21
Electrolyte & Acid-Base Disorders
Nayan Arora, MD
J. Ashley Jefferson, MD
ASSESSMENT OF THE PATIENT
The pathophysiology of all electrolyte disorders is rooted in basic principles of total body water and its distribution across fluid compartments. The optimal evaluation and treatment of fluid and electrolyte disorders requires a careful interpretation of serum and urine chemistries in conjunction with a thorough history and physical examination. While classic teaching has focused on physical examination to determine a patient’s volume status, such an approach can be challenging because of limitations in accurate bedside analysis of volume.
A. Body Water and Fluid Distribution
Total body water depends on the relative proportions of muscle and fat in the body. Total body water is typically estimated as 50% of body weight in women and 60% in men, as women, on average, have a higher proportion of fat to body weight (Table 21–1). Total body water also tends to decrease with age due to declining muscle mass. Approximately two-thirds of total body water is located in the intracellular compartment and one-third is located in the extracellular compartment. The extracellular compartment is further divided into the interstitial fluid volume (15% of body weight) and the plasma fluid volume (5% of body weight). Changes in total body water content are best evaluated by documenting changes in body weight. Extracellular volume (ECV) may be assessed by physical examination (eg, blood pressure, pulse, jugular venous distention, edema). Quantitative assessments of ECV and intravascular volume may be invasive (ie, central venous pressure or pulmonary wedge pressure) or noninvasive (ie, inferior vena cava diameter and right atrial pressure by echocardiography). Intracellular volume (ICV) is assessed using the serum sodium concentration.
Table 21–1. Total body water (as percentage of body weight) in relation to age and sex.

B. Serum Electrolytes
In health, serum electrolytes are maintained within a narrow range by the kidneys (homeostasis). The serum level of an individual electrolyte may be normal, elevated, or decreased, but may not correlate with the total body levels of that electrolyte due to shift of water or electrolytes into and out of cells.
C. Evaluation of Urine
The urine concentration of an electrolyte is helpful to determine whether the kidney is excreting or retaining the electrolyte in response to high or low serum levels. A 24-hour urine collection for daily electrolyte excretion remains the gold standard for assessment of renal electrolyte handling; however, it can be cumbersome, as well as technically challenging in certain patients. A more convenient method to determine renal electrolyte handling is the use of fractional excretion (Fe) of an electrolyte X (Fex) calculated from a spot urine sample and serum sample, using creatinine (Cr):
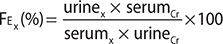
A low fractional excretion indicates renal reabsorption (electrolyte retention), while a high fractional excretion indicates renal wasting (electrolyte excretion). Thus, the fractional excretion helps the clinician determine whether the kidney’s response is appropriate for a specific electrolyte disorder.
D. Serum Osmolality
Total solute concentration is measured by osmolality in millimoles per kilogram. Osmolarity is measured in millimoles of solute per liter of solution. The terms are often used interchangeably in clinical medicine. Plasma osmolality is the total concentration of all the solutes contained in plasma, both electrolytes and nonelectrolytes, and normally ranges between 285 mmol/L and 295 mmol/L. Differences in osmole concentration across cell membranes lead to movement of water to the region of higher osmolality, stimulation of thirst, and secretion of antidiuretic hormone (ADH). Substances that easily permeate cell membranes (eg, urea, ethanol) are ineffective osmoles and do not cause fluid shifts across fluid compartments.
Serum osmolality (Osm) can be estimated using the following formula:
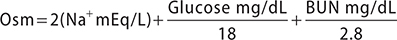
(Note: dividing urea by 2.8 converts mg/dL to mmol/L; dividing glucose by 18 converts mg/dL to mmol/L)
Sodium is the major extracellular cation; doubling the serum sodium in the formula for estimated osmolality accounts for corresponding anions. A discrepancy between measured and estimated osmolality of greater than 10 mmol/kg suggests an osmolal gap, which is the presence of unmeasured osmoles such as ethanol, methanol, isopropanol, and ethylene glycol (see Table 38–5).
DISORDERS OF SODIUM CONCENTRATION
HYPONATREMIA
ESSENTIALS OF DIAGNOSIS

 Must know volume status as well as serum and urine osmolality to determine etiology.
Must know volume status as well as serum and urine osmolality to determine etiology.
 Hyponatremia usually reflects excess water retention rather than sodium deficiency. The serum sodium concentration is not a measure of total body sodium.
Hyponatremia usually reflects excess water retention rather than sodium deficiency. The serum sodium concentration is not a measure of total body sodium.
 The administration of hypotonic fluids commonly cause hyponatremia in hospitalized patients.
The administration of hypotonic fluids commonly cause hyponatremia in hospitalized patients.
 General Considerations
General Considerations
Hyponatremia is defined as a serum sodium concentration less than 135 mEq/L (135 mmol/L) and is the most common electrolyte abnormality encountered in clinical practice. Hyponatremia represents an excess of water relative to sodium in the plasma leading to a reduction in plasma osmolality and subsequent movement of water from the extracellular fluid into the intracellular fluid. Acutely, this movement of water can result in cerebral edema, increasing the risk of seizures and even brain herniation.
Chronic hyponatremia is often asymptomatic or present with mild confusion, nausea, or falls. In these patients, cerebral adaptation has occurred as the brain cells have excreted intracellular osmoles to limit cell swelling. In this setting, over-rapid correction of chronic hyponatremia may produce profound neurologic abnormalities (osmotic demyelination syndrome).
A common misconception is that hyponatremia is secondary to a deficiency in total body sodium, when in reality it reflects an excess of total body water in the vast majority of cases. The basic pathophysiologic principle is the ingestion of water (oral or intravenous) in excess of the amount the kidney can excrete, commonly, but not always, due to the presence of ADH. A diagnostic algorithm using serum osmolality, urine sodium, and volume status separates the causes of hyponatremia into therapeutically useful categories (Figure 21–1).

Figure 21–1. A diagnostic algorithm for the causes of hyponatremia using serum osmolality, urine osmolality, and urine sodium. ADH, antidiuretic hormone; GFR, glomerular filtration rate; SIADH, syndrome of inappropriate antidiuretic hormone.
 Etiology
Etiology
A. Isotonic and Hypertonic Hyponatremia
Hyponatremia is typically associated with hypoosmolality with two exceptions: pseudohyponatremia and hypertonic hyponatremia.
1. Pseudohyponatremia—This represents a laboratory artifact that rarely occurs in patients with marked hypertriglyceridemia or hypergammaglobulinemia. In these settings, there is an increase in the solid components of plasma, relative to plasma water, resulting in a lower sodium per given volume. This issue is becoming less prevalent as most laboratories are now using direct ion selective electrodes without blood dilution. Consultation with the clinical laboratory is necessary if this condition is suspected.
2. Hypertonic hyponatremia—The best clinical examples of this situation are hyperglycemia, and less commonly, mannitol infusion. Both glucose and mannitol are active osmoles, increasing the osmolality of the extracellular fluid, which pulls water from inside cells into the extracellular space. Note, this leads to a reduction in intracellular volume, and cerebral edema is not caused by hyponatremia in this setting; however, cerebral edema may occur in the treatment phase due to over-rapid correction of hyperglycemia and injudicious intravenous fluids. The increased tonicity will also stimulate thirst and vasopressin release, further contributing to water retention. To determine whether the hyponatremia can be entirely attributed to hyperglycemia, a sodium correction factor is often used. Many guidelines recommend using a decrease in the serum sodium concentration of 1.6 mEq/L (or 1.6 mmol/L) for every 100 mg/dL (5.5 mmol/L) rise in plasma glucose above normal.
B. Hypotonic Hyponatremia
Most cases of hyponatremia are hypotonic, highlighting sodium’s role as the predominant extracellular osmole. The presence of hypotonic hyponatremia indicates that water intake exceeds the excretional capacity of the kidney. The next step is to classify hypotonic cases as ADH dependent or independent judged by the kidney’s ability to excrete dilute urine.
1. ADH-independent causes—In rare circumstances, hypotonic hyponatremia can occur when the kidney’s ability to excrete free water is intact (urine osmolality less than 100 mOsm/kg).
a. Psychogenic polydipsia—This condition develops where excess water intake overwhelms the kidney’s capacity to excrete adequate dilute urine. Patients will have appropriately suppressed ADH, reflected by a urine osmolality less than 100 mOsm/kg. Polydipsia occurs primarily in patients with psychiatric disorders. Psychiatric medications may also interfere with water excretion or increase thirst through anticholinergic side effects, further increasing water intake.
b. Beer potomania and the “tea and toast” diet—Malnourished patients who consume a low protein diet with large quantities of beer (beer potomania) or those who consume a low protein (tea and toast) diet may have a marked reduction in free water excretion due to insufficient dietary solute intake. The kidney’s ability to excrete free water is dependent not only on ADH suppression, but also on solute delivery to the distal tubules. With a typical Western diet generating 1000 mOsm of solute per day, normal kidneys can dilute urine to 50 mOsm/kg, allowing a maximum urine volume of 20 L. By contrast, with a low protein diet generating 200 mOsm per day, urinary output would be limited to a maximum of 4 L per day.
c. Renal impairment—Patients with advanced renal impairment (GFR less than 15 mL/min/1.73 m2), whether due to severe chronic kidney disease (CKD) or acute kidney injury, may be unable to dilute their urine (can often only achieve a minimum urine osmolality of 200–250 mOsm/L even with maximal ADH suppression) and are prone to develop water retention and hyponatremia. This can occur in the setting of normal or high plasma osmolality due to accumulation of urea. This differs from hypertonic hyponatremia because urea is an ineffective osmole and is freely permeable across cell membranes, only minimally pulling water into the plasma space.
2. ADH-dependent causes—The most common cause of hypotonic hyponatremia involves a failure to suppress ADH action. This can either be appropriate in the setting of hypovolemia, or a reduced effective arterial volume secondary to cirrhosis or heart failure (hypervolemia), or inappropriate, in the absence of hypovolemia or edematous states, which is known as the syndrome of inappropriate ADH secretion (SIADH).
a. Hypovolemic hyponatremia—Hypovolemic hyponatremia occurs with renal or extrarenal volume loss (sodium and water) and subsequent hypotonic fluid replacement (Figure 21–1). The reduced blood pressure results in an increase in ADH secretion by the pituitary, limiting free water excretion. In this setting, the body sacrifices serum osmolality to preserve intravascular volume.
Cerebral salt wasting is a rare subset of hypovolemic hyponatremia that occurs with intracranial disease (eg, infections, cerebrovascular accidents, tumors, and neurosurgery). Clinical features include refractory hypotension, often in the setting of continuous infusion of isotonic or hypertonic saline. The pathophysiology is unclear but has been attributed to renal sodium wasting, although there is uncertainty as to whether cerebral salt wasting represents a distinct entity or SIADH with desalination of the administered saline.
b. Hypervolemic hyponatremia—Hypervolemic hyponatremia commonly occurs in the edematous states of cirrhosis and heart failure, and rarely in nephrotic syndrome (Figure 21–1). In these settings, a decreased effective arterial blood volume (typically low blood pressure) occurs despite an overall increase in extracellular volume (edema) resulting in ADH secretion. In cirrhosis and heart failure, effective circulating volume is decreased due to systemic vasodilation and reduced cardiac output, respectively.
c. SIADH—In this condition, ADH is secreted in the absence of an appropriate physiologic stimuli such as a decreased effective circulating volume or hyperosmolality. The major causes of SIADH (Table 21–2) are disorders affecting the central nervous system or the lungs (such as cancer or infections) and medications. SIADH is a diagnosis of exclusion, which involves ruling out other causes of hyponatremia (eg, low effective circulating volume, decreased solute intake, cortisol deficiency, and severe hypothyroidism). SIADH is a clinical diagnosis characterized by (1) hyponatremia; (2) decreased plasma osmolality (less than 280 mOsm/kg); (3) absence of heart, kidney, or liver disease; (4) normal thyroid and adrenal function (see Chapter 26); and (5) urine sodium usually over 20 mEq/L. Patients with SIADH may have low blood urea nitrogen (BUN) (less than 10 mg/dL [3.6 mmol/L]) and hypouricemia (less than 4 mg/dL [238 mcmol/L]), which are not only dilutional but result from increased urea and uric acid clearances in response to the volume-expanded state.
Table 21–2. Common causes of syndrome of inappropriate ADH secretion (SIADH).
Central nervous system disorders
Stroke
Hemorrhage
Infection
Trauma
Inflammatory and demyelinating diseases
Pulmonary lesions
Infections (viral, bacterial, fungal)
Malignancies
Many but particularly small cell carcinoma of the lung
Drugs (this is only a partial list as many have been implicated)
Antidepressants: SSRIs, tricyclics, monoamine oxidase inhibitors
Antineoplastics: cyclophosphamide, ifosfamide, methotrexate
Anticonvulsants: carbamazepine, sodium valproate, lamotrigine
Neuroleptics: haloperidol, fluphenazine, trifluoperazine thioridazine, thiothixene
NSAIDs
Methylenedioxymethamphetamine (MDMA; Ecstasy)
Amiodarone
Ciprofloxacin
Opiates
Others
HIV
Pain, postoperative, stress
Hereditary
Idiopathic
NSAIDs, nonsteroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
d. Reset osmostat—This is a rare cause of hyponatremia in which patients regulate vasopressin release around a lower, or hypotonic, set point. Diagnosis involves documentation of dilute urine when serum sodium is lowered by administration of free water, however this is rarely done in clinical practice. The mild hypo-osmolality of pregnancy is a form of reset osmostat.
e. Adrenal insufficiency and hypothyroidism—Cortisol normally provides a negative feedback on ADH release, and therefore cortisol deficiency can lead to uninhibited ADH and hyponatremia. Concomitant mineralocorticoid deficiency may result in hyperkalemia and metabolic acidosis. Hyponatremia due to hypothyroidism only occurs in the context of myxedema coma. Although the exact mechanism is unknown, it may be related to a low cardiac output state and thus decreased effective arterial blood volume.
f. Nausea, pain, and surgery—Nausea and pain are potent stimulators of ADH release. Severe hyponatremia can develop after elective surgery in healthy patients due to excessive use of hypotonic fluids.
g. Exercise-associated hyponatremia—Hyponatremia during or after exercise, especially endurance events such as triathlons and marathons, may be caused by a combination of excessive hypotonic fluid intake and ADH secretion (due to hypovolemia, pain, or nausea). Current guidelines suggest that endurance athletes drink water according to thirst rather than according to specified hourly rates of fluid intake. Electrolyte-containing sport drinks do not protect against hyponatremia since they are markedly hypotonic relative to serum.
h. Thiazide diuretics and other medication—Thiazides may induce hyponatremia, typically in older patients, within a few weeks of initiating therapy, and may be exacerbated by increased thirst and low solute intake. The mechanism appears to be a combination of water intake and a mild diuretic-induced volume contraction leading to ADH secretion. Loop diuretics do not cause hyponatremia as frequently due to impairment of the medullary concentration gradient, limiting the ability of ADH to promote water retention.
Nonsteroidal anti-inflammatory drugs (NSAIDs) increase ADH by inhibiting prostaglandin formation. Prostaglandins and selective serotonin reuptake inhibitors (eg, fluoxetine, paroxetine, and citalopram) can also cause hyponatremia, especially in geriatric patients. Enhanced secretion or action of ADH may result from increased serotonergic tone.
Use of 3,4-methylenedioxymethamphetamine (MDMA, also known as Ecstasy) can lead to hyponatremia and severe neurologic symptoms, including seizures, cerebral edema, and brainstem herniation. MDMA and its metabolites increase ADH release from the hypothalamus. Polydipsia may contribute to hyponatremia since MDMA users typically increase fluid intake to prevent hyperthermia.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Whether hyponatremia is symptomatic depends on both its severity and acuity. Chronic hyponatremia, defined as lasting longer than 48 hours, is often diagnosed on routine electrolyte measurements; patients are often asymptomatic as the brain has adapted to the surrounding hypotonicity. Subtle abnormalities, such as mild concentration and cognitive deficits, as well as gait disturbances that can lead to falls, may be present. Acute hyponatremia (defined as lasting less than 48 hours) can result in marked neurologic symptoms, even with relatively modest hyponatremia, due to acute brain cell swelling and subsequent rise in intracranial pressure. Early symptoms include headache and decrease in attentiveness, which can lead to lethargy, disorientation, and nausea. The most serious symptoms include marked confusion and decreased levels of consciousness, vomiting, seizures, coma, brainstem herniation, and death.
Clinical evaluation starts with a careful history of medications, changes in fluid intake (polydipsia, anorexia, intravenous fluid rates and composition), and fluid output (nausea and vomiting, diarrhea, ostomy output, polyuria, oliguria, insensible losses). The physical examination should attempt to categorize volume status (see Body Water and Fluid Distribution, above). The next determination is why ADH is being released and conditions in which release may be halted abruptly, which can impact the approach to therapy.
B. Laboratory Findings
The initial laboratory assessment should include serum and urine electrolytes and serum and urine osmolality. In clinical practice ADH levels are not measured and urine osmolality is used as a surrogate for ADH activity. Urine osmolality should be checked not only at the time of diagnosis, but may be useful if checked serially during therapy. Bedside assessment of volume status is often insensitive; therefore, a urine sodium may help differentiate between hypovolemia and euvolemia, particularly in nonedematous patients (Figure 21–1). The etiology of most cases of hyponatremia will be apparent by appropriate interpretation of the above laboratory values, in addition to patient history and assessment of volume status. Additional testing, such as thyroid and adrenal function tests may be warranted in the appropriate context.
 Treatment
Treatment
The initial treatment of hyponatremia is contingent on two primary factors, the acuity of onset and the severity of symptoms. In patients with documented acute hyponatremia, ie, onset within 48 hours, sodium can be corrected at the rate at which it fell. In general, the majority of cases are chronic and therefore need to be corrected more slowly to minimize risk of osmotic demyelination.
Pseudohyponatremia from hypertriglyceridemia or hyperproteinemia requires no therapy except confirmation with the clinical laboratory. Translocational hyponatremia from glucose or mannitol can be managed with glucose correction or mannitol discontinuation (if possible). Patients with hypovolemic hyponatremia require fluid resuscitation with either balanced crystalloid solutions or normal saline. Data from 2018 suggest that lactated Ringer’s solution may be a superior choice for resuscitation over normal saline, particularly in ICU patients. The correction of the volume depletion will remove the stimulus for ADH and permit renal excretion of a dilute urine. Great care must be taken because the rapid renal excretion of the excess water may correct the serum sodium level too quickly.
A. Symptomatic Hyponatremia
If a patient has hyponatremia and severe symptoms (eg, seizures, confusion), then emergent treatment with hypertonic saline should commence regardless of etiology. A relatively small increase in serum sodium of 4–5 mEq/L is generally sufficient to promptly reverse severe neurologic symptoms and decrease intracranial pressure. This can be accomplished most effectively by using boluses of 100 mL of 3% NaCl over 10 minutes, which can be repeated twice if needed. Each bolus might be predicted to raise the serum sodium by 1–2 mEq/L. In patients with less severe symptoms, an intravenous infusion of 3% NaCl (0.5–2 mL/kg/h) may be used.
It cannot be overstated that there is no substitute for frequent laboratory monitoring during therapy for hyponatremia, particularly severe cases, which should occur every 1–2 hours.
B. Osmotic Demyelination and Correction Rate
Iatrogenic osmotic demyelination syndrome is the result of overly rapid correction of serum sodium in patients with chronic hyponatremia. Previously called central pontine myelinolysis, osmotic demyelination syndrome may also occur outside the brainstem. Demyelination generally occurs 2–6 days after inappropriate sodium correction and presents with profound neurologic deficits that are often irreversible. Risk factors for osmotic demyelination syndrome include the severity of hyponatremia (majority less than 120 mEq/L), alcoholism, liver disease, malnutrition, and concurrent hypokalemia.
The optimal correction rate for hyponatremia is debated, though consensus guidelines suggest a correction not to exceed 8 mEq/L in a 24-hour period. It should be emphasized that this represents a limit and not a goal. In fact, in patients who are deemed high risk for osmotic demyelination syndrome, based on criteria detailed above, a goal of 4–6 mEq/L/day is appropriate. If rapid discontinuation of the effects of vasopressin are anticipated, particularly in patients who are at high risk for osmotic demyelination syndrome, prophylactic use of intravenous desmopressin acetate (DDAVP) to prevent a water diuresis should be considered. In the event of overcorrection, free water, with or without DDAVP, should be infused to re-lower the serum sodium to an acceptable level.
C. Chronic Hyponatremia
In the majority of patients with chronic hyponatremia, fluid restriction is a cornerstone of therapy, although if the urine remains concentrated (greater than 300 mOsm/kg), it can be difficult to adhere to a strict water restriction that will correct the hyponatremia. Other measures to increase free water excretion should be considered. Options include loop diuretics, with or without salt tablets, which impair the medullary concentration gradient limiting renal concentration, vasopressin receptor antagonists (vaptans), and oral urea.
Vaptans impair ADH action by inhibiting the vasopressin type 2 (V2)–receptors in the collecting duct, thereby inducing a water diuresis. While a logical target for therapy in refractory hyponatremia, these agents have not been shown to improve hard outcomes and are associated with risks of liver toxicity and sodium overcorrection. If these agents are used, they should generally be avoided in patients with cirrhosis, duration limited to 30 days, and fluid restriction should concurrently be lifted in order to reduce the risk of excessive serum sodium correction. An alternative option is the use of oral urea to induce an osmotic diuresis, which enhances free water clearance. The disadvantage of palatability has been addressed by combining urea with sodium bicarbonate, citric acid, and sucrose.
 When to Refer
When to Refer
• Nephrology consultation should be considered in patients with hyponatremia of uncertain cause or in refractory, or complicated cases.
• Aggressive therapies with hypertonic saline, V2-antagonists, or dialysis mandate specialist consultation.
• Consultation may be necessary with severe liver or heart disease.
 When to Admit
When to Admit
Hospital admission is necessary for severe or symptomatic patients or those requiring aggressive therapies (such as hypertonic saline, initiating vaptans) for close monitoring and frequent laboratory testing.
Hoorn EJ et al. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol. 2017 May;28(5):1340–9. [PMID: 28174217]
Semler MW et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018 Mar 1;378(9):829–39. [PMID: 29485925]
Spasovski G et al; Hyponatraemia Guideline Development Group. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014 Feb 25;170(3):G1–47. [PMID: 24569125]
Sterns RH. Treatment of severe hyponatremia. Clin J Am Soc Nephrol. 2018 Apr 6;13(4):641–9. [PMID: 29295830]
Verbalis JG et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013 Oct;126(10 Suppl 1):S1–2. [PMID: 24074529]
HYPERNATREMIA
ESSENTIALS OF DIAGNOSIS

 Increased thirst and water intake are the main defense against hypernatremia.
Increased thirst and water intake are the main defense against hypernatremia.
 Urine osmolality helps differentiate renal from nonrenal water loss.
Urine osmolality helps differentiate renal from nonrenal water loss.
 General Considerations
General Considerations
Hypernatremia is defined as a sodium concentration greater than 145 mEq/L. All patients with hypernatremia have hyperosmolality, unlike hyponatremic patients who can have a low, normal, or high serum osmolality. Hypernatremia develops when there is a relative loss of water that is inadequately compensated for by water ingestion. Rarely, excess sodium intake contributes to hypernatremia when it is associated with an increase in extracellular volume.
The primary responses to hypernatremia are stimulation of thirst (to increase water intake) and increased secretion of ADH (to minimize water loss in the urine). Cells in the hypothalamus are able to sense minimal changes in serum osmolarity, triggering the thirst mechanism and subsequent intake of water. It is nearly impossible to develop hypernatremia in the context of an intact thirst mechanism with appropriate access to water.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
When the patient is dehydrated, orthostatic hypotension and oliguria are typical findings. Because water shifts from the cells to the intravascular space to protect volume status, symptoms may be delayed. Lethargy, irritability, and weakness are early signs. Hyperthermia, delirium, seizures, and coma may be seen with severe hypernatremia (ie, sodium greater than 160 mEq/L). Symptoms in older adults may not be specific.
B. Laboratory Findings
The first steps in evaluating patients with hypernatremia are assessing the urine volume, osmolality, and the osmole excretion rate. The latter can be calculated by multiplying the urine osmolality with urine volume. The copeptin test is discussed below.
C. Etiology
The initial step is to determine whether the patient with hypernatremia is oliguric, ie, urine flow less than 0.5 mL/min, or nonoliguric. Patients who are nonoliguric can be further subdivided by measurement of a urine osmolality.
1. The oliguric patient (urine flow less than 0.5 mL/min)—This is found in several scenarios.
a. Reduced water intake—Hypernatremia will develop in patients with reduced water intake secondary to the inability to communicate and/or limited access to water.
b. Nonrenal water losses—Nonrenal sites of water loss include sweat, gastrointestinal tract, and the respiratory tract. This is most commonly seen in patients with diarrhea or in febrile patients on a ventilator.
c. Shift of water into cells—Rarely, hypernatremia may manifest from a shift of water into cells due to the intracellular gain of an effective osmole. This may be seen with seizures or rhabdomyolysis.
2. The nonoliguric patient (urine flow greater than 0.5 mL/min)—
a. Urine osmolality less than 250 mOsm/kg—Hypernatremia in the setting of dilute urine is characteristic of diabetes insipidus (DI) or release of a vasopressinase. Central DI results from inadequate ADH release from the pituitary (stroke, tumor, infiltration). In nephrogenic DI, ADH levels are normal or even elevated, but the kidneys are less sensitive to the effects. Common causes include lithium therapy, post-relief of urinary obstruction, chronic interstitial nephritis, hypercalcemia, and hypokalemia. Central and nephrogenic DI can be distinguished by the response to exogenous DDAVP administration while hypernatremic. An innovative test to assist in the differentiation of patients with hypotonic polyuria is the measurement of copeptin, a C-terminal peptide synthesized with vasopressin, which mirrors its concentration over a wide range of plasma osmolalities. Elevated levels of copeptin in the setting of hypernatremia suggest the presence of vasopressin and therefore exclude a diagnosis of central DI. Vasopressin is unstable in isolated plasma, and levels are not helpful.
b. Urine osmolality greater than 300 mOsm/kg—Patients with an elevated urine osmolality and a high urine volume have an osmotic diuresis. Both glucose and urea in the urine can promote polyuria associated with an increased free water excretion.
 Treatment
Treatment
Treatment of hypernatremia includes both correcting the cause of the fluid loss, and replacing the water electrolyte deficit.
A. Choice of Fluid for Replacement
In general, the treatment of patients with hypernatremia involves inducing a positive water balance by the administration of hypotonic fluids. This can be accomplished either through the gastrointestinal tract with oral intake or boluses via a feeding tube or intravenously (or a combination of both). Because it can be difficult to correct large water deficits via the gastrointestinal tract alone, the most common strategy is infusion of 5% dextrose in water (distilled water is contraindicated due to the development of hemolysis). Although there appears to be little risk in the rapid correction of hypernatremia, caution should be exercised when infusing large amount of 5% dextrose in water due to the risk of hyperglycemia and subsequent development of an osmotic diuresis, which can aggravate hypernatremia.
In patients who are concurrently volume depleted, priority should be to restore euvolemia via the administration of isotonic fluids.
B. Calculation of Water Deficit
Fluid replacement should include correcting the free water deficit and adding maintenance fluid to replace ongoing and anticipated fluid losses.
1. Chronic hypernatremia—The water deficit is the amount of water calculated to restore the sodium concentration to normal (140 mEq/L). Total body water (TBW) (Table 21–1) correlates with muscle mass and therefore decreases with advancing age, cachexia, and dehydration and, on average, is lower in women than in men. Current TBW equals 40–60% current body weight.

It should be emphasized that the water deficit represents a static period in time and a critical mistake that is often made when considering the volume of water needed to restore sodium balance is failure to incorporate ongoing water loss via urinary output and insensible losses. Insensible losses can be estimated as 500–1000 mL daily; however, they can vary significantly. The proportion of urine volume that is free water can be estimated by calculating the electrolyte free water (EFW) clearance via the equation below.

2. Rate of sodium correction—Although it would be appealing to apply similar principles for patients with hyponatremia to patients with hypernatremia, this practice is not supported by the literature. A slow rate of correction is usually recommended on the basis of osmotic brain adaptation that occurs with chronic hypernatremia and corresponding theoretical risk of cerebral edema if hypernatremia is corrected too quickly. However, a relatively large retrospective study found no evidence of morbidity or mortality with rapid correction of hypernatremia in critically ill patients with admission or hospital-acquired hypernatremia.
3. Treatment of the underlying cause—The underlying cause of the hypernatremia should be identified and addressed. For patients who have central DI, vasopressin deficiency should be replaced by administration of DDAVP.
 When to Refer
When to Refer
Patients with refractory or unexplained hypernatremia should be referred for nephrology consultation.
 When to Admit
When to Admit
• Patients with symptomatic hypernatremia require hospitalization for evaluation and treatment.
• Significant comorbidities or concomitant acute illnesses, especially if contributing to hypernatremia, may necessitate hospitalization.
Chauhan K et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin J Am Soc Nephrol. 2019 May 7;14(5):656–63. [PMID: 30948456]
Fenske W et al. A copeptin-based approach in the diagnosis of diabetes insipidus. N Engl J Med. 2018 Aug 2;379(5):428–39. [PMID: 30067922]
Seay NW et al. Diagnosis and management of disorders of body tonicity-hyponatremia and hypernatremia: Core Curriculum 2020. Am J Kidney Dis. 2020 Feb;75(2):272–86. [PMID: 31606238]
DISORDERS OF POTASSIUM CONCENTRATION
HYPOKALEMIA
ESSENTIALS OF DIAGNOSIS

 Serum potassium level less than 3.5 mEq/L (3.5 mmol/L).
Serum potassium level less than 3.5 mEq/L (3.5 mmol/L).
 Severe hypokalemia may induce arrhythmias and rhabdomyolysis.
Severe hypokalemia may induce arrhythmias and rhabdomyolysis.
 Assessment of urine potassium excretion (urine potassium to creatinine ratio) can distinguish renal from nonrenal loss of potassium.
Assessment of urine potassium excretion (urine potassium to creatinine ratio) can distinguish renal from nonrenal loss of potassium.
 General Considerations
General Considerations
Hypokalemia can result from insufficient dietary potassium intake, intracellular shifting of potassium from the extracellular space, or potassium loss (renal or extra-renal) (Table 21–3). A low dietary potassium intake is usually not sufficient as the kidneys can lower urine potassium excretion to very low levels (less than 15 mEq/L). Shift of potassium into cells is increased by insulin and beta-adrenergic stimulation. Excess potassium excretion by the kidneys is usually due to increased aldosterone action in the setting of preserved delivery of sodium to the distal nephron. Magnesium is an important regulator of potassium handling and low levels lead to persistent renal excretion of potassium such that hypokalemia is often refractory to treatment until the magnesium deficiency is corrected. Loop diuretics (eg, furosemide) cause substantial renal potassium and magnesium losses.
Table 21–3. Causes of hypokalemia.
Decreased potassium intake
Potassium shift into the cell
Alkalosis
Beta-adrenergic agonists
Insulin release (postprandial, exogenous, insulinoma)
Periodic paralysis (hypokalemic)
Renal potassium loss with metabolic acidosis
Proximal RTA
Distal RTA
Hippurate (glue sniffing)
Renal potassium loss with metabolic alkalosis
Normal/low blood pressure
Bartter syndrome
Gitelman syndrome
Magnesium deficiency
Vomiting
Loop/thiazide diuretics
High blood pressure
Elevated renin and aldosterone
Malignant hypertension
Renin producing tumor
Renal artery stenosis
Depressed renin and elevated aldosterone
Adrenal adenoma
Glucocorticoid remediable aldosteronism
Adrenal hyperplasia
Depressed renin and aldosterone
Cushing syndrome
Black licorice
Apparent mineralocorticoid excess
Liddle syndrome
Increased gastrointestinal losses
Diarrhea
Laxative abuse
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Hypokalemia is usually asymptomatic but when severe may lead to muscle weakness and cardiac arrhythmias. Involvement of gastrointestinal smooth muscle may result in constipation or ileus. Rhabdomyolysis with associated acute kidney injury can be seen with potassium levels less than 2.5 mEq/L. Hypokalemia may additionally present as polyuria and polydipsia due to diminished concentrating ability of the kidney (nephrogenic DI) and chronic hypokalemia can lead to kidney disease (tubulointerstitial nephritis).
B. Laboratory Findings
Transient hypokalemia is generally secondary to intracellular shift, while sustained hypokalemia is secondary to potassium wasting or, rarely, inadequate intake. Assessment of renal potassium excretion can help distinguish renal from nonrenal causes of hypokalemia. A 24-hour urine collection is the most accurate method for assessing renal handling of potassium, with a level less than 25 mEq/day compatible with appropriate renal potassium retention, and higher values corresponding to renal potassium wasting. A more immediate assessment can be made by measuring a urine potassium to creatinine ratio (UK/UCr) on a spot urine sample (Figure 21–2). In the setting of hypokalemia, a UK/UCr ratio less than 13 mEq/g (or 1.5 mEq/mmol) is suggestive of a nonrenal etiology, most commonly gastrointestinal losses, intracellular potassium shifts, or inadequate dietary intake, whereas higher values imply renal potassium wasting.

Figure 21–2. Differentiating renal from nonrenal causes of hypokalemia using a spot urine potassium and a spot urine creatinine.
C. Electrocardiogram
Hypokalemia leads to a characteristic progression of electrocardiogram (ECG) changes, initially T wave flattening, subsequently ST depressions and T wave inversions, ultimately leading to U waves as the hypokalemia becomes more severe. There is significant interpatient variability in the degree of hypokalemia and corresponding ECG findings; therefore, typical ECG patterns may not be observed in all patients.
 Etiology
Etiology
1. Inadequate dietary intake—While the kidney can excrete urine that is virtually free of sodium, there continues to be a small amount of potassium excretion even in the setting of diets completely devoid of potassium. With extreme potassium free diets, such as anorexia nervosa and alcoholism, the hypokalemia is worsened by concurrent magnesium depletion.
2. Cellular shift—The most important determinants of intracellular potassium shifts are postprandial insulin and catecholamine release. These physiologic conditions can be exacerbated by beta-adrenergic–agonist administration, as well as high adrenergic states, which can be seen in situations such as alcohol withdrawal and myocardial infarctions. Rare causes include insulinomas and hypokalemic periodic paralysis.
3. Gastrointestinal losses—The most common cause of nonrenal potassium wasting is gastrointestinal loss, both diarrhea and vomiting. Diarrhea may have a high potassium and bicarbonate content resulting in hypokalemia with a concurrent nongap (hyperchloremic) metabolic acidosis. Vomiting leads to hypokalemia with a metabolic alkalosis with most of the potassium loss due to renal wasting (secondary to hypovolemia-induced hyperaldosteronism).
4. Renal wasting—Renal potassium wasting occurs in states where increased distal sodium delivery is coupled to increased aldosterone activity. This most commonly occurs with diuretic use. Rarely, renal tubulopathies (Bartter syndrome or Gitelman syndrome) or renal tubular acidosis may present with hypokalemia.
Primary hyperaldosteronism (Conn syndrome) is due to excess aldosterone production by the adrenal glands, which causes extracellular volume expansion resulting in hypertension associated with hypokalemia and a metabolic alkalosis. Other rare forms of increased mineralocorticoid activity may be identified by measuring plasma renin activity and serum aldosterone levels.
 Treatment
Treatment
Any underlying conditions should be treated and causative drugs discontinued. Magnesium deficiency should be corrected, particularly in refractory hypokalemia. Oral potassium supplementation is the safest treatment for mild to moderate deficiency, although potassium supplements may cause gastrointestinal upset. With mild to moderate diuretic doses, 20 mEq/day of oral potassium is generally sufficient to prevent hypokalemia, whereas with established hypokalemia, 40–100 mEq/day over a period of days to weeks may be needed to treat hypokalemia and fully replete potassium stores.
Intravenous potassium is reserved for severe hypokalemia (less than 3.0 mEq/L) and requires careful monitoring due to the risk of transient hyperkalemia. Potassium chloride may be given through a peripheral intravenous line at rates up to 10–15 mEq/h diluted in 0.5% or 0.9% normal saline, but higher rates (up to 20 mEq/h) require central access due to the risk of peripheral vein irritation. In the event of concurrent metabolic acidosis, potassium repletion should take precedence over alkali administration as correction of the acidosis will result in intracellular shift of potassium, further decreasing extracellular potassium concentration. Similarly, potassium should be given in a saline, rather than dextrose solution since dextrose would stimulate insulin release and hence, intracellular shift.
 When to Refer
When to Refer
Patients with unexplained hypokalemia, refractory or persistent hypokalemia, or suggestive alternative diagnoses (eg, aldosteronism or hypokalemic periodic paralysis) should be referred for nephrology consultation.
 When to Admit
When to Admit
Patients with symptomatic or severe hypokalemia (less than 2.5 mEq/L), especially with cardiac manifestations, require cardiac monitoring, potassium supplementation, and frequent laboratory testing.
Gumz ML et al. An integrated view of potassium homeostasis. N Engl J Med. 2015 Jul 2;373(1):60–72. Erratum in: N Engl J Med. 2015 Sep 24;373(13):1281. [PMID: 26132942]
Palmer BF et al. Physiology and pathophysiology of potassium homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019 Nov;74(5):682–95. [PMID: 31227226]
Wu KL et al. Identification of the causes for chronic hypokalemia: importance of urinary sodium and chloride excretion. Am J Med. 2017 Jul;130(7):846–55. [PMID: 28213045]
HYPERKALEMIA
ESSENTIALS OF DIAGNOSIS

 Serum potassium level greater than 5.2 mEq/L (5.2 mmol/L).
Serum potassium level greater than 5.2 mEq/L (5.2 mmol/L).
 Check medications carefully. Hyperkalemia may develop from angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and potassium-sparing diuretics, most commonly in patients with kidney dysfunction.
Check medications carefully. Hyperkalemia may develop from angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and potassium-sparing diuretics, most commonly in patients with kidney dysfunction.
 The ECG may be normal despite life-threatening hyperkalemia.
The ECG may be normal despite life-threatening hyperkalemia.
 Rule out pseudohyperkalemia and extracellular potassium shift from cells.
Rule out pseudohyperkalemia and extracellular potassium shift from cells.
 General Considerations
General Considerations
Hyperkalemia is a rare occurrence in normal individuals due to adaptive mechanisms designed to prevent accumulation of potassium in the extracellular fluid, mainly via rapid urinary excretion. Persistent hyperkalemia generally requires an impairment in renal potassium excretion due to impaired secretion of or hyporesponsiveness to aldosterone, impaired delivery of sodium and water to the distal nephron, or kidney disease (acute or chronic) (Table 21–4). Transient hyperkalemia suggests shift of potassium from inside cells into the extracellular fluid, which can occur in the context of tissue damage (rhabdomyolysis, tumor lysis, massive hemolysis, and trauma) or metabolic acidosis. Finally, pseudohyperkalemia is a laboratory artifact in which there is an elevation in serum potassium levels in the absence of true electrolyte imbalance as a result of tourniquet application or fist clenching during blood draw, or improper transport or processing of venous samples in patients with marked thrombocytosis or leukocytosis.
Table 21–4. Causes of hyperkalemia.
Pseudohyperkalemia
Marked thrombocytosis or leukocytosis with release of intracellular K+
Repeated fist clenching during phlebotomy, tourniquet application, use of small-bore needles during lab draw
Shift of K+ out of the cell
Metabolic acidosis
Insulin deficiency
Hyperglycemia
α-Adrenergic stimulation
Tissue injury (rhabdomyolysis, hemolysis, tumor lysis)
Hyperkalemic periodic paralysis
Drugs (digoxin overdose, succinylcholine)
Kidney disease, acute and chronic
Renal secretory defects (may or may not have reduced kidney function): kidney transplant, interstitial nephritis, systemic lupus erythematosus, sickle cell disease, amyloidosis, obstructive nephropathy
Hypoaldosteronism
Addison disease
Type IV renal tubular acidosis
Heparin
Ketoconazole
Drugs that inhibit potassium excretion: spironolactone, eplerenone, drospirenone, NSAIDs, ACE inhibitors, angiotensin II receptor blockers, triamterene, amiloride, trimethoprim, pentamidine, cyclosporine, tacrolimus
Excessive intake of K+
Especially in patients with diminished kidney excretion
ACE, angiotensin-converting enzyme; NSAIDs, nonsteroidal anti-inflammatory drugs.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The symptoms of hyperkalemia are a consequence of impaired neuromuscular transmission. The most serious manifestations are cardiac conduction abnormalities and neuromuscular manifestations, such as muscle weakness, which may be profound. This generally occurs with potassium concentrations above 7 mEq/L, though it can vary depending on the acuity in development of hyperkalemia. Hyperkalemic period paralysis is a rare genetic disorder characterized by episodes of painless muscle weakness precipitated by potassium ingestion, rest after heavy exercise, and cold exposure. Hyperkalemia additionally impairs urinary ammonium excretion and may lead to metabolic acidosis.
B. Electrocardiogram
Electrocardiography is an unreliable method for detecting hyperkalemia; clinical studies show poor correlation between serum potassium levels and cardiac manifestations. The rapidity in development of hyperkalemia may correlate with the development of ECG changes. Typical sequential changes on the electrocardiogram are peaking of the T waves, ST-segment depression, and widening of the PR and QRS intervals. As the QRS continues to widen, sine waves may develop, which are concerning for imminent ventricular fibrillation and ultimately asystole.
 Etiology
Etiology
1. Increased potassium release from cells—
a. Pseudohyperkalemia—This condition arises during specimen collection due to fist clenching, application of tourniquets, or using small bore needles. The presence of hemolysis in the processed sample can suggest these etiologies. A more striking example occurs in patients with marked thrombocytosis (greater than 500,000/mcL) or leukocytosis (greater than 100,000/mcL), particularly leukemic cells. Centrifugation or transport via a pneumatic tube system causes significant in vitro cell destruction due to cell fragility. If this condition is suspected a noncentrifuged, a whole blood sample that is walked to the laboratory is required for confirmation.
b. Tissue breakdown—Tissue damage results in release of intracellular potassium to the extracellular space. Common clinical examples include tumor lysis syndrome, crush injuries and severe hemolysis. Hyperkalemia is more common when concurrent renal impairment is present.
c. Hyperglycemia—Patients with uncontrolled diabetes may have hyperkalemia, even in the setting of a low total body potassium, due to a combination of insulin deficiency and hyperosmolarity from hyperglycemia.
d. Metabolic acidosis—Acidosis results in potassium shifting out of the intracellular fluid due to buffering of hydrogen ions into cells. Serum potassium concentration rises approximately 0.7 mEq/L for every decrease of 0.1 pH unit. This effect is not seen with organic acidosis, such as lactic acidosis or ketoacidosis.
2. Impaired renal excretion—
a. Acute kidney injury—A rapid reduction in kidney function leads to poor renal excretion of potassium and does not allow sufficient time for nonrenal adaptive mechanisms to take effect. Hyperkalemia occurs more commonly in oliguric patients.
b. Chronic kidney disease—The ability to maintain normal serum potassium levels is generally preserved until GFR declines to less than 20–30 mL/min/1.73 m2. This is primarily due to adaptive mechanisms, particularly increases in potassium excretion by remaining functioning nephrons and by increase gastrointestinal tract potassium excretion. Hyperkalemia in patients with more modest decreases in GFR are often due to medications that disrupt the renin-angiotensin-aldosterone system, which are commonly used in these patients.
c. Low effective circulating volume—Volume depletion as well as the edematous states of cirrhosis and heart failure can cause hyperkalemia due to a decrease in distal delivery of sodium and water, which impairs potassium excretion.
d. Reduced aldosterone action—Mineralocorticoid deficiency from Addison disease may cause hyperkalemia due to a decreased renal excretion of potassium. Mineralocorticoid resistance due to genetic disorders, interstitial kidney disease, or urinary tract obstruction also leads to hyperkalemia.
3. Medications—A number of medications can be implicated in the development of hyperkalemia and a careful review of a patient’s medication list is imperative. Common medications include ACE inhibitors, angiotensin receptor blockers (ARBs), and NSAIDs, which reduce aldosterone release. Concomitant use of aldosterone antagonists (spironolactone or eplerenone) or medications that directly block the sodium channels of the principal cells (amiloride, triamterene, or trimethoprim) further increase risk of hyperkalemia. Beta-blockers can cause mild hyperkalemia by interfering with potassium uptake by cells, a phenomenon more commonly observed with nonselective beta-blockers. Heparin inhibits aldosterone production in the adrenal glands. Calcineurin inhibitors, such as cyclosporine and tacrolimus, can induce hyperkalemia, partly stimulating the sodium chloride co-transporter in the distal nephron impairing distal sodium delivery. Any of these medications should be used cautiously in patients with renal impairment, and laboratory monitoring is indicated within 1–2 weeks of drug initiation or dosage increase.
 Treatment
Treatment
The diagnosis should be confirmed by repeat laboratory testing to rule out spurious hyperkalemia, especially in the absence of medications that cause hyperkalemia or in patients without kidney disease. The initial treatment is determined by the presence of signs and symptoms as well as the severity in plasma potassium elevation. In all patients, exogenous sources of potassium should be eliminated, medications that can impair potassium excretion discontinued, volume depletion corrected, and metabolic acidosis improved.
In emergency situations (cardiac toxicity, muscle weakness, or potassium greater than 6.5 mEq/L), initial therapy should be intravenous calcium gluconate to stabilize the myocardium in order to protect against arrhythmias, followed by therapies to shift potassium into cells. Insulin and beta-agonists shift potassium intracellularly within 10–15 minutes of administration but have a short duration of action (1–2 hours) (Table 21–5). Sodium bicarbonate may be helpful to shift potassium into cells in patients with a concurrent metabolic acidosis. Once the patient is stabilized, therapies are focused on potassium excretion.
Table 21–5. Treatment of hyperkalemia.

Potassium excretion may be enhanced with the use of loop diuretics. Patiromer and sodium zirconium cyclosilicate are newer potassium-binding drugs that may be used to treat chronic hyperkalemia. Studies have demonstrated that these drugs are both well tolerated and effective in patients with hyperkalemia who have either chronic kidney disease or heart failure and take at least one medication that inhibits the renin-angiotensin-aldosterone system. Neither drug has been studied in acute hyperkalemia. Sodium polystyrene (Kayexalate) has been widely used for decades although its efficacy and safety have been questioned. It may not increase potassium excretion greater than laxatives alone and has been associated with colonic necrosis, both with and without sorbitol coadministration; sodium polystyrene is contraindicated in patients with risk factors for colonic necrosis, such as bowel obstruction, ileus, and postoperative state. Hemodialysis may be necessary to remove potassium in patients with acute or chronic kidney injury, particularly in patients who are oliguric.
 When to Refer
When to Refer
• Patients with hyperkalemia from kidney disease and reduced renal potassium excretion should see a nephrologist.
• Transplant patients may need adjustment of their immunosuppression regimen by transplant specialists.
 When to Admit
When to Admit
Patients with severe hyperkalemia (greater than 6 mEq/L), any degree of hyperkalemia associated with ECG changes, or concomitant illness that may worsen hyperkalemia (eg, tumor lysis, rhabdomyolysis, metabolic acidosis) should be sent to the emergency department for immediate treatment.
Kovesdy CP. Updates in hyperkalemia: outcomes and therapeutic strategies. Rev Endocr Metab Disord. 2017 Mar;18(1):41–7. [PMID: 27600582]
Kovesdy CP et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens. 2017 Dec;11(12):783–800. [PMID: 29030153]
Palmer BF et al. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017 Dec;84(12):934–42. [PMID: 29244647]
DISORDERS OF CALCIUM CONCENTRATION
The normal total plasma (or serum) calcium concentration is 8.5–10.5 mg/dL (or 2.1–2.6 mmol/L). Ionized calcium (normal: 4.6–5.3 mg/dL [or 1.15–1.32 mmol/L]) is physiologically active and necessary for muscle contraction and nerve function.
The calcium-sensing receptor, a transmembrane protein that detects the extracellular calcium concentration, is in the parathyroid gland and the kidney. Functional defects in this protein are associated with diseases of abnormal calcium metabolism such as familial hypocalcemia and familial hypocalciuric hypercalcemia.
HYPOCALCEMIA
ESSENTIALS OF DIAGNOSIS

 Often mistaken as a neurologic disorder.
Often mistaken as a neurologic disorder.
 Decreased serum parathyroid hormone (PTH), vitamin D, or magnesium levels.
Decreased serum parathyroid hormone (PTH), vitamin D, or magnesium levels.
 Despite a low total serum calcium, calcium metabolism is likely normal if ionized calcium level is normal.
Despite a low total serum calcium, calcium metabolism is likely normal if ionized calcium level is normal.
 General Considerations
General Considerations
The most common cause of low total serum calcium is hypoalbuminemia. When serum albumin concentration is lower than 4 g/dL (40 g/L), serum Ca2+ concentration is reduced by 0.8–1 mg/dL (0.20–0.25 mmol/L) for every 1 g/dL (10 g/L) of albumin.
The most accurate measurement of serum calcium is the ionized calcium concentration. True hypocalcemia (decreased ionized calcium) implies insufficient action of PTH or active vitamin D. Important causes of hypocalcemia are listed in Table 21–6.
Table 21–6. Causes of hypocalcemia.
Decreased intake or absorption
Malabsorption
Small bowel bypass, short bowel
Vitamin D deficit (decreased absorption, decreased production of 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D)
Increased loss
Alcoholism
Chronic kidney disease
Diuretic therapy
Endocrine disease
Hypoparathyroidism (genetic, acquired; including hypomagnesemia and hypermagnesemia)
Post-parathyroidectomy (hungry bone syndrome)
Pseudohypoparathyroidism
Calcitonin secretion with medullary carcinoma of the thyroid
Familial hypocalcemia
Associated diseases
Pancreatitis
Rhabdomyolysis
Septic shock
Physiologic causes
Decreased serum albumin1
Decreased end-organ response to vitamin D
Hyperphosphatemia
Aminoglycoside antibiotics, plicamycin, loop diuretics, foscarnet
1Ionized calcium concentration is normal.
The most common cause of hypocalcemia is advanced CKD, in which decreased production of active vitamin D3 (1,25 dihydroxyvitamin D) and hyperphosphatemia both play a role (see Chapter 22). Some cases of primary hypoparathyroidism are due to mutations of the calcium-sensing receptor in which inappropriate suppression of PTH release leads to hypocalcemia (see Chapter 26). Magnesium depletion reduces both PTH release and tissue responsiveness to PTH, causing hypocalcemia. Hypocalcemia in pancreatitis is a marker of severe disease. Elderly hospitalized patients with hypocalcemia and hypophosphatemia, with or without an elevated PTH level, are likely vitamin D deficient.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Hypocalcemia increases excitation of nerve and muscle cells, primarily affecting the neuromuscular and cardiovascular systems. Spasm of skeletal muscle causes cramps and tetany. Laryngospasm with stridor can obstruct the airway. Convulsions, perioral and peripheral paresthesias, and abdominal pain can develop. Classic physical findings include Chvostek sign (contraction of the facial muscle in response to tapping the facial nerve) and Trousseau sign (carpal spasm occurring with occlusion of the brachial artery by a blood pressure cuff). QT prolongation predisposes to ventricular arrhythmias. In chronic hypoparathyroidism, cataracts and calcification of basal ganglia may appear (see Chapter 26).
B. Laboratory Findings
Serum calcium concentration is low (less than 8.5 mg/dL [2.1 mmol/L]). In true hypocalcemia, the ionized serum calcium concentration is also low (less than 4.6 mg/dL [1.15 mmol/L]). Serum phosphate is usually elevated in hypoparathyroidism or in advanced CKD, whereas it is suppressed in early CKD or vitamin D deficiency.
Serum magnesium concentration is commonly low. In respiratory alkalosis, total serum calcium is normal but ionized calcium is low. The ECG shows a prolonged QT interval.
 Treatment1
Treatment1
A. Severe, Symptomatic Hypocalcemia
In the presence of tetany, arrhythmias, or seizures, intravenous calcium gluconate is indicated.1 Because of the short duration of action, continuous calcium infusion is usually required. Ten to 15 milligrams of calcium per kilogram body weight, or six to eight 10-mL vials of 10% calcium gluconate (558–744 mg of calcium), are added to 1 L of D5W and infused over 4–6 hours. By monitoring the serum calcium level frequently (every 4–6 hours), the infusion rate is adjusted to maintain the serum calcium level at 7–8.5 mg/dL.
B. Asymptomatic Hypocalcemia
Oral calcium (1–2 g of elemental calcium) and vitamin D preparations, including active vitamin D sterols, are used. Calcium carbonate is well tolerated and less expensive than many other calcium tablets. A check of urinary calcium excretion is recommended after the initiation of therapy because hypercalciuria (urine calcium excretion greater than 300 mg or 7.5 mmol per day) or urine calcium:creatinine ratio greater than 0.3 may impair kidney function in these patients. The low serum calcium associated with hypoalbuminemia does not require replacement therapy. If serum Mg2+ is low, therapy must include magnesium replacement, which by itself will usually correct hypocalcemia.
 When to Refer
When to Refer
Patients with complicated hypocalcemia from hypoparathyroidism, familial hypocalcemia, or CKD require referral to an endocrinologist or nephrologist.
 When to Admit
When to Admit
Patients with tetany, arrhythmias, seizures, or other symptoms of hypocalcemia require immediate therapy.
Aberegg SK. Ionized calcium in the ICU: should it be measured and corrected? Chest. 2016 Mar;149(3):846–55. [PMID: 26836894]
Rosner MH. Hypocalcemia in a patient with cancer. Clin J Am Soc Nephrol. 2017 Apr 3;12(4):696–9. [PMID: 28274993]
Schafer AL et al. Hypocalcemia: diagnosis and treatment. In: Feingold KR et al, editors. Endotext. 2016 Jan 3. [PMID: 25905251]
HYPERCALCEMIA
ESSENTIALS OF DIAGNOSIS

 Most common causes: primary hyperparathyroidism and malignancy-associated hypercalcemia.
Most common causes: primary hyperparathyroidism and malignancy-associated hypercalcemia.
 Hypercalciuria usually precedes hypercalcemia.
Hypercalciuria usually precedes hypercalcemia.
 Asymptomatic, mild hypercalcemia (above 10.5 mg/dL [2.6 mmol/L]) is usually due to primary hyperparathyroidism.
Asymptomatic, mild hypercalcemia (above 10.5 mg/dL [2.6 mmol/L]) is usually due to primary hyperparathyroidism.
 Symptomatic, severe hypercalcemia (above 14 mg/dL [3.5 mmol/L]) is usually due to hypercalcemia of malignancy.
Symptomatic, severe hypercalcemia (above 14 mg/dL [3.5 mmol/L]) is usually due to hypercalcemia of malignancy.
 General Considerations
General Considerations
Important causes of hypercalcemia are listed in Table 21–7. Primary hyperparathyroidism and malignancy account for 90% of cases. Primary hyperparathyroidism is the most common cause of hypercalcemia (usually mild) in ambulatory patients. Chronic hypercalcemia (over 6 months) or some manifestation such as nephrolithiasis also suggests a benign cause. Tumor production of PTH-related proteins (PTHrP) is the most common paraneoplastic endocrine syndrome, accounting for most cases of hypercalcemia in inpatients. The neoplasm is clinically apparent in nearly all cases when the hypercalcemia is detected, and the prognosis is poor. Granulomatous diseases, such as sarcoidosis and tuberculosis, cause hypercalcemia via overproduction of active vitamin D3 (1,25 dihydroxyvitamin D).
Table 21–7. Causes of hypercalcemia.
Increased intake or absorption
Milk-alkali syndrome
Vitamin D or vitamin A excess
Endocrine disorders
Primary hyperparathyroidism
Secondary or tertiary hyperparathyroidism (usually associated with hypocalcemia)
Acromegaly
Adrenal insufficiency
Pheochromocytoma
Thyrotoxicosis
Neoplastic diseases
Tumors producing PTH-related proteins (ovary, kidney, lung)
Plasma cell myeloma (elaboration of osteoclast-activating factor)
Lymphoma (occasionally from production of calcitriol)
Miscellaneous causes
Thiazide diuretics
Granulomatous diseases (production of calcitriol)
Paget disease of bone
Hypophosphatasia
Immobilization
Familial hypocalciuric hypercalcemia
Complications of kidney transplantation
Lithium intake
PTH, parathyroid hormone.
Milk-alkali syndrome has had a resurgence due to calcium ingestion for prevention of osteoporosis. Heavy calcium carbonate intake causes hypercalcemic acute kidney injury, likely from renal vasoconstriction. The decreased GFR impairs bicarbonate excretion, while hypercalcemia stimulates proton secretion and bicarbonate reabsorption. Metabolic alkalosis decreases calcium excretion, maintaining hypercalcemia.
Hypercalcemia causes nephrogenic DI through activation of calcium-sensing receptors in collecting ducts, which reduces ADH-induced water permeability. Volume depletion further worsens hypercalcemia.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The history and physical examination should focus on the duration of hypercalcemia and evidence for a neoplasm. Hypercalcemia may affect gastrointestinal, kidney, and neurologic function. Mild hypercalcemia is often asymptomatic. Symptoms usually occur if the serum calcium is higher than 12 mg/dL (3 mmol/L) and tend to be more severe if hypercalcemia develops acutely. Symptoms include constipation and polyuria, except in hypocalciuric hypercalcemia, in which polyuria is absent. Other symptoms include nausea, vomiting, anorexia, peptic ulcer disease, renal colic, and hematuria from nephrolithiasis. Polyuria from hypercalciuria-induced nephrogenic DI can result in volume depletion and acute kidney injury. Neurologic manifestations range from mild drowsiness to weakness, depression, lethargy, stupor, and coma in severe hypercalcemia. Ventricular ectopy and idioventricular rhythm occur and can be accentuated by digitalis.
B. Laboratory Findings
The ionized calcium exceeds 1.32 mmol/L. A high serum chloride concentration and a low serum phosphate concentration in a ratio greater than 33:1 (or greater than 102 if SI units are used) suggests primary hyperparathyroidism where PTH decreases proximal tubular phosphate reabsorption. A low serum chloride concentration with a high serum bicarbonate concentration, along with elevated BUN and creatinine, suggests milk-alkali syndrome. Severe hypercalcemia (greater than 15 mg/dL [3.75 mmol/L]) generally occurs in malignancy. More than 300 mg (7.5 mmol) per day of urinary calcium excretion suggests hypercalciuria; less than 100 mg (2.5 mmol) per day suggests hypocalciuria. Hypercalcemia may develop easily in cases of volume depletion in hypercalciuric patients, such as those with malignancy or those receiving oral active vitamin D therapy. Serum phosphate may or may not be low, depending on the cause. Hypocalciuric hypercalcemia occurs in milk-alkali syndrome, thiazide diuretic use, and familial hypocalciuric hypercalcemia.
The chest radiograph may reveal malignancy or granulomatous disease. The ECG can show a shortened QT interval. Measurements of PTH and PTHrP help distinguish between hyperparathyroidism (elevated PTH) and malignancy-associated hypercalcemia (suppressed PTH, elevated PTHrP).
 Treatment
Treatment
Until the primary cause can be identified and treated, renal excretion of calcium is promoted through aggressive hydration and forced calciuresis. The tendency in hypercalcemia is hypovolemia from nephrogenic DI. In volume-depleted patients with normal cardiac and kidney function, 0.45% saline or 0.9% saline can be given rapidly (250–500 mL/h). Loop diuretics to enhance renal calcium excretion should generally be avoided due to possible complications such as nephrolithiasis. These agents were used more commonly in the era of more aggressive volume administration, ie, beyond that needed to achieve euvolemia, and prior to the wide availability of more effective medications, such as bisphosphonates and calcitonin. Loop diuretics may be carefully used in the context of preventing or managing volume overload, particularly in patients with heart failure or kidney dysfunction. Thiazides can worsen hypercalcemia.
Bisphosphonates are the treatment of choice for hypercalcemia of malignancy. Although they are safe, effective, and normalize calcium in more than 70% of patients, bisphosphonates may require up to 48–72 hours before reaching full therapeutic effect. Calcitonin may be helpful in the short-term until bisphosphonates reach therapeutic levels. In emergency cases, dialysis with low calcium dialysate may be needed. Denosumab, a monoclonal antibody against RANKL, inhibits osteoclasts, reducing bone resorption and serum calcium levels; this medication is FDA-approved for malignancy-associated hypercalcemia refractory to bisphosphonate therapy. The calcimimetic agent cinacalcet suppresses PTH secretion and decreases serum calcium concentration; it has been recommended for use in patients with symptomatic or severe primary hyperparathyroidism who are unable to undergo parathyroidectomy and patients with inoperable parathyroid carcinoma. (See Chapters 26 and 39.)
Typically, if dialysis patients do not receive proper supplementation of calcium and active vitamin D, hypocalcemia and hyperphosphatemia develop. On the other hand, hypercalcemia can sometimes develop, particularly in the setting of severe secondary hyperparathyroidism, characterized by high PTH levels and subsequent release of calcium from bone. Therapy may include intravenous vitamin D, which further increases the serum calcium concentration. Another type of hypercalcemia occurs when PTH levels are low. Bone turnover is decreased, which results in a low buffering capacity for calcium. When calcium is administered in calcium-containing phosphate binders or dialysate, or when vitamin D is administered, hypercalcemia results. Hypercalcemia in dialysis patients usually occurs in the presence of hyperphosphatemia, and metastatic calcification may occur. Malignancy should be considered as a cause of the hypercalcemia.
 When to Refer
When to Refer
• Patients may require referral to an oncologist or endocrinologist depending on the cause of hypercalcemia.
• Patients with granulomatous diseases (eg, tuberculosis and other chronic infections, granulomatosis with polyangiitis, sarcoidosis) may require assistance from infectious disease specialists, rheumatologists, or pulmonologists.
 When to Admit
When to Admit
• Patients with symptomatic or severe hypercalcemia require immediate treatment.
• Unexplained hypercalcemia with associated conditions, such as acute kidney injury or suspected malignancy, may require urgent treatment and expedited evaluation.
Ahmad S et al. Hypercalcemic crisis: a clinical review. Am J Med. 2015 Mar;128(3):239–45. [PMID: 25447624]
Bazari H et al. Case records of the Massachusetts General Hospital. Case 24-2016. A 66-year-old man with malaise, weakness, and hypercalcemia. N Engl J Med. 2016 Aug 11;375(6):567–74. [PMID: 27509105]
Minisola S et al. The diagnosis and management of hypercalcaemia. BMJ. 2015 Jun 2;350:h2723. [PMID: 26037642]
Tebben PJ et al. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016 Oct;37(5):521–47. [PMID: 27588937]
Thosani S et al. Denosumab: a new agent in the management of hypercalcemia of malignancy. Future Oncol. 2015;11(21):2865–71. [PMID: 26403973]
DISORDERS OF PHOSPHORUS CONCENTRATION
Plasma phosphorus is mainly inorganic phosphate and represents a small fraction (less than 0.2%) of total body phosphate. Important determinants of plasma inorganic phosphate are renal excretion, intestinal absorption, and shift between the intracellular and extracellular spaces. The kidney is the most important regulator of the serum phosphate level. PTH decreases reabsorption of phosphate in the proximal tubule while 1,25-dihydroxyvitamin D increases reabsorption. Renal proximal tubular reabsorption of phosphate is decreased by volume expansion, corticosteroids, and proximal tubular dysfunction (as in Fanconi syndrome). Fibroblast growth factor 23 (FGF23) is a potent phosphaturic hormone. Intestinal absorption of phosphate is facilitated by active vitamin D. PTH stimulates phosphate release from bone and renal phosphate excretion; primary hyperparathyroidism can lead to hypophosphatemia and depletion of bone phosphate stores. By contrast, growth hormone augments proximal tubular reabsorption of phosphate. Cellular phosphate uptake is stimulated by various factors and conditions, including alkalemia, insulin, epinephrine, feeding, hungry bone syndrome, and accelerated cell proliferation.
Phosphorus metabolism and homeostasis are intimately related to calcium metabolism. See sections on metabolic bone disease in Chapter 26.
HYPOPHOSPHATEMIA
ESSENTIALS OF DIAGNOSIS

 Severe hypophosphatemia may cause tissue hypoxia and rhabdomyolysis.
Severe hypophosphatemia may cause tissue hypoxia and rhabdomyolysis.
 Renal loss of phosphate can be diagnosed by calculating the fractional excretion of phosphate (FePO4).
Renal loss of phosphate can be diagnosed by calculating the fractional excretion of phosphate (FePO4).
 PTH and FGF23 are the major factors that increase urine phosphate.
PTH and FGF23 are the major factors that increase urine phosphate.
 General Considerations
General Considerations
The leading causes of hypophosphatemia are listed in Table 21–8. Hypophosphatemia may occur in the presence of normal phosphate stores. Serious depletion of body phosphate stores may exist with low, normal, or high serum phosphate concentrations.
Table 21–8. Causes of hypophosphatemia.
Diminished supply or absorption
Starvation
Parenteral alimentation with inadequate phosphate content
Malabsorption syndrome, small bowel bypass
Absorption blocked by oral antacids with aluminum or magnesium
Vitamin D–deficient and vitamin D–resistant osteomalacia
Increased loss
Phosphaturic drugs: theophylline, diuretics, bronchodilators, corticosteroids
Hyperparathyroidism (primary or secondary)
Hyperthyroidism
Renal tubular defects with excessive phosphaturia (congenital, Fanconi syndrome induced by monoclonal gammopathy, heavy metal poisoning), alcoholism
Hypokalemic nephropathy
Inadequately controlled diabetes mellitus
Hypophosphatemic rickets
Phosphatonins of oncogenic osteomalacia (eg, FGF23 production)
Intracellular shift of phosphorus
Administration of glucose
Anabolic steroids, estrogen, oral contraceptives, beta-adrenergic agonists, xanthine derivatives
Hungry bone syndrome
Respiratory alkalosis
Salicylate poisoning
Electrolyte abnormalities
Hypercalcemia
Hypomagnesemia
Metabolic alkalosis
Abnormal losses followed by inadequate repletion
Diabetes mellitus with acidosis, particularly during aggressive therapy
Recovery from starvation or prolonged catabolic state
Chronic alcoholism, particularly during restoration of nutrition; associated with hypomagnesemia
Recovery from severe burns
FGF23, fibroblast growth factor 23.
Serum phosphate levels decrease transiently after food intake, thus fasting samples are recommended for accuracy. Moderate hypophosphatemia (1.0–2.4 mg/dL [0.32–0.79 mmol/L]) occurs commonly in hospitalized patients and may not reflect decreased phosphate stores.
In severe hypophosphatemia (less than 1 mg/dL [0.32 mmol/L]), the affinity of hemoglobin for oxygen increases through a decrease in the erythrocyte 2,3-biphosphoglycerate concentration, impairing tissue oxygenation and cell metabolism and resulting in muscle weakness or even rhabdomyolysis. Severe hypophosphatemia is common and multifactorial in alcoholic patients. In acute alcohol withdrawal, increased plasma insulin and epinephrine along with respiratory alkalosis promote intracellular shift of phosphate. Vomiting, diarrhea, and poor dietary intake contribute to hypophosphatemia. Chronic alcohol use results in a decrease in the renal threshold of phosphate excretion. This renal tubular dysfunction reverses after a month of abstinence. Patients with chronic obstructive pulmonary disease and asthma commonly have hypophosphatemia, attributed to xanthine derivatives causing shifts of phosphate intracellularly and the phosphaturic effects of beta-adrenergic agonists, loop diuretics, xanthine derivatives, and corticosteroids. Refeeding or glucose administration to phosphate-depleted patients may cause fatal hypophosphatemia.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Acute, severe hypophosphatemia (less than 1.0 mg/dL [0.32 mmol/L]) can lead to rhabdomyolysis, paresthesias, and encephalopathy (irritability, confusion, dysarthria, seizures, and coma). Respiratory failure or failure to wean from mechanical ventilation may occur as a result of diaphragmatic weakness. Arrhythmias and heart failure are uncommon but serious manifestations. Hematologic manifestations include acute hemolytic anemia from erythrocyte fragility, platelet dysfunction with petechial hemorrhages, and impaired chemotaxis of leukocytes (leading to increased susceptibility to gram-negative sepsis).
Chronic severe depletion may cause anorexia, pain in muscles and bones, and fractures.
B. Laboratory Findings
Urine phosphate excretion is a useful clue in the evaluation of hypophosphatemia. The normal renal response to hypophosphatemia is decreased urinary phosphate excretion to less than 100 mg/day, and a fractional excretion of phosphate (FePO4) less than 5%. The main factors regulating FePO4 are PTH and phosphate intake. Increased PTH or phosphate intake decreases FePO4 (ie, more phosphate is excreted into the urine).
Measurement of plasma PTH or PTHrP levels may be helpful. The clinical usefulness of serum FGF23 levels is undetermined except in uncommon diseases.
Other clinical features may be suggestive of hypophosphatemia, such as hemolytic anemia and rhabdomyolysis. Fanconi syndrome may present with any combination of uricosuria, aminoaciduria, normoglycemic glucosuria, normal anion gap metabolic acidosis, and phosphaturia. In chronic hypophosphatemia, radiographs and bone biopsies show changes resembling osteomalacia.
 Treatment
Treatment
Hypophosphatemia can be prevented by including phosphate in repletion and maintenance fluids. A rapid decline in calcium levels can occur with parenteral administration of phosphate; oral replacement of phosphate is preferable. Moderate hypophosphatemia (1.0–2.5 mg/dL [or 0.32–0.79 mmol/L]) is usually asymptomatic and does not require treatment. The hypophosphatemia in patients with diabetic ketoacidosis (DKA) will usually correct with normal dietary intake. Chronic hypophosphatemia can be treated with oral phosphate repletion. Mixtures of sodium and potassium phosphate salts may be given to provide 0.5–1 g (16–32 mmol) of phosphate per day; an infusion of this mixture should be used for severe, symptomatic hypophosphatemia (less than 1 mg/dL [0.32 mmol/L]). It should provide 279–310 mg/12 h (or 9–10 mmol/12 h) until the serum phosphorus exceeds 1 mg/dL, and the patient can be switched to oral therapy. The infusion rate should be decreased if hypotension occurs. Monitoring of plasma phosphate, calcium, and potassium every 6 hours is necessary because the response to phosphate supplementation is not predictable. Magnesium deficiency often coexists and should be treated.
Contraindications to phosphate replacement include hypoparathyroidism, advanced CKD, tissue damage and necrosis, and hypercalcemia. When an associated hyperglycemia is treated, phosphate accompanies glucose into cells, and hypophosphatemia may ensue.
 When to Refer
When to Refer
• Patients with refractory hypophosphatemia with increased urinary phosphate excretion may require evaluation by an endocrinologist (eg, for hyperparathyroidism and vitamin D disorders) or a nephrologist (eg, for renal tubular defects).
• Patients with decreased gastrointestinal absorption may require referral to a gastroenterologist.
 When to Admit
When to Admit
Patients with severe or refractory hypophosphatemia will require intravenous phosphate.
Kraft MD. Phosphorus and calcium: a review for the adult nutrition support clinician. Nutr Clin Pract. 2015 Feb;30(1):21–33. Erratum in: Nutr Clin Pract. 2015 Jun;30(3):450. [PMID: 25550328]
Manghat P et al. Phosphate homeostasis and disorders. Ann Clin Biochem. 2014 Nov;51(Pt 6):631–56. [PMID: 24585932]
HYPERPHOSPHATEMIA
ESSENTIALS OF DIAGNOSIS

 Advanced CKD is the most common cause.
Advanced CKD is the most common cause.
 Hyperphosphatemia in the presence of hypercalcemia imposes a high risk of metastatic calcification.
Hyperphosphatemia in the presence of hypercalcemia imposes a high risk of metastatic calcification.
 General Considerations
General Considerations
Advanced CKD with decreased urinary excretion of phosphate is the most common cause of hyperphosphatemia. Other causes are listed in Table 21–9.
Table 21–9. Causes of hyperphosphatemia.
Massive load of phosphate into the extracellular fluid
Exogenous sources
Hypervitaminosis D
Laxatives or enemas containing phosphate
Intravenous phosphate supplement
Endogenous sources
Rhabdomyolysis (especially if chronic kidney disease coexists)
Cell lysis by chemotherapy of malignancy, particularly lymphoproliferative diseases
Metabolic acidosis (lactic acidosis, ketoacidosis)
Respiratory acidosis (phosphate incorporation into cells is disturbed)
Decreased excretion into urine
Chronic kidney disease
Acute kidney injury
Hypoparathyroidism
Pseudohypoparathyroidism
Acromegaly
Pseudohyperphosphatemia
Plasma cell myeloma
Hyperbilirubinemia
Hypertriglyceridemia
Hemolysis in vitro
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The clinical manifestations are those of the underlying disorder or associated condition.
B. Laboratory Findings
In addition to elevated phosphate, blood chemistry abnormalities are those of the underlying disease.
 Treatment
Treatment
Treatment is directed at the underlying cause. Exogenous sources of phosphate, including enteral or parenteral nutrition and medications, should be reduced or eliminated. Dietary phosphate absorption can be reduced by oral phosphate binders, such as calcium carbonate, calcium acetate, sevelamer carbonate, lanthanum carbonate, and aluminum hydroxide. Sevelamer, lanthanum, and aluminum may be used in patients with hypercalcemia, although aluminum use should be limited to a few days because of the risk of aluminum accumulation and neurotoxicity. In acute kidney injury and advanced CKD, dialysis will reduce serum phosphate.
 When to Admit
When to Admit
Patients with acute severe hyperphosphatemia require hospitalization for emergent therapy, possibly including dialysis. Concomitant illnesses, such as acute kidney injury or cell lysis, may necessitate admission.
Criscuolo M et al. Tumor lysis syndrome: review of pathogenesis, risk factors and management of a medical emergency. Expert Rev Hematol. 2016;9(2):197–208. [PMID: 26629730]
Felsenfeld AJ et al. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin Dial. 2015 Nov–Dec;28(6):564–77. [PMID: 26303319]
Ketteler M et al. Treating hyperphosphatemia—current and advancing drugs. Expert Opin Pharmacother. 2016 Oct;17(14):1873–9. [PMID: 27643443]
Leaf DE et al. A physiologic-based approach to the evaluation of a patient with hyperphosphatemia. Am J Kidney Dis. 2013 Feb;61(2):330–6. [PMID: 22938849]
DISORDERS OF MAGNESIUM CONCENTRATION
Normal plasma magnesium concentration is 1.8–3.0 mg/dL (or 0.75–1.25 mmol/L), with about one-third bound to protein and two-thirds existing as free cation. Magnesium excretion is via the kidney. Magnesium’s physiologic effects on the nervous system resemble those of calcium.
Altered magnesium concentration usually provokes an associated alteration of Ca2+. Both hypomagnesemia and hypermagnesemia can decrease PTH secretion or action. Severe hypermagnesemia (greater than 5 mg/dL [2.1 mmol/L]) suppresses PTH secretion with consequent hypocalcemia; this disorder is typically seen only in patients receiving magnesium therapy for preeclampsia. Severe hypomagnesemia causes PTH resistance in end organs and eventually decreased PTH secretion in severe cases.
HYPOMAGNESEMIA
ESSENTIALS OF DIAGNOSIS

 Serum concentration of magnesium may be normal even in the presence of magnesium depletion. Check urinary magnesium excretion if renal magnesium wasting is suspected.
Serum concentration of magnesium may be normal even in the presence of magnesium depletion. Check urinary magnesium excretion if renal magnesium wasting is suspected.
 Causes neurologic symptoms and arrhythmias.
Causes neurologic symptoms and arrhythmias.
 Impairs release of PTH.
Impairs release of PTH.
 General Considerations
General Considerations
Causes of hypomagnesemia are listed in Table 21–10. Normomagnesemia does not exclude magnesium depletion because only 1% of total body magnesium is in the extracellular fluid. Hypomagnesemia and hypokalemia share many etiologies, including diarrhea; alcoholism; and diuretic, aminoglycoside, and amphotericin use. Renal potassium wasting also occurs from hypomagnesemia and is refractory to potassium replacement until magnesium is repleted. Hypomagnesemia also suppresses PTH release and causes end-organ resistance to PTH and low 1,25-dihydroxyvitamin D levels. The resultant hypocalcemia is refractory to calcium replacement until the magnesium is normalized. Molecular mechanisms of magnesium wasting have been revealed in some hereditary disorders. There is an FDA warning about hypomagnesemia for patients taking proton pump inhibitors. The presumed mechanism is decreased intestinal magnesium absorption, but it is not clear why this complication develops in only a small fraction of patients taking these medications.
Table 21–10. Causes of hypomagnesemia.
Diminished absorption or intake
Malabsorption, chronic diarrhea, laxative abuse
Proton pump inhibitors
Prolonged gastrointestinal suction
Small bowel bypass
Malnutrition
Alcoholism
Total parenteral alimentation with inadequate Mg2+ content
Increased renal loss
Diuretic therapy (loop diuretics, thiazide diuretics)
Hyperaldosteronism, Gitelman syndrome
Hyperparathyroidism, hyperthyroidism
Hypercalcemia
Volume expansion
Tubulointerstitial diseases
Transplant kidney
Drugs (aminoglycoside, cetuximab, cisplatin, amphotericin B, pentamidine)
Others
Diabetes mellitus
Post-parathyroidectomy (hungry bone syndrome)
Respiratory alkalosis
Pregnancy
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Common symptoms are those of hypokalemia and hypocalcemia, with weakness and muscle cramps. Marked neuromuscular and central nervous system hyperirritability may produce tremors, athetoid movements, jerking, nystagmus, Babinski response, confusion, and disorientation. Cardiovascular manifestations include hypertension, tachycardia, and ventricular arrhythmias.
B. Laboratory Findings
Urinary excretion of magnesium exceeding 10–30 mg/day or a fractional excretion greater than 2% indicates renal magnesium wasting. Hypocalcemia and hypokalemia are often present. The ECG may show prolonged QT interval due to lengthening of the ST segment. PTH secretion is often suppressed (see Hypocalcemia).
 Treatment
Treatment
Magnesium oxide, 250–500 mg orally once or twice daily, is useful for treating chronic hypomagnesemia. Symptomatic hypomagnesemia requires intravenous magnesium sulfate 1–2 g over 5–60 minutes mixed in either dextrose 5% or 0.9% normal saline. Torsades de pointes in the setting of hypomagnesemia can be treated with 1–2 g of magnesium sulfate in 10 mL of dextrose 5% solution pushed intravenously over 15 minutes. Severe, non–life-threatening deficiency can be treated at a rate to 1–2 g/h over 3–6 hours. Magnesium sulfate may also be given intramuscularly in a dosage of 200–800 mg/day (8–33 mmol/day) in four divided doses. Serum levels must be monitored daily and dosage adjusted to keep the concentration from rising above 3 mg/dL (1.23 mmol/L). Tendon reflexes may be checked for hyporeflexia of hypermagnesemia. K+ and Ca2+ replacement may be required, but patients with hypokalemia and hypocalcemia of hypomagnesemia do not recover without magnesium supplementation.
Patients with normal kidney function can excrete excess magnesium; hypermagnesemia should not develop with replacement dosages. In patients with CKD, magnesium replacement should be done cautiously to avoid hypermagnesemia. Reduced doses (50–75% dose reduction) and more frequent monitoring (at least twice daily) are indicated.
Cheungpasitporn W et al. Dysmagnesemia in hospitalized patients: prevalence and prognostic importance. Mayo Clin Proc. 2015 Aug;90(8):1001–10. [PMID: 26250725]
Gommers LM et al. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016 Jan;65(1):3–13. [PMID: 26696633]
HYPERMAGNESEMIA
ESSENTIALS OF DIAGNOSIS

 Often associated with advanced CKD and chronic intake of magnesium-containing drugs.
Often associated with advanced CKD and chronic intake of magnesium-containing drugs.
 General Considerations
General Considerations
Hypermagnesemia is almost always the result of advanced CKD and impaired magnesium excretion. Antacids and laxatives are underrecognized sources of magnesium. Pregnant patients may have severe hypermagnesemia from intravenous magnesium for preeclampsia and eclampsia. Magnesium replacement should be done cautiously in patients with CKD; dose reductions up to 75% may be necessary to avoid hypermagnesemia.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Muscle weakness, decreased deep tendon reflexes, mental obtundation, and confusion are characteristic manifestations. Weakness, flaccid paralysis, ileus, urinary retention, and hypotension are noted. Serious findings include respiratory muscle paralysis and cardiac arrest.
B. Laboratory Findings and Electrocardiogram
Serum Mg2+ is elevated. In the common setting of CKD, BUN, creatinine, potassium, phosphate, and uric acid may all be elevated. Serum Ca2+ is often low. The ECG may show increased PR interval, broadened QRS complexes, and peaked T waves, probably related to associated hyperkalemia.
 Treatment
Treatment
Exogenous sources of magnesium should be discontinued. Calcium antagonizes Mg2+ and may be given intravenously as calcium chloride, 500 mg or more at a rate of 100 mg (4.1 mmol) per minute. Hemodialysis or peritoneal dialysis may be necessary to remove magnesium, particularly with severe kidney disease.
Long-term use of magnesium hydroxide and magnesium sulfate should be avoided in patients with advanced stages of CKD.
Broman M et al. Analysis of hypo- and hypermagnesemia in an intensive care unit cohort. Acta Anaesthesiol Scand. 2018 May;62(5):648–57. [PMID: 29341068]
Chang WT et al. Calcium, magnesium, and phosphate abnormalities in the emergency department. Emerg Med Clin North Am. 2014 May;32(2):349–66. [PMID: 24766937]
Haider DG et al. Hypermagnesemia is a strong independent risk factor for mortality in critically ill patients: results from a cross-sectional study. Eur J Intern Med. 2015 Sep;26(7):504–7. [PMID: 26049918]
ACID-BASE DISORDERS
Assessment of a patient’s acid-base status requires measurement of arterial pH, partial pressure of CO2 (Pco2), and plasma bicarbonate (HCO3–). Blood gas analyzers directly measure pH and Pco2. The HCO3– value is calculated from the Henderson–Hasselbalch equation:
Note on a chemistry panel it is typically the total venous CO2 (TCO2) that is measured as an assessment of serum HCO3–, although this actually measures the sum of dissolved CO2, carbonic acid and serum bicarbonate. Typically, this is within 1–2 mEq/L of the actual serum HCO3– value, and a good approximation in clinical practice.
Venous blood gases can provide useful information for acid-base assessment since the arteriovenous differences in pH and Pco2 are small and relatively constant. Venous blood pH is usually 0.03–0.04 units lower than arterial blood pH, and venous blood Pco2 7 mm Hg or 8 mm Hg higher than arterial blood Pco2. Calculated HCO3– concentration in venous blood is at most 2 mEq/L higher than arterial blood HCO3–. Arterial and venous blood gases will not be equivalent during a cardiopulmonary arrest; arterial samples should be obtained for the most accurate measurements of pH and Pco2.
TYPES OF ACID-BASE DISORDERS
There are two types of acid-base disorders: acidosis and alkalosis. These disorders can be either metabolic (decreased or increased HCO3–) or respiratory (decreased or increased Pco2). Primary respiratory disorders affect blood acidity by changes in Pco2, and primary metabolic disorders are disturbances in HCO3– concentration. A primary disturbance is usually accompanied by a compensatory response, but the compensation does not fully correct the pH disturbance of the primary disorder. If the pH is < 7.40, the primary process is acidosis, either respiratory (Pco2 greater than 40 mm Hg) or metabolic (HCO3– less than 24 mEq/L). If the pH is > 7.40, the primary process is alkalosis, either respiratory (Pco2 less than 40 mm Hg) or metabolic (HCO3– greater than 24 mEq/L). One respiratory or metabolic disorder with its appropriate compensatory response is a simple acid-base disorder.
MIXED ACID-BASE DISORDERS
Two or three simultaneous disorders can be present in a mixed acid-base disorder, but there can never be two primary respiratory disorders. Uncovering a mixed acid-base disorder is clinically important and requires a methodical approach to acid-base analysis (see box Step-by-Step Analysis of Acid-Base Status). Once the primary disturbance has been determined, the clinician should assess whether the compensatory response is appropriate (Table 21–11). An inadequate or an exaggerated response indicates the presence of another primary acid-base disturbance.
Table 21–11. Primary acid-base disorders and expected compensation.

STEP-BY-STEP ANALYSIS OF ACID-BASE STATUS
Step 1: Determine the primary (or main) disorder—whether it is metabolic or respiratory—from blood pH, HCO3–, and Pco2 values.
Step 2: Determine the presence of mixed acid-base disorders by calculating the range of compensatory responses (see Table 21–11).
Step 3: Calculate the anion gap (see Table 21–12).
Step 4: Calculate the corrected HCO3– concentration if the anion gap is increased.
Step 5: Examine the patient to determine whether the clinical signs are compatible with the acid-base analysis.
The anion gap should be calculated for two reasons. First, it is possible to have an abnormal anion gap even if the sodium, chloride, and bicarbonate concentrations are normal. Second, an anion gap larger than 20 mEq/L suggests a primary metabolic acid-base disturbance regardless of the pH or serum bicarbonate level because a markedly abnormal anion gap is never a compensatory response to a respiratory disorder. In patients with an increased anion gap metabolic acidosis, clinicians should calculate the corrected bicarbonate. In increased anion gap acidoses, there should be roughly a millimole for millimole decrease in HCO3– as the anion gap increases. A corrected HCO3– value higher or lower than normal (24 mEq/L) indicates the concomitant presence of metabolic alkalosis or normal anion gap metabolic acidosis, respectively.
Filippatos T et al. Acid-base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin Drug Saf. 2017 Oct;16(10):1121–32. [PMID: 28748724]
Gomez H et al. Understanding acid base disorders. Crit Care Clin. 2015 Oct;31(4):849–60. [PMID: 26410149]
Hamm LL et al. Acid-base homeostasis. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2232–42. [PMID: 26597304]
Scheiner B et al. Acid-base disorders in liver disease. J Hepatol. 2017 Nov;67(5):1062–73. [PMID: 28684104]
Seifter JL. Integration of acid-base and electrolyte disorders. N Engl J Med. 2014 Nov 6;371(19):1821–31. [PMID: 25372090]
Wiener SW. Toxicologic acid-base disorders. Emerg Med Clin North Am. 2014 Feb;32(1):149–65. [PMID: 24275173]
METABOLIC ACIDOSIS
ESSENTIALS OF DIAGNOSIS

 Decreased HCO3– with acidemia.
Decreased HCO3– with acidemia.
 Classified into increased anion gap acidosis and normal anion gap acidosis.
Classified into increased anion gap acidosis and normal anion gap acidosis.
 Lactic acidosis, ketoacidosis, and toxins produce metabolic acidoses with the largest anion gaps.
Lactic acidosis, ketoacidosis, and toxins produce metabolic acidoses with the largest anion gaps.
 Normal anion gap acidosis is mainly caused by gastrointestinal HCO3– loss or renal tubular acidosis (RTA). Urinary anion gap may help distinguish between these causes.
Normal anion gap acidosis is mainly caused by gastrointestinal HCO3– loss or renal tubular acidosis (RTA). Urinary anion gap may help distinguish between these causes.
 General Considerations
General Considerations
The hallmark of metabolic acidosis is decreased HCO3–. Metabolic acidoses are classified by the anion gap, usually normal or increased (Table 21–12). The anion gap is the difference between readily measured anions and cations.
Table 21–12. Anion gap in metabolic acidosis.1
Decreased (< 6 mEq)
Hypoalbuminemia (decreased unmeasured anion)
Plasma cell dyscrasias
Monoclonal protein (cationic paraprotein accompanied by chloride and bicarbonate)
Bromide intoxication
Increased (> 12 mEq)
Metabolic anion
Diabetic ketoacidosis
Alcoholic ketoacidosis
Lactic acidosis
Chronic kidney disease (advanced stages) (PO43–, SO42–)
Starvation
Metabolic alkalosis (increased number of negative charges on protein)
5-oxoproline acidosis from acetaminophen toxicity
Drug or chemical anion
Salicylate intoxication
Sodium carbenicillin therapy
Methanol (formic acid)
Ethylene glycol (oxalic acid)
Normal (6–12 mEq)
Loss of HCO3
Diarrhea
Recovery from diabetic ketoacidosis
Pancreatic fluid loss, ileostomy (unadapted)
Carbonic anhydrase inhibitors
Chloride retention
Renal tubular acidosis
Ileal loop bladder
Administration of HCl equivalent or NH4Cl
Arginine and lysine in parenteral nutrition
1Reference ranges for anion gap may vary based on differing laboratory methods.
In plasma,
Major unmeasured cations are calcium (2.5 mEq/L), magnesium (2 mEq/L), gamma-globulins, and potassium (4 mEq/L). Major unmeasured anions are albumin (2 mEq/L per g/dL), phosphate (2 mEq/L), sulfate (1 mEq/L), lactate (1–2 mEq/L), and other organic anions (3–4 mEq/L). Traditionally, the normal anion gap has been 12 ± 4 mEq/L. With current auto-analyzers, the reference range may be lower (6 ± 1 mEq/L), primarily from an increase in Cl– values. Despite its usefulness, the anion gap can be misleading. Non–acid-base disorders may cause errors in anion gap interpretation; these disorders include hypoalbuminemia, hypernatremia, or hyponatremia. Antibiotics (eg, carbenicillin is an unmeasured anion; polymyxin is an unmeasured cation) may also cause errors in anion gap interpretation. Although not usually associated with metabolic acidosis, a decreased anion gap can occur because of a reduction in unmeasured anions or an increase in unmeasured cations. In hypoalbuminemia, a 2–3 mEq/L decrease in anion gap will occur for every 1 g/dL decline in serum albumin.
INCREASED ANION GAP ACIDOSIS
Normochloremic metabolic acidosis generally results from addition of organic acids such as lactate, acetoacetate, beta-hydroxybutyrate, and exogenous toxins. Other anions such as isocitrate, alpha-ketoglutarate, malate, and D-lactate may contribute to the anion gap of lactic acidosis, DKA, and acidosis of unknown etiology. Uremia causes an increased anion gap metabolic acidosis from unexcreted organic acids and anions (Table 21–13).
Table 21–13. Common causes and therapy for increased anion gap metabolic acidosis.

A. Lactic Acidosis
Lactic acidosis is a common cause of metabolic acidosis, producing an elevated anion gap and decreased serum pH when present without other acid-base disturbances. Lactate is formed from pyruvate in anaerobic glycolysis. Normally, lactate levels remain low (1 mEq/L) because of metabolism of lactate principally by the liver through gluconeogenesis or oxidation via the Krebs cycle.
In lactic acidosis, lactate levels are at least 4–5 mEq/L but commonly 10–30 mEq/L. There are two basic types of lactic acidosis.
Type A (hypoxic) lactic acidosis is more common, resulting from decreased tissue perfusion; cardiogenic, septic, or hemorrhagic shock; and carbon monoxide or cyanide poisoning. These conditions increase peripheral lactic acid production and decrease hepatic metabolism of lactate as liver perfusion declines.
Type B lactic acidosis may be due to metabolic causes (eg, diabetes mellitus, ketoacidosis, liver disease, kidney disease, infection, leukemia, or lymphoma) or toxins (eg, ethanol, methanol, salicylates, isoniazid, or metformin). Propylene glycol can cause lactic acidosis from decreased liver metabolism; it is used as a vehicle for intravenous drugs, such as nitroglycerin, etomidate, and diazepam. Parenteral nutrition without thiamine causes severe refractory lactic acidosis from deranged pyruvate metabolism. Patients with short bowel syndrome may develop D-lactic acidosis with encephalopathy due to carbohydrate malabsorption and subsequent fermentation by colonic bacteria. Nucleoside analog reverse transcriptase inhibitors can cause type B lactic acidosis due to mitochondrial toxicity.
(For treatment of lactic acidosis, see below and Chapter 27.)
B. Diabetic Ketoacidosis (DKA)
DKA is characterized by hyperglycemia and metabolic acidosis with an increased anion gap:
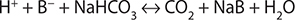
where B– is beta-hydroxybutyrate or acetoacetate, the ketones responsible for the increased anion gap. Diabetics with ketoacidosis may have an additional lactic acidosis from tissue hypoperfusion and increased anaerobic metabolism.
During the recovery phase of DKA, a hyperchloremic non–anion gap acidosis can develop because saline resuscitation results in chloride retention, restoration of GFR, and ketoaciduria.
Patients with DKA and normal kidney function may have marked ketonuria and severe metabolic acidosis but only a mildly increased anion gap. Thus, the size of the anion gap correlates poorly with the severity of the DKA; the urinary loss of Na+ or K+ salts of beta-hydroxybutyrate will lower the anion gap without altering the H+ excretion or the severity of the acidosis. Urine dipsticks for ketones test primarily for acetoacetate and, to a lesser degree, acetone but not the predominant ketoacid, beta-hydroxybutyrate. Dipstick tests for ketones may become more positive even as the patient improves due to the metabolism of beta-hydroxybutyrate. Thus, the patient’s clinical status and pH are better markers of improvement than the anion gap or ketone levels.
C. Alcoholic Ketoacidosis
Chronically malnourished patients who consume large quantities of alcohol daily may develop alcoholic ketoacidosis. Most of these patients have mixed acid-base disorders. Although decreased HCO3– is usual, 50% of the patients may have normal or alkalemic pH. Three types of metabolic acidosis are seen in alcoholic ketoacidosis: (1) Ketoacidosis is due to beta-hydroxybutyratic acid and acetoacetic acid excess. (2) Lactic acidosis: Alcohol metabolism increases the NADH:NAD ratio, causing increased production and decreased utilization of lactate. Accompanying thiamine deficiency, which inhibits pyruvate carboxylase, further enhances lactic acid production in many cases. Moderate to severe elevations of lactate (greater than 6 mmol/L) are seen with concomitant disorders such as sepsis, pancreatitis, or hypoglycemia. (3) Hyperchloremic acidosis from bicarbonate loss in the urine is associated with ketonuria. A concomitant metabolic alkalosis may occur from volume contraction and vomiting. Respiratory alkalosis resulting from alcohol withdrawal, pain, or associated disorders such as sepsis or liver disease may lead to a triple acid-base disorder in these patients.
D. Toxins
(See also Chapter 38.) Multiple toxins and drugs increase the anion gap by increasing endogenous acid production. Common examples include methanol (metabolized to formic acid), ethylene glycol (glycolic and oxalic acid), and salicylates (salicylic acid and lactic acid). The latter can cause a mixed disorder of metabolic acidosis with respiratory alkalosis. In toluene poisoning, the metabolite hippurate is rapidly excreted by the kidney and may present as a normal anion gap acidosis. Isopropanol, which is metabolized to acetone, increases the osmolar gap, but not the anion gap.
E. Uremic Acidosis
As the GFR drops below 15–30 mL/min/m2, the kidneys are increasingly unable to synthesize NH3. The reduced excretion of H+ (as NH4Cl) and accumulation organic anions (eg, phosphate and sulfate) results in an increased anion gap metabolic acidosis.
NORMAL ANION GAP ACIDOSIS
The two major causes are gastrointestinal HCO3– loss and defects in renal acidification (renal tubular acidosis) (Table 21–14). The urinary anion gap can differentiate between these causes.
Table 21–14. Hyperchloremic, normal anion gap metabolic acidoses.

A. Gastrointestinal HCO3– Loss
The gastrointestinal tract secretes bicarbonate at multiple sites. Small bowel and pancreatic secretions contain large amounts of HCO3–; massive diarrhea or pancreatic drainage results in stool HCO3– loss. Hyperchloremia occurs because the ileum and colon secrete HCO3– in exchange for Cl– by countertransport. The resultant volume contraction causes increased Cl– retention by the kidney in the setting of decreased HCO3–. Patients with ureterosigmoidostomies can develop hyperchloremic metabolic acidosis because the colon secretes HCO3– in the urine in exchange for Cl–.
B. Renal Tubular Acidosis
Hyperchloremic acidosis with a normal anion gap and normal (or near normal) GFR, in the absence of diarrhea, defines RTA. The defect is either an inability to excrete H+ as ammonium (inadequate generation of new HCO3–) or inadequate reabsorption of filtered HCO3–. Three major types can be differentiated by the clinical setting, urinary pH, urinary anion gap, and serum K+ level. The pathophysiologic mechanisms of RTA have been elucidated by identifying the responsible molecules and gene mutations.
1. Classic distal RTA (type I)—This disorder is characterized by selective deficiency in H+ secretion by alpha intercalated cells in the collecting tubule. Despite acidosis, the urine cannot be fully acidified (urine pH > 5.5), which retards the binding of H+ to phosphate (H+ + HPO4 2– → H2PO4) and inhibits titratable acid excretion. Furthermore, urinary excretion of NH4Cl is decreased, and the urinary anion gap is inappropriately positive. Enhanced K+ excretion occurs probably due to less competition from H+ in the distal nephron transport system. Nephrocalcinosis and nephrolithiasis are often seen in patients with distal RTA. Hypercalciuria (chronic acidosis decreases tubular calcium reabsorption, and increased mobilization from bone), low urinary citrate and an alkaline urine pH promote calcium phosphate precipitation. Distal RTA develops as a consequence of paraproteinemias, autoimmune disease, and drugs and toxins such as amphotericin.
2. Proximal RTA (type II)—Proximal RTA is due to a defect in the ability of the proximal tubule to reabsorb filtered HCO3–. About 90% of filtered HCO3– is usually reabsorbed by the proximal tubule. A defect in proximal HCO3– reabsorption will overwhelm the distal tubule’s limited capacity to reabsorb HCO3–, resulting in bicarbonaturia and metabolic acidosis. Distal delivery of HCO3– declines as the plasma HCO3– level decreases and when the plasma HCO3– level drops to a threshold of 15–18 mEq/L, the distal nephron can reabsorb the diminished filtered load of HCO3–. At this point bicarbonaturia resolves, and the urinary pH becomes acidic. Thiazide-induced volume contraction can be used to enhance proximal HCO3– reabsorption, leading to the decrease in distal HCO3– delivery and improvement of bicarbonaturia. The increased delivery of HCO3– to the distal nephron enhances K+ secretion, and hypokalemia results when a patient is treated with HCO3– and without adequate K+supplementation. Proximal RTA can exist with other proximal reabsorption defects resulting in glucosuria, aminoaciduria, phosphaturia, and uricosuria (Fanconi syndrome). Causes include plasma cell myeloma and nephrotoxic drugs. Carbonic anhydrase inhibitors (acetazolamide) can cause proximal RTA.
3. Hyporeninemic hypoaldosteronemic RTA (type IV)—Type IV is the most common RTA in clinical practice. This is due to a defect is aldosterone action that impairs distal nephron Na+ reabsorption and therefore K+ and H+ excretion. The clinical presentation is a hyperkalemic nongap metabolic acidosis. Common causes include diabetic nephropathy and tubulointerstitial renal diseases. In patients with these disorders, drugs such as ACE inhibitors, ARBs, spironolactone, and NSAIDs, can worsen the hyperkalemia and acidosis.
C. Other Causes of Nongap Acidosis
A dilutional acidosis may occur when the extracellular volume is rapidly expanded with 0.9% normal saline. Hyperalimentation fluids may contain amino acid solutions that acidify when metabolized, such as arginine hydrochloride and lysine hydrochloride.
1. Urine anion gap—Increased renal NH4Cl excretion to enhance H+ removal is the normal physiologic response to metabolic acidosis. The daily urinary excretion of NH4Cl can be increased from 30 mEq to 200–300 mEq in response to acidosis.
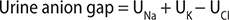
The urinary anion gap is an estimate of the urine excretion of NH4Cl and may help differentiate between gastrointestinal and renal causes of hyperchloremic acidosis. If the cause is gastrointestinal HCO3– loss (diarrhea), renal acidification remains intact and NH4Cl excretion increases appropriately (urinary anion gap will be negative). In a distal RTA, ammonium excretion is impaired and the urinary anion gap is positive. In proximal (type II) RTA, the primary problem is impaired HCO3– reabsorption, leading to increased HCO3– excretion rather than decreased NH4Cl excretion and the urinary anion gap is often negative.
2. Urine osmolal gap—When large amounts of other anions (eg, hippurate in toluene poisoning) are present in the urine, the urinary anion gap may not be reliable. In this situation, NH4+ excretion can be estimated to be 50% of the urinary osmolar gap where urine concentrations and osmolality are in mmol/L.
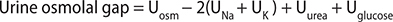
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Symptoms of metabolic acidosis are mainly those of the underlying disorder. Compensatory hyperventilation is an important clinical sign and may be misinterpreted as a primary respiratory disorder; Kussmaul breathing (deep, regular, sighing respirations) may be seen with severe metabolic acidosis.
B. Laboratory Findings
Blood pH, serum HCO3–, and Pco2 are decreased. Anion gap may be normal (hyperchloremic) or increased (normochloremic). Hyperkalemia may be seen.
 Treatment
Treatment
A. Increased Anion Gap Acidosis
Treatment is aimed at the underlying disorder, such as insulin and fluid therapy for diabetes and appropriate volume resuscitation to restore tissue perfusion (see Table 21–13). NaHCO3 therapy is controversial in the treatment of increased anion gap metabolic acidoses and is usually reserved for severe cases (arterial pH < 7.1–7.2). Large amounts of NaHCO3 may have deleterious effects, including hypernatremia, hyperosmolality, volume overload, and worsening of intracellular acidosis.
B. Normal Anion Gap Acidosis
Treatment of RTA is mainly achieved by administration of alkali (either as bicarbonate or citrate) to correct metabolic abnormalities and prevent nephrocalcinosis and CKD.
Large amounts of oral alkali (10–15 mEq/kg/day) (Table 21–14) may be required to treat proximal RTA because much of the administered alkali is excreted into the urine. This can exacerbate hypokalemia and a mixture of sodium and potassium salts is often required. Treatment of type 1 distal RTA requires less alkali (1–2 mEq/kg/day) than proximal RTA and potassium supplementation is typically required.
For type IV RTA, dietary potassium restriction may be necessary and potassium-retaining drugs should be withdrawn. Loop diuretics may be beneficial. Fludrocortisone may be effective in some cases without significant volume expansion. In some cases, oral alkali supplementation (1–2 mEq/kg/day) may be required.
 When to Refer
When to Refer
Most clinicians will refer patients with renal tubular acidoses to a nephrologist for evaluation and possible alkali therapy.
 When to Admit
When to Admit
Patients will require emergency department evaluation or hospital admission depending on the severity of the acidosis and underlying conditions.
Fayfman M et al. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017 May;101(3):587–606. [PMID: 28372715]
Lalau JD et al. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017 Nov;19(11):1502–12. [PMID: 28417525]
Palmer BF et al. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med. 2015 Aug 6;373(6):548–59. [PMID: 26244308]
Seheult J et al. Lactic acidosis: an update. Clin Chem Lab Med. 2017 Mar 1;55(3):322–33. [PMID: 27522622]
Sharma S et al. Comprehensive clinical approach to renal tubular acidosis. Clin Exp Nephrol. 2015 Aug;19(4):556–61. [PMID: 25951806]
Suetrong B et al. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016 Jan;149(1):252–61. [PMID: 26378980]
METABOLIC ALKALOSIS
ESSENTIALS OF DIAGNOSIS

 High HCO3– with alkalemia.
High HCO3– with alkalemia.
 Evaluate effective circulating volume by physical examination.
Evaluate effective circulating volume by physical examination.
 Urinary chloride concentration differentiates saline-responsive alkalosis from saline-unresponsive alkalosis.
Urinary chloride concentration differentiates saline-responsive alkalosis from saline-unresponsive alkalosis.
 General Considerations
General Considerations
Metabolic alkalosis is characterized by high HCO3–. Abnormalities that generate HCO3– are called “initiation factors,” whereas abnormalities that promote renal conservation of HCO3– are called “maintenance factors.” Thus, metabolic alkalosis may persist even after the initiation factors have resolved.
The causes of metabolic alkalosis are classified into two groups based on “saline responsiveness” using the urine Cl– as a marker for volume status (Table 21–15). Saline-responsive metabolic alkalosis is a sign of extracellular volume contraction and is much more commonly encountered than saline-unresponsive alkalosis, which implies excessive total body bicarbonate with either euvolemia or hypervolemia. The compensatory increase in Pco2 rarely exceeds 55 mm Hg; higher Pco2 values imply a superimposed primary respiratory acidosis.
Table 21–15. Metabolic alkalosis.

A. Saline-Responsive Metabolic Alkalosis
Saline-responsive alkalosis is characterized by normotensive extracellular volume contraction (contraction alkalosis) and hypokalemia. Hypotension and orthostasis may be seen. In vomiting or nasogastric suction, loss of acid (HCl) initiates the alkalosis, but volume contraction from Cl– loss maintains the alkalosis (due to increased proximal reabsorption of NaHCO3 and secondary hyperaldosteronsim). Renal Cl– reabsorption is high, and urine Cl– is low (less than 20 mEq/L). In alkalosis, bicarbonaturia may force Na+ excretion as the accompanying cation even if volume depletion is present, and urine Cl– is preferred to urine Na+ as a measure of extracellular volume. Diuretics are a common cause of metabolic alkalosis, but in this setting, the utility of urine Cl– to assess volume is limited.
Metabolic alkalosis is generally associated with hypokalemia due to the direct effect of alkalosis on renal potassium excretion and secondary hyperaldosteronism from volume depletion. Hypokalemia exacerbates the metabolic alkalosis by increasing bicarbonate reabsorption in the proximal tubule and hydrogen ion secretion in the distal tubule. Administration of KCl will correct the disorder.
B. Saline-Unresponsive Alkalosis
1. Hyperaldosteronism—Primary hyperaldosteronism causes extracellular volume expansion and hypertension by increasing distal sodium reabsorption. Aldosterone increases H+ and K+ excretion, producing metabolic alkalosis and hypokalemia. In an attempt to decrease extracellular volume, high levels of NaCl are excreted resulting in a high urine Cl– (greater than 20 mEq/L). Therapy with saline (NaCl) in this setting will only increase volume expansion and hypertension and will not treat the underlying problem of mineralocorticoid excess.
2. Alkali administration with decreased GFR—The normal kidney has a substantial capacity for bicarbonate excretion, protecting against metabolic alkalosis even with large HCO3– intake. However, urinary excretion of bicarbonate is inadequate in CKD. If large amounts of HCO3– are consumed, as with intensive antacid therapy, metabolic alkalosis will occur. Lactate, citrate, and gluconate can also cause metabolic alkalosis because they are metabolized to bicarbonate. In milk-alkali syndrome, sustained heavy ingestion of absorbable antacids and milk causes hypercalcemic kidney injury and metabolic alkalosis. Volume contraction from renal hypercalcemic effects exacerbates the alkalosis.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
There are no characteristic symptoms or signs. Orthostatic hypotension may be encountered. Concomitant hypokalemia may cause weakness and hyporeflexia. Tetany and neuromuscular irritability occur rarely.
B. Laboratory Findings
The arterial blood pH and bicarbonate are elevated. With respiratory compensation, the arterial Pco2 is increased. Serum potassium and chloride are decreased. There may be an increased anion gap. The urine Cl– can differentiate between saline-responsive (less than 25 mEq/L) and unresponsive (greater than 40 mEq/L) causes.
 Treatment
Treatment
Mild alkalosis is generally well tolerated. Severe or symptomatic alkalosis (pH > 7.60) requires urgent treatment.
A. Saline-Responsive Metabolic Alkalosis
Therapy for saline-responsive metabolic alkalosis is correction of the extracellular volume deficit with isotonic saline. Diuretics should be discontinued. H2-blockers or proton pump inhibitors may be helpful in patients with alkalosis from nasogastric suctioning. If pulmonary or cardiovascular disease prohibits adequate resuscitation, acetazolamide will increase renal bicarbonate excretion. Hypokalemia may develop because bicarbonate excretion may induce kaliuresis. Severe cases, especially those with reduced kidney function, may require dialysis with low-bicarbonate dialysate.
B. Saline-Unresponsive Metabolic Alkalosis
Therapy for saline-unresponsive metabolic alkalosis includes surgical removal of a mineralocorticoid-producing tumor or blockage of aldosterone effect a mineralocorticoid antagonist (see Chapter 26).
Feldman M et al. Respiratory compensation to a primary metabolic alkalosis in humans. Clin Nephrol. 2012 Nov;78(5):365–9. [PMID: 22854166]
Peixoto AJ et al. Treatment of severe metabolic alkalosis in a patient with congestive heart failure. Am J Kidney Dis. 2013 May;61(5):822–7. [PMID: 23481366]
Soifer JT et al. Approach to metabolic alkalosis. Emerg Med Clin North Am. 2014 May;32(2):453–63. [PMID: 24766943]
Yi JH et al. Metabolic alkalosis from unsuspected ingestion: use of urine pH and anion gap. Am J Kidney Dis. 2012 Apr;59(4):577–81. [PMID: 22265393]
RESPIRATORY ACIDOSIS (Hypercapnia)
 General Considerations
General Considerations
Respiratory acidosis results from hypoventilation and subsequent hypercapnia. Pulmonary and extrapulmonary disorders can cause hypoventilation.
Acute respiratory failure is associated with severe acidosis and only a small increase in the plasma bicarbonate. After 6–12 hours, the primary increase in Pco2 evokes a renal compensation to excrete more acid and to generate more HCO3–; complete metabolic compensation by the kidney takes several days.
Chronic respiratory acidosis is generally seen in patients with underlying lung disease, such as chronic obstructive pulmonary disease. Renal excretion of acid as NH4Cl results in a compensatory metabolic alkalosis. When chronic respiratory acidosis is corrected suddenly, posthypercapnic metabolic alkalosis may persist until the kidneys excrete the excess bicarbonate over 2–3 days.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
With acute onset, somnolence, confusion, mental status changes, asterixis, and myoclonus may develop. Severe hypercapnia increases cerebral blood flow, cerebrospinal fluid pressure, and intracranial pressure; papilledema and idiopathic intracranial hypertension (pseudotumor cerebri) may be seen.
B. Laboratory Findings
Arterial pH is low and Pco2 is increased. Serum HCO3– is elevated but does not fully correct the pH. If the disorder is chronic, hypochloremia is seen. Respiratory etiologies of respiratory acidosis usually have a wide A-a gradient; a relatively normal A-a gradient suggests a nonpulmonary (eg, central) etiology.
 Treatment
Treatment
If opioid overdose is a possible diagnosis or there is no other obvious cause for hypoventilation, the clinician should consider a diagnostic and therapeutic trial of intravenous naloxone (see Chapter 38). In all forms of respiratory acidosis, treatment is directed at the underlying disorder to improve ventilation.
Bruno CM et al. Acid-base disorders in patients with chronic obstructive pulmonary disease: a pathophysiological review. J Biomed Biotechnol. 2012;2012:915150. [PMID: 22500110]
Chebbo A et al. Hypoventilation syndromes. Med Clin North Am. 2011 Nov;95(6):1189–202. [PMID: 22032434]
Schwartzstein RM et al. Rising PaCO2 in the ICU: using a physiologic approach to avoid cognitive biases. Chest. 2011 Dec;140(6):1638–42. [PMID: 22147823]
RESPIRATORY ALKALOSIS
 General Considerations
General Considerations
Respiratory alkalosis occurs when hyperventilation reduces the Pco2, increasing serum pH. The most common cause of respiratory alkalosis is hyperventilation syndrome (Table 21–16), but bacterial septicemia and cirrhosis are other common causes. In pregnancy, progesterone stimulates the respiratory center, producing an average Pco2 of 30 mm Hg and respiratory alkalosis. Symptoms of acute respiratory alkalosis are related to decreased cerebral blood flow induced by the disorder.
Table 21–16. Causes of respiratory alkalosis.
Hypoxia
Decreased inspired oxygen tension
High altitude
Ventilation/perfusion inequality
Hypotension
Severe anemia
CNS-mediated disorders
Voluntary hyperventilation
Anxiety-hyperventilation syndrome
Neurologic disease
Cerebrovascular accident (infarction, hemorrhage)
Infection
Trauma
Tumor
Pharmacologic and hormonal stimulation
Salicylates
Nicotine
Xanthines
Pregnancy (progesterone)
Liver failure
Gram-negative septicemia
Recovery from metabolic acidosis
Heat exposure
Pulmonary disease
Interstitial lung disease
Pneumonia
Pulmonary embolism
Pulmonary edema
Mechanical overventilation
Adapted, with permission, from Gennari FJ. Respiratory acidosis and alkalosis. In: Maxwell and Kleeman’s Clinical Disorders of Fluid and Electrolyte Metabolism, 5th ed. Narins RG (editor). McGraw-Hill, 1994.
Determination of appropriate metabolic compensation may reveal an associated metabolic disorder (see Mixed Acid-Base Disorders).
As in respiratory acidosis, the metabolic compensation is greater if the respiratory alkalosis is chronic (see Table 21–11). Although serum HCO3– is frequently less than 15 mEq/L in metabolic acidosis, such a low level in respiratory alkalosis is unusual and may represent a concomitant primary metabolic acidosis.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
In acute cases (hyperventilation), there is light-headedness, anxiety, perioral numbness, and paresthesias. Tetany occurs from a low ionized calcium, since severe alkalosis increases calcium binding to albumin.
B. Laboratory Findings
Arterial blood pH is elevated, and Pco2 is low. Serum bicarbonate is decreased in chronic respiratory alkalosis.
 Treatment
Treatment
Treatment is directed toward the underlying cause. In acute hyperventilation syndrome from anxiety, the traditional treatment of breathing into a paper bag should be discouraged because it does not correct Pco2 and may decrease Po2. Reassurance may be sufficient for the anxious patient, but sedation may be necessary if the process persists. Hyperventilation is usually self-limited since muscle weakness caused by the respiratory alkalemia will suppress ventilation. Rapid correction of chronic respiratory alkalosis may result in metabolic acidosis as Pco2 is increased with a previous compensatory decrease in HCO3–. The severity of hypocapnia in critically ill patients has been associated with adverse outcomes.
Batlle D et al. Metabolic acidosis or respiratory alkalosis? Evaluation of a low plasma bicarbonate using the urine anion gap. Am J Kidney Dis. 2017 Sep;70(3):440–4. [PMID: 28599903]
Palmer BF. Evaluation and treatment of respiratory alkalosis. Am J Kidney Dis. 2012 Nov;60(5):834–8. [PMID: 22871240]
FLUID MANAGEMENT
Daily parenteral maintenance fluids and electrolytes for an average adult of 70 kg would include at least 2 L of water in the form of 0.45% saline with 20 mEq/L of potassium chloride. Patients with hypoglycemia, starvation ketosis, or ketoacidosis being treated with insulin may require 5% dextrose-containing solutions. Guidelines for gastrointestinal fluid losses are shown in Table 21–17.
Table 21–17. Replacement guidelines for sweat and gastrointestinal fluid losses.

Weight loss or gain is the best indication of water balance. Insensible water loss should be considered in febrile patients. Water loss increases by 100–150 mL/day for each degree of body temperature over 37°C.
In patients requiring maintenance and possibly replacement of fluid and electrolytes by parenteral infusion, the total daily ration should be administered continuously over 24 hours to ensure optimal utilization.
If intravenous fluids are the only source of water, electrolytes, and calories for longer than a week, parenteral nutrition containing amino acids, lipids, trace metals, and vitamins may be indicated. (See Chapter 29.)
Balanced crystalloid-like lactated Ringer’s solution, rather than normal saline, is the resuscitation fluid of choice in most settings (see Chapter 12). Excessive fluid resuscitation and maintenance are complications in hospitalized patients, especially those with critical illness or acute kidney injury. These complications have been associated with worsened outcomes, such as prolonged mechanical ventilation, dependence on dialysis, and longer hospitalization with increased mortality.
Bentzer P et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016 Sep 27;316(12):1298–309. [PMID: 27673307]
Moritz ML et al. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015 Oct;373(14):1350–60. [PMID: 26422725]
Schindler AW et al. Evidence-based fluid management in the ICU. Curr Opin Anaesthesiol. 2016 Apr;29(2):158–65. [PMID: 26784351]
1See also Chapter 26 for discussion of the treatment of hypoparathyroidism.