The endocannabinoid system (ECS) has only recently begun appearing in anatomy and biology textbooks. We are just beginning to understand the ECS, how it functions, and how it benefits us. Our understanding of the ECS is like a mosaic we are assembling over time. As more of the pieces come together, our understanding grows of how the ECS and cannabis fit into our health and well-being. The mosaic is filling in quite rapidly, considering we didn’t know this system existed 25 years ago.
The ECS sets the baseline tone of well-being in which an organism (you) operates. When healthy, the ECS creates a background signal that all is well and quietly hums as the other systems go about their daily jobs, including the loud and fast branches of the nervous system, the quiet and steady heartbeat, the ever-aware immune system, the slow and steady endocrine system, the intelligent and interactive digestive system, and the hot-blooded reproductive system. Think of the ECS as the wizard behind the curtain, keeping you calm and centered; it is the basal system behind all other systems.
This integral and integrated system influences every system in the body, in both health and disease. It is so important that it has been evolutionarily conserved throughout all three kingdoms of life: plant, animal, and fungi (insects are the only major phyla who do not have the ability to interact with cannabinoids). Despite the fact that the ECS is so ancient, it was discovered only in the 1990s.
The ECS is physiological like the immune system, not anatomical like the cardiovascular or digestive system. While we can trace a blood vessel through the organs of the cardiovascular system or track a bite of food through the digestive system, the endocannabinoid system is the collective functioning of all cells in the body that can either make endocannabinoids or have receptors sensitive to endocannabinoids. At a cellular level, the endocannabinoid system operates like the endocrine system: chemicals are made by cells, then travel to and bind with receptors on other cells to produce a certain outcome.
The chemicals made by the ECS are called endocannabinoids. “Endo” means “within” or “internal.” Our endocannabinoid system is our own internal cannabis system. Cannabinoids are chemicals produced by cannabis that can bind to ECS receptors. If we can learn how endocannabinoid molecules bind to receptors, and learn about the actions such binding stimulates, we can understand how THC, CBD, and other chemical constituents of cannabis affect our bodies.
The bodily effects of consuming the cannabis plant are caused by cannabis constituents interacting with our endocannabinoid system. Plant molecules enter our bodies through our nose, lungs, skin, or digestive system. They then travel to and bind with cannabis receptors, an action that causes myriad physical reactions and sensations. Cannabis receptors permeate nearly every tissue in the body, so cannabis can interface with and cause changes through the whole body. No other plant shares such an intimate chemical relationship with us.
The ECS is complex, like a branching, weblike mycelial network. When looking at the ECS, we must think beyond the one chemical/one receptor/one reaction model; the ECS is a multifaceted, interconnected bodywide cellular network. As we understand this system more and more, we can develop truly integrated methods of approaching health and healing.
Dr. Raphael Mechoulam is credited as the “grandfather” of the endocannabinoid system. An Israeli organic chemist and head of the medicinal chemistry lab at the Hebrew University of Jerusalem in Israel, Mechoulam was among the first to isolate specific cannabinoids (including the main psychoactive constituent of cannabis, delta 9-THC) and elucidate their structures. He discovered the first cannabinoid receptor, called CB1, in 1988 when he was trying to understand how THC worked in the body. He realized that when people consumed cannabis, THC bound to the CB1 receptors in our bodies, causing the blissful feeling of well-being. He wondered if our bodies made a similar chemical of its own that would also bind to the receptor. In 1992, he discovered arachidonoyl ethanolamide (AEA), the body’s own version of THC. AEA was nicknamed “anandamide” by Raphael’s team because ananda means “bliss” in Sanskrit. AEA is our very own bliss molecule!
Mechoulam dubbed the ECS a “global protection system” because the general strategy of the ECS is to help cells, tissues, and organs reestablish a steady state of balance after acute or chronic disruptions, a condition called an “allostatic state.”
Our baseline feeling of safety and well-being is the product of our endocannabinoid system. When this system functions properly, our nervous, endocrine, and immune systems are in safe mode. They’re not sounding the alarm but simply going about their jobs; all is well. Healing, repair, digestion, and reproduction function optimally and we go about our day. Our emotional and psychological states also reflect our “all is well” status, and we experience a sense of well-being and curiosity. This baseline state is the most energy-efficient mode of operating. Animals operate in this mode except when they are chasing or being chased. A problem we humans often face is that as far as our nervous system is concerned, we feel like we are chasing or being chased all day long. It doesn’t matter if it’s actually happening or not — our brains treat it all as real (go ahead and check your pulse while watching a scary movie).
To get a feel for the ECS, let’s go on a little journey to Grandmother’s house. It’s been a long and tiring day, and the sun is going down. A cold wind kicks up, and a driving rain seeps under your collar as you trudge through the gray evening. The sidewalk is crumbling, and a car whips through a puddle and splashes you. Finally you make it to the house. You open the door and walk into a burst of warmth from the fireplace. You can smell a steaming mug of hot chocolate waiting by the fire. You peel off layers of wet outer clothing and sit in a soft chair. Grandma hands you a mug of hot chocolate, drops in a few marshmallows, and looks at you with love. “How are you doing today, my dear?” You are at home; you are safe.
This is a lovely simplification, yes, but it illustrates the complexity of our autonomic nervous system. This system has three branches: the sympathetic (which governs our “fight-or-flight” response), the parasympathetic (which handles rest and digestion), and the enteric (which innervates the gut). These three branches work together to operate all unconscious bodily activities: heartbeat, peristalsis of the digestive system, breathing, pupil dilation, and so on. Thankfully, these functions run automatically, below our conscious level of awareness. The endocannabinoid system operates behind the autonomic nervous system and sets the baseline tone.
If I were to interview a person to determine the health of their ECS, I would begin by asking questions like:
We should be able to answer positively to all of these questions. This state of well-being is our birthright — the baseline we share with all creatures.
In healthy human bodies, the ECS activates in response to injury. Endocannabinoids are released in response to cellular stress in order to reestablish allostasis. The ECS interacts with nearly every cell of the body, and its proper functioning does not require a top-down hierarchy like the nervous system or a centralized chemical factory like the endocrine system. Almost each one of the 50 trillion cells of your body can affect its own health and well-being. We also know that a chronically stressed ECS can become dysfunctional, contributing to pathological conditions.
Before we dive into the biochemical and cellular functions of the endocannabinoid system, let’s address the general functions of the ECS. These include allostasis, protection, perception of well-being, cognition and learning, emotions, feeding, and immunity; the ECS can also act as a reward system. Then we can fill in the mosaic of the ECS at the molecular, receptor, and chemical levels. Understanding how our own internal cannabis system functions will help us understand how cannabis works in us and brings about healing.
Our bodies are able to coordinate 50 trillion cells to keep us healthy and maintain proper body temperature, blood glucose and oxygen levels, heart rate, and breathing rate. When we can’t maintain these parameters, we experience disease or even death. Allostasis is the maintenance of stability through physiological or behavioral change. To reach allostasis, we regulate our body functions as needed to adjust to new or changing environments: when we need to move fast, for example, our heart rate must go up to supply nutrients and oxygen to the muscles that need it. Allostasis (sometimes called homeostasis) is the body’s ability to maintain equilibrium in an ever-changing environment. The endocannabinoid system works to bring about and maintain balance.
The ECS works to reduce inflammation, which is present in all major diseases. The ECS also helps protect against uncontrolled and unregulated cell growth, or cancer (for more on this see Cancer). Pain, which keeps us from hurting ourselves or doing tissue damage, is also regulated by the ECS.
The ECS, with its endocannabinoid “bliss” molecules, signals that all is well. The system also interfaces with other various neurotransmitters (serotonin, dopamine, norepinephrine, and GABA) within the nervous system that are linked to the perception of well-being. A healthy, functioning ECS offers a baseline feeling of safety that allows us to sleep.
The ECS functions within the brain to assist with learning, memory, and expanding consciousness.
Having an overall perception of safety is intricately linked to our feelings of well-being. A healthy ECS decreases anxiety and increases curiosity. When we have a healthy ECS, we are less emotionally reactive, which also has a neurological benefit: we forget to be afraid of situations that are no longer dangerous. People who suffer from PTSD aren’t able to do this. A healthy ECS is also linked to emotional memory and enables us to relax.
The ECS controls feeding behaviors by stimulating our appetite (this is why cannabis gives us the munchies). The ECS also has a hand in controlling cell metabolism and regulating blood sugar levels. Nausea and vomiting are regulated by the ECS in both the brain and digestive lining.
The ECS modulates the immune system by regulating the immune cells themselves (B, T, and natural killer cells). The ECS, like the immune system, also works to decrease inflammation.
Nature gets us to do things like procreate or eat by rewarding us with good feelings via neurotransmitters that hit pleasure centers in our brain and send a signal: Do that again! The endocannabinoid system is directly linked to this neural reward system and plays a role in regulating it.
Before we head into the functions of the ECS by body system, it is helpful to have some of the basic chemical functioning of the system under our belts. We will discuss the chemistry in depth in Chemical Messengers of the ECS. The ECS is made up of (1) chemicals that bind to receptors (for our purposes here, they are AEA and 2AG), (2) receptors that these chemicals bind to (CB1 and CB2), (3) transport molecules for endocannabinoids, and (4) enzymes that create or destroy endocannabinoids. In general, endocannabinoids work by binding to receptors on target cells that have receptors for them, activating the cell to carry out a specific function.
When we understand how a healthy ECS interacts with other body systems, we can begin to understand how the chemicals found in cannabis can help the body achieve a healthier state.
The ECS plays an essential role in early embryonic and prenatal brain development (CB1 receptors have been seen in two-day-old mouse embryos). From our very beginnings, the endocannabinoid system is guiding us home: fertilized human eggs prefer to implant where the uterine lining produces high levels of AEA. Our bliss molecules (AEA) become the chemical flare for the fertilized egg. We implant into bliss! The ECS is deeply involved in many aspects of neural development: proliferation and differentiation of neuronal stem cells, neurodevelopment, creation of functional and effective synapses, orchestration of axonal migration and connection, and modulation of excitatory and inhibitory synaptic transmission in postnatal brain and spinal cord.
The ECS functions within the nervous system to regulate neurogenesis, neural protection, neural plasticity, autonomic tone, stress response, and pain.
The endocannabinoid system is responsible for stimulating growth of new neurons.
When neural tissue (or any tissue really) experiences chemical or physical trauma, it produces AEA and 2AG for protection and forms more CB2 receptors. This enables endocannabinoids to bind to the receptors, which decreases inflammation and prevents neuron damage. Glutamate, an excitatory neurotransmitter, can excite a neuron literally to death. When neurons are damaged by head trauma or a stroke, glutamate production can become unmitigated. The ECS mitigates glutamate excitotoxicity and decreases seizure activity in the brain.
Neuroplasticity is the brain’s ability to reorganize itself by forming new neural connections throughout life. Neuroplasticity enables neurons in the brain to compensate for injury and disease and respond to new situations or changes in the environment. Endocannabinoids stimulate the production of neurons (neurogenesis) in some regions of the brain.
The ECS interacts with all branches of the autonomic nervous system via the countless CB1 receptors on neurons and the neuroglia surrounding them. Within the sympathetic system, endocannabinoids bind to CB1 receptors to slow the release of norepinephrine, a stimulating hormone and neurotransmitter. They also decrease sympathetic mediated pain and modulate the hypothalamic-pituitary-adrenal axis, which is the major pathway for the stress response. Within the parasympathetic nervous system, endocannabinoids bind to receptors to decrease vomiting.
A healthy ECS is key to the body’s ability to adapt to stress and to recover after a stressful event. A healthy ECS helps us mount an appropriate stress response when needed and prevents us from overreacting when it’s not. The ECS also initiates the cessation of the stress response.
Our fight-or-flight stress response is a beautifully orchestrated set of mechanisms that helped our ancestors survive in a tooth-and-claw world. Those who did not have a hypervigilant system didn’t survive an attack, didn’t reproduce, and are no one’s ancestors. Hypervigilance serves us when we are in danger, but it is counterproductive and unhealthy when we are not. The problem is that once our brain has identified a danger, the rest of the body responds. Trying to stop that cascade goes against millions of years of selected-for biology. Given what we know about unmanaged stress causing major disease, the mechanism of the fight-or flight response (and how to mitigate it) warrants further exploration.
How is it possible on one day a loud noise makes you jump and on another day you hardly notice it? The answer is we are able to set and choose the threshold for response. This occurs in the region of the brain known as the amygdala. Alarm reactions begin here. Under stressful conditions, this region compares all incoming information to previous dangerous experiences. If they match closely enough, the amygdala perceives a threat and sounds the alarm. The threshold for sounding the alarm depends on many factors, but an important one is how much influence information from elsewhere has on the amygdala. Information from our higher, conscious brain can override an excited amygdala, calming it down before it goes on full alert. A healthy hippocampus, another brain structure that in part inhibits excitatory responses, can feed the amygdala information without tagging it as dangerous. Ironically, the stress hormone cortisol can shrink the hippocampus; the good news is that when cortisol levels drop, the hippocampus grows. Endocannabinoids binding to CB1 receptors help the hippocampus maintain its size and connections.
The region able to actually override the amygdala is in the higher brain, the prefrontal cortex (affectionately called the “angel lobes,” or the lobes of compassion). Under stressful conditions the amygdala reacts to potential danger before the cortex can process the information, but with practice (creating strong synaptic connections through mindfulness and meditation) the prefrontal cortex can help keep the amygdala from hijacking your emotions. Meditation during nonstressful times strengthens the pathways that will help you keep cool during stressful times.
The heart can also override the amygdala. The heart is neurologically hardwired to every region of the brain, including the amygdala; and the brain can’t shut these signals down. Techniques that focus on breathing and bringing attention and awareness to the heart can help soothe the amygdala during stressful times.
If we constantly perceive the world as threatening, our nervous system remains on high alert. We are neurologically primed to see danger everywhere. Our cortisol levels rise, shrinking the hippocampus and further diminishing our ability to feel secure, and a feedback loop is created.
Corticotropin-releasing hormone (CRH) is a chemical released by the amygdala with one function: to lower AEA levels, allowing or continuing the stress response. Chronic activation by the amygdala lowers AEA levels, which in turn leads to an easier triggering of the fight-or-flight response.
A healthy endocannabinoid system helps to decrease acute stress responses. Healthy AEA levels tamp down stress response in the amygdala, where we perceive threats. When we need to shut down a stress response, rising cortisol levels stimulate an increase in 2AG, diminishing the stress response, extinguishing fear, and enabling us to recover from the stressful event. Endocannabinoid deficiency contributes both to easier activation of the stress response and a diminished ability to recover.
If the ECS is deficient, our ability to perceive threat in a healthy way is diminished; we will overreact and the heightened stress response will continue.
The endocannabinoid system works to decrease pain on multiple levels, beginning at the point of injury all the way up to the perception of pain in the brain. We will more fully discuss the pain pathway in the chapter on conditions (chapter 6), but briefly, the ECS acts to modulate pain at the injury site, nociceptor (pain receptor), immune cells, and central nervous system.
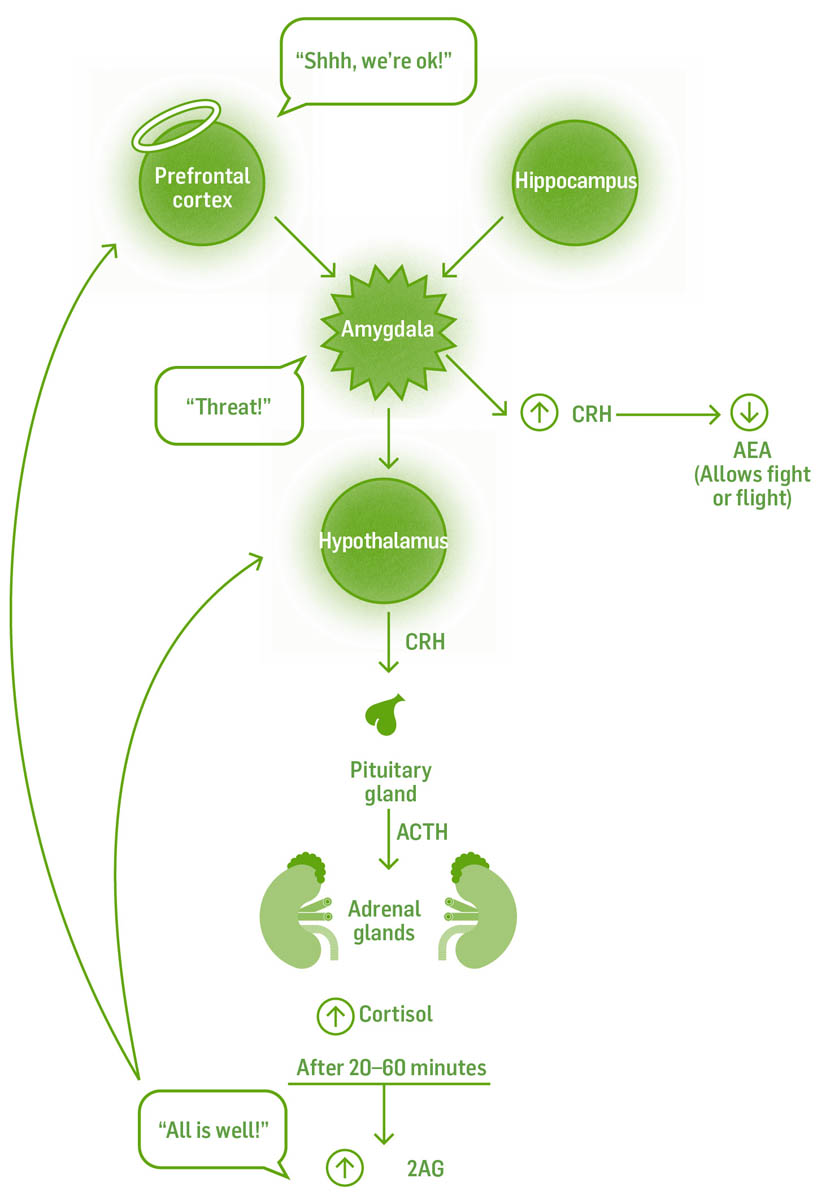
The endocannabinoid system plays a major role in the healthy functioning of the body’s fight-or-flight stress response.
When tissue is injured, cells produce chemicals (including histamine, prostaglandins, bradykinin, norepinephrine, substance P, hydrogen ions, potassium ions, leukotrienes, and adenosine triphosphate) that signal injury and cause the perception of pain. The endocannabinoids 2AG and AEA decrease the production of these chemicals, lessening pain.
When endocannabinoids bind to CB1 receptors on nociceptors (specialized pain receptors), they reduce their rate of firing; fewer pain signals sent to the brain means you feel less pain.
When endocannabinoids bind to CB2 receptors on mast cells and macrophages, it causes these immune cells to slow their production of activating and sensitizing chemicals binding to nociceptors. This again decreases the signal for pain. The body in an effort to regulate pain will increase production of CB2 receptors after an injury to help decrease pain and inflammation as an allostatic function.
There are several mechanisms within the central nervous system through which the ECS helps modulate pain. We will discuss these in detail in the chapter on conditions (see chapter 6).
Some conditions are associated with muscle spasms, like spinal cord injury, multiple sclerosis, cerebral palsy, stroke, brain or head trauma, amyotrophic lateral sclerosis, or hereditary spastic paraplegia. Spasticity sometimes occurs when motor neurons can’t stop firing, always telling a muscle to contract. Endocannabinoids help to increase the neurotransmitter GABA, which inhibits nerve impulses to the muscle, resulting in relaxing the muscle.
CB1 and CB2 receptors are found all over the cardiovascular system. Under normal conditions, the ECS plays a limited role in cardiac function, but under pathological conditions such as septic shock, hemorrhagic shock, myocardial infarction, advanced liver cirrhosis, or doxorubicin-induced heart failure, the ECS lowers blood pressure by decreasing heart contractility. Endocannabinoids binding to CB1 and TRPV1 receptors on vascular tissue causes vasodilation, which also lowers blood pressure. Research on rats shows that the ECS also protects against myocardial tissue ischemia.
The endocannabinoid system functions extensively within the immune system by modulating immune cell function and lowering inflammation. Endocannabinoids bind to CB2 receptors on helper T cells to decrease inflammatory cytokines and increase anti-inflammatory cytokines. The same mechanism also increases T cells, B cells, and natural killer cells.
Endocannabinoids, CB1, and CB2 receptor numbers are upregulated (increased in number) in tumor tissue, making tumors more sensitive to the effects of endocannabinoids. While chemotherapy kills all rapidly dividing cells, both healthy and cancerous, endocannabinoids target only cancer cells; their effects include inducing apoptosis and autophagy, suppressing angiogenesis, and inhibiting cancer migration. We will discuss cancer more fully in the conditions chapter (see chapter 6).
Apoptosis is a genetically directed process of cell self-destruction; it is a normal and controlled part of an organism’s growth and essential to health. The binding of endocannabinoids to the CB1 receptor stimulates apoptosis.
Autophagy is the body’s way of removing damaged cells and replacing them with new ones. It is a natural regenerative process that occurs at a cellular level, reducing the likelihood of contracting some diseases as well as prolonging life span. The binding of endocannabinoids to cannabinoid receptors causes enzymes within the cell to digest the cellular contents. This is especially important in immune cells that have engulfed foreign cells or cancerous cells.
Angiogenesis occurs when tumor cells secrete chemicals that stimulate the growth of blood vessels. The binding of endocannabinoids to the cannabinoid receptors on tumor cells stops the production of these chemicals and shuts off the tumor’s blood supply, starving it to death.
Sometimes cancer cells migrate (metastasize) to other areas of the body to find a better blood supply. The binding of endocannabinoids to the cannabinoid receptors on the tumor cells prevents them from migrating.
Connective tissue includes blood, bone, fascia, ligaments, tendons, and cartilage, together the most abundant category of tissue in our body. The endocannabinoid system interacts with and has beneficial effects on them all.
Bone tissue is made up of osteoblasts (bone builders) and osteoclasts (bone degraders). Both types of cells produce AEA and 2AG and express CB2 receptors. When AEA and 2AG bind to receptors on osteoclasts, bone breakdown is slowed. When AEA and 2AG bind to receptors on osteoblasts, bone building resumes.
AEA and 2AG binding to CB1 receptors in nerves close to the osteoblasts prevents the release of norepinephrine. Norepinephrine, a neurotransmitter of the stress response, decreases bone formation. When you are chronically stressed, your body doesn’t want to allocate resources to building bone. It would rather allocate resources toward escaping from wild animals.
The cells that make up fascia, tendons, and ligaments are called fibroblasts. Cells that make cartilage are called chondroblasts. Both express CB1 and CB2 receptors as well as endocannabinoid metabolic enzymes. It has been shown that cannabinoids (and presumably endocannabinoids) modulate fascial remodeling, prevent cartilage destruction, and decrease connective tissue inflammation. There is much more to be understood about the fascial system itself and the role that endocannabinoids play in it.
Both CB1 and CB2 receptors are found in the digestive system. CB1 receptors are found primarily within the enteric nervous system (the branch of the nervous system coordinating digestion), while CB2 receptors are found in the digestive tissue itself. Hunger and cell metabolism are both regulated by the ECS via regulation of the hormones ghrelin, leptin, orexin, and adiponectin.
When CB1 receptors in the enteric nervous system are bound by 2AG and AEA, digestion slows, gastric acid secretions decrease, the esophageal sphincter relaxes, intestinal motility slows, gastric motility decreases, and gastric emptying is delayed. CB2 receptors are likely involved in the inhibition of inflammation and visceral pain. In pathological conditions such as obesity, adipocytes overproduce CB1 receptors and endocannabinoids, resulting in a positive feedback loop leading to hunger, increased eating, and ultimately metabolic syndrome.
Healthy livers have relatively low levels of CB1 and CB2 receptors and low levels of AEA and 2AG. There are also low levels of the degradation enzymes FAAH and MAGL. All that changes when the liver is injured. Upon injury, CB2 receptor and 2AG levels increase within the liver.
When CB1 receptors are bound by endocannabinoids, fibrogenesis occurs in the liver; when CB2 receptors are activated, fibrosis is prevented. Because CB2 receptor activation is proven to decrease inflammation in connective tissue, it follows that it would also prevent fibrosis in the liver.
The ECS sets an overall tone of safety and well-being in the body. This enables animals (including us) to learn new things, express curiosity, rest, relax, eat, and evolve. The ECS mechanism that creates this state is endocannabinoids binding to CB1, CB2, and TRPV1 receptors. This has the following effects (which we will discuss in depth in the chapter on conditions, chapter 6):
Endocannabinoids begin as a precursor molecule in our cell membranes. The precursor molecule migrates into the cell to interact with synthesizing enzymes that convert it into one of our body’s different endocannabinoids. These molecules are then transported out of the cell to bind with receptors nearby. When they’re done stimulating a receptor, they are transported back into a cell to be broken down and recycled.
The chemical messengers of the ECS are the endocannabinoids. Other body systems employ chemical messengers: the endocrine system has hormones; the nervous system has neurotransmitters; the immune system has chemical messengers called cytokines; and the digestive system has gut peptides. Each chemical messenger has a different name based on its location, but they are actually the same chemicals. Just like in real estate, it’s all about location! Serotonin in your gut is called a gut peptide, but in the nervous system it’s called a neurotransmitter. Endocannabinoids are mainly found in synapses, blood, and extracellular fluid.
Endocannabinoids are unique in that they are not made by a particular gland for use at a distant target, like in the endocrine system. Each and every cell that has synthesizing enzymes has the ability to make them, on demand, when needed. Yes, each and every one of your cells has the ability to maintain its own balance when it deems it necessary.
We’ve recognized seven endocannabinoids: arachidonoyl ethanolamide (AEA), 2-arachidonoylglycerol (2AG), noladin ether, virodhamine, N-arachidonoyl dopamine, palmitoylethanolamide (PEA), and oleoylethanolamide (OEA). AEA and 2AG are the two best known and most studied, and they are the endocannabinoids we will focus our discussion on. Research is just beginning on the others.
AEA, the “bliss molecule,” was the first endocannabinoid discovered. It is found in the brain, spleen, heart, skin, connective tissue, bone, and reproductive organs. It is a partial agonist of the CB1 receptor when found alone and binds with high affinity in the presence of lipoxin A4. AEA also binds to CB2, GRP55, TRPV1, and PPAR receptors. AEA is less abundant than 2AG in the brain, but concentrations quickly increase when needed. AEA is our very own THC. Yay!
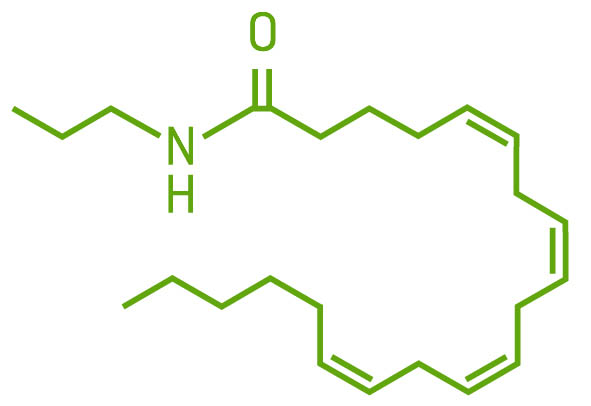
AEA molecule
AEA is made from arachidonic acid, an essential fatty acid in the phospholipid bilayer of the cell, on demand, usually in response to a chemical cue. It is produced through a series of reactions with intermediate chemicals and corresponding enzymes, and is not stored for later use. Endocannabinoids cannot move in or out of the cell on their own; they require transport molecules to move them across cell membranes. AEA is shuttled around by fatty acid–binding protein (FABP). The enzyme fatty acid amide hydrolase (FAAH) breaks down AEA into arachidonic acid and ethanolamine. If FAAH is suppressed or there is too much AEA for FAAH to breakdown, AEA can remain available for further receptor binding or follow a different pathway and be broken down by cyclooxygenase-2 (COX-2) and subsequent enzymes into prostaglandin E2, an inflammatory prostaglandin. CBD is able to increase AEA levels by inhibiting the enzyme FAAH.
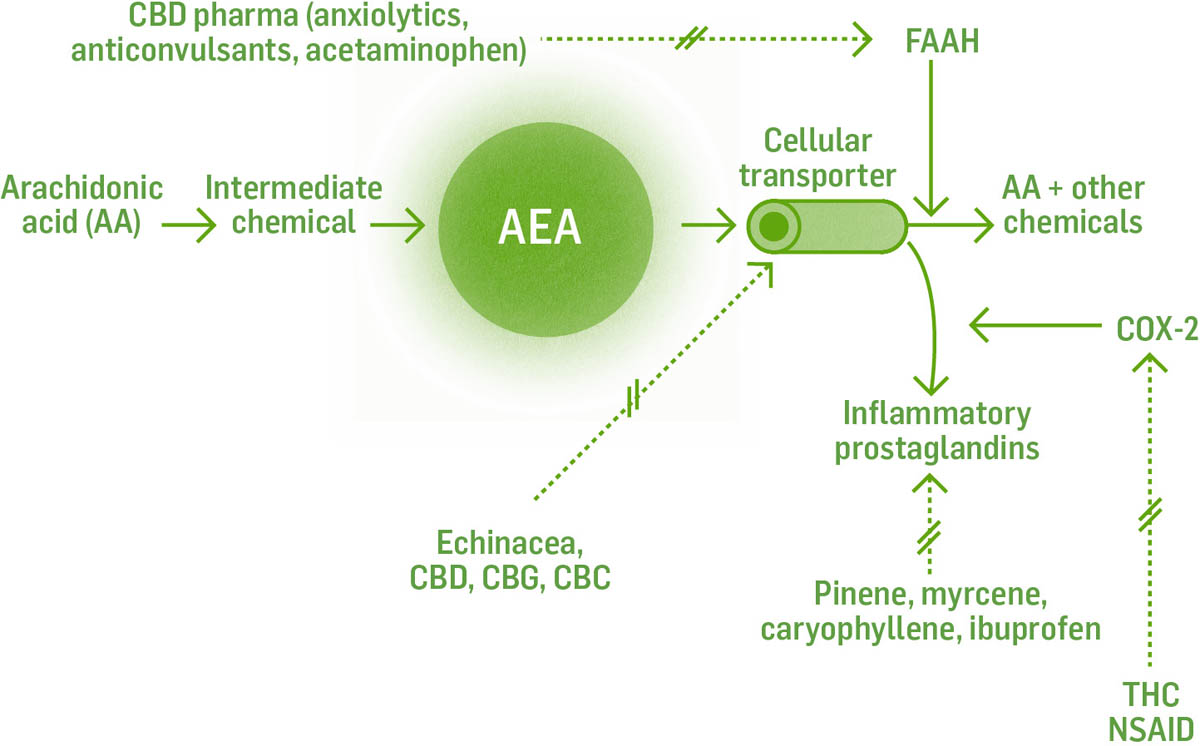
The dotted lines with the double cross through them indicate the place where the constituents/herbs interact and inhibit the reaction.
Double-tap the image to open to fill the screen. Use the two-finger pinch-out method to zoom in. (These features are available on most e-readers.)
The endocannabinoid 2AG is a thousand times more abundant than AEA in the brain and a true agonist for the CB1 receptor; it binds with moderate to low affinity. It binds to the same receptors as AEA: CB1, CB2, GRP55, TRPV1, and PPAR.
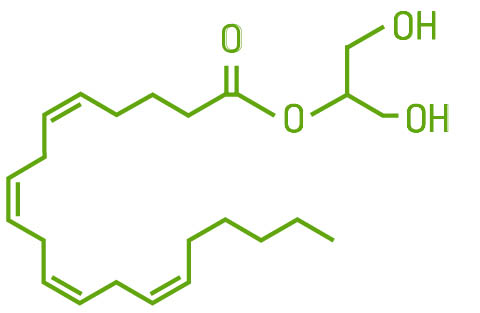
Production of 2AG also begins at the phospholipid bilayer in cell membranes. Through a series of reactions, it is derived from the precursor molecule diacylglycerol (DAG) and leaves the cell via its transport molecule, FABP, to bind to nearby receptors. It is broken down into arachidonate glycerol by the enzyme monoacylglycerol lipase (MAGL). It can also be broken down by COX-2, eventually producing PGE2, an inflammatory cytokine.
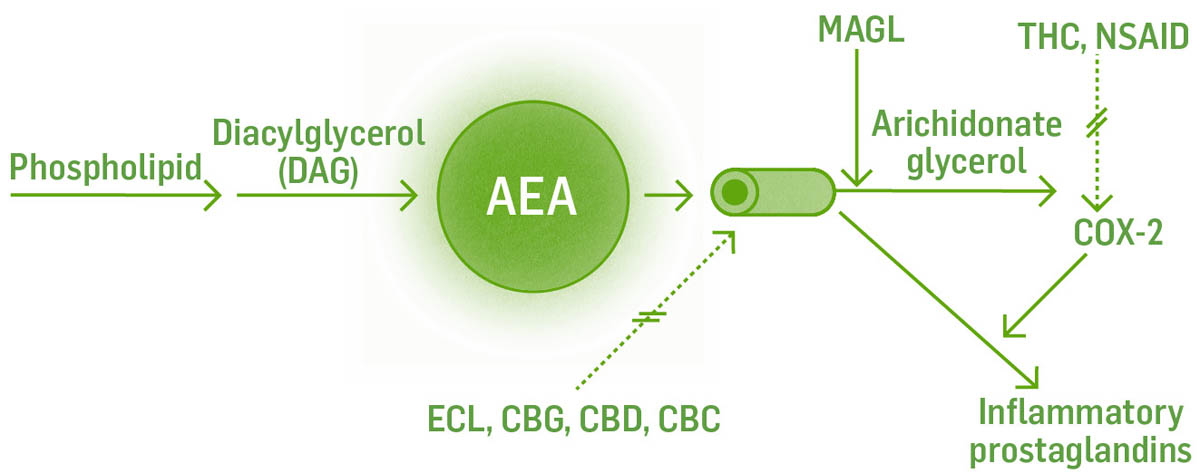
Understanding these biochemical pathways will help us to understand how the multitude of interventions work. For example, ibuprofen and CBD both inhibit FAAH. This leads to less AEA being broken down, more AEA available to bind to receptors, and a decrease in inflammatory cytokine production. THC and CBD block FABP at multiple sites in the transport of AEA specifically, thus increasing AEA available to bind to receptors.
For those of you who want to go deeper into the chemistry of the endocannabinoid system, here is a more detailed look. If you’re not interested in this level of detail, you can see a more general overview of endocannabinoids here.
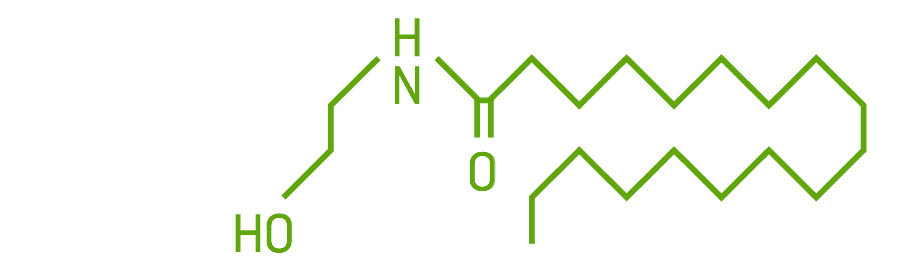
The endocannabinoids PEA and OEA are in the family of chemicals called N-acylethanolamines. They are made from fatty acids. They bind to receptors and are degraded by enzymes. While not considered true endocannabinoids — I call them “adjacents” because they don’t bind to specific cannabinoid receptors — they do bind to some noncannabinoid receptors and function much like endocannabinoids.
PEA binds to the PPAR, GRP55, and TRPV1 receptors; its effects are anti-inflammatory, antinociceptive, neuroprotective, and anticonvulsant.
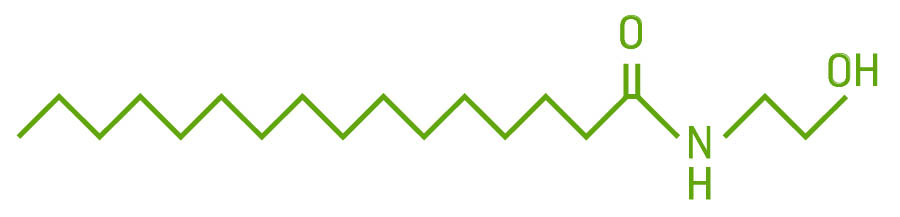
OEA binds to the PPAR and TRPV1 receptors to stimulate fat breakdown and regulate feeding.
In general, endocannabinoids inhibit the release of inflammatory chemicals and the release of neurotransmitters. AEA and 2AG function in similar ways when they bind to receptors. The major differences between them are where they work in the body and how strongly they bind to receptors. If we understand how our endocannabinoids work biochemically, we can apply that understanding to the cannabinoids of cannabis because they bind to the same receptors.
Information travels through the nerves of the nervous system via electrical currents called nerve impulses. Nerves are made of individual cells called neurons. Billions of neurons connect within the brain and spinal cord, but they don’t quite touch each other. The tiny gap between neurons is called the synapse. Electric currents can travel through nerve fibers but cannot jump across synapses. That’s where neurotransmitters come in. Neurotransmitters are chemical messengers released by an excited, “presynaptic” neuron carrying an electric current; these chemicals cross the synapse to bind to receptors on the “postsynaptic” neuron. This can either excite the postsynaptic neuron or inhibit it. The action of all endocannabinoids and cannabinoids happens right here at synapses. Now let’s look at endocannabinoids as neurotransmitters.
In the nervous system both AEA and 2AG are made on demand at the postsynaptic neuron. When they are released from the postsynaptic neuron they travel back to the presynaptic neuron and bind to CB1 receptors on its surface. This prevents neurotransmitters stored in the presynaptic neuron from being released.
Endocannabinoids or cannabinoids binding to presynaptic CB1 receptors can be inhibitory or excitatory depending on which neurotransmitter they are preventing the release of. When they prevent an excitatory neurotransmitter such as glutamate from being released, the effect is inhibition. This is a protective mechanism that prevents the neuron from dying from overfiring (excitotoxicity).
When endocannabinoids or cannabinoids prevent the release of inhibitory molecules, GABA for example, the net effect is stimulation or excitation.
Along with binding to cannabinoid receptors, endocannabinoids can also bind to noncannabinoid receptors and modulate their shape to either increase or decrease their affinity for a particular ligand. For example, AEA is a positive allosteric modulator (enhance) of the glycine receptor and enhances its binding and activation. Glycine, when binding to its receptor, inhibits neuron activation. AEA enhances glycine’s binding and thus its inhibitory function.

Cannabinoids and endocannabinoids play the vital role of chemical messengers; they can jump the tiny gap between nerves (called the synapse) and allow information to travel in the nervous system.
Endocannabinoids made by cells outside the nervous system act as chemical messengers, traveling to other cells and binding to their receptors to have an effect. (Hormones do this, too.) The net effect is decreased production of inflammatory cytokines (chemicals made by and/or for immune cells) and decreased migration of immune cells to inflamed areas. (Immune cells make inflammatory chemicals, which results in more inflammation.)
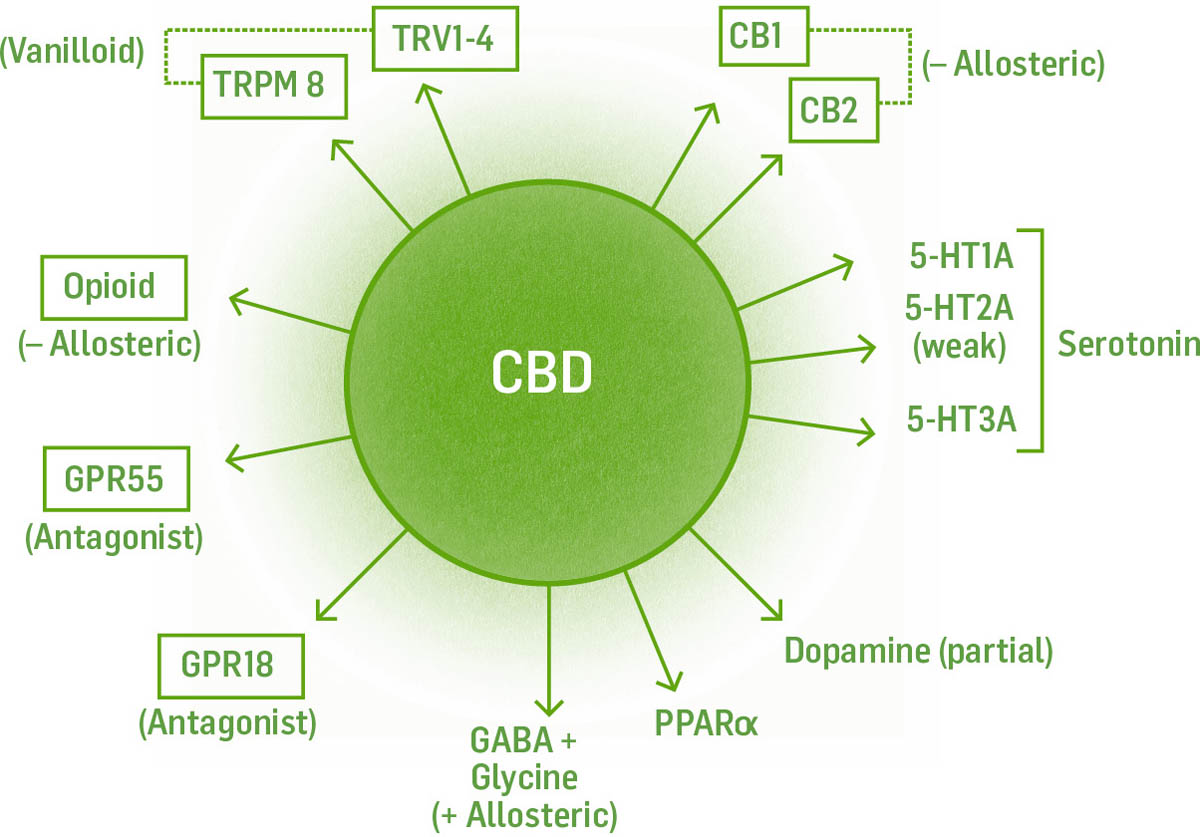
To date, we know of 15 types of receptors in the ECS system. Five are “typical” cannabinoid receptors that work directly with endocannabinoids. The 10 others are not specific to the endocannabinoid system but are affected by endocannabinoids. Endocannabinoids affect these “atypical” receptors by changing the way the receptor interacts with its own specific chemical messenger.
The five typical cannabinoid receptors are CB1, CB2, GPR18, GPR19, and GPR55. The atypical receptors are GABA, serotonin (5-HT1A, 2A, 3), dopamine, adenosine, acetylcholine, glycine, glutamate, PPAR, TRP, and opioid.

In chemistry class, you may have been taught the “lock and key” model for how a chemical messenger binds to a receptor: a specific shape of molecule binds to a receptor specifically shaped to receive it. While this basic premise holds true, the model needs an upgrade. Both the ligand molecules and the receptors are dynamic, vibrating, wiggling, shimmying (dare I say singing?), and changing shape. They are not static; they are constantly moving!
If you’d like to expand your thinking around receptors and binding, there is evidence that it is not even necessary for a chemical messenger and receptor to physically bind. When the vibrating ligand comes close enough to the proper receptor, they entrain to the same vibrations, which activates the receptor. Right now, science has no way to measure receptor entraining because of the limitations of measuring equipment. One day Western science will be able to explain the healing that occurs when we know the specific healthy vibration for each receptor, cell, or tissue and can create that healing vibration and move it close enough to the “unhealthy” tissue so it can entrain the entire body to the song of health. Some healers are already doing this, in fact, and have been doing it for thousands of years in practices like qigong and hands-on healing.
Until science can explain this phenomenon, let’s return to what is understood now. Receptors have more than one place for molecules to bind to. The primary active site is called the orthosteric site; other sites are called allosteric binding sites. Molecules binding to allosteric sites change the receptor’s shape, which can enhance, decrease, or do nothing to the ability of chemical messengers to bind to the orthosteric site.
For example, the shape of the CB1 receptor is perfect for 2AG to bind to. That is why 2AG is an agonist with higher affinity to the CB1 receptor than AEA. AEA, on the other hand, is a partial agonist. It doesn’t bind to the CB1 receptor with as much affinity as 2AG because AEA’s shape is not as close a fit. Lipoxin A4, an anti-inflammatory metabolite of arachidonic acid, binds to a different allosteric site on the receptor that changes the receptor’s shape to allow a better fit for AEA. Lipoxin A4 is, therefore, an allosteric enhancer of AEA. CBD binds to an allosteric site on CB1 and changes its shape so THC does not bind as well, so CBD is a negative allosteric modulator for THC. This will translate to some of the modulatory effects of CBD on THC’s actions in the body.
All chemicals are not created equal in how well they bind to receptors. There is a range of binding strengths; some chemical messengers bind with vigor and high affinity, while others barely hold on. Think of binding as hugging. Some huggers give you a full-body bear hug, while others barely embrace you. An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. Think of an agonist as an “actor.”
Full agonist: a chemical that binds to a receptor to produce a biological response. For example, synthetic THC is a full agonist of CB1 and binds to the receptor so tightly it can cause more intense effects than other partial agonists that don’t bind to the receptor (AEA or THC) as strongly.
Partial agonist: a chemical that binds to receptors but only causes a partial activity. AEA, 2AG, and THC are partial agonists of CB1.
Neutral agonist: a chemical that binds to a receptor and causes no reaction. Neutral agonists work by displacing agonists (or blocking them from binding), causing a decrease of function of the receptor being bound by the agonist.
Inverse agonist: an inverse agonist binds to receptors and causes the opposite reaction as an agonist.
Positive allosteric modulator: a chemical that binds to secondary sites on a receptor and increases agonist signaling by either increasing agonist binding or increasing receptor sensitivity.
Negative allosteric modulator: a chemical that binds to secondary sites on a receptor and decreases agonist signaling by either decreasing agonist binding or increasing receptor sensitivity.
Antagonist: a chemical that blocks the action of the agonist.
The binding of chemicals to cannabinoid receptors is not linear or binary. If you fill the receptors with chemical messengers, the effects are different than if you bind only a few receptors. Adding to the complexity is that the cannabinoid receptors regulate the release of other neurotransmitters and the fact that CB1 receptors can form heterodimers (pair up with) with 10 other receptors to have inhibitory or excitatory effects. This system is vastly more complex than the existing literature would lead us to believe, and the oversimplification of working with isolated constituents could be potentially dangerous given our binary understanding of a mycelial-like multifaceted design (keep this in mind when we discuss the drug rimonabant).
Cannabinoid receptors are found in all vertebrates, as well as many invertebrates and plants, but they are not found in insects (bugs cannot get high!). They are among the oldest type of receptors, dating back 600 million years. They are part of a general class of receptors called G-coupled receptors. All G-coupled receptors span the cell membrane, and the part of the receptor spanning the membrane is made of the same molecules. The difference between the various G-coupled receptors in the body (including CB1, CB2, GABA, dopamine, and serotonin) are the parts that stick out of the membrane to bind with chemical messengers. These parts are like catcher’s mitts that will activate only with the one specifically shaped chemical messenger they can catch. Once a chemical messenger binds to a receptor, a cascade of reactions occurs within the cell to bring about an intended action (the functions we’ve been discussing).
The receptor is where the action is. All the functions of the ECS occur because of endocannabinoids binding to receptors. The effects of cannabis occur here, too. There are 15 different kinds of receptors within the ECS, and these receptors are found in virtually every tissue of the body, which makes the ECS a major regulatory system underlying the nervous, endocrine, immune, digestive, reproductive, and circulatory systems.
Every cell with receptors is a potential target. Chemical messengers that bind with a strong affinity have a strong effect; those with a weak affinity have a lesser effect. Furthermore, the strength of the bond can be affected by allosteric binding molecules.
We can apply what we know about our endocannabinoid system to the workings of cannabis and her chemical constituents. Cannabis communicates to us when her cannabinoids bind to our ECS receptors. The structural similarity of cannabis’s constituents is so close to our own molecules that they bind to our receptors and produce effects like our own endocannabinoids.
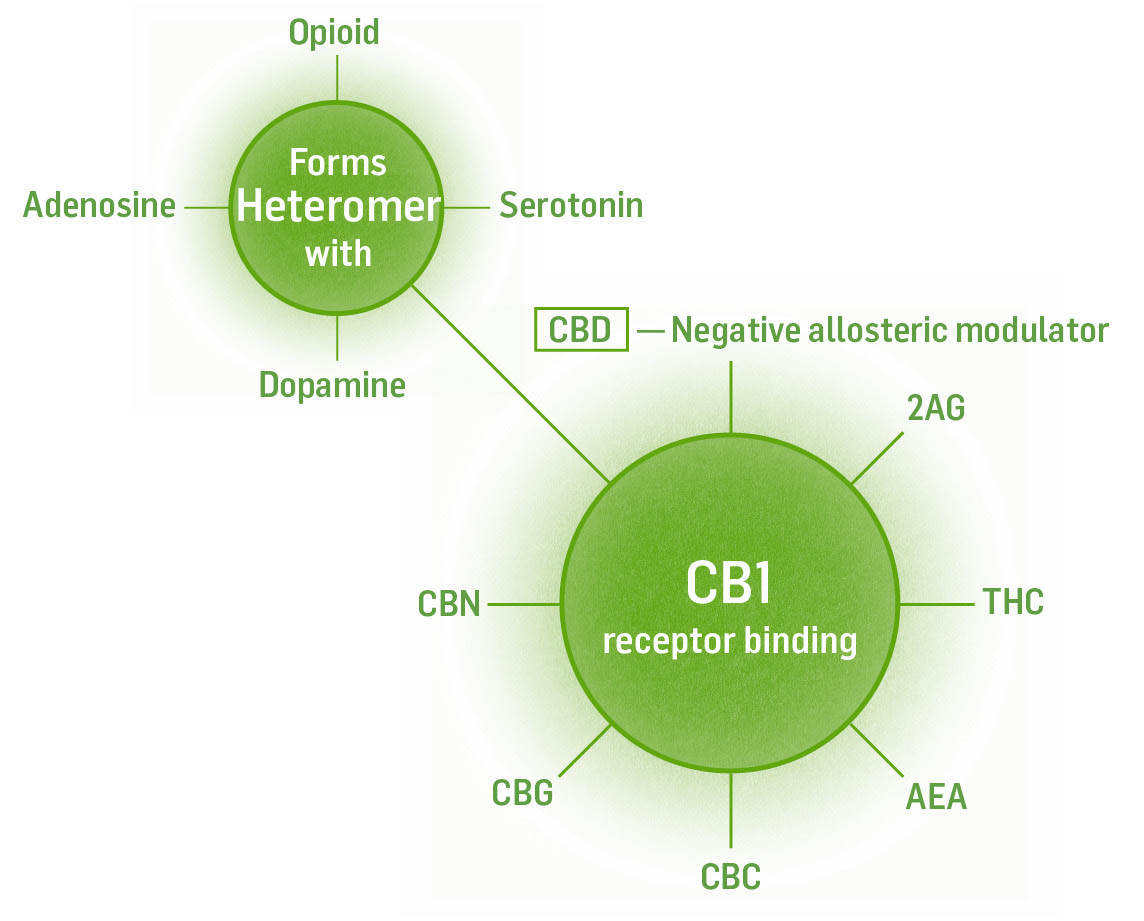
Named CB1 because it was the first receptor discovered in the ECS, CB1 receptors in the brain are found in the regions of the amygdala, hippocampus, cerebellum, cerebral cortex, olfactory bulb, and basal ganglia. No CB1 receptors exist in the brain stem, which controls breathing and heart rate. This is why you cannot die from an overdose of cannabis. Opiates, on the other hand, do bind to receptors in the brain stem. When too many opiate molecules (overdose) bind the receptors here, your breathing and heart will stop.
CB1 receptors are also found in peripheral nerves, the thyroid gland, adipocytes, uterus, pituitary gland, hepatocytes, adrenal gland, reproductive organs, skeletal muscle, lungs, bone marrow, bone tissue, bladder, pancreas, oligodendrocytes, microglia, astrocytes, skin neurons, immune cells, and the gastrointestinal tract.
The CB1 receptor is the most abundant G-coupled protein receptor in the brain, 10 to 50 times more abundant than opiate and dopamine receptors. When bound, the receptor inhibits neurotransmitter release from the neuron. This regulates learning, memory, emotional response, body temperature, motor function, reward, and addiction. In the rest of the body it regulates appetite, metabolism and food intake, bone mass, and tumor cells of neuroglia and endothelium. It also reduces neuroinflammation and pain, and controls differentiation in the developing brain and neuronal survival and synaptic plasticity in the developed brain.
Each receptor has a specific chemical messenger that binds to it. It is important to understand which chemicals bind to which receptors because this will help us create effective medicine for particular conditions.
Partial agonist with higher affinity: 2AG, THC
Partial agonist with weaker affinity: AEA, CBC, CBG, CBN
Negative allosteric modulator: CBD
Inverse agonist: CBD
Heteromeric with: serotonin, dopamine, adenosine, opioid, orexin, and chemokine receptors
To understand the function of a receptor, we need to know where it is.
Cannabinoid receptors exist on the surface of cells within the cell membrane.
Neurons. When CB1 receptors are bound by their ligands on neurons, they inhibit neurotransmitter release from the neuron. CB1 receptors are found in neurons that release GABA, glutamate, serotonin, dopamine, acetylcholine, norepinephrine, corticotropin-releasing factor (CRF), cholecystokinin, dynorphin, and substance P. We will discuss this in detail in the chapter on conditions (chapter 6).
Immune Cells. Chemical messengers binding to CB1 receptors on immune cells usually decreases the production and release of inflammatory cytokines and chemokines that attract more immune cells. The net effect is a decrease in inflammation.
Endothelial Cells. Endothelial cells line structures like blood vessels. Chemical messengers binding to CB1 receptors causes an increase in nitric oxide (NO) production, which causes vasodilation (dilation of the blood vessels, which lowers blood pressure) and decreased platelet aggregation.

In addition to cannabinoid receptors on the surface of cells, receptors have also been discovered within the cell on the mitochondria, lysosome, and the nucleus.
Mitochondria. When ligands bind to mitochondrial receptors, cellular respiration and cyclic AMP function decreases. Because mitochondria use oxygen to produce energy, the cell manufactures less energy. Evidence suggests that this might contribute to memory loss due to neurons’ decreased ability to manufacture energy in the mitochondria.
Lysosomes. CB1 receptors have also been found within the cell on the surface of the tiny organelle called the lysosome. Lysosomes carry degradation enzymes that help the cell break down bacteria or dysfunctional cell parts — think of them as internal cellular recycling centers. They also can cause the release of intracellular calcium from other organelles. Cells undergoing autophagy increase the use of lysosomes.
Nucleus. Ligands binding to CB1 receptors on the nucleus helps regulate cell proliferation, cell death (apoptosis), cell differentiation in a developing brain, and neuronal survival in a developed brain. In neurons, binding protects against nutrient deprivation and neuronal degeneration.
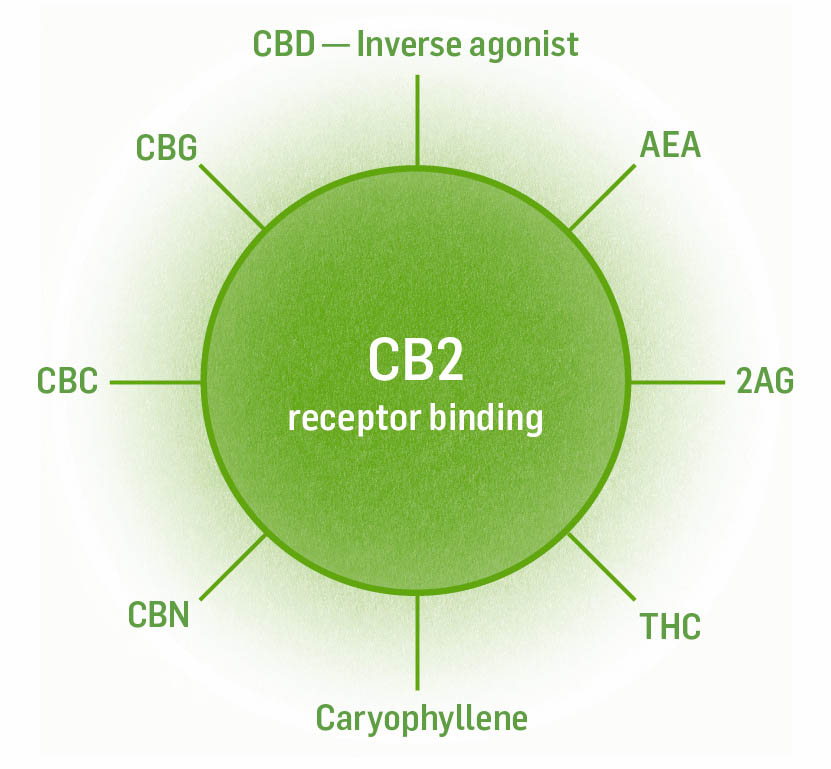
The CB2 receptor is found primarily in the immune system on natural killer cells, B cells, mast cells, macrophages, T cells, tonsils, spleen, thymus, and liver (Kupffer cells). They are also found in cardiovascular tissue (myocardium and endothelium), skin, reproductive tissue, bone, connective tissue, endocrine and exocrine pancreas, tumors, brain, and in the gastrointestinal tract. In the nervous system they are found on microglial cells. Like the CB1 receptors, CB2 receptors are inhibitory when they are bound to by their chemical messenger. CB2 receptors are not commonly found in the nervous system, but when nerve tissue is inflamed or injured, CB2 receptor number increases in neurons, microglia, and astrocytes.
Binding CB2 receptors protects against osteoporosis, atherosclerosis, pain (nociception), chronic liver disease, neurodegeneration, metabolic disorders, inflammation, neuroinflammation, and drug addiction.
Full agonists: AEA, 2AG, THC, caryophyllene (terpene)
Weak agonists: CBN, CBC, CBG
Inverse agonist: CBD
When CB2 receptors are bound to by their chemical messengers, this binding helps protect against pain, atherosclerosis, neuroinflammation, drug addiction, and other disorders.
There are nine “atypical” cannabinoid receptors, so called because they were discovered before the endocannabinoid system and associated with other systems. They are either bound to directly at the orthosteric site (the active site where the chemical messengers bind) by endocannabinoids or cannabinoids or at allosteric sites. In a few instances endocannabinoids/cannabinoids affect transport of the natural chemical messenger of the receptor, which ultimately affects receptor function. These nine receptors add to the depth and complexity of the endocannabinoid system and the places cannabis interfaces with it.
CB2 receptors are not usually found in the nervous system, but when nerve tissue is inflamed or injured, CB2 receptor numbers increase in certain areas.
This is called an “orphan” receptor because it was not understood that GPR18 is a G-protein receptor like CB1 and CB2, that interacts with the endocannabinoids. This receptor was “adopted” in 2006 when it was found that AEA binds to it weakly and N-arachidonoyl glycerine, a metabolite of AEA, binds to it strongly. We also know now that THC binds to the GPR18 receptor and CBD is an antagonist. The GPR18 receptor is found in the spinal cord, small intestine, immune cells, spleen, bone marrow, thymus, lungs, testes, and cerebellum. Its functions include blood pressure regulation, and it aids in immune function as a chemoattractant (binding of it attracts immune cells).
Another orphan, GPR55 receptors were “adopted” in 2007 as endocannabinoid receptors of AEA, 2AG, and THC. CBD antagonizes the receptor. GPR55 receptors are found in the central nervous system, adrenal glands, gastrointestinal tract, lungs, liver, uterus, bladder, and kidney. Binding decreases blood pressure and inflammation, blocks some pain, regulates energy intake and expenditure (it may play a role in obesity and diabetes mellitus), regulates bone cell function, and decreases neurodegeneration.
The last of the orphaned receptors, GPR19 was found in 2006 to bind to AEA and 2AG weakly; OEA is a full agonist. This receptor is limited to the pancreas and the gastrointestinal tract. It functions to decrease food intake, decrease body weight, and improve blood sugar.
Acetylcholine receptors can be either excitatory or inhibitory depending on their location. Two subtypes of the receptor exist: the nicotinic ion-gated channel and the muscarinic G-protein receptor. We will limit our discussion to the nicotinic receptor: this is where the cognitive effects of nicotine are triggered. Acetylcholine receptors are found in the brain (cerebral cortex, thalamus, and hippocampus), neuromuscular junctions, and autonomic nerve ganglia. Acetylcholine receptors affect memory, learning, and attention.
Noncompetitive agonist: AEA
Negative allosteric modulator: CBD
Binding of adenosine receptors promotes rest in neurons of the brain and spinal cord by decreasing nerve transmission, so their binding has an inhibitory effect. Caffeine antagonizes this receptor, blocking adenosine from binding to it (and keeping neurons from getting the break they need!). Adenosine receptors are found in the region of the brain called the dorsal striatum, which is involved in motor activity, cognitive function, and mood.
Adenosine binding causes drowsiness and memory impairment, and it affects cognitive function, mood, and motor activity. (Think of all the things the wonder drug caffeine does for us as it blocks adenosine function — we are able to stay up longer, think faster, and write books!)
The neurotransmitter adenosine supports sleep, suppresses arousal, and is a vasodilator. When it is able to bind to its A2A receptor, it is anti-inflammatory. Increased adenosine is also responsible for the decreased anxiety and better sleep promoted by CBD and THC. They both inhibit adenosine’s transporter and decrease the amount of adenosine being removed from the synapse, allowing it to carry out its functions.
The ECS, through binding glutamate and GABA, affects dopamine levels rather than interacting with dopamine receptors directly. Dopamine is one of the more familiar neurotransmitters, associated with reward and mood. The endocannabinoid system regulates dopamine in a few different ways, and it is dopamine that mediates some of the effects of THC, such as the munchies, lowered cognition, psychosis, amotivation in some heavy users, and the reinforcement behavior of addiction. It is interesting to note that cannabis increases dopamine levels much less than the 10 to 30 percent jump in dopamine levels that cocaine and amphetamines cause. Dopamine receptors are found in the brain in the regions of the midbrain, prefrontal cortex, nucleus accumbens, dorsal striatum, and basal ganglia. Their binding functions in mood, arousal, learning, motivation, and coordinating actions to attain a reward.
CB1 agonists (THC, 2AG, AEA) can either increase or decrease dopamine levels depending on which dopamine-releasing neurons they are connected to. CB1 agonists binding to glutamate receptors cause an increase in the release of dopamine, whereas CB1 agonists binding to GABA receptors cause a decrease in the release of dopamine. Dopamine neurons make endocannabinoids that feed back to glutamate receptors and GABA receptors to regulate the release of dopamine.
CBD is a partial agonist of the dopamine receptor and may be responsible for the effects of drowsiness, fatigue, diarrhea, and decreased appetite.
The neurotransmitter GABA is inhibitory like glycine but primarily functions in the brain, whereas glycine is the primary inhibitory neurotransmitter in the spinal cord. The inhibitory functions associated with GABA are sedation, decreased anxiety, and antiseizure properties. Higher levels of GABA receptors are found in the brain, spinal cord, and pancreas. Lower levels can be found in the intestines, stomach, fallopian tubes, uterus, ovaries, testes, kidney, bladder, lungs, liver, and immune cells.
GABA receptor activation is the major inhibitory mechanism of the central nervous system. Think of it as our neuronal off switch. It regulates pain-signal transduction and causes sedation. Alcohol depresses the nervous system via GABA activation. People with GABA deficits are more susceptible to the psychomimetic effects of THC. Withdrawal from alcohol and benzodiazepines is caused by decreased GABA in the system; when a person consumes external GABA receptor stimulators, the body makes less GABA of its own to maintain balance.
Agonists: CBD and CBDA (less sedation than pharma while maintaining anxiolytic effects), barbiturates, benzodiazepines, alcohol, antiseizure meds
Positive allosteric modulators: CBD and CBDA
THC increases GABA via decreasing reuptake.
Glutamate is the most abundant excitatory neurotransmitter found in the brain. It is involved in most aspects of normal brain function including cognition, memory, learning, and motor activity. Too much glutamate can cause damaging excitotoxicity; interestingly, too little glutamate causes difficulty concentrating and mental exhaustion.
The main receptor for glutamate is the NMDA receptor. When a CB1 receptor is bound by its agonists, on NMDA-containing neurons, a decrease in glutamate binding occurs. Further, binding of CB1 can cause a decrease in NMDA receptors on neurons as well. All this aids in preventing glutamate-induced excitotoxicity.
The glycine receptors are inhibitory ligand-gated ion channels. Glycine receptor channels can be found in the brain (hippocampus, amygdala, cerebral cortex) and spinal cord. Binding the glycine receptor has the effects of neuroprotection, anti-inflammation, decrease in pain signal transmission, and decrease in release of dopamine.
Agonists: glycine, AEA, 2AG, OEA, THC (low dose), and CBD
Positive allosteric modulators: THC and CBD
The binding of ligands to the opioid receptors causes a decrease in pain transmission within the peripheral and central nervous system. There are four receptor subtypes — kappa, delta, gamma, and mu — and they are typically found on presynaptic neurons. Endocannabinoids regulate pain through a variety of ways (addressed in the chapter on conditions, chapter 6); opioid receptors are one such pathway. Opioid receptors can be found in the brain, spinal cord, peripheral nerves, and digestive tract. In general, binding of opioid receptors decreases pain signaling by inhibiting the release of substance P and glutamate.
Binding of the delta-OR is responsible for analgesic, antidepressant, and physical dependence effects of opiates.
Agonists: endorphins, enkephalins, dynorphins
Negative allosteric modulator: CBD/THC (possible mechanism of opiate sparing, prevention of tolerance, and help with opiate withdrawal)
Heterodimer with CB1: inhibit each other’s individual binding functions. Low-dose CB1 agonist increases activity of delta-OR.
Binding of the kappa OR has analgesic and anticonvulsant effects in the body; it also affects depression, hallucinations, diuresis, neuropathic pain, and sedation.
Agonists: endorphins, enkephalins, dynorphins
Heterodimer with CB1
Binding of the mu OR is responsible for analgesia, physical dependence, respiratory depression, euphoria, and decreased gastrointestinal motility.
Agonists: endorphins, enkephalins, dynorphins, opiates (note, these are the only opiate receptor that opiates bind to)
Negative allosteric modulator: CBD/THC
We now move away from the cell-surface receptors and into the nuclear receptors. PPAR receptors are further divided into two subcategories, PPAR alpha and PPAR gamma. All PPAR receptors are found within a cell’s nucleus. PPAR alpha receptors are found in the liver, kidney, heart, skeletal muscle, and adipose tissue. PPAR gamma receptors are found in the heart, muscles, colon, kidney, pancreas, and spleen.
These receptors affect expression by the DNA of regulatory proteins of the cell. Specifically, binding of the PPAR receptors by their agonists regulates lipid metabolism, insulin sensitivity, glucose metabolism, inflammation, pain, cell proliferation, and hepatic enzyme expression.
PPAR alpha weak agonists: fatty acids, eicosanoids, 2AG, AEA, PEA, OEA, THC, CBD, CBC, CBG
PPAR gamma weak agonists: AEA, 2AG, OEA, PEA, THC
Binding of serotonin receptors regulates blood pressure, heart rate, mood, learning, memory, sleep, body temperature, appetite, nausea, vomiting, cerebral blood flow, and acute responses to stress. Binding the receptor is anxiolytic, panicolytic, and antidepressive.
Serotonin receptors are found throughout the body. All of the various functions of serotonin are carried out by the binding of seven different subtypes of the serotonin receptor. The binding of the serotonin receptors by its agonists can either be inhibitory or excitatory for the release of the following neurotransmitters: glutamate, GABA, dopamine, epinephrine and norepinephrine, acetylcholine, oxytocin, prolactin, vasopressin, cortisol, and substance P.
The serotonin (5-hydroxytryptamine or 5-HT) receptors are divided into subclasses of 5-HT1 (A-F), 5-HT2, and 5-HT3. The different types of 5-HT receptors are categorized by location and function.
Pharmaceuticals that target the serotonin receptor are antidepressants (serotonin reuptake inhibitors), antipsychotics (aripiprazole), anorectics, antiemetics, antimigraines, and hallucinogens. CB1 receptor activation at serotonin synapses, GABA, and glutamate receptors increases the release of serotonin.
|
Receptor type |
Where found |
What binds to it |
Functions |
|
5-HT1A |
blood vessels, brain/spinal cord |
agonist: CBD, CBDA; THC partial; antagonist CBG |
antidepressant, anxiolytic, mood, sleep, vasoconstriction |
|
5-HT2A |
blood vessels, CNS, GI, platelets, PNS, smooth muscle |
CBD (weak), LSD, mescaline, DMT, psilocybin, CB1 heterodimer |
addiction, anxiety, appetite, cognition, learning, memory, mood, sleep, vasoconstriction |
|
5-HT3A |
CNS, GI, PNS |
allosteric modulator CBD/THC, AEA antagonist |
anxiety, addiction, emesis, GI motility, learning, memory, nausea |
The 5-HT1A serotonin receptor is found in the brain (cerebral cortex, hippocampus, amygdala, septum, raphe nucleus). Binding of this receptor lowers blood pressure, heart rate, and body temperature. It is antiemetic, analgesic, and antinausea; it increases cerebral blood flow and attenuates acute response to stress; and it is anxiolytic and panicolytic and an antidepressant.
Agonists: CBDA (100 times stronger than CBD), CBD; pharma: buspirone, SSRI, MDMA
Partial agonist: THC
Antagonist: CBG
The 5-HT2A serotonin receptor is also found in the brain’s cerebral cortex, hippocampus, amygdala, septum, and raphe nucleus. Binding of this receptor affects emotions, learning and memory, and pain.
Agonists: entheogens (psychedelics): mescaline, psilocybin, DMT, LSD; pharma: antidepressants and antipsychotics
Weak agonist: CBD
Heterodimer: low-dose THC lowers anxiety; high-dose THC affects memory
The 5-HT3A receptor is found in the brain, spinal cord, peripheral nerves, and gastrointestinal tract. Binding the receptor affects pain, mood, nausea, and emesis.
Antagonist: AEA
Negative allosteric modulators: CBD, THC
The TRP channels are ion channels found on the cell membranes. There are roughly 30 different types of TRP receptors in the body, and depending on the type are found in the central nervous system, on sensory neurons, immune cells (macrophages, dendritic cells, Langerhans cells), endothelium, epithelium, epidermis, hair follicles, keratinocytes (skin cells), perivascular tissue, intestines, kidney, placenta, spleen, lung, and smooth muscle. The vanilloid TRPV group is colocalized with CB1 and CB2 receptors in sensory neurons in the brain and skin; we will focus on this group.
The vanilloid receptors are a class of cell membrane receptors that signal burning pain, cause vasodilation of blood vessels in the area, and lower the temperature of the body.
When the vanilloid receptor is activated you feel a burning pain. Remember the last time you had too much wasabi or cayenne? That’s the burning pain. Notice the redness on your skin when you get a little on it? That’s vasodilation. Dilation of the blood vessel brings in blood. The sensation of pain is protective. It alerts you that something different needs to be done: “Move your hand away from the hot water!” or “Stop rubbing cayenne in your eye!”
The vanilloid receptor can become sensitized/activated by acidic conditions like inflammation and ischemia. This signals pain, helping you protect the area. Inflammatory chemicals such as bradykinin, serotonin, histamine, and prostaglandins also sensitize the receptor. Other agonist/sensitizers of the receptors are heat (above 109°F, or 43°C), menthol, piperine (black pepper), zingerone (ginger), and isothiocyanate (wasabi and mustard).
Vanilloid receptors can also become desensitized/deactivated. People employ this biochemistry when they use a cayenne or menthol cream or salve on painful joints in the body. The continual stimulation by capsaicin in cayenne or menthol quiets the pain signal moving to the brain. Continual stimulation of the vanilloid receptor by capsaicin, AEA, OEA, or CBD all desensitize the receptor and decrease the perception of pain. CBD can also be used internally for pain management at the TRPV1 receptor. An added benefit of cannabis is that it doesn’t cause the burning sensation topically that cayenne does.
Another way to manage pain is to decrease or downregulate the number of receptors. If there are fewer receptors that signal pain, there will be fewer pain signals, which results in less pain. In astrocytes and microglia, chemicals called specialized pro-resolving mediators act on other receptors to decrease the total number of vanilloid receptors that signal pain.
Agonists: AEA, 2AG, THC, THCV, CBD, CBN, CBC, CBG, PEA, and OEA all desensitize at least one TRP receptor. CBD is the only constituent in cannabis that desensitizes all TRP receptors. Other agonists include heat above 109°F or (43°C), low pH, cayenne (capsaicin), mint (menthol), pepper (piperine), ginger (zingerone), and wasabi (isothiocyanate).
|
Vanilloid receptors |
What binds |
Where found |
Functions |
|
TRPV1 |
PEA, OEA, 2AG, AEA, CBD, DRG, PNG, CBC, THCV, CBN, CBG (antagonist) |
dorsal root ganglia (DRG), PNG, trigeminal ganglia (TG), testes, bladder, skin, pancreas |
Pain, nociception, temperature sensation |
|
TRPV2 |
THC, CBD, CBC, CBG (antagonist) |
DRG, brain, spleen, GI, mast cells; smooth, cardiac, and skeletal muscle cells |
Nociception, analgesia, temperature perception, antiproliferation |
|
TRPV3 |
THC, CBD, CBC, THCV, CBDV, CBG |
DRG, TG, CNS, skin, tongue, testes, hair follicles |
Nociception, analgesia, temperature perception, antiproliferation |
|
TRPVA1 |
CBD, CBN, CBG, CBC, THC |
DRG, TG, hair cells, fibroblasts, ovaries, spleen, testes, GI |
Cold sensation, menthol, pathophysiological cold pain, inflammatory and nociceptive pain |
There are a number of ways to support the endocannabinoid system. Much like with the immune system, we can nurture a healthy ECS through diet and lifestyle habits.
Omega-3 fatty acids are beneficial in three ways. They increase CB1 and CB2 receptors, they increase the endocannabinoid synthetic enzymes necessary for making 2AG and AEA, and they are responsible for proper signaling of the ECS. Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), both omega-3 fatty acids, are precursor molecules used by the body to make AEA. By consuming omega-3 fatty acids, we support the ECS in three ways: we increase the number of ligand building blocks, increase synthetic enzymes, and increase receptor numbers! Good sources of omega-3 fatty acids are free-range chicken, eggs, and cold-water fish such as cod and salmon.
It is important to note that in the case of obese individuals, excess omega-3 fatty acids can lower endocannabinoids, especially in dysregulated adipose and liver tissue. Non-obese people tend not to experience a decrease in endocannabinoids with omega-3 supplementation.
Downregulation of CB1 receptors and excess ECS signaling can occur with an excess of omega-6 fatty acids (arachidonic acid is an omega-6 fatty acid). The average American consumes 20 to 30 times more omega-6 fatty acids then omega-3s. This imbalance causes inflammation and pain. One surefire way to reduce omega-6 consumption is to stop eating factory-farmed animals and their eggs and milk. Animals raised in unnatural, unhealthy ways, eating foods they don’t normally eat, like corn, make more omega-6 fatty acids. Animals raised and fed naturally (free ranging and grass grazing) make more omega-3 fatty acids.
A diet high in trans fats and sugar leads to obesity and dysregulation of the ECS. It does so by causing the body to overproduce endocannabinoids, their synthesizing enzymes, and CB1 receptors in visceral adipose, liver, pancreas, and skeletal muscle. (Such a diet also causes diabetes mellitus, metabolic syndrome, atherosclerosis, and heart disease.) This increase in endocannabinoids causes continual CB1 activation, which results in an increase in lipogenesis, decreased insulin sensitivity, and blockage of glucose and fatty acid oxidation, which leads to glucose intolerance. The ECS is responsible for stimulating hunger; when the ECS is dysregulated, it prompts the body to eat more. Maintaining healthy body weight helps regulate the ECS.
A healthy population of gut bacteria is essential. The bacteria in your gut modulate CB1 and CB2 expression and upregulate CB2 receptors. The best first step toward gut health is to get a good quality, one-month supply of probiotic supplement from the refrigerated section of your local health food store. After a month, continue to keep the gut healthy by eating fermented foods and whole plants to give the beneficial bacteria good food to eat. An added benefit of healthy gut flora is an increased expression of CB1 and mu opioid receptors in intestinal epithelium, making us more responsive to endocannabinoids and endorphins. Better responsiveness translates to feeling happier and safer. It makes sense that the bacteria in our bodies would want their host (us) to be happy and healthy so they can continue living the good life in and on us.
In addition to cannabis, there are many herbs and plants that manufacture chemicals that bind to cannabinoid receptors; they act as agonists to CB1 and CB2. CB1 agonists include copal, absinthe, salvia divinorum, camellia sinensis, kava, echinacea, legumes, club moss, algae, liverwort, and helichrysum, as well as some fungi. CB2 agonists include copal, echinacea, and rue.
The insecticide pyrethrum, used in conventional agriculture, antagonizes the CB1 receptor. Phenyl phthalate, an additive used to mold plastics, antagonizes the CB1 receptor; it is also an endocrine disruptor and carcinogen. Simply put, try to eat organic and stop using plastic.
Stress per se has gotten a bad rap. Our stress response is vital and necessary for health and learning. Our inability to manage stress is the problem — and can be a cause of serious disease. Acute stress (and subsequent increase in glucocorticoids and cortisol levels) enhances the ECS, while chronic stress and increased cortisol levels downregulate the ECS. Stress hormones decrease both AEA and 2AG in the corticolimbic circuit, the circuit that helps to regulate the amygdala (and our ability to modulate our emotions and stress response).
Exercise keeps us healthy, helps us manage stress, is the number one intervention for mild depression, and increases ECS signaling by increasing serum AEA and CB1 receptor expression. The experience of “runner’s high” is a combination of increased endorphins (our body’s own opiates) and endocannabinoids. How do we know this? Researchers have chemically blocked endocannabinoids and endorphins both alone and together and found that both are responsible for the good feelings we receive as a reward for exercise.
AEA levels increased 168 percent in healthy individuals (asymptomatic for any disease state) when they received a general relaxation massage.
Anxiolytics increase AEA by inhibiting FAAH. Antidepressants, antipsychotics, and anticonvulsants increase CB1 receptors, which results in an increase in ECS tone and may be responsible for weight gain associated with these medications. Acute opiate use increases ECS function by increasing the synthesis of CB1 receptors. Chronic opiate use downregulates the ECS. Look for a larger discussion of cannabis and opiates in the chronic pain entry in the chapter on conditions (chapter 6).
Acute alcohol use increases ECS signaling. Chronic or binge drinking downregulates the CB1 receptors. There is conflicting evidence of it both increasing and decreasing AEA and 2AG levels.
The brain stimulation that comes with consuming caffeine is a result of caffeine blocking the adenosine receptor. Adenosine acts as a brake for overstimulated neurons. By blocking adenosine from binding to its receptor, caffeine prevents neurons from resting. Adenosine also inhibits CB1 receptors, allowing more dopamine and glutamate to be released — more happy feelings when we drink coffee! Although the ECS may be stimulated, it does not protect the adrenal glands from the detrimental effects of chronic caffeine overconsumption.
The ECS may become dysregulated — meaning too many or too few endocannabinoids are being made by the body — following long-term perturbations. Suboptimal functioning of the ECS, termed endocannabinoid deficiency syndrome (ECDS), can be a factor in many diseases, including migraine headaches, irritable bowel syndrome, fibromyalgia, depression, anxiety, multiple sclerosis, Huntington’s disease, chronic motion sickness, anorexia, schizophrenia, Parkinson’s disease, failure to thrive, and PTSD. (The list is sure to keep growing as we understand more about the ECS.) Because so many people are affected by these conditions, it is useful to know how to improve and increase functioning of the ECS.
In general, for the ECS to work properly, the body must produce sufficient chemical messenger molecules and their receptors and remove the chemical messengers from circulation. When the body makes too much of any chemical messenger or too many receptors, the body is said to be in a hyper or overproductive disease state. The body retains some regulatory function by increasing or decreasing receptor numbers in response to increased or decreased chemical messengers, but the system can’t completely compensate. Too little messenger or too few receptors causes underproductive (hypo) disease states. For example, in thyroid disease, too little thyroid hormone results in hypothyroidism and all the symptoms associated with it.
Because the endocannabinoid system underlies the autonomic nervous system, its dysfunction can be related to many disease states, especially when we consider the role inflammation plays in chronic disease and the role endocannabinoids play in regulating inflammation.
All the steps we discussed for how to support the endocannabinoid system previously would be employed in ECDS. The basic approaches for ameliorating ECDS are much like our efforts to support any other receptor-chemical messenger system.
Increasing chemical messenger synthesis can be accomplished by supplying the nutritional building blocks the body needs to make them. In the case of the endocannabinoids it would be the omega-3 fatty acids. This ensures there is enough raw material to make the endocannabinoids that the body needs. Excess nutritional building blocks are simply used to make something else. This should be the first step for any intervention of increasing ECS functioning.
If we decrease the breakdown of endocannabinoids, they remain free to bind with receptors. We can prevent the breakdown of the endocannabinoids by decreasing their degradation enzymes. There are herbs that are known to prevent the breakdown of both FAAH and MAGL, such as the flavonoids found in red clover, soy, and tea; echinacea; and the CBD in cannabis.
The more receptors for chemical messengers to bind to, the more responsive the system. There are a number of known interventions that increase receptor numbers, such as maintaining a healthy gut biome, increasing omega-3 fatty acids, exercising, and implementing acute cannabis use.
Acute use of cannabis kick-starts the ECS. THC increases CB1 density for up to 14 days, increases sensitivity to all ligands of CB1, decreases transport of AEA and 2AG back into cells for recycling, and stimulates AEA biosynthesis. CBD delays the reuptake of 2AG and AEA at the synapse by inhibiting the transporter and FAAH breakdown of AEA.
Chronic use of high-THC cannabis downregulates and desensitizes CB1 and CB2 receptors, especially at the hippocampus. This downregulation of receptors directly correlates with the number of years using cannabis. Research findings on downregulation are interesting. Downregulation varies by brain region; not all regions are downregulated. One hypothesis is that AEA and 2AG may activate different CB1 receptors in the brain and be affected differently by exogenous THC. Epigenetics of the individual also plays a role in downregulation. In some chronic cannabis users, the CB1 receptor regions on the nucleus are hypermethylated (deactivated), but not in other individuals.
Another thing to consider is THC from whole-plant extracts is a partial agonist of CB1 while synthetic THC is a full agonist. Plant preparations have half the downregulation and desensitization as synthetics; you don’t develop tolerance as easily from whole-plant extracts as from synthetic THC. 2AG is a full agonist of the CB1 receptor, and THC (natural or synthetic) may interfere with 2AG’s binding because when THC is binding to the receptor, 2AG cannot.
One question that comes up when discussing boosting the endocannabinoid system and downregulation is “What about when I’m taking cannabis extracts for other things such as pain? Will I also be downregulating?” The answer is “Yes, and . . .” When we are utilizing cannabis to work with conditions, we know there will be some downregulation, but we also know we will be consuming the plant regularly while we are working with the condition, which essentially replaces the endogenous cannabinoids. Working with specific conditions is different from consuming cannabis to boost the endocannabinoid system. Where we want to be conscientious is when we stop using the cannabis. If we have been using it long term to work with the condition we would then taper down the use over three weeks to allow our own endocannabinoid levels to work back up.