Chapter 9
Avian Mating and Social Behavior
John Alcock
Arizona State University

Opening image: A male Andean Cock‐of‐the‐rock (Rupicola peruvianus) displays in a sunspot. This species is known for its lekking behavior; the most beautifully plumed male with the most appealing display area secures the most matings.
(Photograph by Jason Rothmeyer.)
Imagine that it is springtime in central Arizona (USA), and you are watching a male and female Abert’s Towhee (Melozone aberti) in a suburban backyard (Fig. 9.01). With luck, you will see the female towhee exercising mate choice when she comes around to forage for seeds. She likely will arrive with a male companion by her side, because adult Abert’s Towhees often form long‐lasting social partnerships, and this pair is typical in that regard. The female appears practically attached to her partner as they hurry to the spot where scattered seeds await them. Once the birds have had their fill and have begun to hop toward the nearby shrubs, the female may stop and raise her long tail straight in the air. The male responds promptly to this signal by rushing to his partner and mounting her in a flurry of wing beats. After he succeeds in pressing his cloaca against hers for a few seconds, the mating is over. The two towhees then scurry off together under the shrubbery.

Fig. 9.01 A pair of Abert’s Towhees (Melozone aberti). This species often forms long‐lasting social partnerships.
(Photograph by John Alcock.)
This sequence of social behaviors, which is typical of many songbirds, illustrates the topics covered in this chapter. First, the female towhee clearly takes an active role in determining which male will fertilize her eggs. She does not merely accept the sexual advances of a partner passively, but instead signals her receptivity by elevating her tail, facilitating cloacal access for the male that she has selected as a partner. Abert’s Towhees are representative of birds in general in that females typically accept sperm only from particular males; such choices by females generate a host of questions, such as what are the attributes that females prefer in their mates, and what do they gain from their choices? These questions are at the heart of the first section of this chapter, which addresses the interrelated topics of behavioral mate choice and sexual selection.
Second, birds overall exhibit extremely diverse mating and social behaviors; these towhees provide an example of social monogamy, one of the most common avian mating systems. In Abert’s Towhees, one male associates closely with one female throughout an entire breeding season (if not longer) in a pair‐bond relationship that appears to restrict his reproductive options. Would a male be better off (that is, leave more surviving descendants) if he sought out several sexual partners in each breeding season? Males that fertilize the eggs of more than one female each spring would seem likely to pass on more of their genes than strictly monogamous males in the population. If so, monogamous tendencies in males would be eliminated over time in favor of a different mating system in which some males have several partners rather than only one at a time. This dilemma provides a starting point for the second section of the chapter, which explains why bird species range from purely monogamous to highly polygynous.
Third, these towhees also illustrate issues associated with social behavior in general. The birds clearly are social in the sense of remaining in each other’s company and interacting regularly. They cooperate in the defense of their territory, their private real estate from which they expel other Abert’s Towhees. Both members of this pair feed their young during the breeding season. Furthermore, they permit each other to feed freely on seeds offered to them, but frequently show aggression to the local House Sparrows (Passer domesticus) and other visitors that attempt to eat at the same bird table. What determines why the birds are cooperative in some contexts, but exhibit antisocial aggression in others? This question, along with other key puzzles linked to avian social behavior, is considered in the third and final part of the chapter.
9.1 Female mating preferences
Given what we know now about the sexual give‐and‐take between female and male birds, it is hard to believe that until the mid‐1900s, many biologists believed that females in general (even in our own species!) lacked preferences for particular attributes in their potential mates (Milam 2010). The standard view used to be that males controlled the mating game by competing among themselves for passive females. Charles Darwin, in contrast, argued that females of many bird species had what he termed “aesthetic sensibilities” that they employ when considering would‐be mates. Darwin knew that in pairs of birds where the sexes differed in traits such as singing ability, display dances, or the vividness of their plumage, the male would almost invariably be the sex with the greater capacity for song, the more bizarre courtship displays, and the more striking plumage. Darwin (1871) asked somewhat rhetorically whether “every male of the same species equally excite[s] and attract[s] the female? Or does she exert a choice, and prefer certain males?” He asserted that females do indeed have preferences for certain males.
In recent years, an explosion of research has demonstrated that Darwin was entirely correct in his observation that females are far from neutral bystanders when it comes to selecting mates (Jones and Ratterman 2009). Consider, for example, experiments that have shown how female Zebra Finches (Taeniopygia guttata) assess males via their songs (Woolley and Doupe 2008). When Zebra Finch males are singing in the presence of a female, they tend to produce songs that are faster and more standardized than the songs they sing when no females are nearby. By using two speakers to play back faster and slower songs, researchers can assess which of these variants the females prefer. It turns out that when female Zebra Finches are given this choice, they generally approach the speaker playing the faster song type, thereby demonstrating their preference for this kind of song. The underlying presumption of such an experiment is that under natural conditions, female Zebra Finches seeking mates would tend to approach and choose males able to sing the preferred song quality. This assumption also has been tested in experiments with captive finches: females given a choice between two caged males gave more raised‐tail copulation‐solicitation displays to the individual they visited more often (Witte 2006). In other words, a female’s association preference reveals her sexual preference as well.
Many other experiments on how females respond to the sexual signals of males have shown that females often pay close attention to even subtle differences in male behaviors or other traits. Male vocalizations (Chapter 10) frequently are a component of the sexual displays that female birds use to assess their mates. For example, female Swamp Sparrows (Melospiza georgiana) in eastern North America are attracted to unusually fast trilling songs (Ballentine et al. 2004), whereas female European Serins (Serinus serinus), a small Eurasian finch, prefer songs that incorporate notes of exceptionally high frequency (Cardoso et al. 2007). The speed of sound production correlates with male mating success in the Magnificent Frigatebird (Fregata magnificens) of the tropical Atlantic, a species in which males attract females by generating a drumming sound via bill clacking that then resonates into an inflated, bright red gular pouch, which itself is a sexual signal (Madsen et al. 2007) (Fig. 9.02). In the Brown Skua (Stercorarius antarcticus) of Antarctica, males try to produce calls with many notes per second while also generating notes over a relatively broad range of sound frequencies; it is particularly difficult to do both things at once, and the males best able to call near the upper limits of this performance trade‐off have higher reproductive success than their less adept rivals (Janicke et al. 2007). These and other cases outlined in Chapter 10 illustrate the general rule that female birds prefer vocal performances that are challenging to produce.

Fig. 9.02 Displaying male Magnificent Frigatebird (Fregata magnificens). Males of this species use both visual (inflated red pouch) and auditory signals (calling and bill clacking) to compete for access to reproductively active females.
(Photograph by Gerrit Vyn.)
Although male song in general is a very common sexual signal in many groups of birds, the precise song traits preferred by females vary widely from species to species: some female birds assess males on the basis of their speed of signal production, others by the presence of favored sound frequencies. Still others prefer male vocalizations with a wide frequency range or a large total number of song types in a male’s repertoire. None of these song attributes is favored universally across all birds. More remains to be learned about how a particular kind of signal preference by females of a given species translates into reproductive benefits for those females.
Just as no one acoustic element predominates in female mate preferences, so too no one visual aspect of courtship does the job across all bird species. In some birds, female preferences revolve around the intensity and frequency with which males perform visual displays that show off tail plumes, or wing feathers, or throat pouches. So, when Black Grouse (Tetrao tetrix) gather in groups in Finnish fields to compete for display territories visited by selective females, males differ in their overall activity, and the frequent displayers in relatively large central territories secure more mates than less active, peripheral males (Rintamäki et al. 1995) (Fig. 9.03). This finding suggests that the females evaluate their mates in part by the properties of their visual displays, which determine a male’s territorial success and his ability to stimulate female observers.
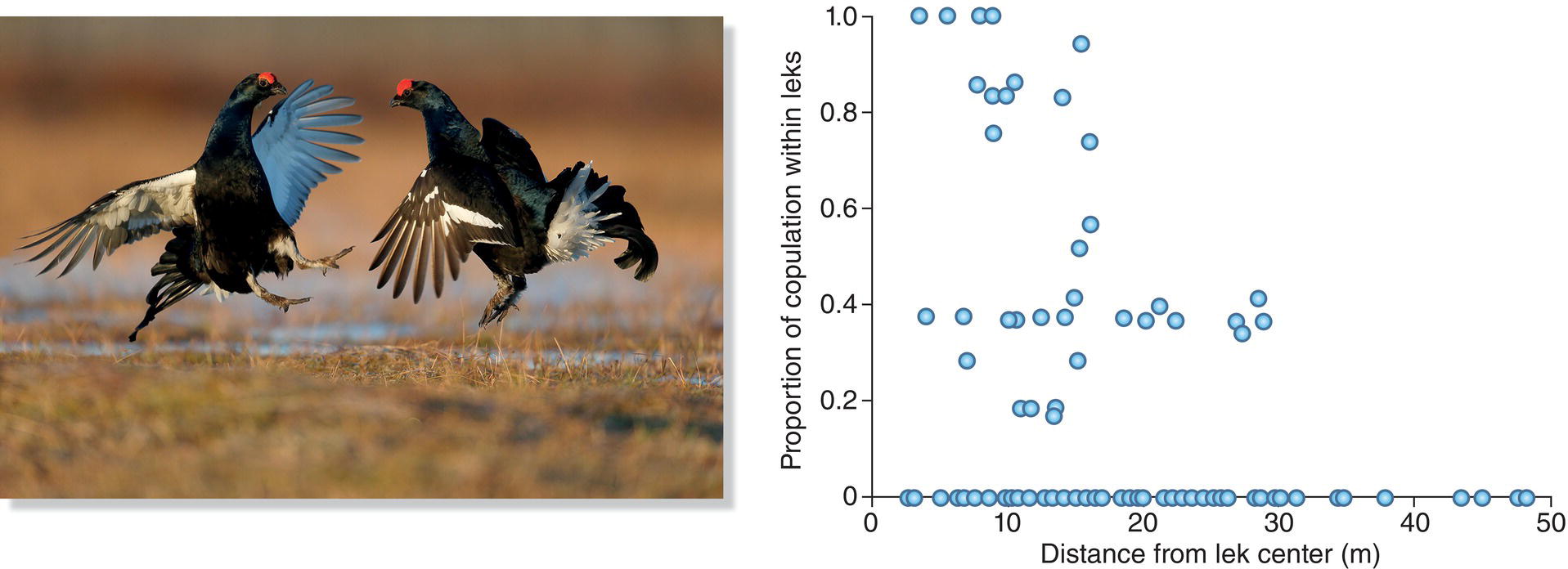
Fig. 9.03 Lekking display of a male Black Grouse (Tetrao tetrix). Males compete for access to mates by displaying their bright ornaments in groups termed leks. In this species, males that have display sites near the center of the lek tend to have higher mating success.
(From Rintamäki et al. 1995. Reproduced with permission from Elsevier. Photograph by Mike Lane.)
For other species, the size of a special male ornament most strongly affects female mate choice. For example, some researchers have found a relationship between the length of the Indian Peafowl’s (Pavo cristatus) amazing train and male reproductive success (Loyau et al. 2008). Peahens may attend to this aspect of the male or some other component of the train, such as the density of eye‐spots, when making a decision about which of several males to select as a mate. Peahens are not the only females with such preferences. In the Eurasian Penduline‐Tit (Remiz pendulinus), choosy females prefer males that have larger dark eye‐stripes (“masks”) over males with less prominent masks. As a result, males with large masks pair off more quickly than their less‐well‐masked rivals (Pogány and Székely 2007).
For other species, female mate choice may revolve around the extent to which a feathered body part reflects ultraviolet (UV) radiation, the yellowness of a patch of feathers, or the redness of a throat pouch (Chapter 4). In particular, carotenoid pigments, which make feathers appear yellow or red, are now believed to be a cue that females of many bird species use to evaluate the attractiveness of potential mates (Hill and McGraw 2006). The general rule—“the brighter, the better”—applies, for example, to the American Redstart (Setophaga ruticilla), an attractive wood‐warbler that breeds across much of northern North America. In this species, males with more brightly colored reddish feathers on their flanks sire more of their mate’s offspring than those with paler flank plumage (Reudink et al. 2009). Females of these songbirds sometimes exercise the option of seeking out redder neighboring males for extra‐pair copulation, a widespread attribute in birds, as we shall see (Westneat et al. 1990). Likewise, when some House Finch (Haemorhous mexicanus) males were artificially made redder by the application of hair dye, these extra‐bright males paired off with females more rapidly than those whose feather color had been made a paler red (Hill 1991) (Fig. 9.04). Studies of this sort make it clear that female mate choice occurs widely in birds (Box 9.01).

Fig. 9.04 House Finch (Haemorhous mexicanus) color variation. Although male House Finches generally have red plumage (left), yellow males (right) also occur in the wild. Color differences are due to dietary carotenoid variation; redder males are directly signaling to females that they have had better access to dietary carotenoids and are in better condition. Females therefore prefer red males over dull or yellow males.
(Photograph by Kevin McGraw.)
9.2 Pair bonds, courtship, and divorce
The sexual signaling that goes on when males court females may serve to induce a female to mate right away, but signals of this sort also can help establish a pair bond between a male and a potential mate as a prelude to eventual copulation and reproduction. In many birds, males and females form a social partnership that lasts for at least one breeding season, and sometimes for many more than one. In the Little Penguin (Eudyptula minor) of Australia and New Zealand, for example, some pairs remain together for as many as 13 years (Nisbet and Dann 2009).
One behavior that in some birds helps form and maintain a pair‐bond relationship is courtship feeding, in which a male brings food to a female during courtship. These gifts may provide information that influences the intensity of the bond between the male and female and thus the female’s sexual receptivity. The quantity of food delivered can have these effects on females, as studies in Poland have shown for the Northern Shrike (Lanius excubitor) (Tryjanowski and Hromada 2005). Male shrikes that offer their partners a large gift of food—that is, a sizeable sparrow or lizard rather than a small cricket—are more likely to be rewarded by a copulation. Likewise, long‐term studies of Black‐legged Kittiwakes (Rissa tridactyla) on the coast of France have found that in successive years females are more likely to re‐pair with males who did a better job of courtship feeding in the previous year (Helfenstein et al. 2003).
Although often it is popularly thought that many pairs of birds “mate for life,” pairs in most species often break up between or even within breeding seasons. These breakups may happen after one member of the pair dies or when one or both members subsequently pair with a different individual. Behavioral ecologists generally refer to this switching between pair‐bonded mates as divorce, and in birds the female usually takes the lead in switching mates. Female birds can initiate a split even in mid‐season, as shown by an experiment involving Black‐capped Chickadees (Poecile atricapillus) in Ontario, Canada. Two researchers, Ken Otter and Laurene Ratcliffe (1996), captured females that had paired with high‐status (dominant) or low‐status (subordinate) males. These females were held in captivity long enough to permit seven neighboring females to abandon their current mate and move to territories with the experimentally “widowed” males. Six of the seven divorcees paired off with a high‐status male; only one accepted a member of the subordinate group. This elegant experiment shows that female birds often are aware of the dominance status of various nearby males, or perhaps are alert to the quality of their territories, and are prepared to divorce their current, lower quality mate should a better opportunity present itself.
In other species, females may eschew divorce, but make the number of eggs they lay dependent upon the physiological state of a male. This is known to occur in Black Wheatears (Oenanthe leucura) (Fig. 9.05), a species found in southwestern Europe and northwestern Africa. In this species, females appear to wait until they have observed their partners carrying stones to their nest cavities before deciding how many eggs they will lay (Soler et al. 1996). Females produce more eggs when their mates have carted in many heavy rocks during the nest‐building period, whereas males that cannot—or are unwilling to—carry large rocks to the nest caves are on average given fewer copulation attempts. Thus, a given male Black Wheatear’s reproductive success appears to depend on his mate’s decisions, which in turn are influenced by his behavioral performance.

Fig. 9.05 Nest stones as an indicator of male quality. As the mating season begins, Black Wheatear (Oenanthe leucura) males carry hundreds of stones to build the base of a nest mound. Females gauge a male’s parental provisioning potential based on the quantity and quality of stones at a nesting site, laying more eggs with males that carry heavier stones.
(Photograph by Manuel Soler.)
Additional evidence helps explain why female wheatears cue in on stone carrying as an indicator of male quality. Males that carry very heavy stones have stronger immune systems (Chapter 7) than do males that only carry smaller stones (Soler et al. 1999). A healthy male may be able to help the female rear a larger clutch. Alternatively, a female with a healthy male may gain by working harder in a year when she has acquired a mate with “good genes” rather than holding back to increase the odds that she will survive to reproduce again in a future year. However, this pattern in which females paired with top males lay more eggs also might arise if the females in the best condition were to acquire stronger males as partners. This outcome would leave the females that are unable to produce a large clutch with lower quality males (Lebigre et al. 2007).
Female birds exhibit many other forms of mate choice, some of which are quite subtle. For example, Sjouke Kingma and colleagues (2009) have found that female Eurasian Blue Tits (Cyanistes caeruleus) at their study site near Groningen in the Netherlands adjust the amount of testosterone they contribute to their eggs in relation to the intensity of the UV coloration of their mate. Eggs containing the sons of high‐UV males receive more testosterone from their mothers. These hormonal adjustments by egg‐laying females may affect the attractiveness of their sons, in turn influencing the reproductive success of their mates, that is, the number of descendants that their partners contribute to the next generation. Females forced to accept lower quality males may gain by withholding testosterone from their eggs if the hormone is expensive to produce and if the sons of these males would be unable to use the biochemical in a developmentally advantageous manner.
At the most general level, we now know that any of a very broad spectrum of male attributes can affect the likelihood that a male will succeed in attracting a mate or in inducing her to lay more eggs, or eggs of higher quality. The cues used by females may even vary between populations of the same species. As described in more detail in Box 3.03, a comparison of mate choice in the North American and European subspecies of the Barn Swallow (Hirundo rustica) showed that females in different populations may focus on different male ornaments. In North American populations of Barn Swallows, males whose ventral plumage is more heavily pigmented secured mates more rapidly than their duller rivals (Safran and McGraw 2004). In contrast, in Europe the males most attractive to females are those with unusually long and symmetrical tail streamers, and differences in plumage coloration play little role in mate choice (Møller 1988). This example illustrates that although female mate choice, broadly defined, may be standard practice in birds, the nature of the preferences can vary markedly from species to species and sometimes from population to population within the same species (Box 9.02).
9.3 Male mating preferences
Many fewer studies have considered whether male birds, like females, are choosy about their partners. As Darwin himself noted, this relative lack of attention to mate choice by males stems from the fact that males are much more likely to court females than the other way around. Therefore, it has made general sense to focus first on the cues females use when evaluating courting males rather than to investigate the intuitively less likely phenomenon of mate choice by males.
Nonetheless, theoretical reasons exist for thinking that male mate choice happens too. Social monogamy is extremely common among birds, and to the extent that males limit themselves to one social and sexual companion per breeding season, they certainly could gain by selecting a superior partner, one with whom a male is likely to rear an above‐average number of surviving offspring. In an impressive diversity of birds, monogamous males cooperate with their mates in sharing most or all of the tasks associated with reproduction (except, of course, egg laying). For such a male to maximize the return on his paternal investments, he may benefit by identifying an especially fertile female or one that is an unusually good parental partner. For example, in the European Pied Flycatcher (Ficedula hypoleuca) population in central Spain, only some females have a white patch on the forehead, and these individuals raise more fledglings on average than do females that lack this patch (Morales et al. 2007) (Fig. 9.06). If males preferred white‐patched females, they might well derive a reproductive benefit from their preference.

Fig. 9.06 Plumage variation as a signal of female quality. In some populations of the European Pied Flycatcher (Ficedula hypoleuca), variation in forehead patch size is a signal of individual quality. For example, females with white forehead patches (right) occupy better territories, where they face fewer parasites and have higher chances of producing more offspring.
(Photographs by Judith Morales‐Fernaz.)
In a good many bird species, females do look rather like males, and this resemblance suggests that the sexes may be engaged in mutual mate choice based on appearance cues. This statement may be particularly true when males vary greatly in their attractiveness so that some males have the option of rejecting certain females in favor of others. One experiment that definitively documented male preferences explored the mating patterns in the Eurasian Blue Tit (Cyanistes caeruleus), whose males tend to associate more with females whose crown feathers reflect UV radiation and associate less with females whose UV plumage has been experimentally diminished (Hegyi et al. 2007). Males in this species therefore select females on the basis of a plumage trait, just as females often choose males with special feather ornaments. Likewise, another study—this one on the Rock Petronia (Petronia petronia), a relative of the exceedingly widespread and more somber House Sparrow (Passer domesticus)—documented that females with an experimentally reduced yellow breast patch were courted less often, and mated at a significantly later date than those with a larger breast patch (Griggio et al. 2005). Such studies demonstrate that male mate choice is a reality in at least some bird species and suggest that overall it is more common than generally realized.
9.4 Adaptive value of mate choice
What might choosy females (and choosy males) gain from their preferences for partners capable of providing them with attractive songs, ornaments, displays, and the like? This question really concerns the adaptive value of mate choice and addresses the long‐term evolutionary causes of a choosy female’s or male’s behavior, not the immediate short‐term physiological causes of a given individual’s behavior. The distinction is considered essential by most behavioral biologists, who distinguish between two kinds of questions (Box 9.03): namely, proximate questions that deal with the internal mechanisms that enable individuals to carry out certain actions, versus ultimate questions that examine the longer term evolutionary explanations for behaviors.
The remainder of this chapter focuses primarily on the ultimate causes of bird behavior; on why, for example, individuals that prefer certain attributes in their partners are more likely to leave surviving descendants than if they had different preferences. Research on the adaptive value of animal characteristics complements—but does not replace—studies on the proximate causes of behavior. Both are needed to paint a complete picture of animal behavior (Box 9.04).
9.4.1 Genetic benefits from mate choice
There are many reasons why a female might derive reproductive benefits from her choice of a partner or partners. When studying female mate choice, we must consider the possibility that female preferences are not actually adaptive, but instead arise via selection that favors those males best able to persuade females to mate with them. This “empty advertisement hypothesis” suggests that females are lured into accepting sperm from persuasive partners that really have nothing of value to offer them, only courtship that activates the proximate mechanisms (Box 9.03) underlying female mating behavior. However, under this scenario, even if the females gain nothing directly from a sexual partner, the females that prefer the most stimulating males may tend to have sons that have inherited that preferred trait and that therefore are especially capable of acquiring mates in the next generation. Given these conditions, the empty advertisement hypothesis is converted into the more widely accepted sexy son hypothesis, with choosy females gaining attractive sons (even if nothing else) from their mate preferences (Weatherhead and Robertson 1979). One example that supports the sexy son hypothesis comes from European Starlings (Sturnus vulgaris) studied in southern Germany, where the sons of polygynous fathers defend more nest boxes and produce more courtship songs than the sons of monogamous fathers (Gwinner and Schwabl 2005). In other words, the sons’ apparent inheritance of their fathers’ mating behaviors would enable females to produce sexy sons by mating with males that are able to acquire several mates rather than just one social partner.
The sexy son hypothesis is not the only adaptive explanation for mate choice that focuses on a possible hereditary benefit from the behavior. For example, a choosy female could get sperm from a hereditarily healthy male whose genes—when passed on to his offspring—will help them develop a strong immune system. Females that made mate choices based on cues of male health could give their progeny a genetic benefit.
Likewise, when male plumage coloration is reflective of the carotenoid content of foods consumed, a female preference for bright reds or yellows in potential partners would lead females to mate with birds that are good foragers. If this ability has a hereditary component, selective females might be securing partners with genes that promote skillful foraging in their offspring.
Some of the clearest evidence for genetic benefits comes from species in which males provide no parental care after mating with the female. Females in these species often are highly selective about which males they choose as mates, presumably to obtain the best genes for their offspring. In the Black Grouse (Tetrao tetrix) of Europe, for example, females carefully observe many displaying males before choosing one to fertilize their eggs (Fig. 9.03). The same male often is chosen by several females, whereas most males in the display area are never selected by any visiting females. The most reproductively successful males are those that dominate other males while keeping their tail ornaments from being damaged in fights. They usually are also the males that occupy central display territories, an achievement that often requires years of intense competition (Höglund et al. 1994; Rintamäki et al. 2001).
The female preference for central‐territory grouse strongly suggests that they gain good genes for their offspring from these males, genes that promote the development of attractive and/or healthy offspring. In the Great Snipe (Gallinago media), a shorebird of Europe and northern Asia, the males chosen most often by females have a stronger immune response than their less successful neighbors, suggesting that they are in the best overall body condition (Ekblom et al. 2005) (Fig. 9.07). Once again, this result suggests an indirect (genetic) benefit from mate choice, although the finding does not absolutely rule out a direct (material) benefit in the form of a reduced risk of acquiring a contagious disease or parasite from preferred sexual partners.

Fig. 9.07 Male Great Snipe (Gallinago media) display. Leks of polygamous species like this one may have 5–30 males competing for females simultaneously.
(Photograph by Dmitry Yakubovich.)
With respect to the benefits to males from mate choice, recall that, as described earlier in this chapter, male Eurasian Blue Tits (Cyanistes caeruleus) prefer females with relatively intense UV‐reflecting crowns. In one study, females of this sort survived better than those with less UV reflectance in their feathers (Doutrelant et al. 2008). Moreover, female Eurasian Blue Tits with brighter yellow (carotenoid‐based) plumage produced larger clutches and had higher fledging success than females with duller yellow coloration. To the extent that males have a choice between bright and dull females as mates, this study suggests that a male preference for brighter females would translate into more progeny for the male. This conclusion also may apply to the Rock Petronia (Petronia petronia), in which females with a larger yellow breast patch weighed more and had more broods per year than conspecifics with a naturally smaller yellow patch (Griggio et al. 2005). Male mate preferences, like mate selection by females, appear to have the potential to raise the reproductive success of choosy individuals.
Moreover, just as females can exercise a kind of mate choice after pairing with a male by modifying their investment in offspring depending on the quality of their partner, so too some male birds appear to adjust their investment in parental care in relation to the quality of their mate. For example, variation in the color of eggs laid by female Spotless Starlings (Sturnus unicolor) in Spain affects male decisions about the care of the resulting offspring. Female starlings that have had some wing feathers removed—thus lowering their condition by making it more difficult for them to fly—lay paler blue eggs than do control birds with a full complement of primaries. Males paired to females that laid pale eggs were less active parents when they started to provision their brood (Soler et al. 2008). Similar male responses to egg color have been documented in the Gray Catbird (Dumetella carolinensis), a North American species that also lays blue eggs (Hanley et al. 2008) (Fig. 9.08). Females in these and other species probably are under pressure to provide signals that encourage their social partners to work hard for their progeny.

Fig. 9.08 Egg color signals mate quality. In the Gray Catbird (Dumetella carolinensis), males are more likely to provide parental care to their offspring when eggs are a vibrant shade of cerulean‐blue (a sign of female condition).
(Photograph by Elizabeth A. Sellers.)
9.4.2 Material benefits from mate choice
In the realm of mating decisions, direct benefits are those that return an advantage directly to the choosy individual. For example, females might prefer males that give them greater nutritional gifts via courtship feeding. In the case of the Yellow‐legged Gull (Larus michahellis) studied on islands off northwestern Spain, females that had been consistently well fed by their courtship partner were less likely to move during copulation, so the male was more likely to transfer sperm during the mating and thus was more likely to sire the offspring of that female (Velando 2004). By making effective copulation contingent upon her mate’s ability to provide her with food, a female could use mate choice to acquire a material benefit, namely, the calories and nutrients needed to enhance her personal condition or her capacity to produce offspring. Likewise, in British Columbia, Canada, the rate at which male Ospreys (Pandion haliaetus) provide their partners with fish during the courtship phase is related to the probability that the female will lay a clutch (Fig. 9.09). Moreover, the frequency of courtship feeding by the male, which is a function of his fish‐catching ability, is correlated with the rate at which he later provisions his offspring. Thus, by evaluating the nature of a male’s courtship feeding, female Ospreys can secure a competent forager as a partner in the feeding of their offspring (Green and Krebs 1995).

Fig. 9.09 Courtship feeding. Particularly common in species for which food resources are inconsistent or hard to find, courtship feeding—a pair‐bonding activity before nestlings hatch—demonstrates to females that a mate is capable of providing food to future young. Here, a male Osprey (Pandion haliaetus) returns with a fish for his mate.
(Photograph by Barbara Rich.)
Moreover, a highly successful forager is likely to be in generally good health and therefore unlikely to pass a contagious debilitating disease to his partner or her offspring. Depending on the bird species, a male’s appearance, the quality of his plumage ornaments, the brightness of the reds and yellows in his feathers, the intensity of his behavioral displays, the number of large rocks he carries to his partner, the nature of his songs, and even the size of the nest he builds for his partner all provide potential indicators of his past, current, and even future physiological condition, and thus of his health and capacity for parental care. For example, in American Kestrels (Falco sparverius) studied in Saskatchewan, Canada, males with bright orange skin (the product of high levels of carotenoids) about the base of the bill (Fig. 9.10) are less likely to have blood parasite infections later during the breeding season when male parental care becomes important to offspring survival. Therefore a preference for males with this coloring would help females acquire capable partners (Dawson and Bortolotti 2006). Likewise, in an experiment in which male House Finches (Haemorhous mexicanus) were inoculated with an infectious bacterial pathogen, birds with redder plumage recovered more quickly than birds with paler, yellower feathers (Hill and Farmer 2005). Because female mate choice in this species is biased in favor of redder males, females could pair off with males able to be better parents because they were resistant to certain diseases.

Fig. 9.10 The Cere can indicate mate quality. In American Kestrels (Falco sparverius), males with the brightest orange cere (bill skin, arrow) are most likely to be the best providers late into the nestling period, when offspring survival is critical but food can be harder to find.
(Photograph by Patricia Donald.)
The quality of a male’s song sometimes serves as another potential indicator of his paternal ability. In this context, it is striking that a male’s ability to learn songs early in his life often is very sensitive to the conditions experienced at that time (Chapter 10). For example, nestlings that receive relatively little food might be handicapped when it comes to imitating their species’ song(s). As a result, a female listening to an adult male sing might learn something about his past developmental history, which in turn might influence his current health and capacity for competent paternal behavior. In this regard, a series of careful laboratory studies have shown that young Song Sparrow (Melospiza melodia) males that are in good condition learn and copy the songs they hear more accurately than do young sparrows that are in poor condition. These better songs later provide information to mating females: when adult female Song Sparrows that have been dosed with hormones to make them sexually responsive listen to taped songs, they are more likely to elevate their tails into the precopulatory position when they hear accurate copies of their species’ song than when they hear the poorer imitations made by disadvantaged males (Nowicki et al. 2002) (Fig. 9.11). This kind of preference could lead the female to mate and bond with a better‐than‐average male, one with a good copy of his species’ song, which correlates with his ability to provide better‐than‐average assistance to the female and her brood.
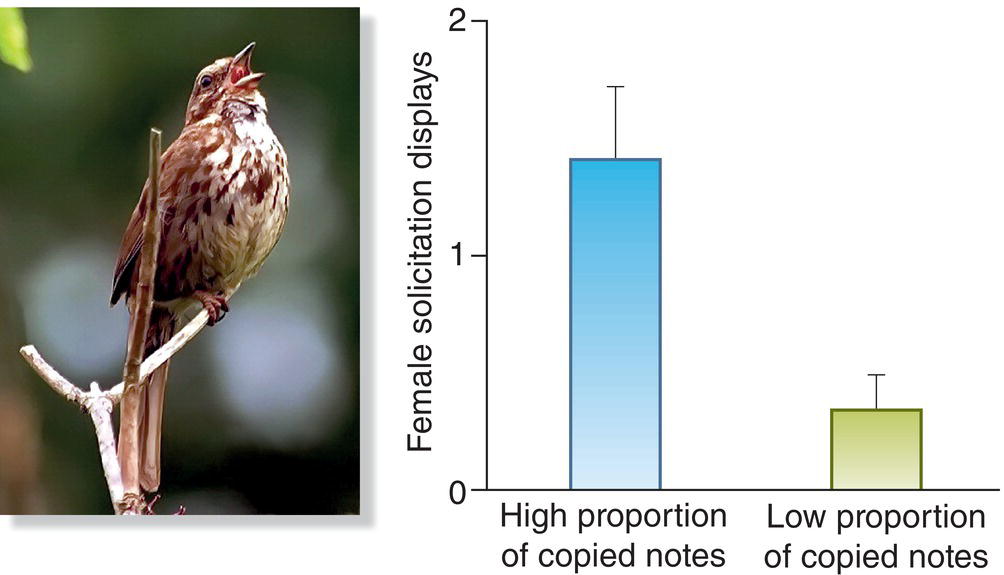
Fig. 9.11 Song production reveals male quality. Male Song Sparrow (Melospiza melodia) nestlings that experience stress during song learning in early development will produce poorer quality songs (with a low proportion of copied notes) compared with non‐stressed males (that produce a high proportion of copied notes). Females prefer songs that are reproduced with accuracy, regardless of the tutor song structure.
(From Nowicki et al. 2002. Reproduced with permission from the Royal Society. Photograph by Hector Chu‐Joy.)
9.5 Sexual selection and mating systems
Mate choice, whether by females or by males, has evolutionary consequences if the differences among the individuals subject to choice are hereditary. Individuals with the favored hereditary attributes may experience greater reproductive success than others of their species. Over time, the genetic basis of the ability to sing a particular kind of song, or to exhibit a particular ornament, or to engage in certain courtship displays, or to supply mates with their favored foods can spread through the species.
Sexually appealing traits generally come with costs: songs can attract predators as well as mates, ornaments make individuals more conspicuous to their enemies, animals focused on displaying to potential partners are less alert to danger, and individuals that offer food to their mates expend valuable time and energy in collecting what they later give away. Charles Darwin (1871) realized that such survival‐reducing traits required a special explanation, which he supplied in the form of his theory of sexual selection. He argued that elaborate ornaments, for example, might put a male at special risk of attack by predators and thus might be thought to be maladaptive, but if these ornaments also increase the odds that a male will acquire a mate and reproduce successfully, then sexual selection favoring the ornaments could trump natural selection against them. The same argument can be applied to any attributes that appear to function strictly in terms of the competition among individuals for mates, whether these are sexually attractive characteristics or traits that enable individuals to prevail over others of the same sex in mating‐related struggles.
As covered in more detail in Chapter 3, sexual selection now is generally considered to be one component of natural selection, in that the conditions required for both forms of selection are fundamentally similar. Both natural and sexual selection act on hereditary variation among individuals in their ability to pass on their genes, but sexual selection does so in the narrow context of competition for access to mates. Nevertheless, sexual selection theory is valuable because it helps focus attention on the significance of pure reproductive competition (Andersson 1994). One of the benefits of this perspective is that researchers alert to the theory can identify puzzles that they otherwise might ignore, particularly those when males or females appear to behave in ways that on first glance might seem to reduce their chances of acquiring mates. As discussed earlier in the chapter, monogamy by males is one example of this sort of puzzle, because the truly monogamous male appears to forego opportunities to mate with more than one female.
9.5.1 Solving the puzzle of male monogamy
Bird mating systems are wonderfully diverse, ranging from monogamy (one male mates with one female) to polyandry (one female mates with several males) to polygyny (one male mates with several females) (Box 9.05). One would think that sexual selection would favor polygynous males, those that acquire more than one mate in a breeding season and thereby increase their chances of fertilizing eggs and leaving many descendants. Because most birds appear to be monogamous, behavioral researchers have wondered whether, under certain special conditions, polygyny actually might reduce, rather than increase, a male’s reproductive success.
One such condition involves reproductive retaliation by abandoned females so that if a male were to leave one partner in order to secure another, his original mate might copulate with another individual. In keeping with this hypothesis, females of many species of birds are sexually receptive to males other than their partners. Indeed, one of the most significant findings in behavioral ornithology during the past several decades is that most bird species previously assumed to be strictly monogamous are not nearly so faithful as researchers had thought. Not so long ago, the British ornithologist David Lack (1968) famously stated, “Well over nine‐tenths (93%) of all passerine subfamilies are normally monogamous … Polyandry is unknown.” Now, however, we know from genetic investigations that in about 90% of all birds, at least some females mate with males other than their social partners. This behavior is termed extra‐pair copulation, with the outcome sometimes resulting in extra‐pair fertilization of eggs (Box 9.06).
Given the generally high prevalence of extra‐pair mating, it is the rare cases of true monogamy that require special explanation. Monogamy would likely be rarer still if males of some species did not attempt to monitor and control their partner’s behavior in order to reduce her opportunities to copulate with other males. Active mate guarding is a common feature of male behavior in many birds, with males following their partner closely, especially during the female’s fertile phase. The fact that male Abert’s Towhees (Melozone aberti) (from the introduction to this chapter) remain so close to their mates is consistent with a guarding function: when the male stays within centimeters of his partner, it must be hard for her to slip away for a clandestine meeting with another male.
The consequences of mate guarding have been assessed experimentally in field studies of many bird species. If active guarding by males does reduce the probability that the female partner will engage in extra‐pair copulations, then temporary removal of the male social mate should lead to a higher probability that his mate will go elsewhere to copulate. This generally turns out to be the case, as shown by experiments on Bluethroats (Luscinia svecica), an attractive Eurasian songbird. When Bluethroat males were held in a cage for only one morning during their mate’s fertile period, the proportion of extra‐pair offspring in her next clutch was twice that of females whose males had not been prevented from guarding their mate (Johnsen et al. 2008).
Jan Komdeur and his colleagues (2001, 2007) have used similar experiments to show that male mate guarding is highly effective in the Seychelles Warbler (Acrocephalus sechellensis). In this island species, the male typically remains vigilant and close to his mate for several days before egg laying, in the period when an egg can be fertilized. Female Seychelles Warblers lay only a single egg, and therefore if a male can sexually monopolize his partner as that egg is being formed and laid, he greatly improves his chances of fertilizing it. Once the egg is laid, however, males no longer guard their partners and instead may attempt to find a neighboring female with whom to mate. Komdeur and company put false eggs into the nests of females that had yet to lay, duping their mates into relaxing their mate guarding even though their females were still fertile. Neighboring males were quick to take advantage of the situation by sneaking into the territory of the unguarded female and copulating with her (Fig. 9.12). As a result, the rate of extra‐pair copulations was much higher for poorly guarded females than for those with an alert protector.
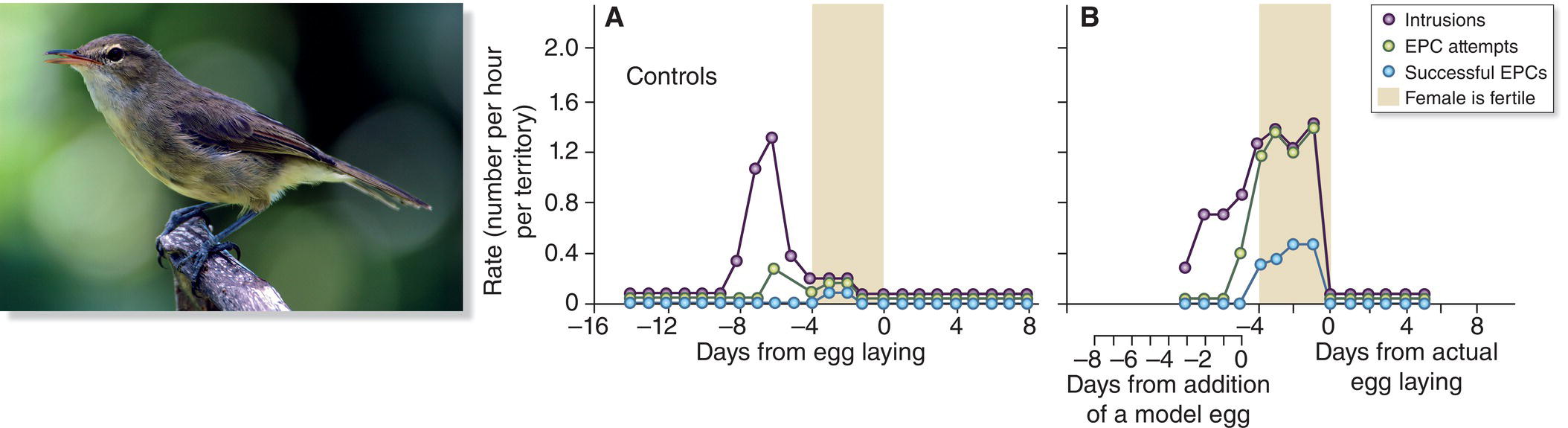
Fig. 9.12 Pre‐breeding mate guarding and extra‐pair copulation (EPC). Male Seychelles Warblers (Acrocephalus sechellensis) fiercely mate guard during the female fertile period to avoid EPCs. (A) In control pairs, the rate of territorial intrusions by other males, EPC attempts, and successful EPCs drops off during the female fertile period, as expected. (B) When males were experimentally fooled into thinking that their mate was past her fertile period (via the addition of a model egg in a newly lined nest), they left her unguarded, allowing other males to engage in more territorial intrusions and gain more EPCs.
(From Komdeur et al. 1999. Reproduced with permission from the Royal Society. Photograph by Jonathan Hornbuckle.)
One point to consider here is that when mate guarding is necessary and effective, the potential for female infidelity can push males towards monogamy, because they must forgo opportunities to mate with other females while guarding their own mates. Another point is that social monogamy—the pair bonding typical of so many birds—does not always, or even often, equate with genetic (true) monogamy in which a socially monogamous couple only has offspring of their own. Such genetic monogamy does occur sometimes, however (Fig. 9.13). For example, Common Loons, the widespread bird known in Eurasia as the Great Northern Diver (Gavia immer), apparently never engage in extra‐pair copulations (Piper et al. 1997). Likewise, in the colonial Dovekie (Alle alle), the species known as the Little Auk in Eurasia, males occasionally attempt an extra‐pair copulation with a neighboring female, but these females make cloacal contact all but impossible and almost never produce offspring with a male other than their social partner (Wojczulanis‐Jakubas et al. 2009). A few songbirds also are represented in the small truly monogamous contingent; for example, a genetic study of a Norwegian population of the Red (aka Common) Crossbill (Loxia curvirostra) found that all offspring were sired by the male that was socially bonded to their mother (Kleven et al. 2008). Similar genetic monogamy occurs in the Florida Scrub‐Jay (Aphelocoma coerulescens), a species whose complex social behavior is discussed later in this chapter (Townsend et al. 2011).
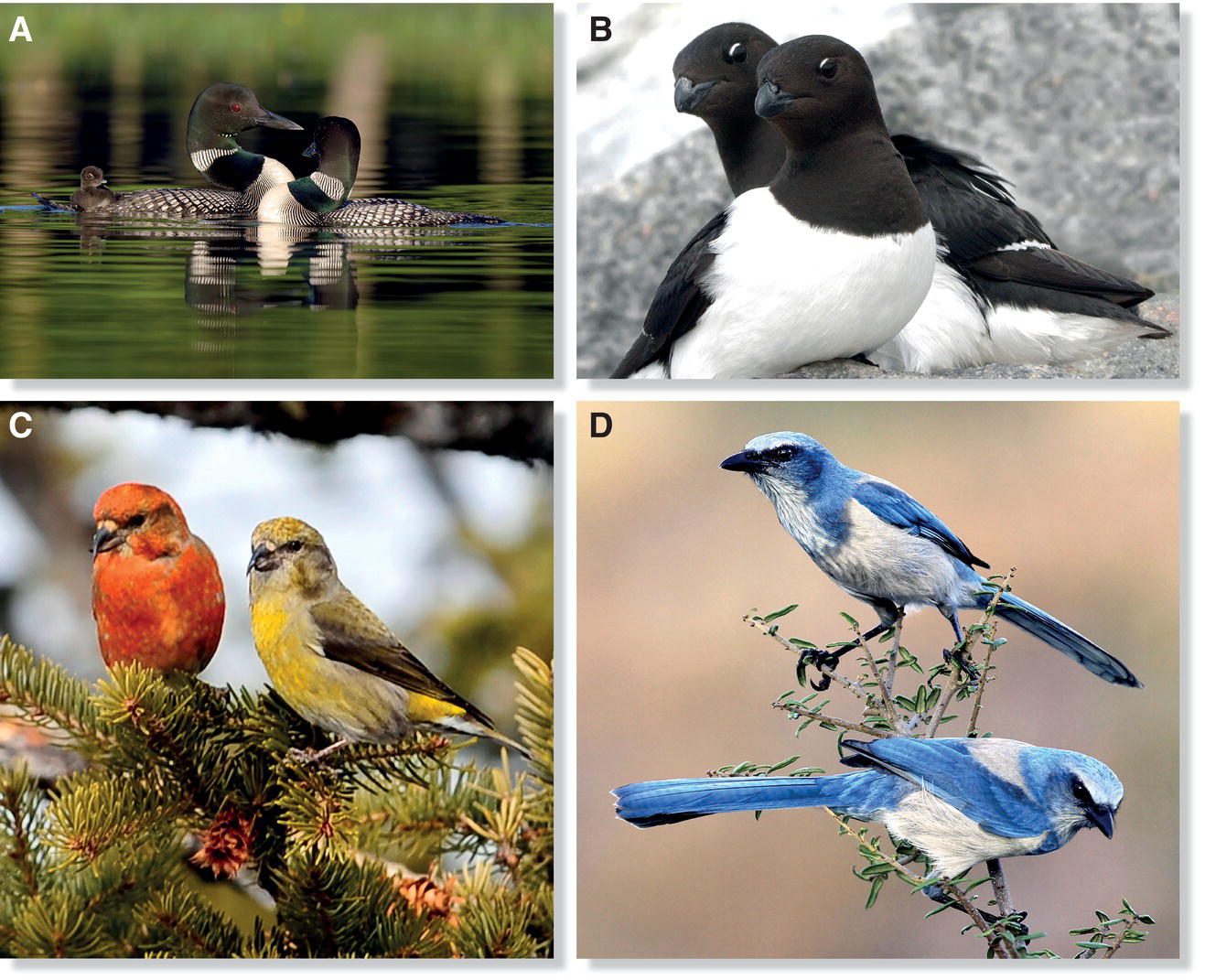
Fig. 9.13 Reproductively monogamous bird species. The following species are among those that form socially and genetically monogamous pair bonds: (A) Common Loons (Gavia immer), (B) Dovekies (Alle alle), (C) Red Crossbills (Loxia curvirostra), and (D) Florida Scrub‐Jays (Aphelocoma coerulescens).
(Photographs by: A, Mary Anne Pfitzinger; B, Leo Roos; C, Christine Pentecost; D, Matthew Studebaker.)
Far more common, however, is the finding that a number of the offspring produced by pair‐bonded males and females have a father other than the female’s social companion. In some species, extra‐pair paternity is relatively low; for example, in a Finnish population of Eurasian Three‐toed Woodpeckers (Picoides tridactylus), fewer than 10% of the nestlings sampled had been sired by a male other than the female’s social partner (Li et al. 2009). On the other hand, fewer than 50% of Reed Bunting (Emberiza schoeniclus) chicks possessed the genes of their mother’s social partner (Dixon et al. 1994). Although not quite so eager for extra‐pair copulations as Reed Buntings, female California Towhees (Melozone crissalis)—a socially monogamous species in which males and females stay in close contact, like their relatives the Abert’s Towhees (Melozone aberti)—nevertheless engage frequently in extra‐pair copulations, with more than 40% of all nests having at least one egg sired by an extra‐pair male (Benedict 2008). In such cases the puzzle of male monogamy can be partly resolved by noting that monogamous males actually are would‐be polygynists who readily take advantage of opportunities to sire additional offspring by mating with extra‐pair females (Box 9.07).
Nonetheless, in explaining why males of so many birds spend most of their time and energy associating with a single female in a pair‐bonded relationship (instead of trying to mate with as many females as possible), we need hypotheses that focus on the benefits of social monogamy. The mate‐guarding hypothesis is not the only such explanation, which is fortunate because it cannot explain, for example, why male Purple‐crowned Fairywrens (Malurus coronatus) in Australia remain close to their females at all times, whether they are fertile or not (Fig. 9.14), nor why they fail to court other females that they happen to encounter (Hall and Peters 2009). In this species and others, monogamy seems driven by the female’s voluntary fidelity to a social partner, not by the male actively preventing his partner from engaging in extra‐pair copulations.

Fig. 9.14 Purple‐crowned Fairywren (Malurus coronatus) pair. Males of this species guard their partners despite the negligible risk of extra‐pair copulation.
(Photograph by Alwyn Simple.)
Let us consider still another explanation for monogamy, that a pair‐bonded male can boost the production of his own surviving offspring by helping his social partner rear a brood of baby birds. This hypothesis makes intuitive sense, because many male birds clearly have the potential to be helpful parents by building a nest, incubating eggs, brooding nestlings, or feeding fledglings. The “mate‐assistance hypothesis” for monogamy generates a number of predictions, one of which is the expectation that the paternal male will have sired a least some of the offspring he helps rear. Genetic studies have upheld this prediction for the great majority of socially monogamous bird species, in which the female’s social partner typically has sired at least some of the offspring of his mate.
A second prediction of the mate‐assistance hypothesis is that females who lose part or all of the assistance of their pair‐bonded partner will rear fewer surviving progeny than females who retain their helpmate through the breeding season. This prediction also has broad support, because the male’s contribution to parenting has been shown to be valuable in many cases. In European Starlings (Sturnus vulgaris), for example, males vary in how much they contribute to the incubation of their mate’s eggs, with some males incubating much of the time and others less so. The males that are the most faithful incubators help their mates keep the eggs at a higher average temperature during the incubation period. These higher egg temperatures promote more rapid development of the embryos within, which is advantageous for the offspring and therefore for both parents (Komdeur et al. 2002).
The importance of males to their mates also has been shown through experimental removal of the partner, which often has a negative effect on the number of fledglings produced at nests cared for by widowed females. If, for example, the male partners of Snow Buntings (Plectrophenax nivalis), a bird of the Arctic, are removed early in the incubation period when males often feed their mates, the widowed females rear fewer offspring than those that are widowed late in the incubation phase (Lyon et al. 1987).
Another kind of test of the mate‐assistance hypothesis for social monogamy comes from a comparative study of a group of European warblers in the genus Acrocephalus. In this genus, monogamous species tend to occupy poor habitats where rearing offspring is difficult, whereas polygynous species occur in resource‐rich environments (Leisler et al. 2002). These trends suggest that, when social monogamy has evolved in this group, it is because having a helpful male partner substantially increases the production of surviving offspring when birds occupy a challenging habitat.
Generally speaking, findings of this sort indicate that paternal males derive benefits from their social monogamy, although it often remains difficult to tell whether these benefits (including reduced partner infidelity and increased offspring production) really outweigh the overall costs (a smaller number of females inseminated).
9.5.2 Polygyny: the puzzle of female participation
It makes intuitive sense from the female perspective to have a socially monogamous, paternal male around to assist in various ways, perhaps especially in the feeding of nestlings and fledglings. In the European Starling (Sturnus vulgaris) and in other species such as the House Wren (Troglodytes aedon) (Soukup and Thompson 1998), females lose paternal care for their offspring when their social partner acquires an extra‐pair mate (Fig. 9.15). Therefore, in the starling and other socially monogamous species, females often react aggressively toward prospecting females that enter their territory. In fact, female European Starlings and House Wrens have been observed destroying the eggs of other females that attempt to breed with their partner. In so doing, a female can try to force monogamy on her partner, making it advantageous for him to care only for her own brood. Given the successful attempts of females of many bird species to monopolize the paternal care offered by a social partner (Slagsvold and Lifjeld 1994), it seems odd that in other species females accept a polygynous male even though the female must share her male’s parental care with his other sexual partners or even lose his assistance altogether.
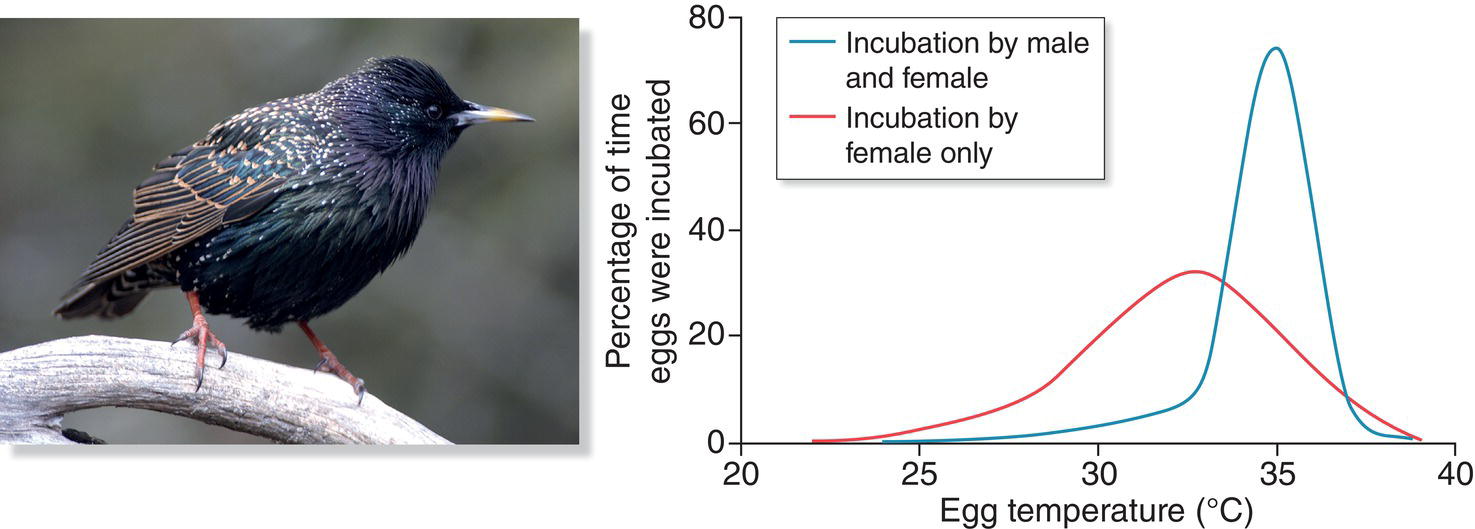
Fig. 9.15 Offspring gain a developmental benefit from attentive fathers. When male European Starlings (Sturnus vulgaris) contribute to incubation, eggs spend more time at temperatures optimal for development (blue line) compared with instances in which only the female incubates (red line).
(From Reid et al. 2002. Reproduced with permission from Springer Science + Business Media. Photograph by Carrie L. Johnson.)
Of course it is possible in some cases that polygynous males may not alert some partners that they have already acquired another mate. For example, this “deceit hypothesis” may apply to the European Pied Flycatcher (Ficedula hypoleuca), in which males that have established a territory and attracted a mate sometimes attempt to acquire a second territory with a second female. These dual territories can be up to 3 kilometers away from one another, and the secondary female may not know that her new‐found male already has a mate elsewhere. Once she begins to lay eggs and incubate, her now polygynous partner typically returns to his primary mate, whose offspring will be the beneficiaries of all or most of his parental care. The secondary female and her brood lose out (Alatalo and Lundberg 1984). Perhaps secondary females are not really deceived by their partners but instead are simply making the best of a bad situation arising from their late arrival on the breeding grounds. Alternatively, they may voluntarily accept a polygynous male whose special attractiveness increases the odds that they will produce sons that inherit his appeal, thereby increasing their number of grand‐offspring (the sexy son hypothesis discussed earlier in the chapter) (Huk and Winkel 2006).
In any event, polygyny is not surprising from the male perspective. Males that can inseminate more than one female successfully typically fertilize more eggs than they would if they restricted themselves, or were restricted by aggressive partners, to a single mate. What conditions enable males to practice polygyny successfully despite the apparent costs to their mates?
Consider the Montezuma Oropendola (Psarocolius montezuma), a Central American species in which several to many females build their long hanging nests from the same tree (Webster 1994) (Fig. 9.16). Under these circumstances, some males can acquire a number of mates by monopolizing the group of females at a nest tree. Indeed, males compete with one another to prevent rivals from approaching the nest tree. Successful males presumably gain more through polygyny than they could by guarding one female or assisting a single partner in the rearing of her brood. To the extent that powerful territorial males can pass their attributes on to their sons, females could gain by accepting top males as their partners, thereby increasing the odds of having reproductively successful sons. The result of male competition for pre‐existing groups of females has been labeled female defense polygyny, a mating system that is common in mammals but relatively rare among birds.

Fig. 9.16 Montezuma Oropendula (Psarocolius montezuma) nest colony. To court females, males spread their wings and make a series of loud trickling‐sounding calls before hanging upside‐down near one of many pendulous nests.
(Photograph by William David Grimes.)
A far more common kind of avian polygyny can occur if some males invest in the defense of resources rather than in direct parental care. Resource defense polygyny is practiced, for example, by the Red‐winged Blackbird (Agelaius phoeniceus) of North America (Fig. 9.17). As males divide up a marsh or abandoned farm field into a set of breeding territories, some individuals may be able to acquire real estate of exceptional value; that is, a territory with unusually large amounts of insect food that could be supplied to nestling blackbirds, or one with unusually safe, well‐concealed nest sites. Unmated female Red‐winged Blackbirds then can be faced with options that include joining an unpaired male who holds a territory with only modest resources or joining an already‐paired male whose territory is of considerably higher quality. Under these circumstances, it can pay for the female to enter into the polygynous arrangement if the difference between the territories of the two males is sufficiently great (Orians 1969).
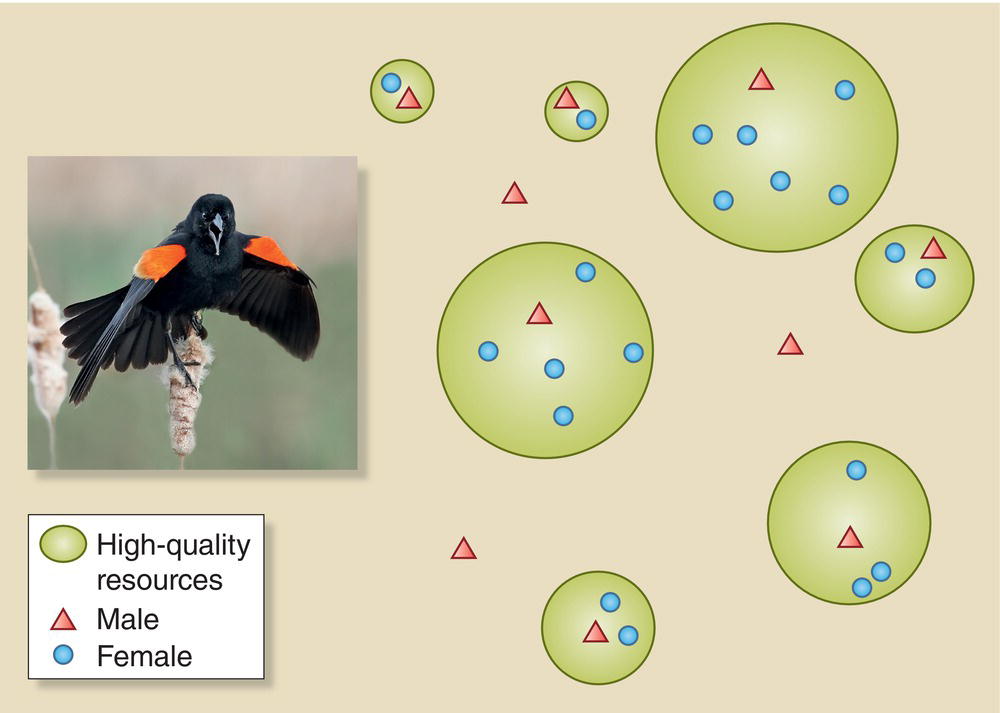
Fig. 9.17 Resource defense polygyny. This mating system arises in Red‐winged Blackbirds (Agelaius phoeniceus) and other species that occupy a patchy nesting habitat with limited resources. Males (red triangles) occupying superior territories with better nest sites and resources (green circles) generally secure more mates (blue circles), while males on inferior territories attract few or none. Some males do not have territories at all as good habitat is limited. This mating system offers a trade‐off between resources and parental care: females on better territories have access to more food but get less help from their mate.
(© Cornell Lab of Ornithology. Photograph by Nick Saunders.)
This explanation for why female Red‐winged Blackbirds often accept polygynous partners has been tested experimentally. Researchers gave some males extra cattail reeds placed over water and others a reed supplement of the same size placed over dry land. Faced with a choice between a safer over‐water location in a territory that already had attracted one female and a less safe one owned by a male without a mate, the female blackbirds in this study almost universally chose the safer over‐water option, even though they had to share a territory with another female and also share whatever parental care was offered by their partner (Pribil and Searcy 2001).
9.5.3 Lek polygyny
The most striking form of polygyny exhibited by birds and other animals occurs at leks, places where males gather in display arenas or guard small display territories, often very close to those of their rivals, in order to court and mate with visiting females. The polygynous males are the fortunate ones that acquire multiple sexual partners during one breeding season. Although only a small fraction of bird species have a lek‐based mating system, this mating behavior has evolved independently in many avian lineages. Lekking species include the Black Grouse (Tetrao tetrix) and Great Snipe (Gallinago media) mentioned earlier in this chapter, as well as some other grouse, sandpipers, and hummingbirds. Lekking also has evolved among the songbirds, where it is exhibited by many manakins, birds‐of‐paradise, bowerbirds, cotingas, and tyrant flycatchers, among others. In leks, the male’s display territory does not contain any resource of direct value to the female: it has no nesting site, no food—only the male himself. Because the only thing a male has to offer a given female is his genes, sexual selection via male–male competition (Chapter 3) generally is acute in lekking species, which in turn have evolved some of the most dramatic and spectacular physical ornaments and display behaviors.
Leks can have many spatial configurations. Males in some species gather only in loose aggregations; in these exploded leks males usually display in the same general area but not necessarily within view of one another. The Satin Bowerbird (Ptilonorhynchus violaceus) (Box 9.01) is an example of this kind of lekking species. Males of that species generally build their bowers about 250–300 meters apart (Reynolds et al. 2009).
At a classic lek, the displaying males are very close together and spend considerable time and energy behaving aggressively toward one another as they compete for spots within the lek favored by visiting females. Black Grouse offer an example of this sort of mating system. In this European species, 10 or 20 males may attend a single display site, with the nearest neighbors spaced only a few meters apart. When female birds visit classic leks, they generally watch the males interacting and displaying before sometimes moving toward a particular individual. As a female approaches a male, he responds with elaborate courtship, often combining both visual and acoustic components. These courtships often involve demonstrations of the extraordinary feather ornaments that many lekking species possess: Greater Sage‐Grouse (Centrocercus urophasianus) in North America fan their tails and puff out the breast feathers that surround their brilliantly orange vocal sacs; Victoria’s Riflebirds (Ptiloris victoriae) in eastern Australia wave their extended wings from one side to the other in a carefully timed ritual; and the Blue Bird‐of‐Paradise (Paradisaea rudolphi) of New Guinea hangs upside down from a limb while shimmering a fan of blue body feathers (Fig. 9.18).

Fig. 9.18 Blue Bird‐of‐Paradise (Paradisaea rudolphi). Males of this species hang upside down and fan out their feathers as part of a lekking courtship display.
(Photograph by Fabien Pekus.)
Subtle differences among males may have big effects on the reproductive success of lekking individuals. In the male Golden‐collared Manakin (Manacus vitellinus), the brightness of the yellow collar greatly affected male reproductive success in one population studied in Panama (Fig. 9.19). Here, as in other brilliantly plumaged birds, the intensity of feather colors is linked to physiological condition, with brighter males tending to be in better physical condition (Stein and Uy 2006). Perhaps because they are in such good shape, bright males may be better performers for visiting females. Indeed, some male Golden‐collared Manakins are faster than others in getting their “beard” of throat feathers extended upon landing on a perch after a period of energetic bouncing from a sapling to the ground and back. In one study, the fastest males resumed their extended throat feather pose in 43 milliseconds, but the slowest males required 63 milliseconds to adopt the correct posture (Schlinger et al. 2001). Needless to say, these and other behavioral differences are tiny in absolute terms, but some ornithologists believe that female manakins can detect the differences as males run through their routines and that they use this information to make decisions about who does and who does not get to mate.

Fig. 9.19 Golden‐collared Manakin (Manacus vitellinus). Males display by rapidly flaring out their golden throat feathers. Females assess males based on their display, throat color (which indicates condition), and position in the lek.
(Photograph by Marie Read.)
There is no question that behavioral differences among males play a critical role in female mate choice in other lekking species. For example, the way in which male Satin Bowerbirds engage in courtship clearly determines their success with females. Males that dance very vigorously, even aggressively, before the female are more likely to mate than those whose actions are less intense. In addition, males that are able to moderate the degree to which they charge at the female do better than males less capable of adjusting their display in response to whether the visiting female flinches, crouches down, or remains in place during a male performance (Patricelli et al. 2002).
From the male’s perspective, lekking represents an investment in courting many females with the possibility of fathering a great many offspring. At most leks, most or all females choose the same male to inseminate them, conferring very high reproductive success on the favored male. The corollary is that the reproductive output of the other, less attractive males is very low (during a given year). For example, at leks of the Golden‐collared Manakin in Panama, three to eight males competed for the attention of visiting females, but only one or two of these males secured almost all the copulations during one breeding period (Schlinger et al. 2008). This pattern of great variation in mating success among individuals is termed high reproductive skew (the alternative pattern where all members of a given sex have equal mating success is termed low reproductive skew). One review of this kind of variation among lekking bird species found that 10–20% of the males at leks customarily account for 70–80% of all matings (Mackenzie et al. 1995) (Box 9.08).
Given that a tiny fraction of the males at most leks monopolize most mating opportunities in any given year, what can the other males do to secure at least some copulations? Males of some species have evolved a behavioral flexibility in which low‐status males employ alternative tactics that help them make the best of a bad situation. For example, at study sites in Sweden, Great Snipe (Gallinago media) males that are excluded from prime territories by highly dominant, highly attractive males sometimes establish territories immediately adjacent to the sites held by their superior rivals. These satellite males occasionally may get a chance to intercept and mate with a female headed toward a prime male on his super‐territory (Höglund and Lundberg 1987).
Another tactic has evolved in the Ruff (Caladris pugnax), a large Eurasian sandpiper, in which actively lek‐displaying males have brightly colored plumage in a variety of color morphs. Other Ruff males lack this distinctive plumage and instead mimic the drab appearance of females. By posing as females, these sneaker males may be permitted to enter a very attractive male’s domain. Yet a third kind of male adopts male plumage but does not hold a territory on the lek; these satellite males linger on the periphery and attempt to mate with females as they are passing by (Fig. 9.20). All three of these strategies are sometimes successful, though the lekking males generally sire the most offspring (Jukema and Piersma 2005).

Fig. 9.20 Reproductive morphs of male Ruffs (Calidris pugnax). Satellite males do not engage in lekking behavior or territory defense. Instead, they linger near the periphery of the lek and try to mate with females as they pass through, or when the lekking males are busy displaying or fighting. Here, the satellite (left) was detected and must suffer the consequences as the dark‐plumed male (right) fiercely defends his territory.
(Photograph by Dominique Halleux.)
Still another tactic that males can use under certain circumstances is to associate with relatives who may concede a few matings to their brothers or nephews. In the Satin Bowerbird, for example, males tend to build their bowers fairly close to that of a relative. These birds avoid attacking the bowers constructed by relatives in favor of going after those owned by unrelated males (Reynolds et al. 2009). The generous behavior exhibited by relatives toward one another makes evolutionary sense in the context of kin selection (Chapter 3). When males help relatives, they help the portion of their own genes present in these other kin.
A final reason why low‐ranking males may participate in lek mating systems requires us to take a long‐term perspective. Males may copulate rarely, if ever, when they are low‐ranking members of a lek, but if they are fortunate to survive a number of years, they eventually may secure an alpha position and all the mating opportunities that go with being a dominant male. However, only males that persistently participate in the lek have a chance to move up the ladder to the top. This argument has been demonstrated to be correct for lekking Long‐tailed Manakins (Chiroxiphia linearis). In this species, some males display to help others attract females. The subordinate males often wait years for the payoff that comes when the more dominant individual dies, enabling the patient helper to move up to the top spot. Only then do they have a chance to leave copies of their genes to the next generation (McDonald and Potts 1994).
9.5.4 Absence of male parental care and the lek paradox
Male Golden‐collared Manakins (Manacus vitellinus) spend many months each year at their lek, with individual manakins returning year after year to exactly the same set of saplings where they reconstruct their display arena. Should a male be so fortunate as to mate, he does not accompany that female when she departs; instead he returns to the dance floor to continue his efforts to persuade other females to copulate with him. The mated females are left to do the hard work of nest building, incubation, nestling feeding, and fledgling chaperoning completely on their own.
Why do male manakins, and males of the many other lekking species, not help their sexual partners rear their offspring? Why do female birds choose to mate with males whose only contribution to the next generation is their sperm? One possibility is that lekking tends to evolve in species in which biparental care is not essential or even is harmful, and hence male care would do little or nothing to boost the reproductive output of either the male or female. This possibility could apply to species such as grouse and sandpipers with precocial offspring (Chapter 11) that are not fed directly by their parents after hatching. Biparental care may be a handicap in some tropical forests and other situations where predators that raid nests are abundant, and where the increased number of visits by an attendant male would increase the chances of a predator finding the nest.
Given that lekking males and their territories provide no parental care or food for females or their offspring (Höglund and Alatalo 1995), it seems reasonable to assume that female mate choice in these birds probably revolves around securing good genes, although, as previously mentioned, females also might benefit from mating with healthy, non‐parasitized, non‐infectious partners. If the ecology of the species is such that male assistance either has little benefit for females or provides few benefits for males, then females may be forced to participate in a system in which the best that they can do is to choose a healthy mate or one with good genes.
The good genes theory, however, does generate a major puzzle, which goes as follows: if females select mates on the basis of their genetic makeup, then in relatively short order, genetic variation in the male population will be drastically reduced. Why? Because if only a small group of males gets to pass on their genes, soon the gene pool will be filled with the particular genetic variants possessed by the small number of popular males of the past. In the absence of hereditary variation among males, females gain nothing by being choosy. But in avian leks today, females of most species continue to be highly selective about their choice of sexual partners. This conundrum is known as the lek paradox.
Many solutions to this paradox have been suggested (Kotiaho et al. 2008), most of which are based on rather arcane mechanisms that could maintain genetic variation in a population even when females consistently choose males with a particular genetic makeup. For example, if female choice favors males that are heterozygous (that is, males that have two different forms of many different genes rather than two copies of the same allele of these genes), then by choosing the most intrinsically diverse males, females select for the maintenance of genetic variation among males over time.
9.5.5 Polyandry: why do males accept polyandrous mates?
Another puzzling feature of avian mating systems involves the rare cases in which males voluntarily participate in polyandrous mating systems in which males mate with females that have more than one sexual partner. Particularly surprising are the instances in which several males act as helpers to the same female, whose sexual favors they must share. In the Galápagos Hawk (Buteo galapagoensis), for example, as many as eight unrelated males may pair bond with a single female on a territory, which all defend jointly. The polyandrous female copulates with all her partners more or less equally, meaning that the males are essentially taking part in a fertilization lottery (DeLay et al. 1996). Why might male Galápagos Hawks tolerate this competition within their territory rather than trying to defend a territory on their own? One possible answer is that suitable hawk territories are in very short supply on some islands in the Galápagos, and generally there are many males competing for each suitable location. If several males join together and cooperate in defending valuable real estate that a female will accept, they can win out over single males that try to defend a solo territory. Under these constraining circumstances, a male in a group has at least some possibility of fertilizing an egg and thus does better than a solo male without a suitable territory.
The Galápagos Hawk is unusual but not unique among birds in engaging in social polyandry, with more than one male behaviorally pair bonded with the same female. An even better known example of polyandry in birds involves the phalaropes, three species of small shorebirds that breed primarily in the Arctic. In the phalaropes, a sex‐role reversal has occurred so that females compete aggressively with one another for mates while courting males (Fig. 9.21). The males they court in turn assume all parental duties once the eggs have been laid in their nests. Some female phalaropes achieve sequential polyandry by abandoning an initial partner after supplying him with a clutch and then securing another mate that will care for a second batch of eggs. When polyandrous females leave their partners, the male’s only good option is to continue to accept the parental role because he can incubate a clutch of eggs successfully by himself. Later, the newly hatched, precocial chicks feed themselves on an abundance of insect food (Reynolds 1987). Males that retaliate against female abandonment by also abandoning their nests would leave no descendants at all. As we might predict given the unusually high male parental investment by phalaropes, genetic studies have shown that only a very small percentage (<2%) of all phalarope chicks are sired by a male other than their caretaker (Schamel et al. 2004). The paternal male phalarope thereby enjoys a direct genetic payoff in return for his attentiveness to his nest.

Fig. 9.21 Sex‐role reversal in Wilson’s Phalaropes (Phalaropus tricolor). In this species, females (right) exhibit brighter plumage and aggressively compete for males (left), which incubate eggs and provide parental care without female help.
(Photograph by Larry Jordan.)
One of the ways male Red Phalaropes (Phalaropus fulicarius) increase the odds that they will produce offspring with their own genes is to increase the frequency with which they copulate with their one partner during her fertile period (Schamel et al. 2004). Male birds that provision offspring also generally make this activity contingent upon sexual access to the mother of those offspring, a fact that some polyandrous females use to “encourage” paternal behavior on the part of all their mates. For example, the Dunnock (Prunella modularis) is a drab little European bird that exhibits a rich diversity of mating systems (Davies 1985). In some cases, a polyandrous female lives with two male partners with whom she copulates at frequent intervals. Socially polyandrous Dunnock females tend to solicit matings from the male that thus far has had fewer copulations, thereby activating the male’s psychological mechanisms that push him (unconsciously of course) toward paternal behavior when the nestlings have hatched and need to be fed. Because copulation frequency correlates with egg fertilizations, males that mate often with a female are likely to have sired at least some of her offspring. A polyandrous female that skillfully partitions matings between two males gains two caretakers for her progeny, a clearly advantageous consequence for the female in this species in which the additional paternal care increases the number of offspring they can rear in a breeding season.
When a polyandrous female gets her partners to care for her offspring, she derives an obvious material benefit from copulating with more than one male. However, many socially monogamous females solicit extra‐pair copulations from males that cannot or will not be available when the eggs hatch. The added parental care hypothesis for polyandry does not apply to these situations. A reproductive benefit that could lead females to mate with multiple partners is that receiving sperm from several males provides fertility insurance against the possibility that their social partner’s sperm are inadequate or defective. Studies of a few species have found that a small but measureable percentage of male songbirds are infertile; the mates of these individuals certainly would benefit by receiving sperm from a fertile extra‐pair partner. Thus, although 2–4% of Eurasian Blue Tit (Cyanistes caeruleus) and related Great Tit (Parus major) males tested in one study were infertile, the females mated to these individuals nonetheless produced six to nine offspring, demonstrating that their extra‐pair copulations provided them with plenty of viable sperm (Krokene et al. 1998).
By far the most popular explanation for why females with a helpful social partner nevertheless mate outside the pair bond is the good genes hypothesis (Chapter 3). Polyandrous females in at least some species make choices that apparently provide them with good genes. For example, in the Reed Bunting (Emberiza schoeniclus), a boldly patterned black, white, and brown European finch, females are more likely to seek out males whose color pattern reveals that they are older than their social partners. To the extent that these older males possess hereditary traits that promote survival, females that select them as sperm donors may gain those valuable attributes for their offspring (Bouwman et al. 2007). Perhaps this is why female Western Bluebirds (Sialia mexicana) whose social mates have been removed temporarily are more likely to accept sexual advances made by neighboring males when those males are considerably older than their missing social partner. Because relatively old males have brighter blue head plumage (Fig. 9.22), females use this as a signal when deciding whether to accept or reject an extra‐pair partner (Budden and Dickinson 2009). Other evidence suggests that these older bluebird males on average sire heavier offspring; heavier nestlings in general have a better chance of surviving to adulthood.
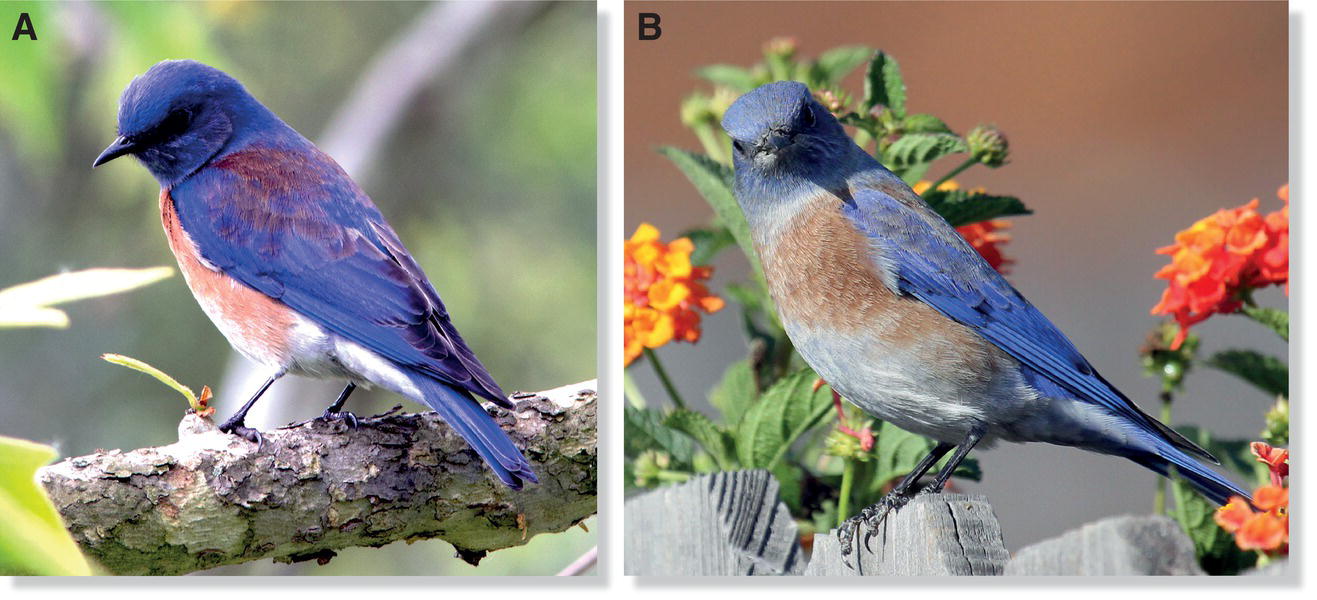
Fig. 9.22 Western Bluebird (Sialia mexicana) plumage differs with age. Females can judge the age of a male by the intensity of his feathers. (A) Males with deeper blue feathers are typically older, demonstrating their potential fitness and ability to survive. (B) Younger males are generally duller and provide less parental care for their broods.
(Photographs by: A, Chris Morrison; B, Andrew A. Reding.)
There are other possible benefits from extra‐pair copulations, some of which may have the function from the female perspective of increasing the genetic diversity of their offspring. Some researchers have suggested that females prefer males that differ from them genetically to a greater extent than their social partners. Indeed, female Reed Buntings have been found to engage in extra‐pair copulations with males that were more genetically dissimilar to them than their partners, with the result that their extra‐pair young were more heterozygous (more likely to have two different copies of a given gene) than the offspring they produced with their social mate (Suter et al. 2007). Greater heterozygosity generally is believed to be developmentally advantageous, and, in fact, the extra‐pair bunting offspring weighed more than their maternal half‐siblings upon fledging.
The Reed Bunting experiment compared the properties of offspring that had different fathers but the same mother and the same rearing conditions, because they grew up in the same nest at the same time. This experimental technique has been applied to other species, including the Bluethroat (Luscinia svecica), the attractive Eurasian songbird mentioned earlier. In one population of this species about a quarter of all offspring are the products of extra‐pair matings. One sample of these extra‐pair offspring was more heterozygous than their maternal half‐siblings who had the mother’s social partner as a father. Moreover, the immune response of the extra‐pair offspring was greater than that of within‐pair offspring (Fossøy et al. 2008). These results support the hypothesis that extra‐pair matings can confer genetic benefits to the resulting offspring, making extra‐pair copulations adaptive for female birds of at least some species. However, results vary across studies, with some not finding any support for the genetic benefits of extra‐pair matings. At present, it remains hard to generalize about the type and impact of the genetic benefits arising from extra‐pair matings across bird species, and this subject therefore remains an active area of research in behavioral ornithology.
9.5.6 Mating systems and sexual conflict
When two male Dunnocks (Prunella modularis) are paired with the same female, the odds are that they both will make parental investments in some offspring that, genetically speaking, are not theirs. The risk of cuckoldry is often substantial in socially monogamous species because, as noted already, the most common form of avian polyandry involves extra‐pair matings by females that have a single social partner with whom they also mate. Although it is possible that females participate willingly in extra‐pair matings and so are voluntary polyandrists, it also is likely that at least in some cases males harass females so severely that they either are forced to mate or do so as a way of ending the harassment. Indeed, some (but not all) behavioral ecologists have concluded that some cases of avian polyandry are entirely the result of male sexual drive and redound to the advantage of the male rather than the female (Arnqvist and Kirkpatrick 2005).
Most birders are likely to have observed male Mallards (Anas platyrhynchos) and other ducks aggressively pursuing apparently reluctant females across the water. Sometimes several sexually motivated male ducks simultaneously clamber onto the back of a harassed female, driving her underwater and potentially even drowning her as they all attempt to copulate (Fig. 9.23). Because these assaults occur more often when females are about to lay their eggs, and because they can result in successful sperm transfer and egg fertilizations, forced copulations of this type are likely to be a part of an adaptive male sexual strategy (McKinney et al. 1983). It seems less likely that they are advantageous to females, which may accept an unwanted mating only to terminate a potentially costly interaction. Cases of this sort have been labeled “convenience polyandry,” with females mating with more than one male because they might be harmed if they refuse.

Fig. 9.23 Forced copulation in the Mallard (Anas platyrhynchos). Males in many duck species often violently force themselves upon females, sometimes even in groups. Females can drown or die of injuries related to these forced copulations.
(Photograph by Don Bantum.)
Note that this example of sexual conflict between males and females of the same species reflects what appears to be a general rule: individuals do what is in their own genetic interest, even if it harms others of their species and even if it reduces the reproductive success of a sexual partner. Such a view is derived from a modern understanding of evolution by natural selection, which occurs when individuals (not species as a whole) differ in their ability to pass on their genes (Chapter 3). As a result, exploitative, manipulative, or aggressive traits that advance individual reproductive success can spread through a population even if they generate severe conflicts of interest within that population.
One such example is seen in the Stitchbird (Notiomystis cincta) of New Zealand, a species in which paired males regularly enter nearby territories in pursuit of other males’ females, which they often grasp in unusual face‐to‐face pairings accompanied by attempted copulation (Fig. 9.24). To this end, the male Stitchbird has a very large cloacal protuberance—the avian intromittent organ analogous to a penis (Chapter 6)—which facilitates the junction of the male cloaca with that of the female, which is necessary for sperm transfer. Researchers studying Stitchbirds have noted that these birds are perfectly capable of mating in the standard avian position, in which the male mounts a cooperative female as she stands still (Low 2005). The face‐to‐face matings involve behavioral and structural adaptations of males that seem designed to circumvent female mate choice and impose polyandry on females.

Fig. 9.24 Forced copulation in the Stitchbird (Notiomystis cincta). This is the only bird species known to copulate face‐to‐face (rather than typical back‐mounting), seeming to negate female choice. Here the arrow points to the tail and belly of the female, positioned on her back on the ground, beneath the male. Several forced copulation postures have been documented in Stitchbirds.
(Photograph by Matthew Low.)
9.6 Costs and benefits of social behavior
Sexual interactions between males and females are merely one of many kinds of social interactions that occur among birds. Avian social behavior embraces a broad spectrum of activities, ranging from highly aggressive acts (as in the forced copulations of the Stitchbird and Mallard) to highly cooperative ones (as in the mutual parenting of a brood of nestlings by a pair of Abert’s Towhees). In analyzing this diversity of behaviors, researchers have been assisted by a cost–benefit approach that is based on the premise that all interactions potentially have both a downside and an upside in terms of their effects on the fitness of the interactors. So, for example, when a male bird slips out of his territory to look for a mating opportunity with another female, his behavior has costs, such as the risk of being attacked by a mate‐guarding male and the chance that his own social partner will mate with a sexual intruder during his absence. Likewise, when a socially paired female accepts or invites an extra‐pair copulation, she may derive a benefit in the form of egg fertilization insurance or better genes, but she also may incur costs including the loss of paternal care that her cuckolded mate now withholds, physical assaults by her disgruntled social partner, or a heightened risk of acquiring sexually transmitted pathogens from her extra‐pair partners.
The application of the cost–benefit approach to sociality has led to insights at several levels. For one thing, researchers now feel confident that highly social species are not inherently superior to more solitary ones; rather, these social species live under conditions in which the benefits of sociality are relatively great compared with the costs. For example, social aggregations are more likely to evolve in bird species whose predators can be better detected, repelled, or overwhelmed by groups of individuals than in species in which the antipredator benefits of sociality are too small to compensate for the costs of social living. Often, these costs are substantial. For example, Cliff Swallows (Petrochelidon pyrrhonota) in North America often nest in large colonies where their mud nests are packed side by side. The high density of these nests facilitates the spread of blood‐sucking swallow bugs, which harm the growth and development of the nestlings they infect (Fig. 9.25). Not only are infestations and contagious diseases more likely to spread in densely packed groups, but competition for food also becomes more intense in an area in which many individuals are foraging (Brown and Brown 1986).
Fig. 9.25 A cost of sociality. (A) Colonies of Cliff Swallows (Petrochelidon pyrrhonota) occur among steep, rocky outcrops (as shown here) and on man‐made structures. High‐density colonies are more susceptible to the spread of nest parasites that impact the fitness and survival of the young. (B) The smaller nestling (left)—from a dense, infested nest colony—was attacked by blood‐sucking swallow bugs, slowing its development and stunting its growth. The larger nestling (right) came from a nest fumigated with insecticide and so developed without the damage caused by swallow bug parasites.
(Photographs by: A, Sharon Jue; B, Charles R. Brown.)
9.6.1 Territoriality and aggressive social behavior
The cost–benefit approach helps explain avian territoriality, which is based on aggressive social interactions as one or more individuals attempt to keep others away from a piece of real estate. The territory may be as small as the square meter display court of a Black Grouse (Tetrao tetrix) or Golden‐collared Manakin (Manacus vitellinus), or as large as the hunting ground of a Golden Eagle (Aquila chrysaetos) or Northern Goshawk (Accipter gentilis), which usually spans thousands of hectares (Fig. 9.26). The costs of territory defense obviously include the time and energy expended in displaying to and chasing off intruders, as well as the risk of injury should a territory holder be hurt in a fight with a determined rival. The display costs alone are not trivial, especially for species in which males sing to deter competitor males from entering their territory (Chapter 10). For example, male Great Reed‐Warblers (Acrocephalus arundinaceus) expend about three times more energy when singing than they do when resting quietly. During spring in central Sweden, some such warblers have been recorded singing nearly continuously for 20 hours a day (Hasselquist and Bensch 2008). Likewise, male Willow Warblers (Phylloscopus trochilus) generally sing from exposed song posts in Scotland where they are subject to heat loss, especially on cool, windy days. They also lose heat as they exhale at an elevated rate when singing, which they do at a rate of five songs a minute for hours at a time. The combined thermoregulatory costs of singing raise these males’ metabolic rates (Chapter 7) by 20% (Ward and Slater 2005).
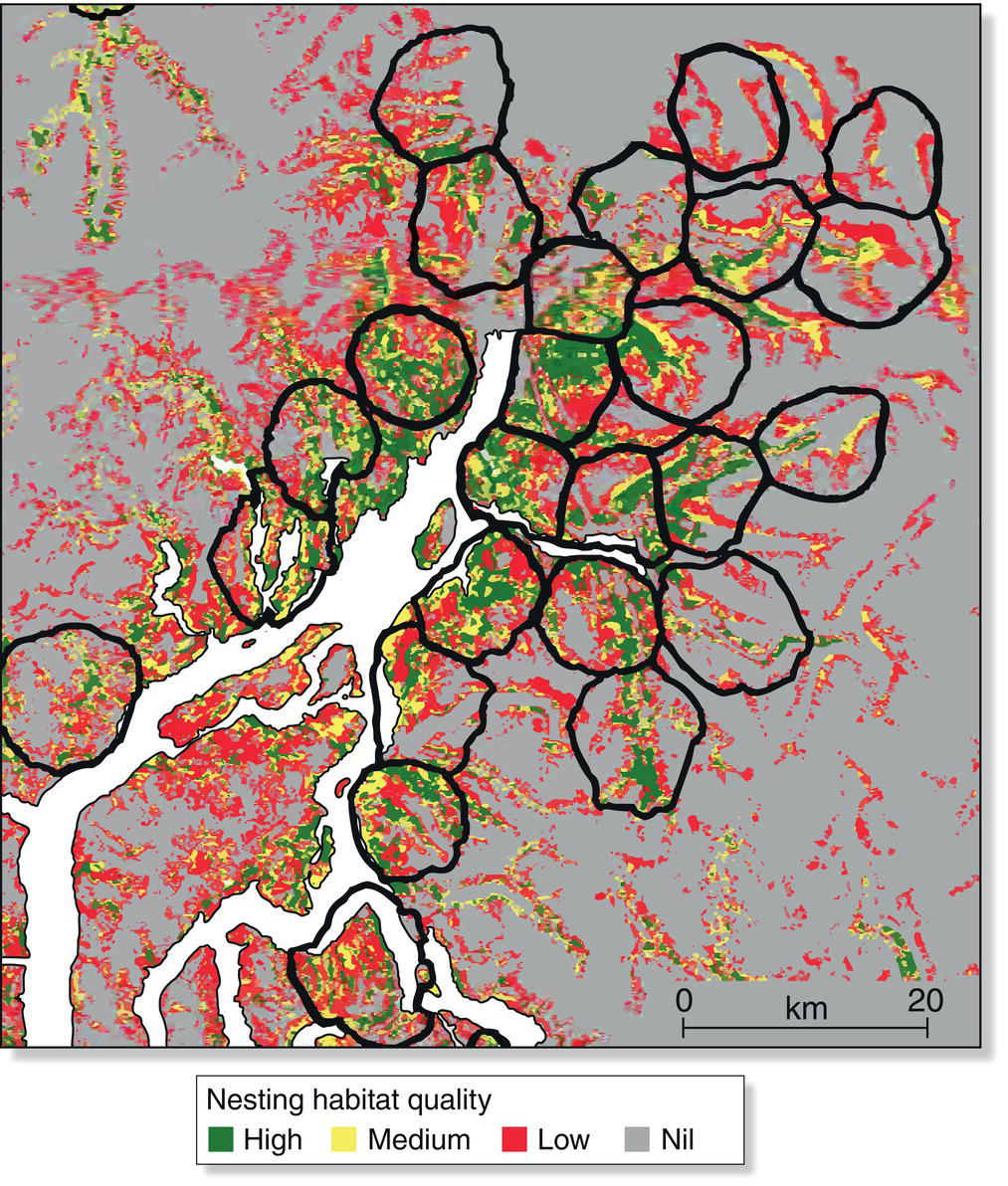
Fig. 9.26 Map of Northern Goshawk (Accipiter gentilis) territories. As typical for a resource‐based territorial species, each nesting pair defends a food‐based territory. Since these large predators need to secure a great deal of food for their nestlings, their territories are very large (often over a kilometer in diameter). Territories (rough black circles) with poorer habitats tend to be larger, as more space is necessary to support the adequate amount of resources.
(From Smith and Sutherland 2008. Reproduced with permission from TimberWest Forest Corporation.)
Many other potential costs are associated with territoriality. A displaying male Vermilion Flycatcher (Pyrocephalus rubinus)—a species found across much of the Americas—flies upwards slowly with its brilliant red plumage fluffed, while singing to draw attention to itself. The fact that this behavior appears to be triggered by a neighbor’s display indicates that males use the flight display as part of their territorial defense system. This display behavior has clear costs in terms of time and energy; moreover, the conspicuousness of the behavior presumably makes the flycatcher at least somewhat more vulnerable to predators such as hawks and falcons, which often are common in the flycatcher’s environment. The same costs may apply to the flashy ornaments of many other male birds, which are used as signals in territorial interactions in part because of their conspicuousness, an attribute that may expose the owner to unwelcome attention (Chapter 3).
Given the costs of territoriality, we can predict that it should evolve only when the fitness benefits more than compensate the territorial individual for the price it pays to monopolize its patch of real estate. Researchers have tested this claim by demonstrating, for example, that in lekking species the possession of a prime display territory boosts male reproductive success. Moreover, when individual males are incapable of acquiring an attractive territory, they often either drop out of the competition altogether for a time (thus saving the costs of trying to be territorial) or employ an alternative mating tactic that does not carry the full costs of territoriality.
Most avian territories are much larger than those of lekking species. In these cases, a potential benefit that comes from owning a territory is access to the food that the defended area contains. We thus might predict that larger species will tend to have larger territories, because a nesting pair of a large species generally requires more food for themselves and their young than two adults and their brood of a small species. This prediction seems to hold across most birds, because it has long been known that large birds, particularly predatory ones, do require very large territories if they are to nest successfully (Schoener 1968).
A prime benefit to males that comes from owning a food‐rich breeding territory is the attraction of a receptive female (or females) to the territory and the male defender. We can predict that males able to control more food‐rich territories will acquire mates more quickly, as has been shown for the Blue Grosbeak (Passerina caerulea) in Alabama (USA). Male grosbeaks in better physical condition are able to produce brighter blue feathers and are more likely to acquire territories with abundant food resources; females prefer these males and pair off with them more quickly (Ballentine and Hill 2003).
Another prediction derived from the cost–benefit approach to territoriality is that individuals in relatively resource‐poor sites should abandon them more often than males in high‐quality territories. In keeping with this expectation, in both Spotted Owls (Strix occidentalis) in western North America and Red‐backed Shrikes (Lanius collurio) in Switzerland, individuals were significantly more likely to leave poor territories than were competitors that secured better sites (Blakesley et al. 2006; Pasinelli et al. 2007).
It also is true that when individuals of a given species are unable to acquire a resource‐rich territory, they often stop trying to do so for a breeding season or two. Although this behavior once was interpreted as an adaptation for the “benefit of the species” because it supposedly helps prevent overutilization of critical resources, dropping out of the breeding competition now is viewed, in light of the cost–benefit approach, as an adaptation that directly benefits the dropouts themselves. Birds that are smaller, less physiologically fit, less experienced, or slightly weaker than average have little to gain from costly attempts to acquire a territory, especially if the good places already have been claimed by stronger rivals and the remaining unoccupied slots are resource poor. These individuals may gain more by conserving their energy so that they can try again when conditions become more favorable or when they are older and more competitive. For example, in migratory populations of the Black Kite (Milvus migrans), a graceful mid‐sized hawk of Europe and Australasia, territories usually are taken by older birds in such good condition that they can arrive early on the breeding grounds. Younger kites become floaters, birds that move about while postponing their territorial and reproductive ambitions (Sergio et al. 2009).
In most territorial bird species, some individuals are non‐territorial floaters. Those that have the good fortune to find a territorial vacancy can be predicted to take advantage of the opportunity that an opening implies. Indeed, floaters in the Eurasian Oystercatcher (Haematopus ostralegus) responded quickly to the experimental removal of established residents by entering a vacant territory and switching tactics, provided that they had intruded into this site previously (Bruinzeel and van de Pol 2004). Presumably, during their earlier secretive incursions, these floaters had acquired information about territory quality that they can use to good advantage if they find that the site now lacks a defender.
9.6.2 Dominance hierarchies
Many species of birds do not defend territories, and even those that do frequently are non‐territorial during some parts of their annual cycle. There are simply times and places where territoriality is precluded by features of the environment, or where the costs of being territorial are much higher than the benefits. For example, when a potentially territorial individual must face large numbers of competitors, the value of being territorial decreases because of the heightened costs of defense. In a large flock of birds, for example, if any one bird were to attempt to keep all the others out of a patch of food, the costs of doing so probably would overwhelm any benefits the would‐be territory holder could generate from its territorial exclusivity. Therefore, traditional territoriality does not occur when groups of birds gather together either for their mutual defense or to forage more safely.
This diminished territoriality does not mean that all competition ends when groups of birds assemble for one or another reason. Indeed, the competition continues but leads to an evolutionary result that is different from the kind of aggressive territoriality exhibited by many nesting birds. Instead, as individuals within a flock jockey for valuable resources such as space and food, the standard result is a dominance hierarchy, which is sometimes labeled a “pecking order.” This secondary term comes from early studies of chickens in which some members of the flock displaced others by administering a peck or two, or simply by approaching or even looking at a subordinate (Schjelderup‐Ebbe 1935).
A special case of dominance hierarchies sometimes occurs among chicks within a nest. These sibling hierarchies are particularly obvious in groups such as herons, seabirds, owls, and raptors, in which successive eggs within a nest hatch on different days (Chapter 11), giving the oldest chicks a decided size advantage over their younger and smaller nest mates. Competition for the food brought by the parents sometimes is acute, and the larger chicks may bully their younger siblings aggressively to the point that these unfortunate subdominant birds simply crouch in the nest rather than begging to be fed. In breeding seasons when food is short, these lower ranked nestlings usually die. In Cattle Egrets (Bubulcus ibis), a bird now found throughout the Americas as well as in much of the rest of the world, the last of the chicks to hatch probably lives longer by not fighting back, since its oldest sibling is three times its weight. Admittedly, such battered subordinates almost always die eventually, sometimes from starvation, or by being forced out of the nest (Fig. 9.27). But on rare occasions, when it becomes the only nestling survivor because of the destruction of its dominant siblings by predators or weather, this once‐subordinate individual will have a chance to be fed. Thus, the last‐hatched nestling may be lucky enough to reach adulthood, but only if it has managed to hold on long enough to take over upon the disappearance of its bullying nest mates (Mock 2004; Mock et al. 1990). Although this system may seem cruel, the nestling dominance hierarchy caused by the asynchronous hatching of eggs is thought to be an adaptation that increases the fitness of the parents: when times are good, more of the chicks survive, but when times are bad there is a way to dispose of the lower ranking members of the brood that allows the more dominant offspring to fledge successfully (Drummond 2006).

Fig. 9.27 Dominance hierarchies in siblings. The first‐born Brown Booby (Sula leucogaster) chick—crouching comfortably in the shade of its parent—has driven its younger and much smaller sibling (circled) into the blaring sun, a lethal act.
(From Alcock 2013. Reproduced with permission from Sinauer Associates.)
Dominance relationships also are apparent in winter‐foraging flocks of songbirds, although their interactions usually are far less dramatic than those that occur in an egret nest. Birds join winter flocks primarily for protection against predators (a benefit), but the birds that live together may experience heightened competition for food (a cost). When there is competition for food, individuals that have become dominant to others displace subordinates from food resources. Thus, in a study of Great Tits (Parus major) in Spain, researchers found that at a cold, high‐elevation site where insect food was scarce, dominant individuals frequently chased low‐ranking members of the winter flock away from feeders (Carrascal et al. 1998).
Given the reality that not all members of a flock of birds are equal competitors, subordinates may avoid overt conflict and concede desired items to their superiors without question. They do so not because the group as a whole benefits from less violent conflict, but rather because the subordinates gain as individuals when the dominant members allow them to remain in the group. There they are more likely to survive long enough to become dominant themselves someday, with all the benefits that high status will confer upon them. In contrast, subordinates that are injured, driven off, or killed outright because of their refusal to accept their low status leave relatively few descendants.
9.6.3 Helpful social behavior
The emphasis in this chapter thus far has been on the competitive aspects of avian interactions, those that occur when individuals attempt to monopolize valuable resources such as food, space, or mates. However, it also is common to observe birds interacting with one another in ways that seem to provide benefits for all parties.
In some cases, birds involved in these mutualisms derive their benefits simply by assembling in groups, the better to reduce the risk for any one flock member of being attacked by a predator. Thus Common Redshanks (Tringa totanus), a Eurasian sandpiper, sometimes are hunted by raptors while foraging or resting on beaches on their wintering grounds. These birds often are found in groups, and if their flocks have formed for defensive purposes, the vulnerability of individuals to predators should decline as group size increases (Fig. 9.28). Field studies have shown that redshanks in larger flocks indeed are less likely to be killed when a predator comes hunting. Because these birds’ foraging success declines as their flocks increase in size, these defensive benefits are likely the main reason that redshanks only form winter flocks (Whitfield 2003).
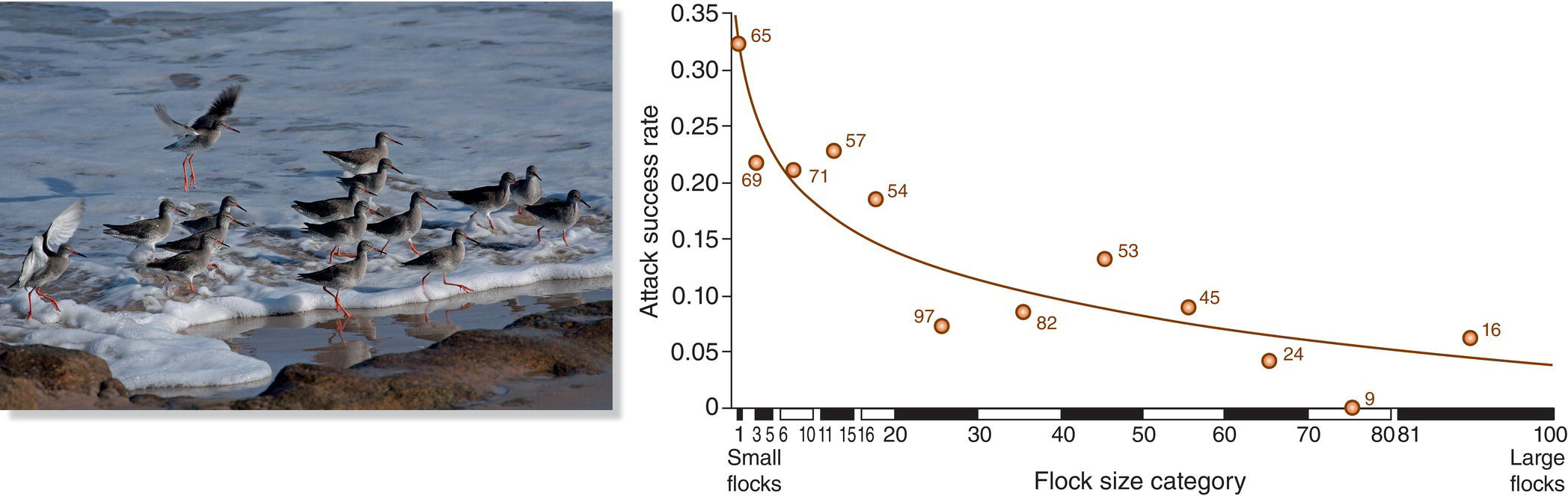
Fig. 9.28 Safety in numbers. A small flock of Common Redshanks (Tringa totanus) on a tidal flat. Predatory hawks are less likely to capture Common Redshanks in larger flocks. The numbers by the data points represent the number of attempted attacks on flocks of a given size range.
(From Cresswell and Quinn 2011. Reproduced with permission from John Wiley and Sons and the authors. Photograph by Rob Woolf.)
When redshanks cluster together, they are being helpful to one another even if they take no mutual action beyond joining others of their species to form a flock. The defensive benefit gained could be as simple as a reduction in the odds of being selected by a predator as a function of the increasing number of prey in the group. If, for example, each group of redshanks is attacked successfully by a raptor on average once per day, regardless of how many redshanks are in the group, then for an individual redshank the risk of dying will be 10 times greater in a flock of 10 birds than it would be in a flock of 100.
Birds may derive other antipredator benefits from flocking, such as the increased vigilance that comes from having many eyes scanning for predators. If one member of a flock spots an enemy and flees, others that had not yet seen the attacker also can react by flying off sooner than they would otherwise. The heightened vigilance hypothesis predicts that members of large groups should be more likely to avoid capture than members of smaller groups when under surprise attack from a predator. Data gathered by Will Cresswell and John Quinn (2010, 2011) on the outcome of sneak attacks by Eurasian Sparrowhawks (Accipiter nisus) on Common Redshanks support this prediction. Sparrowhawks were about three times more likely to make a kill when swooping onto a group of less than 10 sandpipers than when they launched an attack on flocks of 40 or more. The greater protection enjoyed by members of larger groups almost certainly results from the improved combined vigilance of the birds in the bigger flocks.
Of course, mutual benefits also can be gained by individuals engaged in much more active forms of cooperation than merely choosing to be part of a large group of conspecifics. The mobbing of a predator by a group of birds is one of the most familiar examples of avian cooperation, as illustrated by the willingness of some prey species to join others in calling loudly while swooping about a dangerous hawk or owl. The costs of group mobbing include the time and energy spent in the activity and the risk that the predator being mobbed will retaliate by attacking and capturing one of its harassers. However, the substantial benefits of the behavior have been demonstrated as well. In Australia, Powerful Owls (Ninox strenua) mobbed by up to 22 Pied Currawongs (Strepera graculina) sometimes flew from their roost, presumably reducing the chance that the mobbing individuals that lived near that site would be attacked by the predator in the night (Pavey and Smyth 1998). Predators that depend on surprise to make a kill have little to gain by remaining in an area where they have been discovered and are now surrounded by noisy mobbers. Moreover, relatively small predatory species, such as small owls or snakes, also may benefit by departing when noisily mobbed if larger hunters—such as Great Horned Owls (Bubo virginianus) in North America—take advantage of the information provided by mobbers to locate and kill the smaller predators. In any event, the fact that mobbing can cause a formidable predator to flee supports the “move‐on hypothesis,” which seeks to explain why prey species would risk everything to make a scene around a dangerous predator.
No matter who participates in mobbing, the benefits of this behavior are enjoyed by all the birds living in the area from which the roosting owl or hunting snake has departed. This universal benefit raises a fundamental question: why join others in risky mobbing rather than simply letting one’s neighbors do the dirty work? One explanation is that joiners make it more likely that when they begin to mob an enemy, the individuals that they have helped in the past will return the favor and come to their assistance. An ingenious experiment conducted with a Latvian population of European Pied Flycatchers (Ficedula hypoleuca) has provided evidence in support of this “reciprocity hypothesis” (Krams et al. 2008). Working with sets of three pairs of flycatchers whose nest boxes were in close proximity, the researchers caught and temporarily removed one pair before placing a stuffed owl near one of the two other nests. As a result, the captured pair was unable to join the other four birds in mobbing the predator. An hour after releasing the two flycatchers that had been held in captivity, the ornithologists placed a stuffed owl at the nest of the previously captured pair and also at the nest of the earlier “helpful” pair. Under these circumstances, the original pair whose nest had been threatened had a choice of whom to join in harassing an owl, and they consistently chose to go to the aid of the pair that had helped them earlier. This result supports the idea that European Pied Flycatchers favor those that have helped them in the past when making decisions about whether to join others in mobbing a mutual enemy (Box 9.09).
9.6.4 Cooperative breeding and avian altruism
Another form of helpful behavior in birds is termed cooperative breeding, a form of sociality in which some individuals do not reproduce personally but instead assist others in chick rearing. Cooperative breeding is not common, occurring regularly in less than 5% of all bird species, but many species of cooperative breeders have been studied intensely because of their theoretical importance. The broader significance of the behavior stems from the fact that the non‐reproducing helpers appear to be making a sacrifice on behalf of other individuals. Self‐sacrificing behavior—altruism—always is intriguing to biologists familiar with Darwinian theory because it is hard to see how the hereditary basis for altruism can persist if the individuals acting altruistically fail to reproduce (Box 9.10).
Cooperative breeders exhibit a variety of social arrangements, and in some cases all the various group members have at least some chance of reproducing. This occurs, for example, in some joint‐nesting species such as the Greater Anis (Crotophaga major) studied in Panama by Christie Riehl (2010), in which multiple females lay eggs in a communal nest that then is attended by all the males and females in the group. In such cases, helping behaviors that benefit the group also are likely to benefit the helping individual directly, because the helper potentially is the parent of at least some of the offspring. A similar situation occurs when subordinate helper males in a cooperative group occasionally are able to mate with the breeding female and thereby sire some of the offspring. These forms of direct benefit may be hard to document without careful genetic testing to determine which individuals in the group actually are reproducing (Box 9.06), but they clearly are not altruistic.
Direct reproduction by helpers is unusual in most cooperatively breeding birds, so direct benefits cannot fully explain the general evolution of this social system. In nearly all cooperative bird species, most of the helpers within a group are young adults that are earlier offspring of the breeding pair, so they are helping to rear their brothers and sisters. For example, in a cooperatively breeding population of American Crows (Corvus brachyrhynchos) that lives on the campus of Cornell University in New York (USA), the majority of helpers are offspring of the breeding pair, although a very few are brothers of the male breeder (Townsend et al. 2009). Likewise, in the White‐fronted Bee‐eater (Merops bullockoides) of Kenya, older sons help their parents rear more siblings (Emlen et al. 1995). This pervasive pattern of offspring remaining at home to help their parents provides some clues to the various ways that this behavior might be a good strategy for the helpers.
If territories, mates, or other critical resources are in very short supply, young birds actually might not be sacrificing much when they stay and help instead of trying to breed on their own. This general explanation for helping behavior is termed the “ecological constraints hypothesis,” because it focuses on the aspects of the environment that constrain a young bird’s options in ways that render breeding on its own costly, risky, or impossible. Ecological constraints appear to be intense in some cooperative breeders such as the Florida Scrub‐Jay (Aphelocoma coerulescens) (Box 9.10), in which all suitable breeding habitat usually is already occupied by breeding jays, and young adults may have to wait several years before the death of a nearby breeder opens a territorial vacancy.
Another possibility is that by staying in their natal territory, helping birds eventually might inherit their parents’ territory, in which case their temporary period of celibacy may have a big payoff when they secure a location where reproduction is clearly possible. This “delayed payoff hypothesis” deals with cases in which a cooperative individual boosts another’s reproductive success, but only temporarily. This type of benefit seems to apply to species such as the Gray‐crowned Babbler (Pomatostomus temporalis) studied in New South Wales, Australia (Blackmore and Heinsohn 2008), in which territorial openings are usually filled by helpers that are already present in that babbler group. However, one complication for these babblers and other family‐living birds is that only one breeder is likely to die at a time, and if all the helpers are the sons or daughters of the remaining breeder, behavioral incest avoidance may preclude them from pairing with their widowed father or mother. In the Gray‐crowned Babblers and most other cooperative species, helpers pair with a surviving breeder only when they are not closely related.
All the various hypotheses for cooperative breeding discussed thus far involve benefits to helpers via their own direct reproduction. What is the explanation for instances in which helpers have little or no chance of ever reproducing personally? Helpers with no hope of becoming parents would seem be engaged in maladaptive altruism to the extent that their helpful behavior reduces whatever chances they have of breeding independently. In these situations, indirect benefits via kin selection (Chapter 3) are likely to be present. As William D. Hamilton (1964) first pointed out, if altruists direct their assistance to close relatives in ways that raise the reproductive success of these relatives, then, in effect, the altruists have passed on copies of their genes indirectly via the extra relatives they have helped to survive. Hamilton’s genius was to see that genetic information can be copied and transmitted both directly in the bodies of one’s offspring, which in sexually reproducing organisms carry 50% of each parent’s genome, and indirectly via the reproduction of relatives. If individuals have little chance of reproducing themselves, the altruism option can become their most adaptive strategy. Adaptive altruism generally requires that helpers focus their efforts on close relatives, because these relatives share a relatively large proportion of their genes with the helpers. This approach generates an obvious prediction, namely, that helpers will promote the breeding success of their closest relatives.
As noted already, helpers at the nest often are offspring of the parents whose progeny they are feeding or protecting. Sometimes, however, unrelated individuals join cooperatively breeding groups. For example, Carrion Crows (Corvus corone) in Spain leave their natal territory and join other crow families as helpers at their nests. Researchers tracking these crows found that emigrating birds interacted regularly with more than one breeding pair before settling down at one location, a site that rarely was next to the territory in which they were reared. In other words, the birds were not simply selecting a group to join on the basis of the proximity to their natal territory. However, the territory chosen by the dispersing males typically contained a fairly close relative, so that the average genetic similarity between a new helper at the nest and the male resident at this site was about one‐quarter, the same as that shared by an uncle and his nephew. Male Carrion Crows somehow are able to identify their relatives, and they use this ability when searching for individuals with whom to form a cooperative association (Baglione et al. 2003).
Hamilton’s explanation for altruism generates another critical prediction: helpers must have a positive effect on the survival or reproductive success of their close relatives. Some studies have demonstrated that helpers really do increase the survival of their younger siblings. Feedings by helpers at the nest in the Bell Miner (Manorina melanophrys), an Australian honeyeater, increase the body mass and physical condition of the nestlings (te Marvelde et al. 2009) (Fig. 9.29). In the Social Weaver (Philetairus socius) of southern Africa, helpers boost the rate at which nestlings are provisioned; such help may be particularly important during droughts when food is scarce (Covas et al. 2008). In the cooperatively breeding Carrion Crow, the larger the group, the lower the probability that the nest will be parasitized by the Great Spotted Cuckoo (Clamator glandarius) (Fig. 9.30). More directly still, very young nestling crows received more food when cared for by a larger number of helpers, leading to a relatively greater output of fledglings by large groups of these crows (Canestrari et al. 2009).
Fig. 9.29 Helpers improve nestling condition. Bell Miners (Manorina melanophrys) forage and breed in family groups. More food is delivered to each chick when there are more helpers in a group.
(From te Marvelde et al. 2009. Reproduced with permission from Elsevier. Photograph by Marlene Lyell.)
Fig. 9.30 Larger groups experience lower rates of brood parasitism. Carrion Crows (Corvus corone) are cooperative breeders. Larger groups of this species are less likely to fall victim to brood parasitism by Great Spotted Cuckoos (Clamator glandarius), one benefit of avian sociality. Sample sizes of nests per group size category are shown above the bars.
(From Canestrari et al. 2009. Reproduced with permission from Elsevier. Photograph by W. Keith Milne.)
Even when the presence of helpers does not result in more or higher quality offspring, helpers may provide other kinds of benefits. For example, in the Superb Fairywren (Malurus cyaneus) (Box 9.07), helpers are often the sons of the breeding pair, and their work at the nest enables the adult female to bring less food to the nestlings than she would otherwise have to (Cockburn et al. 2008). Helpers in effect do what the breeding female would do, thus permitting her to conserve her energy and avoid risky foraging, potentially translating into a longer life and more breeding attempts for her. When a helped female reproduces again, and again, she generates more siblings of her cooperative sons. In other words, avian altruists that provision their siblings or boost parental survival are doing what evolutionary theorists predict they should be doing: they are making indirect contributions of their genes to the next generation.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Alatalo, R. V. and A. Lundberg. 1984. Polyterritorial polygyny in the Pied Flycatcher Ficedula hypoleuca—evidence for the deception hypothesis. Annales Zoologici Fennici 21:217–228.
- Alcock, J. 2013. Animal Behavior: An Evolutionary Approach, 10th Edition. Sinauer Associates, Inc., Sunderland, MA.
- Andersson, M. 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
- Arnqvist, G. and M. Kirkpatrick. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. American Naturalist 165:S26–S37.
- Baglione, V., D. Canestrari, J. M. Marcos, and J. Ekman. 2003. Kin selection in cooperative alliances of carrion crows. Science 300:1947–1949.
- Ballentine, B. and G. E. Hill. 2003. Female mate choice in relation to structural plumage coloration in Blue Grosbeaks. Condor 105:593–598.
- Ballentine, B., J. Hyman, and S. Nowicki. 2004. Vocal performance influences female response to male bird song: an experimental test. Behavioral Ecology 15:163–168.
- Benedict, L. 2008. Unusually high levels of extrapair paternity in a duetting songbird with long‐term pair bonds. Behavioral Ecology and Sociobiology 62:983–988.
- Blackmore, C. J. and R. Heinsohn. 2008. Variable mating strategies and incest avoidance in cooperatively breeding grey‐crowned babblers. Animal Behaviour 75:63–70.
- Blakesley, J. A., D. R. Anderson, and B. R. Noon. 2006. Breeding dispersal in the California Spotted Owl. Condor 108:71–81.
- Borgia, G. 1985. Bower quality, number of decorations and mating success of male satin bowerbirds (Ptilonorhynchus violaceus). Animal Behaviour 33:266–271.
- Borgia, G. and M. A. Gore. 1986. Feather stealing in the satin bowerbird (Ptilonorhynchus violaceus): male competition and the quality of display. Animal Behaviour 34:727–738.
- Bouwman, K. M., R. E. van Dijk, J. J. Wijmenga, and J. Komdeur. 2007. Older male reed buntings are more successful at gaining extrapair fertilizations. Animal Behaviour 73:15–27.
- Brooker, M. G., I. Rowley, M. Adams, and P. R. Baverstock. 1990. Promiscuity: an inbreeding avoidance mechanism in a socially monogamous species? Behavioral Ecology and Sociobiology 26:191–199.
- Brown, C. R. and M. B. Brown. 1986. Ecto‐parasitism as a cost of coloniality in Cliff Swallows (Hirundo pyrrhonota). Ecology 67:1206–1218.
- Brown, J. L. 1994. Mexican Jay (Aphelocoma ultramarina). Pages 1–16 in The Birds of North America (A. Poole and F. B. Gill, Eds.). American Ornithological Union, Washington, DC.
- Bruinzeel, L. W. and M. van de Pol. 2004. Site attachment of floaters predicts success in territory acquisition. Behavioral Ecology 15:290–296.
- Budden, A. E. and J. L. Dickinson. 2009. Signals of quality and age: the information content of multiple plumage ornaments in male western bluebirds Sialia mexicana. Journal of Avian Biology 40:18–27.
- Canestrari, D., J. M. Marcos, and V. Baglione. 2009. Cooperative breeding in carrion crows reduces the rate of brood parasitism by great spotted cuckoos. Animal Behaviour 77:1337–1344.
- Cardoso, G. C., P. G. Mota, and V. Depraz. 2007. Female and male serins (Serinus serinus) respond differently to derived song traits. Behavioral Ecology and Sociobiology 61:1425–1436.
- Carrascal, L. M., J. C. Senar, I. Mozetich, F. Uribe, and J. Domenech. 1998. Interactions among environmental stress, body condition, nutritional status, and dominance in Great Tits. Auk 115:727–738.
- Cockburn, A., A. H. Dalziell, C. J. Blackmore, M. C. Double, H. Kokko, H. L. Osmond, N. R. Beck, M. L. Head, and K. Wells. 2009. Superb fairy‐wren males aggregate into hidden leks to solicit extragroup fertilizations before dawn. Behavioral Ecology 20:501–510.
- Cockburn, A., R. A. Sims, H. L. Osmond, D. J. Green, M. C. Double, and R. A. Mulder. 2008. Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy‐wrens Malurus cyaneus? Journal of Animal Ecology 77:430–438.
- Covas, R., M. A. du Plessis, and C. Doutrelant. 2008. Helpers in colonial cooperatively breeding sociable weavers Philetairus socius contribute to buffer the effects of adverse breeding conditions. Behavioral Ecology and Sociobiology 63:103–112.
- Cresswell, W. and J. L. Quinn. 2010. Attack frequency, attack success and choice of prey group size for two predators with contrasting hunting strategies. Animal Behaviour 80:643–648.
- Cresswell, W. and J. L. Quinn. 2011. Predicting the optimal prey group size from predator hunting behaviour. Journal of Animal Ecology 80:310–319.
- Dalziell, A. H. and A. Cockburn. 2008. Dawn song in superb fairy‐wrens: a bird that seeks extrapair copulations during the dawn chorus. Animal Behaviour 75:489–500.
- Darwin, C. 1871. The Descent of Man and Selection in Relation to Sex. John Murray, London.
- Davies, N. B. 1985. Cooperation and conflict among dunnocks, Prunella modularis, in a variable mating system. Animal Behaviour 33:628–648.
- Dawson, R. D. and G. R. Bortolotti. 2006. Carotenoid‐dependent coloration of male American kestrels predicts ability to reduce parasitic infections. Naturwissenschaften 93:597–602.
- DeLay, L. S., J. Faaborg, J. Naranjo, S. M. Paz, T. DeVries, and P. G. Parker. 1996. Paternal care in the cooperatively polyandrous Galapagos Hawk. Condor 98:300–311.
- Dixon, A., D. Ross, S. L. C. O'Malley, and T. Burke. 1994. Paternal investment inversely related to degree of extra‐pair paternity in the reed bunting. Nature 371:698–700.
- Doutrelant, C., A. Grégoire, N. Grnac, D. Gomez, M. M. Lambrechts, and P. Perret. 2008. Female coloration indicates female reproductive capacity in blue tits. Journal of Evolutionary Biology 21:226–233.
- Drummond, H. 2006. Dominance in vertebrate broods and litters. Quarterly Review of Biology 81:3–32.
- DuVal, E. H. 2007. Cooperative display and lekking behavior of the Lance‐tailed Manakin (Chiroxiphia lanceolata). Auk 124:1168–1185.
- DuVal, E. H. and B. Kempenaers. 2008. Sexual selection in a lekking bird: the relative opportunity for selection by female choice and male competition. Proceedings of the Royal Society B: Biological Sciences 275:1995–2003.
- Ekblom, R., S. A. Sæther, D. Hasselquist, D. Hannersjö, P. Fiske, J. A. Kålås, and J. Höglund. 2005. Female choice and male humoral immune response in the lekking great snipe (Gallinago media). Behavioral Ecology 16:346–351.
- Emlen, S. T., P. H. Wrege, and N. J. Demong. 1995. Making decisions in the family: an evolutionary perspective. American Scientist 83:148–157.
- Fossøy, F., A. Johnsen, and J. T. Lifjeld. 2008. Multiple genetic benefits of female promiscuity in a socially monogamous passerine. Evolution 62:145–156.
- Green, D. J. and E. A. Krebs. 1995. Courtship feeding in Ospreys Pandion haliaetus: a criterion for mate assessment. Ibis 137:35–43.
- Griggio, M., F. Valera, A. Casas, and A. Pilastro. 2005. Males prefer ornamented females: a field experiment of male choice in the rock sparrow. Animal Behaviour 69:1243–1250.
- Gwinner, H. and H. Schwabl. 2005. Evidence for sexy sons in European starlings (Sturnus vulgaris). Behavioral Ecology and Sociobiology 58:375–382.
- Hall, M. L. and A. Peters. 2009. Do male paternity guards ensure female fidelity in a duetting fairy‐wren? Behavioral Ecology 20:222–228.
- Hamilton, W. D. 1964. The genetical theory of social behaviour, I, II. Journal of Theoretical Biology 7:1–52.
- Hanley, D., G. Heiber, and D. C. Dearborn. 2008. Testing an assumption of the sexual‐signaling hypothesis: does blue‐green egg color reflect maternal antioxidant capacity? Condor 110:767–771.
- Hasselquist, D. and S. Bensch. 2008. Daily energy expenditure of singing great reed warblers Acrocephalus arundinaceus. Journal of Avian Biology 39:384–388.
- Hegyi, G., B. Rosivall, E. Szöllősi, R. Hargitai, M. Eens, and J. Török. 2007. A role for female ornamentation in the facultatively polygynous mating system of collared flycatchers. Behavioral Ecology 18:1116–1122.
- Helfenstein, F., R. H. Wagner, E. Danchin, and J. M. Rossi. 2003. Functions of courtship feeding in black‐legged kittiwakes: natural and sexual selection. Animal Behaviour 65:1027–1033.
- Hill, G. E. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350:337–339.
- Hill, G. E. and K. L. Farmer. 2005. Carotenoid‐based plumage coloration predicts resistance to a novel parasite in the house finch. Naturwissenschaften 92:30–34.
- Hill, G. E. and K. J. McGraw (Eds.). 2006. Bird Coloration, Volume 2: Function and Evolution. Harvard University Press, Cambridge, MA.
- Höglund, J. and R. V. Alatalo. 1995. Leks. Princeton University Press, Princeton, NJ.
- Höglund, J., R. V. Alatalo, A. Lundberg, and O. Rätti. 1994. Context‐dependent effects of tail‐ornament damage on mating success in black grouse. Behavioral Ecology 5:182–187.
- Höglund, J. and A. Lundberg. 1987. Sexual selection in a monomorphic lek‐breeding bird: correlates of male mating success in the great snipe Gallinago media. Behavioral Ecology and Sociobiology 21:211–216.
- Huk, T. and W. Winkel. 2006. Polygyny and its fitness consequences for primary and secondary female pied flycatchers. Proceedings of the Royal Society B: Biological Sciences 273:1681–1688.
- Janicke, T., S. Hahn, M. S. Ritz, and H. U. Peter. 2007. Vocal performance reflects individual quality in a nonpasserine. Animal Behaviour 75:91–98.
- Johnsen, A., H. Pärn, F. Fossøy, O. Kleven, T. Laskemoen, and J. T. Lifjeld. 2008. Is female promiscuity constrained by the presence of her social mate? An experiment with bluethroats Luscinia svecica. Behavioral Ecology and Sociobiology 62:1761–1767.
- Jones, A. G. and N. L. Ratterman. 2009. Mate choice and sexual selection: what have we learned since Darwin? Proceedings of the National Academy of Sciences of the United States of America 106:10001–10008.
- Jukema, J. and T. Piersma. 2005. Permanent female mimics in a lekking shorebird. Biology Letters 2:161–164.
- Kelley, L. A. and J. A. Endler. 2012. Male great bowerbirds create forced perspective illusions with consistently different individual quality. Proceedings of the National Academy of Sciences of the United States of America 109:20980–20985.
- Kingma, S. A., J. Komdeur, O. Vedder, N. von Engelhardt, P. Korsten, and T. G. G. Groothuis. 2009. Manipulation of male attractiveness induces rapid changes in avian maternal yolk androgen deposition. Behavioral Ecology 20:172–179.
- Kleven, O., B. A. Bjerke, and J. T. Lifjeld. 2008. Genetic monogamy in the Common Crossbill (Loxia curvirostra). Journal of Ornithology 149:651–654.
- Koenig, W. D. and R. L. Mumme. 1987. Population Ecology of the Cooperatively Breeding Acorn Woodpecker. Princeton University Press, Princeton, NJ.
- Komdeur, J. 2001. Mate guarding in the Seychelles warbler is energetically costly and adjusted to paternity risk. Proceedings of the Royal Society B: Biological Sciences 268:2103–2111.
- Komdeur, J., T. Burke, and D. S. Richardson. 2007. Explicit experimental evidence for the effectiveness of proximity as mate‐guarding behaviour in reducing extra‐pair fertilization in the Seychelles warbler. Molecular Ecology 16:3679–3688.
- Komdeur, J., F. Kraaijeveld‐Smit, K. Kraaijeveld, and P. Edelaar. 1999. Explicit experimental evidence for the role of mate guarding in minimizing loss of paternity in the Seychelles warbler. Proceedings of the Royal Society B: Biological Sciences 266:2075–2081.
- Komdeur, J., P. Wiersma, and M. Magrath. 2002. Paternal care and male mate‐attraction effort in the European starling is adjusted to clutch size. Proceedings of the Royal Society B: Biological Sciences 269:1253–1261.
- Kotiaho, J. S., N. R. LeBas, M. Puurtinen, and J. L. Tomkins. 2008. On the resolution of the lek paradox. Trends in Ecology and Evolution 23:1–3.
- Krams, I., T. Krama, K. Igaune, and R. Mänd. 2008. Experimental evidence of reciprocal altruism in the pied flycatcher. Behavioral Ecology and Sociobiology 62:599–605.
- Krokene, C., K. Rigstad, M. Dale, and J. T. Lijfeld. 1998. The function of extrapair paternity in blue tits and great tits: good genes or fertility insurance? Behavioral Ecology 9:649–656.
- Lack, D. 1968. Ecological Adaptations for Breeding in Birds. Methuen and Company, London.
- Lebigre, C., R. V. Alatalo, H. Siitari, and S. Parri. 2007. Restrictive mating by females on black grouse leks. Molecular Ecology 16:4380–4389.
- Leisler, B., H. Winkler, and M. Wink. 2002. Evolution of breeding systems in Acrocephaline Warblers. Auk 119:379–390.
- Li, M. H., K. Välimäki, M. Piha, T. Pakkala, and J. Merilä. 2009. Extrapair paternity and maternity in the three‐toed woodpecker, Picoides tridactylus: insights from microsatellite‐based parentage analysis. PLoS One 4:e7895.
- Low, M. 2005. Female resistance and male force: context and patterns of copulation in the New Zealand stitchbird Notiomystis cincta. Journal of Avian Biology 36:436–448.
- Loyau, A., M. Petrie, M. Saint Jalme, and G. Sorci. 2008. Do peahens not prefer peacocks with more elaborate trains? Animal Behaviour 76:E5–E9.
- Lyon, B. E., R. D. Montgomerie, and L. D. Hamilton. 1987. Male parental care and monogamy in snow buntings. Behavioral Ecology and Sociobiology 20:377–382.
- MacFarlane, G. R., S. P. Blomberg, and P. L. Vasey. 2010. Homosexual behaviour in birds: frequency of expression is related to parental care disparity between the sexes. Animal Behaviour 80:375–390.
- Mackenzie, A., J. D. Reynolds, V. J. Brown, and W. J. Sutherland. 1995. Variation in male mating success on leks. American Naturalist 145:633–652.
- Madsen, V., T. Dabelsteen, D. Osorio, and J. L. Osorno. 2007. Morphology and ornamentation in male magnificent frigatebirds: variation with age class and mating status. American Naturalist 169:S93–S111.
- McDonald, D. B. 2007. Predicting fate from early connectivity in a social network. Proceedings of the National Academy of Sciences of the United States of America 104:10910–10914.
- McDonald, D. B. and W. K. Potts. 1994. Cooperative display and relatedness among males in a lek‐mating bird. Science 266:1030–1032.
- McKinney, F., S. R. Derrickson, and P. Mineau. 1983. Forced copulation in waterfowl. Behaviour 86:250–294.
- Milam, E. L. 2010. Looking For a Few Good Males: Female Choice in Evolutionary Biology. Johns Hopkins University Press, Baltimore, MD.
- Mock, D. W. 2004. More Than Kin and Less Than Kind: The Evolution of Family Conflict. Harvard University Press, Cambridge, MA.
- Mock, D. W., H. Drummond, and C. H. Stinson. 1990. Avian siblicide. American Scientist 78:438–449.
- Møller, A. P. 1988. Female choice selects for male sexual tail ornaments in the monogamous swallow. Nature 332:640–642.
- Morales, J., J. Moreno, S. Merino, J. J. Sanz, G. Tomás, E. Arriero, E. Lobato, and J. Martínez de la Puente. 2007. Female ornaments in the Pied Flycatcher Ficedula hypoleuca: associations with age, health and reproductive success. Ibis 149:245–254.
- Mulder, R. A., P. O. Dunn, A. Cockburn, K. A. Lazenby‐Cohen, and M. J. Howell. 1994. Helpers liberate female fairy‐wrens from constraints on extra‐pair mate choice. Proceedings of the Royal Society B: Biological Sciences 255:223–229.
- Nisbet, I. C. T. and P. Dann. 2009. Reproductive performance of little penguins Eudyptula minor in relation to year, age, pair‐bond duration, breeding date and individual quality. Journal of Avian Biology 40:296–308.
- Nowicki, S., W. A. Searcy, and S. Peters. 2002. Quality of song learning affects female response to male bird song. Proceedings of the Royal Society B: Biological Sciences 269:1949–1954.
- Orians, G. H. 1969. On the evolution of mating systems in birds and mammals. American Naturalist 103:589–603.
- Otter, K. and L. Ratcliffe. 1996. Female initiated divorce in a monogamous songbird: abandoning mates for males of higher quality. Proceedings of the Royal Society B: Biological Sciences 263:351–355.
- Pasinelli, G., M. Müller, M. Schaub, and L. Jenni. 2007. Possible causes and consequences of philopatry and breeding dispersal in red‐backed shrikes Lanius collurio. Behavioral Ecology and Sociobiology 61:1061–1074.
- Patricelli, G. L., J. A. C. Uy, G. Walsh, and G. Borgia. 2002. Male displays adjusted to female's response. Nature 415:279–280.
- Pavey, C. R. and A. K. Smyth. 1998. Effects of avian mobbing on roost use and diet of powerful owls, Ninox strenua. Animal Behaviour 55:313–318.
- Piper, W. H., D. C. Evers, M. W. Meyer, K. B. Tischler, J. D. Kaplan, and R. C. Fleischer. 1997. Genetic monogamy in the common loon (Gavia immer). Behavioral Ecology and Sociobiology 41:25–31.
- Pogány, Á. and T. Székely. 2007. Female choice in the penduline tit Remiz pendulinus: the effects of nest size and male mask size. Behaviour 144:411–427.
- Pribil, S. and W. A. Searcy. 2001. Experimental confirmation of the polygyny threshold model for red‐winged blackbirds. Proceedings of the Royal Society B: Biological Sciences 268:1643–1646.
- Pruett‐Jones, S. G. and M. J. Lewis. 1990. Sex‐ratio and habitat limitation promote delayed dispersal in superb fairy‐wrens. Nature 348:541–542.
- Reid, J. M., P. Monaghan, and G. D. Ruxton. 2002. Males matter: the occurrence and consequences of male incubation in starlings (Sturnus vulgaris). Behavioral Ecology and Sociobiology 51:255–261
- Reudink, M. W., P. P. Marra, P. T. Boag, and L. M. Ratcliffe. 2009. Plumage coloration predicts paternity and polygyny in the American redstart. Animal Behaviour 77:495–501.
- Reynolds, J. D. 1987. Mating system and nesting biology of the Red‐necked Phalarope Phalaropus lobatus. Ibis 129:225–242.
- Reynolds, S. M., M. C. Christman, J. A. C. Uy, G. L. Patricelli, M. J. Braun, and G. Borgia. 2009. Lekking satin bowerbird males aggregate with relatives to mitigate aggression. Behavioral Ecology 20:410–415.
- Riehl, C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Current Biology 20:1830–1833.
- Rintamäki, P. T., R. V. Alatalo, J. Höglund, and A. Lundberg. 1995. Male territoriality and female choice on black grouse leks. Animal Behaviour 49:759–767.
- Rintamäki, P. T., J. Höglund, R. V. Alatalo, and A. Lundberg. 2001. Correlates of male mating success on black grouse (Tetrao tetrix L.) leks. Annales Zoologici Fennici 38:99–109.
- Rubenstein, D.R. 2011. Spatiotemporal environmental variation, risk aversion and the evolution of cooperative breeding as a bet‐hedging strategy. Proceedings of the National Academy of Sciences of the United States of America 108:10816–10822.
- Safran, R. J. and K. J. McGraw. 2004. Plumage coloration, not length or symmetry of tail‐streamers, is a sexually selected trait in North American barn swallows. Behavioral Ecology 15:455–461.
- Schamel, D., D. M. Tracy, D. B. Lank, and D. F. Westneat. 2004. Mate guarding, copulation strategies and paternity in the sex‐role reversed, socially polyandrous red‐necked phalarope Phalaropus lobatus. Behavioral Ecology and Sociobiology 57:110–118.
- Schjelderup‐Ebbe, T. 1935. Social behavior in birds. Pages 947–972 in A Handbook of Social Psychology (C. A. Murchison, Ed.). Clark University Press, Worcester, MA.
- Schlinger, B. A., L. B. Day, and L. Fusani. 2008. Behavior, natural history and neuroendocrinology of a tropical bird. General and Comparative Endocrinology 157:254–258.
- Schlinger, B. A., J. D. Schultz, and F. Hertel. 2001. Neuromuscular and endocrine control of an avian courtship behavior. Hormones and Behavior 40:276–280.
- Schoener, T. W. 1968. Sizes of feeding territories among birds. Ecology 49:123–141.
- Sergio, F., J. Blas, and F. Hiraldo. 2009. Predictors of floater status in a long‐lived bird: a cross‐ sectional and longitudinal test of hypotheses. Journal of Animal Ecology 78:109–118.
- Slagsvold, T. and J. T. Lifjeld. 1994. Polygyny in birds: the role of competition between females for male parental care. American Naturalist 143:59–94.
- Smith, J. R. and G. Sutherland. 2008. Northern Goshawk (Accipiter gentilis laingi) habitat and territory models: modelling methodology and implementation and scenario results for coastal British Columbia. Prepared for the Northern Goshawk Habitat Recovery Implementation Group, Ministry of Environment, Victoria, BC.
- Soler, J. J., C. Navarro, T. P. Contreras, J. M. Avilés, and J. J. Cuervo. 2008. Sexually selected egg coloration in spotless starlings. American Naturalist 171:183–194.
- Soler, M., M. Martín‐Vivaldi, J. M. Marín, and A. P. Møller. 1999. Weight lifting and health status in the black wheatear. Behavioral Ecology 10:281–286.
- Soler, M., J. J. Soler, A. P. Møller, J. Moreno, and M. Lindén. 1996. The functional significance of sexual display: stone carrying in the black wheatear. Animal Behaviour 51:247–254.
- Soukup, S. S. and C. F. Thompson. 1998. Social mating system and reproductive success in house wrens. Behavioral Ecology 9:43–48.
- Stein, A. C. and J. A. C. Uy. 2006. Plumage brightness predicts male mating success in the lekking golden‐collared manakin, Manacus vitellinus. Behavioral Ecology 17:41–47.
- Suter, S. M., M. Keiser, R. Feignoux, and D. R. Meyer. 2007. Reed bunting females increase fitness through extra‐pair mating with genetically dissimilar males. Proceedings of the Royal Society B: Biological Sciences 274:2865–2871.
- Tarvin, K. A., M. S. Webster, E. M. Tuttle, and S. Pruett‐Jones. 2005. Genetic similarity of social mates predicts the level of extrapair paternity in splendid fairy‐wrens. Animal Behaviour 70:945–955.
- te Marvelde, L., P. G. McDonald, A. J. N. Kazem, and J. Wright. 2009. Do helpers really help? Provisioning biomass and prey type effects on nestling growth in the cooperative bell miner. Animal Behaviour 77:727–735.
- Tinbergen, N. 1963. On the aims and methods of ethology. Zeitschrift für Tierpsychologie 20:410–433.
- Townsend, A. K., R. Bowman, J. W. Fitzpatrick, M. Dent, and I. J. Lovette. 2011. Genetic monogamy across variable demographic landscapes in the cooperatively breeding Florida scrub‐jays. Behavioral Ecology 22:464–470.
- Townsend, A. K., A. B. Clark, K. J. McGowan, and I. J. Lovette. 2009. Reproductive partitioning and the assumptions of reproductive skew models in the cooperatively breeding American crow. Animal Behaviour 77:503–512.
- Tryjanowski, P. and M. Hromada. 2005. Do males of the great grey shrike, Lanius excubitor, trade food for extrapair copulations? Animal Behaviour 69:529–533.
- Velando, A. 2004. Female control in yellow‐legged gulls: trading paternity assurance for food. Animal Behaviour 67:899–907.
- Ward, S. and P. J. B. Slater. 2005. Raised thermoregulatory costs at exposed song posts increase the energetic cost of singing for willow warblers Phylloscopus trochilus. Journal of Avian Biology 36:280–286.
- Weatherhead, P. J. and R. J. Robertson. 1979. Offspring quality and the polygyny threshold: “the sexy son hypothesis”. American Naturalist 113:201–208.
- Webster, M. S. 1994. Female‐defence polygyny in a Neotropical bird, the Montezuma oropendula. Animal Behaviour 48:779–794.
- Westneat, D. F., P. W. Sherman, and M. L. Morton. 1990. The ecology and evolution of extra‐pair copulations in birds. Current Ornithology 7:331–369.
- Whitfield, D. P. 2003. Redshank Tringa totanus flocking behaviour, distance from cover and vulnerability to sparrowhawk Accipiter nisus predation. Journal of Avian Biology 34:163–169.
- Witte, K. 2006. Time spent with a male is a good indicator of mate preference in female zebra finches. Ethology, Ecology and Evolution 18:195–204.
- Wojcieszek, J. M., J. A. Nicholls, and A. W. Goldizen. 2007. Stealing behavior and the maintenance of a visual display in the sSatin bowerbird. Behavioral Ecology 18:689–695.
- Wojczulanis‐Jakubas, K., D. Jakubas, T. Øigarden, and J. T. Lifjeld. 2009. Extrapair copulations are frequent but unsuccessful in a highly colonial seabird, the little auk, Alle alle. Animal Behaviour 77:433–438.
- Woolfenden, G. E. and J. W. Fitzpatrick. 1984. The Florida Scrub Jay: Demography of a Cooperative‐Breeding Bird. Princeton University Press, Princeton, NJ.
- Woolley, S. C. and A. J. Doupe. 2008. Social context‐induced song variation affects female behavior and gene expression. PLoS Biology 6:525–537.
- Young, L. C., B. J. Zaun, and E. A. VanderWerf. 2008. Successful same‐sex pairing in Laysan Albatross. Biology Letters 4:323–325.