Definitions
The term ‘heart failure’ (HF) is generally used to describe the physiological state in which cardiac function is insufficient to meet the metabolic needs of the tissues. There are detailed criteria for the diagnosis of HF (Framingham criteria and European Society of Cardiology [ESC] guidelines; see Table 5.4, page 72). A complete description of the HF syndrome in every patient should also recognize the following entities:
• diastolic versus systolic HF
• acute versus chronic HF
• right versus left (and biventricular) HF
• high versus low output HF.
Each of these has implications for investigation, management and outcome.
Diastolic versus systolic heart failure. The outcome of these entities is similar, although their treatment is different. The definition of ejection fraction (EF) (the percentage of blood pumped from the ventricles with each heart beat) is fundamental to defining diastolic and systolic HF (Figure 1.1).

Figure 1.1 Description of diastolic versus systolic heart failure (HF), according to left ventricular ejection fraction (LVEF): (a) normal heart function; (b) systolic HF, or HF with reduced ejection fraction (HFrEF); and (c) diastolic HF, or HF with preserved (or normal) ejection fraction (HFpEF/HFnEF). A gray area exists between (b) HFrEF and (c) HFpEF. Patients with an LVEF of 40–49% have been classified by the European Society of Cardiology as having heart failure with mid-range ejection fraction (HFmrEF). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
An EF over 50% is generally regarded as normal, although there remains some debate regarding the specific cut-off point. The latest (2016) ESC guidelines divide patients into three groups according to their left ventricular ejection fraction (LVEF):
• heart failure with reduced EF (HFrEF) when EF is less than 40%
• heart failure with mid-range EF (HFmrEF) when EF is 40–49%
• heart failure with preserved (or normal) EF (HFpEF/HFnEF) when EF is 50% or more.
Systolic heart failure or heart failure with reduced ejection fraction results from myocardial injury, which leads to impaired pumping function of the left ventricle (LV) and significantly reduced LVEF (< 40%). This is the typical manifestation of patients with ischemic heart disease and multiple infarcts.
Patients with HFmrEF have mild LV systolic dysfunction with varying degrees of abnormal diastolic function.
Diastolic heart failure or heart failure with preserved (or normal) ejection fraction. Normally, the driving force for diastolic filling is elastic recoil (suction) and relaxation of the LV due to the coordinated motion of myocardial fibers, which causes the twisting and untwisting of the heart. Diastolic HF (or HFpEF) is caused by abnormal LV suction (from untwisting), relaxation and chamber compliance, with resulting elevation in diastolic pressure. It is the typical manifestation of patients with hypertensive heart disease and small ventricles with reduced stroke volume and filling disturbances.
HFpEF is common in the elderly (affecting more women than men) in whom combined ischemia, hypertrophy and age-related fibrosis may act together to produce increased myocardial stiffness and delayed relaxation. Prevalence increases with age.
Heart failure with normal systolic and diastolic function describes a group of patients with symptomatic HF but no evidence of systolic or diastolic dysfunction on resting echocardiography. In one study, 15% of patients with clinical HF had normal LVEF and normal tests of diastolic function at rest. Historically, these patients would have been diagnosed with HFpEF, but dobutamine stress echocardiography has helped to reclassify about 60% of these patients with various diagnoses, including left ventricular outflow tract obstruction, development of restrictive filling pattern at stress, chronotropic incompetence or underlying ischemic heart disease.
In addition, some symptomatic patients do not have HF. Their symptoms may be caused by fluid overload (due to renal disease) or obesity and physical deconditioning.
Acute versus chronic heart failure. The terminology ‘acute’ and ‘chronic’ in this context relates to time rather than severity. An episode of acute HF can be defined as the sudden onset or gradual worsening of signs and symptoms of HF resulting in hospital admission. The typical presentation of acute HF is pulmonary edema. Acute LV failure elevates LV diastolic pressure, which causes a significant rise in pulmonary venous pressure, resulting in pulmonary congestion (edema) as well as subendocardial ischemia, further LV remodeling and worsening of mitral valve regurgitation. The patient presents with severe dyspnea at rest, pink frothy sputum, sweating and clamminess, and sometimes shock. Raised right-sided filling pressure in response to LV dysfunction contributes to the development of systemic congestion with elevated jugular venous pressure and peripheral edema. The combination of poor cardiac output (in 50% of patients with acute HF) and renal venous hypertension may precipitate a worsening of renal function and could cause cardiorenal syndrome (see Chapter 4). In acute HF, the degree of pulmonary congestion (wet vs dry) and state of tissue perfusion (warm vs cold) varies (see Chapter 5).
HF may often be provoked by an acute illness (e.g. myocardial infarction [MI]) and may not be preceded by chronic symptoms. Conversely, chronic HF has a background of gradually worsening exertional dyspnea, edema, orthopnea and paroxysmal nocturnal dyspnea.
Recognizing the acuity of HF symptoms is an important aspect of managing HF as a chronic disease. Recognizing an exacerbation in a patient with chronic HF should lead to a search for the etiology of this change (see Chapter 3).
Right, left and biventricular failure. Left ventricular HF is the most common type of HF. It is associated with exertional dyspnea, orthopnea and paroxysmal nocturnal dyspnea. Right ventricular HF, most commonly caused by left ventricular HF, is characterized by peripheral edema, abdominal distension (due to ascites), right upper quadrant discomfort (due to liver congestion) and elevated jugular venous pressure, often with prominent v waves due to tricuspid regurgitation (see Chapter 5). Either left- or right-sided HF may occur in the context of low output (fatigue, syncope and hypotension).
High versus low output. The distinction between high and low (cardiac) output has etiologic and therapeutic implications. Low-output HF is associated with pale cool extremities, which reflects vasoconstriction and reduced cardiac output. High-output HF is associated with warm extremities and bounding pulses. In its most extreme state, high-output HF may be due to arteriovenous shunts or diseases associated with severe vasodilation (e.g. vitamin B1 deficiency – beri-beri). The most common cause of high-output HF is marked obesity.
Epidemiology
HF affects an estimated 26 million people worldwide. Although advances in the treatment of acute coronary syndromes have reduced deaths from coronary artery disease (CAD), in the USA in 2014 non-cardiovascular disease was the underlying cause of death for more than one-third of HF-related deaths of adults aged 45 or over. This shift toward less ischemic heart disease is important for HF management approaches.
The enormous impact of HF on individuals and their families is compounded by the huge costs to healthcare budgets around the world. The burden associated with HF is expected to keep rising over the next 20 years due to a number of factors:
• aging populations in developed countries
• an increase in the number of elderly people with CAD and hypertension
• a decrease in case–fatality rates associated with acute coronary syndromes
• improved diagnosis using sensitive techniques such as echocardiography.
The rising prevalence of obesity, metabolic syndrome and diabetes mellitus with associated cardiovascular complications is likely to result in further increases. Some of the factors listed above cannot be modified (e.g. aging of the population), but prevention of those that precede the development of HF (CAD, diabetes mellitus, arterial hypertension, obesity) should be a priority.
Prevalence of HF in developed countries has increased over time; current estimates are shown in Table 1.1. In those with CAD, a 28% decrease in 5-year mortality after MI was associated with a 25% increase in the 5-year rate of HF. About 70% of patients developed HF within 5 years, with two-thirds of clinical cases in the first year.
TABLE 1.1
Prevalence of heart failure – current estimates |
|
• ∼ 26 million people have heart failure (HF) worldwide: 6.5 million in Europe; > 5 million in the USA; ∼ 900 000 in the UK; > 350 000 in Australia
• 8 per 1000 people aged 50–59 years are affected by HF*
• 66 per 1000 men aged 80–89 years are affected by HF*
• 79 per 1000 women aged 80–89 years are affected by HF* |
|
*The known prevalence in African-Americans is around 25% higher. |
Incidence of HF also increases with age. In the Framingham Heart Study, the incidence approximately doubled over each successive decade of life, rising more steeply with age in women than in men. The annual incidence in men rises from 2 per 1000 at 35–64 years of age to 12 per 1000 at 65–94 years. The age-adjusted incidence has not, however, changed significantly over time.
While there are some geographic and demographic variations (reflecting differences in the most common etiologies of HF), about half of all incident cases of HF are either systolic or diastolic HF.
Hospitalization. Although, in the USA, the overall rate of HF-related hospitalization did not change between 2000 and 2010 (Figure 1.2), the proportion of hospitalizations under age 65 increased significantly during this period, from 9.4 to 10.8 per 10 000 population. In 2010, there were over 1 million hospitalizations in the USA with a first-listed discharge diagnosis of HF. The re-hospitalization rate following index admission with HF is high (about 50% at 6 months). However, about 50% of these readmissions are due to non-HF conditions (e.g. respiratory causes) and this rate is even higher in those over 65 years of age.
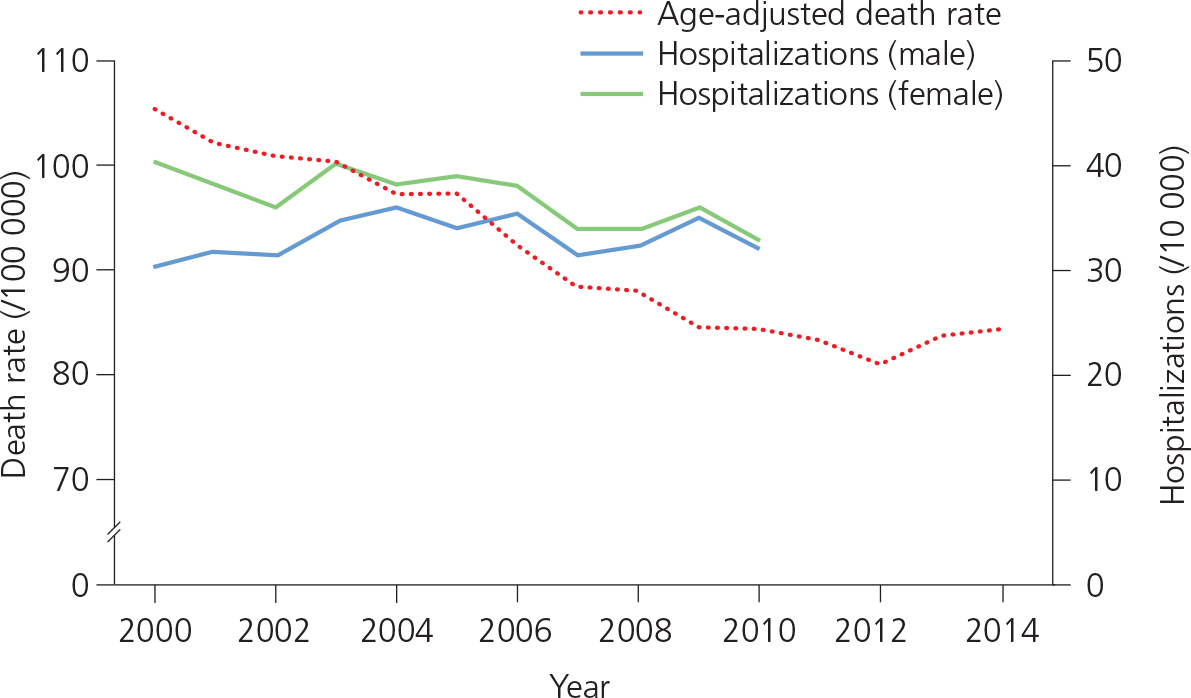
Figure 1.2 Deaths and hospitalizations due to heart failure (HF) in the USA, 2000–2014. Adapted from Centers for Disease Control and Prevention (CDC)/National Center for Health Statistics (NCHS): National Hospital Discharge Survey 2000–2010 and National Vital Statistics System mortality data 2000–2014.
Acute heart failure. While a history of chronic HF can be established in 80% of patients, 20% present with de novo cardiac dysfunction. In 50% of admissions LV systolic function is normal or near normal (HFpEF). Most of this subgroup are elderly women of 75 years and older at presentation.
Mortality. Although comprehensive medical treatment, with attention to target drug doses (especially for beta-blockers), and the advent of device therapy have reduced morbidity and mortality related to HF, mortality still exceeds that reported for most common malignancies. In the USA, the age-adjusted rate for HF-related deaths decreased between 2000 and 2012, then increased slightly between 2012 and 2014 (see Figure 1.2).
HF has an annual mortality of 19%, with a median survival of 2.3 years in men and 1.7 years in women who require hospitalization for a first episode. However, as HF results from heterogeneous causes of cardiac damage, mortality rates vary.
The worst outcomes have been reported with HF related to HIV infection, cardiac infiltration (amyloid) or cardiac cytotoxicity (due to chemotherapy such as anthracyclines), while the mortality for CAD-related HF (ischemic cardiomyopathy) exceeds that of non-CAD-related HF. Conversely, HFpEF appears to have a better prognosis, with an annual mortality of 8–9% in some reports.
Predisposing conditions. CAD, arterial hypertension, valvular heart disease and diabetes mellitus are the four most important predisposing conditions for the development of HF (see Chapter 3). Over the past 50 years, the prevalence of CAD and diabetes mellitus has increased, while rates of valvular heart disease and hypertension have fallen. Nevertheless, aortic valve disease and arterial hypertension remain the commonest causes of HFpEF in elderly people.
Population attributable risk estimates the proportion of HF in the population that is attributable to each predisposing condition. Data suggest that for individuals over 65 years of age CAD (13.1% annual risk), uncontrolled arterial hypertension (12.8%), C-reactive protein as a marker of vascular inflammation (9.7%) and low ankle–brachial index (the ratio of blood pressure in the ankle to that in the upper arm; 9.2%) have the largest effects, while low LVEF (4.1%) and atrial fibrillation (2.2%) have less effect than expected. Table 1.2 shows the risks for some of the most common predisposing conditions.
TABLE 1.2
Risk of heart failure from common predisposing conditions |
| |
Relative risk |
Overall ‘population attributable risk’ (%) |
Coronary heart disease |
8.1 |
62 |
Cigarette smoking |
1.6 |
17 |
Hypertension |
1.4 |
10 |
Overweight |
1.3 |
8 |
Diabetes mellitus |
1.9 |
3 |
Valvular heart disease |
1.5 |
2 |
Somewhat unexpected causes of HF have been found in patients in whom the initial diagnosis was not apparent: idiopathic (50%); myocarditis (9%); ischemic heart disease (7%); infiltrative disease (5%); peripartum cardiomyopathy (4%); hypertension (4%); HIV infection (4%); connective tissue disease (3%); substance abuse (3%); and cytotoxicity (1%).
Additive risk. The presence of two or more predisposing conditions in the same individual increases the risk of developing HF. For example, in patients with hypertension an antecedent MI increases the risk by five to six times, while symptomatic CAD, diabetes mellitus, left ventricular hypertrophy and valve diseases each increase the risk by two to three times.
Key points – definitions and epidemiology
• Heart failure (HF) with reduced ejection fraction (HFrEF) is characterized by an ejection fraction (EF) of less than 40%.
• The European Society of Cardiology has defined a new category of heart failure with mid-range ejection fraction (HFmrEF), characterized by an EF of 40–49%.
• Left ventricular HF is the most common type of HF.
• Symptomatic HF affects 2–3% of the global population.
• The financial burden associated with HF is likely to rise over the next 20 years.
• Coronary artery disease, arterial hypertension, valvular heart disease and diabetes mellitus are the four most important predisposing conditions for the development of HF.
References
Ambrosy AP, Fonarow GC, Butler J et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33.
Bleumink GS, Knetsch AM, Sturkenboom MC et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J 2004;25:1614–19.
Bursi F, Weston SA, Redfield MM et al. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209–16.
Gottdiener JS, Arnold AM, Aurigemma GP et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000;35:1628–37.
Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States 2000–2010. US Department of Health and Human Services, CDC, National Center for Health Statistics: NCHS Data Brief No. 108, October 2012. www.cdc.gov/nchs/data/databriefs/db108.pdf, last accessed 12 May 2017.
Hanyu N, Jiaquan X. Recent trends in heart failure-related mortality: United States, 2000–2014. US Department of Health and Human Services, CDC, National Center for Health Statistics: NCHS Data Brief No. 231, December 2015. www.cdc.gov/nchs/data/databriefs/db231.pdf, last accessed 12 May 2017.
Havranek EP, Masoudi FA, Westfall KA et al. Spectrum of heart failure in older patients: results from the National Heart Failure project. Am Heart J 2002;143:412–17.
He J, Ogden LG, Bazzano LA et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002.
Jhund PS, Macintyre K, Simpson CR et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009;119:515–23.
Lloyd-Jones D, Adams RJ, Brown TM et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 2010;121:e46–215.
Lloyd-Jones DM, Larson MG, Leip EP et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068–72.
Mahadevan G, Davis RC, Frenneaux MP et al. Left ventricular ejection fraction: are the revised cut-off points for defining systolic dysfunction sufficiently evidence based? Heart 2008;94:426–8.
Masoudi FA, Havranek EP, Smith G et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003;41:217–23.
McCullough PA, Philbin EF, Spertus JA et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol 2002;39:60–9.
Stewart S, MacIntyre K, Hole DJ et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315–22.
Thomas MD, Fox KF, Wood DA et al. Echocardiographic features and brain natriuretic peptides in patients presenting with heart failure and preserved systolic function. Heart 2006;92:603–8.
Vasan RS, Larson MG, Benjamin EJ et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999;33:1948–55.

