
In the multiple-choice section, this topic appears in about 9 out of 75 questions. In the free-response section, this topic appears every year.
All bonds occur because of electrostatic attractions. Atoms stick together to form molecules, and atoms and molecules stick together to form liquids or solids because the negatively charged electrons of one atom are attracted to the positively charged nucleus of another atom.
Electrostatic forces are governed by Coulomb’s law, and the entire study of bonding comes down to understanding how Coulomb’s law applies to different chemical situations.
Let’s take a look at Coulomb’s law.

You should be able to extrapolate two things from the formula above.
Atoms join to form molecules because atoms like to have a full outer shell of electrons. This usually means having eight electrons in the outer shell. So atoms with too many or too few electrons in their valence shells will find one another and pass the electrons around until all the atoms in the molecule have stable outer shells. Sometimes an atom will give up electrons completely to another atom, forming an ionic bond. Sometimes atoms share electrons, forming covalent bonds.
An ionic solid is held together by the electrostatic attractions between ions that are next to one another in a lattice structure. They often occur between metals and nonmetals. In an ionic bond, electrons are not shared. Instead, one atom gives up electrons and becomes a positively charged ion while the other atom accepts electrons and becomes a negatively charged ion.
The two ions in an ionic bond are held together by electrostatic forces. In the diagram below, a sodium atom has given up its single valence electron to a chlorine atom, which has seven valence electrons and uses the electron to complete its outer shell (with eight). The two atoms are then held together by the positive and negative charges on the ions.
![]()
The same electrostatic attractions that hold together the ions in a molecule of NaCl hold together a crystal of NaCl, so there is no real distinction between the molecules and the solid. Ionic bonds are strong, and substances held together by ionic bonds have high melting and boiling points.
Coulomb’s law states that more highly charged ions will form stronger bonds than less highly charged ions and smaller ions will form stronger bonds than larger ions. So an ionic bond composed of ions with +2 and −2 charges will be stronger than a bond composed of ions with +1 and −1 charges. Also from Coulomb’s law, we know that the smaller the ions in an ionic bond, the stronger the bond. This is because a small ionic radius allows the charges to get closer together and increases the force between them.
In an ionic solid, each electron is localized around a particular atom, so electrons do not move around the lattice; this makes ionic solids poor conductors of electricity. Ionic liquids, however, do conduct electricity because the ions themselves are free to move about in the liquid phase, although the electrons are still localized around particular atoms. Salts are held together by ionic bonds.
In a covalent bond, two atoms share electrons. Each atom counts the shared electrons as part of its valence shell. In this way, both atoms achieve complete outer shells.
In the diagram below, two fluorine atoms, each of which has seven valence electrons and needs one electron to complete its valence shell, form a covalent bond. Each atom donates an electron to the bond, which is considered to be part of the valence shell of both atoms.
![]()
The number of covalent bonds an atom can form is the same as the number of electrons in its valence shell.
The first covalent bond formed between two atoms is called a sigma (σ) bond. All single bonds are sigma bonds. If additional bonds between the two atoms are formed, they are called pi (π) bonds. The second bond in a double bond is a pi bond and the second and third bonds in a triple bond are also pi bonds. Double and triple bonds are stronger and shorter than single bonds, but they are not twice or triple the strength.

Single bonds have one sigma (σ) bond and a bond order of one.
The single bond has the longest bond length and the least bond energy.
In the F2 molecule shown above, the two fluorine atoms share the electrons equally, but that’s not usually the case in molecules. Usually, one of the atoms (the more electronegative one) will exert a stronger pull on the electrons in the bond—not enough to make the bond ionic, but enough to keep the electrons on one side of the molecule more than on the other side. This gives the molecule a dipole. That is, the side of the molecule where the electrons spend more time will be negative and the side of the molecule where the electrons spend less time will be positive.
The polarity of a molecule is measured by the dipole moment. The larger the dipole moment, the more polar the molecule. The greater the charge at the ends of the dipole and the greater the distance between the charges, the greater the value of the dipole moment.
At some point on the test, you’ll be asked to draw the Lewis structure for a molecule or polyatomic ion. Here’s how to do it.
(a) If the central atom has fewer than eight electrons, remove an electron pair from an outer atom and add another bond between that outer atom and the central atom. Do this until the central atom has a complete octet.
(b) If the central atom has a complete octet, you are finished.
(c) If the central atom has more than eight electrons, that’s okay too.
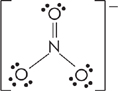
Let’s find the Lewis structure for the ![]() ion.
ion.

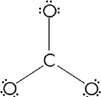

When we put a double bond into the ![]() ion, we place it on any one of the oxygen atoms, as shown below.
ion, we place it on any one of the oxygen atoms, as shown below.

All three resonance forms are considered to exist simultaneously, and the strength and lengths of all three bonds are the same: somewhere between the strength and length of a single bond and a double bond.
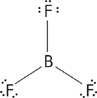
Some atoms can have a complete outer shell with less than eight electrons; for example, hydrogen can have a maximum of two electrons, and beryllium can be stable with only four valence electrons, as in BeH2.
![]()
Boron can be stable with only six valence electrons, as in BF3.
In molecules that have d subshells available, the central atom can have more than eight valence electrons.
Here are some examples.


Molecules almost always have an even number of electrons, allowing electrons to be paired, but there are exceptions, usually involving nitrogen.
![]()

Note that NO2 can be shown with either of two resonance forms.
Electrons repel one another, so when atoms come together to form a molecule, the molecule will assume the shape that keeps its different electron pairs as far apart as possible. When we predict the geometries of molecules using this idea, we are using the valence shell electron-pair repulsion (VSEPR) model.
In a molecule with more than two atoms, the shape of the molecule is determined by the number of electron pairs on the central atom. The central atom forms hybrid orbitals, each of which has a standard shape. Variations on the standard shape occur depending on the number of bonding pairs and lone pairs of electrons on the central atom.
Here are some things you should remember when dealing with the VSEPR model.
The tables on the following pages show the different hybridizations and geometries that you might see on the test.
If the central atom has 2 electron pairs, then it has sp hybridization and its basic shape is linear.

If the central atom has 3 electron pairs, then it has sp2 hybridization and its basic shape is trigonal planar; its bond angles are about 120°.


If the central atom has 4 electron pairs, then it has sp3 hybridization and its basic shape is tetrahedral; its bond angles are about 109.5°.



If the central atom has 5 electron pairs, then it has dsp3 hybridization and its basic shape is trigonal bipyramidal.

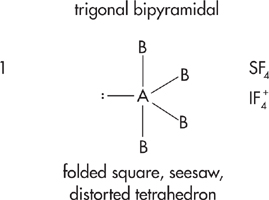


If the central atom has 6 electron pairs, then it has d 2sp3 hybridization and its basic shape is octahedral.

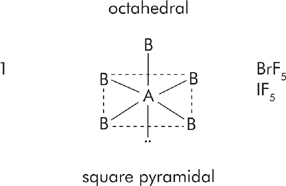

In trigonal bipyrimidal shapes, place the lone pairs in axial position first. In octahedral shapes, place lone pairs in equatorial position first.
Sometimes the bonds that hold the atoms or ions in liquids and solids together are the same strong bonds that hold the atoms or ions together in molecules. Intermolecular forces only exist with covalently bonded molecules. We will also discuss metallic and network forces here.
In a network solid, atoms are held together in a lattice of covalent bonds. You can visualize a network solid as one big molecule. Network solids are very hard and have very high melting and boiling points.
The electrons in a network solid are localized in covalent bonds between particular atoms, so they are not free to move about the lattice. This makes network solids poor conductors of electricity.
The most commonly seen network solids are compounds of carbon (diamond) and silicon (SiO2—quartz). The hardness of diamond is due to the tetrahedral network structure formed by carbon atoms whose electrons are configured in sp3 hybridization. The three-dimensional complexity of the tetrahedral network means that there are no natural seams along which a diamond can be broken.
Metallic substances can be compared with a group of nuclei surrounded by a sea of mobile electrons. As with ionic and network substances, a metallic substance can be visualized as one large molecule. Most metals are very hard, although the freedom of movement of electrons in metals makes them malleable and ductile. All metals, except mercury, are solids at room temperature, and most metals have high boiling and melting points.
Metals composed of atoms with smaller nuclei tend to form stronger bonds than metals made up of atoms with larger nuclei. This is because smaller sized nuclei allow the positively charged nuclei to be closer to the negatively charged electrons, increasing the attractive force from Coulomb’s law.
The electrons in a metallic substance are delocalized and can move freely throughout the substance. The freedom of the electrons in a metal makes it a very good conductor of heat and electricity.
Dipole−dipole forces occur between neutral, polar molecules: the positive end of one polar molecule is attracted to the negative end of another polar molecule.
Molecules with greater polarity will have greater dipole−dipole attraction, so molecules with larger dipole moments tend to have higher melting and boiling points. Dipole−dipole attractions are relatively weak, however, and these substances melt and boil at very low temperatures. Most substances held together by dipole−dipole attraction are gases or liquids at room temperature.
London dispersion forces occur between neutral, nonpolar molecules. These very weak attractions occur because of the random motions of electrons on atoms within molecules. At a given moment, a nonpolar molecule might have more electrons on one side than on the other, giving it an instantaneous polarity. For that fleeting instant, the molecule will act as a very weak dipole.
Since London dispersion forces depend on the random motions of electrons, molecules with more electrons will experience greater London dispersion forces. So among substances that experience only London dispersion forces, the one with more electrons will generally have higher melting and boiling points. London dispersion forces are even weaker than dipole−dipole forces, so substances that experience only London dispersion forces melt and boil at extremely low temperatures and tend to be gases at room temperature.
Hydrogen bonds are similar to dipole–dipole attractions. In a hydrogen bond, the positively charged hydrogen end of a molecule is attracted to the negatively charged end of another molecule containing an extremely electronegative element (fluorine, oxygen, or nitrogen—F, O, N).
Hydrogen bonds are much stronger than dipole–dipole forces because when a hydrogen atom gives up its lone electron to a bond, its positively charged nucleus is left virtually unshielded. Substances that have hydrogen bonds, like water and ammonia, have higher melting and boiling points than substances that are held together by dipole–dipole forces.
Water is less dense as a solid than as a liquid because its hydrogen bonds force the molecules in ice to form a crystal structure, which keeps them farther apart than they are in the liquid form.
Questions 1–4
(A) Metallic bonding
(B) Network covalent bonding
(C) Hydrogen bonding
(D) Ionic bonding
(E) London dispersion forces
1. Solids exhibiting this kind of bonding are excellent conductors of heat.
2. This kind of bonding is the reason that water is more dense than ice.
3. This kind of bonding exists between atoms with very different electronegativities.
4. The stability exhibited by diamonds is due to this kind of bonding.
Questions 5–7
(A) CH4
(B) NH3
(C) NaCl
(D) N2
(E) H2
5. This substance undergoes ionic bonding.
6. This molecule contains two pi (π) bonds.
7. This substance undergoes hydrogen bonding.
Questions 8–10
(A) BF3
(B) CO2
(C) H2O
(D) CF4
(E) PH3
8. The central atom in this molecule forms sp2 hybrid orbitals.
9. This molecule has a tetrahedral structure.
10. This molecule has a linear structure.
11. A liquid whose molecules are held together by which of the following forces would be expected to have the lowest boiling point?
(A) Ionic bonds
(B) London dispersion forces
(C) Hydrogen bonds
(D) Metallic bonds
(E) Network bonds
12. Hydrogen bonding would be seen in a sample of which of the following substances?
(A) CH4
(B) H2
(C) H2O
(D) HI
(E) All of the above
13. Which of the following species does NOT have a tetrahedral structure?
(A) CH4
(B) NH4+
(C) SF4
(D) AlCl4−
(E) CBr4
14. Which form of orbital hybridization can form molecules with shapes that are either trigonal pyramidal or tetrahedral?
(A) sp
(B) sp2
(C) sp3
(D) d2sp
(E) dsp3
15. The six carbon atoms in a benzene molecule are shown in different resonance forms as three single bonds and three double bonds. If the length of a single carbon–carbon bond is 154 pm and the length of a double carbon–carbon bond is 133 pm, what length would be expected for the carbon–carbon bonds in benzene?
(A) 126 pm
(B) 133 pm
(C) 140 pm
(D) 154 pm
(E) 169 pm
16. Which of the following could be the Lewis structure for sulfur trioxide?
| (A) |

|
| (B) |
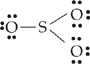
|
| (C) |

|
| (D) |

|
| (E) |
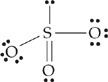
|
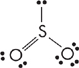
17. In which of the following species does the central atom NOT form sp2 hybrid orbitals?
(A) SO2
(B) BF3
(C) NO3–
(D) SO3
(E) PCl3
18. A molecule whose central atom has d2sp3 hybridization can have which of the following shapes?
I. Tetrahedral
II. Square pyramidal
III. Square planar
(A) I only
(B) III only
(C) I and II only
(D) II and III only
(E) I, II, and III
19. Which of the following molecules will have a Lewis dot structure with exactly one unshared electron pair on the central atom?
(A) H2O
(B) PH3
(C) PCl5
(D) CH2Cl2
(E) BeCl2
20. Which of the following lists of species is in order of increasing boiling points?
(A) H2, N2, NH3
(B) N2, NH3, H2
(C) NH3, H2, N2
(D) NH3, N2, H2
(E) H2, NH3, N2
21. Solid NaCl melts at a temperature of 800°C, while solid NaBr melts at 750°C. Which of the following is an explanation for the higher melting point of NaCl?
(A) A chlorine ion has less mass than a bromine ion.
(B) A chlorine ion has a greater negative charge than a bromine ion.
(C) A chlorine ion has a lesser negative charge than a bromine ion.
(D) A chlorine ion is smaller than a bromine ion.
(E) A chlorine ion is larger than a bromine ion.
22. Which of the compounds listed below would require the greatest energy to separate it into ions in the gaseous state?
(A) NaCl
(B) NaI
(C) MgO
(D) Na2O
(E) MgCl2
23. Which sample of the following compounds contains both ionic and covalent bonds?
(A) H2O2
(B) CH3Cl
(C) C2H3OH
(D) NaNO3
(E) NH2OH
24. Which of the molecules listed below has the largest dipole moment?
(A) Cl2
(B) HCl
(C) SO3
(D) NO
(E) N2
25. Which of the following statements about boiling points is (are) correct?
I. H2O boils at a higher temperature than CO2.
II. Ar boils at a higher temperature than He.
III. Rb boils at a higher temperature than Na.
(A) I only
(B) I and II only
(C) I and III only
(D) II and III only
(E) I, II, and III
1. Use the principles of bonding and molecular structure to explain the following statements.
(a) The boiling point of argon is –186°C, whereas the boiling point of neon is –246°C.
(b) Solid sodium melts at 98°C, but solid potassium melts at 64°C.
(c) More energy is required to break up a CaO(s) crystal into ions than to break up a KF(s) crystal into ions.
(d) Molten KF conducts electricity, but solid KF does not.
2. The carbonate ion ![]() is formed when carbon dioxide, CO2, reacts with slightly basic cold water.
is formed when carbon dioxide, CO2, reacts with slightly basic cold water.
(a) (i) Draw the Lewis electron dot structure for the carbonate ion. Include resonance forms if they apply.
(ii) Draw the Lewis electron dot structure for carbon dioxide.
(b) Describe the hybridization of carbon in the carbonate ion.
(c) (i) Describe the relative lengths of the three C–O bonds in the carbonate ion.
(ii) Compare the average length of the C–O bonds in the carbonate ion to the average length of the C–O bonds in carbon dioxide.
(a) Explain the differences in the properties given in the table above for each of the following pairs.
(i) The bond strengths of N2 and O2
(ii) The bond lengths of H2 and Cl2
(iii) The boiling points of O2 and Cl2
(b) Use the principles of molecular bonding to explain why H2 and O2 are gases at room temperature, while H2O is a liquid at room temperature.
4. H2S, ![]() , XeF2, ICl4–
, XeF2, ICl4–
(a) Draw a Lewis electron dot diagram for each of the molecules listed above.
(b) Use the valence shell electron-pair repulsion (VSEPR) model to predict the geometry of each of the molecules.
5. Use the principles of bonding and molecular structure to explain the following statements.
(a) The angle between the N–F bonds in NF3 is smaller than the angle between the B–F bonds in BF3.
(b) I2(s) is insoluble in water, but it is soluble in carbon tetrachloride.
(c) Diamond is one of the hardest substances on Earth.
(d) HCl has a lower boiling point than either HF or HBr.
1. A In metallic bonding, nuclei are surrounded by a sea of mobile electrons. The electrons’ freedom to move allows them to conduct heat and electricity.
2. C When ice forms, the hydrogen bonds join the molecules in a lattice structure, which forces them to remain farther apart than they were in the liquid form. Because the molecules are farther apart in the solid than in the liquid, the solid (ice) is less dense than the liquid.
3. D Electronegativity is a measure of how much pull an atom exerts on another atom’s electrons. If the difference in electronegativities is large enough (greater than 1.7) then the more electronegative atom will simply take an electron away from the other atom. The two atoms will then be held together by electrostatic attraction (the atom that has gained an electron becomes negative and the atom that has lost an electron becomes positive). That’s an ionic bond.
4. B The carbon atoms in diamond are held together by a network of covalent bonds. The carbon atoms form sp3 hybrid orbitals, resulting in a tetrahedral structure, which is very stable and has no simple breaking points.
5. C NaCl is composed of two elements that have very different electronegativities than each other, so Na gives up an electron to Cl and the two are held together by electrostatic attraction in an ionic bond.
6. D N2 contains a triple bond, so it has one sigma (σ) bond and two pi (π) bonds.
7. B Hydrogen bonding occurs between hydrogen atoms of one molecule and electronegative elements (F, O, or N) of another molecule. So in ammonia, hydrogens from one ammonia molecule will form bonds with nitrogens from another ammonia molecule.
8. A In BF3, boron forms three bonds with fluorine atoms and has no unbonded valence electrons, so it must form sp2 hybrid orbitals.

9. D CF4 forms a tetrahedral structure as shown in the diagram below. The central carbon atom is hybridized sp3.

10. B CO2 forms a linear structure as shown in the diagram below. The central carbon atom is sp hybridized.
![]()
11. B A liquid with a low boiling point must be held together by weak bonds. London dispersion forces are the weakest kind of intermolecular forces.
12. C Hydrogen bonding specifically describes the attraction experienced by a hydrogen atom in one molecule to an extremely electronegative element (F, O, or N) in another molecule. So in water, a hydrogen atom in one water molecule will be attracted to an oxygen atom in another water molecule.

13. C SF4 has 34 valence electrons distributed in the Lewis dot structure and shape shown below.

In this molecule, sulfur forms dsp3 hybrid orbitals, which have a trigonal bipyramid structure. Because SF4 has one unshared electron pair, the molecule takes the “seesaw” or “folded square” shape.
Choices (A) and (B), CH4 and NH4+, each have 8 valence electrons distributed in the same Lewis dot structure and shape, shown below for NH4+.

In these molecules, the central atom forms sp3 hybrid orbitals, which have a tetrahedral structure. There are no unshared electron pairs on the central atom, so the molecules are tetrahedral.
Choices (D) and (E), AlCl4– and CBr4, each have 32 valence electrons distributed in the same Lewis dot structure and shape, shown below for AlCl4–.
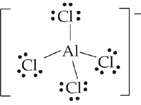
In these molecules, the central atom forms sp3 hybrid orbitals, which have a tetrahedral structure. There are no unshared electron pairs on the central atom, so the molecules are tetrahedral.
14. C The sp3 hybrid orbitals take a tetrahedral shape if the central atom has no unshared electron pairs (CH4, for instance). If the central atom has one unshared electron pair, the molecule takes the trigonal pyramid shape (NH3, for instance).
15. C Resonance is used to describe a situation that lies between single and double bonds, so the bond length would also be expected to be in between that of single and double bonds.
16.
A Choice (A) has the correct number of valence electrons (6 + 6 + 6 + 4 + 2 = 24), and 8 valence electrons on each atom.
About the other choices:
(B) There are only 6 valence electrons on the sulfur atom.
(C) There are too many valence electrons (10) on the sulfur atom.
(D) There are too many valence electrons (26).
(E) There are too many valence electrons (10) on the sulfur atom and not enough (6) on one of the oxygen atoms.
17. E PCl3 is the only one that doesn’t form sp2 hybrid orbitals, forming sp3 orbitals instead. The Lewis dot structures for all of the choices are shown below (note that boron does not need an octet).
| (A) |
|
| (B) |

|
| (C) |
|
| (D) |

|
| (E) |

|
18. D A molecule with d2sp3 hybridization has octahedral structure if the central atom has no unbonded electrons (SF6, for instance).
If the central atom has one unbonded electron pair, the molecule is square pyramidal (IF5, for instance).
If the central atom has two unbonded electron pairs, the molecule is square planar (XeF4, for instance).
A molecule with d2sp3 hybridization can never be tetrahedral.
19. B The Lewis dot structures for the answer choices are shown below. Only PH3 has a single unshared electron pair on its central atom.
| (A) |

|
| (B) |
|
| (C) |

|
| (D) |

|
| (E) |
|
20. A H2 experiences only van der Waals forces and has the lowest boiling point.
N2 also experiences only van der Waals forces, but it is larger than H2 and has more electrons, so it has stronger van der Waals interactions with other molecules.
NH3 is polar and undergoes hydrogen bonding, so it has the strongest intermolecular interactions and the highest boiling point.
21. D The greater the bond strength, the higher the melting point. Both NaCl and NaBr are held together by ionic bonds, and the strength of ionic bonds depends on the strength of the charges and the sizes of the ions. The strength of the charges doesn’t matter in this case because both chlorine and bromine ions have charges of –1. The reason for NaCl’s higher melting point is that chlorine ions are smaller than bromine ions, so the ions can get closer together.
22. C The energy required to separate an ionic compound into gaseous ions is called the lattice energy. The most important factor in determining the lattice energy is the strength of the charges on the two ions. In MgO, the Mg ion is +2 and the O ion is –2. All of the other choices listed contain +1 or –1 ions, so their lattice energies will not be as large.
23. D A sample of NaNO3 contains an Na+ ion and an NO3– polyatomic ion, which are held together with ionic bonds. The atoms in the NO3– ion are held together by covalent bonds. All of the bonds in the other choices are covalent bonds.
24. B The bond that holds HCl together is a covalent bond with a large polarity. The bond that holds NO together is also polar covalent, but its polarity is very small because N and O are so close together on the periodic table. Cl2 and N2 are nonpolar because they share electrons equally, and SO3 is nonpolar because it is symmetrical (trigonal planar). Nonpolar molecules have dipole moments of zero.
25.
B Groups I and II are listed in order of increasing melting points. Remember, stronger bonds or intermolecular forces mean higher melting points.
I. H2O has hydrogen bonding, which makes its intermolecular forces much stronger than those of CO2, which is nonpolar and exhibits only London dispersion forces. So water has a higher boiling point than CO2.
II. These are
nonpolar atoms, so they are held together by weak London dispersion forces. Because London dispersion forces depend on the random movement of electrons, the more electrons in a compound the stronger the London dispersion forces. Ar has more electrons than He, so it has stronger London dispersion forces and a higher boiling point.
III. These are held together by metallic bonding. In general, the smaller the nucleus of a metal, the stronger the metallic bonds. Rb has a
larger nucleus than Na, so it has weaker metallic bonds and a lower boiling point, so this statement is not correct.
1. (a) Molecules of noble gases in the liquid phase are held together by London dispersion forces, which are weak interactions brought about by instantaneous polarities in nonpolar atoms and molecules.
Atoms with more electrons are more easily polarized and experience stronger London dispersion
forces. Argon has more electrons than neon, so it experiences stronger London dispersion forces and boils at a higher temperature.
(b) Sodium and potassium are held together by metallic bonds and positively charged ions in a delocalized sea of electrons.
Potassium is larger than sodium, so the electrostatic attractions that hold the atoms together act at a greater distance, reducing the attractive force and resulting in its lower melting
point.
(c) Both CaO(s) and KF(s) are held together by ionic bonds in crystal lattices.
Ionic bonds are held together by an electrostatic force, which can be determined by using Coulomb’s law.
![]()
CaO is more highly charged, with Ca2+ bonded to O2–. So for CaO, Q1 and Q2 are +2 and –2.
KF is not as highly charged, with K+ bonded to F–. So for KF, Q1 and
Q2 are +1 and –1.
CaO is held together by stronger forces and is more difficult to break apart.
(d) KF is composed of K+ and F– ions. In the liquid (molten) state, these ions are free to move and can thus conduct electricity.
In the solid state, the K+ and F– ions are fixed in a crystal
lattice and their electrons are localized around them, so there is no charge that is free to move and thus no conduction of electricity.
2. (a)

(b) The central carbon atom forms three sigma bonds with oxygen atoms and has no free electron pairs, so its hybridization must be sp2.
(c) (i) All three bonds will be the same length because no particular resonance form is preferred over the others. The actual structure is an average of the resonance structures.
(ii) The C–O bonds in the carbonate ion have resonance forms between single
and double bonds, while the C–O bonds in carbon dioxide are both double bonds.
The bonds in the carbonate ion will be shorter than single bonds and longer than double bonds, so the carbonate bonds will be longer than the carbon dioxide bonds.
3. (a) (i) The bond strength of N2 is larger than the bond strength of O2 because N2 molecules have triple bonds and O2 molecules have double bonds. Triple bonds are stronger and shorter than double
bonds.
(ii) The bond length of H2 is smaller than the bond length of Cl2 because hydrogen is a smaller atom than chlorine, allowing the hydrogen nuclei to be closer together.
(iii) Liquid oxygen and liquid chlorine are both nonpolar substances that experience only London dispersion forces of attraction. These forces are greater for Cl2 because it has more electrons (which makes it more
polarizable), so Cl2 has a higher boiling point than O2.
(b) H2 and O2 are both nonpolar molecules that experience only London dispersion forces, which are too weak to form the bonds required for a substance to be liquid at room temperature.
H2O is a polar substance whose molecules form hydrogen bonds with each other. Hydrogen bonds are
strong enough to form the bonds required in a liquid at room temperature.
4. (a)

(b) H2S has two bonds and two free electron pairs on the central S atom. The greatest distance between the electron pairs is achieved by tetrahedral arrangement. The electron pairs at two of the four corners will cause the molecule to have a bent shape, like water.
SO42– has four bonds around the central S atom and no free electron pairs. The four bonded pairs
will be farthest apart when they are arranged in a tetrahedral shape, so the molecule is tetrahedral.
XeF2 has two bonds and three free electron pairs on the central Xe atom. The greatest distance between the electron pairs can be achieved by a trigonal bipyramidal arrangement. The three free electron pairs will occupy the equatorial positions, which are 120 degrees apart, to minimize repulsion. The two F atoms are at the
poles, so the molecule is linear.
ICl4– has four bonds and two free electron pairs on the central I atom. The greatest distance between the electron pairs can be achieved by an octahedral arrangement. The two free electron pairs will be opposite each other to minimize repulsion. The four Cl atoms are in the equatorial positions, so the molecule is square planar.
5. (a) BF3 has three bonds on the central B atom and no free electron pairs, so the structure of BF3 is trigonal planar, with each of the bonds 120 degrees apart.
NF3 has three bonds and one free electron pair on the central N
atom. The four electron pairs are pointed toward the corners of a tetrahedron, 109.5 degrees apart. The added repulsion from the free electron pair causes the N–F bonds to be even closer together, and the angle between them is more like 107°.
(b) Polar solvents are best at dissolving polar solutes. Nonpolar solvents are best at dissolving nonpolar solutes.
I2(s) is nonpolar, so it dissolves better in carbon
tetrachloride, CCl4, which is nonpolar, than in water, H2O, which is polar.
(c) The carbon atoms in diamond are bonded together in a tetrahedral network, with each carbon atom bonded to three other carbon atoms. The tetrahedral structure of the network bonds does not leave any seams along which the diamond can be broken, so a diamond behaves as one big molecule with no weaknesses.
(d) HBr and HCl are polar
molecules. In liquid form, both substances are held together by dipole–dipole interactions. These interactions are stronger for molecules with more electrons, so HBr has stronger intermolecular bonds and a higher boiling point.
HF has a higher boiling point than HCl because HF undergoes hydrogen bonding, while HCl does not; this causes HF to remain a liquid at higher temperatures than HCl, although HF is a polar molecule with fewer electrons than HCl.