Chapter 12
Avian Migration and Dispersal
David W. Winkler1, Judy Shamoun‐Baranes2, and Theunis Piersma3
1 Cornell University; 2 University of Amsterdam; 3 University of Groningen

(Photograph by Dov E.)
The movements of birds are among their most captivating traits. The regular seasonal movements of avian migrants are remarkably diverse: phalaropes (Phalaropus species) from the high Arctic spend their non‐breeding season in upwelling areas on open tropical seas; Northern Wheatears (Oenanthe oenanthe) traverse most of the northern hemisphere in their back‐and‐forth movements to ancestral wintering areas in Africa; and Resplendent Quetzals (Pharomachrus mocinno) travel from one side of Costa Rica’s mountainous spine to the other and back again in their annual search for fruiting trees. Birds migrate great distances to find suitable habitats, and they also engage in dispersal from the location where they hatched to their adult breeding sites.
Every avian migratory pattern is the result of an adaptive molding of a species’ movements to variation in its environment. Even among similar species in the same habitat, some birds may migrate while others do not. For example, consider the woodpeckers present in summer and winter in the woods outside nearly any town in the northern hemisphere. Woodpeckers in the temperate zone range in size from that of a small thrush to the size of a crow, and in most woods three to five different species occur. The various members of this family share a good deal of their overall biology—chisel‐shaped bills to excavate wood, a brain so well secured that it can stand the blasting that comes with wood chipping, claws that ensure firm grips on trunks and branches, and a stiff tail that helps to support their bodies against the trunks—but the different species have quite different patterns of annual movement. Often only the largest and smallest of these woodpecker species remain in the north for the winter. Closer inspection reveals that the species that leave for the winter are those that have particular food requirements. For example, Northern Flickers (Colaptes auratus) in the northern half of their North American range can no longer excavate ants in snow‐covered frozen ground, and Yellow‐bellied Sapsuckers (Sphyrapicus varius) in northeastern North America can no longer harvest the flowing sap of trees, so both species migrate to areas where their distinctive foraging habits can be pursued for the rest of the year.
Among migrants, differences in migratory routes can involve important differences in physiology. On the northeast coast of North America in fall, two of the songbirds that are captured most commonly in migratory mist‐netting stations are Yellow‐rumped (Setophaga coronata) and Blackpoll (Setophaga striata) Warblers. These closely related songbirds follow very different paths once the breeding season is over. The Yellow‐rumped Warblers will migrate south and winter in the southern USA and Central America, while the Blackpoll Warblers will spend the non‐breeding season in northern South America. The more northern non‐breeding range of the Yellow‐rumped Warbler is reached by fairly short flights over land, whereas the Blackpoll Warbler reaches South America with one long, non‐stop flight that lasts up to 3 days. The Blackpoll Warblers follow prevailing winds out over the Atlantic Ocean past the island of Bermuda until they encounter the trade winds that bring them back to the Caribbean shore of South America. Many ornithologists were very slow to accept the proposition that these small birds were making such long transoceanic flights, but many lines of evidence, including recent tracking of individual birds, now support this conclusion (DeLuca et al 2015). The most striking evidence, visible in the hand when birds are captured before departure, is that the Blackpoll Warbler amasses such huge stores of fat to fuel its journey that it doubles its pre‐departure mass.
Dispersal, the process of discovering and moving to a new breeding home, occurs in all birds, even non‐migratory birds from less seasonal environments. Dispersers must leave their natal territory, where their parents raised them, and get to know their surroundings well enough to find a mate and a breeding locale of their own. Compared with the distances traveled during many migrations, these dispersal movements are small in scale, yet the exploratory movements of dispersal are fraught with uncertainty, and as many birds likely fail to disperse successfully as die in migration. In any particular species, most dispersers stay fairly close to their natal home, yet other individuals may disperse across an entire continent. Dispersal, like migration, comprises a continuum of movements, from the local to the global.
12.1 Types of movements
Avian movements include migration, two‐way trips that bring individual birds back and forth between breeding and wintering sites each year, and dispersal, a departure from the site where birds hatched or bred to find a breeding location elsewhere. In places where a bird species can persist year round, natural selection will likely not favor long‐distance annual movements, with all of its attendant risks. In contrast, birds living in highly seasonal environments may have no choice but to move to distant locations to avoid the harshest seasons or to find a breeding opportunity. Both staying and moving have costs and benefits, and whether an individual bird stays in one place or moves depends on which strategy is more likely to ensure its survival and reproduction.
12.1.1 Philopatry
One of the surprising facts about birds—even the migrants that cover tens of thousands of kilometers in their annual journeys—is that most individuals return annually to the same areas where they bred the year before. This site faithfulness is termed philopatry, a term derived from the ancient Greek words philos (loving) and patria (fatherland).
Most adult birds are philopatric in successive breeding seasons, returning to the same breeding site (or a site nearby) as long as they survive the intervening period. Thus, shearwaters and albatrosses travel the oceans of the world but often return each breeding season to the very same nest scrape or nesting burrow. Some Arctic Terns (Sterna paradisaea) make annual journeys of almost 100,000 kilometers as they migrate from high‐latitude northern breeding sites to non‐breeding areas in the Antarctic region (Fig. 12.01); they return to the same breeding sites more than 98% of the time (Devlin et al. 2008; Fijn et al. 2013). Bobolinks (Dolichonyx oryzivorus) make a round‐trip journey from their grassland breeding areas in northeastern North America to non‐breeding areas in southern South America, and exhibit more than 80% site faithfulness when they return to the breeding grounds (Fajardo et al. 2009).
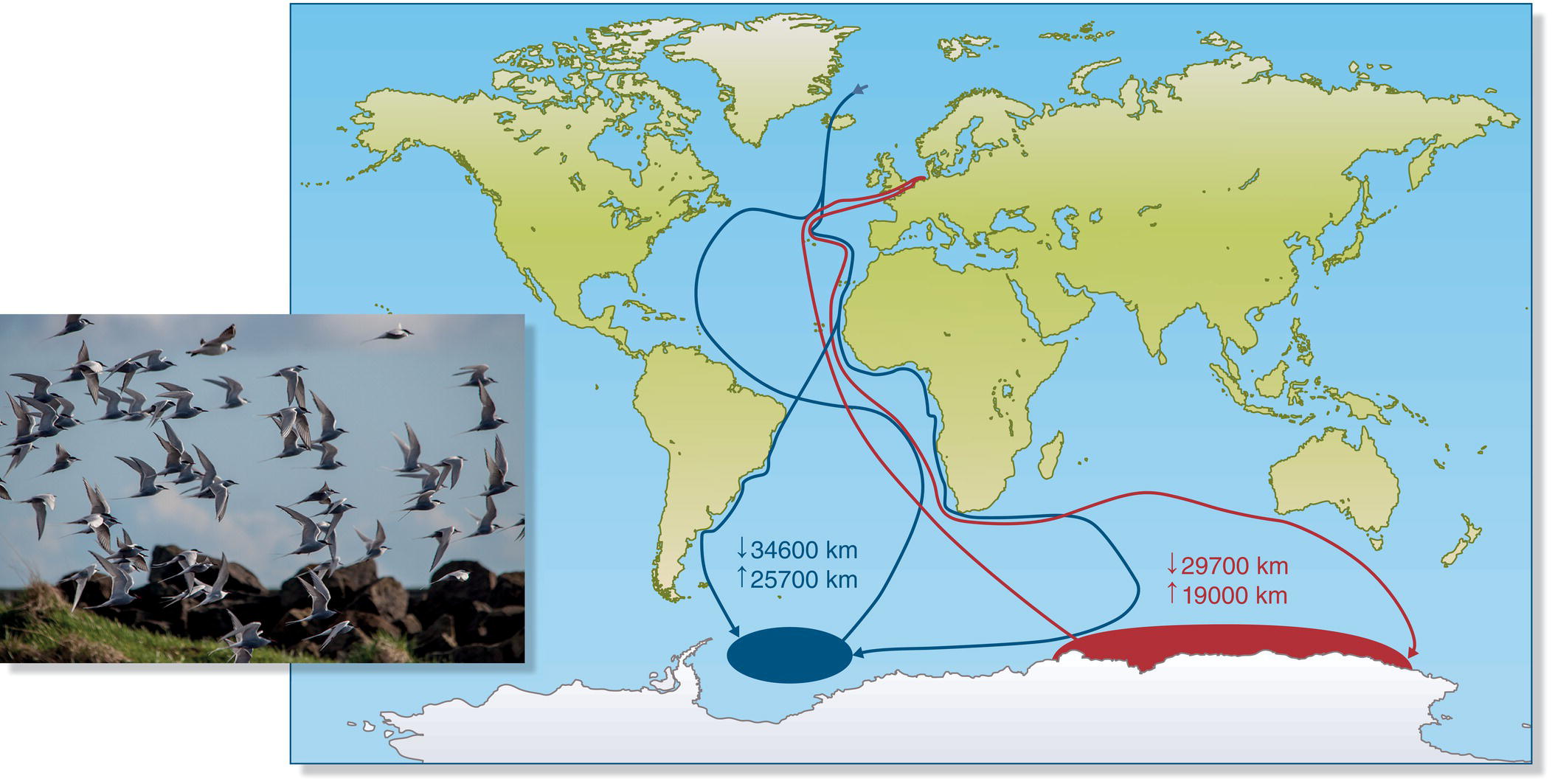
Fig. 12.01 Long‐distance migration. Arctic Terns (Sterna paradisaea) breed in the Arctic and spend their non‐breeding season in or near the Antarctic region. This map depicts the incredibly long migratory tracks (red and blue lines) of two individuals that were tracked throughout their annual cycle.
(From Fijn et al. 2013. Reproduced with permission from ARDEA. Photograph by Örn Óskarsson.)
Philopatry often occurs in the non‐breeding season too. For example, individually marked American Redstarts (Setophaga ruticilla), Black‐throated Blue (Setophaga caerulescens), and Black‐and‐white (Mniotilta varia) Warblers in the Dominican Republic often return to the same territory winter after winter (Wunderle and Latta 2000). Indeed, some early experiments on the homing abilities of wild birds involved transporting them away from their winter home ranges, to which they proved philopatric in later years. For example, ornithologist Richard Mewaldt (1964) displaced some of the White‐crowned Sparrows (Zonotrichia leucophrys) that wintered in his California backyard by sending them east across the North American continent as air cargo to Louisiana and Maryland, where they were released. These displaced sparrows did not return immediately, but many came back to his California yard the next winter, presumably after having flown first to their more northerly breeding locations in the intervening spring (Fig. 12.02).

Fig. 12.02 Winter site fidelity. In a classic experiment, White‐crowned Sparrows (Zonotrichia leucophrys) overwintering in California were transported (dashed lines) and released in two distant locations in Louisiana and Maryland (USA). Despite the unexpected uprooting, these individuals likely migrated north to breed (shaded area) and then returned to their original overwintering sites in California the next year.
(From Mewaldt 1964. Reproduced with permission from AAAS. Photograph by Jack Sutton.)
12.1.2 Local movements, migration, and dispersal
Birds are very mobile creatures and there are many ways to classify their movements. One way to distinguish types of movements is by identifying what they accomplish for the bird. At the short end of the timescale are the local movements associated with daily foraging, flights to and from roost sites, and other daily activities. Migration, by contrast, is an annually repeated seasonal movement away from the breeding area and then back to it, usually over a far greater distance than an individual’s local movements. Birds generally undertake migration to avoid times when resources are scarce and conditions are harsh, or, conversely, to exploit sites and seasons where the benefits of conditions there far outweigh the costs. Migrating birds usually are clearly migrating and doing little else at the same time: a migration is a means to cover distance and reach a new area to live for a season. The final general type of avian movement, dispersal, is a movement to find a place to breed. In most birds, dispersal movements occur on a smaller spatial scale than does migration, and dispersal will generally occur only once or a very few times in an individual bird’s life.
The distinction between local movements and migrations cannot be made solely on the basis of the distance traveled: some types of birds move farther during daily foraging than others do during seasonal migration. For example, Imperial Eagles (Aquila heliaca) in Spain can travel up to 114 kilometers in a single day’s foraging (Fernández et al. 2009) and Short‐tailed Shearwaters (Ardenna tenuirostris) may fly up to 15,000 kilometers during 3‐week‐long foraging trips in the vast Southern Ocean before returning to their nest (Phillips et al. 2008).
The local movements of birds are usually dictated by their habitat and by their need to feed and to avoid being eaten themselves. Many birds stay within a territory or home range when making most of their local movements. Dippers (Cinclus species) that live along fast‐flowing streams worldwide have accordingly long, linear territories along which they move back and forth daily in search of food. Most oystercatchers, and many coastal and riverine birds (some shorebirds, swallows, sungrebes, screamers, etc.), are similarly tied to shoreline habitat, and their daily movements are generally up and down shores rather than across country.
Birds with less restrictive habitat requirements undertake movements that may extend in all directions, up to the edges of their defended space. Still, their movements tend to be concentrated within their territories, spending most of their time in areas that provide abundant food, shelter from predators and the elements, or key areas for displaying and singing. Fairy wrens (Malurus species) in Australia retain small territories for much of the year, and they restrict their movements even closer to the nest when they are feeding nestlings (Tidemann 1990). Swainson’s Warblers (Limnothlypis swainsonii) in Arkansas (USA) (Anich et al. 2012) and Chestnut‐backed Antbirds (Myrmeciza exsul) in Costa Rica (Marcotullio and Gill 1985) concentrate their activities in the most densely vegetated parts of their territories, probably because those areas provide the safest foraging opportunities. Like many other male songbirds, male Cerulean Warblers (Setophaga cerulea) remain close to the trees they favor for singing from during the early part of the breeding season in eastern North America (Barg et al. 2006).
In contrast, other birds may have very little attachment to a single prescribed territory during any part of their annual cycle. Birds that forage on the seas or in the open sky often have feeding conditions that are highly unpredictable and food sources that are diffuse and impossible to defend. Thus these birds often forage over very large distances, and their local movements can occur over a vast scale, as in many pelagic seabirds. Similarly, many swifts and swallows range over broad distances even while feeding young in a nest. Once the constraint of feeding young is removed, these broad foragers become even less limited. Tracking studies of Alpine Swifts (Apus melba) have shown that these birds remain in the air after leaving their breeding sites, flying non‐stop for at least 6 months as they migrate to sub‐Saharan Africa and back (Liechti et al. 2013). When the small seabirds called Black‐legged Kittiwakes (Rissa tridactyla) finish the breeding season in southern Alaska (USA), they show a variety of movements: some kittiwakes stay near the breeding colony all winter, others move up and down the nearby coast, and a third contingent spends much of the winter over the open ocean (McKnight et al. 2011) (Fig. 12.03).
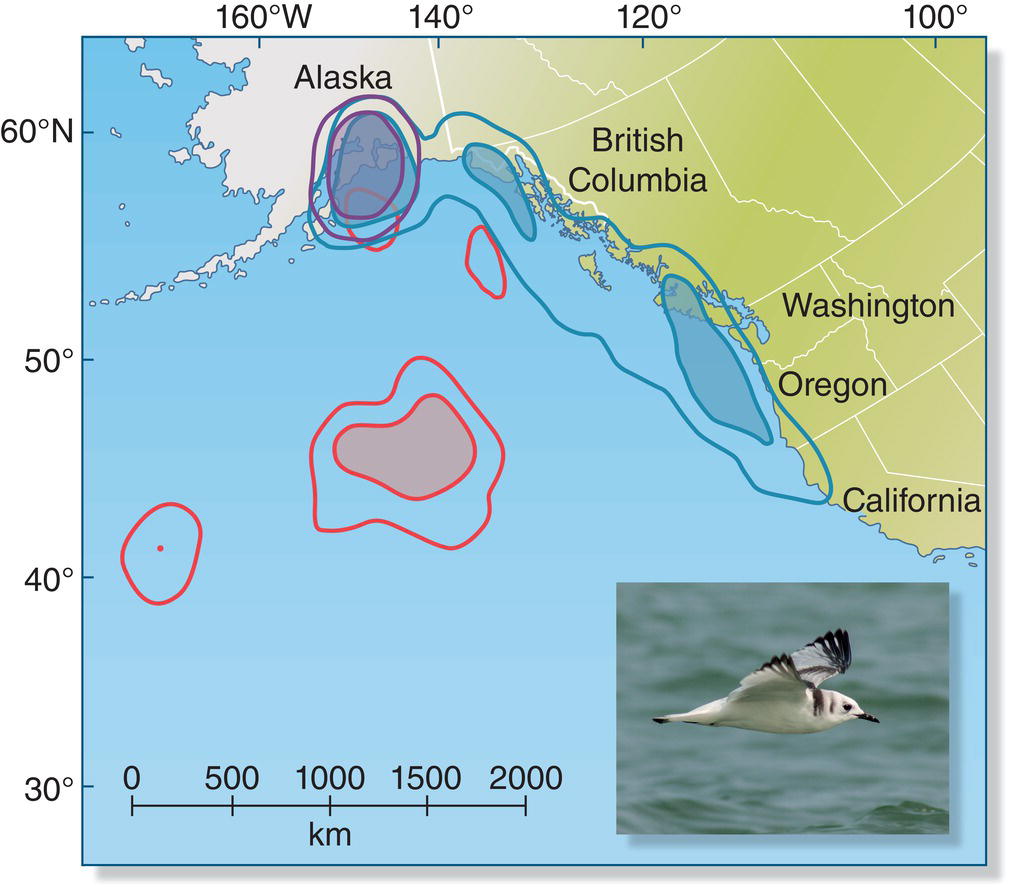
Fig. 12.03 Movements in the non‐breeding season. Individual Black‐legged Kittiwakes (Rissa tridactyla) from the same breeding colony in Alaska (USA) move to different locales in the non‐breeding season: some remain near the colony (purple), others move southeast along the coast (blue), and some overwinter at sea (red).
(From McKnight et al. 2011. © 2011 Inter‐Research. Reproduced with permission. Photograph by Bernie Monette.)
In migratory birds, daily patterns of local movement can vary greatly at different times across the annual cycle. Red Knots (Calidris canutus) range over hundreds of square kilometers of mudflats in the Dutch Wadden Sea (Piersma et al. 1993), but when they arrive on their non‐breeding grounds on the mudflats of Banc d’Arguin, Mauritania, where the availability of food is much more predictabe than in the Netherlands, they limit their ranging to less than a single square kilometer (Leyrer et al. 2012). Recent developments in tracking technology are helping ornithologists explore the local movements of these birds in ever greater detail (Box 12.01).
12.2 Patterns in migration
Each of the world’s species of birds has adapted to the seasonality of resources across the earth’s surface. Some birds find conditions around their breeding area to be sufficient for living year round, and these species often remain in those areas permanently. But for a surprisingly large number of species, conditions are sometimes better elsewhere, and these species engage in various kinds of large‐scale annual movements. Annual migrations can be as long as the globe‐spanning journeys of Arctic Terns (Sterna paradisaea), or as short as the shift of a hummingbird or sunbird a few kilometers up and down a tropical mountain. Patterns in the migrations of birds are affected by many different biological influences, and, in turn, patterns in migration often have fascinating implications for other aspects of avian biology.
Luckily for birds, a large proportion of environmental variation is relatively predictable. The single‐most important driver of environmental variation on earth—seasonal variation—exists because the earth’s axis of rotation is not perpendicular to the sun. It is a cosmic accident that the earth’s present axis of rotation is at an angle of 23.5° to the vertical: because of this tilt, the earth has seasons. In the northern winter, the northern hemisphere leans away from the sun, receiving less solar energy per hour and for fewer hours per day; in the northern summer, the balance is redressed, and the northern hemisphere basks in long days and short, warmer nights. Indeed, towards the North and South Poles, the summer sun does not set for many weeks at a time and winter darkness lasts equally long.
Birds breeding in high latitudes must contend with this seasonal variation, either by remaining throughout the winter or by moving away. To profit from the period of summer abundance, a migratory bird must both arrive and leave at the appropriate time, which requires the ability to move quickly, accurately, and relatively cheaply. With the ability to travel back and forth over large distances, migrating birds can enjoy a suite of favorable environments that occur sequentially at different locations during earth’s annual cycle.
12.2.1 Types of annual movements
One of the most useful ways to categorize migration is by defining different seasonal patterns of movement (Fig. 12.04). Readers who live in the northern or southern temperate zone may take it for granted that many of the birds around them disappear for a large part of every year, often cherishing the residents that can make a living throughout their annual cycle even in locations with harsh winters. These resident birds have the simplest pattern of annual movement—that is, no annual movement at all. Once a resident settles down, it tends to remain in the same general area for the rest of its life, and for some birds this area may be quite small. In the tropics, residency is more common than migration. For example, Chowchillas (Orthonyx spaldingii) (Fig. 12.05) in the wet forests of eastern Australia occupy home ranges of less than 2 hectares (Jansen 1999); individual birds likely remain within an area only a few hundreds of meters across for their entire lives. Many resident tropical birds have home ranges only a little larger than those of the Chowchilla; Ivory‐billed Woodcreepers (Xiphorhynchus flavigaster) in tropical Mexico are on the large end of this range, with home ranges of about 15 hectares (Rivera et al. 2003).
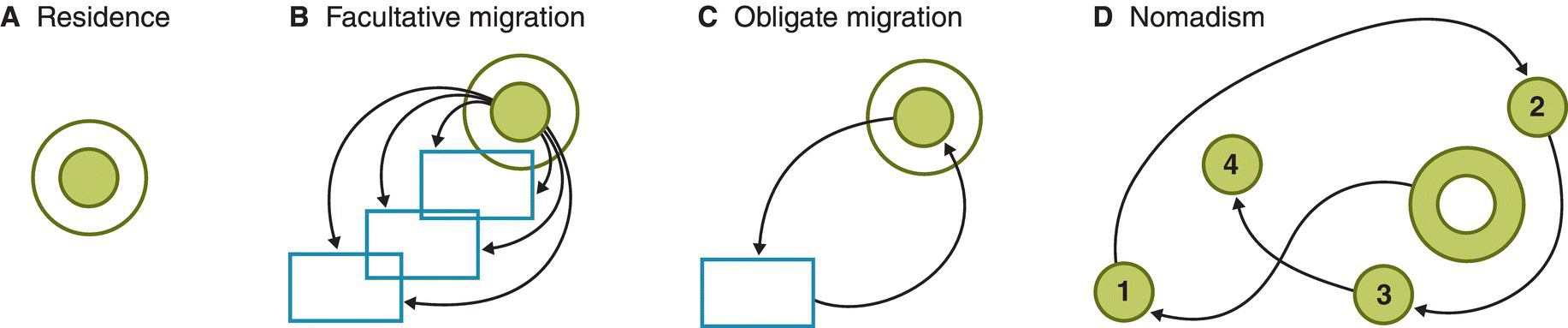
Fig. 12.04 Categories of avian residency and migration. Open shapes represent non‐breeding areas, shading represents breeding areas, and arrows denote movements that link them. (A) Residents remain in the same location throughout the year, and are thus not migratory. (B) Facultative migrants move varying distances in different years, usually depending on conditions in the non‐breeding season. (C) Obligate migrants undertake regular, predictable movements during their annual cycle. (D) Nomadic birds track environmental conditions in all seasons, often breeding in different locations in successive years.
(From Greenberg and Marra 2005. © 2005 Smithsonian Institute. Reproduced with permission from Johns Hopkins University Press.)

Fig. 12.05 Minimal annual movements. Some resident birds like the Chowchilla (Orthonyx spaldingii) of Australia rarely venture more than a few hundred meters from their hatching site.
(Photograph by Gerard Satherley.)
For other tropical birds, especially those that forage by commuting among widely scattered and ephemeral fruiting or flowering trees, daily movements may encompass tens of kilometers. For example, Wreathed Hornbills (Rhyticeros undulatus) in Thailand have a year‐round home range of 2800 hectares (Poonswad and Tsuji 2008). In the tropics, seasonal conditions often vary more in terms of rainfall than in temperature, and most birds do not need to migrate long distances to find suitable year‐round conditions. Thus, over much of the earth, there are more resident bird species than migrants.
Facultative migrants are birds that migrate only under certain conditions, rather than on a more predictable annual cycle. They may sometimes remain on their breeding grounds throughout the winter, moving elsewhere only in years when conditions are particularly harsh. Although their general migratory routes may be similar every year, the distances that facultative migrants travel along those routes can vary from year to year. Facultative migrants show similar variation in the timing of their movements, as they often linger in the breeding areas long after all the obligate migrants have departed on their fall migrations.
Facultative migration is usually triggered by deteriorating environmental conditions, such as a declining food supply or worsening weather. In northern temperate areas, facultative migrants include species such as shorebirds like the Northern Lapwings (Vanellus vanellus) of Eurasia, American Robins (Turdus migratorius) and Eastern Bluebirds (Sialia sialis) of North America, and European Blackbirds (Turdus merula) and European Starlings (Sturnus vulgaris). Members of all of these species sometimes remain in the breeding areas throughout a mild winter, but they move south or coastward when prey become unavailable in extremely cold weather. In North America, some populations of Yellow‐rumped Warblers (Setophaga coronata auduboni) are facultative migrants that track their winter food supply: when a cold front reduces insect availability, densities of Yellow‐rumped Warblers drop in Arizona, and the birds become more abundant to the south in Mexico (Terrill and Ohmart 1984) (Fig. 12.06).

Fig. 12.06 Facultative migrants. Yellow‐rumped Warblers (Setophaga coronata) in some regions move in response to prey availability. As the graph shows, fewer warblers remained at this winter study site when insect abundance declined—in this case, because of cold weather.
(From Terrill and Ohmart 1984. Reproduced with permission from American Ornithologists’ Union. Photograph by Jim Scarff.)
Obligate migrants undertake predictable annual migrations to distant non‐breeding grounds, sometimes traveling thousands of kilometers each season. In obligate migrant species, no individuals stay behind on the breeding grounds, and all of the migrants usually depart at similar times each year, often well before conditions begin to deteriorate. Most songbirds that breed in the northern parts of North America, Europe, and Asia fall into this category.
Many obligate migrants are site faithful in the winter, often showing as much territoriality and predictability in their winter behavior as on the breeding grounds. For example, both male and female Willow Flycatchers (Empidonax traillii) defend small territories throughout the winter in Costa Rica, and they are highly faithful to these sites from year to year (Koronkiewicz et al. 2006). In obligate migrants, many aspects of migration seem to be genetically controlled, resulting in very similar patterns across years. For example, these migrants move by a calendar that appears to be “hard‐wired” in the sense that it is more sensitive to changes in photoperiod than to temporary local variations in weather conditions. Even the smallest bird species can be obligate migrants: for example, Ruby‐throated Hummingbirds (Archilochus colubris) breed as far north as central Canada, then migrate long distances to the southern USA, Mexico, and Central America, sometimes buzzing across the Gulf of Mexico in a single flight.
Partial migrants are species in which some individuals within a population leave long in advance of winter, but others stay behind. This category does not appear in Figure 12.04 because it really represents a mix of different migration patterns within one population of birds. The complexity of partial migration can be seen in the distribution map of the European Blackbird (Fig. 12.07). In the northeastern part of this species’ range, all blackbirds are obligate migrants, with birds present only in the summer months. Conversely, there is a band along the coast of North Africa and in Iran where this species only occurs in the winter months. A broad region occurs between these areas where at least some blackbirds are present in all seasons. Yet in winter, these intermediate areas may harbor a mix of obligate migrants that arrived from the north in early fall, facultative migrants that arrived later in the fall and early winter as conditions deteriorated to the north, and individuals that remained as residents.
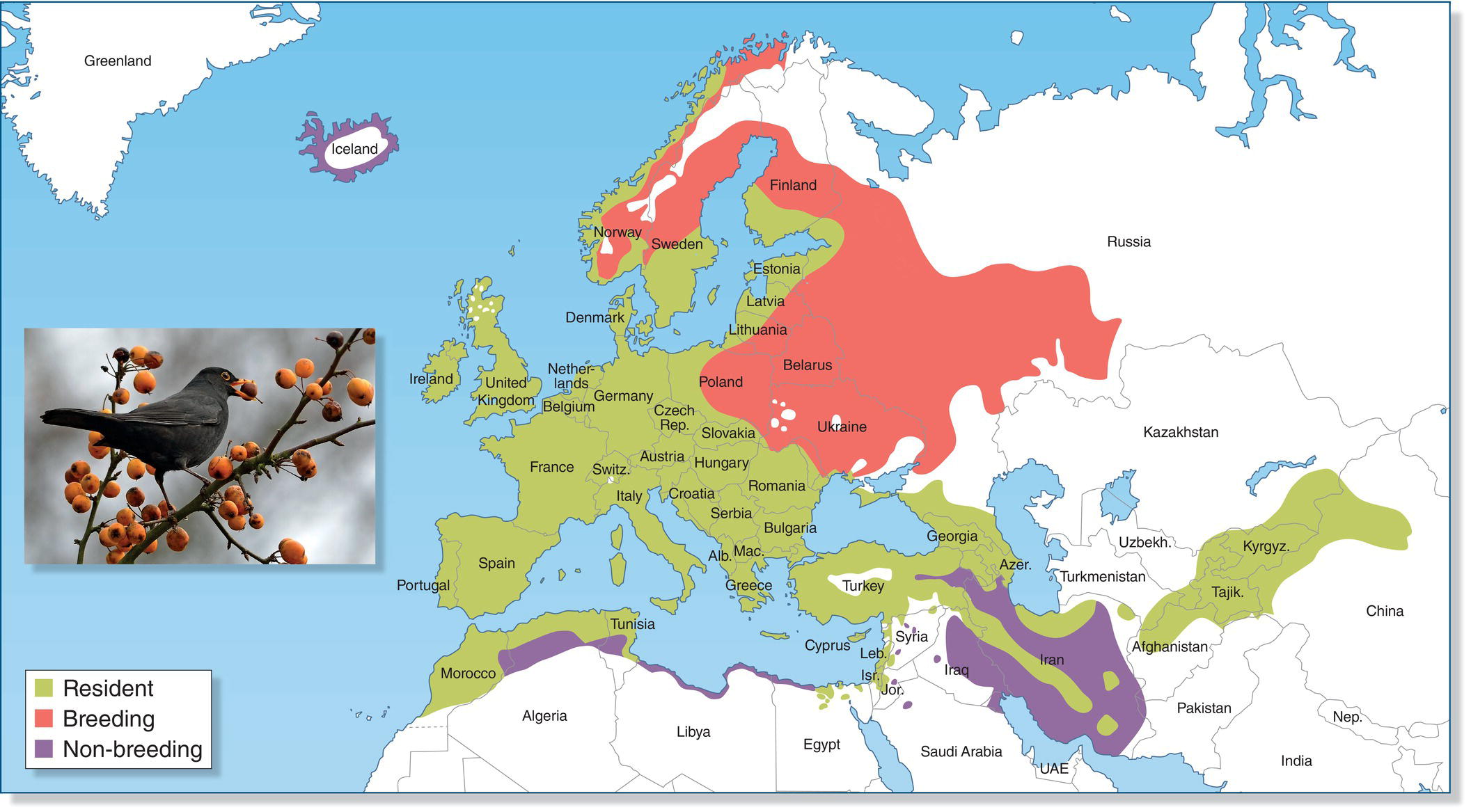
Fig. 12.07 Partial migrants. In different locations, European Blackbirds (Turdus merula) are year‐round residents, facultative migrants, or obligate migrants. Even within the region where blackbirds are present year round (green), the population includes full‐time residents, facultative migrants seeking refuge and resources because conditions farther north became unsuitable, and obligate migrants that migrate south every winter regardless of conditions in the north.
(Map courtesy of www.xeno‐canto.org. Adapted from BirdLife International and NatureServe 2014. Bird species distribution maps of the world. BirdLife International, Cambridge, UK and NatureServe, Arlington, USA. Photograph by Rinus Motmans.)
Nomadic bird species move less predictably from one breeding ground to the next, sometimes in great numbers, and often are quite flexible in where and when they breed. Some birds in this group are on the move for much of their lives. This mode of movement is limited almost entirely to birds of the tundra and coniferous forest of the far north, and to birds of the dry steppes, shrublands, and woods of the arid tropics. In these relatively simple ecosystems, fluctuations in the populations of a single type of food often can drive birds to move long distances between breeding sites. For example, Snowy Owls (Bubo scandiaca) in the high arctic tundras concentrate their foraging on lemmings, the populations of which are highly variable from year to year and from place to place. As a result, these owls have very little attachment to a single breeding site, and they may wander over many thousands of kilometers between breeding sites in successive breeding seasons (Therrien et al. 2014). In Australia in particular, a great variety of birds—from songbirds such as honeyeaters, trillers, and woodswallows, to larger birds such as kites and ducks—are quite flexible in when and where they breed, responding to great variation in water levels and the flowering of key food species of plants. Likewise, the Red‐billed Quelea (Quelea quelea) of Africa—a weaver—is famous both for the massive flocks that breed in ephemeral colonies and for the irregularity of their timing and location (Fig. 12.08). Queleas are nomadic because they feed on the seeds of unpredictable annual grasses that grow lushly only in locations and seasons with good rainfall (Cheke et al. 2007).
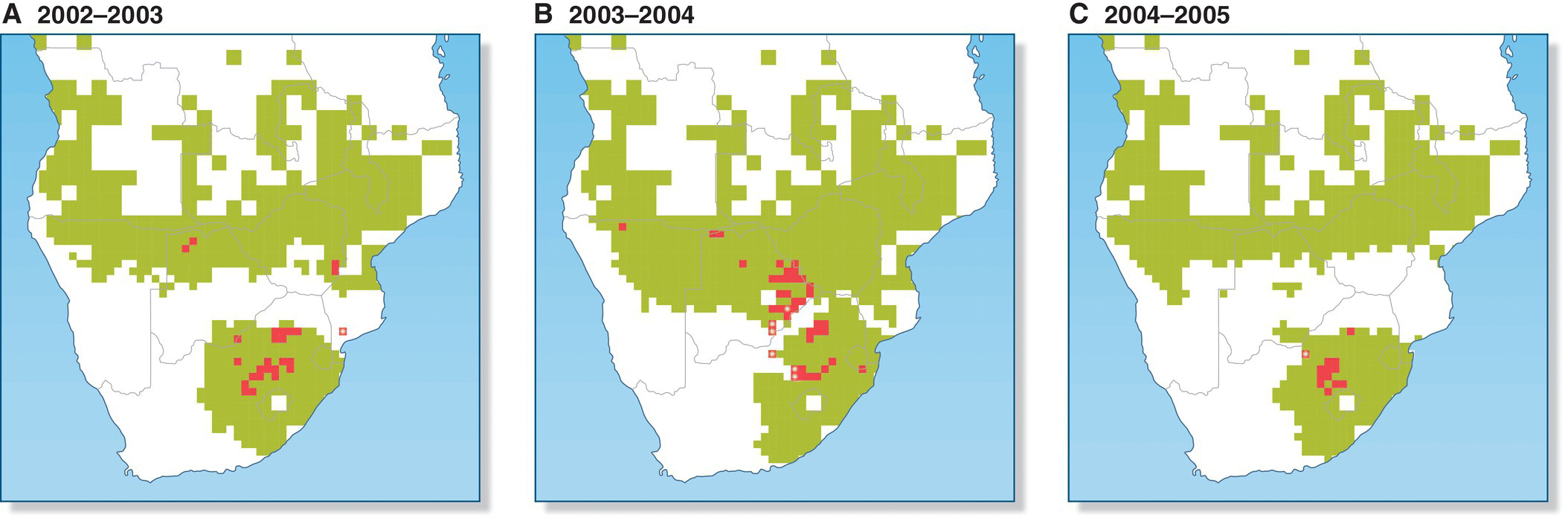
Fig. 12.08 Nomadic breeding. Massive colonies of Red‐billed Queleas (Quelea quelea) breed in locations that receive enough rainfall to produce grass seed (green), which serves as food and nesting material. Quelea colonies (red) move to different areas in different years.
(From Cheke et al. 2007. Reproduced with permission from John Wiley and Sons.)
12.2.2 Migratory connectivity
The term migratory connectivity refers to the degree to which a population of birds breeding in a distinct region remains cohesive as individuals move to a distinct region in the winter (Webster et al. 2002). In species with the strongest migratory connectivity (Fig. 12.09A), all members of a particular breeding population winter in the same well‐defined area. Conversely, where migratory connectivity is low, individuals from different breeding populations share the same non‐breeding sites (Fig. 12.09B). Confusingly, the term population connectivity is also used in reference to migrant birds, and as the tightness of migratory connectivity between breeding and non‐breeding areas increases, the population connectivity between adjacent breeding subpopulations via interactions at the wintering grounds grows weaker.
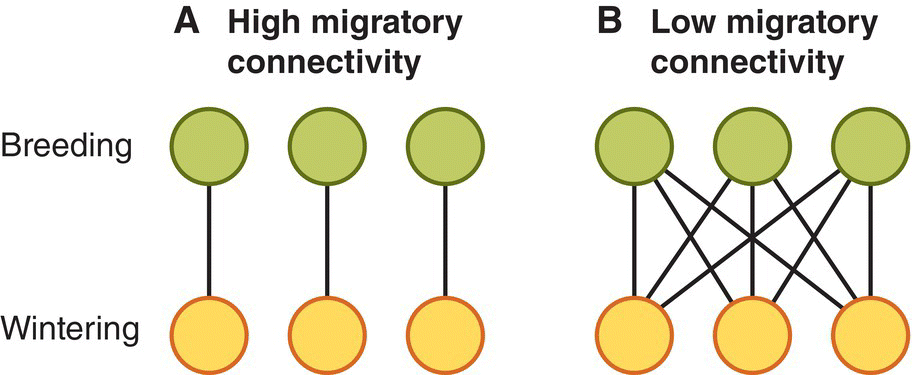
Fig. 12.09 Migratory connectivity. (A) In species with high migratory connectivity, individuals from each breeding region migrate to distinctive non‐breeding areas. (B) When migratory connectivity is low, individuals from different breeding regions intermix during the non‐breeding season.
(© Cornell Lab of Ornithology.)
Studies of two threatened bird species in Europe provide contrasting examples of migratory connectivity patterns. Individual Hoopoes (Upupa epops) from the same breeding population spend the winter in very different parts of Africa (Bächler et al. 2010), showing very weak migratory connectivity (Fig. 12.10). In contrast, Eurasian Wrynecks (Jynx torquilla) from breeding sites in Germany have quite restricted winter ranges in Africa, with greater migratory connectivity compared with Hoopoes (Reichlin et al. 2010).
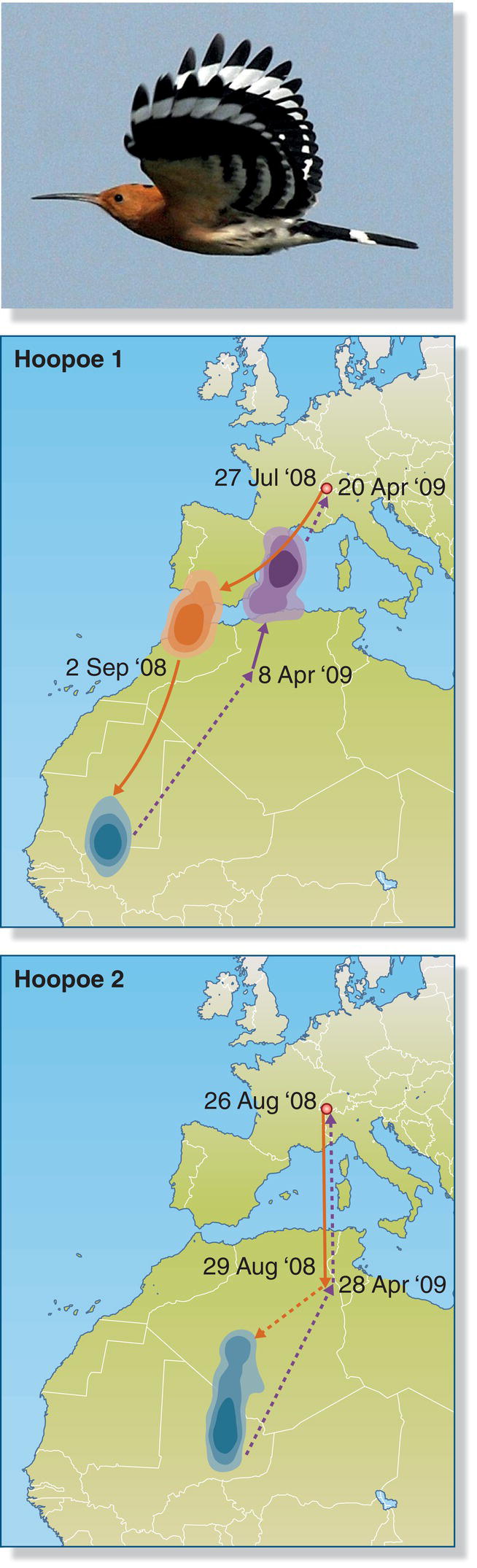
Fig. 12.10 Low migratory connectivity. Two Hoopoes (Upupa epops) originating from the same breeding population (red ring) migrated along different routes to different overwintering locations (blue areas). Orange arrows represent flight paths to the wintering area; purple arrows depict flight paths back to the breeding area. Shading of orange and purple represent stopover locations.
(From Bächler et al. 2010. © 2010 Bächler et al. CC‐BY‐4.0. Photograph by Umang Dutt.)
12.2.3 Migratory paces and paths
Different bird species undertake migratory journeys of varying length and duration. Most species travel in many short bouts of migratory flight interspersed with short periods when they rest and refuel; that is, they “hop.” Others make a few long‐distance non‐stop flights interspersed with periods of refueling; that is, they “skip.” Finally, in some of the most spectacular migratory feats, some species travel in one very long migratory flight—a “jump.”
Each of these strategies has advantages and disadvantages. Traveling in one big jump, as the Bar‐tailed Godwit (Limosa lapponica) does from Alaska to New Zealand, can save flight time during transoceanic flights and reduce the risk of predation in staging areas (Gill et al. 2009). However, such long flights require exceptional physiological adaptations, and, if the weather turns against them, these migrants take great risks making non‐stop flights over large expanses of water. The Hudsonian Godwit (Limosa haemastica)—an only slightly less extreme migrant—usually stops at just one site in central USA on its northward flight from Patagonia to central Alaska, and at three sites on its way back to Patagonia in the northern fall (Fig. 12.11). The longest non‐stop leg of the northward flight, more than 15,000 kilometers from Chile to Nebraska (USA), takes 4–8 days; the birds follow that leg of the migration with a refueling stop of up to 3 weeks before making another multi‐day non‐stop flight to Alaska (Senner et al. 2014).

Fig. 12.11 Long‐distance migration. Researchers attached geolocators (arrow in photo) onto Hudsonian Godwits (Limosa haemastica) to track their movements throughout the year. Individual godwits breed in Alaska, stopping at only a few sites during their annual migrations to and from southern South America. Circles indicate staging and stopover sites where birds rest and refuel, usually for multiple days, awaiting favorable migratory weather conditions.
(From Senner et al. 2014. © 2014 Senner et al. CC‐BY‐4.0. Photograph by Andy Johnson.)
While migrating, most birds make adjustments in their paths in response to changing flight conditions and geography. The Great Snipe (Gallinago media) is an exception in that it migrates from Scandinavia to sub‐Saharan Africa in one rapid bolt, passing non‐stop over habitats both good and bad, in an almost direct path, with seemingly little regard for winds aloft (Klaassen et al. 2011a). It flies fast (with ground speeds nearing 100 kilometers/hour) and covers distances of up to 6800 kilometers in 3–4 days.
Stopping frequently can reduce the risk of exhaustion and the need for extreme physiological adaptations, but it may result in a relatively slow pace of migration and a larger period of vulnerability during frequent refueling stops. For example, Swainson’s Thrushes (Catharus ustulatus) break their northward spring migration across the USA into three or more short legs, stopping at different wooded areas (Wikelski et al. 2003) and thus encountering different predators with different habits at each stop. Individual Lesser Black‐backed Gulls (Larus fuscus) vary greatly in their migratory pace, but many of the individuals tracked from a breeding colony in the Netherlands did not seem to be in a hurry during their fall migration: they stopped often, sometimes only briefly and other times for several weeks (Klaassen et al. 2011b).
Although one might expect birds simply to follow the same back‐and‐forth migration route in the fall and spring, many species clearly choose different paths in different seasons. Migration routes that differ predictably between seasons often are termed “circle” or “loop migrations” and usually result from strikingly different relative wind directions during the south‐bound and north‐bound migrations. For example, several species of soaring migrants flying from Europe to eastern Africa migrate through the Arabian Peninsula in the fall, but further to the west via the Suez and Israel in the spring. Tracking studies have similarly revealed that Red‐backed Shrikes (Lanius collurio) cross the Mediterranean Sea in fall but circumvent it via Arabia in spring (Tøttrup et al. 2012), whereas European Common Cuckoos (Cuculus canorus) execute a tight loop during their annual migrations south and north across the Sahara (Willemoes et al. 2014) (Fig. 12.12).
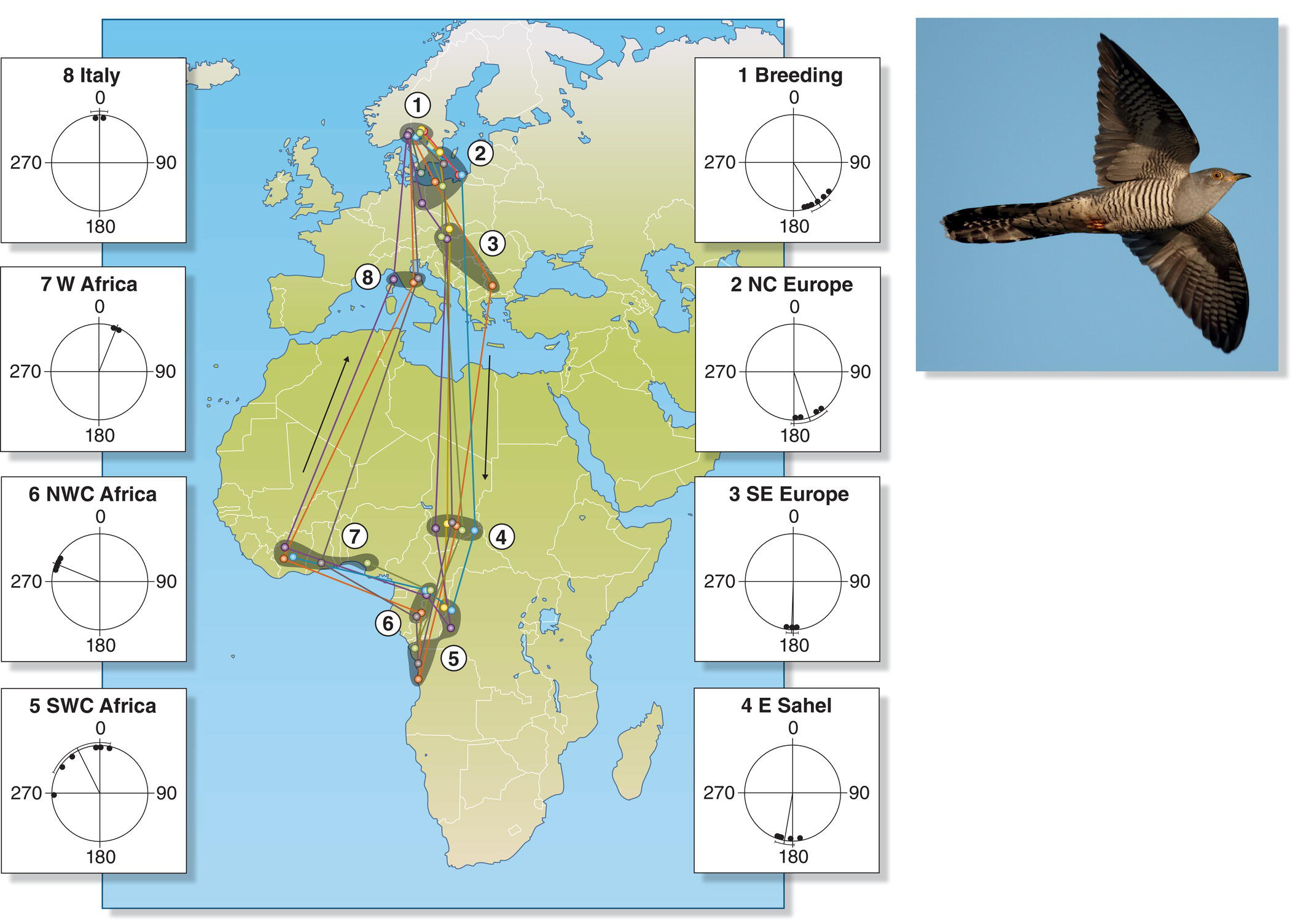
Fig. 12.12 Loop migration. Each year, Common Cuckoo (Cuculus canorus) individuals (colored lines) follow a clockwise path from their breeding grounds in Scandinavia to central Africa and back. The compass circles next to each site show the directional bearings of cuckoos being tracked as they began their flights to the next sites in their migratory circles.
(From Willemoes et al. 2014. © 2014 Willemoes et al. CC‐BY‐4.0. Photograph by Vogelartinfo, https://commons.wikimedia.org/wiki/File:Cuculus_canorus_vogelartinfo.jpg. GFDL 1.2.)
Leapfrog migration occurs in species with a broad breeding range, in which the individuals breeding the farthest north or south migrate to the most distant wintering areas, traveling farther and passing over birds that breed in intermediate areas that move shorter distances to winter. Among songbirds, one of the best known examples of leapfrog migration occurs among the different races of the Fox Sparrow (Passerella iliaca) in western North America. Fox Sparrows are year‐round residents in areas on the central Pacific coast. The subspecies that breeds in central British Columbia (Canada) spends the non‐breeding season far south in Oregon (USA). The subspecies breeding even farther north spend the non‐breeding season farther and farther south (Fig. 12.13). Leapfrog migration is also seen in the Yellow Wagtail (Motacilla flava): northern European breeders migrate to Africa south of 10°N, passing over and beyond populations from the Mediterranean that migrate only to areas north of 11°N (Bell 1996). Leapfrog migration may evolve when conditions in the north become increasingly favorable and the edge of a species’ breeding distribution moves further and further north. Those most northerly breeders may then face greater competition when they return to mix in the non‐breeding areas with birds from southerly populations; since the northern birds are already migrating long distances, natural selection favors their extending their southbound journey to winter in areas with less competition (Buehler et al. 2006).
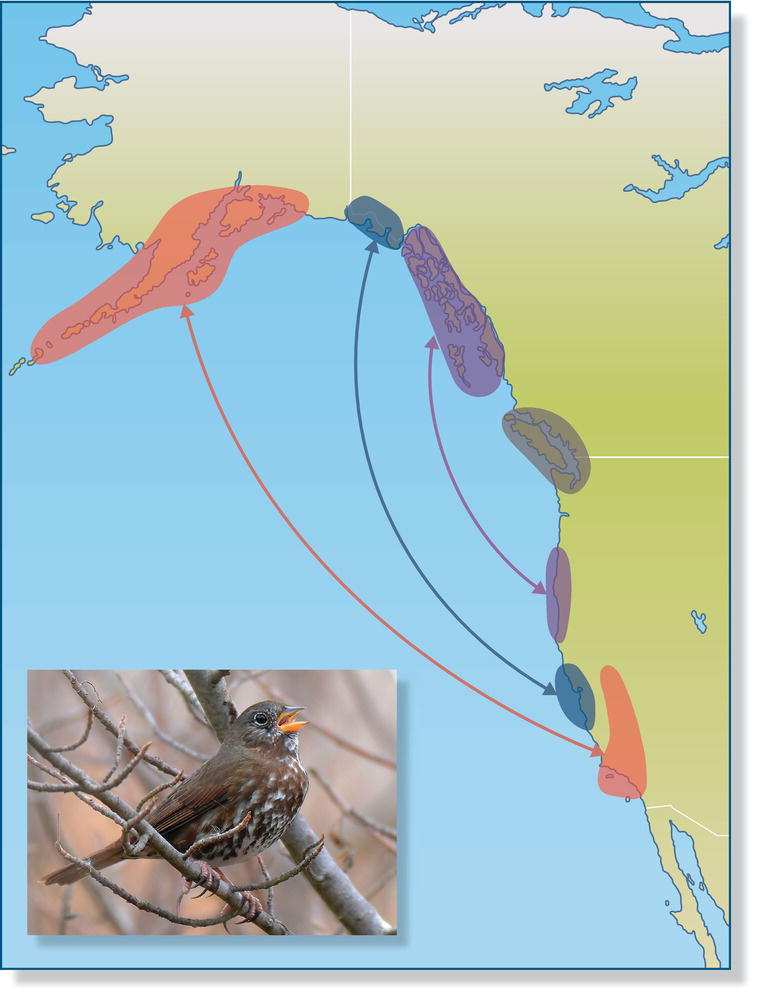
Fig. 12.13 Leapfrog migration. Along the Pacific coast of North America, the most northerly breeding Fox Sparrows (Passerella iliaca) winter farthest south, passing over individuals with intermediate breeding and wintering ranges. Arrows connect summer and winter ranges and do not represent migratory flight paths.
(From Bell 1997. Reproduced with permission from Cooper Ornithological Society. Photograph by Jerry Ting.)
12.2.4 Sex and age differences in migration
Within a bird species, individuals of different ages and sexes often have somewhat different migration routes, non‐breeding areas, and/or migration schedules. In many species, males and females depart from the breeding area at different dates, with departure delayed for the sex that cares for the chicks up to their independence. For example, males of several northern‐breeding species of ducks leave the breeding grounds before the females, which stay until their young have become independent. In some of these same northern habitats, female phalaropes depart before males, since in phalaropes the males provide all parental care to the chicks (Chapter 9).
During the spring migration of many species—especially songbirds—males travel ahead of females and arrive earlier at the breeding grounds, where they can get a jump on competition for breeding territories. In a comparison of 22 species of European landbirds, males returning on their spring migration arrived 3–19 days before females (Saino et al. 2010).
Individual birds often change their patterns of movement with age. Some young shorebirds remain on the non‐breeding grounds during their first year, not returning to the breeding grounds until their second year of life. This pattern of delayed return is even more pronounced in many gannets, gulls, terns, eagles, and condors, all birds that take many years to reach sexual maturity. In most migratory species, adults leave the breeding areas before juveniles. Young Eurasian Spoonbills (Platalea leucorodia) that have just fledged begin their southward migration later than the adults, and spoonbills that fledge late in the season do not migrate as far south as do earlier‐fledging birds (Lok et al. 2011). Similarly, during their first fall migration, young Reed Warblers (Acrocephalus scirpaceus) pass through Israel about 3 weeks later, on average, than do older birds (Merom et al. 2000). In spring migration, birds on their first journey to breeding grounds tend to lag behind birds making repeat migrations; in 12 species of passerines passing through Ontario, Canada, all but one species showed later passage dates for younger birds of both sexes (Stewart et al. 2002).
12.2.5 Movements on the non‐breeding grounds
Once in the non‐breeding areas, birds do not necessarily remain at one site, as most species do during the breeding season. Both White (Ciconia ciconia) and Black (Ciconia nigra) Storks may move around their non‐breeding range in Africa, sometimes spending several weeks in “pre‐wintering areas” before moving farther south for the rest of the northern winter (Van den Bossche 2002; Bobek et al 2008). Gulls of many species also often move from site to site in winter, as typified by a study of the movements of a single Lesser Black‐backed Gull (Larus fuscus) from breeding areas in the Netherlands to non‐breeding areas in France, Portugal, and Spain, and back again (Klaassen et al. 2011b). Similarly, European raptors such as the Lesser Spotted Eagle (Clanga pomarina) often move among several locations in Africa during the winter period (Meyburg et al. 2004). Non‐breeding populations of geese and waterfowl that spend the northern winter in northwestern Europe may suddenly evacuate an area during extremely cold winters with heavy snowfall, or when water bodies freeze, migrating to alternative sites farther south.
Data on the winter movements of smaller birds have become available as tracking devices have grown lighter, and it has become clear that many small birds also shift locations during the winter season. Common Swifts (Apus apus) tracked with tiny geolocators shifted their non‐breeding areas in the Congo Basin of Africa within a single winter (Åkesson et al. 2012). Studies using similar tracking technology found that some Swainson’s Thrushes (Catharus ustulatus) also move among multiple non‐breeding sites in Mexico, Guatemala, and Honduras (Delmore et al. 2012). Similarly, several different species of kingbirds (Tyrannus species) visit a succession of non‐breeding sites in Brazil during the non‐breeding season (Jahn et al. 2013). A large proportion of the Tree Swallows (Tachycineta bicolor) from eastern North America form enormous nocturnal roosts in late fall in sugarcane fields in Louisiana (USA), scattering to non‐breeding sites further south when the cane fields are harvested in November (Laughlin et al. 2013).
12.2.6 Altitudinal migration
Many birds that live in mountainous areas undertake altitudinal migration, usually breeding in higher elevations and moving to lower elevations during the non‐breeding season. They may not travel far in terms of overall distance, but by changing their elevation they can exploit new resources under different environmental conditions, just as another bird might do by migrating much farther to a different latitude. For example, Common Quails (Coturnix coturnix) in Catalonia on the eastern edge of Spain migrate to higher elevations during the summer, following the seasonally rising wave of newly ripened grass seeds, their favored food. These grasses mature later in the season at higher altitudes, and the quail work their way from altitudes of about 200 meters to about 1200 meters over a period of about 2 months before heading downslope again for the winter (Prats et al. 1996).
Altitudinal migration is particularly common in birds of tropical mountains that forage on fruit or nectar. For example, the Resplendent Quetzal (Pharomachrus mocinno) of Central America breeds in montane cloudforests, migrates downslope to pre‐montane wet zones on Pacific slopes, and then moves across the continental divide to low Atlantic slopes (Fig. 12.14), spending several months in each area before returning to its cloudforest breeding areas (Powell and Bjork 1994). Many species that undertake altitudinal migrations are facultative migrants: not all members of the populations migrate, and a given individual may not migrate every year.
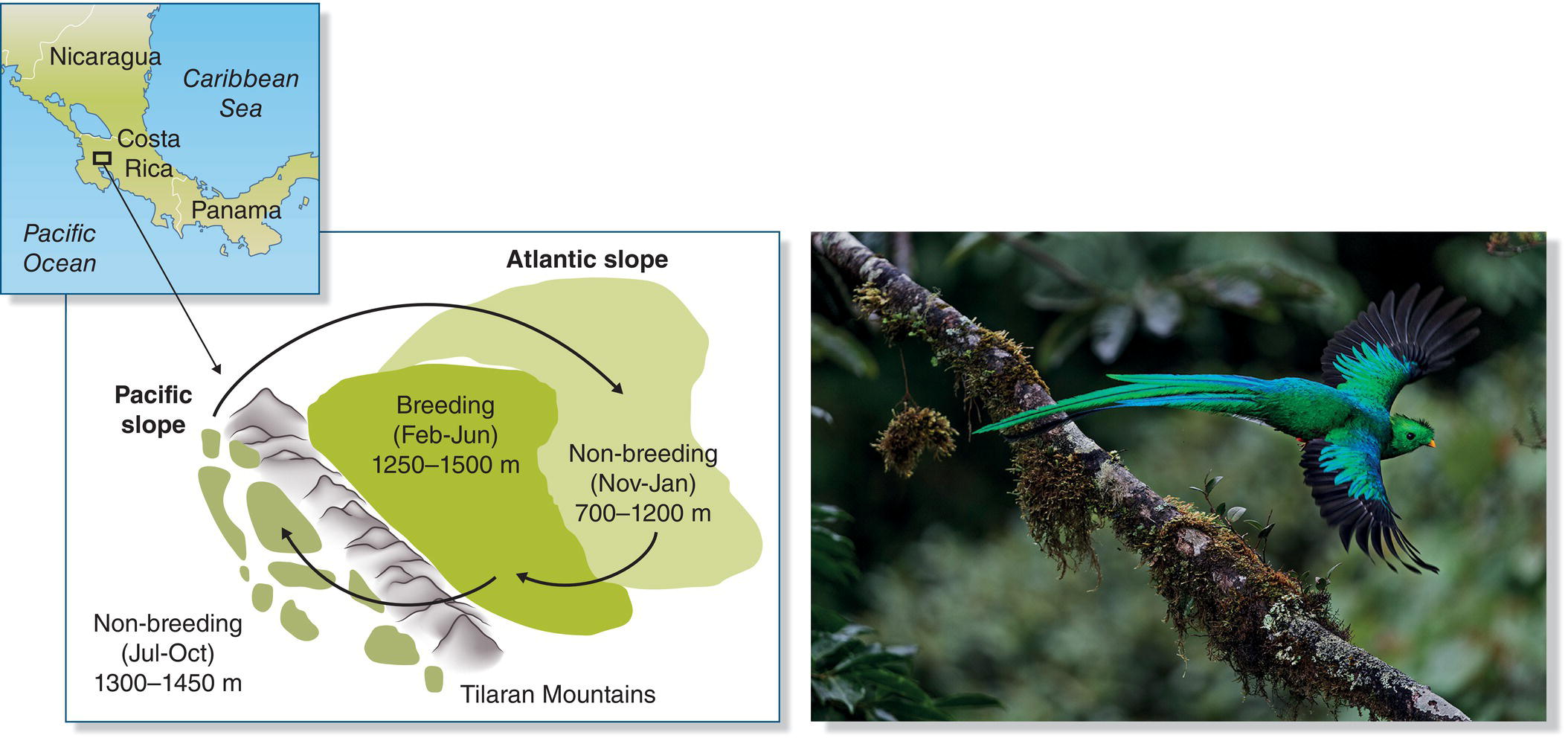
Fig. 12.14 Altitudinal migration. Resplendent Quetzals (Pharomachrus mocinno) migrate to different elevations and slopes in Costa Rica during different parts of the year.
(From Powell and Bjork 1994. Reproduced with permission from Cambridge University Press. Photograph by Jan Pedersen.)
12.2.7 Austral migration
If one of the main drivers of migration is deteriorating living conditions during the winter, one would expect that birds breeding at high latitudes in the southern hemisphere would migrate north to warmer non‐breeding areas, following a migration schedule offset by 6 months from that of the northern hemisphere. Although this kind of austral migration does occur, surprisingly little is known about it because most migration research has focused on the birds that breed in the northern hemisphere.
Compared with their northern counterparts, austral migrants experience some interesting geographic influences on their movements. One of these is visible on any map of the world’s continents and oceans: the landmass area of the southern hemisphere is very heavily weighted toward the tropics, with the land areas of the southern temperate and antarctic zones much smaller than the corresponding zones in the northern hemisphere. Thus, relatively fewer birds in the southern hemisphere live in locations with extreme seasonal variations in temperature. In addition, most austral migrants do not have to cross forbidding ecological barriers to reach adjacent tropical areas. The distances that austral migrants travel are therefore generally much smaller than the distances traveled by long‐distance migrants that breed in the north.
In South America, for example, more than 200 bird species are known to undertake austral migrations. Many migrants originating in temperate South America move north in the austral winter to warmer areas in temperate or subtropical parts of that continent, but some travel farther north to winter in tropical Amazonia. New World flycatcher species account for approximately 33% of these South American austral migrants, and for more than half of all the individual birds that migrate (Chesser 1994).
The migrant birds of both Australia and southern Africa also include species that visit these southern continents in the non‐breeding season and return to breeding areas in the northern hemisphere. Among the birds that breed in these two regions, directed seasonal movements are little known, with most species displaying nomadic or irregular movements in response to variation in environmental conditions. For example, in a review of the movements of honeyeaters in Australia, Keast (1968) estimated that 35% of the species were strict residents, 15% were residents that made local movements, 39% were nomadic, about 6% were altitudinal migrants, and fewer than 5% were latitudinal migrants within Australia. In both Africa (Cumming et al. 2008) and Australia (Roshier et al. 2008), waterfowl do not display large‐scale latitudinal movements as in the northern hemisphere. Rather, their movements appear to be coordinated with unpredictable changes in water levels and habitat availability over regional scales within both continents.
12.2.8 Migratory divides
Migratory divides arise where distinct breeding populations of the same bird species meet, yet birds from each side of the divide have different migration pathways. Migratory divides have been particularly well studied in some European songbirds. For example, a migratory divide across central Scandinavia separates two populations of Willow Warblers (Phylloscopus trochilus) (Bensch et al. 1999). In fall, birds from the southern population migrate to tropical West Africa, whereas birds from the northern population migrate to East and South Africa (Fig. 12.15). Similar migratory divides in thrushes (Box 12.02) and warblers occur across the Rocky Mountains in North America and around the Tibetan Plateau in central Asia.
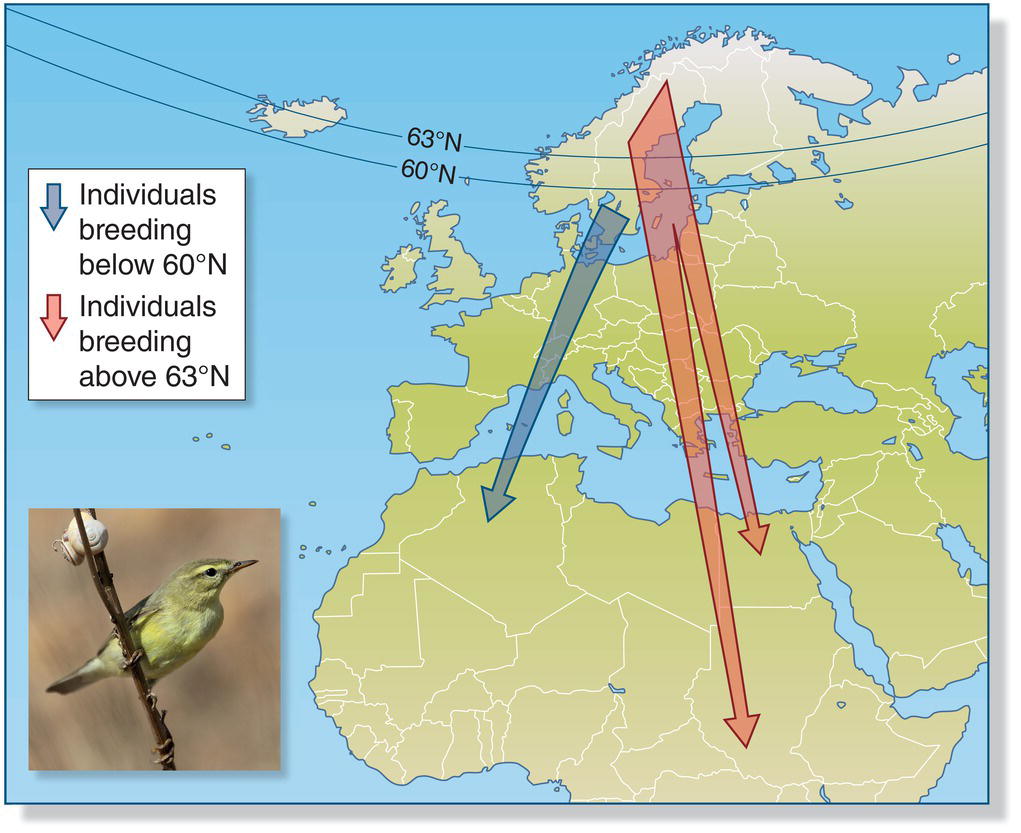
Fig. 12.15 A migratory divide. In their fall migration, Willow Warblers (Phylloscopus trochilus) breeding in Sweden segregate according to the latitude at which they nest: individuals that breed below 60°N (blue) winter in northwestern Africa, whereas breeders above 63°N (red) take a southeastern route to winter in eastern Africa.
(From Bensch et al. 1999. Reproduced with permission from John Wiley and Sons. Photograph by António A. Gonçalves.)
At a migratory divide, parent birds from opposing sides of the divide may sometimes pair up and mate. For example, a pair of White Storks (Ciconia ciconia) breeding along a migratory divide in eastern Germany was tracked for 10 years using satellite telemetry. Every year the female migrated along the eastern route through Turkey and Israel to South Africa, whereas her mate took the western route and migrated a relatively short distance to southern Spain (Kölzsch and Blasius 2008). Tracking the offspring of such partners could provide researchers with the opportunity to dissect the genetic basis for migratory behaviors, since the migratory behaviors of the different parents can be compared with that of their hybrid offspring. Researchers pursued such an opportunity in the lab by captive‐breeding European Blackcaps (Sylvia atricapilla) from either side of their central European migratory divide. Matings of birds from the same side of the divide produced offspring with orientations like their parents, while crosses of parents from across the divide produced hybrid young with intermediate migratory directions (Helbig 1996) (Box 12.03).
12.2.9 Concentration points and staging areas
Many studies of migratory biology have been conducted in places where migrating birds are concentrated by the local topography or other environmental forces. Often these narrow migratory concentration points are utilized by staggering numbers of birds, making them wonderful places to observe and band migrants. One of the largest such migratory concentrations occurs in Veracruz, Mexico, where an aerial “river of raptors” is an annual spectacle (Fig. 12.16). This concentration results from the narrowing of Mexico’s eastern coastal plain near the Isthmus of Tehuantepec. The entire world population of several birds, including the Mississippi Kite (Ictinia mississippiensis) and Swainson’s Hawk (Buteo swainsoni), travel over Veracruz during migration, and count estimates from hawk‐watching stations there are used to estimate the total number of individuals of the entire species (Ruelas Inzunza et al. 2010). Other locations where great numbers of raptors pass overhead during migration include Batumi, Georgia (where the birds are concentrated by the Black Sea); Bosporus, Turkey (where migrants cross from Europe into the Middle East); Israel (where they are concentrated by the Mediterranean Sea to the west and vast deserts to the east); and the Straits of Gibraltar that separate Spain from Morocco (where they are concentrated by the Mediterranean and the Atlantic on either side) (Bildstein 2006) (Fig. 12.16).

Fig. 12.16 Migrating raptors concentrate in narrow overland corridors. This map depicts the five main raptor migration routes worldwide.
(From Bildstein 2006. Reproduced with permission from Cornell University Press.)
Migratory concentrations also occur in areas where food is especially abundant, and many species of migratory birds spend substantial time at staging areas to refuel before continuing their migrations. Well‐known staging sites include Delaware Bay on the mid‐Atlantic coast of North America, which serves as a major staging area for migrating shorebirds that feast on the eggs of horseshoe crabs. This abundance of food allows these shorebirds to increase their weights by 70–80% in a few weeks (Baker et al. 2004). Disrupting these critical areas may cause dramatic population declines of the birds that rely on them for refueling: commercial exploitation has decreased the number of horseshoe crabs in Delaware Bay, resulting in a concomitant decline in numbers of Red Knots (Calidris canutus) (Baker et al. 2004; Niles et al. 2009). Other important staging sites that host huge aggregations of shorebirds include the Copper River Delta in Alaska, the Wadden Sea in western Europe, and the Yellow Sea in eastern Asia. Shorter pauses during migration occur at stopover sites, locations where migrating birds take a break to rest, eat and drink for maintenance, and/or await favorable migratory conditions.
The philopatry (site faithfulness) seen in the more sedentary parts of the annual cycle of many migrants sometimes extends to migratory staging and stopover sites, as some birds use the same route from year to year. Three warbler species (Sylvia atricapilla, Sylvia borin, and Phylloscopus collybita) passing through the Iberian Peninsula during spring and fall migrations show moderate levels of philopatry to their stopover sites (Cantos and Tellería 1994). A fourth species, the Reed Warbler (Acrocephalus scirpaceus), is a specialist of reed beds, a habitat which has a naturally patchy distribution. Likely because of this more specialized habitat requirement, Reed Warblers are more highly philopatric during migration, and some migrating Reed Warblers are known to have stopped over at the same site in Israel for up to five successive migrations (Merom et al. 2000).
12.2.10 Flyways
Flyways are established routes used year after year by large numbers of migrants, with many bird species often converging to use similar flyways. Flyway designations can be useful in bird conservation for establishing international collaborations, coordinating research efforts, and prioritizing areas for conservation. Whether it is for public health concerns about the spread of bird‐borne diseases or conservation concerns about the status of habitat in distant non‐breeding grounds, they serve as a tangible reminder of the way migrating birds create connections between continents and countries. For example, raptors breeding in Europe and Asia use several major flyways, with south‐bound birds in fall funneling through concentration points from the Straits of Gibraltar in the west, through Italy, Israel, the Arabian Peninsula or the peninsulas of southern Asia in the east, to non‐breeding areas in Africa or the Malay Archipelago. Similarly, for the shorebirds of the world, eight major flyways have been described linking North with South America, North America with Europe and Africa, Europe and Asia with Africa, and Asia and Alaska with Australia (Fig. 12.17).
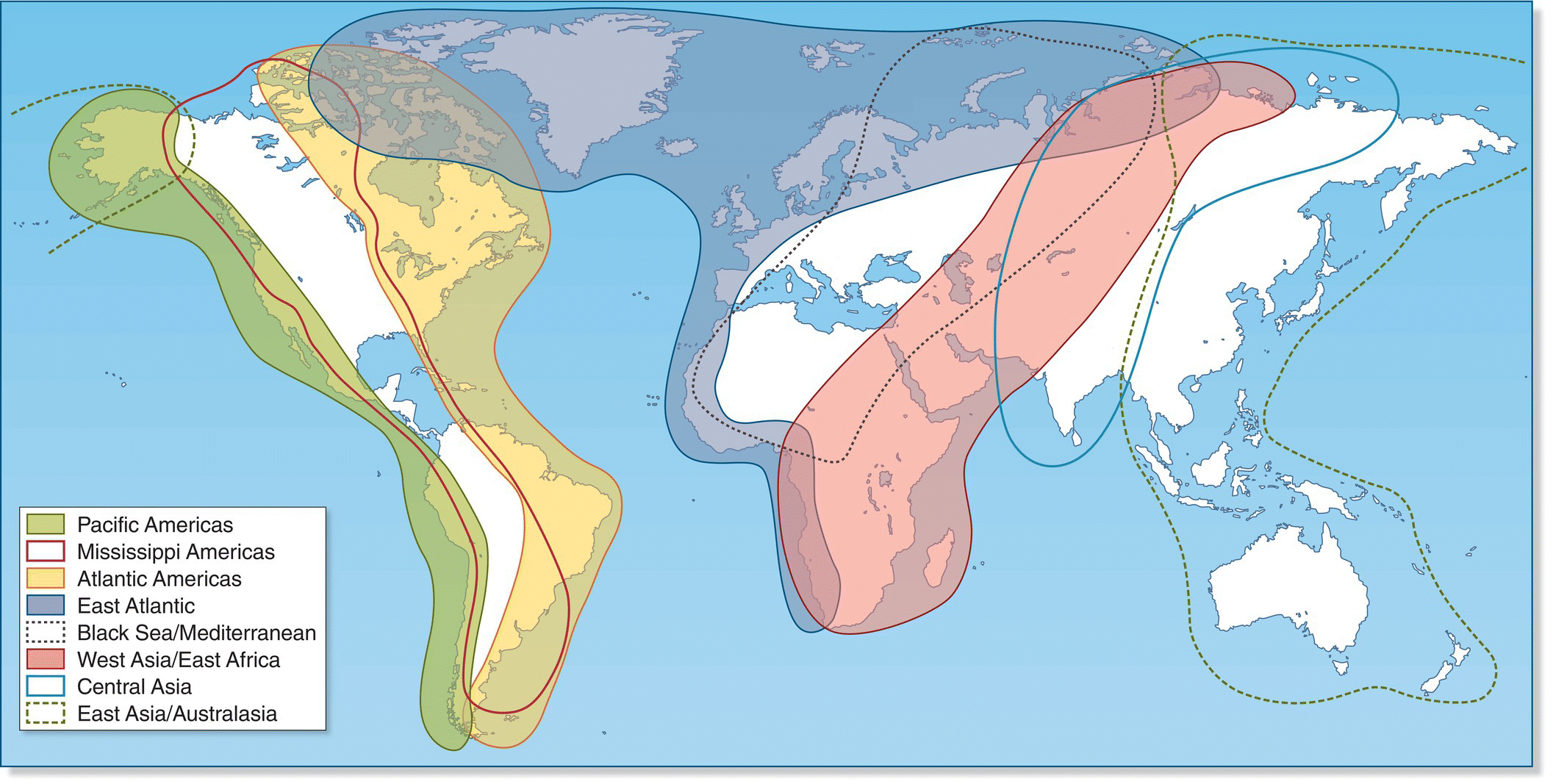
Fig. 12.17 The eight major flyways of migrant shorebirds. Many shorebird species annually migrate along particular flyways between breeding and non‐breeding locations.
(Courtesy of International Wader Study Group.)
The concept of flyways can also be linked to migratory connectivity, as flyways are likely to be more distinct and identifiable for species or populations with strong migratory connectivity. Yet not all birds use clearly defined flyways during migration. In contrast to species that concentrate in huge numbers during migration, many songbirds migrate across very broad areas without clear spatial delineations—a phenomenon termed broad‐front migration. Migratory flyways are more difficult to delineate for such species (La Sorte et al. 2014).
12.2.11 Irruptive migration
Irruptive migrants are usually birds of arctic and boreal regions that generally stay far north during the winter but occasionally “irrupt” into lower latitudes. When they do, birders throughout the northern temperate zone delight in seeing finches and owls in places where they rarely occur. Irruptive species tend to be facultative migrants or nomads that depend on variable crops of tree seeds (mostly birch, alder, or spruce), or on fluctuating populations of northern rodents such as voles and lemmings. When the availability of these foods at northern latitudes fails, the birds wander further south in search of food. These mass movements build anticipation in birders because they often can be predicted on the basis of changes of conditions in the north or the movements of birds elsewhere. These movements are a type of facultative migration because the birds are responding to local declines in food availability rather than engaging in preprogrammed movements away from breeding areas.
These irruptive movements often show remarkable cycling across years. For instance, the irruptions of some northern finches sometimes alternate regularly: every other year, redpolls or siskins are likely to show up at feeders in the northeastern USA (Fig. 12.18). Among the owls and raptors that feed on arctic and boreal rodents, the time between southward irruptions is often closer to 4 years. This periodicity breaks down now and again, and sometimes it is not synchronized among different regions.
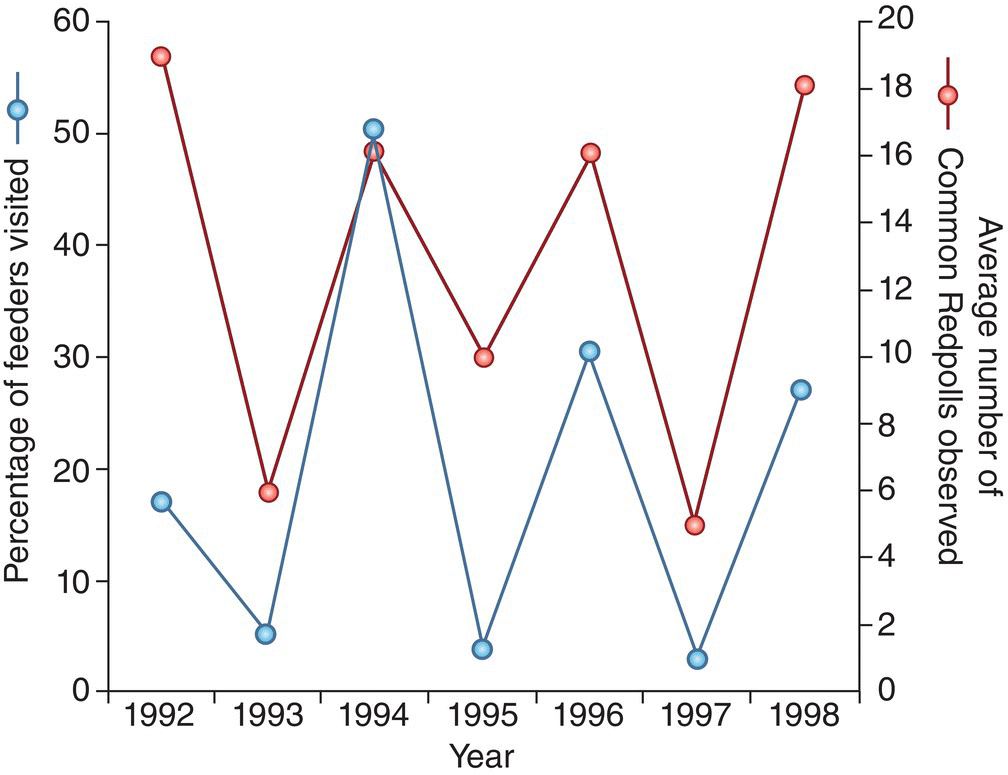
Fig. 12.18 Cyclic abundance of an irruptive migrant. Winter observations of Common Redpolls (Acanthis flammea) at feeders in the northeastern USA reveal that this species appears to follow a 2‐year cycle of alternating abundance and scarcity. In years when redpolls are rare at these feeding sites, they seem to remain farther north during the winter.
(© Cornell Lab of Ornithology.)
12.3 How birds time their migrations
For a migrant to match its movements to its environment, it must make its journeys at the appropriate times of year. A flight across the Sahara or the Pacific Ocean would doom the migrant unless it timed its flight to take advantage of supportive weather conditions (Gill et al. 2014) and arrived at its destination at a time when resources there were plentiful.
12.3.1 Migratory restlessness
When songbirds are ready to migrate, they literally cannot sit still. German ornithologists first noticed this migratory restlessness and gave it a name, Zugunruhe, which migration biologists still use today. Because it is easily recognized and measured, Zugunruhe can serve as a reliable indicator of the strength of migratory preparedness and motivation: migrants, when kept in cages at night, usually jump in the direction that you would expect them to fly if they really were initiating their migratory journeys. Early researchers designed special circular cages that registered the hops of birds in Zugunruhe, using them to study the role of magnetism and cues from constellations in the night sky on bird orientation. Similar approaches using a variety of more advanced recording tools continue to be used today to study songbird orientation. Measurements of Zugunruhe have also played a valuable role in comparative studies of migration, as birds from different populations with different migratory paths and destinations show differing patterns of Zugunruhe intensity (hours per night) and duration (number of nights) that correspond to how long their migrations last under natural conditions.
12.3.2 Biological clocks for migration timing
Birds have internal biological clocks that help them monitor both the time of day and the time of year. Birds can sense the time of day via these internal clocks in the absence of light cues, such as the position of the sun. They similarly have internal biological calendars that tell them about the progression of the seasons even in the absence of a seasonal cue such as a change in day length. Important aspects of these clocks are controlled by changes in hormone levels (Chapter 7).
The time‐sense of birds has been investigated using experiments in which wild birds are brought into captivity and kept under artificial conditions where seasonal cues—such as changing temperatures or changing day length—can be manipulated by the researchers. Studies that artificially manipulate the internal calendars of birds take a very long time, and the Max Planck Institute for Ornithology in southern Germany has been one of the few institutions in the world able to sustain lengthy investigations of this fascinating aspect of bird biology. Work there has concentrated on small migrant songbirds such as the Garden Warbler (Sylvia borin) and European Blackcap (Sylvia atricapilla). The birds used in these experiments often were taken into captivity as young chicks and hand‐raised, and lived their lives in individual cages. To eliminate their ability to measure seasonality by any environmental cue, the temperature and light–dark cycles in their cages were kept constant.
Garden Warblers and Blackcaps monitored for up to 10 years showed persistent seasonal cycles in molt, the size of their internal reproductive organs, and Zugunruhe, even in the absence of external cues about the changing seasons. Surprisingly, however, the complete annual cycle in these birds is not exactly 12 months, but instead is several months shorter. In contrast, the few similar studies of migratory shorebirds showed that these birds have internal cycles of about 14 months.
Under natural conditions, all of these birds use cues from their environment to help calibrate their internal calendars and thereby keep them in synch with the changing seasons. The most important synchronizing cue (or Zeitgeber) from the environment is the photoperiod. Birds are sensitive to the relative length of night and day, and the way that this ratio of light and dark changes with the seasons is an important cue for resetting their biological clocks (Chapter 7). Experiments with captive birds have shown that changing their photoperiod alone can cause them to dramatically recalibrate their annual calendar. For example, by manipulating the schedule of lights in their enclosures, European Starlings (Sturnus vulgaris) can be induced to have complete annual cycles of breeding and molting activity that are as short as 2 months (Gwinner 1986) (Fig. 12.19).
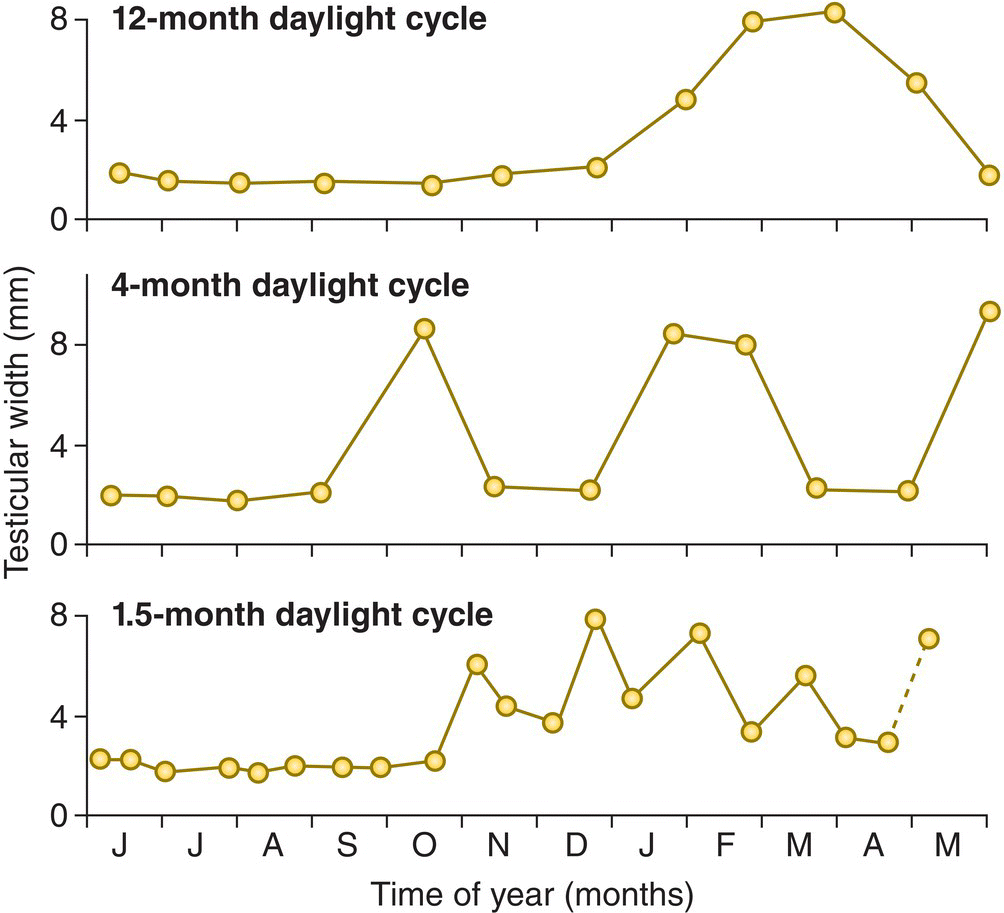
Fig. 12.19 Physiological effect of photoperiod change. The testes of male European Starlings (Sturnus vulgaris) enlarge during the breeding season and shrink during the non‐breeding season. Photoperiod, or the amount of light each day, serves as a cue for the time of year. The top graph shows the testis size—an indicator of breeding condition—of a starling kept under a normal 12‐month daylight cycle. Males kept under artificial daylight cycles that change more rapidly (middle graph) synchronize their annual rhythms to match these faster “years.” Even at a cycle length of 1.5 months (bottom graph), the birds eventually adjust their gonad cycles to such short “annual” periods.
(From Gwinner 1981, 1986. Reproduced with permission from Springer Science + Business Media.)
When birds migrate long distances, they rapidly traverse locations with very different photoperiods, and we still do not fully understand how they are able to synchronize their internal rhythms with these changing external cues from the environment. For instance, in Sanderlings (Calidris alba), individuals originating from the same breeding population may winter over a great range of latitudes (Fig. 12.20). A Sanderling spending the non‐breeding season near the equator must respond to very different day‐length cues than a bird wintering near the southern tips of South America or Africa, yet all of these birds time their northward migrations to arrive on the breeding grounds in the Arctic at the same time. Depending on where they spend the non‐breeding season, different Sanderlings must initiate the migratory flight north in spring under conditions that can vary from lengthening days (the normal condition in the north temperate zone) to very small changes in day length (in the tropics) to shortening day length (if the bird is spending the northern winter in the southern hemisphere).
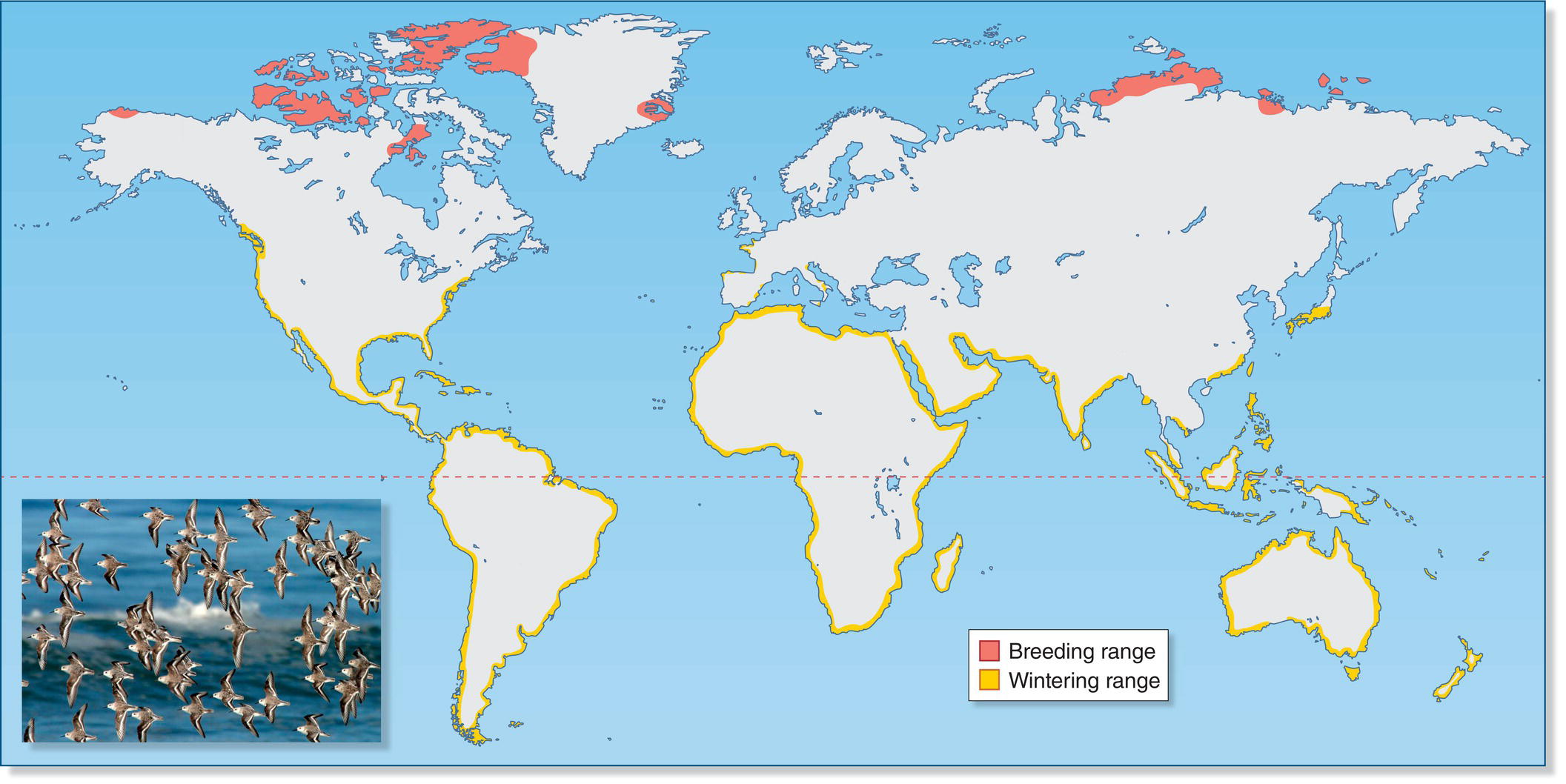
Fig. 12.20 Latitude and photoperiod. All Sanderlings (Calidris alba) breed in the high Arctic (red), but different individuals overwinter along various coasts worldwide (yellow), both north and south of the equator. To time their migrations correctly, birds must tune their internal clocks to different photoperiods, depending on latitude. (Map courtesy of www.xeno‐canto.org.
Adapted from BirdLife International and NatureServe 2011. Bird species distribution maps of the world. BirdLife International, Cambridge, UK and NatureServe, Arlington, USA. Photograph by Eteri Maisuradze. CC‐BY‐SA 2.5.)
Although their internal clock tells birds when to start heading off on a migratory journey, this message can be overridden, at least for a short while, if the bird is not yet in appropriate migratory condition or if environmental conditions are not conducive to migration. Birds preparing to embark on a migratory voyage must integrate a great amount of information, and they can be thought of as running through a preflight checklist prior to departure. For example, when White Storks (Ciconia ciconia) and Honey Buzzards (Pernis apivorus) are leaving their breeding sites to initiate their fall migration, their day of departure is triggered by short periods of locally deteriorating conditions (Shamoun‐Baranes et al. 2006).
12.4 Orientation and navigation
Whenever a bird sets out on a journey, be it a short foraging trip or a migration flight to the other side of the planet, it runs the risk of losing its way. The ability of birds to find their way to precise locations on the earth’s surface after very long journeys must rank as one of the most fascinating aspects of avian biology. Scientists have been investigating the ways that birds achieve this feat for many decades (Box 12.04). One important question has been whether birds are capable of true navigation, estimating their absolute position and planning a route accordingly, or merely orientation, being able to judge compass directions accurately.
This distinction between navigation and orientation raises some interesting parallels in the history of human path finding. Only recently have humans been able to fix easily their absolute location on earth, using technologies like the Global Positioning System (GPS) (Box 12.01). Before GPS, human navigation involved the use of sunrise and sunset times and relied on accurate clocks and maps. Before the invention of accurate clocks, humans were limited to orientation based on celestial cues or long‐distance cues such as the movement of a needle in a magnetic compass, the directions of sea currents, or the flights of shore‐bound birds. As we shall see, there are many similarities in how both birds and humans have found their way.
12.4.1 Magnetic perception of birds
Birds are able to sense the earth’s magnetic field in multiple ways, one of which involves cells in the upper layers of the bill that contain magnetite, a magnetic mineral (Chapter 7). The strength of earth’s magnetic field varies a great deal at both the global scale (being strongest at the poles and weakest at the equator) and on a regional scale (depending on the composition of the local bedrock, sediment thickness, and so forth). Thus, with experience, birds can potentially gather a great deal of positional information from the strength of the magnetic fields that they encounter. For example, male and female Ruffs (Philomachus pugnax), long‐distance migrant shorebirds of the Eurasian Arctic, apparently gather and store geomagnetic information in different ways: when there are larger geomagnetic disturbances during the period of their first southward fall migration, males are displaced from their routes more than females are (Rakhimberdiev et al. 2014).
The other magnetic sense in birds involves a compass‐like ability to detect direction in the earth’s magnetic field. Experiments had long suggested that birds’ eyes are involved in this aspect of their direction finding, even in the absence of visual cues. Experiments have shown that light at the blue and turquoise end of the spectrum interacts with a pigment called cryptochrome in the avian retina. Light excites one of the electrons in a cryptochrome molecule, and the molecule and its associated neuron somehow are able to detect the effect of the magnetic field on the quantum‐mechanic spins of these solo electrons before they return to their stable paired state. Thus, these cryptochromes may be able to produce a visual pattern in the retina that allows birds to “see” direction akin to a magnetic compass (Wiltschko and Wiltschko 2009).
12.4.2 Solar cues and the circadian clock
The sun is a changeable indicator of direction: at noon it is directly overhead, in the morning to the east, and in the afternoon to the west. Birds, like humans, need to know the time of day to make good use of solar position as an indicator of direction. Some early experiments on solar orientation by Gustav Kramer in Germany used large mirrors to shift the apparent location of the sun, but most subsequent research instead has involved shifting the birds’ biological clock. When a domestic homing pigeon (Columba livia) is kept for a few days in a room where the photoperiod is artificially shifted by 6 hours and then released to fly back to its loft, it will orient by the sun but fly in the wrong direction. For example, if the pigeon is kept in a photoperiod that is 6 hours fast and is released at 6:00 a.m., the bird will think it actually is noon, and orient by the sun as if that were the case (Fig. 12.21). In the southern hemisphere, where the noon sun is to the north, clock‐shifted pigeons make the same sensible mistake, of the opposite direction, when released. Similar clock‐shifting experiments have been conducted, with similar results, on a great variety of bird species, including chickadees in the USA and various warblers and robins in Europe. Such studies have shown that some birds use solar orientation even for short daily movements; for example, some jays and nutcrackers use solar cues for spatial direction when returning to the locations where they previously cached food (Wiltschko et al. 1999).
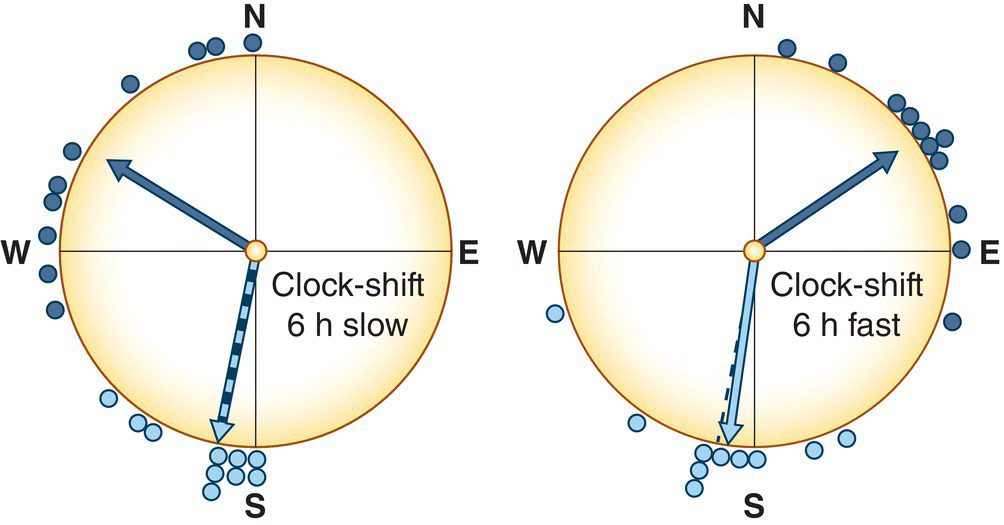
Fig. 12.21 Sun position as a navigational cue. These plots show the orientation of homing pigeons (Columba livia) released north of their loft, to which they were trying to return. Birds kept under normal daylight conditions correctly fly south (light blue circles), whereas birds kept under artificial lighting conditions (with daylight shifted ± 6 hours) instead fly in the direction that would be correct (based on the position of the sun) during the clock‐shifted time of day (dark blue circles).
(From Wiltschko and Wiltschko 2009. Reproduced with permission from American Ornithologists’ Union.)
We have already seen that some frequencies of light interact in interesting ways with the geomagnetic sense of birds, and there is additional evidence that birds can perceive the polarization of light in the sky, but how birds use this ability to orient themselves remains uncertain. For birds that can see patterns in the polarization of light in the sky, these patterns shift through the day in ways that likely accent and augment the information derived from solar position alone. Experimental work has shown that birds use polarized light cues close to the horizon to recalibrate their magnetic compass at sunrise and sunset (Muheim et al. 2006b).
12.4.3 Celestial navigation
Birds are also able to use celestial cues from the night sky to orient their migratory journeys. In experiments with captive‐reared Indigo Buntings (Passerina cyanea) exposed to planetarium skies with either natural or artificial patterns of nocturnal star movement, Emlen (1970) showed that birds do not memorize a map of the stars, but rather use the apparent rotation of the stars in the sky to determine direction. From watching the sky over several nights, birds can determine the location of the poles in the sky around which the other stars seem to move. This orientation cue is one of the most important that nocturnal migrants use during long‐distance migrations.
12.4.4 Other orientation guides
There is much debate about the extent to which birds use their sense of smell (Chapter 7) to find their way, but there is little doubt that tubenosed seabirds effectively use the odors of their colony to trace their way back to the colony and even to their home burrows, and it seems likely that other birds with a keen sense of smell (such as some vultures) likely can acquire olfactory information that could be valuable in finding their way.
Another extraordinary sensory ability of some birds (including domestic pigeons, the species in which this ability has been best studied) is the detection of sound at frequencies as low as 0.05 Hertz, a frequency far below the range of human hearing. Sound at these extremely long wavelengths can travel enormous distances, potentially giving birds information about the direction of distant ocean shores and mountain ranges, or about the approach of distant storms. Accordingly, there is some evidence that homing pigeons using this ability can be seriously disoriented by the intense sonic booms produced by large supersonic aircraft (Hagstrum 2000).
12.4.5 Displacement experiments
Recall that orientation involves having a sense of direction, whereas navigation involves knowing the specific point on the earth where you are located. Experiments in which birds are displaced from their original location and then tracked have consistently suggested that they may be capable of true navigation, at least for parts of their journeys. Over many decades, ornithologists have captured birds on their breeding or wintering territories and moved them far from these sites. Diverse birds, ranging from sparrows to shearwaters and storks to starlings, have returned successfully to their point of origin, often after periods short enough to suggest an almost straight‐line return.
How can these avian travelers achieve such precise feats, especially when they are moved to locations that neither they nor any of their recent ancestors have ever visited? The birds displaced by these experiments may not know precisely where they are; they may sense only some gradient of position and know to travel up that gradient. For instance, they may sense something about the magnetic field or infrasound landscape that gives them a reliable indicator of the direction they should proceed to get home.
An interesting nuance in the results of these displacement experiments is that experienced migrants usually do better in finding their way back home than do first‐time travelers. In displaced White‐crowned Sparrows (Zonotrichia leucophrys), for example, seasoned breeders from the Pacific coast began correcting for their displacement to the opposite side of North America as soon as they were released there, whereas first‐time migrants continued their journeys in what would have been the correct orientation had they still been on the Pacific coast (Thorup et al. 2007). This pattern is consistent with studies of homing pigeons showing that experienced birds are better at considering multiple orientation cues.
12.4.6 Multiple and sometimes conflicting orientation cues
Birds clearly use many kinds of sensory information to guide their journeys. They must manage all this information and handle situations in which these cues are in conflict with one another. As birds get older and gain experience, they generally get better at understanding the situations in which a given sensory mode is most likely to be reliable or unreliable. For example, if a homing pigeon is released on an overcast day when the sun’s position is not clear, it seems natural that the pigeon would shift to its magnetic compass to find its way. Studies of how birds resolve conflicts between celestial cues and shifted magnetic cues indicate that birds sometimes weigh the available information differently, depending on whether they are migrating or not (Muheim et al. 2006a). Thus, these birds are often able to discern which orientation cues are most reliable in different settings and at different scales of travel.
12.5 Migration physiology
One of the remarkable aspects of avian migration is that tiny birds can make such extended journeys under their own power. They can accomplish these feats of migration because they are efficient flying machines.
12.5.1 Fat: the fuel of avian migration
Birds are the only terrestrial organisms (other than humans using machines) that can repeatedly make round trips of many tens of thousands of kilometers. Although flapping flight is hard work, it is unrivaled in its efficacy at covering distance. The energy used by a bird engaged in flapping flight is about eight or nine times greater than the energy that same bird would expend while sleeping (the resting metabolic rate). To achieve these high levels of physical activity, birds need efficient systems for supplying fuel and oxygen to the muscles, and for removing carbon dioxide.
As covered in more detail in Chapter 7, migrating birds use fat as their primary energy source; given the much higher energy density of fat, this adaptation may have pre‐equipped migrant birds with a means of storing sufficient fuel for very long flights. Birds employ special enzymes to mobilize fat out of their fat cells, special transporter proteins to carry the fat through the blood stream, and special enzymes to get the fats into the muscle cells and finally deliver them to the mitochondria, where fats are oxidized to fuel the muscle’s work.
Most birds therefore prepare for migration by storing large amounts of fat within their bodies, some of it just under the skin. Bird‐banders working with migratory songbirds often take fat scores: if one softly blows apart the breast feathers on a passerine in migratory condition, the cream‐colored subcutaneous fat that builds up in the open triangle below the neck (the furcula) is easily visible. As birds grow fatter, so does the extent of this creamy layer under the skin. Birds deposit fat in the abdominal cavity last. Geese and shorebirds that are ready for takeoff on very long‐distance flights can be recognized by their bulging abdomens, loaded with the fat needed to fuel their forthcoming journey.
Migrating birds preparing to depart on long flights can get very fat indeed. Migrating Blackpoll Warblers (Setophaga striata) preparing to depart on a non‐stop transoceanic flight from the coast of Maine (USA) to the north coast of South America routinely double their mass in fat before departure (Fig. 12.22). Just before they depart on a flight to South America, some Wilson’s Phalaropes (Phalaropus tricolor) staging at Mono Lake, California (USA) load up with so much fat that they are unable to fly until after a brief period of weight loss (Jehl 1997). Bar‐tailed Godwits (Limosa lapponica) on the rich intertidal mudflats of southwestern Alaska accumulate similar proportions of fat before their trans‐Pacific journey of more than 11,000 kilometers to New Zealand (Piersma and Gill 1998). Although the fattened godwits still manage to take off, they require a long running start more characteristic of heavy birds such as swans.

Fig. 12.22 Fat storage for migration. The Blackpoll Warbler (Setophaga striata) on the right has stored enough fat (the white visible through the exposed skin of its breast) for a non‐stop flight across the Atlantic Ocean, while the Yellow‐rumped Warbler (Setophaga coronata) on the left needs to store much less fat for a short journey down the coast of North America.
(Photograph by Rebecca L. Holberton.)
Fat storage does not come cheaply. In fact, because fat is so energetically dense, birds that are in the process of adding stores of fat must lengthen their foraging days and double or triple their normal daily food intake. For intake rates to increase so much, the size and capacity of the food‐processing organs, the stomach and the intestine, must increase temporarily as well. The surface area of the intestinal wall and the levels of enzymes that help transport nutrients across this wall into the blood stream go up. As a true biochemical factory, the liver transforms sugars and proteins into the appropriate fats for transport to fat cells. The livers of migrants at the peak of their pre‐migratory foraging activity may be two or three times larger than during the rest of the year.
The physiological demands of migrants change suddenly when they stop their pre‐migratory foraging and set out on long‐distance flights. Godwits and similar transoceanic terrestrial migrants are able to store relatively enormous amounts of fat in part because all the organs that can be dispensed with during flight, especially the gut and the liver, start to shrink during the last days before migration.
12.5.2 Migration and weather
Weather conditions are important for a migrating bird: clouds and fog can obscure orientation cues like the position of the sun and stars, rain can make flight and thermoregulation more difficult, and high temperatures can influence a bird’s water balance. Of all weather variables, wind has the biggest effect on migratory performance. If the wind is blowing in the right direction, it can help a bird save time or energy, but if it is blowing the wrong way, it can blow a bird far off course, cost the bird energy needed for it to stay on course, or cost it days of lost flight time while it waits for better conditions. Helpful wind assistance can double the flight distance of a bird. However, many migrants will make little progress if they are faced with contrary headwinds close to their own flight speeds. Thus, a bird’s decision about how to respond to weather conditions during migration can affect many of the risks involved in these avian journeys. For a bird making a short flight over land, these risks may not be particularly life threatening; however, for a small bird like a Northern Wheatear (Oenanthe oenanthe) that must fly thousands of kilometers over the northern Atlantic Ocean, taking off in the wrong weather conditions can be a matter of life or death.
Migrating birds generally depart from staging and stopover sites on days with favorable winds. Experiments with migrant European Robins (Erithacus rubecula) show that the winds overhead are one of the most important influences on the decision to resume migration (Dänhardt and Lindström 2001; Bulyuk and Tsvey 2013). Alternatively, birds may decide to stop migrating temporarily because of bad weather. However, waiting for good weather or stopping because of bad weather costs time and energy. To make the right choices, birds must weigh different and changing costs and priorities during their journeys.
Broad regional weather conditions also can influence migration routes, because spatial patterns in pressure systems can act as a barrier to migration or create a migratory corridor of beneficial winds. For example, the routes that Eleonora’s Falcons (Falco eleonorae) use to cross the Mozambique Channel are influenced by the location of low‐pressure systems (Mellone et al. 2011). The transoceanic routes and timing of migration in Cory’s Shearwaters (Calonectris diomedea) are shaped by large‐scale wind patterns, resulting in longer but more cost‐effective paths (Felicísimo et al. 2008).
Soaring birds—including many vultures, eagles, large hawks, buzzards, storks, and pelicans—use winds and other air currents in a distinctive way (Chapter 5). Adroitly exploiting atmospheric dynamics enables them to migrate thousands of kilometers while expending very little of their own energy. Instead, they take advantage of rising air currents to gain altitude and then glide slowly down along their migratory route. By climbing and gliding, a soaring migrant can very efficiently cover several hundred kilometers each day. The thermal convection that creates rising air currents happens only during the day and rarely occurs over open water. These restrictions influence the routes that these birds take, as soaring birds often make long detours to avoid flying over large water bodies such as the Mediterranean Sea or the Gulf of Mexico.
12.5.3 Migratory fallouts and mortality during migration
Although birds have extraordinary capacities to traverse huge distances, at times crossing inhospitable environments or traveling through adverse weather conditions, they sometimes misjudge the weather conditions they encounter during their migration. Birds may have to stop migrating suddenly to wait out difficult conditions, backtrack to previously visited locations, or make an unplanned stop to search for food and replenish their energy reserves. When the situation grows extreme and migratory birds are completely exhausted with no options to rest or replenish their energy, they die.
Migratory fallouts are rare occasions when great numbers of migrating birds suddenly land in the same location, too exhausted to continue. Small birds are particularly susceptible to such events, especially during adverse wind conditions when they are blown off course or are stopped by strong headwinds. For example, during periods of adverse wind conditions, tens of thousands of songbirds—including Common Redstarts (Phoenicurus phoenicurus), Northern Wheatears (Oenanthe oenanthe), European Pied Flycatchers (Ficedula hypoleuca), and Garden Warblers—have sometimes appeared suddenly on a small stretch of coast in eastern England (Davis 1966). The most extreme events often occur during water crossings, and similar fallouts occur along the coast of the Gulf of Mexico when northbound migrants in spring encounter strong cold fronts coming from the north with winds directly opposite to the migrants’ flight direction (Gauthreaux 1999).
The fallouts renowned among birders involve great numbers of living birds, but fallout conditions also cause great mortality among the exhausted migrants. Only rarely is this mortality observable, as many migrants perish while migrating over water when they are unable to reach land. On April 8, 1993, a tornado off the coast of Louisiana resulted in the death of 40,000 birds of 45 species (Wiedenfeld and Wiedenfeld 1993), and hurricanes can have a region‐wide impact on populations of migrants birds such as Chimney Swifts (Chaetura pelagica) (Dionne et al. 2008). Mass mortality events also have been observed during extremely cold weather just after arrival at, or just before departure from, breeding areas. Aerial insectivores such as swallows and martins are particularly prone to great losses when temperatures are so cold that insects stop flying. Certain areas along a migration route may be particularly dangerous because of difficult weather conditions. One such area is the vast Sahara desert, crossed by millions of migrants traveling from Europe and Asia to sub‐Saharan Africa and back. The combination of dust storms, strong winds, and limited resources if birds are delayed or blown off‐course can result in very hazardous situations for migrants. On a less dramatic scale, individual migrants may also go far off course and end up far from their species’ normal range; these birds are termed vagrants (Box 12.05). Although finding vagrants is exciting for birders, most of these off‐course birds are not likely to return successfully to their breeding grounds.
Mortality during migration may be caused by many factors other than inclement weather, including collisions with man‐made structures, predation, disease, and habitat loss. A study of Common Chaffinches (Fringilla coelebs) and Bramblings (Fringilla montifringilla) estimated that 10% of the migratory populations of these species is killed by raptors during fall migrations (Lindström 1989). Predation pressure may be such a risk to migrants that it can influence their timing of migration. For example, because of their early southward movements, adult Western Sandpipers (Calidris mauri) precede migratory Peregrine Falcons by almost 1 month, and they complete their annual molt quickly on the non‐breeding grounds before the falcons arrive there. Thus, they stay ahead of falcon predation risk during the most risky period when their flight ability is limited. Juvenile Western Sandpipers migrate later, but they too avoid being exposed to falcons while molting because they do not molt at all during their first winter (Lank et al. 2003).
As these examples show, migration may be a period of heightened risk for almost all migratory birds. For Black‐throated Blue Warblers (Setophaga caerulescens) that breed in New Hampshire (USA) and overwinter in Jamaica, mortality rates are 15 times higher during migration than during stationary breeding and non‐breeding periods (Sillett and Holmes 2002). Similarly, a study of the mortality rates of satellite‐tagged raptors moving between Europe and Africa (Klaassen et al. 2014) found that their daily mortality risk is substantially higher during migration (Fig. 12.23). In contrast, studies on seasonal survival in Red Knots indicate that hardly any mortality occurs during the long migrations to and from the high arctic breeding grounds (Leyrer et al. 2013; Rakhimberdiev et al. 2015).
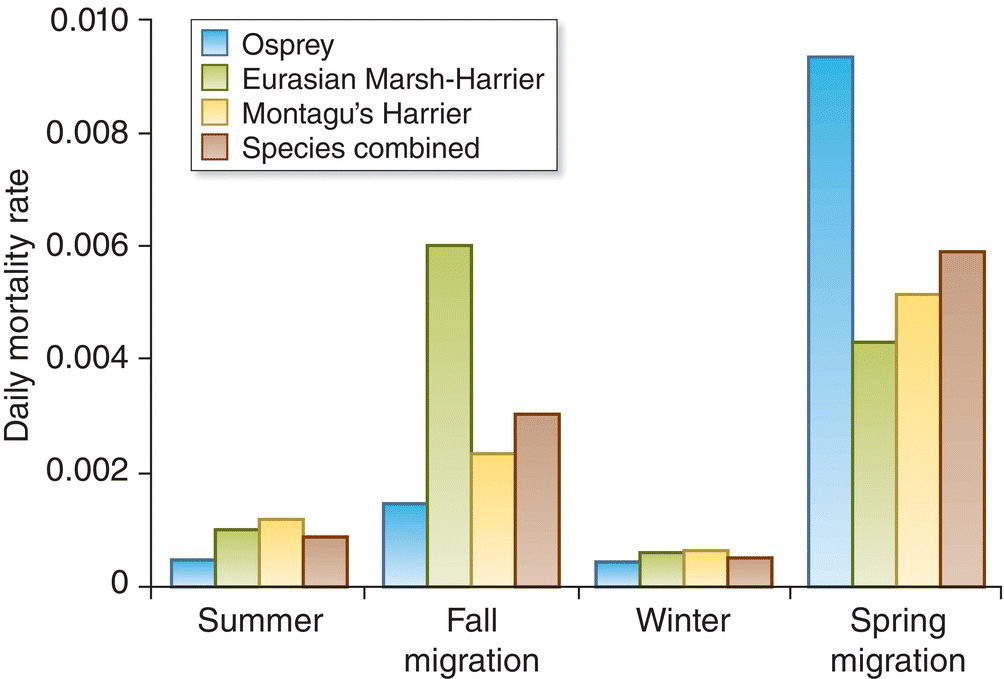
Fig. 12.23 High mortality during raptor migration. This graph depicts the mortality rate for three species of raptors—Montagu’s Harrier (Circus pygargus), Eurasian Marsh‐Harrier (Circus aeruginosus), and Osprey (Pandion haliaetus)—that annually migrate between Europe and Africa. Mortality is greatest during migration, as opposed to summer or winter when movements are much more localized.
(From Klaassen et al. 2014. Reproduced with permission from John Wiley and Sons.)
12.6 Dispersal
There are two general types of dispersal movements made by birds. Breeding dispersal occurs between consecutive breeding seasons when a bird that previously has bred in one location moves to a new breeding location. By contrast, natal dispersal—the first dispersal in any bird’s life—is the movement between the site where a bird is fledged and the site where it first attempts to breed as an adult.
12.6.1 Breeding dispersal and philopatry
Studying dispersal is difficult because it is usually hard to determine the fates of birds that disappear from a study site: did they die, or did they disperse to a new location? Despite this ambiguity, studies of breeding dispersal indicate that long‐distance dispersal is uncommon in most bird species, and instead that most breeding dispersal involves movements of only a short distance. For example, fewer than a third of adult Black Kites (Milvus migrans) in southern Spain switch territories after they have begun breeding. As in many bird species, female kites are more likely than males to move among breeding sites as adults, and members of both sexes are more likely to disperse after nest failure or the death of a mate (Forero et al. 1999). Similarly, Tree Swallows (Tachycineta bicolor) in upstate New York (USA) are unlikely to change their breeding sites in successive years by more than a few hundred meters: in one study, only 4% of male and 14% of female breeders were seen to disperse during their lifetimes. Those that dispersed generally moved a short distance, and the number of birds dispersing fell off exponentially with distance from the previous site (Winkler et al. 2004)
12.6.2 Patterns in natal dispersal
Several generalizations can be made about the natal dispersal of birds. First, natal dispersal is much more common than breeding dispersal. Indeed, very few birds have fledging young that do not disperse from the natal territory before breeding. Second, the distances involved in natal dispersal are usually far greater than in breeding dispersal. For example, Black‐tailed Godwits (Limosa limosa) dispersing in the dairy farmlands of the Netherlands moved on average 2 kilometers during natal dispersal, but only 200 meters during breeding dispersal (Kentie et al. 2014). Similarly, Bobolinks (Dolichonyx oryzivorus) dispersing among grassland patches in Vermont (USA), moved an average of 1522 meters during natal dispersal but only 370 meters during breeding dispersal (Fajardo et al. 2009). Third, as in breeding dispersal, females generally are more likely to disperse, and they tend to disperse longer distances than males. For example, in Tree Swallows in upstate New York, females dispersed on average 8.38 kilometers and males only 2.44 kilometers from their natal site (Fig. 12.24) (Winkler et al. 2005).
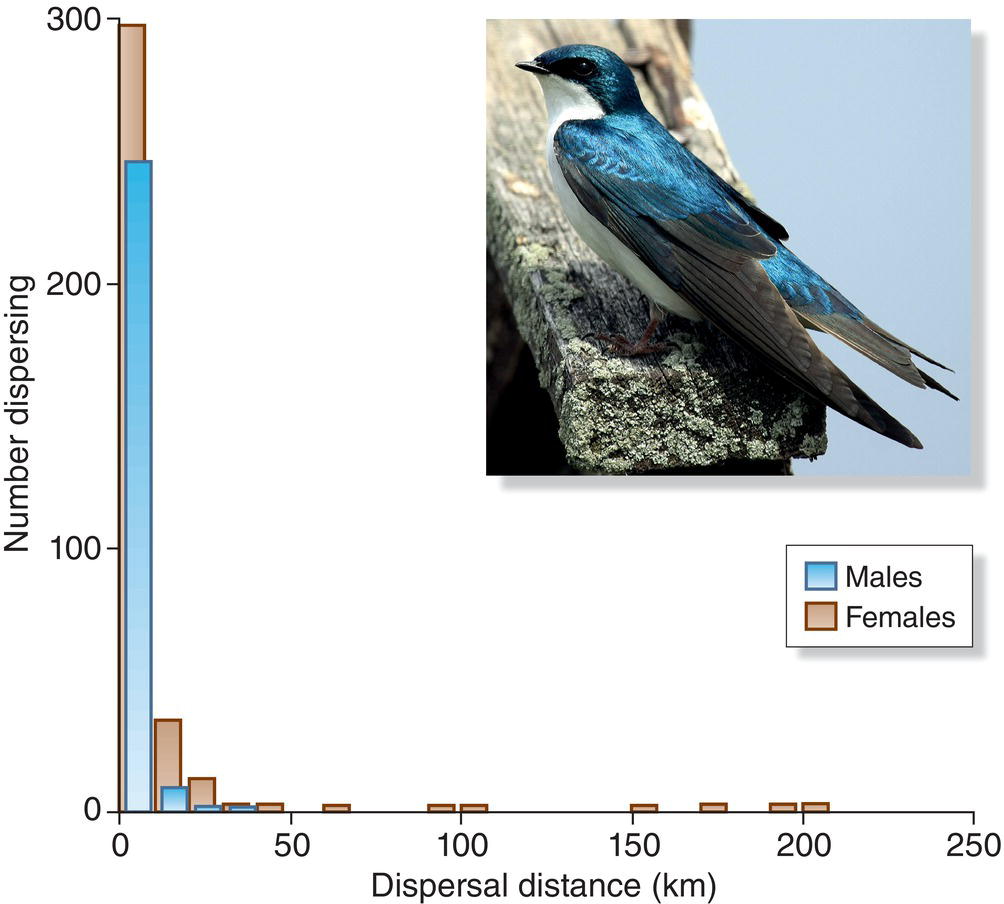
Fig. 12.24 Natal dispersal distances of Tree Swallows (Tachycineta bicolor). In this study, more than half the birds moved only a few kilometers between the site in eastern North America where they were hatched and the site where they later bred as adults. Females (red) tended to move a bit farther than males (blue), and a few birds of both sexes dispersed over much longer distances.
(From Winkler et al. 2005. Reproduced with permission from John Wiley and Sons. Photograph by Tim Lenz.)
Almost all data on natal dispersal distances show skewed distributions with long right‐hand tails, suggesting that most birds disperse short distances but a few move much farther. Some of this pattern, especially for studies with small study areas, may simply reflect the impossibility of detecting dispersal events that take the dispersers very long distances away from the natal site. However, even when studies include the entire species’ range, the pattern seems to be the same: most birds disperse to breeding sites that are not very far from their natal site, with only a relatively small proportion of birds undertaking natal dispersal over much longer distances.
12.6.3 Dispersal and colonization
Dispersal is what drives the expansion of a species’ geographic distribution into new areas. This often occurs very slowly over hundreds or thousands of years, but we have also witnessed many examples of explosive expansions in which birds spread very rapidly into areas where they formerly did not occur (Box 12.06). In most such situations, the early colonists that first make it to a new breeding site stay there; it is their offspring that continue dispersing into unoccupied regions.
This process of colonization and dispersal has been particularly well studied in Western Bluebirds (Sialia mexicana), a small thrush that breeds in cavities and artificial nest boxes throughout much of western North America. Like many cavity‐adopting species, the availability of nesting sites is often a limiting factor for bluebird populations. Before the arrival of humans and human‐made cavities, this species had to rely on clusters of natural cavities, which usually resulted from woodpecker excavations in trees damaged by small forest fires. Therefore, Western Bluebird breeding habitat was patchy and relatively ephemeral, and the ability to colonize new areas by natal dispersal was an important part of their natural history. At present, nesting cavities for Western Bluebirds are becoming more common through the provision of artificial nest boxes and an increase in forest fires, and the species’ range is expanding rapidly (Fig. 12.25).
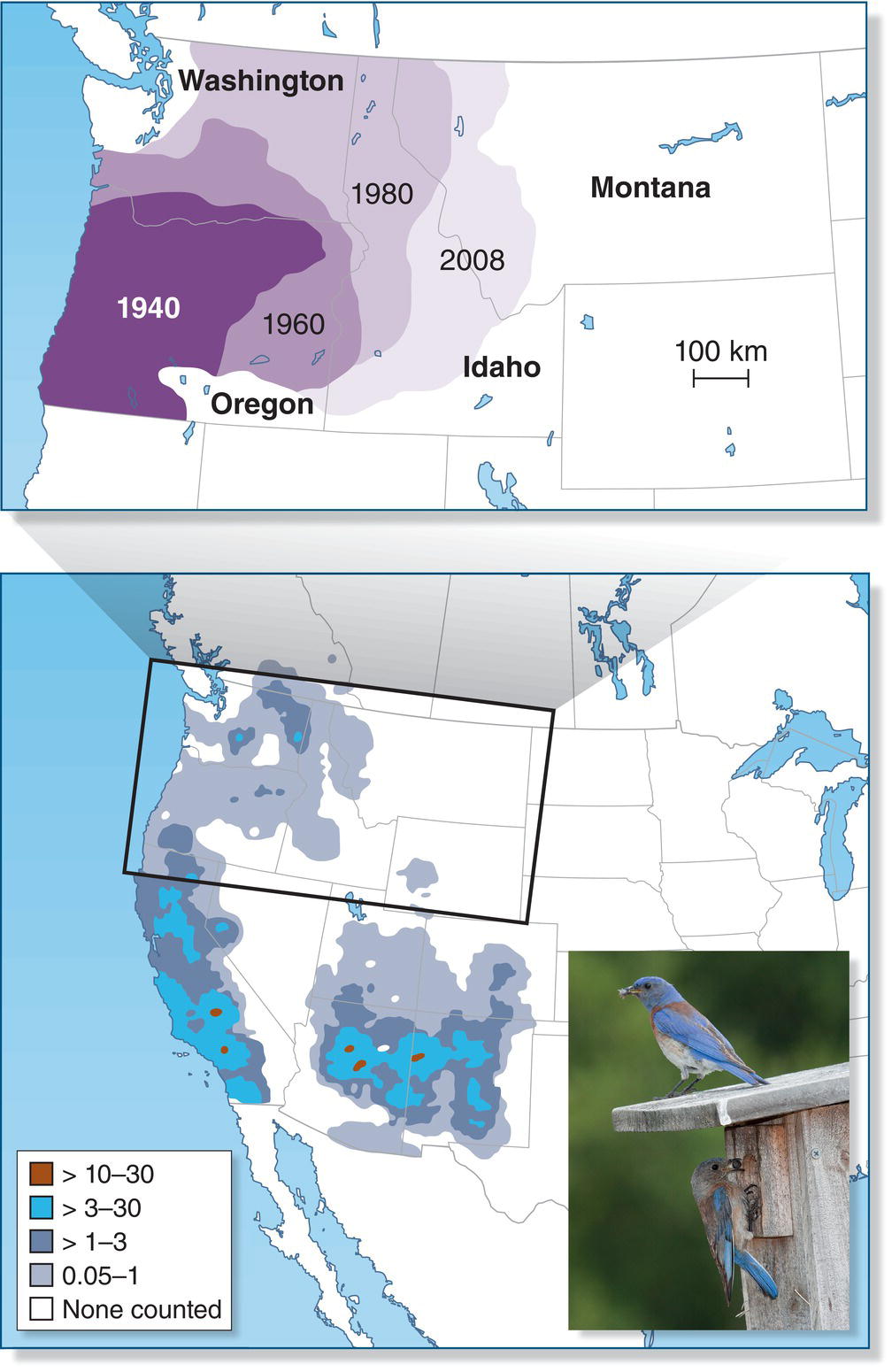
Fig. 12.25 Breeding range expansion. Western Bluebirds (Sialia mexicana) of North America continue to spread eastward into higher elevations where artificial nest boxes provide new breeding sites. Darker colors on the lower map indicate larger population sizes. The top panel shows how this species has expanded its range in four northwestern US states from 1940 to 2008.
(From Duckworth 2009. Reproduced with permission from the Royal Society. Adapted from Sauer et al. 2008. Photograph by Marlin Harms.)
Male Western Bluebirds exhibit a set of different behaviors depending on whether they are dispersers moving into a new habitat, or instead natally philopatric, breeding near the nest where they were themselves hatched. Males that show an aggressive, non‐cooperative set of behaviors tend to disperse farther, and these males are the ones that tend to colonize new areas. Their aggressiveness helps them during colonization by allowing them to win out in competition with the closely related Mountain Bluebird (Sialia currucoides). In places away from the range edge where Western Bluebirds are more abundant, these birds breed cooperatively, and philopatric males benefit from being more social and less aggressive because their tolerance of allowing previously fledged offspring to remain on their territory provides them with a source of helpers to assist in future breeding attempts. Each kind of male—aggressive colonists and more tolerant cooperators—has a higher fitness in its respective setting (Duckworth 2009; Duckworth and Kruuk 2009).
Rapid biological expansions are often portrayed on a map as steady enlargements of a species’ range, with the edges expanding in a uniform fashion. However, as invasive European Starlings (Sturnus vulgaris), House Finches (Carpodacus mexicanus), and Collared Doves (Streptopelia decaocto) expanded their ranges across the USA, small “propagule” or “bridgehead” populations were founded by birds that dispersed far from the main range boundary of the species. After additional time, the population filled in behind the propagules.
When a species is introduced to a new area or initiates its own natural invasion of an area, it may begin to experience very different selection pressures on its movements than it did in its historic range. For example, although they are poor dispersers in their native range, Common Mynahs (Acridotheres tristis) are invasive and expanding in southern Africa, Australia, and elsewhere (Fig. 12.26). The invasion causes spatial sorting in the population whereby individuals that disperse farther accumulate at the front of the expanding range. These longer range dispersers produce more long‐distance dispersers, and a positive feedback can thus be created in which the advancing front of a species’ range accelerates as it spreads across new territory. Thus, because longer dispersal is beneficial when a species is expanding into favorable habitat, invading populations of a species may show a much greater propensity to disperse than populations of the same species in their original range (Berthouly‐Salazar et al. 2012).
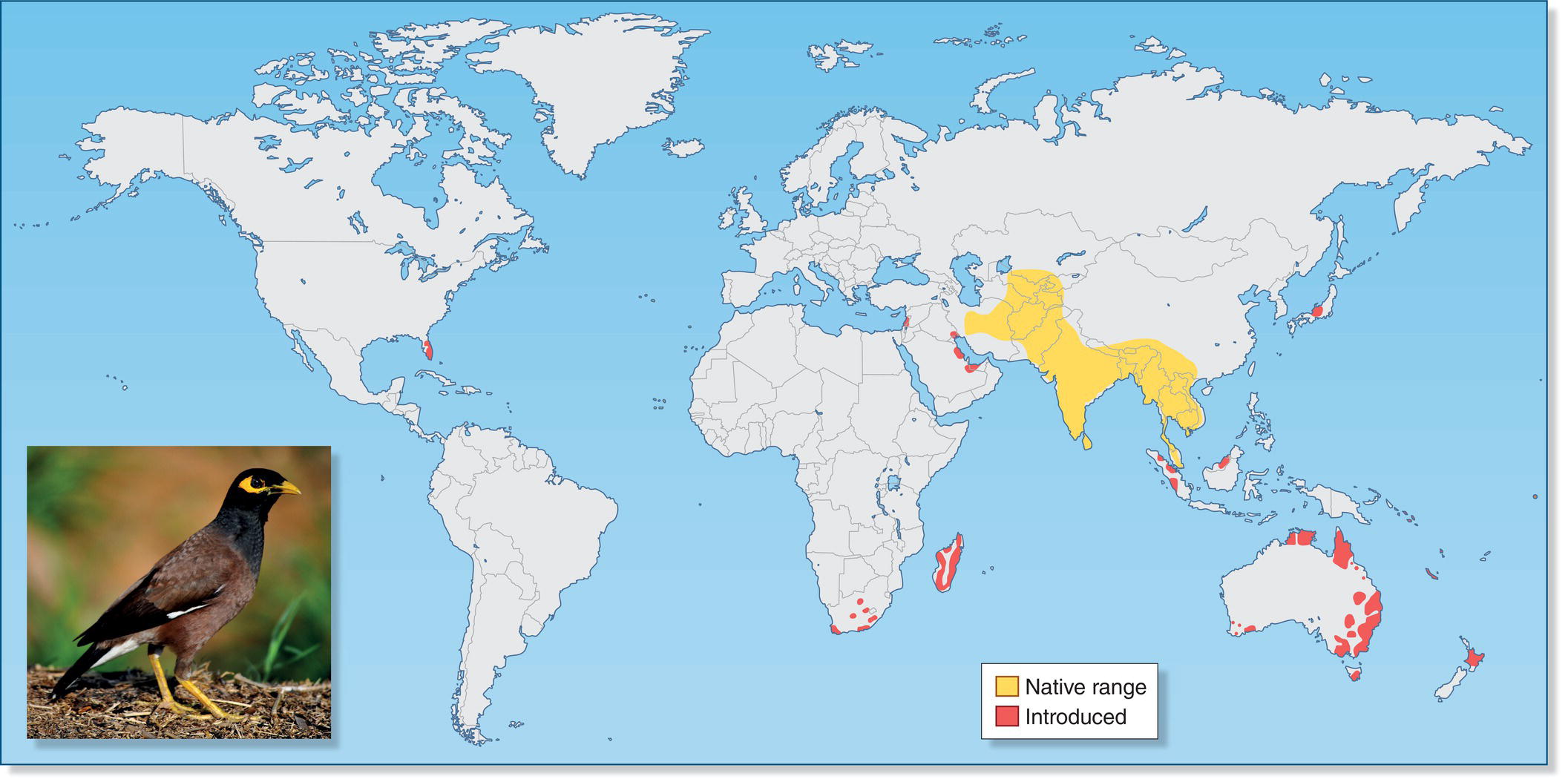
Fig. 12.26 Range expansion in non‐native species. The Common Mynah (Acridotheres tristis) is a poor disperser in its native range (yellow), but tends to disperse much more readily in locations where it has been introduced (red). (Biatch, https://commons.wikimedia.org/wiki/File:Common_Mynah_distribution_map.png.
Photograph by T. G. Santosh, https://commons.wikimedia.org/wiki/File:Indian_Myna.JPG. CC‐BY‐SA 3.0.)
12.6.4 Prospecting for new breeding sites
Birds make specific decisions about which habitats to live in, breed in, and move through. They base these decisions on many different factors, such as vegetation type and structure, food availability, and the abundance of predators. The habitat they prefer may vary by their own age or the season. For example, simply because it survived to fledging, a bird knows that the territory on which it was raised was suitable for breeding, and experienced breeders also have information about specific locations from their own past breeding attempts. Many birds use this kind of personal information to inform their choice of a breeding site. Birds can gain additional cues about the quality of different sites by observing the breeding success or habitat occupancy of other birds. Dispersing individuals therefore may base decisions about where to settle the following year on observations of their neighbors. These observations may be indirect, such as witnessing parental activity around a nest (a cue used by Collared Flycatchers, Ficedula albicollis) (Pärt and Doligez 2003), the song rates of males (which is correlated with breeding success in Black‐throated Blue Warblers, Setophaga caerulescens) (Betts et al. 2008), or direct examination of the contents of other birds’ nests (as documented in Spotless Starlings, Sturnus unicolor) (Parejo et al. 2008).
Birds breeding in colonies can easily gather information from their neighbors. Black‐legged Kittiwakes (Rissa tridactyla) whose nests were experimentally caused to fail were much more likely to return to the same area the next year if their neighbors had been successful (Boulinier et al. 2008). Similarly, the performance of other breeders in a colony of Lesser Kestrels (Falco naumanni) was found to be more important in settlement decisions than the number of kestrels present (Calabuig et al. 2008). Gathering of information by non‐breeders (and breeders who have failed early in the season) also occurs in species that breed in looser aggregations.
Prospecting non‐breeders are fairly common in many species, but this behavior has been particularly well studied in the Collared Flycatcher of northern Europe. Prospecting flycatchers are attracted by conspicuous parental feeding and vigilance activity (both of which are predictors of fledging success) during the mid‐nestling phase. Although prospecting males seemed to be interested primarily in the quality of potential future territories and ignored the quality of individuals living on them, female flycatchers searched for generally high‐quality areas that contained territories held by high‐quality males (Doligez et al. 2004b). Prospecting Collared Flycatchers tended to return the following year to breed close to the sites they had investigated most frequently (Doligez et al. 2004a).
Some birds, such as Eastern Kingbirds (Tyrannus tyrannus) in eastern North America, prospect late in the season after breeding is mostly completed (Redmond et al. 2009). Many other birds that move extensively in the post‐fledging period may be prospecting for information, but in most species it is very difficult to track moving fledglings to their eventual breeding sites the following year, and demonstrate that the place they chose for breeding was one that they explored during the previous summer.
12.6.5 Dispersal and genetic connections among populations
Dispersal also is a key factor in determining how populations are connected genetically through time and space. As discussed in more detail in Chapters 13 and 15, a metapopulation is a collection of distinct subpopulations connected by dispersal. The amount of dispersal among these subpopulations has important implications for the numbers of birds they each support, their persistence through time, and their potential to diverge genetically from one another. In a practical context, the pattern of dispersal of individual birds among subpopulations often has very important consequences for the conservation and management of those subpopulations.
Some avian populations—particularly those in the temperate zone—are extremely well mixed via the natal and breeding dispersal of birds each year. This causes the genetic variation in these species to be fairly homogeneous over large expanses of their range. For example, the Red‐winged Blackbird (Agelaius phoeniceus) has a very broad breeding distribution across North America, yet notably little genetic variation among its many subpopulations (Ball et al. 1988). Similarly, American White Pelicans (Pelecanus erythrorhynchos) show continent‐wide mixing across North America because of their long‐distance dispersal tendencies, an artifact of the ephemeral nature of their colony sites in shallow lakes that fluctuate dramatically in availability from year to year (Reudink et al. 2011). In contrast, many tropical birds are highly sedentary, and populations of tropical birds that are separated into discrete subpopulations are often genetically distinct. This pattern suggests that temperate zone bird populations are often far more interconnected by dispersal than are tropical populations.
12.6.6 Methods for measuring dispersal
Because dispersal is important but hard to study, many creative methods have been developed to determine which birds disperse and where they go. One of the earliest methods used to study dispersal was to recapture breeding birds that were banded (usually as nestlings, thus natal dispersers) some distance from their site of original banding. Dispersal is then measured simply as the straight line distance between these points.
The vast scale of many avian dispersal movements is the greatest challenge when tracking banded birds. Even on small and intensively monitored study areas, researchers can fail to find and capture some dispersing birds that are breeding there. Small study areas also have the problem of not being large enough to include many of the birds that disperse beyond their edges over intermediate and long distances. Most study areas thus produce estimates of dispersal distances that have a sampling bias against longer dispersal movements.
The movements of birds can sometimes be inferred indirectly. For example, when feathers are being grown, they incorporate the stable isotopes of various elements—including hydrogen and carbon—that are derived from the food, water, and air at that site. Since these isotope ratios vary geographically, it is sometimes possible to use the ratios found in a feather to infer where the bird was when that feather was growing. As technological innovations make it increasingly possible to outfit birds with small tracking devices that last for months or years, it is becoming more feasible to track their dispersal directly (Box 12.01).
12.7 Evolution of avian movement patterns
The tremendous variation in the ways that birds move across the seasons and throughout their lives is the result of past evolutionary forces that selected for individuals whose movements gave them the best opportunities to survive and reproduce (Box 12.07). One theory on the evolution of migration is that it may have occurred through an extension of less extreme annual movements in search of food or breeding opportunities. What at one time might have been a simple short‐distance movement to and from seasonal feeding grounds could have become exaggerated as the seasonality of the birds’ environments became more variable or more extreme.
However, it is important to remember that earth has had changing environments throughout its history. One extreme example of environmental change is the recent melting of the glaciers that receded from the northern hemisphere only about 15,000 years ago. Those vast sheets of ice were only the latest in a long cycle of more than 20 Pleistocene glacial cycles that ebbed and flowed over the past 2.5 million years. Migrant birds can be seen as having adapted their patterns of movement to the new opportunities and habitats that receding glaciers left behind. Northern migrants today are the descendants of birds that have been adapting to such changing environments for many millions of years. One can imagine that, with every cycle of Pleistocene glaciation, the migrants retreated to more southern breeding localities and made shorter migrations while the ice ruled the north. Then, when the ice receded, the migrants retained their powers of movement and moved north to take advantage of renewed breeding opportunities there.
It is clear that birds like the warblers, thrushes, and flycatchers that migrate every year to breeding sites all across the northern hemisphere have not evolved this power of migration during the past few thousand years. Instead, they are descendants of avian groups with long and repeated histories of migration. Indeed, a recent study of the evolution of migration in the New World warblers indicates that the earliest‐diverged members of this diverse group already were long‐distance migrants (Winger et al. 2012), and it appears likely that the ancestral ability to migrate may be a shared trait of all birds, if not all vertebrates (Piersma et al. 2005).
12.7.1 How does migration evolve?
The imprint of past evolutionary forces is clearly evident in the present‐day migrations of many birds (Box 12.08). One remarkable example is seen in the migration paths of the Northern Wheatear (Oenanthe oenanthe), a small songbird that breeds over a broad range of latitudes, from the temperate zone to the high Arctic, but always in habitats of sparse vegetation. This species was originally found in Eurasia, but as the northern ice sheets receded after the Last Glacial Maximum, wheatear populations from Siberia spread eastward to colonize Alaska, and wheatears from Europe moved westward across Greenland to breed in the Canadian high Arctic. Amazingly, all of these wheatears that now breed on either side of North America still retrace their ancestral migratory paths to winter in sub‐Saharan Africa. Wheatears in arctic Alaska leave in the fall on a westward track for a migration of many thousands of kilometers to non‐breeding areas in East Africa, and those in the Canadian high Arctic head east in the fall for the long journey to West Africa. Using tiny geolocators to track individual wheatears, researchers have confirmed that birds breeding in Alaska migrate through Asia to reach eastern Africa, traveling some 14,500 kilometers (Bairlein et al. 2012), the longest distance recorded for a migratory songbird (Fig. 12.27). The routes that birds breeding in eastern Canada take are less well studied, although early observations from birds resting on ships (Snow 1953) and banding data suggest that wheatears from Greenland and Iceland cross several thousand kilometers of open ocean to reach the UK or Europe and then continue south to Africa (Delingat et al. 2008).
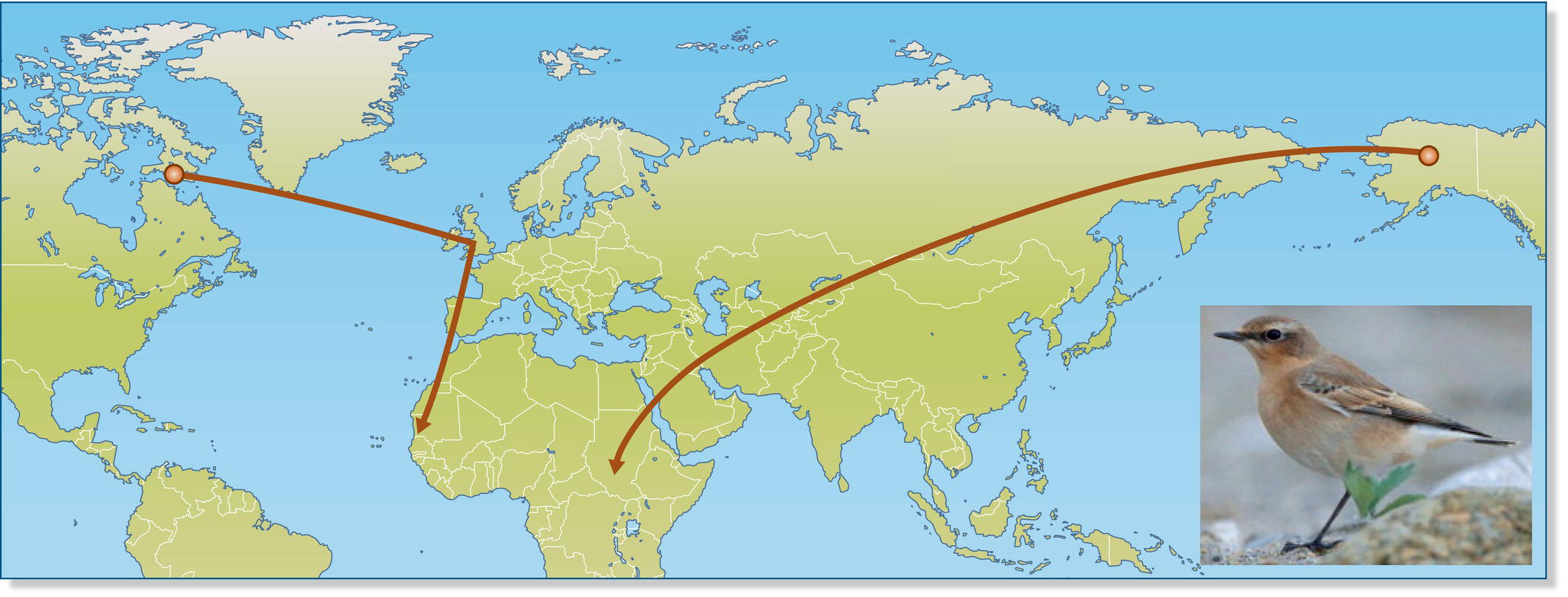
Fig. 12.27 Retracing ancestral migratory paths. Northern Wheatears (Oenanthe oenanthe) have recently expanded into the North American Arctic (orange dots) from both the east and west. Wheatears breeding in Alaska migrate over Asia to reach Africa, while individuals breeding in northeastern Canada migrate to Africa via Greenland and Europe.
(Adapted from Bairlein et al. 2012. Reproduced with permission from the Royal Society.)
The evolutionary results of past movements are sometimes evident over even longer timeframes. For example, the Galápagos Hawk (Buteo galapagoensis) is the only raptor endemic to the Galápagos Archipelago. Since most raptors avoid flying over long stretches of open water, how did the ancestors of these birds come to colonize these remote islands that are 1000 kilometers from the South American continent? It turns out that the Galápagos Hawk is an evolutionary offshoot of the Swainson’s Hawk (Buteo swainsoni), a species that today breeds in northern North America and then migrates, often in large flocks, to the grasslands of southern South America. Genetic evidence for the evolutionary split between the Galápagos and Swainson’s Hawks suggests that their ancestors were undertaking this same migration about 300,000 years ago, when a flock of migrating hawks went off‐course and ended up in the Galápagos to become the founding population of the species we now know as the Galápagos Hawk (Bollmer et al. 2006; Hull et al. 2008).
12.7.2 Migration and ongoing environmental change
Earth’s climate has always been constantly changing, but we are presently in a period of unusually rapid environmental change caused by human activities (Chapter 15). Global temperatures are increasing at an unprecedented rate; as a result, plants are leafing and flowering earlier, insects are emerging on accelerated seasonal schedules, and birds are arriving back from their non‐breeding grounds sooner than before. One potential problem faced by migratory birds is that different organisms are responding to climate change at different rates, resulting in the potential for ecological mismatch between migratory birds and the foods that they eat (Both et al. 2009) (Fig. 12.28).
Fig. 12.28 Ecological mismatch caused by changing seasonality. (A) Many migratory birds have evolved to synchronize their breeding timing (solid red curve) with the period of greatest food availability for their offspring (dotted blue curve) so that they can breed successfully (shaded red area represents frequency of successful breeders). (B) If environmental conditions shift such that peak food availability occurs earlier than peak breeding, early breeders will raise the most offspring sucessfully, but much of the population will breed too late to provide optimal levels of food to their young.
(From Both et al. 2009. Reproduced with permission from John Wiley and Sons.)
Each species of bird has evolved to breed during a time of the year when they can expect enough food to raise their young. In some cases, environmental changes are disrupting this relationship between the timing of breeding and the available food supply. The best studied example of such ecological mismatch is in the European Pied Flycatcher (Ficedula hypoleuca) of northern Europe. Even though these flycatchers have responded to climate change by starting to breed earlier in the year, the key insects with which they feed their chicks have advanced their calendars even more, as the insects are responding to rapidly advancing local conditions that the flycatchers, in their sub‐Saharan non‐breeding areas, do not experience.
It was long thought that these flycatchers were constrained in moving their time of breeding forward because they time their migration based on their internal biological clocks, which are keyed not to short‐term environmental cues but rather to day length. However, it now seems that the birds’ migratory clocks can be adjusted physiologically, meaning that they do not need to wait for various genetic variants to be sifted by natural selection. The photoperiods that young birds experience in the nest before making their first fall migration appear to calibrate their clocks to the earlier setting required by the advancing seasons in the north (Both 2010). It turns out that the birds are arriving in northern Europe later than the spring flush of insects not because their clocks are telling them to leave Africa at a later date, but because they are being held up by poor conditions while migrating through southern Europe, where spring is not advancing at the same pace as it is in northern Europe. This example highlights the general point that migration is both a powerful way for birds to respond to environmental variation and a trait that renders them vulnerable when conditions deteriorate at any point along their annual journey.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Åkesson, S., R. Klaassen, J. Holmgren, J. W. Fox, and A. Hedenström. 2012. Migration routes and strategies in a highly aerial migrant, the common swift Apus apus, revealed by light‐level geolocators. PLoS One 7:e41195.
- Anich, N. M., T. J. Benson, and J. C. Bednarz. 2012. What factors explain differential use within Swainson's Warbler (Limnothlypis swainsonii) home ranges? Auk 129:409–418.
- Arendt, W. J. 1988. Range expansion of the Cattle Egret (Bubulcus ibis) in the Greater Caribbean Basin. Colonial Waterbirds 11:252–262.
- Bächler, E., S. Hahn, M. Schaub, R. Arlettaz, L. Jenni, J. W. Fox, V. Afanasyev, and F. Liechti. 2010. Year‐round tracking of small trans‐Saharan migrants using light‐level geolocators. PLoS One 5:e9566.
- Bairlein, F., D. R. Norris, R. Nagel, M. Bulte, C. C. Voigt, J. W. Fox, D. J. T. Hussell, and H. Schmaljohann. 2012. Cross‐hemisphere migration of a 25 g songbird. Biology Letters 8:505–507.
- Baker, A. J., P. M. González, T. Piersma, L. J. Niles, I. de Lima Serrano do Nascimento, P. W. Atkinson, N. A. Clark, C. D. T. Minton, M. K. Peck, and G. Aarts. 2004. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proceedings of the Royal Society B: Biological Sciences 271:875–882.
- Ball, R. M., Jr, S. Freeman, F. C. James, E. Bermingham, and J. C. Avise. 1988. Phylogeographic population structure of Red‐winged Blackbirds assessed by mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America 85:1558–1562.
- Barg, J. J., D. M. Aiama, J. Jones, and R. J. Robertson. 2006. Within‐territory habitat use and microhabitat selection by male Cerulean Warblers (Dendroica cerulea). Auk 123:795–806.
- Bearhop, S., W. Fiedler, R. W. Furness, S. C. Votier, S. Waldron, J. Newton, G. J. Bowen, P. Berthold, and K. Farnsworth. 2005. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 310:502–504.
- Bell, C. P. 1996. Seasonality and time allocation as causes of leap‐frog migration in the Yellow Wagtail Motacilla flava. Journal of Avian Biology 27:334–342.
- Bell, C. P. 1997. Leap‐frog migration in the fox sparrow: minimizing the coast of spring migration. Condor 99:470–477.
- Bensch, S., T. Andersson, and S. Åkesson. 1999. Morphological and molecular variation across a migratory divide in Willow Warblers, Phylloscopus trochilus. Evolution 53:1925–1935.
- Benvenuti, S. and A. Gagliardo. 1996. Homing behaviour of pigeons subjected to unilateral zinc sulphate treatment of their olfactory mucosa. Journal of Experimental Biology 199:2531–2535.
- Berthold, P. 1991. Genetic control of migratory behaviour in birds. Trends in Ecology and Evolution 6:254–257.
- Berthold, P. and A. J. Helbig. 1992. The genetics of bird migration: stimulus, timing, and direction. Ibis 134:S35–S40.
- Berthold, P. and U. Querner. 1981. Genetic basis of migratory behavior in European warblers. Science 212:77–79.
- Berthouly‐Salazar, C., B. J. van Rensburg, J. J. Le Roux, B. J. van Vuuren, and C. Hui. 2012. Spatial sorting drives morphological variation in the invasive bird, Acridotheris tristis. PLoS One 7:e38145.
- Betts, M. G., A. S. Hadley, N. Rodenhouse, and J. J. Nocera. 2008. Social information trumps vegetation structure in breeding‐site selection by a migrant songbird. Proceedings of the Royal Society B: Biological Sciences 275:2257–2263.
- Bildstein, K. L. 2006. Migrating Raptors of the World: Their Ecology and Conservation. Cornell University Press, Ithaca, NY.
- Billerman, S. M., G. H. Huber, D. W. Winkler, R. J. Safran, and I. J. Lovette. 2011. Population genetics of a recent transcontinental colonization of South America by breeding Barn Swallows (Hirundo rustica). Auk 128:506–513.
- Bobek, M., R. Hampl, L. Peške, F. Pojer, J. Šimek, and S. Bureš. 2008. African Odyssey project—satellite tracking of black storks Ciconia nigra breeding at a migratory divide. Journal of Avian Biology 39:500–506.
- Bollmer, J. L., R. T. Kimball, N. K. Whiteman, J. H. Sarasola, and P. G. Parker. 2006. Phylogeography of the Galápagos Hawk (Buteo galapagoensis): a recent arrival to the Galápagos Islands. Molecular Phylogenetics and Evolution 39:237–247.
- Both, C. 2010. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Current Biology 20:243–248.
- Both, C., M. van Asch, R. G. Bijlsma, A. B. van den Burg, and M. E. Visser. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? Journal of Animal Ecology 78:73–83.
- Boulinier, T., K. D. McCoy, N. G. Yoccoz, J. Gasparini, and T. Tveraa. 2008. Public information affects breeding dispersal in a colonial bird: kittiwakes cue on neighbours. Biology Letters 4:538–540.
- Browder, J. A. 1973. Long‐distance movements of Cattle Egrets. Bird‐Banding 44:158–170.
- Buehler, D. M., A. J. Baker, and T. Piersma. 2006. Reconstructing palaeoflyways of the late Pleistocene and early Holocene Red Knot Calidris canutus. Ardea 94:485–498.
- Bulyuk, V. N. and A. Tsvey. 2013. Regulation of stopover duration in the European Robin Erithacus rubecula. Journal of Ornithology 154:1115–1126.
- Calabuig, G., J. Ortego, J. M. Aparicio, and P. J. Cordero. 2008. Public information in selection of nesting colony by lesser kestrels: which cues are used and when are they obtained? Animal Behaviour 75:1611–1617.
- Cantos, F. J. and J. L. Tellería. 1994. Stopover site fidelity of four migrant warblers in the Iberian Peninsula. Journal of Avian Biology 25:131–134.
- Cheke, R. A., J. F. Venn, and P. J. Jones. 2007. Forecasting suitable breeding conditions for the red‐billed quelea Quelea quelea in southern Africa. Journal of Applied Ecology 44:523–533.
- Chesser, R. T. 1994. Migration in South America: an overview of the austral system. Bird Conservation International 4:91–107.
- Crosby, G. T. 1972. Spread of the Cattle Egret in the western hemisphere. Bird‐Banding 43:205–212.
- Cumming, G. S., P. A. R. Hockey, L. W. Bruinzeel, and M. A. DuPlessis. 2008. Wild bird movements and avian influenza risk mapping in southern Africa. Ecology and Society 13:26.
- Dänhardt, J. and Å. Lindström. 2001. Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Animal Behaviour 62:235–243.
- Davis, P. 1966. The great immigration of early September 1965. British Birds 59:353–376.
- Delingat, J., F. Bairlein, and A. Hedenström. 2008. Obligatory barrier crossing and adaptive fuel management in migratory birds: the case of the Atlantic crossing in Northern Wheatears (Oenanthe oenanthe). Behavioral Ecology and Sociobiology 62:1069–1078.
- Delmore, K. E., J. W. Fox, and D. E. Irwin. 2012. Dramatic intraspecific differences in migratory routes, stopover sites and wintering areas, revealed using light‐level geolocators. Proceedings of the Royal Society B: Biological Sciences 279:4582–4589.
- DeLuca, W. V., B. K. Woodworth, C. C. Rimmer, P. P. Marra, P. D. Taylor, K. P. McFarland, S. A. Mackenzie, and D. R. Norris. 2015. Transoceanic migration by a 12 g songbird. Biology Letters 11(4):20141045.
- Devlin, C. M., A. W. Diamond, S. W. Kress, C. S. Hall, and L. Welch. 2008. Breeding dispersal and survival of Arctic Terns (Sterna paradisaea) nesting in the Gulf of Maine. Auk 125:850–858.
- Dionne, M., C. Maurice, J. Gauthier, and F. Shaffer. 2008. Impact of Hurricane Wilma on migrating birds: the case of the Chimney Swift. Wilson Journal of Ornithology 120:784–792.
- Doligez, B., T. Pärt, and E. Danchin. 2004a. Prospecting in the collared flycatcher: gathering public information for future breeding habitat selection? Animal Behaviour 67:457–466.
- Doligez, B., T. Pärt, E. Danchin, J. Clobert, and L. Gustafsson. 2004b. Availability and use of public information and conspecific density for settlement decisions in the collared flycatcher. Journal of Animal Ecology 73:75–87.
- Duckworth, R. A. 2009. Maternal effects and range expansion: a key factor in a dynamic process? Philosophical Transactions of the Royal Society B: Biological Sciences 364:1075–1086.
- Duckworth, R. A. and L. E. B. Kruuk. 2009. Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63:968–977.
- Emlen, S. T. 1970. Celestial rotation: its importance in the development of migratory orientation. Science 170:1198–1201.
- Fajardo, N., A. M. Strong, N. G. Perlut, and N. J. Buckley. 2009. Natal and breeding dispersal of Bobolinks (Dolichonyx oryzivorus) and Savannah Sparrows (Passerculus sandwichensis) in an agricultural landscape. Auk 126:310–318.
- Felicisímo, Á. M., J. Muñoz, and J. González‐Solis. 2008. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS One 3:e2928.
- Fernández, M., J. Oria, R. Sánchez, L. M. Gonzalez, and A. Margalida. 2009. Space use of adult Spanish Imperial Eagles Aquila adalberti. Acta Ornithologica 44:17–26.
- Fijn, R. C., D. Hiemstra, R. A. Phillips, and J. van der Winden. 2013. Arctic Terns Sterna paradisaea from the Netherlands migrate record distances across three oceans to Wilkes Land, East Antarctica. Ardea 101:3–12.
- Forero, M. G., J. A. Donázar, J. Blas, and F. Hiraldo. 1999. Causes and consequences of territory change and breeding dispersal distance in the Black Kite. Ecology 80:1298–1310.
- Gagliardo, A., J. Bried, P. Lambardi, P. Luschi, M. Wikelski, and F. Bonadonna. 2013. Oceanic navigation in Cory’s shearwaters: evidence for a crucial role of olfactory cues for homing after displacement. Journal of Experimental Biology 216:2798–2805.
- Gauthreaux, S. A., Jr. 1999. Neotropical migrants and the Gulf of Mexico: the view from aloft. Pages 27–50 in Gatherings of Angels: Migrating Birds and their Ecology (K. P. Able, Ed.). Cornell University Press, Ithaca, NY.
- Gill, R.E., Jr, D. C. Douglas, C. M. Handel, T. L. Tibbitts, G. Hufford, and T. Piersma. 2014. Hemispheric‐scale wind selection facilitates bar‐tailed godwit circum‐migration of the Pacific. Animal Behaviour 90:117–130.
- Gill, R.E., Jr, T. L. Tibbitts, D. C. Douglas, C. M. Handel, D. M. Mulcahy, J. C. Gottschalck, N. Warnock, B. J. McCaffery, P. F. Battley, and T. Piersma. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proceedings of the Royal Society B: Biological Sciences 276:447–457.
- Greenberg, R. and Marra, P. P. Eds, 2005.Birds of Two Worlds: The Ecology and Evolution of Migration. Johns Hopkins University Press, Baltimore, MD.
- Gwinner, E. 1981. Circannuale Rhythmen bei Tieren und ihre photoperiodische Synchronisation. Naturwissenschaften 68:542–551.
- Gwinner, E. 1986. Circannual Rhythms: Endogenous Annual Clocks in the Organization of Seasonal Processes. Springer‐Verlag, Berlin.
- Hagstrum, J. T. 2000. Infrasound and the avian navigational map. Journal of Experimental Biology 203:1103–1111.
- Helbig, A. J. 1996. Genetic basis, mode of inheritance and evolutionary changes of migratory directions in Palearctic warblers (Aves: Sylviidae). Journal of Experimental Biology 199: 49–55.
- Hull, J. M., W. K. Savage, J. L. Bollmer, R. T. Kimball, P. G. Parker, N. K. Whiteman, and H. B. Ernest. 2008. On the origin of the Galápagos Hawk: an examination of phenotypic differentiation and mitochondrial paraphyly. Biological Journal of the Linnean Society 95:779–789.
- Jahn, A. E., D. J. Levey, V. R. Cueto, J. P. Ledezma, D. T. Tuero, J. W. Fox, and D. Masson. 2013. Long‐distance bird migration within South America revealed by light‐level geolocators. Auk 130:223–229.
- Jansen, A. 1999. Home ranges and group‐territoriality in Chowchillas Orthonyx spaldingii. Emu 99:280–290.
- Jehl, J. R., Jr. 1997. Fat loads and flightlessness in Wilson's Phalaropes. Condor 99:538–543.
- Keast, A. 1968. Seasonal movement in the Australian honeyeaters (Meliphagidae) and their ecological significance. Emu 67:159–209.
- Kentie, R., C. Both, J. C. E. W. Hooijmeijer, and T. Piersma. 2014. Age‐dependent dispersal and habitat choice in black‐tailed godwits Limosa limosa limosa across a mosaic of traditional and modern grassland habitats. Journal of Avian Biology 45: 396–405.
- Klaassen, R. H. G., T. Alerstam, P. Carlsson, J. W. Fox, and Å. Lindström. 2011a. Great flights by great snipes: long and fast non‐stop migration over benign habitats. Biology Letters 7:833–835.
- Klaassen, R. H. G., B. J. Ens, J. Shamoun‐Baranes, K. M. Exo, and F. Bairlein. 2011b. Migration strategy of a flight generalist, the Lesser Black‐backed Gull Larus fuscus. Behavioral Ecology 23:58–68.
- Klaassen, R. H. G., M. Hake, R. Strandberg, B. J. Koks, C. Trierweiler, K. M. Exo, F. Bairlein, and T. Alerstam. 2014. When and where does mortality occur in migratory birds? Direct evidence from long‐term satellite tracking of raptors. Journal of Animal Ecology 83:176–184.
- Kölzsch, A. and B. Blasius. 2008. Theoretical approaches to bird migration: the white stork as a case study. European Physical Journal: Special Topics 157:191–208.
- Koronkiewicz, T. J., M. K. Sogge, C. Van Riper III, and E. H. Paxton. 2006. Territoriality, site fidelity, and survivorship of Willow Flycatchers wintering in Costa Rica. Condor 108:558–570.
- La Sorte, F. A., D. Fink, W. M. Hochachka, A. Farnsworth, A. D. Rodewald, K. V. Rosenberg, B. L. Sullivan, D. W. Winkler, C. Wood, and S. Kelling. 2014. The role of atmospheric conditions in the seasonal dynamics of North American migration flyways. Journal of Biogeography 41:1685–1696.
- Lank, D. B., R. W. Butler, J. Ireland, and R. C. Ydenberg. 2003. Effects of predation danger on migration strategies of sandpipers. Oikos 103:303–319.
- Laughlin, A. J., C. M. Taylor, D. W. Bradley, D. Leclair, R. C. Clark, R. D. Dawson, P. O. Dunn, A. Horn, M. Leonard, D. R. Sheldon, D. Shutler, L. A. Whittingham, D. W. Winkler, and D. R. Norris. 2013. Integrating information from geolocators, weather radar, and citizen science to uncover a key stopover area of an aerial insectivore. Auk 130:230–239.
- Leyrer, J., T. Lok, M. Brugge, A. Dekinga, B. Spaans, J. A. van Gils, B. K. Sandercock, and T. Piersma. 2012. Small‐scale demographic structure suggests preemptive behavior in a flocking shorebird. Behavioral Ecology 23:1226–1233.
- Leyrer, J., T. Lok, M. Brugge, B. Spaans, B. K. Sandercock, and T. Piersma. 2013. Mortality within the annual cycle: seasonal survival patterns in Afro‐Siberian red knots. Journal of Ornithology 154:933–943.
- Liechti, F., W. Witvliet, R. Weber, and E. Bächler. 2013. First evidence of a 200‐day non‐stop flight in a bird. Nature Communications 4:2554.
- Lindström, Å. 1989. Finch flock size and risk of hawk predation at a migratory stopover site. Auk 106:225–232.
- Lok, T., O. Overdijk, J. M. Tinbergen, and T. Piersma. 2011. The paradox of spoonbill migration: most birds travel to where survival rates are lowest. Animal Behaviour 82:837–844.
- Maddock, M. and D. Geering. 1994. Range expansion and migration of the Cattle Egret. Ostrich 65:191–203.
- Marcotullio, P. J. and F. B. Gill. 1985. Use of time and space by Chestnut‐Backed Antbirds. Condor 87:187–191.
- Martínez, M. M., 1983. Nidificación de Hirundo rustica erythrogaster (Boddaert) en la Argentina. (Aves, Hirundinidae). Neotrópica 29:83–86.
- McKnight, A., D. B. Irons, A. J. Allyn, K. M. Sullivan, and R. M. Suryan. 2011. Winter dispersal and activity patterns of post‐breeding black‐legged kittiwakes Rissa tridactyla from Prince William Sound, Alaska. Marine Ecology Progress Series 442:241–253.
- Mellone, U., P. López‐López, R. Limiñana, and V. Urios. 2011. Weather conditions promote route flexibility during open ocean crossing in a long‐distance migratory raptor. International Journal of Biometeorology 55:463–468.
- Merom, K., Y. Yom‐Tov, and R. McClery. 2000. Philopatry to stopover site and body condition of transient Reed Warblers during autumn migration through Israel. Condor 102:441–444.
- Mewaldt, L. R. 1964. California Sparrows return from displacement to Maryland. Science 146:941–942.
- Meyburg, B. U., C. Meyburg, T. Bĕlka, O. Šreibr, and J. Vrana. 2004. Migration, wintering and breeding of a lesser spotted eagle (Aquila pomarina) from Slovakia tracked by satellite. Journal of Ornithology 145:1–7.
- Muheim, R., F. R. Moore, and J. B. Phillips. 2006a. Calibration of magnetic and celestial compass cues in migratory birds—a review of cue‐conflict experiments. Journal of Experimental Biology 209:2–17.
- Muheim, R., J. B. Phillips, and S. Åkesson. 2006b. Polarized light cues underlie compass calibration in migratory songbirds. Science 313:837–839.
- Niles, L. J., J. Bart, H. P. Sitters, A. D. Dey, K. E. Clark, P. W. Atkinson, A. J. Baker, K. A. Bennett, K. S. Kalasz, N. A. Clark, J. Clark, S. Gillings, A. S. Gates, P. M. González, D. E. Hernandez, C. D. T. Minton, R. I. G. Morrison, R. R. Porter, R. K. Ross, and C. R. Veitch. 2009. Effects of horseshoe crab harvest in Delaware Bay on red knots: are harvest restrictions working? BioScience 59:153–164.
- Parejo, D., T. Pérez‐Contreras, C. Navarro, J. J. Soler, and J. M. Avilés. 2008. Spotless starlings rely on public information while visiting conspecific nests: an experiment. Animal Behaviour 75:483–488.
- Pärt, T. and B. Doligez. 2003. Gathering public information for habitat selection: prospecting birds cue on parental activity. Proceedings of the Royal Society B: Biological Sciences 270:1809–1813.
- Phillips, R. A., J. P. Croxall, J. R. D. Silk, and D. R. Briggs. 2008. Foraging ecology of albatrosses and petrels from South Georgia: two decades of insights from tracking technologies. Aquatic Conservation: Marine and Freshwater Ecosystems 17:S6–S21.
- Piersma, T. and R. E. GillJr. 1998. Guts don't fly: small digestive organs in obese Bar‐tailed Godwits. Auk 115:196–203.
- Piersma, T., R. Hoekstra, A. Dekinga, A. Koolhaas, P. Wolf, P. F. Battley, and P. Wiersma. 1993. Scale and intensity of intertidal habitat use by knots Calidris canutus in the Western Wadden Sea in relation to food, friends and foes. Netherlands Journal of Sea Research 31:331–357.
- Piersma, T., J. Pérez‐Tris, H. Mouritsen, U. Bauchinger, and F. Bairlein. 2005. Is there a “migratory syndrome” common to all migrant birds? Annals of the New York Academy of Sciences 1046:282–293.
- Poonswad, P. and A. Tsuji. 2008. Ranges of males of the Great Hornbill Buceros bicornis, Brown Hornbill Ptilolaemus tickelli and Wreathed Hornbill Rhyticeros undulatus in Khao Yai National Park, Thailand. Ibis 136:79–86.
- Powell, G. V. N. and R. D. Bjork. 1994. Implications of altitudinal migration for conservation strategies to protect tropical biodiversity: a case study of the Resplendent Quetzal Pharomacrus mocinno at Monteverde, Costa Rica. Bird Conservation International 4:161–174.
- Prats, M. T., L. Palacios, S. Gallego, and M. Riera. 1996. Blood oxygen transport properties during migration to higher altitude of wild quail, Coturnix coturnix coturnix. Physiological Zoology 69:912–929.
- Pulido, F. and P. Berthold. 2010. Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proceedings of the National Academy of Sciences of the United States of America 107:7341–7346.
- Pulido, F., P. Berthold, G. Mohr, and U. Querner. 2001. Heritability of the timing of autumn migration in a natural bird population. Proceedings of the Royal Society B: Biological Sciences 268:953–959.
- Rakhimberdiev, E., J. Karagicheva, K. Jaatinen, D.W. Winkler, J.B. Phillips, and T. Piersma. 2014. Naïve migrants and the use of magnetic cues: temporal fluctuations in the geomagnetic field differentially affect male and female Ruff Philomachus pugnax during their first migration. Ibis 156:864–869.
- Rakhimberdiev, E., P. J. van den Hout, M. Brugge, B. Spaans, and T. Piersma. 2015. Seasonal mortality and sequential density dependence in a migratory bird. Journal of Avian Biology 46:332–341.
- Redmond, L. J., M. T. Murphy, A. C. Dolan, and K. Sexton. 2009. Public information facilitates habitat selection of a territorial species: the eastern kingbird. Animal Behaviour 77:457–463.
- Reichlin, T. S., K. A. Hobson, L. I. Wassenaar, M. Schaub, D. Tolkmitt, D. Becker, L. Jenni, and R. Arlettaz. 2010. Migratory connectivity in a declining bird species: using feather isotopes to inform demographic modeling. Diversity and Distributions 16:643–654.
- Reudink, M. W., C. J. Kyle, J. J. Nocera, R. A. Oomen, M. C. Green, and C. M. Somers. 2011. Panmixia on a continental scale in a widely distributed colonial waterbird. Biological Journal of the Linnean Society 102:583–592.
- Rivera, J. H. V., D. Ayala, and C. A. Haas. 2003. Home‐range size, habitat use, and reproduction of the Ivory‐billed Woodcreeper (Xiphorhynchus flavigaster) in dry forest of western Mexico. Journal of Field Ornithology 74:141–151.
- Rolland, J., F. Jiguet, K. A. Jønsson, F. L. Condamine, and H. Morlon. 2014. Settling down of seasonal migrants promotes bird diversification. Proceedings of the Royal Society B: Biological Sciences 281:20140473.
- Roshier, D., M. Asmus, and M. Klaassen. 2008. What drives long‐distance movements in the nomadic Grey Teal Anas gracilis in Australia? Ibis 15:474–484.
- Ruegg, K. 2008. Genetic, morphological, and ecological characterization of a hybrid zone that spans a migratory divide. Evolution 62:452–466.
- Ruegg, K., E. C. Anderson, J. Boone, J. Pouls, and T. B. Smith. 2014. A role for migration‐linked genes and genomic islands in divergence of a songbird. Molecular Ecology 23:4757–4769.
- Ruegg, K., E. C. Anderson, and H. Slabbekoorn. 2012. Differences in timing of migration and response to sexual signalling drive asymmetric hybridization across a migratory divide. Journal of Evolutionary Biology 25:1741–1750.
- Ruelas Inzunza, E., L. J. Goodrich, and S. W. Hoffman. 2010. North American population estimates of waterbirds, vultures, and hawks from migration counts in Veracruz, México. Bird Conservation International 20:124–133.
- Saino, N., D. Rubolini, L. Serra, M. Caprioli, M. Morganti, R. Ambrosini, and F. Spina. 2010. Sex‐related variation in migration phenology in relation to sexual dimorphism: a test of competing hypotheses for the evolution of protandry. Journal of Evolutionary Biology 23:2054–2065.
- Sauer, J. R., J. E. Hines, and J. Fallon. 2008. The North American breeding bird survey, results and analysis 1966–2007. Version 5.15.2008. USGS Patuxent Wildlife Research Center, Laurel, MD.
- Schlichte, H. J. and K. Schmidt‐Koenig. 1971. Zum Heimfindevermögen der Brieftaube bei erschwerter optischer Wahrnehmung. Naturwissenschaften 58:329–330.
- Senner, N. R., W. M. Hochachka, J. W. Fox, and V. Afanasyev. 2014. An exception to the rule: carry‐over effects do not accumulate in a long‐distance migratory bird. PLoS One 9:e86588.
- Shamoun‐Baranes, J., E. van Loon, D. Alon, P. Alpert, Y. Yom‐Tov, and Y. Leshem. 2006. Is there a connection between weather at departure sites, onset of migration and timing of soaring‐bird autumn migration in Israel? Global Ecology and Biogeography 15:541–552.
- Sillett, T. S. and R. T. Holmes. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. Journal of Animal Ecology 71:296–308.
- Snow, D. W. 1953. The migration of the Greenland Wheatear. Ibis 95:376–378.
- Stewart, R. L. M., C. M. Francis, and C. Massey. 2002. Age‐related differential timing of spring migration within sexes in passerines. Wilson Bulletin 114:264–271.
- Terrill, S. B. and R. D. Ohmart. 1984. Facultative extension of fall migration by Yellow‐Rumped Warblers (Dendroica coronata). Auk 101:427–438.
- Therrien, J. F., G. Gauthier, D. Pinaud, and J. Bêty. 2014. Irruptive movements and breeding dispersal of snowy owls: a specialized predator exploiting a pulsed resource. Journal of Avian Biology 45:536–544.
- Thorup, K., I. A. Bisson, M. S. Bowlin, R. A. Holland, J. C. Wingfield, M. Ramenofsky, and M. Wikelski. 2007. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proceedings of the National Academy of Sciences of the United States of America 104:18115–18119.
- Tidemann, S. C. 1990. Factors affecting territory establishment, size and use by three co‐existing species of Fairy‐wrens (Malurus). Emu 90:7–14.
- Tøttrup, A. P., R. H. G. Klaassen, R. Strandberg, K. Thorup, M. W. Kristensen, P. S. Jorgensen, J. Fox, V. Afanasyev, C. Rahbek, and T. Alerstam. 2012. The annual cycle of a trans‐equatorial Eurasian–African passerine migrant: different spatio‐temporal strategies for autumn and spring migration. Proceedings of the Royal Society B: Biological Sciences 279:1008–1016.
- Van den Bossche, W., P. Berthold, M. Kaatz, E. Nowak, and U. Querner. 2002. Eastern European white stork populations: migration studies and elaboration of conservation measures. BfN—Skripten (Bundesamt fur Naturschutz) 66.
- Walcott, C. and R. P. Green. 1974. Orientation of homing pigeons altered by a change in direction of an applied magnetic field. Science 184:180–182.
- Webster, M. S., P. P. Marra, S. M. Haig, S. Bensch and R. T. Holmes. 2002. Links between worlds: unraveling migratory connectivity. Trends in Ecology and Evolution 17:76–83.
- Wiedenfeld, D. J. and M. G. Wiedenfeld. 1993. Large kill of neotropical migrants by tornado and storm in Louisiana, April 1993. Journal of Field Ornithology 66:70–80.
- Wikelski, M., E. M. Tarlow, A. Raim, R. H. Diehl, R. P. Larkin, and G. H. Visser. 2003. Avian metabolism: costs of migration in free‐flying songbirds. Nature 423:704.
- Willemoes, M., R. Strandberg, R. H. G. Klaassen, A. P. Tøttrup, Y. Vardanis, P. W. Howey, K. Thorup, M. Wikelski, and T. Alerstam. 2014. Narrow‐front loop migration in a population of the cuckoo Cuculus canorus, as revealed by satellite telemetry. PloS One 9:e83515.
- Wiltschko, W., R. P. Balda, M. Jahnel, and R. Wiltschko. 1999. Sun compass orientation in seed‐caching corvids: its role in spatial memory. Animal Cognition 2:215–221.
- Wiltschko, R. and W. Wiltschko. 2009. Avian navigation. Auk 126:717–743.
- Winger, B. M., F. K. Barker, and R. H. Ree. 2014. Temperate origins of long‐distance seasonal migration in New World songbirds. Proceedings of the National Academy of Sciences of the United States of America 111:12115–12120.
- Winger, B. M., I. J. Lovette, and D. W. Winkler. 2012. Ancestry and evolution of seasonal migration in the Parulidae. Proceedings of the Royal Society B: Biological Sciences 279:610–618.
- Winkler, D. W., P. H. Wrege, P. E. Allen, T. L. Kast, P. Senesac, M. F. Wasson, P. E. Llambías, V. Ferretti, and P. J. Sullivan. 2004. Breeding dispersal and philopatry in the Tree Swallow. Condor 106:768–776.
- Winkler, D. W., P. H. Wrege, P. E. Allen, T. L. Kast, P. Senesac, M. F. Wasson and P. J. Sullivan. 2005. The natal dispersal of tree swallows in a continuous mainland environment. Journal of Animal Ecology 74:1080–1090.
- Wunderle, J. M., Jr. and S. C. Latta. 2000. Winter site fidelity of Nearctic migrants in shade coffee plantations of different sizes in the Dominican Republic. Auk 117:596–614.