
Our bodies require a constant supply of energy to fuel our working organs, including the brain, heart, lungs and muscles. The major energy currency within the human body is an energy-rich molecule known as adenosine triphosphate, or ATP. In this chapter, we will explore how ATP is produced and the factors that impact how much we need. We will learn about methods used to estimate energy expenditure and how to calculate individual energy requirements. Finally, we will conclude the chapter with a focus on recovery from sport and exercise.
LEARNING OUTCOMES
Upon completion of this chapter, you will be able to:
• define and understand the association between ‘energy’, ‘power’ and ‘work’ and explain their relationship with exercise intensity and duration of exercise and sporting events
• compare and contrast the relative contributions of energy systems in relation to exercise intensity, duration and modality
• discuss methods used to assess energy expenditure and determine daily energy requirements of an individual
• explain the interplay between energy systems that allows physical exercise to occur, as well as those systems’ contribution to recovery.
At rest, the demand for ATP is low; however, sport and exercise can increase this demand as much as a thousandfold, requiring a coordinated metabolic response by the energy systems to replenish ATP levels. The contribution of each energy system is determined by the interaction between the intensity and the duration of exercise, and is regulated by metabolic processes and the central nervous system.
THE RELATIONSHIP BETWEEN ENERGY, WORK AND POWER
Energy exists in many different forms. Although there are many specific types of energy, the two major forms are kinetic energy and potential energy. Kinetic energy is the energy in moving objects or mass, such as mechanical energy and electrical energy. Potential energy is any form of energy that has stored potential and can be put to future use such as nuclear energy and chemical energy (ATP). With exercise, energy is the capacity to do work and is calculated as follows:
Equation 1: Work done (Newton∙metres [N∙m] or Joules [J]) = Force (N) × Distance (m)
Work done, measured in Newton metres or Joules is calculated as force multiplied by distance. For example, the greater the force required to move an object, or the further the distance of the object to be moved, the greater the work done. Power, also known as work rate, is the amount of work done over time:
Equation 2: Power (Watts [W]) = Work done (J) ÷ Time (s)
Therefore, the faster the rate at which work is completed, the higher the power output. With sufficient training, athletes can develop physiological adaptations that allow them to perform a larger amount of work in a short period of time, thus generating higher power outputs (see Table 2.1). Power output is often used in sports such as cycling and rowing to quantify training loads or as a measure of exercise performance. It is not uncommon for professional riders in the Tour de France to produce more than 1600 watts in the final sprint and reach 75 km/h after two weeks of gruelling cycling over the French Alps and having just completed 200 kilometres immediately prior to the sprint!
Table 2.1. Adaptations from aerobic and anaerobic resistance training
| Aerobic training | Anaerobic resistance training |
| Increases in: | Increases in: |
• Aerobic power output |
• Anaerobic power output |
• Muscular endurance at prolonged submaximal intensities |
• Muscular endurance at high power outputs |
• Capillary density |
• Strength production |
• Mitochondrial density and size |
• Muscle fibre size |
• Proportion of Type I muscle fibres |
• Proportion of Type II muscle fibres |
• Aerobic enzymes |
• Anaerobic substrates |
ENERGY IN THE HUMAN BODY
Chemical energy is a form of potential energy that is stored in the bonds of atoms and molecules. Within the body, the major energy currency is the ATP molecule, which comprises three components: An adenine ring (as part of adenosine), ribose sugar and three phosphate groups (triphosphate) (Figure 2.1).
Carbohydrates, protein, fats and alcohol (discussed in more detail in Chapter 4) are sources of energy in the diet. Under normal circumstances, more than 95 per cent of this food energy is digested and absorbed from the gastrointestinal tract, providing the body with its chemical energy needs (see Chapter 3 for more detail on digestion and absorption).

The breakdown of a compound by chemical reaction with water.
Catabolic reactions
Biochemical reactions that result in the breakdown of large molecules and give off energy in the form of ATP.
Anabolic reactions
Small molecules join to form a larger molecule in the presence of energy (ATP).
In the presence of water, ATP can be broken down to form adenosine diphosphate (ADP). This process is known as hydrolysis. Living cells contain ten times more ATP than ADP. When ATP is hydrolysed to ADP, a large amount of energy is released. The release of this free energy from the high-energy bonds is used to drive energy-requiring reactions such as protein synthesis.
Reactions within a cell can be classed as either catabolic or anabolic. Catabolic reactions involve breaking molecules down into their smaller components; energy is released as a by-product of these reactions. Anabolic reactions involve combining simple molecules to form complex molecules, and energy in the form of ATP is required to support these reactions. Energy-yielding reactions (catabolic) within a cell are typically coupled to energy-requiring reactions (anabolic). The high-energy bonds of ATP thus play a central role in cell metabolism by serving as a usable storage form of free energy.
PRODUCTION OF ENERGY: THE ROLE OF METABOLIC PATHWAYS
Given the importance of energy, especially chemical energy in the form of ATP, it is not surprising that the human body has a number of important metabolic pathways to ensure its ATP levels remain relatively constant. A metabolic pathway is a linked series of enzyme-mediated biochemical reactions occurring within a cell.
Enzymes
Proteins that start or speed up a chemical reaction while undergoing no permanent change to their structure. Enzymes perform this function by lowering the minimum energy required (activation energy) to start a chemical reaction. Enzymes are involved in most biochemical reactions; without them, most organisms could not survive.
Cytoplasm
The semifluid substance contained within a cell.
The three main metabolic pathways for ATP resynthesis (Figure 2.2) are: (a) the phosphagen system (ATP-PCr, alactacid), (b) anaerobic glycolysis (lactic acid) and (c) oxidative phosphorylation (mitochondrial ATP production). Both the phosphagen system and glycolysis pathway occur in the cytoplasm (cytosol) of the cell. Oxidative phosphorylation occurs within the mitochondria. Mitochondria are known as the powerhouses of the cell. They are organelles that act like a digestive system to take in nutrients, break them down and create energy-rich molecules for the cell.
The phosphagen system
The phosphagen system is the quickest way to resynthesise ATP, and comprises three reactions (Table 2.2). Phosphocreatine (PCr) donates a phosphate to ADP to produce ATP. Despite its ability to rapidly resynthesise ATP, the total capacity of this high-energy phosphate system to sustain maximal muscle contraction is about four seconds, assuming complete depletion of PCr and ATP. With this in mind, creatine supplementation has been investigated over the past few decades as a way to enhance exercise performance. Creatine supplementation can increase total creatine, specifically PCr levels stored in the muscle, and thus enhance the rephosphorylation of ADP to ATP. Numerous studies have shown the benefits of creatine supplementation on exercise performance, especially that involving short-burst, high-intensity power-type movements, such as power lifting (Cooper et al. 2012).
Supplementation with synthetic creatine can augment the level of creatine in the body and lead to enhanced performance of power activities.
Table 2.2. Three reactions of the phosphagen system
| Reactants | Products | Enzymes Used |
| ATP + Water (H2O) | ADP + Pi + Energy | ATPase |
| PCr + ADP | ATP + Cr | Creatine kinase |
| ADP + ADP | ATP + AMP | Adenylate kinase |
Note: ATP: Adenosine triphosphate; ADP: Adenosine diphosphate: AMP: Adenosine monophosphate; Pi: Inorganic phosphate; PCr: Phosphocreatine; Cr: Creatine
Glycolysis
A major source of cellular energy comes from the breakdown of carbohydrates, particularly glucose (see Chapter 4 for more detail about carbohydrates). The complete oxidative breakdown of glucose to carbon dioxide (CO2) and water (H2O) is written as follows:
C6H12 + 6O2 → 6 CO2 + 6 H2O
Glycolysis
The breakdown of glucose to form two molecules of ATP.
Within cells, glucose is oxidised in a series of steps coupled to the synthesis of ATP. Glycolysis is common to virtually all cells and is the first step in the breakdown of glucose. It increases when oxygen is lacking (anaerobic) and the demand for ATP is high.
The terms ‘aerobic’ and ‘anaerobic’ are used to describe the different conditions by which oxidation of food molecules especially glucose, fatty acids and proteins occur (known as respiration). Aerobic respiration occurs when adequate oxygen is present, anaerobic respiration occurs when lack of oxygen is present and the demand for ATP is high. Check out Box 2.1 for more information.
Anaerobic glycolysis involves a series of ten steps (see Figure 2.2) that utilise glucose, either circulating in the blood or from the stored form of glycogen, to produce two ATP molecules, pyruvate and reduced coenzyme NADH. Glycolysis also produces lactic acid, predominately during exercise performed at high intensities (see Chapter 1 for more information about lactic acid and buffering). Although the production of lactic acid will contribute to the local fatigue of the muscle, it is the only metabolic pathway that can keep up with the high demand for ATP resynthesis and, thus, allow muscle to continue contracting at high intensities. The total capacity of anaerobic glycolysis to sustain maximal contractions is approximately 30 seconds.
Lactic acid
A by-product of anaerobic glycolysis that contributes to fatigue of the muscle.
Coenzyme
A substance that works with an enzyme to initiate or assist the function of the enzyme. It may be considered a helper molecule for a biochemical reaction.
Krebs cycle
A series of biochemical reactions that generate energy from the breakdown of pyruvate (the end-product of glycolysis).
When adequate oxygen is present (aerobic), pyruvate (the end-product of glycolysis) undergoes decarboxylation (a chemical reaction that removes a carboxyl group and releases CO2) in the presence of coenzyme A (CoA) to produce acetyl CoA.
Acetyl CoA then enters the Krebs cycle (also known as the citric acid cycle or TCA cycle), which is the central pathway in oxidative metabolism and the first stage in cellular respiration (Figure 2.2).
Cellular respiration
The Krebs cycle, in conjunction with oxidative phosphorylation, provides the vast majority (more than 95 per cent) of energy used by aerobic cells in humans. The Krebs cycle is a series of eight reactions that break down pyruvate to produce reduced coenzymes NADH+ + H+ and FADH2, carbon dioxide and guanosine triphosphate (GTP), a high-energy molecule (Figure 2.2).
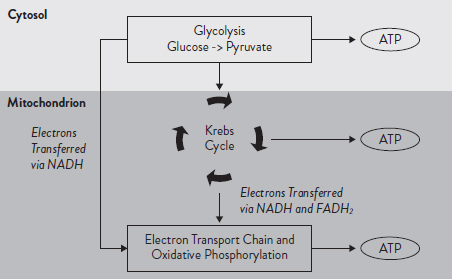
Figure 2.2. Metabolic pathways involved in ATP resynthesis
Box 2.1: Did you know? Aerobic vs anaerobic glycolysis
Before the 1980s, scholars and researchers referred to the complete oxidation of carbohydrate as ‘aerobic glycolysis’, as opposed to ‘anaerobic glycolysis’, which is often referred to now when pyruvate is converted to lactate (a temporary product formed when pyruvate combines with a hydrogen ion, H+). The difference in terminology was based on the assumption that the extent of cell oxygenation was the primary determining factor for the complete oxidation of pyruvate via mitochondrial respiration or production of lactate. This is inconsistent with the biochemistry of glycolysis. We now know that if the intensity of the exercise is high enough, lactate is produced regardless of normal oxygenation, or even hyper-oxygenation such as with the breathing of pure oxygen. Terms—‘lactic glycolysis’ versus ‘alactacid glycolysis’ for intense and steady-state exercise conditions respectively—have been proposed as being more biochemically representative (Baker et al. 2010).
Electron transport chain
Electrons are passed through a series of proteins and molecules in the mitochondria to generate large amounts of ATP.
Cellular respiration
A series of metabolic reactions within the cell that generate energy (ATP) from nutrients.
Electron
Negatively charged subatomic particles.
Oxidative phosphorylation
The electron transport chain (ETC) is the next step in the breakdown of glucose and the final step in cellular respiration. Requiring oxygen to function, reduced coenzymes from the Krebs cycle and glycolysis are re-oxidised with their electrons transferred through the ETC to produce large amounts of ATP (Figure 2.2). Mitochondrial oxidative phosphorylation is the only source of ATP production that has the capacity to support prolonged exercise.
The total yield from the complete oxidation of a glucose molecule is 38 molecules of ATP. This comes from:
• a net gain of two ATP molecules from glycolysis
• an additional two molecules from the conversion of pyruvate to acetyl CoA and subsequent metabolism via the Krebs (citric acid) cycle
• the assumption that the oxidation of the reduced coenzymes, NADH+ + H+ and FADH2, will produce three and two molecules of ATP respectively.
Both glycolysis and the Krebs cycle give rise to ten molecules of NADH+ + H+ and two molecules of FADH2 combined. In the case where two molecules of NADH+ + H+ produced by glycolysis are unable to enter mitochondria directly from the cytosol, the total yield is 36. The pathways involved in glucose degradation also play a central role in the breakdown of other organic molecules (discussed further in Chapter 4), such as nucleotides, amino acids and fatty acids, to form ATP.
INTERACTION AMONG METABOLIC ENERGY SYSTEMS: INFLUENCE OF SPORT AND EXERCISE
The interaction and relative contribution of the three energy systems during different exercise intensities and sporting activities have been of considerable interest to exercise scientists and biochemists. The first attempts to understand these interactions appeared in the literature in the 1960s and 1970s, using incremental exercise and periods of maximal exhaustive exercise. Although energy systems respond differently in relation to the diverse energy demands placed on them during daily and sporting activities, we now know that virtually all physical activities derive some energy from each of the three energy-supplying processes. With this in mind, the energy system most suited (dependent on the energy demands of the exercise) will contribute sequentially, but in an overlapping fashion, to provide energy (see Table 2.3 for examples of which energy system is best suited for various sporting activities).
Compare the demands of a 100-metre sprint to a 42.2-kilometre marathon. The sprint is fast, with minimal oxygen breathed in during its ten-second duration, making the event almost exclusively anaerobic (Newsholme et al. 1994). The marathon, on the other hand, is primarily an aerobic event completed in two to two-and-a-half hours at 80–85 per cent of an elite athlete’s maximal capacity (Newsholme et al. 1994). Despite the different demands of each event, all systems are activated at the start of exercise to maintain ATP levels and ensure adequate supply for maximal power output and intensity. The anaerobic (non-mitochondrial) systems, which are capable of supporting extremely high muscle force application and power outputs such as those during a 100-metre sprint, would be the predominant energy system used at these times. During a marathon race, the anaerobic system, which is limited in its capacity, is unable to meet the energy demands required by extended periods of intense exercise. The aerobic energy system (oxidative metabolism) is the only system that can resynthesise ATP at a rate that can maintain the required power and work output needed during the race. The aerobic system also plays a significant role in performance during high-intensity exercise, with a maximal exercise effort of 75 seconds deriving approximately equal energy from the aerobic and anaerobic energy systems (Baker et al. 2010).
Table 2.3. Energy systems used to support select sporting activities
| Phosphagen (ATP-PCr, alactacid) system | Anaerobic glycolysis (lactic acid) system | Oxidative phosphorylation (mitochondrial ATP production) |
| Sprinting—performance is determined predominantly by the capacity of the ATP-PCr system because of the short distance covered. However, events longer than 100 m would require greater input from anaerobic glycolysis. | Swimming—performance is determined predominantly by the capacity of the ATP-PCr and glycolytic system because of the short distance covered. However, events such as the 1500 m would require input from oxidative phosphorylation. | Marathon running—although all energy systems would be activated, performance is determined predominantly by the capacity of oxidative phosphorylation, with input from anaerobic glycolysis during periods of sprinting. |
| Golf—given the explosive nature of the sport (i.e. club swing), performance is determined predominantly by the capacity of the ATP-PCr system. | Fencing—performance is determined predominantly by the capacity of the ATP-PCr and glycolytic system because of the numerous short, powerful bursts that last around 5–10 seconds. | Basketball—basketball games typically last about 50 minutes, which means performance is determined predominantly by the capacity of oxidative phosphorylation. However, the game also requires short bursts of explosive power and thus would need input from the ATP-PCr and glycolytic systems. |
QUANTIFYING ENERGY EXPENDITURE: APPLICATIONS IN SPORT AND EXERCISE
Regardless of which energy system predominates during exercise, all energy systems contribute to the supply of energy and thus have important implications for performance and recovery. Measurement of an athlete’s energy expenditure helps determine the daily energy requirements for the athlete’s training and competition, to inform dietary requirements to help them achieve body composition and performance goals. For example, a power lifter training to increase muscle mass would aim to consume more energy than is expended to increase his body mass. Alternatively, a boxer attempting to lose weight would aim to consume less energy than he expends. Of course, the composition of the diet can also impact on performance and body composition, as will be discussed in detail in other chapters.
So, how do we measure energy expenditure? We know that the rate of energy metabolism is directly proportional to the amount of heat our whole body produces. As such, the rate of metabolism can be quantified by measuring heat produced by the body. This direct measurement method is known as direct calorimetry. This relationship is represented in Figure 2.3.
Direct calorimetry requires a person to be placed in an insulated chamber, which allows all heat production within the chamber to be measured. Although this method is highly accurate, building a calorimeter is expensive and requires a lot of space in a laboratory. Furthermore, heat that is produced by the exercise equipment when in use may complicate measurements. Therefore, a cheaper and smaller—but still accurate—method known as indirect calorimetry is more widely used for measuring energy expenditure. The most common approach to measuring oxygen consumption is by open-circuit spirometry. This involves collecting all exhaled gases into a mixing chamber, which is then processed and analysed by a metabolic cart (Figure 2.4). The metabolic cart analyses oxygen (O2) consumed and carbon dioxide (CO2) produced to calculate metabolic rate. Metabolic rate can be determined during rest (resting metabolic rate, RMR), or during submaximal or maximal intensity exercise. The maximal amount of oxygen that can be used by the body during high-intensity exercise is termed maximal aerobic capacity (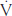 O2max), and is commonly used as an indicator of cardiorespiratory fitness (see Chapter 1 for more information about VO2max and cardiorespiratory fitness).
O2max), and is commonly used as an indicator of cardiorespiratory fitness (see Chapter 1 for more information about VO2max and cardiorespiratory fitness).
Indirect calorimetry
A method of estimating energy expenditure by measuring oxygen consumption and carbohydrate production.
CO2 produced and O2 consumed can also be expressed as a ratio (CO2/O2) to obtain a number that is normally between 0.7 and 1.0. This number is known as the respiratory exchange ratio (RER), and represents the composition of the mixture of lipids (fats) and carbohydrates oxidised through metabolism during submaximal exercise (Peronnet & Massicotte, 1991). These estimations are based on our knowledge of the exact amount of energy produced when metabolising carbohydrates, lipids and proteins with oxygen. Different types of macronutrients produce slightly different amounts of energy per litre of O2 consumed (Table 2.4). However, as protein normally contributes negligible energy to exercise during aerobic exercise of less than two hours, a release of 4.82 kcal·L O2–1 has been observed when burning a mixed macronutrient combination (Lemon & Nagle, 1981). For ease of calculation, 5 kcal of energy per litre of O2 is generally used to calculate energy expenditure during aerobic physical activity. Therefore, a person utilising 3 L·min–1 of oxygen during a run would be expending approximately 15 kcal of energy each minute.
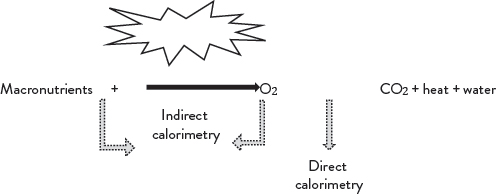
Figure 2.3. Aerobic metabolism pathway for macronutrients
Figure 2.4. Indirect calorimetry using a mouthpiece connected to a metabolic cart
Photo courtesy of Sam Wu
Respiratory exchange ratio
The ratio of carbon dioxide produced to oxygen consumed; used to indicate the relative contribution of substrates oxidised during submaximal exercise.
Table 2.4. Energy produced per litre of O2 when metabolising different macronutrients
| Macronutrient | kcal · L O2–1 |
| Carbohydrate | 5.05 |
| Fat | 4.69 |
| Protein | 4.49 |
*Note: 1 kcal = 4.186 kj
Source: Anonymous 1952.
Ideally, tests to determine aerobic capacity and energy expenditure should be conducted in a controlled environment such as a laboratory to ensure accuracy and precision of results. Equipment specific to the athlete’s sport, such as treadmills, bicycle ergometers, rowing machines and cross-country skis, is commonly used to maximise the relevance of results to the field. However, field tests are sometimes more appropriate, feasible and cheaper to conduct. Such tests, which are maximally exhaustive in nature, include the multistage shuttle run test (also known as the beep test), yo-yo endurance test, or 2.4-kilometre run test (see Table 2.5). At times where a maximal test is not appropriate due to the possible risks of maximal exhaustion, a health or fitness professional may choose to administer a submaximal test. A submaximal test requires a lower intensity of exercise and therefore is associated with a lower medical risk. Physiological data acquired during a submaximal test (commonly heart rate, blood pressure and ratings of perceived exertion) are then used to calculate and estimate the individual’s maximal capacity.
Maximally exhaustive
Exercise that requires the participant to work at their maximal capacity until exhaustion.
RECOVERY FROM SPORT AND EXERCISE
During exercise, oxygen consumption increases to meet demands based on exercise intensity. Upon cessation of exercise, the increased oxygen consumption does not immediately return to pre-exercise levels, but gradually returns to baseline. This recovery period is known as excess post-exercise oxygen consumption (EPOC). Previously termed oxygen debt, it was hypothesised that the increased oxygen uptake post-exercise was to repay the oxygen deficit created at the beginning of exercise, when energy production was not sufficient to meet a sudden increase in energy demands.
Excess post-exercise oxygen consumption
An increased rate of oxygen consumption following high-intensity activity.
Table 2.5. Maximal tests of aerobic capacity and energy expenditure
| The multistage shuttle run test, or beep test, requires participants to run repeats of 20 metres at increasing speeds every minute. |
| The yo-yo endurance test is a variation of the multistage shuttle run test with a higher initial running speed and different increments in speed. |
| The 2.4-kilometre run test, or Cooper 1.5-mile test, involves running 2.4 kilometres on a hard, flat surface in the shortest time possible. VO2max is calculated as (483/time in minutes) + 3.5. |
Box 2.2: Estimating daily energy requirements
The daily energy expenditure for healthy adults can be calculated using the equations below, formulated based on adults 19–78 years of age. It is important to keep in mind that factors other than those accounted for within these equations can also influence resting energy expenditure. These factors include climate, body composition and surface area of the body.
Equations:
For females: resting energy expenditure (kJ/day)
= 9.99 × (weight in kg) +
6.25 × (height in cm) – 4.92 × age – 161
For males: resting energy expenditure (kJ/day)
= 9.99 × (weight in kg) +
6.25 × (height in cm) – 4.92 × age + 5
(Mifflin et al. 1990)
Resting energy expenditure calculated from the above equations can be multiplied by a factor according to the individual’s physical activity level (PAL) for an estimated total daily energy expenditure. These factors are defined as:
| 1.0–1.39: | Sedentary, activities of daily living, sitting in office |
| 1.4–1.59: | Activities of daily living plus 30–60 minutes of light intensity activity (e.g. walking) |
| 1.6–1.89: | Activities of daily living plus standing, carrying light loads, 60 minutes of walking |
| 1.9–2.5: | Activities of daily living plus strenuous work or highly active/ athletic lifestyle (Kerksick & Kulovitz 2014). |
It is important to acknowledge that there is no clear classification for athletes of various fitness levels and training intensity. Therefore, using indirect or direct calorimetry should be encouraged for an accurate measurement of total daily energy expenditure.
The energy required for EPOC is supplied primarily by oxidative pathways and is required to return the body to its resting, dynamically balanced level of metabolism (homeostasis). EPOC can be divided into two portions: a rapid component and a slow component. The metabolic processes that contribute to the rapid component of EPOC include increased body temperature, circulation, ventilation, replenishment of O2 in blood and muscle, resynthesis of ATP and PCr, and lactate shuttling. The underlying mechanisms of the slow component of EPOC are much less understood. Apart from a sustained elevation of circulation, ventilation and body temperature, the slow component has been attributed to the storage of fatty acids as triglycerides, and a shift of substrate use from carbohydrates to lipids. The duration of EPOC depends on various factors, the most important being exercise intensity and duration. Short-duration and low-intensity exercise has been shown to produce short-lasting EPOCs, while high-intensity exercise clearly elicits a more substantial and prolonged EPOC lasting several hours (Borsheim & Bahr 2003). Several hormones released during physical activity also contribute to EPOC and would gradually return to baseline levels (Borsheim & Bahr 2003).
Homeostasis
Processes used by living organisms to maintain steady conditions needed for survival.
Lactate shuttling
Lactate produced at sites of high glycolysis can be shuttled (moved) to other muscles where it can be used as an energy source.
Triglycerides
The main type of fat in our bodies and our diets. They are made up of a glycerol backbone with three fatty acids attached.
SUMMARY AND KEY MESSAGES
Energy systems provide the human body with a continual supply of chemical energy in the form of ATP. Exercise increases the demands for this energy, but it is the intensity and duration of the exercise that ultimately determines the use of ATP and the fuel sources required for its resynthesis.
Key messages
• The two major forms of energy are kinetic energy and potential energy. Energy is the capacity to perform work and power is the rate of work completed.
• Chemical energy within the bonds of a fuel source can be extracted via a series of complex reactions specific to one of three energy systems: the phosphagen system (ATP-PCr, alactacid), anaerobic glycolysis (lactic acid) and oxidative phosphorylation (mitochondrial ATP production).
• The phosphagen system is the quickest of our energy systems, with the capacity to resynthesise ATP for up to six to ten seconds. It is predominantly used during very short, explosive movements.
• Anaerobic glycolysis is second fastest, with the capacity to resynthesise ATP for up to 30 to 60 seconds. It is predominantly used in short-duration, high-intensity ‘speed’ events such as the 400-metre track sprint.
• The aerobic energy system has the slowest rate of ATP resynthesis. Its advantage over the anaerobic energy systems is that it has a much larger capacity and is able to supply energy for hours rather than seconds.
• All activities require an energy contribution from at least two energy systems. Under maximal-effort conditions, all three systems are activated at the beginning of exercise, but one energy system will predominate.
• Metabolic rate and energy expenditure can be assessed by determining heat production from the body or by measuring an individual’s oxygen consumption and carbon dioxide production for a given period.
• EPOC is necessary to return the body to a dynamically balanced resting state and is influenced mainly by exercise intensity and duration.
REFERENCES
Anonymous, 1952, ‘Method of calculating the energy metabolism’, Acta Pædiatrica, vol. 41, pp. 67–76.
Baker, J.S., McCormick, M.C. & Robergs, R.A., 2010, ‘Interaction among skeletal muscle metabolic energy systems during intense exercise’, Journal of Nutrition and Metabolism, vol. 13, doi:10.1155/2010/905612.
Borsheim, E. & Bahr, R., 2003, ‘Effect of exercise intensity, duration and mode on post-exercise oxygen consumption’, Sports Medicine, vol. 33, no. 14, pp. 1037–60.
Cooper, R., Naclerio, F., Allgrove, J. et al., 2012, ‘Creatine supplementation with specific view to exercise/sports performance: An update’, Journal of International Society of Sports Nutrition, vol. 9, no. 1, p. 33, doi:10.1186/1550-2783-9-33.
Kerksick, C.M. & Kulovitz, M., 2014, ‘Requirements of energy, carbohydrates, proteins and fats for athletes,’ in: Bagchi, D., Nair, S. & Sen, C.K., Nutrition and Enhanced Sports Performance: Amsterdam, Elsevier.
Lemon, P. & Nagle, F., 1981, ‘Effects of exercise on protein and amino acid metabolism’, Medicine & Science in Sports & Exercise, vol. 13, no. 3, pp. 141–9.
Mifflin, M.D., St Jeor, S.T., Hill, L.A. et al., 1990, ‘A new predictive equation for resting energy expenditure in healthy individuals’, American Journal of Clinical Nutrition, vol. 51, no. 2, pp. 241–7.
Newsholme, E.A., Leech, A.R. & Duester, G., 1994, Keep on Running: The science of training and performance, Chichester, UK: John Wiley & Sons.
Peronnet, F. & Massicotte, D., 1991, ‘Table of nonprotein respiratory quotient: An update’, Canadian Journal of Sport Science, vol. 16, no. 1, pp. 23–9.