In a Clamshell
The Laurentian Great Lakes have an extensive history of human-mediated biological invasions, beginning at least 150 years ago. During this interval, a number of transitions have occurred with respect to both the types of non-indigenous species (NIS) that established and the mechanisms that vectored them to the lakes. Fish and plants were the most common NIS prior to 1900, with most introductions resulting from deliberate human releases. Algae and invertebrate establishments became more common after transoceanic shipping converted to the use of liquid ballast around 1890. The ship vector was dominant during much of the twentieth century. Since the expanded St. Lawrence Seaway opened in 1959, ships’ ballast water has been the leading vector for approximately 55% of new established species. Eurasia was the source of 68% of NIS that have established in this period, followed by North America (14%), and palearctic/nearctic (7%). We review select NIS that have caused ecological and economic harm in the Great Lakes and, in some cases, spread to inland lakes. We close with a discussion of adaptive management for NIS on the Great Lakes.
Economic and financial factors strongly influence many human decisions and activities, which in turn can affect ecological systems and their associated services. Expansion of global trading networks and trade liberalization policies (e.g., NAFTA, GATT) may inadvertently expose ecosystems to new nonindigenous species (NIS) (Tatum et al. 2006; Tatum and Hay 2007). Levine and D’Antonio (2003) determined that accumulation of mollusk, plant pathogen, and insect NIS were all positively associated with the cumulative value of imported products to the United States. Similarly, Dehnen-Schmutz et al. (2007) noted that frequency of sale and seed price were significant predictors of invasion success for ornamental plants sold in Britain. Once established, NIS may adversely affect a variety of ecosystem services (see, e.g., Cook et al. 2007) and motivate significant expenditure on control activities (e.g., Pimentel et al. 2005; Colautti et al. 2006; Xu et al. 2007; see also chapter 8). Although commercial vectors (e.g., shipping, horticulture, pet trade) are responsible for most NIS introductions, the costs caused by invasive species are generally borne by government, private individuals, or commercial sectors (e.g., agriculture) other than those that introduced the species.
Forty-one percent of humans live in coastal habitats worldwide, and 21 of the world’s largest 33 cities are located within 100 km of the coast (Martinez et al. 2007). Cities are located near coastal areas for a number of reasons, of which ready access to sea ports and marine food sources are particularly important. Costanza et al. (1997) estimated that fully 63% of total global ecosystem services were derived from oceanic ecosystems, principally within coastal zones. Martinez et al. (2007) increased this value to 77%, owing to their inclusion of benefits derived from terrestrial habitats located less than 100 km from the coast.
Cities and coastal ports are often located at the mouth of major river systems. For example, cities and major ports in Europe are located at the outflow of the Rhine (Rotterdam), Danube (Constanta), Schelde (Antwerp), Vistula (Gdansk), Neva (St. Petersburg), Bug (Mykolaiv), and Dnieper (Kherson) rivers. These ports are often linked to other regions by networks of constructed canals. One of the most important of these, the Rhine-Main-Danube canal, was opened in 1992 to link the Black Sea basin with the Rhine River basin. Development of canals facilitates enhanced trade and recreational travel. However, these canals also have been instrumental in the spread of NIS both regionally (Bij de Vaate et al. 2002) and internationally (Ricciardi and MacIsaac 2000), including to the Laurentian Great Lakes. Aquatic ecosystems, in general, are among the most vulnerable to invasion by NIS and attendant externalities (Sala et al. 2000; Connelly et al. 2007). Once invaded, these ecosystems may serve as NIS “hubs” and, by interacting with a global transport network, may facilitate the worldwide spread of invasive species (e.g., Drake and Lodge 2004; Muirhead and MacIsaac 2005; Tatum et al. 2006; Tatum and Hay 2007).
Impacts of invasion of aquatic ecosystems by NIS are numerous and include reduced or enhanced native species diversity (e.g., see Ward and Ricciardi 2007), disease transmission (Indiana Department of Natural Resources 2005), altered nutrient cycling patterns (Mellina et al. 1995), hybridization with native species (see Roman and Darling 2007), and biofouling of industrial and municipal water intake structures (Connelly et al. 2007). Strong adverse impacts associated with NIS establishment and spread have lead to global recognition of the problem, but responses by governments vary widely. Some countries, such as Australia and New Zealand, have implemented programs designed to assess and reduce the likelihood of future invasions and to eliminate or control NIS established within their countries (e.g., Cook et al. 2007; Keller et al. 2007). These efforts can effectively slow invasion rates but come with significant costs for quarantine, risk assessment, and restricted trade patterns.
The Laurentian Great Lakes of North America share many of the attributes described above for oceanic coastal regions, and thus are an ideal model system to examine the history, ecological impacts, economic effects, and societal responses to biological invasions. We review the invasion history of the Great Lakes, identify some major ecological and economic impacts of such invasions, and show how, through risk prevention and control, the biodiversity impacts of invasive species are inextricably linked to the Great Lakes economy.
The Laurentian Great Lakes border Canada and the United States and are among the most heavily utilized water bodies in the world. Containing an estimated 20% of the planet’s surface freshwater, the lakes provide more than 40 million coastal residents with access to drinking and industrial water, hydroelectric supplies, recreation, food, and transportation. Major metropolitan areas and their economies depend on the Great Lakes and the St. Lawrence Seaway, including Chicago, Detroit, Toronto, and Montreal, with more than $200 billion of economic activity conducted annually within the basin (U.S. Policy Committee for the Great Lakes 2002). For the United States alone, the region generates more than 50% of total manufacturing output. Fifty million metric tons of cargo annually passes through the Great Lakes in the international shipping trade, with the main commodities being grain, steel and iron ore, coal, coke, and petroleum products. About one-half of this cargo travels to and from overseas ports, mainly in Europe, the Middle East, and Africa.
In 1829, humans facilitated international commerce in the Great Lakes via construction of the Welland Canal, linking Lakes Erie and Ontario to provide navigable waterways between those water bodies. Subsequent development of the lock system between Lake Ontario and the Saint Lawrence River in 1847, and between Lakes Superior and Huron in 1855, allowed uninterrupted passage from the Atlantic Ocean to Lake Superior (Mills et al. 1993). However, after the Second World War, growth in international trade created the need to deepen the St. Lawrence waterway and allow larger ships to enter the Great Lakes. Canals were later expanded and the modern Saint Lawrence Seaway was opened in 1959. The development of a navigable water network has opened the Great Lakes region both to international trade and to the introduction of NIS. Attendant with the expansion of navigable waters was a major change in the risk of invasion from ships, as ballast utilized to stabilize incoming vessels changed from solid materials (e.g., stone, sand, soil, cobble) to liquid around 1890 (Mills et al. 1993).
In the ground-breaking retrospective study by Mills et al. (1993) of the invasion history of the Great Lakes, clear patterns emerged with respect to the nature of NIS that invaded during different time periods. The initial phase of invasion began in the early 1830s with the introduction of sea lamprey (Petromyzon marinus) to Lake Ontario via the Erie Canal. The Erie Canal was constructed between Lake Erie and the Hudson River to reduce the cost of transporting produce from the East Coast to growing human settlements in the Great Lakes basin and those farther west. Sea lamprey had profound ecological and economic impacts, causing severe declines in whitefish (Coregonus clupeaformis) and lake trout (Salvelinus namaycush) and negatively affecting commercial catches. Eight other NIS became established prior to 1850, all marsh-dwelling plants likely released through the nursery trade or from food cultivars (Mills et al. 1993). Between 1850 and 1900, the rate at which NIS were discovered increased and included a wider variety of species. Although marsh plants continued to be the dominant NIS during this period, shoreline trees, invertebrates, and fishes were also introduced. The dominant vectors of introduction were ships’ solid ballast and accidental releases or food cultivar escapees. Solid ballast used to maintain ship stability during long ocean voyages was often discarded at Great Lakes destination ports, thus enabling the concomitant release of NIS (Mills et al. 1993).
Deliberate release (mainly by government agencies) also was an important vector of introduction (Ricciardi 2006), particularly for chinook salmon (Oncorhynchus tshawytscha), rainbow trout (O. mykiss), and brown trout (Salmo trutta), which were introduced to develop recreational and commercial fisheries. Several aquatic invertebrate NIS were apparently released to increase biological diversity (Mills et al. 1993). Among the “invasive” (i.e., harmful) species discovered between 1850 and 1900 were the submerged macrophytes curly pondweed (Potamogeton crispus) and spiny naiad (Najas marina), the wetland species purple loosestrife (Lythrum salicaria) and narrow-leaved cattail (Typha angustifolia), and the fishes alewife (Alosa pseudoharengus) and common carp (Cyprinus carpio).
Between 1900 and 1958, an additional 54 species were added to the NIS inventory of the Great Lakes (Mills et al. 1993). A recent analysis (see Kelly 2008) indicates that a further five species became established during this interval, for a total of 59 NIS. The taxonomic composition of NIS changed dramatically during the 1900–1958 period, with the first reports (7 species) of algae and a growing importance of invertebrates (17 species) and fishes (11 species). This NIS shift coincided with the gradual replacement of solid by liquid ballast in ships. Water was advantageous as ballast since it was readily available in foreign source ports and its volume could be easily adjusted to maintain ship draft. Thus, it is not surprising that ballast water release was responsible for the first appearance of planktonic invertebrates, including the copepod Eurytemora affinis and the water fleas Daphnia galeata galeata and Eubosmina coregoni. Notable “invasive” species that established in the Great Lakes during this period include the plants fanwort (Cabomba caroliniana), Eurasian watermilfoil (Myriophyllum spicatum), and water chestnut (Trapa natans), all of which outcompete native plants. Mechanisms of introduction varied widely across taxa. Because a particular species may have been introduced by more than one mechanism, in the following section we focus on the dominant vector but recognize that some assignments could be incorrect. Fishes were most often released deliberately (up to five species) or accidentally (up to four species) from fish hatcheries or the aquarium trade. Dominant introduction mechanisms for invertebrates were unintentional release (up to five species) associated with imported ornamental plants or the aquarium trade, while two species each were likely introduced with solid ballast or as cultivation escapees, and finally one by canal. Algae were almost exclusively released from ballast water (six species). Cultivation escapees (up to five species), ships’ solid ballast (four species), and accidental releases (up to three species) were dominant introduction mechanisms of submerged plants, although two species may have entered attached to ship hulls. In summary, the early phase of invasion in the Great Lakes was dominated by wetland plants, while algae, invertebrates, and fishes became far more common additions after 1900. Until 1959, accidental introductions associated with shipping, canals, and other economically motivated activities accounted for up to 37 NIS introductions (16 animals, 21 plants), whereas intentional introductions associated with stocking programs and other activities accounted for up to 26 NIS introductions (19 animals, 6 plants, 1 pathogen) (modified from Mills et al. 1993; Ricciardi 2006).
Determination of the vector (i.e., mode of introduction), pathway (i.e., source), and timing of species invasion is a valuable tool for management and control efforts (Ruiz and Carlton 2003). Invasion histories and vulnerability of ecosystems to future invasions can be re-created and predicted by examining putative vectors (Mills et al. 1993), including international shipping (e.g., Colautti et al. 2003; Drake and Lodge 2004), or by phylogeographic assessments of genetic structure of native and introduced populations (e.g., Cristescu et al. 2001; Kelly et al. 2006). These approaches are particularly relevant to the Laurentian Great Lakes, where vectors, pathways, and the composition of NIS changed markedly after 1959. For instance, a number of studies have explored invasion patterns in the lakes following the opening of the expanded, modern St. Lawrence Seaway in 1959, an event that permitted larger foreign vessels access to the entire Great Lakes system. Grigorovich et al. (2003) and Ricciardi (2006) attributed 67% and 65% of post-seaway introductions, respectively, to ballast water. Ballast water that is loaded in regions outside of the Great Lakes may entrain large numbers of viable freshwater species that are discharged during cargo loading in Great Lakes ports. BOB (“ballast water on board”) ships were recognized as a major vector of NIS introduction to the Great Lakes, and between 1989 and 1993 the United States and Canada introduced ballast water control policies aimed at reducing further introductions (Locke et al. 1991; U.S. Coast Guard 1993). Since 1993, BOB ships have been required to conduct ballast water exchange (BWE) in open-ocean marine waters to purge freshwater NIS from tanks and kill those that remain by exposure to saltwater. Despite these management efforts, 19 new NIS have been reported in the Great Lakes system since 1993, nine of which were most likely introduced by the ship ballast vector (see table 10.1).
Several factors could account for continuing invasions of the Great Lakes. Up until the early 1980s, a large number of Russian ships entered the Great Lakes to collect grain (I. Lantz, Shipping Federation of Canada, personal communication). Most of these vessels are believed to have carried ballast water of Baltic Sea origin, the native or introduced range of many Great Lakes invaders, and these ships may have introduced many NIS. The decline in Russian grain ship visits toward the end of the 1980s was likely due to a need to reduce debts to Western creditors, improved home grain yields, and the imposition of a U.S. embargo on grain sales to the former USSR. During the 1980s, the characteristics of transoceanic ships also changed. An increasing proportion of ships declared no-ballast-on-board status for some or all of their ballast tanks; these “NOBOBs” carried cargo and were exempt from ballast control. However, their tanks still carried large volumes of residual water and sediment. Economically, these vessels experience higher operational efficiency since they backhaul cargoes from the Great Lakes after delivering steel, petrochemicals, or other products to Great Lakes ports (Colautti et al. 2003). Studies have shown that residual ballast harbors large numbers of viable NIS, which pose a risk of discharge to the Great Lakes during multiport operations (Colautti et al. 2003; Bailey et al. 2005). Regulations introduced in Canada in 2006 and the United States in 2008 require that NOBOB tanks be flushed at sea to eliminate freshwater residue (unpublished data; Canada Shipping Act 2006). Since these rules augment those for BOB vessels, and should affect transport of many of the same species, it will take some time before the effectiveness of these policies can be assessed.
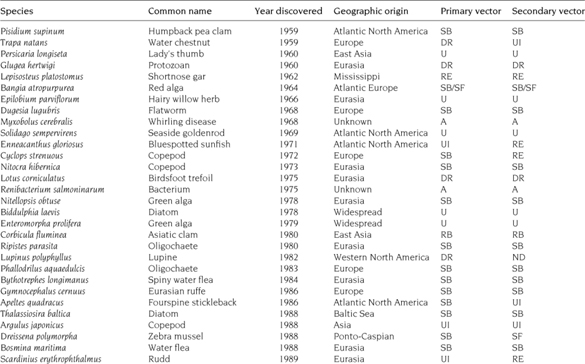
TABLE 10.1. Vectors and origins of NIS reported in the Great Lakes since 1959 (modified from Kelly 2008).
A further problem in determining BWE efficiency for ballasted vessels is time lags (Costello et al. 2007), of which two may occur. First, species may not be detected in the lakes until well after they were introduced. Second, a gap may exist between when species are first detected and when they are first reported (e.g., time to positively identify a species). Time lags almost certainly vary in length, depending on the conspicuousness and invasiveness of the species and the habitats that they colonize.
The high profile of ballast-mediated invasions may also have distracted researchers from consideration of alternative vectors, leading to uncertainty in evaluations. For example, large numbers of fouling organisms—species that usually have sessile adults capable of attaching to the hull, anchor chain, or other external surfaces—have been found attached to ships, a subvector that is dominant in marine environments (Ruiz et al. 2000; Gollasch 2002; Drake and Lodge 2007). Also, the live fish market, aquarium, and aquaculture industries have received less attention, but recent research has indicated that these vectors pose a significant risk to the Great Lakes (Kolar and Lodge 2002; Rixon et al. 2004; Cohen et al. 2007; Keller and Lodge 2007).
In a recent study, Kelly (2008) prioritized alternative vectors to assess the most likely origin and pathway of introduction of NIS since 1959. Eurasia has been the dominant donor region, accounting for 67% of NIS in the Great Lakes since 1959 (figure 10.1, table 10.1). Within Eurasia, Europe and the Black and Caspian Sea basins (Ponto-Caspian) contributed most of the species, with Southeast Asia and the Baltic Sea being of lesser importance. North America has been the next most important donor region, accounting for approximately 14% of NIS, most of which originate in the Northwest Atlantic coastal region. Ship ballast was the strongest vector and accounted for up to 55% of all NIS primary vector assignments (table 10.1). It is interesting that 94% (31 of 33 species) of ship-ballast invasions originated in Eurasia (figure 10.1). Overall, Europe and the Black Sea basin were the main donor regions, which is consistent with coarse measures of propagule pressure—a product of the number of introduced individuals or infective stages and their frequency of introduction—in the Great Lakes. Very little vessel traffic to the Great Lakes originates in Black Sea ports (Colautti et al. 2003), and thus few species—with the exception of quagga mussels (Dreissena bugensis (= D. rostriformis bugensis [Andrusov (1897)]))—were likely brought in directly from this region. Most of the Black Sea species that have established in the Great Lakes also have an invasion history in western European ports, from which most inbound vessel traffic to the Great Lakes does originate (Ricciardi and MacIsaac 2000). Indeed, the opening of the Main Canal in 1992, which connects the Danube with the Rhine system, provided a major westward colonization pathway from the Black, Azov, and Caspian Sea basins (Bij de Vaate et al. 2002). The Main Canal was completed despite concerns regarding possible spread of NIS. It should be noted, however, that concern in Europe regarding NIS has increased dramatically in the past decade, in part due to the spread of Ponto-Caspian species. This invasion pathway, coupled with the impacts of Ponto-Caspian species in western Europe, provided a basis for several key studies that warned of future potential invaders from this region (e.g., Ricciardi and Rasmussen 1998; Grigorovich et al. 2003). However, despite the implementation of ballast control policies between 1989 and 1993, Ponto-Caspian species have continued to invade the Great Lakes (table 10.1). It is possible that such recent invasions were due to a lack of awareness of risks due to residual sediment and water in NOBOB vessels, a ship subvector that has only recently received the attention of policy makers, or to time lags in discovery or reporting.
FIGURE 10.1.
Pathways of ship-ballast–vectored invasions to the Great Lakes since 1959. Arrow width is proportional to the strength of each donor subregion. Numbers in parentheses are the number of species originating in each region. Reprinted from Kelly (2008), with permission of the Transportation Research Board of The National Academy of Sciences of the USA.
The only non-Eurasian species likely introduced to the Great Lakes in ship ballast were of Northwest Atlantic origin. Both G. tigrinus and A. quadracus were likely introduced from the Gulf of St. Lawrence. It is difficult to discern the type of vessel that may have brought these species into the Great Lakes, because it could have been accomplished by “salties” (ships transiting the seaway to conduct international trade), by “lakers” (ships that trade mainly within the Great Lakes but venture into the St. Lawrence River), or by coastal vessels that operate within North America (coastal marine areas and the Great Lakes) and are exempt from BWE regulations. For example, lakers occasionally offload cargo and load ballast water at ports in the St. Lawrence River and estuary (e.g., Quebec City, Sorel, Baie-Comeau) downstream of Montreal (Eakins 1999, 2000; M. Rup, University of Windsor, unpublished data). Vessels with NOBOB tanks also may offload cargo at ports such as Port Cartier, downstream of the seaway, before taking on ballast and proceeding to Great Lakes ports for cargo collection (Colautti et al. 2003). Although both G. tigrinus and A. quadracus are seemingly innocuous NIS, transfer of coastal ballast water, or of infected fishes contained therein, is a possible mechanism (along with naturally migrating fishes) responsible for the recent introduction of viral hemorrhagic septicemia (VHS) into the Great Lakes. Although the origin of VHS is uncertain, molecular studies indicate that it may have originated from the northeast Atlantic. The economic consequences of this disease introduction are not yet known, although it is likely to be profound, because VHS affects more than 40 species of fish.
Since 1959, ship fouling has been implicated in only two species introductions (the algae E. flexuosa and B. atropurpurea), while deliberate release, aquaculture, range extensions, and unauthorized intentional vectors were individually of minor importance, but collectively represented 26–32% of all NIS introductions. Both recreational boating and natural dispersal were of minor importance. Only three introductions have occurred via canals and one via recreational boating (table 10.1). The small proportional contribution of canal-mediated introductions to the total introductions in the Great Lakes is a finding consistent with previous works (see Mills et al. 1993; Ricciardi 2006). However, the unimpeded canal pathways from the Mississippi and Hudson river basins continue to pose a serious risk of future introductions (figure 10.2). This risk is highlighted by the recent construction of an electric fish barrier on the Chicago Sanitary and Ship Canal (CSSC) to prevent spread of round gobies from the Great Lakes to the Mississippi River, but which is now being used to prevent entry by Asian silver and bighead carp into the Great Lakes (Stokstad 2003). These Asian carp, which dispersed via the Mississippi River into the lower Des Plaines River, immediately downstream of the CSSC, pose an additional risk to the Great Lakes since some are infected with carp viremia (Rhabdovirus carpio), a virus of Eurasian origin (Nelson 2003). If Asian carp overcome the electrical barrier, there is a risk that fish in the Great Lakes could become infected with the virus. Herborg et al. (2007) utilized an environmental niche model to identify the Great Lakes as vulnerable to invasion by both carp species, whereas Kolar and Lodge (2002) predicted the opposite based upon life history characteristics of the carp.
Prior to 1959, the Erie Canal was of relatively minor importance for Great Lakes NIS, but it has allowed invasion by several North Atlantic species that have had substantial impacts (Mills et al. 1993). Although only a single species, the blueback herring, invaded via this pathway since 1959, the canal could be an entrance mechanism in the future.
Thus, consideration of all possible vectors since 1959 indicates that ship ballast has been responsible for the greatest numbers of NIS introduced to the Great Lakes. As mentioned above, this continuing risk has focused attention of U.S. and Canadian governments on ballast management policies whose effectiveness is difficult to determine. What is clear is that NIS continue to be discovered in the Great Lakes, the majority of which have a European or Black Sea origin, with ship ballast as the likely vector. Other species are colonizing key port areas in Europe, and so the Great Lakes remain at risk of ballast-mediated introductions from this region. This continuing risk is illustrated by the most recent NIS, the mysid shrimp Hemimysis anomala, which was predicted to pose a risk in recent assessment models (Ricciardi and Rasmussen 1998; Grigorovich et al. 2003). Ballast water of European origin was the most likely vector of this species.
FIGURE 10.2.
Canal and recreational boating pathways of invasion to the Great Lakes since 1959. Reproduced from Kelly (2008), with permission of the Transportation Research Board of the National Academy of Science of the USA.
In the following sections, we highlight case studies of NIS that have had significant ecological and economic effects in the Great Lakes basin. Species considered include the water fleas Bythotrephes longimanus and Cercopagis pengoi, dreissenid mussels D. polymorpha and D. bugensis, and the round goby Apollonia melanostoma (= Neogobius melanostomus). In each case, we consider the vector of invasion, distribution, and consequences in the Great Lakes and secondary spread to other systems.
The water fleas Bythotrephes longimanus and Cercopagis pengoi (figure 10.3) are predators of other zooplankton species and share similar life histories. Both are members of the crustacean family Cercopagidae, both have alternating modes of sexual and asexual reproduction, and both have invasion histories in Europe and the Great Lakes. Bythotrephes has a Eurasian native distribution, whereas Cercopagis is native to the Caspian, Black, Azov, and Aral seas. The first record of Bythotrephes in North America is from Lake Huron (1984), followed by Lakes Ontario and Erie (1985), Lake Michigan (1986), and Lake Superior (1987). Cercopagis was also discovered first in Lake Ontario (1998) and subsequently spread to Lake Michigan (1999) and Lake Erie (2001). Cercopagis has not yet been reported from Lake Superior or Huron. Both species were almost certainly transferred to the Great Lakes in contaminated ballast water from Europe (table 10.1) and subsequently moved within the Great Lakes by internal ballast water transfers by salties or lakers. The latter vessels load and discharge disproportionately more water within the Great Lakes system (M. Rup, unpublished data) and thus are the more likely regional vector.
FIGURE 10.3.
Representative introduced species that have caused significant ecological and economic harm to the Great Lakes include water fleas Cercopagis pengoi and Bythotrephes longimanus (upper and lower, respectively, in the top left image), zebra and quagga mussels (left and right, respectively, in middle row), and round gobies and sea lamprey (left and right, respectively, in lower row). Upper right image highlights water flea fouling (Cercopagis) of commercial gill nets on Lake Erie (Ontario Ministry of Natural Resources). Cercopagis and Bythotrephes image courtesy of Dr. H. Vanderploeg (with permission of the Canadian Journal of Fisheries and Aquatic Sciences, CRC Press); round goby courtesy of Shedd Aquarium.
Bythotrephes has spread rapidly to inland lakes, beginning with Lake Muskoka in central Ontario in 1989. The species has continued to spread, with 108 lakes now reported invaded in the province, including 18 new reports in 2006 (A. Cairns, N.Yan, and J. Muirhead, unpublished data; figure 10.4). Inland lakes have also been invaded in the United States, although reports are seemingly an order of magnitude fewer than in Canada (figure 10.4). In Minnesota, Bythotrephes has been confirmed in 16 inland lakes, mainly on the border with Ontario (U.S. Geological Service Invasive Species Database, unpublished data). Bythotrephes has also been confirmed in at least four lakes in Michigan, two reservoirs in Ohio, and two lakes in Wisconsin (figure 10.4). It is not clear whether the differential occurrence on Canadian and U.S. sides of the Great Lakes is real or the result of differential sampling and reporting. Interestingly, recreational activities and numerous hydraulic connections west of Lake Superior may allow the species to move between the two countries outside of the Great Lakes.
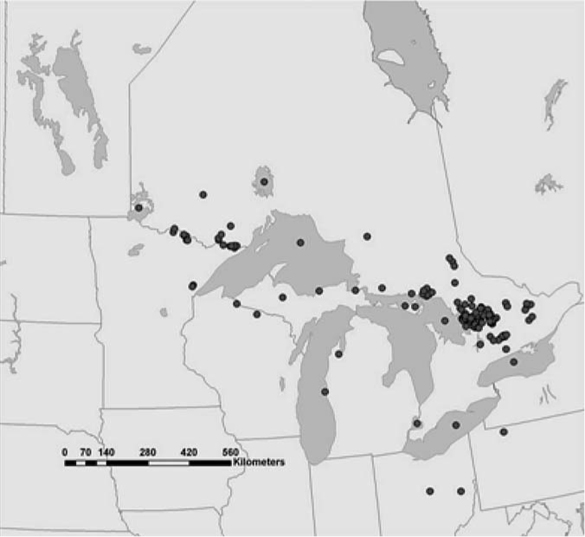
FIGURE 10.4.
Distribution of the spiny water flea Bythotrephes longimanus in Canada and United States (2007). The species colonized the Great Lakes in the early 1980s and spread to inland lakes in the United States and Canada beginning around 1989. Data kindly provided by the Canadian Aquatic Invasive Species Network and Dr. Jim Muirhead.
Bythotrephes has spread much faster to inland systems than has Cercopagis, which, other than the Finger Lakes in New York, has failed to colonize inland systems. The differential occurrence of Bythotrephes in Canada and the United States and the differential rate of spread of the two species are puzzling since both species produce resting stages that foul fishing lines and are believed to be the primary mechanism of spread to and among inland systems (figure 10.3). In addition, boaters in states bordering the Great Lakes are likely as active as those in Canada.
Once lakes are invaded by Bythotrephes or Cercopagis, abundance and diversity of small and midsize zooplankton are reduced. While this nonmarket effect may seem unimportant, it could lead to competition between the invaders and larval fish for food. Future work is required to determine if changes in fish populations are related to food web changes associated with invasion by these two species. These water fleas may also have a more direct impact on sport fisheries and consequently local economies because fouling of fishing lines can hinder recovery of fishing gear, potentially leading to angler frustration and a reduction in the number of recreational anglers visiting invaded lakes.
Zebra mussels (Dreissena polymorpha) and quagga mussels (D. bugensis) (figure 10.3) are mollusks from the Black Sea basin that were reported in the Great Lakes in 1988 and 1989, respectively. D. polymorpha has an extensive invasive range in Europe and was probably introduced to the Great Lakes from northern or western Europe, whereas D. bugensis has a limited distribution and has only recently begun to spread. The species are virtually identical in morphology and seemingly similar in reproductive biology and other traits, although quagga mussels occur in deeper water not inhabited by zebra mussels. Both species were likely introduced as larvae in ballast water from Europe (table 10.1), although fouling of structures such as anchor chains, sea chests, or floating macrophytes by adult mussels may have been responsible (Horvath and Lamberti 1997). It is this fouling ability, coupled with high dispersal ability by natural and human-mediated means, that has likely contributed to the regional spread of zebra and quagga mussels within the Great Lakes and beyond (see chapter 12 for a more comprehensive treatment of establishment and dispersal of zebra mussels). Both species now have extensive histories of spread in temperate eastern North America with ecological effects that are more profound than any other aquatic NIS (for reviews, see MacIsaac 1996; Ward and Ricciardi 2007). The recent discovery of zebra mussels in San Justo Reservoir in San Benito County, California, in January 2008 and quagga mussels in Lake Mead in Nevada and lakes in California highlights the importance of the Great Lakes as a hub for regional spread (see chapter 12).
Both mussels are capable of having significant adverse economic impacts, mainly through fouling of water intake facilities, including hydroelectric units, other power plants, and municipal water supply plants. However, even for these important species, economic data are incomplete; the best evidence comes from hydro plants, which individually spend between $400,000 and $1,500,000 Canadian per year to prevent colonization (Colautti et al. 2006). Connelly et al. (2007) placed the total expenditure of water treatment plants and hydro installations at $267 million U.S. between 1989 and 2004, or about $44,000 per facility per year. Estimates by both Colautti et al. (2006) and Connelly et al. (2007) are far lower than the projections of the U.S. Fish and Wildlife Service’s estimate of $5 billion over 10 years. Colautti et al.’s (2006) value was based on tractable direct expenses, whereas the latter was based upon an extrapolation for all manufacturers and municipalities using raw water from the system.
Damage and control costs associated with the quagga mussel invasion of Lake Mead could be much higher owing to the presence of the massive Hoover Dam hydro works on that system. Leung et al. (2004) estimated that it was cost-effective to spend up to $324,000 U.S. annually to prevent colonization of a single midwestern lake with a large power plant located on it. Many other economic changes wrought by zebra and quagga mussels are nonmarket (e.g., extirpation of native unionid mussels) and not well studied (see chapter 12).
Round gobies [Apollonia melanostoma (= Neogobius melanostomus)] (figure 10.3) were first reported in North America from Lake St. Clair (see figure 10.2) in 1990 (Jude et al. 1992). Another Black Sea native, this species was likely introduced via ballast water (table 10.1). Round gobies have spread to each of the Great Lakes, often forming very large populations. The species has also been found in the lower Gulf of St. Lawrence, in the CSSC, in at least two inland lakes in Michigan, and in Pefferlaw Brook, a tributary of Lake Simcoe, a large inland lake in Ontario. Gobies may disperse naturally or in ballast water within the Great Lakes, or as unrecognized baitfish contaminants to inland systems. A population in Pefferlaw Brook, Canada, was subjected to a $250,000 Canadian eradication campaign during 2005 to protect a recreational fishery valued at approximately $200 million Canadian per year. While the eradication effort seemed successful initially, gobies were found in the same system a year later. Round gobies may have a wide variety of trophic effects, including adverse effects on recruitment of native fishes via predation on their eggs, but also possibly beneficial consumption of smaller size classes of zebra mussels—a preferred prey item (Bauer et al. 2007). Ominously, infected round gobies may have contributed to the spread of VHS (table 10.1) in the Great Lakes owing to the large biomass “reservoir” they represent. If this is correct, it represents a form of “invasional meltdown,” where the dispersal and impact of one invasive species is facilitated by another (Ricciardi 2001).
Round gobies also have been implicated in die-offs of diving waterfowl (e.g., common loons, mergansers), involving a chain of events beginning with growth and consumption of Clostridium botulinum (type E) bacteria by zebra mussels, which in turn are eaten by round gobies, which are consumed by waterfowl, which then fall critically ill (Yule et al. 2006). The economic impact of both VHS and botulism poisoning is not known, though it could be enormous, particularly for VHS because it affects more than 40 species of sport and commercial fishes in the basin.
Largely in response to invasion of the Great Lakes by round gobies, an electrical barrier was constructed in the CSSC to prevent spread of the fish to the Mississippi drainage via the Illinois River (figure 10.2). By the time the $1.3 million U.S. barrier was constructed and operational, round gobies had already passed downstream. This barrier does, however, provide a serendipitous defense against movement of bighead carp (Hypophthalmichthys nobilis) and silver carp (H. molitrix) into the Great Lakes from the Mississippi River. A second barrier, valued at $9.1 million U.S., has been constructed as a backup defense but is not yet operational. While spread of either of these fishes to the Great Lakes is highly undesirable, they are cultured and sold as food elsewhere, and bighead carp are harvested from the Upper Mississippi River for food. There is a high risk that Asian carp could spread via the CSSC to the Great Lakes, although it is uncertain whether the species would thrive in the Great Lakes (Kolar and Lodge 2002; Herborg et al. 2007). Because the species are also sold in Asian food stores in both the United States and Canada, these stores could provide a secondary mechanism of introduction of the species to the Great Lakes. This possibility seems remote, however, because all Great Lakes states and the province of Ontario have implemented bans on live sale, possession, or transport of these fishes.
Biological invasions have clearly wrought irrevocable changes to the nature of food webs in the Great Lakes and how humans interact with those resources. In the United States alone, the total economic loss due to invasive species is estimated to exceed $120 billion annually (Pimentel et al. 2005). No clear estimates exist for the monetary costs of invasive species in the Great Lakes, but the annual total must certainly be in the billions of dollars. Furthermore, the impact of invasions on Great Lakes ecosystems and society clearly have ecological and nonmarket costs, and the latter are difficult to quantify. For example, the extirpation by zebra mussels of native unionid mussels (some of which are of conservation concern) in many inland lakes has left “graveyards” of unionid shells in place of once-thriving native mussel beds; such ecological costs could be quantified using the frameworks outlined in chapter 8, although this has not yet been done.
With the attendant ecological and economic impacts of NIS in the Great Lakes, the system serves as a useful model to illustrate how NIS management can benefit from an adaptive tiered approach. Virtually all experts recognize the inherent value of prevention of invasions (see Ruiz and Carlton 2003; Lodge et al. 2006), and in the current chapter, the focus on vectors, timing, and identity of species invading the Great Lakes has a number of strengths. First, explicit prioritization of vectors allows funds and efforts to be focused on mechanisms most important in transmitting NIS to the lakes. The value of this approach is that we can prevent invasions both by the many species we are aware of and by others not yet identified but that may use a particular vector. Second, the number of species invasions prevented is most likely to be maximized by prioritizing and eliminating the strongest vectors to the lakes. Thus, recent patterns of invasion to the Great Lakes indicate that management of ballast of ships arriving from Europe should reduce the risk of future invasions (e.g., see figure 10.1, table 10.1). Analyses of invasion timelines and the identity of particular NIS can help inform the efficacy of current management programs as well as direct future programs. For example, although midocean BWE policies for transoceanic ships reduces the risk of introducing species intolerant of high salinity, these strategies appear to have been less effective for sediment-dwelling species or those capable of producing resistant resting stages. For example, nine NIS were likely introduced in ballast sediment since ballast water control policies were implemented in 1993 (see table 10.1). This information underpinned recent programs aimed at the management of NOBOB residuals in the Great Lakes (U.S. Coast Guard 2005; Canada Shipping Act 2006). As of 2008, all vessels from non-North American source ports must flush ballast water and/or ballast residuals before entering the Great Lakes if they intend to perform any ballast discharges while operating on the Great Lakes. This policy should effectively eliminate new introductions of European or Asian species via the ballast vector. Because of time lags, however, it might be some time before the efficacy of this policy can be assessed.
Modeling efforts may be useful to identify whether specific organisms pose an invasion risk based upon assessments of life history attributes, propagule pressure, environmental suitability, or a combination of these approaches (e.g., Kolar and Lodge 2002; Muirhead and MacIsaac 2005; Herborg et al. 2007). These approaches will likely be limited to only those species for which excellent background information exists and that are perceived as potentially problematic (e.g., Chinese mitten crabs Eriocheir sinensis, Asian carps). However, managers can utilize the output of these models to discriminate between NIS that may or may not establish and spread, and those likely to have large versus small impacts. Management efforts could be tailored accordingly to guard against introduction of those NIS most likely to survive and become problematic in the Great Lakes.
A focus on prevention cannot be expected to prevent all invasions. In such cases, early detection is desirable, although often difficult. Once new NIS incursions are detected, scientific risk assessments are required to determine the appropriate management response. Some NIS may be perceived as having little potential for establishment, spread, or impact following establishment. These assessments can often be made by examining the life history attributes of the species (e.g., Kolar and Lodge 2002) and interspecific interactions and economic impacts in regions where the species is established. If the risk of establishment and/or the risk of adverse impacts is deemed to be low, then it might be appropriate to take no further action other than managing the vector that was responsible for the introduction. If the species is deemed a moderate to high risk, then additional actions may be warranted. These actions consist of eradication or, failing that, a control-the-spread strategy. The number of invertebrate, aquatic NIS that have been successfully eradicated is quite small (e.g., black striped mussel Mytilopsis in Australia and green alga Caulerpa in California; Bax et al. 2002; Williams and Schroeder 2004). A central problem is detection of the incursion at a sufficiently early stage that the population size and range of an NIS are very small and relatively easy to manage. Nevertheless, cases may occur where it is economically advantageous to establish monitoring programs to facilitate early detection of nascent invasions, particularly where the potential for biofouling is large or the threat to native biodiversity is great. The 100th Meridian Project was designed with this in mind (see chapter 12), although the recent establishment of quagga mussels in the western United States highlights the difficulty in completely eliminating vector activity. Creation of barriers to dispersal, including the electrical field barrier in the CSSC, is an example of a control-the-spread strategy that may be effective not only for target species (e.g., silver and bighead carp) but other NIS, as well.
When prevention and eradication are ineffective, managers and society must adapt to life with the established NIS. At this point, managers are essentially helpless with respect to distribution of the NIS, as for the case with dreissenid mussels in the Great Lakes. Here, management efforts may consist of limiting damage associated with the NIS by controlling its local or regional abundance, as is done on the Great Lakes through chlorination of water intake pipelines to reduce mussel bio-fouling, and application of biocides to specific streams to reduce recruitment of sea lamprey. In a limited number of cases, new markets may be created to exploit the NIS, thereby reducing abundance and economic or ecological impact, as has been done by instituting a bighead carp fishery on the Upper Mississippi River.
In summary, the introduction of NIS has emerged as a critically important form of human-mediated global change. The Great Lakes have been highly receptive to NIS and are now greatly disturbed by them, with society bearing the economic impacts of those invasions. Most evidence points to a small number of vectors, especially ballast contents, as the predominant source of new NIS to the Great Lakes. Development of appropriate strategies to manage NIS in the Great Lakes is clearly a work in progress, but much can be learned from previous invasions both within and outside of the basin to shape management programs of the future.
Acknowledgments We are grateful for funding from the National Science Foundation, the Natural Sciences and Engineering Research Council, and the Canadian Aquatic Invasive Species Network.
Bailey, S. A., I. C. Duggan, P. T. Jenkins, and H. J. MacIsaac. 2005. Invertebrate resting stages in residual ballast sediment of transoceanic ships. Canadian Journal of Fisheries and Aquatic Sciences 62:1090–1103.
Bauer, C. R., A. M. Bobeldyk, and G. A. Lamberti. 2007. Predicting habitat use and trophic interactions of Eurasian ruffe, round gobies, and zebra mussels, in nearshore areas of the Great Lakes. Biological Invasions 9:667–678.
Bax, N., K. Hayes, A. Marshall, D. Parry, and R. Thresher. 2002. Man-made marinas as sheltered islands for alien marine organisms: establishment and eradication of an alien invasive marine species. Pages 26–39 in C. R. Veitch and M. N. Clout, editors. Turning the tide: the eradication of invasive species. Proceedings of the International Conference on Eradication of Island Invasives. IUCN Species Survival Commission No. 27. International Union for Conservation of Nature, Gland, Switzerland.
Bij de Vaate, A., K. Ja d
d ewski, H. A. M. Ketelaars, S. Gollasch, and G Van der Velde. 2002. Geographical patterns in the range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59:1159–1174.
ewski, H. A. M. Ketelaars, S. Gollasch, and G Van der Velde. 2002. Geographical patterns in the range extension of Ponto-Caspian macroinvertebrate species in Europe. Canadian Journal of Fisheries and Aquatic Sciences 59:1159–1174.
Canada Shipping Act. 2006. Ballast water control and management regulations. SOR/2006-129.
Cohen, J., N. Mirotchnick, and B. Leung. 2007. Thousands introduced annually: the aquarium pathway for non-indigenous plants to the St Lawrence Seaway. Frontiers in Ecology and the Environment 5:528–532.
Colautti, R. I., S. A. Bailey, C. D. A. van Overdijk, K. Admunsen, and H. J. MacIsaac. 2006. Characterized and projected costs of nonindigenous species in Canada. Biological Invasions 8:45–59.
Colautti, R. I., A. J. Niimi, C. D. A. van Overdijk, E. L. Mills, K. Holeck, and H. J. MacIsaac. 2003. Spatial and temporal analysis of transoceanic shipping vectors to the Great Lakes. Pages 227–246 in G. M. Ruiz and J. T. Carlton, editors. Invasive species: vectors and management strategies. Island Press, Washington, DC.
Connelly, N. A., C. R. O’Neill Jr., B. A. Knuth, and T. L. Brown. 2007. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environmental Management 40:105–112.
Cook, D. C., M. B. Thomas, S. A. Cunningham, D. L. Anderson, and P. J. De Barro. 2007. Predicting the economic impact of an invasive species on an economic system. Ecological Applications 17:1832–1840.
Costanza, R., R. d’Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, S. Naeem, K. Limburg, J. Paruelo, R. V. O’Neill, R. G. Raskin, P. Sutton, and M. van den Belt. 1997. The value of the world’s ecosystem services and natural capital. Nature 387:253–260.
Costello, C., J. M. Drake, and D. M. Lodge. 2007. Evaluating an invasive species policy: ballast water exchange in the Great Lakes. Ecological Applications 17:655–662.
Cristescu, M. E. A., P. D. N. Hebert, J. D. S. Witt, H. J. MacIsaac, and I. A. Grigorovich. 2001. An invasion history for Cercopagis pengoi based on mitochondrial gene sequences. Limnology and Oceanography 46:224–229.
Dehnen-Schmutz, K., J. Touza, C. Perrings, and M. Williamson. 2007. A century of plant trade and its impact on invasion success. Diversity and Distributions 13:527–534.
Drake, J. M., and D. M. Lodge. 2004. Global hot spots of biological invasions: evaluating options for ballast-water management. Proceedings of the Royal Society of London Series B 271:575–580.
Drake, J. M., and D. M. Lodge. 2007. Hull fouling is a risk factor for intercontinental species exchange in aquatic ecosystems. Aquatic Invasions 2:121–131.
Eakins, N. 1999. Lakers and salties 1998–1999. Canadian Coast Guard, Point Edward, Ontario.
Eakins, N. 2000. Lakers and salties 1999–2000. Canadian Coast Guard, Point Edward, Ontario.
Gollasch, S. 2002. The importance of ship hull fouling as a vector of species introductions into the North Sea. Biofouling 18:105–121.
Grigorovich, I. A., R. I. Colautti, E. L. Mills, K. Holeck, A. Ballert, and H. J. MacIsaac. 2003. Ballast-mediated animal introductions in the Laurentian Great Lakes: retrospective and prospective analyses. Canadian Journal of Fisheries and Aquatic Sciences 60:740–756.
Herborg, L.-M., N. E. Mandrak, B. C. Cudmore, and H. J. MacIsaac. 2007. Comparative distribution and invasion risk of snakehead and Asian carp species in North America. Canadian Journal of Fisheries and Aquatic Sciences 64:1723–1735.
Horvath, T. G., and G. A. Lamberti. 1997. Drifting macrophytes as a mechanism for zebra mussel (Dreissena polymorpha) invasion of lake-outlet streams. American Midland Naturalist 138:29–36.
Indiana Department of Natural Resources 2005. Largemouth bass virus. www.in.gov/dnr/invasivespecies/LMBV.
Jude, D. J., R., H. Reider, and G. R. Smith. 1992. Establishment of Gobiidae in the Great Lakes basin. Canadian Journal of Fisheries and Aquatic Sciences 49:416–421.
Keller, R. P., and D. M. Lodge. 2007. Species invasions and commerce in living aquatic organisms: problems and possible solutions. BioScience 57:428–436.
Keller, R. P., D. M. Lodge, and D. C. Finnoff. 2007. Risk assessment for invasive species produces net economic benefit. Proceedings of the National Academy of Sciences of the United States of America 104:203–207.
Kelly, D. W. 2008. Vectors and pathways for nonindigenous aquatic species in the Great Lakes. Committee on the St. Lawrence Seaway: Options to eliminate introduction of nonindigenous species into the Great Lakes, phase 2. In: Great Lakes Shipping, Trade, and Aquatic Invasive Species, Transportation Research Board Special Report 291, National Research Council of the National Academies, Washington, DC.
Kelly, D. W., J. R. Muirhead, D. D. Heath, and H. J. MacIsaac. 2006. Contrasting patterns in genetic diversity following multiple invasions of fresh and brackish waters. Molecular Ecology 15: 3641–3653.
Kolar, C. S., and D. M. Lodge. 2002. Ecological predictions and risk assessment for alien fishes in North America. Science 298:1233–1236.
Levine, J. M., and C. M. D’Antonio. 2003. Forecasting biological invasions with increasing international trade. Conservation Biology 17:322–326.
Leung, B., J. M. Drake, and D. M. Lodge. 2004. Predicting invasions: propagule pressure and the gravity of Allee effects. Ecology 85:1651–1660.
Locke, A., D. M. Reid, W. G. Sprules, J. T. Carlton, and H. C. van Leeuwen. 1991. Effectiveness of mid-ocean exchange in controlling freshwater and coastal zooplankton in ballast water. Canadian Technical Report of Fisheries and Aquatic Sciences No. 1822, Great Lakes Laboratory for Fisheries and Aquatic Sciences, Burlington, Canada.
Lodge, D. M., S. Williams, H. J. MacIsaac, K. R. Hayes, B. Leung, S. Reichard, R. N. Mack, P. B. Moyle, M. Smith, D. A. Andow, J. T. Carlton, and A. McMichael. 2006. Biological invasions: recommendations for U.S. policy and management. Ecological Applications 16:2035–2054.
MacIsaac, H. J. 1996. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. American Zoologist 36:287–299.
Martinez, M. L., A. Intralawan, G. Vazquez, O. Perez-Maqueo, P. Sutton, and R. Landgrave. 2007. The coasts of our world: ecological, economic and social importance. Ecological Economics 63:254–272.
Mellina, E., J. B. Rasmussen, E. L., and Mills. 1995. Impact of mussel (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Canadian Journal of Fisheries and Aquatic Sciences 52:2553–2573.
Mills, E. L., J. H. Leach, J. T. Carlton, and C. L. Secor. 1993. Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. Journal of Great Lakes Research 19:1–54.
Muirhead, J. R., and H. J. MacIsaac. 2005. Development of inland lakes as hubs in an invasion network. Journal of Applied Ecology 42:80–90.
Nelson, R. 2003. Exotic spring viremia of carp virus confirmed in common carp taken from the Calumet-Sag channel near Chicago, Illinois. U.S. Fish and Wildlife Service. http://news.fws.gov/newsrelease/r3/E5DE11CB-EF51-49E8-A147A9955185C7C9.html. Pimentel, D., R. Zuniga, and D. Morrison. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52:273–288.
Ricciardi, A. 2001. Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? Canadian Journal of Fisheries and Aquatic Sciences 58:2513–2525.
Ricciardi, A. 2006. Patterns of invasion in the Laurentian Great Lakes in relation to changes in vector activity. Diversity and Distributions 12:425–433.
Ricciardi, A., and H. J. MacIsaac. 2000. Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends in Ecology and Evolution 15:62–65.
Ricciardi, A., and J. B. Rasmussen, 1998. Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. Canadian Journal of Fisheries and Aquatic Sciences 55:1759–1765.
Rixon, C. A. M., I. C. Duggan, N. M. N. Bergeron, A. Ricciardi, and H. J MacIsaac. 2004. Invasion risks posed by the aquarium trade and live fish markets on the Laurentian Great Lakes. Biodiversity and Conservation 14:1365–1381.
Roman, J., and J. A. Darling. 2007. Paradox lost: genetic diversity and the success of aquatic invasions. Trends in Ecology and Evolution 22:454–464.
Ruiz, G. M., and J. T. Carlton. 2003. Invasion vectors: a conceptual framework for management. Pages 459–504 in G. M. Ruiz and J. T. Carlton, editors. Invasive species: vectors and management strategies. Island Press, Washington, DC.
Ruiz, G. M., P. W. Fofonoff, J. T. Carlton, M. J. Wonham, and A. H. Hines. 2000. Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annual Review of Ecology and Systematics 31:481–531.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker, and D H. Wall. 2000. Global biodiversity scenarios for the year 2100. Science 287:1770–1774.
Stokstad, E. 2003. Can well-timed jolts keep out unwanted exotic fish? Science 301:157–158.
Tatum, A. J., and S. I. Hay. 2007. Climatic similarity and biological exchange in the worldwide airline transportation network. Proceedings of the Royal Society Series B 274:1489–1496.
Tatum, A. J., D. J. Rogers, and S. I. Hay. 2006. Global transport networks and infectious disease spread. Advances in Parasitology 62:293–343.
U.S. Coast Guard. 1993. Ballast water management for vessels entering the Great Lakes. 33 CFR 151.1510.
U.S. Coast Guard. 2005. Ballast water management practices for NOBOB vessels. 33 CFR Part 151.2035 Fact Sheet. uscg.mil/hq/gm/mso/docs/fact_sheet_nobobbmps.pdf.
U.S. Policy Committee for the Great Lakes. 2002. Great Lakes Strategy 2002: a plan for the new millennium. U.S. Policy Committee for the Great Lakes. http://www.epa.gov/glnpo/gls/gls2002.pdf.
Ward, J. M., and A. Ricciardi. 2007. Impact of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Diversity and Distributions 13:155–165.
Williams, S. L., and S. L. Schroeder. 2004. Eradication of the invasive seaweed Caulerpa taxifolia by chlorine bleach. Marine Ecology Progress Series 272:69–76.
Xu, H., H. Ding, M. Li, S. Qiang, J. Guo, Z. Han, Z. Huang, H. Sun, S. He, H. Wu, and F. Wan. 2007. The distribution and economic losses of alien species invasion to China. Biological Invasions 8:1495–1500.
Yule, A. M., I. K. Barker, J. W. Austin, and R. D. Moccia. 2006. Toxicity of Costridium botulinum type E neurotoxin to Great Lakes fish: implications for avian botulism. Journal of Wildlife Diseases 42:479–493.