23
Urologic Disorders
Mathew Sorensen, MD, MS, FACS
Thomas J. Walsh, MD, MS
Kevin A. Ostrowski, MD
HEMATURIA
ESSENTIALS OF DIAGNOSIS

 Both gross and microscopic hematuria require evaluation.
Both gross and microscopic hematuria require evaluation.
 The upper urinary tract should be imaged, and cystoscopy should be performed if there is hematuria in the absence of an identifiable benign cause.
The upper urinary tract should be imaged, and cystoscopy should be performed if there is hematuria in the absence of an identifiable benign cause.
 General Considerations
General Considerations
An upper tract source (kidneys and ureters) can be identified in 10% of patients with gross or microscopic hematuria. For upper tract sources, stone disease accounts for 40%, medical kidney disease (medullary sponge kidney, glomerulonephritis, papillary necrosis) for 20%, renal cell carcinoma for 10%, and urothelial cell carcinoma of the ureter or renal pelvis for 5%. Medication ingestion and associated medical problems may provide diagnostic clues. Analgesic use (papillary necrosis), cyclophosphamide (chemical cystitis), antibiotics (interstitial nephritis), diabetes mellitus, sickle cell trait or disease (papillary necrosis), a history of stone disease, or malignancy should all be investigated. The lower tract source of gross hematuria (in the absence of infection) is most commonly from bleeding prostatic varices or urothelial carcinoma of the bladder. Microscopic hematuria in the male is most commonly from benign prostatic hyperplasia (13%), kidney stones (6%), or urethral stricture (1.4%). The presence of hematuria in patients receiving antiplatelet or anticoagulation therapy cannot be presumed to be due to the medication; a complete evaluation is warranted consisting of upper tract imaging, cystoscopy, and urine cytology (see Chapter 39 for Bladder Cancer, Cancers of the Ureter & Renal Pelvis, Renal Cell Carcinoma, and Other Primary Tumors of the Kidney).
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
If gross hematuria occurs, a description of the timing (initial, terminal, total) may provide a clue to the localization of disease. Associated symptoms (ie, renal colic, irritative voiding symptoms, or constitutional symptoms) should be investigated. Physical examination should look for signs of systemic disease (fever, rash, lymphadenopathy, abdominal or pelvic masses) as well as signs of medical kidney disease (hypertension, volume overload). Urologic evaluation may demonstrate an enlarged prostate, flank mass, or urethral disease.
B. Laboratory Findings
Initial laboratory investigations include a urinalysis and urine culture. Microhematuria is defined as three or more red blood cells per high-power field on a microscopic evaluation of the urine. A positive dipstick reading for heme merits microscopic examination to confirm or refute the diagnosis of hematuria but is not enough to warrant workup on its own. If urinalysis and culture is suggestive of a urinary tract infection, follow-up urinalysis after treatment of the infection is important to ensure resolution of the hematuria. An estimate of kidney function should be obtained since renal insufficiency may influence the methods of further evaluation and management (eg, ability to obtain contrast imaging) of patients with hematuria. Urine cytology and other urinary-based markers are not routinely recommended in the evaluation of asymptomatic microscopic hematuria.
C. Imaging
The upper tract should be imaged using a CT-intravenous pyelogram (CT-IVP), which is an abdominal and pelvic CT scan without contrast, with contrast, and with excretory delayed imaging. These phases are necessary to identify neoplasms of the kidney or ureter as well as benign conditions such as urolithiasis, obstructive uropathy, papillary necrosis, medullary sponge kidney, or polycystic kidney disease. For patients with relative or absolute contraindications that preclude a CT-IVP (such as kidney dysfunction, intravenous contrast allergy, or pregnancy), a magnetic resonance urogram without and with contrast can be performed. The role of ultrasonographic evaluation of the urinary tract for hematuria is unclear. Although it may provide adequate information for the kidney, its sensitivity in detecting ureteral disease is lower.
D. Cystoscopy
Cystoscopy is necessary to assess for bladder or urethral neoplasm, benign prostatic hyperplasia, and radiation or chemical cystitis; it is indicated for patients with gross hematuria and those over age 35 years with asymptomatic microscopic hematuria.
 Follow-Up
Follow-Up
In patients with negative hematuria evaluations, it is typically recommended that a urinalysis with microscopy be repeated at 6–12 months. If two consecutive annual urinalyses are negative for hematuria (one per year for 2 years), then no further urinalyses are required. If microscopic hematuria persists on annual urinalyses, then repeat evaluation with cystoscopy and upper tract imaging should be considered within 3–5 years. Recurrent gross hematuria may require repeat evaluation with cystoscopy and upper tract imaging 1 year after initial negative evaluation. Urinary cytology may be obtained by the urologist after initial negative evaluation or in persons with risk factors (irritative voiding symptoms, tobacco use, chemical exposures).
 When to Refer
When to Refer
In the absence of a clear benign etiology (such as an infection, menstruation, vigorous exercise, acute stone event, medical renal disease, viral illness, trauma, or recent urologic procedure), hematuria (either gross or microscopic) requires evaluation.
Davis R et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012 Dec;188(6 Suppl):2473–81. [PMID: 23098784]
Georgieva MV et al. Comparison of the harms, advantages, and costs associated with alternative guidelines for the evaluation of hematuria. JAMA Intern Med. 2019;179(10):1352–62. [PMID: 31355874]
Halpern JA et al. Cost-effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Intern Med. 2017 Jun 1;177(6):800–7. [PMID: 28418451]
Sountoulides P et al. Non-visible asymptomatic haematuria: a review of the guidelines from the urologist’s perspective. Expert Rev Anticancer Ther. 2017 Mar;17(3):203–16. [PMID: 28116915]
GENITOURINARY TRACT INFECTIONS
Urinary tract infections are among the most common entities encountered in medical practice. In acute infections, a single pathogen is usually found, whereas two or more pathogens are often seen in chronic infections. Coliform bacteria are responsible for most non-nosocomial, uncomplicated urinary tract infections, with Escherichia coli being the most common. Such infections typically are sensitive to a wide variety of orally administered antibiotics and respond quickly. Nosocomial infections often are due to more resistant pathogens and may require parenteral antibiotics. Renal infections are of particular concern because if they are inadequately treated, loss of kidney function may result. A urine culture is recommended for patients with suspected urinary tract infection and ideally should be obtained prior to the initiation of antibiotic therapy. Previously, a colony count greater than 105/mL was considered the criterion for urinary tract infection, though up to 50% of women with symptomatic infections may have lower counts. In addition, the presence of pyuria correlates poorly with the diagnosis of urinary tract infection, and thus urinalysis alone is not adequate for diagnosis. With respect to treatment, tissue infections (pyelonephritis, prostatitis) require therapy for 1–2 weeks, while mucosal infections (cystitis) require only 1–3 days of therapy.
1. Acute Cystitis
ESSENTIALS OF DIAGNOSIS

 Irritative voiding symptoms.
Irritative voiding symptoms.
 Patient usually afebrile.
Patient usually afebrile.
 Positive urine culture; blood cultures may also be positive.
Positive urine culture; blood cultures may also be positive.
 General Considerations
General Considerations
Acute cystitis is an infection of the bladder, most commonly due to the coliform bacteria (especially E coli) and occasionally gram-positive bacteria (enterococci). The route of infection is typically ascending from the urethra. Viral cystitis due to adenovirus is sometimes seen in children but is rare in immunocompetent adults. Uncomplicated cystitis in men is rare and implies a pathologic process such as infected stones, prostatitis, or chronic urinary retention requiring further investigation.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Irritative voiding symptoms (frequency, urgency, dysuria) and suprapubic discomfort are common. Women may experience gross hematuria, and symptoms may often appear following sexual intercourse. Physical examination may elicit suprapubic tenderness, but examination is often unremarkable. Systemic toxicity is absent.
B. Laboratory Findings
Urinalysis shows pyuria, bacteriuria, and varying degrees of hematuria. The degree of pyuria and bacteriuria does not necessarily correlate with the severity of symptoms. Urine culture is positive for the offending organism, but colony counts exceeding 105/mL are not required for the diagnosis. Patients with asymptomatic bacteriuria or colonization are expected to have positive urine cultures but do not require treatment except in pregnant women. Patients with long-term urinary catheters (indwelling urinary [Foley] or suprapubic catheter) or urostomy urinary diversions are expected to be colonized with bacteria, and thus, urinalysis and urine culture are most helpful in directing therapy rather than determining whether symptomatic infection exists.
C. Imaging
Because uncomplicated cystitis is rare in men, elucidation of the underlying problem with appropriate investigations, such as abdominal ultrasonography, postvoid residual testing, and cystoscopy, is warranted. Follow-up imaging using CT scanning is warranted if pyelonephritis, recurrent infections, or anatomic abnormalities are suspected.
 Differential Diagnosis
Differential Diagnosis
In women, infectious processes such as vulvovaginitis and pelvic inflammatory disease can usually be distinguished by pelvic examination and urinalysis. In men, urethritis and prostatitis may be distinguished by physical examination (urethral discharge or prostatic tenderness).
Noninfectious causes of cystitis-like symptoms include pelvic irradiation, chemotherapy (cyclophosphamide), bladder carcinoma, interstitial cystitis, voiding dysfunction disorders, and psychosomatic disorders.
 Prevention
Prevention
The risk of developing a urinary tract infection can be reduced by drinking plenty of fluid and completely emptying the bladder frequently. Women in whom urinary tract infections tend to develop after intercourse should be advised to void before, and especially after intercourse, and may benefit from a postcoital single-dose of antibiotic. Postmenopausal women with recurrent urinary tract infections (three or more episodes per year) may benefit from a topical estrogen cream. Daily cranberry tablets may reduce the risk of cystitis, though the data are conflicting. Prophylactic antibiotics are generally discouraged. Prior to institution of antibiotic prophylaxis, a thorough urologic evaluation is warranted to exclude any anatomic abnormality (eg, stones, reflux, fistula). An initial course of 6 to 12 months of prophylactic antibiotics can be offered. The benefits of prophylactic antibiotics should be weighed against the risks of developing bacterial resistance.
The risk of acquiring a catheter-associated urinary tract infection in hospitalized patients can be minimized by using indwelling catheters only when necessary, implementing systems to ensure removal of catheters when no longer needed, using antimicrobial catheters in high-risk patients, using external collection devices in select men, identifying significant postvoid residuals by ultrasound, maintaining proper insertion techniques, and utilizing alternatives such as intermittent catheterization.
 Treatment
Treatment
Uncomplicated cystitis in women can be treated with short-term antimicrobial therapy, which consists of single-dose therapy or 1–7 days of therapy. Fosfomycin, nitrofurantoin, and trimethoprim-sulfamethoxazole are the medications of choice for uncomplicated cystitis (Table 23–1). The US Food and Drug Administration (FDA) advises restricting fluoroquinolone use for uncomplicated infections. Local patterns of bacterial resistance should be consulted to identify best treatment options. Some antibiotics may be ineffective because of the emergence of resistant organisms. A review of the literature proposed that acute uncomplicated cystitis in women can be diagnosed without office evaluation or urine culture, and that appropriate first-line therapies include trimethoprim-sulfamethoxazole (160/800 mg twice daily for 3 days), nitrofurantoin (100 mg twice daily for 5–7 days), or fosfomycin trometamol (3 g single dose). In men, uncomplicated urinary tract infection is rare; thus, the duration of antibiotic therapy depends on the underlying etiology. Hot sitz baths or urinary analgesics (phenazopyridine, 200 mg orally three times daily) may provide symptomatic relief. Postmenopausal women with recurrent cystitis can be treated with vaginal estrogen cream 0.5 g nightly for 2 weeks and then twice weekly thereafter.
Table 23–1. Empiric therapy for urinary tract infections.


 Prognosis
Prognosis
Infections typically respond rapidly to therapy, and failure to respond suggests resistance to the selected medication or anatomic abnormalities requiring further investigation.
 When to Refer
When to Refer
• Suspicion or radiographic evidence of anatomic abnormality.
• Evidence of urolithiasis.
• Recurrent cystitis due to bacterial persistence.
Bader MS et al. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017 Mar;129(2):242–58. [PMID: 27712137]
Datta R et al. Nitrofurantoin vs fosfomycin: rendering a verdict in a trial of acute uncomplicated cystitis. JAMA. 2018 May 1;319(17):1771–2. [PMID: 29710273]
Gill CM et al. A review of nonantibiotic agents to prevent urinary tract infections in older women. J Am Med Dir Assoc. 2020 Jan;21(1):46–54. [PMID: 31227473]
Huttner A et al. Effect of 5-day nitrofurantoin vs single-dose fosfomycin on clinical resolution of uncomplicated lower urinary tract infection in women: a randomized clinical trial. JAMA. 2018 May 1;319(17):1781–9. [PMID: 29710295]
Jin J. JAMA patient page. Screening for asymptomatic bacteriuria. JAMA. 2019 Sep 24;322(12):1222. [PMID: 31550033]
Nicolle LE et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019 May 2;68(10):1611–5. [PMID: 31506700]
Shuman EK et al. Urinary catheter-associated infections. Infect Dis Clin North Am. 2018 Dec;32(4):885–97. [PMID: 30241712]
US Food & Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. 2018 Mar 08. http://www.fda.gov/Drugs/DrugSafety/ucm511530.htm
US Food & Drug Administration. FDA News Release: FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. 2018 Jul 10. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse
2. Acute Pyelonephritis
ESSENTIALS OF DIAGNOSIS

 Fever.
Fever.
 Flank pain.
Flank pain.
 Irritative voiding symptoms.
Irritative voiding symptoms.
 Positive urine culture.
Positive urine culture.
 General Considerations
General Considerations
Acute pyelonephritis is an infectious inflammatory disease involving the kidney parenchyma and renal pelvis. Gram-negative bacteria are the most common causative agents including E coli, Proteus, Klebsiella, Enterobacter, and Pseudomonas. Gram-positive bacteria are less commonly seen but include Enterococcus faecalis and Staphylococcus aureus. The infection usually ascends from the lower urinary tract—with the exception of S aureus, which usually is spread by a hematogenous route.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Symptoms include fever, flank pain, shaking chills, and irritative voiding symptoms (urgency, frequency, dysuria). Associated nausea and vomiting and diarrhea are common. Signs include fever and tachycardia. Costovertebral angle tenderness is usually pronounced.
B. Laboratory Findings
Complete blood cell count shows leukocytosis and a left shift. Urinalysis shows pyuria, bacteriuria, and varying degrees of hematuria. White cell casts may be seen. Urine culture demonstrates growth of the offending organism, and blood culture may also be positive.
C. Imaging
In complicated pyelonephritis, renal ultrasound may show hydronephrosis from a stone or other source of obstruction. CT scan may demonstrate decreased perfusion of the kidney or focal areas within the kidney and nonspecific perinephric fat stranding.
 Differential Diagnosis
Differential Diagnosis
The differential diagnosis includes acute cystitis or a lower urinary source. Acute intra-abdominal disease such as appendicitis, cholecystitis, pancreatitis, or diverticulitis must be distinguished from pyelonephritis. A normal urinalysis is usually seen in gastrointestinal disorders; however, on occasion, inflammation from adjacent bowel (appendicitis or diverticulitis) may result in hematuria or sterile pyuria. Abnormal liver biochemical tests or elevated amylase levels may assist in the differentiation. Lower-lobe pneumonia is distinguishable by the abnormal chest radiograph.
In males, the main differential diagnosis for acute pyelonephritis also includes acute epididymitis and acute prostatitis. Physical examination and the location of the pain should permit this distinction.
 Complications
Complications
Sepsis with shock can occur with acute pyelonephritis. In diabetic patients, emphysematous pyelonephritis resulting from gas-producing organisms may be life-threatening if not adequately treated. Healthy adults usually recover complete kidney function, yet if coexistent kidney disease is present, scarring or chronic pyelonephritis may result. Inadequate therapy could result in abscess formation.
 Treatment
Treatment
Urine and blood cultures are obtained to identify the causative agent and to determine antimicrobial sensitivity. In the inpatient setting, intravenous ampicillin and an aminoglycoside are initiated prior to obtaining sensitivity results (Table 23–1). In the outpatient setting, empiric therapy may be initiated (Table 23–1). Antibiotics are adjusted according to sensitivities. If local antibiograms demonstrate local resistance rates for the oral regimen exceed 10%, an initial 24-hour intravenous dose of antibiotic is required. Fevers may persist for up to 72 hours even with appropriate antibiotics; failure to respond within 48 hours warrants imaging (CT or ultrasound) to exclude complicating factors that may require intervention (such as a perinephric abscess or an obstructing stone). Catheter drainage may be necessary in the face of urinary retention and nephrostomy drainage if there is ureteral obstruction. In inpatients, intravenous antibiotics are continued for 24 hours after the fever resolves, and oral antibiotics are then given to complete a 14-day course of therapy.
 Prognosis
Prognosis
With prompt diagnosis and appropriate treatment, acute pyelonephritis carries a good prognosis. Complicating factors, underlying kidney disease, and increasing patient age may lead to a less favorable outcome.
 When to Refer
When to Refer
• Evidence of complicating factors (urolithiasis, obstruction).
• Failure of clinical improvement in 48 hours.
 When to Admit
When to Admit
• Severe infections or complicating factors, evidence of sepsis, or need for parenteral antibiotics.
• Need for radiographic imaging or drainage of urinary tract obstruction.
Bader MS et al. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017 Mar;129(2):242–58. [PMID: 27712137]
Dawson-Hahn EE et al. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews. Fam Pract. 2017 Sept 1;34(5):511–9. [PMID: 28486675]
Johnson JR et al. Acute pyelonephritis in adults. N Engl J Med. 2018 Mar 22;378(12):1162. [PMID: 29562155]
Talan DA et al; EMERGEncy ID Net Study Group. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg Infect Dis. 2016 Sep;22(9). [PMID: 27532362]
Wagenlehner FME et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019 Feb 21;380(8):729–40. [PMID: 30786187]
Yoon YK et al. Role of piperacillin/tazobactam as a carbapenem-sparing antibiotic for treatment of acute pyelonephritis due to extended-spectrum β-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2017 Apr;49(4):410–15. [PMID: 28263710]
3. Acute Bacterial Prostatitis
ESSENTIALS OF DIAGNOSIS

 Fever.
Fever.
 Irritative voiding symptoms.
Irritative voiding symptoms.
 Perineal or suprapubic pain; exquisite tenderness common on rectal examination.
Perineal or suprapubic pain; exquisite tenderness common on rectal examination.
 Positive urine culture.
Positive urine culture.
 General Considerations
General Considerations
Acute bacterial prostatitis is usually caused by gram-negative rods, especially E coli and Pseudomonas species, and less commonly by gram-positive organisms (eg, enterococci). The most likely routes of infection include ascent up the urethra and reflux of infected urine into the prostatic ducts. Lymphatic and hematogenous routes are probably rare.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Perineal, sacral, or suprapubic pain, fever, and irritative voiding complaints are common. Varying degrees of obstructive symptoms may occur as the acutely inflamed prostate swells, which may lead to urinary retention. High fevers and a warm and often exquisitely tender prostate are detected on examination. Care should be taken to perform a gentle rectal examination, since vigorous manipulations may result in septicemia. Prostatic massage is contraindicated.
B. Laboratory Findings
Complete blood count shows leukocytosis and a left shift. Urinalysis shows pyuria, bacteriuria, and varying degrees of hematuria. Urine or expressed prostatic secretions cultures will demonstrate the offending pathogen (Table 23–2).
Table 23–2. Clinical characteristics of prostatitis and chronic pelvic pain syndrome.
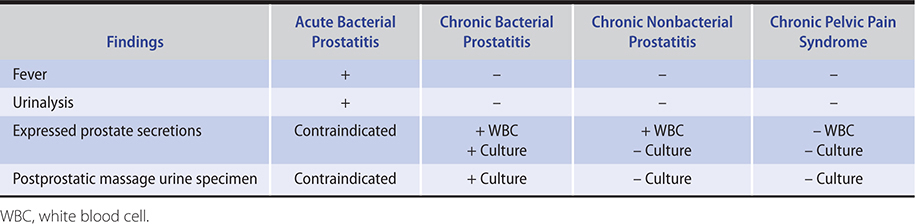
C. Imaging
Acute prostatitis can progress to prostatic abscess and a pelvic CT or transrectal ultrasound is indicated in patients who do not respond to antibiotics in 24–48 hours.
 Differential Diagnosis
Differential Diagnosis
Acute pyelonephritis or acute epididymitis should be distinguishable by the location of pain as well as by physical examination. Acute diverticulitis is occasionally confused with acute prostatitis; however, the history and urinalysis should permit clear distinction. Urinary retention from prostatic enlargement is distinguishable by initial or follow-up rectal examination and postvoid residual bladder scan.
 Treatment
Treatment
Hospitalization may be required, and parenteral antibiotics (ampicillin and aminoglycoside) should be initiated until organism sensitivities are available (Table 23–1). After the patient is afebrile for 24–48 hours, oral antibiotics (eg, quinolones if organism is sensitive) are used to complete 4–6 weeks of therapy. If urinary retention develops, an in-and-out catheterization to relieve the initial obstruction or short-term (12 hours) small indwelling urinary catheter is appropriate.
 Prognosis
Prognosis
Acute bacterial prostatitis is relatively simple to treat, since bacteria are eradicated with appropriate antibiotic therapy. Progression to chronic bacterial prostatitis is rare.
 When to Refer
When to Refer
• Evidence of urinary retention.
• Evidence of chronic prostatitis.
 When to Admit
When to Admit
• Signs of sepsis.
• Need for surgical drainage of bladder or prostatic abscess.
Khan FU et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017 Oct;94:1064–76. [PMID: 28813783]
Lupo F et al. Is bacterial prostatitis a urinary tract infection? Nat Rev Urol. 2019 Apr;16(4):203–4. [PMID: 30700862]
4. Chronic Bacterial Prostatitis
ESSENTIALS OF DIAGNOSIS

 Irritative voiding symptoms.
Irritative voiding symptoms.
 Perineal or suprapubic discomfort, often dull and poorly localized.
Perineal or suprapubic discomfort, often dull and poorly localized.
 Abnormal expressed prostatic secretions and positive culture.
Abnormal expressed prostatic secretions and positive culture.
 General Considerations
General Considerations
Although chronic bacterial prostatitis may evolve from acute bacterial prostatitis or recurrent urinary tract infection, over half of affected men have no history of acute infection. Gram-negative rods are the most common etiologic agents, but only one gram-positive organism (Enterococcus) is associated with chronic infection. Routes of infection are the same as discussed for acute infection.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Clinical manifestations are variable. Most patients have varying degrees of irritative voiding symptoms, urethral pain, and obstructive urinary symptoms. Low back and perineal pain are common. Many patients (25–43%) report a history of urinary tract infections. Physical examination is often unremarkable, although the prostate may feel normal, boggy, or indurated. A postvoid residual urine volume should be measured to evaluate for urinary retention.
B. Laboratory Findings
Urinalysis is normal unless a secondary cystitis is present. Expressed prostatic secretions or a postprostatic massage voided urine or both demonstrate increased numbers of leukocytes (greater than 5–10 per high-power field) and bacterial growth when cultured (Table 23–2). Culture of the secretions and the postprostatic massage urine specimen is necessary to make the diagnosis. Leukocyte and bacterial counts from expressed prostatic secretions do not correlate with severity of symptoms. If no organisms are identified on culture, then nonbacterial prostatitis, chronic pelvic pain, or interstitial cystitis should be suspected.
C. Imaging
Imaging tests are typically not necessary.
 Differential Diagnosis
Differential Diagnosis
Chronic urethritis may mimic chronic prostatitis, though cultures of the fractionated urine may localize the source of infection to the initial specimen, which comes from the urethra. Cystitis may be secondary to prostatitis, but urine samples after prostatic massage may localize the infection to the prostate. Other chronic prostatic conditions, such as nonbacterial prostatitis, chronic pelvic pain, or interstitial cystitis, are distinguished from chronic bacterial prostatitis by examination and culture of prostatic secretions and postprostatic massage urine sample. Anal disease may share some of the symptoms of prostatitis, but physical examination should distinguish between the two.
 Treatment
Treatment
As in acute prostatitis, if patients are febrile or systemically ill, they may require admission and initial intravenous therapy with broad-spectrum antibiotics, such as ampicillin plus gentamicin, a third-generation cephalosporin, or a fluoroquinolone (Table 23–1). Therapy would then continue with oral trimethoprim-sulfamethoxazole, fluoroquinolone, or extended-spectrum beta-lactamase antibiotic based on culture and sensitivities of expressed prostatic secretion or postprostatic massage urine. The optimal duration of therapy remains controversial, ranging from 4 to 6 weeks. Symptomatic relief may be provided by anti-inflammatory agents (indomethacin, ibuprofen), hot sitz baths, and alpha-blockers (tamsulosin, alfuzosin, silodosin).
 Prognosis
Prognosis
Chronic bacterial prostatitis may be recurrent, can be difficult to cure, and often requires repeated courses of therapeutic antibiotics.
 When to Refer
When to Refer
• Persistent symptoms.
• Consideration of enrollment in clinical trials.
Khan FU et al. Comprehensive overview of prostatitis. Biomed Pharmacother. 2017 Oct;94:1064–76. [PMID: 28813783]
Zaidi N et al. Management of chronic prostatitis. Curr Urol Rep. 2018 Aug 31;19(11):88. [PMID: 30167899]
5. Nonbacterial Chronic Prostatitis/Chronic Pelvic Pain Syndrome
ESSENTIALS OF DIAGNOSIS

 Irritative voiding symptoms.
Irritative voiding symptoms.
 Perineal or suprapubic discomfort, similar to that of chronic bacterial prostatitis.
Perineal or suprapubic discomfort, similar to that of chronic bacterial prostatitis.
 Presence of white blood cells in expressed prostatic secretions but negative culture.
Presence of white blood cells in expressed prostatic secretions but negative culture.
 General Considerations
General Considerations
Nonbacterial chronic prostatitis and chronic pelvic pain syndromes are incompletely understood with symptoms due to interrelated cascade of inflammatory, immunologic, endocrine, muscular, neuropathic, and psychologic mechanisms. There are a variety of subtypes based on the most pronounced symptoms. Chronic perineal, suprapubic, or pelvic pain is the most common presenting symptom, though men may complain of pain in the testes, groin, and low back. Pain during or after ejaculation is one of the most prominent and bothersome symptoms in many patients. Psychosocial factors (depression, anxiety, catastrophizing, poor social support, stress) also likely play an important role in the exacerbation of chronic pelvic pain symptoms. Because the cause of nonbacterial prostatitis remains unknown, the diagnosis is usually one of exclusion, and treatment may require multimodal therapy. Quality of life is greatly decreased for many patients with chronic nonbacterial prostatitis and chronic pelvic pain syndrome.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The clinical presentation is identical to that of chronic bacterial prostatitis; however, no history of urinary tract infections is typically present. The National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) (www.prostatitisclinic.com/graphics/questionnaire2.pdf) has been validated to quantify symptoms of chronic nonbacterial prostatitis or chronic pelvic pain syndrome.
B. Laboratory Findings
Increased numbers of leukocytes are typically seen in expressed prostatic secretions, but cultures of both expressed prostatic secretions and postprostatic urine specimens are negative.
 Differential Diagnosis
Differential Diagnosis
The major distinction is from chronic bacterial prostatitis. The absence of positive cultures makes the distinction (Table 23–2). In older men with irritative voiding symptoms and negative cultures, bladder cancer must be excluded. Urinary cytologic examination and cystoscopy are warranted.
 Treatment
Treatment
Multimodal therapy is recommended according to the various phenotypes of patient presentation. Patients with voiding symptoms are treated with an alpha-blocker (tamsulosin, alfuzosin, silodosin). Antibiotics are used to treat newly diagnosed, antimicrobial-naive patients. Psychosocial disorders are treated with cognitive behavioral therapy, antidepressants, anxiolytics, and, if necessary, referral to mental health specialists. Neuropathic pain is treated with gabapentinoids, amitriptyline, neuromodulation, acupuncture, or, if necessary, referral to a pain management specialist. Pelvic floor muscle dysfunction may respond to diazepam, biofeedback techniques, pelvic floor physical therapy (kegel exercises), pelvic shock wave lithotripsy, and heat therapy. Sexual dysfunction with pain symptoms is treated with sexual therapy and phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil). Surgery is not recommended for chronic prostatitis.
 Prognosis
Prognosis
Annoying, recurrent symptoms are common, but serious sequelae have not been identified.
Doiron RC et al. Management of chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2018 Jun;12(6 Suppl 3):S161–3. [PMID: 29875042]
Doiron RC et al. The evolving clinical picture of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a look at 1310 patients over 16 years. Can Urol Assoc J. 2018 Jun;12(6):196–202. [PMID: 29485036]
Franco JV et al. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst Rev. 2018 Jan 26;1:CD012551. Update in: Cochrane Database Syst Rev 2018 May 12;5:CD012551. [PMID: 29372565]
Sandhu J et al. Recent advances in managing chronic prostatitis/chronic pelvic pain syndrome. F1000Res. 2017 Sep 25;6. [PMID: 29034074]
6. Acute Epididymitis
ESSENTIALS OF DIAGNOSIS

 Fever.
Fever.
 Irritative voiding symptoms.
Irritative voiding symptoms.
 Painful enlargement of epididymis.
Painful enlargement of epididymis.
 General Considerations
General Considerations
Most cases of acute epididymitis are infectious and can be divided into one of two categories that have different age distributions and etiologic agents. Sexually transmitted forms typically occur in men under age 35 years, are associated with urethritis, and result from Chlamydia trachomatis or Neisseria gonorrhoeae. Men who practice insertive anal intercourse may have acute epididymitis from sexually transmitted and enteric organisms. Non–sexually transmitted forms typically occur in men age 35 years and older, are associated with urinary tract infections and prostatitis, and are caused by enteric gram-negative rods. The route of infection is probably via the urethra to the ejaculatory duct and then down the vas deferens to the epididymis. Amiodarone has been associated with self-limited epididymitis in a dose-dependent phenomenon.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Symptoms may follow chronic dysfunctional voiding, urinary retention, sexual activity, or trauma. Associated symptoms of urethritis (pain at the tip of the penis and urethral discharge) or cystitis (irritative voiding symptoms) may occur. Pain develops in the scrotum and may radiate along the spermatic cord or to the flank. Scrotal swelling and tenderness are usually apparent. Severe cases may develop systemic symptoms such as fever. Early in the course, the epididymis may be distinguishable from the testis; however, later the two may appear as one enlarged, tender mass. A reactive hydrocele may develop. The prostate may be tender on rectal examination.
B. Laboratory Findings
A complete blood count shows leukocytosis and a left shift. In the sexually transmitted variety, Gram staining of a smear of urethral discharge may be diagnostic of gram-negative intracellular diplococci (N gonorrhoeae). White cells without visible organisms on urethral smear signify nongonococcal urethritis, and C trachomatis is the most likely responsible pathogen. In the non-sexually transmitted variety, urinalysis shows pyuria, bacteriuria, and varying degrees of hematuria. Urine cultures will demonstrate the offending pathogen.
C. Imaging
Scrotal ultrasound may aid in the diagnosis if examination is difficult because of the presence of a large hydrocele or because questions exist regarding the diagnosis.
 Differential Diagnosis
Differential Diagnosis
Tumors generally cause painless enlargement of the testis. Urinalysis is negative, and examination reveals a normal epididymis. Scrotal ultrasound is helpful to define the pathology. Testicular torsion usually occurs in prepubertal males but is occasionally seen in young adults. Acute onset of symptoms and a negative urinalysis favor testicular torsion or torsion of one of the testicular or epididymal appendages. Prehn sign (elevation of the scrotum improves pain from epididymitis) may be suggestive but is not reliable in its diagnosis. A distal ureteral stone often presents with referred pain into the ipsilateral groin and scrotum, but the scrotum is not tender to palpation and a scrotal ultrasound is normal.
 Treatment
Treatment
Bed rest, ice, and scrotal elevation are important in the acute phase. Treatment is directed toward the identified pathogen (Table 23–1). The sexually transmitted variety in patients under age 35 is treated with a single intramuscular injection of ceftriaxone 250 mg plus 10 days of oral doxycycline 100 mg four times daily; in addition, any sexual partners from the preceding 60 days must be evaluated and treated as indicated. Men who practice insertive anal intercourse receive a single intramuscular injection of ceftriaxone 250 mg and 10 days of an oral fluoroquinolone (ciprofloxacin 500 mg twice daily) to cover sexually transmitted and enteric organisms. Non–sexually transmitted forms are treated for 10 days with a fluoroquinolone, at which time evaluation of the urinary tract is warranted to identify underlying disease. Symptoms and signs of epididymitis that do not subside within 3 days require reevaluation of the diagnosis and therapy.
 Prognosis
Prognosis
Prompt treatment usually results in a favorable outcome. If significant scrotal swelling has developed, this may take 4 weeks to resolve. Delayed or inadequate treatment may result in epididymoorchitis, decreased fertility, or abscess formation.
 When to Refer
When to Refer
• Persistent symptoms and infection despite antibiotic therapy.
• Signs of sepsis or abscess formation.
Louette A et al. Treatment of acute epididymitis: a systematic review and discussion of the implications for treatment based on etiology. Sex Transm Dis. 2018 Dec;45(12):e104–8. [PMID: 30044339]
INTERSTITIAL CYSTITIS
ESSENTIALS OF DIAGNOSIS

 Pain with bladder filling; urinary urgency and frequency.
Pain with bladder filling; urinary urgency and frequency.
 Submucosal petechiae or ulcers on cystoscopic examination.
Submucosal petechiae or ulcers on cystoscopic examination.
 Diagnosis of exclusion.
Diagnosis of exclusion.
 General Considerations
General Considerations
Interstitial cystitis (painful bladder syndrome) is characterized by pain with bladder filling that is relieved by emptying and is often associated with urgency and frequency with a dramatic exaggeration of normal sensations. This is a diagnosis of exclusion, and patients must have a negative urine culture and cytology and no other obvious cause such as radiation cystitis, chemical cystitis (cyclophosphamide), vaginitis, urethral diverticulum, or genital herpes. Up to 40% of patients referred to urologists for interstitial cystitis may actually be found to have a different diagnosis after careful evaluation. What was once considered a bladder disorder is now considered a chronic pain syndrome.
Population-based studies have demonstrated a prevalence of between 18 and 40 per 100,000 people. Both sexes are involved, but most patients are women, with a mean age of 40 years at onset. Patients with interstitial cystitis are more likely to report bladder problems in childhood, especially women. Up to 50% of patients may experience spontaneous remission of symptoms, with a mean duration of 8 months without treatment.
The etiology of interstitial cystitis is unknown, and it is most likely not a single disease but rather several diseases with similar symptoms. Associated diagnoses include severe allergies, irritable bowel syndrome, or inflammatory bowel disease. Theories regarding the cause of interstitial cystitis include increased epithelial permeability, neurogenic causes (sensory nervous system abnormalities), and autoimmunity.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Pain, pressure, or discomfort with bladder filling that is relieved with urination, and urgency, frequency, and nocturia are the most common symptoms. Patients should be asked about exposure to pelvic radiation or treatment with cyclophosphamide. Examination should exclude genital herpes, vaginitis, or a urethral diverticulum.
B. Laboratory Findings
Urinalysis, urine culture, and urinary cytology are obtained to examine for infectious causes and bladder malignancy; in interstitial cystitis, they are all normal. Urodynamic testing can be done to assess bladder sensation and compliance and to exclude detrusor instability.
C. Cystoscopy
Cystoscopy may reveal glomerulations (submucosal hemorrhage) with hydrodistention of the bladder. Total bladder capacity should be determined. Biopsy of any suspicious lesions should be performed to exclude other causes such as carcinoma, eosinophilic cystitis, and tuberculous cystitis. The presence of submucosal mast cells is not needed to make the diagnosis of interstitial cystitis.
 Differential Diagnosis
Differential Diagnosis
Exposures to radiation or cyclophosphamide are discovered by the history. Bacterial cystitis, genital herpes, or vaginitis can be excluded by urinalysis, culture, and physical examination. A urethral diverticulum may be suspected if palpation of the urethra demonstrates an indurated mass that results in the expression of pus from the urethral meatus. Urethral carcinoma presents as a firm mass on palpation.
 Treatment
Treatment
There is no cure for interstitial cystitis, but most patients achieve symptomatic relief from one of several approaches, including hydrodistention, which is usually done as part of the diagnostic evaluation. Approximately 20–30% of patients notice symptomatic improvement following this maneuver. Patients with very small bladder capacities (less than 200 mL) are unlikely to respond to medical therapy.
Amitriptyline (10–75 mg/day orally) is often used as first-line medical therapy in patients with interstitial cystitis. Both central and peripheral mechanisms may contribute to its activity. Nifedipine (30–60 mg/day orally) and other calcium channel blockers have also demonstrated some activity in patients with interstitial cystitis. Pentosan polysulfate sodium (Elmiron) is an oral synthetic sulfated polysaccharide that helps restore integrity to the epithelium of the bladder in a subset of patients, and it has been evaluated in a placebo-controlled trial. Other options include intravesical instillation of dimethyl sulfoxide (DMSO) and heparin. Intravesical bacillus Calmette-Guérin (BCG) is not beneficial.
Further treatment modalities include transcutaneous electric nerve stimulation (TENS), acupuncture, stress reduction, exercise, biofeedback, massage, and pelvic floor relaxation. Surgical therapy for interstitial cystitis should be considered only as a last resort and may require cystourethrectomy with urinary diversion.
 When to Refer
When to Refer
Persistent and bothersome symptoms in the absence of identifiable cause.
Daniels AM et al. Interstitial cystitis: an update on the disease process and treatment. J Pain Palliat Care Pharmacother. 2018 Mar;32(1):49–58. [PMID: 30212267]
Giusto LL et al. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 2018 Jul;19(10):1097–108. [PMID: 29972328]
Marcu I et al. Interstitial cystitis/bladder pain syndrome. Semin Reprod Med. 2018 Mar;36(2):123–35. [PMID: 30566978]
Patanaik SS et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. 2017 Jun;295(6):1341–59. [PMID: 28391486]
Zhang W et al. Intravesical treatment of interstitial cystitis/painful bladder syndrome: a network meta-analysis. Int Urogynecol J. 2017 Apr;28(4):515–25. [PMID: 27614759]
URINARY STONE DISEASE
ESSENTIALS OF DIAGNOSIS

 Severe flank pain.
Severe flank pain.
 Nausea and vomiting.
Nausea and vomiting.
 Identification on noncontrast CT or ultrasonography.
Identification on noncontrast CT or ultrasonography.
 General Considerations
General Considerations
Urinary stone disease is exceeded in frequency as a urinary tract disorder only by infections and prostatic disease. It is estimated to afflict 240,000–720,000 Americans per year. The prevalence of kidney stones has increased to 8.8%, or 1 in 11 Americans, representing a 70% increase over the last 15 years. While men are more frequently affected by urolithiasis than women, with a ratio of 1.5:1, the prevalence of stones in women is increasing. Initial presentation usually occurs in the third through fifth decades, and more than 50% of patients will become recurrent stone formers.
Stone formation requires saturated urine that is dependent on solute concentration, ionic strength, pH, and complexation. There are five major types of urinary stones: calcium oxalate, calcium phosphate, struvite (magnesium ammonium phosphate), uric acid, and cystine. The most common types are those composed of calcium oxalate or phosphate (85%), and for that reason most urinary stones are radiopaque on plain abdominal radiographs. Although pure uric acid stones are radiolucent, uric acid stones are frequently composed of a combination of uric acid and calcium oxalate and thus may be radiopaque. Cystine stones frequently have a smooth-edged ground-glass appearance and are radiolucent.
Geographic factors contribute to the development of stones. High humidity and elevated temperatures appear to be contributing factors, and the incidence of symptomatic ureteral stones is greatest in such areas during hot summer months. Higher incidence rates of stones have also been associated with sedentary lifestyle, obesity, hypertension, insulin resistance and poor glycemic control, carotid calcification, and cardiovascular disease.
Many commonly prescribed medications increase the risk of formation of kidney stones, including carbonic anhydrase inhibitors (topiramate, zonisamide, acetazolamide), systemic corticosteroids (prednisone), antiretroviral protease inhibitors (indinavir), gout medications (probenecid), diuretics (furosemide, bumetanide, torsemide, triamterene), decongestants (guaifenesin, ephedrine), and laxatives (if abused for weight loss). The risk of stones from calcium supplementation is controversial. Thus, if calcium supplementation is medically necessary, it is recommended that the calcium supplement be taken with meals, and restricted to no more than 2000 mg of total calcium intake daily (including dietary sources).
Inadequate hydration is another very important dietary factor in the development of urinary stones. Stone formers should be encouraged to drink enough fluid to keep their urine clear or light-yellow at all times with a goal of at least 2500 mL of urine produced daily. Excess animal protein and salt intake (over 3500 mg daily) as well as restricted dietary calcium intake are other important stone risk factors.
Genetic factors may contribute to urinary stone formation. While approximately 50% of calcium-based stones are thought to have a heritable component, other stone types are better characterized genetically. For example, cystinuria is an autosomal recessive disorder. Homozygous individuals have markedly increased excretion of cystine and frequently have numerous recurrent episodes of urinary stones. Distal renal tubular acidosis may be transmitted as a hereditary trait, and urolithiasis occurs in up to 75% of affected patients.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Obstructing urinary stones usually present with acute, unremitting, and severe colic. Pain most often occurs suddenly and may awaken patients from sleep. It is typically localized to the flank and may be associated with nausea and vomiting. In sharp contrast to patients with an acute abdomen, patients with kidney stones are constantly moving trying to find a comfortable position. The pain may occur episodically and may radiate anteriorly over the abdomen. As the stone progresses down the ureter, the pain may be referred into the ipsilateral groin. As the stone traverses the uretero-vesicular junction, patients may complain of marked urinary urgency and frequency and in men, pain may radiate to the tip of the penis. After the stone passes into the bladder, there typically is immediate relief of symptoms, then the stones pass harmlessly through the urethra. Stone size does not correlate with the severity of the symptoms. If the stone fails to pass and obstruction persists, patients may note a deceptive improvement in symptoms. As many as 25% of patients with resolution of pain will have a persistent stone and thus follow-up imaging is recommended in all patients if the stone has not been witnessed to pass.
B. Laboratory Findings
Regardless of symptom severity, urinalysis usually reveals microscopic or gross hematuria (∼90%). However, the absence of microhematuria does not exclude urinary stones. A persistent urinary pH < 5.5 may suggest a uric acid stone, while a persistent urinary pH > 7.2 may suggest a struvite (infection-related) or calcium phosphate stone. Patients with calcium oxalate–based stones typically have a normal urinary pH.
C. Metabolic Evaluation
Stone analysis on recovered stones can facilitate counseling for prevention of recurrence. Patients with uncomplicated first-time stones should undergo dietary counseling as outlined below and can be offered an optional complete metabolic evaluation.
General dietary counseling includes encouraging patients to augment their fluid intake to increase their urine volume (goal urinary output of greater than 2500 mL/day). This typically requires a fluid intake of 3 L/day or more. Stone formers should reduce their sodium intake (goal less than 3500 mg/day), and reduce their animal protein intake (eggs, fish, chicken, pork, and beef). Detailed medical and dietary history, serum chemistries, and urinalysis should be obtained for all patients with newly diagnosed nephrolithiasis. A serum parathyroid hormone level should be checked when hyperparathyroidism is suspected as the cause of calcium oxalate or calcium phosphate stones, and a serum uric acid should be obtained to exclude severe hyperuricemia, which can lead to uric acid stones as well as crystal deposition in the kidneys or heart. A 24-hour urine collection to determine urinary volume, creatinine, pH, calcium, uric acid, oxalate, phosphate, sodium, and citrate excretion is recommended for interested patients with their first stone, for all patients who have recurrent stones, and for patients at high risk for recurrence. Results are used to personalize medical management to individual patient risk factors.
D. Imaging
Noncontrast CT is the most accurate imaging modality for evaluating flank pain given its superior sensitivity and specificity over other tests; however, ultrasonography (which does not use ionizing radiation) is a safe and effective alternative for initial evaluation of renal colic and one that can be used in the emergency department with good accuracy. If the CT scan is used, it should be obtained in the prone position to help differentiate distal ureterovesicular stones from those that have already passed into the urinary bladder. A “low-dose” imaging protocol should be used when available and repeated CT scans should be minimized due to the substantial cumulative radiation exposure that patients with recurrent stones can face. Stone density can be estimated with Hounsfield units (HU) on CT scans to help determine stone type. All stones, whether radiopaque or radiolucent on plain abdominal radiographs, will be visible on noncontrast CT except the rare calculus caused by the protease inhibitor, indinavir. A plain abdominal radiograph (kidney, ureter, and bladder [KUB]) and renal ultrasound examination will diagnose up to 80% of stones. Since more than 60% of patients with acute renal colic will have a stone in the distal 4 cm of the ureter, attention should be directed to that region when examining radiographs and ultrasonographic studies. Pain from a kidney stone is due to the dilatation of the ureter and kidney from the obstruction, and thus small nonobstructing kidney stones are typically not associated with pain.
 Medical Treatment & Prevention
Medical Treatment & Prevention
To reduce the recurrence rate of urinary stones, dietary modification is important. Metabolic evaluation often identifies a modifiable risk factor that can further reduce stone recurrence rates. If no medical treatment is provided, stones will generally recur in 50% of patients within 5 years. Some stone types (eg, uric acid, cystine) are more prone to rapid recurrence than others. An increased fluid intake to dilute the urine and prevent dehydration is the most important dietary risk factor to reduce stone recurrence and may diminish the risk by 50%. Increasing fluid intake to ensure a voided volume of 2.5 L/day is recommended (normal average voided volume is 1.6 L/day). Urine should be clear or light yellow at each void. Medical therapy should be tailored to the patient’s metabolic workup and the activity of their stone disease. Routine follow-up every 6–8 months and annual imaging (preferably with ultrasonography) will help encourage medical compliance, assess for interval stone formation or growth, and permit adjustments in medical therapy based on repeat metabolic studies.
A. General Dietary Recommendations
A 24-hour urinary sodium level of greater than 150 mmol/day indicates excessive sodium intake. Sodium intake should be limited to less than 3500 mg daily. Excessive sodium intake will increase renal sodium and calcium excretion, increase urinary monosodium urates (that can act as a nidus for stone growth), increase the relative saturation of calcium phosphate, and decrease urinary citrate excretion. All of these factors encourage stone growth.
A urinary sulfate level of greater than 20 mEq/day indicates excessive animal protein intake. Animal protein intake should be spread out through the day, not all consumed during any individual meal, and is best limited to 1 g/kg/day. An increased protein load during an individual meal can lead to acidic urine and also increases calcium, oxalate, and uric acid excretion and decrease urinary citrate excretion.
Dietary calcium intake should not be restricted in an effort to decrease stone formation because it may paradoxically lead to increased stone formation due to increased oxalate absorption and consequent hyperoxaluria.
B. Calcium Nephrolithiasis
1. Hypercalciuria—Elevated urinary calcium levels (greater than 4 mg/kg/day or greater than 250 mg/day for males and greater than 200 mg/day for females) lead to hypercalciuric calcium nephrolithiasis. Hypercalciuria can be caused by absorptive, resorptive, and renal disorders. The categorization system provided below was proposed in the 1970s and provides a simplified explanation of the causes of hypercalciuria; however, it is not routinely used in clinical practice. Thiazide diuretics decrease renal calcium excretion; after primary hyperparathyroidism has been excluded, thiazide diuretics should be offered to patients with high urinary calcium and recurrent calcium stones. Chlorthalidone and indapamide are first-line agents since they can be administered once a day, while hydrochlorothiazide for hypercalciuria should be administered twice a day. All patients respond to thiazide diuretics with decreases in urinary calcium unless they have primary hyperparathyroidism or are nonadherent with taking the medication. Clinicians should periodically test patients taking thiazide diuretics for hypokalemia, since they may require potassium supplementation.
Absorptive hypercalciuria is secondary to increased absorption of calcium at the level of the small bowel, predominantly in the jejunum. Absorptive hypercalciuria can be diet-dependent, independent of calcium intake, or due to renal phosphate leak. Oral calcium load testing is no longer performed.
Resorptive hypercalciuria, or primary hyperparathyroidism, is typically due to a parathyroid adenoma. Hypercalcemia, elevated parathyroid hormone, hypophosphatemia, and elevated urinary calcium are present. Appropriate surgical resection of the parathyroid adenoma is curative in 75% of patients with kidney stones due to primary hyperparathyroidism. Medical management is typically reserved for patients who are elderly or frail.
Renal hypercalciuria is the most common form of hypercalciuria and occurs when the renal tubules are unable to efficiently reabsorb filtered calcium. Spilling calcium in the urine may result in secondary hyperparathyroidism with normal serum calcium. A thiazide diuretic is an effective long-term therapy in patients with this disorder because it corrects the urinary calcium losses and is associated with an increase in bone mineral density of approximately 1% per year while receiving therapy.
2. Hyperuricosuria—Hyperuricosuric calcium nephrolithiasis is defined by elevated urinary uric acid levels (greater than 800 mg/day for males and greater than 750 mg/day for females). It is usually secondary to dietary purine excess or endogenous uric acid metabolic defects. Excess uric acid in the urine can lead to uric acid stones if the urine pH is low, or to calcium stones at higher urine pH due to formation of a monosodium urate crystal that then calcifies in a process known as heterogenous nucleation. Dietary purine restriction can reduce hyperuricosuria in 85% of cases. Patients with hyperuricosuria, normocalciuria, and recurrent calcium oxalate stones can be successfully treated with allopurinol. Allopurinol is not first-line treatment of uric acid stones; urinary alkalinization is (see below).
3. Hyperoxaluria—Hyperoxaluric calcium nephrolithiasis (greater than 40 mg/day of urinary oxalate) is usually due to either an intestinal malabsorption disorder or a mismatch in dietary calcium and oxalate intake. When dietary calcium and oxalate intake are consumed concurrently, they are unable to be absorbed systemically as they are bound together in the intestinal tract. If dietary calcium is restricted, or dietary oxalate is excessive, free oxalate is rapidly absorbed and excreted in the urine. Treatment includes adhering to a diet containing moderate calcium intake (1000–1200 mg daily) and avoiding high-oxalate-containing foods (baked potatoes with skins, sweet potatoes. French fries, okra, cocoa powder, grits, Stevia sweetener, beets, spinach, rhubarb, almonds, cashews, and miso soup). Low-dose calcium carbonate (250 mg) can be consumed with meals if dietary calcium increases do not successfully reach 1000 mg daily. Patients with a history of chronic diarrhea, inflammatory bowel disease, malabsorption, supplement use, or gastric bypass surgery are also at risk for hyperoxaluria. In these situations, increased intestinal fat or bile (or both) combine with calcium to form a soap-like product. Calcium is therefore unavailable to bind to oxalate, leading to free oxalate absorption. A small increase in oxalate absorption significantly increases stone formation. If the diarrhea or steatorrhea cannot be effectively curtailed, oral calcium should be increased with meals, either by ingesting dairy products or taking low-dose calcium carbonate supplements (250 mg). Excess ascorbic acid (greater than 2000 mg/day) will substantially increase urinary oxalate levels. Rare enzymatic liver defects can lead to primary hyperoxaluria that is routinely fatal without a combined liver and kidney transplantation.
4. Hypocitraturia—Urinary citrate is the most important inhibitor of stone formation, and urine citrate levels less than 450 mg/day increase the risk of stones. Urinary citrate binds to calcium in solution, thereby decreasing available calcium for precipitation and subsequent stone formation. Hypocitraturic calcium nephrolithiasis is usually idiopathic. Urinary citrate excretion is influenced by systemic acid-base balance and serum potassium levels, and thus, hypocitraturia occurs secondary to any metabolic acidemia (chronic diarrhea, distal renal tubular acidosis), or with systemic potassium losses (long-term treatment with thiazide or loop diuretics). Metabolic acidosis enhances citrate transport into the proximal tubular cells where it is consumed by the citric acid cycle in their mitochondria. Potassium citrate supplements are usually effective treatment in these situations; a typical dose is 40–60 mEq total daily intake, divided into two or three daily doses. Alternatively, oral lemonade has been shown to modestly increase urinary citrate, but this must be consumed several times every day as oral citrate is cleared from the urine in 6–8 hours.
C. Uric Acid Calculil
Urinary pH is the most important contributor to uric acid stone formation, and thus efforts should focus on alkalinizing the urine as first-line therapy (oral potassium citrate or sodium bicarbonate), while efforts to decrease urinary uric acid (allopurinol) should be reserved for patients continuing to form stones despite adequate urinary alkalinization. Urine pH is consistently less than 5.5 in patients who form pure uric acid stones. Increasing the urinary pH dramatically increases uric acid solubility, leading to prevention of stone formation (with urine pH > 6.0) and even stone dissolution (with urine pH > 6.5). Nitrazine pH test strips (which turn blue with alkaline urine pH > 6.0) are often useful to some patients in reinforcing adherence to urinary alkalinization efforts. Less common contributors to uric acid stone formation include hyperuricemia, myeloproliferative disorders, chemotherapy for malignancies with rapid cell turnover or cell death, abrupt and dramatic weight loss, and uricosuric medications (probenecid). If hyperuricemia is present or the patient has a history of recurrent gout, in addition to hyperuricosuria, allopurinol (300 mg/day orally) may be given for stone prevention.
D. Struvite Calculi
Struvite stones are composed of magnesium-ammonium-phosphate and are typically visible on plain radiographs. They are most common in women with recurrent urinary tract infections with urease-producing organisms, including Proteus, Pseudomonas, Providencia, and, less commonly, Klebsiella, Staphylococcus, and Mycoplasma (but not E coli). They rarely present as a first symptomatic ureteral stone. Frequently, a struvite stone is discovered as a large staghorn calculus forming a cast of the renal collecting system. Urinary pH is high, routinely above 7.2. Struvite stones are relatively soft and amenable to percutaneous removal. Appropriate perioperative antibiotics are required. They can recur rapidly, and efforts should be taken to render the patient stone-free.
E. Cystine Calculi
Cystine stones are caused by a genetic metabolic defect resulting in abnormal excretion of cystine. These stones are exceptionally challenging to manage medically. Prevention is centered around marked increased fluid intake during the day and night to achieve a urinary volume of 3–4 L/day, decreased sodium and dietary cystine intake, and increased urinary alkalinization (typically with high-dose potassium citrate) with a goal urinary pH > 7.0. Refractory stone formers may be treated with disulfide inhibitors such as tiopronin (alpha-mercaptoproprionylglycine) or penicillamine. There are no known inhibitors of cystine calculi.
 Medical Expulsion & Surgical Treatment
Medical Expulsion & Surgical Treatment
Signs of infection, including associated fever, tachycardia, hypotension, and elevated white blood cell count, may indicate a urinary tract infection behind the obstructing stone. Any obstructing stone with associated infection is a medical emergency requiring urology consultation and prompt drainage of the kidney with a ureteral stent or a percutaneous nephrostomy tube. Antibiotics alone are inadequate and only used as an adjunct to urinary drainage behind the obstruction. In the acute setting, forcing intravenous fluids will not push stones down the ureter. Forced diuresis can actually be counterproductive and exacerbate pain; instead, a euvolemic state should be achieved.
A. Ureteral Stones
Ureteral stones are usually discovered at three sites: the ureteropelvic junction, the crossing of the ureter over the iliac artery, or the ureterovesicular junction. Stones smaller than 5–6 mm in diameter on a plain abdominal radiograph usually pass spontaneously. Medical expulsive therapy with alpha-blockers (eg, tamsulosin, 0.4 mg orally once daily) in combination with an anti-inflammatory agent (eg, ibuprofen 600 mg orally three times per day), with or without a short course of a low-dose oral corticosteroid (eg, prednisone 10 mg orally daily for 5–10 days), may increase the rate of spontaneous stone passage and appears to be most effective for distal stones greater than 5 mm. Medical expulsive therapy with effective pain medications and imaging follow-up is appropriate for a few weeks. If the stone fails to pass within 4 weeks, the patient has fever, intolerable pain, or persistent nausea or vomiting, or the patient must return to work or anticipates travel, then surgical intervention is indicated.
Stones in the mid and distal ureter that require surgical removal are best managed with ureteroscopic stone extraction, though extracorporeal shock wave lithotripsy (SWL) can be offered as second-line therapy. Ureteroscopic stone extraction involves placement of a small endoscope through the urethra and bladder and into the ureter. Under direct vision, basket extraction or laser fragmentation followed by fragment extraction is performed. A ureteral stent is often placed temporarily to allow drainage of the kidney while the swelling and inflammation from the stone and procedure resolve.
SWL utilizes an external energy source focused on the stone with the aid of fluoroscopy or ultrasonography. SWL is typically performed under anesthesia or sedation as an outpatient procedure with the goal of stone fragmentation. Most stone fragments then pass uneventfully within 2 weeks. Occasionally after SWL, stone fragments obstruct the ureter. Conservative management usually results in spontaneous resolution of the obstruction with eventual passage of the stone fragments. Fragments that have not passed within 6 weeks are unlikely to do so without intervention. SWL is strictly contraindicated in pregnant patients as well as in those with untreated urinary tract infection, in those with an uncorrected coagulopathy, or in those who must continue receiving anticoagulant or antiplatelet therapy. A ureteral stent is typically not necessary with SWL.
Proximal ureteral stones can be treated with SWL or ureteroscopy. SWL is less successful with larger stones and those that are very dense. After cases of SWL failure, ureteroscopic extraction is required.
B. Renal Calculi
Patients with small, asymptomatic, nonobstructing renal calculi, without urinary tract infection, or obstruction may not warrant surgical treatment. If surveillance is elected, the patient should be monitored with serial abdominal radiographs or renal ultrasonographic examinations every 3–12 months. If the calculi grow or become symptomatic, intervention should be undertaken. SWL is most effective for stones less than 1 cm in the lower pole of the kidney or less than 2 cm elsewhere in the kidney. SWL is less effective for stones that are very hard (cystine stones, calcium oxalate stones greater than 1000–1200 Hounsfield units on CT scan) and for obese patients (skin-to-stone distance greater than 10–12 cm). Ureteroscopy and laser lithotripsy is effective for multiple stones and larger stones, though very large stones may require multiple treatment sessions. Stones larger than 15–20 mm and staghorn stones (large branched stones occupying at least two renal calices) are best treated via percutaneous nephrolithotomy. Percutaneous nephrolithotomy is performed by inserting a needle into the appropriate renal calyx and dilating a tract large enough to allow a nephroscope to pass directly into the kidney. In this fashion, larger and more complex renal stones can be inspected, fragmented, and removed. In unusual cases, laparoscopic, robotic-assisted, or open stone removal may be considered. Perioperative antibiotic coverage should be given for any stone procedure, ideally based on preoperative urine culture results.
 When to Refer
When to Refer
• Evidence of urinary obstruction.
• Urinary stone with associated flank pain.
• Anatomic abnormalities, solitary kidney, or chronic kidney disease.
• Concomitant pyelonephritis or recurrent infection.
 When to Admit
When to Admit
• Intractable nausea and vomiting or pain.
• Obstructing stone with fever or other signs of infection.
Alelign T et al. Kidney stone disease: an update on current concepts. Adv Urol. 2018 Feb 4;2018:3068365. [PMID: 29515627]
Corbo J et al. Kidney and ureteral stones. Emerg Med Clin North Am. 2019 Nov;37(4):637–48. [PMID: 31563199]
Diri A et al. Management of staghorn renal stones. Ren Fail. 2018 Nov;40(1):357–62. [PMID: 29658394]
Kim D et al. Hydroxycitrate: a potential new therapy for calcium urolithiasis. Urolithiasis. 2019 Aug;47(4):311–20. [PMID: 30915494]
Li JK et al. Updates in endourological management of urolithiasis. Int J Urol. 2019 Feb;26(2):172–83. [PMID: 30575154]
Mayans L. Nephrolithiasis. Prim Care. 2019 Jun;46(2):203–12. [PMID: 31030821]
Skolarikos A. Medical treatment of urinary stones. Curr Opin Urol. 2018 Sep;28(5):403–7. [PMID: 29939860]
Strilchuk L et al. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf. 2019 Apr;18(4):261–71. [PMID: 30915866]
Zumstein V et al. Surgical management of urolithiasis—a systematic analysis of available guidelines. BMC Urol. 2018 Apr 10;18(1):25. [PMID: 29636048]
MALE ERECTILE DYSFUNCTION & SEXUAL DYSFUNCTION
ESSENTIALS OF DIAGNOSIS

 Erectile dysfunction can have organic and psychogenic etiologies, and the two frequently overlap.
Erectile dysfunction can have organic and psychogenic etiologies, and the two frequently overlap.
 Organic erectile dysfunction may be an early sign of cardiovascular disease and requires evaluation.
Organic erectile dysfunction may be an early sign of cardiovascular disease and requires evaluation.
 Peyronie disease is a common, benign fibrotic disorder of the penis that causes pain, penile deformity, and sexual dysfunction.
Peyronie disease is a common, benign fibrotic disorder of the penis that causes pain, penile deformity, and sexual dysfunction.
 General Considerations
General Considerations
Erectile dysfunction is the consistent inability to attain or maintain a sufficiently rigid penile erection for sexual intercourse. More than half of men aged 40–70 years have erectile dysfunction and its incidence and severity increase with age. Normal male erection is a neurovascular event relying on an intact autonomic and somatic nerve supply to the penis, arterial blood flow supplied by the cavernosal arteries, and smooth and striated musculature of the corpora cavernosa and pelvic floor. Erection is initiated by stimulation of the pelvic nerve plexus leading to release of nitric oxide with resultant increased arterial flow, active relaxation of corpora cavernosal smooth muscle, and increased venous resistance. Contraction of the ischiocavernosus muscle causes further rigidity of the penis with intracavernosal pressures exceeding systolic blood pressure.
Male sexual dysfunction is manifested in a variety of ways, and patient history is critical to the proper classification and treatment. Erectile dysfunction may result from neurogenic, arterial, venous, hormonal, or psychogenic causes. Concurrent medical problems may damage one or more of the mechanisms. The most common cause of erectile dysfunction is a decrease in arterial flow resultant from progressive vascular disease. Endothelial dysfunction results from the decreased bioavailability of nitric oxide with subsequent impairment of arterial vasodilation. Erectile dysfunction may be an early manifestation of endothelial dysfunction, which precedes more severe atherosclerotic cardiovascular disease.
Peyronie disease is a fibrotic disorder of the tunica albuginea of the penis resulting in varying degrees of penile pain, curvature, or deformity. Peyronie disease develops in approximately 5–10% of men and is more common with increased age. While 10% of men improve spontaneously, 50% will stabilize and the remainder will progress if left untreated. Penile deformity can impair normal sexual function and impact self-esteem.
Priapism is the occurrence of penile erection lasting longer than 4 hours resulting in ischemic injury of the corpora cavernosa from venous congestion and cessation of arterial inflow (low flow or “ischemic” priapism). Ischemic priapism is a medical emergency requiring immediate medical or surgical intervention to avoid irreversible penile damage. Ischemic priapism may be caused by red blood cell dyscrasias, drug use, and any of the treatments for erectile dysfunction.
Anejaculation is the loss of seminal emission and may result from androgen deficiency by decreasing prostate and seminal vesicle secretions, or by sympathetic denervation as a result of spinal cord injury, diabetes mellitus or pelvic or retroperitoneal surgery or radiation. Retrograde ejaculation may occur as a result of mechanical disruption of the bladder neck, due to congenital abnormalities, transurethral prostate surgery, pelvic radiation, sympathetic denervation, or treatment with alpha-blockers. Premature ejaculation is the distressful, recurrent ejaculation with minimal stimulation before a person desires. Primary premature ejaculation may be treated with behavioral modification, sexual health counseling, local anesthetic agents, and systemic medications. Secondary premature ejaculation is due to erectile dysfunction and responds to treatment of the underlying disorder.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Erectile dysfunction should be distinguished from problems with penile deformity, libido, orgasm, and ejaculation. The severity, intermittency, and timing of erectile dysfunction should be noted. The history should include inquiries about dyslipidemia, hypertension, depression, neurologic disease, diabetes mellitus, kidney disease, endocrine disorders, and cardiac or peripheral vascular disease. Pelvic trauma, surgery, or irradiation increases a man’s likelihood of erectile dysfunction. Histories of prostate cancer treatment or Peyronie disease should be elicited. The ability to attain but not maintain an erection may be the first sign of endothelial dysfunction and further cardiovascular risk stratification should be considered. Medication use should be reviewed. Special attention should be given to the use of nitrate-containing medications. Alcohol, tobacco, marijuana, and other recreational drug use are associated with an increased risk of sexual dysfunction. The use of pornography to maintain sexual arousal should be elicited.
During the physical examination, vital signs, body habitus (obesity), and secondary sexual characteristics should be assessed. Basic cardiovascular and neurologic examinations should be performed. The genitalia should be examined, noting the presence of Peyronie disease and any abnormalities in size or consistency of either testicle.
B. Laboratory Findings
Laboratory evaluation should be performed in select cases based on patient history and examination. Possible testing includes a lipid profile, glucose, and testosterone. Patients with abnormalities of testosterone may require further evaluation with measurement of free testosterone and luteinizing hormone (LH) to distinguish hypothalamic-pituitary dysfunction from primary testicular failure.
 Treatment
Treatment
Treatment of men suffering from sexual dysfunction should be patient centered and goal oriented. Lifestyle modification and reduction of cardiovascular risk factors are important components of treatment and should include smoking cessation; reduction of alcohol intake; diet; exercise; and treatment of diabetes, dyslipidemia, and hypertension. Men who have a psychogenic component to their erectile dysfunction or who are experiencing emotional distress will benefit from sexual health therapy or counseling.
A. Hormonal Replacement
In men with hypogonadism who have undergone complete endocrinologic evaluation, restoration of normal testosterone levels may improve sexual quality of life (see Male Hypogonadism in Chapter 26.)
B. Vasoactive Therapy
1. Oral agents—Sildenafil, vardenafil, tadalafil, and avanafil inhibit phosphodiesterase type 5 (PDE-5), preventing the degradation of cGMP and increasing blood flow into the penis. While generally similar in their effectiveness, these medications have variable durations of onset, activity, and side effects. Each medication should be initiated at the lowest dose and titrated to achieve the desired effect. There is no impact on libido and priapism is exceedingly rare. These medications are contraindicated in patients taking nitroglycerin or nitrates, since there may be exaggerated cardiac preload reduction causing hypotension and syncope. All patients being evaluated for acute chest pain should be asked about the use of PDE-5 inhibitors before administering nitroglycerin and close monitoring of blood pressure is warranted if there is concern regarding medication overlap.
The combination of PDE-5 inhibitors and alpha-receptor blockers (prescribed for lower urinary tract symptoms) may cause a larger reduction in systemic blood pressure than when PDE-5 inhibitors are used alone. However, these two classes of medication may be safely used in combination if they are initiated and titrated in a stepwise fashion.
2. Injectable or suppository medications—Injection of prostaglandin E2 into the corpora cavernosa is an acceptable form of treatment for many men with erectile dysfunction. Injections are performed using a tuberculin-type syringe or a metered-dose injection device. The base and lateral aspect of the penis is used as the injection site to avoid injury to the superficial blood and nerve supply located dorsally. Complications are rare and include priapism, penile pain, bruising, fibrosis, and infection. Prostaglandin E2 (alprostadil) can also be delivered via an intraurethral suppository. Prostaglandin E2 is often compounded with papaverine, phentolamine, or atropine in order to increase effectiveness. Patients using such compounded agents should be cautioned about the increased risk of priapism and variability of drug effect due to differences in compounding.
C. Vacuum Erection Device
The vacuum erection device creates negative pressure around the penis, drawing blood into the corpora cavernosa. Once tumescence is achieved, an elastic constriction band is placed around the penile base to prevent loss of erection. Such devices are effective but may cause penile discomfort leading to a high rate of disuse. Serious complications are rare.
D. Penile Prosthetic Surgery
Penile prostheses are implanted directly into the paired corpora cavernosa and may be semi-rigid (malleable) or inflatable. The inflatable devices are most commonly used and result in a natural appearance and function because they emulate the tumescence and detumescence of the normal erection. Complications of surgery are rare but include mechanical failure and infection. For men who elect this treatment, personal and partner satisfaction rates are very high due to enhanced spontaneity and reliability of erections.
E. Medical and Surgical Therapy for Peyronie Disease
Oral medications for Peyronie disease have not been approved by the FDA; however, off-label use of multiple vasodilatory, anti-inflammatory, and antioxidant medications is common. Injectable collagenase Clostridium histolyticum is the only FDA-approved medication for the treatment of Peyronie disease. Collagenase enzymatically severs disordered collagen fibers after injection into the central portion of the penile plaque. Surgical treatment is an alternative for men with compromised sexual function due to severe curvature, lesions causing penile instability, or with inadequate results from collagenase. The choice of corrective procedure should be tailored to each patient after a detailed evaluation of disease severity and sexual function.
 When to Refer
When to Refer
• Patients with inadequate response to oral medications, who are unable to tolerate side effects or who are dissatisfied with their current treatment.
• Patients with Peyronie disease or other penile deformity.
• Patients with a history of pelvic or perineal trauma, surgery, or radiation.
• Ischemic priapism is a medical emergency and requires immediate referral to a urologist or the emergency department for intervention to allow restoration of penile blood perfusion.
Chen L et al. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed Pharmacother. 2019 Apr;112:108585. [PMID: 30798136]
Dick B et al. An update on: long-term outcomes of penile prostheses for the treatment of erectile dysfunction. Expert Rev Med Devices. 2019 Apr;16(4):281–6. [PMID: 30898042]
Gabrielson AT et al. Collagenase Clostridium histolyticum in the treatment of urologic disease: current and future impact. Sex Med Rev. 2018 Jan;6(1):143–56. [PMID: 28454897]
Irwin GM. Erectile dysfunction. Prim Care. 2019 Jun;46(2):249–55. [PMID: 31030826]
Yafi FA et al. Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. Sex Med Rev. 2018 Apr;6(2):242–52. [PMID: 28923561]
MALE INFERTILITY
ESSENTIALS OF DIAGNOSIS

 Male infertility is common, contributing to 50% of infertility cases.
Male infertility is common, contributing to 50% of infertility cases.
 Causes include decreased or absent sperm production or function, or obstruction of the male genital tract.
Causes include decreased or absent sperm production or function, or obstruction of the male genital tract.
 Abnormal semen quality may indicate poor health or increased risk of certain health conditions.
Abnormal semen quality may indicate poor health or increased risk of certain health conditions.
 General Considerations
General Considerations
Infertility is the inability of a couple to conceive a child after 1 year of regular, unprotected sexual intercourse. It affects 15–20% of US couples and 50% of cases result from male factors. The evaluation of both partners is critical for treatment success. Following a detailed history and physical examination, a semen analysis should be performed at least twice, on two separate occasions (Figure 23–1). Because spermatogenesis requires approximately 75 days, it is important to review health events and gonadotoxic exposures from the preceding 3 months. Male infertility is associated with a higher risk of testicular germ cell cancer and with a higher rate of medical comorbidity. These men should be counseled and screened appropriately and taught testicular self-examination.

Figure 23–1. Couple-based approach to evaluation and treatment of male factor infertility. FNA, fine-needle aspiration.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The history should include prior testicular insults (torsion, cryptorchidism, trauma), infections (mumps orchitis, epididymitis, sexually transmitted infections), environmental factors (excessive heat, radiation, chemotherapy, prolonged pesticide exposure), medications (testosterone, finasteride, cimetidine, selective serotonin reuptake inhibitors, and spironolactone may affect spermatogenesis; phenytoin may lower FSH; sulfasalazine and nitrofurantoin affect sperm motility; tamsulosin causes retrograde ejaculation), and other drugs (alcohol, tobacco, marijuana). Sexual function, frequency and timing of intercourse, use of lubricants, and each partner’s previous fertility are important. The past medical or surgical history may reveal chronic disease, including obesity; cardiovascular, thyroid, or liver disease (decreased spermatogenesis); diabetes mellitus (decreased spermatogenesis, retrograde or anejaculation); or radical pelvic or retroperitoneal surgery (absent seminal emission secondary to sympathetic nerve injury).
Physical examination should assess features of hypogonadism: underdeveloped sexual characteristics, diminished male pattern hair distribution (axillary, body, facial, pubic), body habitus, gynecomastia, and obesity. Testicular size should be noted (normal size approximately 4.5 × 2.5 cm, volume 18 mL). Varicoceles are abnormally dilated and refluxing veins of the pampiniform plexus that can be identified in the standing position by gentle palpation of the spermatic cord and, on occasion, may only be appreciated with the Valsalva maneuver. The vasa deferentia and epididymides should be palpated (absence of all or part of one or both of the vasa deferentia may indicate the presence of a cystic fibrosis variant, congenital bilateral or unilateral absence of the vasa deferentia).
B. Laboratory Findings
Semen analysis should be performed after 3–5 days of ejaculatory abstinence. The specimen should be analyzed within 1 hour after collection. Normal semen volume should be equal to or greater than 1.5 mL (lesser volumes may be due to retrograde ejaculation, ejaculatory duct obstruction, congenital bilateral absence of the vasa deferentia, or hypogonadism). Abnormal sperm concentrations are less than 15 million/mL (oligozoospermia is the presence of less than 15 million sperm/mL in the ejaculate; azoospermia is the complete absence of sperm). Normal sperm motility and morphology demonstrate greater than 39% motile cells and greater than 3% normal morphology. Abnormal motility (asthenozoospermia) may result from a number of causes, including varicocele, infection, abnormalities of the sperm flagella, or ejaculatory duct obstruction. Abnormal morphology may result from a varicocele, infection, or exposure to gonadotoxins (eg, tobacco, marijuana).
Evaluation of the hypothalamic-pituitary-gonadal axis is warranted if sperm concentration is below 10 million sperm/mL or if the history and physical examination suggest an endocrinologic origin. Initial testing should include serum testosterone and FSH. Specific abnormalities in these hormones should prompt additional testing, including serum LH and prolactin. Elevated FSH and LH levels and low testosterone levels (hypergonadotropic or primary hypogonadism) are associated with primary testicular failure. Low FSH and LH associated with low testosterone (hypogonadotropic or secondary hypogonadism) may be of hypothalamic or pituitary origin. Elevation of serum prolactin may indicate the presence of prolactinoma.
C. Genetic Testing
Genetic testing should be considered after thorough counseling and with shared decision making. Men with sperm concentrations less than 10 million/mL should consider testing for Y chromosome microdeletions and karyotypic abnormalities. Gene deletions from the long arm of the Y chromosome may cause azoospermia or oligozoospermia with age-related decline in spermatogenesis that is transmissible to male offspring. Karyotyping may reveal Klinefelter syndrome. Partial or complete absence of the vasa deferentia should prompt testing for gene mutations associated with cystic fibrosis.
D. Imaging
Scrotal ultrasound can aid in characterizing the testes and may detect a testicular mass or a subclinical varicocele. Men with low ejaculate volume and no evidence of retrograde ejaculation should undergo transrectal ultrasound to evaluate the prostate and seminal vesicles. MRI of the sella turcica should be performed in men with elevated prolactin or hypogonadotropic hypogonadism to evaluate the anterior pituitary gland. MRI of the pelvis and scrotum should be considered in men for whom the testes cannot be identified in the scrotum by physical examination or ultrasound. Men with unilateral absence of the vas deferens should have abdominal imaging to exclude absence of the ipsilateral kidney.
 Treatment
Treatment
A. General Measures
Education about intercourse timing in relation to the woman’s ovulatory cycle as well as the avoidance of spermicidal lubricants should be discussed. In cases of gonadotoxic exposure or medication-related factors, the offending agent should be removed whenever feasible. Patients with active genitourinary tract infections should be treated with appropriate antibiotics. Healthy lifestyle habits, including diet, exercise, and avoidance of gonadotoxins (tobacco, excessive alcohol, and marijuana), should be reinforced.
B. Varicocele
Varicocelectomy is performed to stop retrograde blood flow in abnormal spermatic cord veins. Surgical ligation, which is accomplished via a subinguinal incision with the aid of a surgical microscope and Doppler ultrasound, is the gold standard approach given its high success and low complication rates. Percutaneous venographic embolization of varicoceles is feasible but incurs both radiation and intravenous contrast exposure.
C. Endocrine Therapy
Hypogonadotropic hypogonadism may be treated with human chorionic gonadotropin (2000 international units intramuscularly three times a week) once primary pituitary disease has been excluded or treated. If sperm concentration fails to rise after 12 months, recombinant FSH therapy may be initiated (150 international units subcutaneously three times a week).
D. Ejaculatory Dysfunction Therapy
Patients with retrograde ejaculation may benefit from alpha-adrenergic agonists (pseudoephedrine, 60 mg orally three times a day) or imipramine (25 mg orally three times a day). Medical failures may require the collection of post-ejaculation urine for intrauterine insemination. Anejaculation can be treated with vibratory stimulation or electroejaculation in select cases.
E. Ductal Obstruction
Obstruction of the vas deferens after vasectomy may be successfully managed by microsurgical vasectomy reversal or by surgical sperm retrieval in combination with in vitro fertilization and intracytoplasmic sperm injection.
F. Assisted Reproductive Techniques
Intrauterine insemination and in vitro fertilization (with or without intracytoplasmic sperm injection) are alternatives for patients in whom other means of treating reduced sperm concentration, motility, or functionality have failed. Intrauterine insemination should be performed only when adequate numbers of motile sperm are noted in an ejaculate sample (generally, greater than 10 million motile sperm). With the use of intracytoplasmic sperm injection, some men with azoospermia may still initiate a pregnancy by surgical retrieval of sperm from the testicle, epididymis, or vas deferens.
 When to Refer
When to Refer
• Couples with infertility or who are concerned about their fertility potential.
• Men with known genital insults (cryptorchidism, varicocele) or genetic diagnoses (cystic fibrosis, Klinefelter syndrome) that preclude natural fertility.
• Reproductive-aged men with newly diagnosed cancer or other disease that may require cytotoxic therapies with interest in fertility preservation.
Burnett AL et al. Erectile dysfunction: AUA guideline. J Urol. 2018 Sep;200(3):633–41. [PMID: 29746858]
Duca Y et al. Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin Pharmacother. 2019 Jan;20(1):55–67. Erratum in: Expert Opin Pharmacother. 2019 Jan;20(1):ix. [PMID: 30407872]
Esteves SC et al. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat Rev Urol. 2018 Sep;15(9):535–62. [PMID: 29967387]
Oehninger S et al. Limits of current male fertility testing. Fertil Steril. 2019 May;111(5):835–41. [PMID: 30975387]
Okutman O et al. Genetic evaluation of patients with non-syndromic male infertility. J Assist Reprod Genet. 2018 Nov;35(11):1939–51. [PMID: 30259277]
Rajanahally S et al. The relationship between cannabis and male infertility, sexual health, and neoplasm: a systematic review. Andrology. 2019 Mar;7(2):139–47. [PMID: 30767424]
BENIGN PROSTATIC HYPERPLASIA
ESSENTIALS OF DIAGNOSIS

 Obstructive or irritative voiding symptoms.
Obstructive or irritative voiding symptoms.
 May have enlarged prostate on rectal examination.
May have enlarged prostate on rectal examination.
 Absence of urinary tract infection, neurologic disorder, stricture disease, prostatic or bladder malignancy.
Absence of urinary tract infection, neurologic disorder, stricture disease, prostatic or bladder malignancy.
 General Considerations
General Considerations
Benign prostatic hyperplasia is the most common benign tumor in men, and its incidence is age related. The prevalence of histologic benign prostatic hyperplasia in autopsy studies rises from approximately 20% in men aged 41–50 years, to 50% in men aged 51–60, and to over 90% in men over 80 years of age. Although clinical evidence of disease occurs less commonly, symptoms of prostatic obstruction are also age related. At age 55 years, approximately 25% of men report obstructive voiding symptoms. At age 75 years, 50% of men report a decrease in the force and caliber of the urinary stream.
Risk factors for the development of benign prostatic hyperplasia are poorly understood. Some studies have suggested a genetic predisposition and some have noted racial differences. Approximately 50% of men under age 60 years who undergo surgery for benign prostatic hyperplasia may have a heritable form of the disease. This form is most likely an autosomal dominant trait, and first-degree male relatives of such patients carry an increased relative risk of approximately fourfold.
 Clinical Findings
Clinical Findings
A. Symptoms
The symptoms of benign prostatic hyperplasia can be divided into obstructive and irritative complaints. Obstructive symptoms include hesitancy, decreased force and caliber of the stream, sensation of incomplete bladder emptying, double voiding (urinating a second time within 2 hours), straining to urinate, and postvoid dribbling. Irritative symptoms include urgency, frequency, and nocturia.
The American Urological Association (AUA) symptom index (Table 23–3) is perhaps the single most important tool used in the evaluation of patients with this disorder and should be calculated for all patients before starting therapy. The answers to seven questions quantitate the severity of obstructive or irritative complaints on a scale of 0–5. Thus, the score can range from 0 to 35, in increasing severity of symptoms.
Table 23–3. American Urological Association symptom index for benign prostatic hyperplasia.1
A detailed history focusing on the urinary tract should be obtained to exclude other possible causes of symptoms such as prostate cancer or disorders unrelated to the prostate such as urinary tract infection, neurogenic bladder, or urethral stricture.
B. Signs
A physical examination, digital rectal examination (DRE), and a focused neurologic examination should be performed on all patients. The size and consistency of the prostate should be noted, but prostate size does not correlate with the severity of symptoms or the degree of obstruction. Benign prostatic hyperplasia usually results in a smooth, firm, elastic enlargement of the prostate. Induration, if detected, must alert the clinician to the possibility of cancer, and further evaluation is needed (ie, prostate-specific antigen [PSA] testing, transrectal ultrasound, and biopsy). Examination of the lower abdomen should be performed to assess for a distended bladder.
C. Laboratory Findings
Urinalysis should be performed to exclude infection or hematuria. Clinicians should consider obtaining a serum PSA test, particularly in patients whose life expectancy is longer than 10 years. PSA certainly increases the ability to detect prostate cancer over DRE alone; however, because there is much overlap between levels seen in benign prostatic hyperplasia and prostate cancer, its use remains controversial (see Chapter 39).
D. Imaging
Upper tract imaging (CT or renal ultrasound) is recommended only in the presence of concomitant urinary tract disease or complications from benign prostatic hyperplasia (ie, hematuria, urinary tract infection, chronic kidney disease, history of stone disease). As included in the Choosing Wisely (ABIM Foundation) initiative, neither serum creatinine nor imaging (ultrasound, CT, MRI) should be routinely ordered for patients with benign prostatic hyperplasia.
E. Cystoscopy
Cystoscopy is not recommended to determine the need for treatment but may assist in determining the surgical approach in patients opting for invasive therapy.
F. Additional Tests
Cystometrograms and urodynamic profiles should be reserved for patients with suspected neurologic disease or those who have failed prostate surgery. Flow rates, postvoid residual urine determination, and pressure-flow studies are considered optional.
 Differential Diagnosis
Differential Diagnosis
A history of prior urethral instrumentation, urethritis, sexually transmitted infections, or trauma should be elucidated to exclude urethral stricture or bladder neck contracture. Hematuria and pain are commonly associated with bladder stones. Carcinoma of the prostate may be detected by abnormalities on DRE or an elevated PSA (see Chapter 39). A urinary tract infection can mimic the irritative symptoms of benign prostatic hyperplasia and can be readily identified by urinalysis and culture; however, a urinary tract infection can also be a complication of benign prostatic hyperplasia. Carcinoma of the bladder, especially carcinoma in situ, may also present with irritative voiding complaints; however, urinalysis usually shows evidence of hematuria (see Chapter 39). Patients with a neurogenic bladder may also have many of the same symptoms and signs as those with benign prostatic hyperplasia; however, a history of neurologic disease, stroke, diabetes mellitus, or back injury may be obtained, and diminished perineal or lower extremity sensation or alterations in rectal sphincter tone or the bulbocavernosus reflex might be observed on examination. Simultaneous alterations in bowel function (constipation) might also suggest the possibility of a neurologic disorder.
 Treatment
Treatment
Clinical practice guidelines exist for the evaluation and treatment of patients with benign prostatic hyperplasia. Following evaluation as outlined above, patients should be offered various forms of therapy for benign prostatic hyperplasia. Patients are advised to consult with their primary care clinicians and make an educated decision on the basis of the relative efficacy and side effects of the treatment options (Table 23–4).
Table 23–4. Summary of benign prostatic hyperplasia treatment outcomes.1

Patients with mild symptoms (AUA scores 0–7) should be managed by watchful waiting only. Medical therapy is appropriate for many others. Absolute surgical indications include refractory urinary retention (failing at least one attempt at catheter removal), large bladder diverticula, or any of the following sequelae of benign prostatic hyperplasia: recurrent urinary tract infection, recurrent gross hematuria, bladder stones, or chronic kidney disease.
A. Watchful Waiting
The risk of progression or complications is uncertain. However, in men with symptomatic disease, it is clear that progression is not inevitable and that some men undergo spontaneous improvement or resolution of their symptoms.
Retrospective studies on the natural history of benign prostatic hyperplasia are inherently subject to bias, relating in part to patient selection and also to the type and extent of follow-up. Very few prospective studies addressing the natural history have been reported. One small series demonstrated that approximately 10% of symptomatic men may progress to urinary retention while 50% of patients demonstrate marked improvement or resolution of symptoms. A large randomized study compared finasteride with placebo in men with moderate to severely symptomatic disease and enlarged prostates on DRE. Patients in the placebo arm demonstrated a 7% risk of developing urinary retention over 4 years.
Men with moderate or severe symptoms can also be observed if they so choose. The optimal interval for follow-up is not defined, nor are the specific end points for intervention.
B. Medical Therapy
1. Alpha-blockers—Alpha-blockers can be classified according to their receptor selectivity (Table 23–5) as well as their half-life.
Table 23–5. Alpha-blockade for benign prostatic hyperplasia.

Prazosin is an effective short-acting, nonselective alpha-blocker; however, it requires dose titration and twice daily dosing. Typical side effects include orthostatic hypotension, dizziness, tiredness, retrograde ejaculation, rhinitis, and headache.
Long-acting, nonselective alpha-blockers allow for once-a-day dosing, but dose titration is still necessary because side effects similar to those seen with prazosin may occur. Terazosin improves symptoms and in numerous studies is superior to placebo or finasteride. Terazosin is started at a dosage of 1 mg orally daily for 3 days, increased to 2 mg orally daily for 11 days, then 5 mg orally daily. Additional dose escalation to 10 mg orally daily can be performed if necessary. Doxazosin is started at a dosage of 1 mg orally daily for 7 days, increased to 2 mg orally daily for 7 days, then 4 mg orally daily. Additional dose escalation to 8 mg orally daily can be performed if necessary.
Alpha-1a-receptors are localized to the prostate and bladder neck. Selective blockade of these receptors results in fewer systemic side effects than nonselective alpha-blocker therapy (orthostatic hypotension, dizziness, tiredness, rhinitis, and headache), thus obviating the need for dose titration. The typical dose of tamsulosin is 0.4 mg orally daily taken 30 minutes after a meal. Alfuzosin is a long-acting alpha-1a-blocker; its dose is 10 mg orally once daily with food and does not require titration. Several randomized, double-blind, placebo-controlled trials have been performed comparing terazosin, doxazosin, tamsulosin, and alfuzosin with placebo. All agents have demonstrated safety and efficacy. Floppy iris syndrome, a complication of cataract surgery, can occur in patients taking alpha-blockers and alpha-1a-blockers.
2. 5-alpha-reductase inhibitors—Finasteride and dutasteride block the conversion of testosterone to dihydrotestosterone. These medications impact the epithelial component of the prostate, resulting in reduction in size of the gland and improvement in symptoms. Six months of therapy is required for maximum effects on prostate size (20% reduction) and symptomatic improvement.
Several randomized, double-blind, placebo-controlled trials have been performed comparing finasteride with placebo. Efficacy, safety, and durability are well established. However, symptomatic improvement is seen only in men with enlarged prostates (greater than 40 mL by ultrasonographic examination). Side effects include decreased libido, decrease in volume of ejaculate, and erectile dysfunction. Serum PSA is reduced by approximately 50% in patients receiving finasteride therapy, but the % free PSA is unchanged. Therefore, in order to compare with pre-finasteride PSA levels, the serum PSA of a patient taking finasteride should be doubled.
A report suggests that finasteride therapy may decrease the incidence of urinary retention and the need for operative treatment in men with enlarged prostates and moderate to severe symptoms. The larger the prostate over 40 mL, the greater the relative-risk reduction. However, optimal identification of appropriate patients for prophylactic therapy remains to be determined. Dutasteride is a dual 5-alpha-reductase inhibitor that appears to be similar to finasteride in its effectiveness; its dose is 0.5 mg orally daily.
Both finasteride and dutasteride have been shown to be effective chemopreventive agents for prostate cancer in large, randomized clinical trials. A 25% risk reduction was observed in men with both low and high risk for prostate cancer. However, despite the strength of the evidence for 5-alpha-reductase inhibitors in reducing the risk of prostate cancer, an FDA advisory committee recommended against labeling these agents for prostate cancer chemoprevention, citing the potential increased risk of high-grade tumors in these studies (1.8% vs 1.0% for finasteride and 1% vs 0.5% for dutasteride), isolated risk reduction in low-grade tumors, and inability to apply the findings to the general population. Moreover, the FDA has included the increased risk of being diagnosed with high-grade prostate cancer in the labels of all 5-alpha-reductase inhibitors.
3. Phosphodiesterase-5 inhibitor—Tadalafil is approved by the FDA to treat the signs and symptoms of benign prostatic hyperplasia (Table 23-5); it is also approved for use in men with both urinary symptoms and erectile dysfunction. The data from two randomized, double-blind, placebo-controlled trials demonstrated significant improvements in standardized measurements of urinary function between 2 and 4 weeks after initiating treatment at 5 mg once daily, with minimal adverse effects.
4. Combination therapy—The Medical Therapy of Prostatic Symptoms (MTOPS) trial was a large, randomized, placebo-controlled trial comparing finasteride, doxazosin, the combination of the two, and placebo in 3047 men observed for a mean of 4.5 years. Long-term combination therapy with doxazosin and finasteride was safe and reduced the risk of overall clinical progression of benign prostatic hyperplasia significantly more than did treatment with either medication alone. Combination therapy and finasteride alone reduced the long-term risk of acute urinary retention and the need for invasive therapy. Combination therapy had the risks of additional side effects and the cost of two medications.
C. Conventional Surgical Therapy
1. Transurethral resection of the prostate (TURP)—Over 95% of prostate surgeries can be performed endoscopically (through the urethra). TURP is the gold standard treatment for surgical treatment of benign prostatic hyperplasia, and it often requires a 1- to 2-day hospital stay. While there are only a few head-to-head surgical studies comparing TURP to minimally invasive therapies, most studies show symptom scores and flow rate improvements are superior following TURP compared to any minimally invasive therapy. The risks of TURP include retrograde ejaculation (75%), erectile dysfunction (less than 5%), and urinary incontinence (less than 1%). Potential complications include (1) bleeding; (2) urethral stricture or bladder neck contracture; (3) perforation of the prostate capsule with extravasation; and (4) transurethral resection syndrome, a hypervolemic, hyponatremic state resulting from absorption of the hypotonic irrigating solution. This syndrome was much more prevalent when TURPs were most often performed with monopolar electrocautery but, with the increased use of bi-polar TURPs (using saline irrigation), it is now very rare. Clinical manifestations of the syndrome include nausea, vomiting, confusion, hypertension, bradycardia, and visual disturbances. The risk of transurethral resection syndrome increases with monopolar resection times over 90 minutes. Treatment includes diuresis and, in severe cases, hypertonic saline administration (see Hyponatremia, Chapter 21).
2. Transurethral incision of the prostate (TUIP)—Men with moderate to severe symptoms and small prostates often have posterior commissure hyperplasia or an “elevated bladder neck.” These patients will often benefit from incision of the prostate. The procedure is more rapid and less morbid than TURP. Outcomes in well-selected patients are comparable, though a lower rate of retrograde ejaculation has been reported (25%).
3. Simple prostatectomy (open/robotic)—When the prostate is very large, open or robotic enucleation is considered. What size is “too large” depends upon the surgeon’s experience with TURP. Glands over 100 g are usually considered for enucleation. In addition to size, other relative indications for open prostatectomy include concomitant bladder diverticulum or bladder stone and whether dorsal lithotomy positioning is or is not possible.
Simple prostatectomies can be performed with either a suprapubic or retropubic approach. Simple suprapubic prostatectomy is performed transvesically and is the operation of choice if there is concomitant bladder pathology (eg, bladder stones). These operations can also be performed via robotic-assisted laparoscopic techniques with shorter hospital stays, less blood loss, and decreased need for a suprapubic catheter.
D. Minimally Invasive Therapy
Many minimally invasive therapies are in phase 1 or 2 clinical trials; while some show promise, they have not yet received FDA approval and are not yet on the market. There has been a significant shift toward more minimally invasive therapies due to both cost of care and better data from 3- to 5-year (or more) outcomes. In addition, studies have shown decreased cost compared to medical therapies in as short as 6 months (or as long as 8 years). An overview of all the surgical options and decision making was published by the American Urological Association (Figure 23–2).

Figure 23–2. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia. HoLEP, holmium laser enucleation of the prostate; PUL, prostatic urethral lift; PVP, photoselective vaporization of the prostate; ThuLEP, thulium laser enucleation of the prostate; TUIP, transurethral incision of the prostate; TUMT, transurethral microwave therapy; TURP, transurethral resection of the prostate; TUVP, transurethral vaporization of the prostate. (Reproduced, with permission, from Foster HE et al; Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline Amendment 2019. J Urol. 2019 Sep;202(3):592–8.)
1. Laser therapy—Several coagulation necrosis techniques have been used.
Most urologists prefer to use visually directed laser techniques. Visual coagulative necrosis is performed under cystoscopic control, and the laser fiber is pulled through the prostate at several designated areas depending on the size and configuration of the gland. Coagulative techniques do not create an immediate visual defect in the prostatic urethra—tissue is sloughed over the course of several weeks up to 3 months following the procedure.
Visual contact ablative techniques take longer in the operating room because the laser fiber is placed in direct contact with the prostate tissue, which is vaporized. Photovaporization of the prostate (PVP), an alternative laser technique, uses a high-power KTP laser. An immediate defect is obtained in the prostatic urethra, similar to that seen during TURP.
Interstitial laser therapy places laser fibers directly into the prostate, usually under cystoscopic control. Irritative voiding symptoms may be less in these patients as the urethral mucosa is spared and prostate tissue is resorbed by the body rather than sloughed.
Holmium laser enucleation of the prostate (HoLEP) is a technique of enucleating the adenomatous lobes intact and morcellating the tissue within the bladder. Advantages of HoLEP compared with other methods include ability to treat all prostate sizes, low re-treatment rates, few complications, and shorter duration of catheterization.
Advantages to laser surgery include minimal blood loss, rarity of transurethral resection syndrome, ability to treat patients during anticoagulant therapy, and outpatient surgery. Disadvantages are the lack of tissue for pathologic examination, longer postoperative catheterization time, variable effectiveness, more frequent irritative voiding complaints, and expense of laser fibers and generators.
2. Transurethral needle ablation of the prostate (TUNA)—This procedure uses a specially designed urethral catheter that is passed into the urethra. Interstitial radiofrequency needles are then deployed from the tip of the catheter, piercing the mucosa of the prostatic urethra. Radiofrequencies are then used to heat the tissue, resulting in coagulative necrosis. Subjective and objective improvement in voiding occurs. In randomized trials comparing TUNA to TURP, similar improvement was seen when comparing life scores, peak urinary flow rates, and postvoid residual urine. Bladder neck and median lobe enlargement are not well treated by TUNA.
3. Transurethral electrovaporization of the prostate—This technique uses the standard resectoscope. High current densities result in heat vaporization of tissue, creating a cavity in the prostatic urethra. This procedure usually takes longer than a standard TURP. Long-term comparative data are needed.
4. Hyperthermia—Microwave hyperthermia is most commonly delivered with a transurethral catheter. Some devices cool the urethral mucosa to decrease the risk of injury. However, if temperatures do not go above 45°C, cooling is unnecessary. Symptom score and flow rate improvement are obtained, but (as with laser surgery) large randomized studies with long-term follow-up are needed to assess durability and cost-effectiveness.
5. Implant to open prostatic urethra (UroLift)—A minimally invasive, FDA-approved implant can be used to retract the enlarged lobes of the prostate in symptomatic men age 50 years and older with an enlarged prostate (less than 80 g). This implant staples the prostatic lobes to open the prostatic urethral lumen. This is done in the clinic setting with local analgesia. Short-term data show improved symptoms and voiding flows with minimal impact on erectile or ejaculatory function. Long-term comparative data are needed.
6. Water vapor thermal therapy (Rezum)—This minimally invasive, FDA-approved technique uses a transurethral device to deliver steam into the prostatic tissue and thermally destroy it. This procedure is done in the clinic or ambulatory surgery setting with 3-year data showing improvement in symptoms and voiding flows with minimal impact on erectile or ejaculatory function compared with medical therapy.
 When to Refer
When to Refer
• Progression to urinary retention.
• Patient dissatisfaction with medical therapy.
• Need for surgical intervention or further evaluation (cystoscopy).
Barry MJ et al; Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 2017 Feb;197(2S):S189–97. [PMID: 28012747]
DeWitt-Foy ME et al. Cost comparison of benign prostatic hyperplasia treatment options. Curr Urol Rep. 2019 Jun 19;20(8):45. [PMID: 31218458]
Foster HE et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2019. J Urol. 2019 Sep;202(3):592–8. [PMID: 31059668]
McVary KT et al. Three-year outcomes of the prospective, randomized controlled Rezüm System study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2018 Jan;111:1–9. [PMID: 29122620]
Pattanaik S et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2018 Nov 16;11:CD010060. [PMID: 30480763]
Sun F et al. Transurethral procedures in the treatment of benign prostatic hyperplasia: a systematic review and meta-analysis of effectiveness and complications. Medicine (Baltimore). 2018 Dec;97(51):e13360. [PMID: 30572440]
Villa L et al. Silodosin: an update on efficacy, safety and clinical indications in urology. Adv Ther. 2019 Jan;36(1):1–18. [PMID: 30523608]
CANCERS OF THE GENITOURINARY TRACT
(See Chapter 39 for Cancers of the Genitourinary Tract: Prostate Cancer, Bladder Cancer, Cancers of the Ureter & Renal Pelvis, Renal Cell Carcinoma, & Testicular Cancers.)