Chapter 11
Breeding Biology of Birds
David W. Winkler
Cornell University

(Photograph by Wim Boon.)
Birds are adapted to breed in an amazing diversity of habitats, and avian breeding biology is correspondingly varied and complex. To appreciate some of this wonderful diversity in how birds reproduce, consider for a moment the lives of three kinds of birds from very different environments around the globe.
First, imagine that it is the middle of the winter just outside the French antarctic station in Terre Adélie. A group of male Emperor Penguins (Aptenodytes forsteri) stands huddled together in the freezing, howling wind. These birds will remain at this location for over 100 days, each incubating his single egg atop his feet within a thick insulated flap of flesh and feathers. The egg will hatch just as sunlight is returning in the antarctic spring, giving the new penguin chick most of the highly productive summer to grow, develop, and fledge before the sun disappears again.
Next, imagine yourself in a wet coastal forest as you watch a male Australian Brush‐Turkey (Alectura lathami) poking its bill into the top of a giant pile of dirt and leaves. This male is the builder, tender, and protector of this impressive mound. Inside, the process of decomposition generates heat to incubate the eggs that female brush‐turkeys have laid and buried within the mound. As soon as they hatch, these eggs will produce the most self‐reliant of young birds, chicks that will walk, or fly, off into the forest for a life on their own.
Finally, imagine that you are in a grove of fan palms on the bank of an African river, where you see an African Palm‐Swift (Cypsiurus parvus) returning to its unusual nest. The female palm‐swift has used her sticky saliva to glue a shallow shelf of plant fluff and feathers to the side of a down‐hanging palm leaf, and then she has similarly glued her eggs into this minimal nest. She and her mate take turns incubating these eggs by snuggling up next to them on the palm frond. As soon as they hatch, the chicks will grasp onto this precarious nest and remain there for weeks; meanwhile, their parents will return hundreds of times to provision the chicks as they grow mature enough to forage for flying insects on their own.
These are just three of the many ways that different birds have evolved to raise their offspring in different environments. We arguably know more about the breeding biology of birds than about any other single aspect of their lives, and this chapter outlines some of the generalities shared by all breeding birds along with some of the fascinating variation in the reproductive strategies of different avian groups.
11.1 Timing of breeding
Most birds breed within a limited window of time that depends on the seasonality of the local environment and on aspects of that bird species’ natural history such as its diet, predators, nest type, and habitat. Most birds in temperate regions must breed within the resource‐rich period in the spring that is driven by a seasonal flush in vegetation, the hatching of insects, and the oncoming summer warmth. Seasonal temperature fluctuations become less dramatic towards the equator, and in many tropical habitats it is seasonal variation in rainfall that drives annual cycles in resource availability. Therefore, timing of breeding is often less constrained for birds that breed in the tropics, where some species breed exclusively during a dry season, others find optimal conditions during the transition between dry and wet seasons, and a few are able to breed year‐round.
The timing of availability of food for their young is the factor that seems to have the greatest overall influence on when birds breed. Ideally, nestlings will hatch just when those food resources are most plentiful. Birds often must lay eggs while food supplies are still rising, especially in environments with strong seasonal changes in resource availability. Since many species—particularly passerines—feed primarily insects to their nestlings, these avian parents often lay eggs just before peak insect abundance. In the most extremely seasonal locations towards the earth’s poles, the breeding season of avian insectivores may last only a month or so due to the correspondingly brief period of insect abundance in arctic habitats.
Bird species that feed their chicks on food other than insects often breed at different times than insectivores in the same avian community. For example, American Goldfinches (Spinus tristis) of North America rely almost exclusively on thistle seeds to feed their young (Fig. 11.01), and this species therefore breeds in mid‐summer when thistle seeds ripen, rather than earlier in the spring. Elsewhere, many honeyeater species in Australia breed when and where they can find the abundant blossoms of key Eucalyptus tree species. Likewise, Eleonora’s Falcons (Falco eleonorae) in the eastern Mediterranean lay eggs in late summer, insuring that the peak food demands of their nestlings coincide with the parents’ prime hunting season during the fall landbird migration (Chapter 13).

Fig. 11.01 Breeding season and food availability. American Goldfinches (Spinus tristis) breed later in the summer than most insectivorous birds occupying the same habitat, as they rely on the ripening seeds of thistles and other plants to build their nests and feed their young.
(Photograph by Stan Tekiela, www.NatureSmart.com.)
The more regular the seasonal changes, the easier it is for a potentially breeding bird to predict them. In most environments, year‐to‐year fluctuations are minor enough that the breeding season falls within a predictable period within each year. Most birds’ reproductive systems are primed to be active only during these months. The avian internal clock relies on changes in day length, or photoperiod, which cue a cascade of physiological preparations for breeding (Chapter 7). Birds generally use photoperiod to establish their general breeding period, but often rely on other cues to fine‐tune the initiation of breeding. For example, in the wet forests of Panama, the presence of live insects helps stimulate male Spotted Antbirds (Hylophylax naevioides) into high breeding condition (Hau et al. 2000). The more closely an environmental cue relates to a critical environmental feature, the better it serves as the basis for reproductive scheduling. For example, many birds that nest in arid environments, such as the Red‐billed Quelea (Quelea quelea) of Africa (Chapter 12), are driven to breed after a heavy rainfall (Cheke et al. 2007). The same is true for many desert‐nesting birds, such as Zebra Finches (Taeniopygia guttata) of central Australia and several species of ground finches in the arid lowlands of the Galápagos Islands (Chapter 3). For all of these species, rainfall is a cue that predicts the future availability of seeds and insects related to plants that grow only when water is available.
A few non‐insectivorous birds have evolved to avoid regular breeding seasons altogether. Although they breed most commonly in the spring and summer, Rock Pigeons (Columba livia) of many cities worldwide can breed year'round on a diverse diet of seeds and city scraps. The crossbills of the northern hemisphere initiate reproduction based on the availability of their key resource—pine and spruce seeds. Because the seed production of these trees varies greatly from place to place and from year to year, crossbills may breed as soon as they find an adequate food supply (Hahn 1998), no matter what month of the calendar it happens to be. Birds like crossbills that remain ready to breed upon encountering favorable conditions must pay the costs of maintaining their reproductive systems throughout the year. In contrast, the majority of birds, particularly those in strongly seasonal environments, shut down their reproductive system during the non‐breeding season (Chapter 7).
11.2 Breeding territories
Nearly all birds defend some kind of territory during the breeding season (Chapter 13). Although expensive in terms of time, energy, and personal risk, the benefits of defending a territory often outweigh these costs. The size and richness of a territory’s resources frequently influence mate choice and subsequent breeding success. Males of many species therefore defend large, multipurpose territories to attract one or more females and supply enough food for chick rearing. For example, females of both marsh‐nesting warblers in Eurasia and blackbirds in North America may choose mates on the basis of a male’s territory size. Similarly, female Purple‐throated Caribs (Eulampis jugularis) of the Lesser Antilles prefer males with a higher availability of nectar on their territories. Males in this hummingbird species defend much more nectar than they need for their own use, reserving parts of their territories for females (Temeles and Kress 2010).
Territories also reduce the chance that other individuals of the same species will interfere with the territory owner’s breeding activities. This kind of breeding interference is a large problem for some species. For example, male bowerbirds of Australia and New Guinea craft elaborate structures to attract females, often by stealing rare bower materials from bowers made by neighboring males; if left unguarded by its male owner for just one day, a bower may disappear entirely (Pruett‐Jones and Pruett‐Jones 1994). Many birds may also attempt to destroy the eggs of nearby competitors. For example, in North America, both Marsh Wrens (Cistothorus palustris) and House Wrens (Troglodytes aedon) routinely puncture the eggs of conspecifics and other potential nest‐site competitors; Crimson Rosellas (Platycercus elegans) do the same in Australia (Krebs 1998). Breeding birds often mitigate the risk of this kind of disturbance by guarding their nests. For example, in northern South America, Green‐rumped Parrotlet (Forpus passerinus) pairs guard their eggs diligently. To test the rate of clutch destruction without parental defense, researchers set up experimental nest boxes filled with replicas of this species’ eggs. These false nests were undefended, and nearby Green‐rumped Parrotlet groups destroyed 40% of these clutches within 3 days. By comparison, invading parrotlets destroyed only 5% of clutches in real nests that were actively defended (Beissinger et al. 1998).
11.3 Nests and nest building
Only insects surpass birds in the diversity and sophistication of their nests. A tremendous amount of literature, mostly from the nineteenth century, describes the nest‐building behavior of birds. Yet across the entire spectrum of bird species—from common backyard birds to exotic species in far‐off lands—aspects of the nesting biology of most species have yet to be studied. In terms of construction and placement, each type of bird nest incurs its own costs (such as parental effort and risk of predation or sabotage) and benefits (including greater nest safety and thermoregulatory efficiency). To explore these various costs and benefits, it is necessary to appreciate the functions that nests serve and the diversity of ways these aims are achieved.
11.3.1 Functions of nests
Most birds construct nests primarily to hold and protect their eggs, and to keep them together so that a parent bird can incubate them at the proper temperature for development. A simple scrape in the ground can serve as an adequate nest for some birds. A few birds that lay only one egg at a time have forgone nests altogether and incubate their single egg in other ways. However, most birds build some kind of nest. Nests sometimes serve additional functions: a few birds roost in their nests even beyond the breeding season, whereas others construct nests as a type of display to attract mates. For some species, mutual nest building is a common component of pair formation and bonding (Chapter 9). Nevertheless, nearly all nests must primarily provide a safe haven from predators and the elements.
A successful nest usually thwarts predators via its concealment and/or inaccessibility. The nests of most bird species are therefore purposefully difficult to find. For example, the risk of nest predation is particularly high in many tropical forest habitats, and many tropical passerines accordingly construct notably small nests that are inconspicuous when not attended. Most birds construct nests with common materials, generally avoiding brightly colored substances. To further enhance camouflage, some species affix lichens or bits of bark to a nest’s exterior. The nests of some passerines include a long, trailing pendent, perhaps to break up the visual profile of the nest shape. Most bird species are also quite adept at constructing out'of‐reach nests. For example, birds in groups as diverse as vireos, broadbills, and herons often prefer to nest precariously on the tips of long, high branches—places that most climbing predators like rodents or snakes are unable to reach. Other birds commonly build nests in tree cavities, within cliff nooks, or high on the walls of buildings.
Among the most interesting nest locations are those constructed near animals that deter predators (Chapter 14). Many tropical birds lay their eggs within or very near the nests of aggressive wasps (Joyce 1993) or ants (Young et al. 1990), both of which will attack an approaching predator. In the neotropics, some species of trogons, kingfishers, and parrots dig nest tunnels within active termite or ant mounds (Fig. 11.02). How (or why) these nesting birds are spared the insects’ attacks remains a mystery.

Fig. 11.02 Cavity nesting within active wasp and termite nests. Some birds dig cavities within social insect nests, most likely a strategy to deter predators. (A) A Slaty‐tailed Trogon (Trogon massena) entering a nest hole within an arboreal termite nest. (B) The entrance of a Black‐tailed Trogon (Trogon melanurus) cavity that the birds have excavated within an active arboreal termite nest.
(Photographs by: A, Jenn Sinasac; B, Rebecca Brunner.)
Nests also provide shelter from the elements. The microclimate around and within many nests is much more favorable for eggs and nestlings than is the surrounding environment. For example, temperate‐nesting hummingbirds often choose nest sites under overhanging trees to minimize heat loss at night (Calder 1973). Some gulls place their nests in the shade to reduce the risk of their nestlings overheating or becoming dehydrated (Winnett‐Murray 1980). Similarly, in the desert, where vegetation is scarce and solar radiation intense, many birds build enclosed nests to provide shade for their eggs and themselves. Nests may also prevent eggs and young from rolling out. New World orioles and oropendolas, Old World weavers, and Asian broadbills all build deep, enclosed nests at the tips of long, thin branches. This combination of nest structure and site works in tandem: the structure keeps the eggs from falling out as the nest swings wildly in the wind, and the location prevents access by predators like monkeys.
11.3.2 Diversity of nest locations and nest‐site selection
Birds nest in almost every terrestrial and shallow‐water habitat on Earth—from the surfaces of lakes to rock niches above the timberline, from the howling, frozen barrens of Antarctica to forests and deep caves in the tropics. The sites that birds occupy within these habitats are similarly diverse. Featureless cliff faces, holes and cracks of every type, branches of all diameters, bare ground, and myriad human constructions (skyscrapers, bridges, telephone poles, signs, oil pumps, old boots and hats, and even active ferries)—nearly anything that can support a nest has been used by birds at some time.
Beyond protection from predators and the elements, birds often choose nest sites that are close to a food supply. Nesting sites generally correspond with the ecology of the parent. For example, Crab Plovers (Dromas ardeola) nest in burrows in the sand, not far from the sandy tidal flats on the Indian Ocean where this species prefers to feed; the treeswifts of Asia nest high on thin branches in the midst of their aerial habitat. However, there are many exceptions: both the Secretary‐birds (Sagittarius serpentarius) of Africa and the two long‐legged seriema species of the South American savanna nest atop small trees, despite spending most of their lives running and walking on the ground. Similarly, the Black‐and‐white Warbler (Mniotilta varia) of North America nests on the ground, despite being a specialized forager on the surfaces of tree trunks and branches.
Although some birds have very specific requirements for their nest sites, others show substantial flexibility. Ospreys (Pandion haliaetus) generally nest in treetops, but on islands free of predators they will nest on the ground if no trees are available. Many species with long breeding periods build nests in different sites as their breeding habitat changes across the season. For example, American Robins (Turdus migratorius) breeding in North America often place their first nests of the season low in protected evergreen trees, whereas robins breeding later usually choose higher sites in newly leaved deciduous trees (Sallabanks and James 1999). As the weather becomes progressively hotter in the Arabian Desert, Greater Hoopoe‐Larks (Alaemon alaudipes) similarly shift their nesting preferences from open areas to shadier, shrubby sites (Tieleman et al. 2008).
Increasing evidence suggests that birds pay a great deal of attention to the disturbance and predation risks associated with a given habitat. For example, Rufous‐bellied Thrushes (Turdus rufiventris) in Argentina build a greater proportion of their nests in protective bromeliads when nesting in sites disturbed by human traffic (Lomáscolo et al. 2010). Birds must balance the benefit of a well‐hidden spot with the risk that the same mode of concealment may obscure an approaching predator from view. Playback experiments have shown that when exposed to the recorded calls of larger nest predators, Siberian Jays (Perisoreus infaustus) in northern Sweden chose nest sites in denser vegetation than those chosen by control birds that were not exposed to such recordings (Eggers et al. 2006). Some host species respond similarly to the presence of brood parasites (Chapter 13) that might lay eggs in their nest. For example, in an experiment in Montana (USA), researchers played calls of brood‐parasitic Brown‐headed Cowbirds (Molothrus ater) during the territory establishment periods of their potential host species. The hosts responded by settling in lower densities, compared with other areas where the cowbird calls were not played. Non‐hosts, to whom the presence of cowbirds offered no threat, established territories of equivalent density in both the test and control areas (Forsman and Martin 2009).
Closely related and ecologically similar bird species often compete for nest sites at least as much as they compete for food. Competition for nest holes can be particularly intense in some habitats. Moreover, nest predators often develop a search image after finding multiple nests with similar attributes. Thus, within any given patch of habitat, competition persists both within and among bird species to build nests in less occupied locations.
The males of some species display for a mate from a potential nest site, as do many weavers, herons, and wrens. Others display on a territory where the male has identified likely nest sites, as do marsh‐nesting blackbirds (Fig. 11.03). Species that exhibit these behaviors generally build nests that are demanding to construct or defend, or must nest within sites that are limited in space or resources. In other species, such as gulls, many passerines, and even some polyandrous birds such as jacanas, choosing a nest site appears to require a negotiation between the male and female; each considers sites identified by its mate. This early decision stage of the nesting cycle is hard to study because birds tend to be less committed towards the beginning of a breeding attempt, and slight disturbances caused by human observers may cause the pair to abandon a potential nest site. Therefore, ornithologists do not yet understand as much about this establishment stage of nesting as they do about later parts of the breeding cycle.

Fig. 11.03 Display on a breeding territory. Male birds like this Yellow‐headed Blackbird (Xanthocephalus xanthocephalus) attract mates by defending a high‐quality territory with many potential nest sites.
(Photograph by Herman H. Giethoorn.)
11.3.3 Diversity of nests
Nests vary wonderfully in composition and structure. They can be classified in many different ways; each nest type described below is classified primarily by its shape.
In a number of species, nesting involves site selection but no further nest construction whatsoever. Murres lay their single eggs directly on exposed rock ledges, and New World vultures and condors lay their eggs on the bare floors of shallow caves. White Terns (Gygis alba) of tropical seas lay a single egg on bare branches (Fig. 11.04), as do the potoos of the neotropics. The absence of a nest may be advantageous to these species for different reasons: parents need not waste time and effort nest building, exposed eggs may be less conspicuous to predators, and ectoparasites may be less of a problem when chicks are not restricted to the same soiled spot. Male Emperor Penguins (Aptenodytes forsteri) build no nest, and instead have evolved an incubation pouch that is formed by a loose bulge of skin above their feet; after a female lays her single egg for the season, she passes it to her mate for safe keeping while she leaves for a long period to feed in the distant ocean. All brood parasites also fall into the “nestless” category, as they lay their eggs in nests built by other birds.

Fig. 11.04 Some birds do not build nests. White Terns (Gygis alba) precariously lay a single egg on bare branches.
(Photograph by Duncan Wright, US Fish and Wildlife Service.)
The nests of some species consist of a simple scrape, in which birds displace loose gravel or sand on the ground to provide a slight depression for the eggs (Fig. 11.05). For example, many plovers, terns, and skimmers nest in a very shallow scrape, perhaps lined with a few flat pebbles or nothing at all. Some scrape‐nest species do not even create a depression at all if the substrate at their nesting site is too hard, but they may still arrange flat pebbles and twigs to create a very rudimentary nest.

Fig. 11.05 Scrape nest. Some species like this Ladder‐tailed Nightjar (Hydropsalis climacocerca) scrape an indentation in the substrate for their eggs. In these cases, the incubating adult generally blends in well with its surroundings. Here, the nightjar has just barely modified its nest site, placing this nest type at the boundary between a scrape nest and no nest at all.
(Photographs by Rebecca Brunner.)
Platform nests consist of a mound with a very shallow depression on top. Different birds build platforms on the ground, floating in water, or within trees or shrubs. Platform nests vary tremendously in complexity, from the flimsy platforms of twigs thrown together by most doves (Fig. 11.06) to massive structures—sometimes containing thousands of large sticks—built by storks or large birds of prey like Ospreys (Pandion haliaetus) and eagles. Birds may build platform nests anywhere they find a strong enough support. Many herons, cormorants, storks, and raptors build platform nests in trees, but will use the same types of sticks to nest on the ground if no trees are available. Oilbirds (Steatornis caripensis) build platform nests inside South American caves (Box 11.01). Flamingos build short pedestals of mud, and grebes and some terns build floating rafts of aquatic vegetation to support their shallow nests (Fig. 11.07). The Horned Coot (Fulica cornuta) of South America builds an interesting variant of this type of platform nest: occupying high Andean lakes with limited aquatic vegetation, these birds pile stones in the water, building a shallow nest of vegetation upon the stones at the surface (Fig. 11.08). The accumulation of stones built up by several pairs over a few years can reach up to 1 meter high and 4 meters in diameter, weighing up to a metric ton (Ripley 1957).

Fig. 11.06 Platform nest. Most dove species, such as this Wompoo Fruit‐Dove (Ptilinopus magnificus), build flimsy platform nests made of sticks.
(Photograph by Deane Lewis, http://dl.id.au.)

Fig. 11.07 Vegetation raft nest. Grebes and other aquatic species, like this Black Tern (Chlidonias niger), construct floating rafts of vegetation to support their nests.
(Photograph by Jean Troalen.)
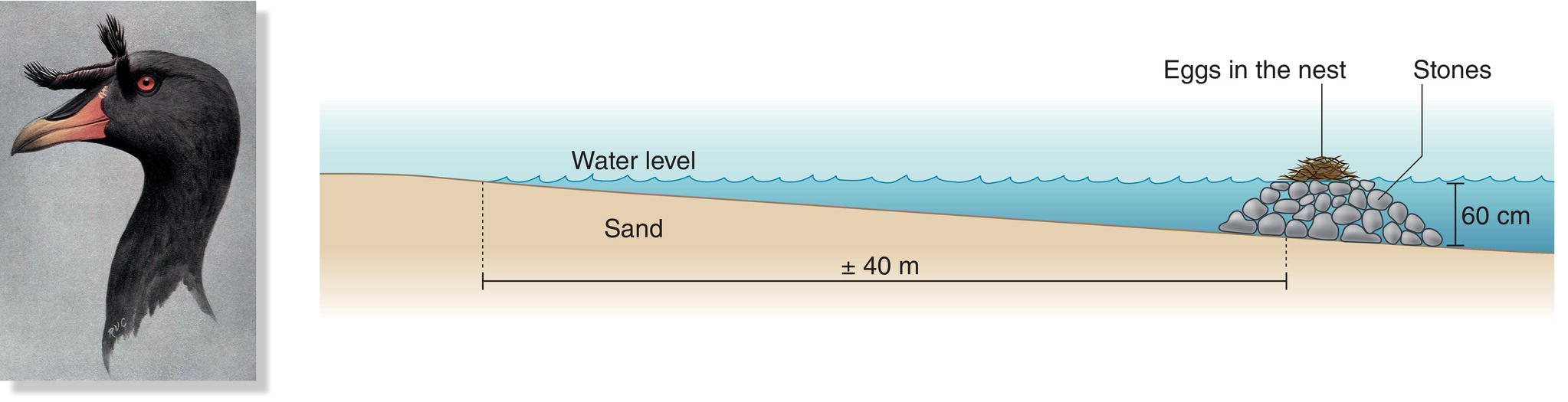
Fig. 11.08 Elevated nesting mounds. Horned Coots (Fulica cornuta) create nest mounds in shallow Andean lakes. These birds gather hundreds of stones until the top of their mound rises above the water surface; they then place debris and vegetation on top as a cushion for their eggs.
(Artwork by Robert V. Clem, from Ripley 1957.)
The majority of bird species build cup nests, structures with depressions on their top surfaces that are at least half as deep as they are wide. Birds place cup nests in a broad variety of sites: on the ground, in trees, beneath waterfalls, and within nest cavities of all kinds. Cup nests come in an equally diverse array of sizes and materials, although they are most commonly made of small twigs, dried grass, or mud. Crows and ravens build the largest cup nests; hummingbirds construct the smallest, often by securing lichens and soft fibers from seeds together with carefully collected spider webs (Fig. 11.09). As outlined below, ornithologists often categorize cup nests based on the nature of their support.

Fig. 11.09 Cup nest. Rufous Hummingbirds (Selasphorus rufus) gather spider webs, lichens, and “vegetable down” from seeds (A) to construct tiny, insulated cup nests (B).
(Photographs by: A, Penny L. Hall; B, David H. Wong, Vancouver, Canada.)
Simple, hard surfaces—the ground, a niche on a cliff, or, most commonly, tree branches—support “statant cups,” structures supported primarily from below. Oftentimes, gravity alone holds the nest in place. Bobolinks (Dolichonyx oryzivorus) of the New World build statant cups directly on the ground. Horned Larks (Eremophila alpestris) do the same in open country, with a slight twist: in order to keep their eggs slightly below ground surface to avoid foot traffic from large mammals, Horned Larks commonly build their statant cups within hollows of hardened cow or horse hoofprints. Most nests built in shrubs and trees are also statant cups, ranging from the loose piles of twigs and grass built by some songbirds to the heavy mud nests built by the Magpie‐larks (Grallina cyanoleuca), White‐winged Choughs (Corcorax melanorhamphos), and Apostlebirds (Struthidea cinerea) of Australia (Fig. 11.10).

Fig. 11.10 Statant cup nest. Constructed with mud, this Apostlebird (Struthidea cinerea) statant cup nest is supported by the tree branch from below.
(Photograph by Irby Lovette.)
“Pensile cups” hang by their rims, which are often securely attached to thin branch tips or reedy vegetation in marshes. New World blackbirds, vireos, and many other songbirds in Australia and Asia build pensile cups. The nest’s belly hangs unsupported, and the lack of sturdy structures surrounding pensile cups often minimizes predator access from below. Pensile nests with deeper centers are considered “pendulous cups.” Birds enter from the top of such nests, which are usually woven from plant strips or fibers. Pendulous cups are characteristic of New World orioles (Fig. 11.11), oropendolas, and caciques, as well as some Old World weaver species.

Fig. 11.11 Pendulous cup nests. The long, sock‐like nest of this Altamira Oriole (Icterus gularis) represents an extreme example of a pensile cup, supported only by its rim.
(Photograph by Greg Page.)
Usually made mainly of mud and/or saliva, “adherent cups” attach securely to vertical surfaces. Barn Swallows (Hirundo rustica), which breed throughout the northern hemisphere, build adherent cups by mixing mud with straw. Asian treeswifts use saliva to glue their tiny nests to the sides of branches (Fig. 11.12). The African Palm‐Swift (Cypsiurus parvus) even goes so far as to glue its eggs to a pad‐like vertical nest, incubating in a vertical position. White‐nest Swiftlets (Aerodramus fuciphagus) of Southeast Asia build perhaps the most extreme adherent cups, which often consist entirely of saliva. As their former common name “Edible‐nest Swiftlet” suggests, the nests of this species are the main ingredient in an Asian delicacy called “bird’s‐nest soup.” People have long harvested these nests in vast caves, climbing rickety skyscraper‐like scaffolds of bamboo to reach them. More recently, harvesters have instead constructed artificial caves for these birds—these structures resemble human houses, with the upper floor open to the air and the comings and goings of swiftlets. A swiftlet pair will often hurriedly build another nest if the first is removed, but these replacement nests usually contain bits of vegetation (as well as saliva) and are thus much less desirable as human food.

Fig. 11.12 Adherent cup nest. Grey‐rumped Treeswifts (Hemiprocne longipennis) and many other treeswift species use saliva to attach their minuscule nests, containing just one egg, to the sides of branches. After hatching, the nestling must balance in the nest or on the adjoining branch for several weeks.
(Photograph by Johnny Wee.)
Some bird species, especially those that nest on the ground amid vegetative cover, build domed nests. The woven dome, integrated with the woven cup, conceals the eggs or nestlings. In North America, meadowlarks build domed nests in grassy areas, and wood‐warblers called Ovenbirds (Seiurus aurocapilla) build them in forests (Fig. 11.13). In the Old World, many grass‐nesting species similarly build domed nests.

Fig. 11.13 Domed nest. The Ovenbird (Seiurus aurocapilla) is named for the domed shape of its nest, which resembles an old‐fashioned oven.
(Photographs by: left, Peter Caulfield; right, Scott R. Loss.)
Globular nests are completely enclosed (with the exception of a small side entrance). Built in shrubs or trees, these nests are characteristic of most wrens. For example, Cactus Wrens (Campylorhynchus brunneicapillus) of North America build grassy spheres, often within the spiny arms of a cholla cactus. In the same desert habitats, Verdins (Auriparus flaviceps) build smaller globe nests with spiny twigs, orienting the spines to the outside for extra protection. Black‐billed (Pica hudsonia) and Eurasian (Pica pica) Magpies build larger globular nests of thorny twigs, sometimes inserting short strands of barbed wire. Broadbills in Asia and some New World flycatchers and South American ovenbirds deter predators by building their globular nests at the ends of long vines or tendrils. Southern Penduline‐Tits (Anthoscopus minutus) of Africa build pendent globular nests with a false entrance to foil predators. A prominent hole on the side of the nest is actually a fake entrance leading to a dead‐end chamber; the true entrance is a concealed slit in the roof of the false entrance that closes behind the parent as it enters and exits the real nest chamber (Fig. 11.14).
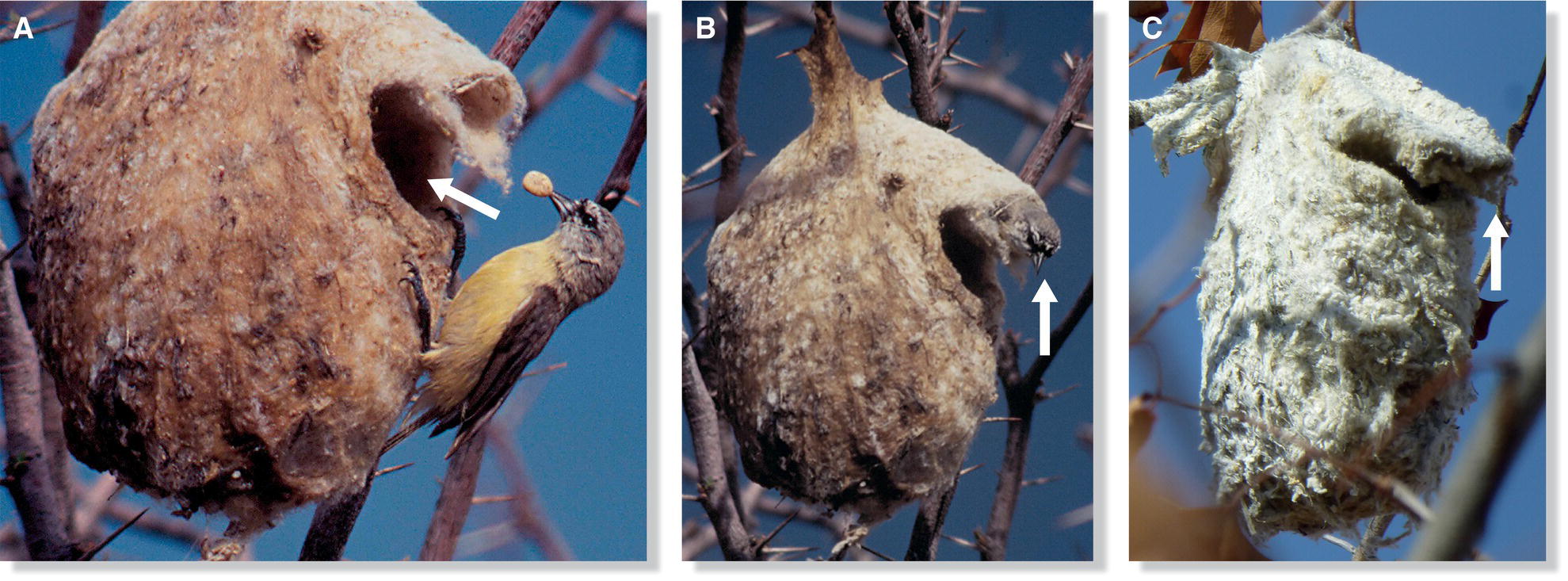
Fig. 11.14 Globular nest with false entrance. (A) Southern Penduline‐Tits (Anthoscopus minutus) build nests in which the larger, more obvious nest opening (arrow) is actually a false entrance. (B) The real nest chamber entrance is located above the false one (arrow). (C) When not occupied, the entrance tunnel lies flat, ending in a concealed slit (arrow).
(Photographs by: A and B, Mike Soroczynski; C, Peter LaBelle.)
A globular nest with an entrance tunnel is called a retort nest (Fig. 11.15). These nests are common among the mud‐nesting swallows, many African weavers, and some swifts. The lengths of these tunnels can vary from a few centimeters to more than a meter. Retort nests built with mud, especially those with very thick‐walled tunnels, can be very difficult for all predators except snakes to access.

Fig. 11.15 Retort nest. Birds like this Baya Weaver (Ploceus philippinus) build retort nests, which have a globular nest chamber with a distinct entrance tunnel.
(Photograph by Con Foley.)
A few species build mound nests, which generally consist of large piles of nest material on the ground or in large trees. Megapodes—the most famous mound nesters—are the only birds that use the nest itself, rather than a parent’s body, as the source of heat for developing embryos (Box 11.02). Only a few passerine species build true mounds: Palmchats (Dulus dominicus) on the island of Hispaniola and several species of African social weavers build colonial mound nests—resembling large, suspended haystacks—in palms or trees. Monk Parakeets (Myiopsitta monachus) use sticks to build colonial mound nests in their native Patagonia. The odd, heron‐like Hamerkop (Scopus umbretta) of Africa builds an enormous nest mound more than 2 meters high and wide, usually in a large tree (Fig. 11.16). Hamerkop nests may contain up to 8000 sticks and weigh up to several hundred kilograms. The nest chamber lies in the center of the mound, connected to the outside by a mud‐lined tunnel.

Fig. 11.16 Mound nests. This Hamerkop (Scopus umbretta) mound nest is in the process of being built in a tree. When finished, these nests can span more than 2 meters. (Batra, https://en.wikipedia.org/wiki/File:Hammerkop_Nest_Kenya_2012.jpg. CC‐BY‐SA 3.0.)
A tree hole is likely the most familiar sort of cavity nest. All woodpeckers are capable of excavating their own nest cavities (Fig. 11.17), sometimes even in healthy tree trunks, thanks to a host of adaptations (Chapter 6). Although woodpeckers are adept at creating nest cavities in trees, many species of parrots, barbets, toucans, nuthatches, and tits are capable of modifying pre‐existing holes to suit their needs. As mentioned earlier, some trogons and other tropical birds dig nest cavities within arboreal termite nests.

Fig. 11.17 Cavity nest. Woodpeckers like this Red‐headed Woodpecker (Melanerpes erythrocephalus) are able to excavate their own nest cavities, sometimes even in healthy trees. Other cavity‐nesting species must find pre‐existing holes (sometimes drilled by a woodpecker in the past); competition over these valuable nesting cavities can be intense.
(Photograph by Stephen Patten.)
When cavities in the ground are longer than they are deep, they are generally termed burrow nests. Numerous species in many different avian families excavate burrows in sandy soil or moist sand. In contrast to the physical characteristics required to excavate in wood, soil excavators do not usually have specialized adaptations for this task. Examples from the temperate zone include the Bank Swallow (Riparia riparia) and the Common Kingfisher (Alcedo atthis); both loosen soil with their bills and clear a tunnel with their weak feet (Fig. 11.18).

Fig. 11.18 Burrow nest. This Common Kingfisher (Alcedo atthis) excavates its nest in a clay bank by loosening soil with its bill and clearing the resulting tunnel with its feet.
(Photograph by Andy Holt.)
Cavity adopters occupy nest cavities created by other animals or through forces such as decay or erosion. Most cavity adopters throughout the world nest in naturally occurring tree holes or rock niches. Some birds that nest in adopted tree cavities rely on other species, usually woodpeckers, to create their holes (Chapter 13). Most artificial nest boxes placed out by humans are intended for cavity‐adopting species that typically nest in old woodpecker cavities. Australia has a particularly high proportion of cavity adopters even though no woodpeckers are found there; the Eucalyptus trees that dominate the Australian landscape develop natural cavities through fungal activity on scars resulting from dropped limbs. All nuthatches are cavity adopters, and many nuthatch species use mud to narrow the entrance to a hole in a tree branch or under a flap of tree bark. True to their name, Rock Nuthatches (Sitta neumayer), and the closely related Persian Nuthatch (Sitta tephronota), nest in rock face niches rather than trees. For added protection, these nuthatches construct a large mud nest within their rock cavity (Fig. 11.19). Many other birds also use mud to modify adopted cavities, and most design the entrance so that the parents may enter and exit with ease. In most hornbill species of Africa and Asia, females use mud and feces to seal themselves inside a nest cavity and remain there for months (Box 11.03). The male is solely responsible for supplying food to the female and their nestlings through a small slit in the mud wall of the cavity opening. In some hornbill species, mother and young emerge together; in others, the female emerges after the chicks are fairly well developed—in which case the nestlings seal themselves back in while both parents continue to feed them.

Fig. 11.19 Cavity adopters. Rock Nuthatches (Sitta neumayer) construct their nests in natural cliff crevices that they expand and enclose using clay and mud. Most other cavity adopters are less capable of modifying their nest sites, requiring fully formed cavities created by woodpeckers or natural weathering.
(Photograph by Amir Ben Dov, Israel.)
Some unusual birds build nests that do not fall cleanly within a single category of nest type. For example, the Rufous Hornero (Furnarius rufus) from South America builds a cup nest of grasses enclosed within a snail‐shaped sphere of hardened mud, complete with separate entrance and egg chambers (Fig. 11.20). In central Africa, Forest Swallows (Petrochelidon fuliginosa) build mud retorts within tree cavities. The Common House‐Martin (Delichon urbicum) of Eurasia builds the walls of its mud cup all the way up to an overhanging surface, incorporating the overhang as the top of its nest. The extremely small entrance presumably limits predator access.
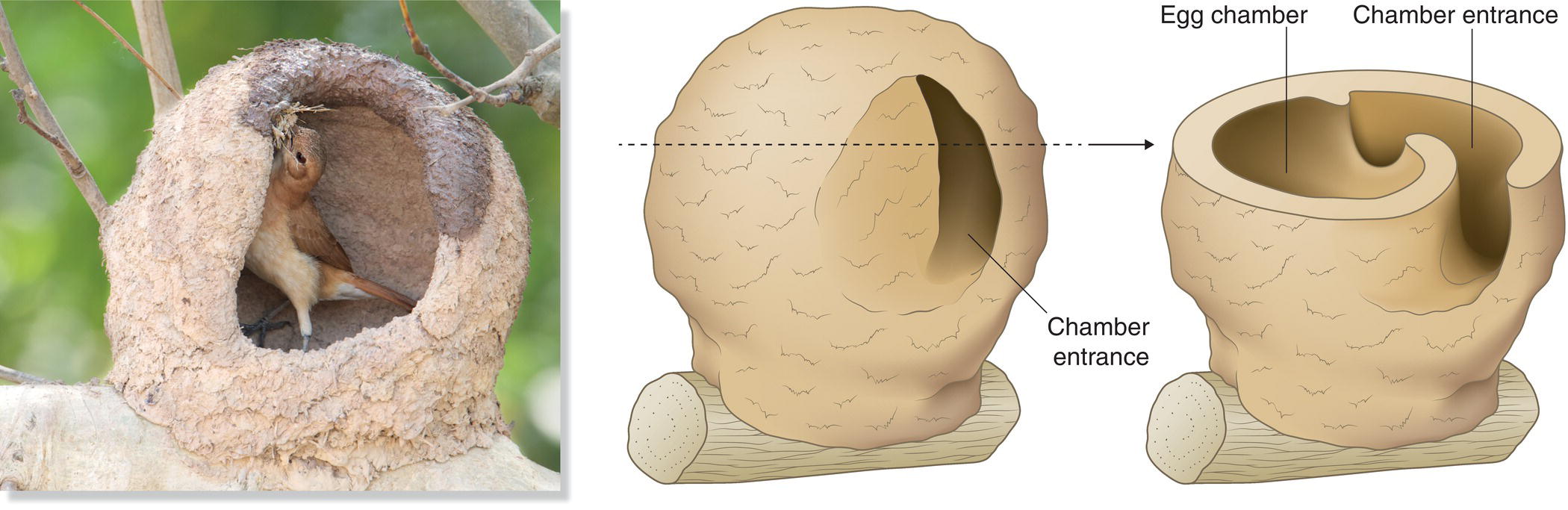
Fig. 11.20 A Rufous Hornero (Furnarius rufus) nest under construction. This species builds a cup nest of grasses enclosed in a statant sphere of hardened mud, creating a separate entrance and egg chambers. These nests often harden to the consistently of cement; they generally last for many years and are sometimes occupied by cavity‐adopting species in subsequent years.
(Photograph by David W. Winkler. Illustration © Cornell Lab of Ornithology.)
11.3.4 Nest linings
Many, if not most, bird nests are lined with materials different than those used in constructing the outer structure. Birds use a great many kinds of materials to line their nests. A bowl of sticks is usually lined with softer materials, such as plant fibers, mammal hair, or dried grass. A tree cavity may have fur, bark strips, moss, or feathers covering the bottom. The likely primary function of most nest linings is to help insulate the nest and its living contents and prevent chicks from getting tangled in the coarser outer nest materials.
Many species line their nests with various combinations of natural materials. Most thrushes build a nest of twigs, grass, and leaves cemented together with mud and lined with soft, fine grasses. The Song Thrush (Turdus philomelos) of Eurasia forms the mud portion of its nest into a hard, smooth lining—sometimes using rotten wood, dung, or peat. Most of the wood‐warblers of the New World line their nests with fine dried grasses, but the Worm‐eating Warbler (Helmitheros vermivorum) of North America lines its nest with the spore capsule stalks of hair moss (Vitz et al. 2013). A surprising number of bird species worldwide line their nests with thin, hair‐like fungus fibers (Fig. 11.21): one survey found filaments of horse‐hair fungus (Marasmius) in the nests of 98 bird species, spanning 27 families throughout tropical and nearctic regions (Aubrecht et al. 2013). Some previous reports citing the use of “animal hair” in nests likely mistook horse‐hair fungus as real animal hair—an easy mistake, given how closely they resemble one another (McFarland and Rimmer 1996).

Fig. 11.21 Nest lining with fungus fibers. (A) Horse‐hair fungus is one of the most common nest lining materials worldwide. (B) For example, Bicknell’s Thrush (Catharus bicknelli) nests are often lined with filaments of this fungus.
(Photographs by K. P. McFarland, Vermont Center for Ecostudies.)
Many birds also use feathers to line their nests. Most female waterfowl line their nests with down plucked from their own breasts, while several songbird species line their nests with feathers that are not their own. The Long‐tailed Tits (Aegithalos caudatus) of Europe could be considered the champions of feather collecting, often lining their oval nests with between 1000 and 2000 feathers (Fig. 11.22).

Fig. 11.22 Feather nest lining. (A) This Long‐tailed Tit (Aegithalos caudatus) has collected the feather of another species to line its nest. (B) A nest of this species has been torn open by a predator, revealing the great number of feathers incorporated into its lining.
(Photographs by: A, R. Zap; B, Ros Wood, LRPS.)
A variety of species, ranging from raptors to starlings, line or adorn their nests with green plant material. Early biologists, failing to see a function for this “decoration,” wondered if these birds simply might be expressing an aesthetic sense. However, research on European Starlings (Sturnus vulgaris) suggests that the green plants used as nest lining contain chemicals that repel or kill avian ectoparasites (Wimburger 1984; Clark and Mason 1988). Subsequent research on Spotless Starlings (Sturnus unicolor) suggests that the compounds in these plants may affect the hormones of the attending female, and perhaps even the sex ratio of the offspring reared in the nest (Polo et al. 2010).
11.4 Nest‐building behaviors
Nest‐building behaviors vary based on the complexity of the nest structure, the availability of materials, and the number of birds involved in the construction of the nest. Clearly some nest types involve more investment and skill to construct than do others. For example, scrape nesters probably spend much more time locating a suitable site than in forming the simple nest depression itself.
When building platform nests, most birds lay a foundation of coarse sticks across branches or another supporting substrate, then add additional sticks until the bulk of the platform is built, and finish with finer sticks and a lining, if any. Building a cup nest often requires more specialized techniques. As in platform construction, birds often start with coarse material for the nest foundation, adding finer materials as they work upward and inward toward the cup. To pack and solidify the nest, the building bird may sit and turn in the center, thrusting its legs and pressing its breast against the surrounding materials. Once the cup is coherent and strong, the bird will often add lining—either by packing it down via continued breast lunges, body rotations, and belly quivers, or by using its bill to weave the lining into existing materials. The aptly named Common Tailorbird (Orthotomus sutorius) of Southeast Asia adds an extra step at the beginning of this process by sewing a funnel‐shaped cradle, and then building its soft cup nest inside, under its cover: first, it pierces a series of small holes near the edges of two large, living leaves. Then, it inserts plant fibers, cobwebs, or cocoon silk through the holes and draws the leaves together (Fig. 11.23).

Fig. 11.23 The stitchwork nest of the Common Tailorbird (Orthotomus sutorius). This bird builds a cup nest within a “cradle” of one or more large leaves, which it sews together using pliable grasses, cobwebs, or silk. In the left photo, note how the bird stitches through the leaf, much as a person using a sewing needle would stitch through a piece of cloth.
(Photographs by: left, Anton Croos, http://commons.wikimedia.org/wiki/File:Nest_of_tailorbird.jpg. CC‐BY‐SA 3.0; right, Adityamadhav83, http://commons.wikimedia.org/wiki/File:(Orthotomus_sutorius)_Common_Tailorbird_nests_at_Madhurawada_04.JPG. CC‐BY‐SA 3.0.)
Pendulous cup nesters exhibit even more intricate building behaviors. With incredible dexterity, these avian weavers often hold onto structural material with their feet, using their bills to weave strands together and form knots. These expert builders flit their heads back and forth, over and under, meticulously positioning supple fibers—usually freshly gathered grass or other green and pliant plant fibers—into a pendent formation. As the fibers dry, the nest walls solidify with considerable integrity and tensile strength. The globular and retort nests of African weavers are created using perhaps the most impressive and elaborate of all avian weaving techniques. These birds use a variety of knots in their constructions, producing nests of remarkable durability and unrivaled intricacy (Fig. 11.24). The orioles and oropendolas of the western hemisphere—quite distant relatives of the African weavers—use most of the same knots (Heath and Hansell 2002).
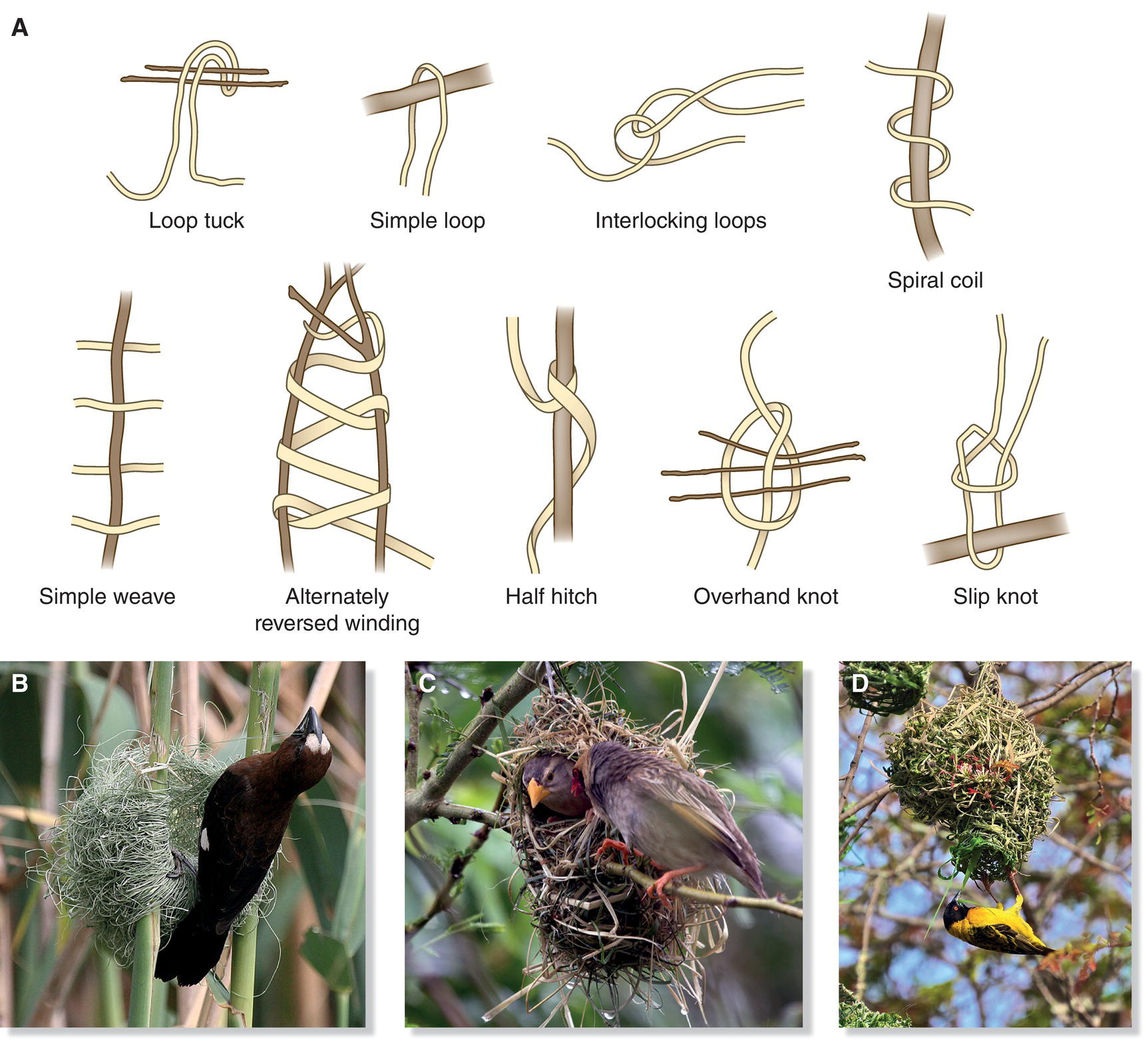
Fig. 11.24 Avian weaving techniques. (A) African weavers employ a variety of patterns and knots during nest construction. (B) The Grosbeak Weaver (Amblyospiza albifrons) constructs its nest with simple loops and tucks. (C) Red‐billed Queleas (Quelea quelea) use spiral coils in their crudely woven nests. (D) The Village Weaver (Ploceus cucullatus) uses more sophisticated stitches, including alternately reversed winding, simple weaves, and half hitches. Overhand and slip knots are even more complex.
(A, from Collias and Collias 1964. Courtesy of Karen Collias Whilden. Photographs by: B, Robert Lewis; C, Susan Knodle; D, Alexander Yates.)
Mud‐nesting birds also employ a great variety of construction methods. The most common technique involves dipping dried vegetation into mud before applying it to the inner nest layers. Many species incorporate mud in this way. Certain swallow species represent the only birds that build adherent nests solely of pure mud (Fig. 11.25). Selecting the right consistency of mud to meet the extraordinary engineering demands of these nests must require considerable discernment.

Fig. 11.25 Mud nests. Cliff Swallows (Petrochelidon pyrrhonota) transport mouthfuls of mud to build and attach their nests to walls and cliffs. Most other mud‐nesting birds use a combination of mud and vegetation.
(Photographs by: left, Cameron Rognan; right, Mike's Birds, https://commons.wikimedia.org/wiki/File:Cliff_Swallow_building_a_nest_(4737045414).jpg. CC‐BY‐SA 2.0.)
11.4.1 Sex roles in nest building
Males and females play various roles in nest construction, depending on the species. However, responsibilities tend to be fairly consistent within certain groups. For example, in hummingbirds and most other lekking avian groups in which there is strong sexual selection on males (Chapters 3 and 9), the female builds the nest entirely on her own. The opposite is true for polyandrous species, such as phalaropes and jacanas, in which the male builds the nest. In many sexually dimorphic species with brighter males, the female often assumes responsibility for most of the nest construction, perhaps because her duller colors help avoid detection of the nest location by predators. When the male and female are similar in appearance, they often each take a nearly equal share in nest building—as in many gulls, corvids, and herons. In these species, the male may gather most of the material while the female builds the nest. In other birds, males take the lead in nest building and the female then adds the lining when the nest is finished.
Many male wrens, and some raptors and weavers, construct surplus nests on their territories. Although the function of these extra nests is open to debate, they may allow rapid re‐nesting if a first nest is lost to predators or competitors. Extra nests may also help attract females: a study of Eurasian Wrens (Troglodytes troglodytes) in the UK showed that the more nests a male had on his territory, the more likely he was to obtain multiple mates—a few such males with multiple nests attracted as many as five females (Evans and Burn 1996). In other species, females evaluate males based on the craftsmanship of their nests. Red‐billed Queleas (Quelea quelea) establish nests and pairs at about the same time, forming enormous colonies very rapidly. To attract a mate, a male builds the first half of a nest and displays from it; if a female deems the half‐nest worthy, the pair builds the rest together.
11.4.2 Duration of nest building
The amount of time required for nest building depends on the type of nest (more elaborate nests take longer), the stage of the breeding season (construction often takes less time as the season progresses), and the weather (rain and snow often cause delays). A songbird in a temperate zone typically spends about 6 days building its simple cup nest—3 days on the outer layer and 3 days on the lining. With shorter breeding seasons, temperate birds must generally build faster than their counterparts in the tropics. For example, a tiny antwren in Panama may spend 10–15 days to build a comparable cup nest. Great Kiskadees (Pitangus sulphuratus), a type of neotropical flycatcher, build bulky, domed nests over several weeks; Chestnut‐headed Oropendolas (Psarocolius wagleri) require about a month to construct their long, pendulous nests. While building a nest, birds also forage, familiarize themselves with their territory and/or mate, and interact with neighbors. Hamerkop (Scopus umbretta) pairs can build their enormous nests in as little as 20–25 days, but they typically spend only a few hours intensively nest building on each of those days (Wilson and Wilson 1986).
11.4.3 Nest appropriation and reuse
Most birds construct a new nest every year, especially if a previous nest supported young all the way through to fledging. After a single use, constructed nests often become flattened and misshapen. Used nests may also be littered with feces, uneaten food, the debris of nestling feather sheaths, and perhaps even the carcass of a chick that did not survive to fledging. These components produce odors that might attract nest predators; used nests also support a wealth of bacteria and invertebrates, some helpful and others harmful. Even most woodpeckers, whose cavities take much effort to excavate, usually create new nests every year. However, starting from scratch each year is not a universal rule; for example, raptors and other large birds with exposed nests may reuse them for generations, and many seabirds will return to the same rock ledge or burrow for their entire lifetimes.
Reusing old nests must provide an advantage for some birds, because many species appropriate the used nests of other species. For example, small gulls and some sandpipers that nest in northern boreal forests appropriate the old nests of landbirds in small spruce trees. Great Horned Owls (Bubo virginianus) of North and South America often adopt large nests of other species such as hawks, crows, or even squirrels. The large, thorny nests of South American thornbirds (Fig. 11.26) are known to be re‐used by at least 11 other bird species (Lindell 1996). Sometimes an old nest is simply used as a physical support for a new nest: in North and Central America, Mourning Doves (Zenaida macroura) may place their flimsy stick nests on almost any elevated support, including the old nests of robins, grackles, or thrashers. The Little Swift (Apus affinis) of Africa adopts old mud retorts originally constructed by swallows, often affixing white feather “decorations” around the entrance.

Fig. 11.26 The well‐defended nest of the Chestnut‐backed Thornbird (Phacellodomus dorsalis). Thornbirds build large globular nests out of sticks; later, these nests are often reused by other species.
(Photograph by Fabrice Schmitt.)
11.5 Eggs
Birds are the only major vertebrate group without at least one live‐bearing species: fish, amphibians, reptiles, and mammals each include at least a few live‐bearing species. Exactly why no live‐bearing birds have evolved remains a bit of a mystery, but one compelling idea is that bearing live young would weigh down the female bird throughout gestation, and thereby either hinder her flight ability or limit her brood size. However, the fact that female bats successfully bear live young while flying competently through their entire pregnancy presents an argument against this hypothesis. Laying external eggs also allows both avian parents to at least potentially aid in their care, as well as the opportunity to abandon the eggs if conditions or circumstances are adverse—thus providing a chance for the parents to survive and rear broods in the future.
All avian eggs have the primary function of protecting the embryo inside as it develops. Yet the eggs of various bird species differ extensively in size and shape, shell coloration and structure, and the relative proportions of albumen and yolk. From the smooth white of some domestic chicken (Gallus gallus) eggs to the granulated, avocado‐like capsule of the Emu (Dromaius novaehollandiae), bird eggs present a dazzling array of diversity. The study of egg diversity is a specialized branch of ornithology termed oölogy (Chapter 13).
11.5.1 Egg structure
The eggs of fish and most amphibians can survive only in water, tying these animals to an existence dependent upon aquatic environments. Bird eggs, in contrast, are able to retain their aqueous contents and develop on dry land across a great range of environmental conditions. Ancestral archosaurs (Chapter 2) first evolved a hard‐shelled egg with internal membranes. Because their eggs contained the watery medium required by embryos to develop, these animals no longer needed to live in or near water; thus began the long evolution of one large branch of land animals, including birds.
Bird eggs are large, especially when compared with shell‐less human eggs, which are about the size of the period at the end of this sentence. The only mammals with eggs at all similar to birds are the monotremes—the four species of echidnas (spiny anteaters) and the Platypus (Ornithorhynchus anatinus)—that lay soft, leathery eggs instead of giving birth to live young. Embryos contained within the large external eggs of birds, reptiles, and monotremes must be entirely resource‐independent during early development—unlike all other mammals, whose mothers internally provide sustenance to the embryo. Thus, a bird egg must be packed with all of the protein, carbohydrates, fats, and water that the embryo will need until it hatches.
One way to understand the structure of an egg is to follow it through the developmental process (Chapter 6). Initially, the ovum is pushed out of its surrounding follicle cells in the ovary, and it is generally fertilized by a sperm as it begins its journey across the infundibulum into the oviduct. As the egg moves down through the oviduct, it passes through a succession of glandular cells that secrete successive layers of albumen and shell membranes; the shell is added last, very near the terminus of the oviduct. Because not one of these added layers involves the addition of new cells with their own nuclei, the egg of an Ostrich (Struthio camelus)—with its single nucleus protected by layers of non‐cellular material—is one of the largest living cells we can observe today.
To acquaint yourself with the major features of an egg, envision or explore a chicken egg from your kitchen (Fig. 11.27). As you crack a chicken egg, notice how a thin membrane holds the brittle mineral shell together, adhering to its inner surface, somewhat akin to the layers of auto safety glass. This membrane sticks tightly to the shell. Another membrane surrounds the albumen, or egg white; this layer can be frustrating to peel off a hard‐boiled egg. Once you have broken through these two outer membranes, you are able to spill the contents of the egg into a bowl; you can see the yolk surrounded by the white, now released from its membrane. Although difficult to see, the yolk itself is surrounded and held together by the vitelline membrane; this membrane is what you rupture if you “break” a yolk. You may notice that the yolk rotates so that a circular, white spot on its surface is upward in the dish. This tissue, termed the blastoderm, would develop into the embryo if the egg were fertilized. If you ever have tried to separate a yolk from the white of a broken egg, you have encountered the gelatinous, stringy parts of the albumen that often are twisted and milky white in color. Hard to separate from the yolk, these tissues are the chalazae. Before the egg was cracked open, the chalazae were connected to the inner face of the shell at the distant ends of the egg, thus suspending the yolk in the center of the albumen, protecting it and holding it in place within the egg.
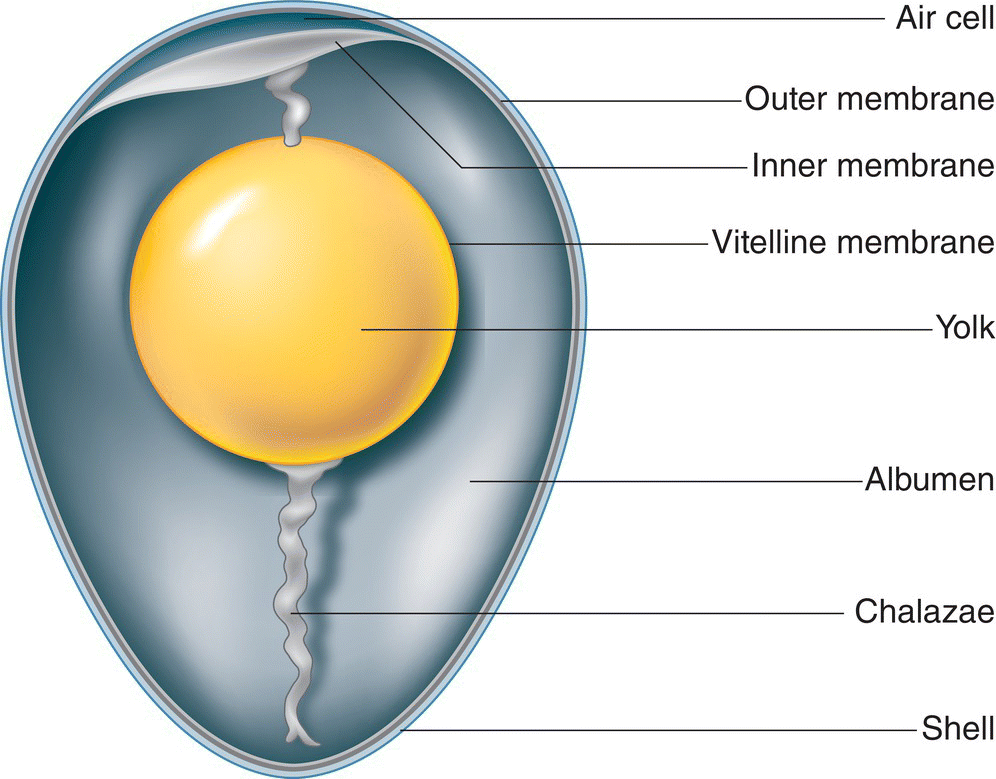
Fig. 11.27 Basic egg anatomy. This cross‐section schematic shows some of the major internal components of an avian egg.
(Illustration by Andrew Leach © Cornell Lab of Ornithology.)
Now we will consider the structure and function of each component in greater detail, beginning with the yolk. The yolk contains all of the lipids (fats) and most of the protein needed to nourish the developing embryo until it hatches. The relative sizes of the yolk and white vary among groups of birds: yolks tend to make up a larger proportion of the total egg mass in species with precocial chicks, which are very well developed at hatching (such as waterfowl, shorebirds, and pheasants), compared to species with altricial young (such as passerines) in which the yolks are smaller. This difference arises from the fact that precocial chicks usually develop in the egg for a longer period of time and therefore require more sustenance, and thus a larger yolk.
The yolk is the first part of the egg produced by the laying female. Before the yolk passes down the oviduct for the addition of albumen and shell, it undergoes rapid growth over a period of about 5 days to 2 weeks. However, many birds—especially songbirds—usually lay one egg per day (and never more). Because yolk preparation requires more than a day, females often prepare yolks in enough ova for a complete clutch before laying their first egg. During this preparation period—sometimes called the “yolking up” stage—the yolk is deposited within the vitelline membrane in alternating bands of darker and lighter yolk, producing a structure that resembles growth rings in a tree when viewed under special laboratory conditions (Fig. 11.28). Because these bands alternate on a daily cycle (dark bands are deposited during the day, when the female ingests food rich in pigments), the yolk ring structure reveals how long each female needs to prepare its yolks for laying. The only structure that interrupts these concentric rings is a cylinder of light‐colored yolk that stretches from the yolk’s core to its surface, where the germinal spot sits. The embryo will develop here, beginning as the flattened disk‐like blastoderm. Since the germinal spot and its column are lighter than the rest of the yolk, the developing embryo always floats to the top, no matter which way the egg is turned.
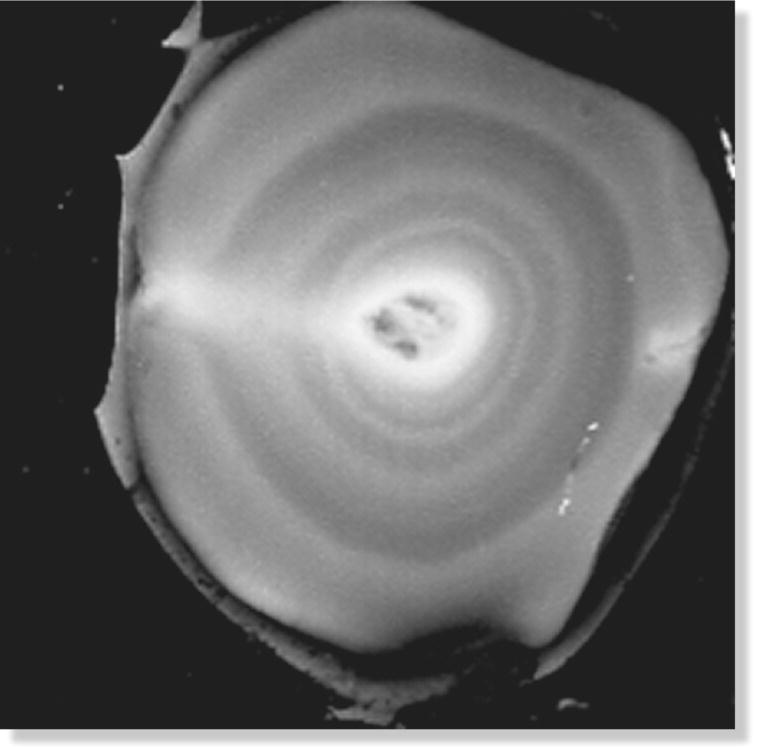
Fig. 11.28 Yolk rings. Over the course of several days, the yolk is deposited within the vitelline membrane in alternating bands of darker and lighter color. In this cross‐section image through a yolk, the paler band cutting across the rings terminates at the germinal spot on the yolk surface, where the embryo will eventually develop.
(Photograph by David W. Winkler.)
The yolk would float up against the shell if the chalazae did not suspend it at the center of the egg. The albumen, which varies in viscosity depending on the proportion of water and protein within it, consists of a series of layers around the yolk. A very thin layer of viscous albumen surrounds the yolk and extends into the chalazae, followed by a thin layer of watery albumen, a thick layer of more viscous and variably fibrous albumen (the largest component), and finally a thin layer of watery albumen right beneath the shell. In addition to providing nearly all of the water and much of the protein for the developing embryo, these layers of albumen serve admirably to protect the embryo from physical damage, provided the shell is not broken.
The shell is the developing embryo’s outer line of defense (Fig. 11.29). Avian eggshells are much thicker and stronger than the shells of other egg‐laying vertebrates. However, this increased thickness renders gas exchange across the shell more difficult. The growth and respiration of a developing embryo require oxygen and produce carbon dioxide. The egg cannot contain all of the oxygen needed to fuel the embryo’s metabolism, nor can it hold all of the carbon dioxide and wastewater produced by the embryo before it hatches. Therefore, the developing embryo must be able to “breathe” through pores in its shell. An egg loses, on average, 18% of its mass between laying and hatching (Rahn and Ar 1974), mostly from water loss across the shell.
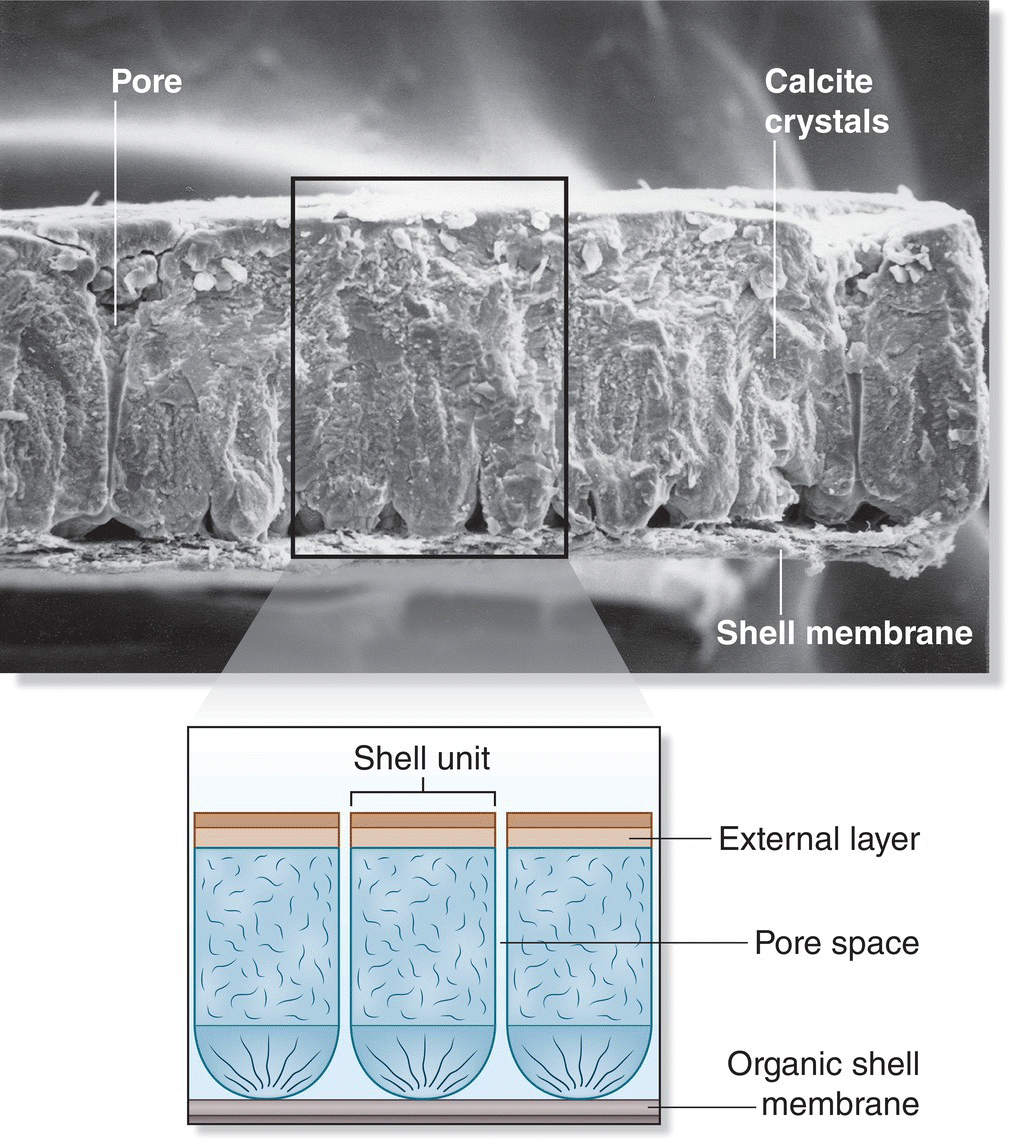
Fig. 11.29 Eggshell composition. This micrograph of an eggshell, paired with an enlarged schematic, shows the location of pores and the internal shell membrane.
(Micrograph courtesy of University of Colorado Museum of Natural History Paleontology Eggshell Collection UCM # HEC 563‐C; illustration by Shaena Montanari.)
The eggshell’s porosity also can put the embryo at risk. Pollutants such as oil can enter the egg and poison the embryo or coat the egg’s surface, cutting off gas exchange. Over 90% of Mallard (Anas platyrhynchos) embryos died when their eggshells were exposed to only one‐tenth of a drop of crude oil (Hoffman 1978).
If you were to open a fertile chicken egg at an advanced stage of embryonic development, the entire inner surface of the shell would be covered with a membrane engorged with blood vessels that facilitate gas exchange. This chorioallantoic membrane (Fig. 11.30) is formed by the fusion of two embryonic sacs, the chorion and the allantois, which are both connected to the core inner organs of the developing embryo—very much analogous to a human embryo’s connection to its mother’s placenta via the umbilical cord. The chorion is the outer membrane that surrounds the entire avian embryo; it is evolutionarily homologous to the membrane of the same name in mammals, which forms much of the mammalian placenta. The allantois holds any metabolic waste that cannot evaporate through the shell, such as uric acid crystals. As a fusion of these membranes, the chorioallantoic membrane serves as the functional equivalent of the embryo’s lungs throughout its development within the egg.
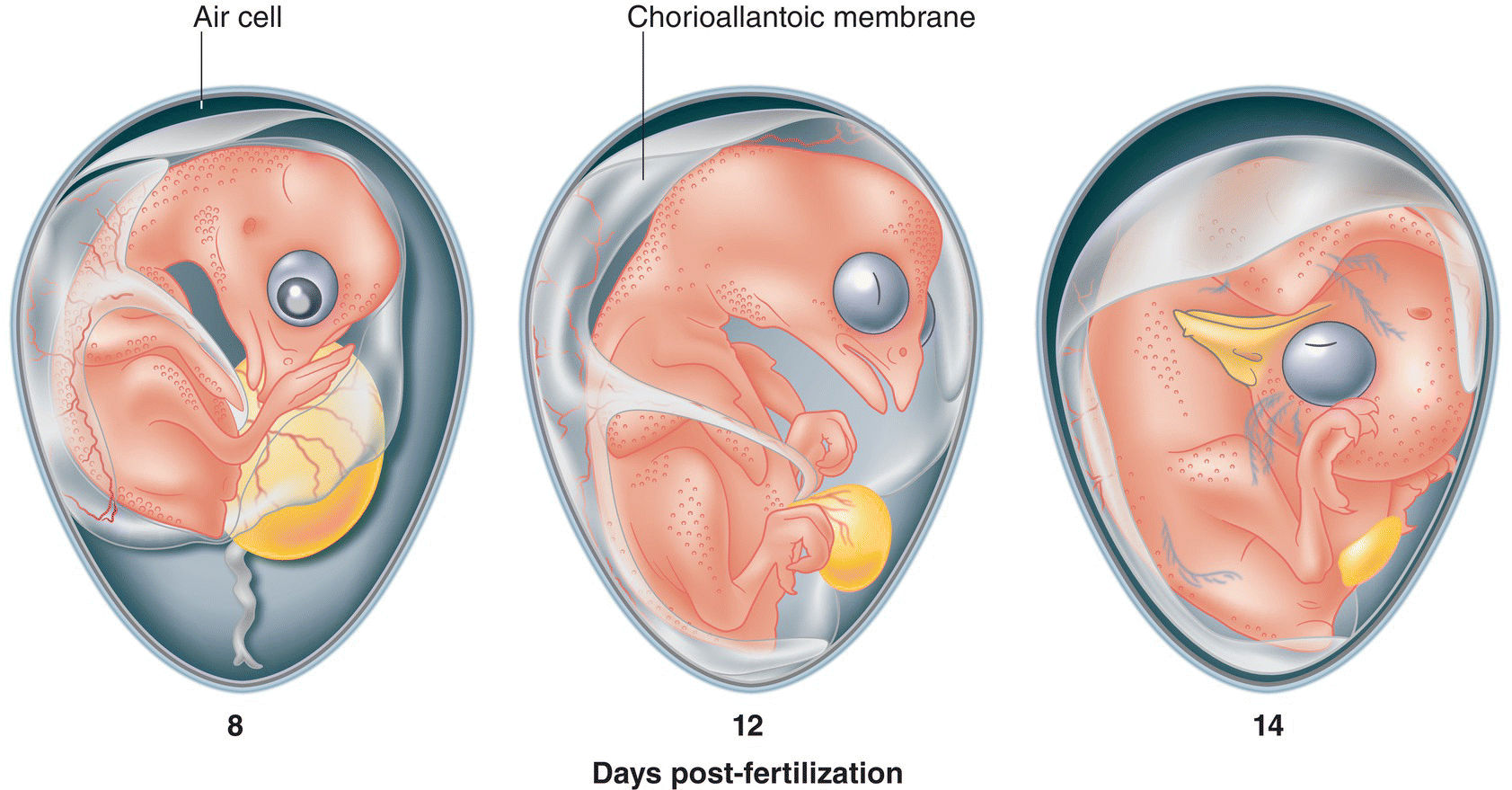
Fig. 11.30 Membrane anatomy changes as the embryo develops. Infused with blood capillaries, the chorioallantoic membrane is connected to the inner organs of this chicken (Gallus gallus) embryo. The air space at the top of the egg gradually expands as water content decreases via consumption by the embryo and evaporation through the permeable shell.
(Illustration by Andrew Leach © Cornell Lab of Ornithology, adapted from Murray et al. 2013.)
The chorioallantoic membrane covers more and more of the shell’s inner surface as the embryo develops. In a newly laid egg, the membrane enclosing the albumen adheres directly to the membrane just below the shell—except at the blunt end of the egg, where a small air space occurs. This is the same air space you may encounter while peeling a fresh hardboiled egg. As the water within the egg evaporates through the shell or is consumed by the developing embryo, this air space between the two membranes gradually expands (Fig. 11.30). The buoyancy imparted by this growing air space is valuable to field ornithologists who need to check the developmental progress of an egg: the extent to which the egg floats or sinks when placed in water often indicates how much longer the embryo must develop before hatching.
11.5.2 Egg size
The egg of the Ostrich (Struthio camelus)—measuring roughly 18 by 14 centimeters and weighing about 1.4 kilograms—is the largest of any living bird. However, Ostrich eggs seem small compared with those of the extinct Elephant Bird (Aepyornis maximus) that roamed Madagascar until only a few hundred years ago—its eggs measured up to 37 by 24 centimeters and weighed as much as 12 kilograms. By way of comparison, at least 150 domestic chicken (Gallus gallus) eggs could have fit inside one Elephant Bird egg. The eggs of the smallest bird in the world, the Bee Hummingbird (Mellisuga helenae) of the West Indies, are only 10–13 millimeters in length and weigh less than 1 gram; approximately 75 of these hummingbird eggs could fit inside one large chicken egg (Fig. 11.31).
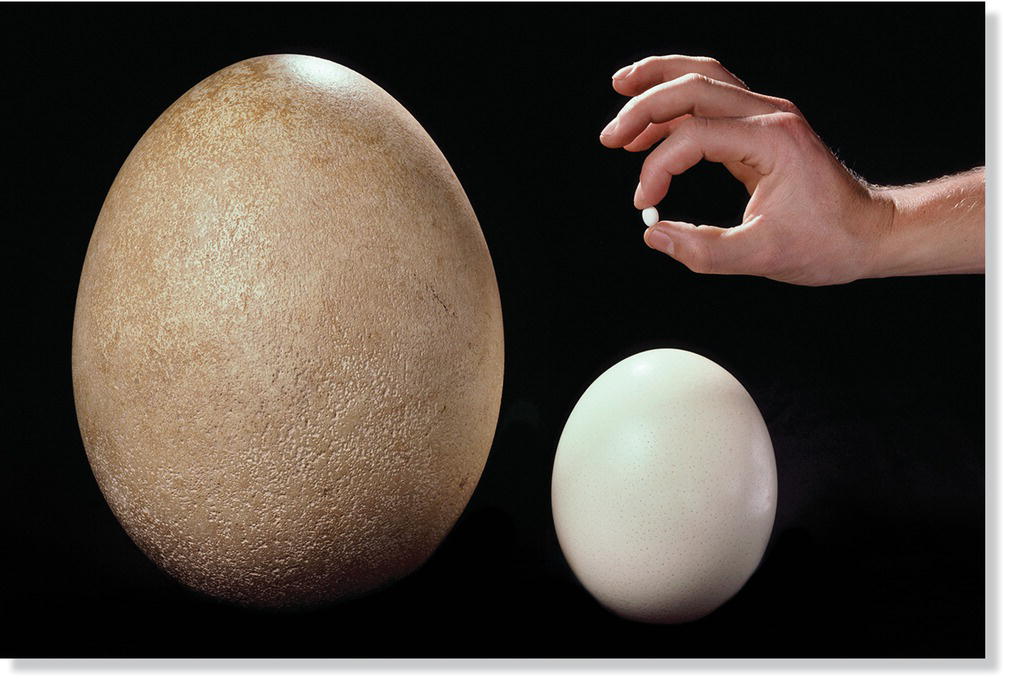
Fig. 11.31 Egg size extremes. The largest egg shown here is from the recently extinct Elephant Bird (Aepyornis maximus). To its right is an Ostrich (Struthio camelus) egg, the largest of all bird species living today. The tiny white egg is from the smallest of living birds, the Bee Hummingbird (Mellisuga helenae).
(© Frans Lanting, www.lanting.com.)
As a general rule, larger birds tend to lay larger eggs. However, when comparing egg size with adult body size, the eggs of larger species are proportionally quite small: an Ostrich egg is only 1.7% of the weight of an adult female Ostrich, whereas a wren’s egg is 13% of the female’s weight. The kiwis of New Zealand lay the largest eggs by relative size, with single eggs that are 25% of the female’s body weight. Not surprisingly, female kiwis generally only lay one egg per season, although on very rare occasions they may lay up to three. Conversely, some brood parasites—the Common Cuckoo (Cuculus canorus) of Eurasia, for example—lay very small eggs relative to their body size. This may be advantageous for multiple reasons: smaller eggs may more closely resemble those of their hosts; it may increase the likelihood that the parasitic chick hatches first, since larger eggs usually take longer to develop; and it may enable brood parasites to lay more eggs per season, increasing the likelihood that some of their young will hatch and survive.
Egg size also varies among birds in other ways. In general, birds that lay large clutches generally produce small eggs relative to their body size. During their first nesting attempts, females of many species lay eggs that are smaller than those of more experienced breeders. The eggs of precocial birds tend to be larger than altricial ones, even when the adults are similar in size. For example, adult cranes and eagles weigh about the same, but the eggs of cranes, which have precocial young, are about 4% of the adult female’s body weight, whereas the altricial eggs of eagles are about 2.8% of the adult’s weight.
11.5.3 Egg shape
Although bird eggs are usually approximately oval, their shapes vary from long and pyriform (pear‐shaped) in some species to nearly spherical in others. The diameter and muscular tension of the oviduct during eggshell formation determine the egg’s shape. Oviducal dimensions and contractions have presumably evolved to match egg shape with the environmental needs of each species. Owls often lay near‐spherical eggs, which may allow efficient packing within their often deep nest cavities. Murres place their single eggs directly on narrow, often slanting, ledges high above the sea. Their eggs are long, with an almost straight side; one end is remarkably pointed, the other forms an obtuse arc. This strange shape ensures that the eggs always roll in a tight circle—a distinct advantage for eggs vulnerable to rolling off perilous ledges (Fig. 11.32). All shorebird eggs also have a pointed shape, even for species nesting in flatlands where the eggs are not in danger of rolling away. This pointed shape may increase incubation efficiency: shorebirds usually lay four eggs, which fit symmetrically and compactly when the pointed ends align toward the center of the clutch. If the eggs become disheveled, the parent shorebird points them all inward again before resuming incubation. Considering that shorebirds lay large eggs in proportion to their body size, and that many nest in cold environments, efficient heat transfer from parent to eggs may be particularly important for these birds.
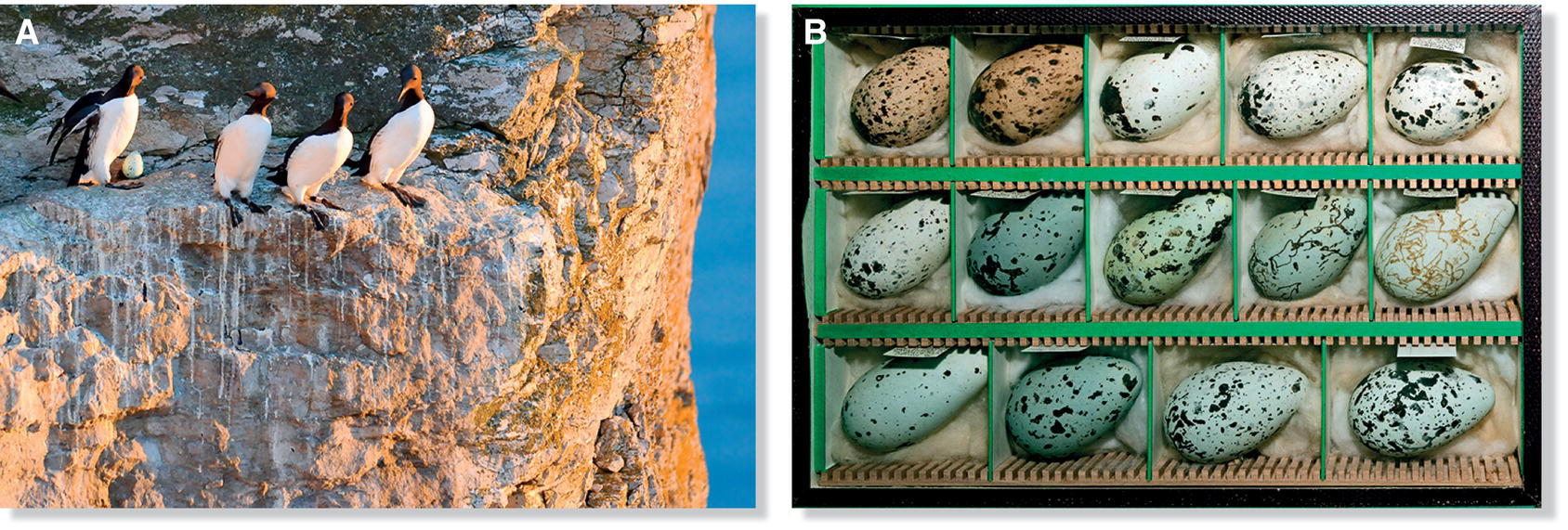
Fig. 11.32 Eggs of cliff‐nesting murres. (A) Common Murres (Uria aalge) nest close together on precarious cliff ledges and lay eggs that are sharply tapered, a shape that serves as a safeguard against rolling off narrow ledges. (B) Murre eggs are also remarkably variable in color and pattern, which may be an adaptation that helps parent murres recognize their own eggs in a tightly packed nesting environment.
(Photographs by: A, David Thyberg, Shutterstock.com; B, Didier Descouens, http://commons.wikimedia.org/wiki/File:Uria_aalge_MHNT_Box_Rouzic.jpg. CC‐BY‐SA 4.0.)
11.5.4 Eggshell color and texture
After the eggs of most species leave the female’s shell gland, pigment glands in the walls of the oviduct deposit successive layers of color on them. The background color of the egg is usually deposited first; any spots, streaks, or other darker markings are added later. The variation in egg coloration and pattern across species is usually correlated with breeding environment. Many birds, particularly those that nest in the open, lay cryptically colored eggs to avoid detection by visual predators. In North and Central America, for example, the color and speckled markings of Killdeer (Charadrius vociferus) eggs, together with the small, flat stones or wood chips used to line the nest, help the nest and its contents blend into the surroundings (Fig. 11.33). In the tropics, Chilean Tinamous (Nothoprocta perdicaria) lay glossy, chocolate‐colored eggs with a purple hue; cassowaries of Australia and New Guinea lay pitted, avocado‐colored eggs. Although striking when seen out of context, these colors actually blend in quite well in their nests on the forest floor.

Fig. 11.33 Well‐camouflaged eggs. Killdeers (Charadrius vociferus) and other birds that nest in the open often lay eggs with colors and patterns that blend in with the surroundings.
(Photograph by William Sproul Sweet.)
Colorful eggs do not always provide camouflage, however. Although tinamou eggs are among the most beautiful in the world (they are shiny and come in many colors), those of some tinamou species do not blend in well with their surroundings. For example, the Great Tinamou (Tinamus major) of Central and South America lays bright, blue‐green eggs that stand out against the generally dark ground. It has been hypothesized that such conspicuous egg coloration deliberately heightens vulnerability to predation or brood parasitism in order to incentivize the male to incubate more attentively. Alternatively, vibrant egg coloration may have evolved to provide benefits to the developing embryo, possibly involving protection from ultraviolet radiation, mediation of photoperiodic synchronization, or antimicrobial defense.
Some birds lay white eggs without any other markings. These species generally nest in dark holes (such as petrels, kingfishers, and woodpeckers), begin incubation with the first egg (such as hawks and owls), or cover their eggs when they leave the nest (such as grebes and some ducks). Because these eggs are hidden from predators hunting by sight, they do not need colors for camouflage. Additionally, pigments may be metabolically expensive to produce or may require dietary precursors that are hard to obtain. For hole‐nesting birds, the white color may provide the added advantage of helping the eggs be more visible to the parents in their dark setting.
Egg coloration and markings are fairly consistent in most bird species. However, there are a few species in which egg color varies considerably—even dramatically—among individuals. Individual Common Murres (Uria aalge), for example, may produce eggs that are deep blue‐green, bright pinkish, warm ocher, pale blue, cream, or white; their egg patterns vary from blotches to lines, with splashes of light yellow‐brown, bright red, dark brown, or black, to no markings whatsoever (Figure 11.32). This variation may help each murre to recognize its own egg among dozens of others within their densely packed breeding colonies. Similarly, the females of several species of African weavers lay eggs in a great variety of colors and spot patterns. This variety seems to facilitate discrimination among a female’s own eggs and those of another of her own species (Jackson 1992) or a brood parasite, such as a cuckoo (Lahti and Lahti 2002).
Bird eggs also vary in texture. Most eggshells have a smooth, matte finish like that of a chicken egg, but there are numerous exceptions. Cassowary and stork eggs are deeply pitted (Fig. 11.34A); grebe and flamingo eggs are chalky (Fig. 11.34B); tinamou eggs are glossy, resembling glazed porcelain (Fig. 11.34C). The greasy surface of most duck and goose eggs may be water resistant. The eggs of the Greater Ani (Crotophaga major) of the neotropics have a chalky white covering that abrades away during incubation to reveal a deep blue color underneath (Fig. 11.35). Anis live in groups in which multiple females lay and incubate eggs in a communal nest, so this change in egg appearance over time provides a ready mechanism by which the incubating caregivers might recognize eggs recently laid by another group member (Riehl 2010).

Fig. 11.34 Variation in egg texture. Although most avian eggshells are smooth, some species lay eggs with (A) pitted, (B) chalky, or (C) glossy surfaces.
(Photographs by: A, Paul André Betancourt; B, Klaus Rassinger and Gerhard Cammerer, Museum Wiesbaden, https://commons.wikimedia.org/wiki/File:Phoenicopterus_roseus_MWNH_0940.JPG. CC‐BY‐SA 3.0; C, Klaus Rassinger and Gerhard Cammerer, Museum Wiesbaden, https://commons.wikimedia.org/wiki/File:Tinamus_major_MWNH_0076.JPG. CC BY‐SA 3.0.)

Fig. 11.35 Chalky eggs of communally nesting Anis. (A) Freshly laid Greater Ani (Crotophaga major) eggs appear completely white; the chalky covering abrades away during incubation to reveal a blue shell underneath. (B) Unrelated females form breeding groups and lay eggs around the same time in one communal nest, sharing incubation duties. Egg color change over time is likely an adaptation that allows incubating individuals to discern new eggs from older ones, which in turn helps them identify eggs laid by other females in the group. Based on color, the eggs in this nest have been incubated for roughly the same amount of time—except for the fresh white one, which was added more recently to the nest.
(From Riehl 2010. Reproduced with permission from Elsevier.)
11.5.5 Egg laying
Once all the membrane layers have been deposited and the shell and pigmentation are complete, the egg passes through the cloaca. Although precise laying times for most species are not known, a pattern seems to hold: the larger the egg, the longer the laying process. Brood parasites have evolved to lay their eggs remarkably quickly, before the host returns to its nest. Common Cuckoos (Cuculus canorus), for instance, can lay an egg in another bird’s nest and disappear in less than 10 seconds (Seel 1973).
The time required for the oviduct to secrete the layers around the ovum determines the interval between laid eggs. Small shorebirds, domestic chickens, woodpeckers, rollers, and most passerines lay eggs about 24 hours apart. In contrast, birds such as ostriches, rheas, herons, storks, cranes, bustards, gulls, doves, owls, hummingbirds, swifts, kingfishers, as well as some accipiter hawks and cuckoos, generally lay eggs every other day. Larger species tend to need more time between eggs, perhaps because the secretion processes in the oviduct take longer for larger eggs. Among waterfowl, for instance, many duck species lay eggs every day, whereas large geese and swans lay every other day. Parrots, even the smaller species, lay their eggs 1–3 days apart. Penguins take 3–6 days between eggs, and the Masked Booby (Sula dactylatra) of tropical seas worldwide lays its two eggs as much as a week apart. Species that lay very large eggs for their size also tend to need longer intervals between eggs: megapodes wait 4–8 days between eggs, whereas kiwis, in the rare event that they lay multiple eggs within a season, need 14–30 days. Most bird species appear to lay successive eggs at approximately the same time of day, usually in the early morning—although some herons, bitterns, and parrots lay at intervals that are decidedly not multiples of 24 hours.
11.6 Clutch size
A clutch is the total number of eggs laid in an uninterrupted series, during a single nesting period, by one female bird. In the early days of ornithology, egg collectors from all over the world amassed large collections of bird eggs (Box 13.04). Clutch size is therefore often the only core avian life history trait for which any quantitative data exist; as a result, there has been considerable interest for almost a century in trying to understand patterns of variation in clutch size. During the 1940s, David Lack at Oxford University (UK) began studies on the clutch sizes and life histories of several local bird species. Many of these studies continue to the present day, providing contributions to avian life history theory that are covered in Chapter 13.
Closely related birds tend to have clutches of similar size. For example, all tubenosed seabirds (such as albatrosses and petrels) lay a single egg, virtually all hummingbirds lay two eggs, shorebirds do not lay more than four eggs, and so forth. Most songbirds lay between two and six eggs, although some tits in Eurasia lay extraordinarily large clutches of up to 17 eggs.
11.6.1 Clutch size and food availability
Food availability may be the most important factor influencing clutch size. Among non‐passerines, birds that feed their young (such as gulls and storks) tend to lay fewer eggs than those whose young forage on their own (such as pheasants, grouse, quail, megapodes, and most shorebirds and waterfowl). The availability of food for the laying female is often an important limitation; since laying an egg is energetically costly for the female—even in places with abundant resources—doing so without an adequate food supply threatens a female’s survival or her subsequent reproductive efforts. Thus, even though females are still physiologically capable of laying more eggs, they often limit their egg production (and thus clutch size) when food is scarce.
The amount of available food for chicks also limits the clutch size of many species. When female birds can anticipate future food conditions at the time when they are laying eggs, they may adjust their clutch size accordingly. Owls and arctic‐nesting raptors are good examples of this flexibility, as their clutch sizes vary dramatically among years with abundant versus scarce prey. For example, female Snowy Owls (Bubo scandiacus) breeding high in the Canadian Arctic lay 7–11 eggs in rich lemming years, and only 3–5 in low lemming years (Parmelee 1992) (Fig. 11.36).

Fig. 11.36 Clutch size flexibility. Snowy Owls (Bubo scandiacus) are among the many bird species that lay larger or smaller clutches depending on prey availability in a given year.
(Photograph by Gerrit Vyn.)
11.6.2 Clutch size, geography, and incubation
Both within and among species, birds that nest in the tropics tend to lay smaller clutches than those at higher latitudes. For example, House Wrens (Troglodytes aedon) lay an average of seven eggs per clutch in Saskatchewan (Canada) but average only three and a half eggs in Costa Rica (Young 1994). This established pattern has generated a number of interesting explanations, most of which involve ecological hypotheses addressing varying resource availability or seasonality across latitudes (Chapter 13). However, other factors may partly explain this pattern. For instance, environmental temperatures during the laying period tend to be warmer for tropical species than for temperate breeders. This difference, it turns out, seems to place many tropical species in a cruel bind. In the tropics, the higher ambient temperature at the nest may induce embryos to start developing even when the eggs are not being actively incubated. Yet embryos that begin development at ambient temperatures are less likely to hatch than those incubated directly by the female at somewhat warmer and better regulated temperatures; thus, birds in the tropics may lay smaller clutches so that they can begin active incubation sooner, rather than waiting until after many eggs are laid (Stoleson and Beissinger 1999).
11.6.3 Clutch size and nest type
In general, cavity nesters lay larger clutches than birds nesting in the open. This pattern has been attributed to the lower predation rates generally experienced by cavity nesters (Slagsvold 1982). Nestlings tend to draw attention to themselves by moving around, begging for food, or simply standing out against their surroundings. Because a smaller number of young in a nest potentially lessens the risk of attracting predators, the more vulnerable open nesters raise fewer young per clutch. The young of open nesters also tend to develop faster, reducing the amount of time they are exposed to predation risk in the nest. If parents can provide only a given amount of food at a given rate, they can either use that food to rear fewer young faster, or a greater number of young more slowly. It logically follows, then, that cavity nesters have larger clutches with longer development times, and open nesters have comparatively smaller clutches with shorter development times.
11.6.4 Clutch size, female age, and time of season
Birds that produce multiple broods each season, such as Eastern Bluebirds (Sialia sialis) in North America and Dunnocks (Prunella modularis) in Europe, tend to lay small clutches early in the season, their largest clutches mid‐season, and then smaller clutches again later in the season. In contrast, for single‐brooded species, clutch size tends to be largest for birds laying at the beginning of the season and declines steadily for birds laying thereafter. Exactly what drives this pattern is still unclear. In some places, it seems likely that food supply declines as the season progresses, and that smaller clutches may therefore be a response to declining food for the laying female or for the forthcoming chicks. However, in other cases, a female may curtail egg production during the warmer parts of the breeding season so that she can incubate her eggs directly rather than have them develop partially under environmental temperatures. Another possibility is that earlier birds lay larger clutches because they are simply in better condition or of higher quality.
In many species, young females lay smaller clutches than older birds. Additionally, younger females often begin nesting later in the season, but even when younger and older females begin laying on the same date, the younger bird often lays a smaller clutch. There are several potential reasons for this pattern. Individual females may gradually improve in their reproductive efficiency, or they may invest more effort into reproduction later in life (Chapter 13). Alternatively, perhaps individuals themselves do not change over their lifetime: at any given time, the younger age classes may include many inferior individuals that will die relatively early and thus are selected out, whereas the older birds in the population are higher quality individuals that have been more effective at both surviving and producing offspring all along. Teasing these possibilities apart requires long‐term studies of many marked individuals throughout their lifetimes. One such study on Black Kites (Milvus migrans) in Spain found evidence for both individual improvement and age class selection (Blas et al. 2009), but a study of Common Terns (Sterna hirundo) in northern Germany concluded that almost all of the perceived reproductive superiority of older females resulted from their steady improvement as they grew older (Rebke et al. 2010) (Box 11.04).
11.6.5 Number of broods per season
When comparing the clutch sizes of birds in temperate zones versus the tropics, it is important to note that most tropical species have much longer breeding seasons than species at higher latitudes. Tropical landbirds often may lay eggs over a period of four or more months (and occasionally all year long), whereas species in temperate zones typically lay within a period of less than 2 months. Arctic species lay within an even shorter timeframe—generally, less than a month. Longer breeding seasons at lower latitudes allow birds living there many more breeding attempts per season than their counterparts at higher latitudes. The same effect appears within temperate latitudes of the northern hemisphere, with birds further north laying larger clutches but fewer clutches per season, and with overall fecundity probably varying little with latitude (Böhning‐Gaese et al. 2000).
11.7 Clutch and egg replacement
Most birds will replace their clutch if it is destroyed, but many single‐brooded species will not re‐lay if the hatched offspring are lost. The laying of a replacement clutch usually requires the female’s hormonal system to cycle back into laying mode, and the rapid yolking up phase of preparing ova for ovulation must be completed—this requires 5–8 days in most passerines and longer in larger birds.
However, if only one egg is removed from the nest, birds respond in diverse ways. Determinate layers lay a fixed number of eggs and will not lay a replacement egg if one is removed from the clutch during the laying period, whereas indeterminate layers will lay replacements until the clutch reaches a particular size. Some indeterminate laying species are capable of prodigious feats: for example, the regular removal of one egg every day from the nest of a Northern Flicker (Colaptes auratus) in Massachusetts (USA) induced the bird to lay 71 eggs in 73 days (Phillips 1887), whereas the normal clutch size of this species is six to eight eggs. Domestic chicken (Gallus gallus) hens, which have been artificially selected over many hundreds of years for egg production, can lay as many as 352 eggs in 359 days. Chicken hens, like all other birds, will stop laying eggs if they are allowed to sit upon a full clutch and begin incubation.
Some species act as indeterminate layers only at a certain point in the laying process. For example, some gulls will lay replacement eggs only if the eggs are removed before the female can sit on two or more. Variation also exists in how different birds determine that a “complete” clutch has been laid; some species seem to count the eggs visually, whereas others seem to rely upon the feel of the eggs beneath them.
The propensity of many birds to replace eggs taken from the nest during the laying period may have helped save some from extinction. Wildlife biologists have been able to “double clutch” many endangered species by removing some eggs to rear in the laboratory while the birds concurrently rear their replacement eggs in the wild. This approach has been an important part of captive‐breeding programs for many endangered bird populations (Chapter 15).
11.8 Incubation
All birds lay external eggs that must be kept in the proper temperature range throughout their development; this temperature is maintained by a process called incubation. The precise temperature necessary for embryonic development varies among bird species, but most avian embryos require temperatures close to 37 °C or 38 °C. Because few environments have constant air temperatures at or above this level, almost all bird species must provide a source of warmth for their eggs. Most species use their own body heat by sitting on top of the eggs, but two groups employ other methods: brood parasites leave the incubation of their eggs to their hosts, and megapodes provide alternative sources of heat through a broad variety of methods (Box 11.02).
In rare cases, birds must cool their eggs to keep them in the appropriate temperature range. Due to their extremely hot breeding habitats along the Gulf of Mexico, Wilson’s Plovers (Charadrius wilsonia) dip their breast feathers in water and run over to cool their eggs with the moisture. Along sandbars of African rivers, Egyptian Plovers (Pluvianus aegyptius) go one step further—in addition to cooling the eggs with wet breast feathers (Fig. 11.37), adults may also bury their eggs (and later sometimes their chicks) in sand to protect them from the sun. One pair of Black‐necked Stilts (Himantopus mexicanus) nesting in hot and arid southern California (USA) soaked their belly feathers and carried the cooling moisture back to the nest 953 times in one day (Grant 1982). Seabirds with open nests on tropical islands or coastal deserts often spend more time standing over their eggs to provide shade than they do incubating.

Fig. 11.37 Cooling eggs in hot temperatures. This Egyptian Plover (Pluvianus aegyptius) has soaked its breast feathers (arrow) and will use the water to cool its eggs.
(© Jabruson, naturepl.com.)
11.8.1 Brood patches and brooding
A few days before a female lays the first egg of her clutch, some of the feathers on her breast and belly fall out; that bare patch of skin develops through egg laying, becoming swollen via the expansion of blood vessels and the retention of water. This area is called a brood patch or an incubation patch (Fig. 11.38). The brood patch can be a single large region that spans much of the area of the belly and breast, or it may consist of a few smaller, distinct bare regions separated by areas that retain their normal feathering. To incubate, birds push the adjoining feathers aside, so that bare skin rests directly on the eggs.
Fig. 11.38 Brood patches. (A) This Hairy Woodpecker (Picoides villosus) is clearly in reproductive condition: its feathers have been gently blown away from its breast to reveal a bare brood patch. Note the redness of the skin—the extensive blood supply to this area helps the parent bird warm its eggs. (B) Shorebirds and gulls often have multiple, separate brood patches associated with each egg they lay in a clutch, as shown in this drawing of a Herring Gull (Larus argentatus). (C) The Magellanic Penguin (Spheniscus magellanicus) has a pouch‐like fold of bare skin on its lower abdomen that encloses, protects, and warms its egg.
(Photographs by: A, Jennifer Scott Schlick; C, R. Roscoe, Photovolcanica.com. B, adapted from Proctor and Lynch 1993. Reproduced with permission from Yale University Press.)
Most birds continue to incubate their young even after they have hatched, providing heat until the chicks have developed the feathers and metabolism necessary to control their own body temperature. This post‐hatching incubation, called brooding, involves much less pressing of the brood patch to the chicks, since newly hatched chicks can be smothered if the parent sits too tightly. Once brooding is over, the feathers in the brood patch grow back (often before the next body molt), the blood vessels shrink to their normal size, and the swelling of the skin subsides.
11.8.2 Incubation period
The incubation period begins when the parents start to incubate the eggs in earnest, with one of the parents incubating most of the time and with the temperature of the eggs being maintained near the optimal temperature, with only brief cooling periods. The incubation period ranges from about 11 days in some of the smaller finches to about 80 days in the larger albatrosses. Bigger eggs generally take longer to hatch. However, considerable variation exists among groups of birds; for example, hummingbird eggs take much longer to hatch (14–23 days) than might be expected from their tiny size. So do the large eggs of storm‐petrels, petrels, and albatrosses: the eggs of Leach’s Storm‐Petrel (Oceanodroma leucorhoa) are similar in size to those of the American Kestrel (Falco sparverius), but the incubation period of the storm‐petrel (38–50 days) is considerably longer than that of the kestrel (about 30 days).
Although incubation periods are generally similar within species, they may vary somewhat from nest to nest in a population. Long bouts of inclement weather sometimes force adults to interrupt incubation to forage to ensure their own survival. After the eggs hatch, the presence of active nestlings acts as a stimulus, indicating the need for adults to switch from “incubation” to “care of the young” behavior. Without that signal, birds sometimes have a hard time knowing when to stop incubating if the clutch is defective: if all the eggs in a clutch fail to hatch, a bird may continue to incubate them for two or even three times its normal period before finally deserting them. For example, Northern Bobwhites (Colinus virginianus) have been known to incubate inviable eggs for as long as 75 days, considerably beyond their normal incubation period of 23–24 days.
11.8.3 Start of incubation
Different species initiate incubation at different times during the laying cycle. Some birds start to incubate after laying their first egg. Although immediate incubation helps protect the egg, this strategy ties the incubating bird to the nest site for a longer period, increasing the adult’s exposure to predators. Additionally, this immediate incubation means that the embryos of the earliest laid eggs will start to develop before the later eggs are laid, resulting in staggered hatching and eventually a brood with young of different ages, a phenomenon called asynchronous hatching. Especially in species with large clutches, this variance in hatching dates can result in a strong hierarchy of sizes and begging abilities among the offspring. Pelicans, cormorants, herons, storks, eagles, hawks, cranes, parrots, and owls are among the birds that use this strategy of immediate incubation (Fig. 11.39).

Fig. 11.39 Asynchronous hatching. In some species like Burrowing Owls (Athene cunicularia), parents begin incubation immediately after laying the first egg; nestlings hatch accordingly at different times. The resulting disparities in age and size often lead to unequal competition among them—older nestlings have an advantage, especially in breeding seasons when food is scarce.
(Photograph by Jan Maguire.)
Raising young of different sizes gives parent birds a systematic way to adjust the number of young to the amount of food available. By first feeding the most vigorously begging chick completely before moving on to another nestling, parents may help ensure that at least some young will survive in years when food is in short supply. If food is scarce, the younger and more recently hatched nestlings, too small to beg competitively, will starve. This phenomenon is known as brood reduction. When food is abundant, however, parents are often able to feed all of their young, even the youngest. Despite the advantages of this strategy, relatively few species have asynchronous hatching; instead, most birds delay the onset of incubation until they lay the last or next‐to‐last egg. For precocial young, synchronized hatching is essential since a full nest of mobile and vocal young birds presents quite a target for predators, and most precocial broods leave the nest very soon after hatching.
11.8.4 Incubation by male and female birds
In many bird species, both members of a pair incubate. Generally, both parents share this task about equally, switching at frequent intervals, although the female most often incubates through the night. Members of a pair may perform a species‐specific “greeting ceremony” when they exchange duties at the nest. These ceremonies can be spectacular among some of the large species such as albatrosses and boobies, but in most birds they are more subdued. For instance, when she hears an approach song from her mate, an incubating female Northern Cardinal (Cardinalis cardinalis) will sing from the nest, a seemingly counterintuitive response that could risk revealing the nest’s location to predators. Her response, however, communicates information regarding whether she is hungry, and if not, the brightly colored male does not approach unnecessarily (Halkin 1997). An incubating bird usually appears more than ready to exchange incubating duties with its mate, but sometimes birds like gulls and plovers engage in pushing matches in which the relieving bird attempts to dislodge the previous incubator from the eggs.
In many other species, only one parent incubates. In groups such as ducks, geese, hummingbirds, most owls, and many passerines, the female usually is the incubating parent. Male geese and owls are fully engaged in defending or gathering food for the female while she incubates, but in the other species the male is emancipated from parental duties and spends his time seeking other breeding opportunities (Chapter 9). On the other hand, only the male incubates the eggs in a few species such as the polyandrous phalaropes and jacanas.
In species in which only a single parent incubates, the roles usually are predictable, with either the male or female always taking care of incubation. The Eurasian Penduline‐Tit (Remiz pendulinus) is an exception. In this species, both sexes are fully capable of incubation, but only one parent generally takes on this duty at a particular nest. Apparently, both parents prefer to leave incubation to their mate in order to pursue other breeding opportunities, and therefore they engage in a serious contest. The female hides her eggs in the lining of the nest to prevent the male from discovering that she has begun laying (Fig. 11.40), because the appearance of a nearly complete clutch may tell the male that his mating opportunities in the current nesting attempt are finished and that this would be the best time for him to desert. By hiding her eggs until she is ready to abandon the nest to the male’s care, the female can be the one to flee the current nest and search out another breeding opportunity elsewhere. In one well‐studied Austrian population, female Eurasian Penduline‐Tits ended up incubating their first clutch about 50% of the time. Although males solely incubated the clutch 20% of the time, it is also common for neither parent to incubate if the female is the one who leaves: both parents abandoned the nest altogether about 30% of the time (Valera et al. 1997).

Fig. 11.40 Conflict over incubation between parents. In the Eurasian Penduline‐Tit (Remiz pendulinus), the female and male compete to shirk incubation duties. Females often bury their eggs in the nest lining (arrow) to prevent male partners from discerning that a clutch is about to be completed—a perfect time to abandon and seek other breeding opportunities, forcing the other sex to provide sole parental care.
(Photograph by Tomasz Przechlewski, http://commons.wikimedia.org/wiki/File:Remiz_pendulinis_6.jpg. CC‐BY‐2.5. Illustration © Cornell Lab of Ornithology.)
11.8.5 Patterns of attentiveness
Incubating birds soon adopt a fairly regular rhythm, alternating periods on the nest (attentive periods) with periods off the nest (inattentive periods). Many birds tend to extend their attentive periods when environmental conditions are harsh, both because the eggs need extra care and because foraging is less productive. Birds tend to leave the nest when they will be able to feed efficiently and return promptly. Thus, most incubating passerines sit on the nest at the beginning and end of the day (the overnight bout usually is uninterrupted) and whenever conditions outside the nest become cooler and wetter. The male Emperor Penguin (Aptenodytes forsteri) endures the longest attentive period, incubating his egg through the latter half of the antarctic winter in one uninterrupted stretch of 64 days, living entirely off his stores of body fat. At the other end of the spectrum, the Karoo Prinia (Prinia maculosa) of southern African deserts has an incubation attentiveness of only about 49% of daylight hours (Chalfoun and Martin 2007).
Species that must forage far from their nest generally have longer attentive periods. In many pelagic seabirds such as fulmars, shearwaters, and many petrels, each parent typically incubates for 1–5 days while its mate forages far away at sea. For Northern Fulmars (Fulmarus glacialis) in the UK and arctic Canada, each shift of incubation can last from 1 to 14 days, depending on the distance and quality of foraging conditions (Mallory et al. 2008). Other birds have adopted unusual incubation rhythms in response to challenging environments. For example, in the high mountains of China, female Blood Pheasants (Ithaginis cruentus) leave their nests unattended each morning for one long period each day—usually lasting more than 6 hours—because it takes such a long time to find the mosses distinctive to their diet (Jia et al. 2010).
11.8.6 Behavior during incubation
The incubating adult usually is very quiet at the beginning of any attentive period, often seeming to doze off for long periods. As the bout wears on, the incubating bird may become more restless, changing its position, turning the eggs, or meddling with the vegetation in or around the nest. When the parent leaves or returns to the nest, it usually does so secretively and deliberately, especially if it is the only bird incubating. Ground‐nesting species leaving their nest often thread their way through the nearby vegetation before flying up; species leaving elevated nests often drop nearly to the ground and fly level for a short distance before rising to their normal height.
In addition to providing warmth, parents care for their eggs in other ways. An incubating bird will sometimes raise its body slightly, reach down among the eggs with its bill, and gently turn them (Fig. 11.41). Although this turning behavior may help prevent the adhesion of embryonic membranes to the shell, embryos of species that do not turn their eggs, such as megapodes and palm‐swifts, seem to suffer no adverse effects. Occasionally, ground‐nesting birds such as gulls and waterfowl will retrieve their eggs if they accidentally roll out of the nest; most birds roll them back with their beaks, but adults with long bills, such as rails, may lift the eggs back into the nest.

Fig. 11.41 Egg‐turning during incubation. Many birds regularly rotate their eggs during incubation, as demonstrated by this Whooper Swan (Cygnus cygnus).
(Photograph by Helen Haden.)
The behavior of the incubating bird often changes as the incubation period advances. Nuthatches, usually noisy, become quiet and secretive in the vicinity of the nest. Defensive birds, such as owls, raptors, gulls, and shorebirds, become much more belligerent as hatching approaches, whereas cryptically colored birds remain on the nest for a longer period in the face of intruders. For example, American Woodcocks (Scolopax minor) in North America sometimes do not flush from the nest until touched. Evolutionary biologists have interpreted these behavioral changes as reflecting the increasing fitness value of the clutch as it nears hatching. Not only are more developed eggs more likely than just‐laid eggs to produce fledglings, but a replacement clutch also becomes less likely to succeed as the breeding season progresses. It therefore makes evolutionary sense that birds are much more likely to abandon a breeding attempt early in the cycle than they are after the young have hatched. In extreme emergencies, however, parent birds may abandon the clutch or brood at any stage; in doing so, adults preserve their capacity to breed again by saving themselves and leaving their young to their fates. When birders and researchers observe nests, it is crucial that they keep disturbances to an absolute minimum, especially early in the nest‐building cycle, and that they consider how visiting a nest at any time can leave scents or other cues that may attract or guide predators to a nest.
You may have seen a pair of raptors perform a food‐exchange ceremony in which the female flies from the nest to receive food from the male in mid‐air, either taking it directly from his talons or catching it as it falls. These characteristic displays have long been considered important for helping these birds establish a pair bond during courtship and maintain it throughout parental care. However, studies of many species also indicate that females who receive more food from their mates lay larger clutches. For example, in both Common Terns (Sterna hirundo) and Black‐legged Kittiwakes (Rissa tridactyla), females that are fed more by their mates during their laying periods lay larger clutches (Nisbet 1977; Helfenstein et al. 2003). Providing food for a female mate most likely improves a pair’s chance of raising more young: because the female does not have to spend as much time foraging, she can spend more time incubating the eggs, and eventually invest more energy into feeding the young, while staying in better condition herself.
11.9 Hatching
In preparation for hatching, bird embryos develop an egg tooth, a short, pointed structure on the tip of the upper beak (and sometimes on the lower beak as well) (Fig. 11.42). While in the egg, the avian embryo generally sits in a “fetal position,” with its head bent forward towards its belly. When it is ready to hatch, the fully developed embryo pulls its neck back and up (often using muscles developed especially for this task), rubbing its egg tooth against the inner wall of the shell, which has already been somewhat weakened by the absorption of calcium from it by the developing embryo. Embryos of most bird species rotate in the egg while repeating this motion many times. Eventually, the chick manages to create a small hole in the eggshell, at which point the egg is pipped. Soon thereafter, the chick begins to puncture the shell with a series of holes—sometimes connected, sometimes not—that nearly encircle the blunt end of the egg. Once the shell is sufficiently weakened, the chick pushes off the end and struggles free, away from the egg membranes. This emergence appears to require a great deal of exertion, and immediately after they hatch most hatchlings lie quite still. Once dry, most nestlings weigh about one‐third less than the fresh, whole egg from which they started. A few days after the young have fully hatched, the egg tooth sloughs off or is reabsorbed.

Fig. 11.42 Egg tooth. To break out of their shells, most bird embryos develop an egg tooth on the tip of the upper beak, visible on this newly hatched Masked Lapwing (Vanellus miles). The egg tooth reabsorbs or rubs off within a few days of hatching.
(Photograph by Kell Nielsen.)
The length of time between pipping and hatching varies among species. Many passerines complete the process in only a few hours, but some larger birds may need as long as 4 days. In many precocial species, siblings coordinate the synchrony of their hatching quite precisely by vocalizing to one another from inside their eggs. Once each soon‐to‐be hatchling breaks the membrane of the air cell in the blunt end of its egg, it begins calling with rapid clicks or peeps produced several times per second, usually from a day or two before hatching until the time of hatching.
11.10 Altricial and precocial young
Newly hatched birds vary widely in their readiness for life outside the egg. Altricial chicks hatch naked or very scantily feathered with down, with no ability to generate enough heat for thermoregulation, and totally dependent on their parents for food. In contrast, precocial chicks are already well feathered when they hatch, with substantial powers of thermoregulation and locomotion, and a considerable degree of independence from their parents for feeding. These two categories—altricial versus precocial—are end‐points on a continuum of developmental types, and quite a few species have chicks that are intermediate blends of these modes of development (Starck and Ricklefs 1998).
11.10.1 Appearance
No one is likely to describe a young altricial bird as attractive. A newly hatched altricial nestling appears to be all abdomen and head, with two large eyes bulging against closed lids. Soon after hatching, it lifts its head on its wobbly neck and opens its mouth (Fig. 11.43). Swollen gape flanges, equipped with nerve endings, extend from the corners on either side of the mouth and taper toward the tip of the bill; touch one of these flanges and the nestling’s mouth is likely to spring open like a mechanical toy. The colors in the area of the mouth are often bright and contrasting, transforming it into a target into which the parent birds place food. The flanges typically are white or vivid yellow; the lining of the mouth is often a vibrant red, orange, or yellow. Some nestlings, especially in estrildid finches and some cuckoos, also have colored patterns on the roofs of their mouths or on their tongues. In nestlings raised in cavity nests, the colors around the mouth tend to be even more intense, presumably to help the parents find the chick’s mouth in low light conditions.
Fig. 11.43 Begging for food. The left‐hand chick in this recently hatched Brown‐headed Nuthatch (Sitta pusilla) brood has started to beg for food. Its bright white gape flanges and inner mouth region serve as a target for food placement by its parents.
(Photograph by c aubrey wiggins iii.)
An altricial nestling’s skin, mostly pink from the muscles and blood vessels beneath it, is very thin and oily in appearance (Fig. 11.44A). The internal organs and an almost empty yolk sac, resorbed back into the embryo as it developed, show through the skin of the distended abdomen. If the nestling hatches with any down, it is usually most abundant on the top of the head and on the back. The color and length of the down varies by species and may or may not follow the outline of the definite feather tracts soon to emerge (Chapter 4). The first true feathers soon push out the down, although a chick often retains wisps of the natal down on the tips of its juvenal feathers. The juvenal feathers begin development beneath the skin, first visible as small bumps on the skin and showing as dark bands before they erupt into the pin feathers that will emerge throughout the chick’s development. In most altricial chicks, pin feathers appear first in the feather tracts of the head, back, and wings, and later on the underparts. For most birds, the wing feathers grow continuously until full powers of flight are achieved at or after fledging.

Fig. 11.44 Altricial versus precocial chicks. (A) Altricial chicks like this Tree Swallow (Tachycineta bicolor) hatch at an early stage of development and are highly dependent on their parents for warmth, protection, and food. (B) Precocial chicks like this Spotted Sandpiper (Actitis macularius) hatch at a later stage of development that allows them to walk and forage within hours or days of hatching.
(Photographs by: A, Chris Gates; B, Navjot Singh.)
Because precocial embryos spend much more time developing in their eggs than do those of altricial species, precocial chicks are much more developed at hatching. Perhaps the most familiar image of a young bird is that of a newly hatched chicken, which well typifies precocial young. The chick’s downy covering, wet from embryonic fluids, dries within 2 or 3 hours, taking on the fluffy appearance that makes the chicks of precocial species so endearing. The motor and sensory capabilities of a newly hatched chicken, after being in the egg for about 22 days, are about as well developed as those of an altricial chick that has spent 12 days in the egg and 10 days in the nest after hatching. As soon as they emerge from the egg, precocial hatchlings, with their eyes already wide open, respond immediately to external stimuli, are remarkably adroit in walking and maintaining their balance, and promptly begin exploring their environment with pecks at unfamiliar objects. This early neuromotor sophistication is one of the hallmarks of precocial chicks.
At hatching, the precocial chick’s abdomen still contains up to one‐third of the original contents of the yolk sac, and its digestive tract is essentially fully functional. The egg tooth is conspicuous, flanges are either reduced or not present along the margin of the bill, and the color of the mouth lining usually is plain—similar to that of the adult—because most precocial young feed themselves and thus have no need to send begging signals to their parents. Precocial chicks generally have large feet and legs along with well‐developed muscles for walking and producing heat for thermoregulation (Fig. 11.44B). The most precocial of all birds, the megapodes (“pod” is a root word meaning “foot”), are the only birds whose embryos break out of their shells using their extremely large and powerful feet instead of an egg tooth (Jones et al. 1995). Megapode chicks are also distinctive in receiving no parental care (Box 11.02). These chicks simply walk off into the surrounding forest soon after they emerge from their incubation mounds, and their wings are fully feathered at hatching, allowing them to flutter away from any threats they encounter outside the mound.
11.10.2 Temperature regulation
A newly hatched altricial nestling is unable to regulate its body temperature. By brooding, the parents keep the nestling’s body temperature high, near the levels at which digestive and growth enzymes work best. As the nestling grows, its surface area‐to‐volume ratio becomes more favorable for heat retention, and it begins to grow its insulating coat of downy and juvenal feathers. Eventually the nestling can convert the food brought by its parents into energy and use that energy for thermoregulation (Chapter 7). In most passerine birds, the parents begin to brood less around the time their nestlings’ eyes open; most altricial young acquire full powers of thermoregulation about the time their wings are half grown.
In contrast, precocial chicks hatch with a thick covering of down, one of nature’s best insulating materials (Chapter 4). Precocial chicks generally are larger than the average altricial nestling; thus, with a more favorable surface area‐to‐volume ratio, precocial chicks lose relatively less heat from their bodies. In fact, they can partly control their body temperature at hatching (well before altricial young can do so), although most still require some brooding. After hatching, however, their temperature control develops much more slowly than in altricial nestlings; some precocial chicks do not attain full temperature control until about 4 weeks of age. The feather coat of each precocial species develops in a pattern suited to its environment. Upland species, such as grouse and shorebirds, generally are more vulnerable to upward radiative heat loss, so they first develop feathers on their back and upper surfaces. Aquatic species, such as loons, grebes, and waterfowl, first develop long, thick feathers on their body’s undersurface that help insulate the chicks when they are floating on cold water.
11.10.3 Weight gain
After an initial lag of a day or two to allow the digestive system to become ready, altricial nestlings develop explosively, often doubling in mass several times during the first 10 days after hatching. For example, the chick of an American Crow (Corvus brachyrhynchos) weighs only 15 grams at hatching, but after 18 days of growth it will have increased to over 300 grams (Ignatiuk and Clark 1991)—a 20‐fold increase. Growth tends to slow as nestling development continues; by the time most passerine young leave the nest, they usually weigh 70–80% as much as their parents. Once they reach independence, most birds are the same size as their parents; a young bird that can fly and behave like an adult may have a distinctive plumage, but there is usually nothing about its body measurements, except perhaps the length of its wing feathers, that distinguishes it from older birds.
In some altricial species, the nestlings put on so much weight late in development that they temporarily weigh more than their parents. This extra stored energy may help to nourish young birds through the challenging period when they must learn to forage on their own. In other altricial species, however, the fat stores are mostly depleted by the time the young leave the nest. Good examples of this weight recession can be found among seabirds. The chicks of the Gray‐headed Albatross (Thalassarche chrysostoma) on South Georgia Island in the Southern Ocean, for example, temporarily weigh up to 30% more than adults before this weight is lost prior to fledging (Reid et al. 2000). In albatrosses, this phenomenon is believed to ensure adequate resources for chicks during the crucial period of feather growth preceding flight. A similar growth pattern occurs in swifts; the excess weight in these nestlings is associated with the developmental costs of exceptionally large and crucial wing feathers along with fine‐tuned adjustments of their mass to optimize flight ability at fledging (Wright et al. 2006).
Most precocial young, particularly those that feed themselves, are somewhat inept at acquiring and ingesting food immediately after they hatch. For their first few days out of the egg, chicks sustain themselves primarily on the large amount of egg yolk still in their abdomens. They may lose weight during this transitional period, but once they begin to feed themselves efficiently, they gain weight in a pattern similar to that of altricial birds. As chicks grow, their metabolic rates also increase. Although overall patterns of weight gain are similar in precocial and altricial birds, their metabolic rates increase in strikingly different ways. The metabolic rate of precocial species increases in two distinct phases: a rapid initial phase that shifts quite abruptly into a slower phase about halfway through development. In contrast, the metabolic rate of altricial chicks increases fairly continuously as they develop.
11.10.4 Sensory and motor development
An altricial nestling’s sensory and motor abilities develop as its body does. At hatching, it is all but helpless, able to stay upright only by leaning against the nest. Nevertheless, it gapes, swallows, digests, and defecates—the four behaviors crucial to obtaining and converting food into a rapidly developing young bird. With most of this energy going into growth, altricial nestlings also need to sleep, which they alternate with feeding. Most altricial chicks cannot even give begging calls during their first day or so, but these calls soon develop, becoming louder and more persistent as the nestling grows. At about one‐fourth of the way through the nestling stage, their eyes open, allowing them to gape at visual stimuli (the parent, a human hand, or even forceps holding food). Then nestlings begin to grasp objects with their feet, although their balance generally is still poor. When defecating, the nestling raises its posterior and moves it from side to side, making it easy for the parents to retrieve the fecal sac; many species also back up to the edge of the nest. A few days after their eyes open, most altricial nestlings develop a crouching response to strange visitors at the nest, and in many species they develop the same reaction to the alarm calls of their parents. By the time most of their feathers have emerged, their begging behavior may have become quite aggressive. As the feathers continue to unfurl from their shafts, the nestlings begin their first preening movements, and they often begin stretching their wings up, to the side, and back.
Precocial chicks, although further along in their sensory and motor development at hatching, still require time to adjust to their new environment outside of the egg. Even megapode chicks must pause underground after hatching, often resting there for more than a day before they begin their ascent up through the layers of mound material above them. For example, the chicks of one well‐studied megapode, the Australian Brush‐Turkey (Alectura lathami), are born with their eyes closed and are unable to raise their heads for several hours. In captivity, once the head is raised, however, chicks quickly begin to preen, removing feather sheaths and making the plumage clean and dry. After 10 hours, chicks begin to respond to light stimuli with open eyes. In the wild, brush‐turkeys begin digging to the surface after this initial period of resting and adjustment, and they develop as they proceed upwards. Along the way, the chicks develop the ability to feed themselves, pecking at soil invertebrates they find in their paths. Within 24 hours, these brush‐turkey chicks have attained thermoregulatory abilities. As digging continues, the young brush‐turkeys become more and more efficient, covering greater distances in shorter amounts of time until finally they emerge ready for life on their own (Göth 2002).
11.10.5 Leaving the nest
Most passerines leave the nest (fledge) about 9–12 days after hatching (the shorter periods are for species nesting in the open). Long before they are capable of sustained flight, many fledgling passerines disperse into denser foliage nearby, communicating their location to their parents through a series of soft calls. By spreading out in vegetation, the chicks are safer than they were in the nest. Altricial fledglings often sit on a protected perch, begging for food only when a parent gets quite close. As their feathers continue to develop, fledglings are fed less and less often until they are independent of parental care. During this transition, fledglings develop many new skills for finding and catching food: they begin pecking at objects, picking up food, catching insects, working at grass seed heads, scratching the ground, and probing in bark furrows. They also begin bathing.
The nestlings of some seabirds like guillemots, murres, and murrelets often leave the nest long before they have functional flight feathers; these young birds continue to develop at sea where the proximity to marine food allows one parent to feed the chick for several more weeks until it can fly (Ydenberg 1989) (Fig. 11.45). In other birds, parents sometimes help fledglings improve their prey‐catching skills; raptors such as Northern Harriers (Circus cyaneus) of North America may drop mice for their young to catch in mid‐air. Some kingfishers may similarly drop recently killed or battered fish into the water as easy targets for their fledglings’ first few dives. American Woodcock (Scolopax minor) chicks, whose bills at first are too short and weak to probe in the soil for earthworms, rely on their mother to obtain them. As she probes and pulls up the worms, the chicks take them directly from her bill. Although the young of many diving species go underwater to escape predators, they are very reluctant at first to dive for food, so much so that the adults initially bring food to the surface for them. Adult grebes, loons, and alcids feed their young for up to 10 weeks, first in the nest and later as the young accompany them on the water’s surface or on one of the parent’s backs.

Fig. 11.45 Fledging before flight. The chicks of some seabirds, like this Guadalupe Murrelet (Synthliboramphus hypoleucus) swimming between its parents, leave the nest long before they can fly. These chicks then move out to sea, where their parents continue to feed them until they reach full independence.
(Photograph by Tim Melling.)
Most precocial chicks leave the nest shortly after they hatch and their feathers dry, reducing the chance that the entire brood will be found and eaten by a predator. The adult parent provides security for its chicks, and a strong social bond often holds them together. Perhaps because they receive essentially no parental care in the nest, chicks of precocial species are generally not referred to as “fledglings.” Various vocalizations keep family members in touch with one another: adults make assembly calls to summon their young, alarm calls voiced by either the adult or young instantly trigger a crouching or freezing response from the chicks, and distress calls made by the young draw adults to their vicinity. As the chicks mature, a social hierarchy usually develops within the group, and siblings often challenge each other. In large precocial species, such as Canada Geese (Branta canadensis), a family may stay together throughout the subsequent winter. Similarly, Upland Geese (Chloephaga picta) in southern South America remain under the protection of their parents from hatching in November until eviction by their parents the following August (Summers 1983). In Tundra Swans (Cygnus columbianus) of northern Europe, the parents defend offspring in two ways: the parents react aggressively when their offspring are threatened, and, more subtly, the very presence of the adults seems to reduce the chances of offspring being threatened by other adult swans. Although second‐ and third‐year offspring are no longer actively defended by their parents, they still may benefit in this way by remaining close to their parents (Scott 1980). In smaller waterfowl like ducks, parental defense is usually less imposing, and the family bond usually is weaker; young may follow their mother at first and a different female later, forming large groups, termed creches, of young from several broods. In these species in which direct defense from the adults is less important, ducklings gain by safety in numbers: the more ducklings there are in a group, the less likely it is that any single duckling will fall prey to a predator (Chapter 13).
In addition to helping avoid predation, leaving the nest soon after hatching allows developing precocial young to move to areas of abundant food. Over the course of their development, for example, goslings (young geese) may walk many kilometers from their nest site in search of rich grass and other non‐woody plants; many species of ducklings gather on productive water bodies; sandpiper and plover chicks congregate with their parents along rich stretches of shore; and groups of young Ostriches (Struthio camelus) in Africa and rheas in South America often wander widely through their respective desert and steppe habitats (Fig. 11.46).

Fig. 11.46 Precocial chicks are able to leave the nest site after hatching. Precocial chicks often follow a parent away from the nest shortly after hatching, decreasing the risk of predation. For example, Lesser Rhea (Rhea pennata) family groups often wander together in search of food shortly after the chicks hatch.
(Photograph by Sergio Bitran Mogilevich.)
11.10.6 Developmental periods and independence
Among altricial birds, the time from hatching to independence ranges from approximately 25 days to several months. The young of most passerine species, however, become efficient fliers at about 17 days and are independent of parental care in about 28 days. Large altricial non‐passerines have long periods of nestling life: young Bald Eagles (Haliaeetus leucocephalus) of North America require 10–12 weeks in the nest before fledging, young Blue‐and‐yellow Macaws (Ara ararauna) in South America require more than 3 months, and young California (Gymnogyps californianus) and Andean (Vultur gryphus) Condors take as long as 5 months before leaving the nest, but may not become entirely independent of parental care for months afterward. Larger penguins brood their young for about 6 weeks, at which point the hatchlings huddle close together with other young in large groups (also called creches) where they continue to be fed by their parents for up to nine additional months.
Generally, altricial birds with short incubation periods tend to have short nestling periods, and those with long incubation periods usually have long nestling periods. It almost seems as though the growth rate of the developing young has a single setting for both within and outside the egg. The length of time that eggs and young spend in the nest is related to the safety of its location. For instance, many ground‐nesting birds have short incubation and nestling periods in general, speeding the young bird through its time of greatest vulnerability to predators.
Young that stay in the nest and receive parental care there for long periods of time are termed nidicolous; those that leave the nest soon after hatching, although usually far from independent, are nidifugous. All altricial young are nidicolous (they are incapable of leaving the nest soon after hatching). In contrast, most precocial young (except many of the seabirds mentioned above) are nidifugous, and their nestling period thus is very short. In altricial birds, the fledging period (the time from hatching to leaving the nest) often can serve as a comparative measure of developmental rate, although even among altricial birds there is considerable variation in how well developed the chicks are when they leave the nest. However, the variation in precocial birds is even greater, with a clear difference between nidicolous and nidifugous species. In any event, the transition to independence may be less jarring for precocial species than for altricial species, because precocial chicks never require as much or as many kinds of parental care as do altricial chicks. As in so many other aspects of young birds’ development, there is considerable variation in the length of dependency of precocial chicks. Young shorebirds and ducks may become independent and ready for a long southward migration without their parents within a few short weeks of hatching; the offspring of large geese and swans may stay with their parents through migration and well into the following winter.
Throughout the transition to independence runs the fundamental parent‐versus‐offspring conflict between what is best for the parent bird and what is best for its offspring. Parents feed the chicks less frequently once they have fledged, and, as the fledglings become more capable of taking care of themselves, the parents become less attentive to their demands. Each offspring eventually finds that it is easier to find food for itself than to try to obtain it from its parents, and then it is off on an independent life.
11.11 Recognition among parents and their young
Even before young birds hatch, they often have social contact with the parent (or parents) attending the nest. Young of some species peep from inside their egg, loudly enough to be audible from the outside. They apparently can hear their parents in return, as many will stop peeping after hearing a vocal response from the adult. Some species, such as Northern Bobwhite (Colinus virginianus), even learn to recognize their own mother’s call while still in the egg (Lickliter and Hellewell 1992).
Nonetheless, only some kinds of parent birds are able to recognize their own young. Researchers can interchange the young of most passerine species without the parents realizing that any change has occurred: the adults usually seem willing to feed any chick that is begging in the nest. Adults of many non‐passerine species, especially colonially nesting birds, however, are not fooled so easily. For example, for 2 or 3 days after their young hatch, most gulls will accept tiny young from other nests as their own, whether the young happen to wander in naturally or arrive as part of a biologist’s experiment. After this short period during which the adults become accustomed to the nestlings in their own territory, they refuse any others; in fact, the adults will chase and peck stray young until they leave the territory or die. This developmental change in recognition likely reflects changes in the probability of getting offspring confused: very young gull chicks are not nearly as likely to wander into foreign territories as are older chicks, and young chicks are therefore far more likely to be the adult’s legitimate offspring.
Most young birds do not recognize their parents for some time after hatching. Altricial nestlings beg from nearly any bird that comes near the nest—even from a predator such as a hawk—unless they are frightened by the size or behavior of the newcomer or the parent gives a warning call. As they grow older, however, nestlings learn to recognize their parents and save their begging energy for the times when it is most likely to lead to a delivery of food. In contrast, the young of many colonially nesting birds recognize the calls of their own parents at an early age. This recognition allows them to prepare quickly to receive food when the parent arrives and, in some species, prevents them from begging from the wrong adults, which may react aggressively. Nestlings recognize their parents’ calls by 6 days of age in Laughing Gulls (Leucophaeus atricilla) of the Atlantic coast of North America (Beer 1969), by 3 days in Thick‐billed Murres (Uria lomvia) being raised on cliffs over the Arctic Ocean (Lefevre et al. 1998), and by at least 14 days (7 days before fledging) in Pinyon Jays (Gymnorhinus cyanocephalus) of the American southwest (Balda and Balda 1978). In virtually all birds, begging vocalizations grow more intense when the chicks are hungrier, but in some colonially nesting passerines, such as Golden‐backed Weavers (Ploceus jacksoni) of Africa, offspring vocalizations carry information about the identity of the chicks as well (Reers and Jacot 2011). For most species whose young gather into creches, the young recognize their parents’ calls by the time these groups form; this is true for Australian Galahs (Eolophus roseicapilla), Adelie Penguins (Pygoscelis adeliae), and American White Pelicans (Pelecanus erythrorhynchos).
11.12 Parental care
All altricial nestlings and most precocial chicks require some form of parental care. From the moment they hatch until they reach independence, altricial young depend heavily on their parents. Parents of altricial young find and feed their nestlings copious amounts of food, often keep the nest clean by disposing of their feces, and defend them against predators. Even most precocial chicks, which usually feed themselves, rely on their parents for vigilance against predators and protection from the elements.
11.12.1 Feeding their young
Parents of altricial young must regularly bring food to their growing offspring. To feed nectar to her bee‐sized young, a female hummingbird thrusts her bill down each of their throats, often probing so vigorously that it seems as though she might poke right through them. The offspring of boobies, penguins, and cormorants often seem to attack their parents when they return home with a load of food, scrambling to ensure access to the fresh harvest.
Some birds bring back large prey items that must be torn up to be fed to nestlings, at least when they are recently hatched. It is no surprise that birds such as raptors bring one large prey item back to the nest at a time, but birds that catch smaller prey often employ a different strategy. Puffins, for example, are adept at bringing a well‐organized file of many small fish, stacked up along the bill, to their nests (Fig. 11.47). Most storm‐petrels gather tiny red crustaceans from the ocean’s surface by day and store them in their esophagus. At night, they return to the nest, when predatory gulls and falcons are much less of a threat. Once safe in their burrows, adult storm‐petrels disgorge a band of crustacean paste—resembling pink toothpaste—to feed their ravenous nestling.

Fig. 11.47 Provisioning chicks. This Atlantic Puffin (Fratercula arctica) returns to its burrow, its beak full of small fish for its single chick.
(Photograph by Harold Moses.)
Birds accomplish some impressive feats while feeding their young. Parents of altricial passerines in the northern temperate zone commonly bring food to their nests hundreds of times per day. For example, Great Tits (Parus major) in Japan, which have broods of seven or eight young, bring one prey item at a time to their nestlings at rates of 300–400 feedings per day (Royama 1966). In North America, one Eastern Phoebe (Sayornis phoebe) with four young made 8942 visits to its nest over the course of 17 days (Kendeigh 1952). If a species brings more than one prey item per visit, it generally comes to its nest fewer times, but it may deliver food at an awe‐inspiring rate.
Most seabird parents forage across vast home ranges. Although many have precocial chicks capable of thermoregulation from an early age, their food is often available only far from the nest and requires great skill to gather; parents therefore must feed their young until they are capable of long‐distance flight and effective foraging. Extraordinary among such seabirds are the albatrosses that nest on tiny islands in the southern oceans and forage for fish, squid, and shrimp for up to 10 days at a time at distances of almost 2000 kilometers from the colony (Pinaud et al. 2005).
A few kinds of avian parents are able to produce food internally for their nestlings rather than harvesting it directly from the environment. For example, for the first week after their chicks hatch, pigeons and doves feed their young crop milk, a rich, thick liquid substance made of epithelial cells from the lining of the parent’s esophagus. Flamingo parents of both sexes also routinely feed their young an esophageal milk‐like substance (Ward et al. 2001). Although it is not always necessary, male Emperor Penguins (Aptenodytes forsteri) can produce a thick milk from their esophagus for the first week or so of their chick’s development if the female is late in returning to the nest to begin feeding.
Some birds carry water as well as food to their young. For example, Common Ravens (Corvus corax) and Anhingas (Anhinga anhinga), among others, deliver water to their nestlings beak‐to‐beak. The sandgrouse of deserts and arid grasslands in Africa and Asia use special sponge‐like breast feathers to transport water from distant water holes to their young (Cade and Maclean 1967).
11.12.2 Defending their young
Parent birds defend their nests and young with varying levels of aggression. Raptors and large ratites may do so with dangerous effectiveness, whereas small passerines may offer only symbolic displays of agitation. Few, if any, birds are more potentially dangerous than an Ostrich (Struthio camelus) or cassowary—with their long and powerful legs—but actual accounts of these birds aggressively defending young are rare. However, few people attacked near the nest of an aggressive raptor or large owl will forget the experience. Large waterfowl, such as swans and large geese, almost always stand and defend their nests or broods, hissing and flapping their powerful wings. If a predator comes too close, they will strike with their wings and scratch with their feet. Smaller waterfowl rely on cryptic behavior to evade predators. The same is true for shorebirds: very large plovers such as lapwings often defend their young aggressively. Smaller shorebird species rely on camouflaged nests, eggs, and young, as well as on distinctive predator distraction displays that often involve the feigning of an injury by the parent bird (Fig. 11.48). The adult may hunch its back and drag one wing half‐open to the side, creating the illusion that it could easily be captured and eaten. As soon as the potential predator is lured far enough away from the eggs or young, the seemingly crippled parent undergoes a transformative “recovery” and runs or flies away with ease.

Fig. 11.48 Predator distraction displays. This Semipalmated Plover (Charadrius semipalmatus) is feigning injury in order to lure a potential predator away from its nest site.
(Photograph by Andy Johnson.)
Some birds remove their young from danger when threatened. If their bills are large enough, rails will carry their young to safety; jacanas transport their young under their wings. Male Sungrebes (Heliornis fulica) of the neotropics carry their young to safety, even in flight, by placing them in a pouch under each wing (Fig. 11.49).

Fig. 11.49 A unique way to transport young. Sungrebes (Heliornis fulica) and other aquatic bird species commonly allow their chicks to ride on their backs as they swim. Male Sungrebes even have special pouches within the folds of their wings that allow them to carry their chicks during flight.
(Photograph by Catalina Castillo.)
Although the young of most species are effectively defenseless, some can simulate a threat or at least promise a disgusting encounter. Within a cavity nest, a brood full of tits or wrynecks can skillfully imitate a hissing snake when a predator comes to investigate their home. Young gulls and many other seabirds may release a barrage of feces and regurgitated food when molested by a predator. Fulmar chicks are notoriously revolting in this respect because their stomachs contain a vile smelling oil; the vomit of fulmar chicks is very difficult for a predator to clean from its fur, feathers, or scales—or for unfortunate researchers to clean from their clothes and hair.
11.12.3 Nest sanitation
Because young altricial birds often spend many days or weeks in the nest, parents often have behaviors that keep the area free of materials that might cause disease or attract predators. As soon as the young hatch, most parents remove the broken eggshells. In addition, the young of most passerines produce their feces, at least initially, in a tough, flexible bag called a fecal sac (Fig. 11.50). These sacs make the removal and disposal of feces easy: many parents simply carry them some distance and drop them. Some species drop these fecal sacs over water; nuthatches and wrens place them on tree branches. The duration of fecal sac production varies among passerines. The young of many species consistently produce their feces in these tidy packets until they leave the nest, so that the nest is nearly without feces even after all the young have fledged. In contrast, the nestlings of other species stop producing their fecal sacs about midway through nestling life, leaving behind a matted mess of feces and nest material after they fledge.

Fig. 11.50 Fecal sac. Most passerine chicks produce packets of waste called fecal sacs that can either be eaten or carried away from the nest by the parent, as demonstrated by this female Red‐winged Blackbird (Agelaius phoeniceus).
(Photograph by Gary Seloff.)
Although sanitation may be a principal reason for fecal sac production, the sacs also allow some birds to recycle scarce resources: parents of some bird species eat the sacs rather than disposing of them. Both the sacs themselves (made of a protein–sugar mixture) and their contents contain valuable nutrients that the parents can consume and potentially reuse (McGowan 1995). Younger chicks, in particular, are relatively inefficient at digesting the food they are fed, and the sac contents thus retain food value that the parents’ more efficient digestive systems can recover.
Spotting a parent bird disposing of a fecal sac is a valuable find for a field ornithologist. A bird carrying a fecal sac is an unequivocal indicator of an active nesting attempt. Because the glistening white sacs are easy to see from a distance, the act of carrying one draws attention to the bird; once spotted, the adult may provide clues regarding the location of its nest, which can be particularly helpful to researchers searching for the nests of cryptic species.
The young of many large bird species rapidly become adept at directing their waste outside of the nest. For example, an Ornate Hawk‐Eagle (Spizaetus ornatus) chick in Guatemala was able to begin defecating over the side of its large nest when it was 3 days old (Lyon and Kuhnigk 1985). Areas around some raptor nests soon become whitewashed with splattered feces—so much so, in fact, that one sometimes can estimate the number of young present without even seeing them. The sheer volume of feces within pelican, cormorant, and heron colonies soon whitewashes and often kills the surrounding vegetation.
11.13 Survival and reproductive trade‐offs
A central idea of life history theory is that adults face a fundamental trade‐off between allocating resources (such as energy and time) for reproduction versus their own self‐maintenance, as covered in more detail in Chapter 13. A Eurasian Blue Tit (Cyanistes caeruleus) may produce more than 15 fledglings in one summer, but that adult likely has (at best) a 50% chance of surviving through to the next year. In contrast, a Royal Albatross (Diomedea epomophora) of the vast Southern Ocean produces one young only every 2 years, but the adult’s annual survival rate is about 95% (Robertson 1993).
11.13.1 Meeting threats to offspring survival
One of the ways that birds can increase their lifetime reproductive output is to increase the survival prospects of their young. The most pervasive threat to the survival of most birds is predation. Most adult birds experience relatively high survival rates for their size because they are able to escape from predators through flight. From this perspective, the act of residing in a single spot to rear young is a risky departure from the typical avian lifestyle. Indeed, for some highly mobile birds, the breeding season is the only significant period they spend on land. Many nest predators will take adults as well as eggs or young, so incubating birds of most species are particularly vulnerable when they are nesting (Fig. 11.51).
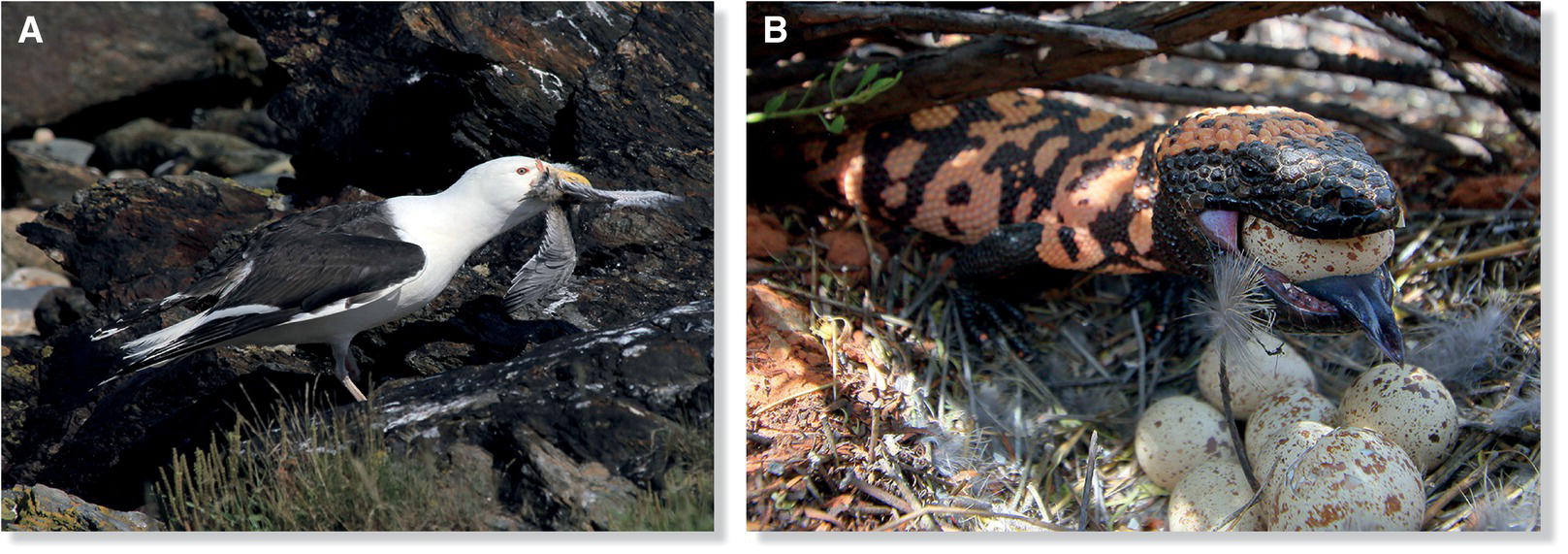
Fig. 11.51 Nest predators. Parent birds—as well as eggs and chicks—are vulnerable to many types of predators while incubating or attending to nestlings. Nest predators like this (A) Great Black‐backed Gull (Larus marinus) and (B) gila monster are a common cause of nesting failure for birds in most habitats.
(Photographs by: A, Brette Soucie, US Fish and Wildlife Service; B, Cameron Rognan.)
Although the loss of an incubating parent is relatively rare, eggs and young often are consumed at a high rate: for example, predators commonly destroy half or more of the nests of most forest‐nesting passerines. Much of the breeding biology of many birds seems to revolve around an effort to evade or fool predators. As we have seen earlier in this chapter, swifts build their nests on cliffs and under waterfalls, Killdeer (Charadrius vociferus) and other plovers lure predators away from their nest by feigning injury, lapwings and geese attack predators that approach too closely, and birds such as penduline‐tits build nests with false openings. Notoriously effective nest predators include raptors, monkeys, raccoons, weasels, cats, opossums, and arboreal snakes; various other birds often can be dangerous nest predators as well. The top nest predators in marine habitats typically are birds such as skuas, jaegers, gulls, and frigatebirds.
Other sources of mortality for young birds tend to be much less constant. Weather is probably the most significant after predation. High rainfall, tides, or winds can cause the wholesale destruction of nests—especially for marsh‐nesting species or colonial birds on low islands. Bad weather also may force parents to leave the nest for extended periods in search of food to maintain their own health, causing them to lose their eggs or young to exposure or starvation. In tropical and desert areas, fledgling production often is severely depressed by unpredictable periods of extended rain or drought, effects often associated with large‐scale climatic oscillations such as El Niño (Jaksic and Fariña 2010). The arrival of El Niño can result in nearly total reproductive failure for seabirds in coastal Chile and Peru.
Brood parasites often cause substantial mortality for birds nesting in a more dispersed fashion (Chapter 13). For example, in a study in Illinois (USA), Brown‐headed Cowbirds (Molothrus ater) were found to parasitize up to 100% of Wood Thrush (Hylocichla mustelina) nests (Robinson and Wilcove 1994), and parasitism by cuckoos in the Old World can foil more than 60% of the nesting attempts of their hosts (Moskát and Honza 2002). Many of these host populations cannot sustain such a high rate of parasitism.
11.13.2 Adult and offspring survival
After considering all the threats they must face, it may come as no surprise that immature birds nearly always experience lower survival rates than birds that have reached reproductive age. For example, in their first few years, most gulls have average annual survival rates of 30–50%, whereas about 80% of older birds survive each year. This pattern likely arises because the transition from being fed to feeding oneself requires the rapid development of foraging skills and predator vigilance. A study of Barn Swallows (Hirundo rustica) in Switzerland by Martin Grüebler and Beat Naef‐Daenzer (2010) found that the extent of post‐fledging parental care had a very large effect on post‐fledging survival: young receiving care for up to 6 days after fledging experienced a survival rate of 23% over 3 weeks, whereas those receiving care for at least 14 days had a survival rate over the same period of 57%.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Aubrecht, G., W. Huber, and A. Weissenhofer. 2013. Coincidence or benefit? The use of Marasmius (horse‐hair fungus) filaments in bird nests. Avian Biology Research 6:26–30.
- Balda, R. P. and J. H. Balda. 1978. The care of young Piñon Jays (Gymnorhinus cyanocephalus) and their integration into the flock. Journal of Ornithology 119:146–171.
- Beer, C. G. 1969. Laughing gull chicks: recognition of their parents' voices. Science 166:1030–1032.
- Beissinger, S. R., S. Tygielski, and B. Elderd. 1998. Social constraints on the onset of incubation in a neotropical parrot: a nestbox addition experiment. Animal Behaviour 55:21–32.
- Blas, J., F. Sergio, and F. Hiraldo. 2009. Age‐related improvement in reproductive performance in a long‐lived raptor: a cross‐sectional and longitudinal study. Ecography 32:647–657.
- Böhning‐Gaese, K., B. Halbe, N. Lemoine, and R. Oberrath. 2000. Factors influencing the clutch size, number of broods and annual fecundity of North American and European land birds. Evolutionary Ecology Research 2:823–839.
- Braasch, A., C. Schauroth, and P. H. Becker. 2009. Post‐fledging body mass as a determinant of subadult survival in Common Terns Sterna hirundo. Journal of Ornithology 150:401–407.
- Cade, T. J. and G. L. MacLean. 1967. Transport of water by adult Sandgrouse to their young. Condor 69:323–343.
- Calder, W. A. 1973. Microhabitat selection during nesting of hummingbirds in the Rocky Mountains. Ecology 54:127–134.
- Chalfoun, A. D. and T. E. Martin. 2007. Latitudinal variation in avian incubation attentiveness and a test of the food limitation hypothesis. Animal Behaviour 73:579–585.
- Cheke, R. A., J. F. Venn, and P. J. Jones. 2007. Forecasting suitable breeding conditions for the red‐billed quelea Quelea quelea in southern Africa. Journal of Applied Ecology 44:523–533.
- Clark, L. and J. R. Mason. 1988. Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77:174–180.
- Collias, N. E. and E. C. Collias. 1964. Evolution of Nest‐building in the Weaverbirds (Ploceidae) . University of California Publications in Zoology, Vol. 73. University of California Press, Berkeley, CA.
- Eggers, S., M. Griesser, M. Nystrand, and J. Ekman. 2006. Predation risk induces changes in nest‐site selection and clutch size in the Siberian jay. Proceedings of the Royal Society B: Biological Sciences 273:701–706.
- Evans, M. R. and J. L. Burn. 1996. An experimental analysis of mate choice in the wren: a monomorphic, polygynous passerine. Behavioral Ecology 7:101–108.
- Forsman, J. T. and T. E. Martin. 2009. Habitat selection for parasite‐free space by hosts of parasitic cowbirds. Oikos 118:464–470.
- Göth, A. 2002. Behavior of Australian Brush‐turkey (Alectura lathami, Galliformes: Megapodiidae) chicks following underground hatching. Journal für Ornithologie 143:477–488.
- Grant, G. S. 1982. Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithological Monographs 30:iii–ix, 1–75.
- Grüebler, M. U. and B. Naef‐Daenzer. 2010. Survival benefits of post‐fledging care: experimental approach to a critical part of avian reproductive strategies. Journal of Animal Ecology 79:334–341.
- Hahn, T. P. 1998. Reproductive seasonality in an opportunistic breeder, the Red Crossbill, Loxia curvirostra. Ecology 79:2365–2375.
- Halkin, S. L. 1997. Nest‐vicinity song exchanges may coordinate biparental care of northern cardinals. Animal Behavior 54:189–198.
- Hau, M., M. Wikelski, and J. C. Wingfield. 2000. Visual and nutritional food cues fine‐tune timing of reproduction in a neotropical rainforest bird. Journal of Experimental Zoology 286:494–504.
- Heath, M. and M. H. Hansell. 2002. Weaving techniques in two species of Icterini, the Yellow Oriole (Icterus nigrogularis) and Crested Oropendola (Psarocolius decumanus). Pages 144–154 in Studies in Trinidad and Tobago Ornithology: Honouring Richard ffrench (F. E. Hayes and S. A. Temple, Eds.). Occasional Paper No. 1 of the Department of Life Sciences. University of the West Indies, St Augustine, Trinidad.
- Helfenstein, F., R. H. Wagner, E. Danchin, and J. M. Rossi. 2003. Functions of courtship feeding in black‐legged kittiwakes: natural and sexual selection. Animal Behaviour 65:1027–1033.
- Hoffman, D. J. 1978. Embryotoxic effects of crude oil in mallard ducks and chicks. Toxicology and Applied Pharmacology 46:183–190.
- Ignatiuk, J. B. and R. G. Clark. 1991. Breeding biology of American crows in Saskatchewan parkland habitat. Canadian Journal of Zoology 69:168–175.
- Jackson, W. M. 1992. Estimating conspecific nest parasitism in the Northern Masked Weaver based on within‐female variability in egg appearance. Auk 109:435–443.
- Jaksic, F. M. and J. M. Fariña. 2010. El Niño and the birds: a resource‐based interpretation of climatic forcing in the southeastern Pacific. Anales Instituto Patagonia 38:121–140.
- Jia. C. X., Y. H. Sun, and J. E. Swenson. 2010. Unusual incubation behavior and embryonic tolerance of hypothermia by the Blood Pheasant (Ithaginis cruentus). Auk 127:926–931.
- Jones, D. N., R. W. R. J. Dekker, and C. S. Roselaar. 1995. The Megapodes . Oxford University Press, New York, NY.
- Joyce, F. J. 1993. Nesting success of rufous‐naped wrens (Campylorhynchus rufinucha) is greater near wasp nests. Behavioral Ecology and Sociobiology 32:71–77.
- Kendeigh, C. S. 1952. Parental Care and its Evolution in Birds . Illinois Biological Monographs No. 22. University of Illinois Press, Urbana, IL.
- Krebs, E. A. 1998. Breeding biology of crimson rosellas (Platycercus elegans) on Black Mountain, Australian Capital Territory. Australian Journal of Zoology 46:119–136.
- Lahti, D. C. and A. R. Lahti. 2002. How precise is egg discrimination in weaverbirds? Animal Behaviour 63:1135–1142.
- Lefevre, K., R. Montgomerie, and A. J. Gaston. 1998. Parent–offspring recognition in thick‐billed murres (Aves: Alcidae). Animal Behaviour 55:925–938.
- Lickliter, R. and T. B. Hellewell. 1992. Contextual determinants of auditory learning in Bobwhite Quail embryos and hatchlings. Developmental Psychobiology 25:7–31.
- Lindell, C. 1996. Benefits and costs to Plain‐fronted Thornbirds (Phacellodomus rufifrons) of interactions with avian nest associates. Auk 113:565–577.
- Lomáscolo, S. B., A. C. Monmany, A. Malizia, and T. E. Martin. 2010. Flexibility in nest‐site choice and nesting success of Turdus rufiventris (Turdidae) in a montane forest in northwestern Argentina. Wilson Journal of Ornithology 122:674–680.
- Lyon, B. and A. Kuhnigk. 1985. Observations on nesting Ornate Hawk‐eagles in Guatemala. Wilson Bulletin 97:141–147.
- Mallory, M. L., J. A. Akearok, D. B. Edwards, K. O’Donovan, and C. D. Gilbert. 2008. Autumn migration and wintering of northern fulmars (Fulmarus glacialis) from the Canadian high Arctic. Polar Biology 31:745–750.
- McFarland, K. P. and C. C. Rimmer. 1996. Horsehair fungus (Marasmius androsaceus) used as nest lining by birds of the subalpine spruce–fir community in the northeastern United States. Canadian Field‐Naturalist 110:541–543.
- McGowan, K. J. 1995. A test of whether economy or nutrition determines fecal sac ingestion in nesting Corvids. Condor 97:50–56.
- Moskát, C. and M. Honza. 2002. European Cuckoo Cuculus canorus parasitism and host's rejection behaviour in a heavily parasitized Great Reed Warbler Acrocephalus arundinaceus population. Ibis 144:614–622.
- Murray, J. R., C. W. Varian‐Ramos, Z. S. Welch, and M. S. Saha. 2013. Embryological staging of the Zebra Finch, Taeniopygia guttata. Journal of Morphology 274:1090–1110.
- Nisbet, I. C. T. 1977. Courtship feeding and clutch size in common terns Sterna hirundo. Pages 101–109 in Evolutionary Ecology (B. Stonehouse and C. M. Perrins, Eds.). Macmillan, London.
- Parmelee, D. F. 1992. Snowy owl (Bubo scandiacus). Pages 1–20 in The Birds of North America , Vol. 10 (A. Poole, P. Stettenheim, and F. Gill, Eds.). National Academy of Sciences, Philadelphia, PA.
- Phillips, C. L. 1887. Egg‐laying extraordinary in Colaptes auratus. Auk 4:346.
- Pinaud, D., Y. Cherel, and H. Weimerskirch. 2005. Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in Yellow‐nosed Albatrosses. Marine Ecology Progress Series 298:295–304.
- Polo, V., I. López‐Rull, D. Gil and J. P. Veiga. 2010. Experimental addition of green plants to the nest increases testosterone levels in female spotless starlings. Ethology 116:129–137.
- Proctor, N. S. and P. J. Lynch. 1993. Manual of Ornithology: Avian Structure and Function . Yale University Press, New Haven, CT.
- Pruett‐Jones, S. and M. Pruett‐Jones. 1994. Sexual competition and courtship disruptions: why do male bowerbirds destroy each other’s bowers? Animal Behaviour 47:607–620.
- Rahn, H. and A. Ar. 1974. The avian egg: incubation time and water loss. Condor 76:147–152.
- Rebke, M., T. Coulson, P. H. Becker, and J. W. Vaupel. 2010. Reproductive improvement and senescence in a long‐lived bird. Proceedings of the National Academy of Sciences of the United States of America 107:7841–7846.
- Reers, H. and A. Jacot. 2011. The effect of hunger on the acoustic individuality in begging calls of a colonially breeding weaver bird. BMC Ecology 11:3.
- Reid, K., P. A. Prince, and J. P. Croxall. 2000. Fly or die: the role of fat stores in the growth and development of Grey‐Headed Albatross Diomedea chrysostoma chicks. Ibis 142:188–198.
- Riehl, C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Current Biology 20:1830–1833.
- Ripley, S. D. 1957. Notes on the horned coot, Fulica cornuta Bonaparte. Postilla 30:1–8.
- Robertson, C. J. R. 1993. Survival and longevity of the northern royal albatross Diomedea epomophora sanfordi at Taiaroa Head 1937–1993. Emu 93:269–276.
- Robinson, S. K. and D. S. Wilcove. 1994. Forest fragmentation in the temperate zone and its effects on migratory songbirds. Bird Conservation International 4:233–249.
- Royama, T. R. 1966. Factors governing feeding rate, food requirement and brood size of nestling Great Tits Parus major. Ibis 108:313–347.
- Sallabanks, R. and F. C. James. 1999. American Robin (Turdus migratorius). The Birds of North America Online (A. Poole Ed.). Cornell Lab of Ornithology, Ithaca, NY.
- Scott, D. K. 1980. Functional aspects of prolonged parental care in Bewick’s swans. Animal Behaviour 28:938–952.
- Seel, D. C. 1973. Egg‐laying by the Cuckoo. British Birds 66:528–535.
- Slagsvold, T. 1982. Clutch size variation in passerine birds: the nest predation hypothesis. Oecologia 54:159–169.
- Starck, J. M. and R. E. Ricklefs (Eds.). 1998. Avian Growth and Development: Evolution within the Altricial–Precocial Spectrum . Oxford University Press, New York, NY.
- Stoleson, S. H. and S. R. Beissinger. 1999. Egg viability as a constraint on hatching synchrony at high ambient temperatures. Journal of Animal Ecology 68:951–962.
- Summers, R. W. 1983. The lifecycle of the Upland Goose (Chloëphaga picta) in the Falkland Islands. Ibis 125:524–544.
- Temeles, E. J. and W. J. Kress. 2010. Mate choice and mate competition by a tropical hummingbird at a floral resource. Proceedings of the Royal Society B: Biological Sciences 277:1607–1613.
- Tieleman, B. I., H. J. Van Noordwijk, and J. B. Williams. 2008. Nest site selection in a hot desert: trade‐off between microclimate and predation risk? Condor 110:116–124.
- Valera, F., H. Hoi, and B. Schleicher. 1997. Egg burial in penduline tits, Remiz pendulinus: its role in mate desertion and female polyandry. Behavioral Ecology 8:20–27.
- Vitz, A. C., L. A. Hanners, and S. R. Patton. 2013. Worm‐eating Warbler (Helmitheros vermivorum). The Birds of North America Online (A. Poole, Ed.). Cornell Lab of Ornithology, Ithaca, NY.
- Ward, A. M., A. Hunt, M. Maslanka, and C. Brown. 2001. Nutrient composition of American flamingo crop milk. Pages 187–193 in Proceedings of the Fourth Conference on Zoo and Wildlife Nutrition, AZA Nutrition Advisory Group (M. Edwards, K. J. Lisi, M. L. Schlegel, R. E. Bray, Eds.). Zoological Society of San Diego, Lake Buena Vista, FL.
- Wilson, R. T. and M. P. Wilson. 1986. Nest building by the Hamerkop Scopus umbretta. Ostrich 57:224–232.
- Wimberger, P. H. 1984. The use of green plant material in bird nests to avoid ectoparasites. Auk 101:615–618.
- Winnett‐Murray, K. 1980. The influence of cover on offspring survival in Western Gulls. Proceedings of the Colonial Waterbird Group 3:33–43.
- Wright, J., S. Markman, and S. M. Denney. 2006. Facultative adjustment of pre‐fledging mass loss by nestling swifts preparing for flight. Proceedings of the Royal Society B: Biological Sciences 273:1895–1900.
- Ydenberg, R. C. 1989. Growth–mortality trade‐offs and the evolution of juvenile life histories in the Alcidae. Ecology 70:1494–1506.
- Young, B. E. 1994. Geographic and seasonal patterns of clutch‐size variation in House Wrens. Auk 111:545–555.
- Young, B. E., M. Kaspari, and T. E. Martin. 1990. Species‐specific nest site selection by birds in ant‐acacia trees. Biotropica 22:310–315.