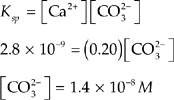
In the multiple-choice section, this topic appears in about 4 out of 75 questions. In the free-response section, this topic appears every year.
Most chemical processes are reversible. That is, reactants react to form products, but those products can also react to form reactants.
A reaction is at equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction.
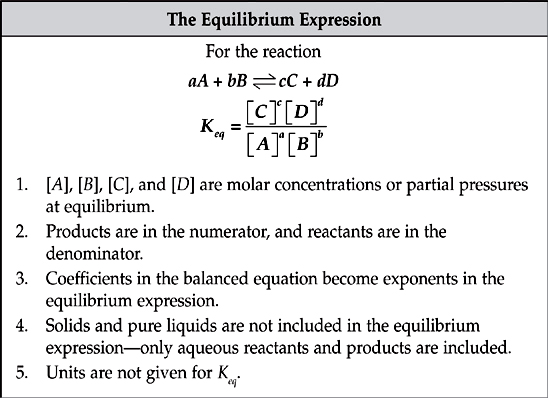
The relationship between the concentrations of reactants and products in a reaction at equilibrium is given by the equilibrium expression, also called the law of mass action.
Let’s look at a few examples:
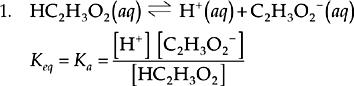
This reaction shows the dissociation of acetic acid in water. All of the reactants and products are aqueous particles, so they are all included in the equilibrium expression. None of the reactants or products have coefficients, so there are no exponents in the equilibrium expression. This is the standard form of Ka, the acid dissociation constant.
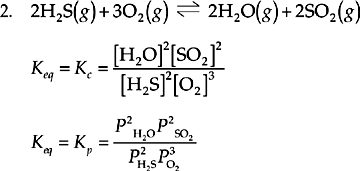
All of the reactants and products in this reaction are gases, so Keq can be expressed in terms of concentration (Kc, moles/liter or molarity) or in terms of partial pressure (Kp, atmospheres). In the next section, we’ll see how these two different ways of looking at the same equilibrium situation are related. All of the reactants and products are included here, and the coefficients in the reaction become exponents in the equilibrium expression.
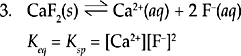
This reaction shows the dissociation of a slightly soluble salt. There is no denominator in this equilibrium expression because the reactant is a solid. Solids are left out of the equilibrium expression because the concentration of a solid is constant. There must be some solid present for equilibrium to exist, but you do not need to include it in your calculations. This form of Keq is called the solubility product, Ksp, which we already saw in Chapter 9.
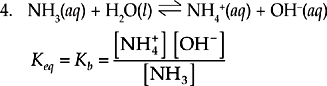
This is the acid-base reaction between ammonia and water. We can leave water out of the equilibrium expression because it is a pure liquid. By pure liquid, we mean that the concentration of water is so large (about 50 molar) that nothing that happens in the reaction is going to change it significantly, so we can consider it to be constant. This is the standard form for Kb, the base dissociation constant.
Here is a roundup of the equilibrium constants you need to be familiar with for the test.
The equilibrium constant has a lot of aliases, but they all take the same form and tell you the same thing. The equilibrium constant tells you the relative amounts of products and reactants at equilibrium.
A large value for Keq means that products are favored over reactants at equilibrium, while a small value for Keq means that reactants are favored over products at equilibrium.
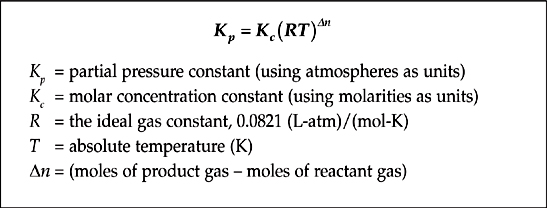
As we saw in the example above, the equilibrium constant for a gas phase reaction can be written in terms of molar concentrations, Kc, or partial pressures, Kp. These two forms of K can be related by the following equation, which is derived from the ideal gas law.
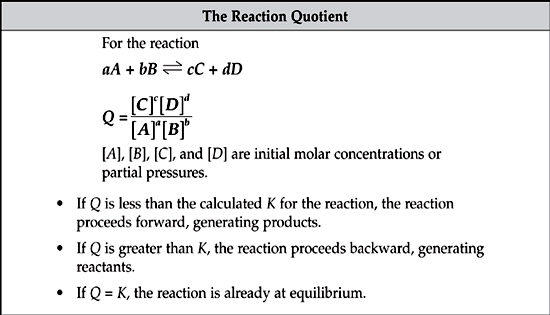
The reaction quotient is determined in exactly the same way as the equilibrium constant, but initial conditions are used in place of equilibrium conditions. The reaction quotient can be used to predict the direction in which a reaction will proceed from a given set of initial conditions.
There is a simple relationship between the equilibrium constants for the steps of a multistep reaction and the equilibrium constant for the overall reaction.
If two reactions can be added together to create a third reaction, then the Keq for the two reactions can be multiplied together to get the Keq for the third reaction.

Le Châtelier’s law says that whenever a stress is placed on a situation at equilibrium, the equilibrium will shift to relieve that stress.
Let’s use the Haber process, which is used in the industrial preparation of ammonia, as an example.
N2(g) + 3 H2(g) ![]() 2 NH3(g) ΔH° = −92.6kJ
2 NH3(g) ΔH° = −92.6kJ
If N2 or H2 is added, the reaction proceeds in the forward direction. If NH3 is added, the reaction proceeds in the reverse direction.
If N2 or H2 is removed, the reaction will proceed in the reverse direction. If NH3 is removed, the reaction will proceed in the forward direction.
When the volume for the Haber process is increased, the reaction proceeds in the reverse direction because the reactants have more moles of gas (4) than the products (2).
When the volume for the Haber process is decreased, the reaction proceeds in the forward direction because the products have fewer moles of gas (2) than the reactants (4).
When the temperature for the Haber process is increased, the reaction proceeds in the reverse direction because the reverse reaction is endothermic (∆H° is positive).
When the temperature for the Haber process is decreased, the reaction proceeds in the forward direction because the forward reaction is exothermic (∆H° is negative).
When an inert gas, like Argon, is added to the system, although the total pressure goes up, the partial pressures of the gases involved in the reaction will remain the same. Therefore, there is no effect on the equilibrium position, because the reaction quotient, Q, is determined by the individual partial pressures of the gases involved in the reaction.
Questions 1–4
(A) Kc
(B) Kp
(C) Ka
(D) Kw
(E) Ksp
1. This equilibrium constant uses partial pressures of gases as units.
2. This equilibrium constant always has a value of 1 × 10–14 at 25°C.
3. This equilibrium constant is used for the dissociation of an acid.
4. The equilibrium expression for this equilibrium constant does not contain a denominator.
5. For a particular salt, the solution process is endothermic. As the temperature at which the salt is dissolved increases, which of the following will occur?
(A) Ksp will increase, and the salt will become more soluble.
(B) Ksp will decrease, and the salt will become more soluble.
(C) Ksp will increase, and the salt will become less soluble.
(D) Ksp will decrease, and the salt will become less soluble.
(E) Ksp will not change, and the salt will become more soluble.
6. 2 HI(g) + Cl2(g)
![]() 2 HCl(g) + I2(g) + energy
2 HCl(g) + I2(g) + energy
A gaseous reaction occurs and comes to equilibrium as shown above. Which of the following changes to the system will serve to increase the number of moles of I2 present at equilibrium?
(A) Increasing the volume at constant temperature
(B) Decreasing the volume at constant temperature
(C) Adding a mole of inert gas at constant volume
(D) Increasing the temperature at constant volume
(E) Decreasing the temperature at constant volume
7. A sealed isothermal container initially contained 2 moles of CO gas and 3 moles of H2 gas. The following reversible reaction occurred:
CO(g) + 2 H2(g)
![]() CH3OH(g)
CH3OH(g)
At equilibrium, there was 1 mole of CH3OH in the container. What was the total number of moles of gas present in the container at equilibrium?
(A) 1
(B) 2
(C) 3
(D) 4
(E) 5
8. 4 NH3(g) + 3 O2(g)
![]() 2 N2(g) + 6 H2O(g) + energy
2 N2(g) + 6 H2O(g) + energy
Which of the following changes to the system at equilibrium shown above would cause the concentration of H2O to increase?
(A) The volume of the system was decreased at constant temperature.
(B) The temperature of the system was increased at constant volume.
(C) NH3 was removed from the system.
(D) N2 was removed from the system.
(E) O2 was removed from the system.
9. A sample of solid potassium nitrate is placed in water. The solid potassium nitrate comes to equilibrium with its dissolved ions by the endothermic process shown below.
KNO3(s) + energy ![]() K+(aq) +
NO3–(aq)
K+(aq) +
NO3–(aq)
Which of the following changes to the system would increase the concentration of K+ ions at equilibrium?
(A) The volume of the solution is increased.
(B) The volume of the solution is decreased.
(C) Additional solid KNO3 is added to the solution.
(D) The temperature of the solution is increased.
(E) The temperature of the solution is decreased.
10. For which of the following gaseous equilibria do Kp and Kc differ the most?
(A) 2H2(g) + O2(g) ![]() 2H2O(g)
2H2O(g)
(B) NH3BH3(g) ![]() NH2BH2(g) + H2(g)
NH2BH2(g) + H2(g)
(C) NO(g) + O3(g) ![]() NO2(g) + O2(g)
NO2(g) + O2(g)
(D) B3N3H6(g) + 3H2(g) ![]() B3N3H12(g)
B3N3H12(g)
(E) BH3(g) + 3HCl(g) ![]() BCl3(g) + 3H2(g)
BCl3(g) + 3H2(g)
11. A 1M solution of SbCl5 in organic solvent shows no noticeable reactivity at room temperature. The sample is heated to 200°C and the following equilibrium reaction is found to occur:
SbCl5
![]() SbCl3 + Cl2
SbCl3 + Cl2
The value of Keq at 200°C was measured to be 10–6. The reaction was then heated to 350°C, and the equilibrium concentration of Cl2 was found to be 0.1 M, which of the following best approximated the value of Keq at 350°C?
(A) 1.5
(B) 1.0
(C) 0.1
(D) 0.01
(E) 0.001
12.
2 NOBr(g)
![]() 2 NO(g) + Br2(g)
2 NO(g) + Br2(g)
The reaction above came to equilibrium at a temperature of 100°C. At equilibrium the partial pressure due to NOBr was 4 atmospheres, the partial pressure due to NO was 4 atmospheres, and the partial pressure due to Br2 was 2 atmospheres. What is the equilibrium constant, Kp, for this reaction at 100°C?
(A) ![]()
(B) ![]()
(C) 1
(D) 2
(E) 4
13. HCrO4– + Ca2+ ↔ H+ + CaCrO4
If the acid dissociation constant for HCrO4– is Ka and the solubility product for CaCrO4 is Ksp, which of the following gives the equilibrium expression for the reaction above?
(A) KaKsp
(B) ![]()
(C) ![]()
(D) ![]()
(E) ![]()
14. Br2(g) + I2(g) ↔ 2 IBr(g)
At 150°C, the equilibrium constant, Kc , for the reaction shown above has a value of 300. This reaction was allowed to reach equilibrium in a sealed container and the partial pressure due to IBr(g) was found to be 3 atm. Which of the following could be the partial pressures due to Br2(g) and I2(g) in the container?
| Br2(g) | I2(g) | |
| (A) | 0.1 atm | 0.3 atm |
| (B) | 0.3 atm | 1 atm |
| (C) | 1 atm | 1 atm |
| (D) | 1 atm | 3 atm |
| (E) | 3 atm | 3 atm |
15. H2(g) + CO2(g) ↔ H2O(g) + CO(g)
Initially, a sealed vessel contained only H2(g) with a partial pressure of 6 atm and CO2(g) with a partial pressure of 4 atm. The reaction above was allowed to come to equilibrium at a temperature of 700 K. At equilibrium, the partial pressure due to CO(g) was found to be 2 atm. What is the value of the equilibrium constant Kp, for the
reaction?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
(E) ![]()
1. BaF2(s) ![]() Ba2+(aq) + 2 F–(aq)
Ba2+(aq) + 2 F–(aq)
The value of the solubility product, Ksp, for the reaction above is 1.0 × 10–6 at 25°C.
(a) Write the Ksp expression for BaF2.
(b) What is the concentration of F– ions in a saturated solution of BaF2 at 25°C?
(c) 500 milliliters of a 0.0060-molar NaF solution is added to 400 ml of a 0.0060-molar Ba(NO3)2 solution. Will there be a precipitate?
(d) What is the value of ∆G° for the dissociation of BaF2 at 25°C?
2. H2CO3
![]() H+ + HCO3– K1 = 4.3 × 10–7
H+ + HCO3– K1 = 4.3 × 10–7
HCO3–
![]() H+ + CO32– K2 = 5.6 × 10–11
H+ + CO32– K2 = 5.6 × 10–11
The acid dissociation constants for the reactions above are given at 25°C.
(a) What is the pH of a 0.050-molar solution of H2CO3 at 25°C?
(b) What is the concentration of CO32– ions in the solution in (a)?
(c) How would the addition of each of the following substances affect the pH of the solution in (a)?
(i) HCl
(ii) NaHCO3
(iii) NaOH
(iv) NaCl
(d) What is the value of Keq for the following reaction?
H2CO3
![]() 2 H+ + CO23
2 H+ + CO23
3. N2(g) + 3 H2(g)
![]() 2 NH3(g) ∆H = –92.4 kJ
2 NH3(g) ∆H = –92.4 kJ
When the reaction above took place at a temperature of 570 K, the following equilibrium concentrations were measured:
[NH3] = 0.20 mol/L
[N2] = 0.50 mol/L
[H2] = 0.20 mol/L
(a) Write the expression for Kc and calculate its value.
(b) What is the value of Kp for the reaction?
(c) Describe how the concentration of H2 will be affected by each of the following changes to the system at equilibrium:
(i) The temperature is increased.
(ii) The volume of the reaction chamber is increased.
(iii) N2 gas is added to the reaction chamber.
(iv) Helium gas is added to the reaction chamber.
4. CaCO3(s) ![]() Ca2+(aq) + CO
Ca2+(aq) + CO![]() (aq) Ksp = 2.8 × 10–9
(aq) Ksp = 2.8 × 10–9
CaSO4(s) ![]() Ca2+(aq) + SO
Ca2+(aq) + SO![]() (aq) Ksp = 9.1 × 10–6
(aq) Ksp = 9.1 × 10–6
The values for the solubility products for the two reactions above are given at 25°C.
(a) What is the concentration of CO![]() ions in a saturated 1.00 liter solution of CaCO3 at 25°C?
ions in a saturated 1.00 liter solution of CaCO3 at 25°C?
(b) Excess CaSO4(s) is placed in the solution in (a). Assume that the volume of the solution does not change.
(i) What is the concentration of the SO![]() ion?
ion?
(ii) What is the concentration of the CO![]() ion?
ion?
(c) A 0.20 mole sample of CaCl2 is placed in the solution in (b). Assume that the volume of the solution does not change.
(i) What is the concentration of the Ca2+ ion?
(ii) What is the concentration of the SO![]() ion?
ion?
(iii) What is the concentration of the CO![]() ion?
ion?
1. B Kp is used for gaseous reactions, and the units used are partial pressures.
2.
D
Kw is the dissociation constant for water.
At 25°C, Kw = [H+][OH–] = 1 × 10–14.
3. C Ka is known as the acid dissociation constant.
4. EKsp is the solubility product. It always has a solid as the reactant. Because the reactant is always in the denominator and solids are ignored in the equilibrium expression, Ksp never has a denominator.
5. A From Le Châtelier’s law, the equilibrium will shift to counteract any stress that is placed on it. Increasing temperature favors the endothermic direction of a reaction because the endothermic reaction absorbs the added heat. So the salt becomes more soluble, increasing the number of dissociated particles, thus increasing the value of Ksp.
6.
E According to Le Châtelier’s law, the equilibrium will shift to counteract any stress that is placed on it. If the temperature is decreased, the equilibrium will shift toward the side that produces energy or heat. That’s the product side where I2 is produced.
Choices (A) and (B) are wrong because there are equal numbers of moles of gas (3 moles) on each side, so changing the volume will not affect the
equilibrium. Choice (C) is wrong because the addition of a substance that does not affect the reaction will not affect the equilibrium conditions.
7. C From the balanced equation:
If 1 mole of CH3OH was created, then 1 mole of CO was consumed and 1 mole of CO remains; and if 1 mole of CH3OH was created, then 2 moles of H2 were consumed and 1 mole of H2 remains. So at equilibrium, there are
(1 mol CH3OH) + (1 mol CO) + (1 mol H2) = 3 moles of gas
8.
D According to Le Châtelier’s law, equilibrium will shift to relieve any stress placed on a system. If N2 is removed, the equilibrium will shift to the right to produce more N2, with the result that more H2O will also be produced.
If the volume is decreased (A), the equilibrium will shift toward the left, where there are fewer moles of gas. If the temperature is increased
(B), the equilibrium will shift to the left. That’s the endothermic reaction, which absorbs the added energy of the temperature increase. If NH3 (C) or O2 (E) is removed, the equilibrium will shift to the left to replace the substance removed.
9.
D According to Le Châtelier’s law, equilibrium will shift to relieve any stress placed on a system. If the temperature is increased, the equilibrium will shift to favor the endothermic reaction because it absorbs the added energy. In this case, the equilibrium will be shifted to the right, increasing the concentration of both K+ and NO3– ions.
Changing the volume of
the solution, (A) and (B), will change the number of K+ ions in solution, but not the concentration of K+ ions. Because solids are not considered in the equilibrium expression, adding more solid KNO3 to the solution (C) will not change the equilibrium. Decreasing the temperature (E) will favor the exothermic reaction, driving the equilibrium toward the left and decreasing the concentration
of K+ ions.
10.
D
Kp and Kc will differ the most in situations where the moles of gas change drastically from the reactants to the products. This relation is given in the equation
Kp = Kc(RT)∆n
The exponential ∆n is the moles of gas in the products minus the moles of gas in the reactants. The biggest change in number of moles of gas is
found in the equation in choice D.
11. D The information about K at 200°C is useless information and can be ignored. Since all the Cl2 found in solution must have come from SbCl5 , we know that at equilibrium [Cl2] = [SbCl3] = 0.1 M, and [SbCl5] = (1.0 – 0.1) M = .99 M. We can then say that K = (0.1)(0.1)/.99 = .0101 which is most closely approximated by choice D.
12.
D
![]()
13.
B We can think of the reaction given in the question as the sum of two other reactions.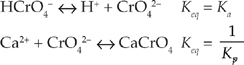
Notice that we are using the reverse reaction for the solvation of CaCrO4, so the reactants and products are reversed and we must take the reciprocal of the solubility product.
When reactions can be added to get another reaction, their
equilibrium constants can be multiplied to get the equilibrium constant of the resulting reaction.![]()
14. A The equilibrium expression for the reaction is as follows:
![]()
When all of the values are plugged into the expression, (A) is the only choice that works.
![]()
15. E Use a table to see how the partial pressures change. Based on the balanced equation, we know that if 2 atm of CO(g) were formed, then 2 atm of H2O(g) must also have formed. We also know that the reactants must have lost 2 atm each.
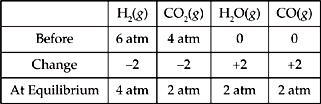
Now plug the numbers into the equilibrium expression.
![]()
1. (a) Ksp = [Ba2+][F–]2
(b) Use the Ksp expression.
Ksp = [Ba2+][F–]2
Two F–s are produced for every Ba2+, so [F–] will be twice as large as [Ba2+].
Let x = [F–]
![]()
x = [F–] = 0.01 M
(c) First we need to find the concentrations of the Ba2+ and F– ions.
Moles = (molarity)(volume)
Moles of Ba2+ = (0.0060 M)(0.400 L) = 0.0024 mol
Moles of F– = (0.0060 M)(0.500 L) = 0.0030 mol
Remember to add the two volumes: (0.400 L) + (0.500 L) = 0.900 L

Now test the solubility expression using the initial values to find the reaction quotient.
Q = [Ba2+][F–]2
Q = (0.0027)(0.0033)2 = 2.9 × 10–8
Q is less than Ksp, so no precipitate forms.
(d) Use the standard free energy expression.
ΔG° = −2.303RT log K
ΔG° = (−2.303)(8.31J/mol − K)(298 K)(log 1.0 × 10−6) = 34,000 J/mol
The positive value of ∆G° means that the reaction is not spontaneous under standard conditions.
2. (a) Use the equilibrium expression.
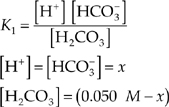
Assume that x is small enough that we can use [H2CO3] = (0.050 M).
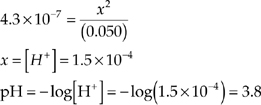
(b) Use the equilibrium expression.

From (a) we know: ![]() .
.
5.6 × 10−11 = 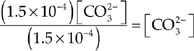
![]() = 5.6 × 10−11
M
= 5.6 × 10−11
M
(c) (i) Adding HCl will increase [H+], lowering the pH.
(ii) From Le Châtelier’s law, you can see that adding NaHCO3 will cause the first equilibrium to shift to the left to try to use up the excess HCO3–. This will cause a decrease in [H+], raising the pH.
You may notice that adding NaHCO3 will also cause the second equilibrium to shift toward the right, which should increase [H+], but because K2 is much smaller than K1, this shift is insignificant.
(iii) Adding NaOH will neutralize hydrogen ions, decreasing [H+] and raising the pH.
(iv) Adding NaCl will have no effect on the pH.
(d) The reaction in (d) is just the sum of the two reactions given. When two reactions can be added to give a third reaction, the equilibrium constants for those reactions can be multiplied to give Keq for the third reaction.
Keq = (K1)(K2) = (4.3 × 10−7)(5.6 × 10−11) = 2.4 × 10−17
3. (a) ![]()
![]()
(b) Use the formula that relates the two constants.
Kp = Kc(RT)Δn
∆n is the change in the number of moles of gas from reactants to products. So ∆n = –2.
Kp = (10)[(0.082)(570)]−2 = (10)(46.7)−2 = 4.7 × 10−3
(c) (i) An increase in temperature favors the endothermic direction. In this case, that’s the reverse reaction, so the concentration of H2 will increase.
(ii) An increase in volume favors the direction that produces more moles of gas. In this case, that’s the reverse direction, so the concentration of H2 will increase.
(iii) According to Le Châtelier’s law, increasing the concentration of the reactants forces the reaction to proceed in the direction that will use up the added reactants. In this case, adding the reactant N2 will shift the reaction to the right and decrease the concentration of H2.
(iv) The addition of He, a gas that takes no part in the reaction, will have no effect on the concentration of H2.
4. (a) Use the solubility product.
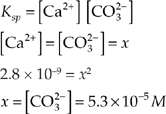
(b) Use the solubility product.
(i) ![]()
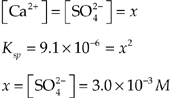
(ii) ![]()
Now use the value of [Ca2+] that you found in (b)(i).

(c)
(i) The CaCl2 dissociates completely, so the solution can be assumed to contain 0.2 moles of Ca2+ ions. We can ignore the ions from CaCO3 and CaSO4 because there are so few of them.
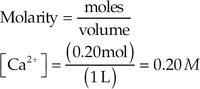
(ii) Use Ksp again with the new value of [Ca2+].
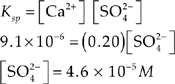
(iii) Use Ksp again with the new value of [Ca2+].