Chapter 4
Feathers and Plumages
Kimberly Bostwick
Cornell University Museum of Vertebrates

(Photograph by Justin Marshall.)
From the radiant, glowing orange plumage of the Andean Cock‐of‐the‐rock (Rupicola peruvianus) to the earth‐toned and exquisitely filagreed patterns on the wing of a Great Argus (Argusianus argus), bird plumage is among the most striking and lovely of animal clothing. But even the simplest of feathers on the plainest bird have fascinating stories to tell: how individual feathers are made of intricately arranged structures; how feathers evolved from simple beginnings in pre‐avian dinosaurs to their modern forms over millions of years; how variations on the basic feather theme led to hundreds of forms expressed among the world’s nearly 10,000 bird species; how different feather types can serve many purposes simultaneously within a single bird; how birds are capable of producing feathers of every color we recognize, and many colors that we do not; how whole plumages are adapted to camouflage or sexually selected to advertise. Clearly, feathers are central to what it means to be a bird, and they represent a fascinating and captivating component of avian biology.
The first three sections of this chapter focus on “how to build” typical individual feathers from three different perspectives—purely anatomical, developmental, and evolutionary. In particular we examine the details of feather structure, how that structure is developed every time a new feather is grown, and how that structure likely evolved in a series of evolutionary steps in theropod dinosaurs. Taken together, these complementary sections provide an introduction to the finer structural details of feathers.
In the next three sections, we examine the practical aspects of wearing feathers at the level of plumages and individual birds. First we learn how ornithologists classify the diversity of different feathers found within and among bird species, and further, how these different feather types function for different purposes. Then, at the level of entire plumages, we investigate the pragmatic business of molting, looking at how birds replace their feather coats throughout their lifetimes. We end with a consideration of hygiene, exploring aspects of day‐to‐day feather care and maintenance.
Finally, in the last three sections we explore one of the most beguiling aspects of feathers: their visual properties, or appearance. We investigate how birds’ feathers use the physics of light to produce all the colors of the rainbow, and a suite of visual textures no rainbow can produce. We close this discussion of feather appearances with a whole‐animal, plumage‐level tour of how bird species customize their appearances using their feathers and plumages to serve their ecological and social needs.
4.1 Structural basics of feathers
Asked to draw a feather, even young children will illustrate three basic features. First, there is the shaft, the thin but stiff, central, rod‐like portion. Second, the vanes extend in opposite directions from the shaft, forming two broad, flat, and more pliable regions of the feather. Third, on close inspection, the vanes—rather than being smooth and solid like a simple sheet of plastic—have a comb‐like substructure. To round out this general description, a biologist would likely direct attention to a few more details of a typical feather (Fig. 4.01). The shaft can be subdivided into two regions, the more medial (or close to the body) portion of the shaft is vane‐less and defines the area called the calamus. The calamus is relatively short and vane‐free primarily because it is the portion of the feather that perforates the bird’s skin, thereby anchoring the feather to the body. The rest of the shaft is distal (further from the body) relative to the calamus, and has the vanes extending from it. This region is called the rachis. Given that the rachis frequently comprises as much as 90–95% of the length of the shaft, the terms rachis and shaft are often used interchangeably.

Fig. 4.01 Basic features of a feather. Anatomically speaking, a feather has a proximal (near) and distal (far) end relative to its attachment site on the wing and/or body. These terms also apply when describing a feather’s component parts.
(© Cornell Lab of Ornithology.)
When most people envision a typical feather, they tend to think of long wing or tail feathers rather than a small fluffy tuft of down—which is also a feather. This contrast draws attention to another fundamental structural feature of feathers, or, more specifically, of feather vanes. A typical wing or tail feather is pennaceous, a feather that has vanes with a relatively smooth, two‐dimensional (flat) surface, and with a distinct shape or outline. A down feather is plumulaceous, a feather with barbs that are so fluffy as to be relatively formless. Plumulaceous feathers are incapable of holding any but the most delicate rounded form, and their overall shape changes drastically in response to the slightest breeze. In order to understand what underlies this difference in vane form (or lack thereof), one needs to understand in more detail the anatomical underpinnings of the firmer, pennaceous feather vane (Fig. 4.01).
Pennaceous vanes are a flexible, breathable, intricate mesh made of hundreds of tiny interlocking fibers. On close inspection, one can see that these vanes are comprised of a dense row of hundreds of long, slender, but stiff filaments, called barbs, branching off from the rachis one after the other, somewhat like teeth on a comb (Fig. 4.02A). At an even finer scale, the barbs themselves have a set of smaller structures, barely visible to the naked eye. That is, along the barbs the overall feather structure—the larger central shaft with many smaller fibers branching off on opposite sides—repeats itself at a smaller scale: each barb consists of a central shaft called the ramus that has its own rows of slender branches, called barbules, extending from either side. Barbules are generally not symmetrical off the two sides of the ramus, and we refer to the barbules that extend away from the bird’s body (or away from the base of the feather) as the distal barbules, and those that extend toward the body as the proximal barbules. The two sides typically have complementary form and function; the distal barbules often have hook‐shaped structures along their ventral (or under) surfaces, called barbicels or hooklets (Fig. 4.02B). The hooklets grab and hold the dorsal surface (or top) of the proximal barbules of the adjacent barb. Thus, although the barbs are long, thin, and can physically disconnect from one another, they are held parallel to neighboring barbs along their entire length by the hooklets on their distal barbules. In this way, barbules generate and maintain the continuous structure of the vanes—causing the feather to appear like one, smooth, continuous surface.

Fig. 4.02 Feather vane and barbule anatomy. (A) Detail of feather vanes with interacting proximal and distal barbules. (B) Detail showing hooklets interact with the barbules on the adjacent barb. (C) Two plumulaceous barbules (i) of a Rock Pigeon (Columba livia), with three regions from the proximal to distal tip (ii–iv) enlarged below. In (ii), the base and proximal nodes are relatively large compared with the central region of the barbule (iii). Distal barbule tips (iv) have substantially reduced nodes.
(A, illustration by Andrew Leach © Cornell Lab of Ornithology. B and C, adapted from Lucas and Stettenheim 1972.)
The fibers that create plumulaceous vanes are also barbs, but with a different structure. Barbs on plumulaceous feathers have proportionally longer and less stiff rami, and their barbules are typically reduced either to fewer, thinner, simple hairs (no hooklets), or even short nodules off the rami (Fig. 4.02C). In comparison to the densely packed, stiff, and hooked fibers of pennaceous vanes, the lack of barbule structure liberates the long thin rami to move independently from one another, and creates the loose, tufted form of a plumulaceous vane. The different functions of these contrasting vane forms are addressed later in this chapter.
All of these feather structures are made of the same material: keratin. Keratin is a proteinaceous connective tissue (the same basic material of human fingernails) produced inside specialized cells called keratinocytes. Among all the species in the animal kingdom, there are a diverse set of genes that produce keratin, and consequently diverse forms of keratin proteins exist. Within vertebrate animals, keratins belong to one of two fundamentally different structural forms (Fig. 4.03A). The alpha‐keratins are found in all vertebrates (including ourselves), are relatively flexible, and make up much of mammalian skin, hair, and nails. Beta‐keratins are unique to reptiles and birds, tend to be tougher, and are found in scales, claws, and feathers (Fig. 4.03B). The specific beta‐keratin found in feathers is a form unique to living birds. The diversity of beta‐keratins within birds is still largely unexplored and—given the importance of the material properties of keratin for feather function, and of feather function for birds in general—promises to tell some interesting stories about the evolution of feathers and birds (Glenn et al. 2008).

Fig. 4.03 Structure and use of keratin. (A) The different molecular structures of alpha‐ and beta‐keratins create distinctive folding (tertiary) structures and properties. (B) Beta‐keratins are unique to birds and reptiles and are the main structural component of feathers and claws, as in the talons of this Bald Eagle (Haliaeetus leucocephalus).
(Photographs by: B (main), Peter Brannon; B (inset), David LaMason.)
4.2 Feather development
One might imagine that the double‐branched feather structure of a typical feather emerges as the feather grows, in a way analogous to how a tree grows branches: by small tips sending shoots off of a growing branch stock. Instead, feathers have their own surprising way of growing into their hallmark branched form.
4.2.1 Follicles
Feather development is a complex process that begins and ends with feather follicles, the specialized structures on the surface of the skin that generate feathers. If you have seen raw chicken, you have probably noticed feather follicles as the little bumps and/or pores on the skin surface (Fig. 4.04). The key to understanding how a follicle makes a feather is to understand its anatomy.

Fig. 4.04 Feather follicles. Each raised bump on the skin of this plucked chicken is a feather follicle. Occurring in distinct tracts called pterylae that follow the contours of the bird, they are separated by regions of bare skin without follicles.
(Photograph by evp82/Shutterstock.com.)
The skin of a bird (like our skin) consists of two major layers, the inner dermis and the outer epidermis. During development within the egg, the surface of the embryo’s skin is visibly dotted with little bumps called feather buds or papillae. Each papilla is formed when a small swelling of dermis, the dermal core, pushes into its overlying thickened epidermal skin (called the epidermal placode) (Fig. 4.05A). As development progresses in the embryo, each papilla transforms into a feather follicle when the epidermal cells at the base of the papilla start growing back into the dermis. The growth of this layer of skin at the perimeter of the papilla is so rapid that the epidermis doubles back on itself, invaginating into the dermis and thereby forming a double wall inside the skin (the internal wall surrounds the dermal core, the outer wall connects to the surface of the skin) (Fig. 4.05B). Thus, a more formal definition of a feather follicle is a double‐walled column of epidermis with a dermal core from which feathers are developed.

Fig. 4.05 Feather growth from dermal papilla. (A) The early bud of a new feather, rising from both the epidermal placode (thickened epidermal skin not shown) and dermal skin layers, forms the papilla. (B) Epidermal cells at the base of the papilla grow rapidly. These cells eventually double‐back into the dermal layer, forming a double layer of invaginated epidermal skin—the feather follicle. (C) A cross‐section of the growing feather within the follicle reveals the arrangement of epidermal skin, creating the epidermal collar and its dermal core.
(Adapted from Prum 1999. Reproduced with permission from John Wiley and Sons.)
Once developed in the embryo, follicles remain in the skin for the rest of the bird’s life. Complex cell growth occurs within the follicle each and every time a feather develops. The cells of the follicle’s inner epidermal layer are the locus of this growth, and they are referred to as the epidermal collar (Fig. 4.05C). Within the epidermal collar, keratinocytes multiply rapidly and transform themselves into cells full of the keratin that becomes the feather. When the development of an individual feather is completed, the cells cease to multiply within the follicle, and it goes dormant. However, the follicle, with its dermal core and epidermal collar, still exists, ready to restart the process when it is time to produce a new feather. New keratinocytes, through their rapid new growth, will then simply push their progenitors upward and outward from the collar, pushing out the old feather and replacing it with the next one. The same follicle can thereby produce several different feather types over a bird’s lifetime.
Two aspects of follicle anatomy make the development of a feather a bit counterintuitive. The first is the fact that feather follicles are tubular structures. How does tissue extruded from a circular growing surface become a “flat” feather? This transformation is described in greater detail later in this section, but as a primer, imagine the growing feather within the follicle being pushed out from the skin as a hollow column of tissue shaped like a straw (Fig. 4.06A). Splitting open the straw along one side and uncurling it (Fig. 4.06B) then produces a long, flattened, but concave structure (Fig. 4.06C). Roughly speaking, feathers grow curled with the outside edges of their vanes touching. This sort of unfurling is the basis for how a flat feather emerges from its initial columnar shape.

Fig. 4.06 Stages of an unfurling feather. (A) As it emerges from the dermal papilla, a feather resembles a straw with a slit in one side (black dashed line). The internal and external surfaces have different curvatures. (B) As it unfurls, the tube interior forms the ventral (underside) surface of the feather. (C) When a feather has unfurled completely, its ventral surface is concave and its dorsal surface is convex.
(© Cornell Lab of Ornithology.)
The next challenge is to figure out how the follicle, which remains fixed on the surface of the skin, can grow an intricately branched structure backwards, from its outer tips to its base. That is, rather than sprouting out barbs from the growing rachis as the rachis grows out (and barbules from the barbs as barbs grow out), feathers develop and emerge from their distal tips first. The branching structure is produced when the finest growing fibers migrate or shift inside the cylindrical follicle with time, to fuse together into larger fibers (like small streams merging into larger rivers) as they grow. Thus, feathers are not so much intricately branched structures like trees, but instead are intricately fused networks of fibers in which the smallest fibers first originate, then merge together, and finally emerge from inside the feather follicle (Fig. 4.07).

Fig. 4.07 Pennaceous feather growth. Growth begins inside the epidermal placode (A) with the initiation of feather barb ridges (B). Inside the feather sheath, the dorsal barb ridges continue to grow down (C, D), eventually meeting the epidermal collar around the placode base. The ventral base of the growing feather forms another barb ridge (E, F), with helical growth leading to the formation of a central rachis ridge of the still sheathed feather (G, H, I). The sheath then deteriorates (J), permitting the barbs of the pennaceous feather to fully unfurl (K, L).
(Courtesy of Matthew Harris, John Fallon, and Richard Prum.)
With these two generalizations in mind—a feather is grown like a thin‐walled straw that becomes unfurled along its long axis, and its branched structure is created by intricate fusing of fine fibers—let us examine the developmental biology of a typical pennaceous feather in more detail.
4.2.2 Generating the branched feather form
The epidermal collar is where everything happens to generate the branching structure of the feather. The geometric organization of the growing and fusing cells within the collar is what creates the classic feather shape of a central shaft (the rachis) with symmetrical side branches (the barbs). Because the position of the keratinocytes within the epidermal collar determines what part of the final feather the cells will become, it is important to understand the relationship between the position of cell growth within the follicle and the position of finished structures on the mature feather.
Just as the whole bird itself has a back, a belly, a head and a tail, the epidermal collar also has an orientation, or sides. Most simply, when unfurled, cells on the inside of the collar (the inside surface of the straw) become the feather’s ventral (or bottom) surface, and those on the outside of the collar (the outside surface of the straw) become the feather’s dorsal (or upper) surface (Fig. 4.08).

Fig. 4.08 Dorsal and ventral surfaces. These Scarlet Macaws (Ara macao) expose both sides of their primary feathers during flight. The dorsal side (top) of each primary is blue; the ventral side (bottom) is red.
(Photograph by Stan Chapman.)
Another basic axis of a feather is proximal/distal (or close to the bird’s body versus further away). As a feather grows, newer cells are generated under the older ones, such that the new growth forces the older growth outward, away from the body (much as our nails grow). Thus, the first cells grown correspond to the distal tip of the feather, and the last cells, the proximal base of the calamus (Fig. 4.01).
The epidermal collar has other visibly differentiated regions that correspond to regions of the mature feather. One area is a distinct swelling or ridge of keratinocytes called the rachis ridge (Fig. 4.09A). As you might expect, cells grown within the rachis ridge produce the rachis. The tissue forming the remainder of the collar is composed of barb ridges: strips of active keratinocytes, packed together in bundles (one bundle of keratinocytes per barb ridge) (Fig. 4.09B) and growing around in a semicircular route toward the rachis ridge. As the barb ridges grow they become the elongate, filamentous barbs that eventually converge upon and fuse with the continuously growing rachis (Fig. 4.10). New barb ridges continually form in the epidermal collar opposite the rachis ridge, and each barb ridge becomes exactly one barb.

Fig. 4.09 Epidermal collar and barb ridge anatomy. (A) Cross‐section of the epidermal collar and the rachis ridge, where the keratin generated by keratinocytes produce the rachis of a feather. (B) An enlargement of the barb ridges, which grow toward the rachis ridge.
(Adapted from Yu et al. 2002. Reproduced with permission from Macmillan Publishers Ltd.)
When the epidermal collar is viewed in cross‐section, it looks like a ring, with blocks of active tissue—the barb ridges—beading the perimeter, and with the rachis ridge forming an enlarged portion on one side of the ring (Fig. 4.09A). On a finer scale, the individual barbs are formed by a process similar to that occurring within the epidermal collar as a whole, with cell migration during growth leading to slender barbules that ultimately fuse with the rami of the barb (Fig. 4.09B).
In summary, although the feather‐growing structure, the follicle, is a hollow cylinder of tissue, the keratin‐growing cells are distributed in strips within that cylinder. These cells migrate and fuse to one another in very specific ways, creating, when it unfurls, the feather’s doubly branched, relatively two‐dimensional structure. One result of this constantly extruded ring of growth is that all fibers at a particular point along the mature feather—whether those of the rachis, the barbs, or the barbules—originated at the same time (Fig. 4.10), and therefore represent a single moment of time during growth. For this reason, the different feather structures do not originate as a simple series. Instead, the distal tip of the rachis originates at the moment of initiation of each feather’s development, while barbs originate over and over again, often in pairs, starting at their tips opposite the rachis, and growing back to merge with the rachis as the rachis ridge grows. Often dozens of barbs will be growing at the same time, each at different points along their length, and each sequentially joining to the rachis. Barbs continually originate as long as the vane is growing. In a process similar to barb growth, barbules originate at their tips in hundreds of pairs as each barb grows, and this process continues until the last barbule on a barb fuses with the rami near its attachment to the rachis.

Fig. 4.10 Progression of feather growth from the epidermal collar. Counterintuitively, distal feather barbs (feather tip) grow out from the epidermal collar before they merge with the central rachis ridge later on during the growth process.
(Adapted from Lucas and Stettenheim 1972.)
As mentioned above, often the hardest part of this process is to visualize how the branching structure of the feather can emerge from a ring of tissue perpetually growing at its base. Yet consider the alternatives. If the feather follicle functioned like a simple pasta maker, squeezing out a hollow tube of keratin, the epidermal collar would simply produce keratinous macaroni or straws. Alternatively, if a feather was to branch into finer subdivisions as it grew from its base outward to its tips, like a plant, vessels and nutrients would be needed to support the tissues growing ever further out away from the body. Given the huge number of feathers on the bird, this would be a difficult undertaking. However, all the growth is in fact generated from one ring of multiplying tissue sitting near the surface of the skin. Thus, the only way for the branching shape to develop is for the many distal tips to grow inward and fuse into larger structures: barbules into barbs, and barbs into the rachi.
The dermal core—the structure that originally precipitated the development of the feather follicle—occupies the space inside the epidermal collar for the duration of feather development. While a feather is developing, blood vessels extend into the base of the growing feather, providing nourishment for the growing cells. As cells multiply, and they are pushed further and further from the epidermal collar, they stop receiving nourishment from the dermal core, and ultimately they dehydrate and die, while younger cells are still growing at the feather’s base. When the feather is fully formed, the blood supply to the entire feather is cut off as the vessels are reabsorbed into the papilla through a hole at the very end of the calamus. On a large feather this hole often can be seen without magnification.
When viewing a living bird, growing feathers can be recognized by the presence of oddly thick, plastic‐looking rods where feathers would otherwise be. These distinctive growing feathers are called pin feathers—newly grown feathers still enclosed within a bluish or grayish waxy covering called a sheath (Fig. 4.11). Developing independently from the rest of the feather, the sheath is produced by the outer layer of the epidermal collar as the feather grows. Functionally, the sheath protects the nascent feather as it grows and then dries and dies. It also holds the feather in its curled, cylindrical form while it continues growing at its base. As the feather matures, and its cells dehydrate and die, the sheath also dries, splits, and falls away or is preened away by the bird.

Fig. 4.11 Pin feathers. This Brown Thrasher (Toxostoma rufum) nestling has conspicuous pin feathers emerging from their waxy, bluish sheaths as it molts from its natal down to its juvenal plumage. Eventually, these pin feather sheaths will either break off or be removed by the bird during preening.
(Photograph by Stephanie Sanchez.)
Although the general process of feather development described here proceeds by tightly programmed and regulated processes, external environmental factors can influence the development of feather structure. For example, brief nutritional deficiencies or short‐term stresses can lead to a sudden reduction of the cross‐sectional area of the barb and barbule fibers, creating visible lines on the mature feather known as a fault bars (Murphy et al. 1989) (Fig. 4.12).

Fig. 4.12 Fault bars in feathers. When a bird experiences high levels of stress (such as severe food limitation) during early development or before an adult molt, fault bars—caused by a temporary reduction in fiber growth—may appear in its new feathers. Often these fault bars are most apparent in the rectrices (tail feathers).
(Photograph by Nicholas Bayly.)
4.3 Evolution of feathers
Among living organisms, only birds have feathers. As we have begun to see, feathers (and their development) are so intricate and complex, they raise questions about how they evolved in the ancestors of modern birds.
One modern hypothesis of the evolutionary steps that led to modern feather structure is summarized in Box 4.01. Feathers were long thought to be essentially a frizzy or frayed version of reptilian scales. However, evidence challenging this hypothesis comes from how feathers grow: rather than developing as plate‐like structures like reptilian scales, feathers are produced by feather follicles, which, as we have seen, are miniature tubular developmental organs distributed over the skin. Although feathers have some features in common with the reptilian scale (like being distributed regularly across the skin, grown from the base, and made of beta‐keratin), the columnar nature of the feather follicle itself is a unique feature. Thus, developmentally, from the creation of the follicle forward, all of the details of feather growth are also novel, with no direct parallels in other animals. In fact, the unique arrangement of the follicle means that a feather can be defined simply as any structure produced by a feather follicle.
This definition may seem trivial given that, at this point in history, all birds and only birds have feathers, but it becomes important when looking back through the fossil record to the origins of feathers in the ancestors of birds (Box 4.01). Over the past several decades, paleontologists working on exceptionally well‐preserved fossils from Liaoning Province in China have filled in many of the details about how various anatomical features of birds, including feathers, evolved in the theropod dinosaurs (Chapter 2). Numerous non‐avian theropod fossils have been found with clear imprints of so‐called “integumentary appendages” surrounding the body—skin‐covering structures like scales, feathers, and hair, presumably grown from the skin itself—forming pigmented halos around fossilized limbs, torsos, heads, necks, and tails (Fig. 4.13). When it was verified that these appendages were composed of beta‐keratin, which is known to occur only in bird feathers and reptile scales, researchers became more comfortable referring to these thin, elongate structures as feathers (Schweitzer 2001).

Fig. 4.13 Proto‐feathers from dinosaur fossils. Integument structures surrounding the body of this small theropod dinosaur from Liaoning Province, China are feather‐like in form and (likely) function. The close‐ups of the six labeled regions on the fossil reveal the filamentous morphology at different areas of the body.
(From Ji et al. 2001. Reproduced with permission from Macmillan Publishers Ltd.)
Together, these fossils contribute to a coherent picture of feather evolution occurring in various steps through many millions of years. The greatest surprise has been how early the hypothesized evolutionary/developmental stages occurred relative to the origin of birds. Until recently, few researchers questioned the idea that feathers are, and have always been, limited to birds. In fact, feathers had long been considered a defining characteristic of birds, as hair is to mammals. But now, relatively simple filamentous feathers—those without the secondary and tertiary structure of barbs and barbules—have been found on some of the earliest fossil theropod dinosaurs that lived long before the first birds evolved. These fossils suggest that many theropod dinosaurs had primitive feathers covering their skin (Fig. 4.14). Similar structures have also been found in even more distantly related dinosaur groups, introducing the possibility that proto‐feathers may have arisen very early in dinosaur evolution and been worn by many diverse dinosaur species.
In addition to the surprisingly ancient origin of primitive feathers, feathers that appear completely modern in form also pre‐date the origin of birds. For example, Mark Norell et al. (2002) published evidence of a modern pennaceous feather from a group of theropod dinosaurs (Fig. 4.15). Other dinosaurs have been found exhibiting a diversity of feather forms all over their bodies, resembling the diversity of feather forms found across the body of a single individual bird today, with distinctive down, body, and wing and/or tail feathers. One particularly exciting fossil discovery was that of a “four‐winged” dinosaur, Microraptor gui (Xu et al. 2003), in which elongated feathers appearing just like modern flight feathers are visible along the hindleg (Chapter 2).

Fig. 4.14 Filamentous dinosaur feathers. (A) This early Beipiaosaurus species fossil provides evidence that filamentous feathers (red arrows) existed on dinosaurs long before the first birds evolved. (B) Close‐up of the filamentous feathers near the spine of the specimen, drawn in monochrome on the right to clearly depict their slender, elongated form.
(From Xu et al. 2009. Reproduced with permission from National Academy of Sciences, USA.)

Fig. 4.15 Fossil with modern feather morphology. (A) The earliest known form of the modern, pennaceous feather comes from this small dromaeosaur skeleton. (B) A magnified view of the boxed area in A) reveals individual feather structures, including rachi and barbs.
(From Norell et al. 2002. Reproduced with permission from Macmillan Publishers Ltd.)
More evidence of modern‐looking feathers in pre‐avian dinosaurs includes a stunning set of publications relating the visual appearance of pre‐birds and their feathers to those of modern birds. Researchers have found fossil evidence that, as in modern birds, ancient feathers had brown and black pigments inside them, including arrangements of these pigments that could indicate the presence of iridescent coloration (Vinther et al. 2008, 2010; Zhang et al. 2010). These discoveries have been used to reconstruct the plumage patterns of a handful of feathered dinosaurs. For example, one study reconstructed a striking coloration pattern that might have existed on the feathered dinosaur Anchiornis huxleyi (Li et al. 2010) (Fig. 4.16).

Fig. 4.16 Feathered dinosaur and its probable plumage coloration. (A) Jurassic troodontid (Anchiornis huxleyi) with the left forelimb in ventral view (inset) and the right in dorsal view. Selected samples (red dots on the fossil wing) show the location of melanosomes (B, C) used to determine the probable coloration of the dinosaur (D).
(A–C, from Li et al. 2010. Reproduced with permission from AAAS and Jacob Vinther. D, illustration by Michael DiGiorgio.)
The original function of feathers, and their role in the origin of flight, has been a contentious issue for more than a century. These recent discoveries of feathers on non‐avian dinosaurs have overturned many of the older arguments about why feathers evolved in the first place. The very ancient origin of feathers, in what appear to be very simple, unbranched forms, argues against feathers originating for aerodynamic functions, as a pennaceous vane is required for feathers to aid in flight. Instead, the variety of feather forms seen across many dinosaur lineages indicates that feathers did not originally evolve for flight at all; instead, they seem to have diversified rapidly from whatever their original function was to become adapted to many functions. That is, as in modern birds, feathers likely performed a variety of functions ranging from protective covering to social advertising or camouflage, with their aerodynamic or flight functions only evolving later, closer in time to the evolutionary radiation of birds themselves (Chapter 2).
4.4 Types of feathers: distribution, forms, and functions
Feather follicles are the sites of feather growth and development. Like hair follicles and their hairs, mature feathers remain attached to the body at the site of the follicle, but unlike hairs, feathers are distributed on the surface of a bird in more widely spaced but tightly regulated patterns. This can be seen on the skin of a whole fresh chicken (Fig. 4.04), turkey, or duck, where the follicles appear as small, distinct, and regularly—almost geometrically—spaced pores. In most birds, the feathers are not distributed uniformly over the body but are grouped into feather tracts, more formally called pterylae, which are defined groupings of feathers. Separating these tracts are regions of bare (unfeathered) skin called apteria (Fig. 4.17). Feathers from the pterylae easily extend over the spaces created by the apteria, and the downy bases of feathers tend to fluff out so that these bare patches are rarely visible on a living bird.

Fig. 4.17 Regions of feather growth. On young or plucked birds, the feather follicles are clearly arranged in feather tracts, or pterylae. Featherless areas are called apteria.
(Adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology.)
The detailed arrangement of feather tracts and bare patches across the body, collectively called the pterylosis, varies among groups of birds, and some birds have unique patterns of pterylosis. For instance, birds in the Corvidae family—which includes ravens, crows, and jays—have a characteristic apterium on their back, along the midline of their spine. On the other hand, a few kinds of birds, especially waterbirds such as penguins, have a continuous pterylosis with no apteria, presumably to help prevent water from penetrating to the birds’ skin and chilling it. Historically, such distinctive patterns provided important clues for identifying some of the major evolutionary groupings of birds (Chapter 2).
Feathers often fulfill different roles on different parts of a bird’s body, and thus the feathers populating the various pterylae come in a great variety of shapes and sizes (Fig. 14.18A). A typical bird might have a fluffy down undercoat for insulation; feathers covering the torso that act as sunscreen, a wind breaker, or a raincoat depending on the weather; vaneless bristles around the mouth for feeding functions; strong pennaceous wing and tail feathers used for flight; and ornamental plumes (that can be located in any number of places) for signaling to potential mates or rivals (Fig. 4.18B).

Fig. 4.18 Types of feathers. (A) Examples of six types of feathers: pennaceous feathers of the wing and tail, semiplume, contour, down, filoplume, and bristle. (B) Feathers used for display: during mate attraction displays, the male King‐of‐Saxony Bird‐of‐Paradise (Pteridophora alberti) thrusts its extraordinary head plumes forward while producing an otherworldly twittering sound.
(A, illustration by Andrew Leach © Cornell Lab of Ornithology. B, photograph by Pete Morris.)
From an engineering standpoint, the various feather types interact beautifully with the two basic physical environments they encounter: fluid media (in the form of air and water) and electromagnetic radiation (in the form of heat and light). By varying the basic feather structures of the rachis, barb, and barbules, birds are able to vary the aerodynamic, hydrodynamic, insulative, repellant, acoustic, and visual properties of their feathers. The resulting diversity of forms underlies the wealth of feather functions. Feather diversity exists within an individual bird at a given time and in a given context, but also throughout an individual’s life, and certainly among species.
Despite the great variety of feather forms and function, they tend to group naturally into six major feather types, each tending to be distributed in specific ways on the body, and each specializing somewhat for different functions. However, be aware that these groupings, despite helping to categorize many important biological properties of feather diversity, are just a useful way of organizing feather diversity, and that the world of feathers is rich with intermediates and exceptions to these general groupings.
4.4.1 Down: insulation
Down feathers are entirely plumulaceous, defined by having a rachis that is shorter than the longest barbs, or with the rachis missing entirely. Structurally, they are relatively simple: their barbs emerge more or less together as a tuft near the base of the feather, where they float free of one another with little or no rachis (Fig. 4.19). As a result, down feathers are fluffy and soft.

Fig. 4.19 Down feather anatomy. Down is an excellent insulator, as its free‐floating barbs trap large quantities of air. (A) A typical down feather; note that the barbs extend substantially farther than the rachis. (B) Detail of down feather structure.
(A, from Lucas and Stettenheim 1972. B, illustration by Andrew Leach © Cornell Lab of Ornithology.)
As warm‐blooded creatures, birds must be able to manage their internal temperatures by thermoregulation: regulating heat loss and heat gain (Chapter 7). Down feathers are excellent insulators, in some ways superior to insulators used in human clothing such as wool and polyester. Down’s role as a lightweight but high‐quality insulator is especially important for small birds living in cold climates, as well as for birds that float on, dive into, or swim in water (Fig. 4.20).

Fig. 4.20 Warm down provides insulation. Like many seabirds, this Chatham Albatross (Thalassarche eremita) chick is well insulated by down feathers. This species incubates its young on a cold, windy, oceanic island where effective thermoregulation is essential.
(Photograph by Lorna Deppe.)
Recent research has attributed the superior insulating abilities of down to its extremely fine fiber structure (Wan et al. 2009): the barbs and barbules form tiny, loose tangles that trap air and its heat. The relatively formless structure of downy feathers is one of the factors that make them great insulators. At the tiny scale of feather barbs and barbules, however, air behaves viscously, so air moving among the tangled barbs can be imagined as a thick substance, such as oil or honey, moving through a fine sieve. Down feathers, rather than relying on a closed barrier to reduce airflow, literally physically trap and tangle warm air in their fine fibers.
Birds have three distinct types of down feathers: natal downs, body downs, and powder downs. Natal downs generally cover the whole bird’s body, are temporary (present only around the time of hatching), and arise from the same feather follicles that later produce contour feathers. In fact, the continuity of the feather follicle (and its epidermal collar) between the growth of subsequent feathers can be seen in some young birds whose natal downs remain attached to the tips of the subsequently growing contour feathers. This weak attachment between the natal down and subsequent contour feather results in the fluffy, unkempt appearance of some juvenile birds like thrushes, robins, and bluebirds (Fig. 4.11).
Precocial and altricial birds typically have distinct strategies of thermoregulation and therefore may have down for more (in the case of precocial birds) or less (in the case of altricial birds) time. Most precocial birds leave the nest shortly after hatching (Chapter 11) and must maintain their own body temperature in the form of insulating natal downs. For instance, newly hatched ducklings or chickens are known for their soft, fluffy look (Fig. 4.21A). In contrast, many altricial birds are much more helpless after they hatch and depend on warmth from a brooding parent bird. While down protects precocial nestlings by acting as an insulator between the nestling’s body temperature and the environment, so too can down prevent desirable heat transfer between the nestling and its parent, and in these cases, too much natal down would prevent heat transfer from the parent’s body to the young. Altricial nestlings therefore often have only sparse natal downs, allowing more efficient heat transfer to the nestling from the warm, naked skin on the belly of their brooding parent (Fig. 4.21B). In some cases, for more slowly developing young that cannot fly for weeks after hatching but who nonetheless spend hours alone in the nest, chicks may have an insulating cover of downy feathers that lasts for much of their flightless period. Young hawks and owls, for example, can have a prolonged downy cover (Fig. 4.21C).

Fig. 4.21 Downy plumage in precocial versus altricial nestlings. (A) Since precocial chicks must immediately maintain their own body temperature, they hatch with a thick layer of down and advanced motor skills. (B) Altricial young hatch completely helpless, with little insulating down (arrow). They rely on their parent(s) to provide warmth during the first stages of their lives. (C) Nestlings in an intermediate category—which includes many slow‐growing species (such as hawks, eagles, and owls)—hatch with little mobility but substantial down, as they must sit exposed for hours awaiting their hard‐to‐capture meals.
(Photographs by: A, Jennifer Hall; B, Lindell Dillon; C, Al Cecere © American Eagle Foundation. All rights reserved. Used by permission.)
Body downs are distinct from natal downs in that the body downs of adult birds arise from follicles that produce only down feathers. These adult down feathers are often distributed throughout the bird’s plumage, and they are most abundant in waterbirds such as penguins, loons, petrels, auks, geese, and ducks (all of whom undoubtedly benefit from the extra thermal insulation). Many birds, such as female waterfowl, pluck body downs from their bellies to line their nests. Of these, the most famous is the Common Eider (Somateria mollissima) (Fig. 4.22). Clothing and bedding filled with eider down is notoriously expensive because the exceptionally soft down must be gathered by hand and each nest typically provides only about 15 grams of down.

Fig. 4.22 Down as nest material. Many waterfowl line their nests with down—often plucked directly from the mother’s breast—to insulate and protect their eggs, as a Common Eider (Somateria mollissima) has done here.
(Photograph by Sergei Korsun.)
Unlike the other two types of downs (and all other feather types, in fact), powder downs are distinctive in that they are never molted, but rather grow continually, disintegrating at their tips to produce a fine keratinous substance that resembles talcum powder in texture. The powder is abundant enough to permeate the bird’s entire plumage, likely helping to make it waterproof. The appearance of powder downs can vary; they look like fluffy contour feathers in some species, or like tiny body downs in others. Powder down occurs only in certain groups of birds, such as herons and pigeons, and these downs may be scattered throughout the plumage or clustered in patches (Fig. 4.23).

Fig. 4.23 Powder down patch. In a few groups of birds, the tips of powder down feathers, shown here on a Snowy Egret (Egretta thula), disintegrate to produce a keratinous water repellant that resembles talcum powder.
(Photograph by Jennifer Miller.)
4.4.2 Contour feathers: feathers for all weather
Named contour feathers because they give a bird its characteristic shape or outline, these feathers cover most of the surface of an adult bird. Contour feathers are defined by having a rachis longer then the longest barb, and at least a portion of the rachis consisting of a pennaceous vane (Fig. 4.24). Frequently there is a plumulaceous portion of the vane at the base of contour feathers. Contour feathers have many roles, often serving a wide variety of functions simultaneously or in different contexts.

Fig. 4.24 Contour feathers. By definition, the rachis of a contour feather must be longer than the longest barb, and at least one portion must be pennaceous. Contour feathers create the characteristic silhouette of a bird.
(Adapted from Lucas and Stettenheim 1972.)
Positioned one atop another but offset like shingles on a roof, the pennaceous portions of contour feather vanes form a surprisingly effective barrier. The plumage can aid in thermoregulation by absorbing or reflecting heat‐generating sunlight and in deflecting wind and/or trapping warm air. In terms of aerodynamics, contour feathers streamline the body during flight (Chapter 5). As demonstrated by the particularly smooth contours of swimming birds such as auks and penguins, the streamlining function is even more critical in water, where drag is much greater than in air.
The ability of feathers to repel water is another important property observed best in contour feathers, one that varies among bird species according to their feather morphology. The same contour feathers that perfect the contours of a diving duck also repel water beautifully, as illustrated by the idiomatic saying “like water off a duck’s back.” Oils from the uropygial gland at the base of the tail have been shown to improve water repellency, but the shape and spacing of the barbs and barbules of a feather are actually more important in conferring its water‐repellent properties. This is because a fundamental physical property of water is that while it will tend to cling to most solid materials, like keratin, it cannot adhere to air spaces in materials (even very small spaces). So, when spanning air spaces in a material, water will form droplets (Fig. 4.25). Thus, if the barbs and barbules on a feather are spaced exactly right, water is forced to bead up and roll away. Alternatively, with barbs and barbules spaced differently (more closely together), water can be pulled in to the feather, saturating or permeating it. Some downs (but not all) therefore tend to be vulnerable to saturation. A few diving birds such as cormorants and anhingas have feathers that are relatively easy to wet (Fig. 4.26). The more water‐absorbent nature of cormorant feathers allows them to dive underwater without carrying along bubbles of air trapped in the feathers, whose buoyancy would work against the diving efforts of the birds (Grémillet et al. 2005).

Fig. 4.25 Water‐repellent feathers. (A) Water beads on the surface of most feathers due to air spaces between the barbs and barbules. (B) When waterfowl such as this Wood Duck (Aix sponsa) resurface from a dive, water pools and slides off their feathers with ease. (C) The uropygial gland (the small yellowish bump at the base of the tail) excretes oils that birds use to maintain and waterproof their feathers. Here a White‐winged Crossbill (Loxia leucoptera) accesses its uropygial oils with its beak, which it will then use to spread the oils over its plumage.
(Photographs by: A, Christine Hall; B, David Nelson; C, Darroch Whitaker, https://en.wikipedia.org/wiki/File:White‐winged_Crossbill_Uropygial.JPG. CC‐BY‐SA 3.0.)

Fig. 4.26 Water‐absorbent feathers. Some diving birds have tighter barb and barbule configurations that facilitate water absorption. Birds with absorbent feathers are faster swimmers, but must dry off before flight. Species such as the Anhinga (Anhinga anhinga) do so by spreading their wings while sunning.
(Photograph by Nathan Corry.)
The plumulaceous portion of the vane at the base of many contour feathers is usually protected from the elements by the pennaceous portion, and tends to contribute an insulating function to contour feathers. Some contour feathers have an afterfeather (also sometimes called an aftershaft), a minor feather that sprouts from the same follicle, but is (usually) smaller than the main feather, and (usually) completely plumulaceous (Fig. 4.27A). Developmentally, afterfeathers are formed inside a feather follicle by sectioning off a portion of the epidermal collar, and creating a second rachis ridge, toward which a subset of the barb ridges then grow. Afterfeathers provide extra insulation, and are especially well developed in grouse (most of which live in seasonally cold or arctic regions). The flightless Emu (Dromaius novaehollandiae) of Australia is unique in having afterfeathers that are as large as the main contour feathers (Fig. 4.27B).

Fig. 4.27 Plumulaceous afterfeathers. (A) A typical contour feather with a plumulaceous afterfeather connected on the right. Afterfeathers, which develop from the same follicle, may serve to protect the main feather. (B) Two double‐shafted plumulaceous feathers of an Emu (Dromaius novaehollandiae), the only species that develops an afterfeather as long as the main contour.
(A, adapted from Lucas and Stettenheim 1972; B, photograph by Lois Hellemond, Mt. Sicker Family Farm.)
Contour feathers, although relatively long, flat, and broad, are attached to the body at a single small point. It would seem that, like leaves in the wind, such feathers would be prone to becoming disheveled or disorganized relative to one another. In order to maintain their neat organization, each contour feather is attached to a set of tiny, specialized muscles. Located beneath the surface of the skin in a perimeter around the follicle, tension in these muscles keeps the feather positioned correctly. These muscles also allow a bird to move its feathers voluntarily. In some cases, muscle sheets immediately beneath the skin act together with the individual feather muscles to coordinate movement of the feathers along an entire feather tract. Most feathers are incapable of being moved individually, and the movement of most feathers is limited to raising and lowering the feathers, alternately fluffing and sleeking them.
The ability to move contour feathers is especially important for signaling in social contexts. The signaling that birds do with their feather movements can be conspicuous and extreme, as in crest movements of Australian cockatoos, or relatively subtle as can be witnessed during interactions among songbirds at birdfeeders worldwide.
The length, coloration, and/or barb and barbule morphology of contour feathers of some bird species are modified to alter their appearance. In such cases, these feathers often act as ornaments, morphological structures whose appearance is modified for the purpose of social signaling and/or display. The curly body feathers of the Curl‐crested Manucode (Manucodia comrii) of New Guinea, the erectile crest feathers of the Palm Cockatoo (Probosciger aterrimus) of Australia (Fig. 4.28A), the stiff, straw‐like crown feathers of the Black Crowned‐Crane (Balearica pavonina) of Africa (Fig. 4.28B), and the long elegant plumes of many herons and egrets worldwide are just a few examples. Male birds‐of‐paradise have made the most of their contour feathers, using them for display as head wires, breast shields, and silhouette‐hiding capes.
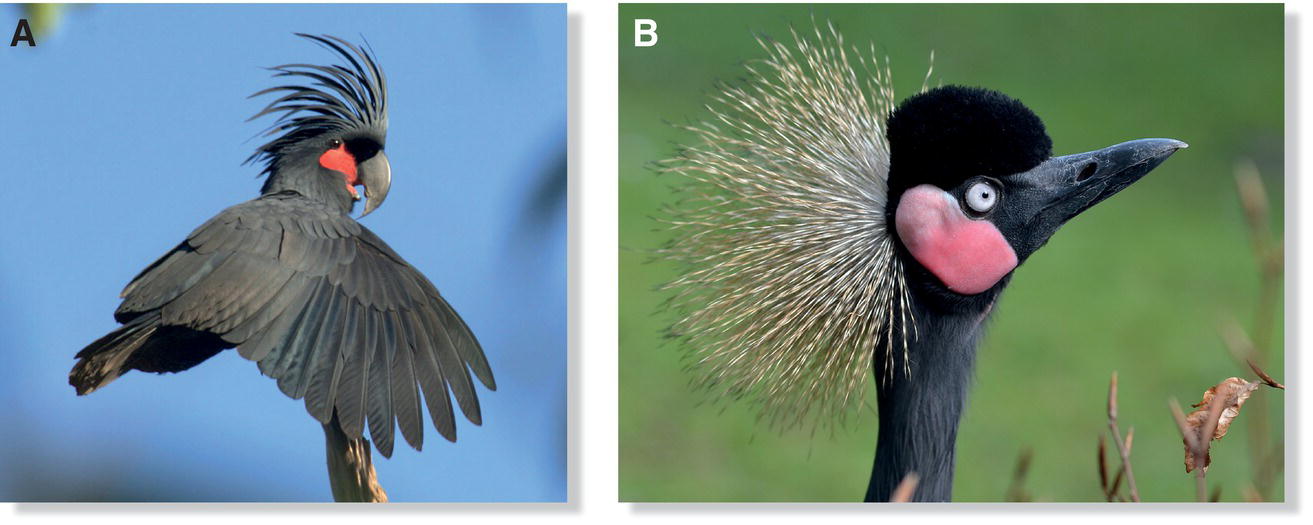
Fig. 4.28 Modified contour feathers. In some species, special contour feathers serve as signaling ornaments, such as (A) the crest feathers of a Palm Cockatoo (Probosciger aterrimus), or (B) the stiffened, golden crown feathers of a Black Crowned‐Crane (Balearica pavonina).
(Photographs by: A, Christina N. Zdenek; B, Olaf Oliviero Riemer, https://commons.wikimedia.org/wiki/File:Balearica_pavonina_(Schwarzer_Kronenkranich_‐_Black_Crowned_Crane)_‐_Weltvogelpark_Walsrode_2012‐0‐120426_0206.jpg. CC‐BY‐SA 3.0.)
4.4.3 Flight feathers: lift and control in air
The flight feathers are an easily defined and identified group, with their own specific locations, structural modifications, and functions. Flight feathers form the majority of the wing and tail surfaces of birds; typically they are the stiffest, largest feathers on a bird, and they are almost completely pennaceous. The width of the two sides of the vanes—lateral and medial, or anterior and posterior, respectively—are often asymmetrical, as the leading edge (or anterior edge, the edge that cuts into air) is typically thinner than the trailing edge (or posterior edge) (Fig. 4.29). This is an adaptation that stabilizes the feather under the pressures of air currents during flight.

Fig. 4.29 Flight feather terminology. As this Crested Caracara (Caracara cheriway) glides down to land, the flight feathers on its wings (remiges) and tail (retrices) are fully extended. Notice that anterior (leading) edges (shaded yellow) face the direction of flight, while the broader posterior edges follow behind (shaded blue).
(Photograph by Alfred Yan.)
There are two sets of flight feathers, those that originate from the wing, called remiges (singular: remige), and those that form the tail, called rectrices (singular: rectrix). Unlike most other feathers, which attach only to the skin, remiges are attached firmly to the bones of the wing, either directly or indirectly via ligaments, and to each other by ligaments connecting their calami. The rectrices form the fan of the tail, and also are connected to one another near their bases by ligaments. The most central pair of retrices attaches directly to the tail bone (pygostyle).
The number of rectrices ranges from 6 to 32 among flying birds, with the higher numbers occurring in larger birds (Box 4.02). The outermost (distal) remiges of the wing are termed the primaries, defined by their attachment to the skeleton of the “hand” or manus (Chapter 6). Depending on the species, birds most commonly have between 9 and 12 primaries. The remainder of the remiges—known as the secondaries—extend from the forearm (more specifically the ulna; Chapter 6) of the bird wing (Fig. 4.30A). Bony bumps on the ulna, called quill knobs, indicate the points of attachment for the secondaries and often are visible on the skeletons of larger flying birds (Fig. 4.30B). Across groups of birds, the number of secondaries varies more than does the number of primaries, ranging from 8 to 32. Birds with long wings, such as albatrosses, have higher numbers of secondaries, giving them a larger wing surface. To identify and discuss the biology of important flight feathers, ornithologists have developed a standardized numbering system for individual feathers (Fig. 4.31).

Fig. 4.30 Quill knobs. A Turkey Vulture’s (Cathartes aura) secondary flight feathers attach directly to the ulna (A) via bony protrusions called quill knobs (B), shown here without the attached feathers.
(From Turner et al. 2007. Reproduced with permission from AAAS.)

Fig. 4.31 Numbering system for remiges and rectices. Ornithologists subcategorize remiges based on their attachment site: primary feathers extend from the metacarpal bone, while secondary feathers attach to the ulna. Each remige is assigned a number, starting at the meeting point of the two wing bones; therefore, primaries are numbered from right to left, secondaries from left to right. Numbering of rectices begins at the center of the tail and extends outward in both directions. Note that flight feather counts vary widely among species.
(Adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology.)
Bordering and overlying the remiges and rectrices on both the dorsal and ventral sides of the wing and tail are rows of contour feathers called coverts. Coverts are particularly important in contributing to the streamlined shape of the wing and tail; the aerodynamics of the wing are extremely sensitive to the details of wing shape (Chapter 5).
The crucial importance of flight feathers for generating lift and establishing control in the air is discussed in greater detail in Chapter 5. Suffice it to say here that remiges are the primary sources of lift generation, and while rectrices also generate lift, they are particularly important for stability and control in flight. Beyond their roles in the aerodynamics of flight, it is worth noting the specific material properties of flight feathers that make them so excellent at manipulating and managing the fluid media of both air and water. Most obviously, in order to generate lift in the very thin medium of air, the feathers of the wing and tail need to form long and broad surfaces that are at once firm, flexible, and lightweight. Feather keratin, as a material, is both firm and flexible, and the woven structure of the pennaceous vane augments these properties by creating a very specific shape while reducing weight and contributing even greater flexibility than could be provided by a solid sheet of keratin.
One incidental effect of the wing feathers moving through air is that their movements tend to generate sounds. Microstructural modifications that intensify or minimize sound production during flight are widespread. In most owls, for instance, the leading edge of the first several primary feathers has a loose fringe; the dorsal surface of the inner vanes of most flight feathers has a soft pile composed of elongated distal barbules (Fig. 4.32). These modifications allow owls to fly very quietly, creating little noise even within the high‐frequency range that their rodent prey can hear quite well. In contrast, many species of New World flycatchers, as well as pigeons and doves worldwide, have specializations of the primary feathers that exaggerate the sounds produced by incidental wing movements into sounds that function as signals (Hingee and Magrath 2009). As Charles Darwin himself noted, males of many bird species have feather modifications that have evolved for sound production associated with display behaviors and sexual selection (Darwin 1871) (Box 4.03).

Fig. 4.32 Owls have specialized flight feathers. Owls are extremely quiet fliers, thanks to two main differences in their flight feather microstructure. The inner vanes develop a soft pile of elongated distal barbules (boxed area) that reduces the noise of feathers moving over one another. The leading edges of their primaries have a comb‐like fringe that reduces air turbulance and its associated noise.
(Photograph by Kersti, https://commons.wikimedia.org/wiki/File:EulenfederTeil3.jpg. CC‐BY‐SA 3.0.)
4.4.4 Semiplumes: insulation around the edges
Semiplumes represent an intermediate form between down and contour feathers (Fig. 4.33). Unlike contour feathers, the barbules of semiplumes often lack hooks, and thus the barbs do not cling together to form a pennaceous vane. This results in a vane that is not completely formless like down, but that nonetheless is not connected tightly together like the pennaceous portions of contour feathers. Unlike down feathers, the rachi of semiplumes are longer than the longest barb. Semiplumes often occur at the edge of contour feather tracts, sometimes visible and sometimes hidden beneath the contour feathers, and at the inside of the wings (essentially in the bird’s armpit) or similarly at the inside of the thigh. Semiplumes are probably contour feathers that have lost the pennaceous portion of their vanes, as they are located in areas relatively protected from the elements by the contours of the bird’s body. They likely complement the insulating function of down.

Fig. 4.33 Semiplumes are intermediate feather forms. Semiplumes lack barbule hooks like down but have barbs shorter than the rachis, as in contour feathers.
(Photograph by Jennifer Layton.)
4.4.5 Bristles: detection and protection
Bristles are a highly specialized feather type in which the rachis is relatively stiffened, more strongly tapered, and lacking barbs along most of its length. Bristles are essentially contour feathers that have been simplified for functional purposes, and they are found almost exclusively in the region of the avian head (Fig. 4.34). The best‐known bristles are rictal bristles, which project from the base of the beak. Many birds, especially those that catch insects in the air such as flycatchers and nightjars, have rictal bristles (Chapter 8). Other regions of the head may also sport bristles, and bristles themselves are categorized by their location, for example as facial bristles (from the sides of the head), narial bristles (on the cere or behind the nares), and loral bristles (from the lores).

Fig. 4.34 Bristle feather variations. Bristle points of origin are shown here on a Wild Turkey (Meleagris gallopavo). Bristles often have a shaft and an aftershaft, both used to collect sensory information.
(Adapted from Lucan and Stettenheim 1972.)
Some ornithologists have suggested that bristles might help birds funnel insects into the mouth, but few studies have been conducted to test this supposition. In a study of Willow Flycatchers (Empidonax traillii), Michael Conover and Don Miller (1980) found that experimentally taping down or removing the rictal bristles did not affect the birds’ success in capturing insects. By using a wind tunnel, however, these researchers demonstrated that the bristles do protect the birds’ eyes, as more particles struck the eyes of birds whose rictal bristles had been removed. Rictal bristles are particularly prominent in certain groups (such as the neotropical puffbirds) that regularly capture scaly moths, butterflies, and other large, noxious insects; these bristles may protect the face and eyes of these birds. Similarly, “eyelashes” in birds are often bristles, and these are particularly well developed in birds such as the Ostrich (Struthio camelus) and Emu (Dromaius novaehollandiae), and in various species of rheas, hawks, turkeys, hornbills, and cuckoos (Fig. 4.35). Another possible but basically unstudied potential function of rictal bristles is detecting the motion of prey held in the beak, providing a sensory function akin to the sensitive whiskers of some mammals (Cunningham et al. 2011).

Fig. 4.35 Bristle feathers (“eyelash”). Presumably these bristle feathers provide the eyes with extra protection, which may be particularly important for open country birds such as (A) the Southern Ground‐Hornbill (Bucorvus leadbeateri) and (B) the Secretary‐bird (Sagittarius serpentarius).
(Photographs by: A, Debbie Aird Photography; B, Cynthia Mammoser.)
4.4.6 Filoplumes: feather kinesthetics
Scattered among and usually hidden by the contour feathers of most birds is a distinctive but rarely visible type of feather. These filoplumes are the smallest feathers, with an extremely slender, bare rachis and a tiny tuft of barbs (if any) only on the tip (Fig. 4.36). Otherwise reminiscent of the much larger bristles, filoplumes are distinct in lacking feather muscles, instead having distinctive sensory receptors in the skin next to their follicles. These miniscule feathers are thought to monitor movement within the feather coat (Necker 1985). Birds presumably use this information to monitor and detect changes in feather positions that might be caused by wind or body movements (Brown and Fedde 1993).

Fig. 4.36 Filoplumes: the smallest feather type. Subtle changes in filoplume position—caused by a bird’s motions or surroundings rather than muscles near the follicle—may provide important sensory information relevant to processes such as thermoregulation.
(Illustration by Charles L. Ripper © Cornell Lab of Ornithology.)
4.5 Molts and plumages
Although feathers are strong and flexible for their size, the fine structures of which feathers are composed deteriorate with use. Like a hair, a mature feather is a dead structure; it cannot be repaired if it becomes worn or broken. Unlike hair, however, feathers do not grow continuously, but instead develop to maturity fairly rapidly and then remain on the bird’s body. Use, sun, and the elements ensure that over time feathers become brittle, faded, and frayed. To restore their feather coats, birds must replace them regularly, usually annually or semiannually, with new feathers. This regular practice of replacing all or part of the feather coat, usually over a relatively short period of time, is called molt or molting.
In a complete molt, all feathers of the body are replaced; in a partial molt, only some subsets of feathers are replaced during that particular molt. Not all feather replacement constitutes a molt; for example, if a whole feather is entirely lost between molts, it usually grows back right away. However, partially damaged, broken, or worn feathers do not necessarily stimulate the feather follicle, and therefore these feathers are replaced only during the regular molt cycle.
An individual bird generally experiences two series of molts: those that occur as the bird matures from chick to adulthood, and those that occur in annual cycles throughout the bird’s adult life. The molts that accompany the maturing of an individual from a hatchling to an adult are fairly variable across species, but because the appearances of the resulting plumages vary predictably within a species, they can often be used by researchers to determine an individual bird’s age. The molts that occur once an individual achieves adulthood are annual changeovers of major parts of the plumage, and in some bird species they provide a semiannual opportunity to change appearance. Accordingly, some birds’ plumages appear the same molt after molt, whereas others alternate between different plumage patterns in different seasons.
4.5.1 Subadult versus definitive plumages
During all molts, growth of the new feathers pushes the old feathers from their follicles. As mentioned above, in juvenile birds, each natal down feather may be physically attached to the tip of its successor, the juvenal feather, which is the first true contour feather grown by a young bird (Fig. 4.11). Note that the term “juvenal” is applied to feathers and plumages, whereas “juvenile” designates a young bird itself; these two similar words often are confused.
For a variety of reasons, young birds usually pass through one or more subadult (immature) plumages, eventually reaching the definitive plumages of a mature bird. Subadult plumages are defined as those plumages that come before the definitive plumage, and usually only differ from definitive plumages in the coloration or patterning of the feathers. The age at which birds reach definitive plumage varies greatly among bird species. Many songbirds take less than a year, although in many species there may be subtle differences in the definitive plumages of younger and older birds. In general, long‐lived species such as large raptors, gulls, and ocean‐going seabirds retain their subadult plumages for a longer period of time. For example, the Bald Eagle (Haliaeetus leucocephalus) of North America does not attain its definitive plumage until its fourth or fifth year. Before acquiring a clean white head and tail, young birds go through several subadult plumages that are much less tidy, with a blotchy or mottled brown‐and‐white appearance. Many gulls also take several years to reach definitive plumage. In so‐called “three‐year gulls” such as the Ring‐billed Gull (Larus delawarensis) and Mew Gull (Larus canus), birders can distinguish first‐winter, second‐winter, and adult birds. Four‐year gulls, such as the Herring Gull (Larus argentatus), Great Black‐backed Gull (Larus marinus), and Lesser Black‐backed Gull (Larus fuscus), sport at least four different plumages on their way to maturity (Fig. 4.37). Male Steller’s Eiders (Polysticta stelleri) acquire definitive plumage in their third fall; the males of the larger Common (Somateria mollissima) and King (Somateria spectabilis) Eiders do so in their fourth fall. Slowly maturing birds such as albatrosses may take as long as 7 or 8 years to reach their definitive plumage.

Fig. 4.37 Complete plumage cycle of the Herring Gull (Larus argentatus). Many gull species follow a similar plumage trajectory, involving 3 years of subadult plumage—each year slightly different from the last—before reaching a definitive (adult) plumage. Plumages here are labeled using the Humphrey–Parkes system.
(Illustration by N. John Schmitt.)
In some species, one sex remains in subadult plumage longer than the other, a situation termed delayed plumage maturation. The male American Redstart (Setophaga ruticilla) of North America, for instance, does not wear his orange‐and‐black definitive breeding plumage until his second breeding season. His first breeding plumage is nearly identical to that of the adult female, who has yellowish patches and who acquires her definitive plumage by her first breeding season.
4.5.2 Annual cycles: molt and wear
Once birds obtain their definitive plumages as adults, they continue molting in annually driven cycles, which vary in frequency, type, and timing across species. Some birds undergo only one complete molt each year. Many songbirds that breed in temperate regions undergo a complete molt after the breeding season and then a partial molt in winter or spring. This partial molt may include all of the contour feathers, but not the wings and tail, and provides brighter colors for the breeding season. Only a few species are known to have two complete molts per year; most of these live in harsh habitats in which feathers wear out quickly. Examples include Marsh Wrens (Cistothorus palustris) and Bobolinks (Dolichonyx oryzivorus) of North America, which move within abrasive vegetation, and several species of African larks that dwell in windy, sandy deserts.
Some songbird species that have only one complete molt per year may nevertheless be able change their appearance seasonally by taking advantage of the natural process of feather wear. For example, European Starlings (Sturnus vulgaris) have prominent buff‐colored tips on their belly and breast feathers after they molt in mid‐ to late summer. By the time they are ready to breed in the following spring, these buff‐colored tips have worn off, and the birds’ underparts are dark and glossy. Similarly, adult male Purple Finches (Haemorhous purpureus) in North America have dull red heads after their annual molt in late summer (Fig. 4.38A), but feather wear slowly removes the outer barbules, revealing the bright, cherry‐red color below (Fig. 4.38B). The plumages of male Northern Cardinals (Cardinalis cardinalis) in North America and Common Chaffinches (Fringilla coelebs) in Eurasia have a grayish or buffy cast in fresh fall plumage, but these drab‐toned feather edges wear away; by late spring both species sport their brightest colors, although neither has undergone an intervening molt.

Fig. 4.38 Feather wear affects seasonal plumage. Male Purple Finches (Haemorhous purpureus) have (A) a dull plumage in the fall, but as the gray barbules wear away over winter they reveal (B) the bright cherry‐red breeding plumage of the underlying barbs.
(Photographs by: A, Kevin Bolton; B, Christian Dionne.)
4.5.3 Plumage naming systems
In the most general sense, plumage is a bird’s entire feather coat at a given point in time. A molt produces new plumage, and molts are named for the type of plumage they produce (rather than for the plumage they are changing from). To study how and why molts and plumages evolved, ornithologists require a system for naming and comparing them among species.
Historically, one common practice was to name plumages for the presumed presence or absence of breeding activity at the time the bird wore that particular plumage. Thus, birds were said to be in nuptial (breeding) plumage in the spring and summer, and in postnuptial or winter plumage in the fall and winter. This system is inherently problematic, however, because many birds do not fit into this simple breeding/non‐breeding plumage dichotomy. First, many birds never change into anything that could be called a distinct breeding plumage; they appear the same year‐round. Second, different plumages are not necessarily linked with reproductive activities or seasons of the year. In many tropical regions, for instance, there often is no annual season analogous to winter.
As part of a larger effort to identify similarities among the plumages of different bird species, Phil Humphrey and Kenneth Parkes (1959) developed a system for classifying plumages that still bears their names: the Humphrey–Parkes or H‐P plumage nomenclature (Table 4.01). Under this system, an adult bird’s main plumage each year—the one it wears the longest and the one usually produced by a complete molt—is termed the basic plumage. This term can be confusing for bird enthusiasts in the temperate zone, who generally think of a bird’s nuptial or breeding plumage as the “main” plumage because it usually is the most noticeable and colorful. However, the breeding plumage is rarely worn for as long as the non‐breeding plumage, and it often follows only a partial molt. Instead, the basic plumage of many long‐distance migrant birds is a duller non‐breeding or winter plumage, and the molt producing it occurs in the late summer or fall.
Table 4.01 The Humphrey–Parkes nomenclature for plumages and molts. Note that molts are named for the feathers they produce, not for the feathers they shed. Subadult plumages are numbered as first, second, third, and so forth. After a bird molts into its definitive plumage, this numbering is no longer used.

How might we explain the beautiful breeding plumages of many birds? Humphrey and Parkes reasoned that other plumages are likely inserted as needed between basic plumages depending on a species’ social system and ecology. Thus, when a partial molt occurs before breeding, it produces an additional plumage, the alternate plumage. This is the plumage that temperate zone bird enthusiasts usually think of as the breeding plumage. It is this molt, for example, that produces the brilliant red of the male Scarlet Tanager (Piranga olivacea) of North America (Fig. 4.39), the rosy breast and crown of the Eurasian Linnet (Carduelis cannabina) of Eurasia, and the contrasting rust, black, and white patches on dozens of species of migrating shorebirds worldwide. If, in addition to the basic and alternate plumages, the bird produces another plumage within an annual cycle, as do ptarmigans and certain New World buntings, that plumage is termed supplemental. Supplemental plumages are relatively uncommon.

Fig. 4.39 Breeding plumage in a seasonal temperate migrant. The alternate plumage of a male Scarlet Tanager (Piranga olivacea) is a combination of its basic molt and a partial molt before breeding.
(Photograph by Larry Gridley.)
The H‐P system is simple but flexible, and it works well even for molt and plumage sequences that are highly specialized, such as those of ducks. In early summer after mating, many ducks, such as the Wood Duck (Aix sponsa) of North America, have a complete prebasic molt, producing a dull‐colored basic plumage termed eclipse plumage. Most male ducks in eclipse plumage resemble females (Fig. 4.40A). Female Wood Ducks also undergo a prebasic molt a bit later in summer, after their young are independent—although their appearance does not change much. The basic plumage of both sexes is soon lost in a molt of the body feathers—a partial prealternate molt—which produces the brightly colored head and other distinctive features of the male (Fig. 4.40B). The occurrence of the prealternate molt immediately after the prebasic molt (much sooner than in most species) is related to the timing of courtship in these ducks, which begins in the fall. Thus, like the colorful Wood Duck, many male ducks are in basic plumage for only a few weeks of the year.

Fig. 4.40 Eclipse versus alternate molts in adult waterfowl. (A) Shortly after breeding, both male and female Wood Ducks (Aix sponsa) complete their similarly drab prebasic eclipse molt. (B) As courtship begins weeks later, the male completes a partial prealternate molt into flashy mate‐attracting plumage. In both photos, the male can be distinguished by his bright red iris.
(Photographs by: A, Walter P. Szymanski; B, Steve Patterson.)
4.5.4 The four molting strategies
The H‐P system has long provided the standard ornithological terminology for describing avian plumages, with some useful later variants that incorporate new findings. For example, the original H‐P nomenclature defined the first molt after the juvenal plumage (which follows the natal down) as the first prebasic molt that generated the first basic plumage. However, this first post‐juvenal molt occurs at different times in various species and results in plumages with different functions, so this assignment effectively threw off the alignment of the subsequent definitive basic molts. More recently, some researchers (Howell and Corben 2000; Howell et al. 2003) have suggested that the juvenal plumage itself be considered the first basic plumage, as it is a complete molt that tends to be timed in a way that establishes the annual basic molt cycle most accurately. Under this modification to the terminology, any plumages intervening between this initial juvenal/first basic plumage and the second basic plumage are recognized as alternate, supplemental, or formative plumages.
With this modification in place, four basic strategies of plumage development become evident. These four strategies result from different combinations of two patterns. The first dichotomy is whether the species has only one molt each year, and thus employs a “basic” molt strategy, or has two molts each year, and thus uses an “alternate” molt strategy. The second dichotomy is whether any plumages (such as alternate, supplemental, or formative plumages) are inserted between the juvenal plumage and the next, or second, basic molt. If so, the species has a “complex” molt strategy; if not, it has a “simple” one. Thus, the four molt strategies are simple basic, complex basic, simple alternate, and complex alternate.
4.5.5 Progression and timing of molt
In most birds, especially landbirds, a molt occurs through an orderly, sequential replacement of feathers along a feather tract, so that in any particular tract only a subset of the feathers are being replaced at any one time (Fig. 4.41). This strategy is particularly important for the flight feathers; rarely does a landbird molt more than a few wing or tail feathers at a time. In many woodpeckers, for example, the stiffened rectrices act as a prop to support the bird while it searches up and down tree trunks. Molting of these feathers starts with the second innermost rectrices and progresses to the outside. The long central pair of rectrices is replaced only after the other tail feathers have grown in, so the tail can continue to serve as a sturdy brace throughout the molt cycle.

Fig. 4.41 Molt progression in flight feathers. Most birds molt a few feathers at a time over several weeks. Each feather is lost and subsequently regrown in the order of the arrows. Wing molt often starts at the boundary between the primaries and secondaries, while tail molt proceeds outward from the center. In the illustration above, the first retrix pair has regrown to its fullest extent; pair number three has re‐emerged most recently and must develop almost completely (like the second pair) before the fourth pair drops.
(Adapted from illustration by N. John Schmitt © Cornell Lab of Ornithology.)
On the other hand, many waterbirds—including loons, grebes, anhingas, swans, geese, and ducks—drop all their remiges simultaneously, leaving the birds unable to fly for as long as a month during their prebasic molt. Similarly, some of the smaller owls, such as the Northern Pygmy‐Owl (Glaucidium gnoma), the Little Owl (Athene noctua), and the Burrowing Owl (Athene cunicularia), molt all their rectrices at once, as do some other short‐tailed birds that use their tails very little in flying, such as alcids and rails.
Females of a number of hornbill species also molt abruptly. In these Old World cavity nesters, females are sealed inside the nest cavity by a mixture of mud and saliva that nearly closes the cavity entrance, likely an adaptation to exclude predators such as climbing snakes and monkeys from the nest (Chapter 11). Females remain cavity‐bound throughout incubation and the early part of their nestlings’ life; during this time, female hornbills undergo a full molt, which creates an extended flightless period.
Occasionally, a bird will shed many feathers all at once in response to some external stress. This shock molt or fright molt has been reported for dozens of species in numerous avian families but occurs only in unusual circumstances. For instance, in a clearly adaptive strategy, a bird may shed its tail feathers when its tail is grabbed by a predator. Fright molt occasionally occurs when a bird is handled by people. The mechanism of shock molt has not been determined, but clearly some relaxation of the muscles holding each feather shaft must be involved, because under normal circumstances substantial force is required to pull out even a single feather.
The rate of a molt varies with latitude and the extent of seasonal change in the climate. Molt often is more rapid, for example, in arctic‐nesting gulls than in closely related gulls of the temperate zone. Birds breeding at polar latitudes in the arctic simply do not have time for a multi‐week molt after breeding, because soon they must head south for the winter. The abundance of food and continual daylight for foraging in the arctic undoubtedly help these birds in gathering the resources they need to molt rapidly. In some tropical species, on the other hand, molts may be prolonged. For example, Rufous‐collared Sparrows (Zonotrichia capensis) in northern South America take 2 months to molt. Breeding seasons in the tropics also may last a long time, and the energy demands of some species are low enough that they are able to overlap molt and breeding activity.
Birds generally molt when they are not engaged in other energetically demanding activities, such as feeding young or migrating. Long‐distance migrants may molt before they migrate, after they migrate, or partly before and partly after—but rarely, if ever, during migration. In the north temperate zone, adults of many species of songbird molt during late summer, when they no longer are caring for young and before the rigors of fall migration.
4.6 Feather care
Feathers are essential but delicate structures, so birds spend a good deal of time caring for them. Because the mature feather is a dead structure, it has no circulatory system for internal maintenance. Therefore, a bird provides all feather care from the outside.
In preening, a bird grasps a feather near its base and then slides its bill along the shaft toward the tip of each feather (Fig. 4.42), smoothing the barbs to re‐establish any broken connections and remove stale oil and dirt. The bird fluffs the feathers in the section of the body it is preening and turns and twists its head and body as needed to reach the desired area. Preening keeps the feathers neat and clean, preserving their streamlining and insulating effects. Preening also helps remove external parasites, or ectoparasites, such as flies, ticks, and lice.

Fig. 4.42 Feather preening. A Swallow‐tailed Kite (Elanoides forficatus) preens its rectrices. Birds spend a great deal of time maintaining their feathers to remove dirt and ecotoparasites, spread protective oils, and re‐zip barbs and barbules.
(Photograph by JimGrayImages.com.)
When preening, many species use the beak to spread oily secretions from their uropygial gland (also called the oil gland or preen gland) over their feathers. The uropygial gland, one of the few glands in a bird’s skin, is located on the rump at the base of the rectrices. A preening bird frequently nibbles on a specialized tuft of feathers on the uropygial gland, which transfers the gland’s oils onto the bill. The oil becomes distributed throughout the plumage as the bird preens its feathers. Oil gland secretions help keep feathers water repellent but are not essential; when the oil glands of ducks were removed, their feathers did not lose their waterproofing ability immediately. Instead, the secretion acts like a conditioner that prevents feather barbs and barbules from becoming brittle and breaking prematurely (such breakage changes the surface characteristics of the feather vane, and thus wetability of the feather) (Jacob and Ziswiler 1982). As described earlier, the actual waterproofing of feathers seems to result primarily from the microstructural arrangement of the barbs and barbules, which provide an evenly spaced surface of ridges with narrow gaps between them that sheds water very effectively. Thus, spreading oil gland secretions seems to facilitate waterproofing only indirectly; feathers provide the waterproofing, but oil gland secretions make feathers last longer.
To condition the feathers on the head (which cannot be preened with the beak), a bird can rub its head directly on the gland, or wipe oil on its feet with its beak and then scratch its head. Birds use one of two scratching techniques, either a direct method where they bring their foot forward under the wing (Fig. 4.43A), or through an indirect method by stretching their leg and foot over the wing (Fig. 4.43B). Virtually all species use only one of these two techniques. Of course, birds can also scratch their heads when not applying secretions, and do so as a general means of preening feathers they cannot reach. Birds that cannot scratch their heads with their feet, either because of accidental injuries or the experimental use of neck collars, tend to have more ectoparasites living on their heads (Clayton 1991).

Fig. 4.43 Scratching techniques. To maintain out‐of‐reach feathers (such as those at the top of the head), many birds apply preening oils with their feet, either by (A) raising the leg and foot under the wing while bending down, as demonstrated by this Yellow‐crowned Night‐Heron (Nyctanassa violacea), or (B) bringing the leg and foot over and above the wing, as demonstrated by this Rufous Hummingbird (Selasphorus rufus).
(Photographs by: A, Andrew McCullough; B, Robert T. Moore.)
Bathing is another feather‐maintaining behavior that complements preening. Birds may bathe in water, snow, or even dust. The manner of bathing tends to be quite consistent within various avian families, with most species prefering water baths. Swallows and swifts drop down repeatedly and skim the surface of a lake, pond, or stream to wet their feathers. Terns plunge into the water from the air, and kingfishers do so from an overhanging branch. Many forest birds bathe in rainwater or dew that has collected on leaves. Most songbirds stand or squat in shallow puddles, vigorously ruffling their feathers and shaking both wings, creating a veritable shower. Hummingbirds may dart in and out of the spray from a garden hose on a hot day. Common Nighthawks (Chordeiles minor) may bathe on the wing during intense downpours, alternately flapping rapidly and ruffling the body feathers amid the deluge. However, wetting the body, even for bathing, must occur within limits; a thoroughly wet bird can be quite helpless, and many are unable to fly efficiently when soaked, making them particularly vulnerable to predators. Because birds must dry as soon as possible, certain drying behaviors are commonplace: after bathing, a bird typically shakes its body to throw off extra water, whirs its wings, fluffs its feathers to promote evaporation, and preens vigorously. In favorable circumstances, a soaked bird can dry itself in a matter of minutes.
Dust bathing is reported most often for species that spend a lot of time on the ground, such as House Sparrows (Passer domesticus) and Wild Turkeys (Meleagris gallopavo). This behavior is not a substitute for water bathing, and many species of birds do both. In dust bathing, a bird usually squats or lies down in a dusty area, often a dirt road, and drives fine particles through its plumage by beating its wings in the dust, rolling its body, fluffing its feathers, and wiping with its head and beak (Fig. 4.44). The Greater Rhea (Rhea americana) of South America goes as far as picking up dust and throwing it over its body. The bird removes some of the dust by violent shaking, but often much dust remains, staining the bird’s plumage the color of the soil. Presumably dust bathing can help reduce ectoparasites, but this possibility has not been studied experimentally. Many ectoparasites are arthropods, which breathe through small holes in their outer skeletons that are vulnerable to clogging and/or desiccating from dust exposure. Comparisons of birds with and without opportunities for dust bathing have shown that dust bathing also helps to remove substances coating the feathers, such as old oil gland secretions.

Fig. 4.44 Dust bathing. A male Gambel’s Quail (Callipepla gambelii) fluffs its feathers in powdery desert soil, a practice that may remove excess oils that attract ectoparasites. Alternatively, dust bathing may desiccate or clump ectoparasites already in the oils, making it easier to preen them off.
(Photograph by Eric Gofreed.)
Sunning also is thought to maintain feathers (Simmons 1986), but exactly how it does so is not well understood. Sunning birds adopt varied and sometimes comical postures: commonly the bird’s feathers are fluffed, the tail is spread against the ground, and a wing is extended on at least one side, sometimes on both. Sunning birds frequently lie on the ground in a warm place, with the head lowered and tipped to the side, remaining nearly motionless for many seconds or minutes. Although sunning has been described for numerous species of birds, few experimental studies have tested its functions. Suggested purposes include conditioning the feathers by keeping them supple through limited heating, harming or repositioning ectoparasites, and saving energy by taking up solar heat through the feather coat.
Many large birds, including cormorants, anhingas, herons, pelicans, storks, and New World vultures, stand for extended periods with their wings extended to the side in a pose known as the spread‐wing posture (Fig. 4.26). Cormorants (also known as shags) and anhingas (also known as snakebirds) are closely related. Both types of bird prey on fish and use the spread‐wing posture to dry their feathers after swimming. However, the feathers of anhingas are more permeable to water. This permeability reduces buoyancy and allows them to swim for long periods with only their necks and heads above the surface. Because their permeable feathers take up extra water, anhingas take longer to dry. Furthermore, anhingas generate less heat internally than do cormorants, and in cooler areas anhingas may use the spread‐wing posture to absorb sunlight. The difference in anhinga and cormorant energy production is reflected in their geographic distributions: cormorants can live in much cooler regions than can anhingas.
Like bathing and sunning, anting is believed to help control ectoparasites. In passive anting, a bird simply stations itself among a swarm of ants, permitting them to run all over its body and move in and out among the feathers (Fig. 4.45). In active anting, a bird picks up an ant or other chemically potent object, such as a millipede, and deliberately rubs it on its feathers. Objects rubbed in the feathers during anting (in its broad sense) include insects, plant material, and even cigarette butts. Ornithologists assume that the rubbed materials contain chemicals that are noxious to ectoparasites. Because birds sometimes eat the arthropods used in anting, it is possible that the process also helps rid a potential food item of its most noxious chemical protection.

Fig. 4.45 Passive anting. Similar to sunning and dusting, some birds remove ectoparasites by allowing ants to crawl through their feathers. This American Crow (Corvus brachyrhynchos) sits among a group of ants (look closely on the back and neck), wings slightly outstretched to enable access.
(Photograph by Sean McCann.)
4.7 Coloration: form and function at the scale of light
Birds exhibit a spectacular range of colors and patterns with their feathers—consider the sparkling, metallic iridescence of a hummingbird’s throat, or the earthy brown, leaf‐like patterning that renders a frogmouth virtually indistinguishable from its surroundings. More than just decoration, coloration is another level of feather form and function. That is, just as feathers are able to use basic properties of air and water to generate lift, trap air or shed water, they can also—at an even finer scale—manipulate light to produce a magnificent array of colors and patterns. These colors and patterns in turn play important roles in the ecology and behavior of birds, through things like predator avoidance and social signaling. However, no matter what level of biological function you are interested in, the key to unlocking the many fascinating secrets of color in feathers is to have a basic understanding of light.
4.7.1 Light, perception, and color
Light is one form of electromagnetic radiation: a complex but basic physical force that can be thought of as an energy‐carrying wave. One general property of waves is that they maintain a specific measurable distance between successive peaks of the wave—the wavelength. Electromagnetic radiation can manifest itself in a broad continuum of wavelengths in what is called the electromagnetic spectrum (Fig. 4.46). The wavelengths of the electromagnetic radiation in the electromagnetic spectrum range from very long radio waves (many kilometers in length) to very short gamma rays (with wavelengths on the scale of atomic nuclei).

Fig. 4.46 The electromagnetic spectrum. Visible light is one of the many types of electromagnetic energy we use, and is only a small portion of the full spectrum. The full spectra of other energy forms and their relative wavelengths are shown in Hertz (Hz).
(Courtesy of NASA.)
Our sun emits electromagnetic radiation across a wide range of wavelengths from all over the electromagnetic spectrum. Some of this radiation we feel (heat), some of it we see (light), some of it we are affected by but do not directly perceive with our senses (ultraviolet or UV), and some of it we are mostly oblivious to (X‐rays and gamma rays, which are powerful and destructive but which we are largely protected from by our atmosphere). Most of the energy from the sun is emitted in the portion of the electromagnetic spectrum that our eyes sense directly; this portion of the electromagnetic spectrum is called the visible spectrum.
While visible light is defined as the portion of the electromagnetic spectrum that human eyes are sensitive to, other animals can perceive a lesser or greater portion of the overall electromagnetic spectrum. Birds’ eyes are sensitive to a greater portion of the spectrum than humans—birds can see both slightly longer and shorter wavelengths. In other words, birds literally see some of the energy that we can only feel as heat, and they can also see color that is invisible to us in the UV portion of the spectrum.
The property that we recognize as “color” represents the ability of our human eyes to distinguish among the different wavelengths of light across the visible spectrum. Whereas the spectrum is continuous, our eyes have three types of cone cells—color‐sensitive receptors—each of which is sensitive to a different part of the visible spectrum, corresponding to what we perceive as red, blue, and green wavelengths. By interpolating between these receptors, we are able to break the continuous spectrum up into about six basic colors (red, orange, yellow, green, blue, and violet) plus their various shades and combinations.
The eyes of birds have more types of color receptors than ours do (Chapter 7), and additionally birds have the anatomical equivalents of color filters that can further fine‐tune how light is received by their color receptors. This means that a bird’s ability to resolve different parts of the visible spectrum is much better than ours. Thus birds not only see a greater portion of the overall electromagnetic spectrum than we do, but they also see more colors within the spectrum than we can discern (Box 4.04). For this reason, it is important to remember that the color of any object, including a feather, is determined by a combination of two things: (1) what happens to light when it interacts with the object; and (2) how this information is interpreted in the visual system of the observer. For now, we will ignore the differences between bird and human visual systems, and instead focus on how light interacts with objects like feathers.
In order to “see” the independent spectral colors present in sunlight (cumulatively defined as white light) we can use a prism to spread the individual wavelengths out to form the discreet bands of color as seen in any rainbow. In a rainbow, you are witnessing a sequence of light wavelengths from long (red) to short (indigo). Feathers, skin, and other objects appear the color that they do according to the subset of wavelengths that emerge from their surfaces after interacting with light. For example, white feathers reflect the full spectrum of long to short wavelengths visible in sunlight, and therefore appear to be white in color. Alternatively, when only a subset of wavelengths (e.g. red) return to the viewer after hitting a bird’s feathers, those wavelengths stimulate specific receptors that correspond to our recognition of red. Feathers will appear black when little or no light escapes from them, and thus few visual receptors are triggered in our eyes. In addition to the colors we perceive, birds see a wider rainbow, with more colors in between; what we see as the “blue” part of the rainbow would be several distinct colors to a bird.
Light is often affected by the specific properties of the object with which it interacts, being reflected, transmitted, absorbed, and/or emitted (Box 4.05). Ornithologists have categorized two basic mechanisms by which light produces colors in skin and feathers. The first mechanism is influenced by pigments (molecules, or assemblages of atoms) that absorb and interact with light at the atomic level, and result in subtractive coloration. Subtractive coloration occurs when pigments change or remove light. In contrast, additive coloration involves mechanisms that are influenced by structural arrangements of materials (e.g. keratin) that can reflect and transmit light in specific ways. Birds may use both of these mechanisms separately or in concert to produce the various colors that humans see on them, and to produce the additional colors that birds can perceive but humans cannot.
4.7.2 Producing color in feathers
At first the distinction between pigment colors and structural colors may seem too subtle to matter for an ornithologist, but actually this dichotomy is fundamental to understanding avian coloration. Certain categories of avian colors tend to be produced entirely by pigments, others by structures, and many shades of colors can be produced only by combining the two methods. Further, how and where different colors are deposited within a growing feather can be limited depending on how the colors are generated. These differences add up to broad evolutionary and ecological patterns of where, how, and why birds create color in their feathers. (The same is true for other areas of their bodies; note that although this chapter is focused on feathers, similar coloration mechanisms apply to avian skin and irises.)
For example, the lovely plumages of New World orioles are heavily pigmented (Fig. 4.47A). Most generally, pigments can be thought of as colored substances—often large and complex molecules—that can absorb white light, interact with its energy at an atomic level, and then emit only certain wavelengths, or colors. The specific wavelengths emitted depend on the light’s effect on the pigment molecule’s molecular bonds. Roughly speaking, light can shake or twist atoms within a molecule, or promote electrons to higher orbital levels.
Fig. 4.47 Pigmented versus structural colors. (A) The hues of this Venezuelan Troupial (Icterus icterus) are mostly an effect of feather pigments, which absorb certain wavelengths and reflect others. These pigments are independent of feather structure. (B) In contrast, the sky‐blue hues of this Mountain Bluebird (Sialia currucoides) are almost entirely structural, created by scattering only the blue wavelengths of incoming light with bubbles of keratin and air.
(Photographs by: A, Sébastien Clermont‐Petit; B, Brenda Sand.)
In contrast, the Mountain Bluebird (Sialia currucoides) has an almost entirely unpigmented plumage (Fig. 4.47B). Its structural colors are created instead by very specific physical arrangements of keratin and air. For structural colors, usually very few (two or three) different substances (such as melanin, keratin, and air) cause light to behave in such a way that only certain wavelengths of light are reflected and reinforced, while others are just transmitted or are lost. Thus, just as the water droplets that create a rainbow are not themselves colored, structural colors in feathers often are produced by the arrangement of uncolored objects (such as keratin and air) that interact with light but have no inherent color themselves.
4.7.3 Pigmentary colors: absorbing and transforming light
Pigments are given their characteristic colors by atomic‐level processes that occur when light hits and excites a molecule that has specific light‐responsive properties. Without going into the details of the chemistry, energy in the form of white light can be differentially absorbed and/or re‐emitted by these specialized pigment molecules in a specific subset of wavelengths. The two main classes of pigments found in birds are melanins and carotenoids.
Melanins are complex molecules found in all vertebrates and give mammals their hair and skin colors. Melanins are the most abundant pigments in birds and primarily occur in melanosomes, melanin‐containing bodies embedded within skin cells and feathers (Fig. 4.48). Birds synthesize their own melanins from amino acids, which they obtain from the proteins in their diet. In general, the melanins are excellent light absorbers and are mainly used to produce the earth tones in bird plumages. Depending on the concentration and distribution of specific melanins in skin and feather tissues, their colors can vary from the darkest blacks through subtle grays, and from muddy browns, to red‐browns, yellow‐browns, and pale yellows. There are two classes of melanins: the eumelanins and the phaeomelanins. Eumelanins tend to be blacks and grays; for example, they color the all‐black members of the genus Corvus (crows and ravens) found worldwide. Pheaomelanins create browner hues, such as the reddish browns of many owls, shorebirds, and pheasants. The antbirds of the neotropics create beautiful patterns by combining various earth‐toned melanin pigments. Because melanins are synthesized within cells, their deposition can be very tightly controlled and used to create extremely intricate patterns (Fig. 4.49).

Fig. 4.48 Melanosome variations. Micrographs (photographs taken by a microscope) reveal a diversity of melanosome structures across species. The different melanin molecules within such melanosomes create different feather hues: (A) brown in a Tufted Titmouse (Baeolophus bicolor), (B) brown‐black in a Macaroni Penguin (Eudyptes chrysolophus), (C) gray in a Double‐crested Cormorant (Phalacrocorax auritus), (D) black in a Palm Cockatoo (Probosciger aterrimus), and (E) iridescence in a Brazilian Teal (Amazonetta brasiliensis). (F) Melanosome structure in a fossil microraptor. The dot on each bird indicates the approximate sampling location.
(From Li et al. 2012. Reproduced with permission from AAAS. Insets A–E from del Hoyo et al. 2016. Reproduced with permission from Lynx Edicions.)

Fig. 4.49 Melanins can create elaborate feather patterning. Melanin pigments form stripes and eyespots in the feathers of a Great Argus (Argusianus argus); here a male is displaying for an observant female. Cell‐by‐cell level control allows extremely intricate patterning within a tail feather.
(Photographs by Jeremy Johnson, Meddling with Nature: left, CC‐BY‐SA 4.0, retrieved March 15, 2015, www.meddlingwithnature.com; right, CC‐BY‐SA 3.0, retrieved November 5, 2014, Wikimedia Commons.)
Carotenoids are the other major class of avian pigments. Generally made of long carbon chains, carotenoids re‐emit light from very specific parts of the visible spectrum, often producing bright reds, oranges, and yellows. Unlike melanins, carotenoids are synthesized only by plants, algae, and fungi; therefore birds must acquire them in their diet, either directly by eating plants or indirectly by eating something that has eaten plants. Many bird species consume a diversity of plant‐generated carotenoid molecules (or carotenoid precursor molecules that are not fully formed) and change them into a specific form that they then embed in their plumage (Fig. 4.50A). In some species, differences in the specific types of carotenoids in the diets of individuals can cause color variations in their plumage. For example, some Cedar Waxwings (Bombycilla cedrorum) in North America have tail‐feather tips that are more peachy red than the typical yellow (Fig. 4.50B)—these individuals have eaten berries of the japanese honeysuckle (an invasive plant species) that contain an unusual reddish carotenoid pigment that the waxwings are unable to metabolize fully to their preferred yellow pigment type (Mulvihill et al. 1992).

Fig. 4.50 Carotenoid pigments. Obtained from the diet, carotenoids create warm red, orange, and yellow hues, as featured in (A) the facial feathers of this Golden Palm Weaver (Ploceus bojeri). (B) Cedar Waxwings (Bombycilla cedrorum) typically have yellow‐tipped tail feathers.
(Photographs by: A, Peter S. A. Baker, DPAGB, LRPS; B, Dave Spier.)
Currently, although hundreds of carotenoids have been found in nature, only a few dozen have been identified in bird diets, blood serum, and/or feathers. In general, most terrestrial birds seem to have diets relatively rich in the carotenoid pigments lutein and zeaxanthin, whereas aquatic birds have greater access to gazaniaxanthin and rubixanthin pigments. The roles of rarer carotenoids in avian coloration are still largely unknown.
In addition to the very common, widespread pigment groups of melanins and carotenoids, some birds use other types of more exotic pigments. For example, porphyrins are complex, nitrogen‐containing molecules related to hemoglobin, which birds (and other living things) make by modifying amino acids. Porphyrins are most striking in the purplish reds and greens of the turacos, a family of colorful African birds (Fig. 4.51A). The porphyrin pigment turacoverdin, which produces the spectacular green in the plumage of many turacos, is one of the few green pigments known in birds. Most greens in other birds are thought to be produced by yellow carotenoid pigments overlying blue structural colors. Porphyrins produce interesting shades of red, browns, greens, or pinks in a number of different avian orders. They also can interact with melanins to produce the browns of many owls, and they are found in various pigeons and gallinaceous birds. In eggshells, porphyrins may appear pink or red, or they may be masked by melanins. Although the exact chemical structure varies from pigment to pigment, all porphyrins fluoresce bright pink under UV light (Fig. 4.51B).

Fig. 4.51 Porphyrin pigments. (A) Most of the green and violet‐blue feathers on this Hartlaub’s Turaco (Tauraco hartlaubi) are a product of porphyrin pigments, which are rare in birds. (B) Easily degraded by UV sunlight, porphyrins are useful markers of feather age in the nocturnal migrant species in which they occur. Recently molted feathers of Northern Saw‐whet Owls (Aegolius acadicus) fluoresce bright pink under UV light.
(Photographs by: A, derekkeats, https://commons.wikimedia.org/wiki/File:Hartlaub%27s_Turaco.jpg. CC‐BY‐SA 2.0; B, Joe Subolefsky.)
Another unusual group of pigments are the psittacofulvoids, which, as their name suggests, are found only in parrots. These pigments, which are fundamentally different from the carotenoids, produce many of the vivid hues found in parrots (Fig. 4.08).
Pigment molecules are used extensively in avian visual signaling; because pigments are obtained in different manners (some are manufactured within the body whereas birds obtain others from their diet) they have the potential to carry information about the health and vigor of an individual bird. This information can help birds advertise their overall desirability as healthy mates, or undesirability as strong competitors.
4.7.4 Structural colors: bending light
To understand structural colors, it is necessary to understand a few more aspects of the physics of light. First, light refracts, or bends from its straight path, when it passes between one kind of material and another; for example, when it passes from air to glass, water, or keratin (Fig. 4.52A). However, all the wavelengths of light do not bend at the same angle as they move between materials. Different wavelengths refract at different angles, with longer wavelengths bending less and shorter wavelengths bending more. Therefore, if white light hits a material boundary, the different wavelengths separate. Light moving through the flat, parallel surfaces of window glass is bent and separated when it enters the glass; however, it is bent back exactly in reverse when it moves from the glass to the air again, so the original structure of the light is restored, and no separation of color is visible to us. In contrast, the glass surfaces of a classic prism are not parallel; light traveling through is bent both at the entering and exiting surfaces, but the non‐parallel surfaces cause the light to become separated into its complete spectrum of colors. Using a rainbow as a real‐life illustrative example once more, refraction is how white light can become separated by the structure of materials. Under certain lighting conditions, the curved surfaces of water droplets falling in rain act like a prism, separating the white light passing through and creating the rainbow (Fig. 4.52B).

Fig. 4.52 Light properties that can produce color. (A) Light bends as it passes through distinct materials. Here, wavelengths separate inside the glass but bend in reverse as they contact air again, reuniting once more as white light. (B) When white light passes through a falling raindrop at a 42‐degree angle, it refracts and separates into independent wavelengths (forming a spectral rainbow, as in a prism).
(Illustrations by: A, ajizai. http://wikimediafoundation.org/wiki/File:Refraction_photo.png; B, KES47. https://commons.wikimedia.org/wiki/File%3ARainbow1.svg.)
Refraction in feathers
Birds have the materials necessary to use this property of light to make color in their feathers. The degree to which light refracts when moving between materials depends on the difference in the refractive index (a quantification of the light‐bending property) of the materials. Feathers are made of keratin, which has a refractive index sufficiently different from air to cause substantial refraction. Other common materials in feathers include pigments, especially melanins, which have a refractive index distinctive from both air and keratin. Thus, the ability to refract light is one of the material properties of feathers. With the necessary properties of their constituent materials in place, two fundamentally different forms of light scattering potentially could occur in feathers, incoherent and coherent scattering.
Incoherent scattering
For many years, blue colors in birds were erroneously attributed to a group of related physical mechanisms termed Raleigh, Tyndall, or Mie scattering. To achieve Raleigh scattering, air bubbles inside the keratin matrix of the feather were modeled as being small enough to allow the passage of larger wavelengths of light around them (the reds, oranges, yellows, and greens) but large enough to scatter all wavelengths of a certain size and smaller in random directions (blues and purples). The defining property of such incoherent scattering is the true randomness of the air bubbles within the keratin, and thus the randomness of the scattering of larger wavelengths of light. Although incoherent scattering does produce some of the subtle blues and bright whites found in nature, such as the sky and blue ice (Fig. 4.53A), there is no evidence of this process producing a blue color in birds or other living animals. Instead, the only “color” produced using incoherent scattering in bird feathers is white (Fig. 4.53B).

Fig. 4.53 Incoherent scattering of light. (A) Icebergs appear to have a blue hue due to the random arrangement of trapped air bubbles, which scatter shorter wavelengths of light. (B) In feathers, incoherent scattering within the keratin matrix can only produce white (reflectance of all hues cumulatively), as on this Rock Ptarmigan (Lagopus muta).
(Photographs by: A, Christopher Michel, http://wikimediafoundation.org/wiki/File:Alaska_(3733278715).jpg. CC‐BY‐2.0; B, Perhols, https://commons.wikimedia.org/wiki/File%3ASvalbard_rock_ptarmigan_svalbardrype_pho_w_5266.jpg. CC‐BY‐SA‐3.0.)
Coherent scattering
Research during the past several decades has shown that all known structural colors beyond white in birds are produced through coherent scattering. Coherent scattering occurs in feathers when the air bubbles, melanin, and keratin are arranged in very specific patterns relative to one another in ways that separate and reinforce only certain wavelengths of light. Two fundamentally different kinds of structural colors are produced from coherent scattering: structural blues and iridescent colors. In order to understand how these two forms of structural colors differ from one another, it is necessary to consider one more physical property of light—interference—that contributes to the behavior of light inside a feather.
Interference
Interference is what occurs when waves interact with one another. If two light waves of the same wavelength are added together, they will continue to oscillate at that same wavelength, but their amplitudes—perceived by us as their intensities—will add together differently depending on how the individual waves are lined up. If waves are timed to be exactly in phase (oscillating together so that their peaks and troughs coincide), their amplitude will double; this is called constructive interference (Fig. 4.54A). If they are timed exactly so that one wave peaks as the other bottoms out, their intensity sums to zero, and they cancel each other out—a phenomenon called destructive interference (Fig. 4.54B). In these ways, light of the same wavelength may interact with itself in predictable ways so that its waves can either strengthen or weaken each other.
Sunlight provides a constant stream of light containing manifold wavelengths. Some subset of all this light shares the same wavelength, and this light has the opportunity to interact constructively to increase the intensity of the light at that specific wavelength. Coherent scattering occurs when a feather’s keratin (and embedded materials such as air or melanin) are arranged in such a way that a large proportion of the incoming light of one specific frequency, determined by the spacing of the keratin itself, is scattered outward from the surface of the feather in phase and thus is reinforced and intensified. All other wavelengths are absorbed or transmitted backward towards the bird’s body, thus minimizing or canceling out their effects.

Fig. 4.54 Two extremes of wave interaction. (A) Constructive interference occurs when two waves of equal frequency rise and fall at the same time (occuring “in phase”); their energy combines and the amplitude doubles. (B) Conversely, when two waves of equal magnitude occur in exact opposition (one wave hits its peak while the other hits its valley), they cancel each other out. This is called destructive interference. (Adapted from Haade, http://wikimediafoundation.org/wiki/File:Interference_of_two_waves.svg. CC‐BY‐SA 3.0.)
Structural blues
Structural blues are produced when the air bubbles within the feather’s keratin matrix are found in a specific spatial distribution, termed quasi‐ordered (Fig. 4.55). When the air bubbles within the keratin are distributed this way, they scatter only the shortest wavelengths of light back out, and they scatter specific wavelengths back out in phase, thus reinforcing and intensifying them. Colors produced in this way are limited to those at the short end of the spectrum, and thus are primarily blues and frequencies in the UV end of the spectrum that humans cannot see.

Fig. 4.55 Quasi‐ordered keratin and visible light. (A) The electric‐blue color of this Plum‐throated Cotinga (Cotinga maynana) is purely structural. (B) In this species, the quasi‐ordered arrangement of keratin and air spaces—shown here at a microscopic level—is isotropic (uniform in all directions), scattering light in a way that produces these particular blue wavelengths.
(A, photograph by Félix Uribe. B, from Noh et al. 2010. Reproduced with permission from John Wiley and Sons.)
It is worth noting that pigments are often used in conjunction with structural colors in bird plumage. For example, black and dark brown melanins are often used as background layers under structural blue feather surfaces. As such, these pigments absorb unwanted colors of light that otherwise would wash out the purer structural colors that are being isolated and reinforced. Alternatively, colorful carotenoid pigments are often combined with structural colors to create hues of color that neither carotenoids nor structure can make by themselves. For instance, in a pet store you might see Budgerigars (Melopsittacus undulatus) of different colors. Wild‐type (green) Budgerigars have a matrix of keratin and air that scatters blue wavelengths, sandwiched between a top layer of yellow pigment and a bottom layer of melanin (Fig. 4.56A). To produce a saturated green, the yellow pigment reflects and emits yellow wavelengths while the blue wavelengths are reflected by the scattering matrix, and the melanin absorbs whatever other wavelengths make it through the first two layers. The other Budgerigar color morphs occur when one or more of these elements is missing. Blue Budgerigars lack the yellow pigment layer, allowing the reflection of the blue wavelengths (Fig. 4.56B); yellow Budgerigar feathers lack both the coherently scattering blue layer and the bottom melanin layer (Fig. 4.56C). The absence of both pigments, which leaves only the incoherent scattering layer, results in white feathers (Fig. 4.56D).

Fig. 4.56 Budgerigar (Melopsittacus undulatus) color morphs. (A) Three elements create the green color in the barbs of wild‐type Budgerigar feathers: coherent scattering of blue‐green frequencies via the keratin–air matrix, yellow pigment embedded in the keratin (above), and a layer of melanin on the underside of the barb. A change in any of these properties results in a different color morph. (B) Blue Budgerigar feathers maintain the structural components of the wild‐type, but lack the yellow pigment. (C) Pure yellow morphs maintain the top layer of yellow carotenoids, but lack the coherently structured blue layer and melanin layer below. (D) White Budgerigar feathers lack the yellow pigment, the coherent blue, and bottom melanin layers.
(Photographs by: A, Benjamint444, https://commons.wikimedia.org/wiki/File:Budgerigar‐male‐strzelecki‐qld.jpg. GFDL 1.2; B, Isidro Vila Verde; C, Kelsey Lee; D, Brandi Brussel.)
Structural blues in feathers have some distinctive observable properties. With a microscope you can see that the keratin–air arrays that make the blue are found only in the rami of feathers (not the rachis or barbules). When lit from underneath (the ventral surface), a blue feather will look brownish because the melanin layer underlying it filters out much of the light before it can reach the “blue” structures. This direction‐dependent effect is not seen in a pure pigment‐produced color: if a pigment‐produced yellow feather is held up to light, it still looks yellow, because the color is produced by pigments, which are not directionally sensitive.
Iridescence
Informally, observers of birds recognize iridescence as a certain set of optical properties—usually, bright and intense colors, often with a metallic shininess, and often more intense if viewed from a certain direction. Formally, however, the most defining property of iridescence is that the exact hue (Box 4.04) of color observed shifts depending on the angle at which it is viewed. Thus, the colors of the “eyes” of a peacock’s plumes, the shine of a sunbird gorget, and the sheen on the back of many black birds shift hue with the angle of view. As often seen in hummingbird gorgets, hues can switch abruptly by going from a bright, saturated color to the complete absence of color, or black. In optical physics, iridescence is defined as a phenomenon created by specific micro‐ or nanostructures. In birds, the nanostructures necessary to create iridescent colors are produced only inside barbules by various combinations of keratin, air, and melanin.
Iridescence is typically produced when the melanin inside the feather barbules is arranged in more highly ordered configurations than the quasi‐ordered air‐in‐keratin matrix used to produce structural blues. In principle, any wavelength of light (not just blue) can be reflected and reinforced simply by varying the spacing of these melanin structures. This allows for the diversity of iridescent colors observed among and within species of birds. While the arrangement of melanosomes can vary greatly, all conform in some way to a geometric system of layers, and they always occur in the barbules of the feather. The layered or stacked structures of melanin near the surface of the barbules force the incoming light not only to interfere constructively to intensify certain colors (as with the isotropic blues discussed above) but also to do so in such a way that, as an observer’s angle changes relative to the barbule surface and light source, the distance the light travels between the reflecting layers effectively changes, and therefore the hue of the color that is reinforced changes accordingly.
Because iridescence has evolved numerous times among different groups of birds, there is quite a bit of nanoscale morphological diversity underlying the phenomenon. Consequently, variation in the hue and intensity of iridescence can produce a subtle purple‐blue or green sheen via a thick film of keratin over a layer of melanin (Fig. 4.57A) or a display of intense and saturated colors created by many layers of melanin organized in “quarter wave stacks” (Fig. 4.57B). However, the basic principle is always the same: to use the internal nanostructural geo‐metry to generate refraction and interference that separates and reinforces only a subset of wavelengths of light. An added condition is that the subset of wavelengths that is reinforced changes with the relative angle between the surface of the object, the light source, and the viewer. The glistening and shimmering metallic effect typically associated with iridescence is an almost incidental by‐product of the very smooth surface and subsurface structures generally needed to generate iridescence.
Fig. 4.57 Nanostructural varieties of iridescence. (A) Light refracts through a thick matrix of keratin, situated above a layer of melanin, to generate the midnight‐blue sheen in the neck and breast feathers of this Black Grouse (Tetrao tetrix). (B) Layers of melanin organized in “quarter wave stacks” reflect light at different angles, creating the spectacular rainbow iridescence of this Himalayan Monal (Lophophorus impejanus).
(Photographs by: A, Conrad Mackinnon; B, Dibyendu Ash, https://commons.wikimedia.org/wiki/File%3AHimalayan_Monal_Adult_Male_East_Sikkim_Sikkim_India_11.05.2014.png. CC‐BY‐SA 3.0.)
4.8 Feather and plumage texture: light at the scale of barbules, barbs, and whole feathers
The appearance of the plumage involves more than just the color of the feathers. Sun glinting off the wing of a jet‐black raven can appear almost like white patches. Sun reflecting off a European Starling’s (Sturnus vulgaris) “black” breast might have a purple, blue, or green iridescent hue. In contrast, no sunlight at all seems to reflect off from the velvety black breast feathers of a Carola’s Parotia (Parotia carolae) of New Guinea, no matter the angle from which they are examined. These variations in black birds can be seen at relatively far viewing distances. Closer at hand—at the distances at which birds often interact with one another—there is often even more to see, and these effects can be quite spectacular. Brilliant reds can be very different, from the plastic‐looking, opaque reds of the Cut‐throat (Amadina fasciata) of Africa to the glassy, transparent reds of the Black‐necked Red‐Cotinga (Phoenicircus nigricollis) of Amazonian South America. Some feathers, even when uniformly colored or patterned, form a plumage that looks distinctly scaled or plated, as in the African Emerald Cuckoo (Chrysococcyx cupreus), whereas in other birds individual feathers appear to merge together and create a continuous silky surface over the entire bird, as in the Purple Glossy‐Starling (Lamprotornis purpureus) of Africa (Harvey et al. 2013).
What causes these differences in feather textures? To answer this question, we need to consider the effect of the surfaces of the rachis, barbs, and barbules of feathers—structures that are large relative to the nanostructures of pigments and structural colors.
Plumulaceous feathers invariably create an overall soft, fluffy appearance, like that of a downy chick. Their relatively fine barbs and barbules are long, loose, and therefore are not aligned or organized so that light can reflect from them in any structured way. Light scatters equally in all directions, and thus there is no shininess or directionality to it.
One variation of a soft, downy, look can be caused by a different mechanism: a velvety feather surface in adult birds can be created by exceptionally long, upright, distal barbules sticking out from the otherwise flat surface of a pennaceous feather vane. One great example of this is evident in the velvety‐appearing feather surfaces of owl feathers (Fig. 4.32). If you examine them very closely with a magnifying glass, you can actually see these barbule hairs coating the feather surface like a sea of grass. Although evolved to silence the owl’s flight, the visual effect is to reduce the glossiness of the feather. Many of the black birds‐of‐paradise, on the other hand, have broad areas of velvety black feathers with similarly protruding barbules capturing any and all light sent in their direction. This light‐absorbing background is used to set the stage for their showy iridescent ornaments.
A hairy appearance can be created in various ways. For example, the New Zealand kiwis have feathers with relatively long, thin rachi and very narrow vanes created by short barbs. Alternatively, the seemingly hair‐like neck feathers of many herons have relatively stiff, open pennaceous vanes in which the barbules are so reduced as to leave the barbs running parallel but free from one another. In these cases, the barbs of the vane are stiff enough that they lie relatively parallel to one another even without barbules to hold them together. The parallel‐lying fibers then create a hair‐like appearance.
At the other end of the continuum, birds can use barb and barbule orientations and surface shape to create almost mirror‐like reflections of light. An overall glossy or shiny effect can be generated in various ways, including by a smooth planar rachis and/or barb surfaces in a firm pennaceous feather. Rachi in general usually have a very smooth surface; thus, when they are viewed at a particular angle, light often reflects off the surface of the rachi in a shiny manner—the wider the rachis, the stronger this effect. Likewise, broader, flatter barb surfaces can generate a feather that is more likely to reflect light in a shiny way. For that reason, the wings and tails of birds tend to appear shinier than other parts of their bodies, where the larger feathers have broader rachi and barbs.
A suite of other feather textures and appearances exists: transparent versus opaque, glassy versus plastic‐like, scaled versus silky. All are caused by variations in the rachi, barb, and barbule surfaces and internal structures, but many of these are only beginning to be studied. Finally, although individual feathers contribute enormously to the apparent surface texture of birds, all the feathers constituting a plumage ultimately overlap and thus can interact with one another to create a great diversity of visual effects.
4.9 Visual functions of plumage
Coloration and patterning play important roles in the behavioral ecology of birds. All animals must strike an evolutionary balance between hiding from predators and being conspicuous for social signaling. For many animals, concealment is prioritized over advertising. Thus, just as the earthy, cryptic colors of many mammals, reptiles, and amphibians make them relatively inconspicuous, so too, many birds sport brown, gray, and olive‐green tones that render them harder to see. On the other hand, many birds are brightly colored, perhaps because flight makes them less vulnerable to predators, perhaps because bright colors are concealing in some habitats (in sunlit, fruit‐ and flower‐laden forest canopies, for instance), or perhaps because colors are so crucial for birds’ social interactions. Birds’ plumages declare the identity of their species, their age and status as individuals, advertise their health, and are the centerpieces of a lot of sexual selection (Chapter 3). The inherent ability of feathers to form complex colors and patterns, when coupled with the social and communicative nature of birds themselves, has made birds some of the most ornate animals on earth.
4.9.1 Predator avoidance
Predation is a significant threat for most birds, and avoiding predation appears to be the main reason so many birds have evolved cryptic coloration. There are numerous ways for a bird to create crypsis, or patterning that generally causes an animal to blend in with its background.
Many birds use background matching: their plumages include colors and patterns that mimic the substrates on which they are most often found. The mottled patterns on birds of the scrub or forest floor are excellent examples; the woodcocks of the northern hemisphere, button‐quails of Australia, and many other birds worldwide resemble the leafy litter in which they are frequently found. Nearly all shorebirds, from sandpipers to curlews, have mottled brownish backs that conceal these ground nesters during incubation as well as during foraging on beaches, mud flats, or in grasses (Chapter 11). Worldwide, birds of grasses and reeds—the bitterns, many Old World warblers and Old World buntings, most New World sparrows, and the cisticolas of Africa—often have patterns with many shades of brown arranged in long streaks to help them blend into the stripy vegetation they live within. The northern‐dwelling ptarmigans molt from their mottled‐brown summer plumage to a coat of pure white that blends with heavy snow cover in winter; similarly, the Snowy Owl (Bubo scandiacus), Snow Goose (Chen caerulescens), and Snow Bunting (Plectrophenax nivalis) of the arctic tundra all wear snow‐matching white plumages. Even birds that look gaudily colored in pictures may blend perfectly into their natural surroundings; many birders visiting the tropics have had the humbling experience of scrutinizing a well‐lit, bright green, and visually “birdless” tree that is nonetheless squawking with birds, before finally glimpsing one well‐camouflaged leaf‐green parrot just as the flock of a hundred takes off!
Some birds use disruptive coloration. Like the camouflage of military and hunting gear, disruptive plumage coloration uses larger patches, streaks, or other bold color patterns to break up the outline of the bird—distracting an observer from noticing the whole bird as one form (Fig. 4.58A). For example, the bold, black neckbands of many plovers make it more difficult to distinguish the whole bird as one form (Fig. 4.58B). Similarly, the eyelines—lines of feathers above or through the eye that contrast with the base head color—found in so many birds are thought to help distract from their otherwise conspicuous dark, round eyes, by either drawing out the round eye with a dark lengthening line extending back from it, or with a brighter, usually white, line above the eye. Disruptive coloration provides the added advantage of camouflage on a variety of backgrounds.

Fig. 4.58 Two types of disruptive coloration. (A) This Egyptian Nightjar’s (Caprimulgus aegyptius) plumage—with its irregular steaks, mottled blotches, and general hue—almost perfectly matches its surroundings (arrow denotes the virtually indistinguishable nestling beneath the adult). Camouflage is essential for this species, which nests in exposed rocky areas. (B) The neckbands of this Madagascar Plover (Charadrius thoracicus) break up the outline of its body, disrupting the focus of an onlooker. Different areas of its body also blend in with the landscape: its belly and face match the sand, while its back and head match the vegetation.
(Photographs by: A, “K K,” https://commons.wikimedia.org/wiki/File%3ACaprimulgus_aegyptius.jpg. CC‐BY‐SA 3.0; B, © bryanjsmith.)
Employing another strategy to minimize detectability, many birds are hard to see because they are darker on top than below, a pattern known as countershading. Many bird species, including smaller plovers, sandpipers, and songbirds, exhibit this pattern. With the darker back lit by the bright sun overhead and the lighter underparts in the shade of the bird’s own body, the bird appears to be nearly one color, and until it moves, predators have difficulty seeing it as a three‐dimensional object. In addition, white underparts may reflect the color of the ground, producing even more effective camouflage. Countershading is especially widespread in open‐country birds that are vulnerable to distant predators hunting by sight. It also is common in birds that spend much time on or flying above water, such as loons, grebes, ducks, auks, gulls, terns, and many pelagic birds (Fig. 4.59).

Fig. 4.59 Adaptive function of countershading. A Great Black‐backed Gull (Larus marinus) attacks a Ring‐necked Duck (Aythya collaris), thanks in part to countershading: from below, the gull’s plumage (dorsally dark, ventrally light) blends into the white sky, camouflaging its presence and movements.
(Photograph by Ajay Sequeira.)
Birds may further increase the benefits of cryptic plumage by using behaviors that add to their crypsis. Simply holding still is a good way to avoid attracting the attention of predators. By freezing and flattening against the beach, young terns and gulls eliminate their shadows and look just like bumps on the sand. The nocturnal potoos of the neotropics complement their mottled brown‐and‐gray plumage with behavior; they spend most of the day just sitting, often perched on the limb of a dead tree, with neck stretched up and large eyes closed, looking like an extension of the limb. Many small owls adopt similar stick postures. The strategy of remaining relatively still also works for many predators, such as herons prospecting for fish or frogs along a shoreline. In some contrasting cases, subtle movement can actually enhance concealment. For example, bitterns—which accentuate their streaked pattern by standing in a marsh with their beaks pointed skyward—will sway slightly from side to side with the surrounding reeds as the wind blows.
In a creative twist regarding the use of behavior to enhance concealing plumage, many birds have conspicuous tail or wing markings that draw attention only once the bird flies off. The barred black‐and‐tan upperparts of New World flickers and the black‐and‐white upperparts of the Eurasian Hoopoe (Upupa epops) are easily overlooked against the ground on which they forage. Yet in flight, the bright yellow wings and white rump of the flicker and the bold, black‐and‐white wing pattern of the Eurasian Hoopoe are visually striking (Chapter 14). When such attention‐grabbing plumage patterns are revealed suddenly, they may startle an approaching predator. Similarly, many passerines have distracting contrasting (usually white) outer tail feathers or feather tips that may cause a predator to hesitate or to strike the bird’s tail and miss the primary target of its body. Because these conspicuous markings can be made to disappear when the bird lands, suddenly concealing the bird’s position, they may further confuse predators that had focused primarily on the bold markings during pursuit.
Predation avoidance via feathers is not restricted to their appearance: at least one group of birds sequesters toxins in its feathers that make them distasteful—and potentially even poisonous—to predators (Box 4.06).
One final note on predation and avian coloration: bear in mind that different predators have different visual capabilities, so one cannot assume that all prey appear as conspicuous or as cryptic to predators as they do to humans. For instance, most mammalian predators and nocturnal avian predators (such as owls) lack color vision. Many bright yellow or red birds thus may be relatively inconspicuous to these predators, which see the colors as shades of gray. On the other hand, hawks, eagles, and other avian predators that are active by day generally have full color vision including some UV wavelengths. Thus, markings on a bird may be cryptic for one kind of predator but not for another.
4.9.2 Social recognition and signaling
Birds use external appearance in the form of color and pattern to distinguish species, age classes, sexes, and/or individuals. These forms of recognition are outlined below.
Many of the most important interactions in the life of a bird occur between an individual and its conspecifics, the other members of its own species, including its parents, offspring, potential mates, and competitors. At a basic level, birds must recognize conspecifics to choose an appropriate mate and/or exclude appropriate competitors. Plumage, along with other signals and cues such as song and behavior, plays an obvious role in distinguishing the relevant parties.
It generally is true that individuals who mate solely with their own species likely will be more successful at producing young, and this tendency will be favored strongly by natural selection (Chapter 3). As a result, the appearance of an individual can play a role in species recognition, literally the ability of members of a species to recognize other members of their own species. However, whether species recognition has been a driving force of plumage evolution per se is a harder question to answer. That is, although researchers have long assumed that both color and pattern function in species recognition, devising experiments that clearly demonstrate a selective role in species recognition is difficult, and few supporting data exist for this idea (Mendelson and Shaw 2012). For instance, when conspicuous species‐specific markings, such as the red epaulettes of the male Red‐winged Blackbird (Agelaius phoeniceus) of North America (Chapter 9), are experimentally covered, the sexes nevertheless continue to establish pairs (Searcy and Yasukawa 1983), and these experimentally altered males are recognized as conspecifics by other males. Clearly, species recognition involves a variety of cues acting together; songs and behavior play key roles, and species‐specific color patterns also influence how conspecifics treat one another.
Likewise, patterns exhibited in different plumages may be used for age recognition, the ability of individuals to distinguish between age classes. Many birds have distinctive subadult plumages before they become sexually mature. Further, in many species the males continue to wear these subadult plumages for one or more years after they are capable of reproducing. Territorial adult males are often more tolerant of males in subadult plumages on their territories than they would be of definitive males. Additionally, given a choice, females usually select mates in their adult, definitive plumages. Yet it can be difficult to assess whether these interactions are mediated solely on the basis of the age‐indicating plumage itself, rather than some other indicators of age.
Perhaps more critically, plumage coloration likely plays a role in sex recognition: ascertaining the gender of a conspecific. Many species of birds show sexual dimorphism (Chapter 3); males and females are distinctive from one another in some aspect of their morphology. Sexual dichromatism, in which one sex is colored differently from the other, is a particularly common form of sexual dimorphism in birds. To a bird looking for a mate, knowing the sex of another individual is key, but sex recognition also is critical in numerous other social interactions, including defending resources, territories, or mates. In species in which the sexes differ dramatically in appearance, sex recognition is likely straightforward. However, plumage differences between sexes can be very subtle. In many woodpeckers, for example, the amount of red on the head reveals the sex (Fig. 4.60). In one classic experiment, a researcher painted malar stripes onto female woodpeckers that made them look more like males, and their mates then chased them away (Noble 1936).

Fig. 4.60 Sexual dichromatism. Male and female White‐backed Woodpeckers (Dendrocopos leucotos) can be distinguished by their head color; males have red crowns, while female crowns are black.
(Photograph by Koichi Saito.)
Note that birds do not necessarily require different plumages to recognize the opposite sex. Even when UV reflectance is taken into account, many songbirds are monomorphic, or have the same plumages in both sexes. In these species, males and females likely recognize each other by some other cue, such as song or behavior. Therefore sexual differences in plumage may arise for a variety of reasons other than sex recognition, including the different roles played by each sex in courtship, nest attendance, and territorial defense, for example. Sexual selection—the differential fitness that arises from competition within and between the sexes—also plays an important role in creating sexual dimorphism. Along with predation, sexual selection has been a predominant force driving the evolution of the incredible diversity of avian plumage (Chapter 3).
Lastly, birds use plumage differences for individual recognition, because many birds clearly recognize other individual birds, especially their mates. How do they do so? Birds probably identify familiar individuals the same way humans recognize other people, by subtle differences in color, head and body shape, posture, and voice. Several studies have demonstrated that birds use color and pattern to recognize individuals. When Ruddy Turnstones (Arenaria interpres)—arctic‐breeding shorebirds with highly variable plumage—were shown models that mimicked their neighbors or strangers, they reacted aggressively only toward the strangers (Whitfield 1986), strong evidence that they could distinguish their neighbors by appearance alone. In addition, several colonially nesting species, including Ring‐billed Gulls (Larus delawarensis) in North America, can pick their own young from a crowd of young by their facial markings.
Visit birdbiology.org for many online resources on the topics covered in this chapter.
References
- Ammann, G. A. 1937. Number of contour feathers of Cygnus and Xanthocephalus. Auk 54:201–202.
- Bostwick, K. S., D. O. Elias, A. Mason, and F. Montealegre‐Z. 2010. Resonating feathers produce courtship song. Proceedings of the Royal Society B: Biological Sciences 277:835–841.
- Bostwick, K. S. and R. O. Prum. 2003. High‐speed video analysis of wing‐snapping in two manakin clades (Pipridae: Aves). Journal of Experimental Biology 206:3693–3706.
- Bostwick, K. S. and R. O. Prum. 2005. Courting bird sings with stridulating wing feathers. Science 309:736.
- Bostwick, K. S., M. L. Riccio, and J. M. Humphries. 2012. Massive, solidified bone in the wing of a volant courting bird. Biology Letters 8:760–763.
- Brown, R. E. and M. R. Fedde. 1993. Air‐flow sensors in the avian wing. Journal of Experimental Biology 179:13–30.
- Clark, C. J. 2009. Courtship dives of Anna's hummingbird offer insights into flight performance limits. Proceedings of the Royal Society B: Biological Sciences 276:3047–3052.
- Clark, C. J. and T. J. Feo. 2008. The Anna's hummingbird chirps with its tail: a new mechanism of sonation in birds. Proceedings of the Royal Society B: Biological Sciences 275:955–962.
- Clayton, D. H. 1991. Coevolution of avian grooming and ectoparasite avoidance. Pages 258–289 in Bird–Parasite Interactions: Ecology, Evolution and Behaviour (J. E. Loye and M. Zuk, Eds.). Oxford University Press, Oxford.
- Conover, M. R. and D. E. Miller. 1980. Rictal bristle function in Willow Flycatcher. Condor 82:469–471.
- Cunningham, S. J., M. R. Alley, and I. Castro. 2011. Facial bristle feather histology and morphology in New Zealand birds: implications for function. Journal of Morphology 272:118–128.
- Darwin, C. 1871. The Descent of Man, and Selection in Relation to Sex. John Murray, London.
- del Hoyo, J., A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana (eds.) 2016. Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
- Dumbacher, J. P., B. M. Beehler, T. F. Spande, H. M. Garraffo, and J.W. Daly. 1992. Homobatrachotoxin in the genus Pitohui: chemical defense in birds? Science 258:799–801.
- Dumbacher, J. P., K. Deiner, L. Thompson, and R. C. Fleischer. 2008. Phylogeny of the avian genus Pitohui and the evolution of toxicity in birds. Molecular Phylogenetics and Evolution 49:774–781.
- Glenn, T. C., J. O. French, T. J. Heincelman, K. L. Jones, and R. H. Sawyer. 2008. Evolutionary relationships among copies of feather beta (β) keratin genes from several avian orders. Integrative and Comparative Biology 48:463–475.
- Grémillet, D., C. Chauvin, R. P. Wilson, Y. Le Maho, and S. Wanless. 2005. Unusual feather structure allows partial plumage wettability in diving great cormorants Phalacrocorax carbo. Journal of Avian Biology 36:57–63.
- Harvey, T. A., K. S. Bostwick, and S. Marschner. 2013. Directional reflectance and milli‐scale feather morphology of the African Emerald Cuckoo, Chrysococcyx cupreus. Journal of the Royal Society Interface 10:20130391.
- Hingee, M. and R. D. Magrath. 2009. Flights of fear: a mechanical wing whistle sounds the alarm in a flocking bird. Proceedings of the Royal Society B: Biological Sciences 276:4173–4179.
- Howell, S. N. G. and C. Corben. 2000. A commentary on molt and plumage terminology: implications from the Western Gull. Western Birds 31:50–56.
- Howell, S. N. G., C. Corben, P. Pyle, and D. I. Rogers. 2003. The first basic problem: a review of molt and plumage homologies. Condor 105:635–653.
- Humphrey, P. S. and K. C. Parkes. 1959. An approach to the study of molts and plumages. Auk 76:1–31.
- Jacob, J. and V. Ziswiler. 1982. The uropygial gland. Pages 199–314 in Avian Biology, Vol. 6 (D. S. Farner, J. R. King, and K. C. Parkes, Eds.). Academic Press, New York, NY.
- Ji, Q., M. A. Norell, K. Q. Gao, S. A. Ji, and D. Ren. 2001. The distribution of integumentary structures in a feathered dinosaur. Nature 410:1084–1088.
- Li, Q., K. Q. Gao, Q. Meng, J. A. Clarke, M. D. Shawkey, L. D’Alba, R. Pei, M. Ellison, M. A. Norell, and J. Vinther. 2012. Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335:1215–1219.
- Li, Q., K. Q. Gao, J. Vinther, M. D. Shawkey, J. A. Clarke, L. D'Alba, Q. Meng, D. E. G. Briggs, and R. O. Prum. 2010. Plumage color patterns of an extinct dinosaur. Science 327:1369–1372.
- Lucas, A. M. and P. R. Stettenheim. 1972. Avian Anatomy: Integument (Agriculture Handbook 362). US Department of Agriculture, Washington, DC.
- Mendelson, T. C., and K. L. Shaw. 2012. The (mis)concept of species recognition. Trends in Ecology and Evolution 27:421–427.
- Mulvihill, R. S., K. C. Parkes, R. C. Leberman, and D. S. Wood. 1992. Evidence supporting a dietary basis for orange‐tipped rectrices in the Cedar Waxwing. Journal of Field Ornithology 63:212–216.
- Murphy, M. E., B. T. Miller, and J. R. King. 1989. A structural comparison of fault bars with feather defects known to be nutritionally induced. Canadian Journal of Zoology 67:1311–1317.
- Necker, R. 1985. Observations on the function of a slowly‐adapting mechanoreceptor associated with filoplumes in the feathered skin of pigeons. Journal of Comparative Physiology A: Sensory, Neural and Behavioral Physiology 156:391–394.
- Noble, G. K. 1936. Courtship and sexual selection of the Flicker (Colaptes auratus luteus). Auk 53:269–282.
- Noh, H., S. F. Liew, V. Saranathan, S. G. J. Mochrie, R. O. Prum, E. R. Dufresne, and H. Cao. 2010. How non‐iridescent colors are generated by quasi‐ordered structures of bird feathers. Advanced Materials 22:2871–2880.
- Norell, M., Q. Ji, K. Gao, C. Yuan, Y. Zhao, and L. Wang. 2002. Palaeontology: 'modern' feathers on a non‐avian dinosaur. Nature 416:36–37.
- Prum, R. O. 1998. Sexual selection and the evolution of mechanical sound production in manakins (Aves: Pipridae). Animal Behaviour 55:977–994.
- Prum, R. O. 1999. Development and evolutionary origin of feathers. Journal of Experimental Zoology 285:291–306.
- Schweitzer, M. H. 2001. Evolutionary implications of possible protofeather structures associated with a specimen of Shuvuuia deserti. Pages 181–192 in New Perspectives on the Origin and Early Evolution of Birds (J. Gauthier and L. F. Gall, Eds.). Yale Peabody Museum, New Haven, CT.
- Searcy, W. A. and K. Yasukawa. 1983. Sexual selection and red‐winged blackbirds. American Scientist 71:166–174.
- Simmons, K. E. L. 1986. The Sunning Behaviour of Birds. Short Run Press, Exeter.
- Turner, A. H., P. J. Makovicky, and M. A. Norell. 2007. Feather quill knobs in the dinosaur Velociraptor. Science 317:1721.
- Vinther, J., D. E. G. Briggs, J. Clarke, G. Mayr, and R. O. Prum. 2010. Structural coloration in a fossil feather. Biology Letters 6:128–131.
- Vinther, J., D. E. G. Briggs, R. O. Prum, and V. Saranathan. 2008. The colour of fossil feathers. Biology Letters 4:522–525.
- Wan, X., J. Fan, and H. Wu. 2009. Measurement of thermal radiative properties of penguin down and other fibrous materials using FTIR. Polymer Testing 28:673–679.
- Wetmore, A. 1936. The number of contour feathers in passeriform and related birds. Auk 53:159–169.
- Whitfield, D. P. 1986. Plumage variability and territoriality in breeding turnstone Arenaria interpres: status signaling or individual recognition? Animal Behaviour 34:1471–1482.
- Xu, X., X. Zheng, and H. You. 2009. A new feather type in a nonavian theropod and the early evolution of feathers. Proceedings of the National Academy of Sciences of the United States of America 106:832–834.
- Xu, X., Z. Zhou, X. Wang, X. Kuang, F. Zhang, and X. Du. 2003. Four‐winged dinosaurs from China. Nature 421:335–340.
- Yu, M., P. Wu, R. B. Widelitz, and C. M. Chuong. 2002. The morphogenesis of feathers. Nature 420:308–312.
- Zhang, F., S. L. Kearns, P. J. Orr, M. J. Benton, Z. Zhou, D. Johnson, X. Xu, and X. Wang. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463:1075–1078.