Science is a way of thinking much more than it is a body of knowledge.
Carl Sagan
We humans know a lot about the world in which we live. The origins of this quest for knowledge predate writing, as early man’s very survival depended on an intimate knowledge of the natural world of seasons and plants, of tools and fire. Sheer pragmatism required that humans be keen observers. Almost certainly, there were early thinkers who wondered about deeper mysteries: those who wondered Why? as well as What? and How? We will never know just how deep ran the thoughts of these early scientists; however, we do know for certain that by 2,500 years ago, people were asking thoroughly modern questions.
On their craggy peninsula in the Aegean Sea, the early Greek philosophers debated long and hard about whether the natural state of matter was resting or moving and whether there existed a smallest particle of matter. Just as important, they recorded their thoughts so that others, separated by both space and time, could appreciate and build on their ideas and debates. In the recording, they tacitly laid claim to the origins of fundamental science.
Much has been written of these long- dead thinkers, but this book is not concerned with their specific thoughts. After all, their ideas were only generally correct and wrong in many specifics. However, we are concerned with their intellectual legacy.
Although the early Greeks may be credited with the start of the journey, the picture has been clarified in the intervening centuries. Our mastery of the natural world includes curing deadly diseases, learning to fly, and taking the first steps toward recreating the hot, all- consuming nuclear flame that fuels the sun.
In 1803, the British poet William Blake wrote “The Auguries of Innocence,” which began
To see a world in a grain of sand
And a heaven in a wild flower,
Hold infinity in the palm of your hand
And eternity in an hour.
To see the world in a grain of sand is surely a metaphor, but it is not without an element of truth. By considering a single grain of sand and attempting to understand all of its fundamental pieces, one can learn a great deal about the laws that govern the greater universe. For instance, is there a smallest bit of sand? Under a microscope, sand looks a lot like a very small rock. If we crush the grain of sand, we are left with what appears to be even smaller rocks. If we crush those, do we have an infinite chain of ever- smaller rocks?
Asking this question for all the disparate substance of the world—rocks, water, air, food, and so on—led scientists to realize that all the matter of the universe could be created by combining different amounts of a little more than one hundred substances. We call these primordial substances elements, and some of their names are likely familiar from chemistry class, such as hydrogen, oxygen, and carbon. Combine hydrogen and oxygen, and you get water. Combine sodium and chlorine, and you get salt. In fact, if you mix the right elements in just the right way, you can make anything.
So one might ask whether these elements could be subdivided into individual units, that is to say, Is there a smallest unit of oxygen? And, indeed, it turns out to be true, with each element having a smallest piece. We call these smallest pieces atoms and have determined that the atoms of each element are distinct. If you want to have a basic mental picture of elements and atoms, think of an old- style toy store that specializes in selling marbles. One bin contains yellow marbles, while another has big red marbles and in yet another there are tiny green ones. So, each bin contains marbles of a distinct size and color. All the marbles within each bin are identical, and no two bins have marbles identical to those in any other bin.
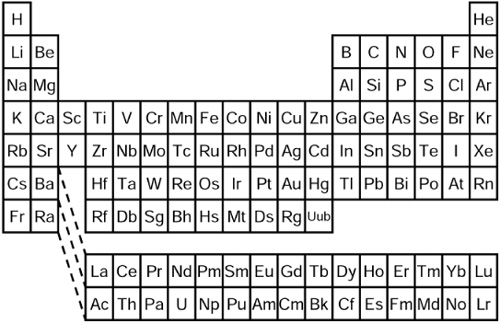
So too it is with elements and atoms. All of the atoms of a given element are identical, and the atoms of different elements are distinct. And anything on Earth can be made by arranging the right combination of atoms in the right configuration. While the details of how you do the mixing are quite complex, one can learn a lot of chemistry just by using this simple analogy of marbles. Figure 1.1 lists the elements we’ve identified thus far in a chart known as the Periodic Table of the Elements, or just the periodic table for short. Each block denotes a particular element. Elements that react similarly when combined with other elements are grouped together in columns.
Figure 1.1. The periodic table, showing the currently discovered chemical elements. All observed matter in the universe can be constructed by combinations of these hundred or so elements.
Although what I’ve told you about atoms is true in important ways, in the early 1900s physicists came to realize that atoms could themselves be broken down. By 1932, physicists discovered that all atoms could be assembled through the right mix of three even smaller particles called protons, neutrons, and electrons. All protons were identical and so were all neutrons and electrons. On the face of it, this was a spectacular improvement in our quest for simplicity. The discovery that with about a hundred different kinds of atoms one could make anything in the world was an astounding simplification. But we now knew that the elements themselves could be made from the right combinations of these three simpler ingredients. For example, a hydrogen atom could be made from one proton and one electron, while helium atoms required two protons, two neutrons, and two electrons. The patterns for atoms of other elements were eventually determined.
As it became clear that atoms could be constructed of smaller particles, there naturally was interest in trying to figure out how the particles were arranged inside the atom. For instance, were the protons, neutrons, and electrons all clumped together in a tapioca- like mass? Or perhaps they were lined up like beads on a string. Logic really couldn’t guide us to decide what an atom looked like. For that we needed experiments.
It was Ernest Rutherford, working at the turn of the twentieth century, who figured out the rough structure of the atom. He found that the atom is somewhat like a little solar system. From his and others’ work, it was shown that each atom has equal numbers of electrons and protons. The protons are all clumped together with the neutrons in a tiny ball that is called the nucleus of the atom. The electrons swirl around the nucleus at a relatively great distance. Following the solar system analogy, the nucleus is equivalent to the sun and the electrons are more like the planets. The protons were found to have a positive electrical charge and the electrons had precisely the same amount of charge, but negative. Exactly why this should be so is not known even today. The neutrons were electrically neutral. Each atom had equal numbers of electrons and protons. The number of neutrons doesn’t follow such simple rules, but, with the exception of hydrogen, the number of neutrons in an atom is similar to the number of protons but usually a bit higher.
After the basics of the atom were discovered, scientists learned other facts about its components. Even though the protons and electrons have equal electrical charge (although opposite in sign), they have radically different mass. The proton has about two thousand times more mass than does the electron. The neutron’s mass is a smidge larger than the proton’s mass. This disparity in the masses of the atom’s components means that something like 99.95% of the mass of an atom is in the nucleus.
Protons and neutrons inhabit the nucleus of the atom, with the electrons swirling around at a relatively large distance, but this doesn’t give us an accurate idea of the size of an atom. Atoms are really, really tiny. If you were to line up atoms “edge to edge,” it would take 10 million to make up a single millimeter or 250 million to make up a single inch. Even after one realizes just how small the atom is, not even that truly gives the full picture. The atom consists of mostly empty space, with the diameter of the nucleus of the atom being about ten thousand times smaller than the atom itself.
One can perhaps get an idea of just how mind- bogglingly empty an atom is by analogy. Consider a carbon atom, one of the building blocks of life. A carbon atom consists of six protons and six neutrons in the nucleus, with six electrons swirling in a sphere, far from the nucleus. Let’s imagine we blew up each proton or neutron to be a sphere the size of a printed “o” on this page. We could think of the nucleus as six of these red spheres (the protons) and six blue spheres (the neutrons) all clumped together. Let’s further put this analogous nucleus at the 50- yard line of Soldier Field, home of the Chicago Bears football team. If we did this, the rest of the atom would consist of six electrons, each much smaller than a printed period on this page, swirling like frenzied bees in a sphere the size of the football stadium. The atom is almost entirely empty space (Figure 1.2). Even so, these tiny, empty atoms of a hundred different elements, each consisting of only protons, neutrons, and electrons, form the building blocks of the entire universe.
Figure 1.2. If protons and neutrons were blown up to the size of the letter “o” on this page, a single atom would fill a football stadium and yet most of this space would be empty. The relative size of the nucleus and atom are not drawn to scale. Courtesy Dan Claes.
You’d think that scientists would celebrate the realization that with three tiny particles, they could explain the universe—and that they’d then leave well enough alone. But we physicists are a curious lot, and the scientists of the time kept poking at the question. In the 1940s and 1950s, physicists studied the data coming from their new toys, such as the “atom smashers,” and from cosmic rays, which seemed to be raining down on Earth from space itself. They discovered particles in their data that did not fit neatly into the “proton, neutron, electron, or atom” classification scheme. In fact, they found nearly a hundred different particles that seemed to have similarities with the primordial protons, neutrons, and electrons. These particles were given names: pions, kaons, lambdas, and Vs. Scientists scratched their heads.
The scratching went on for quite a few years until 1964, when a very clever proposal was made. Maybe the primordial protons and neutrons weren’t so fundamental after all. Perhaps they themselves were made of even smaller objects. These objects have come to be called quarks (pronounced “kworks”), after an inconsequential line from James Joyce’s Finnegans Wake (“Three quarks for Muster Mark!”). Unlike earlier choices for the names of fundamental particles (both the words “atom” and “proton” have Greek antecedents: atomos, meaning “not able to be cut,” and protos, meaning “first”), the word “quark” has no such academic inspiration and fits well with modern physics’ tradition of whimsical names.
Figure 1.3. The six quarks (top), with their fanciful names. The fraction indicates the charge held by that quark, where +1 is the charge of a proton. Protons and neutrons (bottom) are made by a suitable combination of up and down quarks.
Originally only three quarks were proposed, but we now know of six. Their names are up, down, charm, strange, top, and bottom. These names don’t really have any deeper meaning. Of all the quarks, two are by far the most prevalent: the up and down quarks. These two make up the proton (consisting of two ups and one down) and neutron (one up and two downs). The others are necessary to fully explain the plethora of particles discovered in particle accelerators (the pions, kaons, lambdas, and Vs listed above, as well as many others). Figure 1.3 lists the six quarks and shows how they make up the proton and the neutron.
The first three quarks proposed were the up, down, and strange quarks. The names “up” and “down” come from an older theory of the nucleus in which the protons and neutrons were treated as essentially the same thing. “Up” and “down” had a technical meaning but the words can be thought of as being similar to the two sides of a coin. The language of this older theory was carried over to the quarks. The name “strange” also is a historical holdover. Some of the particles discovered in the early accelerator and cosmic ray experiments acted oddly and people said, “Huh! That’s strange.” It turned out that the unusual behavior was related to the fact that they contained a strange quark within them, so the name migrated from the strange particles to the quark.
So it’s a bit tricky to say when the up, down, and strange quarks were discovered, as scientists saw them in the first six or so decades of the 1900s. However, it was only in 1964 that they were recognized for what they were. The up quark has an electrical charge two- thirds that of a proton (+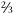 ), while both the down and strange quarks have only one- third the charge of the proton but with the opposite sign (–
), while both the down and strange quarks have only one- third the charge of the proton but with the opposite sign (–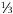 ). It seemed odd to have two quarks with –
). It seemed odd to have two quarks with –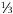 charge and only one with +
charge and only one with +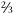 charge, but that was how the theory was initially formulated.
charge, but that was how the theory was initially formulated.
The charm quark supposedly got its name because somebody said, “Wouldn’t it be charming if there were a fourth quark, this one with +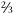 charge like the up quark?” It’s hard to tell whether this is true or merely physics folklore, but the charm quark was simultaneously discovered in 1974 by two experiments, each based on one of America’s coasts, at the Brookhaven National Laboratory on Long Island in New York state and the Stanford Linear Accelerator Laboratory in California. The bottom quark was discovered in 1977 at Fermi National Accelerator Laboratory (Fermilab) in Illinois, as was the top quark in 1995. I was one of the discoverers of the top quark as part of two competing teams of physicists, each comprising some five hundred scientists. The names top and bottom have no real meaning, although for a while “truth” and “beauty” competed for the honor of names for the two heaviest quarks. The use of these two alternative terms has declined over the past decade and is now pretty rare. That’s kind of a shame, as I liked to tell people who came to my public lectures that I was “searching for truth.”
charge like the up quark?” It’s hard to tell whether this is true or merely physics folklore, but the charm quark was simultaneously discovered in 1974 by two experiments, each based on one of America’s coasts, at the Brookhaven National Laboratory on Long Island in New York state and the Stanford Linear Accelerator Laboratory in California. The bottom quark was discovered in 1977 at Fermi National Accelerator Laboratory (Fermilab) in Illinois, as was the top quark in 1995. I was one of the discoverers of the top quark as part of two competing teams of physicists, each comprising some five hundred scientists. The names top and bottom have no real meaning, although for a while “truth” and “beauty” competed for the honor of names for the two heaviest quarks. The use of these two alternative terms has declined over the past decade and is now pretty rare. That’s kind of a shame, as I liked to tell people who came to my public lectures that I was “searching for truth.”
With the introduction of quarks, we are approaching one boundary of the current frontier of knowledge. Thus it is important to pause to learn something of the nature of quarks. As best as we currently know, quarks are one class of fundamental particles. There are other types, and we’ll discuss them shortly. In a physics context, “fundamental” means that to the best of our knowledge, quarks have no size and contain nothing smaller within them (i.e., they have no internal structure). Basically, in the journey into the heart of matter, we are made of molecules, which are in turn made of atoms. Atoms are made of protons, neutrons, and electrons, while protons and neutrons are made of quarks. But when we get to quarks, it’s the end of the road. That’s it. Quarks are as small as things get, or at least so goes current thinking. Figure 1.4 illustrates the various levels of the microworld for which we have some knowledge.
Now naturally, it may well be true that quarks actually are made of even smaller things. Such a possibility is just one of the exciting questions on which the Large Hadron Collider (from now on referred to as the LHC) might shed some light. We’ll explore this possibility in the next chapter, but for the moment, let’s concentrate on what we know about quarks.
Of the six types of quarks, two of them (the up and down quarks) are needed to make up protons and neutrons and, consequently, are stable, which means they don’t decay. The other four quark types (charm, strange, top, and bottom) have very short lifetimes, existing for just a fraction of a second before decaying quickly into the more mundane up and down type quarks.
Figure 1.4. The study of nature involves looking at ever- smaller things to find the smallest and most fundamental constituents. Courtesy Fermilab.
Quarks have special rules governing how they can combine. As stated earlier, it takes three quarks to make a proton (two ups and a down) or a neutron (two downs and an up). We now know that to make any particle of the class that includes protons and neutrons requires exactly three quarks. There is another class of particles that can be made by combining one matter quark with one antimatter quark, but these are mentioned here only for completeness. Antimatter is a concept that will be described toward the end of this chapter.
To appreciate quarks, we have to peek ahead to the idea of forces. Although most people have at least a passing familiarity with gravity and electricity, far fewer people are aware that there are two other forces: the strong and the weak nuclear forces. These two forces, whose names we shorten to simply the strong and weak forces, have an appreciable effect only in the nucleus of an atom, with the strong force holding the nucleus together and the weak force governing some types of radioactive decay.
The strong force plays an important role in how quarks behave. Originally, the strong force was understood only as that which holds protons and neutrons together in the nucleus of the atom. There were earlier theories on how this force worked, but the picture was greatly simplified by the realization that quarks inhabit the protons and neutrons. It turns out that just as quarks have electrical charge and consequently feel the electrical force, they also have a new type of charge that governs the strong force. This strong force keeps the quarks in the protons and neutrons and holds the nucleus of an atom together.
Although this new type of charge is properly called the strong nuclear force charge, we colloquially call it “color.” Color in this context has absolutely no relationship to the ordinary meaning of the word. We use the word “color” simply because of a convenient analogy. If you take red, blue, and green lights and simultaneously shine them on a wall, the resulting light will be white, which one might colloquially call no color at all. Similarly, individual quarks have color charge, but if you take them three at a time and put them in a proton, that proton has no net color charge. So we say that quarks can have three types of strong nuclear charge: red, blue, and green. Further, it is true that each proton and neutron always contain three quarks, each with a different color. It is not possible to have a proton with two or three red quarks, because protons have no net color, and only by combining red, blue, and green can one get white.
In Figure 1.5, we see that the color (strong charge) is unrelated to the quark type. For example, we can see that the down quark may have any color. To make a proton, all that is required is two up quarks and one down quark, each of which must randomly have one of the three strong force colors (red, green, or blue). Maybe this is easiest to see if we compare it to positive and negative numbers. For numbers, (+1) + (–1) = 0. For quarks, red + blue + green = 0 (or, equivalently, white).
In discussing color, we are led to another interesting feature of quarks. No quark has ever been directly observed. This doesn’t mean that there is no evidence for quarks; indeed, the evidence for their existence is simply overwhelming. But it turns out to be impossible to pull a quark out of the atom and study it. Unlike a sandbox, from which you can pull out a single grain of sand to look at, quarks are locked firmly in their respective protons and neutrons. This fact is a consequence of how the strong force acts. The strong force is similar to a spring, in that as you stretch a spring, it gets harder and harder to stretch it more. Contrast this to the electric or magnetic forces, which get weaker as two charged particles are pulled apart. Think of two magnets, which get harder and harder to keep apart (or push together) the closer you bring them to one another. Conversely, when the magnets are far apart, they don’t have any appreciable effect on one another. The springlike nature of the strong force has a very real effect on how quarks interact, but the most important feature is that quarks are generally stuck inside protons and neutrons. Technically, we say that quarks are “confined,” which means that, under normal conditions, quarks cannot leave the proton or neutron that contains them.
Figure 1.5. In quarks the colors red, blue, and green combine to make white. Similarly it takes three quarks, with three distinct strong-force charges to combine to make the strong-force neutral proton. The use of the word “color” for quark charges is purely metaphorical and has nothing to do with visible color.
The analogy between the strong force and a spring can be extended further. If you pull a spring or a rubber band hard enough, it will break. The strong force acts similarly. If you pull two quarks apart, the strong force resists more and more. But if you pull hard enough, the strong force “spring” will break. The distance at which the strong force spring breaks is about the size of the proton, which explains why the proton has the size it does. When the spring breaks, the quarks are then no longer connected and can move apart. Because of details beyond the scope of this book, these quarks are not “bare” quarks and cannot be seen like an electron that is knocked out of an atom. The idea is discussed in a little more detail in the text surrounding Figure 2.4. Briefly, in the “breaking” of the strong force spring, the energy originally stored in the spring creates more quarks and antimatter quarks. (This is a consequence of Einstein’s oft- quoted but rarely understood equation: E = mc2. Since the equation can literally be read as “energy equals matter,” we see this as an example of energy converting directly to matter.) In the end, quarks always travel in pairs or triplets, safely ensconced in particles like protons.
The property of quarks that is most frequently mentioned is their mass, which spans a large range. The up and down quarks have a mass about 0.004 that of the proton, and the super- heavy top quark has a mass of 170 times that of a proton. We have only a hazy idea as to what gives the quarks their respective masses and, indeed, why they have any mass at all. The study of that particular question is perhaps the LHC’s chief goal. In the next chapter, we will explore current thinking on this interesting question.
One thing that is very striking about quarks is that there seems to be a recurring pattern in their appearance. For instance, the up, charm, and top quarks all have the same electrical charge, as do the down, strange, and bottom quarks. Further, the up and down quarks are natural partners, in that they are the only quarks present in the stable proton and neutron. For this reason, as well as others, it is natural to group the quarks into three distinct pairs. We call these pairs generations and give each generation a number. The up and down quarks are generation I, charm and strange quarks form generation II, and top and bottom quarks form generation III. The reason for three similar groups of quarks is quite mysterious and is probably telling us something profound, if we only had the wits to understand it. Perhaps the LHC might teach us why this recurring pattern is present. We will get back to this question again in chapter 2. Table 1.1 summarizes what we know about quarks.
We have identified protons, neutrons, and electrons as components of atoms and have identified quarks as components of protons and neutrons. So far, we’ve not discussed the role of quarks in the electron. That’s because there are no quarks in electrons. In fact, like the quark, the electron is thought to be fundamental, which is to say that the electron contains no smaller particles within it. Electrons have electrical charge like quarks do, but they do not have color charge. Because of this they do not experience the “springy force” that quarks do and, consequently, each electron is not confined in the manner of quarks. This explains why they are not stuck in the nucleus but rather are free to orbit in the outskirts of the atom.
We said earlier that the universe can be built up by a proper mixture of up and down quarks and electrons. But we also know that there are two additional “carbon copies” (i.e., generations) of these quarks (e.g., the charm and strange and top and bottom quarks). Are there counterparts to the electron that might accompany these heavier quarks? Indeed there are. We have discovered two additional particles, called the muon and the tau, which have the same electrical charge and general characteristics as the electron but are heavier. Like the word “candy,” which we use generically when we don’t need to specify exactly what sugary food we’re talking about, there is a word that allows us to refer to all electrons and electron counterparts. This word is “lepton,” which stems from the Greek leptos for light. We generally refer to electrons, muons, and taus as charged leptons to remind us that these particles carry an electrical charge. Like much of physics, Greek letters are used to symbolize these objects. The symbol for the muon is ? (the Greek letter mu), while the symbol for the tau is ? (for the Greek letter tau). Table 1.1 and Figure 1.6 show how these charged leptons fit in to the scheme of subatomic particles. Some items in the table will be explained further below.
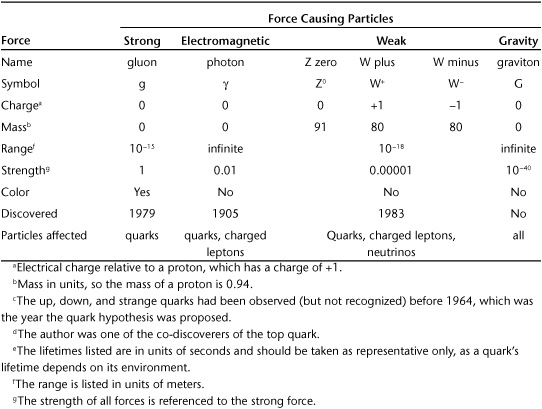
Figure 1.6. Full list of currently known subatomic particles. Courtesy Fermilab.
Although the electron (an electrically charged lepton) is a familiar particle, there also is a class of leptons that isn’t so familiar. In the early 1900s, the study of radioactivity was all the rage. But there was a type of radioactivity that perplexed physicists. Radioactivity is the decay, or transmutation, of the nucleus of the atom of one element into the nucleus of another element. The confusion stemmed from when physicists looked at the energy involved in the process of decay, they found that the energy after the decay seemed to be lower than before. This fact flabbergasted physicists, as it was a fundamental tenet of physics at the time (and still is) that energy cannot be created or destroyed. Clearly something was awry.
The conundrum was solved in 1930 when Wolfgang Pauli realized that the radioactivity mystery could be explained if in the process of radioactive decay a particle was emitted that both had a very tiny mass and was electrically neutral. A name was proposed for the particle, neutrino, from the Italian for “little neutral one,” because it was a neutral lepton. (Actually the name came from the Italian scientist Enrico Fermi, not the Austrian Pauli. Pauli’s term “neutron” came to mean something else.) The neutrino was first experimentally observed in 1956. The symbol for a neutrino is v, the Greek letter nu.
When Pauli proposed the neutrino, he didn’t fully appreciate just how peculiar a particle it was. The reason the energy budget didn’t add up in these peculiar radioactivity experiments was because the neutrino was carrying away some of the energy. Later experiments showed that neutrinos can pass through lots of matter without being detected. Although the penetrating power of neutrinos depends somewhat on their energy, neutrinos of the energies typically seen in radioactive decay could pass through five light- years of solid lead with just a 50% probability of being detected. Five light- years equals more than 48 million kilometers, or 30 million miles. So it’s not at all surprising that the physicists doing those early radioactive decay experiments were unable to see the neutrino and were therefore confused.
Pauli spoke of only a single kind of neutrino, but a 1962 experiment showed that there is more than one kind of neutrino, with an electron- type and a muon- type clearly identified. Naturally, physicists wondered if there was a tau- type as well, a hypothesis confirmed in 2000. To distinguish the three types of neutrinos, we write them with a subscript (see Figure 1.6 for examples).
With the realization that there are three distinct types of neutrinos, each with an affinity for a particular charged lepton, our catalogue of the known types of matter particles is complete. Ordinary matter is made exclusively of up and down quarks, plus electrons and electron- type neutrinos. Why there should be two carbon copies (charm, strange, muon, and muon- type neutrino) and (top, bottom, tau, and tau- type neutrino) is not understood, but these 12 particles (six quarks, three charged leptons, and three neutral leptons) is the entire list of matter particles that we've discovered thus far.
We’ve listed the particles of which we’re aware, but we’ve entirely neglected a crucial part of the story. After all, something keeps the planets circling the sun, electrons surrounding the nucleus of atoms, and the protons and neutrons firmly ensconced in their safe nuclear cocoon. These phenomena are governed by an idea called a force.
Forces can be simply defined as that which governs the motion of a particle. The force can attract or repel. Forces can even govern phenomena like radioactivity, which is kind of weird given the normal meaning of the word. In fact, we should use the word “interaction” instead of forces, so as to cover the radioactivity case. But the word “force” is so ingrained that we’ll stick with it.
Physicists currently know of four forces (Figure 1.7). The most familiar of the forces is gravity, which keeps us solidly planted on Earth and governs the motions of the heavens. Ironically, this familiar phenomenon has most jealously guarded its secrets and remains the most mysterious force in the subatomic realm.
The second most familiar force is electromagnetism, which explains electricity of course but also magnetism, light, and all of chemistry. The electromagnetic force is much, much stronger than gravity and can cause both attraction and repulsion between two objects, while gravity is only attractive.
The other two forces are much less familiar. The strong force is responsible for holding the nucleus of the atom together, while the weak nuclear force is responsible for some kinds of radioactivity. As their names suggest, they have wildly different strengths.
Two important properties that distinguish the various forces are their ranges and relative strengths. Both gravity and electromagnetism have infinite range. In principle, every atom feels the effects of gravity from every other atom in the universe. In contrast, the strong and weak forces are only relevant over a very small distance and become essentially zero when the distances under consideration become larger than a proton.
With such different behaviors, there is no single number that characterizes the forces’ relative strengths. After all, two quarks separated by a distance just larger than the nucleus of an atom would feel no effect from the strong or weak force but would feel the effects of both gravity and electromagnetism. But once we get two particles close enough that all four forces come into play, we can compare their strengths. In doing so, we find that these strengths span an enormous range.
Figure 1.7. The four forces have distinct characteristic strengths and ranges over which they work. Courtesy the Particle Data Group, Lawrence Berkeley Laboratory.
For instance, if we take the strong force to be the standard against which we compare the other three, we find the second strongest force, electromagnetism, is about a hundred times weaker. The third strongest force, the weak force, is about a hundred thousand times weaker than the strong force. The most familiar of the forces, gravity, is weak enough to be in a class of its own, about 10–40 times smaller than the strong force. For those readers whose math is a bit rusty, remember that 10–2 is the same as 0.01. Thus 10–40 is a zero, followed by a decimal point, then 39 zeros and a one. That’s small! Gravity is so weak that we’ve never been able to see any effect caused by gravity in modern particle physics experiments. Consequently, a quantum theory of gravity has eluded us. We simply don’t know how gravity works in the realm of the ultrasmall. Further, the relative weakness of gravity is very troubling to physicists, and working out the reason for this weakness is something to which it is hoped the LHC might contribute.
People have a feel for how gravity works, but at the subatomic level, forces reveal a funny behavior. For “big” sizes, say about the size of a molecule, gravity is simply everywhere. Wherever you walk, gravity always pulls you downward, and there is no place where there is no gravity. In the quantum realm, things act differently. It turns out that, in the same way that atoms are small bits of elements, there are smallest bits of force. Each force has a characteristic particle associated with it.
The idea that a force like gravity could consist of small particles is somewhat counterintuitive, so let’s explore it. Consider wind. Wind blows in your face, keeps a kite in the air, or pushes an empty can down the road. Wind exerts a force and is therefore analogous to other forces, like gravity or electromagnetic force. And, like gravity, air is something that is everywhere.
In addition to the forces, we also know something about chemistry. We know that air consists of molecules of oxygen, nitrogen, carbon dioxide, and the like. Thus the wind in your face is actually caused by uncounted molecules hitting you. Similarly, all of the forces at the subatomic level are treated as consisting of little particles of force.
As with much of particle physics, the names of the force- carrying particles are silly or simply mysterious. The particle causing the strong force is called the gluon, because it “glues” the nucleus together. The photon, familiar as light, is the particle carrying electromagnetism. Both the gluon and the photon have zero mass, but this isn’t true for the weak force. Indeed, there are three types of particles that cause the weak force: the electrically neutral Z0 (simply called “the Z boson”) and two particles with electrical charge, W+ and W–, which are pronounced “W plus” and “W minus” (showing that they have the electrical charge of a proton [+] or an electron [–], respectively). These three particles are very heavy, with each one carrying a mass in the range spanned by bromine and zirconium atoms, or nearly a hundred times heavier than a proton.
The fourth force, the quantumly mysterious gravity, is thought to be caused by a particle, too. This particle is called the graviton. The graviton has never been observed, and you should regard with suspicion any claim to its properties. However if it does exist, we are able to work out what some of its properties must be. For instance it must be electrically neutral and have zero mass. Some day the graviton might be observed, and there’s a Nobel Prize in it for the clever person who manages it. However, given gravity’s weak nature, this prize is not likely to be claimed any time soon. Table 1.1 lists the details of the force- causing particles.
While the table lists the known quarks, leptons, and force- causing particles and brings us to the very frontier of knowledge, there is one little wrinkle that has not yet been mentioned. Even though we think the handful of particles and forces we’ve mentioned thus far is all that’s needed to describe our universe, it turns out that there is a duplicate for every particle listed. Our next frontier topic concerns a mirror image of our familiar matter. This mirror matter is called antimatter, and it is one of the phrases popular with science fiction buffs that is science and isn’t fiction.
Figure 1.8. A paper clip made of matter combined with a paper clip of antimatter would release energy comparable to that released in the atomic detonation at Hiroshima.
The simplest description of antimatter is that it is the opposite of matter. Take some antimatter, add an identical amount of matter, and they both disappear in a blinding release of energy. The amount of energy released is huge compared with the amount of matter and antimatter involved. To give you a sense of size, if you took a paper clip made of matter and let it touch a paper clip of antimatter, the energy release is about the same as the 1945 atomic explosion at Hiroshima (see Figure 1.8).
Antimatter was predicted in 1928 by Paul Dirac. Does it really exist? The answer is a most emphatic Yes! The antimatter electron (called the positron) was discovered in 1932. The antiproton was observed in 1955, while the antimatter neutron waited until 1956. Protons and neutrons are made of quarks, but their antimatter counterparts are made of antiquarks. For example, the antiproton consists of two antimatter up quarks and one antimatter down quark. Antimatter particles have the opposite electrical charge from their matter counterpart; for instance, the proton has an electrical charge of +1, the antimatter proton (the antiproton) has an electrical charge of –1.
We have now observed antimatter counterparts for every type of quark and lepton. The simple existence of antimatter is interesting, but antimatter presents to us a truly fascinating mystery. To appreciate this mystery requires that you know two facts. First, you need to know that when we make antimatter in our laboratories, it always comes with an identical amount of matter. Always. Make an antimatter up quark, and you must simultaneously make an up quark. We never make an antimatter particle without a corresponding matter particle.
The reason for this is a thing called a conservation law. When there was only energy, there was no matter and no antimatter. Conservation means to keep something unchanged, so when antimatter is created, an identical amount of matter needs to be created to “cancel it out.” Both the matter and antimatter are “created from nothing” or, more accurately, created from pure energy.
The second fact that one must consider is perhaps obvious, but extremely mysterious. This fact is the simple observation that in everyday life, we just don’t see antimatter anywhere. There’s nothing in our understanding of antimatter that excludes antimatter stars, antimatter planets, or even antimatter people. As long as these things were kept isolated from matter, they should exist. And yet they don’t. Nowhere in the universe, as deep as our telescopes can see, do we see any substantial chunk of antimatter.
So why is that? Nobody really knows. This doesn’t mean that we know nothing about the subject, but rather that the experiments done to date have not told us the entire story. We expect the experiments of the LHC to shed light on the mystery, particularly the LHCb experiment (described in chapter 4).
With the introduction of the quarks, leptons, force- causing particles, and now antimatter, we have discussed everything we know about subatomic particles. If we take the particles from generation I and tosses in the force- causing particles, we can build everything we see in the universe from galaxies to ice cream. Toss in the particles from generations II and III, and we can explain the results of all experiments ever conducted in our huge particle accelerators, too. We call the theory that includes all these ideas the Standard Model of particle physics.
With such a broad set of phenomena that we understand as well as we do, scientists are justifiably proud. To be able to take a handful of different types of particles and paint the tapestry of the cosmos is not a small feat. However, one should not be left with the impression that such an accomplishment has not left profound mysteries. In fact, for all our achievements, there’s still a lot to do. Having focused our efforts on describing what we know, in the next chapters we shift our attention to some things we don’t know and how the Large Hadron Collider is expected to shed light on these mysteries.