Chapter 14
Cellular Determination
Introduction
There are a huge variety of cell types in the nervous system with completely different shapes, patterns of connectivity, neurochemistry, and physiological characteristics. The distribution of different types of neuron follows a highly invariant pattern in a given species. Neurons located at a given position within the nervous system are always the same type. In vertebrates, this is true on the level of populations of neurons (e.g., cells of layer III of the cortical area 17 are glutaminergic pyramidal cells projecting to cortical areas 18 and 19); in many invertebrates, it is true even on the level of individual, uniquely identifiable cells. Experimental studies carried out over the last few decades have provided substantial progress in understanding how the right types of neuron develop in the right place in the brain.
Neurons are specified gradually by a series of fate choices that begin with their distant progenitors who make the first decision between neural and ectodermal fates. Neural progenitors, those dividing cells that are determined to give rise to neurons and glia, arise from a specialized region of the ectoderm called the neurectoderm, known in vertebrates as the neural plate (Fig. 14.1). A highly conserved molecular network, discussed in Chapter 13, specifies the size and position of this region. The neural plate rolls or folds into the neural tube and detaches from the overlying epidermis. The neural tube consists of a continuous pseudostratified neuroepithelium that lines, on its apical surface, an inner lumen, called the ventricle (Fig. 14.1C, D). Neuroepithelial cells of the neural tube begin dividing symmetrically at the apical surface or ventricular zone, producing two more cells that divide (Fig. 14.1C′). After a period of time, during which this doubling process leads to an exponential expansion of cell numbers, these neural progenitors may begin to divide asymmetrically or spin off postmitotic daughters that differentiate and other daughters that divide again. Finally, at the end of neurogenesis, cell divisions lead only to differentiating daughters. The number of cells in any part of the nervous system is thus largely governed by the proliferative potential and division patterns of the neural progenitors.
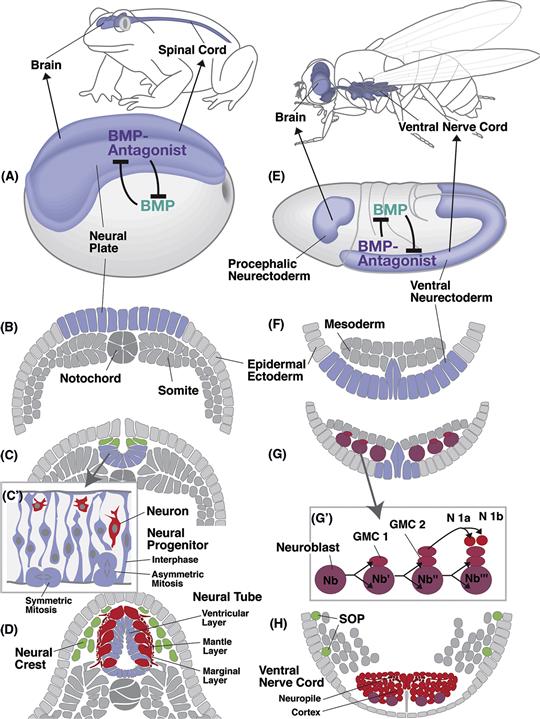
Figure 14.1 Synopsis of early neural development in vertebrates (example: the amphibian Xenopus laevis; A–D) and insects (example: the fruitfly Drosophila melanogaster; E–H). (A and E) Dorsolateral views of embryos at the onset of neurulation. The neurectoderm is shaded light blue. The expression of the signal Bone Morphogenetic Protein (BMP) and its antagonists is indicated. (B and F) Schematic cross sections of embryos depicted in (A and E). (C and G) Cross sections at a later stage when neurulation is well under way. In vertebrates, the neural plate folds in to become the neural tube. Cells at the junction between the neural tube and the epidermal ectoderm (green) form the neural crest, which gives rise to the peripheral and autonomic nervous systems. The neural tube is a proliferating neuroepithelium (C’); symmetric mitoses increase the number of epithelial neural progenitor cells, asymmetric mitoses result in daughter cells that delaminate from the epithelium and become neurons (red). In insects, individual neural progenitors (neuroblasts; purple) delaminate from the neurectoderm. They divide in a stem cell mode (G’), producing stacks of daughter cells called ganglion mother cells (GMC). Each ganglion mother cell divides into two neurons (e.g., N1a and N1b). (D and H) Cross sections of late embryos in which some neurons (red) have differentiated. In vertebrates, these neurons form the mantle layer that surrounds the neuroepithelium, now called ventricular layer. Neurites gather at the outside of the neural tube (marginal layer). In insects neuronal cell bodies form an outer layer (cortex); neurites gather in the center, forming the neuropile. Progenitors of the peripheral nervous system [sensory organ progenitors (SOPs); green] segregate from different locations in the epidermis.
Once a cell has exited the cell cycle, a newly born precursor must make a number of other decisions: whether to become a neuron or a glial cell, whether to be excitatory or inhibitory, what shape to take, what partners to connect with, and so forth. It is important to understand that much of what we know about this topic comes from work in invertebrate species such as C. elegans and especially the fruit fly, Drosophila melanogaster. Progenitors of the central nervous system of Drosophila, called neuroblasts (the equivalent of neuroepithelial cells in vertebrates), separate from the neurectoderm and move inside the embryo as individual cells (Fig. 14.1G). Mitotic divisions of Drosophila neuroblasts are asymmetric throughout development. Thus, each neuroblast divides unequally into one large and one small daughter cell. The large cell remains as a neuroblast and continues to divide asymmetrically for a number of rounds (Fig. 14.1G′). The small cell, called the ganglion mother cell (GMC), typically divides one more time and both of its daughter cells differentiate into mature neurons.
In this chapter, we begin to explore the molecular and cellular aspects of determination, such as the genes that specify neural versus nonneural fate, and how position and time of birth shape a neuron’s fate as well as the mode of division of its parental cell. Running through all this is a network of transcription factors that influence almost every aspect of a neuron’s specific identity. We will then discuss how these factors might work together and go through several well studied examples of neuronal determination in which we see them at work. These include neurons versus glia, the sensory cells of the peripheral nervous system, the retina, the spinal cord, and finally the cerebral cortex.
Neurogenesis
Neurogenesis proceeds quite differently in different parts of the neural primordium. For example, neuroepithelial cells in the anterior part of the vertebrate neural tube (which includes the forebrain) undergo many more rounds of division than those of the posterior part (spinal cord). In addition, the maturing neurons have distinct phenotypes and establish different types of connections depending on where they are located. When pieces of neural tube are transplanted to new positions, features such as proliferative potential and cellular phenotype are often carried to the new position, implying that, in the neural primordium, intrinsic determinants profoundly influence the fate of neural progenitors. These determinants subdivide the neural primordium into a “geographic map” with many different domains, each one characterized by a unique identity. The mechanisms underlying the spatial regionalization, discussed in more detail in Chapter 13, are intricately involved in neural fate specification.
Along its anterior-posterior axis the neural tube is subdivided into transverse domains that give rise to morphologically defined partitions of the CNS, including the spinal cord, hindbrain, midbrain, and forebrain (Fig. 14.2B). Each of these major sections of the neural tube becomes further partitioned into neuromeres, each neuromere delineated by its own peripheral nerve. In the spinal cord of mammals, one distinguishes 7 cervical (neck) neuromeres whose neurons innervate the upper extremity, 12 thoracic neuromeres, 5 lumbar neuromeres belonging to the lower extremity, and a variable number of sacral neuromeres. Seven or eight neuromeres are present in the hindbrain, where they are called rhombomeres. Similar to spinal neuromeres, rhombomeres are characterized by the pattern of peripheral nerves they emit. The midbrain is thought to represent a single neuromere. The forebrain possesses no peripheral nerves; however, based upon other morphological landmarks, as well as the expression of molecular markers, it is subdivided into six prosomeres. The posterior three prosomeres (P4, P5, and P6) give rise to the diencephalon, while prosomeres 1, 2, and 3 form the telencephalon. The vertebrate neural tube is divided in the dorsoventral axis into four longitudinal domains, called the floor plate, basal plate, alar plate, and roof plate. The basal plate next to the floor plate includes all motor neurons. In the lateral wall of the neural tube, a furrow called sulcus limitans separates the basal plate ventrally from the alar plate dorsally. The alar plate later gives rise to the dorsal sensory column. Both the anterior-posterior and the dorsal-ventral axes of the neurectoderm are regulated by the expression of conserved homeodomain transcription factors (see Chapter 13). Thus, in both vertebrates and invertebrates, these spatially arrayed transcription factors of the anterior-posterior and the dorsal-ventral axes collaborate to divide the developing nervous system into grids of molecular coordinates that specify the positional identity of neural progenitors down to the level of small groups or, in some invertebrates, even single cells.
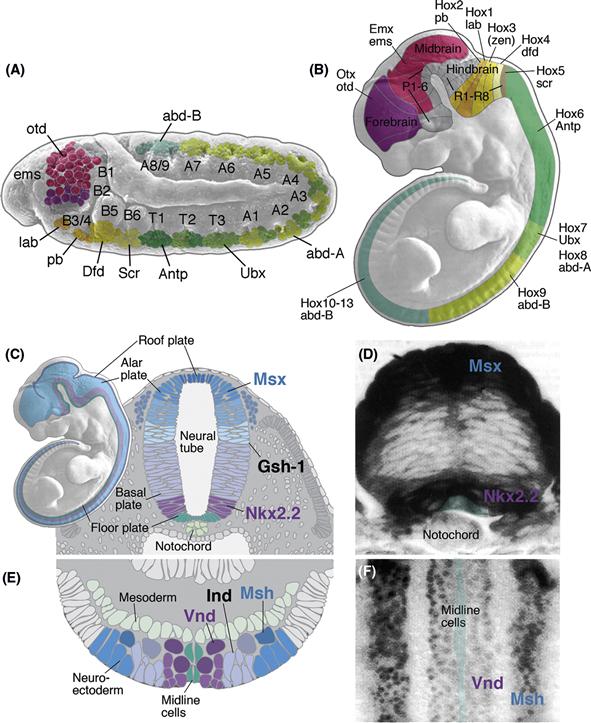
Figure 14.2 Specification of regional identity of the neural primordium. (A and B) Homeobox genes control regional identity along the antero-posterior axis. (A) Drosophila. The neurectoderm and neuroblast layer is subdivided into a series of neuromeres, each of which gives rise to a segmental ganglion. Transcription factors of the Hox family are expressed in blocks of neuromeres and provide neuroblasts with a segment-specific identity. In the segments of the abdomen (A1–A9), thorax (T1–T3), and posterior head (B3–B6) genes of the Hox complex are expressed (lab, pb, Dfd, Scr, Antp, Ubx, AbdA, AbdB); head gap genes (otd, ems) are found in the anterior head (B1–2). B: Mouse. Homologous Hox genes and Head gap genes are expressed in sets of vertebrate neuromeres in the same antero-posterior sequence (P1–P6: prosomeres of forebrain; R1–7: rhombomeres of hindbrain). Otx is expressed in the dorsal telencephalon (P1–3) that gives rise to cerebral cortex. Emx defines alar plate of prosomeres 4–6, that will form the dorsal thalamus and ventral thalamus, as well as the dorsal midbrain. Hox genes are expressed in hindbrain and spinal cord. Note that for the Hox genes, the level indicated represents the anterior boundary of a wider domain that extends posteriorly for an unspecified distance. (C–F) Regional identity along the dorso-ventral axis. (C and D) Vertebrate. The neural tube (shown in C for mouse) is subdivided into four domains, the floor plate, basal plate, alar plate, and roof plate. Floor plate and roof plate do not give rise to neurons. The basal plate produces motoneurons and interneurons. Interneurons formed by the alar plate are the target of sensory input to the spinal cord and brain. The Homeobox gene Nkx2.2 is expressed in the ventral part of the basal plate; Msx is expressed in the dorsal part of the alar plate. The expression of these two genes in a cross section of the zebrafish spinal cord are shown in (D). A third homeobox gene, Gsh-1, is expressed at a lateral level in between Msx and Nkx2.2. (E and F) Homologs of these genes are expressed in the same dorso-ventral succession in Drosophila. The schematic cross section of the fly neurectoderm (E) shows that the Nkx2.2 homolog Vnd forms a medial stripe adjacent to the midline cells; Ind (homolog of Gsh-1) and Msh (homolog of Msx) are expressed at an intermediate and lateral level, respectively. Panel F shows a ventral view of a whole mount in situ preparation demonstrating the expression of Vnd and Msh.
Neurogenesis is patterned in time as well as space. This is exemplified in Drosophila neuroblasts, which go through a program of proliferation where each neuroblast gives rise to a series of distinct GMCs. The first GMCs of a neuroblast lineage tend to lie deeper in the CNS and usually generate neurons with long axons, whereas the later arising GMCs stay closer to the edge of the CNS and produce neurons with short axons. In the stages when the first GMCs are generated, most neuroblasts express Hunchback (Hb), and GMCs that arise at this time inherit this Hb expression. Later, the same neuroblasts turn off Hb and express Krueppel (Kr) instead, and GMCs generated at this stage inherit Kr expression. Experiments in which Hb or Kr is eliminated or expressed at the wrong time lead to predictable switches in the fates of early and later- born descendants of these neuroblasts (Isshiki, Pearson, Holbrook, & Doe, 2001). It is likely that a temporal succession of transcription factors like those in Drosophila also patterns neurogenesis in vertebrates (Elliott, Jolicoeur, Ramamurthy, & Cayouette, 2008).
In many CNS tissues there is a clear relationship between cell birth and cell fate. For example, during the development of the Drosophila nervous system, GMCs and immature neurons form a stack on top of the neuroblast from which they originated. Postmitotic neurons generally do not migrate, so the progeny of a neuroblast remain spatially close to one another, with the position of each neuron dependent on the position of the neuroblast and the time at which it was born. Thereby, a two-layered cortex-neuropil architecture typical of the mature ganglion is generated (Fig. 14.1H). The histogenesis of the six-layered, mammalian cerebral cortex is also easy to describe. It is “inside out” with the earliest cells to exit the cell cycle forming the deepest layers and the last cells to be born forming the most superficial layers. While intrinsic temporally controlled transcription factors play a key role in regulating histogenesis, it is also becoming clear that environmental factors can also influence histogenesis. For example, in the retina, the first cells to be born are retinal ganglion cells, which by negative feedback influence the next born cells to choose a different fate—by downregulating the transcription factors that are important for the determination of retinal ganglion cells. There is also a rich dialogue between factors that drive cell cycle exit and cell fate, such that some transcription factors (see below) have direct effects on the cell cycle, and some cell cycle exit factors influence cell fate (Ohnuma & Harris, 2003).
The Proneural and Neurogenic Genes
In the developing nervous system of Drosophila, the functions of the genes known as the proneural and the neurogenic genes, which have proved to be so important for the initial differentiation of neurons in all animals, were first elucidated. The neurectoderm of the Drosophila embryo is a mixed population of cells. Only some of these cells become neuroblasts; others develop as dermoblasts. Cell–cell interactions that take place within the neurectoderm define the number and pattern of neuroblasts. Experimental and genetic studies suggest a two-step mechanism for this process (Campos-Ortega, 1995). First, discrete groups of neurectodermal cells are made competent to become neuroblasts. These groups of cells, called proneural clusters, represent “equivalence groups” in which all cells initiate a neural rather than an epidermal fate (Fig. 14.3A, B). In Drosophila, a group of proneural genes expressed in the proneural clusters is responsible for making these cells competent to become neural progenitors. In a second step, cells of the proneural cluster interact to sort out which of them will become neurons and which will fall back to become dermoblasts (Fig. 14.3A). As the first cell in each cluster begins to differentiate as a neuroblast, it sends out inhibitory signals to its neighbors, inhibiting them from also becoming neuroblasts. This can be demonstrated by ablating a developing neuroblast with a laser micro-beam, as what happens next is that a neighboring cell of the equivalence group, which would otherwise have developed into dermoblast, switches its developmental program and replaces the lost neuroblast. It is the neurogenic genes that mediate this inhibitory cell–cell interaction.
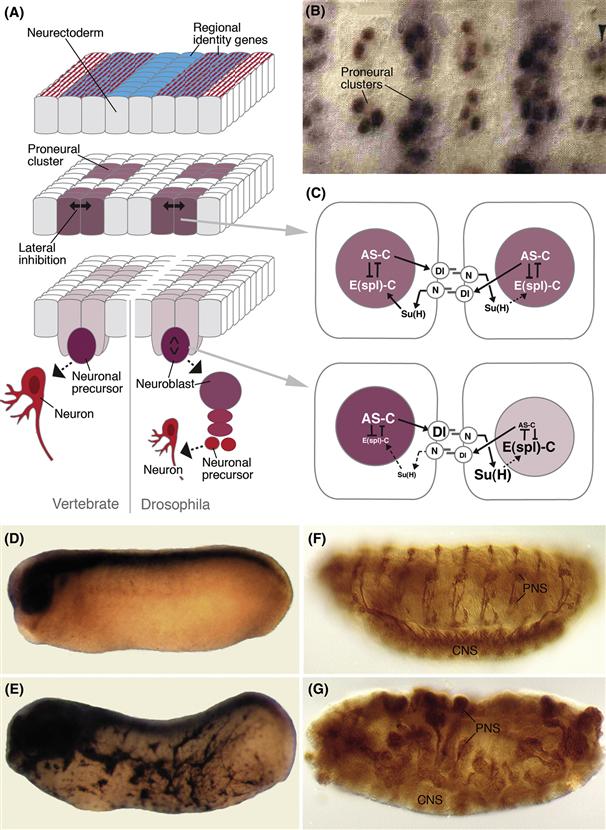
Figure 14.3 Function of proneural and neurogenic genes in neural development. (A) The sequence of events that leads to the segregation of individual neural progenitors (in insects) or neurons (in vertebrates). First, proneural genes are turned on in discrete cell populations, called proneural clusters (purple). In Drosophila, a number of genes (called “regional identity genes” in the diagram) have been identified. They are expressed in a distinct pattern of transverse and longitudinal stripes before proneural genes appear and are proven or likely factors to control the expression of proneural genes. Proneural genes in turn trigger different events in the proneural clusters. One outcome of their expression is a cell–cell interaction process, mediated by the neurogenic genes, that selects a single cell (or a smaller group of cells) from each proneural cluster. Only this cell continues along the neural pathway and segregates from the neurectoderm as a neuroblast (right side of panel; intense purple), which divides and produces a neural lineage (red); the other cells of the proneural cluster remain within the neurectoderm. In vertebrates (left side of panel), the entire neurectoderm (i.e., the neural plate/neural tube) is formed by neural progenitors. Here, the proneural/neurogenic gene cassette seems to select cells that become postmitotic and differentiate from other (neural progenitor) cells, which remain in the neuroepithelium and continue to proliferate. (B) Detail of proneural gene expression in the neurectoderm of the Drosophila embryo (from Skeath & Carroll, 1992). (C) Interaction between proneural and neurogenic genes in the Drosophila neurectoderm. The upper panel shows two cells of a proneural cluster in which proneural genes of the achaete-scute complex (AS-C) are expressed. Genes of the AS-C activate the expression of the signaling molecule Delta (Dl). Dl activates the receptor Notch (N) in neighboring cells; this leads to cleavage of the N molecule and the translocation of the cytoplasmic domain of N along with the transcription factor Suppressor of Hairless [Su(H)] to the nucleus, where they upregulate the expression of proteins of the Enhancer of split complex [E(spl)-C]. E(spl) initiates or maintains the development of an undifferentiated neurectodermal cell; at the same time, it directly inhibits the expression of AS-C genes, which promote differentiation of a cell as a neural progenitor or neuron. Imbalances introduced by an unknown mechanism into the levels of AS-C or E(spl)-C expression, respectively, are rapidly amplified (lower half of C) and lead to the segregation of a single neural progenitor (high levels of AS-C) from other cells that stay epithelial [high levels of E(spl)-C]. (D–G) The proneural gene turns epidermal cells into neurons. (D) A normal Xenopus embryo stained for the neural marker NCAM shows no staining in the epidermis. (E) A neuroD-injected embryo has NCAM-stained cells with neuronal morphology in the entire epidermis. (F) Wild-type Drosophila embryo labeled with the neuronal marker 22C10 contains discrete clusters of neurons forming the central nervous system (CNS) and peripheral nervous system (PNS). (G) Loss of the signaling molecule Dl causes a massive increase in neurons at the expense of epidermis.
Most proneural genes are transcription factors of the basic helix-loop-helix (bHLH) family. Loss of proneural gene function leads to a reduction in neuron production. Misexpression of proneural genes often has the opposite phenotype. For example, misexpression of proneural gene mRNA in vertebrates leads to an increased number of neural progenitor cells in the neural tube or transforms of epidermal cells into neurons (Fig. 14.3D, E). The neurogenic genes encode a cell communication mechanism that acts to restrict the number of developing neurons. The neurogenic genes are also known as the Notch signaling pathway. The Delta and Notch genes encode membrane proteins that function as signal and receptor, respectively. Activation of Notch by Delta sets in motion a signal transduction cascade (Fig. 14.3C) that involves another neurogenic gene called Suppressor of Hairless in Drosophila or CBF-1 in mammals. Su(H)/CBF-1 forms a complex with the intracellular domain of Notch, which is released from the membrane when the pathway is activated. This complex moves into the nucleus and activates the E(spl)-C genes in Drosophila or the ESR and Hairy genes in mammals. These are bHLH transcription factors that antagonize and repress the proneural bHLH genes, completing an inhibitory feedback loop. In Drosophila loss of function of any of the neurogenic genes results in a higher number of neural progenitors, at the expense of dermoblasts (Fig. 14.3F, G). In mammals, loss of the Notch signaling pathway usually results in a higher number of a specific type of neuron at the expense of other types or at the expense of glial cells, depending on when and where this function is lost.
Transcriptional Hierarchies and Networks
As we have seen above with respect to spatial and temporal determination as well as in the proneural and neurogenic pathways, many of the key players in neuronal determination are transcription factors. Transcription factors are protein products of genes that turn on or repress the transcription of other genes or themselves. We find again and again, whether in simple worms or mammals, that transcription factors have a central role in cell-fate determination. Often we find complex transcriptional hierarchies whereby one transcription factor induces the expression of another, which induces the expression of yet another. Such linear hierarchies often make fate decisions progressive and incremental. Such a drive forward in the cell fate progression is sometimes accomplished by a more complex network of transcription factors such that each one activates the next one and represses the previous one so that once a progenitor turns on a particular set of intrinsic factors, it can no longer revert to a more primitive state. Once a particular state is reached, transcription factors can work in interactive circuits that reinforce decisions by mutual cross-activation. But, as we shall also see, some networks of transcription factors work by cross-repression to sharpen boundaries between domains and to make sure that neighboring cells have distinct fates. We also note that a single progenitor will express a variety of transcription factors at any one time and that these can often act together as a combinatorial transcription factor code. Theoretically, a set of just 4 transcription factors on or off in a cell could specify one of 16 distinct fates. The developing nervous system expresses hundreds or even thousands of different transcription factors, so it isn’t difficult to imagine how transcriptional codes could finely specify the fate of billions of distinct neuronal types.
Asymmetric Cell Division and Cell Fate
Neural progenitors, as we mentioned above, can divide symmetrically to increase geometrically in number; or they divide asymmetrically, whereby one daughter cell retains progenitor fate and keeps dividing, whereas the other one differentiates as a neuron. To produce these two distinct fates, intrinsic determinants expressed in the neural progenitors are often differentially distributed during asymmetric divisions. The mechanism controlling asymmetric divisions has been elucidated in great detail for the Drosophila neuroblasts, which, as we know, generate one large daughter neuroblast that will divide again and a small GMC that divides just once more to produce postmitotic neurons.
The machinery involved in asymmetric cell division (Betschinger & Knoblich, 2004) involves a number of proteins at the apical and basal cortex of the neuroblast (Fig. 14.4A). At the apical side of the neuroblast is a highly conserved complex of localized proteins called the “Par-complex.” Members of this complex include the so-called Par proteins (Par3, Par-6) and atypical protein kinase C (aPKC). As the neuroblast delaminates from the neurectoderm, the Par complex maintains its polarized localization, defining the apical pole of the cell (Fig. 14.4A). During neuroblast divisions, the Par-complex recruits another group of proteins, Inscuteable (Insc) and Partner of Inscuteable (aka Pins), to the apical pole (Fig. 14.4B). The importance of the apically localized Par/Insc complex for asymmetric cell division is twofold: (1) Pins locally activates a G-protein-coupled pathway that attracts one of the centrioles toward this complex. The result of this is that the axis of the mitotic spindle is apicobasal (Fig. 14.4B). An apicobasally directed spindle, resulting in an apical daughter cell and a basal daughter cell, constitutes a general feature of asymmetric divisions in the developing nervous system; by contrast, symmetric divisions are generally characterized by horizontal spindles. (2) Proteins of the Par-complex interact with the cytoskeleton in such a way that a group of other proteins, which are also highly conserved among different animal groups, are transported toward the basal pole of the cell. These basal proteins include Miranda (Mir), Numb, and Prospero (Fig. 14.4B, D). As a result of the apicobasal mitotic spindle, only the basal daughter (i.e., the GMC) cell will receive these proteins. Prospero is a homeodomain transcription factor whose function is to switch on genes required for neuronal differentiation and switch off genes that are crucial for continued proliferation (Choksi et al., 2006). Numb acts as a negative regulator of the Notch pathway (discussed above), which keeps Notch pathway activity low in the GMC. Low Notch activity causes the de-repression of proneural genes, as well as the arrest of proliferation (after one more mitoses) of the GMC. By contrast, in the basal daughter cell, Notch pathway activity is kept high by proteins of the apical complex (Par-3, Insc), which promotes continued division of this cell.
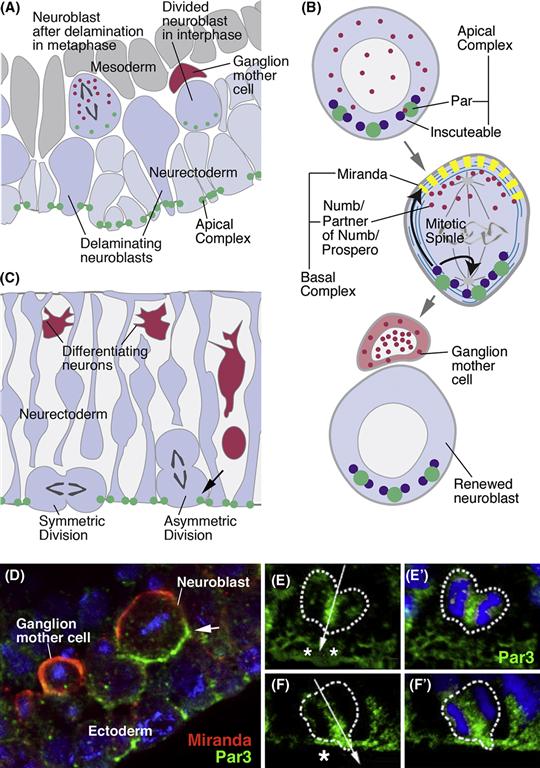
Figure 14.4 Control of asymmetric cell division in neural progenitors. (A) Schematic section of Drosophila neurectoderm at the stage of neuroblast delamination. Proteins of the apical complex (e.g., Par3, Inscuteable) are expressed apically in the neurectoderm and are carried interiorly by delaminating neuroblasts. (B) Schematic of neuroblast before (top), during (middle), and after (bottom) mitosis. The apical complex controls asymmetric distribution of intrinsic fate determinants, such as Numb and Prospero (red), by orienting the mitotic spindle vertically and by localizing the Miranda protein (yellow) basally. Miranda “traps” Numb at the basal pole of the dividing neuroblast (middle panel) and thereby channels it into the ganglion mother cell (bottom panel). (C) Schematic cross section of the mouse neuroepithelium. The mouse Par complex is also expressed apically in neural progenitors (green). In asymmetric divisions, Par 3 is maintained only in the epithelial daughter cell and not the delaminating neural precursor (arrow). (D) Confocal section of Drosophila neurectoderm (from Wodarz & Huttner, 2003). Expression of apical complex (represented by Par3, green) in ectoderm (arrowhead) and delaminated neuroblast (arrow). Miranda (red) is expressed basally; following neuroblast mitosis, it is restricted to ganglion mother cell. (E–F’) Confocal sections of dividing neural progenitors in mouse neural tube (neurectoderm), showing expression of Par3 (from Bultje et al., 2009). (E, E’) Symmetric division. Apical expression of Par3 (small asterisks) is inherited by both daughter cells. (F, F’) Asymmetric division. Par3 ends up only in the one daughter cell to the left; the other daughter cell, lacking Par3, delaminates and adopts a neural fate.
In the vertebrate CNS, neuroepithelial cells first tend to divide symmetrically, giving rise to two dividing progenitor cells, but as neurogenesis proceeds, some neuroepithelial cells begin to divide asymmetrically—in this case giving rise to one neuroepithelial progenitor and one differentiated neuron. The mechanism responsible for vertebrate asymmetric divisions, including many of the genes involved, is similar to what has been described above for Drosophila.
In vertebrates, the orientation of the spindle axis is also thought to play a decisive role in switching between symmetric and asymmetric divisions. Divisions along the horizontal plane produce two cells that stay in contact with the ventricular (apical) surface, whereas more apicobasal divisions produce a basal daughter that migrates away from the ventricular surface, while the other daughter remains in contact, consistent with it retaining a neuroepithelial fate (Fig. 14.4C) (Chenn & McConnell, 1995). Recent findings show that mitotic spindles that are only slightly tilted away from the horizontal axis are sufficient to generate daughters with different fates. Neuroepithelial cells are highly columnar and have only a small apical membrane domain contacting the ventricular surface. As in Drosophila, the Par-complex proteins are restricted to the apical domain. What appears to matter in determining the fate of a daughter cell is whether or not it inherits the apical domain (Wodarz & Huttner, 2003). Only perfectly apicobasal cleavage planes will cut through the apical domain, such that both daughters will receive half of it. In this case, the mitosis is symmetric, producing two progenitors that remain epithelial and continue to divide. If the plane is tilted, even if only slightly, one daughter cell will inherit all of the apical domain and remain proliferative; the other one will delaminate and differentiate (Fig. 14.4.E, F).
Among the vertebrate homologues of the Drosophila genes involved in asymmetric cell divisions are several members of the apical complex. Par-3 acts as an activator of Notch pathway activity in the neuroepithelium, maintaining progenitor proliferation. mInsc is the mammalian homologue of Insc, and ASG3 is the mammalian homolog of Pins. Interfering with the expression or function of these homologues leads to the loss of apicobasal spindle orientation in the mammalian nervous system, resulting in too many cells dividing in the horizontal plane. In the mouse retina this causes the production of large clones arising from neuroepithelial cells, which can be explained if these cells divide symmetrically to produce two progenitors rather than one. Several members of the basal complex, such as Numb and Prospero, may also play a key role in the distinct fates of daughter cells, for example, knockout of the mammalian homolog of Numb is essential for cortical neuroepithelial cells to remain in a progenitor state (Petersen, Zou, Hwang, Jan, & Zhong, 2002).
The orientation of division may thus have an important role in determining cell fate by the uneven segregation of determinants between the daughter cells, but it can also cause the two daughter cells to inhabit slightly different chemical niches—for example, one daughter might remain closer to a source of a secreted growth factor or an extracellular differentiation factor.
Neurons and Glia
The nervous system is composed of two main classes of cells: neurons and glia. Glial cells of the vertebrate CNS include three major types, microglia, astrocytes, and oligodendrocytes. Microglia are derived from the bone marrow and will not be further considered here. Astrocytes are multipolar cells whose processes interact with neuronal synapses, as well as the capillary network; astrocytes form the blood-brain barrier. Oligodendrocytes produce large, lamellar processes that make up the myelin sheath around axons.
Lineage tracing experiments show that neurons and glial cells are often produced from a common progenitor, which we can call a “neuroglioblast” in Drosophila. Neuroglioblasts form part of the neuroblast population that arises in the embryo (Fig. 14.5A, B). Two generic determinants of glial fate have been identified. These include, glial cells missing (gcm) and reversed polarity (repo), both of them expressed at an early stage in neuroglioblast development (Jones, 2005). Their crucial involvement in glial fate is attested to by the fact that ectopic expression of these genes converts neurons into glial cells and that loss-of-function mutants lack glial cells. Interestingly, neither repo nor gcm appear to play a role in glial development in vertebrates.
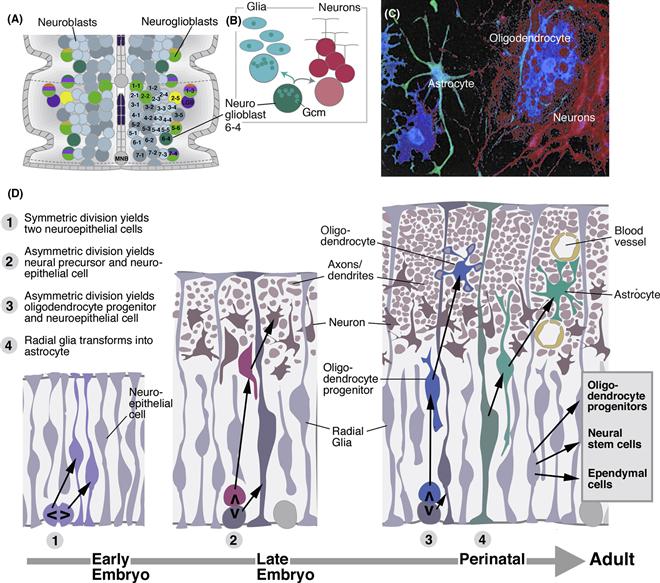
Figure 14.5 Specification of glial lineages. (A) Neuroblast map of the ventral nerve cord with glioblasts and neuroglioblasts indicated. Neuroblasts/neuroglioblasts of one hemineuromere are identified alphanumerically. (B) Neuroglioblast Nb6-4 expresses the glial regulatory protein Gmc (green). When this cell divides into two daughter cells, 6-4 G and 6-4 N, the Inscuteable complex and Miranda segregate Gmc into 6-4 G, which thereby becomes specified as glioblast. 6-4 N generates neurons. (C) Photograph of cell lineage obtained by injecting an individual neuroepithelial cell from mouse cortical progenitor cultured in a dish. Neural and glial-specific antibodies reveal the presence of both neurons (purple) and glia (astrocytes: green; oligodendrocytes: blue) in the lineage (from Qian et al., 2000). (D) Schematic cross section of neural tube of early embryo (left), late embryo (middle), and around birth (right; see time line at bottom). At early stage, symmetric divisions of neuroepithelial cells (1) result in an increased number of neural progenitors. At later stages, neuroepithelial cells (now synonymous with radial glial cells) start to divide asymmetrically, producing neurons (red), and maintaining their own number. Perinatally, production of neurons ceases, and neuroepithelial cells/radial glia switch to the production of oligodendrocyte progenitors (blue). Many radial glial cells delaminate and become astrocytes (green). Radial glia also give rise to the ependymal cells lining the ventricles of the adult CNS, to more oligodendrocyte progenitors, and to neural stem cells that remain active throughout adult life (after Kriegstein & Alvarez-Buylla, 2009).
In the central nervous system of vertebrates, as in Drosophila, multipotent neural progenitors give rise to glia, as well as neurons (Fig. 14.5C). It is usually the case that neurons arise first and glia later. The first rounds of division of the neural progenitors are symmetric and lead to an increase of the progenitor pool (Fig. 14.5D1). Subsequently, asymmetric divisions give rise to neurons (Fig. 14.5D2). Neurons move away from the ventricular surface and form a mantle layer of increasing thickness. As a result, toward the end of neurogenesis, the neuroepithelial cells that are left become elongated cells, called radial glia (Fig. 14.5D2). These cells still have their original apicobasal polarity; with their apical poles they form the lining of the ventricle; the long, basal process (that serves migrating neurons as guidance cue; see Chapter 15) is in touch with the basal surface, where blood vessels and meninges (the connective tissue layers surrounding the CNS) appear. Radial glia keep dividing, now mostly producing oligodendroctye progenitors (OLPs; Fig. 14.5D3). OLPs migrate throughout the central nervous system and differentiate into oligodendrocytes; many OLPs remain mitotically active throughout adult life. At the same time, most of the radial glia withdraw their apical attachments to the ventricular layer and differentiate into multipolar, mature astrocytes (Fig. 14.5D4). Astrocytes continue to divide throughout life; they react to neural injury by strong proliferation, forming glial scars (gliosis). A few radial glia, at specific locations, form a reservoir of adult neural stem cells (see Box 14.1).
Box 14.1 Neural Stem Cells
The proliferating progenitor cells or neuroblasts that give rise to more differentiated progeny but themselves remain in the cell cycle are called neural stem cells (NSCs) (Gage, 2000). Some frogs and fish continue to grow bigger throughout their lifetimes, and their brains grow with their bodies, adding new cells from localized stem cell populations. However, in most animals, including humans, the majority of proliferating neural cells use themselves up during development so the sources of new neurons in an adult animal are extremely limited. This is why damage to the central nervous system is medically much more serious than damage to the other organs, such as the skin or liver, where stem cells that persist into adulthood can replace injured tissue. Nevertheless, it has been found that even in adult mammals, NSCs persist in certain “niches” near ventricular layers. Interestingly, one pocket of rich stem cell activity is in the hippocampus, where certain aspects of learning take place. It is thought that a turnover of pyramidal cells in the hippocampus may be important for replacing old memory traces with new ones.
There is excitement about the potential of using NSCs in a replacement strategy for brain damage due to injury, stroke, or degenerative diseases such as Parkinson’s disease, or retinal degeneration. One could imagine using NSCs to treat patients suffering from Parkinson’s disease or Retinitis Pigmentosa. The cells could be expanded in culture under conditions where they begin to differentiate along particular pathways such as dopaminergic neurons or photoreceptors, and these new neurons could be transplanted into the brain or retina to replace ones lost due to damage or disease.
Important questions about neural stem cells in the adult brain abound. Why do these cells remain undifferentiated and capable of division when their neighbors have exited the cell cycle and differentiated? What signals do these cells need in order to stimulate their proliferation and differentiation? How can their differentiation toward particular types of neurons or glia be controlled experimentally? While there is much speculation on the role of various signaling and growth factors in regulating these processes, more work needs to be done to identify the molecules that prevent stem cells from differentiating and the signals that release their potential.
Interestingly, the environment of the organism plays a key role in regulating NSCs (Kempermann, 2011). Exercise is clearly important for the generation of new neurons in the adult rat hippocampus, and an enriched environment is important for the survival of new generated hippocampal cells. Rats put in simple cages without exercise wheels generate fewer new neurons, and these neurons have a higher chance of dying than in rats that are given exercise regimes and who are held in complex cages with a variety of toys. Stress works in the other direction and seems to inhibit stem cell proliferation and survival in the hippocampus. The identification of the regulatory factors that regulate these NSC activities might prove invaluable in treating neural or glial degenerative conditions without the need for transplants.
Another source of stem cells in mammals is the inner cell mass of the early conceptus. These are the cells that give rise to all the tissues of the embryo proper (Vallier & Pedersen, 2005). When these embryonic stem cells (ESCs) are grown in culture under certain conditions, they can produce neural precursors. It seems that the lessons learned about the mechanisms of neural induction are paying off in this context, because neural inducing signals increase the probability that ESCs will follow a neural pathway. ESCs may have an advantage over neural stem cells from adults because they come from a stage in development when their potential fates are less restricted by the inheritance of intrinsic determinants. More recently, using a cocktail of transcription factors that are normally expressed in ESCs, it has become possible to turn almost any cell in the body back to a pluripotent stem cell (Takahashi & Yamanaka, 2006). Stem cells produced in this way are called induced Pluripotent Stem Cells (iPSCs) and can be taken from the skin or blood of patients. Human iPSCs, besides having a potential role in therapy for neuronal injury and disease, are also very useful for the cellular modeling of disease processes, such as certain forms of autism (Marchetto et al., 2010).
Various laboratories are trying to turn ESCs or iPSCs into various types of neurons. Since a great deal is known about the inductive signals that are critical to the generation of motor neurons in the vertebrate spinal cord, it may be asked whether this knowledge can be used to direct stem cells to a motor neuron fate. Jessell and colleagues (Wichterle et al., 2002) combined inducers that first turn mouse ESCs into neural progenitors with factors such as retinoic acid that drives posterior regional identity such as spinal cord and Shh that drives ventral fates in the spinal cord to push these ESCs to differentiate into neural then spinal and finally into motor neurons that express the same combinatorial codes of transcription factors as motor neurons in vivo. These ESC-derived motor neurons can be labeled by Green Fluorescent Protein (GFP) under the control of the HB9 promoter (expressed only in motor neurons) and when these fluorescents cells are transplanted into the embryonic spinal cord of a chick embryo, they extend axons, and form synapses with target muscles (Fig. B14.1). Moreover such ES-derived motor neurons develop appropriate transmitter enzymes and receptors, and electrical properties, such that they can make perfectly functional synapses with muscle fibers. Thus, by using signaling pathways discovered by studying normal cell determination in the developing nervous system, biologists may be able to direct stem cells to form specific types of neurons.
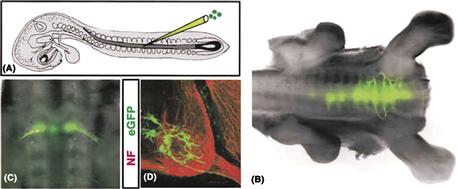
Figure B14.1 Integration of transplanted ESC-derived motor neurons into the spinal cord. (A) Implantation of fluorescently labeled ESC-derived motor neurons into an embryonic chick spinal cord. (B) Fluorescence image showing GFP-labeled motor neurons in thoracic and lumbar spinal cord. (C and D) Location of ES-cell derived GFP-labeled motor neurons clustered in the ventral spinal cord and sending axons out the ventral root. (From Wichterle et al., 2002.)
Clearly, the more we know about neuronal determination, the more likely we will be able to direct stem cells down appropriate developmental pathways that will be useful in treating the damaged nervous system.
Oligodendrocytes arise during several waves. During the first wave, high levels of the ventrally expressed Shh morphogen activate transcription factor determinants of oligodendrocyte fate, called Olig 1 and Olig 2, in the ventral spinal cord (Kessaris, Pringle, & Richardson, 2001; Zhou, Choi, & Anderson, 2001). At later developmental times, oligodendrocyte and astrocyte formation occurs at more dorsal levels of the neural tube. Early expressed transcriptional regulators akin to Olig have not yet been identified for astrocytes. However, we do know that BMPs and some cytokines (e.g., ciliary neurotrophic factor, CNTF) promote astrocyte fate. BMPs and Shh act in an antagonistic manner in the decision between astrocyte and oligodendrocyte formation: BMP activates astrocyte formation and inhibits oligodendrocytes, while Shh does the reverse.
The Notch signaling pathway also plays an important role in glial development. First, high Notch activity in the neuroepithelium acts as a permissive factor for gliogenesis. Thus, activation of the Notch pathway suppresses the transcription of proneural bHLH transcription factors. Lowering Notch levels will result in premature conversion of neuroepithelium into neurons, thereby depleting the pool of cells that would normally give rise to glia at a later stage. Zebrafish embryos with reduced Notch activity in the spinal cord show increased numbers of neurons, at the expense of glia. In addition, at a later stage, Notch may also play a direct role in promoting the expression of glial specific genes. Introducing a constitutively active form of Notch into cultured neural progenitors triggers astrocyte differentiation.
Sensory Neurons of the Peripheral Nervous System
With the exception of the eye, all sensory organs of the vertebrate are derived from sensory placodes and neural crest. A placode is a morphologically visible thickening of an epithelium that often subsequently invaginates into the interior and transforms into a distinct tissue or organ (Schlosser, 2006). Sensory placodes appear during the late stages of neurulation in the dorsal ectoderm that flanks the invaginating neural tube (Fig. 14.6A–C). The most anterior placode is the olfactory placode that gives rise to the olfactory sensory neurons of the nose. Further posterior is the otic placode that forms the inner ear. Slightly more laterally is the placode of the trigeminal system that produces the sensory neurons innervating the receptors of the skin and muscles of the face and the head. Developing later and at a more lateral level in contact with the pharyngeal clefts (the gill slits in fish and amphibians) are the epibranchial placodes. They give rise to sensory neurons that innervate the taste receptors of the mouth, and receptors in the inner organs, such as the heart, large blood vessels, and digestive tract.
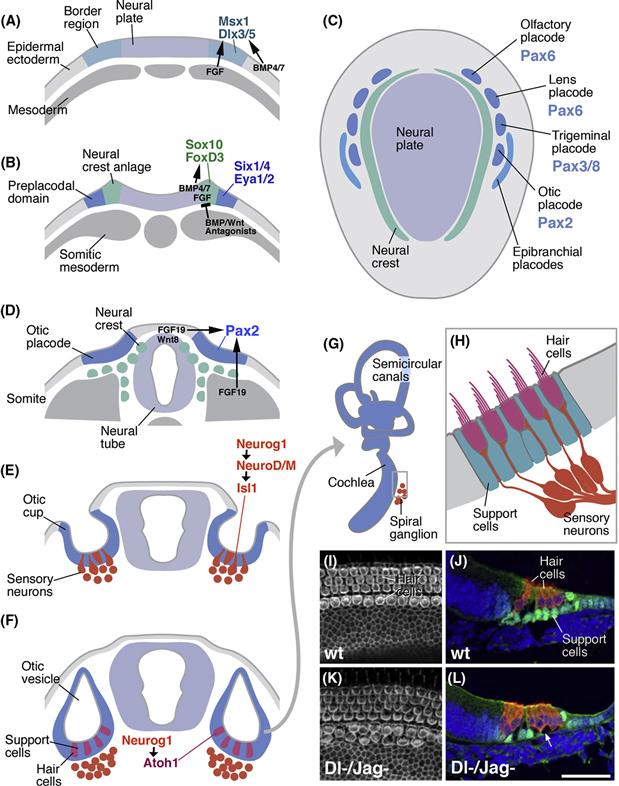
Figure 14.6 Sensory organ development in vertebrates. (C) Schematic dorsal view of neurula stage embryo, showing neural plate (light blue) flanked by sensory placodes (dark blue; anteriorly) and neural crest (green). Placodes express specific transcription factors of the Pax family. (A, B, D–F) Schematic cross sections of vertebrate embryo at successive stages, showing specification of the neural crest and sensory placodes (A, B) and development of the otic placode (D–F). (A) BMP signals from the lateral (epidermal) ectoderm and FGF signals from the mesoderm specify border region in between neural plate and epidermal ectoderm. (B) Peak levels of BMP and Wnt at the lateral boundary of the neural plate specify neural crest. Further laterally, these signaling pathways are inhibited, allowing for the appearance of the preplacodal ectoderm, from which all sensory placodes are derived. (D) Otic placode is induced from dorsal ectoderm by inductive signals (e.g., FDG19, Wnt8c) from neural tube and somites. (E) Otic placode invaginates. Precursors of sensory neurons (red) delaminate from placodal epithelium. (F) Cells of the placodal epithelium differentiate. Precursors of hair cells (sensory receptors; magenta) and support cells (green) appear. (G) Otic placode grows and forms cochlea and semicircular canals. (H) Magnified view of cross section of part of cochlea. Dendrites of sensory neurons (spiral ganglion) form synaptic contacts with sensory hair cells. These specialized, ciliated cells are surrounded by support cells. (I and J) Surface view (I) and section (J) of mouse cochlea, showing normal number and pattern of hair cells and support cells. (K and L) In Dl/Jag double mutants, hair cells are increased in number, and support cells are decreased. (From Kiernan, Cordes, Kopan, Gossler, & Gridley, 2005.)
Sensory neurons of the trunk are derived from the neural crest (Fig. 14.6B–D). The neural crest is a transient population of cells that arises along the lateral edges of the neural plate (Chapter 13). Crest cells become localized to the dorsal part of the neural tube as it folds up, and then leave the neural tube and migrate along several well-defined pathways. Cells destined as sensory neurons aggregate in segmentally reiterated masses that form the dorsal root ganglia. Long peripheral dendrites extend from the dorsal root ganglia toward the skin, muscles, and skeleton. Here, dendritic endings of sensory neurons interact with specialized, mesodermally derived support cells, forming small “corpuscules” that are tuned to a specific type of stimulus, including touch, vibration, stretch, temperature, and pain.
Classical embryological experiments in which parts of donor embryos were transplanted to different sites in host embryos indicated that as they invaginate, placodes come in contact with the neural tube, the archenteron, the mesoderm, and the epidermal ectoderm. These structures all have inductive capacities and influence the fate of the placodal cells. In some instances, specific signaling molecules have been identified. The picture that emerges is that neural crest and sensory placodes are specified in a step-wise manner from the border territory in between neural plate and epidermal ectoderm. (1) At an early stage, prior to neurulation, BMP signals expressed in the epidermal ectoderm and FGF from mesoderm underlying the ectoderm trigger the expression of “border genes,” including the homeobox transcription factors Msx1 and Dlx3/5 (Fig. 14.6A). (2) As neurulation sets in, the border territory splits into neural crest, medially, and preplacodal ectoderm, laterally (Fig. 14.6B). BMP expression reaches peak levels in, and is required for, neural crest ectoderm; at the same time, BMPs inhibit placode formation. Coinciding with high BMP activity are Wnt signals. Both signaling pathways promote the expression of a new set of transcription factors required for neural crest development, including Sox10 and FoxD3. (3) Antagonists of BMP and Wnt are secreted from the mesoderm underlying the lateral part of the former border territory. In response, cells in this region turn on the transcription factors Six1/4 and Eya1/2, which specify the lateral border territory as preplacodal ectoderm (Fig. 14.6B). (4) The preplacodal ectoderm splits into the different sensory placodes as a result of signals from adjacent neural tube, mesoderm, and ectoderm. For example, FGF3 and Wnt8c from the hindbrain and FGF19 from the axial mesoderm work together to induce the otic placode (Fig. 14.6C, D). Each placode acquires its own characteristic set of transcription factors. Different members of the Six and Eya family, initially expressed in an overlapping pattern in the entire preplacodal ectoderm, become restricted to individual placodes. Another class of transcription factors, the Pax proteins, is added as well. Notably, pax2 expression is strongly correlated with the otic placode; pax6 appears in the olfactory and lens placode, and pax3 and pax8 in the trigeminal placode (Fig. 14.6C). Absence or downregulation of these genes results in loss of the corresponding sensory organ.
Homologues of most of the molecules involved in specification of sensory placodes also operate in the Drosophila sensory system. Indeed, molecular evidence shows that the proteins encoded by the pax, eya, and six genes form complexes that as a whole unit bind to DNA and regulate transcription. Genes that in this manner encode cooperating proteins are called gene networks. The fact that their function is dependent on all members of the complex explains why entire gene networks are often conserved throughout evolution. The pax/six/eya network, activated by BMP and/or Wnt signals, is one of the best-studied examples of such a conserved gene network.
Specification of the sensory placode is just the first step in a long chain of events leading up to the mature sensory organ. Let us take a look at this process for the otic placode that gives rise to all cells of the inner ear. Following invagination from the dorsal ectoderm, the otic placode forms a hollow vesicle, the otic vesicle or otocyst (Fig. 14.6E, F). The lumen of the otocyst ultimately becomes the endolymph filled cavity of the inner ear. Initially, the wall of the otocyst is formed by a homogenous epithelial layer. Subsequently, the otocyst folds in a complex manner to give rise to the cochlea for sensing sound, the utricle for sensing gravity, and the semicircular canals for sensing rotational head movements in the three dimensions (Fig. 14.6G). Again, signals acting upon the otic placode from the outside induce these different structures. For example, Shh from the floorplate of the neighboring neural tube acts on the ventral otocyst and specifies this territory as cochlea; ectopic expression of Shh in the entire otocyst causes lack of semicircular canals, and expansion of the cochlea.
As the inner ear takes shape, the cells that form the octocyst wall diversify. In the same way that we learned about for the neural tube, different cell types delaminate from the epithelium and form additional layers basally adjacent to the epithelium (Fig. 14.6D). The anterior part of the otocyst forms a neurogenic region that sequentially, in two phases, produces the sensory neurons and hair cells of the inner hair. During the first phase of neurogenesis, expression of the proneural genes Neurogenin 1 and NeuroD promotes the formation of sensory neurons which innervate the cochlea and semicircular canals (Fig. 14.6E, H). During the second phase, cells of the neurogenic part of the otocyst switch on another proneural gene, Ath1 (homolog of the Drosophila atonal gene; see below), which prompts cells to adopt the fate of sensory hair cells (Fig. 14.6F, H). Cells of the neurogenic region that become neither sensory neurons nor hair cells will form support cells that anchor the hair cells in the epithelium lining the inner ear.
During the first phase of neurogenesis, Notch pathway activity restricts the number of cells that become sensory neurons. Notch activity is also required during the second phase of neurogenesis to distinguish between hair cells and support cells. Thus, precursors of hair cells appear at regular intervals, separated by support cell precursors (Fig. 14.6I–L). A Notch dependent lateral inhibition mechanism operates to determine the spacing of hair cells. Thus, at an early stage, the Notch receptor and the Delta ligand are expressed in all cells of the otic epithelium. Subsequently, Delta becomes restricted to the developing hair cells, which thereby inhibit their neighbors from choosing the same fate; these neighbors become the support cells. Genetic studies, using mouse and zebrafish, show that loss of Delta in the developing inner ear results in an increased number of hair cells (Takebayashi et al., 2007) (Fig. 14.6K, L).
In Drosophila and many other invertebrates, sensory organs are comprised of small clusters of sensory neurons and support cells, which together are called sensilla (Fig. 14.7). Sensilla are scattered over the entire body surface of the larval and adult fly. The cells that compose each sensillum are clonal descendants of a single Sensory Organ Progenitor cell (SOP) (Lai & Orgogozo, 2004). The fate of sensory neurons and their support cells is determined by factors expressed in the SOP and delivered through a series of asymmetric divisions to the individual cells. In most cases, all cells of a sensillum are born in three consecutive divisions. The SOP, also called the primary precusor cell or pI, divides into two pII daughters called pIIa and pIIb (Fig. 14.7B). PIIa divides one more time and gives rise to the outer support cells, which form processes in the shape of hairs or bristles. PIIb gives rise to the sensory neuron and inner support cells in two consecutive divisions (Fig. 14.7B). The first step in sensillum development, the determination of SOPs, is controlled in a manner similar to that regulating neuroblast determination. Thus, the expression of proneural genes defines proneural clusters in the ectoderm that are competent to form SOPs (Fig. 14.7C, D). At the same time, proneural genes activate the Notch pathway, mediated by the neurogenic genes, which single out one SOP and prompt all other cells of the proneural cluster to abort neural development. The identities of individual SOPs are specified by different proneural genes. For example, Poxn and Amos are expressed and required in precursors of chemosensory receptors. Atonal (ato) is expressed and required in the proneural clusters that give rise to stretch receptors (chordotonal organs). Loss of ato function results in the absence of these cell types; likewise ectopic expression of ato in other SOPs induces the production of chordotonal organs.
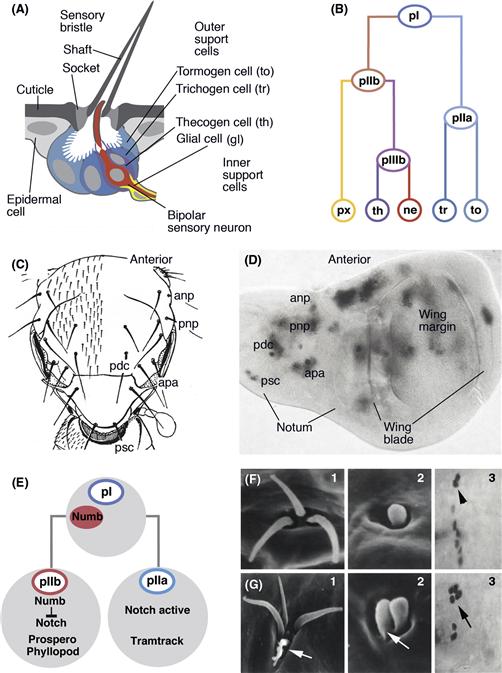
Figure 14.7 Sensory organ development in Drosophila. (A) Structure of mature sensilla. Schematic section of external mechanoreceptor (microchaete). Bipolar sensory neuron (red) is surrounded by two inner support cells, the thecogen cell (purple) and glial cell (yellow). Two outer support cells, the tormogen cell (light blue) and trichogen cell (dark blue), form the socket and shaft of the bristle-shaped cuticular component of the sensillum. (B) Tree diagram of canonical cell lineage of mechanoreceptive sensillum (microchaete). Progenitor of sensillum (SOP or pI; top) divides into two daughter cells, pIIa and pIIb. Division of pIIa gives rise to outer support cells (trichogen and tormogen cell). PIIb divides into progenitor pIIIb that then produces sensory neuron and thecogen cell; the other daughter of pIIb develops as glial cell (as shown here for the microchaete) or expresses a number of other different fates (not shown). (C and D) Sensillum patterning. (C) Schematic dorsal view of Drosophila thorax (notum) indicating invariant pattern of mechanoreceptive bristles. Some of the large bristles (macrochaetes) are identified individually. Anp, anterior notopleural; apa, anterior postalar; pdc, posterior dorsocentral; pnp, posterior notopleural; psc, posterior scutellar. (D) Expression of the proneural gene scute (grey) in wing imaginal disc. In the proximal region of the disc which gives rise to the notum, scute is expressed in proneural clusters which can be assigned to individual macrochaetes. (E–J) Distribution of fate determinants and Notch signaling in the sensillum lineage. (E) During the pI mitosis, Numb, an inhibitor of Notch signaling, is distributed to pIIb. This activates determinants of neurons like Prospero and Phyllopod. Loss of Numb in pI causes transformation of pIIb into pIIa, with a concomitant doubling of outer support cells and absence of neuron/inner support cells. (F, G) Examples of this transformation are the mechanoreceptive Keilin’s organ that, in wild type (F1), has three sets of shaft/socket, and, in numb mutant (G1), has four to five sets of these outer support cells. Likewise, duplication of shaft/socket occurs in a basiconical sensillum (F2: wild-type; G2: numb mutant). F3 shows molecular marker Cut expressed in two outer support cells of a mechanoreceptive sensory organ of the embryo (arrowhead). In the numb mutant (G3), these cells are doubled in number (arrow).
Once determined, SOPs divide asymmetrically, using the same mechanism discussed above for neural progenitors of the CNS. A crucial factor in generating the asymmetry of SOP divisions is the basal complex member, Numb (Fig. 14.7E). After the the first SOP mitosis, the cell receiving Numb becomes pIIb. Numb disrupts the Notch signaling pathway in pIIb, allowing for the expression of genes that are required for sensory neurons, including Prospero and phyllopod. If Numb is deleted, Notch can act in both pII cells, which changes the fate of pIIb to that of pIIa, and causes a duplication of outer support cells (the descendants of pIIa) and loss of neuron/inner support cells (the descendants of pIIb; Fig. 14.7G). It is clear that recurring themes, such as spatially restricted homeobox containing transcription factors, proneural bHLH genes, asymmetric cell divisions, and Notch signaling play a role in sensory neuron determination in vertebrates and invertebrates just as they do in the central nervous system of these animals.
The Retina
The retina is a sensory organ with different origins. The Drosophila retina arises from an imaginal disc, whereas the vertebrate retina arises from an outpocketing of the anterior neural plate. Studies of how specific neurons arise and diversify in the retinas of these distinct animals reveal surprising similarities despite their very different embryological origins, betraying a shared molecular evolution of visual organs that predates the separation of vertebrates and invertebrates (Lamb, Collin, & Pugh, 2007).
The insect compound eye is built of a large number of identical facets, called ommatidia. Drosophila possesses approximately 800 ommatidia in each eye. Each ommatidium contains eight identifiably unique photoreceptor neurons and several accessory cells. Six of the photoreceptors (R1–R6) form an outer trapezoidal array, whereas two (R7 and R8) are located in the center (Fig. 14.8A). Among the accessory cells are cone cells, which form the lens of each ommatidium, and pigment cells, which surround the photoreceptors and optically shield the ommatidia from one another.
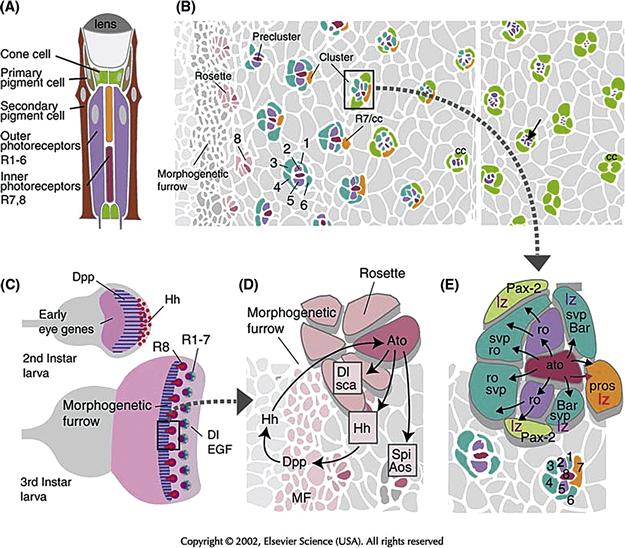
Figure 14.8 Determination of ommatidial cell fate in the Drosophila compound eye. (A) Schematic longitudinal section of ommatidium depicting cell types in different colors. (B) Surface view of part of the eye imaginal disc at a stage when photoreceptor clusters become assembled in a sequential fashion. Gray profiles indicate the apical surface of undifferentiated cells; colored profiles demarcate different types of photoreceptors and cone cells. At the left margin, all cells are undifferentiated. This is followed by a phase in which eye disc cells become more closely packed and constricted at their apical pole, thereby forming the morphogenetic furrow. As they leave the furrow, cells form more or less regularly spaced rosettes (light red). Within each rosette, a single cell becomes singled out as the photoreceptor R8 (dark red). At the time when it becomes distinguishable morphologically, four other photoreceptors (R2, R5: lilac; R3, R4: blue) have joined R8. Together, these five cells form the so-called preclusters. Three more photoreceptors (R1, R6: blue; R7: orange) join the preclusters, leading up to complete photoreceptor clusters. This step is followed by the appearance of four cone cells (green) that surround the photoreceptors in a circular fashion. On the right side of the panel, photoreceptors can be seen to segregate from the surface. Their apical membranes (all shown in purple) become increasingly smaller (arrow) and finally disappear altogether. (C) Initiation of compound eye development. During midlarval stages (Top), the signal Hedgehog (Hh; red) is expressed at the posterior tip of the eye disc. Hh activates the expression of another signaling protein, Dpp (blue), which turns on several “early eye genes,” which commit the undifferentiated, proliferating cells that comprise the eye disc to an eye fate. During late larval stages, Hh and other factors initiate differentiation of the first ommatidial cells, the R8 photoreceptors, by turning on the proneural gene atonal (ato). This sets in motion other signaling pathways (EGFR signaling; Notch/Delta signaling) required for the proper spacing of ommatidia and the determination of other ommatidial cell fates. (D) Determination and spacing of R8 are controlled by the proneural gene atonal (ato), as well as the inhibitory signals Delta (Dl) and Scabrous (Sca). Initially switched on in the entire morphogenetic furrow, ato expression then becomes restricted to a mosaic of regularly spaced cells, which subsequently differentiate as R8. Continued secretion of Hh, which signals across the MF toward the more anterior cells of the eye disc, drives the morphogenetic furrow across the eye disc. (E) Determination of photoreceptors R1–R7 and cone cells. Secretion of the TGF β homologue Spitz (Spi) from R8 stimulates cells surrounding R8 via the Drosophila EGF receptor homologue (DER), the Ras signaling pathway (symbolized by black arrows). In conjunction with other signaling events, including Notch/Delta, photoreceptors are induced to express specific transcription factors, which are required for their respective fates. The lozenge (lz) gene is expressed in R1, R6, R7, and cone cells; seven-up (svp) is expressed in R1, 6, 3, and 4; rough (ro) in R2, 3, 4, and 5; BarI in R1, and 6; prospero (pros) in R7, and Pax-2 in cone cells.
Cells of the ommatidia are formed from clonally unrelated, uncommitted precursor cells that are generated from the proliferation of cells in the eye imaginal disc (Mollereau & Domingos, 2005). The ommatidial cells do not appear all at once, but follow a reproducible temporal sequence (Fig. 14.8B). During late larval life, a wave of differentiation passes over the eye disc in a posterior-to-anterior direction. In front of the wave, cells are still dividing and uncommitted, whereas behind the wave front, which is visible as a depression in the disc called the “morphogenetic furrow” (MF), cells are mostly postmitotic and are beginning to differentiate. As they leave the MF, cells of the eye disc become arranged in a pattern of “rosettes” that foreshadow the regular ommatidial pattern. Soon afterward, one cell is singled out in each rosette to become R8. Being the first cells to differentiate, R8 cells can be considered as “crystallization centers” around which the other ommatidial cells aggregate in a stereotyped pattern.
R8 cells express the proneural gene atonal (ato). Atonal is turned on by the signaling protein Hedgehog (Hh, a homologue of vertebrate Sonic hedgehog, Shh). Atonal thus first turns on in a continuous band of cells, similar to a preneural cluster, within the morphogenetic furrow, which then initiate lateral inhibition through Delta Notch signaling (Fig. 14.8C, D). By this mechanism, ato expression becomes restricted to a set of spaced cells, which subsequently differentiate as R8 photoreceptors. Atonal expression causes Hh to be produced and released. Thus, these R8 precursors continue emitting Hh, which signals across the morphogenetic furrow toward the more anterior cells of the eye disc to induce the next set of R8 cells (Ma, Zhou, Beachy, & Moses, 1993). This Hh-mediated feedback mechanism drives the wave of differentiation—that is, the morphogenetic furrow, across the eye disc, leaving in its wake a beautifully spaced mosaic of R8s, each crystallizing an ommatidium. Deleting ato results in the total failure of retinal development, which indicates that R8 plays a pivotal inductive role, triggering the determination of other cells that join the ommatidial clusters. The first cells to join the R8 cell shortly after its determination are R2, R3, R4, and R5 (Fig. 14.8A). Together with R8 they make up an ommatidial “precluster.” The next cells to join the cluster are R1 and R6, followed by R7 and four cone cells; the last cells to join the cluster are the different types of pigment cells.
Shortly following its own determination, R8 puts out signals that activate two different signaling pathways: Notch and Ras. The Notch pathway, as we know, is activated by Delta. The Ras pathway represents a highly conserved biochemical cascade of cytoplasmic kinases (Ras, Raf, MPK), which in this case are activated by the epidermal growth factor receptor (EGFR). R8 emits a signal, Spitz (Spi), that activates this receptor and another signal, Argos (Aos), that acts as an inhibitor. Activation of these signaling cascades spreads concentrically from R8 to the precluster cells (R2, R3, R4, and R5) and then the remainder of the ommatidial cells (Fig. 14.8D). The precise, temporally regulated activation of EGFR and Notch signaling pathways assigns distinct phenotypes to the cells that join the ommatidial clusters.
The way in which differential signaling pathway activation controls ommatidial cell fates has been studied most closely for two cell types, R7 and the cone cells, which appear at a relatively late stage when all other photoreceptors are already in place. The Pax-2 gene specifies cone cells (Fig. 14.8E). To express Pax-2, the cone-cell-to-be depends on three inputs (Flores et al., 2000): (1) the previous expression of the transcription factor lozenge (lz); (2) activation of the Notch pathway; and (3) activation of the EGFR signaling pathway. If any one of these prerequisites is not met, the cells will fail to express Pax-2 and will not differentiate as cone cells. Conversely, if a set of cells that normally does not receive all three inputs is exposed to them experimentally, these cells will express Pax-2 and become cone cells. R7 cells normally express lz, but do not activate the Notch pathway, and experimental misexpression of activated Notch in the R7 precursor will turn this cell into a cone cell.
The determination of the R7 cell deserves special mention in view of the pivotal role it has played in opening up the molecular-genetic study of signaling pathways (Zipursky & Rubin, 1994). The important advantage a genetic model system like Drosophila has to offer is that developmentally relevant molecules, such as signaling proteins, receptors, and signal transducers, can be identified by mutant screens. One of the first of such screens in the field of cell determination took advantage of the fact that only one of the photoreceptor cells of each ommatidium, R7, is sensitive to ultraviolet (UV) light. Thus, mutagenizing flies and screening for offspring that are blind to UV light yielded a fly line that lacked the R7 cell in every ommatidium and was therefore aptly called sevenless (Harris, Stark, & Walker, 1976) (sev) (Fig. 14.9). The close study of this gene showed that it encodes a receptor with tyrosine kinase activity and that this receptor is expressed (among other cells of the eye disc) in the cell that will become R7. Lack of the receptor causes the cell that would normally become R7 to develop as a cone cell instead. In several follow-up screens, many signal transducing molecules and transcription factors activated by the Sev receptor were identified. Among them were the Drosophila homologues of Ras, Raf, and MAPK. One of the most rewarding findings was the identification of the signal that binds to Sev. Given the widespread expression of Sev, it was clear that a signal emanating from a point source must exist, in order to ensure that Sev would become active only in one cell in each ommatidium. The logical candidate to emit such a signal would be R8, the only cell in broad contact with the presumptive R7. A genetic screen for R7-less flies yielded identification of a membrane bound signaling molecule, called Bride of sevenless (Boss), which is expressed specifically in R8 cells and serves as the ligand for Sev (Reinke & Zipursky, 1988). These experiments provided an impressive demonstration of the awesome power of genetic screens combined with precise knowledge of the developing system in identifying new genes and proteins controlling development.
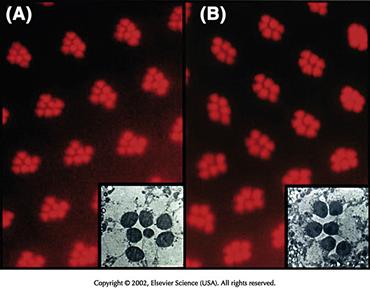
Figure 14.9 Photoreceptors in the eye of normal and sevenless mutants. (A) If a light is shone from the back of a fly’s head and focused in the facets of the eye, individual photoreceptors can be seen because of their ability to pipe light. The wild-type animal has the normal pattern of seven photoreceptors visible in each facet. The small one in the center is photoreceptor R7. (B) The same technique used in a sevenless mutant shows only the six large photoreceptors R1–6 in each facet. R7 is missing. Insets show electron micrographs through single facets.
The vertebrate retina, like the Drosophila retina, develops from a population of pluripotent neuroepithelial progenitors, which produce a diversity of neurons and glia (Fig. 14.10A). As in Drosophila, vertebrate neurogenesis and determination in the retina follows a temporal order (Livesey & Cepko, 2001). Thus, at any given time during retinogenesis, only a few fates are available to differentiating cells. Such temporal fate restrictions are likely to be caused by transiently expressed transcription factors that switch on over particular periods of neurogenesis, such as Ikaros, a vertebrate homologue of Hb, which plays a similar role in Drosophila histogenesis. Cells born early in the vertebrate retina generally adopt fates as retinal ganglion cells (Fig. 14.11A–C), followed sequentially by horizontals and amacrines, bipolars, photoreceptors, and Müller glia. This is also similar to Drosophila in which first R8s and then sequentially other cell types differentiate. This progressive shift in cell type genesis in vertebrates is supported by dissociation experiments in which cells are put into culture at low density at various stages of development. If progenitors are isolated at the time when retinal ganglion cells (RGCs) are normally born, they tend to turn into ganglion cells in culture. Later progenitors in the same culture medium become mainly photoreceptors (Watanabe & Raff, 1990).
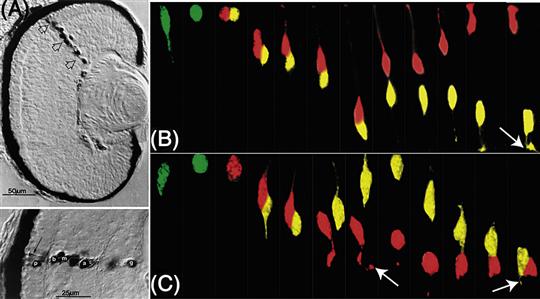
Figure 14.10 Clones of cells in the Xenopus and zebra fish retina. (A top) Daughters of a single retina progenitor in Xenopus injected with horseradish peroxidase are seen to form a column that spans the retinal layers and contributes many distinct cell types, (A bottom) p, photoreceptor; b, bipolar cell; m, Muller cell; a, amacrine cell; and g, ganglion cell. (B) Time-lapse images of an RGC progenitor in zebra fish dividing at the apical surface of the retina to produce two cells one of which (yellow) becomes an RGC. (C) A similar RGC progenitor in a mutant retina where there are no host RGCs because it is mutant for the ath5 gene. This cell divided to produce two daughters, both of which become RGCs (arrows point to emerging axons).
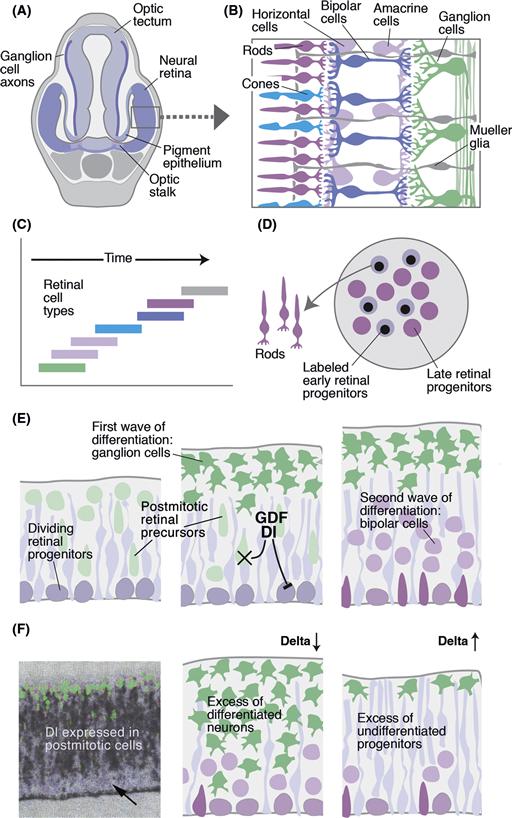
Figure 14.11 Vertebrate retina development. (A) The neural retina bulges out of the ventral neural tube at the level of the diencephalon. It is joined to the brain by the optic stalk along which the axons of retinal ganglion cells will course on their way to the tectum. (B) The seven major cell types in the retina coded by color. Their laminar arrangement by cell type is evident. (C) Birth dating studies in the retina show that different cell types are born in different but overlapping periods of development. (D) When early generated retinal precursor cells (labeled by a pulse of BrdU) are mixed with older cells in culture, they show an increased probability of turning into late cells, such as rods. (E) Development of the retina. Schematic section of retinal neuroepithelium at three different stages (early: left; late: right). Dividing retinal progenitors (purple) produce different cell types depending on the developmental stage. Differentiation inhibitors (Dl) released from postmitotic neural precursors inhibit retinal progenitors from producing more ganglion cells. Postmitotic cells also secrete differentiation factors such as GDF which limit the time window during which postmitotic cells can become retinal ganglion cells. (F) Expression and function of Dl in the developing retina. Left panel: expression of Dl in postmitotic neural precursor; dividing progenitors of the ventricular layer (arrow) or differentiated neurons of the basal mantle layer (green) do not express Dl. Middle: Loss of Dl results in increased number of early-born retinal cells (retinal ganglion cells), at the expense of undifferentiated retinal progenitors. Right: Overexpression of Dl causes the opposite phenotype.
There is an intrinsic element to the succession of cellular fates in the vertebrate retina (Cayouette, Poggi, & Harris, 2006), which is revealed when rat retinal progenitors are isolated and allowed to divide on their own in culture. These isolated progenitors give rise to clones of cells of the same general birth order, size, and composition as clones in vivo. If one follows these clones as they develop, there is another surprising similarity to Drosophila retinal determination, which is that cells seem to choose their cell type in a stochastic manner—that is, lineage does not predict fate.
So how do vertebrate retinal cells become determined so that there are the right number of all the different cell types? As in Drosophila, vertebrate homologue of ato, ath5, is turned on early, when the first retinal cells are being born. Delta-Notch signaling then ensures that not too many cells become RGCs. If ath5 expressing progenitors are followed by time-lapse analysis in the zebra fish retina (Poggi, Vitorino, Masai, & Harris, 2005), they appear to divide to generate one RGC that expresses ath5 at high levels and one other cell in which ath5 levels are reduced (Fig. 14.10B). Several other transcription factors are expressed at about this time in various populations of cells as they begin to differentiate. One such factor, Ptf1a, is turned on in the subset of newly postmitotic cells that will become inhibitory neurons in the retina (i.e., the amacrine and horizontal cells). Ath5 is necessary for the determination of RGCs, while Ptf1a is essential for the generation of inhibitory neurons. Ptf1a also controls inhibitory neuron differentiation in the spinal cord (see the following section). In the absence of Ath5 cells that would have become RGCs and in the absence of Ptf1a, cells that would have become amacrine or horizontal cells choose excitatory fates instead. All horizontal cells, it turns out, express both Ath5 and Ptf1a. This begins to look like a combinatorial code. Indeed, other studies have shown that particular combinations of retinal transcription factors, sometimes two or three together, drive cells to become specific cell types, while single factors are much less effective (Ohsawa & Kageyama, 2008).
How these different intrinsic determinants are turned on in these combinations is unknown, but it is clear that fate determination in the retina is not just an intrinsic affair. When retinal cells are removed at the stage when RGCs are being born, mixed into aggregates, and cultured in vitro, they differentiate into RGCs as one would expect. However, if the same cells are mixed with an excess of retinal cells several days older (i.e., when photoreceptors are generated), they generally become photoreceptors (Fig. 14.11D). This work shows that individual cells have the capacity to differentiate into different cell types, and the fate they choose depends on the environment in which they are born. This makes good sense, for as retinal development proceeds, the environment changes simply as a result of various retinal cell types being generated, much as in the Drosophila retina. Indeed, there is strong evidence for negative feedback in influencing the fate of RGCs. Young embryonic chick progenitors are inhibited in their ability to produce RGCs when cultured adjacent to older retinas in which there are lots of RGCs, and depletion of the RGCs from these older retinal cell populations abolishes this inhibition (Waid & McLoon, 1998). When normal retinal progenitor cells that express a fluorescent protein under the control of the ath5 promoter are transplanted to normal embryos, the cells that turn on ath5 usually generate only a single RGC daughter (see above), but these same cells when transplanted into mutant retinas that lack RGCs, they often generate two RGC daughters, providing evidence that such lineages are sensitive to feedback signals (Fig. 14.10C) (Poggi et al., 2005). It seems that Ath5 itself is negatively regulated by such feedback signals. Similarly normal progenitors transplanted into Ptf1a mutant retinas are more likely to turn on Ptf1a to try to compensate for the missing cell types, suggesting again that negative feedback normally keeps the level of Ptf1a expression appropriate to generate the correct proportion of inhibitory neurons in the retina (Jusuf et al., 2011).
What could these feedback signals be? First, as mentioned above, the Notch signaling pathway contributes to retinal diversity in the vertebrate retina (Fig. 14.11E, F). When Notch signaling is compromised in early retinal progenitors, too many cells differentiate as early cell types, whereas later interference with Notch signaling leads to an increase in later-born cell types. The Notch signaling pathway, by allowing only a certain number of cells to express particular proneural genes and thus differentiate at any one time, is important in creating cellular diversity in the vertebrate retina (Dorsky, Chang, Rapaport, & Harris, 1997). RGCs also express Shh, and the Shh knockout mouse contains increased number of RGCs. These results suggest that Shh provides a feedback inhibition signal in the developing mouse retina, echoing what happens in the Drosophila retina where R8 cells express Hh, which regulates the expression of ato. The growth differentiation factor 11 (GDF11) is another factor secreted by RGCs, and it acts by limiting the temporal window during which progenitors are competent to express ath5 and thus produce RGCs.
The Spinal Cord
The vertebrate spinal cord plays a central role in conveying motor commands to the periphery via somatic and autonomic motor neurons. It also harbors neurons that receive and transmit cutaneous, proprioceptive, and visceral sensory information from specialized receptors in the body and limbs. This information is used to activate protective reflexes such as the nociceptive withdrawal reflex, and to shape motor activity needed for locomotion. The component neurons of these spinal sensorimotor circuits are generated by the patterning events that specify neuronal identity in the embryonic spinal cord. Antero-posterior positioning contributes to neural patterning in the cord as evidenced by differences in the number and types of specialized cells that are found at different axial levels. This is most apparent for branchiomotor, phrenic, preganglionic, and lateral motor column motor neuron cell types that are patterned by the Hox genes (Dasen, Liu, & Jessell, 2003; Dasen, De Camilli, Wang, Tucker, & Jessell, 2008). The Hox genes are also likely to regulate the diversity and abundance of specialized interneuron cell types that are similarly distributed in different numbers along the neuroaxis.
The dorso-ventral position in which a neuronal progenitor is located within the neural tube is one of the major determinants of neuronal cell fate in the spinal cord. Signaling centers at the ventral and dorsal midline establish opposing gradients of Shh and BMP signaling, respectively. As a result of this signaling the ventricular zone is subdivided into many discrete progenitor domains that in turn produce motor neurons and all the classes of spinal interneuron. The ventral-most region of the spinal cord, the “floor plate” is induced by a Shh signal emitted from the underlying notochord (Chapter 13). Once induced, the floor plate serves as a secondary source of Shh, which percolates dorsally through the cord to provide graded Shh signaling to ventral neural tube progenitors (Fig. 14.12A). Differential Shh signaling partitions the ventral basal plate into five discrete progenitor domains that in turn generate six neuronal cell types (Jessell, 2000).
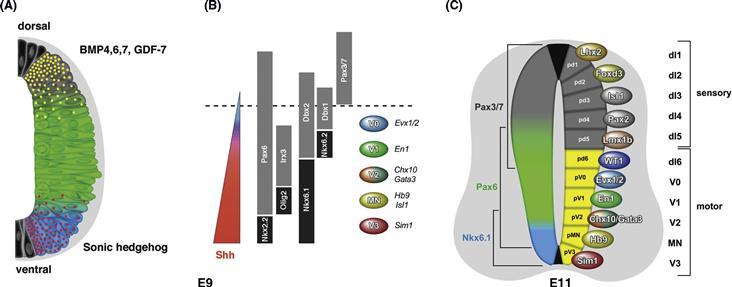
Figure 14.12 Specification of neurons in the vertebrate spinal cord. (A) Schematic cross section of the mouse neural tube. Shh protein (red) is released ventrally from the notochord and floor plate (black cells). It forms a gradient with high concentrations ventrally and low concentrations dorsally. BMPs and other TGF-B-related molecules are released from the dorsal epidermis and dorsal neural tube to form an opposing gradient. These opposing gradients lead to the expression of Nkx6.1 (blue) ventrally, Pax6 (green) medially, and Pax3/Pax7 (dorsally). These expression patterns are refined further (see C) and they contribute to the subdivision of the ventricular zone (inner band of cells) into eleven distinct progenitor domains (pd1–pd6 and pV0 –pV3). (B) A Shh gradient (red) in the ventral half of the neural tube establishes the expression domains of the class I and class II homeodomain genes (see text for details). This in turn leads to the development of five ventral progenitor domains that give rise to V0, V1, V2, MN, and V3 neurons. Cross-repressive interactions between class I (gray) and class II (black) homeodomain genes establish the boundaries between these progenitor domains. (C) Representative cross section through the mouse spinal cord at E11.5 showing the primary classes of neuron that are generated in the embryonic spinal cord. Neurons that arise ventrally (dI6 –V3) are thought to become components of the spinal motor circuitry, while more dorsal cell types (dI1–dI5) contribute to the reception and transduction of sensory information from the body.
How do cells at different dorso-ventral levels interpret their exposure to different levels of Shh in order to acquire different fates? Threshold levels of Shh activate homeobox transcription factors such as Nkx2.2 and Nkx6.1 (Class II) ventrally, while at the same time turning off other factors such as Pax6, Dbx2, and Pax3/7 (Class I) dorsally (Goulding, Lumsden, & Gruss, 1993; Jessell, 2000). Thus, neural tube progenitors in different dorso-ventral regions express specific homeodomain proteins, which either are turned on or off at particular Shh thresholds (Fig. 14.12B). The boundaries between these domains are sharpened through reciprocal repression of the two classes of transcription factor. The ventral border of the class I Pax-6 gene initially overlaps the dorsal border of the class II Nkx2.2 gene, but then reciprocal repression sets in, so that only one of these factors is expressed in any particular progenitor cell. By this process, specific domains uniquely express particular combinations of Class I and Class II homeodomain transcription factors.
Each domain that is generated by cross-repression produces a single generic class of neuron. V3 interneurons that arise close to the floor plate are generated from progenitors that express Nkx2.2, but not Pax6 or Olig2. Motor neuron progenitors express Nkx6.1 but not Irx3 and Nkx2.2. Nkx6.1 turns on Olig2, a bHLH transcription factor that is required for motor neuron differentiation, and Olig2 in turn activates the expression of motor neuron specific transcription factors, such as HB9, Lim3, and Isl1/2, that are each involved in motor neuron differentiation (Fig. 14.12B, C).
A variety of interneuron cell types are generated from the other progenitor domains, with each domain producing a single primary generic class of interneuron (Fig. 14.12C). The V2 progenitor domain is unique in that it generates two interneuron classes: V2a and V2b INs. Notch signaling controls the specification of these two cell neuronal types, which results in the production of equivalent numbers of V2a and V2b neurons (Peng et al., 2007). Dll4 is selectively expressed in a subset of V2 progenitors and these cells activate the Notch pathway in sibling V2 neurons, which in turn induces the expression of Scl and the V2a differentiation program (Del Barrio et al., 2007). The V1 interneuron class that arises just dorsal to the V2 progenitor domain exemplifies many of the features of these early interneuron classes. While these cells share a number of similar attributes, rostral directed ipsilateral axons and inhibitory neurotransmitter phenotype, they are comprised of multiple specialized cell types, possibly five or more. This specialization of neuronal phenotype appears to be regulated by a strict temporal program that overlays the DV spatial program (Stam et al., 2012).
In the dorsal embryonic cord, the alar plate can be coarsely subdivided into two major progenitor regions: a more dorsal region of the neural tube comprising three progenitor domains that generate Class A dI1–dI3 neurons and a second intermediate region that generates Lbx1-expressing Class B neurons (Fig. 14.13). Class A neurons (dI1–dI3) are generated in a BMP and Wnt-dependent manner, whereas Class B (dI4–dI6) interneuron differentiation is independent of BMP/Wnt signaling. The downstream targets of the dorsal BMP/Wnt pathway include the bHLH transcription factors Olig3, Atoh1, Neurog1/2, and Ascl1, which function as cell type specific determinants in Class A progenitors. Olig3 is expressed in all Class A progenitors, where it is required for dI1–dI3 neuron specification. It does so by repressing the expression of Lbx1 (Muller et al., 2005), an essential postmitotic regulator of Class B neuronal identity. Within the Class A region, the proneural bHLH transcription factors Atoh1, Neurog1/2, and Ascl1 function at two levels. They act as proneural determinants for dI1, dI2, and dI3 neurons, respectively, and they regulate boundary formation between the dI1, dI2, and dI3 progenitor domains via a cross repression mechanism (Gowan et al., 2001), thereby subdividing the Class A region into three distinct progenitor domains.
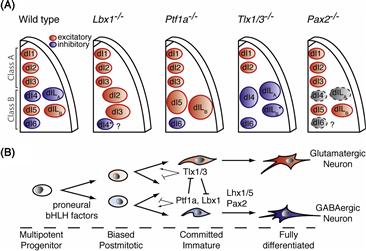
Figure 14.13 Genetic control of neuronal cell types and neurotransmitter phenotype in the dorsal spinal cord. (A) Six early (dI) and two late (dIL) populations are generated in the embryonic spinal cord. dI1–3, dI5, and dILB neurons are excitatory glutamatergic neurons (red), and dI4, dI6, and dILA differentiate into inhibitory cell types (blue). From left to right: In Lbx1 mutant mice, Class B neurons fail to form, and cells are converted to Class A fates. Excess dI2–dI3 glutamatergic cells are generated. In Ptf1a mutant mice, dI4 and dILA interneurons transfate to glutamatergic dI5 and dILB neurons. This results in the complete loss of inhibitory neurons in the dorsal spinal cord and an excess of excitatory neurons. In Tlx1/3 mutants, dI3, dI5, and dILB excitatory interneuron formation is impaired, and there is a concomitant upregulation of GABAergic markers. Mutant dILB cells (*) maintain Lmx1b expression, but they switch to an inhibitory phenotype. In Pax2 mutants, inhibitory neuron markers are lost, but dI4/dILA neurons do not adopt the identity of excitatory dorsal neurons. (B) Model for the specification of excitatory or inhibitory transmitter phenotype in the dorsal spinal cord. Crucial genetic interactions occur in early postmitotic neurons between HD and bHLH transcription factors. Gain or loss of these factors produces a fate switch, with additional factors such as Pax2 operating in postmitotic neurons to consolidate the neurotransmitter program.
A number of homeodomain transcription factors, including Pax3/Pax7, Msx1/3, and Gsx1/Gsx2, also have essential roles in patterning the alar plate. Pax3 and Pax7 are broadly expressed in alar plate progenitors where they appear to act as general determinants of dorsal identity (Fig. 14.12C). Gsx1 and Gsx2 are expressed in dI3–dI5 progenitors, where they control the specification of dI3, dI5, and dILB neurons (Mizuguchi et al., 2006) by upregulating the expression of Ascl1, which is an essential proneural determinant for all three cell types. Gsx2, which is required for the differentiation of dI3 interneurons, promotes Ascl1 expression in dI3 progenitors by blocking the expression of Neurog1/2. This suggests that homeodomain patterning factors like Gsx2 promote specific differentiation programs by functioning as permissive factors for proneural determinants such as Ascl1.
Class B Lbx1-expressing neurons come in two flavors: GABAergic inhibitory interneurons and excitatory glutamateregic interneurons. These two populations are marked by the mutually exclusive expression of Pax2, Lhx1/5, Gbx1 (inhibitory dI4 and dILA neurons), or Lmx1b, Tlx1/3 (dI5 and dILB excitatory neurons). This dichotomy suggests neurotransmitter choice and neuronal identity are closely linked. Indeed, the aquisition of a glutamatergic phenotype depends on the activity of the Tlx1 and Tlx3 selector HD transcription factors that determine dI5 and dILB identity (Cheng et al., 2004). Tlx1/3 in turn represses the Pax2-dependant inhibitory differentiation program. Conversely, the bHLH factor Ptf1a drives Class B neurons to differentiate as GABAergic INs by repressing Tlx1/3 expression (Glasgow, Henke, MacDonald, Wright, & Johnson, 2005; Mizuguchi et al., 2006). While the Tlx1/3 and Ptf1a transcription factors appear to be the primary determinants of Class B neurotransmitter phenotype, Pax2 and Lhx1/5 function downstream of Ptf1a to consolidate the GABAergic neurotransmitter program. Inactivation of the Pax2 gene results in the loss of inhibitory neurotransmitter markers from dorsal dILA neurons. In this instance, Tlx1/3 and glutamatergic markers are not upregulated, which indicates that these cells do not convert to excitatory sensory neurons.
Motor Neurons
Motor neurons in the spinal cord are organized in columns at different anterior-posterior levels. At limb levels, three major columns are evident: the medial motor column that innervates the axial muscles of the back, the hypaxial motor column that projects to the ventral body wall muscles, and the lateral motor column that innervates the appendicular musculature muscle (Dasen et al., 2003, 2008; Jessell, 2000). In the cervical region and lumbar spinal cord, the Lateral Motor Column (LMC) innervates forelimb and hindlimb muscles, respectively. In the midthoracic region, the Preganglionic Motor Column (PGC) innervates the sympathetic chain (Fig. 14.14A–C). A gradient of FGF and retinoic acid signaling selectively establishes differential Hox gene expression along the AP axis at different levels, boundaries between which are sharpened by reciprocal inhibition. This anterior-posterior logic of motor neuron identity is thus similar to that governing the positioning of the neurons in the dorsal ventral axis of the spinal cord. FoxP1, which acts as a co-factor for the Hox transcription factors is required for the specification of LMC type motor neurons, and the PGC or Column of Terni as it is otherwise known (Dasen et al., 2008; Rousso, Gaber, Wellik, Morrisey, & Novitch, 2008).
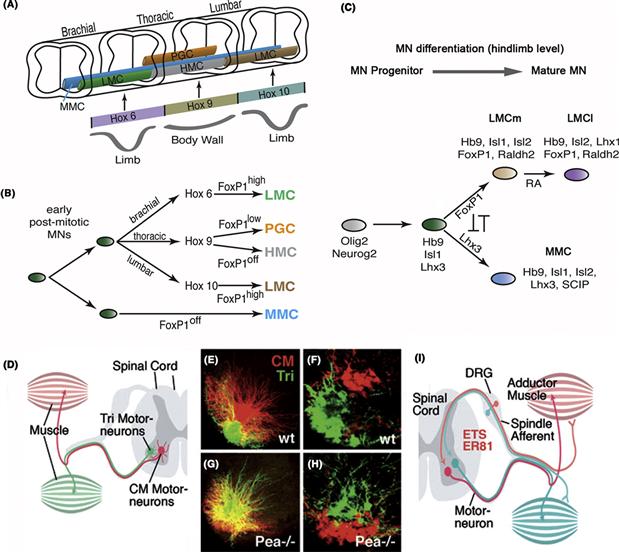
Figure 14.14 Establishment of motor neuron identity along the antero-posterior axis of the spinal cord. (A) Polarized expression of FGF and retinoic acid (RA) is responsible for the regionally specific expression of Hox genes along the antero-posterior axis of the neural tube. In addition, inhibitory interactions among the Hox genes themselves sharpen the boundaries within which a given Hox gene is expressed. Hox genes are differentially expressed along the AP axis of the spinal cord and function in conjunction with the forkhead domain gene FoxP1 to establish motor neuron columnar identity. Motor columns are patterned in a manner that corresponds to the arrangement of their targets at brachial, thoracic, and lumbar levels. The MMC innervates muscles of the body wall and therefore extends along the length of the spinal cord. The LMC innervates limb muscles and is present at brachial and lumbar levels. The HMC and PGC innervate muscles of the body wall and autonomic neurons of the sympathetic ganglia, respectively, at thoracic levels. (B) MNs acquire columnar identity based on differential expression of Hox6/9/10 and FoxP1 off/low/high along the AP axis. In FoxP1 mutants, MNs assume MMC or HMC fates along the entire AP axis and other motor columns do not form. (C) Proposed model of the genetic specification of MN progenitors in lumbar cord. The actions of FoxP1, Lhx3, and retinoic acid (RA) establish the combinatorial expression profiles associated with each of the motor columns in lumbar spinal cord. (D) The motor column is subdivided into pools of motor neurons innervating specific muscles. These pools, including the Tri motor neuron pool and the CM motor neuron pool, occupy discrete locations in the ventral horn of the spinal cord and show distinct dendritic branching patterns. These properties, which depend on the expression of specific transcription factors such as the LIM gene PEA3, can be visualized by backfilling the motor neurons with fluorescent labels injected in the corresponding muscles. (E and F) Two different markers visualize the dendritic arborization (E) and cell body position (F) of the Tri motor neurons (green) and CM motor neurons (red). (G and H) Knockout of the PEA3 gene results in ventral shift of CM motor neuron location, and dorsal expansion of Tri motor neuron dendrites. (I) Reflex circuits between sensory afferents (from muscle spindles) and motor neurons innervating the corresponding muscle are formed because both spindle afferents and motor neurons targeted by them express a common transcription factor, such as ETS ER81 shown here. Among the downstream targets of ETS ER81 are homophilic adhesion molecules that allow the ingrowing sensory axon to “recognize” the proper motor neuron target. Abbreviations: MMC, median motor column; LMC, lateral motor column; HMC, hypaxial motor column; PGC, preganglionic autonomic motor column; RA, retinoic acid.
A–C adapted from Dasen et al. (2008); Rousso et al. (2008). E–H adapted from Vrieseling and Arber (2006).
The lateral motor columns are subdivided into pools of motor neurons that innervate different muscles. One major subdivision within the LMC is between motor neurons that innervate dorsal limb muscles and motor neurons that innervate ventral limb muscles. Transcription factors of the LIM homeodomain (LIM-HD) family are involved in specification, as motor neurons expressing different combinations of LIM-HD factors such as Isl1, Isl2, Lhx1, and Lhx3 are expressed in different motor neuron classes that innervate different muscle groups (Fig. 14.14C). This is particularly well illustrated in the primary motor neurons (Rop, Mip, and Cap) of zebra fish, which can easily be distinguished by their relative rostral to caudal position in each spinal segment, as well as their axon trajectories to distinct muscle regions. These three neurons express different combinations of LIM transcription factors. The identity of these neurons, as judged by their projection to different muscle regions, is influenced by the position in which they develop. These projection patterns can be changed by transplantation in which the position of these neurons is changed prior to axon extension. Transplantations that lead to such switches in projection correlate with induced changes in LIM-HD code suggesting a causal link (Appel et al., 1995).
Motor neurons that innervate specific muscles are grouped into motor pools, which are distinguished by the expression of distinct members of the ETS family of transcription factors. For example, the ETS gene ER81 is expressed in the motor neurons that innervate the limb adductor muscle in chicks, whereas the iliotrochanter motor neurons express the PEA3 ETS gene (Fig. 14.14D–I). The initial expression of these ETS genes depends on peripherally derived signals such as GDNF and coincides with the arrival of motor neuron terminals in the vicinity of their specific targets. In PEA3 knockout mice, the motor neurons that are normally PEA3 positive fail to develop normally so muscles that are normally targets of these neurons are hypoinnervated and become severely atrophic. Not only do these motor neuron pools fail to branch normally within their target muscles, but the cell bodies and dendrites of these motor neurons also are mispositioned within the spinal cord, which is critical for the control of their reflexive and descending innervation (Fig. 14.14E–H). Recent work suggests that these ETS genes may regulate the expression of cell adhesion and axon guidance factors that help motor neurons recognize their target. Interestingly, the sensory afferents that innervate the stretch receptors in a particular muscle express the same ETS gene as the motor neurons that innervate that muscle, suggesting this may help the sensory neuron axons find the dendrites of these motor neurons to complete the connection that is the basis of the monosynaptic stretch reflex.
The motor neuron system in the ventral nerve cord of the Drosophila larva, which is responsible for crawling, displays a relatively simple repetitive organization (Landgraf & Thor, 2006). In each half segment, there are approximately 36 motor neurons arising from about 15 neuroblasts (Fig. 14.15A, B). Some project through the intersegmental nerve (ISN) to innervate the more interior muscles that span the length of each segment and cause the animal to shorten. Others project through the segmental nerve (SN) to innervate the more exterior set of muscles that are more radial in orientation and cause the animal to lengthen. This is somewhat comparable to the division in vertebrate limbs, where the sets of (usually antagonistic) muscles derived from the ventral and dorsal muscle masses are innervated by pools of motor neurons whose axons project along divergent paths. ISN and SN motor neurons are further subdivided into subclasses of motor neurons that innervate distinct muscle territories.
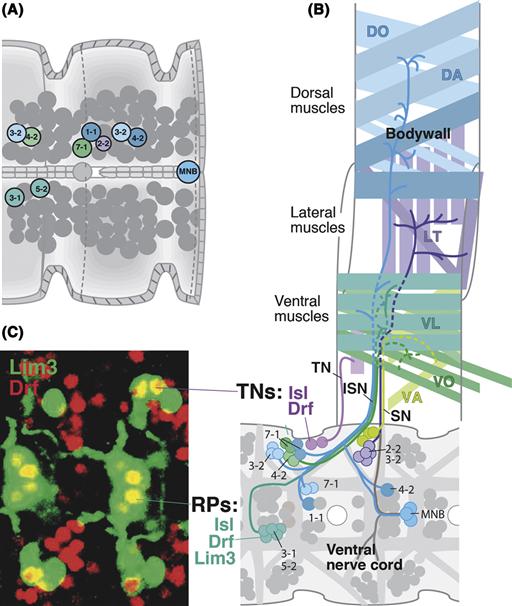
Figure 14.15 Specification of motor neurons in the Drosophila ventral cord. (A) Neuroblast map of two embryonic segments, showing in color the subset of neuroblasts that give rise to motor neurons. (B) Schematic flattened view of one body segment, showing in different colors the pattern of bodywall muscles and motor neurons innervating these muscles. The specific motor neuron(s) innervating each of the 30 muscle fibers comprising the musculature has been mapped. Motor neurons are color coded such that their color matches the one of the neuroblast from which it descends (see A) and the muscle it innervates. As in vertebrates, distinct groups of motor neurons are specified by expressing transcription factors of the LIM and POU family. Shown here and, photographically, in panel C are the RP neurons, expressing the combination Isl, Drf, and Lim3, and the transverse nerve (TN) motor neurons that express only Isl and Drf. Mutations in any of these genes result in motor neuronal abnormalities.
B from Certel and Thor (2004).
No master/selector gene has been identified that specifies generic motor neuron fate in Drosophila, although combinatorial codes of transcription factors specify different motor neuronal subtypes (Landgraf & Thor, 2006). The first subdivision in Drosophila is between ventrally projecting motor neurons, vMNs, and dorsally projecting motor neurons, dMNs. Interestingly, vMN identity is controlled by the combinatorial action of the Nkx6 and HB9 homeobox genes (Broihier & Skeath, 2002; Cheesman, Layden, Von Ohlen, Doe & Eisen, 2004), a similar combination to that used to specify generic motor neuron fate in vertebrates. The vMNs in Drosophila, like the motor neurons in vertebrates, are then specified into subclasses that project along specific nerves to innervate specific muscles, by the further expression of combinatorial codes of LIM-HD factors (Fig. 14.15C). dMNs depend upon other sets of transcription factors: even-skipped (eve) and grain (grn) (Garces & Thor, 2006; Landgraf, Roy, Prokop, VijayRaghavan, & Bate, 1999). In contrast to the LIM-HD factors, Nkx6 and HB9, the vertebrate orthologues of even-skipped (Evx1/2) and grain (Gata2/3) are not involved in vertebrate motor neuron specification. Thus, Drosophila vMNs appear to be evolutionary more similar to vertebrate motor neurons.
What aspects of motor neuron phenotype do combinatorial codes of transcription factors control? This issue has remained elusive, but more recent studies have pointed to two important motoneuron properties: axon pathfinding molecules and electrophysiological aspect of identity. Specifically, Dfr has been found to be involved in the regulation of a specific immunoglobulin-containing cell-adhesion molecule called Beat-1c, and the beat-ic gene is likely a direct target of this transcription factor (Certel & Thor, 2004). Similarly, eve controls Unc-5, a receptor for the pathfinding guidance molecule netrin, in dorsally projecting motor neurons. Eve also controls the expression of genes involved in generating voltage- and ligand-gated currents in dorsally projecting motor neurons (Pym, Southall, Mee, Brand, & Baines, 2006).
The Cerebral Cortex
The cerebral cortex of mammals is the neural structure that is important for conscious thought, perception, and associative learning, as well as providing executive control over voluntary movement. The overall architecture of the cortex is relatively simple both in terms of its areal structure and the general composition of cells that make up the cortex. The neocortex, which forms most of the cerebral cortex in humans, is a six-layered structure, with each layer having its own particular array of neuronal cell types. Differences in the thicknesses and cellular densities of particular layers have enabled histologists to distinguish as many as 100 cytoarchitectonically distinct cortical areas in humans. There are two primary classes of neurons in the cortex. The majority of these cells, approximately 80%, are glutamatergic pyramidal neurons that originate from neuroepithelial cells in the ventricular zone of the telencephalic vesicles. Asymmetric divisions of these cells generate one daughter that remains neuroepithelial and apical, and another daughter that migrates basally. In most instances, the migrating daughter becomes a postmitotic cortical neuron, but as development proceeds, an increasing number of migrating daughter cells travel only a short distance to the subventricular zone, where they divide symmetrically to generate two neurons. The second major class of neurons in the cortex, GABAergic inhibitory interneurons, arise from the ventral telencephalon or ganglionic eminence (Anderson, Eisenstat, Shi & Rubenstein, 1997).
Cortical precursors, once generated, migrate away from the ventricular and subventricular zones, through an intermediate zone that will later be filled with axons, until they arrive at the cortical plate where they stop migrating and begin to differentiate into an enormous variety of specialized cortical cell types. This basal migration is coupled with an inside-out pattern of histogenesis in which the firstborn cells take up the deep layers of the cortical plate and the later-born cells migrate past the earlier generated neurons to occupy more superficial layers (Fig. 14.16A) (Angevine & Sidman, 1961). Cortical cells are specified with respect to their laminar fates before they migrate, for if postmitotic cells from the ventricular zone of a young embryo (destined for deep layers) are transplanted into the ventricular zone of an older one that is generating superficial cells, then the transplanted cells will still migrate to deep layers and have deep-layer fates (Fig. 14.16B, top panel). However, if progenitors from a young ventricular zone are in their last mitotic cycle when they are transplanted to the older environment, they can change their laminar identity, suggesting that cortical cell fate is still undetermined until the cells exit the cell cycle (Fig. 14.16B, middle panel). Interestingly, the reverse experiment of transplanting premitotic progenitors of old donors to younger ventricular zones does not result in these cells assuming earlier, deeper-layer fates (Fig. 14.16B, bottom panel), suggesting that progression of determination is a one-way street (McConnell, 1995).
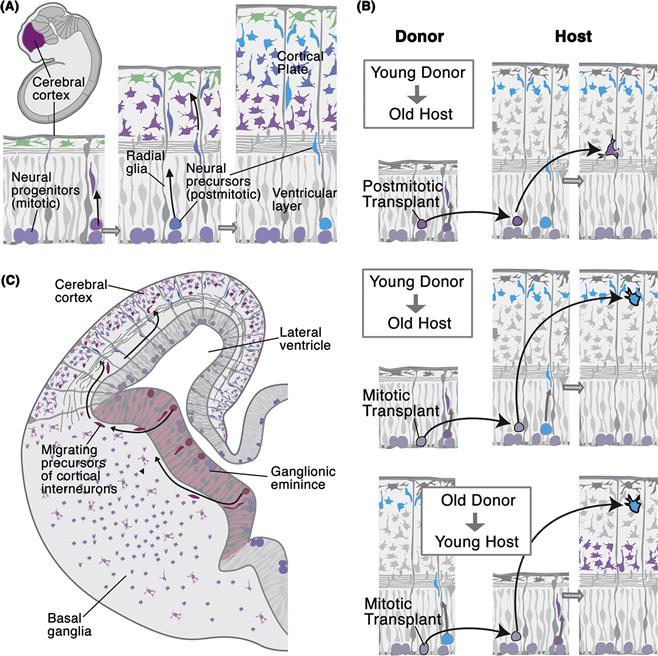
Figure 14.16 Laminar fate determination in the cerebral cortex. (A) Morphogenesis of the mammalian cerebral cortex. Neural precursors are born in the ventricular layer and migrate away from the ventricular surface, following tracks provided by radial glial cells. The firstborn cells are the Cajal-Retzius neurons (left in figure). Later-born neurons accumulate in a dense matrix of cells, the cortical plate (middle of figure). In this plate, neurons are ordered by birth date in such a way that older neurons (magenta) remain in deep layers, and younger neurons (blue) migrate through the deep layers to attain a superficial position (right in figure). (B) Top: If postmitotic ventricular cells from young donors (which normally would become deep cells) are transplanted into an old host, they do not adapt to their new environment and develop as deep layer neurons (arrow). However, if ventricular cells that have not yet exited the cell cycle are transplanted from young donors into an old host (middle panel), they adapt to their new environment and develop as superficial neurons (arrow). If, on the other hand, premitotic progenitors from old donors are transplanted into young brains, they can take later fates and become upper layer neurons. (C) Migration route of GABAergic interneurons from the ganglionic eminences in the ventral telencephalon into the cortical plate in a mammalian embryo.
The specification of excitatory neuron cell types appears to be largely under the control of intrinsic determinants, many of which are transcription factors. Pax6, Emx1, FoxG1, and Lhx2 function at early times to regulate arealization and to broadly specify cortical progenitors. Cell type specification is closely linked to the laminar position, and while a number of genes have been identified that mark neurons in different laminae, in most instances the programs that specify neuronal cell identity are still poorly defined. Nonetheless, some key components of the developmental switch that controls the differentiation of subcortically versus intracortically projecting pyramidal neurons have been identified (Leone, Srinivasan, Chen, Alcamo, & McConnell, 2008). Neurons in laminar V project subcortically to the superior colliculus, hindbrain, and spinal cord, and they are derived from cells that express the Fezf2 transcription factor. Fezf2 in turns activates expression of the Ctip2 zinc-finger factor, while repressing expression of Satb2, a chromatin remodeling transcription factor. In the absence of Fezf2, glutamatergic pyramidal neurons, neurons in lamina V do not express Ctip2 and instead of sending their axons subcortically, they instead extend axons across the corpus callosum to other cortical areas. Interestingly, Satb2, is markedly upregulated in Fezf2 mutants. Satb2 is required for neurons in the cortex to cross the corpus callosum. Overexpression of Satb2 represses Ctip2 expression and results in fewer lamina V cells sending axons subcortically to the hindbrain and spinal cord. Conversely, overexpression of Fezf2 generates an excess of subcortically projecting neurons.
As noted previously, GABAergic neurons arise from the medial and caudal ganglionic eminences (MGE and CGE). They then migrate tangentially to the cortex, where they intercalate into the developing cortical plate and form connections with glutamatergic pyramidal neurons. Multiple inhibitory interneuron cell types are derived from these two domains (Anderson et al., 1997; Batista-Brito and Fishell, 2009). The MGE produces somatostatin-positive (SST+) interneurons and fast spiking interneurons that express the calcium-binding protein parvalbumin (PV) and the potassium channel Kv3.1, which enable neurons to generate trains of high-frequency action potentials. While fast spiking PV neurons arise from ventral regions of the MGE, there is also a temporal gradient of PV versus SST interneuron differentiation, revealing spatial and temporal cues that regulate the development of these two cell types. Bipolar neuron cell types (calretinin and VIP cells) that are generated from the CGE develop later than their MGE-derived counterparts. Nkx2.1 is a key early determinant of GABAergic interneuron identity, by promoting MGE fates over LGE and CGE fates. This function may reflect an early role for Nkx2.1 in patterning the subpallium. Loss of Nkx2.1 results in a marked depletion of MGE derived neuronal cell types including PV+ and SST+ interneurons. Dlx1/2 and Mash1, which is regulated by Dlx1/2, are required for the specification of GABAergic cell types in the forebrain. These genes are global determinants of GABA neuron differentiation, as they are also required for the development of GABAergic neurons in the striatum, including medium spiny neurons.
Extrinsic signaling contributes to the laminar specification of cortical progenitors as indicated by experiments showing that BDNF is able to induce late-born progenitors to acquire early-born laminar fates. Extrinsic signals also appear to control the migration and distribution of inhibitory interneurons in the cortex. The loss of subcortically projecting pyramidal neurons in mice that lack Fezf2 is associated with changes in the laminar distribution of PV+ and SST+ interneurons, with reduced numbers of both cell types in lamina V (Lodato et al., 2011). Neuronal activity also regulates the development of subsets of inhibitory interneurons, including migration and their axonal and dendritic morphology. It therefore appears that neuronal differentiation in the cortex is regulated by time of birth, intrinsic transcriptional determinants (some of which may be activated in a time dependent manner—e.g., Fezf2 is expressed in deep-layer pyramidal neurons and is downregulated when upper-layer neurons that are born later are generated), and by extrinsic signals and activity as these cells migrate and intercalate into the cortical circuitry. Neuronal activity also plays a prominent role in regulating neuronal connectivity in the cortex. This is perhaps best exemplified by the activity-dependent refinement of the ocular dominance columns in the visual cortex (see Chapter 19).
Conclusions
The generation of neurons involves a variety of extrinsic signals and intrinsic transcription factors that confer individual identities on postmitotic neurons. Whether vertebrate or invertebrate, this process of determination involves a similar set of molecular and cellular mechanisms, such as regional and temporal identity genes, proneural genes, signaling molecules and receptors, the Notch pathway, and asymmetric cell division. All these conspire, over the course of neuronal generation, to create progenitors with distinct combinations of transcription factors that uniquely specify the fates of the differentiating neurons by regulating the expression of genes that affect specific features of the cell such as axon and dendrite growth and targeting, the topic of the next chapter.
References
1. Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476.
2. Angevine JBJ, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768.
3. Appel B, Korzh V, Glasgow E, et al. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125.
4. Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Current Topics in Developmental Biology. 2009;87:81–118.
5. Betschinger J, Knoblich JA. Dare to be different: Asymmetric cell division in Drosophila, C elegans and vertebrates. Current Biology. 2004;14:R674–R685.
6. Broihier HT, Skeath JB. Drosophila homeodomain protein dHb9 directs neuronal fate via cross-repressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50.
7. Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202.
8. Campos-Ortega JA. Genetic mechanisms of early neurogenesis in Drosophila melanogaster. Molecular Neurobiology. 1995;10:75–89.
9. Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends in Neurosciences. 2006;29:563–570.
10. Certel SJ, Thor S. Specification of Drosophila motoneuron identity by the combinatorial action of POU and LIM-HD factors. Development. 2004;131:5429–5439.
11. Cheesman SE, Layden MJ, Von Ohlen T, Doe CQ, Eisen JS. Zebrafish and fly Nkx6 proteins have similar CNS expression patterns and regulate motoneuron formation. Development. 2004;131:5221–5232.
12. Cheng L, Arata A, Mizuguchi R, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nature Neuroscience. 2004;7:510–517.
13. Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641.
14. Choksi SP, Southall TD, Bossing T, et al. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Developmental Cell. 2006;11:775–789.
15. Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933.
16. Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertiores for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316.
17. Del Barrio MG, Taveira-Marques R, Muroyama Y, et al. A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development. 2007;134:3427–3436.
18. Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70.
19. Elliott J, Jolicoeur C, Ramamurthy V, Cayouette M. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron. 2008;60:26–39.
20. Flores GV, Duan H, Yan H, et al. Combinatorial signaling in the specification of unique cell fates. Cell. 2000;103:75–85.
21. Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438.
22. Garces A, Thor S. Specification of Drosophila aCC motoneuron identity by a genetic cascade involving even-skipped, grain and zfh1. Development. 2006;133:1445–1455.
23. Glasgow SM, Henke RM, MacDonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469.
24. Goulding MD, Lumsden A, Gruss P. Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development. 1993;117:1001–1016.
25. Gowan K, Helms AW, Hunsaker TL, et al. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232.
26. Harris WA, Stark WS, Walker JA. Genetic dissection of the photoreceptor system in the compound eye of Drosophila Melanogaster. Journal of Physiology. 1976;256:415–439.
27. Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521.
28. Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1:20–29.
29. Jones BW. Transcriptional control of glial cell development in Drosophila. Developmental Biology. 2005;278:265–273.
30. Jusuf PR, Almeida AD, Randlett O, Joubin K, Poggi L, Harris WA. Origin and determination of inhibitory cell lineages in the vertebrate retina. Journal of Neuroscience. 2011;31:2549–2562.
31. Kempermann G. Seven principles in the regulation of adult neurogenesis. The European Journal of Neuroscience. 2011;33:1018–1024.
32. Kessaris N, Pringle N, Richardson WD. Ventral neurogenesis and the neuron-glial switch. Neuron. 2001;31:677–680.
33. Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362.
34. Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience. 2009;32:149–184.
35. Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Developmental Biology. 2004;269:1–17.
36. Lamb TD, Collin SP, Pugh Jr EN. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience. 2007;8:960–976.
37. Landgraf M, Thor S. Development and structure of motoneurons. International Review of Neurobiology. 2006;75:33–53.
38. Landgraf M, Roy S, Prokop A, VijayRaghavan K, Bate M. Even-skipped determines the dorsal growth of motor axons in Drosophila. Neuron. 1999;22:43–52.
39. Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Current Opinion in Neurobiology. 2008;18:28–35.
40. Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nature Reviews Neuroscience. 2001;2:109–118.
41. Lodato S, Rouaux C, Quast KB, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779.
42. Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–938.
43. Marchetto MC, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539.
44. McConnell SK. Constructing the cerebral cortex: Neurogenesis and fate determination. Neuron. 1995;15:761–768.
45. Mollereau B, Domingos PM. Photoreceptor differentiation in Drosophila: From immature neurons to functional photoreceptors. Developmental Dynamics. 2005;232:585–592.
46. Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nature Neuroscience. 2006;9:770–778.
47. Muller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes & Development. 2005;19:733–743.
48. Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208.
49. Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Research. 2008;1192:90–98.
50. Peng CY, Yajima H, Burns CE, et al. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827.
51. Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934.
52. Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. Journal of Cell Biology. 2005;171:991–999.
53. Pym EC, Southall TD, Mee CJ, Brand AH, Baines RA. The homeobox transcription factor Even-skipped regulates acquisition of electrical properties in Drosophila neurons. Neural Development. 2006;1:3.
54. Qian X, Shen Q, Goderie SK, et al. Timing of CNS cell generation: A programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80.
55. Reinke R, Zipursky SL. Cell–cell interaction in the Drosophila retina: The bride of sevenless gene is required in photoreceptor cell R8 for R7 cell development. Cell. 1988;55:321–330.
56. Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240.
57. Schlosser G. Induction and specification of cranial placodes. Developmental Biology. 2006;294:303–351.
58. Skeath JB, Carroll SB. Regulation of proneural gene expression and cell fate during neuroblast segregation in Drosophila. Development. 1992;114:939–946.
59. Stam FJ, Hendricks TJ, Zhang J, et al. Renshaw cell interneuron specialization is controlled by a temporally restricted transcription factor program. Development 2012; [Epub ahead of print].
60. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126 663–76.
61. Takebayashi S, Yamamoto N, Yabe D, et al. Multiple roles of Notch signaling in cochlear development. Developmental Biology. 2007;307:165–178.
62. Vallier L, Pedersen RA. Human embryonic stem cells: an in vitro model to study mechanisms controlling pluripotency in early mammalian development. Stem Cell Reviews. 2005;1:119–130.
63. Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452.
64. Waid DK, McLoon SC. Ganglion cells influence the fate of dividing retinal cells in culture. Development. 1998;125:1059–1066.
65. Watanabe T, Raff MC. Rod photoreceptor development in vitro: Intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–467.
66. Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397.
67. Wodarz A, Huttner WB. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mechanisms of Development. 2003;120 1297–309.
68. Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807.
69. Zipursky SL, Rubin GM. Determination of neuronal cell fate: Lessons from the R7 neuron of Drosophila. Annual Review of Neuroscience. 1994;17:373–397.