
FIG 79. Teleogryllus oceanicus, male. (© D. Rentz)
Calling, chorusing and competing: insect songsters
This chapter is mainly concerned with the first of the eight phases of the mating sequence outlined at the end of the previous chapter. This phase – rapprochement – involves the location and coming together of potential mates. Across all the main groups of Orthoptera, communication by sound is the principal means by which this is effected. In some species sound communication did not evolve, while in others it has been lost. Generally, these are species that have changed little from the ancestral forms of the Orthoptera, or ones that live in aggregations that make location and attraction at a distance unnecessary. The mating systems of some of these species will be discussed in the next chapter.
For those species that make use of sound communication in pair formation, it is usually the male that advertises his presence and readiness to mate by a calling, or advertisement song. As well as playing a role in attracting a willing female, and, perhaps, in influencing her choice of potential mates, the calling song is also involved in mediating the competitive interactions among rival males. Further, both the calling male, and the potential mate, of whichever sex, that travels to the other will be exposing themselves to increased risk of predatory or parasitic enemies. The different patterns of calling song and response that have evolved are thus the outcome of complex and cross-cutting combinations of both natural and sexual selection pressures (Hoy 1992).
THE MALE CALLING SONG
Since it is almost always the male who utters the calling song, and the female who (initially at least) moves towards the male, it is supposed that singing entails higher risks and costs than phonotaxis towards the singer, although considerable risks and costs must be involved in both. Viewed from the standpoint of its role in the mating system of a species, the function of song is generally thought to be to bring the female to the male. However, the male’s broadcasting of his song has other consequences. It makes him highly vulnerable to predators and parasites that locate prey or hosts by sound, but also the stridulatory movements themselves may provide a cue for visual predators. The male’s song also puts him into a competitive relationship with other males who may be present, either singing or not, who are potential rivals for the attentions of arriving females. Both of these aspects, along with selective pressures exerted by the females, are likely to have played a significant part in the formation of the great variety of singing behaviour and song characteristics to be found among the Orthoptera.
Both male songsters and females moving towards them are more vulnerable to visually hunting predators and parasites than they otherwise might be. Darwin’s advocacy of sexual selection in the case of the peacock and birds of paradise provided an explanation of the gaudy colour patterns and vivid displays of the males. However, where the medium of communication is sound, as in many orthopterans, the tendency has been towards cryptic coloration in both sexes, and relatively slight sex differences in appearance. If sexual selection favours development of singing ability in males, natural selection favours adaptation to offset the greater exposure to predation that the advertisement song brings with it.
An astonishing example of rapid evolutionary change in the balance of sexual and (survival-related) natural selection is provided by a population of the Polynesian field cricket Teleogryllus oceanicus. On the Hawaiian island of Kauai, where males were subject to attacks by an acoustically orienting parasitic fly (Ormia ochracea), a flat-winged mutation that rendered the crickets unable to sing spread to 90 per cent of the males in 15 to 20 generations, despite strong selective pressure in favour of songsters from female preferences (Zuk et al., 2006; Bailey & Zuk, 2008; Tinghitella et al., 2009).

FIG 79. Teleogryllus oceanicus, male. (© D. Rentz)
Where and when to sing
The conflicting pressures from predation risk and the reproductive interest in being heard by a receptive female combine to affect the male’s preference for his song perch. Males of many species that live among herbaceous vegetation tend to sing from the cover of dense tufts of grasses or small shrubs. In this way they can increase the distance over which their song carries, compared with ground-level emission, while benefiting from concealment. The great green bush-cricket (Tettigonia viridissima) tends to sing from perches at a greater height than surrounding vegetation. One study showed that the transmission of the song of this species increased with the height of its song perch above the ground (Römer, 1992). However, despite their large size and loud song, male great green bush-crickets are astonishingly difficult to locate visually. Males of the meadow grasshopper (Chorthippus parallelus) generally sing from much lower (less than 20 cm above ground) perches, presumably to avoid predation from birds and spiders (Gardiner & Hill, 2005b). In some species, males sing from the outer edges of grass tufts, but are ready to freeze and/or suddenly dive down into tangled vegetation when alarmed. British examples of these strategies include the wartbiter (Decticus verrucivorus), the grey bush-cricket (Platycleis albopunctata) and the bog bush-cricket (Metrioptera brachyptera). Other species, especially those ground-living species that inhabit more open and sparsely vegetated habitats, may typically utter one bout of song only from each perch, quickly moving on to another. This is a common behaviour among males of the British mottled grasshopper (Myrmeleotettix maculatus) and the common field grasshopper (Chorthippus brunneus). The common green grasshopper (Omocestus viridulus) and stripe-winged grasshopper (Stenobothrus lineatus) also usually utter just one burst of song before moving on to another perch. Species that typically inhabit bushes or trees combine cryptic colour patterns and shapes with a tendency to sing from the cover of dense vegetation (see the DVD for several examples of these tactics).
However, in all these cases it seems likely that the adaptation to avoidance of predation has been achieved at some cost to the reproductive function of the calling song. Where males move position after each burst of song, or where they sing from dense and structurally complex vegetation cover, the difficulties faced by females in locating the sound source and then moving towards it are considerable. Often, deviations from the standard model of male calling and female phonotaxis have evolved in response to these challenges. For example, males that call and search simultaneously are generally ones that take on the risks associated with both calling and searching while the females remain static. In a small number of other examples, notably the speckled bush-cricket (Leptophyes punctatissima), females reply to the male’s chirps, and the male’s location of and movement towards, the female is achieved by a finely adjusted call-and-response sequence.
This means of pair-formation is rather rare. It appears to be limited to a small number of bush-crickets. These include members of three European genera of ephippigerine bush-crickets and some phaneropterine bush-crickets, notably the British speckled bush-cricket. Among the ephippigerines, in some species the female moves to the male, while in others either sex may undertake the risks of phonotaxis (Hartley 1993). In Steropleurus stali the male approaches the female if she delays phonotaxis, which she is more likely to do the more previous matings she has had (Bateman, 2001). This is presumably a case of different male/female reproductive interests shifting the burden of phonotaxis between them. Among the phaneropterine bush-crickets, it is usually the male that moves to the female, a reversal of the more general pattern among signalling Orthoptera.
The signalling system of the speckled bush-cricket has been the subject of extensive observational and experimental research (e.g. Hartley & Robinson, 1976; Robinson, 1980, 1990; Robinson et al., 1986; Zimmermann et al., 1989; Robinson & Hall, 2002; Rheinlaender et al., 2007; Ofner et al., 2007; Kostarakos et al., 2007). The calling song of the male consists of an extremely brief chirp, emitted at irregular intervals. It consists of 5 to 8 rapid pulses (each lasting approximately one millisecond), the whole chirp lasting for no more than 10 to 13 milliseconds. A responsive female answers with a chirp at the same high frequency (in the ultrasonic range of around 40 kH), that is even shorter than the chirp of the male, often consisting of only a single pulse. Experimental studies carried out by Robinson and others have demonstrated that a male begins to move towards a female only if she replies within a definite time window of 35 milliseconds, placed between 25 and 55 milliseconds after the start of the male call. Subsequent location of the female is enabled by exchanges of chirps between the pair. The use of ultrasonic frequencies means that communication can take place only at quite close quarters, as these sounds attenuate with distance faster than those of lower frequency. In fact, it seems that although the female can hear and respond to the call of the male at a distance of up to seven metres, her song is more subdued and cannot be heard by the male at that distance. Male phonotaxis takes place only when the pair are separated by quite small distances of up to five metres (M. Hall, pers. comm.), the male response being limited by the intensity of the female’s response, or by a combination of that together with the length of the pause before he hears her response.

FIG 80. Stridulatory apparatus of the speckled bush-cricket: male and female.

FIG 81. Tibial ‘ears’ of the speckled bush-cricket: male and female.
It seems likely that this system has evolved under powerful predation pressures, with call and response kept to a minimum to limit the ability of enemies to eavesdrop. Males also increase their calling rate once a female responds, confining energy expenditure and predation risk to times when the chances of mating are increased. It seems likely that the critical time window for the male to respond to the female chirp helps in identifying her response by enhancing the signal-to-noise ratio. Not only is the speed of sound-processing in this species quite exceptional, but the ability of the male to locate the female despite the brevity of her responses is also remarkable. As this species may occupy perches at widely varying heights up to at least 10 metres (Ash & Robinson, unpub.; Robinson et al., 2009) in herbaceous or woody vegetation, males have to locate the source of the female chirps both vertically and horizontally. Detailed studies of phonotaxis in the males of this species have shown that they use a combination of their binaural hearing system with regular stops to alter their bodily posture, rolling from side to side, turning on the spot, and tilting their head and thorax downwards (Kostarakos et al., 2007; Ofner et al., 2007; Rheinlaender et al., 2007. See also Chapter 2).
The time of day when the males sing may also be seen as a compromise between reproductive advantage and predation risk and other cost factors. In general, we would expect males to sing in areas and at times when they are most likely to attract willing females, consistent with minimising the costs and risks of calling. One frequent compromise is to sing at night when visually hunting predators and diurnal sound orienting ones are not active. This has the benefit for the males of both enhancing the transmission of their song, as the sound conductivity of the air is greater at night, and reducing the energetic cost of calling. This latter cost is very significant. The calorific cost of calling averages ten times the normal metabolic rate in orthopterans, but in some cases it has been estimated to be as much as 35 times that of a resting male (Prestwich, 1994; Walker, 1983a). Females, too, may benefit from reduced predation risk as they move towards singing males under cover of darkness. Calling early in the night has the added advantage that ambient temperatures have cooled only slightly.
However, this strategy also has its downside: the threat from nocturnal predators! A detailed study by Belwood of nocturnal bush-crickets in the forests of Peru and Panama gives excellent insights into the complexity of the interactions involved (Belwood, 1990). Forest-gleaning bats, ovenbirds and tropical ant birds were all found to be major predators on bush-crickets, along with tropical screech owls, titi monkeys and spiders. In the face of such powerful selective pressures from nocturnal predators it seems that a variety of adaptations to song and singing behaviour have evolved. Some highly palatable species sing later in the night when bats are less active while those species that sing just after sunset formed the bulk of the prey in her study. The character of the song itself also appears to be adaptive to nocturnal predator avoidance. Individual chirps are very short (up to one second), and issued infrequently. The song is at a very high (ultrasonic) frequency, and the insects actually sing for only a small part of the song period. It seems that the sporadic nature of the emission of sound does limit the ability of bats to locate the calling bush-cricket, as does the tendency of these species to sing in choruses. Belwood suggests that chorusing might confuse predators and so make it more difficult for them to locate individual prey (see later in this chapter).
But there is a trade-off between predator avoidance and effective calling of potential mates. Adaptations that make songsters difficult for predators to locate may also have the disadvantage of making them difficult to locate by approaching females. Belwood suggests that there are two sorts of counter-adaptation that address this. One is fine-tuning of ear morphology and song structure to the detection of sporadic calls. The other is the use of vibratory signals that are not detectable by the bats. In two groups of bush-crickets she studied (copiphorine and pseudophylline species) calling was accompanied by complex up/down movements of the body with all legs firmly planted on the substrate. This tremulation produces airborne vibrations and vibrations in the substrate that females can use to locate the calling male. However, an added layer of complexity is provided by the fact that although bats cannot detect the vibratory signals, predatory spiders can. But, as bush-crickets are provided with highly sensitive detectors of vibration (the tibial subgenual organs) it seems likely that they can detect the approaching spiders (as well as other enemies) by vibration. Belwood also notes that the very long antennae of pseudophylline bush-crickets are kept in constant circular motion round their bodies while they are active, with the possibility that this might help to detect approaching spiders (see also Heller, 1995).
Song structure and what it communicates
As a form of communication the calling song conveys information both to potential mates and to other males. There are five variable aspects of the sound produced by orthopterans that, either separately or in combination, can convey such information. These are: the frequency or pitch of the sound; the pattern of rise and fall of pitch (‘frequency modulation’); sound intensity or loudness; the pattern of increase or decrease in sound intensity (‘amplitude modulation’); and the sequencing of sound patterns through time (different sorts and lengths of buzzes, chirps, trills, etc. and the intervals between them). However, the nature of the environmental conditions when the song is emitted and the relative location of the hearer both have consequences for the effectiveness of the communication of information. In general, the interference provided by the songs of other males of the same species, sounds made by members of other species (see clips of male songs of stripe-winged and common green grasshoppers on the DVD), as well as non-biotic sounds, such as the wind, can all serve to obscure aspects of the song structure. Also, the greater the distance between source and receiver, the more likely it is that the signal will be degraded. Although song intensity may make a difference to how far it transmits, other things being equal, the information content carried by it is limited by the fact that it varies with distance. Similarly, pitch may be varied by conditions of transmission, so it seems likely that for most species the most reliable information is carried by the sequential patterns of sounds through time.
Unfortunately there is no international consensus among researchers on how to classify the various elements of the song structure. The most basic element is the sound produced by a single up-and-down stroke of the hind leg against the wing in grasshoppers, or by the opening and/or closing of the tegmina in crickets and bush-crickets. This is sometimes referred to as a ‘phonotome’, but Ragge and Reynolds (1998) prefer the term ‘syllable’. A series of syllables forming a single unit of sound is termed an ‘echeme’, and, in turn, a series of these forming a repeating unit is termed an ‘echeme sequence’. However, the analysis of a song involves more complexities – for example, some species produce sounds in both directions of movement of the stridulatory mechanism, others only one (‘diplosyllables’, ‘hemisyllables’) and there are numerous different ways of sequencing echemes through time. The complexity of the song may be even greater than this – with some species interspersing incomplete syllables among the complete ones, and sometimes combining echemes into second-order repeated units. For some purposes, too, it is useful to include the silence between buzzes or chirps in the definition of the song unit – so the repeated unit is the period taken up by the chirp together with the interval of silence before the onset of the next chirp.
Finally, of course, some species have prolonged, continuous calling songs, and in cases such as these – for example, the great green bush-cricket (T. viridissima), long-winged conehead (Conocephalus discolor), and Roesel’s bush-cricket (Metrioptera roeselii) – the song can be given structure by variations in intensity or frequency. On a longer time scale, most species have favoured periods of day or night during which they sing. The daily pattern of these periods is known as the ‘duty cycle’. Generally, of course, males do not sing continually throughout even their duty cycle. The timing of duty periods during day or night varies very much among orthopteran species, in relation to predation risks, maturation times of females, temperature, and other factors as noted above (see, for example, Walker 1983a). For deeper understanding of these issues than can be provided here, see Ragge and Reynolds (1998), who also describe the techniques for producing and interpreting oscillograms to represent the structures of songs visually. For many more informal purposes, descriptive terms that indicate the pattern of sounds as they are heard by humans (unaided or with the use of such devices as bat detectors) – such as ‘buzz’, ‘trill’ or ‘chirp’ – have a reasonably clear meaning.
The issuing of the calling song by the male indicates to any female in hearing distance that he is ready to mate, and also provides cues to his location. A receptive female will turn towards the sound and make her way to its source. With hearing organs on both sides of the body, comparison of inputs from the two sides provides the female with cues as to the general direction of the sound. However, this is no simple matter, as both horizontal and vertical dimensions of location have to be combined, and she may have to surmount intervening obstacles, or deal with the branching structure of a bush or other complex perch. Experimental studies have shown that orthopterans cannot locate a sound from a source that is head-on to them, and so their movement towards the sound source takes the form of a succession of turns with subsequent corrections. The resulting course, even in the absence of obstacles, has a zig-zag character, as the insect responds to the relative direction of successive pulses of sound (Rheinlaender & Römer, 1990; Oldfield, 1980; Bailey & Stephen, 1984). The localisation of calling males by females attracted to them seems to improve with the height of their path above the ground, where there are fewer echoes and so improved directionality of sound (Rheinlaender & Römer, 1990), but this is likely to render them more vulnerable to predation. The neural processes involved in both recognition of the species-specific song pattern by a female, and location of the singing male, have been studied experimentally by Hedwig and colleagues. Directional location requires processing of inputs from each ear separately, while recognition of the song involves summation of inputs, but both aspects have to be integrated for phonotaxis to operate. Considerable progress has been made in identifying the neural structures involved in these feats in the case of crickets – especially Gryllus bimaculatus (see Hedwig, 2001, 2006, 2007; Hedwig & Poulet, 2004, 2005; Poulet & Hedwig, 2005; see also Chapter 2).
In many species, especially where males sing from perches in shrubs or dense vegetation, the fine tuning of location is achieved by the female detecting the vibrations of his perch that are caused by the male’s stridulation. Kalmring et al. (1990) report experiments showing that the locating ability of females of two European bush-cricket species (Tettigonia cantans and Ephippiger ephippiger) is enhanced if vibratory signals are given in addition to auditory ones. In the case of E. ephippiger the males cease stridulating when they detect an approaching female. Greenfield (1990) describes the use of vibratory signals by the males of the genus Neoconocephalus in addition to stridulation as an aid to females in locating their perches at close quarters. British coneheads of the genus Conocephalus also use vibratory signals (see the example of C. dorsalis on the DVD). There is evidence that visual clues are also helpful in mate location for some species. For example, the bush-cricket Poecilimon affinis is a species in which the male approaches a female who responds to his brief chirp within a definite time window. A study by von Helversen and Wendler (2000) showed that males were able to locate a responding female by sound alone, but only by way of a round-about route. However, when visual cues were added, their performance was much improved. It seems that they first establish the direction of the female from her response chirp, but then use visual landmarks to maintain a direct route to her.

FIG 82. A female Ephippiger ephippiger, a species that makes use of vibratory as well as auditory cues in phonotaxis.

FIG 83. European species of Poecilimon: P. thoracicus, female, and P. brunneri, male. (Bulgaria).
In those species that locate potential mates by way of acoustic communication, the calling song must carry information about not only the location of the caller, but also his species. In crickets, whose songs often include ‘pure’ notes, and in some bush-crickets, it seems that the frequency spectrum of the song plays an important part both in locating the singing male and in recognising his species (e.g. Oldfield, 1980). In bush-cricket species such as Tettigonia cantans, T. viridissima and Ephippiger ephippiger and E. discoidalis, in which the males sing from tall grasses or shrubs, females use the frequency composition of the song in identifying the singer. This is possible as the different sound frequencies attenuate at different rates with distance, so that the changing relative intensities of the different frequency components of the song will provide clues to both the distance of the singer and his species (Kalmring et al., 1990). However, for grasshoppers, and other bush-crickets, whose songs tend to include broad frequency bands, it seems that the song structure is the main feature used by females to identify the species of singing males. For many species it has been shown that the calling song of the male is the main means by which females recognise the species of potential mates, so that song plays an important part in reproductive isolation.
The distinctiveness of the male calling song is particularly significant where several species occur in the same habitat, and sing at the same times. Ability to occupy a distinct acoustic transmission channel in such orthopteran communities may be an important source of competition between species, and so may influence the species composition in the habitat (Bukhvalova, 2006). The reverse may also be true: the species composition of a habitat may influence song structures. Jang and Gerhardt (2006) compared aspects of song structure in two species of American crickets (Gryllus fultoni and G. vernalis). In areas where both species occurred, chirp rate and pulse rate of the songs were divergent. In locations occupied by only one of the species, both aspects of song overlapped. The characteristics of the calling song are likely to have played a significant part in the formation of new species (see Heller, 2006), and comparison of the songs of closely related forms gives useful evidence for classification, as well as providing the basis for speculation about their evolutionary origins. Ragge (1987) and Ragge and Reynolds (1998) have shown the value of oscillograms representing the song structures of European species for both these purposes. Ragge argues that given the role of song in species recognition, it is probable that where two forms have identical or very closely similar calling songs they should be regarded as belonging to the same species, even if there are minor morphological differences. Equally, differences in the calling song can justify separation of forms as distinct species even though they may be inseparable on the basis of morphological characters. Puzzles in the taxonomy of closely related species of Chorthippus and Euchorthippus among European grasshoppers, as well as bush-crickets in the genus Metrioptera, have been resolved on the basis of song comparisons (Ragge, 1987).

FIG 84. A male Tettigonia cantans singing from his perch (Swiss Alps).
However, the calling song of the male does not always provide definitive clues to its identification, and there are instances where females may be attracted by the songs of males of species other than their own. Often this does not result in wasted effort under natural conditions, as many such species pairs do not inhabit the same localities (even though their distributional range may overlap considerably). An experimental study by Morris and Fullard (1983) of song recognition in three American species of the genus Conocephalus produced some interesting results. Males of all three species aggregate and sing in unsynchronised choruses, and have similar broad frequency bands in their songs. Females of one species, C. nigropleurum, moved towards recordings of random noise, noise combined with songs of single males or choruses of males of their own species. They also appeared not to discriminate between songs of males of their own species and those of C. attenuatus. However, they did not respond to males of C. brevipennis, whether presented singly or along with males of their own species, even when the song of C. brevipennis was modified to make it more similar to the male of their own species. Although all three species have overlapping ranges, only C. nigropleurum and C. brevipennis occur in the same habitats (although as adjacent populations rather than intermingled).
Morris and Fullard suggest that females of C. nigropleurum may have undergone selection to recognise and ignore the songs of male C. brevipennis, rather than to identify the song of their own species. The failure to discriminate between conspecific males and those of C. attenuatus is not mal-adaptive as the two species do not occur together. According to Morris and Fullard, a wide range of continuous sounds of roughly the right frequency range will be sufficient to induce positive phonotaxis, with more precise species-recognition taking place at close quarters. They cite a parallel with the two related crickets, Gryllus campestris and G. bimaculatus, where females of the latter species appear to discriminate against songs of male G. campestris, rather than in favour of the songs of conspecific males.
In general it seems that where the songs of two species are very similar they tend to occur in different habitats, or call in different seasons or at different times of day (Schatral 1990). However, this is not always the case, and in the example of two North American ground crickets (Allonemobius fasciatus and A. socius), females of each species seem to be unable to distinguish the songs of the other from their own, despite actual differences in the songs. Doherty and Howard (1996) argue that in this case heterospecific mating is not necessarily mal-adaptive as there appear to be post-insemination barriers to hybridisation, costs to the females involved in mating are likely to be low, and they gain from nuptial feeding whichever male they mate with (see Chapter 6). The females of the bush-cricket Tettigonia cantans will respond to the songs of males of T. viridissima, especially if they are closer and so perceived as louder (Schul et al., 1998). This phenomenon of imperfect separation of the reproductive behaviour of closely related species (called ‘reproductive interference’) is of considerable interest, and we will return to it in the following chapter with reference to three groundhoppers (Tetrix species).

FIG 85. (a) A male Allonemobius fasciatus, singing (© D. Funk); (b) A male Tettigonia viridissima, showing stridulatory apparatus; (c) A female Tettigonia cantans, liable to confuse the song of T. viridissima with that of her own species.
Female preference and the calling song
These exceptions aside, the calling song of the male typically enables females to locate the singer, and to identify his species as the appropriate one. If a female is willing to mate, the song also stimulates her to move towards her potential mate. However, in many species there is evidence that the song has a third function: that of allowing the female to discriminate between rival males. As we saw in the previous chapter, the question of female choice in non-human animals has long been a source of controversy. However, if there are systematic differences in appearance or behaviour between the two sexes in a species, it seems likely that sexual selection has played a part in their evolution. As we saw, sexual selection can take the form of competition between males for access to females or of the exercise of choice on the part of females. Often, however, it is difficult to distinguish between these when we analyse the mating systems of particular species. As we shall see, males of some species of Orthoptera form aggregations and sing in chorus, so that it is difficult to decide whether mating success is a function of competition within the aggregation, or of female choice from among the assembled songsters.
However, there is now good evidence to suggest that females of at least some species show preferences for certain features of the song performance of some males, as against others (see reviews in Zuk & Simmons, 1997; Gerhardt & Huber, 2002; Robinson & Hall, 2002). Female choice may be classified as active or passive. Passive choice refers to the tendency of females to move towards the male that happens to be closest or sounds the loudest, and can be considered simply as contingent (i.e. the ‘choice’ just reflects the chance spatial relationship between singer and hearer) or as an aspect of competition among the males to project their song to receptive females. Where choice is active, however, females show preferences for the song characteristics of one male rather than another, in the absence of other means of differentiating them. Such preferences have been demonstrated experimentally for a range of species across the different sub-groups of the Orthoptera, and in some cases backed up by field observation. The calling songs of a New Zealand weta, Hemideina crassidens, are delivered from the entrances to holes, or ‘galleries’, in tree trunks, and are reported to have patterns specific to each individual (Field & Sandlant, 1983). It may be that these differences inform female choice of potential mates.
Theoretical considerations suggest that these female preferences may have evolved because they correlate with immediate or with genetic benefits to the female of mating with a male with the preferred trait, rather than with a rival. Immediate benefits might include mating with minimal predation risk (mating with the closest male if phonotaxis is risky might confer this benefit), mating with a large male who can provide a substantial nuptial gift (see next chapter), or with an energetic male whose powerful song implies that he is free of parasites. Genetic benefits, as we saw in the previous chapter, can be of two main sorts – the probability of producing offspring with ‘good genes’, or the likelihood of producing male offspring that will be attractive to females (‘sexy sons’).
One commonly reported example of female preference is for the first song heard when more than one male is singing within earshot of the female. This leading call preference has been shown to be widespread among bush-crickets, as well as some crickets and grasshoppers – extending even to frogs. The central American bush-cricket Neoconocephalus spiza, for example, exhibits this preference, even when the second male sings for longer and (within limits) more loudly. Snedden and Greenfield (1998) established that this is a result of a neurological mechanism that temporarily suppresses the animal’s response to a second sound. However, although this preference may not have evolved as part of the mating system, it does have consequences for it: if males’ mating success is influenced by their ability to be the lead caller, then males calling close to each other will be constrained to compete to be perceived as the lead caller, or adopt an alternative strategy. Studying a species of Mecopoda bush-cricket in southern India, in which females prefer leading male callers, Nityananda and Balakrishnan (2008) explored the availability of such alternative male strategies. They found that generally leading callers sang louder than followers, and that spacing did not compensate for the disadvantage of being a follower. However, a majority of follower males did sing when leaders were silent, and it was found that leading was a function of chirp rate, itself a consequence of contingent factors such as, in their view, male condition (but see Hartbauer et al., 2006). As leader and follower roles were not played consistently from night to night, it seems likely that this is not a heritable trait.
In some species the males stake out and defend song perches as territories. Occupants of such territories inhibit singing in males that cannot acquire or have not yet gained a territory, and such silent males are sometimes termed ‘satellite’ males. In her study of the north American meadow bush-cricket Orchelimum nigripes, Feaver (1983) observed that females only mate with singing males. In this species, the male territories are grouped together, and females show a preference for males among groups, as against lone singers. Even among grouped singers, the females (who mate only once) move from male to male over many hours before finally making their choice of male. Greater choosiness on the part of females is to be expected in species where the females mate only once.
Although experimental studies have revealed female preferences in a wide range of species, the nature of the preferences varies greatly from species to species. As well as lead callers, males may be preferred because they sing more loudly, or with higher, or in some cases, lower, frequency, greater rate of syllable repetition, more syllables per chirp, higher chirp rate and other song features (Gwynne & Bailey, 1988; Simons, 1988). A further influence on female choice in some species is an indication from the male call of structural asymmetry. In the European bow-winged grasshopper Chorthippus biguttulus, females discriminate against male songs that include gaps of more than 4 milliseconds. This generally is the outcome of loss of one of the male’s hind legs, and is thought to indicate some form of developmental deficiency (von Helversen & von Helversen, 1994).
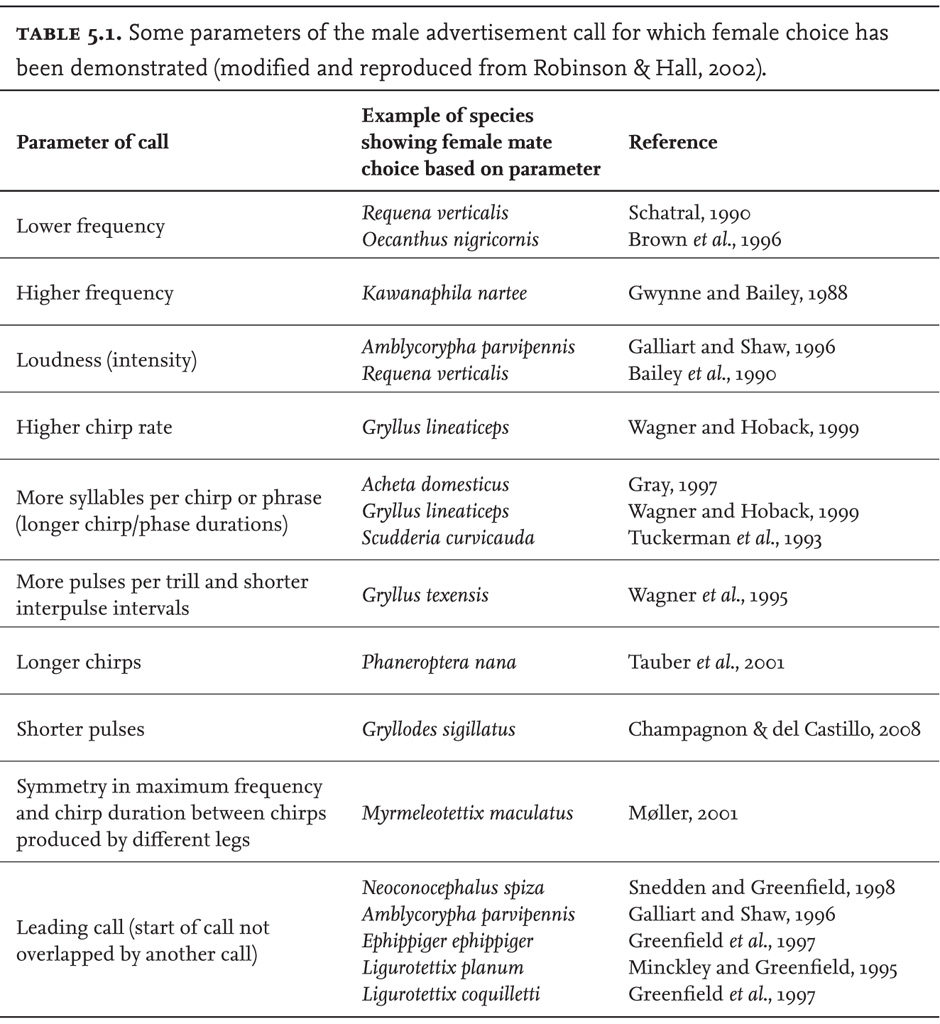
Some of these features of the calling song can be construed as reliable indicators of male fitness – both in the common-sense meaning of the term and in its more technical meaning in evolutionary theory (genetic constitution conferring high probability of survival and reproduction). Other things being equal, ability to sing loudly, as lead caller, with more signal content per unit time, as well as ability to sing for prolonged periods, indicate an energetic male, one that has out-competed rival males, is resistant to parasites and, in general, possesses ‘good genes’.
Females of Orchelimum nigripes use song characteristics to discriminate in favour of larger and older males (Feaver, 1983; Gwynne, 2001). Favouring older males might seem adaptive for the females since this is a species with a high rate of mortality among young males, suggesting that any male that has lived long enough and succeeded in gaining and holding a territory has good genes. However, in other species, a preference for younger males has been observed. Reasons for this may have to do with expected reductions in the fertility of older males through declining sperm quality, or insufficient sperm numbers (Velando et al., 2008). Verburgt et al. (2011) showed that, in Gryllus bimaculatus, various aspects of song performance changed with age, probably because of declining power in the flight muscles. These changes in the song would enable females to detect the age of potential mates and to exercise the preference of the females of this species for younger males.
There is more recent evidence that female preferences and the intensity of selection may be to some extent a function of female acoustic experience, rather than being under direct genetic control (Wagner et al., 2001; West-Eberhard, 2003). The case of the Polynesian field-cricket Teleogryllus oceanicus, in which the majority of males in one population lost their ability to call, provided a unique opportunity to explore phenotypic plasticity in female responses to male song. In this species, females generally show a preference to a song structure containing more long chirps. Bailey and Zuk (2008) conducted a rearing experiment in which some females were reared in silence (replicating common acoustic experience on Kauai) while others were played recordings of a range of male songs. When mature, the former group were both more responsive and less discriminating in their responses to male songs than females reared with relevant acoustic experience. This suggests that variability in female response to male song characteristics on the basis of experience during development may enable adaptation in a situation of radical environmental change, as on Kauai. Another implication is that the intensity of sexual selection on male song may also vary in relation to the acoustic experience of females. In general, it may be that mate-choice is likely to be less significant in low-density populations, or in complex environments, where locating any mate at all is relatively rare (Robinson, pers.comm.).

FIG 86. Orchelimum nigripes, male (© D. Funk); stridulatory apparatus of Gryllus bimaculatus.
Findings on female preference for large males are more clear-cut. Generally, there is a close relationship between the vigour of the male song – its chirp rate, intensity, persistence, etc. – and male size. In turn, size is a good indicator, for many species, of fecundity (the female can expect sufficient sperm to fertilise her eggs), and also of male competitive success against other males. This is particularly so in the case of males, such as those of Orchelimum, that defend territories. Tuckerman et al. (1993) showed female preference for songs to be indicative of male size in the north American bush-cricket Scudderia curvicauda. Similarly, Champagnon and del Castillo (2008) found that in the widespread tropical cricket Gryllodes sigillatus, females preferred males who delivered songs with short pulses. This trait is associated with large size, but males did not show significant genetic variance in those size parameters that were measured. As the authors point out, this could be the outcome of sexual selection depleting genetic variation in a characteristic relevant to male mating success. In the tree cricket, Oecanthus nigricornis, females show a preference for lower-frequency songs which are, in turn, indicative of larger male size. In this species, larger males are the ones that tend to win fights with other males, and females who mate with larger males produce more eggs (Brown, 1999). Some burrowing species, such as mole-crickets, use specially shaped ‘horns’ at the entrance to their burrows that amplify the sounds of their songs. Some South African tree crickets are also reported to amplify their songs by making holes in leaves of the right shape to fit their vibrating tegmina (cited in Ragge & Reynolds, 1998: 33).

FIG 87. Male Gryllodes sigillatus, singing. (© D. Funk)
A further reason for thinking that females might have evolved a preference for larger males is that in some species the larger males are able to supply greater direct benefits – such as access to good egg-laying sites, protective shelters, or, as in some bush-crickets, a nutritious nuptial gift. There is some evidence to suggest that female preference for song features that indicate large size may be associated with variations in the size of the male nuptial gift in those species that have this as a part of their mating system. In the European bush-cricket Poecilimon zimmeri, for example, females use song to locate and choose males. Where they have a choice, they prefer song features that are associated with male size, and this, in turn, is associated with spermatophylax size (Lehmann & Lehmann, 2008). In the tree cricket Oecanthus nigricornis, there is no correlation between female choice and spermatophylax size. However, larger males had more protein in the relevant glands, indicating that nutritional quality of the spermatophylax may be important, not just size (Bussière et al., 2005a, 2005b). However, for one well-studied species, the Australian bush-cricket Requena verticalis, the evidence is equivocal, and there may be no correlation between male size and either quality or size of the nuptial gift (see Schatral, 1990; Bailey et al., 1990. See also Chapter 6 for further discussion).
An experimental study of two US species of mole-crickets reported by Forrest (1983) indicated a strong female preference for loud-calling males. Males were placed in soil-filled buckets, surrounded by traps to collect others attracted by their songs. In fact, both males and females were attracted, but in both species the louder of two males placed within 20 metres of one another was far more successful in attracting females. In one calling bout, a male of Scapteriscus ascletus attracted 24 females, while his less vocal competitor attracted only one. In the case of the other species, S. vicinus, the figures were even more stark: 27 to none. However, as the acoustic horns these species construct to amplify their songs are more effective in damp soil, it could be that loudness of song is an indicator of a suitable site for oviposition, rather than (or perhaps as well as?) the size and fitness of the male.
Evidence of female preferences on the basis of male song has generally come from experimental designs that vary particular aspects of song that are associated with size, fecundity or some other male trait. However, some recent work has shown that at least in some species, female preferences are more complex than this assumes. Studies of the calling and phonotactic behaviour of the Australian field cricket Teleogryllus commodus analysed variation in five distinct features of song structure (Brooks et al., 2005; Bentsen et al. 2006). Females showed preferences for specific combinations of values of these aspects of song structure. These preferences are considered ‘stabilising’, as selection is away from extreme values of the structural features concerned. However, in field trials it was also shown that females preferred males that repeated their bouts of calling more frequently. As this is an open-ended variable, female selection on this aspect of male performance (indicative of male effort or condition) is directional. To make matters even more complex, it seems that there is an interaction between female preferences for particular structural characteristics of song and for male calling effort. Bentsen et al. conclude that studies that manipulate only a single dimension of male signalling may underestimate both the strength and complexity of selection. A study of female preferences of components of male song in Teleogryllus oceanicus by Bailey (2008) even showed that female preferences for different song components exercise selection pressures in contrasting directions!
Most of the studies of female preferences and sexual selection cued by male song have examined the effects of features of the male calling, or advertisement song. However, in many species of crickets, males have two versions of their song – a calling song and a courtship song, delivered at close quarters. There is evidence that for at least some species the calling song functions mainly to indicate the location and species of the male, while the courtship song may give more particular information about the quality of the male. A study by Zuk et al. (2008) gives support to this in Teleogryllus oceanicus. Like Bentsen et al., these authors distinguished a number of structural elements in the song – in their case, eight in number. As would be expected on the hypothesis that female choice is related to features of the courtship song, it was discovered that, among different males, the courtship song varied more than the calling song did in five out of eight elements.
CALLING SONG AS A MESSAGE TO OTHER MALES
The calling songs of males do seem to play an important part in the formation of pairs for courtship and mating in many species. However, in others, as we have seen, the calling songs appear to play a lesser role in male-to-female communication, and much research attention has been paid to the role of song in the mediation of the relationships among males. Indeed, sound communication between males appears to be no less significant a component of orthopteran mating systems than that between males and females.
It is commonly observed that populations of various species of orthopterans appear to be concentrated in small areas within wider stretches of apparently suitable habitat. It may be that this pattern of distribution is related to subtle differences in food availability, micro-climate, soil properties, or physical aspect. However, whatever their underlying causes, the existence of such aggregations invites questions as to how individual insects distribute themselves spatially within them. There is considerable evidence that the calling song of males is the main mechanism by which such spatial relationships are established and maintained. In the case of the mole-crickets studied by Forrest (1983), the male calling song also attracts other males and, as with the females, the louder song attracts the most males. Forrest’s hypothesis is that males may benefit from being drawn to other singing males either because this brings them to superior sites already colonised by other males, or because as satellite males they may be able to intercept females that are attracted to the initial songster. A further possibility, to be considered further below, is that aggregations as such confer benefits on the males that participate in them.
Like those of the mole-crickets, the calling songs of male field crickets also attract both males and females. However, within a certain distance adjacent males repel one another (Boake, 1983). The effect of these mutual responses to song on the part of males is to distribute them spatially into what are sometimes called ‘acoustic territories’. Within an aggregation, the spacing of singing males can be very uniform, and, at least in some species, it is mediated wholly by sound communication. This was demonstrated for the western Australian copiphorine bush-cricket Mygalopsis marki by Bailey and Thiele (1983). Experimentally deafened males were introduced into an area that had been cleared of normal males and their distribution compared to that of a control group over a period of six days. The spatial distribution of the control group was similar to that of undisturbed populations, but the deafened males remained in close proximity to one another. In another experiment, males with tegmina trimmed to reduce the intensity of their songs were released, and their spatial distribution measured. In this experiment, the males distributed themselves evenly, but their mean distance apart was significantly less than that of undamaged males. The study also revealed that in addition to moving closer or further apart in response to the sound intensity of their neighbours, males could also move up or down in the shrubby vegetation to adjust their relative positions. This response enabled males to maintain their acoustic distance while remaining on the same bush. In this species, males show strong fidelity to their song sites, with some males retaining their positions on the same bush for more than 30 days.
A similar study of the spatial distribution of males of a Colombian subtropical bush-cricket, Panacanthus pallicornis, in two types of forest habitat demonstrated that male spacing behaviour is mediated by sound communication. Artifically deafened males tended to aggregate, while the control group, with hearing intact, spread through the forest (Chamorro-R et al., 2007)
Like males of Mygalopsis, those of the mainly tropical and sub-tropical species of the genus Neoconocephalus also show strong fidelity to particular song sites, which they may occupy for several weeks (Greenfield, 1990). Regular spacing of singing males within a wider aggregation is also a feature of these species, and intruding males are repelled by a distinct contact call. Greenfield argues that discontinuous song structures may have evolved in some species with dense populations so that males have an ‘acoustic window’ between chirps to monitor the songs of adjacent males. In the case of species with a continuous song, it is possible that males are able to periodically close off the broad-spectrum tracheal hearing input so as to focus on the more finely tuned inputs from their tibial hearing organs (Bailey & Thiele, 1983). In common with other orthopteran groups, non-territorial, non-singing satellite males are often interspersed among the songsters. Among British species of the related genus Conocephalus there is evidence of territorial defence of song perches. Males of the short-winged conehead Conocephalus dorsalis appear to scent-mark leaves, and intruding males are driven off. However, in this species the song is continuous, and contact between males takes the form of intense antennation, aggressive bodily postures and kicking with the hind legs (see DVD). Males of another British species, Roesel’s bush-cricket (Metrioptera roeselii) also compete for favoured song perches. Rival males produce short bursts of song, unlike the continuous stridulation of the calling song, and lunge at one another until one retreats (personal observation; see DVD). Males of the meadow grasshopper (Chorthippus parallelus) also compete for favoured song-perches by posturing together with brief bursts of stridulation (see DVD).
It seems likely that spacing behaviour among males in many species represents a compromise between whatever benefits may accrue to individuals from being part of an aggregation, on the one hand, and avoidance of the costs of possible damage and heightened risks of predation that would be associated with direct physical conflict, on the other (Bailey & Thiele, 1983; Greenfield, 1990). However, the spacing of males within aggregations need not be even. If there are definite advantages to be gained from occupying some particular song perches rather than others, perhaps because of greater height, better protection from predators, or greater likelihood of encountering females, there may be intense competition between males within an aggregation to occupy particular sites. This will also be true where there is significant variation among males in their fitness, energy or aggressiveness. In these cases, the mutual monitoring of calling songs may provide information that enables each male to assess the likelihood of success in direct conflict. However, in many species, males also have a distinct aggressive song. In the New Zealand stenopelmatid Hemideina crassidens, the aggressive song is emitted when two males encounter one another, but it may also be used when courtship is interrupted, and before, during or after actual conflict between males (Field & Sandlant, 1983; Field, 2001). Greenfield and Minckley (1993) describe the alternation of graded aggressive songs between rival males of the American tarbrush grasshopper Ligurotettix planum. Males of this species compete to call from shrubs whose foliage is a favoured food source for females, and females may use the male call either as an indicator of his quality, or as a short cut to assessing the shrub he occupies as a food source (or both). For males to establish relations of dominance in this way, each must have a reliable indicator of the combat fitness of his rival. Greenfield (1997) argues that neither intensity nor frequency of the song will satisfy this criterion, and in fact individual males that win such stand-offs are normally ones that call faster or call for longer. This is the case in L. planum, and other species such as Gryllus bimaculatus and Hemideina crassidens.
Singing together
In addition to the male calling song’s role in mediating the spatial relationships and relations of dominance between males, there appear to be other sorts of information conveyed, and other dimensions of behaviour altered. By far the most dramatic and puzzling of these forms of behaviour are the ‘awesome’ chorusing episodes referred to in a classic article by R. D. Alexander (1975). These included ‘a massive synchrony extending several hundred meters, from one end of the forest to the other’ produced by cicadas, and another of ‘synchronised alternation’ exhibited by a population of katydids (bush-crickets) in the Appalachians. However, as Alexander acknowledges, these events are rare, and often not observed even by close students of the species concerned. It is thus unlikely that they form part of the evolved mode of life of the species, but rather should be seen as incidental effects of their normal behaviour under unusual conditions. Nevertheless, there are many species that do have as a regular feature of their behaviour periods when numerous males sing more or less simultaneously, and often modify their song in response to that of others in the same general area. This phenomenon of chorusing in orthopterans, as in some other groups of insects, amphibians and birds, has long been a topic of discussion and controversy. Wynne-Edwards (1962) offered a group-level explanation in terms of males monitoring and regulating population size, and Alexander himself in his earlier work offered a variety of explanations, some depending on physiological constraints that rendered some male song as an unavoidable response to that of a neighbour.
Alternating or synchronising
However, the more recent focus on the operation of selective pressures at or below the level of individual organisms, and the associated tendency to look for adaptive explanations of behaviour, have shifted attention from the group level of interaction to that of pairs of interacting individuals. These are now generally thought to form the basis of the larger-scale chorusing phenomena.
Alexander provides some interesting experimental evidence that, in the case of at least some species, the response of one male to the song of a calling neighbour is an incidental effect. Such ‘captive phonoresponders’ may be trapped into responding to the song of another by the way they have evolved to maintain the rhythm of their own song by monitoring it through their own hearing organs. However, he acknowledges that regular interaction in the singing of adjacent males is such a widespread and ingrained feature of the calling behaviour of many species that it is likely to have arisen, or at least become intensified, by selection – whether natural or sexual. In line with much contemporary thinking, Alexander postulates that these interactions are best understood as forms of male rivalry in the competition for mating opportunities.
If we consider the situation of two males singing within hearing distance of one another, Alexander argues that two of the obvious responses are likely to be maladaptive. Retreating to another song site is likely to incur risk of predation, may lead to a less favourable location, and will involve a loss of time and energy that might have been put into effective calling. The alternative of staying and fighting is also likely to be very costly, also involving risk of damage or even death, increased risk of predation, and diversion of time and energy. The adaptive alternative is likely to be to stay in position but out-sing the rival: ‘acoustic competition’.
Sometimes this results in the superior singer inhibiting the song of his rival. Greenfield (1990) gives the example of the inhibition of the discontinuous song of Neoconocephalus spiza by any continuous sound within the frequency range of its song. In this species, subordinate males confine their chirps to gaps in the song of continuous singers, or sing at another time of day.
Alternatively, male songsters may stimulate the competitive responses of others. The consequences of this will differ as between different sorts of song structure. Alexander discusses the likely outcome of male-to-male competitive phonoresponse for three common types of song structure. Where the normal song is a prolonged trill, composed of rapidly delivered pulses, a competing male can either start first, or continue after his rival has stopped. The result would be extended and overlapping songs produced by adjacent males. Alexander gives the tree crickets Oecanthus species as examples, and among the British species we might include the long-winged conehead (Conocephalus discolor) and Roesel’s bush-cricket (Metrioptera roeselii).
In species whose male calling song is a series of short chirps, and in which the key recognition pattern for females is the pulse rate, number or pattern within the chirp, the maximally effective strategy for a male will be to chirp in the interval between his rival’s chirps. This pattern of alternation is widespread among male Orthoptera. The north American bush-cricket Pterophylla camellifolia is often cited (e.g. Greenfield & Shaw, 1983), as are such British species as the grey bush-cricket (Platycleis albopunctata), the dark bush-cricket (Pholidoptera griseoaptera) and grasshoppers such as the common field (Chorthippus brunneus) and the common meadow (Chorthippus parallelus) (see DVD). In the case of the dark bush-cricket, the temporal frequency of chirps is increased during bouts of alternation, and when alternating at close quarters (less than three metres) the length of each chirp is increased (Young, 1971, cited in Greenfield & Shaw, 1983). As Robinson and Hall, (2002) point out, this raises interesting questions about how species in which mutual location and phonotaxis depends on exchange of chirps between males and females manage to avoid obscuring the responding chirps of females by acoustic interactions between males. In the European bush-cricket Phaneroptera nana the frequency spectra and temporal patterns of the male and female songs are different, and males respond in a very brief time window to the female reply, but delay their response to an alternating male (Tauber, 2001).

FIG 88. Male long-winged conehead singing – note the partly exposed mirror of the right tegmen.

FIG 89. Male grey bush-cricket singing.
If the key communicative unit is the pattern formed by slowly repeated chirps and the interval between each and the next, then alternation on the part of males would produce the effect of a more rapidly repeating pattern than is characteristic of the species. In such a situation, the species-specific pattern for female recognition could only be preserved if the males synchronise their chirps. Presumably sexual selection would tend to produce synchronised chirping in such species.
However, some authors (unlike Alexander) postulate ‘spoiling’ tactics on the part of competing males. As we have seen, even in synchronising species chirps are not delivered exactly in unison. There is a leader/follower pattern in Neoconocephalus, in which the follower, in beginning the repeating unit of the song slightly later than the leader, will obscure the ending of the latter’s chirp or buzz (Greenfield, 1990). This may be significant if the ending of song units is used by females or other males in identifying the caller (Greenfield & Shaw, 1983; Greenfield, 1990). Greenfield et al. (1997) have provided a model which would explain both synchronisation and alternation of male calls as consequences of a mechanism in the central nervous system that regulates the rate of signalling. This might have evolved as a male competitive strategy in response to female preferences for leading calls, as in the south Indian bush-cricket Mecopoda elongata (Hartbauer et al., 2006; Nityananda et al., 2007). However, models provided by Nityananda and Balakrishnan (2009) for the sister species, Mecopoda ‘chirper’, suggest that in this species, in which males alter their chirp rate in response to one another, synchrony could not have evolved as a stable strategy unless aggregation preceded it. They argue that, at least in this lineage, aggregation may have conferred advantages in terms of protection from predation, or communication of intra-specific cues, with synchronised calling as a cooperative strategy being selected subsequently.
There are other, more complex interactions in which parts of rival male songs are obscured. Heller’s study of a subdivision of phaneropterine bush-crickets (the Barbitistini) illustrates the diversity in this group of complex male song structures, including changes of amplitude of single syllables, combinations of different (‘hard and ‘soft’) syllable types and fixed temporal sequences of syllables of each type. Females respond to specific elements in the male song, and Heller suggests that complexity has evolved in response to competitive interactions between males to obscure significant elements in one another’s calls (Heller, 1990; Greenfield & Roizen, 1993; Minckley et al., 1995; see also Bailey (2006) for a more detailed discussion of the evolution of signal complexity).

FIG 90. Neoconocephalus retusus, male. (© D. Funk)
Alexander, too, argues that selective pressures arising from a range of different strategies used by males in addition to simple competitive calling may explain the evolution of more complex song structures. Since the formation of pairs is frequently disrupted by the intervention of competing males, more complex calling songs may have evolved to signal differentially to rival males, and/or to females at various distances. The latter may respond to different parameters of the song (intensity, lead frequency, rapidity of pulses, number of pulses in a chirp, and so on) at different distances. For example, males of the eastern US bush-cricket Microcentrum rhombifolium alternate two calls (‘ticks’ and ‘lisps’). The ‘lisp’ when emitted at high intensity is a long-range signal, while at low intensity, females move towards the male. Females also reply to the series of ticks in the male song, also with ticks, and these enable the male to locate the female at short range. Males also use the ticking signal when attempting to interrupt a communicating pair (Alexander, 1960 and Grove, 1959, cited in Alexander, 1975).

FIG 91. Barbitistes obtusus, male (southern France), a species with a complex song structure (see Ragge & Reynolds 1998: 120–122).
The British short-winged conehead (Conocephalus dorsalis) also has a complex prolonged song in which two different sounds – described by Ragge (1965) as a ‘hiss’ and a rapid ‘ticking’ sound – alternate with one another every few seconds. His more detailed analysis (Ragge & Reynolds, 1998) reveals an alternating pattern of repeated echemes, each of which is made up of four syllables (the ‘hiss’ or ‘rustle’ component), and repeated single-syllable ‘ticks’, each sequence lasting several seconds. The significance of this degree of complexity is not clear. The mating system of this species does seem to involve territorial defence, and there is frequent antagonistic interaction between males. However, it is also the case that this species and the long-winged conehead (Conocephalus discolor) overlap in their habitat requirements and are frequently found in mixed communities. Superficially the songs of the two species are very similar (although hardly audible to the human ear without amplification), but in C. discolor the ‘hiss’ echemes are tri-syllabic. In general the song of C. discolor is uniform, without the regular alternation of ticking phases – although as Ragge & Reynolds show, some males of C. discolor do include brief interludes of ticking sounds in their song. It may be that this is a case where the key function of song complexity has to do with species recognition (see sequences of both species on the DVD).
Aggregation and chorusing
Alexander argues that many chorusing phenomena may simply be incidental consequences of competitive interactions between individual males. However, even this implies some degree of spatial aggregation of singing males and clearly aggregation and chorusing are closely related to one another. Aggregations, in the sense of patches of more densely concentrated subpopulations, may be, and usually are, based on the spatial distribution of some important resource. Aggregations of sexually active males are frequently associated with zones that contain resources important for females, especially safety from predators, feeding or egg-laying sites, or with areas where females are more likely to be encountered for other reasons, such as emergence sites. Groupings of males in such locations are referred to as ‘resource-based aggregations’. Such aggregations may be the result of passive movements of males, in the sense that the aggregation is merely an incidental effect of a number of males independently finding or being drawn to them on the basis of the likelihood of finding willing mates. However, the evidence in many species is that males are attracted by the songs of males that are already aggregated. This could be understood as a behavioural disposition that has evolved from an original basis in passive aggregation.
More difficult to explain are aggregations of chorusing males that appear not to be centred around competition for or control over areas that are otherwise more likely to be favoured by females. As Alexander rightly points out, it can often be difficult for a human observer to be sure that such areas really do not include resources important for females. However, in those cases of chorusing that are presumed not to be resource based, this appears to be a form of cooperative behaviour among the males that are in other respects rivals. The current orthodoxy in evolutionary theory demands an explanation of how such cooperation could have evolved (and could persist) in terms of the reproductive interests of individual males (or their genes). There are two strong candidates to meet this challenge. One is that non-resource based aggregations offer protection from predation in some way. This could be because an approaching predator might be more easily detected, or, perhaps, because in some way potential predators are confused or intimidated by the size of the aggregation, or the volume of the sound, or the difficulty of locating individual sound sources. On the other hand, it might also be the case that aggregations of chorusing insects could actually attract the attention of predators and so increase predation risk, and there seems to be little evidence either way.
The other possible explanation advanced by Alexander draws on the idea of sexual selection by female preference. It could be in the reproductive interest of individual males to approach and call from an aggregation only if by doing so they were more likely to have mating (and fertilisation) success, than if they were to remain aloof and sing solo. This could be the case if chorusing groups attracted into their midst more sexually responsive females per member from outside their ‘arena’ than the average solo performer. In turn, this would be a likely scenario if females had evolved preferences for males singing in aggregations, compared with soloists. Females would be likely to evolve such preferences in polygynous species where the males offer little in the way of parental investment (such as control over resources, as in resource-based aggregations). In such species, females would be likely to express preferences for inheritable traits that are generally preferred by females of the species, or for phenotypic indicators of male ‘quality’. Either way, female preferences for male traits could have selective effects only if females are able to compare the performances of a number of rival males, and also if this could be achieved with minimal additional cost or risk to the female over simply mating with the first male she encounters. Given these conditions, there would be strong sexually selective pressures on males to form chorusing aggregations. Such pressures would become still more powerful if females simply refused to mate with solo males, and they might even override any costs to both sexes from increased risk of predation.
This analysis draws on the analogy between insect chorusing and the lekking behaviour of some vertebrate species (see the previous chapter). Despite some disanalogies, such as the reuse of the same arena year after year by relatively long-lived individuals in the case of birds such as grouse and European ruff, the model does seem to work quite well for at least some chorusing behaviour of insects. However, there still is only limited empirical evidence of the relative mating success of different males within such insect choruses, or of any advantage accruing to male members of choruses compared with soloists.
T. J. Walker (e.g. 1983a) took this argument further, and provided some empirical confirmation. Using essentially the same model of sexual selection as Alexander, he emphasised the distinction between spatial aggregation and chorusing. While spatial aggregation is the key characteristic of leks, the relevant feature of orthopteran choruses is that they are concentrated in time. They are likely to occur during time periods when the maximum number of females is available, and, reciprocally, females are most likely to be active when numerous males are performing, enabling their displays to be compared. These are likely to be relatively brief periods during the day, and choruses as ‘concurrent display’ are the temporal equivalent of a lek – for which Walker coined the term ‘spree’. Walker gives the examples of American short-tailed crickets (Anurogryllus arboreus) and mole-crickets of the genus Scapteriscus which chorus without aggregation, although it is difficult to see how chorusing could occur in the absence of at least some spatial aggregation (or indeed, how lekking could work without temporally simultaneous displays!).
In the case of Scapteriscus species, males chorus briefly just after sunset, and females fly to them. Flight entails potentially increased risk of predation, as well as significant energetic costs to the females. The adaptive ‘tradeoff’ in this example is that males call and females fly to them at night, so avoiding visual predators, but this occurs just for a short period after sunset when ambient temperature is still high enough to minimise energetic costs. The spree is thus reinforced by males calling more profitably during this period, and females responding at a time when they have the best chance of comparing male calls. This pattern of chorusing – where most or all of the males in a local population sing together for limited periods, followed by extended periods of silence, is termed ‘unison bout singing’. Where there is no consistent temporal relationship between song units delivered by individual males within the chorus, this is referred to as ‘unsynchronised unison singing’ (Greenfield & Shaw, 1983) or ‘unsynchronised chorusing’ (Otte, 1977). Examples mentioned in the literature include field crickets (Gryllus species), stripe-winged grasshopper (Stenobothrus lineatus) and Neoconocephalus exiliscanorus (see Robinson & Hall, 2002).
There appears to be rather little evidence that bears directly on the question whether individual males singing in choruses actually encounter more females, or acquire more mating opportunities than they would if they sang solo. Morris et al. (1978) reported a preference on the part of females of the north American bush-cricket Conocephalus nigropleurum for grouped males. However, studies on other species have failed to find evidence of this (Robinson & Hall, 2002).
In the absence of such evidence, the most likely explanation of much orthopteran chorusing is that it is an incidental effect of interactions among males that have become spatially aggregated. As noted above, resource-based aggregations, whether formed passively or by attraction of males to the calls of others already aggregated, are relatively easy to explain. Questions concerning the frequency and interpretation of purely mating aggregations and ‘sprees’ remain open to further research.
INSTEAD OF CALLING: OTHER MALE STRATEGIES
As we have seen, some groups of orthopterans do not use sound as a mode of communication. These are usually species that live in dense aggregations such that a distinct phase of rapprochement is unnecessary: males and females readily encounter one another in pursuit of their non-reproductive activities. However, even among species that do communicate by sound, and in which males have a distinct calling song, the calling song is by no means the only way that males find themselves potential mates.
One alternative male strategy, especially for smaller individuals or less powerful singers, is to adopt the role of silent satellite close by a male songster, and attempt to intercept females drawn to his neighbour. This is particularly common in males of species where there is male conflict over song perches or control of resources important to females, such as the British short-winged conehead and field cricket.
Defeated males in song contests over occupancy of bushes in the American tarbush grasshopper Ligurotettix coquilletti may increase their chances of mating by remaining on the bush even if the cost of doing so is to avoid direct conflict with the dominant male by remaining silent (Greenfield, 1997; see the next chapter for a fuller account of this mating system). Studies of satellite behaviour in several species suggest that playing this role is a consequence of prevailing conditions, and individual males may adopt either strategy, according to circumstances. For example, a subordinate male in a high-quality location may switch to calling when he shifts to a lower-quality location.
In at least some grasshopper species, it seems that the male advertisement has a relatively small role in the formation of courting or mating pairs. The males of the US band-winged oedipodine grasshopper Trimerotropis maritima, for example, engage in dramatic flights, in which they display the bright colouring of their hind wings and as they do so emit ‘crepitation’ sounds. However, a detailed observational study by Steinberg and Willey (1983) offered no evidence of females being attracted to such displays. As many as 162 instances of pair formation were observed, and all of them resulted from the searching activities of males on the ground.
In some circumstances, especially where population density is high, it may benefit males to modulate between singing and active visual searching. Males of the lesser mottled grasshopper (Stenobothrus stigmaticus), lesser marsh grasshopper (Chorthippus albomarginatus) and the mottled grasshopper (M. maculatus) are among those that often adopt this method of mate searching, spending much of their time moving rapidly around favoured areas of their habitat, emitting occasional bursts of stridulation (see the DVD).
In studies of a zone of overlap between the nominate sub-species of the meadow grasshopper and the Iberian sub-species, C. parallelus erythropus (Ritchie, 1990; Butlin & Ritchie, 1991; Neems & Butlin, 1992, 1995), females of each subspecies showed statistically significant preferences for males of their own subspecies. This suggests that female choice is influenced by aspects of male calling or courtship behaviour that differ as between the two sub-species. Behavioural studies in the zone of overlap between the two did show differences in the patterns of calling, courtship and search behaviour. C. p. parallelus males sang in longer bouts, with more songs per bout, than did C. p. erythropus. In addition, male erythropus songs had longer intervals between echemes, and between bouts of singing they moved actively in search of females. Males of C. p. parallelus more frequently remained inactive between bouts of singing. Although not discussed in Neems and Butlin, sub-species erythropus, unlike parallelus, has a well developed and distinctive courtship song (see oscillograms in Ragge & Reynolds 1998). The implication of these studies is that female discrimination and response in C. parallelus parallelus is primarily to acoustic cues in the male song, while in erythropus, the greater devotion to searching as against singing suggests a greater role for visual and chemical cues at close quarters (Ritchie, 1990). It seems likely that chemical cues in the form of pheromones play a significant role in orthopteran courtship generally. This topic has recently become the focus of more research, as we shall see in the following chapter.