Chapter 15
Bird Conservation
John W. Fitzpatrick and Amanda D. Rodewald
Cornell Lab of Ornithology

(Photograph by Gerrit Vyn.)
Until the mid‐1800s, travelers in the springtime woods of eastern North America would marvel as multitudes of large, long‐tailed doves swirled into dark clouds overhead during the spring migration. Millions of beating wings resonated like strong winds through pine boughs while the immense horde streamed northward. The passage of such a flock might continue all day, with straggler flocks following thereafter for weeks.
The Passenger Pigeon (Ectopistes migratorius) was then almost certainly the most abundant bird on earth (Fig. 15.01). Accounts of their numbers written between 1630 and 1880 read like science fiction (Schorger 1955). John James Audubon wrote of a flock passing for three successive days near Louisville, Kentucky (USA) in 1813, stating that “the light of the noonday sun was obscured as by an eclipse.” He estimated the mile‐wide flock to contain a minimum of 1.1 billion birds. Alexander Wilson, often called the father of North American ornithology, considered his own 1806 estimate of a single flock—2,230,272,000 birds—to be well below its actual number.

Fig. 15.01 Passenger Pigeons (Ectopistes migratorius). At the time of the European settlement of North America, this species was considered to be the most abundant bird on earth. Audubon reported a flock estimated to contain over 1 billion individuals. Flocks once migrated across eastern North America, settling to breed in huge colonies where they found bounties of tree‐nuts (such as acorns, chestnuts, and beechnuts).
(Illustration by N. John Schmitt.)
How many Passenger Pigeons existed across the entire range of the species? Nobody knows for sure, but the number may have been 5 billion or more. For perspective, only one wild bird species in the world today, the Red‐billed Quelea (Quelea quelea) of sub‐Saharan Africa, likely reaches 1 billion individuals. Yet by 1914 the Passenger Pigeon was extinct, just 100 years after Audubon and his contemporaries wondered at its incredible abundance. Accounts of Passenger Pigeons provide deeply moving glimpses of a world that was very different from today, yet existed only a few generations ago. This contrast reminds us of the astonishing changes caused by humans across the world’s landscapes and across bird populations.
No doubt exists about why Passenger Pigeons disappeared. As for so many other species, this gregarious dove was extinguished by the deadly one‐two‐three punch that already had become a signature of human expansion across the world: (1) uncontrolled exploitation (in this case, hunting for food); (2) advances in technology that aided exploitation as numbers decreased (firearms and railroads); and (3) large‐scale alteration of the landscape for agriculture and human settlement (clearing of mature, seed‐producing oak and beech forests).
Many contemporary accounts of Passenger Pigeon nesting colonies emphasized the frenzied slaughter of the birds by hunters more than the natural wonder of the birds themselves. Even at the last large colony documented—in 1878 near Petoskey, Michigan (USA)—300 tons of Passenger Pigeons killed by professional market hunters were shipped by railroad to restaurants in Chicago, New York, and Boston. Because the species nested in dense colonies and nestlings were easy and delicious prey, a colony’s entire reproductive output could be wiped out during a single season. Last‐ditch efforts to avert the Passenger Pigeon’s extinction in the 1890s through publicity and legal protection came too late: Martha, the world’s last individual Passenger Pigeon, died at the Cincinnati Zoo (USA) on September 1, 1914.
Preserved as a specimen at the National Museum of Natural History in Washington, DC, Martha invites reflection on humans’ relationship with the earth. She died just as the global conservation movement was being born. This new movement would go on to establish the revolutionary laws, organizations, and actions that serve to protect birds and other emblems of wildness today.
15.1 History of bird conservation
The roots of bird conservation lie in the many past tragedies of overexploitation that are exemplified by the Passenger Pigeon. The earliest bird protection laws were efforts to curb overuse of hunted species. Laws establishing hunting seasons and regulations for particular groups of birds, such as upland game birds, were passed in the early 1700s in North America. Likewise, in both Britain and Newfoundland (now Canada), some of the first laws protecting birds were passed in 1775 to stem the exploitation of the Great Auk (Pinguinus impennis). In response to an already staggering level of market hunting in eastern North America, the state of New York passed legislation in 1791 to restrict hunting seasons for Heath Hen (Tympanuchus cupido cupido), and Massachusetts (USA) passed an Act to Protect Useful Birds in 1818. As early as the mid‐1800s Michigan passed several laws attempting to limit the hunting of Passenger Pigeons, although they were weakly enforced.
New Zealand enacted its first law to protect native birds with the Wild Birds Protection Act of 1864 (earlier misguided regulations had instead protected introduced bird species). Shortly thereafter, the UK passed the Sea Birds Preservation Act of 1869, which restricted shooting and egg collecting during the breeding season. This act was followed by a series of Wild Birds Protection Acts in the UK between 1880 and 1896 that prohibited harvesting outside of certain seasons and taking the eggs of wild birds. These initial legislative efforts proved insufficient to curtail the loss of certain species but did fuel a more organized and widespread conservation movement.
Commercial plume hunting in the late 1800s was a turning point for early bird conservation. At this time, feathers (and sometimes entire birds) were considered fashionable adornments on women's hats (Fig. 15.02). Gaudiest were the plumes of several herons, such as the Great Egret (Ardea alba) and Snowy Egret (Egretta thula), which bred in large rookeries. By the 1890s, the scale and biological effects of commercial plume hunting rivaled the wholesale slaughter of Passenger Pigeons two decades earlier. In North America, concern over plume hunting and the decline of birds led, in part, to the founding of the American Ornithologists’ Union (AOU) in 1883, along with its influential Bird Protection Committee. In 1886 a charter committee member, George Bird Grinnell, established the Audubon Society dedicated to the protection of birds. In 1889 in the UK, the Royal Society for the Protection of Birds (RSPB) was similarly created in response to the feather trade; today the RSPB is one of the world’s largest conservation organizations.

Fig. 15.02 Feathers as fashion accessories. The widespread use of feathers—and sometimes entire birds—as fashion accessories (A) led to the near extinction of some populations of egrets and other charismatic birds, thereby sparking some of the earliest bird conservation movements such as these early supporters of the Royal Society for the Protection of Birds in the United Kingdom (B).
(Photographs courtesy of the Royal Society for the Protection of Birds.)
Despite laws regulating the commercial harvest of certain birds, widespread hunting and trading continued largely unchecked around the world until the early 1900s, when activists began focusing on trade rather than harvest. Catapulted by the social activism of local Audubon societies, the US government passed the landmark Lacey Act, which banned interstate shipments of birds killed in violation of any state or local law. Revisions to this act ultimately gave rise to the Migratory Bird Treaty Act in 1918, which implemented elements of the International Migratory Bird Convention Act signed in 1916 by Great Britain, Canada, and the USA. Later, other international treaties were incorporated into the act, including the Migratory Bird and Game Mammal Treaty with Mexico (1936), and the Convention on Nature Protection and Wildlife Preservation in the Western Hemisphere (1940). International treaties that protect birds continued to be ratified around the world throughout the twentieth century; among the most important were the African Convention on the Conservation of Nature and Natural Resources (1968) and the Agreement on the Conservation of African‐Eurasian Migratory Waterbirds (1995).
Expanding the targets of conservation beyond the regulation of hunting and trade, major efforts to conserve birds by protecting their habitats came in the late 1800s and early 1900s. Preserves were created in many countries around the globe; in the late 1800s, for example, several New Zealand islands were named as sanctuaries for native birds such as the Kakapo (Strigops habroptila) and Kiwi (Apteryx species).
This period also saw the birth of broader, organized systems of protected areas. Partly in response to the plume trade, Theodore Roosevelt designated the first federal refuge in 1903 at Pelican Island, Florida, thereby launching the National Wildlife Refuge System within the USA. Not long afterward, Mexico’s first wildlife refuge was established at Isla Guadalupe. The National Park concept remains a foundation of habitat conservation around the globe. In the USA, after establishment of the first National Park in Yellowstone (1872), a trove of other areas received similar protection through the newly created system of National Forests, National Parks, and National Monuments. Australia followed (1879), then Canada (1885), and New Zealand (1887). Today nearly 100 countries boast internationally recognized national parks. Among the most recent is Afghanistan, which created its first national park, Band‐e‐Amir, in 2009.
Habitat protection remains among the most important elements in bird conservation and involves every level of society. In addition to government‐led efforts, a variety of non‐governmental organizations promote protection and management of habitats for birds. An increasing number of places around the world are officially recognized for their international importance to birds. The Ramsar List of Wetlands of International Importance and the UNESCO World Heritage List are two examples of multinational conventions that have identified places of global importance for conservation. BirdLife International now recognizes more than 11,000 Important Bird Areas (IBAs) around the globe, although legal recognition and protection of these areas vary widely among countries.
Bird conservation in the twentieth century was shaped profoundly by the public’s increasing awareness of environmental problems and growing sense of collective responsibility for environmental protection. In an influential collection of essays titled A Sand County Almanac, Aldo Leopold (1949) established both a philosophical and a practical framework for treating the natural landscape as a long‐term resource to be used carefully and held in safekeeping for future generations. Leopold introduced the concept of the “land ethic,” whereby natural resources are seen to warrant the level of ethical consideration typically given to humans and human culture. A few decades later, the book Silent Spring by Rachel Carson (1962) exposed the devastating environmental effects of pesticides, which later were confirmed to be the cause of drastic declines in Peregrine Falcons (Falco peregrinus) and other raptors (Chapter 13).
In the 1970s, growing environmental concerns spawned the landmark legislation and international treaties that today provide strong legal mechanisms for bird protection. In 1973, the USA enacted its most far‐reaching piece of environmental legislation, the Endangered Species Act (ESA). For the first time, individual species were legally recognized as possessing intrinsic value and rights to protection under law, regardless of how trivial or irrelevant any species might seem to human society. In 1972 the United Nations Conference on the Human Environment helped frame environmental policies and global treaties that eventually led to the Kyoto Protocol for greenhouse gas emissions. Conservation legislation in the European Union also continues to be shaped by milestone legislation that occurred in the 1970s, when the Birds Directive (1979) established a framework for conserving all birds throughout the European Union, banned activities that directly threaten birds, required that hunting be managed sustainably, eliminated non‐selective and large‐scale killing, and closely regulated the trade of live and dead birds.
Today, the most important global treaty for bird protection is the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), drafted in 1973 by the World Conservation Union (now the International Union for the Conservation of Nature and Natural Resources, IUCN). This international agreement, to which 175 countries now voluntarily subscribe, binds participating parties to monitor, regulate, or prohibit the international import and export of species deemed in need of global protection. The legal framework for enforcement within each participating country is provided by domestic laws. The heart of CITES lies in three appendices constituting the list of species for which international trade is restricted. Appendix I includes the most endangered species (including for example all macaws, many other parrots, and a few falcons), for which all commercial trade is strictly prohibited and special import/export permits are required for scientific transport. Appendix II lists species that are not in immediate danger of extinction but that could become so in the absence of regulation (for example, all parrots, hawks, and falcons not in Appendix I). Species may be added to or removed from Appendices I and II, or moved between them, only via discussion and vote at periodic CITES conferences. Appendix III lists species added by individual countries that, for any reason, request international assistance in regulating their trade. Without doubt, the international protection afforded by being listed as a CITES species has prevented many birds species from going extinct: until the loss of the last wild Spix’s Macaw (Cyanopsitta spixii) in 2000 (Fig. 15.03), not a single bird species regulated by CITES had become extinct in the wild as a result of international trade.

Fig. 15.03 CITES regulations help preserve wild birds. One of the main benefits of CITES regulations is to ban the import and export of protected bird species. The Spix’s Macaw (Cyanopsitta spixii) is one of the few CITES‐protected bird species that has been extirpated in the wild owing to the captive bird trade. Hope remains that regulated captive breeding of this species will allow its future reintroduction into its former range.
(Photograph by Alain Breyer.)
15.2 Conservation biology
As the disciplines of ecology, population biology, evolutionary biology, animal behavior, and genetics matured during the twentieth century, scientists also began to study the human‐generated threats and biological attributes that were causing many species around the world to decline and go extinct. Gradually, these disciplines coalesced into efforts to derive solutions to conservation problems, giving rise to the applied science now known as conservation biology. Today the field of conservation biology continues to grow in its number of practicing professionals, in the breadth and scope of its research, and in the global importance of its contributions to conservation issues.
Applied sciences like conservation biology generally combine basic principles of several fields to solve everyday problems and to accomplish specific, real‐world tasks. Just as engineers use mathematics, physics, and chemistry to design bridges or fuel‐efficient automobiles, conservation biologists employ basic biological disciplines in the effort to stabilize ecosystems and restore wild populations. Conservation biologists often face grim realities of impending disaster, and they work within ecological systems that differ vastly from one another and contain hundreds or even thousands of species that are engaged in myriad physical and biological interactions. The complexity of interactions among natural populations and the relative youth of the sciences engaged to understand them help explain why so many bird species went extinct between the sixteenth and twenty‐first centuries, before humans could fully recognize the underlying problems and work to ameliorate the threats.
Successful bird conservation involves five integrated steps: (1) understanding the native functions and fluctuations characterizing bird populations, species, and ecological communities; (2) determining whether and how these natural conditions have been, or could be, perturbed by human impacts; (3) projecting how the desired conditions could be restored; (4) taking action for protection and management; and (5) measuring the consequences of alternative management scenarios. Understanding the decline of a bird population requires knowing basic facts about the species’ evolutionary history, its degree of habitat specialization, and what regulates its populations. At the most basic level, all population declines fundamentally reflect changes in birth rates, death rates, and the area of available habitat, or combinations of these three parameters. The task of conservation biology is to pinpoint what has changed for the worse and to devise strategies for eliminating or mitigating those changes.
15.3 Recent avian extinctions
Bird species have been evolving and going extinct throughout their existence (Chapter 2). These past origins and disappearances have been driven by naturally occurring, long‐term processes across the earth’s surface. Continents split apart and came together. Volcanic islands arose from the sea and then eroded away. Earth’s climate fluctuated between hot and cold, wet and dry. Such slow but enormous events made the extinction of species a natural feature of evolutionary change, as local environments shifted and species of different regions encountered and competed with one another. In fact, it is estimated that 99% of all bird species that have ever lived on earth are now extinct. So, why should we be concerned about bird species becoming extinct today?
The answer is simple: the wave of extinction currently underway is occurring at a pace and scale fully comparable to the five greatest extinction episodes in the earth’s history, but this sixth mass extinction is directly caused by human activities across the globe. The physical and biological changes humans have caused on earth are so profound and widespread that a new expression—the Anthropocene—has been coined to describe this epoch in geological terms (Ellis 2011; Steffen et al. 2011). The worldwide spread of modern Homo sapiens has resulted in the extinction of about 8000 species of landbirds, according to recent estimates based on fossil deposits (Martin and Steadman 1999). Most biologists agree that human‐caused extinctions now rival those associated with the events that extinguished the dinosaurs about 65 million years ago (Chapter 2). Such enormous ecological impact is outside the bounds of natural processes and therefore should be a matter of intense concern for all people who care about the natural environment.
15.3.1 Avian extinctions in the Anthropocene
On an evolutionary timescale, modern humans have spread across the globe only very recently from our origins in Africa, but we have had enormous impacts on birds everywhere we have colonized. A great wave of extinctions in Australia, for example, coincided with the arrival of humans on that continent about 50,000 years ago, apparently resulting in part from vastly increased fire frequency (Miller et al. 2005). Similar extinction patterns occurred near the time of human colonization on every land mass on earth except Africa. We cleared forests, replaced grasslands with crops, built towns, drained marshes, filled swamplands, irrigated dry areas, and suppressed natural fire in some places and increased its frequency in others. Everywhere we went, we hunted birds for food, clothing, and ornamentation. In each newly colonized area we encountered birds that had evolved no defenses against our evermore advanced hunting techniques and our steadily increasing numbers. On remote islands filled with uniquely adapted species, we introduced new avian diseases and new predators. On the continents we did the reverse, systematically destroying the largest predators—wolves, bears, and big cats—and thereby releasing cascades of herbivores and middle‐sized predators that altered ecosystems in numerous ways (Prugh et al. 2009). Everywhere, we introduced domesticated herbivores into habitats that had evolved without them. By steadily making the world more accommodating for ourselves, we profoundly altered every natural system we encountered. The effects on birds have been devastating.
Humans began documenting the world’s birds in modern scientific terms—with written records, paintings, and preserved specimens—around AD 1500. By that time, tropical islands the world over already had been ravaged, resulting in the loss of many hundreds of bird species now known only from a few bones or descriptions by early explorers. Many birds on predator‐free islands had lost the need for flight, resulting in the evolutionary reduction of their wings. Included among the pre AD 1500 extinctions are some of the most unusual and spectacular birds ever to have evolved, including the 400‐kilogram Elephant Bird (Aepyornis maximus) on Madagascar (the largest modern bird known) (Fig. 15.04), at least 10 species of moas in New Zealand (Chapter 2), flightless ibises on Hawaii, flightless owls on Caribbean islands, flightless pigeons on Indian Ocean islands, and probably thousands of other island‐dwelling bird species, including numerous flightless rails, worldwide.
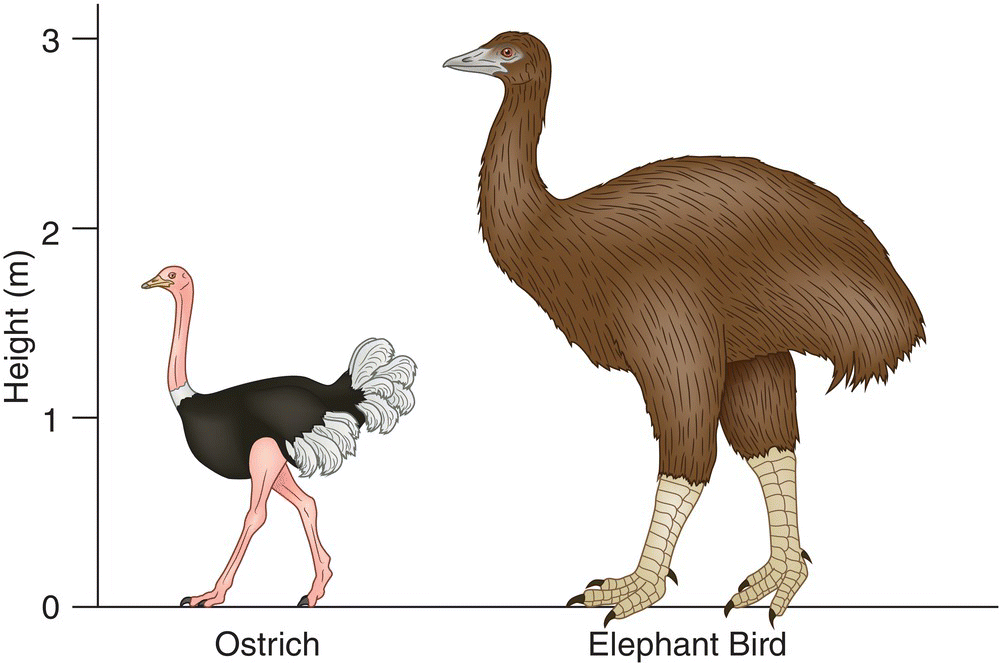
Fig. 15.04 The extinct Elephant Bird (Aepyornis maximus) of Madagascar. This was the largest known bird in history, and it briefly coexisted with humans: Elephant Bird remains have been found with traces of human butchery and many eggshells have been found in archaeological fire pits. Single eggs could feed entire human families, and this may be one reason this species went extinct around 1000 years ago—eggs would have been the most vulnerable stage of an Elephant Bird’s life. An Elephant Bird (right) is shown next to an Ostrich (Struthio camelus) (left) for scale.
(© Cornell Lab of Ornithology.)
Humans began populating North and South America less than 20,000 years ago and reached the Caribbean Islands around 6500 years ago. These earliest Native Americans spread rapidly across the hemisphere, introducing wholly new ecological forces that coincided with a spectacularly swift and catastrophic disappearance of animals. Most dramatic was the nearly complete extinction of the New World megafauna, a varied assemblage of large mammals that included horses, camels, giant sloths, mammoths and mastodons, glyptodonts, gomphotheres, saber‐toothed cats, and many others. Why such a large and diverse animal fauna disappeared so suddenly between 12,000 and 8000 years ago remains hotly debated. The tight coincidence between human arrival and the disappearance of the megafauna strongly suggests that humans were a primary cause, perhaps setting in motion a cascade of extinctions compounded by rapid habitat changes, disappearance of keystone species, direct predation by humans, and human‐borne diseases to which the native fauna had no immunity.
Extinction of the western hemisphere megafauna included dozens of species of birds as well as mammals. The most spectacular of these birds included many species of scavengers that lived on the carcasses of large mammals, as African vultures do today. These extinct vultures, condors, and teratorns (giant, soaring scavengers apparently only distantly related to vultures) included some of the largest flying birds ever to have lived. They once occurred throughout the western hemisphere and dwarfed today’s raptors (Fig. 15.05).
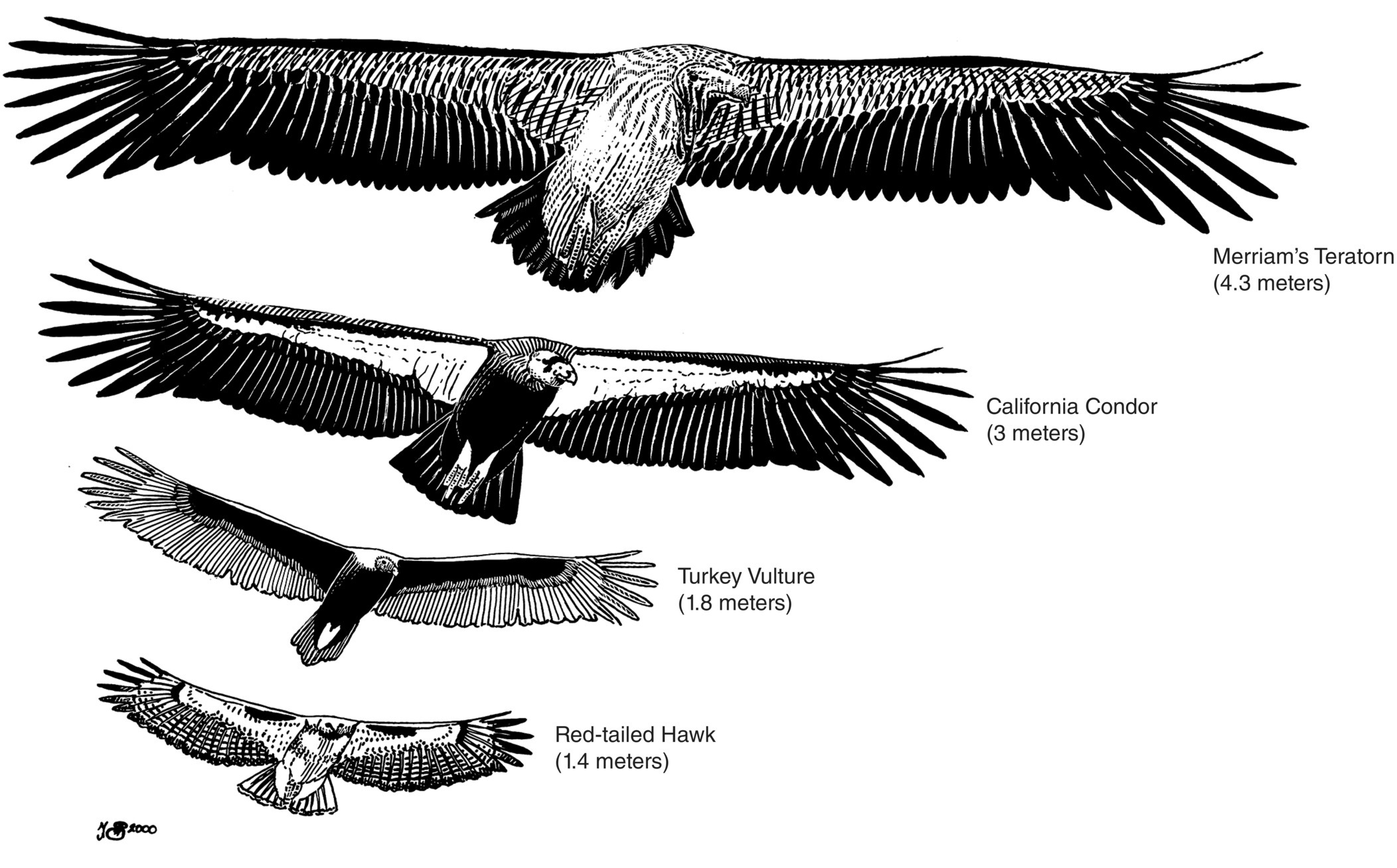
Fig. 15.05 Wingspan of Merriam’s Teratorn (Teratornis merriami) compared with living raptors. Similar to modern vultures, the teratorn group (now extinct) scavenged carcasses in open landscapes. However, when the prehistoric megafauna that composed the teratorn diet went extinct around 10,000 years ago, teratorns soon followed. Here, the wingspan of Merriam’s Teratorn is compared with the wingspans of some modern raptors and vultures (three bottom species).
(Illustration by N. John Schmitt.)
Until a few thousand years ago the Caribbean Islands also harbored remarkably diverse assemblages of now‐vanished landbirds, including crows, large flightless owls, numerous macaws, giant parakeets, and nightjars. Bones of these species are found at human archaeological sites dating back only 3000–4000 years (Pregill et al. 1994). Just as they did later across the islands of the Pacific, humans caused a steady disappearance of birds in these fragile tropical islands, both by cutting down the forests and by killing and eating the birds directly. Today, only a small fraction of the original bird fauna persists in the Caribbean, and many of these—including parrots, doves, nightjars, woodpeckers, thrashers, and orioles—are among the most seriously threatened in the world.
15.3.2 Historical extinctions on oceanic islands
As of 2014, the IUCN Red List for birds tallied 130 bird species known to have gone extinct since AD 1500, plus four species classified as “extinct in the wild.” The 198 additional species classified as Critically Endangered include many that have not been sighted in recent decades and which also may be extinct. These modern extinctions demonstrate much about the impacts of humans on the world’s birds. The most obvious pattern is that all but a handful of extinct species were restricted to oceanic islands where the earlier absence of humans and other mammals had left birds especially vulnerable to predation and habitat change.
Flightless birds were especially easy prey for humans and their domesticated cats. For example, on Mauritius Island in the Indian Ocean, sailors drove the naïvely fearless Dodos (Raphus cucullatus) (Fig. 15.06) aboard their ships to serve as food for ensuing weeks at sea. The species was last observed in 1662, and not a single whole specimen has been preserved. A close relative of the Dodo, the Rodrigues Solitaire (Pezophaps solitaria), met a similar fate. On another nearby island lived a large and poorly documented bird once called the Reunion “Solitaire” (Threskiornis solitarius); recently uncovered bones show that this extinct bird was not related to the Dodo but rather was an ibis (Mourer‐Cauviré et al. 1995). In the North Atlantic, sailors feasted on the abundant Great Auk (Pinguinus impennis) (Fig. 15.07A), and fishermen even chopped them up to use as bait; the last Great Auk was killed in 1844. In the Bering Sea, Pallas’s Cormorant (Phalacrocorax perspicillatus) (Fig. 15.07B), the largest member of its family, vanished at about the same time, and for similar reasons as the whaling industry expanded across the North Pacific. In the islands of the South Pacific, numerous species disappeared as introduced mammals spread among the islands. Notable among the dozens of species lost from Pacific islands were several species of New Zealand wren (suborder Acanthisitti), an ancient and enigmatic lineage of tiny insectivorous songbirds that had included three flightless species (Fig. 15.08).
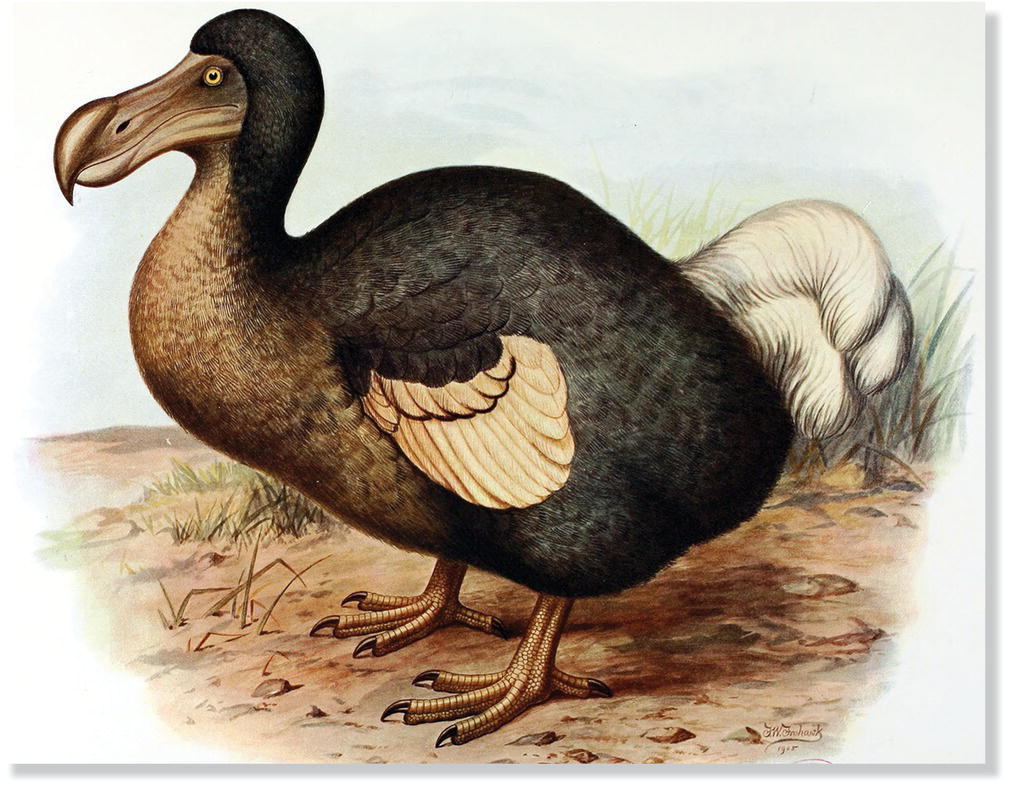
Fig. 15.06 Dodo (Raphus cucullatus). This flightless inhabitant of Mauritius Island was an easy target for hungry sailors and the introduced pigs, cats, and rats they left behind. Dodos, along with now‐extinct native tortoises, may have dispersed and enabled the hard‐shelled seeds of several trees to germinate, as their digestive tracts scoured the hard seed exteriors. Introduced tortoises are now employed to help conserve native tree species in place of these extinct species.
(Artwork by Frederick William Frohawk, from Rothschild 1907, public domain.)
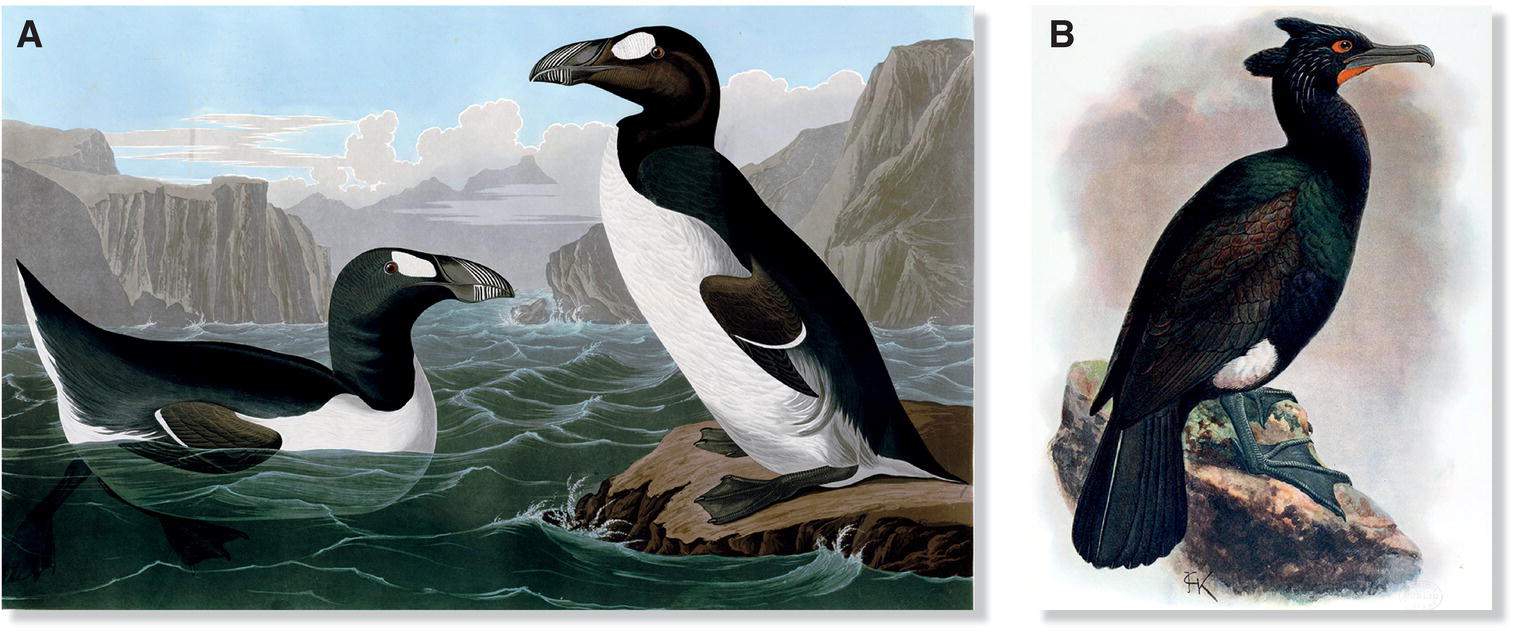
Fig. 15.07 Extinct seabirds. (A) Prehistoric Great Auk (Pinguinus impennis) bones, recovered from middens and caves in much of northwest Europe, suggest that they were heavily hunted long before sailors and fisheries destroyed populations off northeastern North America starting in the 1700s. The Great Auk was extinct by 1844. (B) Pallas’s Cormorant (Phalacrocorax perspicillatus), the largest species of cormorant ever known, met a similar fate. Whalers and fur trappers began overhunting populations off the northeastern Russian coast and Rocky Islands around 1750. The species became extinct about 100 years later.
(Artwork by: A, John James Audubon, from Audubon 1827–38, public domain; B, John Gerrard Keulemans, from Rothschild 1907, public domain.)

Fig. 15.08 Bush Wren (Xenicus longipes). Once widespread across New Zealand, populations crashed by the early twentieth century owing to introduced mammalian predators. Small numbers of a distinct subspecies persisted into the 1960s, but the final attempt to relocate them to a predator‐free island failed in 1972.
(Artwork by John Gerrard Keulemans, from Buller 1888, public domain.)
The full list of modern extinctions is dominated by rails, parrots, pigeons, and Hawaiian honeycreepers (Box 15.01). This highly non‐random taxonomy of avian extinction is largely a consequence of the propensity for certain bird groups to colonize oceanic islands and evolve into unique—and vulnerable—island endemics.
15.3.3 Historical extinctions on continents
Continents largely have been spared the massive wave of recent avian extinctions that have occurred on islands, although this trend may be reversing (Butchart et al. 2006b). Avian extinctions are so rare on continents that it is possible to list them all here.
North America has lost the most bird species among continental land masses, with at least three and probably five or six species disappearing since 1850. The Labrador Duck (Camptorhynchus labradorius) apparently bred on rocky islands off the Gulf of St. Lawrence, where it was exposed to plume hunting, egg robbing, and introduced mammalian predators. The last Labrador Ducks were shot in the 1870s, just as Passenger Pigeons (Ectopistes migratorius) were becoming history’s most spectacular example of overexploitation. The Carolina Parakeet (Conuropsis carolinensis) declined rapidly throughout the 1800s because of forest clearing and hunting (Fig. 15.09). By 1890 only the most remote forests of central Florida harbored a few parakeets; small flocks were seen through 1915, but reliable reports ceased in the early 1920s. Immense flocks of Eskimo Curlews (Numenius borealis) migrated north through the Great Plains in spring and then southeastward in fall across eastern Canada and the Atlantic Ocean to winter in Patagonia. During spring migration curlews depended on prairie insects, especially in areas recovering after wildfires or bison trampling. By the late 1800s such critical habitat patches were virtually eliminated by agricultural conversion, suppression of wildfire, and annihilation of the great bison herds. Some accounts suggest that migrating Eskimo Curlews relied on enormous swarms of Rocky Mountain grasshoppers, which also mysteriously went extinct around 1900 (Lockwood and DeBrey 1990). A few Eskimo Curlews were collected in South America through the early 1920s, and the last documented occurrence was a single bird photographed near Galveston, Texas in 1962.
Hopes are now dim for the continued survival of two specialized denizens of the forests and bottomlands of southeastern North America, the Ivory‐billed Woodpecker (Campephilus principalis) and Bachman’s Warbler (Vermivora bachmanii). Ivory‐billed Woodpeckers lived in the treetops of old‐growth pine and bottomland hardwood forests, foraging on beetle larvae by chipping the bark from large, dying trees. These great forests experienced wildfires, floods, hurricane damage, and destruction by massive wintering colonies of Passenger Pigeons, thereby providing copious dead and dying wood, the critical resource for this powerful woodpecker. By the early 1900s the old‐growth forests had been logged, fires were being suppressed, and remnant Ivory‐billed Woodpeckers were being collected as specimens whenever spotted. No Ivory‐billed Woodpecker nest has been found in North America since the 1930s, and the last photographic record from Cuba was in 1956. Credible reports did persist, however, and an intriguing series of encounters in 2004/2005 (Fitzpatrick et al. 2005) spawned a multi‐year, range‐wide search involving hundreds of participants. Nevertheless, no indisputable evidence exists that the species survives today. Bachman’s Warblers preferred dense forest openings and cane thickets, perhaps favoring habitats regenerating after hurricanes. The species declined as forests were converted to farmlands, and the rich floodplains were channelized and drained. Bachman’s Warbler experienced double jeopardy because its sole wintering grounds (Cuba and the Isle of Youth) also underwent rapid habitat conversion into sugar cane plantations. The last confirmed Bachman’s Warbler was a singing male that returned to the same location in South Carolina for three successive breeding seasons in 1958, 1959, and 1960.

Fig. 15.09 Carolina Parakeets (Conuropsis carolinensis). The Carolina Parakeet was the only species of parrot native to the USA and Canada. Though the precise reasons for its extinction remain unknown, extensive hunting, deforestation, and disease are all implicated as potential factors.
(Watercolor by Francis Lee Jaques, courtesy of Madelyn Morey.)
Avian diversity reaches its global peak in Central and South America, but together these continental land masses are known to have lost only five bird species: one resulting from habitat destruction, one from illegal trade, and three from degradation of local wetland ecosystems. The colossal‐sized Imperial Woodpecker (Campephilus imperialis) (a close relative of the Ivory‐billed Woodpecker) occupied old‐growth pine forests of the Cordillera Occidental in western Mexico until these forests were obliterated by logging (Fig. 15.10). The species has not been documented since 1956. The Colombian Grebe (Podiceps andinus) was endemic to a wetland system near Bogotá (especially Lake Tota). Systematic drainage, increased concentration of pollutants, dramatically altered aquatic vegetation, and introduced rainbow trout combined to cause a grebe population collapse in the mid‐twentieth century, and the last individuals were seen in 1977. The flightless Atitlan Grebe (Podilymbus gigas), endemic to Lake Atitlan, Guatemala, met a similar fate during the same time period, apparently accelerated by competition for food from introduced bass. The Slender‐billed Grackle (Quiscalus palustris) was endemic to marshlands in a tiny area of central Mexico which over hundreds of years have been systematically ditched and drained for agricultural uses and urban development. The last free‐flying Spix’s Macaw (Cyanopsitta spixii) disappeared from southeastern Brazil in 2000 (Fig. 15.03). Its decline throughout the twentieth century began with widespread clearing of its forest habitat but was hastened by persistent capturing for illegal trade. Fortunately, this spectacular species persists in captivity, providing hope that someday it can be successfully reintroduced into its native habitat.

Fig. 15.10 Imperial Woodpeckers (Campephilus imperialis). Now extinct, this was the world’s largest woodpecker. The species depended on large tracts of mid‐montane pine‐oak forests in western Mexico. Before the mid‐twentieth century, small social groups were seen foraging on dead trees, although the species was poorly known. The last confirmed birds were observed before logging operations destroyed much of their preferred habitat; hunting and harvesting young were additional factors leading to their extinction.
(Illustration by N. John Schmitt.)
Remarkably, the continental mainlands of Europe, Asia, Africa, and Australia appear to have lost only two bird species among them during recorded history. The mysterious, non‐migratory Pink‐headed Duck (Rhodonessa caryophyllacea) was always rare across the marshlands of the Indian subcontinent (Fig. 15.11). Wetland drainage throughout its limited range hastened its disappearance. Although occasional unsubstantiated reports continue, reliable sightings ceased after 1935. The colorful Paradise Parakeet (Psephotus pulcherrimus) (Chapter 14) once was common in grassy woodlands within a limited range in northeastern Australia but began declining rapidly near the end of the nineteenth century as a result of land clearing, overgrazing, hunting, and predation by introduced mammals. The bird was believed to be extinct for a time and then was rediscovered, but the last confirmed sighting was in 1927. Similarly, the mysterious, nocturnal Night Parrot (Pezoporus occidentalis) of Australia has been reported only a few times in recent decades, and has long hovered around the list of modern avian extinctions. Recent evidence suggests the existence of at least a tiny population in western Queensland (Fig. 15.12).
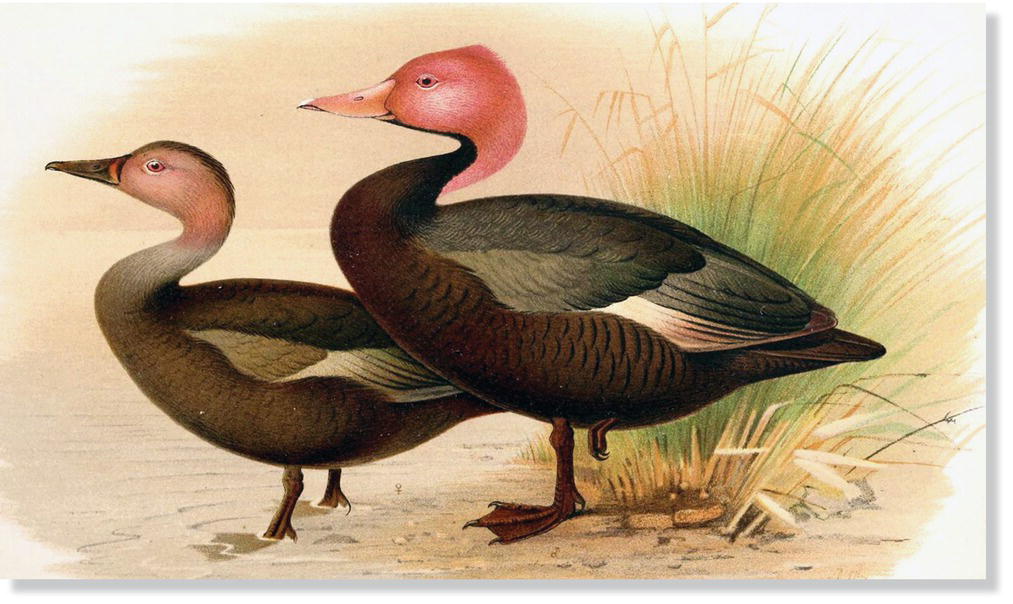
Fig. 15.11 Pink‐headed Duck (Rhodonessa caryophyllacea). Always considered rare, this species was native to India, Bangladesh, and Myanmar; the last confirmed sighting was in 1935.
(Artwork by Henrik Grönvold, from Wall 1908, public domain.)

Fig. 15.12 Night Parrot (Pezoporus occidentalis). This is an extremely rare, nocturnal parrot that inhabits grasslands and swamps of western Queensland (Australia), feeding mainly on grass seed. Its population status remains a mystery, but a few live birds have been observed recently.
(Photograph by Peter W. Lindenburg.)
15.4 Causes of avian population declines
Except through cataclysmic geological events, extinctions do not happen overnight. Instead, species decline from forces acting over time—from just years in exceptional cases, as with naïve or flightless island birds faced with introduced predators, to decades or longer when the cause is slow conversion of essential habitat. Bird populations are extremely sensitive to changes in the composition, function, and stability of their natural communities and ecosystems. Devising conservation solutions requires first identifying the forces that are causing the declines, most of which fall into two interacting categories: extrinsically imposed stressors or threats (nowadays mostly brought about by humans) and intrinsic biological attributes that provide evolutionary advantages under natural conditions but can make a species vulnerable as environmental conditions change.
15.4.1 Demography, habitat, and life histories
All populations fluctuate year to year, but average numbers can remain stable over time because average birth rates and death rates are balanced. As covered in more detail in Chapter 13, the scientific study of birth rates and death rates, called demography, plays an enormous role in conservation biology. Age‐specific birth rates and death rates specify, for individuals at each age, their average number of offspring produced and their probability of dying. Age‐specific death rates compound to produce a schedule of survivorship, the probability of being alive at a given age. Population stability over any given time period requires that, on average, the total accumulated reproductive output from all age classes matches the number of deaths occurring during that same period. Therefore, understanding the factors that affect birth rates and death rates is essential in conservation research.
The word habitat also holds a crucial place in conservation biology and refers to the comprehensive range of physical, biological, and geographic conditions required by individuals of a given population in order to survive and/or reproduce. As discussed later, bird species vary considerably in their tolerance limits for environmental conditions. Conservation biologists devote enormous effort to understanding in detail the extent and nature of these habitat tolerances. Conservation practitioners devote similarly huge efforts to ensuring that sufficient habitat area exists and is properly managed.
All bird populations have an evolutionary history. In adapting to the range of environmental conditions characterizing its habitat, every population evolves numerous life history traits that provide survival and reproductive advantages within that habitat (Chapter 13). For birds, some of the most important variables relevant to conservation are the period of nest dependency, period of juvenile dependency, age at first breeding, mating system, age‐specific clutch size, reproductive lifespan, and dispersal/migration pattern. Understanding long‐term changes in population numbers requires identifying how environmental changes interact with these life history attributes to cause changes in birth rate, death rate, and distribution of suitable habitat, or a combination of these factors.
15.4.2 Habitat specialization and the six forms of rarity
Outright habitat loss obviously leads to population decline for any species that depends on that habitat, but not all species are affected equally. Certain species are more vulnerable than others when native habitats disappear. Rare species generally are more vulnerable than common ones, but what exactly does “rare” mean? Most species thought of as naturally rare actually are (or once were) reasonably common in one or a few localized areas and may have very specific habitat requirements. Protecting their local geographic centers or special habitats may be sufficient to ensure these species’ survival, but if these primary centers of occurrence have been destroyed, then protecting such species is extremely challenging. Identifying the nature of a target species’ rarity is a critical step in designing its protection and recovery.
Three features of a species’ distribution affect its vulnerability: its overall geographic distribution (range size); the overall numbers of the species within its range (population density); and the spectrum of habitats in which the species can live and breed (habitat specificity). Deborah Rabinowitz et al. (1986) categorized British plants according to these three variables to produce a simple framework for identifying seven different forms of rarity, six of which are relevant to birds (Fig. 15.13). Below we describe these six forms of rarity and give examples of endangered birds that fit each category:
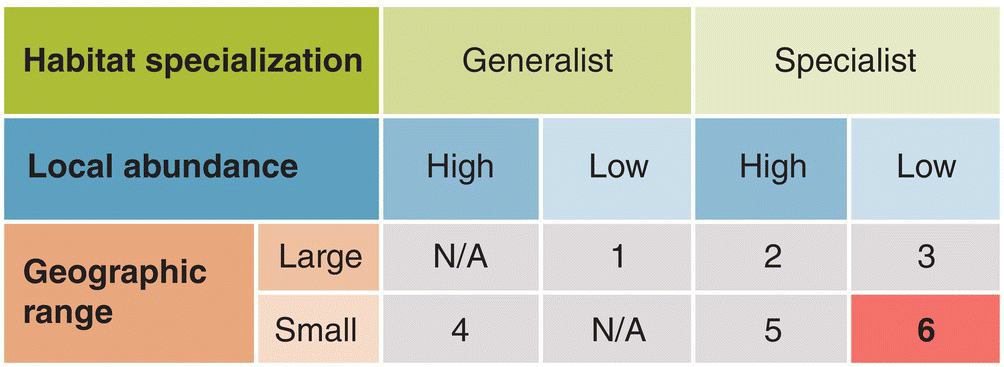
Fig. 15.13 Six forms of rarity in birds. Geographic distribution (large or small), local abundance (high or low), and habitat specialization (generalist or specialist) are all factors that influence vulnerability. Species in category 6—which are highly specialized with small populations and a narrowly local range—typically have the greatest risk of extinction.
(From Rabinowitz 1981. Reproduced with permission from John Wiley and Sons.)
Rarity form 1: Widely distributed, small local populations with broad habitat tolerance
Most continental raptors fit this category, which explains why few other than the very largest eagles appear on endangered lists. Being at the top of the food chain, hawks and owls generally have large home ranges and consequently low population densities. The Crowned Hawk‐Eagle (Stephanoaetus coronatus) (Fig. 15.14) of sub‐Saharan Africa is the second largest eagle on that continent and recently was moved to a higher risk category by the IUCN because, although the bird occurs across a wide range of forested habitats, its populations suffer persecution from local farmers. The Lesser Kestrel (Falco naumanni) breeds in a variety of open habitats across Europe and Asia, and winters broadly across Africa. Once listed as Vulnerable because intensifying agriculture and pesticide use caused sharp declines across its entire breeding range, the species has rebounded since 2000 and recently was reclassified as Least Concern (BirdLife International 2016a).

Fig. 15.14 Crowned Hawk‐Eagle (Stephanoaetus coronatus). As one of the largest and most powerful eagles of Africa, the Crowned Hawk‐Eagle is a species of increasing concern because of habitat destruction, hunting, and trapping. Although this species is a widespread, generalist predator, its population density is low even in patches of preferred habitat, which makes it vulnerable.
(Photograph by Peter R. Steward.)
Rarity form 2: Widely distributed, large local populations with narrowly specialized habitat requirements
Numerous declining bird species fit this category as humans reduce and fragment populations of once‐widespread ecological specialists. Red‐cockaded Woodpeckers (Picoides borealis) originally were common in old‐growth pine savannas across the entire southeastern USA (Fig. 15.15). This cooperative‐breeding woodpecker nests in cavities excavated high on the trunks of living pines and protects its nests from predation by snakes and small mammals by drilling tiny “resin‐wells” into the surrounding bark, producing a barrier of sticky pine pitch. Red‐cockaded Woodpeckers nest almost exclusively in mature long‐leaf pine stands in which fungus renders the center of certain trunks soft enough to hollow out. They also require an open, grassy understory and gradually disappear from pine forests that are not burned regularly. Therefore, populations persist today only in well‐managed locations where mature pine stands remain uncut and are burned every 2–3 years. Oilbirds (Steatornis caripensis) are found broadly across the tropical forested regions of northern South America, but they are locally common only at their widely scattered breeding colonies. These nocturnal birds have a highly specialized breeding habitat, as they nest only within large, dark caves (Chapter 12).

Fig. 15.15 Red‐cockaded Woodpecker (Picoides borealis). An endemic of the southeastern USA, this species is locally common, but highly specialized on long‐leaf pine forests that depend on frequent burn cycles to remain healthy. As primary cavity excavators, Red‐cockaded Woodpeckers provide a service to numerous other secondary cavity‐nesting species.
(Photograph by Juli Wells.)
Rarity form 3: Widely distributed, small local populations with narrowly specialized habitat requirements
Small population size combined with habitat specialization render species in this category vulnerable despite their broad geographic ranges. Piping Plovers (Charadrius melodus) (Fig. 15.16) breed on barren sandy beaches from central Canada and the Great Lakes east to the Atlantic coast of North America, but even the largest of these beaches rarely harbors more than two or three pairs. Its narrow habitat tolerance places the species directly in competition with humans, who also love sandy beaches. Consequently, Piping Plovers always will be vulnerable simply because of insufficient breeding habitat free of human disturbance, and the species now depends on active protection of nest sites by humans. A recent analysis suggests that this species will be particularly vulnerable to rising sea levels and storm surges resulting from climate change (Seavey et al. 2011). The endangered White‐headed Duck (Oxyura leucocephala) (Chapter 3) breeds in scattered populations across Europe and western Asia on small, semipermanent or temporary lakes that have extensive areas of shallow water with fringes of dense emergent vegetation and pondweeds. Numbers have plummeted because of wetland draining, pollution, and hybridization with Ruddy Ducks (Oxyura jamaicensis) introduced from North America (Muñoz‐Fuentes et al. 2007) (Chapter 3).

Fig. 15.16 Piping Plovers (Charadrius melodus). A recently hatched Piping Plover nestling is guarded by its parent. This species exists only in small, local populations. It prefers to nest in sparsely vegetated sandy beaches, making it vulnerable to coastal development, introduced predators, and rising sea levels related to climate change.
(Photograph by Brendan Toews Photography.)
Rarity form 4: Small geographic range, large local populations with broad habitat tolerance
This form of rarity is often seen in bird species that are restricted to islands, where they may have small global population sizes despite being common within their restricted range. For example, the Gray Trembler (Cinclocerthia gutturalis), an odd member of the mockingbird family that gets its name from its habit of quivering its body and tail while singing, is found only on the islands of Martinique and St. Lucia in the Lesser Antilles (Fig. 15.17). The global range of the Gray Trembler is therefore small, but within those islands the species is relatively common across a range of habitat types, from lowland dry scrub to high‐elevation tropical forest.

Fig. 15.17 Gray Trembler (Cinclocerthia gutturalis). Island species like the Gray Trembler may be quite common in their native range and occupy a variety of habitats, but they are vulnerable because their global range is small.
(Photograph by Ed Schneider.)
Rarity form 5: Small geographic range, large local populations with narrowly specialized habitat requirements
This category probably contains the largest proportion of at‐risk bird species around the world. Most geographically restricted species, even those with narrow habitat tolerances, can be locally common in places where their specific habitat needs are met. However, they easily become threatened if those habitats are altered. For example, the spectacular Strange‐tailed (Alectrurus risora) and Cock‐tailed (Alectrurus tricolor) Tyrants remain locally common within their limited ranges in grasslands of southeastern South America (Fig. 15.18). However, global numbers of both species are plummeting, as are those of many other grassland specialists, because of agricultural conversion and persistent burning of grasslands for livestock foraging (Di Giacomo et al. 2011). All around the world, bird species in this important category of rarity depend on the protection and long‐term management of habitat patches within their respective native ranges.

Fig. 15.18 Two at‐risk South American flycatchers. Species around the globe are vulnerable to extinction when they are locally common but highly specialized and limited to a small geographic range. (A) The Strange‐tailed Tyrant (Alectrurus risora) requires tall‐grass marsh habitat. (B) Similarly, the Cock‐tailed Tyrant (Alectrurus tricolor) occurs in limited areas of humid and seasonally dry tall‐grassland habitats. Both are threatened by rapid agricultural and grazing habitat conversion.
(Photographs by: A, Cláudio Dias Timm; B, Scott Olmstead, https://en.wikipedia.org/wiki/File:Cock‐tailed_Tyrant_(Alectrurus_tricolor)_perched.jpg. CC‐BY‐SA 2.0.)
Rarity form 6: Small geographic range, small local populations with narrowly specialized habitat requirements
Species in this category are the world’s most vulnerable, because local perturbations or catastrophes can extinguish them immediately. Temperate zone habitats of North America and Eurasia contain few avian examples, because most birds on these large continents have large ranges compared with other, more sedentary and vulnerable groups of organisms such as plants, stream fishes, freshwater mollusks, and cave‐dwelling arthropods (Stein et al. 2000). Throughout the world’s tropical realms, however—both on islands and on continents—many ecologically specialized and locally endemic birds also have naturally low population densities. Such species are vulnerable to every threat discussed in this chapter. Examples include numerous island‐endemic species of hawks, owls, and nightjars, such as the Mauritius Kestrel (Falco punctatus), Seychelles Scops‐Owl (Otus insularis), and Puerto Rican Nightjar (Antrostomus noctitherus). Many bird species similarly occupy tiny ranges on isolated mountain ridges across the world’s tropics, and those having low population densities, such as Bannerman’s Turaco (Tauraco bannermani) (Fig. 15.19) of western Cameroon, are especially at risk as forests are cleared.

Fig. 15.19 Bannerman’s Turaco (Tauraco bannermani). Among the rarest and most vulnerable species in the world, Bannerman’s Turaco is range restricted, locally rare, and highly specialized. Tropical species such as this turaco are particularly at risk because of their dependence on habitats that occur only in a single geographic area, which are easily lost to habitat conversion.
(Photograph by Paul Ellis.)
15.5 Major threats to bird populations
The IUCN recognizes 11 categories of threats facing the world’s living species (IUCN 2012). These IUCN categories differ widely from one another in how and where they affect birds (Box 15.02). Several of the IUCN categories lump extremely important human‐caused threats to birds together under a single designation. For example, direct hunting and killing are lumped together with timber extraction, under the heading “Biological resource use.” Here we describe the most important threats causing bird population declines.
15.5.1 Habitat loss
The most pervasive cause of avian population declines worldwide is loss of suitable habitat. All birds have unique adaptations that allow them to take advantage of specific and predictable features of their habitat. Humans have continually changed the structure, composition, and distribution of habitats, eliminating some and grossly changing others, but we cannot change the fundamental requirements of the bird species that use these habitats. For example, across North America, Europe, and Asia native prairies and steppes have been converted to grow grain crops or introduced forage grasses, thereby reducing population sizes of the grassland larks, pipits, shrikes, sparrows, and buntings that rely on native grassland habitats. By draining wetlands to create more areas for grazing or cultivation, humans similarly have reduced the total number of ducks, grebes, and shorebirds. Forests have been cleared for timber, agriculture, and livestock; coastlines and shorelines have become centers for residential and commercial development; and native scrubs have been replaced with pines and eucalyptus. All such cases of widespread habitat modification have resulted in dramatic declines among the birds adapted to the orginal habitats.
Worldwide, forest habitat continues to be lost at an unsustainable rate, especially in the tropics, and the loss of forest is a particularly prominent conservation concern (Fig. 15.20). Indonesia, for example, supports the third‐largest expanse of tropical forest in the world, at almost 100 million hectares; only the forests of Amazonia and the Congo Basin are larger. A recent report found that Indonesia's rates of forest clearing and conversion to oil palm plantations are accelerating, placing it among the five countries with the highest percentage of primary forest loss (Wich et al. 2011). Indonesia’s numerous tropical islands harbor hundreds of locally endemic forest birds, almost all of which are declining as their habitat is converted. On Madagascar a number of birds are similarly poised on the brink of extinction because of the centuries‐long conversion of the island’s habitats into vast grazing lands and rice paddies. The Madagascar Serpent‐Eagle (Eutriorchis astur) (Fig. 15.21), suspected to be extinct since the 1930s, was rediscovered in the early 1990s but exists only as a tiny population in a remote forest tract at the northeastern corner of the island. Owing to the evolutionary uniqueness of the flora and fauna of Madagascar (Chapter 3), considerable international assistance has been focused on helping its government identify the best remaining patches of native habitat and secure them as ecological preserves. Today, however, a tremendous increase in illegal logging threatens to undermine these efforts (Randriamalala and Liu 2010).
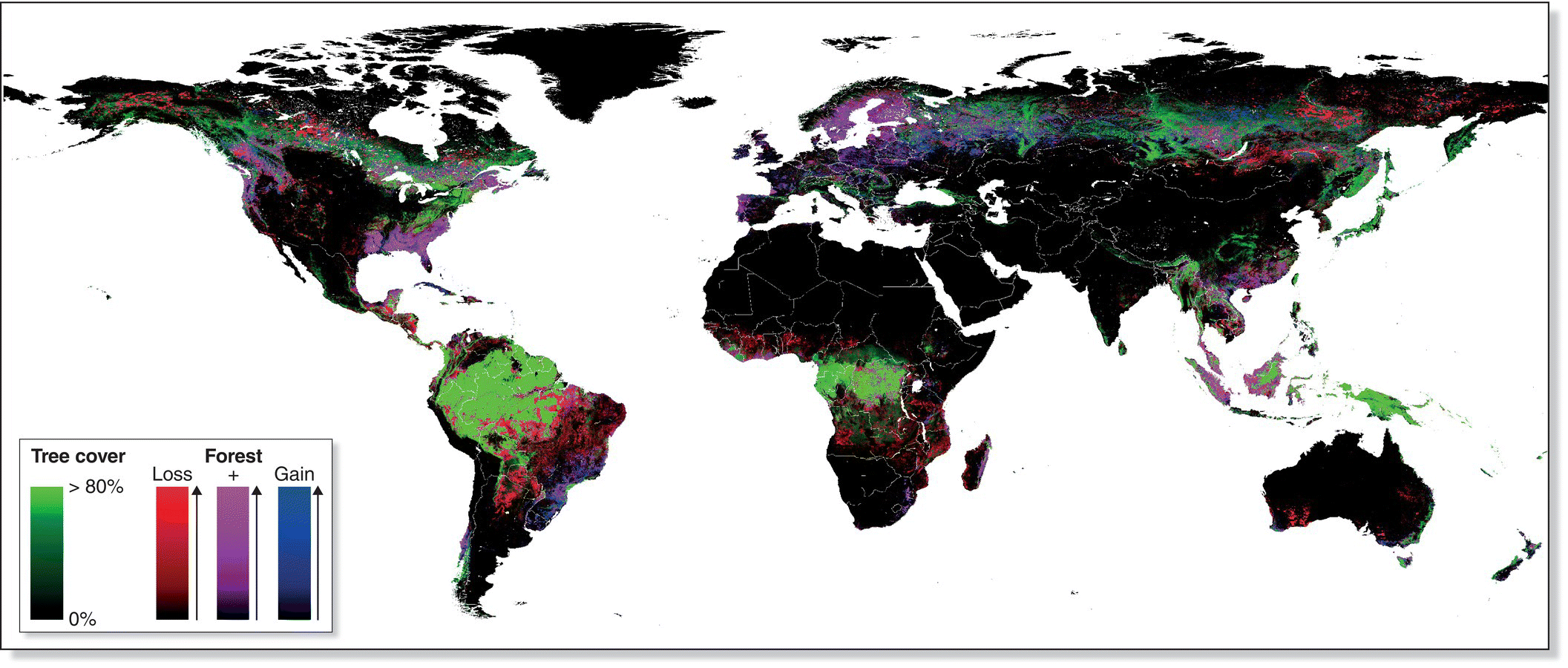
Fig. 15.20 Forest turnover worldwide. Using high‐resolution satellite imagery, this map characterizes forest extent (green), gain (blue), loss (red), and both gain and loss (magenta) from 2000 to 2012. During this 12‐year study, 2.3 million square kilometers of forest were lost, and 0.8 million square kilometers were gained (through regrowth and planting initiatives). Deforestation in the tropics is of particular conservation concern; the extent of tropical forest at the turn of the twentieth century was twice that of today.
(From Hansen et al. 2013. Reproduced with permission from AAAS.)

Fig. 15.21 Madagascar Serpent‐Eagle (Eutriorchis astur). This rare eagle persists only because some of its important lowland Madagascar forest habitats have been protected by international conservation efforts. Unfortunately, illegal logging continues throughout Madagascar, leaving this bird’s future uncertain.
(Photograph by Russell Thorstrom.)
Non‐forest habitats are mentioned less frequently than forest in most discussions of habitat loss, but many such habitats are also at the highest level of conservation concern. For example, arid habitats in the wheatbelt region of Western Australia, part of which is recognized formally as a National Biodiversity Hotspot, face continued pressure from agriculture and other stressors that threaten its many endemic species. The Brazilian cerrado similarly ranks among the 25 global biodiversity hotspots (Myers et al. 2000), and almost 5% of its more than 770 bird species are endemics (Fig. 15.22). Two‐thirds of the cerrado’s original extent has been converted to agriculture (mainly soybeans) over the past 40 years (Cavalcanti and Joly 2002). Estimates suggest that the cerrado could be gone entirely by 2030, and progressive extinctions of its avifauna will have detrimental effects on the functional ecology of central Brazil (Batalha et al. 2010).
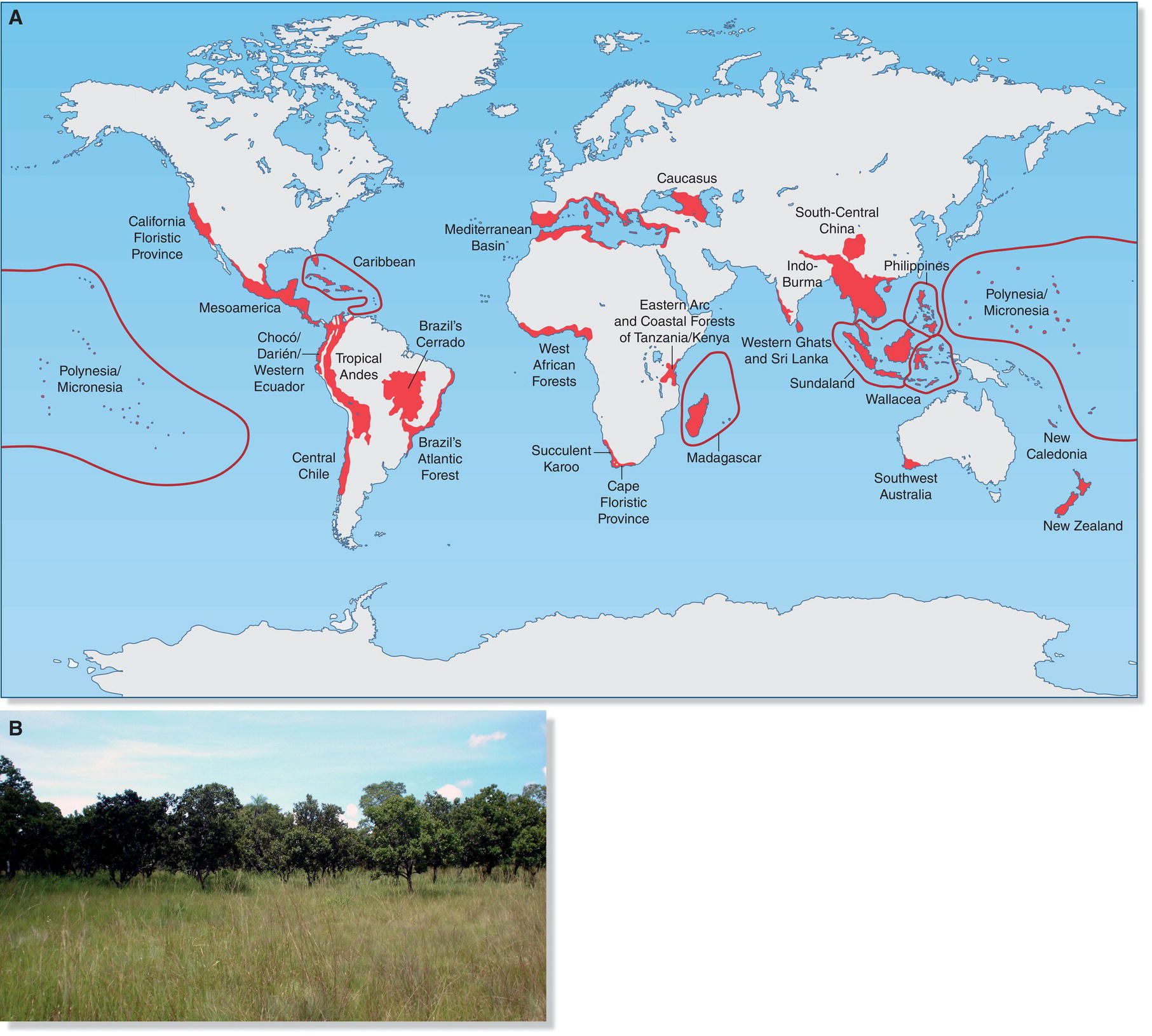
Fig. 15.22 Biodiversity hotspots. (A) Habitats with a large proportion of endemic species—those found nowhere else on earth—are considered the highest priority for conservation action. Saving these hotspot habitats protects species that are at high extinction risk. (B) Many hotspots, including this Brazilian cerrado, are threatened because of agricultural conversion and human encroachment.
(A, from Myers et al. 2000. Reproduced with permission from Macmillan Publishers Ltd. B, photograph by Benjamin G. Freeman.)
15.5.2 Habitat fragmentation
One of the most widespread patterns in nature is that larger islands hold more species than smaller islands, in part because population size tends to mirror island size, and because small populations tend to go extinct from random fluctuations more easily than large ones. In addition, larger islands contain a greater diversity of habitat types than smaller islands, thereby providing niches for more kinds of species. The importance of this species–area relationship (Chapter 14) goes far beyond its explanatory power for biodiversity on islands: virtually all continental habitats around the world now are subdivided into a mosaic of island‐like patches.
By converting native forests, grasslands, scrubs, and deserts into patchworks of landscapes designed for our own use, humans create habitat islands of different sizes, at differing distances from one another, and separated by different intervening habitats than would occur naturally. Persisting as islands, remnant patches of native habitat can be isolated as thoroughly as if they were surrounded by water. Just as on oceanic islands, habitat islands inevitably begin to lose their bird species.
Loss of species from habitat patches following isolation has been demonstrated by hundreds of studies around the world over the past 50 years. One of the most consistent patterns is that species with the smallest populations disappear first. In Java, for example, after the Bogor Botanical Garden arboretum had been isolated for 50 years, it had lost 75% of the bird species with originally small populations, but the species that were more common largely persisted (Diamond et al. 1987). In Panama, nearly 50% of the original bird community disappeared from the island of Barro Colorado after it was formed by the flooding of Gatun Lake during construction of the Panama Canal (Chapter 14). The birds that disappeared first were those with naturally low population density, such as ground‐cuckoos, raptors, and woodpeckers (Willis 1974; Robinson 1999).
Species that require the largest tracts of habitat in order to breed successfully and persist are termed area‐sensitive species. In eastern North American forests, Wood Thrushes (Hylocichla mustelina), Eastern Wood‐Pewees (Contopus virens), and Red‐eyed Vireos (Vireo olivaceus) are sensitive only in regard to tracts below about 20 hectares and occur in most tracts above this size. In contrast, species such as Pileated Woodpeckers (Dryocopus pileatus) and Worm‐eating Warblers (Helmitheros vermivorum) require much larger tracts, and their frequency increases steadily with patch size, even above 1000 hectares (Fig. 15.23). Therefore, tracts of considerable size are required to preserve the complete community of woodland bird species.
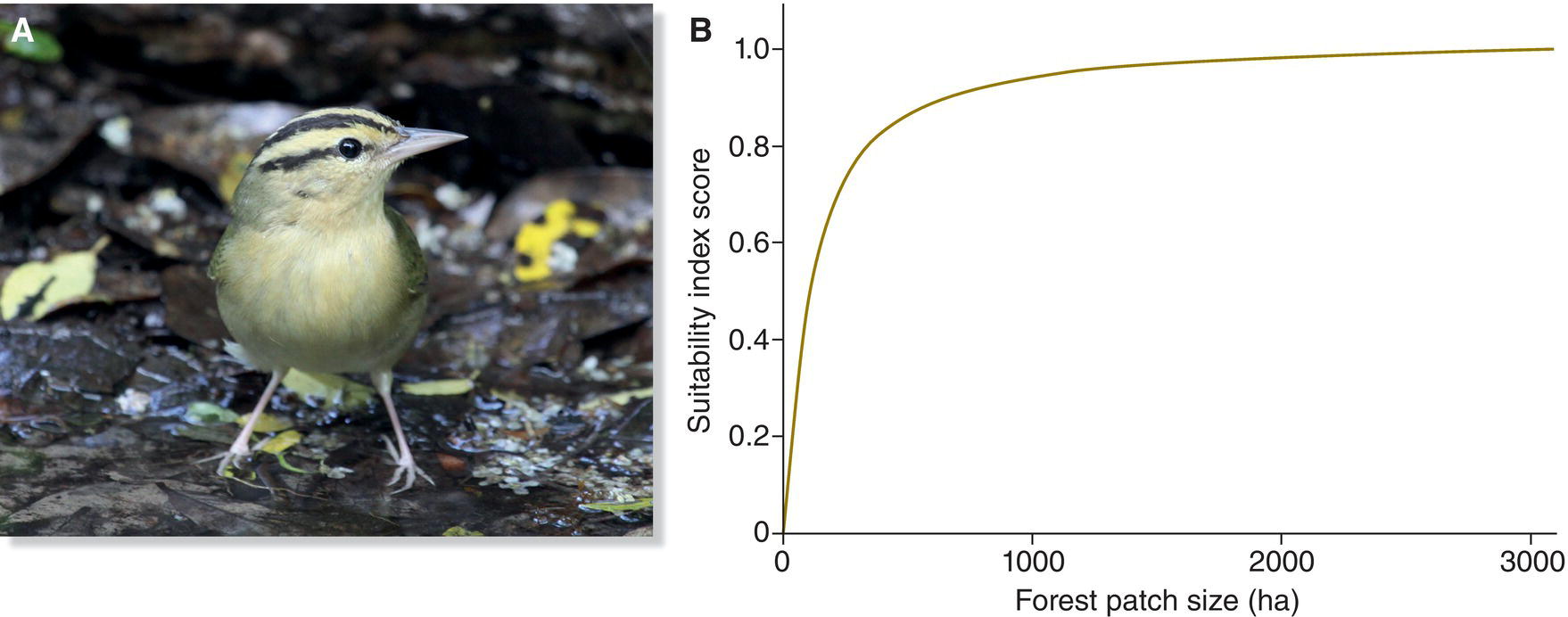
Fig. 15.23 Some species require large forest tracts. (A) The Worm‐eating Warbler (Helmitheros vermivorum) is one of many species that requires large areas of intact, primary forest to sustain healthy breeding populations. (B) The larger the area, the greater the habitat suitability index for this species.
(A, photograph by Andrew Jordan. B, from Tirpak et al. 2009.)
Grassland birds also exhibit area sensitivity. In agricultural regions, remnant patches of native grassland may form habitat archipelagos amid encroaching oceans of alfalfa, grain crops, or soybeans. As a result, grassland species all over the world are deeply threatened. Species having the lowest population densities are threatened most severely, a problem compounded by the fact that density itself is area sensitive—that is, many grassland birds occur at the highest densities within the largest tracts of good habitat (Ribic et al. 2009). Birds such as stone‐curlews and sandgrouse in Europe (Goriup et al. 1991), prairie‐chickens and Sprague’s Pipits (Anthus spragueii) in North America, and seriemas and rheas in South America are failing to persist in any but the very largest islands of native grassland. Almost all the world’s bustards (Fig. 15.24) and other birds of the Asian shrub‐steppes are declining, even in areas where they are protected (Bota et al. 2005). Protecting the largest remaining expanses of native grassland has emerged as an urgent global priority for bird conservation.

Fig. 15.24 Grassland birds are declining worldwide. This Indian Bustard (Ardeotis nigriceps) is one of the many bustards that face extinction due to hunting and habitat destruction. Fewer than 250 individuals of this species remain in the wild.
(Photograph by Nitin Vyas.)
Compounding problems of habitat fragmentation are edge effects, or factors causing habitat near the edge of a patch to be less suitable for survival or reproduction than habitat in the middle. For birds, the most important edge effects are: (1) changes in microclimate (sunlight, temperature, and humidity) near a habitat edge, which can affect plant composition, habitat structure, and prey abundance; (2) introduced plants and animals, which penetrate habitat fragments and alter their characteristics near the edge; (3) increased frequency of habitat disturbance such as fire or wind damage; (4) greater numbers of generalist or edge‐using mammalian and avian predators; and (5) elevated brood parasitism, especially by cuckoos (in the Old World) and cowbirds (in the New World). For example, in forest fragments of southern Illinois (USA), 50–100% of songbird nests are parasitized by cowbirds, and 70–99% are destroyed by predators before hatching (Brawn and Robinson 1996). Edge effects are especially pronounced in tropical forests, where they can be the dominant influence affecting community composition in fragmented habitats (Laurance 2008).
15.5.3 Introduced predators
The introduction of new predators into habitats previously lacking them ranks among the most pervasive threats to birds all over the world. Rats, cats, mongooses, stoats, and ferrets are the most widespread and notorious culprits, but the full list is long and even includes reptiles such as monitor lizards and snakes. As noted elsewhere, the problem is especially acute on islands where many native birds evolved flightlessness, have few defensive behaviors against predators, or nest on the ground. Even on continents, however, non‐native predators (especially domestic cats) kill millions of birds annually, causing population declines and local extirpations (Loss et al. 2013).
Oceanic islands across the Pacific once provided predator‐free breeding places for millions of seabirds comprising dozens of species of petrels and shearwaters, albatrosses, terns, boobies, frigatebirds, tropicbirds, and cormorants. During recent decades, huge populations of invasive rats and cats have caused precipitous population declines, endangerment, and extinction on these islands. On Kiritimati (formerly called Christmas Island), cat eradication measures have failed, and black rats recently arrived. Seabird numbers have dropped by half, and the largest remaining population of the endangered Phoenix Petrel (Pterodroma alba) is in grave danger of extinction (BirdLife International 2016b). A comprehensive analysis of seabird–rat interactions worldwide shows that storm‐petrels and other burrow‐nesting seabirds are by far the most vulnerable, and that gulls and other ground nesters are less so (Jones et al. 2008).
In Madagascar, the introduction of exotic fish into Lake Aloatra is blamed for the loss of the endemic Alaotra Grebe (Tachybaptus rufolavatus), which has not been seen since 1985 and was declared extinct in 2010. A similar fate appeared to befall the Madagascar Pochard (Aythya innotata), but a tiny population was rediscovered in 2006 on Lake Matsaborimena in northern Madagascar, and a captive‐rearing program has been undertaken to restore its numbers.
15.5.4 Direct exploitation
The most blatant cases of human‐induced population declines are those in which adults were killed at such a scale that the birth rate of new recruits could not compensate for the death rate imposed by humans. Familiar examples involved killing of adults for food—as for the Dodo (Raphus cucullatus), Passenger Pigeon (Ectopistes migratorius), and Eskimo Curlew (Numenius borealis)—or ornamentation, as for egrets and herons. At least 857 bird species are currently at risk from direct hunting or trapping (IUCN 2012).
During the late twentieth century, global trade in exotic pets introduced a new element to the birth rate–death rate story. Many large tropical parrots became extremely rare in their native ranges because humans were stealing young parrots from their nests. This predation did not change the biological birth rate, because surviving adults continued to produce chicks, but it vastly reduced the effective birth rate, because most chicks were stolen from the wild and smuggled out to foreign countries. In the Lesser Antilles, for example, the illegal sale of Imperial Parrots (Amazona imperialis) (Fig. 15.25) reduced the effective birth rate to nearly zero in the mountaintop forests of Dominica. In 1979 the population of this spectacular parrot dropped further to about 50 individuals after a devastating hurricane. In the decades since, organized guarding of parrot nest sites and protection of mountaintop forests across Dominica has allowed offspring once again to recruit into the breeding population, effectively reversing the decline; as of 2008 the Imperial Parrot population had increased to about 350 individuals (Reillo and Durand 2008). Similar actions have greatly reduced the illegal parrot trade throughout the other islands of the Lesser Antilles.

Fig. 15.25 Imperial Parrot (Amazona imperialis). This species is found only on the island of Dominica in the West Indies. Populations were already in sharp decline from the illegal parrot trade when tropical storms killed many adults in 1979. This event galvanized efforts to more actively guard the species’ preferred nesting areas. Populations are now beginning to recover.
(Photograph by Mikko Pyhälä.)
The beautiful, somewhat nomadic Red Siskin (Spinus cucullatus) originally was common in open, semiarid areas of northern South America but has fallen to critically low numbers as a result of massive pressure from trappers for global trade (Fig. 15.26). This species has a pleasant song, and captive siskins will mate with domestic canaries to produce highly sought‐after rose‐colored hybrids. Tiny siskin populations persist in northern Venezuela, where it is among the country’s four bird species with the highest priority for conservation (Rodríguez et al. 2004). A recently discovered population in Guyana supplies additional hope, although it too is under pressure from illegal trapping (Robbins et al. 2003).

Fig. 15.26 Red Siskin (Spinus cucullatus). This species is prized in the pet trade because it will breed readily with domestic canaries. A common sight in the grasslands of Venezuela and Colombia during the early twentieth century, it now is endangered, as entire populations have been extirpated by illegal capture.
(Photograph by Siskini, http://en.wikipedia.org/wiki/File:Cucullatamachocolombia.jpg.)
15.5.5 Chemical toxins and pollution
The publication of Rachel Carson’s Silent Spring in 1962 alerted the world to the ecological dangers of chemical herbicides and insecticides, and her fears proved well‐founded when many raptor species began disappearing from long‐occupied habitats all over the world. Declines were especially pronounced for Bald Eagles (Haliaeetus leucocephalus), Ospreys (Pandion haliaetus), and Peregrine Falcons (Falco peregrinus) in the USA and for Eurasian Sparrowhawks (Accipiter nisus) in Europe. The primary cause turned out to be reproductive failure from eggs being crushed in the nest. The resulting flurry of studies became some of the most famous in the history of conservation biology. By comparing the fragments of crushed eggs with egg specimens stored in museum collections (Chapter 13), scientists discovered that modern eggshells were up to 30% thinner than normal (Ratcliffe 1967; Hickey and Anderson 1968; Cade et al. 1988). The problem was traced to contamination of adult birds by chlorinated hydrocarbon pesticides, in particular the common organic pesticide DDT (dichlorodiphenyltrichloroethane) and its stable form DDE. At that time, DDT had become one of the most widely used pesticides in the world. Migratory birds of prey were eating birds, mammals, and fish that had been accumulating DDE in their tissue, thereby accumulating even higher concentrations of DDE than did species lower in the food chain. Physiological effects on raptors included higher death rates among adults, but the reduced birth rate from eggshell thinning was an even bigger problem. Populations of most northern hemisphere hawks and falcons plummeted in places where DDT use was widespread, but they began to recover almost immediately in places where DDT was banned.
Vast quantities of organic pesticides continue to be used around the world, and their long‐term effects on birth rates and death rates among birds remain poorly documented. The toxic insecticide monocrotophos is now banned in the USA, much of Europe, and Australia because of its impact on birds. Highly publicized deaths of nearly 6000 Swainson's Hawks (Buteo swainsoni) in Argentina during the late 1990s (Goldstein et al. 1999) brought global attention to its dangers, but monocrotophos remains widely used in many countries.
Vultures are especially susceptible to environmental toxins (Box 15.03). Many factors contributed to the steady decline of California Condors (Gymnogyps californianus) throughout the twentieth century, but the chief cause was lead poisoning. Virtually all condors found dead in the wild had numerous lead pellets in their crops, and the species is unusually sensitive to elevated levels of lead in its blood. In 1982 all 21 remaining wild condors were captured in order to protect the species from further exposure to environmental lead and to launch a captive‐breeding program. Today, lead poisoning continues to be the chief cause of illness and death among free‐flying condors, and experts agree that the species’ true recovery depends on elimination or substantial reduction of lead in the environment (Finkelstein et al. 2012).
The release of sulfur‐containing compounds into the atmosphere as a by‐product of coal‐fueled power plants and steel manufacturing causes a widespread form of environmental pollution. Acid rain is caused by the condensation of water droplets that incorporate atmospheric gases such as hydrogen sulfide in areas downwind of major industrial centers in North America and eastern Europe. The environmental effects of acid rain vary geographically, depending largely on the availability of chemical buffers in the soil such as calcium carbonate from dissolving limestone. In particular, in granitic landscapes that lack buffers, lakes and soils can become acidified, causing zooplankton and mollusks to become scarce. The ensuing ecological chain reaction often culminates in the loss of snail‐eating and fish‐eating birds such as loons, ducks, and raptors. Acid rain also is correlated with widespread deposition of methylmercury, an extremely toxic form of mercury produced by burning fossil fuels for power generation. The combination of food‐chain disruption and mercury accumulation is blamed for dramatic declines in the reproductive success of Common Loons (Gavia immer) in both North America and Europe (Burgess and Meyer 2008).
Declines in calcium uptake as a consequence of acid rain deposition have been linked to significantly impaired reproductive success among European songbirds, in part because they cannot obtain enough calcium to produce viable eggs (Graveland 1998). In the northern Appalachian Mountains of the USA, acid rain has caused a 10‐fold reduction in land snails, and Bicknell’s Thrush (Catharus bicknelli) numbers are declining within otherwise intact forest (Lambert et al. 2008) (Fig. 15.27).
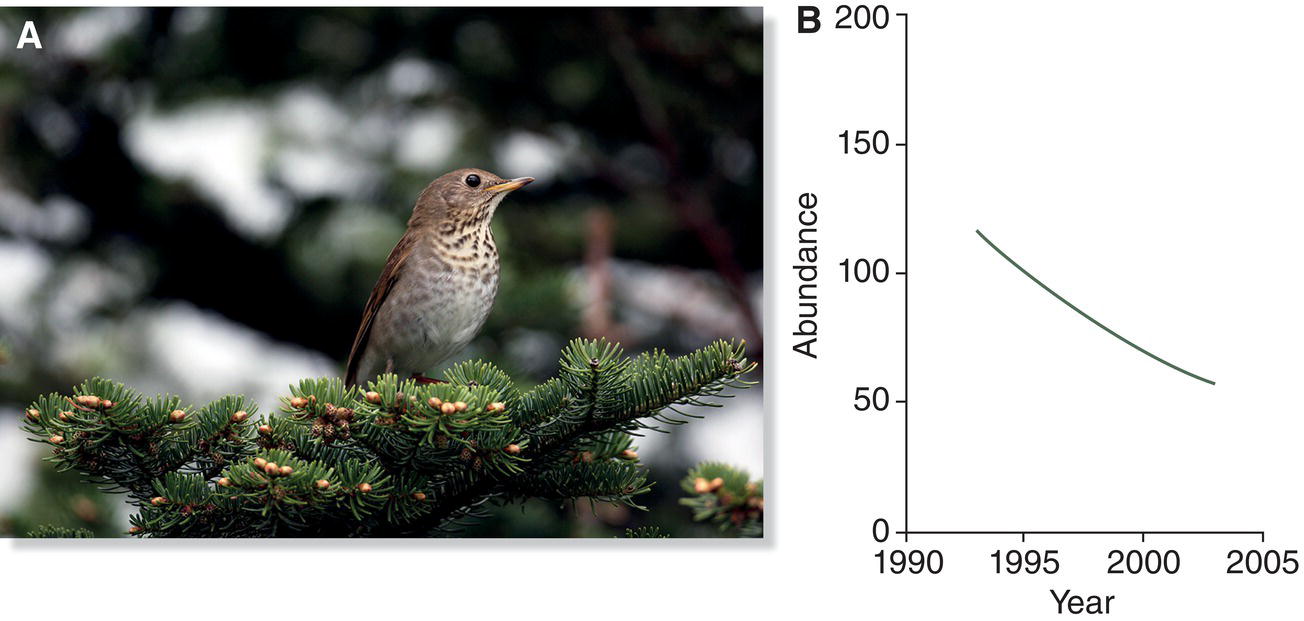
Fig. 15.27 Population decline due to acid rain. (A) Bicknell’s Thrush (Catharus bicknelli) breeds exclusively in dense spruce‐fir thickets in northeastern USA and adjacent Canada. (B) Surveys of the species over the last few decades have revealed strong declines in abundance, particularly in the White Mountains of Vermont. Since their forest habitat remains largely intact, researchers believe these declines are due to acid rain.
(A, photograph by Gerard R. Dewaghe. B, from King et al. 2008. Reproduced with permission from Springer Science + Business Media.)
Since the late 1800s, environmental pollution from human sewage, manufacturing plants, oil refineries, coal‐powered energy plants, and agricultural fertilizers has changed the chemistry of the air, water, and soils throughout the industrialized and agricultural worlds. Pollution reduces prey diversity and alters vegetation structure in lakes, marshes, rivers, and streams, in turn reducing the reproductive success of aquatic birds such as ducks, grebes, bitterns, and loons. Some of the worst effects are caused by eutrophication, when excess nutrients in the water allow one or a few species of algae or cyanobacteria to explode and take over the entire system, reducing dissolved oxygen and choking out the native food web.
15.5.6 Introduced disease
By the time of Captain Cook’s arrival in the Hawaiian Islands in 1778, the resident Polynesians already had caused the extinction of numerous large bird species, but about 70 species of small native birds continued to occupy the forests from sea level to mountaintop. During the second half of the 1800s, a baffling wave of disappearances overtook Hawaiian songbirds, island by island. In 1902, ornithologist H. W. Henshaw described the phenomenon: “large areas of forest, which are yet scarcely touched by the axe save on the edges and except for a few trails, have become almost absolute solitude. One may spend hours in them and not hear the note of a single native bird. Yet a few years ago these areas were abundantly supplied with native birds.” Across the entire archipelago, dozens of bird species went extinct almost simultaneously, and throughout the twentieth century additional species steadily met the same fate.
Many factors were responsible for these losses, but the introduction of disease‐bearing mosquitoes on Maui in 1826 certainly was the single‐most devastating event for Hawaii’s bird fauna since the original arrival of humans 1000 years earlier. Avian malaria and avian pox are the two most virulent agents. Both are carried and transmitted by a complex of mosquito species originally brought to Hawaii from Mexico in the water casks of merchant ships. These two diseases occur worldwide among continental birds, which have evolved resistance, and no doubt were carried annually to Hawaii by migratory birds for hundreds of thousands of years. Before the mosquito’s introduction, however, no vector existed to transfer these deadly pathogens from resistant migrants to naïve native birds. Consequently, no native Hawaiian bird had evolved resistance to malaria or pox (Fig. 15.28).
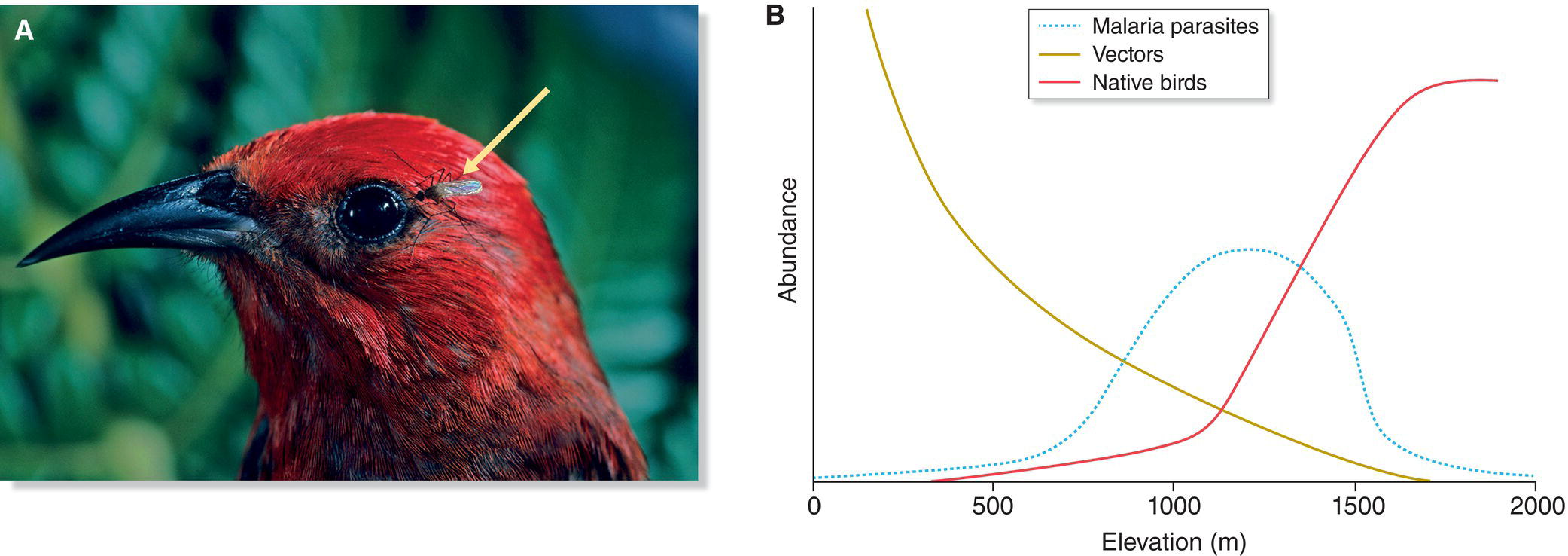
Fig. 15.28 Hawaiian birds and avian malaria. (A) Mosquito vectors (arrow), which transmit avian malaria to birds, are relatively recent introductions to Hawaii. Endemic Hawaiian birds like this Apapane (Himatione sanguinea) lack resistance to the disease. (B) Their highland habitat keeps birds isolated from the vectors that prefer warmer, lower elevations. Many lower‐elevation endemic birds of Hawaii have gone extinct because of avian malaria and compounding factors including habitat destruction, invasive species, and other diseases. Global warming puts all remaining species at risk as the mosquitoes that transmit malaria are able to move into higher elevations.
(A, photograph by Jack Jeffrey Photography. B, from van Riper et al. 1986. Reproduced with permission from the Ecological Society of America.)
As humans began transferring mosquitoes among the Hawaiian Islands, avian death rates soared. Because mosquitoes are scarce in the colder habitats above 1300 meters, a remnant native avifauna could persist above this elevation, but at lower elevations mosquitoes have been impossible to control because they breed in tiny water reservoirs, including tree‐fern trunks hollowed out by introduced pigs. In many areas of Hawaii today, 50% of the free‐flying mosquitoes below 1000 meters elevation carry avian malaria, an infection rate that is vastly higher than any known in continental sites. In the future, warming climates might expand the elevational range of mosquitoes, slowly eliminating the high‐elevation refuges essential to the survival of some Hawaiian bird species. The long‐term hope for the few remaining native forest birds is the gradual development of resistance through natural selection, as apparently is underway in the Hawaii Amakihi (Hemignathus virens) (Woodworth et al. 2005). The statistics are grim, however: over the Hawaiian Islands as a whole, 17 of the 42 recently living native songbird species already have gone extinct, and most of the remaining species are poised on the brink (Pratt et al. 2009). The most recent to disappear were the Poo‐uli (Melamprosops phaeosoma) of Maui (the last male died in captivity in 2004), and the Kauai Oo (Moho braccatus) (a single, long‐lived male in the Alakai Swamp disappeared in 1989) (Fig. 15.29).

Fig. 15.29 Recent species extinctions in Hawaii. (A) In 2004, Maui lost its last remaining captive Poo‐uli (Melamprosops phaeosoma). (B) In 1989 the last male Kauai Oo (Moho braccatus) was heard in Alakai Swamp singing without a mate to hear him.
(Photographs by: A, US Fish and Wildlife Service; B, Robert Shallenberger.)
15.5.7 Climate change
Direct links are often difficult to document conclusively, but climate change increasingly is implicated as a threat to bird populations around the world. Northward‐shifting distributions and progressively earlier spring arrival and nesting dates among northern hemisphere birds are well documented (Thomas and Lennon 1999; Parmesan and Yohe 2003). An ambitious study comparing distributions of California birds across a century has documented numerous changes, with almost every species tracking its climatic niche through shifts along elevational or rainfall gradients (Tingley et al. 2009). Some northern hemisphere birds are no longer breeding at the time of maximal food abundance because of failure to keep pace with progressively earlier peaks in arthropod prey. Mismatched migration timing is not always species‐wide. Hudsonian Godwits (Limosa haemastica), which winter in southern South America and breed in the high Arctic, have arrived progressively earlier near Anchorage, Alaska, where spring has shifted earlier very steadily. Elsewhere, the godwits’ arrival is now mistimed in places where spring temperatures are cooler and more variable than in previous decades (Senner 2012). Birds such as the Kittlitz’s Murrelet (Brachyramphus brevirostris) and Ivory Gull (Pagophila eburnea), which breed at the edge of polar and glacial ice, appear to be undergoing especially rapid population declines as climate change alters the distribution and conditions of their specialized habitats.
15.5.8 Stochastic extinction
A major objective of conservation biologists is to reduce the probability that a target species will experience stochastic extinction, that is, disappearance resulting from random population fluctuations (Chapter 13). Even the most stable bird populations in nature always fluctuate through time because of year‐to‐year variation in physical and biological factors such as rainfall, storms, food supply, parasites, disease, and predation. These normal fluctuations in population size are referred to as stochastic variation (Fig. 15.30). Large and widespread populations have great capacity to persist despite this stochastic variation, in part because of their sheer numbers (after all, half of a huge population is still huge) but also because at any one time different parts of the population may be affected by different variables, so numbers may increase in some areas while decreasing in others. Because birds usually can disperse into areas where density is reduced, larger populations are buffered against perturbations, both natural and human generated. For smaller populations, even minor perturbations can cause a population to disappear entirely. For example, on the island of Hawaii, a small population of the endangered, fruit‐eating Ou (Psittirostra psittacea) survived until 1984. Unfortunately, in that year the Mauna Loa volcano erupted and, by chance, sent a small lava flow directly through the Ou’s forested refuge. The last confirmed sighting of an individual was in 1987: reduced to a tiny population, the Ou had become highly vulnerable and finally succumbed to an isolated, natural, random event.
Fig. 15.30 Stochastic variation in population size. Over the years, the size of Redhead (Aythya americana) and Canvasback (Aythya valisineria) duck breeding populations show the stochastic variation that is normal in healthy populations. Population estimates were calculated using point counts.
(From US Fish and Wildlife Service 2012.)
15.5.9 Tipping points in extinction: Allee effects
The Passenger Pigeon (Ectopistes migratorius) went extinct mainly because humans killed them for food in vast numbers while also systematically destroying their breeding and wintering habitats. The final years of the Passenger Pigeon’s disappearance, however, still present a biological mystery. The species had become rare over most of its range, but for a decade or more thousands of adults still migrated northward in scattered bands, too few in number to attract market hunters. Why, then, did these scattered individuals not begin to recover, instead of vanishing completely within a few years? The Allee effect (named for its discoverer, W. C. Allee) refers to a decline in per capita reproductive success as population density decreases (Chapter 13). Small numbers of pigeons remained after hunting mostly had stopped, but this species was adapted to breed in huge colonies where local predators could feast on young pigeons yet still leave millions of young birds to fledge. Passenger Pigeon numbers may have fallen below a critical threshold required for finding suitable mates and stimulating hormonal pathways required for successful reproduction. Isolated individuals and small flocks instinctively may have searched for large colonies rather than nesting on their own. It is speculated that when the Passenger Pigeon population fell below a critical density, a manifestation of the Allee effect took the species the rest of the way to extinction. Species vary widely in their susceptibility, but strong Allee effects are important to discover. In certain species, falling below a density threshold can accelerate a previously gradual population decline that might be occurring for other reasons, thereby rendering such species more extinction‐prone than others.
15.5.10 Multiple stressors
Many bird species are declining because of multiple factors that have compounding effects on both birth and death rates. For example, the extraordinary disappearance of native birds from the Hawaiian islands resulted from combinations of habitat loss, introduced grazing mammals, introduced predatory mammals, introduced diseases and disease‐bearing insects, and the spread of exotic vegetation, among other stressors. Many migratory birds experience threats on both their breeding and wintering grounds, as well as in migratory transit. The most extreme examples may be the long‐distance migratory shorebirds (Chapter 12). These species breed in high arctic habitats of North America and Asia, where they face habitat degradation and mismatched timing of food resources resulting from climate change. Twice a year, they migrate thousands of kilometers, stopping only at traditional stopover sites on food‐rich interior marshes and coastal tidal flats, many of which are being depleted of natural food resources or converted for agricultural and commercial development. They overwinter on the tropical coastlines and grasslands of South America, Africa, Australia, and southeastern Asia, where many are hunted, trapped, and netted for food. Recent, dramatic population crashes among dozens of once‐common migratory shorebird species represent one of the most challenging conservation dilemmas facing avian conservation biologists (Fig. 15.31).
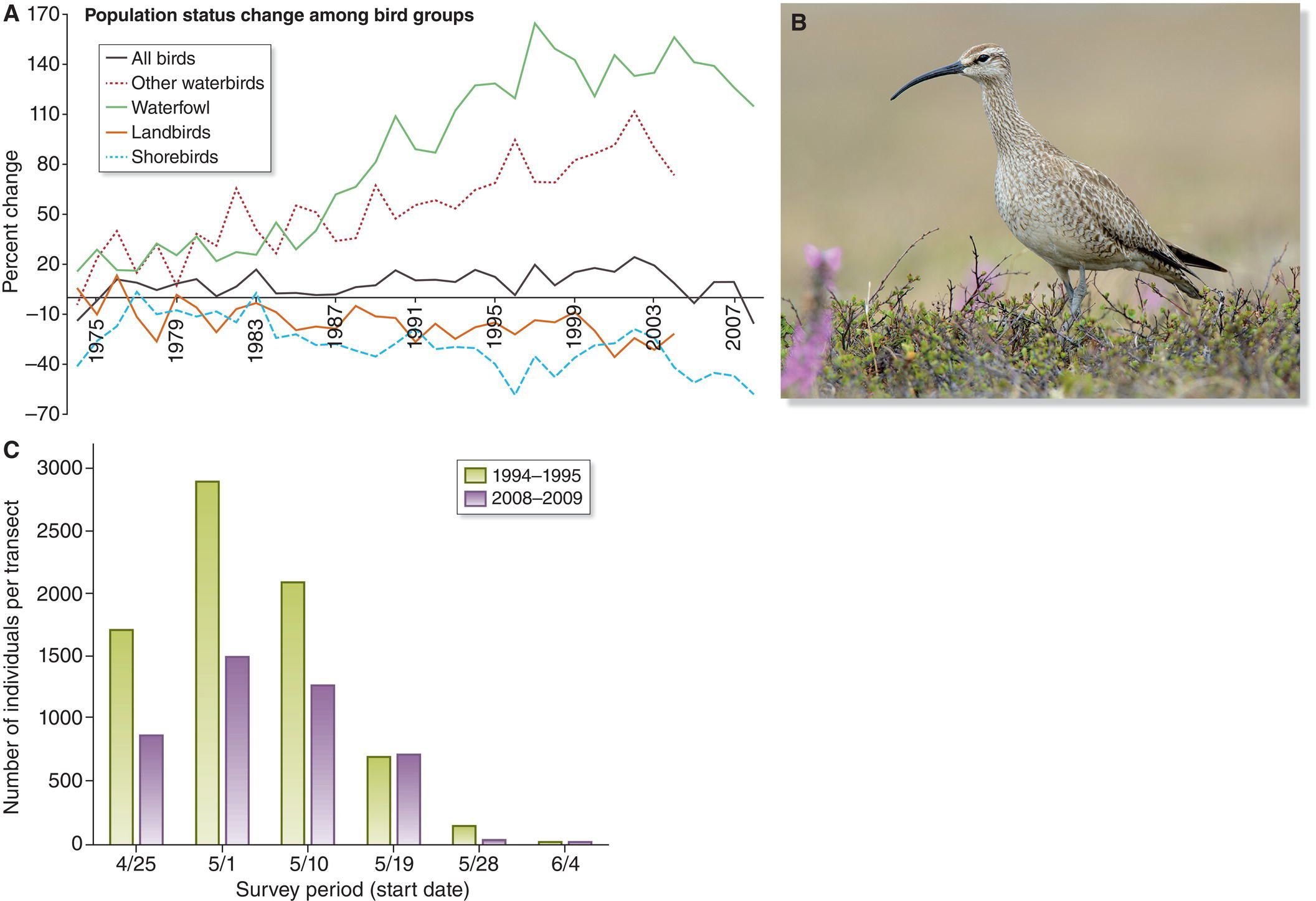
Fig. 15.31 Shorebirds are in worldwide decline. (A) Compared with other bird groups, migratory shorebird populations are declining most severely. (B) The Whimbrel (Numenius phaeopus) is one shorebird species facing population declines, although the reasons remain ambiguous. (C) Surveys of Whimbrels conducted along Virginia Beach (USA) transects in the 1990s (green bars) compared with the 2000s (violet bars) show significant declines.
(A, adapted from North American Bird Conservation 2012. B, photograph by Gerrit Vyn. C, from Watts and Truitt 2011. Reproduced with permission from the Waterbird Society and authors.)
15.5.11 Genetics and the extinction vortex
When populations are reduced to very small sizes, they face risks not only from environmental fluctuations and chance perturbations but also from genetic effects. Because close inbreeding increases the chances that offspring will have low genetic diversity, the incest taboo is exceptionally strong among birds: individuals typically avoid pairing and breeding with members of their immediate genetic family.
Through many generations of sexual reproduction, genetic mutations and recombination permit the accumulation of vast pools of genetic diversity among bird populations that are relatively large. When a population becomes very small, three related factors cause genetic variation to decline. First, genetic drift occurs as a result of chance sampling errors during mating: some genetic variants, beginning with the rarest ones, disappear altogether while others increase in frequency. Second, the occurrence of new genetic variants declines, because fewer individuals exist in which new mutations can appear. Finally, inbreeding occurs more frequently, because breeding adults in a small population are more likely to be related to one another than in a large one. The resulting inbreeding depression is of particular conservation concern when endangered birds have been reduced to tiny population sizes. The Stitchbird (Notiomystis cincta), recently discovered to represent its own distinctive family (Notiomystidae), was once common across the vast North Island (New Zealand) but was extirpated everywhere except on small Little Barrier Island. Recent translocations intended to establish several new populations were severely hampered by hatching failure and poor survival associated with inbreeding (Brekke et al. 2010).
Extreme reduction in genetic diversity following a population reduction, known as a genetic bottleneck, constrains the population’s ability to adapt to changing environmental conditions and progressively lowers average fitness by reducing the birth rate and increasing the death rate. One thing to remember is that not all birds in a population will reproduce successfully during their lifetimes; therefore, the number of individuals passing along their genes is always lower than the total number of individuals alive at any one time. Thus, extremely small populations with even fewer active breeders may be subject to an ever‐worsening spiral, known as the extinction vortex (Fig. 15.32). As each succeeding generation fails to replace itself fully, more alleles are lost, and the population becomes ever more genetically homogeneous, further lowering reproduction and survival. All these genetic effects are compounded by the ever‐worsening demographic risks as the population grows smaller and smaller.
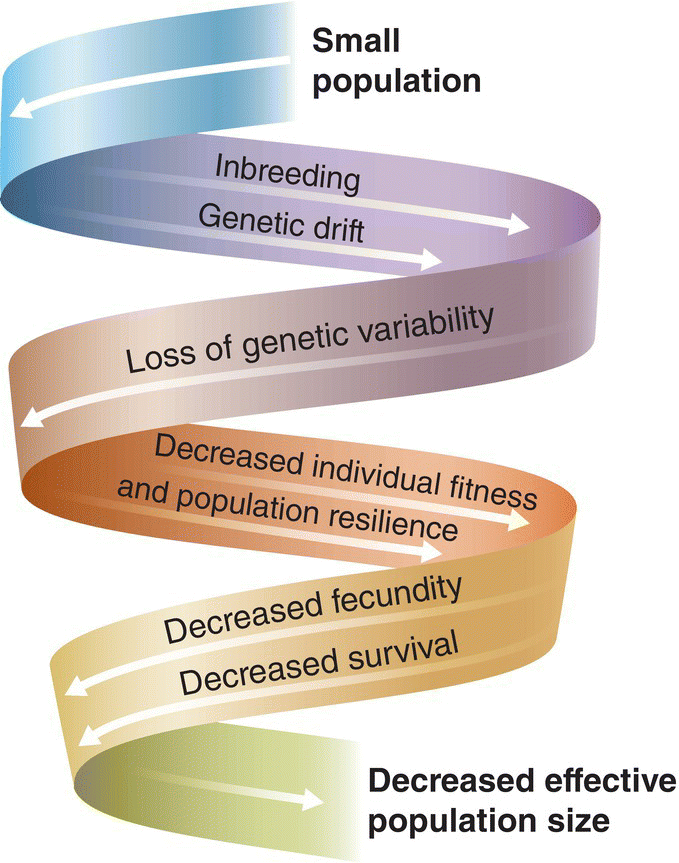
Fig. 15.32 The extinction vortex. Compounding negative effects on population demographics occur more rapidly in small populations, as overall genetic diversity steadily declines.
(© Cornell Lab of Ornithology, adapted from Krebs 2008.)
15.6 Avian population increases
Not all environmental changes cause population declines. Across eastern North America, for example, forest‐inhabiting birds such as the Northern Cardinal (Cardinalis cardinalis), Tufted Titmouse (Baeolophus bicolor), and Red‐bellied Woodpecker (Melanerpes carolinus) have increased and spread northward as humans increased the availability of winter food (sunflower seeds from bird feeders) and shelter (ornamental plantings), and as landscapes once cleared for farmlands have reverted to forest. A century earlier, populations of birds associated with farmlands and successional habitat, such as the Bobolink (Dolichonyx oryzivorus), Brown Thrasher (Toxostoma rufum), and Eastern Towhee (Pipilo erythrophthalmus), surged with the clearing of eastern forests. These open‐land birds now are declining in parallel with the increases in tree cover.
Introduced species often undergo major population expansions after long periods of being restricted to a small area, as birth rates and death rates adjust to the new environment. Beginning in the early 1900s, Eurasian Collared‐Doves (Streptopelia decaocto) suddenly began expanding from their original Mediterranean range, eventually colonizing most of Europe and Asia before being introduced to the Bahamas in the 1970s. From there, the species continued a spectacular expansion across much of North America (Fig. 15.33). This species represents a case in which birth rates have exceeded death rates for a long time across its entire range.

Fig. 15.33 The invasion of Eurasian Collared‐Doves (Streptopelia decaocto). Over the past several decades, this invasive species has spread from Europe into the Bahamas, through Florida, the USA, and Canada. Currently it is spreading from Mexico into Central America. The maps represent sightings across North America from 1998 to 2013 as logged into the eBird citizen science portal. The probability of detection is higher in areas with darker shades of purple.
(Maps © Cornell Lab of Ornithology. Photograph by Horia Varlan, https://en.wikipedia.org/wiki/File:Streptopelia_decaocto_‐balcony_‐two‐8.jpg. CC‐BY‐2.0.)
Increases in certain species can have cascading ecological consequences affecting many other species. During the late twentieth century, Snow Goose (Chen caerulescens) numbers across arctic Canada began burgeoning, with some populations increasing at the phenomenal rate of 8% per year (US Fish and Wildlife Service 2012). Large‐scale farms in the migration and wintering areas of North America provided huge quantities of remnant rice, corn, and other grains left on the ground after harvesting (lowering goose death rates), and adult geese arrived on their breeding grounds in such excellent condition that they could breed earlier and lay larger clutches (increasing goose birth rates). These human‐induced environmental changes caused a Snow Goose population explosion. Ironically, this change threatens many other tundra‐breeding bird species because of an ecological chain reaction: Snow Geese forage by snipping and tugging up the roots of native tundra vegetation, and their numbers have grown so large that vast areas of once‐verdant tundra are being reduced to sterile, hypersaline (extra salty) dirt. Consequently, certain migrant shorebirds such as Hudsonian Godwits (Limosa haemastica), Lesser Yellowlegs (Tringa flavipes), and American Golden‐Plovers (Pluvialis dominica) are losing breeding habitat, and their numbers in the affected areas appear to be declining.
15.7 Conservation solutions
Accomplishing lasting conservation in real‐world settings is not easy. Conservation is a time‐consuming, long‐term, often messy effort requiring many steps, most of which are social, political, and economic. Accomplishing the steps necessary both to reverse population declines and to recover the underlying ecosystems almost always requires addressing the human social and economic realities that caused the problems in the first place. Indeed, engaging all stakeholders in the process of accomplishing lasting conservation usually is much harder than identifying and resolving the biological issues. Most aspects of this human element in conservation are outside the scope of this chapter, but it must be recognized that fully integrated conservation solutions are extraordinarily complex undertakings in balancing the needs of both humans and the natural world (Kareiva and Marvier 2011).
Successful conservation depends on good scientific information. This section summarizes how principles of conservation biology are applied in efforts to prioritize, stabilize, restore, and recover declining bird populations around the world. Important steps include: (1) discovering and accurately documenting specific population declines; (2) setting priorities for research and action; (3) understanding the biological underpinnings of the problem; (4) deriving steps that could resolve the problem for the long term; (5) identifying the relevant socioeconomic contexts, opportunities, and constraints; (6) implementing the prescribed steps; (7) measuring the outcomes; and (8) repeating as necessary until desired conditions are achieved and demonstrated to be lasting.
Given these general approaches, no universally effective solutions exist for conservation issues. Countries and cultures may differ in the conservation strategies they regard as being the most useful and successful.
For birds, the most commonly identified problems involve population declines, but resolving every genuine problem is impossible, so prioritization is crucial. Understanding the biological underpinnings of conservation challenges means applying basic principles of genetics, evolutionary biology, life history theory, population biology, community ecology, animal behavior, ecological modeling, and computer simulations to interpret the decline and prescribe steps to reverse it. The necessary steps are as diverse as the myriad reasons for decline and include efforts such as reducing illegal trade and hunting, establishing reserves to protect native habitats and watersheds, controlling introduced mammals, promoting sustainable harvesting of tropical forest resources, and developing ecotourism in and around protected areas as an incentive for local and indigenous people to maintain these habitats in their natural state. Once such steps are undertaken, follow‐up monitoring is essential, because the prescribed steps may be incorrect or insufficient, or because environmental conditions and threats might continue to change.
15.7.1 Monitoring populations
Not every population decline represents a problem. For example, dramatic declines in certain forest songbirds on long‐term study plots in New Hampshire (USA) between 1969 and 1998 resulted not from ecosystem degradation or alarming demographic imbalances, but rather from steady forest succession following the end of the region’s agricultural era. It turns out that other species in this area that use older forests were increasing in number for exactly the same reason, but it took careful analysis of a 30‐year dataset to comprehend the reasons for these changes (Holmes and Sherry 2001). This example illustrates a vital ingredient for bird conservation the world over: identifying population declines that warrant conservation attention often depends upon the availability and dispassionate statistical scrutiny of carefully gathered long‐term data (Chapter 13).
15.7.2 Setting priorities
Limited resources make it impossible to devote equal attention to every conservation problem in the world. Priority setting is essential. Several different approaches exist by which to compare bird species and/or their habitats in terms of their conservation priority. No one of these approaches is necessarily better than the others, so it pays to consider all of them when evaluating where to invest time, energy, and resources.
Taxonomic distinctiveness
Species that are taxonomically distinct (such as species that are the sole members of their genus, family, or order) have especially high conservation priority, because their disappearance would mean permanent loss of a globally unique evolutionary lineage. An excellent example is the Kagu (Rhynochetos jubatus) (Fig. 15.34), a distinctive terrestrial bird restricted to New Caledonia in the southwest Pacific. This species is an ancient evolutionary relict, and its relationship to its closest living relative—the Sunbittern (Eurypyga helias) of the American tropics—likely dates to the period when the southern continents were united (Chapter 2). The only member of its family (Rhynochetidae), the Kagu is highly endangered as a consequence of habitat loss and depredation by dogs.

Fig. 15.34 The Kagu (Rhynochetos jubatus) is a taxonomically distinctive species. Losing relictual species like the Kagu, endemic to New Caledonia, means the loss of an entire evolutionary branch on the avian tree of life.
(Photograph by Tony Palliser.)
Genetic distinctiveness
The advent of DNA‐sequencing technology revolutionized our capacity to detect the previously hidden genetic distinctiveness of local populations, often resulting in their new recognition as fully separate species. In many cases this recognition elevates the conservation priority placed on the newly elevated species, especially when it represents a flagship for a distinctive ecological community. For example, the California Gnatcatcher (Polioptila californica), only recently recognized as a full species, instantly galvanized attention, money, conservation planning, and controversy as an indicator species for the coastal sage scrub habitat that is imperiled by explosive human population growth throughout southern California (USA) (Chase et al. 2001).
Endemism
Concentrations of locally endemic bird species represent regions of extremely high conservation priority, where investment can save multiple species with no other geographic opportunities for protection. Some of the most distinctive and seriously threatened areas of endemism receiving worldwide conservation attention include Madagascar, the Hawaiian Islands, various Indonesian archipelagos, the Atlantic forests of southeastern Brazil, and the fynbos shrublands of the Western Cape of South Africa.
Hotspots
Regions of high overall biological diversity, or biodiversity hotspots (Myers et al. 2000) (Fig. 15.22), command high conservation priority because of the sheer number (richness) of species that can benefit from a given unit of investment. Birds are good indicators of overall biodiversity in most terrestrial habitats. Tropical forests rank highest in species richness, but many non‐forest hotspots are of particular importance because they have undergone proportionally greater habitat loss than forests have.
Endangerment (vulnerability)
Of all the criteria for prioritizing conservation investment, arguably the most important is the degree to which a species or ecosystem faces the threat of disappearance. Categories of endangerment are defined in all official lists of species at risk, such as the US Endangered Species List and the IUCN Red List, and the very term “Critically Endangered” all but demands global attention for the 147 species currently bearing this rank (BirdLife International 2013).
Indicators, flagships, and umbrellas
Birds are beacons of broader environmental conditions. Many kinds of birds serve particularly well as indicator species for ecological communities, because increases or decreases in these species correlate with the health and status of hundreds of other species of animals and plants. California Gnatcatchers were viewed as good indicator species for coastal sage scrub in California (USA) and Baja California (Mexico); hence they were given special significance in a landmark multi‐species habitat conservation plan to protect this ecosystem. The Northern Spotted Owl (Strix occidentalis) is an indicator species that is especially charismatic to humans; such species are called flagship species because they draw our collective attention and attract special resources toward conservation of their habitat. Umbrella species are those whose successful conservation would ensure the long‐term security of a host of others species or whole ecological communities, by virtue of their special needs. The critically endangered Great Philippine Eagle (Pithecophaga jefferyi)—the largest raptor in the Asia–Pacific region—is both flagship and umbrella for the rapidly disappearing, endemic tropical evergreen forests of the Philippine Islands (Fig. 15.35). Because of its large home range size, low population density, and dependence on large mammals as prey, this charismatic eagle requires very large and ecologically intact forest tracts to remain secure in the presence of a growing human population. Ensuring its protection would secure countless other less noticeable species, including many birds.

Fig. 15.35 Great Philippine Eagle (Pithecophaga jefferyi). This species is the world’s largest and most endangered eagle. Due to habitat destruction, hunting, and low reproductive rates, its continued survival depends upon nation‐wide conservation actions to protect remnant tracts of tropical forest.
(Photograph by Kike Arnal © Cornell Lab of Ornithology.)
Ecological role
Some bird species play such pivotal ecological roles that their decline or elimination would have a disproportionate impact on community or ecosystem function. Examples include parrots, pigeons, and hornbills, which disperse seeds and nuts across their respective landscapes, and in so doing provide such a vital service to shrubs and trees that the plants and the birds have coevolved and are now dependent on one another. The loss of intact parrot communities because of capture and illegal international trade (Fig. 15.36) therefore threatens not only the birds but also the long‐term health of their forest habitats.

Fig. 15.36 Illegal capture and trade of parrots worldwide. Illegally caught in the wild, (A) Burrowing Parakeets (Cyanoliseus patagonus) of Patagonia and (B) Turquoise‐fronted Parrots (Amazona aestiva) of Brazil—in addition to many other species—are shipped long distances in poor conditions to be sold as pets. (C) These nestlings were confiscated en route after being robbed from their nests. With no way of returning them to their wild nests, these individuals became part of a Brazilian public education program. Most parrots captured illegally either die or enter the worldwide pet trade.
(Photographs by: A and B, © World Parrot Trust. All rights reserved; C, Gláucia Seixas, Blue Fronted Amazon Project–Brasil, www.fundacaoneotropica.org.br.)
Utilitarian role
The importance of native bird species in the daily lives of humans might not have ranked highly in conservation priority setting until the recent, sudden, and dramatic declines in the vulture community of India and southeastern Asia (Box 15.03). The functional disappearance of an entire scavenger community in a region with the world’s highest human densities reminds us of the importance of intact native systems in supplying ecological services to humans. In this case, loss of the native vultures even threatens centuries‐old funeral and burial traditions of groups such as the Zoroastrian Parsis. Perhaps the best example of establishing conservation priorities based on the economic impact of native birds is the tri‐national, public–private partnership represented by the North American Waterfowl Management Plan. This agreement coordinates wetland protection across Canada, the USA, and Mexico to enhance migratory waterfowl. It originally was motivated by the economic impact of sport hunting and more recently was strengthened by the even greater public investment in passive wildlife viewing. Many non‐profit organizations raise millions of dollars annually to further the conservation of wildlife habitats based on their economic and cultural importance.
Gap analysis
With the advent of sophisticated computer mapping and geographic information system (GIS) technology, conservation scientists can overlay distribution maps of threatened and endangered species with maps of protected areas. This crucial exercise is known as gap analysis, because its goal is to reveal important gaps in landscape protection that leave certain species with few or no refuges under conservation management. Gap analysis provides a useful tool for prioritizing the establishment of new protected areas, from local to global scales.
15.7.3 Population viability analysis and metapopulations
Small populations of virtually any species eventually will become isolated from the rest of their kind. Random population fluctuations, depressed reproduction and survival resulting from edge effects, and unpredictable catastrophic disease or weather events make small populations highly vulnerable to extinction. The general observation that all bird populations naturally fluctuate through time is of particular relevance to their conservation and management, because a central goal of avian conservation programs is to reduce the probability that a bird population will decline to zero—that is, go extinct. A typical (although arbitrary) goal may be to sustain a population that has a 95% chance of persisting over a long period of time, for example 100 years. Such a metric is known as a minimum viable population (Shaffer and Samson 1985).
How does one estimate the probability of a population going extinct over the course of a century? To provide some precision in measuring population vulnerability, conservation biologists in the 1980s and 1990s developed population viability analysis (PVA) as a tool for predicting the likelihood of extinction for populations of different sizes. The PVA is a computer simulation of the trajectory and fate of populations undergoing annual cycles of reproduction, dispersal, and mortality, and represents an exercise that today serves as a cornerstone of conservation biology. The simplest PVAs are single‐population models, which simulate numbers through time, based on assumed initial and maximum allowable population sizes, key demographic variables (especially age‐specific birth rates and death rates), and the degree and nature of annual variability in these demographic variables. Typically, each run of a PVA model is allowed to proceed until the simulated population either goes extinct or survives to an arbitrary end‐point, perhaps 500 or 1000 years in the future. Runs are repeated many times using identical starting population sizes and demographic assumptions to gain an understanding of the range of possible outcomes, and then the process is repeated for different starting population sizes. Typical outputs are various forms of graphic representation of extinction probabilities or mean projected population sizes as a function of both time and starting population size (Fig. 15.37). In most real‐world cases, population sizes are limited by the sizes of extant habitat patches, so the PVA is used to set minimum requirements for how big a habitat preserve must be to ensure an acceptably low probability that a target species within it will go extinct over a given time period (often 100 years).
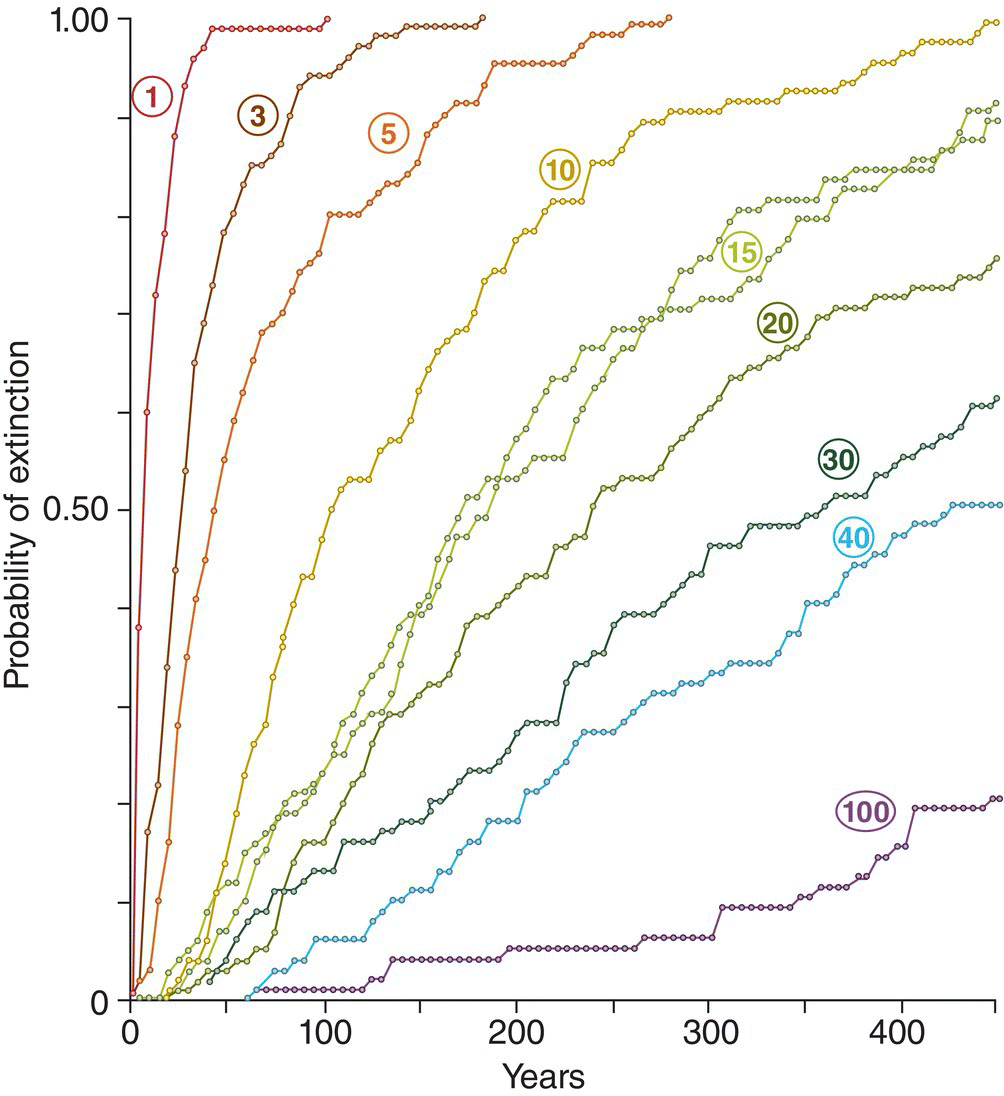
Fig. 15.37 Population viability analysis. Here various starting population sizes (in circles beside each plotline) of the threatened Florida Scrub‐Jay (Aphelocoma coerulescens) were used in a simulation model to determine extinction probabilities over time, given well‐documented demographic information about the species’ population biology. In each set of simulations, each population size was repeated for 100 runs, yielding predicted extinction probabilities through time.
(From Fitzpatrick et al. 1991. Reproduced with permission from Florida Fish and Wildlife Conservation Commission.)
A PVA conducted for Lammergeiers (Gypaetus barbatus) shows the value of this method. Lammergeiers were exterminated from the Alps of Europe in the late nineteenth century, but recently have been reintroduced there through the release of captive‐bred young birds. By 2006, the reintroduced population had increased to nine breeding pairs. Based on a population viability analysis involving age‐specific survival probability and observed fecundity rates, Michael Schaub et al. (2009) showed that the population was likely to increase in the future even if no further captive birds were released. Such information provides data critical for making informed decisions about where to invest future conservation efforts and, in this case, suggested that efforts should be redirected from captive breeding to demographic surveillance of the wild population.
Today, most PVAs involve complex models that integrate factors such as density dependence, habitat succession cycles, and genetic considerations. Many modern PVAs incorporate advances in which the hypothetical population is modeled as a collection of separately tracked individuals, each having its own lifespan and reproductive history. In the most realistic models, individuals disperse across the simulated landscape according to a set of rules, which themselves are subject to explicit assumptions built into the model. Therefore a common challenge for field studies of target species is to document when and how individuals disperse between birth and breeding.
Of course, even if a bird population goes extinct locally, it does not necessarily mean the end of the line for that species, because nearby conspecific populations potentially could recolonize the empty site through dispersal and thereby “rescue” the population in that habitat patch. Indeed, it is now recognized that one of the most important demographic processes in natural bird populations is the exchange of individuals among relatively discrete subpopulations. A network of populations that have some level of connectivity via dispersal is termed a metapopulation.
The metapopulation concept recognizes that many bird populations in the real world are subdivided into more or less discrete subpopulations, each occupying a patch of suitable habitat within a larger area of poor or wholly unsuitable matrix habitat. Each suitable habitat patch may be large enough to accommodate a potentially independent population of birds, but except in the very largest patches, local extinctions followed by recolonizations still can occur simply because of demographic fluctuations. Metapopulation modeling is especially useful in conservation planning for species that are highly susceptible to local extinction because they breed in naturally patchy or artificially fragmented habitats. For example, the Golden‐cheeked Warbler (Setophaga chrysoparia) breeds only in the state of Texas (USA) in mature oak‐juniper forests, a specialized breeding habitat that has always been naturally patchy. The configuration of appropriate oak‐juniper patches further changes over time as individual patches burn in both natural and human‐caused fires, becoming poor warbler habitat until they regenerate into mature forest. Metapopulation models for the Golden‐cheeked Warbler therefore must take into account factors like the size of oak‐juniper habitat patches, their likelihood of persistence as appropriate habitat into the future, the number and reproductive success of the warbler pairs breeding in each patch, and the dispersal of warblers among patches (Alldredge et al. 2004; Horne et al. 2011) (Fig. 15.38).

Fig. 15.38 Habitat loss and conservation planning. (A) The Golden‐cheeked Warbler (Setophaga chrysoparia) is an endangered species that prefers to breed in large patches of mature oak‐juniper forest. (B) Habitat alteration from 1999 through 2011, where the areas shown in red no longer support breeding warblers and green indicates areas in which the species has persisted, and (C) the general decline of suitable breeding habitat in eight monitored areas, demonstrates that landscape‐scale planning for conservation of remaining suitable habitats is vital.
(A, photograph by Gil Eckrich. B and C, from Duarte et al. 2013. CC‐BY‐SA 3.0.)
The related concept of source–sink dynamics recognizes that even within more or less continuous habitat, patches may differ from one another in size, topography, resource abundance, or predator communities, so that factors affecting local population density or growth rate are not necessarily the same from patch to patch. Therefore, bird populations in different patches may differ from one another in overall productivity. In a source population more offspring are produced than can be accommodated in that patch, whereas sink populations experience high mortality or low reproduction and will not produce enough young to replace dying individuals. Source populations tend to export dispersing birds, which may settle in less productive patches that have vacant space for immigrants to settle. Dispersers also can colonize uninhabited patches that either experienced a local extinction or became newly suitable because of succession, fire, or some other change in habitat.
Even though flying birds have the capability to disperse widely, avian populations often are subdivided by the patchiness of their habitat. This is especially true within modern landscapes where humans have fragmented many habitats that formerly were continuous over large geographic areas. Indeed, metapopulation dynamics may be particularly important in populations of many bird species in areas—from islands to tropical forest fragments—where small patches of habitat support remnant bird populations.
Landscape structure is likely to have a strong effect on the degree of independence or connectivity of different subpopulations in a metapopulation. In many cases connectivity between habitat patches is enhanced if they are linked by habitat corridors, or strips of suitable habitat that connect two or more larger patches. Although corridors generally are thought to facilitate the dispersal of individuals from one patch of habitat to another, this idea rarely has been tested. Douglas Levey et al. (2005) used small (approximately 1 hectare) clear‐cuts within a matrix of a South Carolina (USA) pine forest to test the importance of corridors to the movement of Eastern Bluebirds (Sialia sialis). They tracked the birds in an innovative way, using seeds marked with fluorescent dyes that were easily detected at night to find the remains of seeds eaten and then defecated by the birds. They found that seed dispersal, and thus movement of the birds themselves, was strongly facilitated by the existence of a narrow corridor connecting two of the clear‐cuts, primarily because the bluebirds tended to follow habitat edges as they moved through the landscape.
15.7.4 Managing exploitation
Perhaps the most complete recovery of any bird in the world is that of the Wood Duck (Aix sponsa), once hunted so extensively across eastern North America that by 1900 ornithologist Joseph Grinnell, editor of Forest and Stream magazine, wrote “Being shot at all seasons of the year [Wood Ducks] are becoming very scarce and are likely to be exterminated before long.” Instead, this beautiful duck became the first clear beneficiary of regulated hunting. In 1918, the hunting season for Wood Ducks was completely closed. Thanks to its high reproductive rate, by the 1930s the Wood Duck had recovered across much of its former range, and by 1941 its numbers were high enough that hunting was permitted broadly again. By the 1960s, population estimates ranged between 1 and 2 million, and during the 1970s and 1980s the Wood Duck began spreading into previously unoccupied areas of the Great Plains, northwestern Canada, and northern Mexico (Hepp and Bellrose 1995).
The early phase of the Wood Duck’s recovery depended on complete cessation of hunting as an extra source of mortality for several decades. This ban was followed by careful population monitoring by wildlife personnel and strict management of hunting limits throughout the USA and Canada. One wonders what North America would be like today if the same management strategies had been imposed for Passenger Pigeons (Ectopistes migratorius) only a few decades earlier.
15.7.5 Reserve design
Few species can persist if their range is reduced to a single patch of habitat, especially if that patch is small. The key to conserving rare species and vanishing ecological communities is to secure habitat reserve networks that are designed to accomplish long‐term protection, ideally by securing multiple local populations and permitting movements among them. Single‐population modeling helps us project the minimum size required to reduce within‐patch extinctions, and metapopulation modeling can help estimate extinction probabilities for alternative network configurations. Such exercises have produced a series of general rules that guide the ideal design of reserves and reserve networks (Fig. 15.39). The following are the most important of these rules:
- Bigger is better, because larger reserves contain larger total population sizes of birds, reducing vulnerability to stochastic extinctions.
- Round or square is better than long and thin, to minimize the proportion of the bird population that is subject to edge effects.
- Create multiple reserves whenever possible to reduce the chances that a catastrophe could wipe out an entire species.
- Ensure at least one large reserve in the network to provide a reliable source of dispersers to recolonize smaller patches after local extinctions.
- Minimize distances among reserves to facilitate local movements of birds among patches and reduce genetic isolation within them.
- Add “stepping stones” and “corridors” between reserves, because these small pieces of habitat often greatly facilitate the movement of birds among reserves, thus allowing a fragmented reserve system to act more as if it were larger and more continuous.
- A two‐dimensional landscape configuration is better than a linear one because it promotes opportunities for dispersal and recolonization among all habitat patches.

Fig. 15.39 Guidelines for habitat reserve networks. Several widely accepted rules guide the ideal design of reserves and reserve networks, in order to maximize their capacity for preserving target species or regional biodiversity.
(From Diamond 1975. Reproduced with permission from Elsevier.)
Protecting networks of habitat reserves by securing and properly managing islands of habitat remains the single most important means for protecting the world’s rarest birds and most endangered ecological communities. The Brazilian Atlantic forest, for example, contains one of the highest concentrations of locally endemic birds on earth, but these birds also are among the most endangered because of habitat loss. Comprehensive analysis of remnant patches and protected areas shows that only 11.7% of the original forest remains, and fully 83% of this remainder exists in tracts that are 50 hectares or smaller in size (Ribeiro et al. 2009). Clearly, protecting the largest remaining tracts is essential, and three of these contiguous tracts contain more than 13% of the remaining forest. With more than 40% of the remaining forest located more than 50 kilometers from its nearest protected nature reserve (Fig. 15.40), it is equally crucial to identify and protect the smaller tracts that are the most vital corridors and stepping stones connecting the larger reserves.
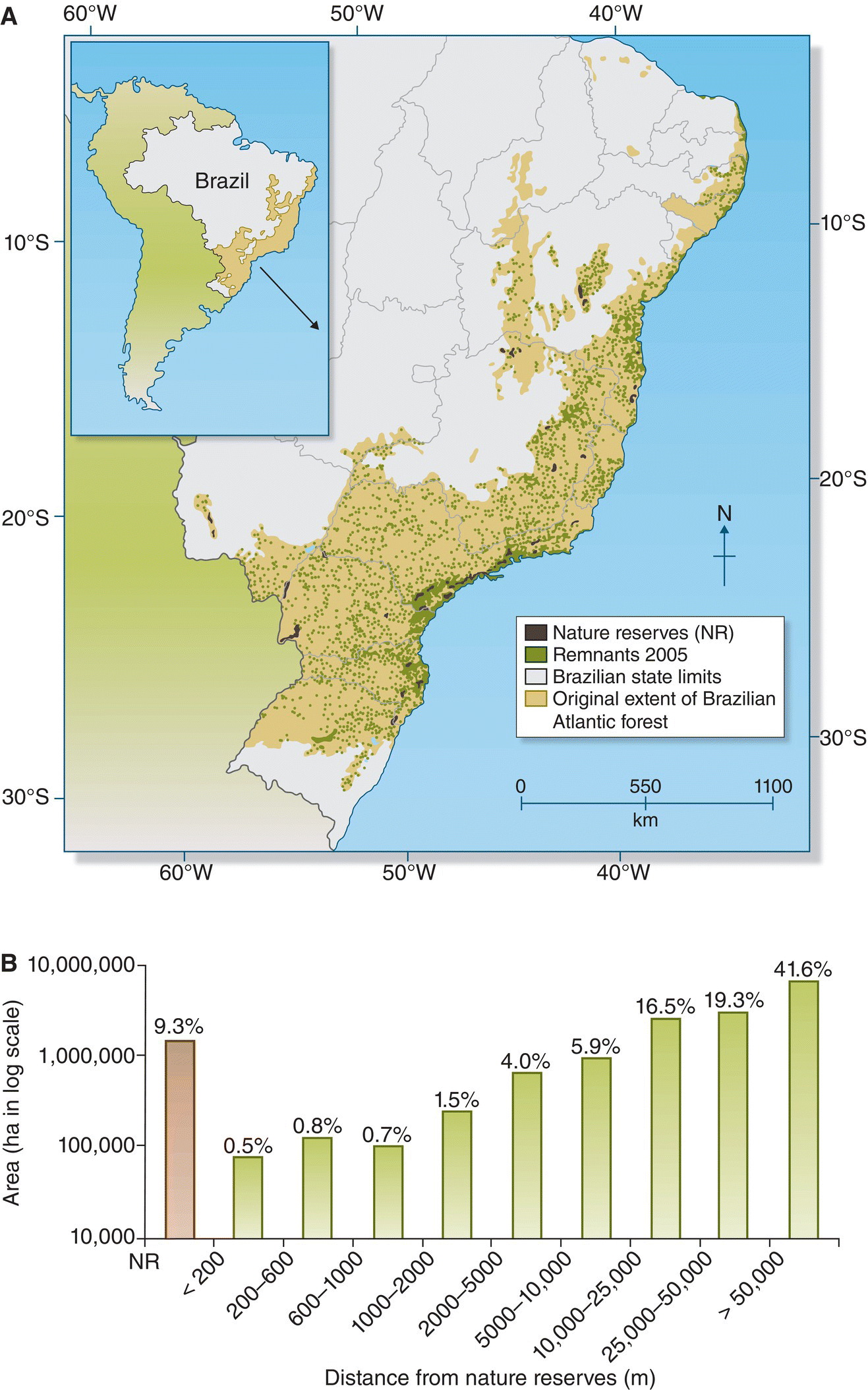
Fig. 15.40 Remnant Brazilian Atlantic forest. (A) Only 11% of the original Brazilian Atlantic forest (tan‐shaded area) remains. Nature reserves are shown in black and remnant patches of forest in green. (B) The area (height of bars) and percentage (given above bars) of remnant Atlantic forest patches are shown for distinct classes of distances from nature reserves. Note that over 40% of remnant patches occur more than 50,000 meters from the nearest nature reserve, leaving them isolated and vulnerable to species loss without recolonization.
(From Ribeiro et al. 2009. Reproduced with permission from Elsevier.)
15.7.6 Genetic augmentation
Close inbreeding and genetic bottlenecking probably explain why some of the most endangered bird species begin failing to breed successfully as they reach critically low numbers. In the Hawaiian Crow (Corvus hawaiiensis), for example, the last few pairs of wild individuals failed to fledge any young between 1991 and 2001, when the last wild pair disappeared (Fig. 15.41). A number of the eggs they produced appeared to be infertile when examined after the breeding season, and the inbreeding hypothesis was reinforced when DNA fingerprinting of the small captive population revealed the survivors to be unusually similar to one another genetically. To maximize successful reproduction within the captive flock, project managers did their best to pair individual crows with mates as genetically dissimilar as possible. Analysis of genetic similarity, plus carefully tracking of the pedigrees of all breeders and their offspring, allows keepers to avoid sib–sib or parent–offspring pairings and also to promote pairings likely to keep the existing genetic diversity from declining further (Hoeck et al. 2015). This pairing is accomplished by keeping a studbook, now a standard practice in zoos around the world and one that is especially important in captive‐rearing programs involving rare birds. This process has been highly successful for the captive flock of Hawaiian Crows, which grew from only 12 birds in the early 1990s to 114 by 2014.
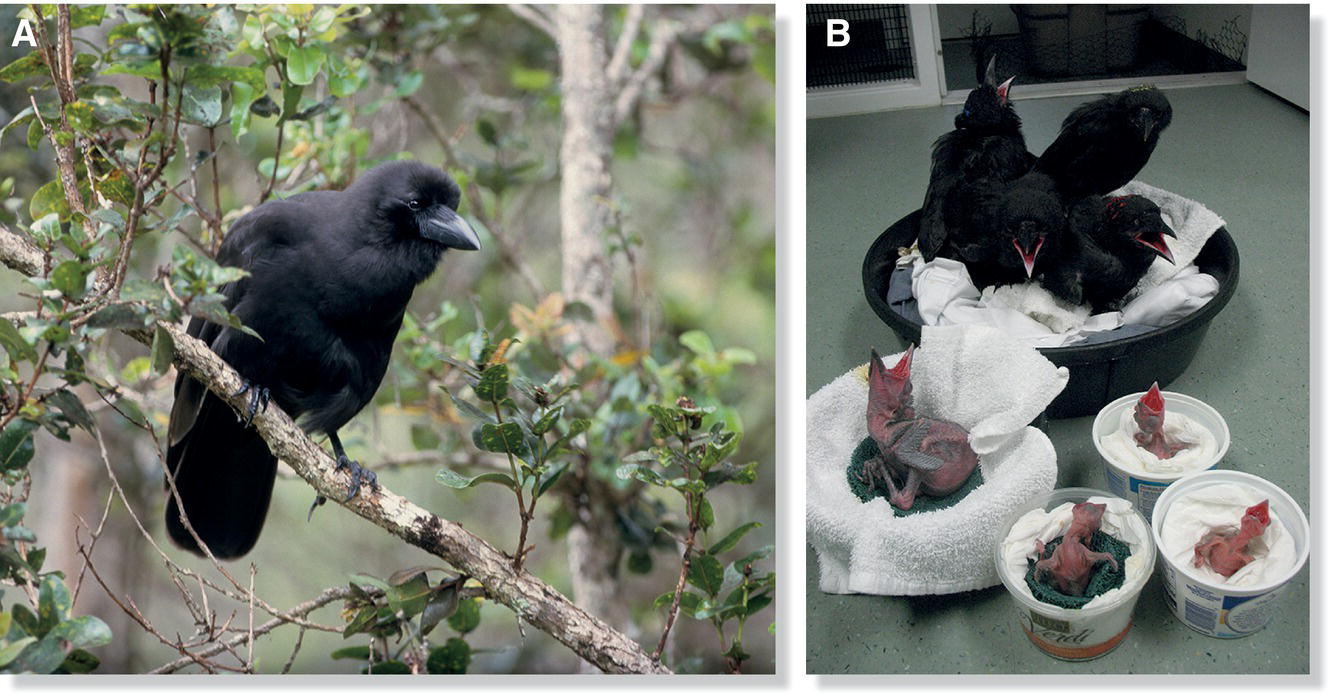
Fig. 15.41 Captive rearing to recover endangered species demands careful genetic record‐keeping. (A) The few remaining Hawaiian Crows (Corvus hawaiiensis) are part of ongoing captive‐breeding programs aimed at eventual reintroduction into the wild. Paired birds must have minimal genetic similarity to reduce infertility of eggs and lethal birth defects in offspring. (B) Successfully hatched young birds are potential breeders for future generations, providing a glimmer of hope for this critically endangered species.
(Photographs: A, by Jack Jeffrey Photography; B, courtesy of Zoological Society of San Diego © 2014 DSZG.)
Human intervention sometimes can relieve genetic bottlenecks within tiny wild populations, thereby avoiding the associated extinction spiral caused by the loss of genetic variability. A dramatic example of genetic rescue was seen in an isolated population of Greater Prairie‐Chickens (Tympanuchus cupido) in southern Illinois (USA) that was declining in the 1980s. By 1993, fewer than 50 individuals remained, and these possessed only about two‐thirds of the genetic diversity found in the larger populations in nearby Nebraska, Kansas, and Minnesota. Only about half of the eggs laid by the Illinois females were hatching, compared with 93% hatchability elsewhere (Westemeier et al. 1991), strongly suggesting inbreeding depression. When birds from other populations were experimentally introduced into the declining population, the hatching rate increased to pre‐bottleneck levels (Fig. 15.42), and the population was stabilized (Bouzat et al. 1998).
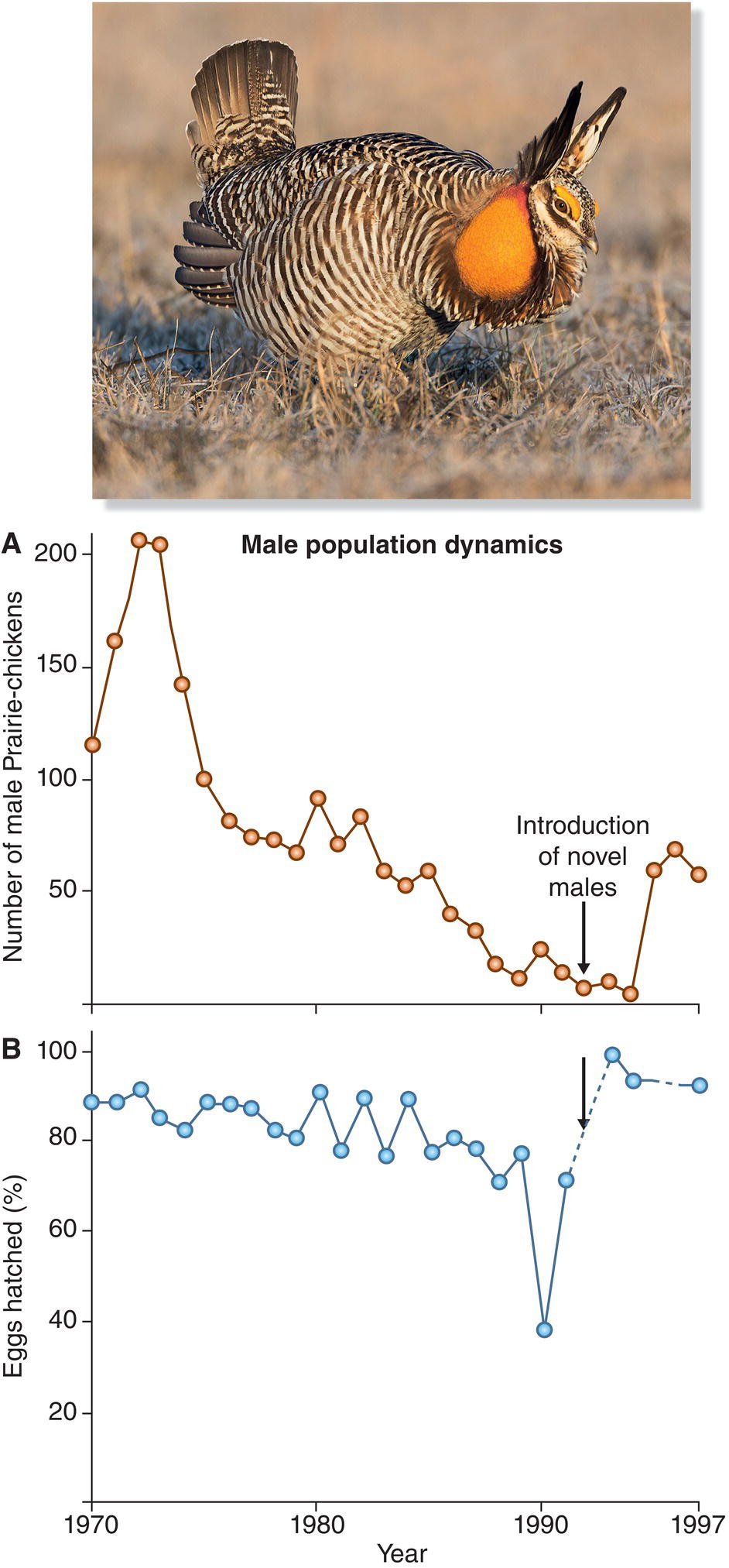
Fig. 15.42 Genetic rescue. (A) By the 1980s, a small isolated population of Greater Prairie‐Chickens (Tympanuchus cupido) in southern Illinois was declining, and fewer than 50 individuals remained in 1993. Biologists introduced males (indicated by arrows) from larger populations in an attempt to “rescue” the genetic diversity of the population. (B) One sign of a genetic bottleneck is egg‐hatching failure, a clear trend in the early 1990s. After genetic rescue, both hatching success and number of breeding males immediately began to increase.
(From Westemeier et al. 1998. Reprinted with permission from AAAS. Photograph by Jeff Dyck.)
Maintaining genetic variability in small populations across many generations is a difficult challenge. Recently, in a sobering review, one of the original architects of population viability analysis argued that species management in zoos is failing to achieve this goal for a large proportion of target species (Lacy 2013). On the other hand, captive‐rearing programs increasingly are being combined successfully with conservation efforts in the field, effectively blurring the distinction between in situ (field‐based) and ex situ (captivity‐based) conservation strategies. Some examples of these hybrid efforts are described in the following sections.
15.7.7 Captive rearing and reintroduction
Captive rearing to protect critically endangered animals began early in the twentieth century, with the breeding by European nobility of the last few Pére David’s deer (which were extinct in their native China) often cited as the first example. In the UK, the Wildfowl and Wetlands Trust was founded in 1946 to breed endangered waterfowl, and in 1959 a breeding program for a broad array of globally endangered birds and mammals was initiated at the Jersey Zoo, later called the Durrell Wildlife Park. In the 1960s biologists in the USA began collecting the eggs of wild Whooping Cranes (Grus americana) to establish a captive‐breeding flock in Patuxent, Maryland (Box 15.04), and in 1970 the Peregrine Fund was established to breed, rear, and release Peregrine Falcons (Falco peregrinus). Today the conservation practice of captive rearing is widespread and often embraced as an important mission of zoos and aviaries around the world. Broadly speaking, the goals of all such programs are similar: to use modern incubation and husbandry techniques, often supplemented with eggs collected from wild individuals, to increase the overall birth rate and juvenile survival rate of rare birds, resulting in an increasing population of healthy adults. Then, employing strategically designed, species‐specific release strategies, to use these captive‐reared individuals to establish or augment free‐ranging natural populations in appropriate habitat, while still maintaining genetically healthy stocks in captivity.
Dramatic success stories illustrate the conservation value of captive rearing and reintroduction, and some failures illustrate the most important pitfall. By 1972, when DDT and related compounds were banned from widespread use, the Peregrine Falcon had functionally disappeared from most of North America. Using eggs from tundra‐breeding subspecies, conservation biologists reared and began releasing young falcons from wooden boxes that served as artificial nests, constructed to provide human access from the rear for feeding. Soon, falcons were being hatched successfully from the convenient and predator‐free “cliffs” provided by urban skyscrapers across eastern North America (Chapter 13). Eventually, released birds began returning and pairing with one another, establishing new territories and new nest sites. The population recovered steadily, and in 1999, the Peregrine Falcon was removed from the US Endangered Species List. Recent population estimates suggest that the species has achieved or surpassed its pre‐1900 numbers.
Similar efforts began in the late 1970s to rescue the critically endangered Mauritius Kestrel (Falco punctatus) (Fig. 15.43) on the island where the Dodo (Raphus cucullatus) had gone extinct two centuries earlier. Habitat loss and pesticides had reduced this distinctive small falcon to only four individuals by 1974. Biologists removed the first eggs laid in several nests and supplemented the kestrels’ diet to promote their ability to lay replacement clutches, effectively doubling the population’s birth rate. Young birds were released back into the wild and supplied with food. The population recovered dramatically and currently numbers more than 800 individuals occupying essentially all of the remaining forest habitat on the island. The species’ long isolation on a small oceanic island probably explains why inbreeding has not hampered this recovery: deleterious genetic variants were likely already eliminated by the functionally perpetual “bottleneck” imposed by the small size of the island. Other species on Mauritius that appear to have escaped extinction thanks to captive breeding include the Echo Parakeet (Psittacula eques) and the Pink Pigeon (Nesoenas mayeri).

Fig. 15.43 Mauritius Kestrel (Falco punctatus). This species recovered from the brink of extinction in the mid‐1970s thanks to the timely intervention of biologists. Eggs were removed and raised in a captive‐breeding program, while parents raised their own replacement clutches in the wild. The population today is healthy and at carrying capacity in remaining forest habitats.
(Photograph by Sam Cartwright.)
Following a century‐long population collapse and predictions of imminent extinction, all the 21 remaining wild California Condors (Gymnogyps californianus) were taken into captivity between 1982 and 1987, thereby launching a successful captive‐breeding program. The first captive‐bred young hatched in 1988. Nestlings were fed by keepers hidden behind barricades, via hand puppets resembling condor heads to minimize imprinting on humans (Fig. 15.44). Experimental releases using Andean Condors (Vultur gryphus) as surrogates allowed biologists to hone techniques for tracking and monitoring released birds by practicing with a biologically similar but less endangered species—female Andean Condors were released into the wild, and later were recaptured and transported back to South America. Captive‐bred California Condors were first reintroduced in 1992, and by 2014 the total free‐flying population numbered 228 in four separate populations (Fig. 15.45), with an additional 193 in captivity. Despite this success, free‐flying condors continue to suffer excessive illness and death, mainly from lead poisoning caused by the ingestion of lead ammunition in mammal carcasses (Church et al. 2006). As a result, individuals throughout the range need to be recaptured regularly for veterinary treatment to remove lead from their systems.

Fig. 15.44 California Condor (Gymnogyps californianus) puppets help rear captive‐bred chicks. By using barriers and puppets to minimize imprinting on humans during captive rearing, the San Diego Zoo (USA) has successfully raised and reintroduced captive‐bred chicks into the wild.
(Photograph courtesy of Zoological Society of San Diego © 2014 SDZG.)
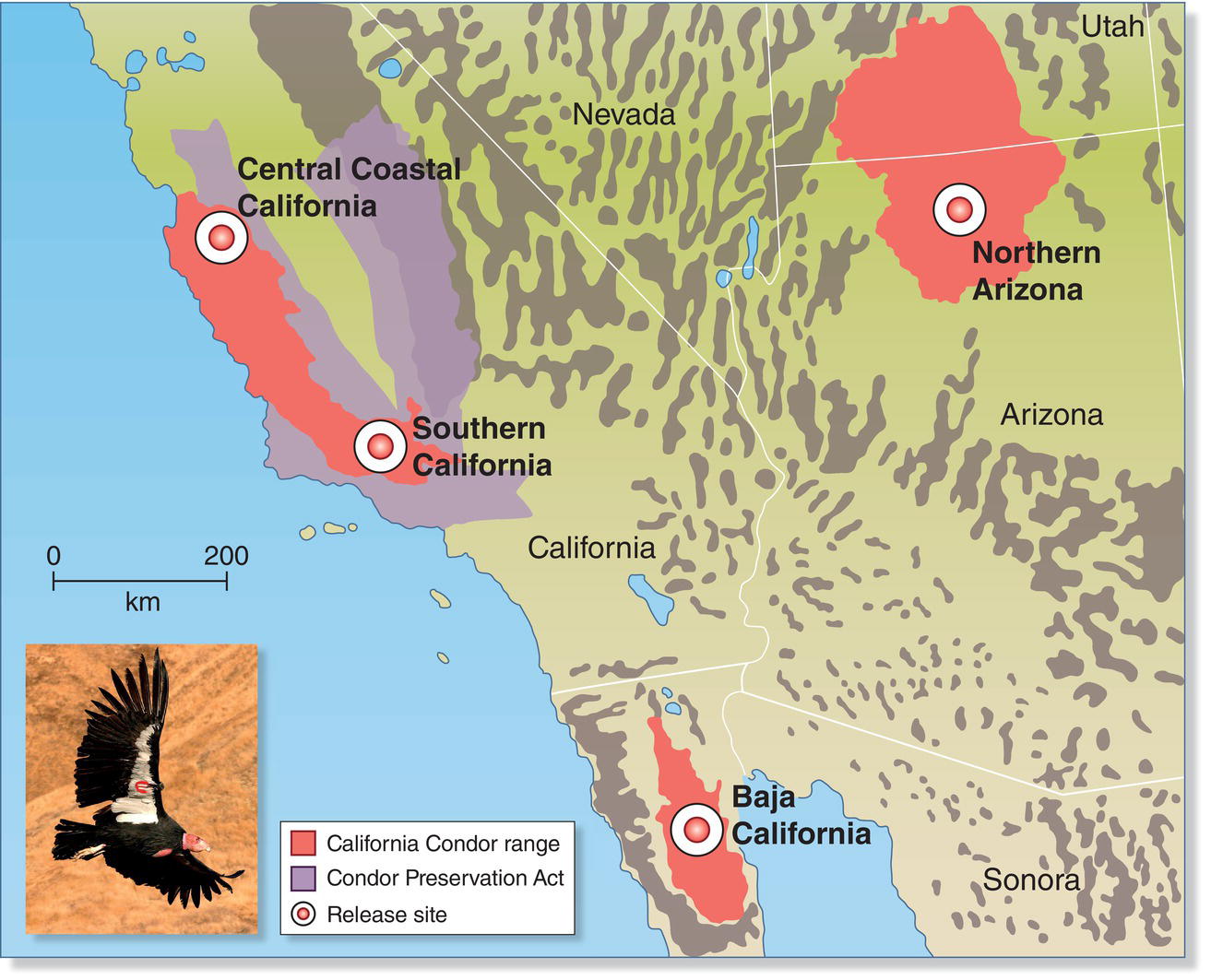
Fig. 15.45 Reintroduction of California Condors (Gymnogyps californianus). Although captive‐breeding programs have released individuals into the wild successfully, many birds become ill after ingesting meat from animals killed with lead ammunition.
(Photograph courtesy of US Fish and Wildlife Service/Pacific Southwest Region.)
For the California Condor, the original problem still has not been solved, and the population is growing only because of persistent augmentation from the captive‐breeding program (Walters et al. 2010). Failing to remedy the original problems can cause captive rearing and reintroduction to fail entirely. Expensive and well‐executed efforts during the 1990s to augment the dwindling wild population of Hawaiian Crows (Corvus hawaiiensis) with young birds raised in captivity failed because the habitat in the mid‐elevation Kona forests still suffered from the suite of challenges originally threatening the species. These threats included avian pox, avian malaria, introduced rats and feral cats (and associated toxoplasmosis), a degraded forest understory grazed by feral cattle, and—ironically—predation by the native Hawaiian Hawk (Buteo solitarius), itself on the US Endangered Species List but now thriving. Success of a new Hawaiian Crow reintroduction (tentatively scheduled for 2016) will depend upon controlling at least some of the continuing problems in the release zone.
15.7.8 Eliminating introduced mammals
Birds that evolved in the absence of grazing and/or predatory mammals are especially vulnerable to a vicious combination of threats from introduced mammals: habitat degradation from grazing and browsing, reduced nesting success caused by rat predation, and elevated predation on juveniles and adults by everything from rats (Fig. 15.46) and cats to mongooses and pigs (Box 15.05). Beginning in earnest in the 1980s and now rapidly gaining momentum all over the world, the eradication of introduced rats, goats, sheep, pigs, deer, and cattle from oceanic islands has begun to recover some of these unique ecosystems and reverse population declines among their birds. New Zealand is widely recognized as the world leader in rat eradication, having developed and applied a method using bait poisoned with the anticoagulant brodifacoum, which proved to be the most effective eradication agent (Taylor and Thomas 1989, 1993). In recent decades rats have been eliminated on dozens of New Zealand islands. In one of the largest eradication projects ever undertaken, Campbell Island, with an area of 113 square kilometers, was declared rat‐free in 2005, 5 years after the project began, and it now supports populations of several endemic New Zealand birds.

Fig. 15.46 Invasive mammalian predators. Numerous invasive predators have caused the decline and extinction of native avifauna. The black rat is infamous the world over for its skill at accessing and eating nestling birds.
(Photograph courtesy of Nga Manu Images.)
Off the coast of Baja California (Mexico), tiny Socorro Island harbors a diverse community of landbirds and breeding seabirds, including a number of endemic species and subspecies. Introduced sheep altered the vegetation composition and structure, leaving every one of the island’s specialties threatened, critically endangered, or extinct. Removal of sheep by the Mexican navy and replanting of some native shrubs and forbs have steadily improved the habitat, and the critically endangered Socorro Mockingbird (Mimus graysoni) appears to be recovering. The Socorro Dove (Zenaida graysoni), extinct in the wild since 1972, might be reintroduced if current plans to control the significant feral cat population move forward.
Ground‐nesting seabirds, so vulnerable to rat depredation on oceanic islands all over the world, now have been shown to respond rapidly to predator removal. On the island of Anacapa, off the coast of southern California (USA), Scripps's Murrelet (Synthliboramphus scrippsi) had declined severely for decades because of predation by black rats. During 2001 and 2002 all rats were eradicated via helicopter broadcasts of poison pellets. By 2005, nest‐site occupancy, nesting attempts, and hatching rates had increased substantially, and the nest‐depredation rate had dropped from 52% to 7% (Whitworth et al. 2005). Predator control (first rats, then cats) has similarly also prevented extinction of Europe’s most endangered seabird, Zino’s Petrel (Pterodroma madeira) of Madeira Island.
On the Hawaiian island of Maui, one of the world’s wettest rainforests is the site of at least a partial success story. About 500 critically endangered Maui Parrotbills (Pseudonestor xanthophrys) and several thousand Akohekohes (Palmeria dolei) persist in ohia forests largely above the elevations of malaria‐bearing mosquitoes, where another critically endangered native honeycreeper, the Poo‐uli (Melamprosops phaeosoma), was first discovered in 1972. Amid the billowy mosses were more than a dozen endangered plants, including spectacular lobelias that had not flowered in decades. Feral pigs were rooting the soil, eating young emerging plants, and reducing overall plant diversity. In part because of the forest’s importance in protecting and purifying the watershed for the human population along the coast, a consortium of owners formed the East Maui Watershed Preserve. Their most controversial management decision was to fence the lower boundary of the preserve and eliminate feral pigs by lethal snaring, which produced the desired effects almost instantly (Fig. 15.47). The lobelias are flowering again, and Maui Parrotbills and Akohekohes thrive above them, augmented by a captive‐breeding program. Unfortunately, this habitat restoration came too late to save the Poo‐uli, as the last individual died in captivity in 2004.

Fig. 15.47 East Maui Watershed Preserve and the effects of excluding feral pigs. In order to restore the ecological integrity of forest undergrowth after decades of devastation by feral pigs (left image), this preserve was fenced and the pigs were systematically exterminated. This action was controversial, but it achieved the desired outcome, as understory regrowth occurred in just a few years (right image). Maintaining fenced mammal exclosures and removing invasive exotic plants remain essential management actions for the persistence of rare native plants and birds in this watershed.
(Photographs courtesy of The Nature Conservancy of Hawaii.)
15.7.9 Translocation
Among the most important strategies for rescuing critically endangered species is translocation, or moving individuals from one location to another area where threats have been reduced or eliminated. As with predator eradication, the conservation biologists of New Zealand have been global pioneers in using translocation, often coupled with intensive habitat management, to prevent the extinction of extremely rare bird species. Much of lowland New Zealand has been converted into grazing land, which has resulted in the loss of all but the most resilient native species. Furthermore, because the main islands are teeming with introduced rats, cats, ferrets, and stoats, most native landbird species are threatened or endangered. During recent decades, New Zealanders have produced truly dramatic conservation success stories, teaching the rest of the world much about what is possible even in the face of daunting odds.
A particularly compelling conservation success involves the recovery of the Chatham Robin (Petroica traversi) (Fig. 15.48), which by 1980 was extinct except for three males and two females, one of which was infertile. All five survivors were translocated to predator‐free Mangere Island, and each year the first clutches of the final breeding female (nicknamed “Old Blue”) were transferred to be cross‐fostered by Tomtits (Petroica macrocephala). Old Blue re‐nested each year, and the population began recovering after she paired with the breeding male, “Old Yellow.” All surviving black robins are descended from these two individual birds. Key to this landmark project’s success is the ongoing absence of predators on Mangere Island and the larger South East Island, where today the Chatham Robin population numbers more than 200. Although genetic diversity is exceedingly low after this dramatic population bottleneck, the effects of inbreeding depression have not been observed, suggesting that this population has faced several dramatic crashes over evolutionary time, potentially eliminating deleterious recessive alleles.

Fig. 15.48 Chatham Robin (Petroica traversi). Reduced to five individuals by the mid‐1980s, human intervention was the last option for species recovery. This was accomplished by transferring the few remaining individuals to offshore islands that were free of predators and carefully cross‐fostering eggs within nests of other small passerines. The population currently has recovered to approximately 250 individuals.
(Photograph by Leon Berard.)
The charismatic Kakapo (Strigops habroptila) (Fig. 15.49), a huge, flightless, nocturnal parrot that roosts and breeds in burrows, was once widespread but is now entirely gone from all three of New Zealand’s main islands. With the species at the brink of extinction, it was widely feared that only males still existed, but in 1977 a small population was discovered on Stewart Island, where they were being devoured by cats. In 1989 the historic Kakapo Recovery Plan moved 65 individuals to small, nearly predator‐free offshore islands, where any arriving cats, rats, or stoats could be systematically eliminated. Some islands proved unsuitable, but on others the population expanded, aided by intensive care and captive rearing by humans. As of 2014, 130 Kakapo remain.

Fig. 15.49 Kakapo (Strigops habroptila). A large, flightless, nocturnal parrot, this New Zealand endemic was an easy target of introduced feral cats and was nearly extinct by the late 1970s. In 1989, the Kakapo Recovery Plan moved 65 individuals to several small, virtually predator‐free islands. With extensive volunteer efforts and successful captive rearing, current populations include 130 monitored individuals.
(Photograph by Stephen Jaquiery, Otago Daily Times.)
A number of other New Zealand endemics, all highly vulnerable to mammal predation, have been similarly rescued. Notable among these is the Takahe (Porphyrio mantelli) (Fig. 15.50), a giant, flightless rail once believed extinct but rediscovered in 1948 and today numbering well over 200. Between 1983 and 1991, Takahe were transferred successfully to four different predator‐free islands (Maud, Mana, Kapiti, and Tiritiri Matangi). Importantly, a remnant Takahe population now is expanding within its native range in the Murchison Mountains of South Island in a large preserve where the concerted elimination of red deer and stoats has restored the tussock grass habitat to nearly pristine condition.

Fig. 15.50 Takahe (Porphyrio mantelli). This large, flightless rail was presumed extinct until a small population was rediscovered in 1948. Similar to other island endemics that are threatened by invasive mammal predators, this species recovered strongly with efforts to eliminate invasive animals, restore native grassland habitat, transfer individuals to several predator‐free islands, and monitor populations with intensive radio‐tagging.
(Photograph by Janice McKenna.)
Other distinctive New Zealand endemics now being rescued via translocation to predator‐free or predator‐controlled islands include the Little Spotted Kiwi (Apteryx owenii), Saddleback (Philesturnus carunculatus), Kokako (Callaeas cinereus), and Stitchbird (Notiomystis cincta). Especially notable is the Maungatauri Restoration Project on North Island, New Zealand’s largest and most ambitious example of “mainland” restoration. Beginning in 2004 and completed in 2006, this 34‐square‐kilometer forested ancient volcano is now surrounded by a remarkable, 47‐kilometer fence designed to prohibit entry by rodents, cats, stoats, brush‐tailed possums, and ungulates. Systematic poisoning, trapping, and hunting of pests began once the fence was closed, and very soon thereafter a number of rare species were rediscovered. A pair of Takahe was introduced in 2006, followed by a series of other rare endemics. The intent is to have a full complement of surviving North Island birds free‐flying and reproducing within this landmark project. One of the world’s rarest seabirds—the Magenta Petrel (Pterodroma magentae) of Chatham Island—has been saved by within‐island translocation of chicks prior to fledging to the Sweetwater Secure Breeding Site, a 3‐hectare area fenced against predators.
15.7.10 Conservation‐reliant species
In 2005, a group of well‐known conservation biologists published a landmark paper announcing that a large proportion of today’s threatened and endangered species likely will never be able to survive on their own in the absence of human investment of energy, time, and resources (Scott et al. 2005). They further proposed that our twenty‐first century definition of “recovery” for endangered species explicitly acknowledges that many are bound to remain conservation‐reliant species because they will require some form of continuing active management. Their proposal, which met only casual, mostly philosophical, resistance, makes a profound statement about human aspirations for conservation. We must no longer expect or hope that our planet might someday achieve a state in which natural systems persist sustainably, with an essentially complete array of native species, unless we continue to invest actively in their well‐being.
“Conservation‐reliant” bird species exist all over the world, with two types now broadly recognized (Goble et al. 2012). Population management‐reliant species require continual human intervention for local populations to persist. For example, predator‐proof cages and/or human exclosures have become essential for successful breeding of endangered shorebirds such as Black Stilts (Himantopus novaezelandiae) in New Zealand, Hooded Plovers (Thinornis cucullatus) in Australia, Piping Plovers (Charadrius melodus) in eastern North America, and perhaps African Oystercatchers (Haematopus moquini) in South Africa. California Condors (Gymnogyps californianus) probably always will require capture and chelation therapy to keep lead poisoning from greatly reducing the free‐living population. Periodic genetic augmentation from captive populations may be essential for a variety of species.
“Threat management‐reliant” species are those capable of persisting as wild populations as long as their habitat is managed to reduce or eliminate otherwise recurring threats. For example, the Palila (Loxioides bailleui) and numerous other endangered Hawaiian birds can persist indefinitely on their own, but only if introduced mammals are actively and continuously controlled throughout sufficient areas of habitat (Reed et al. 2012).
15.7.11 Habitat management and restoration
Habitat is the essential requirement for the conservation of any species, community, or ecosystem. Because habitat loss and degradation are the leading causes of avian population declines worldwide, efforts to protect or restore habitat always will be the cornerstones of conservation solutions for most species. As discussed earlier, conservation is accomplished most effectively by protecting large habitat patches, ideally with multiple patches configured across the landscape to promote functional metapopulations for target species. However, simply establishing a habitat patch as a protected area without attending to its long‐term management is usually insufficient to ensure successful conservation of its species and natural ecosystem functions. Almost everywhere on earth, habitats experience continuing human influences, either directly (as through exposure to exotic predators such as cats) or indirectly (as through altered water regimes or fire cycles). Therefore, most natural area preserves—even the very largest ones—require active habitat management to remain suitable for the species and ecological systems they are designed to protect.
Mimicking natural disturbance is often a vital form of habitat management. Many grassland habitats, for example, support higher species diversity when subjected to periodic disturbances that mimic the passing of a herd of grazing ungulates. Consequently, short‐rotation grazing by domestic cattle actually can improve grasslands as a habitat for some birds. On the other hand, cattle tend to loaf and forage near streams, causing erosion of stream banks and degradation of the riparian thickets that are so important for breeding bird communities. Habitat management in grazing lands may require the construction and maintenance of fencing to permit control of stocking rates, to limit exposure periods within each grazing unit, and to reduce or prohibit access of cattle to riparian habitats (Fig. 15.51).

Fig. 15.51 Riparian restoration. The San Pedro River is the last free‐flowing (undammed) river of the southwestern USA, providing habitat for over 300 bird species throughout the year. Cattle attracted to water can destroy such habitats (top images), as they compact the soil and overgraze native plants. The Nature Conservancy now owns and manages several portions of this critical habitat corridor, actively keeping cattle out and restoring damaged plant communities (bottom images) essential to birds and numerous other animals.
(From Krueper et al. 2003. Reproduced with permission from John Wiley and Sons.)
The influence of fire on bird species diversity has become a major focus of conservation research and management experiments in ecosystems around the world. Especially in tropical dry forests, human‐caused fires at unnaturally high frequencies degrade forest structure and composition and sometimes eliminate forest altogether. Vast areas of interior Madagascar, for example, once supported dry forests but are now dominated by introduced grass because of purposeful annual burning by humans. The severely endangered Van Dam’s Vanga (Xenopirostris damii) is among many Madagascar endemics whose dry forest habitat has been reduced to tiny fragments by uncontrolled brush fires. Similar threats face dry‐forest endemics in Western Australia, where birds such as the Mallee Emuwren (Stipiturus mallee) (Fig. 15.52) are reduced to tiny forest fragments with a dense understory of Spinifex vegetation. These areas are extremely vulnerable to fire, and emuwrens strongly favor patches that have not burned in decades (Brown et al. 2009). For such habitats and all their threatened birds, the most crucial form of habitat management is prevention and active suppression of fire. In fact, inappropriate fire‐management regimes have been identified as the greatest single threat to Australia’s birds after direct habitat destruction (Olsen and Weston 2005). The same may be true for the cerrado and campo bird communities of central Brazil (Cavalcanti and Alves 1997).

Fig. 15.52 Mallee Emuwren (Stipiturus mallee). Although this species strongly prefers habitat that has not burned recently, the scrub they prefer is highly vulnerable to fire. Habitat management that includes frequent burning can have strong negative influences on such species.
(Photograph by Colin Cock.)
On the other hand, a surprising number of the most globally threatened bird species now are understood to respond positively to periodic wildfire, and some actually depend on it. Most are tightly associated with plant communities where lightning‐caused fires are natural and frequent, and local populations can decline to extinction if fire is suppressed or excluded. Indeed, many post‐fire specialists currently may be endangered more by fire suppression than by outright habitat loss. In these cases, successful habitat management requires prescribed burning to maintain the ecological conditions under which the species evolved. Florida Scrub‐Jays (Aphelocoma coerulescens), for example, gradually decline within otherwise optimal scrub oak habitat where fire has been suppressed. Their numbers rebound quickly once fire is reintroduced to their habitat (Fig. 15.53), and recruitment of new breeders reaches its peak in habitat tracts managed by prescribed burning every 5–10 years (Breininger and Oddy 2004).
Fig. 15.53 Florida Scrub‐Jay (Aphelocoma coerulescens). A highly specialized bird of lighting‐prone scrub oak habitats, the persistence of this species depends on frequent (every 5–10 years) prescribed or permitted natural burns. This graph shows a dramatic population increase after a prescribed burn at a study site in central Florida (USA), but in the absence of another fire, the population declined again after 10 years. Without such natural fire cycles producing early successional habitat, populations of this species disappear.
(From Fitzpatrick and Bowman 2016. Reproduced with permission from Cambridge University Press. Photograph by Jane Luxton Photography.)
Many ecosystems around the world are so degraded (from overgrazing, excessive ditching and draining, introduction of exotic plants, intensive logging, and other causes) that they may be unable to recover on their own. Such patches may require habitat restoration in the form of aggressive, often recurring, treatments intended to bring back some or all of the original species, plant and animal community structures, and ecosystem functions.
Restoring freshwater wetlands is a major priority worldwide. Restoration strategies may include fencing out grazing herbivores, filling agricultural drainage ditches, removing dams and diversions, and even using bulldozers to knock down levees and redirect stream flows into former channels. Such actions help restore water levels and riparian habitats, renew seasonal marshes, and bring back annual wet/dry cycles to which herons, migratory shorebirds, and waterfowl are adapted. The largest wetland restoration project in the world involves the massive Everglades watershed in southern Florida (USA), a colossal, multibillion dollar effort to re‐establish wetlands and seasonal water flow originating in the central peninsula and extending south to Florida Bay. Until humans ditched and drained this region during the 1950s and 1960s, annual summer rains produced vast sheet flows covering nearly 30,000 square kilometers of wet prairies and marshlands. Spring dry seasons contracted many of these wet areas, concentrating fish, snakes, and amphibians into shallow pools that supported huge nesting colonies of wading birds such as Wood Storks (Mycteria americana), Roseate Spoonbills (Platalea ajaja), and various herons, egrets, and ibises. Restoring native biological functions to this system involves purchasing ranches and farmlands from private landowners, securing conservation easements from others, bulldozing down massive levees bordering channelized rivers (Fig. 15.54), and constructing huge culverts that allow seasonal flood waters once again to inundate large areas, including lands that have been farmed or grazed for decades. Wetland bird populations will flourish as a result.

Fig. 15.54 Kissimmee River restoration. (A) Channelization of the Kissimmee River in Florida in the 1960s destroyed the ecological values of one of the world’s largest river–floodplain–wetland networks. (B) In 1992, the Kissimmee River Restoration project began to restore the ecological role of the original river by backfilling the channel and re‐meandering the river.
(Photographs courtesy of South Florida Water Management District.)
Restoration projects in tropical areas of the world teach important lessons. In western Costa Rica, the restoration of bird‐rich tropical dry forest habitats is enhanced by actively reducing fire frequency. Habitat management that limited grazing, reduced fire, and controlled nest‐parasitic Shiny Cowbirds (Molothrus bonariensis) has prevented the likely extinction of the Pale‐headed Brush‐Finch (Atlapetes pallidiceps) in southern Ecuador, which was reduced to 14 pairs in 1994 but now numbers in the hundreds. Forest restoration has helped prevent the extinction of the Rodrigues Warbler (Acrocephalus rodericanus) and Rodrigues Fody (Foudia flavicans) on the island of Mauritius, the Waldrapp (Geronticus eremita) of Morocco, the Grenada Dove (Leptotila wellsi) in the Caribbean, and the Cerulean Paradise‐flycatcher (Eutrichomyias rowleyi) in Indonesia.
In Australia, a widespread region of subtropical forest in northern New South Wales called the “Big Scrub” was largely cleared for agriculture, endangering numerous locally endemic bird species, among them the Black‐breasted Buttonquail (Turnix melanogaster). Public awareness of the biological importance of this area, combined with active replanting of native plants on both private and public lands, has begun to ameliorate this habitat loss. In other temperate forests of southeastern Australia, bird species of conservation concern, such as the locally threatened Hooded Robin (Melanodryas cucullata), were found to be much more numerous in younger forests under restoration than in nearby stands of old growth, perhaps because of the presence of aggressive Noisy Miners (Manorina melanocephala) in the old‐growth forests (Lindenmayer et al 2012).
15.7.12 Adaptive management
Some of the biggest challenges in conserving birds and their habitats occur because management actions must be taken before full knowledge of the system has been gained. In the long run, therefore, effective ecosystem management requires not only defining a conservation target but also acknowledging the gaps in knowledge about how to achieve it. The best approach is a three‐step process known as adaptive management. First, specific goals are defined as the desired outcomes of management actions (perhaps to sustain a specified population size or density of an indicator species, or a specified abundance target for a suite of different species). Second, by experimenting with management alternatives and constantly monitoring, evidence about how the target species respond to different management actions is gathered continually. Third, management plans are modified periodically as a consequence of the new information, and the process is repeated. As time goes on, therefore, we adapt management techniques to incorporate new understandings about the system. The approach stresses the importance of “learning as we go” through landscape‐scale experiments (for example, different burn regimes, grazing frequencies, water depths, or forest thinning), accompanied by monitoring of key species and processes. Adaptive management demands that managers be ready, willing, and able to modify management techniques as new information comes in. Such an approach can be challenging to carry out, especially by public land‐management bureaucracies such as park services, wildlife agencies, forestry divisions, and local governments that are accustomed to operating with fixed and longstanding management formulae. Often, such agencies also lack the funding and scientific infrastructure for designing and conducting experiments and subsequent monitoring. For these reasons, adaptive management represents an important opportunity for public agencies to partner with private conservation groups and research institutions to accomplish the most effective long‐term management of their ecological landscapes and conservation of priority species.
15.7.13 Ecosystem management is complicated
During the past half‐century, conservation‐minded ecologists began recognizing that relationships among native species and communities are complex, have evolved across large landscapes and long timescales, and exist within a complex milieu of human social and economic realities. Because ecological relationships occur at many scales, the protection of a target species (such as an endangered or declining bird) or an assemblage of species (such as a grassland bird community) requires ensuring the continued functioning of a host of ecological processes: fire, periodic flooding, nutrient cycling, plant succession, and seasonal migrations. Such processes may occur unpredictably (for example, damaging storms), or in cycles that may be daily (activity patterns of insectivorous birds and their prey), annual (seasonal flooding of vernal pools), regular multi‐year (predator–prey cycles), or irregular multi‐year (floods or fires). All these processes, in turn, differ across the landscape and among different ecological regions (Fig. 15.55). For this reason, large‐scale conservation efforts break the challenges down into geographically separate, locally tailored planning efforts called ecoregional planning (Box 15.06).
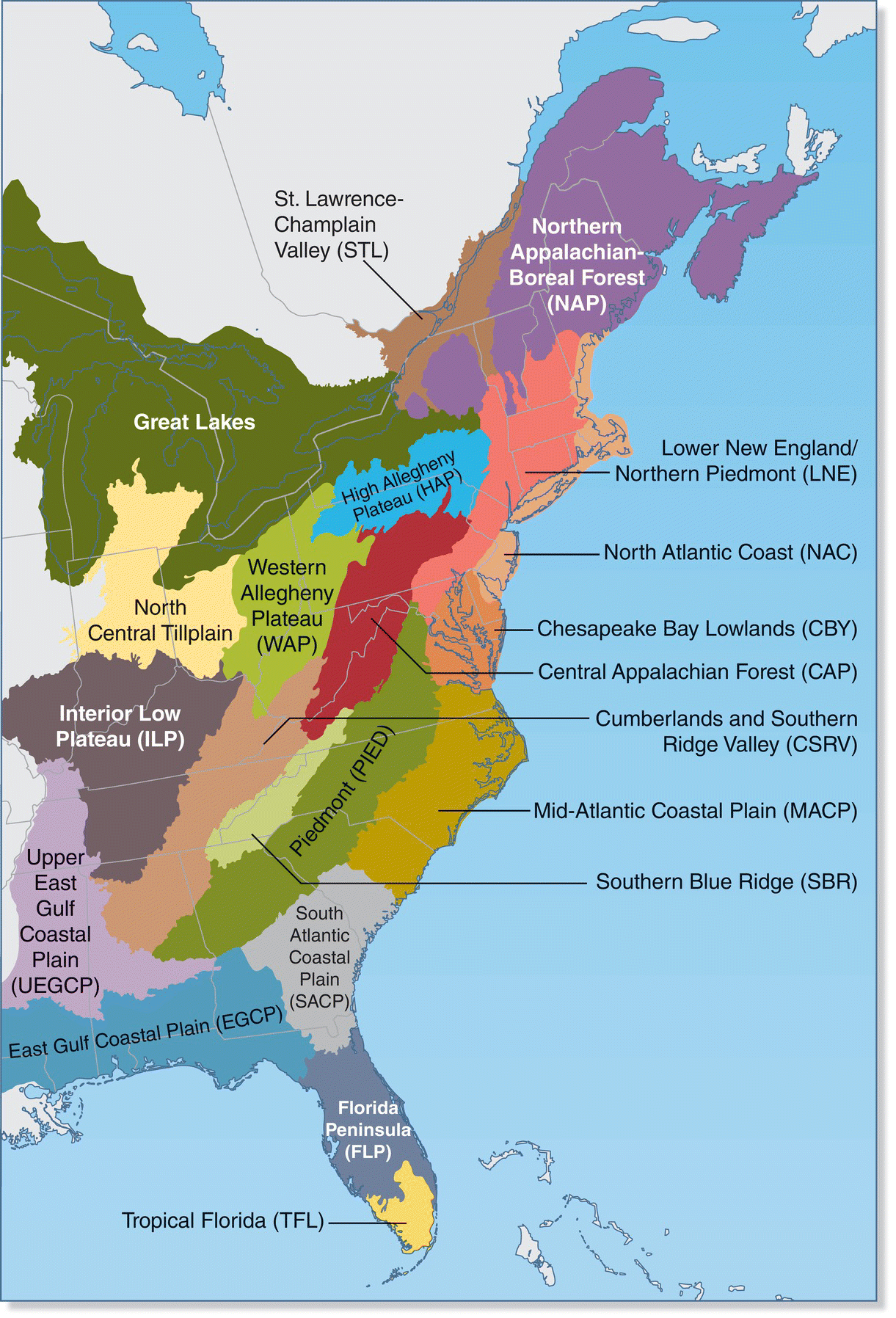
Fig. 15.55 Ecoregional conservation planning. Instead of using political borders to define areas, ecoregional conservation planning uses watersheds, soils, topography, climate, and dominant vegetation formations to delineate different ecosystems across the landscape, as in this ecoregional map of the eastern USA. Conservation plans for each region begin by identifying viable populations of rare species, the best examples of characteristic natural communities, and the list of threats facing these places. The result is a set of priorities for conservation actions in each ecoregion.
(From The Nature Conservancy 2015. Reproduced with permission from The Nature Conservancy.)
If the human factor could be ignored, the overriding management challenge for preserving native bird species would be to ensure that all the above‐mentioned processes continue to function within landscapes of sufficient size so that extinctions would not occur simply because populations were too small. Today, however, meaningful conservation success can be achieved only by recognizing that the human factor does exist. Although human social needs complicate the challenges even further, they must be incorporated into any long‐term management solution. Socioeconomic needs can be addressed in the context of conservation planning through a number of tools, including community development via ecotourism, economic incentives for land management, payments for ecosystem services, and education.
Understanding and maintaining the full range of natural processes across landscapes, together with conserving their ecological services and natural resources that meet the human political and cultural communities in which they are nested, has become known as ecosystem management. This term is inexact and sometimes controversial, but it acknowledges that human cultures and ecological systems are now inescapably woven together. Birds in their native habitats will not be conserved without embracing a holistic approach that identifies how conservation management strategies engage with, and even serve, the human societies that surround them.
15.8 Value of wild birds
Why should humans care about protecting birds, given how expensive and difficult it is to do so? What are birds worth to society? What values are gained by investing in their protection? Is bird conservation worth the cost?
Such questions can be asked about any aspect of biodiversity, not only birds; yet as in so many other areas of biology, birds provide a popular framework for addressing these wider issues. Practical as well as ethical and spiritual answers exist to these questions, and the subject is worth reviewing here for two reasons. First, questions about the values of biodiversity often are debated in public and political forums but rarely get resolved, because the subject involves not only facts but also experiences and emotions. Second, conservation requires a collective willingness to protect shared resources from experiencing the tragedy of the commons; that is, the loss of communally valuable assets as a result of individually selfish behavior. Appreciating the values of communally shared assets is essential for gauging the community’s willingness to invest in their protection.
Values of biodiversity fall into at least four distinct categories: direct, indirect, emotional, and ethical. Direct benefits, such as food, other commercially traded products, and direct economic gains, are the benefits that are most measurable and material. Indirect benefits, such as environmental services, biological indicators, genetic information, and research values, are more diffuse. For many people, the emotional benefits are even more important than the previous two, because birds capture our attention, feed our imagination, stimulate our aesthetic senses, and even populate our spiritual lives. Finally, on ethical grounds, it has been argued that birds, as components of the natural world, possess an intrinsic right to exist, so failing to invest in their protection constitutes a selfish and short‐sighted violation of the moral imperative that we share to preserve the natural world.
15.8.1 Direct benefits
Many humans all over the world still harvest wild birds for food. In many regions this tradition damages bird populations and is illegal, but the practice continues. On the other hand, dozens of species of game birds, including ducks, grouse, quail, tinamous, cranes, and rails, and upland shorebirds, such as woodcocks and snipe, are legally regulated for sport and subsistence hunting in various countries. Hunting regulations are usually intended to ensure the long‐term sustainability of these bird populations, and the practice generates huge economic value. In the USA, for example, expenditures related to equipment and trips for waterfowl hunting produced a total economic output of $2.3 billion in 2006 (Carver 2008). In addition, taxes on ammunition used by duck hunters, augmented by private charitable giving, have resulted in the protection of more than 40.5 million hectares of wetland and adjacent upland habitat for the benefit of game birds, especially ducks and geese. Such protection efforts simultaneously provide habitat for non‐game bird species, as well as for thousands of other species of plants and animals.
In southeastern Asia, nests of the White‐nest Swiftlet (Aerodramus fuciphagus), which are made of hardened saliva, are such a favored human food that these birds are encouraged to nest in artificial houses to facilitate the harvest, and the taking of nests from natural caves is tightly controlled (Chapter 11). Elsewhere, down feathers from wild geese and ducks have been used for centuries as insulation and cushioning in pillows and blankets. Nitrogen‐rich guano from cormorants and boobies also still is harvested from tropical oceanic islands around the world for the production of agricultural fertilizer.
Recreational bird watching is among the most rapidly growing outdoor pastimes in the world. During 2006 in the USA, about 48 million people participated in bird watching, spent $36 billion in travel and equipment, and generated $82 billion in total industry output (Carver 2009). The global ecotourism market is even larger, and it is beginning to provide strong local incentives for the conservation of natural ecosystems. For example, an economic analysis of ecotourism in one region of Amazonian Peru concluded that ecotourism and conservation concessions, as a replacement for logging concessions, are justified on narrow economic grounds alone (Kirkby et al. 2010). In this sense, bird conservation can be viewed as essential in maintaining an important and rapidly expanding economic influence in the world.
15.8.2 Indirect benefits: ecosystem services
Birds provide crucial ecosystem services in maintaining healthy and diverse ecological systems and processes throughout the world. For example, by eating or caching fruits, seeds, and nuts, birds provide an environmental courier service promoting dispersal, gene flow, and colonization opportunities for thousands of species of plants in all kinds of forests and shrublands. Indeed, these interactions have been instrumental in the very evolution of sugar‐ and nutrient‐laden fruits and seeds. In western North America and in Eurasia, numerous pine species have coevolved with birds such as the Pinyon Jay (Gymnorhinus cyanocephalus), Clark’s Nutcracker (Nucifraga columbiana), and Eurasian Nutcracker (Nucifraga caryocatactes) to produce large seeds that are dispersed and cached by these corvids.
By creating nest holes in dead trees, cacti, river banks, and even flat prairie ground, birds such as woodpeckers, bee‐eaters, kingfishers, swallows, and even terrestrial owls create cavities that last for decades, providing shelter and breeding places for whole communities of insects, mammals, reptiles, and other birds. Because birds are mid‐level and top‐level predators in the food chains of almost every ecosystem in the world, they influence the evolution of mimicry in butterflies, cryptic coloration in insects, schooling behavior in fishes, and sentinel behavior in social birds and mammals.
Birds serve human economic interests by controlling insect populations. In Salt Lake City, Utah (USA) a gold statue of two California Gulls (Larus californicus) celebrates the role played by this gull in devouring huge swarms of crickets that threatened to destroy the crops of early farmers. Both migrant and resident songbirds maintain the health and condition of the world’s forests by consuming untold billions of leaf‐eating insect larvae, and many bird populations respond rapidly to regional outbreaks of damaging insect pests such as gypsy moths, tent caterpillars, and spruce budworms (Holmes et al. 1979). Other ecological services provided by birds include the scavenging of animal carcasses by vultures, caracaras, crows, and gulls, and the pollination of flowering plants by hummingbirds, sunbirds, and honeyeaters.
The idiomatic expression “canary in the coal mine” symbolizes one of the most important services provided to humans by birds. Until recently, coal miners around the world descended into their risky underground workplaces carrying caged canaries (Serinus canaria) (Fig. 15.56), to which miners paid close attention. Deadly methane gas is odorless, but a gasping or dying canary warned miners to escape before accumulating gas threatened them with the same fate. Today we understand that birds can reveal environmental changes and dangers in far more diverse ways. Their position atop natural food chains makes hawks, eagles, and falcons highly susceptible to accumulations of environmental poisons, as experienced by the Peregrine Falcon (Falco peregrinus) in the 1960s. Cadmium poisoning of White‐tailed Ptarmigan (Lagopus leucura) in the Rocky Mountains of western North America revealed the lethal impacts of heavy metals in mining areas worldwide (Larison et al. 2000). Population declines of birds occupying freshwater habitats signal problems stemming from poor water quality or invasive species. In a wide variety of habitats around the world, ecologically specialized birds, as well as other animals, can serve as indicator species that allow us to evaluate the success of habitat management and restoration efforts.

Fig. 15.56 The “canary in the coal mine.” This expression originated from the practice of bringing domesticated canaries (Serinus canaria) into active underground coal mines. Since toxic methane gas affects these birds faster than it does humans, the canaries were used as biological indicators of this invisible risk to the miners. Today many wild bird species serve as sensitive indicators of changing environmental conditions.
(Photograph courtesy of the Queensland Mines Rescue Service.)
15.8.3 Indirect benefits: genetic information and scientific study
A major reason for protecting the world’s biodiversity is that we are only beginning to explore the wonderful storehouses of genetic information represented by wild organisms. Information contained in the DNA of every individual bird has survived millions of years of evolutionary challenges. All these complex evolutionary stories still are largely untold, and some could be directly useful to humans. For example, we possess at best only a rudimentary understanding of the genetic bases for disease resistance, protein synthesis, detoxification of natural compounds, complex behavior patterns, neural pathways, and many other natural functions of organisms. Every time we allow an organism to go extinct, we lose potential information about its history, evolutionary potential, and capacity for helping us lead our own lives more fully.
As subjects of scientific research all over the world, birds continue to teach us how nature works. They are mostly diurnal and are relatively easy to observe. They occur in virtually every habitat on land and sea. They are extremely diverse, both socially and structurally. They migrate from pole to pole and from mountaintop to valley bottom. They exhibit remarkable feats of both short‐term problem solving and long‐term memory. They are environmentally sensitive, and their birth rates and death rates reveal much about the world around them. For all these reasons and more, birds provide raw materials for never‐ending scientific inquiry into virtually all aspects of the living world.
Many of the foundational discoveries in the modern science of biology have involved birds. Charles Darwin and Alfred Russel Wallace used birds to derive the theory of evolution by natural selection. By studying closely related bird species on islands in the South Pacific, Ernst Mayr deduced how allopatric speciation gives rise to new species (Chapter 2). David Lack developed time‐tested principles of life history theory based on birds (Chapter 13). Using birds on islands as principal examples, Robert MacArthur and Edward O. Wilson and their followers derived ground‐breaking principles of ecology, island biogeography, population biology, and community structure (Chapter 14). Konrad Lorenz and Niko Tinbergen won Nobel prizes for their studies of bird behavior (Chapter 9). The most successful game‐management theories in the world were derived from studies of birds. Modern studies of environmental toxicology and wildlife disease often focus on birds as bio‐indicators. The biology of migration, navigation, and homing behavior, and the neurophysiology of memory are all illuminated through studies of birds both within and outside of the laboratory (Chapters 7 and 12). Many key principles of social evolution and animal communication stem from research on birds (Chapter 9). Accordingly, hundreds of scientific journals now regularly publish technical research on bird biology.
15.8.4 Emotional benefits
Rachel Carson chose the title Silent Spring for her landmark 1962 book that called attention to the explosion of global threats from pesticides and pollution and helped launch the modern environmental movement. Carson intended this title to shock the public world into contemplating a landscape so impoverished as to lack bird songs in the spring—she chose it because she appreciated the power of bird song for all of us.
By representing beauty in nature, by providing spiritual and even religious experiences for millions of humans, birds deliver emotional enjoyment to people all over the world. It is impossible to estimate the collective value of our pleasure in seeing migratory bird flocks, hearing the chorus of bird songs in a forest, watching gulls wheel at the seashore, enjoying the kaleidoscope of gaudy birds at a feeder, or admiring an astoundingly nimble hummingbird at a flower. The true monetary value of these and countless other encounters with birds cannot be known, but without doubt they contribute enormously to the flood of pleasures that humans derive from nature. Moreover, these very pleasures increasingly are demonstrated to have remarkable physical and psychological healing power (Buzzell and Chalquist 2009; Selhub and Logan 2012). This connection is reflected further in the symbolic bird names we give ourselves—from New Zealander “kiwis” to sport teams like the “Pittsburgh Penguins” and “Baltimore Orioles” to innumerable school mascots.
15.8.5 Ethical considerations
In progressively dominating the earth, humans have demonstrated phenomenal (and ever‐increasing) power to destroy life. It frequently is argued, on both theological and secular grounds, that humans have a moral obligation to protect the natural and evolutionary heritage of the earth, including all of its species, not just the ones that provide us economic value. In this view, all non‐human life forms such as birds have intrinsic value by virtue of their unique evolutionary histories, their modern ecological roles, their structural and behavioral complexity, and their aesthetic beauty, even if they have no “purpose.” Every bird, like the entirety of nature—arising over 3 billion years of evolutionary history—can be viewed as a work of natural art every bit as exquisitely complex, mysterious, and beautiful as the cathedrals, paintings, literature, and poetry of humankind, if not more so. For anyone holding such a view of nature and its birds, it is especially easy and important to answer the question “Why should we save them?”
15.9 What each of us can do
Most conservation projects require the involvement of many people working together, including communities, public agencies, research groups, land‐management specialists, not‐for‐profit organizations, education programs, fund‐raising efforts, and even political initiatives. Besides participating actively in any of these activities, individual citizens also can contribute on their own towards keeping common birds common and keeping rare birds from going extinct. Birds and biological diversity exist everywhere, and their long‐term protection depends just as much on managing millions of small places across the landscape as it does on establishing and managing large parks and preserves. Every little bit helps.
15.9.1 Backyard conservation
Every piece of outdoor space, from city parks and suburban backyards to rural farmlands and forests, can serve as habitat that helps conserve birds. The elements that birds need to survive—shelter, nesting habitat, food, and water—can be maintained in all kinds of backyards, schoolyards, and parks (Fig. 15.57). As is true for natural ecosystems, managed landscapes will support more bird species if they have a varied habitat structure, including grassy open spaces; gardens interspersed with boulders, ferns, and shrubs; and trees of different species and heights. Crucial to any bird‐friendly yard is dense shrubbery to provide both nesting habitat and protection from predators for a wide variety of songbirds. Trees attract both year‐round residents and migratory songbirds. Water helps, even if supplied by a simple birdbath placed near protective shrubbery. Pesticides and lawn chemicals should not be used, because they reduce both insect and plant diversity, and some are even directly harmful or fatal to birds.

Fig. 15.57 Bird‐friendly yards are easy and effective. All available outdoor spaces can be transformed from ecological wastelands into diverse, wildlife‐friendly habitat. For instance, this yard in Ohio (USA) started out with little plant and structural diversity (left image) and was transformed into a rich habitat for birds (right image) with dense shrubs, birdbaths, feeders, and a diversity of native plant species.
(Photographs courtesy of www.backyardhabitat.info.)
Healthy forests are filled with dead and dying trunks and limbs. It is important to fight the common tendency to remove these elements. Allowing dead limbs and trees to remain in place whenever possible makes a yard much more attractive to a wide variety of bird species. In addition to being essential for woodpeckers, dead and dying wood provides important foraging habitat for many songbirds, nesting habitat for even more species, and vital perch sites for raptors, shrikes, and countless songbirds that sally after flying insects.
Domestic cats should remain indoors at all times (Fig. 15.58). This precaution is especially important during the spring and summer, when recently fledged and naïve juvenile birds are most vulnerable to predation. A recent, comprehensive review suggests that, in the USA alone, about 2.4 billion birds are killed annually by free‐roaming house cats (Loss et al. 2013). Therefore, “cats indoors” must be a year‐round rule for anybody seriously committed to bird conservation.
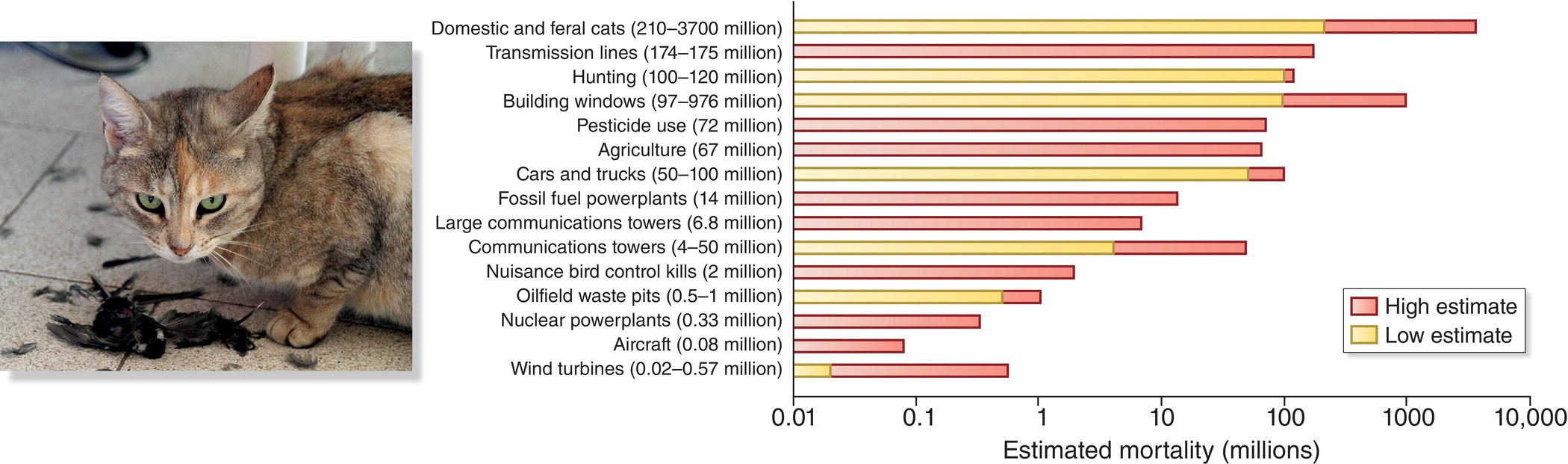
Fig. 15.58 Cats and bird mortality. Domestic and feral cats are the leading source of human‐caused avian mortality in the USA, and this is probably true in many other places as well. Studies have shown that regardless of the use of bells, claw removal, and other forms of cat control, cats remain effective bird killers if permitted to roam free. The only way to protect avian wildlife from cats is to keep all domestic cats indoors.
(Data from Nigelj, http://en.wikipedia.org/wiki/File:Bird_mortality.svg#file. CC‐BY‐SA 3.0. Photograph by Egisto Mannini.)
15.9.2 Citizen science
Population monitoring is essential for bird conservation, because it provides data for detecting population declines, setting priorities, and measuring the success of management practices. However, public agencies and professional scientists alone cannot possibly monitor every species, habitat preserve, and important bird area, even though doing so at both local and continental scales is essential for detecting changes and planning action. In short, monitoring is crucial, but the task seems impossibly enormous. This dilemma increasingly is being resolved by the extraordinary rise of citizen science as a tool for environmental research. Bird watchers and ornithologists have led the way in the explosion of citizen science, and bird‐monitoring projects involving volunteers are being carried out all over the world. Through involvement in these projects, legions of amateur bird enthusiasts now contribute crucial information of direct relevance to conservation while simultaneously having fun and enriching their lives. In recent years the most accessible, powerful, and informative global citizen science project involving birds is eBird (http://ebird.org), an internet‐based global database managed by the Cornell Lab of Ornithology (Sullivan et al. 2014). Tens of thousands of eBird participants from around the world now regularly submit checklists that specify species, numbers, location, date, time of day, and minutes of effort during the observation period. We invite you to join us in this endeavor.
Detailed statistical analysis of citizen science data has long been an accepted method for determining both local and range‐wide population trends. Citizen science data also provide the basis for bird atlases that document the distribution of birds throughout states and countries. Recent advances in machine learning and high‐performance computing, combined with data‐intensive citizen science projects such as eBird, have yielded spectacularly fine‐scale modeling of bird distributions and movements (Fig. 15.59). Contributing observations to any citizen science project that connects to a globally available database is one of the easiest and most important ways that an individual can contribute to bird conservation.
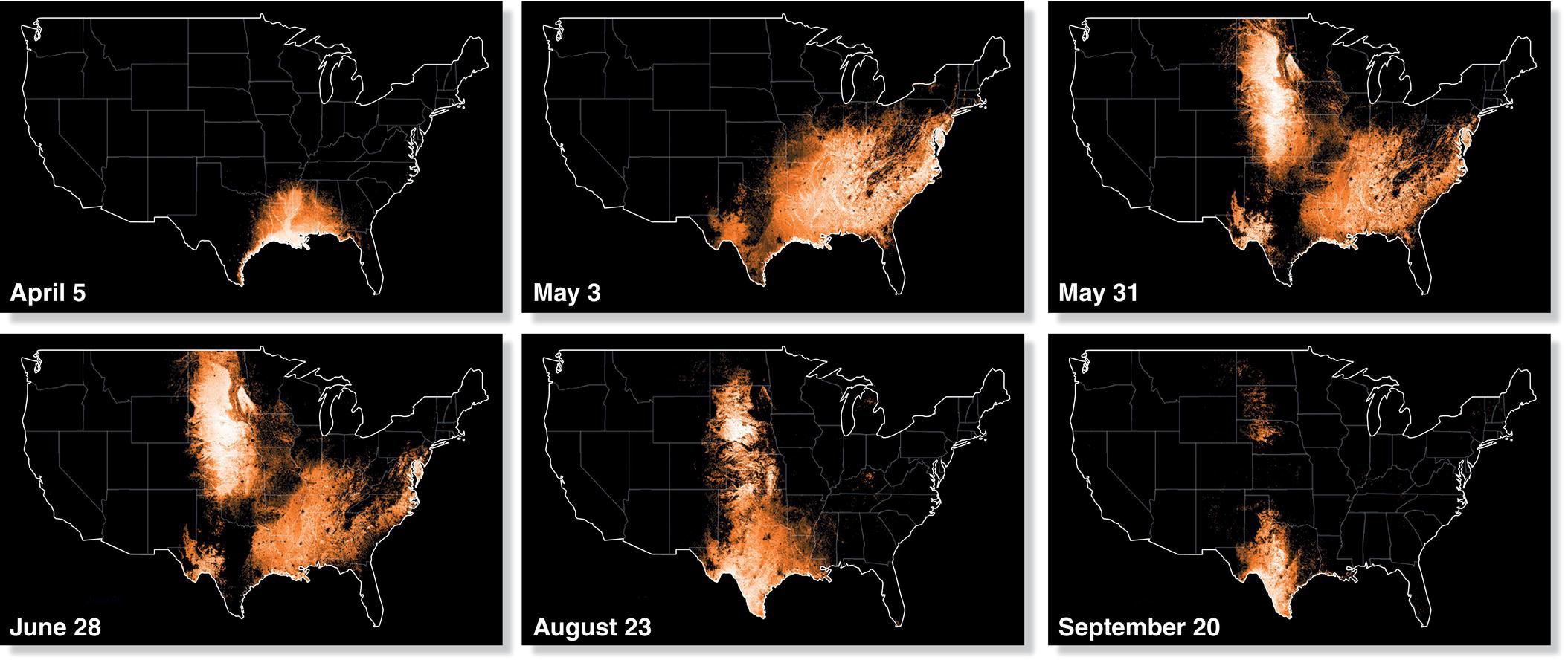
Fig. 15.59 Citizen science brings bird distributions to life. These spectacularly fine‐scale maps show the seasonal distribution of Orchard Orioles (Icterus spurius) across eastern North America. Data were provided via hundreds of thousands of citizen scientist records from free eBird software, which permits people to record their bird observations worldwide. These maps use eBird data to generate probabilistic models, predicting the likelihood that a species will be present at a given date and location (white, high probability of observing; deep orange, low probability). Such maps are expanding our understanding of animal movements by providing precise information at continental scales at relatively low cost.
(© Cornell Lab of Ornithology.)
15.9.3 Adopting a place
Sustaining bird populations and biodiversity so that future generations can enjoy them requires the recognition that humans now manage the earth. Whether or not we like this complicated role, we are stewards of the world’s species and natural systems. Doing a good job will require widespread cultural adoption of conservation ethics into our morally and spiritually driven prescriptions for human behavior. Adoption of this land ethic means that each individual must strive to allow natural systems and species to coexist with us within our human‐dominated world. A powerful route to this long‐term result is for individuals to choose a favorite outdoor place and actively invest in its well‐being. Our important places need not be large, spectacular, or rare, because keeping common birds common must go hand‐in‐hand with rescuing the endangered ones. Besides, many of the big, spectacular places, such as national parks and wildlife refuges, already are equipped for long‐term conservation, and it is the smaller local places that need our help.
Individuals can contribute to conservation by personally committing to a local park, schoolyard, recreation area, commercial woodlot, beachfront dune, or grounds of a corporate headquarters. Becoming familiar with the ownership, management plans, and real‐world challenges of a place can reveal numerous opportunities for personal contributions, such as volunteer work, teacher training, or fund raising. Encouraging owners to examine management plans that favor native birds often reveals simple changes that can have positive impacts on bird populations, such as planting native shrubs, lengthening mowing cycles, altering drainage regimes, or removing invasive plants. Financial investments, especially endowments for land management, improve the chances that favorable management practices will continue indefinitely.
BirdLife International’s Important Bird Areas (IBAs) program recognizes more than 11,000 specific sites that play unusually important roles for birds in 200 different countries and territories around the world (Fig. 15.60). Through input from birders and scientists, specialists within participating countries have identified these as key sites for conservation because they harbor notable numbers of threatened species, represent concentrations of endemic or ecologically specialized species, or support unusually large numbers of migratory or colonial species. Recognition of these sites promotes both public and private investment in their long‐term protection, management, and monitoring. At any scale, an important feature of every IBA is its capacity to attract conservation action from public agencies and to motivate private individuals to invest their own time and energy in its future through vigilance, grassroots activism, and advocacy.
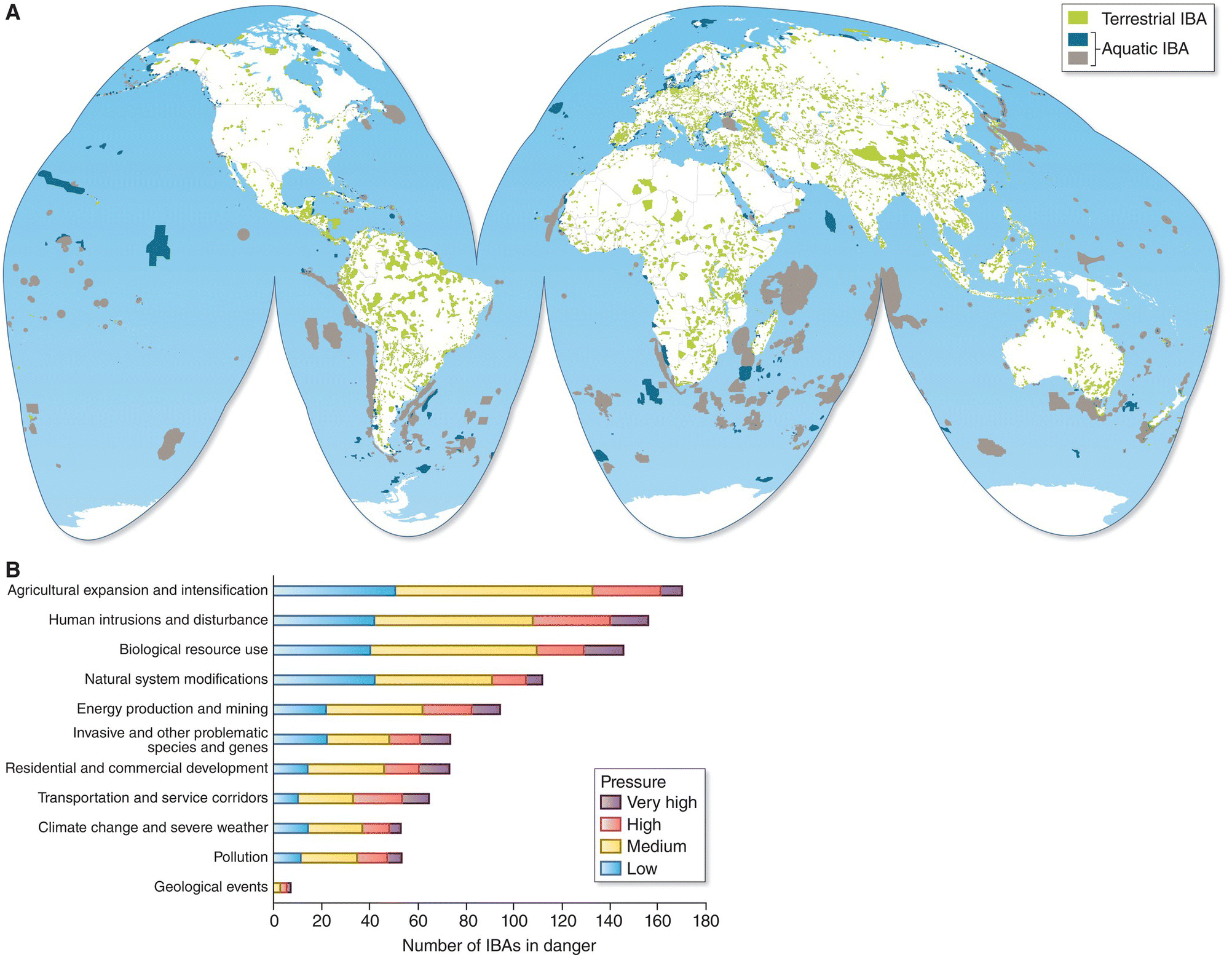
Fig. 15.60 BirdLife International’s Important Bird Areas (IBAs). This program identifies key habitat areas for protecting birds and bird populations around the world. (A) IBAs are recognized worldwide in both terrestrial (green) and marine (brown and dark blue) habitats. By identifying the world’s most important bird conservation areas we can help to promote investments to conserve, manage, and monitor them. (B) Types of disturbances that endanger IBAs, ranked by number and risk level.
(Case study 80 (A) and case study 545 (B) from www.birdlife.org. Courtesy of Birdlife International.)
15.9.4 Vigilance, grassroots activism, and advocacy
Every individual can help promote bird conservation by exercising vigilance (such as noticing and reporting violations of wetlands or endangered species laws), political activism in elections (such as supporting public referendums that support the conservation of open space and biodiversity), and advocacy (such as working to convince local planning or zoning authorities to adopt open space provisions or to monitor key local indicator species). Advocacy that promotes long‐term protection of key parcels of land will always be a backbone of conservation action, but public advocacy about laws, policies, and industrial practices may be equally important in protecting the welfare of bird populations. Examples include advocating against recreational uses of lead in fishing and hunting, against certain pesticides that kill non‐target species, for the use of streamers by the long‐line fishing industry (these greatly reduce seabird bycatch), and for legal restrictions on siting and lighting of communication towers and wind turbines. Local and regional conservation organizations often produce excellent information on these and many other issues for which individual advocacy can make a real difference.
15.9.5 Consumer choices
Some consumer products support conservation. An excellent example is shade‐grown coffee, which is grown in the shaded understory of managed forests that provide other forest products (such as fruits and firewood), reduce erosion, and support a wide diversity of birds. In Central and South America, millions of migratory birds, including some rapidly declining species such as the Cerulean Warbler (Setophaga cerulea), prefer shade‐coffee plantations. Moreover, shade‐coffee areas represent most of the remaining forest cover in some countries, such as El Salvador. Worldwide, shade‐coffee ground is being rapidly converted to less ecologically valuable habitats, such as sun‐coffee plantations in which coffee is grown as an intensively managed monoculture. In response, conservation‐minded groups have created a market and certification standards for shade‐grown coffee. Similarly, in both South America and in southeastern Asia and Indonesia, spectacularly diverse native rainforests are being replaced by single‐species oil‐palm plantations. Production of “certified sustainable palm oil” more than tripled between 2009 and 2011, reaching 4.8 million tons.
15.9.6 Environmental education
Promoting and participating in local environmental education helps broaden the community of people who grasp the importance of conserving birds and biodiversity. Half of the world’s human population now live in cities, and the proportion will increase to 70% by 2050 (Cohen 2003). This trend, combined with the sweeping popularity of digital entertainment, means that fewer and fewer of the world’s citizens will grow up in direct contact with nature. Because humans are more likely to protect what they understand and love, all possible forms of environmental education—via books, media, museums, nature centers, and classroom curricula—will be even more crucial in the future than they are today.
Every individual motivated to protect birds and habitats can help pass that passion on to others in his or her community. Thousands of interpretive nature centers, museums, and zoos rely on volunteers to help provide interpretation and hands‐on experiences for their visitors. Classroom teachers often seek local experts to assist with environmental material, both in the classroom and on field trips. Outstanding supplementary materials for environmental education are now available for elementary and middle‐school teachers. Donating such materials to schools—and volunteering to help teach them—can help the next generation learn about saving birds, wildlife, and habitats.
15.9.7 Birding with youngsters
Most people who are passionate about birds and conservation can identify the mentors who helped spark their initial interest in the natural world, sometimes even with just a single event or outing during childhood. Conservation will endure only if each generation contributes, and one of the best ways to help the next generation embrace the need to conserve birds and habitat is to share one’s own knowledge and passion with today’s children. Lifelong passions can begin as a consequence of remarkably simple experiences. The singular thrill of seeing a beautiful bird, or studying a complex display, through binoculars or a telescope can be just such an experience for a young person (Fig. 15.61). Seeing birds in real life and being taught how to use optics and field guides can open a child’s mind to a lifelong appreciation of diversity and beauty in nature. This appreciation is the all‐important first step on the pathway toward commitment to conserving such wonderful things.

Fig. 15.61 Promoting environmental ethics, continued respect, and responsibility. Respect and continued conservation interest will only occur if today’s concerned citizens transmit their knowledge and values to tomorrow’s citizens. (A) Fewer and fewer people are going outside, visiting natural parks, and permitting their children to experience nature. This graph shows that visitation rates to national parks in the USA have steadily declined in the last two decades. (B) Showing children the beauty of a bird in its natural setting is a wonderful, easy way to share environmental values with future generations.
(A, from Pergams and Zaradic 2006. Reproduced with permission from Elsevier. B, photograph by Molly Fitzpatrick.)
15.9.8 Contribute to conservation organizations
Not‐for‐profit organizations (also known as non‐governmental organizations, or NGOs) play vital roles in the conservation of birds and biodiversity all around the world. These charitable institutions depend upon gifts and bequests from the public, and in many countries such gifts can provide tax advantages to the donor. Most conservation organizations are staffed by mission‐driven, hard‐working individuals who earn only modest salaries and operate via four principal sources of revenue: charitable gifts, membership dues, and bequests from the public; grants from private or publicly funded foundations; contracts with public agencies or industry to carry out specific projects; and proceeds from permanently invested endowments.
The best NGOs have clear mission statements describing what they do and why they do it. Programs remain tightly focused to these missions and typically are described succinctly in an annual report providing the potential contributor an excellent way to learn about and compare different organizations. Discretionary resources such as public gifts help support essential operating costs, such as salaries for management, development, and clerical staff, building or rental costs, utilities, travel, computer hardware and software, and communications.
Hundreds of conservation‐oriented NGOs exist around the world, and their roles in accomplishing conservation vary widely. Local land trusts, country‐wide organizations, and a select few, large, international NGOs specialize in land acquisition or land management via conservation easements, often leveraging their own resources by forming partnerships with governmental agencies on public land. Many NGOs have strong programs in environmental education, delivered through publications, websites, citizen science projects, teacher‐training workshops, and interpretive nature centers. Some organizations concentrate on advocacy, strategically lobbying local, state, and federal legislators to influence public policy, public investment, or environmental regulations. Still others devote substantial resources to legal research and environmental litigation. Finally, many conservation organizations invest in scientific research to advance understanding in conservation biology, some by developing strong partnerships with research universities or natural history museums. All these roles are vital, because the challenges facing the natural world are far too great to be met solely by governments or by any organization acting in isolation. Therefore, contributing financially to conservation NGOs constitutes one of the most tangible ways in which an individual can participate in the process.
15.9.9 Never give up
The idea of personally investing in protecting birds and conserving nature may be new to some readers of this Handbook, while others may have been passionately involved since childhood. For both groups, the most important message of all is to do what you can, persist at it, and never give up. It is essential never to resign oneself to the notion that conservation battles cannot be won.
To be sure, many environmental losses caused by humans are now irreversible. We cannot bring back the Dodo (Raphus cucullatus), Elephant Bird (Aepyornis maximus), or moas. Sightings of Labrador Ducks (Camptorhynchus labradorius) and Kona Grosbeaks (Chloridops kona) ended forever more than a century ago. The Pink‐headed Duck (Rhodonessa caryophyllacea) appears to be gone from Asia, and the Slender‐billed Curlew (Numenius tenuirostris) may be lost from Europe, thus joining its North American cousin, the Eskimo Curlew (Numenius borealis), as testaments to overexploitation. Most sobering of all, despite abundances that defied imagination, Passenger Pigeons (Ectopistes migratorius) remain only as museum specimens and as a history lesson. All these species still call to us, but only about the immorality and immortality of our mistakes in allowing them to disappear.
Today, however, we have unprecedented capacity for learning and for change. If we retain the resolve to do so, we have the knowledge and ability to repair ecosystems, even ones damaged for centuries by cultures less aware than our own about the values that such places provide. We have more power than ever before to model the trajectories of populations and ecosystems and to predict with remarkable precision the ecological consequences of our actions before we commit to them. Instantaneous global communication has spawned revolutions, but it also provides the imperative to convince new generations of humans in every culture—the next managers of the earth—to avoid committing the mistakes made by our forebears. A growing list of conservation success stories proves that we really do have the power to bring bird species back from the very brink of extinction. In short, humans have extraordinary power to rescue and retain nature simply by deciding that we value other species, that we can protect natural systems if we put our minds to it, and that we can improve our behavior as managers of the earth.
It is by no means too late to imagine that human societies can coexist peacefully and gainfully with flourishing and intact natural systems. Such a day, however, will not be achieved without effort. We must put our collective minds to the task. Fortunately, enjoying and learning from birds will always be part of the process.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Alldredge, M. W., J. S. Hatfield, D. D. Diamond, and C. D. True. 2004. Golden‐cheeked warbler (Dendroica chrysoparia) in Texas: importance of dispersal toward persistence in a metapopulation. Pages 371–383 in Species Conservation and Management: Case Studies (H. R. Akcakaya, M. A. Burgman, O. Kindvall, C. C. Wood, P. Sjögren‐Gulve, J. S. Hatfield, and M. A. McCarthy, Eds.). Oxford University Press, New York, NY.
- Audubon, J. J. 1827–38. The Birds of America; from Original Drawings by John James Audubon, 4 vols. Published by the author, London.
- Batalha, M. A., M. V. Cianciaruso, and J. C. MottaJr. 2010. Consequences of simulated loss of open cerrado areas to bird functional diversity. Natureza and Conservação 8:34–40.
- BirdLife International. 2013. State of the World’s Birds: Indicators for our Changing World. BirdLife International, Cambridge, UK.
- BirdLife International. 2016a. Species factsheet: Falco naumanni. http://www.birdlife.org (last accessed February 2016).
- BirdLife International. 2016b. Species factsheet: Pterodroma alba. http://www.birdlife.org (last accessed February 2016).
- Bota, G., M. B. Morales, S. Mañosa, and J. Camprodon, Eds. 2005. Ecology and Conservation of Steppe‐land Birds. Lynx Edicions, Barcelona.
- Bouzat, J. L., H. H. Cheng, H. A. Lewin, R. L. Westemeier, J. D. Brawn, and K. N. Paige. 1998. Genetic evaluation of a demographic bottleneck in the Greater Prairie Chicken. Conservation Biology 12:836–843.
- Brawn, J. D. and S. K. Robinson. 1996. Source–sink population dynamics may complicate the interpretation of long‐term census data. Ecology 77:3–12.
- Breininger, D. R. and D. M. Oddy. 2004. Do habitat potential, population density, and fires influence Scrub‐Jay source sink dynamics? Ecological Applications 14:1079–1089.
- Brekke, P., P. M. Bennett, J. Wang, N. Pettorelli, and J. G. Ewen. 2010. Sensitive males: inbreeding depression in an endangered bird. Proceedings of the Royal Society B: Biological Sciences 277:3677–3684.
- Brown, S., M. Clarke, and R. Clarke. 2009. Fire is a key element in the landscape‐scale habitat requirements and global population status of a threatened bird: the Mallee Emu‐wren (Stipiturus mallee). Biological Conservation 142:432–445.
- Buller, W. L. 1888. A History of the Birds of New Zealand, 2nd Edition. Judd & Co., London.
- Burgess, N. M. and M. W. Meyer. 2008. Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology 17:83–91.
- Butchart, S. H. M., A. J. Stattersfield, and T. M. Brooks. 2006a. Going or gone: defining ‘possibly extinct’ species to give a truer picture of recent extinctions. Bulletin of the British Ornithologists’ Club 126A:7–24.
- Butchart, S. H. M., A. J. Stattersfield, and N. J. Collar. 2006b. How many bird extinctions have we prevented? Oryx 40:266–278.
- Buzzell, L. and C. Chalquist. 2009. Ecotherapy: Healing with Nature in Mind. Sierra Club Books, San Francisco, CA.
- Cade, T. J., J. H. Enderson, C. G. Thelander, and C. M. White, Eds. 1988. Peregrine Falcon Populations: Their Management and Recovery. Peregrine Fund, Inc., Boise, ID.
- Carson, R. 1962. Silent Spring. Houghton Mifflin, Boston, MA.
- Carver, E. 2008. Economic Impact of Waterfowl Hunting in the United States. Addendum to the 2006 National Survey of Fishing, Hunting, and Wildlife‐Associated Recreation Report 2006‐2. US Fish and Wildlife Service, Washington, DC.
- Carver, E. 2009. Bird‐Watching in the United States: A Demographic and Economic Analysis. Addendum to the 2006 National Survey of Fishing, Hunting, and Wildlife‐Associated Recreation Report 2006‐2. US Fish and Wildlife Service, Washington, DC.
- Cavalcanti, R. B. and M. A. S. Alves. 1997. Effects of fire on savanna birds in central Brazil. Ornitologia Neotropical 8:85–87.
- Cavalcanti, R. B. and C. A. Joly. 2002. Biodiversity and conservation priorities in the Cerrado Region. Pages 351–367 in The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna (P. S. Oliveira and R. J. Marquis, Eds.). Columbia University Press, New York, NY.
- Chase, M. K., W. B. Kristan III, A. J. Lynam, M. V. Price, and J. T. Rotenberry. 2001. Single species as indicators of species richness and composition in California coastal sage scrub birds and small mammals. Conservation Biology 14:474–487.
- Church, M. E., R. Gwiazda, R. W. Risebrough, K. Sorenson, C. P. Chamberlain, S. Farry, W. Heinrich, B. A. Rideout, and D. R. Smith. 2006. Ammunition is the principal source of lead accumulated by California Condors re‐introduced to the wild. Environmental Science and Toxicology 40:6143–6150.
- Cohen, J. E. 2003. Human population: the next half century. Science 302:1172–1175.
- Di Giacomo, A. G., A. S. Di Giacomo, and J. C. Reboreda. 2011. Effects of grassland burning on reproductive success of globally threatened Strange‐tailed Tyrants Alectrurus risora. Bird Conservation International 21:411–422.
- Diamond, J. M. 1975. The island dilemma: lessons of modern biogeographic studies for the design of natural reserves. Biological Conservation 7:129–146.
- Diamond, J. M., K. D. Bishop, and S. Van Balen. 1987. Bird survival in an isolated Javan Indonesian woodland: island or mirror? Conservation Biology 1:132–142.
- Duarte, A., J. L. R. Jensen, J. S. Hatfield, and F. W. Weckerly. 2013. Spatiotemporal variation in range‐wide Golden‐cheeked Warbler breeding habitat. Ecosphere 4:152.
- Ellis, E. C. 2011. Anthropogenic transformation of the terrestrial biosphere. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369:1010–1035.
- Finkelstein, M. E., D. F. Doak, D. George, J. Burnett, J. Brandt, M. Church, J. Grantham, and D. R. Smith. 2012. Lead poisoning and the deceptive recovery of the critically endangered California condor. Proceedings of the National Academy of Sciences of the United States of America 109:11449–11454.
- Fitzpatrick, J. W. and R. Bowman. 2016. Florida scrub‐jays: oversized territories and group defense in a fire‐maintained habitat. Pages 77–96 in Cooperative Breeding in Vertebrates (W.D. Koenig, J. L. Dickinson, Eds.). Cambridge University Press, Cambridge, UK.
- Fitzpatrick, J. W., M. Lammertink, M. D. Luneau Jr, T. W. Gallagher, B. R. Harrison, G. M. Sparling, K. V. Rosenberg, R. W. Rohrbaugh, E. C. H. Swarthout, P. H. Wrege, S. B. Swarthout, M. S. Dantzker, R. A. Charif, T. R. Barksdale, J. V. Remsen Jr, S. D. Simon, and D. Zollner. 2005. Ivory‐billed Woodpecker (Campephilus principalis) persists in continental North America. Science 308:1460–1462.
- Fitzpatrick, J. W., G. E. Woolfenden, and M. T. Kopeny. 1991. Ecology and development‐related habitat requirements of the Florida Scrub‐Jay (Aphelocoma coerulescens coerulescens). Page 49 in Florida Nongame Wildlife Program Technical Report No. 8. Florida Game and Fresh Water Fish Commission, Tallahassee, FL.
- Goble, D. D., J. A. Wiens, J. M. Scott, T. D. Male, and J. A. Hall. 2012. Conservation‐reliant species. BioScience 62:869–873.
- Goldstein, M. I., T. E. Lacher Jr, B. Woodbridge, M. J. Bechard, S. B. Canavelli, M. E. Zaccagnini, G. P. Cobb, E. J. Scollon, R. Tribolet, and M. J. Hooper. 1999. Monocrotophos‐induced mass mortality of Swainson’s Hawks in Argentina, 1995–96. Ecotoxicology 8:201–214.
- Goriup, P. D., J. Norton, L. A. Batten, and Joint Nature Conservation Committee. 1991. The Conservation of Lowland Dry Grassland Birds in Europe. Joint Nature Conservation Committee, Peterborough.
- Graveland, J. 1998. Effects of acid rain on bird populations. Environmental Reviews 6:41–54.
- Hansen, M. C., P. V. Potapov, R. Moore, M. Hancher, S. A. Turubanova, A. Tyukavina, D. Thau, S. V. Stehman, S. J. Goetz, T. R. Loveland, A. Kommareddy, A. Egorov, L. Chini, C. O. Justice, and J. R. G. Townsend. 2013. High‐resolution global maps of 21st‐century forest cover change. Science 342:850–853.
- Hepp, G. R. and F. C. Bellrose. 1995. Wood duck (Aix sponsa). In The Birds of North America, No. 169. (A. Poole and F. Gill, Eds.). Academy of Natural Sciences, Philadelphia, PA and American Ornithologists’ Union, Washington, DC.
- Hickey, J. J. and D. W. Anderson. 1968. Chlorinated hydrocarbons and eggshell changes in raptorial and fish‐eating birds. Science 162:271–273.
- Hoeck, P. E. A., M. E. Wolak, R. A. Switzer, C. M. Kuehler, and A. A. Lieberman. 2015. Effects of inbreeding and parental incubation on captive breeding success in Hawaiian crows. Biological Conservation 184:357–364.
- Holmes, R. T., J. C. Schultz, and P. Nothnagle. 1979. Bird predation on forest insects: an exclosure experiment. Science 206:462–463.
- Holmes, R. T. and T. W. Sherry. 2001. Thirty‐year bird population trends in an unfragmented temperate deciduous forest: importance of habitat change. Auk 118:589–609.
- Horne, J. S., K. M. Strickler, and M. Alldredge. 2011. Quantifying the importance of patch‐specific changes in habitat to metapopulation viability of an endangered songbird. Ecological Applications 21:2478–2486.
- IUCN (International Union for Conservation of Nature and Natural Resources). 2012. IUCN Red List of Threatened Species. Version 2012.1. IUCN, Cambridge, UK.
- Jones, H. P., B. R. Tershy, E. S. Zavaleta, D. A. Croll, B. S. Keitt, M. E. Finkelstein, and G. R. Howald. 2008. Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22:16–26.
- Kareiva, P. M. and M. Marvier. 2011. Conservation Science: Balancing the Needs of People and Nature. Roberts and Company, Greenwood Village, CO.
- King, D. I., J. D. Lambert, J. P. Buonaccorsi, and L. S. Prout. 2008. Avian population trends in the vulnerable montane forests of the northern Appalachians, USA. Biodiversity Conservation 17:2691–2700.
- Kirkby, C. A., R. Giudice‐Granados, B. Day, K. Turner, L. M. Velarde‐Andrade, A. Dueñas‐Dueñas, J. C. Lara‐Rivas, and D. W. Yu. 2010. The market triumph of ecotourism: an economic investigation of the private and social benefits of competing land uses in the Peruvian Amazon. PLoS One 5:e13015.
- Krebs, C. J. 2008. Ecology: The Experimental Analysis of Distribution and Abundance, 6th Edition. Benjamin Cummings, San Francisco, CA.
- Krueper, D., J. Bart, and T. D. Rich. 2003. Response of vegetation and breeding birds to the removal of cattle on the San Pedro River, Arizona (USA). Conservation Biology 17:607–615.
- Lacy, R. C. 2013. Achieving true sustainability of zoo populations. Zoo Biology 32:19–26.
- Lambert, J. D., D. I. King, J. P. Buonaccorsi, and L. S. Prout. 2008. Decline of a New Hampshire Bicknell's Thrush population, 1993–2003. Northeastern Naturalist 15:607–618.
- Larison, J. R., G. E. Likens, J. W. Fitzpatrick, and J. G. Crock. 2000. Cadmium toxicity among wildlife in the Colorado Rocky Mountains. Nature 406:181–183.
- Laurance, W. F. 2008. Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biological Conservation 141:1731–1744.
- Leopold, A. 1949. The Sand County Almanac. Oxford University Press, Oxford.
- Levey, D. J., B. M. Bolker, J. J. Tewksbury, S. Sargent, and N. M. Haddad. 2005. Effects of landscape corridors on seed dispersal by birds. Science 309:146–148.
- Lindenmayer, D. B., A. R. Northrop‐Mackie, R. Montague‐Drake, M. Crane, D. Michael, S. Okada, and P. Gibbons. 2012. Not all kinds of revegetation are created equal: revegetation type influences bird assemblages in threatened Australian woodland ecosystems. PLoS One 7:e34527.
- Lockwood, J. A. and L. D. DeBrey. 1990. A solution for the sudden and unexplained extinction of the Rocky Mountain Grasshopper (Orthoptera: Acrididae). Environmental Entomology 19:1194–1205.
- Loss, S. R., T. Will, and P. P. Marra. 2013. The impact of free‐ranging domestic cats on wildlife of the United States. Nature Communications 4:1396.
- Martin, P. S. and D. W. Steadman. 1999. Prehistoric extinctions on islands and continents. Pages 17–55 in Extinctions in Near Time: Causes, Contexts, and Consequences (R. D. E. MacPhee and H. D. Sues, Eds.). Springer, New York, NY.
- Miller, B. and K. J Mullette. 1985. Rehabilitation of an endangered Australian bird: the Lord Howe Island woodhen Tricholimnas sylvestris (Sclater). Biological Conservation 34:55–95.
- Miller, G. H., M. L. Fogel, J. W. Magee, M. K. Gagan, S. J. Clarke, and B. J. Johnson. 2005. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 309:287–290.
- Mourer‐Chauviré, C., R. Bour, and S. Ribes. 1995. Was the solitaire of Réunion an ibis? Nature 373:568.
- Muñoz‐Fuentes, V., C. Vilá, A. J. Green, J. J. Negro, and M. D. Sorenson. 2007. Hybridization between white‐headed ducks and introduced ruddy ducks in Spain. Molecular Ecology 16:629–638.
- Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca, and J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858.
- North American Bird Conservation Initiative Canada. 2012. The State of Canada's Birds, 2012. Environment Canada, Ottawa.
- Olsen, P. and M. Weston. 2005. Fire and Birds: Fire Management for Biodiversity. Birds Australia, Hawthorn East, Victoria.
- Parmesan, C. and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42.
- Pergams, O. R. W. and P. A. Zaradic. 2006. Is love of nature in the US becoming love of electronic media? 16‐year downtrend in national park visits explained by watching movies, playing video games, internet use, and oil prices. Journal of Environmental Management 80:387–393.
- Pratt, T. K., C. T. Atkinson, P. C. Banko, J. D. Jacobi, and B. L. Woodworth, Eds. 2009. Conservation Biology of Hawaiian Forest Birds: Implications for Island Avifauna. Yale University Press, New Haven, CT.
- Pregill, G. K., D. W. Steadman, and D. R. Watters. 1994. Late quaternary vertebrate faunas of the Lesser Antilles: historical components of Caribbean biogeography. Bulletin of the Carnegie Museum of Natural History 30:1–51.
- Prugh, L. R., C. J. Stoner, C. W. Epps, W. T. Bean, W. J. Ripple, A. S. Laliberte, and J. S. Brashares. 2009. The rise of the mesopredator. BioScience 59:779–791.
- Rabinowitz, D. 1981. Seven forms of rarity. Pages 205–217 in The Biological Aspects of Rare Plant Conservation (H. Synge, Ed.). Wiley, Chichester.
- Rabinowitz, D., S. Cairns, and T. Dillon. 1986. Seven forms of rarity and their frequency in the flora of the British Isles. Pages 182–204 in Conservation Biology: The Science of Scarcity and Diversity (M. E. Soule, Ed.). Sinauer Associates, Sunderland, MA.
- Randriamalala, H. and Z. Liu. 2010. Rosewood of Madagascar: between democracy and conservation. Madagascar Conservation and Development 5:11–22.
- Ratcliffe, D. A. 1967. Decrease in eggshell weight in certain birds of prey. Nature 215:208–210.
- Reed, J. M., D. D. DesRochers, E. A. VanderWerf, and J. M. Scott. 2012. Long‐term persistence of Hawaii's endangered avifauna through conservation‐reliant management. BioScience 62:881–892.
- Reillo, P. R. and S. Durand. 2008. Parrot conservation on Dominica: successes, challenges, and technological innovations. Journal of Caribbean Ornithology 21:52–58.
- Ribeiro, M. C., J. P. Metzger, A. C. Martensen, F. J. Ponzoni, and M. M. Hirota. 2009. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142:1141–1153.
- Ribic, C. A., R. R. Koford, J. R. Herkert, D. H. Johnson, N. D. Niemuth, D. E. Naugle, K. K. Bakker, D. W. Sample, and R. B. Renfrew. 2009. Area sensitivity in North American grassland birds: patterns and processes. Auk 126:233–244.
- Robbins, M. B., M. J. Braun, and D. W. Finch. 2003. Discovery of a population of the endangered Red Siskin (Carduelis cucullata) in Guyana. Auk 120:291–298.
- Robinson, W. D. 1999. Long‐term changes in the avifauna of Barro Colorado Island, Panama, a tropical forest desolate. Conservation Biology 13:85–97.
- Rodríguez, J. P., F. Rojas‐Suárez, and C. J. Sharpe. 2004. Setting priorities for the conservation of Venezuela's threatened birds. Oryx 38:373–382.
- Rothschild, L. W. R. 1907. Extinct Birds: An attempt to unite in one volume a short account of those Birds which have become extinct in historical times—that is, within the last six or seven hundred years. To which are added a few which still exist, but are on the verge of extinction. Hutchinson, London.
- Schaub, M., R. Zink, H. Beissmann, F. Sarrazin, and R. Arlettaz. 2009. When to end releases in reintroduction programmes: demographic rates and population viability analysis of bearded vultures in the Alps. Journal of Applied Ecology 46:92–100.
- Schorger, A. W. 1955. The Passenger Pigeon: Its Natural History and Extinction. University of Oklahoma Press, Norman, OK.
- Scott, J. M., D. D. Goble, J. A. Wiens, D. S. Wilcove, M. Bean, and T. Male. 2005. Recovery of imperiled species under the Endangered Species Act: the need for a new approach. Frontiers in Ecology and the Environment 3:383–389.
- Seavey, J. R., B. Gilmer, and K. M. McGarigal. 2011. Effect of sea‐level rise on Piping Plover (Charadrius melodus) breeding habitat. Biological Conservation 144:393–401.
- Selhub, E. M. and A. C. Logan. 2012. Your Brain on Nature: The Science of Nature's Influence on Your Health, Happiness and Vitality. John Wiley and Sons Canada, Mississauga, Ontario.
- Senner, N. R. 2012. One species but two patterns: populations of the Hudsonian Godwit (Limosa haemastica) differ in spring migration timing. Auk 129:670–682.
- Shaffer, M. L. and F. B. Samson. 1985. Population size and extinction: a note on determining critical population sizes. American Naturalist 125:144–152.
- Steffen, W., J. Grinevald, P. Crutzen, and J. McNeill. 2011. The Anthropocene: conceptual and historical perspectives. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 369:842–867.
- Stein, B. A., L. S. Kutner, G. A. Hammerson, L. L. Master, and L. E. Morse. 2000. State of the states: geographic patterns of diversity, rarity, and endemism. Pages 119–158 in Precious Heritage: The Status of Biodiversity in the United States (B. A. Stein, L. S. Kutner, and J. S. Adams, Eds.). Oxford University Press, Oxford.
- Sullivan, B. L., J. L. Aycrigg, J. H. Barry, R. E. Bonney, N. Bruns, C. B. Cooper, T. Damoulas, A. A. Dhondt, T. Dietterich, A. Farnsworth, D. Fink, J. W. Fitzpatrick, T. Fredericks, J. Gerbracht, C. Gomes, W. M. Hochachka, M. J. Iliff, C. Lagoze, F. A. La Sorte, M. Merrifield, W. Morris, T. B. Phillips, M. Reynolds, A. D. Rodewald, K. V. Rosenberg, N. M. Trautmann, A. Wiggins, D. W. Winkler, W. K. Wong, C. L. Wood, J. Yu, and S. Kelling. 2014. The eBird enterprise: an integrated approach to development and application of citizen science. Biological Conservation 169:31–40.
- Taylor, R. H. and B. W. Thomas. 1989. Eradication of Norway rats (Rattus norvegicus) from Hawea Island, Fiordland, using brodifacoum. New Zealand Journal of Ecology 12:23–32.
- Taylor, R. H. and B. W. Thomas. 1993. Rats eradicated from rugged Breaksea Island (170 ha), Fiordland, New Zealand. Biological Conservation 65:191–198.
- The Nature Conservancy. 2015. Eastern Ecoregions Map. The Nature Conservancy Eastern Regional Office, Boston, MA. https://www.conservationgateway.org/ConservationByGeography/NorthAmerica/UnitedStates/edc/reportsdata/terrestrial/ecoregional/Pages/default.aspx (last accessed January 2016).
- Thomas, C. D. and J. J. Lennon. 1999. Birds extend their ranges northwards. Nature 399:213.
- Tingley, M. W., W. B. Monahan, S. R. Beissinger, and C. Moritz. 2009. Birds track their Grinnellian niche through a century of climate change. Proceedings of the National Academy of Sciences of the United States of America 106:19637–19643.
- Tirpak, J. M., D. T. Jones‐Farrand, F. R. Thompson III, D. J. Twedt, and W. B. Uihlein III. 2009. Multiscale Habitat Suitability Index Models for Priority Landbirds in the Central Hardwoods and West Gulf Coastal Plain/Ouachitas Bird Conservation Regions. General Technical Report NRS‐49. US Department of Agriculture, Forest Service, Northern Research Station, Newtown Square, PA.
- US Fish and Wildlife Service. 2012. Waterfowl Population Status, 2012. US Department of the Interior, Fish and Wildlife Service, Washington, DC.
- van Riper III, C., S. G. van Riper, M. L. Goff, and M. Laird. 1986. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs 56:327–344.
- Wall, F. 1908. A popular treatise of the common Indian snakes, Part VIII. Journal of the Bombay Natural History Society 18(4):710–735.
- Walters, J. R., S. R. Derrickson, D. M. Fry, S. M. Haig, J. M. Marzluff, and J. M. Wunderle Jr. 2010. Status of the California Condor (Gymnogyps californianus) and efforts to achieve its recovery. Auk 127:969–1001.
- Watts, B. D. and B. R. Truitt. 2011. Decline of Whimbrels within a Mid‐Atlantic staging area (1994–2009). Waterbirds 34:347–351.
- Westemeier, R. L., J. D. Brawn, S. A. Simpson, T. L. Esker, R. W. Jansen, J. W. Walk, E. L. Kershner, J. L. Bouzat, and K. N. Paige. 1998. Tracking the long‐term decline and recovery of an isolated population. Science 282:1695–1698.
- Westemeier, R. L., S. A. Simpson, and D. A. Cooper. 1991. Successful exchange of prairie‐chicken eggs between nests in two remnant populations. Wilson Bulletin 103:717–720.
- Whitworth, D. L., H. R. Carter, R. J. Young, J. S. Koepke, F. Gress, and S. Frangman. 2005. Initial recovery of Xantus’s Murrelets following rate eradication on Anacapa Island, California. Marine Ornithology 33:131–137.
- Wich, S., A. Riswan, J. Jenson, J. Refisch, and C. Nellemann, Eds. 2011. Orangutans and the Economics of Sustainable Forest Management in Sumatra. UNEP/GRASP/PanEco/YEL/ICRAF/GRID‐Arendal, Birkeland Trykkeri AS, Norway.
- Willis, E. O. 1974. Population and local extinctions of birds of Barro Colorado Island, Panama. Ecological Monographs 44:153–169.
- Woodworth, B. L., C. T. Atkinson, D. A. LaPointe, P. J. Hart, C. S. Spiegel, E. J. Tweed, C. Henneman, J. LeBrun, T. Denette, R. DeMots, K. L. Kozar, D. Triglia, D. Lease, A. Gregor, T. Smith, and D. Duffy. 2005. Host population persistence in the face of introduced vector‐borne diseases: Hawaii amakihi and avian malaria. Proceedings of the National Academy of Sciences of the United States of America 102:51531–1536.