Disorders of the thyroid gland result primarily from autoimmune processes that stimulate the overproduction of thyroid hormones (thyrotoxicosis) or cause glandular destruction and underproduction of thyroid hormones (hypothyroidism). Neoplastic processes in the thyroid gland can lead to benign nodules or thyroid cancer.
Thyroidal production of the hormones thyroxine (T4) and triiodothyro-nine (T3) is controlled via a classic endocrine feedback loop (see Fig. 179-1). Some T3 is secreted by the thyroid, but most is produced by deiodination of T4 in peripheral tissues. Both T4 and T3 are bound to carrier proteins [thyroid-binding globulin (TBG), transthyretin (binds T4 only), and albumin] in the circulation. Increased levels of total T4 and T3 with normal free levels are seen in states of increased carrier proteins (pregnancy, estrogens, cirrhosis, hepatitis, and inherited disorders). Conversely, decreased total T4 and T3 levels with normal free levels are seen in severe systemic illness, chronic liver disease, and nephrosis.
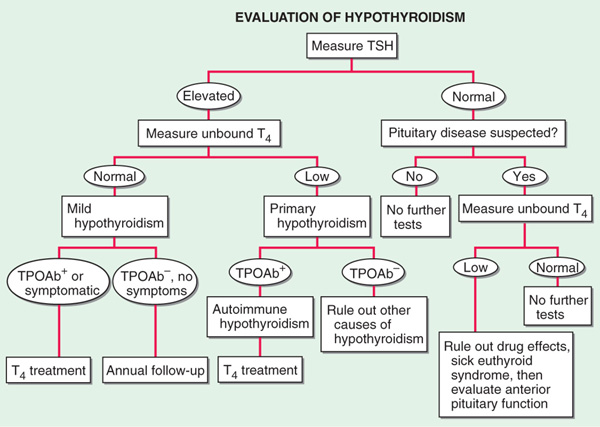
FIGURE 181-1 Evaluation of hypothyroidism. TPOAb+, thyroid peroxidase antibodies present; TPOAb–, thyroid peroxidase antibodies not present; TSH, thyroid-stimulating hormone.
Deficient thyroid hormone secretion can be due to thyroid failure (primary hypothyroidism) or, less commonly, pituitary or hypothalamic disease (secondary hypothyroidism) (Table 181-1). Transient hypothyroidism may occur in silent or subacute thyroiditis. Subclinical (or mild) hypothyroidism is a state of normal free thyroid hormone levels and mild elevation of TSH; despite the name, some pts may have minor symptoms. With higher TSH levels and low free T4 levels, symptoms become more readily apparent in clinical (or overt) hypothyroidism. In areas of iodine sufficiency, autoimmune disease and iatrogenic causes are the most common causes of hypothyroidism. The peak age of occurrence is around 60 years, and prevalence increases with age. Congenital hypothyroidism is present in 1 of 4000 newborns; the importance of its recognition and prompt treatment for child development has led to the adoption of neonatal screening programs.
TABLE 181-1 CAUSES OF HYPOTHYROIDISM


Symptoms of hypothyroidism include lethargy, dry hair and skin, cold intolerance, hair loss, difficulty concentrating, poor memory, constipation, mild weight gain with poor appetite, dyspnea, hoarse voice, muscle cramping, and menorrhagia. Cardinal features on examination include bradycardia, mild diastolic hypertension, prolongation of the relaxation phase of deep tendon reflexes, and cool peripheral extremities. Goiter may be palpated, or the thyroid may be atrophic and nonpalpable. Carpal tunnel syndrome may be present. Cardiomegaly may be present due to pericardial effusion. The most extreme presentation is a dull, expressionless face, sparse hair, periorbital puffiness, large tongue, and pale, doughy, cool skin. The condition may progress into a hypothermic, stuporous state (myxedema coma) with respiratory depression. Factors that predispose to myxedema coma include cold exposure, trauma, infection, and administration of narcotics. In mild hypothyroidism, the classic findings of overt hypothyroidism may not be present, and the clinical picture may be dominated by fatigue and ill-defined symptoms.
Decreased serum free T4 is common to all varieties of hypothyroidism. An elevated serum TSH is a sensitive marker of primary hypothyroidism but is not found in secondary hypothyroidism. A summary of the investigations used to determine the existence and cause of hypothyroidism is provided in Fig. 181-1. Thyroid peroxidase (TPO) antibodies are increased in >90% of pts with autoimmune-mediated hypothyroidism. Elevated cholesterol, increased creatine phosphokinase, and anemia may be present; bradycardia, low-amplitude QRS complexes, and flattened or inverted T waves may be present on ECG.
FIGURE 181-2 Evaluation of thyrotoxicosis. aDiffuse goiter, positive TPO antibodies, ophthalmopathy, dermopathy; bcan be confirmed by radionuclide scan. TSH, thyroid-stimulating hormone.
TREATMENT Hypothyroidism
Adult pts <60 years without evidence of heart disease may be started on 50–100 μg of levothyroxine (T4) daily. In the elderly or in pts with known coronary artery disease, the starting dose of levothyroxine is 12.5–25 μg/d. The dose should be adjusted in 12.5- to 25-μg increments every 6–8 weeks on the basis of TSH levels, until a normal TSH level is achieved. The average daily replacement dose is 1.6 μg/kg/d, but dosing should be individualized and guided by TSH measurement. In secondary hypothyroidism, TSH levels cannot be used, and therapy needs to be guided by free T4 measurement. Women on levothyroxine replacement should have a TSH level checked as soon as pregnancy is diagnosed, as the replacement dose typically increases by 30–50% during pregnancy. Failure to recognize and treat maternal hypothyroidism may adversely affect fetal neural development. Therapy for myxedema coma should include levothyroxine (500 μg) as a single IV bolus followed by daily treatment with levothyroxine (50–100 μg/d), along with hydrocortisone (50 mg every 6 h) for impaired adrenal reserve, ventilatory support, space blankets, and treatment of precipitating factors.
Causes of thyroid hormone excess include primary hyperthyroidism (Graves’ disease, toxic multinodular goiter, toxic adenoma, iodine excess); thyroid destruction (subacute thyroiditis, silent thyroiditis, amiodarone, radiation); extrathyroidal sources of thyroid hormone (thyrotoxicosis factitia, struma ovarii, functioning follicular carcinoma); and secondary hyper-thyroidism [TSH-secreting pituitary adenoma, thyroid hormone resistance syndrome, human chorionic gonadotropin (hCG)-secreting tumors, gestational thyrotoxicosis]. Graves’ disease, caused by activating TSH-receptor antibodies, is the most common cause of thyrotoxicosis and accounts for 60–80% of cases. Its prevalence in women is 10-fold higher than in men; its peak occurrence is at age 20–50 years.
Symptoms include nervousness, irritability, heat intolerance, excessive sweating, palpitations, fatigue and weakness, weight loss with increased appetite, frequent bowel movements, and oligomenorrhea. Pts are anxious, restless, and fidgety. Skin is warm and moist, and fingernails may separate from the nail bed (Plummer’s nails). Eyelid retraction and lid lag may be present. Cardiovascular findings include tachycardia, systolic hypertension, systolic murmur, and atrial fibrillation. A fine tremor, hyperreflexia, and proximal muscle weakness also may be present. Long-standing thyrotoxicosis may lead to osteopenia. In the elderly, the classic signs of thyrotoxicosis may not be apparent, the main manifestations being weight loss and fatigue (“apathetic thyrotoxicosis”).
In Graves’ disease, the thyroid is usually diffusely enlarged to two to three times its normal size, and a bruit or thrill may be present. Infiltrative ophthalmopathy (with variable degrees of proptosis, periorbital swelling, and ophthalmoplegia) and dermopathy (pretibial myxedema) also may be found; these are extrathyroidal manifestations of the autoimmune process. In subacute thyroiditis, the thyroid is exquisitely tender and enlarged with referred pain to the jaw or ear, and sometimes accompanied by fever and preceded by an upper respiratory tract infection. Solitary or multiple nodules may be present in toxic adenoma or toxic multinodular goiter.
Thyrotoxic crisis, or thyroid storm, is rare, presents as a life-threatening exacerbation of hyperthyroidism, and can be accompanied by fever, delirium, seizures, arrhythmias, coma, vomiting, diarrhea, and jaundice.
Investigations used to determine the existence and causes of thyrotoxicosis are summarized in Fig. 181-2. Serum TSH is a sensitive marker of thyrotoxicosis caused by Graves’ disease, autonomous thyroid nodules, thyroiditis, and exogenous levothyroxine treatment. Associated laboratory abnormalities include elevation of bilirubin, liver enzymes, and ferritin. Thyroid radioiodine uptake may be required to distinguish the various etiologies: high uptake in Graves’ disease and nodular disease vs. low uptake in thyroid destruction, iodine excess, and extrathyroidal sources of thyroid hormone. (Note: Radioiodine is the nuclide required for quantitative thyroid uptake, whereas technetium is sufficient for imaging purposes.) The ESR is elevated in subacute thyroiditis.
TREATMENT Thyrotoxicosis
Graves’ disease may be treated with antithyroid drugs or radioiodine; subtotal thyroidectomy is rarely indicated. The main antithyroid drugs are methimazole or carbimazole (10–20 mg two to three times a day initially, titrated to 2.5–10 mg/d) and propylthiouracil (100–200 mg every 8 h initially, titrated to 50 mg once or twice a day). Methimazole is preferred in most pts because of easier dosing. Thyroid function tests should be checked 3–4 weeks after initiation of treatment, with adjustments to maintain a normal free T4 level. Since TSH recovery from suppression is delayed, serum TSH levels should not be used for dose adjustment in the first few months. The common side effects are rash, urticaria, fever, and arthralgia (1–5% of pts). Uncommon but major side effects include hepatitis, an SLE-like syndrome, and, rarely, agranulocytosis (<1%). All pts should be given written instructions regarding the symptoms of possible agranulocytosis (sore throat, fever, mouth ulcers) and the need to stop treatment pending a complete blood count to confirm that agranulocytosis is not present. Propranolol (20–40 mg every 6 h) or longer-acting beta blockers such as atenolol (50 mg/d) may be useful at the start of treatment to control adrenergic symptoms until euthyroidism is reached. Anticoagulation with warfarin should be considered in all pts with atrial fibrillation. Radioiodine can also be used as initial treatment or in pts who do not undergo remission after a 1- to 2-year trial of antithyroid drugs. Antecedent treatment with antithyroid drugs should be considered in elderly pts and those with cardiac problems, with cessation of antithyroid drugs 3–5 days prior to radioiodine administration. Radioiodine treatment is contraindicated in pregnancy; instead, symptoms should be controlled with the lowest effective dose of propylthiouracil (PTU). (Methimazole is not recommended in pregnancy because of reports of fetal agenesis cutis.) Corneal drying may be relieved with artificial tears and taping the eyelids shut during sleep. Progressive exophthalmos with chemosis, ophthalmoplegia, or vision loss is treated with large doses of prednisone (40–80 mg/d) and ophthalmologic referral; orbital decompression may be required.
In thyroid storm, large doses of PTU (600-mg loading dose) should be administered orally, per nasogastric tube, or per rectum, followed 1 h later by 5 drops saturated solution of KI (SSKI) q6h. PTU (200–300 mg every 6 h) should be continued, along with propranolol (40–60 mg PO q4h or 2 mg IV every 4 h) and dexamethasone (2 mg every 6 h). Any underlying precipitating cause should be identified and treated.
Radioiodine is the treatment of choice for toxic nodules. Subacute thyroiditis in its thyrotoxic phase should be treated with NSAIDs and beta blockade to control symptoms, with monitoring of the TSH and free T4 levels every 4 weeks. Antithyroid drugs are not effective in thyroiditis. The clinical course of subacute thyroiditis is summarized in Fig. 181-3. Transient levothyroxine replacement (50–100 μg/d) may be required if the hypothyroid phase is prolonged. Silent thyroiditis (or postpartum thyroiditis if within 3–6 months of delivery) should be treated with beta blockade during the thyrotoxic phase and levothyroxine in the hypothyroid phase, with withdrawal after 6–9 months to assess recovery.
FIGURE 181-3 Clinical course of subacute thyroiditis. The release of thyroid hormones is initially associated with a thyrotoxic phase and suppressed TSH levels. A hypothyroid phase then ensues, with T4 and TSH levels that are initially low but gradually increase. During the recovery phase, increased TSH levels combined with resolution of thyroid follicular injury leads to normalization of thyroid function, often several months after the beginning of the illness. ESR, erythrocyte sedimentation rate; UT4, unbound T4.
Any acute, severe illness can cause abnormalities of circulating thyroid hormone levels or TSH, even in the absence of underlying thyroid disease. Therefore, the routine testing of thyroid function should be avoided in acutely ill pts unless a thyroid disorder is strongly suspected. The most common pattern in sick euthyroid syndrome is a decrease in total and free T3 levels, with normal levels of TSH and T4. This is considered an adaptive response to a catabolic state. More ill pts may additionally have a fall in total T4 levels, with normal free T4 levels. TSH levels may range from <0.1 to >20 mU/L, with normalization after recovery from illness. The patho-genesis of this condition is not fully understood but may involve altered binding of T4 to TBG and effects of high glucocorticoid and cytokine levels. Unless there is historic or unequivocal clinical evidence of hypothyroidism, thyroid hormone should not be administered and thyroid function tests should be repeated after recovery.
Amiodarone is a type III antiarrhythmic agent that has some structural similarity to thyroid hormone and has a high iodine content. Amiodarone treatment leads to substantial iodine overload and is associated with (1) acute, transient suppression of thyroid function, (2) hypothyroidism, or (3) thyrotoxicosis. These effects are only partially attributable to iodine overload. Hypothyroidism can occur in pts with preexisting thyroid disease, with an inability to escape from the suppressive effect of excess iodine. Pts with hypothyroidism can be easily managed with levothyroxine replacement therapy, without a need to stop amiodarone. There are two major forms of amiodarone-induced thyrotoxicosis (AIT). Type 1 AIT is associated with an underlying thyroid abnormality (preclinical Graves’ disease or nodular goiter). Thyroid hormone synthesis becomes excessive as a result of increased iodine exposure. Type 2 AIT occurs in pts with no intrinsic thyroid abnormalities and is the result of destructive thyroiditis. Differentiation between type 1 and type 2 AIT may be difficult as the high iodine load interferes with thyroid scans. The drug should be stopped, if possible, although this is often difficult to achieve without compromising the arrhythmia management. Amiodarone has a long biologic half-life, and its effects persist for weeks following discontinuation. Therapy of type 1 AIT consists of high-dose antithyroid drugs, but efficacy may be limited. In type 2 AIT, sodium ipodate (500 mg/d) or sodium tyropanoate (500 mg, 1–2 doses/d) can be used to rapidly lower thyroid hormone levels. Potassium perchlorate (200 mg every 6 h) can be used to deplete the thyroid of iodine, but long-term use carries a risk of agranulocytosis. Glucocorticoids in high doses are partially effective. Lithium can be used to block thyroid hormone release. In some cases, subacute thyroidectomy may be necessary to control thyrotoxicosis.
Goiter refers to an enlarged thyroid gland (>20–25 g), which can be diffuse or nodular. Goiter is more common in women than men. Biosynthetic defects, iodine deficiency, autoimmune disease, dietary goitrogens (cabbage, cassava root), and nodular diseases can lead to goiter. Worldwide, iodine deficiency is the most common etiology of goiter. Nontoxic multinodular goiter is common in both iodine-deficient and iodine-replete populations, with a prevalence of up to 12%. The etiology, other than iodine deficiency, is usually not known and may be multifactorial. If thyroid function is preserved, most goiters are asymptomatic. Substernal goiter may obstruct the thoracic inlet and should be evaluated with respiratory flow measurements and CT or MRI in pts with obstructive signs or symptoms (difficulty swallowing, tracheal compression, or plethora). Thyroid function tests should be performed in all pts with goiter to exclude thyrotoxicosis or hypothyroidism. Ultrasound is not generally indicated in the evaluation of diffuse goiter, unless a nodule is palpable on physical exam.
Iodine or thyroid hormone replacement induces variable regression of goiter in iodine deficiency. Thyroid hormone replacement is rarely effective in significantly shrinking a nontoxic goiter that is not due to iodine deficiency or a biosynthetic defect. Radioiodine reduces goiter size by about 50% in the majority of pts. Surgery is rarely indicated for diffuse goiter but may be required to alleviate compression in pts with nontoxic multinodular goiter.
In addition to features of goiter, the clinical presentation of toxic MNG includes subclinical hyperthyroidism or mild thyrotoxicosis. The pt is usually elderly and may present with atrial fibrillation or palpitations, tachycardia, nervousness, tremor, or weight loss. Recent exposure to iodine, from contrast dyes or other sources, may precipitate or exacerbate thyrotoxicosis; this may be prevented by prior administration of an antithyroid drug. The TSH level is low. T4 may be normal or minimally increased; T3 is often elevated to a greater degree than T4. Thyroid scan shows heterogeneous uptake with multiple regions of increased and decreased uptake; 24-h uptake of radioiodine may not be increased. Cold nodules in a multinodular goiter should be evaluated in the same way as solitary nodules (see below). Antithyroid drugs, often in combination with beta blockers, can normalize thyroid function and improve clinical features of thyrotoxicosis but do not induce remission. A trial of radioiodine should be considered before subjecting pts, many of whom are elderly, to surgery. Subtotal thyroidectomy provides definitive treatment of goiter and thyrotoxicosis. Pts should be rendered euthyroid with antithyroid drugs before surgical intervention.
A solitary, autonomously functioning thyroid nodule is referred to as toxic adenoma. Most cases are cause by somatic activating mutations of the TSH receptor. Thyrotoxicosis is typically mild. A thyroid scan provides a definitive diagnostic test, demonstrating focal uptake in the hyperfunctioning nodule and diminished uptake in the remainder of the gland, as activity of the normal thyroid is suppressed. Radioiodine ablation with relatively large doses (e.g., 10–29.9 mCi 131I) is usually the treatment of choice.
Thyroid neoplasms may be benign (adenomas) or malignant (carcinomas). Carcinomas of the follicular epithelium include papillary, follicular, and anaplastic thyroid cancer. Thyroid cancer incidence is ~9/100,000 per year. Papillary thyroid cancer is the most common type of thyroid cancer (70–90%). It tends to be multifocal and to invade locally. Follicular thyroid cancer is difficult to diagnose via fine-needle aspiration (FNA) because the distinction between benign and malignant follicular neoplasms rests largely on evidence of invasion into vessels, nerves, or adjacent structures. It tends to spread hematogenously, leading to bone, lung, and CNS metastases. Anaplastic carcinoma is rare, highly malignant, and rapidly fatal. Thyroid lymphoma often arises in the background of Hashimoto’s thyroiditis and occurs in the setting of a rapidly expanding thyroid mass. Medullary thyroid carcinoma arises from parafollicular (C) cells producing calcitonin and may occur sporadically or as a familial disorder, sometimes in association with multiple endocrine neoplasia type 2.
Features suggesting carcinoma include recent or rapid growth of a nodule or mass, history of neck irradiation, lymph node involvement, hoarseness, and fixation to surrounding tissues. Glandular enlargement may result in compression and displacement of the trachea or esophagus and obstructive symptoms. Age <20 or >45, male sex, and larger nodule size are associated with a worse prognosis.
An approach to the evaluation of a solitary nodule is outlined in Fig. 181-4.
FIGURE 181-4 Approach to the pt with a thyroid nodule. *About one-third of nodules are cystic or mixed solid-cystic. FNA, fine-needle aspiration; TSH, thyroid-stimulating hormone; US, ultrasound.
Benign nodules should be monitored via serial examination. TSH suppression with levothyroxine results in decreased nodule size in about 30% of pts. Suppressive therapy should not exceed 6–12 months if unsuccessful.
Follicular adenomas cannot be distinguished from follicular carcinomas on the basis of cytologic analysis of FNA specimens. The extent of surgical resection (lobectomy vs. near-total thyroidectomy) should be discussed prior to surgery.
Near-total thyroidectomy is required for papillary and follicular carcinoma and should be performed by a surgeon who is highly experienced in the procedure. If risk factors and pathologic features indicate the need for radioiodine treatment, the pt should be treated for several weeks postoperatively with liothyronine (T3, 25 μg two to three times a day), followed by withdrawal for an additional 2 weeks, in preparation for postsurgical radioablation of remnant tissue. A therapeutic dose of 131I is administered when the TSH level is >50 IU/L. Alternatively, recombinant TSH can be used to raise the preablation TSH level. This appears to be equally effective as thyroid hormone withdrawal for radioablation therapy. Subsequent levothyroxine suppression of TSH to a low, but detectable, level (0.1–0.5 IU/L) should be attempted in pts with a low risk of recurrence, and to a completely suppressed level in those with a high risk of recurrence. In the latter case, free T4 should be monitored to avoid overtreatment. Follow-up scans and serum thyroglobulin levels (acting as a tumor marker in an athyreotic pt) should be performed at regular intervals after either thyroid hormone withdrawal or administration of recombinant human TSH.
The management of medullary thyroid carcinoma is surgical, as these tumors do not take up radioiodine. Testing for RET mutations should be performed to assess for the presence of MEN-2, and the family should be screened if testing is positive. Following surgery, serum calcitonin provides a marker of residual or recurrent disease.

For a more detailed discussion, see Jameson JL, Weetman AP: Disorders of the Thyroid Gland, Chap. 341, p. 2911, in HPIM-18.