
FIG 1. The Gresham grasshopper: (a) Lombard Street, City of London; (b) Gresham House, Cambridge; (c) Gresham Palace, Budapest; (d) The Royal Exchange, City of London.
And here, if you like, the Cricket DID chime in! … The kettle had had the last of its solo performance. It persevered with undiminished ardour; but the cricket took first fiddle and kept it. Good Heaven, how it chirped! Its shrill, sharp, piercing voice resounded through the house, and seemed to twinkle in the outer darkness like a star … Yet they went very well together, the Cricket and the kettle. The burden of the song was still the same; and louder, louder, louder still they sang it in their emulation … There was all the excitement of a race about it. Chirp, chirp, chirp! Cricket a mile ahead. Hum, hum, hum-m-m! Kettle making play in the distance like a great top. (Dickens, 2004 [1845])
Many of Dickens’s readers would have recognised from their own experience his description of the singing of the ‘cricket on the hearth’. Unfortunately this once-familiar songster is now rarely heard, except, perhaps, in the homes of keepers of reptiles, who use them to feed their pets. However, another group of insects, the grasshoppers, continue to contribute their songs to the soundscape of the British summer. Although the use of sound for communication is not universal among grasshoppers, crickets and their allies, it is very widespread. And it is this feature that above all others attracts the attention of passers-by, as well as poets, musicians and scientists. An ear-opening introduction to contemporary creative uses of insect sounds can be accessed from the website www.pestival.org, a festival celebrating insects in art and the art of being an insect. Particularly interesting is Chris Watson’s installation ‘whispering in the leaves’ at the Royal Botanical Gardens, Kew (for related events and recordings, visit: www.chriswatson.net).
Scientists have been interested in the nature of the insects’ musical instruments, the messages they communicate in their songs, and the responses of their intended listeners. Although the song of the male may play its part in making known to any interested female both his presence and his readiness to mate, it may also give away his location to a less welcome eavesdropper in the shape of a potential predator or parasite. Dickens’s emphasis on the competitive performance of his cricket is more accurate than he may have realised. It is now recognised that the calling songs of grasshoppers and crickets communicate information between the males themselves and are involved in their competitive interactions and spatial distribution. The extraordinary complexity and diversity exhibited by grasshoppers and crickets in their reproductive behaviour has stimulated a great body of research, some of which will be introduced in Chapters 4, 5 and 6.
The songs of crickets and bush-crickets, especially, have proved so attractive in some cultures, notably in China and Japan, that these insects are caged and kept for their song (Jin, 1994, in Gwynne, 2001; Pemberton, 1990). In China, not only is there a long tradition of breeding crickets that are selected for their aggression and fighting ability (Jin & Yen, 1998), but they were also a symbol of fecundity (Meng, 1993, in Gwynne, 2001). Elsewhere, grasshoppers and crickets are assigned a great variety of other symbolic meanings. According to Kevan (1979) species of bush-crickets were feared in Navaho native American culture as they were thought to be associated with spirits and attracted to corpses. The great Cuban dancer, Carlos Acosta, tells us that while he was a disruptive schoolboy his father had told him of a dream.
Last night I had a magnificent dream … You were dancing in a majestic theatre in another country and suddenly a cricket landed on your shoulders … Don’t you realise, the cricket represents hope. It means you are going to be great one day. (Acosta, 2007: 76)
In stark contrast, Arundhati Roy uses the image of swarms of grasshoppers as an omen of genocide. She reports the account given by a friend of the sole survivor of the massacre of Armenians that took place in Anatolia in 1915.
She was ten years old in 1915. She remembered the swarms of grasshoppers that arrived in her village, Dubne, which was north of the historic Armenian city of Dikranagert, now Diyarbakir. The village elders were alarmed, she said, because they knew in their bones that the grasshoppers were a bad omen. They were right; the end came in a few months, when the wheat in the fields was ready for harvesting. (Roy, 2009: 133–4)
A rather different moral is perpetuated in the story of the ant and the cicada, originally told by Aesop, but later transmuted into the encounter of ant and grasshopper. Either way, the message is the same: the grasshopper/cicada spends the summer in song, with no thought of tomorrow, while the industrious ant makes preparation for the bitter weather to come. Then, the destitute songster comes to beg or borrow from the ant, who replies: ‘You used to sing! I’m glad to know it. Well, try dancing for a change’ (Fabre, 1917: 1). Not every commentator finds the moral of this tale convincing. Fabre disputes its claims on behalf of the ant, while, in one of his short stories, Somerset Maugham (1963: 101–4) declares: ‘… I could never quite reconcile myself to the lesson. My sympathies were with the grasshopper and for some time I never saw an ant without putting my foot on it.’ To underline the point, the story goes on to describe the contrasting lives of two brothers, Tom, a carefree and disgraceful hedonist, and George, a hard-working, prudent and faithful husband and father. Just as George is expecting to get his reward and watch the descent of his profligate brother into the gutter, Tom marries a rich and elderly lady, who dies and leaves him everything.
Visitors to the City of London might be surprised to see a number of representations of grasshoppers distributed in this citadel of international finance: on the weather vane and embossed on a wall of the Royal Exchange, and suspended over the pavement in Lombard Street. In apparent defiance of the fabled image of the grasshopper as hedonistic songster, these representations refer us to the long association of the Gresham family with finance and banking. Perhaps the best-known Gresham is Sir Thomas (1519–71), merchant and financier, and financial agent to several English monarchs, including Elizabeth I. He was founder of the Royal Exchange, and his legacy was devoted to the establishment of Gresham College. The grasshopper motif figures above the Gresham family coat of arms, and legend has it that the founder of the family, Roger de Gresham, was abandoned as a new-born baby in long grass, only to be discovered by a woman whose attention was drawn to him by a grasshopper. The grasshopper motif is to be found on the exchange in Boston, in the bar of the spectacular art nouveau Gresham Palace in Budapest (originally owned and commissioned by the Gresham Life Assurance Company), in the car park of Gresham House in Cambridge, and elsewhere. Apparently, in the system of English heraldry the grasshopper symbolised wisdom and nobility. In view of our current experience of the financial services, perhaps the original association of the grasshopper with short-term profligacy might be reinstated!

FIG 1. The Gresham grasshopper: (a) Lombard Street, City of London; (b) Gresham House, Cambridge; (c) Gresham Palace, Budapest; (d) The Royal Exchange, City of London.
More material relations between human societies and grasshoppers and crickets include actual and potential uses of orthopterans as food sources, and, more dramatically, the economic impact of locusts and other migratory species. Fabre (1917) extols the virtues of the ‘locusts’ (that is, what we would call grasshoppers) as important food for turkeys, guinea fowl and red-legged partridge, and so, indirectly, as contributing to human diets. He is more hesitant about the desirability of grasshoppers as direct culinary fare, but cites the gospel according to St. Matthew as evidence that the diet of St. John the Baptist was ‘locusts and wild honey’ during his time in the desert, and an Arab author to the effect that the wives of the Prophet were sent grasshoppers as gifts, and that the Caliph of Omar once declared: ‘would that I had a basket of them to eat’. Gwynne (2001) mentions 4,000-year-old cave deposits in Wyoming that contained evidence of cooked bush-crickets, as well as more recent accounts of the roasts, soups and ‘cricket cakes’ prepared by native Americans. Still more recently, farming and eating insects has been advocated as a contribution to the growing crisis of global food production. A feature in The Guardian Weekend (Bailey, 2010) claimed that 1,400 insect species are eaten in over 80 per cent of nations. The UN’s Food and Agriculture Organisation held a workshop in Thailand in 2008 to highlight the idea, and our own Oxford Museum of Natural History hosted a ‘Banquet of Bugs’ in April 2011 to promote insects as an environmentally sustainable alternative to meat. Grasshopper salsa tacos, cricket tostados, and cricket fried rice were among the delicacies on offer.
Less benign are the ravages to crops that orthopterans can cause in some parts of the world as a result of the formation of dense migratory aggregations. Locusts are especially notorious for their recurrent outbreaks in parts of Africa and Australasia. Two species in particular (Schistocerca gregaria and Locusta migratoria) have been the most frequent research subjects. In these species a change of ‘phase’, from solitary to gregarious forms, underlies the tendency to form massive aggregations. Gregarious forms often differ in colour and structure as well as in their behaviour. The change of developmental pathway that produces the gregarious forms may be triggered by degenerating habitat conditions, or by increased population density, and is mediated by pheromonal communication. Plagues and outbreaks of dense clouds of flying insects that descend to feed as they move can cover tens to hundreds of square kilometres. A radar station in south eastern Australia monitored the passage over a 1 km line of two million locusts per night for six nights in November 1979 (Farrow, 1990). A similar pattern of aggregation and migration is found in other groups of orthopterans. Gwynne (2001) describes huge bands of so-called mormon crickets (Anabrus simplex) and coulee crickets (Peranabrus scabricollis) that form in western USA. The nymphs of these bush-crickets (or ‘katydids’ as they are known in America) form dense marching bands numbering many millions, up to 16 km long and 2 km wide. Although, as Gwynne points out, these species are less damaging to crops than has been assumed, drastic control measures have been used against them. Other bush-crickets that form aggregations under some conditions cause economically significant damage to forest trees in south and central America, to coconut palms and banana crops in Papua New Guinea and to grain in Ethiopa (Barrientos & Montes, 1997; Solulu et al., 1998; Rentz & Gurney, 1985). The variegated grasshopper (Zonocerus variegatus) of western and central Africa periodically forms large aggregations and was known as a minor pest of plantation crops such as coffee, pineapple and banana from early in the 20th century. However, in the 1970s and 1980s it became a much more abundant and serious pest, possibly owing to a combination of deforestation and increased cultivation of cassava as a subsistence crop (Chapman et al., 1986).

FIG 2. Locusta migratoria.
THE GRASSHOPPERS AND CRICKETS AND THEIR RELATIVES
Although, as we have seen, the lives of humans and those of grasshoppers and crickets are and have been intertwined in many ways, the lives and interactions of these insects have a fascination in their own right and this will be the focus of the rest of this book.
The insect order to which the grasshoppers and crickets belong is the Orthoptera (from the Greek, ‘straight winged’). Often the orthopterans are treated alongside closely related groups (as in Ragge, 1965 and Marshall & Haes, 1988). These relatives of the Orthoptera include cockroaches and mantids (order Dictyoptera), earwigs (order Dermaptera) and stick insects (order Phasmida). Taken together, these groups are often known as ‘orthopteroid’ insects. In this book we will be concerned more narrowly with the Orthoptera in the strict sense, but first it is necessary to give brief indications of those characteristics that mark out the Orthoptera from their relatives.
The stick insects (Phasmida) will be familiar to most readers, with their long, roughly tubular bodies, and three pairs of legs that are more or less equal in length. The tarsi have five segments. There are no native species, although a small number of species, probably escapees from domestication, or deliberate introductions, have established themselves in England – mostly in the south-west peninsula. Some tropical species are winged or elaborately spined, but those found in England conform to the characteristic stick shape.
The cockroaches (Dictyoptera) are flattened dorsoventrally, like beetles. However, in winged forms the fore wings overlap along the midline of the insects’ dorsal surface, and are not shell-like as in beetles. The cerci that project from the rear end of the abdomen are simple, and the tarsi have five segments. Although the hind legs may be longer than the fore and mid legs, they show no obvious adaptation for jumping. The large common or oriental cockroach (Blatta orientalis) is probably the best known, but, owing to its pest status, it is now rarely encountered in Britain. It and other non-native species are often kept as pet food, and may occasionally establish temporary colonies outdoors. However, they do not generally persist except in artificially heated buildings or refuse tips.

FIG 3. A stick insect, Parachymorpha zomproi.

FIG 4. The Australian cockroach, Periplaneta australasiae, an alien species sometimes found in artificially heated buildings in Britain.
There are three native cockroaches. These are much smaller than the oriental cockroach, and rather inconspicuous. The dusky cockroach (Ectobius lapponicus) inhabits scrubby habitats, and is found mainly in south central England; the tawny cockroach (Ectobius pallidus) inhabits both heath and downland in southern England; while the lesser cockroach (Ectobius panzeri) is a predominantly coastal species of southern England, west Wales and East Anglia.
The mantids are included with cockroaches in the order Dictyoptera. The image of the praying mantis (Mantis religiosa) is well-known and quite representative of the group, with its powerful and spiny fore legs adapted for catching its prey. There are no native British species, although occasional escapees may be seen. There are up to 15 European species (Battiston et al., 2010), but, with the exception of the praying mantis, these are mainly southern in distribution.

FIG 5. Two native British cockroaches: the dusky cockroach, Ectobius lapponicus, and the tawny cockroach, Ectobius pallidus.

FIG 7. The common earwig, Forficula auricularia.
Earwigs (order Dermaptera) are small, dorsoventrally flattened insects. Their distinguishing feature is the modification of the cerci at the tip of the abdomen to form curved pincers, or forceps, that can be used as defensive weapons. They may be with or without wings. In the winged forms the fore wings are reduced to short, hardened flaps that cover the folded-up hind wings when the insects are at rest. As in the other groups discussed so far, the hind legs are not modified for jumping. There are four probably native species, together with a small number of aliens that have become established in Britain (see Marshall & Haes, 1988).
Now to the Orthoptera! Fossil evidence indicates that this is one of the oldest of the insect orders, dating from the late Carboniferous period as much as 300 million years ago (Sharov, 1968; Gorochov et al., 2006). Perhaps the most well-known feature of the grasshoppers, crickets and their allies is that their hind legs are longer than the other two pairs, and adapted for jumping. This is indicated in an older name for them: the ‘saltatoria’. Another very widespread, but not quite universal, characteristic is the use of sound communication. The males of most species (and the females of some) have specially adapted structures for the production of sound. These are generally body parts so modified that when they are rubbed together a sound is produced, the repetition rate or frequency composition of which is usually distinctive for each species. In some groups there are structures modified to act as resonators which amplify the sound, while some others use vegetation or a chamber in a burrow for this. Most species also have specialised hearing organs, sometimes tuned to the dominant frequency of the song. Receptive females use the song of the male to move towards him, an activity known as ‘phonotaxis’. In a few species it is the male that moves to the female. The structures concerned in both sound production and hearing differ very markedly among the different groups of Orthoptera, suggesting that sound communication may have evolved independently several times in the order.

FIG 8. The male stridulatory apparatus of the speckled bush-cricket, Leptophyes punctatissima, and the southern field cricket, Gryllus bimaculatus.

FIG 9. The tibial ‘ear’ of a bush-cricket, Phaneroptera falcata.
Like other orthopteroids, orthopterans pass through a series of stages in their development from egg to adult. Unlike orders such as Lepidoptera (butterflies and moths) and Coleoptera (beetles), which have a distinct larval (caterpillar) stage followed by a ‘resting’ pupal stage, the juvenile developmental stages of the orthopteroids resemble smaller versions of the adults. The wings develop externally, and can be seen as small flaps in the nymphal stages (hence the term ‘exopterygote’ which is used to describe this sort of developmental pattern). What distinguishes the Orthoptera is that in the winged forms (including most of the short-winged, or brachypterous species) the developing wing buds are inverted in the final two nymphal stages. This does not occur in the development of the winged forms in the other orthopteroid orders.
There are believed to be over 25,000 species of Orthoptera worldwide (Orthoptera Species File: http://Orthoptera.SpeciesFile.org), and some 650 in Europe (www.ortheur.org gives 1040, but the geographical scope of this site is wider than that of other estimates). However, partly because of our northerly climate, and partly because of the brief period during which the insects were able to colonise the British Isles, the number of British native species is small: some 28 species. There are also two long-established aliens that remain closely associated with human activity, and a small number of recent additions that appear to be establishing themselves. However, despite the small number of species, the British orthopteran fauna is very diverse both in the range of taxonomic groups that are represented, and in terms of habitat and mode of life.

FIG 10. A final instar nymph of the grey bush-cricket, Platycleis albopunctata, showing inverted wing-stubs.
THE SUBDIVISIONS OF THE ORTHOPTERA
The Orthoptera are divided into two main suborders: the Ensifera and the Caelifera.
The Ensifera includes the true crickets (Gryllidae), bush-crickets (Tettigoniidae) and mole-crickets (Gryllotalpidae), as well as more exotic groupings including the ambidextrous crickets (Haglidae), Jerusalem crickets (Stenopelmatidae), king crickets, ground and tree weta (Anostostomatidae), camel-crickets and cave crickets (Rhaphidophoridae), cooloola monsters (Cooloolidae), raspy and leaf-rolling crickets (Gryllacrididae), scaly crickets (Mogoplistidae), splay-footed crickets (Schizodactylidae) and ant-crickets, or ant’s nest crickets (Myrmecophilidae). The earliest orthopterans were Ensiferans belonging to groups that are now extinct.
Various authors differ on the higher-level classification of these groupings, and as the majority of families do not have British representatives, it is not necessary to enter too far into taxonomic controversy here. However, several families usually grouped together in the superfamily Stenopelmatoidea, although strictly beyond the scope of this book, are especially interesting, and exhibit characteristics relevant to one of our principal themes. This large and diverse grouping includes predominantly flightless cricket-like species with vernacular names such as cooloolee and dingo monsters, Jerusalem crickets, king crickets and raspy crickets (see Field (Ed.), 2001). As a group they are very widely distributed globally, and have radiated especially in the southern hemisphere. Research on these insects has been slow to develop, but knowledge of them is rapidly expanding. One particularly well-researched grouping are the ‘weta’ of New Zealand. The term weta is the Maori name given to them. The group shows great disparities between males and females, with males often having enlarged heads and tusk-like mandibles. The ground weta (Hemiandrus species) are distinctive in their extensive use of chemical communication, but it is the tree weta (Hemideina species) that have attracted most research attention by virtue of their distinctive reproductive behaviour. Males are aggressive, and occupy holes in trees where they gather together ‘harems’ of females. We will return to a detailed discussion of their extraordinary lifestyle in the chapters on mating systems and sexual selection (Chapters 4, 5 and 6).
The term Ensifera refers to the sword shaped ovipositors possessed by the females of the suborder, although it is not an accurate characterisation of the ovipositors of female crickets. These are variously described as spear or needle shaped. Nevertheless, the general point is valid. Prominent and elongated ovipositors projecting from the rear of the abdomen are almost universal among the females of the suborder. Another characteristic feature, and one present in both males and females, is the pair of long, flexible filamentous antennae. In many species these are as long as or even much longer than the body. As well as providing multi-modal sensory information, the antennae are used extensively in courtship. There are internal anatomical features and DNA evidence that support the view that the different subdivisions of the Ensifera belong together as a natural grouping (Gwynne, 2001).
The other suborder of the Orthoptera is the Caelifera. This suborder evolved later than the Ensifera, probably during the early Triassic, and is likely to have evolved from earlier ensiferans (Gorochov et al., 2006). The term Caelifera may refer to the inconspicuous chisel-shaped ovipositors of the females (in contrast to the sword shaped ones of the Ensifera) (Marshall, pers. corr.). The Caelifera also have shorter, relatively stiff, parallel sided or clubbed antennae, quite different in appearance to the long filamentous and tapering antennae of the Ensifera. By far the greatest number of species worldwide are grasshoppers, or grasshopperlike insects that are assigned together under the superfamily grouping ‘Acridomorpha’. This grouping is further subdivided into seven superfamilies, only two of which are represented in the European fauna. These are the Acridoidea and the Pyrgomorphoidea. The latter superfamily is represented in Europe by one genus only: Pyrgomorpha, characterised by a distinctive profile, in which the dorsal surface of the head rises from its junction with the pronotum, rather than continuing in the same plane as is usual in grasshoppers.
The superfamily Acridoidea includes eleven families, only two of which are represented in Europe. One of these, the Pamphagidae, includes some of the largest orthopterans in Europe, but their distribution is limited to the extreme south.
The other family is the Acrididae, the principal grasshopper family. Worldwide some 25 subfamilies are distinguished (Orthoptera Species File: http://Orthoptera.SpeciesFile.org), but most of the European species and all of those that are native to Britain belong to just two: the Oedipodinae and the Gomphocerinae.

FIG 11. The grasshopper Pyrgomorpha conica, from southern Europe.

FIG 12. The Egyptian grasshopper, Anacridium aegyptium.

FIG 13. A melanopline grasshopper, Miramella alpina, and an acridine grasshopper, Acrida ungarica.
Other subfamilies with some European species include the subfamily Cyrtacanthacridinae to which belongs the well-known Egyptian grasshopper (Anacridium aegyptium), vagrants or escapees of which are frequently reported in Britain.
The subfamily Melanoplinae includes a distinctive group of several mostly brachypterous (short-winged) species that occur in the European mountain ranges, and the subfamily Acridinae includes the elongated, twiglike Acrida ungarica.
In addition to the Acridomorpha, there are two further extant superfamiles of the suborder Caelifera. These are the Tridactyloidea and the Tetrigoidea. The Tridactyloidea includes sandhoppers and pygmy mole-crickets and is represented in Europe by just two species (in the family Tridactylidae), Xya pfaendleri and X. variegata. These are scarce, small southern species that inhabit unvegetated margins of rivers and lakes. They feed on algae, and dig long, winding galleries in the earth where they take refuge in bad weather (Bellmann & Luquet, 2009).
The other caeliferan superfamily is the Tetrigoidea, and it is represented in both Britain and Europe by several members of the family Tetrigidae. In English they are known variously as groundhoppers, pygmy grasshoppers and grouse-locusts. They look superficially like very small grasshoppers, but are distinguished by the extension of the pronotum back over the dorsal surface of the abdomen. Britain has three species, all included in the genus Tetrix.
ORTHOPTERANS AND THEIR ENEMIES
One stimulus to the study of diseases, parasites and predators of grasshoppers and crickets has been the hope of biological control over the populations of pest species such as locusts. However, despite the fact that some of their enemies can cause high death rates, the scientific view seems to be that the population dynamics of orthopterans are not, in general, greatly affected (see Chapter 9).
Predators of orthopterans include many species of birds, small mammals (especially bats) and reptiles, but they are also highly vulnerable to a range of invertebrate predators, most notably spiders and robber-flies (Diptera: Asilidae), as well as other orthopterans, such as the carnivorous wartbiter bush-cricket. Belwood (1990) describes predation on bush-crickets by forest gleaning bats in South America (see Chapter 5), while Robinson (1980) mentions four British species of bats that pick insects from vegetation, and whose hearing is in the frequency range of the speckled bush-cricket. Gwynne (2001) describes the remarkable convergence of predators on marching bands of mormon crickets in western USA: gulls, lizards, rodents, badgers, over two dozen bird species – and even fish, as the ‘katydids’ raft over rivers. Even in the absence of such huge concentrations of orthopterans in temperate climates, orthopterans can still constitute important components of the diet of birds – including threatened farmland species, and game birds such as pheasant and partridge (see Chapter 9).
Spiders are serious predators of orthopterans at all developmental stages. Early instar nymphs suffer very high mortality from ‘wandering’ spiders of family Lycosidae (see Oedekoven & Joern, 1998; Cherrill & Begon, 1989b). Adults and late instar nymphs frequently fall victim to large web-weaving spiders such as the labyrinth spider (Agelena labyrinthica), the garden spider (Araneus diadematus) and the four-spotted spider (Araneus quadratus). The wasp spider (Argiope bruennichi), a recent addition to the British spider fauna, is another effective predator of orthopterans.
The females of several species of solitary wasp, the most widespread of which is Tachysphex pompiliformis, collect and paralyse grasshoppers. These are carried back to a burrow, where the wasp lays an egg on the underside of the thorax between the first and second pairs of legs. Early in the season up to ten grasshopper nymphs may be used to provision a single brood cell, but later a single large nymph may be sufficient. The newly hatched wasp larva feeds on the grasshopper body contents, and emerges from the burrow about a week later. A range of grasshopper species are attacked, including Chorthippus species, Stenobothrus lineatus and Myrmeleotettix maculatus (Edwards (Ed.), 1998; Baldock, 2010; Owens, pers. corr.).

FIG 14. A wasp spider, Argiope bruennichi, with grasshopper prey.

FIG 15. A solitary wasp, Tachysphex pompiliformis, with field grasshopper, Chorthippus brunneus nymph as prey. (© N. Owens)
Grasshoppers and bush-crickets are also threatened by a range of invertebrate parasites and parasitoids. One of the most fully studied is the parasitoid fly Blaesoxipha plumicornis (Diptera: Sarcophagidae). The female fly gives birth to tiny larvae which she deposits on the abdomen of a grasshopper. The fly larva burrows through the cuticle and feeds on the host’s body tissues until it is full grown. It then emerges from the membrane between the grasshopper’s head and pronotum, and pupates in soil. Up to four fly larvae have been recorded from a single grasshopper, but more usually only one larva is deposited per host (Richards & Waloff, 1954). Usually it is females that are targeted by the fly, possibly because they are more readily detected as they move toward singing males, or because they are a more nutritious host for the larva. The host grasshopper invariably dies when the fly larva has completed its development.
The eggs of grasshoppers and bush-crickets are also vulnerable to parasitism, especially by wasps of the genus Scelio (Hymenoptera: Scelionidae). Richards and Waloff (1954) estimated the level of parasitism in eggs of Chorthippus parallelus and C. brunneus at their study site as between 19 and 28 per cent of egg pods, with most eggs in each parasitised pod being affected (see Chapter 9 for more detail). A study cited by Brown (1983) reported four out of ten pods of C. brunneus, and three out of thirteen of C. parallelus affected, but only seven or eight percent of eggs were parasitised. A chalcid wasp (Hymenoptera: Chalcidae) is reported as an egg parasite of the short-winged conehead (Conocephalus dorsalis) (Blair, 1948).
Orthopterans are also parasitized by nematode worms, and are very vulnerable to various pathogenic fungi and bacterial diseases. Grasshopper victims of the fungus Entomophthera can sometimes be seen with their legs wrapped around the stems of grasses (Marshall & Haes, 1988). Streett & McGuire (1990) give a wide-ranging review of the diseases of grasshoppers.
The calling song of male orthopterans provides one important means by which potential predators and parasites are able to locate them. Such ‘acoustically orienting’ enemies have hearing that is sensitive to the frequency range of their host species. Belwood’s study, mentioned above, details predation by acoustically orienting bats, and birds such as screech owls, as well as carnivorous bush-crickets and mantids, which prey on bush-crickets of the subfamily Pseudophyllinae in South American forests. Flies of the widespread family Tachinidae are well-known parasitoids of other insects, and some (tribe Ormiini) parasitise crickets, mole-crickets and bush-crickets. Like the females of Blaesoxipha, the flies deposit their young larvae on the abdomen of their host. The larva goes on to consume the body tissues of the host ensiferan, emerging when fully grown, and pupating outside the host’s body. The flies use the calling song of the male orthopteran to locate it, so it is mostly males that are parasitised (Cade ,1975; Allen, 2000; review in Robinson & Hall, 2002).
Under selection pressure from intense predation and parasitism, orthopterans have evolved numerous defensive strategies. Primary defences are ones that operate independently of the actual presence of a potential threat. Most grasshoppers and crickets are cryptically coloured, and often have shapes that mimic the structures of vegetation such as leaves or twigs (for example, the leaf mimics of the Pseudophyllinae, see Gwynne, 2001, plates 10 to 13). A few species (but none of the British ones) have adopted bright ‘aposematic’ colour patterns that are believed to act as a warning to potential predators, and a few are mimics of other invertebrates – notably spiders and ants. Marshall & Haes (1988) give as an example of the former the similarity of early nymphs of the dark bush-cricket (P. griseoaptera) to a lycosid spider, and the greenhouse camel-cricket (D. asynamorus) is also remarkably spider-like. The early instars of the south east Asian conocephaline bush-cricket Macroxyphus sumatranus look and behave in ways that closely resemble ants (Gwynne, 2001). These are presumably adaptations that offer some protection against visual predators.

FIG 16. Spider-like early instar nymph of the dark bush-cricket, Pholidoptera griseoaptera.
If a potential predator or parasite is detected, one option is to remain perfectly still on the expectation that camouflage will be effective. However, if the threat appears urgent (as when approached by large mammals such as humans) the main alternatives are to dive deep into long grasses or other deep vegetation, or to jump or fly. The first of these is available only in some microhabitats, but it is very effective. Jumping or flying has several disadvantages. First, it attracts attention, offsetting its value as a means of escape; second, it is energetically costly; and, third, it runs the risk of blundering into a spider’s web. However, the jump/fly response is often effective if, as very often seems to be the case, the fugitive is able to ‘disappear’ by landing on a suitable background and remaining motionless. In a few species, potential predators are greeted by a defensive ‘startle’ behaviour, often combining a distinctive threat posture with an aggressive stridulation. Some of the most striking examples are provided by the giant and tree weta of New Zealand (Field & Glasgow, 2001).
Acoustically orienting predators and parasitoids exert selective pressures on songsters – predominantly males. However, singing is likely to have been evolved by sexual selection through competition with other males and the need to attract potential mates. There is thus some tension between pressures to enhance reproductive success and the heightened vulnerability to predation and parasitism that song brings with it. Possible defences include developing the ability to detect a potential enemy before it becomes an immediate threat, singing at times when potential enemies are not active, or switching to a different call frequency (or even to an alternative channel of communication, such as vibration (‘tremulation’)). Ceasing to call as a potential predator approaches is another tactic (ter Hofstede et al., 2008), as is simply calling briefly and intermittently, so that an enemy does not have time to effectively locate the caller.
Assuming that there is a trade-off between the reproductive success of males and the increased vulnerability to predation or parasitism imposed by their calling song, it is to be expected that natural selective pressures will have influenced the pattern of acoustic communication in species that suffer from acoustically orienting enemies.
It may be speculated that the very brief and intermittent chirps of the speckled bush-cricket (Leptophyes punctatissima), together with the call-and-response pattern established when a willing female answers a male chirp, could be a system that has evolved in response to predation or parasitism from acoustically orienting hunters (Robinson, 1980; Robinson et al., 1986; Robinson, 1990). A recent case of ‘evolution in action’ is reported from a Hawaiian island on which males of the cricket Teleogryllus oceanicus have suffered intense selective pressure from a parasitoid tachinid fly (Ormia ochracea). In a few generations most of the males have evolved as flat-winged forms, no longer able to sing. This is despite the fact that females continue to show preference for calling males (Zuk et al., 2006; Bailey and Zuk, 2008. See Chapter 5 for more details).
THE BRITISH ORTHOPTERA
The 28 British native species, together with the two long-established aliens and two recent colonists, include representatives of five families of the Ensifera (Rhaphidophoridae, Tettigoniidae, Gryllidae, Mogoplistidae and Gryllotalpidae) and, among the Caelifera, the Tetrigidae and two subfamilies of the Acrididae. Detailed accounts of the appearance, life history, behaviour, habitat and distribution of the individual species will be given in Chapters 7 and 8. This section will provide an introduction to the main distinctive features of each family or subfamily grouping.
ENSIFERA
1. Rhaphidophoridae (camel-crickets)
The greenhouse camel-cricket (Diestrammena (formerly Tachycines) asynamorus) is a long-established alien, now occurring rarely in heated greenhouses. It is the only established breeding species of this family in Britain. The camel-crickets are wingless and also lack either hearing organs or means of stridulation. However, they do have vibration receptors (subgenual organs) in the tibiae. The females have long, slightly up-turned ovipositors. The antennae are especially long, often several times the length of the body, and the legs are also long and spindly, giving a spider-like appearance. They live in caves or other dark, humid places, and are often gregarious, interacting frequently by stroking one another with their antennae.

FIG 17. The greenhouse camel-cricket, Diestrammena asynamorus, female.
2. Tettigoniidae (bush-crickets)
The bush-crickets are also sometimes referred to as long-horned grasshoppers, and in America and Australasia are commonly called ‘katydids’, a word thought to be an onomatopoeic representation of the song of an American species, Pterophylla camellifolia (Gwynne, 2001: 18–19). Fossil remains of ancestral bush-crickets date from at least the mid Triassic (although Gwynne (2001), following Sharov (1968), places them as early as the late Permian – some 250 million years ago). There are ten native British species, and two recent colonists.
The bush-cricket body is roughly tubular (that is, not dorsoventrally flattened), the antennae are long and filamentous, the hind legs are long and adapted for jumping, and the tarsi have four segments. The pronotum is saddle-shaped, with the rear edge of the dorsal surface curved. In fully winged species the wings are folded back over the dorsal surface of the abdomen when the insect is at rest. The males of most species produce sound by rubbing together their fore wings (tegmina). These are structurally modified for this function, and in the males of some species with reduced wings the stridulatory apparatus comprises most of the wing. In bush-crickets the sound is produced by scraping the left tegmen over the right. In just two species that occur in Britain there is no stridulation, but the males produce a sound by drumming with one leg against a substrate. The specialised hearing organs consist of a pair of ‘ears’ each located in a fore tibia, just below the knee. The tibial ears are linked internally by extended tracheal tubes to a pair of auditory spiracles located on the sides of the first segment of the thorax (see Chapter 2 for more details).

FIG 18. Female great green bush-cricket, Tettigonia viridissima, a fully winged species; male dark bush cricket, Pholidoptera griseoaptera, showing fore wings (tegmina) reduced to stridulatory apparatus only.
The females have conspicuous ovipositors that project from the rear of the abdomen. Although the ancestors of bush-crickets are believed to have laid their eggs in the soil, the majority now lay their eggs (singly or in small batches) in plant tissue. The straight or sickle-shaped ovipositors are used to insert the eggs into the appropriate substrate. The ovipositor is made up of three pairs of articulated valves, through which the eggs pass, singly, into the selected medium. In a few species that lay their eggs in tough plant tissue (such as the bark of trees – e.g. M. thalassinum) the material may be excavated or softened by chewing first. Some species that lay their eggs in plant stems, such as Roesel’s bush-cricket (M. roeselii) and the short-winged conehead (C. dorsalis), nibble holes in the outer cuticle of the stem prior to piercing it with their ovipositors (see the DVD selections on these species).
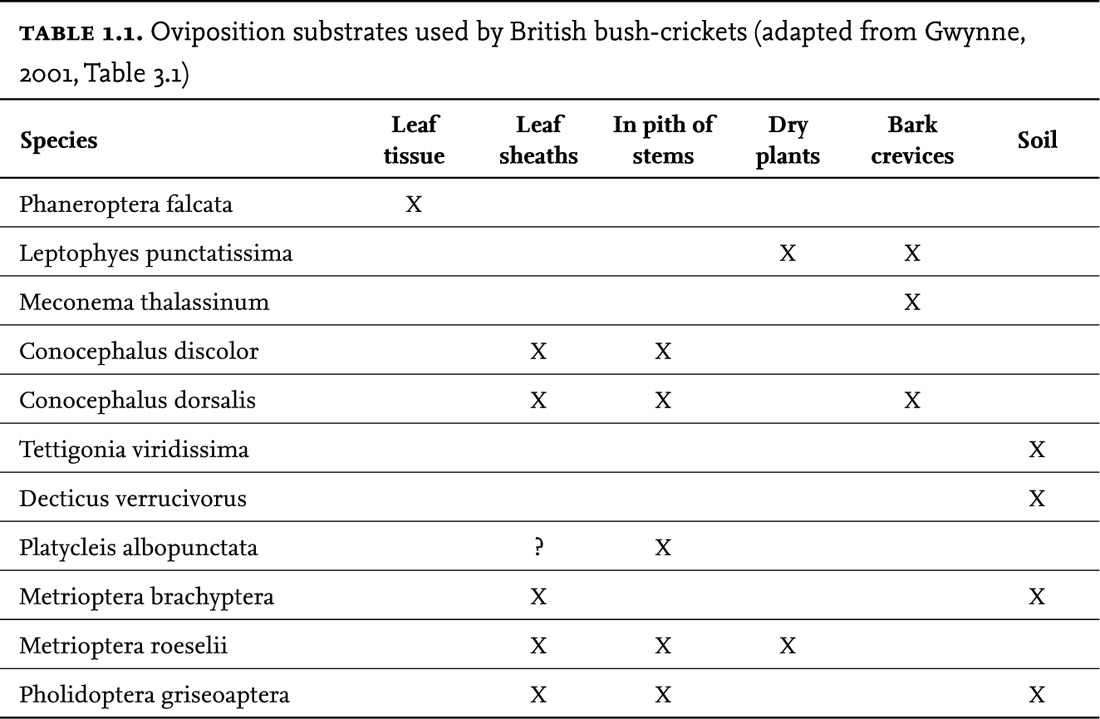
Variation in the shapes of ovipositors may be related to the kind of substrate used and also the ovipositing technique employed. The robust, straight ovipositors of the great green bush-cricket (T. viridissima) and the wartbiter (D. verrucivorus) are supposed to be an adaptation to their habit of ovipositing in soil. The short, stubby ovipositor of the sickle-bearing bush-cricket (P. falcata) is adapted for its habit of laying its eggs between the epidermal layers of leaves.
The males have a pair of cerci that are used to grip the female during mating, and, in some cases, to prolong copulation by constraining her movements. A general feature of the mating systems of the bush-crickets (shared with a few other orthopteran groups) is that during mating the male transfers not just the sperm and associated fluid to the female, but also a gelatinous mass, known as the spermatophylax. The female subsequently eats this, and the function of these ‘nuptial gifts’ has inspired a great deal of fascinating research and controversy (see Chapters 4 and 6). The bush-crickets are usually omnivorous or vegetarian, but a few are predatory carnivores, feeding on small invertebrates. As the English name implies, the bush-crickets generally live among vegetation, although a few have become secondarily adapted for life on the ground. Depending on species, there are from four to eight nymphal instars, and the life cycle may be annual or, in some species, may be prolonged by an extended embryonic period in the egg for two or more years (up to seven years). The wing stubs in winged species are inverted for the final two instars (see Chapter 3).

FIG 19. (a) Ovipositor of a female warbiter, Decticus verrucivorus; (b) female wartbiter egg-laying in the ground; (c) eggs of the short-winged conehead in stem of the sea clubrush, Bolboschoenus maritimus; (d) ovipositor of the short-winged conehead, Conocephalus discolor.
3. Crickets (Gryllidae)
Although ancestral gryllids were present from the mid- to late-Triassic period, the true crickets did not make an appearance until the early Cretaceous (some 130 million years ago). The British gryllid cricket fauna is very limited, with only two native species, and one established alien. The antennae are long and filamentous, the body is flattened dorsoventrally, and the dorsal surface of the pronotum is rectangular. The hind legs are longer than the fore and mid legs, but not so markedly as in the bush-crickets. The hind legs are also sturdy, and not spindly, as in the bush-crickets. Both males and females have long straight cerci, and the ovipositor of the females is straight with a pointed swelling at the tip, and projects backwards from the rear end of the abdomen. Males have rows of spines facing outwards from their hind tibiae.
In fully winged species the hardened tegmina are folded back and around the sides of the abdomen when the insect is at rest. Males of most species are able to produce sounds by stridulation. As in the bush-crickets, the sound is produced by scraping one tegmen over the other, but in the gryllid crickets, it is the right tegmen that is moved over the left. The ‘ears’ of the crickets are, like those of the bush-crickets, located on the fore tibiae, and linked to auditory spiracles on the first thoracic segment. However, there are some structural differences, and it is believed that hearing evolved independently in the two families.
The life cycles differ greatly from species to species, and sometimes within a species, depending on climate. Eggs are usually laid in the soil rather than in plant material, and there may be from eight to eleven nymphal instars. As in other orthopterans, the wing stubs (in winged species) are inverted for the final two instars. Several of the structural features of the crickets can be seen as adaptive for life on the ground, rather than in vegetation, and most species take shelter in holes or under leaf litter, while others (such as the field cricket, Gryllus campestris) dig burrows.

FIG 20. Female field cricket, Gryllus campestris.
4. Scaly crickets (Mogoplistidae)
The family is widespread globally, but only one British species is known. Formerly considered a subfamily of the Gryllidae, the scaly crickets are so called because their body surface is covered with minute fragile scales, giving them a rough, granular appearance. The body is flattened dorsoventrally, and wings are entirely absent in most species. There are no stridulatory organs, and no tibial hearing organs. The antennae are used constantly in communication with conspecifics and in monitoring the environment. Both sexes have long, tapering cerci, and there are three tarsal segments. The hind legs are shorter than in most other Ensifera, but the crickets retain the ability to jump. The female ovipositor is spear-shaped, as in the true crickets.

FIG 21. Female scaly cricket, Pseudomogoplistes vicentae.
5. Mole-crickets (Gryllotalpidae)
It seems that the mole-crickets evolved somewhat later than the Gryllidae, during the Cretaceous period. There is just one British species, which is close to extinction, although there are relatively frequent reports of accidental imports of alien species in plant material. The most striking feature of the mole-crickets is the enlargement and structural modification of the fore legs for digging. Together, the head and pronotum form a smoothly tapering egg shape, and the hind legs are secondarily reduced in size, so that the overall shape of the insect is modified for its mainly subterranean habit. The antennae are short, compared with those of the other ensiferan groups, and there is a pair of long, straight cerci at the tip of the abdomen. The hind tarsi have three segments. The short tegmina are hardened and, in the males, structurally modified for stridulation, with the hind-wings furled over the abdomen as in winged species of true crickets. The females lack the prominent ovipositor characteristic of other ensiferans. Females remain in their burrows with the eggs and are almost unique among the Orthoptera in showing maternal care of the eggs and early instar nymphs.

FIG 22. The mole cricket, Gryllotalpa gryllotalpa. (© B. Pinchen)
CAELIFERA
1. Groundhoppers (Tetrigidae)
The groundhoppers are small relatives of the grasshoppers. The antennae are short and the long hind legs are adapted for jumping. The most obvious difference between them and the grasshoppers is the backward extension of the pronotum over the dorsal surface of the abdomen, and in some species beyond its tip. The fore wings are reduced to small lateral flaps, while the hind wings are fan-folded under the pronotum. There are two segments in the tarsi of the fore and mid legs, three in the tarsi of the hind legs, and there is no arolium (small pad or swelling) between the claws. There appear to be no stridulatory or hearing organs. The eggs are laid in batches in the soil or among low-growing plants, and the resulting nymphs pass through five instars in the male, six in the female. There are three British species.

FIG 23. The common groundhopper, Tetrix undulata.
2. Grasshoppers (Acrididae)
The grasshoppers are the most familiar of the orthopteran groups. They are common, and sometimes abundant, in a range of grassland types. The antennae are short and either parallel-sided or thickened towards the tip – sometimes club-tipped (as in butterflies). The pronotum is saddle shaped and has several features that are important for identification. When viewed from above, the rear edge of the pronotum is curved outwards, but does not project back over the abdomen as in the groundhoppers.
There is a fine longitudinal ridge, or keel, along the midline of the dorsal surface of the pronotum (the ‘discus’). There are two more longitudinal keels, one on each side of the discus of the pronotum. These may be straight and almost parallel, more or less strongly curved inwards, or angled inwards (‘indented’). There is also a fine groove (‘median suture’) that runs transversely across the discus.
Most species are fully winged, with the tegmina hardened, and both pairs of wings folded back over the abdomen when the insect is at rest. Some species (including one British species, Chorthippus parallelus) have their wings markedly reduced, although in some such species there are long-winged forms. The hind legs are long, and grasshoppers are generally very effective jumpers. Fully winged species are able to supplement their jumps with flights of ten metres or more.
Most species have a distinctive song produced by the male, and sometimes also by the female. There are two subfamilies with representatives among the British species. In subfamily Oedipodinae sound is usually produced by scraping a ridge on the hind femur over a prominent vein in the tegmen. In the one native British oedipodine grasshopper (Stethophyma grossum) a series of sharp clicking sounds is made by flicking a hind tibia against the adjacent wing tip (see DVD). Most British species belong to subfamily Gomphocerinae, and in this group sound is produced by scraping a row of tiny pegs on the hind femur against a prominent (radial) vein on the flexed tegmen. Both hind femora are used, often to some extent out of synchronisation with one another. Some species have elaborate courtship performances, including both song and dance. The hearing organs are a pair of distinct auditory cavities located at the sides of the first abdominal segment.

FIG 24. The common field grasshopper, Chorthippus brunneus, male.

FIG 25. Dorsal surface of the pronotum of grasshoppers, showing the shape of the lateral keels: (a) lesser marsh grasshopper, Chorthippus albomarginatus; (b) meadow grasshopper, Chorthippus parallelus; (c) common green grasshopper, Omocestus viridulus; (d) field grasshopper, Chorthippus brunneus.
The female ovipositor is short, with two pairs of valves, whose shape is useful in the identification of some species. Males have an up-turned subgenital plate at the tip of the abdomen, and different profiles of the abdomen tips are useful in distinguishing males from females. Both sexes have a pair of short cerci.
The eggs are laid in batches among grasses or in the soil. They are surrounded by a secretion that hardens into a pod. In temperate climates the winter is usually spent in the egg stage, the nymphs hatching the following spring and passing through four or five nymphal instars. As in other winged orthopterans, the developing wing pads are inverted in the final two nymphal stages. There are eleven British species (with a further two that occur in the Channel Islands).

FIG 26. Tip of the abdomen of male and female of the lesser marsh grasshopper, Chorthippus albomarginatus.
WHERE AND HOW TO FIND GRASSHOPPERS AND CRICKETS
A glance at the distribution maps for the British species reveals one very clear pattern: more than a third of the native species occur only south of a line between southern coastal districts of Wales, the Bristol Channel and the Wash. The distribution of others reaches not much further north, while even the hardy species that are widespread throughout mainland Britain often become noticeably more localised northwards. Ireland, while not significantly different climatically from the rest of the British Isles, has only twelve species. D. Ragge (1963, 1965, 1988) has provided a powerful explanatory framework for this pattern. His analysis combines historical climates, geological formations and vegetation cover, together with understanding of the habitat requirements and climatic tolerances of our orthopteran fauna. Conditions prior to the retreat of the ice at the end of the last glaciation (approximately 12,000 years ago) were too cold for all of the species that currently occur here. At that time, the lowering of the sea level due to the formation of the ice cap had allowed the formation of a land bridge between the British and continental mainlands, and another between the west of northern Britain and the north of Ireland.
As the climate continued to warm, and the ice to retreat, orthopteran species began to colonise what eventually became southern Britain. Cold-tolerant species such as the common field grasshopper (C. brunneus), the common green grasshopper (O. viridulus), the common groundhopper (T. undulata), the speckled bush-cricket (L. punctatissima) and the oak bush-cricket (M. thalassinum) are likely to have been the first to colonise. These would have spread northwards and, together with the mottled grasshopper (M. maculatus), the large marsh grasshopper (S. grossum), the lesser marsh grasshopper (C. albomarginatus), and the mole-cricket (G. gryllotalpa), would then have crossed the land bridge between mainland Britain and Ireland. By the onset of the Boreal period (some 9,000 years ago) sea-level rise cut off Ireland and the Isle of Man from the British mainland, preventing any more orthopteran species from colonising Ireland from the rest of the British Isles. Some time later (Ragge suggests between 9,000 and 8,000 years ago) further sea-level rise isolated the British mainland from continental Europe, preventing further colonisation of Britain from the rest of Europe. This historical sequence helps to explain why species that endure more hostile climates in northern Europe, and probably could survive in Britain, still do not occur here. It seems that their post-glacial northward migration through what is now France took place too late for them to cross the land bridge.
Ragge’s account makes good sense of the absence of the meadow grasshopper (C. parallelus) from Ireland and the Isle of Man: its inability to fly may have slowed down its range expansion into northern Britain, so that it arrived at the land bridge too late to colonise them. However, as he acknowledges, his account has difficulty in making sense of the isolated population of the lesser mottled grasshopper (S. stigmaticus) on the Isle of Man. Did it formerly have a more widespread distribution in England? If so, what explains its extinction everywhere else? On the assumption of a north-western land bridge to Ireland, the presence there of highly localised populations of the dark bush-cricket (P. griseoaptera) and Roesel’s bush-cricket (M. roeselii) also seems puzzling. Although Roesel’s bush-cricket has recently extended its British range both northwards and eastwards, its known historical range was far distant from the land bridge to Ireland. The dark bush-cricket’s current range does reach to south-west Scotland, but its known distribution in Ireland is confined to restricted areas in the south and west. It is, of course, possible that the geographical gaps in the currently known distribution of these species were filled in previous, warmer epochs, with subsequent contraction leaving isolated relict populations.
With the climatic warming that took place following the arrival of the native orthopteran fauna came also a rapid spread of forest cover. This would have severely limited range extensions on the part of almost all species, and it seems likely that they remained confined to small areas of open ground, perhaps on the coasts, river margins, and adjacent to early human settlements. On Ragge’s account, deforestation would have begun in earnest from around 5,000 years ago in Neolithic times, when the East Anglian Brecks and southern downlands were cleared. By the end of the Roman occupation only half of the forest remained, and orthopterans would have had much greater opportunities for dispersal. However, their subsequent patterns of dispersal would have been limited both by a climate that was by now cooler in the summers, and by features of geology, land forms and vegetation cover.
Ragge’s analysis focuses on the Hampshire basin – including the New Forest, the Isle of Purbeck and the Isle of Wight. This remains the richest area in Britain for Orthoptera. The sands and clays of the New Forest and the heaths of north Purbeck are fringed by calcareous downland ridges. The acidic sandy soils are nutrient poor, and so do not support luxurious vegetation, while the valley bottoms have become waterlogged because of the underlying clay. These conditions support the boggy hollows where species such as the large marsh grasshopper (S. grossum) and bog bush-cricket (M. brachyptera) thrive, while on drier heathland and scrubby woodland occur the woodland grasshopper (O. rufipes) and the wood cricket (N. sylvestris), as well as small populations of the rare heath grasshopper (C. vagans). On the warm, south-facing slopes of the downland can be found the great green bush-cricket (T. viridissima) and stripe-winged grasshopper (S. lineatus). A similar pattern of wet and dry heathland and chalk downland is to be found to the north and east, in the London basin, in the shape of the North Downs, South Downs and Surrey heaths.
These areas remain prime hunting grounds for Orthoptera enthusiasts, but there are many opportunities for the study of these fascinating insects elsewhere. Some species are to be found in a wide range of coastal habitats, while others can still be found in the wider countryside, and in urban locations where intensive agriculture or excessive tidy-mindedness have so far failed to eliminate them. As will be discussed at greater length in Chapter 9, the post-war transition to more intensive agriculture over large areas of southern and central England must have devastated populations of the commoner grasshoppers and bush-crickets. Orthoptera are virtually absent from intensive arable cropland, while the nutrient enrichment and commercially dictated cutting regimes of hay meadows allow the survival of no more than two or three very resilient species of grasshopper. Lightly grazed pasture seems to be favourable to a few more common species. Loss of hedgerows and copses, too, will have eradicated much of the habitat of species such as the oak, speckled and dark bush-crickets on farmland.
However, there are some compensations. Fragments of suitable habitat do persist – often unintentionally – in intensively managed farmland. Some hedgerows and woodland have survived, and are increasingly valued by many farmers and other land managers. Alongside hedgerows there are often footpaths, bridleways and cart tracks, and these, together with ditches, continue to provide habitat for as many as eight or nine species. Other patches of suitable habitat for several species are incidental products of modern infrastructures such as coastal flood defences, the cuttings and embankments of motorways, the verges of lanes and roads, open areas in forestry plantations and margins of reservoirs. Special mention here should be made of many so-called brownfield sites, former industrial land that has benefited from neglect of formal management and has acquired a rich botanical diversity and invertebrate fauna. Such sites are frequent targets for developers, and need to be actively advocated and defended.

FIG 27. New Forest wet heath, habitat of the large marsh grasshopper, Stethophyma grossum, and the bog bush-cricket, Metrioptera brachyptera.
Many areas set aside for public recreation, such as urban parks and gardens, country parks, and rural ‘visitor attractions’ such as stately homes and gardens, have a good range of Orthoptera species, and it is well worth surveying them and highlighting their value to owners and managers. Finally, alarm about the loss of biodiversity associated with agricultural intensification and urbanisation has led to a shift of policy in the direction of conservation. Land managers can apply for government funding to set aside land from commercial production in favour of habitat provision. In addition both statutory and voluntary bodies have established nature reserves where the interests of biodiversity conservation are given (or should be given) priority in the management regime. Such measures have undoubtedly been to some degree beneficial, but newer approaches that conceptualise nature conservation at the level of whole interconnected landscapes may offer more promise for the future if adequate funding can be gained.

FIG 28. A so-called ‘brownfield’ site on Canvey Island, Essex, with an exceptionally rich invertebrate fauna.
The above discussion should provide some suggestions as to where to look for grasshoppers and crickets, but not necessarily how to do so! Often, if the day is warm, and the observer is possessed of good hearing, the general location of a suitable site to survey can be made on the basis of the songs of the grasshoppers. Otherwise, experience soon enables one to spot a potentially suitable patch of habitat. Having found such a place, perhaps the simplest method is to walk slowly through the area, watching out for the insects to jump or briefly fly as they attempt to escape. Even then, locating the insect is not always easy. A grasshopper will often touch a grass stem before it lands, and while its hunter is distracted by the movement, it will have disappeared a few inches further on. While some species remain motionless and perfectly camouflaged once they land, others dive down into the depths of vegetation.
Even when located, grasshoppers and bush-crickets keep a close watch, and even a slow, gentle approach to get a close look at structure or markings will often trigger another jump. Alternatively, the insect will swivel around the grass stem so that only its feet are visible. A hand passed around the other side of the stem will sometimes persuade it to swivel back again – but only sometimes! The method of walking slowly and searching visually when they jump or fly is probably the best way of locating groundhoppers, as they make no sound. Here, some experience of their favoured habitats and geographical distribution is a most useful aid.
With experience, and good hearing, it is possible to locate and identify many species by noting distinctive features of their song. For those of us with a deteriorating ability to pick up high-frequency sounds, a bat detector is invaluable. Again, some experience is necessary to recognise the modified sounds of the different species, but when this can be done the detector is a great help with surveying for bush-crickets, especially. Speckled bush-crickets, the coneheads, grey bush-crickets, wartbiters and, for some of us, even Roesel’s bush-crickets, are very difficult or outright impossible to hear unaided. Surveying in Essex using a bat detector has revealed that the speckled bush-cricket, for example, is far more widespread then would have been guessed from visual sightings. Even those with exceptionally acute hearing still may benefit from the use of bat detectors (Baldock, 1999; Gardiner et al., 2010).
The oak and southern oak bush-crickets do not stridulate, and I have no evidence that the sound of their drumming can be used by humans to locate them. Several methods are recommended. They fly at night, and the oak bush-cricket comes to light, often entering lighted rooms, or intervening during moth-trapping sessions. It is said they may be found as road casualties after being dislodged from their perches by high winds. Another method, which is hard work but moderately successful for both species, is beating the lower branches of deciduous trees, while holding a purpose-made beating tray, or inverted umbrella, below. This seems to be more effective as a means of finding nymphs than adults – either simply because they are more numerous, or, possibly, because the adults tend to live in higher foliage. Finally, shining a torch on the trunks of trees at night is said to be an effective method of finding the females (Hawkins, 2001) – although in some areas this might put one at some personal risk from other humans!
Surveying the range of Orthoptera inhabiting particular sites can be very valuable if evidence is needed to defend an area from unsuitable development, or give advice on management. Despite their lack of gaudy decoration, grasshoppers and crickets are very beautiful animals, and make excellent photographic subjects. Photography is also an extremely useful aid to identification for the beginner. Although very high-quality photographs can now be taken using low-priced cameras, it is helpful to be able to obtain a large image while working at some distance from the subject. A macro lens (e.g. 200 mm) or camera with a macro setting is therefore advisable. For identification from photographs it is important to get a clear view of the diagnostic features. So, for example, a dorsal view of the side keels of the pronotum together with a lateral view showing the leading edge of the fore wing in focus are required for the identification of many grasshoppers. A clear view of the shape of the ovipositor in female bush-crickets is another example.
Filming the fascinating behaviour of orthopterans can also be a great source of interest and information. As I found, for someone without experience of filming, there is initially a very steep learning curve! Even with a degree of ability to work the technology, the problems of filming in the field are legion. As the subjects necessitate use of macro settings, filming without a tripod is impossible, even for those with a very steady hand. This means that filming has to be done from a static viewpoint, given the disturbance caused by attempting to move a tripod around in long grass, scrub or heath. This problem can only be addressed by getting to know the site very well before beginning filming. With experience it is possible to get a sense of exactly where and when during the day certain species come to bask, or sing, or carry out their courtship. Then it is a matter of setting up the tripod and camera, and waiting quietly in the hope that something will happen. Often it doesn’t, and the main reward of a day’s fieldwork will be aching legs and a liberal covering of insect bites. However, the satisfaction to be gained when things do happen, and one can view the results on a TV screen in the evening, is ample compensation. I guess the required attitude of mind is very similar to that needed for angling.
For more help with identification as well as scientific research projects, sound recording of orthopteran songs has a most important role to play. Pioneering work by D. W. Ragge in field recording of the songs of grasshoppers and crickets has contributed to the taxonomy of European species by showing how song is often a key element in the development of reproductive isolation between populations and the differentiation of species. In grasshoppers and bush-crickets the frequency spectrum of the song seems to be less significant than the rhythmic pattern of sound intensity. This has been represented by Ragge and others in the form of oscillograms which are distinctive for each species, and also show some revealing differences within species. Ragge describes the technique, and provides song analyses and oscillograms for the western European species in Ragge & Reynolds (1998).
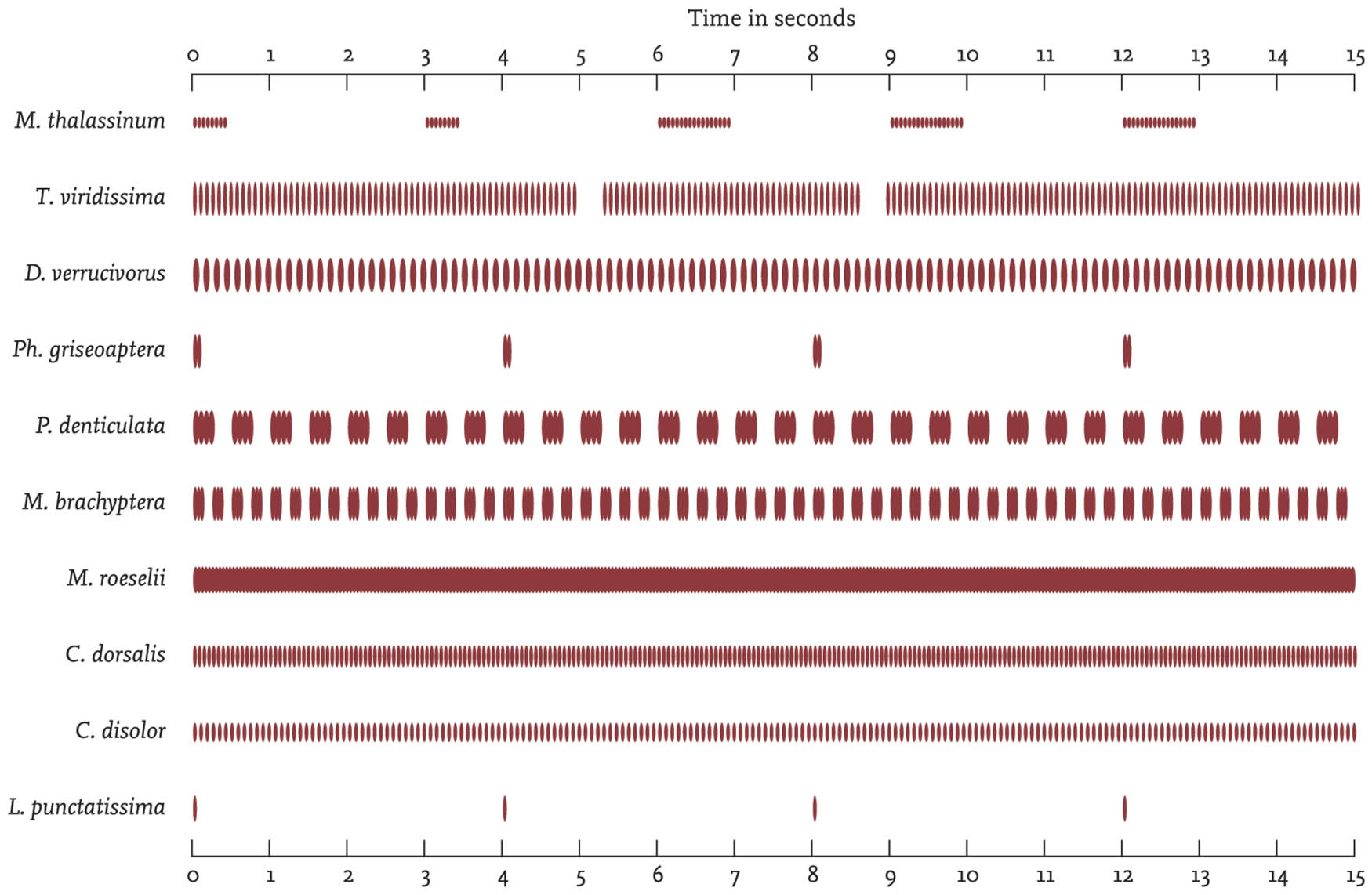
FIG 29. Song diagrams of the British Orthoptera, drawn from oscillograms by D. Ragge (reproduced from Ragge 1965, figs 38 and 58, with permission).
Finally, for some purposes it is important to be able to estimate the size of orthopteran populations in a given area. Several methods have been used. These include catching, marking and releasing insects. Subsequently samples are recaptured, and overall population size can be calculated from the proportions of marked to non-marked individuals among the new sample. Other methods include using a sweep-net and passing it through vegetation in a standardised way, counting insects in quadrats laid out at intervals across a site and walking a standard transect across a site, counting the insects flushed as one walks. Pit-fall traps are also sometimes used, although these are more useful for indicating the presence or absence of species than for estimating population sizes. Each of these survey techniques has its advantages and disadvantages, and these vary according to the purpose of the research and the nature of the habitat under study. An excellent critical review is Gardiner et al., 2005.
ABOUT THIS BOOK
Chapter 2 is about ‘mechanics’. It gives an account of the main external features of the orthopteran body, together with brief introductions to the basic mechanisms underlying sight, hearing, and the chemical senses. The processes involved in generating the distinctive motor responses to these sensory inputs are also described, for example, how orthopterans achieve their extraordinary leaps, fly and steer their phonotactic movements.
Chapter 3 introduces some of the diversity of orthopteran life cycles. Two key themes are the focus of the chapter. One is the various ways in which grasshoppers and crickets can vary the timing of the different phases of their life cycle in relation to the seasons, or to deal with adverse climatic conditions. The other theme is the phenomenon of alternative developmental pathways that are available within populations of some species. The most widely known examples are, of course, the phase transitions of locusts, but the production of minorities of long-winged forms in normally short-winged species is observed in a few British species. The coexistence in populations of many species of a variety of colour forms also poses puzzles that have been addressed in the scientific literature.
Chapters 4, 5 and 6 should be taken together, as they deal with aspects of a common theme: the reproductive behaviour of orthopterans. As almost all species reproduce sexually, reproduction involves at least these necessary stages: locating a potential mate, getting up close, persuading him or her to cooperate, and mating (in fact, as we shall see, there are more). It is commonly assumed that sexual reproduction is a cooperative process, in which both partners share an interest. However, it has long been recognised that conflict is also involved: males often compete for females, and females frequently reject the advances of males. Darwin went so far as to suggest that inheritable differences that conferred advantages in the struggle for sexual partners constituted a distinct evolutionary mechanism – sexual selection – alongside natural selection. Chapter 4 traces some of the history of debate about this idea, and more recent developments which emphasise the asymmetry of male and female interests and the evolutionary significance of sexual conflict. Chapter 5 is concerned mainly with the first steps in the reproductive process: how males signal their presence to potential mates, how rival males react to this ‘advertisement’, and how receptive females recognise and respond to it. The focus, then, is on the use of song, and the extent to which females exhibit preferences on the basis of its quality or persistence. Chapter 6 reports on further phases in the encounter between male and female, including courtship, mating, the transfer of sperm and the methods by which males attempt to ensure that it is their sperm that fertilises the female’s eggs. The diversity of behaviour exhibited across the different orthopteran groups is astonishing – from the elaborate song-and-dance routines of some grasshoppers, to the provision of gelatinous ‘nuptial gifts’ by male bush-crickets and the guarding of harems in holes in trees by male tree weta of New Zealand. The chapter draws on a selection from the vast international literature that describes and attempts to interpret these ‘mating systems’ in terms of the ideas of sexual selection and conflicts of interest between males and females.
Chapters 7, 8 and 9 deal exclusively with British species (including a small number that are not, or not yet, fully resident, but may occasionally be found here). Species and varieties found in the Channel Islands but not in mainland Britain are not included (see Evans & Edmondson, 2007). Preceding this group of chapters is an identification key to the British species. The aim is to enable identification on the basis of clear photographs, without the need to catch or kill specimens. A few structural features that may be difficult to see in a photograph are mentioned for confirmation of the identification of some species. Chapter 7 consists of species accounts for the British Ensifera (bush-crickets, crickets and the mole-cricket). Photographs and descriptions are given for each species, together with accounts of the main variant forms, methods for distinguishing it from lookalikes, and details of behaviour, life cycle, habitat, British distribution and any conservation issues. Chapter 8 repeats the exercise for the Caelifera (groundhoppers and grasshoppers).
Chapter 9 brings together material from previous chapters to inform a general discussion of the habitats, distribution and conservation issues concerning the British Orthoptera.