Implantable cardiac defibrillators
Death from heart failure (HF) is usually related to pump failure or ventricular arrhythmia (Figure 8.1). Left ventricular ejection fraction (LVEF) is the major predictor of outcome and sudden death. The majority of clinical trials have shown an increased risk of ventricular arrhythmias when LVEF is reduced to 30–35% or lower. A number of clinical trials have shown a mortality benefit in patients receiving an implantable cardiac defibrillator (ICD) either as primary or secondary prevention (Table 8.1).

Figure 8.1 Data from the MERIT-HF trial show that the risk of sudden death is greatest when patients are NYHA class II and that death from pump failure is greater in advanced symptomatic heart failure (NYHA class IV). CHF, chronic heart failure; NYHA, New York Heart Association. Source: Lancet 1999;353:2001–7, reproduced with permission from Elsevier © 1999.
|
TABLE 8.1
Recommendations for ICD implantation* |
Primary prevention
• LV dysfunction (LVEF ≤ 35%), prior MI/IHD and at least 40 days after acute MI
• LV dysfunction (LVEF ≤ 35%) of non-ischemic etiology
• High risk of sudden cardiac death (e.g. familial long QT, some cases of hypertrophic myopathy)
Secondary prevention
• Survivor of cardiac arrest due to VF/VT, after excluding reversible cause
• Structural heart disease with sustained VT
• Syncope, LVEF ≤ 35% and inducible sustained VT/VF at electrophysiological study
*Assuming patient has an overall life expectancy > 1 year, with good functional status. ICD, implantable cardiac defibrillator; IHD, ischemic heart disease; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia. |
Approximately 10% of patients who have an ICD for primary prevention receive an appropriate shock in the first 2 years after the device is implanted. Patients who receive an ICD for secondary prevention have a high risk of recurrence without treatment, with a rate of sudden death of up to 40% 1 year after presentation. Pharmacological therapy (e.g. amiodarone) is not effective at preventing sudden death and an ICD is superior therapy.
ICDs are usually implanted in the left prepectoral area (they can, if required, be implanted on the right) and are inserted under conscious sedation. The battery life of a modern device is about 5–10 years. The complications are similar to those experienced with pacemakers. There is an increasing trend to avoid intravascular leads. Subcutaneous defibrillators, placed in the adipose tissue over the sternum, with the shock lead outside the venous system, are clinically available (see Fast Facts: Cardiac Arrhythmias).
Defibrillators in non-ischemic cardiomyopathies. Although there is a clear mortality benefit for prophylactic ICDs in patients with systolic HF related to coronary artery disease (CAD), evidence for benefit in HF that is not due to CAD has been less than convincing. Since the early ICD trials, optimal medical management as well as cardiac resynchronization therapy (CRT) for HF has significantly improved outcome. In a recent large scale study, patients with LVEF ≤ 35% without CAD were randomized to receive either an ICD or optimal clinical care (including CRT) and were followed for a median of 67.6 months. Sudden cardiac death rates were low in both groups and the ICD did not confer a significant benefit compared with standard care. This study suggests that the decision to implant a prophylactic automated ICD can be safely delayed in patients with non-ischemic cardiomyopathy in favor of 6–12 months’ intensive pharmacological management. Not infrequently, the significant reversal of LV remodeling may obviate the need for device therapy.
Cardiac resynchronization therapy
Hypertension, myocardial infarction (MI) and ischemia can all cause myocardial cell damage and areas of fibrosis. Ischemia and fibrosis can damage the conduction system of the heart, slowing ventricular depolarization by promoting cell-to-cell conduction, a much slower depolarizing process. This results in a widening of the QRS complex on the surface ECG, often with a left bundle branch block type morphology. In many cases this results in a differential effect in the contractile function of the ventricles known as ventricular dyssynchrony, in which parts of the ventricle no longer achieve maximum contractility and move at different rates and with a different contractile force to other parts. There are two types of ventricular dyssynchrony:
• intraventricular dyssynchrony, in which the muscle fibers within the left ventricle contract at differing rates and speeds
• interventricular dyssynchrony in which the left and right ventricles no longer contract simultaneously.
Dyssynchrony results in a loss of efficient ventricular contraction and impairs stroke volume and cardiac output. Often there is also dyssynchrony of contraction of the papillary muscles that control mitral valve function, resulting in mitral valve regurgitation which further impairs cardiac output (see page 29).
To improve overall cardiac function and resynchronize the heart, the right and left ventricles need to be paced simultaneously. Right ventricular (RV) pacing is a well-established, safe and effective technology. Placing a pacing lead within the left ventricular (LV) cavity is also feasible, but it is fraught with potential problems, particularly thromboembolism to the systemic circulation. Instead, a pacing lead is placed into the coronary sinus, which runs under the heart between the left atrium and ventricle, and then into the peripheral veins under the left ventricle so that the myocardium can be paced reliably. Accessing these veins has been a technical challenge but with better delivery tools the success rate of placing a lead in a satisfactory position is now high.
The technology. In patients with sinus rhythm, three pacing leads are inserted: one to the right atrium, one to the right ventricle and one within the coronary sinus to access a posterolateral vein underneath the left ventricle (Figure 8.2). (In patients with permanent atrial fibrillation there is no requirement for an atrial lead.) The coronary sinus ostium is accessed using a specialized sheath system. Once the sheath is placed within the body of the sinus a balloon occlusive catheter is inserted. The balloon is expanded to occlude the vein and contrast is injected through the catheter and a venogram taken (Figure 8.3). This allows the anatomic details of the side veins of the coronary sinus to be documented (see Figure 8.3). A pacing lead and guide wire are then inserted into the sheath and the lead is advanced over the wire into its final position (Figure 8.4).
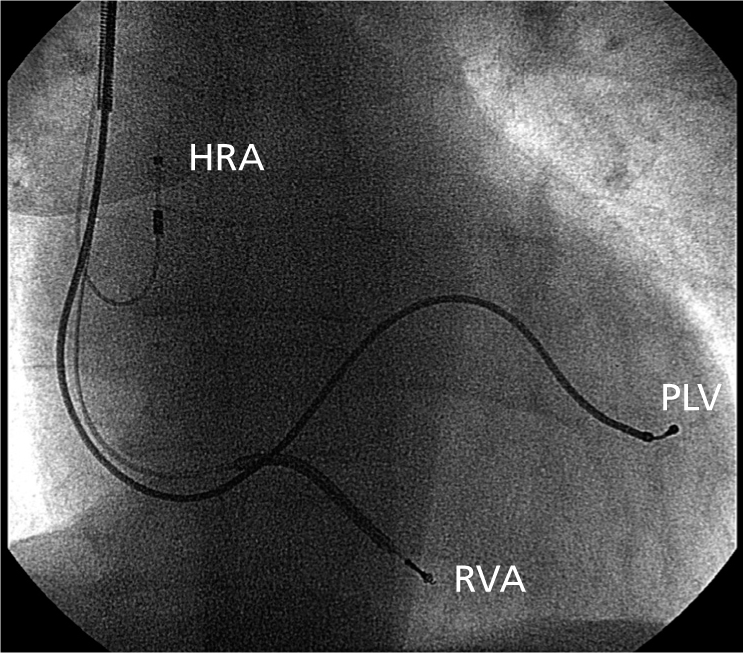
Figure 8.2 Cardiac resynchronization therapy. Anterior–posterior chest X-ray showing final lead positions: a pacing lead to the high right atrium (HRA), a defibrillating lead at the right ventricular apex (RVA) and a pacing lead within the posterolateral coronary sinus vein (PLV).

Figure 8.3 Venogram showing the positioning of a balloon occlusive catheter in the coronary sinus and the location of the posterolateral coronary side vein.

Figure 8.4 Chest X-ray showing the position of the pacing lead within a sheath, which slides over the guide wire. Once the lead is in position and stable the sheath is removed by cutting it away from the lead.
The leads are connected to a pacing generator. During sinus rhythm, the device senses the underlying p wave and via a short atrioventricular (AV) delay (this must be shorter than intrinsic AV nodal conduction) ‘force’ paces the ventricles. The ventricles are continuously driven or paced by the device and intrinsic conduction is suppressed.
Types of device. The basic requirement for CRT is to be able to pace both ventricles simultaneously. It is recommended that the device should pace the ventricles at least 98% of the time to exert its full benefit. A device may be either a pacemaker (CRT-P) or a pacemaker and defibrillator (CRT-D), but there is some controversy about which device is best. Patients with less symptomatic HF (NYHA class II or III) are at relatively greater risk of sudden death, whereas patients with ambulatory class IV are more likely to suffer pump failure. There is therefore an argument to implant a CRT-D in patients with fewer symptoms and a CRT-P in those with advanced HF.
The landmark CARE-HF study showed that most of the survival benefit of the device was related to CRT-P, while the COMPANION study showed no significant difference between CRT-P and CRT-D in terms of reduction in sudden deaths. Although there are no convincing data of a significant benefit of CRT-D, in the real world a CRT-D device is usually implanted because all patients will have impaired LV function.
Cost implications. CRT is considerably more expensive than standard pacing, often three to four times the cost; there is also a significant cost difference between the CRT-P (AUS$15 000) and CRT-D (AUS$35 000) devices (see above). As the incidence of HF increases, there will be a need for more implants, substantially increasing the cost to society.
Does CRT reduce mortality? The CARE-HF trial studied 813 patients with symptomatic NYHA class II or III HF despite optimal medical treatment; 50% were randomized to continue optimal medical treatment with angiotensin-converting enzyme (ACE) inhibitors/beta-blockers and diuretics, and 50% additionally underwent implantation of a CRT pacemaker. Mortality was reduced by 32% in the CRT group (Figure 8.5). The readmission rate with decompensated HF was also significantly reduced, as were sudden deaths and overall mortality. As a result, CRT is now standard therapy for patients with HF, broad QRS complexes and symptoms despite optimal medical treatment (Table 8.2).

Figure 8.5 The results of the CARE-HF study show a significant mortality reduction (p < 0.002) in patients with HF implanted with a CRT pacemaker compared with those who received optimal pharmacological treatment only (see text). Adapted from Cleland JGF et al. N Engl J Med 2005;352:1539–49.
|
TABLE 8.2
Inclusion criteria for cardiac resynchronization therapy |
|
• Impaired LV function: LVEF < 35%
• NYHA class II, III and ambulatory class IV symptoms of HF despite optimal medical treatment with ACE inhibitors or ARBs/beta-blockers/diuretics and spironolactone
• Wide QRS complexes > 120 ms, preferably with a LBBB morphology (> 150 ms with non-LBBB QRS morphology)
• Reasonable expectation of life > 1 year
• Sinus rhythm or atrial fibrillation*
• CRT-D (defibrillation) or CRT-P (pacemaker) depends on clinical judgment |
|
*CRT for patients with atrial fibrillation, provided pacing occurs for more than 92% of the time. If not, consider atrioventricular nodal ablation. ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; HF heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association. |
Does CRT improve morbidity? Many studies have consistently shown an improvement in NYHA symptoms, usually decreasing by at least one class, sometimes two or even three. These changes are maintained for at least 2 years and there is evidence that the improvements occur over the duration of the device.
Exercise capacity is significantly increased, as is quality of life. There is also a significant reduction in the readmission rate for decompensated HF. There is also some evidence that CRT slows down the progression of HF.
Does everyone benefit? Virtually all studies have shown that 20–30% of patients fail to demonstrate a positive outcome (‘non-responders’). Failure is defined as little change in symptoms or outcome or in a change in LVEF, but in many studies the definition of non-response is controversial. As these devices are expensive, identifying patients who may not benefit is critically important. There are a number of factors which make the device less likely to be beneficial but none of these is currently felt to be absolute. Cardiac MRI can show a full-thickness posterior MI, and has been used to decide if placing a lead in the posterior coronary sinus veins is worthwhile; often it is not. Echocardiography has been used to look at a number of variables of dyssynchrony but there has been a lack of reproducibility.
Possible contraindications are shown in Table 8.3. Cost-effectiveness for ICDs is significantly reduced in patients over 75–80 years old (Figure 8.6), and clinical judgment should be used in very elderly patients. The results of the Reverse and MADIT-CRT trials suggest that early implantation of the device slows down the progression of HF and allows positive LV remodeling. Increasingly, CRT is used earlier in treatment.
|
TABLE 8.3
Possible contraindications for CRT device implantation |
|
• Poor ventricular rate control in atrial fibrillation (AV nodal ablation is recommended to ensure pacing is constant)
• Right bundle branch block
• Full-thickness posterior MI (can affect lead positioning)
• Narrow QRS complexes (< 120 ms)
• Mild symptoms |
|
AV, atrioventricular; CRT, cardiac resynchronization therapy; MI, myocardial infarction. |
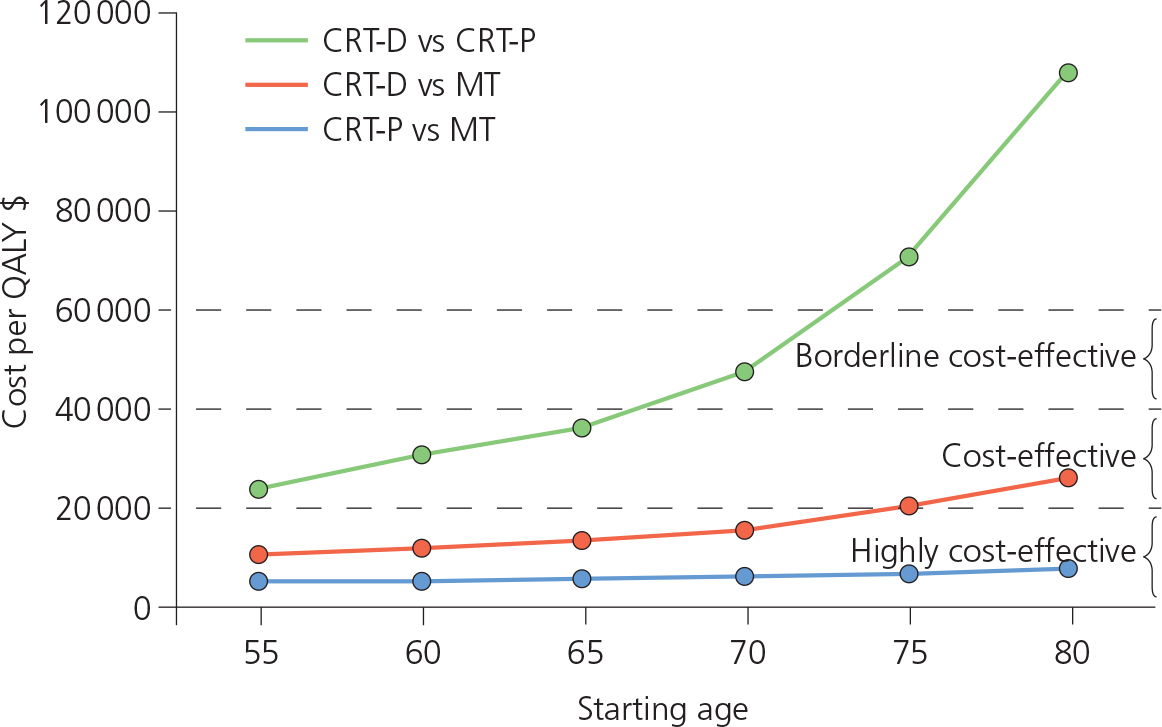
Figure 8.6 Comparison of the cost-effectiveness of a CRT pacemaker (CRT-P) versus a CRT combined pacemaker and defibrillator (CRT-D), and each device compared with medical treatment. Both devices (CRT-P and CRT-D) are cost-effective compared with medical treatment only in patients under 75 years old. CRT, cardiac resynchronization therapy; MT, medical treatment; QALY, quality-adjusted life-year.
Patients with systolic HF and a QRS greater than 120 ms with optimal medical treatment should be referred for assessment. Patients with left bundle branch block (LBBB) and QRS duration greater than 150 ms, as well as those with non-ischemic cardiomyopathy, respond best to CRT. Although non-responders are a significant issue, some patients respond very significantly (‘super-responders’). Identifying these patients has also been a challenge. Often the only way to know for sure whether the patient will respond is to implant the device.
Do these systems need regular maintenance? All devices need to be interrogated on a regular basis throughout their life, usually every 6 months to test lead integrity and battery life. Not all patients respond positively to CRT; across the major trials a consistent 20–40% of patients are deemed non-responders. Maximizing the hemodynamic benefit of CRT for patients in sinus rhythm has been shown to improve outcome. This involves better synchronization of atrial contraction to maximize ventricular filling (AV optimization), and determining the best timing between left and right ventricular contraction (VV optimization).
AV and VV optimization. Until recently, echocardiography has been the ‘gold standard’ approach; however, because it is time consuming and requires a specific skill set it is not routinely performed in all centers. In addition, serious doubt has been cast on its reproducibility.
Sensors that can automatically determine the optimal setting for CRT devices, and electrical algorithms based upon changes in intracardiac electrograms, have been developed. Recent studies have shown a positive outcome comparable to echocardiographic assessment. The SonR device uses a contractility sensor within the atrial lead of a CRT device to automatically reprogram the AV and VV intervals. Results from the Respond trial showed equivalence with echocardiography but also suggested a significant reduction in HF-related hospital readmission rates using weekly measurements from the sensor. Another device (CardioMEMS™) is a small pressure sensor implanted into the pulmonary arteries via a catheter-based percutaneous approach, which is capable of continuously measuring pulmonary artery pressures (Figure 8.7). A recent study found up to a 46% reduction in HF-related hospitalizations within 6 months of implant and a 34% reduction at 12 months. Future developments will allow the device to transmit data directly to an implanted CRT device.

Figure 8.7 The CardioMEMS™ HF system, seen here in relation to a dime, is a miniaturized wireless monitoring sensor, deployed within a peripheral pulmonary artery via a percutaneous catheter approach, that transmits changes in pulmonary artery pressures. Image provided courtesy of St. Jude Medical, Inc.
Advances in LV lead technology such as multipolar pacing, which allows programming of the different pacing vectors in the left ventricle, have also improved outcome and responder rates. Mapping of the lead position at implant to find the greatest electrical delay between the right and left ventricle also improves the results of CRT.
Is implantation safe? Current systems are safe to implant, with overall mortality less than 0.5%. Specific risks relating to CRT include:
• failure to implant the left ventricular leads (< 5%)
• late lead displacement (8%)
• phrenic nerve stimulation (< 10%).
Other risks are similar to those of any standard pacemaker implant:
• pneumothorax (1%)
• infection (early < 1%; late 1.5%)
• lead dislodgment (1–5%).
Cardiac contractility modulation
Cardiac contractility modulation via a pacemaker-like device is a relatively new development for the treatment of HF. A high-voltage biphasic signal is delivered through one of three intracardiac pacing leads to the RV septum (a newer device has only two leads). The signal is timed to coincide with the absolute refractory period of the ventricle. Although it does not result in cardiac contraction, it enhances myocardial function by improving myocyte calcium handling and mitochondrial function. It also has a positive effect on cardiac protein modification, including enhancing myocardial gene expression.
In general, clinical studies have indicated a sustained improvement in quality of life, exercise capacity and LVEF. There are few randomized studies, but recent evidence suggests that the patients who benefit the most are those with an ejection fraction of 25–40% and a narrow QRS duration, who remain symptomatic on optimal medical treatment and whose LV function does not require a prophylactic defibrillator. These patients are not eligible for CRT and therefore represent an additional group who do not respond to medical therapy alone.
The device needs to deliver a signal for 5–12 hours per day; the battery can be recharged externally through a specialized power supply. Over 2500 devices have been implanted in Europe but as yet the device has not achieved mainstream application worldwide, although the accumulating clinical data are promising.
Cardiac surgery
The aim of specific surgery for HF over and above valve replacement and coronary bypass is to reduce the LV size and volume, and remove ‘redundant’ or scar tissue (surgical ventricular restoration, variants are known eponymously as the Batista and Dor procedures). The STICH trial compared routine bypass surgery in patients with impaired LV function with an additional procedure to reduce LV volume in patients with large anterior wall scars. Outcomes were similar in both groups; the reduction in LV volumes did not confer an outcome advantage. Thus, reductive LV surgery should only be considered in patients with severe LV dilation and HF, but needs to be individualized and balanced against the risks.
Mitral valve surgery is covered in Chapter 3.
Left ventricle assist device. Although contemporary medical therapy with vasodilators, beta-blockers and aldosterone antagonists (see Chapter 7) has a significant effect on the mortality and morbidity of advanced HF, it is not sufficient in patients with severe symptoms. Cardiac transplantation in this setting is very effective, with 10-year survival rates approaching 50%, but in view of the shortage of organs it remains trivial. Mechanical pumps that take over the function of the damaged ventricle have therefore been developed to stabilize the patient’s condition when hemodynamic compromise ensues, either as a result of acute illness (MI, myocarditis, postpartum) or in the process of ongoing LV remodeling (in chronic HF). These devices unload the damaged ventricle (mainly LV) either by redirecting the blood from the ventricle via inflow and outflow cannulas to the ascending aorta or by providing continuous support from inside the ventricular cavity or aorta. Available LV assist devices include:
• extracorporeal (pulsatile and non-pulsatile)
• implantable (pulsatile and non-pulsatile) (Figure 8.8)
• total artificial heart.

Figure 8.8 The Heartmate implantable left ventricle assist device (Thoratec Corporation, Pleasanton, CA, USA).
The pumps are either hydraulic or electromagnetic, and based on either an axial or centrifugal design. Most of the available systems operate at either fixed rates or in an automatic mode; the latter ejects when the pump is 90% full or when it senses decreased filling (more physiological). The aortic valve rarely opens when the heart is being supported, so pump output is synonymous with cardiac output. The devices can deliver cardiac output of up to 10 L/minute. The pump rhythm is completely dissociated from ventricular rhythm. The newest LV assist devices use a textured biological surface that does not require long-term anticoagulation.
The effect of LV assist devices on LV function has been extensively studied. At the cellular level the mechanical unloading attenuates myocardial histological abnormalities, improves the efficiency of mitochondria and decreases the extent of myocyte apoptosis.
Dramatic decreases in plasma renin, angiotensin II, epinephrine, norepinephrine, vasopressin and atrial natriuretic peptide have been observed, with inflammatory markers such as tumor necrosis factor (TNF), interleukin (IL)-6 and IL-8 beneficially affected by chronic support. At the organ level, there is reversal of LV remodeling with reduction in LV size, improvement in LVEF and regression of myocyte hypertrophy, and better hemodynamics, exercise tolerance and functional state.
Complications are frequent and potentially life-threatening. They differ according to the time of implantation (Table 8.4). Pre-existing mitral stenosis or aortic regurgitation may require correction before implantation. Patients with inoperable coronary artery disease (CAD) sometimes continue to have angina.
|
TABLE 8.4
Complications associated with left ventricle assist devices |
In early postoperative period
• Bleeding (30%)
• Right-sided heart failure (10%)
• Air embolism |
In late postoperative period
• Infection (25%)
• Thromboembolism
• Failure of the device |
The REMATCH trial compared the long-term effect of LV assist devices with medical therapy. The results supported the use of the devices for patients with advanced HF, but at the cost of device-related complications, including:
• 35% failure rate at 2 years (none after 1 year)
• 28% device infection rate after 3 months
• 42% bleeding rate after 6 months.
Median number of days spent both in and out of the hospital were higher in the device group.
Patient selection. There are three groups of patients who should be considered for LV assist devices.
• Patients who are not expected to recover adequate cardiac function and who require mechanical support as a bridge to transplantation.
• Patients who require ventricular assistance to allow the heart to rest and recover its function before explantation (bridge to recovery). (There have been a number of case reports where myocarditis-induced HF has recovered after a period of mechanical support.)
• Patients who require permanent LV support and are not eligible for cardiac transplantation (destination therapy).
Destination therapy enables long-term myocardial replacement therapy and in some cases, the patient’s hemodynamic profile may improve to such an extent that heart replacement therapy could be reconsidered.
Extracorporeal membrane oxygenation (ECMO) has been a major step forward in the management of acute severe pulmonary and cardiac failure. ECMO is a modified heart–lung machine that can support life for days or weeks, thereby allowing adequate time for treatment (including a bridge to implantation of an LV assist device, a total artificial heart [TAH] or cardiac transplantation) or myocardial recovery. There is strong evidence of the positive impact of ECMO on outcomes in patients with refractory cardiogenic shock after surgery and following acute MI.
The total artificial heart is a mechanical device that entirely replaces the heart’s function. It is currently used as a bridge to transplantation, but work continues to make it ready for potential destination therapy. A synthetic fully functional mechanical heart is one of the holy grails of medicine. While transplant recipients outnumber organ donors there will be a drive to develop this technology. The first mechanical heart was used as a bridge to transplant in 1969. A number of versions of the device have been made since. Studies show that up to 80% of patients survive to transplant. Newer prototypes using electronic sensors and ‘biomaterials’ such as chemically treated animal tissues are in the advanced stages of development, and it is projected that in the long term totally implantable hearts will be available within the next 5–10 years.
Currently in use, the SynCardia TAH is a biventricular, pneumatic pulsatile blood pump that completely replaces the patient’s native ventricles. It is lined with polyurethane and has a pneumatically driven diaphragm (Figure 8.9). The device is used as a bridge to transplantation in patients for whom LV assist and CRT devices are contraindicated, including those with aortic regurgitation, cardiac arrhythmias, an LV thrombus, an aortic prosthesis, an acquired ventricular septal defect, or irreversible biventricular failure requiring high pump outputs. The device is powered by a large console that limits the patient’s movement. Portable drivers that will enable patients to return home are in the testing phase. Over 1500 such devices have been implanted worldwide, although there are still significant technical and patient-related challenges; a fully functioning chronically implanted device is still some years away.

Figure 8.9 SynCardia total artificial heart (SynCardia Systems Inc., Tucson, AZ, USA), a pulsatile blood pump that completely replaces the patient’s right and left ventricles.
Complications. As with all mechanical devices the two major complications are thromboembolism and infection. Infection is common (up to 20% of cases are device related) but treatable and does not necessarily mandate device removal. Pulmonary infection is also common (up to 20%), as are neurological events (15%). Despite these complications, overall success has been increasing and these devices are becoming increasingly acceptable.
Cardiac transplantation outcomes have been steadily improving over the last 30 years. Currently, over 85% of patients will survive 12 months and 50% of transplant recipients are still alive at 10 years. Although highly successful, the major limitation is the availability of donor hearts. In the USA there are approximately 2500 transplants per year, 3500 worldwide, against an estimated 800 000 eligible people with end-stage HF. This mismatch in demand has spurred the development of other approaches to support a failing heart (see above). A new technique acquiring donor hearts shortly after death has been pioneered in Australia and, if viable, may help to increase donor availability.
Patient selection. Patients with severe HF who may benefit from transplantation (Table 8.5) should be referred to a heart transplant center for assessment of the severity of the disease process, to ensure that all other therapeutic options have been exhausted and to evaluate potential hazards or contraindications to the treatment. Patients who are considered for transplantation have to undergo a battery of tests.
|
TABLE 8.5
Indications for heart transplantation |
|
• NYHA class III or IV heart failure refractory to optimal medical and surgical therapy as evidenced by:
– peak oxygen consumption (VO2max) < 14 mL/kg/min during cardiopulmonary exercise testing
• Refractory angina despite maximum therapy
• Life-threatening arrhythmias despite maximum therapy |
Age is one of the most controversial exclusion criteria for transplantation (Table 8.6). The upper age limit for recipients is determined by the patient’s physiological rather than chronological age, as older patients have a greater probability of occult systemic disease that may complicate their postoperative course and limit their survival.
|
TABLE 8.6
Exclusion criteria for heart transplantation |
|
• Age > 70 years
• TPG > 15 mmHg or PVR > 6 Woods units if not reversible on vasodilator challenge
• Morbid obesity BMI ≥ 35, or cachexia BMI < 20
• Current alcohol, smoking or drug abuse
• Active malignancy
• Active systemic infection or peptic ulcer disease
• Uncontrolled hyperlipidemia
• Personality/behavioral disorder likely to affect treatment adherence
• Severe peripheral and cerebrovascular disease
• Severe osteoporosis
• Other medical condition likely to cause death within 5 years
• Diabetes mellitus with end-organ damage.
• Irreversible secondary organ disease (unless combined treatment is considered) |
|
BMI, body mass index (kg/m2); PVR, pulmonary vascular resistance; TPG, transpulmonary gradient. |
Eligible patients are placed on the waiting lists. Those who are hemodynamically unstable are referred for mechanical support. Improved preoperative care has meant that the death rate for patients awaiting cardiac transplantation has declined considerably.
The criteria for matching potential recipients with the appropriate donor are based primarily on ABO blood group compatibility and patient size (donor weight should be within 30% of the recipient). The panel of reactive antibodies (PRA) representing the major histocompatibility antigens is used to screen the recipient for antibodies that may mediate hyperacute rejection. If the PRA is greater than 15%, suggesting recipient presensitization, a prospective negative cross match between the recipient and donor sera is mandatory before transplantation.
After surgery patients are placed on an intensive immunosuppressive regimen, including a calcineurin inhibitor (ciclosporin or tacrolimus), antimetabolite (mycophenolate mofetil or azathioprine) and corticosteroid. Prophylactic therapy against pneumocystis and cytomegalovirus (CMV)/toxoplasmosis infections is also given if the patient has been exposed previously. Transplantation is a major psychological trauma, and family and social support are key to a successful outcome. A life-long commitment to therapy and follow-up is required.
The commonest causes of morbidity and mortality after transplantation are listed in Table 8.7. Significant morbidity is also attributed to other conditions resulting from the use of immunosuppressive therapy (mainly calcineurin inhibitors and steroids). These include arterial hypertension (∼90% of recipients), diabetes mellitus (∼30%), renal dysfunction (∼30%) and hyperlipidemia (∼85%).
|
TABLE 8.7
Commonest causes of morbidity and mortality after transplantation |
Within 30 days of transplant
• Rejection
• Primary graft failure
• Infection (mainly bacterial)
Within 1 year of transplant
• Infection
• Rejection
• Graft failure |
After 1 year
• Coronary artery vasculopathy (in 50% at 5 years)
• Infection (mainly cytomegalovirus and fungal)
• Malignancy (mainly skin and lymphomas) |
Key points – non-pharmacological management
• Patients with poor left ventricular systolic function (ejection fraction < 35%), who are symptomatic on optimal medical treatment, with broad QRS complexes (> 120 ms) and left bundle branch block should be referred for cardiac resynchronization therapy.
• With evolving technology, the complication rate of left ventricle assist devices and total artificial hearts will decline and their use will significantly increase as a bridge to transplantation and as destination therapy.
• Assuming that the candidate pool (patients with end-stage heart failure [HF]) continues to grow, the burden on healthcare systems worldwide could become significant.
• Cardiac transplantation remains a viable option in selected individuals with end-stage HF despite the shortage of donor organs; steady improvement in survival after cardiac transplantation has been observed.
References
Abi-Samra F, Gutterman D. Cardiac contractility modulation: a novel approach for the treatment of heart failure. Heart Fail Rev 2016;21: 645–60.
Abraham WT, Adamson PB, Bourge R et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66.
Barghash M, Reyentovich A. The use of implantable HF monitoring systems and the CHAMPION trial. J Am Coll Cardiol 12 Jan 2016 (online). www.acc.org/latest-in-cardiology/articles/2016/01/11/12/44/the-use-of-implantable-hf-monitoring-systems-and-the-champion-trial, last accessed 12 May 2017.
Dhital KK, Iyer A, Connellan M et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015;385:2585–91.
European Society of Cardiology. Guidelines on Cardiac Resynchronisation therapy and Pacing: www.escardio.org/guidelines
Jones RH, Velazquez EJ, Michler RE et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705–17.
Kaye G, Lemery R. Fast Facts: Cardiac Arrhythmias, 3rd edn. Oxford: Health Press Limited, 2017.
Lars Køber L, Thune JT, Nielsen JT et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30.
Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy provides an effective overview of the effect of CRT since the first trials. J Am Coll Cardiol 2014;64:1047–58.
Liu X, Yang HJ, Ping HQ et al. The safety and efficacy of cardiac contractility modulation in heart failure: a meta-analysis of clinical trials. Herz 2017;Jan 18 [Epub ahead of print].
Moss AJ, Hall WJ, Cannom DS et al. Cardiac resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38.
Nicholls M. Respond-CRT trial. Eur Heart J 2016;37:3128–9. [Editorial]
Rose EA, Gelijns AC, Moskowitz AJ et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–43.
Solomon SD, Foster E, Bourgoun M et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 2010;122:985–92.








