Chapter 10
Avian Vocal Behavior
Bruce E. Byers and Donald E. Kroodsma
University of Massachusetts Amherst

(Photograph by Sushyue Liao.)
Birds are noisy animals. This noisiness is a boon to birdwatchers, who often rely on sounds to detect birds that would otherwise remain unnoticed as they forage in dense foliage, lurk in tall grass, or fly high overhead. Birders also benefit from the specificity of birds’ sounds, as birds of almost any species can be identified solely by the sounds they make. These practical benefits of bird sounds, however, pale in comparison with the aesthetic ones. Who can fail to be impressed by the symphonic beauty of the dawn chorus in a temperate woodland, the overwhelming cacophony of a seabird nesting colony, the mysterious mood music of a tropical forest as dusk falls, or the virtuoso individual performances of many songbirds?
Bird sounds did not, of course, evolve for the convenience and pleasure of humans. Birds use sounds to communicate, and scientific investigation of bird vocalizations has focused on questions related to this function. What information is transmitted by vocalizations? Why do individual birds produce so many different sounds? Why are some bird vocalizations so complex and elaborate? How and why do the vocalizations of a given species vary? How do birds produce their sounds? How does a developing bird gain the ability to produce the sounds of its species? The aim of this chapter is to address these questions about bird vocalizations.
Throughout this chapter you will find references to songs and calls that will help you understand vocal behavior in birds. To get the most of out of this material, it is important to listen to the associated sounds while reading it. To animate and listen to these sounds online, go directly to the following site: birdbiology.org/vocalbehavior.
10.1 Studying bird sounds: the investigator’s toolbox
In the not too distant past, ornithologists interested in studying bird vocalizations undertook their research equipped with nothing more than their own ears and perhaps the ability to transcribe the sounds they heard into musical notation. Today, scientific investigation of bird vocalizations is aided by digital recorders, sensitive microphones, and computerized tools that augment our senses and allow us to make measurements that otherwise would be impossible. We describe some of these tools below, after a brief description of the physical properties that they measure.
10.1.1 Physical basis of sound
Consider the way in which a drum produces sound (Fig. 10.01). Pounding on the tightly stretched membrane of the drumhead causes the membrane to vibrate. As the vibrating membrane moves outward, the air molecules near it compress. When the vibrating membrane moves inward, nearby air molecules move to fill the space vacated by the membrane, thereby spreading further apart. Thus, the air near the drumhead is alternately compressed and thinned out, forming alternating high‐pressure and low‐pressure bands. As air molecules near the drumhead interact with those a bit further away, the pattern of alternating high‐ and low‐pressure bands is transmitted to air increasingly distant from the drum and thus forms a sound wave traveling through the air.

Fig. 10.01 Sound waves are generated by moving air molecules. Just before a stationary drum membrane is struck (left), air molecules are more or less evenly distributed. Once struck, the drum membrane moves down (downward arrows) and air molecules spread out to fill the low‐pressure space. When the membrane oscillates back up (upward arrows), it adds pressure to the air above, forcing air molecules to compact. This process repeats, creating waves of pressure changes that are detected and translated into audible sound by the ear.
(© Cornell Lab of Ornithology.)
If a sound wave reaches a human eardrum, the eardrum vibrates in response, initiating a chain of events that converts the vibrations into an electrical signal that the brain interprets as a sound. Our sound perception capabilities allow us to discriminate among different sounds; for example, some sounds are high pitched and others are low pitched (Box 10.01). These differences in perceived pitch result from differences in how fast the object that generated the initial sound wave was vibrating. For example, on a piano the heavy wires that produce the lowest C vibrate only 32.7 times per second, and the thin wires that produce the highest C vibrate more than 4000 times per second. These vibrations generate sound waves; the higher the rate at which waves are generated, the higher the resulting sound’s perceived pitch. The rate at which a sound’s waves are produced is called its frequency, which is measured in cycles (waves) per second, or Hertz (Hz), named after Heinrich Hertz, a pioneer in the study of sound.
10.1.2 Visualizing sounds as spectrograms
One way to supplement our hearing and enhance our listening skills is to use computer programs that convert bird sounds to visual representations that we can see and study (Box 10.02). Traditionally, ornithologists often visualized bird vocalizations in the form of a spectrogram (also called a sonogram), illustrated here using a simple whistled song of North America’s Black‐capped Chickadee (Poecile atricapillus) (Fig. 10.02A). The spectrogram’s vertical axis represents sound frequency, measured in kilohertz (kHz); the horizontal axis represents time. This chickadee song is often described in field guides as sounding like “hey‐sweetie,” with the first part slightly higher in frequency than the second. Sure enough, the spectrogram shows two whistled notes separated by about 0.2 seconds of silence. The first whistle begins at about 4.0 kHz, roughly the frequency of the highest C on a piano, and descends to about 3.5 kHz. The second note is about 400 Hz lower, at approximately 3.6 kHz.
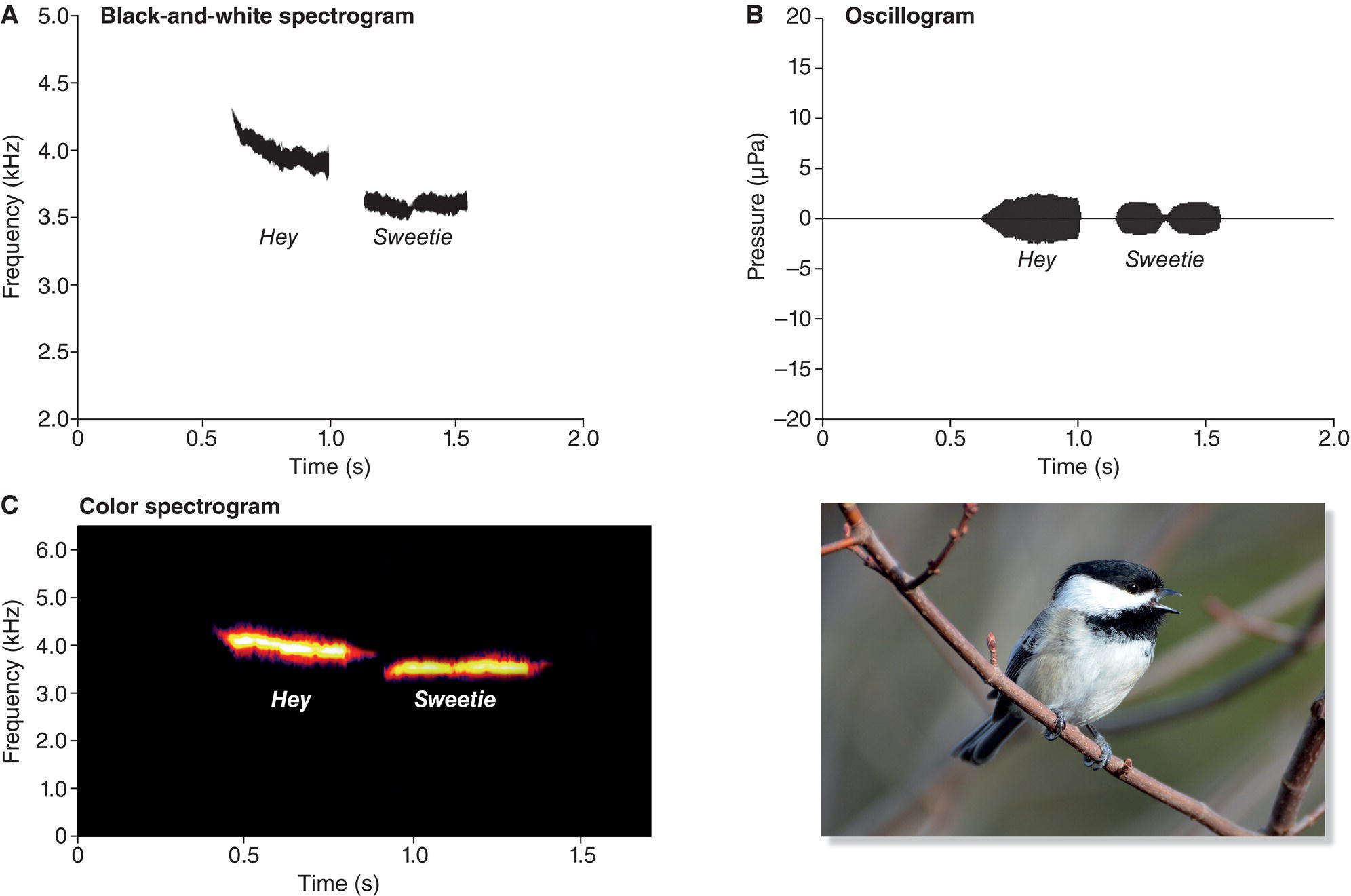
Fig. 10.02 Three ways to visualize a vocalization. Here, the “hey‐swee‐tie” song of a Black‐capped Chickadee (Poecile atricapillus) is represented three different ways. (A) In a black‐and‐white spectrogram, a sound’s frequency is plotted against time. (B) An oscillogram displays changes in a vocalization’s loudness over time; the vertical axis represents sound intensity (the louder the song, the more vertical space the plot will occupy). (C) Modern spectrograms often use color to represent sound intensity (the brighter the color, the higher the intensity). Listen online.
(Recordist: Matthew D. Medler. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Peter Brannon.)
In a black‐and‐white spectrogram, however, relative loudness (sound intensity) is not demonstrated clearly. For example, in this chickadee song, there is a very brief break in the second whistle where there is a subtle but clear drop in the loudness (sound intensity) near the middle of the whistle. The black‐and‐white spectrogram does not clearly reveal this drop in loudness. The relative loudness of these sounds is shown more clearly in a second type of graph, an oscillogram (Fig. 10.02B). As in a spectrogram, the horizontal axis of an oscillogram represents time, but an oscillogram’s vertical axis shows sound intensity: the louder a sound, the greater the height of the wave on its oscillogram.
Today, ornithologists often use a colorized spectrogram that merges these two kinds of information to help us visualize both patterns of frequency and patterns of sound intensity (Fig. 10.02C). The spectrograms used throughout this chapter show time on the horizontal axis, sound frequency on the vertical axis, and relative loudness using colors: from white/gold (loud) to purple/pink (less loud). Experienced researchers can often look at a spectrogram and imagine clearly the associated song, but this skill requires practice. Be sure to take advantage of the web resources associated with this chapter, where you can watch animated versions of most spectrograms depicted while simultaneously listening to the sounds they portray (Box 10.02).
10.1.3 Using your eyes to assist your ears
By using spectrograms to help us “see” bird sounds, we can begin to appreciate the finer details of what we hear. Consider another example of a song with pure whistles, the “poor old sam peabody peabody peabody” song of the White‐throated Sparrow (Zonotrichia albicollis) (Fig. 10.03). This song, evocative of the northern North American forests in which this species breeds, is introduced by two or three relatively long whistles, each on a slightly different frequency, denoted by the different words “poor old sam.” In some individuals, successive notes rise in frequency, and in some they fall, but the overall pattern is unmistakable. Look at the last portion of the spectrogram and find the triplet notes represented by the three syllables in the word “pea‐bo‐dy.” When you listen to the song, it is hard to distinguish the three notes in “peabody,” but their separation is considerably more apparent in the spectrogram.
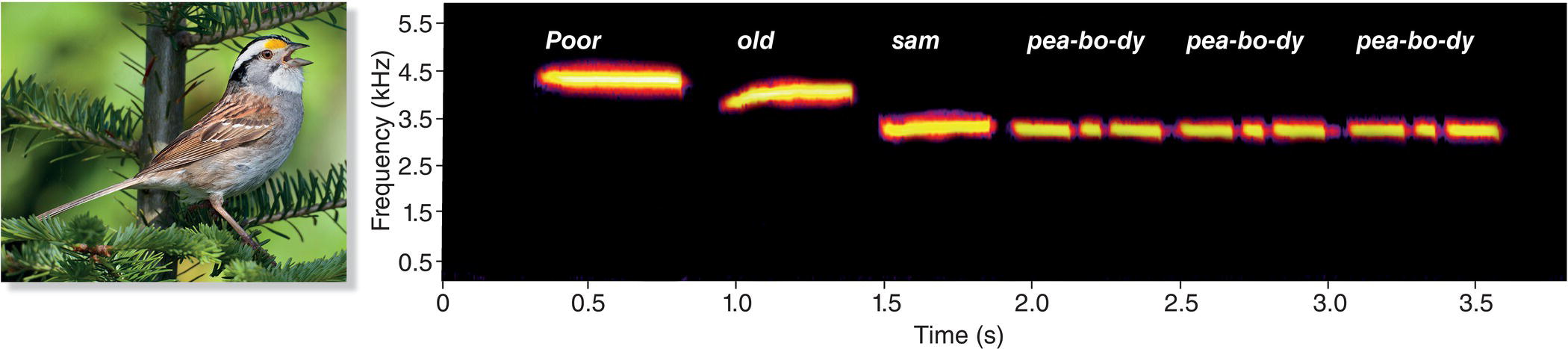
Fig. 10.03 Pure whistles in the song of the White‐throated Sparrow (Zonotrichia albicollis). Males sing whistled notes of distinct frequencies that are traditionally transcribed as “poor old sam peabody peabody peabody.” The individual triplet notes within each “pea‐bo‐dy” are easy to distinguish in the spectrogram, but more difficult to hear. Listen online.
(Recordist: Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Jim Zipp.)
In sharp contrast to whistled sounds, such as the chickadee’s “hey‐sweetie” and sparrow’s “peabody,” are “noisy” sounds, which include a broad span of simultaneously produced frequencies. Noisy sounds appear on a spectrogram as vertical lines, rather than horizontal ones. The snap of a finger and the slam of a door are examples of noisy sounds. Similarly, a woodpecker tapping on a tree produces noise because different parts of the tree vibrate at different frequencies.
The drumming sounds of three North American woodpecker species nicely illustrate this and show how patterns of noise can be highly distinctive. The first two species—the Downy Woodpecker (Picoides pubescens) (Fig. 10.04A) and Hairy Woodpecker (Picoides villosus) (Fig. 10.04B)—are similar in appearance but can be distinguished by their different sizes or by their distinct vocalizations. They differ, too, in the rate at which they drum. Surprisingly, the larger Hairy Woodpecker drums faster than the Downy. Counting the number of pulses in a second shows that the Hairy Woodpecker has about 26 drums per second and the Downy only 15. The third species, the Yellow‐bellied Sapsucker (Sphyrapicus varius) (Fig. 10.04C), adds a unique rhythm to its drumming, delivering taps that start fast and end in a slower rate, unlike the Downy and Hairy Woodpeckers.
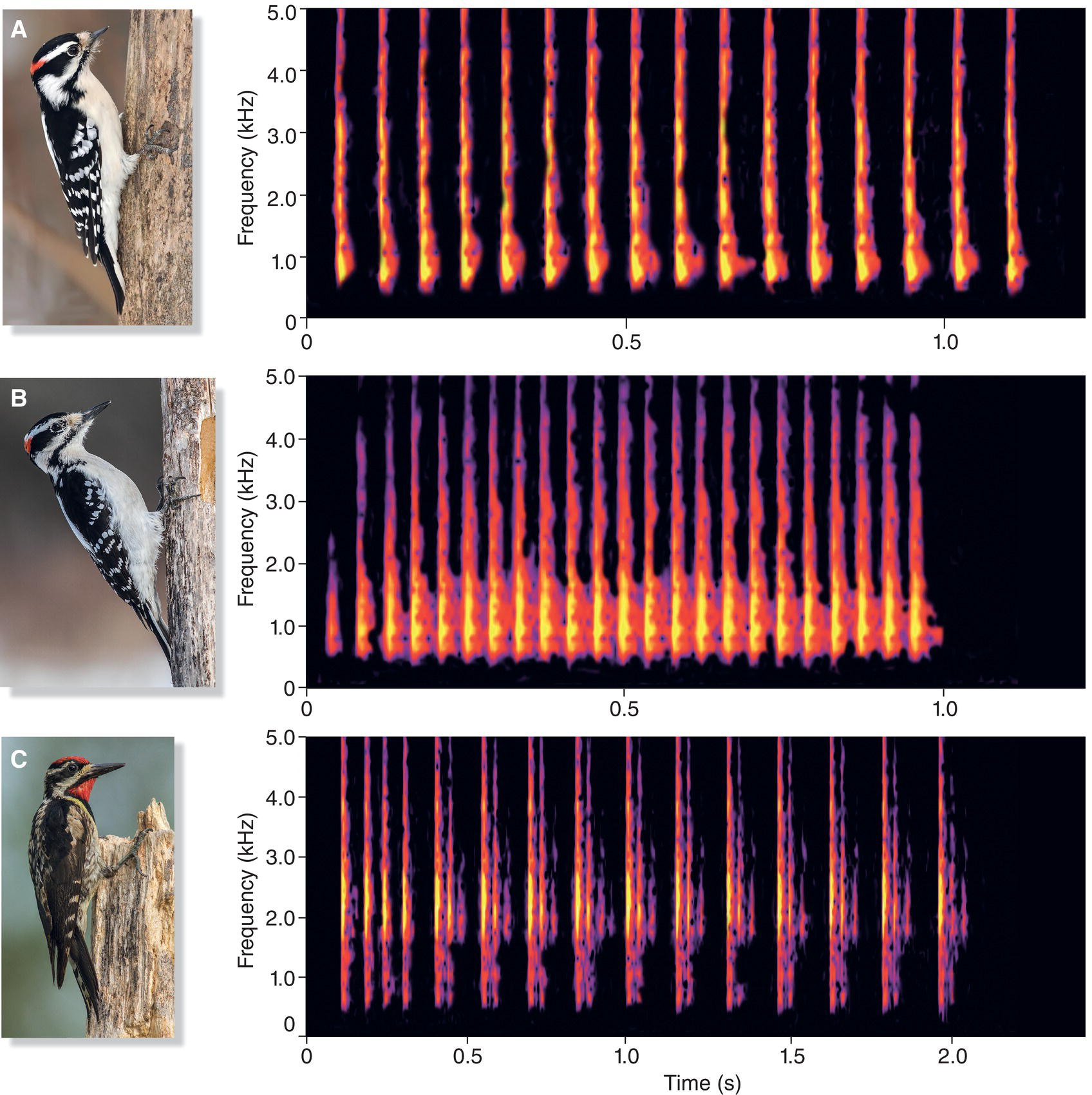
Fig. 10.04 Woodpecker drumming differs by species. Many woodpeckers drum at a species‐specific rate that is easily discernible in spectrograms. Note the wide frequency range of the noisy sounds in the territorial drums of three different species: (A) Downy Woodpeckers (Picoides pubescens) drum at a rate of about 15 drums per second. (B) Hairy Woodpeckers (Picoides villosus) drum at a faster rate of 26 drums per second. (C) Yellow‐bellied Sapsuckers (Sphyrapicus varius) have variable drumming rates, often slowing down at the end of a series of drums. Listen online.
(Recordists: A and C, Gregory F. Budney; B, William W. H. Gunn. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photographs by: A, Rafael Garciá Sánchez; B and C, Bill McMullen.)
Spectrograms can help us visualize more complex sounds, too. Consider, for example, the song of the Common Yellowthroat (Geothlypis trichas), a wood‐warbler species found throughout most of North America. The field guides render the yellowthroat’s song as “witchity‐witchity‐witchity,” suggesting that we should search a spectrogram for a repeating pattern of individual notes. In line with our expectation, spectrograms of yellowthroat songs show that each “witch‐i‐ty” consists of three or four notes, and successive “witchities” appear to be identical (Fig. 10.05). This pattern occurs in almost every yellowthroat song. When the songs are slowed to one‐half or one‐quarter speed, we can more easily hear each note, especially while following along in the spectrogram. Halving the speed also halves the frequency, so the song sounds lower pitched.
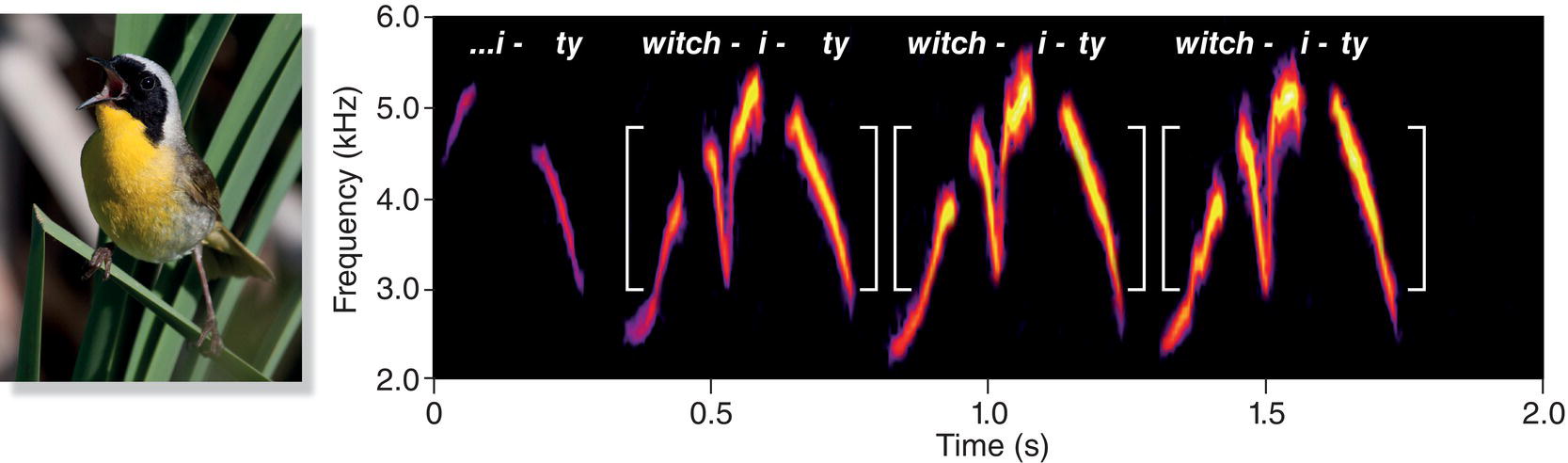
Fig. 10.05 Dissecting the components of a Common Yellowthroat (Geothlypis trichas) song. In the spectrogram, repeated “witchity” phrases (white brackets) are easy to discern. Each phrase can be further divided into three notes, “witch‐i‐ty.” Online you can hear these features even more clearly by comparing the normal song to recordings that have been slowed to half and quarter speeds. Note that slowing the speed causes the pitch to drop. Listen online.
(Recordist: Wilbur L. Hershberger. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Bill Bouton. CC‐BY‐SA 2.0.)
One final example illustrates the usefulness of a spectrogram in seeing the diverse components of song. The song of the Pacific Wren (Troglodytes pacificus) of western North America has been described as “the pinnacle of song complexity” (Kroodsma 1980) (Fig. 10.06). The songs of these wrens are truly remarkable; they can be 10 seconds long or even longer, but during that time the wren sings successive notes so rapidly that all we hear is a blur. Once we slow the song down, however, we can appreciate all the fine details and begin to hear what we can see in the spectrogram. At one‐half and especially at one‐quarter normal speed, the listener can identify each note the wren produces.
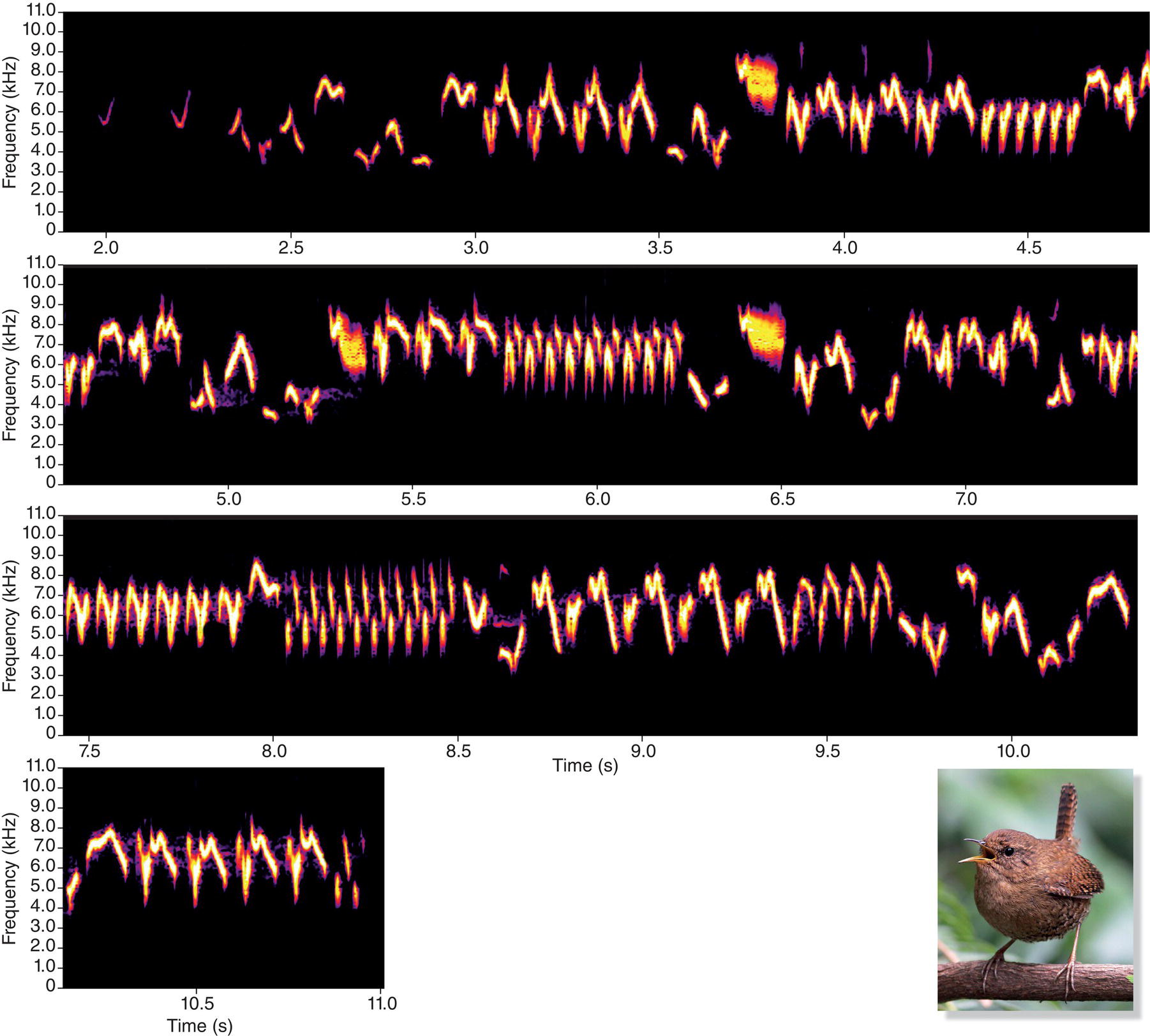
Fig. 10.06 Pacific Wren (Troglodytes pacificus) songs are remarkably complex. This spectrogram shows the diversity of elements in this tiny wren’s long and complicated song. Web content includes both regular and slowed (half and quarter) speeds, which reveal even more astounding detail. Listen online.
(Recordist: Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Liron Gertsman.)
10.2 Vocal repertoires
Our experience tells us that birds produce a tremendous variety of sounds (Box 10.03). How can we begin to make sense of this great diversity? The best way is to start with an individual bird. Researchers who study bird sounds often mark a bird, usually with a distinctive combination of colored leg bands, and then follow it, often for days at a time and sometimes year after year. Researchers record sounds that the bird makes and carefully document the context in which each sound was recorded. Next, they make spectrograms of each recording so that the anatomy of each sound can be studied in more detail. Finally, they sort all the spectrograms by shape and combination of notes to establish the entire vocal repertoire of that individual.
This approach has revealed that an individual bird usually has a relatively limited selection of vocal signals. A signal is a trait that influences the behavior of other individuals and that evolved for that function. Consider again the Black‐capped Chickadee (Poecile atricapillus). Extensive research has shown that chickadees have about 14 different kinds of vocalizations (Hailman 1989; Hailman and Ficken 1996). Three of these sounds are frequently used, are highly distinctive, and are readily recognized by anyone who watches chickadees for even a short time. One of these is the “hey‐sweetie” whistle song (Fig. 10.02), usually sung loudly from an exposed perch. Another vocalization, the one for which the bird was named, is the “chick‐a‐dee” call, often heard in winter flocks and near birdfeeders (Fig. 10.07A). These birds give this call in a variety of contexts, including when they seem mildly alarmed. The third common vocalization is the “gargle,” a complex sound used mostly by males during aggressive interactions (Fig. 10.07B).
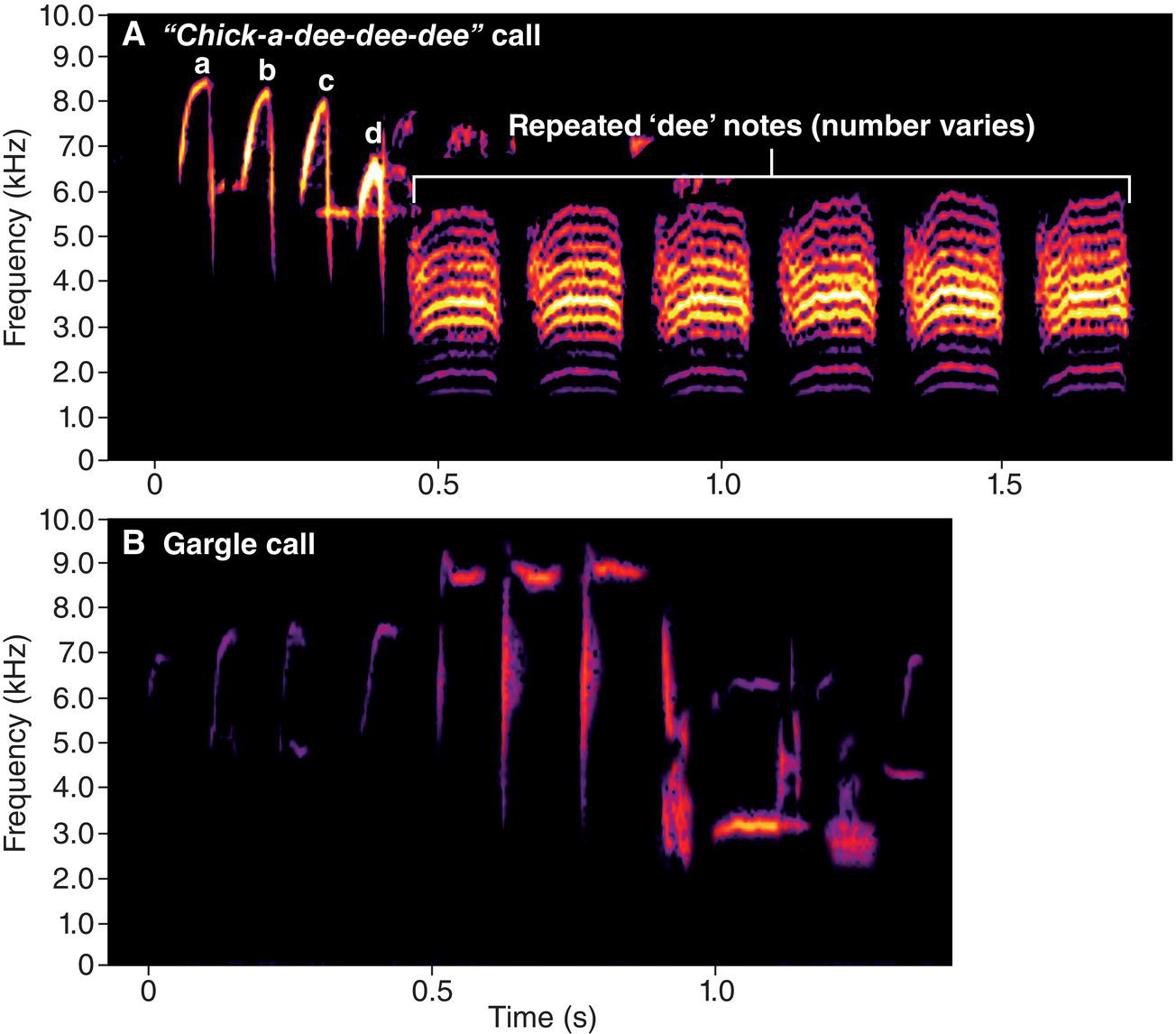
Fig. 10.07 Calls of the Black‐capped Chickadee (Poecile atricapillus). In addition to the “hey‐sweetie” song illustrated in Fig. 10.02, chickadees have other vocal signals. (A) The “chick‐a‐dee” call is often associated with flock identity. Although this call consists of the same component notes (a–d) in addition to the characteristic “dee” sequence, individuals modify the note order and/or repetition according to the call variety specific to their flock. (B) Gargle calls are typically given by males during aggressive interactions. Listen online.
(Recordists: A, Matthew D. Medler; B, Gerrit Vyn. Courtesy of Macaulay Library © Cornell Lab of Ornithology.)
10.2.1 Songs
One type of avian vocal signal—the song—has received special attention from researchers. Song has been defined in numerous ways, but it is generally accepted that songs are long, loud vocalizations, typically delivered from an exposed perch, with the presumed function of attracting mates or repelling territorial intruders. This definition of song is not very precise, and indeed the distinction between songs and other types of bird vocalizations is somewhat arbitrary. Nonetheless, this general definition is appropriate in many situations because most birds use special, conspicuous vocalizations to attract mates or defend territories. Because songs are often sung loudly and persistently, they are often the most noticeable of the vocalizations that we hear from birds.
The complexity of song varies widely among bird species. In some, such as the Superb Lyrebird (Menura novaehollandiae) of eastern Australia (Fig. 10.08), the song is by far the most complex vocalization produced. In other species, however, the song can be less complex than other vocalizations. For example, the “hey‐sweetie” song of Black‐capped Chickadees (Poecile atricapillus) is much simpler than their gargle call.

Fig. 10.08 The Superb Lyrebird (Menura novaehollandiae). Lyrebirds are famous for their vocal mimicry of both biotic (other animals) and abiotic (including chainsaws and car alarms) sounds. Listen online.
(Photograph by Alex C. Maisey.)
Song is often thought of as a behavior of male birds, but females and males both sing in many bird species, especially in tropical habitats. For example, females sing even more than males in the Streak‐backed Oriole (Icterus pustulatus) (Fig. 10.09A) and the Stripe‐headed Sparrow (Peucaea ruficauda) (Fig. 10.09B), both of which are found in Mexico and Central America (Price et al. 2008; Illes and Yunes‐Jimenez 2009). Ways in which female birds use song are covered later in this chapter.

Fig. 10.09 Both sexes may sing. Females sing more than males in (A) the Streak‐backed Oriole (Icterus pustulatus) and (B) the Stripe‐headed Sparrow (Peucaea ruficauda). Both sexes sing in many tropical bird species.
(Photographs by: A, Jay Ailworth; B, Jean‐François Hic.)
Songs are especially prominent among the 4600 or so species in the oscine subgroup of the order Passeriformes (Chapter 2). The oscines, known informally as the songbirds, are renowned for their singing ability. Unlike most other birds, songbirds learn many components of their songs, which therefore can be quite complex. One aspect of this complexity is the number of different song types an individual sings, a number that varies immensely among songbird species. Many songbird species have multiple song types in their repertoires. A European male Common Chaffinch (Fringilla coelebs), for example, may sing up to six different “chip chip chip chooee chooee cheeoo” song types (Slater et al. 1980). Each song type has a distinctive structure and sequence of notes; the differences are visible on spectrograms and are audible to anyone who listens carefully (Fig. 10.10). A male Common Chaffinch typically sings 2 to 15 or so renditions of one song type, but then he switches abruptly to another type, and eventually to another, until he returns to sing, with great fidelity, the initial type (Riebel and Slater 1999). Song Thrushes (Turdus philomelos), another European songbird, are more impressive: each male has about 100 different song types (Ince and Slater 1985). Likewise, Common Nightingales (Luscinia megarhynchos) may sing 100–200 different song types (Kiefer et al. 2006) (Fig. 10.11A). The largest repertoire found so far, however, is that of the Brown Thrasher (Toxostoma rufum) (Fig. 10.11B) of North America. When thousands of spectrograms of one male Brown Thrasher were sorted into different categories, he was estimated to sing well over 2000 different songs (Kroodsma and Parker 1977).
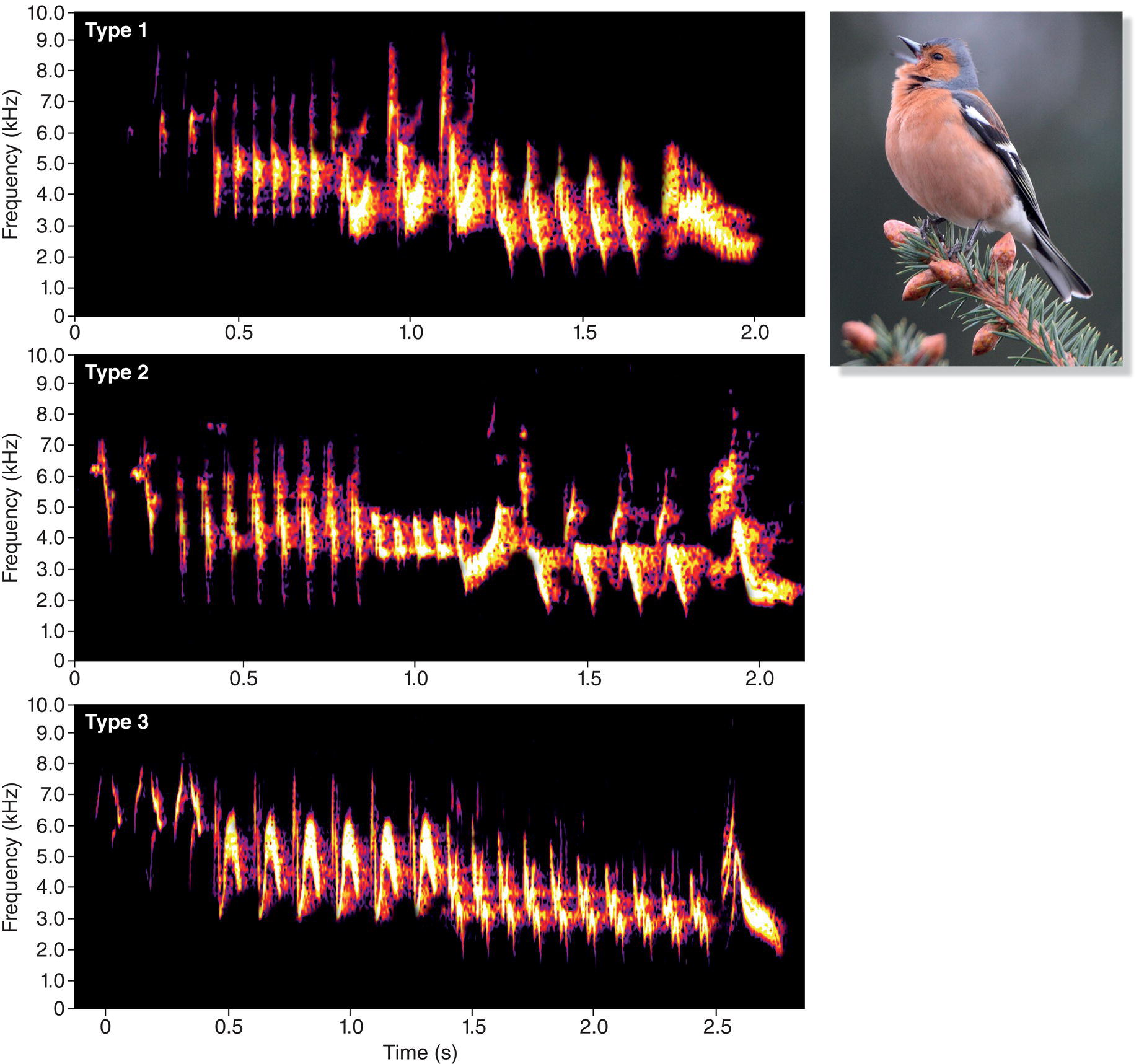
Fig. 10.10 Repertoires. Many species are capable of singing several song types, which together form a repertoire. Compare the spectrograms of three Common Chaffinch (Fringilla coelebs) song types; each has a distinctive structure and sequence of notes. Listen online.
(Recordist: Krzysztof Zyskowski. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Jim Almond.)
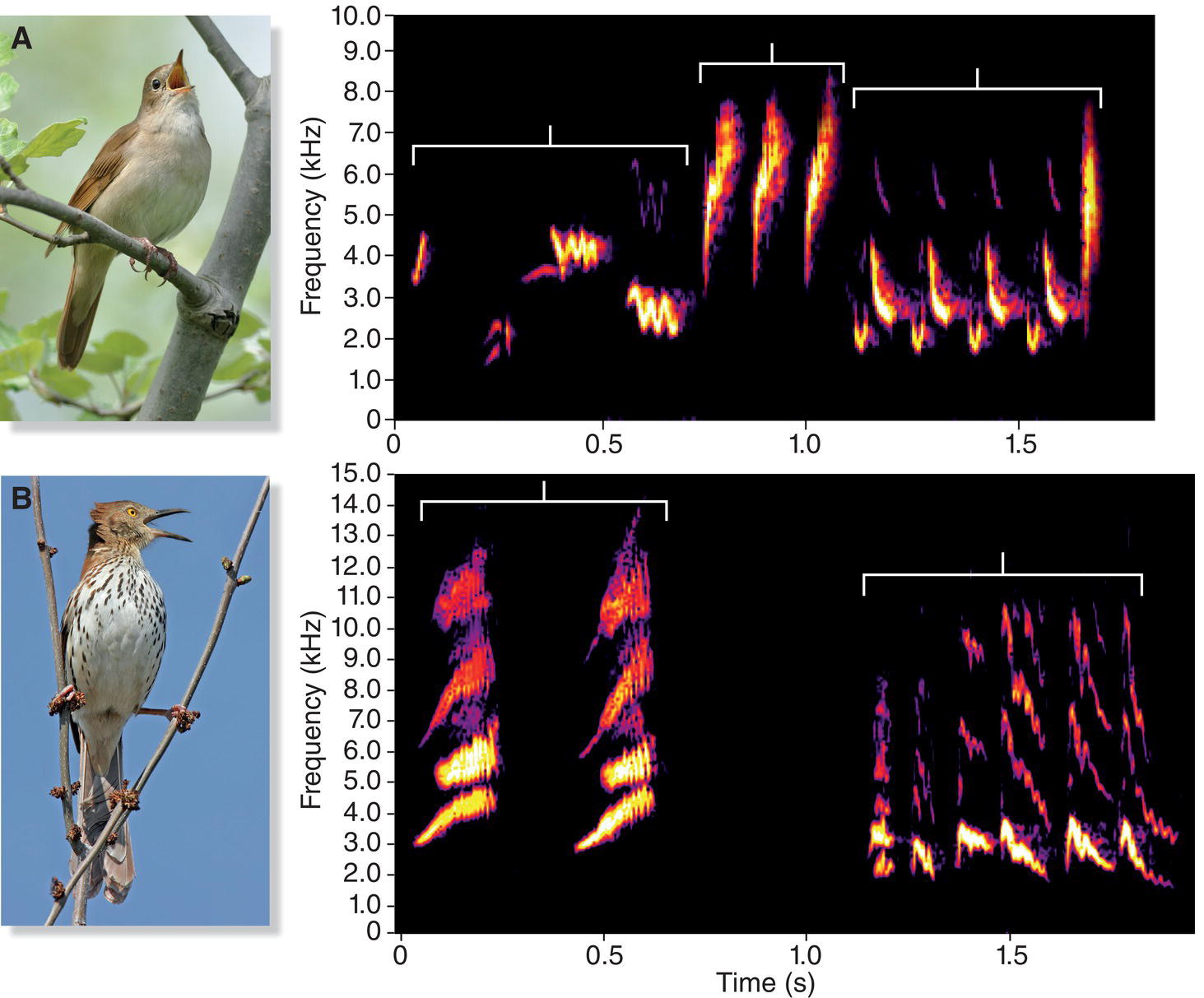
Fig. 10.11 Species with large repertoires. (A) Common Nightingales (Luscinia megarhynchos) can sing hundreds of song types. Each bracket indicates a phrase; together the phrases form a song. (B) Brown Thrashers (Toxostoma rufum) hold the record for the most song types: one male may sing more than 2000 song types. Each bracket indicates a song. Listen online.
(Recordists: A, Arnoud B. van den Berg; B, Wilbur L. Hershberger. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photographs by: A, Wouter Marck; B, Nick Saunders.)
Although songbird song repertoires can be large, songbirds of some species use very simple song repertoires. For example, as noted earlier in this chapter, the Black‐capped Chickadee uses one basic “hey‐sweetie” song. Except for slight differences in frequency, all “hey‐sweetie” songs look basically the same on spectrograms. Other North American species with simple song repertoires include Chipping Sparrows (Spizella passerina), Indigo Buntings (Passerina cyanea), and White‐crowned Sparrows (Zonotrichia leucophrys). Eurasian species with similarly basic repertoires include Redwings (Turdus iliacus), Short‐toed Treecreepers (Certhia brachydactyla), Meadow Pipits (Anthus pratensis), and Willow Tits (Poecile montanus). Members of all these species use one basic song type, and the sequence of notes in their songs remains essentially unchanged from one song to the next.
10.2.2 Calls
The term call is applied to any bird vocalizations that—compared with songs—are shorter, generally simpler, and less likely to function exclusively in mate attraction or territory defense. Although the distinction between calls and songs is not always clear‐cut, in practice a given vocalization usually can be readily classified as either a song or a call. Most bird species have vocal repertoires that include both calls and songs.
Overall, calls play a large role in facilitating the myriad interactions that characterize a bird’s social life. Calls have a variety of communicative functions, with different calls of a species frequently serving different roles (Box 10.04). Contact calls help members of a flock or pair maintain contact with their companions. The flight calls of nighttime migrants probably serve a similar function. Food calls attract offspring or flock mates to a newly discovered source of food. A nestling’s begging calls induce its parents to feed it. Alarm calls warn other birds of approaching predators. Mobbing calls attract other birds to join in harassment of a predator. Aggressive calls help resolve conflicts between birds.
10.2.3 Influences on vocal structure
Bird song researchers often refer to the “structure” of a bird vocalization, by which they mean a combination of all the features that form the sound, many of which can be seen on a spectrogram. These features include the duration of the sound, its main frequency, whether the sound is a relatively pure whistle or reaches across a broad spectrum of frequencies at once, whether individual sound units are repeated, and so on.
To some extent, the structure of a vocalization is dictated by its function. For example, the alarm calls that small birds produce when a hawk flies overhead are usually high, narrow‐bandwidth sounds that begin softly, grow louder, and then fade away. Sounds with these characteristics, such as the high “zee” of many birds, are difficult for a predator to locate (Marler 1955; Klump and Shalter 1984) (Fig. 10.12). In contrast, the mobbing calls that songbirds broadcast as they dive and swoop at a predator often consist of harsh, broadband noise; these sounds are more easily located, thereby revealing the location of the predator and perhaps encouraging other birds to join the mobbing attack (Ficken and Popp 1996) (Fig. 10.13). More generally, sounds associated with aggression are often broadband noise emphasizing low frequencies, whereas appeasing sounds are higher in frequency (Morton 1977).
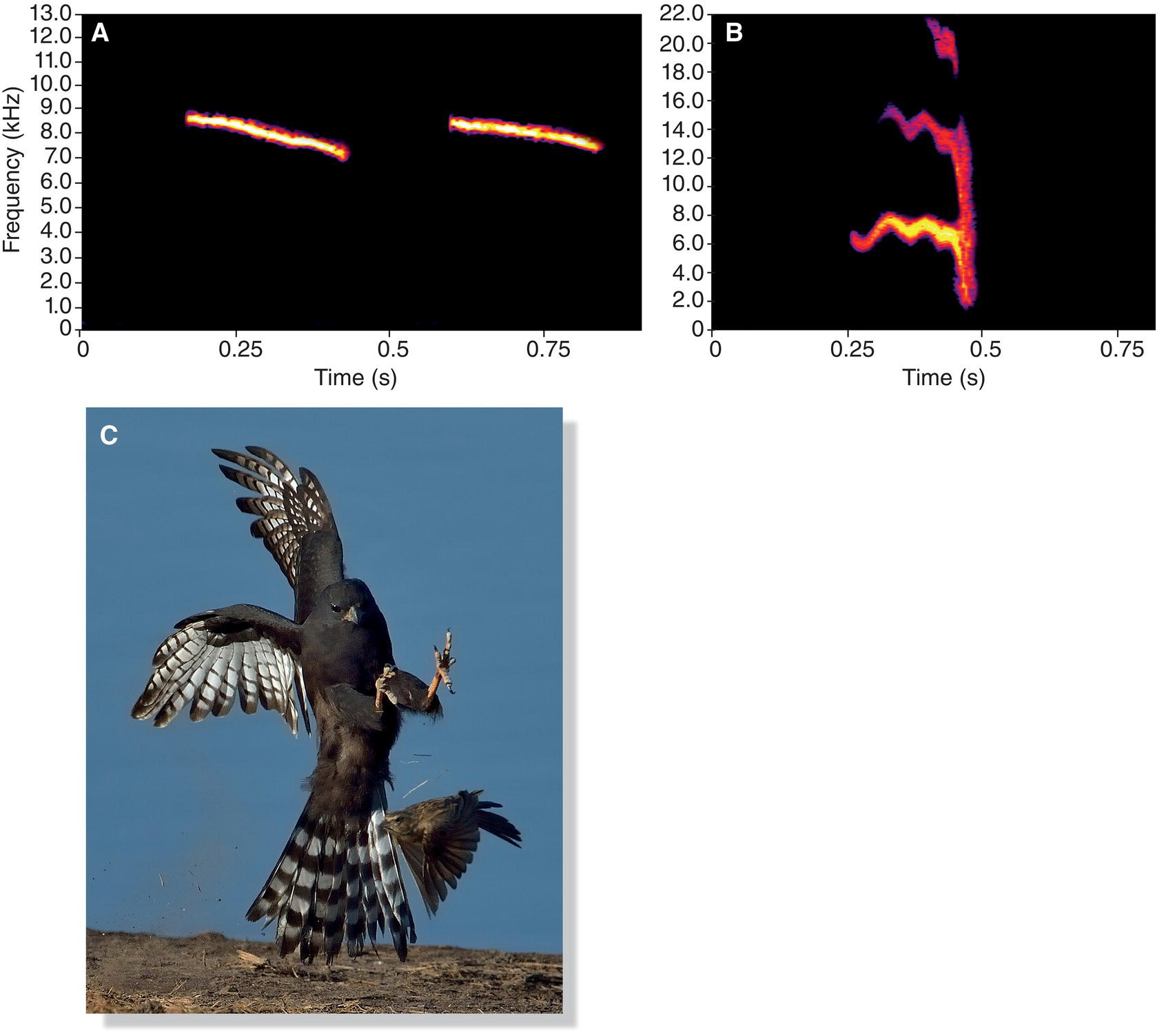
Fig. 10.12 Structure and function of alarm calls. (A) The high frequency and narrow bandwidth of Tufted Titmouse (Baeolophus bicolor) “zee” alarm calls make them difficult for a predator to locate. (B) The “shree” alarm calls of an American Robin (Turdus migratorius) fade from loud to soft and cover a range of frequencies. (C) Aerial predators like this Gabar Goshawk (Micronisus gabar) sometimes hunt by targeting birds with a delayed response to alarm calls, separating them from the flock. Listen online.
(Recordists: A, Wilbur L. Hershberger; B, Matthew D. Medler. Courtesy of Macaulay Library © Cornell Lab of Ornithology. C, photograph by Duncan Blackburn.)

Fig. 10.13 Mobbing. Many songbird species (here, European Starlings, Sturnus vulgaris) will mob—chase and harass—larger birds perceived as a threat (here, an Osprey, Pandion haliaetus). In contrast to alarm calls, mobbing calls are harsh, broadband sounds that reveal the location of the calling birds, presumably to induce others to join the mobbing group.
(Photograph by John Dustan.)
The structure of complex songs may also be influenced by the type of habitat in which a bird sings. The sound characteristics that are optimal for long‐distance transmission of vocal signals vary among different habitats, and song structure may evolve to maximize transmission effectiveness in a particular habitat. For example, songs of the Great Tits (Parus major) that breed in Great Britain differ consistently in dense forests versus more open woodlands; forest songs are simpler, lower in frequency, and contain purer tones (Hunter and Krebs 1979). Similarly, Satin Bowerbirds (Ptilonorhynchus violaceus) living in dense rainforests in Australia use vocalizations that are lower pitched and have fewer trills compared with bowerbirds living in more open habitats (Nicholls and Goldizen 2006).
Bird species that live near the ground often use songs dominated by low‐frequency, fairly pure tones, as reflections off the ground cause sounds that are higher than 1 or 2 kilohertz to transmit poorly. Thus, the low frequency, often tremulous whistles that are conspicuous components of tropical forest soundscapes are, in many cases, voiced by ground‐dwelling birds such as tinamous (Fig. 10.14).

Fig. 10.14 Ground‐dwelling species use low‐frequency vocalizations. In dense forest habitats, ground‐dwelling birds like this Great Tinamou (Tinamus major) have evolved low‐frequency songs, optimized for transmission near the ground. Listen online.
(Photograph by Jacques Erard.)
10.2.4 What do bird sounds mean?
Many of the researchers who document and study the vocal repertoires of birds worry that the way humans classify birds’ signals may not match the classifications used by the birds themselves. Our main tools of analysis, such as spectrograms, are relatively crude ways to picture a sound, showing only selected features of this extremely rich form of communication. The visualized features may be important, but in reality we usually do not fully understand which features the birds themselves really attend to.
In fact, birds may make use of a far greater variety of sounds than we appreciate. Reconsider the Black‐capped Chickadee’s (Poecile atricapillus) seemingly simple “chick‐a‐dee” call (Fig. 10.07A). The initial part of this call consists of four different notes: ‘a’, ‘b’, ‘c’, and ‘d’. Most “chick‐a‐dee” calls contain these four notes, but the birds deliver the notes in different combinations, and with differing number of repetitions, to form hundreds of qualitatively different kinds of calls (Hailman et al. 1985). In addition, each note’s structure is variable, so each different note combination itself has dozens of potential variants.
Do chickadees send different messages with all these different combinations and variations? This is a challenging but intriguing question. Building on an earlier discovery that chickadees produce “chick‐a‐dee” mobbing calls in response to the presence of stationary or perched (but not flying) predators (Ficken et al. 1978), researchers have discovered that the number of notes in a call differs depending on the size of the predator (Templeton et al. 2005). In an experiment, smaller predators such as Northern Pygmy‐Owls (Glaucidium gnoma) and Merlins (Falco columbarius) elicited “chick‐a‐dee” calls that contained more “dee” notes than did the calls produced in response to larger predators such as Great Horned Owls (Bubo virginianus) and Gyrfalcons (Falco rusticolus). Thus, longer calls with more “dee” notes might serve to indicate the greater threat posed by smaller predators (which are more likely than larger predators to prey on small birds such as chickadees). Do chickadees actually use variation in the number of “dee” notes to communicate this kind of information? Perhaps so: when experimenters played recorded “chick‐a‐dee” calls through a hidden speaker, responding birds approached more closely and stayed longer (as if aggressively mobbing) in response to calls with many “dee” notes than to calls with fewer “dee” notes.
Our understanding of the meanings of avian vocalizations to other birds is truly in its infancy. Our current classifications may bear little resemblance to how birds actually hear and use their vocal signals, and we must remember that our ultimate goal is to ascertain how they do this.
10.3 Vocal development
Birds produce a variety of sounds, but how do they acquire the ability to do so? The first sounds made by most newly hatched nestlings are soft peeps, given perhaps in response to siblings or to parents who provide warmth and food. The nestling grows and eventually becomes a fledgling, a yearling, and finally an adult. During this development, the bird’s vocal repertoire also develops, with various vocalizations appearing in turn to enable the growing bird to manage its social environment. How does the bird know each vocalization and when to use it? Are its vocalizations encoded in its genes, to be uttered automatically in the appropriate circumstances? Or is development more complex, with the young bird learning much of what it knows from other individuals, much as young humans learn language from adults?
Birds in a few groups—parrots, hummingbirds, and songbirds—clearly do learn during vocal development. This learning process is intriguing to researchers who have studied it intensively, primarily in songbirds. In all songbirds that have been studied, researchers have discovered some kind of vocal learning. Just as in humans, this learning involves listening to a model sound, memorizing the model, and practicing until the sound matches with great fidelity the young bird’s memory of the original sound.
10.3.1 Song development in songbirds
Among birds, the songbirds are especially diverse. Comprising about 4600 of the world’s roughly 10,000 bird species, they are one of two subgroups within the order Passeriformes (Chapter 2). The songbirds are well known for their complex, often beautiful (at least to human ears) songs (Fig. 10.15). Not all songbirds produce conventionally beautiful songs—consider the unmelodious songs of most species of crows, jackdaws, or ravens—but most songbirds earn their name by singing frequently and conspicuously.
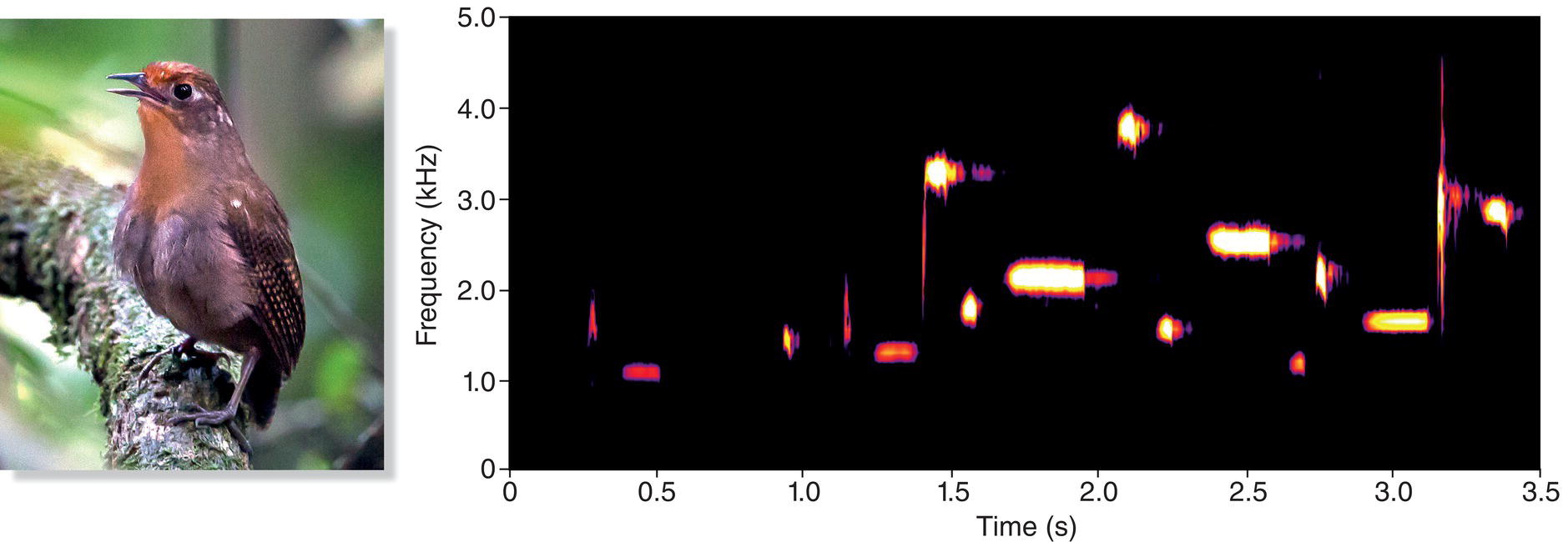
Fig. 10.15 A complex song. The song of the Musician Wren (Cyphorhinus arada) is among the most melodious of bird songs. Listen online.
(Recordist: Curtis A. Marantz. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by João Sérgio Barros Freitas de Souza.)
In the laboratory, we can readily demonstrate the song‐learning potential of developing songbirds. In North America, the White‐crowned Sparrow (Zonotrichia leucophrys) has been a favorite study species for researchers interested in how songbirds learn their vocalizations. When researchers remove a male sparrow from its nest at 8 or 9 days of age and keep him in a laboratory where he cannot hear adult males, he develops a highly abnormal or “isolate” song (Fig. 10.16A). However, a lab‐reared male exposed at an early age to recordings of wild adults will learn to sing the details of the tutor song (Marler and Tamura 1964) (Fig. 10.16B). Regardless of where the young bird was born, he can learn a wide range of White‐crowned Sparrow songs, taken from anywhere in the geographic range of the species (Petrinovich 1985). The youngster’s song matches the song with which he was tutored, detail for detail.
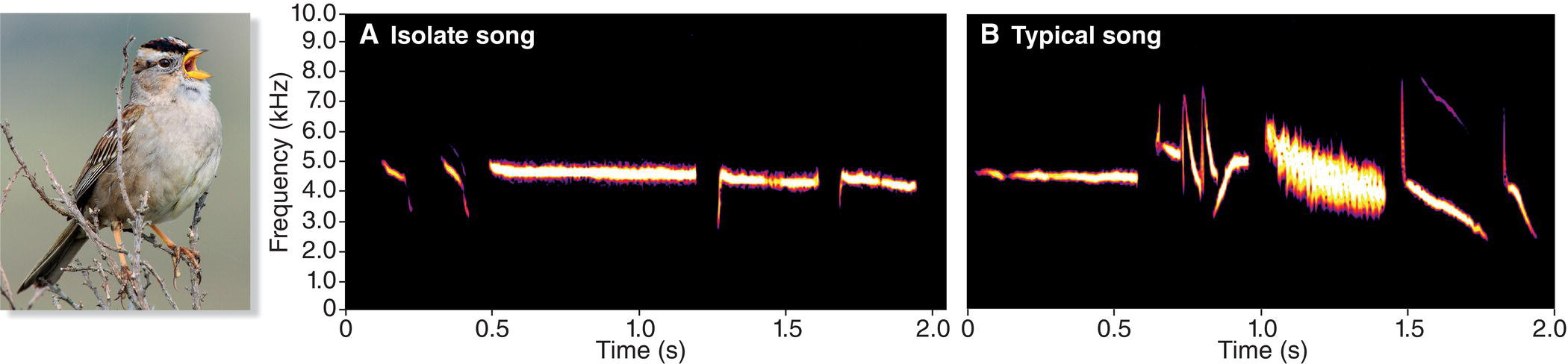
Fig. 10.16 Songbirds learn their songs. (A) White‐crowned Sparrow (Zonotrichia leucophrys) nestlings raised without exposure to adult songs develop abnormal songs. (B) Adult sparrows sing songs that they memorized early in life. Listen online.
(Recordists: A, Masakazu Konishi; B, Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Pat Ulrich.)
Most songbirds have a sensitive period for song learning, a relatively brief time during which they are best equipped to memorize the details of a tutor’s song. For a White‐crowned Sparrow, model songs broadcast from a loudspeaker in the laboratory have the greatest influence from day 15 of a bird’s life to about day 50; the birds have a much more difficult time learning songs heard after day 50 (Marler 1970). If the young bird also is provided with a live bird to interact with, learning after day 50 becomes somewhat easier but never as easy, it seems, as during those early weeks of life (Petrinovich and Baptista 1987). Other songbirds that learn best during a sensitive period early in life include Swamp Sparrows (Melospiza georgiana) (Marler and Peters 1988) and Marsh Wrens (Cistothorus palustris) (Kroodsma and Pickert 1980) in North America; Common Chaffinches (Fringilla coelebs) (Thorpe 1958; Slater and Ince 1982) in Eurasia; and Zebra Finches (Taeniopygia guttata) (Immelmann 1969; Braaten 2010) in Australia. Generally, birds of species with a well‐defined sensitive period develop a repertoire of sounds during their first year of life and then rarely, if ever, modify it in later years.
Some birds, however, are much less constrained by sensitive periods and retain the ability to learn new songs well into adulthood or even throughout their lives. These open‐ended learners include the Northern Mockingbird (Mimus polyglottos) of North America (Derrickson 1987), the Common Nightingale (Luscinia megarhynchos) and European Pied Flycatcher (Ficedula hypoleuca) of Eurasia and Africa (Todt and Geberzahn 2003; Eriksen et al. 2011), the Village Indigobird (Vidua chalybeata) of Africa (Payne 1985), the Saddleback (Philesturnus carunculatus) of New Zealand (Jenkins 1978), and the cosmopolitan European Starling (Sturnus vulgaris) (Eens et al. 1992).
Songbirds are selective in what they learn. A young male White‐crowned Sparrow, for example, does not learn just any bird song it hears. Instead, the young bird tends to imitate only sounds that match, to some extent, his innate knowledge of the characteristics of his own species’ song. If he hears both Song Sparrow (Melospiza melodia) and White‐crowned Sparrow songs, for example, he will learn the White‐crowned Sparrow songs (Marler 1970). This inherent bias toward songs of his own species helps prevent him from mistakenly learning the songs of other species that may be singing in his vicinity. This protection mechanism is not perfect, however. For example, when experimenters housed a young White‐crowned Sparrow only with a singing adult Song Sparrow, the young bird’s physical and vocal interactions with the adult overrode his inborn tendencies, and the White‐crowned Sparrow learned to sing Song Sparrow songs (Baptista and Petrinovich 1986).
10.3.2 Process of song development
The process by which songbirds learn to sing unfolds in stages. A young songbird first memorizes a song, which it can do as early as 15–20 days of age. Days, months, or even a year later, the bird recalls that early memory as it practices singing and tries repeatedly to produce an imitation of what it remembers. Listening carefully to its own practice songs, the bird makes successive corrections to the practice sounds that eventually result in a near‐perfect copy of the remembered song.
Nestlings of many species begin to practice singing shortly after leaving the nest, often at about 3 weeks of age. The earliest practicing, known as subsong, is barely detectable, even by someone listening very carefully from a short distance away. Among birds hand‐raised in the laboratory, subsong often occurs in a fledgling that recently has been fed. The youngster perches, resting comfortably, even appearing to doze with eyes closed and head tilting to one side. All the bird’s systems appear to be completely at rest, but soft whispers, accompanied by barely perceptible throat movements, reveal that inwardly the future singer has begun to practice. If this resting state is disturbed by a sibling (or a curious human), the young bird breaks abruptly from its apparent slumber, becomes fully alert, and halts its practice singing.
As the fledgling grows older, its practice songs gradually become louder, more persistent, and more structured. The sounds begin to resemble the adult song. This transition was evident in a study of the non‐migratory Bewick’s Wren (Thryomanes bewickii) in Oregon (USA) (Kroodsma 1974). In the study population, a typical young male wren left his parents’ territory at 4–5 weeks of age, and a few weeks later was already defending the territory he would hold for the rest of his life. On that territory, the quality of his practice singing gradually improved (Fig. 10.17A) until his songs matched, detail for detail, the songs of his adult neighbors. Although the young male was capable of learning his father’s songs during the first 4 or 5 weeks of his life (when he was cared for by his parents), he instead learned the songs of males adjacent to his later territory (Fig. 10.17B).
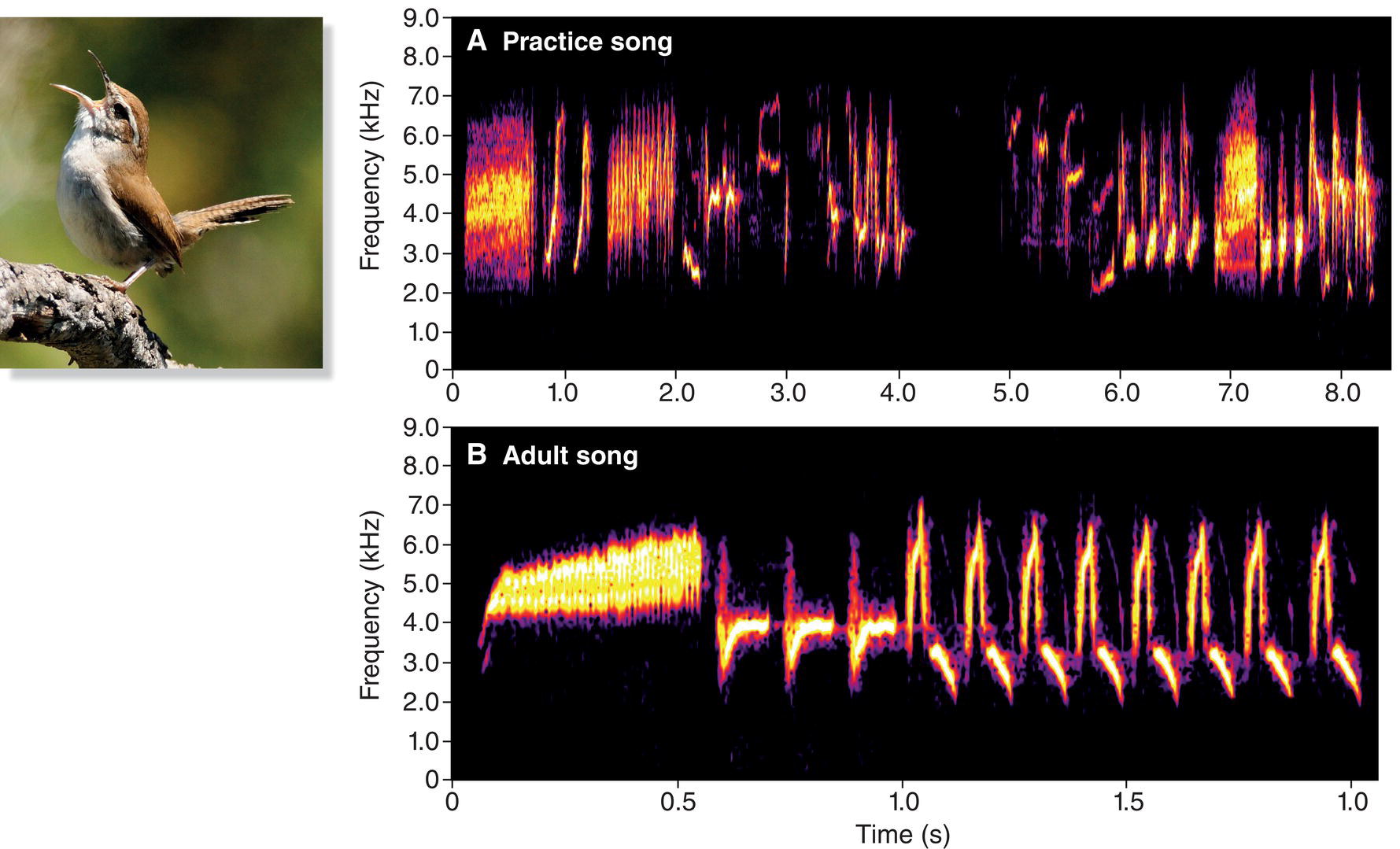
Fig. 10.17 Oscine song development requires exposure and practice. In songbirds, song learning often begins in the nest, but song development is not completed until later. In Bewick’s Wrens (Thryomanes bewickii), newly fledged males choose a territory, where they are exposed to the songs of their neighbors. (A) Young males begin practicing the songs of their new neighbors shortly after dispersing. (B) After much practice, an individual’s song matches those of his neighbors. Listen online.
(Recordist: Donald E. Kroodsma. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Susan Teefy.)
The practice singing (subsong) of a young Bewick’s Wren or other songbird is remarkably similar to the practice speaking (babbling) through which humans progress as toddlers. Each young male Bewick’s Wren must master a vocabulary of about 16 different song types, copied from his adult male neighbors. Eventually, he will be able to choose one of those 16 song types and sing it 20–50 times in succession, giving each rendition crisply and consistently, with no mistakes or wavering. Then he will introduce another of the 16 songs, and eventually another, until slowly, methodically, over several hours, he has worked his way through his entire song repertoire.
But the practicing young wren gets much of this wrong. Like a babbling human toddler, he takes bits of sound out of context and strings them together in a continuous, nonsensical sequence. The sounds lack the crispness and confidence of an adult wren’s vocalizations, and no two attempts at the same sound are alike. Eventually, however, this young wren will be as competent as his father, just as we master spoken language.
Wherever you live, chances are you will be able to hear young birds practicing if you listen very carefully during times of the year when juvenile birds are becoming independent. Practice songs are less structured and more rambling than the adult male song.
10.3.3 Diversity of song development in songbirds
With well over 4000 species of songbirds, trying to generalize about how “the songbird” develops its song is difficult. Perhaps the only valid general statement we can make is that evidence of learning has been found in all songbird species studied to date. But how this learning occurs varies considerably among species.
Natural selection (Chapter 3) has molded the developmental process so that individual birds develop vocalizations that function appropriately in the communication system of their own species. In some cases, this evolutionary process has yielded developmental programs characterized by high‐fidelity imitation. For example, young Swamp Sparrows (Melospiza georgiana) must hear adult songs in order to later produce normal songs of their own, which generally are near‐perfect imitations of the songs they heard earlier (Marler and Sherman 1985). The young sparrows fail to develop normal songs if they are prevented from hearing adult songs to imitate. In other species, however, imitation plays a much smaller role. A laboratory study of young Gray Catbirds (Dumetella carolinensis), for example, showed that they do not require exposure to normal catbird song (Kroodsma et al. 1997). Catbirds isolated from adult songs, even starting at the young age of 8 days, later developed seemingly normal repertoires of hundreds of typical catbird song types (Fig. 10.18).
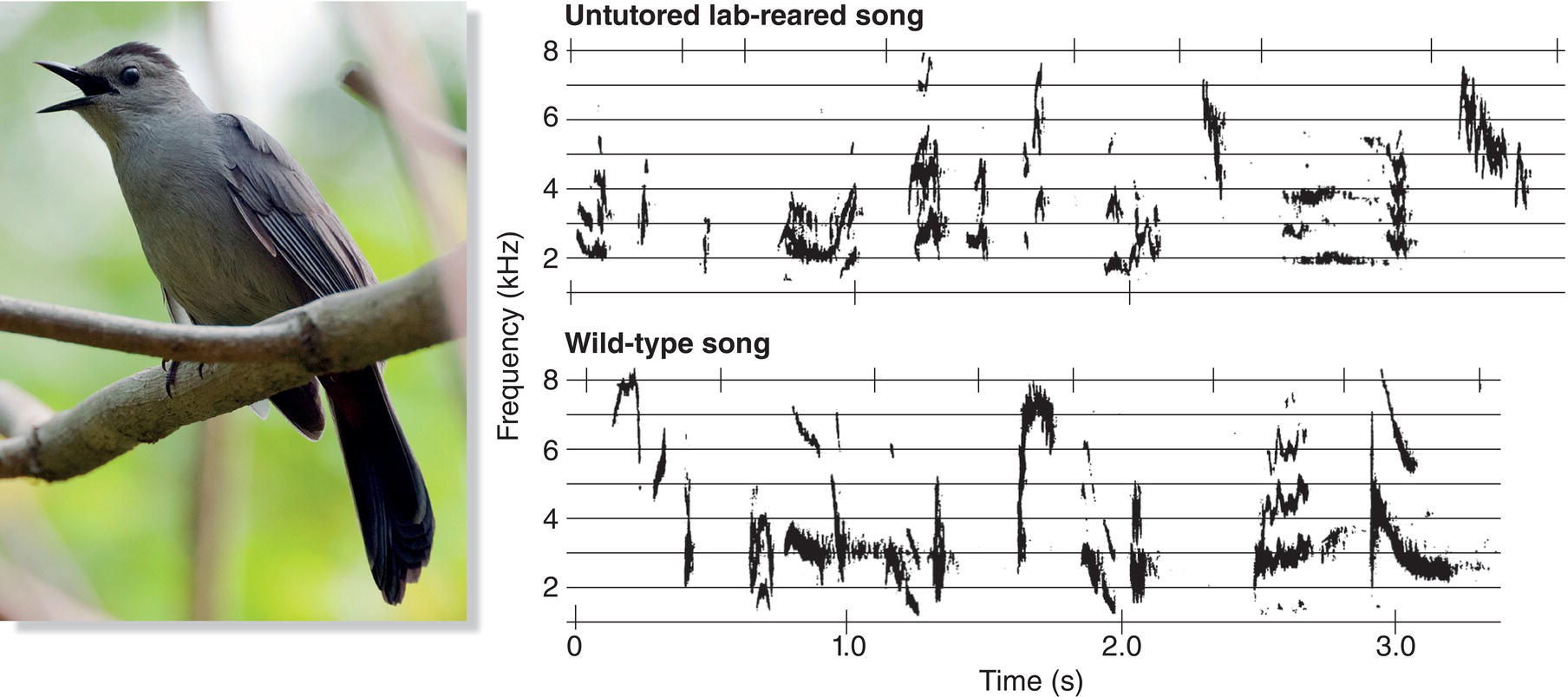
Fig. 10.18 Not all species learn by imitation. Some species, like the Gray Catbird (Dumetella carolinensis), do not require exposure to adult song. Compare the songs of an untutored, lab‐reared male (above) to those of a wild bird (below). The untutored bird sings a complete wild‐type catbird song without exposure to conspecific recordings.
(From Kroodsma et al. 1997. Reproduced with permission from Elsevier. Photograph by Michael Hogan, hoganphoto.com.)
Nevertheless, observations in the laboratory and of wild birds suggest that Gray Catbirds can imitate the songs of both their own and other species, so vocal learning certainly does occur in catbirds; however, imitating other adult catbirds does not dominate the song development process as it does in many other species. Overall, we can picture song developmental programs as falling along a continuum, from species at one extreme that acquire songs strictly by precise imitation during an early sensitive period, to species at the other extreme that rely almost exclusively on invention and improvisation of songs that continues throughout life. Between these extremes lies a range of developmental programs that vary in the relative importance and fidelity of imitation, in the timing of and limits on song acquisition, and in the number of different songs acquired.
Why do young birds of some species imitate their neighbors so perfectly, while the young of other species improvise unique repertoires? To better understand these differences, we need to know something about the social life of the species in question and how they use songs during social interactions.
In a number of studies of how songbirds acquire and use songs, young birds have been found to be especially good at learning the songs of other adults in their immediate neighborhood. For example, research on Indigo Buntings (Passerina cyanea) in Michigan (USA) has shown that these birds live in small “neighborhoods” in which the resident males share the same song type (Payne 1981, 1983). Young males and females typically breed in neighborhoods other than the one in which they were hatched. When a young male settles on a territory during his first breeding attempt, he usually has an odd song, one that is unique to him and that typically bears little resemblance to his father’s song. Over time, however, he changes his song to match the song details of an immediate territorial neighbor, thereby perpetuating the local neighborhood of songs (Fig. 10.19). A young male Indigo Bunting must gain some social advantage by using the same song type as his immediate neighbors, and his song development program ensures that he is able to sing the local shared song type.
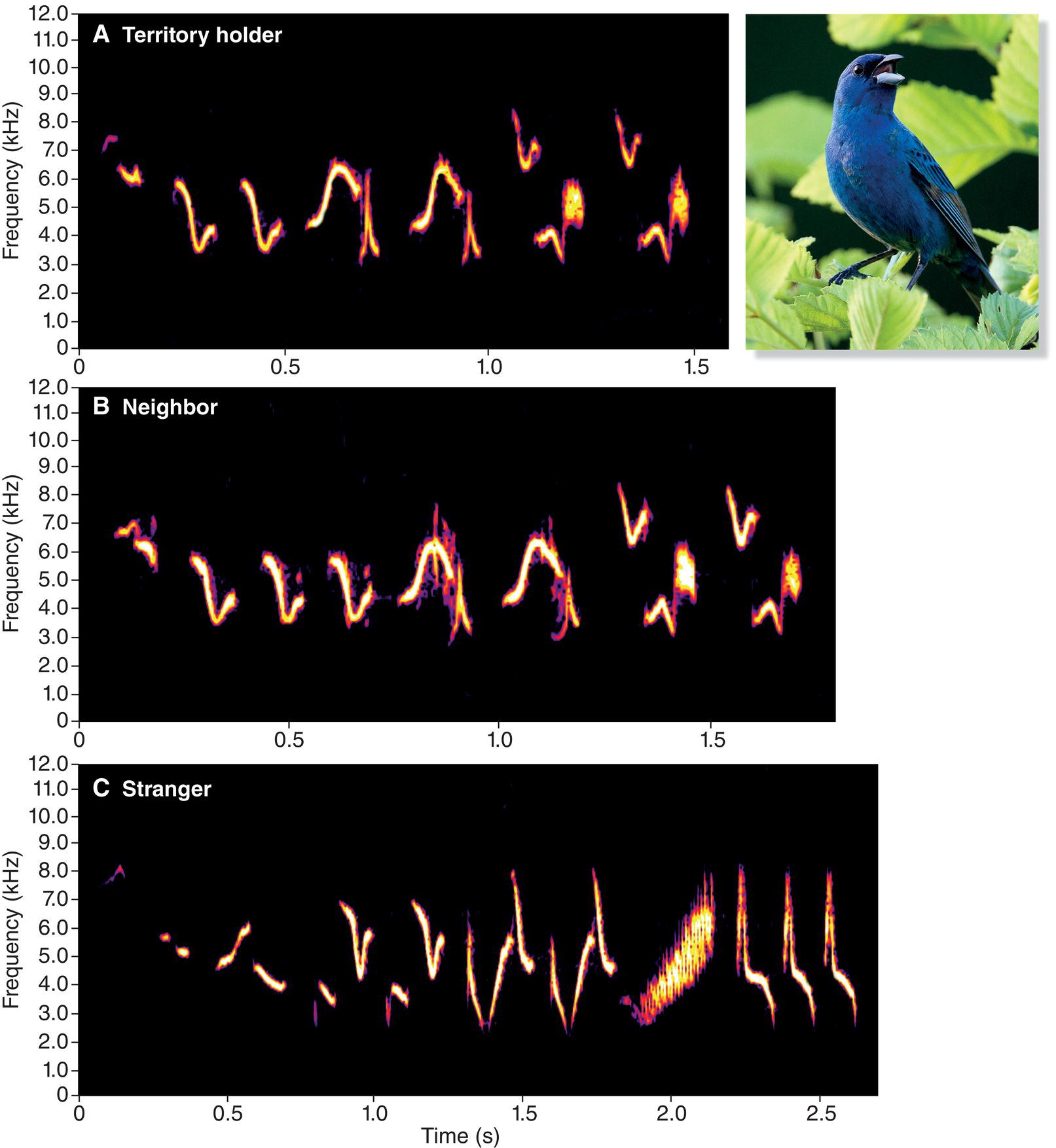
Fig. 10.19 Local dialects and song development. Young male Indigo Buntings (Passerina cyanea) gradually transition from singing song components developed on their natal territories to songs that match the details of their territorial neighbors. Compare the virtually identical songs of (A) one territorial adult and (B) his neighbor with (C) a conspecific recorded in a different neighborhood. Listen online.
(Recordists: A and B, William W. H. Gunn; C, Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Mike Martin.)
Young Song Sparrows (Melospiza melodia) develop their songs in much the same fashion as Indigo Buntings, but the process is more complex because each sparrow has a repertoire of 8–10 different song types. In non‐migratory populations, young male Song Sparrows do not settle down immediately, but rather spend some time late in their hatch year ranging over an area that includes the territories of four or five adults (Arcese 1989). In a well‐studied population in Washington State (USA), a young male learns songs from the adults in his small neighborhood and seems to follow two rules in his learning process (Beecher 2008). First, each song type he acquires is a complete song copied from a particular bird; unlike birds of some other species, a young Song Sparrow does not create amalgamated songs by linking together pieces from different songs or different males. Second, he preferentially learns the song types most common in his neighborhood, so if all five adults in the neighborhood share a particular song type, the youngster is especially likely to learn it; songs unique to a single adult are far less likely to be learned (Fig. 10.20).
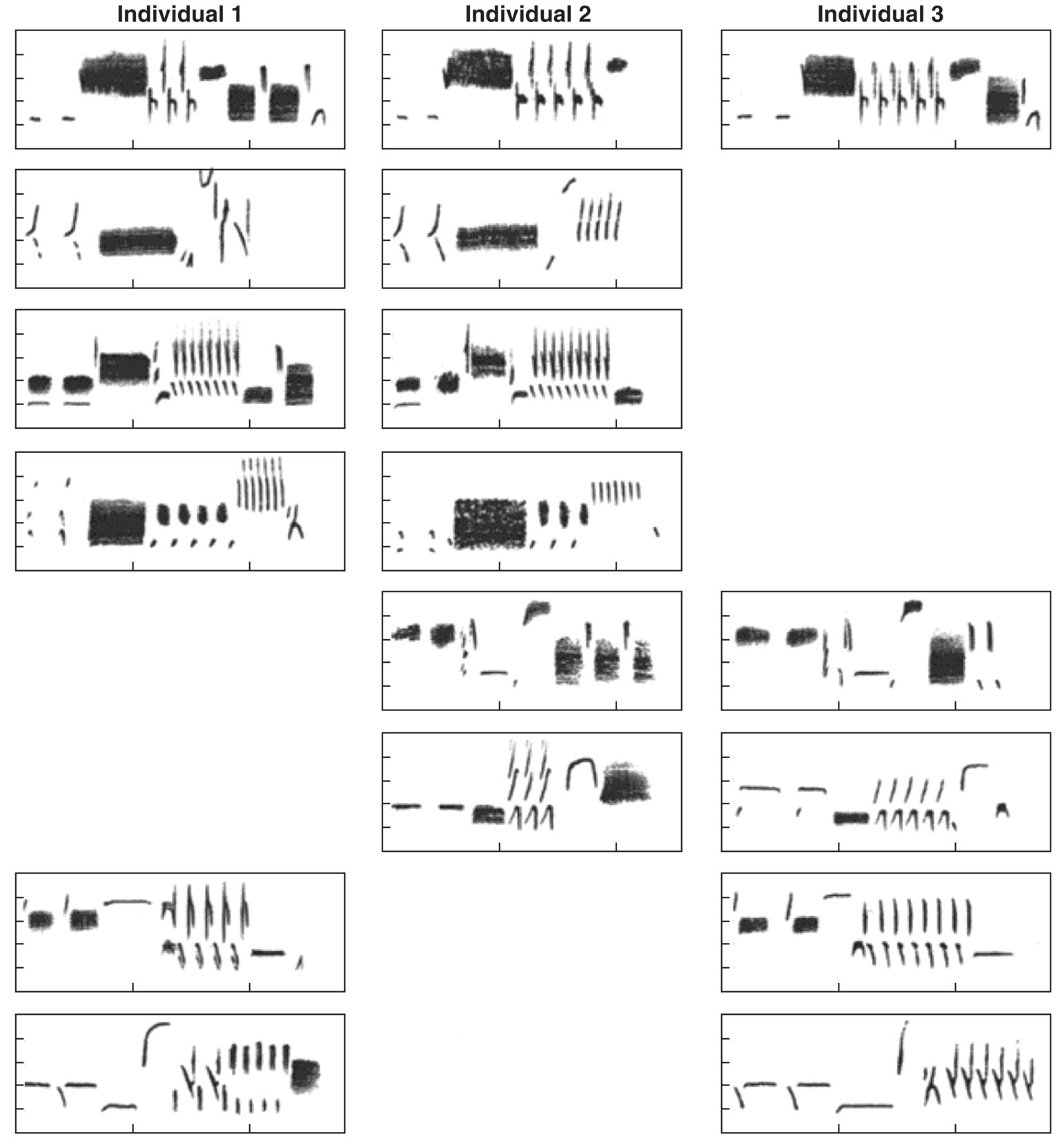
Fig. 10.20 Song learning can lead to song type sharing. Young male Song Sparrows (Melospiza melodia) acquire repertoires of several song types by imitating their neighbors. After establishing a territory, they learn some of the common neighborhood song types, thus ensuring that they share some song types with all or most of their territorial neighbors. The three columns in the figure contain the song repertoires of three neighboring birds. Rows show similar (shared) song types.
(From Beecher 2008. Reproduced with permission from Elsevier.)
These rules for learning maximize the chance that a Song Sparrow male settling in for his first breeding season will share some song types with all or most of his neighbors. Sharing song types with neighbors enhances a Song Sparrow’s ability to interact with and influence other birds in his social environment, in part because male Song Sparrows in this population resolve conflicts by means of a communication system that requires that a singer selectively use song types of varying degrees of similarity to those used by his opponent (Beecher et al. 1996; Burt et al. 2001).
A comparison of Marsh (Cistothorus palustris) and Sedge (Cistothorus platensis) Wrens provides an additional illustration of how song development programs ensure the acquisition of a signal repertoire appropriate to a species’ communication system. Laboratory studies have shown that Marsh Wrens acquire their large song repertoires (more than 100 song types per male) by imitation (Kroodsma and Pickert 1984; Kroodsma 1986). In western North America, many Marsh Wren populations are resident year round and, as in the Song Sparrows of Washington State, imitation tends to yield neighbors with similar song repertoires. But in western Marsh Wrens, the vocal similarity between neighboring males is more extreme, and neighbors may have nearly identical repertoires. They often use these repertoires to perform a display called matched countersinging, in which two males engage in a kind of call‐and‐response battle of matching songs (Verner 1976). A bout of matched countersinging might start with one male singing song type A, which his neighbor counters with A; the first male immediately switches to type B, and the neighbor replies with B; type C is followed by C, and so on through many dozens of song types. Successive song types in the exchange are different enough that a human listener can readily distinguish them and follow the matching interplay (Fig. 10.21).

Fig. 10.21 Matched countersinging. Male Marsh Wrens (Cistothorus palustris) engage in singing contests in which one male matches each song type sung by the other, while cycling rapidly through multiple song types. Listen online.
(Photograph by Ron Dudley.)
In contrast to Marsh Wrens, Sedge Wrens in North America generally do not engage in this intricate countersinging. They communicate not with matching song types but with generalized songs that declare their identity as a “Sedge Wren.” In fact, matched countersinging usually is impossible for Sedge Wrens in North America, because no two males in a population share identical song repertoires (Kroodsma and Verner 1978). This lack of song‐type sharing is related to the Sedge Wren’s different mode of song development. In the laboratory, Sedge Wrens do not imitate songs the way Marsh Wrens do, but rather they mostly improvise, with each bird inventing 100 or more of its own song types (Kroodsma et al. 1999a).
Why have Sedge Wrens evolved improvisation and unshared song types, whereas closely related Marsh Wrens evolved imitation and matched countersinging? These differences may stem from differences in the two species’ lifestyles. The Sedge Wrens of North America are somewhat nomadic, unpredictable in their breeding locations in different years and sometimes moving even within a year. As a result, neighboring males are often strangers to one another and are not involved in the kind of long‐term social relationships that favor communication with matching sounds. In these circumstances, improvisation may be the most effective mode of developing a repertoire of many song types. In contrast, Marsh Wrens resident in western North America live in stable communities whose members interact with one another repeatedly over many months or years. Such repeated interactions with the same individuals may foster communication with shared identical songs, which in turn favors careful imitation of those songs.
A connection between social stability and song imitation is also apparent in Sedge Wren populations resident in the tropics. Unlike their nomadic North American relatives, tropical Sedge Wrens are non‐migratory and remain on their territories year round. Research on resident Sedge Wrens in Costa Rica and Brazil has shown that, in contrast to the absence of song‐type sharing among North American Sedge Wrens, neighboring males in those places tend to share many song types, an indication that the birds have learned their songs through imitation of their neighbors. Birds in Brazilian populations even exhibit matched countersinging, a sure sign of song imitation (Kroodsma et al. 1999b, 2002).
10.3.4 Song development in suboscine passerines
We can better appreciate song development in songbirds by understanding how songs develop in other bird groups. Consider the close relatives of the oscine songbirds, the suboscine passerines. Suboscines and oscines together form the order Passeriformes (Chapter 2). Only a relative handful of suboscine groups—such as the broadbills and the pittas—live in the Old World. About a thousand suboscine species, however, reside in the New World, including groups such as the antbirds, antpittas, woodcreepers, cotingas, and flycatchers.
The striking differences in song development between the oscine songbirds and suboscines are clearly illustrated by studies of song development in two very similar species found in eastern North America, the Alder (Empidonax alnorum) and Willow (Empidonax traillii) Flycatchers. These two species are essentially identical in appearance, and the only reliable way to identify them in the field is by their songs and calls: the Alder Flycatcher sings a song that sounds like “fee‐BEE‐o” (Fig. 10.22A), and the Willow Flycatcher sings a “FITZ‐bew” song (Fig. 10.22B).
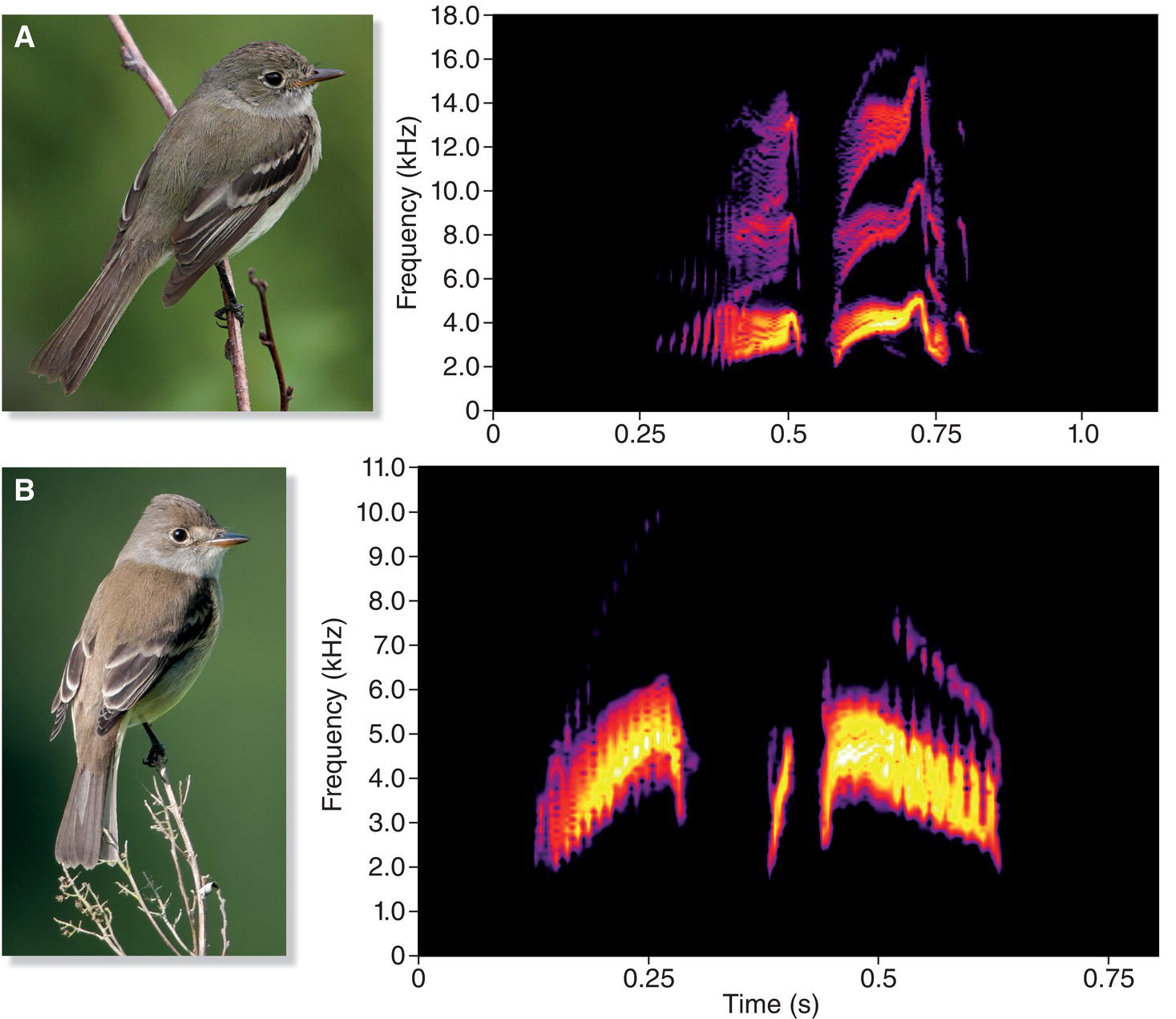
Fig. 10.22 Suboscine song development. In contrast to oscines, suboscine passerines typically develop normal songs without learning or experience. For example, (A) Alder Flycatchers (Empidonax alnorum) and (B) Willow Flycatchers (Empidonax traillii) develop songs typical of their species even when exclusively exposed to songs of a different species as young birds. Although the two species look very similar, their songs are quite distinct. Listen online.
(Recordists: A, Michael J. Andersen; B, Wilbur L. Hershberger. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photographs by: A, Simon Pierre Barrette, http://en.wikipedia.org/wiki/File:Empidonax_alnorum_CT2.jpg. CC‐BY‐3.0; B, Wes Aslin.)
In an experiment to determine how these suboscine flycatchers develop their songs, young Alder and Willow Flycatchers were taken from their nests at about 10 days of age and moved to the laboratory (Kroodsma 1984). The young Willow Flycatchers were then trained with Alder Flycatcher songs played over loudspeakers, and Alder Flycatchers were trained with Willow Flycatcher songs. Although the young Willow Flycatchers heard only Alder “fee‐BEE‐o” songs, and the young Alder Flycatchers heard only Willow “FITZ‐bew” songs, the birds were not confused. Unlike songbirds, these young flycatchers developed perfectly normal songs of their own species. The young flycatchers did not imitate the songs they heard, and their experience with the wrong songs caused no problems.
In the wild, young Alder Flycatchers usually remain near each other in a small family flock during their first few days out of the nest. If the fledgling brothers and sisters get separated, they use a particular vocalization as a contact call, as if to keep tabs on each other and perhaps announce their location to their parents, who are still feeding them. This call is an unmistakable, although scratchy and uncertain, rendition of what will eventually become the adult song, the “fee‐BEE‐o.” Thus, as soon as young Alder Flycatchers leave the nest, they are already uttering what clearly will become their adult song. In the laboratory, the process is the same: a young bird that has just left the nest gives this call repeatedly when separated from its siblings. This call is already being used at 14 days of age, the age at which young songbirds are just beginning to memorize the sounds they will later produce.
An additional experiment shows just how different suboscine flycatchers are from songbirds (Kroodsma and Konishi 1991). Eastern Phoebes (Sayornis phoebe) were brought into the laboratory at 10–12 days of age, before the birds were old enough to sing. The young birds were anesthetized and surgically deafened. The deafened birds developed normal song, even though they could not hear themselves practice. In contrast, songbirds must hear themselves vocalize in order to compare their practice sounds with their remembered model sounds. Phoebes, with their innate songs, do not require this kind of auditory feedback.
Eastern Phoebes, Alder Flycatchers, and Willow Flycatchers are only three of the more than 1000 species of suboscine birds, so generalizing from them to the whole suborder might be unwise. Nonetheless, clues from other suboscine species, such as the contact calls used by young birds and the lack of geographic variation in song form, suggest that the song development of most suboscines may resemble that of these flycatchers. At the same time, however, research has revealed evidence of song learning in at least one suboscine species, the Three‐wattled Bellbird (Procnias tricarunculatus) (Fig. 10.23). These bellbirds are rather long‐lived—wild bellbirds can live more than 20 years—and careful study of marked birds in Costa Rica has shown that individuals alter their songs from year to year, with all the birds in an area making the same change in concert (Kroodsma 2005). This observation strongly suggests that the birds are copying songs from one another, although no one knows which bird first changes its song, or why.
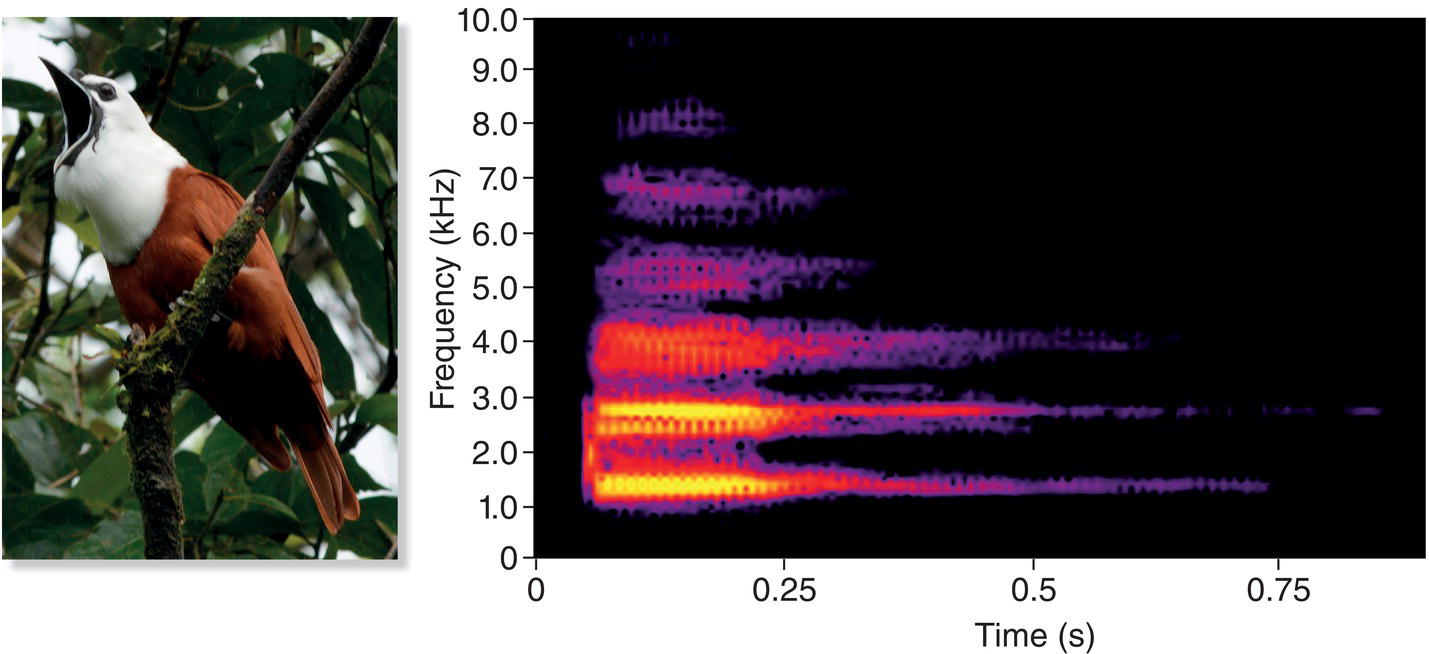
Fig. 10.23 Some suboscines may learn their songs. Long‐lived Three‐wattled Bellbirds (Procnias tricarunculatus) modify their songs from year to year. These changes occur in concert among all birds in a population, strongly suggesting that the birds are imitating one another. Bellbird songs contain some incredibly loud elements. Listen online.
(Recordist: David L. Ross, Jr. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Eduardo Chang.)
10.3.5 Vocal development in non‐passerines
Vocal development has been less well studied in the other orders of birds. Extensive vocal learning seems to occur in only two other orders, the parrots (order Psittaciformes) and the hummingbirds (order Apodiformes).
Parrots, of course, are renowned for their ability to imitate human speech in captivity, and captive parrots of some species, such as Budgerigars (Melopsittacus undulatus), modify their calls to match those of their mates or other flock members (Farabaugh et al. 1994; Hile et al. 2000). In addition, captive Budgerigars have been induced to imitate calls played through a loudspeaker (Manabe et al. 2008). Experiments have shown that wild parrots also may imitate the vocalizations of other individuals of their species. For example, in the Galah (Eolophus roseicapilla), an Australian cockatoo, many wild birds responded to playback of unfamiliar calls by using calls that changed steadily over successive utterances, becoming progressively more similar to the playback call (Scarl and Bradbury 2009). Wild and captive Orange‐fronted Parakeets (Eupsittula canicularis) in Costa Rica also changed their calls in response to playback, but in this species the changes sometimes made the calls increasingly similar to the playback, sometimes increasingly dissimilar, and sometimes by turns increasingly similar and dissimilar (Balsby and Bradbury 2009). Both parrot species clearly alter their vocalizations in response to the vocalizations they hear, and many of the changes are imitative. What is the function of these rapid vocal changes? Perhaps the ability to modify calls quickly to match (or “anti‐match”) other birds facilitates the social interactions and negotiations that ensue when a flock encounters another, perhaps unfamiliar, flock.
Vocal imitation by hummingbirds was first demonstrated in California (USA) by raising young Anna’s Hummingbirds (Calypte anna) (Fig. 10.24A) in the laboratory (Baptista and Schuchmann 1990). In this experiment, the songs of a hand‐reared hummingbird that had been raised in isolation were compared with the songs of three other hand‐reared birds, one of which had been housed with singing adults for 8 weeks before being moved to live with the other two youngsters. The isolated bird ultimately developed only highly abnormal songs, but the songs of birds in the other group were fairly normal and very similar among group members, suggesting that they had imitated one another. Additional evidence of song imitation comes from the existence of hummingbird dialects, in which birds in an area sing similar songs that differ from those of birds in other areas. For example, among Sparkling Violetears (Colibri coruscans) in Ecuador, the songs of territorial neighbors are very similar, but the songs of non‐neighbors are not, and each bird’s songs are more similar to those of birds in its geographic area than to those of birds that live farther away (Gaunt et al. 1994) (Fig. 10.24B). This kind of song‐type clustering caused by imitation also has been documented in Wedge‐tailed Sabrewings (Campylopterus curvipennis) in Mexico (González and Ornelas 2009). There, a breeding group of sabrewings was found to be arranged in three adjacent “song neighborhoods,” each with it own distinctive songs. The song neighborhoods persisted for at least 4 years, suggesting that a neighborhood’s song types might be passed to successive generations by song learning.
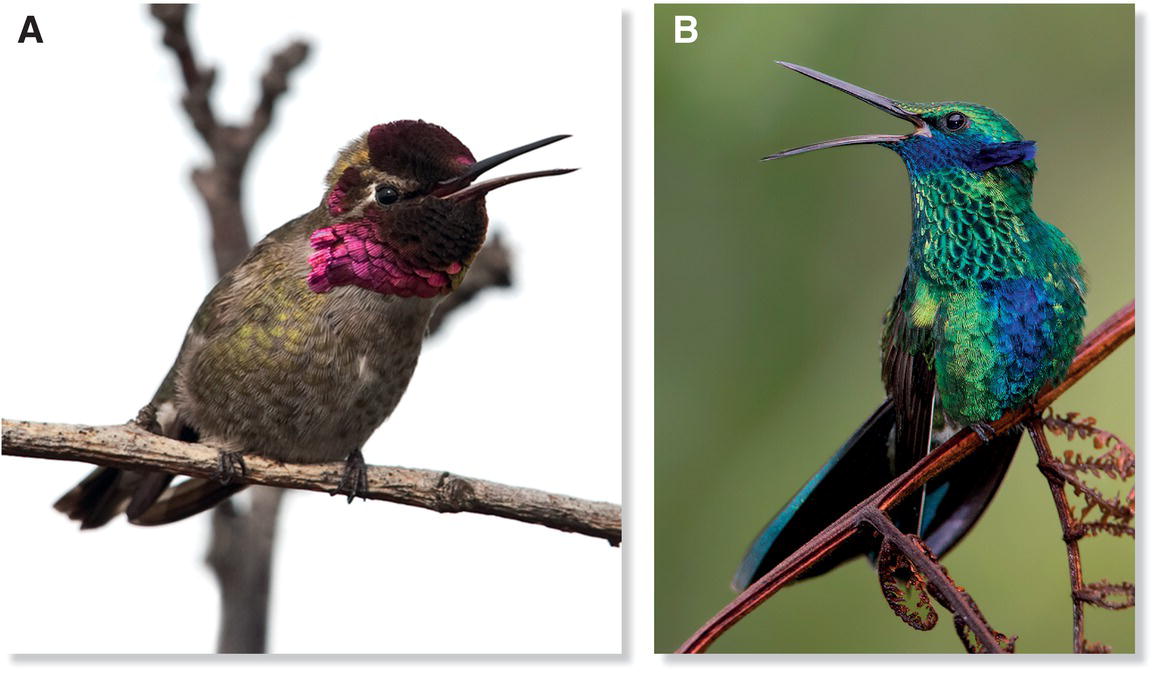
Fig. 10.24 Hummingbirds learn their songs. (A) Anna’s Hummingbirds (Calypte anna) and (B) Sparkling Violetears (Colibri coruscans) acquire their songs through imitation. Many hummingbird species have regional dialects, an indicator of song learning.
(Photographs by: A, Johanna van de Woestijne; B, George Scott.)
Vocal development in other groups of birds is more like that of suboscines and seems to involve little, if any, imitation. Doves and pigeons, for example, develop normal vocalizations without learning them from adults (Nottebohm and Nottebohm 1971). In addition, the hybrid offspring of mating between members of different dove species produce vocalizations intermediate to those of the two parents, again suggesting that vocal development is encoded in the genes and does not involve imitation of adults (Lade and Thorpe 1964). Similarly, hybrid offspring of two Common Quail (Coturnix coturnix) subspecies with different crowing calls produce intermediate calls (Derégnaucourt 2010). Roosters (male domestic chickens, Gallus gallus) develop normal crowing calls even if they cannot hear, suggesting that no vocal learning is taking place (Konishi 1963). Although researchers have studied vocal development in relatively few non‐passerine groups, most believe that vocal learning is unlikely to be found outside of the passerines, hummingbirds, and parrots.
10.4 Production and control of song
A bird may produce a variety of calls and sometimes hundreds or even thousands of different songs. How are these vocal sounds produced? How does the brain acquire and store knowledge of them? These questions have inspired substantial research and some fascinating discoveries.
10.4.1 Sound production apparatus
The organ that birds use to produce vocal sounds is known as the syrinx (Fig. 10.25). This avian equivalent of the mammalian voice box lies within a bird’s chest, located at the junction where the trachea (windpipe) splits to form the two tubular bronchi that lead to the lungs (Chapter 6).
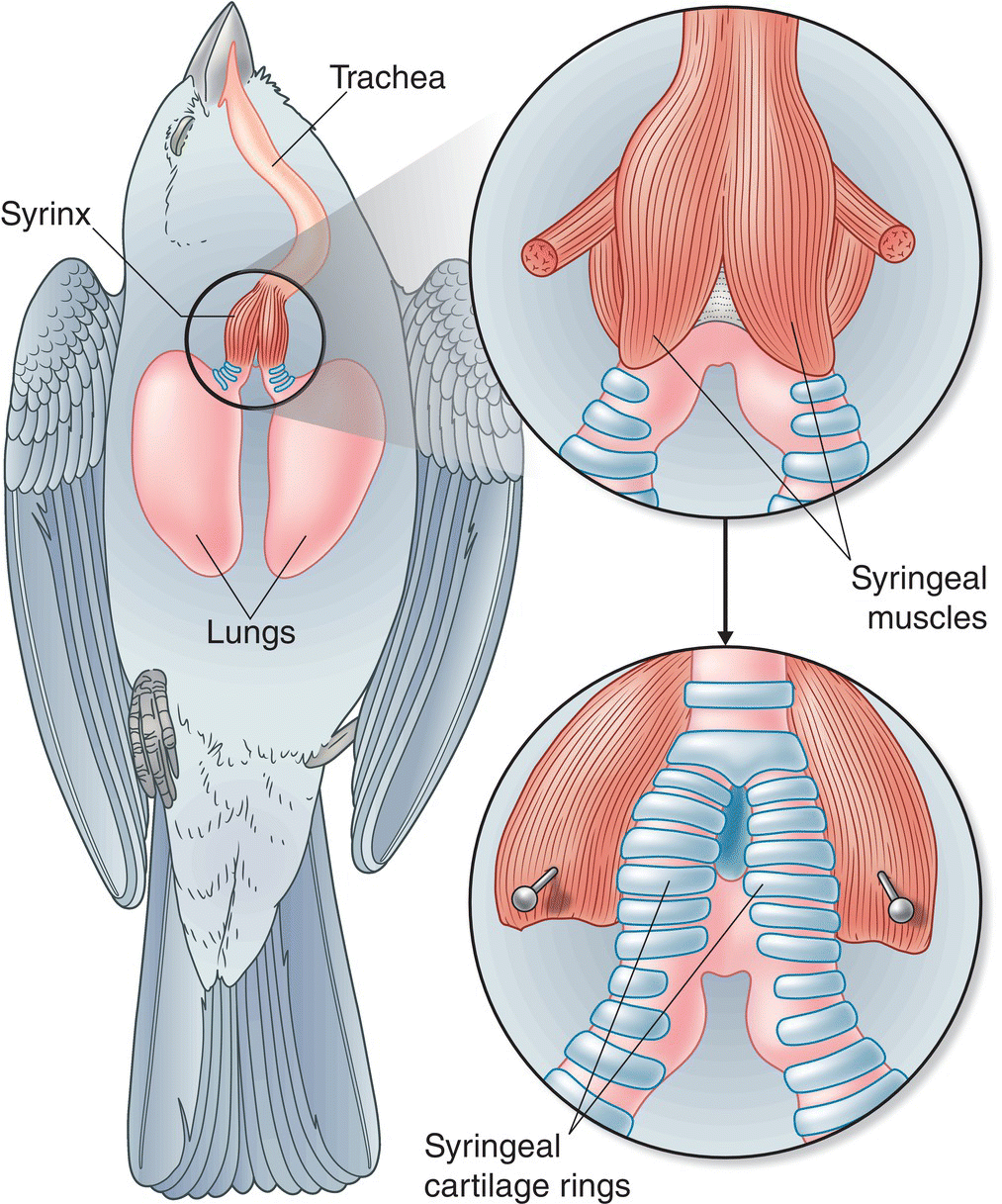
Fig. 10.25 The syrinx. Situated near the junction of the trachea and the bronchi, the avian voice organ typically consists of a pair of chambers held open by rings of cartilage. Songbirds can control the muscles on each side of the syrinx independently, allowing them to produce two sounds simultaneously.
(Adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology.)
The details of the syrinx’s structure differ among groups of birds (Chapter 6), but, as you might expect, the anatomical complexity of the syrinx is greatest in the songbirds. In the songbirds, the syrinx includes small pads of tissue, known as labia, located in each bronchus. Six pairs of muscles in the syrinx control the movement of the labia, and when the labia are extended into the bronchial cavity, exhaled air passing over them causes them to vibrate. These vibrations are the source of the sound waves that constitute bird vocalizations. The pitch, timing, and other aspects of the sound are altered and modulated by movements of the labia, trachea, and beak, which change the rate of airflow through the syrinx and the shape of the space that the sound waves pass through on their way to the outside environment.
The multiple muscles of the songbird syrinx provide very fine control of the sounds produced, making possible the extraordinary vocal gymnastics of many species. In addition, the songbird nervous system controls the muscles on each side of the syrinx independently. Thus, the two sides can produce different sounds at the same time. Some bird songs clearly demonstrate this dual control of the two sides of the syrinx. In the songs of the Wood Thrush (Hylocichla mustelina) of North America, for example, one side of the syrinx produces the higher pitched sounds and the other side produces the lower pitched sounds (Borror and Reese 1956); in essence, a Wood Thrush can sing a duet with itself. Other species in which researchers have found vocalizations generated by a similar “two voice” mechanism include the Brown‐headed Cowbird (Molothrus ater), domestic Island Canary (Serinus canaria), Brown Thrasher (Toxostoma rufum), and Australasian Magpie (Gymnorhina tibicen) (Suthers and Zollinger 2004; Suthers et al. 2011) (Fig. 10.26).
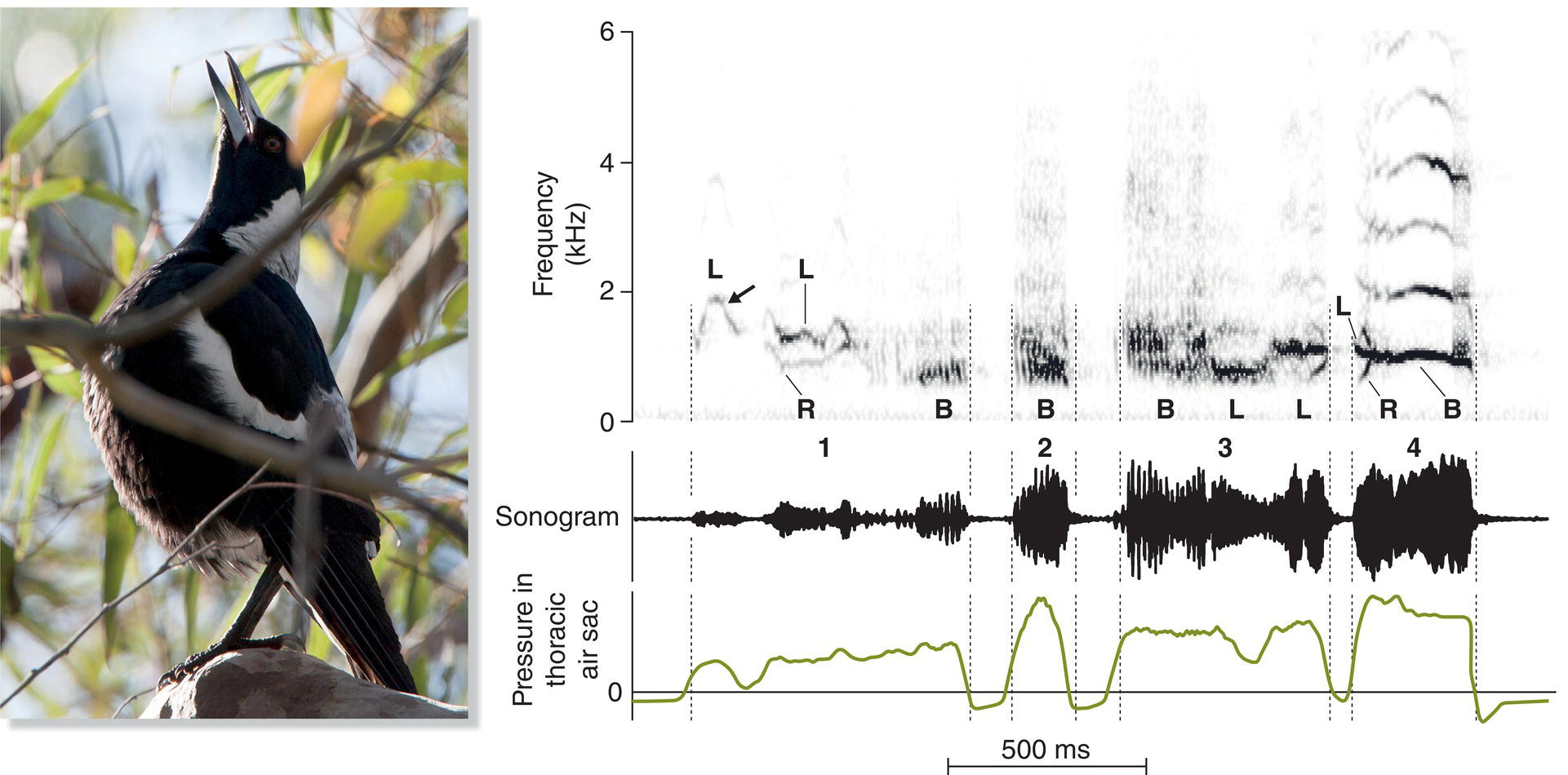
Fig. 10.26 Dual syringeal control. Australasian Magpie (Gymnorhina tibicen) songs include sounds produced using both sides of the syrinx together (B), as well as by the left (L) or right (R) syrinx alone. The high variability in each of the song syllables (labeled 1–4) relates to changes in the thoracic air‐sac pressure before and after syllable production, as new air passes through the thoracic sac before reaching the lungs. Listen online.
(From Suthers et al. 2011. Reproduced with permission from Springer Science + Business Media. Photograph by Thomas Cullen.)
10.4.2 Neural basis of song perception and production
One of the great success stories in contemporary neuroscience has been the identification of songbird brain pathways involved in learning and producing songs. As a result of this success, songbirds (or at least a few species of songbird) have become important model systems for studying the neural basis of a complex, learned behavior. The domestic Zebra Finch (Taeniopygia guttata), a cage bird whose wild counterparts are widely distributed in Australia, is the model species in which the neurobiology of singing behavior has been studied most intensively.
Neurobiologists have identified two key brain pathways associated with songbird singing. One of these is known as the motor pathway; the other is the anterior forebrain pathway. Each pathway includes a set of brain nuclei (groups of interconnected neurons at particular locations in the brain). Neural connections between the nuclei constitute the pathway. Both the motor pathway and the anterior forebrain pathway include the nucleus known as HVC (Fig. 10.27).
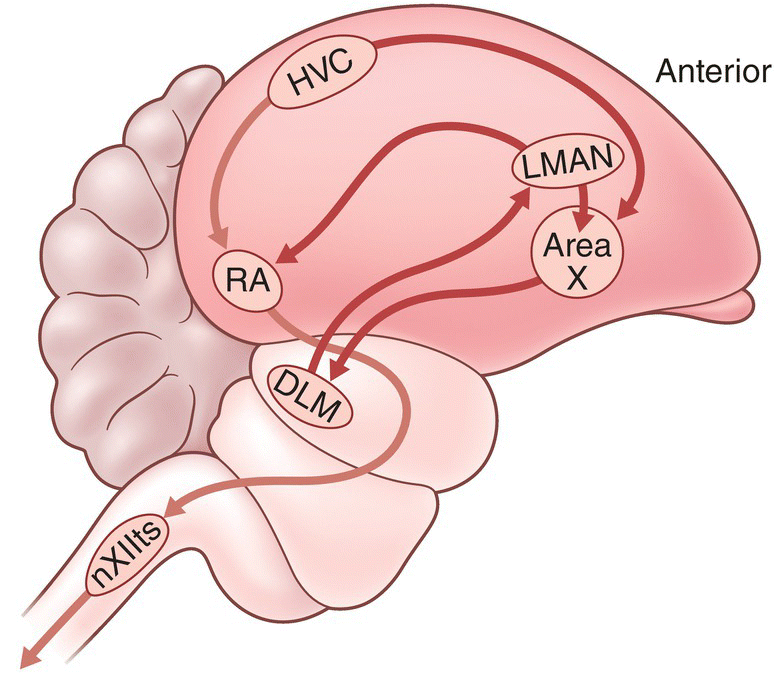
Fig. 10.27 Brain pathways for bird song learning and production. Two main pathways are highlighted in this schematic of a passerine bird brain. In the motor pathway, song is produced when signals from the nucleus called HVC are transmitted to the archistriatum (RA) and ultimately to the 12th cranial nerve (nXIIts), which controls the muscles that produce songs. The anterior forebrain pathway processes and evaluates the songs that a bird hears, and it guides song acquisition as signals from the HVC are transmitted to area X, the dorsolateral medial nucleus (DLM), and the lateral magnocellular nucleus of the neostriatum (LMAN).
(From Nottebohm 2005. © 2005 Fernando Nottebohm. CC BY 4.0.)
In the motor pathway, information specifying how to produce a learned song travels from HVC through several other nuclei to the nuclei that control the nerves of the syrinx and respiratory system. Thus, output from HVC guides the motor processes that produce songs. In the anterior forebrain pathway, HVC and other nuclei process the sounds that the bird hears and, in developing birds, store memorized sounds. Subsequently, the pathway evaluates the sounds of the bird’s own singing and guides an appropriate adjustment of motor output until the developing bird perfects its adult song. In adult birds, the pathway may evaluate and classify the sounds that the bird hears and also helps ensure that the bird’s own song types stay stereotyped and do not deteriorate.
In many species, the overall size of the song‐control nuclei in the brain is proportional to the size of the bird’s song repertoire. For example, in Cassin’s Finches (Haemorhous cassinii) of western North America, females sing very little, and the song‐control centers of the female are less than half the size of those of the male (MacDougall‐Shackleton et al. 2005). Male Carolina Wrens (Thryothorus ludovicianus) of eastern North America have robust song‐control nuclei, but in females of the species, which do not sing, the nuclei are so minuscule that researchers had difficulty detecting them (Nealen and Perkel 2000). In species in which females do sing extensively, these brain nuclei are correspondingly larger, although usually they still are smaller than those of males. For example, in the Forest Weaver (Ploceus bicolor) of southern Africa, females sing just as much as males and with similarly sized repertoires, but a male’s brain nuclei nonetheless are about 1.5 times as large as a female’s (Gahr et al. 2008). In addition to such between‐sex differences, the size of song nuclei may vary among males of a species, so that males with more songs have larger nuclei. Thus, for example, male Sedge Warblers (Acrocephalus schoenobaenus) with larger song repertoires tend to have larger song‐control nuclei than do males with smaller repertoires (Airey et al. 2000). In addition, species with huge song repertoires such as the Brown Thrasher (Toxostoma rufum) also tend to have enormous song‐control nuclei (DeVoogd et al. 1993).
In many songbirds, the song‐control centers change with the seasons, shrinking during the non‐breeding season and enlarging again during the breeding season when singing increases dramatically (Nottebohm 1981; Brenowitz 2008). Careful work has also shown that new neurons are created in some of these song‐control nuclei, even in adults (Goldman and Nottebohm 1983; Paton and Nottebohm 1984). This genesis of new neurons in the brains of adult songbirds was a surprising and important discovery, because neurobiologists had previously thought that the brains of adult vertebrates were incapable of developing new neurons. Additional research that followed this finding in birds has revealed that adult neurogenesis occurs in all vertebrates, including humans.
The main song‐control nuclei have been found in all major groups within the songbirds. What about other types of birds? The brains of suboscines that do not learn their songs lack the song‐control nuclei found in songbirds. Of course, some neural network must control suboscine vocalizations, but nothing similar to the pathways of the songbird brain has been found. Parrots and hummingbirds have brain structures analogous to those of the songbird pathways, but their brain anatomy is sufficiently different from that of songbirds that vocal learning and its neural machinery likely arose independently in these three groups (Striedter 1994; Jarvis et al. 2000).
10.5 Song variation in space and time
Bird songs vary in many ways: among species, among places, among individual birds, and even among the songs of a single individual. You can hear a lot of this variation yourself (Box 10.05). Song researchers have long been intrigued by this variation. Why do species differ in how their signals vary from one population to the next (over space) and in how their signals vary from one generation to the next (through time)? To answer such questions, researchers must first document patterns of variation and then try to understand the relationship between the observed patterns and song function. The goal is to understand both proximate questions of how sounds come to vary and ultimate questions of why they vary the way they do (Chapter 9, Box 9.03).
10.5.1 Differences among species
Why does each bird species have its own distinctive songs? During a bird’s normal daily activities, it typically interacts mainly with other members of its own species, because they are the ones with which it must cooperate or compete most directly to survive and reproduce successfully. Producing signals that influence members of other species would often be a waste of time and energy.
Humans take advantage of these differences between species in a variety of ways. The sounds are indispensable in bird survey work, for example. In many habitats, especially in dense forests, few birds are seen but many are heard. Thus it is the birds’ voices that enable us to determine the relative abundance of different species in different habitats. Or consider those who survey migrating birds. Most small birds migrate at night, when they are invisible, at least to our eyes, but many of them call, and the calls they use in flight are distinctive enough that they can be used to identify species (Evans and O'Brien 2002; Lanzone et al. 2009).
10.5.2 Individual distinctiveness
We humans can identify each other easily, as is to be expected, given that recognizing individuals is the foundation for social relationships. Birds, too, rely on social relationships, so we might expect that they, too, can recognize individuals. One way that people can recognize one another is by our distinctive voices, and research shows that many birds can recognize each other by voice, too.
The first evidence that songbirds might have this ability came from research on the Ovenbird (Seiurus aurocapilla), a wood‐warbler that breeds in forested areas of eastern North America. Each male Ovenbird has his own unique rendition of the species’ characteristic “tea‐CHER” song. Differences in the songs of individual Ovenbirds can be seen clearly in spectrograms, and a field playback experiment demonstrated that the birds use those differences to distinguish familiar from unfamiliar individuals (Weeden and Falls 1959). In the experiment, a speaker was placed on the territory of a male Ovenbird. When the songs of a neighboring male were broadcast, the resident male acknowledged them with a few songs in return. But when songs from an unfamiliar male were broadcast from the same location, the territorial male responded much more aggressively, presumably because a newly arrived, unfamiliar male poses a bigger threat than does a neighbor going about his usual activities.
The Ovenbird experiment showed that a male can distinguish the familiar songs of his neighbor from the unfamiliar songs of a stranger. To determine if a bird’s abilities go beyond distinguishing neighbors from strangers and extend to true individual recognition, a more sophisticated experimental design was required. An ingenious extension of the individual recognition experiment was first performed on White‐throated Sparrows (Zonotrichia albicollis) (Falls and Brooks 1975). Territorial sparrows were tested twice (Fig. 10.28). When a neighbor’s song and a stranger’s song were broadcast on the boundary between the territories of the focal bird and the neighbor, the focal bird responded more aggressively to the stranger’s song. However, when the neighbor/stranger playback test was repeated at a different territorial boundary, away from the normal location of the neighbor, the focal bird responded very aggressively to both playbacks, presumably because the abnormal location of the simulated out‐of‐place neighbor makes him just as threatening as a stranger. Because the White‐throated Sparrow’s responses to its neighbor’s songs differed depending on the location from which the songs were broadcast, we can infer that that the sparrows recognized that the songs belonged to a particular individual (the neighbor). Apparently, males defending territories come to know their neighbors and the songs they sing.
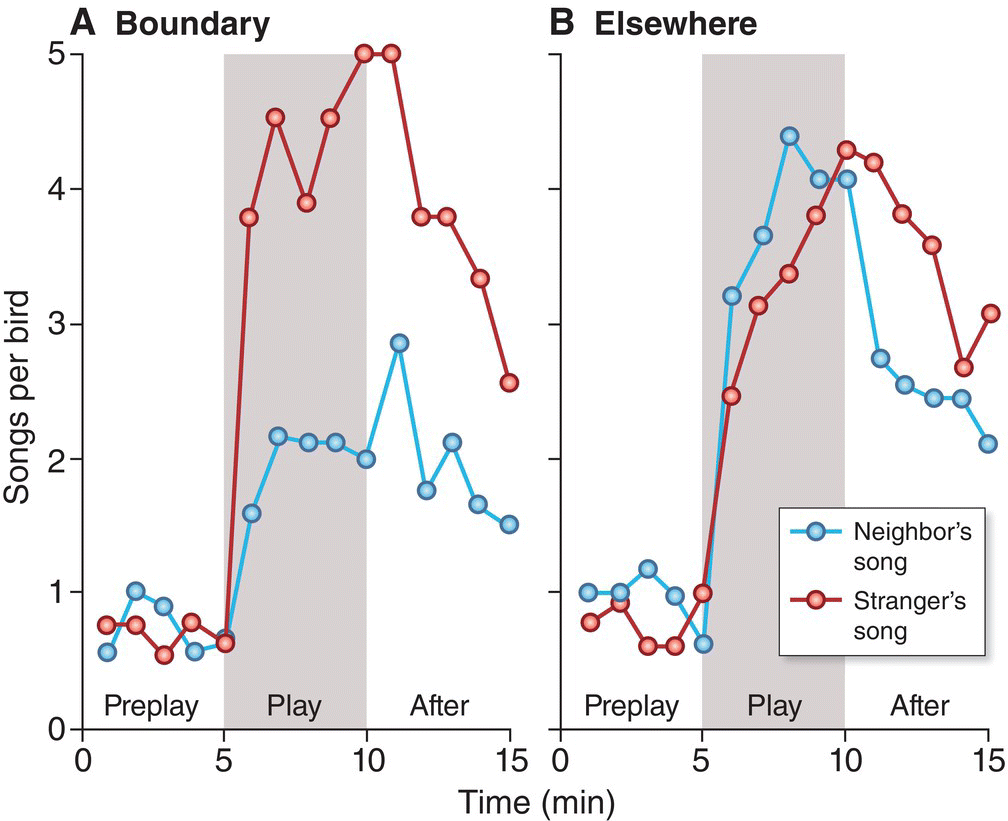
Fig. 10.28 Testing individual recognition in White‐throated Sparrows (Zonotrichia albicollis). (A) Territorial males respond more aggressively (with greater song output) to playback of a stranger’s song (red) than to playback of a neighbor’s song (blue) when the songs are played on the territory border between the focal male and his neighbor. (B) However, the focal male responds with equal aggression to neighbor playback if the songs are played at a location other than the border, presumably because the defending male realizes that his neighbor—now singing from a different location—seems to be encroaching on his territory. The focal bird’s ability to respond differently to his neighbor’s song when presented in different contexts suggests that he can recognize and identify his neighbor by song alone.
(From Falls and Brook 1975. © Canadian Science Publishing or its licensors. Reproduced with permission.)
Individual recognition is especially easy for White‐throated Sparrows, because each male has a single, distinctive song type. Thus, the memory needed to recognize all of one’s neighbors is limited, and distinguishing neighbors is straightforward. However, individual recognition also has been demonstrated in species with much larger repertoires. Song Sparrows (Melospiza melodia), for example, may have up to 10 different songs apiece, but they still can recognize their neighbors, even though collectively the neighbors may have more than 50 song types (Stoddard et al. 1991). Overall, playback experiments have detected neighbor–stranger discrimination or individual recognition by song in about three dozen songbird species of varying repertoire size, as well as in the North American suboscine Alder Flycatcher (Empidonax alnorum) (Lovell and Lein 2005) and in some non‐passerine species such as Little Owls (Athene noctua) in France (Hardouin et al. 2006) and Green Woodhoopoes (Phoeniculus purpureus) in South Africa (Radford 2005).
Birds also use vocalizations other than territorial song to recognize individuals. Bank Swallows (Riparia riparia) live in colonies, and once the young leave the nest, they mix with other young (Fig. 10.29). Parents use the distinctive calls of their offspring to locate them within large flocks of other young Bank Swallows (Beecher et al. 1986).
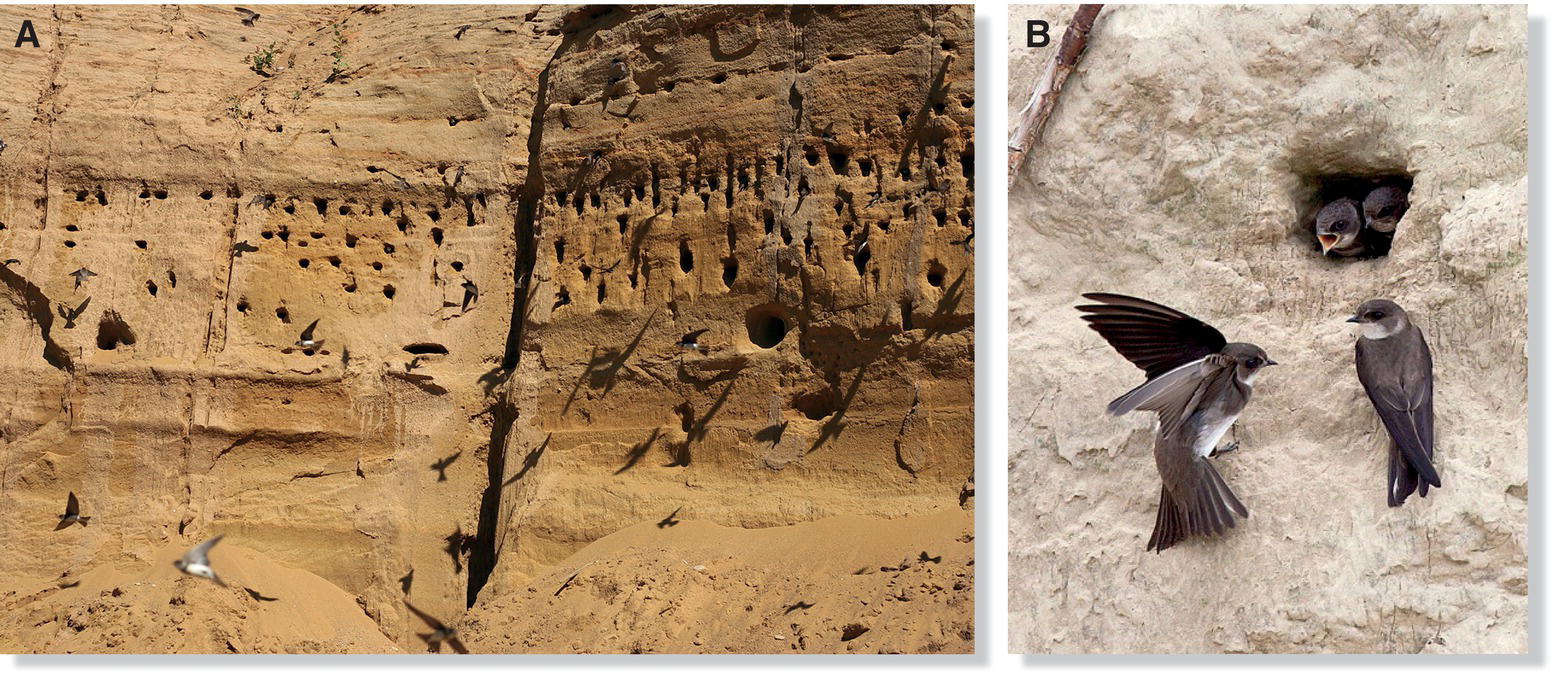
Fig. 10.29 Call recognition in colonial birds. (A) Bank Swallows (Riparia riparia) nest in large colonies. (B) Parents learn the distinctive calls of their offspring during the nesting period and later use them to identify their young when they gather in large flocks after fledging.
(Photographs by: A, Shkumbin on Flickr, https://commons.wikimedia.org/wiki/File%3ARiparia_riparia_colony.jpg. CC‐BY‐2.0; B, Bruce Van Valen.)
Numerous experiments show that in many species, mates recognize each other. Sometimes they do so under conditions that seem overwhelmingly difficult to us, such as in a huge colony of seabirds or penguins. Consider, for example, the King Penguins (Aptenodytes patagonicus) of Antarctica. Instead of building nests, they incubate a single egg as it rests atop their feet. These penguins breed in dense colonies that may contain tens of thousands of birds, and an individual may move around the colony as it incubates. Thus, when a member of a pair returns from a foraging trip and is ready to take over the incubation duties, finding its mate can be a challenge. The returning bird calls and awaits a return call from its mate, suggesting that pair members are able to recognize one another’s calls. Field experiments have confirmed this ability: a King Penguin is much more likely to call in response to playback of the calls of its mate than to playback of the calls of other colony members (Lengagne et al. 2000). Similar experiments on colonial Magellanic Penguins (Spheniscus magellanicus) show that females of this species respond more strongly to recordings of their mate’s display calls than to such calls recorded from other males (Clark et al. 2006).
10.5.3 Geographic variation
One distinctive feature of the vocalizations of most songbird species is their tendency to differ from place to place. One particular form of geographic variation in the songs of songbirds—dialects—has attracted special attention from researchers. We humans have dialects in our speech, of course, and astute listeners can often pinpoint a person’s place of origin by the subtleties of their accent. Songbirds have dialects as well, and dialects in both human speech and bird song are a consequence of vocal learning. If individuals generally remain near the location where they learned their vocal signals, then the individuals in that geographic area come to use the same local dialect.
In reference to birds, the term dialect signifies any spatial clustering of similar vocalizations. Song dialects thus can include only a few birds or many thousands, depending on how learning and dispersal affect the distribution of vocalizations in a particular species. Each small neighborhood of like‐singing Indigo Buntings (Passerina cyanea) could be called a dialect, as could the entire population of millions of Black‐capped Chickadees (Poecile atricapillus) living from the eastern USA to western Canada who all sing very similar versions of the “hey‐sweetie” song.
Distinguishing song dialects requires a good ear, but a skillful listener can hear dialect differences in the songs of many songbird species. Dialects have been especially well studied in the White‐crowned Sparrows (Zonotrichia leucophrys) of the coastal chaparral in California (USA). There, boundaries between dialects are so sharp that in some places a listener can stand facing the Pacific Ocean and hear songs of one dialect to the left and another to the right (Baptista 1975). Distinguishing dialects is especially easy in this species because each male uses a single song form, and one song thus identifies a male’s dialect (Fig. 10.30).
Fig. 10.30 Distinguishing dialects. White‐crowned Sparrow (Zonotrichia leucophrys) songs occur in dialects; the form of a male’s song depends on where he lives. Each song form occurs within one particular area. Spectrograms A through D illustrate four different White‐crowned Sparrow dialects. Listen online.
(Recordists: A, Arthur A. Allen and Peter Paul Kellog; B, Arthur A. Allen and David G. Allen; C, Leonard J. Peyton; D, Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology.)
Just how do these sparrow dialects form? The chaparral in California is a fire climax community. Vast stretches of the habitat routinely burn, temporarily destroying good sparrow habitat. The destruction is part of normal renewal, however, and when pockets of suitable habitat again become available, the sparrows re‐inhabit them. Founding birds can have songs that are incompletely learned or in some way different, and these odd songs then can become the basis for a new dialect. As suitable habitat areas expand, they eventually contact other nearby areas of suitable habitat. Boundaries between dialects form at these areas of secondary contact (Fig. 10.31).
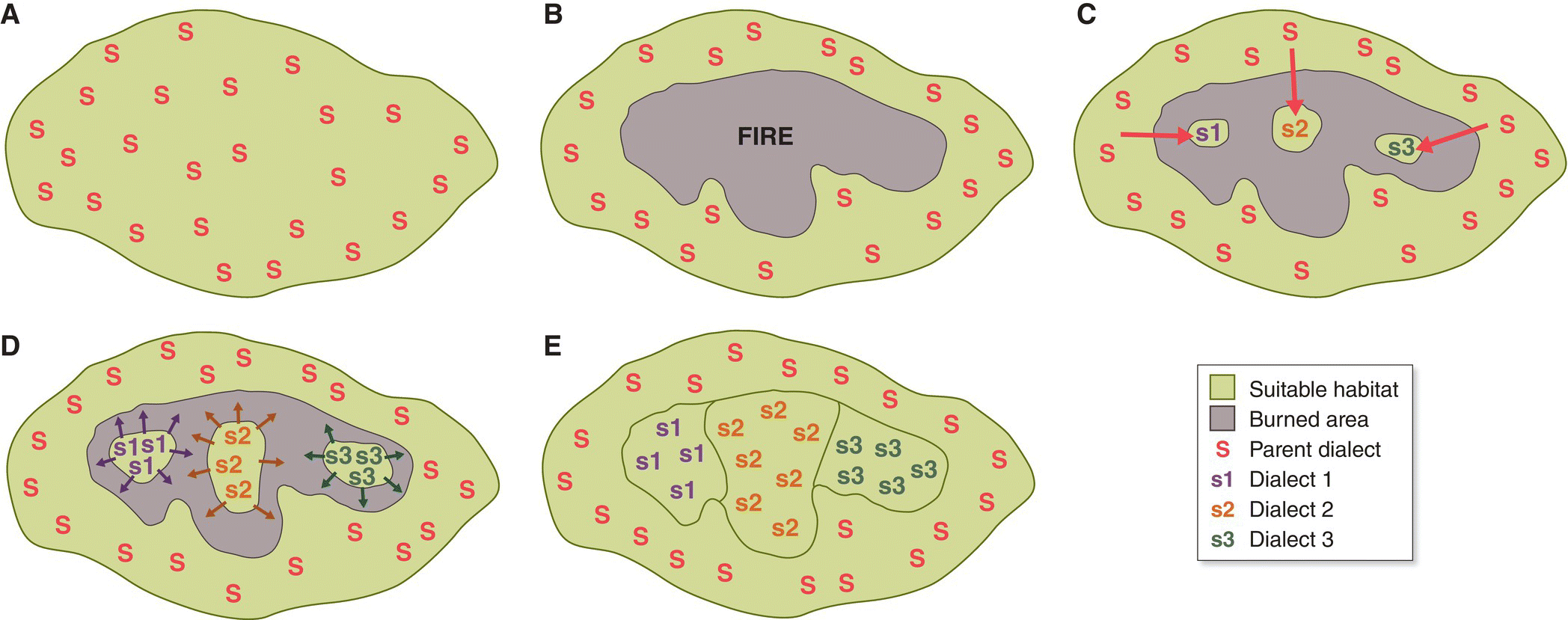
Fig. 10.31 Local dialect formation in White‐crowned Sparrows (Zonotrichia leucophrys). When some of the vegetation in an area containing White‐crowned Sparrows (A) that share a song dialect (represented as “S”) burns down (B), new dialects (s1, s2, s3) may form as small areas of suitable habitat regenerate (C). If the new areas are colonized by birds singing distinctive song types, these dialects may be spread by learning as the population in the new area grows (D). As the vegetation in the burned area continues to regenerate, formerly isolated populations come into contact, forming new dialect boundaries (E).
(© Cornell Lab of Ornithology.)
The formation of dialects in this fashion is relatively straightforward. More controversial is the issue of how dialect boundaries are maintained once they have formed. The central question is: How socially and genetically isolated are the birds in different dialects? The degree of isolation is determined in large part by when and where young sparrows enter a breeding population and learn their songs. We know that many dialects tend to remain stable (Trainer 1983; Nelson et al. 2004), so two possibilities exist. First, males may learn the songs of their home area early in life and later settle there, within their fathers’ dialect region. Alternatively, young males may disperse to new dialect areas and then modify their songs to match the local dialect.
Exactly what young White‐crowned Sparrows do, however, remains unclear. Laboratory experiments show that young males learn most readily early in life, during a period in which wild birds still live on their parents’ territory. However, laboratory experiments also show that, under the right social conditions, birds can modify their songs later in life. Banding studies in the field show that some young males remain in their natal dialect area, but some move across dialect boundaries to settle (Baker and Mewaldt 1978; Petrinovich et al. 1981). Merely knowing that a young male may opt for either choice does not address the critical question: Does the dialect boundary in any way inhibit dispersal? Genetic analyses suggest that birds in some White‐crowned Sparrow populations disperse freely out of their home dialect area but that dispersal may be restricted in other populations (Baker et al. 1982; MacDougall‐Shackleton and MacDougall‐Shackleton 2001; Soha et al. 2004). Additional research is necessary before we can fully understand how dialect boundaries affect dispersal and mating opportunities in the White‐crowned Sparrow.
Dialect patterns often occur at geographic scales larger than the local dialects found in White‐crowned Sparrows. The Marsh Wren (Cistothorus palustris) provides a good example. Field guides typically identify a single species of Marsh Wren across most of North America, but careful listening reveals two distinctly different Marsh Wren song variants (Kroodsma 1989). In eastern North America, male Marsh Wrens typically introduce their songs with a nasal buzz and then produce a relatively musical series of repeated notes (Fig. 10.32A). Among eastern birds, this basic song pattern is used consistently; even though a male sings 50 or more different song types, each type is of this basic formula. The songs of western Marsh Wrens are very different from those of their eastern relatives (Fig. 10.32B). The songs of these birds are raucous, buzzy, noisy, and coarse, and some include loud, penetrating whistles modulated rapidly in frequency. Western songs are never introduced by the faint buzzy note of the east, and they include or end with a raspy, noisy note.
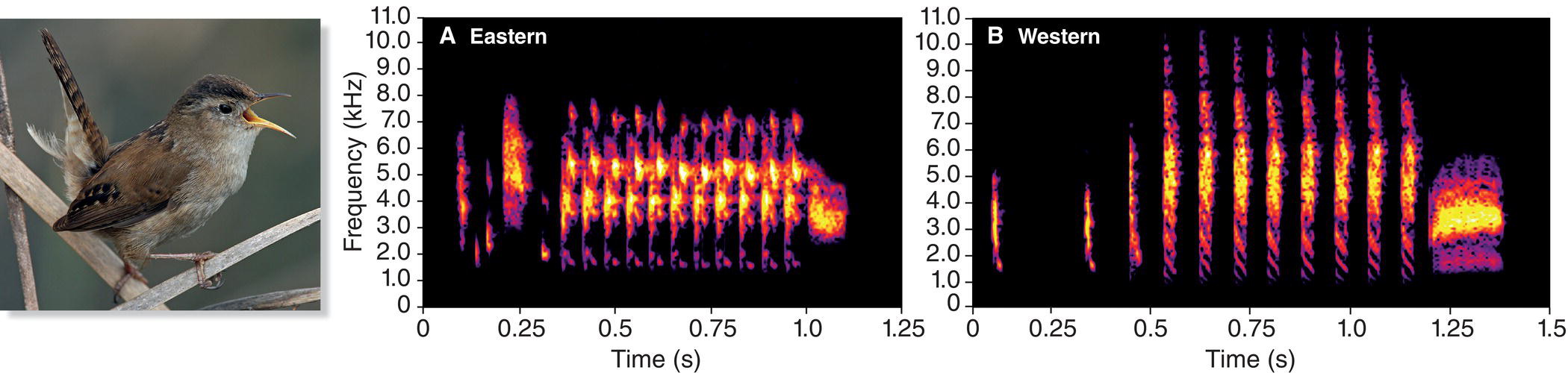
Fig. 10.32 Regional dialects. (A) Marsh Wrens (Cistothorus palustris) in eastern North America sing songs that begin with a nasal buzz, followed by repeated melodious notes. (B) In the western half of their range, Marsh Wrens produce coarse, buzzy songs that often contain raspy notes. Listen online.
(Recordists: A, William W. H. Gunn; B, Kevin J. Colver. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Ron Wolf.)
The simplest explanation for the origin of these regional differences lies in the distribution of habitats during past periods of North American glaciation. When glaciers advanced, they may have isolated two Marsh Wren populations, one in the west and the other in the east. Over thousands of years, the song differences we hear today developed in these two isolated populations. As the glaciers receded, the available habitat increased, and the two populations then met in the central Great Plains. Even though males of the two groups can learn each other’s songs, as has been shown in the laboratory (Kroodsma and Canady 1985), in nature they tend to maintain their distinctiveness, and few hybrid singers occur.
10.5.4 Diversity of geographic patterns
In some songbird species, the same song types recur throughout the geographic range of the species. Within any population of Blyth’s Reed‐Warblers (Acrocephalus dumetorum), for example, many different song types exist with little sharing among males, but the song types found in one population generally occur in other populations across the species’ wide breeding range in Asia and Europe (Marova et al. 2010) (Fig. 10.33). Also widely distributed are the hundred or so components from which Indigo Bunting (Passerina cyanea) songs are constructed; local dialects in buntings are formed not so much by the types of elements used, but by their particular combinations (Baker and Boylan 1995).
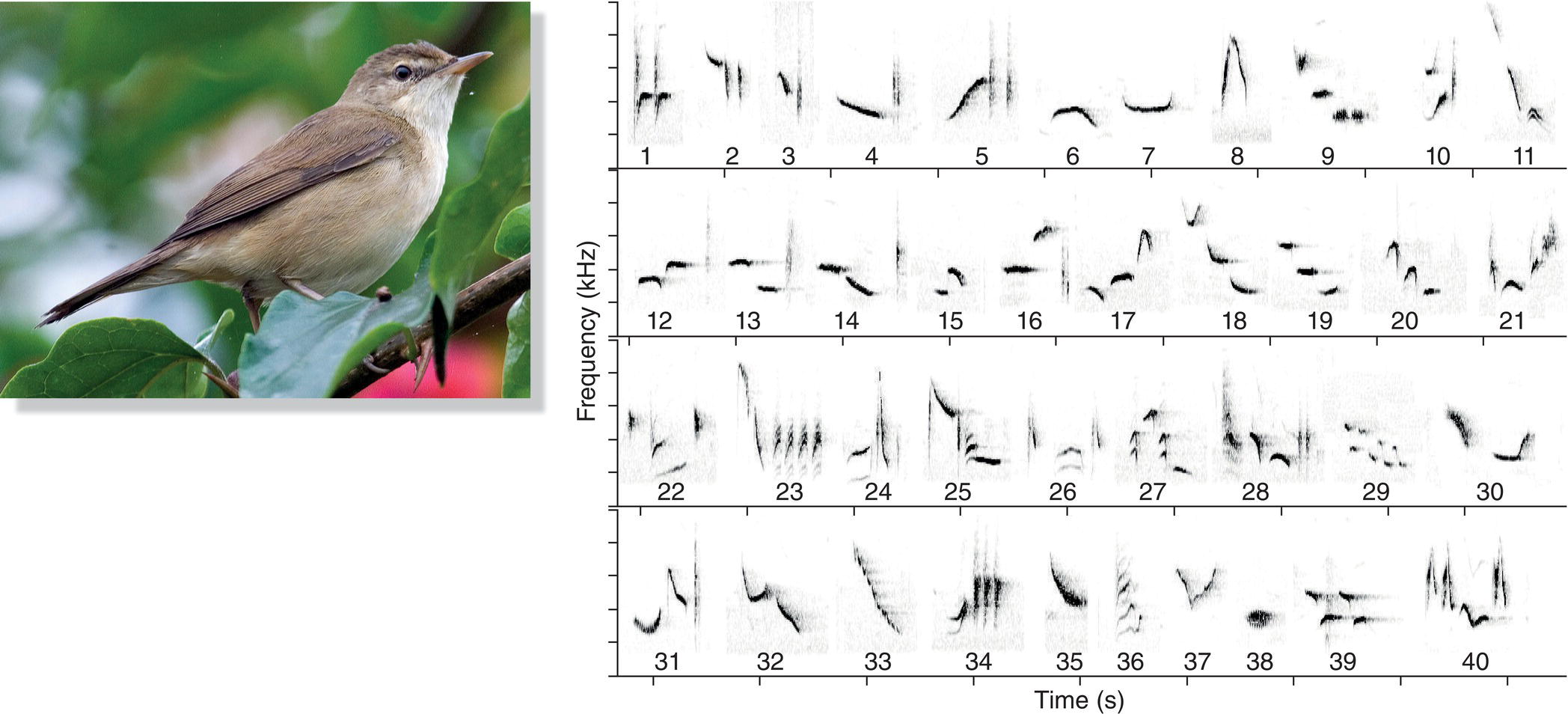
Fig. 10.33 Widespread song types. Some species sing song types that recur across their entire geographic range. For example, even though Blyth’s Reed‐Warblers (Acrocephalus dumetorum) within a local neighborhood do not typically share songs, the individual song types found in that area may also occur in distant populations. Each number here corresponds to a different song type found throughout this species’ range.
(From Marova et al. 2010. Reproduced with permission from Springer Science + Business Media. Photograph by Shankar70, https://commons.wikimedia.org/wiki/File:Blyth%27s_Reed_Warbler.jpg. CC BY‐SA 3.0.)
The whistled “hey‐sweetie” of the Black‐capped Chickadee (Poecile atricapillus) has an especially puzzling geographic distribution. The chickadee uses the same song across continental North America. Across this huge geographic area, patterns of whistles are the same in both frequency and sound intensity, and birds use the same rules to transpose their songs in frequency (Ratcliffe and Weisman 1985; Weisman et al. 1990; Horn et al. 1992). Such consistency in a learned song across an entire continent is remarkable. How the chickadees maintain such a stereotyped learned signal over such a vast geographic expanse remains a mystery. The more typical songbird pattern is to show at least some form of local variation.
Some intriguing local chickadee populations do have song forms other than the “hey‐sweetie,” however. One is on Martha’s Vineyard, a small island off the coast of Massachusetts (USA). There, males also whistle, but the two whistles are on the same frequency, with no drop between the first and second whistles in each phrase (Kroodsma et al. 1999c). Furthermore, song dialects occur on the island, so that males in the eastern and western parts of the island use different songs. A second pocket of chickadee song differentiation occurs near Fort Collins, Colorado (USA), where three different non‐standard whistle songs occur, and each male uses a repertoire that includes two or all three of the local song types (Gammon and Baker 2004). In addition, males in coastal Oregon and Washington (USA) sing a variety of whistled songs (Kroodsma et al. 1999c).
How do these regional differences in song arise? Martha’s Vineyard is about 11 kilometers off the coast of New England, so birds there may be relatively isolated from mainland North America. Perhaps isolated pockets of chickadees also occurred in some western states, so songs in those areas had a chance to diverge from the standard “hey‐sweetie.” We do not know whether these vocally distinct populations are genetically distinct or if a female of one song tradition would accept a male from another.
Work with Black‐capped Chickadees in the laboratory has only enhanced the mysterious nature of chickadee song. If male chickadees from normal “hey‐sweetie” populations are tutored with normal “hey‐sweetie” songs in the laboratory, they nonetheless develop highly abnormal songs, unlike anything one would expect from a chickadee in nature (Kroodsma et al. 1995). Although the whistled tonal quality remains, the songs consist of from one to seven or more different whistles on a variety of frequencies. Furthermore, males develop repertoires of up to three different song types. Groups of laboratory males isolated from one another even develop different “dialects.” Over most of the North American continent, some social factors must restrict chickadee song variation to the simple “hey‐sweetie” form; those factors are absent in the highly artificial laboratory environment. What are these social factors? Perhaps it is the female chickadee that sets standards for what songs are to be sung, as happens in other birds. For example, female Brown‐headed Cowbirds (Molothrus ater) respond differently to the various songs of males, and in that way “instruct” males about which songs to sing to be especially successful in acquiring a mate (West and King 1988). Perhaps the female chickadee also has certain inviolate standards for her mate’s song.
The diverse patterns of geographic variation are puzzling, but some hints as to why songs vary in these ways are found in certain North American wood‐warblers. In a number of wood‐warbler species, males have two categories of songs (Spector 1992). One category contains songs that seem to be used especially to attract females. For example, both the “beee‐buzzzz” of the Blue‐winged Warbler (Vermivora cyanoptera) (Fig. 10.34A1) and the “pleased‐pleased‐pleased‐to‐MEETCHA” of the Chestnut‐sided Warbler (Setophaga pensylvanica) (Fig. 10.34B1) are used to attract mates. Spectrogram analyses show that these mate‐attraction song forms vary little over the entire geographic ranges of these species (Byers 1996; Kroodsma 1981). These species also use a second category of song at dawn and in highly aggressive situations, when males are countersinging with one another, especially near territory boundaries (Fig. 10.34A2, B2). These songs occur in dialect patterns, with songs often changing over short distances.

Fig. 10.34 Different song types can serve different functions. In some species, such as (A) Blue‐winged Warblers (Vermivora cyanoptera) and (B) Chestnut‐sided Warblers (Setophaga pensylvanica), songs in the first category (type 1) function in mate attraction, whereas songs in the second category (type 2) function in male–male aggression. Listen online.
(Recordists: type 1, Geoffrey A. Keller; type 2, Wilbur L. Hershberger and Matthew D. Medler, respectively. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photographs by: A, Jim Zipp; B, Larry Gridley.)
Among these wood‐warblers, it seems that a male attempting to attract a female succeeds best if he has a highly stereotyped signal that all females can recognize. Females of many songbird species tend to disperse further than males (Chapter 12), so an especially stereotyped male signal will be unmistakably recognizable to all females, no matter where they fledged. When males address other males on neighboring territories, however, some advantage must accrue to having a local signal. Vocalizations employed by wood‐warblers in more aggressive contexts accordingly vary from place to place, in dialects.
10.5.5 Geographic variation in suboscine vocalizations
Dialects occur widely in learned vocalizations such as the songs of songbirds, but how much do non‐learned vocalizations change over geographic space? In most species vocalizations stay relatively constant over space. For example, the songs of the Pale‐edged Flycatcher (Myiarchus cephalotes) are very similar throughout the species’ range in South America, and the same is true for many other species of Myiarchus flycatchers (Lanyon 1978). No detectable local variation occurs in the songs of Long‐tailed Manakins (Chiroxiphia linearis) in Costa Rica (Trainer and Parsons 2001) or Acadian Flycatchers (Empidonax virescens) in Maryland (USA) (Payne and Budde 1979). The same general pattern is found among most other suboscines. The songs of these birds are not learned but instead seem to be innate, developing mainly under genetic control.
Because suboscine songs are a good indicator of a bird’s genetic background, the songs of suboscine birds can help us determine which populations of birds belong to which species. During the 1960s, for example, investigators discovered that the North American species then known as “Traill’s Flycatcher” actually includes two subgroups whose songs differ consistently, suggesting that the two groups do not interbreed (Stein 1963). This song difference helped researchers recognize that “Traill’s Flycatcher” was actually two species, the Alder Flycatcher (Empidonax alnorum) and the Willow Flycatcher (Empidonax traillii). More recently, researchers recognized that the closely related “Western Flycatcher” similarly consists of two different groups, each with its own distinctive songs (Johnson 1980). Further investigation of genetic variation showed that the song differences identified genetically distinct populations (Johnson and Marten 1988), which subsequently were recognized as distinct species, the Pacific‐slope Flycatcher (Empidonax difficilis) and the Cordilleran Flycatcher (Empidonax occidentalis).
Song differences still serve as indicators of the limits of suboscine species, especially when used in conjunction with information on the genetic differences between populations that sing different songs. For example, researchers who investigated geographic variation in the calls of a neotropical antbird noticed that the songs of birds living in Panama and Costa Rica sounded different than those of similar‐looking birds living further east and south in Colombia (Cuervo et al. 2007). The researchers suspected that the song differences indicated that the two populations in fact were different species. Analyses of genetic differentiation supported this suspicion, as the genetic differences between the populations are large enough to suggest that each population should be classified as a separate species, the Dull‐mantled Antbird (Myrmeciza laemosticta) and the Magdalena Antbird (Myrmeciza palliata) (Chaves et al. 2010). In addition, the researchers identified song characteristics that are diagnostic of each proposed species. Thus, songs can serve as valuable clues to direct the efforts of systematists, who often are faced with the challenge of defining species in suboscine groups such as antbirds (Fig. 10.35), woodcreepers, and flycatchers in which different species can be very similar in appearance.
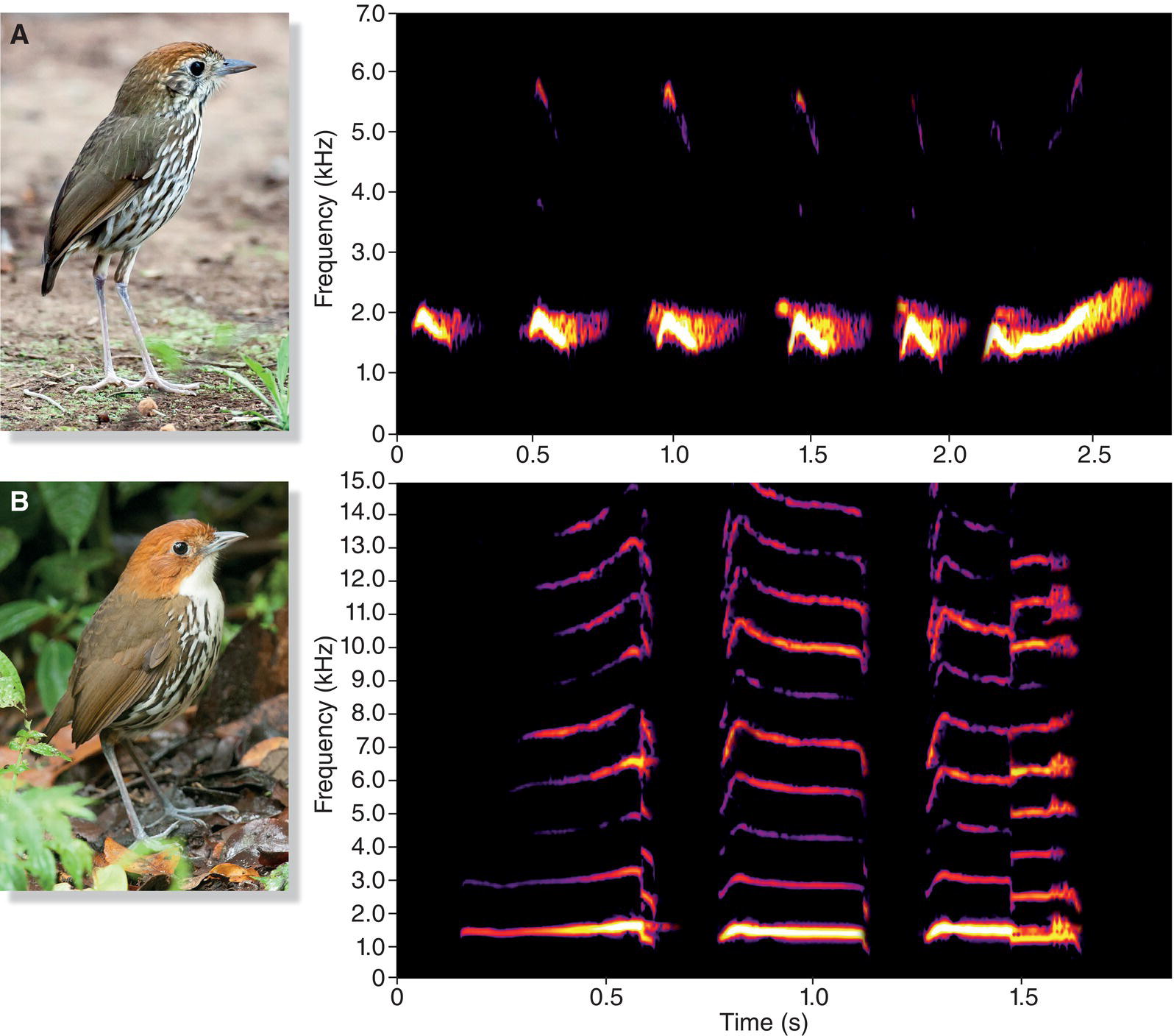
Fig. 10.35 Songs help us recognize suboscine species. Differences in song can help systematists identify cryptic species. For example, although (A) Watkins’s Antpittas (Grallaria watkinsi) and (B) Chestnut‐crowned Antpittas (Grallaria ruficapilla) occur in overlapping geographic ranges and are similar in appearance, they can be distinguished by their distinct songs. Listen online.
(Recordists: A, Linda R. Macaulay; B, Paul A. Schwartz. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photographs by: A, Robert Lewis; B, Arthur Grosset.)
10.5.6 Change over time
Because oscine songs are learned, they have a high potential to change over time as well as over space (Box 10.06). The Indigo Bunting (Passerina cyanea) of eastern North America is among the few species in which song changes in a population have been tracked over a long period (Payne et al. 1981, 1988). As you may recall from earlier in this chapter, each male Indigo Bunting sings a single song type, which he acquires by copying an adult male in his neighborhood. However, not all buntings make perfect copies of their tutor’s song. Thus, as songs are copied from male to male, changes accumulate in the songs present in a given area. In addition, new songs may be introduced to an area when older males from other locations immigrate into the population. In Indigo Buntings, these factors contribute to a rapid turnover of song forms within local populations. Over time, many songs are introduced, and many songs are lost, and only a few are copied and maintained for long periods of years. Over 10 years, the song types in a population turn over almost completely. Similarly steady, rapid change over time also has been found in the songs of Corn Buntings (Emberiza calandra) (Holland et al. 1996), European Starlings (Sturnus vulgaris) (Adret‐Hausberger 1986), and Common Chaffinches (Fringilla coelebs) (Ince et al. 1980) in Europe, and in Singing Honeyeaters (Gavicalis virescens) in Australia (Baker et al. 2001).
In some other songbird species, song form does not change at such a rapid pace. For example, in a population of Wood Thrushes (Hylocichla mustelina), song elements remained unchanged over a 9‐year period (Whitney 1992). Similarly, the trills that define White‐crowned Sparrow (Zonotrichia leucophrys) song dialects have been shown to persist for up to 34 years in some sparrow populations (Trainer 1983; Chilton and Lein 1996; Nelson et al. 2004). In the Galápagos Archipelago of Ecuador, the well‐studied population of Medium Ground‐Finches (Geospiza fortis) on the island of Daphne Major (Chapter 3) used the same set of four song types for six consecutive years (Gibbs 1990) and in a population of the same species on nearby Santa Cruz Island, some song types persisted for 38 years (Goodale and Podos 2010).
Some species even exhibit these rapid‐turnover and slow‐change patterns simultaneously. In Chestnut‐sided Warblers (Setophaga pensylvanica) of eastern North America, songs of one category (the category used in aggression between males) changed rapidly and continuously during a 20‐year study, but songs of a second category (the category used by males to attract females) did not change appreciably over that span (Byers et al. 2010). In fact, some Chestnut‐sided Warbler songs from this second category recorded in 2010 are identical to those recorded in the 1950s.
10.6 Functions of bird song
What do birds use their vocalizations for? Most investigations of the functions of bird vocalizations have focused on songbird songs. These are among the most extravagant and noticeable sounds birds make, and it is no wonder that they have attracted so much attention. Researchers now generally agree that singing has two main functions: to help defend a territory and to help attract and stimulate a mate.
10.6.1 Territorial defense
Territoriality is common among birds and song often plays an important role in avian territorial behavior. One indication of this role is the manner in which a territorial male songbird responds to the songs of another male. If a recording of a species’ song is broadcast within the territory of a conspecific, the resident male often responds aggressively; he may even go so far as to attack the loudspeaker or an associated decoy (Fig. 10.36). Such song playback has been shown to elicit aggressive behavioral responses in many species. This aggressive response suggests that a territorial male interprets a rival’s song as a signal of his intention to claim some turf. But would singing in return help keep an intruder out?

Fig. 10.36 Simulated intrusion experiment with Townsend’s Warblers (Setophaga townsendi). Here, a male (top) attacks a decoy (below, on branch) during a territorial intrusion experiment; recorded songs were broadcast through speakers near the decoy, within the pictured male’s territory, to assess his aggressive response.
(Photographs by Scott Pearson.)
Evidence that songs help to keep other males out of a territory comes from “speaker replacement” experiments. In these experiments, researchers temporarily remove a male from his territory and replace him with recordings played through a series of speakers. For example, experimenters in Pennsylvania (USA) removed male Song Sparrows (Melospiza melodia) from their territories, performing the removals two at a time to create pairs of non‐adjacent, unoccupied territories (Nowicki et al. 1998). One of the empty territories in each pair served as a silent control. On the other territory, the experimenters placed two speakers from which Song Sparrow songs were broadcast, alternating between the two speakers to simulate the presence of the territorial male. These simulated males, consisting only of songs, deterred potential interlopers; speaker‐inhabited territories were much less likely to be reoccupied by intruders than were the silent controls. Song does seem to send the message to “keep out” to other birds that are prospecting for potential territories.
The role of song in territorial defense is further confirmed by muting experiments. In one such experiment, territorial male Seaside Sparrows (Ammodramus maritimus) in Florida (USA) were temporarily muted by surgery on one of their air sacs (McDonald 1989). A male with a disabled air sac cannot maintain enough pressure in his respiratory system to produce songs. In the experiment, the muted males lost their ability to defend their territories; intruders were far more likely to invade and successfully claim all or part of a muted male’s territory than to invade the territory of a control male who could sing. When the air sacs healed and the muted males regained their songs, most were then able to regain their temporarily lost territory.
Vocalizations other than songs also can serve as keep‐out signals. For example, birds of species that defend territories during the non‐breeding season, when singing declines or disappears in some species, may then use a simpler call to broadcast territorial rights. For example, migratory Yellow Warblers (Setophaga petechia) may defend individual feeding territories on their non‐breeding grounds in Mexico. When neighboring warblers confront one another at a territorial border, they utter a rapid succession of short “chip” calls (Greenberg and Ortiz 1994). Additional evidence that chip calls play a role in territorial conflict among Yellow Warblers comes from a field playback experiment (Neudorf and Tarof 1998) in which non‐breeding, territorial Yellow Warblers in Panama mounted an aggressive response to a playback of Yellow Warbler chips but not to chips of the Northern Waterthrush (Parkesia noveboracensis), another wood‐warbler species present in the area.
10.6.2 Mate attraction
Bird song also functions in mate attraction. One piece of circumstantial evidence that male songbirds use songs to attract females is that males generally sing more frequently before they acquire a mate than they do afterwards. At a study site in France, for example, male Common Nightingales (Luscinia megarhynchos) sing extensively at night when they are unpaired but cease nocturnal singing once they acquire a mate (Amrhein et al. 2002). During the day, unpaired nightingales sing steadily throughout the breeding season, but a paired male substantially decreases his singing once his mate lays eggs and is thus no longer reproductively available to other males. In Sedge Warblers (Acrocephalus schoenobaenus) breeding in Great Britain, a male’s copious singing declines to almost none immediately after pairing (Catchpole 1973). Studies of numerous other species have shown that, typically, unpaired males sing all day long and paired males sing far less often, and that if a paired male loses his female, he reverts to abundant singing. The amount of song coming from a male is therefore often a good indication of his pairing status.
Additional evidence that male song attracts females comes from field experiments with species such as European Starlings (Sturnus vulgaris), European Pied Flycatchers (Ficedula hypoleuca), and House Wrens (Troglodytes aedon) (Eriksson and Wallin 1986; Mountjoy and Lemon 1991; Johnson and Searcy 1996). In these experiments, speakers were placed near the openings of nest boxes, and songs of the focal species were played through the speakers at some boxes, while the speakers at other boxes remained silent. In all three species, females were far more likely to approach or enter nest boxes with songs than silent boxes. This result seems to indicate that females used songs to identity potential mating opportunities.
In the laboratory, female birds often seem to find male songs appealing. To test the attractiveness of a song to females, researchers can monitor the females’ copulation‐solicitation display, the tail‐up posture that initiates copulation in many species (Fig. 10.37). In copulation‐solicitation experiments, females typically display more in response to songs of their own species than to songs of other species. For example, in a laboratory experiment with female Red‐winged Blackbirds (Agelaius phoeniceus), researchers compared the number of displays performed in response to playback of typical blackbird songs with the number performed in response to a mockingbird’s imperfect imitation of blackbird songs (Searcy and Brenowitz 1988). The mockingbird songs induced far fewer displays than did the songs of the females’ own species. The strong sexual response to own‐species song suggests that females regard the songs as sexual signals.

Fig. 10.37 Copulation‐solicitation display. A male (top) Lark Sparrow (Chondestes grammacus) responds to a female’s tail‐up solicitation display (bottom) by initiating copulation. In the lab, females will sometimes display in response to male song.
(Photograph by Ron Dudley.)
10.7 Signal values of differences among singers
As noted earlier in this chapter, individual songbirds of a given species tend to have individually distinctive songs and singing behavior. Thus, birds listening to a singer’s attempts to defend a territory or attract a mate might be reading cues more subtle than the simple presence or absence of singing. For example, an avian listener can probably judge the location of the singer (how distant it is) by the loudness and degree of degeneration in the sound quality of its songs. If a singer is of a species that uses different song types or different styles of singing in different contexts, its songs give clues about its motivational state. When songs vary geographically, a bird’s song identifies the region in which it acquired its songs. Similarly, if a male’s songs match those of his local neighborhood, he reveals that he probably has lived in the neighborhood long enough to establish his local identity. In species in which birds add to their song repertoires in successive years, singing reveals a bird’s age. Differences in singing may also reveal to listeners differences in the intrinsic qualities of the singer. The singing emanating from a territory might indicate the relative health or genetic quality of its owner, information that might be useful to potential mates or rivals.
10.7.1 Song output
Differences among singers could be revealed by something as simple as the number of songs produced in a day. A male in possession of a territory rich in food might be able to acquire energy more quickly than a male on a poorer territory and therefore be able to sing more actively. One way to test this hypothesis is to provide wild birds with extra food and see how their singing is affected. For example, when researchers provided mealworms to territorial New Zealand Robins (Petroica australis), the birds’ song output was greater on the days on which they received this extra food than on the days just before and just after (Barnett and Briskie 2011). A similar experiment on Australian Reed‐Warblers (Acrocephalus australis) evoked a similar result: territorial males sang more songs when experimenters supplied blowfly maggots than when none were provided (Berg et al. 2005). Black‐capped Chickadees (Poecile atricapillus) provided with a daily helping of mealworms produced more songs and began singing earlier in the day than did comparable chickadees that received no extra food (Grava et al. 2009). These experiments, and others like them, suggest that a female seeking a mate could use the amount of song produced by a male to judge his ability to obtain a good territory, or perhaps to indirectly judge the quality of the territory itself, which might be important as a food supply for herself and her young. A male seeking to establish a territory could use the amount of song to judge the likelihood that a rival male is too healthy and robust to be challenged successfully.
There is some evidence that avian listeners do attend to quantity of song output. For example, in a Swedish population of Snow Buntings (Plectrophenax nivalis), males that produced more songs nested earlier, an indication that females chose them ahead of males with lower song output (Hofstad et al. 2002). Early mating also was associated with high song output in an Austrian population of Blackcaps (Sylvia atricapilla) (Hoi‐Leitner et al. 1995). In this case, the researchers also showed that males with higher song output had higher quality territories with denser vegetation and better‐protected nest sites. Thus, singing a lot of songs helped male Blackcaps both to secure a better territory and to attract a better (or at least an earlier) mate. In comparisons between males on territories of similar quality, high‐output males still reproduced earlier, suggesting that females based their mate choice on the song output rather than by directly assessing the territory.
10.7.2 Song performance
In addition to differences in the quantity of songs produced, males may differ in more subtle aspects of song performance. For example, the songs of different males can differ in the rate at which similar syllables are repeated within a song (the trill rate), in the frequency bandwidth of their songs, or in the pitch (frequency) at which a shared song type is produced. Males also may differ in their ability to sing consistently; researchers have documented between‐male variability in ability to maintain consistent pitch across consecutive syllables in a song, in the ability to maintain a consistent time interval between the songs in a bout of singing, and in the ability to maintain a constant between‐syllable frequency ratio across the songs in a bout.
Research findings suggest that such differences in how songs are performed influence the ability of males to attract mates and win contests with other males. For example, male Chestnut‐sided Warblers (Setophaga pensylvanica) in eastern North America differ in their ability to maintain constant pitch and timing across successive songs in a bout, and the most consistent singers have the largest number of extrapair offspring (Byers 2007). Moreover, when a female has an offspring fathered by a male other than her social mate (Chapter 9), the genetic father almost always sings more consistently than the female’s social mate. These findings suggest that a female seeking an extrapair partner makes her selection by comparing males’ song performance consistency. In a population of Tropical Mockingbirds (Mimus gilvus) in Colombia, dominant males sang more consistently than subordinate males. In dominant “alpha male” mockingbirds, a bird’s rendition of a given syllable type varied only a little (Botero et al. 2009), and these consistent singers had more offspring than did more variable singers.
A different kind of song performance seems to be important in other species whose songs include rapid trills. Trills require rapid, coordinated movements of the beak, syrinx, and respiratory system, and these movements are especially hard to execute when the trill spans a high bandwidth, with a big difference between its highest and lowest frequencies. A bird that sings an especially rapid trill with a high bandwidth is executing a physically challenging task. For example, female Swamp Sparrows (Melospiza georgiana) judge males on the basis of their ability to sing such high‐performance trills: in a laboratory test, female sparrows performed more sexual displays in response to playback of high‐performance songs than to playback of low‐performance songs (Ballentine et al. 2004).
Why would avian listeners be impressed by a male that sings rapid, high‐bandwidth trills or bouts of highly consistent songs? Probably because performing those difficult vocal feats may require unusually good stamina and coordination. Thus, males whose singing performance is excellent may be especially healthy or vigorous, and song performance therefore may be a good indicator of a mate worth choosing or a rival worth avoiding.
10.7.3 Song repertoire size
A species’ mode of song development influences the size of its song repertoire. Species that do not learn songs, such as most suboscine passerines, tend to have small repertoires. In contrast, song learning has enabled some songbirds to evolve huge repertoires. A Five‐striped Sparrow (Amphispiza quinquestriata) has 75 different song types, a Tropical Mockingbird (Mimus gilvus) has 130 types, a Common Nightingale (Luscinia megarhynchos) has 200 types, and a Brown Thrasher (Toxostoma rufum) has 2000 types. Why do some songbird species have huge song repertoires, whereas in other species each male uses only a single song type? Efforts to answer to this question have focused, not surprisingly, on what we presume to be the two main functions of bird song: territory defense and mate attraction and stimulation.
In some species, larger repertoires might help males display their prowess and defend their territories. Marsh Wrens (Cistothorus palustris) in western North America, for example, seem to duel with their large repertoires. The complexity of interactions can be dizzying. Marsh Wrens have been shown to match a sequence of as many as 10 different song types broadcast from a loudspeaker, but because males in the local marsh sang songs in a predictable sequence, the males could sing each song type in the sequence even before the tape recorder played it (Verner 1976); the male could anticipate which song would come next and sing it before the simulated intruder did. The singing of Common Nightingales in Europe also includes predictable song type sequences and anticipatory matching (Todt and Hultsch 1996). This kind of matched countersinging might be important in directing threats to particular individuals or in mediating interactions. Likewise, females somehow might be impressed by the way a singer uses his repertoire.
Some evidence supports the idea that large repertoires function in mate attraction. In the laboratory, females of some species respond more strongly to a greater variety of song. In one experiment, captive female European Pied Flycatchers (Ficedula hypoleuca) could simultaneously hear recordings of two singing males, one of which used a larger repertoire of song types than the other (Lampe and Sætre 1995). The females who built nests placed them closer to the large‐repertoire speaker than to the small‐repertoire speaker. In other species, however, a female’s response in laboratory experiments does not seem to depend on the diversity of songs to which she is exposed. For example, researchers found no preference for larger repertoires among female European Starlings (Sturnus vulgaris) that could choose to elicit playback of one of two different male song bouts by choosing between two different perches (Gentner and Hulse 2000).
Measuring a female’s responses in nature is more difficult, and most field studies instead test for an association between the properties of a male’s songs and his success in attracting or fertilizing a female. Some field studies have found a relationship between a male’s repertoire size and his mating success. A study of a German population of polygynous Great Reed‐Warblers (Acrocephalus arundinaceus), for example, found that the number of mates a male acquired was correlated with the size of his song repertoire (Catchpole 1986). However, a later study of the same population found no correlation between repertoire size and number of mates (Forstmeier and Leisler 2004). More generally, most field studies of repertoire size and female mate choice have found no effect of repertoire size. Overall, the inconsistent results of research on song repertoires and female mate choice suggest that we cannot yet reach a firm conclusion about whether females use variation in repertoire sizes to evaluate potential mates.
In at least two species, the Great Tit (Parus major) of Eurasia and the Song Sparrow (Melospiza melodia) of North America, males with larger repertoires survive longer than males with smaller repertoires (Lambrechts and Dhondt 1986; Reid et al. 2005). One is tempted to conclude that having a large song repertoire somehow causes a male to live longer, but perhaps it is more likely that good health permits both the longer life and the development of a larger repertoire in youth. Young male Bewick’s Wrens (Thryomanes bewickii), for example, learn more songs if they hatch early in the breeding season than if they hatch late; early‐hatching birds have more time to learn songs before their first winter, and they may have a chance to select the best territory too (Kroodsma 1974). In some species, birds with larger repertoires are older, because males continue to add songs to their repertoires throughout life. For these reasons, males with larger repertoires are more likely to be healthier and better at surviving, and both males and females might be attentive to these vocal indicators of success.
10.8 Other remarkable features of singing behavior
No overview of bird vocalizations would be complete without mention of some especially striking and intriguing manifestations of avian vocal behavior that long have delighted naturalists. In this section, we describe the pre‐sunrise burst of singing that greets early‐rising bird watchers, the singing abilities of many female birds, the coordinated singing performance conducted by mated pairs of some species, the propensity of some birds to mimic the sounds of species other than their own, and the gymnastic efforts of birds that sing in flight.
10.8.1 Dawn chorus
Some of the most remarkable avian singing occurs at dawn (Fig. 10.38). Most day‐active birds are silent throughout the night, presumably roosting quietly somewhere on their territory. Beginning an hour or so before sunrise, birds of many species begin their dawn chorus. Typically, the chorus begins with a burst of songs from the earliest singing species. As the dawn progresses, other species join the chorus in a rather predictable sequence. The order in which species join may be determined by differences in how well species can see in dim light. According to this hypothesis, a bird will not begin singing until there is enough light for it to see well enough to do whatever it must be able to do while singing, such as detecting predators that might notice a singing bird. Evidence in support of the hypothesis comes from a study that measured the size of the eyes of representative individuals of each of 57 passerine species (Thomas et al. 2002). Birds with larger eyes have larger pupils to let in light, so birds with bigger eyes are able to see better in dim light. The researchers also measured the time, relative to sunrise, at which each species began singing at several sites in Europe. These data revealed that species with larger eyes began singing earlier, presumably because they were able to see well earlier. A similar study of a tropical dawn chorus in an Ecuadorian forest revealed a somewhat more complex pattern (Berg et al. 2006). There, the earliest passerine singers were species that lived high in the forest canopy, where light first penetrated at dawn. Singing began progressively later at lower levels in the forest vegetation, and began latest in species that foraged on the heavily shaded forest floor. Within each level of the forest, however, start time and eye size were correlated, just as in the temperate forest.

Fig. 10.38 Dawn chorus. Each day, birds begin to sing in the darkness before dawn. Try to pick out different species in this dawn chorus, recorded on a spring morning in western Oregon (USA). Listen online.
(Photograph by Janet Dodson.)
The quantity and often the quality of singing during the dawn chorus differ markedly from singing during the rest of the day. In many species, singers use the same kind of songs that they sing later in the day but sing them at a markedly faster rate. For example, a Buff‐breasted Flycatcher (Empidonax fulvifrons) in a neotropical forest sings the same song types throughout the day, but its dawn singing is four times faster than its singing later in the day (Lein 2007). Other species, such as the Chipping Sparrow (Spizella passerina), give dawn performances that are qualitatively different from their daytime singing (Liu and Kroodsma 2007). At dawn, a male sparrow often sits on the ground near a male from a neighboring territory and delivers bursts of song as if they were being shot from a machine gun. He will sing two or three elements of a song, pause very briefly, sing another burst followed by a pause, and so on. This dawn vocal display seems highly energized, even frantic. In contrast, during daytime singing, a male Chipping Sparrow sits on an exposed perch high in a tree and produces a longer version of his song that lasts about 2 seconds. Between songs, the sparrow pauses for 10 seconds or more, producing a more leisurely, relaxed display.
Reed Buntings (Emberiza schoeniclus) in Europe also sing differently at dawn than they do later in the day (Suter et al. 2009; Brunner and Pasinelli 2010). At dawn, a paired male sings a nearly continuous stream of 2.5‐second songs, with only a brief, 0.5‐second pause, or even no pause at all, between songs. Later in the morning, this fast‐paced dawn bout gives way to singing at a more relaxed pace. During daytime singing, males sing much more slowly (pausing 5–10 seconds between songs) and also sing shorter songs (about 1.5 seconds long).
Other species use entirely different songs at dawn than during the day. Certain North American wood‐warblers that have two song categories use their aggressive songs for the first 30–60 minutes of the morning and then lapse into a slower‐paced delivery of their other song. A male Yellow Warbler (Setophaga petechia), for example, uses about 12 song types delivered with immediate variety during his dawn chorus, and after half an hour or so he switches to his single daytime song type (Spector 1991). The male reverts to songs of his aggressive dozen during daytime encounters with other males, but the singing is never as energized as at dawn. Some New World flycatchers, such as the Eastern Wood‐Pewee (Contopus virens) (Fig. 10.39) and the Vermilion Flycatcher (Pyrocephalus rubinus), also use qualitatively different songs at dawn (Allard 1934).
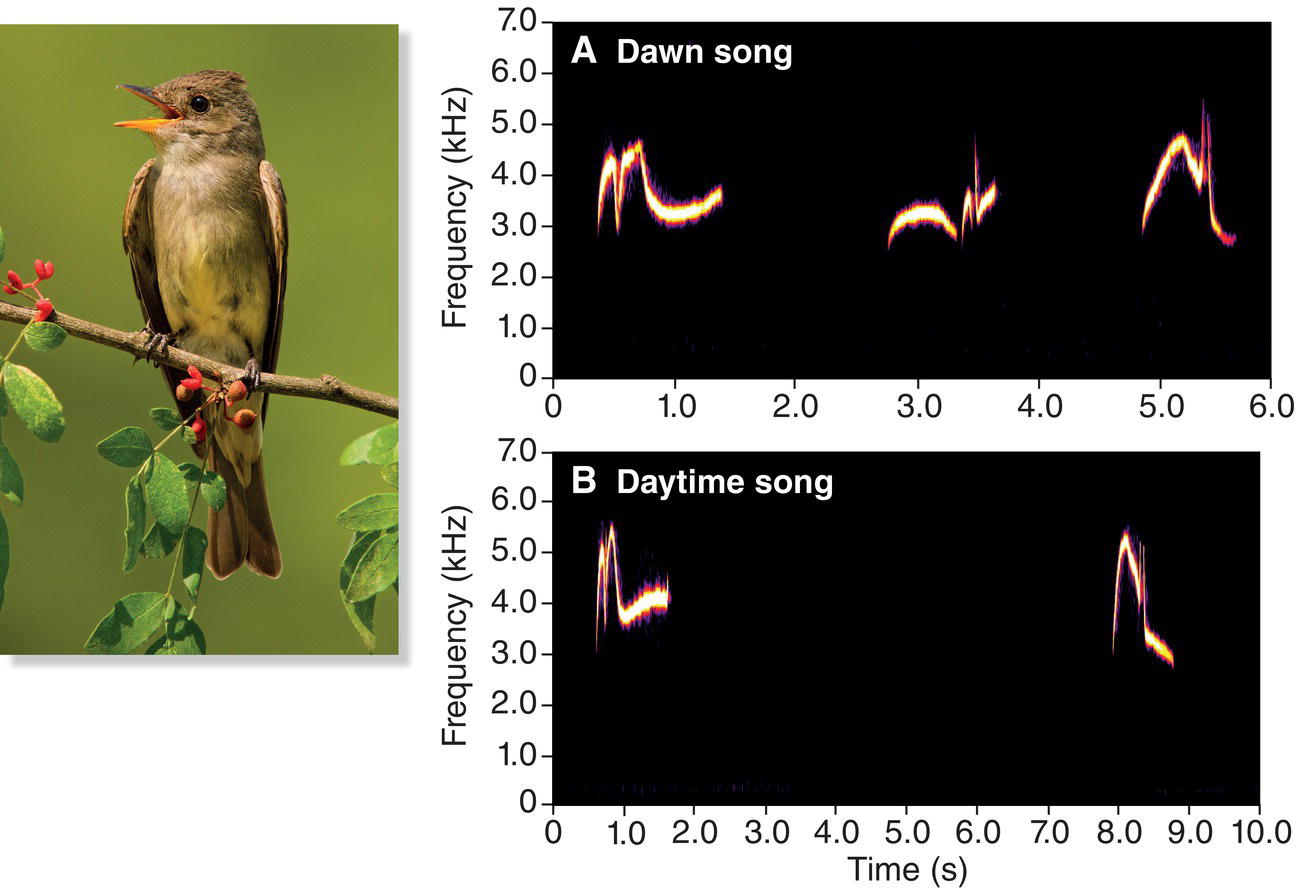
Fig. 10.39 Temporal variation in suboscine song. Some New World flycatchers, such as Eastern Wood‐Pewees (Contopus virens), sing (A) distinctive song types at dawn compared with (B) the rest of the day. Listen online.
(Recordist: Geoffrey A. Keller. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by Bob Dunlap.)
The dawn bout is a truly extraordinary display. Why is there such a burst of energetic singing at dawn? Researchers have proposed many ideas for why dawn singing is so dramatic. Perhaps males sing at dawn because that is the best time to attract females. Also, conditions at dawn are often calm, so song at that time may carry over the maximum possible distance. Furthermore, the dawn chorus occurs at a time of day too dark for effective foraging, so singing then rather than later is an efficient use of time. Or perhaps singing at dawn is especially important for territory defense; dawn follows the longest period of inactivity, and predation occurs at night, too, so dawn might be an important time for a bird to proclaim, “I am still alive” and “This territory is still mine.” The dawn chorus probably serves multiple purposes, with the relative emphases undoubtedly varying from species to species. More generally, the dawn chorus may serve as a ritualized display that establishes hierarchies and broadcasts information about them. Avian listeners can glean the information they need to establish and monitor the social relationships that form the basis of their complex societies.
10.8.2 Female song
A common misperception—especially among people who spend most of their time in the north temperate zone—is that song is primarily a behavior of male birds. This incorrect perception that song is solely a male trait also arises in part because most bird song research has been conducted on species that breed in the north temperate zone, where male‐only song is more prevalent. Overall, song is indeed more common in male birds, but females of hundreds of bird species also sing as part of their normal activities. Many of the functions of female song parallel those of males: females use song to defend territories against members of both sexes, for attracting and communicating with their mates, and for advertising their availability and condition. For example, studies of Alpine Accentors (Prunella collaris) in France have shown that females use song to attract male mates, and that the males can distinguish between songs of females and males (Langmore et al. 1996). Female song is particularly prevalent in birds in tropical regions around the world. In many such species, females sing less frequently or more quietly than males, but in other species the songs of females are common and conspicuous (Langmore 1998).

Fig. 10.40 Coordinated duets. Pairs of Bearded Barbets (Lybius dubuis) produce duets so well synchronized that they sound as if they are being made by a single bird.
(Photograph by Nathan Rupert.)
Some functions of song are particular to females. Female birds may use song to communicate about their reproductive state by letting potential mates (both their own social mate and other nearby males) know when they are in their fertile egg‐laying period. For example, studies of the Pied Bush‐Chat (Saxicola caprata) in Uttarakhand (India) have shown that females sing in various contexts, including in duets with their mate and during antagonistic interactions with other females. Their songs are most common during the early parts of the breeding cycle, with female Pied Bush‐Chats singing only rarely once they are incubating eggs (Sethi et al. 2012).
At a later state of the nesting cycle, some female birds may similarly use song to communicate with multiple fledglings that have left the nest. In Black‐headed Grosbeaks (Pheucticus melanocephalus) at a breeding site in Utah (USA), family groups of parents and fledglings start to wander away from their home territory about 3 weeks after the young birds leave the nest. These family groups use vocalizations—including songs from both parents—to stay together as they move through thick vegetation, and experiments have shown that the young birds are more responsive to songs of their own parents than to songs of unrelated grosbeaks (Ritchison 1983).
10.8.3 Duetting
The members of a mated pair of birds communicate with one another frequently, and in many species the communication includes vocal signals. In some species these vocal exchanges are simply overlapping bouts of calling, but in others they involve coordinated singing (Fig. 10.40). Such coordinated duets differ among the species in which they occur. The difference can be in how often mates join in duets, in the relative complexity of the male and female sounds, in how precisely the vocal exchanges are timed, and in the nature of the overlap between the male and female songs (from synchrony to antiphony, in which the male and female rapidly alternate their contributions).
The Carolina Wren (Thryothorus ludovicianus), a common songbird of the eastern USA, has a simple form of duet (Shuler 1965). Male Carolina Wrens sing a loud, resounding “teakettle‐teakettle‐teakettle,” each song consisting of a complex syllable (“teakettle”) repeated several times. On occasion, the female appends to this song a coarse rattle or “churr”—unmistakably the female Carolina Wren announcing her social bond to her mate (Fig. 10.41). The quality of male and female utterances differs markedly: females are only occasional participants, and the timing of the male and female contributions does not appear to be well coordinated.
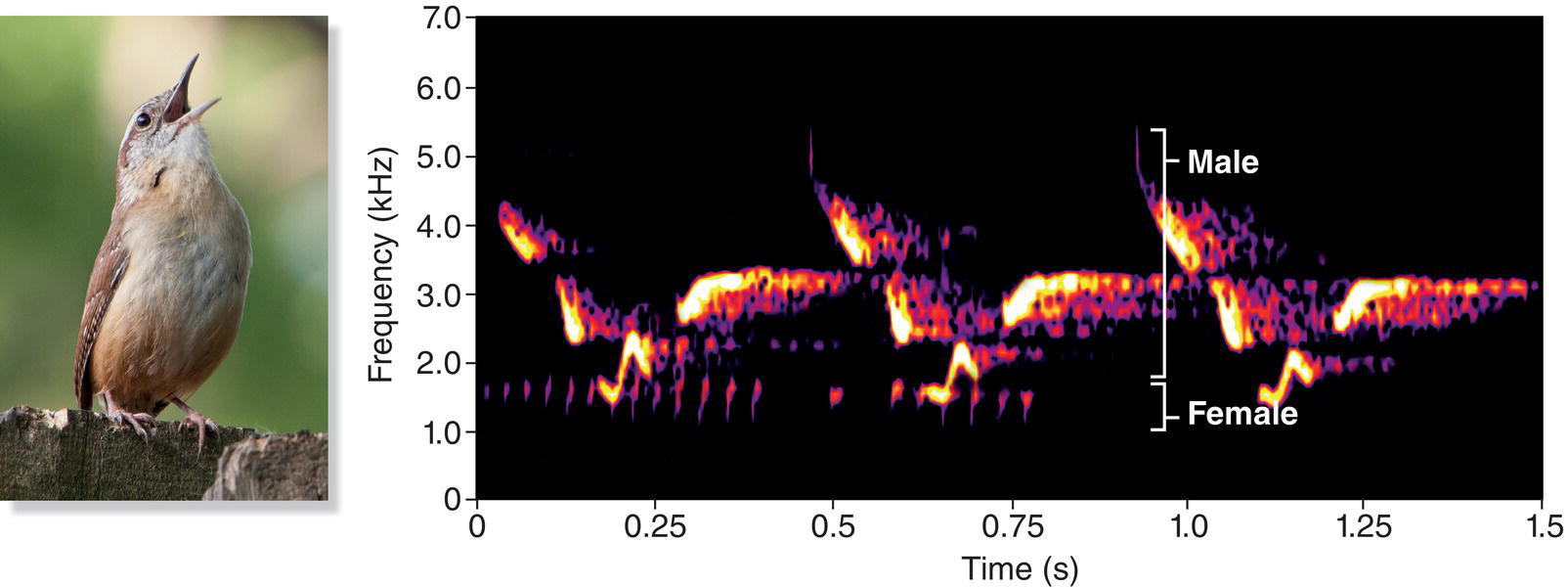
Fig. 10.41 A simple duet. Male and female Carolina Wrens (Thryothorus ludovicianus) perform a simple duet without tight choreography. A male repeats a loud phrase several times, while his mate occasionally adds a low‐frequency rattle. Listen online.
(Recordist: Gerrit Vyn. Courtesy of Macaulay Library © Cornell Lab of Ornithology. Photograph by David Mozzoni.)
Far more extraordinary are the complex, highly coordinated duets sung by some related wren species. So coordinated are some of these singing efforts that it is impossible for a human listener to differentiate between the male and female contributions unless the two singing birds are separated by a considerable distance. Among the Cantorchilus wrens of the neotropics, the Buff‐breasted (Cantorchilus leucotis), Bay (Cantorchilus nigricapillus), Riverside (Cantorchilus semibadius), and Plain (Cantorchilus modestus) Wrens sing duets of this sort (Levin 1996; Mann et al. 2003, 2009). In these duets, male and female contributions are equally complex, exchanges are highly coordinated and precise, and both sexes participate frequently.
Most duetting bird species live in the tropics, where duetting also occurs in various species of parrots, woodpeckers, bushshrikes, fairy‐wrens, whipbirds, drongos, antbirds, flycatchers, monarch flycatchers, shrikes, and weavers, among others. The occurrence of duetting in these settings is almost certainly related to the long‐term pair‐bonds of many tropical species. In these species, pairs maintain prolonged monogamous bonds and remain on their territory year round. The two members of a pair thus have a long‐lasting relationship and a long‐term shared investment in a territory; these factors may foster a communication system in which both partners benefit from sending highly coordinated signals that help defend their joint territory and maintain their pair bond.
10.8.4 Mimicry
Mimicry is a fascinating consequence of song learning: birds of some species learn not only their own songs but also the songs of other species. In North America, the Northern Mockingbird (Mimus polyglottos) is perhaps the best‐known example of an accomplished mimic: male mockingbirds sing up to 200 different song types, and a considerable number are clearly copied from other bird species. Renowned mimics elsewhere include the lyrebirds of Australia and Lawrence’s Thrush (Turdus lawrencii) in South America (Fig. 10.42).

Fig. 10.42 Vocal mimicry. Lawrence’s Thrush (Turdus lawrencii) is a skilled mimic of other forest birds in South America. Listen online.
(Photograph by Daniel Lane.)
Species not typically thought of as mimics also occasionally learn the vocalizations of other species. In North America, Blue Jays (Cyanocitta cristata) imitate the calls of various species of hawks, for example. European Starlings (Sturnus vulgaris) are excellent mimics. A study of starlings in five European populations documented high‐fidelity mimicry of more than 70 different sounds, including a cat’s meow, a frog’s call, a squeaky door, and the vocalizations of bird species ranging across the taxonomic spectrum, from owls, gulls, ducks, and sandpipers to thrushes, warblers, finches, cuckoos, and more (Hausberger et al. 1991).
In some species, mimicry occurs only very rarely and seems to be a maladaptive side effect of song learning. Well‐documented examples from North America include a Vesper Sparrow (Pooecetes gramineus) singing songs of the Bewick’s Wren (Thryomanes bewickii) (Kroodsma 1972), a Chestnut‐sided Warbler (Setophaga pensylvanica) singing Indigo Bunting (Passerina cyanea) songs (Payne et al. 1984), and a Cerulean Warbler (Setophaga cerulea) singing Hooded Warbler (Setophaga citrina) songs (Boves et al. 2010). In all of these cases, the males who learned the wrong species’ songs remained unpaired, thereby paying a fitness price for their learning error.
For species in which mimicry is routine and normal, researchers have proposed a number of hypotheses to explain its occurrence. One hypothesis is that mimics use other species’ songs to help exclude those species from the mimics’ territories. This possibility has not yet been thoroughly investigated. Or perhaps learning the songs of other species is simply an easy way for a songbird to develop a diverse repertoire of novel sounds, which might be used to impress a female. This idea is supported by studies of Marsh Warblers (Acrocephalus palustris) at breeding sites in Belgium; these warblers learn and mimic both the songs of other local breeding birds and those of species that they encounter only on their overwintering sites in Africa during their non‐breeding season (Dowsett‐Lemaire 1979).
10.8.5 Flight songs
Although most birds sing while perched, some sing in flight. Many shorebirds produce long, complex songs in flight above their breeding grounds (Miller 1983; Miller et al. 1988). Sky Larks (Alauda arvensis) impress us by delivering their seemingly unending soliloquy from great heights (Fig. 10.43). Hummingbirds, too, often combine flight and sounds—which can combine vocalizations with sounds produced by their wings and tail (Clark 2011)—in their impressive courtship displays (Fig. 10.44).

Fig. 10.43 Flight song. In lengthy displays, Eurasian Sky Larks (Alauda arvensis) spiral into the sky while repeatedly singing whistled notes. At the peak height of their display, they turn and rapidly descend into their territory while continuing to sing. Listen online.
(Photograph by Susanne Rintelen.)
Fig. 10.44 Aerial hummingbird displays. Hummingbirds of many species produce both vocal and non‐vocal sounds during aerial courtship displays. (A) This male Calliope Hummingbird (Selasphorus calliope) fans his neck feathers (arrow) in mid‐air, which creates vibrations in addition to a spectacular visual display—all while simultaneously singing—to court a nearby female. (B) Male Ruby‐throated Hummingbirds (Archilochus colubris) perform U‐shaped flight displays that include a wing buzz at the bottom of each arc. This auditory and visual display can deter competing males or attract receptive females.
(A, photograph by Tania Simpson. B, adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology.)
Flying is a physically demanding activity, so singing in flight is undoubtedly more difficult than singing from a perch. Why do some birds do it? In open country, birds may have few high perches from which to broadcast their songs, so singing in the air may be the best available option. Hence, most birds with flight songs are birds of grasslands or the arctic steppe. The extra energy required to deliver the songs in flight must be rewarded by an increase in the area over which the song can be heard.
However, some flight‐singing species do not quite fit this neat explanation for flight songs. The Ovenbird (Seiurus aurocapilla), a common warbler of forests in northeastern North America, is a prime example (Lein 1981). Its flight song may be heard near dawn and dusk, sometimes even in the middle of the night, when an Ovenbird launches itself from near the forest floor up into the canopy and sometimes beyond. Elements of the normal Ovenbird song are embedded in the long, jumbled stream of the flight song, but this bird’s vocal performance in flight is very different from its normal earthbound sounds.
The Ovenbird’s flight song is just one of a multitude of astounding avian vocalizations. The evolutionary “goal” of a bird—to survive and leave offspring—is rather simple, but the role of sound in achieving this mission is extraordinary. A human listener hears short songs, long songs; simple songs, complex songs; musical songs, harsh songs; duets and solo performances; simple repertoires, huge repertoires; and much, much more. Young songbirds are learning, practicing, and babbling as they copy older birds, which pass traditions on to future generations, creating sounds that we have come to recognize and love.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Adret‐Hausberger, M. 1986. Temporal dynamics of dialects in the whistled songs of Starlings. Ethology 71:140–152.
- Airey, D. C., K. L. Buchanan, T. Szekely, C. K. Catchpole, and T. J. DeVoogd. 2000. Song, sexual selection, and a song control nucleus (HVc) in the brains of European sedge warblers. Journal of Neurobiology 44:1–6.
- Allard, H. A. 1934. The twilight song of the wood pewee, Myiochanes virens Linnaeus. American Naturalist 68:266–274.
- Amrhein, V., P. Korner, and M. Naguib. 2002. Nocturnal and diurnal singing activity in the nightingale: correlations with mating status and breeding cycle. Animal Behaviour 64:939–944.
- Arcese, P. 1989. Territory acquisition and loss in male song sparrows. Animal Behaviour 37:45–55.
- Baker, M. C., E. M. Baker, and M. S. A. Baker. 2001. Island and island‐like effects on vocal repertoire of singing honeyeaters. Animal Behaviour 62:767–774.
- Baker, M. C. and J. T. Boylan. 1995. A catalog of song syllables of Indigo and Lazuli Buntings. Condor 97:1028–1040.
- Baker, M. C. and L. R. Mewaldt. 1978. Song dialects as barriers to dispersal in white‐crowned Sparrows, Zonotrichia leucophrys nuttalli. Evolution 32:712–722.
- Baker, M. C., D. B. Thompson, G. L. Sherman, M. A. Cunningham, and D. F. Tomback. 1982. Allozyme frequencies in a linear series of song dialect populations. Evolution 36:1020–1029.
- Ballentine, B., J. Hyman, and S. Nowicki. 2004. Vocal performance influences female response to male bird song: an experimental test. Behavioral Ecology 15:163–168.
- Balsby, T. J. S. and J. W. Bradbury. 2009. Vocal matching by orange‐fronted conures (Aratinga canicularis). Behavioural Processes 82:133–139.
- Baptista, L. F. 1975. Song dialects and demes in sedentary populations of the White‐crowned Sparrow (Zonotrichia leucophrys nuttalli). University of California Publications in Zoology 105:1–52.
- Baptista, L. F. and L. Petrinovich. 1986. Song development in the white‐crowned sparrow: social factors and sex differences. Animal Behaviour 34:1359–1371.
- Baptista, L. F. and K. L. Schuchmann. 1990. Song learning in the Anna Hummingbird (Calypte anna). Ethology 84:15–26.
- Barnett, C. A. and J. V. Briskie. 2011. Strategic regulation of body mass and singing behavior in New Zealand Robins. Ethology 117:28–36.
- Beecher, M. D. 2008. Function and mechanisms of song learning in Song Sparrows. Advances in the Study of Behavior 38:167–225.
- Beecher, M. D., M. B. Medvin, P. K. Stoddard, and P. Loesche. 1986. Acoustic adaptations for parent–offspring recognition in swallows. Experimental Biology 45:179–193.
- Beecher, M. D., P. K. Stoddard, S. E. Campbell, and C. L. Horning. 1996. Repertoire matching between neighbouring song sparrows. Animal Behaviour 51:917–923.
- Beletsky, L. D., B. J. Higgins, and G. H. Orians. 1986. Communication by changing signals: call switching in red‐winged blackbirds. Behavioral Ecology and Sociobiology 18:221–229.
- Beletsky, L. D. and G. H. Orians. 1985. Nest‐associated vocalizations of female red‐winged blackbirds, Agelaius phoeniceus. Zeitschrift für Tierpsychologie 69:329–339.
- Berg, K. S., R. T. Brumfield, and V. Apanius. 2006. Phylogenetic and ecological determinants of the neotropical dawn chorus. Proceedings of the Royal Society B: Biological Sciences 273:999–1005.
- Berg, M. L., N. H. Beintema, J. A. Welbergen, and J. Komdeur. 2005. Singing as a handicap: the effects of food availability and weather on song output in the Australian reed warbler Acrocephalus australis. Journal of Avian Biology 36:102–109.
- Bermúdez‐Cuamatzin, E., A. A. Ríos‐Chelén, D. Gil, and C. M. Garcia. 2009. Strategies of song adaptation to urban noise in the house finch: syllable pitch plasticity or differential syllable use? Behaviour 146:1269–1286.
- Borror, D. J. and C. R. Reese. 1956. Vocal gymnastics in Wood Thrush songs. Ohio Journal of Science 56:177–182.
- Botero, C. A., R. J. Rossman, L. M. Caro, L. M. Stenzler, I. J. Lovette, S. R. de Kort, and S. L. Vehrencamp. 2009. Syllable type consistency is related to age, social status and reproductive success in the tropical mockingbird. Animal Behaviour 77:701–706.
- Boves, T. J., D. A. Buehler, and P. C. Massey. 2010. Interspecific song imitation by a Cerulean Warbler. Wilson Journal of Ornithology 122:583–587.
- Braaten, R. F. 2010. Song recognition in zebra finches: are there sensitive periods for song memorization? Learning and Motivation 41:202–212.
- Brenowitz, E. A. 2008. Plasticity of the song control system in adult birds. Pages 332–349 in Neuroscience of Birdsong (H. P. Zeigler and P. Marler, Eds.). Cambridge University Press, Cambridge, UK.
- Brumm, H. 2004. The impact of environmental noise on song amplitude in a territorial bird. Journal of Animal Ecology 73:434–440.
- Brunner, P. and G. Pasinelli. 2010. Variation in singing style use in the reed bunting Emberiza schoeniclus: influencing factors and possible functions. Journal of Avian Biology 41:388–397.
- Burt, J. M., S. E. Campbell, and M. D. Beecher. 2001. Song type matching as threat: a test using interactive playback. Animal Behaviour 62:1163–1170.
- Byers, B. E. 1996. Geographic variation of song form within and among Chestnut‐sided Warbler populations. Auk 113:288–299.
- Byers, B. E. 2007. Extrapair paternity in chestnut‐sided warblers is correlated with consistent vocal performance. Behavioral Ecology 18:130–136.
- Byers, B. E., K. L. Belinsky, and R. A. Bentley. 2010. Independent cultural evolution of two song traditions in the chestnut‐sided warbler. American Naturalist 176:476–489.
- Catchpole, C. K. 1973. The functions of advertising song in the sedge warbler (Acrocephalus schoenobaenus) and the reed warbler (A. scirpaceus). Behaviour 46:300–320.
- Catchpole, C. K. 1986. Song repertoires and reproductive success in the great reed warbler Acrocephalus arundinaceus. Behavioral Ecology and Sociobiology 19:439–445.
- Chaves, J. C., A. M. Cuervo, M. J. Miller, and C. D. Cadena. 2010. Revising species limits in a group of Myrmeciza Antbirds reveals a cryptic species within M. laemosticta (Thamnophilidae). Condor 112:718–730.
- Chilton, G. and M. R. Lein. 1996. Long‐term changes in songs and song dialect boundaries of Puget Sound White‐crowned Sparrows. Condor 98:567–580.
- Clark, C. J. 2011. Wing, tail, and vocal contributions to the complex acoustic signals of courting Calliope Hummingbirds. Current Zoology 57:187–196.
- Clark, J. A., P. D. Boersma, and D. M. Olmsted. 2006. Name that tune: call discrimination and individual recognition in Magellanic penguins. Animal Behaviour 72:1141–1148.
- Cuervo, A. M., A. Hernández‐Jaramillo, J. O. Cortés‐Herrera, and O. Laverde. 2007. Nuevos registros de aves en la parte alta de la Serranía de las Quinchas, Magdalena medio, Colombia. Ornitología Colombiana 5:94–98.
- Derégnaucourt, S. 2010. Interspecific hybridization as a tool to understand vocal divergence: the example of crowing in quail (genus Coturnix). PLoS One 5:e9451.
- Derrickson, K. C. 1987. Yearly and situational changes in the estimate of repertoire size in Northern Mockingbirds (Mimus polyglottos). Auk 104:198–207.
- DeVoogd, T. J., J. R. Krebs, S. D. Healy, and A. Purvis. 1993. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proceedings of the Royal Society B: Biological Sciences 254:75–82.
- Díaz, M., A. Parra, and C. Gallardo. 2011. Serins respond to anthropogenic noise by increasing vocal activity. Behavioral Ecology 22:332–336.
- Dooling, R. J. 2002. Avian Hearing and the Avoidance of Wind Turbines . National Renewable Energy Laboratory, Golden, CO.
- Dooling, R. J., M. R. Leek, O. Gleich, and M. L. Dent. 2002. Auditory temporal resolution in birds: discrimination of harmonic complexes. Journal of the Acoustical Society of America 112:748–759.
- Dowsett‐Lemaire, F. 1979. The imitative range of the song of the Marsh Warbler Acrocephalus palustris, with special reference to imitations of African birds. Ibis 121:453–468.
- Eberhardt, L. S. 1997. A test of an environmental advertisement hypothesis for the function of drumming in Yellow‐bellied Sapsuckers. Condor 99:798–803.
- Eens, M., R. Pinxten, and R. F. Verheyen. 1992. Song learning in captive European starlings, Sturnus vulgaris. Animal Behaviour 44:1131–1143.
- Eriksen, A., T. Slagsvold, and H. M. Lampe. 2011. Vocal plasticity—are pied flycatchers, Ficedula hypoleuca, open‐ended learners? Ethology 117:188–198.
- Eriksson, D. and L. Wallin. 1986. Male bird song attracts females—a field experiment. Behavioral Ecology and Sociobiology 19:297–299.
- Evans, W. R. and M. O'Brien. 2002. Flight Calls of Migratory Birds: Eastern North American Landbirds (CD‐ROM) . Old Bird, Inc., Ithaca, NY.
- Falls, J. B. and R. J. Brooks. 1975. Individual recognition by song in white‐throated sparrows II. Effects of location. Canadian Journal of Zoology 53:1412–1420.
- Farabaugh, S. M., A. Linzenbold, and R. J. Dooling. 1994. Vocal plasticity in budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. Journal of Comparative Physiology 108:81–92.
- Ficken, M. S., R. W. Ficken, and S. R. Witkin. 1978. Vocal repertoire of the Black‐capped Chickadee. Auk 95:34–48.
- Ficken, M. S. and J. Popp. 1996. A comparative analysis of passerine mobbing calls. Auk 113:370–380.
- Forstmeier, W. and B. Leisler. 2004. Repertoire size, sexual selection, and offspring viability in the great reed warbler: changing patterns in space and time. Behavioral Ecology 15:555–563.
- Gahr, M., R. Metzdorf, D. Schmidl, and W. Wickler. 2008. Bi‐directional sexual dimorphisms of the song control nucleus HVC in a songbird with unison song. PLoS One 3:e3073.
- Gammon, D. E. and M. C. Baker. 2004. Song repertoire evolution and acoustic divergence in a population of black‐capped chickadees, Poecile atricapillus. Animal Behaviour 68:903–913.
- Gaunt, S. L. L., L. F. Baptista, J. E. Sánchez, and D. Hernandez. 1994. Song learning as evidenced from song sharing in two hummingbird species (Colibri coruscans and C. thalassinus). Auk 111:87–103.
- Gentner, T. Q. and S. H. Hulse. 2000. Female European starling preference and choice for variation in conspecific male song. Animal Behaviour 59:443–458.
- Gibbs, H. L. 1990. Cultural evolution of male song types in Darwin's medium ground finches, Geospiza fortis. Animal Behaviour 39:253–263.
- Goldman, S. A. and F. Nottebohm. 1983. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proceedings of the National Academy of Sciences of the United States of America 80:2390–2394.
- González, C. and J. F. Ornelas. 2009. Song variation and persistence of song neighborhoods in a lekking hummingbird. Condor 111:633–640.
- Goodale, E. and S. W. Kotagama. 2005. Alarm calling in Sri Lankan mixed‐species bird flocks. Auk 122:108–120.
- Goodale, E. and S. W. Kotagama. 2006. Context‐dependent vocal mimicry in a passerine bird. Proceedings of the Royal Society B: Biological Sciences 273:875–880.
- Goodale, E. and J. Podos. 2010. Persistence of song types in Darwin's finches, Geospiza fortis, over four decades. Biology Letters 6:589–592.
- Grava, T., A. Grava, and K. A. Otter. 2009. Supplemental feeding and dawn singing in Black‐capped Chickadees. Condor 111:560–564.
- Greenberg, R. and J. S. Ortiz. 1994. Interspecific defense of pasture trees by wintering Yellow Warblers. Auk 111:672–682.
- Hailman, J. P. 1989. The organization of major vocalizations in the Paridae. Wilson Bulletin 101:305–343.
- Hailman, J. P. and M. S. Ficken. 1996. Comparative analysis of vocal repertoires, with reference to Chickadees. Pages 136–159 in Ecology and Evolution of Acoustic Communication in Birds (D. E. Kroodsma and E. H. Miller, Eds.). Cornell University Press, Ithaca, NY.
- Hailman, J. P., M. S. Ficken, and R. W. Ficken. 1985. The ‘chick‐a‐dee’ calls of Parus atricapillus: a recombinant system of animal communication compared with written English. Semiotica 56:191–224.
- Halfwerk, W. and H. Slabbekoorn. 2009. A behavioural mechanism explaining noise‐dependent frequency use in urban birdsong. Animal Behaviour 78:1301–1307.
- Hardouin, L. A., P. Tabel, and V. Bretagnolle. 2006. Neighbour–stranger discrimination in the little owl, Athene noctua. Animal Behaviour 72:105–112.
- Hausberger, M., P. F. Jenkins, and J. Keene. 1991. Species‐specificity and mimicry in bird song: are they paradoxes? A reevaluation of song mimicry in the European starling. Behaviour 117:53–81.
- Hile, A. G., T. K. Plummer, and G. F. Striedter. 2000. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Animal Behaviour 59:1209–1218.
- Hofstad, E., Y. Espmark, A. Moksnes, T. Haugan, and M. Ingebrigtsen. 2002. The relationship between song performance and male quality in snow buntings (Plectrophenax nivalis). Canadian Journal of Zoology 80:524–531.
- Hoi‐Leitner, M., H. Nechtelberger, and H. Hoi. 1995. Song rate as a signal for nest site quality in blackcaps (Sylvia atricapilla). Behavioral Ecology and Sociobiology 37:399–405.
- Holland, J., P. K. McGregor, and C. L. Rowe. 1996. Changes in microgeographic song variation of the Corn Bunting Miliaria calandra. Journal of Avian Biology 27:47–55.
- Horn, A. G., M. L. Leonard, L. Ratcliffe, S. A. Shackleton, and R. G. Weisman. 1992. Frequency variation in songs of Black‐capped Chickadees (Parus atricapillus). Auk 109:847–852.
- Howell, J. C. 1972. Communicative behavior in the cedar waxwing (Bombycilla cedrorum) and the bohemian waxwing (Bombycilla garrulus) (Doctoral dissertation). University of Michigan, Ann Arbor, MI.
- Hu, Y. and G. C. Cardoso. 2009. Are bird species that vocalize at higher frequencies preadapted to inhabit noisy urban areas? Behavioral Ecology 20:1268–1273.
- Hunter, M. L. and J. R. Krebs. 1979. Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. Journal of Animal Ecology 48:759–785.
- Illes, A. E. and L. Yunes‐Jimenez. 2009. A female songbird out‐sings male conspecifics during simulated territorial intrusions. Proceedings of the Royal Society B: Biological Sciences 276:981–986.
- Immelmann, K. 1969. Song development in the zebra finch and other estrildid finches. In Bird Vocalizations: Their Relations to Current Problems in Biology and Psychology (R. A. Hinde, Ed.). Cambridge University Press, Cambridge, UK.
- Ince, S. A. and P. J. B. Slater. 1985. Versatility and continuity in the songs of thrushes (Turdus spp.). Ibis 127:355–364.
- Ince, S. A., P. J. B. Slater, and C. Weismann. 1980. Changes with time in the songs of a population of Chaffinches. Condor 82:285–290.
- Jarvis, E. D., S. Ribeiro, M. L. da Silva, D. Ventura, J. Vielliard, and C. V. Mello. 2000. Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature 406:628–632.
- Jenkins, P. F. 1978. Cultural transmission of song patterns and dialect development in a free‐living bird population. Animal Behaviour 26:50–78.
- Johnson, L. S. and W. A. Searcy. 1996. Female attraction to male song in house wrens (Troglodytes aedon). Behaviour 133:357–366.
- Johnson, N. K. 1980. Character variation and evolution of sibling species in the Empidonax difficilis‐flavescens complex (Aves: Tyrannidae). University of California Publications in Zoology 112:1–151.
- Johnson, N. K. and J. A. Marten. 1988. Evolutionary genetics of Flycatchers. II. Differentiation in the Empidonax difficilis complex. Auk 105:177–191.
- Kiefer, S., A. Spiess, S. Kipper, R. Mundry, C. Sommer, H. Hultsch, and D. Todt. 2006. First‐year common nightingales (Luscinia megarhynchos) have smaller song‐type repertoire sizes than older males. Ethology 112:1217–1224.
- Klump, G. M. and M. D. Shalter. 1984. Acoustic behavior of birds and mammals in the predator context. I. Factors affecting the structure of alarm signals. II. The functional significance and evolution of alarm signals. Zeitschrift für Tierpsychologie 66:189–226.
- Konishi, M. 1963. The role of auditory feedback in the vocal behavior of the domestic fowl. Zeitschrift für Tierpsychologie 20:349–367.
- Kroodsma, D. E. 1972. Variations in songs of Vesper Sparrows in Oregon. Wilson Bulletin 84:173–178.
- Kroodsma, D. E. 1974. Song learning, dialects, and dispersal in the Bewick's wren. Zeitschrift für Tierpsychologie 35:352–380.
- Kroodsma, D. E. 1980. Winter Wren singing behavior: a pinnacle of song complexity. Condor 82:357–365.
- Kroodsma, D. E. 1981. Geographical variation and functions of song types in Warblers (Parulidae). Auk 98:743–751.
- Kroodsma, D. E. 1984. Songs of the Alder Flycatcher (Empidonax alnorum) and Willow Flycatcher (Empidonax traillii) are innate. Auk 101:13–24.
- Kroodsma, D. E. 1986. Song development by castrated marsh wrens. Animal Behavior 34:1572–1575.
- Kroodsma, D. E. 1989. Two North American song populations of the Marsh Wren reach distributional limits in the central Great Plains. Condor 91:332–340.
- Kroodsma, D. E. 2005. The Singing Life of Birds: The Art and Science of Listeniing to Birdsong . Houghton Mifflin Harcourt, Boston, MA.
- Kroodsma, D. E., D. J. Albano, P. W. Houlihan, and J. A. Wells. 1995. Song development by Black‐capped Chickadees (Parus atricapillus) and Carolina Chickadees (P. carolinensis). Auk 112:29–43.
- Kroodsma, D. E., B. E. Byers, S. L. Halkin, C. Hill, D. Minis, J. R. Bolsinger, J. A. Dawson, E. Donelan, J. Farrington, F. B. Gill, P. Houlihan, D. Innes, G. Keller, L. Macaulay, C. A. Marantz, J. Ortiz, P. K. Stoddard, and K. Wilda. 1999c. Geographic variation in Black‐capped Chickadee songs and singing behavior. Auk 116:387–402.
- Kroodsma, D. E. and R. A. Canady. 1985. Differences in repertoire size, singing behavior, and associated neuroanatomy among Marsh Wren populations have a genetic basis. Auk 102:439–446.
- Kroodsma, D. E., P. W. Houlihan, P. A. Fallon, and J. A. Wells. 1997. Song development by grey catbirds. Animal Behaviour 54:457–464.
- Kroodsma, D. E. and M. Konishi. 1991. A suboscine bird (eastern phoebe, Sayornis phoebe) develops normal song without auditory feedback. Animal Behavior 42:477–487.
- Kroodsma, D. E., W. C. Liu, E. Goodwin, and P. A. Bedell. 1999a. The ecology of song improvisation as illustrated by North American Sedge Wrens. Auk 116:373–386.
- Kroodsma, D. E. and L. D. Parker. 1977. Vocal virtuosity in the Brown Thrasher. Auk 94:783–785.
- Kroodsma, D. E. and R. Pickert. 1980. Environmentally dependent sensitive periods for avian vocal learning. Nature 288:477–479.
- Kroodsma, D. E. and R. Pickert. 1984. Sensitive phases for song learning: effects of social interaction and individual variation. Animal Behavior 32:389–394.
- Kroodsma, D. E., J. Sánchez, D. W. Stemple, E. Goodwin, M. L. Da Silva, and J. M. E. Vielliard. 1999b. Sedentary life style of Neotropical sedge wrens promotes song imitation. Animal Behaviour 57:855–863.
- Kroodsma, D. E. and J. Verner. 1978. Complex singing behaviors among Cistothorus wrens. Auk 95:703–716.
- Kroodsma, D. E., R. W. Woods, and E. A. Goodwin. 2002. Falkland Island Sedge Wrens (Cistothorus platensis) imitate rather than improvise large song repertoires. Auk 119:523–528.
- Lade, B. I. and W. H. Thorpe. 1964. Dove songs as innately coded patterns of specific behaviour. Nature 202:366–368.
- Lambrechts, M. M. and A. A. Dhondt. 1986. Male quality, reproduction, and survival in the great tit (Parus major). Behavioral Ecology and Sociobiology 19:57–63.
- Lampe, H. M. and G. P. Sætre. 1995. Female Pied Flycatchers prefer males with larger song repertoires. Proceedings of the Royal Society B: Biological Sciences 262:163–167.
- Langmore, N. E. 1998. Functions of duet and solo songs of female birds. Trends in Ecology and Evolution 13:136–140.
- Langmore, N. E., N. B. Davies, B. J. Hatchwell and I. R. Hartley. 1996. Female song attracts males in the alpine accentor Prunella collaris. Proceedings of the Royal Society B: Biological Sciences 263:141–146.
- Lanyon, W. E. 1978. Revision of the Myiarchus Flycatchers of South America. Bulletin of the American Museum of Natural History 161:427–628.
- Lanzone, M., E. Deleon, L. Grove, and A. Farnsworth. 2009. Revealing undocumented or poorly known flight calls of Warblers (Parulidae) using a novel method of recording birds in captivity. Auk 126:511–519.
- Lein, M. R. 1981. Display behavior of Ovenbirds (Seiurus aurocapillus) II. Song variation and singing behavior. Wilson Bulletin 93:21–41.
- Lein, M. R. 2007. Patterns of dawn singing by Buff‐breasted Flycatchers. Journal of Field Ornithology 78:343–351.
- Lengagne, T., T. Aubin, P. Jouventin, and J. Lauga. 2000. Perceptual salience of individually distinctive features in the calls of adult king penguins. Journal of the Acoustical Society of America 107:508–516.
- Levin, R. N. 1996. Song behaviour and reproductive strategies in a duetting Wren, Thryothorus nigricapillus. I. Removal experiments. Animal Behaviour 52:1093–1106.
- Liu, W. C. and D. E. Kroodsma. 2007. Dawn and daytime singing behavior of Chipping Sparrows (Spizella passerina). Auk 124:44–52.
- Lohr, B. and R. J. Dooling. 1998. Detection of changes in timbre and harmonicity in complex sounds by zebra finches (Taeniopygia guttata) and budgerigars (Melopsittacus undulatus). Journal of Comparative Psychology 112:36–47.
- Lohr, B., R. J. Dooling, and S. Bartone. 2006. The discrimination of temporal fine structure in call‐like harmonic sounds by birds. Journal of Comparative Psychology 120:239–251.
- Lovell, S. F. and M. R. Lein. 2005. Individual recognition of neighbors by song in a suboscine bird, the alder flycatcher Empidonax alnorum. Behavioral Ecology and Sociobiology 57:623–630.
- MacDougall‐Shackleton, E. A. and S. A. MacDougall‐Shackleton. 2001. Cultural and genetic evolution in mountain white‐crowned sparrows: song dialects are associated with population structure. Evolution 55:2568–2575.
- MacDougall‐Shackleton, S. A., G. F. Ball, E. Edmonds, R. Sul, and T. P. Hahn. 2005. Age‐ and sex‐related variation in song‐control regions in Cassin's finches, Carpodacus cassinii. Brain Behavior and Evolution 65:262–267.
- Manabe, K., R. J. Dooling, and E. F. Brittan‐Powell. 2008. Vocal learning in budgerigars (Melopsittacus undulatus): effects of an acoustic reference on vocal matching. Journal of the Acoustical Society of America 123:1729–1736.
- Mann, N. I., K. A. Dingess, F. K. Barker, J. A. Graves, and P. J. B. Slater. 2009. A comparative study of song form and duetting in neotropical Thryothorus wrens. Behaviour 146:1–43.
- Mann, N. I., L. Marshall‐Ball, and P. J. B. Slater. 2003. The complex song duet of the Plain Wren. Condor 105:672–682.
- Marler, P. 1955. Characteristics of some animal calls. Nature 176:6–8.
- Marler, P. 1970. A comparative approach to vocal learning: song development in white‐crowned sparrows. Journal of Comparative and Physiological Psychology 71:1–25.
- Marler, P. and S. Peters. 1988. Sensitive periods for song acquisition from tape recordings and live tutors in the swamp sparrow, Melospiza georgiana. Ethology 77:76–84.
- Marler, P. and V. Sherman. 1985. Innate differences in singing behaviour of sparrows reared in isolation from adult conspecific song. Animal Behaviour 33:57–71.
- Marler, P. and M. Tamura. 1964. Culturally transmitted patterns of vocal behavior in sparrows. Science 146:1483–1486.
- Marova, I. M., V. V. Ivanitskii, and O. D. Veprintseva. 2010. Individual, population, and geographic differentiation in advertising song of the Blyth's Reed Warbler, Acrocephalus dumetorum (Sylvidae). Biology Bulletin 37:846–860.
- McDonald, M. V. 1989. Function of song in Scott's seaside sparrow (Ammodramus maritimus peninsulae). Animal Behaviour 38:468–485.
- Miller, E. H. 1983. The structure of aerial displays in three species of Calidridinae (Scolopacidae). Auk 100:440–451.
- Miller, E. H., W. W. H. Gunn, and B. N. Veprintsev. 1988. Breeding vocalizations of Baird's Sandpiper Calidris bairdii and related species, with remarks on phylogeny and adaption. Ornis Scandinavica 19:257–267.
- Mockford, E. J. and R. C. Marshall. 2009. Effects of urban noise on song and response behaviour in great tits. Proceedings of the Royal Society B: Biological Sciences 276:2979–2985.
- Morton, E. S. 1977. On the occurrence and significance of motivation‐structural rules in some bird and mammal sounds. American Naturalist 111:855–869.
- Mountjoy, D. J. and R. E. Lemon. 1991. Song as an attractant for male and female European starlings, and the influences of song complexity of their response. Behavioral Ecology and Sociobiology 28:97–100.
- Nealen, P. M. and D. J. Perkel. 2000. Sexual dimorphism in the song system of the Carolina wren Thryothorus ludovicianus. Journal of Comparative Neurology 418:346–360.
- Nelson, D. A., K. I. Hallberg, and J. A. Soha. 2004. Cultural evolution of Puget Sound white‐crowned sparrow song dialects. Ethology 110:879–908.
- Nemeth, E. and H. Brumm. 2009. Blackbirds sing higher‐pitched songs in cities: adaptation to habitat acoustics or side‐effect of urbanization? Animal Behaviour 78:637–641.
- Neudorf, D. L. and S. A. Tarof. 1998. The role of chip calls in winter territoriality of Yellow Warblers. Journal of Field Ornithology 69:30–36.
- Nicholls, J. A. and A. W. Goldizen. 2006. Habitat type and density influence vocal signal design in satin bowerbirds. Journal of Animal Ecology 75:549–558.
- Nottebohm, F. 1981. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science 214:1368–1370.
- Nottebohm, F. 2005. The neural basis of birdsong. PLoS Biology 3(5):e164.
- Nottebohm, F. and M. E. Nottebohm. 1971. Vocalizations and breeding behaviour of surgically deafened Ring Doves (Streptopelia risoria). Animal Behaviour 19:313–327.
- Nowicki, S., W. A. Searcy, and M. Hughes. 1998. The territory defense function of song in song sparrows: a test with the speaker occupation design. Behaviour 135:615–628.
- Paton, J. A. and F. N. Nottebohm. 1984. Neurons generated in the adult brain are recruited into functional circuits. Science 225:1046–1048.
- Payne, R. B. 1981. Song learning and social interaction in indigo buntings. Animal Behaviour 29:688–697.
- Payne, R. B. 1983. The social context of song mimicry: song‐matching dialects in indigo buntings (Passerina cyanea). Animal Behaviour 31:788–805.
- Payne, R. B. 1985. Behavioral continuity and change in local song populations of village indigobirds Vidua chalybeata. Zeitschrift für Tierpsychologie 70:1–44.
- Payne, R. B. and P. Budde. 1979. Song differences and map distances in a population of Acadian Flycatchers. Wilson Bulletin 91:29–41.
- Payne, R. B., L. L. Payne, and S. M. Doehlert. 1984. Interspecific song learning in a wild Chestnut‐sided Warbler. Wilson Bulletin 96:292–294.
- Payne, R. B., L. L. Payne, and S. M. Doehlert. 1988. Biological and cultural success of song memes in indigo buntings. Ecology 69:104–117.
- Payne, R. B., W. L. Thompson, K. L. Fiala, and L. L. Sweany. 1981. Local song traditions in indigo buntings: cultural transmission of behavior patterns across generations. Behaviour 77:199–221.
- Petrinovich, L. 1985. Factors influencing song development in the white‐crowned sparrow (Zonotrichia leucophrys). Journal of Comparative Psychology 99:15–29.
- Petrinovich, L. and L. F. Baptista. 1987. Song development in the white‐crowned sparrow: modification of learned song. Animal Behaviour 35:961–974.
- Petrinovich, L., T. Patterson, and L. F. Baptista. 1981. Song dialects as barriers to dispersal: a re‐evaluation. Evolution 35:180–188.
- Price, J. J., L. Yunes‐Jimenez, M. Osorio‐Beristain, K. E. Omland, and T. G. Murphy. 2008. Sex‐role reversal in song? Females sing more frequently than males in the Streak‐backed Oriole. Condor 110:387–392.
- Radford, A. N. 2005. Group‐specific vocal signatures and neighbour–stranger discrimination in the cooperatively breeding green woodhoopoe. Animal Behaviour 70:1227–1234.
- Ratcliffe, L. and R. G. Weisman. 1985. Frequency shift in the fee bee song of the Black‐capped Chickadee. Condor 87:555–556.
- Reid, J. M., P. Arcese, A. L. Cassidy, S. M. Hiebert, J. N. M. Smith, P. K. Stoddard, A. B. Marr, and L. F. Keller. 2005. Fitness correlates of song repertoire size in free‐living song sparrows (Melospiza melodia). American Naturalist 165:299–310.
- Riebel, K. and P. J. B. Slater. 1999. Song type switching in the chaffinch, Fringilla coelebs: timing or counting? Animal Behaviour 57:655–661.
- Ritchison, G. 1983. The function of singing in female Black‐Headed Grosbeaks (Pheucticus melanocephalus): family‐group maintenance. Auk 100:105–116.
- Satischandra, S. H. K., P. Kodituwakku, S. W. Kotagama, and E. Goodale. 2010. Assessing “false” alarm calls by a drongo (Dicrurus paradiseus) in mixed‐species bird flocks. Behavioral Ecology 21:396–403.
- Scarl, J. C. and J. W. Bradbury. 2009. Rapid vocal convergence in an Australian cockatoo, the galah Eolophus roseicapillus. Animal Behaviour 77:1019–1026.
- Searcy, W. A. and E. A. Brenowitz. 1988. Sexual differences in species recognition of avian song. Nature 332:152–154.
- Sethi, V. K., D. Bhatt, and A. Kumar. 2012. Structure and context of female song in a tropical bird, the Pied Bush Chat. Current Science 103:827–832.
- Shuler, J. 1965. Duet singing in the Carolina Wren. Wilson Bulletin 77:405.
- Slabbekoorn, H. and A. den Boer‐Visser. 2006. Cities change the songs of birds. Current Biology 16:2326–2331.
- Slabbekoorn, H. and M. Peet. 2003. Birds sing at a higher pitch in urban noise. Nature 424:267.
- Slater, P. J. B. and S. A. Ince. 1982. Song development in Chaffinches: what is learnt and when? Ibis 124:21–26.
- Slater, P. J. B., S. A. Ince, and P. W. Colgan. 1980. Chaffinch song types: their frequencies in the population and distribution between repertoires of different individuals. Behaviour 75:207–218.
- Smith, W. J. 1969. Displays of Sayornis phoebe (Aves, Tyrannidae). Behaviour 33:283–322.
- Soha, J. A., D. A. Nelson, and P. G. Parker. 2004. Genetic analysis of song dialect populations in Puget Sound white‐crowned sparrows. Behavioral Ecology 15:636–646.
- Spector, D. A. 1991. The singing behavior of yellow warblers. Behaviour 117:29–52.
- Spector, D. A. 1992. Wood‐warbler song systems: a review of paruline singing behaviors. Current Ornithology 9:199–238.
- Stein, R. C. 1963. Isolating mechanisms between populations of Traill's Flycatchers. Proceedings of the American Philosophical Society 107:21–50.
- Stoddard, P. K., M. D. Beecher, C. L. Horning, and S. E. Campbell. 1991. Recognition of individual neighbors by song in the song sparrow, a species with song repertoires. Behavioral Ecology and Sociobiology 29:211–215.
- Striedter, G. F. 1994. The vocal control pathways in budgerigars differ from those in songbirds. Journal of Comparative Neurology 343:35–56.
- Suter, S. M., D. Ermacora, N. Rieille, and D. R. Meyer. 2009. A distinct reed bunting dawn song and its relation to extrapair paternity. Animal Behaviour 77:473–480.
- Suthers, R. A., J. M. Wild, and G. Kaplan. 2011. Mechanisms of song production in the Australian magpie. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 197:45–59.
- Suthers, R. A. and S. A. Zollinger. 2004. Producing song: the vocal apparatus. Annals of the New York Academy of Sciences 1016:109–129.
- Templeton, C. N., E. Greene, and K. Davis. 2005. Allometry of alarm calls: black‐capped chickadees encode information about predator size. Science 308:1934–1937.
- Thomas, R. J., T. Szekely, I. C. Cuthill, D. G. C. Harper, S. E. Newson, T. D. Frayling, and P. D. Wallis. 2002. Eye size in birds and the timing of song at dawn. Proceedings of the Royal Society B: Biological Sciences 269:831–837.
- Thorpe, W. H. 1958. Further studies of the process of song learning in the Chaffinch (Fringilla coelebs gengleri). Nature 182:554–557.
- Todt, D. and N. Geberzahn. 2003. Age‐dependent effects of song exposure: song crystallization sets a boundary between fast and delayed vocal imitation. Animal Behaviour 65:971–979.
- Todt, D. and H. Hultsch. 1996. Acquisition and performance of song repertoires: ways of coping with diversity and versatility. Pages 79–96 in Ecology and Evolution of Acoustic Communication in Birds (D. E. Kroodsma and E. H. Miller, Eds.). Cornell University Press, Ithaca, NY.
- Trainer, J. M. 1983. Changes in song dialect distributions and microgeographic variation in song of white‐crowned sparrows (Zonotrichia leucophrys nuttalli). Auk 100:568–583.
- Trainer, J. M. and R. J. Parsons. 2001. Uniformity of Long‐tailed Manakin songs from three localities in Costa Rica. Wilson Bulletin 113:431–434.
- van Casteren, A., J. R. Codd, J. D. Gardiner, H. McGhie, and A. R. Ennos. 2010. Sonation in the male common snipe (Capella gallinago gallinago L.) is achieved by a flag‐like fluttering of their tail feathers and consequent vortex shedding. Journal of Experimental Biology 213:1602–1608.
- Verner, J. 1976. Complex song repertoire of male long‐billed Marsh Wrens in eastern Washington. Living Bird 14:263–300.
- Weeden, J. S. and J. B. Falls. 1959. Differential responses of male Ovenbirds to recorded songs of neighboring and more distant individuals. Auk 76:343–351.
- Weisman, R., L. Ratcliffe, I. Johnsrude, and T. A. Hurly. 1990. Absolute and relative pitch production in the song of the Black‐capped Chickadee. Condor 92:118–124.
- West, M. J. and A. P. King. 1988. Female visual displays affect the development of male song in the cowbird. Nature 334:244–246.
- Whitney, C. L. 1992. Temporal stability of song in a local population of Wood Thrushes. Wilson Bulletin 104:516–520.
- Wood, G. A. 1984. Tool use by the Palm Cockatoo Probosciger aterrimus during display. Corella 8:94–95.