Peter B. Rosenquist and W. Vaughn McCall
Clinicians who care for patients with treatment-resistant depression (TRD) have a number of challenges, not the least of which are determining what treatments to offer and in what order and ensuring that these treatments are administered using evidenced-based protocols. While this chapter focuses primarily on transcranial magnetic stimulation (TMS), it is written from the perspective of the ideal “full-service” mental health provider. This type of provider offers a full range of services and US Food and Drug Administration (FDA)–approved treatments, including assessment, psychotherapy, pharmacotherapy, TMS, electroconvulsive therapy (ECT), and vagus nerve stimulation (VNS), for patients with mood disorders.
Which patients are ideal candidates for TMS and what are the factors that determine a successful course of TMS? The outcome of TMS is determined to varying degrees by patient factors (ie, severity of illness, adequacy and resistance to prior therapeutic trials) and by the TMS technique used. In this chapter we explore these factors as they relate to where TMS should be positioned in the range of therapeutic interventions.
While TRD patients may be self-referred, more often their regular treating psychiatrist who has exhausted the available pharmacologic or psychotherapeutic options refers them. In the face of complex presentations in individuals with TRD, it should be evident that caring for severe and treatment-resistant conditions begins with a careful evaluation. Competent practicing clinicians have learned to gather history, assess mental status, and construct a formulation and plan.
Table 5.1 lists the essential elements for the assessment, which are used to define the patient’s condition in such a way as to make very clear the basis for decision-making. Questions to be presented include the following: why this treatment, at what frequency or intensity, when to stop or amend the treatment plan, how to proceed at the point of achieving response or remission, and with what alternatives?
TABLE 5.1 Essential Elements in a Framework for Assessment
Element |
Considerations |
Diagnosis |
The primary diagnosis |
Documentation of past treatment |
Treatments that have been tried Adequacy of therapeutic trials Number of failed trials Adverse effects associated with prior treatments |
Measurement of symptoms |
Baseline severity of illness Monitoring of response |
Measurement of adverse effects |
Systemic Psychiatric Cognitive |
Multiple frames of reference |
Provider rating Patient rating Caregiver rating |
The primary diagnosis is an essential component of the FDA’s labeled indications for a given treatment. The vast majority of patients receive neuromodulation therapies for the treatment of a major depressive disorder (MDD), and this constitutes the common denominator of labeled indications. Antidepressant medications and psychotherapy, the most common first-line therapies for depression, are effective for many patients; however, a sizable minority of patients (10%–40%) are refractory to these interventions.
Of considerable importance in treatment selection and planning are medical, psychiatric, and psychosocial comorbid conditions that may affect the efficacy or safety of a given treatment. MDD with comorbid axis I disorders is associated with more chronic course, functional impairment, and treatment resistance (Petersen et al., 2001). Similarly, axis II personality disorders, specifically borderline personality, are associated with a poor prognosis in the treatment of MDD (Newton-Howes, Tyrer, & Johnson, 2006), although there is insufficient evidence specifically for neurostimulation therapies in the treatment of personality disorders (Gescher, Cohen, Ruttmann, & Malevani, 2011; Alino, Jimenez, Flores, & Alcocer, 2010).
Ideally, a record of prior treatments is available to construct a chronology of doses, durations, and combinations of medications, psychotherapies, and neuromodulation trials. Despite the probability of missing data, there are a number of useful tools that can be used to systematize this history-taking and characterize the degree of treatment resistance in terms of the number of failed trials (Ruhe, van Rooijen, Spijker, Peeters, & Schene, 2012). Recognition memory typically outstrips spontaneous recall, so a checklist of medications given to the patient prior to and reviewed during the initial visit will help in this evaluation. It can be a challenge to define specific episodes of care for some patients. However, a simple timeline similar to the National Institute of Mental Health Life Chart Manual (Leverich & Post, 1993) can be used to clarify the onset of the present illness and the adequacy of each medication trial.
The antidepressant treatment history form (ATHF; Prudic, Sackeim, & Devanand, 1990) has been used in clinical trials (including the pivotal trials in TMS) to define the degree of treatment resistance by the number of failed adequate medication trials in the present episode. Because the ATHF systematically evaluates multiple sources of data on prior treatment, it can be somewhat cumbersome to use in its fully validated form. However, the ATHF is a helpful point of reference when summarizing treatment resistance, for example, ATHF level 2 refers to a patient’s depression that was not relieved by two antidepressants administered at an adequate dose and duration.
As discussed in Chapter 3, the FDA-labeled indication for TMS is restricted to patients who have failed only one adequate trial of antidepressant medications (ADMs) during the present depressive episode (O’Reardon et al., 2007), and repetitive TMS (rTMS) is less effective in patients with multiple antidepressant failures (Lisanby et al., 2009). The clinician should assess the patient’s degree of treatment resistance in order to adequately define the potential treatment response, given the fact that patients with significant TRD (i.e., failure of two or more antidepressant treatments in the current episode) are being treated off label. Chapter 3 outlines the data in primarily open-label trials that support the use of rTMS is TRD. However, the off-label use of rTMS should be discussed prior to treatment in patients who have failed multiple antidepressant trials.
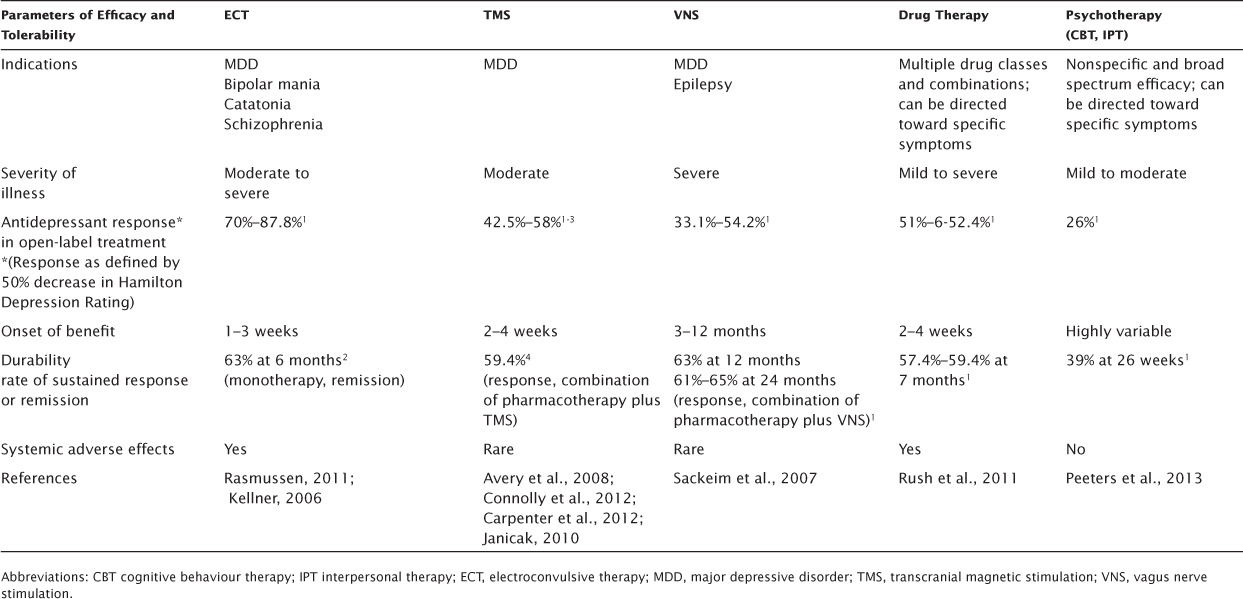
Tables 5.2 and 5.3 outline the advantages and disadvantages of TMS compared with other antidepressant therapies. Pharmacotherapy and psychotherapy are included in the table, most often as first-line treatments. ECT and VNS are usually reserved for more severely ill or treatment-resistant patients.
When discussing available options, major treatment considerations include patient factors such as diagnosis, severity of illness, psychiatric comorbidities, and general medical conditions that may be relative contraindications for a given treatment. Other advantages and disadvantages derive from specific characteristics of the treatment. These include intrinsic aspects such the range of efficacy, rate of response in a clinical setting, onset and durability of therapeutic benefit, and side effects. In addition, practical considerations such as length of an adequate trial, availability of the treatment, cost, standardization of the treatment across providers, and caregiver considerations should be discussed.
While TMS has shown promise in a number of neuropsychiatric conditions (Slotema, Bloom, Hoek, & Sommer, 2010), the FDA-approved indication is for the treatment of moderately treatment-resistant MDD. By contrast, there are data that support the efficacy of ECT in a number of severe psychiatric conditions, including MDD, manic episodes, MDD with psychotic features, catatonia, and schizophrenia (American Psychiatric Association Task Force, 2001). VNS is approved for the treatment of epilepsy and MDD. In addition, pharmacotherapy has been the mainstay for treatment of major depression and a range of comorbid conditions, including anxiety disorders and psychosis. Though limited to patients with adequate insight, willingness, and ability to participate, evidence-based psychotherapies are effective treatments for major depression (Thase et al., 1997) and also may ameliorate comorbid conditions, as patients generally improve their state of mind or change maladaptive behavior. While all treatments appear to be affected to some extent by medication resistance, the available evidence would support ECT and VNS in the most treatment-resistant patients (Prudic et al., 1996; Rasmussen et al., 2007).
TABLE 5.2 Comparative Advantages and Disadvantages of Antidepressant Therapies—Clinical Efficacy and Tolerability

TABLE 5.3 Comparative Advantages and Disadvantages of Antidepressant Therapies—Practical Considerations
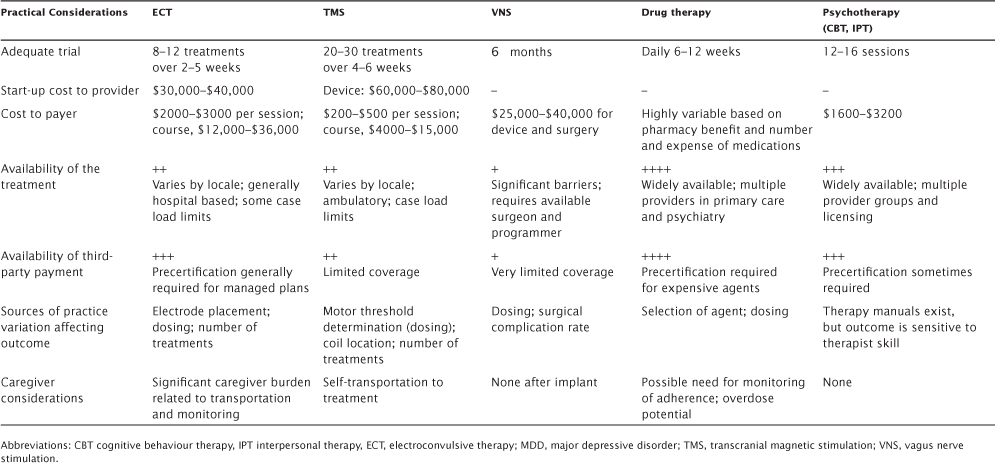
The evidence in support of TMS efficacy in MDD comes from more than 30 randomized, controlled trials (RCTs) that were FDA approved in 2008 and based on results of an industry-sponsored, large-sample (n = 301) RCT (O’Reardon et al., 2007), as well as independent replication in an National Institutes of Health–sponsored, large-sample (n = 190) RCT (George et al., 2010). These data show a rather modest remission rate for TMS, primarily in patients who were younger, minimally treatment resistant, had a less chronic course of depression, and were without psychosis.1
However, the data that are perhaps most comparable to the effectiveness of TMS in the treatment of MDD in a typical private practice are the open-label data from the pivotal trials. Open-label data are arguably more comparable to those from a community TRD clinic or private practice. The open-label crossover and 3-week continuation of the industry-sponsored pivotal clinical trial were encouraging: the cumulative remission was 27.1% and response rates was 42.4% after 6 weeks of treatment and 36.5% and 45.9% after 9 weeks (Avery et al., 2008).
To the obvious interpretive caveats associated with open label treatment, it must be added that the 9 week outcomes included the addition of antidepressant monotherapy during the three weeks of taper. Many would argue, however, that data from the “real-world” of clinical practice are essential in evaluating a treatment. Two recent post marketing studies provide a glimpse into how TMS has performed in clinical practice. These open label studies differed from the controlled industry sponsored and NIH trials in a number of ways. A broader range of patients were enrolled at the discretion of the treating clinician: those with, bipolar depression, depression not otherwise specified, and those with variable severity and treatment resistance including previous ECT non-responders. These studies furthermore employed variable technique including right sided and bilateral stimulation, flexible and therefore at times more intensive dosing, variable continuation and maintenance treatments, and allowed for the use of concomitant antidepressant medications.
Connolly et al. published a retrospective review of the first 100 patients (85 acute and 15 maintenance cases) treated at the University of Pennsylvania over three years following FDA approval in 2008. Patients receiving an index course of treatment were flexibly dosed with up to 30 treatments over six weeks. A range of outcome assessments were employed in the study, with primary outcome of the CGI-I at 6 weeks. Accordingly, the CGI response rate in this study was 49.5%, HRDS response and remission rates were 42.5% and 40%.
Carpenter et al. reported on a study of 307 outpatients treated in 42 different clinical TMS centers including 32 private practice, 7 academic and 3 non-academic institutions. Patients were treated with open label left DLPFC stimulation according to standard protocol of up to six weeks of treatment 5 days a week, 3000 pulses per session. Outcomes were assessed using clinician rated (CGI) and patient rated scales (PHQ-9, IDS-SR.) The authors reported that categorical response and remission rates were consistent in clinical magnitude on all outcome measures used in the study (CGI-S, PHQ-9, and IDS-SR). They note a response rate of 58% as defined by CGI-S ≤ 3 “mildly ill or better” at the end of acute treatment, with 37.1 achieving remission as defined by CGI-S of ≤ “borderline mentally ill: or “normal not at all ill”. In contrast to the results from multisite sham-controlled trials, treatment resistance was a less robust predictor of response and remission, as 54% of the patients in the study population met criteria for resistance to more than one adequate antidepressant medication trial during the current illness episode, and patients who had failed a minimum of one adequate antidepressant trial were as likely to be TMS responders as those who had failed two or more trials in the current episode.
Figure 5.1 shows effect sizes for TMS versus antidepressants and ECT, comparing results from the pivotal trial versus recent metaanalyses. In this context, TMS demonstrates favorable efficacy compared with ADMs, especially for the labeled indication of treatment resistance, but fails to measure up to the more robust effect size for ECT.
In an open-label setting, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial demonstrated that depressed patients unremitted after two antidepressant trials are increasingly unlikely to respond to subsequent medications (Rush et al., 2006). Specifically, in STAR*D, crossover to a different ADM yielded remission rates of 21% after one failed trial, 16% after two failed trials, and only 6.9% after three failed trails. Data from the open label extension of the TMS pivotal trial show that TMS treatment compares favorably with ADMs, with 25.6% remitting after one failed ADM trial in the present episode and 17.9% and 18.2% after two and three failures, respectively (Avery et al., 2008).
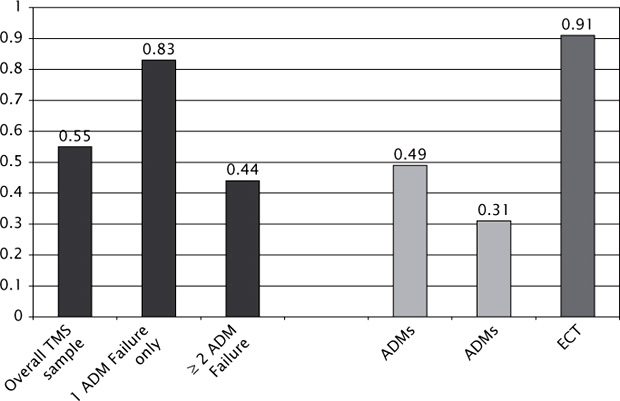
FIGURE 5.1 Comparative Effect Sizes of TMS (Transcranial Magnetic Stimulation), ADMs (Anti-Depressant Medication) and ECT (Electroconvulsive Therapy) on the HAM-D scale (Hamilton Depression Rating Scale).
Results for psychotherapy are similar to those for TMS and ADM for patients who failed one antidepressant trial. In the STAR*D trial, patients unresponsive to citalopram discontinued this medication and switched to 16 cognitive behavior therapy sessions over 12 weeks (N = 36); sessions in stage 2 showed a remission rate of 25% (Thase et al., 2007). A recent comparative effectiveness review by the Agency for Healthcare Research and Quality on Nonpharmacologic Treatments for Major Depression did not identify eligible studies gauging the effect of psychotherapy in patients with higher levels of treatment resistance (Gaynes et al., 2011).
How does the efficacy of TMS in MDD compare to that of ECT? Surprisingly, the majority of head-to-head studies have failed to demonstrate a statistical advantage for either treatment (Figure 5.2). However, most of the studies have generally been too small to determine noninferiority or equivalence. The exception thus far was a randomized trial of 46 depressed patients treated alternately with TMS (15 treatments left dorsolateral prefrontal cortex (DLPFC) totaling 15,000 pulses, 110% of motor threshold) versus titrated ECT (82% bilateral 1.5X threshold/18% unilateral 2.5X threshold, flexibly dosed [mean 6.3 treatments]; Eranti et al., 2007). Ham-D scores at the end of treatment were significantly lower in the ECT group, and the number of remitted patients also favored ECT (13/22; 68.4%) over TMS (4/24; 16.7%).
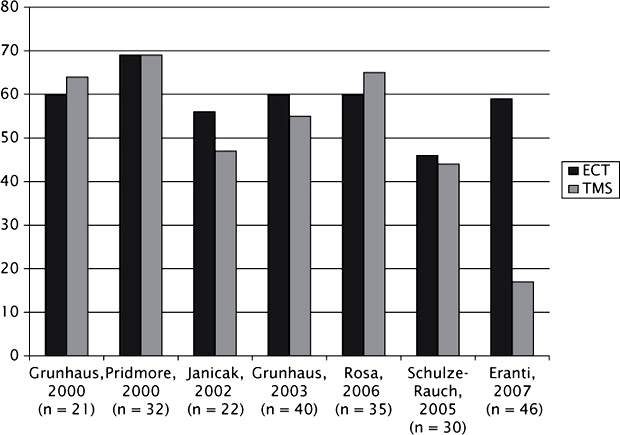
FIGURE 5.2 TMS versus ECT efficacy outcomes in depression without psychotic features. Reprinted from Avery (2008).
Patients and caregivers invariably want to understand the treatment plan. What constitutes an adequate trial? Of the treatments listed in Table 5.3, ECT has the most rapid onset of treatment response, followed by TMS, pharmacotherapy, psychotherapy, and VNS. This same order applies to the duration of an effective treatment course. For ECT, this is on the order of 6–12 treatments given over a 2- to 5-week period, followed by TMS and pharmacotherapy with daily treatments or doses given over a 4- to 6-week period. Although it is difficult in practice to find therapists trained to provide the manual-driven, evidence-based psychotherapies that range from 12 to 16 sessions, most treatments are offered at weekly intervals and require at least that many weeks to achieve an outcome. Finally, those individuals who have received a VNS implant need to understand that improvements develop slowly over many months and require gradual titration of the stimulus parameters to promote tolerability.
Two things are generally required for a treatment to be widely available, presuming an adequate demand already exists: trained providers who have access to the technology and mechanisms for reimbursement; both predict whether the treatment is available to meet demand. Currently data are limited on the number of neurostimulation practitioners in the United States. With FDA approval of the TMS device and the fact that it can be administered in an office setting, the number of practices offering TMS appears to be growing steadily.
The second predictor, availability of reimbursement, continues to affect the growth of TMS, although the number of insurers that reimburse for TMS services is increasing. This is very different from reimbursement for VNS. Although VNS has an FDA indication for the treatment of MDD, the Centers for Medicare and Medicaid Services and other private insurers have denied coverage for VNS for the treatment of MDD in contrast to the coverage for epilepsy (McCall & Rosenquist, 2007).
As discussed earlier, the strongest predictor of efficacy to date appears to be the level of treatment resistance in the TRD patient, with fewer prior antidepressant treatment failures in the present illness associated with better outcomes (Lisanby et al., 2009). In other studies, positive predictors have included a shorter duration of the present illness, younger age, greater baseline sleep disturbance, and lack of comorbid anxiety or psychosis (Eranti et al., 2007; Lisanby et al., 2009; Aguirre et al., 2011; Brakemeier et al., 2007).
What about patients with bipolar disorder? Limited data suggest that patients with bipolar depression respond somewhat less robustly than those with unipolar depression. Twenty of the 100 patients in the Connolly et al., 2012 effectiveness study who were treated with TMS had bipolar disorder (11 bipolar I, 4 bipolar II, and 5 bipolar not otherwise specified). A subgroup analysis showed that the 6-week Clinical Global Impression-Improvement scale (Guy, 1976) response and remission rates for patients with bipolar depression was 35% and 15%, respectively, compared with 50.6% and 24.7% for the overall sample.
Results of TMS treatment of mania are mixed. An early blinded, controlled trial demonstrated significant improvements in mood for patients treated with right, rather than left, prefrontal TMS at 20 Hz (Grisaru, Chudakov, Yaroslavsky, & Belmaker, 1998). A subsequent open-label study using right high-frequency prefrontal TMS in the treatment of bipolar mania found that TMS was associated with a reduction in manic symptoms (Michael & Erfurth, 2004). However, Kaptsan et al. (2003) could not replicate this result in a sham-controlled study. Reports of TMS triggering cycle into the manic state are rare (Dolberg, Schreiber, & Grunhaus, 2001). Li et al. (2004) reported that three of seven bipolar depressed acute responders to TMS were successfully maintained without relapse for 1 year with weekly maintenance treatment. However, there are no studies specifically examining the long-term management of bipolar disorder or those with rapid cycling type.
Post-stroke depression presents a unique challenge in terms of efficacy and safety because of the elevated risk for seizure following cerebrovascular accident (Burn et al., 1997). TMS has been used widely, safely, and successfully in post-stroke rehabilitation. Both low (contralateral)–and high (ipsilateral)–frequency TMS stimulation of motor cortex appear to be of benefit in the treatment of motor dysfunction and disability in patients with ischemic stroke (Emara et al., 2010). A small study of treatment-resistant post-stroke depression (N = 20) demonstrated efficacy for 10 sessions of active (10 Hz, 110% of motor threshold) versus sham stimulation of left DLPFC (Jorge et al., 2004). Reduction in depressive symptoms in this study was not influenced by patient age, type or location of ischemic stroke, volume of left frontal leukoaraiosis, or distance from coil to cortex; however, it did correlate positively with greater frontal gray and white matter volumes. Similarly, 15 sessions of left DLPFC TMS (10 Hz; 110% of motor threshold) was effective compared with sham stimulation in a group of elderly patients (N = 92) with vascular depression; again, frontal gray matter volume was a positive predictor (Jorge, Moser, Acion, & Robinson, 2008). In both studies, adverse effects were mild and consistent with other depressed populations.
A number of treatment-related factors may affect the antidepressant response to TMS. These include stimulation intensity, frequency, number of pulses administered, and duration of the treatment course (Gershon, Dannon, & Grunhaus, 2003; Padberg et al., 2002; Sachdev et al., 2002). Over time, TMS studies have used steadily higher intensity stimuli relative to motor threshold and extended the number of stimulations per session, as well as the duration of the treatment course. These modifications appear to improve the efficacy of the treatment (Gershon et al., 2003). Concerns regarding the potential for inducing seizures through excessive stimulation have largely been addressed with the safety screening, which excludes high-risk individuals (Keel, Smith, & Wassermann, 2001), and the inclusion of adequate intertrain interval between pulse trains.
One study examined the safety, tolerability, and effectiveness of a reduced intertrain interval, thereby increasing the number of stimulations per treatment session (10 Hz, 5-second pulse train with 10-second intertrain interval, 120 trains per session for a total of 6800 pulses over 34-minute session and 34,000 pulses weekly; Hadley et al., 2011). In open-label treatment, these higher doses were well tolerated by the 21 patients treated, with no seizures or serious adverse events recorded. Twelve patients achieved remission with a Beck Depression Inventory score of 10 or lower, with a mean of 15.3 (14.2 standard deviation) sessions (range 3–45 sessions), and six patients showed remission after 1 week (five sessions). If these results are representative, it appears that a more aggressive approach to treatment may produce a more robust and rapid response.
Based on the manner of construction of the electromagnet, there are four coil types that have been engineered for research and treatment purposes. These include the circular coil, the figure-8, the C-shaped coil, and, most recently, the so-called H or deep coil (Brainsway), recently approved by the FDA for use in the treatment of depression. At the time of this writing, at least four manufacturers produce TMS devices, while only two (Neurostar, Malvern PA, USA and Brainsway, Jerusalem, Israel) have received FDA approval for depression. Some manufacturers market a range of products depending upon the intended use. For high-frequency TMS, a cooling system is required to prevent overheating of the electromagnet with repeated stimulation. While the neurophysiological responses to TMS vary according to coil and stimulator type, waveform shape and polarity, coil position, and orientation relative to target cortex (Davey, Epstein, George, & Bohning, 2003; Kammer, Beck, Erb, & Grodd, 2001; Kammer, Beck, Thielscher, Laubis-Herrmann, & Topka, 2001; Thielscher & Kammer, 2004), currently there are no clinical trials that directly compare the type of coil or the manufacturer.
Magnetic field strength decreases rapidly with distance from the coil (Dolberg, Schreiber, & Grunhaus, 2001). Therefore, increasing the distance from the coil to the target cortex decreases the intensity of the stimulation reaching the brain, which is in turn negatively correlated with the degree of stimulation-induced brain activation and antidepressant response (Kozel et al., 2000; Mosimann et al., 2002; Nahas et al., 2001). The reduced efficacy seen in the elderly may be due in part to age-related atrophy, leading to an increased distance between the coil and cortex (Mosimann et al., 2002, 2004; Hadley et al., 2011; Manes et al., 2001; Fregni et al., 2006). Nahas et al. (2004) tested these ideas by adjusting the TMS dosage by the distance to prefrontal cortex in a group of older adults. This resulted in a higher rate of responders than in earlier studies, suggesting that one might increase dosage intensity to compensate in elderly patients. Further research is required to determine whether specific coil types designed for deep stimulation would similarly improve outcomes in the elderly.
Arguably, the main advantage of TMS is its lack of systemic side effects, compared with more invasive treatments. However, the potential for TMS to induce a seizure requires careful attention to patient selection and preparedness for this eventuality. In a recent review focusing on seizure risk, the authors concluded that the risk of seizures is low, with some caveats (Loo, Mcfarquhar, & Mitchell, 2008). Most cases of accidental seizures have occurred in patients with preexisting neurological disorder; however, they may also occur in the absence of predisposing factors at high stimulation intensities. The transcranial magnetic stimulation adult safety screen (TASS) is a useful instrument for identifying individuals who might be at greater risk for seizure and other adverse effects of TMS (Keel et al., 2001). The TASS includes questions about predisposing neurological history, such as head injury, epilepsy or family history of seizure, medications, and neurosurgical procedures, and also screens for the presence of ferrometallic implants (within 30 cm of the treatment coil), which is arguably the only absolute contraindication for TMS treatment. Other, relative contraindications include heart disease, cardiac pacemakers, and medication pumps.
Bae et al. (2007) reviewed 30 publications that described the use of TMS in 280 patients with epilepsy and found four instances of seizures occurring during treatment (crude risk, 1.4%). In most instances, the seizure was similar to the patient’s typical seizure, and no instances of status epilepticus or life-threatening seizures were reported. Thirteen studies have reported seizure frequencies before and after TMS. Overall, it appears that TMS may actually reduce seizure frequency for a period of 2 to 8 weeks following treatment (Bae et al., 2007). TMS has been successfully reintroduced without recurrence to a patient with no known risk factors who experienced a seizure after the fourth treatment with the addition of valproate (Bagati et al., 2012).
Nevertheless, the TMS treatment suite should have trained and vigilant staff and all necessary equipment available to manage the patient who experiences a seizure during the course of treatment. A licensed health professional should be in the treatment room at all times during the delivery of a treatment, and a responsible physician should be close enough to the treatment area to respond immediately should there be an emergency.
Figure 5.3 lists equipment and measures to take should a seizure occur, which include immediately stopping TMS treatment and basic airway and cardiovascular management and monitoring. If the treatment facility lacks access to a code paging system, licensed personnel with basic a cardiac life support training should be available to manage the patient until emergency technicians arrive.
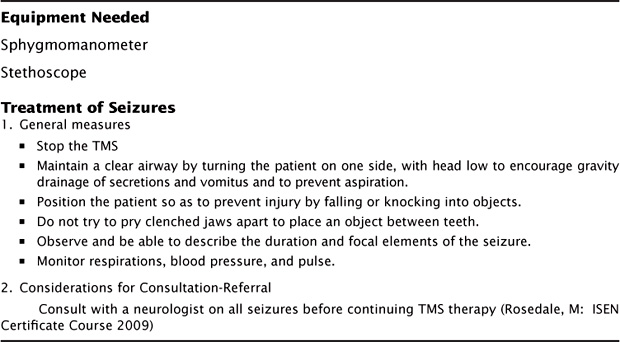
FIGURE 5.3 Safety Measures In the TMS Treatment Room
High-frequency TMS is associated with intensity-related discomfort at the site of stimulation, presumably related to direct stimulation of the superficial nerves in the scalp. Methods to diminish this include minor repositioning of the coil, use of topical lidocaine, and specialized shields that are placed over the surface of the treatment coil to attenuate the magnetic field where it makes contact with the scalp (Borckardt et al., 2008). TMS may also produce more lasting headache and scalp soreness, which are generally treated with analgesics. Treatment dropout is rare, but some individuals may require a diminished intensity of stimulation, at least initially, in order to build up tolerance for the unpleasant sensation. Headache and application-site pain declines over the course of treatment as patients become tolerant (Janicak et al., 2008), and this may help to reassure patients who experience these adverse effects. Patients and staff in the treatment room must wear earplugs with 30-dB protection to prevent auditory damage from the loud clicks associated with each TMS pulse.
Of note, TMS does not appear to have a direct effect on sleep, that is, it produces neither sedation nor activation, as may be seen with some ADMs (Rosenquist, Krystal, Heart, Demitrack, & Vaughn McCall, 2012). Instead, sleep improves as depression improves generally. For this reason, the TMS practitioner may need to consider a hypnotic for those patients with this problem, at least early in the course of treatment.
TMS appears to have gained a place in the treatment of major depression and compares favorably with other treatment alternatives for patients with a moderate degree of treatment resistance or where there are concerns about the ability of the patient to tolerate general anesthetic or drug side effects. The reimbursement environment for TMS may be improving over time, with local coverage decisions having been realized in some Medicare jurisdictions. There is an ample and expanding literature describing techniques of administration and continued technological development in the field that may ultimately lead to better outcomes.
Aguirre, I., Carretero, B., Ibarra, O., Kuhalainen, J., Martínez, J., Ferrer, A., & Garcia-Toro, M. (2011). Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. Journal Affective Disorders, 130(3), 466–469.
Alino, J. J., Jimenez, J. L., Flores, S. C., & Alcocer, M. I. (2010). Efficacy of transcranial magnetic stimulation (TMS) in depression: naturalistic study. Actas Espanolas de Psiquiatria, 38(2), 87–93.
American Psychiatric Association Task Force on ECT. (2001). The practice of ECT: recommendations for treatment, training and privileging. Washignton, DC: American Psychiatric Press.
Avery, D. H., Isenberg, K. E., Sampson, S. M., Janicak, P. G., Lisanby, S. H., Maixner, D. F., … George, M.S. (2008). Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. Journal Clinical Psychiatry, 69(3), 441–451.
Bae, E., Schrader, L., Machii, K., Alonso-Alonso, M., Riviello Jr, J., Pascual-Leone, A., … Er (2007). Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behavior, 10(4), 521–528.
Bagati, D., Mittal, S., Praharaj, S. K., Sarcar, M., Kakra, M., & Kumar, P. (2012). Repetitive transcranial magnetic stimulation safely administered after seizure. Journal of ECT, 28(1), 60–61.
Borckardt, J. J., Smith, A. R., Hutcheson, K., Johnson, K., Nahas, Z., Anderson, B., … George, M. S. (2008). Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. Journal of ECT, 22, 259–264.
Brakemeier, E., Luborzewski, A., Danker-Hopfe, H., Kathmann, N., & Bajbouj, M. (2007). Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). Journal Psychiatric Research, 41(5), 395–403.
Burn, J., Dennis, M., Bamford, J., S, Ercock, P., Wade, D., & Warlow, C. (1997). Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. British Medical Journal, 315(7122), 1582.
Carpenter, L. L., Janicak, P. G., Aaronson, S. T., Boyadjis, T., Brock, D. G., Cook, I. A., Dunner, D. L., Lanocha, K., Solvason, H. B., Demitrack, M. A. (2012). Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and Anxiety, 29(7), 587–596.
Connolly, R., Helmer, A., Cristancho, M., Cristancho, P., O’Reardon, J. (2012). Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. Journal of Clinical Psychiatry, 73(4), e567–e573.
Davey, K., Epstein, C., George, M., & Bohning, D. (2003). Modeling the effects of electrical conductivity of the head on the induced electric field in the brain during magnetic stimulation. Clinical Neurophysiology, 114(11), 2204–2209.
Dolberg, O., Schreiber, S., & Grunhaus, L. (2001). Transcranial magnetic stimulation-induced switch into mania: a report of two cases. Biological Psychiatry, 49(5), 468–470.
Emara, T., Moustafa, R., Elnahas, N., Elganzoury, A., Abdo, T., Mohamed, S., & Eletribi, M. (2010). Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. European Journal Neurology, 17(9), 1203–1209.
Eranti, S., Mogg, A., Pluck, G., Landau, S., Purvis, R., Brown, R. G., … McLoughlin, D. M. (2007). A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. American Journal Psychiatry, 164(1), 73–81.
Fregni, F., Marcolin, M., Myczkowski, M., Amiaz, R., Hasey, G., Rumi, D., … Pascual-Leone, A. (2006). Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. International Journal Neuropsychopharmacology, 9(06), 641–654.
Gaynes, B., Lux, L., Lloyd, S., Hansen, R., Gartlehner, G., Keener, P., & Lohr, K. N. (2011). Nonpharmacologic interventions for treatment-resistant depression in adults. Rockville, MD, Agency for Healthcare Research and Quality (US); 2011 Sep. Report No.: 11-EHC056-EF. AHRQ Comparative Effectiveness Reviews.
George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., … Sackeim, H. A. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of General Psychiatry, 67, 507–516.
Gershon, A. A., Dannon, P. N., & Grunhaus, M. D. (2003). Transcranial magnetic stimulation in the treatment of depression. American Journal Psychiatry, 160, 835–845.
Gescher, D. M., Cohen, S., Ruttmann, A., & Malevani, J. (2011). ECT revisited: impact on major depression in borderline personality disorder. Australian New Zealand Journal Psychiatry, 45(11), 1003–1004.
Grisaru, N., Chudakov, B., Yaroslavsky, Y., & Belmaker, R. (1998). Transcranial magnetic stimulation in mania: a controlled study. American Journal Psychiatry, 155(11), 1608–1610.
Grunhaus, L., Schreiber, S., Dolberg, O. T., Polak, D., & Dannon, P. N. (2003). A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biological Psychiatry, 53, 324–331.
Guy, W. (1976). ECDEU Assessment Manual for Psychopharmacology-Revised. US Department of Health, Education and Welfare publication (ADM 76-338) Rockville, MD; National Institute of Mental Health. 218–222.
Hadley, D., Anderson, B. S., Borckardt, J. J., Arana, A., Li, X., Nahas, Z., & George, M. S. (2011). Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. The Journal of ECT, 27(1), 18–25.
Janicak, P., O’Reardon, J., Sampson, S., Husain, M., Lisanby, S., Rado, J., … Demitrack, M. (2008). Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. Journal of Clinical Psychiatry, 69(2), 222–232.
Janicak, P. G., Nahas, Z., Lisanby, S. H., Solvason, H. B., Sampson, S. M., McDonald, W. M., Marangell, L. B., Rosenquist, P., McCall, W. V., Kimball, J., O’Reardon, J. P., Loo, C., Husain, M. H., Krystal, A., Gilmer, W., Dowd, S. M., Demitrack, M. A., Schatzberg, A. F. (2010). Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimulation, 3(4), 187–199.
Jorge, R., Moser, D., Acion, L., & Robinson, R. (2008). Treatment of vascular depression using repetitive transcranial magnetic stimulation. Archives of General Psychiatry, 65(3), 268.
Jorge, R., Robinson, R., Tateno, A., Narushima, K., Acion, L., Moser, D., … Chemerinski, E. (2004). Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biological Psychiatry, 55(4), 398–405.
Kammer, T., Beck, S., Erb, M., & Grodd, W. (2001). The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clinical Neurophysiology, 112(11), 2015–2021.
Kammer, T., Beck, S., Thielscher, A., Laubis-Herrmann, U., & Topka, H. (2001). Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clinical Neurophysiology, 112(2), 250–258.
Kaptsan, A., Yaroslavsky, Y., Applebaum, J., Belmaker, R., & Grisaru, N. (2003). Right prefrontal TMS versus sham treatment of mania: a controlled study. Bipolar Disorders, 5(1), 36–39.
Keel, J. C., Smith, M. J., & Wassermann, E. M. (2001). A safety screening questionnaire for transcranial magnetic stimulation. Clinical Neurophysiology, 112(4), 720.
Kellner, C., Knapp, R., Petrides, G., Rummans, T., Husain, M., Rasmussen, K., Mueller, M., Bernstein, H., O’Connor, K., Smith, G., Biggs, M., Bailine, S., Malur, C., Yim, E., McClintock, S., Sampson, S., Fink, M. (2006). Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Archives of General Psychiatry, 63(12), 1337–1344.
Kozel, F., Nahas, Z., deBrux, C., Molloy, M., Lorberbaum, J., Bohning, D., … George, M. S. (2000). How coil–cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(3), 376–384.
Li, X., Nahas, Z., Anderson, B., Kozel, F., & George, M. (2004). Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression?. Depression Anxiety, 20(2), 98–100.
Lisanby, S. H., Husain, M. M., Rosenquist, P. B., Maixner, D., Gutierrez, R., Krystal, A.,… George, M. S. (2009). Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology, 34, 522–534.
Loo, C., Mcfarquhar, T., & Mitchell, P. (2008). A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. The International Journal of Neuropsychopharmacology, 11(1), 131–147.
Manes, F., Jorge, R., Morcuende, M., Yamada, T., Paradiso, S., & Robinson, R. (2001). A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. International Psychogeriatrics, 13(02), 225–231.
McCall, W. V., & Rosenquist, P. B. (2007). Beware the IDEs of March. The Journal of ECT, 23(3), 137–138.
Michael, N., & Erfurth, A. (2004). Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. Journal Affective Disorders, 78(3), 253–257.
Mosimann, U., Marré, S., Werlen, S., Schmitt, W., Hess, C., Fisch, H., & Schlaepfer, T. (2002). Antidepressant effects of repetitive transcranial magnetic stimulation in the elderly: correlation between effect size and coil-cortex distance. Archives General Psychiatry, 59(6), 560–561.
Mosimann, U., Schmitt, W., Greenberg, B., Kosel, M., Müri, R., Berkhoff, M., … Schlaepfer, T. (2004). Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Research, 126(2), 123–133.
Nahas, Z., Li, X., Kozel, F. A., Mirzki, D., Memon, M., Miller, K., … George, M. S. (2004). Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depression Anxiety, 19(4), 249–256.
Nahas, Z., Teneback, C. C., Kozel, A., Speer, A. M., DeBrux, C., Molloy, M., … George, M. S. (2001). Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. J Neuropsychiatry Clinical Neuroscience, 13(4), 459–470.
Newton-Howes, G., Tyrer, P., & Johnson, T. (2006). Personality disorder and the outcome of depression: meta-analysis of published studies. British Journal Psychiatry, 188, 13–20.
O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., … Sackeim, H. A. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological Psychiatry, 62, 1208–1216.
Padberg, F., Zwanzger, P., Keck, M., Kathmann, N., Mikhaiel, P., Ella, R., … Möller, H. J. (2002). Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology, 27(4), 638–645.
Peeters, F., Huibers, M., Roelofs, J., van Breukelen, G., Hollon, S. D., Markowitz, J. C., van Os, J., Arntz, A. (2013). The clinical effectiveness of evidence-based interventions for depression: a pragmatic trial in routine practice. Journal of Affective Disorders, 145(3), 349–355.
Petersen, T., Gordon, J. A., Kant, A., Fava, M., Rosenbaum, J. F., & Nierenberg, A. A. (2001). Treatment resistant depression and axis I co-morbidity. Psychological Medicine, 31(7), 1223–1229.
Prudic, J., Haskett, R. F., Mulsant, B., Malone, K. M., Pettinati, H. M., Stephens, S., … Sackeim, H. A. (1996). Resistance to antidepressant medications and short-term clinical response to ECT. American Journal Psychiatry, 153(8), 985–992.
Prudic, J., Sackeim, H. A., & Devanand, D. P. (1990). Medication Resistance and Clinical Response to Electroconvulsive Therapy. Psychiatry Research, 31, 287–296.
Rasmussen, K. G., Mueller, M., Knapp, R. G. Husain, M. M., Rummans, T. A., Sampson, S. M., … Kellner, C. H. (2007). Antidepressant medication treatment failure does not predict lower remission with ECT for major depressive disorder: a report from the consortium for research in electroconvulsive therapy. Journal Clinical Psychiatry, 68(11), 1701–1706.
Rosenquist, P., Krystal, A., Heart, K., Demitrack, M., & Vaughn McCall, W. (2012). Left dorsolateral prefrontal transcranial magnetic stimulation (TMS): Sleep factor changes during treatment in patients with pharmacoresistant major depressive disorder. Psychiatry Research, 205(1–2), 67–73.
Ruhe, H. G., van Rooijen, G., Spijker, J., Peeters, F. P., & Schene, A. H. (2012). Staging methods for treatment resistant depression. A systematic review. Journal Affective Disorders, 137(1–3), 35–45.
Rush, A. J., Kraemer, H. C., Sackeim. H. A., Fava, M., Trivedi, M. H., Frank, E., … ACNP Task Force. (2006). Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology, 31(9), 1841–1853.
Rush, A. J., Trivedi, M. H., Stewart, J. W., Nierenberg, A. A., Fava, M., Kurian, B. T., Warden, D., Morris, D. W., Luther, J. F., Husain, M. M., Cook, I. A., Shelton, R. C., Lesser, I. M., Kornstein, S. G., Wisniewski, S. R. (2011). Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. American Journal of Psychiatry, 168(7), 689–701.
Sachdev, P., Mcbride, R., Loo, C., Mitchell, P., Malhi, G., & Croker, V. (2002). Effects of different frequencies of transcranial magnetic stimulation (TMS) on the forced swim test model of depression in rats. Biological Psychiatry, 51(6), 474–479.
Sackeim, H. A., Brannan, S. K., Rush, A. J., George, M. S., Marangell, L. B., Allen, J. (2007). Durability of antidepressant response to vagus nerve stimulation (VNS). International Journal of Neuropsychopharmacology, 10(6), 817–826.
Slotema, C. W., Bloom, J. D., Hoek, H. W., & Sommer, I. E. (2010). Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. Journal Clinical Psychiatry, 71(7), 873–884.
Thase, M. E., Friedman, E. S., Biggs, M. M. Thase, M. E., Friedman, E. S., Biggs, M. M., … Rush, A. J. (2007). Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry, 164(5), 739–752.
Thase, M. E., Greenhouse, J. B., Frank, E. Reynolds, C. F. III, Pilkonis, P. A., Hurley, K., … Kupfer, D. J. (1997). Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Archives General Psychiatry, 54(11), 1009–1015.
Thielscher, A., & Kammer, T. (2004). Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clinical Neurophysiology, 115(7), 1697–1708.
UK ECT Review Group. (2003). Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet, 361, 799–808.