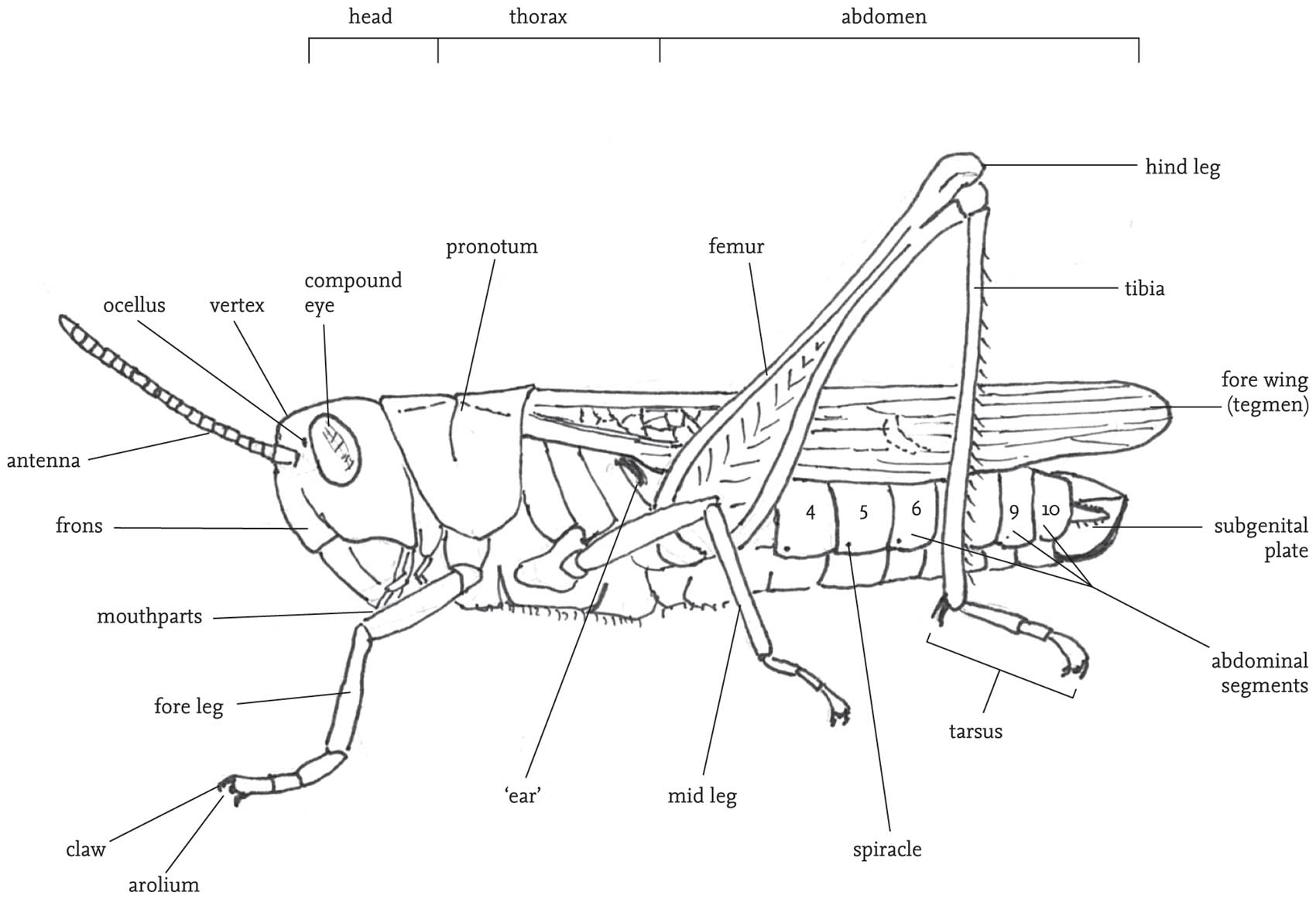
FIG 30. External structures of a male grasshopper.
Orthoptera: structure and function
Singing, hearing, jumping and flying
Subsequent chapters will be devoted to the natural history of orthopterans – their adaptations to their habitats and modes of life, their reproductive interactions, methods of avoiding predators and parasites, and so on. This chapter aims to provide a brief outline of the mechanisms underlying these different activities. Grasshoppers, crickets and their relatives are equipped with specialised sensory organs that detect vibration, sound, moisture, taste and smell. They also have well-developed visual acuity. Sensory inputs from this range of sources are processed in the nervous system and issue in nervous and hormonal messages, which in turn trigger motor activities such as feeding, flying, mating, egg-laying and jumping. There is space here to deal with only some of these sensory and motor activities, and only in a summary way. However, there should be enough information to give the reader a sense not only of how these insects achieve such remarkable feats of, for example, direction-finding, thermoregulation, or escaping, but also of their limitations and vulnerabilities. Because of the special place of sound communication in the lives of most orthopterans, the mechanisms involved in this will be explored in a dedicated section.
The chapter begins with a brief guide to the main bodily features of grasshoppers and crickets, and this will be referred to in other chapters. Some knowledge of this is essential for identifying specimens, as well as for understanding many descriptions of their behaviour.
EXTERNAL FEATURES
There are features of structure and associated sensory and motor abilities that are widely shared across the order.
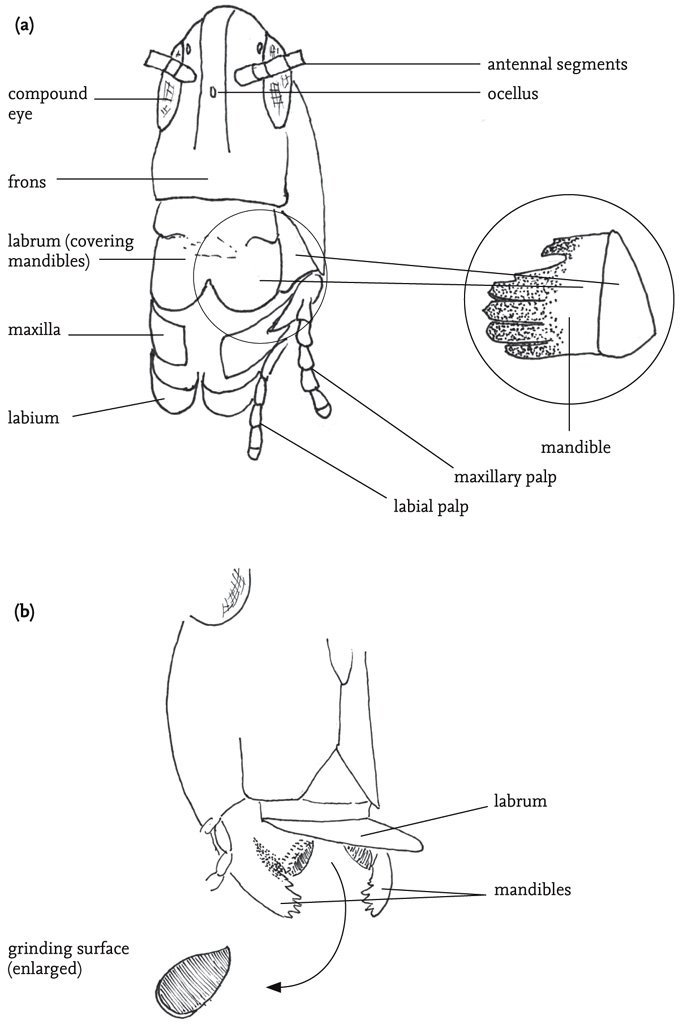
FIG 31. (a) Head and mouthparts of a grasshopper, with (right hand side) right mandible (hidden behind the labrum in the main drawing); (b) Mouthparts of a groundhopper, with labrum raised to reveal mandibles, with flattened grinding surface.
The body is divided into three sections: head, thorax and abdomen. The main features carried on the head are the two large, prominent compound eyes, the antennae, and the mouthparts with their associated sensory organs, the palps. Most species also have three tiny simple eyes situated on the ‘forehead’ (frons), between the compound eyes. The mouthparts are adapted for biting and chewing, and consist of an upper ‘lip’ or labrum that covers a pair of mandibles. The mandibles are used for biting. They open and close laterally, and usually have a serrated outer edge. Below these are the maxillae, used for chewing. The maxillae and the ‘lower lip’ (labium) bear fine, jointed projections, the palps. These are important sense organs, and are closely involved in detecting and selecting food, as well as being involved in chemical communication between the sexes, especially in bush-crickets. The antennae project from the forehead. They consist of a variable number of ring-like segments, and are covered with sensory hairs (sensilla) that give the insect both tactile and chemical information about its environment. The head also contains the brain (‘cerebrum’), and another neural complex, the suboesophageal ganglion. These receive sensory inputs directly from the eyes, antennae, mouthparts and alimentary canal, as well as receiving and processing neural inputs from the rest of the body. Close by in the head are two pairs of endocrine glands, the corpora allata and corpora cardiaca, whose secretions play a range of crucial roles in metabolism, behavioural readiness and development.
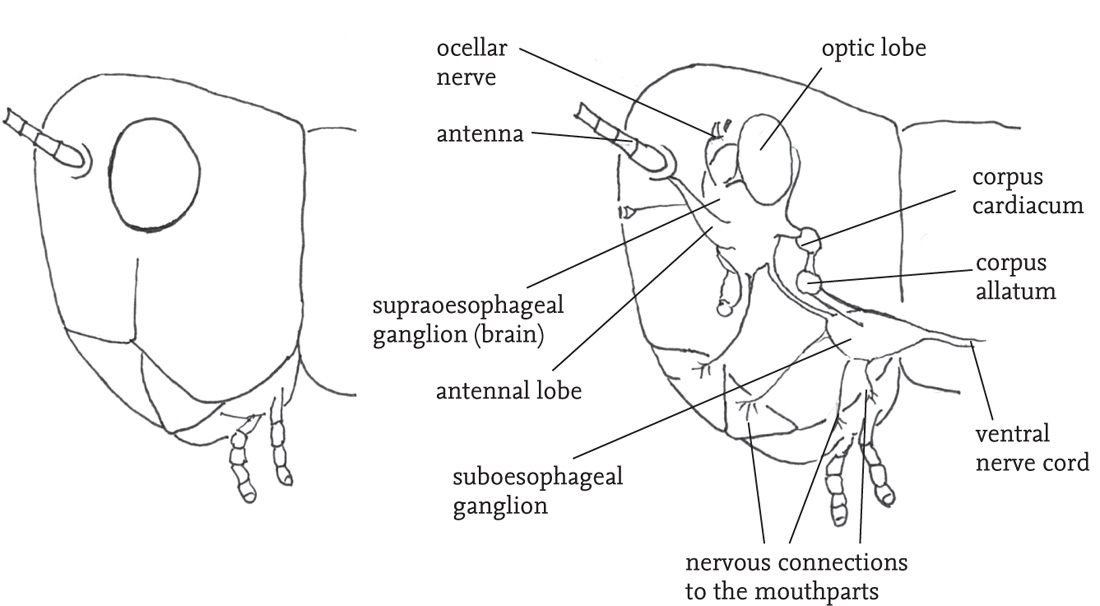
FIG 32. Side view of the head of a grasshopper, showing (right hand side) the internal nervous and endocrine organs (schematic).
The thorax is composed of three segments. A pair of legs is attached to the lower part of each of these thoracic segments, and, in winged species, the wing-bases stem from the upper part of the second and third thoracic segments. The dorsal plate on the first thoracic segment (the pronotum) is saddle-shaped (except in the mole-crickets), and covers the wing-bases. The shape and structural features of the pronotum give useful clues to the identification of species, especially in the grasshoppers. The legs articulate with their respective thoracic segment by way of short sections known as the coxa and trochanter, while the longest sections (the femur and tibia) are joined to one another by knee-like joints. Each leg ends in a foot, or ‘tarsus’, which usually consists of three or four segments, the terminal one of which bears a small claw. The legs are used in locomotion, but also play a role in regulating body temperature, enabling the insect to lift itself from, or press its body down on its substrate. The fore legs are also used for manipulating food items, and may be extensively modified for digging, especially in burrowing species. In most groups of the Orthoptera the hind legs are much longer than the other two pairs, and the femora are packed with powerful muscles that are involved in jumping. In some groups, notably the mole-crickets, the hind legs are secondarily reduced, but in almost all species some ability to jump is retained. In some species there are sharp spines on the hind legs that are used in aggressive encounters, or for defence.
In winged species there is usually a marked structural differentiation between the fore and hind wings. The former are relatively narrow and to some degree hardened, and termed the ‘tegmina’ (singular: tegmen). When the insect is at rest the fore wings are held back over the abdomen, and provide a protective cover for the more delicate hind wings, which are folded fan-like between the tegmina and the abdomen. The thorax in fully winged orthopterans is packed with powerful flight muscles, and each segment has a complex of nerve cells (ganglion) that is involved in the control of flight and the movements of the legs, sometimes directly responding to external stimuli, but also under the control of the coordinating function of the brain. Except in populations undergoing range extension, and in migratory species, such as the locusts, orthopterans rarely fly or jump spontaneously. These activities are costly in energetic terms, and tend to be used only in cases of emergency, typically in escape from predators. Some species, notably the groundhoppers, but also some grasshoppers (see Gardiner, 2009e) are able to use their legs to swim.

FIG 34. Hind legs of tree weta, Hemideina crassidens, showing spines on the tibiae (collection of the Natural History Museum, London).
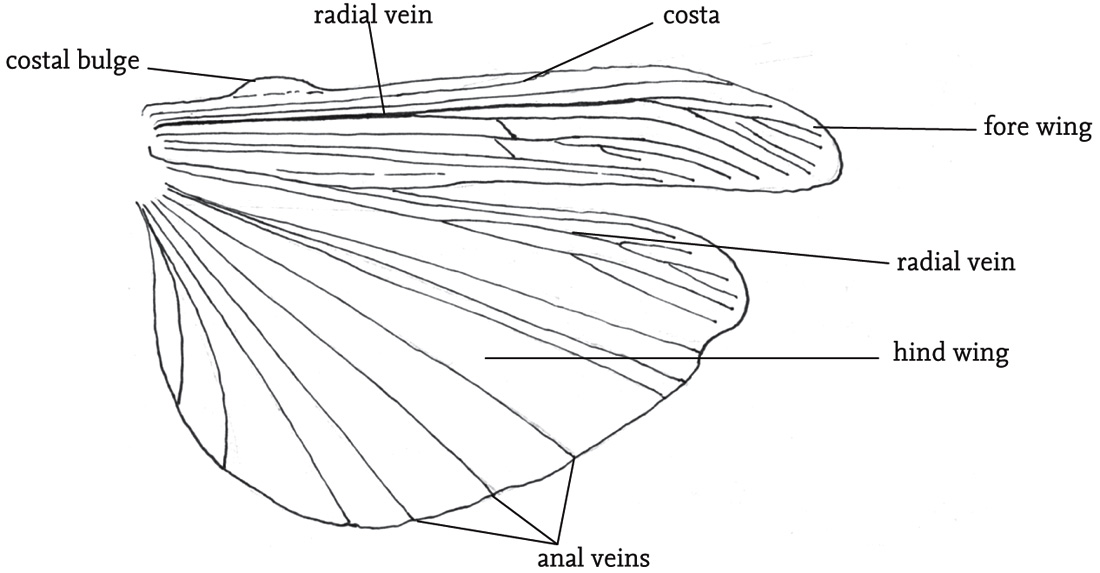
FIG 35. Extended wings (right-hand side) of the field grasshopper, Chorthippus brunneus, showing the principal veins and costal bulge.

FIG 36. Groundhopper with wings extended (© Roy Cornhill); slender groundhopper, Tetrix subulata, swimming.
The abdomen consists of eleven segments, but these are not always clearly visible, as terminal structures are considerably modified for reproductive functions. Each segment has pair of spiracles, or breathing pores, which allow the exchange of gases involved in respiration, but also play a role in maintaining the water-balance of the body. Towards the tip of the abdomen is a pair of projections (cerci), which may be modified in the case of males to constrain females during mating (see the Key, Fig K5). Towards the tip of the female abdomen is a genital opening into which sperm pass during or after mating, and a complex structure used for egg-laying (the ovipositor). The shape and structure of the ovipositor can be a useful aid to identification, especially among the bush-crickets (see the Key, Fig K3). At the tip of the abdomen, the ventral portion of the segment is termed the subgenital plate. The shape of this is also useful in the identification of some species. The contents of the abdomen include the hindmost sections of the digestive system, terminating in the anus at the tip of the abdomen, the rear portion of the longitudinal ventral nerve chord, together with a series of ganglia distributed along it, and some glandular structures associated with the formation of the eggs (and in some groups egg pods) in females, and sperm, plus ancillary substances secreted along with the sperm, in males.
ORTHOPTERAN SENSES
Orthopterans are able to monitor a wide range of aspects of their environment by means of both specialised organs and numerous minute projections from the body surface known as sensilla. The main specialised organs are the eyes, hearing organs and vibration detectors. The sensilla are distributed over the whole body surface, but are especially concentrated in certain areas such as the palps, mouthparts, antennae and, in females, the ovipositor.
The reception of sound and vibration will be dealt with separately below. The organs of visual perception are the two compound eyes, combined with three simple ocelli arranged on the front of the head. The compound eyes give a 360° field of vision, with a relatively small area of frontal overlap. This enables binocular vision in front of the insect, and so the distance of a fixed object can be judged by parallax. Each of the facets and associated structures (ommatidia) that form the compound eye receives light from a narrow field, and the associated sensory cells are linked to neurones that synthesise the multitude of sensory inputs into a single image. This does not give the insect clear vision of detail, but broad patterns of shape, contrast, and especially movement can be detected. Light receptor cells grouped within each ommatidium contain specialised areas (‘rhabdomes’) that contain pigments, and variations in these are responsible for colour vision. The ocelli are believed to function mainly to monitor light-levels, and may be important when light intensity is low. Orthopterans such as locusts that engage in long-distance flights, as well as others involved in dispersal during range extension, or simply moving to favoured parts of a local habitat, require directional information to aid navigation. This is provided by specialised ommatidia in the dorsal rim of each compound eye, enabling detection of light polarisation. This has been studied in the locust (Schistocerca gregaria), but the adaptation seems to be present in a wide range of other species (Zufall et al., 1989; Labhart, 1999; Labhart & Meyer, 2002)
The senses of touch, taste and smell, as well as the ability to monitor temperature and moisture levels, are all mediated by sensilla. These are variously shaped outgrowths of the cuticle that enclose a variable number of nerve-cells whose dendrites are adapted to respond to specific stimuli and which transmit sensory information via their axons to the ganglions of the central nervous system. Sensilla that respond to chemical stimuli, as well as those used to detect moisture levels, have pores in their cuticles. The olfactory sensilla (responsible for the sense of smell) have numerous pores that allow in airborne molecules, while those responsible for the sense of taste (contact chemoreceptors) have a pore at the tip. Chemically sensitive sensilla are especially densely concentrated on the antennae, and, as the antennae are used especially for distance reception of scents, the olfactory sensilla predominate. However, there are also numerous touch- and taste-sensitive sensilla on the antennae.
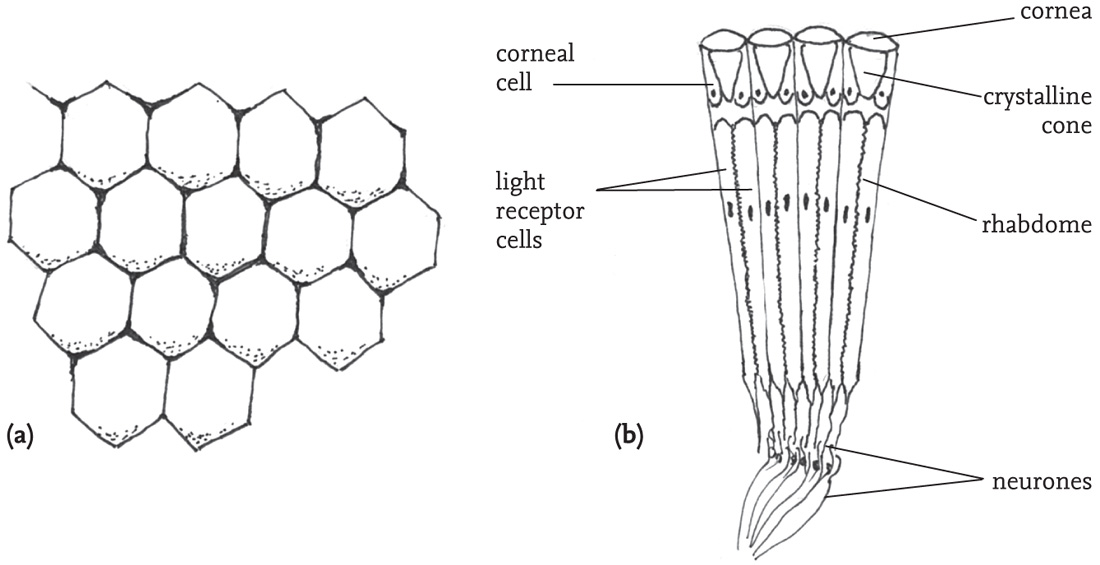
FIG 38. (a) Surface of part of a compound eye, showing hexagonal facets; (b) section through a compound eye showing four ommatidia (simplified).
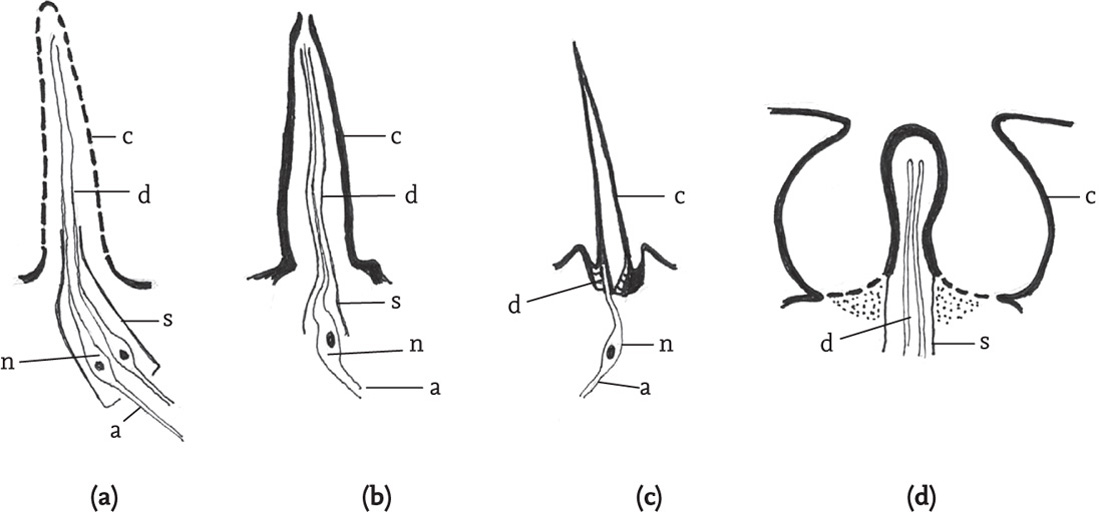
FIG 39. Cuticular sensilla (diagrammatic): (a) olfactory sensillum; (b) contact chemosensory (‘taste’) sensillum; (c) mechanoreceptor; (d) coeloconic thermo/hygro receptor. (Key: a: axon; c: cuticle; d: dendrite; n: nerve cell; s: sheath.) (Adapted from Chapman & Joern 1990.)
The concentrations of sensilla on the palps and mouthparts are mainly concerned with providing sensory information relevant to choosing and processing food items. The tips of the palps are equipped with large numbers of sensilla that are sensitive to taste, the physical properties and surface odours of potential foods. These sensilla continue to be involved in transmitting information as the palps repeatedly tap a food item (palpation) while the insect bites and chews (Blaney & Duckett, 1975; Chapman & Sword, 1993). The mouthparts themselves are also provided with dense patches of contact chemoreceptors and these continue the process of evaluating food as it is being consumed. The fore and mid legs are also endowed with sensilla that are sensitive to the surface chemistry of food substances, as well as with helping to hold and guide the food to the relevant mouthparts (see the DVD).
Most research on the chemical senses of orthopterans has focused on selection of food and feeding behaviour. Comparative research has shown that there are variations in the numbers and role of sensilla that are related to the dietary patterns of the species concerned (e.g. Chapman & Thomas, 1978). In general, grass-feeders have relatively fewer sensilla than species that feed on broad-leaved plants, and those with highly specialised diets also have fewer than food-generalists, in ways that relate to the respective differences in the requirements to identify plants that are either good to eat or not good to eat. Even species thought to be food-generalists (‘polyphagous’) reject some plants, and demonstrate preferences between the range of plant species that they will eat. An experimental study on feeding and survival among last instar nymphs of Schistocerca americana showed that rejection of plant materials on the basis of detection of deterrent chemicals played an important part in food selection, along with learnt aversions resulting from ingestion of toxins (Chapman & Sword, 1994).
Much of the above information has come from the study of acridids, especially locusts, but broadly similar sensory patterns are present in the various groups of ensiferans (Gwynne, 2001; Bland & Rentz, 1991). The role of chemical communication in other aspects of the lives of grasshoppers and crickets has been less thoroughly researched. In locusts, pheromones are known to play a part in aggregation, maturation, oviposition and phase-transition (Fuzeau-Braesch et al., 1988; Norris, 1970; Okelo, 1979; Schmidt & Osman, 1988; Whitman, 1990), while a role for pheromones in mate-recognition in grasshoppers is suggested by the presence of greater numbers of chemoreceptors in the antennae of males (Blaney & Simmonds, 1990). More recent research has established a crucial role for chemical communication in the reproductive behaviour of crickets (see Chapter 6 for more detail).
The relatively impermeable cuticle of orthopterans presents problems for maintaining physiologically optimal body temperatures and fluid content. Regulation of body temperature is critical both for development (see Chapter 3) and for maintenance of normal functioning in adults. Orthopterans appear to have little ability to generate heat internally, but are seriously affected by overheating and by cooling. To some extent the insect’s body temperature is regulated by convection currents and by heat transfers from surrounding air or solid substrates. Evaporation through the cuticle and spiracles also may have a small effect. Colour, too, may affect the ability of insects to absorb or reflect heat energy (Joern, 1982). At least one species has the ability to change colour in response to changes in ambient temperature (Key & Day, 1954). Perhaps the most important means of maintaining body temperature within the necessary range is by various behavioural strategies – the body can be pressed down onto the substrate or raised up on stretched legs as appropriate; the body can be oriented to incident solar radiation (‘flanking’: Chappell & Whitman, 1990) so as to maximise heat gain, or the insect can avoid overheating by retreating to the shelter of dense vegetation. The ability of different species of grasshopper to raise their body temperature above the ambient by these means varies considerably and is a significant determinant of their habitat requirements and geographical distribution (Willott, 1997). Water regulation is achieved mainly by food selection and by varying the water content of faeces, although direct drinking of water has also been noted.
LOCOMOTION: JUMPING AND FLYING
Jumping
The ability to jump long distances relative to their body-size is very widespread in the Orthoptera, and especially well-developed in the grasshoppers and groundhoppers, although most crickets and bush-crickets are also able to jump if provoked, and the long-established British alien, the greenhouse camel-cricket (Diestrammena asynamorus) has a prodigious leap. However, jumping is highly expensive energetically, and tends to be used only as a response to disturbance. Even then, it is frequently augmented by flight once the jump has commenced.
The mechanics of jumping in grasshoppers have constituted a challenge to researchers, and our understanding of the anatomical and physiological processes involved owes much to the extensive studies of the locust (Schistocerca gregaria) by H. C. Bennet-Clark (e.g. Bennet-Clark, 1975, 1976, 1990). The initial take-off is powered by opening of the angle between femur and tibia of each hind leg. The muscles involved are packed into the swollen femur, and are attached at their distal end (the ‘knee’) to the tip of the tibia. The largest of the muscles in the femur is called the ‘extensor tibiae’, and its contraction pulls on the ligament by which it is attached to a flexible area of cuticle at the tip of the tibia, so straightening the leg and exerting force on the substrate, via the tarsi.
However, this is by no means the whole story, as the power required to propel the insect into the air for a distance up to 0.8 metres is far more than can be mobilised by the simple contraction of the extensor muscle. To understand the source of this extra power, other features of the femur’s structure have to be introduced. Running alongside the extensor muscle, ventrally to it, is another muscle, the ‘flexor tibiae’. This is attached ventrally to the joint between tibia and femur, and has a pocket close to the point of attachment that fits over an inward projection of the cuticle of the femur (‘Heitler’s lump’). When the flexor muscle is contracted, the angle between femur and tibia is locked closed by the connection between the lump and pocket, but when it is relaxed, the femur and tibia can swing apart. Two other types of structure are also present in the femur. Towards the tip of the femur are two ‘semilunar’ processes, attached at one end to the wall of the femur and at the other to a ligament of the extensor muscle. The extensor muscle, as well as being attached along the inner and dorsal walls of the femur, also has attachments along a blade-like structure, the ‘apodeme’, which, at the distal end, forms the ligament that attaches the muscle to the tip of the tibia.
Now, if the extensor muscle is flexed while the flexor muscle is also flexed, locking the tibia in place, the semilunar processes, the flexible cuticle of the tibial attachment, and the apodeme of the extensor muscle, are subject to powerful stresses. If the flexor muscle is then relaxed, the energy stored in the apodeme and sublunary processes is released, as in the action of a steel spring, or catapult. This ‘storage-release’ mechanism augments the power of the extensor muscle tenfold. According to Bennet-Clark, the tensile stress of the apodeme material is similar to that of silk, and its energy storage about five times that of steel (Bennet-Clark, 1990: 182)! Anatomical studies by Bennet-Clark (1975) of other grasshoppers as well as the common groundhopper (Tetrix undulata) indicate that this mechanism is common to at least the Caelifera.
Studies of the nervous pathways between the metathoracic ganglion and the muscles of the femora, and the patterns of their excitation during jumping, have given support to the above account of the mechanisms involved in jumping in locusts (Hoyle & Burrows, 1973; Heitler & Burrows, 1977a, 1977b).
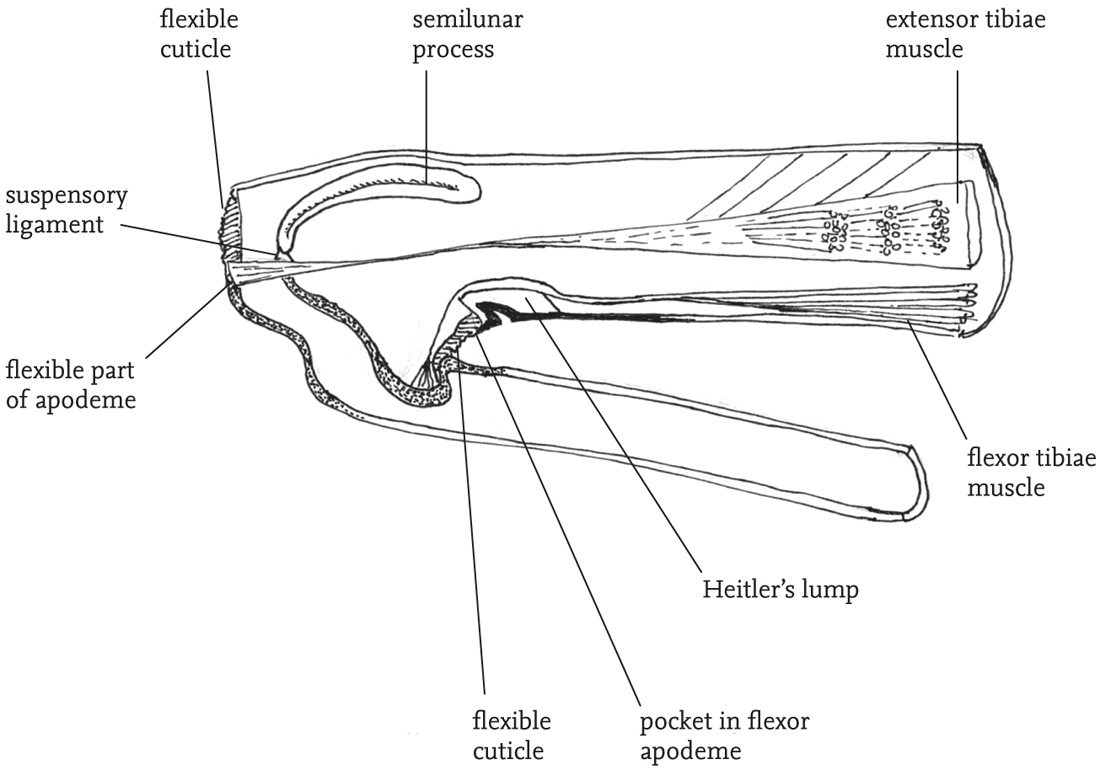
FIG 40. Longitudinal section of femoro-tibial joint of the locust Schistocerca gregaria showing structures involved in jumping (based on Bennet-Clark 1975, fig. 1:53, with permission).
Flying
In fully winged grasshoppers and crickets jumping is frequently supplemented by flight. It seems that ancestral orthopterans were flightless, but the ability to fly has evolved as a feature of many species of both Caelifera and Ensifera. However, in numerous species the wings of one or both sexes have become reduced, with associated loss of the ability to fly. In some of these (‘brachypterous’) species varying proportions of some populations develop as fully winged (‘macropterous’) forms that are capable of flight. These macropterous forms are responsible for colonisation of new habitat patches, and are probably important for range-extension in response to favourable climatic or general environmental changes (see Chapters 3 and 9).
As flight is dependent on high thoracic temperatures, and is very demanding in terms of energy expenditure, it tends to be used rather sparingly in cooler, temperate climates. In some species of grasshopper, there are short flight-displays that play a part in their reproductive behaviour, and in other species flight is commonly a ‘last-resort’ means of escape from predators when temperatures are sufficiently high. British species such as the common field grasshopper, stripe-winged grasshopper, large marsh grasshopper and common green grasshopper fly readily when alarmed, but their flights are relatively short (10 to 15 metres) and show only slight manoeuvrability.
As an escape tactic this relies on the ability of the insect to adjust its landing position. The landing process itself appears to be achieved by a coordinated set of reflexes (and is not dependent on visual inputs), and may be adapted to lessen the physical impact of the landing on a hard surface. However, it also could be that in facing the original source of disturbance the insect may benefit from being able to visually monitor the predator (Kral, 2010).
It also seems likely that there is considerable spontaneous flight activity between local habitat patches, but much greater publicity and research attention has been paid to larger-scale migratory and swarming phenomena that are more characteristic of species of the tropics and subtropics. Although large-scale migratory activity is usually associated with locusts, it is also a feature of the behaviour of numerous other acridid species, and some ensiferans such as the mormon cricket, Anabrus simplex (see Gwynne, 2001). The ability to migrate by flying considerable distances is an adaptation to a variety of environmental exigencies – most notably to ephemeral habitats and to regular climatic and seasonal changes that render particular habitat patches unfavourable for survival or development at certain times of year. In semi-arid grassland systems of tropical and subtropical Africa, Australia, the Middle East and India, large-scale grasshopper and locust migrations are common in relation to seasonal changes. The insects generally take to the wing at dusk and continue their flights for several hours into the night and, with ground-speeds of from 20 to 50 km per hour, can cover from 60 to 250 km in a night.
Brief flights initiated by the threat of predation, or performed as part of a visual display, require availability of immediately accessible energy sources to feed the intense activity of the flight muscles. In grasshoppers the initial fuel is carbohydrate in the haemolymph, and glycogen deposits in the muscles themselves. However, because the circulatory system is ‘open’, and the haeomolymph washes over internal organs (rather than being carried in vessels as in vertebrate systems) diffusion is relatively inefficient. An adaptation to this situation is that carbohydrate is present in the haemolymph in very high concentrations in the form of trehalose, which is broken down into glucose at the muscle site by enzyme activity. However, in species such as locusts, which are capable of sustaining prolonged flights, a supplementary source of energy is needed. For longer-distance flights (i.e. after 4 to 5 minutes), locusts draw on energy stored in their fat-body in the form of triacylglycerides. These are metabolised under hormonal control to form diglycerides that are then oxidised to provide energy over prolonged periods of muscle activity (Goldsworthy, 1990).
SINGING AND HEARING: SOUND COMMUNICATION IN ORTHOPTERANS
The songs of grasshoppers on summer days are among the most evocative of wildlife sounds. Many other groups of animals make use of sound communication, but no other insect group compares with the Orthoptera for the sheer variety and complexity of their uses of sound. Because of this, and because of the deep embedding of sound communication in their modes of life, this has been overwhelmingly the most popular topic in scientific research on the order (see, for example, Hartley & Robinson, 1976; Hartley, 1993; Greenfield, 1997; Ragge & Reynolds, 1998; Hartley et al., 2000; Robinson & Hall, 2002; Gerhardt & Huber, 2002; Drosopoulos & Claridge, 2006; Bailey & Zuk, 2008; Nityananda & Balakrishnan, 2009).
Some groups of Orthoptera, such as groundhoppers (Tetrigidae) and camel-crickets (Raphidophoridae), lack specialised hearing organs. In some species this seems to be the ancestral condition, while in others the ability to hear has been lost in the course of their evolution. However, all species have the ability to detect low frequency vibration by means of specialised organs (the subgenual organs) located in the tibia of all three pairs of legs, and in the Ensifera (crickets and their allies) the organs that are specialised for hearing may have evolved from the subgenual organs in the fore tibia. It seems likely that hearing evolved prior to the ability to produce sounds, probably as a means of detecting predators. Although the neural basis for hearing is very similar in all groups of Orthoptera, it is thought that the specialised hearing organs evolved separately in the Ensifera and the Caelifera (grasshoppers and their relatives). It also seems likely that the ability to produce sound by stridulation also evolved independently several times in various orthopteran groups as the mechanisms involved differ considerably (Gwynne, 1995, 2001).
If sound communication evolved separately several times during the evolution of the Orthoptera, and remains almost universal across the main subdivisions of the order, this raises the question of why this particular channel of communication is favoured against other possible ones (see Robinson 1990).
First, orthopterans are relatively heavy, large-bodied insects that are vulnerable to a range of parasites and predators. Their ability to jump or fly is an important means of escape, but is generally used as a tactic of last resort. Compared, for example, to relatively small-bodied groups, such as butterflies, the agility of orthopterans in flight is very limited. Butterflies use flight not only as a means of escape, but also in mate location. Moreover, they use the colour patterns of their large wings in courtship, to communicate species identity and to deter or distract predators from vulnerable body parts. Relatively sedentary orthopterans tend to rely on cryptic colour patterns and body structures and the ability to remain motionless when approached by predators. If showy colours are ruled out, then some other channel of communication is needed.
Second, many species (especially among the ensiferans) are active at night as a predator-avoidance adaptation and this, too, rules out visual communication. Of course there are nocturnal predators, but a variety of defensive adaptations have evolved in response to these. Also, chemical communication can be used effectively at night over long distances, as demonstrated by night-flying moths. There is a growing recognition that chemical communication plays a significant part in the reproductive activities of many orthopterans, although usually for interactions at close quarters.
Third, they are relatively immobile insects with dispersed populations living in structurally complex environments, so visual communication is likely to be ineffective. Orthopterans that lack, or have lost, the ability to communicate using sound (such as camel-crickets and groundhoppers) are generally ones that live in dense aggregations, and therefore can rely on tactile or chemical communication.
Fourth, the medium of sound has several variable parameters, enabling a large amount of information to be encoded – the location, species, sex, mating readiness and quality of the caller.
Fifth, sound can be switched off as soon as a potential predator is detected (ter Hofstede et al., 2008), although something comparable is possible with visual stimuli as when brightly coloured butterflies snap their wings sharply together to expose cryptic undersides.
Orthopterans may use sound to deter predators, and many aspects of sound communication are likely to have been shaped by selective pressure from parasites and predators. However, the main uses of sound communication across the different subdivisions of the order are linked to their reproductive activity, in ordering the interactions between males, in attracting potential mates and in close-quarters courtship behaviour. These aspects of the behaviour of grasshoppers and crickets will be the focus of Chapters 4, 5 and 6, and the natural history of acoustic communication, especially, in Chapter 5.
In this section the emphasis will be on the various mechanisms that have evolved for hearing and for the production of sound in the different orthopteran groups. The ways in which acoustic information is processed and responded to by the insects concerned is a fascinating topic that is being addressed in current research. Unfortunately there is space here to offer only a brief glimpse of this.
Hearing
In bush-crickets (Tettigoniidae) the ‘ears’ are located in the tibiae of the fore legs, just below the joint between tibiae and femora. Each ear consists of two thinned areas of cuticle (the tympana). In some species (such as, for example, the speckled bush-cricket, Leptophyes punctatissima) the tympana are exposed on the surface of the tibia, but more often they are partially enclosed by folds in the cuticle, so that from the external view they are hidden behind slits in the tibial wall. There are numerous grades of elaboration of the surrounding structure, including, in some species, an external structure almost resembling the mammalian ear (Bailey, 1990).
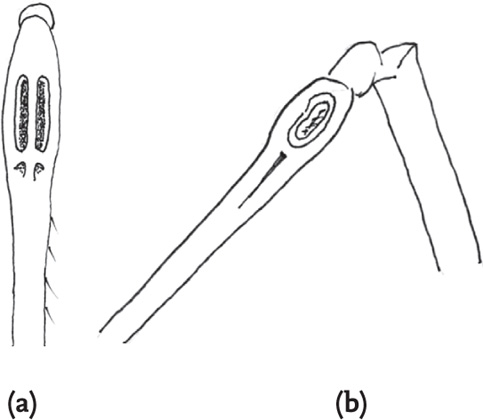
FIG 42. Fore tibiae of bush-crickets, showing tibial ‘ears’: (a) long-winged conehead, Conocephalus discolor, anterior view (tympana hidden); (b) speckled bush-cricket, Leptophyes punctatissima, side view (tympana exposed).
Internally, each pair of tympana is linked by an elongated tracheal tube to a spiracle on the side of the first thoracic segment, close to, but distinct from, the ventilatory spiracle. The auditory spiracle varies considerably in shape and size both within and between species, and acuity of hearing in females has been shown to be linked to size of the spiracle in some species (Bailey, 1998).
It is generally accepted that the main point of entry of sound is the spiracle. Interior to the spiracle, the trachea is expanded to form an auditory vesicle (the ‘bulla’), which acts to amplify the incoming sound. The sound waves are transmitted along the trachea to the tibia. Here, the trachea divides into two sections, each terminating at one of the paired tympana. The resulting vibrations of the tympana are mediated by the two parts of the trachea and the membrane separating them, and registered by a row of receptor cells that make up an organ called the crista acoustica, attached to the dorsal surface of the anterior of the two sections of the trachea. Neither the tympana nor the tracheae are directly responsible for perception of the frequency spectrum of incident sound and it seems that this is achieved by the arrangement of the auditory receptor cells on the crista acoustica – the largest cells, toward the proximal end of the organ, being sensitive to lower frequencies.
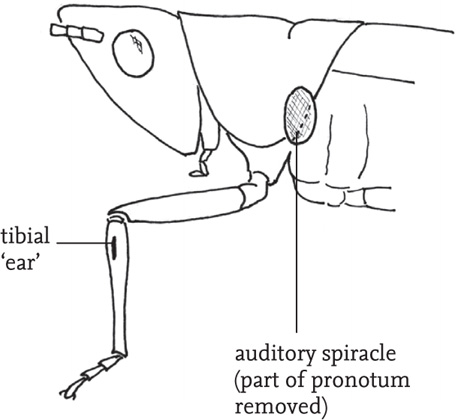
FIG 43. External features of the hearing system of a bush-cricket, Conocephalus dorsalis.
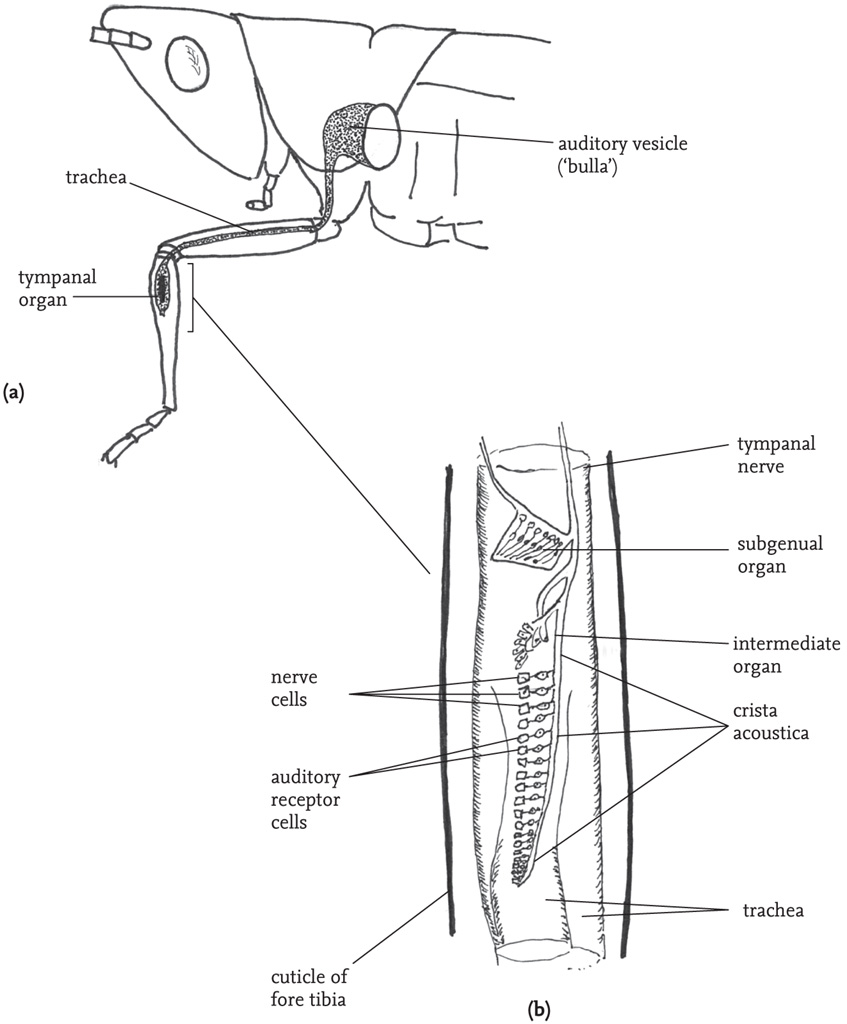
FIG 44. Internal structures of the hearing system of a bush-cricket, Conocephalus dorsalis: (a) route of the auditory trachea; (b) longitudinal section of basal area of the fore tibia, showing the tympanal organ and associated structures, greatly enlarged and simplified (adapted from Ragge in Ragge & Reynolds 1998, fig. 21, with permission).
Although it seems to be generally agreed that the main point of entry of sounds is via the acoustic spiracle, there remains some uncertainty about the role of the tympana and their exposure to incoming sound through the tibial slits. This may vary among different bush-cricket groups, and resonance in the cavity formed around the tympana may play a role in directional perception (Robinson & Hall, 2002: 14).
All three pairs of legs bear tibial organs, although only the fore legs have tympana and sensory receptors for sound. However, the tibial organs of all three pairs of legs possess a subgenual organ, which is sensitive to substrate vibration, and an ‘intermediate’ organ, which harbours vibratory receptor cells.
The hearing organs of crickets are believed to have evolved independently from those of the bush-crickets from common origins in the subgenual organs of their common ancestor. Like bush-crickets, crickets also have a ‘tracheal-tibial’ hearing system, but there are some differences of structure and function. The anterior tympanum in each tibial ear is reduced in size and not functional in hearing, and the septum between the left and right tracheae is thin, giving good sound transmission between the two sides of the system.
Other ensiferan groups, including Gryllotalpidae, Haglidae and Anostostomatidae, share broadly similar tibial-tracheal hearing systems, but with considerable variation in details of structure. However, some groups appear to have no hearing organs and are presumed deaf to airborne sounds.
In grasshoppers the hearing organs are located on each side of the first abdominal segment. The thin membranous tympana lie within depressions in the abdominal cuticle, and are in some species partially covered by flaps. Internally, the tympana are connected to closed air-sacs that are linked, so that sound waves from each side of the insect are transmitted to both tympana. Unlike the bush-cricket tympana, those of grasshoppers show different modes of vibration at different points on their surface, and these are detected by different groups of sensory cells (scolopidia) which are tuned to different sound frequencies. It seems that the receptor cells respond to a complex combination of inputs from different parts of the tympanum and the associated motions of the relevant ganglion (Müller’s organ) (Robinson & Hall, 2002).
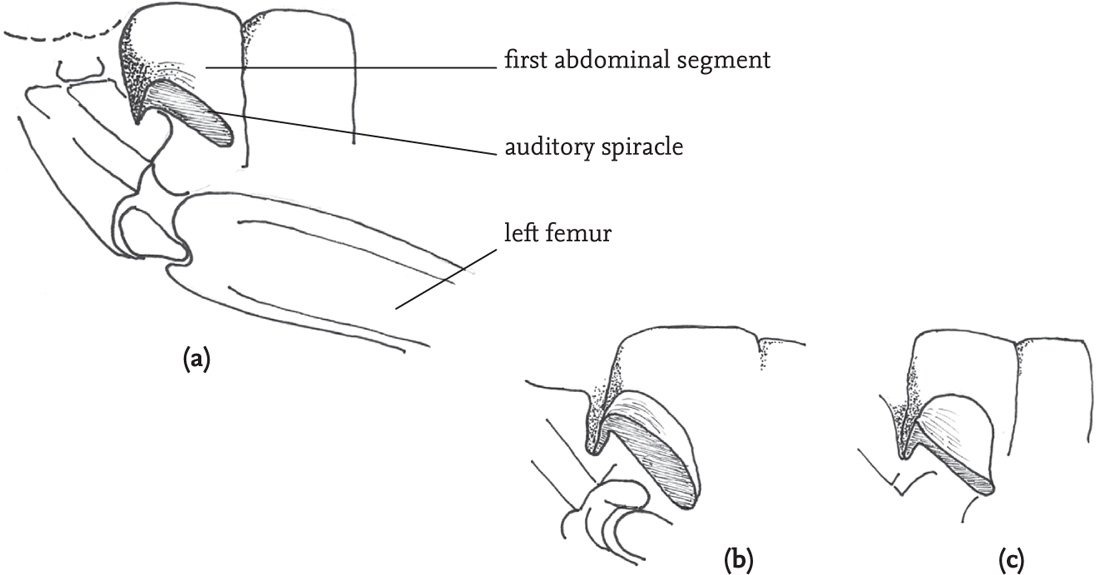
FIG 45. Side view of the auditory spiracle and nearby structures of two grasshopper species: (a) lesser marsh grasshopper, Chorthippus albomarginatus, female; (b) stripe-winged grasshopper, Stenobothrus lineatus, male; (c) stripe-winged grasshopper, female.
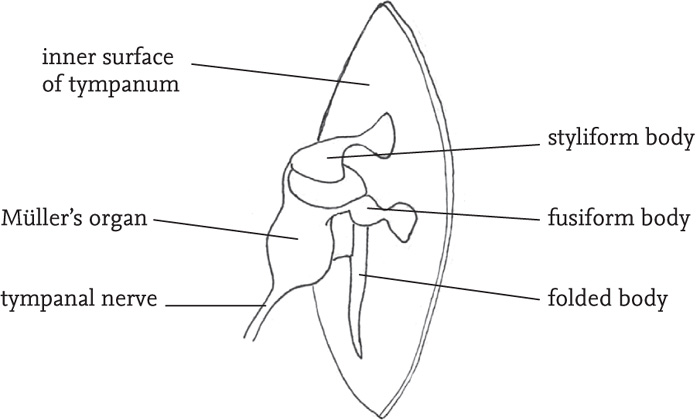
FIG 46. Inside view of the tympanum and associated structures of a grasshopper (redrawn from Ragge, in Ragge & Reynolds 1998, fig 23, with permission).
Production of sound
With some exceptions, the mechanism used for sound production is the scraping of one part of the hardened cuticle against another, and this is termed ‘stridulation’. However, the various subgroupings within the Orthoptera have rather different structural modifications for stridulation, indicating that sound-communication may have evolved independently several times.
Stridulation in male bush-crickets (Tettigoniidae) involves scraping the modified left fore wing (tegmen) over the right. The right tegmen has an area of thin, resonating membrane, the mirror, near the wing-base, and, on its dorsal surface, a projection called the scraper or plectrum. The left tegmen has a toothed vein, called the file, on its lower surface. As the wings are opened and closed, the file is drawn over the plectrum and vibrations are communicated to the frame of the mirror on the right tegmen, so amplifying the sound. In many species the tegmina are reduced or absent, and in the males of some species, such as the speckled bush-cricket (L. punctatissima), they are retained as little more than the sound-producing mechanism. Prior to stridulation, the insect warms up, with rhythmic muscular contractions. The wings are raised to allow the file and scraper to come into contact, and repeatedly opened and closed. In most cases, sound is produced mainly or wholly by the wing closure, to yield a single sound pulse, or ‘syllable’. In some species, however, sound is produced by both opening and closing the tegmina, and, in a few, the sound is produced by the opening movement. A sequence of several rapidly repeated syllables followed by an interval will be heard by humans as a single chirp or buzz, sometimes termed an ‘echeme’. Echemes may be further organised into more complex song structures in many species. The sounds produced by bush-crickets have carrier frequencies that vary from 600Hz at the lower end, up to as high as 135 kHz. Although most have broad frequency spectra, with peaks at several points within the range, some sing with relatively pure tones. As well as the role of the mirror in amplifying sound, the air space between the tegmina and the abdomen also has a role, although precisely what this is remains controversial (Robinson & Hall, 2002).
In most bush-crickets only the males stridulate. However, in some subfamilies (notably Phaneropterinae and Ephippigerinae) the females have a responding chirp. In the speckled bush-cricket, the very brief female chirp is produced, as in the male, by left-over-right movements of the greatly reduced tegmina, but the plectrum is on the lower surface of the left tegmen and the toothed structure that serves as the file is on the upper surface of the right tegmen.
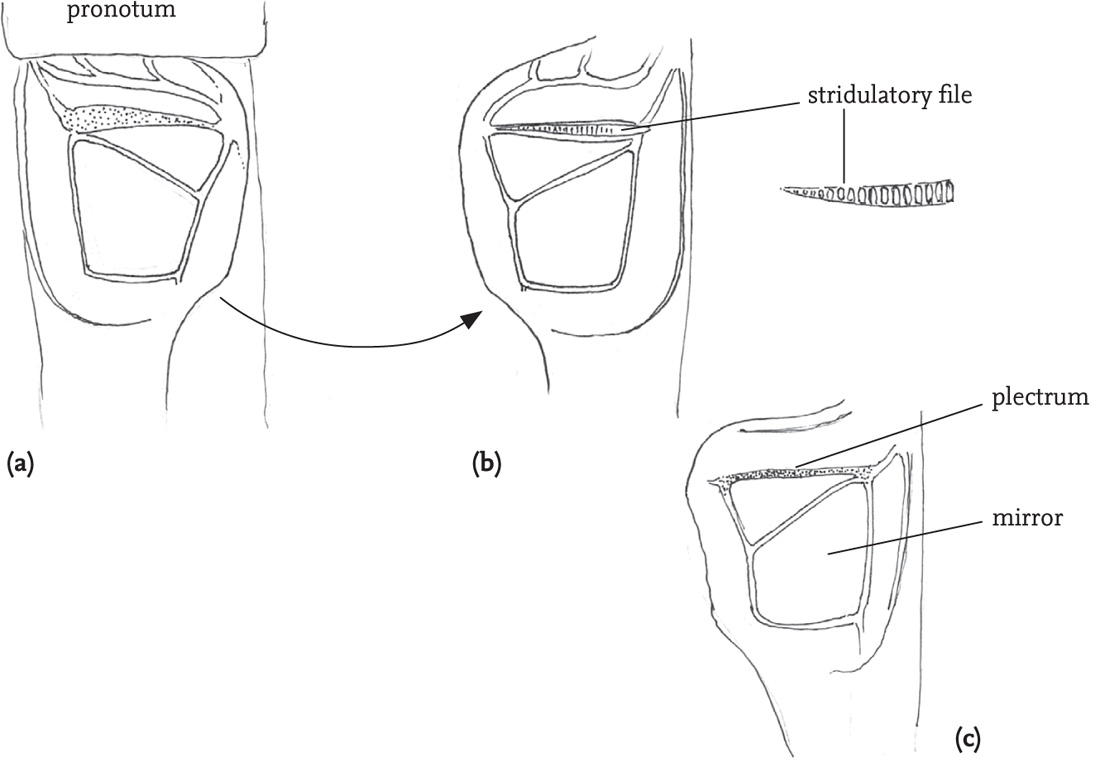
FIG 47. Stridulatory apparatus of a male short-winged conehead, Conocephalus dorsalis: (a) base of left tegmen from above; (b) the same, viewed from below, and showing stridulatory file; (c) the right tegmen, showing the mirror and roughened ‘plectrum’.
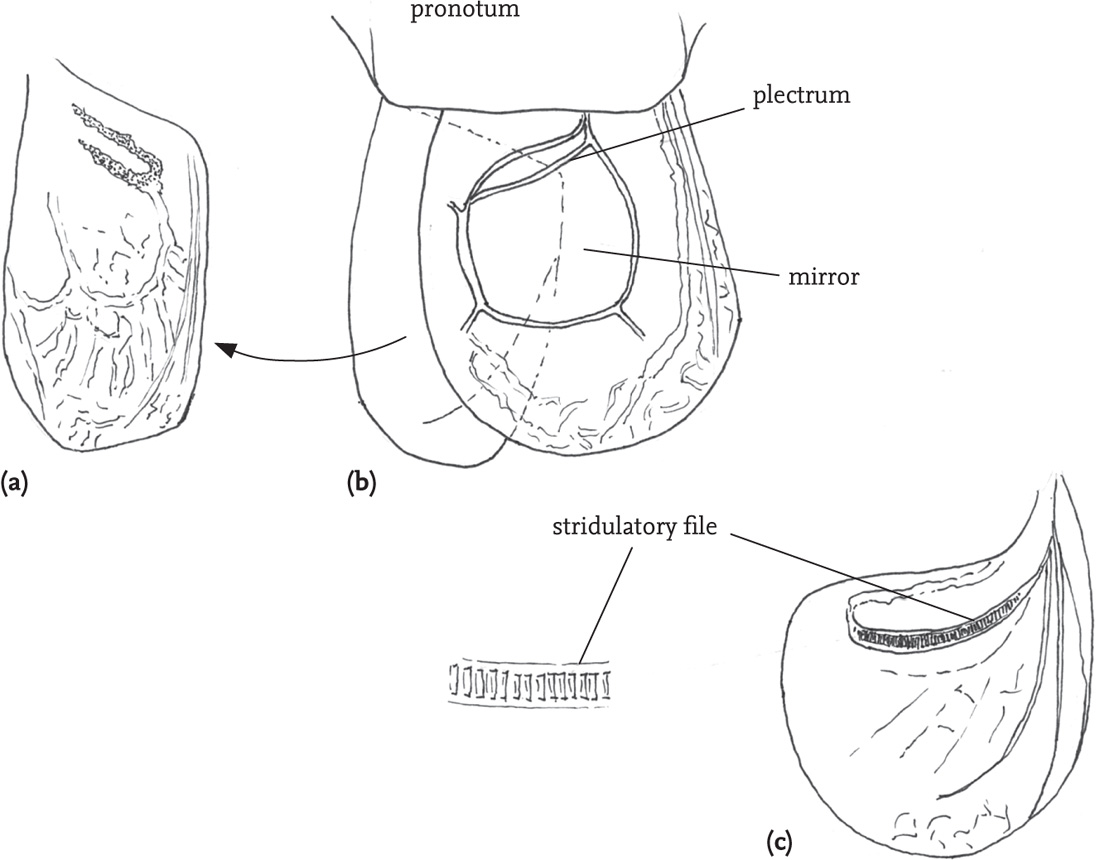
FIG 48. Stridulatory apparatus of a male dark bush-cricket, Pholidoptera griseoaptera, in which the tegmina are greatly reduced: (a) left tegmen from above; (b) right tegmen with left tegmen displaced as in stridulation; (c) left tegmen viewed from below, showing the stridulatory file.
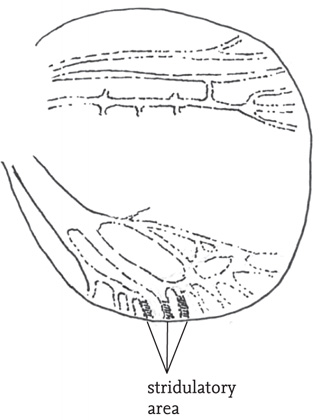
FIG 49. Right tegmen of a female speckled bush-cricket, Leptophyes punctatissima, showing stridulatory area (redrawn from Ragge in Ragge & Reynolds 1998, fig. 10, with permission).
In some bush-cricket subfamilies the ability to produce sound by tegminal stridulation has been lost, but in one group (Australasian giant leaf bush-crickets, Phyllophora species) sound can be produced by a quite different mechanism – scraping a file on the hind coxa (first leg segment) against a ridge on one of the ventral plates of the thorax.
The British species, the oak bush-cricket (Meconema thalassinum), produces sound by drumming with one hind leg on the leaf it is standing on. The resulting sounds are said to be audible some metres away, but the action also produces substrate vibration which may be important in enabling females to locate a drumming male.
Secondary loss of the ability to stridulate has occurred in a number of cricket (Gryllidae) species, but in the ones that have retained it, sound is also produced by rubbing together modified basal areas of the tegmina. However, in crickets, the usual mechanism is movement of the right over the left tegmen. Both wings are similar in structure, with a file of teeth formed from a vein (the Cu2 vein, as in bush-crickets) on both tegmina, and each forming one side of a roughly triangular resonating membrane: the harp. During stridulation the file of teeth on the underside of the right tegmen is passed over a plectrum on the upper side of the left tegmen. The file communicates vibrations to the harp and an adjacent thin membranous area, the mirror, on the right tegmen, while the similar harp and mirror on the left wing also resonate in phase with those on the right. The frequency of the sound produced is determined by a combination of the rate at which the teeth of the file are plucked by the plectrum, and the resonating frequency of the harp and mirror areas on the wings. The larger these are, in general, the lower the frequency of the song. Compared with most bush-crickets, the resulting song is more musical, with relatively pure tones. As with the bush-crickets, there is some dispute as to the role played by the semi-enclosed airspace that is formed below the tegmina when the cricket raises them in order to sing. It is noticeable that the males of the field cricket (Gryllus campestris) produce their calling song with the wings held high, but lower them when producing the distinct, quieter courtship chirps and clicks at close quarters (see the DVD).
Mole-crickets (Gryllotalpidae) also usually stridulate by passing the right over the left tegmen. The structural adaptations for stridulation are less developed than in the true crickets, with a less clearly defined harp, and no mirror. However, mole-crickets are able to amplify their songs by using their burrow as an effective secondary resonator, tuned to the frequency of their song.
The New Zealand weta (Anostostomatidae) have evolved their astonishingly diverse modes of sound production in isolation from the other groups of Orthoptera. Systematic research by L. H. Field and co-workers over more than thirty years has revealed much of this structural diversity and its related behavioural complexity (see, for example, Field & Bailey, 1997; Field, 1982; Field & Sandlant, 1983; Field & Rind, 1992; Field (ed.), 2001; McVean & Field, 1996; Kelly, 2006, 2008; see also Chapter 6 for more detail on the reproductive behaviour of some of these insects).
Lacking wings, the weta share a common elementary mode of sound production that is complemented in some species by a range of other methods. The common pattern is patches or rows of projections on the sides of one or more abdominal segments that are rubbed or scraped against variously shaped projections on the inner surfaces of the hind femora. Although this basic femoro-abdominal mechanism is shared by all weta species, it has undergone great diversification in the different genera and species. The ground and tusked weta (genus Hemiandrus) have the most elementary version, with patches of pegs or short spines on three abdominal tergites, and patches or rows of pegs on the femora. As these insects appear to have no ability to hear airborne sounds, it seems that their sound production functions solely in defence against would-be predators. The tree weta (Hemideina) generally possess a file on each side of the second abdominal tergite, with patches of blunt pegs on the femora. The latter project towards the leading edge of the file so that the two structures engage when femora and abdomen move against one another. The femoro-abdominal structures are very diverse in the giant weta (Deinacrida), including, in one group, crescent-shaped and grooved ridges on the second abdominal tergite that connect with a radial arrangement of pegs and spines on the femora, making multiple engagements with the ridges as they are drawn over them.
Stridulation in the giant weta appears to be used solely as a defensive response, but the tree weta (Hemideina species) also use stridulation in aggressive encounters with other males, in response to rejection of mating attempts and as a calling song. In defensive stridulation, the hind legs are drawn down against projections on the abdomen, while in other contexts the insect rocks forwards and draws the abdomen upwards against the hind femora.
Three other sound-producing structures have been evolved in a few species of weta, in addition to the shared femoro-abdominal one. The tusked weta have curved forward-pointing ‘tusks’ on the mandibles. These cross over towards the tips, and have lines of oval tubercles on mutually facing surfaces of the tusks. Sound is produced as these scrape against one another when the mandibles are opened. Another mechanism (also present in a few bush-crickets) is pleuro-coxal stridulation, in which patches of spines on the underside of abdominal segments one to three rub against small spines on the dorsal surface of the coxal segments of the hind legs. The third mechanism is ‘tergal-tergal’ stridulation. Several species of Deinacrida have blunt pegs on the underside of the rear edge of some dorsal plates of the abdomen that engage with dense patches of spines on the upperside of the leading edge of the one behind. Sounds are produced when the insect ‘telescopes’ its abdomen, scraping the abdominal plates against one another.
Most grasshoppers use a quite different mechanism. The hind femora of the male each have a file formed by a row of tiny pegs on the inner surface. In stridulation the hind legs are moved up and down so that the file on each hind femur rubs against a prominent vein (the radial vein) in the (flexed) fore wing on that side of the body, usually producing a louder sound on the down-stroke. In most species the legs move independently of one another and slightly out of phase during stridulation. Among British species this is most marked in the stripe-winged grasshopper (Stenobothrus lineatus) (see DVD). The file in this species is exceptionally long and dense, and the amplitude of the leg movement very wide. The stridulatory movement is thus unusually slow, and the lag between left and right leg-movements is obvious (see DVD). However, males of this species, along with most others, frequently lose one of their hind legs, and continue to stridulate quite effectively with the other.
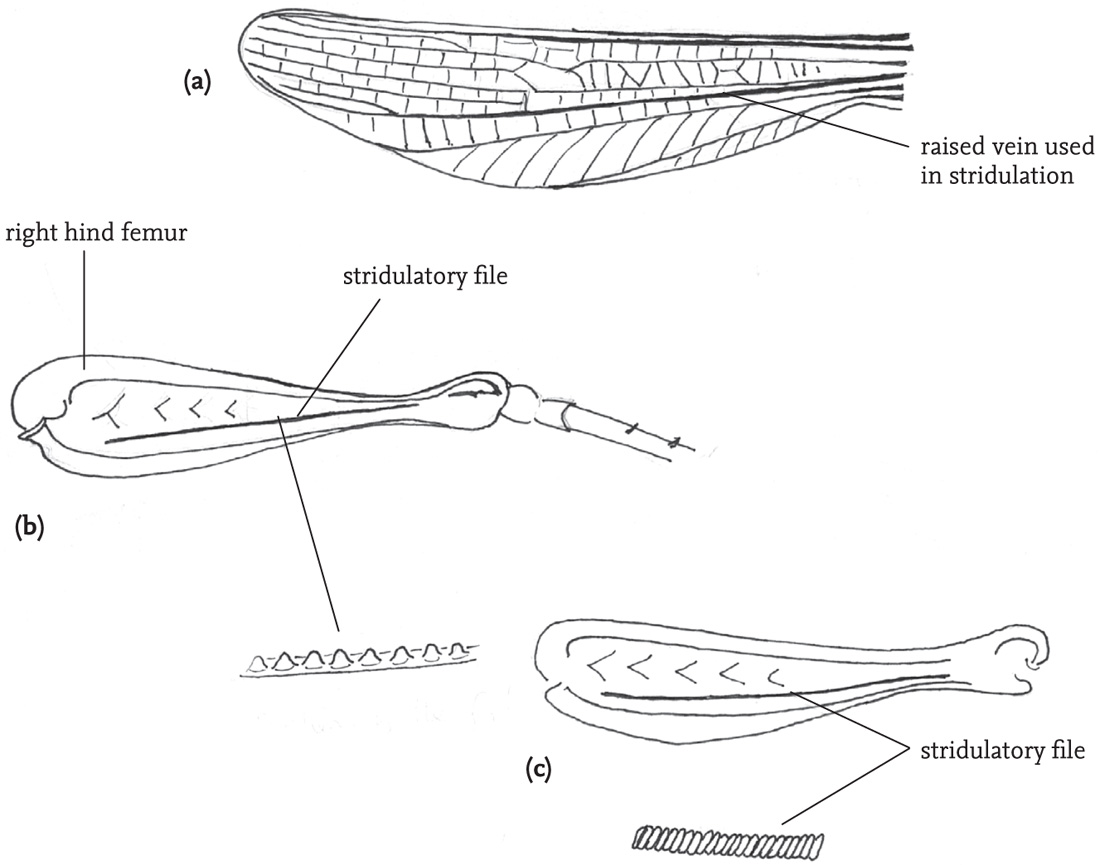
FIG 50. Stridulatory apparatus of two grasshopper species: (a) right fore wing (tegmen) of a male woodland grasshopper, Omocestus rufipes; (b) right femur of the same insect, showing the stridulatory file; (c) right femur of a male stripe-winged grasshopper, Stenobothrus lineatus, showing more densely toothed file.
Females of many grasshopper species are able to stridulate, and the stridulatory mechanism and the pattern of the song usually resemble that of the male. However, the stridulatory pegs on the hind femora are formed from the surrounding cuticle of bristles, rather than the bristles themselves, as in males. Female stridulation is usually much quieter than that of males, and much less often heard, as sexually receptive females are very quickly located by males (see brief female stridulation in the courtship sequence of the mottled grasshopper on the DVD).
Grasshoppers belonging to the subfamily Oedipodinae are represented in Britain only by the large marsh grasshopper (Stethophyma grossum), although another, the blue-winged grasshopper (Oedipoda caerulescens) occurs on the Channel Islands, and could well colonise mainland Britain. In this group, there are no stridulatory pegs on the hind femora, and stridulation is usually effected by the scraping of a ridge on the hind femur over a file of teeth on a prominent vein in the fore wing, although the large marsh grasshopper has yet another method.
PATTERN RECOGNITION, LOCALISATION OF SOUND AND PHONOTAXIS
Bush-crickets have several ways of determining the direction of a sound source. First, the delay in reception of sound travelling through the trachea and across the septum dividing right and left sides causes a difference in phase between sound waves making contact with the tympana externally and from within. Second, with sounds of sufficiently high frequency relative to the size of the insect’s body, a ‘sound shadow’ is produced on the side of the body facing away from the sound source which can be detected in species with enlarged tracheal vesicles. In crickets, the thin septum between the tracheae on each side of the body allows for effective transmission of sound impulses between the two sides. Detection of phase and pressure differences impacting on the tympana from the two sides of the body provides a further means to locate the source of sound.
Hedwig and his co-workers are conducting on-going research to analyse the neural processing of sound information in relation to the phonotaxis of female crickets, notably the southern field cricket, Gryllus bimaculatus (Hedwig & Poulet, 2004, 2005; Poulet & Hedwig, 2005; Hedwig, 2001, 2006). Impulses from acoustic receptors in the ears are transmitted by afferent neurons to the first thoracic ganglion. One pair of neurons (two pairs in bush-crickets), termed omega neurons because of their similarity in shape to the Greek letter 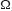 , play a key role in processing the incoming information, and are, in the species studied, most sensitive to the carrier frequency of the species-specific song. The anterior pair of omega neurons inhibit one another in ways that enhance the contrast between sensory inputs from the two sides of the body, supporting directional auditory processing. Other neurons are involved in transmitting information both backwards and forwards. The ascending auditory neurons transmit nerve impulses forwards to the brain for further processing.
, play a key role in processing the incoming information, and are, in the species studied, most sensitive to the carrier frequency of the species-specific song. The anterior pair of omega neurons inhibit one another in ways that enhance the contrast between sensory inputs from the two sides of the body, supporting directional auditory processing. Other neurons are involved in transmitting information both backwards and forwards. The ascending auditory neurons transmit nerve impulses forwards to the brain for further processing.
However, for (usually) females to be able to respond effectively to the (usually) male calling song, it is necessary not only for the source of the sound to be located, but also for the identity of the caller to be recognised (Gerhardt & Huber 2002). The processing requirements of these two achievements are different, and it seems that different neural pathways are involved. Sound localisation requires comparison of inputs from the two sides, whereas pattern recognition involves integration of information from both sides.
Early work (e.g. Huber, 1964) supposed that pattern recognition preceded the phonotactic response. However, more recent experimental work on crickets has demonstrated that the steering response of the receptive insect begins in response to the first syllable of the male song and then continues as a series of reflex-like responses to successive sound pulses. The neural pathway here seems to be a circuit involving the first thoracic ganglion independently of any higher level processing of sound in the brain. The processing involved in pattern recognition takes place in the brain on the basis of impulses transmitted forwards from the first thoracic ganglion, with walking and steering commands then being transmitted back to the thoracic ganglion. The interaction between these processes is not yet fully understood (Hedwig, pers. corr.).
In the speckled bush-cricket (L. punctatissima) the problems posed by sound location and phonotaxis are still more challenging than for ground-dwelling crickets. In this species it is the male that moves towards the female. The first extra problem is that localisation of the source of sound has to be made on the basis of extremely brief and infrequent chirps. The second is that the species inhabits structurally complex environments, in which the caller may be located either above or below the hearer, or to the left or right. The first problem is addressed by the male calling and the female responding within a narrow time-window, followed by an increased rate of calling and responding (see Chapter 5 for more detail).
The problem posed by the requirement to locate a sound source in three-dimensional space is solved very effectively by male speckled bush-crickets. In experiments conducted by Rheinlaender et al. (2007) using a walking frame and artificial female calls located on the horizontal as well as at 45° above or below males, the latter chose a path very close to the shortest one in all trials. The researchers hypothesised that the information underlying this ability must be detection of changes in the differential sound intensity across the two ears as the insect approached the sound-source. However, this would not enable discrimination between a source located above the approaching insect and one located below. It seems that this discrimination is achieved by a twisting movement of the body, which the researchers term ‘tilting’, that is performed at the start of phonotaxis (Kostarakos et al., 2007). The ability of the males to locate sound sources at various displacements from the horizontal becomes less reliable above 60°, and confusion sets in towards a 90° elevation or depression (Ofner et al., 2007).