56 | MAGNETIC RESONANCE SPECTROSCOPY STUDIES IN SUBSTANCE ABUSERS
LINDA CHANG, CHRISTINE C. CLOAK, AND JOHN L. HOLT
Magnetic resonance spectroscopy (MRS) is a quantitative technique that has been used in chemistry laboratories to identify chemical structures since the 1950s. More recently, MRS has become a useful imaging modality that can be applied both in vivo noninvasively and ex vivo in tissue extracts. Therefore, MRS is particularly useful as a clinical translational tool for evaluations and longitudinal monitoring of brain abnormalities associated with various brain disorders, including addiction to drugs. When performed in vivo, MRS can be performed in a select voxel (or volume of interest), which is typically referred to as localized or single voxel MRS, or across a grid of voxels in a slice to form an image of the metabolite of interest as in MRS imaging (MRSI) (Fig. 56.1). More advanced techniques also allow multiple slices of metabolite maps to be obtained with MRSI, so that a larger portion of the brain can be assessed.
Although the overwhelming majority of MRS studies in brain disorders focus on the proton (1H) resonances associated with various brain metabolites and chemicals, resonances from other atomic nuclei also can be measured with MRS. Molecules with other nuclei include many that are relevant to neurobiology and neuropharmacology (e.g., lithium [7Li], carbon [13C], fluorine [19F], sodium [23Na], and phosphorus [31P]). Owing to the requirement of specialized MRS equipment (e.g., specific coils), costs, and limited expertise, however, only few studies have employed the other nuclei, specifically 31P MRS or 13C MRS, to study brain abnormalities associated with drug abuse. We will discuss these MRS studies and how they can provide additional insights into the neuropathophysiology associated with major categories of drugs abused. At the end of the chapter, we will also compare and contrast how the brain may be affected by these different types of drugs, and some technical issues to be considered in the use of MRS to assess brain changes and to monitor treatment effects.
BRAIN METABOLITES MEASURED WITH MAGNETIC RESONANCE SPECTROSCOPY
Proton MRS can measure several major brain metabolites (see Fig. 56.1). The concentrations of these metabolites represent neuronal or glial cellular density or health, and hence are useful for assessing possible brain alterations associated with drug exposure or abuse. The metabolites observable with MRS are different from those measured in most other neurochemical assays. Specifically, 1H-MRS detects proton resonances from metabolites that are found in large (millimolar) quantities, whereas most of the neurochemicals measured in basic science research laboratories are in much smaller quantities (e.g., less than micromolar concentrations). Typical brain metabolites observed include N-acetylaspartate (NAA) at 2.02 ppm, glutamate (Glu) at 2.1–2.5 ppm with overlapping resonances from glutamine (Gln) and gamma amino butyric acid (GABA), total creatine (tCR) at 3.03 and 3.94 ppm, choline-containing compounds (Cho) at 3.2 ppm, myoinositol (MI) at 3.56 ppm, and less often lactate (Lac) at 1.35 ppm.
NAA is a putative neuronal marker, because it is found primarily in mature neurons and is reduced in conditions of neuronal damage or loss (Birken and Oldendorf, 1989). NAA is localized primarily in the neuronal cytosol and is the second most abundant amino acid in the brain. Although NAA is not known to have specific neurophysiological properties, it serves as an acetyl donor, an initiator of protein synthesis or a carbon transfer source across the mitochondrial membrane (Tsai and Coyle, 1995). Therefore, lower NAA has been reported in brain disorders that are associated with neuronal injury or mitochondrial dysfunction. Lower than normal brain NAA levels were reported in hypoxia, cerebral infarction, head trauma, dementias, chronic HIV infection, neurodegenerative disorders, and some individuals with substance abuse (Schuff et al., 2006) as described in the following.
Glu is an amino acid involved in the tricarboxylic acid cycle and is the major excitatory neurotransmitter in the CNS. Since the majority of the neurons in the brain are glutamatergic, Glu levels typically correlate well with NAA levels on MRS. Excess amounts of Glu in the extracellular spaces have been associated with neurotoxicity and cell death (McDonald and Johnston, 1990; Plaitakis and Shashidharan, 2000). However, Glu levels measured on MRS typically represent the neuronal cytosolic Glu pool, which is much larger than the extracellular pool. The proton resonances from Glu overlaps with those of glutamine and GABA, forming an overlapping range of resonances often reported as Glx; therefore, specialized MRS techniques are often required to measure Glu. These techniques include two-dimensional MRS or echo time (TE)-averaged point resolved spectroscopy (PRESS). The proton resonances from Glu are readily observed with shorter echo times (<50 ms) on MRS, which is more easily performed with localized MRS, rather than MRSI.
GABA, which is derived from Glu, is the predominant inhibitory neurotransmitter in the CNS and is often altered in psychiatric or neurological disorders (Chang et al., 2003). Specialized editing techniques (e.g., J-resolved spectroscopy or MEGA-PRESS) are also needed to subtract out the much larger overlapping resonances from the total creatine resonances (at 3.0 ppm) or from Glx (~1.9 ppm) in order to detect the very small GABA resonances. Therefore, very few studies have been performed to evaluate GABA levels in substance-dependent individuals.
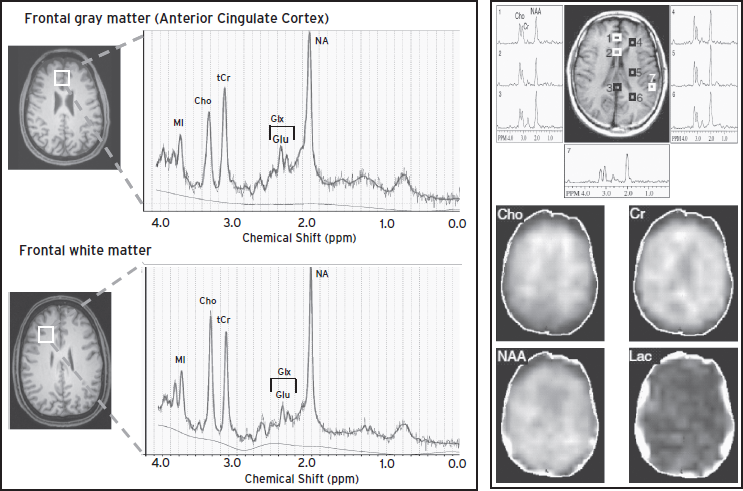
Figure 56.1 Left: Localized MR spectroscopy from a 40-year-old healthy man, showing voxel locations in the frontal gray matter (top) and frontal white matter (bottom) and the corresponding MR spectra with the major brain metabolite levels from these brain regions. Note higher N-acetyl compounds (NA) levels in the frontal gray matter, but higher choline (Cho) levels in the frontal white matter. Right: 2D MRSI data showing regional variability of metabolite concentrations in the different spectra (top) and spectroscopic images of choline-containing compounds (Cho); creatine (Cr); NA, which includes N-acetylaspartate (NAA); and lactate (Lac) across the brain slice (bottom). (Right panel is modified from Barker P.B., Lin D.D. (2006). In vivo proton MR spectroscopy of the human brain. Prog Nucl Mag Res Sp 49:99–128; permission to use obtained from Dr. Peter Barker and from Elsevier.)
The Cho peak on MRS comprises the water-soluble choline-containing compounds, mainly free choline, phosphocholine, and glycerophosphocholine. These compounds are found in the pathways of synthesis and breakdown of choline-containing phospholipids; therefore, Cho is often increased in conditions associated with increased cell membrane turnover and breakdown, such as neoplasms, demyelinating conditions (e.g., multiple sclerosis), cerebral infarction, and even in neuroinflammatory or neuroinfectious conditions (e.g., infections with HIV or JCV). Cho is also found to be three times higher in glia than in neurons (Brand et al., 1993). Therefore, in conditions that glial activation are prominent, such as in psychostimulant users, Cho may be elevated as well.
MI is an organic osmolyte and a putative glial marker (Ross et al., 1992). In vitro studies showed that MI is present in high levels in glia, particularly astrocytes, but lower levels are found in neuronal cells (Brand et al., 1993). Therefore, gliosis, with reactive glial proliferation including microglia, during brain degeneration or neuroinflammation, might lead to increased MI concentrations, often along with increased Cho and tCR. MI also is involved in plasma membrane metabolism and is enzymatically converted to inositol triphosphate (IP3), an important intracellular second messenger (Worley et al., 1987). A defect or interference of this pathway also might result in a change in the MI concentration. Elevated MI levels have been noted in brain injury, infections, and degeneration (Mader et al., 2008). However, the proton resonances from MI are not visible on MRS studies performed with long echo-times (>100 ms). Therefore, short-echo time MRS studies are preferable in most instances and are more easily performed with localized MRS than with MRSI.
The proton resonance for tCR (at 3.0 ppm) includes free creatine and phosphocreatine, which likely reflects high-energy phosphate metabolism because phosphocreatine is involved in adenosine triphosphate (ATP) synthesis (Miller, 1991). tCR is often used as a reference metabolite in MRS studies because it was once thought to be relatively constant even in disease states; however, changes in tCR levels have been reported in multiple brain disorders as well as in substance abusers, as noted in the following.
Phosphocreatine (PCr) can also be measured with 31P MRS, which additionally can assess phosphomonoesters (PME), inorganic phosphate, phosphodiesters (PDE), and three resonances associated with ATP (i.e., gamma-ATP, alpha-ATP, and beta-ATP). These phosphorous metabolites provide insights to the integrity of cellular energy stores, as well as cellular membrane composition and turnover. Several studies used 31P MRS to evaluate these energetic metabolites in individuals during methadone-maintenance (see discussion following). Last, 13C MRS can be used to study the metabolism of particular neurochemicals because the precursors of these chemicals can be enriched with 13C, and the levels of the metabolic products can be measured. One example is 13C-glutamate, which has been used to assess the neuronal health in individuals dependent on stimulants.
MAGNETIC RESONANCE SPECTROSCOPY STUDIES IN PSYCHOSTIMULANT ABUSE
Psychostimulants typically refer to several drugs that are commonly abused; these include but are not limited to cocaine, amphetamines (i.e., amphetamine, methamphetamine, and MDMA), and methylphenidate. MRS studies of stimulant users demonstrated abnormalities in brain metabolites associated with neurons, glia, and cellular metabolism. Findings often vary by the amount used, time since last use, and age of exposure to the stimulant drug.
MRS STUDIES IN COCAINE USERS
Several MRS studies of adult cocaine users have reported cerebral metabolite alterations (Table 56.1). Specifically, lower levels of the neuronal marker NAA were found in the anterior cingulate cortex (ACC) (Chang et al., 1999b) and thalamus (Li et al., 1999). Because the majority of neurons are glutamatergic, it is not surprising that Glu/tCR was also lower in the ACC of cocaine users, especially in those with a longer duration of cocaine use (Yang et al., 2009). In the same frontal region (ACC), cocaine users showed higher glial response with elevated MI, which also correlated with the duration of cocaine use (Chang et al., 1997). However, elevated tCR levels were found in both parietal (Chang et al., 1997; O’Neill et al., 2001b) and frontal white matter (Chang et al., 1997), as well as the ACC of cocaine users, especially in men (Chang et al., 1999b). The etiology of the elevated tCR is unclear, but similarly elevated tCR was also observed in children with prenatal cocaine exposure (Smith et al., 2001a). Although glial cells contain higher levels of tCR than neurons, the elevated tCR did not always coincide with the same brain regions that showed higher glial marker MI. Elevated tCR in the frontal lobe has been correlated with the frequency of cocaine use (Chang et al., 1997). In children with prenatal cocaine exposure, the higher frontal white matter tCR suggest aberrant cellular metabolism; however, these children had normal NAA, which indicated normal neuronal function and density (Smith et al., 2001a).
Although metabolites measured with MRS typically reflect cellular changes, one study found that acute cocaine administration induced increases in basal ganglia Cho and NAA, showing a transient effect at lower doses, but greater and more sustained increases in these metabolites at higher doses (Christensen et al., 2000). The investigators of this study posited that cocaine might have induced osmotic stress, which led to increased intracellular water and hence increased transverse relaxation times (T2*) of the Cho and NAA, via cocaine’s action as an indirect dopamine D1 receptor agonist. However, since cocaine causes vasoconstriction, probably via its effects on the dopamine receptors on the blood vessels, the tissue concentrations of these metabolites may be increased within the voxel accordingly.
Studies that evaluated individuals who abused both cocaine and alcohol found even greater neurotoxic effects than those who abused cocaine only. For instance, prefrontal cortex GABA concentrations were lower than normal in cocaine users, and were even lower in those who also used alcohol (Ke et al., 2004). Another study found that cocaine using alcoholics had higher frontal white matter choline, indicative of cellular damage or turnover, but non-alcoholic cocaine users showed normal Cho levels. Furthermore, lower NAA in the dorsolateral prefrontal cortex of cocaine users partially normalized with abstinence from alcohol (Meyerhoff et al., 1999), again suggesting that some of the metabolite abnormalities may reflect transient neuronal dysfunction rather than neuronal loss. Because alcohol abuse is very common among cocaine-dependent subjects, and cocaine is not known to be neurotoxic from preclinical studies, findings from these studies suggest that the metabolite abnormalities observed in prior MRS studies may primarily be caused by alcohol abuse or the synergistic effects of alcohol and cocaine (e.g., the neurotoxic effects of cocaethylene).
Few MRS studies evaluated the effects of pharmacological treatment for cocaine dependence. One small study found that the GABA levels in the prefrontal cortex of cocaine users increased after pramipexole treatment, but the GABA levels were not related to treatment success (Streeter et al., 2005). A preliminary study also found that cocaine users showed normalized glutamate levels after N-acetylcysteine treatment (Schmaal et al., 2012).
MRS STUDIES IN METHAMPHETAMINE-DEPENDENT INDIVIDUALS
MRS studies of methamphetamine (METH) users (Table 56.2) consistently reported metabolite alterations that suggest neuronal or cell membrane injury, but the findings vary depending on the age of the subjects, amount METH used, and duration of abstinence. Abstinent METH-dependent individuals showed lower NAA in the basal ganglia regions (Chang et al., 2005; Ernst et al., 2000), and those with the greatest cumulative METH usage had the lowest NAA in their frontal white matter (Ernst et al., 2000; Sung et al., 2007). Glial activation with elevated MI was also found in the frontal lobe regions (Chang et al., 2005; Ernst et al., 2000; Sung et al., 2007). Some of these metabolite alterations might normalize with longer duration of abstinence, since subjects with longer duration of abstinence had higher NAA (Sung et al., 2007). The one small study that used 13C MRS to evaluate the incorporation of 13C in the tricarboxylic acid cycle found lower glial 13C-bicarbonate production in METH users, but the sample size is too small to yield conclusive interpretations (Sailasuta et al., 2010).
TABLE 56.1. MRS studies in cocaine users
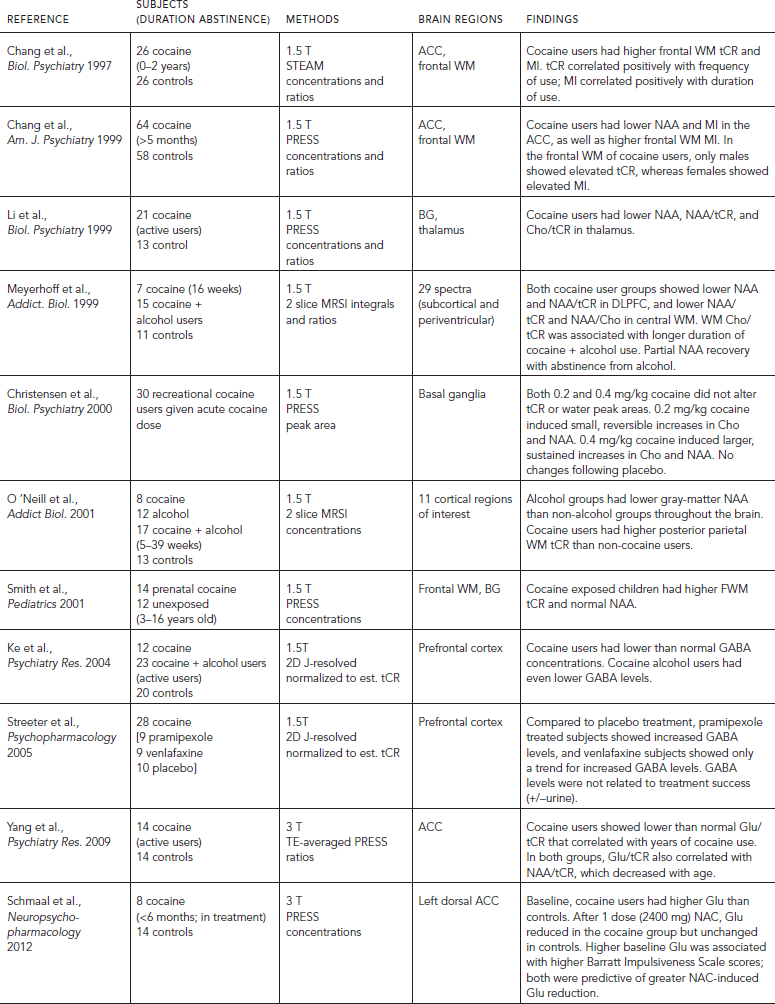
TABLE 56.2. MRS studies in methamphetamine users
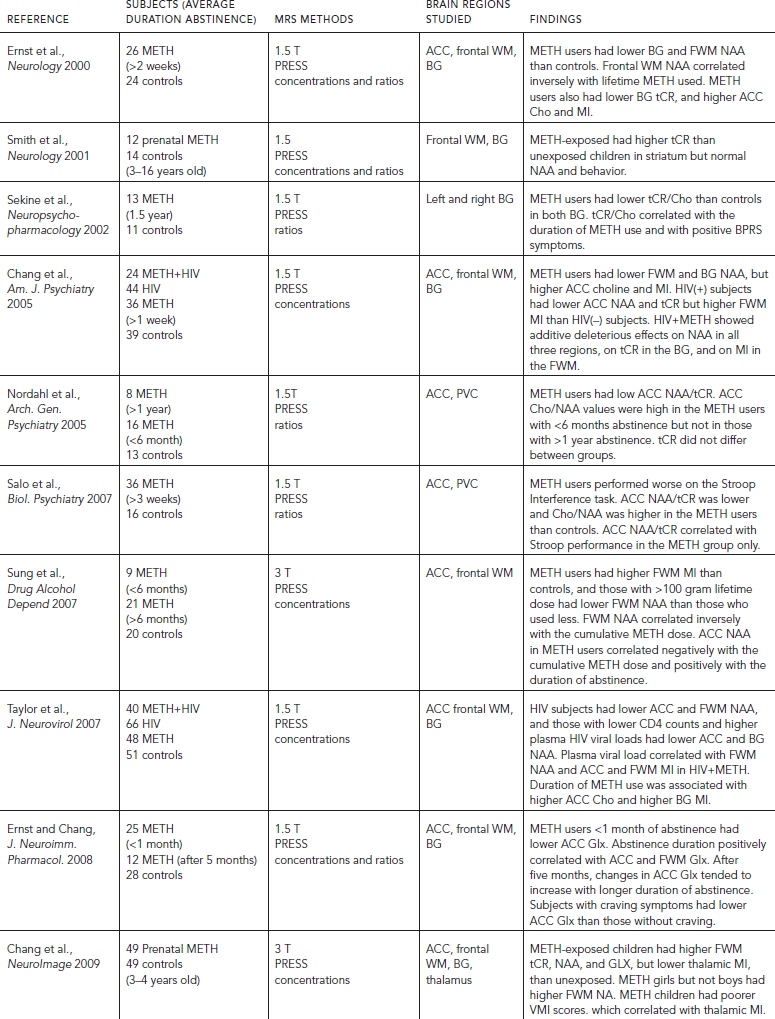
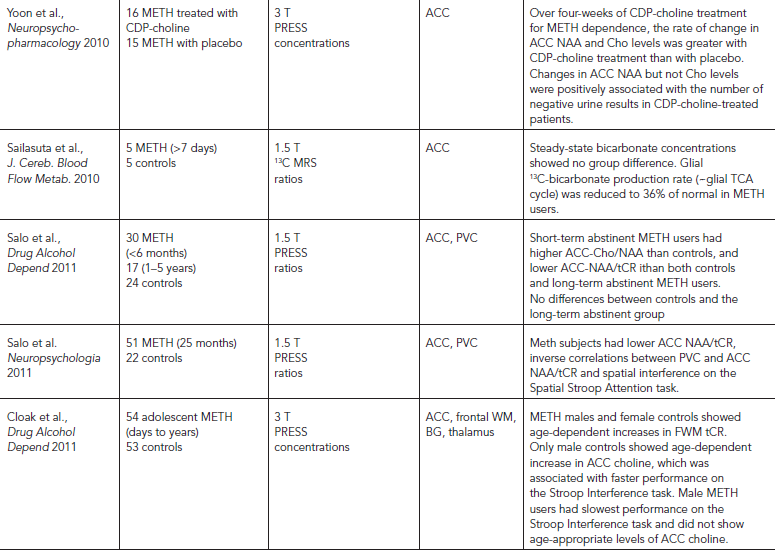
Methamphetamine use is prevalent among HIV-infected individuals. MRS studies that evaluated the combined effects of METH and HIV consistently found additive effects of lower NAA levels in the frontal white matter (WM) and basal ganglia, as well as elevated myoinositol (Chang et al., 2005; Taylor et al., 2007). These findings demonstrate that METH use may exacerbate the neurotoxic effects of HIV, especially in the striatum.
A few studies evaluated METH subjects longitudinally to assess for possible treatment effects on brain metabolite abnormalities. Cell membrane turnover with elevated Cho/NAA in recently abstinent METH subjects may normalize with longer duration of abstinence (>one year) (Nordahl et al., 2005). In addition, Glx, which is the sum of Glu, Gln, and GABA, was lower in those during early abstinence but may normalize or become elevated with more than five months of abstinence (Ernst and Chang, 2008). In addition, METH users treated with cytidine-5′-diphosphate choline, but not those given placebo, for one month showed increased NAA and choline levels, and greater increased NAA levels were associated with more negative urine results (Yoon et al., 2010). These findings suggest that alteration in the neuronal marker NAA may be reversible and that MRS may be useful for monitoring treatment effects.
These metabolite alterations might be related to behavioral consequences in METH users. For example, low basal ganglia tCR/Cho levels were associated with more psychiatric symptoms, even in abstinent METH users (Sekine et al., 2002). Lower ratios of NAA/tCR in the ACC also correlated with poorer executive function (Salo et al., 2007). In addition, abstinent METH subjects with the lowest ACC Glx had the greatest craving symptoms (Ernst and Chang, 2008). Because craving is an important symptom for relapse, Glx levels may be a useful marker for predicting relapse.
Unlike the consistent findings of possible neurotoxicity of chronic long-term METH abuse, acute dextroamphetamine administration to humans showed no metabolite changes in the ACC (McGrath et al., 2008), but increased glial metabolite ratio MI/tCR and PME/bATP in the temporal lobe (Silverstone et al., 2002). These changes may reflect a glial response to the drug, which may be transient or reversible.
Although many chronic METH users first used METH during their teenage years, only one MRS study evaluated brain metabolite levels in adolescent METH users. In contrast to the lower NAA found in the adults, these younger subjects showed normal NAA levels, which may be because of their lower cumulative lifetime doses. However, these young METH users showed less steep age-dependent increases in their frontal lobe metabolites (frontal white matter tCR in the females and ACC choline in the males), and the young male METH users also showed poorer executive function (Cloak et al., 2011). In contrast, children with prenatal METH exposure showed elevated tCR in the basal ganglia (Smith et al., 2001b) and the frontal white matter (Chang et al., 2009), which is similar to children with prenatal cocaine exposure (Smith et al., 2001a). The girls with prenatal METH exposure additionally showed elevated frontal white matter NAA. These children also showed lower glial metabolite MI in the thalamus that correlated with poorer visual motor integration performance (Chang et al., 2009). Taken together, the neurotoxic effects of METH on the developing brain varied based on age of exposure and sex.
ECSTASY (3,4-METHYLENEDIOXY-N- METHYLAMPHETAMINE)
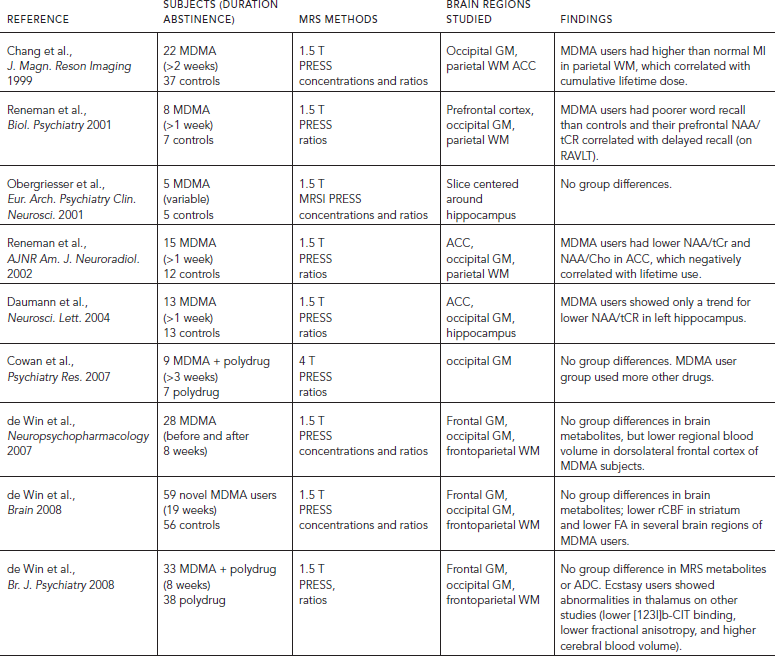
Findings from MRS studies of ecstasy users are somewhat conflicting (Table 56.3). Early MRS studies reported higher glial marker MI in the parietal white matter of ecstasy users that correlated with estimated lifetime usage of the drug (Chang et al., 1999a), as well as lower frontal gray matter NAA/tCR and NAA/Cho, both also correlated negatively with the estimated lifetime use (Reneman et al., 2002). Because lower ratios of NAA/tCR and NAA/Cho may reflect normal NAA and higher tCR and Cho, these findings may suggest glial activation or response in the brains of ecstasy users. Such glial response may or may not represent toxic effects to the neurons. However, another study found that lower NAA/tCR was associated with impaired memory in ecstasy users (Reneman et al., 2001), which suggested that the drug might have “neurotoxic” effects with cognitive consequences. However, more recent prospective studies, with larger sample size and similar comparison subjects who used multiple substances but not ecstasy, failed to find metabolite abnormalities on MRS in those who used ecstasy, despite alterations in other imaging measures (de Win et al., 2007, 2008a, 2008b). Even small studies that focused on metabolite levels in the hippocampus found little (Daumann et al., 2004) or no (Obergriesser et al., 2001) differences between the ecstasy users and comparison subjects. Two studies of polydrug users who used ecstasy also found no differences between the ecstasy users and comparison subjects (Cowan et al., 2007), but noted an association between lower frontal cortex NAA/tCR and more lifetime marijuana use in this population (Cowan et al., 2009). Taken together, the brain metabolite levels are relatively normal in ecstasy users, except for possible increased glial metabolites in some individuals. Given the extremely common co-abuse of other psychostimulants and marijuana in ecstasy users, these glial responses also might have resulted from other drugs abused. Because preclinical studies of MDMA showed that the drug primarily affects the serotonergic nerve terminals, MRS does not appear to be the most sensitive imaging method for detecting the neurotoxic effects of ecstasy.
MRS STUDIES OF INHALANT (TOLUENE, ORGANIC SOLVENTS) ABUSE OR EXPOSURE
Few studies have addressed the neurotoxic effects of inhalant or solvent abuse using MRS. This is partly due to the transient usage pattern of inhalants or solvents by youths. Toluene and organic solvents are readily accessible to adolescents because they are common ingredients in household cleaning solutions, paint thinner, or glue. These inhalant or solvent abusers tend to progress to abusing other illicit drugs as adults. A few MRS studies evaluated individuals who abused inhalants, while others assessed individuals who had environmental (i.e., work place) exposure to chemicals that have abuse potential. Similar to other drugs of abuse, MRS studies showed metabolite abnormalities in inhalant users that reflect both neuronal and glial abnormalities. In addition, oxygen deprivation during the inhalation process also may exacerbate the neurotoxicity. A study of workers exposed to organic solvents on a daily basis found higher Cho/tCR in the parietal white matter, thalamus, and basal ganglia, and their duration of exposure correlated with metabolite abnormalities in the basal ganglia (Alkan et al., 2004). A case study of a woman who had abused toluene showed lower NAA/tCR in the centrum semiovale (Noda et al., 1996). This finding was validated in a larger study of individuals who abused toluene (paint-thinner), and showed lower NAA/tCR in the parietal and cerebellar white matter, as well as higher than normal MI/tCR in the same brain regions, especially in those with longer duration of abuse (Aydin et al., 2003). A similar study of toluene abusers found elevated Cho/tCR and Cho/NAA in the basal ganglia, and those with higher Cho/tCR ratios in the left basal ganglia had more psychiatric symptoms (Takebayashi et al., 2004). Therefore, findings from these few MRS studies clearly demonstrated the neurotoxic effects of toluene abuse, with evidence for glial activation and possible neuronal injury in the white matter and the striatum.
MRS STUDIES OF OPIATE USERS
Multiple MR studies of opiate-dependent individuals found brain abnormalities, including altered brain activation, structural neuroimaging abnormalities, and neuropsychological deficits. In particular, these brain abnormalities were typically found in the prefrontal brain regions of opiate-dependent individuals (Licata and Renshaw, 2010). However, only a few MRS studies evaluated opiate-dependent subjects (Table 56.4).
TABLE 56.3. MRS studies in ecstasy (MDMA) users
Patients enrolled in opioid maintenance therapy (OMT) with either methadone or buprenorphine have been studied with both 13P MRS and 1H MRS. 13P MRS studies showed that methadone maintenance patients had elevated percentage of phosphomonoester in the white matter after seven days of treatment (Christensen et al., 1996; Kaufman et al., 1999), peaking between 15 and 28 days on treatment (Silveri et al., 2004), and normalized compared with controls following an average of 137 weeks of treatment (Kaufman et al., 1999). Reduced percentage of phosphocreatine was observed after seven days on treatment (Kaufman et al., 1999; Silveri et al., 2004), with continued decreases as a function of treatment duration up to 28 days (Silveri et al., 2004), and remained lower than controls after 137 weeks of treatment (Kaufman et al., 1999). Additionally, percentage of nucleotide triphosphates (β-NTP) and percentage of total nucleotide phosphates were lower than normal in the brains of cocaine and heroin-dependent individuals (Christensen et al., 1996). These findings indicated that cerebral energy metabolism is significantly altered during opiate withdrawal and methadone maintenance.
Studies using 1H MRS further showed evidence of neuronal injury, with lower frontal gray matter (GM) NAA (Haselhorst et al., 2002) and lower dorsal ACC NAA and Glx (Yucel et al., 2007). Possible cell membrane turnover was also implicated with higher Cho in frontal WM of OMT subjects (Hermann et al., 2012a). Such cell membrane turnover may result from neurotransmitter release or adaptation associated with neuroplasticity. ACC Glu concentration also correlated with the number of previous withdrawals, whereas Glx decreased with age in controls but increased with age in opiate-dependent subjects (Hermann et al., 2012a). Investigators from this study suggested that these findings may be related to a hyperglutamatergic state associated with more frequent withdrawals, which might result in kindling of withdrawal symptoms after repeated withdrawal episodes.
TABLE 56.4. Brain metabolite abnormalities associated with opiate use or dependence
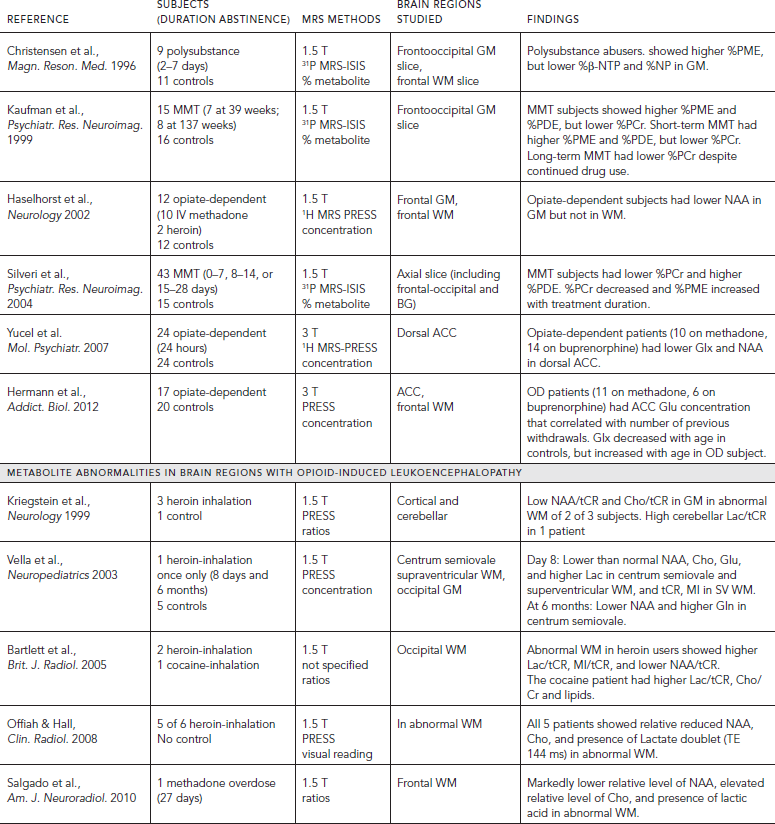
In addition to the MRS studies in opiate-dependent individuals without gross structural brain abnormalities, several case reports or small series have used MRS to evaluate heroin-induced spongiform leukoencephalopathy, which is a rare condition resulting from inhalation of heroin vapors (aka “chasing the dragon”). These patients typically showed lactate and lower brain metabolite in the abnormal white matter regions. Specifically, lower levels of NAA (Kriegstein et al., 1999; Offiah and Hall, 2008; Vella et al., 2003), NAA/tCR (Bartlett and Mikulis, 2005); tCR (Vella et al., 2003); Cho, Glu, and MI (Vella et al., 2003); as well as higher Lac (Offiah and Hall, 2008; Kriegstein et al., 1999; Vella et al., 2003), or Lac/tCR (Bartlett and Mikulis, 2005) were found in brain regions showing leukoencephalopathy. A longitudinal case study of a 16-year-old boy who inhaled heroin only once showed bilateral leukoencephalopathy with reduced levels of brain metabolites and presence of lactate eight days later, and the reduced NAA and elevated Gln persisted at six months (Vella et al., 2003). Similarly, methadone-induced leukoencephalopathy showed relatively lower NAA, elevated Cho and presence of Lac (Salgado et al., 2010), whereas a case of cocaine-induced leukoencephalopathy showed only elevated Lac/tCR, Cho/tCR and lipids (Bartlett and Mikulis, 2005).
Taken together, findings from these 13P MRS and 1H MRS studies suggest opiate abuse may lead to increased cell membrane turnover (elevated percentage of PDE and percentage of PME, and Cho), possibly related to neuronal damage (lower NAA) and alterations in cerebral metabolism (lower percentage of PCr and presence of lactate). However, interpretations of these findings are confounded by several factors. First, with the exception of patients with drug-induced leukoencephalopathy, most patients were enrolled in OMT. Variability in scan days from dosing of methadone may affect cerebral metabolism and/or phospholipids, especially in early treatment prior to methadone tolerance. Additionally, many subjects had active polydrug abuse or dependence (cocaine, amphetamine, barbiturates, alcohol, etc.), which may additionally alter MRS detectable metabolites. Finally, the lifestyles of these patients may make accurate histories difficult to obtain (Yucel et al., 2007). More studies are needed to determine whether MRS may be useful for monitoring the efficacy of treatments in these individuals.
BRAIN METABOLITES ABNORMALITIES IN ALCOHOL USE DISORDERS
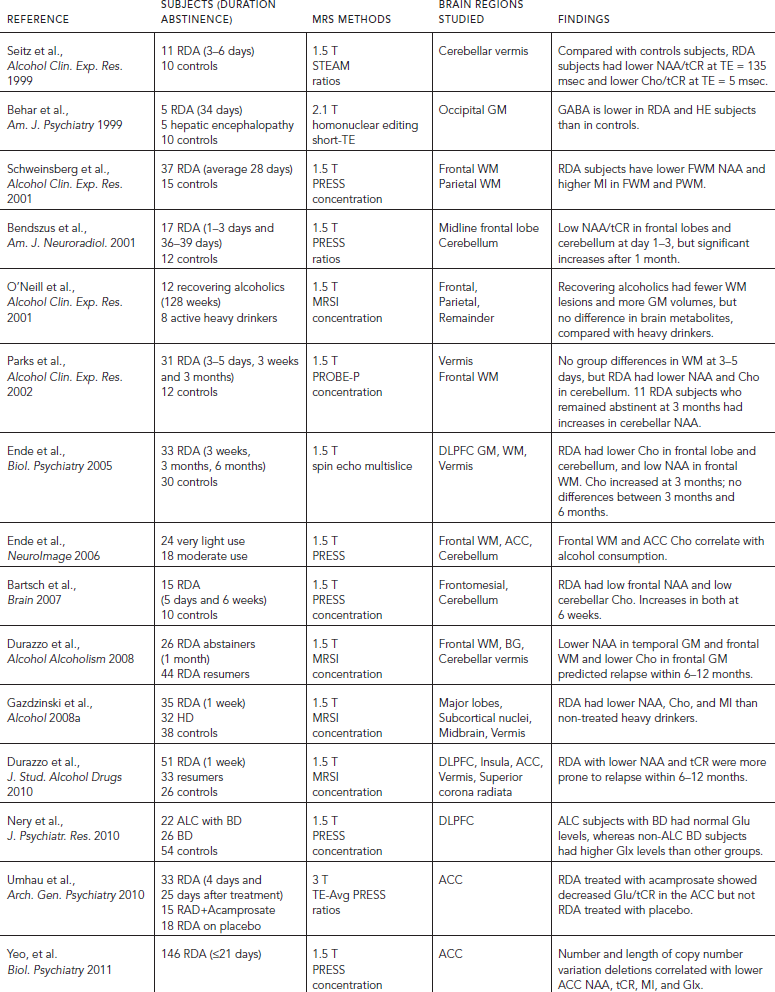
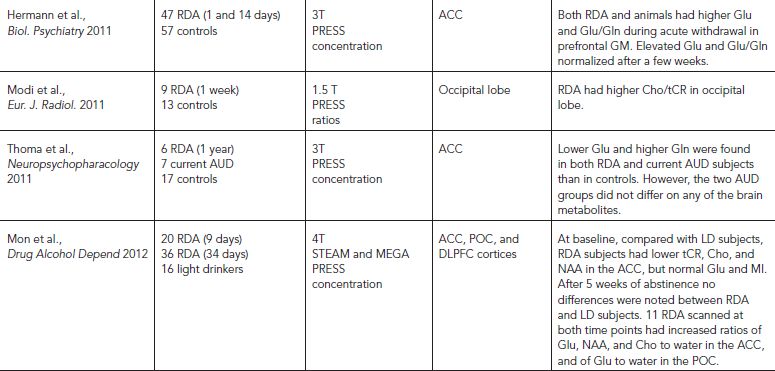
The neuropathological consequences of alcohol use disorders (AUD) include neuronal loss in the dorsolateral frontal cortex, cerebellum, and hypothalamus (Harding et al., 1997; Korbo, 1999; Kril and Halliday, 1999), glial abnormalities in the hippocampi, and dorsolateral prefrontal cortex, (Harper, 2009; Miguel-Hidalgo et al., 2002), as well as reductions in density of both neuronal and glial cells in the orbitofrontal cortex (Miguel-Hidalgo et al., 2006) (Table 56.5). Based on these neuropathological findings, most 1H MRS studies were evaluated for neuronal loss (NAA), cellular (Cho), glial (MI), and metabolic (tCR) alterations in the frontal lobes, cerebellum, and subcortical regions. Almost all 1H MRS studies of recently detoxified alcohol-dependent (RDA) individuals within two weeks of detoxification found consistent evidence of neuronal loss or injury, with lower NAA levels, lower NAA/tCR or NAA/Cho in the frontal lobe GM (Fein et al., 1994; O’Neill et al., 2001a), frontal WM (Bartsch et al., 2007; Durazzo et al., 2004; Durazzo et al., 2008; Ende et al., 2005; Gazdzinski et al., 2008a; Jagannathan et al., 1996; Schweinsburg et al., 2001), cerebellum (Bendszus et al., 2001; Jagannathan et al., 1996; Parks et al., 2002; Seitz et al., 1999), anterior cingulate cortex (Mon et al., 2012), parietal lobe (O’Neill et al., 2001a), and thalamus (Jagannathan et al., 1996). Only one study of individuals during early (three to five days) abstinence reported normal levels of frontal WM NAA (Parks et al., 2002). Another major metabolite abnormality in RDA subjects is lower Cho levels and lower Cho/Cr or Cho/NAA, which were found in the cerebellum (Bartsch et al., 2007; Ende et al., 2005; Seitz et al., 1999), frontal WM (Ende et al., 2005), anterior cingulate cortex (Lee et al., 2007; Mon et al., 2012), and the occipital region (Modi et al., 2011). The lower Cho or Cho/tCR ratios suggest alterations in myelin or cell membrane, or decreased cellular synthesis. Additionally, elevated MI concentrations, suggestive of glial activation, have been reported in the thalamus and ACC (Schweinsburg et al, 2000), as well as in the frontal and parietal WM (Schweinsburg et al., 2001).
MRS also may be useful for predicting relapse because the odds of relapse to alcohol use within 6–12 months were greater in those with lower NAA in the temporal GM and frontal WM and lower Cho in the frontal GM (Durazzo et al., 2008). A more recent study further confirmed that relapsers had significantly lower NAA than non-relapsers in many brain regions, including the dorsolateral prefrontal cortex (DLPFC), ACC, insula, cerebellar vermis, and the corona radiata; therefore, the authors speculated that connectivity of components of the rewards system may be impaired in those at risk for relapse (Durazzo et al., 2010). A small study that evaluated patients with bipolar disorder with and without alcoholism found only higher Glx levels in the DLPFC in the bipolar subjects, whereas those with alcoholism actually had normal brain metabolites despite their worse outcome and treatment refractoriness for their bipolar symptoms (Nery et al., 2010).
Recent studies have investigated the role of Glu and its metabolite Gln in AUD. Glu has been implicated in both AUD neuropathology and in the development of alcohol dependence. Young (<40 years old) RDA subjects abstinent for two weeks, without gross brain atrophy on MRI (GM, WM, and CSF volumes), showed significantly lower than normal ACC tCR, Cho, and Glu/tCR (Lee et al., 2007). Some of these metabolite abnormalities might be transient because older subjects (>40 years) with longer histories of AUD showed lower than normal NAA, Glu, and tCR in the ACC at baseline, mild elevation of Glu during early abstinence, and normalization of these metabolite abnormalities four weeks later (Mon et al., 2012). Furthermore, despite lower than normal Glu and Gln in RDA subjects, those who had been abstinent for at least one year also showed normal brain metabolite levels. Finally, in a placebo-controlled study of acamprosate, decreased Glu/tCR ratios were found only in the RDA subjects who were on acamprosate after four weeks, but not in the placebo treated RDA subjects. Because acamprosate is a medication approved for the treatment of alcohol dependence that reduces craving and relapse, the decreased Glu/tCR ratio after treatment may be a potential surrogate marker for future studies. Unfortunately, craving and relapse measures were not evaluated in relation to the changes in Glu/tCR in the subjects studied, and no correlations with CSF glutamate or other clinical measures were performed. Nevertheless, the various studies that evaluated brain GLX or glutamate demonstrated that MRS may be useful to evaluate the altered brain glutamatergic system in alcoholics, which may represent the effects of alcohol consumption or a predisposing susceptibility to AUD.
Several studies suggested that much of the neuropathological damage associated with AUD is reversible. With sustained abstinence, recovery has been observed in WM volume (Monnig et al., 2012), GM volume (Sullivan and Pfefferbaum, 2005) and cognitive function (see following). When compared with control subjects after 35 to 39 days (Bendszus et al., 2001; Mon et al., 2012), or three months of abstinence (Ende et al., 2005), RDA subjects showed relatively normal levels of frontal (ACC) NAA, Glu, and Cho (Mon et al., 2012), NAA/tCR (Bendszus et al., 2001), frontal GM and WM Cho (Ende et al., 2005), cerebellar Cho/NAA (Martin et al., 1995), NAA/tCR and Cho/tCR (Bendszus et al., 2001), and Cho levels (Ende et al., 2005). In longitudinal follow-up studies of RDA subjects who were scanned after three days, three weeks, and three months of abstinence, frontal WM metabolites were normal at three days, whereas the cerebellar NAA and volume were lower at baseline but continued to increase toward normalization at three months, suggesting that the cerebellum is particularly sensitive, and may recover slower from the alcohol induced brain injury (Parks et al., 2002).
TABLE 56.5. Brain changes associated with AUD: selected MRS studies
BRAIN METABOLITE ABNORMALITIES IN AUD ARE RELATED TO COGNITIVE PERFORMANCE
Alcohol may induce cognitive impairments, particularly in the learning and memory domains, which may be associated with alterations in brain metabolites measured with 1H MRS. For example, deficits in delayed recall on the Hopkins Verbal Learning Test were negatively correlated with occipital GABA plus homocarnosine in RDAs (Behar et al., 1999), and improvements in verbal learning assessed on day 2 and day 38 of abstinence were correlated with increases in frontal NAA/tCR (Bendszus et al., 2001). Furthermore, levels of the glial marker MI in either the ACC or the parietal cortex of RDA subjects negatively correlated with verbal learning, delayed visual memory, visuomotor scanning speed, and visuospatial memory, after 2 days of abstinence, and with auditory-verbal learning after 35 days of abstinence (Mon et al., 2012). Additionally, poorer short-term memory function was associated with lower Glu/tCR in the ACC of young RDA who had no evidence of GM or WM atrophy (Lee et al., 2007). Furthermore, poorer visuospatial learning was associated with higher DLPFC Glx after 2 days of abstinence, but with higher Cho on day 35 of abstinence, whereas poorer working memory was associated with higher glial marker MI also on day 35 (Mon et al., 2012). However, the degree of improvement on these cognitive measures from day 2 to day 35 did not correlate with changes in metabolite concentrations (Mon et al., 2012). Moreover, the neuronal marker NAA also may predict cognitive performance. In one study, cerebellar NAA/tCR in RDA subjects correlated with their performance on the Concentration Load test both after 2 days and after 38 days of abstinence (Bendszus et al., 2001). In heavy drinkers, the lower than normal frontal WM NAA also correlated with poorer executive and working memory functioning (Meyerhoff et al., 2004).
BRAIN METABOLITE ABNORMALITIES IN TREATMENT-NAIVE AUD
Most studies were performed on RDA subjects, who may not be representative of the majority (85%) of people with AUD who do not seek treatment (Gazdzinski et al., 2008a). RDA subjects typically have more severe medical and psychiatric comorbidities (Meyerhoff et al., 2013), and may have up to 50% greater lifetime alcohol consumption (Fein and Landman, 2005). Lifetime consumption of alcohol is an important consideration, because even light to moderate drinkers without AUD showed reductions in frontal Cho levels that correlated with alcohol consumption within 90 days of their MRS (Ende et al., 2006). The few studies that compared current heavy drinkers (HD) to RDA and healthy controls reported conflicting results. Surprisingly, RDA participants typically showed lower brain metabolite levels than HD subjects. For example, although NAA, Cho, tCR, or MI were typically lower in HD subjects than in controls (Gazdzinski et al., 2008a), including HD subjects with a negative family history of AUD, the brain metabolite abnormalities in these HD subjects were less severe than those observed in RDA individuals (Meyerhoff et al., 2004). In contrast, although both current drinkers and AUD subjects who had been abstinent for one year showed lower brain Glu and higher Gln than controls, both AUD subject groups showed no differences in NAA, tCR, Cho, MI, Gln, or Glx in their ACC (Thoma et al., 2011). Similarly, despite group differences in regional brain volumes and the amount of white matter lesions, recovering alcoholics (median abstinence 128 weeks) and active heavy drinkers showed no differences in their brain metabolites in any brain regions studied (O’Neill et al., 2001a). Therefore, MRS may not be sensitive for differentiating the brain health of HD and RDAs. However, the lack of differences between RDA, HD, and control subjects in these two studies might have resulted from normalization of the brain metabolites in the RDAs following abstinence.
ALCOHOL USE WITH TOBACCO SMOKING
Cigarette smoking is the most common comorbidity among drug users, including those with AUD. Up to 80% of alcohol-dependent individuals regularly smoke (Romberger and Grant, 2004) and 50–90% are dependent on nicotine (Daeppen et al., 2000). Only a few MRS studies specifically evaluated brain metabolite abnormalities in tobacco smokers. One study of current tobacco smokers showed lower hippocampal NAA, and their ACC Cho correlated with lifetime nicotine exposure (Gallinat et al., 2007). Another study of tobacco smokers who “slipped” or relapsed from smoking cessation (with nicotine replacement therapy) showed lower baseline Glu/tCR, GABA/tCR, and Cho/tCR than those who were able to remain abstinent from the treatment (Mashhoon et al., 2011). Therefore, these metabolite ratios may be useful for predicting treatment outcomes (Table 56.6).
Smoking appeared to have additive effects with alcohol use in that RDA subjects who smoked had the lowest metabolite concentrations. RDA subjects with greater alcohol consumption also smoked more and had worse neuropsychological performance (Durazzo et al., 2004). Furthermore, after one month of abstinence, compared with RDA subjects who smoked (sRDA), non-smoking RDA (nsRDA) subjects showed greater increases (normalization) in medial temporal lobe Cho and NAA (Gazdzinski et al., 2008b), frontal WM NAA and Cho, and thalamic Cho (Durazzo et al., 2006). Additionally, diffusion tensor imaging indicated lower than normal fractional anisotropy in brain regions of RDA subjects who showed lower NAA concentrations (Durazzo et al., 2006), as well as in the frontal WM, superior corona radiata, and adjacent WM in sRDA, but not nsRDA subjects (Wang et al., 2009). Therefore, comorbid tobacco smoking with alcohol use may lead to greater brain metabolite abnormalities, which may be related to the direct neurotoxic effects from these comorbid conditions, or indirect effects from hypoxia associated with smoking. Prior studies of individuals with AUD often did not account for concurrent tobacco smoking. Because NAA and Cho may normalize in alcohol users who became abstinent, but not in AUD with concurrent tobacco smoking, some of the metabolite abnormalities found in subjects with AUD may be caused by comorbid tobacco smoking.
MRS STUDIES IN MARIJUANA SMOKERS
Marijuana or cannabis smoking is also very common among drug users and is often used for medicinal purposes. Medicinal marijuana use is now legal in more than half of the United States. Few studies have used MRS to measure brain metabolites in chronic marijuana users (see Table 56.6). Because marijuana use is common among HIV-infected individuals, one study evaluated the independent and combined effects of chronic marijuana use in subjects with or without HIV-infection. Regardless of HIV-infection status, abstinent marijuana users showed lower levels of basal ganglia NAA, Cho, and glutamate, but elevated thalamic tCR (Chang et al., 2006). Similarly, young men who smoked marijuana daily showed mild memory and attention impairments and lower levels of NAA/tCR in the dorsolateral prefrontal cortex, and the NAA/tCR levels in their lentiform nuclei positively correlated with hair cannabidiol levels (indicative of amount of marijuana smoked recently) (Hermann et al., 2007). A small 2D MRS study of marijuana-dependent young men reported lower than normal global MI/tCR levels (from one large voxel that included basal ganglia, thalamus, hippocampus, and surrounding regions) (Silveri et al., 2011). Furthermore, young regular marijuana users showed lower levels of glutamate, NAA, tCR, and MI in the ACC; however, some of these marijuana users were also heavy alcohol users and/or were medicated for depression (Prescot et al., 2011). Last, a study of polydrug users who used ecstasy and marijuana found only an association between lower frontal cortex NAA/tCR and more lifetime marijuana use but not with other drug use (Cowan et al., 2009). Taken together, the few studies that evaluated brain metabolite changes in chronic marijuana users found decreased neuronal and/or glial metabolites in the basal ganglia and in the frontal lobe. Longitudinal studies are needed to evaluate the relationship between marijuana use and brain metabolite abnormalities, and whether these alterations are reversible or may normalize with longer duration of abstinence. Because marijuana is often used by adolescents, when their brains are still undergoing development, future evaluations should assess the impact of marijuana usage on brain metabolites or cognitive changes during brain development.
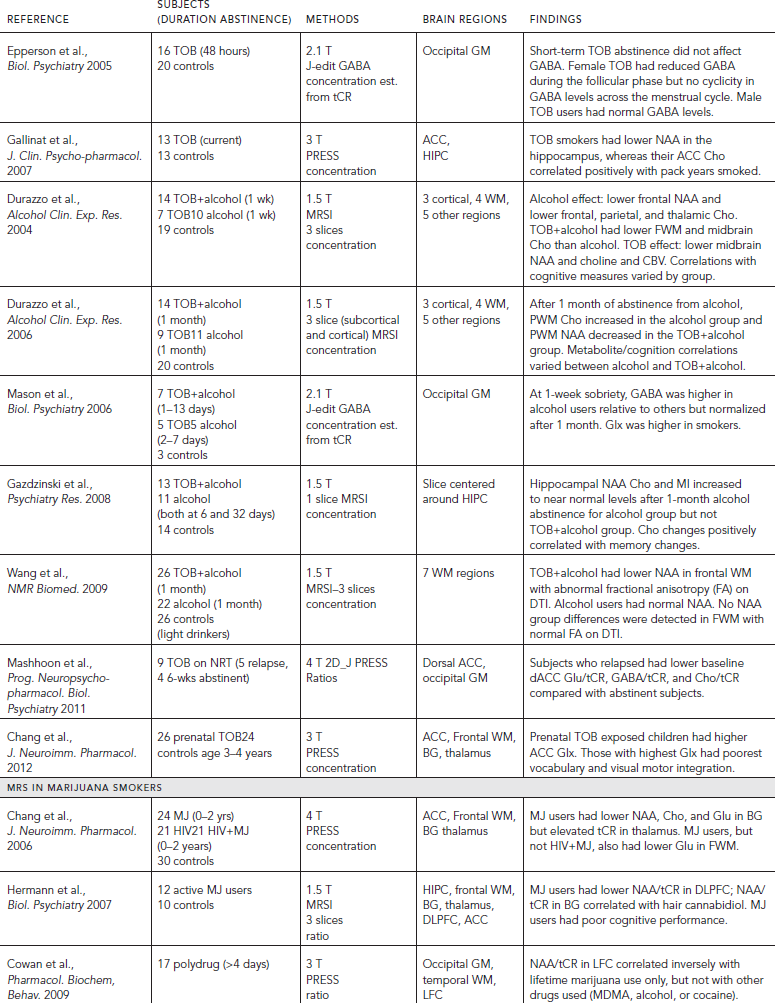
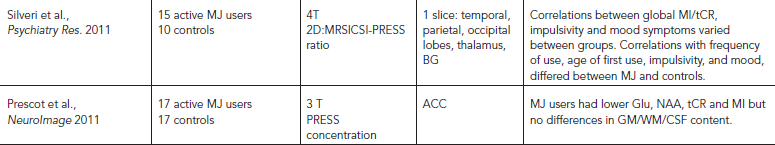
TABLE 56.6. MRS studies in tobacco (TOB) or marijuana (MJ) smokers or exposure
SUMMARY MRS FINDINGS IN VARIOUS DRUGS OF ABUSE AND DRUG DEPENDENCE
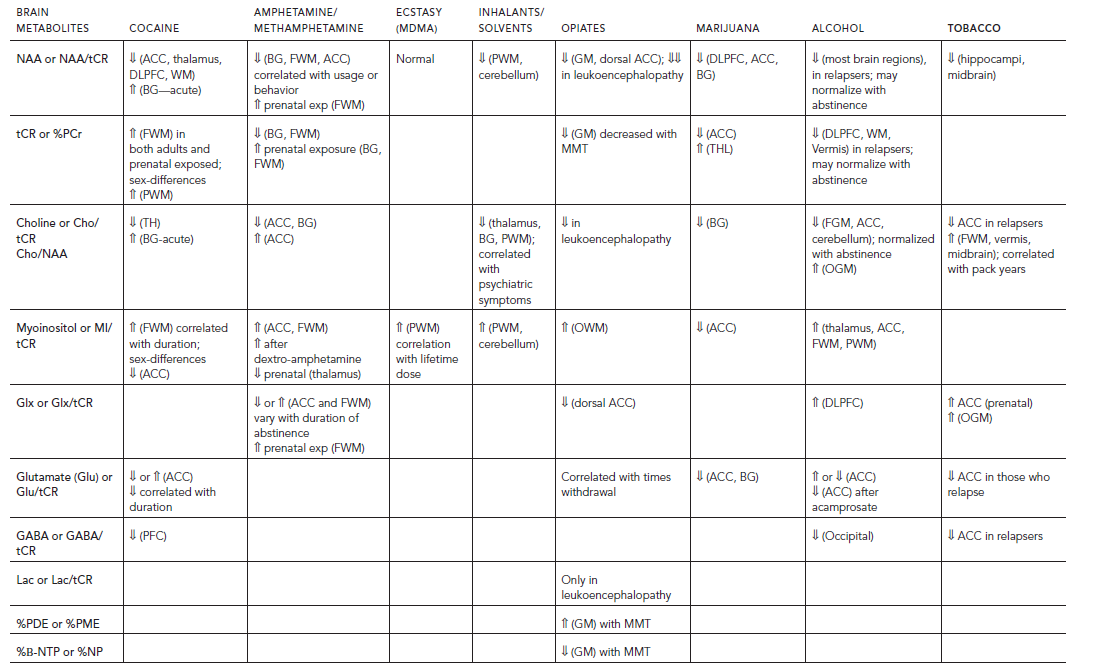
MRS can detect neurochemical alterations that reflect neuronal loss, dysfunction, or glial activation associated with various drugs of abuse. Specifically, neuronal markers such as NAA and Glu or Glx are shown to be lower than normal in many brain regions of the drug users discussed in the preceding, except for those who used ecstasy. Decreased NAA often correlated with the cumulative exposure of the drug, cognitive performance, severity of psychiatric symptoms, and may even predict the likelihood of relapse (e.g., in alcoholics). However, NAA levels also may normalize with longer duration of abstinence from alcohol or methamphetamine use, which indicates that some of the neuronal injury may be reversible. The magnitude of the lower NAA levels are typically in the range of 5–7%, which are less than those found in neurodegenerative disorders, such as frontotemporal dementia (>25%) and Alzheimer’s disease (>10%), or in neuroinflammatory conditions such as multiple sclerosis (up to >30%, especially in secondary progressive disease) and chronic HIV infection (5–20%). However, such decreased NAA may differ across brain regions and may vary greatly depending on the stage of the disease (Table 56.7).
Similarly, the glial marker MI is often elevated in the white matter of drug users which may reflect glial activation or neuroinflammation associated with drug abuse. Specifically, elevated MI has been reported in the white matter of psychostimulant (cocaine, methamphetamine, and ecstasy) users, inhalant users, opiate-dependent individuals, and alcoholics. The magnitude of the elevated MI in drug users (10–15%) are only slightly less than those observed in neuroinflammatory responses associated with other chronic brain disorders. For example, MI is often elevated in patients with chronic HIV infection (up by 10–20%) or neurodegenerative disorders such as Alzheimer’s disease (also 10–20%, depending on the brain region), but may be higher in those with more acute neuroinflammation such as progressive multiple sclerosis (10–35%). Limited data on cocaine or marijuana users reported lower MI in the ACC, but these subjects might have had other confounding variables (e.g., depression).
Because tCR and choline compounds are in both neurons and glia, their levels are more variable depending on the brain regions assessed, usage patterns, or duration of abstinence. tCR was consistently found to be elevated in the white matter of adult cocaine users and in children with prenatal exposure to either cocaine or methamphetamine, although most of these individuals also had tobacco exposure. In contrast, adult methamphetamine users showed lower tCR levels in both the basal ganglia and white matter, and alcoholics also showed lower tCR in the DLPFC and WM, especially during early abstinence and in those who relapsed. Since tCR reflects energetic metabolites, its level may reflect the energy states of the brains of drug users during various states of recovery.
Glutamate or Glx levels, which reflect primarily Glu, tended to be lower during early abstinence (e.g., in methamphetamine users and RDA), but may normalize with longer duration of abstinence, with pharmacotherapy (e.g., RDA treated with acamprosate), and may also correlate with number of withdrawals (e.g., in opiate users). Glx is also elevated in the frontal lobes of tobacco smokers, individuals with AUD, and in those with prenatal stimulant exposure. However, quantitative measurements of Glx and Glu are often less reliable than other metabolites, and are not possible with studies performed using longer echo times. Therefore, better techniques are needed for future analyses (see the following). The few studies that evaluated GABA also found reduced levels in cocaine and alcohol dependent individuals, and the lower levels may predict relapse (e.g., tobacco smoking). This plethora of studies demonstrate that MRS provides valuable in vivo neuropathological assessment of the brains of drug addicted individuals, which may be useful both for initial assessments but may also be used as a surrogate marker to predict treatment outcome or relapse to drug use.
TECHNICAL CONSIDERATIONS AND FUTURE DIRECTIONS
MRS complements other neuroimaging techniques well because it can often be acquired with structural imaging, which is required for voxel placements for MRS studies. This approach was used in a study of alcohol users (Wang et al., 2009); the investigators used diffusion tensor imaging to assess axonal integrity, which demonstrated abnormal fractional anisotropy, and selected brain regions for MRS evaluation from previously collected MRSI. Therefore, MRSI has the advantage that once the data are collected, selected brain regions can be queried retrospectively. However, because of the magnetic field inhomogeneity from different tissues across the brain, localized MRS often allows better assessments of brain metabolite concentrations in selected brain regions, especially at short-echo time acquisitions (<100 ms). This is important because some of the metabolite resonances are visible only at shorter echo time MRS (e.g., myoinositol, glutamate, lactate, lipids).
TABLE 56.7. Brain metabolite abnormalities (in selected brain regions) associated with drugs of abuse or exposure
Aside from technical issues related to the MRS acquisition, many clinical variables may influence the metabolite measurements. For example, the duration of abstinence from the drug use, the cumulative amount of drug exposure, age of the drug exposure, and comorbid drug use (e.g., tobacco or marijuana smoking) all may affect the brain metabolite levels. Therefore, study designs and interpretations of the metabolite levels from MRS need to include these variables. Furthermore, normal variations in brain metabolites can be caused by sex differences (e.g., men have higher choline than women in the frontal WM) and age-dependent changes (e.g., tCR increase and Glu decrease with age), which need to be controlled when evaluating brain metabolite abnormalities in drug users.
Future studies with advanced MRS techniques may further assess other neurochemicals that may be affected with drug dependence. For example, glutathione levels can be measured to assess the oxidative capacity of brain tissue using MEGA-PRESS or 2-D MRS techniques. Similarly, glutamate levels are difficult to delineate and may be better assessed using these same techniques. Given the abundance of glutamatergic neurons and the important role of glutamate in addiction, more MRS studies to evaluate this neurochemical are needed. More studies are also needed to determine whether MRS may be a useful surrogate marker for predicting treatment outcomes. MRS may be used along with other neuroimaging techniques as endophenotypes to evaluate genetic variations or deletions on how the brain may be impacted by drugs of abuse (e.g., rare copy number deletions in AUD [Pettersson-Yeo et al., 2011]) and to predict the likelihood of relapse, which may guide treatment approaches. Because MRS can be performed noninvasively in both humans and in animals, it can be a powerful research tool for clinical-translational studies, especially those that require longitudinal assessments (e.g., MRS were performed in both humans and rodents during alcohol withdrawal and abstinence [Hermann et al., 2012b]).
DISCLOSURES
Dr. Chang has no conflicts of interest to disclose. Dr. Chang is funded by the National Institutes of Health (K24-DA016170, R24-DA027318, R01-DA035659, U54-NS056883).
Dr. Cloak has no conflicts of interest to disclose. Dr Cloak is funded by the National Institutes of Health (K01-DA021203), and the Hawaii CommunityFoundation (Geist Foundation: 12ADVC-51341).
Dr. Holt has no conflicts of interest to disclose.
REFERENCES
Alkan, A., Kutlu, R., et al. (2004). Occupational prolonged organic solvent exposure in shoemakers: brain MR spectroscopy findings. Magn. Reson. Imaging 22:707–713.
Aydin, K., Sencer, S., et al. (2003). Single-voxel proton MR spectroscopy in toluene abuse. Magn. Reson. Imaging 21:777–785.
Bartlett, E., and Mikulis, D.J. (2005). Chasing “chasing the dragon” with MRI: leukoencephalopathy in drug abuse. Br. J. Radiol. 78:997–1004.
Bartsch, A.J., Homola, G., et al. (2007). Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130:36–47.
Behar, K.L., Rothman, D.L., et al. (1999). Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am. J. Psychiatry 156:952–954.
Bendszus, M., Weijers, H.G., et al. (2001). Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Am. J. Neuroradiol. 22:1926–1932.
Birken, D.L., and Oldendorf, W.H. (1989). N-acetyl-l-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci. Biobehav. Rev. 13:23–31.
Brand, A., Richter-Landsberg, C., et al. (1993). Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 15:289–298.
Chang, L., Cloak, C.C., et al. (2003). Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J. Clin. Psychiatry 64 (Suppl 3):7–14.
Chang, L., Cloak, C., et al. (2009). Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage 48:391–397.
Chang, L., Cloak, C., et al. (2006). Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J. Neuroimmune Pharmacol. 1:65–76.
Chang, L., Ernst, T., et al. (1999a). Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J. Magn. Reson. Imaging 10:521–526.
Chang, L., Ernst, T., et al. (2005). Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am. J. Psychiatry 162:361–369.
Chang, L., Ernst, T., et al. (1999b). Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am. J. Psychiatry 156:716–722.
Chang, L., Mehringer, C.M., et al. (1997). Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol. Psychiatry 42:1105–1114.
Christensen, J.D., Kaufman, M.J., et al. (2000). Proton magnetic resonance spectroscopy of human basal ganglia: response to cocaine administration. Biol. Psychiatry 48:685–692.
Christensen, J.D., Kaufman, M.J., et al. (1996). Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn. Reson. Med. 35:658–663.
Cloak, C.C., Alicata, D., et al. (2011). Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug Alcohol Depend. 119:207–215.
Cowan, R.L., Bolo, N.R., et al. (2007). Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 155:179–188.
Cowan, R.L., Joers, J.M., et al. (2009). N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3 T magnetic resonance spectroscopy study. Pharmacol. Biochem. Behav. 92:105–110.
Daeppen, J.B., Smith, T.L., et al. (2000). Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women: the Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 35:171–175.
Daumann, J., Fischermann, T., et al. (2004). Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci. Lett. 362:113–116.
de Win, M.M., Jager, G., et al. (2008a). Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain 131:2936–2945.
de Win, M.M., Jager, G., et al. (2008b). Neurotoxic effects of ecstasy on the thalamus. Br. J. Psychiatry 193:289–296.
de Win, M.M., Reneman, L., et al. (2007). A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology 32:458–470.
Durazzo, T.C., Gazdzinski, S., et al. (2004). Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin. Exp. Res. 28:1849–1860.
Durazzo, T.C., Gazdzinski, S., et al. (2006). Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin. Exp. Res. 30:539–551.
Durazzo, T.C., Gazdzinski, S., et al. (2008). Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol Alcohol. 43:683–691.
Durazzo, T.C., Pathak, V., et al. (2010). Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J. Stud. Alcohol Drugs 71:278–289.
Ende, G., Walter, S., et al. (2006). Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. NeuroImage 32:740–746.
Ende, G., Welzel, H., et al. (2005). Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol. Psychiatry 58:974–980.
Epperson, C.N., O’Malley, S., et al. (2005). Sex, GABA, and Nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol. Psychiatry. 57:44–48.
Ernst, T., Chang, L., et al. (2000). Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology 54:1344–1349.
Ernst T, Chang L. (2008). Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J. Neuroimmune Pharmacol. 3(3):165–172.
Fein, G., and Landman, B. (2005). Treated and treatment-naive alcoholics come from different populations. Alcohol 36:19–26.
Fein, G., Meyerhoff, D.J., et al. (1994). 1H magnetic resonance spectroscopic imaging separates neuronal from glial changes in alcohol-related brain atrophy. In: Lancaster, F.E., ed. Alcohol and Glial Cells. Bethesda, MD: National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, pp. 227–241.
Gallinat, J., Lang, U.E., et al. (2007). Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J. Clin. Psychopharmacol. 27:80–84.
Gazdzinski, S., Durazzo, T.C., et al. (2008a). Are treated alcoholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol 42:67–76.
Gazdzinski, S., Durazzo, T.C., et al. (2008b). Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 162:133–145.
Harding, A.J., Wong, A., et al. (1997). Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7:78–87.
Harper, C. (2009). The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 44:136–140.
Haselhorst, R., Dursteler-MacFarland, K.M., et al. (2002). Frontocortical N-acetylaspartate reduction associated with long-term i.v. heroin use. Neurology 58:305–307.
Hermann, D., Frischknecht, U., et al. (2012a). MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict. Biol. 17:659–667.
Hermann, D., Sartorius, A., et al. (2007). Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCR) loss in male recreational cannabis users. Biol. Psychiatry 61:1281–1289.
Hermann, D., Weber-Fahr, W., et al. (2012b). Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol. Psychiatry 71:1015–1021.
Jagannathan, N.R., Desai, N.G., et al. (1996). Brain metabolite changes in alcoholism: an in vivo proton magnetic resonance spectroscopy (MRS) study. Magn. Reson. Imaging 14:553–557.
Kaufman, M.J., Pollack, M.H., et al. (1999). Cerebral phosphorus metabolite abnormalities in opiate-dependent polydrug abusers in methadone maintenance. Psychiatry Res. 90:143–152.
Ke, Y., Streeter, C.C., et al. (2004). Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 130:283–293.
Korbo, L. (1999). Glial cell loss in the hippocampus of alcoholics. Alcohol Clin. Exp. Res. 23:164–168.
Kriegstein, A.R., Shungu, D.C., et al. (1999). Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”). Neurology 53:1765–1773.
Kril, J.J., and Halliday, G.M. (1999). Brain shrinkage in alcoholics: a decade on and what have we learned? Prog. Neurobiol. 58:381–387.
Lee, E., Jang, D.P., et al. (2007). Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport 18:1511–1514.
Li, S.J., Wang, Y., et al. (1999). Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol. Psychiatry 45:1481–1487.
Licata, S.C., and Renshaw, P.F. (2010). Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann. NY Acad. Sci. 1187:148–171.
Mader, I., Rauer, S., et al. (2008). (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. Eur. J. Radiol. 67:250–257.
Martin, P.R., Gibbs, S.J., et al. (1995). Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clin. Exp. Res. 19:1078–1082.
Mashhoon, Y., Janes, A.C., et al. (2011). Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Prog. Neuropsychopharmacol. Biol. Psychiatry 35:1709–1713.
Mason, G.F., Petrakis, I.L., et al. (2006). Cortical Gamma-Aminobutyric acid levels and the recovery from ethanol dependence: Preliminary evidence of modification by cigarette smoking. Biol. Psychiatry. 59:85–93.
McDonald, J.W., and Johnston, M.V. (1990). Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res. Brain Res. Rev. 15:41–70.
McGrath, B.M., McKay, R., et al. (2008). Acute dextro-amphetamine administration does not alter brain myo-inositol levels in humans and animals: MRS investigations at 3 and 18.8 T. Neurosci. Res. 61:351–359.
Meyerhoff, D.J., Bloomer, C., et al. (1999). Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addict. Biol. 4:405–419.
Meyerhoff, D.J., Blumenfeld, R., et al. (2004). Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin. Exp. Res. 28:650–661.
Meyerhoff, D.J., Durazzo, T.C., et al. (2013). Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr. Top. Behav. Neurosci. 13:511–540.
Miguel-Hidalgo, J.J., Overholser, J.C., et al. (2006). Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin. Exp. Res. 30:1845–1855.
Miguel-Hidalgo, J.J., Wei, J., et al. (2002). Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol. Psychiatry 52:1121–1133.
Miller, B.L. (1991). A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed. 4:47–52.
Modi, S., Bhattacharya, M., et al. (2011). Brain metabolite changes in alcoholism: localized proton magnetic resonance spectroscopy study of the occipital lobe. Eur. J. Radiol. 79:96–100.
Mon, A., Durazzo, T.C., et al. (2012). Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 125:27–36.
Monnig, M.A., Tonigan, J.S., et al. (2012). White matter volume in alcohol use disorders: a meta-analysis. Addict. Biol. 10.1111/j.1369–1600.2012.00441.x. [Epub ahead of print.]
Nery, F.G., Stanley, J.A., et al. (2010). Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J. Psychiatr. Res. 44:278–285.
Noda, S., Yamanouchi, N., et al. (1996). Proton MR spectroscopy in solvent abusers. Ann. NY Acad. Sci. 801:441–444.
Nordahl, T.E., Salo, R., et al. (2005). Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch. Gen. Psychiatry 62:444–452.
O’Neill, J., Cardenas, V.A., et al. (2001a). Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin. Exp. Res. 25:1673–1682.
O’Neill, J., Cardenas, V.A., et al. (2001b). Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 6:347–361.
Obergriesser, T., Ende, G., et al. (2001). Hippocampal 1H-MRSI in ecstasy users. Eur. Arch. Psychiatry Clin. Neurosci. 251:114–116.
Offiah, C., and Hall, E. (2008). Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin. Radiol. 63:146–152.
Parks, M.H., Dawant, B.M., et al. (2002). Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin. Exp. Res. 26:1368–1380.
Plaitakis, A., and Shashidharan, P. (2000). Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson’s disease. J. Neurol. 247(Suppl 2):II25–II35.
Prescot, A.P., Locatelli, A.E., et al. (2011). Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage 57:69–75.
Reneman, L., Majoie, C.B., et al. (2002). Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. Am. J. Neuroradiol. 23:231–237.
Reneman, L., Majoie, C.B., et al. (2001). Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol. Psychiatry 50:550–554.
Romberger, D.J., and Grant, K. (2004). Alcohol consumption and smoking status: the role of smoking cessation. Biomed. Pharmacother. 58:77–83.
Ross, B., Kreis, R., et al. (1992). Clinical tools for the 90s: magnetic resonance spectroscopy and metabolite imaging. Eur. J. Radiol. 14:128–140.
Sailasuta, N., Abulseoud, O., et al. (2010). Glial dysfunction in abstinent methamphetamine abusers. J. Cereb. Blood Flow Metab. 30:950–960.
Salgado, R.A., Jorens, P.G., et al. (2010). Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. Am. J. Neuroradiol. 31:565–566.
Salo, R., Nordahl, T.E., et al. (2007). Attentional control and brain metabolite levels in methamphetamine abusers. Biol. Psychiatry 61:1272–1280.
Salo, R., Buonocore, M.H., et al. (2011). Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend. 113:133–138.
Salo, R., Nordahl, T.E., et al. (2011). Spatial inhibition and the visual cortex: a magnetic resonance spectroscopy imaging study. Neuropsychologia. 49:830–838.
Schmaal, L., Veltman, D.J., et al. (2012). N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology 37:2143–2152.
Schuff, N., Meyerhoff, D.J., et al. (2006). N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv. Exp. Med. Biol. 576:241–262; discussion 361–363.
Schweinsburg, B.C., Taylor, M.J., et al. (2001). Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin. Exp. Res. 25:924–934.
Schweinsburg, B.C., Taylor, M.J., et al. (2000). Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: a preliminary MR spectroscopy study. Alcohol Clin. Exp. Res. 24:699–705.
Seitz, D., Widmann, U., et al. (1999). Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin. Exp. Res. 23:158–163.
Sekine, Y., Minabe, Y., et al. (2002). Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology 27:453–461.
Silveri, M.M., Jensen, J.E., et al. (2011). Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res. 191:201–211.
Silveri, M.M., Pollack, M.H., et al. (2004). Cerebral phosphorus metabolite and transverse relaxation time abnormalities in heroin-dependent subjects at onset of methadone maintenance treatment. Psychiatry Res. 131:217–226.
Silverstone, P.H., O’Donnell, T., et al. (2002). Dextro-amphetamine increases phosphoinositol cycle activity in volunteers: an MRS study. Hum. Psychopharmacol. 17:425–429.
Smith, L.M., Chang, L., et al. (2001a). Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics 107:227–231.
Smith, L.M., Chang, L., et al. (2001b). Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology 57:255–260.
Streeter, C.C., Hennen, J., et al. (2005). Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl.) 182:516–526.
Sullivan, E.V., and Pfefferbaum, A. (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl.) 180:583–594.
Sung, Y.H., Cho, S.C., et al. (2007). Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 88:28–35.
Takebayashi, K., Sekine, Y., et al. (2004). Metabolite alterations in basal ganglia associated with psychiatric symptoms of abstinent toluene users: a proton MRS study. Neuropsychopharmacology 29:1019–1026.
Taylor, M.J., Schweinsburg, B.C., et al. (2007). Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J. Neurovirol. 13:150–159.
Thoma, R., Mullins, P., et al. (2011). Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology 36:1359–1365.
Tsai, G., and Coyle, J.T. (1995). N-acetylaspartate in neuropsychiatric disorders. Prog. Neurobiol. 46:531–540.
Umhau, J.C., Momenan, R., et al. (2010) Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals. Arch. Gen. Psychiatry 67(10):1069–77.
Vella, S., Kreis, R., et al. (2003). Acute leukoencephalopathy after inhalation of a single dose of heroin. Neuropediatrics 34:100–104.
Wang, J.J., Durazzo, T.C., et al. (2009). MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 22:516–522.
Worley, P.F., Baraban, J.M., et al. (1987). Inositol trisphosphate receptor localization in brain: variable stoichiometry with protein kinase C. Nature 325:159–161.
Yang, S., Salmeron, B.J., et al. (2009). Lower glutamate levels in rostral anterior cingulate of chronic cocaine users: A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 174:171–176.
Yeo, R.A., Gangestad, S.W., et al. (2011). Rare copy number deletions predict individual variation in human brain metabolite concentrations in individuals with alcohol use disorders. Biol. Psychiatry 70:537–544.
Yoon, S.J., Lyoo, I.K., et al. (2010). Neurochemical alterations in methamphetamine-dependent patients treated with cytidine-5′-diphosphate choline: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology 35:1165–1173.
Yucel, M., Lubman, D.I., et al. (2007). A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol. Psychiatry 12:611, 691–702.