Indicated when acute blood loss is sufficient to produce hypovolemia, whole blood provides both oxygen-carrying capacity and volume expansion. In acute blood loss, hematocrit may not accurately reflect degree of blood loss for 48 h until fluid shifts occur.
Indicated for symptomatic anemia unresponsive to specific therapy or requiring urgent correction. Packed red blood cell (RBC) transfusions may be indicated in pts who are symptomatic from cardiovascular or pulmonary disease when Hb is between 70 and 90 g/L (7 and 9 g/dL). Transfusion is usually necessary when Hb is <70 g/L (<7 g/dL). One unit of packed RBCs raises the Hb by approximately 10 g/L (1 g/dL). In the setting of acute hemorrhage, packed RBCs, fresh-frozen plasma (FFP), and platelets in an approximate ratio of 3:1:10 units are an adequate replacement for whole blood. Removal of leukocytes reduces risk of alloimmunization and transmission of cytomegalovirus. Washing to remove donor plasma reduces risk of allergic reactions. Irradiation prevents graft-versus-host disease in immunocompromised recipients by killing alloreactive donor lymphocytes. Avoid related donors.
(1) Hypertransfusion therapy to block production of defective cells, e.g., thalassemia, sickle cell anemia; (2) exchange transfusion—hemolytic disease of newborn, sickle cell crisis; (3) transplant recipients—decreases rejection of cadaveric kidney transplants.
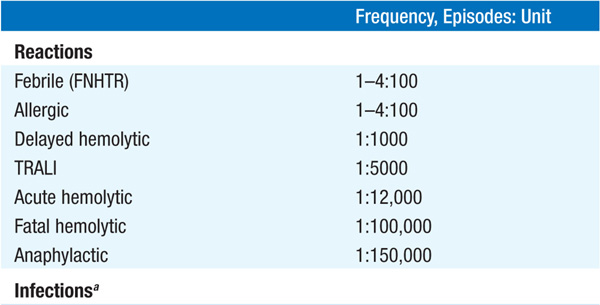
TABLE 9-1 RISKS OF TRANSFUSION COMPLICATIONS

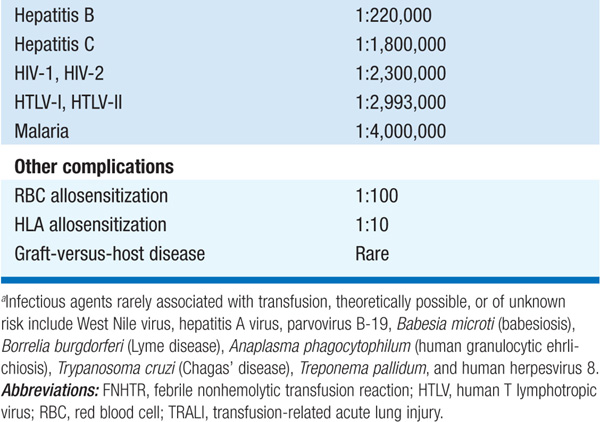
(1) Transfusion reaction—immediate or delayed, seen in 1–4% of transfusions; IgA-deficient pts at particular risk for severe reaction; (2) infection—bacterial (rare); hepatitis C, 1 in 1,800,000 transfusions; HIV transmission, 1 in 2,300,000; (3) circulatory overload; (4) iron overload—each unit contains 200–250 mg iron; hemochromatosis may develop after 100 U of RBCs (less in children), in absence of blood loss; iron chelation therapy with deferoxamine indicated; (5) graft-versus-host disease; (6) alloimmunization.
Use of pt’s own stored blood avoids hazards of donor blood; also useful in pts with multiple RBC antibodies. Pace of autologous donation may be accelerated using erythropoietin (50–150 U/kg SC three times a week) in the setting of normal iron stores.
The main goal of red cell exchange transfusions is to remove sickle cells and replace them with normal red cells to interrupt the vicious cycle of sickling, stasis, vasoocclusion, and hypoxia that propagate sickle cell crises. The usual target is 70% hemoglobin A.
Prophylactic transfusions usually reserved for platelet count <10,000/μL (<20,000/μL in acute leukemia). One unit elevates the count by about 10,000/μL if no platelet antibodies are present as a result of prior transfusions. Efficacy assessed by 1-h and 24-h posttransfusion platelet counts. HLA-matched single-donor platelets may be required in pts with platelet alloantibodies.
Fresh frozen plasma (FFP) is a source of coagulation factors, fibrinogen, antithrombin, and proteins C and S. It is used to correct coagulation factor deficiencies, rapidly reverse warfarin effects, and treat thrombotic thrombocytopenic purpura (TTP). Cryoprecipitate is a source of fibrinogen, factor VIII, and von Willebrand factor; it may be used when recombinant factor VIII or factor VIII concentrates are not available.
Hemapheresis is removal of a cellular or plasma constituent of blood; the specific procedure is referred to by the blood fraction removed.
Removal of WBCs; most often used in acute leukemia, esp. acute myeloid leukemia (AML) in cases complicated by marked elevation (>100,000/μL) of the peripheral blast count, to lower risk of leukostasis (blast-mediated vasoocclusive events resulting in central nervous system or pulmonary infarction, hemorrhage). Leukapheresis is replacing bone marrow aspiration to obtain hematopoietic stem cells. After treatment with a chemotherapeutic agent and granulocyte-macrophage colony-stimulating factor, hematopoietic stem cells are mobilized from marrow to the peripheral blood; such cells are leukapheresed and then used for hematopoietic reconstitution after high-dose myeloablative therapy. A third emerging medical use of leukapheresis is to harvest lymphocytes to use as adoptive immunotherapy.
Used in some pts with thrombocytosis associated with myeloproliferative disorders with bleeding and/or thrombotic complications. Other treatments are generally used first. Plateletpheresis also enhances platelet yield from blood donors.
(1) Hyperviscosity states— e.g., Waldenström’s macroglobulinemia; (2) TTP; (3) immune-complex and autoantibody disorders—e.g., Goodpasture’s syndrome, rapidly progressive glomerulonephritis, myasthenia gravis; possibly Guillain-Barré, systemic lupus erythematosus, idiopathic thrombocytopenic purpura; (4) cold agglutinin disease, cryoglobulinemia. In plasma exchange, abnormal proteins are removed and normal plasma or plasma components are replaced; useful in TTP to remove anti-ADAMTS13 antibody and provide normal ADAMTS13 levels.

For a more detailed discussion, see Dzieczkowski JS, Anderson KC: Transfusion Biology and Therapy, Chap. 113, p. 951, in HPIM-18.