I
Indole-3-acetic acid (IAA)
Indole-3-acetic acid (IAA) is the major form of the plant hormone, auxin, a key regulator of cell division, elongation, and differentiation in higher plants (Goldsmith, 1993). Recent studies showed that a combination of IAA and horseradish peroxide (HRP) was cytotoxic to cancer cells and could be used as a novel cancer therapy (Folkes et al., 1998; Greco and Dachs, 2001; Wardman, 2002). IAA must undergo oxidative decarboxylation by HRP before it becomes cytotoxic (Folkes and Wardman, 2001). Since the activated form of IAA produced free radicals, including peroxy radicals, the combination of IAA and horseradish peroxidase could be used to enhance cellular oxidative stress and bring about apoptosis (Candeias et al., 1995). Kim and coworkers (2004) showed that the combination of IAA and horseradish produced free radicals in a dose-dependent manner and induced apoptosis in G361 human melanoma cells. The presence of 1.2 μg/mL of HRP, 100 and 500 μM IAA, caused 50 percent and 100 percent of the cells to die, respectively (Figure I.54). The mechanism involved activation of caspase-8 and caspase-9, which in turn led to activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase. Another examination of the mechanism of IAA cytotoxicity by de Melo et al. (2004) suggested induction of cell death by IAA involved the production of reactive-oxygen species by HRP.
Indole-3-acetic acid. (Wu et al., Sens. Actuat., B96:658–662, 2003. With permission.)
References
Candeias, L.P., Folkes, L.K., Porssa, M., Patrick, J., and Wardman, P., Enhancement of lipid peroxidation by indole-3-acetic acid derivatives: Substituent effects, Free Rad. Res., 23:403– 418, 1995.
de Melo, M.P., de Lima, T.M., Pithon-Curi, T.C., and Curi, R., The mechanism of indole acetic acid cytotoxicity, Toxicol. Lett., 148:103–111, 2004.
Folkes, L.K., Candeias, L.P., and Wardman, P., Toward targeted “oxidation therapy” of cancer; peroxidasecatalysed cytotoxicity of indole-3-acetic acid, Int. J. Radiat. Oncol. Biol. Phys., 42:917–920, 1998.
Folkes, L.K. and Wardman, P., Oxidative activation of indole-3-acetic acids to cytotoxic species—a potential new role for plant auxins in cancer therapy, Biochem. Pharmacol., 61:129–136, 2001.
Goldsmith, M.H.M., Cellular signaling: New insights into the action of the plant growth hormone auxin, Proc. Natl. Acad. Sci., 90:11442–11445, 1993.
Greco, O. and Dachs, G.U., Gene directed enzyme/prodrug therapy of cancer; Historical appraisal and future prospectives, J. Cell Physiol., 187:22–36, 2001.
Kim, D.-S., Jeon, S.-E., and Park, K.-C., Oxidation of indole-3-acteic acid by horseradish peroxidase induces apoptosis in G361 human melanoma cells, Cell Signal, 16:81–88, 2004.
Wardman, P., Curr. Pharm. Des., 8:1376–1374, 2002. Wu, K., Sun, Y., and Hu, S., Development of an amperometric indole-3-acetic acid sensor based on carbon nanotubes film coated glassy carbon electrode, Sens. Actual., B96:658–662, 2003.
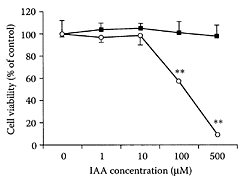
FIGURE I.54 Cytotoxic effect of IAA/HRP in G361 human melanoma cells. After serum-starvation cells were treated with varying concentrations (1–500 μM) of IAA in the absence (■) and presence (○) of HRP (1.2 μg/mL). Each experiment was repeated at least twice, independently and representative results shown. ** p<0.01 compared to the untreated control. (From Kim et al., Cell Signal, 16:81–88,2004. With permission.)
Indole-3-carbinol
see Diindoylmethane
Inulin
Inulin, a naturally occurring, complex polysaccharide present in many plants, is obtained primarily from the roots of chicory (Cicorium intybus L.) (De Bruyn et al., 1992) or the tubers of Jerusalem artichoke (Baldini et al., 2003). Chicory inulin is composed of mixtures of linear β 2–1 fructans varying in length from 2 to approximately 65 fructose residues. In comparison, Jerusalem artichoke inulin has a much shorter chain length. The linear 1,2-β-linked D-frutofuranoside chains of inulin are attached via an α1-β2 type sucrose linkage to a terminal glucose molecule. The inability of digestive enzymes to digest these 1,2-β-link-ages ensures inulin reaches the gut intact, where it is fermented by the gut flora. In fact, inulin is a prebiotic, stimulating the growth of bifidobacteria and inhibiting colon carcinogenesis in animal models (Reddy et al., 1997; Roberfroid et al., 1998; Reddy, 1999). Causey and coworkers (2000) showed inulin significantly lowered serum triglycerides in hypercholesterolemic men, as well as improved the gut flora. Koo etal. (2003) found inulin-stimulated NO synthesis via activation of protein kinase C (PKC)-α and protein tyrosinase kinase, which activated NF-κB in RAW 264.7 cells. The release of NO is important for its tumoricidal effects and may explain the anticarcinogenic effects associated with inulin. These results point to inulin having considerable promise as a functional ingredient in the diet.
Videla and coworkers (2001) showed dietary inulin reduced the severity of dextran sodium sulfate colitis in rats. Oral inulin prevented colonic mucosal inflammation by dextran sodium sulfate (DSS) that histologically resembles human ulcerative colitis (Okayasu et al., 1990). In addition to improving histological scores and decreasing the release of inflammatory mediators, it also lowered tissue myeloperoxidase accumulation in DSS colitis in rats. These results suggested inulin may be a useful dietary or pharmacological intervention in patients suffering from ulcerative colitis.
References
Baldini, M., Danuso, F., Turi, M., and Vannozzi, G.P., Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosum L.) for inulin and sugar yield from stalk and tuber, Ind. Crops Prod., 19:25–40, 2003.
Causey, J.L., Feirtag, J.M., Gallaher, D.D., Tungland, B.C., and Slavin, J.L., Effects of dietary inulin on serum lipid, blood glucose and gastrointestinal environment in hypercholesterolemic men, Nutr. Res., 20:191–201, 2000.
De Bruyn, A., Alvarez, A.P., Sandra, P., and DeLeenheer, L., Isolation and identification of O-βD-fructofuranosyl-( 2–1)-O-βD-O-βD-fructofuranosyl-(2-l)-D fructose: A product of the enzymatic hydrolysis of the inulin from Cicorium intybus, Carbohydr. Res., 235:303–308, 1992.
Koo, H.-N., Hong, S.-H., Seo, H.-G., Yoo, T.-S., Lee, K.-N., Kim, N.-S., Kim, C.-H., and Kim, H.- M., Inulin stimulates NO synthesis via activation of PKC-α and protein tyrosine kinase, resulting in the activation of NF-κB by IFN-γ-primed RAW 264.7 cells, J. Nutr. Biochem., 14:598–605, 2003.
Okayasu, I., Hatakeyama, S., and Yamada, M., A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice, Gastroenterology, 98:694–702, 1990.
Reddy, B.S., Possible mechanisms by which pro- and prebiotics influence colon carcinogenesis and tumor growth, J. Nutr., 129:1478S–1482S, 1999.
Reddy, B.S., Hamid, R., and Rao, C.V., Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition, Carcinogenesis, 18:1371–1374, 1997.
Roberfroid, M.B., Van Loo, J.A.E., and Gibson, G.R., The bifidogenic nature of chicory inulin and its hydrolysis products, J. Nutr., 128:11–19, 1998.
Videla, S., Vilaseca, J., Antolin, M., Garcia-Lafuente, A., Guarner, F., Crespo, E., Casalots, J., Salas, A., and Malagelada, J.R., Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat, Am. J. Gastroenterol., 96:1486–1493, 2001.
Isoflavones
see also Daidzein and Genistein Isoflavones represent a group of phytoestrogens that are chemically strikingly similar to mammalian estrogens. Phytoestrogens mainly bind to the second subtype of the estrogen receptor (ERβ), while mammalian estradiol has a higher binding affinity for the “classic” estrogen receptor ERα (Kuiper et al., 1998; Casanova et al., 1999). They can thus act as either estrogen agonists or antagonists (Setchell, 1998; Setchell and Cassidy, 1999). Legumes are good sources of isoflavones, with soybeans and soy products being the most abundant, containing approximately 0.2–1.6 mg/g dry weight (Kurzer and Xu, 1997). Fitpatrick (2003) recently reviewed the literature on the effects of soy isoflavones on lipid metabolism, osteoblasts and osteoclasts, bone markers, bone-mineral density, and cognition. Based on very limited human clinical data, it was hard to make definitive recommendations to clinicians other than moderate use for postmenopausal women. The low incidence of breast cancer, cardiovascular disease, and climacteric symptoms in Japanese women compared to Caucasians has been attributed to higher soybean intake. Watanabe et al. (2002) showed a slight improvement in elongation of the menstrual cycle in young women taking an isoflavone-rich tablet. Climacteric women also showed improvement in bone density, hypertension, and climacteric symptoms when maintained on these tablets.
References
Casanova, M., You, L., Gaido, K.W., ArchibequeEngle, S., Janszen, D.B., and Heck, H.A., Developmental effects of dietary phytoestrogens in SpragueDawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro, Toxicol. Sci., 51:236– 244, 1999.
Fitzpatrick, L.A., Soy isoflavones: Hope or hype? Maturitas, 44(1):S21−S29, 2003.
Kuiper, G.G.J.M., Lemmen, J.G., Carlsson, B., Corton, J.C., Safe, S.H., van der Saag, P.T., Van der Burg, B., and Gustafsson, J.A., Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β, Endocrinology, 139:4252–4263, 1998.
Kurzer, M.S. and Xu, X., Dietary phytoestrogens, Ann. Rev. Nutr., 17:353–381, 1997.
Setchell, K.D., Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones, Am. J. Clin. Nutr., 68:1333S-1346S, 1998.
Setchell, K.D. and Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health, J. Nutr., 129:758S-767S, 1999.
Watanabe, S., Uesugi, S., and Kikuchi, Y., Isoflavones for prevention of cancer, cardiovascular diseases, gynecological problems and possible immune potentiation, Biomed. Pharmacother., 56:302–312, 2002.
Isohomohalichondrin B (From Litaudon et al., Tetrahedron Lett., 35:9435–9438, 1994. With permission.)
Isohomohalichondrin B
Isohomohalichondrin B (IHB), a member of the halichondrin group, was isolated in New Zealand from the deep-water sponge Lissodendoryx sp. It was shown by Litaudon et al. (1994) to be highly toxic towards P388 (murine leukemia) cells. Bergamaschi and coworkers (1999) found IHB to be a potent, antitumor agent delaying cell cycle S-phase progress, mitotic block, tetraploidy, and inducing apoptosis in a human cancer-cell line.
References
Bergamaschi, D., Ronzoni, S., Taverna, S., Faretta, M., De Feudis, P., Faircloth, G., Jimeno, J., Erba, E., and D’Incalci, M., Cell cycle perturbations and apoptosis induced by isohomohalichondrin B (IHB), a nairal marine compound., Br. J. Cancer, 79:267–277, 1999.
Litaudon, M., Hart, J.B., Blunt, J.W., Lake, R.J., and Munro, M., Isohomohalichondrin B, a new antitumor polyethermacrolide from New Zealand deep-water sponge Lissodendoryx sp., Tetrahedron Lett., 35: 9435–9438, 1994.
Isoliquiritigenin
The chalcone isoliquiritigenin (ISL), isolated from licorice and shallots, was shown by Yamamoto et al. (1991) to inhibit the formation of skin papilloma induced by 7,12-dimethylbenz[α]-anthracene (DMBA) and TP (phorbol acetate). Maggliolini et al. (2002) reported ISL exhibited both estrogenic and antiproliferative effects on MCF7 breastcancer cells. High concentrations of this phytoestrogen inhibited proliferation of MCF7 cells, while low concentrations stimulated progression of estrogen-dependent breast tumors. Based on these results, they cautioned that the level of ISL taken by menopausal women be carefully monitored.
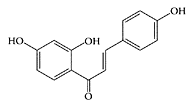
Isoliquiritigenin. (From Cao et al., J. Chromatogr., A. 1042:203–209, 2004. With permission.)
The ability of ISL to suppress metastasis of mouse renal-cell carcinoma was reported by Yamazaki and coworkers (2002). The number of metastatic lung nodules was significantly reduced in the presence of ISL. Kanzawa and coworkers (2003) recently reported ISL effectively inhibited prostate cancer. They found that the cell growth of prostate-cancer cell line DU145 was significantly reduced by ISL in a dose- and time-dependent manner (Figure I.55). The mechanism of action appeared to involve induction of S- and G2/M-phase arrest and was associated with enhanced expression of GADD153. These results suggested ISL was a potential candidate for treating prostate cancer.
The potential of ISL in the treatment of lung cancer was recently demonstrated by Ii et al. (2004). They found ISL inhibited cell proliferation of a human lung-cancer cell line in a doseand time-dependent manner. Cell cycle progression was arrested at the G2/M phase, which was associated with enhanced expression of p21CIP1/WAF1, a universal inhibitor of cyclicdependent kinases.
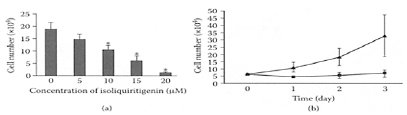
FIGURE I.55 Effect of ISL on the growth of the prostate cancer DU145 cell lines, (a) Dose-dependent effect: cells were exposed for 48 h to various concentrations of ISL or DMSO alone (control), p<0.05 versus control. (b) Time-kinetics study: cells were exposed to 15 μM of ISL for 24, 48, and 72 h. ((▲) DMSO alone (control) (■)) (From Kanzawa et al., Eur. Urol., 43:580–586, 2003. With permission.)
References
Cao, Y., Wang, Y., Ji, C., and Ye, J., Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection, J. Chromatogr., A. 1042:203–209, 2004.
Ii, T., Satomi, Y., Katoh, D., Shimada, J., Baba, M., Okuyama, T., Nishimo, H., and Kitamura, N., Induction of cell cycle arrest and p21CIPI/WAFI expression in human lung cancer cells by isoliquiritigenin, Cancer Lett., 207:27–35, 2004.
Kanzawa, M., Satomi, Y., Mitzutani, Y., Ukimura, O., Kawauchi, A., Sakai, T., Baba, M., Okuyama, T., Nishino, H., and Miki, T., Isoliquiritigenin inhibits the growth of prostate cancer, Eur. Urol., 43:580–586, 2003.
Maggliolini, M., Statti, G., Vivacqua, A., Gabriele, S., Rago, V., Loizzo, M., Menichini, F., and Amdo, S., Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells, J. Steroid Biochem. Mol. Biol., 82:315–322, 2002.
Yamamoto, S., Aizu, E., Jiang, H., Nakadate, T., Kiyoto, I., Wang, J.C., and Kato, R., The potent antitumor-promoting agent isoliquiritigenin, Carcinogenesis, 12:317–323, 1991.
Yamazaki, S., Morita, T., Endo, H., Hamamoto, T., Baba, M., Joichi, S., Kaneko, S., Okada, Y., Okuyama, T., Nishino, H., and Tokue, A., Isoliquiritigenin suppresses pulmonary mertastasis of mouse renal cell carcinoma, Cancer. Lett., 183:23–30, 2002.