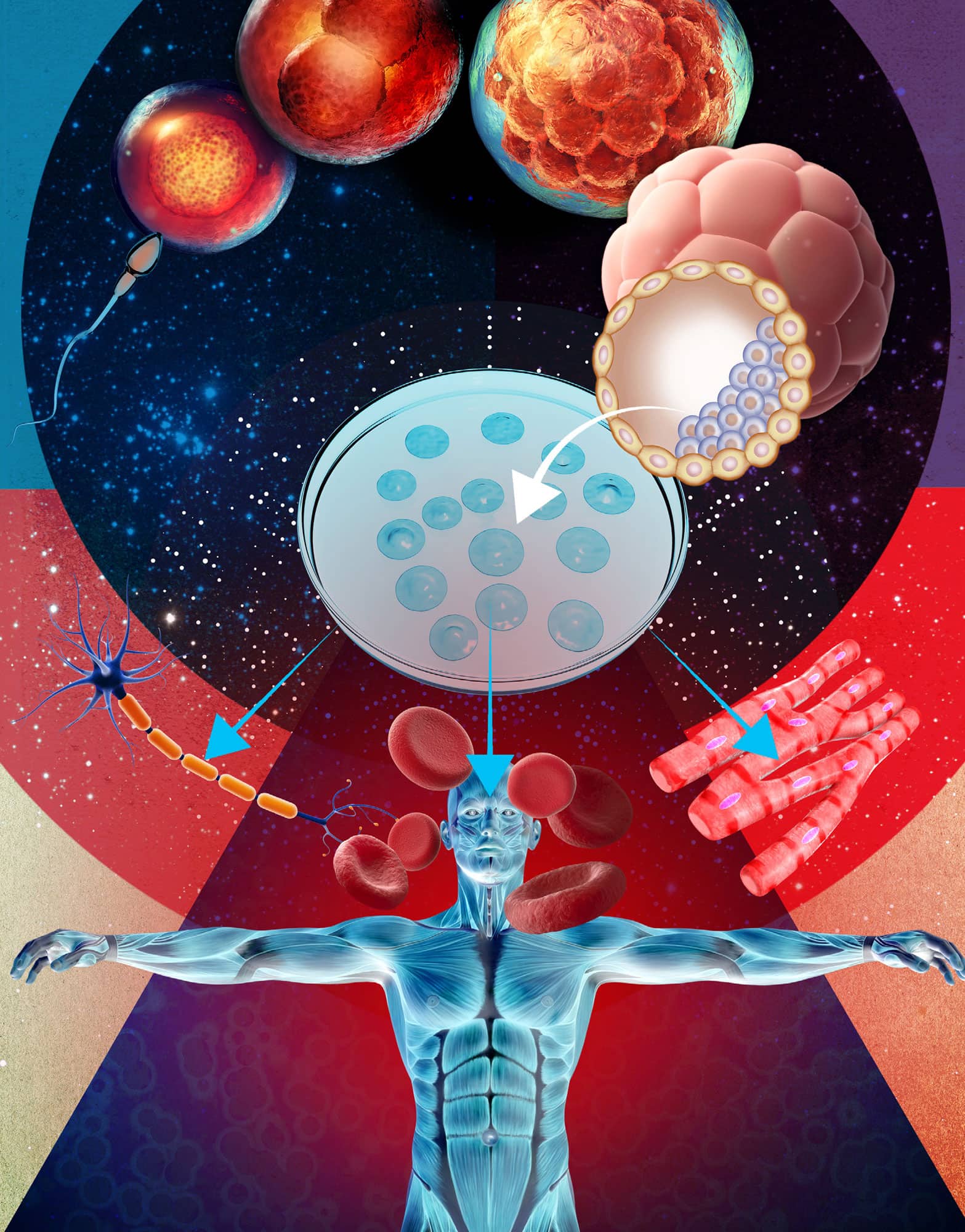
STEM CELLS & REPROGRAMMING
the 30-second theory
All organisms have specialized stem cells that have the capacity to make many different cell types. Stem cells replenish organs when cells die or need to be replaced. For example, most of the cells in our intestines are lost and replaced every few days. But can any cell in our bodies change itself into another cell type or are cells programmed for just one cell type? Researchers were surprised to discover that almost all the cells in our bodies have the capacity to change into all other cells and have a spectacular capacity to be reprogrammed. Studies in the 1950s and 60s demonstrated that the nucleus of a cell could be reprogrammed by transferring it into an unfertilized egg that has had its nucleus removed. Indeed, these ‘cloned’ eggs can even develop into embryos and sometimes to a fully grown adult. In 2006 scientists discovered the special conditions for cell reprogramming. They identified a cocktail of just four proteins that, when introduced into a cell, could generate ‘induce pluripotent stem’ (iPS) cells. These stem cells have tremendous potential because they can be differentiated in a dish to create many types of cells and tissues. These iPS cells are used to study how development works, to model human disease and to produce cells and organs for regenerative medicine and tissue therapy.
3-SECOND THRASH
Stem cell research hopes to recreate specialized organs from any cell in the body and regenerate new tissues to restore functions affected by the ageing process or disease.
3-MINUTE THOUGHT
One of the most important conclusions from stem cell experiments was that the genome is present and intact, in almost every somatic cell, not just in the germ line that normally transmits it to the next generation. However, what has really caught public attention is the idea that induced pluripotent stem cells and adult stem cells are potential elixirs of life that we can use to replace tissues worn out by old age or destroyed by disease.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
JOHN GURDON
1933–
British developmental biologist who demonstrated that the nuclei of differentiated intestinal cells can generate into all of the cell types when reintroduced into an enucleated egg
SHINYA YAMANAKA
1962–
Nobel Prize-winning Japanese stem cell researcher who created ‘induced pluripotent stem (iPS) cells’ after introducing four reprogramming factors into mouse fibroblasts
30-SECOND TEXT
Edith Heard
Stem cell research may lead to promising new treatments for diseases and major injuries.
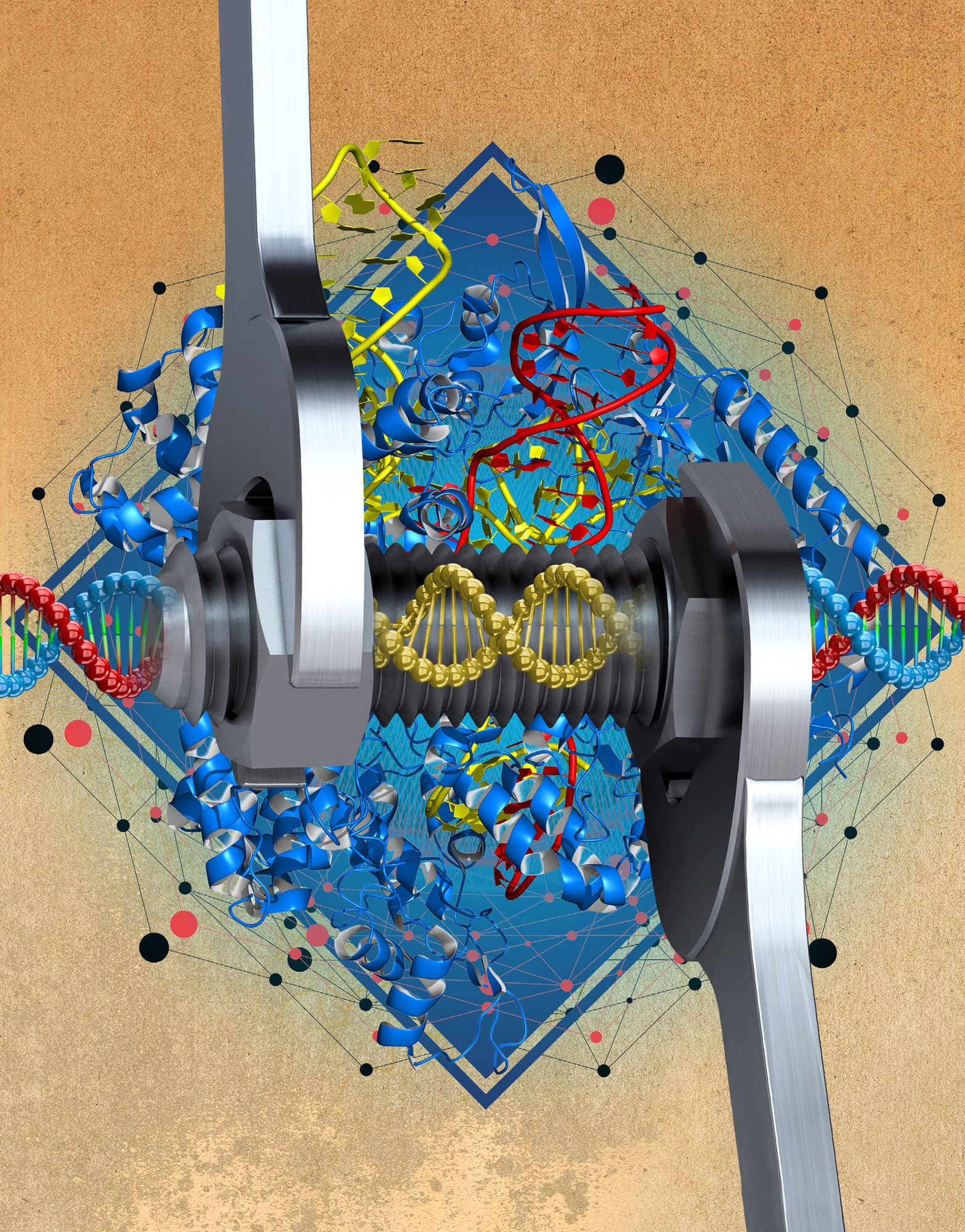
GENOME EDITING
the 30-second theory
Using engineered enzymes to edit genomes is a new approach that promises to transform genetic studies and treatment of genetic diseases. It uses molecular tools to modify the genome at targeted points. This is achieved by creating site-specific enzymes called ‘nucleases’ that establish DNA breaks at defined sequences in pieces of DNA: these include targeted Zinc finger nucleases (ZFNs) and transcription-activator-like effector nucleases (TALEN). These approaches link a non-specific DNA cutting enzyme to proteins that recognize specific DNA sequences. An alternative technology is based on the microbial CRISPR-Cas9 system that harnesses RNA-programmed targeting of a nuclease to a specific DNA sequence. All these approaches introduce a specific double-strand break in DNA at a defined point in the genome. Once the genome is broken, repair enzymes can disrupt or replace DNA sequences at or near the point where the cut was made. The ability to modify the DNA sequence of a single cell or even whole organism in a targeted fashion allows studies that assess the impact of the change on the phenotype. Targeted nucleases also facilitate gene therapy for inherited disorders, the goal being to replace defective genes with normal alleles at the same natural location to correct the genetic mutation.
3-SECOND THRASH
The revolutionary technology of genome editing engineers the genome by targeting modifying enzymes to specific DNA sequences.
3-MINUTE THOUGHT
Researchers can now cut and paste genetic material with unprecedented precision. Enzymes targeted to specific DNA sequences in the genome can be used as ‘molecular scissors’ to generate site-specific breaks. These breaks allow insertion or deletion of genomic sequences that can inactivate genes. Alternatively, new DNA can be provided to replace the endogenous gene with a targeted modification.
RELATED TOPICS
See also
3-SECOND BIOGRAPHIES
EMMANUELLE CHARPENTIER & JENNIFER DOUDNA
1968– & 1964–
French microbiologist and American chemist who in 2012 adapted the CRISPR system to take advantage of a synthetic guide RNA that directs the Cas9 enzyme
FENG ZHANG
1982–
Chinese-born biomedical scientist who in 2013 harnessed the CRISPR-Cas9 system for genome editing in eukaryotic cells
30-SECOND TEXT
Matthew Weitzman
Using engineered enzymes to edit genomes is an exciting new approach that could lead to major medical advances.