30
Common Problems in Infectious Diseases & Antimicrobial Therapy
Peter V. Chin-Hong, MD
B. Joseph Guglielmo, PharmD
COMMON PROBLEMS IN INFECTIOUS DISEASES
FEVER OF UNKNOWN ORIGIN (FUO)
ESSENTIALS OF DIAGNOSIS

 Illness of at least 3 weeks in duration.
Illness of at least 3 weeks in duration.
 Fever over 38.3°C on several occasions.
Fever over 38.3°C on several occasions.
 Diagnosis has not been made after three outpatient visits or 3 days of hospitalization.
Diagnosis has not been made after three outpatient visits or 3 days of hospitalization.
 General Considerations
General Considerations
The intervals specified in the criteria for the diagnosis of FUO are arbitrary ones intended to exclude patients with protracted but self-limited viral illnesses and to allow time for the usual radiographic, serologic, and cultural studies to be performed. The criteria for FUO are met when a diagnosis has not been made after three outpatient visits or 3 days of hospitalization.
The recently added categories of FUO include complications of current health care scenarios: (1) Hospital-associated FUO refers to the hospitalized patient with fever of 38.3°C or higher on several occasions, due to a process not present or incubating at the time of admission, in whom initial cultures are negative and the diagnosis remains unknown after 3 days of investigation (see Health Care–Associated Infections below); (2) neutropenic FUO includes patients with fever of 38.3°C or higher on several occasions with less than 500 neutrophils per microliter in whom initial cultures are negative and the diagnosis remains uncertain after 3 days (see Chapter 2 and Infections in the Immunocompromised Patient, below); (3) HIV-associated FUO pertains to HIV-positive patients with fever of 38.3°C or higher who have been febrile for 4 weeks or more as an outpatient or 3 days as an inpatient, in whom the diagnosis remains uncertain after 3 days of investigation with at least 2 days for cultures to incubate (see Chapter 31). Although not usually considered separately, FUO in solid organ transplant recipients and FUO in the returning traveler are common scenarios, each with a unique differential diagnosis, and are also discussed in this chapter.
For a general discussion of fever, see the section on fever and hyperthermia in Chapter 2.
A. Common Causes
Most cases represent unusual manifestations of common diseases and not rare or exotic diseases—eg, tuberculosis, endocarditis, gallbladder disease, and HIV (primary infection or opportunistic infection) are more common causes of FUO than Whipple disease or familial Mediterranean fever.
B. Age of Patient
In adults, infections (25–40% of cases) and cancer (25–40% of cases) account for the majority of FUOs. In children, infections are the most common cause of FUO (30–50% of cases) and cancer a rare cause (5–10% of cases). Autoimmune disorders occur with equal frequency in adults and children (10–20% of cases), but the diseases differ. Juvenile rheumatoid arthritis is particularly common in children, whereas systemic lupus erythematosus, granulomatosis with polyangiitis (formerly Wegener granulomatosis), and polyarteritis nodosa are more common in adults. Still disease, giant cell arteritis, and polymyalgia rheumatica occur exclusively in adults. In adults over 65 years of age, multisystem immune-mediated diseases such as temporal arteritis, polymyalgia rheumatica, sarcoidosis, rheumatoid arthritis, and granulomatosis with polyangiitis account for 25–30% of all FUOs.
C. Duration of Fever
The cause of FUO changes dramatically in patients who have been febrile for 6 months or longer. Infection, cancer, and autoimmune disorders combined account for only 20% of FUOs in these patients. Instead, other entities such as granulomatous diseases (granulomatous hepatitis, Crohn disease, ulcerative colitis) and factitious fever become important causes. One-fourth of patients who say they have been febrile for 6 months or longer actually have no true fever or underlying disease. Instead, the usual normal circadian variation in temperature (temperature 0.5–1°C higher in the afternoon than in the morning) is interpreted as abnormal. Patients with episodic or recurrent fever (ie, those who meet the criteria for FUO but have fever-free periods of 2 weeks or longer) are similar to those with prolonged fever. Infection, malignancy, and autoimmune disorders account for only 20–25% of such fevers, whereas various miscellaneous diseases (Crohn disease, familial Mediterranean fever, allergic alveolitis) account for another 25%. Approximately 50% of cases remain undiagnosed but have a benign course with eventual resolution of symptoms.
D. Immunologic Status
In the neutropenic patient, fungal infections and occult bacterial infections are important causes of FUO. In the patient taking immunosuppressive medications (particularly organ transplant patients), cytomegalovirus (CMV) infections are a frequent cause of fever, as are fungal infections, nocardiosis, Pneumocystis jirovecii pneumonia, and mycobacterial infections.
E. Classification of Causes of FUO
Most patients with FUO will fit into one of five categories.
1. Infection—Both systemic and localized infections can cause FUO. Tuberculosis and endocarditis are the most common systemic infections associated with FUO, but mycoses, viral diseases (particularly infection with Epstein-Barr virus and CMV), toxoplasmosis, brucellosis, Q fever, cat-scratch disease, salmonellosis, malaria, and many other less common infections have been implicated. Primary infection with HIV or opportunistic infections associated with AIDS—particularly mycobacterial infections—can also present as FUO. The most common form of localized infection causing FUO is an occult abscess. Liver, spleen, kidney, brain, and bone abscesses may be difficult to detect. A collection of pus may form in the peritoneal cavity or in the subdiaphragmatic, subhepatic, paracolic, or other areas. Cholangitis, osteomyelitis, urinary tract infection, dental abscess, or paranasal sinusitis may cause prolonged fever.
2. Neoplasms—Many cancers can present as FUO. The most common are lymphoma (both Hodgkin and non-Hodgkin) and leukemia. Posttransplant lymphoproliferative disorders may also present with fever. Other diseases of lymph nodes, such as angioimmunoblastic lymphoma and Castleman disease, can also cause FUO. Primary and metastatic tumors of the liver are frequently associated with fever, as are renal cell carcinomas. Atrial myxoma is an often forgotten neoplasm that can result in fever. Chronic lymphocytic leukemia and multiple myeloma are rarely associated with fever, and the presence of fever in patients with these diseases should prompt a search for infection.
3. Autoimmune disorders—Still disease, systemic lupus erythematosus, cryoglobulinemia, and polyarteritis nodosa are the most common causes of autoimmune-associated FUO. Giant cell arteritis and polymyalgia rheumatica are seen almost exclusively in patients over 50 years of age and are nearly always associated with an elevated erythrocyte sedimentation rate (greater than 40 mm/h).
4. Miscellaneous causes—Many other conditions have been associated with FUO but less commonly than the foregoing types of illness. Examples include thyroiditis, sarcoidosis, Whipple disease, familial Mediterranean fever, recurrent pulmonary emboli, alcoholic hepatitis, drug fever, and factitious fever.
5. Undiagnosed FUO—Despite extensive evaluation, the diagnosis remains elusive in 15% or more of patients. Of these patients, the fever abates spontaneously in about 75% with no diagnosis; in the remainder, more classic manifestations of the underlying disease appear over time.
 Clinical Findings
Clinical Findings
Because the evaluation of a patient with FUO is costly and time-consuming, it is imperative to first document the presence of fever. This is done by observing the patient while the temperature is being taken to ascertain that fever is not factitious (self-induced). Associated findings that accompany fever include tachycardia, chills, and piloerection. A thorough history—including family, occupational, social (sexual practices, use of injection drugs), dietary (unpasteurized products, raw meat), exposures (animals, chemicals), and travel—may give clues to the diagnosis. Repeated physical examination may reveal subtle, evanescent clinical findings essential to diagnosis.
A. Laboratory Tests
In addition to routine laboratory studies, blood cultures should always be obtained, preferably when the patient has not taken antibiotics for several days, and should be held by the laboratory for 2 weeks to detect slow-growing organisms. Cultures on special media are requested if Legionella, Bartonella, or nutritionally deficient streptococci are possible pathogens. “Screening tests” with immunologic or microbiologic serologies (“febrile agglutinins”) are of low yield and should not be done. If the history or physical examination suggests a specific diagnosis, specific serologic tests with an associated fourfold rise or fall in titer may be useful. Because infection is the most common cause of FUO, other body fluids are usually cultured, ie, urine, sputum, stool, cerebrospinal fluid, and morning gastric aspirates (if one suspects tuberculosis). Direct examination of blood smears may establish a diagnosis of malaria or relapsing fever (Borrelia).
B. Imaging
All patients with FUO should have a chest radiograph. Studies such as sinus CT, upper gastrointestinal series with small bowel follow-through, barium enema, proctosigmoidoscopy, and evaluation of gallbladder function are reserved for patients who have symptoms, signs, or a history that suggest disease in these body regions. CT scan of the abdomen and pelvis is also frequently performed and is particularly useful for looking at the liver, spleen, and retroperitoneum. When the CT scan is abnormal, the findings often lead to a specific diagnosis. A normal CT scan is not quite as useful; more invasive procedures such as biopsy or exploratory laparotomy may be needed. The role of MRI in the investigation of FUO has not been evaluated. In general, however, MRI is better than CT for detecting lesions of the nervous system and is useful in diagnosing various vasculitides. Ultrasound is sensitive for detecting lesions of the kidney, pancreas, and biliary tree. Echocardiography should be used if one is considering endocarditis or atrial myxoma. Transesophageal echocardiography is more sensitive than surface echocardiography for detecting valvular lesions, but even a negative transesophageal study does not exclude endocarditis (10% false-negative rate). The usefulness of radionuclide studies in diagnosing FUO is variable. Some experts use positron emission tomography (PET) in conjunction with CT scans early in the investigation of FUO. However, more studies are needed before this practice can be more fully integrated into clinical practice. Theoretically, a gallium or PET scan would be more helpful than an indium-labeled white blood cell scan because gallium and fluorodeoxyglucose may be useful for detecting infection, inflammation, and neoplasm, whereas the indium scan is useful only for detecting infection. Indium-labeled immunoglobulin may prove to be useful in detecting infection and neoplasm and can be used in the neutropenic patient. It is not sensitive for lesions of the liver, kidney, and heart because of high background activity. In general, radionuclide scans are plagued by high rates of false-positive and false-negative results that are not useful when used as screening tests and, if done at all, are limited to those patients whose history or examination suggests local inflammation or infection.
C. Biopsy
Invasive procedures are often required for diagnosis. Any abnormal finding should be aggressively evaluated: Headache calls for lumbar puncture to rule out meningitis; skin rash should be biopsied for cutaneous manifestations of collagen vascular disease or infection; and enlarged lymph nodes should be aspirated or biopsied for neoplasm and sent for culture. Bone marrow aspiration with biopsy is a relatively low-yield procedure (15–25%; except in HIV-positive patients, in whom mycobacterial infection is a common cause of FUO), but the risk is low and the procedure should be done if other less invasive tests have not yielded a diagnosis, particularly in persons with hematologic abnormalities. Liver biopsy will yield a specific diagnosis in 10–15% of patients with FUO and should be considered in any patient with abnormal liver tests even if the liver is normal in size. CT scanning and MRI have decreased the need for exploratory laparotomy; however, surgical visualization and biopsies should be considered when there is continued deterioration or lack of diagnosis.
 Treatment
Treatment
Although an empiric course of antimicrobials is sometimes considered for FUO, it is rarely helpful and may impact infectious diseases diagnoses (eg, by reducing the sensitivity of blood cultures).
 When to Refer
When to Refer
• Any patient with FUO and progressive weight loss and other constitutional signs.
• Any immunocompromised patient (eg, transplant recipients and HIV-infected patients).
• Infectious diseases specialists may also be able to coordinate and interpret specialized testing (eg, Q fever serologies) with outside agencies, such as the US Centers for Disease Control and Prevention.
 When to Admit
When to Admit
• Any patient who is rapidly declining with weight loss where hospital admission may expedite workup.
• If FUO is present in immunocompromised patients, such as those who are neutropenic from recent chemotherapy or those who have undergone transplantation (particularly in the previous 6 months).
Fusco FM et al. Fever of unknown origin (FUO): which are the factors influencing the final diagnosis? A 2005–2015 systematic review. BMC Infect Dis. 2019 Jul 22;19(1):653. [PMID: 31331269]
Mulders-Manders CM et al. Long-term prognosis, treatment, and outcome of patients with fever of unknown origin in whom no diagnosis was made despite extensive investigation: a questionnaire based study. Medicine (Baltimore). 2018 Jun;97(25):e11241. [PMID: 29924054]
Zhai YZ et al. Clinical analysis of 215 consecutive cases with fever of unknown origin: A cohort study. Medicine (Baltimore). 2018 Jun;97(24):e10986. [PMID: 29901588]
INFECTIONS IN THE IMMUNOCOMPROMISED PATIENT
ESSENTIALS OF DIAGNOSIS

 Fever and other symptoms may be blunted because of immunosuppression.
Fever and other symptoms may be blunted because of immunosuppression.
 A contaminating organism in an immunocompetent individual may be a pathogen in an immunocompromised one.
A contaminating organism in an immunocompetent individual may be a pathogen in an immunocompromised one.
 The interval since transplantation and the degree of immunosuppression can narrow the differential diagnosis.
The interval since transplantation and the degree of immunosuppression can narrow the differential diagnosis.
 Empiric broad-spectrum antibiotics may be appropriate in high-risk patients whether or not symptoms are localized.
Empiric broad-spectrum antibiotics may be appropriate in high-risk patients whether or not symptoms are localized.
 General Considerations
General Considerations
Immunocompromised patients have defects in their natural defense mechanisms resulting in an increased risk for infection. In addition, infection is often severe, rapidly progressive, and life threatening. Organisms that are not usually problematic in the immunocompetent person may be important pathogens in the compromised patient (eg, Staphylococcus epidermidis, Corynebacterium jeikeium, Propionibacterium acnes, Bacillus species). Therefore, culture results must be interpreted with caution, and isolates should not be disregarded as solely contaminants. Although the type of immunodeficiency is associated with specific infectious disease syndromes, any pathogen can cause infection in any immunosuppressed patient at any time. Thus, a systematic evaluation is required to identify a specific organism.
A. Impaired Humoral Immunity
Defects in humoral immunity are often congenital, although hypogammaglobulinemia can occur in multiple myeloma, chronic lymphocytic leukemia, small lymphocyte lymphoma, and in patients who have undergone splenectomy. Patients with ineffective humoral immunity lack opsonizing antibodies and are at particular risk for infection with encapsulated organisms, such as Haemophilus influenzae, Neisseria meningitides, and Streptococcus pneumoniae. Although rituximab is normally thought of as being linked to impaired cellular immunity, it has been associated with the development of Pneumocystis jirovecii infection and progressive multifocal leukoencephalopathy (PML) as well as with hepatitis B reactivation.
B. Granulocytopenia (Neutropenia)
Granulocytopenia is common following hematopoietic cell transplantation (“stem cell transplantation”) and among patients with solid tumors—as a result of myelosuppressive chemotherapy—and in acute leukemias. The risk of infection begins to increase when the absolute granulocyte count falls below 1000/mcL, with a dramatic increase in frequency and severity when the granulocyte count falls below 100/mcL. The infection risk is also increased with a rapid rate of decline of neutrophils and with a prolonged period of neutropenia. The granulocytopenic patient is particularly susceptible to infections with gram-negative enteric organisms, Pseudomonas, gram-positive cocci (particularly Staphylococcus aureus, S epidermidis, and viridans streptococci), Candida, Aspergillus, and other fungi that have recently emerged as pathogens such as Trichosporon, Scedosporium, Fusarium, and the mucormycoses.
C. Impaired Cellular Immunity
Patients with cellular immune deficiency encompass a large and heterogeneous group, including patients with HIV infection (see Chapter 31); patients with lymphoreticular malignancies, such as Hodgkin disease; and patients receiving immunosuppressive medications, such as corticosteroids, cyclosporine, tacrolimus, and other cytotoxic medications. This latter group—those who are immunosuppressed as a result of medications—includes patients who have undergone solid organ transplantation, many patients receiving therapy for solid tumors, and patients receiving prolonged high-dose corticosteroid treatment (eg, for asthma, temporal arteritis, systemic lupus erythematosus). Patients taking tumor necrosis factor (TNF) inhibitors, such as etanercept and infliximab, are also included in this category. Patients with cellular immune dysfunction are susceptible to infections by a large number of organisms, particularly ones that replicate intracellularly. Examples include bacteria, such as Listeria, Legionella, Salmonella, and Mycobacterium; viruses, such as herpes simplex, varicella, and CMV; fungi, such as Cryptococcus, Coccidioides, Histoplasma, and Pneumocystis; and protozoa, such as Toxoplasma.
D. Hematopoietic Cell Transplant Recipients
The length of time it takes for complications to occur in hematopoietic cell transplant recipients can be helpful in determining the etiologic agent. In the early (preengraftment) posttransplant period (days 1–21), patients will become severely neutropenic for 7–21 days. Patients are at risk for gram-positive (particularly catheter-related) and gram-negative bacterial infections, as well as herpes simplex virus, respiratory syncytial virus, and fungal infections. In contrast to solid organ transplant recipients, the source of fever is unknown in 60–70% of hematopoietic cell transplant patients. Between 3 weeks and 3 months posttransplant, infections with CMV, adenovirus, Aspergillus, and Candida are most common. P jirovecii pneumonia is possible, particularly in patients who receive additional immunosuppression for treatment of graft-versus-host disease. Patients continue to be at risk for infectious complications beyond 3 months following transplantation, particularly those who have received allogeneic transplantation and those who are taking immunosuppressive therapy for chronic graft-versus-host disease. Varicella-zoster is common, and Aspergillus and CMV infections are increasingly seen in this period as well.
E. Solid Organ Transplant Recipients
The length of time it takes for infection to occur following solid organ transplantation can also be helpful in determining the infectious origin. Immediate postoperative infections often involve the transplanted organ. Following lung transplantation, pneumonia and mediastinitis are particularly common; following liver transplantation, intra-abdominal abscess, cholangitis, and peritonitis may be seen; after kidney transplantation, urinary tract infections, perinephric abscesses, and infected lymphoceles can occur.
Most infections that occur in the first 2–4 weeks posttransplant are related to the operative procedure and to hospitalization itself (wound infection, intravenous catheter infection, urinary tract infection from an indwelling urinary [Foley] catheter) or are related to the transplanted organ. In rare instances, donor-derived infections (eg, West Nile virus, tuberculosis) may present during this time period. Compensated organ transplants obtained abroad through “medical tourism” can introduce additional risk of infections, which vary by country and by transplant setting. Infections that occur between the first and sixth months are often related to immunosuppression. During this period, reactivation of viruses, such as herpes simplex, varicella-zoster, and CMV is quite common. Opportunistic infections with fungi (eg, Candida, Aspergillus, Cryptococcus, Pneumocystis), Listeria monocytogenes, Nocardia, and Toxoplasma are also common. After 6 months, if immunosuppression has been reduced to maintenance levels, infections that would be expected in any population occur. Patients with poorly functioning allografts receiving long-term immunosuppression therapy continue to be at risk for opportunistic infections.
F. Tumor Necrosis Factor Inhibitor Recipients
Patients taking TNF inhibitors have specific defects that increase risk of bacterial, mycobacterial (particularly tuberculosis), viral (HBV reactivation and HCV progression), and fungal infections (Pneumocystis, molds, and endemic mycoses). Infection risk may be highest shortly after therapy is initiated (within the first 3 months) and with a higher dose of medications.
G. Recipients of Other Biologics
In addition to TNF inhibitors, other biologics target a variety of immunologic pathways that are involved in immunologic mediated disease and in cancer replication. Disruption of these pathways include, but are not limited to impact on B cells, T cells, complement, and leukocytes. This may result in not only serious infections, but the development of autoimmune disease and malignancies as well. Some medications have been observed to have specific associations with opportunistic infections (eg, natalizumab and PML, or eculizumab and meningococcal disease). Other biologics such as chimeric antigen receptor T (CAR-T) cells may have unintended infectious risks that are currently unknown, or may have adverse effects that mimic infection (eg, cytokine release syndrome). Checkpoint inhibitors (eg, anti-PD-1 and CTLA antibodies) used for the treatment of advanced malignancies also may have effects that mimic infection via immune enhancement. Prolonged immunosuppression used to treat immune-associated adverse events in CAR-T and checkpoint inhibitor therapy (eg, TNF inhibitors and corticosteroids) can then result in opportunistic and other infections. As more biologics are developed and used, clinicians must remain vigilant for the possibility of serious infectious disease risk.
H. Other Immunocompromised States
A large group of patients who are not specifically immunodeficient are at increased risk for infection due to debilitating injury (eg, burns or severe trauma), invasive procedures (eg, chronic central intravenous catheters, indwelling urinary [Foley] catheters, dialysis catheters), central nervous system dysfunction (which predisposes patients to aspiration pneumonia and decubitus ulcers), obstructing lesions (eg, pneumonia due to an obstructed bronchus, pyelonephritis due to nephrolithiasis, cholangitis secondary to cholelithiasis), and use of broad-spectrum antibiotics. Patients with diabetes mellitus have alterations in cellular immunity, resulting in mucormycosis, emphysematous pyelonephritis, and foot infections.
 Clinical Findings
Clinical Findings
A. Laboratory Findings
Routine evaluation includes complete blood count with differential, chest radiograph, and blood cultures; urine and respiratory cultures should be obtained if indicated clinically or radiographically. Any focal complaints (localized pain, headache, rash) should prompt imaging and cultures appropriate to the site.
Patients who remain febrile without an obvious source should be evaluated for viral infection (serum CMV antigen test or polymerase chain reaction), abscesses (which usually occur near previous operative sites), candidiasis involving the liver or spleen, or aspergillosis. Serologic evaluation may be helpful if toxoplasmosis or an endemic fungal infection (coccidioidomycosis, histoplasmosis) is a possible cause. Antigen-based assays may be useful for the diagnosis of aspergillosis (detected by galactomannan level in serum or bronchoalveolar lavage fluid), or other invasive fungal disease, including Pneumocystis infection (serum [1 → 3]-beta-D-glucan level).
B. Special Diagnostic Procedures
Special diagnostic procedures should also be considered. The cause of pulmonary infiltrates can be easily determined with simple techniques in some situations—eg, induced sputum yields a diagnosis of Pneumocystis pneumonia in 50–80% of patients with AIDS with this infection. In other situations, more invasive procedures may be required (bronchoalveolar lavage, transbronchial biopsy, open lung biopsy). Skin, liver, or bone marrow biopsy may be helpful in establishing a diagnosis. Next generation DNA-sequencing analysis (eg, of plasma, bronchoalveolar lavage, cerebrospinal fluid) is an increasingly used and validated option for diagnosis of infectious diseases in immunocompromised persons.
 Differential Diagnosis
Differential Diagnosis
Transplant rejection, organ ischemia and necrosis, thrombophlebitis, and lymphoma (posttransplant lymphoproliferative disease) may all present as fever and must be considered in the differential diagnosis.
 Prevention
Prevention
While prophylactic antimicrobial medications are used commonly, the optimal medications or dosage regimens are debated. Hand washing is the simplest and most effective means of decreasing hospital-associated infections, especially in the compromised patient. Invasive devices such as central and peripheral lines and indwelling urinary (Foley) catheters are potential sources of infection. Some centers use laminar airflow isolation or high-efficiency particulate air (HEPA) filtering in hematopoietic cell transplant patients. Rates of infection and episodes of febrile neutropenia, but not mortality, are decreased if colony-stimulating factors are used (typically in situations where the risk of febrile neutropenia is 20% or higher) during chemotherapy or during stem-cell transplantation.
A. Pneumocystis & Herpes Simplex Infections
Trimethoprim-sulfamethoxazole (TMP-SMZ), one double-strength tablet orally three times a week, one double-strength tablet twice daily on weekends, or one single-strength tablet daily for 3–6 months, is frequently used to prevent Pneumocystis infections in transplant patients. In patients allergic to TMP-SMZ, dapsone, 50 mg orally daily or 100 mg three times weekly, is recommended. Glucose-6-phosphate dehydrogenase (G6PD) levels should be assessed before dapsone is instituted. Acyclovir prevents herpes simplex infections in bone marrow and solid organ transplant recipients and is given to seropositive patients who are not receiving ganciclovir or valganciclovir for CMV prophylaxis. The usual dose is 200 mg orally three times daily for 4 weeks (hematopoietic cell transplants) to 12 weeks (other solid organ transplants).
B. CMV
No uniformly accepted approach has been adopted for prevention of CMV. Prevention strategies often depend on the serologic status of the donor and recipient and the organ transplanted, which determines the level of immunosuppression after transplant. In solid organ transplants (liver, kidney, heart, lung), the greatest risk of developing CMV disease is in seronegative recipients who receive organs from seropositive donors. These high-risk patients usually receive oral valganciclovir, 900 mg daily for 3–6 months (longer in lung transplant recipients). Other solid organ transplant recipients (seropositive recipients) are at lower risk for developing CMV disease, but still usually receive oral valganciclovir for 3 months. The lowest-risk group for the development of CMV disease is in seronegative patients who receive organs from seronegative donors. Typically, no CMV prophylaxis is used in this group. Ganciclovir and valganciclovir also prevent herpes virus reactivation. Because immunosuppression is increased during periods of rejection, patients treated for rejection usually receive CMV prophylaxis during rejection therapy. Alternatively, in a preemptive approach, patients can be monitored without specific prophylaxis by having blood sampled weekly to look for CMV by polymerase chain reaction techniques. If CMV is detected, then therapy is instituted with oral valganciclovir, 900 mg orally twice daily for a minimum of 2–3 weeks.
Recipients of hematopoietic cell transplants are more severely immunosuppressed than recipients of solid organ transplants, are at greater risk for developing serious CMV infection (usually CMV reactivation), and thus usually receive more aggressive prophylaxis. Like in solid organ transplant recipients, two approaches have been used: universal prophylaxis or preemptive therapy. In the former, all high-risk patients (seropositive patients who receive allogeneic transplants) may receive oral valganciclovir, 900 mg daily to day 100. However, valganciclovir is associated with significant bone marrow toxicity. Letermovir is being used increasingly, and it is not associated with bone marrow toxicity. Universal prophylaxis may be costly. Because of the possibility of bone marrow toxicity and the expense, many clinicians traditionally preferred the preemptive approach over the universal prophylaxis approach for recipients of hematopoietic stem cell transplants. However, while this preemptive approach is effective, it does miss a small number of patients in whom CMV disease would have been prevented had prophylaxis been used. Other preventive strategies include use of CMV-negative or leukocyte-depleted blood products for CMV-seronegative recipients.
C. Other Organisms
Routine decontamination of the gastrointestinal tract to prevent bacteremia in the neutropenic patient is not recommended. The use of prophylactic antibiotics in the afebrile, asymptomatic neutropenic patient is debated, although many centers have adopted this strategy. Rates of bacteremia are decreased, but overall mortality is not affected and emergence of resistant organisms takes place. Use of intravenous immunoglobulin is reserved for the small number of patients with severe hypogammaglobulinemia following hematopoietic stem cell transplantation and should not be routinely administered to all transplant patients.
Prophylaxis with antifungal agents to prevent invasive mold (primarily Aspergillus) and yeast (primarily Candida) infections is routinely used, but the optimal agent, dose, and duration are also debated. Lipid-based preparations of amphotericin B, aerosolized amphotericin B, intravenous and oral fluconazole or voriconazole, and oral posaconazole solution and tablets are all prophylactic options in the neutropenic patient. Because voriconazole is superior to amphotericin for documented Aspergillus infections and because posaconazole prophylaxis (compared with fluconazole) has been shown to result in fewer cases of invasive aspergillosis among allogeneic stem cell transplant recipients with graft-versus-host disease, one approach to prophylaxis is to use oral fluconazole (400 mg/day) for patients at low risk for developing fungal infections (those who receive autologous stem cell transplants) and oral voriconazole (200 mg twice daily) or oral posaconazole (200 mg suspension three times daily or 300 mg [three 100-mg tablets] sustained-release tablets once daily) for those at high risk (allogeneic transplants, graft-versus-host disease) at least until engraftment (usually 30 days). In solid organ transplant recipients, the risk of invasive fungal infection varies considerably (1–2% in liver, pancreas, and kidney transplants and 6–8% in heart and lung transplants). Whether universal prophylaxis or observation with preemptive therapy is the best approach has not been determined. Although fluconazole is effective in preventing yeast infections, emergence of fluconazole-resistant Candida and molds (Fusarium, Aspergillus, Mucor) has raised concerns about its routine use as a prophylactic agent in the general solid organ transplant population. However, liver transplant recipients with additional risk factors, such as having undergone a choledochojejunostomy, having had a high transfusion requirement or having developed kidney disease, may benefit from abbreviated postoperative Candida prophylaxis.
Given the high risk of reactivation of tuberculosis in patients taking TNF inhibitors, all patients should be screened for latent tuberculosis infection (LTBI) with a tuberculin skin test or an interferon-gamma release assay prior to the start of therapy. If LTBI is diagnosed, treatment with the TNF inhibitors should be delayed until treatment for LTBI is completed. There is also a marked risk of reactivation of hepatitis B and hepatitis C in patients taking TNF inhibitors; patients should also be screened for these viruses when TNF inhibitor treatment is being considered. Providers should also ensure that patients’ vaccinations are up-to-date before starting TNF inhibitors therapy.
 Treatment
Treatment
A. General Measures
Because infections in the immunocompromised patient can be rapidly progressive and life-threatening, diagnostic procedures must be performed promptly, and empiric therapy is usually instituted.
While reduction or discontinuation of immunosuppressive medication may jeopardize the viability of the transplanted organ, this measure may be necessary if the infection is life-threatening. Hematopoietic growth factors (granulocyte and granulocyte-macrophage colony-stimulating factors) stimulate proliferation of bone marrow stem cells, resulting in an increase in peripheral leukocytes. These agents shorten the period of neutropenia and have been associated with reduction in infection.
B. Specific Measures
Antimicrobial medication therapy ultimately should be tailored to culture results. While combinations of antimicrobials are used with the intent of providing synergy or preventing resistance, the primary reason for empiric combination therapy is broad-spectrum coverage of all likely pathogens.
Empiric therapy is often instituted at the earliest sign of infection in the immunosuppressed patient because prompt therapy favorably affects outcome. The antibiotic or combination of antibiotics used depends on the degree of immune compromise and the site of infection. For example, in the febrile neutropenic patient, an algorithmic approach to therapy is often used. Febrile neutropenic patients should be empirically treated with broad-spectrum agents active against selected gram-positive bacteria, Pseudomonas aeruginosa, and other aerobic gram-negative bacilli (such as cefepime 2 g every 8 hours intravenously). The addition of vancomycin, 10–15 mg/kg/dose intravenously every 12 hours, should be considered in those patients with suspected infection due to methicillin-resistant Staphylococcus aureus (MRSA), S epidermidis, enterococcus, and resistant viridans streptococci. Continued neutropenic fever necessitates broadening of antibacterial coverage from cefepime to agents such as imipenem 500 mg every 6 hours or meropenem 1 g every 8 hours intravenously with or without tobramycin 5–7 mg/kg intravenously every 24 hours. Antifungal agents (such as voriconazole, 200 mg intravenously or orally every 12 hours, or caspofungin, 50 mg daily intravenously) should be added if fevers continue after 5–7 days of broad-spectrum antibacterial therapy. Regardless of whether the patient becomes afebrile, therapy is usually continued until resolution of neutropenia. There is some evidence to support earlier discontinuation of antibiotics in the neutropenic patient who becomes afebrile if no signs or symptoms of infection persist.
Patients with fever and low-risk neutropenia (neutropenia expected to persist for less than 10 days, no comorbid complications requiring hospitalization, and cancer adequately treated) can be treated with oral antibiotic regimens, such as ciprofloxacin, 750 mg every 12 hours, plus amoxicillin-clavulanic acid, 500 mg every 8 hours. Antibiotics are usually continued as long as the patient is neutropenic even if a source is not identified. In the organ transplant patient with interstitial infiltrates, the main concern is infection with Pneumocystis or Legionella species, so that empiric treatment with a macrolide or fluoroquinolone (Legionella) and TMP-SMZ, 15 mg/kg/day orally or intravenously, based on trimethoprim component (Pneumocystis) would be reasonable in those patients not receiving TMP-SMZ prophylaxis. If the patient does not respond to empiric treatment, a decision must be made to add more antimicrobial agents or perform invasive procedures (see above) to make a specific diagnosis. By making a definite diagnosis, therapy can be specific, thereby reducing selection pressure for resistance and superinfection.
 When to Refer
When to Refer
• Any immunocompromised patient with an opportunistic infection.
• Patients with potential drug toxicities and drug interactions related to antimicrobials where alternative agents are sought.
• Patients with latent tuberculosis, HBV, and HCV infection in whom therapy with TNF inhibitors is planned.
 When to Admit
When to Admit
Immunocompromised patients who are febrile, or those without fevers in whom an infection is suspected, particularly in the following groups: solid-organ or hematopoietic stem cell transplant recipient (particularly in the first 6 months), neutropenic patients, patients receiving TNF inhibitors, and transplant recipients who have had recent rejection episodes (including graft-versus-host disease).
Beyar-Katz O et al. Empirical antibiotics targeting gram-positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database Syst Rev. 2017 Jun 3;6:CD003914. [PMID: 28577308]
Drayson MT et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Oncol. 2019 Dec;20(12):1760–72. [PMID: 31668592]
Fung M et al. Plasma cell-free DNA Next-generation sequencing to diagnose and monitor infections in allogeneic hematopoietic stem cell transplant patients. Open Forum Infect Dis. 2018 Nov 16;5(12):ofy301. [PMID: 30581881]
Hamandi B et al. Voriconazole and squamous cell carcinoma after lung transplantation: a multicenter study. Am J Transplant. 2018 Jan;18(1):113–24. [PMID: 28898527]
Marty FM et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017 Dec 21;377(25):2433–44. [PMID: 29211658]
Reese PP et al. Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Ann Intern Med. 2018 Sep 4;169(5):273–81. [PMID: 30083748]
Selhorst P et al. Longer-term outcomes of HIV-positive-to-HIV-positive renal transplantation. N Engl J Med. 2019 Oct 3;381(14):1387–9. [PMID: 31577883]
Van de Wyngaert Z et al. Discontinuation of antimicrobial therapy in adult neutropenic haematology patients: a prospective cohort. Int J Antimicrob Agents. 2019 Jun;53(6):781–8. [PMID: 30831232]
Wilk AR et al. National landscape of HIV+ to HIV+ kidney and liver transplantation in the United States. Am J Transplant. 2019 Sep;19(9):2594–605. [PMID: 31207040]
HEALTH CARE–ASSOCIATED INFECTIONS
ESSENTIALS OF DIAGNOSIS

 Health care–associated infections are acquired during the course of receiving health care treatment for other conditions.
Health care–associated infections are acquired during the course of receiving health care treatment for other conditions.
 Hospital-associated infections are a subset of health care–associated infections defined as those not present or incubating at the time of hospital admission and developing 48 hours or more after admission.
Hospital-associated infections are a subset of health care–associated infections defined as those not present or incubating at the time of hospital admission and developing 48 hours or more after admission.
 Most health care–associated infections are preventable.
Most health care–associated infections are preventable.
 Hand washing is the most effective means of preventing health care–associated infections and should be done routinely even when gloves are worn.
Hand washing is the most effective means of preventing health care–associated infections and should be done routinely even when gloves are worn.
 General Considerations
General Considerations
Worldwide, approximately 10% of patients acquire a health care–associated infection, resulting in prolongation of the hospital stay, increase in cost of care, and significant morbidity and mortality. The most common infections are urinary tract infections, usually associated with indwelling urinary (Foley) catheters or urologic procedures; bloodstream infections, most commonly from indwelling catheters but also from secondary sites, such as surgical wounds, abscesses, pneumonia, the genitourinary tract, and the gastrointestinal tract; pneumonia in intubated patients or those with altered levels of consciousness; surgical wound infections; MRSA infections; and Clostridioides difficile colitis.
Some general principles are helpful in preventing, diagnosing, and treating health care–associated infections:
1. Many infections are a direct result of the use of invasive devices for monitoring or therapy, such as intravenous catheters, indwelling urinary (Foley) catheters, shunts, surgical drains, catheters placed by interventional radiology for drainage, nasogastric tubes, and orotracheal or nasotracheal tubes for ventilatory support. Early removal of such devices reduces the possibility of infection.
2. Patients in whom health care–associated infections develop are often critically ill, have been hospitalized for extended periods, and have received several courses of broad-spectrum antibiotic therapy. As a result, health care–associated infections are often due to multidrug resistant pathogens and differ from those encountered in community-acquired infections. For example, S aureus and S epidermidis (a frequent cause of prosthetic device infection) are often resistant to methicillin and most cephalosporins (ceftaroline is active against MRSA) and require vancomycin for therapy; Enterococcus faecium resistant to ampicillin and vancomycin; gram-negative infections caused by Pseudomonas, Citrobacter, Enterobacter, Acinetobacter, Stenotrophomonas, extended-spectrum beta-lactamases (ESBL)–producing E coli, and Klebsiella may be resistant to most antibacterials. When choosing antibiotics to treat the seriously ill patient with a health care–associated infection, antimicrobial history and the “local ecology” must be considered. In the most seriously ill patients, broad-spectrum coverage with vancomycin and a carbapenem with or without an aminoglycoside is recommended. Once a pathogen is isolated and susceptibilities are known, the most narrow-spectrum, least toxic, most cost-effective regimen should be used.
Widespread use of antimicrobial medications contributes to the selection of drug-resistant organisms; thus, every effort should be made to limit the spectrum of coverage and unnecessary duration. All too often, unreliable or uninterpretable specimens are obtained for culture that result in unnecessary use of antibiotics. The best example of this principle is the diagnosis of line-related or bloodstream infection in the febrile patient. To avoid unnecessary use of antibiotics, thoughtful consideration of culture results is mandatory. A positive wound culture without signs of inflammation or infection, a positive sputum culture without pulmonary infiltrates on chest radiograph, or a positive urine culture in a catheterized patient without symptoms or signs of pyelonephritis are all likely to represent colonization, not infection.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
Catheter-associated infections have a variable presentation, depending on the type of catheter used (peripheral or central venous catheters, nontunneled or tunneled). Local signs of infection may be present at the insertion site, with pain, erythema, and purulence. Fever is often absent in uncomplicated infections and, if present, may indicate more disseminated disease such as bacteremia, cellulitis and septic thrombophlebitis. Often signs of infection at the insertion site are absent.
1. Fever in an intensive care unit patient—Fever complicates up to 70% of patients in intensive care units, and the etiology of the fever may be infectious or noninfectious. Common infectious causes include catheter-associated infections, hospital-acquired and ventilator-associated pneumonia (see Chapter 9), surgical site infections, urinary tract infections, and sepsis. Clinically relevant sinusitis is relatively uncommon in the patient in the intensive care unit.
An important noninfectious cause is thromboembolic disease. Fever in conjunction with refractory hypotension and shock may suggest sepsis; however, adrenal insufficiency, thyroid storm, and transfusion reaction may have a similar clinical presentation. Drug fever is difficult to diagnose and is usually a diagnosis of exclusion unless there are other signs of hypersensitivity, such as a typical maculopapular rash (most common with beta-lactams).
2. Fever in the postoperative patient—Postoperative fever is very common and noninfectious fever resolves spontaneously. Timing of the onset of the fever in relation to the surgical procedure may be of diagnostic benefit.
a. Immediate fever (in the first few hours after surgery)—Immediate fever can be due to medications that were given perioperatively, to surgical trauma, or to infections that were present before surgery. Necrotizing fasciitis due to group A streptococci or mixed organisms may present in this period. Malignant hyperthermia is rare and presents 30 minutes to several hours following inhalational anesthesia and is characterized by extreme hyperthermia, muscle rigidity, rhabdomyolysis, electrolyte abnormalities, and hypotension. Aggressive cooling and dantrolene are the mainstays of therapy. Aspiration of acidic gastric contents during surgery can result in a chemical pneumonitis (Mendelson syndrome) that develops rapidly, is transient, and does not require antibiotics. Fever due to surgical trauma usually resolves in 2–3 days; however, it may be longer in more complicated operative cases and in patients with head trauma.
b. Acute fever (within 1 week of surgery)—Acute fever is usually due to common causes of hospital-associated infections, such as ventilator-associated pneumonia (including aspiration pneumonia in patients with decreased gag reflex) and line infections. Noninfectious causes include alcohol withdrawal, gout, pulmonary embolism, and pancreatitis. Atelectasis following surgery is commonly invoked as a cause of postoperative fever but there is no good evidence to support a causal association between the presence or degree of atelectasis and fever.
c. Subacute fever (at least 1 week after surgery)—Surgical site infections commonly present at least 1 week after surgery. The type of surgery that was performed predicts specific infectious etiologies. Patients undergoing cardiothoracic surgery may be at higher risk for pneumonia and deep and superficial sternal wound infections. Meningitis without typical signs of meningismus may complicate neurosurgical procedures. Postoperative deep abdominal abscesses may require drainage.
B. Laboratory Findings
Blood cultures are universally recommended, and chest radiographs are frequently obtained. A properly prepared sputum Gram stain and semi-quantitative sputum cultures may be useful in selected patients where there is a high pretest probability of pneumonia but multiple exclusion criteria probably limit generalizability in most patients, such as immunocompromised patients and those with drug resistance. Other diagnostic strategies will be dictated by the clinical context (eg, transesophageal echocardiogram in a patient with S aureus bacteremia).
Any fever in a patient with a central venous catheter should prompt the collection of blood. The best method to evaluate bacteremia is to gather at least two peripherally obtained blood cultures. Blood cultures from unidentified sites, a single blood culture from any site, or a blood culture through an existing line will often be positive for coagulase-positive staphylococci, particularly S epidermidis, often resulting in the inappropriate use of vancomycin. Unless two separate venipuncture cultures are obtained—not through catheters—interpretation of results is impossible, and unnecessary therapy often results. Each “pseudobacteremia” increases laboratory costs, antibiotic use, and length of stay. Microbiologic evaluation of the removed catheter can sometimes be helpful, but only in addition to (not instead of) blood cultures drawn from peripheral sites. The differential time to positivity measures the difference in time that cultures simultaneously drawn through a catheter and a peripheral site become positive. A positive test (at least 120 minutes’ difference in time) supports a catheter-related bloodstream infection, while a negative test suggests catheters may be retained.
 Complications
Complications
Complications such as septic thrombophlebitis, endocarditis, or metastatic foci of infection (particularly with S aureus) may be suspected in patients with persistent bacteremia and fever despite removal of the infected catheter. Additional studies such as venous Doppler studies, transesophageal echocardiogram, and chest radiographs may be indicated, and 4–6 weeks of antibiotics may be needed. In the case of septic thrombophlebitis, anticoagulation with heparin is also recommended if there are no contraindications.
 Differential Diagnosis
Differential Diagnosis
Although most fevers are due to infections, about 25% of patients will have fever of noninfectious origin, including drug fever, nonspecific postoperative fevers (tissue damage or necrosis), hematoma, pancreatitis, pulmonary embolism, myocardial infarction, and ischemic bowel disease.
 Prevention
Prevention
The concept of universal precautions emphasizes that all patients are treated as though they have a potential blood-borne transmissible disease, and thus all body secretions are handled with care to prevent spread of disease. Body substance isolation requires use of gloves whenever a health care worker anticipates contact with blood or other body secretions. Even though gloves are worn, health care workers should routinely wash their hands, since it is the easiest and most effective means of preventing hospital-associated infections. Application of a rapid drying, alcohol-based antiseptic is simple, takes less time than traditional hand washing with soap and water, is more effective at reducing hand colonization, and promotes compliance with hand decontamination. For prevention of transmission of C difficile infection, hand washing is more effective than alcohol-based antiseptics. Consequently, even after removing gloves, providers should always wash hands in cases of proven or suspected C difficile infection.
Peripheral intravenous lines should be replaced no more frequently than every 3–4 days. Some clinicians replace only when clinically indicated or if the line was put in emergently. Arterial lines and lines in the central venous circulation (including those placed peripherally) can be left in place indefinitely and are changed or removed when they are clinically suspected of being infected, when they are nonfunctional, or when they are no longer needed. Using sterile barrier precautions (including cap, mask, gown, gloves, and drape) is recommended while inserting central venous catheters. Antibiotic-impregnated (minocycline plus rifampin or chlorhexidine plus silver sulfadiazine) venous catheters reduce line infections. Silver alloy–impregnated indwelling urinary (Foley) catheters reduce the incidence of catheter-associated bacteriuria, but not consistently catheter-associated urinary tract infections. Best practices to prevent ventilator-associated pneumonia include avoiding intubation if possible, minimizing and daily interruption of sedation, pooling/draining of subglottic secretions above the tube cuff, and elevating the head of the bed. Silver-coated endotracheal tubes may reduce the incidence of ventilator-associated pneumonia but has limited impact on hospital stay duration or mortality, so they are not generally recommended. Catheter-related urinary tract infections and intravenous catheter-associated infections are not Medicare-reimbursable conditions in the United States. Preoperative skin preparation with chlorhexidine and alcohol (versus povidone-iodine) reduces the incidence of infection following surgery. Another strategy that can prevent surgical-site infections is the identification and treatment of S aureus nasal carriers with 2% mupirocin nasal ointment and chlorhexidine soap. Daily bathing of ICU patients with chlorhexidine-impregnated washcloths versus soap and water results in lower incidence of health care–associated infections and colonization. Selective decontamination of the digestive tract with nonabsorbable or parenteral antibiotics, or both, may prevent hospital-acquired pneumonia and decrease mortality but is in limited use because of the concern of the development of antibiotic resistance. Prevention bundles (implementing more than one intervention concomitantly) are commonly used as a practical strategy to enhance care in the healthcare setting.
Attentive nursing care (positioning to prevent pressure injuries, wound care, elevating the head during tube feedings to prevent aspiration) is critical in preventing hospital-associated infections. In addition, monitoring of high-risk areas by hospital epidemiologists is critical in the prevention of infection. Some guidelines advocate rapid screening (active surveillance cultures) for MRSA on admission to acute care facilities among certain subpopulations of patients (eg, those recently hospitalized, admission to the intensive care unit, patients undergoing hemodialysis). However, outside the setting of an MRSA outbreak, it is not clear whether this strategy decreases the incidence of hospital-associated MRSA infections.
Vaccines, including hepatitis A, hepatitis B, and the varicella, pneumococcal, and influenza vaccinations, are important adjuncts. (See section below titled Immunization Against Infectious Diseases.)
 Treatment
Treatment
A. Fever in an Intensive Care Unit Patient
Unless the patient has a central neurologic injury with elevated intracranial pressure or has a temperature higher than 41°C, there is less physiologic need to maintain euthermia. Empiric broad-spectrum antibiotics (see Table 30–5) are recommended for neutropenic and other immunocompromised patients and in patients who are clinically unstable.
B. Catheter-Associated Infections
Factors that inform treatment decisions include the type of catheter, the causative pathogen, the availability of alternate catheter access sites, the need for ongoing intravascular access, and the severity of disease.
In general, catheters should be removed if there is purulence at the exit site; if the organism is S aureus, gram-negative rods, or Candida species; if there is persistent bacteremia (more than 48 hours while receiving antibiotics); or if complications, such as septic thrombophlebitis, endocarditis, or other metastatic disease exist. Central venous catheters may be exchanged over a guidewire provided there is no erythema or purulence at the exit site and the patient does not appear to be septic. Methicillin-resistant, coagulase-negative staphylococci are the most common pathogens; thus, empiric therapy with vancomycin, 15 mg/kg/dose intravenously twice daily, should be given assuming normal kidney function. Empiric gram-negative coverage should be used in patients who are immunocompromised or who are critically ill (see Table 30–5).
Antibiotic treatment duration depends on the pathogen and the extent of disease. For uncomplicated bacteremia, 5–7 days of therapy is usually sufficient for coagulase-negative staphylococci, even if the original catheter is retained. Fourteen days of therapy is generally recommended for uncomplicated bacteremia caused by gram-negative rods, Candida species, and S aureus. Antibiotic lock therapy involves the instillation of supratherapeutic concentrations of antibiotics with heparin in the lumen of catheters. The purpose is to achieve adequate concentrations of antibiotics to kill microbes in the biofilm. Antibiotic lock therapy can be used for catheter-related bloodstream infections caused by both gram-positive and gram-negative bacterial pathogens and when the catheter is being retained in a salvage situation.
 When to Refer
When to Refer
• Any patient with multidrug-resistant infection.
• Any patient with fungemia, S aureus bacteremia, or persistent bacteremia of any organism.
• Patients whose catheters cannot be removed.
• Patients with multisite infections.
• Patients with impaired or fluctuating kidney function for assistance with dosing of antimicrobials.
• Patients with refractory or recurrent C difficile colitis.
Baur D et al. Effect of antibiotic stewardship on the incidence of infection and colonization with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017 Sep;17(9):990–1001. [PMID: 28629876]
DeFilipp Z et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019 Nov 21;381(21):2043–50. [PMID: 31665575]
Harris PNA et al; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018 Sep 11;320(10):984–94. [PMID: 30208454]
Kao D et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017 Nov 28;318(20):1985–93. [PMID: 29183074]
McDonald LC et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):e1–48. [PMID: 29462280]
Radonovich LJ Jr et al; ResPECT Investigators. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019 Sep 3;322(9):824–33. [PMID: 31479137]
Wilcox MH et al; MODIFY I and MODIFY II Investigators. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017 Jan 26;376(4):305–17. [PMID: 28121498]
Yahav D et al; Bacteremia Duration Study Group. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019 Sep 13;69(7):1091–8. [PMID: 30535100]
INFECTIONS OF THE CENTRAL NERVOUS SYSTEM
ESSENTIALS OF DIAGNOSIS

 Central nervous system infection is a medical emergency.
Central nervous system infection is a medical emergency.
 Symptoms and signs common to all central nervous system infections include headache, fever, sensorial disturbances, neck and back stiffness, positive Kernig and Brudzinski signs, and cerebrospinal fluid abnormalities.
Symptoms and signs common to all central nervous system infections include headache, fever, sensorial disturbances, neck and back stiffness, positive Kernig and Brudzinski signs, and cerebrospinal fluid abnormalities.
 General Considerations
General Considerations
Infections of the central nervous system can be caused by almost any infectious agent, including bacteria, mycobacteria, fungi, spirochetes, protozoa, helminths, and viruses.
 Etiologic Classification
Etiologic Classification
Central nervous system infections can be divided into several categories that usually can be readily distinguished from each other by cerebrospinal fluid examination as the first step toward etiologic diagnosis (Table 30–1).
Table 30–1. Typical cerebrospinal fluid findings in various central nervous system diseases.

A. Purulent Meningitis
Patients with bacterial meningitis usually seek medical attention within hours or 1–2 days after onset of symptoms. The organisms responsible depend primarily on the age of the patient as summarized in Table 30–2. The diagnosis is usually based on the Gram-stained smear (positive in 60–90%) or culture (positive in over 90%) of the cerebrospinal fluid.
Table 30–2. Initial antimicrobial therapy for purulent meningitis of unknown cause.
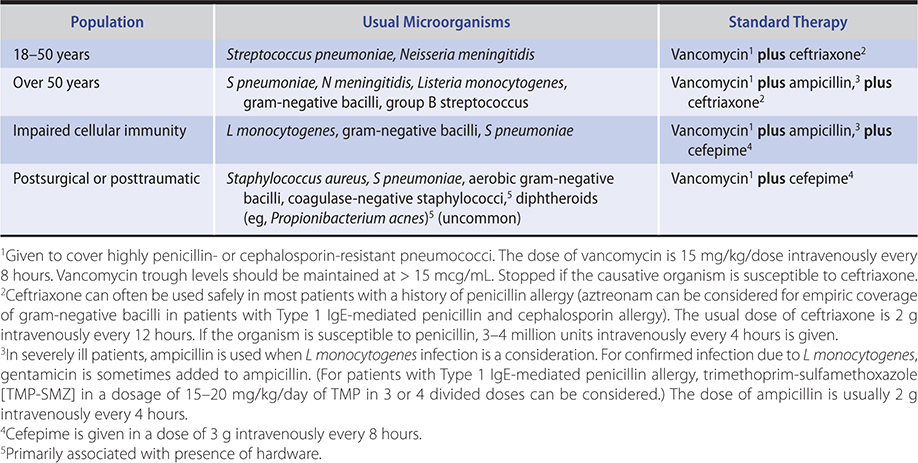
B. Chronic Meningitis
The presentation of chronic meningitis is less acute than purulent meningitis. Patients with chronic meningitis usually have a history of symptoms lasting weeks to months. The most common pathogens are Mycobacterium tuberculosis, atypical mycobacteria, fungi (Cryptococcus, Coccidioides, Histoplasma), and spirochetes (Treponema pallidum and Borrelia burgdorferi). The diagnosis is made by culture or in some cases by serologic tests (cryptococcosis, coccidioidomycosis, syphilis, Lyme disease).
C. Aseptic Meningitis
Aseptic meningitis—a much more benign and self-limited syndrome than purulent meningitis—is caused principally by viruses, especially herpes simplex virus and the enterovirus group (including coxsackieviruses and echoviruses). Infectious mononucleosis may be accompanied by aseptic meningitis. Leptospiral infection is also usually placed in the aseptic group because of the lymphocytic cellular response and its relatively benign course. This type of meningitis also occurs during secondary syphilis and disseminated Lyme disease. Prior to the routine administration of measles-mumps-rubella (MMR) vaccines, mumps was the most common cause of viral meningitis. Drug-induced aseptic meningitis has been reported with nonsteroidal anti-inflammatory drugs, sulfonamides, and certain monoclonal antibodies.
D. Encephalitis
Encephalitis (due to herpesviruses, arboviruses, rabies virus, flaviviruses [West Nile encephalitis, Japanese encephalitis], and many others) produces disturbances of the sensorium, seizures, and many other manifestations. Patients are more ill than those with aseptic meningitis. Cerebrospinal fluid may be entirely normal or may show some lymphocytes and, in some instances, (eg, herpes simplex) red cells as well. Influenza has been associated with encephalitis, but the relationship is not clear. An autoimmune form of encephalitis associated with N-methyl-D-aspartate receptor antibodies should be suspected in younger patients with encephalitis and associated seizures, movement disorders, and psychosis.
E. Partially Treated Bacterial Meningitis
Previous effective antibiotic therapy given for 12–24 hours will decrease the rate of positive cerebrospinal fluid Gram stain results by 20% and culture by 30–40% but will have little effect on cell count, protein, or glucose. Occasionally, previous antibiotic therapy will change a predominantly polymorphonuclear response to a lymphocytic pleocytosis, and some of the cerebrospinal fluid findings may be similar to those seen in aseptic meningitis.
F. Neighborhood Reaction
As noted in Table 30–1, this term denotes a purulent infectious process in close proximity to the central nervous system that spills some of the products of the inflammatory process—white blood cells or protein—into the cerebrospinal fluid. Such an infection might be a brain abscess, osteomyelitis of the vertebrae, epidural abscess, subdural empyema, or bacterial sinusitis or mastoiditis.
G. Noninfectious Meningeal Irritation
Carcinomatous meningitis, sarcoidosis, systemic lupus erythematosus, chemical meningitis, and certain medications—nonsteroidal anti-inflammatory drugs, OKT3, TMP-SMZ, and others—can also produce symptoms and signs of meningeal irritation with associated cerebrospinal fluid pleocytosis, increased protein, and low or normal glucose. Meningismus with normal cerebrospinal fluid findings occurs in the presence of other infections such as pneumonia and shigellosis.
H. Brain Abscess
Brain abscess presents as a space-occupying lesion; symptoms may include vomiting, fever, change of mental status, or focal neurologic manifestations. When brain abscess is suspected, a CT scan should be performed. If positive, lumbar puncture should not be performed since results rarely provide clinically useful information and herniation can occur. The bacteriology of brain abscess is usually polymicrobial and includes S aureus, gram-negative bacilli, streptococci, and mouth anaerobes (including anaerobic streptococci and Prevotella species).
I. Health Care–Associated Meningitis
This infection may arise as a result of invasive neurosurgical procedures (eg, craniotomy, internal or external ventricular catheters, external lumbar catheters), complicated head trauma, or hospital-acquired bloodstream infections. Outbreaks have been associated with contaminated epidural or paraspinal corticosteroid injections. In general, the microbiology is distinct from community-acquired meningitis, with gram-negative organisms (eg, Pseudomonas), S aureus, and coagulase-negative staphylococci and, in the outbreaks associated with contaminated corticosteroids, mold and fungi (Exserohilum rostratum and Aspergillus fumigatus) playing a larger role.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The classic triad of fever, stiff neck, and altered mental status has a low sensitivity (44%) for bacterial meningitis. However, nearly all patients with bacterial meningitis have at least two of the following symptoms—fever, headache, stiff neck, or altered mental status.
B. Laboratory Tests
Evaluation of a patient with suspected meningitis includes a blood count, blood culture, lumbar puncture followed by careful study and culture of the cerebrospinal fluid, and a chest film. The fluid must be examined for cell count, glucose, and protein, and a smear stained for bacteria (and acid-fast organisms when appropriate) and cultured for pyogenic organisms and for mycobacteria and fungi when indicated. Latex agglutination tests can detect antigens of encapsulated organisms (S pneumoniae, H influenzae, N meningitidis, and Cryptococcus neoformans) but are rarely used except for detection of Cryptococcus or in partially treated patients. Polymerase chain reaction (PCR) testing of cerebrospinal fluid has been used to detect bacteria (S pneumoniae, H influenzae, N meningitidis, M tuberculosis, B burgdorferi, and Tropheryma whipplei) and viruses (herpes simplex, varicella-zoster, CMV, Epstein-Barr virus, and enteroviruses) in patients with meningitis. The greatest experience is with PCR for herpes simplex, varicella-zoster, and JC virus. These tests are very sensitive (greater than 95%) and specific. In addition to its use in meningitis, molecular methods such as PCR and next-generation sequencing are being used increasingly for the diagnosis of encephalitis, transverse myelitis, and brain abscess. In general, molecular diagnostic tests may provide a more sensitive and rapid alternative to traditional culture and serology methods. However, it is difficult to ascertain the true sensitivity of many molecular tests for CNS infections given the absence of a gold standard. In some cases, tests to detect several organisms may not be any more sensitive than culture (or serology), but the real value is the rapidity with which results are available, ie, hours compared with days or weeks.
C. Lumbar Puncture and Imaging
Since performing a lumbar puncture in the presence of a space-occupying lesion (brain abscess, subdural hematoma, subdural empyema, necrotic temporal lobe from herpes encephalitis) may result in brainstem herniation, a CT scan is performed prior to lumbar puncture if a space-occupying lesion is suspected on the basis of papilledema, seizures, or focal neurologic findings. Other indications for CT scan are an immunocompromised patient or moderately to severely impaired level of consciousness. If delays are encountered in obtaining a CT scan and bacterial meningitis is suspected, blood cultures should be drawn and antibiotics and corticosteroids administered even before cerebrospinal fluid is obtained for culture to avoid delay in treatment (Table 30–1). Antibiotics given within 4 hours before obtaining cerebrospinal fluid probably do not affect culture results. MRI with contrast of the epidural injection site and surrounding areas is recommended (sometimes repeatedly) for those with symptoms following a possibly contaminated corticosteroid injection to exclude epidural abscess, phlegmon, vertebral osteomyelitis, discitis, or arachnoiditis.
 Treatment
Treatment
Although it is difficult to prove with existing clinical data that early antibiotic therapy improves outcome in bacterial meningitis, prompt therapy is still recommended. In purulent meningitis, the identity of the causative microorganism may remain unknown or doubtful for a few days and initial antibiotic treatment as set forth in Table 30–2 should be directed against the microorganisms most common for each age group.
The duration of therapy for bacterial meningitis varies depending on the etiologic agent: H influenzae, 7 days; N meningitidis, 3–7 days; S pneumoniae, 10–14 days; L monocytogenes, 14–21 days; and gram-negative bacilli, 21 days.
For adults with pneumococcal meningitis, dexamethasone 10 mg administered intravenously 15–20 minutes before or simultaneously with the first dose of antibiotics and continued every 6 hours for 4 days decreases morbidity and mortality. Patients most likely to benefit from corticosteroids are those infected with gram-positive organisms (S pneumoniae or S suis), and those who are HIV negative. It is unknown whether patients with meningitis due to N meningitidis and other bacterial pathogens benefit from the use of adjunctive corticosteroids. Increased intracranial pressure due to brain edema often requires therapeutic attention. Hyperventilation, mannitol (25–50 g intravenously as a bolus), and even drainage of cerebrospinal fluid by repeated lumbar punctures or by placement of intraventricular catheters have been used to control cerebral edema and increased intracranial pressure. Dexamethasone (4 mg intravenously every 4–6 hours) may also decrease cerebral edema.
Therapy of brain abscess consists of drainage (excision or aspiration) in addition to 3–4 weeks of systemic antibiotics directed against organisms isolated. An empiric regimen often includes metronidazole, 500 mg intravenously or orally every 8 hours, plus ceftriaxone, 2 g intravenously every 12 hours, with or without vancomycin, 10–15 mg/kg/dose intravenously every 12 hours. Vancomycin trough serum levels should be greater than 15 mcg/mL in such patients. In cases where abscesses are smaller than 2 cm, where there are multiple abscesses that cannot be drained, or if an abscess is located in an area where significant neurologic sequelae would result from drainage, antibiotics for 6–8 weeks can be used without drainage.
In addition to antibiotics, in cases of health care–associated meningitis associated with an external intraventricular catheter, the probability of cure is increased if the catheter is removed. In infections associated with internal ventricular catheters, removal of the internal components and insertion of an external drain is recommended. After collecting cerebrospinal fluid, epidural aspirate, or other specimens for culture, empiric antifungal therapy with voriconazole as well as routine empiric treatment for other pathogens (as above) is recommended until the specific cause of the patient’s central nervous system or parameningeal infection has been identified. In addition, early consultation with a neurosurgeon is recommended for those found to have an epidural abscess, phlegmon, vertebral osteomyelitis, discitis, or arachnoiditis to discuss possible surgical management (eg, debridement).
Therapy of other types of meningitis is discussed elsewhere in this book (fungal meningitis, Chapter 36; syphilis and Lyme borreliosis, Chapter 34; tuberculous meningitis, Chapter 33; herpes encephalitis, Chapter 32).
 When to Refer
When to Refer
• Patients with acute meningitis, particularly if culture negative or atypical (eg, fungi, syphilis, Lyme disease, M tuberculosis), or if the patient is immunosuppressed.
• Patients with chronic meningitis.
• All patients with brain abscesses and encephalitis.
• Patients with suspected hospital-acquired meningitis (eg, in patients who have undergone recent neurosurgery or epidural or paraspinal corticosteroid injection).
• Patients with recurrent meningitis.
 When to Admit
When to Admit
• Patients with suspected acute meningitis, encephalitis, and brain or paraspinous abscess should be admitted for urgent evaluation and treatment.
• There is less urgency to admit patients with chronic meningitis; these patients may be admitted to expedite diagnostic procedures and coordinate care, particularly if no diagnosis has been made in the outpatient setting.
Fitzgerald D et al. Invasive pneumococcal and meningococcal disease. Infect Dis Clin North Am. 2019 Dec;33(4):1125–41. [PMID: 31668194]
Morens DM et al. Eastern equine encephalitis virus—another emergent arbovirus in the United States. N Engl J Med. 2019 Nov 21;381(21):1989–92. [PMID: 31747726]
Tunkel AR et al. 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017 Mar 15;64(6):e34–65. [PMID: 28203777]
Wilson MR et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019 Jun 13;380(24):2327–40. [PMID: 31189036]
ANIMAL & HUMAN BITE WOUNDS
ESSENTIALS OF DIAGNOSIS

 Cat and human bites have higher rates of infection than dog bites.
Cat and human bites have higher rates of infection than dog bites.
 Hand bites are particularly concerning for the possibility of closed-space infection.
Hand bites are particularly concerning for the possibility of closed-space infection.
 Antibiotic prophylaxis indicated for noninfected bites of the hand and hospitalization required for infected hand bites.
Antibiotic prophylaxis indicated for noninfected bites of the hand and hospitalization required for infected hand bites.
 All infected wounds need to be cultured to direct therapy.
All infected wounds need to be cultured to direct therapy.
 General Considerations
General Considerations
About 1000 dog bite injuries require emergency department attention each day in the United States, most often in urban areas. Dog bites occur most commonly in the summer months. Biting animals are usually known by their victims, and most biting incidents are provoked (ie, bites occur while playing with the animal or after surprising the animal while eating or waking it abruptly from sleep). Failure to elicit a history of provocation is important, because an unprovoked attack raises the possibility of rabies. Human bites are usually inflicted by children while playing or fighting; in adults, bites are associated with alcohol use and closed-fist injuries that occur during fights.
The animal inflicting the bite, the location of the bite, and the type of injury inflicted are all important determinants of whether they become infected. Cat bites are more likely to become infected than human bites—between 30% and 50% of all cat bites become infected. Infections following human bites are variable. Bites inflicted by children rarely become infected because they are superficial, and bites by adults become infected in 15–30% of cases, with a particularly high rate of infection in closed-fist injuries. Dog bites, for unclear reasons, become infected only 5% of the time. Bites of the head, face, and neck are less likely to become infected than bites on the extremities. “Through and through” bites (eg, involving the mucosa and the skin) have an infection rate similar to closed-fist injuries. Puncture wounds become infected more frequently than lacerations, probably because the latter are easier to irrigate and debride.
The bacteriology of bite infections is polymicrobial. Following dog and cat bites, over 50% of infections are caused by aerobes and anaerobes and 36% are due to aerobes alone. Pure anaerobic infections are rare. Pasteurella species are the single most common isolate (75% of infections caused by cat bites and 50% of infections caused by dog bites). Other common aerobic isolates include streptococci, staphylococci, Moraxella, and Neisseria; the most common anaerobes are Fusobacterium, Bacteroides, Porphyromonas, and Prevotella. The median number of isolates following human bites is four (three aerobes and one anaerobe). Like dog and cat bites, infections caused by most human bites are a mixture of aerobes and anaerobes (54%) or are due to aerobes alone (44%). Streptococci and S aureus are the most common aerobes. Eikenella corrodens (found in up to 30% of patients), Prevotella, and Fusobacterium are the most common anaerobes. Although the organisms noted are the most common, innumerable others have been isolated—including Capnocytophaga (dog and cat), Pseudomonas, and Haemophilus—emphasizing the point that all infected bites should be cultured to define the microbiology.
HIV can be transmitted from bites (either from biting or receiving a bite from an HIV-infected patient) but has rarely been reported.
 Treatment
Treatment
A. Local Care
Vigorous cleansing and irrigation of the wound as well as debridement of necrotic material are the most important factors in decreasing the incidence of infections. Radiographs should be obtained to look for fractures and the presence of foreign bodies. Careful examination to assess the extent of the injury (tendon laceration, joint space penetration) is critical to appropriate care.
B. Suturing
If wounds require closure for cosmetic or mechanical reasons, suturing can be done. However, one should never suture an infected wound, and wounds of the hand should generally not be sutured since a closed-space infection of the hand can result in loss of function.
C. Prophylactic Antibiotics
Prophylaxis is indicated in high-risk bites and in high-risk patients. Cat bites in any location and hand bites by any animal, including humans, should receive prophylaxis. Individuals with certain comorbidities (diabetes, liver disease) are at increased risk for severe complications and should receive prophylaxis even for low-risk bites, as should patients without functional spleens who are at increased risk for overwhelming sepsis (primarily with Capnocytophaga species). Amoxicillin-clavulanate (Augmentin) 500 mg orally three times daily for 5–7 days is the regimen of choice. For patients with serious allergy to penicillin, a combination of clindamycin 300 mg orally three times daily together with one of the following is recommended for 5–7 days: doxycycline 100 mg orally twice daily, or double-strength TMP-SMZ orally twice daily, or a fluoroquinolone (ciprofloxacin 500 mg orally twice daily or levofloxacin 500–750 mg orally once daily). Moxifloxacin, a fluoroquinolone with good aerobic and anaerobic activity, may be suitable as monotherapy at 400 mg orally once daily for 5–7 days. Agents such as dicloxacillin, cephalexin, macrolides, and clindamycin should not be used alone because they lack activity against Pasteurella species. TMP-SMZ has poor activity against anaerobes and should only be used in combination with clindamycin.
Because the risk of HIV transmission is so low following a bite, routine postexposure prophylaxis is not recommended. Each case should be evaluated individually and consideration for prophylaxis should be given to those who present within 72 hours of the incident, the source is known to be HIV infected, and the exposure is high risk.
D. Antibiotics for Documented Infection
For wounds that are infected, antibiotics are clearly indicated. How they are given (orally or intravenously) and the need for hospitalization are individualized clinical decisions. The most commonly encountered pathogens require treatment with ampicillin-sulbactam (Unasyn), 1.5–3.0 g intravenously every 6–8 hours; or amoxicillin-clavulanate (Augmentin), 500 mg orally three times daily; or with ertapenem, 1 g intravenously daily. For the patient with severe penicillin allergy, a combination of clindamycin 600–900 mg intravenously every 8 hours plus a fluoroquinolone (ciprofloxacin, 400 mg intravenously every 12 hours; levofloxacin, 500–750 mg intravenously once daily) or TMP-SMZ (10 mg/kg of trimethoprim daily in two or three divided doses) is indicated. Duration of therapy is usually 2–3 weeks unless complications such as septic arthritis or osteomyelitis is present; if these complications are present, therapy should be extended to 4 and 6 weeks, respectively.
E. Tetanus and Rabies
All patients must be evaluated for the need for tetanus (see Chapter 33) and rabies (see Chapter 32) prophylaxis.
 When to Refer
When to Refer
• If septic arthritis or osteomyelitis is suspected.
• For exposure to bites by dogs, cats, reptiles, amphibians, and rodents.
• When rabies is a possibility.
 When to Admit
When to Admit
• Patients with infected hand bites.
• Deep bites, particularly if over joints.
Bula-Rudas FJ et al. Human and animal bites. Pediatr Rev. 2018 Oct;39(10):490–500. [PMID: 30275032]
Dhillon J et al. Scoping decades of dog evidence: a scoping review of dog bite-related sequelae. Can J Public Health. 2019 Jun;110(3):364–75. [PMID: 30378009]
Kheiran A et al. Cat bite: an injury not to underestimate. J Plast Surg Hand Surg. 2019 Dec;53(6):341–6. [PMID: 31287352]
SEXUALLY TRANSMITTED DISEASES
ESSENTIALS OF DIAGNOSIS

 All sexually transmitted diseases (STDs) have subclinical or latent periods, and patients may be asymptomatic.
All sexually transmitted diseases (STDs) have subclinical or latent periods, and patients may be asymptomatic.
 Simultaneous infection with several organisms is common.
Simultaneous infection with several organisms is common.
 All patients who seek STD testing should be screened for syphilis and HIV.
All patients who seek STD testing should be screened for syphilis and HIV.
 Partner notification and treatment are important to prevent further transmission and reinfection of the index case.
Partner notification and treatment are important to prevent further transmission and reinfection of the index case.
 General Considerations
General Considerations
The most common STDs are gonorrhea,* syphilis,* human papillomavirus (HPV)–associated condyloma acuminatum, chlamydial genital infections,* herpesvirus genital infections, trichomonas vaginitis, chancroid,* granuloma inguinale, scabies, louse infestation, and bacterial vaginosis (among women who have sex with women). However, shigellosis*; hepatitis A, B, and C*; amebiasis; giardiasis*; cryptosporidiosis*; salmonellosis*; and campylobacteriosis may also be transmitted by sexual (oral-anal) contact, especially in men who have sex with men. Ebola virus and Zika virus have both been associated with sexual transmission. Both homosexual and heterosexual contact are risk factors for the transmission of HIV (see Chapter 31). All STDs have subclinical or latent phases that play an important role in long-term persistence of the infection or in its transmission from infected (but largely asymptomatic) persons to other contacts. Simultaneous infection by several different agents is common.
Infections typically present in one of several ways, each of which has a defined differential diagnosis, which should prompt appropriate diagnostic tests.
A. Genital Ulcers
Common etiologies include herpes simplex virus, primary syphilis, and chancroid. Other possibilities include lymphogranuloma venereum (see Chapter 33), granuloma inguinale caused by Klebsiella granulomatis (see Chapter 33), as well as lesions caused by infection with Epstein-Barr virus and HIV. Noninfectious causes are Behçet disease (see Chapter 20), neoplasm, trauma, drugs, and irritants.
B. Urethritis With or Without Urethral Discharge
The most common infections causing urethral discharge are Neisseria gonorrhoeae and Chlamydia trachomatis. N gonorrhoeae and C trachomatis are also frequent causes of prostatitis among sexually active men. Other sexually transmitted infections that can cause urethritis include Mycoplasma genitalium and, less commonly, Ureaplasma urealyticum and Trichomonas vaginalis. Noninfectious causes of urethritis include reactive arthritis with associated urethritis.
C. Vaginal Discharge
Common causes of vaginitis are bacterial vaginosis (caused by overgrowth of anaerobes such as Gardnerella vaginalis), candidiasis, and T vaginalis (see Chapter 18). Less common infectious causes of vaginitis include HPV-associated condylomata acuminata and group A streptococcus. Noninfectious causes are physiologic changes related to the menstrual cycle, irritants, and lichen planus. Even though N gonorrhoeae and C trachomatis are frequent causes of cervicitis, they rarely produce vaginal discharge.
 Screening & Prevention
Screening & Prevention
All persons who seek STD testing should undergo routine screening for HIV infection, using rapid HIV testing (if patients may not follow up for results obtained by standard methods) or nucleic acid amplification followed by confirmatory serology (if primary HIV infection may be a possibility) as indicated. Most algorithms now start with an antigen/antibody combination HIV-1/2 immunoassay with a confirmatory HIV-1/HIV-2 antibody differentiation immunoassay. Patients in whom certain STDs have been diagnosed and treated (chlamydia or gonorrhea, and trichomonas in women) are at a high risk for reinfection and should be encouraged to be rescreened for STDs at 3 months following the initial STD diagnosis.
Asymptomatic patients often request STD screening at the time of initiating a new sexual relationship. Routine HIV testing and hepatitis B serology testing should be offered to all such patients. In sexually active women who have not been recently screened, cervical Papanicolaou testing and nucleic acid amplification testing of a urine specimen for gonorrhea and chlamydia are recommended. Among men who have sex with men, additional screening is recommended for syphilis; hepatitis A; urethral, pharyngeal, and rectal gonorrhea; as well as urethral and rectal chlamydia. Nucleic acid amplification testing is recommended for gonorrhea or chlamydia. There are no recommendations to screen heterosexual men for urethral chlamydia, but this could be considered in STD clinics, adolescent clinics, or correctional facilities. The periodicity of screening thereafter depends on sexual risk, but most screening should be offered at least annually to sexually active adults (particularly to those 25 years old and under). Clinicians should also evaluate transgender men and women for STD screening, based on current anatomy and behaviors practiced. If not immune, hepatitis B vaccination is recommended for all sexually active adults, and hepatitis A vaccination in men who have sex with men. Persons between the ages of 9 and 26 should be routinely offered vaccination against HPV (9-valent).
The risk of developing an STD following a sexual assault is difficult to accurately ascertain given high rates of baseline infections and poor follow-up. Victims of assault have a high baseline rate of infection (N gonorrhoeae, 6%; C trachomatis, 10%; T vaginalis, 15%; and bacterial vaginosis, 34%), and the risk of acquiring infection as a result of the assault is significant, but is often lower than the preexisting rate (N gonorrhoeae, 6–12%; C trachomatis, 4–17%; T vaginalis, 12%; syphilis, 0.5–3%; and bacterial vaginosis, 19%). Victims should be evaluated within 24 hours after the assault, and nucleic acid amplification tests for N gonorrhoeae and C trachomatis should be performed. Vaginal secretions are obtained for Trichomonas wet mount and culture, or point-of-care testing. If a discharge is present, if there is itching, or if secretions are malodorous, a wet mount should be examined for Candida and bacterial vaginosis. In addition, a blood sample should be obtained for immediate serologic testing for syphilis, hepatitis B, and HIV. Follow-up examination for STDs should be repeated within 1–2 weeks, since concentrations of infecting organisms may not have been sufficient to produce a positive test at the time of initial examination. If prophylactic treatment was given (may include postexposure hepatitis B vaccination without hepatitis B immune globulin; treatment for chlamydial, gonorrheal, or trichomonal infection; and emergency contraception), tests should be repeated only if the victim has symptoms. If prophylaxis was not administered, the individual should be seen in 1 week so that any positive tests can be treated. Follow-up serologic testing for syphilis and HIV infection should be performed in 6, 12, and 24 weeks if the initial tests are negative. The usefulness of presumptive therapy is controversial, with some feeling that all patients should receive it and others that it should be limited to those in whom follow-up cannot be ensured or to patients who request it.
Although seroconversion to HIV has been reported following sexual assault when this was the only known risk, this risk is believed to be low. The likelihood of HIV transmission from vaginal or anal receptive intercourse when the source is known to be HIV positive is 1 per 1000 and 5 per 1000, respectively. Although prophylactic antiretroviral therapy has not been studied in this setting, the Department of Health and Human Services recommends the prompt institution of postexposure prophylaxis with antiretroviral therapy if the person seeks care within 72 hours of the assault, the source is known to be HIV positive, and the exposure presents a substantial risk of transmission.
In addition to screening asymptomatic patients with STDs, other strategies for preventing further transmission include evaluating sex partners and administering preexposure vaccination of preventable STDs to individuals at risk; other strategies include the consistent use of male and female condoms and male circumcision. Adult male circumcision has been shown to decrease the transmission of HIV by 50%, and of herpes simplex virus and HPV by 30% in heterosexual couples in sub-Saharan Africa. For each patient, there are one or more sexual contacts who require diagnosis and treatment. Prompt treatment of contacts by giving antibiotics to the index case to distribute to all sexual contacts (patient-delivered therapy) is an important strategy for preventing further transmission and to prevent reinfection of the index case.
Note that vaginal spermicides and condoms containing nonoxynol-9 provide no additional protection against STDs. Early initiation of antiretroviral therapy in HIV-infected individuals can prevent HIV acquisition in an uninfected sex partner. Also, preexposure prophylaxis with a once-daily pill containing emtricitabine plus tenofovir disoproxil fumarate (TDF) has been shown to be effective in preventing HIV infection among high-risk men who have sex with men, heterosexual women and men, transgender women, and persons who inject drugs.
 When to Refer
When to Refer
• Patients with a new diagnosis of HIV.
• Patients with persistent, refractory, or recurrent STDs, particularly when drug resistance is suspected.
Chou R et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019 Jun 11;321(22):2214–30. [PMID: 31184746]
MacGowan RJ et al. Effect of internet-distributed HIV self-tests on HIV diagnosis and behavioral outcomes in men who have sex with men: a randomized clinical trial. JAMA Intern Med. 2019 Nov 18. [Epub ahead of print] [PMID: 31738378]
Marcus JL et al. Risk compensation and clinical decision making—the case of HIV preexposure prophylaxis. N Engl J Med. 2019 Feb 7;380(6):510–2. [PMID: 30726699]
Price JC et al. Sexually acquired hepatitis C infection in HIV-uninfected men who have sex with men using pre-exposure prophylaxis against HIV. J Infect Dis. 2019 Apr 16;219(9):1373–76. [PMID: 30462305]
Sonawane K et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017 Nov 21;167(10):714–24. [PMID: 29049523]
Unemo M et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017 Aug;17(8):e235–79. [PMID: 28701272]
Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017 Feb 23;376(8):765–73. [PMID: 28225683]
INFECTIONS IN PERSONS WHO INJECT DRUGS
ESSENTIALS OF DIAGNOSIS

 Common infections that occur with greater frequency in persons who inject drugs include:
Common infections that occur with greater frequency in persons who inject drugs include:
– Skin infections, aspiration pneumonia, tuberculosis.
– Hepatitis A, B, C, D; STDs; HIV/AIDS.
– Pulmonary septic emboli, infective endocarditis.
– Osteomyelitis and septic arthritis.
 General Considerations
General Considerations
There is a high incidence of infection among persons with opioid use disorder, particularly among people who inject drugs. Increased risk of infection is likely associated with poor hygiene and colonization with potentially pathogenic organisms, contamination of drugs and equipment, increased sexual risk behaviors, and impaired immune defenses. The use of parenterally administered recreational drugs has increased enormously in recent years, fueled in part by an epidemic of prescription opioid misuse and abuse. More than 2 million persons in North America are estimated to have used injection drugs in the past year.
Skin infections are associated with poor hygiene and use of nonsterile technique when injecting drugs. S aureus (including community-acquired methicillin-resistant strains) and oral flora (streptococci, Eikenella, Fusobacterium, Peptostreptococcus) are the most common organisms, with enteric gram-negatives generally more likely seen in those who inject into the groin. Cellulitis and subcutaneous abscesses occur most commonly, particularly in association with subcutaneous (“skin-popping”) or intramuscular injections and the use of cocaine and heroin mixtures (probably due to ischemia). Myositis, clostridial myonecrosis, and necrotizing fasciitis occur infrequently but are life-threatening. Wound botulism in association with black tar heroin occurs sporadically but often in clusters.
Aspiration pneumonia and its complications (lung abscess, empyema, brain abscess) result from altered consciousness associated with drug use. Mixed aerobic and anaerobic mouth flora are usually involved.
Tuberculosis also occurs in persons who use drugs, and infection with HIV has fostered the spread of tuberculosis in this population. Morbidity and mortality rates are increased in HIV-infected individuals with tuberculosis. Classic radiographic findings are often absent; tuberculosis is suspected in any patient with infiltrates who does not respond to antibiotics.
Hepatitis is very common among persons who inject drugs and is transmissible both by the parenteral (hepatitis B, C, and D) and by the fecal-oral route (hepatitis A). Multiple episodes of hepatitis with different agents can occur. Hepatitis C has also been associated with non-injection heroin use as well as intranasal use of other drugs, likely secondary to blood on shared straws.
Pulmonary septic emboli may originate from venous thrombi or right-sided endocarditis.
STDs are not directly related to drug use, but the practice of exchanging sex for drugs has resulted in an increased frequency of STDs. Syphilis, gonorrhea, and chancroid are the most common.
HIV/AIDS has a high incidence among persons who inject drugs and their sexual contacts and among the offspring of infected women (see Chapter 31).
Infective endocarditis in persons who inject drugs is most commonly caused by S aureus, Candida (usually C albicans or C parapsilosis), Enterococcus faecalis, other streptococci, and gram-negative bacteria (especially Pseudomonas and Serratia marcescens). See Chapter 33.
Other vascular infections include septic thrombophlebitis and mycotic aneurysms. Mycotic aneurysms resulting from direct trauma to a vessel with secondary infection most commonly occur in femoral arteries and less commonly in arteries of the neck. Aneurysms resulting from hematogenous spread of organisms frequently involve intracerebral vessels and thus are seen in association with endocarditis.
Osteomyelitis and septic arthritis involving vertebral bodies, sternoclavicular joints, the pubic symphysis, the sacroiliac joints, and other sites usually results from hematogenous distribution of injected organisms or septic venous thrombi. Pain and fever precede radiographic changes, sometimes by several weeks. While S aureus—often methicillin-resistant—is most common, Serratia, Pseudomonas, Candida (often not C albicans), and other pathogens rarely encountered in spontaneous bone or joint disease are found in persons who inject drugs.
 Treatment
Treatment
A common and difficult clinical problem is management of a person known to inject drugs who presents with fever. In general, after obtaining appropriate cultures (blood, urine, and sputum if the chest radiograph is abnormal), empiric therapy is begun. If the chest radiograph is suggestive of a community-acquired pneumonia (consolidation), therapy for outpatient pneumonia is begun with ceftriaxone, 1 g intravenously every 24 hours, plus either azithromycin (500 mg orally or intravenously every 24 hours) or doxycycline (100 mg orally or intravenously twice daily). If the chest radiograph is suggestive of septic emboli (nodular infiltrates), therapy for presumed endocarditis is initiated, usually with vancomycin 15 mg/kg/dose every 12 hours intravenously (due to the high prevalence of MRSA and the possibility of enterococcus). If the chest radiograph is normal and no focal site of infection can be found, endocarditis is presumed. While awaiting the results of blood cultures, empiric treatment with vancomycin is started. If blood cultures are positive for organisms that frequently cause endocarditis in drug users (see above), endocarditis is presumed to be present and treated accordingly. If blood cultures are positive for an organism that is an unusual cause of endocarditis, evaluation for an occult source of infection should go forward. In this setting, a transesophageal echocardiogram may be quite helpful since it is 90% sensitive in detecting vegetations and a negative study is strong evidence against endocarditis. If blood cultures are negative and the patient responds to antibiotics, therapy should be continued for 7–14 days (oral therapy can be given once an initial response has occurred). In every patient, careful examination for an occult source of infection (eg, genitourinary, dental, sinus, gallbladder) should be done. Clinicians also can have a significant role to play in integrating treatment of opioid use disorder when patients present with infectious disease complications. This includes screening for opioid use disorder, undergoing specific training for and prescribing opioid use disorder medications, treatment of withdrawal symptoms, and linkage to community-based treatment after hospital discharge.
 When to Refer
When to Refer
• Any patient with suspected or proven infective endocarditis.
• Patients with persistent bacteremia.
 When to Admit
When to Admit
• Persons who inject drugs with fever.
• Patients with abscesses or progressive skin and soft tissue infection that require debridement.
Jackson KA et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs—six sites, 2005–2016. MMWR Morb Mortal Wkly Rep. 2018 Jun 8;67(22):625–8. [PMID: 29879096]
Larney S et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiatry. 2019 Dec 26. [Epub ahead of print] [PMID: 31876906]
Schranz AJ et al. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019 Jan 1;170(1):31–40. [PMID: 30508432]
Springer SA et al. Integrating treatment at the intersection of opioid use disorder and infectious disease epidemics in medical settings: a call for action after a national academies of sciences, engineering, and medicine workshop. Ann Intern Med. 2018 Sep 4;169(5):335–6. [PMID: 30007032]
Zhang AY et al. The changing epidemiology of candidemia in the United States: injection drug use as an increasingly common risk factor—active surveillance in selected sites, United States, 2014–17. Clin Infect Dis. 2019 Nov 2. [Epub ahead of print] [PMID: 31676903]
ACUTE INFECTIOUS DIARRHEA
ESSENTIALS OF DIAGNOSIS

 Acute diarrhea: lasts less than 2 weeks.
Acute diarrhea: lasts less than 2 weeks.
 Chronic diarrhea: lasts longer than 2 weeks.
Chronic diarrhea: lasts longer than 2 weeks.
 Mild diarrhea: 3 or fewer stools per day.
Mild diarrhea: 3 or fewer stools per day.
 Moderate diarrhea: 4 or more stools per day with local symptoms (abdominal cramps, nausea, tenesmus).
Moderate diarrhea: 4 or more stools per day with local symptoms (abdominal cramps, nausea, tenesmus).
 Severe diarrhea: 4 or more stools per day with systemic symptoms (fever, chills, dehydration).
Severe diarrhea: 4 or more stools per day with systemic symptoms (fever, chills, dehydration).
 General Considerations
General Considerations
Acute diarrhea can be caused by a number of different factors, including emotional stress, food intolerance, inorganic agents (eg, sodium nitrite), organic substances (eg, mushrooms, shellfish), medications, and infectious agents (including viruses, bacteria, and protozoa) (Table 30–3). From a diagnostic and therapeutic standpoint, it is helpful to classify infectious diarrhea into syndromes that produce inflammatory or bloody diarrhea and those that are noninflammatory, nonbloody, or watery. In general, the term “inflammatory diarrhea” suggests colonic involvement by invasive bacteria or parasites or by toxin production. Patients complain of frequent bloody, small-volume stools, often associated with fever, abdominal cramps, tenesmus, and fecal urgency. Common causes of this syndrome include Shigella, Salmonella, Campylobacter, Yersinia, invasive strains of Escherichia coli, and other Shiga-toxin–producing strains of E coli (STEC), Entamoeba histolytica, and C difficile. Tests for fecal leukocytes or the neutrophil marker lactoferrin are frequently positive, and definitive etiologic diagnosis requires stool culture. Noninflammatory diarrhea is generally milder and is caused by viruses or toxins that affect the small intestine and interfere with salt and water balance, resulting in large-volume watery diarrhea, often with nausea, vomiting, and cramps. Common causes of this syndrome include viruses (eg, rotavirus, norovirus, astrovirus, enteric adenoviruses), vibriones (Vibrio cholerae, Vibrio parahaemolyticus), enterotoxin-producing E coli, Giardia lamblia, cryptosporidia, and agents that can cause food-borne gastroenteritis. In developed countries, viruses (particularly norovirus) are an important cause of hospitalizations due to acute gastroenteritis among adults.
Table 30–3. Acute bacterial diarrheas and “food poisoning” (listed in alphabetical order).

The term “food poisoning” denotes diseases caused by toxins present in consumed foods. When the incubation period is short (1–6 hours after consumption), the toxin is usually preformed. Vomiting is usually a major complaint, and fever is usually absent. Examples include intoxication from S aureus or Bacillus cereus, and toxin can be detected in the food. When the incubation period is longer—between 8 hours and 16 hours—the organism is present in the food and produces toxin after being ingested. Vomiting is less prominent, abdominal cramping is frequent, and fever is often absent. The best example of this disease is that due to Clostridium perfringens. Toxin can be detected in food or stool specimens.
The inflammatory and noninflammatory diarrheas discussed above can also be transmitted by food and water and usually have incubation periods between 12 and 72 hours. Cyclospora, cryptosporidia, and Isospora are protozoans capable of causing disease in both immunocompetent and immunocompromised patients. Characteristics of disease include profuse watery diarrhea that is prolonged but usually self-limited (1–2 weeks) in the immunocompetent patient but can be chronic in the compromised host. Epidemiologic features may be helpful in determining etiology. Recent hospitalization or antibiotic use suggests C difficile; recent foreign travel suggests Salmonella, Shigella, Campylobacter, E coli, or V cholerae; undercooked hamburger suggests STEC; outbreak in long-term care facility, school, or cruise ship suggests norovirus (including newly identified strains, eg, GII.4 Sydney); and fried rice consumption is associated with B cereus toxin. Prominent features of some of these causes of diarrhea are listed in Table 30–3.
 Treatment
Treatment
A. General Measures
In general, most cases of acute gastroenteritis are self-limited and do not require therapy other than supportive measures. Treatment usually consists of replacement of fluids and electrolytes and, very rarely, management of hypovolemic shock and respiratory compromise. In mild diarrhea, increasing ingestion of juices and clear soups is adequate. In more severe cases of dehydration (postural light-headedness, decreased urination), oral glucose-based rehydration solutions can be used (Ceralyte, Pedialyte).
B. Specific Measures
In immunocompetent adults, empiric antimicrobial therapy for bloody diarrhea while waiting for results is recommended only with the following circumstances: (1) documented fever, abdominal pain, bloody diarrhea, and bacillary dysentery (frequent scant bloody stools, fever, abdominal cramps, tenesmus) presumptively due to Shigella; and (2) returning travelers with a temperature of at least 38.5°C or signs of sepsis.
Either a fluoroquinolone or azithromycin should be used as empiric antimicrobial therapy for bloody diarrhea. Empiric antibacterial treatment should be considered in immunocompromised people with severe illness and bloody diarrhea. Loperamide may be given to immunocompetent adults with acute watery diarrhea, but should be avoided with Shigella infection or in suspected or proven toxic megacolon. Therapeutic recommendations for specific agents can be found elsewhere in this book.
Bányai K et al. Viral gastroenteritis. Lancet. 2018 Jul 14;392(10142):175–86. [PMID: 30025810]
Guery B et al. Clostridioides difficile: diagnosis and treatments. BMJ. 2019 Aug 20;366:l4609. [PMID: 31431428]
Nelson RL et al. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev. 2017 Mar 3;3:CD004610. [PMID: 28257555]
Newman KL et al. Gastroenteritis in men who have sex with men in Seattle, Washington, 2017–2018. Clin Infect Dis. 2019 Oct 17. [Epub ahead of print] [PMID: 31621824]
Shane AL et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017 Nov 29;65(12):e45–80. [PMID: 29053792]
INFECTIOUS DISEASES IN THE RETURNING TRAVELER
ESSENTIALS OF DIAGNOSIS

 Most infections are common and self-limited.
Most infections are common and self-limited.
 Identify patients with transmissible diseases that require isolation.
Identify patients with transmissible diseases that require isolation.
 The incubation period may be helpful in diagnosis.
The incubation period may be helpful in diagnosis.
 Less than 3 weeks following exposure may suggest dengue, leptospirosis, and yellow fever; more than 3 weeks suggest typhoid fever, malaria, and tuberculosis.
Less than 3 weeks following exposure may suggest dengue, leptospirosis, and yellow fever; more than 3 weeks suggest typhoid fever, malaria, and tuberculosis.
 General Considerations
General Considerations
The differential diagnosis of fever in the returning traveler is broad, ranging from self-limited viral infections to life-threatening illness. The evaluation is best done by identifying whether a particular syndrome is present, then refining the differential diagnosis based on an exposure history. The travel history should include directed questions regarding geography (rural versus urban, specific country and region visited), time of year, animal or arthropod contact, unprotected sexual intercourse, ingestion of untreated water or raw foods, historical or pretravel immunizations, and adherence to malaria prophylaxis.
 Etiologies
Etiologies
The most common infectious causes of fever—excluding simple causes such as upper respiratory infections, bacterial pneumonia and urinary tract infections—in returning travelers are malaria (see Chapter 35), diarrhea (see next section), and dengue (see Chapter 32). Others include mononucleosis (associated with Epstein-Barr virus or cytomegalovirus), respiratory infections, including seasonal influenza, influenza A/H1N1 “swine” influenza, and influenza A/H5N1 or A/H7N9 “avian” influenza (see Chapter 32); leptospirosis (see Chapter 34); typhoid fever (see Chapter 33); and rickettsial infections (see Chapter 32). In recent years coronaviruses have emerged as particularly significant regional and global outbreaks of various sizes (SARS-CoV, MERS-CoV, and SARS-CoV-2). Foreign travel is increasingly recognized as a risk factor for colonization and disease with resistant pathogens, such as ESBL-producing gram-negative organisms. Systemic febrile illnesses without a diagnosis also occur commonly, particularly in travelers returning from sub-Saharan Africa or Southeast Asia.
A. Fever and Rash
Potential etiologies include dengue, Ebola, Chikungunya, and Zika viruses, viral hemorrhagic fever, leptospirosis, meningococcemia, yellow fever, typhus, Salmonella typhi, and acute HIV infection.
B. Pulmonary Infiltrates
Tuberculosis, ascaris, Paragonimus, and Strongyloides can all cause pulmonary infiltrates.
C. Meningoencephalitis
Etiologies include N meningitidis, leptospirosis, arboviruses, rabies, and (cerebral) malaria.
D. Jaundice
Consider hepatitis A, yellow fever, hemorrhagic fever, leptospirosis, and malaria.
E. Fever Without Localizing Symptoms or Signs
Malaria, typhoid fever, acute HIV infection, rickettsial illness, visceral leishmaniasis, trypanosomiasis, and dengue are possible etiologies.
F. Traveler’s Diarrhea
See next section.
 Clinical Findings
Clinical Findings
Fever and rash in the returning traveler should prompt blood cultures and serologic tests based on the exposure history. The workup of a pulmonary infiltrate should include the placement of a PPD or use of an interferon-gamma release assay, examination of sputum for acid-fast bacilli and possibly for ova and parasites. Patients with evidence of meningoencephalitis should receive lumbar puncture, blood cultures, thick/thin smears of peripheral blood, history-guided serologies, and a nape biopsy (if rabies is suspected). Jaundice in a returning traveler should be evaluated for hemolysis (for malaria), and the following tests should be performed: liver biochemical tests, thick/thin smears of peripheral blood, and directed serologic testing. The workup of traveler’s diarrhea is presented in the following section. Finally, patients with fever but no localizing signs or symptoms should have blood cultures performed. Routine laboratory studies usually include complete blood count with differential, electrolytes, liver biochemical tests, urinalysis, and blood cultures. Thick and thin peripheral blood smears should be done (and repeated in 12–24 hours if clinical suspicion remains high) for malaria if there has been travel to endemic areas. Other studies are directed by the results of history, physical examination, and initial laboratory tests. They may include stool for ova and parasites, chest radiograph, HIV test, and specific serologies (eg, dengue, leptospirosis, rickettsial disease, schistosomiasis, Strongyloides). Bone marrow biopsy to diagnose typhoid fever could be helpful in the appropriate patient. Increasingly, next-generation sequencing of plasma or body fluids such as cerebrospinal fluid is used as an adjunctive modality for diagnosis when traditional methods have not yielded a diagnosis.
 When to Refer
When to Refer
Travelers with fever, particularly if immunocompromised.
 When to Admit
When to Admit
Any evidence of hemorrhage, respiratory distress, hemodynamic instability, and neurologic deficits.
Huang C et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. [PMID: 31986264]
Polen KD et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for men with possible Zika virus exposure—United States, August 2018. MMWR Morb Mortal Wkly Rep. 2018 Aug 10;67(31):868–71. [PMID: 30091965]
Thwaites GE et al. Approach to fever in the returning traveler. N Engl J Med. 2017 Feb 9;376(6):548–60. [PMID: 28177860]
TRAVELER’S DIARRHEA
ESSENTIALS OF DIAGNOSIS

 Usually a benign, self-limited disease occurring about 1 week into travel.
Usually a benign, self-limited disease occurring about 1 week into travel.
 Prophylaxis not recommended unless there is a comorbid disease (inflammatory bowel syndrome, HIV, immunosuppressive medication).
Prophylaxis not recommended unless there is a comorbid disease (inflammatory bowel syndrome, HIV, immunosuppressive medication).
 Single-dose therapy of a fluoroquinolone usually effective if significant symptoms develop.
Single-dose therapy of a fluoroquinolone usually effective if significant symptoms develop.
 General Considerations
General Considerations
Whenever a person travels from one country to another—particularly if the change involves a marked difference in climate, social conditions, or sanitation standards and facilities—diarrhea may develop within 2–10 days. Bacteria cause 80% of cases of traveler’s diarrhea, with enterotoxigenic E coli, Shigella species, and Campylobacter jejuni being the most common pathogens. Less common are Aeromonas, Salmonella, noncholera vibriones, E histolytica, and G lamblia. Contributory causes include unusual food and drink, change in living habits, occasional viral infections (adenoviruses or rotaviruses), and change in bowel flora. Chronic watery diarrhea may be due to amebiasis or giardiasis or, rarely, tropical sprue.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
There may be up to ten or even more loose stools per day, often accompanied by abdominal cramps and nausea, occasionally by vomiting, and rarely by fever. The stools are usually watery and not associated with fever when caused by enterotoxigenic E coli. With invasive bacterial pathogens (Shigella, Campylobacter, Salmonella), stools can be bloody and fever may be present. The illness usually subsides spontaneously within 1–5 days, although 10% remain symptomatic for 1 week or longer, and symptoms persist for longer than 1 month in 2%. Traveler’s diarrhea is also a significant risk factor for developing irritable bowel syndrome.
B. Laboratory Findings
In patients with fever and bloody diarrhea, stool culture is indicated, but in most cases, cultures are reserved for those who do not respond to antibiotics.
 Prevention
Prevention
A. General Measures
Avoidance of fresh foods and water sources that are likely to be contaminated is recommended for travelers to developing countries, where infectious diarrheal illnesses are endemic.
B. Specific Measures
Because not all travelers will have diarrhea and because most episodes are brief and self-limited, the currently recommended approach is to provide the traveler with a supply of antimicrobials. Prophylaxis is recommended for those with significant underlying disease (inflammatory bowel disease, AIDS, diabetes mellitus, heart disease in older adults, conditions requiring immunosuppressive medications) and for those whose full activity status during the trip is so essential that even short periods of diarrhea would be unacceptable.
Prophylaxis is started upon entry into the destination country and is continued for 1 or 2 days after leaving. For stays of more than 3 weeks, prophylaxis is not recommended because of the cost and increased toxicity. For prophylaxis, several oral antimicrobial once-daily regimens are effective, such as ciprofloxacin, 500 mg, or rifaximin, 200 mg. Bismuth subsalicylate is effective but turns the tongue and the stools black and can interfere with doxycycline absorption, which may be needed for malaria prophylaxis; it is rarely used.
 Treatment
Treatment
For most individuals, the affliction is short-lived, and symptomatic therapy with loperamide is all that is required, provided the patient is not systemically ill (fever 39°C or higher) and does not have dysentery (bloody stools), in which case antimotility agents should be avoided. Packages of oral rehydration salts to treat dehydration are available over the counter in the United States (Infalyte, Pedialyte, others) and in many foreign countries.
When treatment is necessary, in areas where toxin-producing bacteria are the major cause of diarrhea (Latin America and Africa), loperamide (4 mg oral loading dose, then 2 mg after each loose stool to a maximum of 16 mg/day) with a single oral dose of ciprofloxacin (750 mg), levofloxacin (500 mg), or ofloxacin (200 mg) cures most cases of traveler’s diarrhea. If diarrhea is associated with bloody stools or persists despite a single dose of a fluoroquinolone, 1000 mg of azithromycin should be taken. In pregnant women and in areas where invasive bacteria more commonly cause diarrhea (Indian subcontinent, Asia, especially Thailand where fluoroquinolone-resistant Campylobacter is prevalent), azithromycin is the medication of choice. Rifaximin, a nonabsorbable agent, is also approved for therapy of traveler’s diarrhea at a dose of 200 mg orally three times per day or 400 mg twice a day for 3 days. Because luminal concentrations are high, but tissue levels are insufficient, it should not be used in situations where there is a high likelihood of invasive disease (eg, fever, systemic toxicity, or bloody stools).
 When to Refer
When to Refer
• Cases refractory to treatment.
• Persistent infection.
• Immunocompromised patient.
 When to Admit
When to Admit
Patients who are severely dehydrated or hemodynamically unstable should be admitted to the hospital.
Eckbo EJ et al. New tools to test stool: managing travelers’ diarrhea in the era of molecular diagnostics. Infect Dis Clin North Am. 2019 Mar;33(1):197–212. [PMID: 30712762]
Schweitzer L et al. Emerging concepts in the diagnosis, treatment, and prevention of travelers’ diarrhea. Curr Opin Infect Dis. 2019 Oct;32(5):468–74. [PMID: 31361658]
ANTIMICROBIAL THERAPY
SELECTED PRINCIPLES OF ANTIMICROBIAL THERAPY
Specific steps (outlined below) are required when considering antibiotic therapy for patients. Medications within classes, medications of first choice, and alternative medications are presented in Table 30–4.
Table 30–4. Medication of choice for suspected or documented microbial pathogens (listed in alphabetical order, within classes).

A. Etiologic Diagnosis
Based on the organ system involved, the organism causing infection can often be predicted. See Tables 30–5 and 30–6.
Table 30–5. Examples of initial antimicrobial therapy for acutely ill, hospitalized adults pending identification of causative organism (in alphabetical order).

Table 30–6. Examples of empiric choices of antimicrobials for adult outpatient infections (in alphabetical order).
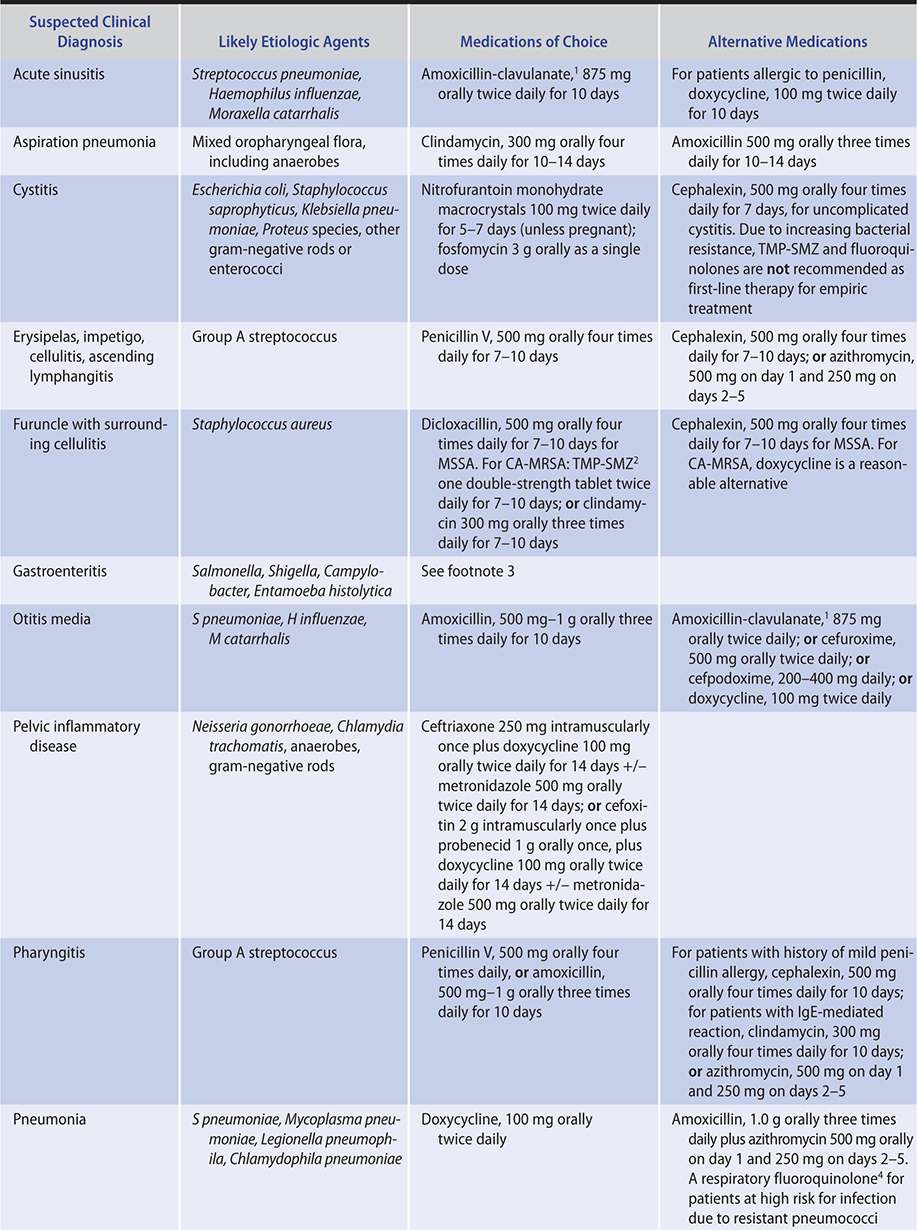
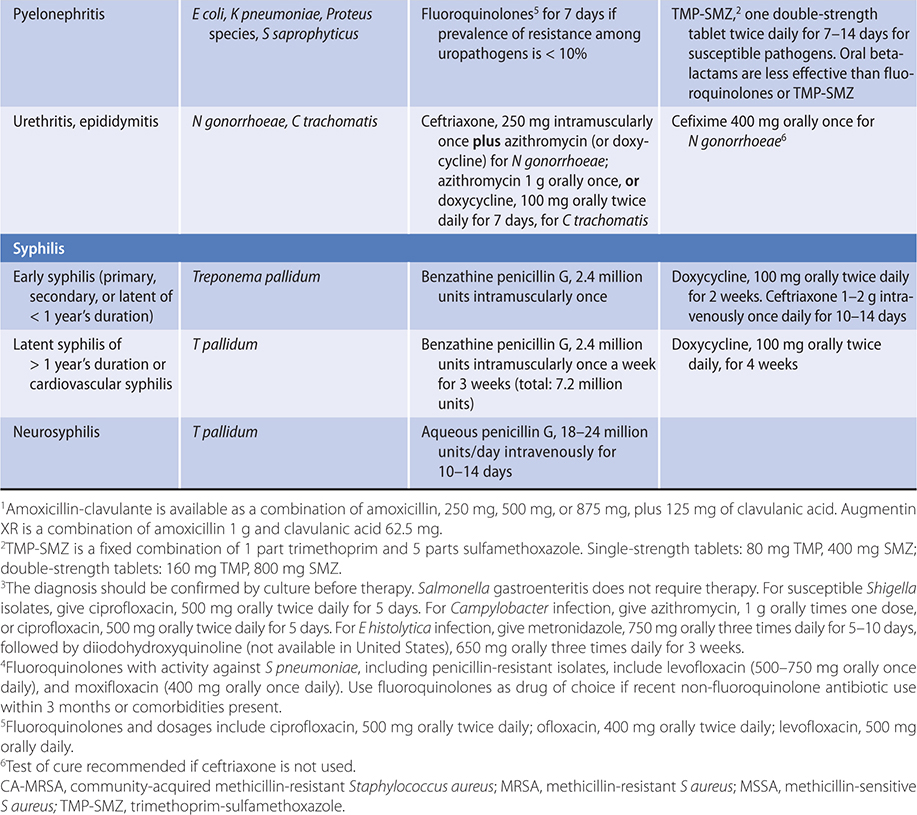
B. “Best Guess”
Select an empiric regimen that is likely to be effective against the suspected pathogens.
C. Laboratory Control
Specimens for laboratory examination should be obtained before institution of therapy to determine susceptibility.
D. Clinical Response
Based on clinical response and other data, the laboratory reports are evaluated and then the desirability of changing the regimen is considered. If the specimen was obtained from a normally sterile site (eg, blood, cerebrospinal fluid, pleural fluid, joint fluid), the recovery of a microorganism in significant amounts is meaningful even if the organism recovered is different from the clinically suspected agent, and this may force a change in treatment. Isolation of unexpected microorganisms from the respiratory tract, gastrointestinal tract, or surface lesions (sites that have a complex flora) may represent colonization or contamination, and cultures must be critically evaluated before medications are abandoned that were judiciously selected on a “best guess” basis.
E. Drug Susceptibility Tests
Some microorganisms are predictably inhibited by certain medications; if such organisms are isolated, they need not be tested for drug susceptibility. For example, all group A hemolytic streptococci are inhibited by penicillin. Other organisms (eg, enteric gram-negative rods) are variably susceptible and generally require susceptibility testing whenever they are isolated. Organisms that once had predictable susceptibility patterns are now associated with resistance and require testing. Examples include the pneumococci, which may be resistant to multiple medications (including penicillin, macrolides, and TMP-SMZ); the enterococci, which may be resistant to penicillin, aminoglycosides, and vancomycin; and ESBL producing–E coli resistant to third-generation cephalosporins and fluoroquinolones.
When culture and susceptibility results have been finalized, clinicians must use the most narrow-spectrum agent and the shortest duration possible to decrease the selection pressure for antibacterial resistance.
Antimicrobial drug susceptibility tests may be performed on solid media as disk diffusion tests, in broth, in tubes, in wells of microdilution plates, or as E-tests (strips with increasing concentration of antibiotic). The latter three methods yield results expressed as MIC (minimal inhibitory concentration). In most infections, the MIC is the appropriate in vitro test to guide selection of an antibacterial agent. When there appear to be marked discrepancies between susceptibility testing and clinical response, the following possibilities must be considered:
1. Selection of an inappropriate medication, medication dosage, or route of administration.
2. Failure to drain a collection of pus or to remove a foreign body.
3. Failure of a poorly diffusing drug to reach the site of infection (eg, central nervous system) or to reach intracellular phagocytosed bacteria.
4. Superinfection in the course of prolonged chemotherapy.
5. Emergence of drug resistance in the original pathogen or superinfection with a new more resistant organism.
6. Participation of two or more microorganisms in the infectious process, of which only one was originally detected and used for medication selection.
7. Inadequate host defenses, including immunodeficiencies and diabetes mellitus.
8. Noninfectious causes, including drug fever, malignancy, and autoimmune disease.
F. Promptness of Response
Response depends on a number of factors, including the patient (immunocompromised patients respond slower than immunocompetent patients), the site of infection (deep-seated infections such as osteomyelitis and endocarditis respond more slowly than superficial infections such as cystitis or cellulitis), the pathogen (virulent organisms such as S aureus respond more slowly than viridans streptococci; mycobacterial and fungal infections respond slower than bacterial infections), and the duration of illness (in general, the longer the symptoms are present, the longer it takes to respond). Thus, depending on the clinical situation, persistent fever and leukocytosis several days after initiation of therapy may not indicate improper choice of antibiotics but may be due to the natural history of the disease being treated. In most infections, either a bacteriostatic or a bactericidal agent can be used. In some infections (eg, infective endocarditis and meningitis), a bactericidal agent should be used. When potentially toxic medications (eg, aminoglycosides, flucytosine) are used, serum levels of the medication are measured to minimize toxicity and ensure appropriate dosage. In patients with altered renal or hepatic clearance of medications, the dosage or frequency of administration must be adjusted; it is best to measure levels in older adults, in morbidly obese patients, or in those with altered kidney function when possible and adjust therapy accordingly.
G. Duration of Antimicrobial Therapy
Generally, effective antimicrobial treatment results in reversal of the clinical and laboratory parameters of active infection and marked clinical improvement. However, varying periods of treatment may be required for cure. Key factors include (1) the type of infecting organism (bacterial infections generally can be cured more rapidly than fungal or mycobacterial ones), (2) the location of the process (eg, endocarditis and osteomyelitis require prolonged therapy), and (3) the immunocompetence of the patient.
H. Adverse Reactions and Toxicity
These include hypersensitivity reactions, direct toxicity, superinfection by drug-resistant microorganisms, and drug interactions. If the infection is life-threatening and treatment cannot be stopped, the reactions are managed symptomatically or another medication is chosen that does not cross-react with the offending one (Table 30–4). If the infection is less serious, it may be possible to stop all antimicrobials and monitor the patient closely.
I. Route of Administration
Intravenous therapy is preferred for acutely ill patients with serious infections (eg, endocarditis, meningitis, sepsis, severe pneumonia) when dependable levels of antibiotics are required for successful therapy. Certain medications (eg, doxycycline, fluconazole, voriconazole, rifampin, metronidazole, TMP-SMZ, and fluoroquinolones) are so well absorbed that they generally can be administered orally in seriously ill—but not hemodynamically unstable—patients.
Food does not significantly influence the bioavailability of most oral antimicrobial agents. However, the tetracyclines (particularly tetracycline) and the quinolones chelate multivalent cations resulting in decreased oral bioavailability. Posaconazole suspension should always be administered with food.
A major complication of intravenous antibiotic therapy is infection due to the manipulation of the intravenous catheter. Peripheral catheters are changed every 48–72 hours to decrease the likelihood of catheter-associated infection, and antimicrobial-coated central venous catheters (minocycline and rifampin, chlorhexidine and sulfadiazine) have been associated with a decreased incidence of these infections. Most of these infections present with local signs of infection (erythema, tenderness) at the insertion site. In a patient with fever who is receiving intravenous therapy, the catheter must always be considered a potential source. Small-gauge (20–23F) peripherally inserted silicone or polyurethane catheters (Per Q Cath, A-Cath, Ven-A-Cath, and others) are associated with a low infection rate and can be maintained for 3–6 months without replacement. Such catheters are ideal for long-term outpatient antibiotic therapy.
J. Cost of Antibiotics
The cost of these agents can be substantial. In addition to acquisition costs and monitoring costs (drug levels, liver biochemical tests, electrolytes, etc), the cost of treating adverse reactions, the cost of treatment failure and superinfection, and the costs associated with drug administration must be considered.
K. Antimicrobial Stewardship
Antimicrobial stewardship is a critically important tool intended to optimize clinical outcomes while minimizing unintended consequences of antimicrobial use. These consequences include drug toxicity, superinfection, emergence of bacterial resistance, and impact upon the human microbiome. The Infectious Diseases Society of America recommends establishment of an antimicrobial stewardship team at all acute care facilities. The core members of a stewardship team should include an infectious diseases physician and a clinical pharmacist with infectious diseases training. If possible, the addition of a clinical microbiologist, an information system specialist, an infection control professional, and a hospital epidemiologist would be preferable. Key strategies for a stewardship team, as well as the individual prescriber, should include questions associated with the “Four Moments of Antibiotic Decision Making”: (1) Does this patient have an infection that requires antibiotics? (2) Have the appropriate cultures been ordered before starting antibiotics? (3) After a few days of empiric antibiotics have passed, can antibiotics be stopped?; Can therapy be narrowed?; Can therapy be switched from intravenous to oral? (4) What duration of antibiotic therapy is necessary for this patient’s diagnosis? Stewardship interventions centered upon one or more of the above questions have been demonstrated to decrease the risk of C difficile and Candida superinfection as well as attenuate the negative impact of antibiotics on the human microbiome.
Ray MJ et al. Antibiotic prescribing without documented indication in ambulatory care clinics: national cross sectional study. BMJ. 2019 Dec 11;367:l6461. [PMID: 31826860]
Tamma PD et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017 Sep 1;177(9):1308–15. [PMID: 28604925]
Tamma PD et al. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2019 Jan 15;321(2):139–40. [PMID: 30589917]
HYPERSENSITIVITY
 Penicillin Allergy
Penicillin Allergy
All penicillins are cross-sensitizing and cross-reacting. Skin tests using penicilloyl-polylysine and undegraded penicillin can identify most individuals with IgE-mediated reactions (hives, bronchospasm). In those patients with positive reaction to skin tests, the incidence of subsequent immediate severe reactions associated with penicillin administration is high. A history of a penicillin reaction in the past is often not reliable. Only a small proportion (less than 5%) of patients with a stated history of penicillin allergy experience an adverse reaction when challenged with the medication. The decision to administer penicillin or related medications (other beta-lactams) to patients with an allergic history depends on the severity of the reported reaction, the severity of the infection being treated, and the availability of alternative medications. For patients with a history of severe reaction (anaphylaxis), alternative medications should be used. In the rare situations when there is a strong indication for using penicillin (eg, syphilis in pregnancy) in allergic patients, desensitization can be performed. If the reaction is mild (nonurticarial rash), the patient may be rechallenged with penicillin or may be given another beta-lactam antibiotic.
Allergic reactions include anaphylaxis, serum sickness (urticaria, fever, joint swelling, angioedema 7–12 days after exposure), skin rashes, fever, interstitial nephritis, eosinophilia, hemolytic anemia, other hematologic disturbances, and vasculitis. The incidence of hypersensitivity to penicillin is estimated to be 1–5% among adults in the United States. Life-threatening anaphylactic reactions are very rare (0.05%). Ampicillin produces maculopapular skin rashes more frequently than other penicillins, but many ampicillin (and other beta-lactam) rashes are not allergic in origin. The nonallergic ampicillin rash usually occurs after 3–4 days of therapy, is maculopapular, is more common in patients with coexisting viral illness (especially Epstein-Barr infection), and resolves with continued therapy. The maculopapular rash may or may not reappear with rechallenge. Beta-lactams can induce nephritis with primary tubular lesions associated with anti-basement membrane antibodies.
If the intradermal test described below is negative, desensitization is not necessary, and a full dose of the penicillin may be given. If the test is positive, alternative medications should be strongly considered. If that is not feasible, desensitization is necessary.
Patients with a history of allergy to penicillin are also at an increased risk for having a reaction to cephalosporins or carbapenems. A common approach to these patients is to assess the severity of the reaction. If an IgE-mediated reaction to penicillin can be excluded by history, a cephalosporin can be administered. When the history justifies concern about an immediate-type reaction, penicillin skin testing should be performed. If the test is negative, the cephalosporin or carbapenem can be given. If the test is positive, there is a 5–10% chance of cross reactivity with cephalosporins, and the decision whether to use cephalosporins depends on the availability of alternative agents and the severity of the infection. While carbapenems historically have been considered highly cross reactive with penicillins, the cross reactivity appears to be minimal (1%).
Leis JA et al. Point-of-care beta-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Infect Dis. 2017 Oct 1;65(7):1059–65. [PMID: 28575226]
Sacco KA et al. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017 Sep;72(9):1288–96. [PMID: 28370003]
IMMUNIZATION AGAINST INFECTIOUS DISEASES
RECOMMENDED IMMUNIZATION FOR ADULTS
Immunization is one of the most important tools (along with sanitation) used to prevent morbidity and mortality from infectious diseases. In general, the administration of most vaccinations induces a durable antibody response (active immunity). In contrast, passive immunization occurs when preformed antibodies are given (eg, immune globulin from pooled serum), resulting in temporary protection which is a less durable response. The two variants of active immunization are live attenuated vaccines (which are believed to result in an immunologic response more like natural infection), and inactivated or killed vaccines.
The schedule of vaccinations varies based on the risk of the disease being prevented by vaccination, whether a vaccine has been given previously, the immune status of the patient (probability of responding to vaccine) and safety of the vaccine (live versus killed product, as well as implications for the fetus in pregnant women). Recommendations for healthy adults as well as special populations based on medical conditions are summarized in Table 30–7, which can be accessed online at https://www.cdc.gov/vaccines/schedules.
Table 30–7. Recommended adult immunization schedule—United States, 2020.
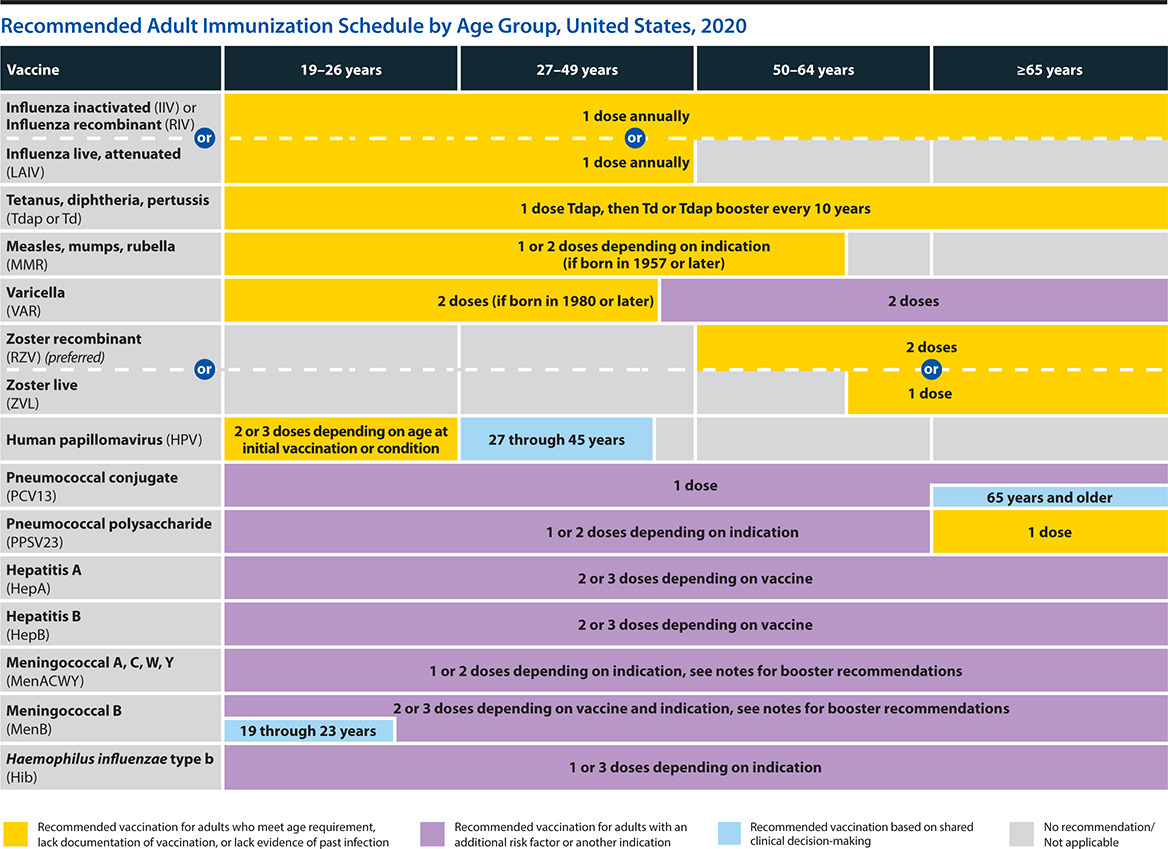
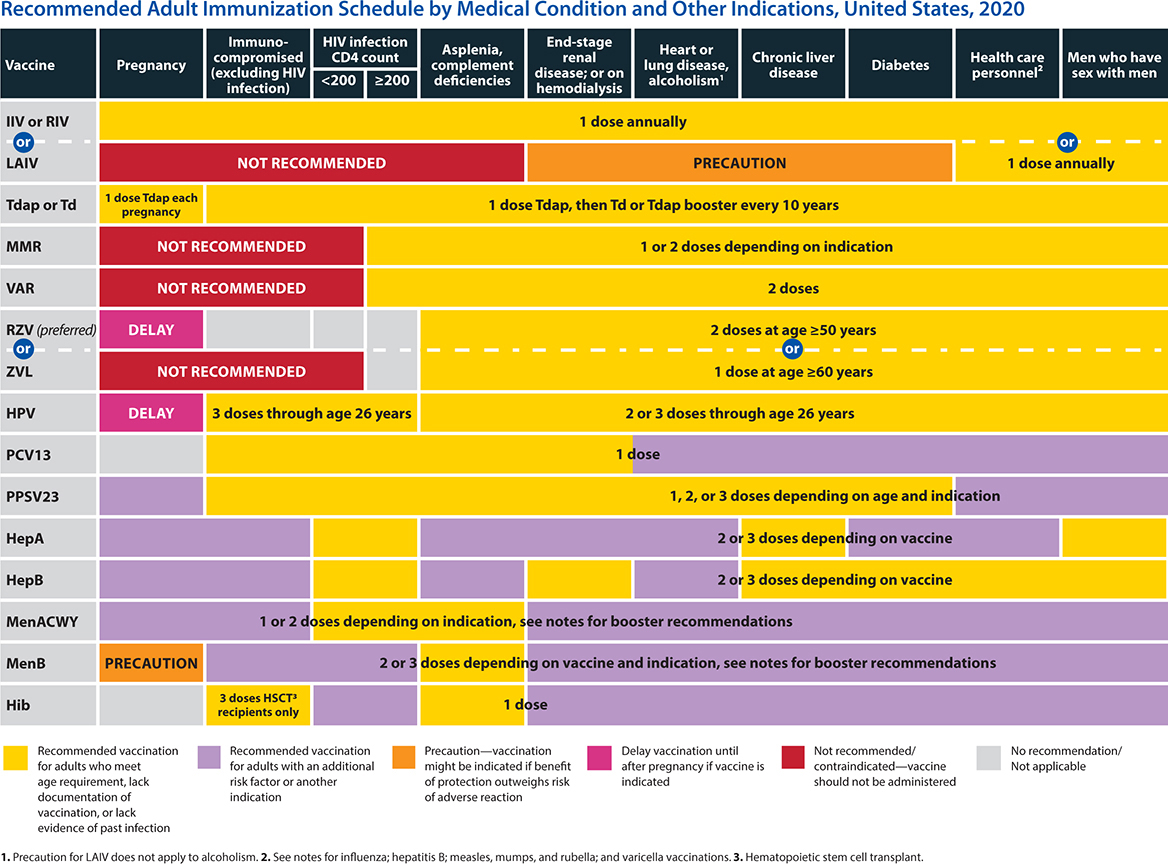




1. Healthy Adults
Vaccination recommendations are made by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (Table 30–7).
2. Pregnant Women
Given the uncertainty of risks to the fetus, vaccination during pregnancy is generally avoided with the following exceptions: tetanus (transfer of maternal antibodies across the placenta is important to prevent neonatal tetanus), diphtheria, and influenza. Live vaccines are avoided during pregnancy.
Influenza can be a serious infection if acquired in pregnancy, and all pregnant women should be offered influenza (inactivated) vaccination. The live attenuated (intranasal) influenza vaccine is not recommended during pregnancy.
3. HIV-Infected Adults
HIV-infected patients have impaired cellular and B cell responses. Inactivated or killed vaccinations can generally be given without any consequence, but the recipient may not be able to mount an adequate antibody response. Live or attenuated vaccines are generally avoided with some exceptions (ie, in patients with CD4+ T lymphocytes greater than 200 cells/mcL). Guidelines for vaccinating HIV-infected patients have been issued jointly by the Centers for Disease Control and Prevention, the US National Institutes of Health, and the HIV Medical Association of the Infectious Diseases Society of America. Timing of vaccination is important to optimize response. If possible, vaccination should be given early in the course of HIV disease or following immune reconstitution.
4. Hematopoietic Cell Transplant Recipients
Hematopoietic cell transplant (HCT) recipients have varying rates of immune reconstitution following transplantation, depending on (1) the type of chemotherapy or radiotherapy used pretransplant (in autologous HCT), (2) the preparative regimen used for the transplant, (3) whether graft-versus-host disease is present, and (4) the type of immunosuppression used posttransplantation (in allogeneic HCT). Vaccines may not work immediately in the posttransplant period. B cells may take 3–12 months to return to normal posttransplant, and naïve T cells that can respond to new antigens appear only 6–12 months posttransplant. B cells of posttransplant patients treated with rituximab may take up to 6 months to fully recover after the last dose of the medication. Vaccines are therefore administered 6–12 months following transplantation with a minimum of 1 month between doses to maximize the probability of response.
5. Solid Organ Transplant Recipients
Solid organ transplant recipients demonstrate a broad spectrum of immunosuppression, depending on the reason for and type of organ transplantation and the nature of the immunosuppression (including T-cell-depleting agents during treatment of organ rejection). These factors affect the propensity for infection posttransplantation and the ability to develop antibody responses to vaccination. In many cases, the time between placing a patient on a transplant list and undergoing the transplantation takes months or years. Providers should take this opportunity to ensure that indicated vaccines are given during this pretransplant period to optimize antibody responses. If this is not possible, most experts give vaccines 3–6 months following transplantation. Live vaccines are contraindicated in the posttransplant period.
RECOMMENDED IMMUNIZATIONS FOR TRAVELERS
Individuals traveling to other countries frequently require immunizations in addition to those routinely recommended and may benefit from chemoprophylaxis against various diseases. Vaccinations against yellow fever and meningococcus are the only ones required by certain countries. These and other travel-specific vaccines are listed at http://wwwnc.cdc.gov/travel/destinations/list.
Various vaccines can be given simultaneously at different sites. Some, such as cholera, plague, and typhoid vaccine, cause significant discomfort and are best given at different times. In general, live attenuated vaccines (measles, mumps, rubella, yellow fever, and oral typhoid vaccine) should not be given to immunosuppressed individuals or household members of immunosuppressed people or to pregnant women. Immunoglobulin should not be given for 3 months before or at least 2 weeks after live virus vaccines, because it may attenuate the antibody response.
Chemoprophylaxis of malaria is discussed in Chapter 35.
VACCINE SAFETY
Most vaccines are safe to administer. In general, it is recommended that the use of live vaccines be avoided in immunocompromised patients, including pregnant women. Vaccines are generally not contraindicated in the following situations: mild, acute illness with low-grade fevers (less than 40.5°C); concurrent antibiotic therapy; soreness or redness at the site; and family history of adverse reactions to vaccinations. Absolute contraindications to vaccines are rare (Table 30–8).
Table 30–8. Adverse effects and contraindications to commonly used vaccines in adults (listed in alphabetical order).

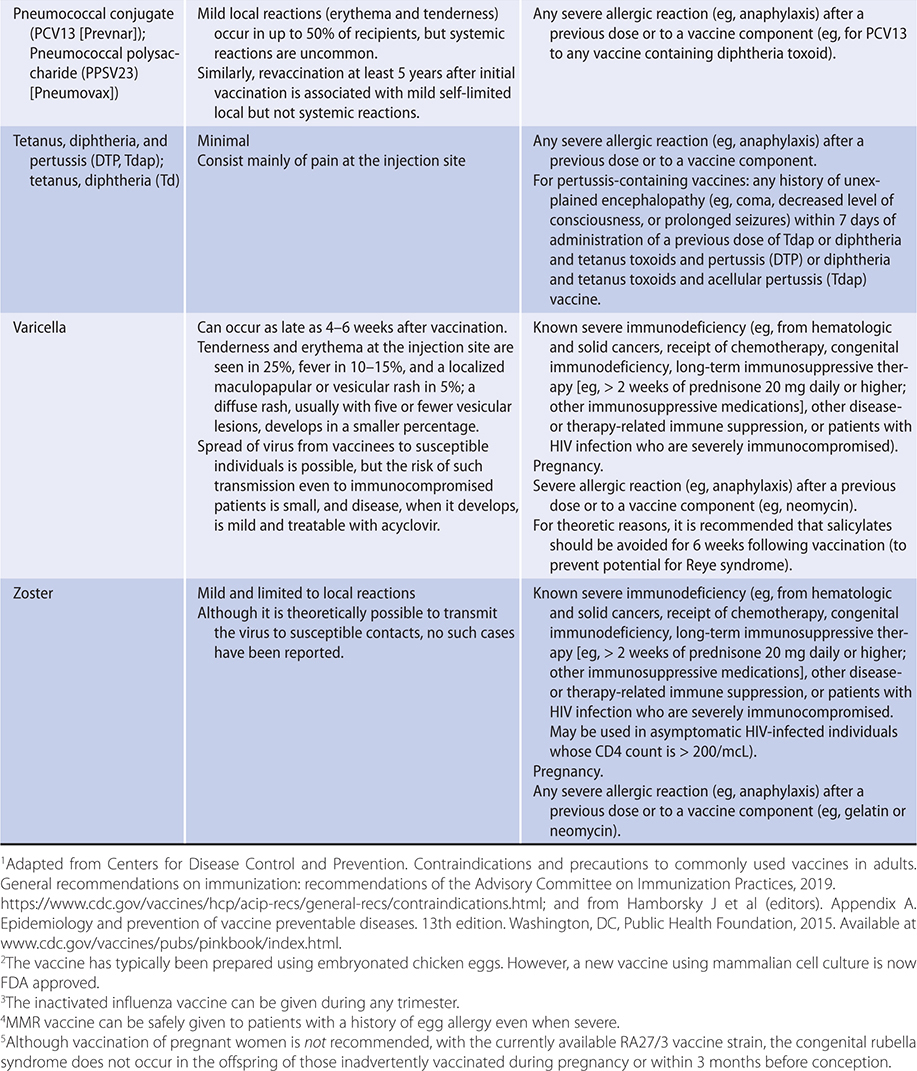
Centers for Disease Control and Prevention (CDC). Adult immunization schedules—United States, 2020. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html
Centers for Disease Control and Prevention (CDC). Health Information for International Travel. https://wwwnc.cdc.gov/travel/page/yellowbook-home
Centers for Disease Control and Prevention (CDC). Vaccine safety. https://www.cdc.gov/vaccinesafety/index.html
Danziger-Isakov L et al. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019 Sep;33(9):e13563. [PMID: 31002409]
Freedman MS et al. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020 Feb 7;69(5):133–5. [PMID: 32027627]
Grohskopf LA et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep. 2019 Aug 23;68(3):1–21. [PMID: 31441906]
Leidner AJ et al. Cost-effectiveness of adult vaccinations: a systematic review. Vaccine. 2019 Jan 7;37(2):226–34. [PMID: 30527660]
Paules CI et al. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med. 2018 Jan 4;378(1):7–9. [PMID: 29185857]
*Reportable to public health authorities.