
IN CONTEXT
Chemistry
1770s Antoine Lavoisier and others show that water and salt can return to their former state after heating, but sugar or wood cannot.
1807 Jöns Jakob Berzelius suggests a fundamental difference between organic and inorganic chemicals.
1852 British chemist Edward Franklin suggests the idea of valency, the ability of atoms to combine with other atoms.
1858 British chemist Archibald Couper suggests the idea of bonds between atoms, explaining how valency works.
1858 Couper and August Kekulé propose that organic chemicals are made by chains of bonded carbon atoms with side branches of other atoms.
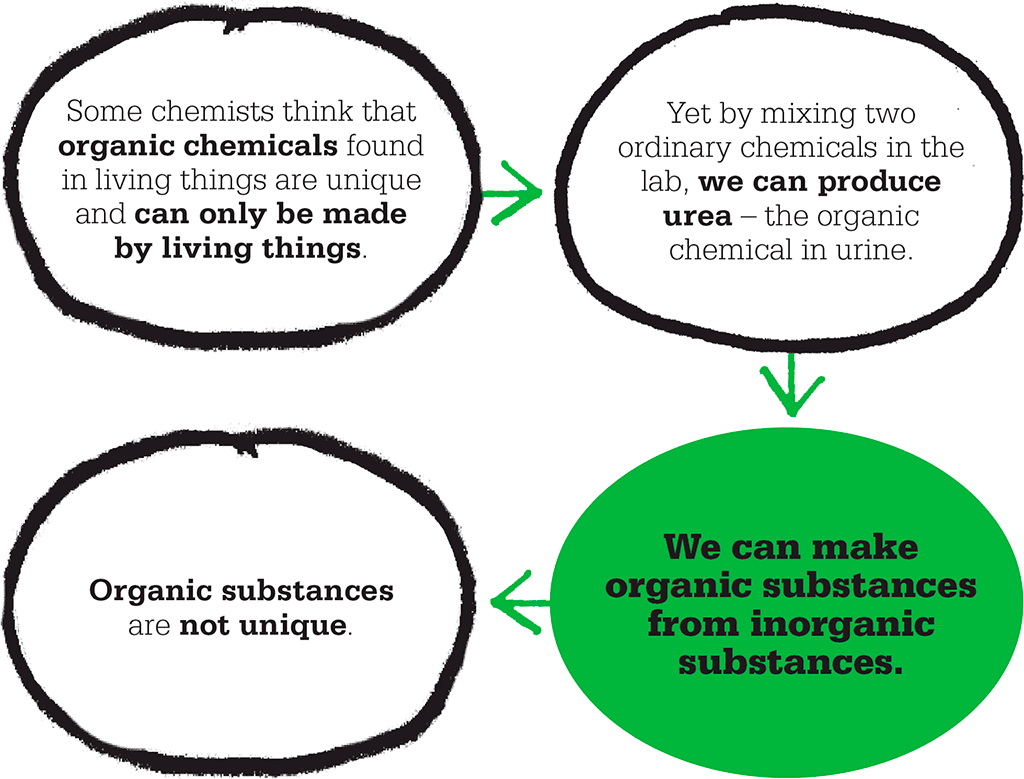
In 1807, the Swedish chemist Jöns Jakob Berzelius suggested that a fundamental difference existed between the chemicals involved in living things and all other chemicals. These unique, “organic” chemicals, Berzelius argued, could only be assembled by living things themselves and, once broken down, could not be remade artificially. His idea chimed with the prevailing theory known as “vitalism”, which held that life was special and that living things were endowed with a “life force” beyond the understanding of chemists. So it came as a surprise when the pioneering experiments of a German chemist called Friedrich Wöhler showed that organic chemicals are not unique at all, but behave according to the same basic rules as all chemicals.
We now know that organic chemicals comprise a multitude of molecules based on the element carbon. These carbon-based molecules are indeed essential components of life, but many can be synthesized from inorganic chemicals – as Wöhler discovered.
Chemistry rivals
Wöhler’s breakthrough came about because of a scientific rivalry. In the early 1820s, Wöhler and fellow chemist Justus von Liebig both came up with identical chemical analyses for what seemed to be two very different substances – silver fulminate, which is explosive, and silver cyanate, which is not. Both men assumed that the other had got his results wrong, but after corresponding, they found they were both right. This group of compounds led chemists to realize that substances are defined not just by the number and kinds of atoms in the molecule but also by the atoms’ arrangement. The same formula may apply to different structures with different properties – these different structures were later named isomers by Berzelius.
Wöhler and Liebig went on to forge a brilliant partnership, but it was Wöhler alone who, in 1828, stumbled upon the truth about organic chemicals.

Widely used in fertilizers, urea is rich in nitrogen, which is essential to the growth of plants. Synthetic urea, first made by Wöhler, is now a key raw material in the chemical industry.
The Wöhler synthesis
Wöhler was mixing silver cyanate with ammonium chloride, expecting to get ammonium cyanate. Instead, he got a white substance that had different properties from ammonium cyanate. The same powder appeared when he mixed lead cyanate with ammonium hydroxide. Analysis showed the white powder to be urea – an organic substance that is a key component of urine, and has the same chemical formula as ammonium cyanate. According to Berzelius’s theory, it could be made only by living things – yet Wöhler had synthesized it from inorganic chemicals. Wöhler wrote to Berzelius: “I must tell you that I can make urea without the use of kidneys”, explaining that urea was in fact an isomer of ammonium cyanate.
The significance of Wöhler’s discovery took many years to sink in. Even so, it showed the way for the development of modern organic chemistry, which not only reveals how all living things depend on chemical processes, but enables the artificial synthesis of valuable organic chemicals on a commercial scale. In 1907, a synthetic polymer called Bakelite was produced from two such chemicals and ushered in the “Age of Plastics” that shaped the modern world.
FRIEDRICH WÖHLER

Born in Eschersheim, near Frankfurt in Germany, Friedrich Wöhler trained in obstetrics at the University of Heidelberg. But chemistry was his passion and, in 1823, he went to study with Jöns Jakob Berzelius in Stockholm. On his return to Germany, he embarked on a remarkable and varied career in chemical research and innovation.
Besides the first artificial synthesis of an organic substance, Wöhler’s many discoveries – often made with Justus von Liebig – included aluminium, beryllium, yttrium, titanium, and silicon. He also helped to develop the idea of “radicals” – basic molecular groups from which other substances are built. Although later disproved, this theory paved the way for today’s understanding of how molecules assemble. In later years, Wöhler became an authority on the chemistry of meteorites and helped set up a factory for purifying nickel.
Key works
1830 Summary of Inorganic Chemistry
1840 Summary of Organic Chemistry
See also: Antoine Lavoisier • John Dalton • Jöns Jakob Berzelius • Leo Baekeland • August Kekulé