Chapter 8
Avian Food and Foraging
Thomas W. Sherry
Tulane University

(Photograph by Magnus Elander.)
Watching even common birds feed, as naturalists have done for centuries, can provoke insightful questions and lead to new observations about their diets, behaviors, and biology. Watching a thrush forage along the ground, you might wonder how it decides where to probe for an earthworm. At the seashore, witnessing an osprey suddenly stop mid‐flight and plummet to the water, you might ask how it determines when and where to fish.
In a tropical rainforest, some birds fly out to capture insects in the air, some specialize in probing in clusters of dead vegetation, while others restrict themselves to flipping fallen leaves. Still other birds consume mostly fruit, some feed only on large insects and small lizards, and others dive for fish in small streams. Why do bird species eat such different types of food? Why do some specialize on just a few kinds of feeding locations or prey types?
If you scrutinize the ground below a jacamar roost in a New World tropical rainforest, you might notice that in consuming its diet of large insects, the bird leaves behind a pile of butterfly wings, but never those of the locally abundant Heliconius butterflies, a group of slow‐flying species that should be easy for a bird to catch. Why do these birds avoid some types of insects but not others? You might notice that small, foliage‐gleaning birds such as warblers pop their heads up, scanning their surroundings even more often than they scan for food. How do birds avoid becoming prey themselves, and avoid other dangers associated with feeding?
While watching large flocks of terns, gannets, or boobies out over the ocean, or birds following army ants in a tropical rainforest, you might wonder how birds track their changing food patches from day to day and sort out who feeds where. You might observe that individual birds of the same species feed differently from one another. They possess the same species‐typical morphology, so why do they not feed similarly?
These are examples of fruitful observations and questions that arise when watching foraging birds. The purpose of this chapter is to summarize the vast observations and literature on birds’ diets, feeding behavior, and anatomical and morphological adaptations for foraging.
8.1 Basics: meeting energy and nutritional demands
A bird’s metabolism largely determines how much food it requires (Chapter 7). All birds are homeotherms with a generally high metabolic rate. Homeothermy is energetically costly, necessitating frequent eating. Because of this high cost, most birds either eat high‐energy plant foods—such as seeds, fruit, and nectar—or they gain high‐energy protein and lipids from eating animals. The metabolic rate typically scales with body size, so that larger birds consume more food in total than smaller birds, but consume less food per unit of body mass (Chapter 7). Conversely, small birds have a higher metabolism per unit of mass, requiring a higher feeding rate; this in turn makes small birds more sensitive to feeding interruptions caused by challenges such as inclement weather.
8.1.1 Calories and nutrients
Aside from these general patterns, birds vary considerably in their energy needs. For example, birds that live in cold environments may need to elevate their metabolic rate just to maintain body temperature. Hot environments also may require increased energy expenditure as birds use evaporative cooling via panting and the wetting of body parts. For example, a pelican sitting in the sun on a hot day may visibly flutter the neck membranes of its gular pouch, an effective but energetically expensive form of evaporative cooling (Chapter 7).
Birds living in seasonal environments undergo annual changes in energy‐demanding activities such as migration. Fats are the most efficient fuel for long‐distance migration (Chapter 12). Many birds put on fat reserves to buffer themselves against inhospitable or unpredictable circumstances—such as blizzards, monsoons, or droughts—that reduce feeding opportunities. Birds that rapidly accumulate fat reserves by increasing their consumption rate are described as going through a period of hyperphagia that is often accompanied by increased metabolism. Some birds may also conserve energy by decreasing their metabolic rate and simultaneously dropping body temperature, entering a state of torpor (Chapter 7).
Scientists measure food consumption in energetic units of calories. However, certain nutrients can constitute a disproportional currency in birds’ diets (Chapter 7). Willow Ptarmigans (Lagopus lagopus) of northern Europe and North America, for example, increase their intake of valuable nitrogen and phosphorus via their choice of particular plant foods. Geese and some ducks also feed selectively on the most digestible and high‐nutrient plants and plant parts. Calcium is a chemical element that birds often seek in particular food types. Calcium is often in short supply for female birds that require it to create eggshells for their young. Birds frequently obtain calcium by eating snails, bones, or other calcium‐rich foods that otherwise are not a major dietary component. A variety of seed‐ and plant‐eating birds additionally consume soil at clay licks (a habit known as geophagy). Studies in South America, where clay licks are known as “colpas,” have suggested that birds like macaws and their relatives visit them primarily as a source for sodium, an element that is rare in plants but important for animal physiology (Fig. 8.01). Evidence supporting this hypothesis includes the low sodium content of plants forming a major part of these birds’ diet, the high‐sodium clays selected by the birds, the location of colpas far inland and thus far from other natural sources of sodium (such as the ocean), the increased use of colpas by parent birds when feeding chicks who need dietary sodium for early growth (Brightsmith and Muñoz‐Najar 2004; Powell et al. 2009), and signs of sodium limitation in other animals like ants living in the same regions (Dudley et al. 2012). The colpa clays consumed by birds may also help them detoxify the alkaline compounds contained in some seeds (Powell et al. 2009).

Fig. 8.01 Clay as a nutritional supplement. Birds that regularly visit clay licks, such as these Red‐and‐green Macaws (Ara chloropterus), probably consume clay for its high sodium content.
(Photograph © Frans Lanting, www.lanting.com.)
8.1.2 Feeding rates and food abundance
Given a nutritious and available food source, how does a bird’s feeding rate change as prey availability, or concentration, increases? The simplest model of this feeding process, or functional response to the prey’s availability, is direct and linear. Figure 8.02A shows prey (or victim) mortality corresponding to a linear increase in predator feeding rate with prey density. This curve is flat because more prey are eaten by the predator as prey density increases, so the percent eaten does not change with density until the individual is satiated and feeding stops. This type of functional response results from passive feeders, which are rare among birds.

Fig. 8.02 Three alternative functional responses to prey availability. Arrows indicate satiation point. (A) Individuals may feed at a linear rate, regardless of prey availability, until they are satiated. (B) Alternatively, individuals may initially feed at high rates, then gradually become more selective as they near satiation and prey become easier to find. (C) Still other individuals may feed at low rates (reflective of low prey density), then rapidly increase their feeding rate as prey availability increases, and finally return to lower levels of prey exploitation as prey become highly abundant.
(From Holling 1965. Reproduced with permission from Cambridge University Press.)
A humped functional response curve is far more typical in birds and other predators (Fig. 8.02B). Such a curve indicates a rapid increase in consumption rate as prey density increases, but then a decreased feeding rate up to the point of satiation. This kind of relationship has been documented in Eurasian Oystercatchers (Haematopus ostralegus) feeding on mussels (Goss‐Custard et al. 1996). The humped functional response to prey density typically arises when birds respond rapidly to visible or easily detected prey, but slow their consumption rate as handling time per prey item increases. In other words, searching is important at low prey densities, but prey‐handling time takes over more of the consumer’s time as prey density increases. Under these circumstances, the percentage of prey consumed declines with prey density.
Ecologists have observed a third type of functional response in bird foraging, one depicted graphically as an S‐shaped or sigmoidal curve (Fig. 8.02C). This curve represents infrequent use of a rare resource, then a rapid increase in its exploitation with increasing density, and finally diminishing use as satiation is reached. Correspondingly, the percentage of prey consumed first increases, then decreases with increased prey density. One explanation for this pattern centers on the cognitive abilities of feeding birds, and proposes that as a bird increasingly encounters a cryptic prey type—such as a well‐camouflaged caterpillar or moth—it develops a mental representation of that prey type that helps guide its search. With increased consistent exposure, a specific search image can make the bird far more efficient in finding it. A similar process occurs when human birders first scan an unfamiliar habitat with binoculars. Cryptic birds often are hard to spot, but after you discover a few, you develop a mental search image for them and start to see them “everywhere.”
Alexandra Pietrewicz and Alan Kamil (1979) tested this experimentally in foraging birds. They projected images of cryptic moths to caged Blue Jays (Cyanocitta cristata) and measured the time it took for each bird to detect and peck at the image, getting a mealworm reward when each did so. With consecutive exposure to images of the same moth species, the Blue Jays detected the images increasingly efficiently. However, when the researchers shuffled images of differently colored moth species, the birds showed little improvement in detecting any particular moth type. In effect, the sequential encounter of various different moth types prevented the development of an efficient search image for any particular moth.
This classic example illustrates a trade‐off in which efficiency in detecting certain kinds of prey decreases the efficient detection of other types. A more recent example comes from Herring Gulls (Larus argentatus) and Great Black‐backed Gulls (Larus marinus) hunting crabs in New England intertidal and subtidal zones (Ellis et al. 2012). Both gull species detect and consume Jonah crabs far more frequently than predicted based on abundance, probably because this crab is far easier for the gulls to capture and handle than two other much more common crab species. The Blue Jay and gull examples both illustrate the widespread phenomenon of birds specializing to increase their foraging efficiency, a pattern illustrated by the classic proverb that a “jack of all trades is master of none.”
8.2 Optimizing what, when, where, and how to forage
All birds must make foraging choices, such as deciding where to feed, what search path to follow, how long to persist in a food patch, what foods to pursue or bypass, whether to join a group, and how to balance feeding with other considerations such as avoiding predators. These decisions constitute the fundamental questions of foraging behavior. Researchers create models as tools to describe how animals make decisions, given assumptions and constraints about the foraging process, and then test these models using observations and experiments.
8.2.1 Optimal Foraging Theory
Optimization models are an important way to understand animal behaviors such as foraging, habitat use, and communication. Optimal Foraging Theory is an important subdiscipline of this general field. The underlying assumption of such models is that natural selection favors those individuals that perform best—as foragers, in the context of this chapter—because efficient foraging leads to higher survival and more offspring (Chapter 3). Optimal Foraging Theory thereby helps researchers make explicit predictions about how birds should behave.
Models of optimal foraging decisions typically have three components. First, what is the form of natural selection at work, and over what time period does it occur? For example, what matters more: to maximize the energy harvested over short time periods such as hours or days, or to minimize the likelihood of starvation or death over a longer season? Second, what behavioral options or choices are available to, and being exercised by, the bird? These options could include whether or not to attack a particular prey individual or how long to persist foraging in a resource patch. Third, what constraints are limiting the variety of foraging options? Limitations can arise from many sources, for example, gape size limits the maximal size of prey that birds can swallow, whereas lung capacity for oxygen constrains dive (search) times in diving birds. In fact, all factors that influence what birds actually consume relative to the food available are potential constraints in optimal foraging models.
One of the most basic decisions foraging birds make is whether to spend time and energy attacking a particular prey item they have already encountered, or instead to continue searching for more profitable prey. The very first optimal foraging models looked at the inherent trade‐offs between the energy in each prey type, the search time needed to find it, and the handling time required to catch and consume it, comparing the costs and benefits of feeding as a generalist (attacking all items encountered) versus a specialist (passing over some items). In these models it is generally assumed that natural selection favors optimizing short‐term energy gains, and the choice available to the forager is whether or not to pursue a particular prey item when encountered. The constraints include the overall abundance of the prey (which influences search time) and the profitability of the prey (which usually is assessed using handling time since more profitable items provide more energy per item and require less handling time). These models, formulated with simple algebra, predict, among other things, that foragers should ignore less profitable items as long as more profitable items are sufficiently abundant. The original prey choice models and their later variants now have been tested hundreds of times through observations of wild birds. These field studies typically support the prediction that greater overall food abundance favors greater diet selectivity.
Occasionally, predictions from optimal foraging models are not supported. This could mean either that birds do not always forage optimally, or that some assumptions of a particular model were not met under the circumstances in which it was tested. The real world is not nearly as simple as optimal foraging models assume: prey often are not encountered sequentially, foragers often lack the perfect knowledge of all prey types needed to make optimal decisions, and foragers are selected for traits other than optimizing feeding rate over short time intervals. For example, birds might forage, seemingly suboptimally, on low‐quality prey found near safe cover if moving out to forage on better quality prey would cause the foraging birds to become more vulnerable to predators.
In a classic study of optimal foraging, Reto Zach (1979) tested aspects of prey choice and harvesting behavior in Northwestern Crows (Corvus caurinus) along the coast of British Columbia, Canada. He noticed that the crows selected and broke open whelks by flying up and repeatedly dropping these marine snails onto rocks. A variety of birds drop food items onto hard substrates to access food: Golden Eagles (Aquila chrysaetos) drop tortoises, American Crows (Corvus brachyrhynchos) drop black walnuts, and Lammergeiers (Gypaetus barbatus) drop bones (Davenport et al. 2014). In this case Zach observed that the birds selected only the largest whelks and that the drop height was consistently about 5 meters. Zach questioned why the crows bypassed smaller whelks containing perfectly good meat and why the drop height was not lower, which would use less energy. To mimic this behavior, he performed his own whelk drops using a long pole equipped with a pulley that hoisted a small, tippable platform. Assuming the crows were foraging optimally, he predicted that the shells of larger whelks would break after fewer drops than those of smaller whelks, and that the likelihood of a shell breaking would increase little as the drop height rose above 5 meters. His results confirmed these predictions: larger whelks required fewer drops to break (and thus required less effort) than smaller whelks, regardless of drop height, and drops above 5 meters provided little improvement in breakage, regardless of whelk size (Fig. 8.03). These results support the idea that the crows adopted whelk‐foraging tactics that optimize their short‐term energy harvest. John Davenport et al. (2014) extended our understanding of this behavior in a study of Carrion Crows (Corvus corone) and Hooded Crows (Corvus cornix) in the UK. Their results similarly showed that the birds based foraging decisions on efficiency of mussel shell breakage rather than travel costs.
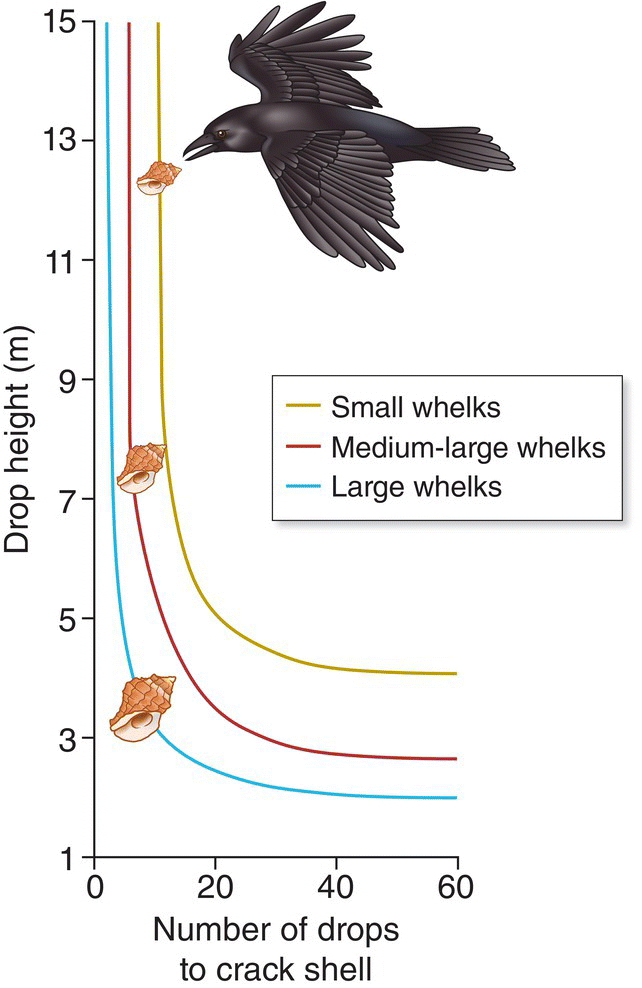
Fig. 8.03 Optimal foraging behavior. Northwestern Crows (Corvus caurinus) almost exclusively prefer to forage on larger shelled whelks (a type of mollusk). They drop shells onto rocks to crack them. Smaller whelks require more and higher drops (gold), whereas larger whelks can be dropped from lower heights with a higher rate of opening success (blue). There is an additional trade‐off in the effort necessary to carry, drop, and retrieve various whelks; larger whelks are often stolen by other birds. For these crows, dropping medium–large whelks (red) from a height of 5 meters is optimal.
(Adapted from Zach 1979. © Cornell Lab of Ornithology.)
8.2.2 Optimal prey searching strategies
Optimal Foraging Theory also addresses how birds should optimize the way they search for prey. For example, many birds capable of foraging over large areas of open ocean, like albatrosses and penguins, face the problem of detecting sparsely scattered prey patches. A major constraint facing such foragers is a paucity of information to guide them to high‐quality feeding locations. One potential strategy animals employ in such circumstances is Lévy flight—named after a French mathematician who introduced the concept in 1937. Lévy flight is characterized by short, randomly oriented searches interspersed by occasional longer flights, and it provides a useful way to understand how some birds forage (Ornes 2013).
Several recent studies have explored how seabirds search for food over vast distances of open ocean. In one, researchers used GPS satellite data to track Wandering Albatrosses (Diomedea exulans) over antarctic waters. The locational data were coupled with temperature data‐loggers placed in the birds’ stomachs that allowed the biologists to determine the mass and timing of prey eaten (Humphries et al. 2012). This integrated approach showed that some albatrosses used Lévy‐like flight patterns (Fig. 8.04), whereas others used random search paths, and some used a mixture of the two. A different research group (Watanabe and Takahashi 2013) examined the search paths of Adelie Penguins (Pygoscelis adeliae) foraging on krill in the open ocean near Antarctica using cameras, data‐loggers, and motion detectors attached to the penguins (Fig. 8.05). They found that these birds also had search patterns characteristic of Lévy foraging paths to optimize the discovery of patchily and sparsely distributed prey.
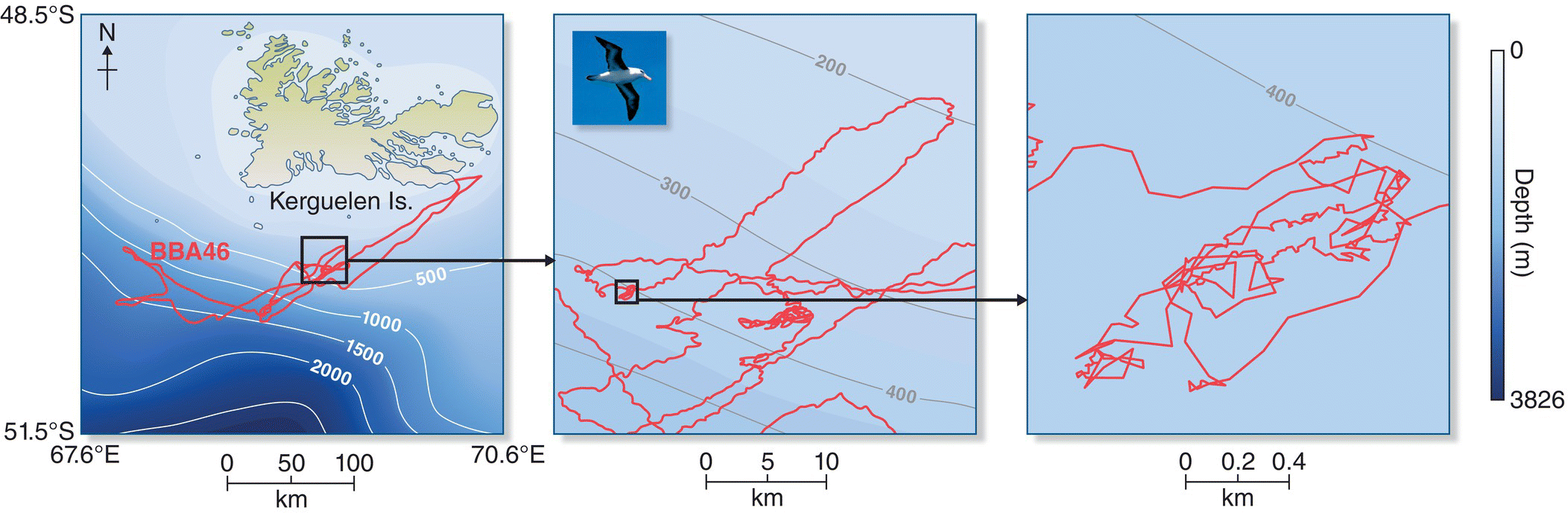
Fig. 8.04 Lévy flight foraging hypothesis. Black lines trace the flight path of an individual Black‐browed Albatross (Thalassarche melanophris) foraging off the Kerguelen Islands in the southern Indian Ocean. These tracks reveal a Lévy‐congruent flight pattern: short, randomly oriented searches interspersed with longer flights. Background color denotes depth (meters); areas within red squares are enlarged in adjacent right panels.
(From Humphries et al. 2012. Reproduced with permission from National Academy of Sciences, USA.)
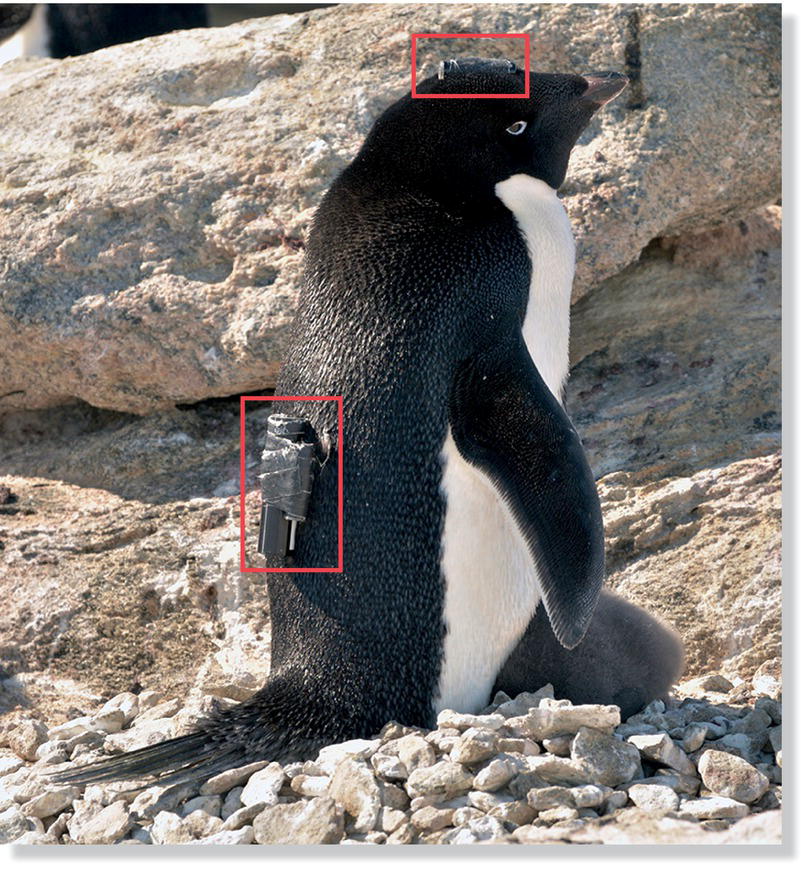
Fig. 8.05 Tracking foraging penguins. Researchers identified foraging patterns by fitting two motion detectors and a video camera (red rectangles) to Adelie Penguins (Pygoscelis adeliae). When hunting at sea, these penguins use a combination of long search trips with numerous short forays to find patchily distributed prey.
(Photograph by Yuuki Watanabe.)
Optimal Foraging Theory also addresses how long a forager should stay in a particular patch of food. This situation is faced by foraging animals that discover that finding food becomes harder, with diminishing returns the longer they stay in a patch (Fig. 8.06A). Given the three components of such models, natural selection should cause the bird to maximize its long‐term food gain rate. The bird’s behavioral choice is how long to stay in the patch as determined by experience. The bird should adopt a “give‐up time” rule that causes it to leave the patch when its rate of feeding diminishes to the average rate in the environment overall. The third component involves constraints, which in this model include both the diminishing return as the bird stays in a particular patch (Fig. 8.06A) and the time cost of leaving a patch to search for a new one. One prediction from this model, seen by comparing Fig. 8.06B and Fig. 8.06C, is that the longer the search‐and‐travel time span between patches, the longer the foraging bird should remain within a particular patch.
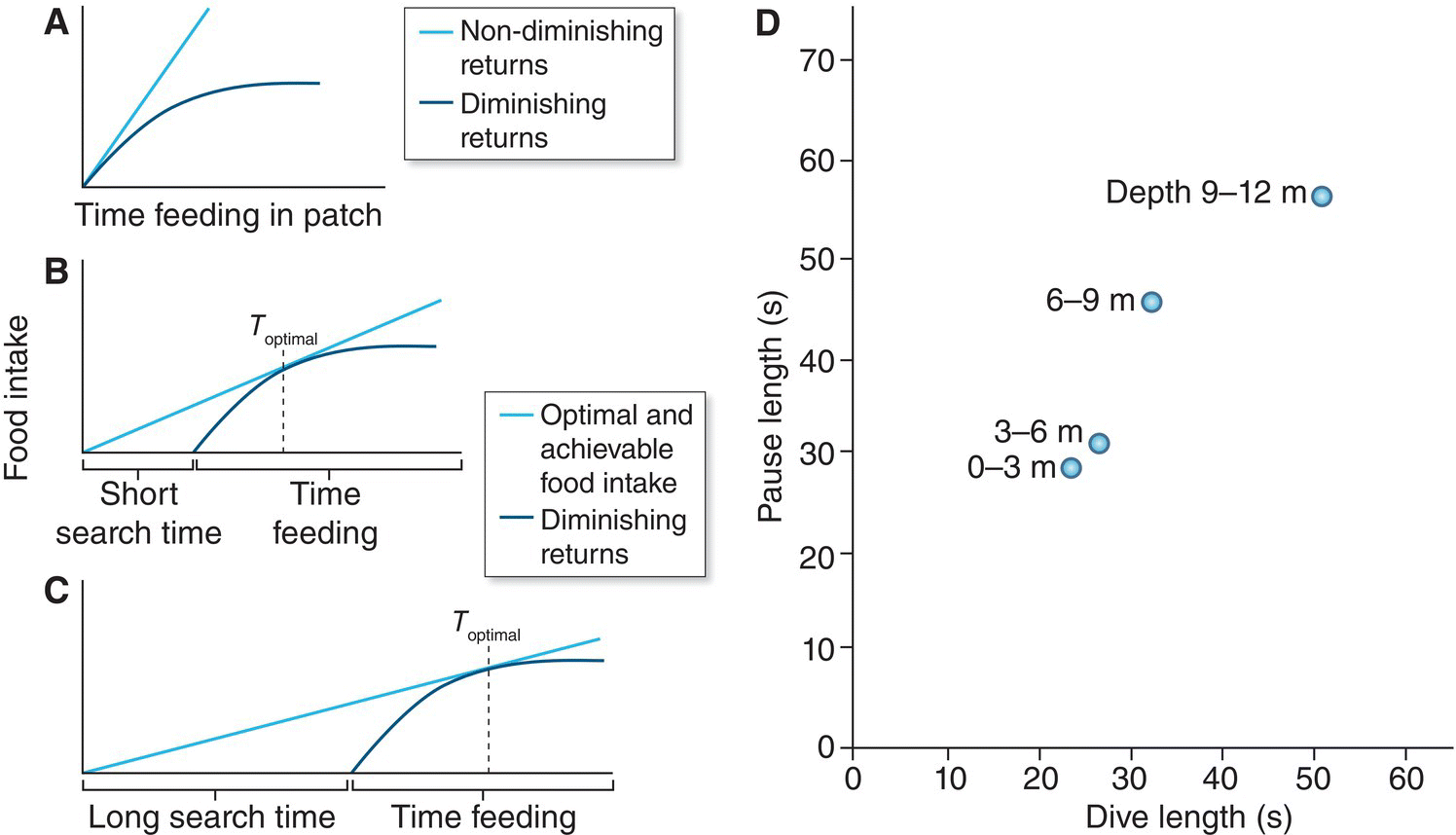
Fig. 8.06 An optimal foraging model for patch occupancy. (A) Food gain curves for feeding in a patch, with and without diminishing returns on time and effort. (B) Overall (net) rate of food gain: time feeding plus short search time. (C) Overall (net) rate of food gain: time feeding plus long search time. Note that despite identical diminishing returns curves in B and C, the optimal time to keep feeding in the patch is longer with a longer search time. (D) Actual dive times of Common Eiders (Somateria mollissima) foraging on bottom‐dwelling shellfish are directly associated with the pause length (seconds) after foraging dives. As diving time increases, so does the subsequent pause between dives, showing diminishing returns of deeper dives. Error bars denote means and standard errors of the mean for grouped dive times (10‐second groupings, x‐axis). Circles represent mean pause lengths for dives at specific depths (meters).
(A–C, reproduced with permission from Thomas W. Sherry. D, From Ydenberg and Guillemette 1991. Reproduced with permission from John Wiley and Sons.)
Working in the Gulf of St. Lawrence (Quebec, Canada), Ron Ydenberg and Magella Guillemette (1991) tested this model with Common Eiders (Somateria mollissima), which dive for invertebrates on the ocean bottom. The birds have to decide how long to stay on the bottom to forage in the food patch. In this example, unlike many cases of patch feeding in nature, the time between dives is spent coping with the physiological constraint of loading oxygen at the water’s surface rather than searching for a new patch. The diminishing returns arise because longer dives require proportionately longer recovery times on the surface as well as longer travel times between the surface and the food. The graphic models (Fig. 8.06B, C) predict most simply that optimal foraging time should increase with dive depth. This prediction of the model was supported: the pause (or recovery) time at the surface indeed corresponds with dive time, most of which is spent foraging on the bottom (Fig. 8.06D).
8.2.3 Foraging in groups
Birds foraging in groups may face additional decisions involving trade‐offs between benefits and costs. Group‐living individuals face questions including: (1) whether to join the group, taking into account the number of birds already in the group and food abundance; (2) how to forage and compete within the group; and (3) how to take advantage of or collaborate with other group members. Group living is complicated by the fact that the optimal behavior for an individual bird almost always depends on the choices made by other individuals, a scenario that can be modeled using evolutionary game theory. Consider, for example, a bird’s choice either to locate its own food or to steal food from another individual in the group. If all other individuals in the group are locating food, then a thief does well in the absence of competition from other thieves; conversely, if all other individuals are thieves, then locators do best. Neither pure strategy is an evolutionarily stable strategy because an individual adopting a particular strategy does better when more individuals in a group use the other strategy. This situation should lead to an equilibrium that balances the frequencies of individuals using different strategies: the best foraging decision varies depending on the frequency of individuals in the group making alternative decisions.
One example of such scrounging behavior comes from aviary experiments involving Common Ravens (Corvus corax). Individual birds made decisions about when to recover cached food depending on whether or not potential competitor individuals had witnessed the food being cached (Box 8.01). In a laboratory study, Kieron Mottley and Luc‐Alain Giraldeau (2000) constrained Scaly‐breasted Munias (Lonchura punctulata), small Southeast Asian seed‐eaters that typically feed in small flocks, to be either food finders or scroungers. In each flock of six individuals, the proportion of finders and scroungers was changed systematically from 6:0 (finders to scroungers) to 0:6. Although all individuals were trained to act as finders by pushing a bar that dumped seeds into a communal feeding dish, only the birds on one side of a partition—the finders—had access to the bar and could generate new resources. The birds on the other side were effectively scroungers, and relied on the finders to push the bar and make seeds available to the birds on both sides of the partition. As predicted, scroungers fared worse in terms of feeding rate when there were more scroungers, whereas the finders’ feeding rates changed little under the different ratios of feeding roles (Fig. 8.07). Notably, scroungers did best when they were rare, yet did not have a large effect on finders. Finders did best when they were rare, and the cost of competing with other finders did not change significantly as the number of scroungers increased. This result parallels that in nature, which tends to maintain a balanced equilibrium of both finders and scroungers. In a follow‐up experiment, the researchers found that under less restricted conditions, individual Scaly‐breasted Munias did in fact switch strategies to reach an equilibrium.

Fig. 8.07 Optimal producer and scrounger strategies. Laboratory flocks of Scaly‐breasted Munias (Lonchura punctulata) were divided into two foraging strategies: “producers” that fed by jumping on a bar (which spilled out food) and “scroungers” that only fed on seed spilled by the producers. As predicted by theory, producers or finders (orange circles) fed at a consistent rate regardless of the number of scroungers. However, scroungers (blue circles) fed at high rates when few other individuals were scroungers, but experienced dramatically lower feeding success as the number of competing scroungers increased. A similar process may apply to wild birds that find or follow other individuals to new food sources.
(From Mottley and Giraldeau 2000. Reproduced with permission from Elsevier. Photograph by Ravivaidya, https://en.wikipedia.org/wiki/File:Scaly_breasted_munia_feeding.jpg. CC‐BY‐SA‐3.0.)
Optimal Foraging Theory has been used to explore many other aspects of foraging behavior. For example, the threat of predation means that birds often cannot simultaneously maximize feeding efficiency and safety (Cresswell 2008; Bonter et al. 2013). Consistent with this idea, Black‐capped Chickadees (Poecile atricapillus) of North America carry food from an exposed birdfeeder back to cover (safety) more often when they perceive that staying at the feeder exposes them to predation risk and when carrying the food entails relatively little cost. This idea was first tested experimentally by simulating the presence of a predator using a model hawk “flown” past a birdfeeder on a wire (Lima 1985). For these chickadees, moving to cover had short‐term costs, because the added transport time decreased their feeding efficiency, but it increased their long‐term survival. Predators pose a threat to most birds, which are often particularly vulnerable or conspicuous during foraging.
8.3 Diversity of foods and foraging behaviors
Optimal foraging models attempt to understand how birds might generally optimize different aspects of feeding behavior given available choices and constraints. However, another way to understand avian diets and feeding is to look at the many differences among birds. Birds eat virtually every kind of food imaginable, even some that are inedible to most other organisms. Generalist bird species eat diverse foods, but most bird species are specialists and eat only a narrow array of available foods. The diversity of birds’ diets and foraging reflects selectivity by birds with different attributes and skills. However, an improved ability to detect, capture, handle, swallow, or digest one type of food often decreases a bird's ability to use other types.
8.3.1 New technologies for studying diets
Ornithologists use diverse methods to test hypotheses about foraging and determine what birds eat. Two new technologies deserve special mention because they are being applied widely to understand what birds eat when their diet is difficult to observe directly, such as when birds feed over vast ocean areas or feed on small items. The first such technology tracks diets using stable isotopes—chemical signatures derived from birds’ food and stored in their tissues such as blood and bone. Stable isotopes are forms of an element that differ in atomic weight, and their relative frequencies after being incorporated into bird tissues provide signals about feeding. For example, stable isotopes of carbon vary naturally depending on latitude and plant productivity, and among types of plants with different types of photosynthesis. Stable nitrogen isotopes differ in ocean versus terrestrial environments, and change with trophic level. For example, researchers used carbon and nitrogen isotopes in the collagen (connective) tissue within bones (both fossil and recent) to show that endangered Hawaiian Petrels (Pterodroma sandwichensis) nesting on different islands forage in different regions of the Northeast Pacific Ocean, and that their diet has changed over time (Wiley et al. 2013).
The second technology uses DNA sequencing of organic fragments in the gut or feces of a bird, matched to unique DNA sequences for diverse prey types such as insect species. For example, Daniel Karp et al. (2014) used these genetic methods to identify which small insectivorous bird species in Costa Rica had consumed coffee berry borer beetles, the most economically important pest of coffee beans.
8.3.2 Diversity of avian foraging strategies
The diversity of birds’ diets and foraging behaviors is evident in aspects of their anatomy—especially their beaks, feet, wings, and tails—that reflect feeding adaptations. Since “function follows form” in feeding, general physical characteristics of birds underline how distinctively different birds obtain prey. For example, various foliage‐gleaning birds capture insects in and near vegetation; these birds typically hop rapidly, facilitated by long tarsi, through leafy vegetation, capture prey with short, tweezer‐like beaks, and use small, rounded wings to move rapidly and hover within vegetation. In contrast, woodpeckers pound dead trees to disturb insects, and remove wood or flake off bark to reveal prey hidden within trunks and branches. Their strong, chisel‐like bills, strong tarsi and toes for gripping tree trunks, tails for props, and shock‐absorbing heads and necks are all adaptations that assist in these feeding behaviors. A very different set of adaptations is required for capturing fish and other aquatic animals in shallow water; for example, herons, egrets, and their relatives use long legs for wading; stalking, foot‐raking, or stamping to reveal prey at the bottom of the water; and long necks with straight, pointed beaks for stabbing prey.
Birds do not simply pick items from a natural buffet table. Instead, essentially everything they ingest is living, or the product thereof, and thus what birds eat reflects not only their own anatomy and behavior, but also the behavioral and evolutionary responses of the food they consume (Box 8.02). Some plants have easily accessible nectar, fruit, and seeds, but other plant products and all animal prey are defended against birds in diverse ways. Birds generally have to work at foraging and feeding, and their adaptations to feeding must take into account their ecological and evolutionary interactions with their food. Hummingbirds provide one clear example: flowers of a particular shape favor the evolution of an appropriately shaped hummingbird bill (Chapter 14). When two species—in this case, predator and prey (or food source)—evolve traits in response to the other, the process is called coevolution.
A bird species’ adaptation for food acquisition can be envisioned using the metaphor of “filters” between the total food potentially available to all birds in a particular environment and what each bird species actually consumes. Such filters operate at all stages of foraging, from the identification of food to feeding (Fig. 8.08), and involve all other species and the environment. For example, a specialized seed‐eater’s selection of a preferred food removes from the environment (filters out) foods otherwise available to a less specialized seed‐eater.
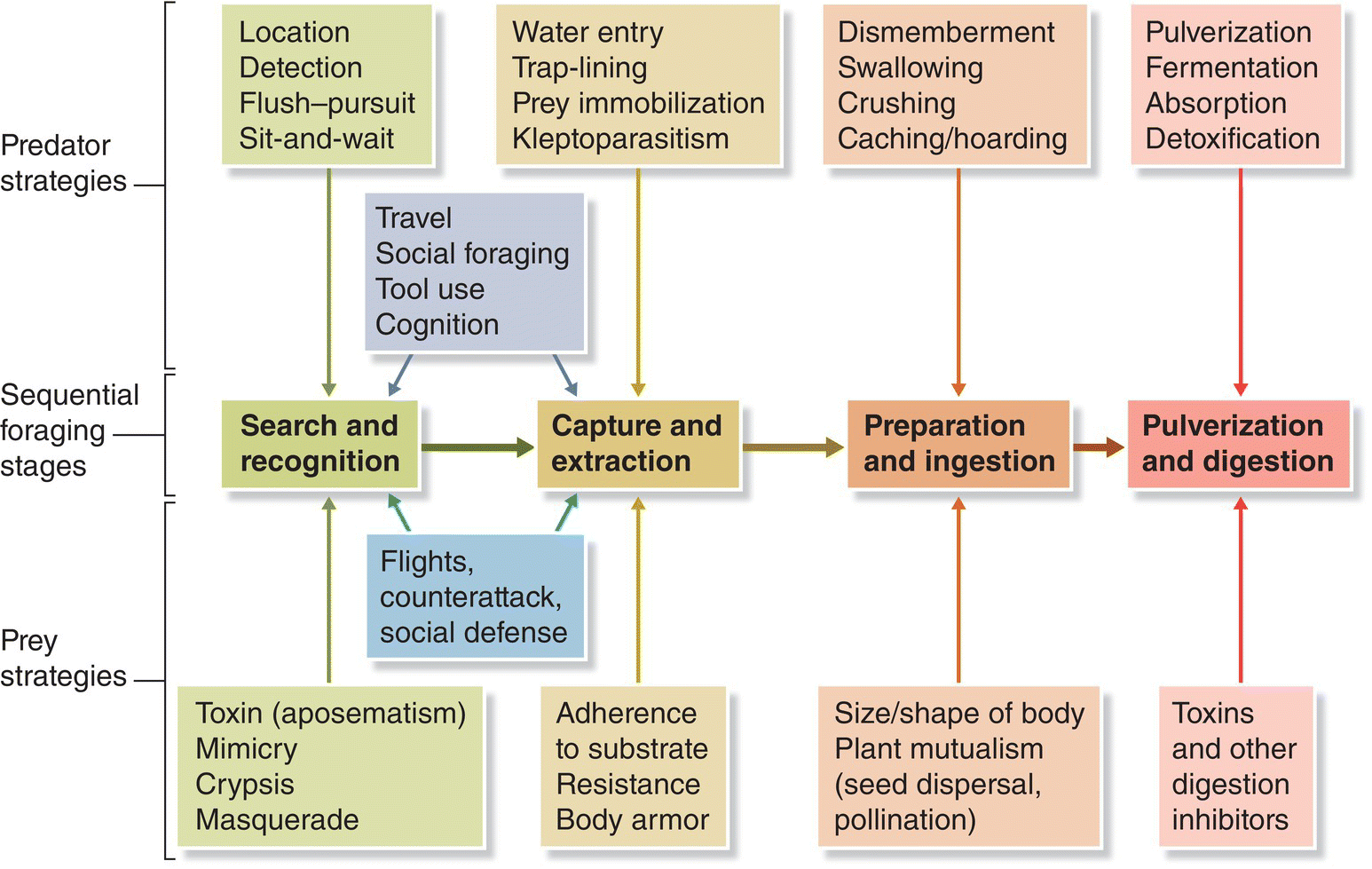
Fig. 8.08 Sequential stages of the foraging process. This schematic shows how both predators and prey use stage‐specific ecological and evolutionary strategies (and counterstrategies) to forage, feed, and avoid becoming prey.
(Reproduced with permission from Thomas W. Sherry.)
Another kind of filter comes from the coevolutionary arms race between birds and their animal prey: a bird species’ adaptations leading to improved prey detection or capture also impose selection on their prey. Accordingly, birds’ adaptations for capturing prey can result in adaptations such as stings or toxins in their prey. Similarly, birds’ adaptations for handling and swallowing prey can put pressure on prey to develop anti‐swallowing defenses like spines.
Insectivorous birds rummaging for prey in vegetation must remain continually vigilant for hawks, snakes, or other predators. The same fear response that helps protect these birds from predators has imposed selection for the eyespots seen in hundreds of species of butterflies and moths in Costa Rica alone (Janzen et al. 2010). These eyespots serve as a deceitful defense that helps these prey species appear to be predators (Fig. 8.09). Various prey also masquerade as inedible or innocuous objects to avoid predation, such as caterpillars—which are generally relished by birds—that mimic twigs or bird excrement. Recently, domestic chickens (Gallus gallus) were used as predators in laboratory experiments to show that chicks exposed to real twigs were more reluctant and took longer to attack two twig‐mimicking caterpillar species than non‐exposed chicks, even when the caterpillar prey were in plain sight (Skelhorn et al. 2010). Masquerade thus effectively deceives some birds, and its widespread manifestation by prey suggests a strong survival advantage in nature. Each successful counteradaptation by prey or a food plant filters out those birds that are not capable of detecting, capturing, handling, or digesting those prey or food sources.

Fig. 8.09 Antipredator defense in arthropods. These caterpillars trigger avian flight (fear) responses with their large eyespots, which mimic those of snakes and other predators so well that the caterpillars often escape unscathed.
(Adapted from Janzen et al. 2010. Reproduced with permission from National Academy of Sciences, USA.)
8.3.3 Stages of foraging and consumption
Delineating stages within the feeding process helps to illustrate the diverse and subtle purposes of specific adaptations that birds use for particular food types. Although these stages may be delineated arbitrarily, because one stage grades into the next, many feeding adaptations are clearly stage‐specific and integrate aspects of birds’ anatomy, cognition, and behavior.
Search and recognition
The first problem that hungry birds face is locating appropriate foods. This process can involve active search, waiting for prey to come to the bird, provoking prey to move and disclose itself, or a combination of these and other search strategies.
Flying birds are able to search widely and so have an advantage in finding patchily or widely distributed foods. One obvious morphological adaptation to long‐distance searching is high aspect ratio (pointed) wings, which enable efficient long‐distance flights (Chapter 5). For example, albatrosses and other seabirds search large areas of ocean surface for small fish, squid, and crustaceans. Terrestrial birds such as swifts and swallows also have high aspect ratio wings and efficiently travel long distances seeking rich aggregations of flying insects such as termite swarms. Swallows often seek bodies of water to feed on ephemeral prey such as mayflies, midges, and caddis flies. Swifts and martins feed opportunistically on arthropods that constitute “aerial plankton” and are concentrated by updrafts associated with passing storm fronts (Russell 1999). Southern Carmine Bee‐eaters (Merops nubicoides) of Africa often specialize on grasshoppers and other orthopteran insects disturbed into the open by bush fires, grazing mammals, people, or tractors.
For other birds, the food‐searching process often involves scanning substrates such as leaves, tree trunks, and the ground. Small birds scan in different ways, with subtle—and sometimes not‐so‐subtle—variations in morphology linked to their search methods. For example, foliage‐gleaning warblers scan foliage with occasional hops and short flights, tree creepers creep up tree trunks clinging with large feet and a prop‐like tail, and nuthatches walk headfirst down tree trunks.
Walking and running are also efficient ways for birds to search, chase, and subdue prey. Ratites such as the Ostrich (Struthio camelus) and Emu (Dromaius novaehollandiae) have long legs and strong toes that are well adapted for traveling long distances. Other long‐legged birds that hunt primarily on foot include the bustards of Africa, Eurasia, and Australia; the Secretary‐bird (Sagittarius serpentarius) of Africa; and the seriemas of South America. All of these birds live in relatively open grassland or desert environments that facilitate travel on foot.
One widespread avian search strategy is flush–pursuit foraging, a behavior that takes advantage of the innate startle response of insect prey such as flies and plant‐hoppers. In this strategy, a bird hops and pirouettes animatedly through vegetation, simultaneously revealing brightly contrasting plumage patterns by suddenly spreading its wings and tail. A startled prey then flushes from its hiding place, and the bird pursues it in flight. A variety of Old World and New World flycatchers and redstarts use this behavior. Some of the best studied are the neotropical Painted (Myioborus pictus) and Slate‐throated (Myioborus miniatus) Redstarts. Piotr Jabloński (1999) and Ronald Mumme (2002) tested the efficacy of this foraging mode by experimentally darkening these birds’ white tail feathers to eliminate the advantage of contrasting plumage. They found that the artificially darkened redstarts located prey less frequently and fed their nestlings less food than control birds with normal plumage (Fig. 8.10). These experiments thereby confirmed that the contrasting plumage pattern increases the effectiveness of these redstarts’ flush–pursuit foraging strategy.
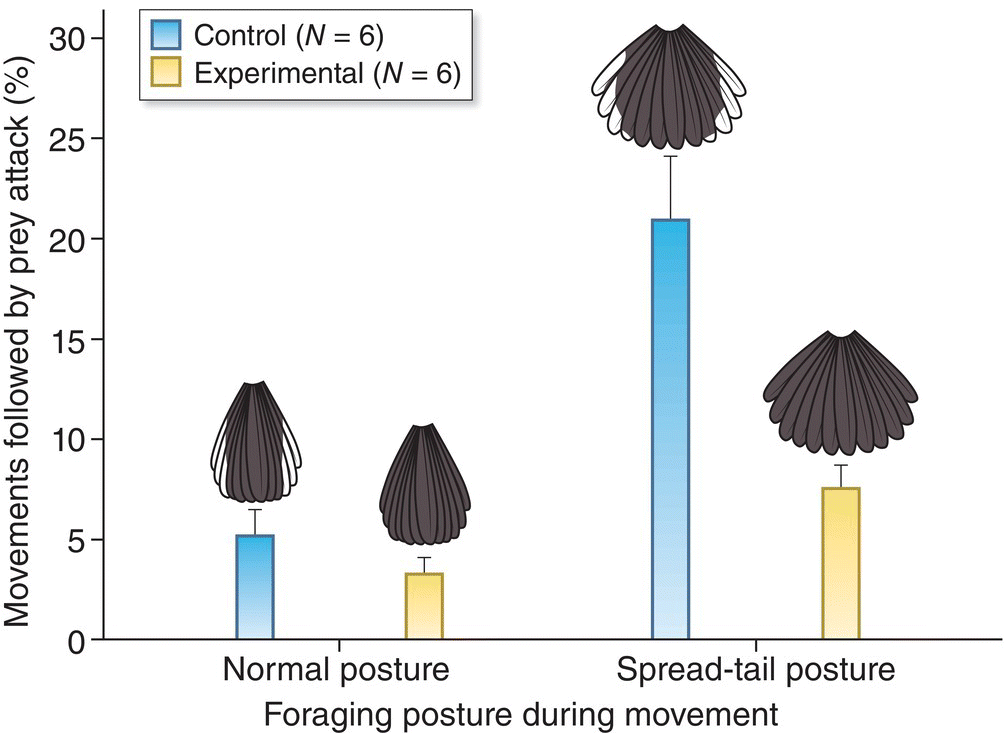
Fig. 8.10 Artificially darkened tails lower foraging success. Slate‐throated Redstarts (Myioborus miniatus) flash their black‐and‐white tails to startle and subsequently catch prey. When the tails of some redstarts were experimentally darkened, typical flushing movements resulted in fewer attacks, suggesting that the white patterning enhances the ability to startle prey.
(From Mumme 2002. Reproduced with permission from Auk.)
Other birds use a “sit‐and‐wait” strategy to capture prey that move infrequently. Examples of birds that wait patiently for an opportune moment include fish‐eating birds such as kingfishers, wading birds such as herons that slowly stalk prey, and many raptors that perch and watch the ground closely. Some birds that specialize on relatively large insects—such as the puffbirds of Central and South America—also use a sit‐and‐wait strategy. This strategy is a bit perplexing because many of the puffbirds’ prey are nocturnally active and hide during the day. What causes prey to move and reveal themselves to day‐hunting birds? One possibility is that heat from a moving patch of sunlight within the rainforest can cause an insect to change its position. Another is that ants moving continually through rainforest foliage occasionally irritate insects into making small motions that are then detected by the keen‐eyed birds.
In an unusual but effective variation of the sit‐and‐wait strategy, some birds use tool‐like enhancements to attract prey within striking distance. For example, herons may set out baits for fish (Fig. 8.11A), and Snowy Egrets (Egretta thula) vibrate their beaks rapidly just beneath the water surface to attract mosquito fish (Gambusia) (Kushlan 1973). Burrowing Owls (Athene cunicularia) surround their burrow entrances with bits of mammal dung, but are not slovenly housekeepers: the dung attracts dung beetles, which are a favored prey of the owls (Levey et al. 2004) (Fig. 8.11B). If one removes the dung piles experimentally, the Burrowing Owls replace them.
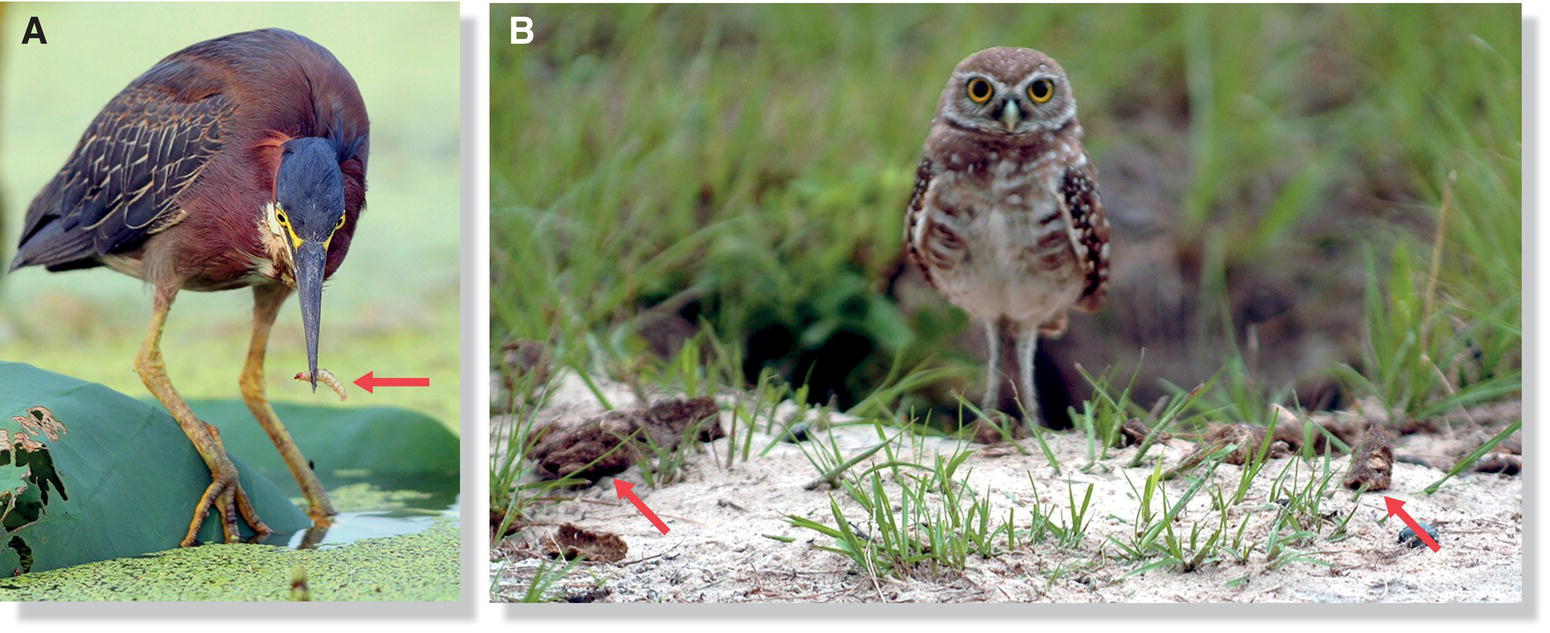
Fig. 8.11 Prey‐attractant tools. (A) Green Herons (Butorides virescens) sometime use bait, such as insects (arrow), to lure fish within striking distance. (B) Similarly, Burrowing Owls (Athene cunicularia) place mammal dung (arrows) near their burrows to attract dung beetles.
(A, photograph by Greg Lavaty. B, from Levey et al. 2004; photograph by R. C. Wolff.)
In addition to using diverse search strategies to locate prey, birds also employ a variety of sensory modes. Birds, like humans, rely heavily on color vision in feeding, but they also use a variety of other senses, and their sensory world differs significantly from ours (Chapter 7). For example, avian vision can be more acute than ours, allowing birds to detect prey from great distances, a trait well applied by raptors such as eagles and falcons. Eurasian Kestrels (Falco tinnunculus) perceive ultraviolet wavelengths, allowing them to detect the urine paths of voles that run through meadow grasses (Viitala et al. 1995). Nocturnal birds such as owls and nightjars have extremely acute vision in low light conditions, but even with this ability, most species are generally crepuscular, foraging at dawn and dusk except when moonlight extends feeding activity times.
Owls have a particularly acute sense of hearing, and some have anatomically offset ears that allow the precise localization of the source of a sound using a two‐dimensional map in the brain (Chapter 7). Great Gray (Strix nebulosa) and Snowy (Bubo scandiacus) Owls can detect sounds and successfully capture prey through as much as 30 centimeters of snow cover. To help them fly stealthily as they approach prey, most owls also have feathers that dampen the sound of air currents passing across them, giving potential prey less warning (Chapter 4). All these adaptations make owls formidable nighttime predators. Other birds, such as thrushes and the antpittas of Central and South America, have an acute sense of hearing to detect underground animals, and woodpeckers similarly listen for wood‐boring insects.
Although most birds have a poorly developed sense of smell (Chapter 7), especially as compared with mammals, some birds do use olfaction to find food, sometimes over great distances. For example, some seabirds search over large ocean areas, and once they discover a smell, move upwind to find its source. Researchers and birders wishing to attract such pelagic birds often take advantage of this behavior by leaving a slick of fish oil behind their boat; with luck, the smell of the oil lures in petrels, shearwaters, albatrosses, and other seabirds from downwind (Fig. 8.12A). Terrestrial birds that feed on rotting carcasses might be expected to have acute senses of smell, but the Old World vultures do not appear to locate their prey through olfaction. In contrast, some New World vultures, including King (Sarcoramphus papa) and Turkey (Cathartes aura) Vultures, have a strong olfactory sense (Fig. 8.12B). Turkey Vultures can smell their way to rotting meat even when it is completely covered by leaves in dense tropical forest (Houston 1986). The nocturnally adapted kiwis of New Zealand also use olfaction for feeding (Fig. 8.12C). They are the only birds with nostrils at the very tip of the beak, and are capable of detecting scents at concentrations of only a few parts per million. Kiwis typically feed on invertebrates, especially earthworms, which they detect by probing their beaks into leaf litter and soil.

Fig. 8.12 Olfaction in foraging. (A) Bonin Petrels (Pterodroma hypoleuca)—like many petrels, shearwaters, and albatrosses—use their keen sense of smell to locate prey on the ocean surface. (B) King Vultures (Sarcoramphus papa), along with most New World vultures, use olfaction to locate carrion when foraging in forested habitats. (C) Great Spotted Kiwis (Apteryx haastii) and other kiwi species are nocturnal foragers, employing smell to locate earthworms and other prey underground.
(Photographs by: A, Chandler S. Robbins; B, E. J. Peiker; C, Sharon Richards.)
Some birds use tactile senses to locate prey at close range, employing pits near the bill end that are packed with cell receptors (Cunningham et al. 2010). Shorebirds such as snipe and woodcock use their long, sensitive beaks to detect earthworms and other invertebrates via touch in muddy spots. Some ibises detect crayfish with their long, decurved beaks, which they deploy in up‐and‐down, sewing‐machine‐like movements that both detect and grab prey. The Shovel‐billed Kookaburra (Clytoceyx rex) of New Guinea rainforests plows its beak laterally through the soil to detect invertebrates.
Many birds have stiff, pin‐like feathers called facial “bristles” that help them sense or capture prey. Rictal bristles are specialized rigid, whisker‐like feathers that are usually evenly spaced at the base of the bill (Chapter 4). These bristles are most conspicuous in birds that pursue insects, including nightjars, flycatchers, and some New World warblers such as redstarts (Fig. 8.13A). Because many birds with bristles forage in dark locations, the bristles may have a tactile function to help the bird sense its prey’s location as it closes in (Fig. 8.13B). Alternative hypotheses for the bristles’ function are that they funnel prey into the bird’s mouth (both physically and by providing tactile information, as mammalian whiskers do); that they help hold onto prey for manipulation once it has been caught; and that they protect the eyes and face from spiny appendages and other threats from prey. For example, the White‐necked Puffbird (Notharchus hyperrhynchus) (Fig. 8.13A) of Central America feeds on many large insects, and correspondingly has a long, deep beak as well as well‐developed bristles between the beak and eyes that likely protect its face from the large and sometimes spine‐bearing prey species these birds catch and handle (Sherry and McDade 1982). Woodpeckers also have bristles that appear to protect their nostrils and face from woody debris. Surprisingly few experiments have tested the function of rictal bristles, although studies by Michael Conover and Don Miller (1980) suggested that the bristles of Willow Flycatchers (Empidonax traillii) serve a protective rather than a prey‐capture function. The role of bristles likely varies among bird species, and more experimental studies are needed to clarify how rictal bristles function.

Fig. 8.13 Facial bristles. (A) Many species have facial bristles (arrows), but the type and function of these bristles differ. The American Redstart (Setophaga ruticilla) (left) and Royal Flycatcher (Onychorhynchus coronatus) (middle) likely use their bristles for detecting insect prey during aerobatic foraging. The White‐necked Puffbird (Notharchus hyperrhynchus) (right) is thought to use its long bristles to protect its face and eyes from the spines of its large insect prey. (B) Other species use bristles for gathering sensory information. For example, North Island Brown Kiwis (Apteryx mantelli) (left) use facial bristles for sensing the movement of subterranean prey, as evidenced by the vibration and pressure‐sensing Herbst corpuscles (black arrows), nerve bundles (black star), and muscles (white stars) near the bristle follicle (right). Scale bar = 5 mm in histological view.
(Photographs by: A, left, Jennifer Malpass; middle, Andrew Snyder; right, Thore Noernberg; B, left, Tui De Roy, AUSCAPE; right, from Cunningham et al. 2011. Reproduced with permission from John Wiley and Sons.)
Cognitive skills help foraging birds learn what, where, and when to forage for the best foods. Individual hummingbirds may encounter hundreds to thousands of flowers that differ in nectar quality, renewal rates, and availability. Using both aviary and field experiments with Green‐backed Firecrowns (Sephanoides sephaniodes) in Chile, Paulina González‐Gómez and colleagues (2011a, 2011b) showed that most individual territorial male hummingbirds could distinguish and recall nectars of different quality (sucrose concentration), their location within a patch, and renewal intervals (to which they adjusted their flower visitation rates) even many hours after their original foraging experience.
Sometimes birds learn important foraging skills from their parents. For example, Common Ravens (Corvus corax) go through a juvenile phase of highly exploratory (“curious”) behavior that helps them learn both from their parents and by trial and error about which foods are edible (Box 8.01). This apparently innate tendency to approach novel objects or environments appears most frequently in birds that forage broadly on many different types of food. In addition, parents may teach their young how to hunt, a behavior found most commonly in raptors and other carnivores requiring complex skills to capture prey. For example, parent Ospreys (Pandion haliaetus) sequentially bring dead prey to their youngest offspring, then bring live but incapacitated prey, and—as the young develop skills—live prey that requires recapture (Adams‐Hunt and Jacobs 2007). In Costa Rica, Alexander Skutch (1976) described White‐fronted Nunbird (Monasa morphoeus) parents holding a large insect conspicuously for their juvenile to practice snatching while in flight. The importance of learning to feed is suggested by local feeding traditions within a species; for example, a population of Peregrine Falcons (Falco peregrinus) in New Mexico (USA) specializes in capturing bats as they emerge from their roosting caves (Skutch 1976).
In addition to recognizing edible foods, searching birds must recognize and avoid inedible or dangerous items. Birds use both innate recognition mechanisms and learning to avoid dangerous prey. An experiment with Turquoise‐browed Motmots (Eumomota superciliosa) (Fig. 8.14) and Great Kiskadees (Pitangus sulphuratus) demonstrated a dramatic case of innate recognition of danger. Both species are liable to encounter potentially deadly coral snakes while foraging. Coral snakes and a variety of other animals that are dangerous or noxious to potential predators display aposematic coloration: brightly colorful warning coloration that has evolved to get a predator’s attention and thereby reduce unnecessarily injurious or life‐threatening attacks. Susan Smith (1975, 1977) presented painted wooden dowels to hand‐reared motmots and kiskadees that had no previous experience with venomous snakes. She found that both species innately feared most the combinations of red, yellow, and black rings that resembled the most common coral snake species in their area. In fact, young Great Kiskadees cowered in the farthest corner of their cages when presented with wooden models that even crudely resembled coral snakes. Other experiments showed that the birds exhibited no aversion to longitudinal stripes of the same color combinations or to alternating rings of other colors. Thus, even without firsthand experience, these birds appear to recognize and fear the particular aposematic color patterns of the snake species that are most dangerous to them.
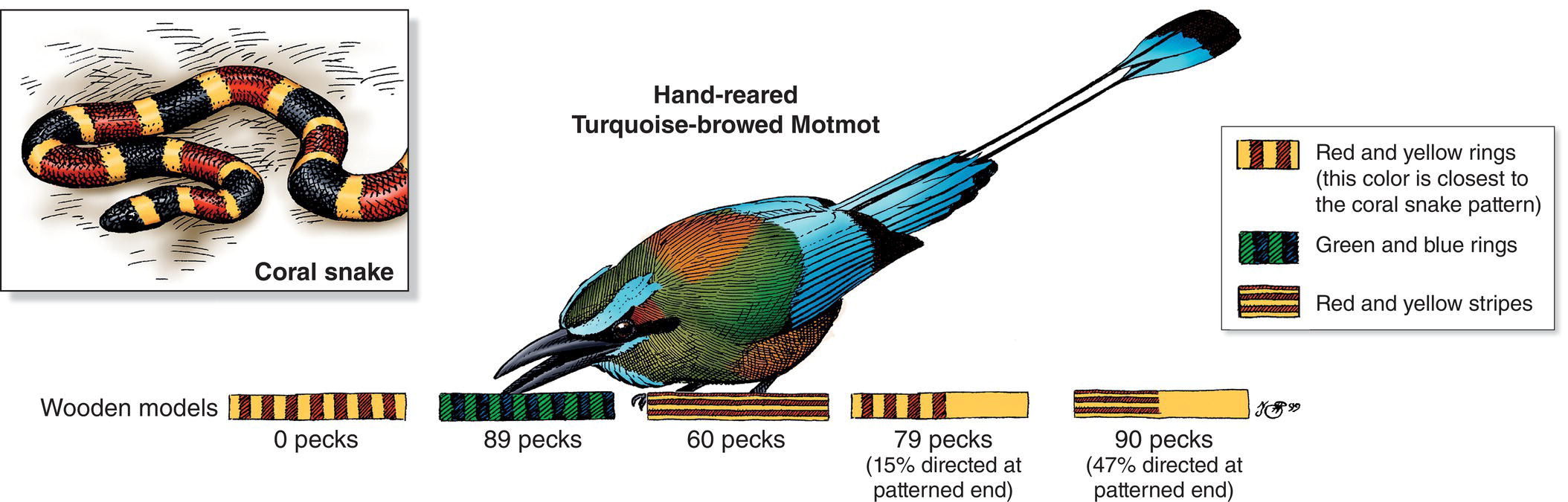
Fig. 8.14 Innate responses to coral snake warning colors. Turquoise‐browed Motmots (Eumomota superciliosa) sometimes capture prey from the leaf litter, where they are vulnerable to venomous coral snakes. Experiments revealed that naïve motmots (hand‐reared, with no experience in the wild) recognized and completely avoided (0 pecks) wooden models that resembled coral snakes, but pecked at other color and pattern combinations.
(Adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology, and Smith 1975.)
A variety of venomous or potentially toxic prey, though not as dangerous as coral snakes, are readily handled by birds that have become specialized on those prey types. Some bee‐eaters, for example, have an innate beating behavior by which they de‐venom and de‐sting these insects. An unknown physiological mechanism allows several species of Pitohui in New Guinea to tolerate dietary toxins (Chapter 4) and then incorporate those toxins into their own plumage (Dumbacher et al. 2004; Jønsson et al. 2008).
Some birds are reluctant to approach or feed on food sources that they have not experienced previously, perhaps sensing a general threat from unknown and dangerous hazards. For example, although young Common Ravens (Corvus corax) exhibit curiosity, older birds in contrast may exhibit generalized neophobia (Box 8.01).
Learning also is clearly important in many birds’ responses to noxious prey. A classic study of such learning comes from studies of hand‐reared captive Blue Jays (Cyanocitta cristata). The birds were presented with a meal of distasteful monarch butterflies (Brower 1969, 1984), which caused them to vomit (Fig. 8.15). This strong negative experience resulted in the jays later avoiding both monarchs and other similarly colored butterflies. Other animals that are not distasteful can benefit by evolving similar colors and gaining protection by deceit. This process can lead to elaborate mimicry complexes, in which toxic and non‐toxic prey evolve similar appearances. In tropical forests, diverse predators and prey interact year‐round (Chapter 14). The wealth of both model and mimetic insects at tropical latitudes poses a problem for bird species such as the New World jacamars that rely on these insects as prey, as they have to distinguish edible prey from very similar‐looking inedible species (Box 8.03).

Fig. 8.15 Learning response to noxious prey. A captive Blue Jay (Cyanocitta cristata) consumes a toxic monarch butterfly for the first time and vomits in response to its unpalatable alkaloids. After just one bad experience, jays and other birds learn to avoid eating butterflies with the monarch’s coloration and pattern.
(Photographs by Lincoln P. Brower, Sweet Briar College.)
Capture and extraction
For many birds, locating food is a minor challenge compared with capturing the prey once it is found.
Just as wing structure can help birds find prey, it can enhance aerodynamic performance for capturing prey (Chapter 5). For example, Accipiter hawks use their short, rounded wings, coupled with a long tail, to maneuver through tight woodland spots to surprise and attack songbirds. Analogously, various insectivorous birds have converged on a set of traits that facilitate rapid acceleration from rest in order to have the advantage of attacking relatively wary insects by surprise. These traits include small body size; short, rounded wings; short tails that further reduce aerodynamic drag; long legs, probably to help jump at takeoff; and relatively long and flat‐tipped beaks to extend reach at the moment of capture. Insectivorous birds with this combination of attributes include the todies of the Caribbean region, the phylogenetically distant tody‐flycatchers of Central and South America, and the African Dwarf Kingfisher (Ispidina lecontei).
Pouncing birds take advantage of perches in order to scan the surrounding ground for potential prey to attack. An example of such birds’ dependence on appropriate perches is illustrated by a study of perch limitation in Florida (USA), where Loggerhead Shrikes (Lanius ludovicianus) fly on average 6.5 meters to the ground from fencerows and 9.2 meters from higher perches on palmettos. These search distances were deemed to be the optimal search radius because shorter distances do not provide as much food, and longer distances involve lowered attack success and reduced efficiency. These presumed optimal search distances were used to show that a shortage of available perches was contributing to population decline in this species (Yosef and Grubb 1992, 1994); when high‐quality perches were added experimentally to territories, birds decreased their territory size, more birds settled in those areas, and their overall body condition improved.
A predator’s body size constrains what it can attack successfully, and most predatory birds select prey substantially smaller than themselves. However, some birds of prey can successfully attack and subdue prey much larger than their own body size. For example, Golden Eagles (Aquila chrysaetos) in the western USA sometimes chase adult pronghorn antelope, land on their backs, paralyze them, and pull them down. In Spain, these eagles can pull young ibex goats off cliff edges, using gravity and the rocks below to complete the kill.
Birds foraging in open water have special opportunities for capturing prey, but also encounter challenges including water’s high viscosity, increased pressure and darkness associated with deeper water, and the reflectance and refraction of surface light that obscures objects below. Dabbling ducks avoid most of these problems by feeding on foods at or near the surface. Other birds, such as mergansers and puffins, have serrations on their bills for grabbing slippery fish (Fig. 8.16A, B). American White Pelicans (Pelecanus erythrorhynchos) scoop up fish from just below the surface along with the surrounding water (Fig. 8.16C), and then eject the water while sitting at the surface, leaving the fish trapped in the extendable pouch below their lower mandible. Herons use their spear‐like bills to stab prey using a sit‐and‐wait strategy. They can also clamp down and use their bills as tongs to maneuver prey items into the most effective position before gulping them down in one seamless motion (Fig. 8.16D). Pied Kingfishers (Ceryle rudis) of Africa have wings adapted for hovering, which allow them to hunt far from shore without trees or other high perches from which to detect fish. Some pelagic birds of the southern oceans, such as the White‐faced Storm‐Petrel (Pelagodroma marina), feed far out at sea by facing into the strong winds and using rounded, outstretched wings; long, dangling legs that seem to walk on the water; and a small beak to pick tiny items such as crustacean plankton from the surface. Frigatebirds snatch flying fish and squid from the water surface during forward motion, often by adeptly tipping their heads back so that their long beaks stay in contact long enough to secure their prey. Black Skimmers (Rynchops niger) fly just above calm water, allowing their lower mandible to skim through the water; when they touch a fish, they snap their mandible shut, pinning the prey against the upper mandible (Fig. 8.17). Black Skimmers frequently cruise back through the ripples created by their first flight across water to snatch fish attracted to the disturbance.

Fig. 8.16 Bills of fish‐eating birds. (A) The serrated bill of Red‐breasted Mergansers (Mergus serrator) grips fish for easy handling. (B) The ridges of Atlantic Puffin (Fratercula arctica) bills have backward‐pointing spikes, allowing them to hold many small fish at once. (C) American White Pelicans (Pelecanus erythrorhynchos) gulp large quantities of water into their enlarged pouches, pushing out excess water and retaining any fish. (D) Great Blue Herons (Ardea herodias) use their spear‐like bills to pierce or clamp down on prey in shallow water. (A, © Cornell Lab of Ornithology.
Photographs by: B, Sandy A. Flint; C, Lawrence I. Finkel; D, Marie Read.)

Fig. 8.17 Specialized bill and foraging strategy of the Black Skimmer (Rynchops niger). (A) This species captures fish by efficiently skimming the water surface with its lower mandible. (B) A dorsal–ventral view of its extremely laterally compressed bill. (C) In lateral view, the lower mandible is visibly longer than the upper, providing more surface area with which to contact fish near the water surface.
(Photographs by: A, Tony Mills, www.photoartbytonymills.com; B, Dane G. Adams; C, © Laura L. Erickson.)
Many seabirds plunge‐dive to capture fish at great depths. Boobies and gannets are adapted to dive from tens of meters in the air, achieving water‐entry speeds up to 95 kilometers per hour and dives as deep as 30 meters. Just before entering the water, they pull their wings back behind their streamlined bodies (Fig. 8.18). When diving seabirds such as gannets forage for fish they also have to overcome the physical properties of light reflection and refraction at the water surface. Because light reflected off a fish and into the air is refracted at an angle, to a flying predatory bird the fish appears to be in a different location than it actually is. Diving seabirds appear to learn from experience how to take into account the challenge of light refraction in determining where to dive.

Fig. 8.18 Plunge‐diving. To hunt, Blue‐footed Boobies (Sula nebouxii) fly high into the air before plunging into the ocean, achieving great speeds both above and below water. Note that individuals near the surface have folded their wings tightly, giving their bodies a streamlined, torpedo‐like shape that decreases drag upon water entry.
(Photograph © Frans Lanting, www.lanting.com.)
Birds that actively pursue fish below the water surface must overcome the simultaneous problems of buoyancy and viscosity (resistance to movement). Solutions to both of these problems involve trade‐offs with aerial flight ability, because the very adaptations that facilitate efficient flapping flight in air—the reduced body density from hollow bones and large wings—increase buoyancy and drag when birds are submerged. Thus, a variety of birds dive successfully via adaptations that sacrifice some or all of their flight ability. At one end of this spectrum are anhingas and cormorants, which fly well and dive to pursue fish in relatively shallow water using their long, snake‐like necks. These birds have relatively dense bones but their large wings and tails help them retain a strong ability to fly. Cormorants and anhingas reduce their buoyancy by wetting their flight feathers; these must be dried out after a dive, and these birds are often observed with their wings outstretched in the sun (Chapter 4). Loons and grebes use webbed or fringed feet for rear propulsion, and also have heavy bones. Trade‐offs are implicit in all compromises, and because loons’ wings are relatively small for their body size, they experience difficulty taking off in flight from the water surface. The struggle sometimes causes loons to patter across a lake’s surface for hundreds of meters while paddling with both feet and wings to gain sufficient take‐off speed. Alcids such as auks, murres, and guillemots are even more specialized divers; they reach depths of more than 200 meters, stay down for several minutes at a time, swallow prey underwater, and propel themselves by paddling with their wings. Alcids have sacrificed efficient flight to dive well, and species such as guillemots must flap their wings vigorously and continuously to stay aloft—an energetic cost. Penguins are the most specialized of all diving birds: their wings are highly modified into efficient paddles that preclude flight altogether. Emperor Penguins (Aptenodytes forsteri) are pre‐eminent divers even among penguins and can dive to a depth of 535 meters and remain underwater for 18 minutes. Their streamlined shape, heavy bones, and ability to reduce their metabolic rate (and thus conserve oxygen; Chapter 7) help them accomplish these underwater feats.
Birds that feed in shallow water also are well adapted for detecting and capturing prey. To reach aquatic invertebrates, dippers counteract the buoyancy from air bubbles trapped in their feathers by gripping the bottom of fast‐flowing streams with their large feet and walking underwater. Herons and egrets fish from above, stalking shallow‐water prey on long legs and using various feeding tactics such as sit‐and‐wait foraging. These birds also use their feet in a variety of ways to assist feeding, such as stirring up bottom muck to reveal invertebrates. The brightly colored feet of Snowy Egrets (Egretta thula) provide a contrasting backdrop that silhouettes and exposes their prey (Fig. 8.19A). Some waders like the Reddish Egret (Egretta rufescens) of the Americas and the Black Heron (Egretta ardesiaca) (Fig. 8.19B) of Africa use shade from their own outstretched wings to overcome the problem of light reflected from the water surface. This behavior is called canopy feeding, and might also attract fish that tend to hide in shade and shallow water.
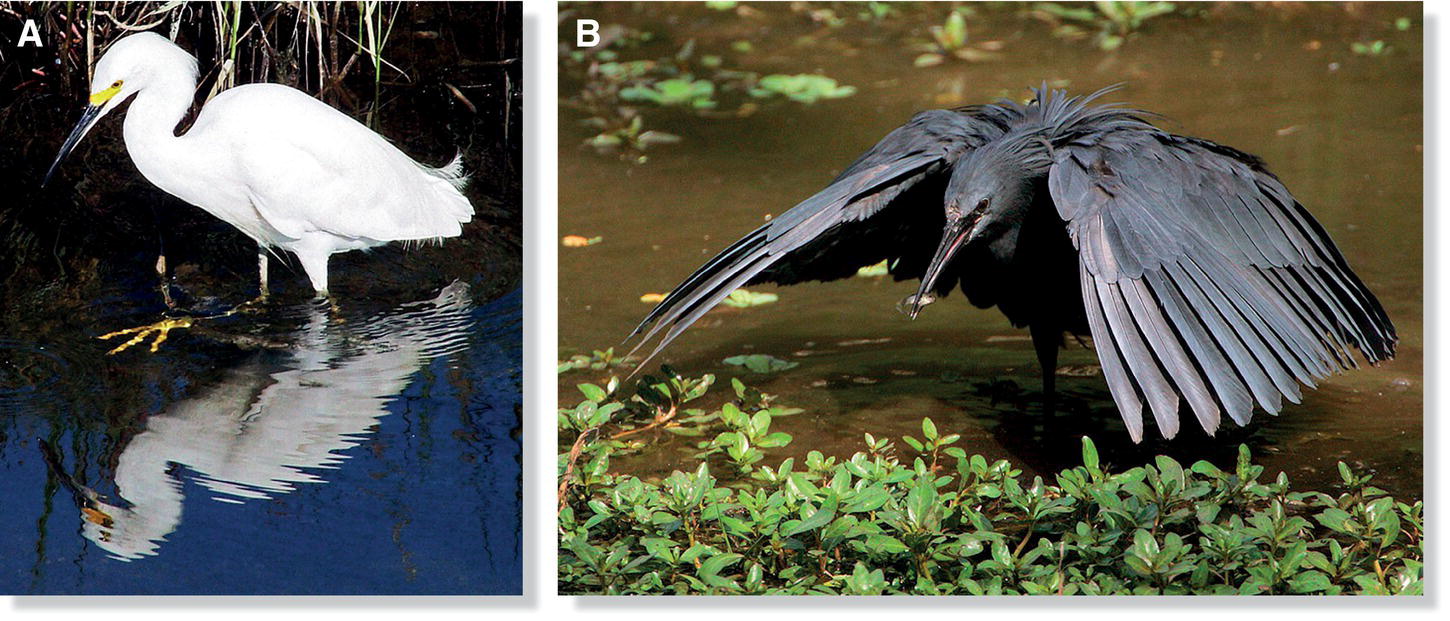
Fig. 8.19 Egret foraging strategies. (A) Snowy Egrets (Egretta thula) use their bright yellow feet to spot silhouettes of their prey in shallow water. (B) Black Herons (Egretta ardesiaca) outstretch their wings while foraging to disguise their shadow and reduce surface reflection. This technique, sometimes called canopy feeding, also tricks fish that seek shade for protection.
(Photographs by: A, Hugh Ryono, Aquarium of the Pacific; B, Steve Garvie, https://en.wikipedia.org/wiki/File:Flickr_‐_Rainbirder_‐_Black_Egret_%28Egretta_ardesiaca%29.jpg. CC‐BY‐2.0.)
An important challenge in capturing prey from water involves surface tension—the smaller the prey, the greater the pull. Some shorebirds that feed in shallow water, such as phalaropes, use the forces of surface tension to extract tiny invertebrates trapped in rows of water droplets and transport them up their beak (Rubega and Obst 1993). Manu Prakash and colleagues (2008) showed how these birds can vary the surface tension of the droplets by alternately opening and closing their beak, drawing the droplets up toward their mouth.
The problem of extracting prey from harder, protective substrates such as soil, trees, bones, and shells provides a diverse set of opportunities for avian feeding adaptations. For example, Oriental Honey‐buzzards (Pernis ptilorhynchus) extract wasp grubs with a straightened claw while a well‐feathered face protects them from stings. Snail Kites (Rostrhamus sociabilis) have long, decurved upper mandibles, an adaptation for extracting mollusks from their shells (as their name implies). A few species of birds use tools to extract prey from tough substrates. The Woodpecker Finch (Camarhynchus pallidus) of the Galápagos Archipelago uses cactus thorns or twigs to pry insect larvae and other arthropods from holes and crevices in dead wood or mossy tangles (Fig. 8.20). Carrion Crows (Corvus corone) in Sendai City, Japan turn automobiles into nutcrackers, swooping down when stoplights at busy intersections are red to deposit an intact walnut or to harvest a cracked one. Under more natural settings, Northwestern Crows (Corvus caurinus) in Washington State (USA) drop shellfish onto rocks, whereas Egyptian Vultures (Neophron percnopterus) drop rocks to break open ostrich eggs (Adams‐Hunt and Jacobs 2007; Bluff et al. 2007). New Caledonian Crows (Corvus moneduloides) demonstrate even more sophisticated cognitive abilities in the variety of tools they select and manufacture (Box 8.04).

Fig. 8.20 Foraging extraction tool. Woodpecker Finches (Camarhynchus pallidus) use small twigs or spines to extract grubs from decaying wood. These birds carefully choose twigs depending on the dimension of the cavity and may reuse a preferred tool several times.
(Photograph by Susan B. Wright.)
Birds’ beaks often have conspicuous adaptations for capturing foods. Shorebirds feed on a wide variety of food types in water, soil, mud, and sand. Their beaks correspondingly differ in their stoutness, length, curvature, and other features (Fig. 8.21) according to each species’ probing method and foraging habitat.

Fig. 8.21 Resource partitioning and bill variation. Shorebirds have bills adapted to access prey within specific substrate types and depths. (A) Marbled Godwits (Limosa fedoa) and (B) Whimbrels (Numenius phaeopus) use their long bills to forage for large invertebrates that often live far below the surface. The Whimbrel’s down‐curved bill also fits into the burrows of fiddler crabs, their primary food in the winter. (C) The American Oystercatcher (Haematopus palliatus) drills at shellfish attached to rocks with its chisel‐shaped bill. (D) Dunlins (Calidris alpina) forage on coastal mudflats and sandbars via methodical probing, often compared to the motion of a sewing machine. (E) Semipalmated Plovers (Charadrius semipalmatus) hunt by sight on wet substrates, quickly snatching marine invertebrates and crustaceans with their small, blunt bills. (F) Least Sandpipers (Calidris minutilla) forage with their short, sharp bills by rapidly pecking the surface of wet substrates and/or quickly probing into mud.
(© Cornell Lab of Ornithology.)
Many shorebirds have a remarkable ability to seize and extract invertebrates with their beaks. Some can separate their bill tips (while the rest of the bill remains closed) like tweezers, an ability called rhynchokinesis. A variety of shorebirds have recently been shown to use rhynchokinesis to capture and transport small prey in both soil and open water (Estrella and Masero 2007) (Fig. 8.22). Other birds use cranial kinesis, defined as the movement of one component of the skull relative to another (Chapter 6). One of the most unusual cases involves hummingbirds, for although they are thought to feed largely on nectar, many hummingbirds also feed extensively on arthropods for added protein. Hummingbirds take prey in flight by flexing their upper beak upwards (via rhynchokinesis) and their lower beak downwards (via cranial kinesis) to increase their fly‐catching efficiency (Yanega and Rubega 2004).
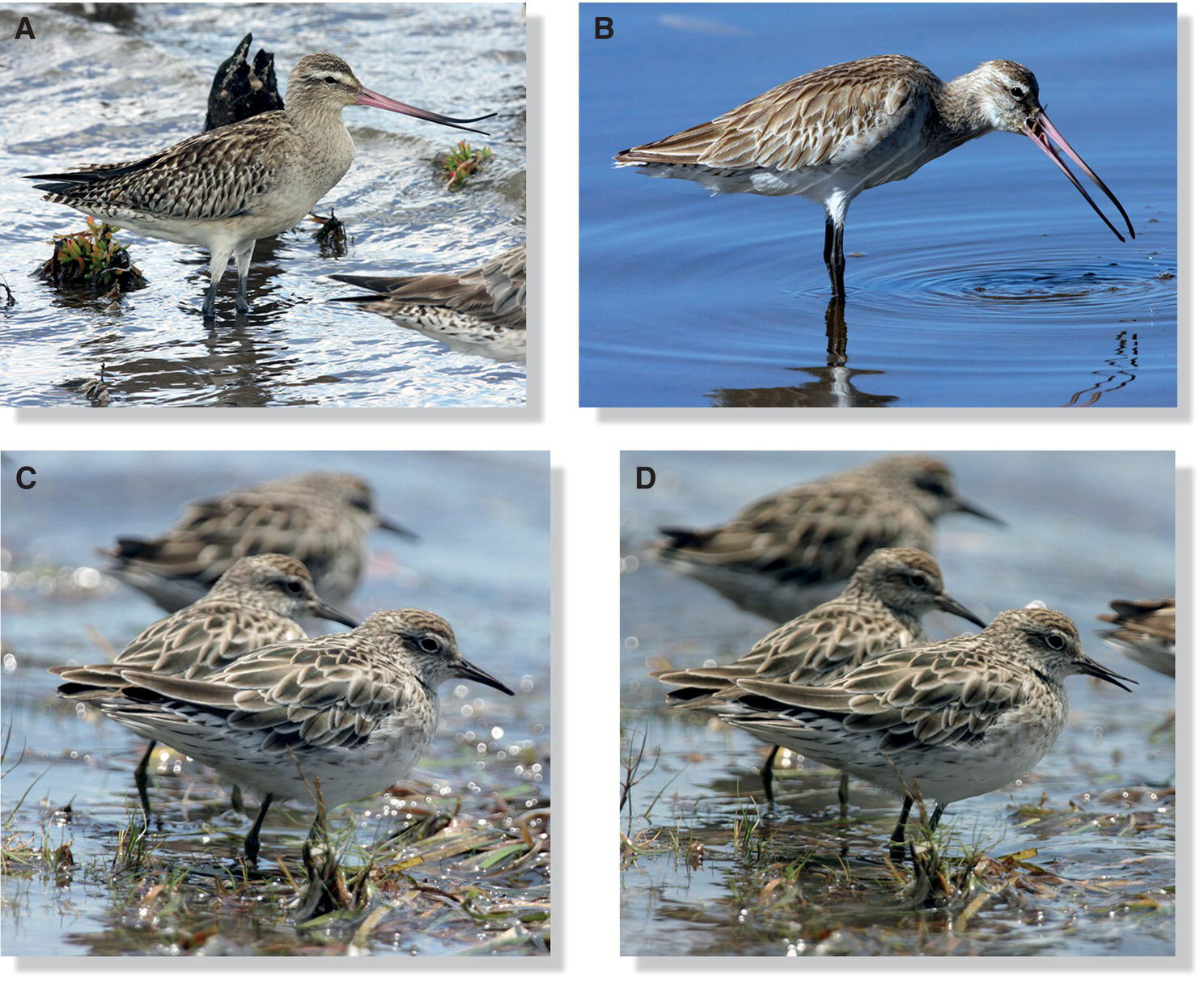
Fig. 8.22 Rhynchokinesis. Bar‐tailed Godwits (Limosa lapponica) can bend their upper mandibles up (A) and down (B) to aid in food handling and capture. (C) Sharp‐tailed Sandpipers (Calidris acuminata) can also flex their upper bill tip (D).
(Photographs by Robert Inglis.)
Woodpeckers are pre‐eminent extractors, and most species are able to chisel into wood to get at carpenter ants, wood‐boring insect larvae, and other arthropods. Other woodpeckers, including flickers, use their long, extrusible tongue to forage for ants and other insects in subterranean tunnels (Fig. 8.23). The highly specialized Campo Flicker (Colaptes campestris) of southern South America feasts primarily on termites from large terrestrial mounds, which it extracts delicately using its tongue.

Fig. 8.23 Tongue protrusion in woodpeckers. The hyoid bone serves as an attachment site for muscles along the throat, head, and tongue in all birds. (A) Woodpecker hyoids have two bony extensions called “horns.” Sheathed in powerful muscle, these hyoid horns are so long that they wrap around the back of the skull—sometimes into the nostril cavity, as shown in this Northern Flicker (Colaptes auratus). (B) To protrude their long tongues, woodpeckers contract the horn muscles, which hug the skull and push the hyoid forward. Equipped with such specialized tongue anatomy, woodpeckers adeptly extract prey from deep cavities.
(© Cornell Lab of Ornithology.)
Some birds use beaks and tongues in tandem to extract food efficiently from various substrates (Fig. 8.24). For example, the Eurasian Nutcracker (Nucifraga caryocatactes) has an unusual bifurcated tongue that is a specialized adaptation for both prying open conifer cones to extract their seeds and for manipulating those seeds. The tongues of ducks and geese are often fringed with comb‐like structures for straining small food items from water. Birds including sapsuckers and lorikeets have adaptations for nectar absorption, such as brush‐ or feather‐like tongues, to increase the surface area for nectar uptake. Woodpeckers have long, narrow tongues with barbs at the ends to extract insects from deep within wood cavities after drilling into the cavities to get closer access. Flamingos have highly specialized beaks and tongues to filter large quantities of water, extracting planktonic algae and small invertebrates such as insects, mollusks, and crustaceans (Fig. 8.25).

Fig. 8.24 Diversity of specialized bird tongues. (A) White‐headed Woodpeckers (Picoides albolarvatus) have a bifurcated tongue tip for extracting seeds from pine cones. (B) Canada Geese (Branta canadensis) have tongues with spiny projections to help them sift through food in water. (C) Purple‐crowned Lorikeets (Glossopsitta porphyrocephala) have tongues with specialized brush tips to capture nectar and pollen from flowers. (D) Hairy Woodpeckers (Picoides villosus) quickly extend their sticky, barbed tongues deep into excavated holes to impale and extract invertebrate prey.
(© Cornell Lab of Ornithology.)
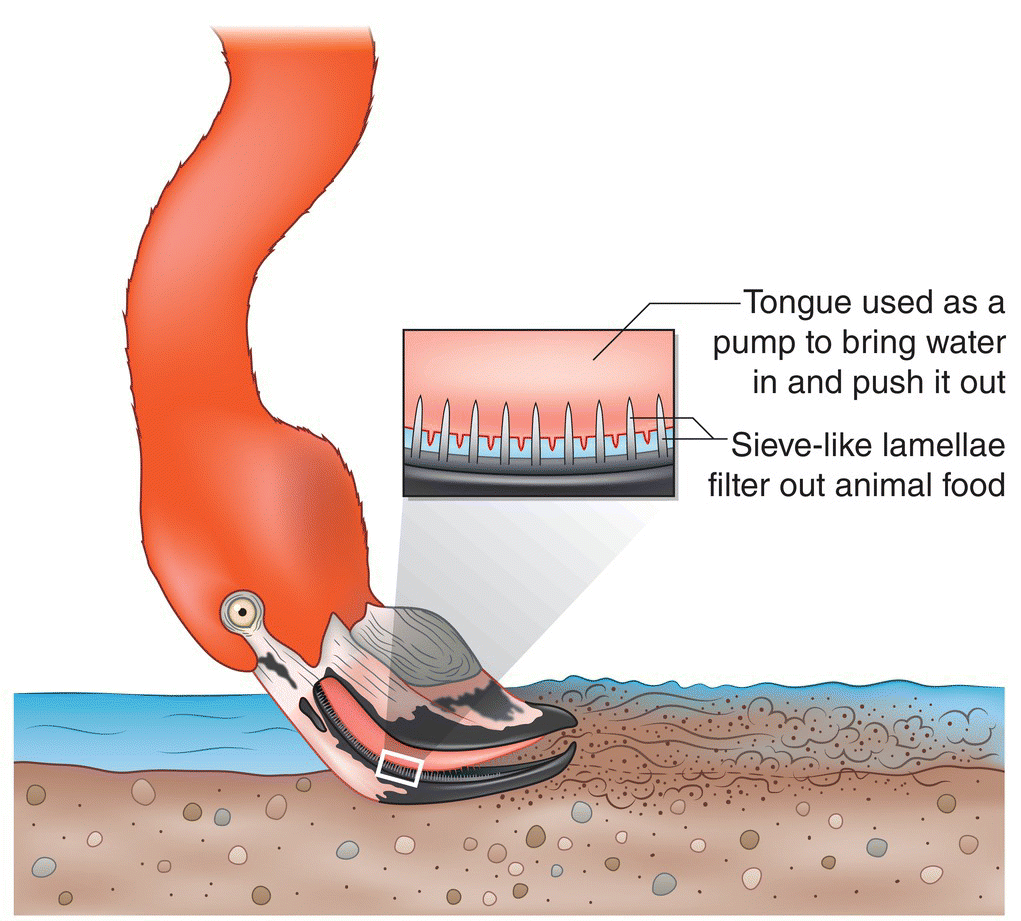
Fig. 8.25 Filter foraging. Equipped with a complex beak–tongue filtration system, Greater Flamingos (Phoenicopterus roseus) forage on tiny drifting animals in shallow water. Flamingos suck in muddy water and force it out with their muscular tongues via piston‐like pumping. In the process, the water passes through numerous hair‐like projections (lamellae) on the upper and lower mandibles, which capture prey like a sieve.
(© Cornell Lab of Ornithology.)
Novel specializations of the beak and tongue in shorebirds like Western Sandpipers (Calidris mauri) and Dunlin (Calidris alpina) permit them to graze on foods too small to pick up individually (Elner et al. 2005; Mathot et al. 2010). Both of these species feed on biofilm, a thin layer of microbes, their secretions, and other organic matter found at the bottom of shallow, intertidal flats. Denticles, tooth‐like structures that line the roof of the maxilla, are a specialized anatomical feature of these birds’ beaks that may aid in scraping biofilm from the tongue. The tongue itself traps biofilm in water with microscopic, mucus‐covered papillae (bumps) covered with microvilli. The dense bristles fringing and tipping the tongue work to trap biofilm just as the fringed tongues of nectar‐feeding birds trap nectar.
The most specialized nectar‐feeders, hummingbirds, take nectar feeding one step further. Their tongues are forked, tubular‐like straws, and have flattish coils to increase the surface area for nectar absorption (Fig. 8.26). Until recently, scientists believed that capillary action sopped up liquids adhering to hummingbirds’ delicately bifurcated, curled, and fringe‐tipped tongues. Alejandro Rico‐Guevara and Margaret Rubega (2011) discovered an alternative mechanism using high‐speed (stop–action) videography of hummingbirds feeding through clear artificial tubes. They found that, when inserted into nectar, the curved lamellae (parallel hair‐like structures) along the tongue tips of hummingbirds unfurl from their supporting pair of rods, while the elasticity of the rods and the opposing forces of the nectar’s surface tension cause the rods themselves to separate (Fig. 8.27). The process of retracting the tongue and its lamellae past the liquid surface into the air causes the lamellae to refurl and trap a tube of nectar into each of the bifurcated tongue tips, which adhere to each other due to surface tension. This mechanism also explains why hummingbirds prefer sugary, viscous nectar, which is not easily extracted using capillary action (Kim et al. 2011). After collecting these nectar tubes, the bird unloads the nectar by squeezing (flattening) its tongue as it extrudes it back through its flattened beak.

Fig. 8.26 Bifurcated tongue. (A) The tongue of this Saw‐billed Hermit (Ramphodon naevius) splits into two parts when exiting the bill. (B) A close‐up view of its forked tongue reveals tiny hair‐like plates (lamellae). As the tongue retracts, the lamellae fold in and funnel captured nectar into the mouth.
(Photographs by Alejandro Rico‐Guevara.)

Fig. 8.27 Nectar extraction by a Ruby‐throated Hummingbird (Archilochus colubris) tongue. (A) Dorsal views show the tongue tips fully immersed in nectar and then extracted from the nectar in stages, from totally immersed (top) at 0 milliseconds (ms), to outside the liquid (bottom). Arrows show the same reference point on the tongue in each image. (B) Cross‐sectional diagrams indicate the changes in lamellae position at the reference point over time. From top to bottom: inside rotation of the entire structure (blue and red colors represent portions of visible lamellae along each side of the rod); tongue tips joining; and lamellae closing and closed. In the first two diagrams, the lamellae are inside the nectar; in the last two, the lamellae have been withdrawn but contain nectar trapped inside the grooves. Scale bars = 0.5 mm.
(From Rico‐Guevara and Rubega 2011. Reproduced with permission from National Academy of Sciences, USA.)
Hummingbirds have a variety of other adaptations for exploiting nectar, including their unique wing and shoulder anatomy for hovering flight (Chapter 5), and diverse beak lengths and curvature, adapted to different flower sizes and shapes (Fig. 8.28). Some hummingbirds and other species also engage in traplining—visiting the same set of food sources on a regular basis to minimize competition and maximize availability. Research on the Purple‐throated Carib (Eulampis jugularis) on eastern Caribbean islands (Temeles et al. 2006) illustrates the potential for competition within and among species. In this hummingbird, the relatively larger males outcompete females for the densest patches of flowers, defending these territories and thus forcing females to move around more widely. Experiments support the expectation that females employ traplining and are more efficient than males, minimizing travel costs by visiting flowers at longer intervals, thereby allowing the flowers to accumulate more nectar, and feeding from different flower types. Increasing levels of competition from other birds has the opposite effect: when female Purple‐throated Caribs encounter flowers depleted by other hummingbirds (or experimentally depleted by scientists), they return more quickly so as to try and compete pre‐emptively.
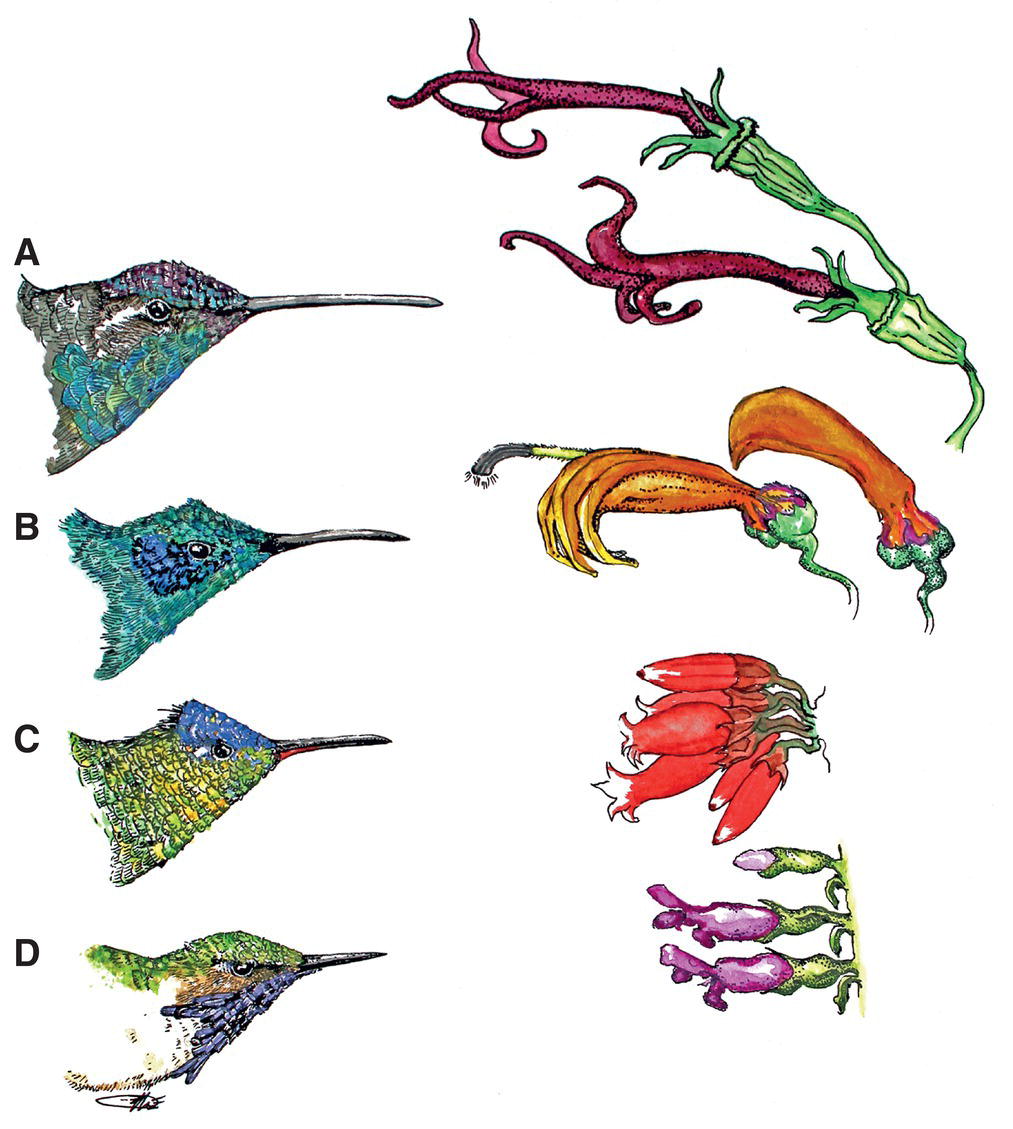
Fig. 8.28 Bill size corresponds to flower shape. The (A) Magnificent Hummingbird (Eugenes fulgens), (B) Green Violetear (Colibri thalassinus), (C) Fiery‐throated Hummingbird (Panterpe insignis), and (D) Volcano Hummingbird (Selasphorus flammula) coexist in the mountains of Costa Rica, where each species has a bill adapted to different flower sizes and shapes.
(© Cornell Lab of Ornithology, adapted from Wolf et al. 1976.)
Predatory birds must hold onto and immobilize prey prior to ingestion. To hold onto different prey types, raptors and owls have talons. Other birds have bills that are either hooked (as in raptors, shrikes, ant shrikes, and some puffbirds) or serrated (mergansers and puffins) (Fig. 8.16A, B). Fish‐eaters such as cormorants and penguins have backwardly pointing projections on their mandibles to help direct their slippery prey towards their esophagus. Birds such as jacamars and bee‐eaters chase aerially fleeing insects and often have long, thin beaks that can close quickly at the tip and reach beyond an insect’s fragile wings to secure the prey by its body.
Preparation and ingestion
Once a bird has successfully captured and secured food, processing often is needed to facilitate swallowing or digestion. Mammals use teeth to tear, chew, and grind their food before it enters the gut, but birds do not have teeth. Nonetheless, many birds process food extensively before ingestion and digestion. For example, raptors, vultures, and buzzards use their feet and beaks to dismember prey. Fish‐eaters often numb or kill their prey by whacking it against a perch, and they generally swallow fish headfirst to avoid choking on spines that run along the dorsal fins. Insect‐eating birds usually swallow their prey headfirst for the same reason.
Most birds other than parrots and raptors simply use their beak to immobilize and prepare food. With only a beak to handle prey, most birds are limited to food types that are small enough to fit through the gape of their mouth unless they can break up or soften larger food items. Good evidence for gape limitation comes from a study of nightjar species that found a correlation between the prey size and the size of the birds’ mouths, rather than with the birds’ overall body size (Holyoak 2001). As you might expect, the nightjar species with the largest gapes ate the largest insects. Gape size also limits the size of seeds and fruits consumed by many frugivores (Wheelwright 1985) and insectivores (Box 8.02).
In addition to shredding food with a beak and talons or softening it up with the beak, birds have additional ways of processing food. Once food is swallowed, many seed‐eaters and some insect‐feeders pulverize the food physically within their highly muscular gizzard (Chapter 6). Birds that feed on fruits containing seeds too large to pass through the gut may regurgitate the seeds after separating them from the surrounding fruit pulp, sometimes long after swallowing them. When birds transport viable seeds, the parent plant benefits, and plants have evolved nutritious fruits so that birds will be attracted to find, harvest, and disperse their seeds. For example, the Resplendent Quetzal (Pharomachrus mocinno), a spectacular iridescent green member of the Trogon family, plucks fruits of the avocado family while in flight and often regurgitates the large, uneaten seeds some distance from the parent plant. Seed dispersal by birds is an important ecological service in many tropical habitats.
Birds that feed directly on seeds often must process them by breaking open the seed coat or extracting them from cones or other protective structures. Perhaps the most pre‐eminent avian seed‐eaters are the blue macaws. The Hyacinth Macaw (Anodorhynchus hyacinthinus) of arid parts of Brazil specializes on palm nuts and other seeds with exocarps so hard that humans need sledgehammers to open them. These birds accomplish this feat by using the mechanical advantage from the short lever‐arms of their powerful, deep beaks. The heavy‐beaked Hawfinch (Coccothraustes coccothraustes) of Eurasia uses more than 45 kilograms of force to open cherry and olive stones. Other adept seed‐crackers include large‐billed species of bullfinches, grosbeaks, and cardinals.
Some birds that specialize on the seeds of conifer trees have evolved specialized morphologies to extract seeds from cones, leading to coevolution between the birds and trees. Some of the most compelling examples of this process come from studies of Clark’s Nutcrackers (Nucifraga columbiana) and Red Crossbills (Loxia curvirostra) (Box 8.05).
Nectar‐feeders are similar to seed‐eaters in that they must work to extract food from plants. Thus, they have also evolved both mutualistic and parasitic relationships with plants (Chapter 14). Nectarivory has evolved in diverse bird groups along with striking convergences in beak shape. Nectar specialists include the New World hummingbirds, the African and Asian sunbirds, different groups of honeycreepers in Hawaii and Latin America, and the Australasian honeyeaters. Birds in all these groups participate in mutualisms with plants in which the bird is dusted with pollen while extracting nectar, and thereby moves pollen among flowers. These birds often are highly specialized in their choice of flowers. Plants have adapted to their avian pollinators in various ways. For example, New World plants pollinated by hovering hummingbirds often have flowers that are inaccessible to other birds, whereas Old World plants pollinated by sunbirds often have structures for the birds to perch on while feeding.
A wealth of evidence supports the idea that the beaks of hummingbirds are adapted to flower length and shape, based primarily on correlations between the two—for example, the tendency for short‐beaked hummingbirds to visit short flowers. The longer term evolutionary question is whether flower length and shape actually cause specialized bird morphology by selectively restricting access to nectar rewards. This requires researching whether hummingbirds with different bill anatomy are more or less efficient when foraging for nectar on different flower species. A recent study tested the impact of different flowers on the Purple‐throated Carib (Eulampis jugularis) (Temeles et al. 2009). These hummingbirds are sexually dimorphic, meaning that females and males have different morphology. In this case females have longer, more curved beaks than males (Fig. 8.29), and the sexes tend to feed on different flower types. By experimenting with both real and artificial flowers, these researchers showed that when females fed on long, curved flowers, they extracted nectar more efficiently than males. Males, on the other hand, used their shorter, straighter beaks more efficiently on short artificial flowers, particularly while hovering. This study provides a clear example of a trade‐off in which one adaptation outcompetes another under one set of circumstances, and vice versa.
Fig. 8.29 Sexual dimorphism in bill anatomy. (A) Female Purple‐throated Caribs (Eulampis jugularis) have longer, more curved bills compared with (B) males. Each sex is most efficient at extracting nectar from flowers that match its bill shape, and both employ different strategies to guard or search for flowers.
(Photographs by: A, Stephen Daly; B, Fred D. Canter.)
Although we typically associate nectar feeding with birds like hummingbirds that extract nectar from tubular flowers, birds have found other solutions for accessing nectar. For example, a group of neotropical tanagers called flowerpiercers are nectar robbers that poke a hole through the flower base to draw off nectar without helping the flower to transport pollen. Some hummingbirds with short, pointed bills do the same thing—so one cannot assume that hummingbirds with short bills feed only on shallow flowers. Sapsuckers in North America feed on sweet plant fluids in a different way: they drill neat rows of holes in tree bark to stimulate oozing sap (Fig. 8.30). It is still debated whether the sapsuckers feed primarily on the plant sap itself or on the insects that it attracts. Ruby‐throated Hummingbirds (Archilochus colubris) are able to migrate north in spring into areas in eastern North America before flowers are available because they parasitize both the sap and insects attracted to the holes made by Yellow‐bellied Sapsuckers (Sphyrapicus varius). The hummingbirds even follow the hole‐makers through the woods to learn the locations of the sapsuckers’ feeding areas (Flaspohler and Grosshuesch 1996).

Fig. 8.30 Tree sap foraging. Yellow‐bellied Sapsuckers (Sphyrapicus varius) drill and maintain a series of holes to access tree sap. Other species, like warblers and hummingbirds, have been known to visit these holes for the sugar and the insects attracted to it.
(Photograph by Cameron Rognan.)
Finally, some birds process the foods they catch or find, and cache non‐perishable seeds or frozen meat for later use. The most general definition of caching is handling food to save it for future use. This behavior usually has three components: deferring the immediate consumption of the food item; handling so as to deter its use by most other animals; and the diverse behaviors necessary to select, prepare, transport, place, conceal, and later retrieve the food (Vander Wall 1990). Birds that cache obviously benefit from having access to foods long after they were originally available. The caching of seeds can also benefit plants via the dispersal of occasional seeds that the birds never retrieve. Caching, hoarding, or storing food (all are synonyms) are beneficial enough to birds that this behavior has evolved in at least 15 bird families within four orders. Hoarding species can store food items either individually (scatter hoarders) or grouped (larder hoarders).
Two basic questions are important to understand hoarding behavior: (1) why is it beneficial; and (2) how do birds manage it? One of the most obvious advantages of hoarding is the ability to control the distribution of food in time and space, and accordingly most hoarding behavior follows natural rhythms: diurnal, tidal, or seasonal. Hoarding can reduce a bird’s vulnerability to environmental fluctuations in food and reduce its vulnerability to worsening weather conditions that impede foraging. Some birds hide food early in the morning for use later in the day, hedging bets against inclement weather and making sure they have enough energy to survive cold temperatures during a long night. For example, New Zealand Robins (Petroica australis) store earthworms and insects in tree branch crotches and stump ends (Powlesland 1980). Other birds cache when food is only temporarily available, such as Northwestern Crows (Corvus caurinus) in British Columbia that cache shellfish during low tide to feed on later when the intertidal zone is inundated (James and Verbeek 1984).
Other birds hoard food for much longer periods, as a resource for future survival and reproduction. For example, Clark’s Nutcrackers (Nucifraga columbiana) and Pinyon Jays (Gymnorhinus cyanocephalus) in the Rocky Mountains of North America hoard when pine seeds are abundant in the fall. The thousands of seeds that they cache keep well for months and provide up to 70–100% of their food in winter and early spring (Giuntoli and Mewaldt 1978). These saved food resources allow the birds both to persist in locations that otherwise would be inhospitable and to start breeding far earlier in spring than their potential competitors. Similarly, Eurasian titmice such as Willow Tits (Poecile montanus) and Crested Tits (Lophophanes cristatus) hoard in the fall. Their stored provender constitutes up to 50% of their diet later in the year when food is scarce and improves their chances of overwinter survival (Haftorn 1954; Jansson et al. 1981).
Some raptors, particularly falcons, may hoard the relatively large food items they catch, and use them weeks or months later. For example, American Kestrels (Falco sparverius) may store multiple mice in small trees or grass clumps (Stendell and Waian 1968; Toland 1984). Eleonora’s Falcons (Falco eleonorae) store migratory songbirds they catch during the prey’s fall flights across the Mediterranean Sea in shrubs, and even imprison them live in rock crevices, keeping them fresh (Vaughan 1961; Walter 1979; Qninba et al. 2015). A variety of owls hoard to supplement food both while breeding and during cold periods, times when energetic costs of survival increase and when food keeps well by freezing. Eurasian Pygmy‐Owls (Glaucidium passerinum), for example, hoard more food during cold winter periods (Solheim 1984). Other predatory birds known for caching prey include the butcherbirds of Australia and New Guinea and the widely distributed shrikes. Many species in both of these groups impale animals—such as small mammals, birds, lizards, and grasshoppers—on thorns or on barbed‐wire fences. This impaling behavior may have originated as a way for these birds to handle and anchor prey so that it could be torn and dismembered more easily (Fig. 8.31). These birds now use cached items as part of courtship, as supplemental food for other reproductive activities, and for surviving inclement weather. Some desert shrikes in North Africa have adopted an unusual variant of this impaling behavior and similarly cache date‐palm fruits that help them survive dry, food‐lean summer months (Parrott 1980).

Fig. 8.31 Impaling prey. Long‐tailed Shrikes (Lanius schach) and other members of the family Laniidae impale prey on thorns or other sharp objects. Impaled prey is easier to dismember; some shrike species also impale prey as a form of caching.
(Photograph by Eddy Lee Kam Pang.)
When food is limiting, pre‐caching competition provides another compelling advantage for hoarding. Caching food that is only temporarily available—such as a seed crop or a large animal carcass (Box 8.01)—helps keep it away from competitors. A variety of seasonally fruiting pine species with large and wingless seeds are esteemed by diverse corvid species in both the New and Old World temperate latitudes. These species show varying dependence as well as corresponding behavioral, morphological, and spatial cognition adaptations for caching and recovering seeds (Box 8.06). Competition for these seasonally abundant, nutritious, and long‐keeping foods can be intense, so some bird species travel long distances to conceal their seed caches. The Gray Jay (Perisoreus canadensis) even uses special salivary gland secretions to form compact food balls containing multiple seeds and fruits that they scatter on conifer foliage and bark (Dow 1965; Vander Wall 1990).
A final advantage of hoarding is to redistribute food for convenience, perhaps minimizing time spent foraging instead of other activities. In New Guinea, for example, male Macgregor’s Bowerbirds (Amblyornis macgregoriae) store fresh, ripe fruit in shrubs and understory trees near their bowers, thereby increasing the time they can spend in courtship later (Pruett‐Jones and Pruett‐Jones 1985).
Pulverization and digestion
The final stages of feeding begin when food enters the esophagus from the oral cavity and continue into the processes of digestion. Since every stage of feeding influences what is eaten, physiology and digestion influence diet. Because the fate of foods in the digestive tract is the domain of physiology, this topic is covered in greater detail in Chapters 6 and 7.
One of the most obvious ways birds break down food to make it digestible is to pulverize hard items such as seeds in their gizzard. Since birds do not have molar teeth as mammals do, many birds ingest small stones to aid the strong muscles of their gizzard in grinding food (Chapters 6 and 7). Few birds eat plant leaves, probably because of the low nutritive value relative to birds’ high metabolic demands, but the exceptions are informative about the trade‐offs involved in specializing on leaves. The Hoatzin (Opisthocomus hoazin) of South America has a particularly unusual specialization of its digestive tract, which contains symbiotic microbes that break down the cellulose in leaves via fermentation (Chapters 6 and 7), similar to the rumenary action of cows and other ungulates. The plantcutters (Phytotoma species) of South America eat diets comprised of over 90% leaves. They benefit from feeding year‐round on a few plant species with relatively high protein to fiber ratios, coupled with special enzyme activity throughout the length of their intestine that helps them digest proteins and sugars (Meynard et al. 1999; Bucher et al. 2003).
When considering feeding choices by frugivorous birds, it is important to recall that the evolutionary goal of fruiting plants is to make fruits attractive to seed‐dispersing frugivores while deterring seed‐destroying frugivores. Some plants appear to have accomplished this goal by producing compounds that are toxic to seed‐destroying frugivores. For example, wild chili peppers use the compound capsaicin, which humans sense as the chili peppers’ “heat,” to influence which animals consume their fruits, and how long their gut retains the seeds (Tewksbury and Nabhan 2001; Levey et al. 2006; Tewksbury et al. 2008). These pepper plants pack their fruits with capsaicin and relatively high concentrations of oils and proteins. Mammals, which tend to macerate and thus destroy seeds with their molar teeth, can taste capsaicin, but birds lack the molecular receptor that capsaicin binds to, and so do not sense this pungency. Video observations of plants in nature document that mammals avoid eating these peppers, whereas some bird species (including thrushes, thrashers, and flycatchers) consume many. These same bird species are excellent seed dispersers for the plants, defecating the seeds unharmed after gut passage times of several hours.
8.4 Benefits and costs of social foraging
Birds join groups for many purposes, including nesting, roosting, mating, and feeding (Chapter 9). Even in groups formed only for feeding, such as foraging flocks, individuals benefit in the locating, subduing, and handling of prey. The diversity of flock types and circumstances raises questions about when it is advantageous for an individual to join a group. At what group size are diminishing returns from competition reached? Or when does the increased risk of predation outweigh the group benefits? Benefits typically derive from finding food and feeding more successfully and safely. Group feeding also carries unique risks, however, such as having food items stolen by other group members. It is also complex in that it involves decision processes that depend on what other group members do, and involves complex interactions with the prey or other food resources. The diverse benefits and costs of group foraging also depend on whether the other group members belong to the same or different species; whether they are strangers or relatives; whether the resource is permanent, seasonal, or ephemeral; and on the balance between competition versus cooperation.
The simplest types of avian feeding groups are aggregations that form temporarily around resources too abundant, ephemeral, or otherwise difficult for a single individual or mated pair to defend. Dominance hierarchies often form in these kinds of foraging groups. Such hierarchies occur among the individual House Sparrows (Passer domesticus) feeding on crumbs in an urban plaza as well as among the diverse bird species visiting a fruiting tree in a tropical forest. Such aggregations may form simply because individuals are attracted to sites where other individuals are foraging.
A well‐studied class of avian aggregations involves seabirds diving into a frothing mass of prey below the water surface. Because schooling fish simultaneously try to evade predatory fish attacking from below and seabirds diving from above, they are easier to attack from both directions, creating a mutualism between the different fish‐eating groups. Many species of seabirds—including petrels, shearwaters, gulls, terns, boobies and gannets, pelicans, cormorants, and frigatebirds—may join these foraging groups (Fig. 8.32). Some of the raucous screaming at such aggregations is not excitement, but rather fear from birds with prey being pursued by birds who would rather steal than catch their own food. Acquisition of food by force is known as kleptoparasitism (or piracy) and is a characteristic behavior of frigatebirds (so‐named because they behave like aerial pirates), which harass boobies and tropicbirds in warm waters. Skuas and jaegers similarly steal food from terns and kittiwakes at higher latitudes (Chapter 14). This behavior is frequent in nature because the parasite is at an advantage while on the chase since it is unencumbered and has an aerodynamic edge over a victim slowed by carrying a heavy prey item. One consequence of kleptoparasitism is that it creates both an incentive for the parasite to join flocks and for the victim to avoid them.

Fig. 8.32 Mixed‐species foraging. Seabirds, such as cormorants and gulls, often forage for fish in mixed‐species groups. As group size increases, so does hunting success and overall consumption.
(Illustration by N. John Schmitt.)
Diverse bird species may aggregate at animal carcasses. These ephemeral banquets of carrion are traditionally provisioned by various large carnivores (Fig. 8.33). Humans supplement natural kills both inadvertently (by road kills and hunting) and deliberately (as in the case of lead‐free carcasses provisioned to California Condors (Gymnogyps californianus) as part of a conservation effort; Chapter 15). The most species‐rich feeding assemblages of avian scavengers involve eight or more species of African vultures in places where carcasses are continually generated by large mammalian carnivores such as lions. The scavenging birds typically arrive within hours of a kill, locating it from cues such as the presence of hyenas or other scavenging birds. Studies have documented that larger bodied species such as White‐headed (Trigonoceps occipitalis) and Lappet‐faced (Torgos tracheliotos) Vultures arrive first, because their larger and stronger beaks are capable of tearing through skin to get at the meat (Kruuk 1967; Houston 1975). These two larger African species also have relatively long necks that can reach deep into carcass holes and orifices, and tongues with tooth‐like projections to help rasp meat off the bones. This creates a type of positive interaction between species known as trophic advantage, since smaller species later gain better access to food they can handle (Cortés‐Avizanda et al. 2012). In contrast, a study of 639 cheetah kills of various animals in Serengeti National Park, Tanzania (Hunter et al. 2007) found that mammalian scavengers (lions, hyenas, and jackals) generally arrived within half an hour of a kill, but that vulture species did not arrive in a standard order. The arrival times of the vultures in this area may not have been distinguished by species because the abundant mammalian scavengers opened the carcasses quickly enough to make food promptly accessible to vultures of all sizes.

Fig. 8.33 Carcass aggregation. Different vulture species often congregate at the same large carcass, provisioned either by carnivores or humans (as a conservation measure). Here, critically endangered White‐rumped (Gyps bengalensis) and Slender‐billed (Gyps tenuirostris) Vultures flock to the carcass of a water buffalo in Cambodia.
(Photograph by Yula Kapetanakos.)
These and the many other avian feeding aggregations illustrate the benefits and costs of foraging in groups, especially competition within and among species. Competition within species contributes to broadening diets (Box 8.01). Competition among species leads to niche partitioning (Chapter 14), illustrated by the various depths different seabirds dive for prey, the existence of birds that catch their own prey and those that kleptoparasitize other birds, and carrion‐feeders that specialize on different parts of a kill. These niche differences attest to the importance of interspecific competition; indeed, competition both among and within species is common in feeding aggregations simply because the individuals that feed most efficiently (often in groups) have the greatest fitness, and thus tend to survive and reproduce.
Feeding in a group also raises the question: When do the benefits of joining these groups outweigh the costs arising from competition? An experiment involving aggregations of multiple species of carrion‐feeders in northern Spain addressed both costs and benefits of feeding on large carcasses (Cortés‐Avizanda et al. 2012). Dead livestock carcasses were used to create “vulture restaurants” as part of a vulture conservation initiative. The study compared how birds used these predictable carrion sources versus experimentally reproduced ephemeral (unpredictable) carcasses more similar to those occurring naturally from unpredictable kills by predatory mammals. Although the same number of carrion‐feeding bird species visited carcasses of both types, most of the spoils from the predictable treatment went to the largest species, the Eurasian Griffon (Gyps fulvus), because these socially dominant birds were easily able to find and exclude other competitor species from the vulture restaurants. At the ephemeral carcasses, by contrast, other carrion‐feeder species often discovered the carcass first, and were less likely to be excluded from feeding by Eurasian Griffons. This study clearly showed how the unpredictability of prey occurrence contributes to the coexistence of multiple vulture species in this feeding assemblage.
Joining a flock potentially provides another benefit to the individual by increasing the time it can spend foraging (Chapter 9). At first glance, this benefit might seem counterintuitive, because squabbling and competing with flock members tend to reduce rather than increase foraging time. In many situations, however, birds in flocks are able to share the sentinel duties of watching for predators. Overall, a flock can have many more eyes looking for danger even if each individual bird spends less of its own time being vigilant. The enhanced vigilance benefit of foraging in a group probably is responsible for the various combinations of mixed‐species flocks (Chapter 14) that occur in most parts of the world. Although mixed‐species foraging flocks differ in their patterns of seasonality and in their social organization, they are widespread because most small birds are vulnerable to attacks by raptors and other predators. At north temperate latitudes, these flocks tend to form in fall when food becomes scarce and the relative benefit of increased surveillance contributes to feeding efficiency. In fall, groups of titmice, nuthatches, creepers, and woodpeckers travel actively together, scanning tree trunks and branches for small arthropods. In many tropical areas, mixed‐species flocks form year‐round and can comprise dozens of species each searching different substrates for prey.
Some bird species take on specialized roles within flocks, such as sentinels. This is most common in relatively open areas where predators can be scanned for and easily spotted, and an alarm call broadcast. In South America, White‐winged Shrike‐Tanagers (Lanio versicolor) serve as a sentinel species for canopy flocks. However, they occasionally use their alarm calls deceptively to obtain food from other flock members; this species essentially “cries wolf” to signal the need to dive for cover, even though no predator is present, thereby allowing the sentinel to retrieve the morsel (Munn 1986) (Fig. 8.34A). Fork‐tailed Drongos (Dicrurus adsimilis), songbirds that forage in mixed‐species flocks in the Kalahari Desert in Africa, use alarm calls honestly to protect other bird species from predators—as well as dishonestly—but also take deceptive alarm calls one step further. They take advantage of their ability to mimic different target bird species’ alarm calls and change them regularly so that their victims do not become habituated to and ignore a particular call type (Flower et al. 2014) (Fig. 8.34B). The drongo can even change the alarm call used on a targeted individual—especially when a particular alarm call type has failed to elicit a prey drop by the victim (Chapter 10).

Fig. 8.34 Real and deceptive alarm calls. (A) White‐winged Shrike‐Tanagers (Lanio versicolor) often serve as sentinels in mixed‐species flocks, alerting other species to avian predators—although sometimes they also sound false alarms to steal prey found by fleeing flock members. (B) Similarly, Fork‐tailed Drongos (Dicrurus adsimilis) use drongo‐specific “chink” alarm calls to alert other drongos of predators or to deceive conspecifics and steal their prey (three individuals in a, b, and c were recorded using their alarm calls in true and false contexts). Drongos also mimic heterospecific alarm calls that cause foraging birds to flee for cover, abandoning found prey. Drongos have been observed and recorded mimicking heterospecific alarm calls while watching other species forage, for example: d, Asian Glossy Starlings (Aplonis panayensis); e, Crowned Lapwing (Vanellus coronatus); and f, Southern Pied‐Babbler (Turdoides bicolor).
(A, illustration by N. John Schmitt. B, from Flower 2011. Reproduced with permission from the Royal Society/Tom Flower and Martina Boerner.)
Sentinel species are often boisterous and provide flock cohesion for other species. The sentinel function of these boisterous species provides an incentive for non‐sentinel species to join a flock. This is particularly important for birds like shorebirds and ducks that are vulnerable when feeding with their heads down, or for those like the antwrens in neotropical forests that search for prey hidden within clusters of curled, dead leaves. In a study of Amazonian mixed‐species flock assemblages in Peru (Martínez and Zenil 2012), it was found that different types of foragers depend on sentinel benefits. In upland tierra firme habitat, a different Thamnomanes antshrike species served as the alarm caller to the one in nearby inundated forests, and different species of flycatchers and dead‐leaf foragers were parts of these flocks in the two forest types. As predicted, the more vigilant species in each flock type, the flycatchers, responded more weakly and recovered more quickly in response to experimental playbacks of the antshrikes’ alarm calls. The more vigilant flycatchers therefore seemed less reliant on the antshrike alarm calls compared with their more vulnerable flock‐mates who were dead‐leaf foraging specialists.
When food is abundant enough to sustain a large feeding group but is unpredictable relative to individuals’ searching capacity, the ability to locate food using knowledgeable individuals—informants—minimizes the risk of starvation. This idea raises the question of whether some birds form breeding aggregations, such as a heron rookery or booby colony, or other kinds of social groups to gain information about where to feed. This idea is known as the “information center hypothesis.” Despite its appeal, this idea has been well documented in only a few cases, including Cliff Swallows (Petrochelidon pyrrhonota) (Brown 1986), Common Ravens (Corvus corax), and Ocellated Antbirds (Phaenostictus mcleannani) (Box 8.07).
Cooperative hunting as a means to improve prey‐capture efficiency is yet another benefit of group foraging in birds. This ability is exploited by some raptors and fish‐eaters, and by at least one passerine songbird. In the simplest case, members of a mated pair or a small group hunt in a coordinated way to capture more or larger prey than would be possible otherwise, even after food is shared between the hunters. Ravens (the one passerine known to hunt cooperatively) often hunt in tandem (Box 8.01), as do Golden Eagles (Aquila chrysaetos) and Aplomado Falcons (Falco femoralis). Harris’s Hawks (Parabuteo unicinctus) in New Mexico hunt in groups of two to six individuals, and capture animals such as jackrabbits that single hawks cannot subdue. For these hawks, hunting efficiency increases directly with hunting group size (Bednarz 1988). One tactic is for one or two group members to penetrate shrubbery cover to flush prey into the open, where other group members are waiting to make the kill. Among fish‐eating birds, groups of American White Pelicans (Pelecanus erythrorhynchos) may form a line or semicircle of a dozen or more individuals and work to scare fish into shallow water where they can be more easily scooped up (Fig. 8.35).

Fig. 8.35 Prey‐capture efficiency in groups. (A) When a group of American White Pelicans (Pelecanus erythrorhynchos) comes upon a school of fish, they will form a line and/or splash with their wings to corral their prey into shallower water. (B) Sometimes pelicans will form a circle around their prey to increase fishing efficiency under water.
(Illustration by N. John Schmitt.)
8.5 Feeding specialization and generalization
Feeding specialization and generalization are two ends of a behavior spectrum in which individuals range, respectively, from being highly selective in what they eat to being non‐discriminating. A hummingbird that feeds from the flowers of only a few plant species is clearly a specialist, and most ravens are clearly generalists (Box 8.01), as is the Cocos Finch (Pinaroloxias inornata) (Box 8.08). Understanding a species’ specialization is important for interpreting feeding adaptations, because the more specialized a species is, the more likely it is that a particular adaptation is evolutionarily fine‐tuned to help the bird exploit a particular resource. The beaks of crossbills (Box 8.05) and many hummingbirds are good illustrations of specialized morphologies that have evolved to help these species gain access to particular food resources that they could not use otherwise.
Feeding specialization also is important in the context of conservation, because particular food resources can disappear or become less available. Species with specialized diets are dependent on specific, potentially unreliable, resources and so are at higher risk of population decline or extinction (Chapter 15). Specialist feeders are even more vulnerable if their specialization involves evolutionary commitment via adaptations specific to a particular food or foraging substrate. The Ivory‐billed Woodpecker (Campephilus principalis) of the southeastern USA provides a poignant example of how a specialization that is highly successful under some circumstances can quickly become a liability when conditions change. With their large beaks, these big woodpeckers could chisel into huge dead trees and extract large beetle larvae and other energy‐rich invertebrates. Sadly, nearly all old‐growth forests within its range in the southern USA were logged between 1850 and 1950. Although much of this area remained forested, the newly regenerating forest offered few of the dead and dying trees required by the Ivory‐billed Woodpeckers. The shortage of mature trees precipitated rapid, habitat‐induced range contraction and population decline, resulting in the woodpecker’s widespread extirpation and ultimate extinction. Meanwhile, the superficially similar Pileated Woodpecker (Dryocopus pileatus), which lives in the same habitat but has a far more generalist diet and foraging behavior, remains abundant.
Thus, feeding specialization can be defined more formally as the consistent use and dependency on one or a few food types because of foraging adaptations evolved specifically for such foods. Conversely, feeding generalization, or opportunism, is the absence of specialization. These seemingly simple definitions are not always easy to apply, and many birds cannot be classified easily. For example, a species can eat a narrow range of foods but do so using diverse behaviors, or conversely can eat a variety of foods but in a consistent, stereotypical way. Another complexity arises from the multiplicity of skills required to feed successfully, including encountering, recognizing, catching, handling, and finally digesting foods (Fig. 8.08): a bird may be specialized in one of these skills but not in others. With these confounders, one needs to define specialization carefully in a particular context.
8.5.1 Conditions favoring diet specialization
No general theory yet links the different components of specialization discussed above. Ecological conditions favoring specialization include a stable environment, competition from other species, and the availability of a distinctive resource.
One assumption underlying most cases of foraging and diet specialization in birds is termed the ecological principle of allocation; it holds that the energy or other resources an organism allocates to one aspect of its life history are unavailable for other aspects. In the present context, the more specialized an organism is for one resource, the less efficiently it can exploit other resources. Thus, for a bird to gain efficiency in a particular feeding technique, it generally needs to specialize in that technique, and that specialization decreases its efficiency in other techniques. For example, a thin beak with a forceps‐like tip that is well adapted to gleaning small insects efficiently is likely to be incapable of cracking hard seeds, and, conversely, birds with massive bills that easily crack seeds are not likely efficient at gleaning small insects. Trade‐offs are inherent to the principle of allocation: skill in one aspect of an organism’s performance comes at the expense of skill in others. We already have encountered examples of trade‐offs in which a bird gains efficiency in recognizing and handling particular prey types with the downside of reducing its efficiency in using other co‐occurring prey types.
The most important general condition for specialization is a reliable, year‐round food source, so that the specialist is not penalized by the disappearance of its preferred food. Conversely, highly seasonal or unpredictable environments are poor places to specialize. Not surprisingly, many of the most dramatic examples of avian foraging specialization come from the tropics, which are relatively predictable compared with seasonally variable, higher latitude environments. For example, groups of tropical forest insectivores in Peru have narrower foraging niches (thus, greater specialization) than do ecologically similar groups at temperate latitudes (Marra and Remsen 1997). This finding stems from a tendency for many year‐round resident tropical insectivores to feed on a specific substrate, a behavior known as substrate‐specific foraging. For example, many Central American woodcreepers and many Malaysian woodpeckers use only certain tree types (Sillett et al. 1997; Styring and Zakaria bin Hussin 2004). The year‐round availability of hanging dead‐leaf substrates colonized by hiding insects such as cockroaches and crickets supports entire foraging guilds of tropical New World antwrens that specialize in foraging in this aerial leaf litter (Rosenberg 1990, 1993) (Chapter 14). Similarly, a large guild of Costa Rican flycatchers contains many species with unusually high diet selectivity and specialization, especially as compared with migratory flycatchers that feed seasonally in the same rainforest habitats (Sherry 1984).
Reliably constant, year‐round resources likely explain the tendency for resident birds at higher latitudes, as well as in the tropics, to feed as specialists on particular substrates. Understanding why some resources are more constant than others in a given environment thus helps us understand not only the nature of resource specialization but also why some birds migrate and others do not. In any environment, some resources tend to be relatively buffered from fluctuations, whereas others vary across space and time. For terrestrial birds, buffered resources tend to be hidden and cryptic, or relatively difficult to reach, such as wood‐boring beetle grubs in trees and arthropods under leaves or deep underground. Such prey tend to be relatively constant in abundance and require methodological searching strategies to find and extract, so their use favors specialized searching behaviors. Birds that reside on a feeding territory year‐round are well suited to exploit such foods. In contrast, exploiting resources that are conspicuous and variable in space and time—such as foliage and flying insects, and many kinds of fruit and nectar—requires a different set of skills related to opportunistic search and foraging behaviors.
Sophisticated cognitive abilities also may help resident birds cope with temporary resource shortages as compared with the more opportunistic and plastic behavior of migrants. For example, Daniel Sol et al. (2005) showed that year‐round resident birds in Europe, which must cope with shortages even of buffered foods, are particularly successful in exploiting novel foods, perhaps due to their methodological searching. Moreover, they found that resident birds tend to be relatively larger brained than migratory European songbirds, a trait that may facilitate the kinds of learning needed to find and exploit novel foods. A study of a group of Great Tits (Parus major) that systematically search out and kill bats wintering in a Hungarian cave, documented a novel foraging behavior (Estók et al. 2010). When these researchers experimentally provisioned these birds with alternative foods, their predation rate on bats decreased, supporting the idea that hunger provides a stimulus for such novel behaviors. A resident European bird, the Eurasian Blue Tit (Cyanistes caeruleus), provides another particularly famous example of novel foraging behavior. A population of these birds in the UK learned to open milk bottle caps to get at the cream, a trait that then spread culturally among nearby groups of tits. Intense competition for scarce winter food may even have contributed to the evolution of sex differences in cognitive, specifically memory, skills, illustrated in Great Tits: females exceed males in remembering the locations of food cached by another local titmouse species, which is thought to help females cope with losing out to socially dominant males in direct competition for food (Brodin and Urhan 2015).
The second general condition for feeding specialization is competition from other animals. The importance of interspecific competition in structuring species’ niches has been debated (often contentiously) among ecologists, but today little doubt exists that competition is a critical factor in causing species to specialize (Chapter 14). The importance of interspecific competition is supported by the phenomenon of ecological release, in which species expand their feeding niche—becoming more generalized—when competing with fewer species. One of the best documented examples of ecological release involving many species comes from the Caribbean Islands. In these islands, there are fewer bird species and those that inhabit the islands can expand their range of habitats as compared with the same species residing in species‐rich Panamanian forests where they must compete with many competitors (Ricklefs 2001) (Fig. 8.36).

Fig. 8.36 Ecological release and habitat breadth. As both the local (blue line) and regional number of species increases—here comparing species‐poor St. Kitts with biodiverse Panama—the breadth of habitats occupied per species (yellow line) and the local abundance of species within particular habitats (red line) decreases. This pattern is consistent with the idea that species are “released” from interspecific competition in species‐poor regions and thus become more abundant in a wider range of habitats (as in St. Kitts).
(Adapted from Ricklefs and Cox 1977, reproduced with permission from John Wiley and Sons; and Wunderle 1985, reproduced with permission from The Wilson Bulletin.)
A conspicuous feature of many foraging guilds—sets of coexisting bird species that feed on similar resources in similar ways—is that each species becomes specialized in a way that reflects competition with the other guild species (Chapter 14). Dominant species may specialize on a specific resource, leaving others to feed opportunistically as social subordinates on leftovers or on resources that the specialists cannot monopolize completely. A classic example of such a guild is birds that feed on piñon pine seeds and cache them for future use (Vander Wall and Balda 1981) (Box 8.05). Other avian foraging guilds that are structured by competitive interactions include professional versus opportunistic army‐ant followers (Boxes 8.08 and 14.08) and guilds of vultures and other carrion‐feeders.
A third situation that promotes the evolution of specialists is food that requires particular anatomical adaptations for handling. As mentioned earlier in this chapter, the Snail Kite (Rostrhamus sociabilis) has a specialized bill that allows it to rely on one species of abundant, marsh‐dwelling snail. As discussed in Box 8.05, different Red Crossbill (Loxia curvirostra) morphs have bill shapes and sizes that are fine‐tuned to extract seeds from particular types of conifer cones. Both the African Harrier‐Hawk (Polyboroides typus) and the Crane Hawk (Geranospiza caerulescens) from the New World tropics have double‐jointed legs that allow them to reach deeply into tree holes and other crevices to extract amphibians and the nestlings of other birds (Fig. 8.37).

Fig. 8.37 Foraging adaptation convergence in raptor legs. (A) The African Harrier‐Hawk (Polyboroides typus) and (B) the Crane Hawk (Geranospiza caerulescens) from the neotropics have independently evolved the ability to bend their knees in multiple directions. This adaptation permits them to grab prey from otherwise difficult‐to‐reach places, including cavity nests, burrows, and narrow crevices.
(Photographs by: A, Marietjie Froneman; B, Andreas M. Schmidt.)
8.5.2 Individual feeding specializations
Some of the most surprising specializations arise when members of the same bird population feed on different resources, a phenomenon observed in a number of species (Bolnick et al. 2003). Some of these differences arise from genetic differences, but others are learned as the individuals mature. Within species, sexual dimorphism for feeding (as opposed to secondary sexual trait dimorphism driven by sexual selection—Chapters 3 and 9) is the most familiar and widespread genetically based feeding specialization. Examples of species in which the sexes feed on different resources include many woodpeckers, raptors, and the dramatically sexually divergent (and sadly extinct) Huia (Heteralocha acutirostris) of New Zealand (Fig. 8.38). In these and many other birds, sexual dimorphism in beak and/or body size results in sexual differences in prey types, sizes, or foraging habitats.

Fig. 8.38 Sexual dimorphism in bill shape. The two sexes of the extinct Huia (Heteralocha acutirostris) of New Zealand were once thought to be separate species, owing to the extreme differences in their bills. Pairs were reported to work together to optimize foraging: males had chisel‐shaped bills, perfect for drilling holes in wood, while females used their long, decurved bills to probe under bark for insects.
(Artwork by John Gerrard Keulemans, from Buller 1888, public domain.)
Learning underlies many other feeding specializations by individual birds. Learning by experience that improves feeding skills is widespread, especially in species requiring skill to catch and handle prey. Learned feeding skills especially benefit individuals in communities with few species. This circumstance favors broad diets—resulting from intraspecific competition—but diminishes feeding efficiency, as illustrated by the Cocos Finch (Pinaroloxias inornata) (Box 8.08). One of the best studied examples of learned feeding specialization within a species involves Eurasian Oystercatchers (Haematopus ostralegus) (Sutherland et al. 1996). Individual oystercatchers often feed on only one species of intertidal invertebrate, such as mussels, cockles, crabs, or polychaete worms. Oystercatcher individuals that feed on the same prey—such as mussels—may specialize further by technique as “stabbers” (inserting their beaks to pry the shell open) or “hammerers” (pounding a shell’s weak spot until it breaks to expose the meat inside). Hammerers may specialize even further on either the dorsal or ventral surface of a valve, and ventral hammerers may even specialize on a right or left valve. Why do oystercatcher individuals specialize to this high degree and pass over prey that other individuals find profitable? Part of the answer may be that these prey types are difficult for oystercatchers to find and handle, and the optimal search behavior for one prey type may cause individuals to overlook another. Likewise, oystercatchers must learn how to exploit each type of prey efficiently. Once learned, a particular feeding technique may require commitment, because a bird’s beak becomes shaped to some extent by abrasion and makes a particular type of feeding more efficient. Individual feeding specializations allow birds to increase their efficiency while competing for resources, a battle where even the slightest increase in foraging efficiency might be the difference between life and death.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Adams‐Hunt, M. M. and L. F. Jacobs. 2007. Cognition for foraging. Pages 104–138 in Foraging: Behavior and Ecology (D. W. Stephens, J. S. Brown, and R. C. Ydenberg, Eds.). University of Chicago Press, Chicago, IL.
- Bednarz, J. C. 1988. Cooperative hunting in Harris’ hawks (Parabuteo unicinctus). Science 239:1525–1527.
- Benkman, C. W. 1993. Adaptation to single resources and the evolution of crossbill (Loxia) diversity. Ecological Monographs 63:305–325.
- Benkman, C. W. 1999. The selection mosaic and diversifying coevolution between crossbills and lodgepole pine. American Naturalist (Supplement) 153:S75–S91.
- Benkman, C. W., W. C. Holimon, and J. W. Smith. 2001. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55:282–294.
- Benkman, C. W., T. L. Parchman, A. Favis, and A. M. Siepielski. 2003. Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. American Naturalist 162:182–194.
- Bluff, L. A., A. A. S. Weir, C. Rutz, J. H. Wimpenny, and A. Kacelnik. 2007. Tool‐related cognition in New Caledonian crows. Comparative Cognition and Behavior Reviews 2:1–25.
- Bolnick, D. I., R. Svanbäck, J. A. Fordyce, L. J. H. Yang, J. M. David, C. D. Hulsey, and M. L. Forister. 2003. The ecology of individuals: incidence and implications of individual specialization. American Naturalist 161:1–28.
- Bonter, D. N., B. Zuckerberg, C. W. Sedgwick, and W. M. Hochachka. 2013. Daily foraging patterns in free‐living birds: exploring the predation‐starvation trade‐off. Proceedings of the Royal Society B: Biological Sciences 280:20123087.
- Brightsmith, D. J. and R. A. Muñoz‐Najar. 2004. Avian geophagy and soil characteristics in southeastern Peru. Biotropica 36:534–543.
- Brodin, A. and A. U. Urhan. 2015. Sex differences in learning ability in a common songbird, the great tit—females are better observational learners than males. Behavioral Ecology and Sociobiology 69:237–241.
- Brower, L. P. 1969. Ecological chemistry. Scientific American 220:22–29.
- Brower, L. P. 1984. Chemical defence in butterflies. Pages 109–134 in The Biology of Butterflies (R. I. Vane‐Wright and P. R. Ackery, Eds.). Academic Press, London.
- Brown, C. R. 1986. Cliff swallow colonies as information centers. Science 234:83–85.
- Bucher, E. H., D. Tamburini, A. Abril, and P. Torres. 2003. Folivory in the white‐tipped plantcutter Phytotoma rutila: seasonal variations in diet composition and quality. Journal of Avian Biology 34:211–216.
- Buller, W. L. 1888. A History of the Birds of New Zealand, Volume 1, 2nd Edition. Self‐published, London.
- Chai, P. 1996. Butterfly visual characteristics and ontogeny of responses to butterflies by a specialized tropical bird. Biological Journal of the Linnean Society 59:37–67.
- Chai, P. and R. B. Srygley. 1990. Predation and the flight, morphology, and temperature of Neotropical rain‐forest butterflies. American Naturalist 135:748–765.
- Chaves‐Campos, J. 2011. Ant colony tracking in the obligate army ant‐following antbird Phaenostictus mcleannani. Journal of Ornithology 152:497–504.
- Chaves‐Campos, J., Y. Araya‐Ajoy, C. A. Lizana‐Moreno, and K. N. Rabenold. 2009. The effect of local dominance and reciprocal tolerance on feeding aggregations of ocellated antbirds. Proceedings of the Royal Society B: Biological Sciences 276:3995–4001.
- Chaves‐Campos, J. and J. A. DeWoody. 2008. The spatial distribution of avian relatives: do obligate army‐ant‐following birds roost and feed near family members? Molecular Ecology 17:2963–2974.
- Conover, M. R. and D. E. Miller. 1980. Rictal bristle function in Willow Flycatcher. Condor 82:469–471.
- Cortés‐Avizanda, A., R. Jovani, M. Carrete, and J. A. Donázar. 2012. Resource unpredictability promotes species diversity and coexistence in an avian scavenger guild: a field experiment. Ecology 93:2570–2579.
- Cresswell, W. 2008. Non‐lethal effects of predation in birds. Ibis 150:3–17.
- Cunningham, S. J., M. R. Alley, and I. Castro. 2011. Facial bristle feather histology and morphology in New Zealand birds: implications for function. Journal of Morphology 272:118–128.
- Cunningham, S. J., M. R. Alley, I. Castro, M. A. Potter, M. Cunningham, and M. J. Pyne. 2010. Bill morphology of Ibises suggests a remote‐tactile sensory system for prey‐detection. Auk 127:308–316.
- Davenport, J., M. J. A. O’Callaghan, J. L. Davenport, and T. C. Kelly. 2014. Mussel dropping by Carrion and Hooded Crows: biomechanical and energetic considerations. Journal of Field Ornithology 85:196–205.
- Dow, D. D. 1965. The role of saliva in food storage by the Gray Jay. Auk 82:139–154.
- Dudley, R., M. Kaspari, and S. P. Yanoviak. 2012. Lust for salt in the western Amazon. Biotropica 44:6–9.
- Dumbacher, J. P., A. Wako, S. R. Derrickson, A. Samuelson, T. S. Spande, and J. W. Daly. 2004. Melyrid beetles (Choresine): a putative source for the batrachotoxin alkaloids found in poison‐dart frogs and toxic passerine birds. Proceedings of the National Academy of Sciences of the United States of America 101:15857–15860.
- Ellis, J. C., K. E. Allen, M. S. Rome, and M. J. Schulman. 2012. Choosing among mobile prey species: why do gulls prefer a rare subtidal crab over a highly abundant intertidal one? Journal of Experimental Marine Biology and Ecology 416–417:84–91.
- Elner, R. W., P. G. Beninger, D. L. Jackson, and T. M. Potter. 2005. Evidence of a new feeding mode in western sandpiper (Calidris mauri) and dunlin (Calidris alpina) based on bill and tongue morphology and ultrastructure. Marine Biology 146:1223–1234.
- Estók, P., S. Zsebök, and B. M. Siemers. 2010. Great tits search for, capture, kill and eat hibernating bats. Biology Letters 6:59–62
- Estrella, S. M. and J. A. Masero. 2007. The use of distal rhynchokinesis by birds feeding in water. Journal of Experimental Biology 210:3757–3762.
- Flaspohler, D. and D. Grosshuesch. 1996. Ruby‐throated Hummingbirds observed following Yellow‐bellied Sapsucker: evidence for keystone bird species in northern hardwood forests. Passenger Pigeon 58:237–240.
- Flower, T. 2011. Fork‐tailed drongos use deceptive mimicked alarm calls to steal food. Proceedings of the Royal Society B: Biological Sciences 278:1548–1555.
- Flower, T. P., M. Gribble, and A. R. Ridley. 2014. Deception by flexible alarm mimicry in an African bird. Science 344:513–516.
- Giuntoli, M. and L. R. Mewaldt. 1978. Stomach contents of Clark’s Nutcrackers collected in western Montana. Auk 95:595–598.
- González‐Gómez, P. L., F. Bozinovic, and R. A. Vásquez. 2011a. Elements of episodic‐like memory in free‐living hummingbirds, energetic consequences. Animal Behaviour 81:1257–1262.
- González‐Gómez, P. L., R. A. Vásquez, and F. Bozinovic. 2011b. Flexibility of foraging behavior in Hummingbirds: the role of energy constraints and cognitive abilities. Auk 128:36–42.
- Goss‐Custard, J. D., A. D. West, and W. J. Sutherland. 1996. Where to feed. Pages 105–132 in The Oystercatcher: From Individuals to Populations (J. D. Goss‐Custard, Ed.). Oxford University Press, New York, NY.
- Haftorn, S. 1954. Contributions to the food biology of tits especially about storing of surplus food. I. The Crested Tit (Parus c. cristatus L.). Det Kongelige Norske videnskabers selskabs skrifter 4:1–123.
- Heinrich, B. 1989. Ravens in Winter. Simon and Schuster, New York, NY.
- Heinrich, B. 1999. Mind of the Raven: Investigations and Adventures with Wolf‐Birds. HarperCollins Publishers, New York, NY.
- Holling, C. S. 1965. The functional response of predators to prey density and its role in mimicry and population regulation. Memoirs of the Entomological Society of Canada 97:5–60.
- Holyoak, D. T. 2001. Food and feeding ecology. Pages 55–65 in Nightjars and their Allies: The Caprimulgiformes. Oxford University Press, Oxford.
- Houston, D. C. 1975. Ecological isolation of African scavenging birds. Ardea 63:55–64.
- Houston, D. C. 1986. Scavenging efficiency of Turkey Vultures in tropical forest. Condor 88:318–323.
- Humphries, N. E., H. Weimerskirch, N. Queiroz, E. J. Southall, and D. W. Sims. 2012. Foraging success of biological Lévy flights recorded in situ. Proceedings of the National Academy of Sciences of the United States of America 109:7169–7174.
- Hunt, G. R. 2000. Tool use by the New Caledonian crow Corvus moneduloides to obtain Cerambycidae from dead wood. Emu 100:109–114.
- Hunter, J. S., S. M. Durant, and T. M. Caro. 2007. Patterns of scavenger arrival at cheetah kills in Serengeti National Park Tanzania. African Journal of Ecology 45:275–281.
- Jabloński, P. G. 1999. A rare predator exploits prey escape behavior: the role of tail‐fanning and plumage contrast in foraging of the painted redstart (Myioborus pictus). Behavioral Ecology 10:7–14.
- James, P. C. and N. A. M. Verbeek. 1984. Temporal and energetic aspects of food storage in Northwestern Crows. Ardea 72: 207–216.
- Jansson, C., J. Ekman, and A. von Brömssen. 1981. Winter mortality and food supply in tits Parus spp. Oikos 37:313–322.
- Janzen, D. H., W. Hallwachs, and J. M. Burns. 2010. A tropical horde of counterfeit predator eyes. Proceedings of the National Academy of Sciences of the United States of America 107:11659–11665.
- Jønsson, K. A., R. C. K. Bowie, J. A. Norman, L. Christidis, and J. Fjeldså. 2008. Polyphyletic origin of toxic Pitohui birds suggests widespread occurrence of toxicity in corvoid birds. Biology Letters 4:71–74.
- Karp, D. S., S. Judson, G. C. Daily, and E. A. Hadly. 2014. Molecular diagnosis of bird‐mediated consumption in tropical farmland. Springerplus 3:630.
- Kim, W., T. Gilet, and J. W. M. Bush. 2011. Optimal concentrations in nectar feeding. Proceedings of the National Academy of Sciences of the United States of America 108:16618–16621.
- Kruuk, H. 1967. Competition for food between vultures in East Africa. Ardea 55:171–192.
- Kushlan, J. A. 1973. Bill‐vibrating: a prey‐attracting behavior of the Snowy Egret, Leucophoyx thula. American Midland Naturalist 89:509–512.
- Langham, G. M. 2004. Specialized avian predators repeatedly attack novel color morphs of Heliconius butterflies. Evolution 58:2783–2787.
- Levey, D. J., R. S. Duncan, and C. F. Levins. 2004. Use of dung as a tool by burrowing owls. Nature 431:39.
- Levey, D. J., J. J. Tewksbury, M. L. Cipollini, and T. A. Carlo. 2006. A field test of the directed deterrence hypothesis in two species of wild chili. Oecologia 150:61–68.
- Lima, S. L. 1985. Maximizing feeding efficiency and minimizing time exposed to predators: a trade‐off in the black‐capped chickadee. Oecologia 66:60–67.
- Marden, J. H. and P. Chai. 1991. Aerial predation and butterfly design: how palatability, mimicry, and the need for evasive flight constrain mass allocation. American Naturalist 138:15–36.
- Marra, P. P. and J. V. Remsen Jr. 1997. Insights into the maintenance of high species diversity in the Neotropics: habitat selection and foraging behavior in understory birds of tropical and temperate forests. Ornithological Monographs 48:445–483.
- Martínez, A. E. and R. T. Zenil. 2012. Foraging guild influences dependence on heterospecific alarm calls in Amazonian bird flocks. Behavioral Ecology 23:544–550.
- Mathot, K. J., D. R. Lund, and R. W. Elner. 2010. Sediment in stomach contents of Western sandpipers and Dunlin provide evidence of biofilm feeding. Waterbirds 33:300–306.
- Meynard, C., M. V. López‐Calleja, F. Bozinovic, and P. Sabat. 1999. Digestive enzymes of a small avian herbivore, the Rufous‐tailed Plantcutter. Condor 101:904–907.
- Mottley, K. and L. A. Giraldeau. 2000. Experimental evidence that group foragers can converge on predicted producer–scrounger equilibria. Animal Behaviour 60:341–350.
- Mumme, R. L. 2002. Scare tactics in a Neotropical warbler: white tail feathers enhance flush–pursuit foraging performance in the Slate‐throated Redstart (Myioborus miniatus). Auk 119:1024–1035.
- Munn, C. A. 1986. Birds that ‘cry wolf.’ Nature 319:143–145.
- Ornes, S. 2013. Foraging flights. Proceedings of the National Academy of Sciences of the United States of America 110:3202–3204.
- Parrott, J. 1980. Frugivory by Great Grey Shrikes Lanius excubitor. Ibis 122:532–533.
- Pietrewicz, A. T. and A. C. Kamil. 1979. Search image formation in the blue jay (Cyanocitta cristata). Science 204:1332–1333.
- Powell, L. L., T. U. Powell, G. V. N. Powell, and D. J. Brightsmith. 2009. Parrots take it with a grain of salt: available sodium content may drive collpa (clay lick) selection in southeastern Peru. Biotropica 41:279–282.
- Powlesland, R. G. 1980. Food‐storing behaviour of the South Island Robin. Mauri Ora 8:11–20.
- Prakash, M., D. Quéré, and J. W. M. Bush. 2008. Surface tension transport of prey by feeding shorebirds: the capillary ratchet. Science 320:931–934.
- Pruett‐Jones, M. A. and S. G. Pruett‐Jones. 1985. Food caching in the tropical frugivore, MacGregor’s Bowerbird (Amblyornis macgregoriae). Auk 102:334–341.
- Qninba, A., A. Benhoussa, M. Radi, A. El Idrissi, H. Bousadik, B. Badaoui, and M. A. El Agbani. 2015. Mode de prédation très particulier du Faucon d’Éléonore Falco eleonorae sur l’Archipel d’Essaouira (Maroc Atlantique). Alauda 83:149–150.
- Ricklefs, R.E. 2001. The Economy of Nature, 5th Edition. W. H. Freeman and Company, New York, NY.
- Ricklefs, R. E. and G. W. Cox. 1977. Morphological similarity and ecological overlap among passerine birds on St. Kitts, British West Indies. Oikos 29:60–66.
- Rico‐Guevara, A. and M. A. Rubega. 2011. The hummingbird tongue is a fluid trap, not a capillary tube. Proceedings of the National Academy of Sciences of the United States of America 108:9356–9360.
- Rosenberg, K. V. 1990. Dead‐leaf foraging specialization in tropical forest birds: measuring resource availability and use. Pages 360–368 in Avian Foraging: Theory, Methodology, and Applications (M. L. Morrison, C. J. Ralph, J. Verner, and J. R. Jehl Jr, Eds.). Studies in Avian Biology 13. Cooper Ornithological Society.
- Rosenberg, K. V. 1993. Diet selection in Antwrens: consequences of substrate specialization. Auk 110:361–375.
- Rubega, M. A. and B. S. Obst. 1993. Surface‐tension feeding in phalaropes: discovery of a novel feeding mechanism. Auk 110:169–178.
- Russell, R. W. 1999. Precipitation scrubbing of aerial plankton: inferences from bird behavior. Oecologia 118:381–387.
- Rutz, C., L. A. Bluff, N. Reed, J. Troscianko, J. Newton, R. Inger, A. Kacelnik, and S. Bearhop. 2010. The ecological significance of tool use in New Caledonian crows. Science 329:1523–1526.
- Sherry, T. W. 1984. Comparative dietary ecology of sympatric, insectivorous Neotropical flycatchers (Tyrannidae). Ecological Monographs 54:313–338.
- Sherry, T. W. and L. A. McDade. 1982. Prey selection and handling in two Neotropical hover‐gleaning birds. Ecology 63:1016–1028.
- Sillett, T. S., A. James, and K. B. Sillett. 1997. Bromeliad foraging specialization and diet selection of Pseudocolaptes lawrencii (Furnariidae). Ornithological Monographs 48:733–742.
- Skelhorn, J., H. M. Rowland, M. P. Speed, and G. D. Ruxton. 2010. Masquerade: camouflage without crypsis. Science 327:51.
- Skutch, A. F. 1976. Parent Birds and their Young. University of Texas Press, Austin, TX.
- Smith, S. M. 1975. Innate recognition of coral snake pattern by a possible avian predator. Science 187:759–760.
- Smith, S. M. 1977. Coral‐snake pattern recognition and stimulus generalization by naïve great kiskadees (Aves: Tyrannidae). Nature 265:535–536.
- Sol, D., L. Lefebvre, and J. D. Rodríquez‐Teijeiro. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proceedings of the Royal Society B: Biological Sciences 272:1433–1441.
- Solheim, R. 1984. Caching behavior, prey choice and surplus killing by Pygmy Owls Glaucidium passerinum during winter, a functional response of a generalist predator. Annales Zoologici Fennici 21:301–308.
- Stendell, R. and L. Waian. 1968. Observations on food caching by an adult female sparrow hawk. Condor 70:187.
- Styring, A. R. and M. Zakaria bin Hussin. 2004. Foraging ecology of woodpeckers in lowland Malaysian rain forests. Journal of Tropical Ecology 20:487–494.
- Sutherland, W. J., B. J. Ens, J. D. Goss‐Custard, and J. B. Hulscher. 1996. Specialization. Pages 57–75 in The Oystercatcher: From Individuals to Populations (J. D. Goss‐Custard, Ed.). Oxford University Press, New York, NY.
- Temeles, E. J., C. R. Koulouris, S. E. Sander, and W. J. Kress. 2009. Effect of flower shape and size on foraging performance and trade‐offs in a tropical hummingbird. Ecology 90:1147–1161.
- Temeles, E. J., K. C. Shaw, A. U. Kudla, and S. E. Sander. 2006. Traplining by purple‐throated carib hummingbirds: behavioral responses to competition and nectar availability. Behavioral Ecology and Sociobiology 61:163–172.
- Tewksbury, J. J., D. J. Levey, M. Huizinga, D. C. Haak, and A. Traveset. 2008. Costs and benefits of capsaicin‐mediated control of gut retention in dispersers of wild chilies. Ecology 89:107–117.
- Tewksbury, J. J. and G. P. Nabhan. 2001. Directed deterrence by capsaicin in chilies. Nature 412:403–404.
- Toland, B. 1984. Unusual predatory and caching behavior of American Kestrels in central Missouri. Raptor Research 18:107–110.
- Vander Wall, S. B. 1990. Food Hoarding in Animals. University of Chicago Press. Chicago, IL.
- Vander Wall, S. B. and R. P. Balda. 1981. Ecology and evolution of food‐storage behavior in conifer‐seed‐caching corvids. Zeitschrift für Tierpsychologie 56:217–242.
- Vaughan, R. 1961. Falco eleonorae. Ibis 103:114–128.
- Viitala, J., E. Korpimäki, P. Palokangas, and M. Koivula. 1995. Attraction of Kestrels to vole scent marks visible in ultraviolet light. Nature 373:425–427.
- Walter, H. 1979. Eleonora's Falcon: Adaptations to Prey and Habitat in a Social Raptor. University of Chicago Press, Chicago, IL.
- Watanabe, Y. Y. and A. Takahashi. 2013. Linking animal‐borne video to accelerometers reveals prey capture variability. Proceedings of the National Academy of Sciences of the United States of America 110:2199–2204.
- Werner, T. K. and T. W. Sherry. 1987. Behavioral feeding specialization in Pinaroloxias inornata, the ‘Darwin's Finch' of Cocos Island, Costa Rica. Proceedings of the National Academy of Sciences of the United States of America 84:5506–5510.
- Wheelwright, N. T. 1985. Fruit size, gape width, and the diets of fruit‐eating birds. Ecology 66:808–818.
- Wiley, A. E., P. H. Ostrom, A. J. Welch, R. C. Fleischer, H. Gandhi, J. R. Southon, T. W. StaffordJr, J. F. Penniman, D. Hu, F. P. Duvall, and H. F. James. 2013. Millennial‐scale isotope records from a wide‐ranging predator show evidence of recent human impact to oceanic food webs. Proceedings of the National Academy of Sciences of the United States of America 110:8972–8977.
- Willis, E. O. 1973. The behavior of Ocellated Antbirds. Smithsonian Contributions to Zoology 144:1–57.
- Wolf, L. L., F. G. Stiles, and F. R. Hainsworth. 1976. Ecological organization of a tropical, highland hummingbird community. Journal of Animal Ecology 45:349–379.
- Wunderle Jr, J. M. 1985. An ecological comparison of the avifaunas of Grenada and Tobago, West Indies. Wilson Bulletin 97:356–365.
- Yanega, G. M. and M. A. Rubega. 2004. Hummingbird jaw bends to aid insect capture. Nature 428:615.
- Ydenberg, R. and M. Guillemette. 1991. Diving and foraging in the Common Eider. Ornis Scandinavica 22:349–352.
- Yosef, R. and T. C. GrubbJr. 1992. Territory size influences nutritional condition in non‐breeding Loggerhead Shrikes (Lanius ludovicianus): a ptilochronology approach. Conservation Biology 6:447–449.
- Yosef, R. and T. C. GrubbJr. 1994. Resource dependence and territory size in Loggerhead Shrikes (Lanius ludovicianus). Auk 111:465–469.
- Zach, R. 1979. Shell dropping: decision‐making and optimal foraging in Northwestern crows. Behaviour 68:106–117.