Brucellae are small, gram-negative, unencapsulated, nonsporulating, nonmotile rods or coccobacilli that can persist intracellularly. The genus Brucella includes four major clinically relevant species: B. melitensis (acquired by humans most commonly from sheep, goats, and camels), B. suis (from swine), B. abortus (from cattle or buffalo), and B. canis (from dogs).
Brucellosis is transmitted via ingestion, inhalation, or mucosal or percutaneous exposure; the disease in humans is usually associated with exposure to infected animals or their products in either occupational settings (e.g., slaughterhouse work, farming) or domestic settings (e.g., consumption of contaminated foods, especially dairy products). The global prevalence of brucellosis is unknown because of difficulties in diagnosis and inadequacies in reporting systems.
Regardless of the specific infecting species, brucellosis often presents with one of three patterns: a febrile illness similar to but less severe than typhoid fever; fever and acute monarthritis, typically of the hip or knee, in a young child (septic arthritis); or long-lasting fever, misery, and low-back or hip pain in an older man (vertebral osteomyelitis).
• An incubation period of 1 week to several months is followed by the development of undulating fever; sweats; increasing apathy, fatigue, and anorexia; and nonspecific symptoms such as headache, myalgias, and chills.
• Brucella infection can cause lymphadenopathy, hepatosplenomegaly, epididymoorchitis, neurologic involvement, and focal abscess.
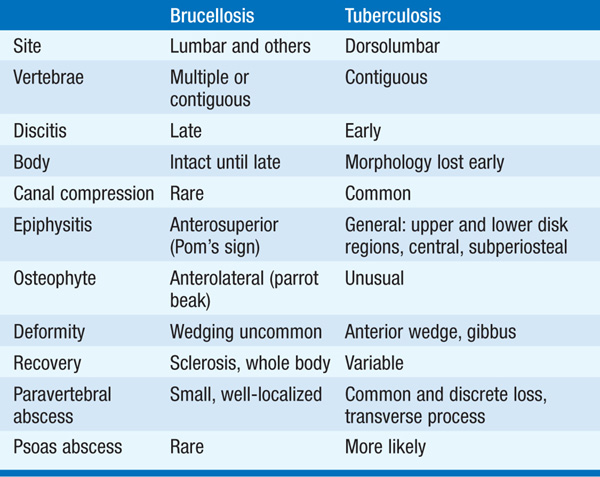
• Given the persistent fever and similar symptoms, tuberculosis is the most important differential diagnosis (Table 100-1).
TABLE 100-1 RADIOLOGY OF THE SPINE: DIFFERENTIATION OF BRUCELLOSIS FROM TUBERCULOSIS

Laboratory personnel must be alerted to the potential diagnosis to ensure that they take precautions to prevent occupational exposure.
• The organism is successfully cultured in 50–70% of cases. Cultures using the BACTEC systems usually become positive in 7–10 days and can be deemed negative at 3 weeks.
• PCR analysis of blood or tissue samples is more sensitive, faster, and safer than culture.
• Agglutination assays for IgM are positive early in infection. Single titers of ≥1:160 and ≥1:320 are diagnostic in nonendemic and endemic areas, respectively.
• The recommended regimen is streptomycin at a dosage of 0.75–1 g/d (or gentamicin at 5–6 mg/kg qd) for 14–21 days plus doxycycline at a dosage of 100 mg bid for 6 weeks.
– Rifampin (600–900 mg/d) plus doxycycline (100 mg bid) for 6 weeks constitute an alternative regimen (the current World Health Organization [WHO] recommendation).
– Significant neurologic disease requires at least 3–6 months of treatment, with ceftriaxone supplementation of a standard regimen.
– Endocarditis requires a four-drug regimen (an aminoglycoside, rifampin, a tetracycline, and ceftriaxone or a fluoroquinolone) for at least 6 weeks.
– Relapse rates range from 5 to >20% and depend on the specific antibiotic regimen used; pts should be monitored for at least 2 years.
Tularemia is the only disease caused by Francisella tularensis, a small, gram-negative, aerobic bacillus that is a potential agent of bioterrorism (Chap. 33).
• Human infection occurs via interaction with biting or blood-sucking insects (especially ticks and tabanid flies in the spring and summer), wild or domestic animals (e.g., wild rabbits, squirrels), or the environment.
– The organism gains entry into the skin or mucous membranes through bites or inapparent abrasions or is acquired via inhalation or ingestion.
– As few as 10 organisms can result in infection when injected into the skin or inhaled.
• More than half of U.S. cases occur in Arkansas, Oklahoma, South Dakota, and Missouri.
After an incubation period of 2–10 days, tularemia generally starts with an acute onset of fever, chills, headache, and myalgias. The ulceroglandular/glandular forms of tularemia affect 75–85% of pts, but several other syndromes involving systemic manifestations can occur.
• Ulceroglandular/glandular tularemia: The hallmark of ulceroglandular tularemia is an indurated, erythematous, nonhealing ulcer lasting 1–3 weeks that begins as a pruritic or tender lesion, ulcerates, has sharply demarcated edges and a yellow exudate, and develops a black base.
– Inguinal/femoral lymphadenopathy is most common in adults; nodes can become fluctuant and drain spontaneously.
– In glandular tularemia (5–10% of cases), no primary skin lesion is apparent.
• Oculoglandular tularemia: In 1% of pts, infection of the conjunctiva—usually by contact with contaminated fingers—results in purulent conjunctivitis with regional lymphadenopathy and debilitating pain. Painful preauricular lymphadenopathy distinguishes tularemia from other diseases.
• Oropharyngeal and GI tularemia: Acquired through oral inoculation (via contaminated foods or fingers), the infection can present as pharyngitis and cervical adenopathy, intestinal ulcerations, mesenteric lymphadenopathy, diarrhea, nausea, vomiting, and abdominal pain.
• Pulmonary tularemia: Infection is acquired via inhalation or via hematogenous spread from ulceroglandular or typhoidal tularemia. Pts present with signs and symptoms similar to those of pneumonia of other etiologies (e.g., nonproductive cough, dyspnea, pleuritic chest pain, CXR with bilateral patchy or lobar infiltrates or cavitary lesions).
• Typhoidal tularemia: Due to pharyngeal or GI inoculation or to bacteremic disease, typhoidal tularemia consists of fever and signs of sepsis, generally without skin lesions or lymphadenopathy. This form is the result of a large inoculum or a preexisting compromising condition.
The diagnosis of tularemia is most frequently confirmed by serology, although up to 30% of pts infected for 3 weeks have negative results in serologic tests.
• Cultures are positive in only 10% of cases; organisms in culture pose a significant risk to laboratory personnel.
• PCR has been used to detect F. tularensis DNA in clinical specimens, mainly for ulceroglandular disease.
TREATMENT Tularemia
• Gentamicin (2.5 mg/kg IV bid for 7–10 days) is considered the drug of choice; pts who defervesce within the first 48–72 h of treatment may receive a 5- to 7-day course.
– Streptomycin (1 g IM q12h for 10 days) is also effective, but tobramycin is not.
– Doxycycline is another alternative, but it must be given for at least 14 days because it is only bacteriostatic against F. tularensis.
– Healing of skin lesions and lymph nodes may take 1–2 weeks. Late lymph-node suppuration can occur, with sterile necrotic tissue.
Yersinia pestis causes plague, a systemic zoonosis that primarily affects small rodents in rural areas of Africa (where 80% of all human cases worldwide occur), Asia, and the Americas. As the rodent population succumbs to disease, fleas (the arthropod vector) search for a new host and can transmit the bacteria to humans.
• In addition to fleabites, direct contact with infected tissues or airborne droplets can cause human infections. Given the possibility of airborne transmission, Y. pestis is a potential agent of bioterrorism (Chap. 33).
• There are a median of 7 cases per year in the U.S., most of them occurring near the “Four Corners” (the junction point of New Mexico, Arizona, Colorado, and Utah) and further west in California, southern Oregon, and western Nevada.
Worldwide, bubonic plague accounts for 80–95% of all plague cases, with primary septicemic plague occurring in 10–20% of cases and primary pulmonary plague in only a small minority of cases.
• Bubonic plague: After an incubation period of 2–6 days, the onset of bubonic plague is sudden and is characterized by fever (>38°C), malaise, myalgia, dizziness, and increasing pain due to progressive lymphadenitis in the regional lymph nodes near the fleabite or other inoculation site.
– The tender, swollen lymph node (bubo) has a boggy consistency with an underlying hard core when palpated.
– With treatment, fever resolves within 2–5 days; without treatment, infection can disseminate and cause serious illness (e.g., secondary pneumonic plague, meningitis).
• Primary septicemic plague: Pts present with gram-negative septicemia not preceded by lymphadenopathy. Persons >40 years old are at greater risk, although this form of the disease can occur in all age groups.
• Pneumonic plague: After a short incubation period averaging a few hours to 3 days, pts experience a sudden onset of fever, nonspecific signs and symptoms (e.g., headache, myalgias, vomiting), and respiratory manifestations (e.g., cough, chest pain, sputum production with hemoptysis).
– Pneumonitis that is initially segmental can progress to lobar pneumonia and then to bilateral lung involvement.
– The mortality rate is nearly 100% without treatment and is still >50% with effective treatment.
The WHO recommends an initial presumptive diagnosis followed by confirmation in a reference laboratory.
• The appropriate specimens for diagnosis of bubonic, pneumonic, and septicemic plague are bubo aspirate (after injection of 1 mL of normal saline), bronchoalveolar lavage fluid or sputum, and blood, respectively. Gram’s, Wayson, or Wright-Giemsa staining of these samples may reveal bipolar gram-negative rods.
• Given the potential risk to laboratory workers, culture of Y. pestis should be performed only at reference laboratories, which use direct immunofluorescence, PCR, and/or specific bacteriophage lysis as confirmatory tests for identification. The optimal growth temperature is 25–29°C.
• In the absence of other positive diagnostic testing, a serologic diagnosis can be made.
• Streptomycin (1g IM q12h) or gentamicin (5 mg/kg IV q24h) is the drug of choice. Doxycycline (200 mg/d PO/IV in 1 or 2 doses) and chloramphenicol (12.5 mg/kg PO/IV q6h) are alternative agents.
• For pts who are hospitalized with pneumonic plague or in whom this disease is suspected, respiratory droplet precautions should be implemented until treatment has been given for at least 48 h.
• Postexposure antimicrobial prophylaxis lasting 7 days is recommended after household, hospital, or other close (<2 m) contact with persons with untreated pneumonic plague. Doxycycline (200 mg/d PO/IV in 1 or 2 doses), ciprofloxacin (1 g PO q12h), or TMP-SMX (320 mg of the TMP component PO q12h) are effective agents for prophylaxis.
• Bartonella species are fastidious, facultative intracellular, gram-negative bacteria that cause an array of infectious disease syndromes in humans.
• Most Bartonella species have successfully adapted to survival in specific domestic or wild mammals, creating a reservoir for human infection. The exceptions are B. bacilliformis and B. quintana, which are not zoonotic.
• Clinical presentation generally depends on both the infecting Bartonella species and the immune status of the infected individual.
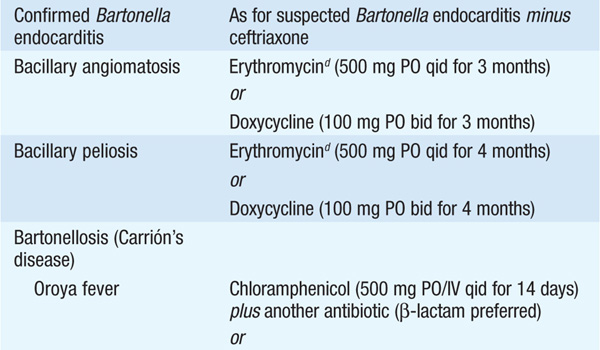
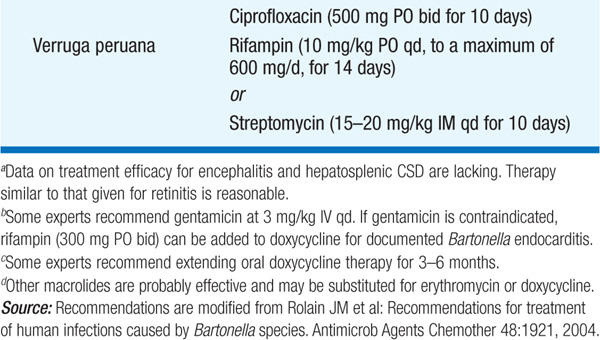
• Therapy for syndromes caused by Bartonella is summarized in Table 100-2.
TABLE 100-2 ANTIMICROBIAL THERAPY FOR DISEASE CAUSED BY BARTONELLA SPECIES IN ADULTS
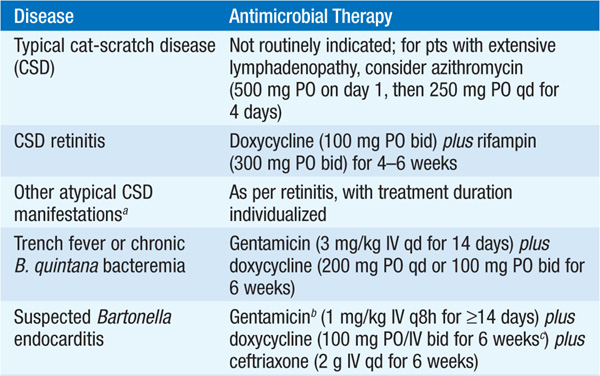
B. henselae is the principal etiologic agent of CSD, although other Bartonella species may rarely be involved. Consistent with the disease’s name, contact (being scratched, bitten, or licked) with apparently healthy cats, and especially with kittens, is the primary source of infection. Adults are affected nearly as frequently as children. In the U.S., the estimated incidence is ~10 cases per 100,000 population.
Of pts with CSD, 85–90% have typical disease consisting of a localized lesion (papule, vesicle, or nodule) at the site of inoculation with subsequent painful regional lymphadenopathy ≥1–3 weeks after cat contact.
• Axillary and epitrochlear nodes are most commonly involved and suppurate in 10–15% of cases.
• Low-grade fever, malaise, and anorexia develop in ~50% of pts.
• Atypical disease involves extranodal manifestations (e.g., fever of unknown origin, ophthalmologic manifestations, neurologic involvement, osteomyelitis).
• In immunocompetent pts, the disease resolves spontaneously without treatment, although its resolution takes weeks or months.
Serologic testing is most commonly used but is variably sensitive and specific. It is noteworthy that seroconversion may take a few weeks. Bartonella species are difficult to culture, but PCR analysis of lymph node tissue, pus, or the primary inoculation lesion is highly sensitive and specific.
Bacillary angiomatosis is caused by B. henselae and B. quintana, while peliosis is caused only by the former species. These diseases occur most often in HIV-infected pts with CD4+ T cell counts of <100/μL.
• Pts with bacillary angiomatosis present with one or more painless skin lesions that may be tan, red, or purple in color. Subcutaneous masses or nodules, ulcerated plaques, and verrucous growths also occur. B. quintana is also associated with lytic bone lesions, primarily in the long bones.
• Peliosis is an angioproliferative disorder characterized by blood-filled cystic structures that affects primarily the liver but also the spleen and lymph nodes. Hypodense hepatic areas are usually evident on imaging.
• Both diseases are diagnosed on histologic grounds. Blood cultures may be positive.
• Trench fever (5-day fever) is caused by B. quintana, which is spread by the human body louse among its only animal reservoir: humans.
• Much less common today than in the trenches of World War I, the disease now primarily affects homeless people.
• After a usual incubation period of 15–25 days, disease classically ranges from a mild febrile illness to a recurrent or protracted and debilitating disease. Fever is often periodic, with episodes of 4–5 days separated by ~5-day afebrile periods.
• Diagnosis requires identification of B. quintana in blood cultures.
• Untreated, the disease usually lasts 4–6 weeks. Death is rare.
Bartonella species (typically B quintana or B. henselae) are an important cause of culture-negative endocarditis. The disease’s manifestations are similar to those of subacute endocarditis of any etiology (Chap. 89). Even if incubated for prolonged periods (up to 6 weeks), blood cultures are positive in only ~25% of cases. Serologic or PCR testing for Bartonella in cardiac valve tissue can help establish the diagnosis in pts with negative blood cultures.
Bartonellosis is a biphasic disease caused by B. bacilliformis, which is transmitted by a sandfly vector found in the Andes valleys of Peru, Ecuador, and Colombia.
• Oroya fever is the initial, bacteremic, systemic form, and verruga peruana is its late-onset, eruptive manifestation.
• Oroya fever may present as a nonspecific bacteremic febrile illness without anemia or as acute, severe hemolytic anemia with hepatomegaly and jaundice of rapid onset.
– In verruga peruana, red, hemangioma-like, cutaneous vascular lesions of various sizes appear either weeks to months after systemic illness or with no previous suggestive history. The lesions persist for months up to 1 year.
• In systemic illness, Giemsa-stained blood films show typical intraerythrocytic bacilli, and blood and bone marrow cultures are positive. Serologic assays may be helpful. Biopsy may be required to confirm the diagnosis of verruga peruana.

For a more detailed discussion, see Corbel MJ, Beeching NJ: Brucellosis, Chap. 157, p. 1296; Jacobs RF, Schutze GE: Tularemia, Chap. 158, p. 1301; Prentice MB: Plague and Other Yersinia Infections, Chap. 159, p. 1305; and Giladi M, Ephros M: Bartonella Infections, Including Cat-Scratch Disease, Chap. 160, p. 1314, in HPIM-18.