3. Beaver biology and ecology
3.1 Taxonomy and distribution
The Eurasian beaver and the North American beaver are the only surviving members of the once larger family of Castoridae. Both modern beaver species are physically very similar, making them hard to distinguish in the field. They have very similar ecological requirements and behavioural patterns, and were once considered to be a single species. Chromosome analysis has identified that the Eurasian beaver possesses 48 pairs of chromosomes, while the North American beaver has 40 (Lavrov and Orlov 1973). As a result, the two species will not interbreed and produce viable offspring, even when attempted through captive breeding. Through differences in tail shape and subtle differences in their pelage, beaver species can be determined on closer inspection. Examination of the anal gland secretions provides reliable differences in colour and viscosity which can be used to determine beaver species and sex (Rosell and Sun 1999). It is now believed that divergence occurred about 7.5 million years ago when beavers first colonised North America from Eurasia across the land bridge of the Bering Strait (Horn et al. 2011). From the Eurasian fossil record of the Early and Middle Pleistocene (~2.4–0.13 Ma ago), in mature rivers and wetlands, modern beavers appear to have lived alongside, or possibly to have been locally excluded by, the slightly larger extinct beaver Trogontherium cuvieri; the prevalence of the two forms at archaeological sites demonstrates an inverse relationship (Mayhew 1978).
By the 16th century, Eurasian beavers were largely extinct across most of Europe and Asia. At its lowest population point, it is believed that the Eurasian beaver in its western range was reduced to 200 animals on the River Elbe in Germany, 30 on the River Rhone in France and ~100 in Telemark, Norway (Nolet and Rosell 1998). Since the 1900s, beaver numbers have recovered throughout much of their former European range as a result of a combination of legal protection, hunting regulation, proactive reintroductions/translocations and natural recolonisations. Breeding farms that produced beavers for commercial fur farming, and later for release into the wild for restocking, were established at Voronezh (Russia) and Popielno (Poland) in 1933 and 1958, respectively (Jaczewski et al. 1995). The first official conservation translocation occurred from Norway to Sweden in 1922, and since then there have been more than 200 recorded translocations of beavers (Halley et al. 2012). Eurasian beavers have now been restored to over 24 countries in Europe (Halley and Rosell 2002) and are currently estimated to number over 1 million individuals globally (Halley et al. 2012).

Figure 3.1 Current distribution of Eurasian beaver (red) and North American beaver (green). Black represents refugia where Eurasian beavers were never extinct (numbered west to east); ‘F’ indicates countries where reintroduction feasibility studies have been conducted. (Updated from Halley, Rosell and Saveljev 2012)
Also notable is a sizeable population (~8,000) of introduced North American beavers in Finland (Kauhala and Turkia 2013) and northwest Russia. In 1937, seven North American beavers from New York State, USA, were introduced to Finland to supplement an ongoing reintroduction of the nearly extinct Eurasian beaver there (Lahte and Helminen 1974). At that time, most zoologists recognised only one species of beaver worldwide (Parker et al. 2012). North American beavers are now also present in small numbers in parts of Belgium, western Germany and Luxembourg, as a result of escapes from a zoo and game parks (Dewas et al. 2012). Due to the significant biological and ecological similarities of these two species, identification and removal of the non-native North American beaver is important, requiring active management and resource investment (Parker et al. 2012).
3.2 Anatomy and appearance
Eurasian beavers are large (adults >20 kg), semi-aquatic, herbivorous rodents with a head and body length of ~80–100 cm and a tail length of ~30 cm on average in adults (Żurowski and Kasperczyk 1988), making them the second largest rodent species in the world. Beavers do not attain adult size until around three years of age. Adults of both sexes have similar head and body lengths, although females are on average 1–1.5 kg heavier than males, and are impossible to distinguish visually unless a female is heavily pregnant or lactating, during which time nipples will be visible (four under the forelegs).

Figure 3.2 Adult Eurasian beaver. (I. Sargent)
Coat colour is usually brown but can range from pale golden to black. The beaver’s flattened tail is probably its most distinctive feature, covered in visible scales and dark grey in colour. When moving on land, beavers normally walk on all fours, although they can walk upright on their hind legs for very short distances when carrying mud or vegetation, particularly when building or restoring lodges for winter. During feeding sessions, they can be observed resting on their back legs while holding food in their forepaws. Beavers have small ears but have good hearing. Their eyesight is poor and largely functions to identify and respond to movements. They have an excellent sense of smell, and their chemical communication abilities are highly developed (Campbell-Palmer and Rosell 2010). While swimming, they utilise their powerful, webbed hind feet in a coordinated kicking motion for propulsion through the water and their tail as a steering rudder (Wilsson 1971; Novak 1987).
3.3 Breeding and young
Beavers live in family groups, usually comprising an adult breeding pair with their offspring from the current and the previous year’s litters. Once paired, beavers tend to remain together until one partner either dies or is displaced by another individual of the same sex in territorial disputes. Mating usually takes place late December to February (depending on location). After a gestation of 105–107 days, the female gives birth to one litter per year, typically of one to four kits, in spring/early summer (with some geographical variation). The number of kits produced, and their survival, is affected by various factors such as the age of parents, the surrounding population density and habitat quality and altitude (Novak 1987; Campbell 2010; Campbell et al. 2013). Beaver kits are born fully furred, and usually weigh between 300 and 700 g. Their eyes are open soon after birth; and, although they will feed on their mother’s milk for 2–3 months, they can consume vegetation after their first week. The kits remain in the lodge for approximately the first 1–2 months of their lives while their parents and older siblings bring leafy twigs and other vegetation for them to eat. By the time they are approximately 2 years old, they have become sexually mature and usually disperse in order to attempt to establish territories and partners of their own (Wilsson 1971).
Although some beavers remain with their family units as non-breeding individuals for many years, particularly if there is a lack of suitable habitat to move to (Campbell et al. 2005), most 2 year olds will begin to search for territories of their own in the spring. During this period, they are capable of travelling long distances along water bodies and may undertake shorter trips overland. Beavers do not like to be far from the water or to travel over open land for large distances, though reports of individuals up to 11.7 km away from water exist these are rare (Saveljev et al. 2002); the barrier effect of watershed divides on beaver colonisation varies depending on topography (Halley et al. 2012). There is no consistent difference in dispersal between the sexes (Saveljev et al. 2002; McNew and Woolf 2005), and individuals will commonly make exploratory excursions into neighbouring areas (Campbell et al. 2005). After dispersal, they have been recorded taking up residence in some unconventional aquatic habitats, such as small ornamental ponds or sewage-treatment plants. The dispersal process can be hazardous for beavers, with deaths via road traffic accidents or through infections from wounds caused by other beavers. Beavers from one family are highly intolerant of those from other families and will fight aggressively with intruders. During these fights, deep, penetrating wounds are frequently inflicted by biting to the shoulders, flank and tail (Figure 3.4). Such wounds can result in severe injuries and/or become septic, and can result in death (Piechocki 1977).

Figure 3.3 Mother and ~3-month-old kit. (S. Gardner)

Figure 3.4 Crescent-shaped scarring from beaver bites inflicted during territorial disputes on the underside of a tanned beaver pelt. (R. Needham)
3.4 Habitat and territoriality
Eurasian beavers occupy freshwater bodies (ponds, streams, rivers, marshes and lakes) throughout much of Europe. Populations are still scattered in western and southern regions of this range. In Asia, there are relict populations in China (Xinjiang) and in Mongolia. The species is expanding rapidly in both distribution and numbers (Halley et al. 2012). Although they are highly adaptable and can modify many types of natural, cultivated and artificial habitats, they prefer still or slow-moving water with stable depths of at least 60 cm (Gurnell et al. 2009). Where these habitats are unavailable or are already colonised by other beavers, they will colonise narrower watercourses and construct dams to create suitable habitat.
Although the average size of a beaver territory is approximately 3 km of shore length, size can vary greatly depending on habitat quality, particularly winter food resources, from 0.5 to 20 km of shore length (Macdonald et al. 1995; Herr and Rosell 2004; Campbell et al. 2005). During the Scottish Beaver Trial the total length of waters’ edge used by each beaver pair or family ranged from 1.8 to 4.7 km (Harrington et al. 2015). The extent and character of a territory is also affected by the surrounding density of the beaver population.

Figure 3.5 Newly created wetland through beaver dam-building activity at the Devon Wildlife Trial site. (D. Plummer)
It is a common misconception that suitable beaver habitat solely comprises large tracts of wet woodland. In developed landscapes, they will readily exploit any palatable vegetation in close proximity to water bodies such as mown amenity bankings, grass verges, grazing pastures or agricultural crops. In climates that experience prolonged winters below freezing, access to woody browse in order to collect a cached food reserve is, however, an essential, limiting factor in survival and population growth.
3.5 Diet and feeding
Beavers are entirely herbivorous and will readily consume a wide range of bark, shoots and leaves of woody plants (majority broadleaf species), as well as herbaceous and aquatic vegetation. During spring and summer, up to 90% of their diet is composed of terrestrial, semi-emergent and aquatic species of plants (Nolet et al. 1995), though it can be under 30% in Norway (Campbell et al. 2013). Although beavers can fell quite large trees (>1 m in diameter), they tend to favour smaller saplings (<5 cm diameter) in order to obtain their bark, side branches and leafy stems (Haarberg and Rosell 2006). Favoured woody plant species include aspen and poplar (Populus spp.), willow (Salix spp.) and rowan (Sorbus aucuparia), while alder (Alnus glutinosa) is generally avoided (Fustec et al. 2001; Haarberg and Rosell 2003; Iason et al. 2014). Foraging generally takes place close to the bank; in Denmark, for example, 95% of beaver-cut stems were within 5 m of water (Elmeros et al. 2003); in Norway, 70% of cut stems were within 10 m and 90% within 20 m (Haarberg and Rosell 2006); in Russia, 90% of cut stems were within 13 m of water and 99% within 20 m (Baskin and Sjöberg 2003). Similarly, findings from the Scottish Beaver Trial determined that most foraging activity on trees occurred within 10 m from the waters’ edge (Iason et al. 2014).
Beavers will forage a few hundred metres away from water to obtain preferred forage species such as aspen or poplar where these are not available in the vicinity of the water’s edge. In flatter landscapes, beavers will create or extend their dams to flood the surrounding land in order to access desirable foraging sites. In landscapes with expanding beaver populations, competition for territories means that beavers will utilise faster-flowing watercourses on steeper gradients, though not normally above a 2.5% incline (Howard and Larson 1985; Webb et al. 1997; Schulte 1998). Some of the territories they establish in these low-quality environments may only be tenable for a single season when the woody resource they depend upon becomes depleted and the small dams built on fast-flowing streams are swept away by the spring floods.

Figure 3.6 Beaver feeding on browse at the water’s edge. (I. Sargent)
3.6 Behaviours
Beavers spend large amounts of time in fresh water foraging and maintaining their territories, which they mark with castoreum and anal gland secretion (Rosell et al. 1998). They defend these established territories as a family against other beavers (Wilsson 1971; Rosell and Thomsen 2006). They do not hibernate, but reduce their activities and establish underwater caches of woody vegetation in front of occupied lodges during autumn to tide them through the winter months (Wilsson 1971; Hartman and Axelsson 2004). Food caches have been observed in Britain, within the Scottish Beaver Trial and Tayside beaver populations (Campbell et al. 2012; Harrington et al. 2014). The material in caches which remains unused can, on occasion, rapidly regenerate by sending out new side shoots, particularly where it contains willow. Beavers are strong and able diggers and can excavate burrows, chambers and canals (Wilsson 1971; Richard 1967). Earth, sticks and branches, stones and vegetation are used for construction purposes. Although beavers often live in purpose-built lodges constructed from compacted mud and cut branches, they may also utilise a number of burrows in the same territory (Campbell et al. 2012a). Where the banks of a watercourse allow the construction of large burrows, lodge-building is uncommon. Burrows can be re-roofed with sticks and mud where sections collapse.

Figure 3.7 Beaver lodge with extended food cache (branches in water to left of main structure), Bavaria. (R. Campbell-Palmer)
Next to tree-felling, beavers are perhaps best known for their dam-building behaviour. Beavers normally only build dams when necessary to retain and manage water levels. For example, no dams exist on any of the main river channels in an area in Telemark, Norway, in which several of the authors have spent time investigating the behaviour and ecology of beavers. The creation of dams by beavers depends on habitat characteristics. On lakes or wide rivers (>10 m), damming is largely unknown. Beavers living on narrower water bodies (<6 m wide and in 97% of cases in water originally <0.7 m deep: Hartman and Törnlöv 2006) often build dams, and can create extensive systems of multiple impoundments. It appears that a depth of ~70 cm behind the dam is the ‘target’, though higher dams have been observed, especially on streams with high banks. A 2012 survey across the Tayside catchment found 7 dams with an average height of 0.75cm, with 32 dams (majority short-lived) then recorded between September 2013 and November 2014 (TBSG 2015). The incidence of damming varies according to the characteristics of a watershed. On one river system in Norway, ~10% of the beaver territories had actively maintained dams on river tributaries (Parker and Rønning 2007); in a Polish study, 19.5% of beaver territories had active damming (Żurowski and Kasperczyk 1986), while Russian studies have reported figures ranging from 19 to 53% (Danilov and Kan’shiev 1983).
Dams provide deeper water and a safer environment for beavers to move through, and create deeper and constant water levels concealing the entrance of their main lodge. Beavers strongly prefer to move through water as opposed to travelling overland, which may involve more energy expenditure and leaves them vulnerable to predators. Where they do travel overland on a regular basis, they prefer to use short routes. Over time, these features, for example across a narrow banking separating one watercourse from another, are commonly excavated to create canals. The deeper water retained by a dammed beaver pond affords submerged access to food caches should the surface become frozen in winter. The length of time that dams persist in the environment varies and can be relatively short, particularly if the food resources being utilised become depleted or the dams are not worth maintaining (Halley et al. 2009; Rosell et al. 2005).

Figure 3.8 Typical beaver dam on narrow watercourse, Scotland. (D. Gow)
A few modern records, all from North America, exist of the collapse of beaver dams resulting in downstream damage to land or travel infrastructure (Butler and Malanson 2005). The materials released by most breached dams are most commonly trapped elsewhere in the watercourse further downstream. Beaver dams rarely collapse as entire structures, but rather breach at a limited point which then erodes through water action. If beavers are still present at the site, they will maintain the dam and rapidly repair any breaches. Such breaches typically occur in autumn and spring at high water discharge (Halley et al. 2009).
Beavers are renowned for using their tails to generate alarm signals to warn other family members of danger, but additionally let potential predators know they have been spotted. ‘Tail-slapping’, as the name suggests, involves a beaver raising its tail above the water surface, then bringing it down sharply to slap the water’s surface. Often the beaver dives immediately afterwards. Like most social mammals, interactions between individuals serve to reinforce family bonds. They are more common in younger animals and tend to decline with age. Beavers can produce a range of vocalisations; and family members will commonly produce whining calls when they meet, with young animals tending to be much more vocal. Juveniles will communicate with a range of mews, short, soft squeaking calls or repetitive crying noises, to which the adults respond (Wilsson 1971). Territorial display behaviours, involving the repeated lifting of a cut branch or other object, have been reported in the well-studied Norwegian populations (Thomsen et al. 2006) and likely occur elsewhere.

Figure 3.9 Tail-slapping in beavers. (Rachael Campbell-Palmer)
Grooming is an important behaviour which maintains the beavers’ fur in good condition and removes parasites. Beavers have two distinct layers of fur: the soft underfur, which is very dense; and the outer, coarser guard-hair layer. This structure ensures that, when they submerge in water, a layer of air is trapped next to their skin which helps to repel water and offers very effective insulation properties in cold climates. This prevents saturation and allows beavers to dry themselves quickly when they emerge from the water. Beavers can submerge for up to 15 minutes when they feel threatened or attempt to avoid predators; but most dives are to collect aquatic plants, are much shorter in duration (a few minutes), and are repeated frequently when foraging (Novak 1987).
3.7 Parasites and diseases
The range of pathogens that can be harboured by the Eurasian beaver has been reviewed and health-screening recommendations made for importation of beavers to Scotland (Goodman et al. 2012; Campbell-Palmer et al. 2015). Beavers can carry host-specific parasites not currently present in Britain, though these are not known to infect or harm other species. These include the beaver beetle Platypsyllus castoris, a stomach nematode Travassosius rufus, and a specialised trematode or intestinal fluke Stichorchis subtriquetrus. These species have now all been recorded in wild beavers in Scotland (Campbell-Palmer et al. 2012; Goodman et al. 2012; Duff et al. 2013). Non-native, host-specific parasites are not of concern to human, livestock or other wildlife health, so no active management for these species is presumed to be required. Other parasites such as Giardia spp. and Cryptosporidium spp. are already present in British wildlife, livestock and humans, and therefore it is possible beavers may contract them and, like other species, contaminate raw water sources. A risk assessment undertaken by the Centre of Expertise on Animal Disease Outbreaks determined that these other sources of infection (humans, livestock and other wildlife) are likely to pose a more significant risk to water contamination, though they recommend appropriate risk assessment for future beaver releases. In conclusion, the likelihood of beavers being important sources of contamination for these two parasites is considered unlikely (EPIC 2015). There are no published reports of Mycobacterium bovis infections in the Eurasian beaver and therefore it is not viewed as a major risk factor for domestic livestock. However, as with any other mammal, beavers could theoretically become infected with bovine TB if exposed to an animal actively shedding the organism.
Any current beaver population of unknown origin may carry non-native diseases and parasites, in particular Echinococcus multilocularis (also known as the fox tapeworm), rabies and tularaemia. Any health-screening programme involving beavers should include the sampling of any cadavers and/or a live-trapping programme to collect blood and faecal samples as required. E. multilocularis is a zoonotic parasite of serious health concern, and is regarded as one of the most pathogenic parasitic zoonoses in the northern hemisphere (North America, northern and central Eurasia) (Eckert et al. 2000; Vuitton et al. 2003). Although it is established in many countries across Central Europe, other European countries are presently deemed free of this parasite – including the United Kingdom, which employs strict measures to prevent entry, i.e. the Pet Travel Scheme (DEFRA 2012).
Barlow et al. (2011) diagnosed E. multilocularis in a captive beaver at post-mortem. This individual was held in an English captive collection but had been directly wild-caught and imported from Bavaria (in 2007), Germany. Sample screening across the Tay and Earn catchments, and ongoing post-mortem examination of beaver cadavers, has demonstrated no evidence of E. multilocularis in free-living beavers in Scotland. The rabies virus has not been reported in Eurasian beavers but theoretically may affect any mammal. Screening of the live animal is not currently possible, so any imported beavers should be sourced from rabies-free areas or quarantined according to the current Rabies Importation Order (as amended). Like all other rodents, beavers may harbour common European rodent pathogens (Goodman et al. 2012). It is recommended that any imported, wild-caught beavers are screened for the following as a minimum: E. multilocularis, hantavirus, tularaemia, Yersinia spp., leptospirosis, Salmonella spp., Campylobacter spp. and Toxoplasma gondii, and quarantined for rabies.
3.8 Population biology
Beaver survival, population establishment, development and distribution in Britain will directly impact on the management requirements of this species. At low densities, beavers have the ability to blend unobtrusively into an environment, with any conflicts tending to be localised. During this initial phase of colonisation, they select the most favourable sites, typically larger rivers and lakes, where dam-building activity is rare. As beaver populations grow and the population density increases, successive generations occupy less-favoured habitats (i.e. those more likely to be modified by beavers) in minor watercourses or anthropogenic environments. In such locations, their presence can become more obvious as environments are modified, often through a process of dam creation to increase water levels for protection of natal lodges and access to food resources, and often with more obvious feeding impacts. It is generally at this point that conflicts with human land-use interests become more likely. Dam creation and its attendant landscape alteration is the most common cause of conflicts.
Findings from the Scottish Beaver Trial, the Tayside Beaver Study Group, River Otter and various reports on both free-living beavers and enclosure projects throughout Britain clearly demonstrate that Eurasian beavers can survive and adapt well to the British landscape (Campbell et al. 2012a; Campbell-Palmer et al. 2015; Harrington et al. 2015; TBSG 2015). Although their established predators, such as the European Wolf (Canis lupus lupus), European lynx (Lynx lynx) and brown bear (Ursus arctos), are now absent from Britain, young beavers and particularly kits can still be predated by red fox (Vulpes vulpes), domestic dogs (Canis lupus familiaris), pine marten (Martes martes), birds of prey and even large pike (Esox lucius) (Kile et al. 1996; Rosell and Hovde 1998; Rosell et al. 2005). There are anecdotal reports that otters (Lutra lutra), American mink (Neovison vison) and badgers (Meles meles) may also be opportunistic predators, particularly of lone kits.
As beaver populations increase in density, beavers will often be wounded or even killed in territorial conflicts with non-related individuals. Mortality rates across Europe vary greatly depending on a range of factors such as habitat quality, population density, varying climatic conditions, extremes of weather (particularly spring flooding), hunting and trapping, predators (particularly wolves) and disease (Novak 1987; Rosell et al. 2005). Many studies demonstrate that mortality rates are highest in the first two years of life (e.g. Payne 1984; Campbell et al. 2012b), though survival during the first year after birth can be very high (e.g. 92% vs. 87% for breeding adults, Campbell et al. 2012b). Deaths due to road accidents may also be a significant cause of mortality, with small numbers of beaver fatalities already reported on roads in Perthshire (H. Dickinson, personal communication 2014; Campbell-Palmer et al. 2015). Other causes of mortality in Europe include flooding and associated drowning (mainly kits), and an inability to wean fully and to cope with dietary changes to vegetation. While captive beavers have been recorded as surviving to 28 years of age, the mean adult life expectancy in the wild is believed to be 12–14 years (Nolet et al. 1997), though older territory-holding and reproducing individuals have been recorded in Norwegian study populations (Campbell et al. in press).
As with all wildlife, beaver population density varies considerably in time and space and with habitat quality. In addition, beaver density is influenced by their territorial behaviour, with mean territory size tending to decline as habitat quality increases (Parker and Rosell 2012). Since beavers rarely move more than 60 m from water (Barnes and Dibble 1988; Donkor and Fryxell 1999), and most activity is within 20 m of the shore, they occupy landscapes along banksides in a linear fashion. Most landscapes include lengths of unsuitable beaver habitat, e.g. stretches of rapids. Therefore, at the landscape scale, the distribution of beaver territories is often highly discontinuous (Parker et al. 2001a). Growth rates in newly established beaver populations are often initially slow, as individuals disperse into a large river system with relatively few potential mates, reducing the rate at which they are encountered. As dispersing offspring may travel dozens of kilometres from their family territories, the process of population establishment creates a ‘patchwork’ pattern of beaver territories. This can produce a ‘lag phase’ of slow growth after reintroduction or colonisation of an area, which is then followed by rapid expansion. The length of time required for rapid population expansion varies depending on the characteristics of the river system and may take 15–20 years on larger river systems (Hartman 1995). In release projects, if large (~40+) numbers of animals are released, the chances that dispersing offspring will meet each other sooner are greater and therefore initial population growth rates usually higher.
After this phase of population growth, a decrease in territorial sizes may become evident (Campbell et al. 2005). At this point, the availability of habitat becomes a limiting factor, and territorial disputes become more common. Mortality rates increase directly through fighting and indirectly through the stresses of living in smaller territories that need to be defended more vigorously. This whole process can become physically evident by a decrease in breeding rates, delayed dispersal and lighter individual body weights (Busher et al. 1983). While a developing beaver population with abundant habitat can display growth rates of 15–20% per annum (Hartman 1995), populations will level off with no demonstrable growth once their whole environment is occupied. However, as long as there is suitable unused habitat available, beaver populations will grow and expand.
Another aspect of beaver ecology that exerts considerable influence on the spatial and temporal distribution, and density of occupied territories, is the alternating pattern of site occupation and abandonment in lower-quality habitat which emerges in mature populations. Following a period of occupation, sites may be abandoned for a number of years if food resources become depleted. Once preferred food species have grown back in sufficient quantity, a new period of colonisation of the site will occur. Thus, a dynamic source-sink pattern of site occupation and abandonment becomes established, with rotation times varying depending on habitat quality and harvest levels (Fryxell 2001). In these populations, measured at a landscape scale large enough to include both good and poor beaver habitats (e.g. >~100 km2), the proportion of sites occupied at any time, i.e. the site occupation rate, tends to vary between 0.33 and 0.50. Thus, after levelling off at an initial population peak, populations may decline somewhat before roughly stabilising. In such populations, between a third and half of the potential beaver habitat within a larger area will be in use at any particular time (Parker and Rosell 2012).
Finally, it is worth noting that a lack of genetic diversity in the initial population may result in inbreeding depression that could limit the rate of population growth and geographic expansion.
The mean litter size for the Eurasian beaver based on fetus counts is approximately 2.5 and based on young born approximately 2.0 (Danilov and Kan’shiev 1983; Mörner 1990; Danilov et al. 2011; Parker et al. 2012); however, delayed sexual maturity beyond the age of 3 years is common, and sexually mature females may not breed in all years, a reproductive response apparently linked to persistently high densities and dwindling food supplies (Campbell 2010). In southern Scandinavian landscapes ≥100 km2 in area and encompassing beaver habitats of varying quality, the mean density of occupied sites in four studies was 0.26/km2 (range = 0.32–0.20). On a smaller scale, the highest densities tended to occur in agricultural landscapes with a prevalence of low-gradient streams (Parker et al. 2013).
In management terms, after reintroduction, slow establishment may therefore be expected, followed by a period of rapid growth and dispersal, before becoming more stable at a lower population size and density (Figure 3.10). Culling both during the period of rapid growth or rough stability following is likely to be followed by rapid recolonisation by surplus animals. Once beaver territories become fully occupied, nonlethal management, which permits beaver presence within tolerable limits, should slow or prevent further colonisation and significant increase in density due to the territoriality of this species. In reintroduced populations in areas of Sweden and Norway, it has taken several decades for beavers to attain typical population densities and start to occupy suboptimal habitats (Hartman 1994; Halley 1995). It is typically not until this stage that a higher incidence of human–wildlife conflict occurs (Bhat et al. 1993; Deblinger et al. 1999).
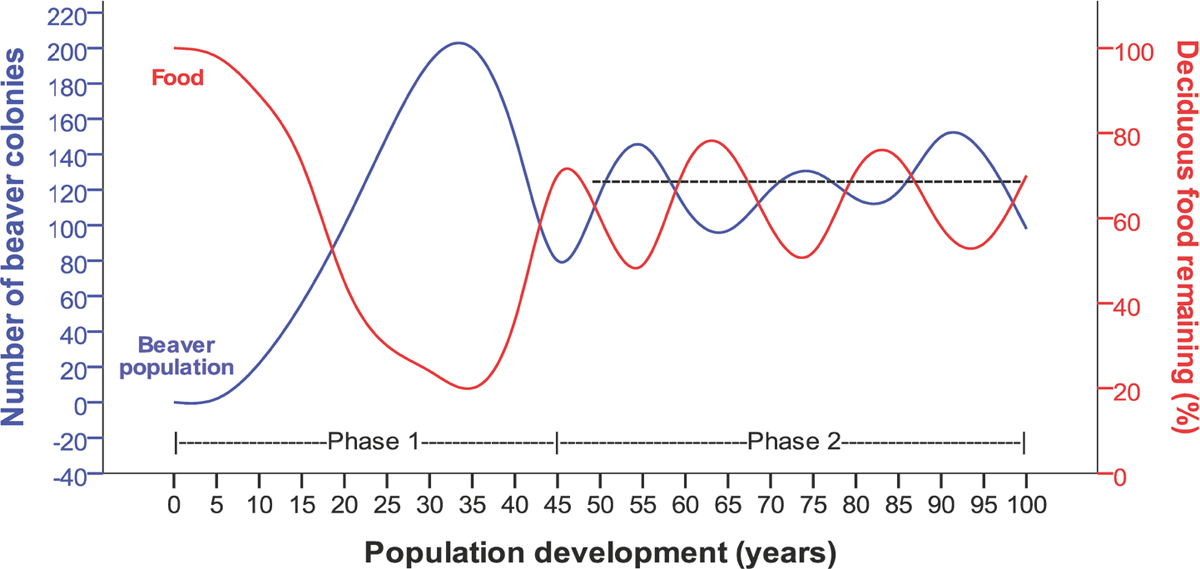
Figure 3.10 A graphical presentation of the predicted relationship between a developing beaver population and its prime winter food source of deciduous trees and bushes. Assumptions for the model are an area of 500 km2 of typical beaver habitat in Fennoscandian boreal forest with a peak in colony density occurring at 0.40 active territories/km2 after 35 years, and a mean active territory density of 0.25/km2 (dotted black line) following population recovery. Culling pressure is light or non-existent. The basic shape of the curve for colony number in ‘phase 1’ is relatively well founded in field studies. The shape in ‘phase 2’ is based on limited anecdotal observation. (Parker and Rosell 2012)
3.9 North American beaver introductions
Since their release, North American beavers have spread throughout southwest and central Finland and into Karelian Russia. They have established a presence in parts of Belgium, western Germany and Luxembourg as a result of zoo escapes (Dewas et al. 2012).
Expanding populations of both species have converged on two fronts in Finland and northwest Russia (Parker et al. 2012). The body size of both species is similar, though the litter size of the North American beaver is slightly larger. Only minor differences in life history, ecology and behaviour exist, suggesting a nearly complete niche overlap. The question is whether coexistence or competitive exclusion will ultimately result, with the possible regional extirpation or eventual extinction of one of the species. This would be of concern if the outcompeted species was the Eurasian beaver. Though extirpation of North American beaver from continental Eurasia is still possible (Parker et al. 2012), it may never happen given a lack of willingness to accept the seriousness of the problem and limited resources for wildlife management.
Preventative measures incorporated in the beaver-management plan for the Czech Republic include a database of all the captive collections holding North American beavers, the establishment of cooperation to ensure prompt attempts to recapture any escaped animals, the elimination of any identified wild-living North American beaver as a non-native species, and the promotion to captive collections of the replacement of North American individuals with Eurasian Beavers (Vorel et al. 2013). Given this experience and the documented ability of beavers to escape from captivity, it would be prudent to reduce the potential for introduction of North American beavers to Britain. Very few captive facilities in Britain currently hold North American beavers. The species can, however, be readily imported. Greater consideration should be given to the purpose of such animal movements, to sterilisation, or to replacement with Eurasian beavers. The registration and permanent individual identification of any captive North American beavers in Britain would be sensible.
Key concepts
•There are two species of beaver – Eurasian and North American/Canadian – which can be difficult to tell apart in the field. North American beavers have been introduced or have escaped in parts of Europe, presenting a management issue. Preventative action should ensure this does not occur in Britain. Currently, there are no known wild-living North American/Canadian beavers in Britain.
•Beavers are large (20 kg+), semi-aquatic, completely herbivorous rodents.
•Sexing beavers without handling is difficult unless the nipples of heavily pregnant or lactating females are visible. This is because both sexes conceal their sexual organs within a cloaca. With experience and appropriate handling beavers can be sexed according to the colour and viscosity of their anal gland secretions.
•Beavers live in family units, with one dominant pair of breeding adults and their offspring from 1–2 breeding seasons. They produce one litter of typically 2–4 kits per year. Families are highly territorial.
•In the wild beaver mortality can be high during the first 2 years of life, but if they survive this period they can live on average to 12–14 years.
•Beavers live in freshwater habitats, with the majority of their on-land activity occurring within 20 m of the shoreline.
•They are subject to the same range of parasites and diseases as other wildlife, though directly imported animals should be screened for non-native pathogens.
•Initial population growth on a river system is typically relatively slow, then rapid for a period. As long as there is available suitable unoccupied habitat, beaver populations will grow and expand.
•Populations then peak and may decline somewhat as lower quality habitat which cannot sustain beavers permanently goes out of use for a period while the vegetation recovers. Thereafter populations reach rough stability, regulated by the territorial nature of beavers, and with lower quality sites cycling in and out of use.
•Kits in Britain will be subject to predation pressure, and roadkill may be a significant cause of mortality for dispersers and adults.









