Rabies is a zoonosis generally transmitted to humans by the bite of a rabid animal and caused by rabies virus—a nonsegmented, negative sense, single-stranded RNA virus in the family Rhabdoviridae. Each animal reservoir harbors distinct rabies virus variants.
Worldwide, canine rabies causes ~55,000 human deaths each year, most of them in Asia and Africa.
• Endemic canine rabies has been eliminated in the United States and most other resource-rich countries but persists in bats, raccoons, skunks, and foxes. In 2008, there were 6841 confirmed animal cases of rabies in the U.S.
• Bats (especially silver-haired and eastern pipistrelle bats) cause most human cases in North America, although there may be no known history of a bat bite or other bat exposure.
The incubation period can range from a few days to >1 year but is usually 20–90 days. During most of this period, rabies virus is present at or close to the site of the bite.
• The virus binds to postsynaptic nicotinic acetylcholine receptors and spreads centripetally along peripheral nerves toward the CNS at a rate up to ~250 mm/d. Establishment of CNS infection is followed by centrifugal spread along peripheral nerves to other tissues, including salivary glands—hence the excretion of virus in the saliva of rabid animals.
• The most characteristic pathologic CNS finding is the Negri body—an eosinophilic cytoplasmic inclusion that is composed of rabies virus proteins and viral RNA and is found primarily within Purkinje cells of the cerebellum and in pyramidal neurons of the hippocampus.
Rabies usually presents as atypical encephalitis with preservation of consciousness; the disease may be difficult to recognize after the onset of coma. This disease, which usually leads to death despite aggressive therapy, has three phases.
• Prodrome: Pts have fever, headache, malaise, nausea, vomiting, and anxiety or agitation lasting 2–10 days. Paresthesias, pain, or pruritus near the site of exposure (which has usually healed at this point) is found in 50–80% of cases and strongly suggests rabies.
• Acute neurologic phase: Pts present with the encephalitic (furious) form of rabies in 80% of cases and with the paralytic form in 20%.
– Encephalitic form: Pts develop symptoms common to other viral encephalitides (e.g., fever, confusion, hallucinations, combativeness, and seizures) that last 2–10 days. Autonomic dysfunction is common and includes hypersalivation, gooseflesh, cardiac arrhythmia, and/or priapism.
• A distinguishing feature of rabies is prominent early brainstem dysfunction resulting in hydrophobia and aerophobia (involuntary, painful contraction of the diaphragm and the accessory respiratory, laryngeal, and pharyngeal muscles in response to swallowing liquid or exposure to a draft of air).
• Hypersalivation and pharyngeal dysfunction produce characteristic foaming at the mouth.
• Death usually occurs within days of brainstem involvement. With aggressive supportive care, late complications include cardiopulmo-nary failure, disturbances of water balance (syndrome of inappropriate antidiuretic hormone secretion or diabetes insipidus), and GI hemorrhage.
– Paralytic form: For unknown reasons, muscle weakness predominates but cardinal features of rabies encephalitis (hyperexcitability, hydro-phobia, aerophobia) are lacking. Muscle weakness usually begins in the bitten extremity and proceeds to quadriparesis.
• Coma and death: Even with aggressive supportive measures, recovery is rare. Death usually occurs within 2 weeks.
In North America, the diagnosis is often considered relatively late in the clinical course. Rabies should be considered for pts with acute atypical encephalitis or acute flaccid paralysis (including those in whom Guillain-Barré syndrome is suspected).
• Most routine laboratory tests in rabies are normal or nonspecific; it is important to test for alternative, potentially treatable diagnoses.
• Negative antemortem rabies-specific laboratory tests never exclude a diagnosis of rabies, and tests may need to be repeated after an interval for diagnostic confirmation.
– In a previously unimmunized pt, serum neutralizing antibodies to rabies virus are diagnostic, but these antibodies may not be present until late in the disease course. The presence of rabies virus–specific antibodies in CSF suggests rabies encephalitis, regardless of immunization status.
– Reverse-transcription PCR (RT-PCR) can detect virus in fresh saliva samples, CSF, and skin and brain tissues.
– Direct fluorescent antibody testing is highly sensitive and specific and can be applied to brain tissue or skin biopsy samples from the nape of the neck (where virus is found in cutaneous nerves at the base of hair follicles).
Management is palliative and supportive. There is no established treatment for rabies.
Rabies is almost uniformly fatal but is nearly always preventable with appropriate postexposure prophylaxis during the incubation period. Only 7 pts have survived infection with rabies virus, and only 1 of these pts had not received rabies vaccine before disease onset.
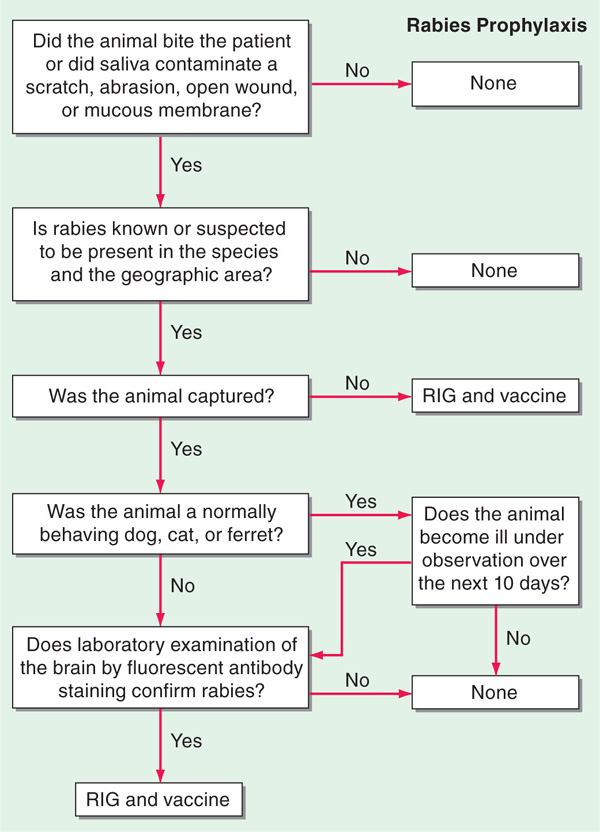
• An algorithm for rabies postexposure prophylaxis is depicted in Fig. 113-1.
FIGURE 113-1 Algorithm for rabies postexposure prophylaxis. RIG, rabies immune globulin. [From L Corey, in Harrison’s Principles of Internal Medicine, 15th ed. E Braunwald et al (eds): New York, McGraw-Hill, 2001, adapted with permission.]
– Local wound care (e.g., thorough washing, debridement of devitalized tissue) can greatly reduce the risk of rabies.
– All previously unvaccinated pts should receive human rabies immune globulin (RIG, 20 IU/kg; 40 IU/kg for equine RIG) no later than 7 days after the first vaccine dose. The entire dose should be infiltrated at the site of the bite; if not anatomically feasible, the residual RIG should be given IM at a distant site.
– Inactivated rabies vaccine should be given as soon as possible (1 mL IM in the deltoid region) and repeated on days 3, 7, and 14 for previously unvaccinated pts; previously vaccinated pts require booster doses only on days 0 and 3.
• Preexposure prophylaxis is occasionally given to persons at high risk (including certain travelers to rabies-endemic areas). A primary vaccine schedule is given on days 0, 7, and 21 or 28.
Most zoonotic viruses only incidentally infect and produce disease in humans; only a few agents are regularly spread among humans by arthropods.
• The Arenaviridae, Bunyaviridae, and Flaviviridae—all RNA viruses—are among the major families of arthropod- and rodent-borne viruses.
• Arthropod-borne viruses infect the vector after a blood meal from a viremic vertebrate; after spreading throughout the vector and ultimately reaching the salivary glands, the viruses can be transmitted to another vertebrate during a blood meal.
• Humans become infected with rodent-borne viruses by inhalation of aerosols containing the viruses and through close contact with chronically infected rodents and their excreta.
Infection is usually subclinical; when disease does occur, it generally does so in one of four occasionally overlapping clinical syndromes: fever and myalgia, encephalitis, arthritis and rash, or hemorrhagic fever (HF).
Fever and Myalgia This is the most common syndrome associated with zoonotic viruses. Typically, pts have an acute onset of fever, severe myalgia, malaise, and headache. Complete recovery after 2–5 days of illness is usual. Important examples include the following.
• Lymphocytic choriomeningitis (LCM): This infection is transmitted from chronically infected mice and pet hamsters via aerosols of excreta and secreta. About one-fourth of infected pts have a 3- to 6-day febrile phase, a brief remission, and then recurrent fever, headache, nausea, vomiting, and meningeal signs lasting ~1 week.
– Other manifestations include transient alopecia, arthritis, pharyngitis, cough, maculopapular rash, and orchitis.
– Pregnant women can have mild infection yet pass on the virus to the fetus, who can develop hydrocephalus and chorioretinitis.
– The diagnosis should be considered when an adult has aseptic meningitis and any of the following: autumn seasonality, a well-marked febrile prodrome, a low CSF glucose level, or CSF mononuclear cell counts >1000/μL.
– LCM viremia is most likely in the initial febrile phase of illness. LCM can also be diagnosed by IgM-capture ELISA of serum or CSF or by RT-PCR of CSF.
• Dengue fever: The 4 serotypes of dengue viruses are all transmitted by the mosquito Aedes aegypti, which is also a vector for yellow fever. After an incubation period of 2–7 days, pts experience the sudden onset of fever, headache, retroorbital pain, back pain, severe myalgia (break-bone fever), adenopathy, palatal vesicles, and scleral injection.
– The illness usually lasts 1 week, and a maculopapular rash often appears near the time of defervescence.
– A second infection with a different dengue serotype can lead to dengue hemorrhagic fever (DHF; see “Hemorrhagic Fever,” below).
– The diagnosis is made by IgM ELISA or paired serologic tests during recovery or by antigen-detection ELISA or RT-PCR during the acute phase. Virus is easily isolated from blood during the acute phase by inoculation of mosquitoes or mosquito cell culture. Leukopenia, thrombocytopenia, and increased serum aminotransferase levels may be documented.
Encephalitis Depending on the causative virus, there is much variability in the ratio of clinical to subclinical disease, the mortality rate, and residua (Table 113-1). The pt usually presents with a prodrome of nonspecific symptoms that is followed quickly by headache, meningeal signs, photo-phobia, and vomiting; involvement of deeper structures leads to lethargy, cognitive deficits, focal neurologic signs, and coma. Acute encephalitis usually lasts from a few days to 2–3 weeks, and recovery may be slow and incomplete. Treatable causes of encephalitis (e.g., HSV) should be ruled out promptly. Some important examples of arboviral encephalitides follow.
TABLE 113-1 PROMINENT FEATURES OF ARBOVIRAL ENCEPHALITIS
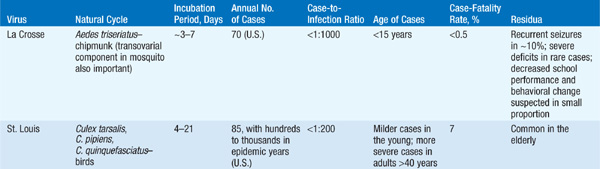

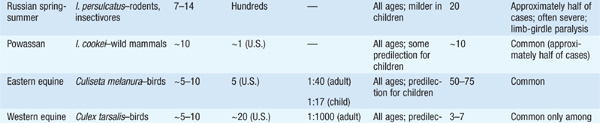

• Japanese encephalitis: This infection is present throughout Asia and the western Pacific islands. Spinal and motor neuron disease can be documented in addition to encephalitis. An effective vaccine (ideally given on days 0, 7, and 30) is available and is indicated for summer travelers to rural Asia, where the risk can be as high as 2.1 cases per 10,000 per week.
• West Nile encephalitis: Usually a mild or asymptomatic disease, West Nile virus infection can cause aseptic meningitis or encephalitis and is present throughout the Western Hemisphere. Encephalitis, serious sequelae, and death are more common among elderly pts, diabetic and hypertensive pts, and pts with previous CNS disease. Unusual clinical features include chorioretinitis and flaccid paralysis.
• Eastern equine encephalitis (EEE): EEE occurs primarily within endemic swampy foci along the eastern coast of the United States during the summer and early fall. EEE is one of the most severe arboviral diseases and is characterized by rapid onset, rapid progression, high mortality risk, and frequent residua. PMN-predominant pleocytosis of the CSF within the first 3 days of disease is common.
Arthritis and Rash Alphaviruses are common causes of true arthritis accompanied by a febrile illness and maculopapular rash. Examples include the following.
• Sindbis virus: Found in northern Europe and the independent states of the former Soviet Union, this virus causes a maculopapular rash that often vesiculates on the trunk and extremities. The arthritis of this condition is multiarticular, migratory, and incapacitating, with resolution of the acute phase in a few days; joint pains may persist for months or years.
• Chikungunya virus: Found in rural Africa and Asia, this virus results in the abrupt onset of fever, severe arthralgias, migratory polyarthritis mainly affecting small joints, and a rash that begins coincident with defervescence at day 2–3 of illness.
• Ross River virus: A cause of epidemic polyarthritis in Australia and the eastern Pacific Islands, this virus causes rash and persistent joint involvement, typically in the absence of other constitutional symptoms. Because of joint pain, only ~50% and 10% of pts can resume normal activities at 4 weeks and 3 months, respectively.
Hemorrhagic Fever The viral HF syndrome is a constellation of findings based on vascular instability and decreased vascular integrity. All HF syndromes begin with the abrupt onset of fever and myalgia and can progress to severe prostration, headache, dizziness, photophobia, abdominal and/or chest pain, anorexia, and GI disturbances. On initial physical examination, there is conjunctival suffusion, muscular or abdominal tenderness to palpation, hypotension, petechiae, and periorbital edema. Laboratory examination usually reveals elevated serum aminotransferase levels, proteinuria, and hemoconcentration. Shock, multifocal bleeding, and CNS involvement (encephalopathy, coma, convulsions) are poor prognostic signs. Early recognition is important; appropriate supportive measures and, in some cases, virus-specific therapy can be instituted.
• Lassa fever: Endemic and epidemic in West Africa, Lassa fever, which is caused by a rodent-borne virus, has a more gradual onset than other HF syndromes. Bleeding is evident in 15–30% of cases. A maculopapular rash is often noted in light-skinned pts with Lassa fever.
– Pregnant women have higher mortality rates, and the fetal death rate is 92% in the last trimester.
– Pts with high-level viremia or a serum aspartate aminotransferase level of >150 IU/mL are at an elevated risk of death, and the administration of ribavirin (32 mg/kg IV × 1 dose, followed by 16 mg/kg q6h for 4 days and then 8 mg/kg q8h for 6 days), which appears to reduce this risk, should be considered.
• South American HF syndromes (Argentine, Bolivian, Venezuelan, Brazilian): These syndromes resemble Lassa fever; however, thrombocytopenia, bleeding, and CNS dysfunction are common.
– Passive antibody treatment for Argentine HF is effective, and an effective vaccine exists.
– Ribavirin is likely to be effective in all South American HF syndromes.
• Rift Valley fever: Although Rift Valley fever virus typically causes fever and myalgia, HF can occur with prominent liver involvement, renal failure, and probably disseminated intravascular coagulation (DIC).
– Retinal vasculitis can occur in ~10% of otherwise mild infections, and pts’ vision can be permanently impaired.
– There is no proven therapy for Rift Valley fever. A live attenuated vaccine is in trials.
• HF with renal syndrome: This entity is most often caused in Europe by Puumala virus (rodent reservoir, the bank vole) and in Asia by Hantaan virus (rodent reservoir, the striped field mouse).
– Severe cases of HF with renal syndrome caused by Hantaan virus evolve in identifiable stages: the febrile stage with myalgia, lasting 3 or 4 days; the hypotensive stage, often associated with shock and lasting from a few hours to 48 h; the oliguric stage with renal failure, lasting 3–10 days; and the polyuric stage with diuresis and hyposthenuria.
– Infections with Puumala virus result in a much-attenuated picture but the same general presentation.
– IgM-capture ELISA is positive within 2 days of admission and confirms the diagnosis.
– The mainstay of therapy is expectant management of shock and renal failure. Ribavirin may reduce rates of mortality and morbidity in severe cases if treatment is begun within the first 4 days of illness.
• Hantavirus pulmonary syndrome (HPS): After a prodrome of ~3–4 days, pts enter a cardiopulmonary phase marked by tachycardia, tachypnea, and mild hypotension. Over the next few hours, the illness may rapidly progress to severe hypoxemia and respiratory failure; the mortality rate is ~30–40% with good management. Pts surviving the first 2 days of hospitalization usually recover with no residua.
– The disease is linked to rodent exposure. Sin Nombre virus infects the deer mouse and is the most important virus causing HPS in the United States.
– Thrombocytopenia (an important early clue), hemoconcentration, proteinuria, and hypoalbuminemia are typical.
– IgM testing of acute-phase serum may give positive results, even during the prodromal stage, and can confirm the diagnosis. RT-PCR of blood clots or tissue usually gives a positive result in the first 7–9 days of illness.
– Treatment is nonspecific and requires intensive respiratory management and other supportive measures.
• Yellow fever: A former cause of major epidemics, yellow fever causes a typical HF syndrome with prominent hepatic necrosis, most commonly in urban South America and Africa. Pts are viremic for 3–4 days and can have jaundice, hemorrhage, black vomit, anuria, and terminal delirium. Vaccination of visitors to endemic areas and control of the mosquito vector A. aegypti prevent disease.
• Dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS): Previous infection with a heterologous dengue virus serotype may elicit non-protective antibodies and enhanced disease if pts are reinfected. In mild cases, lethargy, thrombocytopenia, and hemoconcentration occur 2–5 days after typical dengue fever, usually at the time of defervescence. In severe cases, frank shock occurs, with cyanosis, hepatomegaly, ascites and pleural effusions, and GI bleeding.
– The risk decreases considerably after age 12; DHF/DSS is more common among females than among males, more severe among whites than among blacks, and more common among well-nourished than among malnourished persons.
– With good care, the overall mortality rate is as low as 1%. Control of A. aegypti, the mosquito vector, is the key to control of the disease.
The family Filoviridae contains two genera, Marburgvirus and Ebolavirus, that consist of negative-sense, single-stranded RNA viruses. Ebolavirus has 5 species named for their original site of recognition.
• With the exception of Reston virus (an Ebola virus), all Filoviridae are African viruses that cause severe disease with high mortality rates.
• Both Marburg virus and Ebola virus are biosafety level 4 pathogens because of the high mortality rate from infection and the aerosol infectivity of the agents.
Marburg virus was first identified in 1967; in 2004–2005, a Marburg virus epidemic occurred in Angola, with >250 cases and a case-fatality rate of 90%. Ebola virus was first identified in 1976 and has been associated with several epidemics of severe HF; the mortality rate ranges from 50 to 90%, depending on the species.
• Human-to-human transmission occurs, but epidemiologic studies have failed to yield evidence for an important role (like that documented in Ebola disease in monkeys) of airborne particles in human Ebola disease.
• The reservoir is unknown, but speculation currently centers on bats.
Both viruses replicate well in virtually all cell types, and viral replication is associated with cellular necrosis. Acute infection is associated with high levels of circulating virus and viral antigen. Fatal cases are associated with the lack of an antibody response, but clinical recovery is probably mediated by the cellular immune response since convalescent-phase plasma is not protective.
After a 7- to 10-day incubation period, pts experience an abrupt onset of fever, severe headache, myalgia, nausea, vomiting, diarrhea, prostration, and depressed mentation.
• A maculopapular rash may appear at day 5–7 and is followed by desquamation. Bleeding may occur at this time and is apparent from any mucosal site and into the skin.
• The fever may break after 10–12 days, and the pt may eventually recover.
• Recrudescence and secondary bacterial infection may occur.
• Leukopenia is common early on and is followed by neutrophilia. Thrombocytopenia, transaminitis, and jaundice are common.
High concentrations of virus in blood can be documented by antigen-detection ELISA, virus isolation, or RT-PCR. Antibodies can be detected in recovering pts.
TREATMENT Ebola and Marburg Virus Infections
• No virus-specific therapy is available, and supportive measures may not be as useful as had been hoped.
• Studies in rhesus monkeys suggest that treatment with an inhibitor of factor VIIa/tissue factor or with activated protein C may improve survival rates.
• Barrier nursing precautions can greatly decrease the spread of filoviruses.