17
Breast Disorders
Armando E. Giuliano, MD, FACS, FRCSEd
Sara A. Hurvitz, MD, FACP
BENIGN BREAST DISORDERS
FIBROCYSTIC CONDITION
ESSENTIALS OF DIAGNOSIS

 Painful breast masses; often multiple and bilateral.
Painful breast masses; often multiple and bilateral.
 Rapid fluctuation in mass size is common.
Rapid fluctuation in mass size is common.
 Pain often worsens during premenstrual phase of cycle.
Pain often worsens during premenstrual phase of cycle.
 Most common age is 30–50. Rare in postmenopausal women not receiving hormonal replacement.
Most common age is 30–50. Rare in postmenopausal women not receiving hormonal replacement.
 General Considerations
General Considerations
Fibrocystic condition is the most frequent lesion of the breast. Although commonly referred to as “fibrocystic disease,” it does not, in fact, represent a pathologic or anatomic disorder. It is common in women 30–50 years of age but rare in postmenopausal women who are not taking hormonal replacement. Estrogen is considered a causative factor. There may be an increased risk in women who drink alcohol, especially women between 18 and 22 years of age. Fibrocystic condition encompasses a wide variety of benign histologic changes in the breast epithelium, some of which are found so commonly in normal breasts that they are probably variants of normal but have nonetheless been termed a “condition” or “disease.”
The microscopic findings of fibrocystic condition include cysts (gross and microscopic), papillomatosis, adenosis, fibrosis, and ductal epithelial hyperplasia. Although fibrocystic condition has generally been considered to increase the risk of subsequent breast cancer, only the variants with a component of epithelial proliferation (especially with atypia), papillomatosis, or increased breast density on mammogram represent true risk factors.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Fibrocystic condition may produce an asymptomatic mass in the breast that is discovered by accident, but pain or tenderness often calls attention to it. Discomfort often occurs or worsens during the premenstrual phase of the cycle, at which time the cysts tend to enlarge. Fluctuations in size and rapid appearance or disappearance of a breast mass are common with this condition as are multiple or bilateral masses and serous nipple discharge. Patients will give a history of a transient lump in the breast or cyclic breast pain.
B. Diagnostic Tests
Mammography and ultrasonography should be used to evaluate a mass in a patient with fibrocystic condition. Ultrasonography alone may be used in women under 30 years of age. Because a mass due to fibrocystic condition is difficult to distinguish from carcinoma on the basis of clinical findings, suspicious lesions should be biopsied. Core needle biopsy, rather than fine-needle aspiration (FNA), is the preferable technique. Excisional biopsy is rarely necessary but should be done for lesions with atypia or where imaging and biopsy results are discordant. Surgery should be conservative, since the primary objective is to exclude cancer. Occasionally, FNA cytology will suffice. Simple mastectomy or extensive removal of breast tissue is rarely, if ever, indicated for fibrocystic condition.
 Differential Diagnosis
Differential Diagnosis
Pain, fluctuation in size, and multiplicity of lesions are the features most helpful in differentiating fibrocystic condition from carcinoma. If a dominant mass is present, the diagnosis of cancer should be assumed until disproven by biopsy. Mammography may be helpful, but the breast tissue in young women is usually too radiodense to permit a worthwhile study. Sonography is useful in differentiating a cystic mass from a solid mass, especially in women with dense breasts. Final diagnosis, however, depends on analysis of a biopsy specimen.
 Treatment
Treatment
When the diagnosis of fibrocystic condition has been established by previous biopsy or is likely because the history is classic, aspiration of a discrete mass suggestive of a cyst is indicated to alleviate pain and, more importantly, to confirm the cystic nature of the mass. The patient is reexamined at intervals thereafter. If no fluid is obtained by aspiration, if fluid is bloody, if a mass persists after aspiration, or if at any time during follow-up a persistent or recurrent mass is noted, biopsy should be performed.
Breast pain associated with generalized fibrocystic condition is best treated by avoiding trauma and by wearing a good supportive brassiere during the night and day. Hormone therapy is not advisable because it does not cure the condition and has undesirable side effects. Danazol (100–200 mg orally twice daily), a synthetic androgen, is the only treatment approved by the US Food and Drug Administration (FDA) for patients with severe pain. This treatment suppresses pituitary gonadotropins, but androgenic effects (acne, edema, hirsutism) usually make this treatment intolerable; in practice, it is rarely used. Similarly, tamoxifen reduces some symptoms of fibrocystic condition, but because of its side effects, it is not useful for young women unless it is given to reduce the risk of cancer. Postmenopausal women receiving hormone replacement therapy may stop or change doses of hormones to reduce pain. Oil of evening primrose, a natural form of gamolenic acid, has been shown to decrease pain in 44–58% of users. The dosage of gamolenic acid is six capsules of 500 mg orally twice daily. Studies have also demonstrated a low-fat diet or decreasing dietary fat intake may reduce the painful symptoms associated with fibrocystic condition. Topical treatments such as nonsteroidal anti-inflammatory drugs are rarely of value.
The role of caffeine consumption in the development and treatment of fibrocystic condition is controversial. Some studies suggest that eliminating caffeine from the diet is associated with improvement while other studies refute the benefit entirely. Many patients are aware of these studies and report relief of symptoms after giving up coffee, tea, and chocolate. Similarly, many women find vitamin E (400 international units daily) helpful; however, these observations remain anecdotal.
 Prognosis
Prognosis
Exacerbations of pain, tenderness, and cyst formation may occur at any time until menopause, when symptoms usually subside, except in patients receiving hormonal replacement. The patient should be advised to examine her own breasts regularly just after menstruation and to inform her clinician if a mass appears. The risk of breast cancer developing in women with fibrocystic condition with a proliferative or atypical epithelial hyperplasia or papillomatosis is higher than that of the general population. These women should be monitored carefully with physical examinations and imaging studies.
Chetlen AL et al. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017 Mar;24:345–9. [PMID: 27916596]
Groen JW et al. Cyclic and non-cyclic breast pain: a systematic review on pain reduction, side effects, and quality of life for various treatments. Eur J Obstet Gynecol Reprod Biol. 2017 Dec;219:74–93. [PMID: 29059585]
Qu P et al. Detection rate is not higher for women with BBD history in breast cancer screening. J Public Health (Oxf). 2019 Nov;pii:fdz147. [PMID: 31774529]
FIBROADENOMA OF THE BREAST
This common benign neoplasm occurs most frequently in young women, usually within 20 years after puberty. It is somewhat more frequent and tends to occur at an earlier age in black women. Multiple tumors are found in 10–15% of patients.
The typical fibroadenoma is a round or ovoid, rubbery, discrete, relatively movable, nontender mass 1–5 cm in diameter. Clinical diagnosis in young patients is generally not difficult. In women over 30 years, fibrocystic condition of the breast and carcinoma of the breast must be considered. Cysts can be identified by aspiration or ultrasonography. Fibroadenoma does not normally occur after menopause but may occasionally develop after administration of hormones.
No treatment is usually necessary if the diagnosis can be made by core needle biopsy. Excision with pathologic examination of the specimen is performed if the diagnosis is uncertain or the lesion grows significantly. Cryoablation, or freezing of the fibroadenoma, appears to be a safe procedure if the lesion is a biopsy-proven fibroadenoma prior to ablation. Cryoablation is not appropriate for all fibroadenomas because some are too large to freeze or the diagnosis may not be certain. There is no obvious clinical advantage to cryoablation of a histologically proven fibroadenoma except that some patients may feel relief that a mass is gone. However, at times a mass of scar or fat necrosis replaces the mass of the fibroadenoma. Reassurance seems preferable. It is usually not possible to distinguish a large fibroadenoma from a phyllodes tumor on the basis of needle biopsy results or imaging alone and histology is usually required. Presumed fibroadenomas larger than 3–4 cm should be excised to rule out phyllodes tumors.
Phyllodes tumor is a fibroadenoma-like tumor with cellular stroma that grows rapidly. It may reach a large size and, if inadequately excised, will recur locally. The lesion can be benign or malignant. If benign, phyllodes tumor is treated by local excision. The treatment of malignant phyllodes tumor is more controversial, but complete removal of the tumor with a margin of normal tissue avoids recurrence. Because these tumors may be large, total mastectomy is sometimes necessary. Lymph node dissection is not performed, since the sarcomatous portion of the tumor metastasizes to the lungs and not the lymph nodes.
Co M et al. Mammary phyllodes tumour: a 15-year multicentre clinical review. J Clin Pathol. 2018 Jun;71(6):493–7. [PMID: 29146885]
Koh VCY et al. Size and heterologous elements predict metastases in malignant phyllodes tumours of the breast. Virchows Arch. 2018 Apr;472(4):615–21. [PMID: 29127495]
Krings G et al. Fibroepithelial lesions; the WHO spectrum. Semin Diagn Pathol. 2017 Sep;34(5):438–52. [PMID: 28688536]
Meng X et al. Giant fibroadenoma of the breast: a rare case in a mature woman. Int J Surg Case Rep. 2019;63:36–9. [PMID: 31561187]
NIPPLE DISCHARGE
In order of decreasing frequency, the following are the most common causes of nipple discharge in the nonlactating breast: duct ectasia, intraductal papilloma, and carcinoma. The important characteristics of the discharge and some other factors to be evaluated by history and physical examination are listed in Table 17–1.
Table 17–1. Characteristics of nipple discharge in the nonpregnant, nonlactating woman.
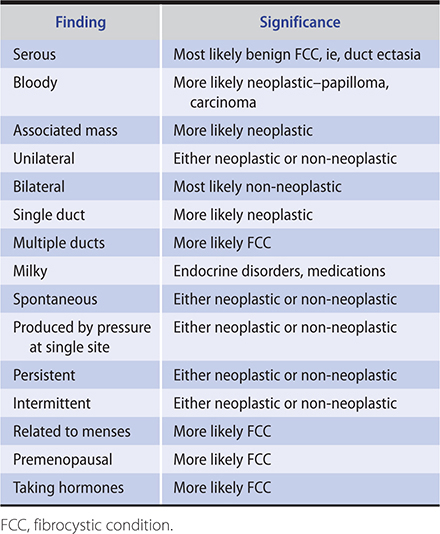
Spontaneous, unilateral, serous or serosanguineous discharge from a single duct is usually caused by an ectatic duct or an intraductal papilloma or, rarely, by an intraductal cancer. A mass may not be palpable. The involved duct may be identified by pressure at different sites around the nipple at the margin of the areola. Bloody discharge is suggestive of cancer but is more often caused by a benign papilloma in the duct. Cytologic examination may identify malignant cells, but negative findings do not rule out cancer, which is more likely in older women. In any case, the involved bloody duct—and a mass if present—should be excised. A ductogram (a mammogram of a duct after radiopaque dye has been injected), like cytology, is of limited value since excision of the suspicious ductal system is indicated regardless of findings. Ductoscopy, evaluation of the ductal system with a small scope inserted through the nipple, has been attempted but is not effective management.
In premenopausal women, spontaneous multiple duct discharge, unilateral or bilateral, most noticeable just before menstruation, is often due to fibrocystic condition. Discharge may be green or brownish. Papillomatosis and ductal ectasia are usually detected only by biopsy. If a mass is present, it should be removed.
A milky discharge from multiple ducts in the nonlactating breast may occur from hyperprolactinemia. Serum prolactin levels should be obtained to search for a pituitary tumor. Thyroid-stimulating hormone (TSH) helps exclude causative hypothyroidism. Numerous antipsychotic medications and other medications may also cause a milky discharge that ceases on discontinuance of the medication.
Oral contraceptive agents or estrogen replacement therapy may cause clear, serous, or milky discharge from a single duct, but multiple duct discharge is more common. In the premenopausal woman, the discharge is more evident just before menstruation and disappears on stopping the medication. If it does not stop, is from a single duct, and is copious, exploration should be performed since this may be a sign of cancer.
A purulent discharge may originate in a subareolar abscess and require removal of the abscess and the related lactiferous sinus.
When localization is not possible, no mass is palpable, and the discharge is nonbloody, the patient should be reexamined every 3 or 4 months for a year, and a mammogram and an ultrasound should be performed. Although most discharge is from a benign process, patients may find it annoying or disconcerting. To eliminate the discharge, proximal duct excision can be performed both for treatment and diagnosis.
Castellano I et al. The impact of malignant nipple discharge cytology (NDc) in surgical management of breast cancer patients. PLoS One. 2017 Aug 14;12(8):e0182073. [PMID: 28806416]
Çetin K et al. Evaluation and management of pathological nipple discharges without using intraductal imaging methods. Ir J Med Sci. 2020 May;189(2):451–60. [PMID: 31631245]
Li GZ et al. Evaluating the risk of underlying malignancy in patients with pathologic nipple discharge. Breast J. 2018 Jul;24(4):624–7. [PMID: 29520933]
Zacharioudakis K et al. Can we see what is invisible? The role of MRI in the evaluation and management of patients with pathological nipple discharge. Breast Cancer Res Treat. 2019 Nov;178(1):115–20. [PMID: 31352554]
FAT NECROSIS
Fat necrosis is a rare lesion of the breast but is of clinical importance because it produces a mass (often accompanied by skin or nipple retraction) that is usually indistinguishable from carcinoma even with imaging studies. In the past, most fat necrosis has been seen after trauma, but now fat necrosis is commonly seen after fat injections to augment breast size or fill defects after breast surgery. The resultant mass may be confused with cancer. If untreated, the mass gradually disappears. If imaging is not typical of fat necrosis, the safest course is to obtain a biopsy. Core needle biopsy is usually adequate. Fat necrosis is also common after segmental resection, radiation therapy, or flap reconstruction after mastectomy.
Ellis LJ et al. How should we manage women with fat necrosis following autologous breast reconstruction: an algorithmic approach. Breast J. 2020 Apr;26(4):711–5. [PMID: 31602711]
Nakada H et al. Fat necrosis after breast-conserving oncoplastic surgery. Breast Cancer. 2019 Jan;26(1):125–30. [PMID: 30151780]
BREAST ABSCESS
During nursing, an area of redness, tenderness, and induration may develop in the breast. The organism most commonly found in these abscesses is Staphylococcus aureus (see Puerperal Mastitis, Chapter 19).
Infection in the nonlactating breast is rare. A subareolar abscess may develop in young or middle-aged women who are not lactating. These infections tend to recur after incision and drainage unless the area is explored during a quiescent interval, with excision of the involved lactiferous duct or ducts at the base of the nipple. In the nonlactating breast, inflammatory carcinoma must always be considered. Thus, incision and biopsy of any indurated tissue with a small piece of erythematous skin is indicated when suspected abscess or cellulitis in the nonlactating breast does not resolve promptly with antibiotics. Often needle or catheter drainage is adequate to treat an abscess, but surgical incision and drainage may be necessary.
Barron AU et al. Do acute-care surgeons follow best practices for breast abscess management? A single-institution analysis of 325 consecutive cases. J Surg Res. 2017 Aug;216:169–71. [PMID: 28807202]
Saboo A et al. Trends in non-lactation breast abscesses in a tertiary hospital setting. ANZ J Surg. 2018 Jul–Aug;88(7–8):739–44. [PMID: 29045009]
DISORDERS OF THE AUGMENTED BREAST
At least 4 million American women have had breast implants. Breast augmentation is performed by placing implants under the pectoralis muscle or, less desirably, in the subcutaneous tissue of the breast. Most implants are made of an outer silicone shell filled with a silicone gel, saline, or some combination of the two. Capsule contraction or scarring around the implant develops in about 15–25% of patients, leading to a firmness and distortion of the breast that can be painful. Some require removal of the implant and surrounding capsule.
Implant rupture may occur in as many as 5–10% of women, and bleeding of gel through the capsule is noted even more commonly. Although silicone gel may be an immunologic stimulant, there is no increase in autoimmune disorders in patients with such implants. The FDA has advised symptomatic women with ruptured silicone implants to discuss possible surgical removal with their clinicians. However, women who are asymptomatic and have no evidence of rupture of a silicone gel prosthesis do not require removal of the implant. Women with symptoms of autoimmune illnesses often undergo removal, but no benefit has been shown.
Studies have failed to show any association between implants and an increased incidence of breast cancer. However, breast cancer may develop in a patient with an augmentation prosthesis, as it does in women without them. Detection in patients with implants may be more difficult because mammography is less able to detect early lesions. Mammography is better if the implant is subpectoral rather than subcutaneous. Prostheses are usually placed retropectorally after mastectomy, but prepectoral placement is used more commonly. Local recurrence is usually cutaneous or subcutaneous and is easily detected by palpation. Rarely, lymphoma of the breast with silicone implants has been reported.
If a cancer develops in a patient with implants, it should be treated in the same manner as in women without implants. Such women should be offered the option of mastectomy or breast-conserving therapy, which may require removal or replacement of the implant. Radiotherapy of the augmented breast often results in marked capsular contracture. Adjuvant treatments should be given for the same indications as for women who have no implants.
Clemens MW et al. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017 Mar 1;37(3):285–9. [PMID: 28184418]
Doren EL et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017 May;139(5):1042–50. [PMID: 28157769]
Elston JB et al. Complications and recurrence in implant-sparing oncologic breast surgery. Ann Plast Surg. 2017 Jun;78(6S Suppl 5):S269–74. [PMID: 28328633]
CARCINOMA OF THE FEMALE BREAST
ESSENTIALS OF DIAGNOSIS

 Risk factors: Age, nulliparity, childbirth after age 30, family history of breast cancer or genetic mutations (BRCA1, BRCA2, or others), and personal history of breast cancer or some types of proliferative conditions.
Risk factors: Age, nulliparity, childbirth after age 30, family history of breast cancer or genetic mutations (BRCA1, BRCA2, or others), and personal history of breast cancer or some types of proliferative conditions.
 Early findings: Single, nontender, firm to hard mass with ill-defined margins; mammographic abnormalities and no palpable mass.
Early findings: Single, nontender, firm to hard mass with ill-defined margins; mammographic abnormalities and no palpable mass.
 Later findings: Skin or nipple retraction; axillary lymphadenopathy; breast enlargement, erythema, edema, pain; fixation of mass to skin or chest wall.
Later findings: Skin or nipple retraction; axillary lymphadenopathy; breast enlargement, erythema, edema, pain; fixation of mass to skin or chest wall.
 Incidence & Risk Factors
Incidence & Risk Factors
Breast cancer will develop in one of eight American women. Next to skin cancer, breast cancer is the most common cancer in women; it is second only to lung cancer as a cause of death. In 2019, there were approximately 268,600 new cases and 41,760 deaths from breast cancer in the United States. Worldwide, breast cancer is diagnosed in approximately 2.1 million women, and about 626,679 die of breast cancer each year.
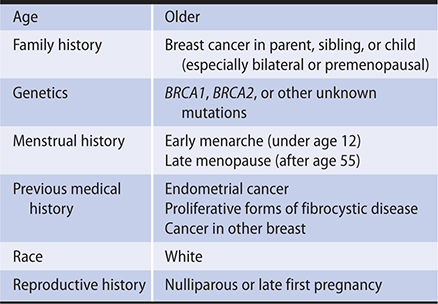
The most significant risk factor for the development of breast cancer is age. A woman’s risk of breast cancer rises rapidly until her early 60s, peaks in her 70s, and then declines. A significant family history of breast or ovarian cancer may also indicate a high risk of developing breast cancer. Germline mutations in the BRCA family of tumor suppressor genes or other breast cancer susceptibility genes accounts for approximately 5–10% of breast cancer diagnoses and tend to cluster in certain ethnic groups, including women of Ashkenazi Jewish descent. Women with a mutation in the BRCA1 gene, located on chromosome 17, have an estimated 85% chance of developing breast cancer in their lifetime. Other genes associated with an increased risk of breast and other cancers include BRCA2 (associated with a gene on chromosome 13); ataxia-telangiectasia mutation (ATM), BARD1, CHEK2, PALB2, RAD51D; and mutation of the tumor suppressor gene p53. Primary care clinicians should assess a woman’s personal and family history for breast, ovarian, tubal or peritoneal cancer using a familial risk assessment tool. Those with a positive result should receive genetic counseling in order to decide whether genetic testing is indicated.
Even when genetic testing fails to reveal a predisposing genetic mutation, women with a strong family history of breast cancer are at higher risk for development of breast cancer. Compared with a woman with no affected family members, a woman who has one first-degree relative with breast cancer has double the risk of developing breast cancer and a woman with two first-degree relatives with breast cancer has triple the risk of developing breast cancer. The risk is further increased for a woman whose affected family member was premenopausal at the time of diagnosis or had bilateral breast cancer. Lifestyle and reproductive factors also contribute to risk of breast cancer. Nulliparous women and women whose first full-term pregnancy occurred after the age of 30 have an elevated risk. Early menarche (under age 12) and late natural menopause (after age 55) are associated with an increase in risk, especially for hormone receptor–positive breast cancer. Combined oral contraceptive pills also appear to increase the risk of breast cancer, with longer use associated with higher risk. Several studies show that concomitant administration of progesterone and estrogen to postmenopausal women may increase the incidence of breast cancer, compared with the use of estrogen alone or with no hormone replacement treatment. Alcohol consumption, high dietary intake of fat, and lack of exercise may also increase the risk of breast cancer. Fibrocystic breast condition is also associated with an increased incidence of cancer when it is accompanied by proliferative changes, papillomatosis, atypical epithelial hyperplasia, or increased breast density on mammogram. A woman who had cancer in one breast is at increased risk for cancer developing in the other breast. In these women, a contralateral cancer develops at rate of 1% or 2% per year. Women with cancer of the uterine corpus have a risk of breast cancer significantly higher than that of the general population, and women with breast cancer have a comparably increased risk of endometrial cancer. Socioeconomic and racial factors have also been associated with breast cancer risk. Breast cancer tends to be diagnosed more frequently in women of higher socioeconomic status.
Women at greater than average risk for developing breast cancer (Table 17–2) should be identified by their clinicians and monitored carefully. Several risk assessment models have been validated (most extensively the Gail 2 model) to evaluate a woman’s risk of developing cancer. Some women at high risk may consider prophylactic mastectomy, oophorectomy, tamoxifen, or an aromatase inhibitor (AI).
Table 17–2. Factors associated with increased risk of breast cancer (listed in alphabetical order).
Women with genetic mutations in whom breast cancer develops may be treated in the same way as women who do not have mutations (ie, lumpectomy), though there is an increased risk of ipsilateral and contralateral breast cancer after lumpectomy for these women. One study showed that of patients with a diagnosis of breast cancer who were found to be carriers of a BRCA mutation, approximately 50% chose to undergo bilateral mastectomy.
Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. [PMID: 30207593]
Couch FJ et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017 Sep 1;3(9):1190–6. [PMID: 28418444]
Frey MK et al. Prevalence of nonfounder BRCA1/2 mutations in Ashkenazi Jewish patients presenting for genetic testing at a hereditary breast and ovarian cancer center. Cancer. 2019 Mar 1;125(5):690–7. [PMID: 30480775]
Jin J. JAMA patient page. Should I be tested for BRCA mutations? JAMA. 2019 Aug 20;322(7):702. [PMID: 31429898]
Siegel RL et al. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7–34. [PMID: 3062040]
US Preventive Services Task Force; Owens DK et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019 Aug 20;322(7):652–65. [PMID: 31429903]
 Prevention
Prevention
Several clinical trials have evaluated the use of selective estrogen receptor modulators (SERMs), including tamoxifen and raloxifene, for prevention of breast cancer in women with no personal history of breast cancer but at high risk for developing the disease. A meta-analysis of nine of these studies including 83,399 women with a median follow-up of 65 months demonstrated a 38% reduction in breast cancer incidence (hazard ratio [HR], 0.62; 95% CI, 0.56, 0.69) with a 10-year cumulative incidence of 6.3% in control groups and 4.2% in SERM-treated groups. An increased risk of endometrial cancer, cataracts, and venous thromboembolic events but a reduced risk of vertebral fractures was seen in SERM groups. While SERMs have been shown to be effective at reducing the risk of breast cancer and saving costs, the use of this intervention by women has been relatively low, possibly due to the perceived risks and side effects of therapy.
Similar to SERMs, AIs, such as exemestane and anastrozole, have shown success in preventing breast cancer with a lower risk of uterine cancer and thromboembolic events, although bone loss is a significant side effect of this treatment.
US Preventive Services Task Force; Owens DK et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019 Sep 3;322(9):857–66. [PMID: 31479144]
 Early Detection of Breast Cancer
Early Detection of Breast Cancer
A. Screening Programs
Screening detects breast cancer before it has spread to the lymph nodes in about 80% of the women evaluated. This increases the chance of survival to about 85% at 5 years.
Substantial evidence supports the use of routine screening mammography; however, recommendations relating to timing and frequency vary by different agencies and countries. About one-third of the abnormalities detected on screening mammograms will be found to be malignant when biopsy is performed. The probability of cancer on a screening mammogram is directly related to the Breast Imaging Reporting and Data System (BIRADS) assessment, and workup should be performed based on this classification. The sensitivity of mammography varies from approximately 60% to 90%. This sensitivity depends on several factors, including patient age, breast density, tumor size, tumor histology (lobular versus ductal), location, and mammographic appearance. In young women with dense breasts, mammography is less sensitive than in older women with fatty breasts, in whom mammography can detect at least 90% of malignancies. Smaller tumors, particularly those without calcifications, are more difficult to detect, especially in dense breasts. The lack of sensitivity and the low incidence of breast cancer in young women have led to questions concerning the value of mammography for screening in women 40–50 years of age. The specificity of mammography in women under 50 years varies from about 30% to 40% for nonpalpable mammographic abnormalities to 85% to 90% for clinically evident malignancies. Guidelines from at least six separate organizations exist in the United States and each differs slightly, making it somewhat complex for clinicians and patients to navigate. While the American College of Radiology, American Medical Association, and National Comprehensive Cancer Network (NCCN) recommend starting mammography screening at age 40, the US Preventive Services Task Force (USPSTF) recommends starting screening at age 50. Most guidelines recommend annual screening; however, the American Cancer Society recommends decreasing the frequency of screening to every 1–2 years starting at age 55 years and the USPSTF recommends routine mammography be done no more than every 2 years beginning at age 50 years. It is generally agreed that mammography should continue until life expectancy is shorter than 7–10 years, although the USPSTF recommends stopping screening after age 74 years regardless of life expectancy. Thus, clinicians should have an informed discussion with patients about screening mammography regarding its potential risks (eg, false positives, overdiagnosis, radiation exposure) and benefits (eg, early diagnosis), taking into consideration a patient’s individual risk factors.
B. Imaging
1. Mammography—Mammography is the most reliable means of detecting breast cancer before a mass can be palpated. Most slowly growing cancers can be identified by mammography at least 2 years before reaching a size detectable by palpation.
Indications for mammography are as follows: (1) screening at regular intervals asymptomatic women at risk for developing breast cancer; (2) evaluating each breast when a diagnosis of potentially curable breast cancer has been made, and at regular intervals thereafter; (3) evaluating a questionable or ill-defined breast mass or other suspicious change in the breast; (4) searching for an occult breast cancer in women with metastatic disease in axillary nodes or elsewhere from an unknown primary; (5) screening women prior to cosmetic operations or prior to biopsy of a mass, to examine for an unsuspected cancer; (6) monitoring women with breast cancer who have been treated with breast-conserving surgery and radiation; and (7) monitoring the contralateral breast in women with breast cancer treated with mastectomy.
Film mammography has largely been replaced by digital mammography. Although full-field digital mammography provides an easier method to maintain and review mammograms and may improve image quality, it has not been proven to improve overall cancer detection and is less economical. It may offer screening benefits to women younger than age 50 years and to women with dense breasts. The benefits of computer-assisted detection are debatable with studies failing to consistently show it adds improved sensitivity for invasive cancer detection. No study has shown computer-assisted detection improves mortality rates. Tomosynthesis creates tomographic “slices” of the breast volume with a single acquisition. This technique may improve the sensitivity of mammography especially in patients with dense breast tissue and reduces the number of callbacks but increases the radiation dose and has not yet been shown in prospective studies to improve long-term patient outcomes.
Calcifications are the most easily recognized mammographic abnormality. The most common findings associated with carcinoma of the breast are clustered pleomorphic microcalcifications. Such calcifications are usually at least five to eight in number, aggregated in one part of the breast and differing from each other in size and shape, often including branched or V- or Y-shaped configurations. There may be an associated mammographic mass density or, at times, only a mass density with no calcifications. Such a density usually has irregular or ill-defined borders and may lead to architectural distortion within the breast, but may be subtle and difficult to detect.
Patients with a dominant or suspicious mass on examination must undergo biopsy despite mammographic findings. The mammogram should be obtained prior to biopsy so that other suspicious areas can be noted and the contralateral breast can be evaluated. Mammography is never a substitute for biopsy because it may not reveal clinical cancer, especially in a very dense breast.
Communication and documentation among the patient, the referring clinician, and the interpreting physician are critical for high-quality screening and diagnostic mammography. The patient should be told about how she will receive timely results of her mammogram; that mammography does not “rule out” cancer; and that she may receive a correlative examination such as ultrasound at the mammography facility if referred for a suspicious lesion. She should also be aware of the technique and need for breast compression and that this may be uncomfortable. The mammography facility should be informed in writing by the clinician of abnormal physical examination findings. The Agency for Health Care Policy and Research (AHCPR) Clinical Practice Guidelines strongly recommend that all mammography reports be communicated in writing to the patient and referring clinician. Legislation has been passed in a number of US states that requires imaging facilities to report to patients the density of their breasts. This may prompt women with dense breasts to discuss with their clinician whether or not additional screening options would be appropriate in addition to mammogram.
2. Other imaging—MRI and ultrasound may be useful screening modalities in women who are at high risk for breast cancer but not for the general population. The sensitivity of MRI is much higher than mammography; however, the specificity is significantly lower and this results in multiple unnecessary biopsies. The increased sensitivity despite decreased specificity may be considered a reasonable trade-off for those at increased risk for developing breast cancer but not for normal-risk population. The NCCN guidelines recommend MRI in addition to screening mammography for high-risk women, including those with deleterious mutations, those who have a lifetime risk of breast cancer of at least 20%, and those with a personal history of lobular carcinoma in situ (LCIS).
Women who received radiation therapy to the chest in their teens or twenties are also known to be at high risk for developing breast cancer and screening MRI may be considered in addition to mammography. A Netherlands study (Dense Tissue and Early Breast Neoplasm Screening “DENSE”) involving over 40,000 women with extremely dense breast tissue demonstrated that the addition of annual MRI to screening mammography was associated with a lower rate of cancers being diagnosed in 2 years.
C. Clinical Breast Examination and Self-Examination
Breast self-examination has not been shown to improve survival. Because of the lack of strong evidence demonstrating value, the American Cancer Society no longer recommends monthly breast self-examination. While breast self-examination is not a recommended practice, patients should recognize and report any breast changes to their clinicians as it remains an important facet of proactive care.
Welch HG et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016 Oct 13;375(15):1438–47. [PMID: 27732805]
 Clinical Findings Associated With Early Detection of Breast Cancer
Clinical Findings Associated With Early Detection of Breast Cancer
A. Symptoms and Signs
The presenting complaint in about 70% of patients with breast cancer is a lump (usually painless) in the breast. About 90% of these breast masses are discovered by the patient. Less frequent symptoms are breast pain; nipple discharge; erosion, retraction, enlargement, or itching of the nipple; and redness, generalized hardness, enlargement, or shrinking of the breast. Rarely, an axillary mass or swelling of the arm may be the first symptom. Back or bone pain, jaundice, or weight loss may be the result of systemic metastases, but these symptoms are rarely seen on initial presentation.
The relative frequency of carcinoma in various anatomic sites in the breast is shown in Figure 17–1.
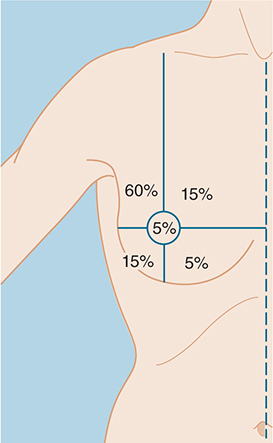
Figure 17–1. Frequency of breast carcinoma at various anatomic sites.
Inspection of the breast is the first step in physical examination and should be carried out with the patient sitting, arms at her sides and then overhead. Abnormal variations in breast size and contour, minimal nipple retraction, and slight edema, redness, or retraction of the skin can be identified (Figure 17–2). Asymmetry of the breasts and retraction or dimpling of the skin can often be accentuated by having the patient raise her arms overhead or press her hands on her hips to contract the pectoralis muscles. Axillary and supraclavicular areas should be thoroughly palpated for enlarged nodes with the patient sitting (Figure 17–3). Palpation of the breast for masses or other changes should be performed with the patient both seated and supine with the arm abducted. Palpation with a rotary motion of the examiner’s fingers as well as a horizontal stripping motion has been recommended.
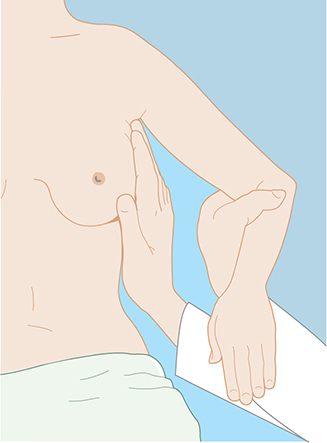
Figure 17–2. Skin dimpling. (Used, with permission, from Armando E. Giuliano, MD.)
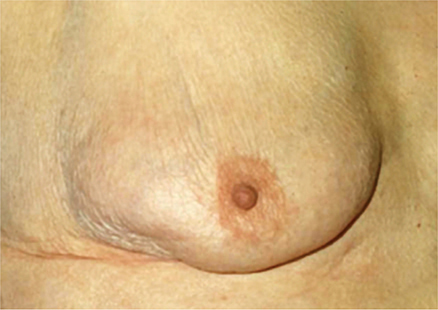
Figure 17–3. Palpation of axillary region for enlarged lymph nodes.
Breast cancer usually consists of a nontender, firm or hard mass with poorly delineated margins (caused by local infiltration). Very small (1–2 mm) erosions of the nipple epithelium may be the only manifestation of Paget disease of the breast (Figure 17–4). Watery, serous, or bloody discharge from the nipple is an occasional early sign but is more often associated with benign disease.
Figure 17–4. Paget disease. (Used, with permission, from Armando E. Giuliano, MD.)
A lesion smaller than 1 cm in diameter may be difficult or impossible for the examiner to feel but may be discovered by the patient. She or he should always be asked to demonstrate the location of the mass; if the clinician fails to confirm the patient’s suspicions and imaging studies are normal, the examination should be repeated in 2–3 months, preferably 1–2 weeks after the onset of menses. During the premenstrual phase of the cycle, increased innocuous nodularity may suggest neoplasm or may obscure an underlying lesion. If there is any question regarding the nature of an abnormality under these circumstances, the patient should be asked to return after her menses.
Metastases tend to involve regional lymph nodes, which may be palpable. One or two movable, nontender, not particularly firm axillary lymph nodes 5 mm or less in diameter are frequently present and are generally of no significance. Firm or hard nodes larger than 1 cm are typical of metastases. Axillary nodes that are matted or fixed to skin or deep structures indicate advanced disease (at least stage III). On the other hand, if the examiner thinks that the axillary nodes are involved, that impression will be borne out by histologic section in about 85% of cases. The incidence of positive axillary nodes increases with the size of the primary tumor. Noninvasive cancers (in situ) do not metastasize. Metastases are present in about 30% of patients with clinically negative nodes.
In most cases, no nodes are palpable in the supraclavicular fossa. Firm or hard nodes of any size in this location or just beneath the clavicle should be biopsied. Ipsilateral supraclavicular or infraclavicular nodes containing cancer indicate that the tumor is in an advanced stage (stage III or IV). Edema of the ipsilateral arm, commonly caused by metastatic infiltration of regional lymphatics, is also a sign of advanced cancer.
B. Laboratory Findings
Liver or bone metastases may be associated with elevation of serum alkaline phosphatase. Hypercalcemia is an occasional important finding in advanced cancer of the breast. Serum tumor markers such as carcinoembryonic antigen (CEA) and CA 15-3 or CA 27-29 are not recommended for diagnosis of early lesions or for routine surveillance for recurrence after a breast cancer diagnosis.
C. Imaging
1. For lesions felt only by the patient—Ultrasound is often valuable and mammography essential when an area is felt by the patient to be abnormal but the clinician feels no mass. MRI should not be used to rule out cancer because MRI has a false-negative rate of about 3–5%. Although lower than mammography, this false-negative rate cannot permit safe elimination of the possibility of cancer. False-negative results are more likely seen in infiltrating lobular carcinomas and ductal carcinoma in situ (DCIS) than invasive ductal carcinoma.
2. For metastatic lesions—For patients with suspicious symptoms or signs (bone pain, abdominal symptoms, elevated liver biochemical tests) or locally advanced disease (clinically abnormal lymph nodes or large primary tumors), staging scans are indicated prior to surgery or systemic therapy. Chest imaging with CT or radiographs may be done to evaluate for pulmonary metastases. Abdominal imaging with CT or ultrasound may be obtained to evaluate for liver metastases. Bone scans using 99mTc-labeled phosphates or phosphonates are more sensitive than skeletal radiographs in detecting metastatic breast cancer. Bone scanning has not proved to be of clinical value as a routine preoperative test in the absence of symptoms, physical findings, or abnormal alkaline phosphatase or calcium levels. The frequency of abnormal findings on bone scan parallels the status of the axillary lymph nodes on pathologic examination. Positron emission tomography (PET) scanning alone or combined with CT (PET-CT) may also be used for detecting soft tissue or visceral metastases in patients with locally advanced disease or with symptoms or signs of metastatic disease.
D. Diagnostic Tests
1. Biopsy—The diagnosis of breast cancer depends ultimately on examination of tissue or cells removed by biopsy. Treatment should never be undertaken without an unequivocal histologic or cytologic diagnosis of cancer. The safest course is biopsy examination of all suspicious lesions found on physical examination or imaging, or both. About 60% of lesions clinically thought to be cancer prove on biopsy to be benign, while about 30% of clinically benign lesions are found to be malignant. These findings demonstrate the fallibility of clinical judgment and the necessity for biopsy.
All breast masses require a histologic diagnosis by biopsy with one probable exception: a nonsuspicious, presumably fibrocystic mass, in a premenopausal woman. Rather, these masses can be observed through one or two menstrual cycles. However, if the mass is not cystic and does not completely resolve during this time, it must be biopsied. Figures 17–5 and 17–6 present algorithms for management of breast masses in premenopausal and postmenopausal patients.

Figure 17–5. Evaluation of breast masses in premenopausal women. (Adapted, with permission, from Chang S, Haigh PI, Giuliano AE. Breast disease. In: Berek JS, Hacker NF [editors], Practical Gynecologic Oncology, 4th ed, Philadelphia: Lippincott Williams & Wilkins, 2004.)
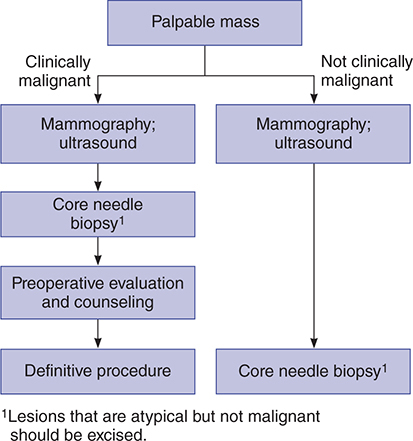
Figure 17–6. Evaluation of breast masses in postmenopausal women. (Adapted, with permission, from Chang S, Haigh PI, Giuliano AE. Breast disease. In: Berek JS, Hacker NF [editors], Practical Gynecologic Oncology, 4th ed, Philadelphia: Lippincott Williams & Wilkins, 2004.)
The simplest biopsy method is needle biopsy, either by aspiration of tumor cells (FNA cytology) or by obtaining a small core of tissue with a hollow needle (core needle biopsy). Core needle biopsy is preferable.
Large-needle (core) biopsy removes a core of tissue with a large cutting needle and is the diagnostic procedure of choice for both palpable and image-detected abnormalities. Handheld biopsy devices make large-core needle (14-gauge) biopsy of a palpable mass easy and cost effective in the office with local anesthesia. As in the case of any needle biopsy, the main problem is sampling error due to improper positioning of the needle, giving rise to a false-negative test result. This is extremely unusual with image-guided biopsies. Core biopsy allows the tumor to be tested for the expression of biological markers, such as estrogen receptor (ER), progesterone receptor (PR), and HER2.
FNA cytology is a technique whereby cells are aspirated with a small needle and examined cytologically. This technique can be performed easily with virtually no morbidity and is much less expensive than excisional or open biopsy. The main disadvantages are that it requires a pathologist skilled in the cytologic diagnosis of breast cancer and it is subject to sampling problems. Furthermore, noninvasive cancers usually cannot be distinguished from invasive cancers. The incidence of false-positive diagnoses is extremely low, perhaps 1–2%. The false-negative rate is as high as 10%. Most experienced clinicians would not leave a suspicious dominant mass in the breast even when FNA cytology is negative unless the clinical diagnosis, breast imaging studies, and cytologic studies were all in agreement, such as a fibrocystic lesion or fibroadenoma. Given the stated limitations, FNA is not the modality of choice for sampling an abnormal breast mass and has been largely replaced by core needle biopsy.
Open biopsy under local anesthesia as a separate procedure prior to deciding upon definitive treatment has become less common with the increased use of core needle biopsy. Needle biopsy, when positive, offers a more rapid approach with less expense and morbidity, but when nondiagnostic it must be followed by open biopsy. It generally consists of an excisional biopsy, which is done through an incision with the intent to remove the entire abnormality, not simply a sample. Intraoperative frozen section examination of a breast biopsy has generally been abandoned unless there is a high clinical suspicion of malignancy in a patient well prepared for the diagnosis of cancer and its treatment options.
2. Biopsy with ultrasound guidance—Ultrasonography is performed primarily to differentiate cystic from solid lesions but may show signs suggestive of carcinoma. Ultrasonography may show an irregular mass within a cyst in the rare case of intracystic carcinoma. If a tumor is palpable and feels like a cyst, an 18-gauge needle can be used to aspirate the fluid and make the diagnosis of cyst. If a cyst is aspirated and the fluid is nonbloody, it does not have to be examined cytologically. If the mass does not recur, no further diagnostic test is necessary. Nonpalpable mammographic densities that appear benign should be investigated with ultrasound to determine whether the lesion is cystic or solid. These may even be needle biopsied with ultrasound guidance.
3. Core needle biopsy with mammographic guidance (“stereotactic biopsy”)—When a suspicious abnormality is identified by mammography alone and cannot be palpated by the clinician, the lesion should be biopsied under mammographic guidance. In the computerized stereotactic guided core needle technique, a biopsy needle is inserted into the lesion with mammographic guidance, and a core of tissue for histologic examination can then be examined. Vacuum assistance increases the amount of tissue obtained and improves diagnosis.
Mammographic localization excisional biopsy is performed by obtaining a mammogram in two perpendicular views and placing a needle, hook-wire, or radioactive or radar-detectable seed localizer near the abnormality so that the surgeon can locate the lesion intraoperatively. After mammography confirms the position of the localizer in relation to the lesion, an incision is made and the localizer is identified. Often, the abnormality cannot even be palpated through the incision—as is the case with microcalcifications—and thus it is essential to obtain a mammogram of the specimen to document that the lesion was excised. At that time, a second marker needle can further localize the lesion for the pathologist. Image-guided core needle biopsies have proved equivalent to mammographic localization biopsies. Core biopsy is preferable to mammographic localization for accessible lesions since an operation can be avoided. A metal clip should be placed after any image-guided core biopsy to facilitate finding the site of the lesion if subsequent treatment is necessary.
4. Other imaging modalities—Other modalities of breast imaging have been investigated for diagnostic purposes. Automated breast ultrasonography is useful in distinguishing cystic from solid lesions but should be used only as a supplement to physical examination and mammography. MRI is highly sensitive but not specific and should not be used for screening except in highly selective cases. For example, MRI is useful in differentiating scar from recurrence postlumpectomy and is valuable to screen high-risk women (eg, women with BRCA mutations). It may also be of value to examine for multicentricity when there is a known primary cancer; to examine the contralateral breast in women with cancer; to examine the extent of cancer, especially lobular carcinomas; or to determine the response to neoadjuvant chemotherapy. Moreover, MRI-detected suspicious findings that are not seen on mammogram or ultrasound may be biopsied under MRI guidance. PET scanning does not appear useful in evaluating the breast itself but is useful to examine for distant metastases.
5. Cytology—Cytologic examination of nipple discharge or cyst fluid may be helpful on rare occasions. As a rule, mammography (or ductography) and breast biopsy are required when nipple discharge or cyst fluid is bloody or cytologically questionable.
 Differential Diagnosis
Differential Diagnosis
The lesions to be considered most often in the differential diagnosis of breast cancer are the following, in descending order of frequency: fibrocystic condition of the breast, fibroadenoma, intraductal papilloma, lipoma, and fat necrosis.
 Staging
Staging
The American Joint Committee on Cancer and the International Union Against Cancer have a joint TNM (tumor, regional lymph nodes, distant metastases) staging system for breast cancer. The eighth edition is a landmark change in the TNM staging system because it adds biologic markers to modify the anatomic staging.
 Pathologic Types
Pathologic Types
Numerous pathologic subtypes of breast cancer can be identified histologically (Table 17–3).
Table 17–3. Histologic types of breast cancer.

Except for the in situ cancers, the histologic subtypes have only a slight bearing on prognosis when outcomes are compared after accurate staging. The noninvasive cancers by definition are confined by the basement membrane of the ducts and lack the ability to spread. Histologic parameters for invasive cancers, including lymphovascular invasion and tumor grade, have been shown to be of prognostic value. Immunohistochemical analysis for expression of hormone receptors and for overexpression of HER2 in the primary tumor offers prognostic and therapeutic information.
 Special Clinical Forms of Breast Cancer
Special Clinical Forms of Breast Cancer
A. Paget Carcinoma
Paget carcinoma is not common (about 1% of all breast cancers). Over 85% of cases are associated with an underlying invasive or noninvasive cancer, usually a well differentiated infiltrating ductal carcinoma or a DCIS. The ducts of the nipple epithelium are infiltrated, but gross nipple changes are often minimal, and a tumor mass may not be palpable.
Because the nipple changes appear innocuous, the diagnosis is frequently missed. The first symptom is often itching or burning of the nipple, with superficial erosion or ulceration. These are often diagnosed and treated as dermatitis or bacterial infection, leading to delay or failure in detection. The diagnosis is established by biopsy of the area of erosion. When the lesion consists of nipple changes only or an associated DCIS, the incidence of axillary metastases is extremely low, and the prognosis is excellent. When a breast mass is also present, the incidence of axillary metastases rises, with an associated marked decrease in prospects for cure by surgical or other treatment.
B. Inflammatory Carcinoma
This is the most malignant form of breast cancer and constitutes less than 3% of all cases. The clinical findings consist of a rapidly growing, sometimes painful mass that enlarges the breast. The overlying skin becomes erythematous, edematous, and warm. Often there is no distinct mass, since the tumor infiltrates the involved breast diffusely. The inflammatory changes, often mistaken for an infection, are caused by carcinomatous invasion of the subdermal lymphatics, with resulting edema and hyperemia. If the clinician suspects infection but the lesion does not respond rapidly (1–2 weeks) to antibiotics, biopsy should be performed. The diagnosis should be made when the redness involves more than one-third of the skin over the breast and biopsy shows infiltrating carcinoma with invasion of the subdermal lymphatics. Metastases tend to occur early and widely, and for this reason inflammatory carcinoma was considered rarely curable. However, radiation, hormone therapy (if hormone receptor positive), anti-HER2 therapy (if HER2 overexpressing or amplified), surgery, and chemotherapy have resulted in some long-term cures. Mastectomy is indicated when chemotherapy and radiation have resulted in clinical remission with no evidence of distant metastases. In these cases, residual disease in the breast may be eradicated.
 Breast Cancer Occurring During Pregnancy or Lactation
Breast Cancer Occurring During Pregnancy or Lactation
Breast cancer complicates up to one in 3000 pregnancies. The diagnosis is frequently delayed, because physiologic changes in the breast may obscure the lesion and screening mammography is not done in young or pregnant women. Data are insufficient to determine whether interruption of pregnancy improves the prognosis of patients who are identified to have potentially curable breast cancer and who receive definitive treatment during pregnancy. The decision whether or not to terminate the pregnancy must be made on an individual basis, taking into account the patient’s wishes, the clinical stage of the cancer and overall prognosis, the gestational age of the fetus, and the potential for premature ovarian failure in the future with systemic therapy.
It is important for primary care and reproductive specialists to aggressively work up any breast abnormality discovered in a pregnant woman. Pregnancy (or lactation) is not a contraindication to operation or treatment, and therapy should be based on the stage of the disease as in the nonpregnant (or nonlactating) woman. Women with early-stage gestational breast cancer who choose to continue their pregnancy should undergo surgery to remove the tumor and systemic therapy if indicated. Retrospective reviews of patients treated with anthracycline-containing regimens for gestational cancers (including leukemia and lymphomas) have established the relative safety of these regimens during pregnancy for both the patient and the fetus. Taxane-based and trastuzumab-based regimens have not been evaluated extensively, however. Radiation therapy should be delayed until after delivery. Overall survival rates have improved, since cancers are now diagnosed in pregnant women earlier than in the past and treatment has improved.
 Bilateral Breast Cancer
Bilateral Breast Cancer
Bilateral breast cancer occurs in less than 5% of cases, but there is as high as a 20–25% incidence of later occurrence of cancer in the second breast. Bilaterality occurs more often in familial breast cancer, in women under age 50 years, and when there is a deleterious mutation. The incidence of second breast cancers increases directly with the length of time the patient is alive after her first cancer—about 1–2% per year. Tamoxifen or aromatase inhibitors decrease the risk of a contralateral hormone receptor–positive cancer.
In patients with breast cancer, mammography should be performed before primary treatment and at regular intervals thereafter, to search for occult cancer in the opposite breast or conserved ipsilateral breast.
 Noninvasive Cancer
Noninvasive Cancer
Noninvasive cancer can occur within the ducts (DCIS) or lobules (LCIS). DCIS tends to be unilateral and is believed to progress to invasive cancer if untreated. In approximately 40–60% of women who have DCIS treated with biopsy alone, invasive cancer develops within the same breast. LCIS is generally agreed to be a marker of an increased risk of breast cancer rather than a direct precursor of breast cancer itself. In the eighth edition of The American Joint Committee on Cancer (AJCC) Cancer Staging Manual, LCIS is no longer considered a cancer. The probability of breast cancer (DCIS or invasive cancer in either breast) in a woman in whom LCIS has been diagnosed is estimated to be 1% per year. If LCIS is detected on core needle biopsy, an excisional biopsy without lymph node sampling may be performed to rule out DCIS or invasive cancer, but NCCN guidelines suggest observation alone is satisfactory. The incidence of LCIS is rising, likely due to increased use of screening mammography. In addition, the rate of mastectomy after the diagnosis of LCIS is increasing in spite of the fact that mastectomy is only recommended in those patients who otherwise have an increased risk of breast cancer through family history, genetic mutation, or past exposure to thoracic radiation. Pleomorphic LCIS may behave more like DCIS and may be associated with invasive carcinoma. For this reason, pleomorphic LCIS should be surgically removed with clear margins.
The treatment of intraductal lesions is controversial. DCIS can be treated by wide excision with or without radiation therapy or with total mastectomy. Conservative management is advised in patients with small lesions amenable to lumpectomy. Patients in whom LCIS is diagnosed or who have received lumpectomy for DCIS may discuss chemoprevention (with hormonal blockade therapy) with their clinician, which is effective in reducing the risk of invasive breast cancer in DCIS that has been completely excised by breast-conserving surgery and in LCIS. Axillary metastases from in situ cancers should not occur unless there is an occult invasive cancer. Because a sentinel lymph node biopsy after mastectomy cannot be performed, it should be performed in a patient undergoing mastectomy for DCIS in case an invasive component is discovered.
Vapiwala N et al. No impact of breast magnetic resonance imaging on 15-year outcomes in patients with ductal carcinoma in situ or early-stage invasive breast cancer managed with breast conservation therapy. Cancer. 2017 Apr 15;123(8):1324–32. [PMID: 27984658]
 Biomarkers & Gene Expression Profiling
Biomarkers & Gene Expression Profiling
Determining the ER, PR, and HER2 status of the tumor at the time of diagnosis of early breast cancer and, if possible, at the time of recurrence is critical, both to gauge a patient’s prognosis and to determine the best treatment regimen. In addition to ER status and PR status, the rate at which tumor divides (assessed by an immunohistochemical stain for Ki67) and the grade and differentiation of the cells are also important prognostic factors. These markers may be obtained on core biopsy or surgical specimens, but not reliably on FNA cytology. Patients whose tumors are hormone receptor-positive tend to have a more indolent disease course than those whose tumors are receptor-negative. Moreover, treatment with an anti-hormonal agent is an essential component of therapy for hormone-receptor positive breast cancer at any stage. While up to 60% of patients with metastatic breast cancer will respond to hormonal manipulation if their tumors are ER-positive, less than 5% of patients with metastatic, ER-negative tumors will respond to hormone therapy.
Another key element in determining treatment and prognosis is the amount of the HER2 oncogene present in the cancer. HER2 overexpression (HER2-oncogene–positive breast cancer) is generally more aggressive than breast cancer with normal HER2 expression (HER2-negative breast cancer). Individually these biomarkers are predictive and thus provide insight to guide appropriate therapy. Moreover, when combined they provide useful information regarding risk of recurrence and prognosis. In general, tumors that lack expression of HER2, ER, and PR (“triple negative”) have a higher risk of early recurrence and metastases and are associated with a worse survival compared with other types. Neither endocrine therapy nor HER2-targeted agents are useful for this type of breast cancer. Chemotherapy has been the primary treatment option for triple-negative breast cancer. In contrast, patients with early stage, hormone receptor–positive breast cancer may not benefit from the addition of chemotherapy to hormonally targeted treatments. Several molecular tests have been developed to assess risk of recurrence and to predict which patients are most likely to benefit from chemotherapy for early-stage disease. Oncotype DX (Genomic Health) evaluates the expression of 21 genes relating to ER, HER2, and proliferation in a tumor specimen and categorizes a patient’s risk of recurrence (recurrence score) as high, intermediate, or low risk. Patients in low- or intermediate-risk categories do not benefit from chemotherapy. This test is primarily indicated for ER-positive, lymph node–negative tumors, but an ongoing study (RxPONDER” NCT01272037) is addressing its predictive value in lymph node–positive, ER–positive breast cancer in a prospective manner. MammaPrint (Agendia) is an FDA-approved 70-gene signature assay that is available for evaluating prognosis. This test classifies patients into good and poor prognostic groups to predict clinical outcome and may be used on patients with hormone receptor–positive or –negative breast cancer. American Society of Clinical Oncology (ASCO) guidelines indicate this assay may be best used to help determine whether chemotherapy may be safely withheld in patients with hormone receptor–negative, HER2-negative, node-positive breast cancer at high clinical risk. ASCO does not recommend using this assay in hormone receptor–negative or HER2-positive breast cancer. The eighth edition of the AJCC staging system has incorporated genomic assays to provide a prognostic stage. Patients with low-risk genomic assays may be downstaged from their TNM stage. For example, a patient who has a TNM stage of II but has a low Oncotype Recurrence Score could be classified as having stage I disease per the eighth edition.
Ibraheem AF et al. Community clinical practice patterns and mortality in patients with intermediate Oncotype DX recurrence scores: who benefits from chemotherapy. Cancer. 2019 Jan 15;125(2):213–22. [PMID: 30387876]
Krop I et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017 Aug 20;35(24):2838–47. [PMID: 28692382]
Sparano JA et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018 Jul 12;379(2):111–21. [PMID: 29860917]
Sparano JA et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019 Jun 20;380(25):2395–2405. [PMID: 31157962]
Wolff AC et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018 Nov;142(11):1364–82. [PMID: 29846104]
 Curative Treatment
Curative Treatment
Not all breast cancer is systemic at the time of diagnosis, and a pessimistic attitude concerning the management of breast cancer is unwarranted. Most patients with early breast cancer can be cured. Treatment with a curative intent is advised for clinical stage I, II, and III disease (see Table 39–2). Patients with locally advanced (T3, T4) and even inflammatory tumors may be cured with multimodality therapy. When metastatic disease is diagnosed in most patients, palliation becomes the goal of therapy. Treatment with palliative intent is appropriate for all patients with stage IV disease and for patients with unresectable local cancers.
A. Choice and Timing of Primary Therapy
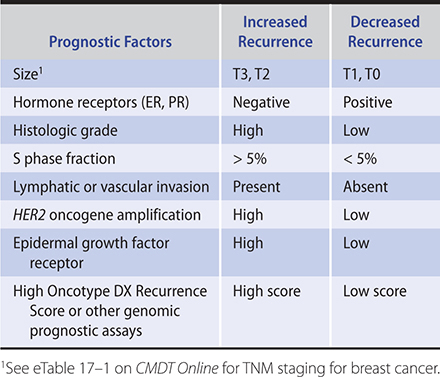
The extent of disease and its biologic aggressiveness are the principal determinants of the outcome of primary therapy. Clinical and pathologic staging help in assessing extent of disease, but each is to some extent imprecise. Other factors such as tumor grade, hormone receptor assays, HER2 oncogene amplification, and genomic assays are of prognostic value and are key to determining systemic therapy but are not as relevant in determining the type of local therapy.
Controversy has surrounded the choice of primary therapy of stage I, II, and III breast carcinoma. Currently, the standard of care for stage I, stage II, and most stage III cancer is surgical resection followed by adjuvant radiation or systemic therapy, or both, when indicated. Neoadjuvant therapy has become popular since large tumors may be shrunk by chemotherapy prior to surgery, making some patients who require mastectomy candidates for lumpectomy. In addition, in some cases of triple-negative and HER2-amplified breast cancer, the response to neoadjuvant therapy may determine the need for additional postoperative systemic therapy. It is important for patients to understand all of the surgical options, including reconstructive options, prior to having surgery. Patients with large primary tumors, inflammatory cancer, or palpably enlarged lymph nodes should have staging scans performed to rule out distant metastatic disease prior to definitive surgery. In general, adjuvant systemic therapy is started when the breast has adequately healed, ideally within 4–8 weeks after surgery. While no prospective studies have defined the appropriate timing of adjuvant chemotherapy, a single- institution study of over 6800 patients suggests that systemic therapy should be started within 60 days of surgery, especially in women with stage II or III breast cancer, triple-negative breast cancer, or HER2-positive disease.
B. Surgical Resection
1. Breast-conserving therapy—Multiple, large, randomized studies including the Milan and NSABP trials show that disease-free and overall survival rates are similar for patients with stage I and stage II breast cancer treated with partial mastectomy (breast-conserving lumpectomy or “breast conservation”) plus axillary dissection followed by radiation therapy and for those treated by modified radical mastectomy (total mastectomy plus axillary dissection).
Tumor size is a major consideration in determining the feasibility of breast conservation. The NSABP lumpectomy trial randomized patients with tumors as large as 4 cm. To achieve an acceptable cosmetic result, the patient must have a breast of sufficient size to enable excision of a 4-cm tumor without considerable deformity. Therefore, large tumor size is only a relative contraindication. Subareolar tumors, also difficult to excise without deformity, are not contraindications to breast conservation. Clinically detectable multifocality is a relative contraindication to breast-conserving surgery, as is fixation to the chest wall or skin or involvement of the nipple or overlying skin. The patient—not the surgeon—should be the judge of what is cosmetically acceptable. Given the relatively high risk of poor outcome after radiation, concomitant scleroderma is a contraindication to breast-conserving surgery. A history of prior therapeutic radiation to the ipsilateral breast or chest wall (or both) is also generally a contraindication for breast conservation, although accelerated partial breast irradiation may permit a second breast irradiation.
Axillary dissection is primarily used to properly stage cancer and plan radiation and systemic therapy. Intraoperative lymphatic mapping and sentinel node biopsy identify lymph nodes most likely to harbor metastases if present (Figure 17–7). Sentinel node biopsy is a proven alternative to axillary dissection in patients without clinical evidence of axillary lymph node metastases. If sentinel node biopsy reveals no evidence of axillary metastases, it is highly likely that the remaining lymph nodes are free of disease and axillary dissection may be omitted. An important study from the American College of Surgeons Oncology Group randomized women with sentinel node metastases to undergo completion of axillary dissection or to receive no further axillary-specific treatment after lumpectomy; no difference in 10-year survival was found. Omission of axillary dissection is acceptable for women with tumor-free sentinel nodes or those with involvement of one or two sentinel nodes who are treated with lumpectomy, whole breast irradiation, and adjuvant systemic therapy.
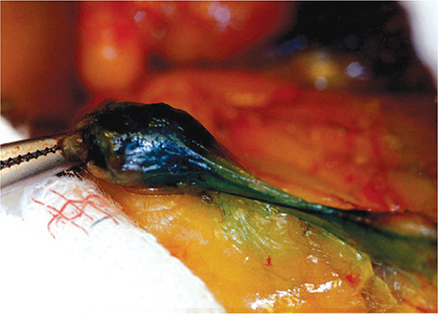
Figure 17–7. Sentinel node. (Used, with permission, from Armando E. Giuliano, MD.)
Breast-conserving surgery with sentinel node biopsy and radiation is the preferred form of treatment for patients with early-stage breast cancer. Despite the numerous randomized trials showing no survival benefit of mastectomy over breast-conserving partial mastectomy with irradiation or of axillary dissection over sentinel node biopsy, these conservative procedures appear to be underutilized.
2. Mastectomy—Modified radical mastectomy was previously the standard therapy for most patients with early-stage breast cancer. This operation removes the entire breast, overlying skin, nipple, and areolar complex usually with underlying pectoralis fascia with the axillary lymph nodes in continuity. The major advantage of modified radical mastectomy is that radiation therapy may not be necessary, although radiation may be used when lymph nodes are involved with cancer or when the primary tumor is 5 cm or larger. The disadvantage of mastectomy is the cosmetic and psychological impact associated with breast loss. Radical mastectomy, which removes the underlying pectoralis muscle, should be performed rarely, if at all. Axillary node dissection is not indicated for noninvasive cancers because nodal metastases are rarely present. Skin-sparing and nipple-sparing mastectomy is available but is not appropriate for all patients. Breast reconstruction, immediate or delayed, should be discussed with patients who choose or require mastectomy. Patients should have an interview with a reconstructive plastic surgeon to discuss options prior to making a decision regarding reconstruction. Time is well spent preoperatively in educating the patient and family about these matters.
C. Radiotherapy
Radiotherapy after partial mastectomy consists of 5–7 weeks of five daily fractions to a total dose of 5000–6000 cGy. Most radiation oncologists use a boost dose to the cancer location. Shorter fractionation schedules may be reasonable for women with low-risk, early-stage breast cancer. Accelerated partial breast irradiation, in which only the portion of the breast from which the tumor was resected is irradiated for 1–2 weeks, appears effective in achieving local control for selected patients; however, the prospective randomized NSABP B-39/RTOG 0413 trial failed to demonstrate noninferiority of partial breast irradiation compared to whole breast irradiation. With 4216 patients randomized and treated on this study, cumulative ipsilateral breast tumor recurrences at 10 years were slightly higher in the partial breast irradiation arm (4.6% vs 3.9%). Toxicity also tended to be less with whole breast irradiation. Another randomized study (“RAPID”) that only allowed patients with node-negative disease enrolled 2135 patients and demonstrated noninferiority. Although the conclusions of these two noninferiority studies were discordant, the rates of in-breast tumor recurrence in both arms was quite low. Guidelines by the American Society of Radiation Oncology (ASTRO) and the European Society for Radiotherapy (ESTRO) indicate that it is appropriate to discuss partial breast radiation for women over the age of 50 with node-negative, hormone receptor–positive, small (T1) tumors with surgical margins of at least 2 mm. Moreover, in women over the age of 70 with small (less than 2 cm), lymph node–negative, hormone receptor–positive cancers, radiation therapy may be avoided. The recurrence rates after intraoperative radiation, while low, appear significantly higher than postoperative whole breast radiation therapy. However, in all of these situations, a balanced discussion with a radiation oncologist to weigh the risks and benefits of each approach is warranted.
Current studies suggest that radiotherapy after mastectomy may improve recurrence rates and survival in younger patients and those with tumors 5 cm or larger or positive lymph nodes.
Giuliano AE et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017 Sep 12;318(10):918–26. [PMID: 28898379]
Grover S et al. Survival after breast-conserving surgery with whole breast or partial breast irradiation in women with early stage breast cancer: a SEER data-base analysis. Breast J. 2017 May;23(3):292–8. [PMID: 27988987]
Lyman GH et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017 Feb 10;35(5):561–4. [PMID: 27937089]
Vicini FA et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019 Dec 14;394(10215):2155–64. [PMID: 31813636]
Whelan TJ et al; RAPID Trial Investigators. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet. 2019 Dec 14;394(10215):2165–72. [PMID: 31813635]
D. Adjuvant Systemic Therapy
The goal of systemic therapy, including hormone-modulating medications (endocrine therapy), cytotoxic chemotherapy, and HER2-targeted agents such as trastuzumab, is to kill cancer cells that have escaped the breast and axillary lymph nodes as micrometastases before they become macrometastases (ie, stage IV cancer). Systemic therapy improves survival and is advocated for most patients with curable breast cancer. In practice, most medical oncologists use adjuvant chemotherapy for patients with either node-positive or higher-risk (eg, hormone receptor–negative or HER2-positive) node-negative breast cancer and use endocrine therapy for all hormone receptor–positive invasive breast cancer unless contraindicated. Prognostic factors other than nodal status that are used to determine the patient’s risks of recurrence are tumor size, ER and PR status, nuclear grade, histologic type, proliferative rate, oncogene expression (Table 17–4), and patient’s age and menopausal status. In general, systemic chemotherapy decreases the chance of recurrence by about 30%, hormonal modulation decreases the relative risk of recurrence by 40–50% (for hormone receptor–positive cancer), and HER2-targeted therapy decreases the relative risk of recurrence by approximately 40% (for HER2-positive cancer). Systemic chemotherapy is usually given sequentially, rather than concurrently with radiation. In terms of sequencing, typically chemotherapy is given before radiation and endocrine therapy is started concurrent with or after radiation therapy.
Table 17–4. Prognostic factors for recurrence in node-negative breast cancer.
The long-term advantage of systemic therapy is well established. All patients with invasive hormone receptor–positive tumors should consider the use of hormone-modulating therapy. Most patients with HER2-positive tumors should receive trastuzumab-containing chemotherapy regimens. In general, adjuvant systemic chemotherapy should not be given to women who have small node-negative breast cancers with favorable histologic findings and tumor biomarkers. The ability to predict more accurately which patients with HER2-negative, hormone receptor–positive, lymph node–negative tumors should receive chemotherapy is improving with the advent of prognostic tools, such as Oncotype DX and MammaPrint (see Biomarkers and Gene Expression Profiling above). These tests are thus validated tools to enable clinicians to better select patients who can safely omit chemotherapy.
1. Chemotherapy—The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis involving over 28,000 women enrolled in 60 trials of adjuvant polychemotherapy versus no chemotherapy demonstrated a significant beneficial impact of chemotherapy on clinical outcome in non–stage IV breast cancer. This study showed that adjuvant chemotherapy reduces the risk of recurrence and breast cancer–specific mortality in all women and also showed that women under the age of 50 derive the greatest benefit.
a. Anthracycline- and cyclophosphamide-containing regimens—On the basis of the superiority of anthracycline-containing regimens in metastatic breast cancer, both doxorubicin and epirubicin have been studied extensively in the adjuvant setting. Studies comparing Adriamycin (doxorubicin) and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) to cyclophosphamide-methotrexate-5-fluorouracil (CMF) have shown that treatments with anthracycline-containing regimens are at least as effective as treatment with CMF. The EBCTCG analysis including over 14,000 patients enrolled in trials comparing anthracycline-based regimens to CMF, showed a small but statistically significant improved disease-free and overall survival with the use of anthracycline-based regimens. It should be noted, however, that most of these studies included a mixed population of patients with HER2-positive and HER2-negative breast cancer and were performed before the introduction of trastuzumab. Retrospective analyses of a number of these studies suggest that anthracyclines may be primarily effective in tumors with HER2 overexpression or alteration in the expression of topoisomerase IIa (the target of anthracyclines and close to the HER2 gene). Given this, for HER2-negative, node-negative breast cancer, four cycles of AC or six cycles of CMF are probably equally effective.
b. Taxanes—When taxanes (paclitaxel and docetaxel) emerged in the 1990s, multiple trials were conducted to evaluate their use in combination with anthracycline-based regimens. The majority of these trials showed an improvement in disease-free survival and at least one showed an improvement in overall survival with the taxane-based regimen. A meta-analysis of taxane versus non-taxane anthracycline-based regimen trials showed an improvement in disease-free and overall survival for the taxane-based regimens. Several regimens have been reported including AC followed by paclitaxel (AC-P) or docetaxel (Taxotere) (AC-T), docetaxel concurrent with AC (TAC), 5-fluorouracil-epirubicine-cyclophosphamide (FEC)-docetaxel, and FEC-paclitaxel.
The US Oncology trial 9735 compared four cycles of AC with four cycles of docetaxel (Taxotere) and cyclophosphamide (TC). With a median of 7 years’ follow-up, this study showed a statistically significantly improved disease-free survival and overall survival in the patients who received TC.
The three Anthracyclines in early Breast Cancer (ABC) (USOR 06-090, NSABP B-46, NSABP B-49) trials (total N = 4242) each compared the TC regimen to anthracycline/taxane-based chemotherapy regimens (TaxAC) in HER2-negative early-stage breast cancer. An interim joint analysis showed that the invasive disease-free survival at 4 years was improved by 2.5% with the addition of an anthracycline (P = 0.04) but that the benefit of an anthracycline was primarily seen in triple-negative disease (HR 1.42, 95% CI 1.04, 1.94). No survival difference was observed at the time of interim reporting. In contrast, the West German Study Group phase III PlanB study compared six cycles of adjuvant TC to four cycles of EC followed by four cycles of docetaxel (EC-D) in 2449 patients with intermediate to high risk, HER2-negative breast cancer. Dose reductions were higher in the anthracycline arm (19.7% vs 6.6%, P < 0.001) and grade 3/4 toxicities were consistently and significantly higher in the anthracycline arm. TC demonstrated noninferiority to EC-D with a 5-year disease-free survival of 90% for each arm (HR TC vs EC-D = 0.996; 95% CI 0.77–1.29). In contrast to the ABC study, subset analysis indicated a similar disease-free survival in each treatment arm regardless of recurrence score, nodal status, Ki67 reactivity, grade, or triple-negative subtype. A Danish phase III study was conducted to compare six cycles of TC to six cycles of EC-D in 2012 high-risk patients with TOP2A normal breast cancer. The 5-year disease-free survival was 87.9% for EC-D vs 88.3% for DC (HR, 1.00; P = 1.00). While it is generally agreed that taxanes should be used for most patients receiving chemotherapy for early breast cancer, data relating to the benefits of anthracyclines are conflicting; thus, a balanced discussion regarding the potential risks versus benefits of the addition of anthracyclines is warranted, especially in hormone receptor–positive disease.
c. Duration and dose of chemotherapy—The ideal duration of adjuvant chemotherapy still remains uncertain. However, based on the meta-analysis performed in the Oxford Overview (EBCTCG), the current recommendation is for 3–6 months of the commonly used regimens. Data suggest that the timing and sequencing of anthracycline-taxane–based chemotherapy may be important. Multiple trials beginning in the 1980s sought to demonstrate whether dose-intensification of adjuvant chemotherapy by shortening the intervals between cycles (“dose-dense”), or by giving chemotherapy at full dose sequentially rather than concurrently at reduced doses is associated with better outcomes. The EBCTCG meta-analysis included 37,298 patients treated on 26 trials and showed a significant 3.4% absolute decrease and 14% relative risk reduction in breast cancer recurrences with dose-intensification. Moreover, the absolute 10-year breast cancer mortality was improved by 2.4%. While impressive, the benefit of dose-intensification appeared to be strongest in node-positive disease. Its benefit, if any, in HER2-positive disease in the era of HER2-targeted therapy has not been validated. Additionally, the use of dose-intensification in (non-anthracycline) taxane-based regimens has not been evaluated.
d. Chemotherapy side effects—Chemotherapy side effects, which are discussed in Chapter 39, are generally well controlled.
2. Targeted therapy—Targeted therapy refers to agents that are directed specifically against a protein or molecule expressed uniquely on tumor cells or in the tumor microenvironment.
a. HER2 overexpression—Approximately 20% of breast cancers are characterized by amplification of the HER2 oncogene leading to overexpression of the HER2 oncoprotein. The poor prognosis associated with HER2 overexpression has been drastically improved with the development of HER2-targeted therapy. Trastuzumab (Herceptin [H]), a monoclonal antibody that binds to HER2, is effective in combination with chemotherapy in patients with HER2-overexpressing metastatic and early breast cancer. In the adjuvant setting, the first and most commonly studied chemotherapy backbone used with trastuzumab is AC-T. Subsequently, the BCIRG006 study showed similar efficacy for AC-TH and a nonanthracycline-containing regimen, TCH (docetaxel, carboplatin, trastuzumab). Both were significantly better than AC-T in terms of disease-free and overall survival and TCH had a lower risk of cardiac and bone marrow (leukemia/myelodysplasia) toxicity. Both AC-TH and TCH are FDA-approved for nonmetastatic, HER2-positive breast cancer. In these regimens, trastuzumab is given with chemotherapy and then continues beyond the course of chemotherapy with a goal, in general, to complete a full year. Neoadjuvant chemotherapy plus dual HER2-targeted therapy with trastuzumab and pertuzumab (also a HER2-targeted monoclonal antibody that prevents dimerization of HER2 with HER3 and has been shown to be synergistic in combination with trastuzumab) is a standard of care option available to patients with stage II/III HER2-positive breast cancer (see Neoadjuvant Therapy). The phase III randomized placebo-controlled adjuvant “APHINITY” study evaluated 1 year of adjuvant pertuzumab in combination with trastuzumab. With a median follow up of 74 months, the 6-year invasive disease-free survival was 2.8% better with the year of pertuzumab (90.6% vs 87.8%, stratified HR 0.76, 0.64, 0.91). This benefit appeared to be restricted to those with node-positive disease where the absolute invasive disease-free survival benefit was 4.5% (87.9% vs 83.4%). However, no significant overall survival advantage has been demonstrated. Its use in the adjuvant setting is primarily restricted to patients with high-risk, node-positive disease. Neratinib, an orally bioavailable dual HER1 (EGFR), HER2 tyrosine kinase inhibitor, is FDA-approved as adjuvant therapy. The phase III placebo-controlled EXTENET study demonstrated that neratinib improves invasive disease-free survival when given for 1 year after completion of a year of standard adjuvant trastuzumab-based therapy (median follow-up 5.2 years, stratified HR 0.73, 95% CI 0.57, 0.92, P = 0.0083). Neratinib is associated with gastrointestinal toxicity, most notably moderate to severe diarrhea in approximately 40% of patients who did not use antidiarrheal prophylaxis. Measures to mitigate this side effect are being evaluated in the CONTROL clinical trial (NCT02400476). Pending those final results, patients should be treated with upfront prophylaxis with loperamide.
Patients who undergo neoadjuvant trastuzumab-based chemotherapy and have residual disease remaining at the time of surgery have a comparatively poor outcome compared to those who achieve a pathologic complete response. In the phase III randomized KATHERINE trial, 1486 patients with residual disease after standard neoadjuvant trastuzumab/taxane-based therapy (18% of whom also received neoadjuvant pertuzumab) were randomized to receive the antibody-drug conjugate trastuzumab emtansine or standard trastuzumab for 14 cycles after surgery. Patients treated with trastuzumab emtansine had a statistically significantly improved 3-year invasive disease-free survival (88% vs 77%), associated with a 50% relative risk reduction. These data led to the FDA approval of adjuvant trastuzumab emtansine in 2019 for patients with residual disease after standard trastuzumab-containing neoadjuvant therapy.
Retrospective studies have shown that even small (stage T1a,b) HER2-positive tumors have a worse prognosis compared with same-sized HER2-negative tumors and may thus be appropriate for trastuzumab-based regimens. The NSABP B43 study is ongoing to evaluate whether the addition of trastuzumab to radiation therapy is warranted for DCIS.
Cardiomyopathy develops in a small but significant percentage (0.4–4%) of patients who receive trastuzumab-based regimens. For this reason, anthracyclines and trastuzumab are rarely given concurrently and cardiac function is monitored periodically throughout therapy.
b. Endocrine therapy—Adjuvant hormone modulation therapy is highly effective in decreasing relative risk of recurrence by 40–50% and mortality by 25% in women with hormone receptor–positive tumors regardless of menopausal status.
(1) Tamoxifen—The traditional regimen had been 5 years of the estrogen-receptor antagonist/agonist tamoxifen until the 2012 reporting of the Adjuvant Tamoxifen Longer Against Shorter (ATLAS) trial in which 5 versus 10 years of adjuvant tamoxifen were compared. In this study, disease-free and overall survival were significantly improved in women who received 10 years of tamoxifen, particularly after year 10. Though these results are impressive, the clinical application of long-term tamoxifen use must be discussed with patients individually, taking into consideration risks of tamoxifen (such as secondary uterine cancers, venous thromboembolic events, and side effects that impact quality of life). Ovarian suppression in addition to tamoxifen has been shown to significantly improve 8-year disease-free survival (83.2% vs 78.9%) and 8-year overall survival (93.3% vs 91.5%) compared to tamoxifen alone in the randomized Suppression of Ovarian Function Trial [SOFT] study, though the benefits appeared to be seen primarily in chemotherapy-treated patients with higher risk disease.
(2) Aromatase inhibitors—AIs, including anastrozole, letrozole, and exemestane, reduce estrogen production and are also effective in the adjuvant setting for postmenopausal women. AIs should not be used in a patient with functioning (premenopausal) ovaries since they do not block ovarian production of estrogen. At least seven large randomized trials enrolling more than 24,000 postmenopausal patients with hormone receptor–positive nonmetastatic breast cancer have compared the use of AIs with tamoxifen or placebo as adjuvant therapy. All of these studies have shown small but statistically significant improvements in disease-free survival (absolute benefits of 2–6%) with the use of AIs. In addition, AIs have been shown to reduce the risk of contralateral breast cancers and to have fewer associated serious side effects (such as endometrial cancers and thromboembolic events) than tamoxifen. However, they are associated with accelerated bone loss and an increased risk of fractures as well as a musculoskeletal syndrome characterized by arthralgias or myalgias (or both) in the majority of patients. The American Society of Clinical Oncology and the NCCN have recommended that postmenopausal women with hormone receptor–positive breast cancer be offered an AI either initially or after tamoxifen therapy. HER2 status should not affect the use or choice of hormone therapy. A combined analysis of the SOFT and Tamoxifen and Exemestane Trial (TEXT) studies showed for the first time that exemestane plus ovarian suppression with triptorelin was associated with a reduced risk of relapse compared to tamoxifen, making this a viable adjuvant therapy option for premenopausal women with high-risk ER-positive breast cancers.
3. Bisphosphonates—Multiple randomized studies have evaluated the use of adjuvant bisphosphonates in addition to standard local and systemic therapy for early-stage breast cancer and have shown, in addition to improvement in bone density, a consistent reduction in the risk of metastatic recurrence in postmenopausal patients. A meta-analysis evaluating more than 18,000 women with early-stage breast cancer treated with bisphosphonates or placebo showed that bisphosphonates reduce the risk of cancer recurrence (especially in bone) and improve breast cancer–specific survival primarily in postmenopausal women. Side effects associated with bisphosphonate therapy include bone pain, fever, osteonecrosis of the jaw (rare, less than 1%), esophagitis or ulcers (for oral bisphosphonates), and kidney injury. Although the FDA has not yet approved the adjuvant use of bisphosphonates to reduce the risk of breast cancer recurrence, the 2017 jointly published guidelines of the Cancer Care Ontario and American Society of Clinical Oncology recommend that bisphosphonate use (zoledronic acid or clodronate) be considered in the adjuvant therapy plan for postmenopausal breast cancer patients. In addition, denosumab (another bone stabilizing medication), which is an antibody directed against receptor activator of nuclear factor kappa B ligand (RANK-L), was evaluated in two phase III adjuvant trials with discordant results. The “D-CARE” study randomized patients with early-stage breast cancer (all biologic subtypes) to receive denosumab or placebo and failed to demonstrate a reduction in breast cancer recurrences or deaths. It is speculated that one possible reason for this negative result may be due to the fact that premenopausal patients (who do not have a demonstrated metastatic recurrence benefit from bisphosphonates) were included in the study. In contrast, the ABCSG-18 trial restricted enrollment to postmenopausal women and did show an improvement in disease-free survival with denosumab.
4. Adjuvant therapy in older women—Data relating to the optimal use of adjuvant systemic treatment for women over the age of 65 are limited. Results from the EBCTCG overview indicate that while adjuvant chemotherapy yields a smaller benefit for older women compared with younger women, it still improves clinical outcomes. Moreover, individual studies do show that older women with higher risk disease derive benefits from chemotherapy. One study compared the use of oral chemotherapy (capecitabine) to standard chemotherapy in older women and concluded that standard chemotherapy is preferred. Another study (USO TC vs AC) showed that women over the age of 65 derive similar benefits from the taxane-based regimen as women who are younger. The benefits of endocrine therapy for hormone receptor–positive disease appear to be independent of age. In general, decisions relating to the use of systemic therapy should take into account a patient’s comorbidities and physiological age, more so than chronologic age.
E. Neoadjuvant Therapy
The use of systemic therapy prior to resection of the primary tumor (neoadjuvant) is a standard option that in many cases should be discussed with patients prior to surgery. This enables the assessment of in vivo sensitivity of the tumor to the selected systemic therapy. Patients with hormone receptor–negative, triple-negative, or HER2-positive breast cancer are more likely to have a pathologic complete response (meaning no residual invasive cancer at the time of surgery) to neoadjuvant chemotherapy than those with hormone receptor–positive breast cancer. A complete pathologic response at the time of surgery, especially in hormone receptor–negative tumors, is associated with improvement in event-free and overall survival. Neoadjuvant chemotherapy also increases the chance of breast conservation by shrinking the primary tumor in women who would otherwise need mastectomy for local control. Survival after neoadjuvant chemotherapy is similar to that seen with postoperative adjuvant chemotherapy.
1. HER2-positive breast cancer—Dual targeting of HER2 with two monoclonal antibodies, trastuzumab and pertuzumab, showed positive results in two clinical trials in the neoadjuvant setting, the TRYPHAENA and the NEOSPHERE studies.
Based on these clinical trials, three regimens are FDA-approved in the HER2-positive neoadjuvant setting: docetaxel (T), cyclophosphamide (C), trastuzumab (H), and pertuzumab (P) (TCHP) for six cycles; 5-fluorouracil, epirubicin, cyclophosphamide (FEC) for 3 cycles followed by THP for 3 cycles; or THP for 4 cycles (followed by three cycles of postoperative FEC). After completing surgery, patients should resume HER2-targeted therapy (trastuzumab emtansine if there is residual disease at the time of surgery, trastuzumab with or without pertuzumab if pathologic complete response is achieved with consideration for the use of neratinib as extended adjuvant therapy for high-risk disease).
2. Hormone receptor–positive, HER2-negative breast cancer—Patients with hormone receptor–positive breast cancer have a lower chance of achieving a pathologic complete response with neoadjuvant therapy than those patients with triple-negative or HER2-positive breast cancers. Studies indicate similar response rates with neoadjuvant endocrine therapy compared to neoadjuvant chemotherapy. Typically, responses are not appreciated unless 4–6 or more months of therapy are given. Outside of the clinical trial setting, the use of neoadjuvant hormonal therapy is generally restricted to postmenopausal patients who are unable or unwilling to tolerate chemotherapy.
3. Triple-negative breast cancer—No targeted therapy has yet demonstrated meaningful improvements in long-term outcomes for patients with curable breast cancer that is lacking in HER2 amplification or hormone receptor expression. Neoadjuvant chemotherapy leads to pathologic complete response in up to 40–50% of patients with triple-negative breast cancer. Patients who achieve a pathologic complete response seem to have a similar prognosis to other breast cancer subtypes with pathologic complete response. However, those patients with residual disease at the time of surgery have a poor prognosis. Based on the theory that triple-negative breast cancers may be more vulnerable to DNA-damaging agents, several studies have evaluated whether the addition of platinum salts to a neoadjuvant chemotherapy regimen is beneficial in this disease subtype, several of which have shown improved outcomes (pathologic complete response and disease-free survival) with platinum use. Other studies are ongoing to evaluate the pathologic complete response rates and long-term outcomes associated with incorporating platinums into standard chemotherapy regimens. One randomized phase III study, “CREATE-X,” indicated that the use of eight cycles of adjuvant capecitabine improved disease-free survival and overall survival in patients with residual disease after standard neoadjuvant therapy. This benefit was primarily observed in those with triple-negative breast cancer. As such, it has become a standard option available to patients who do not experience a complete response after neoadjuvant therapy.
4. Timing of sentinel lymph node biopsy in neoadjuvant setting—There is considerable concern about the timing of sentinel lymph node biopsy, since the chemotherapy may affect cancer present in the lymph nodes. Several studies have shown that sentinel node biopsy can be done after neoadjuvant therapy. However, a large multicenter study, ACOSOG 1071, demonstrated a false-negative rate of 10.7%, well above the false-negative rate outside the neoadjuvant setting (less than 1–5%). If three nodes are removed and isotope and dye are used, the false-negative rate falls to an acceptable level. Some physicians recommend performing sentinel lymph node biopsy before administering the chemotherapy in order to avoid a false-negative result and to aid in planning subsequent radiation therapy. Others prefer to perform sentinel lymph node biopsy after neoadjuvant therapy to avoid a second operation and assess post-chemotherapy nodal status. If a complete dissection is desired, this can be performed at the time of the definitive breast surgery. The SENTINA trial showed similarly poor results for sentinel node biopsy after neoadjuvant therapy. An effective approach for sentinel node biopsy for patients who had involved nodes pre-chemotherapy is to place a clip in the positive node and be sure to excise it at the time of sentinel node biopsy. No study has evaluated the survival impact of no axillary treatment for node-positive patients who become node-negative after neoadjuvant therapy. Important questions remain to be answered involving the use of neoadjuvant therapy, including which neoadjuvant agents to use, duration of treatment, and additional postoperative therapy.
 Palliative Treatment
Palliative Treatment
Palliative treatments are aimed to manage symptoms, improve quality of life, and even prolong survival, without the expectation of achieving cure. Even with cure of the disease not expected, palliative treatments are appropriate for breast cancer metastatic to distant sites. In the United States, it is uncommon to have distant metastases at the time of diagnosis (de novo metastases). However, most patients who have a breast cancer recurrence after initial local and adjuvant therapy have metastatic rather than local (in breast) disease. Breast cancer most commonly metastasizes to the liver, lungs, and bone, causing symptoms such as fatigue, change in appetite, abdominal pain, cough, dyspnea, or bone pain. Headaches, imbalance, vision changes, vertigo, and other neurologic symptoms may be signs of brain metastases. Triple-negative (ER-, PR-, HER2-negative) and HER2-positive tumors have a higher rate of brain metastases than hormone receptor–positive, HER2-negative tumors.
A. Radiotherapy and Bisphosphonates
Palliative radiotherapy may be advised for primary treatment of locally advanced cancers with distant metastases to control ulceration, pain, and other manifestations in the breast and regional nodes. Irradiation of the breast and chest wall and the axillary, internal mammary, and supraclavicular nodes should be undertaken in an attempt to cure locally advanced and inoperable lesions when there is no evidence of distant metastases. A small number of patients in this group are cured in spite of extensive breast and regional node involvement.
Palliative irradiation is of value also in the treatment of certain bone or soft-tissue metastases to control pain or avoid fracture. Radiotherapy is especially useful in the treatment of isolated bony metastases, chest wall recurrences, brain metastases and sometimes, in lieu of the preferred option of orthopedic surgery for acute spinal cord compression.
In addition to radiotherapy, bisphosphonate therapy has shown excellent results in delaying and reducing skeletal events in women with bony metastases. Pamidronate and zoledronic acid are FDA-approved intravenous bisphosphonates given for bone metastases or hypercalcemia of malignancy from breast cancer. Denosumab is FDA-approved for the treatment of bone metastases from breast cancer, with data showing that it reduced the time to first skeletal-related event (eg, pathologic fracture) compared to zoledronic acid.
Caution should be exercised when combining radiation therapy with chemotherapy because toxicity of either or both may be augmented by their concurrent administration. In general, only one type of therapy should be given at a time unless it is necessary to irradiate a destructive lesion of weight-bearing bone while the patient is receiving chemotherapy. Systemic therapy should be changed only if the disease is clearly progressing or if intolerable side effects have developed. This is especially difficult to determine for patients with destructive bone metastases, since changes in the status of these lesions are difficult to determine radiographically.
B. Targeted Therapy
1. Hormonally based therapy for metastatic disease—The following therapies have all been shown to be effective in hormone receptor–positive metastatic breast cancer: administration of medications that block or downregulate estrogen receptors (such as tamoxifen or fulvestrant, respectively) or medications that block the synthesis of hormones (such as AIs); ablation of the ovaries, adrenals, or pituitary; and the administration of hormones (eg, estrogens, androgens, progestins); see Table 17–5. Because only 5–10% of women with ER-negative tumors respond, they should not receive endocrine therapy. Women within 1 year of their last menstrual period are arbitrarily considered to be premenopausal and should have surgical (bilateral oophorectomy) or chemical ovarian ablation (using a gonadotropin-releasing hormone [GnRH] analog such as leuprolide [Lupron], goserelin [Zoladex], or triptorelin). Premenopausal women who have had chemical or surgical ovarian ablation are then eligible to receive the same hormonally targeted therapies that are available to postmenopausal women. Current guidelines indicate that sequential hormonal therapy is the preferred treatment for hormone receptor–positive metastatic breast cancer except in the rare case when disease is immediately threatening visceral organs.
Table 17–5. Hormonal agents commonly used for management of metastatic breast cancer (listed in alphabetical order).

a. First-line treatment options
(1) Hormonally targeted agents—Single-agent hormonally targeted therapy options include fulvestrant (500 mg intramuscularly days 1 and 15, then every month), tamoxifen (20 mg orally daily), or an AI (anastrozole, letrozole, or exemestane). The average time to progression associated with single agent first-line tamoxifen is 5–8 months and with AI is approximately 8–12 months. The side effect profile of AIs differs from tamoxifen. The main side effects of tamoxifen are nausea, skin rash, and hot flushes. Rarely, tamoxifen induces hypercalcemia in patients with bony metastases. Tamoxifen also increases the risk of venous thromboembolic events and uterine hyperplasia and cancer. The main side effects of AIs include hot flushes, vaginal dryness, and joint stiffness; however, osteoporosis and bone fractures are significantly higher than with tamoxifen. Results from the phase III FALCON study (comparing first-line treatment with the pure estrogen antagonist fulvestrant to anastrozole) confirmed that the use of first-line fulvestrant improves progression-free survival by almost 3 months (HR 0.79, 95% CI 0.637, 0.999, P = 0.0486) with the largest treatment effect observed in patients without visceral disease. Overall survival data are needed.
(2) Hormonally targeted therapy plus cyclin dependent kinase inhibition—Hormonally driven breast cancer may be particularly sensitive to inhibition of cell cycle regulatory proteins, called cyclin dependent kinases (CDK). A phase III randomized, placebo-controlled study (PALOMA-2) of letrozole plus an oral CDK 4/6 inhibitor (palbociclib) for the first-line treatment of postmenopausal women with hormone receptor–positive advanced breast cancer demonstrated a striking and highly significant 10-month improvement in progression-free survival associated with the use of palbociclib. Two additional CDK4/6 inhibitors, ribociclib and abemaciclib, were also evaluated in placebo-controlled phase III trials (MONALEESA-2 and MONARCH-3, respectively) in the same disease setting and demonstrated a similar improvement in progression-free survival when added to an AI. These three studies all demonstrated a median progression-free survival of over 2 years; the longest median progression-free survival reported in metastatic ER-positive breast cancer to date. Similar progression-free survival benefits were achieved with ribociclib in younger women in the phase III randomized trial (MONALEESA-7) that exclusively enrolled premenopausal women (treated with goserelin to suppress ovarian function in combination with endocrine therapy). This study was the first to report a significant improvement in overall survival with the addition of a CDK4/6 inhibitor to endocrine therapy. At 42 months, 70.2% of patients in the ribociclib arm were alive compared with 46.0% of patients in the placebo arm (HR 0.71%; 95% CI, 0.54, 0.95; P = 0.00973). Collectively, these results provide support for the use of a CDK4/6 inhibitor plus AI as the gold standard treatment in the first-line setting. Importantly, these therapies yield objective response rates as good as or better than that seen with chemotherapy. All three agents are FDA-approved in the first-line setting in combination with an AI. In general, CDK4/6 inhibitors are well tolerated, though monitoring patients for grade 3/4 neutropenia (especially with ribociclib and palbociclib) and management of diarrhea (especially with abemaciclib) are necessary. Febrile neutropenia and infections are rare and use of growth factors is not required; however, palbociclib and ribociclib are given for 3 consecutive weeks, stopping for 1 week to allow white cell count to recover. Abemaciclib is given twice daily continuously (28-day cycles).
b. Treatment options when disease progresses after hormonal-based therapy
(1) Secondary or tertiary hormonal therapy—Patients who have disease progression following first-line hormonal therapy may be offered a different form of endocrine therapy. For example, if a patient has been treated with an AI as first-line therapy, fulvestrant or tamoxifen should be considered at the time of disease progression as second-line therapy.
(2) Fulvestrant plus CDK4/6 inhibitor—All three CDK4/6 inhibitors have also been evaluated in phase III trials (PALOMA-3, MONALEESA-3, MONARCH-2) in patients whose disease has progressed on prior endocrine therapy and all have shown a significant improvement in median progression-free survival with the addition of CDK4/6 inhibitor to fulvestrant compared to placebo plus fulvestrant. Palbociclib (125 mg orally daily), ribociclib (600 mg orally daily), and abemaciclib (150 mg orally twice daily) are all FDA approved in combination with fulvestrant for this indication. In 2019, overall survival was shown to be significantly improved with the addition of ribociclib (MONALEESA-3) or abemaciclib (MONARCH-2) to fulvestrant, thus underscoring the importance of these agents for this disease. Abemaciclib is also FDA-approved as a single agent (200 mg orally twice daily) for patients with advanced ER-positive breast cancer who have received prior endocrine therapy and chemotherapy. It should be noted that there is no evidence to date that use of a CDK4/6 inhibitor benefits patients whose disease has progressed after therapy with a CDK4/6 inhibitor. Thus, at this time, use of any CDK4/6 inhibitor after disease progression on a CDK4/6 inhibitor is not appropriate outside of a clinical trial.
(3) Everolimus plus endocrine therapy—Everolimus (Afinitor) is an oral inhibitor of the mammalian target of rapamycin (MTOR)—a protein whose activation has been associated with the development of endocrine resistance. A phase III, placebo-controlled trial (BOLERO-2) evaluated the AI exemestane with or without everolimus in 724 patients with AI-resistant, hormone receptor–positive metastatic breast cancer and found that patients treated with everolimus had a significantly improved progression-free survival (7.8 months vs 3.2 months; HR, 0.45; 95% CI, 0.38–0.54; P < 0.0001) but no significant difference in overall survival. Everolimus has also been evaluated in combination with fulvestrant and shown to have similar improvements in progression-free survival compared to single agent fulvestrant. The main side effect of everolimus is stomatitis (mouth sores). This can be avoided, almost completely, by the prophylactic use of oral steroid mouthwash starting with cycle 1.
(4) Phosphatidylinositol-3-kinase (PI3K) inhibitors plus endocrine therapy—Approximately 40% of hormone receptor–positive breast cancers have activation of the PI3K-AKT-mTOR pathway, most commonly due to an activating mutation of PI3K on the PIK3CA gene. Alpelisib is an oral alpha-isoform selective inhibitor of PI3K with clinical activity in PIK3CA-mutated breast cancer. It was evaluated in a randomized, placebo-controlled, phase III clinical trial (SOLAR-1) in combination with fulvestrant in patients with hormone receptor–positive breast cancer who had been previously treated with an AI and demonstrated over a 5-month improvement in median progression-free survival (11.0 months vs 5.7 months) in patients with tumor PIK3CA mutations. No benefit was observed with alpelisib in wild-type tumors. These data led to the FDA approval of alpelisib for PIK3CA-mutated hormone receptor–positive breast cancer in 2019. Side effects of alpelisib include hyperglycemia, diarrhea, rash, and transaminitis.
2. HER2-targeted agents—For patients with HER2-positive tumors, trastuzumab plus chemotherapy significantly improves clinical outcomes, including survival compared to chemotherapy alone. Pertuzumab is an FDA-approved monoclonal antibody that targets the extracellular domain of HER2 at a different epitope than targeted by trastuzumab and inhibits receptor dimerization. A phase III placebo-controlled randomized study (CLEOPATRA) showed that patients treated with the combination of pertuzumab, trastuzumab, and docetaxel had a significantly longer progression-free survival (18.5 months vs 12.4 months; HR, 0.62; 95% CI, 0.51–0.75; P < 0.001) compared with those treated with docetaxel and trastuzumab. Longer follow-up revealed a significant overall survival benefit associated with pertuzumab as well.
Lapatinib, an oral targeted medication that inhibits the intracellular tyrosine kinases of the epidermal growth factor and HER2 receptors, is FDA-approved for the treatment of trastuzumab-resistant HER2-positive metastatic breast cancer in combination with capecitabine, thus, a completely oral regimen. The combination of trastuzumab plus lapatinib has been shown to be more effective than lapatinib alone for trastuzumab-resistant metastatic breast cancer. Moreover, several trials have shown a significant clinical benefit for continuing HER2-targeted agents beyond progression. T-DM1 ado-trastuzumab emtansine (Kadcyla) is an FDA-approved novel antibody-drug conjugate in which trastuzumab is stably linked to a derivative of maytansine, enabling targeted delivery of the cytotoxic chemotherapy to HER2-overexpressing cells. T-DM1 is associated with an improved progression-free and overall survival compared to lapatinib plus capecitabine in patients with HER2-positive, trastuzumab-pretreated advanced disease (EMILIA). Another antibody drug conjugate, trastuzumab deruxtecan (DS8201, TDxd) in which a HER2-targeted antibody is linked to a novel toxic payload (topoisomerase-I inhibitor) has demonstrated striking activity in heavily pretreated HER2-positive metastatic breast cancer. In a nonrandomized phase II trial (“DESTINY-BREAST”), the objective response rate was 60.9% and median progression-free survival was 16.4 months. The FDA provided accelerated approval for this medication in 2019. A novel HER2-selective oral tyrosine kinase inhibitor, tucatinib, has also been evaluated for HER2-positive metastatic breast cancer. This medication is of particular interest given its ability to penetrate the blood-brain barrier, potentially improving outcomes for those with brain metastases. A phase III trial (“HER2CLIMB”) compared capecitabine plus trastuzumab plus either tucatinib or placebo in patients with pretreated, HER2-positive advanced disease and demonstrated an improved progression-free survival in the overall population, improved progression-free survival in those with central nervous system metastases and, importantly, a significantly improved overall survival. These data are expected to lead to the FDA approval of this agent in 2020. Several other medications targeting HER2 and its associated signaling pathways are in development, including neratinib, margetuximab, pyrotinib, and HER2-targeted vaccines.
3. Targeting “triple-negative” breast cancer—Breast cancers lacking expression of the hormone receptors ER, PR, and HER2 behave more aggressively and have traditionally been amenable only to therapy with cytotoxic chemotherapy. However, in 2019, the FDA approved a novel therapy (atezolizumab) for metastatic triple-negative breast cancer that is positive for PD-L1 expression (based on the SP-142 antibody test of tumor immune cells). This approval was based on results from a phase III randomized trial (IMPASSION 130) that demonstrated the anti-PD-L1 antibody atezolizumab significantly improved the median progression-free survival in the intent to treat population (7.2 months vs 5.5 months) and in the population with PD-L1-positive tumors (7.5 months vs 5.0 months) when added to nab-paclitaxel. While median overall survival was not significantly improved in the intent to treat population, there was almost a 10-month improvement in overall survival in patients with PD-L1-positive tumors (25.0 months vs 15.5 months). These are the first results to demonstrate effectiveness of immune modulation therapy in breast cancer and have been practice changing. Another therapy garnering attention for triple-negative disease is sacituzumab govitecan, an antibody-drug conjugate that delivers SN-38 (active metabolite of the chemotherapy irinotecan) to Trop-2 overexpressing breast cancer cells. This antibody-drug conjugate has demonstrated significant clinical activity in heavily pretreated triple-negative breast cancer, leading to its priority review designation by the FDA. An ongoing phase III trial, ASCENT, is underway. A phase II placebo-controlled randomized trial (LOTUS) demonstrated a significantly improved progression-free survival (6.2 months vs 4.9 months, stratified HR 0.60, P = 0.037) by adding the oral AKT inhibitor ipatasertib to first-line paclitaxel. These results need to be confirmed in a larger phase III study but are the first promising results of an AKT inhibitor for triple-negative breast cancer.
4. Targeting PARP in BRCA1/2 mutation-associated breast cancer—Poly (adenosine diphosphate-ribose) polymerase (PARP) is an enzyme important in single-strand DNA repair. Patients who carry germline mutations in BRCA1 or BRCA2 have tumors with deficient double-strand DNA repair mechanisms. Experts have theorized that inhibiting PARP selectively kills BRCA1/2- mutated cancers. A phase III clinical trial (OlympiAD) that compared olaparib (an oral PARP inhibitor) to treatment of physician’s choice (single-agent chemotherapy) demonstrated a significantly improved progression-free survival (7.0 months vs 4.2 months, HR 0.58; P < 0.001), an improved response rate, and a lower rate of adverse events than standard therapy. Talazoparib, a second PARP inhibitor, has also been shown to improve outcomes similarly in the phase III EMBRACA study. Both olaparib and talazoparib are FDA-approved for BRCA-mutated metastatic breast cancer as single agents.
C. Palliative Chemotherapy
Cytotoxic medications should be considered for the treatment of metastatic breast cancer (1) if life- or organ-threatening visceral metastases are present (especially brain, liver, or lymphangitic pulmonary), (2) if hormonal treatment is unsuccessful or the disease has progressed after an initial response to hormonal manipulation (for hormone receptor–positive breast cancer), or (3) if the tumor is ER-negative or HER2-positive. Prior adjuvant chemotherapy does not seem to alter response rates in patients who relapse. A number of chemotherapy medications (including vinorelbine, paclitaxel, docetaxel, gemcitabine, ixabepilone, carboplatin, cisplatin, capecitabine, albumin-bound paclitaxel, eribulin, and liposomal doxorubicin) may be used as single agents with first-line objective response rates ranging from 30% to 50%.
Combination chemotherapy yields statistically significantly higher response rates and progression-free survival rates, but has not been conclusively shown to improve overall survival rates compared with sequential single-agent therapy. Combinations that have been tested in phase III studies and have proven efficacy compared with single-agent therapy include capecitabine/docetaxel, gemcitabine/paclitaxel, and capecitabine/ixabepilone (see Tables 39–3 and 39–13). Various other combinations of medications have been tested in phase II studies, and a number of clinical trials are ongoing to identify effective combinations. Patients should be encouraged to participate in clinical trials given the number of promising targeted therapies in development. It is generally appropriate to treat willing patients with multiple sequential lines of therapy as long as they tolerate the treatment and as long as their performance status is good (eg, at least ambulatory and able to care for self, up out of bed more than 50% of waking hours).
André F et al; SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019 May 16;380(20):1929–40. [PMID: 31091374]
Bardia A. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019 Feb 21;380(8):741–51. [PMID: 30786188]
Burstein HJ et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2019 Feb 10;37(5):423–38. [PMID: 30452337]
Cameron D et al; Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017 Mar 25;389(10075):1195–205. [PMID: 28215665]
Caudle AS et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016 Apr 1;34(10):1072–8. [PMID: 26811528]
Caudle AS et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of American Society of Breast Surgeons Members. Ann Surg Oncol. 2017 Oct;24(10):2925–34. [PMID: 28766207]
Coleman R et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020 Jan;21(1):60–72. [PMID: 31806543]
Dhesy-Thind S et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017 Jun 20;35(18):2062–81. [PMID: 28618241]
Diéras V et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017 Jun;18(6):732–42. [PMID: 28526536]
Earl H et al; PERSEPHONE Steering Committee and Trial Investigators. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019 Jun 29;393(10191):2599–2612. [PMID: 31178152]
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37,298 women with early breast cancer in 26 randomised trials. Lancet. 2019 Apr 6;393(10179):1440–52. [PMID: 30739743]
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018 Jan;19(1):27–39. [PMID: 29242041]
Ejlertsen B et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer: DBCG 07-READ, an open-label, phase III, randomized trial. J Clin Oncol. 2017 Aug 10;35(23):2639–46. [PMID: 28661759]
Finn RS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016 Nov 17;375(20):1925–36. [PMID: 27959613]
Francis PA et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018 Jul 12;379(2):122–37. [PMID: 29863451]
Gnant M et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019 Mar;20(3):339–51. [PMID: 30795951]
Goetz MP et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017 Nov 10;35(32):3638–46. [PMID: 28968163]
Im SA et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019 Jul 25;381(4):307–16. [PMID: 31166679]
Litton J et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018 Aug 23;379(8):753–63. [PMID: 30110579]
Martin M et al; ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 Dec;18(12):1688–1700. [PMID: 29146401]
Masuda N et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017 Jun 1;376(22):2147–59. [PMID: 28564564]
Modi S et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020 Feb 13;382(7):610–21. [PMID: 31825192]
Murthy RK et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020 Feb 13;382(7):597–609. [PMID: 31825569]
National Comprehensive Cancer Network. NCCN guidelines: breast cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
Nitz U et al. West German study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019 Apr 1;37(10):799–808. [PMID: 30785826]
Pivot X et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicenter, open-label, phase 3 randomised trial. Lancet. 2019 Jun 29;393(10191):2591–8. [PMID: 31178155]
Robson M et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017 Aug 10;377(6):523–33. Erratum in: N Engl J Med. 2017 Oct 26;377(17):1700. [PMID: 28578601]
Schmid P et al. Atezolizumab and nab-paclitaxel in advanced triple negative breast cancer. N Engl J Med. 2018 Nov 29;379(22):2108–21. [PMID: 30345906]
Slamon DJ et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020 Feb 6;382(6):514–24. [PMID: 31826360]
Sledge GW Jr et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017 Sep 1;35(25):2875–84. [PMID: 28580882]
Sledge GW Jr et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019 Sept 29. [Epub ahead of print] [PMID: 31563959]
Tripathy D et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor–positive advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 Jul;19(7):904–15. [PMID: 29804902]
von Minckwitz G et al; KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019 Feb 14;380(7):617–28. [PMID: 30516102]
 Prognosis
Prognosis
Stage of breast cancer is the most reliable indicator of prognosis (Table 17–6). Axillary lymph node status is the best-analyzed prognostic factor and correlates with survival at all tumor sizes. When cancer is localized to the breast with no evidence of regional spread after pathologic examination, the clinical cure rate with most accepted methods of therapy is 75% to more than 90%. In fact, patients with small mammographically detected biologically favorable tumors and no evidence of axillary spread have a 5-year survival rate greater than 95%. When the axillary lymph nodes are involved with tumor, the survival rate drops to 50–70% at 5 years and probably around 25–40% at 10 years. The use of biologic markers, such as ER, PR, grade, and HER2, helps identify high-risk tumor types as well as direct treatment used (see Biomarkers & Gene Expression Profiling). Gene analysis studies can predict disease-free survival for some subsets of patients. The eighth edition of the AJCC Staging Manual has incorporated these factors into staging.
Table 17–6. Approximate survival of patients with breast cancer by TNM stage.
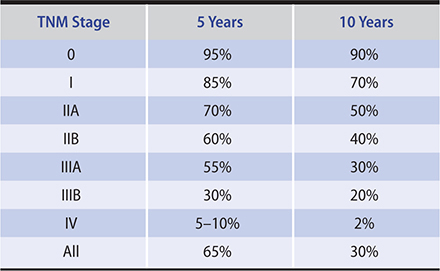
Five-year statistics do not accurately reflect the final outcome of therapy. The mortality rate of breast cancer patients exceeds that of age-matched normal controls for nearly 20 years. Thereafter, the mortality rates are equal, though deaths that occur among breast cancer patients are often directly the result of tumor.
In general, breast cancer appears to be somewhat more aggressive and associated with worse outcomes in younger than in older women, and this may be related to the fact that fewer younger women have ER-positive tumors. Disparities in treatment outcome for different racial and ethnic backgrounds have been reported by several studies. These differences appear to be not only due to different socioeconomic factors (and a resulting difference in access to healthcare) but also due to differences in the subtype of breast cancer diagnosed.
For those patients whose disease progresses despite treatment, some studies suggest supportive group therapy may improve survival. Especially as they approach the end of life, such patients will require meticulous palliative care (see Chapter 5).
DeSantis CE et al. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019 May;69(3):211–33. [PMID: 30762872]
Giuliano AE et al. Eighth edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. 2018 Jul;25(7):1783–5. [PMID: 29671136]
Jemal A et al. Factors that contributed to black-white disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018 Jan 1;36(1):14–24. [PMID: 29035645]
Miller KD et al. Cancer statistics for hispanics/latinos, 2018. CA Cancer J Clin. 2018 Nov;68(6):425–45. [PMID: 30285281]
Pan H et al; EBCTCG. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017 Nov 9;377(19):1836–46. [PMID: 29117498]
 Follow-Up Care
Follow-Up Care
After primary therapy, patients with breast cancer should be monitored long term in order to detect recurrences and to observe the opposite breast for a second primary carcinoma. Local and distant recurrences occur most frequently within the first 2–5 years. During the first 2 years, most patients should be examined every 6 months, then annually thereafter. Special attention is paid to the contralateral breast because a new primary breast malignancy will develop in 20–25% of patients. In some cases, especially in hormone receptor–positive breast cancer, metastases are dormant for long periods and may appear 20 years or longer after removal of the primary tumor. Although studies have failed to show an adverse effect of hormonal replacement in disease-free patients, it is rarely used after breast cancer treatment, particularly if the tumor was hormone receptor–positive. Even pregnancy has not been associated with shortened survival of patients rendered disease free—yet many oncologists are reluctant to advise a young patient with breast cancer that it is safe to become pregnant. The use of estrogen replacement for conditions such as osteoporosis, vaginal dryness, and hot flushes may be considered for a woman with a history of breast cancer after discussion of the benefits and risks; however, it is not routinely recommended, especially given the availability of nonhormonal agents for these conditions (such as bisphosphonates and denosumab for osteoporosis).
A. Local Recurrence
The incidence of local recurrence correlates with tumor size, the presence and number of involved axillary nodes, the histologic type of tumor, the presence of skin edema or skin and fascia fixation with the primary tumor, and the type of definitive surgery and local irradiation. Local recurrence on the chest wall after total mastectomy and axillary dissection develops in as many as 8% of patients. When the axillary nodes are not involved, the local recurrence rate is less than 5%, but the rate is as high as 25% when they are heavily involved. A similar difference in local recurrence rate was noted between small and large tumors. Factors such as multifocal cancer, in situ tumors, lymphovascular invasion, positive resection margins, chemotherapy, and radiotherapy have an effect on local recurrence in patients treated with breast-conserving surgery. Adjuvant systemic therapy greatly decreases the rate of local recurrence.
Chest wall recurrences usually appear within the first several years but may occur as late as 15 or more years after mastectomy. All suspicious nodules and skin lesions should be biopsied. Local excision or localized radiotherapy may be feasible if an isolated nodule is present. If lesions are multiple or accompanied by evidence of regional involvement in the internal mammary or supraclavicular nodes, the disease is best managed by radiation treatment of the entire chest wall including the parasternal, supraclavicular, and axillary areas as well as systemic therapy.
Local recurrence after mastectomy usually signals the presence of widespread disease and is an indication for studies to search for metastases. Distant metastases will develop within a few years in most patients with locally recurrent tumor after mastectomy. When there is no evidence of metastases beyond the chest wall and regional nodes, irradiation for cure after complete local excision should be attempted. After partial mastectomy, local recurrence does not have as serious a prognostic significance as after mastectomy. However, those patients in whom a recurrence develops have a worse prognosis than those who do not. It is speculated that the ability of a cancer to recur locally after radiotherapy is a sign of aggressiveness and resistance to therapy. Completion of the mastectomy should be done for local recurrence after partial mastectomy; some of these patients will survive for prolonged periods, especially if the breast recurrence is DCIS or occurs more than 5 years after initial treatment. Systemic chemotherapy or hormonal treatment should be used for women in whom disseminated disease develops or those in whom local recurrence occurs.
B. Breast Cancer Survivorship Issues
Given that most women with non-metastatic breast cancer will be cured, a significant number of women face survivorship issues stemming from either the diagnosis or the treatment of the breast cancer, or both. These challenges include psychological struggles, cognitive dysfunction (also called “chemo brain”), upper extremity lymphedema, weight management problems, cardiovascular issues, bone loss, postmenopausal side effects, and fatigue. One randomized study reported that survivors who received psychological intervention from the time of diagnosis had a lower risk of recurrence and breast cancer–related mortality. A randomized study in older, overweight cancer survivors showed that diet and exercise reduced the rate of self-reported functional decline compared with no intervention.
1. Edema of the arm—Significant edema of the arm occurs in about 10–30% of patients after axillary dissection with or without mastectomy. It occurs more commonly in obese women, in women who had radiotherapy, and in women who had postoperative infection. Partial mastectomy with radiation to the axillary lymph nodes is followed by chronic edema of the arm in 10–20% of patients. Sentinel lymph node dissection has proved to be an accurate form of axillary staging without the side effects of edema or infection. Judicious use of radiotherapy, with treatment fields carefully planned to spare the axilla as much as possible, can greatly diminish the incidence of edema, which will occur in only 5% of patients if no radiotherapy is given to the axilla after a partial mastectomy and lymph node dissection.
Late or secondary edema of the arm may develop years after treatment, as a result of axillary recurrence or infection in the hand or arm, with obliteration of lymphatic channels. When edema develops, a careful examination of the axilla for recurrence or infection is performed. Infection in the arm or hand on the dissected side should be treated with antibiotics, rest, and elevation. If there is no sign of recurrence or infection, the swollen extremity should be treated with rest and elevation. A mild diuretic may be helpful. If there is no improvement, a compressor pump or manual compression decreases the swelling, and the patient is then fitted with an elastic glove or sleeve. Most patients are not bothered enough by mild edema to wear an uncomfortable glove or sleeve and will treat themselves with elevation or manual compression alone. Rarely, edema may be severe enough to interfere with use of the limb. A prospective randomized study has shown that twice weekly progressive weight lifting improves lymphedema symptoms and exacerbations and improves extremity strength.
2. Breast reconstruction—Breast reconstruction is usually feasible after total or modified radical mastectomy. Reconstruction should be discussed with patients prior to mastectomy, because it offers an important psychological focal point for recovery. Reconstruction is not an obstacle to the diagnosis of recurrent cancer. The most common breast reconstruction has been implantation of a silicone gel or saline prosthesis in the subpectoral plane between the pectoralis minor and pectoralis major muscles. Alternatively, autologous tissue can be used for reconstruction.
Autologous tissue flaps have the advantage of not feeling like a foreign body to the patient. The most popular autologous technique currently is reconstruction using abdominal tissue flaps. This includes the deep inferior epigastric perforator (DIEP) flap and the more traditional transrectus abdominis muscle (TRAM) flap. A latissimus dorsi flap can be swung from the back but offers less volume than the TRAM flap and thus often requires supplementation with an implant. Reconstruction may be performed immediately (at the time of initial mastectomy) or may be delayed until later, usually when the patient has completed adjuvant therapy. When considering reconstructive options, concomitant illnesses should be considered, since the ability of an autologous flap to survive depends on medical comorbidities. In addition, the need for radiotherapy may affect the choice of reconstruction as radiation may increase fibrosis around an implant or decrease the volume of a flap.
3. Risks of pregnancy—Clinicians are often asked to advise patients regarding the potential risk of future pregnancy after definitive treatment for early-stage breast cancer. To date, no adverse effect of pregnancy on survival of women who have had breast cancer has been demonstrated. When counseling patients, oncologists must take into consideration the patients’ overall prognosis, age, comorbidities, and life goals.
In patients with inoperable or metastatic cancer (stage IV disease), induced abortion may be advisable because of the possible adverse effects of hormonal treatment, radiotherapy, or chemotherapy upon the fetus in addition to the expectant mother’s poor prognosis.
Asdourian MS et al. Association between precautionary behaviors and breast cancer-related lymphedema in patients undergoing bilateral surgery. J Clin Oncol. 2017 Dec 10;35(35):3934–41. [PMID: 28976793]
Carroll JE et al. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer. 2019 Jan 15;125(2):298–306. [PMID: 30474160]
Dieli-Conwright CM et al. Hispanic ethnicity as a moderator of the effects of aerobic and resistance exercise in survivors of breast cancer. Cancer. 2019 Mar 15;125(6):910–20. [PMID: 30500981]
Gerstl B et al. Pregnancy outcomes after a breast cancer diagnosis: a systematic review and meta-analysis. Clin Breast Cancer. 2018 Feb;18(1):e79–88. [PMID: 28797766]
Hummel SB et al. Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2017 Apr 20;35(12):1328–40. [PMID: 28240966]
Janelsins MC et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017 Feb 10;35(5):506–14. [PMID: 28029304]
Lambertini M et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018 Apr 1;110(4):426–9. [PMID: 29087485]
Mandelblatt JS et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol. 2018 Nov 10;36(32):3211–22. [PMID: 30281396]
Van Dyk K et al. The cognitive effects of endocrine therapy in survivors of breast cancer: a prospective longitudinal study up to 6 years after treatment. Cancer. 2019 Mar 1;125(5):681–9. [PMID: 30485399]
CARCINOMA OF THE MALE BREAST
ESSENTIALS OF DIAGNOSIS

 A painless lump beneath the areola in a man usually over 50 years of age.
A painless lump beneath the areola in a man usually over 50 years of age.
 Nipple discharge, retraction, or ulceration may be present.
Nipple discharge, retraction, or ulceration may be present.
 Generally poorer prognosis than in women.
Generally poorer prognosis than in women.
 General Considerations
General Considerations
Breast cancer in men is a rare disease; the incidence is only about 1% of all breast cancer diagnoses. The average age at occurrence is about 70 years, and there may be an increased incidence of breast cancer in men with prostate cancer. As in women, hormonal influences are probably related to the development of male breast cancer. There is a high incidence of both breast cancer and gynecomastia in Bantu men, theoretically owing to failure of estrogen inactivation by associated liver disease. It is important to note that first-degree relatives of men with breast cancer are considered to be at high risk. This risk should be taken into account when discussing options with the patient and family. In addition, BRCA2 mutations are common in men with breast cancer. Men with breast cancer, especially with a history of prostate cancer, should receive genetic counseling.
 Clinical Findings
Clinical Findings
A painless lump, occasionally associated with nipple discharge, retraction, erosion, or ulceration, is the primary complaint. Examination usually shows a hard, ill-defined, nontender mass beneath the nipple or areola. Gynecomastia not uncommonly precedes or accompanies breast cancer in men. Nipple discharge is an uncommon presentation for breast cancer in men but is an ominous finding associated with carcinoma in nearly 75% of cases.
Breast cancer staging is the same in men as in women. Gynecomastia and metastatic cancer from another site (eg, prostate) must be considered in the differential diagnosis. Benign tumors are rare, and biopsy should be performed on all males with a defined breast mass.
 Treatment
Treatment
Treatment consists of modified radical mastectomy in operable patients, who should be chosen by the same criteria as women with the disease. Breast conserving therapy is rarely performed. Irradiation is the first step in treating localized metastases in the skin, lymph nodes, or skeleton that are causing symptoms. Examination of the cancer for hormone receptors and HER2 overexpression is of value in determining adjuvant therapy. Over 95% of men have ER-positive tumors and less than 10% have overexpression of HER2. Androgen receptor is also commonly overexpressed in male breast cancer. Adjuvant systemic therapy and radiation are used for the same indications as in breast cancer in women.
Because breast cancer in men is frequently hormone receptor positive, diagnosed late, and is a disseminated disease, endocrine therapy is of considerable importance in its management. Tamoxifen is the main medication for management of advanced breast cancer in men. Tamoxifen (20 mg orally daily) should be the initial treatment. There is little data regarding the use of AIs in men, but they are being used more frequently. Castration in advanced breast cancer is a successful measure and more beneficial than the same procedure in women but is rarely used. Objective evidence of regression may be seen in 60–70% of men with endocrine therapy for metastatic disease—approximately twice the proportion in women. Bone is the most frequent site of metastases from breast cancer in men (as in women), and endocrine therapy relieves bone pain in most patients so treated. The longer the interval between mastectomy and recurrence, the longer the remission following treatment is likely.
Chemotherapy should be administered for the same indications and using the same dosage schedules as for women with metastatic disease or for adjuvant treatment.
 Prognosis
Prognosis
A large population-based, international breast cancer study reported that after adjustment for prognostic features (age, stage, treatment), men have a similar relative survival stage for stage compared to women. For node-positive disease, 5-year survival is approximately 69%, and for node-negative disease, it is about 88%.
For those patients whose disease progresses despite treatment, meticulous efforts at palliative care are essential (see Chapter 5).
Giordano SH. Breast cancer in men. N Engl J Med. 2018 Jun 14;378(24):2311–20. [PMID: 29897847]