In the multiple-choice section, this topic appears in about 9 out of 75 questions. In the free-response section, this topic appears almost every year.
Molarity (M) expresses the concentration of a solution in terms of volume. It is the most widely used unit of concentration, turning up in calculations involving equilibrium, acids and bases, and electrochemistry, among others.
When you see a chemical symbol in brackets on the test, that means they are talking about molarity. For instance, “[Na+]” is the same as “the molar concentration (molarity) of sodium ions.”
![]()
Molality (m) expresses concentration in terms of the mass of a solvent. It is the unit of concentration used for determining the effect of most colligative properties, where the number of moles of solute is more important than the nature of the solute.
![]()
Molarity and molality differ in two ways: Molarity tells you about moles of solute per volume of the entire solution (that is, the solute and the solvent), whereas molality tells you about moles of solute per mass of the solvent. Keeping in mind that one liter of water weighs one kilogram, and that for a dilute solution, the amount of solution is about the same as the amount of solvent, you should be able to see that for dilute aqueous solutions, molarity and molality are basically the same.
Mole fraction (Xs) gives the fraction of moles of a given substance (S) out of the total moles present in a sample. It is used in determining how the vapor pressure of a solution is lowered by the addition of a solute.
![]()
There is a basic rule for remembering which solutes will dissolve in which solvents.
Like dissolves like
That means that polar or ionic solutes (like salt) will dissolve in polar solvents (like water). That also means that nonpolar solutes (like organic compounds) are best dissolved in nonpolar solvents. When an ionic substance dissolves, it breaks up into ions. That’s dissociation. Free ions in a solution are called electrolytes because they can conduct electricity.
The van’t Hoff factor (i) tells how many ions one unit of a substance will dissociate into in solution. For instance
Colligative properties are properties of a solution that depend on the number of solute particles in the solution. For colligative properties, the identity of the particles is not important.
When a solute is added to a solution, the boiling point of the solution increases.
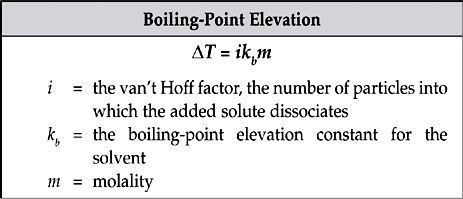
When solute is added to a solution, the freezing point of the solution decreases.

When a solute is added to a solution, the vapor pressure of the solution will decrease. You may want to note that a direct result of the lowering of vapor pressure of a solution is the raising of its boiling point.

When a pure solvent and a solution are separated by a membrane that only allows solvent to pass through, the solvent will try to pass through the membrane to dilute the solution. The pressure that must be applied to stop this process is called the osmotic pressure. The greater the concentration of solute in the solution, the greater the osmotic pressure. The equation for osmotic pressure takes a form that is similar to the ideal gas equation, as shown below.

Density is the measure of mass per unit volume. Density can be used to describe liquids, solids, or gases. Because density relates mass and volume, it is useful if you need to convert between molarity, which deals with volume, and molality, which deals with mass.

Roughly speaking, a salt can be considered “soluble” if more than 1 gram of the salt can be dissolved in 100 milliliters of water. Soluble salts are usually assumed to dissociate completely in aqueous solution. Most, but not all, solids become more soluble in a liquid as the temperature is increased.
Salts that are “slightly soluble” and “insoluble” still dissociate in solution to some extent. The solubility product (Ksp) is a measure of the extent of a salt’s dissociation in solution. The Ksp is one of the forms of the equilibrium expression, which we’ll discuss in Chapter 10. The greater the value of the solubility product for a salt, the more soluble the salt.
Solubility Product
For the reaction
AaBb(s) ![]() a Ab+(aq) + b Ba–(aq)
a Ab+(aq) + b Ba–(aq)
The solubility expression is
Ksp = [Ab+]a[Ba–]b
For example:
CaF2(s) ![]() Ca2+(aq) + 2 F–(aq) Ksp = [Ca2+][F–]2
Ca2+(aq) + 2 F–(aq) Ksp = [Ca2+][F–]2
Ag2CrO4(s) ![]() 2 Ag+(aq) + CrO42–(aq) Ksp = [Ag+]2[CrO42–]
2 Ag+(aq) + CrO42–(aq) Ksp = [Ag+]2[CrO42–]
CuI(s) ![]() Cu+(aq) + I–(aq) Ksp = [Cu+][I–]
Cu+(aq) + I–(aq) Ksp = [Cu+][I–]
Let’s look at the solubility expression for AgCl.
Ksp = [Ag+][Cl–] = 1.6 × 10–10
If we throw a block of solid AgCl into a beaker of water, we can tell from the Ksp what the concentrations of Ag+ and Cl– will be at equilibrium. For every unit of AgCl that dissociates, we get one Ag+ and one Cl–, so we can solve the equation above as follows:
[Ag+][Cl–] = 1.6 × 10–10
(x)(x) = 1.6 × 10–10
x2 = 1.6 × 10–10
x = [Ag+] = [Cl–] = 1.3 × 10–5 M
So there are very small amounts of Ag+ and Cl– in the solution.
Let’s say we add 0.10 mole of NaCl to 1 liter of the AgCl solution. NaCl dissociates completely, so that’s the same thing as adding 1 mole of Na+ ions and 1 mole of Cl– ions to the solution. The Na+ ions will not affect the AgCl equilibrium, so we can ignore them; but the Cl– ions must be taken into account. That’s because of the common ion effect.
The common ion effect says that the newly added Cl– ions will affect the AgCl equilibrium, although the newly added Cl– ions did not come from AgCl.
Let’s look at the solubility expression again. Now we have 0.10 mole of Cl– ions in 1 liter of the solution, so [Cl–] = 0.10 M.
[Ag+][Cl–] = 1.6 × 10–10
[Ag+](0.10 M) = 1.6 × 10–10
[Ag+] = 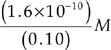
[Ag+] = 1.6 × 10–9 M
Now the number of Ag+ ions in the solution has decreased drastically because of the Cl– ions introduced to the solution by NaCl. So when solutions of AgCl and NaCl, which share a common Cl– ion, are mixed, the more soluble salt (NaCl) can cause the less soluble salt (AgCl) to precipitate. In general, when two salt solutions that share a common ion are mixed, the salt with the lower value for Ksp will precipitate first.
You should have a good working knowledge of the solubilities of common salts. This is especially useful for the part in Section II in which you are asked to predict the outcome of chemical reactions.
• Alkali Metals: Li+, Na+, K+, Rb+, Cs+
All salts of the alkali metals are soluble.
• Ammonium: NH4+
All ammonium salts are soluble.
• Alkaline Earths and Transition Metals
The solubility of these elements varies depending on the identity of the anion.
The following are mostly soluble:
• Nitrate: NO3–
All nitrate salts are soluble.
• Chlorate: ClO3–
All chlorate salts are soluble.
• Perchlorate: ClO4–
All perchlorate salts are soluble.
• Acetate: C2H3O2–
All acetate salts are soluble.
• Chloride, Bromide, Iodide: Cl–, Br–, I–
Salts containing Cl–, Br–, and I– are soluble
EXCEPT for those containing: Ag+, Pb2+, and Hg![]() .
.
• Sulfate: SO![]()
Sulfate salts are soluble
EXCEPT for those containing: Ag+, Pb2+, Hg![]() , Ca2+, Sr2+, and Ba2+.
, Ca2+, Sr2+, and Ba2+.
The following are mostly insoluble:
• Hydroxide: OH–
Hydroxide salts are insoluble
EXCEPT for those containing alkali metals, which are soluble
AND those containing Ca2+, Sr2+, and Ba2+, which fall in the gray area of moderate solubility.
• Carbonate: CO![]()
Carbonate salts are insoluble
EXCEPT for those containing alkali metals and ammonium, which are soluble.
• Phosphate:
![]()
Phosphate salts are insoluble
EXCEPT for those containing alkali metals and ammonium, which are soluble.
• Sulfite: SO![]()
Sulfite salts are insoluble
EXCEPT for those containing alkali metals and ammonium, which are soluble.
• Chromate: CrO![]()
Chromate salts are insoluble
EXCEPT for those containing alkali metals and ammonium, which are soluble.
• Sulfide: S2–
Sulfide salts are insoluble
EXCEPT for those containing alkali metals, the alkaline earths, and ammonium, which are soluble.
The lower the temperature and the higher the pressure of the gas, the more soluble the gas will be. Think of what happens when you open a bottle of warm seltzer. The gas suddenly escapes from the warm liquid when you release the pressure.
Questions 1–4
(A) Molarity (M)
(B) Molality (m)
(C) Density
(D) pH
(E) pOH
1. Has the units moles/kg.
2. This is the negative logarithm of the hydrogen ion concentration.
3. Can have the units grams/liter.
4. Has the units moles per liter.
5. Which of the following is (are) colligative properties?
I. Freezing-point depression
II. Vapor pressure lowering
III. Boiling-point elevation
(A) I only
(B) I and II only
(C) I and III only
(D) II and III only
(E) I, II, and III
6. Which of the following aqueous solutions has the highest boiling point?
(A) 0.5 m NaCl
(B) 0.5 m KBr
(C) 0.5 m CaCl2
(D) 0.5 m C6H12O6
(E) 0.5 m NaNO3
7. When sodium chloride is added to a saturated aqueous solution of silver chloride, which of the following precipitates would be expected to appear?
(A) Sodium
(B) Silver
(C) Chlorine
(D) Sodium chloride
(E) Silver chloride
8. A substance is dissolved in water, forming a 0.50-molar solution. If 4.0 liters of solution contains 240 grams of the substance, what is the molecular mass of the substance?
(A) 60 grams/mole
(B) 120 grams/mole
(C) 240 grams/mole
(D) 480 grams/mole
(E) 640 grams/mole
9. The solubility product, Ksp, of AgCl is 1.8 × 10–10. Which of the following expressions is equal to the solubility of AgCl?
(A) (1.8 × 10–10)2 molar
(B) ![]() molar
molar
(C) 1.8 × 10–10 molar
(D) (2)(1.8 × 10–10 molar
(E) ![]() molar
molar
10. A 0.1-molar solution of which of the following acids will be the best conductor of electricity?
(A) HC2H3O2
(B) H2CO3
(C) H2S
(D) HF
(E) HNO3
11. When 31.0 grams of a nonionic substance is dissolved in 2.00 kg of water, the observed freezing-point depression of the solution is 0.93°C. If kf for water is 1.86°C/m, which of the following expressions is equal to the molar mass of the substance?
(A) ![]() g/mol
g/mol
(B) ![]() g/mol
g/mol
(C) ![]() g/mol
g/mol
(D) ![]() g/mol
g/mol
(E) (31.0)(0.93)(1.86)(2.00) g/mol
12. What is the boiling point of a 2 m solution of NaCl in water? (The boiling point elevation constant, kb, for water is 0.5°C/m.)
(A) 100°C
(B) 101°C
(C) 102°C
(D) 103°C
(E) 104°C
13. When an aqueous solution of potassium chloride is compared with water, the salt solution will have
(A) a higher boiling point, a lower freezing point, and a lower vapor pressure.
(B) a higher boiling point, a higher freezing point, and a lower vapor pressure.
(C) a higher boiling point, a higher freezing point, and a higher vapor pressure.
(D) a lower boiling point, a lower freezing point, and a lower vapor pressure.
(E) a lower boiling point, a higher freezing point, and a higher vapor pressure.
14. If 46 grams of MgBr2 (molar mass 184 grams) are dissolved in water to form 0.50 liters of solution, what is the concentration of bromine ions in the solution?
(A) 0.25-molar
(B) 0.50-molar
(C) 1.0-molar
(D) 2.0-molar
(E) 4.0-molar
15. A solution contains equal masses of glucose (molecular mass 180) and toluene (molecular mass 90). What is the mole fraction of glucose in the solution?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
(E) ![]()
16. When benzene and toluene are mixed together, they form an ideal solution. If benzene has a higher vapor pressure than toluene, then the vapor pressure of a solution that contains an equal number of moles of benzene and toluene will be
(A) higher than the vapor pressure of benzene.
(B) equal to the vapor pressure of benzene.
(C) lower than the vapor pressure of benzene and higher than the vapor pressure of toluene.
(D) equal to the vapor pressure of toluene.
(E) lower than the vapor pressure of toluene.
17. How many moles of Na2SO4 must be added to 500 milliliters of water to create a solution that has a 2-molar concentration of the Na+ ion? (Assume the volume of the solution does not change).
(A) 0.5 moles
(B) 1 mole
(C) 2 moles
(D) 4 moles
(E) 5 moles
18. Given that a solution of NaCl (molar mass 58.5 g/mole) in water (molar mass 18 g/mole) has a molality of 0.5 m, which of the following can be determined?
I. The mass of the NaCl in the solution
II. The total mass of the solution
III. The mole fraction of the NaCl in the solution
(A) I only
(B) III only
(C) I and II only
(D) II and III only
(E) I, II, and III
19. How many liters of water must be added to 4 liters of a 6-molar HNO3 solution to create a solution that is 2-molar?
(A) 2 liters
(B) 4 liters
(C) 6 liters
(D) 8 liters
(E) 12 liters
20. Which of the following expressions is equal to the Ksp of Ag2CO3?
(A) Ksp = [Ag+][CO32–]
(B) Ksp = [Ag+][CO32–]2
(C) Ksp = [Ag+]2[CO32–]
(D) Ksp = [Ag+]2[CO32–]2
(E) Ksp = [Ag+]2[CO32–]3
21. If the solubility of BaF2 is equal to x, which of the following expressions is equal to the solubility product, Ksp, for BaF2?
(A) x2
(B) 2x2
(C) x3
(D) 2x3
(E) 4x3
22. A beaker contains 50.0 ml of a 0.20 M Na2SO4 solution. If 50.0 ml of a 0.10 M solution of Ba(NO3)2 is added to the beaker, what will be the final concentration of sulfate ions in the solution?
(A) 0.20 M
(B) 0.10 M
(C) 0.050 M
(D) 0.025 M
(E) 0.012 M
23. The solubility of strontium fluoride in water is 1 × 10–3 M at room temperature. What is the value of the solubility product for SrF2?
(A) 2 × 10–3
(B) 4 × 10–6
(C) 2 × 10–6
(D) 4 × 10–9
(E) 2 × 10–9
24. The bottler of a carbonated beverage dissolves carbon dioxide in water by placing carbon dioxide in contact with water at a pressure of 1 atm at room temperature. The best way to increase the amount of dissolved CO2 would be to
(A) increase the temperature and increase the pressure of CO2.
(B) decrease the temperature and decrease the pressure of CO2.
(C) decrease the temperature and increase the pressure of CO2.
(D) increase the temperature without changing the pressure of CO2.
(E) increase the pressure of CO2 without changing the temperature.
25. When 300. ml of a 0.60 M NaCl solution is combined with 200. ml of a 0.40 M MgCl2 solution, what will be the molar concentration of Cl– ions in the solution?
(A) 0.20 M
(B) 0.34 M
(C) 0.68 M
(D) 0.80 M
(E) 1.0 M
26. Silver hydroxide will be LEAST soluble in a solution with a pH of
(A) 3
(B) 5
(C) 7
(D) 9
(E) 11
27. Copper (II) chloride will be LEAST soluble in a 0.02-molar solution of which of the following compounds?
(A) NaCl
(B) CuNO3
(C) CaCl2
(D) NaCO3
(E) KI
28. A student added 0.10 mol of NaBr and 0.20 mol of BaBr2 to 2 liters of water to create an aqueous solution. What is the minimum number of moles of Ag(C2H3O2) that the student must add to the solution to precipitate out all of the Br– ions as AgBr?
(A) 0.20
(B) 0.30
(C) 0.40
(D) 0.50
(E) 1.00
29. A student added 1 liter of a 1.0 M KCl solution to 1 liter of a 1.0 M Pb(NO3)2 solution. A lead chloride precipitate formed, and nearly all of the lead ions disappeared from the solution. Which of the following lists the ions remaining in the solution in order of decreasing concentration?
(A) [NO3–] > [K+] > [Pb2+]
(B) [NO3–] > [Pb2+] > [K+]
(C) [K+] > [Pb2+] > [NO3–]
(D) [K+] > [NO3–] > [Pb2+]
(E) [Pb2+] > [NO3–] > [K+]
30. The solubility of PbS in water is
3 × 10–14 molar. What is the solubility product constant, Ksp, for PbS?
(A) 2 × 10–7
(B) 9 × 10–7
(C) 3 × 10–14
(D) 3 × 10–28
(E) 9 × 10–28
1. The molecular weight and formula of a hydrocarbon are to be determined through the use of the freezing-point depression method. The hydrocarbon is known to be 86 percent carbon and 14 percent hydrogen by mass. In the experiment, 3.72 grams of the unknown hydrocarbon were placed into 50.0 grams of liquid benzene, C6H6. The freezing point of the solution was measured to be 0.06°C. The normal freezing point of benzene is 5.50°C, and the freezing-point depression constant for benzene is 5.12°C/m.
(a) What is the molecular weight of the compound?
(b) What is the molecular formula of the hydrocarbon?
(c) What is the mole fraction of benzene in the solution?
(d) If the density of the solution is 875 grams per liter, what is the molarity of the solution?
2. The value of the solubility product, Ksp, for calcium hydroxide, Ca(OH)2, is 5.5 × 10–6, at 25°C.
(a) Write the Ksp expression for calcium hydroxide.
(b) What is the mass of Ca(OH)2 in 500 ml of a saturated solution at 25°C?
(c) What is the pH of the solution in (b)?
(d) If 1.0 mole of OH– is added to the solution in (b), what will be the resulting Ca2+ concentration? Assume that the volume of the solution does not change.
3. Explain the following statements in terms of the chemical properties of the substances involved.
(a) A 1-molal aqueous solution of sodium chloride has a lower freezing point than a
1-molal aqueous solution of ethanol.
(b) NaCl is a strong electrolyte, whereas PbCl2 is a weak electrolyte.
(c) Propanol is soluble in water, but propane is not.
(d) In a dilute aqueous solution, molarity and molality will have the same value.
4. For sodium chloride, the solution process with water is endothermic.
(a) Describe the change in entropy when sodium chloride dissociates into aqueous particles.
(b) Two saturated aqueous NaCl solutions, one at 20°C and one at 50°C, are compared. Which one will have higher concentration? Justify your answer.
(c) The solubility product of Ce2(SO4)3 decreases as temperature increases. Is the solution process for this salt endothermic or exothermic? Justify your answer.
(d) When equal molar quantities of HF and HCl are added to separate containers filled with the same amount of water, the HCl solution will freeze at a lower temperature. Explain.
1. B Molality is the measure of moles of solute per kilograms of solvent.
2. D pH = –log[H+]
3. C Density is a measure of mass per unit volume (e.g., grams per liter).
4. A Molarity is the measure of moles of solute per liter of solution.
5. E All of the choices are colligative properties, which means they depend only on the number of particles in solution, not on the identity of those particles.
6. C Boiling-point elevation is a colligative property. That is, it depends only on the number of particles in solution, not on the specific particles.
Remember the formula: ∆T = kmx.
All of the solutions have the same molality, so the one with the greatest boiling-point elevation will be the one that breaks up into the most ions in solution. CaCl2 breaks up into 3 ions, C6H12O6 doesn’t break up into ions, and the other three break up into 2 ions.
7. E Sodium chloride is much more soluble than silver chloride. Because of the common ion effect, the chloride ions introduced into the solution by sodium chloride will disrupt the silver chloride equilibrium, causing silver chloride to precipitate from the solution.
8. B First find the number of moles.
Moles = (molarity)(volume)
Moles of substance = (0.50 M)(4.0 L) = 2 moles
Moles = ![]()
So MW = ![]() = 120 g/mol
= 120 g/mol
9. E The solubility of a substance is equal to its maximum concentration in solution.
For every AgCl in solution, we get one Ag+ and one Cl–, so the solubility of AgCl—let’s call it x—will be the same as [Ag+], which is the same as [Cl–].
So for AgCl, Ksp = [Ag+][Cl–] = 1.8 × 10–10 = x2.
x = ![]()
10. E The best conductor of electricity (also called the strongest electrolyte) will be the solution that contains the most charged particles. HNO3 is the only strong acid listed in the answer choices, so it is the only choice where the acid has dissociated completely in solution into H+ and NO3– ions. So a 0.1-molar HNO3 solution will contain the most charged particles and, therefore, be the best conductor of electricity.
11. B We can get the molality from the freezing-point depression with the expression ∆T = kfmx. Because the substance is nonionic, it will not dissociate, and x will be equal to 1, so we can leave it out of the calculation.
m = ![]()
We know the molality and the mass of the solvent, so we can calculate the number of moles of solute.
Moles = (molality)(kilograms of solvent) = ![]() (kg)
(kg)
Now we use one of our stoichiometry relationships.
Moles = ![]()
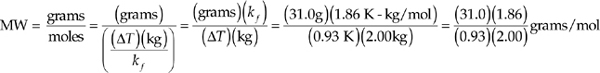
12. C ∆T = kbmx.
Each NaCl dissociates into two particles, so x = 2.
∆T = (0.5°C/m)(2 m)(2) = 2°C
So the boiling point of the solution is 102°C.
13. A Particles in solution tend to interfere with phase changes, so the boiling point is raised, the freezing point is lowered, and the vapor pressure is lowered.
14. C First we’ll find the molarity of the MgBr2 solution.
Moles = ![]()
Moles of MgBr2 added = ![]() = 0.25 moles
= 0.25 moles
Molarity = ![]() = 0.50-molar
= 0.50-molar
For every MgBr2 in solution, 2 Br– ions are produced, so a 0.50-molar MgBr2 solution will have twice the concentration of Br– ions, so the bromine ion concentration is 1.0-molar.
15.
B Let’s say the solution contains 180 grams of glucose and 180 grams of toluene. That’s 1 mole of glucose and 2 moles of toluene. So that’s 1 mole of glucose out of a total of 3 moles, for a mole fraction of ![]() .
.
16. C From Raoult’s law, the vapor pressure of an ideal solution depends on the mole fractions of the components of the solution. The vapor pressure of a solution with equal amounts of benzene and toluene will look like as follows.
![]()
That’s just the average of the two vapor pressures.
17. A Let’s find out how many moles of Na+ we have to add.
Moles = (molarity)(volume)
Moles of Na+ = (2 M)(0.5 L) = 1 mole
Because we get 2 moles of Na+ ions for every mole of Na2SO4 we add, we only need to add 0.5 moles of Na2SO4.
18. B We can’t determine (I) and (II) because we don’t know how much solution we have. We can figure out (III) because molality tells us the number of moles of NaCl found in 1 kilogram of water. We can figure out how many moles of water there are in 1 kilogram. So if we have a ratio of moles of NaCl to moles of water, we can figure out the mole fraction of NaCl.
19. D The number of moles of HNO3 remains constant.
Moles = (molarity)(volume)
Moles of HNO3 = (6 M)(4 L) = (2 M)(x)
x = 12 liters, but that’s not the answer.
To get a 2-molar solution we need 12 liters, but the solution already has 4 liters, so we need to add 8 liters of water. That’s the answer.
20. C Ksp is just the equilibrium constant without a denominator.
When Ag2CO3 dissociates, we get the following reaction:
Ag2CO3(s) ![]() 2 Ag+ + CO32–
2 Ag+ + CO32–
In the equilibrium expression, coefficients become exponents, so we get
Ksp = [Ag+]2[CO32–]
21. E For BaF2, Ksp = [Ba2+][F–]2.
For every BaF2 that dissolves, we get one Ba2+ and two F–.
So if the solubility of BaF2 is x, then [Ba2+] = x, and [F–] = 2x
So Ksp = (x)(2x)2 = (x)(4x2) = 4x3
22. C The Ba2+ ions and the SO4– ions will combine and precipitate out of the solution, so let’s find out how many of each we have.
Moles = (molarity)(volume)
Moles of SO4– = (0.20 M)(0.050 L) = 0.010 mole
Moles of Ba2+ = (0.10 M)(0.050 L) = 0.0050 mole
To find the number of moles of SO4– left in the solution, subtract the moles of Ba2+ from the moles of SO4–.
0.010 mole – 0.0050 mole = 0.0050 mole
Now use the formula for molarity to find the concentration of SO4– ions. Don’t forget to add the volumes of the two solutions.
![]()
23. D Use the formula for Ksp. For every SrF2 in solution, there will be one strontium ion and two fluoride ions, so [Sr2+] will be 1 × 10–3 M and [F–] will be 2 × 10–3 M.
Ksp = [Sr2+][F–]2
Ksp = (1 × 10–3
M)(2 × 10–3
M)2
Ksp = 4 × 10–9
24. C The lower the temperature, the more soluble a gas will be in water. The greater the pressure of the gas, the more soluble it will be.
25. C The Cl– ions from the two salts will both be present in the solution, so we need to find the number of moles of Cl– contributed by each salt.
Moles = (molarity)(volume)
Each NaCl produces 1 Cl–
Moles of Cl– from NaCl = (0.60 M)(0.300 L) = 0.18 mole
Each MgCl2 produces 2 Cl–
Moles of Cl– from MgCl2 = (2)(0.40 M)(0.200 L) = 0.16 mole
To find the number of moles of Cl– in the solution, add the two together.
0.18 mole + 0.16 mole = 0.34 mole
Now use the formula for molarity to find the concentration of Cl– ions. Don’t forget to add the volumes of the two solutions.
Molarity = ![]() = 0.68 M
= 0.68 M
26. E Silver hydroxide will be least soluble in the solution with the highest hydroxide concentration. That would be the solution with the highest pH.
27. C According to the common ion effect, ions already present in a solution will affect the solubility of compounds that also produce those ions. So a solution containing Cu+ ions or Cl– ions will inhibit the solubility of CuCl.
A 0.02-molar solution of NaCl will have a 0.02-molar concentration of Cl– ions and a 0.02-molar solution of CuNO3 will have a 0.02-molar concentration of Cu+ ions, so choices (A) and (B) will affect the solubility of CuCl to the same extent.
The correct answer is choice (C), CaCl2, because a 0.02-molar solution of CaCl2 will have a 0.04-molar concentration of Cl– ions, so this solution will do the most to inhibit the solubility of CuCl.
Choices (D) and (E) have no effect on the solubility of CuCl.
28. D The solution contains 0.50 moles of Br– ions, 0.10 from NaBr and 0.40 from BaBr2 (each BaBr2 provides 2 Br– ions). Each Ag+ ion will remove 1 Br– ion, so the student needs to add 0.50 moles of Ag(C2H3O2).
29. A At the start, the concentrations of the ions are as follows:
[K+] = 1 M
[Cl–] = 1 M
[Pb2+] = 1 M
[NO3–] = 2 M
After PbCl2 forms, the concentrations are as follows:
[K+] = 1 M
[Cl–] = 0.5 M
[Pb2+] = 0 M
[NO3–] = 2 M
So from greatest to least
[NO3–] > [K+] > [Pb2+]
30. E The solubility of a substance is equal to its maximum concentration in solution. For every PbS in solution, we get one Pb2+ and one S2–, so the concentration of PbS, 3 × 10–14 M, will be the same as the concentrations of Pb2+ and S2–.
Ksp = [Pb2+][S2–]
Ksp = (3 × 10–14
M) (3 × 10–14
M) = 9 × 10–28
1. (a) First we’ll find the molality of the solution. The freezing point depression, ∆T, is
5.50°C – 0.06°C = 5.44°C.
∆T = km
Solve for m
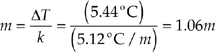
From the molality of the solution, we can find the number of moles of unknown hydrocarbon.
Molality = ![]()
Solve for moles.
Moles = (molality)(kg of solvent)
Moles of hydrocarbon = (1.06 m)(0.050 kg) = 0.053 moles
Now we can find the molecular weight of the hydrocarbon.
MW = ![]() = 70.2 g/mol
= 70.2 g/mol
(b) You can use the percent by mass and the molecular weight.
For carbon
(86%)(70 g/mol) = 60 g/mol
Carbon has an atomic weight of 12, so there must be ![]() = 5 moles of carbon in 1 mole of the hydrocarbon.
= 5 moles of carbon in 1 mole of the hydrocarbon.
For hydrogen
(14%)(70 g/mol) = 10 g/mol
Hydrogen has an atomic weight of 1, so there must be ![]() = 10 moles of hydrogen in 1 mole of the hydrocarbon.
= 10 moles of hydrogen in 1 mole of the hydrocarbon.
So the molecular formula for the hydrocarbon is C5H10.
(c) We know that there are 0.053 moles of hydrocarbon. We need to find the number of moles of benzene.
Moles = ![]()
Moles of benzene = ![]() = 0.64 mol
= 0.64 mol
Total moles = 0.64 mol + 0.053 mol = 0.69 mol
Mole fraction of benzene = ![]()
(d) Remember the definition of molarity.
Molarity = ![]()
We know that the moles of solute is 0.053. We need to find the liters of solution.
The weight of the solution is
50.00 g + 3.72 g = 53.72 g
Density = ![]()
Solve for liters.
Liters of solution = ![]() = 0.0614 L
= 0.0614 L
Molarity = ![]() = 0.863 M
= 0.863 M
2. (a) The solubility product is the same as the equilibrium expression, but because the reactant is a solid, there is no denominator.
Ksp = [Ca2+][OH–]2
(b) Use the solubility product.
Ksp = [Ca2+][OH–]2
5.5 × 10–6 = (x)(2x)2 = 4x3
x = 0.01 M for Ca2+
One mole of calcium hydroxide produces 1 mole of Ca2+, so the concentration of Ca(OH)2 must be 0.01 M.
Moles of Ca(OH)2 = (0.01 M)(0.500 L) = 0.005 moles
Grams = (moles)(MW)
Grams of Ca(OH)2 = (0.005 mol)(74 g/mol) = 0.37 g
(c) We can find [OH–] from (b).
If [Ca2+] = 0.01 M, then [OH–] must be twice that, so [OH–] = 0.02 M
pOH = –log[OH–] = 1.7
pH = 14 – pOH = 14 – 1.7 = 12.3
(d) Find the new [OH–]. The hydroxide already present is small enough to ignore, so we’ll use only the hydroxide just added.
Molarity = ![]()
[OH–] = ![]() = 2.0 M
= 2.0 M
Now use the Ksp expression.
Ksp = [Ca2+][OH–]2
5.5 × 10–6 = [Ca2+](2.0 M)2
[Ca2+] = 1.4 × 10–6
M
3. (a) Freezing-point depression is a colligative property, which means that it depends on the number of particles in solution, not their identity.
Sodium chloride dissociates into Na+ and Cl–, so every unit of sodium chloride produces two particles in solution. Ethanol does not dissociate, so sodium chloride will put twice as many particles in solution as ethanol.
(b) An electrolyte is a substance that ionizes in solution, thus causing the solution to conduct electricity.
Both of the salts dissociate into ions, but PbCl2 is almost insoluble, so it will produce very few ions in solution, while NaCl is extremely soluble and produces many ions.
(c) Water is best at dissolving polar substances.
Propanol (C3H7OH) has a hydroxide group, which makes it polar, and thus soluble in water. Propane (C3H8) is nonpolar and is best dissolved in nonpolar solvents.
(d) Remember the definitions, and remember that a dilute solution has very little solute.
Molarity = ![]()
Molality = ![]()
For water, 1 liter weighs 1 kilogram, so for a dilute solution this distinction disappears.
If there is very little solute, the mass and volume of the solution will be indistinguishable from the mass and volume of the solvent.
4. (a) Entropy increases when a salt dissociates because aqueous particles have more randomness than a solid.
(b) Most salt solution processes are endothermic, and endothermic processes are favored by an increase in temperature, therefore increasing temperature will increase the solubility of most salts.
(c) Ce2(SO4)3 becomes less soluble as temperature increases, so the solution process for this salt must be exothermic.
(d) Freezing-point depression is a colligative property, which means that it depends on the number of particles in solution, not their identity.
HCl is a strong acid, which means that it dissociates completely. This means that 1 mole of HCl in solution will produce 2 moles of particles. HF is a weak acid, which means that it dissociates very little. This means that 1 mole of HF in solution will remain at about 1 mole of particles in solution.
Therefore, the HCl solution will have more particles than the HF solution.