27
Diabetes Mellitus & Hypoglycemia
Umesh Masharani, MB, BS, MRCP (UK)
DIABETES MELLITUS
ESSENTIALS OF DIAGNOSIS

Type 1 diabetes
 Polyuria, polydipsia, and weight loss associated with random plasma glucose of 200 mg/dL (11.1 mmol/L) or more.
Polyuria, polydipsia, and weight loss associated with random plasma glucose of 200 mg/dL (11.1 mmol/L) or more.
 Plasma glucose of 126 mg/dL (7.0 mmol/L) or more after an overnight fast, documented on more than one occasion.
Plasma glucose of 126 mg/dL (7.0 mmol/L) or more after an overnight fast, documented on more than one occasion.
 Ketonemia, ketonuria, or both.
Ketonemia, ketonuria, or both.
 Islet autoantibodies are frequently present.
Islet autoantibodies are frequently present.
Type 2 diabetes
 Many patients are over 40 years of age and are obese.
Many patients are over 40 years of age and are obese.
 Polyuria and polydipsia. Ketonuria and weight loss are uncommon at time of diagnosis. Candidal vaginitis may be an initial manifestation.
Polyuria and polydipsia. Ketonuria and weight loss are uncommon at time of diagnosis. Candidal vaginitis may be an initial manifestation.
 Plasma glucose of 126 mg/dL or more after an overnight fast on more than one occasion. Two hours after 75 g oral glucose, diagnostic values are 200 mg/dL (11.1 mmol) or more.
Plasma glucose of 126 mg/dL or more after an overnight fast on more than one occasion. Two hours after 75 g oral glucose, diagnostic values are 200 mg/dL (11.1 mmol) or more.
 HbA1c 6.5% or more.
HbA1c 6.5% or more.
 Hypertension, dyslipidemia, and atherosclerosis are often associated.
Hypertension, dyslipidemia, and atherosclerosis are often associated.
 Epidemiologic Considerations
Epidemiologic Considerations
An estimated 34.2 million people (10.5%) in the United States have diabetes mellitus, of which approximately 5–10% have type 1 diabetes and most of the rest have type 2 diabetes. A third group designated as “other specific types” by the American Diabetes Association (ADA) (Table 27–1) number in the thousands.
Table 27–1. Other specific types of diabetes mellitus.
Genetic defects of pancreatic B cell function
MODY 1 (HNF-4alpha); rare
MODY 2 (glucokinase); less rare
MODY 3 (HNF-1alpha); accounts for two-thirds of all MODY
MODY 4 (PDX1); very rare
MODY 5 (HNF-1beta); very rare
MODY 6 (neuroD1); very rare
Mitochondrial DNA
Genetic defects in insulin action
Type A insulin resistance
Leprechaunism
Rabson-Mendenhall syndrome
Lipoatrophic diabetes
Diseases of the exocrine pancreas
Endocrinopathies
Drug- or chemical-induced diabetes
Other genetic syndromes (Down, Klinefelter, Turner, others) sometimes associated with diabetes
MODY, maturity-onset diabetes of the young; PDX1, pancreatic duodenal homeobox 1.
 Classification & Pathogenesis
Classification & Pathogenesis
Diabetes mellitus is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either a deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate for the resistance.
A. Type 1 Diabetes Mellitus
This form of diabetes is due to pancreatic islet B cell destruction predominantly by an autoimmune process in over 95% of cases (type 1A) and idiopathic in less than 5% (type 1B). The rate of pancreatic B cell destruction is quite variable, being rapid in some individuals and slow in others. It occurs at any age but most commonly arises in children and young adults with a peak incidence at age 10–14 years. Type 1 diabetes is usually associated with ketosis in its untreated state. Exogenous insulin is therefore required to reverse the catabolic state, prevent ketosis, reduce the hyperglucagonemia, and reduce blood glucose.
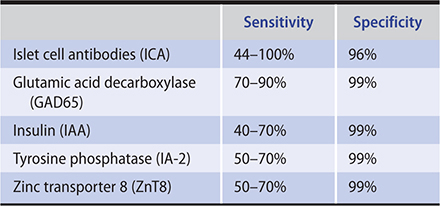
1. Immune-mediated type 1 diabetes mellitus (type 1A)—Approximately one-third of the disease susceptibility is due to genes and two-thirds to environmental factors. Genes that are related to the HLA locus contribute about 40% of the genetic risk. About 95% of patients with type 1 diabetes possess either HLA-DR3 or HLA-DR4, compared with 45–50% of white controls. HLA-DQ genes are even more specific markers of type 1 susceptibility, since a particular variety (HLA-DQB1*0302) is found in the DR4 patients with type 1, while a “protective” gene (HLA-DQB1*0602) is often present in the DR4 controls. The other important gene that contributes to about 10% of the genetic risk is found at the 5′ polymorphic region of the insulin gene. Mutations in genes associated with T cell tolerance can also cause autoimmune diabetes. The autoimmune regulatory gene (AIRE) product regulates the expression of several proteins in the thymus causing the deletion of self-reactive T cells. Type 1 diabetes mellitus as well as other autoimmune disorders (autoimmune polyglandular syndrome 1) develop in 20% of individuals with homozygote mutations in AIRE. Most patients with type 1 diabetes mellitus have circulating antibodies to islet cells (ICA), glutamic acid decarboxylase 65 (GAD65), insulin (IAA), tyrosine phosphatase IA2 (ICA-512), and zinc transporter 8 (ZnT8) at the time the diagnosis is made (Table 27–2). These antibodies facilitate screening for an autoimmune cause of diabetes, particularly screening siblings of affected children, as well as adults with atypical features of type 2 diabetes mellitus. Also, low levels of anti-insulin antibodies develop in almost all patients once they are treated with insulin.
Table 27–2. Diagnostic sensitivity and specificity of autoimmune markers in patients with newly diagnosed type 1 diabetes mellitus.
Family members of diabetic probands are at increased lifetime risk for developing type 1 diabetes mellitus. A child whose mother has type 1 diabetes has a 3% risk of developing the disease and a 6% risk if the child’s father has it. The risk in siblings is related to the number of HLA haplotypes that the sibling shares with the diabetic proband. If one haplotype is shared, the risk is 6% and if two haplotypes are shared, the risk increases to 12–25%. The highest risk is for monozygotic twins, where the concordance rate is 25–50%.
Some patients with a milder expression of type 1 diabetes mellitus initially retain enough B cell function to avoid ketosis, but as their B cell mass diminishes later in life, dependence on insulin therapy develops. Islet cell antibody surveys among northern Europeans indicate that up to 15% of “type 2” diabetic patients may actually have this mild form of type 1 diabetes (latent autoimmune diabetes of adulthood; LADA). Evidence for environmental factors playing a role in the development of type 1 diabetes include the observation that the disease is more common in Scandinavian countries and becomes progressively less frequent in countries nearer and nearer to the equator. Also, the risk for type 1 diabetes increases when individuals who normally have a low risk emigrate to the Northern Hemisphere. For example, Pakistani children born and raised in Bradford, England, have a higher risk for developing type 1 diabetes compared with children who lived in Pakistan all their lives.
Which environmental factor is responsible for the increased risk is not known. Breastfeeding in the first 6 months of life appears to be protective. There is accumulating evidence that improvements in public health and reduced infections (especially parasitic) lead to immune system dysregulation and development of autoimmune disorders such as asthma and type 1 diabetes.
Check point inhibitor immunotherapies for advanced malignancies, such as nivolumab, pembrolizumab, and ipilimumab, can precipitate autoimmune disorders, including type 1 diabetes. The onset of diabetes can be rapid and the patients frequently have diabetic ketoacidosis at presentation. Autoantibodies against islet antigens are only present in about 50% of patients. Patients receiving these drugs should be carefully monitored for the development of diabetes.
2. Idiopathic type 1 diabetes mellitus (type 1B)—Approximately 5% of subjects have no evidence of pancreatic B cell autoimmunity to explain their insulinopenia and ketoacidosis. This subgroup has been classified as “idiopathic type 1 diabetes” and designated as “type 1B.” Although only a minority of patients with type 1 diabetes fall into this group, most of these individuals are of Asian or African origin. About 4% of the West Africans with ketosis-prone diabetes are homozygous for a mutation in PAX-4 (Arg133Trp)—a transcription factor that is essential for the development of pancreatic islets.
B. Type 2 Diabetes Mellitus
This represents a heterogeneous group of conditions that used to occur predominantly in adults, but it is now more frequently encountered in children and adolescents. Circulating endogenous insulin is sufficient to prevent ketoacidosis but is inadequate to prevent hyperglycemia in the face of increased needs owing to tissue insensitivity (insulin resistance).
Genetic and environmental factors combine to cause both the insulin resistance and the beta cell loss. Most epidemiologic data indicate strong genetic influences, since in monozygotic twins over 40 years of age, concordance develops in over 70% of cases within a year whenever type 2 diabetes develops in one twin. So far, more than 30 genetic loci have been associated with an increased risk of type 2 diabetes. A significant number of the identified loci appear to code for proteins that have a role in beta cell function or development. One of the genetic loci with the largest risk effect is TCF7L2. This gene codes for a transcription factor involved in the WNT signaling pathway that is required for normal pancreatic development.
Early in the disease process, hyperplasia of pancreatic B cells occurs and probably accounts for the fasting hyperinsulinism and exaggerated insulin and proinsulin responses to glucose and other stimuli. With time, chronic deposition of amyloid in the islets may combine with inherited genetic defects progressively to impair B cell function.
Obesity is the most important environmental factor causing insulin resistance. The degree and prevalence of obesity varies among different racial groups with type 2 diabetes. While obesity is apparent in no more than 30% of Chinese and Japanese patients with type 2, it is found in 60–70% of North Americans, Europeans, or Africans with type 2 and approaches 100% of patients with type 2 among Pima Indians or Pacific Islanders from Nauru or Samoa.
Visceral obesity, due to accumulation of fat in the omental and mesenteric regions, correlates with insulin resistance; subcutaneous abdominal fat seems to have less of an association with insulin insensitivity. There are many patients with type 2 diabetes who, while not overtly obese, have increased visceral fat; they are termed the “metabolically obese.” Exercise may affect the deposition of visceral fat as suggested by CT scans of Japanese wrestlers, whose extreme obesity is predominantly subcutaneous. Their daily vigorous exercise program prevents accumulation of visceral fat, and they have normal serum lipids and euglycemia despite daily intakes of 5000–7000 kcal and development of massive subcutaneous obesity.
C. Other Specific Types of Diabetes Mellitus
1. Maturity-onset diabetes of the young (MODY)—This subgroup of monogenic disorders is characterized by noninsulin requiring diabetes with autosomal dominant inheritance and an age at onset of 25 years or younger. Patients are nonobese, and their hyperglycemia is due to impaired glucose-induced secretion of insulin. Six types of MODY have been described (Table 27–1). Except for MODY 2, in which a glucokinase gene is defective, all other types involve mutations of a nuclear transcription factor that regulates islet gene expression. Patients younger than 30 years with endogenous insulin production (urinary C-peptide/creatinine ratio of 0.2 nmol/mmol or higher) and negative autoantibodies are candidates for genetic screening for MODY. The enzyme glucokinase is a rate-limiting step in glycolysis and determines the rate of adenosine triphosphate (ATP) production from glucose and the insulin secretory response in the beta cell. MODY 2, due to glucokinase mutations, is usually quite mild, associated with only slight fasting hyperglycemia and few if any microvascular diabetic complications. MODY 3, due to mutations in hepatic nuclear factor 1 alpha is the most common form, accounting for two-thirds of all MODY cases. Initially, patients with MODY 3 are responsive to sulfonylurea therapy but the clinical course is of progressive beta cell failure and eventual need for insulin therapy. Mutations in both alleles of glucokinase present with more severe neonatal diabetes. Mutation in one allele of the pancreatic duodenal homeobox 1 (PDX1) causes diabetes usually at a later age (~ 35 years) than other forms of MODY; mutations in both alleles of PDX1 cause pancreatic agenesis.
2. Diabetes mellitus associated with a mutation of mitochondrial DNA—Since sperm do not contain mitochondria, only the mother transmits mitochondrial genes to her offspring. Diabetes due to mutations of mitochondrial DNA occurs in less than 2% of patients with diabetes. The most common cause is the A3243G mutation in the gene coding for the tRNA (Leu, UUR). Diabetes usually develops in these patients in their late 30s, and characteristically, they also have hearing loss (maternally inherited diabetes and deafness [MIDD]).
3. Wolfram syndrome—Wolfram syndrome is an autosomal recessive neurodegenerative disorder first evident in childhood. It consists of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, hence the acronym DIDMOAD. It is due to mutations in a gene named WFS1, which encodes a 100.3 KDa transmembrane protein localized in the endoplasmic reticulum. Cranial diabetes insipidus and sensorineural deafness develop during the second decade in 60–75% of patients. Ureterohydronephrosis, neurogenic bladder, cerebellar ataxia, peripheral neuropathy, and psychiatric illness develop later in many patients.
4. Autosomal recessive syndromes—Homozygous mutations in a number of pancreatic transcription factors, NEUROG3, PTF1A, RFX6, and GLI-similar 3 (GLIS3), cause neonatal or childhood diabetes. Homozygous PTF1A mutations result in absent pancreas and cerebellar atrophy; NEUROG3 mutations cause severe malabsorption and diabetes before puberty. Homozygous mutations in RFX6 cause the Mitchell-Riley syndrome characterized by absence of all islet cell types apart from pancreatic polypeptide cells, hypoplasia of the pancreas and gallbladder, and intestinal atresia. GLIS3 gene plays a role in transcription of insulin gene, and homozygous mutations cause neonatal diabetes and congenital hypothyroidism. The gene EIF2AK3 encodes PKR-like ER kinase (PERK), which controls one of the pathways of the unfolded protein response. Absence of PERK leads to inadequate response to ER stress and accelerated beta cell apoptosis. Patients with mutation in this gene have neonatal diabetes, epiphyseal dysplasia, developmental delay, and liver and kidney dysfunction (Wolcott-Rallison syndrome).
5. Diabetes mellitus secondary to other causes—Endocrine tumors secreting growth hormone, glucocorticoids, catecholamines, glucagon, or somatostatin can cause glucose intolerance (Table 27–3). In the first four of these situations, peripheral responsiveness to insulin is impaired. With excess of glucocorticoids, catecholamines, or glucagon, increased hepatic output of glucose is a contributory factor; in the case of catecholamines, decreased insulin release is an additional factor in producing carbohydrate intolerance, and with somatostatin, inhibition of insulin secretion is the major factor. Diabetes mainly occurs in individuals with underlying defects in insulin secretion, and hyperglycemia typically resolves when the hormone excess is resolved.
Table 27–3. Secondary causes of hyperglycemia.
Hyperglycemia due to tissue insensitivity to insulin
Medications (corticosteroids, sympathomimetic drugs, niacin, alpelisib)
Hormonal tumors (acromegaly, Cushing syndrome, glucagonoma, pheochromocytoma)
Liver disease (cirrhosis, hemochromatosis)
Muscle disorders (myotonic dystrophy)
Adipose tissue disorders (lipodystrophy, truncal obesity)
Hyperglycemia due to reduced insulin secretion
Medications (thiazide diuretics, phenytoin, pentamidine, calcineurin inhibitors)
Hormonal tumors (somatostatinoma, pheochromocytoma)
Pancreatic disorders (pancreatitis, hemosiderosis, hemochromatosis)
High-titer anti-insulin receptor antibodies that inhibit insulin binding cause a clinical syndrome characterized by severe insulin resistance, glucose intolerance or diabetes mellitus, and acanthosis nigricans. These patients usually have other autoimmune disorders. There are reports of spontaneous remission or remission with cytotoxic therapy.
Many drugs are associated with carbohydrate intolerance or frank diabetes (Table 27–3). The drugs act by decreasing insulin secretion or by increasing insulin resistance or both. Cyclosporine and tacrolimus impair insulin secretion; sirolimus principally increases insulin resistance. These agents contribute to the development of new-onset diabetes after transplantation. Corticosteroids increase insulin resistance but may also have an effect on beta cell function; in a case control study and a large population cohort study, oral corticosteroids doubled the risk for development of diabetes. Thiazide diuretics and beta-blockers modestly increase the risk for diabetes. Treating the hypokalemia due to thiazides may reverse the hyperglycemia. Atypical antipsychotics, particularly olanzapine and clozapine, are associated with increased risk of glucose intolerance. These drugs cause weight gain and insulin resistance but may also impair beta cell function; an increase in rates of diabetic ketoacidosis (DKA) has been reported. Alpelisib is a phosphatidylinositol-3-kinase (PI3K) inhibitor and is approved for use in combination with fulvestrant for hormone receptor–positive, HER2-negative, PIK3CA-mutated breast cancer. PI3K is a component of the insulin signaling pathway, and hyperglycemia is a common side effect of alpelisib treatment.
Chronic pancreatitis or subtotal pancreatectomy reduces the number of functioning B cells and can result in a metabolic derangement very similar to that of genetic type 1 diabetes except that a concomitant reduction in pancreatic A cells may reduce glucagon secretion so that relatively lower doses of insulin replacement are needed.
 Insulin Resistance Syndrome (Metabolic Syndrome)
Insulin Resistance Syndrome (Metabolic Syndrome)
Twenty-five percent of the general nonobese, nondiabetic population has insulin resistance of a magnitude similar to that seen in type 2 diabetes. These insulin-resistant nondiabetic individuals are at much higher risk for developing type 2 diabetes than insulin-sensitive persons. These individuals also tend to have other risk factors for atherosclerotic disease: elevated plasma triglycerides and small, dense, low-density lipoproteins (LDLs); lower high-density lipoproteins (HDLs); higher blood pressure; hyperuricemia; abdominal obesity; prothrombotic state with increased levels of plasminogen activator inhibitor type 1 (PAI-1); and proinflammatory state with increased levels of proinflammatory cytokines such as IL-6 and TNF-alpha.
It has been postulated that hyperinsulinemia and insulin resistance play a direct role in these metabolic abnormalities, but supportive evidence is inconclusive. The main value of grouping these disorders as a syndrome, however, is to remind clinicians that the therapeutic goals are not only to correct hyperglycemia but also to manage the elevated blood pressure and dyslipidemia that result in increased cerebrovascular and cardiac morbidity and mortality in these patients.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
1. Type 1 diabetes—A characteristic symptom complex of hyperosmolality and hyperketonemia from the accumulation of circulating glucose and fatty acids typically presents in patients with type 1 diabetes. When absolute insulin deficiency is of acute onset, the following symptoms develop abruptly: increased urination and thirst, blurred vision, weight loss, paresthesias, and altered level of consciousness. Ketoacidosis exacerbates the dehydration and hyperosmolality by producing anorexia and nausea and vomiting, interfering with oral fluid replacement.
a. Increased urination and thirst—These symptoms are consequences of osmotic diuresis secondary to sustained hyperglycemia. The diuresis results in a loss of glucose as well as free water and electrolytes in the urine.
b. Blurred vision—As the lenses are exposed to hyperosmolar fluids, blurred vision often develops.
c. Weight loss—Despite normal or increased appetite, weight loss is a common feature of type 1 when it develops subacutely. The weight loss is initially due to depletion of water, glycogen, and triglycerides; thereafter, reduced muscle mass occurs as amino acids are diverted to form glucose and ketone bodies. Loss of subcutaneous fat and muscle wasting are features of more slowly developing insulin deficiency. Lowered plasma volume produces symptoms of postural hypotension, which is a serious prognostic sign. Total body potassium loss and the general catabolism of muscle protein contribute to the weakness.
d. Paresthesias—Parethesias may be present at the time of diagnosis, particularly when the onset is subacute. They reflect a temporary dysfunction of peripheral sensory nerves, which clears as insulin replacement restores glycemic levels closer to normal, suggesting neurotoxicity from sustained hyperglycemia.
e. The level of consciousness shown by the patient—The patient’s level of consciousness can vary depending on the degree of hyperosmolality. When insulin deficiency develops relatively slowly and sufficient water intake is maintained, patients remain relatively alert and physical findings may be minimal. When vomiting occurs in response to worsening ketoacidosis, dehydration progresses and compensatory mechanisms become inadequate to keep serum osmolality below 320–330 mOsm/L. Under these circumstances, stupor or even coma may occur. The fruity breath odor of acetone further suggests the diagnosis of DKA.
2. Type 2 diabetes—While increased urination and thirst may be presenting symptoms in some patients with type 2 diabetes, many other patients have an insidious onset of hyperglycemia and are asymptomatic initially. This is particularly true in obese patients, whose diabetes may be detected only after glycosuria or hyperglycemia is noted during routine laboratory studies. Occasionally, when the disease has been occult for some time, patients with type 2 diabetes may have evidence of neuropathic or cardiovascular complications at the time of presentation. Hyperglycemic hyperosmolar coma can also be present when the serum osmolality exceeds 320–330 mOsm/L; in these cases, patients are profoundly dehydrated, hypotensive, lethargic, or comatose but without the Kussmaul respirations of ketoacidosis.
a. Skin manifestations—Chronic skin infections are common. Generalized pruritus and symptoms of vaginitis are frequently the initial complaints of women. Diabetes should be suspected in women with chronic candidal vulvovaginitis. Balanoposthitis (inflammation of the foreskin and glans in uncircumcised males) may occur.
Other skin findings include acanthosis nigricans. which is associated with significant insulin resistance. The skin in the axilla, groin, and back of neck is hyperpigmented and hyperkeratotic (Figure 27–1) Eruptive xanthomas on the flexor surface of the limbs and on the buttocks and lipemia retinalis due to hyperchylomicronemia can occur in patients with uncontrolled type 2 diabetes who also have a familial form of hypertriglyceridemia.
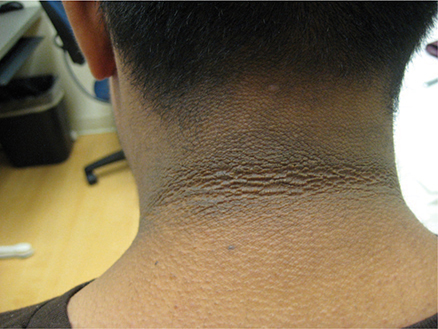
Figure 27–1. Acanthosis nigricans of the nape of the neck, with typical dark and velvety appearance. (Used, with permission, from Umesh Masharani, MB, BS, MRCP [UK].)
b. Body habitus—Overweight or obese patients frequently have type 2 diabetes. Even those who are not significantly obese often have characteristic localization of fat deposits on the upper segment of the body (particularly the abdomen, chest, neck, and face) and relatively less fat on the appendages, which may be quite muscular. This centripetal fat distribution is characterized by a high waist circumference; a waist circumference larger than 40 inches (102 cm) in men and 35 inches (88 cm) in women is associated with an increased risk of diabetes. Mild hypertension is often present in obese patients with diabetes.
c. Obstetrical complications—Type 2 diabetes should be considered in women who have delivered babies larger than 9 lb (4.1 kg) or have had polyhydramnios, preeclampsia, or unexplained fetal losses.
B. Laboratory Findings
1. Urine glucose—A convenient method to detect glucosuria is the paper strip impregnated with glucose oxidase and a chromogen system (Clinistix, Diastix), which is sensitive to as little as 100 mg/dL (5.5 mmol) glucose in urine. A normal renal threshold for glucose as well as reliable bladder emptying is essential for interpretation.
Nondiabetic glycosuria (renal glycosuria) is a benign asymptomatic condition wherein glucose appears in the urine despite a normal amount of glucose in the blood, either basally or during a glucose tolerance test. Its cause may vary from mutations in the SGLT2 gene coding for sodium-glucose transporter 2 (familial renal glycosuria) to one associated with dysfunction of the proximal renal tubule (Fanconi syndrome, chronic kidney disease), or it may merely be a consequence of the increased load of glucose presented to the tubules by the elevated glomerular filtration rate (GFR) during pregnancy. As many as 50% of pregnant women normally have demonstrable sugar in the urine, especially during the third and fourth months. This sugar is practically always glucose except during the late weeks of pregnancy, when lactose may be present.
2. Urine and blood ketones—Qualitative detection of ketone bodies can be accomplished by nitroprusside tests (Acetest or Ketostix). Although these tests do not detect beta-hydroxybutyric acid, which lacks a ketone group, the semiquantitative estimation of ketonuria thus obtained is nonetheless usually adequate for clinical purposes. Many laboratories measure beta-hydroxybutyric acid, and there are meters available (Precision Xtra; Nova Max Plus) for patient use that measures beta-hydroxybutyric acid levels in capillary glucose samples. Beta-hydroxybutyrate levels greater than 0.6 mmol/L require evaluation. Patients with levels greater than 3.0 mmol/L, equivalent to very large urinary ketones, require hospitalization.
3. Plasma or serum glucose—The glucose concentration is 10–15% higher in plasma or serum than in whole blood because structural components of blood cells are absent. A plasma glucose level of 126 mg/dL (7 mmol/L) or higher on more than one occasion after at least 8 hours of fasting is diagnostic of diabetes mellitus (Table 27–4). Fasting plasma glucose levels of 100–125 mg/dL (5.6–6.9 mmol/L) are associated with increased risk of diabetes (impaired fasting glucose tolerance).
Table 27–4. Criteria for the diagnosis of diabetes.
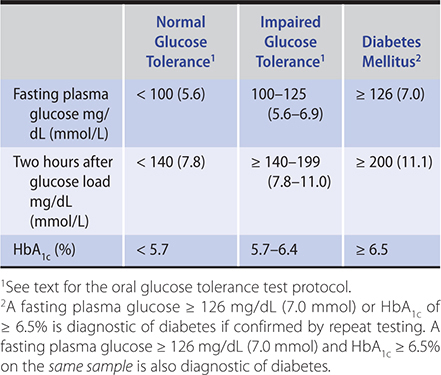
4. Oral glucose tolerance test—If the fasting plasma glucose level is less than 126 mg/dL (7 mmol/L) when diabetes is nonetheless suspected, then a standardized oral glucose tolerance test may be done (Table 27–4). In order to optimize insulin secretion and effectiveness, especially when patients have been on a low-carbohydrate diet, a minimum of 150–200 g of carbohydrate per day should be included in the diet for 3 days preceding the test. The patient should eat nothing after midnight prior to the test day. On the morning of the test, patients are then given 75 g of glucose in 300 mL of water. The glucose load is consumed within 5 minutes. The test should be performed in the morning because there is some diurnal variation in oral glucose tolerance, and patients should not smoke or be active during the test.
Blood samples for plasma glucose are obtained at 0 and 120 minutes after ingestion of glucose. An oral glucose tolerance test is normal if the fasting venous plasma glucose value is less than 100 mg/dL (5.6 mmol/L) and the 2-hour value falls below 140 mg/dL (7.8 mmol/L). A fasting value of 126 mg/dL (7 mmol/L) or higher or a 2-hour value of greater than 200 mg/dL (11.1 mmol/L) is diagnostic of diabetes mellitus. Patients with 2-hour value of 140–199 mg/dL (7.8–11.1 mmol/L) have impaired glucose tolerance. False-positive results may occur in patients who are malnourished, bedridden, or afflicted with an infection or severe emotional stress.
5. Glycated hemoglobin (hemoglobin A1) measurements—Hemoglobin becomes glycated by ketoamine reactions between glucose and other sugars and the free amino groups on the alpha and beta chains. Only glycation of the N-terminal valine of the beta chain imparts sufficient negative charge to the hemoglobin molecule to allow separation by charge dependent techniques. These charge-separated hemoglobins are collectively referred to as hemoglobin A1 (HbA1). The major form of HbA1 is hemoglobin A1c (HbA1c) where glucose is the carbohydrate. HbA1c comprises 4–6% of total hemoglobin A.
Since HbA1c circulates within red blood cells whose life span lasts up to 120 days, it generally reflects the state of glycemia over the preceding 8–12 weeks, thereby providing an improved method of assessing diabetic control. The HbA1c value, however, is weighted to more recent glucose levels (previous month) and this explains why significant changes in HbA1c are observed with short-term (1 month) changes in mean plasma glucose levels. Measurements should be made in patients with either type of diabetes mellitus at 3- to 4-month intervals. In patients monitoring their own blood glucose levels, HbA1c values provide a valuable check on the accuracy of monitoring. In patients who do not monitor their own blood glucose levels, HbA1c values are essential for adjusting therapy. The A1c Derived Average Glucose Study reported that the relationship between average glucose in the previous 3 months and HbA1c was (28.7 × HbA1c) – 46.7. There is, however, substantial individual variability; for HbA1c values between 6.9% and 7.1%, the glucose levels range from 125 mg/dL to 205 mg/dL (6.9–11.4 mmol/L; 95% CIs). For HbA1c of 6%, the mean glucose levels range from 100 mg/dL to 152 mg/dL (5.5–8.5 mmol/L); and for 8% they range from 147 mg/dL to 217 mg/dL (8.1–12.1 mmol/L). For this reason, caution should be exercised in estimating average glucose levels from measured HbA1c.
The accuracy of HbA1c values can be affected by hemoglobin variants or traits. In patients with high levels of hemoglobin F, immunoassays give falsely low values of HbA1c. The National Glycohemoglobin Standardization Program website (www.ngsp.org) has information on the impact of frequently encountered hemoglobin variants and traits on the results obtained with the commonly used HbA1c assays.
Any condition that shortens erythrocyte survival or decreases mean erythrocyte age (eg, recovery from acute blood loss, hemolytic anemia) will falsely lower HbA1c, irrespective of the assay method used because of the extended time that it takes circulating hemoglobin to be glycosylated. Intravenous iron and erythropoietin therapy for treatment of anemia in chronic kidney disease also falsely lower HbA1c levels. Alternative methods such as fructosamine should be considered for these patients. Vitamins C and E are reported to falsely lower test results possibly by inhibiting glycation of hemoglobin. Conditions that increase erythrocyte survival such as splenectomy for hereditary spherocytosis will falsely raise HbA1c levels. Iron deficiency anemia is also associated with higher HbA1c levels.
HbA1c is endorsed by the ADA as a diagnostic test for type 1 and type 2 diabetes (Table 27–4). A cutoff value of 6.5% (48 mmol/mol) was chosen because the risk for retinopathy increases substantially above this value. The advantages of using the HbA1c to diagnose diabetes is that there is no need to fast; it has lower intraindividual variability than the fasting glucose test and the oral glucose tolerance test; and it provides an estimate of glucose control for the preceding 2–3 months. People with HbA1c levels of 5.7–6.4% (39–46 mmol/mol) should be considered at high risk for developing diabetes (prediabetes). The diagnosis should be confirmed with a repeat HbA1c test, unless the patient is symptomatic with plasma glucose levels greater than 200 mg/dL (11.1 mmol/L). This test is not appropriate to use in populations with high prevalence of hemoglobinopathies or in conditions with increased red cell turnover.
6. Serum fructosamine—Serum fructosamine is formed by nonenzymatic glycosylation of serum proteins (predominantly albumin). Since serum albumin has a much shorter half-life than hemoglobin, serum fructosamine generally reflects the state of glycemic control for only the preceding 1–2 weeks. Reductions in serum albumin (eg, nephrotic state, protein-losing enteropathy, or hepatic disease) will lower the serum fructosamine value. When abnormal hemoglobins or hemolytic states affect the interpretation of glycohemoglobin or when a narrower time frame is required, such as for ascertaining glycemic control at the time of conception in a diabetic woman who has recently become pregnant, serum fructosamine assays offer some advantage. Normal values vary in relation to the serum albumin concentration and are 200–285 mcmol/L when the serum albumin level is 5 g/dL. HbA1c values and serum fructosamine are highly correlated. Serum fructosamine levels of 300, 367, and 430 mcmol/L approximate to HbA1c values of 7%, 8%, and 9%, respectively. Substantial individual variability exists, though, when estimating the likely HbA1c value from the fructosamine measurement.
7. Self-monitoring of blood glucose—Capillary blood glucose measurements performed by patients themselves, as outpatients, are extremely useful. In type 1 patients in whom “tight” metabolic control is attempted, they are indispensable. A large number of blood glucose meters are available. All are accurate, but they vary with regard to speed, convenience, size of blood samples required, reporting capability, and cost. Popular models include those manufactured by LifeScan (One Touch), Bayer Corporation (Contour), Roche Diagnostics (Accu-Chek), and Abbott Laboratories (Precision, FreeStyle). These blood glucose meters are relatively inexpensive, ranging from $20 to $80 each. Test strips remain a major expense, costing about $0.25 to $1.50 apiece. Each glucose meter also comes with a lancet device and disposable 26- to 33-gauge lancets. Most meters can store from 100 to 1000 glucose values in their memories and have capabilities to download the values into a computer or smartphone. Some meters are designed to communicate with a specific insulin pump. Contour Next Link meter, for example, communicates with the MiniMed Medtronic pump. The accuracy of data obtained by home glucose monitoring does require education of the patient in sampling and measuring procedures as well as in properly calibrating the instruments.
The clinician should be aware of the limitations of the self-monitoring glucose systems. The strips have limited lifespans and improper storage (high temperature; open vial) can affect their function. Patients should also be advised not to use expired strips. Increases or decreases in hematocrit can decrease or increase the measured glucose values. Meters and the test strips are calibrated over the glucose concentrations ranging from 60 mg/dL (3.3 mmol/L) to 160 mg/dL (8.9 mmol/L) and the accuracy is not as good for higher and lower glucose levels. When the glucose is less than 60 mg/dL (3.3 mmol/L), the difference between the meter and the laboratory value may be as much as 20%. Glucose oxidase–based amperometric systems underestimate glucose levels in the presence of high oxygen tension. This may be important in the critically ill who are receiving supplemental oxygen; under these circumstances, a glucose dehydrogenase–based system may be preferable. Glucose-dehydrogenase pyrroloquinoline quinone (GDH-PQQ) systems may report falsely high glucose levels in patients who are receiving parenteral products containing nonglucose sugars such as maltose, galactose, or xylose or their metabolites. Some meters have been approved for measuring glucose in blood samples obtained at alternative sites such as the forearm and thigh. There is, however, a 5- to 20-minute lag in the glucose response on the arm with respect to the glucose response on the finger. Forearm blood glucose measurements could therefore result in a delay in detection of rapidly developing hypoglycemia. Impaired circulation to the fingers (for example, in patients with Raynaud disease) will artificially lower fingerstick glucose measurements (pseudohypoglycemia).
8. Continuous glucose monitoring systems—Patients are increasingly using continuous glucose monitoring systems. These systems, manufactured by Medtronic MiniMed, DexCom systems, and Abbott Diagnostics, involve inserting a subcutaneous sensor (rather like an insulin pump cannula) that measures glucose concentrations continuously in the interstitial fluid for 7–14 days. The DexCom and MiniMed systems transmit glucose data wirelessly to smartphones or to the screens of insulin pumps. Directional arrows indicate rate and direction of change of glucose levels, and alerts can be set for dangerously low or high glucose values. The FreeStyle Libre (Abbott Diagnostics) sensor system requires the patient to hold a reading device or a smartphone close to the sensor patch for about a second to see the real time glucose value. The MiniMed system requires calibration with periodic fingerstick glucose levels, which is not necessary for the Dexcom and Freestyle Libre systems. A 6-month randomized controlled study of type 1 patients showed that adults (25 years and older) using these continuous glucose monitoring systems had improved glycemic control without an increase in the incidence of hypoglycemia. A randomized controlled study of continuous glucose monitoring during pregnancy showed improved glycemic control in the third trimester, lower birth weight, and reduced risk of macrosomia. The individual glucose values are not that critical—what matters is the direction and the rate at which the glucose is changing, allowing the user to take corrective action. The wearer also gains insight into the way particular foods and activities affect their glucose levels. The other main benefit is the low glucose alert warning. Summaries of the continuous glucose monitoring data collected over 2–12 weeks can be very helpful. The percentage of “time in range” (glucose levels 70–180 mg/day [3.9–10 mmol/L]), glucose levels that are low or high, and their variability can be assessed. There is a strong correlation between glucose levels that are 70% “time in range” and an HbA1c of approximately 7%.
Many of these systems are covered by insurance. The initial cost is about $800 to $1000, and the sensor, which has to be changed every 7 to 14 days, costs $35 to $60; the out-of-pocket expense is about $4000 annually.
9. Lipoprotein abnormalities in diabetes—Circulating lipoproteins are just as dependent on insulin as is the plasma glucose. In type 1 diabetes, moderately deficient control of hyperglycemia is associated with only a slight elevation of LDL cholesterol and serum triglycerides and little if any change in HDL cholesterol. Once the hyperglycemia is corrected, lipoprotein levels are generally normal. However, in patients with type 2 diabetes, a distinct “diabetic dyslipidemia” is characteristic of the insulin resistance syndrome. Its features are a high serum triglyceride level (300–400 mg/dL [3.4–4.5 mmol/L]), a low HDL cholesterol (less than 30 mg/dL [0.8 mmol/L]), and a qualitative change in LDL particles, producing a smaller dense particle whose membrane carries supranormal amounts of free cholesterol. These smaller dense LDL particles are more susceptible to oxidation, which renders them more atherogenic. Measures designed to correct the obesity and hyperglycemia, such as exercise, diet, and hypoglycemic therapy, are the treatment of choice for diabetic dyslipidemia, and in occasional patients in whom normal weight was achieved, all features of the lipoprotein abnormalities cleared. Since primary disorders of lipid metabolism may coexist with diabetes, persistence of lipid abnormalities after restoration of normal weight and blood glucose should prompt a diagnostic workup and possible pharmacotherapy of the lipid disorder. Chapter 28 discusses these matters in detail.
American Diabetes Association. Standards of medical care in diabetes—2020. Diabetes Care. 2020 Jan;43(Suppl 1):S1–212 [PMID: 31862760]
 Clinical Trials about Optimum Diabetic Glucose Control
Clinical Trials about Optimum Diabetic Glucose Control
Findings of the Diabetes Control and Complications Trial and of the United Kingdom Prospective Diabetes Study have confirmed the beneficial effects of improved glycemic control in both type 1 and type 2 diabetes.
A. Type 1 Diabetes
The Diabetes Control and Complications Trial (DCCT), a long-term therapeutic study involving 1441 patients with type 1 diabetes mellitus, reported that “near” normalization of blood glucose resulted in a delay in the onset and a major slowing of the progression of established microvascular and neuropathic complications of diabetes during a follow-up period of up to 10 years. Multiple insulin injections (66%) or insulin pumps (34%) were used in the intensively treated group, who were trained to modify their therapy in response to frequent glucose monitoring. The conventionally treated groups used no more than two insulin injections, and clinical well-being was the goal with no attempt to modify management based on HbA1c determinations or the glucose results.
In half of the patients, a mean hemoglobin A1c of 7.2% (normal: less than 6%) and a mean blood glucose of 155 mg/dL (8.6 mmol/L) were achieved using intensive therapy, while in the conventionally treated group HbA1c averaged 8.9% with an average blood glucose of 225 mg/dL (12.5 mmol/L). Over the study period, which averaged 7 years, there was an approximately 60% reduction in risk between the two groups in regard to diabetic retinopathy, nephropathy, and neuropathy. The intensively treated group also had a nonsignificant reduction in the risk of macrovascular disease of 41% (95% CI, –10% to 68%). Intensively treated patients had a threefold greater risk of serious hypoglycemia as well as a greater tendency toward weight gain. However, there were no deaths definitely attributable to hypoglycemia in any persons in the DCCT study, and no evidence of posthypoglycemic cognitive damage was detected.
Subjects participating in the DCCT study were subsequently enrolled in a follow-up observational study, the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Even though the between-group differences in mean HbA1c narrowed over 4 years, the group assigned to intensive therapy had a lower risk of retinopathy at 4 years, microalbuminuria at 7 to 8 years, and impaired GFR (less than 60 mL/min/1.73 m2) at 22 years of continued study follow-up. Moreover, by the end of the 11-year follow-up period, the intensive therapy group had significantly reduced their risk of any cardiovascular disease events by 42% (95% CI, 9% to 23%; P = 0.02). Thus, it seems that the benefits of good glucose control persist even if control deteriorates at a later date.
The general consensus of the ADA is that intensive insulin therapy associated with comprehensive self-management training should be standard therapy in patients with type 1 diabetes mellitus after the age of puberty. Exceptions include those with advanced chronic kidney disease and older adults since in these groups the detrimental risks of hypoglycemia outweigh the benefits of tight glycemic control.
B. Type 2 Diabetes
The United Kingdom Prospective Diabetes Study (UKPDS), a multicenter study, was designed to establish, in type 2 diabetic patients, whether the risk of macrovascular or microvascular complications could be reduced by intensive blood glucose control with oral hypoglycemic agents or insulin and whether any particular therapy was of advantage.
Intensive treatment with either sulfonylureas, metformin, combinations of those two, or insulin achieved mean HbA1c levels of 7%. This level of glycemic control decreased the risk of microvascular complications (retinopathy and nephropathy) in comparison with conventional therapy (mostly diet alone), which achieved mean levels of HbA1c of 7.9%. Weight gain occurred in intensively treated patients except when metformin was used as monotherapy. No adverse cardiovascular outcomes were noted regardless of the therapeutic agent. In the overweight or obese subgroup, metformin therapy was more beneficial than diet alone in reducing the number of patients who suffered myocardial infarctions and strokes. Hypoglycemic reactions occurred in the intensive treatment groups, but only one death from hypoglycemia was documented during 27,000 patient-years of intensive therapy.
Tight control of blood pressure (median value 144/82 mm Hg vs 154/87 mm Hg) substantially reduced the risk of microvascular disease and stroke but not myocardial infarction. In fact, reducing blood pressure by this amount had substantially greater impact on microvascular outcomes than that achieved by lowering HbA1c from 7.9% to 7%. An epidemiologic analysis of the UKPDS data showed that every 10 mm Hg decrease in mean systolic blood pressure was associated with 11% reduction in risk for myocardial infarction. More than half of the patients needed two or more medications for adequate therapy of their hypertension, and there was no demonstrable advantage of angiotensin-converting enzyme (ACE) inhibitor therapy over therapy with beta-blockers with regard to diabetes end points. Use of a calcium channel blocker added to both treatment groups appeared to be safe over the long term in this diabetic population despite some controversy in the literature about its safety in patients with diabetes.
Like the DCCT trialists, the UKPDS researchers performed post-trial monitoring to determine whether there were long-term benefits of having been in the intensively treated glucose and blood pressure arms of the study. The intensively treated group had significantly reduced risk of myocardial infarction (15%, P = 0.01) and death from any cause (13%, P = 0.007) during the follow-up period. The subgroup of overweight or obese subjects who were initially randomized to metformin therapy showed sustained reduction in risk of myocardial infarction and death from any cause in the follow-up period. Unlike the sustained benefits seen with glucose control, there were no sustained benefits from having been in the more tightly controlled blood pressure group. Both blood pressure groups were at similar risk for microvascular events and diabetes-related end points during the follow-up period.
Thus, the follow-up of the UKPDS type 2 diabetes cohort showed that, as in type 1 diabetes, the benefits of good glucose control persist even if control deteriorates at a later date. Blood pressure benefits, however, last only as long as the blood pressure is well controlled.
 Clinical Trials about How to Prevent Diabetes
Clinical Trials about How to Prevent Diabetes
A. Prevention of Type 1 Diabetes
At the time of diagnosis of type 1 diabetes, there remains significant B cell pancreatic function. This explains why soon after diagnosis, the diabetes goes into partial clinical remission and little or no insulin is required (“honeymoon”). The clinical remission is short-lived, however, and eventually patients lose all B cell function and have more labile glucose control. Studies have been performed to prolong this partial clinical remission using immunomodulatory agents. The CD3 complex is the major signal-transducing element of the T cell receptor, and the anti-CD3 antibodies are believed to modulate the autoimmune response by selectively inhibiting the pathogenic T cells or by inducing regulatory T cells. Phase I/II and II/III clinical trials of humanized monoclonal antibodies against CD3, hOKT3gamma (Ala-Ala) (teplizumab), and ChAglyCD3 (otelixizumab) delayed but did not completely arrest the decline in insulin production in patients with newly diagnosed type 1 diabetes. A similar phase 2 clinical trial using teplizumab was undertaken in nondiabetic relatives of patients with type 1 diabetes who had two or more diabetes-related antibodies and glucose intolerance. In the 5 years after randomization, 43% of the patients receiving teplizumab and 72% of the placebo group developed diabetes.
B. Prevention of Type 2 Diabetes
The Diabetes Prevention Program studied whether treatment with either diet and exercise or metformin could prevent the onset of type 2 diabetes in overweight men and women aged 25–85 years who had impaired glucose tolerance. Intervention with a low-fat diet and 150 minutes of moderate exercise (equivalent to a brisk walk) per week reduced the risk of progression to type 2 diabetes by 71%. Participants who took metformin 850 mg twice daily reduced their risk of developing type 2 diabetes by 31%, but this intervention was relatively ineffective in those who were either less obese or in the older age group. Eighty-eight percent of the persons in the Diabetes Prevention Program elected to continue followup in the Diabetes Prevention Program Outcome Study. At 15 years of followup, the cumulative incidence of diabetes was 55% in the lifestyle group and 62% in the control group.
 Treatment
Treatment
A. Diet
A well-balanced, nutritious diet remains a fundamental element of therapy. There is no specific recommendation on the percentage of calories that should come from carbohydrate, protein, and fat. The macronutrient proportions should be individualized based on the patient’s eating patterns, preferences, and metabolic goals. In general, most patients with diabetes consume about 45% of their total daily calories in the form of carbohydrates, 25–35% in the form of fat, and 10–35% in the form of protein. In patients with type 2 diabetes, limiting the carbohydrate intake and substituting some of the calories with monounsaturated fats, such as olive oil, rapeseed (canola) oil, or the oils in nuts and avocados, can lower triglycerides and increase HDL cholesterol. A Mediterranean-style eating pattern (a diet supplemented with walnuts, almonds, hazelnuts, and olive oil) has been shown to improve glycemic control and lower combined endpoints for cardiovascular events and stroke. In those patients with obesity and type 2 diabetes, weight reduction by caloric restriction is an important goal of the diet (see Chapter 29). Patients with type 1 diabetes or type 2 diabetes who take insulin should be taught “carbohydrate counting,” so they can administer their insulin bolus for each meal based on its carbohydrate content.
The current recommendations for saturated fats and dietary cholesterol intake for people with diabetes are the same as for the general population. Saturated fats should be limited to less than 10% of daily calories and dietary cholesterol intake should be less than 300 mg/day. For those patients with kidney disease, dietary protein should be maintained at the recommended daily allowance of 0.8 g/kg/day. Exchange lists for meal planning can be obtained from the American Diabetes Association and its affiliate associations or from the American Dietetic Association (http://www.eatright.org), 216 W. Jackson Blvd., Chicago, IL 60606 (312-899-0040).
1. Dietary fiber—Plant components such as cellulose, gum, and pectin are indigestible by humans and are termed dietary “fiber.” Insoluble fibers such as cellulose or hemicellulose, as found in bran, tend to increase intestinal transit and may have beneficial effects on colonic function. In contrast, soluble fibers such as gums and pectins, as found in beans, oatmeal, or apple skin, tend to retard nutrient absorption rates so that glucose absorption is slower and hyperglycemia may be slightly diminished. Although its recommendations do not include insoluble fiber supplements such as added bran, the ADA recommends food such as oatmeal, cereals, and beans with relatively high soluble fiber content as staple components of the diet in diabetics. High soluble fiber content in the diet may also have a favorable effect on blood cholesterol levels.
2. Glycemic index—The glycemic index of a carbohydrate containing food is determined by comparing the glucose excursions after consuming 50 g of test food with glucose excursions after consuming 50 g of reference food (white bread):

Eating low glycemic index foods results in lower glucose levels after meals. Low glycemic index foods have values of 55 or less and include many fruits, vegetables, grainy breads, pasta, and legumes. High glycemic index foods have values of 70 or greater and include baked potato, white bread, and white rice. Glycemic index is lowered by the presence of fats and protein when food is consumed in a mixed meal. Even though it may not be possible to accurately predict the glycemic index of a particular food in the context of a meal, it is reasonable to choose foods with low glycemic index.
3. Artificial and other sweeteners—Saccharin (Sweet N Low), sucralose (Splenda), acesulfame potassium (Sweet One), and rebiana (Truvia) are “artificial” sweeteners that can be used in cooking and baking. Aspartame (NutraSweet) lacks heat stability, so it cannot be used in cooking. None of these sweeteners raise blood glucose levels.
Fructose represents a “natural” sugar substance that is a highly effective sweetener, induces only slight increases in plasma glucose levels, and does not require insulin for its metabolism. However, because of potential adverse effects of large amounts of fructose on raising serum cholesterol, triglycerides, and LDL cholesterol, it does not have any advantage as a sweetening agent in the diabetic diet. This does not preclude, however, ingestion of fructose-containing fruits and vegetables or fructose-sweetened foods in moderation.
Sugar alcohols, also known as polyols or polyalcohol, are commonly used as sweeteners and bulking agents. They occur naturally in a variety of fruits and vegetables but are also commercially made from sucrose, glucose, and starch. Examples are sorbitol, xylitol, mannitol, lactitol, isomalt, maltitol, and hydrogenated starch hydrolysates (HSH). They are not as easily absorbed as sugar, so they do not raise blood glucose levels as much. Therefore, sugar alcohols are often used in food products that are labeled as “sugar free,” such as chewing gum, lozenges, hard candy, and sugar-free ice cream. However, if consumed in large quantities, they will raise blood glucose and can cause bloating and diarrhea.
B. Medications for Treating Hyperglycemia
The medications for treating type 2 diabetes are listed in Table 27–5.
Table 27–5. Drugs for treatment of type 2 diabetes mellitus.
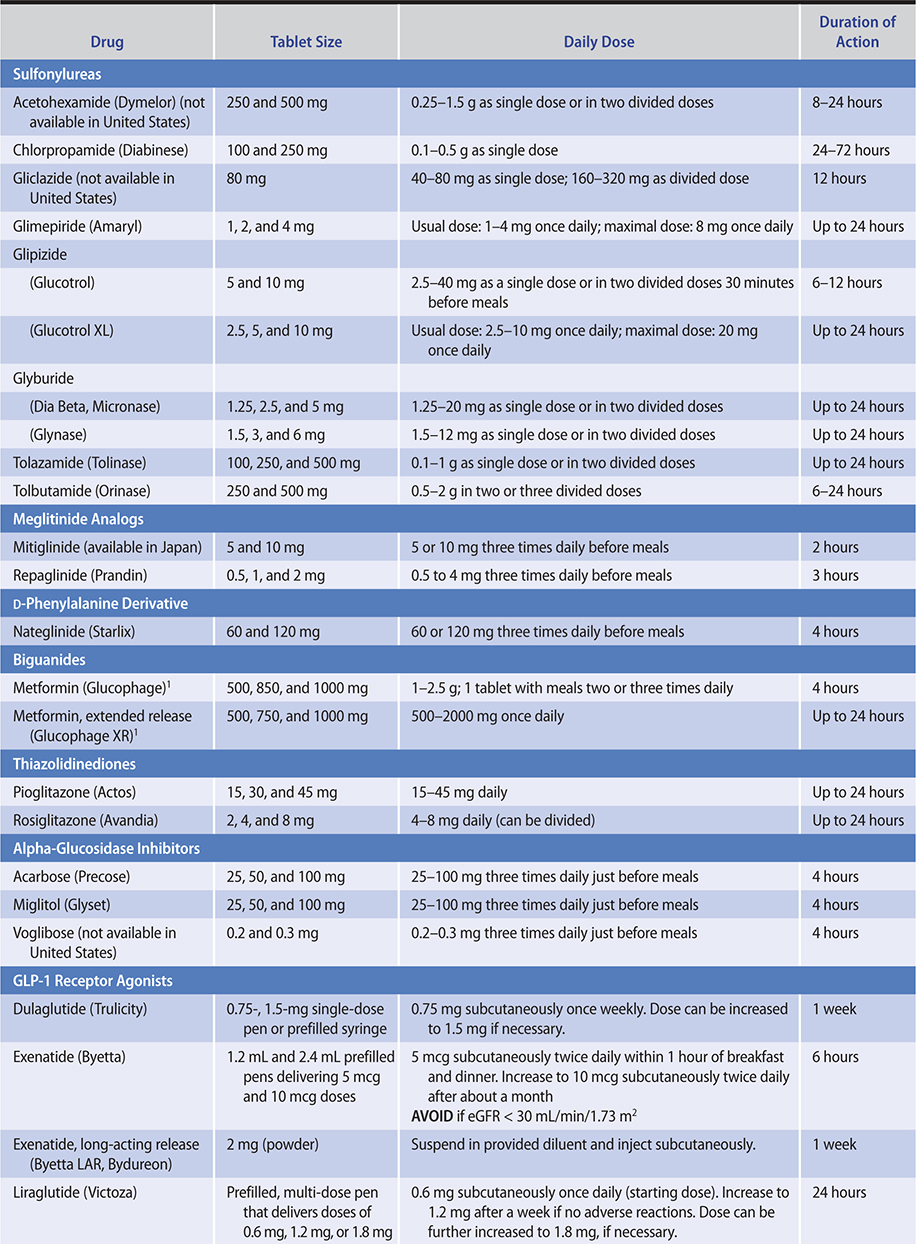

1. Medications that primarily stimulate insulin secretion by binding to the sulfonylurea receptor on the beta cell—
a. Sulfonylureas—The primary mechanism of action of the sulfonylureas is to stimulate insulin release from pancreatic B cells.
Sulfonylureas are used in patients with type 2 but not type 1 diabetes, since these medications require functioning pancreatic B cells to produce their effect on blood glucose. Sulfonylureas are metabolized by the liver and apart from acetohexamide, whose metabolite is more active than the parent compound, the metabolites of all the other sulfonylureas are weakly active or inactive. The metabolites are excreted by the kidney and, in the case of the second-generation sulfonylureas, partly excreted in the bile.
Hypoglycemia is a common adverse reaction with the sulfonylureas. Weight gain is also common, especially in the first year of use. The mechanisms of the weight gain include improved glucose control and increased food intake in response to hypoglycemia.
Idiosyncratic reactions are rare, with skin rashes or hematologic toxicity (leukopenia, thrombocytopenia) occurring in less than 0.1% of users.
(1) First-generation oral sulfonylureas (tolbutamide, tolazamide, acetohexamide, chlorpropamide)—Tolbutamide is probably best administered in divided doses (eg, 500 mg before each meal and at bedtime); however, some patients require only one or two tablets daily with a maximum dose of 3000 mg/day. Because of its short duration of action (about 6–10 hours, which is independent of kidney function), tolbutamide is relatively safe to use in kidney disease. Prolonged hypoglycemia has been reported rarely with tolbutamide, mostly in patients receiving antibacterial sulfonamides (sulfisoxazole), phenylbutazone for arthralgias, or the oral azole antifungal medications to treat candidiasis. These medications apparently compete with tolbutamide for oxidative enzyme systems in the liver, resulting in maintenance of high levels of unmetabolized, active sulfonylurea in the circulation.
Tolazamide, acetohexamide, and chlorpropamide are rarely used. Chlorpropamide has a prolonged biologic effect, and severe hypoglycemia can occur especially in older adults as their renal clearance declines with aging. Its other side effects include alcohol-induced flushing and hyponatremia due to its effect on vasopressin secretion and action.
(2) Second-generation sulfonylureas (glyburide, glipizide, gliclazide, glimepiride)—Glyburide, glipizide, gliclazide, and glimepiride are 100–200 times more potent than tolbutamide. These medications should be used with caution in patients with cardiovascular disease or in elderly patients, in whom prolonged hypoglycemia would be especially dangerous.
The usual starting dose of glyburide is 2.5 mg/day, and the average maintenance dose is 5–10 mg/day given as a single morning dose; maintenance doses higher than 20 mg/day are not recommended. Some reports suggest that 10 mg is a maximum daily therapeutic dose, with 15–20 mg having no additional benefit in poor responders and doses over 20 mg actually worsening hyperglycemia. A “Press Tab” formulation of “micronized” glyburide—easy to divide in half with slight pressure if necessary—is available. Glyburide is metabolized in the liver and the metabolic products of glyburide have hypoglycemic activity. This probably explains why assays specific for the unmetabolized compound suggest a plasma half-life of only 1–2 hours, yet the biologic effects of glyburide are clearly persistent 24 hours after a single morning dose in diabetic patients. Glyburide is unique among sulfonylureas in that it not only binds to the pancreatic B cell membrane sulfonylurea receptor but also becomes sequestered within the B cell. This may also contribute to its prolonged biologic effect despite its relatively short circulating half-life.
Glyburide has few adverse effects other than its potential for causing hypoglycemia, which at times can be prolonged. Flushing has rarely been reported after ethanol ingestion. It does not cause water retention, as chlorpropamide does, but rather slightly enhances free water clearance. Glyburide should not be used in patients with liver failure and chronic kidney disease because of the risk of hypoglycemia. Elderly patients are at particular risk for hypoglycemia even with relatively small daily doses.
The recommended starting dose of glipizide is 5 mg/day, with up to 15 mg/day given as a single daily dose before breakfast. When higher daily doses are required, they should be divided and given before meals. The maximum dose recommended by the manufacturer is 40 mg/d, although doses above 10–15 mg probably provide little additional benefit in poor responders and may even be less effective than smaller doses. For maximum effect in reducing postprandial hyperglycemia, glipizide should be ingested 30 minutes before meals, since rapid absorption is delayed when the medication is taken with food.
At least 90% of glipizide is metabolized in the liver to inactive products, and 10% is excreted unchanged in the urine. Glipizide therapy should therefore not be used in patients with liver failure. Because of its lower potency and shorter duration of action, it is preferable to glyburide in elderly patients and for those patients with kidney disease. Glucotrol-XL provides extended release of glipizide during transit through the gastrointestinal tract with greater effectiveness in lowering prebreakfast hyperglycemia than the shorter-duration immediate-release standard glipizide tablets. However, this formulation appears to have sacrificed its lower propensity for severe hypoglycemia compared with longer-acting glyburide without showing any demonstrable therapeutic advantages over glyburide.
Gliclazide (not available in the United States) is another intermediate duration sulfonylurea with a duration of action of about 12 hours. The recommended starting dose is 40–80 mg/day with a maximum dose of 320 mg. Doses of 160 mg and above are given as divided doses before breakfast and dinner. The medication is metabolized by the liver; the metabolites and conjugates have no hypoglycemic effect. An extended release preparation is available.
Glimepiride has a long duration of effect with a half-life of 5 hours allowing once or twice daily dosing. Glimepiride achieves blood glucose lowering with the lowest dose of any sulfonylurea compound. A single daily dose of 1 mg/day has been shown to be effective, and the maximal recommended dose is 8 mg. It is completely metabolized by the liver to relatively inactive metabolic products.
b. Meglitinide analogs—Repaglinide is structurally similar to glyburide but lacks the sulfonic acid-urea moiety. It acts by binding to the sulfonylurea receptor and closing the adenosine triphosphate (ATP)-sensitive potassium channel. It is rapidly absorbed from the intestine and then undergoes complete metabolism in the liver to inactive biliary products, giving it a plasma half-life of less than 1 hour. The medication therefore causes a brief but rapid pulse of insulin. The starting dose is 0.5 mg three times a day 15 minutes before each meal. The dose can be titrated to a maximum daily dose of 16 mg. Like the sulfonylureas, repaglinide can be used in combination with metformin. Hypoglycemia is the main side effect. Like the sulfonylureas, repaglinide causes weight gain. Metabolism is by cytochrome P450 3A4 isoenzyme, and other medications that induce or inhibit this isoenzyme may increase or inhibit (respectively) the metabolism of repaglinide. The medication may be useful in patients with kidney impairment or in older adults.
Mitiglinide is a benzylsuccinic acid derivative that binds to the sulfonylurea receptor and is similar to repaglinide in its clinical effects. It is approved for use in Japan.
c. D-phenylalanine derivative—Nateglinide stimulates insulin secretion by binding to the sulfonylurea receptor and closing the ATP-sensitive potassium channel. It is rapidly absorbed from the intestine, reaching peak plasma levels within 1 hour. It is metabolized in the liver and has a plasma half-life of about 1.5 hours. Like repaglinide, it causes a brief rapid pulse of insulin, and when given before a meal it reduces the postprandial rise in blood glucose. For most patients, the recommended starting and maintenance dose is 120 mg three times a day before meals. Use 60 mg in patients who have mild elevations in HbA1c. Like the other insulin secretagogues, its main side effects are hypoglycemia and weight gain.
2. Medications that primarily lower glucose levels by their actions on the liver, muscle, and adipose tissue—
a. Metformin—Metformin is the first-line therapy for patients with type 2 diabetes. It can be used alone or in conjunction with other oral agents or insulin in the treatment of patients with type 2 diabetes. It is ineffective in patients with type 1 diabetes.
Metformin’s therapeutic effects primarily derive from the increasing hepatic adenosine monophosphate-activated protein kinase activity, which reduces hepatic gluconeogenesis and lipogenesis. Metformin has a half-life of 1.5–3 hours and is not bound to plasma proteins or metabolized, being excreted unchanged by the kidneys.
The current recommendation is to start metformin at diagnosis. A side benefit of metformin therapy is its tendency to improve both fasting and postprandial hyperglycemia and hypertriglyceridemia in obese patients with diabetes without the weight gain associated with insulin or sulfonylurea therapy. Patients with chronic kidney disease should not be given this medication because failure to excrete it would produce high blood and tissue levels of metformin that could stimulate lactic acid overproduction. In the United States, metformin use is not recommended at or above a serum creatinine level of 1.4 mg/dL in women and 1.5 mg/dL in men. In the United Kingdom, the recommendations are to review metformin use when the serum creatinine exceeds 130 mcmol/L (1.5 mg/dL) or the estimated glomerular filtration rate (eGFR) falls below 45 mL/min/1.73 m2. The medication should be stopped if the serum creatinine exceeds 150 mcmol/L (1.7 mg/dL) or the eGFR is below 30 mL/min/1.73 m2. Patients with liver failure or persons with excessive alcohol intake should not receive this medication because of the risk of lactic acidosis.
The maximum dosage of metformin is 2550 mg, although little benefit is seen above a total dose of 2000 mg. It is important to begin with a low dose and increase the dosage very gradually in divided doses—taken with meals—to reduce minor gastrointestinal upsets. A common schedule would be one 500-mg tablet three times a day with meals or one 850- or 1000-mg tablet twice daily at breakfast and dinner. Up to 2000 mg of the extended-release preparation can be given once a day. Lower doses should be used in patients with eGFRs between 30 and 45 mL/min/1.73 m2 and in the elderly who are at higher risk for acute kidney injury from reduced renal functional reserve.
The most frequent side effects of metformin are gastrointestinal symptoms (anorexia, nausea, vomiting, abdominal discomfort, diarrhea), which occur in up to 20% of patients. These effects are dose-related, tend to occur at onset of therapy, and often are transient. However, in 3–5% of patients, therapy may have to be discontinued because of persistent diarrheal discomfort. Patients switching from immediate-release metformin to comparable dose of extended-release metformin may experience fewer gastrointestinal side effects. At the time of this publication in June, 2020, five companies had withdrawn metformin from the market because of contamination with N-nitrosodimethylamine. Other companies do not appear to have a problem with contamination and continue to manufacture metformin.
Hypoglycemia does not occur with therapeutic doses of metformin, which permits its description as a “euglycemic” or “antihyperglycemic” medication rather than an oral hypoglycemic agent. Dermatologic or hematologic toxicity is rare. Metformin interferes with the calcium dependent absorption of vitamin B12-intrinsic complex in the terminal ileum; vitamin B12 deficiency can occur after many years of metformin use. Periodic screening with vitamin B12 levels should be considered, especially in patients with peripheral neuropathy or if a macrocytic anemia develops. Increased intake of dietary calcium may prevent the metformin-induced B12 malaborption.
Lactic acidosis has been reported as a side effect but is uncommon with metformin in contrast to phenformin. Almost all reported cases have involved persons with associated risk factors that should have contraindicated its use (kidney, liver, or cardiorespiratory insufficiency and alcoholism). Acute kidney injury can occur rarely in certain patients taking metformin who receive radiocontrast agents. Metformin therapy should therefore be temporarily halted on the day of radiocontrast administration and restarted a day or two later after confirmation that kidney function has not deteriorated.
b. Thiazolidinediones—Two medications of this class, rosiglitazone and pioglitazone, are available for clinical use. These medications sensitize peripheral tissues to insulin. They bind the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-gamma) and affect the expression of a number of genes. Like the biguanides, this class of medications does not cause hypoglycemia.
Both rosiglitazone and pioglitazone are effective as monotherapy and in combination with sulfonylureas or metformin or insulin, lowering HbA1c by 1–2%. When used in combination with insulin, they can result in a 30–50% reduction in insulin dosage, and some patients can come off insulin completely. The oral dosage of rosiglitazone is 4–8 mg daily and of pioglitazone, 15–45 mg daily, and the medications do not have to be taken with food. Rosiglitazone is primarily metabolized by the CYP 2C8 isoenzyme and pioglitazone is metabolized by CYP 2C8 and CYP 3A4.
The combination of a thiazolidinedione and metformin has the advantage of not causing hypoglycemia. Patients inadequately managed on sulfonylureas can do well on a combination of sulfonylurea and rosiglitazone or pioglitazone.
These medications have some additional effects apart from glucose lowering. Rosiglitazone therapy is associated with increases in total cholesterol, LDL cholesterol (15%), and HDL cholesterol (10%). There is a reduction in free fatty acids of about 8–15%. The changes in triglycerides are generally not different from placebo. Pioglitazone in clinical trials lowered triglycerides (9%) and increased HDL cholesterol (15%) but did not cause a consistent change in total cholesterol and LDL cholesterol levels. A prospective randomized comparison of the metabolic effects of pioglitazone and rosiglitazone showed similar effects on HbA1c and weight gain. Small prospective studies have demonstrated that treatment with these medications leads to improvements in the biochemical and histologic features of nonalcoholic fatty liver disease. The thiazolidinediones also may limit vascular smooth muscle proliferation after injury, and there are reports that pioglitazone can reduce neointimal proliferation after coronary stent placement. In one double-blind, placebo-controlled study, rosiglitazone was shown to be associated with a decrease in the ratio of urinary albumin to creatinine excretion.
Safety concerns and some troublesome side effects limit the use of this class of medication. Rosiglitazone use declined when a meta-analysis of 42 randomized clinical trials suggested that this medication increases the risk of angina pectoris or myocardial infarction; the European Medicines Agency suspended the use of rosiglitazone in Europe. In the United States, the FDA established a restricted distribution program. A subsequent large prospective clinical trial (the RECORD study) failed to confirm the meta-analysis finding and the restrictions were lifted in the United States.
Edema occurs in about 3–4% of patients receiving monotherapy with rosiglitazone or pioglitazone. The edema occurs more frequently (10–15%) in patients receiving concomitant insulin therapy and may result in heart failure. The medications are contraindicated in diabetic individuals with New York Heart Association class III and IV cardiac status. Thiazolidinediones have also been reported as being associated with new onset or worsening macular edema. Apparently, this is a rare side effect, and most of these patients also had peripheral edema. The macular edema resolved or improved once the medication was discontinued.
Troglitazone, the first medication in this class, was withdrawn from clinical use because of medication-associated fatal liver failure. Although rosiglitazone and pioglitazone have not been reported to cause liver injury, the FDA recommends that they should not be used in patients with clinical evidence of active liver disease or pretreatment elevation of the alanine aminotransferase (ALT) level that is 2.5 times greater than the upper limit of normal. Liver biochemical tests should be performed on all patients prior to initiation of treatment and periodically thereafter.
An increase in fracture risk in women (but not men) has been reported with both rosiglitazone and pioglitazone. The fracture risk is in the range of 1.9 per 100 patient-years with the thiazolidinedione as opposed to 1.1 per 100 patient-years on comparison treatment. In at least one study of rosiglitazone, the fracture risk was increased in premenopausal as well as postmenopausal women.
Other side effects include anemia, which occurs in 4% of patients treated with these medications; it may be due to a dilutional effect of increased plasma volume rather than a reduction in red cell mass. Weight gain occurs, especially when the medication is combined with a sulfonylurea or insulin. Some of the weight gain is fluid retention, but there is also an increase in total fat mass. Clinical studies have reported conflicting results regarding an association of bladder cancer with pioglitazone use. A 10-year observational cohort study of patients taking pioglitazone failed to find an association with bladder cancer. A large multipopulation pooled analysis (1.01 million persons over 5.9 million person-years) also failed to find an association between cumulative exposure of pioglitazone or rosiglitazone and incidence of bladder cancer. Another population-based study, however, generating 689,616 person-years of follow-up did find that pioglitazone but not rosiglitazone was associated with an increased risk of bladder cancer.
3. Medications that affect absorption of glucose—Alpha-glucosidase inhibitors competitively inhibit the alpha-glucosidase enzymes in the gut that digest dietary starch and sucrose. Two of these medications—acarbose and miglitol—are available for clinical use in the United States. Voglibose, another alpha-glucosidase inhibitor is available in Japan, Korea, and India. Acarbose and miglitol are potent inhibitors of glucoamylase, alpha-amylase, and sucrase but have less effect on isomaltase and hardly any on trehalase and lactase.
a. Acarbose—The recommended starting dose of acarbose is 50 mg orally twice daily, gradually increasing to 100 mg three times daily. For maximal benefit on postprandial hyperglycemia, acarbose should be given with the first mouthful of food ingested. In diabetic patients, it reduces postprandial hyperglycemia by 30–50%, and its overall effect is to lower the HbA1c by 0.5–1%.
The principal adverse effect, seen in 20–30% of patients, is flatulence. This is caused by undigested carbohydrate reaching the lower bowel, where gases are produced by bacterial flora. In 3% of cases, troublesome diarrhea occurs. This gastrointestinal discomfort tends to discourage excessive carbohydrate consumption and promotes improved compliance of type 2 patients with their diet prescriptions. When acarbose is given alone, there is no risk of hypoglycemia. However, if combined with insulin or sulfonylureas, it might increase the risk of hypoglycemia from these agents. A slight rise in hepatic aminotransferases has been noted in clinical trials with acarbose (5% vs 2% in placebo controls, and particularly with doses greater than 300 mg/day). The levels generally return to normal on stopping the medication.
b. Miglitol—Miglitol is similar to acarbose in terms of its clinical effects. It is indicated for use in diet- or sulfonylurea-treated patients with type 2 diabetes. Therapy is initiated at the lowest effective dosage of 25 mg orally three times a day. The usual maintenance dose is 50 mg three times a day, although some patients may benefit from increasing the dose to 100 mg three times a day. Gastrointestinal side effects occur as with acarbose. The medication is not metabolized and is excreted unchanged by the kidney. Miglitol should not be used in end-stage chronic kidney disease, when its clearance would be impaired.
4. Incretins—Oral glucose provokes a threefold to fourfold higher insulin response than an equivalent dose of glucose given intravenously. This is because the oral glucose causes a release of gut hormones, principally glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP1), that amplify the glucose-induced insulin release. This “incretin effect” of GLP-1 secretion (but not GIP1 secretion) is reduced in patients with type 2 diabetes and when GLP-1 is infused in patients with type 2 diabetes, it stimulates insulin secretion and lowers glucose levels. GLP-1, unlike the sulfonylureas, has only a modest insulin stimulatory effect at normoglycemic concentrations. This means that GLP-1 has a lower risk for hypoglycemia than the sulfonylureas.
In addition to its insulin stimulatory effect, GLP-1 also has a number of other pancreatic and extrapancreatic effects. It suppresses glucagon secretion and so may ameliorate the hyperglucagonemia that is present in people with diabetes and improve postprandial hyperglycemia. GLP-1 acts on the stomach delaying gastric emptying; the importance of this effect on glucose lowering is illustrated by the observation that antagonizing the deceleration of gastric emptying markedly reduces the glucose lowering effect of GLP-1. GLP-1 receptors are present in the central nervous system and may play a role in the anorectic effect of the drugs. Type 2 diabetic patients undergoing GLP-1 infusion are less hungry; it is unclear whether this is mainly due to a deceleration of gastric emptying or whether there is a central nervous system effect as well.
a. GLP-1 receptor agonists—GLP-1’s half-life is only 1–2 minutes. It is rapidly proteolyzed by dipeptidyl peptidase 4 (DPP-4) and by other enzymes, such as endopeptidase 24.11, and is also cleared quickly by the kidney. The native peptide, therefore, cannot be used therapeutically. Five GLP-1 receptor agonists with longer half-lives, exenatide, liraglutide, dulaglutide, lixisenatide, and semaglutide, are available for clinical use.
Exenatide (Exendin 4) is a GLP-1 receptor agonist isolated from the saliva of the Gila monster (a venomous lizard) that is more resistant to DPP-4 action and cleared by the kidney. Its half-life is 2.4 hours, and its glucose lowering effect is about 6 hours. Exenatide is dispensed as two fixed-dose pens (5 mcg and 10 mcg). It is injected 60 minutes before breakfast and before dinner. Patients with type 2 diabetes should be prescribed the 5 mcg pen for the first month and, if tolerated, the dose can then be increased to 10 mcg twice a day. The medication is not recommended in patients with eGFR less than 30 mL/min/1.73 m2. In clinical trials, adding exenatide therapy to patients with type 2 diabetes already taking metformin or a sulfonylurea, or both, further lowered the HbA1c value by 0.4% to 0.6% over a 30-week period. These patients also experienced a weight loss of 3–6 pounds. Exenatide LAR is a once-weekly preparation that is dispensed as a powder (2 mg). It is suspended in the provided diluent just prior to injection. In comparative clinical trials, the long-acting drug lowers the HbA1c level a little more than the twice daily drug. Low-titer antibodies against exenatide develop in over one-third (38%) of patients, but the clinical effects are not attenuated. High-titer antibodies develop in a subset of patients (~6%), and in about half of these cases, an attenuation of glycemic response has been seen.
Liraglutide is a soluble fatty acid acylated GLP-1 analog. The half-life is approximately 12 hours, allowing the medication to be injected once a day. The dosing is initiated at 0.6 mg daily, increased after 1 week to 1.2 mg daily. Some patients may benefit from increasing the dose to 1.8 mg. In clinical trials lasting 26 and 52 weeks, adding liraglutide to the therapeutic regimen (metformin, sulfonylurea, thiazolidinedione) of patients with type 2 diabetes further lowered the HbA1c value. Depending on the dose and design of the study, the HbA1c decline was in the range of 0.6% to 1.5%. The patients had sustained weight loss of 1–6 pounds. Liraglutide at a dose of 3 mg daily has been approved for weight loss.
In a postmarketing multinational study of 9340 patients with type 2 diabetes with known cardiovascular disease, the addition of liraglutide was associated with a lower primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio 0.87, P = 0.01). Patients taking liraglutide had lower HbA1c levels, weight loss of 2.3 kg, lower systolic blood pressure, and fewer episodes of severe hypoglycemia.
Dulaglutide consists of two GLP-1 analog molecules covalently linked to an Fc fragment of human IgG4. The GLP-1 molecule has amino acid substitutions that resist DPP-4 action. The half-life of dulaglutide is about 5 days. The usual dose is 0.75 mg weekly by subcutaneous injection. The maximum recommended dose is 1.5 mg weekly. Dulaglutide monotherapy and combination therapy lowers HbA1c by about 0.7% to 1.6%. Weight loss ranged from 2 pounds to 7 pounds.
Lixisenatide is a synthetic analog of exendin 4 (deletion of a proline and addition of 6 lysines to the C-terminal region) with a half-life of 3 hours. It is dispensed as two fixed-dose pens (10 mcg and 20 mcg). The 10-mcg dose is injected once daily before breakfast for the first 2 weeks, and if tolerated, the dose is then increased to 20 mcg daily. Its clinical effect is about the same as exenatide with HbA1c lowering in the 0.4–0.6% range. Weight loss ranges from 2 pounds to 6 pounds. Antibodies to lixisenatide occur frequently (70%) and ~2.4% with the highest antibody titers have attenuated glycemic response.
Semaglutide is a synthetic analog of GLP-1 with a drug half-life of about 1 week. It has an alpha-aminoisobutyric acid substitution at position 8 that makes the molecule resistant to DPP4 action and a C-18 fatty di-acid chain attached to lysine at position 26 that binds to albumin, which accounts for the drug’s long half-life. Semaglutide is dispensed as two pens: one pen delivers a 0.25-mg or 0.5-mg dose and the other pen delivers a 1-mg dose. The recommended dosing is 0.25 mg weekly for 4 weeks and if tolerated the dose is then increased to 0.5 mg per week. The 1-mg per week dose can provide additional glucose lowering effect. Semaglutide monotherapy and combination therapy lowers HbA1c from 1.5% to 1.8%.
Semaglutide is an oral medication; the patient must take it fasting with a glass of water and then wait half an hour before eating, drinking, or taking other medicines. The recommended starting dose is 3 mg daily for the first month and increased to 7–14 mg daily as tolerated and as needed for glucose control.
The most frequent adverse reactions of the GLP-1 receptor agonists are nausea (11–40%), vomiting (4–13%), and diarrhea (9–17%). The reactions are more frequent at the higher doses. In clinical trials about 1–5% of participants withdrew from the studies because of the gastrointestinal symptoms.
The GLP-1 receptor agonists have been associated with increased risk of pancreatitis. The pancreatitis was severe (hemorrhagic or necrotizing) in 6 instances, and 2 of these patients died. In the liraglutide and dulaglutide clinical trials, there were 13 and 5 cases of pancreatitis in the drug-treated groups versus 1 and 1 case in the comparator groups, respectively. This translates to about 1.4–2.2 vs 0.6–0.9 cases of pancreatitis per 1000 patient-years. Patients taking GLP-1 receptor agonists should be advised to seek immediate medical care if they experience unexplained persistent severe abdominal pain.
There have been rare reports of acute kidney injury in patients taking exenatide. Some of these patients had preexisting kidney disease, and others had one or more risk factors for kidney disease. A number of the patients reported nausea, vomiting, and diarrhea, and it is possible that these side effects caused volume depletion and contributed to the development of the kidney injury. For this reason, the GLP-1 receptors agonists should be prescribed cautiously in patients with kidney impairment. Liraglutide, semaglutide, and dulaglutide are metabolized by proteolysis and are preferred choices in patients with kidney failure.
GLP-1 receptor agonists stimulate C-cell neoplasia and cause medullary thyroid carcinoma in rats. Human C-cells express very few GLP-1 receptors, and the relevance to human therapy is unclear. The medications, however, should not be used in patients with personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia (MEN) syndrome type 2.
b. DPP-4 inhibitors—An alternate approach to the use of GLP-1 receptor agonists is to inhibit the enzyme DPP-4 and prolong the action of endogenously released GLP-1 and GIP. Four oral DPP-4 inhibitors, sitagliptin, saxagliptin, linagliptin, and alogliptin, are available in the United States for the treatment of type 2 diabetes. An additional DPP-4 inhibitor, vildagliptin, is available in Europe. Other DPP-4 inhibitors—gemigliptin, anagliptin, teneligliptin, trelagliptin, omarigliptin, evogliptin, and gosogliptin—have been approved outside the United States and European Union (Korea, India, Thailand, Japan, Russia, and several South American countries).
Sitagliptin, when used alone or in combination with other diabetes medications, lowers HbA1c by approximately 0.5%. The usual dose of sitagliptin is 100 mg once daily, but the dose is reduced to 50 mg daily if the calculated creatinine clearance is 30–50 mL/min and to 25 mg for clearances less than 30 mL/min. Saxagliptin, when added to the therapeutic regimen (metformin, sulfonylurea, thiazolidinedione) of patients with type 2 diabetes, further lowered the HbA1c value by about 0.7–0.9%. The dose is 2.5 mg or 5 mg orally once a day. The 2.5-mg dose should be used in patients with eGFR less than 50 mL/min/1.73 m2.
Alogliptin lowers HbA1c by about 0.5–0.6% when added to metformin, sulfonylurea, or pioglitazone. The usual dose is 25 mg orally daily. The 12.5-mg dose is used in patients with eGFR of 30–60 mL/min/1.73 m2; and 6.25 mg for clearance less than 30 mL/min/1.73 m2. Linagliptin lowers HbA1c by about 0.4–0.6% when added to metformin, sulfonylurea, or pioglitazone. The dose is 5 mg orally daily, and since, it is primarily excreted unmetabolized via the bile, no dose adjustment is needed in patients with kidney disease. Vildagliptin lowers HbA1c by about 0.5–1% when added to the therapeutic regimen of patients with type 2 diabetes. The dose is 50 mg once or twice daily.
The main adverse effect of DPP-4 inhibitors appears to be a predisposition to nasopharyngitis or upper respiratory tract infection. Hypersensitivity reactions, including anaphylaxis, angioedema, and exfoliative skin conditions (such as Stevens-Johnson syndrome), have been reported. There have also been reports of pancreatitis, but the frequency of the event is unclear. Cases of liver failure have been reported with the use of alogliptin, but it is uncertain if alogliptin was the cause. The medication, however, should be discontinued in the event of liver failure. Rare cases of hepatic dysfunction, including hepatitis, have been reported with the use of vildagliptin; and liver biochemical testing is recommended quarterly during the first year of use and periodically thereafter. Saxagliptin may increase the risk of heart failure. In a post-marketing study of 16,492 patients with type 2 diabetes, heart failure occurred in 3.5% in the saxagliptin group and 2.8% in the placebo group (hazard ratio 1.27). Patients with the highest risk of heart failure were those who had a history of heart failure or had elevated levels of N-terminal of the prohormone B-type natriuretic peptide (NT-pBNP) or had kidney impairment. In a large post-marketing study, alogliptin, like saxagliptin, was associated with a slightly increased rate of heart failure. The FDA has issued a warning that the DPP-4 inhibitors can occasionally cause joint pains that resolve after stopping the drug.
5. Sodium-glucose co-transporter 2 inhibitors—Glucose is freely filtered by the kidney glomeruli and is reabsorbed in the proximal tubules by the action of sodium-glucose co-transporters (SGLT). Sodium-glucose co-transporter 2 (SGLT2) accounts for about 90% of glucose reabsorption and its inhibition causes glycosuria in people with diabetes, lowering plasma glucose levels. The oral SGLT2 inhibitors canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin are approved for clinical use in the United States. These agents reduce the threshold for glycosuria from a plasma glucose threshold of about a 180 mg/dL to about 40 mg/dL; and lower HbA1c by 0.5–1% when used alone or in combination with other oral agents or insulin. The efficacy is higher at higher HbA1c levels when more glucose is excreted as a result of SGLT2 inhibition. The loss of calories results in modest weight loss of 2–5 kg.
The usual dose of canagliflozin is 100 mg daily but up to 300 mg daily can be used in patients with normal kidney function. The dose of dapagliflozin is 10 mg daily but 5 mg daily is the recommended initial dose in patients with hepatic failure. The usual dosage of empagliflozin is 10 mg daily but a higher dose of 25 mg daily can be used. The recommended starting dose of ertugliflozin is 5 mg, but the dose can be increased to 15 mg daily if additional glucose lowering is needed.
In a postmarketing multinational study of 7020 patients with type 2 diabetes with known cardiovascular disease, the addition of empagliflozin was associated with a lower primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio 0.86, P = 0.04). The mechanisms regarding the benefit remain unclear. Weight loss, lower blood pressure, and diuresis may have played a role since there were fewer deaths from heart failure in the treated group whereas the rates of myocardial infarction were unaltered. A similar multinational study was performed with the addition of canagliflozin. This was a study of 10,142 patients with type 2 diabetes with known or at increased risk for cardiovascular disease. The canagliflozin treated group had a lower primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio 0.86, P = 0.02). In a 2019 heart failure study of 4744 patients with NYHA class II, III, IV heart failure and ejection fraction of less 40%, dapagliflozin reduced the cumulative incidence of worsening heart failure or cardiovascular death (hazard ratio 0.74, P < 0.001). Forty-two percent of the patients had diabetes; the findings in patients with and without diabetes were the same. Both empagliflozin and canagliflozin show benefit in terms of progression of albuminuria and kidney injury, possibly by lowering glomerular hyperfiltration. In a 2019 multinational study of 4401 patients with type 2 diabetes and albuminuric chronic kidney disease (eGFR 30–89 mL/min/1.73 m2 with albumin [mg] to creatinine [g] ratio > 300 to 5000) and taking an ACE inhibitor or angiotensin receptor blocker, canagliflozin reduced the risk of end-stage renal disease, the doubling of serum creatinine, and of renal death.
As might be expected, the efficacy of the SGLT2 inhibitors is reduced in chronic kidney disease. They can also increase creatinine and decrease eGFR, especially in patients with kidney impairment. Their use is generally not recommended in patients with eGFR less than 45 mL/min/1.73 m2 and are contraindicated in patients with eGFR less than 30 mL/min/1.73 m2. The main side effects are increased incidence of genital mycotic infections and urinary tract infections affecting ~8–9% of patients. Cases of necrotizing fasciitis of the perineum (Fournier gangrene) have been reported. There have also been reports of cases of pyelonephritis and septicemia requiring hospitalization. The glycosuria can cause intravascular volume contraction and hypotension.
The multinational study with canagliflozin showed an increased risk of amputations, especially of the toes (hazard ratio 1.97). This finding has not been observed in other studies using this drug or with the other SGLT2 inhibitors.
Canagliflozin has been reported to cause a decrease in bone mineral density at the lumbar spine and the hip. In a pooled analysis of eight clinical trials (mean duration 68 weeks), a 30% increase in fractures was observed in patients taking canagliflozin. It is likely that the effect on the bones is a class effect and not restricted to canagliflozin. All the SGLT2 inhibitors cause a modest increase in LDL cholesterol levels (3–8%). Also, in clinical trials, patients taking dapagliflozin had higher rates of breast cancer (nine cases vs none in comparator arms) and bladder cancer (10 cases vs 1 in placebo arm). These cancer rates exceeded the expected rates in age-matched reference diabetes population.
Cases of DKA have been reported with off-label use of SGLT2 inhibitors in patients with type 1 diabetes. Type 1 patients are taught to give less insulin if their glucose levels are not elevated. SGLT2 inhibitors lower glucose levels by changing the renal threshold and not by insulin action. Type 1 patients taking an SGLT2 inhibitor, because the glucose levels are not elevated, may either withhold or reduce their insulin doses to such a degree as to induce ketoacidosis. SGLT2 inhibitors should not be used in patients with type 1 diabetes and in those patients labeled as having type 2 diabetes but who are very insulin deficient and ketosis-prone.
6. Others—Pramlintide is a synthetic analog of islet amyloid polypeptide (IAPP or amylin). When given subcutaneously, it delays gastric emptying, suppresses glucagon secretion, and decreases appetite. It is approved for use both in type 1 diabetes and in insulin-treated type 2 diabetes. In 6-month clinical studies with type 1 and insulin-treated type 2 patients, those taking the medication had an approximately 0.4% reduction in HbA1c and about 1.7 kg weight loss compared with placebo. The HbA1c reduction was sustained for 2 years but some of the weight was regained. The medication is given by injection immediately before the meal. Hypoglycemia can occur, and it is recommended that the short-acting or premixed insulin doses be reduced by 50% when the medication is started. Since the medication slows gastric emptying, recovery from hypoglycemia can be a problem because of delay in absorption of fast-acting carbohydrates. Nausea is the other main side effect, affecting 30–50% of persons, but tends to improve with time. In patients with type 1 diabetes, the initial dose of pramlintide is 15 mcg before each meal and titrated up by 15-mcg increments to a maintenance dose of 30 mcg or 60 mcg before each meal. In patients with type 2 diabetes, the starting dose is 60 mcg premeals increased to 120 mcg in 3 to 7 days if no significant nausea occurs.
Bromocriptine, a dopamine 2 receptor agonist, has been shown to modestly lower HbA1c by 0.1–0.5% when compared to baseline and 0.4–0.5% compared to placebo. Common side effects are nausea, vomiting, dizziness, and headache.
Colesevelam, the bile acid sequestrant, when added to metformin or sulfonylurea or insulin, lowered HbA1c 0.3–0.4% when compared to baseline and 0.5–0.6% compared to placebo. HbA1c lowering, however, was not observed in a single monotherapy clinical trial comparing colesevelam to placebo. Colesevelam use is associated with ~20% increase in triglyceride levels. Other adverse effects include constipation and dyspepsia.
With their modest glucose lowering and significant side effects, using bromocriptine or colesevelam to treat diabetes is not recommended.
7. Medication combinations—Several medication combinations are available in different dose sizes, including glyburide and metformin (Glucovance); glipizide and metformin (Metaglip); repaglinide and metformin (Prandi-Met); rosiglitazone and metformin (Avandamet); pioglitazone and metformin (ACTOplusMet); rosiglitazone and glimepiride (Avandaryl); pioglitazone and glimepiride (Duetact); sitagliptin and metformin (Janumet); saxagliptin and metformin XR (Kombiglyze XR); linagliptin and metformin (Jentadueto); alogliptin and metformin (Kazano); alogliptin and pioglitazone (Oseni); dapagliflozin and metformin (Xigduo); canagliflozin and metformin (Invokamet); empagliflozin and metformin (Synjardy); empagliflozin and linagliptin (Glyxambi); ertugliflozin and metformin (Segluormet); ertugliflozin and sitagliptin (Steglujan); insulin degludec and liraglutide (Xultophy); and insulin glargine and lixisenatide (Soliqua). These medication combinations, however, limit the clinician’s ability to optimally adjust dosage of the individual medications and for that reason are not recommended.
C. Insulin
Insulin is indicated for type 1 diabetes as well as for type 2 diabetic patients with insulinopenia whose hyperglycemia does not respond to diet therapy either alone or combined with other hypoglycemic medications.
1. Characteristics of available insulin preparations—Human insulin is dispensed as either regular (R) or NPH (N) formulations. Six analogs of human insulin—three rapidly acting (insulin lispro, insulin aspart, insulin glulisine) and three long-acting (insulin glargine, insulin detemir, and insulin degludec)—are approved by the FDA for clinical use. Commercial insulin preparations differ with respect to the time of onset and duration of their biologic action (Table 27–6). All currently available insulins contain less than 10 ppm of proinsulin and are labeled as “purified.” These purified insulins preserve their potency, so that refrigeration is recommended but not crucial. During travel, reserve supplies of insulin can be readily transported for weeks without losing potency if protected from extremes of heat or cold. All the insulins in the United States are available in a concentration of 100 units/mL (U100) and dispensed in 10-mL vials or 0.3-mL cartridges or prefilled disposable pens. Several insulins are available at higher concentrations: insulin glargine, 300 units/mL (U300); insulin degludec, 200 units/mL (U200); insulin lispro, 200 units/mL (U200); and regular insulin, 500 units/mL (U500).
Table 27–6. Summary of bioavailability characteristics of the insulins.
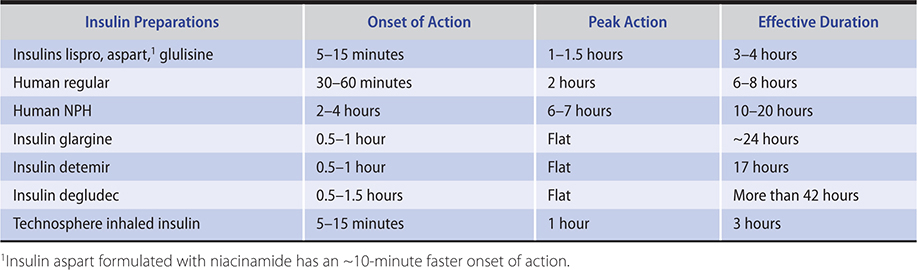
2. Insulin preparations—See Table 27–7. The rapidly acting insulin analogs and the long-acting insulins are designed for subcutaneous administration, while regular insulin can also be given intravenously.
Table 27–7. Insulin preparations available in the United States.1
Rapidly acting human insulin analogs
Insulin lispro (Humalog, Lilly; Admelog, Sanofi)
Insulin aspart (Novolog, FiAsp, Novo Nordisk)
Insulin glulisine (Apidra, Sanofi Aventis)
Short-acting regular insulin
Regular insulin (Lilly, Novo Nordisk)
Technosphere inhaled regular insulin (Afrezza)
Intermediate-acting insulins
NPH insulin (Lilly, Novo Nordisk)
Premixed insulins
70% NPH/30% regular (70/30 insulin—Lilly, Novo Nordisk)
70% NPL/25% insulin lispro (Humalog Mix 75/25—Lilly)
50% NPL/50% insulin lispro (Humalog Mix 50/50—Lilly)
70% insulin aspart protamine/30% insulin aspart (Novolog Mix 70/30—Novo Nordisk)
70% insulin degludec/30 insulin aspart (Ryzodeg, Novo Nordisk)
Long-acting human insulin analogs
Insulin glargine (Lantus (U100), Toujeo (U300), Sanofi Aventis; Basaglar (U100), Lilly)
Insulin detemir (Levemir, Novo Nordisk)
Insulin degludec (Tresiba, Novo Nordisk)
1All insulins available in the United States are recombinant human or human insulin analog origin. All the above insulins are dispensed at U100 concentration. There is an additional U500 preparation of regular insulin; U300 preparation of insulin glargine; U200 preparation of insulin lispro; U200 preparation of insulin degludec.
NPH, neutral protamine Hagedorn.
a. Short-acting insulin preparations—
(1) Regular insulin—Regular insulin is a short-acting soluble crystalline zinc insulin whose effect appears within 30 minutes after subcutaneous injection and lasts 5–7 hours when usual quantities are administered. Intravenous infusions of regular insulin are particularly useful in the treatment of DKA and during the perioperative management of patients with diabetes who require insulin. For markedly insulin-resistant persons who would otherwise require large volumes of insulin solution, a U500 preparation of human regular insulin is available both in a vial form and a disposable pen. A U500 insulin syringe should be used if the vial form is dispensed. U500 regular insulin is much more expensive than the U100 concentration and is rarely needed.
(2) Rapidly acting insulin analogs—Insulin lispro (Humalog, Admelog) is an insulin analog where the proline at position B28 is reversed with the lysine at B29. Insulin aspart (Novolog) is a single substitution of proline by aspartic acid at position B28. In insulin glulisine (Apidra) the asparagine at position B3 is replaced by lysine and the lysine in position B29 by glutamic acid. These three analogs have less of a tendency to form hexamers, in contrast to human insulin. When injected subcutaneously, the analogs quickly dissociate into monomers and are absorbed very rapidly, reaching peak serum values in as soon as 1 hour—in contrast to regular human insulin, whose hexamers require considerably more time to dissociate and become absorbed. The amino acid changes in these analogs do not interfere with their binding to the insulin receptor, with the circulating half-life, or with their immunogenicity, which are all identical with those of human regular insulin. An insulin aspart formulation (FiAsp) that contains niacinamide (vitamin B3) has a more rapid initial absorption and its onset of action is about 10 minutes faster than the standard insulin aspart formulation. Because of this more rapid onset of action, the 1-hour (but not 2-hour) postprandial glucose excursions are lower compared to the standard formulation.
Clinical trials have demonstrated that the optimal times of preprandial subcutaneous injection of comparable doses of the rapidly acting insulin analogs and of regular human insulin are 20 minutes and 60 minutes, respectively, before the meal. The quicker onset of action with the rapidly acting insulin analogs allows the patient to inject insulin just before eating rather than wait for 60 minutes as needed for regular insulin. Another desirable feature of rapidly acting insulin analogs is that their duration of action remains at about 4 hours for most commonly used dosages. This contrasts with regular insulin, whose duration of action is significantly prolonged when larger doses are used.
The rapidly acting analogs are commonly used in pumps. In a double-blind crossover study comparing insulin lispro with regular insulin in insulin pumps, persons using insulin lispro had lower HbA1c values and improved postprandial glucose control with the same frequency of hypoglycemia. In the event of pump failure, however, users of the rapidly acting insulin analogs will have more rapid onset of hyperglycemia and ketosis.
While insulin aspart has been approved for intravenous use (eg, in hyperglycemic emergencies), there is no advantage in using insulin aspart over regular insulin by this route. A U200 concentration of insulin lispro is available in a disposable prefilled pen. The only advantage of the U200 over the U100 insulin lispro preparation is that it delivers the same dose in half the volume.
b. Long-acting insulin preparations—
(1) NPH (neutral protamine Hagedorn or isophane) insulin—NPH is an intermediate-acting insulin whose onset of action is delayed to 2–4 hours, and its peak response is generally reached in about 6–7 hours. The onset of action is delayed by combining 2 parts soluble crystalline zinc insulin with 1 part protamine zinc insulin. This produces equivalent amounts of insulin and protamine, so that neither is present in an uncomplexed form (“isophane”). Because its duration of action is often less than 24 hours (with a range of 10–20 hours), most patients require at least two injections daily to maintain a sustained insulin effect. Occasional vials of NPH insulin have tended to show unusual clumping of their contents or “frosting” of the container, with considerable loss of bioactivity. This instability is rare and occurs less frequently if NPH human insulin is refrigerated when not in use and if bottles are discarded after 1 month of use.
(2) Insulin glargine—In this insulin, the asparagine at position 21 of the insulin A chain is replaced by glycine and two arginines are added to the carboxyl terminal of the B chain. The arginines raise the isoelectric point of the molecule closer to neutral making it more soluble in an acidic environment. In contrast, human insulin has an isoelectric point of pH 5.4. Insulin glargine is a clear insulin, which, when injected into the neutral pH environment of the subcutaneous tissue, forms microprecipitates that slowly release the insulin into the circulation. This insulin lasts for about 24 hours without any pronounced peaks and is given once a day to provide basal coverage. This insulin cannot be mixed with the other human insulins because of its acidic pH. When this insulin was given as a single injection at bedtime to type 1 patients in clinical trials, fasting hyperglycemia was better controlled with less nocturnal hypoglycemia when compared to NPH insulin.
A more concentrated form of insulin glargine (U300) is available as an insulin pen. In clinical trials in type 1 patients, U300 use did not result in better control or reduce the rates of hypoglycemia. Although limited clinical data suggest that insulin glargine is safe in pregnancy, it is not approved for this use.
(3) Insulin detemir—In this insulin analog, the threonine at position B30 has been removed and a 14-C fatty acid chain (tetradecanoic acid) is attached to the lysine at position 29 by acylation. Its prolonged action is due to dihexamerization and binding of hexamers and dimers to albumin at the injection site as well as binding of the monomer via its fatty acid side chain to albumin in the circulation. The affinity of insulin detemir is fourfold to fivefold lower than that of human soluble insulin and therefore the U100 formulation of insulin detemir has an insulin concentration of 2400 nmol/mL compared with 600 nmol/mL for NPH. The duration of action for insulin detemir is about 17 hours at therapeutically relevant doses. It is recommended that the insulin be injected once or twice a day to achieve a stable basal coverage. It has been approved for use during pregnancy.
(4) Insulin degludec—In this insulin analog, the threonine at position B30 has been removed, and the lysine at position B29 is conjugated to hexdecanoic acid via a gamma-L-glutamyl spacer. In the vial, in the presence of phenol and zinc, the insulin is in the form of dihexamers but when injected subcutaneously, it self associates into large multihexameric chains consisting of thousands of dihexamers. The chains slowly dissolve in the subcutaneous tissue and insulin monomers are steadily released into the systemic circulation. The half-life of insulin degludec is 25 hours. Its onset of action is in 30–90 minutes and its duration of action is more than 42 hours. It is recommended that the insulin be injected once or twice a day to achieve a stable basal coverage. Insulin degludec is available in two concentrations, U100 and U200, and dispensed in prefilled disposable pens.
c. Mixed insulin preparations—Patients with type 2 diabetes can sometimes achieve reasonable glucose control with just prebreakfast and predinner injections of mixtures of short acting and NPH insulins. The regular insulin or rapidly acting insulin analog is withdrawn first, then the NPH insulin and then injected immediately. Stable premixed insulins (70% NPH and 30% regular) are available as a convenience to patients who have difficulty mixing insulin because of visual problems or impairment of manual dexterity (Table 27–7). Premixed preparations of insulin lispro and NPH insulins are unstable; stability is achieved by replacing the NPH insulin with NPL (neutral protamine lispro). This insulin has the same duration of action as NPH insulin. Premixed combinations of NPL and insulin lispro (75% NPL/25% insulin lispro mixture [Humalog Mix 75/25] and 50% NPL/50% insulin lispro mixture [Humalog Mix 50/50]) are available for clinical use. Similarly, a 70% insulin aspart protamine/30% insulin aspart (NovoLog Mix 70/30) is available. The main advantages of these mixtures are that they can be given within 15 minutes of starting a meal and they are superior in controlling the postprandial glucose rise after a carbohydrate rich meal. These benefits have not translated into improvements in HbA1c levels when compared with the usual 70% NPH/30% regular mixture. The longer-acting insulin analogs, insulin glargine and insulin detemir, cannot be mixed with either regular insulin or the rapidly acting insulin analogs. Insulin degludec, however, can be mixed and is available as 70% insulin degludec/30% insulin aspart and is injected once or twice a day.
3. Methods of insulin administration—
a. Insulin syringes and needles—Plastic disposable syringes are available in 1-mL, 0.5-mL, and 0.3-mL sizes. The “low-dose” 0.3-mL syringes are popular because many patients with diabetes do not take more than 30 units of insulin in a single injection except in rare instances of extreme insulin resistance. Three lengths of needles are available: 6 mm, 8 mm, and 12.7 mm. Long needles are preferable in obese patients to reduce variability of insulin absorption. The needles are of 28, 30, and 31 gauges. The 31-gauge needles are almost painless. “Disposable” syringes may be reused until blunting of the needle occurs (usually after three to five injections). Sterility adequate to avoid infection with reuse appears to be maintained by recapping syringes between uses. Cleansing the needle with alcohol may not be desirable since it can dissolve the silicone coating and can increase the pain of skin puncturing.
b. Sites of injection—Any part of the body covered by loose skin can be used, such as the abdomen, thighs, upper arms, flanks, and upper buttocks. Preparation with alcohol is not required prior to injection as long as the skin is clean. Rotation of sites is recommended to avoid delayed absorption when fibrosis or lipohypertrophy occurs from repeated use of a single site. Regular insulin is absorbed more rapidly when injected in the deltoid or abdomen compared to thighs and buttocks. Exercise can increase absorption when the injection site is adjacent to the exercise muscle. For most patients, the abdomen is the recommend region for injection because it provides adequate area in which to rotate sites. The effect of anatomic regions appears to be much less pronounced with the analog insulins.
c. Insulin pen injector devices—Insulin pens eliminate the need for carrying insulin vials and syringes. Cartridges of insulin lispro and insulin aspart are available for reusable pens (Novo Nordisk, and Owen Mumford). Disposable prefilled pens are also available for regular insulin (U100 and U500), insulin lispro, insulin aspart, insulin glulisine, insulin detemir, insulin glargine, insulin degludec, NPH, 70% NPH/30% regular, 75% NPL/25% insulin lispro, 50% NPL/50% insulin lispro, 70% insulin aspart protamine/30% insulin aspart, and 70% insulin degludec/30% insulin aspart. Pen needles are available in 29, 31, and 32 gauges and 4-, 5-, 6-, 8-, and 12.7-mm lengths (Novofine; BD).
d. Insulin pumps—In the United States, Medtronic Mini-Med, Insulet, and Tandem make battery operated continuous subcutaneous insulin infusion (CSII) pumps. These pumps are small (about the size of a pager) and easy to program. They offer many features, including the ability to set a number of different basal rates throughout the 24 hours and to adjust the time over which bolus doses are given. They also are able to detect pressure build-up if the catheter is kinked. The catheter connecting the insulin reservoir to the subcutaneous cannula can be disconnected, allowing the patient to remove the pump temporarily (eg, for bathing). Ominpod (Insulet Corporation) is an insulin infusion system in which the insulin reservoir and infusion set are integrated into one unit (pod), so there is no catheter (electronic patch pump). The pod, placed on the skin, delivers subcutaneous basal and bolus insulin based on wirelessly transmitted instructions from a personal digital assistant. The great advantage of continuous subcutaneous insulin infusion (CSII) is that it allows for establishment of a basal profile tailored to the patient allowing for better overnight and between meals glucose control. The ability to adjust the basal insulin infusion makes it easier for the patient to manage glycemic excursions that occur with exercise. The pumps have software that can assist the patient to calculate boluses based on glucose reading and carbohydrates to be consumed. They keep track of the time elapsed since the last insulin bolus; the patient is reminded of this when he or she attempts to give additional correction bolus before the effect of the previous bolus has worn off (“insulin on board” feature). This feature reduces the risk of overcorrecting and subsequent hypoglycemia.
CSII therapy is appropriate for patients with type 1 diabetes who are motivated, mechanically inclined, educated about diabetes (diet, insulin action, treatment of hypoglycemia and hyperglycemia), and willing to monitor their blood glucose four to six times a day. Known complications of CSII include ketoacidosis, which can occur when insulin delivery is interrupted, and skin infections. Another disadvantage is its cost and the time needed of the clinician and staff to initiate therapy. Almost all patients use rapid-acting insulin analogs in their pumps.
V-go (Valeritas) is a mechanical patch pump designed specifically for people with type 2 diabetes who use a basal/bolus insulin regimen. The device is preset to deliver one of three fixed and flat basal rates (20, 30, or 40 units) for 24 hours (at which point it must be replaced) and there is a button that delivers two units per press to help cover meals.
e. Closed loop systems—Algorithms have been devised to use glucose data from the continuous glucose monitoring systems to automatically deliver insulin by continuous subcutaneous insulin infusion pump. These closed loop systems (artificial pancreas) have been shown in clinical studies to improve nighttime glucose control, modestly lower HbA1c levels, and reduce the risk of nocturnal hypoglycemia. The MiniMed 670 G and the Tandem Control-IQ, have been approved for clinical use. The MiniMed 670 G closed loop system uses glucose data from a sensor to automatically adjust basal insulin doses every 5 minutes, targeting a sensor glucose level of 120 mg/dL (6.7 mmol/L). Insulin delivery is suspended when the sensor glucose level falls below or is predicted to fall below target level. The glucose target can be adjusted up to 150 mg/dL (8.3 mmol/L) for physical activity. The Tandem Control-IQ targets a sensor glucose level of 112.5 mg/dL (6.25 mmol/L). The patient is still responsible for bolusing insulin for meals and snacks. There are also Do-It-Yourself closed loop systems using free open source software. One such system, called the “Loop,” uses the Dexcom G6 sensor, the iPhone, and the Omnipod insulin pump. The “Loop” controller is downloaded on to the iPhone, and it uses the Dexcom G6 sensor glucose measurements (also on the iPhone) to automatically adjust basal insulin delivery on the Omnipod pump. Increasing numbers of type 1 patients use these Do-It-Yourself systems, but they are not approved for use by the FDA. Successful use, however, requires the patient to be proficient at using both the insulin pump and continuous glucose monitor. The systems are expensive; the insulin pump, which needs to be replaced every 4 years, costs about $6000 and the pump supplies are $1500 per year. The continuous glucose monitoring system costs approximately $4000 per year.
f. Inhaled insulin—Technosphere insulin (Afrezza) is a dry-powder formulation of recombinant human regular insulin that can be inhaled. It consists of 2- to 2.5-mcm crystals of the excipient fumaryl diketopiperazine that provide a large surface area for adsorption of proteins like insulin. The technosphere insulin is rapidly absorbed with peak insulin levels reached in 12–15 minutes and declining to baseline in 3 hours; the median time to maximum effect with inhaled insulin is approximately 1 hour and declines to baseline by about 3 hours. In contrast, the median time to maximum effect with subcutaneous insulin lispro is about 2 hours and declines to baseline by 4 hours. In clinical trials, technosphere insulin combined with basal insulin was as effective in glucose lowering as rapid-acting insulin analogs combined with basal insulin. It is formulated as a single-use, color-coded cartridge delivering 4, 8, or 12 units immediately before the meal. The manufacturer provides a dose conversion table; patients injecting up to 4 units of rapid-acting insulin analog should use the 4-unit cartridge. Those injecting 5 to 8 units should use the 8-unit cartridge. If the dose is 9–12 units of rapid-acting insulin premeal then one 4-unit cartridge and one 8-unit cartridge or one 12-unit cartridge should be used. The inhaler is about the size of a referee’s whistle.
The most common adverse reaction of the inhaled insulin is a cough, affecting about 27% of patients. A small decrease in pulmonary function (forced expiratory volume in 1 second [FEV1]) is seen in the first 3 months of use, which persists over 2 years of follow-up. Inhaled insulin is contraindicated in patients who smoke and in those with chronic lung disease, such as asthma and chronic obstructive pulmonary disease. Spirometry should be performed to identify potential lung disease prior to initiating therapy. During clinical trials, there were two cases of lung cancer in patients who were taking inhaled insulin and none in the comparator-treated patients. All the patients in whom lung cancer developed had a history of prior cigarette smoking. There were also two cases of squamous cell carcinoma of the lung in nonsmokers exposed to inhaled insulin; these cases occurred after completion of the clinical trials. Cases of lung cancer were also reported in cigarette smokers using a previously available inhaled insulin preparation (Exubera). The incidence rate in the Exubera treated group was 0.13 per 1000 patient-years and 0.03 per 1000 patient-years in the comparator-treated group.
D. Transplantation
1. Pancreas transplantation—All patients with end-stage kidney disease and type 1 diabetes who are candidates for a kidney transplant should be considered potential candidates for a pancreas transplant. Eligibility criteria include age younger than 55 and minimal cardiovascular risk. Contraindications include noncorrectable coronary artery disease, extensive peripheral vascular disease, and significant obesity (weight greater than 100 kg). The pancreas transplant may occur at the same time as kidney transplant or after kidney transplant. Patients undergoing simultaneous pancreas and kidney transplantation have an 83% chance of pancreatic graft survival at 1 year and 69% at 5 years. Solitary pancreatic transplantation in the absence of a need for kidney transplantation is considered only in those rare patients who do not respond to all other insulin therapeutic approaches and who have frequent severe hypoglycemia, or who have life-threatening complications related to their lack of metabolic control. Solitary pancreas transplant graft survival is 78% at 1 year and 54% at 5 years.
2. Islet transplantation—Total pancreatectomy is curative for severe pain syndrome associated with chronic pancreatitis. The pancreatectomy, however, results in surgical diabetes. Harvesting islets from the removed pancreas and autotransplanting them into the liver (via portal vein) can prevent the development of diabetes or result in “mild” diabetes (partial islet function) that is easier to manage. Since the islets are autologous no immunosuppression is required. The number of islets transplanted is the main predictor of insulin independence.
People with type 1 diabetes can become insulin independent after receiving islets isolated from a donor pancreas (alloislet transplant). The islets are infused into the portal vein using a percutaneous transhepatic approach, and they lodge in the liver releasing insulin in response to physiologic stimuli. Long-term immunosuppression is necessary to prevent allograft rejection and to suppress the autoimmune process that led to the disease in the first place. Insulin independence for more than 5 years has been demonstrated in patients who got anti-CD3 antibody or anti-thymocyte globulin induction immunosuppression and calcineurin inhibitors, mTor inhibitors, and mycophenolate mofetil as maintenance immunosuppression. One major limitation is the need for more than one islet infusion to achieve insulin independence. This is because of significant loss of islets during isolation and the period prior to engraftment. Widespread alloislet transplantation will depend on improving insulin independence rates with one infusion and also demonstrating that the long-term outcomes are as good as those of pancreas transplant alone.
DeFronzo RA et al. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017 Jan;13(1):11–26. [PMID: 27941935]
McMurray JJV et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019 Nov 21;381(21):1995–2008. [PMID: 31535829]
Perkovic V et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295–306. [PMID: 30990260]
Qaseem A et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017 Feb 21;166(4):279–90. [PMID: 28055075]
 Steps in the Management of the Diabetic Patient
Steps in the Management of the Diabetic Patient
A. Diagnostic Examination
An attempt should be made to characterize the diabetes as type 1 or type 2 or other specific types such as MODY, based on the clinical features present and on whether or not ketonuria accompanies the glycosuria. Features that suggest end-organ insulin insensitivity to insulin, such as visceral obesity, acanthosis nigricans, or both, must be identified. The family history should document not only the incidence of diabetes in other members of the family but also the age at onset, association with obesity, the need for insulin, and whether there were complications. For the occasional patient, measurement of GAD65, IAA, ICA 512, and zinc transporter 8 antibodies can help distinguish between type 1 and type 2 diabetes (Table 27–2). Many patients with newly diagnosed type 1 diabetes still have significant endogenous insulin production, and C peptide levels do not reliably distinguish between type 1 and type 2 diabetes. Other factors that increase cardiac risk, such as smoking history, presence of hypertension or hyperlipidemia, or oral contraceptive pill use, should be recorded.
Laboratory diagnosis of diabetes should document fasting plasma glucose levels above 126 mg/dL (7 mmol/L) or postprandial values consistently above 200 mg/dL (11.1 mmol/L) or HbA1c of at least 6.5% and whether ketonuria accompanies the glycosuria. An HbA1c measurement is also useful for assessing the effectiveness of future therapy. Baseline values include fasting plasma triglycerides, total cholesterol and HDL cholesterol, electrocardiography, kidney function studies, peripheral pulses, and neurologic, podiatric, and ophthalmologic examinations to help guide future assessments.
B. Patient Education (Self-Management Training)
Since diabetes is a lifelong disorder, education of the patient and the family is probably the most important obligation of the clinician who provides care. The best persons to manage a disease that is affected so markedly by daily fluctuations in environmental stress, exercise, diet, and infections are the patients themselves and their families. The “teaching curriculum” should include explanations by the clinician or nurse of the nature of diabetes and its potential acute and chronic hazards and how they can be recognized early and prevented or treated. Self-monitoring of blood glucose should be emphasized, especially in insulin-requiring diabetic patients, and instructions must be given on proper testing and recording of data.
Patients taking insulin should have an understanding of the actions of basal and bolus insulins. They should be taught to determine whether the basal dose is appropriate and how to adjust the rapidly acting insulin dose for the carbohydrate content of a meal. Patients and their families and friends should be taught to recognize signs and symptoms of hypoglycemia and how to treat low glucose reactions. Strenuous exercise can precipitate hypoglycemia, and patients must therefore be taught to reduce their insulin dosage in anticipation of strenuous activity or to take supplemental carbohydrate. Injection of insulin into a site farthest away from the muscles most involved in the exercise may help ameliorate exercise-induced hypoglycemia, since insulin injected in the proximity of exercising muscle may be more rapidly mobilized. Exercise training also increases the effectiveness of insulin, and insulin doses should be adjusted accordingly. Infections can cause insulin resistance, and patients should be instructed on how to manage the hyperglycemia with supplemental rapidly acting insulin.
Advice on personal hygiene, including detailed instructions on foot and dental care, should be provided. All infections (especially pyogenic ones) provoke the release of high levels of insulin antagonists, such as catecholamines or glucagon, and thus bring about a marked increase in insulin requirements. Patients who are taking oral agents may decompensate and temporarily require insulin. Patients should be told about community agencies, such as Diabetes Association chapters, that can serve as a continuing source of instruction.
Finally, vigorous efforts should be made to persuade patients with newly diagnosed diabetes who smoke to give up the habit, since large vessel peripheral vascular disease and debilitating retinopathy are less common in nonsmoking diabetic patients.
C. Therapy
Treatment must be individualized on the basis of the type of diabetes and specific needs of each patient. However, certain general principles of management can be outlined for hyperglycemic states of different types.
1. Type 1 diabetes—Traditional once- or twice-daily insulin regimens are usually ineffective in type 1 patients without residual endogenous insulin. If near-normalization of blood glucose is attempted, at least four measurements of capillary blood glucose and three or four insulin injections are necessary.
A combination of rapidly acting insulin analogs and long-acting insulin analogs allows for more physiologic insulin replacement. Table 27–8 illustrates a regimen with a rapidly acting insulin analog and insulin detemir or insulin glargine that might be appropriate for a 70-kg person with type 1 diabetes eating meals providing standard carbohydrate intake and moderate to low fat content.
Table 27–8. Examples of intensive insulin regimens using rapidly acting insulin analogs (insulin lispro, aspart, or glulisine) and insulin detemir, or insulin glargine or degludec in a 70-kg man with type 1 diabetes.1–3

Insulin glargine or insulin degludec is usually given once in the evening to provide 24-hour coverage. There are occasional patients in whom insulin glargine does not last for 24 hours, and in such cases, it needs to be given twice a day. Insulin detemir usually has to be given twice a day to get adequate 24-hour basal coverage. Alternatively, small doses of NPH (~3–4 units) can be given with each meal to provide daytime basal coverage with a larger dose at night.
CSII by portable battery-operated “open loop” devices allow the setting of different basal rates throughout the 24 hours and permit bolus dose adjustments by as little as 0.05-unit increments. The 24-hour basal dosage is usually based on age and body weight. An adolescent might need as much as 0.4 unit/kg/day; young adult (less than 25 years), 0.35 unit per/kg/day; and older adults, 0.25 unit/kg/day. For example, a 70-kg, 30-year-old person may require a basal rate of 0.7 unit per hour throughout the 24 hours with the exception of 3 am to 8 am, when 0.8 unit per hour might be appropriate (given the “dawn phenomenon”—reduced tissue sensitivity to insulin between 5 am and 8 am). The meal bolus varies based on the time of day and the person’s age. Adolescents and young adults usually require 1 unit for about 10 g of carbohydrate. Older adults usually require about 1 unit for 15 g of carbohydrate. The correction factor—how much insulin is needed to lower glucose levels by 50 mg/dL—can be calculated from the insulin-to-carbohydrate ratios. For example, if 1 unit is required for 15 g of carbohydrate, then 1 unit will lower glucose levels by 50 mg/dL. If 1.5 units of insulin are required for 15 g of carbohydrate (that is, 1 unit for 10 g carbohydrate), then 1.5 units of insulin will lower glucose levels by 50 mg/dL (that is, 1 unit will lower glucose level by 33 mg/dL). For a 70-kg 30-year-old person, bolus ratios of 1 unit for 12–15 g of carbohydrate plus 1 unit for 50 mg/dL of blood glucose over a target value of 120 mg/dL would be reasonable starting point. Further adjustments to basal and bolus dosages would depend on the results of blood glucose monitoring. One of the more difficult therapeutic problems in managing patients with type 1 diabetes is determining the proper adjustment of insulin dose when the prebreakfast blood glucose level is high. Occasionally, the prebreakfast hyperglycemia is due to the Somogyi effect, in which nocturnal hypoglycemia leads to a surge of counterregulatory hormones to produce high blood glucose levels by 7 am. However, a more common cause for prebreakfast hyperglycemia is the waning of circulating insulin levels by the morning.
The diagnosis of the cause of prebreakfast hyperglycemia can be facilitated by self-monitoring of blood glucose at 3 am in addition to the usual bedtime and 7 am measurements or by analyzing data from the continuous glucose monitor. This is required for only a few nights, and when a particular pattern emerges from monitoring blood glucose levels overnight, appropriate therapeutic measures can be taken. The Somogyi effect can be treated by lowering the basal insulin dose at bedtime or by eating a snack at bedtime. When a waning insulin level is the cause, then either increasing the evening basal insulin dose or shifting it from dinnertime to bedtime (or both) can be effective. If this fails, insulin pump therapy may be required. The currently available closed loop systems enable patients to achieve close to normal glucose levels in the morning with a low risk of nocturnal hypoglycemia.
2. Type 2 diabetes—Therapeutic recommendations are based on the relative contributions of beta cell insufficiency and insulin insensitivity in individual patients. The possibility that the individual patient has a specific etiologic cause for their diabetes should always be considered, especially when the patient does not have a family history of type 2 diabetes or does not have any evidence of central obesity or insulin resistance. Such patients should be evaluated for other types of diabetes such as LADA or MODY (Table 27–1). Patients with LADA should be prescribed insulin when the disease is diagnosed and treated like patients with type 1 diabetes. It is also important to note that many patients with type 2 diabetes mellitus have a progressive loss of beta cell function and will require additional therapeutic interventions with time.
a. Weight reduction—One of the primary modes of therapy in the obese patient with type 2 diabetes is weight reduction. Normalization of glycemia can be achieved by weight loss and improvement in tissue sensitivity to insulin. A combination of caloric restriction, increased exercise, and behavior modification is required if a weight reduction program is to be successful. Understanding the risks associated with the diagnosis of diabetes may motivate the patient to lose weight.
For selected patients, medical or surgical options for weight loss should be considered. Orlistat, phentermine/topiramate, lorcaserin, naltrexone/extended-release bupropion, and high-dose liraglutide (3 mg daily) are weight loss medications approved for use in combination with diet and exercise (see Chapter 29).
Bariatric surgery (Roux-en-Y, gastric banding, gastric sleeve, biliopancreatic diversion/duodenal switch) typically results in substantial weight loss and improvement in glucose levels. A meta-analysis examining the impact of bariatric surgery on patients with diabetes and BMI of 40 kg/m2 or greater noted that 82% of patients had resolution of clinical and laboratory manifestations of diabetes in the first 2 years after surgery and 62% remained free of diabetes more than 2 years after surgery. The improvement was most marked in the procedure that caused the greatest weight loss (biliopancreatic diversion/duodenal switch). There was, however, a high attrition of patients available for follow-up, and there was little information about different ethnic types. Weight regain does occur after bariatric surgery, and it can be expected that 20–25% of the lost weight will be regained over 10 years. The impact of this weight gain on diabetes recurrence depends principally on the degree of beta cell dysfunction.
Nonobese patients with type 2 diabetes frequently have increased visceral adiposity—the so-called metabolically obese normal weight patient. There is less emphasis on weight loss, but exercise remains an important aspect of treatment.
b. Glucose-lowering agents—Figure 27–2 outlines the treatment approach based on the consensus algorithm proposed by the American Diabetes Association and the European Association for the Study of Diabetes. The current recommendation is to start metformin therapy at diagnosis and not wait to see whether the patient can achieve target glycemic control with weight management and exercise. See discussion of the individual medications, above.
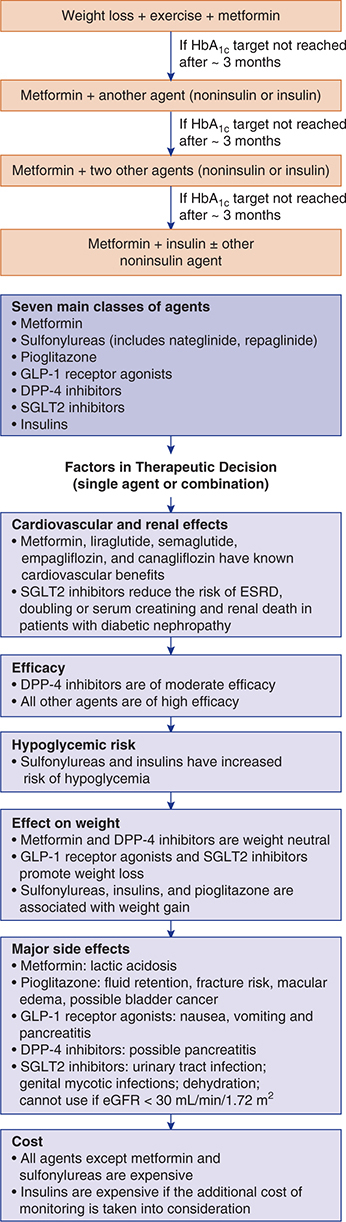
Figure 27–2. Algorithm for the treatment of type 2 diabetes based on the 2018 recommendations of the consensus panel of the American Diabetes Association/European Association for the Study of Diabetes.
When diabetes is not well controlled with initial therapy (usually metformin), then a second agent should be added. Presence of cardiovascular or kidney disease, or both, will determine the choice of the second agent. Liraglutide, semaglutide, empagliflozin, canagliflozin, and dapagliflozin have improved cardiovascular outcomes. The SGLT2 inhibitors may be especially beneficial in patients with heart failure or diabetic nephropathy, or both. The GLP1 receptor agonists and the SGLT2 inhibitors promote weight loss, and it may not be necessary to add a third agent. Sulfonylureas have been available for many years and their use in combination with metformin is well established. They do, however, have the propensity of causing hypoglycemia and weight gain. In patients who experience hyperglycemia after a carbohydrate-rich meal (such as dinner), a short-acting secretagogue (repaglinide or nateglinide) before meals may suffice to get the glucose levels into the target range. Patients with severe insulin resistance may be candidates for pioglitazone. Pioglitazone may also reduce the risk for recurrent stroke in patients who have a history of stroke or transient ischemic attack. If two agents are inadequate, then a third agent is added, although data regarding efficacy of such combined therapy are limited.
When the combination of oral agents (and injectable GLP-1 receptor agonists) fails to achieve euglycemia in patients with type 2 diabetes, then insulin treatment should be instituted. Various insulin regimens may be effective. One proposed regimen is to continue the oral combination therapy and then simply add a bedtime dose of NPH or long-acting insulin analog (insulin glargine or insulin detemir) to reduce excessive nocturnal hepatic glucose output and improve fasting glucose levels. If the patient does not achieve target glucose levels during the day, then daytime insulin treatment can be initiated. A convenient insulin regimen under these circumstances is a split dose of 70/30 NPH/regular mixture (or Humalog Mix 75/25 or NovoLogMix 70/30) before breakfast and before dinner. If this regimen fails to achieve satisfactory glycemic goals or is associated with unacceptable frequency of hypoglycemic episodes, then a more intensive regimen of multiple insulin injections can be instituted as in patients with type 1 diabetes. Metformin principally reduces hepatic glucose output, and it is reasonable to continue with this medication when insulin therapy is instituted. Pioglitazone, which improves peripheral insulin sensitivity, can be used together with insulin but this combination is associated with more weight gain and peripheral edema. The sulfonylureas, the GLP-1 receptor agonists, the DPP-4 inhibitors, and the SGLT2 inhibitors also have been shown to be of continued benefit. Weight-reducing interventions should continue even after initiation of insulin therapy and may allow for simplification of the therapeutic regimen in the future.
D. Acceptable Levels of Glycemic Control
A reasonable aim of therapy is to approach normal glycemic excursions without provoking severe or frequent hypoglycemia. Table 27–9 summarizes blood glucose and HbA1c goals for different patient groups. The UKPDS study demonstrated that blood pressure control was as significant or more significant than glycemic control in patients with type 2 diabetes regarding the prevention of microvascular as well as macrovascular complications.
Table 27–9. Glycemic targets for different groups of patients with diabetes.

Mingrone G et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012 Apr 26;366(17):1577–85. [PMID: 22449317]
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. N Engl J Med. 1993 Sep 30;329(14):977–86. [PMID: 8366922]
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998 Sep 12;352(9131):837–53. [PMID: 9742976]
E. Complications of Insulin Therapy
1. Hypoglycemia—Hypoglycemic reactions are the most common complications that occur in patients with diabetes who are treated with insulin. The signs and symptoms of hypoglycemia may be divided into those resulting from stimulation of the autonomic nervous system and those from neuroglycopenia (insufficient glucose for normal central nervous system function). When the blood glucose falls to around 54 mg/dL (3 mmol/L), the patient starts to experience both sympathetic (tachycardia, palpitations, sweating, tremulousness) and parasympathetic (nausea, hunger) nervous system symptoms. If these autonomic symptoms are ignored and the glucose levels fall further (to around 50 mg/dL [2.8 mmol/L]), then neuroglycopenic symptoms appear, including irritability, confusion, blurred vision, tiredness, headache, and difficulty speaking. A further decline in glucose can lead to loss of consciousness or even a seizure. With repeated episodes of hypoglycemia, there is adaptation, and autonomic symptoms do not occur until the blood glucose levels are much lower and so the first symptoms are often due to neuroglycopenia. This condition is referred to as “hypoglycemic unawareness.” It has been shown that hypoglycemic unawareness can be reversed by keeping glucose levels high for a period of several weeks. Except for sweating, most of the sympathetic symptoms of hypoglycemia are blunted in patients receiving beta-blocking agents. Though not absolutely contraindicated, these medications must be used with caution in patients with diabetes who require insulin, and beta-1-selective blocking agents are preferred.
Hypoglycemia can occur in a patient taking sulfonylureas, repaglinide, and nateglinide, particularly if the patient is elderly, has kidney or liver disease, or is taking certain other medications that alter metabolism of the sulfonylureas (eg, phenylbutazone, sulfonamides, or warfarin). It occurs more frequently with the use of long-acting sulfonylureas than with shorter-acting agents. Otherwise, hypoglycemia in insulin-treated patients occurs as a consequence of three factors: behavioral issues, impaired counterregulatory systems, and complications of diabetes.
Behavioral issues include injecting too much insulin for the amount of carbohydrates ingested. Drinking alcohol in excess, especially on an empty stomach, can also cause hypoglycemia. In patients with type 1 diabetes, hypoglycemia can occur during or even several hours after exercise, and so glucose levels need to be monitored and food and insulin adjusted. Some patients do not like their glucose levels to be high, and they treat every high glucose level aggressively. These individuals who “stack” their insulin—that is, give another dose of insulin before the first injection has had its full action—can develop hypoglycemia.
Counterregulatory issues resulting in hypoglycemia include impaired glucagon response, sympatho-adrenal responses, and cortisol deficiency. Patients with diabetes of greater than 5 years in duration lose their glucagon response to hypoglycemia. As a result, they are at a significant disadvantage in protecting themselves against falling glucose levels. Once the glucagon response is lost, their sympatho-adrenal responses take on added importance. Unfortunately, aging, autonomic neuropathy, or hypoglycemic unawareness due to repeated low glucose levels further blunts the sympatho-adrenal responses. Occasionally, Addison disease develops in persons with type 1 diabetes mellitus; when this happens, insulin requirements fall significantly, and unless insulin dose is reduced, recurrent hypoglycemia will develop.
Complications of diabetes that increase the risk for hypoglycemia include autonomic neuropathy, gastroparesis, and end-stage chronic kidney disease. The sympathetic nervous system is an important system alerting the individual that the glucose level is falling by causing symptoms of tachycardia, palpitations, sweating, and tremulousness. Failure of the sympatho-adrenal responses increases the risk of hypoglycemia. In addition, in patients with gastroparesis, if insulin is given before a meal, the peak of insulin action may occur before the food is absorbed causing the glucose levels to fall. Finally, in end-stage chronic kidney disease, hypoglycemia can occur presumably because of decreased insulin clearance as well as loss of renal contribution to gluconeogenesis in the postabsorptive state.
To prevent and treat insulin-induced hypoglycemia, the diabetic patient should carry glucose tablets or juice at all times. For most episodes, ingestion of 15 grams of carbohydrate is sufficient to reverse the hypoglycemia. The patient should be instructed to check the blood glucose in 15 minutes and treat again if the glucose level is still low. A parenteral (1 mg) or nasal inhalation (3 mg) glucagon emergency kit should be provided to every patient with diabetes who is receiving insulin therapy. Family or friends should be instructed how to inject it subcutaneously or intramuscularly into the buttock, arm, or thigh or administer a nasal dose in the event that the patient is unconscious or refuses food. The medication can occasionally cause vomiting, and the unconscious patient should be turned on his or her side to protect the airway. Glucagon mobilizes glycogen from the liver, raising the blood glucose by about 36 mg/dL (2 mmol/L) in about 15 minutes. After the patient recovers consciousness, additional oral carbohydrate should be given. People with diabetes receiving hypoglycemic medication therapy should also wear an identification MedicAlert bracelet or necklace or carry a card in his or her wallet (1-800-ID-ALERT, www.medicalert.org).
Medical personnel treating severe hypoglycemia can give 50 mL of 50% glucose solution by rapid intravenous infusion. If intravenous access is not available, 1 mg of glucagon can be injected intramuscularly or 3 mg given by nasal spray.
2. Immunopathology of insulin therapy—At least five molecular classes of insulin antibodies are produced during the course of insulin therapy in diabetes, including IgA, IgD, IgE, IgG, and IgM. With the switch to human and purified pork insulin, the various immunopathologic syndromes such as insulin allergy, immune insulin resistance, and lipoatrophy have become quite rare since the titers and avidity of these induced antibodies are generally quite low.
a. Insulin allergy—Insulin allergy, or immediate-type hypersensitivity, is a rare condition in which local or systemic urticaria is due to histamine release from tissue mast cells sensitized by adherence of anti-insulin IgE antibodies. In severe cases, anaphylaxis results. When only human insulin has been used from the onset of insulin therapy, insulin allergy is exceedingly rare. Antihistamines, corticosteroids, and even desensitization may be required, especially for systemic hypersensitivity. There have been case reports of successful use of insulin lispro in those rare patients who have a generalized allergy to human insulin or insulin resistance due to a high titer of insulin antibodies.
b. Immune insulin resistance—A low titer of circulating IgG anti-insulin antibodies that neutralize the action of insulin to a small extent develops in most insulin-treated patients. With the old animal insulins, a high titer of circulating antibodies sometimes developed, resulting in extremely high insulin requirements—often more than 200 units daily. This is now rarely seen with the switch to human or highly purified pork insulins and has not been reported with the analogs.
c. Lipodystrophy—Atrophy of subcutaneous fatty tissue leading to disfiguring excavations and depressed areas may rarely occur at the site of injection. This complication results from an immune reaction, and it has become rarer with the development of human and highly purified insulin preparations. Lipohypertrophy, on the other hand, is a consequence of the pharmacologic effects of insulin being deposited in the same location repeatedly. It can occur with purified insulins as well. Rotation of injection sites will prevent lipohypertrophy.
Rodriguez-Gutierrez R et al. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ. 2019 Nov 5;367:l5887. [PMID: 31690574]
 Chronic Complications of Diabetes
Chronic Complications of Diabetes
Late clinical manifestations of diabetes mellitus include a number of pathologic changes that involve small and large blood vessels, cranial and peripheral nerves, the skin, and the lens of the eye. These lesions lead to hypertension, end-stage chronic kidney disease, blindness, autonomic and peripheral neuropathy, amputations of the lower extremities, myocardial infarction, and cerebrovascular accidents. These late manifestations correlate with the duration of the diabetic state subsequent to the onset of puberty. In type 1 diabetes, end-stage chronic kidney disease develops in up to 40% of patients, compared with less than 20% of patients with type 2 diabetes. Proliferative retinopathy ultimately develops in both types of diabetes but has a slightly higher prevalence in type 1 patients (25% after 15 years’ duration). In patients with type 1 diabetes, complications from end-stage chronic kidney disease are a major cause of death, whereas patients with type 2 diabetes are more likely to have macrovascular diseases leading to myocardial infarction and stroke as the main causes of death. Cigarette use adds significantly to the risk of both microvascular and macrovascular complications in diabetic patients.
A. Ocular Complications
1. Diabetic cataracts—Premature cataracts occur in diabetic patients and seem to correlate with both the duration of diabetes and the severity of chronic hyperglycemia. Nonenzymatic glycosylation of lens protein is twice as high in diabetic patients as in age-matched nondiabetic persons and may contribute to the premature occurrence of cataracts.
2. Diabetic retinopathy—There are two main categories of diabetic retinopathy: nonproliferative and proliferative (see Chapter 7). Diabetic macular edema can occur at any stage. Nonproliferative (“background”) retinopathy represents the earliest stage of retinal involvement by diabetes and is characterized by such changes as microaneurysms, dot hemorrhages, exudates, and retinal edema. The prevalence of nonproliferative retinopathy in patients with type 2 diabetes is 60% after 16 years.
Proliferative retinopathy involves the growth of new capillaries and fibrous tissue within the retina and into the vitreous chamber. It is a consequence of small vessel occlusion, which causes retinal hypoxia; this in turn stimulates new vessel growth. New vessel formation may occur at the optic disk or elsewhere on the retina. Prior to proliferation of new capillaries, a preproliferative phase often occurs in which arteriolar ischemia is manifested as cotton-wool spots (small infarcted areas of retina). Vision is usually normal until vitreous hemorrhage or retinal detachment occurs.
Proliferative retinopathy can occur in both types of diabetes but is more common in type 1, developing about 7–10 years after onset of symptoms, with a prevalence of 25% after 15 years’ duration. Vision-threatening retinopathy virtually never appears in type 1 patients in the first 3–5 years of diabetes or before puberty. Up to 20% of patients with type 2 diabetes have retinopathy at the time of diagnosis, because many were probably diabetic for an extensive period of time before diagnosis. Annual consultation with an ophthalmologist should be arranged for patients who have had type 1 diabetes for more than 3–5 years and for all patients with type 2 diabetes. Chapter 7 describes the treatment of retinopathy and macular edema. There is no contraindication to using aspirin in patients with proliferative retinopathy.
3. Glaucoma—Glaucoma occurs in approximately 6% of persons with diabetes. It is responsive to the usual therapy for open-angle disease. Neovascularization of the iris in patients with diabetes can predispose to closed-angle glaucoma, but this is relatively uncommon except after cataract extraction, when growth of new vessels has been known to progress rapidly, involving the angle of the iris and obstructing outflow.
B. Diabetic Nephropathy
Diabetic nephropathy is initially manifested by albuminuria; subsequently, as kidney function declines, urea and creatinine accumulate in the blood (see Chapter 22). Conventional 24-hour urine collections, in addition to being inconvenient for patients, also show wide variability of albumin excretion, since several factors such as sustained erect posture, dietary protein, and exercise tend to increase albumin excretion rates. For these reasons, an albumin-creatinine ratio in an early morning spot urine collected upon awakening is preferable. In the early morning spot urine, a ratio of albumin (mcg/L) to creatinine (mg/L) of less than 30 mcg/mg creatinine is normal, and a ratio of 30–300 mcg/mg creatinine suggests abnormal microalbuminuria. At least two early morning spot urine collections over a 3- to 6-month period should be abnormal before a diagnosis of microalbuminuria is justified. Short-term hyperglycemia, exercise, urinary tract infections, heart failure, and acute febrile illness can cause transient albuminuria and so testing for microalbuminuria should be postponed until resolution of these problems.
Subsequent end-stage chronic kidney disease can be predicted by persistent urinary albumin excretion rates exceeding 30 mcg/mg creatinine. Glycemic control as well as a protein diet of ~0.8 g/kg/day may reduce both the hyperfiltration and the elevated microalbuminuria in patients in the early stages of diabetes and those with incipient diabetic nephropathy. Antihypertensive therapy also decreases microalbuminuria. Evidence from some studies supports a specific role for ACE inhibitors in reducing intraglomerular pressure in addition to their lowering of systemic hypertension. An ACE inhibitor (captopril, 50 mg twice daily) in normotensive diabetic patients impedes progression to proteinuria and prevents the increase in albumin excretion rate. Since microalbuminuria has been shown to correlate with elevated nocturnal systolic blood pressure, it is possible that “normotensive” diabetic patients with microalbuminuria have slightly elevated systolic blood pressure during sleep, which is lowered during antihypertensive therapy. This action may contribute to the reported efficacy of ACE inhibitors in reducing microalbuminuria in “normotensive” patients. SGLT2 therapy should be instituted in patients with type 2 diabetes who have progression of kidney disease despite taking optimal antihypertensive therapy, which includes an ACE inhibitor or angiotensin receptor blocker.
C. Diabetic Neuropathy
Diabetic neuropathies are the most common complications of diabetes, affecting up to 50% of older patients with type 2 diabetes.
1. Peripheral neuropathy—
a. Distal symmetric polyneuropathy—This is the most common form of diabetic peripheral neuropathy where loss of function appears in a stocking-glove pattern and is due to an axonal neuropathic process. Longer nerves are especially vulnerable, hence the impact on the foot. Both motor and sensory nerve conduction is delayed in the peripheral nerves, and ankle jerks may be absent.
Sensory involvement usually occurs first and is generally bilateral, symmetric, and associated with dulled perception of vibration, pain, and temperature. The pain can range from mild discomfort to severe incapacitating symptoms. The sensory deficit may eventually be of sufficient degree to prevent patients from feeling pain. Patients who have a sensory neuropathy should therefore be examined with a 5.07 Semmes-Weinstein filament and those who cannot feel the filament must be considered at risk for unperceived neuropathic injury.
The denervation of the small muscles of the foot can result in clawing of the toes and displacement of the submetatarsal fat pads anteriorly. These changes, together with the joint and connective tissue changes, alter the biomechanics of the foot and increase plantar pressures. This combination of decreased pain threshold, abnormally high foot pressures, and repetitive stress (such as from walking) can lead to calluses and ulcerations in the high-pressure areas such as over the metatarsal heads (Figure 27–3). Peripheral neuropathy, autonomic neuropathy, and trauma also predisposes to the development of Charcot arthropathy. An acute case of Charcot foot arthropathy presents with pain and swelling, and if left untreated, leads to a “rocker bottom” deformity and ulceration. The early radiologic changes show joint subluxation and periarticular fractures. As the process progresses, there is frank osteoclastic destruction leading to deranged and unstable joints particularly in the midfoot. Not surprisingly, the key issue for the healing of neuropathic ulcers in a foot with good vascular supply is mechanical unloading. In addition, any infection should be treated with debridement and appropriate antibiotics; healing duration of 8–10 weeks is typical. Occasionally, when healing appears refractory, platelet-derived growth factor (becaplermin [Regranex]) should be considered for local application. Once ulcers are healed, therapeutic footwear is key to preventing recurrences. Custom molded shoes are reserved for patients with significant foot deformities. Other patients with neuropathy may require accommodative insoles that distribute the load over as wide an area as possible. Patients with foot deformities and loss of their protective threshold should get regular care from a podiatrist. Patients should be educated on appropriate footwear and those with loss of their protective threshold should be instructed to inspect their feet daily for reddened areas, blisters, abrasions, or lacerations.
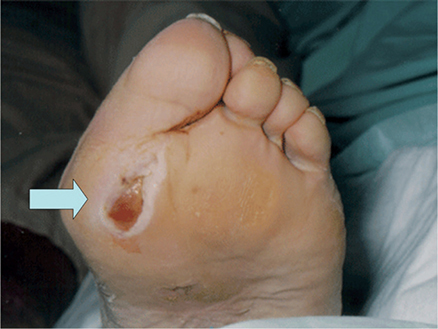
Figure 27–3. Diabetic foot ulcer over head of first metatarsal (arrow). (Used, with permission, from Dean SM, Satiani B, Abraham WT. Color Atlas and Synopsis of Vascular Diseases. McGraw-Hill, 2014.)
In some patients, hypersensitivity to light touch and occasionally severe “burning” pain, particularly at night, can become physically and emotionally disabling. Nortriptyline or desipramine in doses of 25–150 mg/day orally may provide dramatic relief for pain from diabetic neuropathy, often within 48–72 hours. This rapid response is in contrast to the 2 or 3 weeks required for an antidepressive effect. Patients often attribute the benefit to having a full night’s sleep. Mild to moderate morning drowsiness is a side effect that generally improves with time or can be lessened by giving the medication several hours before bedtime. This medication should not be continued if improvement has not occurred after 5 days of therapy. Amitriptyline, 25–75 mg orally at bedtime, can also be used but has more anticholinergic effects. Tricyclic antidepressants, in combination with fluphenazine (3 mg daily in three divided doses) have been shown in two studies to be efficacious in painful neuropathy, with benefits unrelated to relief of depression. Gabapentin (900–1800 mg orally daily in three divided doses) has also been shown to be effective in the treatment of painful neuropathy and should be tried if the tricyclic medications prove ineffective. Pregabalin, a congener of gabapentin, has been shown in an 8-week study to be more effective than placebo in treating painful diabetic peripheral neuropathy. However, this medication was not compared with an active control. Also, because of its abuse potential, it has been categorized as a schedule V controlled substance. Duloxetine (60–120 mg), a serotonin and norepinephrine reuptake inhibitor, is approved for the treatment of painful diabetic neuropathy. Capsaicin, a topical irritant, is effective in reducing local nerve pain; it is dispensed as a cream (Zostrix 0.025%, Zostrix-HP 0.075%) to be rubbed into the skin over the painful region two to four times daily. Gloves should be used for application since hand contamination could result in discomfort if the cream comes in contact with eyes or sensitive areas such as the genitalia. Application of a 5% lidocaine patch over an area of maximal pain has been reported to be of benefit. It is approved for treatment of postherpetic neuralgia.
Diabetic neuropathic cachexia is a syndrome characterized by a symmetric peripheral neuropathy associated with profound weight loss (up to 60% of total body weight) and painful dysesthesias affecting the proximal lower limbs, the hands, or the lower trunk. Treatment is usually with insulin and analgesics. The prognosis is generally good, and patients typically recover their baseline weight with resolution of the painful sensory symptoms within 1 year.
b. Isolated peripheral neuropathy—Involvement of the distribution of only one nerve (“mononeuropathy”) or of several nerves (“mononeuropathy multiplex”) is characterized by sudden onset with subsequent recovery of all or most of the function. This neuropathology has been attributed to vascular ischemia or traumatic damage. Cranial and femoral nerves are commonly involved, and motor abnormalities predominate. The patient with cranial nerve involvement usually has diplopia and single third, fourth, or sixth nerve weakness on examination but the pupil is spared. A full recovery of function occurs in 6–12 weeks. Diabetic amyotrophy presents with onset of severe pain in the front of the thigh. Within a few days or weeks of the onset of pain, weakness and wasting of the quadriceps develops. As the weakness appears, the pain tends to improve. Management includes analgesia and improved diabetes control. The symptoms improve over 6–18 months.
2. Autonomic neuropathy—Neuropathy of the autonomic system occurs principally in patients with diabetes of long duration. It affects many diverse visceral functions including blood pressure and pulse, gastrointestinal activity, bladder function, and erectile dysfunction. Treatment is directed specifically at each abnormality.
Involvement of the gastrointestinal system may be manifested by nausea, vomiting, postprandial fullness, reflux or dysphagia, constipation or diarrhea (or both), and fecal incontinence. Gastroparesis should be considered in type 1 diabetic patients in whom unexpected fluctuations and variability in their blood glucose levels develops after meals. Metoclopramide has been of some help in treating diabetic gastroparesis. It is given in a dose of 10 mg orally three or four times a day, 30 minutes before meals and at bedtime. Drowsiness, restlessness, fatigue, and lassitude are common adverse effects. Tardive dyskinesia and extrapyramidal effects can occur, especially when used for longer than 3 months, and the FDA has cautioned against the long-term use of metoclopramide.
Erythromycin appears to bind to motilin receptors in the stomach and has been found to improve gastric emptying over the short term in doses of 250 mg three times daily, but its effectiveness seems to diminish over time. In selected patients, injections of botulinum toxin into the pylorus can reduce pylorus sphincter resistance and enhance gastric emptying. Gastric electrical stimulation has been reported to improve symptoms and quality of life indices in patients with gastroparesis refractory to pharmacologic therapy.
Diarrhea associated with autonomic neuropathy has occasionally responded to broad-spectrum antibiotic therapy (such as rifaximin, metronidazole, amoxicillin/clavulanate, ciprofloxacin, or doxycycline), although it often undergoes spontaneous remission. Refractory diabetic diarrhea is often associated with impaired sphincter control and fecal incontinence. Therapy with loperamide, 4–8 mg daily, or diphenoxylate with atropine, two tablets up to four times a day, may provide relief. In more severe cases, tincture of paregoric or codeine (60-mg tablets) may be required to reduce the frequency of diarrhea and improve the consistency of the stools. Clonidine has been reported to lessen diabetic diarrhea; however, its usefulness is limited by its tendency to lower blood pressure in these patients who already have autonomic neuropathy, resulting in orthostatic hypotension. Constipation usually responds to stimulant laxatives such as senna.
Incomplete emptying of the bladder can sometimes occur. Bethanechol in doses of 10–50 mg orally three times a day has occasionally improved emptying of the atonic urinary bladder. Catheter decompression of the distended bladder has been reported to improve its function, and considerable benefit has been reported after surgical severing of the internal vesicle sphincter.
Use of Jobst fitted stockings, tilting the head of the bed, and arising slowly from the supine position can be helpful in treating symptoms of orthostatic hypotension. When such measures are inadequate, then treatment with fludrocortisone 0.1–0.2 mg orally daily can be considered. This medication, however, can result in supine hypertension and hypokalemia. Midodrine (10 mg orally three times a day), an alpha-agonist, can also be used.
Insulin neuritis or treatment-induced neuropathy of diabetes occurs occasionally in patients with poor glucose control and whose glucose levels improve rapidly in days or a few weeks. Symptoms include severe sensory neuropathic pains and sometimes autonomic functions. These symptoms improve over a few months.
Erectile dysfunction can result from neurologic, psychological, or vascular causes, or a combination of these causes. The phosphodiesterase type 5 (PDE5) inhibitors sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis) have been shown in placebo-controlled clinical trials to improve erections in response to sexual stimulation. The recommended dose of sildenafil for most patients is one 50-mg tablet taken approximately 1 hour before sexual activity. The peak effect is at 1.5–2 hours, with some effect persisting for 4 hours. Patients with diabetes mellitus using sildenafil reported 50–60% improvement in erectile function. The maximum recommended dose is 100 mg. The recommended dose of both vardenafil and tadalafil is 10 mg. The doses may be increased to 20 mg or decreased to 5 mg based on efficacy and side effects. Tadalafil has been shown to improve erectile function for up to 36 hours after dosing. Low doses are available for daily use. In clinical trials, only a few adverse effects have been reported—transient mild headache, flushing, dyspepsia, and some altered color vision. Priapism can occur with these medications, and patients should be advised to seek immediate medical attention if an erection persists for longer than 4 hours. The PDE5 inhibitors potentiate the hypotensive effects of nitrates and their use is contraindicated in patients who are concurrently using organic nitrates in any form. Caution is advised for men who have suffered a heart attack, stroke, or life-threatening arrhythmia within the previous 6 months; men who have resting hypotension or hypertension; and men who have a history of heart failure or have unstable angina. Rarely, a decrease in vision or permanent visual loss has been reported after PDE5 inhibitor use.
Intracorporeal injection of vasoactive medications causes penile engorgement and erection. Medications most commonly used include papaverine alone, papaverine with phentolamine, and alprostadil (prostaglandin E1). Alprostadil injections are relatively painless, but careful instruction is essential to prevent local trauma, priapism, and fibrosis. Intraurethral pellets of alprostadil avoid the problem of injection of the medication.
External vacuum therapy (Erec-Aid System) is a nonsurgical treatment consisting of a suction chamber operated by a hand pump that creates a vacuum around the penis. This draws blood into the penis to produce an erection that is maintained by a specially designed tension ring inserted around the base of the penis and which can be kept in place for up to 20–30 minutes. While this method is generally effective, its cumbersome nature limits its appeal.
Surgical implants of penile prostheses remain an option for those patients in whom the nonsurgical approaches are ineffective.
D. Cardiovascular Complications
1. Heart disease—Microangiopathy occurs in the heart of patients with diabetes and may explain the etiology of congestive cardiomyopathies in those who do not have demonstrable coronary artery disease. More commonly, however, heart disease in patients with diabetes is due to coronary atherosclerosis. Myocardial infarction is three to five times more common in diabetic patients and is the leading cause of death in patients with type 2 diabetes. Cardiovascular disease risk is increased in patients with type 1 diabetes as well, although the absolute risk is lower than in patients with type 2 diabetes. Premenopausal women who normally have lower rates of coronary artery disease lose this protection once diabetes develops. The increased risk in patients with type 2 diabetes reflects the combination of hyperglycemia, hyperlipidemia, abnormalities of platelet adhesiveness, coagulation factors, hypertension, oxidative stress, and inflammation. Large intervention studies of risk factor reduction in diabetes are lacking, but it is reasonable to assume that reducing these risk factors would have a beneficial effect. Lowering LDL cholesterol reduces first events in patients without known coronary disease and secondary events in patients with known coronary disease. These intervention studies included some patients with diabetes, and the benefits of LDL cholesterol lowering was apparent in this group. The National Cholesterol Education Program clinical practice guidelines have designated diabetes as a coronary risk equivalent and have recommended that patients with diabetes should have an LDL cholesterol goal of less than 100 mg/dL (2.6 mmol/L). Lowering LDL cholesterol to 70 mg/dL (1.8 mmol/L) may have additional benefit and is a reasonable target for most patients with type 2 diabetes who have multiple risk factors for cardiovascular disease.
Aspirin at a dose of 81–325 mg daily is effective in reducing cardiovascular morbidity and mortality in patients who have a history of myocardial infarction or stroke (secondary prevention). For primary prevention, a 2018 randomized study of 15,480 persons with diabetes but no evident cardiovascular disease observed that 100 mg of aspirin reduced the first vascular event of myocardial infarction, stroke or transient ischemic attack or death from vascular event (excluding intracranial hemorrhage) (rate ratio 0.88; 95% confidence interval 0.79 to 0.97). There were, however, more major bleeding events, especially gastrointestinal, in the aspirin group (rate ratio 1.29; 95% confidence interval 1.09 to 1.52). Thus, for primary prevention, the use of aspirin should only be considered for patients with high cardiovascular risk and low bleeding risk and generally not for adults older than 70 years. Based on the Early Treatment Diabetic Retinopathy Study (ETDRS), there does not appear to be a contraindication to aspirin use to achieve cardiovascular benefit in diabetic patients who have proliferative retinopathy. Aspirin also does not seem to affect the severity of vitreous/preretinal hemorrhages or their resolution.
2. Hypertension—The ADA also recommends lowering systolic blood pressure to less than 140 mm Hg and diastolic pressure to less than 90 mm Hg in patients with diabetes. The systolic target of 130 mm Hg or less and diastolic target of 80 mm Hg or less are recommended for the younger patient if they can be achieved without undue treatment burden. The Systolic Blood Pressure Intervention Trial (SPRINT) reported that treating to a systolic blood pressure of less than 120 mm Hg reduced cardiovascular events by 25% and death from cardiovascular causes by 43% during 3.26 years of follow-up. People with diabetes, however, were excluded from this study, and it is unclear if the results are applicable to this population. Patients with type 2 diabetes who already have cardiovascular disease or microalbuminuria should be considered for treatment with an ACE inhibitor. More clinical studies are needed to address the question of whether patients with type 2 diabetes who do not have cardiovascular disease or microalbuminuria would specifically benefit from ACE inhibitor treatment.
3. Peripheral vascular disease—Atherosclerosis is markedly accelerated in the larger arteries. It is often diffuse, with localized enhancement in certain areas of turbulent blood flow, such as at the bifurcation of the aorta or other large vessels. Clinical manifestations of peripheral vascular disease include ischemia of the lower extremities, erectile dysfunction, and intestinal angina.
The incidence of gangrene of the feet in patients with diabetes is 30 times that in age-matched controls. The factors responsible for its development, in addition to peripheral vascular disease, are small vessel disease, peripheral neuropathy with loss of both pain sensation and neurogenic inflammatory responses, and secondary infection. In two-thirds of patients with ischemic gangrene, pedal pulses are not palpable. In the remaining one-third who have palpable pulses, reduced blood flow through these vessels can be demonstrated by plethysmographic or Doppler ultrasound examination. Prevention of foot injury is imperative. Agents that reduce peripheral blood flow such as tobacco should be avoided. Control of other risk factors such as hypertension is essential. Beta-blockers are relatively contraindicated because of presumed negative peripheral hemodynamic consequences but data that support this are lacking. Cholesterol-lowering agents are useful as adjunctive therapy when early ischemic signs are detected and when dyslipidemia is present. Patients should be advised to seek immediate medical care if a diabetic foot ulcer develops. Improvement in peripheral blood flow with endarterectomy and bypass operations is possible in certain patients.
E. Skin and Mucous Membrane Complications
Chronic pyogenic infections of the skin may occur, especially in poorly controlled diabetic patients. Candidal infection can produce erythema and edema of intertriginous areas below the breasts, in the axillas, and between the fingers. It causes vulvovaginitis in women with chronically uncontrolled diabetes who have persistent glucosuria and is a frequent cause of pruritus. While antifungal creams containing miconazole or clotrimazole offer immediate relief of vulvovaginitis, recurrence is frequent unless glucosuria is reduced.
In some patients with type 2 diabetes, poor glycemic control can cause a severe hypertriglycemia, which can present as eruptive cutaneous xanthomas and pancreatitis. The skin lesions appear as yellow morbilliform eruptions 2–5 mm in diameter with erythematous areolae. They occur on extensor surfaces (elbows, knees, buttocks) and disappear after triglyceride levels are reduced.
Necrobiosis lipoidica diabeticorum is usually located over the anterior surfaces of the legs or the dorsal surfaces of the ankles. They are oval or irregularly shaped plaques with demarcated borders and a glistening yellow surface and occur in women two to four times more frequently than in men. Pathologically, the lesions show degeneration of collagen, granulomatous inflammation of subcutaneous tissues and blood vessels, capillary basement membrane thickening and obliteration of vessel lumina. The condition is associated with type 1 diabetes, although it can occur in patients with type 2 diabetes, and also in patients without diabetes. First-line therapy includes topical and subcutaneous corticosteroids. Improving glycemic control may help the condition.
“Shin spots” are not uncommon in adults with diabetes. They are brownish, rounded, painless atrophic lesions of the skin in the pretibial area.
F. Bone and Joint Complications
Long-standing diabetes can cause progressive stiffness of the hand secondary to contracture and tightening of skin over the joints (diabetic cheiroarthropathy), frozen shoulder (adhesive capsulitis), carpal tunnel syndrome, and Dupuytren contractures. These complications are believed to be due to glycosylation of collagen and perhaps other proteins in connective tissue. There may also be an inflammatory component.
Data on bone mineral density and fracture risk in people with diabetes are contradictory. Patients with type 2 diabetes do appear to be at increased risk for nonvertebral fractures. Women with type 1 diabetes have an increased risk of fracture when compared with women without diabetes. Other factors, such as duration of diabetes, and diabetes complications, such as neuropathy and kidney disease, likely affect both the bone mineral density and fracture risk.
Diffuse idiopathic skeletal hyperostosis (DISH) is characterized by ossification of the anterior longitudinal ligaments of the spine and various extraspinal ligaments. It causes stiffness and decreased range of spinal motion. The peripheral joints most commonly affected are the metacarpophalangeal joints, elbows, and shoulders. Diabetes, obesity, hypertension, and dyslipidemia are risk factors for this condition.
Hyperuricemia and acute and tophaceous gout are more common in type 2 diabetes.
Bursitis, particularly of the shoulders and hips, occurs more frequently than expected in patients with diabetes.
ASCEND Study Collaborative Group et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018 Oct 8;379(16):1529–39. [PMID: 30146931]
Grennan D. Diabetic foot ulcers. JAMA. 2019 Jan 1;321(1):114. [PMID: 30620372]
Hinchliffe RJ et al; International Working Group on the Diabetic Foot (IWGDF). Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020 Mar;36 Suppl 1:e3276. [PMID:31958217]
Salutini E et al. The complexity of diabetic foot management: from common care to best practice. The Italian Expert Opinion by Delphi Survey. Int J Low Extrem Wounds. 2019 Dec 15:1534734619890814. [PMID: 31838925]
Selvarajah D et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019 Dec;7(12):938–48. [PMID: 31624024]
Shen JI et al. Evidence for and against ACC/AHA 2017 guideline for target systolic blood pressure of < 130 mm Hg in persons with type 2 diabetes. Curr Cardiol Rep. 2019 Nov 23;21(11):149. [PMID: 31760494]
 Special Situations
Special Situations
A. Diabetes Management in the Hospital
Most patients with diabetes are hospitalized for reasons other than their diabetes. Indeed, up to 10–15% of all hospitalized patients have diabetes. It is challenging using outpatient oral therapies or insulin regimens in the hospital because patients are not eating as usual and they are often fasting for procedures. Clinical events increase adverse reactions associated with diabetes medicines, eg, thiazolidinediones can cause fluid retention and worsen heart failure; metformin should not be used in patients with significant chronic kidney or liver disease or those getting contrast for radiographic studies; and SGLT2 inhibitors may be associated with increased risk of diabetic ketoacidosis. Subcutaneous or intravenous insulin therapy is frequently substituted for other diabetes medicines because the insulin dose can be adjusted to match changing inpatient needs, and it is safe to use insulin in patients with heart, kidney, and liver disease. The data on the use of continuous glucose monitors, insulin pumps, and hybrid closed loop systems in hospitalized patients are insufficient. Whether patients stay on these systems in the hospital will depend on their severity of illness and access to specialist care. In general, decisions regarding insulin dosing should be made based on capillary blood glucose measurements and not on the data from continuous glucose monitors. For selected patients, it may be preferable to have them stay on their hybrid closed loop system. Patients should be transitioned to a conventional basal bolus subcutaneous insulin regimen if they are unable to manage their pump and/or continuous glucose monitor because of their illness or if they refuse to follow the institutional guidelines on using the pump or continuous monitor (eg, giving themselves insulin boluses and not informing the clinical staff). The systems have to be removed if the patient is getting an MRI.
On the general medical and surgical inpatient services, most patients are treated with subcutaneous insulin regimens. Limited cross-sectional and prospective studies suggest that the best glucose control is achieved on a combination of basal and bolus regimen with 50% of daily insulin needs provided by intermediate- or long-acting insulins. Standardized order sets can reduce errors, and they often include algorithms for recognition and treatment of hypoglycemia (see http://ucsfinpatientdiabetes.pbworks.com for examples). Oral medicines, especially metformin and sulfonylureas, can be resumed as the patient is being prepared for hospital discharge.
In the intensive care units (ICUs), glucose levels are controlled most frequently using insulin infusions. Patients receiving total parenteral nutrition can have insulin added to the bag. Standard total parenteral nutrition contains 25% dextrose so an infusion rate of 50 mL/h delivers 12.5 g of dextrose per hour.
Based on the evidence available, ICU patients with diabetes and new-onset hyperglycemia with blood glucose levels above 180 mg/dL (10 mmol/L) should be treated with insulin, aiming for target glucose levels between 140 mg/dL (7.8 mmol/L) and 180 mg/dL (10 mmol/L). In the ICU setting, aiming for blood glucose levels close to 100 mg/dL (5.6 mmol/L) is not beneficial and may even be harmful. When patients leave the ICU, target glucose values between 100 mg/dL (5.6 mmol/L) and 180 mg/dL (10 mmol/L) may be appropriate, although this view is based on clinical observations rather than conclusive evidence.
See Chapter 3 for a discussion of preoperative and perioperative diabetic management.
The morbidity and mortality in diabetic patients are twice those of nondiabetic patients. Those with new-onset hyperglycemia (ie, those without a preadmission diagnosis of diabetes) have even higher mortality—almost eightfold that of nondiabetic patients in one study. These observations have led to the question of whether tight glycemic control in the hospital improves outcomes.
Kansagara D et al. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011 Feb 15;154(4):268–82. [PMID: 21320942]
B. Pregnancy and the Diabetic Patient
See Chapter 19. Tight glycemic control with normal HbA1c levels is very important during pregnancy. Early in pregnancy, poor control increases the risk of spontaneous abortion and congenital malformations. Late in pregnancy, poor control can result in polyhydramnios, preterm labor, stillbirth, and fetal macrosomia with its associated problems. Diabetes complications can impact both maternal and fetal health. Diabetic retinopathy can first develop during pregnancy or retinopathy that is already present can worsen. Diabetic women with microalbuminuria can have worsening albuminuria during pregnancy and are at higher risk for preeclampsia. Low-dose (81 mg) aspirin can reduce the risk of preeclampsia and should be prescribed after 12 weeks of gestation. Patients who have preexisting kidney failure (prepregnancy creatinine clearance less than 80 mL/min) are at high risk for further decline in kidney function during the pregnancy, and this may not reverse after delivery. Diabetic gastroparesis can severely exacerbate the nausea and vomiting of pregnancy and some patients may require fluid and nutritional support.
Although there is evidence that glyburide is safe during pregnancy, the current practice is to control diabetes with insulin therapy. Every effort should be made, utilizing multiple injections of insulin or a continuous infusion of insulin by pump, to maintain near-normalization of fasting and preprandial blood glucose values while avoiding hypoglycemia.
Regular and NPH insulin and the insulin analogs lispro, aspart, and detemir are labeled pregnancy category B. Insulin glargine, glulisine, and degludec are labeled category C because of lack of clinical safety data. A small study using insulin glargine in 32 pregnancies did not reveal any problems.
Unless there are fetal or maternal complications, diabetic women should be able to carry the pregnancy to full-term, delivering at 38 to 41 weeks. Induction of labor before 39 weeks may be considered if there is concern about increasing fetal weight. See Chapter 19 for further details.
American Diabetes Association. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020 Jan;43(Suppl 1):S183–92. PMID: 31862757
 Prognosis
Prognosis
The DCCT showed that the previously poor prognosis for as many as 40% of patients with type 1 diabetes is markedly improved by optimal care; there is evidence that survival of people with type 1 diabetes relative to the general population has improved over the recent years. Renal and cardiovascular disease are the main contributors to the excess mortality.
For type 2 diabetes, the UKPDS documented a reduction in microvascular disease with glycemic control, although this was not apparent in the obese subgroup. Cardiovascular outcomes were not improved by glycemic control, although antihypertensive therapy showed benefit in reducing the number of adverse cardiovascular complications as well as in reducing the occurrence of microvascular disease among hypertensive patients.
In addition to poorly understood genetic factors relating to differences in individual susceptibility to development of long-term complications of hyperglycemia, it is clear that in both types of diabetes, the diabetic patient’s awareness of the potential complications of the disease and motivation contribute significantly to the ultimate outcome.
 When to Refer
When to Refer
• All patients should receive self-management education when diabetes is diagnosed and at intervals thereafter. The instructional team should include a registered dietitian and registered nurse; they must be Certified Diabetes Educators (CDEs).
• Patients with type 1 diabetes should be comanaged by an endocrinologist and a primary care provider.
• Patients with type 2 diabetes should be referred to an endocrinologist if treatment goals are not met or if the patient requires an increasingly complex regimen to maintain glycemic control.
• Patients with type 2 diabetes should be referred to an ophthalmologist or optometrist for a dilated eye examination when the diabetes is diagnosed, and patients with type 1 diabetes should be referred 5 years after the diagnosis is made.
• Patients with peripheral neuropathy, especially those with loss of protective threshold (unable to detect 5.07 Semmes-Weinstein filament) or structural foot problems, should be referred to a podiatrist.
• Referrals to other specialists may be required for management of chronic complications of diabetes.
Academy of Nutrition and Dietetics. https://www.eatright.org
American Association of Diabetes Educators. https://www.diabeteseducator.org
American Diabetes Association. https://www.diabetes.org
American Diabetes Association. Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015 Jan;38(Suppl 1):S11–90. [PMID: 25537706]
Juvenile Diabetes Research Foundation. https://www.jdrf.org
DIABETIC COMA
Coma may be due to a variety of causes not directly related to diabetes. Certain causes directly related to diabetes require differentiation: (1) Hypoglycemic coma resulting from excessive doses of insulin or oral hypoglycemic agents. (2) Hyperglycemic coma associated with either severe insulin deficiency (DKA) or mild to moderate insulin deficiency (hyperglycemic hyperosmolar state). (3) Lactic acidosis associated with diabetes, particularly in patients with diabetes who have severe infections or with cardiovascular collapse.
DIABETIC KETOACIDOSIS
ESSENTIALS OF DIAGNOSIS

 Hyperglycemia greater than 250 mg/dL (13.9 mmol/L).
Hyperglycemia greater than 250 mg/dL (13.9 mmol/L).
 Metabolic acidosis with blood pH < 7.3; serum bicarbonate less than 15 mEq/L.
Metabolic acidosis with blood pH < 7.3; serum bicarbonate less than 15 mEq/L.
 Serum positive for ketones.
Serum positive for ketones.
 General Considerations
General Considerations
Diabetic ketoacidosis (DKA) is a disorder primarily in patients with type 1 diabetes but can occur in patients with type 2 diabetes who have severe illness. DKA may be the initial manifestation of type 1 diabetes or may result from increased insulin requirements in type 1 diabetes patients during the course of infection, trauma, myocardial infarction, or surgery. It is a life-threatening medical emergency with a mortality rate just under 5% in individuals under 40 years of age, but with a more serious prognosis in older adults, who have mortality rates over 20%. The National Data Group reports an annual incidence of five to eight episodes of DKA per 1000 diabetic persons. Ketoacidosis may develop in patients with type 2 diabetes when severe stress such as sepsis or trauma is present. DKA is one of the more common serious complications of insulin pump therapy, occurring in approximately 1 per 80 patient-months of treatment. Many patients who monitor capillary blood glucose regularly ignore urine ketone measurements, which signals the possibility of insulin leakage or pump failure before serious illness develops. Poor compliance, either for psychological reasons or because of inadequate education, is one of the most common causes of recurrent DKA.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The appearance of DKA is usually preceded by a day or more of polyuria and polydipsia associated with marked fatigue, nausea, and vomiting. If untreated, mental stupor ensues that can progress to coma. Drowsiness is fairly common, but frank coma only occurs in about 10% of patients. On physical examination, evidence of dehydration in a stuporous patient with rapid deep breathing and a “fruity” breath odor of acetone strongly suggests the diagnosis. Hypotension with tachycardia indicates profound fluid and electrolyte depletion, and mild hypothermia is usually present. Abdominal pain and even tenderness may be present in the absence of abdominal disease. Conversely, cholecystitis or pancreatitis may occur with minimal symptoms and signs.
B. Laboratory Findings
Typically, the patient with moderately severe DKA has a plasma glucose of 350–900 mg/dL (19.4–50 mmol/L), serum ketones at a dilution of 1:8 or greater or beta-hydroxybutyrate more than 4 nmol/L, hyperkalemia (serum potassium level of 5–8 mEq/L), slight hyponatremia (serum sodium of approximately 130 mEq/L), hyperphosphatemia (serum phosphate level of 6–7 mg/dL [1.9–2.3 mmol/L]), and elevated blood urea nitrogen and serum creatinine levels (Table 27–10). Acidosis may be severe (pH ranging from 6.9 to 7.2 and serum bicarbonate ranging from 5 mEq/L to 15 mEq/L); PCO2 is low (15–20 mm Hg) related to compensatory hyperventilation. Fluid depletion is marked, typically about 100 mL/kg. In euglycemic ketoacidosis, the patient can have severe acidosis and fluid depletion but the plasma glucose levels are only modestly elevated, usually less than 250 mg/day (13.9 mmol/L). This condition is seen in patients in whom diabetic ketoacidosis develops while receiving treatment with SGLT2 inhibitors. Ketoacidosis with lower glucose levels also occur in pregnancy and may reflect the expanded plasma volume and the increased glomerular filtration rate.
Table 27–10. Laboratory diagnosis of coma in diabetic patients.
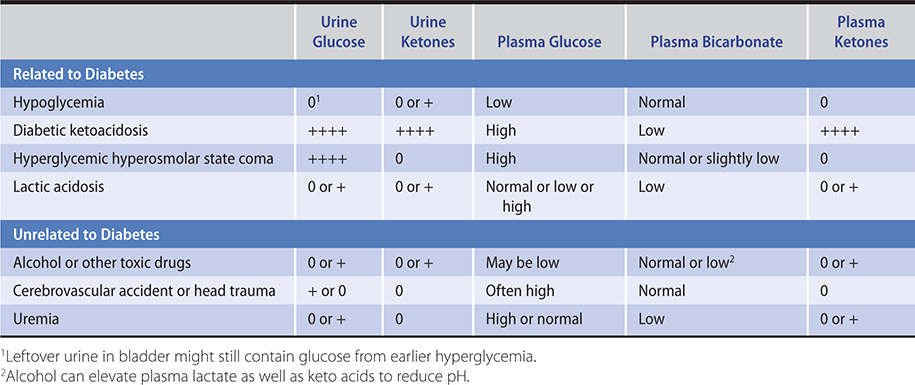
The difference between venous and arterial pH is 0.02 to 0.15 pH units and venous and arterial bicarbonate is 1.88 mEq/L. These small differences will not affect either the diagnosis or the management of DKA, and there is no need to collect arterial blood for measuring the acid-base status.
The hyperkalemia occurs despite total body potassium depletion because of the shift of potassium from the intracellular to extracellular spaces that occurs in systemic acidosis. The average total body potassium deficit resulting from osmotic diuresis, acidosis, and gastrointestinal losses is about 3–5 mEq/kg. Similarly, despite the elevated serum phosphate, total body phosphate is generally depleted. Serum sodium is generally reduced due to loss of sodium ions (7–10 mEq/kg) by polyuria and vomiting and because severe hyperglycemia shifts intracellular water into the interstitial compartment. For every 100 mg/dL of plasma glucose, serum sodium decreases by 1.6 mEq/L (5.56 mmol/L). The decrease in serum sodium may be greater when patients have more severe hyperglycemia (greater than 400 mg/dL, 22.2 mmol/L) and a correction factor of 2.4 mEq/L may be used. Hypertriglyceridemia should be considered if the corrected sodium is very low. Serum osmolality can be directly measured by standard tests of freezing point depression or can be estimated by calculating the molarity of sodium, chloride, and glucose in the serum. A convenient method of estimating effective serum osmolality is as follows (normal values in humans are 280–300 mOsm/kg):

These calculated estimates are usually 10–20 mOsm/kg lower than values measured by standard cryoscopic techniques. Central nervous system depression or coma occurs when the effective serum osmolality exceeds 320–330 mOsm/L. Coma in a diabetic patient with a lower osmolality should prompt a search for the cause of coma other than hyperosmolality (see Table 27–10 and Chapter 24).
Ketoacidemia represents the effect of insulin lack at multiple enzyme loci. Insulin lack associated with elevated levels of growth hormone, catecholamines, and glucagon contributes to increases in lipolysis from adipose tissue and in hepatic ketogenesis. In addition, reduced ketolysis by insulin-deficient peripheral tissues contributes to the ketoacidemia. The only true “keto” acid present is acetoacetic acid which, along with its by-product acetone, is measured by nitroprusside reagents (Acetest and Ketostix). The sensitivity for acetone, however, is poor, requiring over 10 mmol/L, which is seldom reached in the plasma of ketoacidotic patients—although this detectable concentration is readily achieved in urine. Thus, in the plasma of ketotic patients, only acetoacetate is measured by these reagents. The more prevalent beta-hydroxybutyric acid has no ketone group and is therefore not detected by conventional nitroprusside tests. This takes on special importance in the presence of circulatory collapse during DKA, wherein an increase in lactic acid can shift the redox state to increase beta-hydroxybutyric acid at the expense of the readily detectable acetoacetic acid. Bedside diagnostic reagents are then unreliable, suggesting no ketonemia in cases where beta-hydroxybutyric acid is a major factor in producing the acidosis. Combined glucose and ketone meters (Precision Xtra, Nova Max Plus) that measure blood beta-hydroxybutyrate concentration on capillary blood are available. Many clinical laboratories also offer direct blood beta-hydroxybutyrate measurement.
Nonspecific elevations of serum amylase and lipase occurs in about 16–25% of cases of DKA, and an imaging study may be necessary if the diagnosis of acute pancreatitis is being seriously considered. Leukocytosis as high as 25,000/mcL with a left shift may occur with or without associated infection. The presence of an elevated or even a normal temperature can suggest the presence of an infection, since patients with DKA are generally hypothermic if uninfected.
 Treatment
Treatment
Patients with mild DKA are alert and have pH levels between 7.25 and 7.30 and beta-hydroxybutyrate levels of 3–4 mmol/L; those with moderate ketoacidosis are either alert or a little drowsy and have pH levels between 7.0 and 7.24 and beta-hydroxybutyrate levels of 4–8 mmol/L; and those with severe ketoacidosis are stuporose and have a pH < 7.0 and beta-hydroxybutyrate levels of greater than 8 mmol/L. Those with mild ketoacidosis can be treated in the emergency department, but those with moderate or severe ketoacidosis require admission to the ICU or step-down unit. Therapeutic goals are to restore plasma volume and tissue perfusion, reduce blood glucose and osmolality toward normal, correct acidosis, replenish electrolyte losses, and identify and treat precipitating factors. Gastric intubation is recommended in the comatose patient to prevent vomiting and aspiration that may occur as a result of gastric atony, a common complication of DKA. An indwelling urinary catheter may also be necessary. In patients with preexisting heart or kidney failure or those in severe cardiovascular collapse, a central venous pressure catheter should be inserted to evaluate the degree of hypovolemia and to monitor subsequent fluid administration.
A comprehensive flow sheet that includes vital signs, serial laboratory data, and therapeutic interventions (eg, fluids, insulin) should be meticulously maintained by the clinician responsible for the patient’s care. Plasma glucose should be recorded hourly and electrolytes and pH at least every 2–3 hours during the initial treatment period. Bedside glucose meters should be used to titrate the insulin therapy. The patient should not receive sedatives or opioids in order to avoid masking signs and symptoms of impeding cerebral edema.
A. Fluid Replacement
In most patients, the fluid deficit is 4–5 L. Initially, 0.9% saline solution is the solution of choice to help reexpand the contracted vascular volume and should be started in the emergency department as soon as the diagnosis is established. The saline should be infused rapidly to provide 1 L/h over the first 1–2 hours. After the first 2 L of fluid have been given, the intravenous infusion should be at the rate of 300–400 mL/h. Use 0.9% (“normal”) saline unless the serum sodium is greater than 150 mEq/L, when 0.45% (“half normal”) saline solution should be used. The volume status should be very carefully monitored clinically. Failure to give enough volume replacement (at least 3–4 L in 8 hours) to restore normal perfusion is one of the most serious therapeutic shortcomings adversely influencing satisfactory recovery. Excessive fluid replacement (more than 5 L in 8 hours) may contribute to acute respiratory distress syndrome or cerebral edema. When blood glucose falls to approximately 250 mg/dL (13.9 mmol/L), the fluids should be changed to a 5% glucose-containing solution to maintain serum glucose in the range of 250–300 mg/dL (13.9–16.7 mmol/L). This will prevent the development of hypoglycemia and will also reduce the likelihood of cerebral edema, which could result from too rapid decline of blood glucose.
B. Insulin Replacement
Immediately after initiation of fluid replacement, regular insulin can be given intravenously in a loading dose of 0.1 unit/kg as a bolus to prime the tissue insulin receptors. Following the initial bolus, intravenous doses of insulin as low as 0.1 unit/kg/h are continuously infused or given hourly as an intramuscular injection; this is sufficient to replace the insulin deficit in most patients. A prospective randomized study showed that a bolus dose is not required if patients are given hourly insulin infusion at 0.14 unit/kg. Replacement of insulin deficiency helps correct the acidosis by reducing the flux of fatty acids to the liver, reducing ketone production by the liver, and also improving removal of ketones from the blood. Insulin treatment reduces the hyperosmolality by reducing the hyperglycemia. It accomplishes this by increasing removal of glucose through peripheral utilization as well as by decreasing production of glucose by the liver. This latter effect is accomplished by direct inhibition of gluconeogenesis and glycogenolysis as well as by lowered amino acid flux from muscle to liver and reduced hyperglucagonemia.
The insulin infusion should be “piggy-backed” into the fluid line so the rate of fluid replacement can be changed without altering the insulin delivery rate. If the plasma glucose level fails to fall at least 10% in the first hour, a repeat loading dose (0.1 or 0.14 unit/kg) is recommended. Rarely, a patient with immune insulin resistance is encountered, and this requires doubling the insulin dose every 2–4 hours if hyperglycemia does not improve after the first two doses of insulin. The insulin dose should be adjusted to lower the glucose concentration by about 50–70 mg/dL/h (2.8–3.9 mmol/L). If clinical circumstances prevent use of an insulin infusion, then the insulin can be given intramuscularly. An initial 0.15 unit/kg of regular insulin is given intravenously, and at the same time, the same size dose is given intramuscularly. Subsequently, regular insulin is given intramuscularly hourly at a dose of 0.1 unit/kg until the blood glucose falls to around 250 mg/dL, when the insulin can be given subcutaneously. Patients who normally take insulin glargine or insulin detemir can be given their usual maintenance doses during the initial treatment of their DKA. The continuation of their subcutaneous basal insulins means that lower doses of intravenous insulin will be needed, and there will be a smoother transition from intravenous insulin infusion to the subcutaneous regimen.
C. Potassium
Total body potassium loss from polyuria and vomiting may be as high as 200 mEq. However, because of shifts of potassium from cells into the extracellular space as a consequence of acidosis, serum potassium is usually normal to slightly elevated prior to institution of treatment. As the acidosis is corrected, potassium flows back into the cells, and hypokalemia can develop if potassium replacement is not instituted. If the patient is not uremic and has an adequate urinary output, potassium chloride in doses of 10–30 mEq/h should be infused during the second and third hours after beginning therapy as soon as the acidosis starts to resolve. Replacement should be started sooner if the initial serum potassium is inappropriately normal or low and should be delayed if serum potassium fails to respond to initial therapy and remains above 5 mEq/L, as in cases of chronic kidney disease. Occasionally, a patient may present with a serum potassium level less than 3.5 mEq/L, in which case insulin therapy should be delayed until the potassium level is corrected to greater than 3.5 mEq/L. An ECG can help monitor the patient’s potassium status: High peaked T waves are a sign of hyperkalemia, and flattened T waves with U waves are a sign of hypokalemia. Foods high in potassium content should be prescribed when the patient has recovered sufficiently to take food orally. Tomato juice has 14 mEq of potassium per 240 mL, and a medium-sized banana provides about 10 mEq.
D. Sodium Bicarbonate
The use of sodium bicarbonate in the management of DKA has been questioned since clinical benefit was not demonstrated in one prospective randomized trial and because of the following potentially harmful consequences: (1) development of hypokalemia from rapid shift of potassium into cells if the acidosis is overcorrected; (2) tissue anoxia from reduced dissociation of oxygen from hemoglobin when acidosis is rapidly reversed (leftward shift of the oxygen dissociation curve); and (3) cerebral acidosis resulting from lowering of cerebrospinal fluid pH. It must be emphasized, however, that these considerations are less important when very severe acidosis exists. Therefore, it is recommended that bicarbonate be administered in DKA if the arterial blood pH is 7.0 or less, with careful monitoring to prevent overcorrection. One or two ampules of sodium bicarbonate (one ampule contains 44 mEq/50 mL) should be added to 1 L of 0.45% saline with 20 mEq KCl or to 400 mL of sterile water with 20 mEq KCl and infused over 1 to 2 hours. (Note: Addition of sodium bicarbonate to 0.9% saline would produce a markedly hypertonic solution that could aggravate the hyperosmolar state already present.) It can be repeated until the arterial pH reaches 7.1, but it should not be given if the pH is 7.1 or greater since additional bicarbonate would increase the risk of rebound metabolic alkalosis as ketones are metabolized. Alkalosis shifts potassium from serum into cells, which could precipitate a fatal cardiac arrhythmia.
E. Phosphate
Phosphate replacement is seldom required in treating DKA. However, if severe hypophosphatemia of less than 1 mg/dL (0.32 mmol/L) develops during insulin therapy, a small amount of phosphate can be replaced per hour as the potassium salt. Three randomized studies, though, in which phosphate was replaced in patients with DKA did not show any apparent clinical benefit from phosphate administration. Moreover, attempts to use potassium phosphate as the sole means of replacing potassium have led to a number of reported cases of severe hypocalcemia with tetany. To minimize the risk of inducing tetany from too-rapid replacement of phosphate, the average deficit of 40–50 mmol of phosphate should be replaced intravenously at a rate no greater than 3–4 mmol/h in a 60- to 70-kg person. A stock solution (Abbott) provides a mixture of 1.12 g KH2PO4 and 1.18 g K2HPO4 in a 5-mL single-dose vial (this equals 22 mmol of potassium and 15 mmol of phosphate). One-half of this vial (2.5 mL) should be added to 1 L of either 0.45% saline or 5% dextrose in water. Two liters of this solution, infused at a rate of 400 mL/h, will correct the phosphate deficit at the optimal rate of 3 mmol/h while providing 4.4 mEq of potassium per hour. (Additional potassium should be administered as potassium chloride to provide a total of 10–30 mEq of potassium per hour, as noted above.) If the serum phosphate remains below 2.5 mg/dL (0.8 mmol/L) after this infusion, a repeat 5-hour infusion can be given.
F. Hyperchloremic Acidosis During Therapy
Because of the considerable loss of keto acids in the urine during the initial phase of therapy, substrate for subsequent regeneration of bicarbonate is lost and correction of the total bicarbonate deficit is hampered. A portion of the bicarbonate deficit is replaced with chloride ions infused in large amounts as saline to correct the dehydration. In most patients, as the ketoacidosis clears during insulin replacement, a hyperchloremic, low-bicarbonate pattern emerges with a normal anion gap. This is a relatively benign condition that reverses itself over the subsequent 12–24 hours once intravenous saline is no longer being administered. Using a balanced electrolyte solution with a pH of 7.4 and 98 mEq/L chloride such as Plasma-lyte instead of normal saline (pH ~5.5; chloride 154 mEq/L) has been reported to prevent the hyperchloremic acidosis.
G. Treatment of Associated Infection
Antibiotics are prescribed as indicated (Table 30–5). Cholecystitis and pyelonephritis may be particularly severe in these patients.
H. Transition to Subcutaneous Insulin Regimen
Once the DKA is controlled and the patient is awake and able to eat, subcutaneous insulin therapy can be initiated. The patient with type 1 diabetes may have persistent significant tissue insulin resistance and may require a total daily insulin dose of approximately 0.6 unit/kg. The amount of insulin required in the previous 8 hours can also be helpful in estimating the initial insulin doses. Half the total daily dose can be given as a long-acting basal insulin and the other half as short-acting insulin premeals. The patient should receive subcutaneous basal insulin and rapid-acting insulin analog with the first meal and the insulin infusion discontinued an hour later. The overlap of the subcutaneous insulin action and insulin infusion is necessary to prevent relapse of the DKA. In patients with preexisting diabetes, giving their basal insulin by subcutaneous injection at initiation of treatment simplifies the transition from intravenous to subcutaneous regimen. The increased insulin resistance is only present for a few days, and it is important to reduce both the basal and bolus insulins to avoid hypoglycemia. A patient with new-onset type 1 diabetes usually still has significant beta cell function and may not need any basal insulin and only very low doses of rapid-acting insulin before meals after recovery from the ketoacidosis. Patients with type 2 diabetes and DKA due to severe illness may initially require insulin therapy but can often transition back to oral agents during outpatient follow-up.
 Prognosis
Prognosis
Low-dose insulin infusion and fluid and electrolyte replacement combined with careful monitoring of patients’ clinical and laboratory responses to therapy have dramatically reduced the mortality rates of DKA to less than 5%. However, this complication remains a significant risk in the aged who have mortality rates greater than 20% and in patients in profound coma in whom treatment has been delayed. Acute myocardial infarction and infarction of the bowel following prolonged hypotension worsen the outlook. A serious prognostic sign is end-stage chronic kidney disease, and prior kidney dysfunction worsens the prognosis considerably because the kidney plays a key role in compensating for massive pH and electrolyte abnormalities. Symptomatic cerebral edema occurs primarily in the pediatric population. Risk factors for its development include severe baseline acidosis, rapid correction of hyperglycemia, and excess volume administration in the first 4 hours. Onset of headache or deterioration in mental status during treatment should lead to consideration of this complication. Intravenous mannitol at a dosage of 1–2 g/kg given over 15 minutes is the mainstay of treatment. Excess crystalloid infusion can precipitate pulmonary edema. Acute respiratory distress syndrome is a rare complication of treatment of DKA.
After recovery and stabilization, patients should be instructed on how to recognize the early symptoms and signs of ketoacidosis. Urine ketones or capillary blood beta-hydroxybutyrate should be measured in patients with signs of infection or in insulin pump-treated patients when capillary blood glucose remains unexpectedly and persistently high. When heavy ketonuria and glycosuria persist on several successive examinations, supplemental rapid-acting insulin should be administered and liquid foods such as lightly salted tomato juice and broth should be ingested to replenish fluids and electrolytes. The patient should be instructed to contact the clinician if ketonuria persists, and especially if there is vomiting and inability to keep down fluids. Recurrent episodes of severe ketoacidosis often indicate poor compliance with the insulin regimen, and these patients will require intensive counseling.
Fayfman M et al. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017 May;101(3):587–606. [PMID: 28372715]
Islam T et al. Guidelines and controversies in the management of diabetic ketoacidosis—a mini-review. World J Diabetes. 2018 Dec 15;9(12):226–29. [PMID: 30588284]
Karslioglu French E et al. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ. 2019 May 29;365:l114. [PMID: 31142480]
Modi A et al. Euglycemic diabetic letoacidosis: a review. Curr Diabetes Rev. 2017;13(3):315–21. [PMID: 27097605]
HYPERGLYCEMIC HYPEROSMOLAR STATE
ESSENTIALS OF DIAGNOSIS

 Hyperglycemia greater than 600 mg/dL (33.3 mmol/L).
Hyperglycemia greater than 600 mg/dL (33.3 mmol/L).
 Serum osmolality greater than 310 mOsm/kg.
Serum osmolality greater than 310 mOsm/kg.
 No acidosis; blood pH > 7.3.
No acidosis; blood pH > 7.3.
 Serum bicarbonate greater than 15 mEq/L.
Serum bicarbonate greater than 15 mEq/L.
 Normal anion gap (less than 14 mEq/L).
Normal anion gap (less than 14 mEq/L).
 General Considerations
General Considerations
This second most common form of hyperglycemic coma is characterized by severe hyperglycemia in the absence of significant ketosis, with hyperosmolality and dehydration. It occurs in patients with mild or occult diabetes, and most patients are typically middle-aged to elderly. Accurate figures are not available as to its true incidence, but from data on hospital discharges it is rarer than DKA even in older age groups. Underlying chronic kidney disease or heart failure is common, and the presence of either worsens the prognosis. A precipitating event such as infection, myocardial infarction, stroke, or recent operation is often present. Certain medications such as phenytoin, diazoxide, corticosteroids, and diuretics have been implicated in its pathogenesis, as have procedures associated with glucose loading such as peritoneal dialysis.
 Pathogenesis
Pathogenesis
A partial or relative insulin deficiency may initiate the syndrome by reducing glucose utilization of muscle, fat, and liver while inducing hyperglucagonemia and increasing hepatic glucose output. With massive glycosuria, obligatory water loss ensues. If a patient is unable to maintain adequate fluid intake because of an associated acute or chronic illness or has suffered excessive fluid loss, marked dehydration results. As the plasma volume contracts, kidney function becomes impaired, limiting the urinary glucose losses and exacerbating the hyperglycemia. Severe hyperosmolality develops that causes mental confusion and finally coma. It is not clear why ketosis is virtually absent under these conditions of insulin insufficiency, although reduced levels of growth hormone may be a factor, along with portal vein insulin concentrations sufficient to restrain ketogenesis.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Onset may be insidious over a period of days or weeks, with weakness, polyuria, and polydipsia. The lack of features of DKA may retard recognition of the syndrome and delay therapy until dehydration becomes more profound than in ketoacidosis. Reduced intake of fluid is not an uncommon historical feature, due to either inappropriate lack of thirst, nausea, or inaccessibility of fluids to elderly, bedridden patients. A history of ingestion of large quantities of glucose-containing fluids, such as soft drinks or orange juice, can occasionally be obtained. Lethargy and confusion develop as serum osmolality exceeds 310 mOsm/kg, and convulsions and coma can occur if osmolality exceeds 320–330 mOsm/kg. Physical examination confirms the presence of profound dehydration in a lethargic or comatose patient without Kussmaul respirations.
B. Laboratory Findings
Severe hyperglycemia is present, with blood glucose values ranging from 800 mg/dL to 2400 mg/dL (44.4 mmol/L to 133.2 mmol/L) (Table 27–10). In mild cases, where dehydration is less severe, dilutional hyponatremia as well as urinary sodium losses may reduce serum sodium to 120–125 mEq/L, which protects to some extent against extreme hyperosmolality. However, as dehydration progresses, serum sodium can exceed 140 mEq/L, producing serum osmolality readings of 330–440 mOsm/kg. Ketosis and acidosis are usually absent or mild. Prerenal azotemia is the rule, with serum urea nitrogen elevations over 100 mg/dL (35.7 mmol/L) being typical.
 Treatment
Treatment
A. Fluid Replacement
Fluid replacement is of paramount importance in treating nonketotic hyperglycemic coma. The onset of hyperosmolarity is more insidious in elderly people without ketosis than in younger individuals with high serum ketone levels, which provide earlier indicators of severe illness (vomiting, rapid deep breathing, acetone odor, etc). Consequently, diagnosis and treatment are often delayed until fluid deficit has reached levels of 6–10 L.
If hypovolemia is present as evidenced by hypotension and oliguria, fluid therapy should be initiated with 0.9% saline. In all other cases, 0.45% saline appears to be preferable as the initial replacement solution because the body fluids of these patients are markedly hyperosmolar. As much as 4–6 L of fluid may be required in the first 8–10 hours. Careful monitoring of the patient is required for proper sodium and water replacement. An important end point of fluid therapy is to restore urinary output to 50 mL/h or more. Once blood glucose reaches 250 mg/dL (13.9 mmol/L), fluid replacement should include 5% dextrose in either water, 0.45% saline solution, or 0.9% saline solution. The rate of dextrose infusion should be adjusted to maintain glycemic levels of 250–300 mg/dL (13.9–16.7 mmol/L) in order to reduce the risk of cerebral edema.
B. Insulin
Less insulin may be required to reduce the hyperglycemia in nonketotic patients as compared to those with diabetic ketoacidotic coma. In fact, fluid replacement alone can reduce hyperglycemia considerably by correcting the hypovolemia, which then increases both glomerular filtration and renal excretion of glucose. Insulin treatment should therefore be delayed unless the patient has significant ketonemia (beta-hydroxybutyrate more than 1 mmol/L). Start the insulin infusion rate at 0.05 unit/kg/h (bolus is not needed) and titrate to lower blood glucose levels by 50–70 mg/dL per hour (2.8–3.9 mmol/L/h). Once the patient has stabilized and the blood glucose falls to around 250 mg/dL (13.9 mmol/L), insulin can be given subcutaneously.
C. Potassium
With the absence of acidosis, there may be no initial hyperkalemia unless associated end-stage chronic kidney disease is present. This results in less severe total potassium depletion than in DKA, and less potassium replacement is therefore needed. However, because initial serum potassium is usually not elevated and because it declines rapidly as a result of insulin’s effect on driving potassium intracellularly, it is recommended that potassium replacement be initiated earlier than in ketotic patients, assuming that no chronic kidney disease or oliguria is present. Potassium chloride (10 mEq/L) can be added to the initial bottle of fluids administered if the patient’s serum potassium is not elevated.
D. Phosphate
If severe hypophosphatemia (serum phosphate less than 1 mg/dL [0.32 mmol/L]) develops during insulin therapy, phosphate replacement can be given as described for ketoacidotic patients (at 3 mmol/h).
 Prognosis
Prognosis
The severe dehydration and low output state may predispose the patient to complications such as myocardial infarction, stroke, pulmonary embolism, mesenteric vein thrombosis, and disseminated intravascular coagulation. Fluid replacement remains the primary approach to the prevention of these complications. Low-dose heparin prophylaxis is reasonable but benefits of routine anticoagulation remain doubtful. Rhabdomyolysis is a recognized complication and should be looked for and treated.
The overall mortality rate of hyperglycemic hyperosmolar state coma is more than ten times that of DKA, chiefly because of its higher incidence in older patients, who may have compromised cardiovascular systems or associated major illnesses and whose dehydration is often excessive because of delays in recognition and treatment. (When patients are matched for age, the prognoses of these two hyperglycemic emergencies are reasonably comparable.) When prompt therapy is instituted, the mortality rate can be reduced from nearly 50% to that related to the severity of coexistent disorders.
After the patient is stabilized, the appropriate form of long-term management of the diabetes must be determined. Insulin treatment should be continued for a few weeks but patients usually recover sufficient endogenous insulin secretion to make a trial of diet or diet plus oral agents worthwhile. When the episode occurs in a patient who has known diabetes, then education of the patient and caregivers should be instituted. They should be taught how to recognize situations (nausea and vomiting, infection) that predispose to recurrence of the hyperglycemic, hyperosmolar state, as well as detailed information on how to prevent the escalating dehydration that culminates in hyperosmolar coma (small sips of sugar-free liquids, increase in usual hypoglycemic therapy, or early contact with the clinician).
Fayfman M et al. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017 May;101(3):587–606. [PMID: 28372715]
Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS hyperosmolar hyperglycaemic guidelines group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med. 2015 Jun;32(6):714–24. [PMID: 25980647]
LACTIC ACIDOSIS
ESSENTIALS OF DIAGNOSIS

 Severe metabolic acidosis with compensatory hyperventilation.
Severe metabolic acidosis with compensatory hyperventilation.
 Blood pH < 7.30.
Blood pH < 7.30.
 Serum bicarbonate less than 15 mEq/L.
Serum bicarbonate less than 15 mEq/L.
 Anion gap greater than 15 mEq/L.
Anion gap greater than 15 mEq/L.
 Absent serum ketones.
Absent serum ketones.
 Serum lactate greater than 5 mmol/L.
Serum lactate greater than 5 mmol/L.
 General Considerations
General Considerations
Lactic acidosis is characterized by accumulation of excess lactic acid in the blood. Normally, the principal sources of this acid are the erythrocytes (which lack enzymes for aerobic oxidation), skeletal muscle, skin, and brain. Conversion of lactic acid to glucose and its oxidation principally by the liver but also by the kidneys represent the chief pathways for its removal. Hyperlactatemia and acidosis occur when lactate production exceeds lactate consumption. Causes include tissue hypoxia (global or local), disorders that increase epinephrine levels (severe asthma with excess beta-adrenergic agonist use, cardiogenic or hemorrhagic shock, pheochromocytoma), and drugs that impair oxidative phosphorylation (antiretroviral agents and propofol). Most cases of metformin-associated lactic acidosis occur in patients in whom there were contraindications to the use of metformin, in particular kidney failure. Metformin levels are usually greater than 5 mcg/L when metformin is implicated as the cause of lactic acidosis. Other causes of lactic acidosis include several inborn errors of metabolism and the MELAS syndrome (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes). D-lactic acidosis can occur in patients with short bowel syndrome when unabsorbed carbohydrates are presented as substrate for fermentation by colonic bacteria.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The main clinical feature of lactic acidosis is marked hyperventilation. When lactic acidosis is secondary to tissue hypoxia or vascular collapse, the clinical presentation is variable, being that of the prevailing catastrophic illness. However, in the idiopathic, or spontaneous, variety, the onset is rapid (usually over a few hours), blood pressure is normal, peripheral circulation is good, and there is no cyanosis.
B. Laboratory Findings
Plasma bicarbonate and blood pH are quite low, indicating the presence of severe metabolic acidosis. Ketones are usually absent from plasma and urine or at least not prominent. The first clue may be a high anion gap (serum sodium minus the sum of chloride and bicarbonate anions [in mEq/L] should be no greater than 15). A higher value indicates the existence of an abnormal compartment of anions. If this cannot be clinically explained by an excess of keto acids (diabetes), inorganic acids (uremia), or anions from medication overdosage (salicylates, methyl alcohol, ethylene glycol), then lactic acidosis is probably the correct diagnosis. (See also Chapter 21.) In the absence of azotemia, hyperphosphatemia may be a clue to the presence of lactic acidosis for reasons that are not clear. The diagnosis is confirmed by a plasma lactic acid concentration of 5 mmol/L or higher (values as high as 30 mmol/L have been reported). Normal plasma values average 1 mmol/L, with a normal lactate/pyruvate ratio of 10:1. This ratio is greatly exceeded in lactic acidosis.1
 Treatment
Treatment
Aggressive treatment of the precipitating cause of lactic acidosis is the main component of therapy, such as ensuring adequate oxygenation and vascular perfusion of tissues. Empiric antibiotic coverage for sepsis should be given after culture samples are obtained in any patient in whom the cause of the lactic acidosis is not apparent (Table 30–5).
Alkalinization with intravenous sodium bicarbonate to keep the pH above 7.2 has been recommended by some in the emergency treatment of lactic acidosis; as much as 2000 mEq in 24 hours has been used. However, there is no evidence that the mortality rate is favorably affected by administering bicarbonate, and its use remains controversial. Hemodialysis may be useful in cases where large sodium loads are poorly tolerated and in cases associated with metformin toxicity.
 Prognosis
Prognosis
The mortality rate of spontaneous lactic acidosis is high. The prognosis in most cases is that of the primary disorder that produced the lactic acidosis.
DeFronzo R et al. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016 Feb;65(2):20–9. [PMID: 26773926]
THE HYPOGLYCEMIC STATES
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin at plasma glucose levels in the range of 60 mg/dL (3.3 mmol/L) and impairment of brain function at approximately 50 mg/dL (2.8 mmol/L). Fasting hypoglycemia is often subacute or chronic and usually presents with neuroglycopenia as its principal manifestation; postprandial hypoglycemia is relatively acute and is often heralded by symptoms of neurogenic autonomic discharge (sweating, palpitations, anxiety, tremulousness).
 Differential Diagnosis (Table 27–11)
Differential Diagnosis (Table 27–11)
Table 27–11. Common causes of hypoglycemia in adults.1
Fasting hypoglycemia
Pancreatic B cell tumor
Surreptitious administration of insulin or sulfonylureas
Extrapancreatic tumors
Postprandial hypoglycemia
Gastric surgery
Occult diabetes mellitus
Alcohol-related hypoglycemia
Immunopathologic hypoglycemia
Idiopathic anti-insulin antibodies (which release their bound insulin)
Antibodies to insulin receptors (which act as agonists)
Drug-induced hypoglycemia
1In the absence of clinically obvious endocrine, kidney, or liver disorders and exclusive of diabetes mellitus treated with hypoglycemic agents.
Fasting hypoglycemia may occur in certain endocrine disorders, such as hypopituitarism, Addison disease, or myxedema; in disorders related to liver malfunction, such as acute alcoholism or liver failure; and in instances of end-stage chronic kidney disease, particularly in patients requiring dialysis. These conditions are usually obvious, with hypoglycemia being only a secondary feature. When fasting hypoglycemia is a primary manifestation developing in adults without apparent endocrine disorders or inborn metabolic diseases from childhood, the principal diagnostic possibilities include (1) hyperinsulinism, due to either pancreatic B cell tumors, iatrogenic or surreptitious administration of insulin or sulfonylurea; and (2) hypoglycemia due to extrapancreatic tumors.
Postprandial (reactive) hypoglycemia may be seen after gastrointestinal surgery and is particularly associated with the dumping syndrome after gastrectomy and Roux-en-Y gastric bypass surgery. Occult diabetes very occasionally presents with postprandial hypoglycemia. Rarely, it occurs with islet cell hyperplasia—the so-called noninsulinoma pancreatogenous hypoglycemia syndrome.
Alcohol-related hypoglycemia is due to hepatic glycogen depletion combined with alcohol-mediated inhibition of gluconeogenesis. It is most common in malnourished individuals with excessive alcohol intake but can occur in anyone who is unable to ingest food after an acute alcoholic episode followed by gastritis and vomiting.
Immunopathologic hypoglycemia is an extremely rare condition in which anti-insulin antibodies or antibodies to insulin receptors develop spontaneously.
HYPOGLYCEMIA DUE TO PANCREATIC B CELL TUMORS
ESSENTIALS OF DIAGNOSIS

 Hypoglycemic symptoms—often neuroglycopenic (confusion, blurred vision, anxiety, convulsions).
Hypoglycemic symptoms—often neuroglycopenic (confusion, blurred vision, anxiety, convulsions).
 Immediate recovery upon administration of glucose.
Immediate recovery upon administration of glucose.
 Blood glucose less than 45 mg/dL (2.5 mmol/L) with a serum insulin level of 6 microunits/mL or more.
Blood glucose less than 45 mg/dL (2.5 mmol/L) with a serum insulin level of 6 microunits/mL or more.
 General Considerations
General Considerations
Fasting hypoglycemia in an otherwise healthy, well-nourished adult is rare and is most commonly due to an adenoma of the islets of Langerhans. Ninety percent of such tumors are single and benign, but multiple adenomas can occur as well as malignant tumors with functional metastases. Adenomas may be familial, and multiple adenomas have been found in conjunction with tumors of the parathyroids and pituitary (MEN type 1 [MEN 1]). About 30% of sporadic insulinoma tumors have a somatic mutation in the YY1 gene (T372R) that encodes the transcriptional repressor YY1. Over 99% of insulinomas are located within the pancreas and less than 1% in ectopic pancreatic tissue.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The most important prerequisite to diagnosing an insulinoma is simply to consider it, particularly in relatively healthy-appearing persons who have fasting hypoglycemia associated with some degree of central nervous system dysfunction such as confusion or abnormal behavior. A delay in diagnosis can result in unnecessary treatment for psychomotor epilepsy or psychiatric disorders and may cause irreversible brain damage. In long-standing cases, obesity can result as a consequence of overeating to relieve symptoms.
The so-called Whipple triad is characteristic of hypoglycemia regardless of the cause. It consists of (1) a history of hypoglycemic symptoms, (2) an associated low plasma glucose level (40–50 mg/dL), and (3) relief of symptoms upon ingesting fast-acting carbohydrates in approximately 15 minutes. The hypoglycemic symptoms in insulinoma often develop in the early morning or after missing a meal. Occasionally, they occur after exercise.
Patients typically complain of neuroglycopenic symptoms such as blurred vision or diplopia, headache, feelings of detachment, slurred speech, and weakness. Personality and mental changes vary from anxiety to psychotic behavior, and neurologic deterioration can result in convulsions or coma. Hypoglycemic unawareness is very common and adrenergic symptoms of palpitations and sweating may be blunted. With the ready availability of home blood glucose–monitoring systems, patients sometimes present with documented fingerstick blood glucose levels in 40s and 50s at time of symptoms. Access to diabetic medications (sulfonylureas or insulin) should be explored—does a family member have diabetes, or does the patient or family member work in the medical field? Medication-dispensing errors should be excluded—has the patient’s prescription medication changed in shape or color? Patients with insulinoma or factitious hypoglycemia usually have a normal physical examination.
B. Laboratory Findings
B cell adenomas do not reduce secretion of insulin in the presence of hypoglycemia, and the critical diagnostic test is to demonstrate inappropriately elevated serum insulin, proinsulin, and C-peptide levels, at a time when plasma glucose level is below 45 mg/dL.
The diagnostic criteria for insulinoma after a 72-hour fast are listed in Table 27–12. Other causes of hyperinsulinemic hypoglycemia include factitious administration of insulin or sulfonylureas. Factitious use of insulin will result in suppression of endogenous insulin secretion and low C-peptide levels. In patients who have injected insulin, the insulin/C-peptide ratio (pmol/L) will be greater than 1. An elevated circulating proinsulin level in the presence of fasting hypoglycemia is characteristic of most B cell adenomas and does not occur in factitious hyperinsulinism. Thus, C-peptide levels (by immunochemiluminometric assays [ICMA]) of greater than 200 pmol/L and proinsulin levels (by radioimmunoassay [RIA]) of greater than 5 pmol/L are characteristic of insulinomas. In patients with insulinoma, plasma beta-hydroxybutyrate levels are suppressed to 2.7 mmol/L or less. No single hormone measurement (insulin, proinsulin, C-peptide) is 100% sensitive and specific for the diagnosis of insulinoma, and insulinoma cases have been reported with insulin levels below 3 microunits/mL (ICMA assay) or proinsulin level below 5 pmol/L. These hormonal assays are also not standardized, and there can be significant variation in the test results. Therefore, the diagnosis should be based on multiple biochemical parameters.
Table 27–12. Diagnostic criteria for insulinoma after a 72-hour fast.
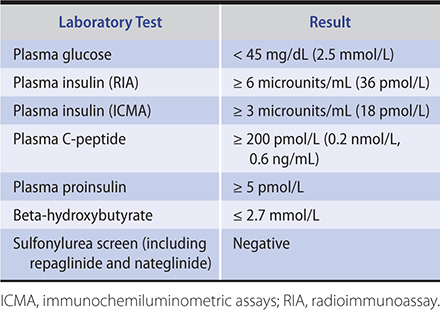
In patients with epigastric distress, a history of renal calculi, or menstrual or erectile dysfunction, a serum calcium, gastrin, or prolactin level may be useful in screening for MEN 1 associated with insulinoma.
C. Diagnostic Tests
If the history is consistent with episodic spontaneous hypoglycemia, patients should be given a home blood glucose monitor and advised to monitor blood glucose levels at the time of symptoms and before consumption of carbohydrates, if this can be done safely. Patients with insulinomas frequently report fingerstick blood glucose levels between 40 mg/dL (2.2 mmol/L) and 50 mg/dL (2.8 mmol/L) at the time of symptoms. The diagnosis, however, cannot be made based on a fingerstick blood glucose. It is necessary to have a low laboratory glucose concomitantly with elevated plasma insulin, proinsulin, and C-peptide levels and a negative sulfonylurea screen. When patients give a history of symptoms after only a short period of food withdrawal or with exercise, then an outpatient assessment can be attempted. The patient should be brought by a family member to the office after an overnight fast and observed in the office. Activity such as walking should be encouraged and fingerstick blood glucose measured repeatedly during observation. If symptoms occur or fingerstick blood glucose is below 50 mg/dL (2.8 mmol/L) then samples for plasma glucose, insulin, C-peptide, proinsulin, sulfonylurea screen, serum ketones, and antibodies to insulin should be sent. If outpatient observation does not result in symptoms or hypoglycemia and if the clinical suspicion remains high, then the patient should undergo an inpatient supervised 72-hour fast. A suggested protocol for the supervised fast is shown in Table 27–13.
Table 27–13. Suggested hospital protocol for supervised fast in diagnosis of insulinoma.
(1) Place intravenous cannula and obtain baseline plasma glucose, insulin, proinsulin, beta-hydroxybutyrate, and C-peptide measurements at onset of fast.
(2) Permit only calorie-free and caffeine-free fluids and encourage supervised activity (such as walking).
(3) Obtain fingerstick glucose measurements every 4 hours until values < 60 mg/dL are obtained. Then increase the frequency of fingersticks to each hour, and when capillary glucose value is < 45 mg/dL send a venous blood sample to the laboratory for plasma glucose.1 Check frequently for manifestations of neuroglycopenia.
(4) At 48 hours into the fast, send a venous blood sample for plasma glucose, insulin, proinsulin, C-peptide, beta-hydroxybutyrate, and sulfonylurea measurement.
(5) If symptoms of hypoglycemia occur or if a laboratory value of serum glucose is < 45 mg/dL, or if 72 hours have elapsed, conclude the fast with a final blood sample for plasma glucose,1 insulin, proinsulin, C-peptide, beta-hydroxybutyrate, and sulfonylurea measurements. Then give oral fast-acting carbohydrate followed by a meal. If the patient is confused or unable to take oral agents, administer 50 mL of 50% dextrose intravenously over 3–5 minutes. Do not conclude a fast based simply on a capillary blood glucose measurement—wait for the laboratory glucose value—unless the patient is very symptomatic and it would be dangerous to wait.
1Glucose sample should be collected in sodium fluoride containing tube on ice to prevent glycolysis and the plasma separated immediately upon receipt at the laboratory. Arrange for the laboratory to run the glucose samples “stat.”
In 30% of patients with insulinoma, the blood glucose levels often drop below 45 mg/dL (2.5 mmol/L) after an overnight fast, but some patients require up to 72 hours to develop symptomatic hypoglycemia. However, the term “72-hour fast” is actually a misnomer in most cases since the fast should be immediately terminated as soon as symptoms appear and laboratory confirmation of hypoglycemia is available. In normal male subjects, the blood glucose does not fall below 55–60 mg/dL (3.1–3.3 mmol/L) during a 3-day fast. In contrast, in normal premenopausal women the plasma glucose can reach values as low as 35 mg/dL (1.9 mmol/L). In these cases, however, the women are not symptomatic, presumably owing to the development of sufficient ketonemia to supply energy needs to the brain. Insulinoma patients, on the other hand, become symptomatic when plasma glucose drops to subnormal levels, since inappropriate insulin secretion restricts ketone formation. Moreover, the demonstration of a nonsuppressed insulin level of 3 microunits/mL or more using an ICMA assay (greater than 6 microunits/mL using an RIA assay) in the presence of hypoglycemia suggests the diagnosis of insulinoma. If hypoglycemia does not develop in a male patient after fasting for up to 72 hours—and particularly when this prolonged fast is terminated with a period of moderate exercise—insulinoma must be considered an unlikely diagnosis.
Stimulation tests with pancreatic B cell secretagogues such as tolbutamide, glucagon, or leucine have been devised to demonstrate exaggerated and prolonged insulin secretion in the presence of insulinomas. However, because insulin-secreting tumors have a wide range of granule content and degrees of differentiation, they are variably responsive to these secretagogues; and a negative response does not necessarily rule out an insulinoma. For these reasons, stimulation tests are not recommended in the diagnostic workup of insulinoma.
An oral glucose tolerance test is of no value in the diagnosis of insulin-secreting tumors. HbA1c levels may be low but there is considerable overlap with normal patients and no particular value is diagnostic.
D. Preoperative Localization of B Cell Tumors
After the diagnosis of insulinoma has been unequivocally made by clinical and laboratory findings, studies to localize the tumor should be initiated. Most tumors are in the pancreas, and ectopic cases are rare.
Because of the small size of these tumors (averaging 1.5 cm in diameter in one large series), imaging studies do not necessarily identify all of them. A pancreatic dual phase helical CT scan with thin section can identify 82–94% of the lesions. MRI scans with gadolinium can be helpful in detecting a tumor in 85% of cases. One case report suggests that diffusion-weighted MRI can be useful for detecting and localizing small insulinomas, especially for those with no hypervascular pattern. 111In-octreotide scans for insulinomas, which typically express somatostatin receptor type 3, are positive in only 50–70% of cases. PET/CT scans using gallium-labeled somatostatin analogs such as DOTA-1-NaI3-octreotide (DOTA-NOC), which have a higher affinity for somatostatin receptor subtypes 2, 3, and 5, have been reported to be useful in localizing the tumors. Insulinomas express GLP-1 receptors, and radiolabeled GLP-1 receptor agonists such as Lys(40)(Ahx-hydrazinonicotinamide [HYNIC]-[(99m)Tc)NH(2)]-exendin-4 for SPECT/CT have also been reported to visualize the tumors. The imaging study used will depend on local availability and local radiologic skill. If the imaging study is normal, then an endoscopic ultrasound should be performed. In experienced hands, about 80–90% of tumors can be detected with this procedure. Fine-needle aspiration of the identified lesion can be attempted to confirm the presence of a neuroendocrine tumor. If the tumor is not identified or the imaging result is equivocal, then the patient should undergo selective calcium-stimulated angiography, which has been reported to localize the tumor to a particular region of the pancreas approximately 90% of the time. In this test, angiography is combined with injections of calcium gluconate into the gastroduodenal, splenic, and superior mesenteric arteries, and insulin levels are measured in the hepatic vein effluent. The procedure is performed after an overnight fast. Calcium gluconate 10% solution diluted to a volume of 5 mL with 0.95% saline is bolused into the selected artery at a dose of 0.0125 mmol calcium/kg (0.005 mmol calcium/kg for obese patients). Small samples of blood (5 mL) are taken from the hepatic effluent at times 0, 30, 60, 90, 120, and 180 seconds after the calcium injection. Fingerstick blood glucose levels are measured at intervals and a dextrose infusion is maintained throughout the procedure to prevent hypoglycemia. Calcium stimulates insulin release from insulinomas but not normal islets, and so a step-up from baseline in insulin levels at 30 or 60 seconds (twofold or greater) regionalizes the source of the hyperinsulinism to the head of the pancreas for the gastroduodenal artery, the uncinate process for the superior mesenteric artery, and the body and tail of the pancreas for the splenic artery calcium infusions. A less than twofold elevation of insulin in the 120-second sample may represent effects of recirculating calcium and is not considered a positive localization. In a single insulinoma, the response is in one artery alone unless the tumor resides in an area fed by two arteries or if there are multiple insulinomas (eg, in MEN 1). Patients who have diffuse islet hyperplasia (the noninsulinoma pancreatogenous hypoglycemia syndrome) will have positive responses in multiple arteries. Because diazoxide may interfere with this test, it should be discontinued for at least 48–72 hours before sampling. Patients should be closely monitored during the procedure to avoid hypoglycemia (as well as hyperglycemia, which could affect insulin gradients). These studies combined with careful intraoperative ultrasonography and palpation by a surgeon experienced in insulinoma surgery identifies up to 98% of tumors.
 Treatment
Treatment
The treatment of choice for insulin-secreting tumors is surgical resection. While waiting for surgery, patients should be given oral diazoxide. Divided doses of 300–400 mg/day usually suffice, although an occasional patient may require up to 800 mg/day. Side effects include edema due to sodium retention, gastric irritation, and mild hirsutism. Hydrochlorothiazide, 25–50 mg daily, can be used to counteract the sodium retention and edema as well potentiate diazoxide’s hyperglycemic effect.
In patients with a single benign pancreatic B cell adenoma, 90–95% have a successful cure at the first surgical attempt when intraoperative ultrasound is used by a skilled surgeon. Diazoxide should be administered on the day of the surgery because it reduces the risk of hypoglycemia during surgery. Typically, it does not mask the glycemic rise indicative of surgical cure. Blood glucose should be monitored throughout surgery, and 5% or 10% dextrose infusion should be used to maintain euglycemia. In cases where the diagnosis has been established but no adenoma is located after careful palpation and use of intraoperative ultrasound, it is no longer advisable to blindly resect the body and tail of the pancreas, since a nonpalpable tumor missed by ultrasound is most likely embedded within the fleshy head of the pancreas that is left behind with subtotal resections. Most surgeons prefer to close the incision and schedule a selective arterial calcium stimulation with hepatic venous sampling to locate the tumor site prior to a repeat operation. Laparoscopy using ultrasound and enucleation has been successful with a single tumor of the body or tail of the pancreas, but open surgery remains necessary for tumors in the head of the pancreas.
In patients with inoperable functioning islet cell carcinoma with and without hepatic metastasis and in approximately 5–10% of MEN 1 cases when subtotal removal of the pancreas has failed to produce cure, the treatment approach is the same as for other types of pancreatic neuroendocrine tumors (pNETs). Diazoxide is the treatment of choice in preventing hypoglycemia. Frequent carbohydrate feedings (every 2–3 hours) can also be helpful, although weight gain can become a problem. Somatostatin analogs, octreotide or lanreotide, should be considered if diazoxide is ineffective or if there is tumor progression. Surgery or embolization (bland, chemo- and radio-) or thermal ablation (radiofrequency, microwave, and cryoablation) can be used to reduce tumor burden and also provide symptomatic relief. Chemotherapy regimens that can be considered include combination of streptozocin, 5-fluorouracil, and doxorubicin; capecitabine and oxaliplatin; and capecitabine and temozolomide (Table 39–3). Targeted therapies against multiple steps in PI3K/AKT/mTor pathway have been shown to be helpful. Everolimus, an inhibitor of mTor, is approved for treatment of advanced pNETs. Sunitinib, a monoclonal antibody against VEGF receptors 2 and 3, PDGFR alpha and beta, and c-kit, has been shown to slow growth of pNETs. Treatment with radioisotopes (indium-111 or yttrium-90 or lutetium-177) linked to a somatostatin analog have been reported to show benefit in a proportion of patients.
NONISLET CELL TUMOR HYPOGLYCEMIA
These rare causes of hypoglycemia include mesenchymal tumors such as retroperitoneal sarcomas, hepatocellular carcinomas, adrenocortical carcinomas, and miscellaneous epithelial-type tumors. The tumors are frequently large and readily palpated or visualized on CT scans or MRI.
In many cases the hypoglycemia is due to the expression and release of an incompletely processed insulin-like growth factor 2 (IGF-2) by the tumor.
The diagnosis is supported by laboratory documentation of serum insulin levels below 5 microunit/mL with plasma glucose levels of 45 mg/dL (2.5 mmol/L) or lower. Values for growth hormone and IGF-1 are also decreased. Levels of IGF-2 may be increased but often are “normal” in quantity, despite the presence of the immature, higher-molecular-weight form of IGF-2, which can be detected only by special laboratory techniques.
Not all the patients with nonislet cell tumor hypoglycemia have elevated pro-IGF-2. Ectopic insulin production has been described in bronchial carcinoma, ovarian carcinoma, and small cell carcinoma of the cervix. Hypoglycemia due to IgF-1 released from a metastatic large cell carcinoma of the lung has also been reported. GLP-1–secreting tumors (ovarian and pNETs) can also cause hypoglycemia by stimulating insulin release from normal pancreatic islets.
The prognosis for these tumors is generally poor, and surgical removal should be attempted when feasible. Dietary management of the hypoglycemia is the mainstay of medical treatment, since diazoxide is usually ineffective.
Bodnar TW et al. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014 Mar;99(3):713–22. [PMID: 24423303]
POSTPRANDIAL HYPOGLYCEMIA
1. Hypoglycemia Following Gastric Surgery
Hypoglycemia sometimes develops in patients who have undergone gastric surgery (eg, gastrectomy, vagotomy, pyloroplasty, gastrojejunostomy, Nissan fundoplication, Billroth II procedure, and Roux-en-Y), especially when they consume foods containing high levels of readily absorbable carbohydrates. This late dumping syndrome occurs about 1–3 hours after a meal and is a result of rapid delivery of high concentration of carbohydrates in the proximal small bowel and rapid absorption of glucose. The hyperinsulinemic response to the high carbohydrate load causes hypoglycemia. Excessive release of gastrointestinal hormones such as GLP-1 likely play a role in the hyperinsulinemic response. The symptoms include lightheadedness, sweating, confusion and even loss of consciousness after eating a high carbohydrate meal. To document hypoglycemia, the patient should consume a meal that leads to symptoms during everyday life. An oral glucose tolerance test is not recommended because many normal persons have false-positive test results. There have been case reports of insulinoma and noninsulinoma pancreatogenous hypoglycemia syndrome in patients with hypoglycemia post Roux-en-Y surgery. It is unclear how often this occurs. A careful history may identify patients who have a history of hypoglycemia with exercise or missed meals, and these individuals may require a formal 72-hour fast to rule out an insulinoma.
Treatment for secondary dumping includes dietary modification, but this may be difficult to sustain. Patients can try more frequent meals with smaller portions of less rapidly digested carbohydrates. Alpha-glucosidase therapy may be a useful adjunct to a low carbohydrate diet. Octreotide 50 mcg administered subcutaneously two or three times a day 30 minutes prior to each meal has been reported to improve symptoms due to late dumping syndrome. Treatment with exendin 9-39, a GLP-1 receptor agonist, may prevent post gastric bypass hypoglycemia. SGLT2 inhibitors may ameliorate the postprandial glucose rise, the subsequent insulin response, and hypoglycemia. There is a report of a patient with Roux-en-Y surgery who had complete resolution of both hyperglycemia and hypoglycemia when she was given canagliflozin. Various surgical procedures to delay gastric emptying have been reported to improve symptoms but long-term efficacy studies are lacking.
2. Functional Alimentary Hypoglycemia
Patients have symptoms suggestive of increased sympathetic activity, including anxiety, weakness, tremor, sweating or palpitations after meals. Physical examination and laboratory tests are normal. It is not recommended that patients with symptoms suggestive of increased sympathetic activity undergo either a prolonged oral glucose tolerance test or a mixed meal test. Instead, the patients should be given home blood glucose monitors (with memories) and instructed to monitor fingerstick glucose levels at the time of symptoms. Only patients who have symptoms when their fingerstick blood glucose is low (less than 50 mg/dL) and who have resolution of symptoms when the glucose is raised by eating rapidly released carbohydrate need additional evaluation. Patients who do not have evidence for low glucose levels at time of symptoms are generally reassured by their findings. Counseling and support should be the mainstays in therapy, with dietary manipulation only an adjunct.
3. Occult Diabetes
This condition is characterized by a delay in early insulin release from pancreatic B cells, resulting in initial exaggeration of hyperglycemia during a glucose tolerance test. In response to this hyperglycemia, an exaggerated insulin release produces a late hypoglycemia 4–5 hours after ingestion of glucose. These patients are often obese and frequently have a family history of diabetes mellitus.
Patients with this type of postprandial hypoglycemia often respond to reduced intake of refined sugars with multiple, spaced, small feedings high in dietary fiber. In the obese, treatment is directed at weight reduction to achieve ideal weight. These patients should be considered to have prediabetes or early diabetes (type 1 or 2) and advised to have periodic medical evaluations.
4. Autoimmune Hypoglycemia
Patients with autoimmune hypoglycemia have early postprandial hyperglycemia followed by hypoglycemia 3–4 hours later. The hypoglycemia is attributed to a dissociation of insulin-antibody immune complexes, releasing free insulin.
The disorder is associated with methimazole treatment for Graves disease, although it can also occur in patients treated with various other sulfhydryl-containing medications (captopril, penicillamine) as well as other drugs such as hydralazine, isoniazid, and procainamide. In addition, it has been reported in patients with autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, and polymyositis as well as in plasma cell myeloma (formerly multiple myeloma) and other plasma cell dyscrasias where paraproteins or antibodies cross-react with insulin. There is also an association with the HLA class II alleles (DRB1*0406, DQA1*0301, and DQB1*0302). These alleles are 10 to 20 times more common in Japanese and Korean populations, which explains why the disorder has been reported mostly in Japanese patients.
High titers of insulin autoantibodies, usually IgG class, can be detected. Insulin, proinsulin, and C-peptide levels may be elevated, but the results may be erroneous because of the interference of the insulin antibodies with the immunoassays for these peptides.
In most cases, the hypoglycemia is transient and usually resolves spontaneously within 3–6 months of diagnosis, particularly when the offending medications are stopped. The most consistent therapeutic benefit in management of this syndrome has been achieved by dietary treatment with small, frequent low-carbohydrate meals. Prednisone (30–60 mg orally daily) has been used to lower the titer of insulin antibodies.
FACTITIOUS HYPOGLYCEMIA
Factitious hypoglycemia may be difficult to document. A suspicion of self-induced hypoglycemia is supported when the patient is associated with the health professions or has access to insulin or sulfonylurea medications taken by a diabetic member of the family. The triad of hypoglycemia, high immunoreactive insulin, and suppressed plasma C-peptide immunoreactivity is pathognomonic of exogenous insulin administration. Insulin and C-peptide are secreted in a 1:1 molar ratio. A large fraction of the endogenous insulin is cleared by the liver, whereas C-peptide, which is cleared by the kidney, has a lower metabolic clearance rate. For this reason, the molar ratio of insulin and C-peptide in a hypoglycemic patient should be less than 1.0 in cases of insulinoma and is greater than 1.0 in cases of exogenous insulin administration. When sulfonylureas, repaglinide, and nateglinide are suspected as a cause of factitious hypoglycemia, a plasma level of these medications to detect their presence may be required to distinguish laboratory findings from those of insulinoma.
HYPOGLYCEMIA DUE TO INSULIN RECEPTOR ANTIBODIES
Hypoglycemia due to insulin receptor autoantibodies is an extremely rare syndrome; most cases have occurred in women often with a history of autoimmune disease. Almost all of these patients have also had episodes of insulin-resistant diabetes and acanthosis nigricans. Their hypoglycemia may be either fasting or postprandial and is often severe and is attributed to an agonistic action of the antibody on the insulin receptor. Balance between the antagonistic and agonistic effects of the antibodies determines whether insulin-resistant diabetes or hypoglycemia occurs. Hypoglycemia was found to respond to corticosteroid therapy but not to plasmapheresis or immunosuppression.
Kim CH et al. Autoimmune hypoglycemia in a type 2 diabetic patient with anti-insulin and insulin receptor antibodies. Diabetes Care. 2004 Jan;27(1):288–9. [PMID: 14694017]
MEDICATION- & ETHANOL-INDUCED HYPOGLYCEMIA
A number of medications apart from the sulfonylureas can occasionally cause hypoglycemia. Common offenders include the fluoroquinolones such as gatifloxacin and levofloxacin, pentamidine, quinine, ACE inhibitors, salicylates and beta-adrenergic blocking agents. The fluoroquinolones, particularly gatifloxacin, have been associated with both hypoglycemia and hyperglycemia. It is thought that the drug acts on the ATP sensitive potassium channels in the beta cell. Hypoglycemia is an early event, and hyperglycemia occurs several days into therapy. Intravenous pentamidine is cytotoxic to beta cells and causes acute hyperinsulinemia and hypoglycemia followed by insulinopenia and hyperglycemia. Fasting patients taking noncardioselective beta-blockers can have an exaggerated hypoglycemic response to starvation. The beta-blockade inhibits fatty acids and gluconeogenesis substrate release and reduces plasma glucagon response. Therapy with ACE inhibitors increases the risk of hypoglycemia in patients who are taking insulin or sulfonylureas presumably because these drugs increase sensitivity to circulating insulin by increasing blood flow to the muscle. Some opioids cause hypoglycemia. Tramadol use has been associated with increased risk of hospitalization for hypoglycemia. Methadone overdose has also been reported to cause hypoglycemia and a rapid dose escalation of methadone in cancer patients can lower glucose levels.
Ethanol-associated hypoglycemia may be due to hepatic alcohol dehydrogenase activity depleting NAD. The resultant change in the redox state—increase in NADH to NAD+ ratio—causes a partial block at several points in the gluconeogenic pathway. With prolonged starvation, glycogen reserves become depleted within 18–24 hours and hepatic glucose output becomes totally dependent on gluconeogenesis. Under these circumstances, a blood concentration of ethanol as low as 45 mg/dL (9.8 mmol/L) can induce profound hypoglycemia by blocking gluconeogenesis. Neuroglycopenia in a patient whose breath smells of alcohol may be mistaken for alcoholic stupor. Prevention consists of adequate food intake during ethanol ingestion. Therapy consists of glucose administration to replenish glycogen stores until gluconeogenesis resumes.
When sugar-containing soft drinks are used as mixers to dilute alcohol in beverages (gin and tonic, rum and cola), there seems to be a greater insulin release than when the soft drink alone is ingested and a tendency for more of a late hypoglycemic overswing to occur 3–4 hours later. Prevention would consist of avoiding sugar mixers while ingesting alcohol and ensuring supplementary food intake to provide sustained absorption.
Vue MH et al. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectrum. 2011 Aug;24(3):171–7.
1In collecting samples, it is essential to rapidly chill and separate the blood in order to remove red cells, whose continued glycolysis at room temperature is a common source of error in reports of high plasma lactate. Frozen plasma remains stable for subsequent assay.