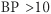 mmHg usually indicates >20% reduction in blood volume (± syncope, light-headedness, nausea, sweating, thirst).
mmHg usually indicates >20% reduction in blood volume (± syncope, light-headedness, nausea, sweating, thirst).1. Hematemesis : Vomiting of blood or altered blood (“coffee grounds”) indicates bleeding proximal to ligament of Treitz.
2. Melena : Altered (black) blood per rectum (>100 mL blood required for one melenic stool) usually indicates bleeding proximal to ligament of Treitz but may be as distal as ascending colon; pseudomelena may be caused by ingestion of iron, bismuth, licorice, beets, blueberries, charcoal.
3. Hematochezia: Bright red or maroon rectal bleeding usually implies bleeding beyond ligament of Treitz but may be due to rapid upper GI bleeding (>1000 mL).
4. Positive fecal occult blood test with or without iron deficiency.
5. Symptoms of blood loss: e.g., light-headedness or shortness of breath.
Orthostatic drop in 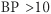 mmHg usually indicates >20% reduction in blood volume (± syncope, light-headedness, nausea, sweating, thirst).
mmHg usually indicates >20% reduction in blood volume (± syncope, light-headedness, nausea, sweating, thirst).
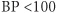 mmHg systolic usually indicates <30% reduction in blood volume (± pallor, cool skin).
mmHg systolic usually indicates <30% reduction in blood volume (± pallor, cool skin).
Hematocrit may not reflect extent of blood loss because of delayed equilibration with extravascular fluid. Mild leukocytosis and thrombocytosis. Elevated blood urea nitrogen is common in upper GI bleeding.
Age >60, associated illnesses, coagulopathy, immunosuppression, presentation with shock, rebleeding, onset of bleeding in hospital, variceal bleeding, endoscopic stigmata of recent bleeding [e.g., “visible vessel” in ulcer base (see below)].
Peptic ulcer (accounts for ~50%), gastropathy [alcohol, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), stress], esophagitis, Mallory-Weiss tear (mucosal tear at gastroesophageal junction due to retching), gastroesophageal varices.
Swallowed blood (nosebleed); esophageal, gastric, or intestinal neoplasm; anticoagulant and fibrinolytic therapy; hypertrophic gastropathy (Ménétrier’s disease); aortic aneurysm; aortoenteric fistula (from aortic graft); arteriovenous malformation; telangiectases (Osler-Rendu-Weber syndrome); Dieulafoy lesion (ectatic submucosal vessel); vasculitis; connective tissue disease (pseudoxanthoma elasticum, Ehlers-Danlos syndrome); blood dyscrasias; neurofibroma; amyloidosis; hemobilia (biliary origin).
After hemodynamic resuscitation (see below and Fig. 47-1).
FIGURE 47-1 Suggested algorithm for pts with acute upper GI bleeding. Recommendations on level of care and time of discharge assume pt is stabilized without further bleeding or other concomitant medical problems. ICU, intensive care unit; PPI, proton pump inhibitor.
• History and physical examination: Drugs (increased risk of upper and lower GI tract bleeding with aspirin and NSAIDs), prior ulcer, bleeding history, family history, features of cirrhosis or vasculitis, etc. Hyperactive bowel sounds favor upper GI source.
• Nasogastric aspirate for gross blood, if source (upper versus lower) not clear from history; may be falsely negative in up to 16% of pts if bleeding has ceased or duodenum is the source. Testing aspirate for occult blood is meaningless.
• Upper endoscopy: Accuracy >90%; allows visualization of bleeding site and possibility of therapeutic intervention; mandatory for suspected varices, aortoenteric fistulas; permits identification of “visible vessel” (protruding artery in ulcer crater), which connotes high (~50%) risk of rebleeding.
• Upper GI barium radiography: Accuracy ~80% in identifying a lesion, though does not confirm source of bleeding; acceptable alternative to endoscopy in resolved or chronic low-grade bleeding.
• Selective mesenteric arteriography: When brisk bleeding precludes identification of source at endoscopy.
• Radioisotope scanning (e.g., 99Tc tagged to red blood cells or albumin); used primarily as screening test to confirm bleeding is rapid enough for arteriography to be of value or when bleeding is intermittent and of unclear origin.
Anal lesions (hemorrhoids, fissures), rectal trauma, proctitis, colitis (ulcerative colitis, Crohn’s disease, infectious colitis, ischemic colitis, radiation), colonic polyps, colonic carcinoma, angiodysplasia (vascular ectasia), diverticulosis, intussusception, solitary ulcer, blood dyscrasias, vasculitis, connective tissue disease, neurofibroma, amyloidosis, anticoagulation.
FIGURE 47-2 Suggested algorithm for pts with acute lower gastrointestinal bleeding. *Some suggest colonoscopy for any degree of rectal bleeding in pts <40 years as well. †If massive bleeding does not allow time for colonic lavage, proceed to angiography.
• History and physical examination.
• In the presence of hemodynamic changes, perform upper endoscopy followed by colonoscopy. In the absence of hemodynamic changes, perform anoscopy and either flexible sigmoidoscopy or colonoscopy: Exclude hemorrhoids, fissure, ulcer, proctitis, neoplasm.
• Colonoscopy: Often test of choice, but may be impossible if bleeding is massive.
• Barium enema: No role in active bleeding.
• Arteriography: When bleeding is severe (requires bleeding rate >0.5 mL/min; may require prestudy radioisotope bleeding scan as above); defines site of bleeding or abnormal vasculature.
• Surgical exploration (last resort).
Often small-bowel source. Consider small-bowel enteroclysis x-ray (careful barium radiography via peroral intubation of small bowel), Meckel’s scan, enteroscopy (small-bowel endoscopy), or exploratory laparotomy with intraoperative enteroscopy.
• Venous access with large-bore IV (14–18 gauge); central venous line for major bleed and pts with cardiac disease; monitor vital signs, urine output, Hct (fall may lag). Gastric lavage of unproven benefit but clears stomach before endoscopy. Iced saline may lyse clots; room-temperature tap water may be preferable. Intubation may be required to protect airway.
• Type and cross-match blood (6 units for major bleed).
• Surgical standby when bleeding is massive.
• Support blood pressure with isotonic fluids (normal saline); albumin and fresh-frozen plasma in cirrhotics. Packed red blood cells when available (whole blood if massive bleeding); maintain Hct >25–30. Fresh-frozen plasma and vitamin K (10 mg SC or IV) in cirrhotics with coagulopathy.
• IV calcium (e.g., up to 10–20 mL 10% calcium gluconate IV over 10–15 min) if serum calcium falls (due to transfusion of citrated blood). Empirical drug therapy (antacids, H2 receptor blockers, omeprazole) of unproven benefit.
• Specific measures: Varices: octreotide (50-μg bolus, 50-μg/h infusion for 2–5 days), Sengstaken-Blakemore tube tamponade, endoscopic sclerosis, or band ligation; propranolol or nadolol in doses sufficient to cause beta blockade reduces risk of recurrent or initial variceal bleeding (do not use in acute bleed) (Chap. 166); ulcer with visible vessel or active bleeding: endoscopic bipolar, heater-probe, or laser coagulation or injection of epinephrine; gastritis: embolization or vasopressin infusion of left gastric artery; GI telangiectases: ethinylestradiol/norethisterone (0.05/1.0 mg PO qd) may prevent recurrent bleeding, particularly in pts with chronic renal failure; diverticulosis: mesenteric arteriography with intra-arterial vasopressin; angiodysplasia: colonoscopic bipolar or laser coagulation, may regress with replacement of stenotic aortic valve.
• Indications for emergency surgery: Uncontrolled or prolonged bleeding, severe rebleeding, aortoenteric fistula. For intractable variceal bleeding, consider transjugular intrahepatic portosystemic shunt (TIPS).

For a more detailed discussion, see Laine L: Gastrointestinal Bleeding, Chap. 41, p. 320, in HPIM-18.