CHAPTER 123
Valvular Heart Disease
MITRAL STENOSIS (MS)
Etiology
Most commonly rheumatic, although history of acute rheumatic fever is now uncommon; congenital MS is rare cause, observed primarily in infants.
History
Symptoms most commonly begin in the fourth decade, but MS often causes severe disability at earlier ages in developing nations. Principal symptoms are dyspnea and pulmonary edema precipitated by exertion, excitement, fever, anemia, paroxysmal tachycardia, pregnancy, sexual intercourse, etc.
Physical Examination
Right ventricular lift; palpable S1; opening snap (OS) follows A2 by 0.06–0.12 s; OS–A2 interval inversely proportional to severity of obstruction. Diastolic rumbling murmur with presystolic accentuation in sinus rhythm. Duration of murmur correlates with severity of obstruction.
Complications
Hemoptysis, pulmonary embolism, pulmonary infection, systemic embolization; endocarditis is uncommon in pure MS.
Laboratory ECG
Typically shows atrial fibrillation (AF) or left atrial (LA) enlargement when sinus rhythm is present. Right-axis deviation and RV hypertrophy in the presence of pulmonary hypertension.
CXR
Shows LA and RV enlargement and Kerley B lines.
Echocardiogram
Most useful noninvasive test; shows inadequate separation, calcification and thickening of valve leaflets and subvalvular apparatus, and LA enlargement. Doppler flow recordings provide estimation of transvalvular gradient, mitral valve area, and degree of pulmonary hypertension (Chap. 121).
TREATMENT Mitral Stenosis (See Fig. 123-1)

FIGURE 123-1 Management of mitral stenosis (MS). †There is controversy as to whether pts with severe MS (MVA <1.0 cm2) and severe pulmonary hypertension (PH) (PASP >60 mmHg) should undergo percutaneous mitral balloon valvotomy (PMBV) or mitral valve replacement (MVR) to prevent right ventricular failure. CXR, chest x-ray; ECG, electrocardiogram; echo, echocardiography; LA, left atrial; MR, mitral regurgitation; MVA, mitral valve area; MVG, mean mitral valve pressure gradient; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; PAWP, pulmonary artery wedge pressure; 2D, 2-dimensional. (From RO Bonow et al: J Am Coll Cardiol 48:e1, 2006; with permission.)
At-risk pts should receive prophylaxis for recurrent rheumatic fever (penicillin V 250–500 mg PO bid or benzathine penicillin G 1–2 M units IM monthly). In the presence of dyspnea, sodium restriction and oral diuretic therapy; beta blockers, digitalis, or rate-limiting calcium channel antagonists (i.e., verapamil or diltiazem) to slow ventricular rate in AF. Warfarin (with target INR 2.0–3.0) for pts with AF and/or history of systemic and pulmonic emboli. For AF of recent onset, consider reversion (chemical or electrical) to sinus rhythm, ideally after >3 weeks of anticoagulation. Mitral valvotomy in the presence of symptoms and mitral orifice < ~1.5 cm2. In uncomplicated MS, percutaneous balloon valvuloplasty is the procedure of choice; if not feasible, then open surgical valvotomy (Fig. 123-1).
MITRAL REGURGITATION (MR)
Etiology
Mitral valve prolapse (see below), rheumatic heart disease, ischemic heart disease with papillary muscle dysfunction, LV dilatation of any cause, mitral annular calcification, hypertrophic cardiomyopathy, infective endocarditis, congenital.
Clinical Manifestations
Fatigue, weakness, and exertional dyspnea. Physical examination: sharp upstroke of arterial pulse, LV lift, S1 diminished: wide splitting of S2; S3; loud holosystolic murmur and often a brief early-mid-diastolic murmur due to increased transvalvular flow.
Echocardiogram
Enlarged LA, hyperdynamic LV, identifies mechanism of MR; Doppler analysis helpful in diagnosis and assessment of severity of MR and degree of pulmonary hypertension.
TREATMENT Mitral Regurgitation (See Fig. 123-2)
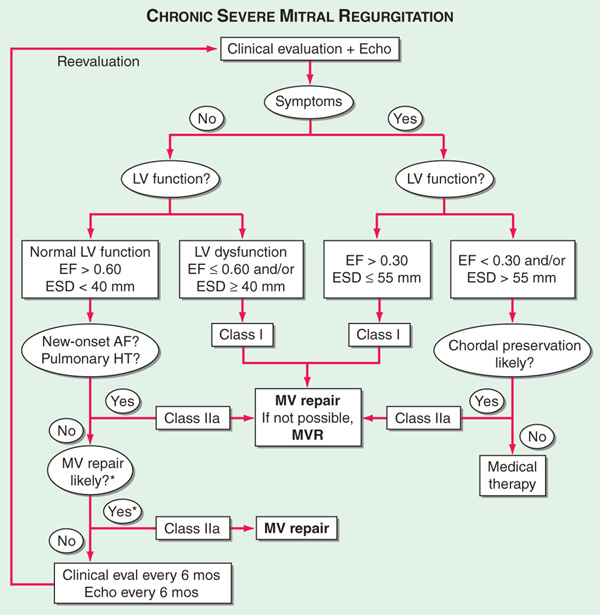
FIGURE 123-2 Management of advanced mitral regurgitation. *Mitral valve (MV) repair may be performed in asymptomatic pts with normal left ventricular (LV) function if performed by an experienced surgical team and if the likelihood of successful MV repair is >90%. AF, atrial fibrillation; Echo, echocardiography; EF, ejection fraction; ESD, end-systolic dimension; eval, evaluation; HT, hypertension; MVR, mitral valve replacement. (From RO Bonow et al: J Am Coll Cardiol 48:e1, 2006; with permission.)
For severe/decompensated MR, treat as for heart failure (Chap. 133). IV vasodilators (e.g., nitroprusside) are beneficial for acute, severe MR. Anticoagulation is indicated in the presence of atrial fibrillation. Surgical treatment, either valve repair or replacement, is appropriate if pt has symptoms or evidence of progressive LV dysfunction [LV ejection fraction (LVEF) <60% or end-systolic LV diameter by echo >40 mm]. Operation should be carried out before development of chronic heart failure symptoms.
MITRAL VALVE PROLAPSE (MVP)
Etiology
Most commonly idiopathic; may accompany rheumatic fever, ischemic heart disease, atrial septal defect, Marfan syndrome, Ehlers-Danlos syndrome.
Pathology
Redundant mitral valve tissue with myxedematous degeneration and elongated chordae tendineae.
Clinical Manifestations
More common in females. Most pts are asymptomatic and remain so. Most common symptoms are vague chest pain and supraventricular and ventricular arrhythmias. Most important complication is severe MR resulting in LV failure. Rarely, systemic emboli from platelet-fibrin deposits on valve. Sudden death is a very rare complication.
Physical Examination
Mid or late systolic click(s) followed by late systolic murmur at the apex; exaggeration by Valsalva maneuver, reduced by squatting and isometric exercise (Chap. 119).
Echocardiogram
Shows posterior displacement of one or both mitral leaflets late in systole.
TREATMENT Mitral Valve Prolapse
Asymptomatic pts should be reassured. Beta blockers may lessen chest discomfort and palpitations. Prophylaxis for infective endocarditis is indicated only if prior history of endocarditis. Valve repair or replacement for pts with severe mitral regurgitation; aspirin or anticoagulants for pts with history of TIA or embolization.
AORTIC STENOSIS (AS)
Etiologies
Most common are (1) degenerative calcification of a congenitally bicuspid valve, (2) chronic deterioration of a trileaflet valve, and (3) rheumatic disease (almost always associated with rheumatic mitral disease).
Symptoms
Exertional dyspnea, angina, and syncope are cardinal symptoms; they occur late, after years of obstruction and aortic valve area ≤1.0 cm2.
Physical Examination
Weak and delayed (parvus et tardus) arterial pulses with carotid thrill. Double apical impulse (palpable S4); A2 soft or absent; S4 common. Diamond-shaped systolic murmur ≥ grade 3/6, often with systolic thrill. Murmur is typically loudest at second right intercostal space, with radiation to carotids and sometimes to the apex (Gallavardin effect).
ECG
Often shows LV hypertrophy, but not useful for predicting gradient.
Echocardiogram
Shows LV hypertrophy, calcification and thickening of aortic valve cusps with reduced systolic opening. Dilatation and reduced contraction of LV indicate poor prognosis. Doppler quantitates systolic gradient and allows calculation of valve area.
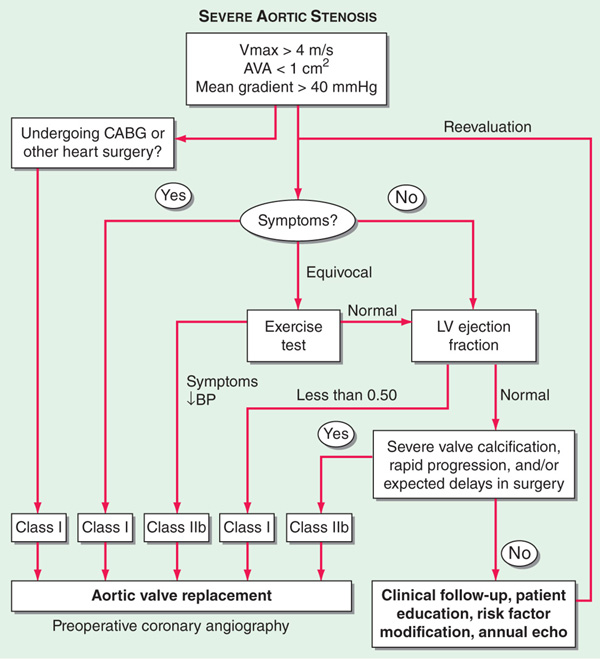
FIGURE 123-3 Algorithm for the management of aortic stenosis (AS). AVA, aortic valve area; BP, blood pressure; CABG, coronary artery bypass graft surgery; LV, left ventricle; Vmax, maximal velocity across aortic valve by Doppler echocardiography. (Modified from CM Otto: J Am Coll Cardiol 47:2141, 2006.)
Avoid strenuous activity in severe AS, even in asymptomatic phase. Treat heart failure in standard fashion (Chap. 133), but use vasodilators with caution in pts with advanced disease. Valve replacement is indicated in adults with symptoms resulting from AS and hemodynamic evidence of severe obstruction. Transcatheter aortic valve implantation (TAVI) is an investigational approach for pts at excessive surgical risk that has demonstrated favorable results.
AORTIC REGURGITATION (AR)
Etiology
Valvular: Rheumatic (especially if rheumatic mitral disease is present), bicuspid valve, endocarditis. Dilated aortic root: dilatation due to cystic medial necrosis, aortic dissection, ankylosing spondylitis, syphilis. Three-fourths of pts are male.
Clinical Manifestations
Exertional dyspnea and awareness of forceful heartbeat, angina pectoris, and signs of LV failure. Wide pulse pressure, water hammer pulse, capillary pulsations (Quincke’s sign), A2 soft or absent, S3 common. Blowing, decrescendo diastolic murmur along left sternal border (along right sternal border with aortic dilatation). May be accompanied by systolic murmur of augmented blood flow.
Laboratory ECG and CXR
LV enlargement.
Echocardiogram
LA enlargement, LV enlargement, high-frequency diastolic fluttering of mitral valve. Failure of coaptation of aortic valve leaflets may be present. Doppler studies useful in detection and quantification of AR.
TREATMENT Aortic Regurgitation
Standard therapy for LV failure (Chap. 133). Vasodilators (long-acting nifedipine or ACE inhibitors) are recommended if hypertension present. Surgical valve replacement should be carried out in pts with severe AR when symptoms develop or in asymptomatic pts with LV dysfunction (LVEF <50%, LV end-systolic volume >55 mL/m2, end-systolic diameter >55 mm, or LV diastolic dimension >75 mm) by imaging studies.
TRICUSPID STENOSIS (TS)
Etiology
Usually rheumatic; most common in females; almost invariably associated with MS.
Clinical Manifestations
Hepatomegaly, ascites, edema, jaundice, jugular venous distention with slow y descent (Chap. 119). Diastolic rumbling murmur along left sternal border increased by inspiration with loud presystolic component. Right atrial and superior vena caval enlargement on chest x-ray. Doppler echocardiography demonstrates thickened valve and impaired separation of leaflets and provides estimate of transvalvular gradient.
TREATMENT Tricuspid Stenosis
In severe TS, surgical relief is indicated, with valvular repair or replacement.
TRICUSPID REGURGITATION (TR)
Etiology
Usually functional and secondary to marked RV dilatation of any cause and often associated with pulmonary hypertension.
Clinical Manifestations
Severe RV failure, with edema, hepatomegaly, and prominent v waves in jugular venous pulse with rapid y descent (Chap. 119). Systolic murmur along lower left sternal edge is increased by inspiration. Doppler echocardiography confirms diagnosis and estimates severity.
TREATMENT Tricuspid Regurgitation
Intensive diuretic therapy when right-sided heart failure signs are present. In severe cases (in absence of severe pulmonary hypertension), surgical treatment consists of tricuspid annuloplasty or valve replacement.

For a more detailed discussion, see O’Gara P, Loscalzo J: Valvular Heart Disease, Chap. 237, p. 1929, in HPIM-18.



