
IN CONTEXT
Physics
1754 Benjamin Franklin proves that lightning is natural electricity with his famous kite experiment.
1767 Joseph Priestley publishes a comprehensive account of static electricity.
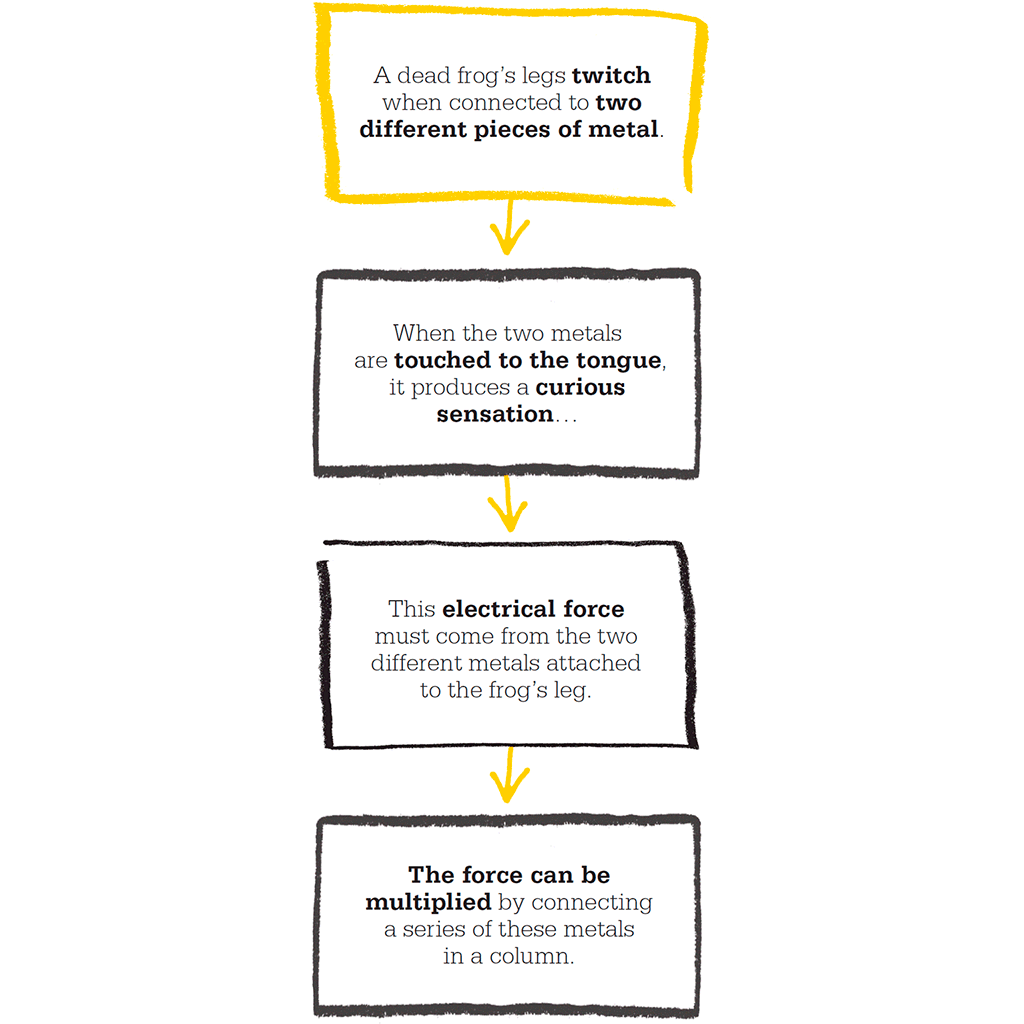
1780 Luigi Galvani conducts his frog’s legs experiments with “animal electricity”.
1800 English chemists William Nicholson and Anthony Carlisle use a Voltaic pile to split water into its two elements, oxygen and hydrogen.
1807 Humphry Davy isolates the elements potassium and sodium using electricity.
1820 Hans Christian Ørsted reveals the link between magnetism and electricity.
For centuries, philosophers had wondered at the terrifying power of lightning, and also at the way in which sparks can be drawn from solids such as amber when rubbed with a silk cloth. The Greek word for amber was “electron”, and the sparking phenomenon became known as static electricity.
In an experiment of 1754, Benjamin Franklin flew a kite into a thunderstorm and showed that these two phenomena were closely related. When he saw sparks flying from a brass key tied to the kite’s line, he proved that the clouds were electrified, and that lightning is also a form of electricity. Franklin’s work inspired Joseph Priestley to publish a comprehensive work on The History and Present State of Electricity in 1767. But it was the Italian Luigi Galvani, a lecturer in anatomy at the University of Bologna, who, in 1780, took the first major steps towards understanding electricity when he noticed a frog’s leg twitch.
Galvani was investigating a theory that animals are driven by “animal electricity”, whatever that was, and was dissecting frogs to look for evidence of this. He noticed that if there was a machine nearby generating static electricity, a frog’s leg lying on the bench suddenly twitched, even though the frog was long dead. The same thing happened when a frog’s leg was hung on a brass hook that came into contact with an iron fence. Galvani believed this evidence supported his belief that electricity was coming from the frog itself.

Luigi Galvani is shown here conducting his famous frog’s legs experiment. He believed that animals were driven by an electrical force, which he called “animal electricity”.
Volta’s breakthrough
Galvani’s younger colleague Alessandro Volta, a professor of natural philosophy, was intrigued by Galvani’s observations and was initially convinced by his theory.
Volta himself had a notable background in electricity experiments. In 1775, he had invented the “electrophorus”, a device that provided an instant source of electricity for an experiment (the modern equivalent is a capacitor). It consisted of a resin disc rubbed with cat fur to give it a static electric charge. Each time a metal disc was placed over the resin, the charge was transferred, electrifying the metal disc.
Volta stated that Galvani’s animal electricity was “among the demonstrated truths”. But he soon began to have his doubts. He came to the conclusion that the electricity causing the frog’s legs to twitch on the hook came from the touching of the two different metals (the brass and the iron). He published his ideas in 1792 and 1793, and set about investigating the phenomenon.
Volta found that a single junction of two different metals did not produce much electricity, although there was enough for him to feel a curious sensation with his tongue. But then he had the brilliant idea of multiplying the effect by making a series of such junctions connected by salt water. He took a small disc of copper, then placed a disc of zinc on top, then a piece of cardboard soaked in salt water, then another disc of copper, zinc, salty wet cardboard, copper, zinc, and so on, until he had a column, or stack. In other words, he created a pile, or “battery”. The point of the salty wet cardboard was to carry the electricity without letting the metals either side of it come into contact with each other.
The result was, literally, electrifying. Volta’s crude battery probably produced only a few volts (the electrical unit named after him), but that was enough to make a tiny spark when the two ends were connected by a piece of wire, and enough to give him a mild electric shock.
"Each metal has a certain power, which is different from metal to metal, of setting the electric fluid in motion."
Alessandro Volta
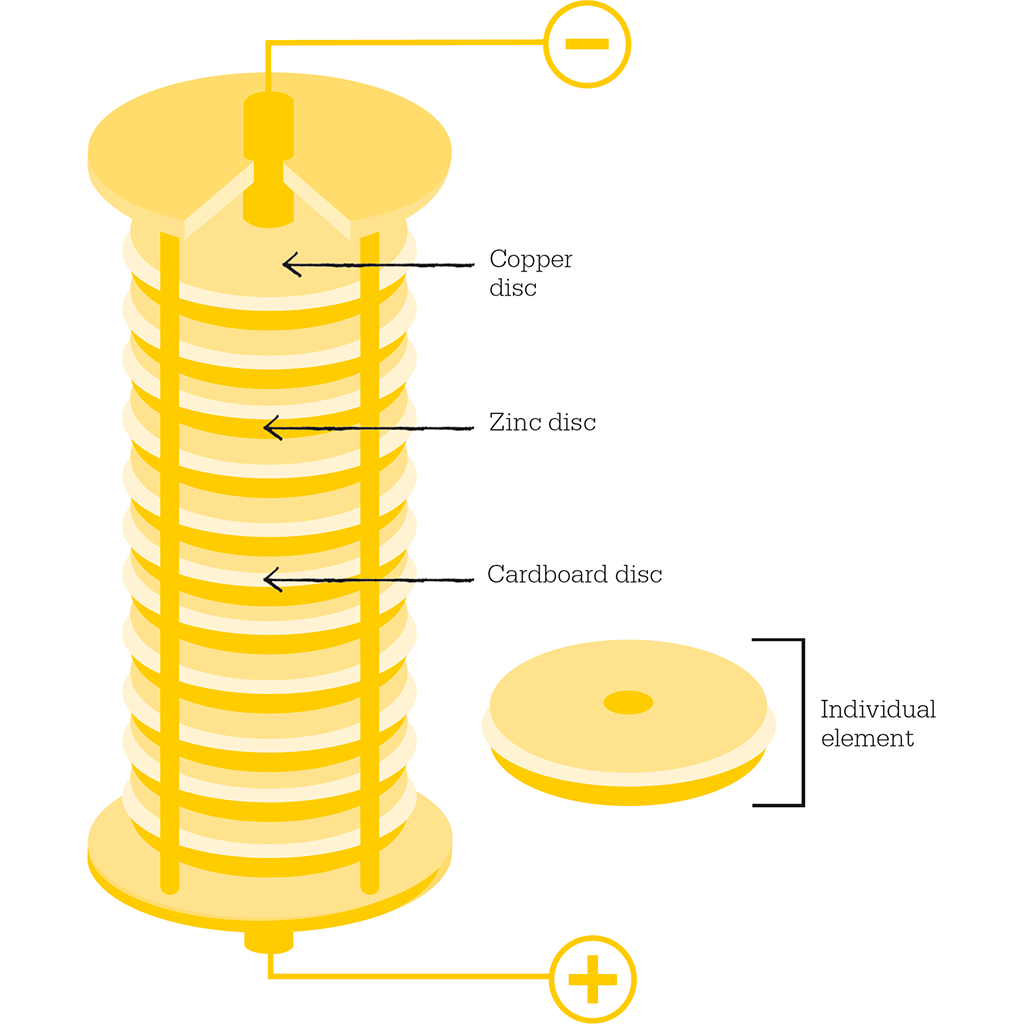
This diagram of a voltaic pile shows the copper and zinc discs separated by cardboard soaked in salt water. Volta’s original piles contained an additional zinc disc at the bottom, and an additional copper disc at the top. These were later shown to be unnecessary to produce the electrical current.
The news spreads
Volta made his discovery in 1799, and news spread rapidly. He demonstrated the effect to Napoleon Bonaparte in 1801, but more importantly, in March 1800, he had reported his results in a long letter to Sir Joseph Banks, president of the Royal Society in Britain. The letter was entitled
“On the electricity excited by the mere Contact of conducting Substances of different Kinds”, and in it Volta describes his apparatus: “I place then horizontally, on a table or any other stand, one of the metallic pieces, for example one of silver, and over the first I adapt one of zinc; on the second I place one of the moistened discs, then another plate of silver followed immediately by another of zinc…I continue to form…a column as high as possible without any danger of its falling.”
Without a buzzer or a semiconductor to detect voltage, Volta used his body as a detector, and did not seem to mind getting electric shocks: “I receive from a column formed of twenty pairs of pieces (not more) shocks which affect the whole finger with considerable pain.” He then describes a more elaborate apparatus, consisting of a series of cups or drinking glasses, each containing salt water, arranged in a line or a circle. Each pair is connected by a piece of metal that dips into the liquid in each cup. One end of this metal is silver, the other zinc, and these metals may be soldered together or connected by a wire of any metal, provided that only the silver dips into the liquid in one cup, and only the zinc into the next. He explains that this is in some ways more convenient than the solid pile, albeit more cumbersome.
Volta describes in detail the various unpleasant sensations that result from putting one hand in the bowl at one end of the chain and touching a wire attached to the other end to the forehead, eyelid, or tip of the nose: “I feel nothing for some moments; afterwards, however, there begins at the part applied to the end of the wire, another sensation, which is a sharp pain (without shock), limited precisely to the point of contact, a quivering, not only continued, but which always goes on increasing to such a degree, that in a little time it becomes insupportable, and does not cease till the circle is interrupted.”

Volta demonstrated his electric pile to Napoleon Bonaparte at the French National Institute in Paris in 1801. Napoleon was sufficiently impressed to make Volta a count the same year.
Battery mania
That his letter reached Banks at all is surprising, since the Napoleonic wars were in progress, but Banks immediately spread the word to anyone who might be interested. Within weeks, people all over Britain were making electric batteries and investigating the properties of current electricity. Before 1800, scientists had had to work with static electricity, which is difficult and unrewarding. Volta’s invention allowed them to find out how a range of materials – liquids, solids, and gases – react to a live electrical current.
Among the first to work with Volta’s discovery were William Nicholson, Anthony Carlisle, and William Cruickshank, who, in May 1800, made their own “pile of thirty-six half crowns with the correspondent pieces of zinc and pasteboard” and passed the current through platinum wires into a tube filled with water. The bubbles of gas that appeared were identified as two parts of hydrogen and one part of oxygen. Henry Cavendish had shown that the formula of water is H2O, but this was the first time anyone had split water into its separate elements.
Volta’s pile was the ancestor of all modern batteries, used in everything from hearing aids to trucks and aircraft. Without batteries, many of our everyday devices would not work.
"The language of experiment is more authoritative than any reasoning: facts can destroy our ratiocination [logical argument] – not vice versa."
Alessandro Volta
Reclassifying metals
In addition to kick-starting the study of current electricity, and thereby not only creating a new branch of physics but rapidly advancing the development of modern technology, Volta’s pile led to a whole new chemical classification of metals, for he tried a variety of pairs of metals in his pile, and found that some worked much better than others. Silver with zinc made an excellent combination, as did copper with tin, but if he tried silver and silver, or tin and tin, he got no electricity at all; the metals had to be different. He showed that metals could be arranged in a sequence such that each became positive when placed in contact with the one next below it in the series. This electrochemical series has been invaluable to chemists ever since.
Who was right?
An ironic aspect of this story is that Volta started investigating the touching of different metals only because he doubted Galvani’s hypothesis. Yet Galvani was not entirely wrong – our nerves do indeed work by sending electrical impulses around the body – while Volta himself did not get his theory entirely right. He believed that the electricity arose from just the touching together of two different metals, whereas Humphry Davy later showed that something could not come from nothing. When electricity is being generated, something else must be consumed. Davy suggested that there was a chemical reaction going on, and this led him to further important discoveries about electricity.
ALESSANDRO VOLTA

Born in 1745 in Como, northern Italy, Alessandro Giuseppe Antonio Anastasio Volta was brought up in an aristocratic, religious family who hoped that he would become a priest. Instead he became interested in static electricity, and, in 1775, he made an improved device for generating it, called the “electrophorus”. He discovered methane in the atmosphere at Lake Maggiore in 1776, and investigated its combustion by the novel method of igniting it with an electrical spark inside a sealed glass vessel.
In 1779, Volta was appointed professor of physics at the University of Pavia, a post he held for 40 years. Towards the end of his life, he pioneered the remotely operated pistol, whereby an electric current travelled 50km (30 miles) from Como to Milan and fired a pistol. This was the forerunner of the telegraph, which uses electricity to communicate. The unit of electrical potential, the volt, is named after him.
Key work
1769 On the Attractive Force of Electrical Fire
See also: Henry Cavendish • Benjamin Franklin • Joseph Priestley • Humphry Davy • Hans Christian Ørsted • Michael Faraday