
IN CONTEXT
Chemistry
1871 Charles Darwin suggests that life might have begun in “some warm little pond”.
1922 Russian biochemist Alexander Oparin proposes that complex compounds might have formed in a primitive atmosphere.
1952 In the USA, Kenneth A Wilde passes 600-volt sparks through a mixture of carbon dioxide and water vapour, and obtains carbon monoxide.
1961 Spanish biochemist Joan Oró adds further likely chemicals to the Urey–Miller mix and obtains molecules vital for DNA, among others.
2008 Miller’s former student Jeffrey Bada and others use newer, more sensitve techniques to obtain many more organic molecules.
Scientists have long pondered the origin of life. In 1871, Charles Darwin wrote in a letter to his friend Joseph Hooker, “But if…we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, lights, heat, electricity etc present, that a protein compound was chemically formed ready to undergo still more complex changes…” In 1953, American chemist Harold Urey and his student Stanley Miller found a way to replicate Earth’s early atmosphere in the laboratory, and generated from inorganic matter organic (carbon-based) compounds that are essential to life.
Before the Urey–Miller experiment, advances in chemistry and astronomy had analysed the atmospheres on the other, lifeless planets in the Solar System. In the 1920s, Soviet biochemist Alexander Oparin and British geneticist J B S Haldane independently suggested that if conditions on prebiotic (pre-life) Earth resembled those planets, then simple chemicals could have reacted together in a primordial soup to form more complex molecules, from which living things might have evolved.
Recreating Earth’s early atmosphere
In 1953, Urey and Miller carried out the first prolonged experiment to test the Oparin–Haldane theory. In a closed series of connected glass flasks, sealed from the atmosphere, they placed water and a mixture of gases thought to have been present in Earth’s primitive atmosphere – hydrogen, methane, and ammonia. The water was heated so that water vapour formed and wafted its way round all the flasks in the closed loop. In one of the flasks was a pair of electrodes, between which sparks were passed continuously to represent lightning – one of the hypothetical triggers for primordial reactions. The sparks provided enough energy to break up some of the molecules, and so generate highly reactive forms that would go on to react with other molecules.
Within a day, the mixture had turned pink, and after two weeks Urey and Miller found that at least 10 per cent of the carbon (from the methane) was now in the form of other organic compounds. Two per cent of the carbon had formed amino acids, which are the vital building blocks of the proteins in all living things. Urey encouraged Miller to send a paper about the experiment to the journal Science, which published it as “Production of amino acids under possible primitive earth conditions”. The world could now imagine how Darwin’s “warm little pond” may have generated the first life forms.
In an interview, Miller said that “just turning on the spark in a basic prebiotic experiment will yield amino acids”. Scientists later found, using better equipment than was available in 1953, that the original experiment had produced at least 25 amino acids – more than are found in nature. Since Earth’s early atmosphere almost certainly contained carbon dioxide, nitrogen, hydrogen sulphide, and sulphur dioxide released from volcanoes, a much richer mixture of organic compounds might well have been created then – and was indeed formed in subsequent experiments. Meteorites containing dozens of amino acids, some found on Earth and others not, have also spurred on the search for signs of life on planets beyond the Solar System.
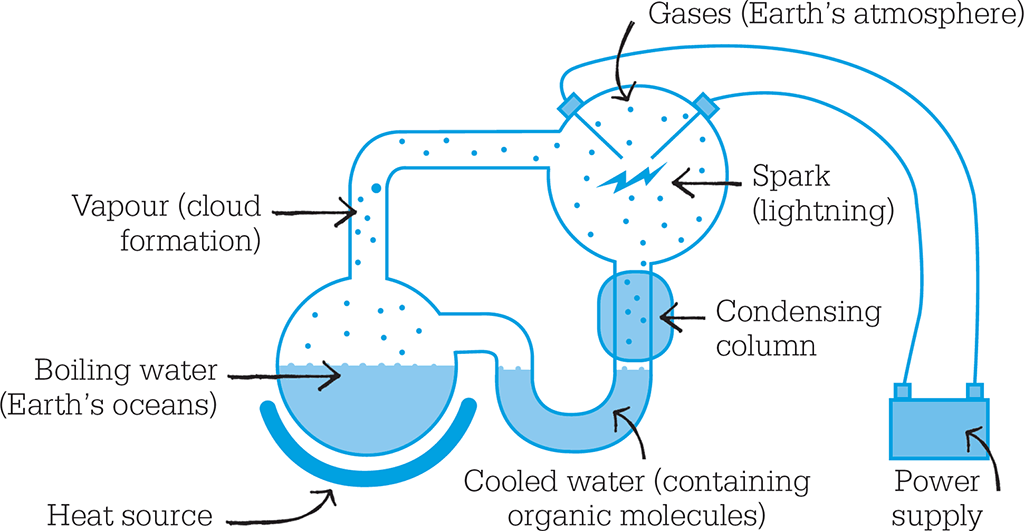
Laboratory apparatus replicated the effect of lightning on early Earth’s primitive atmosphere, in a continual loop of chemical reactions.
"My study [of the Universe] leaves little doubt that life has occurred on other planets. I doubt if the human race is the most intelligent form of life."
Harold C Urey
HAROLD UREY AND STANLEY MILLER
Harold Clayton Urey was born in Walkerton, Indiana, USA. His work on the separation of isotopes led to the discovery of deuterium, which won him the Nobel Prize in Chemistry in 1934. He went on to develop enrichment of uranium-235 by gaseous diffusion, which was crucial for the Manhattan Project’s development of the first atomic bomb. After his prebiotic experiments with Stanley Miller in Chicago, he later moved to San Diego, and studied the Moon rocks brought back by Apollo 11.
Stanley Lloyd Miller was born in Oakland, California. After studying chemistry at the University of California at Berkeley, he was a teaching assistant at Chicago, and worked with Harold Urey. He later became a professor at San Diego.
Key work
1953 Production of Amino Acids under Possible Primitive Earth Conditions
See also: Jöns Jakob Berzelius • Friedrich Wöhler • Charles Darwin • Fred Hoyle