In a Clamshell
Invasive species are now recognized worldwide as a serious side effect of international trade. They often spread irreversibly, and damages increase over time. To reduce such damages, private and public investments are increasing in an effort to prevent the arrival of species or eradicate them early in an invasion, control their local abundance once they have become established, or slow their spread. Most often, however, the damages of invasive species are accepted as a new cost of doing business, and humans change their behavior to minimize the impact. In this chapter, we argue that integrating ecological and economic analyses is essential to guide policy development in support of more cost-effective management. A key goal is to describe quantitatively the feedbacks between economic and ecological systems and to provide answers to such questions as how many dollars should be invested in prevention versus control, and what benefits are derived from such investments. This chapter describes the impacts of some high-profile invasive species, explains the extent to which ecological and economic systems are integrated, and looks to epidemiology for a model of how research and management could be better integrated to inform policy.
In the last two decades, experts and the public have recognized two important things about many anthropogenic environmental changes: first, these changes are increasingly global in scope, and second, they are hard to reverse. These characteristics apply with special force to harmful nonindigenous species, which we refer to as “invasive species” throughout this book. Both the global scope and the difficulty of reversing invasions impart considerable urgency to increasing our understanding of this problem. Invading organisms reproduce and spread, even if we cease introducing more individuals. The problem of harmful invasive species gets worse without management.
Research to better understand invasions comes naturally to scientists and social scientists, especially to those of us in universities. We also, however, believe it is urgent to focus our research on questions important to natural resource managers and policy makers, given society’s explicit desire to reduce the current and future damages caused by invasive species. We want our research and its implementation to increase social welfare. Using the perspectives and tools of economists is appropriate because invasive species are, by definition, driven by human activities, usually commercial enterprises. Solutions will derive from changes in industry practices and consumer behavior.
Humans are as much the target of our study as the species that humans move around the globe. If research is to inform natural resource management and policy, it must be conducted collaboratively by natural and social scientists, and in the context of possible management and policy responses to invasive species. We elaborate on these general points after considering some specific examples of invasive species, their environmental and economic costs, and societal responses to them.
Aquarium keepers, like owners of all sorts of plants and animals, sometimes tire of the organisms under their care and release them. In 2000, populations of the invasive seaweed Caulerpa taxifolia were discovered in two Southern California coastal embayments. This species, including a very invasive strain, has been sold widely in aquarium shops because it is fast growing, hardy, and beautiful (Walters et al. 2006). Some of these same characteristics have caused a well-documented history of harmful invasions. In various invaded marine ecosystems, including the Mediterranean Sea, commercial and recreational fishing, recreational activities like scuba diving, and tourism have all suffered (Meinesz 1999). When the species was discovered in California, a consortium of private and government agencies launched a concerted eradication effort using chlorine applications under anchored tarps. The effort cost at least $3.7 million over 5 years (Woodfield and Merkel 2005), and it was successful.
Without policy responses to prevent additional Caulerpa introductions, however, the need for many similarly expensive management situations would probably occur in the future as other naive aquarium owners dispose of unwanted plants (Walters et al. 2006). The U.S. Department of Agriculture (USDA) used its authority under the Plant Protection Act of 2000 to declare the Mediterranean aquarium strain of C. taxifolia a federal noxious weed. Such a designation gives the USDA authority to prohibit importation, exportation, or movement of the species in interstate commerce. In 2001, the state of California went a step further and made it illegal to possess C. taxifolia and nine other Caulerpa species. Nevertheless, various species and strains of Caulerpa remain easy to purchase in all states (Walters et al. 2006). The story of Caulerpa eradication near San Diego, then, is a success story. It is an example of successful implementation of a strategy referred to as “early detection, rapid response, and eradication,” supported by additional efforts (of minimal success thus far) to prohibit future introductions.
Across the continent and about a century earlier, the construction of the Welland Canal by-passed Niagara Falls and allowed sea lamprey (Petromyzon marina), along with ships and barges, access to the upper Great Lakes. Despite the fact that most sea lamprey previously lived their adult lives in the Atlantic Ocean, large and self-sustaining populations soon thrived in the upper lakes. While the increased navigation fostered commercial activities that were beneficial to humans, the invasion by sea lamprey was not. Adult sea lamprey are parasitic on other fish species, using their rasping and suckerlike mouth to feast on the blood of commercially valuable species such as lake trout (Salvelinus namaycush) and whitefish (Coregonus spp.). The result was declining fisheries and a public outcry.
Fortunately, larval sea lamprey are confined to the tributaries of the Great Lakes, where they reside for about 7 years before assuming their adult bloodsucking habits. The larvae are easy to locate and are highly susceptible to TFM (3-trifluoromethyl-4-nitrophenol), a chemical discovered in 1955. When applied at appropriate concentrations in tributaries, TFM kills sea lamprey larvae with acceptably low effects on other species. Since 1956 the United States and Canada have together spent about $15 million annually on monitoring and poisoning sea lamprey. Sea lamprey populations plummeted, and harm to the fisheries is kept tolerably low with these continuous expenditures. The management efforts directed at sea lamprey constitute a remarkably successful “control” effort, the ongoing expense of which is justified by even larger benefits in the protection of Great Lakes fisheries.
In 1869, gypsy moth (Lymantria dispar), which had been imported from its native range in Europe, escaped an unsuccessful attempt at silk production in Massachusetts. Thus began an invasion of North America that is ongoing today. Gypsy moth infestations can completely defoliate vast forests of oak and other trees and can achieve such abundance that their excrement and bodies are sometimes a serious nuisance in urban areas. Outbreaks of gypsy moths are often controlled with an aggressive integrated pest management program. In areas where the gypsy moth is now a permanent resident, expenditures to keep their populations acceptably low are very high when the periodic population outbreaks are treated with pesticides. As for sea lamprey, the best that can be hoped for in these areas is successful control, not eradication. Therefore, for every acre that becomes infested as the invasion progresses, future control costs will be high (perhaps forever) if pesticide treatments are chosen. Otherwise, humans must simply adapt (sensu economics, not evolution) to the periodic damage to urban and natural forests.
Because of the damage and/or control costs once gypsy moths become established, the USDA and states from Wisconsin south to North Carolina spend about $12 million annually to slow the southwestward march of gypsy moths across the country. A combination of trapping, aerial spraying of insecticides, and mating-disrupting pheromones has slowed by 50% the advance of the invasion front, from about 13 miles per year to about 6 miles per year (Sharov et al. 2002). Although this effort is expensive, it is cost-effective because damages are avoided, at least for a year, in the area in advance of the invasion front—an area of roughly 9,000 square miles (1,500 miles × 6 miles). The avoided damages are much higher than the costs of the slow-the-spread program (Sharov 2004). Preventing long-distance, especially human-mediated, dispersal ahead of the advancing invasion front remains a challenge for this program, but overall the scientific and management responses to the gypsy moth are a successful example of a slow-the-spread strategy.
Stories that end in at least some level of success—eradication of Caulerpa, control of sea lamprey, slowing the spread of the gypsy moth—are rare and unfortunately are vastly outnumbered by harmful invasions that proceed apace to a grim and often irreversible outcome. Some of the most visible, dramatic, and widespread examples come from forests.
In the United States, a combination of nonindigenous insects, fungi, and other parasites and pathogens have essentially extirpated American chestnut (Castanea dentata) and American elm (Ulmus americana), previously two of the dominant trees in eastern natural and urban forests, respectively (Burnham 1988; Gilbert 2002). Many other beloved and valuable species seem likely to face a similar demise from ongoing invasions: flowering dogwood (Cornus florida), destroyed by the anthracnose pathogen, has declined in abundance by more than 90% in some forest types over the last two decades (Holzmueller et al. 2006); American beech (Fagus grandifolia) is succumbing to beech bark blister; Eastern hemlock (Tsuga canadensis) is declining as the hemlock wooly adelgid spreads across the East and Midwest; butternut (Juglans cinerea) invariably dies after infection by butternut canker, which is common and spreading in the Northeast and Midwest (Ostry and Woeste 2004); mortality of ashes (Fraxinus spp.) hovers near 100% as the emerald ash borer advances across the Midwest (BenDor et al. 2006); and several species of oak (Quercus spp.) are vulnerable to sudden oak death, the spread of which has only recently begun but has already jumped from the West Coast to the East Coast in the nursery trade (Gilbert 2002). All the responsible pests and pathogens are nonindigenous, with many arriving in the United States as hitchhikers in shipments of plants, wood products, or wood packing material.
It is not just accidentally introduced pests and pathogens that damage forestry production and damage natural and urban forests. Deliberately introduced plants, such as the kudzu vine (Pueraria lobata), are also outcompeting native vegetation for light, nutrients, and space. And, like the gypsy moth, they can seem like a good thing at first. The American public first saw the fast-growing, attractively purple-flowered kudzu vine from Japan at the 1876 Centennial Exposition in Philadelphia (Forseth and Innis 2004). For decades thereafter, particularly in the southeastern United States, it served well as an ornamental plant that also provided summer shade under overgrown porches. Later, especially during the first half of the twentieth century, as justifiable concerns grew about the severe soil erosion and nutrient depletion that accompanied intensive cotton agriculture, the U.S. government distributed 85 million seedlings, paying southern farmers to plant them (Forseth and Innes 2004). As for so many introduced species, only later did the downsides to kudzu become apparent, especially as other economic forces caused the decline of row cropping and livestock operations that had included management of kudzu. Millions of kudzu plants began to escape control altogether (Forseth and Innes 2004).
By mid-century, the costs of kudzu had become painfully obvious. Kudzu now occurs from Texas to Florida and north to New York, covering over 3 million hectares, which increases by about 50,000 hectares per year (Forseth and Innes 2004). Forest productivity losses are between $100 million and $500 million per year, power companies spend about $1.5 million annually to control kudzu, and a 6-year effort was required to eradicate kudzu from the Chickamauga and Chattanooga National Military Park. The best that can be hoped for is locally successful eradication efforts, whose long-term success depends on continued monitoring and control, as the species continues to expand its geographic range from the southeastern United States. Unfortunately, the list of deliberately introduced plants like kudzu that have become very harmful to agriculture, livestock, forestry, and natural ecosystems is long, including hundreds of species. It also continues to grow.
In addition to lost productivity and increased expenditures for control efforts in human-managed landscapes, the result of these invasive species is an ongoing shift in the composition of forests that is similar in magnitude to that of a nationwide forest fire, only slower. Large negative consequences exist for industries involving horticulture, landscaping, wood products, recreation, and tourism, as well as for natural ecosystems. Forest ecosystems provide the most obvious examples of damaging, unreversed invasions, but the same patterns characterize other terrestrial, marine, and freshwater ecosystems.
Zebra mussels (Dreissena polymorpha) and quagga mussels (D. bugensis (=D. rostriformis bugensis [Andrusov (1897)])) are the best-documented examples of similar phenomena in freshwater ecosystems in North America. Both are small striped bivalve mollusks. Zebra mussel was discovered in Lake St. Clair, between lakes Erie and Huron, in the mid-1980s, with quagga mussels following within a few years. These mussels were released when ships discharged ballast water that had been taken up in a port in northern Europe, where zebra and quagga mussels had previously invaded from their native ranges around the Black Sea. With those ballast water releases, Lake St. Clair, and quickly other Great Lakes, became the beachhead for ongoing invasions of freshwater ecosystems of North America. From the Great Lakes, two major human-driven vectors of dispersal allowed zebra and quagga mussels to spread. First, the Chicago Sanitary and Ship Canal provided a ready conduit for the mussels to escape Lake Michigan (crossing a former watershed divide) and colonize the Illinois and Mississippi rivers downstream. From the Mississippi River proper, the mussels, especially zebra mussel so far, hitched rides upstream on barges to colonize tributaries, including the Ohio, Tennessee, and Missouri rivers.
Second, recreational boaters, who often visit multiple rivers and lakes, inadvertently carried mussels overland on their boat trailers and boats to inland lakes that are not connected by water to initial sites of infestation. Within a decade, zebra mussel colonized much of the Great Lakes–St. Lawrence River and Mississippi River drainage basins. In 2007 and 2008, colonization of the West Coast by quagga and zebra mussels, respectively, began. Quagga mussel was discovered in Lake Mead, the Colorado River, and the California Aqueduct (Stokstad 2007), while zebra mussel was discovered in a California reservoir. Much suitable habitat for zebra and quagga mussels remains to be colonized east of the Appalachians and in the West, including the Columbia and Sacramento-San Joaquin rivers (Drake and Bossenbroek 2004). While the probability of transport of live mussels to those regions from the Midwest is lower than to waterways in the Midwest, mussels are being transported, and without increased slow-the-spread efforts, these regions almost surely will be colonized and suffer damages in the future (Bossenbroek et al. 2007), especially with new sources of invasion in the western waterways.
Efforts to slow the spread of mussels are occurring at regional, state, and federal levels, but their efficacy is poorly documented, and they are almost certainly underfunded (Leung et al. 2002; Lodge et al. 2006). Additional investments in such efforts are warranted because the damages caused by zebra mussels are large, including at least $150 million annually in the Great Lakes region by clogging up water intake pipes in power plants, municipal water supplies, and industrial facilities that withdraw raw surface water (O’Neill 1996). In addition, sharp zebra mussel shells foul beaches, hinder recreation, extirpate native clam species, increase harmful algal blooms, and likely contribute to botulism outbreaks that devastate migrating waterfowl and fishes in the Great Lakes region (Yule et al. 2006). Zebra mussels are successfully (if expensively) controlled inside industrial facilities, and have been eradicated from one quarry lake in Virginia, but no technique exists to reduce the population of zebra mussel in an entire lake or waterway without killing many other organisms.
The zebra mussel invasion, like those described above for terrestrial ecosystems, will continue, more slowly perhaps if a more effective slow-the-spread campaign is implemented, but humans in North America are stuck with zebra and quagga mussels. Forevermore in North America, they will be abundant, and native clams and many other native species will be less abundant, some perhaps extinct (Strayer and Malcom 2007). The changes in our behavior to cope with these changes, and the expenditures necessary to control them in power plants, will likely grow over time until zebra and quagga mussels occupy all suitable waterways in North America. And many other invasive species already in the Great Lakes are following the mussels across the country.
The invasive species vignettes above bring up a very important question: is prevention a management option? Though prevention is little practiced in North America, the answer is yes, of course, prevention is possible. Slow-the-spread programs show that, on a regional scale, prevention is possible even if only temporary. Prevention is also possible at the continent’s borders. Anyone who has returned to North America from a trip abroad knows not to try to bring any fresh fruit, or the insects or pathogens that it might harbor, into the country. And some rare rigorous inspection programs at borders show how much potential damage could be avoided with rigorous screening and interdiction programs. For example, comprehensive inspections of air cargo at Kahului Airport, Hawaii, during 20 weeks in 2000–2001 revealed 279 insect species, 125 of which were not known from Hawaii, and 47 plant pathogen species, 16 of which were not known from Hawaii (Hawaii Department of Agriculture 2002). Most of the time at this and other airports in North America, however, inspections are far fewer. Such organisms ordinarily go undetected and are released into the environment. Some will cause great harm.
Prevention is possible, then, but it is reasonable to wonder how much prevention would cost, and whether it would be cheaper than the damages that occur in the absence of prevention. The vignettes above illustrate how costly invasions can be, either through damages suffered or the expenditures to support eradication or control efforts, but would prevention be equally costly? These sorts of questions motivate much of this book. Despite the slowness of these and many other unfolding invasion disasters, they should be regarded with urgency because the costs are high, grow over time as the populations of harmful species spread, and are too often irreversible. Are we simply stuck with such costs, or are prevention and more aggressive control approaches viable alternatives? In this book, we focus on freshwater examples to illustrate the causes, consequences, and potential management responses to invasive species. We combine ecological modeling with economic modeling to answer questions about management and policy.
Human values determine both which environmental changes we call damages and what investments in management responses seem appropriate. The positive and negative values that humans assign to species or other characteristics of ecosystems are appropriately informed by various financial, scientific, religious, and ethical considerations, but inescapably it is humans that do the valuing and responding (Hamlin and Lodge 2006). Invasions occurred before humans appeared, but the rate at which global commerce now causes them is orders of magnitude higher than natural background rates (Lodge and Shrader-Frechette 2003). More and increasingly international transportation of goods causes invasions, and human behavior will either continue to increase invasions or rein them in. The combination of natural and social science represented in this book is essential to both diagnose invasions and respond to them.
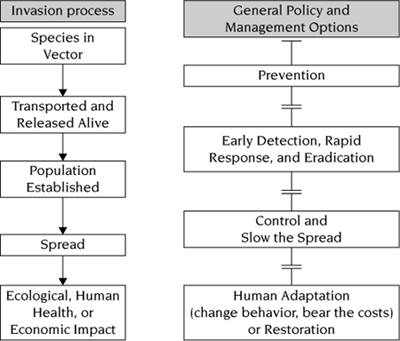
Following the vignettes above, we could continue to illustrate the issue of invasive species with thousands of additional examples, replete with idiosyncratic biological details. Such catalogs of examples, however, can obscure the processes that are common to all invasions (figure 1.1, left column). Understanding the processes, in turn, is essential to prescribing appropriate management responses (figure 1.1, right column).
Species are carried in a vector, which transports the species either overtly (e.g., the pet and horticultural trades) or incidentally (e.g., insect pests in lumber shipments, ballast water of ships, viruses carried by humans themselves) (figure 1.2). Depending on the traits of the species, and the conditions and the duration in the vector, some proportion of the organisms may be alive when they are released or escape at a location outside their native range.
Depending on the taxonomic group of organisms, many to most species subsequently go extinct in a new location, but a proportion—on the order of 5% for plants (Keller et al. 2007) and up to 50% for animals (Jeschke and Strayer 2005)—establish a self-sustaining population. While some of these established species remain localized, perhaps not even detected by humans, a proportion, again about 5–50%, spread widely and become abundant at many new locations. Such species—roughly 0.3% of introduced plants and up to 25% of introduced animals (as calculated from the numbers above)—cause undesirable environmental and/or economic changes and are categorized as invasive. By definition, invasive species, which are a subset of nonindigenous species, are bad.

FIGURE 1.1.
The stages of biological invasion (left column) and the management and policy options available to society (right column) at each stage of invasion. The desire to reduce the negative impacts of species (bottom left) motivates the study of biological invasions. Reprinted from Lodge et al. (2006), with permission of the Ecological Society of America.
FIGURE 1.2.
Vectors by which nonindigenous species enter the United States and are transported within the United States. Reprinted from Lodge et al. (2006), with permission of the Ecological Society of America.
Policy and management implications become clear when these underlying processes and probabilistic transitions during invasion are recognized. The possible human management responses narrow as any invasion progresses (figure 1.1). As illustrated by the above vignettes, prevention is possible only early in the process, before a species arrives in a new range or at the point of entry. Eradication depends on the rapid convergence of appropriate technology, political will, and resources. Once a species is well established, eradication is costly and sometimes impossible. When the opportunity for eradication has passed, only two options remain: control of populations in selected locations, and adaptation by humans.
In most countries, including those in North America, adaptation has been vastly more typical than any other response, except when pests or pathogens have threatened either humans directly or highly valuable agricultural crops. Apart from these exceptions, we passively suffer the consequences of invasions. In the last decade, however, investments in eradication, control, and finally prevention have increased for natural ecosystems, and policy discussions in the United States and elsewhere increasingly feature prevention efforts.
In this book, we assess current scientific capability to forecast the identity, spread, and impact of potential invasive species. In chapters 3–6 we address the series of transitions represented in the left column of figure 1.1. Furthermore, we explore how ecological forecasting can be used in risk assessment and risk management of invasive species, testing especially whether cost-effective approaches, including prevention, can be identified.
Interest in prevention necessarily focuses attention on vectors (figure 1.2). Vectors are commercial activities driven by human desires for the benefits from increased trade. In the absence of strong efforts to prevent invasions, increasing trade will increase invasions. The numbers of nonindigenous plant pathogens, insects, and mollusks discovered in the United States since 1920 are strongly correlated with importation of goods over the same time period (figure 1.3). Trade with many countries is increasing (figure 1.4), and documented invasions are increasing in marine, terrestrial, and aquatic ecosystems (Ricciardi 2006; figure 1.5). Different vectors operate at different spatial scales and with different potential management interventions. Detailed knowledge of vectors, as well as of different taxonomic groups of organisms, must be combined in biological and economic models if they are to guide management and policy to cost-effectively reduce damages from invasive species.
A circle of feedbacks exists between ecological processes and economic processes (figure 1.6): the economic benefits of trade drive invasions, invasions cause negative economic and environmental impacts, and human perception of those impacts feeds back as management or policy initiatives to reduce trade or at least reduce the negative side effects of trade. Another way to look at this situation is as an adaptive loop, among risk assessment, risk perception, and risk management, that changes the risks to be assessed. A distinctive strength of this book lies in applying a combination of ecology and economics, with strong mathematical and statistical foundations, to management and policy questions.
FIGURE 1.3.
Total imports into the United States since 1920 (measured in dollars) as a potential driver of cumulative invasions by terrestrial insects in the United States since 1920. Modified from Levine and D’Antonio (2003).
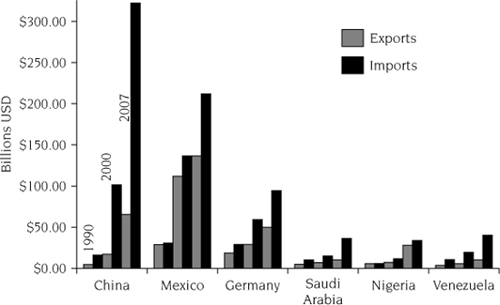
FIGURE 1.4.
Changes in total trade volume between selected countries and the United States, 1900–2007. First bars for each country are imports/exports during 1990; subsequent pairs of bars are for 2000 and 2007, respectively. Data from the U.S. Department of Commerce (2008).
Economics and the biological sciences have many similarities. Both are disciplines of limits—both examine how species deal with scarcity. Whether it is a human’s reaction to a limited budget and unlimited wants or a squirrel’s response to limited food and unlimited appetite for reproduction, all species deal with limits. These limiting factors, as defined within both economics and the biological sciences, drive research efforts. Yet failure to account for joint influences on these limits in economic systems and biological systems can cause inaccurate perceptions of how each system works and provide misleading policy guidance. The idea of joint determination applies: links between the biological and economic systems create a progression of natural and human actions and reactions, in which a feedback loop emerges. Disturbances in one system set off repercussions in the other system, and these repercussions feed back into the system where the disturbances originated (e.g., Daly 1968; Clark 1990; Crocker and Tschirhart 1992; Sohngen and Mendelsohn 1998; Wilson 1998; Shogren and Crocker 1999; Dasgupta et al. 2000; Finnoff and Tschirhart 2003).
FIGURE 1.5.
Cumulative number of nonindigenous species that have been discovered in three major aquatic ecosystems in the last 150+ years. It is not known how many species remain undiscovered in each ecosystem, or how long the discovered species had been present before they were discovered (Costello et al. 2007). Nevertheless, the data suggest strongly that trade and/or other mechanisms by which humans cause the movement of species (e.g., canal construction) have caused an increasing number of invasions. Data from Cohen and Carlton (1998), Ricciardi (2001), and http://http://www.corpi.ku.lt/nemo/).
The impact of invasive species is a good example of joint determination. Thresholds for expansion of invasive species are functions of the present distributions and trends of their populations, their interactions with habitats, and the economic circumstances that cause introductions of additional individuals and the quality of potential habitat (e.g., fragmentation). Important economic circumstances include the relative prices of alternative sites for economic development and relative wealth of the landholders in the area. Sites with low relative returns in their “highest and best” use are more likely to be left undisturbed. Moreover, the rich can better afford to set aside undisturbed habitat that may be less susceptible to invasions.
FIGURE 1.6.
Feedback between ecological processes (left column) and economic processes. The lightface text indicates the variety of tools, many recently developed or applied, that we use to model and forecast different stages of invasion. The bold arrows indicate possible feedback pathways in which damages from an invasion cause humans to change investments to reduce future damages: the impact of a species is expressed in increased control costs; in response, humans increase prevention expenditures that reduce the number of organisms entrained in the responsible vector. Modified from Leung et al. (2002).
These interactions demonstrate that invasive species establishment and spread are determined by both economic and biological parameters. Effective models of the spread and impact of invasive species require natural and social scientists to integrate their respective tools and their indicators of success and failure. Integration across disciplinary boundaries is especially crucial when a proposed policy may trigger a political feud fueled by misperceptions of benefits and costs imposed on natural and social systems. The resulting challenge is to integrate models, methods, and mind-sets to help researchers and decision makers better understand and manage the delicate balance between private rights of self-determination and social rights to environmental protection.
The most straightforward and pragmatic method is to form a research team that includes both economists and ecologists to construct an explicit model to estimate the trade-offs associated with alternative policy options. Models are always abstractions and must never be mistaken for reality. Nevertheless, the integrative thought process of model construction focuses attention on the most important links between systems. The differences and similarities between economics and ecology can be addressed directly by forcing researchers to construct and link the human and natural sectors of the model. A linked model can then provide informed guidance for pragmatic choices among the trade-offs necessarily involved in policy making.
We illustrate this approach using a model that captures the risks posed by one invasive species, lake trout (Salvelinus namaycush), on one endangered species, the cutthroat trout (Oncorhynchus clarki bouvieri) in Yellowstone Lake in Yellowstone National Park, Wyoming. Settle et al. (2002) explored how feedbacks between humans and nature affect the likelihood of the desired result—an increased population of cutthroat trout, because many more anglers prefer to catch cutthroat. In a dynamic modeling framework, Settle et al. incorporated both economic and ecological flows and reciprocal flows between the two systems. To test the importance of the economic-ecological feedbacks, the authors compared the modeling results with and without the reciprocal flows between the two systems. They considered two scenarios: (1) a remove-all-lake-trout scenario, in which lake trout are immediately removed from Yellowstone Lake; and (2) a leave-the-lake-trout-be scenario, in which lake trout are left alone to reach a steady state within the Yellowstone Lake ecosystem.
Under the remove-all-lake-trout scenario, the steady-state population of cutthroat trout is about 2.7 million and 3.4 million, without and with feedbacks. Without feedback between the economic and ecological systems, park visitors continue to fish as before, putting constant pressure on the cutthroat. With feedback, visitors react to declining cutthroat populations by fishing less and visiting other attractions more. This behavioral reaction by park visitors, which reflects an increase in what economists call the shadow price of fishing, now affects the ecosystem because a decline in fishing time produces an increase in the population of cutthroat. Incorporating feedbacks between the economic and ecological system produced estimates of a 26% larger population of cutthroat, the desired species.
Under the leave-the-lake-trout-be scenario, Settle et al. (2002) found a different result. Now a no-feedback model (fishing continued as before) suggested a more desirable outcome than would be likely to occur—almost 1 million cutthroat trout remain versus zero cutthroat trout when feedbacks were included. Without feedbacks, visitors continued to fish and acted as a control on the population of lake trout, even though it is an incidental catch. With feedbacks, visitors shifted away from fishing as the cutthroat trout population declined and the lake trout population increased, leaving the lake trout to take over as cutthroat were extirpated. Without incorporating feedbacks, policy advice might have led park officials to adopt the cheaper leave-the-lake-trout-be policy, satisfied that at least the cutthroat would continue to exist in Yellowstone Lake. According to the model by Settle et al. (2002), such a policy would likely have resulted in the disappearance of cutthroat. The National Park Service currently uses a policy of gill netting lake trout. (See chapter 2 for additional discussion of this example.)
This example illustrates how integrating the feedbacks between economics and ecology can be essential to provide appropriate advice for management and policy for invasive species. Technical integrated models can be a powerful tool to make the linkages among disciplines transparent and workable. Failure to account for the specific links between ecosystems and economic systems might lead to inappropriate management of either the ecosystem or the economic system. Integration of economics and ecology is fundamental both for science and policy. For science, integration implies more accurate estimates of both economic and ecological phenomena. For policy, integration means a better appreciation of the alternative viewpoints that arise when attempting to address a difficult challenge like invasive species management. Societal responses to infectious disease, including research and the way it informs disease management and policy, provide a useful analogy through which to approach the similar intellectual challenges posed by invasive species.
With the spread of such pathogens as SARS and West Nile virus into new continents, such as North America, distinctions between disease and invasive species become blurred; indeed, some diseases are caused by nonindigenous pathogens and parasites. Perhaps due to clear human impacts and well-publicized public health costs, great investments have been made into bioeconomic research, policy, and management of human infectious disease (Roberts 2006). Such responses to infectious disease provide useful parallels for bioeconomic analysis, management, and policy of ecological invasions, which remain in their infancy.
One essential quantity for characterizing dynamics of an infectious disease is the basic reproduction number, the number of secondary infections arising from direct contact with a single infective organism that is introduced into an otherwise susceptible population (Diekmann et al. 1990). This single statistic has proved a convenient metric for assessing methods of disease control. For example, Wonham et al. (2004) estimated that mosquito control that reduced mosquito populations to 30–60% of endemic levels would have prevented the 1999 outbreak of West Nile virus in New York, an outbreak that eventually lead to the spread of this disease across North America. In the context of a biological population, the basic reproduction number is the number of surviving offspring produced during the lifetime of a single individual (Caswell 2001). Although widely applicable to biological invasions, the actual application of this simple statistic to the control of invading populations remains in its infancy (but see de Camino Beck and Lewis 2007).
Infectious diseases may establish in one city and then jump to another, much the same way as aquatic invasive species can spread from one lake to another. One class of models, successful in predicting these jumps in disease contagion, borrows from physics and transportation theory. Here, modifications of the empirical gravity law are used to define the level of attraction of contagion among cities in a network. Cities are like planets—attractiveness is positively related to city size and negatively related to distance between cities. Sets of rules, based on this principle, have been fitted to observed infection data for diseases such as measles (Xia et al. 2004). When incorporated in a network model, the rules can then be used to track or predict spatial spread of infectious disease among cities. As we show in chapter 7, these so-called gravity models have also been used successfully in modeling the spread of invasive species in networks of lakes.
Investment in the modeling and analysis of infectious disease control measures has extended to the realm of livestock and agriculture (Morris 1999), particularly in cases where the diseases can have devastating market impacts. Modeling of the spread of foot and mouth disease in 2001 in the United Kingdom guided the use of different control measures, including culling, prophylactic vaccination, and vaccination strategies that target key spatial transmission foci (Keeling et al. 2003). For this disease, focal units are the individual livestock farms housing infected cattle. Unfortunately, there is no simple nondestructive action analogous to prophylactic vaccination for the control of noninfectious invasive species. Such an action amounts to wholesale manipulation of the biotic resources available to the invader (analogous to decreasing the density of susceptible individuals). For example, tree thinning has been used as a management strategy to stem the spread of the invasive mountain pine beetle into new areas of pine forest (Steeger and Smith 1999). However, for most invasions, such control methods are considered a method of last resort because their costs, both economic and ecological, are so high.
Economic costs of human disease are an area of active current research (Roberts 2006), and an increasing motivation for public health efforts. The economic impact of animal infectious diseases can also be high and a strong motivator for improved management and policy. For example, botulinum infection of Canadian salmon in the 1980s devastated the salmon fish industry. Livestock diseases such as bovine spongiform encephalopathy (“mad cow disease”), found to be capable of crossing species barriers, and foot and mouth, which is capable of very rapid spread, have played havoc with the British beef industry. Methods of economic impact assessment are well developed at the level of the production unit (herd or farm) (Rushton et al. 1999) but are more elusive at national and international levels (Riviere-Cinnamond 2006). For humans, public health costs of infectious disease are typically measured by cost-of-illness studies, which calculate the implications of illness on the use of resources. Many economists prefer to measure disease impacts through surveying the population’s willingness to pay for treatment or prevention services (Mangtani and Shah 2006). This easily translates benefits of treatment or prevention into monetary terms. Analogous methods, outlined in chapter 8, are also employed in the study of the economics of invasive species.
As we emphasized in the preceding section, the coupling between biological dynamics and economics is a two-way street: economic conditions can also affect infectious disease outbreaks. Immune status of a person is affected by living conditions, by the quality and quantity of food consumed, and by access to clean water (World Health Organization 2002). Furthermore, trade activity can spread disease from one place to the next (Narasimham 2006). Evaluation of economics of infectious disease can require such two-way coupling (Roberts 2006). In this book, we demonstrate the necessity of a similar two-way coupling between invasion dynamics and economics.
As economists and ecologists, we also learn from the methods of economic analysis applied to disease. The most common is based on cost-benefit analysis. While many cost-benefit analyses employ a static approach, dynamic analyses have been applied to subjects such as HIV intervention policy (Kumaranayake 2006). Even over 5- or 10-year spans, the abundance of an invasive species can increase by orders of magnitude. This means that dynamical models are needed for invasive species, even more than for disease bioeconomics. In this book, we put a premium on the development of dynamical models, illustrated especially in chapter 9, which can be connected directly to policy and management decision making. In the next section, we briefly consider how current policies at various levels are or are not informed by the integration of economic and ecological analyses.
Important arenas in which the feedbacks between the biological and economic systems are adjudicated are international agreements. Movements of species within countries can also cause great damage (Perry et al. 2002), but a large focus of ongoing policy development is international. Once a species is introduced to one country, dispersal to neighboring countries and to countries strongly connected by trade becomes much more likely. Decisions about importation or exportation by one country affect the interests of many other countries. While national policy often focuses on importation, international agreements are the usual venue for more explicitly recognizing steps that should be taken to prevent exportation, as well as importation, of harmful species.
Although more than 50 international and regional legal instruments address invasive species, few of these are binding (Shine et al. 2005). Of these, the binding agreement most directly aimed at preventing environmental harm is the Convention on Biological Diversity (CBD), ratified by more than 170 parties (not including the United States). Under the CBD, however, the obligation for compliance lies with each signatory country, and the repercussions for noncompliance are virtually nonexistent.
In contrast, international trade agreements have exerted the strongest influence over invasive species policy because the costs of noncompliance are high. Globally, the most relevant agreements are those based in the World Trade Organization (WTO), although the following comments apply also to binational and regional agreements, such as the North American Free Trade Agreement. Because the overarching goal of WTO is to increase international trade (which increases the probability of biological invasions), there is an inherent tension between promoting trade and preventing the introduction of invasive species.
Under WTO, the International Plant Protection Convention specifies standards (through the Agreement on the Application of Sanitary and Phytosanitary Measures [SPS Agreement]) that national laws must meet if a nation wishes to reduce the introduction of invasive species (Hedley 2004). These standards apply to invasive species of all kinds, including plants, plant pests, animals, and animal parasites. Any regulations to reduce the introduction of unwanted species must minimize the impact on trade. The initial burden in demonstrating the need for protection is on the importing nation, which must demonstrate via a scientific risk assessment that an import is likely to cause a harmful introduction. While the role of scientific risk analysis appears preeminent in the SPS Agreement, it remains largely unclear what constitutes a scientific risk assessment that can meet the SPS standards. Most of the cases that have been adjudicated have been decided in favor of the exporting country (Pauwelyn 1999). Countries are under pressure to quickly open their borders to imports rather than take precautionary measures to prevent the introduction of invasive species. The difficult balancing act, not yet achieved, is to provide adequate safeguards to prevent invasive species while not unduly hindering the high-speed, high-volume international flow of goods (Jenkins 2002).
Most national policies, including those in the United States, have responded very little to the threat of invasive species, for at least two related reasons. First, while the costs of invasions have been estimated as $120 billion annually for the United States (Pimentel et al. 2005), such aggregate estimates are certainly incomplete and are difficult to parse with respect to policy options for specific vectors, and few specific rigorous economic analyses exist (Lovell et al. 2006; Olson 2006). Second, policy responses aimed at reducing invasions and increasing human welfare could instead lower human welfare and cause unanticipated economic distortions if their costs (in lower trade or shifts in the economy) outweigh their benefits (in decreased damages from invasive species) (Lovell et al. 2006; Olson 2006). Rational policies depend on better quantification of the externalities of trade manifesting as damage costs of invasive species, the vectors by which they move around (otherwise policies might be misdirected), and the costs of alternative policies.
Fortunately, research progress is rapid at this nexus of biology, economics, and policy. For example, a recent analysis demonstrated that the Australian Weed Risk Assessment, under which any plant proposed for importation intoAustralia is allowed only if it survives a risk assessment, brings net economic benefits to Australia (Keller et al. 2007). In this book, we explore in more detail under which circumstances of costs, benefits, and spatial scales alternative policy and management strategies are warranted.
Determining the expected total benefits from a management action or policy is not straightforward and requires the expertise of both ecologists and economists. Additionally, because this work requires extensive modeling of the outcomes from alternative scenarios, mathematicians are needed to synthesize models from ecology and economics into a unified framework. Only when the expertise from these fields is combined does it become possible to answer the questions asked by managers and policy makers (figure 1.7). In the following paragraphs, we present two of these questions, and explain how expertise from the three disciplines can be used to provide solutions.
How do we rationally spend on prevention versus control for a species that is not yet established? When faced with species that are predicted by ecologists and/or economists to be damaging, the possible responses are to prevent its arrival, to manage it once it arrives, or to simply live with the impacts. Deciding among these options requires knowledge of the economic costs of prevention (e.g., removing a fish species from the aquarium trade), the costs of managing the species if it arrives (e.g., seasonal pesticide applications to reduce population densities), the benefits from not having the species, and the benefits from controlling the species versus no management. Usually, it will also be necessary to consider multiple methods for both preventing and managing the species. Extracting answers from such potentially complex series of scenarios requires a combination of rigorous economic and ecological models.
FIGURE 1.7.
Three areas of research that must be jointly considered to understand biological invasions and reduce their impact. The goal of this book is to increase the area of overlap among the three circles.
What level of resources is it rational to spend on one versus another vector of invasive species transport? Most ecosystems have received invasive species from multiple vectors (see chapter 10 for an analysis of the Great Lakes ecosystem). It is necessary to be mindful of multiple routes of trade and travel when determining how best to reduce the risk from new invasions. Determining the appropriate expenditure on each vector will require knowledge of the total value of that vector, the costs that restrictions on it would cause, and the degree to which the risk of new invasions would be reduced considering any compensatory responses from other vectors. It would also require forecasts of the likely economic and ecological impacts of invasive species from each vector. Scenarios covering the range of management responses would need to be rigorously assessed to determine which approach is optimal.
In the following chapters, we present methods for answering these and related questions. Collectively, the authors of this book come from the disciplines of economics, ecology, and mathematical biology. We have worked together for more than 7 years under the auspices of the Integrated Systems for Invasive Species (ISIS) project, funded by the U.S. National Science Foundation and Canadian Natural Sciences and Engineering Research Council. Over this time, we have identified many important scientific questions and attempted to provide tools for answering them in ways that are relevant to addressing the issues of invasive species. The conceptual frameworks presented in this book have been developed through extensive collaboration, such that all chapters are heavily influenced by each discipline. In each chapter, we critically review the work of both the ISIS project and of the many other researchers working on similar problems.
Chapter 2 presents a more theoretical analysis of the ways in which ecological and economic systems interact, and how invasive species affect these interactions. This chapter shows how the problems of managing invasions can be addressed through a unified bioeconomic framework.
Chapters 3–6 are organized according to the ecological progression of invasions (figure 1.1). Chapter 3 describes the methods available for predicting the identity and the economic and ecological impacts of invaders before they are introduced. If this can be done successfully, information becomes available to determine which species or vectors it is rational to control to prevent invasive species from arriving. Another component in such a decision, however, is the potential geographic range of a species. Chapter 4 reviews recent developments for environmental niche models, tools that describe where a species is able to survive. These models can be used before a species is established to determine the value of prevention and after it is established to determine the value of control, slow-the-spread efforts, and, if possible, eradication.
Because the likelihood that a species will become established is positively related to the number of individuals released—propagule pressure—it may be possible to prevent invasions by controlling the rate and location of releases of individuals. Chapter 5 reviews the ecological and mathematical theory behind this approach and describes the combinations of vectors and species for which management based on it will be appropriate.
Once a species is introduced (e.g., through the pet trade vector), it is often dispersed secondarily by additional human vectors, such as recreational boating. Chapter 6 reviews the available models for predicting such secondary dispersal and illustrates with case studies how these models can inform management responses.
Chapters 7–9 address more general issues for bioeconomic modeling of invasive species. Chapter 7 provides a rigorous analysis of the types of uncertainty that exist and the general issues that they present for modeling. It provides context for many of the models and methods presented in other chapters and suggests research strategies for the future.
Although the market costs of invasive species are generally easy to resolve, the nonmarket costs are extremely difficult to assess, as they are for many other environmental issues. Despite this, there is ample reason to believe that nonmarket costs are often substantial. Chapter 8 describes and reviews methods for determining these costs.
Chapter 9 ties together the models and theory from earlier chapters and presents a framework for integrating economic and ecological data to determine the optimal type and timing of invasive species management. This chapter emphasizes the ways that management efforts affect ecological and economic systems and how the state of those systems feeds back and affects optimal management.
Chapter 10 analyzes for one ecosystem, the North American Great Lakes, the many ways that economic forces and ecology have interacted to create the current state of invasions. This ecosystem is the focus of much economic activity, including canal construction and navigation, commercial and recreational angling, aquaculture, and the ornamental plant and animal trades. Activities such as these have led to the establishment of at least 183 nonindigenous species (Ricciardi 2006), many of which have become invasive.
Chapters 11 and 12 present case studies for two well-known invasive species and show how the bioeconomic framework presented throughout the book has been applied to prescriptions for their management. Chapter 11 focuses on the rusty cray-fish (Orconectes rusticus), an invader of lakes and streams in the U.S. Upper Midwest with large economic and ecological impacts, and discusses methods for control and eradication. Chapter 12 focuses on the zebra mussel, a well-studied invader across Europe and North America. This species has received much management effort and provides a good case study to demonstrate the necessity for, and effectiveness of, management that is based on a rigorous bioeconomic understanding of an invasive species.
Finally, in chapter 13, we take a step back from the more technical issues and critically assess the contribution that bioeconomic modeling has made to effective management of invasive species (figure 1.7). Anumber of examples from the authors’ own work are described, along with the management responses that have come from them.
BenDor, T. K., S. S. Metcalf, L. E. Fontenot, B. Sangunett, and B. Hannon. 2006. Modeling the spread of the emerald ash borer. Ecological Modelling 197:221–236.
Bossenbroek, J. M., L. E. Johnson, B. Peters, and D. M. Lodge. 2007. Westward expansion of the zebra mussel in North America: forecasting a low probability-high impact event. Conservation Biology 21:800–810.
Burnham, C. R. 1988. The restoration of the American chestnut. American Scientist 76:478–487.
Caswell, H. 2001. Matrix population models: construction, analysis, and interpretation, 2nd edition. Sinauer Associates, Sunderland, MA.
Clark, C. W. 1990. Mathematical bioeconomics: the optimal management of renewable resources, 2nd edition. John Wiley and Sons, New York.
Cohen, A. N., and J. T. Carlton. 1998. Accelerating invasion rate in a highly invaded estuary. Science 279:555–558.
Costello, C., J. M. Drake, and D. M. Lodge. 2007. Evaluating the effectiveness of an environmental policy: ballast water exchange and invasive species in the North American Great Lakes. Ecological Applications 17:655–662.
Crocker, T., and J. Tschirhart. 1992. Ecosystems, externalities and economics. Environmental and Resource Economics 2:551–567.
Daly, H. 1968. On economics as a life science. Journal of Political Economy 76:392–406.
Dasgupta, P., S. Levin, and J. Lubchenco. 2000. Economic pathways to ecological sustainability. BioScience 50:339–345.
de Camino Beck, T., and M. A. Lewis. 2007. A new method for calculating net reproductive value from graph reduction with applications to the control of invasive species. Bulletin of Mathematical Biology 69:1341–1354.
Diekmann, O., J. A. P. Heesterbeek, and J. A. J. Metz. 1990. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology 28:365–382.
Drake, J. M., and J. M. Bossenbroek. 2004. The potential distribution of zebra mussels in the United States. BioScience 54:931–941.
Finnoff, D., and J. Tschirhart. 2003. Protecting an endangered species while harvesting its prey in a general equilibrium ecosystem model. Land Economics 79:160–180.
Forseth, I. N., and A. F. Innis. 2004. Kudzu (Pueraria montana): history, physiology, and ecology combine to make a major ecosystem threat. Critical Reviews in Plant Sciences 23:401–413.
Gilbert, G. S. 2002. Evolutionary ecology of plant diseases in natural ecosystems. Annual Review of Phytopathology 40:13–43.
Hamlin, C., and D. M. Lodge. 2006. Ecology and religion for a post natural world. Pages 279–309 in D. M. Lodge and C. Hamlin, editors. Religion and the new ecology: environmental responsibility in a world in flux. University of Notre Dame Press, South Bend, IN.
Hawaii Department of Agriculture. 2002. Kahului Airport risk assessment. Hawaii Department of Agriculture, Plant Quarantine Division, Honolulu, HI.
Hedley, J. 2004. The international plant protection convention and invasives. Pages 185–202 in M. L. Miller and R. N. Fabian, editors. Harmful invasive species: legal responses. Environmental Law Institute, Washington, DC.
Holzmueller, E., S. Jose, M. Jenkins, A. Camp, and A. Long. 2006. Dogwood anthracnose in eastern hardwood forests: what is known and what can be done? Journal of Forestry 104:21–26.
Jenkins, P. T. 2002. Paying for protection from invasive species. Issues in Science and Technology 19:67–72.
Jeschke, J. M., and D. L. Strayer. 2005. Invasion success of vertebrates in Europe and North America. Proceedings of the National Academy of Sciences of the United States of America 102:7198–7202.
Keeling, M. J., M. E. J. Woolhouse, R. M. May, G. Davies, and B. T. Grenfell. 2003. Modelling vaccination strategies against foot-and-mouth disease. Nature 421:136–142.
Keller, R. P., D. M. Lodge, and D. C. Finnoff. 2007. Risk assessment for invasive species produces net bioeconomic benefits. Proceedings of the National Academy of Sciences of the United States of America 104:203–207.
Kumaranayake, L. 2006. Trade and infectious disease outbreaks: ensuring public health without compromising free trade. Pages 341–354 in J. A. Roberts, editor. The economics of infectious disease. Oxford University Press, Oxford.
Leung, B., D. M. Lodge, D. Finnoff, J. F. Shogren, M. A. Lewis, and G. Lamberti. 2002. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings of the Royal Society of London Series B Biological Sciences 269:2407–2413.
Levine, J. M., and C. M. D’Antonio. 2003. Forecasting biological invasions with increasing international trade. Conservation Biology 17:322–326.
Lodge, D. M., and K. Shrader-Frechette. 2003. Nonindigenous species: ecological explanation, environmental ethics, and public policy. Conservation Biology 17:31–37.
Lodge, D. M., S. Williams, H. MacIsaac, K. Hayes, B. Leung, S. Reichard, R. N. Mack, P. B. Moyle, M. Smith, D. A. Andow, J. T. Carlton, andA. McMichael 2006. Biological invasions: recommendations for U.S. policy and management. Ecological Applications 16:2035–2054.
Lovell, S. J., S. F. Stone, and L. Fernandez. 2006. The economic impacts of aquatic invasive species: a review of the literature. Agricultural and Resource Economics Review 35:195–208.
Mangtani, P., and A. Shah. 2006. The socio-economic burden of influenza: costs of illness and “willingness to pay” in a publicly funded health care system. Pages 159–180 in J. A. Roberts, editor. The economics of infectious disease. Oxford University Press, Oxford.
Meinesz, A. 1999. Killer Algae. University of Chicago Press, Chicago.
Morris, R. S. 1999. The application of economics to animal health programmes: a practical guide. Revue Scientifique et Technique, Office International des Epizooties 18:305–314.
Narasimham, V. 2006. Trade and infectious disease outbreaks: ensuring public health without compromising free trade. Pages 341–354 in J. A. Roberts, editor. The economics of infectious disease. Oxford University Press, Oxford.
Olson, L. J. 2006. The economics of terrestrial invasive species: a review of the literature. Agricultural and Resource Economics Review 35:178–194.
O’Neill, C. R. 1996. National zebra mussel information clearinghouse infrastructure economic impact survey—1995. Dreissena! 7(2):1–5.
Ostry, M. E., and K. Woeste. 2004. Spread of butternut canker in North America, host range, evidence of resistance within butternut populations and conservation genetics. Pages 114–120 in C. H. Michler, P. M. Pijut, J. W. Van Sambeek, M. V. Coggeshall, J. Seifert, K. Woeste, R. Overton, and F. Ponder, Jr., editors. Black walnut in a new century: proceedings of the 6th Walnut Council Research Symposium. U.S. Department of Agriculture, Forest Service, North Central Research Station. St. Paul, MN.
Pauwelyn, J. 1999. The WTO agreement on sanitary and phytosanitary (SPS) measures, as applied in the first three SPS disputes: EC—hormones, Australia—salmon and Japan—varietals. Journal of International Economic Law 2:641–664.
Perry, W. L., D. M. Lodge, and J. L. Feder. 2002. Importance of hybridization between indigenous and nonindigenous freshwater species: an overlooked threat to NorthAmerican biodiversity. Systematic Biology 51:255–275.
Pimentel, D., R. Zuniga, and D. Morrison. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52:273–288.
Ricciardi, A. 2001. Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? Canadian Journal of Fisheries and Aquatic Sciences 58:2513–2525.
Ricciardi, A. 2006. Patterns of invasion in the Laurentian Great Lakes in relation to changes in vector activity. Diversity and Distributions 12:425–433.
Riviere-Cinnamond, A. 2006. Economics of animal health: implications for public health. Pages 215–236 in J. A. Roberts, editor. The economics of infectious disease. Oxford University Press, Oxford.
Roberts, J. A., editor. 2006. The economics of infectious disease. Oxford University Press, Oxford.
Rushton, J., P. K. Thornton, and M. J. Otte. 1999. Methods of economic impact assessment. Revue Scientifique et Technique, Office International des Epizooties 18:315–338.
Settle, C., T. D. Crocker, and J. F. Shogren. 2002. On the joint determination of biological and economic systems. Ecological Economics 42:301–311.
Sharov, A. A. 2004. Bioeconomics of managing the spread of exotic pest species with barrier zones. Risk Analysis 24:879–892.
Sharov A., D. Leonard, A. M. Liebhold, E. A. Roberts, and W. Dickerson. 2002. Slow the spread: a national program to contain the gypsy moth. Journal of Forestry 100(5):30–36.
Shine, C., N. Williams, and F. Burhenne-Guilmin. 2005. Legal and institutional frameworks for invasive alien species. Pages 233–284 in H. A. Mooney, R. N. Mack, J. A. McNeely, L. E. Neville, P. J. Schei, and J. K. Waage, editors. Invasive alien species: a new synthesis. Island Press, Washington, DC.
Shogren, J., and T. Crocker. 1999. Risk and its consequences. Journal of Environmental Economics and Management 37:44–51.
Sohngen, B., and R. Mendelsohn. 1998. Valuing the impact of large-scale ecological change in a market: the effect of climate change on U.S. timber. American Economic Review 88:686–709.
Steeger, C., R. Holt, and J. Smith. 1999. Enhancing biodiversity through partial cutting. British Columbia Ministry of Forests report. Pandion Ecological Research, Nelson, British Columbia. Stokstad, E. 2007. Invasive species—feared quagga mussel turns up in western United States. Science 315:453–453.
Strayer, D. L., and H. Malcom. 2007. Effects of zebra mussels (Dreissena polymorpha) on native bivalves: the beginning of the end or the end of the beginning? Journal of the North American Benthological Society 26:111–122.
U.S. Department of Commerce. 2008. TradeStats Express. Available at http://tse.export.gov/.
Walters, L. J., K. R. Brown, W. T. Stam, and Olsen J. L. 2006. E-commerce and Caulerpa: unregulated dispersal of invasive species. Frontiers in Ecology and the Environment 4:75–79.
Wilson, E. O. 1998. Consilience. Alfred Knopf, New York.
Wonham, M. J., T. de Camino-Beck, and M. A. Lewis. 2004. An epidemiological model for West Nile Virus: invasion analysis and control applications. Proceedings of the Royal Society of London Series B Biological Sciences 271:501–507.
Woodfield, R., and K. Merkel. 2005. Eradication and surveillance of Caulerpa taxifolia within Agua Hedionda Lagoon, Carlsbad, California, Fourth Year Status Report. Report prepared for Southern California Caulerpa Action Team.
World Health Organization. 2002. World health report. World Health Organization, Geneva.
Xia, Y., O. N. Bjørnstad, and B. T. Grenfell. 2004. Measles metapopulation dynamics: a gravity model for pre-vaccination epidemiological coupling and dynamics. American Naturalist 164:267–281.
Yule, A. M., J. W. Austin, I. K. Barker, B. Cadieux, and R. D. Moccia. 2006. Persistence of Clostridium botulinum neurotoxin type E in tissues from selected freshwater fish species: implications to public health. Journal of Food Protection 69:1164–1167.