Michelle L. Moyer, Mario A. Cristancho, and John P. O’Reardon
First emerging as a potential tool for noninvasive neuronal stimulation in the early 20th century (Thompson, 1910), repetitive transcranial magnetic stimulation (rTMS) has undergone multiple stages of development. Anthony Barker invented the first modern-day rTMS device in 1985 as a way to study electrophysiology (Barker, Freeston, Jalinous, Merton, and Morton, 1985). In 1995, George et al. (1995) used rTMS to target specific prefrontal brain regions thought to be involved in the etiology or pathophysiology of major depression in an open-label study. Since then, the rTMS literature has accumulated more than 15 years of research and at least 30 published randomized controlled trials (RCTs). Although most of the research has supported the antidepressant properties of rTMS, the degree of clinical benefit has been variable and, in some cases, marginal. However, a clear trend toward more robust effects has been seen as both stimulation technique (e.g., dose, coil placement, and course duration) and research quality (e.g., better sham stimulation and larger sample sizes) improve. rTMS has become a recognized, accepted, and clinically available therapeutic intervention. The US Food and Drug Administration (FDA) cleared TMS for the treatment of adults with nonpsychotic major depression in October 2008.
In this chapter we review the efficacy of left dorsolateral TMS as a treatment for major depression, focusing on the large-scale RCTs underpinning its efficacy. We also review the broader synthesis of efficacy data in the metaanalyses that have been conducted and reported in the literature. Now that TMS is available in clinical practice in the United States, effectiveness data have emerged, and it is reviewed here. Last, emerging data on efficacy in special populations (e.g., adolescent depression and depression during pregnancy) is examined.
There have been three large-scale studies (sample size >100) of rTMS in major depression: one industry-sponsored study that led to FDA approval, a National Institutes of Health (NIH)–funded study with dosage parameters similar to those in the industry-sponsored study but with design enhancements, and a European study of the augmentation effects of rTMS when used in combination with pharmacotherapy (Herwig et al., 2007; O’Reardon et al., 2007; George et al., 2010).
In the industry-sponsored,1 multicenter (23 sites) RCT, 301 medication-free patients with treatment-resistant depression (TRD) were randomized to either active rTMS or sham stimulation (O’Reardon et al., 2007). The trial was intended to provide the necessary data for premarket approval by the FDA and required that the company show the efficacy and safety of the device in a sham-controlled trial. Total score change in the Montgomery–Asberg depression rating scale (MADRS) after 4 weeks of treatment was used as the primary outcome measure. Secondary outcomes included MADRS score at week 6 and Hamilton depression scale (HAMD) 17- and 24-item scores at weeks 4 and 6. For the primary outcome measure, active rTMS was superior to sham stimulation (P = 0.038), but only following correction for a baseline difference in MADRS scores between the two groups.
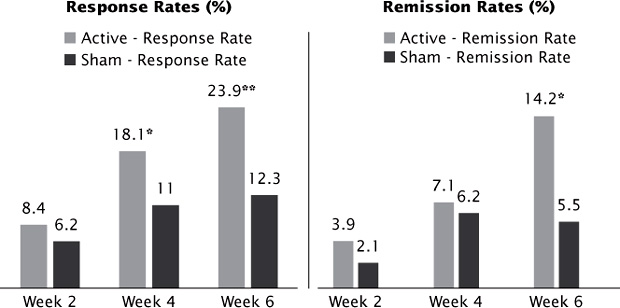
Active stimulation was also statistically superior to sham based on categorical outcomes with response rates (≥50% reduction from baseline) almost two-fold higher on all three scales at week 4 (MADRS, 18.1% active versus 11.0% sham; HAMD-17, 20.5% active versus 11.6% sham; and HAMD-24, 19.4% active versus 11.6% sham). This difference in response rates was sustained and statistically significant at week 6. Remission (MADRS <10, HAMD-24 <11, and HAMD-17 <8) rates were approximately three-fold higher with active stimulation at week 6 (14.2% active versus 5.5% [P < 0.05] by MADRS; Figure 3.1). Of note, the study sample was moderately treatment resistant, with up to four failed adequate antidepressant trials in the current episode (mean = 1.6 failed antidepressant trials). In addition, the stimulation dose was 3000 pulses per day (high frequency over the left dorsolateral prefrontal cortex [DLPFC] that, although less than the number of pulses used in off-label clinical practice (up to 8000 pulses per day), was higher than the doses used in previous studies (O’Reardon et al., 2007).
Although the categorical results in terms of response and remission rates were reassuring, albeit somewhat low, the failure to show a statistically superior response of active versus sham treatment on the a priori primary measure (continuous change on the MADRS scale) created some disagreement among those on the FDA advisory panel who evaluated the effectiveness of rTMS for the treatment of depression. In 2007, the FDA advisory panel did not approve rTMS for the treatment of depression, stating that the device was safe but that the efficacy was based on a post hoc analysis and therefore subject to potential bias (Scudiero, 2007). However, in October 2008, the FDA reevaluated Neuronetics’ application for the rTMS device under Section 510(k) of the Federal Food, Drug, and Cosmetic Act. This provision allows approval of a device if the device is sufficiently similar to existing devices and requires that the company demonstrate that the device is safe but does not require that the company demonstrate superiority to sham in an RCT. The Neuronetics Neurostar rTMS device was approved under Section 510(K) (Melkerson, 2008) for “the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.”
FIGURE 3.1 Acute response and remission rates from the pivotal trial that resulted in FDA approval. *P < .05 versus sham; **P < .01 versus sham, LOCF analysis. LOCF = Last Observation Carried Forward.
The FDA approval specifically cited the use of rTMS in patients who had failed to achieve satisfactory improvement from one prior adequate antidepressant medication in the current episode. This approval was based on the post hoc analysis of patients in the pivotal trial who failed only one prior antidepressant trial (n = 164) that resulted in a clear superiority of active rTMS versus sham as early as week 2, with response rates by MADRS of 10.2% versus 4.0%, at week 4 of 20.5% versus 9.2%, and at week 6 of 25.0% versus 9.2% (Lisanby et al., 2009). This narrow label provides guidance for clinicians about patients who are most likely to respond to rTMS. However, in a practical sense, few patients present as possible rTMS candidates at that relatively low level of treatment resistance. Off-label rTMS is frequently used in patients with more TRD, a practice that is supported by the effectiveness data from clinical practice that has recently been published (Carpenter et al., 2012; Connolly, Helmer, Cristancho, Cristancho, and O’Reardon, 2012).
Patients whose HAMD-17 score did not decrease by more than 25% of their baseline score after 4 weeks of blinded treatment during the acute phase entered an open-label extension phase (Avery et al., 2008). Of the 158 patients who enrolled in the open-label phase, 85 had originally been assigned to the sham arm and 73 had originally been assigned to the active arm (the extended rTMS group). The primary outcome measure for the extension phase was the baseline-to-endpoint (i.e., 6 week) change in MADRS score. Those who had been originally assigned to the active arm showed a response rate of 26% and a remission rate of 11%. Those who had originally been assigned to sham showed a response rate of 42.4% and a remission rate of 20%. During the 3-week taper phase (six sessions of TMS over 3 weeks with transition to antidepressant medication), remission rates by MADRS parameters increased further to 17.6% for the extended TMS group and to 30.6% for the sham-to-rTMS group. Numbers were slightly higher but comparable for the HAMD-24 scores (Avery et al., 2008). These data support the concept that some patients may require extended rTMS sessions in order to attain remission, although the data are limited by the open-label study design.
A second large RCT (n = 190) sponsored by the NIH replicated the results from the industry-sponsored trial after 3 weeks of treatment (George et al., 2010). In this study, medication-free TRD patients (mean = 1.5 failed antidepressant trials) received either active rTMS or sham stimulation. The study procedures were similar to those used in the industry-sponsored trial with the differences being a better placebo control (i.e., improved sham coil) and more accurate targeting with magnetic resonance imaging (MRI). The sham coil was an active scalp stimulation, which ensured fully adequate blinding, and scalp location could be adjusted anteriorly by 1 cm depending on MRI results to ensure accurate targeting on the DLPFC. The primary outcome measure was remission rate, which was defined as HAMD score ≤3 or two consecutive HAMD scores ≤10. Remission rate in patients receiving active stimulation was 14%, which was superior to those receiving sham stimulation (5%) at week 3 (P = 0.02), and the number needed to treat (NNT)2 was 12 (George et al., 2010). Of note, these remission rates are similar to the rates reported in the industry-sponsored trial at week 6.
Those patients who did not improve significantly (defined as ≥30% reduction in HAMD score) after 3 weeks of blind treatment in the optimization of TMS (OPT-TMS) trial were given the option to enter an open-label phase trial (phase 2) and received 3 weeks of high-frequency rTMS at 120% motor threshold (MT) over the left DLPFC for a total of 3000 pulses per session. Those who did not respond after 3 weeks of open-label, high-frequency rTMS (n = 81) received up to 4 weeks of slow-frequency rTMS at 120% MT over the right DLPFC for 1800 pulses per session; 26% subsequently achieved remission. Of all the patients in the OPT-TMS phase 2 trial (n = 141), 30.5% met criteria for remission, while 41% met criteria for response; original treatment assignment (active versus sham in phase 1) was not statistically associated with remission (McDonald et al., 2011).
The third large-scale RCT was conducted in Europe (n = 127). This study did not examine rTMS as a stand-alone therapy but rather tested for an accelerating antidepressant effect when a patient was randomized to simultaneously start on an antidepressant or placebo. The investigators measured outcomes after 3 weeks of rTMS at 110% MT over the left DLPFC for 2000 pulses per session. Response rates were not statistically different in the two treatment groups. Thus, rTMS in combination with an antidepressant did not increase antidepressant response (Herwig et al., 2007).
Despite the publication of results from relatively large sham-controlled trials, systematic reviews of the extant literature are important to gain a more comprehensive picture of rTMS efficacy. Metaanalyses that include good-quality and homogeneous RCTs are categorized at level 1 evidence of efficacy according to the Oxford 2011 Levels of Evidence guidance (2012). Briefly, levels of evidence range from 1 to 5, with 1 being the most scientifically reliable and 5 being the least scientifically reliable (e.g., expert opinion). Levels can be graded up or down depending on variables such as the quality or consistency of the study in question. In general, cohort studies are level 2, case-control studies are level 3, and case series are level 4 (Oxford 2011 Levels of Evidence, 2012).
The major benefit of a metaanalysis is to synthesize the results of many smaller studies into one large study, which then increases study power. In this case, “power” is defined as the probability that the outcome or change in the dependent variable (i.e., improvement in depression) is actually related to the intervention or independent variable (i.e., rTMS). Therefore, higher power equals higher confidence in the effect of an intervention. In addition, metaanalyses gather the available evidence in a single study so that a clinician need not look up every trial on a particular intervention before making a clinical decision. Still, this methodology is not perfect and can suffer from significant issues of bias, both due to selection and statistical methods (Gavaghan, Moore, and McQuay, 2000; Chan, Hrobjartsson, Haahr, Gotzsche, and Altman, 2004; Wood et al., 2008).
To date, at least 11 metaanalyses have been published, with most of them finding that rTMS is statistically superior to sham for the treatment of major depression. Initial metaanalyses were published in early 2000. McNamara et al. (2001) included five studies and reported a NNT of 2.3. Holtzheimer et al. (2001) included 12 studies in their analysis and obtained an effect size of 0.81. These results are in line with those from Burt et al. (2002) and Kozel and George (2002) who reported effect sizes of 0.62 and 0.53, respectively.3
More recent and larger metaanalyses continue to support the role of rTMS as an effective and safe tool in the treatment of major depression. Lam et al. (2008) focused on TRD and analyzed 21 RCTs (n = 899) in terms of response (≥50% reduction in HAMD or MADRS) and remission (HAMD <7 and MADRS <12). Both response and remission rates were significantly superior with active stimulation when compared with sham (25% versus 9% and 17% versus 6%, respectively). Additionally, an effect size of 0.48 and an NNT of 6 for response and 7 for remission were reported. Fourteen of the 21 studies used a stricter definition of TRD (those who failed at least two antidepressant medications [ADM] in the current episode) and still yielded an NNT of 6 for both response and remission.
Similarly, Schutter (2009) analyzed 30 RCTs (n = 1164) in which high-frequency stimulation over the left DLPFC was compared with sham stimulation. The results favored active rTMS, with an overall effect size of 0.39 (95% confidence interval [CI], 0.25–0.54). Schutter (2010) also evaluated low-frequency stimulation, and in a pooled sample of 252 depressed patients from nine RCTs, found an effect size of 0.63 (95% CI, 0.03–1.24), favoring active treatment with low-frequency rTMS.
In the most recent metaanalysis, Slotema et al. (2010) pooled data from 34 RCTs, with a total sample of 1383 patients with depression (751 received active rTMS and 632 received sham stimulation). The results favored active rTMS, with a weighted mean effect size of 0.55 (P < 0.001). When broken down by type of stimulation, effect sizes remained medium to large: 0.53 (P < 0.001) for stimulation over the left DLPFC, 0.82 (P < 0.001) for stimulation over the right DLPFC, and 0.47 (P = 0.03) for sequential bilateral stimulation.
TMS has not been directly compared with pharmacotherapy to date, but indirect comparisons are made possible by metaanalyses. Turner et al. (2008) obtained trial data for 12 antidepressant medications from the FDA (constituting 12,564 patients). The overall effect size for published studies was 0.41. However, when all studies from the FDA database were included, the overall effect size was 0.31. As can be seen in Figure 3.2, the efficacy of rTMS is comparable to medications. However, there are many other reasons one might favor rTMS over medication.
According to a recent metaanalysis, the 1-year relapse rate with maintenance ADM treatment was 23% (Williams, Simpson, Simpson, and Nahas, 2009). However, Janicak et al. (2010) reported a 10% relapse rate during a 6-month observational period of acute rTMS responders, and Mantovani et al. (2012) reported a relapse rate of only 13.5% during a 3-month observation period of the OPT-TMS remitters.
FIGURE 3.2 Comparative effect sizes of rTMS and antidepressant medications. ADM = antidepressant medications, ECT = Electroconvulsive Therapy, HAM-D = Hamilton Depression Rating Scale.
As mentioned earlier, Avery et al. (2008) reported that remission rates in rTMS after failure of one or two ADMs were comparable to those reported in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial based on the HAMD-17 data (Rush et al., 2006b). However, remission rates were two to three times higher after three prior medication failures for rTMS (18.2%) versus medication change (6.9%). Additionally, in the open-label phase of the OPT-TMS study, McDonald et al. (2011) reported a remission rate of 30.5% (again, based on HAMD scores). The most comparable data to the STAR*D trial, though, would be observational data from “real world” clinical practice.
In the study by Connolly et al. (2012) of 100 consecutive patients to receive rTMS in one outpatient setting, a 35% remission rate after 6 weeks of acute treatment was reported. Notably, the mean number of failed medication trials in the current episode was 3.4 for this population. In addition, the most recent naturalistic study of 307 outpatients reported remission rates of 39.9% in those who failed one or more medications during their current major depressive episode and 34.9% in those who failed two or more medications in their current episode (Carpenter et al., 2012).
Finally, side effects can be a significant problem for patients on antidepressant medications, leading to high discontinuation rates. STAR*D data noted discontinuation rates ranging from 23.1% to 41.4% (McGrath et al., 2006; Rush et al., 2006b). Dropout rates for rTMS are consistently less than 10% in both RCTs and obervational settings (Herwig et al., 2007; O’Reardon et al., 2007; George et al., 2010; Connolly et al., 2012).
Due to the high tolerability and relatively benign side effect profile of rTMS, as well as the convenience of being an office-based procedure that does not require anesthesia, rTMS has become an attractive alternative to electroconvulsive therapy (ECT) for some patients (Figure 3.3). To date, seven small trials have compared the two treatments (Grunhaus et al., 2000; Pridmore, Bruno, Turnier-Shea, Reid, and Rybak, 2000; Janicak et al., 2002; Grunhaus, Schreiber, Dolberg, Polak, and Dannon, 2003; Schulze-Rauschenbach et al. 2005; Rosa et al., 2006; Eranti et al., 2007).
Although rTMS was a statistical tie in six of the seven trials (Grunhaus et al., 2000, Grunhaus et al., 2003; Pridmore et al., 2000; Janicak et al., 2002; Schulze-Rauschenbach et al., 2005; Rosa et al., 2006), the sample sizes are too small to determine noninferiority or equivalence of rTMS relative to ECT. Additionally, ECT has effect sizes almost two-fold higher than those reported for rTMS (see Figure 3.2; UK ECT Review Group, 2003).
A metaanalysis by Slotema et al. (2010) pooled data from six trials comparing ECT (both unilateral and bilateral) with rTMS (high-frequency to left DLPFC). The pooled sample tallies 215 patients (113 received rTMS and 102 received ECT). A weight effect size of −0.47 (P = 0.004) favored ECT. Although that is an expected result, it is worth noting that the rTMS sample received suboptimal treatment (compared with current practice): the total number of pulses per session ranged from 1000 to 2500 and the stimulation was delivered at or below MT in three out of six studies. Despite ECT’s superiority, rTMS offers an effective alternative to patients in whom ECT is contraindicated or not tolerated.
It is true that technique and dosing parameters have been maximized over the last several years, but there is still a wide variety of individual response to rTMS. Lisanby et al. (2009) investigated predictive factors for the 301 participants from the O’Reardon et al. (2007) study. For the acute phase of the trial, statistical analysis showed that a major depression duration of less than 2 years and decreased evidence of antidepressant treatment resistance were each independently associated with improved response to rTMS. In fact, the effect size of rTMS for the subset of participants who had failed only one medication was 0.83 (but it was still 0.42 for those who had failed two to four medications in their current episode). When participants from the open-label phase were evaluated, less treatment resistance was also a predictor of positive outcomes for those originally assigned to sham, but duration of episode had no bearing on either group (sham-to-open or active-to-open). For all open-label participants, higher baseline severity score on the MADRS and absence of a comorbid anxiety disorder were positive predictors of response. Interestingly, older age (often cited as a negative predictor of response) showed no significant difference for either acute or open-label response. However, it should be noted that the study did not include anyone aged >70 years.
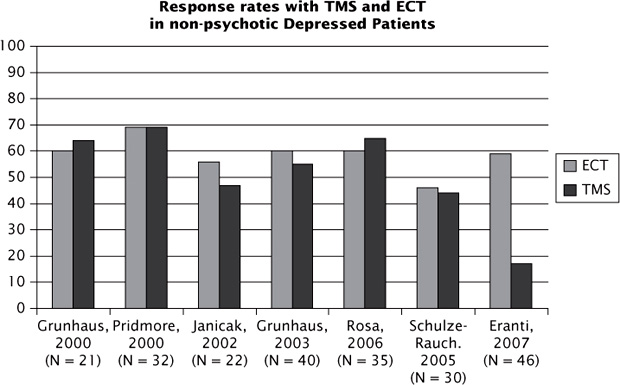
FIGURE 3.3 Comparative outcomes from the ECT versus rTMS trials.
In the 24-week follow-up phase of the O’Reardon et al. (2007) study, a more robust response to rTMS in the acute trial phase was associated with a lower risk of relapse; however, the findings of Lisanby et al. were not statistically associated with time to relapse. The authors posited that this could be due to the small sample size or because positive predictors of acute response might not be the same as the positive predictors of long-term response.
Subsequent studies have verified medication resistance as a negative predictor of response (George et al., 2010; Connolly et al., 2012; Janicak et al., 2010). However, a large naturalistic study of 307 patients showed only a moderate effect of medication resistance on remission (39.9% of patients who failed one or fewer medications during their current episode remitted, while 34.9% achieved remission who failed two or more medications). In addition, comorbid anxiety was not a negative predictor of response (Carpenter et al., 2012).
Other factors that have shown greater responsiveness to rTMS are the absence of psychotic symptoms (indirectly assumed because ECT has shown superiority to rTMS in psychotic depression) and the absence of benzodiazepines or anticonvulsants as adjunctive treatment (Slotema et al., 2010; Rodriguez-Martin et al., 2001; Li et al., 2011).
With the availability of rTMS as a clinical treatment in the United States, the first large reports of its real-world effectiveness have emerged (Carpenter et al., 2012; Connolly et al., 2012). Although these nonresearch samples have a high degree of treatment resistance (2.5–3.4 adequate antidepressant trials on average), results are encouraging. Carpenter et al. (2012) pooled data from 42 clinical practices treating unipolar patients in a current major depressive episode (n = 307). The clinical global impression of severity scale was the primary outcome, and response and remission rates were 58% and 37.1% at either 6 weeks or at the point of maximal clinical benefit, respectively. These rates were congruent with those from self-report outcome measures. Those with less severe symptoms at baseline, of younger age, and showing less treatment resistance did better. Of note, a single seizure occurred in a patient with sleep deprivation who was also taking a combination of bupropion and stimulants (Carpenter et al., 2012).
The Connolly et al. (2012) study differs from the Carpenter study in several respects. The sample size is smaller (n = 100), and all patients come from a single academic medical center. It also includes cases of bipolar depression (n = 20) as well as more patients who had failed ECT (36% versus 15%). The overall results, however, were similar to those from the Carpenter et al. study: the response rate and remission rate, as measured by the investigator-reported clinical global impression scale, were 50.6% and 24.7%, respectively, and congruent with self-report measures. Treatment resistance was not found to predict treatment outcomes negatively, as might have been expected. For those patients who previously failed ECT, the response and remission rates were 47% and 20% at 6 weeks, suggesting that a course of rTMS in patients who failed to tolerate or respond to an ECT course is a reasonable consideration in the treatment algorithm (Figure 3.4).
In the bipolar subgroup (n = 20), response and remission rates were lower than in the unipolar group (a response rate of 35% and a remission rate of 15%). This may suggest that current treatment parameters for unipolar patients may be less effective in those with bipolar depression, though caution is warranted in drawing that inference in light of the small sample size. Overall, the study reported no seizures and a low dropout rate of 3% due to adverse events (headache and scalp discomfort). This study also had a maintenance phase with about half of the sample entering 6 months of maintenance rTMS in combination with medications. The overall durability was acceptable and comparable to ECT maintenance, with 62% maintaining responder status at the last observation carried forward or at 6 months.
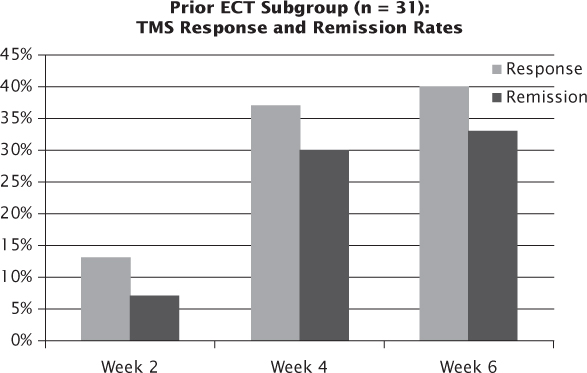
FIGURE 3.4 Outcomes with rTMS following a prior unsuccessful trial of ECT.
The proven clinical efficacy of rTMS has inspired researchers to investigate it as a possible treatment for populations where medication dosing has historically been limited due to side effects, intolerance, or the risk of significant drug–drug interactions. Those populations include adolescents, pregnant women, and the elderly.
The open-label study by Wall et al. (2011) of 8 adolescents had a 71% remission rate using the Children’s Depression Rating Scale—Revised when the adolescents were treated with 3000 pulses per day for 30 treatments. Furthermore, over the course of acute treatment and for 6 months follow-up, cumulative improvement was seen and results were maintained. Other researchers have also shown some promising results in small trials, although dosing parameters and sample sizes varied widely from those of the Wall et al. study. Bloch et al. (2008) had a response rate of 30%, but only treated his patients at 400 pulses a day for 14 days, and Loo et al. (2006) had promising results for only two patients, precluding reliable statistical analysis.
The first open-label study of rTMS in 10 pregnant women with major depression was recently completed (Kim et al., 2011). Dosing parameters were 1 Hz at 100% MT on the right DLPFC at 300 pulses a day for 20 days; right-sided treatment was chosen for its lower seizure risk. The response rate was 70%, and all mothers had healthy babies with no perinatal or postnatal complications.
Finally, Figiel et al. (1998) first reported a difference in response rates to rTMS between those who were aged ≤65 years (56%) and those who were aged >65 years (23%) and noted that this could be due to prefrontal atrophy seen on MRI. A recent pilot study of rTMS in 18 patients aged 55–75 years adjusted motor threshold dosing intensity from 103% to 141% based on MRI-measured prefrontal atrophy (Nahas et al., 2004). Acute treatment was only given for 3 weeks, but the response rate was 27%. The authors concluded that dosing of 120% MT could overcome increased skull-to-cortex distance due to prefrontal atrophy in most elderly patients. It is also reasonable to conclude that a longer course of acute treatment might have resulted in increased response/remission rates (Avery et al., 2008; Gershon, Dannon, & Grunhaus, 2003).
While rTMS is a safe and effective treatment, it is not time efficient, as it requires daily sessions lasting 30–60 minutes over a 4- to 6-week period. An interesting pilot study by Holtzheimer et al. (2010) addressed this deficiency by giving a relatively large number of pulses (15,000) over 2 days. rTMS at these higher dosing levels was safe and well tolerated. At day 3, results were impressive, with a response rate of 43%. Good durability was observed at the week 3 and week 6 follow-up points (with response rates of 36% at both time points). Remission rates reported at the three time points were 29%, 36%, and 29%, respectively. This so-called accelerated rTMS approach may be quite helpful in patients where access or distance to an rTMS center is problematic. It may also have implications for the future delivery of rTMS on an in-patient basis. More research of a controlled nature is needed to validate this approach. Even higher doses have been delivered to healthy volunteers (38,880) in 1 week (Anderson et al., 2006), and an open label study by Hadley et al. (2011) safely delivered 30,000 pulses per week in 20 patients, with encouraging outcomes.
Other forms of rTMS may become of increasing clinical importance in the future. An exciting development is deep rTMS where the magnetic field has a depth of about 6 cm and is capable of reaching frontal subcortical structures as well as the orbitofrontal cortex. At the time of going to press, an rTMS machine by Brainsway (Jerusalem, Israel), which is purported to deliver deep rTMS, had just received FDA clearance.
Theta burst rTMS offers the potential of having more robust effects on neuroplasticity than standard rTMS and is in the early clinical trial phase of development. Finally, there is great interest in combining rTMS with psychotherapy techniques such as cognitive behavioral therapy and mindfulness. Such approaches conducted during rTMS sessions themselves may bring “on line” prefrontal cortex activation and thus have the potential to further enhance rTMS’s efficacy.
Anderson, B., Mishory, A., Nahas, Z., Borckardt, J. J., Yamanaka, K., Rastogi, K., & George, M. S. (2006). Tolerability and safety of high daily doses of repetitive transcranial magnetic stimulation in healthy young men. Journal of ECT, 22(1), 49–53.
Arroll, B., Elley, C. R., Fishman, T., Goodyear-Smith, F. A., Kenealy, T., Blashki, G., Kerse, N., & Macgillivray, S. (2009). Antidepressants versus placebo for depression in primary care. Cochrane Database Systematic Review, 8(3).
Avery, D. H., Isenberg, K. E., Sampson, S. M., Janicak, P. G., Lisanby, S. H., Maixner, D. F., Loo, C., Thase, M. E., Demitrack, M. A., & George, M.S. (2008). Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. Journal Clinical Psychiatry, 69(3), 441–451.
Barker, A. T., Freeston, I. L., Jalinous, R., Merton, P. A., & Morton, H. B. (1985). Magnetic stimulation of the human brain. Journal Physiology, 369, 1–3.
Bloch, Y., Grisaru, N., Harel, E. V., Beitler, G., Faivel, N., Ratzoni, G., Stein, D., & Levkovitz, Y. (2008). Repetitive trascranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. Journal of ECT, 24(2), 156–159.
Burt, T., Lisanby, S. H., & Sackheim, H. A. (2002). Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal Neuropsychopharmacology, 5(1), 73–103.
Carpenter, L. L., Janicak, P. G., Aaronson, S. T., Boyadjis, T., Brock, D. G., Cook, I. A., Dunner, D. L., Lanocha, K., Solvason, H. B.&Demitrack, M. A. (2012). Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and Anxiety, 29, 587–596.
Chan, A. W., Hrobjartsson, A., Haahr, M. T., Gotzsche, P. C., & Altman, D. G. (2004). Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. Journal American Medical Association, 291(20), 2457–2465.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates.
Connolly, R. K., Helmer, A., Cristancho, M. A., Cristancho, P., & O’Reardon, J. P. (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. Journal Clinical Psychiatry, 73(4), 567–573.
Eranti, S., Mogg, A., Pluck, G., Landau, S., Purvis, R., Brown, R. G., Howard, R., Knapp, M., Philpot, M., Rabe-Hesketh, S., Romeo, R., Rothwell, J., Edwards, D., & McLoughlin, D. M. (2007). A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. American Journal Psychiatry, 164(1), 73–81.
Figiel, G. S., Epstein, C., McDonald, W. M., Amazon-Leece, J., Figiel, L., Saldivia, A., & Glover, S. (1998). The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. Journal Neuropsychiatry Clinical Neuroscience, 10, 20–25.
Gavaghan, D. J., Moore, R. A., & McQuay, H. J. (2000). An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain, 85(3), 415–424.
George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., Anderson, B., Nahas, Z., Bulow, P., Zarkowski, P., Holtzheimer, P. E., 3rd, Schwartz, T.&Sackeim, H. A. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of General Psychiatry, 67, 507–516.
George, M. S., Wassermann, E. M., Williams, W. A., Callahan, A., Ketter, T. A., Basser, P., Hallett, M.&Post, R. M. (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport, 6(14), 1853–1856.
Gershon, A. A., Dannon, P. N., & Grunhaus, M. D. (2003). Transcranial magnetic stimulation in the treatment of depression. American Journal Psychiatry, 160, 835–845.
Grunhaus, L., Dannon, P.N., Schreiber, S., Dolberg, O. H., Amiaz, R., Ziv, R., & Lefkifker, E. (2000). Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biological Psychiatry, 47, 314–324.
Grunhaus, L., Schreiber, S., Dolberg, O. T., Polak, D., & Dannon, P. N. (2003). A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biological Psychiatry, 53, 324–331.
Hadley, D., Anderson, B. S., Borckardt, J. J., Arana, A., Li, X., Nahas, Z., & George, M. S. (2011). Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. The Journal of ECT, 27(1), 18–25.
Herwig, U., Fallgatter, A. J., Hoppner, J., Eschweiler, G. W., Kron, M., Hajak, G., … Schönfeldt-Lecuona, C. (2007). Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. British Journal Psychiatry, 191, 441–448.
Holtzheimer, P. E., McDonald, W. M., Mufti, M., Kelley, M. E., Quinn, S., Corso, G., & Epstein, C. M. (2010). Accelerated repetitive transcranial magnetic stimulation (aTMS) for treatment-resistant depression. Depression and Anxiety, 27, 960–963.
Holtzheimer, P. E., Russo, J., & Avery, D. H. (2001). A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bulletin, 35(4), 149–169.
Janicak, P. G., Dowd, S. M., Martis, B., Alam, D., Beedle, D., Krasuski, J., … Viana, M. (2002). Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biological Psychiatry, 51, 659–667.
Janicak, P. G., Nahas, Z., Lisanby, S. H., Solvason, H. B., Sampson, S. M., McDonald, W. M., … Schatzberg, A. F. (2010). Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimulation, 3, 187–199.
Kim, D. R., Epperson, N., Pare, E., Gonzalez, J. M., Parry, S., Thase, M. E, … O’Reardon, J. P. (2011). An open label pilot study of transcranial magnetic stimulation for pregnanct women with major depressive disorder. Journal Womens Health, 20(2), 255–261.
Kozel, F. A., & George, M. S. (2002) Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. Journal Psychiatric Practice, 8(5), 270–275.
Lam, R. W., Chan, P., Wilkins-Ho, M., & Yatham, L. N. (2008). Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Canadian Journal Psychiatry, 53(9), 621–631.
Li, X., Large, C. H., Ricci, R., Taylor, J. J., Nahas, Z., Bohning, D. E., … George MS. (2011). Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor and prefrontal effective connectivity. Psychiatry Research, 194(2), 141–148.
Lisanby, S. H., Husain, M. M., Rosenquist, P. B., Maixner, D., Gutierrez, R., Krystal, A.,… George, M. S. (2009). Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology, 34, 522–534.
Loo, C., McFarquhar, T., & Walter, G. (2006). Transcranial magnetic stimulation in adolescent depression. Australasian Psychiatry, 14(1), 81–85.
Mantovani, A., Pavlicova, M., Avery, D., Nahas, Z., McDonald, W. M., Wajdik, C. D., … Lisanby, S. H. (2012). Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (Tms) in treatment-resistant depression. Depression and Anxiety, 29, 883–890.
McDonald, W. M., Durkalski, V., Ball, E. R. 3rd, Holtzheimer, P. E., Pavlicova, M., Lisanby, S. H., … George, M. S. (2011). Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment locations in treatment-resistant depression. Depress Anxiety, 28, 973–980.
McGrath, P. J., Stewart, J. W., Fava, M., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., … Rush, A. J. (2006). Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. American Journal Psychiatry, 163(9), 1531–1541.
McNamara, B., Ray, J. L., Arthurs, O. J., & Boniface, S. (2001). Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychological Medicine, 31(7), 1141–1146.
Melkerson, M. N. (2008). Special premarket 510(k) notification for NeuroStar TMS Therapy System for major depressive disorder. Retrieved from Food and Drug Administration. http://www.accessdata.fda.gov/cdrh_docs/pdf8/K083538.pdf
Morris, S. B., & DeShon, R. P. (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7, 105–125.
Nahas, Z., Li, X., Kozel, F. A., Mirzki, D., Memon, M., Miller, K., … George, M. S. (2004). Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depression Anxiety, 19(4), 249–256.
O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., … Sackeim, H. A. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological Psychiatry, 62, 1208–1216.
Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine Web site. 2012. Retrieved from http://www.cebm.net/index.aspx?o=5653
Pridmore, S., Bruno, R., Turnier-Shea, Y., Reid, P., & Rybak, M. (2000). Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. International Journal Neuropsychopharmacology, 3(2), 129–134.
Ray, J. W., & Shadish, W. R. (1996). How interchangeable are different estimators of effect size? Journal Consulting Clinical Psychology, 64, 1316–1325. (See also “Correction to Ray and Shadish (1996),” Journal Consulting Clinical Psychology, 66(532): 1998.)
Rodriguez-Martin, J. L., Barbanoj, J. M., Schlaepfer, T. E., Clos, S., Perez, V., Kulisevsky, J., & Gironell, A. (2001). Transcranial magnetic stimulation for treating depression. Cochrane Database Systematic Review, (4). doi:10.1002/14651858.CD003493.
Rosa, M. A., Gattaz, W. F., Pascual-Leone, A., Fregni, F., Rosa, M. O., Rumi, D. O., … Marcolin, M. A. (2006). Comparison of repetitive transcranial magnetic stimulation and electroconvulsive therapy in unipolar non-psychotic refractroy depression: a randomized, single-blind study. International Journal Neuropsychopharmacology, 9(6), 667–676.
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., … Fava, M. (2006a). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal Psychiatry, 163(11), 1905–1917.
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Stewart, J. W., Nierenberg, A. A., Thase, M. E., … Fava, M.; STAR*D Study Team. (2006b). Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal Medicine, 354(12), 1231–1242.
Schulze-Rauschenbach, S. C., Harms, U., Schlaeper, T. E., Maier, W., Falkai, P., & Wagner, M. (2005). Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. British Journal Psychiatry, 186, 410–416.
Schutter, D. (2009). Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological Medicine, 39(01), 66–75.
Schutter, D. (2010). Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychological Medicine, 40(11), 1789–1795.
Scudiero, J. L. (2007). Brief summary from the neurological devices panel meeting. Published January 26, 2007. vailable from: Food and Drug Administration. Accessed July 14, 2010. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/ucm124779.htm
Slotema, C. W., Bloom, J. D., Hoek, H. W., & Sommer, I. E. (2010). Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. Journal Clinical Psychiatry, 71(7), 873–884.
Thompson, S. P. (1910). A physiological effect of an alternating magnetic field. Proceedings of the Royal Society B: Biological Sciences, 82(557), 396–398.
Turner, E. H., Matthews, A. M., Linardatos, E., Tell, R. A., & Rosenthal, R. (2008). Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal Medicine, 358(3), 252–260.
UK ECT Review Group. (2003). Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet, 361, 799–808.
Wall, C. A., Croarkin, P. E., Sim, L. A., Husain, M. M., Janicak, P. G., Kozel, F. A., … Sampson, S. M. (2011). Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. Journal Clinical Psychiatry, 72(9), 1263–1269.
Williams, N., Simpson, A. N., Simpson, K., & Nahas, Z. (2009). Relapse rates with long-term antidepressant drug therapy: a meta-analysis. Human Psychopharmacology, 24(5), 401–408.
Wood, L., Egger, M., Gluud, L. L., Schulz, K. F., Jüni, P., Altman, D. G., … Sterne, J. A. (2008). Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. British Medical Journal, 336, 601–605.