 between 1985 and 1995, coinciding with AIDS awareness campaigns. However, from 1997 to 2015 there has been a steady
between 1985 and 1995, coinciding with AIDS awareness campaigns. However, from 1997 to 2015 there has been a steady  in incidence.
in incidence.Gonorrhoea—‘flow of seed’ as named by Galen, Greek physician, in the 2nd century ad—has probably been known to be sexually transmitted for several millennia, as shown by references in the Old Testament (Leviticus 15), and the attribution of ‘strangury’ to ‘pleasures of Venus’ by Hippocrates.
Gram –ve kidney-shaped cocci about 1 µm in diameter; appear in pairs (diplococci) with concave aspects facing each other, typically inside PMNLs.
Fastidious growth requirements: temperature 35–37°C, pH 6.5–7.5, atmosphere containing 5–7% carbon dioxide, selective and enriched culture media (e.g. Modified New York City) supplemented with iron, essential amino acids, glucose, and antimicrobials to inhibit other organisms.
Humans are the only natural host. Primarily infects the columnar epithelium of lower genital tract, rectum, pharynx, and conjunctiva with transluminal spread to epididymis and prostate in ♂ and endometrium and pelvic organs in ♀ with occasional haematogenous dissemination.
WHO estimates 78 million cases annually worldwide. The UK incidence peaked around the early 1970s, followed by a marked  between 1985 and 1995, coinciding with AIDS awareness campaigns. However, from 1997 to 2015 there has been a steady
between 1985 and 1995, coinciding with AIDS awareness campaigns. However, from 1997 to 2015 there has been a steady  in incidence.
in incidence.
Highest incidence in the UK is in MSM, ages 15–29 years, urban and deprived populations, and black Carribeans. N. gonorrhoeae infection is regarded as a surrogate marker of high-risk sexual behaviour and is closely associated with other STIs, especially Chlamydia trachomatis (co-infection in up to 40% of ♀ and 25% of ♂ with gonorrhoea).
N. gonorrhoeae does not confer immunity as outer membrane proteins vary and re-infection is common.
• Anogenital and pharyngeal infections: almost exclusively sexually transmitted. Evidence of transmission from toilet seats is contentious, as the organism loses viability on drying. Fomite transmission is very unusual, but anecdotal cases have been reported following the shared use of a portable male urinal and inflatable sex doll.
• Adult conjunctivitis: often associated with anogenital infection due to auto-inoculation, but non-sexual transmission is possible as reported in sporadic epidemics and isolated cases attributed to poor hygiene, accidental inoculation, or irrigation with urine (folk remedy). Flies have been implicated as vectors in an Australian outbreak.
• Neonatal infection: vertical transmission due to exposure in birth canal.
• 60–80% transmission of gonorrhoea from ♂ to ♀ after one episode of sex. Risk reduced by ~40% with condom use.
• 20% transmission from ♀ to ♂ after one episode of sex. Risk reduced by 75% with condom use.
• Transmission from pharynx to urethra occurs in 26% of partners.
• 30% vertical transmission risk.
• Spontaneous clearance ~5% from pharynx in 10 days (no data for other sites of infection).
See Table 8.1.
Table 8.1 Sites of infection. Infection commonly co-exists at several different sites
| Heterosexual ♂ (%) | MSM (%) | ♀ (%) | |
| Urethra | >90 | 60–70 | 65–75 |
| Cervix | — | — | 80–90 |
| Pharynx + other site(s) | 3–10 | 10–30 | 5–15 |
| Pharynx only | <5 | 10–15 | <5 |
| Rectum + other site(s) | — | 25–50 | 25–40 |
| Rectum only | — | 20–40 | 5 |
• Incubation period: 2–5 days, range 1–14.
• Urethral discharge in 80% and/or dysuria in 50%. May be scanty and mucoid initially, but becomes profuse and purulent, yellow/green within 24 hours.
• Mucopurulent or purulent urethral discharge, typically profuse, but if scanty can be elicited by urethral massage.
• Erythema of the urethral meatus sometimes with oedema.
• Urine ‘threads’—plugs of pus from urethral (Littré’s) glands in FVU, indicating anterior urethritis.
• Rarely epididymal tenderness or balanitis.
• Asymptomatic in ~50%. If symptomatic, may be due to co-infection.
• Symptoms, when present, appear within 10 days of infection.
•  vaginal discharge in up to 50% (most common symptom).
vaginal discharge in up to 50% (most common symptom).
• Lower abdominal pain in up to 25%.
• Dysuria without frequency ~12%.
• Intermenstrual bleeding or menorrhagia (unusual).
• Commonly no abnormal findings.
• Mucopurulent endocervical discharge and easily induced cervical bleeding (<50%).
• Pelvic/lower abdominal tenderness (<5%).
Usually asymptomatic, but may cause anal discharge (12%), pain, discomfort, or pruritus (<7%), and less frequently rectal bleeding, tenesmus, and constipation. Proctoscopy may show mucoid or purulent discharge, erythema, oedema, and friability. In ♀, the positive correlation with duration of cervical infection suggests transmucosal spread of genital secretions into the anal canal as the main route, with anal intercourse implicated in ~10%.
Asymptomatic in >90%. Occasional mild pharyngitis and/or cervical lymphadenopathy. Almost 100% spontaneous clearance within 12 weeks; significant association with disseminated gonococcal infection.
Adult infection, which is uncommon, presents with purulent discharge and inflammation affecting one or both eyes. If untreated, complications, such as keratitis and pan-ophthalmitis, can lead to blindness.
In girls, the vulval and vaginal epithelium are vulnerable to infection. Although, theoretically, infection may be acquired accidentally from infected secretions, especially with poor sanitation and hygiene, gonorrhoea is a strong indicator of sexual abuse. It usually presents as a purulent oedematous vulvovaginitis.
Gonococcal urethritis in boys, or pharyngeal and rectal infection in both sexes, is almost always the result of sexual abuse.
Complications are uncommon in both men and women.
• Infection of the median raphe: linear erythematous swelling.
• Tysonitis: painful swelling of parafrenal gland.
• Meatal para-urethral gland abscess.
• Peri-urethral cellulitis and abscess: inflammation of Littré’s glands with duct obstruction produces small cysts and abscesses causing tender swelling in fossa navicularis or bulb. Urine flow may be re-stricted. Painful erections +/– ventral angulation if corpus spongiosum is affected.
• Urethral strictures and fistulae: sequelae of peri-urethral abscess in untreated infection.
• Cowperitis and abscess: Cowper’s (bulbo-urethral) glands at the base of the prostate are affected, causing fever, pain in perineum, particularly on defecation, and urinary frequency or retention. Abscesses usually point to one side of the perineum or are palpable rectally.
• Prostatitis and seminal vesiculitis: acute features include fever, malaise, perineal discomfort, tenesmus, suprapubic pain, urgency of micturition or retention, haematuria, and painful erections. Swollen prostate, tender on rectal examination. Chronic prostatitis may develop.
• Epididymitis (<1%):  Chapter 13, ‘Epididymo-orchitis’, pp. 193–200.
Chapter 13, ‘Epididymo-orchitis’, pp. 193–200.
• Inflammation of para-urethral (Skene’s) glands.
• Bartholinitis +/– Bartholin’s abscess: single or bilateral. Vulval pain and erythema with tender cystic swelling of the posterior half of the labium majora. Pus may be seen or expressed from the duct orifice.
• Pelvic inflammatory disease (PID  Chapter 11, ‘Pelvic inflammatory disease, pp. 169–179) may occur in 10–20% of untreated infections.
Chapter 11, ‘Pelvic inflammatory disease, pp. 169–179) may occur in 10–20% of untreated infections.
Usually found in ♀ with associated PID, suggesting intra-abdominal spread, but also rarely reported in ♂ implicating lymphatic or haematogenous dissemination ( Chapter 11, ‘Complications and sequelae’, p. 174).
Chapter 11, ‘Complications and sequelae’, p. 174).
• Occurs in <1% with mucosal infection.
• Host factors: 4-fold  in ♀ (especially during or just after menstruation, or in pregnancy, particularly with pharyngeal infection). Complement deficiency predisposes to recurrent episodes in <10% with DGI.
in ♀ (especially during or just after menstruation, or in pregnancy, particularly with pharyngeal infection). Complement deficiency predisposes to recurrent episodes in <10% with DGI.
• auxotype AHU– (arginine, hypoxanthine, and uracil dependent)
• antibiotic resistance, which varies with time and region.
The preceding mucosal infection tends to be asymptomatic. Usually presents with mild fever, skin rash, and arthralgia +/– arthritis.
• Skin (gonococcal dermatitis) in 67%: initial macules develop into papules, vesicles with petechiae, and then typical necrotic pustules surrounded by erythema. Usually at extremities (especially hands).
• Skeletal: tenosynovitis (in ~33%) producing migratory arthralgia, mostly in wrists, fingers, toes, and ankles. Arthritis (single joint), in ~50%, with effusion typically involving the knee, wrist, or metacarpophalangeal joints.
• Endocarditis in 1–3% leading to aortic incompetence and cardiac failure; also pericarditis and myocarditis.
• Meningitis, similar to meningococcal (very rare).
Screening for gonorrhoea and chlamydia in 16–25-year-olds has become part of antenatal care in some areas, and is likely to reduce complications and adverse pregnancy outcomes due to both infections.
In pregnancy the proportion of pharyngeal infection is  by 15–35%, presumably due to
by 15–35%, presumably due to  in oral sex. Genital infection is less likely to be complicated by PID (because of thickening of cervical mucus), but cases of salpingitis have been reported in the 1st trimester.
in oral sex. Genital infection is less likely to be complicated by PID (because of thickening of cervical mucus), but cases of salpingitis have been reported in the 1st trimester.
• Infection of chorio-amnion can cause septic abortion, but there is no consistent evidence for  risk.
risk.
• Preterm delivery and low birth weight  3–6-fold.
3–6-fold.
• Premature rupture of membrane (PROM) is more frequent.
• Post-partum/intra-partum or post-abortal endometritis and pyrexia illness are  3-fold and occur in ~42% with gonorrhoea.
3-fold and occur in ~42% with gonorrhoea.
• Some studies suggest that risk of DGI is  , reflecting
, reflecting  pharyngeal infection.
pharyngeal infection.
• Screening in pregnancy advisable in symptomatic women, PROM, septic abortion, intra/post-partum fever, or those at  risk.
risk.
Occurs because of exposure in birth canal during labour with  risk if PROM or preterm delivery. Antenatal screening and treatment is the best way to prevent neonatal infection. The most severe manifestations are ophthalmia neonatorum and sepsis, with milder infections causing rhinitis, pharyngitis (in 33% with ophthalmia), vaginitis, urethritis, and infection at site of foetal monitoring. Meningitis is a rare complication.
risk if PROM or preterm delivery. Antenatal screening and treatment is the best way to prevent neonatal infection. The most severe manifestations are ophthalmia neonatorum and sepsis, with milder infections causing rhinitis, pharyngitis (in 33% with ophthalmia), vaginitis, urethritis, and infection at site of foetal monitoring. Meningitis is a rare complication.
A notifiable disease in the UK, defined as conjunctivitis with a purulent discharge in an infant arising within 21 days of birth. Occurs in 30–40% of those exposed. Typically develops within 2–5 days of delivery and presents with oedema of the conjunctiva and eyelids with profuse discharge. Without treatment infection may extend to sub-conjunctival connective tissue and the cornea leading to ulceration. If ulcers perforate, anterior synechiae formation or panophthalmitis may follow, which can result in blindness.
The rate of infection in at-risk infants is  to 2–5% by prophylaxis with 0.5% erythromycin ointment applied once to both eyes soon after birth (other topical treatments are no longer recommended).
to 2–5% by prophylaxis with 0.5% erythromycin ointment applied once to both eyes soon after birth (other topical treatments are no longer recommended).
Associated with vulvovaginitis, proctitis, or ophthalmia neonatorum. Usually polyarticular, presenting as pseudoparalysis and is rarely progressive.
See Box 8.1.
Box 8.1 Frequently asked questions
In ♂, urethral symptoms usually appear within 10 days of exposure to gonorrhoea, although 5–10% are asymptomatic when diagnosed. Symptoms are much less common with rectal and throat infection. Up to 70% of ♀ diagnosed with gonorrhoea are asymptomatic. Therefore, although most people are probably diagnosed shortly after being infected, it is possible that it may have been present for weeks or months.
Yes, gonorrhoea can be cured by antibiotics. When swabs are taken for gonorrhoea culture, the laboratory also tests for antibiotic sensitivities, so that the appropriate antibiotic is identified. It is recommended that, while awaiting these results, an antibiotic be used to which >95% of the local stains of N. gonorrhoeae are sensitive.
• In ♀: PID may occur in 10–20% of untreated cases of gonorrhoea. Infertility may occur as a result of PID.
• In ♂: urethral strictures and fistulae are sequelae of peri-urethral abscesses in untreated gonorrhoea and it may also cause epididymitis.
Yes. With increasing emergence of antibiotic resistance it is important to have a test of cure about 2 weeks after treatment. Abstinence is advised until you and your partner(s) have a negative test of cure result.
Gonorrhoea can survive for up to 24 hours on surfaces such as toilet seats and there has been a report of acquisition by an 8-year-old girl from a dirty aeroplane toilet. However, it is very rare and, in children, infection should always raise concerns of sexual abuse.
There is a risk of neonatal infection due to exposure in the birth canal during labour. Gonococcal ophthalmia neonatorum is acquired by 30–40% of infants exposed to N. gonorrhoeae. Without treatment, there are  risks of preterm delivery, low birth weight, premature rupture of membranes, and post-partum/post-abortion endometritis. Prompt treatment significantly reduces these risks and, if necessary, your baby will receive prophylaxis or treatment.
risks of preterm delivery, low birth weight, premature rupture of membranes, and post-partum/post-abortion endometritis. Prompt treatment significantly reduces these risks and, if necessary, your baby will receive prophylaxis or treatment.
Sampling depends on sexual history. NAATs are recommended for screening as they have higher sensitivity than culture. If asymptomatic, but a contact of gonorrhoea infection, culture is also recommended.
• Women: health professional or self-taken vulvovaginal swab for NAAT testing. Consider pharyngeal and rectal swab depending on history.
• Heterosexual ♂: FVU (or urethral swab) for NAAT testing (pharyngeal swab not necessary, even if history of cunnilingus).
• MSM: FVU, pharyngeal and rectal swabs recommended for all (these can be self-taken). ~20% with rectal infection do not report receptive anal intercourse.
• Endocervical: microscopy. Culture if Gram –ve intracellular diplococci seen or gonorrhoea suspected (e.g. contact of gonorrhoea).
• Rectal: depending on sexual history and symptoms, NAAT, microscopy of rectal slide if proctitis, consider culture if gonorrhoea suspected.
• Pharyngeal swab for NAAT depending on history (+/– culture).
Microscopy of urethral smear, FVU for NAAT, culture of urethral swab if gonorrhoea suspected.
• Urethral sample: microscopy if symptoms of urethritis, and culture of urethral swab if gonorrhoea suspected.
• Offer rectal NAAT for all (depending on history), with proctoscopy and microscopy if rectal symptoms and rectal gonorrhoea culture if gonorrhoea suspected.
• Pharyngeal swab for NAAT ± culture if gonorrhoea suspected.
Swab from eyelid and test potential 1º infection sites with NAAT and culture.
• Test potential 1º infection sites with NAAT and culture.
• Material from skin lesions and joint aspirate for NAAT and culture.
Microscopy (×1000) of Gram-stained genital specimens shows N. gonorrhoeae as Gram –ve diplococci within PMNLs (Plate 4). Smear sensitivity compared with culture:
• ♂: urethra 90–95% (symptomatic) and 50–75% (asymptomatic)
• ♀: cervix 37–50% and urethra 20%
• rectum: blind swab 40%, using proctoscope 70–80%.
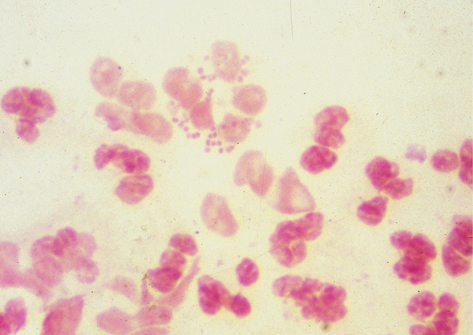
Plate 4 Gram stain gonorrhoea (8).
Conjunctival specimens are suitable for Gram stain and microscopy, but pharyngeal specimens are not suitable as other neisserial commensals are commonly seen.
NAAT testing is the recommended first-line test for gonorrhoea and is widely available, commonly in combination with chlamydia NAAT. NAATs are significantly more sensitive from all sites compared with culture, with sensitivities >96% for both symptomatic and asymptomatic infection. They are suitable for ♂ urine, vulvo-vaginal (including self-collected), and endocervical samples (♀ urine sample has lower sensitivity). NAATs are recommended for rectal and pharyngeal testing as sensitivities are superior to culture, although they are unlicensed for testing at these sites, so local validation of testing platforms is required. Confirmatory testing of reactive NAAT using a second NAAT with a different target is required (unless already culture positive), where population prevalence is low and positive predictive value of reactive test is <90%.
Gonorrhoea culture less sensitive than NAAT, but is still required for detection of isolates with  antimicrobial sensitivity (becoming an increasing concern). Sensitivity predicts success in >95% with intermediate sensitivity suggesting failure in 5–15%, although
antimicrobial sensitivity (becoming an increasing concern). Sensitivity predicts success in >95% with intermediate sensitivity suggesting failure in 5–15%, although  dosage may be effective. Resistance may be due to plasmids (circular DNA fragments independent of chromosome) or chromosomal mutation.
dosage may be effective. Resistance may be due to plasmids (circular DNA fragments independent of chromosome) or chromosomal mutation.
▶ Caution with lubricants during examination, as they may inhibit the growth of N. gonorrhoeae.
Specimens for culture may be directly inoculated onto culture medium and incubated, or transported in a non-nutrient medium (e.g. Amies, Stuart) or a CO2-producing culture medium.
Transport media should be kept at 4°C after inoculation (check product information or with local lab). Provided that the material in the transport medium is processed within 48 hours, there is only ~5% loss in sensitivity compared with direct inoculation.
The culture medium is examined for growth at 24 and 48 hours. Presumptive identification is by positive cytochrome oxidase reaction and microscopy. Definitive identification by a combination of further tests, e.g. carbohydrate utilization (N. gonorrhoeae utilizes glucose only), monoclonal antibody test (e.g. Phadebact), and enzyme substrate degradation.
Further information
British Association for Sexual Health and HIV Gonorrhoea guideline  https://www.bashh.org/guidelines
https://www.bashh.org/guidelines
▶ Advise avoidance of any sexual contact until patient and partner(s) have completed treatment, and received negative test of cure result.
▶ Offer full STI screen, including HIV. Repeat screening after the window periods should be recommended.
Treatment before antibiotic sensitivity is known should be with an antibiotic to which >95% of local strains are sensitive.
Ceftriaxone 1g IM single dose (NB Addition of azithromycin no longer recommended due to increasing resistance). Ciprofloxacin 500mg oral single dose if known to be sensitive. To treat gonococcal PID: Ceftriaxone 1g IM single dose plus chosen treatment regimen for PID (see Chapter 11). To treat gonococcal epididymo-orchitis: Ceftriaxone 1g IM single dose plus chosen treatment regimen for epididymo-orchitis (See Chapter 13)
• Cefixime 400mg oral single dose plus azithromycin 2g oral single dose
• Gentamycin 240mg IM single dose plus azithromycin 2g oral single dose
• Spectinomycin 2g IM single dose plus azithromycin 2g oral single dose (not effective for treatment of pharyngeal infection)
• Azithromycin 2g oral single dose (high levels of resistance worldwide)
To treat PID  Chapter 11, ‘Pelvic inflammatory disease’, pp. 169–179, and
Chapter 11, ‘Pelvic inflammatory disease’, pp. 169–179, and  Chapter 13, ‘Epididymo-orchitis’ pp. 193–200.
Chapter 13, ‘Epididymo-orchitis’ pp. 193–200.
 Quinolone and tetracycline antimicrobials are contraindicated:
Quinolone and tetracycline antimicrobials are contraindicated:
• Ceftriaxone 1g IM single dose
• Spectinomycin 2g IM single dose• Azithromycin 2g oral single dose
Systemic treatment for 3 days recommended in case of corneal involvement as the cornea is relatively avascular.
• Recommended: ceftriaxone 1g IM single dose
• Alternative: spectinomycin 2 g IM daily for 3 days or azithromycin 2 g oral single dose plus doxycycline 100 mg bd for 7 days plus ciprofloxacin 250 mg daily for 3 days
Hospital admission is recommended. Assess for endocarditis or meningitis. Initial therapy should be parenteral:
• ceftriaxone 1 g IM/IV daily or cefotaxime 1 g IV 8-hourly or
• ciprofloxacin 500 mg IV 12-hourly or
• spectinomycin 2 g IM 12-hourly.
The parenteral antibiotic should be continued for 24–48 hours until improvement, and then replaced with one of the following oral antibiotics, to complete 7 days treatment: cefixime 400 mg; ciprofloxacin 500 mg; or ofloxacin 400 mg bd.
Unlicensed in the UK ( Chapter 7, ‘Unlicensed medications’, p. 131). Guidelines advise as alternative treatment for uncomplicated anogenital and disseminated gonorrhoea infections.
Chapter 7, ‘Unlicensed medications’, p. 131). Guidelines advise as alternative treatment for uncomplicated anogenital and disseminated gonorrhoea infections.
Reconstitute spectinomycin powder, 2 g with 3.2 mL of sterile water for injection, to make up 5 mL solution. Shake vigorously before drawing up into disposable syringe. Administer by injecting into the upper outer quadrant of the gluteal muscle using a 20 gauge needle.
IV antibiotic for at least 2 weeks and 4 weeks, respectively, with ceftriaxone 2 g daily or 1 g 12-hourly plus single dose azithromycin 2 g oral. Seek specialist advice.
• Ophthalmia neonatorum: single dose of ceftriaxone IV/IM 25–50 mg/kg up to a maximum of 125 mg.
• Sepsis, scalp abscess, meningitis, or arthritis in neonate or prepubertal child <45 kg: ceftriaxone 25–50 mg/kg daily IV/IM for 7–14 days.
• Neonatal prophylaxis: if mother is not treated before delivery, a single dose of ceftriaxone 25–50 mg/kg to a maximum of 125 mg.
• For uncomplicated anogenital or pharyngeal infection if weight <45 kg: single dose ceftriaxone 25–50 mg/kg IV/IM (max 125 mg).
• Treatment of children who weight >45 kg is as for adults.
PN is essential. Contact tracing period:
• Men with symptomatic urethritis: 2 weeks prior to onset of symptoms or until last sexual partner, if longer.
• Asymptomatic men and women, or infection at other sites: 3 months prior to diagnosis or until the last sexual partner.
• All sexual contacts should be offered full STI screen and epidemiological treatment. Similar principles should apply to the mother of a neonate with gonococcal infection and her sex partner(s).
TOC is recommended for all. If asymptomatic, a NAAT test should be performed >2 weeks after treatment. TOC is particularly important following treatment of pharyngeal infections, alternative treatment regimen or persisting symptoms. Culture (>72 hours after treatment complete) is recommended in addition if still symptomatic, alternative treatment regimen used or resistance detected on initial culture.
Gonorrhoea facilitates HIV transmission, producing  in detectable virus in genital secretions. This is reversed following antibiotic treatment. Infection should be treated as for people without HIV infection.
in detectable virus in genital secretions. This is reversed following antibiotic treatment. Infection should be treated as for people without HIV infection.