CASE 49
A 66-year-old man presents for evaluation of skin growths on his face. For several years he has had scaly, rough growths on his face, forehead, and scalp. He has had individual lesions removed by previous physicians, but keeps getting more and more. He has never been diagnosed with skin cancer. He has a long history of sun exposure and multiple sunburns, primarily as a consequence of working outdoors and playing golf. He takes an aspirin a day and pravastatin for high cholesterol. He has no other significant medical history. On examination of his skin, you note multiple 4- to 7-mm lesions on the face and scalp that are flat, pink, and scaly. They feel rough on palpation. They are all in areas that would be exposed to the sun. He has several on the dorsal surfaces of his hands and forearms. You diagnose him as having multiple actinic keratoses. Along with recommending skin protection from the sun, you prescribe topical 5-fluorouracil (5-FU).
 What is the mechanism of action of 5-FU?
What is the mechanism of action of 5-FU?
 What are the adverse effects of 5-FU when given systemically?
What are the adverse effects of 5-FU when given systemically?
ANSWERS TO CASE 49:
Alkylating and Antimetabolite Agents
Summary: A 66-year-old man with multiple actinic keratoses is prescribed 5-FU.
 Mechanism of action of 5-FU: Pyrimidine antagonist that, after a complex conversion to 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), covalently inhibits thymidylate synthetase and thus impairs DNA synthesis, thereby preventing cell proliferation and inducing cell death.
Mechanism of action of 5-FU: Pyrimidine antagonist that, after a complex conversion to 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), covalently inhibits thymidylate synthetase and thus impairs DNA synthesis, thereby preventing cell proliferation and inducing cell death.
 Adverse effects of systemic 5-FU: Myelosuppression, nausea, vomiting, and hair loss.
Adverse effects of systemic 5-FU: Myelosuppression, nausea, vomiting, and hair loss.
CLINICAL CORRELATION
Actinic keratoses are premalignant skin lesions that frequently occur as a result of excessive sun exposure. Untreated, actinic keratoses may progress to become squamous cell carcinomas of the skin. Persons with multiple lesions are often treated with topical 5-FU. Other pharmacologic treatments for actinic keratosis include topical imiquod cream and ingenol mebutate gel. Systemic 5-FU is given parenterally primarily for the treatment of certain solid tumors. Systemic 5-FU is myelosuppressive and causes frequent GI disturbances and hair loss. Topical 5-FU does not have the systemic side effects but can cause significant local redness, itching, and burning of the skin.
APPROACH TO:
Pharmacology of Alkylating and Antimetabolite Agents
OBJECTIVES
1. Outline the principles of cancer chemotherapy and the development of resistance to chemotherapeutic agents.
2. List the antimetabolite and alkylating chemotherapeutic agents and describe their mechanisms of action, therapeutic uses, and adverse effects.
DISCUSSION
Class
Appropriate cancer chemotherapy demands a thorough understanding of the kinetics of tumor cell growth, including its control and regulation, a thorough understanding of the pharmacologic properties of available anticancer agents, and an appreciation of the interactions between them.
Combination chemotherapy early in therapy increases the likelihood of destroying drug-resistant populations of cells that are refractory to treatment and therefore is generally more effective than monotherapy. To be most effective, the drugs used in combination chemotherapy should each have therapeutic activity with different dose-limiting toxicities and should be administered during several cycles of treatment to allow recovery from acute adverse effects.
The drugs used to treat cancer are classified as alkylating agents, antimetabolites, cytotoxic antibiotics, plant alkaloids, hormonal agents, and miscellaneous agents. This case focuses primarily on the alkylating agents and antimetabolites (Table 49–1). Depending on the tumor type, they are often used in combinations or as adjunct therapy to surgical and radiation procedures.
TABLE 49–1 • ANTICANCER DRUGS: ALKYLATING AGENTS AND ANTIMETABOLITES (MAY BE COMBINED WITH OTHER ANTICANCER AGENTS)
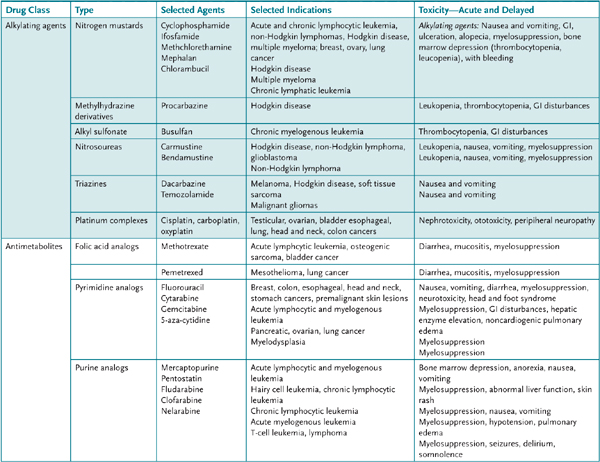
Primary resistance to anticancer drugs is thought to occur because of some inherent genetic characteristics of tumor cells. Acquired resistance of tumor cells to a specific anticancer drug may occur via several different mechanisms that usually involve either amplification or overexpression of one or more genes. For example, resistance to methotrexate is caused by either decreased drug transport into tumor cells, a modification of the target enzyme dihydrofolate reductase (DHFR) that results in a decreased affinity for methotrexate, or an increased level of DHFR in tumor cells. Resistance to the chemotherapeutic effects of alkylating agents may develop because of decreased cell permeability, increased cell thiol content that serves as a “decoy” target for alkylation, increased activity of glutathione transferases, and modification of DNA repair mechanisms. Alternatively, after exposure of a tumor cell to a number of structurally different agents, a so-called multidrug, or pleiotropic, resistance may develop to chemotherapeutic agents because of decreased uptake or retention of the drugs. This is a result of either increased expression of the constitutively expressed multidrug resistance gene (MDR-1), which codes for a surface cell membrane P-glycoprotein involved in drug efflux, or by overproduction of one of a number of other multidrug resistance proteins, for example, MRP-1, that are involved in the transmembrane export of drugs. Multidrug resistance is the major form of resistance to vinca alkaloids, etoposide, paclitaxel, anthracyclines, and dactinomycin.
Other Classes of Selected Anticancer Drugs Cytotoxic antibiotics: Dactinomycin (actinomycin D), bleomycin, doxorubicin.
Plant alkaloids: Vinblastine, vincristine, vinorelbine, etoposide, paclitaxel, topotecan.
Hormonal agents: Steroid hormones: megestrol acetate, hydrocortisone, prednisone.
Antiandrogens: Flutamide.
Antiestrogens: Tamoxifen.
Gonadotropic-releasing hormone (GRH) agonists: Goserelin acetate, leuprolide.
Aromatase inhibitors: Aminoglutethimide, anastrozole, exemestane, letrozole.
Growth factor receptor inhibitors: Cetuximab, gefitinib, erlotinib, bevacizumab.
Miscellaneous agents: Cisplatin, imatinib, hydroxyurea, mitotane, arsenic trioxide, procarbazine.
Mechanism of Action
Alkylating Agents The cytotoxic effects of alkylating agents result from the transfer of their alkyl groups to numerous cellular components, most notably the bases of DNA, particularly the N7 position of guanine, which in replicating cells (G1 and S phase) results in either miscoding or strand breakage.
Antimetabolites Methotrexate (MTX): Folic acid antagonist that binds the catalytic site of DHFR to reduce the synthesis of tetrahydrofolate that results in downstream reduction of thymidylate and an indirect inhibition of DNA synthesis as well as RNA and protein synthesis.
Fluorouracil (5-FU): A prodrug that is converted to FdUMP by a multistep process. FdUMP covalently forms an inhibitory ternary complex with the enzyme thymidylate synthetase and reduced folate N5,10-methylene tetrahydrofolate, which are essential to the synthesis of thymidylate and the production of DNA. Through other metabolic conversions, 5-FU is also incorporated into DNA as 5-fluorodeoxyuridine-5-triphosphate (FdUTP) and into RNA as 5-fluorouridine-5-triphosphate (FUTP), which results in further inhibition of DNA function as well as inhibition of RNA processing and mRNA activity.
Mercaptopurine (6-MP): The precise mechanism of action of mercaptopurine, a modified purine, is unknown. Like the natural purines, hypoxanthine and guanine, it is converted to a nucleotide by hypoxanthine guanine phosphoribosyltransferase (HGPRT). The product, in this case 6-thioinosinic acid, inhibits purine nucleotide interconversion.
Pharmacokinetics
Cyclophosphamide is not itself cytotoxic but must first be converted by hepatic microsomal enzymes to form the cytotoxic agents, phosphoramide mustard and acrolein.
COMPREHENSION QUESTIONS
49.1 Resistance to methotrexate is a result of which of the following?
A. Increased activity of glutathione transferases
B. Increased cell thiol content
C. Modification of DNA repair mechanisms
D. Modification of the target enzyme DHFR
49.2 Which of the following agent forms an inhibitory ternary complex with the enzyme thymidylate synthetase?
A. Cyclophosphamide
B. Fluorouracil (5-FU)
C. Mercaptopurine (6-MP)
D. Methotrexate (MTX)
49.3 Which of the following is true in general of combination cancer chemotherapy?
A. It is administered during several cycles of treatment.
B. It is less effective than monotherapy.
C. It includes at least two drugs with similar dose-limiting toxicities.
D. It includes one drug that has no inherent therapeutic activity.
ANSWERS
49.1 D. Resistance to methotrexate may be a result of a modification of the target enzyme DHFR. It may also be a consequence of decreased drug transport into tumor cells or an increased level of DHFR in tumor cells. Resistance to the chemotherapeutic effects of alkylating agents may develop because of decreased cell permeability; increased cell thiol content, which serves as a “decoy” target for alkylation; increased activity of glutathione transferases; and modification of DNA repair mechanisms.
49.2 B. Fluorouracil (5-FU) is a prodrug that is converted to FdUMP, which covalently forms an inhibitory ternary complex with the enzyme thymidylate synthetase and reduced folate N5,10-methylene tetrahydrofolate, both of which are essential to the synthesis of thymidylate and the production of DNA. Mercaptopurine (6-MP) is thought to inhibit purine nucleotide interconversion. Methotrexate (MTX) is a folic acid antagonist that binds the catalytic site of DHFR to reduce the synthesis of tetrahydrofolate that results in downstream reduction of thymidylate and an indirect inhibition of DNA synthesis as well as RNA and protein synthesis. The cytotoxic effects of alkylating agents like cyclophosphamide are a result of the transfer of their alkyl groups to numerous cellular components, most notably the bases of DNA that in replicating cells (G1 and S phase) results in either miscoding or strand breakage.
49.3 A. Combination chemotherapy early in therapy increases the likelihood of destroying drug-resistant populations of cells that are refractory to treatment and therefore is generally more effective than monotherapy. To be most effective, the drugs used in combination chemotherapy should each have therapeutic activity with different dose-limiting toxicities and should be administered during several cycles of treatment to allow recovery from acute adverse effect.
PHARMACOLOGY PEARLS
 Smaller tumors are generally more responsive to chemotherapy than larger tumors because of the increased probability of drug-resistant mutations in the larger tumors.
Smaller tumors are generally more responsive to chemotherapy than larger tumors because of the increased probability of drug-resistant mutations in the larger tumors.
 Development of a mild leukopenia is evidence of the adequate absorption of orally administered alkylating agents.
Development of a mild leukopenia is evidence of the adequate absorption of orally administered alkylating agents.
 Leucovorin (citrovorum factor), a folic acid analog that does not require reduction by DHFR, can be used to “rescue” patients from MTX overdose or high-dose MTX therapy.
Leucovorin (citrovorum factor), a folic acid analog that does not require reduction by DHFR, can be used to “rescue” patients from MTX overdose or high-dose MTX therapy.
REFERENCES
Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy, 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2005.
DeVita VT Jr, Hellman S, Rosenberg SA. Cancer: Principles and Practices of Oncology, 7th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2004.
Perry MD. The Chemotherapy Source Book, 3rd ed. Baltimore (MD): Lippincott Williams & Wilkins, 2001.
 What is the mechanism of action of 5-FU?
What is the mechanism of action of 5-FU? What are the adverse effects of 5-FU when given systemically?
What are the adverse effects of 5-FU when given systemically? Mechanism of action of 5-FU: Pyrimidine antagonist that, after a complex conversion to 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), covalently inhibits thymidylate synthetase and thus impairs DNA synthesis, thereby preventing cell proliferation and inducing cell death.
Mechanism of action of 5-FU: Pyrimidine antagonist that, after a complex conversion to 5-fluoro-2’-deoxyuridine-5’-monophosphate (FdUMP), covalently inhibits thymidylate synthetase and thus impairs DNA synthesis, thereby preventing cell proliferation and inducing cell death.