
In a Clamshell
Since the discovery of zebra mussels and quagga mussels in North America, dreissenid mussels have driven recent U.S. policy development on invasive species. Combining past studies on dreissenid mussels with our current research, we present an unusually (perhaps uniquely) complete synthesis of the entire invasion sequence from both ecological and economic perspectives. This chapter demonstrates models of potential spread, ecological niche models, assessments of the factors that influence establishment, estimates of economic impacts, integrated optimization modeling of the value of slowing the spread, and assessment of the behavior of decision makers. To bring all these ideas into focus, this chapter asks what it is worth to keep dreissenid mussels from becoming established in western states and provinces of North America, as a focal point to our research. Answering this question relies not only on objective ecological and economic estimates of critical variables at the intersection of the intertwined systems, but on the perspectives and attitudes of policy makers about investments in prevention and control. Given limited financial resources, there is no single dollar amount to answer the question; the answer lies in the accuracy of the models and the behavior and priorities of managers and decision makers. This chapter demonstrates a piece-by-piece development of a framework for a comprehensive bioeconomic assessment, which should be useful for assessing the risks of other invasive species.
January 2007 began a new chapter in the invasion of dreissenid mussels in North America. For the first time, dreissenid mussels were not restricted to the eastern United States and Canada. On January 6, 2007, quagga mussels (Dreissena bugensis (= D. rostriformis bugensis [Andrusov (1897)])) were discovered in a marina in Lake Mead, a large reservoir near Las Vegas, Nevada, on the Colorado River (National Park Service 2007). Preventing this invasion from happening has been the goal of the 100th Meridian Initiative, a cooperative organization including state, federal, and provincial agencies, which was established in 1998. For the past 4 years, we (in collaboration with David Lodge and many of the other contributors in this book) have been working to shed light on two basic questions: What is it worth to keep dreissenid mussels from becoming established in the western states and provinces of North America? How much information and/or modeling is needed to make a policy recommendation given all the uncertainties in the invasion process? Answering these questions requires ecological predictions of dispersal, potential habitat, probability of establishment, and likely abundance and also estimates of direct and indirect economic impacts, the incorporation of policy time horizons, and the behavior of those individuals making decisions. Given these multifaceted and interlinked components, the insights we have generated are incomplete, yet provide a clear example of the research necessary to (eventually) integrate ecology and economics for improved decision making. This integration apparently has been lacking, as agencies of the western United States may now find out sooner than we expected if dreissenid mussels will have the similar economic and ecological impacts as they have had in eastern North America.
In 1986 zebra mussels (Dreissena polymorpha) were discovered in Lake St. Clair (Herbert et al. 1989) near Detroit, Michigan, and Windsor, Ontario, and in 1991 quagga mussels were introduced in Lake Ontario (May and Marsden 1992). These introductions occurred even though the dispersal capabilities of dreissenid mussels were known as far back as 1893 and their potential economic impact as early as 1959, including a report for the Environmental Protection Service of Canada in 1981 (for a list of predictions, see Carlton 1991). Despite these warnings, there was no coordinated effort to prevent these invasions from occurring. Dreissenid mussels are estimated to currently cost U.S. industries millions/year (O’Neill 1997). Additionally, they have caused the local extinction of many native mollusks (Strayer and Smith, 1996), have changed the structure of fish communities in the Hudson River (Strayer et al. 2004), and have been implicated in the demise of valuable sport fish populations (Dermott 2001). Given that this invasion was predicted, what would it have been worth to invest to keep dreissenid mussels out of North America?
Early in the North American invasion, Ludyanskiy et al. (1993) went as far as to predict, “Within the next few years, and certainly by the turn of the century, the zebra mussel will be found in almost all parts of the United States and southern Canada.” The rapid spread of zebra mussels through the Great Lakes and shipping routes of the United States soon after their introduction in Lake St. Clair supported such predictions (Allen and Ramcharan 2001). However, from 1993 to 2006, the range of dreissenid mussels did not change much (Johnson et al. 2006). The distribution of dreissenid mussels in 2006 was primarily limited to the Great Lakes, rivers with active shipping connected to the Great Lakes (e.g., Mississippi, Kentucky, Tennessee, and Hudson rivers), and inland lakes within 150 km of the distribution as of 1993 (figure 12.1). As of 2007, this distribution has markedly changed with the discovery of quagga mussels in Lake Mead and other reservoirs of the lower Colorado River.
FIGURE 12.1.
The known distribution of dreissenid mussels in North America in 1989, 1993, and 2007. Data from the U.S. Geological Survey Nonindigenous Aquatic Species information resource (http://nas.er.usgs.gov/).
Though the rate of spread had declined, there was still substantial concern recently as to the future distribution of dreissenid mussels in North America. The State of Minnesota has a major public outreach campaign to limit the number of lakes invaded by dreissenid mussels. As of 2007, only three lakes in Minnesota were known to contain dreissenid mussels, despite the presence of dreissenid mussels in the Mississippi River and Lake Superior for previous 15 years. Likewise, the 100th Meridian Initiative focuses on preventing the westward spread of dreissenid mussels and other aquatic nuisance species in North America. Within the context of this concern, the objective of this chapter is to synthesize the research of this poster child of invasive species within the bioeconomic framework discussed in the central portion of this book. We place particular emphasis on how these tools have been used to assess the risk dreissenid mussels pose to the western United States.
FIGURE 12.2.
General framework of the bioeconomics of the dreissenid invasion of North America, including the mechanisms by which dreissenid mussels transfer between stages and examples of models to predict the success of these transfers. GARP, genetic algorithm for rule-set production; SVM, support vector machine modeling; CGE, computable general equilibrium modeling; SDP, stochastic dynamic programming.
In addition, we hope this chapter provides a synthetic framework by which the bioeconomics of other invasive species can be assessed. This chapter is designed to mimic the overall structure of this book, from predicting the introduction of dreissenid mussels to assessing their regional economic impacts (figure 12.2). Our focus in the chapter is primarily on the later stages of invasion (i.e., secondary spread and impact) and can be compared to the overall invasion process presented in chapter 1 (see figure 1.1). In this chapter, we highlight many of the mechanisms and the tools/models that have been used to understand the dreissenid invasion; specific details of these models can be found in their corresponding chapters in the rest of the book. It should be noted that several stages of the dreissenid mussel invasion have been studied more thoroughly than others, which will be evident in the range and specificity of the research presented in the following sections. This merely suggests that there is plenty of opportunity to further study this process and, more important, the availability of interdisciplinary research opportunities. We expect that through this chapter it becomes apparent that achieving an acceptable answer about the benefits of stopping or slowing the spread of an invasive species requires the integration of ecology, economics, and mathematics at all the stages of an invasion.
Natural history traits can be used to predict whether a species will become invasive in a certain region. Dreissenid mussels have several traits that make them highly suitable as invasive species, including a history of invasion, high fecundity, an ability to withstand aerial exposure, and several unique traits compared with other freshwater bivalves of North America. From a historical analysis of successful and unsuccessful mollusk invaders in this region, it is known that high fecundity is strongly linked to invasion (Keller et al. 2007). Dreissenid mussels have extremely high fecundity relative to other mollusk species; a single female mussel can release > 1,000,000 eggs during her life span (Sprung 1993). The high fecundity rate facilitated rapid range expansion, and due to their fecundity rates, dreissenid mussels have a natural history strongly amenable to invasion.
A second trait that has enabled dreissenid mussels to spread via overland transport in North America is their ability to withstand aerial exposure. In the laboratory, zebra mussels can survive out of water for several days in temperate summer conditions. Quagga mussels were able to survive some aerial exposure, but their percent weight loss was higher and percent survival was lower than those of zebra mussels (Ricciardi et al. 1995). This difference in aerial tolerance fit with the known patterns of zebra and quagga mussels as of 2006. Through 2006, quagga mussels were known only in the Great Lakes, and all the inland lake infestations had been reported as zebra mussels, although we suspect that few of these populations were examined closely to determine if the mussels were zebra mussels or quagga mussels. Contrary to the lower aerial exposure tolerances of quagga mussels, they were the species that made the longest overland jump of more than 1,500 km from the Great Lakes region to the Colorado River. Adult dreissenid mussels, subjected to aerial exposure, have been found on several occasions on boats in many western states (100th Meridian Initiative 2007). It should also be noted that many boats have live wells, bilge tanks, and other compartments that are capable of transporting water, and thus also living mussels or juveniles, eliminating the need for dreissenid mussel to survive aerial exposure. In general, the aerial tolerance of dreissenid mussels has evidently been important in their capabilities for long-distance dispersal.
Beyond their high fecundity and ability to withstand aerial exposure, dreissenid mussels have several unique traits compared with other freshwater bivalves in North America (Mackie 1991). Native freshwater bivalves of North America live their entire adult lives partially buried in sediments in lakes and streams (i.e., infaunal), and the larvae are either brooded (ovoviviparous, Sphaeriidae clams) or parasitic (glochidium, Unionidae clams). The glochidium larvae usually exist on the skin, fins, or gill of fish, and many species are host specific (Parmalee and Bogan 1998). As opposed to native unionids, dreissenids have a pelagic larval stage that enables passive dispersal via water currents. Dreissenid mussels are also epifaunal; that is, they live attached to and above the substrate. The epifaunal nature of dreissenid mussels allows these new species to utilize habitat not used by native bivalves in the freshwaters of North America. Not only do dreissenid mussels colonize natural substrates, such as rock outcroppings, but they also are abundant on human structures, including buoys, boats, docks, and locks. A trait-based assessment of dreissenid mussels would thus have been sufficient to identify dreissenid mussels as being extremely high-risk species long before they became established in the Great Lakes (Carlton 1991).
In addition to species traits increasing the likelihood of invasion success, species that have a history of invasion elsewhere tend to be successful invaders in other similar regions (Kolar and Lodge 2001). Dreissenid mussel are native to the Ponto-Caspian basin in Eastern Europe; during the 1700s and 1800s this region was connected to waterways across Europe by the construction of canals, and by the 1830s zebra mussels had expanded their range to include much of Europe and Britain. Likewise, after their introduction into the Great Lakes, dreissenid mussels quickly expanded their range throughout the navigable waterways that are connected to the Great Lakes. The most recent “island” to become invaded by zebra mussels was Ireland, in 1994, even though Britain has had zebra mussels since 1824 (Pollux et al. 2003). Given their past success as invaders throughout Europe and eastern North America and the similarities of western North America to these previously invaded locations, a clear warning of the potential for dreissenid mussels to become established, and rapidly spread, in western North America should have been heeded.
Dreissenid mussels have the right traits to be good invaders, but are all the water bodies across North America equally suitable habitat? Many techniques have been used to model the ecological niches of invasive species (see chapter 4), including several attempts to forecast the potential distribution of zebra mussels in North America, with no specific efforts on predicting the potential habitat of quagga mussels (but see Thorp et al. 2002). These forecasts have included nonspatial models based on water-quality parameters (Ramcharan et al. 1992; Mellina and Rasmussen 1994), regional models that use specific lake and geology parameters (e.g., Neary and Leach 1992; Koutnik and Padilla 1994), and national models based on low-resolution data such as air temperature, frost frequency, and geology (Strayer 1991; Drake and Bossenbroek 2004). The consistent theme through all of these predictions is that water quality or a surrogate, such as bedrock geology, is essential to predicting what bodies of water would allow dreissenids to thrive if they were introduced.
To assess the potential habitat for dreissenid mussels west of the 100th meridian, analyses have been conducted at both regional and local scales. On a national scale, the suitable habitat for zebra mussels has been predicted using a genetic algorithm for rule-set production (GARP; see chapter 4) based on 11 environmental and geologic variables, which were at a resolution of 0.1 decimal degrees (Drake and Bossenbroek 2004), and solely using data on calcium concentration in water (Whittier et al. 2008). The final GARP model was based on five variables thought to be biologically relevant to the distribution of zebra mussels, including frost frequency, maximum annual temperature, slope, bedrock geology, and surface geology. The results suggest that much of the western United States may not be as susceptible to zebra mussel invasion as previously thought, or compared to predictions of previous models based on temperature variables alone (Strayer 1991). The results, however, do predict that certain areas of the Columbia, Colorado, and Sacramento-San Joaquin river basins are at significant risk (figure 4.3; Drake and Bossenbroek 2004). Whittier et al. (2008) predicted the potential distribution of zebra mussels for each of approximately 60 ecoregions in the United States based on calcium concentrations. The results of this effort were fairly similar to the results of the GARP model, particularly in the Great Plains and Midwest. The differences between the Whittier model and the GARP model were most evident in the Southwest, where the Whittier model predicts higher risk of suitable habitat, and in the Southeast and Northeast, where it predicts a lower risk. The Whittier et al. (2008) results are important because they show that the suitability of Lake Mead and other southwestern reservoirs was predictable.
On a more localized scale, detailed water-quality data were collected to predict the potential densities of zebra mussels if they were introduced in lakes Mead and Roosevelt (Bossenbroek et al. 2007). Population density, the effect of each individual, and the overall range of a species are three key indicators of the overall impact (both ecological and economic) of an invasive species (Parker et al. 1999). Using a previously published model (Ramcharan et al. 1992), predicted abundances of zebra mussels were compared to those of water bodies with reported zebra mussel density. Based on these data and model, density predictions suggest that both Lake Mead (Colorado River) and Roosevelt Lake (Columbia River) could support substantial population densities of zebra mussels (Bossenbroek et al. 2007). Lake Mead is expected to have densities reaching hundreds of thousands per square meter, whereas Roosevelt Lake would most likely maintain more moderate populations in the thousands per square meter. High densities of zebra mussels in lakes Mead and Roosevelt would likely lead to substantial economic impacts because these bodies of water contain more infrastructure than most lakes and rivers in the Midwest, including hydropower dams, municipal water supply systems, and irrigation pumps.
As demonstrated, several tools have now been used to predict the potential distribution and niche boundary of zebra mussels. The compilation of these studies suggests that the areas most at risk to future invasions include the southeastern and southwestern United States and the regions already invaded by dreissenid mussels, that is, the Great Lakes region. These models do acknowledge that suitable habitat for dreissenid mussels exists in many areas throughout the country that have not yet been invaded.
The availability of suitable habitat does not guarantee that a lake will become invaded, which requires the introduction of viable propagules and establishment of a population. Dreissenid mussels have several modes by which they can disperse, both natural and human mediated (Johnson and Padilla 1996). The arrival of dreissenid mussels within North America is believed to be the result of ballast discharge from ships originating from the Ponto-Caspian sea region (Mills et al. 1993), originally in Lake St. Clair. From this initial point of introduction, natural dispersal of dreissenid mussels occurred primarily due to the movement of water, which has been shown in large river systems and coupled lake-stream systems. Coupled lake-stream systems constitute a source-sink model of zebra mussel dispersal (Horvath et al. 1996; Bobeldyk et al. 2005), such that in-stream dreissenid mussel populations are often not self-sustaining but are dependent on continuous recruitment from source populations of the upstream lake. Although populations may not easily establish in moving water, live veligers (the planktonic juvenile stage of dreissenid mussels) can travel more than 300 km in larger rivers, such as the Illinois River (Stoeckel et al. 1997). The downstream dispersal of zebra mussels through streams results in establishment of populations in streams and downstream lakes (Bobeldyk et al. 2005) and is thought to be the source of approximately one-third of all the inland lake invasions (Johnson et al. 2006). The presence of wetlands in streams, however, can inhibit this downstream spread (Bodamer and Bossenbroek 2008). Downstream movement of quagga mussels has already occurred in the lower Colorado River. Lakes Mohave and Havasu have quagga mussels and are downstream of Lake Mead, which is assumed is the initial point of introduction in the Colorado system.
Without the aid of flowing water, long-distance dispersal of dreissenid mussels requires a human vector. After their initial introduction into the Great Lakes, ships and barges played a substantial role in the dispersal of zebra mussels. By 1993, almost all of the navigable waters of eastern North America were invaded with zebra mussels (Allen and Ramcharan 2001). The overland transport to inland lakes also requires an additional human vector, most notably recreational boating (Johnson and Carlton 1996). The spread of zebra mussels via recreational boaters has been modeled on several occasions with the use of gravity models (Schneider et al. 1998; Bossenbroek et al. 2001; Leung et al. 2006; Bossenbroek et al. 2007; for detailed description, see chapters 6 and 7). Linking the spread of invasive species with the movement of people enables integration with geography and economics. Thus far, several invasive species studies have used gravity models, frequently employed by geographers, but there is abundant opportunity to use recreational demand models, which economists use to analyze recreation choices, including consumer behavior.
Based on an understanding of the mechanisms of spread, Bossenbroek et al. (2007) constructed a gravity model to explore the movement patterns of recreational boaters from areas with zebra mussels on a national scale (figure 12.3). The parameters of the model were estimated by comparing model results with survey data collected via the 100th Meridian Initiative. The model results are consistent with the observed slow range expansion of dreissenid mussels in recent years, with the exception of the newly discovered population of quagga mussels in Lake Mead. The model would have predicted the invasion of Lake Mead to be a low probability event, yet more probable than the invasion of most other reservoirs in the western United States and even more probable than many lakes and reservoirs in the eastern United States. The uncertainty involved with these predictions is assessed in detail in chapter 7. Although the Bossenbroek et al. (2007) article was focused on zebra mussels, we believe the model is indicative of the movement patterns that should be exhibited by quagga mussels.
We have updated the national gravity model of Bossenbroek et al. (2007) by considering lakes Mead, Havasu, and Mohave on the Colorado River and the Lake of the Ozarks in Missouri as additional sources of dreissenid mussels (J. M. Bossenbroek unpublished data). Because there are now more sources of dreissenid mussels, the probability of invasion for every lake in the country has increased. The question becomes whether these new sources substantially increase the risk of invasion to noninvaded lakes, such as Roosevelt Lake in the Columbia River. The original gravity model prediction was that 89 boats (on a relative scale) from dreissenid-infested waters would travel to Roosevelt Lake. The additional sources increased the number of potentially infested boats traveling to Roosevelt Lake by 25% to 114 boats. Thus, Roosevelt Lake is not predicted to have a high risk of introduction compared to many other watersheds in the Great Lakes region (see figure 12.3). The introduction of quagga mussels in Lake Mead was also surprising based on an assessment of the current and past distribution of dreissenid mussels at several scales, which quantified the slow range expansion and the low occurrence of long-overland dispersal events (Kraft and Johnson 2000). As of 2003, only six states had more than 10 inland invasions observed, and they account for 97% of the 293 lakes reported to be invaded in the United States (Johnson et al. 2006). For the four states surrounding Lake Michigan, <8% of suitable lakes (based on Drake and Bossenbroek 2004) larger than 25 hectares had been invaded by 2003. Although the number of invaded lakes has increased over time, the rate of invasions has decreased (figure 12.4). The reasons for this decline in invasion rate in the past several years, despite hundreds of available lakes, could be the result of education efforts, the limited attractiveness to boaters of many lakes, or some indication of a necessary threshold of propagules required for a population to become established.
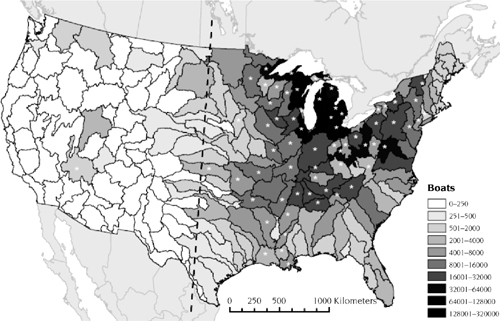
FIGURE 12.3.
Relative predicted number of boats from water bodies invaded by dreissenid mussels traveling to each watershed within the United States as calculated with a gravity model. An asterisk indicates that dreissenid mussels have been observed in the watershed on at least one occasion. The dashed line specifies the 100th meridian. Modified from Bossenbroek et al. (2007).
Little is known about how many dreissenid mussels (juveniles or adults) must be introduced to begin a new population in a previously uninfested and unconnected water body. Dreissenid mussels dispersed by recreational boaters can be transported by several mechanisms, including adult mussels attached to parts of boats or attached to macrophytes entangled in a boat or a trailer, and juveniles contained in any pocket of water remaining in a boat, such as a live well or bilge (Johnson and Carlton 1996). In just one example, Johnson and Carlton (1996) examined boats leaving Lake St. Clair and found between 0% and 31% of boats carrying macrophytes with zebra mussels, depending on the day. Depending on the ambient temperature and humidity (Ricciardi et al. 1995) and rate of transport, dreissenid mussels have the ability to survive transport across the entire country. Indeed, quagga mussels in Lake Mead were not the first discovery of dreissenid mussels west of the 100th meridian. On several occasions, boats arrived at Lake Mead with mussels attached (100th Meridian Initiative 2007) but were cleaned prior to launch.
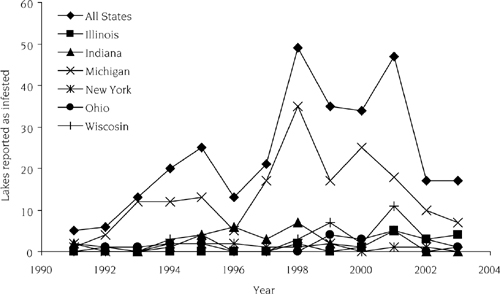
FIGURE 12.4.
Annual numbers of invasions in the six states known to have 10 or more invasions of inland waters and for the entire United States as of 2004. Reproduced from Johnson et al. (2006), with permission of Springer Science and Business Media.
A single boat launched into a lake with dreissenid mussels does not ensure that a population will become established. The probability of a lake becoming invaded with zebra mussels is not linearly related to the number of propagules introduced. Instead, zebra mussels exhibit Allee effects (Courchamp et al. 1999; Leung et al. 2004), which cause inverse density dependence at low densities. Thus, a threshold number of propagules exists above which establishment is much more likely and below which a population is likely to go extinct. There are several possible sources of Allee effects in natural populations, including genetic inbreeding, demographic stochasticity, or a reduction in cooperative interactions or the ability of mates or gametes to encounter. Several aspects of dreissenid mussel life history, including gamete production, gamete release and survival, the external fertilization process, veliger state, and status of settled adults, may be subject to Allee effects. Dreissenid mussels are sexual in their reproduction, thus requiring that gametes be released in close proximity in both space and time. Analyses by A. B. Potapov (unpublished data) suggest that there is a maximum distance of about 10–20 cm between male and female mussels necessary for successful fertilization of gametes. Such a short critical distance thus requires a large population or the introduction of both male and female mussels within close proximity. The existence of Allee effects in dreissenid mussels and the long distance between infested midwestern waters and the large reservoirs of the western United States suggest that the introduction of sufficient numbers of quagga mussels into Lake Mead was a rare event, but one that could be expected based on our gravity modeling described in the preceding section.
Understanding establishment is essential for risk assessment and for policy making. Meshing dispersal and establishment success with the associated economic impacts allows an accurate assessment of the risk invaders pose to society and whether strategies directed toward the problem are worthwhile.
The establishment of dreissenid mussels in the water bodies of North America has caused substantial economic impacts in term of both nonmarket and market costs. Nonmarket costs include direct and indirect impacts on the ecology and are not readily quantifiable because they are linked to environmental goods and services not exchanged in the marketplace. The nonmarket effects of dreissenid mussels include changes in water quality and impacts on other species. Dreissenid mussels alter concentrations of nutrients (e.g., Mellina et al. 1995), increase water clarity (e.g., Fahnenstiel et al. 1995), and can negatively affect native mussels by colonizing their shells and inhibiting filter feeding (Schloesser et al. 1996; Ricciardi et al. 1998). The observed nonmarket impacts of dreissenid mussels are one reason the 100th Meridian Initiative is concerned about their western spread.
Despite the paucity of native mussels in the western United States (NatureServe 2006), there are several nonmarket effects that dreissenid mussels could impose on western rivers and reservoirs. One major concern in the Columbia River basin is the potential risk to the native salmonid species that pass through fish ladders. If fish ladders become encrusted with dreissenid mussels, salmonids could be damaged by rubbing against the sharp shells of the mussels (Northwest Natural Resource Group 2003). Dreissenid mussels have also been associated with changes in the distribution of fish communities in river systems, including declines in open-water species and increases in littoral species (Strayer et al. 2004). The lower Colorado River is home to several endangered fish species (Dobson et al. 1997), which are already threatened by several nonindigenous fish species (Stohlgren et al. 2006). The introduction of dreissenid mussels could further imperil these species. Although nonmarket impacts of dreissenid mussels may be high, we concentrate the remainder of the chapter on market impacts given the complete lack of nonmarket impact estimates. Thus, the potential impacts discussed are likely underestimates of the total value of the impacts (if all impacts are assumed to be detrimental).
In relation to the regions of eastern North America currently affected by dreissenid mussel invasions, the West is more dependent on surface-water supplies for power, drinking water, and irrigation. This dependence highlights the importance of understanding the chances of and potential impacts of a dreissenid mussel invasion. While it has been estimated that the one-time cost to install systems to control zebra mussels at Columbia River hydroelectric projects could range from hundreds of thousands to more than a million dollars each (Phillips et al. 2005), a more complete estimate of the consequences has been lacking. Similar to the assessments of O’Neill (1997) and Pimentel et al. (2005), Phillips et al. (2005) assess only the likely direct impacts of dreissenid mussels on an industry, without examining the opportunity costs of the impacts (as described in chapter 8) and how these impacts propagate throughout the entire economy.
To assess the economy wide impacts of dreissenid mussels on the entire economy of the Columbia River basin, we used a computable general equilibrium (CGE) model to capture both primary and secondary (indirect) economic impacts (as described in Warziniack et al. 2008). In the CGE model, the economy consists of households and producing sectors, linked to one another and the rest of the world through commodity and factor markets (figure 12.5). It is through imports into commodity and factor markets that the CGE model can be linked to the transportation of dreissenid mussels, via a gravity model or random-utility model (see chapter 6). Other potential linkages with the biology of dreissenid mussels include habitat suitability models of water sources (e.g., reservoirs), regional spread models, and population dynamics, including growth and abundance models. These explicit linkages will be incorporated into future work on the CGE model. Because the ecological linkages have yet to be made, our analysis was limited to a binary problem: no dreissenid mussels or a “complete” dreissenid mussel invasion. The “complete” scenario is not unfounded, however, because of the dreissenid mussel invasion dynamics that occurred in eastern North America.
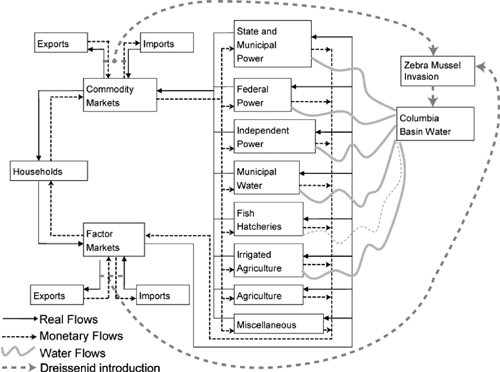
FIGURE 12.5.
Diagram of the Columbia River basin economy used in a computable general equilibrium (CGE) modeling.
In the CGE model, the invasion of dreissenid mussels was incorporated as affecting the production process of waters users. The consequences to these water users were defined based on the observed impacts to independent power companies and municipal water users in the eastern United States (Deng 1996) and estimated impacts on federal hydropower facilities (Phillips et al. 2005). Impacts on irrigated agriculture were also expected and included in the analysis at a similar magnitude as the observed water users. This is a potentially important impact because the Columbia River basin contains more than 5.1 million acres of irrigated farmland and 73.5% ($8.5 billion) of agricultural operating expenditures in the region (U.S. Department of Agriculture 2004). Although impacts on fish hatcheries were also to be expected, no relevant analyses were found to use them in the model, and thus we excluded them (indicated by a dashed gray line in figure 12.5). The industry-specific consequences of a dreissenid mussel invasion are captured in the CGE model by including productivity shocks, reflected by increases in the per-unit output costs of effected industries. Dreissenid mussels cover surfaces and clog intake pipes for industries dependent on water of the Columbia River basin, resulting in costly cleaning and reduced capacity for production.
In the CGE model, industries affected by the zebra mussel invasion were presumed to respond by installing mitigation equipment and hiring people to monitor and control the effects. These responses lead to increases in the cost per unit of output of affected industries (where the estimates range from 0.1% to 0.3% increases and are detailed in Warziniack et al. 2008), which result in declines in productivity of each industry and to efficiency losses. Thus, inputs in the production process, termed “factors of production” by economists, are not as productive in the face of an invasion as they would be without an invasion. Affected industries are not able to produce as much output per unit of input(s) as they were before the invasion, making each unit of output more expensive to produce. The consequences are seen in terms of altered production and input choices by firms and associated changes in all prices, incomes, and choices throughout the regional economy as it adjusts to these changes. The net of all the adjustments across the economy is then evaluated in relation to the noninvaded condition. The procedure extends the single market valuation discussion of chapter 8 into operation in a multimarket, economywide setting that accounts for all direct and indirect effects of the invasion. In this setting, the reciprocal price and income effects from simultaneously shifting demand and supply curves throughout the regional economy are taken into account (although shifts occur in differing degrees and directions). In addition, the method provides aggregate measures of the welfare changes in terms of equivalent variations, or what households would have been willing to pay to not experience the invasion (calculated in relation to the benchmark equilibrium of the noninvaded 2001 data and similar to the consumer surplus measures discussed in chapter 8). The CGE model for the noninvaded economy was based on 2001 IMPLAN data for the Columbia River basin (IMPLAN is the industry standard source of detailed regional economic data developed by the Minnesota IMPLAN Group, Inc.; see www.implan.com).
Given the estimates in changes of unit costs, the potential impacts of dreissenid mussels on the Columbia River basin was estimated to result in an aggregate mean annual welfare loss of roughly $3.31 million, based the CGE model (Warziniack et al. 2008). The standard deviations range from $0.5 million to $1.5 million for that estimate, depending on the precision of the unit-cost impact estimates, where the method of Harrison and Vinod (1992) was employed to account for uncertainty in the unit-cost impact estimates. The method generates unbiased and asymptotically consistent estimators of the welfare change in terms of household equivalent variations, where the only source of uncertainty is the cost impact to industries.
Within the predicted distributions of welfare losses, there are significant differential impacts across households. Households with incomes of $50,000–75,000 bear the largest proportion of mean welfare losses, while those households with incomes of $10,000–15,000 have the smallest mean welfare change (in aggregate). On a per-household basis, welfare impacts range from a low of $1.18 per household (for households with incomes of $25,000–35,000) to a high of $5.22 per household (for households with incomes >$150,000). These results (note that only the mean estimates are reported here) demonstrate a low proportional expected severity of impact from dreissenid mussel invasion of the Columbia River basin and a wide variation in impacts across households. Of course, while a $3 million annual welfare loss may seem substantial to some and not to others, whether it induces a policy response depends not only on what the policy itself might cost and how effective it might be (as noted in chapter 9), but also on how these preferences influence responses of policy makers over time and with differing perceptions of risk.
While the above ecological and economic analyses provide insight into the management of dreissenid mussel invasions, they do not provide specific prescriptions for policy makers. Optimal prescriptions can, however, be provided by merging the population ecology and the potential economic impacts of dreissenid mussels with the economic theory of endogenous risk. Endogenous risk captures the risk-benefit trade-offs created by jointly determined ecosystem conditions, species characteristics, and economic circumstances (as noted in chapter 8 and in Crocker and Tschirhart 1992; Settle et al. 2002). The theory of endogenous risk assumes that people and firms invest scarce resources to change risk. People mitigate risk through prevention (self-protection) efforts to reduce the likelihood of an invasion, and they adapt to risk through control (self-insurance) efforts to reduce the severity of an invasion if it occurs. This framework has been used to examine the risks of biodiversity loss, environmental, and economic damages that dreissenid mussels pose to society using stochastic dynamic programming (SDP; Shogren 2000; Leung et al. 2002).
The optimal decision making of a policy maker (i.e., a resource manager) and (in turn) a private firm (i.e., a power plant) to a potential dreissenid mussel invasion in a Midwest lake was analyzed with an SDP model (Finnoff et al. 2005, 2006, 2007; summarized in part in chapter 9). Optimal decisions were characterized by those of a policy maker that maximized social welfare given the privately optimal choices of the firm (choices that maximize private profit). The manager is the primary decision maker in balancing the risks and benefits of prevention and control, while realizing that private individuals may also make investments to reduce risk. The ecological component of the model incorporates the introduction, establishment, and growth of dreissenid mussels. The SDP model demonstrates (at least) two critical issues relating to human behavior that must be considered in developing integrated optimization models for policy analysis. The first critical issue is to what degree complexity is important for sound policy analysis (as demonstrated in chapter 2). The second critical issue is the attitudes of human decision makers toward time and overt risk, which can have as much influence on optimal decision making as the consequences of the risk.
Variations in a manager’s preferences concerning time and risk will influence the choices on the mix of prevention and control. This is a pertinent issue because although scientists have argued that invasive species can be managed most cost-effectively with greater investments in prevention (Leung et al. 2002; Simberloff 2003), investments in prevention are not typically done (Bossenbroek et al. 2005). In many cases, private and public resources are invested primarily to control existing invaders rather than to prevent new invasions. Managers frequently wait until after invaders have arrived and then scramble to limit the damages. For example, farmers often limit investment in prevention efforts because they perceive the introduction of weeds to be outside of their control, while they are comfortable with control methods such as herbicides (Wilson et al. 2008), even though these decisions may not be economically efficient. These paradoxical decisions can be understood by recognizing the link between typical human preferences over time and for risk bearing with the technology of risk reduction (for complete details, see Finnoff et al. 2006, 2007).
The SDP model applied to the aforementioned zebra mussel invasion of a Midwest lake was used to assess a resource manager’s preferences over time. Variations in preferences across managers were assessed by varying the discount rate for several types of resource managers differentiated by their level of risk aversion, from risk neutral to highly risk averse. Risk preferences are represented in the SDP model by the curvature of the von Neumann-Morgenstern utility function (Holt and Laury 2002), and risk attitudes were varied from risk neutral to highly risk averse.
The key results of the SDP analysis are somewhat counterintuitive: an increase in the discount rate (i.e., shorter time horizon) causes prevention to fall and control to increase (figure 12.6). Managers with greater preferences toward the present decrease their investment in prevention and increase their investment in control of invasive species. This choice of responses to zebra mussels with less prevention and more control causes the probability of invasion to increase, which is followed by larger populations. The resulting damages require increased levels of adaptation by the power plant, which ultimately lowers overall welfare.
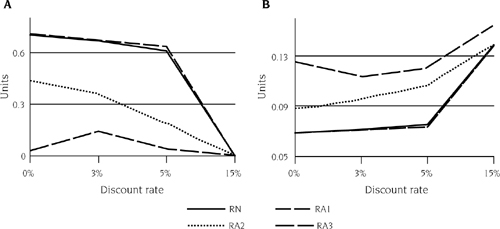
FIGURE 12.6.
An example of the impacts of the discount rate and levels of risk aversion in the endogenous risk framework in mean annual collective prevention (A) and mean annual collective control (B). Units are the average number of prevention (A) or control (B) events that take place on an annual basis. In this example, discount rates were examined at levels of 0%, 3%, 5%, and 15%, with four levels of risk perception, from risk neutral (RN) to very risk averse (RA3).
The results for risk attitudes mirror those for the discount rate, where a more risk-averse manager will choose a less risky alternative for managing invasive species (Finnoff et al. 2007) and will tend to invest more in control than in prevention (figure 12.6). In theory, greater risk aversion has two effects on the portfolio choice of prevention and control. First, if one is more risk averse, holding onto a dollar is more attractive (i.e., a sure bet) than spending it on either prevention or control, which are both affected by random events, that is, the probability of invasion and stochastic population growth. Second, attenuating indirect effects reflect the technical relationship between the two strategies, but it is ambiguous whether the indirect effect works with or against the direct effect.
In our analysis, risk-averse managers selected their less risky combination of strategies, a portfolio with less prevention and more control. Again, this finding seems counterintuitive. But to a more risk-averse manager, a dollar spent on control is worth more than a dollar spent on prevention because control is a sure bet while prevention is risky. The intuition is that control is relatively more attractive because its expected marginal effectiveness exceeds the expected marginal effectiveness of prevention. There is less uncertainty in the application of control—it removes existing invaders from the system; there is more uncertainty in prevention because it only reduces the chance of invasion and does not eliminate it. For this reason, the direct effect on prevention dominates the indirect effect; more risk-averse managers use less prevention. Since prevention and control act as substitutes, less prevention implies more control. As with increases in the discount rate, mean annual prevention employment is lower for higher degrees of risk aversion. This serves to increase the probability of invasion, with resulting abundance, adaptation, and control increases that ultimately lower overall welfare.
In summary, the theory of endogenous risk can be used to frame the question of how to manage the prevention and control of invasive species. The approach accounts for both biological and economic circumstances of invasions, as well as the feedbacks between the two systems. Within this framework, one can investigate whether the integration is worth the effort and how changes in managers’preferences over time and for bearing risk influence the optimal mix of public prevention and public control, and how that affects private adaptation.
By integrating the economic and ecological models for dreissenid mussels, reviewed in this chapter, there will be many opportunities to provide recommendations for managers and industries likely to be affected by a dreissenid mussel invasion. In the context of an invasion, many managers and policy makers are often interested in what will be the “cost” of an invasion into a particular location; that is, they want a dollar figure. For the most part, when ecologists have attempted to provide this figure, the exercise has been one of summing up potential (or actual) direct impacts (e.g., Phillips et al. 2005; Pimentel et al. 2005). Unfortunately, as we have shown in this chapter, understanding the bioeconomics of an invasion is much more complex. Defining a dollar figure requires not only ecological predictions of establishment, likely abundance, suitable habitat, and dispersal, but also economic predictions of direct and indirect impacts to the larger economy, the behavior of decision makers, and the incorporation of policy time horizons. Thus, the answer to our first question—what it is worth to keep dreissenid mussels from becoming established in the western states and provinces of North America—is “it depends.” As unsatisfactory as this answer may be, we still believe that our bioeconomic assessment of the western spread of dreissenid mussels does provide policy and management prescriptions.
Currently, our recommendations for the Columbia River basin are based on two separate analyses. First, the CGE model suggests that a full-blown invasion of the Columbia River basin would reduce welfare of the regional economy by approximately $3.3 million/year. Second, gravity model predictions suggest that the probability of dreissenid mussels being introduced into Lake Mead was four times more likely than an introduction into Roosevelt Lake (the largest reservoir on the Columbia River; see chapter 7). Considering that Lake Mead was invaded after dreissenid mussels had been in North America for almost 20 years, the probability of a dreissenid invasion into Roosevelt Lake in any given year is low. The challenge that still remains is to combine these predictions for the Columbia River within an integrated optimization model that also includes an assessment of uncertainty, the discount rate that policy makers in this region work from, and the influence of risk perception.
As with our first question, the answer to our second question—how much information and/or modeling is needed to make a policy recommendation given all the uncertainties in the invasion process—may seem unsatisfactory: at the current state of research into the bioeconomics of invasive species, we do not know exactly how much information or modeling is needed. For most stages of the invasion pathway, only a handful of studies exist on any particular species (even for dreissenid mussels), and even fewer studies exist on their economic impacts, let alone studies that fully integrate economics and ecology in a sophisticated manner. As this chapter shows, those ecological studies that include “economics” do so in an elementary manner, such as simply accounting for direct impacts, and those economic studies that included “ecology” do so in simplistic ways, such as assuming a full invasion or simplifying the movement patterns of human vectors. This chapter, however, does layout the framework and the steps needed to identify satisfactory answers concerning the bioeconomics of invasive species.
The dreissenid invasion of North America has been used time and again as the poster child for aquatic invasive species, as has been demonstrated in this chapter and book. Research on dreissenid mussels has been extensive and has covered many disciplines beyond what has been covered here, including population genetics, bioenergetics, species interactions including competition and facilitation, nutrient dynamics, and so forth. Dreissenid mussels have also been the model organism for efforts to develop or adapt new techniques and models in the field of ecology and economics, including the use of gravity models, niche modeling, and assessments of risk aversion by managers. The integration of invasive species biology and economics has also taken advantage of this base of knowledge and led to understanding the value of spending on prevention and control of invasive species, and dreissenid mussels in particular (Leung et al. 2002; Finnoff et al. 2006, 2007). Our continued goal is to integrate these disciplines to provide advice and quantifiable results to agencies, managers, and other research scientists to efficiently and effectively manage and predict the continuing spread of dreissenid mussels and other invasive species in North America.
Acknowledgments The chapter was substantially improved by the editors of this book and reviews by A. Bobeldyk, C. Jerde, and three anonymous reviewers. This material is based on work supported by the Integrated Systems for Invasive Species project (D. M. Lodge, principal investigator) funded by the National Science Foundation (DEB 02-13698) and by the National Sea Grant. J.M.B. was supported in part by an award from the National Sea Grant and the U.S. Fish and Wildlife Service (awarded to D. M. Lodge). This is publication 2009-004 from the University of Toledo Lake Erie Center.
Allen, Y. C., and C. W. Ramcharan. 2001. Dreissena distribution in commercial waterways of the US: using failed invasions to identify limiting factors. Canadian Journal of Fisheries and Aquatic Sciences 58:898–907.
Bobeldyk, A. M., J. M. Bossenbroek, M. A. Evans-White, D. M. Lodge, and G. A. Lamberti. 2005. Secondary spread of zebra mussels (Dreissena polymorpha) in coupled lake-stream systems. Ecoscience 12:339–346.
Bodamer, B., and J. M. Bossenbroek. 2008. Wetlands as barriers: effects of vegetated waterways on downstream dispersal of zebra mussels (Dreissena polymorpha). Freshwater Biology 53: 2051–2060.
Bossenbroek, J. M., L. E. Johnson, B. Peters, and D. M. Lodge. 2007. Forecasting the expansion of zebra mussels in the United States. Conservation Biology 21:800–810.
Bossenbroek, J. M., C. E. Kraft, and J. C. Nekola. 2001. Prediction of long-distance dispersal using gravity models: zebra mussel invasion of inland lakes. Ecological Applications 11: 1778–1788.
Bossenbroek, J. M., J. McNulty, and R. P. Keller. 2005. Can ecologists heat up the discussion on invasive species risk? Risk Analysis 25:1595–1597.
Carlton, J. T. 1991. Predictions of the arrival of the zebra mussel in North America. Dreissena polymorpha Information Review 2(2):1.
Courchamp, F., T. Clutton-Brock, and B. Grenfell. 1999. Inverse density dependence and the Allee effect. Trends in Ecology and Evolution 14:405–410.
Crocker, T. D., and J. Tschirhart. 1992. Ecosystems, externalities, and economies. Environmental Resource Economics 2:551–567.
Deng, Y. 1996. Present and expected economic costs of zebra mussel damages to water users with Great Lakes water intakes. Ph.D. thesis, Ohio State University.
Dermott, R. 2001. Sudden disappearance of the amphipod Diporeia from eastern Lake Ontario, 1993–1995. Journal of Great Lakes Research 27:423–433.
Dobson, A. P., J. P. Rodriguez, W. M. Roberts, and D. S. Wilcove. 1997. Geographic distribution of endangered species in the United States. Science 275:550–553.
Drake, J. M., and J. M. Bossenbroek. 2004. The potential distribution of zebra mussels (Dreissena polymorpha) in the U.S.A. BioScience 54:931–941.
Fahnenstiel, G. L., G. A. Lang, T. F. Nalepa, and T. H. Johengen. 1995. Effects of zebra mussel (Dreissena polymorpha) colonization on water quality parameters in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21:435–448.
Finnoff, D., J. F. Shogren, B. Leung, and D. Lodge. 2005. The importance of bioeconomic feedback in invasive species management. Ecological Economics 52:367–381.
Finnoff, D, J. F. Shogren, B. Leung, and D. M. Lodge. 2006. Prevention versus control in invasive species management. Pages 166–202 in A. Kontonlean, U. Pascual, and T. Swanson, editors. Biodiversity economics. Cambridge University Press, Cambridge, UK.
Finnoff, D., J. F. Shogren, B. Leung, and D. Lodge. 2007. Take a risk: preferring prevention over control of biological invaders. Ecological Economics 62:216–222.
Harrison, G. W., and H. D. Vinod. 1992. The sensitivity analysis of applied general equilibrium models: completely randomized factorial sampling designs. Review of Economics and Statistics 74:357–362.
Herbert, P. D. N., B. W. Muncaster, and G. L. Mackie. 1989. Ecological and genetic-studies on Dreissena polymorpha (Pallas)—a new mollusk in the Great-Lakes. Canadian Journal of Fisheries and Aquatic Sciences 46:1587–1591.
Holt, C. A., and Laury, S. K., 2002. Risk aversion and incentive effects. American Economic Review 92:1644–1655.
Horvath, T. G., G. A. Lamberti, D. M. Lodge, and W. L. Perry. 1996. Zebra mussel dispersal in lake-stream systems: source-sink dynamics? Journal of the North American Benthological Society 15:564–575.
Johnson, L. E., J. M. Bossenbroek, and C. E. Kraft. 2006. Patterns and pathways in the post-establishment spread of non-indigenous aquatic species: the slowing invasion of North American inland lakes by the zebra mussel. Biological Invasions 8:475–489.
Johnson, L. E., and J. T. Carlton. 1996. Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77:1686–1690.
Johnson, L. E., and D. K. Padilla. 1996. Geographic spread of exotic species: ecological lessons and opportunities from the invasion of the zebra mussel Dreissena polymorpha. Biological Conservation 78:23–33.
Keller, R. P., J. M. Drake, and D. M. Lodge. 2007. Fecundity as a basis for risk assessment of nonindigenous freshwater molluscs. Conservation Biology 21:191–200.
Kolar, C. S., and D. M. Lodge. 2001. Progress in invasion biology: predicting invaders. Trends in Ecology and Evolution 16:199–204.
Koutnik, M. A., and D. K. Padilla. 1994. Predicting the spatial distribution of Dreissena polymorpha (zebra mussel) among inland lakes of Wisconsin—modeling with a GIS. Canadian Journal of Fisheries and Aquatic Sciences 51:1189–1196.
Kraft, C. E., and L. E. Johnson. 2000. Regional differences in rates and patterns of North American inland lake invasions by zebra mussels (Dreissena polymorpha). Canadian Journal of Fisheries and Aquatic Sciences 57:993–1001.
Leung, B., J. M. Bossenbroek, and D. M. Lodge. 2006. Boats, pathways, and aquatic biological invasions: estimating dispersal potential with gravity models. Biological Invasions 8:241–254.
Leung, B., J. M. Drake, and D. M. Lodge. 2004. Predicting invasions: propagule pressure and the gravity of Allee effects. Ecology 85:1651–1660.
Leung, B., D. M. Lodge, D. Finnoff, J. F. Shogren, M. A. Lewis, and G. Lamberti. 2002. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings of the Royal Society of London Series B Biological Sciences 269:2407–2413.
Ludyanskiy, M. L., D. McDonald, and D. Macneill. 1993. Impact of the zebra mussel, a bivalve invader. BioScience 43:533–544.
Mackie, G. L. 1991. Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219:251–268.
May, B., and J. E. Marsden. 1992. Genetic identification and implications of another invasive species of dreissenid mussel in the Great-Lakes. Canadian Journal of Fisheries and Aquatic Sciences 49:1501–1506.
Mellina, E., and J. B. Rasmussen. 1994. Patterns in the distribution and abundance of zebra mussel (Dreissena polymorpha) in rivers and lakes in relation to substrate and other physicochemical factors. Canadian Journal of Fisheries and Aquatic Sciences 51:1024–1036.
Mellina, E., J. B. Rasmussen, and E. L. Mills. 1995. Impact of zebra mussel (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Canadian Journal of Fisheries and Aquatic Sciences 52:2553–2573.
Mills, E. L., R. M. Dermott, E. F. Roseman, D. Dustin, E. Mellina, D. B. Conn, and A. P. Spidle. 1993. Colonization, ecology, and population-structure of the quagga mussel (Bivalvia, Dreissenidae) in the lower Great-Lakes. Canadian Journal of Fisheries and Aquatic Sciences 50:2305–2314.
National Park Service. 2007. Live zebra mussels found at Lake Mead; Resource agencies initiate program to assess extent and prevent spread. Press release. Available at http://www.100thmeridian.org/mead.asp.
NatureServe. 2006. NatureServe Explorer: an online encyclopedia of life [web application]. Version 4.7. NatureServe, Arlington, VA. Available at www.natureserve.org/explorer (accessed June 13, 2006).
Neary, B. P., and J. H. Leach. 1992. Mapping the potential spread of the zebra mussel (Dreissena-polymorpha) in Ontario. Canadian Journal of Fisheries and Aquatic Sciences 49:406–415.
Northwest Natural Resource Group. 2003. Preparing to meet the challenge: an assessment of invasive species management in Idaho. Prepared for the Idaho Invasive Species Council. Northwest Natural Resource Group, Boise, Idaho.
100th Meridian Initiative. 2007. News and Announcements. Available at http://100thmeridian.org/news.asp.
O’Neill, C. R. 1997. Economic impact of zebra mussels—results of the 1995 National Zebra Mussel Information Clearinghouse study. Great Lake Research Review 3:35–44.
Parker, I. M., D. Simberloff, W. M. Lonsdale, K. Goodell, M. Wonham, P. M. Kareiva, M. H. Williamson, B. Von Holle, P. B. Moyle, and J. E. Byers. 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1:3–19.
Parmalee, P. W., and A. E. Bogan. 1998. The freshwater mussels of Tennessee. University of Tennessee Press, Knoxville.
Phillips, S., T. Darland, and M. Sytsma. 2005. Potential economic impacts of zebra mussels on the hydropower facilities in the Columbia River basin. Prepared for the Bonneville Power Administration. Pacific States Marine Fisheries Commission, Portland, OR.
Pimentel, D., R. Zuniga, and D. Monison. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52: 273–288.
Pollux, B., D. Minchin, G. Van Der Velde, T. Van Alen, S. Y. Moon-Van der Staay, and J. Hackstein. 2003. Zebra mussels (Dreissena polymorpha) in Ireland, AFLP-fingerprinting and boat traffic both indicate an origin from Britain. Freshwater Biology 48:1127–1139.
Ramcharan, C. W., D. K. Padilla, and S. I. Dodson. 1992. Models to predict potential occurrence and density of the zebra mussel, Dreissena-polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 49:2611–2620.
Ricciardi, A., R. J. Neves, and J. B. Rasmussen. 1998. Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. Journal of Animal Ecology 67:613–619.
Ricciardi, A., R. Serrouya, and F. G. Whoriskey. 1995. Aerial exposure tolerance of zebra and quagga mussels (Bivalvia, Dreissenidae)—implications for overland dispersal. Canadian Journal of Fisheries and Aquatic Sciences 52:470–477.
Schloesser, D. W., T. F. Nalepa, and G. L. Mackie. 1996. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. American Zoologist 36:300–310.
Schneider, D. W., C. D. Ellis, and K. S. Cummings. 1998. A transportation model assessment of the risk to native mussel communities from zebra mussel spread. Conservation Biology 12:788–800.
Settle, C., T. D. Crocker, and J. F. Shogren. 2002. On the joint determination of biological and economic systems. Ecological Economics 42:301–311.
Shogren, J. F. 2000. Risk deductions strategies against the “explosive invader.”In C. Perrings, M. Williamson, and S. Dalmazzone, editors. The economics of biological invasions. Edward Elgar, Northhampton, MA.
Simberloff, D. 2003. How much information on population biology is needed to manage introduced species? Conservation Biology 17:83–92.
Sprung, M. 1993. The other life: an account of present knowledge of the larval phase of Dreissena polymorpha. Pages 39–53 in T. F. Nalepa and D. Schloesser, editors. Zebra mussels: biology, impacts, and control. CRC Press, Boca Raton, FL.
Stoeckel, J. A., D. W. Schneider, L. A. Soeken, K. D. Blodgett, and R. E. Sparks. 1997. Larval dynamics of a riverine metapopulation: implications for zebra mussel recruitment, dispersal, and control in a large-river system. Journal of the North American Benthological Society 16: 586–601.
Stohlgren, T. J., D. Barnett, C. Flather, P. Fuller, B. Peterjohn, J. Kartesz, and L. L. Master. 2006. Species richness and patterns of invasion in plants, birds, and fishes in the United States. Biological Invasions 8:427–447.
Strayer, D. L. 1991. Projected distribution of the zebra mussel, Dreissena polymorpha, in North America. Canadian Journal of Fisheries and Aquatic Sciences 48:1389–1395.
Strayer, D. L., K. A. Hattala, and A. W. Kahnle. 2004. Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Canadian Journal of Fisheries and Aquatic Sciences 61:924–941.
Strayer, D. L., and L. C. Smith. 1996. Relationships between zebra mussels (Dreissena polymorpha) and unionid clams during the early stages of the zebra mussel invasion of the Hudson River. Freshwater Biology 36:771–779.
Thorp, J. H., J. E. Alexander, and G. A. Cobbs. 2002. Coping with warmer, large rivers: a field experiment on potential range expansion of northern quagga mussels (Dreissena bugensis). Freshwater Biology 47:1779–1790.
U.S. Department of Agriculture. 2004. 2002 Census of Agriculture. NationalAgricultural Statistics Service. Washington D.C. Available at http://www.agcensus.usda.gov/Publications/2002/index.asp.
Warziniack, T. W., D. Finnoff, and J. F. Shogren. 2008. Evaluating the 100th Meridian Initiative: assessing the impacts of zebra mussel invasion on the Columbia River basin. Working paper, Department of Economics and Finance, University of Wyoming, Laramie.
Whittier, T. R., P. L. Ringold, A. T. Herlihy, and S. M. Pierson. 2008. A calcium-based invasion risk assessment for zebra and quagga mussels (Dreissena spp). Frontiers in Ecology and the Environment 6:180–184.
Wilson, R. S., M. Tucker, N. Hooker, J. LeJeune, and D. J. Doohan. 2008. Perceptions and beliefs about weed management: perspectives of Ohio grain and produce farmers. Weed Technology 22:339–350.