37
Disorders Related to Environmental Emergencies
Jacqueline A. Nemer, MD, FACEP
Marianne A. Juarez, MD
COLD & HEAT
The human body maintains a steady temperature through the balance of internal heat production and environmental heat loss. Heat exchange between the body and environment occurs via four common processes: radiation, evaporation, conduction, and convection. In extreme temperatures, the body’s thermoregulation may fail, resulting in the core body temperature moving toward the temperature of the external environment. Cold and heat exposure may cause a wide spectrum of conditions ranging in severity from mild to potentially life threatening to death. Many of these conditions are preventable with appropriate education and planning. Preventive measures must be implemented on an individual and population level.
The likelihood and severity of extreme temperature-related conditions depend on physiologic and environmental factors. Physiologic risk factors include extremes of age; cognitive impairment; poor physical conditioning, sedentary lifestyle or immobility; poor acclimatization; concurrent injury; prior temperature-related injury; and numerous underlying medical conditions, especially those affecting cognition and thermoregulation. Pharmacologic risk factors include medications, holistic or alternative treatments, illicit drugs, tobacco, and alcohol. There is a subset of medications associated with a particularly high likelihood of worsening temperature-related conditions, such as those that impact sweating and the central nervous system and those that affect cutaneous blood flow such as peripheral vasoconstrictors or vasodilators. Environmental risk factors include changing weather conditions, inadequate clothing or housing (homelessness, or housing with inadequate temperature control), and occupational or recreational exposure.
DISORDERS DUE TO HEAT
ESSENTIALS OF DIAGNOSIS

 Spectrum of preventable heat-related illnesses: heat cramps, heat exhaustion, heat syncope, and heat stroke.
Spectrum of preventable heat-related illnesses: heat cramps, heat exhaustion, heat syncope, and heat stroke.
 Heat stroke: hyperthermia with cerebral dysfunction in a patient with heat exposure.
Heat stroke: hyperthermia with cerebral dysfunction in a patient with heat exposure.
 Best outcome: early recognition, initiation of rapid cooling, and avoidance of shivering during cooling; delays in cooling result in higher morbidity and mortality in heat stroke victims.
Best outcome: early recognition, initiation of rapid cooling, and avoidance of shivering during cooling; delays in cooling result in higher morbidity and mortality in heat stroke victims.
 Best choice of cooling method: whichever can be instituted the fastest with the least compromise to the patient.
Best choice of cooling method: whichever can be instituted the fastest with the least compromise to the patient.
 General Considerations
General Considerations
Heat-related illnesses are among the most commonly seen environmental emergencies presenting to emergency departments. The amount of heat retained in the body is determined by internal metabolic function and environmental conditions, including temperature and humidity. Hyperthermia results from the body’s inability to maintain normal internal temperature through heat loss. Hyperthermia results from either compromised heat dissipation mechanisms or abnormally high heat production. Increased metabolic rate is the most important factor in elevation of body temperature. Heat loss occurs primarily through sweating and peripheral vasodilation. The direct transfer of heat from the skin to the surrounding air, by convection or conduction, occurs with diminishing efficiency as ambient temperature rises, especially above 37.2°C (the point at which heat transfer reverses direction). At normal temperatures, evaporation accounts for approximately 20% of the body’s heat loss, but at high temperatures it becomes the major mechanism for dissipation of heat. This mechanism diminishes as humidity rises.
Heat stress can be caused by a combination of environmental and metabolic heat. Climate change may significantly contribute to the risk of heat-related conditions.
There is a spectrum of preventable heat stress conditions, ranging from mild forms, such as heat cramps, to severe forms, such as heat stroke. Risk factors include longer duration of exertion, hot environment, insufficient acclimatization, and dehydration. Additional risk factors include skin disorders or other medical conditions that inhibit sweat production or evaporation, obesity, prolonged seizures, hypotension, reduced cutaneous blood flow, reduced cardiac output, the use of drugs that increase metabolism or muscle activity or impair sweating, and withdrawal syndromes. Illicit drugs can cause increased muscle activity and thus generate increased body heat.
Classic (nonexertional) heat-related illness may occur in any individual in a hot, relaxing environment with increased severity in individuals with the risk factors mentioned above, despite minimal physical activity.
Heat cramps are exercise-associated painful involuntary muscle contractions during or immediately after exercise. They result from dilutional hyponatremia as sweat losses are replaced with water alone. Heat exhaustion is characterized by dehydration, sodium depletion, or isotonic fluid loss with accompanying cardiovascular changes. It results from prolonged strenuous activity in a hot environment without adequate water or salt intake.
Heat syncope is defined as a transient loss of consciousness with spontaneous return to normal mentation. It results from volume depletion and cutaneous vasodilation with subsequent systemic and cerebral hypotension. Exercise-associated postural hypotension is usually the cause of heat syncope and may occur during or immediately following exercise. Heat stroke is a severe form of heat-related illness resulting in cerebral dysfunction with core body temperature over 40°C. It may present in one of two forms: classic and exertional. Classic (nonexertional) heat stroke occurs in patients with impaired thermoregulatory mechanisms or in extreme environmental conditions. Exertional heat stroke occurs in healthy persons undergoing strenuous exertion in a hot or humid environment. Persons at greatest risk are those who are at the extremes of age, chronically debilitated, and taking medications that interfere with heat-dissipating mechanisms.
 Clinical Findings
Clinical Findings
When diagnosing and treating heat-related illnesses, it is necessary to use an internal (rectal, Foley, or esophageal) thermometer since the skin temperature may not accurately reflect core body temperature. Heat cramps are painful skeletal muscle contractions and severe muscle spasms with onset during or shortly after exercise. Examination findings typically include stable vital signs; normal or slightly increased core body temperature; moist and cool skin; and tender, hard, lumpy, painful muscles that may be twitching. The diagnosis is made clinically.
Heat exhaustion is diagnosed based on symptoms and clinical findings of a core body temperature slightly elevated but less than 40°C, tachycardia, and moist skin. Symptoms are similar to those of heat cramps and heat syncope. Additional symptoms include nausea, vomiting, malaise, myalgias, hyperventilation, thirst, and weakness. Central nervous system symptoms include headache, dizziness, fatigue, anxiety, paresthesias, impaired judgment, and occasionally psychosis. Heat exhaustion may progress to heat stroke if sweating ceases and mental status declines.
Heat syncope generally occurs in the setting of prolonged vigorous physical activity or prolonged standing in a hot humid environment followed by a sudden collapse. Physical examination may reveal cool and moist skin, a weak pulse, and low systolic blood pressure.
Heat stroke is a life-threatening emergency. The hallmark of heat stroke is cerebral dysfunction when the core body temperature is over 40°C. Presenting symptoms include all findings seen in heat exhaustion with additional neurologic symptoms such as dizziness, weakness, emotional lability, confusion, delirium, blurred vision, convulsions, collapse, and unconsciousness. Physical examination findings may be variable and therefore unreliable. Exertional heat stroke may present with sudden collapse and loss of consciousness followed by irrational behavior. Sweating may not be present. Clinicians must be vigilant in monitoring for kidney injury, liver failure, metabolic derangements, respiratory compromise, coagulopathy, and ischemia, since initial laboratory findings may be nonspecific.
 Treatment
Treatment
A. Heat Cramps
Move the patient to a shaded, cool environment and provide oral isotonic or hypertonic rehydration solution to replace both electrolytes and water. Oral salt tablets are not recommended. Advise the patient to rest for at least 2 days with continued dietary supplementation before returning to work or resuming strenuous activity in the heat.
B. Heat Exhaustion
Move the patient to a shaded, cool environment, provide adequate fluid and electrolyte replacement, and initiate active cooling measures if necessary. Physiologic saline or isotonic glucose solution may be administered intravenously when oral administration is not appropriate. At least 48 hours of rest and rehydration are recommended.
C. Heat Syncope
Treatment is essentially the same as for heat exhaustion: rest and recumbency in a shaded, cool place, and fluid and electrolyte replacement by mouth, or intravenously if necessary.
D. Heat Stroke
Initially, the patient’s ABCs (airway, breathing, circulation) must be addressed and stabilized, then treatment is aimed at rapidly reducing the core body temperature within 1 hour while supporting circulation and perfusion. Patients should be placed on pulse oximetry and cardiac monitors while continuing to measure core body temperature, fluid intake and output. The patient should be observed for complications such as hypovolemic or cardiogenic shock, metabolic abnormalities, cardiac arrhythmias, coagulopathy, acute respiratory distress syndrome (ARDS), hypoglycemia, rhabdomyolysis, seizures, organ dysfunction, infection, and severe edema that can progress to a compartment syndrome. Circulatory failure in heat-related illness is mostly due to shock from relative or absolute hypovolemia. Oral or intravenous fluid administration must be provided to ensure adequate urinary output. Clinicians must also assess for and treat concurrent conditions such as infection, trauma, and drug effects.
Choice of cooling method depends on which can be instituted the fastest with the least compromise to the overall care of the patient. Evaporative cooling is preferred for nonexertional heat stroke and conductive-based cooling for exertional heat stroke. Evaporative cooling is a noninvasive, effective, quick, and easy way to reduce temperature. This is accomplished by placing the undressed patient in lateral recumbent position or supported in a hands-and-knees position to expose maximum skin surface to the air while the entire undressed body is sprayed with lukewarm water (20°C) and cooled by large fans circulating room air. Addition of inhaled cool air or oxygen may aid in cooling but must not be used alone. Conductive-based cooling involves cool fluid infusion, gastric or bladder lavage, ice packs, and immersion into ice water or cool water. When immersion in ice water or cold water is available in the field, it is the preferred method of cooling for exertional heat stroke. Ice packs are most effective when covering the whole body, as opposed to the traditional method of placing them in the axilla and groin only. Intravascular heat exchange catheter systems as well as hemodialysis using cold dialysate (30–35°C) have also been successful in reducing core body temperature.
Shivering must be avoided because it inhibits the effectiveness of cooling by increasing internal heat production. Medications can be used to suppress shivering including magnesium, quick-acting opioid analgesics, benzodiazepines, and quick-acting anesthetic agents. Skin massage is recommended to prevent cutaneous vasoconstriction. Antipyretics (aspirin, acetaminophen) have no effect on environmentally induced hyperthermia and are contraindicated. Treatment must be continued until the core body temperature drops to 39°C.
 Prevention
Prevention
Education is necessary to improve prevention and early recognition of heat-related disorders. Individuals may take steps to reduce personal risk factors and to gradually acclimatize to hot environments. For prevention of occupational heat-related illness, a comprehensive preventive program should assess personal risk factors, estimated wet-bulb globe temperature, workload, acclimatization status, and early symptom recognition.
Coaches, athletic trainers, athletes, and parents of young athletes must be educated about heat-related illness, specifically about prevention, risks, symptoms and signs, and treatment. Medical evaluation and monitoring should be used to identify the individuals and the weather conditions that increase the risk of heat-related disorders.
Those who are physically active in a hot environment must increase fluid consumption before, during, and after physical activities. Fluid consumption should include balanced electrolyte fluids and water. Water consumption alone may lead to electrolyte imbalance, particularly hyponatremia. It is not recommended to have salt tablets available for use because of the risk of hypertonic hypernatremia. Close monitoring of fluid and electrolyte intake and early intervention are recommended in situations necessitating exertion or activity in hot environments.
 Prognosis
Prognosis
Mortality is high from heat stroke, most frequently secondary to multiorgan dysfunction. The patient is also at risk for rhabdomyolysis, ARDS, and inflammation even after temperature has normalized. Following heat stroke, immediate reexposure to ambient heat must be avoided.
 When to Refer
When to Refer
Potential consultants include a surgeon for suspicion of compartment syndrome, nephrologist for kidney injury, and transplant surgeon for fulminant liver failure.
 When to Admit
When to Admit
All patients with suspected heat stroke must be admitted to a hospital with intensive care capability for close monitoring.
Epstein Y et al. Heatstroke. N Engl J Med. 2019 Jun 20;380(25):2449–59. [PMID: 31216400]
Gauer R et al. Heat-related illnesses. Am Fam Physician. 2019 Apr 15;99(8):482–9. [PMID: 30990296]
King MA et al. Influence of prior illness on exertional heat stroke presentation and outcome. PLoS One. 2019 Aug 20;14(8):e0221329. [PMID: 31430332]
Lipman GS et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat illness: 2019 update. Wilderness Environ Med. 2019 Dec;30(4S):S33–46. [PMID: 31221601]
ACCIDENTAL SYSTEMIC HYPOTHERMIA
ESSENTIALS OF DIAGNOSIS

 Systemic hypothermia is a core body temperature below 35°C.
Systemic hypothermia is a core body temperature below 35°C.
 Accurate core body temperature measurement must be obtained using a low-reading core temperature probe that measures as low as 25°C.
Accurate core body temperature measurement must be obtained using a low-reading core temperature probe that measures as low as 25°C.
 Core body temperature must be over 32°C before terminating resuscitation efforts.
Core body temperature must be over 32°C before terminating resuscitation efforts.
 Extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass may be considered in hypothermic patients with hemodynamic instability or cardiac arrest.
Extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass may be considered in hypothermic patients with hemodynamic instability or cardiac arrest.
 General Considerations
General Considerations
Systemic hypothermia is defined as core body temperature below 35°C. This may be primary, from exposure to prolonged ambient, extremely low temperature, or secondary, due to thermoregulatory dysfunction. Both may be present at the same time.
Hypothermia must be considered in any patient with prolonged exposure to an ambient cold environment, especially in any patients with prior cold weather injury as well as the risk factors listed in the Cold & Heat section. In prolonged or repetitive cold exposure, hypothermia ensues if the body’s thermoregulatory responses become impaired.
 Clinical Findings
Clinical Findings
Symptoms and signs of hypothermia are typically nonspecific and markedly variable based on the patient’s underlying health and circumstances of cold exposure. Laboratory studies must assess acid-base status; electrolytes, particularly potassium and glucose; kidney, liver, and pancreas function; coagulation; and rhabdomyolysis. Inaccurate laboratory values will occur if the blood sample is warmed to 37°C for testing. All patients must be evaluated for associated conditions including hypoglycemia, trauma, infection, overdose, and peripheral cold injury.
Accurate core body temperature measurements must be obtained using a low-reading core temperature probe that measures as low as 25°C. Stage I hypothermia is typically seen when the core body temperature is between 32°C and 35°C and is defined by shivering and possibly poor judgment or coordination but with hemodynamic stability and a normal level of consciousness. Stage II hypothermia correlates with core body temperature 28–32°C. Shivering stops; bradycardia, dilated pupils, slowed reflexes, cold diuresis, and confusion and lethargy ensue. The electrocardiogram (ECG) may reveal a J wave or Osborn wave (positive deflection in the terminal portion of the QRS complex, most notable in leads II, V5, and V6) (Figure 37–1). When the core body temperature is below 28°C, the likelihood of hemodynamic instability and cardiac arrest increase dramatically. Stage III hypothermia (core body temperature 24–28°C) is characterized by loss of consciousness but present vital signs. Stage IV hypothermia (core body temperature less than 24°C) is the loss of vital signs. Coma, loss of reflexes, asystole, or ventricular fibrillation may falsely lead the clinician to assume the patient is dead despite reversible hypothermia.
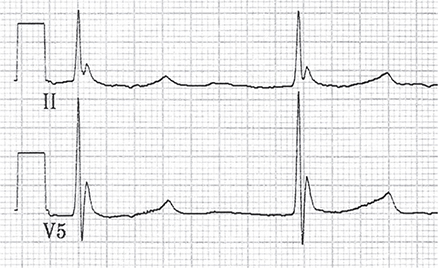
Figure 37–1. Electrocardiogram shows leads II and V5 in a patient whose body temperature is 24°C. Note the bradycardia and Osborn waves. These findings become more prominent as the body temperature lowers and gradually resolve with rewarming. Osborn waves have an extra positive deflection in the terminal portion of the QRS complex and are best seen in the inferior and lateral precordial leads (most notably in leads II, V5, and V6).
 Treatment
Treatment
Rewarming is the initial, imperative treatment for all hypothermic patients. Resuscitation begins with rapid assessment and support of airway, breathing, and circulation, simultaneously with the initiation of rewarming, and prevention of further heat loss. All cold, wet clothing must be removed and replaced with warm, dry clothing and blankets.
Mild or stage I hypothermia can be treated with passive external rewarming (eg, removing and replacing wet clothes with dry ones) or by active external rewarming. In contrast to those with more severe hypothermia, it is safe and recommended for the uninjured patient with mild hypothermia to become physically active to generate heat. Active external rewarming is noninvasive, highly effective, and safe for mild hypothermia. It involves applying external heat to the patient’s skin. Examples include warm bedding, heated blankets, heat packs, and immersion into a 40°C bath. Warm bath immersion limits the ability to monitor the patient or treat other coexisting conditions. Patients with mild hypothermia and previous good health usually respond well to passive and active external warming.
Stage II and III hypothermia are treated as above with the addition of more aggressive rewarming strategies. This requires close monitoring of vital signs and cardiac rhythm during rewarming. Warm intravenous fluids (38–42°C) are considered minimally invasive and effective.
As hypothermia becomes more severe, there are increased complications of both hypothermia itself and of rewarming. Complications of rewarming occur as colder peripheral blood returns to central circulation. This may result in core temperature afterdrop, rewarming lactic acidosis from shunting lactate into the circulation, rewarming shock from peripheral vasodilation, and hypovolemia, ventricular fibrillation, and other cardiac arrhythmias. Afterdrop can be lessened by active external rewarming of the trunk but not the extremities and by avoiding any muscle movement by the patient. Extreme caution must be taken when handling the hypothermic patient to avoid triggering potentially fatal arrhythmias in a phenomenon known as rescue collapse.
Patients with hemodynamic instability or cardiac arrest should be transferred to a facility with ECMO or cardiopulmonary bypass capability.
Early recognition and advanced management guidelines are needed for patients with stage IV hypothermia. For hypothermic patients in cardiac arrest, high-quality CPR must be initiated and continued until the patient’s core body temperature is at least 32°C. Below 30°C, arrhythmias and asystole may be refractory to drug therapy until the patient has been rewarmed; therefore, treatment should focus on excellent CPR technique in conjunction with aggressive rewarming of the patient. Epinephrine or vasopressin may be given in cardiac arrest of the severely hypothermic patient. International Commission for Mountain Emergency Medicine recommends extracorporeal life support as the treatment of choice for patients at high risk for hypothermic cardiac arrest. Extracorporeal life support has been shown to substantially improve survival of patients with unstable circulation or cardiac arrest.
Any hypothermic patient with return of spontaneous circulation must be monitored very closely because of the high likelihood of subsequent multiorgan system failure.
 When to Admit
When to Admit
Hypothermia patients must undergo close monitoring for potential complications. This is typically done during an inpatient admission or prolonged emergency department observation.
Haverkamp FJC et al. The prehospital management of hypothermia—an up-to-date overview. Injury. 2018 Feb;49(2):149–64. [PMID: 29162267]
Pasquier M et al. An evaluation of the Swiss staging model for hypothermia using hospital cases and case reports from the literature. Scand J Trauma Resusc Emerg Med. 2019 Jun 6;27(1):60. [PMID: 31171019]
Zafren K. Out-of-hospital evaluation and treatment of accidental hypothermia. Emerg Med Clin North Am. 2017 May; 35(2):261–79. [PMID: 28411927]
HYPOTHERMIA OF THE EXTREMITIES
ESSENTIALS OF DIAGNOSIS

 “Keep warm, keep dry, and keep moving” to prevent cold-induced injury.
“Keep warm, keep dry, and keep moving” to prevent cold-induced injury.
 Rewarming of the extremity suffering cold-induced injury must be performed as soon as possible once there is no risk of refreezing; exercise, rubbing, or massage must be avoided during rewarming.
Rewarming of the extremity suffering cold-induced injury must be performed as soon as possible once there is no risk of refreezing; exercise, rubbing, or massage must be avoided during rewarming.
 Clinical Findings
Clinical Findings
Cold exposure of the extremities produces immediate localized and then generalized vasoconstriction, which may result in a wide range of injuries. Tissue damage occurs because of ischemia and intravascular thromboses, endothelial damage, or actual freezing. Freezing (frostbite) may occur when skin temperatures drop or in the presence of wind, water, immobility, malnutrition, or vascular disease.
For all forms of cold-induced injury to an extremity, caution must be taken to avoid rubbing or massaging the injured area and to avoid applying moisture, ice, or heat. The cold-injured extremity must be protected from trauma, secondary infection, and further cold exposure.
 Prevention
Prevention
“Keep warm, keep dry, and keep moving.” For optimal prevention of frostbite, individuals must wear warm, dry clothing. Arms, legs, fingers, and toes must be exercised to maintain circulation. Wet clothing, socks, and shoes must be replaced with dry ones. Risk factors include underlying diseases or medications that decrease tissue perfusion and prolonged cold environmental exposure. Caution must be taken to avoid cramped positions; wet or constrictive clothing; prolonged dependency of the feet; use of tobacco, alcohol, and sedative medications; and exposure to wet, muddy ground and windy conditions.
FROSTNIP & CHILBLAIN (Erythema Pernio)
Frostnip is a superficial nonfreezing injury causing local paresthesias of the involved area that completely resolve with passive external rewarming.
Chilblains or erythema pernio are inflammatory skin changes caused by exposure to cold without actual freezing of the tissues. These skin lesions may be red or purple papular lesions, which are painful or pruritic, with burning or paresthesias. They may be associated with edema or blistering and aggravated by warmth. With continued exposure, ulcerative or hemorrhagic lesions may appear and progress to scarring, fibrosis, and atrophy. Treatment consists of elevating and passively externally rewarming the affected part.
IMMERSION FOOT OR TRENCH FOOT
Immersion foot (or hand) is caused by prolonged immersion in cold water or mud, usually below 10°C. Prehyperemic stage is marked by early symptoms of cold and anesthesia of the affected area. Hyperemic stage follows with a hot sensation, intense burning, and shooting pains. Posthyperemic stage occurs with ongoing cold exposure; the affected part becomes pale or cyanotic with diminished pulsations due to vasospasm. This may result in blistering, swelling, redness, ecchymoses, hemorrhage, necrosis, peripheral nerve injury, or gangrene.
Treatment consists of air drying and gradual rewarming by exposure to air at room temperature. Affected parts are elevated to aid in removal of edema fluid. Pressure sites are protected with cushions. Bed rest is required until all ulcers have healed.
FROSTBITE
Frostbite is injury from tissue freezing and formation of ice crystals in the tissue. Most tissue destruction follows reperfusion of the frozen tissues resulting in further tissue damage. In mild cases, only the skin and subcutaneous tissues are involved. Symptoms include numbness, prickling, itching, and pallor. With increasing severity, deeper structures become involved. The skin appears white or yellow, loses elasticity, and becomes immobile. Edema, hemorrhagic blisters, necrosis, gangrene, paresthesias, and stiffness may occur.
 Treatment
Treatment
A. Immediate Treatment
Evaluate and treat the patient for associated systemic hypothermia, concurrent conditions, and injury. Early use of systemic analgesics is recommended for nonfrozen injuries. Hydrate patients to avoid hypovolemia and to improve perfusion.
1. Rewarming—Rapid rewarming at temperatures slightly above normal body temperature may significantly decrease tissue necrosis and reverse the tissue crystallization. If there is any possibility of refreezing, the frostbitten part must not be thawed. Ideally, the frozen extremity must not be used, but if required for evacuation, the affected frozen extremity must be padded and splinted to avoid additional injury. Rewarming is best accomplished by warm bath immersion. The frozen extremity is immersed in a moving water bath heated to 37–39°C for approximately 30 minutes until the area becomes soft and pliable to the touch. Water in this temperature range feels warm but not hot to the normal hand or wrist. If warm water is not available, then passive thawing in a warm environment must be allowed. Dry heat is not recommended because it is more difficult to regulate and increases the likelihood of accidental burns. Thawing may cause tenderness and burning pain. Once the frozen part has thawed and returned to normal temperature, discontinue external heat. In the early stage, rewarming by exercise, rubbing, or friction is contraindicated. The patient must be kept on bed rest with the affected parts elevated and uncovered at room temperature. Avoid application of casts, occlusive dressings, or bandages. Blisters must be left intact unless signs of infection supervene.
2. Anti-infective measures and wound care—Frostbite increases susceptibility to tetanus and infection. Tetanus prophylaxis status must be verified and updated as needed. Infection risk may be reduced by aseptic wound care. Nonadherent sterile gauze and fluffy dressing must be loosely applied to wounds and cushions used for all areas of pressure. Topical aloe vera cream or gel should be applied to the thawed tissue before application of dressings. Antibiotics should not be administered empirically.
B. Medical and Surgical Treatment Options
Telemedicine may be used so that specialists can provide advice on early field treatment of cold-injured patients in remote areas, thereby improving outcomes. Nonsteroidal anti-inflammatory drugs should be administered (in the absence of contraindications) until frostbite wounds are healed or surgical management occurs. Clinicians must watch for evidence of compartment syndrome and need for fasciotomy. Eschar formation without evidence of infection may be conservatively treated. The underlying skin may heal spontaneously with the eschar acting as a biologic dressing. Rates of amputation have been reduced with the use of intravenous infusions of synthetic prostaglandins and of tissue plasminogen activators, and with intra-arterial administration of a thrombolytic within 24 hours of exposure. The rate of tissue salvage decreases with every hour of delay from rewarming to thrombolytic therapy.
C. Follow-Up Care
Patient education must include ongoing care of the cold injury and prevention of future hypothermia and cold injury. Gentle, progressive physical therapy to promote circulation should be instituted as tolerated.
 Prognosis
Prognosis
Recovery from frostbite depends on the underlying comorbidities, the extent of initial tissue damage, the rewarming reperfusion injury, and the late sequelae. The involved extremity may be at increased susceptibility for discomfort and injury upon re-exposure to cold. Neuropathic sequelae include pain, numbness, tingling, hyperhidrosis, and cold sensitivity of the extremities. Nerve conduction abnormalities may persist for many years after a cold injury.
 When to Admit
When to Admit
• Management of tissue damage, comorbidities, associated injuries.
• Need for hospital-based interventions.
• Psychosocial factors that could compromise patient safety or recovery.
Drinane J et al. Thrombolytic salvage of threatened frostbitten extremities and digits: a systematic review. J Burn Care Res. 2019 Aug 14;40(5):541–9. [PMID: 31188429]
Khan SL et al. Barriers to frostbite treatment at an academic medical center. Am J Emerg Med. 2019 Aug;37(8):1601.e3–5. [PMID: 31088748]
McIntosh SE et al. Wilderness Medical Society Practice Guidelines for the Prevention and Treatment of Frostbite: 2019 Update. Wilderness Environ Med. 2019 Dec;30(4S):S19–32. [PMID: 31326282]
Shenaq DS et al. Urban frostbite: strategies for limb salvage. J Burn Care Res. 2019 Aug 14;40(5):613–9. [PMID: 30990527]
DROWNING
ESSENTIALS OF DIAGNOSIS

 The first requirement of rescue is immediate rescue breathing and CPR.
The first requirement of rescue is immediate rescue breathing and CPR.
 Clinical manifestations include hypoxemia, pulmonary edema, and hypoventilation.
Clinical manifestations include hypoxemia, pulmonary edema, and hypoventilation.
 Patients must be assessed for hypothermia, hypoglycemia, alcohol intake, concurrent injuries, and medical conditions.
Patients must be assessed for hypothermia, hypoglycemia, alcohol intake, concurrent injuries, and medical conditions.
 General Considerations
General Considerations
Drowning, as defined by the World Health Organization, is any “process resulting in primary respiratory impairment from submersion in a liquid medium.” Drowning may result in asphyxiation (from fluid aspiration or laryngospasm), hypoxemia, hypothermia, and acidemia. Outcomes from drowning range from life without morbidity to death. Morbidity may be immediate or delayed. A patient may be deceptively asymptomatic during the initial recovery period only to deteriorate or die as a result of acute respiratory failure within the following 12–24 hours. Disseminated intravascular coagulation may also lead to bleeding after asphyxiation from drowning.
Drowning is a leading cause of death in children and highly preventable in all ages with implementation of educational and safety measures. Clinicians must provide patient education and guidance about drowning prevention.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The patient’s appearance may vary from asymptomatic to marked distress with abnormal vital signs. Symptoms and signs include respiratory difficulty, chest pain, dysrhythmia, hypotension, cyanosis, and hypothermia (from cold water or prolonged submersion). A pink froth from the mouth and nose indicates pulmonary edema. The patient may experience headache, neurologic deficits, and altered level of consciousness.
B. Laboratory Findings
Metabolic acidosis is common and arterial blood gas results may be helpful in determining the degree of injury since initial clinical findings may appear benign. PaO2 is usually decreased; PaCO2 may be increased or decreased; pH is decreased. Bedside blood sugar must be checked rapidly. Other testing is based on clinical scenario.
 Prevention
Prevention
Education and prevention are critical given the high burden of disease from drowning.
Preventive measures must be taken to reduce morbidity and mortality from drowning. Conditions that increase risk of submersion injury include the use of alcohol, psychotropics, and other drugs, inadequate water safety skills, poor physical health, hyperventilation, sudden acute illness, acute trauma, decompression sickness, dangerous water conditions, and environmental exposures.
 Treatment
Treatment
A. First Aid
1. The first requirement of rescue is immediate basic life support treatment and CPR. At the scene, immediate airway management and measures to combat hypoxemia are critical to improve outcome.
2. Patient must be assessed for hypothermia, hypoglycemia, concurrent medical conditions, and associated trauma.
3. Rescuer must not attempt to drain water from the victim’s lungs.
4. Resuscitation and basic life support efforts must be continued until core body temperature reaches 32°C.
B. Subsequent Management
1. Ensure optimal ventilation and oxygenation—The onset of hypoxemia exists even in the alert, conscious patient who appears to be breathing normally. Oxygen must be administered immediately at the highest available concentration. Oxygen saturation must be maintained at 90% or higher via some form of supplemental oxygen.
Serial physical examinations and chest radiographs must be performed to detect possible pneumonitis, atelectasis, and pulmonary edema. Bronchodilators may be used to treat wheezing. Nasogastric suctioning may be necessary to decompress the stomach.
2. Cardiovascular support—Intravascular volume status must be monitored and supported by vascular fluid replacement, vasopressors, or diuretics as needed.
3. Correction of blood pH and electrolyte abnormalities—Metabolic acidosis is present in the majority of drowning victims, but this typically corrects through adequate ventilation and oxygenation. Glycemic control improves outcome.
4. Cerebral and spinal cord injury—Central nervous system damage may progress despite apparently adequate treatment of hypoxia and shock.
5. Hypothermia—Core body temperature must be measured and managed as appropriate (see Accidental Systemic Hypothermia, above).
 Course & Prognosis
Course & Prognosis
As the duration of submersion lengthens, the probability of worse outcomes increases. Favorable prognosis is related to a duration of submersion less than 5 minutes. Respiratory damage is often severe in the minutes to hours following a drowning. With appropriate respiratory supportive treatment, patients may improve rapidly over the first few days following the drowning. Long-term complications of drowning may include neurologic impairment, seizure disorder, and pulmonary or cardiac damage. Prognosis is directly correlated with the patient’s age, submersion time, rapidity of prehospital resuscitation and subsequent transport to a medical facility, clinical status at time of arrival to hospital, Glasgow Coma Scale score, pupillary reactivity, and overall health assessment (APACHE II score).
 When to Admit
When to Admit
Most patients with significant drowning or concurrent medical or traumatic conditions require inpatient monitoring following the event. This includes continuous monitoring of cardiorespiratory, neurologic, renal, and metabolic function. Pulmonary edema may not appear for 24 hours.
Parenteau M et al. Drowning management. Mil Med. 2018 Sep 1;183(Suppl 2):172–9. [PMID: 30189074]
Reynolds JC et al. Long-term survival after drowning-related cardiac arrest. J Emerg Med. 2019 Aug;57(2):129–39. [PMID: 31262547]
Sulovic LS et al. Accidental drowning: the importance of early measures of resuscitation for a successful outcome. Case Rep Emerg Med. 2018 Jun 4;2018:7525313. [PMID: 29974001]
THERMAL BURNS
ESSENTIALS OF DIAGNOSIS

 Estimates of the burn location, size, and depth greatly determine the treatment plan.
Estimates of the burn location, size, and depth greatly determine the treatment plan.
 The first 48 hours of burn care offer the greatest impact on morbidity and mortality of a burn victim.
The first 48 hours of burn care offer the greatest impact on morbidity and mortality of a burn victim.
Worldwide, burns are a common cause of injury and potential morbidity and mortality. Burn prognosis is affected by the type of environment where the burn occurred. Low-resource settings (wilderness or low-income areas) are associated with delays in and suboptimal access to standard burn treatments.
The first 48 hours after thermal burn injury offer the greatest opportunity to impact the survival of the patient. Early surgical intervention, wound care, enteral feeding, glucose control and metabolic management, infection control, and prevention of hypothermia and compartment syndrome have contributed to significantly lower mortality rates and shorter hospitalizations. Research utilizing several different well-established burn severity scores has shown the importance of patient comorbidities to the prognosis of patients with severe burn injuries.
 General Considerations
General Considerations
A. Classification
Burns are classified by extent, depth, patient age, and associated illness or injury. Accurate estimation of burn size and depth is necessary to quantify the parameters of resuscitation.
1. Extent—In adults, the “rule of nines” (Figure 37–2) is useful for rapidly assessing the extent of a burn. It is important to view the entire patient to make an accurate assessment of skin findings on initial and subsequent examinations. One rule of thumb is that the palm of an open hand in adult patients constitutes 1% of total body surface area (TBSA). TBSA is calculated for partial- and full-thickness burns.

Figure 37–2. Estimation of body surface area in burns.
2. Depth—Judgment of depth of injury is difficult. Superficial burns may appear red or gray but will demonstrate excellent capillary refill and are not blistered initially. If the wound is blistered and appears pink and wet, this represents a superficial partial-thickness burn. Deep partial-thickness burns appear white and wet, and bleed if poked; cutaneous sensation is maintained. Full-thickness burns result in a loss of adnexal structures and may appear white-yellow in color, or may have a black charred appearance. This stiff, dry skin does not bleed when poked and cutaneous sensation is lost.
Deep partial-thickness and full-thickness burns are treated in a similar fashion. Both require early debridement and grafting to heal appropriately, without which the skin becomes thin and scarred.
B. Survival After Burn Injury
Transfer to a burn unit is determined by large burn size, circumferential burn, or burn involving a joint or high-risk body part, and by comorbidities. Mortality rates have been significantly reduced due to treatment advances including improvements in wound care, treatment of infection, early burn excision, skin substitute usage, and early nutritional support.
C. Associated Injuries or Illnesses
Smoke inhalation, associated trauma, and electrical injuries are commonly associated with burns. Severe burns from any source may result in similar complications (eg, infections, respiratory compromise, multiorgan dysfunction, venous thromboembolism, and gastrointestinal complications).
D. Systemic Reactions to Burn Injury
Burns greater than approximately 20% of TBSA may lead to systemic metabolic derangements requiring intensive support. The inflammatory cascade can result in shock and coagulopathy.
 Treatment
Treatment
A. Initial Resuscitation
1. Primary survey—Burn patients require a full trauma assessment, starting with “ABCDE” (airway, breathing, circulation, disability, exposure).
a. Airway control—Serial assessments of airway and breathing are necessary because airway compromise and ARDS may develop, particularly in those with inhalation injury.
b. Vascular access—Vascular access must be obtained on all burn patients.
c. Fluid resuscitation—Patients with burns greater than 15% of TBSA require intravascular fluid administration of large volumes of crystalloid. The most widely recognized guideline for fluid resuscitation is the Parkland formula (https://www.mdcalc.com/parkland-formula-burns) in which the fluid requirement in the first 24 hours is estimated as 4 mL/kg × body weight per percent of body surface area burned. Half the calculated fluid is given in the first 8-hour period from the time of injury, not the time of arrival to medical care. The remaining fluid is delivered over the next 16 hours. An extremely large volume of fluid may be required. Crystalloid solutions alone may be insufficient to restore cardiac preload during the period of burn shock. Conversely, clinicians must watch for clinical signs of volume overload as it may lead to pulmonary complications or to a compartment syndrome from edema. Electrical burns and inhalation injury may increase the fluid requirement.
B. Management
1. Pain control—Pain control is critical in burn injury patients. Treatment is with (oral or intravenous) nonsteroidal anti-inflammatory drugs and opioids (see Chapter 5).
2. Chemoprophylaxis—
a. Tetanus immunization—Verify and update tetanus prophylaxis status in all burn patients. (See Chapter 33.)
b. Antibiotics—All nonsuperficial wounds need to be covered with topical antibiotics. Prophylaxis with systemic antibiotics is not indicated.
3. Surgical management—
a. Escharotomy—As tissue swelling occurs, ischemia may develop under any constricting eschar of an extremity, neck, chest, or in circumferential full-thickness burns of the trunk. Escharotomy incisions can be life- and limb-saving.
b. Fasciotomy—Fasciotomy is indicated for any compartment syndrome. Clinicians must frequently monitor patients for development of early signs of a compartment syndrome, particularly in those with circumferential burns.
c. Debridement, dressings, and topical and systemic antibiotic therapy—Minor burn wounds must be debrided to determine the depth of the burn and then thoroughly cleansed. Thereafter, daily wound care must consist of debridements as needed, topical antibiotics, and wound dressings. Patient compliance and adequate pain control is essential for successful outpatient treatment. The wound must be reevaluated by the treating clinician within 24–72 hours to evaluate for signs of infection.
The goal of burn wound management is to protect the wound from desiccation and avoid further injury or infection. Regular and thorough cleansing of burned areas is of critical importance. Topical antibiotics may be applied after wound cleansing. Silver sulfadiazine is no longer recommended.
It is imperative to closely monitor for and treat systemic infection, since this remains a leading cause of morbidity among patients with major burn injuries. Health care–associated infections are increasingly common.
d. Wound management—The goal of therapy after fluid resuscitation is rapid and stable closure of the wound. Wounds that do not heal spontaneously in 7–10 days (ie, deep partial-thickness or full-thickness burns) are best treated by a specialist through excision and autograft to avoid development of granulation and infection. The quality of the skin in regenerated deep partial-thickness burns is marginal because of the very thin dermis that emerges.
Cultured allogeneic keratinocyte grafts can provide rapid early coverage for superficial burn injuries. Skin substitution with cultured grafts may be life-saving for severe burns. Although the replaced dermis has nearly normal histologic dermal elements, there are no adnexal structures present, and very few, if any, elastic fibers.
e. Abdominal compartment syndrome—Abdominal compartment syndrome is a potentially lethal condition that may develop in severely burned patients, with mortality rates of approximately 60% despite surgical intervention. Diagnosis is confirmed by bladder pressures greater than 30 mm Hg in at-risk patients. Surgical abdominal decompression may improve ventilation and oxygen delivery but may not impact survival.
C. Patient Support
Burn patients require extensive supportive care, both physiologically and psychologically. It is important to maintain normal core body temperature and avoid hypothermia, by maintaining environmental temperature at or above 30°C, in patients with burns over more than 20% of TBSA. Burn patients are at risk for many complications such as respiratory injury, ARDS or respiratory failure unresponsive to maximal ventilatory support, sepsis, multiorgan failure, and venous thromboembolism.
Burn patients have increased metabolic and energy needs for wound healing and require careful assessment and provision of optimal nutrition. Early aggressive nutrition (by parenteral or enteral routes) reduces infections, recovery time, noninfectious complications, length of hospital stay, long-term sequelae, and mortality.
Prevention of long-term scars remains a formidable problem in seriously burned patients.
 Prognosis
Prognosis
Prognosis depends on the extent and location of the burn tissue damage, associated injuries, comorbidities, and complications. Hyperglycemia is a predictor of worse outcomes. Common complications include sepsis; gangrene requiring limb amputation; or neurologic, cardiac, cognitive, or psychiatric dysfunction. Psychiatric support may be necessary following burn injury.
 When to Refer
When to Refer
Transfer to a burn unit is indicated for large burn size (for partial-thickness burns greater than 10% of TBSA or for full-thickness burns greater than 5% of TBSA), circumferential burn, inhalation injury, or burn involving a joint or high-risk body part (face, hands, feet, genitalia), and for patients with comorbidities.
 When to Admit
When to Admit
• All severe burn patients require extensive supportive care, both physiologically and psychologically.
• Significant burns (based on location and extent).
• Patients with significant comorbidities and suboptimal home situations.
• Burn center consultation can advise which patients require transfer and which can be managed via telemedicine/telephone consultation.
• Monitoring includes vital signs, wound care, and observation for potential complications of electrolyte abnormalities, acute kidney injury, hepatic failure, cardiopulmonary compromise, hyperglycemia, and infection.
Chao KY et al. Respiratory management in smoke inhalation injury. J Burn Care Res. 2019 Jun 21;40(4):507–12. [PMID: 30893426]
Otterness K et al. Emergency department management of smoke inhalation injury in adults. Emerg Med Pract. 2018 Mar;20(3):1–24. [PMID: 29489306]
Smith RR et al. Analysis of factors impacting length of stay in thermal and inhalation injury. Burns. 2019 Nov;45(7):1593–9. [PMID: 31130323]
ELECTRICAL INJURY
ESSENTIALS OF DIAGNOSIS

 Extent of injury is determined by the type, amount, duration, and pathway of electrical current.
Extent of injury is determined by the type, amount, duration, and pathway of electrical current.
 Clinical findings of death are unreliable; therefore, resuscitation efforts must be attempted before assuming the electrical injury victim is dead.
Clinical findings of death are unreliable; therefore, resuscitation efforts must be attempted before assuming the electrical injury victim is dead.
 Skin findings may be misleading and are not indicative of the degree of deeper tissue injury.
Skin findings may be misleading and are not indicative of the degree of deeper tissue injury.
 General Considerations
General Considerations
Electricity-induced injuries are common and yet most are preventable. These injuries occur by exposure to electrical current of low voltage, high voltage, or lightning. Electrical current type is either alternating current (AC) or direct current (DC) and is measured in volts (V). Electricity causes acute injury by direct tissue damage, muscle tetany, direct thermal injury and coagulation necrosis, and associated trauma.
Alternating current (AC) is an electric current that periodically reverses direction in a sine wave pattern and may cause muscle tetany, which prolongs the duration and amount of current exposure. AC can be low voltage or high voltage. Most households and businesses use electric power in the form of AC at low voltages (less than 1000 V). Low voltage electrical injuries can range from minor to significant damage and death. High voltage (greater than 1000 V) AC electrical injuries are often related to occupational exposure and associated with deep tissue damage and higher morbidity and mortality. Direct current (DC) is unidirectional electrical flow (ie, lightning, batteries, and automotive electrical systems). It is more likely to cause a single intense muscle contraction and asystole. Lightning differs from other high-voltage electrical shock in that lightning delivers a direct current of millions of volts in a fraction of a second.
The extent of damage from electrical injuries depends on the following factors: voltage, current type, tissue resistance, moisture, pathway, duration of exposure, associated trauma, and comorbidities. Current is the most important determinant of tissue damage. Current passes through the tissues of least resistance as energy, which produces heat and causes direct thermal injury. Tissue resistance varies throughout the body with nerve cells being the most vulnerable and bone the most resistant to electrical current.
 Clinical Findings
Clinical Findings
Electrical burns are of three distinct types: flash (arcing) burns, flame (clothing) burns, and the direct heating effect of tissues by the electrical current.
Skin damage does not correlate with the degree of injury. Not all electrical injuries cause skin damage; very minor skin damage may be present with massive internal injuries. Symptoms and signs may range from very subtle to death. The presence of entrance and exit burns signifies an increased risk of deep tissue damage. Current passing through skeletal muscle can cause muscle necrosis and contractions severe enough to result in bone fracture. If the current passes through the heart or brainstem, death may be immediate due to ventricular fibrillation, asystole, or apnea.
Resuscitation must be initiated on all victims of electrical injury since clinical findings are deceptive and unreliable.
 Complications
Complications
Complications include cardiac or respiratory arrest; dysrhythmias; neurologic dysfunction, such as autonomic dysfunction (resulting in pupils that are fixed, dilated, or asymmetric); altered mental status; seizures; paralysis; headache; vascular injury; tissue edema and necrosis; compartment syndrome; associated traumatic injuries (ruptured ear drums, fractures); pneumothorax; rhabdomyolysis; acute kidney injury; hypovolemia; infections; ocular complications; sepsis; gangrene; and cognitive or psychiatric dysfunction. Psychiatric support may be necessary following electrical injury.
 Treatment
Treatment
A. Emergency Measures
The patient must be assessed and treated as a trauma victim. The patient must be safely separated from the electrical current prior to initiation of CPR or other treatments. Resuscitation must then be initiated since clinical findings of death are unreliable.
B. Hospital Measures
The initial assessment involves airway, breathing, and circulation followed by a full trauma protocol. Fluid resuscitation is important to maintain adequate urinary output. Initial evaluation includes cardiac monitoring and ECG, complete blood count, electrolytes, kidney function tests, liver chemistries, creatine phosphokinase or urine myoglobin, urinalysis, and cardiac enzymes. ECG does not show typical patterns of ischemia since the electrical damage is epicardial. Victims must be evaluated for hidden injury, organ injury, blunt trauma, dehydration, skin burns, hypertension, acid-base disturbances, and neurological as well as psychological damage.
Electrical burn wounds are an underrecognized, yet devastating form of burn injury with wide-ranging and significant complications.
When electrical injury occurs, there must be a high suspicion for extensive deep tissue necrosis. Superficial skin may appear deceivingly benign, leading to a delayed or completely overlooked diagnosis of deep tissue injury. Deep tissue necrosis leads to profound tissue swelling, resulting in a high risk of a compartment syndrome. Early debridement of devitalized tissues and tetanus prophylaxis may reduce the risks of infection.
Pain management is important before, during, and after initial treatment and subsequent rehabilitation.
 Prognosis
Prognosis
Prognosis depends on the degree and location of electrical injury, initial tissue damage, associated injuries, comorbidities, and complications.
 When to Refer
When to Refer
• Specialists may be needed to perform fasciotomy for compartment syndrome, to perform debridement of devitalized tissue, or to perform microvascular reconstruction.
• Ophthalmologists should evaluate patients for possible ocular complications, and subsequently ENT physicians should evaluate for tympanic membrane rupture or hearing loss.
 When to Admit
When to Admit
Indications for hospitalization include high-voltage exposure; dysrhythmia or ECG changes; large burn size; neurologic, pulmonary, or cardiac symptoms; suspicion of significant deep tissue or organ damage; transthoracic current pathway; history of cardiac disease or other significant comorbidities or injuries; and need for surgery.
Gentges J et al. Electrical injuries in the emergency department: an evidence-based review. Emerg Med Pract. 2018 Nov;20(11):1–20. [PMID: 30358379]
Gille J et al. Electrical injury—a dual center analysis of patient characteristics, therapeutic specifics and outcome predictors. Scand J Trauma Resusc Emerg Med. 2018 May 31;26(1):43. [PMID: 29855384]
Pilecky D et al. Risk of cardiac arrhythmias after electrical accident: a single-center study of 480 patients. Clin Res Cardiol. 2019 Aug;108(8):901–8. [PMID: 30771067]
Stockly OR et al. The impact of electrical injuries on long-term outcomes: a Burn Model System National Database study. Burns. 2020 Mar;46(2):352–9. [PMID: 31420267]
Waldmann V et al. Electrical cardiac injuries: current concepts and management. Eur Heart J. 2018 Apr 21;39(16):1459–65. [PMID: 28444167]
RADIATION EXPOSURE
ESSENTIALS OF DIAGNOSIS

 Damage from radiation is determined by the source, type, quantity, duration, bodily location, and susceptibility and accumulation of exposures of the person.
Damage from radiation is determined by the source, type, quantity, duration, bodily location, and susceptibility and accumulation of exposures of the person.
 Clinicians and patients must be educated regarding the risks of medical diagnostic radiation. These radiation risks must be weighed against the benefits of the medical imaging needed.
Clinicians and patients must be educated regarding the risks of medical diagnostic radiation. These radiation risks must be weighed against the benefits of the medical imaging needed.
 General Considerations
General Considerations
Radiation exposure may occur from environmental, occupational, medical care, accidental, or intentional causes. The extent of damage from radiation exposure depends on the type, quantity, and duration of radiation exposure; the organs exposed; the degree of disruption to DNA; metabolic and cellular function; and the age, underlying condition, susceptibility, comorbidities, and accumulative exposures of the victim.
Radiation occurs from both nonionizing and ionizing radiation sources. Nonionizing radiation is low energy, resulting in injuries related to local thermal damage (ie, microwave, ultraviolet, visible light, and radiowave). Ionizing radiation is high energy, causing bodily damage in several ways. Ionizing radiation is either electromagnetic (x-rays and gamma rays) or particulate (alpha or beta particles, neutrons, and protons). Exposure may be external, internal, or both.
The International Commission on Radiological Protection (ICRP) website provides the most up-to-date recommendations for protection against ionizing radiation (http://www.icrp.org/index.asp). The World Health Organization (WHO) publishes guidelines on radiation emergencies (https://www.who.int/ionizing_radiation/a_e/en/). These guidelines include recommended interventions during the early, intermediate, and late emergency phases and the management of their psychosocial impact.
 Clinical Findings
Clinical Findings
Radiation exposure results in acute and delayed effects. It is important to obtain the event history in order to assess the amount of radiation exposure, and the possibility of coexisting injuries or conditions. Acute effects involve damage of the rapidly dividing cells (ie, the mucosa, skin, and bone marrow). This may be manifested as mucositis, nausea, vomiting, gastrointestinal edema and ulcers, skin burns, and bone marrow suppression over hours to days after exposure. Delayed effects include malignancy, reproduction abnormalities, and liver, kidney, and central nervous system and immune system dysfunction.
Clinicians must be educated to recognize and treat acute radiation sickness also referred to as acute radiation syndrome. Acute radiation syndrome is due to an exposure to high doses of ionizing radiation over a brief time course. The symptom onset is within hours to days depending on the dose. Symptoms include anorexia, nausea, vomiting, weakness, exhaustion, lassitude and, in some cases, prostration; these symptoms may occur singly or in combination. Dehydration, anemia, and infection may follow. The Centers for Disease Control and Prevention offers web-based information regarding acute radiation syndrome (https://www.cdc.gov/nceh/radiation/emergencies/arsphysicianfactsheet.htm).
In acute radiation exposure, medical care includes close monitoring of the gastrointestinal, cutaneous, hematologic, cardiopulmonary, and cerebrovascular symptoms and signs from initial exposure and over time.
 Therapeutic Radiation Exposure
Therapeutic Radiation Exposure
Radiation therapy has been a successful component in the treatment of many malignancies. These radiation-treated cancer survivors have a higher risk of development of a second malignancy; obesity; and pulmonary, cardiac, and thyroid dysfunction as well as an increased overall risk for chronic health conditions and mortality.
 Medical Imaging Radiation Exposure
Medical Imaging Radiation Exposure
Medical imaging with ionizing radiation exposure has dramatically increased over the past few decades. Medical imaging radiation exposure is a growing concern for health care professionals and the public. With the rising use of medical imaging, there is international focus on improving the safety by standardization and regulation of radiation dosing in medical diagnostics and education for clinician and the public about this issue.
The American College of Radiology (ACR) provides “ACR Appropriateness Criteria,” which are evidence-based guidelines created by expert panels to serve as a reference for best practices for imaging decisions by health care providers (https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria). Clinicians and patients must carefully weigh the risks and benefits of radiation exposure when deciding on an imaging test.
 Occupational & Environmental Radiation Exposure
Occupational & Environmental Radiation Exposure
The National Nuclear Security Administration’s Radiation Emergency Assistance Center provides 24-hour access to expert consultation services over the telephone or via email (telephone: 1-865-576-1005 or email: https://orise.orau.gov/reacts/). The Centers for Disease Control and Prevention “Radiation Emergencies” website (https://www.cdc.gov/nceh/radiation/emergencies/index.htm) is a useful resource for professionals.
 Treatment
Treatment
Treatment is focused on decontamination, symptomatic relief, supportive care, and psychosocial support and management of coexisting conditions or injuries. Specific supportive treatments are determined by the dose, route, and effects of exposure and associated conditions present.
 Prognosis
Prognosis
Prognosis is determined by the radiation dose, duration, and frequency as well as by the underlying condition of the victim. Death is usually due to hematopoietic failure, gastrointestinal mucosal damage, central nervous system damage, widespread vascular injury, or secondary infection.
Carcinogenesis is related to the radiation type, total dose, duration, and accumulation of exposure, and to the susceptibility of the victim. Radiation-related cancer risks persist throughout the exposed person’s life span.
With the increased use of ionizing radiation for medical diagnostics and treatments, there is a growing concern for the iatrogenic increase in radiation-induced cancer risks. There are age-related sensitivities to radiation; prenatal and younger age victims are more susceptible to carcinogenesis.
 When to Admit
When to Admit
Most patients with significant ionizing radiation exposure require admission for close monitoring and supportive treatment.
Dainiak N. Medical management of acute radiation syndrome and associated infections in a high-casualty incident. J Radiat Res. 2018 Apr 1;59(Suppl 2):ii54–64. [PMID: 29509947]
Oren O et al. Curbing unnecessary and wasted diagnostic imaging. JAMA. 2019 Jan 22;321(3):245–6. [PMID: 30615023]
Parrish JS et al. Disasters resulting from radiologic and nuclear events. Crit Care Clin. 2019 Oct;35(4):619–31. [PMID: 31445609]
Subcommittee on Appropriateness Criteria Radiation Exposure; Jordan DW et al. Validation of adult relative radiation levels using the ACR Dose Index Registry: report of the ACR Appropriateness Criteria Radiation Exposure Subcommittee. J Am Coll Radiol. 2019 Feb;16(2):236–9. [PMID: 30245216]
ENVIRONMENTAL DISORDERS RELATED TO ALTITUDE
DYSBARISM & DECOMPRESSION SICKNESS
ESSENTIALS OF DIAGNOSIS

 Symptoms temporally related to recent altitude or pressure changes.
Symptoms temporally related to recent altitude or pressure changes.
 Early recognition and prompt treatment of decompression illness are extremely important.
Early recognition and prompt treatment of decompression illness are extremely important.
 Patient must also be assessed for hypothermia, hypoglycemia, concurrent injuries, and medical conditions.
Patient must also be assessed for hypothermia, hypoglycemia, concurrent injuries, and medical conditions.
 Consultation with diving medicine or hyperbaric oxygen specialist is indicated.
Consultation with diving medicine or hyperbaric oxygen specialist is indicated.
 General Considerations
General Considerations
Dysbarism and decompression illness are physiologic problems that result from altitude changes and the effects of environmental pressure on the gases in the body during underwater descent and ascent. These are most likely to occur when scuba diving is followed closely by travel to high altitudes, or when the scuba diver is not adherent to the conservative dive guidelines for dive duration, course, depth, and surface times.
As a diver begins to descend, the gases in the body compress and dissolve in the blood and the tissues. These gases dissolve throughout areas of the body that are both compressible (lungs, gastrointestinal tract) and noncompressible (sinuses, joints). When the diver descends further, there is increased pressure on the gases to dissolve even more into the bloodstream and these tissues. During the subsequent ascent, the dissolved gases expand. The gas compression and expansion depends on the difference between the atmospheric pressure and the partial pressure of the gas dissolved in the tissues.
Dysbarism results from barotrauma when gas compression or expansion occurs in parts of the body that are noncompressible or have limited compliance. Pulmonary overinflation syndrome is one of the most serious and potentially fatal results of barotrauma. This syndrome is due to an inappropriately rapid ascent causing alveoli rupture and air bubble extravasation into tissue planes or even the cerebral circulation.
Decompression illness occurs when the ascent is too rapid and gas bubbles form and cause damage depending on their location (ie, coronary, pulmonary, spinal or cerebral blood vessels, joints, soft tissues). Decompression illness symptoms depend on the size, number, and location of released gas bubbles (notably nitrogen). Risk of decompression illness depends on multiple factors: the dive details (depth, duration, number of dives, interval surface time between dives, and water conditions) as well as the diver’s age, weight, physical condition, physical exertion, the rate of ascent, and the length of time between the low altitude (scuba dive) and high altitude (air travel or ground ascent). Predisposing factors include obesity, injury, hypoxia, lung or cardiac disease, right to left cardiac shunt, diver’s overall health, dehydration, alcohol and medication effects, and panic attacks. Decompression illness may also occur in those who take hot showers after cold dives. There have been clinical case reports of delayed presentations of decompression illness following post-dive exercise.
Preventive measures include diver education; pre-dive medical screening and dive planning; strict adherence to dive course, timing, and depths; and a slow and controlled ascent plus proper control of buoyancy. Conservative recommendation is to avoid high altitudes (air travel or ground ascent) for at least 24 hours after surfacing from the dive, especially following multiple dives.
 Clinical Findings
Clinical Findings
The range of clinical manifestations varies depending on the location of the gas bubble formation or the compressibility of gases in the body. Symptom onset may be immediate or within minutes or hours (in the majority), up to 48 hours later. Decompression illness symptoms include pain in the joints (“the bends”); skin pruritus or burning (skin bends); cardiac symptoms (acute coronary syndrome, conduction abnormalities); spinal cord or cerebral symptoms (focal neurologic dysfunction or “dissociation” symptoms that do not follow typical distribution neuroanatomy patterns); labyrinthine decompression illness (“the staggers,” central vertigo); pulmonary decompression illness (“the chokes,” inspiratory pain, cough, and respiratory distress); arterial gas embolism (cerebral, pulmonary); barotrauma of the lungs, ear, and sinus; and coma and death.
Decompression illness involving the brain and spinal cord may occur by different mechanisms due to air bubbles causing arterial occlusion, venous obstruction, or in situ toxicity.
The clinician must assess for associated conditions of hypothermia, hypoglycemia, hypovolemia, drowning, trauma, envenomation, or concurrent medical conditions.
 Treatment
Treatment
Early recognition and prompt treatment are extremely important. Decompression illness must be considered if symptoms are temporally related to recent diving or altitude or pressure changes within the past 48 hours. Continuous administration of 100% oxygen is indicated and beneficial for all patients. Hyperbaric oxygen treatment is commonly recommended for decompression illness symptoms. Immediate consultation with a diving medicine or hyperbaric oxygen specialist is indicated even if mild decompression illness symptoms resolve. Nonsteroidal anti-inflammatory drugs, acetaminophen, or aspirin may be given for pain control if there are no contraindications. Opioids must be used very cautiously, since these may obscure the response to recompression.
 When to Admit
When to Admit
Rapid transportation to a hyperbaric treatment facility for recompression is imperative for decompression illness. The Divers Alert Network is an excellent worldwide resource for emergency advice 24 hours daily for the management of diving-related conditions (https://www.diversalertnetwork.org/).
Gore MD et al. Is it safe to SCUBA dive with asthma? Expert Rev Respir Med. 2019 Sep 11:1–9. [PMID: 31509025]
Mallen JR et al. SCUBA medicine for otolaryngologists: Part II. Diagnostic, treatment, and dive fitness recommendations. Laryngoscope. 2020 Jan;130(1):59–64. [PMID: 30776095]
Pollock NW et al. Updates in decompression illness. Emerg Med Clin North Am. 2017 May;35(2):301–19. [PMID: 28411929]
HIGH-ALTITUDE ILLNESS
ESSENTIALS OF DIAGNOSIS

 The severity of the high-altitude illness is affected by the rate and height of ascent and the individual’s susceptibility.
The severity of the high-altitude illness is affected by the rate and height of ascent and the individual’s susceptibility.
 Prompt recognition and medical treatment of early symptoms of high-altitude illness may prevent progression.
Prompt recognition and medical treatment of early symptoms of high-altitude illness may prevent progression.
 Clinicians must assess other conditions that may coexist or mimic symptoms of high-altitude illness.
Clinicians must assess other conditions that may coexist or mimic symptoms of high-altitude illness.
 Immediate descent is the definitive treatment for high-altitude cerebral edema and high-altitude pulmonary edema.
Immediate descent is the definitive treatment for high-altitude cerebral edema and high-altitude pulmonary edema.
 General Considerations
General Considerations
As altitude increases, there is a decrease in both barometric pressure and oxygen partial pressure resulting in hypobaric hypoxia. High-altitude illnesses are due to hypobaric hypoxia at high altitudes (usually greater than 2000 meters or 6562 feet). High-altitude illness includes a spectrum of disorders categorized by end-organ effects (mostly cerebral and pulmonary) and exposure duration (acute and long-term). Acute high-altitude illnesses are acute hypoxia, acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE).
Acclimatization occurs as a physiologic response to the increasing altitude and increasing hypobaric hypoxia. High-altitude illness results when the hypoxic stress is greater than the individual’s ability to acclimatize. Risk factors for high-altitude illness include increased physical activity with insufficient acclimatization, inadequate education and preparation, individual susceptibility, and previous high-altitude illness. The key determinants of high-altitude illness risk and severity include both individual susceptibility factors and altitudinal factors, such as rate and height of ascent and total change in altitude over time.
Individual susceptibility factors include underlying conditions such as cardiac and pulmonary disease, patent foramen ovale, blood disorders, pregnancy, neurologic conditions, smoking, recent surgery, diabetes, and many other chronic medical conditions. Those with symptomatic neurologic, cardiac, or pulmonary disease must avoid high altitudes.
Patient assessment for high-altitude illness must also include evaluation for other conditions, which may coexist or may present in a similar manner.
1. High-Altitude–Associated Neurologic Conditions: AMS & HACE
There is a spectrum of neurologic conditions caused by high altitude, ranging from acute mountain sickness (AMS) to the more serious form, high-altitude cerebral edema (HACE).
AMS includes symptoms such as headache (most severe and persistent symptom), lassitude, drowsiness, dizziness, chilliness, nausea and vomiting, and difficulty sleeping. Later symptoms include irritability, difficulty concentrating, anorexia, insomnia, and increased headaches.
HACE includes the severe symptoms of AMS and results from cerebral vasogenic edema and cerebral cellular hypoxia. It usually occurs at elevations above 2500 meters (8202 feet) but may occur at lower elevations. Hallmarks are altered mental status, ataxia, severe lassitude, and encephalopathy. Examination findings may include confusion, ataxia, urinary retention or incontinence, focal neurologic deficits, papilledema, and seizures. Symptoms may progress to obtundation, coma, and death.
 Treatment
Treatment
Definitive treatment is immediate descent of at least 610 meters (2001 feet), and descent must continue until symptoms improve. Descent is essential if the symptoms are persistent, severe, or worsening, or if HACE or HAPE is present. If immediate descent is not possible, portable hyperbaric chambers can provide symptomatic relief, but this must not delay descent.
Initial treatment involves oxygen administration to keep the pulse oximetry SpO2 to greater than 90%.
Acetazolamide is an effective medication for both prophylaxis and treatment of mild symptoms of AMS.
Dexamethasone is given for moderate to severe AMS. Dexamethasone is the primary treatment for HACE. Acetazolamide can be added as an adjunct in severe HACE cases. In most individuals, symptoms clear within 24–48 hours. HACE treatment must continue until 24 hours after resolution of symptoms or until descent is completed. Dexamethasone should not be given for longer than 7 days.
It is imperative that the clinician also assess for other conditions that may mimic or coexist with AMS and HACE.
If HAPE symptoms and signs are present along with HACE, nifedipine or a selective phosphodiesterase inhibitor may be added for pulmonary vasodilation. The clinician must be cautious when using combinations of vasodilators.
2. Acute HAPE
HAPE is the leading cause of death from high-altitude illness. The hallmark is markedly elevated pulmonary artery pressure followed by pulmonary edema. Early symptoms may appear within 6–36 hours after arrival at a high-altitude area. These include incessant dry cough, shortness of breath disproportionate to exertion, headache, decreased exercise performance, fatigue, dyspnea at rest, and chest tightness. Recognition of the early symptoms may enable the patient to descend before incapacitating pulmonary edema develops. Strenuous exertion must be avoided. As pulmonary edema worsens, wheezing, orthopnea, and hemoptysis may develop.
Physical findings may include tachycardia, mild fever, tachypnea, cyanosis, prolonged respiration, rales, wheezing, and rhonchi. The clinician must assess for other potential medical conditions because the clinical picture may resemble other entities. Diagnosis is usually clinical; ancillary tests are nonspecific or unavailable on site. Prompt recognition and medical attention of early symptoms of HAPE may prevent progression.
 Treatment
Treatment
Immediate descent (at least 610 meters [2000 feet]) is essential, although this may not be immediately possible and may not alone improve symptoms.
The patient must be placed at rest, reclined with head elevated. Supplemental oxygen must be administered to maintain pulse oximetry reading SpO2 greater than 90%. Recompression in a portable hyperbaric bag will temporarily reduce symptoms if rapid or immediate descent is not possible, but must not delay descent.
Nifedipine can be used as an adjunct if the other therapies (descent, oxygen, or portable hyperbaric chambers) are not successful or available. Selective phosphodiesterase inhibitors may be used for HAPE prevention and may also provide effective symptom relief as an alternative or if nifedipine is not available. Administering nifedipine plus a phosphodiesterase inhibitor as pulmonary vasodilators is not recommended because this combination may also lower the mean arterial pressure and decrease cerebral perfusion. Treatment for ARDS (see Chapter 9) is required for some patients. If neurologic symptoms are present concurrently with HAPE and do not resolve with improved oxygenation, dexamethasone may be added according to HACE treatment guidelines.
There is an international effort to advance the understanding of high-altitude pulmonary edema through the research and database registry (https://www.altitude.org).
 Prevention of High-Altitude Disorders
Prevention of High-Altitude Disorders
Pre-trip preventive measures include participant education, medical preparticipation evaluation, pre-trip planning, optimal physical conditioning before travel, and adequate rest and sleep the day before travel and during the trip. Preventive efforts during ascent include reduced food intake and avoidance of alcohol, tobacco, and limiting any unnecessary physical activity during travel.
Gradual ascent is the most effective way to allow acclimatization. Low-risk ascension rate is 2 or more days to arrive at 2500–3000 meters. The altitude reached during waking hours is not as important as the altitude at which the hiker sleeps. Mountaineering parties at altitudes of 3000 meters or higher must carry a supply of oxygen and medical equipment sufficient for several days.
Drug prophylaxis may be prescribed for AMS and HACE if no contraindications exist. Prophylactic low-dose acetazolamide has been shown to reduce the incidence and severity of AMS and HACE when started 3 days prior to ascent and continued for 48–72 hours at high altitude. Dexamethasone is an alternative prophylactic medication for AMS and HACE.
Individuals with a past history of HAPE should use drug prophylaxis to reduce the risk of recurrence. Nifedipine started the day before ascent and continued through the fourth day at target elevation, or through the seventh day if the ascent rate was faster, is recommended. Salmeterol can be added beginning 24 hours prior to ascent. Salmeterol is used as an adjunct to nifedipine but not as monotherapy.
Phosphodiesterase inhibitors may be beneficial in the treatment of HAPE based on their physiologic effects of decreased pulmonary arterial pressures and pulmonary vasodilation.
 When to Admit
When to Admit
• All patients with HACE or HAPE must be hospitalized for further observation.
• Hospitalization must also be considered for any patient who remains symptomatic after treatment and descent.
• Pulmonary symptoms and hypoxia may be worsened by complications such as pulmonary embolism, secondary respiratory infection, bronchospasm, mucous plugging, or acute coronary syndrome.
Luks AM et al. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2019 update. Wilderness Environ Med. 2019 Dec;30(4S):S3–18. [PMID: 31248818]
Meier D et al. Does this patient have acute mountain sickness? The Rational Clinical Examination Systematic Review. JAMA. 2017 Nov 14;318(18):1810–19. [PMID: 29136449]
Nieto Estrada VH et al. Interventions for preventing high altitude illness: Part 1. Commonly-used classes of drugs. Cochrane Database Syst Rev. 2017 Jun 27;6:CD009761. [PMID: 28653390]
SAFETY OF AIR TRAVEL & SELECTION OF PATIENTS FOR AIR TRAVEL
The medical safety of air travel depends on the nature and severity of the traveler’s preflight condition and factors such as travel duration and frequency of travel, use and frequency of inflight exercise, cabin altitude pressure, availability of medical supplies, infectious diseases of other travelers, and the presence of health care professionals on board. Air travel passengers are susceptible to a wide range of flight-related problems: pulmonary (hypoxemia, spontaneous pneumothorax), venous thromboembolism (VTE), infections, cardiac, gastrointestinal, ocular, immunologic, syncope, neuropsychiatric, metabolic, trauma, and illicit substance-related conditions. Air-travel risks are higher for those air travelers with preexisting medical conditions.
Hypobaric hypoxia is the underlying etiology of most serious medical emergencies in flight due to cabin altitude. Despite commercial aircraft pressurization requirements, there is significant hypoxemia, dyspnea, gas expansion, and stress in travelers, particularly in those with underlying pulmonary disease.
Air travel has been the main focus of medical reviews on travel-related VTE; however, any form of prolonged travel involving immobilization is associated with increased risk of VTE (now referred to as “traveler’s thrombosis”). VTE risk is more relevant for those passengers with additional VTE risk factors. Risks for VTE in long-distance travelers include the following: (1) travel involving immobilization for 4 or more hours, (2) hypercoagulable disorders, and (3) acquired risks. Prevention measures may include wearing graduated compression stockings; frequent exercise and position changes during travel; and the use of thromboprophylaxis, such as low-molecular-weight heparin (LMWH) or direct-acting oral anticoagulant (DOAC) (see Chapter 14).
Air travel is not advised for anyone who is “incapacitated” or has any “unstable conditions.” The Air Transport Association of America defines an incapacitated passenger as “one who is suffering from a physical or mental disability and who, because of such disability or the effect of the flight on the disability, is incapable of self-care; would endanger the health or safety of such person or other passengers or airline employees; or would cause discomfort or annoyance of other passengers.” Unstable conditions include active pneumothorax, advanced pulmonary hypertension, acute worsening of an underlying lung disease, poorly controlled hypertension, dysrhythmias, angina, valvular disease, heart failure, or acute psychiatric condition; severe anemia or symptomatic sickle cell disease; recent myocardial infarction; cerebrovascular accident; poorly controlled seizure disorder; deep venous thrombosis; postsurgery, especially heart surgery (unless approved by surgeon); and any active communicable disease. Public Health Travel Restrictions have shown efficacy in preventing commercial air or international travel of persons with certain communicable diseases that pose public health threat.
 Pregnancy
Pregnancy
Pregnant travelers have unique travel-related and location-specific risks. A clinician’s authorization is required if travel is essential during the ninth month of pregnancy or earlier in a complicated or high-risk pregnancy.
Long travel increases risk of VTE for the pregnant traveler. Pregnant travelers are at higher risk from infection transmission and air travel radiation exposure.
 Prevention
Prevention
Air travel complications may be reduced by the following preventive measures: passenger prescreening, passenger education, and in-flight positioning and activity. Prescreening evaluation is recommended for all high-risk patients including preexisting requirement for oxygen, continuous positive airway pressure or ventilator support, underlying restrictive or obstructive lung disease, comorbidities worsened by hypoxemia, previous respiratory distress during air travel, recent pneumothorax, and recent (within 6 weeks) acute respiratory illness. Patients at risk for hypoxia must be assessed prior to air travel to determine if there is a need for supplemental in-flight oxygen.
Air travel education must include risk reduction measures for VTE, infectious diseases, and exacerbations of underlying medical conditions. Patients and clinicians can check the World Health Organization website for the most updated information on travel health risks and infectious diseases (https://www.who.int/travel-advice).
All long-distance travelers can reduce VTE risk by avoiding constrictive clothing, staying well-hydrated, changing position frequently, avoiding cramped position, avoiding leg crossing, engaging in frequent (at least every hour) in-flight leg stretching exercises, and walking for 5 minutes every hour. Clinicians must assess those with high risk of VTE prior to air travel to determine whether anticoagulation is indicated (see Table 14–14).
ACOG Committee Opinion No. 746 summary: air travel during pregnancy. Obstet Gynecol. 2018 Aug;132(2):533–4. [PMID:30045206]
Czuprynska J et al. Annotation: travel and thrombosis. Br J Haematol. 2020 Mar;188(6):838–43. [PMID: 31372991]
Ergan B et al. Should I stay or should I go? COPD and air travel. Eur Respir Rev. 2018 Jun 13;27(148):180030. [PMID: 29898904]