The British species, part 1: Ensifera
Bush-crickets, crickets and their relatives
This large subdivision of the Orthoptera includes nine (or ten; see Gwynne, 2001 and Chapter 2) orthopteran families worldwide, of which only five are represented in the British fauna. These are: the Tettigoniidae (the bush-crickets or ‘katydids’); the Gryllidae (the true crickets); Mogoplistidae (scaly crickets); Gryllotalpidae (mole-crickets); and the Rhaphidophoridae (camel-crickets and cave weta).
The derivation of the word ‘Ensifera’ suggests a reference to the sword-shaped external ovipositor that is a characteristic feature of the families belonging to the suborder (although the true crickets have more needle-shaped ones!). Another characteristic feature is the long – often much longer than the body – pair of filamentous antennae possessed by both sexes. There are also internal structural differences and characteristics of DNA shared by all groups within the suborder that justify their treatment as a single ‘clade’ (i.e. as sharing a common ancestor). The two families Raphidophoridae and the Tettigoniidae are generally classified together in the super-family Tettigoniodea, while the Gryllidae, Mogoplistidae and Gryllotalpidae are included together in the Grylloidea.
FAMILY RHAPHIDOPHORIDAE: THE CAMEL CRICKETS
Members of this family have much in common with the Tettigoniidae (bush-crickets), having long, filamentous antennae, four-segmented tarsi and swordlike ovipositors. They also resemble most bush-cricket species in their mating behaviour. During copulation the male transfers to the female a gelatinous spermatophore. This mass (which varies in size between species) contains both a sperm sac (ampulla) and an attached mass of edible material (the spermatophylax), which the female consumes while the sperm are transferred to her spermatheca (see Chapter 6).
The Rhaphidophoridae differ from most of the bush-crickets in being entirely wingless, and lack the hearing organ on the fore tibia. However, they do have a structure on the fore tibiae, called the subgenual organ, that enables the insects to detect substrate vibration. The pronotum is smoothly rounded, and both antennae and the cerci are much longer than is usual among the Tettigoniidae. There may be ten or more nymphal instars. Only one species has been established in Britain, probably introduced along with plants imported from southern China, although other accidental introductions of related species are occasionally reported.
THE GREENHOUSE CAMEL-CRICKET
Diestrammena (formerly Tachycines) asynamorus (Adelung, 1902)
MEASUREMENTS: Male: 12–14 mm in length; female: 14–18 mm in length (excluding the ovipositor); ovipositor: 10–12 mm; antennae: 65–70 mm.


FIGS A–C. Male; male, dorsal view; female.
Description
The pronotum is smoothly rounded, and the convex outline of the back when viewed from the side may explain the vernacular name. The ground colour is pale brown, with darker mottling and banding, especially on the rear margins of the pronotum and abdominal segments. The legs are also pale with dark banding. There are no wings, and no hearing organs on the fore tibiae. The legs, antennae, abdominal cerci and palps are also very long in relation to the size of the body. The four segments of each tarsus are also elongated and there are sparsely distributed long bristles on the legs and cerci.
Similar species
There are no similar British species, although there are reports of occasional accidental introductions of related species such as Dolichopoda bormansi from Corsica (Evans & Edmondson, 2007).
Life cycle
In the artificially heated environments where this species breeds in Britain, the life cycle is continuous through the year. The eggs are approximately 2–5 mm in length by 1–1.2 mm, and are laid singly or in small batches of up to 8 in soil. The eggs hatch after 8 to 10 weeks, depending on temperature, and the resulting nymphs pass through 8 to 10 or more instars to reach adulthood. This takes 6 to 10 months. As breeding is not governed by seasons, and the rate of nymphal development varies considerably in the same population, adults and all nymphal stadia may be found together. Nymphs are whitish in colour immediately after moulting, but acquire the characteristic brown-blotched pattern in a day or so. The cast-off skin is rapidly eaten after each moult. The ovipositor appears as a very small projection at the third instar, and becomes longer at each successive stage (see Chapter 3, Fig 59 for images of key developmental stages).
Habitat
In Britain this species is confined to artificially heated environments, such as greenhouses, warehouses or even garages. However, most British records are from garden centres or glasshouses with exotic plants. The two recently reported populations were living in plant nurseries, under wooden staging or paving slabs close to hot-water pipes, as well as in storage cupboards. The mid-winter temperature in one case was between 13°C and 17°C (Panter, 2007; Sutton, 2007b; own observations). During daylight hours the insects tended to be found hidden away in groups in dark, moist and warm locations.
Behaviour
Camel-crickets are nocturnal, and hide in dark corners during the day. In the absence of stridulation and developed hearing organs, it seems likely that they communicate by vibrations of the substrate, but they also cluster together in dark and damp places during daylight hours, constantly touching one another with their exceptionally long antennae. Courtship and mating seem to occur mainly at night, and male and female adults have been observed with the male resting a fore leg on the pronotum of the female. Ragge (1965) describes the male as attempting to push his abdomen under the female from various angles until he is able to crawl backwards underneath her. Mating lasts for 2 to 4 minutes, and during it a spermatophore is placed at the base of the female’s ovipositor. The spermatophore is white and gelatinous, approximately 3–4 mm in diameter, and is rapidly eaten by the female. The palps are used during feeding and general ‘searching’ behaviour, as well as by females in selecting suitable ovipositing sites. The female draws her ovipositor under her body so that it points directly down to the soil, moves slightly forwards and presses the tip into the ground. When it is fully inserted into the soil, egg-laying is indicated by a pumping motion of the abdomen (T. Kettle, pers. comm.).
Both sexes have very powerful back legs and can jump long distances when disturbed. They are therefore very difficult to catch. They are reputed to feed on other insects, including insect pests. However, staff at one plant nursery reported their penchant for the flesh of dead rats, and in captivity they ate rabbit flesh. Close observation of captive crickets by several observers produced no examples of them taking live prey. They appear to be generalised scavengers, feeding on a variety of plant material such as lettuce (where they bite holes in the leaf lamina), groundsel, carrot, apple, nectarine, as well as dead woodlice, mealworms and rabbit flesh. One instance was noted of a female feeding from the carcase of another camel-cricket. However, it seems likely that the ‘victim’ was either dead or moribund prior to being eaten! Early instars feed on small plants, including soil algae. At all stages, if provided with a range of ‘options’ for cover during the day, they will tend to roost together, and opt for the dampest situation.
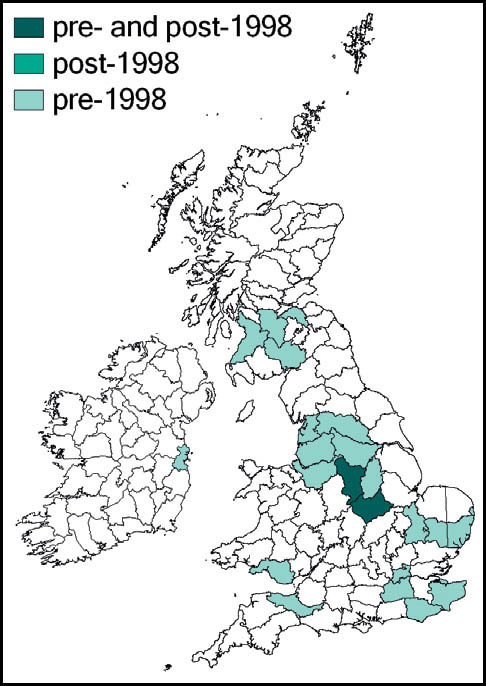
History
Greenhouse camel-crickets were probably introduced into heated greenhouses in Britain (and much of the rest of Europe) in the latter part of the 19th century. In England the species has been recorded from widely scattered locations in Somerset, Sussex, Kent, Surrey, Middlesex, Suffolk, Cambridgeshire, Leicestershire, Derbyshire, Cheshire and Lancashire. It has also been recorded from Glamorgan, Glasgow, Edinburgh, Ayr and Dumfries. There are Irish records from Dublin. Post-1960 records given by Marshall & Haes (1988) include: plant nurseries at Canterbury (1962–5) and near Woking (1970–3); in the garage of a private house near Fleetwood, Lancashire (1975); and Dublin zoological gardens (1975).
The two most recent reports appear to be from a garden centre at Clowne, Derbyshire, where it was recorded in 2006 (and had been present, according to staff, for at least 10 years), and from another at Leicester (where it had been present for at least 15 years). In the face of the impending closure of the Leicester site, rescue attempts were made in December 2006 and January 2007 by G. Panter, H. Ikin, M. Frankum and P. Sutton. Small numbers of the crickets were retained in the hope that the population could be kept going in captivity, but these attempts proved unsuccessful in the long term. However, the Derbyshire population was fully recognised and positively valued by the centre management. John Kramer and the author visited the site on 8 December 2009, only to find that it, too, was due to close down. With the help and support of staff we collected as many of the crickets as could be found (causing some amusement and curiosity from customers). In all, we collected some 15 insects, several of them in various juvenile stages. These were kept in two old aquaria, in which they reproduced continuously, allowing several independent ‘colonies’ to be established during 2010, including a sample taken by Bristol Zoo. At the time of writing, one sample has reached a range of instars including adults in the third generation from the original stock.
Status and conservation
Accidental introductions with exotic plants imported from the Far East resulted in the spread of the camel-cricket throughout much of Europe in the 19th century. According to the owners of the garden centre in Leicester, their crickets had come in from plant stock imported mainly from Belgium, Holland and Denmark.
It seems quite possible that other populations of this species still survive in heated glasshouses somewhere in Britain, and efforts should be made to locate any other remaining populations. Meanwhile, a case could be made for a captive breeding programme to be established, using specimens from the known surviving British population.
References: Marshall & Haes, 1988; Ragge, 1965; Panter, 2007; Sutton, 2007b, 2011.
FAMILY TETTIGONIIDAE: THE BUSH-CRICKETS
Also known as ‘long-horned grasshoppers’ and, especially in North America and Australasia, as ‘katydids’, this family includes some of the most thoroughly studied of orthopteran groups. They number over 6,000 known species, and are distributed over the whole of the world, apart from Antarctica. The majority of species are nocturnal, and spend most of their time in vegetation. The females possess external sword-like ovipositors, which may be used for piercing plant tissue or, less commonly, for digging into soil. The antennae are long and filamentous and are used in an elaborate ‘fencing’ performance during courtship.
Along with crickets and grasshoppers, most species communicate by sound (see Chapters 2 and 5). In most species, males transfer an edible ‘nuptial gift’ to the female during mating, and this has provided researchers with a valuable way of exploring controversial issues in evolutionary theory, notably sexual selection and reproductive conflict between the sexes (see Chapters 4, 5 and 6).
The males sing by rubbing together their modified fore wings (or tegmina), as do the true crickets, but the bush-crickets are distinctive in scraping the left over the right tegmen. The songs of bush-crickets tend to be of a higher frequency than those of grasshoppers, close to, or above the limits of human hearing. The bush-crickets also have distinctive hearing organs, tuned to the frequencies of their songs, located on the basal area of the tibiae of their fore legs, and linked via an enlarged auditory vesicle to a spiracle on the thorax (see Chapter 2).
Many species in Britain have a life cycle lasting two or more years, with the first year (or more) spent in the egg stage. Most species pass through five or six nymphal instars, usually emerging as adults during mid to late summer. In winged species, the wing buds are inverted for the final two juvenile instars. In some species the wings are reduced, often, in males, consisting almost wholly of the stridulatory apparatus. Some species have both long-and short-winged forms.
Bush cricket (© Tim Bernhard)
THE OAK BUSH-CRICKET
Meconema thalassinum (De Geer, 1773)
MEASUREMENTS: Male: 12–17 mm in length; female: 11–17 mm in length.
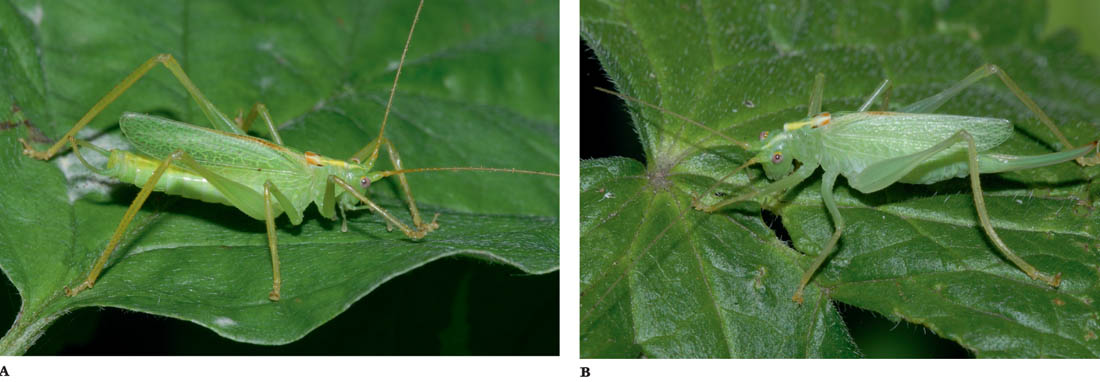
FIGS A–B. Male; female.
Description
The oak bush-cricket is pale green in colour and rather delicately built. Both sexes have an indistinct yellowish stripe along the middle of the dorsal surface of the abdomen that is continued onto the pronotum and head. However, on the dorsal surface of the pronotum it is flanked by two small black and two chestnut brown markings. The hind margins of the wings (i.e. the ‘ridge’ of the tent-like shape formed when the wings are folded) are often narrowly brownish, and the eyes are pale lilac. In mature specimens, the tibiae and tarsi are yellowish green. The adults are fully winged, the wings reaching back to approximately the tip of the abdomen. The wings of the male are not modified for stridulation. The male has a pair of long, curved cerci at the tip of his abdomen, and the female has a long, slightly up-curved ovipositor, which is green, usually shading to yellow and finally brown towards the tip (see the Key, Figs K3e and K5c).
Similar species
The great green bush-cricket (Tettigonia viridissima) is a much bigger and more sturdy insect, and is found in quite different habitats. The speckled bush-cricket (Leptophyes punctatissima) often occurs in the same haunts, but is a more compact insect, has only very tiny wing stubs, and, as the name suggests, is covered by minute black points. The southern oak bush-cricket (Thalassium meridionale) is a newcomer to Britain and a close relative of the oak bush-cricket. The two species look very similar, except that the southern oak has only very tiny wing stubs in the adult.
Life cycle
The eggs are laid from late summer to autumn, in crevices in the bark of trees, or under mosses or lichens. The eggs are buff-coloured, and approximately 3 mm by 1 mm in size. They may pass one or two winters in the egg stage, and hatch in late spring. The resulting nymphs pass through five nymphal instars to reach adulthood by late July or early August. The adults survive well into November in most years.
Habitat
They live among the foliage of trees – most commonly, as their vernacular name implies, in oak trees – but they can also be found in a wide range of other tree species as well as garden hedges and shrubs. There are even reports of their occurrence in reed beds (Haes, 1976). They are commonly found in urban and suburban parks and gardens.

FIG C. Characteristic ‘Hindu’ resting pose.
Behaviour
They are nocturnal, and during the day they rest on the underside of leaves, body pressed close to the leaf-lamina, and legs spread out in an angular pattern – rather resembling the image of a Hindu god. Even when present in gardens, they are extremely difficult to locate during daytime, but at night they are often attracted to light, and commonly enter houses, only to settle motionless on the ceiling.
When they are active, from dusk onwards, they walk about from leaf to leaf and onto the trunks or larger branches of trees. They can hop and also fly short distances. They feed mainly on small invertebrates, such as caterpillars and other larvae, aphids and even members of their own species. They will also consume some plant material but in captivity do not thrive on this alone.
There are some literature references to the males being able to produce a soft stridulation, but it seems that the main mode of communication is vibratory. The males issue a series of bursts of drumming on the substrate with one hind foot. This is Ragge’s description of the process:
Both pairs of wings are raised upwards until they are perpendicular to the body, and one of the hind feet is drummed on to the substrate (e.g. the surface of a leaf). The other hind leg is extended backwards to act as a support. The drumming is extremely rapid and is in very short bursts of less than a second. There are usually a number of these bursts, the first ones being of shorter duration than the remainder. The whole process lasts no more than a few seconds. During the drumming the abdomen also vibrates, but is held clear of the surface on which the insect is resting. (Ragge, 1965: 98)
Presumably females can detect the vibrations produced in this way and move towards the drumming male. During mating, the male encircles the female’s abdomen with his long, curved cerci.
Oak bush-crickets can be found by shining a torch beam on tree trunks in the evening, when females may be seen laying their eggs. Alternatively, sharply beating the lower branches of trees during the daytime with a stick while holding a specially designed beating tray (or an up-turned umbrella) beneath will usually dislodge them from their resting places. This technique is more successful for finding nymphs during June or early July. After high winds they can often be found on the ground under trees, and they can also often be found in spiders’ webs around outhouses and sheds. In gardens, if clippings from trees and shrubs are collected in garden bags, bush-crickets will find their way to the top within an hour or so and can be easily detected.
Enemies
It may be that they fall victim to nocturnal predators such as bats. In gardens, cats find and catch them, and they are frequently found in spiders’ webs. Smith (1972) gave an account of a pair of goldcrests bringing 31 oak bush-crickets to feed their young in just three hours of observation (Ragge, 1973).
Distribution
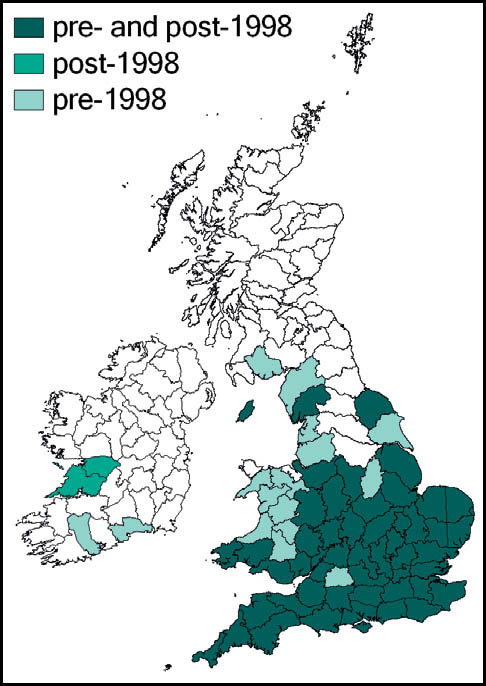
The species is widespread throughout almost the whole of the European mainland. In Britain it is common and widespread in the southern and midland counties of England, becoming more localised further north, towards Yorkshire, Lancashire and Cumbria (Sutton, 2008a). It is widespread in Wales, and has a small number of known localities in Ireland.
Status and conservation
Although generally considered to be common and widespread, at least in the more southerly parts of Britain, some observers report that it has been less easy to find in recent years. It is difficult to see why this should be so, and it may simply represent a temporary cyclical contraction of the population.
References: Haes, 1976; Ragge, 1965, 1973; Smith, 1972; Sutton, 2008a.
THE SOUTHERN OAK BUSH-CRICKET
Meconema meridionale (Costa, 1860)
MEASUREMENTS: Male: 11–15 mm in length; female: 11–17 mm in length.
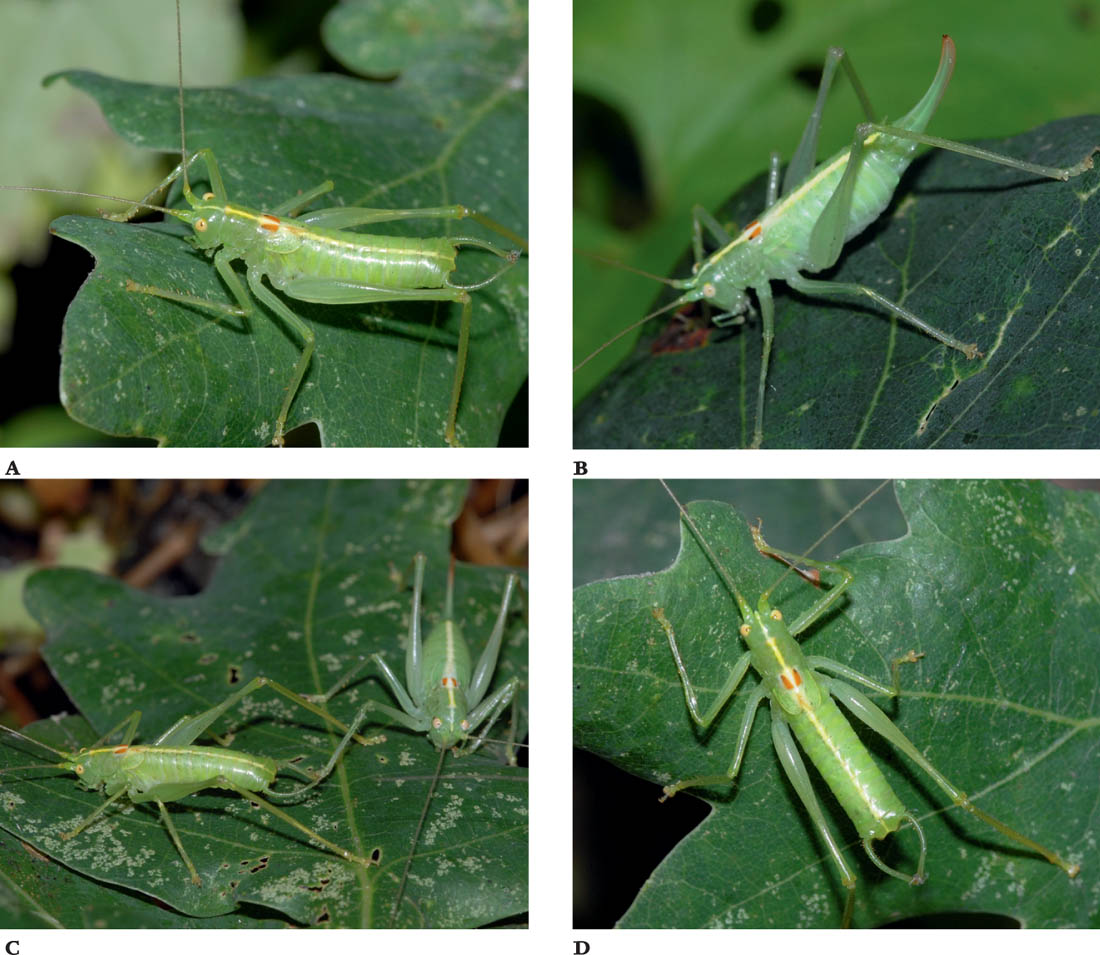
FIGS A–D. Male; female; male and female; male, dorsal view.

FIG E. Dorsal view, female.
Description
This species is very similar in appearance to the oak bush-cricket, but the most obvious difference is the lack of developed wings. In M. meridionale the wings are reduced to small stubs in both sexes. The body is mainly pale green, with a poorly defined yellowish stripe along the dorsal surface of the body. This is flanked by two wedge-shaped brown markings on the dorsal surface of the pronotum. In this species, where pairs of black spots are also present, they are merged into the brown markings at their anterior tips. The ovipositor is more distinctly up-curved towards the tip.
Similar species
The speckled bush-cricket (Leptophyes punctatissima) also has reduced wings and a pale green ground colour, but it is a more robust insect, and is covered with minute black points. The ovipositor of the female of the speckled bush-cricket is wider and more strongly up-curved.
The oak bush-cricket (M. thalassinum) is fully winged. There are other, minor differences: the eyes are usually cream to pinkish in M. meridionale, lilac in M. thalassinum, and the ovipositor is more sharply up-turned towards the tip in M. meridionale, regularly curved and slightly longer in M. thalassinum. Also, the patterns on the rear of the dorsal surface of the pronotum differ. In M. meridionale the paired black spots (where they are present) are fused with the brown wedges, but they are distinct in M. thalassinum. The shape of the tip of the male abdomen between the two cerci is also different in the two species, and the cerci of M. meridionale are slightly longer than those of M. thalassinum (see the Key, Figs K5c and e).
Life cycle
The adults are found from late July onwards and it is believed that the life cycle is closely similar to that of M. thalassinum. The eggs are laid in crevices in bark.
Habitat
The southern oak bush-cricket lives in oak trees as well as sycamore, elder, birch, maple, bay, and a range of garden shrubs.
Behaviour
During the day they are inactive and remain hidden among foliage. Although they cannot fly, they can jump well and can successfully avoid capture. Like the oak bush-cricket, they are nocturnal, and feed on small insects. In captivity they are cannibalistic, in particular taking advantage of nymphs when these are vulnerable after moulting. The males drum with one hind foot, although the pattern of taps (from 3 to 7 in succession (Hawkins, 2001)) is somewhat different from that of the oak bush-cricket. Hawkins describes the mating posture: with the male on his back, facing away from the female, but with his head raised to grip the ovipositor of the female with his mandibles. As in other bush-crickets, an edible spermatophore is transferred to the female during mating.
They can be found by beating lower branches of trees as described for the oak bush-cricket, by shining a torch beam onto tree trunks, and also by waiting for them to climb up to the surface of garden bags after pruning.
Enemies
Given the similarity between this and the oak bush-cricket it seems likely that they are vulnerable to the same predators.
History
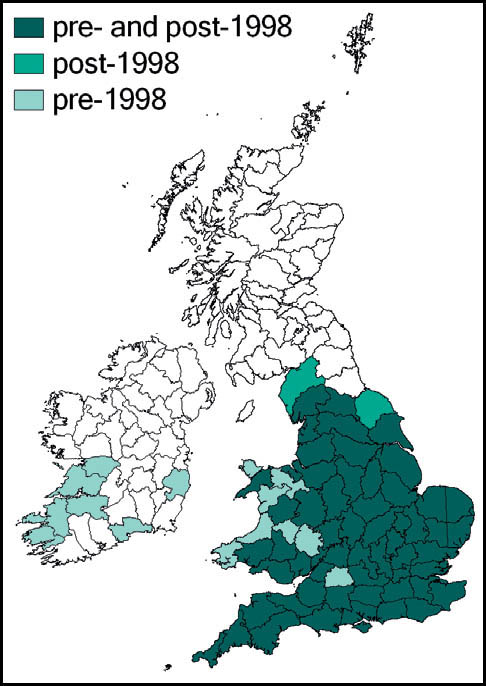
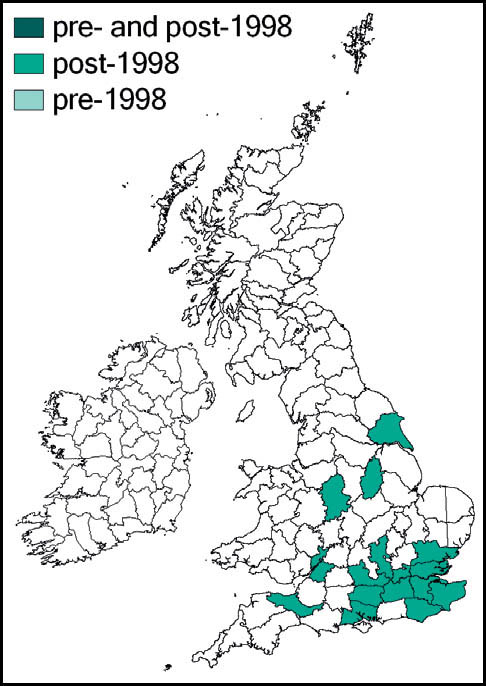
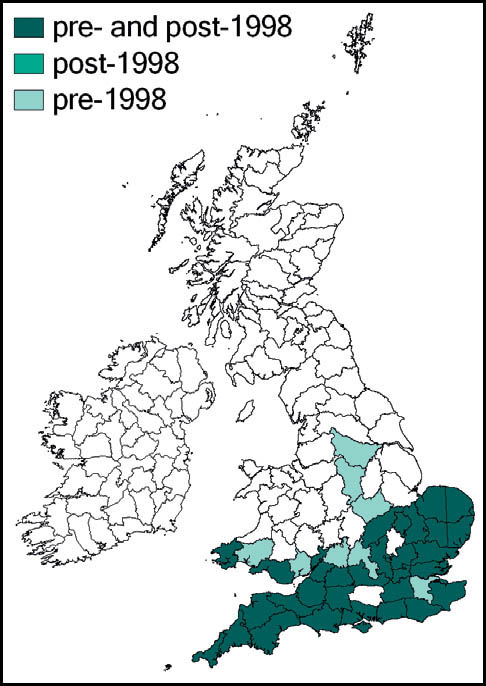
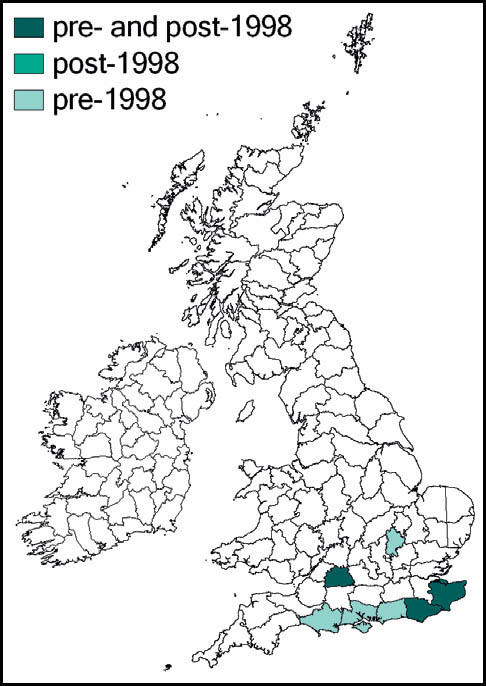
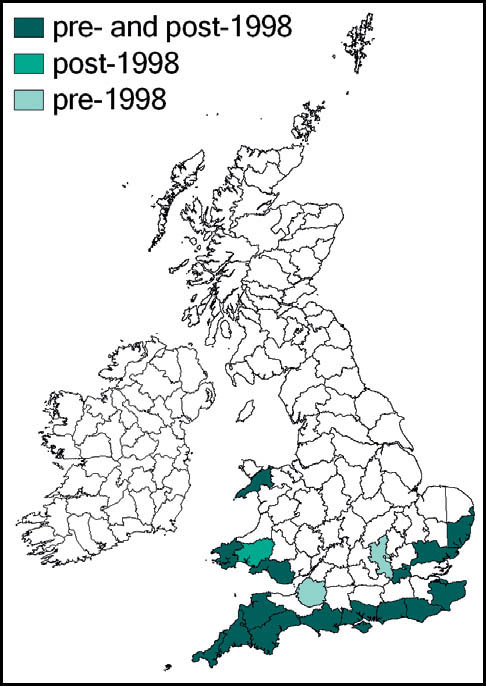
The species was first described from Italy, by Costa, in 1860. Prior to 1960 it was known also from eastern France and parts of Yugoslavia. Since 1960 its northward spread has been noted: from southern Germany and Austria and northern and eastern France to the Netherlands, Belgium and northern Germany in the 1990s. Between 1995 and 2001 it was recorded from more than 20 sites in Normandy. Its imminent arrival in Britain was predicted (e.g. Widgery, 2001a: 360) and the first British specimen was found by R. D. Hawkins by beating a birch tree growing in a garden near Thames Ditton railway station on 15 September 2001. Alerted by this discovery, D. Coleman found the insect at another Surrey site in the London suburbs, and this time it was confirmed that an established breeding colony was present. On 20 October 2001 yet another discovery of the species, this time in a garden in Maidenhead, Berkshire, was made (Hawkins, 2001). Retrospectively, the insect was identified as having been present in a London garden in 2001 (Sutton, 2004d).
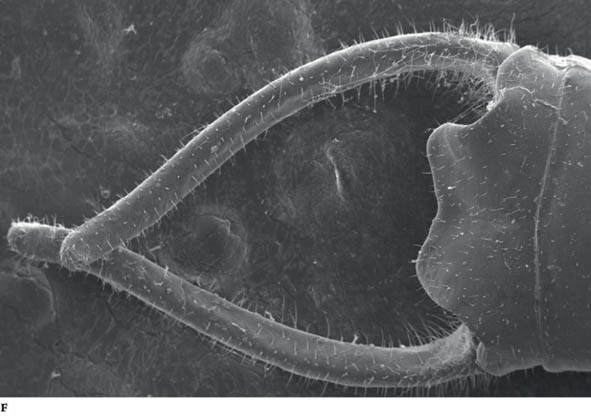
FIG F. Scanning electron microscope photo of male cerci, used to constrain the female during mating (see Chapter 6) (© K. Vahed).
Since 2001, the spread of reported British sightings has been remarkable:
Distribution
The southern oak bush-cricket now appears to be an established breeding species at numerous localities in Greater London, Avon, Somerset, Berkshire, Buckinghamshire, Hampshire, Surrey, Sussex, Kent, and Essex, with outlying records from Nottinghamshire, Staffordshire and Yorkshire.
Status and conservation
It is generally accepted that this species was incidentally imported into Britain in horticultural produce, and may have established itself in several localities independently. However, the pattern of records suggests that it is also spreading out from its original introduction. As there appears to be no winged form, the general assumption is that its rapid spread is aided by its hitching lifts on motorised transport. Support for this is provided by an observation of an especially tenacious specimen that attached itself to a car and remained in place over a 150-mile drive (Edmondson, 2011). It seems likely that this species will continue to spread out from its strongholds in several southern and south-eastern counties of England.
References: Edmondson, 2011; Hawkins, 2001; Sutton, 2003a, 2004d, 2005b, 2006b, 2007a, 2007d, 2008a, 2008c, 2010a; Widgery 2001a.
THE GREAT GREEN BUSH-CRICKET
Tettigonia viridissima (Linnaeus, 1758)
MEASUREMENTS: Male: 40–50 mm in length; female: 40–55 mm in length.

FIGS A–C. Male; female; male, dorsal view.
Description
This is the largest of the British Orthoptera, green in ground colour, and fully winged. The wings extend back well beyond the tip of the abdomen in males, and as far as, sometimes beyond, the tip of the long, straight ovipositor in the females. The male fore wings are modified for stridulation, with a ridged basal area on the left fore wing and a toothed ‘file’ on the underside. The right fore wing has a clear ‘mirror’ basally, that is covered by the left fore wing when the insect is at rest.
There is an ill-defined brownish dorsal stripe along the abdomen, and another along the leading edges of the fore wings, including the stridulatory area in the male. The yellow to brownish stripe is continued on the dorsal surface of the pronotum, and on the head often becomes lilac in colour. The eyes, too, are lilac. Most abdominal segments have a narrow yellow lateral ‘dash’ marking towards the ventral surface, just above the spiracle. Often the abdominal segments are indistinctly bordered laterally with brownish or purple tints. The legs frequently are flecked with tiny purple-black points.
Similar species
This species is quite distinctive, but superficially resembles the oak bush-cricket. However, that species is much smaller and more delicately built, paler in colour, and unlikely to be found in the same habitat as the great green.
In the very few localities where the wartbiter (Decticus verrucivorus) still occurs, the great green bush-cricket may sometimes also be found. However, the wartbiter has a much more compact, ‘chunky’ appearance, shorter wings (relative to its body length), and a markedly angled pronotum (smoothly saddle-shaped in the great green bush-cricket).
The recently established sickle-bearing bush-cricket (Phaneroptera falcata) has, as its vernacular name implies, a very differently shaped ovipositor in the female. In general, this species has a more frail appearance, with relatively longer and more ‘spindly’ legs. It is also is covered in minute black spots.
Nymphs of the great green bush-cricket are also minutely covered in tiny purple-black spots and could be confused with nymphs or adults of the speckled bush-cricket (Leptophyes punctatissima), but the body shapes of the two species are quite different.
Life cycle
The eggs are laid in soil during the summer, and pass two or more winters before hatching in late April or May. The number of nymphal instars varies, with a range from six to nine recorded. The nymphs are bright green with an indistinct brownish dorsal stripe and are densely marked with tiny purple-black points, especially on the legs. In early stages the wing stubs appear as small lobes pointing down behind the rear edge of the pronotum. In the final two instars the wing-stubs are reversed, and slight indications of the future venation are perceptible. The ovipositor is visible in the final three or four instars of the female, increasing in length with each moult. The adults are usually present by mid-July (4 July in East Sussex in 2009 (Sutton, 2009) is exceptionally early) and can be heard until the end of October (see Chapter 3, Fig 53 for images of selected developmental stages).
Habitat
The characteristic habitat is rank grassland in the process of succession to scrub, with clumps of bramble, or low hawthorn bushes providing a favoured song perch for the males. Females, too, use the upper branches of bushes to bask on warm days. The insect may also be found on south-facing slopes of downland, in hedgerows, and in gardens. In coastal and estuarine sites it is often abundant among Phragmites reeds along ditches and dykes, and in neglected ‘foldings’ on the landward side of sea defences.
Behaviour
The great green bush-cricket is nocturnal, but the males often begin to sing from mid-afternoon onwards. The song is one of the loudest emitted by any British orthopteran, and can be heard at distances of several meters (Ragge, 1965, says 200 metres, but this is presumably for those with better hearing than mine!). The tone is slightly metallic and rasping, and is produced in continuous, prolonged bursts. While singing, the male appears to ‘pump’ his abdomen rhythmically – presumably to enhance respiration. Even when singing, the cryptic coloration of a male perched among vegetation makes it very difficult to locate. There is some competition between males for song perches, and where the population is dense one may nip an intruder with his mandibles. The latter will usually dive down into lower vegetation. The females are inactive during the day, spending much of their time basking on sunny days. They, too, are surprisingly difficult to find for such large insects. When approached, they tend to rely on their cryptic appearance for protection, and remain static. If disturbed in a more determined way, they tend, unlike most other bush-crickets, to climb upwards through the vegetation. Even then, their movements are slow and awkward. They can fly, but do so rather reluctantly, and usually only for short distances.
They are omnivorous, including in their diet both vegetation and invertebrates such as caterpillars, aphids, flies and other orthopterans – including members of their own species. The great French entomologist Henri Fabre (1823–1915) recorded the feeding and courtship activities of this species in the south of France. This is his vivid description of the predation of the great green bush-cricket on a cicada.
In the dense branches of the plane-trees, a sudden sound rings out like a cry of anguish, strident and short. It is the desperate wail of the Cicada, surprised in his quietude by the Green Grasshopper, that ardent nocturnal huntress, who springs upon him, grips him in the side, opens and ransacks his abdomen. An orgy of music followed by butchery. (Fabre, 1917: 276)
Fabre found that, in captivity, they would eat a range of vegetable matter, including fruit, insects such as cicadas, and other delicacies: ‘The Green Grasshopper resembles the English: she dotes on underdone rump steak seasoned with jam’ (Fabre, 1917: 289).
Fabre also described the courtship activity of specimens in captivity. Head-to-head they ‘fenced’ with their long antennae (as do most bush-cricket species), while the male gave brief stridulations occasionally. Fabre notes that the process seemed interminable, but when he looked the following morning, mating had clearly taken place and a ‘green bladder-like arrangement’ (the spermatophore) was hanging from the base of the female’s ovipositor. After a couple of hours she consumed this, bit by bit.
Vahed & Gilbert (1996) measured the spermatophore in this species as weighing more than 22 per cent of the body weight of the male.
Enemies
Ragge (1973) refers to reports of this species being attacked by yellow buntings and by a house sparrow.
Distribution
The great green bush-cricket is widely distributed in Europe, North Africa and temperate Asia. In Britain it has a mainly southerly distribution, south of a line from the south Wales coast to the Wash.
Status and conservation
This species is very localised and is vulnerable to ‘tidy minded’ management of grassland. Its requirement for late succession patches and fringes with tall grasses and scrub can be accommodated by rotational management with long cycles.
References: Fabre, 1917; Ragge, 1965, 1973; Sutton, 2009; Vahed & Gilbert, 1996.
THE LARGE CONEHEAD
Ruspolia nitidula (Scopoli, 1786)
MEASUREMENTS: Male: 25–37 mm in length; female: 25–45 mm in length; ovipositor: 17–26 mm in length (Harz, 1969).

FIGS A–B. Male; male, showing stridulatory apparatus (both photos © B. Thomas).
Description
The large conehead is bright green, with a pale whitish line across the tip of its sharply conical head, and running back through the eyes. There is a fine black line along each tibia. The wings are very long, usually reaching back beyond the tip of the very long, straight ovipositor in females, and giving the insect a slender appearance. Brown and reddish forms are reported from mainland Europe.
Similar species
The great green bush-cricket (T. viridissima) is comparable in size, but the head shape of the large conehead is distinctive. The great green bush-cricket also has relatively shorter wings, and a dark band along the dorsal surface.
The long-winged conehead (C. discolor) is much smaller, and, like the great green bush-cricket, it has dark brown coloration on the dorsal surface, especially on the head and pronotum.
The song is loud and distinctive, enabling discovery and identification of the first British arrivals.
Life cycle
In southern Europe it has an annual life cycle, over-wintering in the egg stage. There is so far no clear evidence of its breeding in Britain, but occasional sightings indicate that the adults emerge relatively late in the year, from August onwards.
Habitat
In southern Europe its habitat is given as moist meadows and waste ground, but also dry fields with long grass (Bellmann, 1985; Bellmann & Luquet, 2009).
Behaviour
The spermatophylax ‘nuptial gift’ to the female is extremely small in this species (approximately 0.3 per cent of the male weight), and females, which mate only once, delay consumption of the ampulla (Vahed & Gilbert, 1996; Gwynne, 2001; see also Chapter 6).
The male song is a prolonged, continuous buzz that can last for 10 minutes or more. In this respect the song resembles that of M. roeselii, but it is an almost pure tone of about 13 to 20 kHz (Hathway et al. report a somewhat higher lead frequency), and is delivered almost exclusively at night (Ragge & Reynolds, 1998).
History
There are occasional reports of specimens incidentally imported in plant material (e.g. Nottingham and Northamptonshire, 2001 (Widgery, 2002)) but the first reports of the species arriving in the British Isles apparently unaided were from the Isles of Scilly in August and September 2003. Males were heard singing and subsequently captured and definitively identified on two islands: St Marys and St Agnes (Hathway et al., 2003; Sutton, 2003c).
A single male was located on Canford Cliffs, Poole, Dorset, in September 2005 and another at the same site in 2006 – suggesting the possibility of a breeding population (Sutton, 2006b).
Distribution
South and central Europe, Eastern Europe, North Africa and eastwards to Palaearctic Asia. It has been spreading northwards in mainland Europe in recent years, and was established in Normandy by 2002 (Sutton, 2003c).
Status and conservation
Given continued warming of the climate, it seems likely that this species will establish a breeding population in the British Isles (and it may have already done so).
References: Bellmann, 1985; Bellmann & Luquet, 2009; Evans & Edmundson, 2007; Gwynne, 2001; Hathway et al., 2003; Harz, 1969; Ragge & Reynolds, 1998; Vahed & Gilbert, 1996; Sutton, 2003c, 2006b; Widgery, 2002.
THE WARTBITER
Decticus verrucivorus (Linnaeus, 1758)
MEASUREMENTS: Male: 26–34 mm in length; female: 27–42 mm in length.

FIGS A–D. Male; plain green female; male, showing dorsal surfaces of pronotum and stridulatory apparatus; female, dorsal view.

FIGS E–F. Female with black markings; rare yellow and purple form (© P. Sutton).
Description
The wartbiter is a robust, ‘chunky’ insect. The adults are fully winged, but the wings barely reach back to the tip of the abdomen. The wings of the males are modified basally for stridulation. The head and pronotum have a smooth, shiny appearance. The pronotum is strongly angled at the sides, and has a fine median longitudinal ridge. The ovipositor is long, relatively narrow and slightly up-turned.
The typical form is bright green, often with variable amounts of dark brown to black blotching on the wings. There is also often a black mark on the rear margin of some of the abdominal segments. The eyes are very dark – contrasting with the green of the head. The base of the ovipositor in females is beige, shading to dark brown towards the tip. The stridulatory area of the left fore wing in the male is irregularly ridged and brown in colour in fully mature individuals. The cerci are yellowish, and have an inward-projecting tooth. The ventral surfaces of the hind femora are yellow, and the hind tibiae are pale lilac.
There are other colour forms, which are very rare in British populations: a grey form (f. bingleii – sometimes described as ‘brown’) was discovered in the main Sussex population in 1987, while a remarkable form with purple sides and yellowish wings also formerly occurred there. Neither has been seen since, although a partially purple form does occasionally occur (Cherrill & Brown, 1991a; Sutton & Browne, 1992; Sutton, 2009).
Similar species
It would be difficult to confuse this species with any other. Our only other species of similarly large size is the great green bush-cricket (T. viridissima), which does occur on some of the few remaining sites for the wartbiter. However, the great green bush-cricket is rather more slender in build, and has significantly longer wings relative to its body. Also the great green’s pronotum is smoothly rounded and saddle-shaped, contrasting with the strongly angled pronotum of the wartbiter.
The sickle-bearing bush-cricket (Phaneroptera falcata) is another large and fully-winged green bush-cricket. However, it is much more delicately built than the wartbiter, with long, spindly legs, and, in the female, a short, strongly curved ovipositor. So far, only one British locality is known for this species – one not shared with the wartbiter.
Life cycle
The greyish brown eggs are laid singly or in small batches in bare soil, or in short turf, to a depth of 0.5 to 2 cm from July onwards. They soon enter a resting stage, and remain in diapause until this is broken by cold winter weather. Embryonic development usually continues, with a further diapause which is broken by the onset of the next cold winter temperatures. The eggs thus usually hatch in the spring (early to mid-April) after their second winter. However, both in Britain and in mainland Europe this pattern is variable, and embryonic development can take up to seven years. There are seven nymphal instars. The rate of nymphal development is strongly dependent on sunny and warm weather, and the date at which development is completed varies from year to year. Usually adults appear from about the beginning of July and continue to emerge until the last week in July. However, adults have been recorded as early as mid-June, and in 1989 at the main Sussex locality, no nymphs were observed after 28 June. Development is slightly faster in males, which also have lower body weight. After the final moult both sexes increase in weight, especially females, which more than double their weight to an average of 2.1 grammes by late August. The weight gain in females is an indication of their fecundity, and as their weight increases with age, it is likely that they do not achieve their full reproductive potential if they encounter inclement weather in the latter part of their adult lives, or if poor weather in spring slows nymphal development. Depending on the weather in late summer and autumn, the adults can survive until mid-October.
Habitat
The wartbiter survives in a wide variety of habitat types further south in Europe, but in Britain it is at the northern edge of its range, and so has much more specialised requirements. The British populations are concentrated in a very small number of unimproved calcareous downland slopes, with one very precarious tiny population on grass/heather heathland. The downland population that has been most closely studied by A. Cherrill and colleagues has been shown to require a mosaic of dense tufts of tall grasses (Brachypodium pinnatum and Bromus erectus at the main study site) with areas of short turf and some bare ground. The short turf and bare ground are required for oviposition, and are also the habitat for early-instar nymphs. Sixth and seventh instar nymphs and adults are found more frequently associated with the tall grass tufts. In the few downland sites where they occur, population densities are greatest on south-facing slopes, compared with more easterly aspects where they also survive.
Their diet includes other invertebrates, and, not surprisingly, they are found in sites that are rich in other species of Orthoptera. On the south-facing slopes of their strongest British population they coexist with particularly large numbers of M. roeselii and C. discolor, along with T. viridissima, S. lineatus, C. parallelus, C. brunneus and O. viridulus.
In its heathland habitat the mosaic vegetation structure may be comparable to that on downland, with taller heathers interspersed with sedges and fine grasses.
Behaviour
In captivity, females have been observed to lay their eggs in soil, some of them showing a preference for short turf over bare ground. During oviposition the female inserts her ovipositor vertically into the soil, apparently testing for suitability of the substrate. When a suitable site is found, she presses the ovipositor fully into the soil, and usually lays one egg quickly afterwards. Most frequently only one egg is laid in one session but sometimes a small batch is laid (up to 13 have been recorded). After the egg (or final egg in a batch) has been laid, the female raises and lowers her ovipositor to fill in the space above the eggs, and, after removing her ovipositor, scrapes the soil surface around the oviposition site, so concealing it. There is evidence that warmer sites are selected for oviposition.
The adults are bulky animals, apparently rather poor climbers, whose typical responses to disturbance are to remain still and rely on their excellent camouflage, or to jump out of the way – usually into deep grass tufts. When they do this they have the appearance of small, plump green frogs. They are omnivorous, feeding on both vegetation and other invertebrates, including beetles and grasshoppers (see DVD sequence).
The adults, while tending to favour the shelter of the dense grass tufts, will bask in sunshine on the warmer aspect of the tussocks. However, as the microclimate is cooler in the tufts than in the shorter turf, it seems likely that the association with this vegetation structure on the part of adults and late instars is related to avoidance of predation. The tufts are also used by the males as song perches. Unlike many other bush-crickets, they sing most in the early part of the day – from mid-morning to early afternoon, and only during warm weather. The song is usually delivered from a perch on a grass tussock, and takes the form of prolonged bursts of repeated high-pitched clicks. The rate of repetition increases to about 10 clicks per second within a minute or so of the start of the burst. The volume of the song is quite low, and for many observers a bat detector is needed to locate it at more than a few metres distance. Males are also reported to emit short isolated chirps.

FIG G. Head and pronotum of this female show signs of predator damage: possibly beak-markings of a bird.
Like most bush-crickets, the males produce a substantial spermatophore which includes a ‘nuptial gift’, the spermatophylax, which is bitten off and consumed by the female while sperm from the ampulla are transferred to her. In the wartbiter, literature sources give measurements of the mass of the spermatophore varying from approximately 6 per cent (Cherrill & Brown, 1990a) to 11 per cent of the body-weight of the male (Vahed & Gilbert, 1996).
Enemies
Cherrill & Brown (1992a) noted the presence at their study site of foraging foxes, badgers, kestrels, magpies, crows and starlings. The large size of the late instars and adults probably makes them an attractive food item for predators, and Cherrill (1997) found numbers of adults with severed legs, or hind femora, tibiae or ovipositors, and one female with a beak mark on her abdomen. Insects showing signs of past predator attacks were more common among adults and late instars.
However, mortality rates were shown to be much higher among the earlier nymphal instars, with population densities declining by 99.3 per cent from egg hatch to adult emergence, but remaining quite constant after that. Mortality arising from defective moulting between instars is known to be high, but various other causes including predation probably contributed to the high levels of pre-adult mortality (Cherrill & Brown, 1990a).
Distribution
The wartbiter is widely distributed in Europe and temperate Asia. As a species close to the northern edge of its range in Britain and northern Europe, it is especially vulnerable here to habitat change and inclement weather. There is evidence of decline in recent decades in the Netherlands, Belgium, Denmark and southern Sweden as well as in Britain.
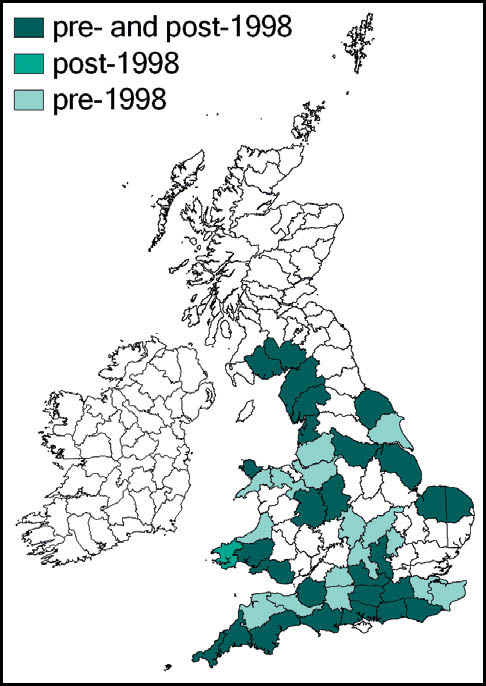
In Britain it has always been a rare and highly localised species, confined to a few localities south of the Bristol Channel. These localites were in east Kent, Sussex, Hampshire (New Forest), the Isle of Wight, Dorset and Wiltshire. The majority of these populations have been lost as a result of urban development, agricultural change and changes in habitat management (in some cases enacted for conservation purposes!). Currently it is believed that the wartbiter is confined to no more than four of its original localities: two in east Sussex (including the largest, at Castle Hill National Nature Reserve), one on downland in Wiltshire, and the other a very small heathland population near Wareham. There is, however, at least one site, in Kent, where re-introduction of the species appears to have been successful.
Status and conservation
The wartbiter was scheduled in the Wildlife and Countryside Act, 1981, and included as ‘Vulnerable’ in the Nature Conservancy Council’s Red Data Books: 2. Insects (Shirt, 1987). It was the subject of a Nature Conservancy Council/English Nature Species Recovery programme from 1987, with substantial research conducted by Andrew Cherrill, Valerie Brown and colleagues (the main basis of the above account), together with captive breeding and attempts at reintroduction. The captive breeding programme proved very demanding for several reasons. The nymphs had to be reared in separate containers because of cannibalism, they suffered from protozoan and fungal infections, as well as a high frequency of failed moults, and they required high-quality food (Pearce-Kelly et al., 1998). The process was labour intensive, but produced enough late instars and adults for release into three sites, one of which, at least, still has a population of the species.
Adults of the British population are smaller than those from further south in Europe, and our populations are also much less dense. In Cherrill’s view (1993), the species is capable of high reproductive success in Britain only in years when weather conditions are particularly favourable. Even then, it is dependent on appropriate habitat management on its predominantly south-facing grassland sites. A complex sward structure, with tufts of coarse grasses interspersed on a fine scale with short turf and bare ground, appears to be an essential combination of requirements for wartbiters during their developmental stages, and for thermo-regulation, shelter from predators, song perches, oviposition, and mate location for the adults (see also Chapter 9).
References: Cherrill, 1993, 1997; Cherrill & Brown, 1990a, 1990b, 1991a, 1991b, 1992a, 1992b; Cherrill et al., 1991; Haes et al., 1990; Ingrisch, 1984a; Pearce-Kelly et al., 1998; Sutton, 2009; Sutton & Browne, 1992; Vahed & Gilbert, 1996.
THE DARK BUSH-CRICKET
Pholidoptera griseoaptera (De Geer, 1773)
MEASUREMENTS: Male: 13–20 mm in length; female: 13–20 mm in length.

FIGS A–C. Male; female; male, dorsal view.

FIGS D–F. Grey form; chestnut form; underside.
Description
This species has a robust build. The dorsal surface of the pronotum is smoothly curved with a fine median longitudinal ridge that extends forwards on the head. The side flanges (‘paranota’) of the pronotum are continuous with the dorsal surface, but form a definite angle with it. The males have small inner projections near the base of their cerci. The ovipositor in the female is relatively long, broad, and up-curved. There are no hind wings, and the fore wings in the male are greatly reduced to function solely in stridulation. The wings in the female are even more vestigial, being reduced to tiny flaps just visible at the rear of the pronotum.
The ground colour varies from pale grey to grey-brown, with darker grey or brown fine flecks and indistinct markings. There are often darker patches of grey-black, especially on the sides of the pronotum and on the outer surface of the hind femora. One striking form has sandy or chestnut brown coloration on the dorsal surface of head, pronotum and abdomen, contrasting with the paler sides. The ovipositor is usually darker than the rest of the body, especially towards the tip. The underside of the abdomen is yellow to yellowish green.
Similar species
There are three other medium-sized bush-crickets that could be confused with P. griseoaptera.
Life cycle
The eggs are buff-coloured, 4 mm by 1 mm in size, and laid in crevices in bark or in rotting wood. They may hatch the following spring (in mid-to late April), but are more likely to over-winter twice, to give a two-year life cycle. There are six nymphal stages, with the tiny wing stubs of the males visible in the final two. The nymphs are more strikingly contrasting in their colour patterns than the adults. They are commonly pale brown with darker brown patterns and blotches, the latter frequently along the sides of abdomen and pronotum, and on the hind femora. They settle on exposed lower leaves of bramble and other shrubs on sunny days. The adults emerge from mid-July onwards, and survive later into the autumn and winter than most other species.
Habitat
Although sometimes found among tall grasses, they are rarely far from patches of low scrub, especially bramble clumps and rough hedgerows. They are apparently also found on exposed cliffs in the south-west (Marshall & Haes, 1988).
Behaviour
Like most other bush-crickets they are omnivorous and include other insects as well as small spiders in their diet. In captivity they will also consume earthworms (A. Kettle, pers. comm.).
They are mainly nocturnal, skulking among the branches of bramble scrub during the day. From mid-afternoon, especially late in the season, the females, especially, spend long periods basking in sun spots. They alternate their posture between exposing each side to the sun, with the hind leg facing the sun lowered to expose more of the abdomen, and exposing their dorsal surface to the sun, hind legs stretched out behind. Both sexes frequently run their antennae through their mouthparts, presumably to clean them, although it is also possible that this behaviour is linked to the ‘fencing’ with their antennae during courtship, and involves chemical communication. Active preening of the fore legs with the mouthparts is also frequent. In cloudy or even wet weather the males may begin singing quite early in the day. Usually, however, they are heard from mid-afternoon onwards, and continue through the night.
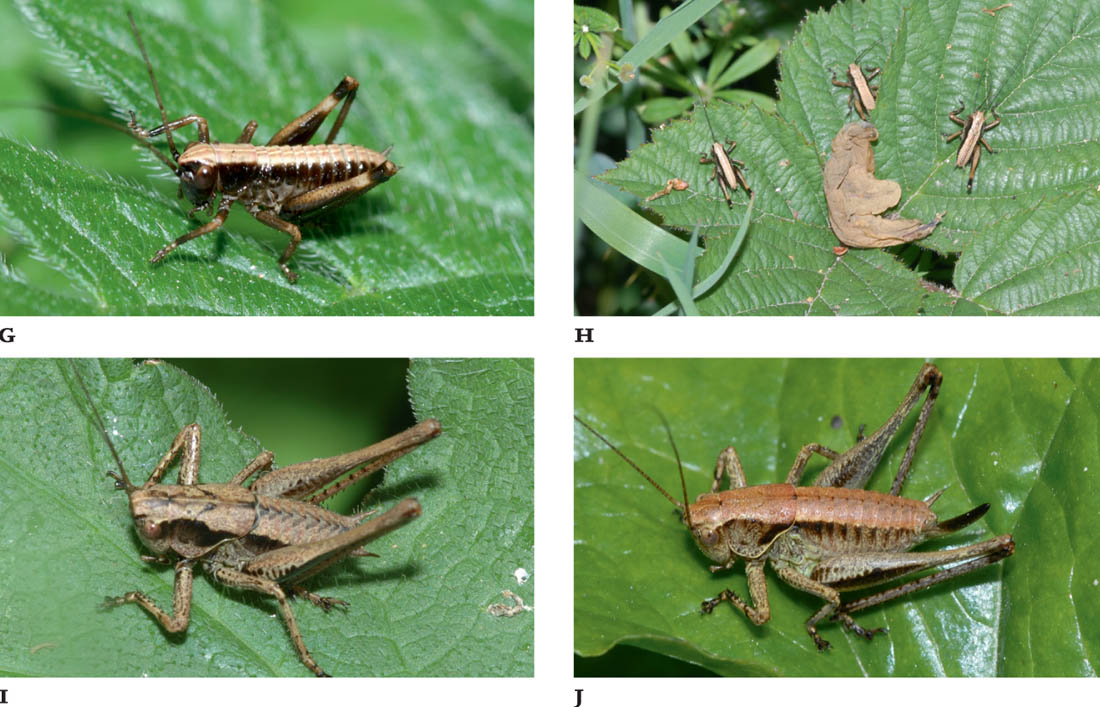
FIGS G–J. Early instar nymph; basking nymphs; late instar nymph, male; late instar nymph, female.
They are gregarious, and from mid-afternoon into the evening often gather in groups of a dozen or more within a square metre on lower leaves and branches of the bramble. The group may include four or more males, each chirping, with females gathering from inner branches of the scrub. Although the females approach singing males they do not, at this time, show any interest in mating. However, prolonged ‘fencing’ with their antennae is noticeable.
The ‘solo’ song of the male is a brief, high pitched chirp repeated at variable intervals of a few seconds. However, several males are often singing in close proximity to one another, and sometimes this results in a regular pattern of alternation between two adjacent males. When more than two males sing together, their chirps still are generally emitted separately to produce a non-synchronous ‘chorus’ (Jones, 1963, 1966). The males can also emit a more prolonged chirp lasting approximately a second. This is considered to signify an aggressive interaction between males, but it is also occasionally emitted by males on the approach of females. In general, males do not appear to interact aggressively with one another. However, experimental work does show that the onset, termination, and timing of chirps are all affected by the stridulation of other males (see Chapter 5).
Courtship involves prolonged ‘fencing’ with the antennae between a male and female pair at close proximity. Eventually the female mounts the male from the rear, actively palpating the dorsal segments of his abdomen, presumably imbibing a chemical secretion. The male raises his hind legs to allow the female to mount, but may still reject her at this stage by a powerful back-kick of the hind legs. Males who do this continue to chirp, indicating continuing readiness to mate (see DVD). This somewhat puzzling behaviour is explained by Gwynne (2001) in relation to the mormon cricket (Anabrus simplex) in terms of the ‘choosiness’ of the male in view of the cost to him involved in the ‘nuptial gift’. Apparently the males assess the weight of the females that mount them, accepting only the heaviest ones (presumably as these are most likely to lay more eggs). In the dark bush-cricket, the spermatophore weighs some 10.7 per cent of the male body weight (Vahed & Gilbert, 1996).
Enemies
They can be caught in the webs of larger spiders, most notably the wasp spider (Argiope bruennichi).
Distribution
The dark bush-cricket is widely distributed and generally common throughout most of Europe. In southern Britain it is perhaps our commonest bush-cricket, and has been recorded in almost every 10 km square south of a line from south-west Wales to the Wash. North of this, its known distribution is more scattered, but reaches as far north as north Yorkshire and south-west Scotland. It is also known from the south and west of Ireland.
Status and conservation
The dark bush-cricket remains common within its geographical range and thrives on small, neglected patches of habitat. Its only serious threat seems to be excessive tidy-mindedness that might disrupt that neglect.
References: Gwynne, 2001; Jones, 1963, 1966; Hartley, 1967; Vahed & Gilbert, 1996.
THE GREY BUSH-CRICKET
Platycleis albopunctata (Goeze, 1778)
MEASUREMENTS: Male: 20–25 mm in length; female: 20–28 mm in length.

FIGS A–C. Male; female; dorsal view, male.
Description
The grey bush-cricket is sturdily built and medium sized. It is fully winged, with the wings extending back to a few millimetres beyond the tip of the abdomen. In the males, the anterior part of the leading edge of each fore wing is modified for stridulation. The male cerci have inwardly directed spines about halfway along.
The ovipositor of the female is broad, up-turned and black in fully mature specimens. In both sexes there is a slight median ridge on the rear part of the dorsal surface of the pronotum, which divides, with the front end forming a ‘y’ shape. The adults vary from grey to light brown in ground colour, with fine blackish flecks that coalesce into patches in places, especially the sides of the pronotum (paranota). The paranota have pale borders, but these are obscured by darker flecks in adults. The outer surfaces of the hind femora frequently have a black ‘fish-bone’ marking. The underside is usually a very pale green or yellow. A form with green rather than grey or brown coloration of the pronotum is reported (e.g. Ragge, 1965), and the subspecies (jerseyana) that occurs in the Channel Islands often has more greenish coloration (Evans & Edmondson, 2007).
Similar species
There are three similar species.
In Britain the grey bush-cricket is mainly found in coastal sites, and not usually in association with any of the above (although occasionally with M. roeselii (B. Pinchen, pers. comm.). The song of the male is also distinctive.
Life cycle
The eggs are laid singly or in small batches by the female in sand or soil, or sometimes in plant stems or crevices. They hatch in April or May, and there are six nymphal instars. The nymphs are brown, or green with brown head and pronotum. They have pale cream-yellow borders to the paranota, and variable black markings. The up-turned wing stubs are noticeable in the final two nymphal instars. Adults emerge from early July onwards, but do not usually survive much beyond the end of September or early October.
Habitat
In mainland Europe the grey bush-cricket inhabits rough grassland, but in Britain it can be found in a variety of coastal habitats such as among marram grass (Ammophila arenaria) tussocks on sand dunes, on grassy fringes of coastal cliffs, on stabilised shingle and among low scrub. Patches of loose soil or sand, interspersed with dense, low vegetative cover exposed to the sun seem to be characteristic of all habitat types.
Behaviour
The adults are very secretive, and sensitive to disturbance (a colleague remarked they should be re-named ‘the shy bush-cricket’). They are active mainly in the warmer part of the day, and the patient observer can sometimes see them basking in sunny weather on taller vegetation, such as shrubby seablite (Suaeda vera) or sea purslane (Halimione portulacoides), and even on driftwood. Even here, however, they are never far from cover, and quickly descend into the depths of the vegetation if they detect the slightest movement. If alarmed when this is not a feasible option, they are effective in jumping and flying for the nearest cover. They are omnivorous, including other insects such as grasshopper nymphs in their diet, but, according to Ragge (1965), can be kept successfully in captivity on vegetable matter. In sand-dune habitats they feed on marram grass, often selecting desiccated stems. One was observed by the author lunging at a gatekeeper butterfly that settled some 5 cm away from it!
The males usually sing from cover and are very difficult to locate, even with the aid of a bat detector. The song consists of a brief chirp, repeated at a rate of 2 to 4 times per second, and continued in prolonged bursts lasting several minutes. The sound is rather quiet, and often obscured by the sound of the wind in its exposed habitat. However, when picked up with the aid of a bat detector it is quite distinctive. Males do not congregate, as males of the dark bush-cricket do, but ‘duets’ can often be heard when the song of one male interacts with that of another. However, in laboratory experiments such alternating duets have been shown to break down after some time, and the interaction may simply function to enable the males to space themselves out across the available habitat (Latimer, 1981a, 1981b). There is some evidence of at least temporary territoriality among the males, as there are brief skirmishes when they encounter one another (see DVD). The male delivers a spermatophore to the female on mating. This is given as approximately 5.5 per cent of the male’s body weight (Vahed & Gilbert, 1996).

FIGS D–E. Singing male with ‘mirror’ partly exposed; underside of female with remains of spermatophore visible.
The females are more frequently found than the males away from deep cover on patches of unvegetated soil or sand, presumably seeking oviposition sites.
Enemies
None is reported in the literature, but their habit of retreating into cover suggests adaptation to avoid diurnal visual predators – possibly birds (such as ringed plover, dunlin, turnstone or kestrel) or mammals.
Distribution
This species is widespread in southern, south-western and central Europe, as well as north Africa. In Britain it is at the northern edge of its range, and this may explain its rather specialised coastal and southerly distribution. Its distribution is almost continuous along the south coasts of England and Wales, and the southwest peninsula, with outliers further north along the east coast to Suffolk and, on the west, to north Wales. There is a small scattering of inland and more northerly reports. A site 14 km inland was reported from Ringwood, Hampshire (Widgery, 1998, 2001a) in the 1990s, but extension of an adjacent industrial estate may have been the cause of its subsequent demise (B. Pinchen, pers. corr.).
Status and conservation
The main threat to the grey bush-cricket is development for housing or tourism on its coastal habitats. On the east coast, in Essex and Suffolk, it is found in a small number of sand dune systems where it had been supposed extinct. Recent survey work suggests that it is currently increasing, and further colonisations along the east coast seem likely (Harvey et al., 2005; Gardiner et al., 2010). This might be aided by climate change, but coastal erosion might offset any such gains.
References: Evans & Edmondson, 2007; Harvey et al., 2005; Gardiner et al., 2010; Latimer, 1981a, 1981b; Ragge, 1965; Vahed & Gilbert, 1996; Widgery, 1998, 2001a.
ROESEI’S BUSH-CRICKET
Metrioptera roeselii (Hagenbach, 1822)
MEASUREMENTS: Male: 13–22 mm in length; female: 14–22 mm in length.

FIGS A–D. Male; female; green female; male, dorsal view.

FIG E. The macropterous f. diluta, male.
Description
Roesel’s bush-cricket is a medium-sized, sturdily built insect, in which the wings are typically only part-developed – reaching back over only the first five or six abdominal segments in the male, fewer in the female. The fore wing stubs of the male are partially modified for stridulation, and the long cerci have an inner projection halfway along. The ovipositor is broad and up-turned, brown shading to black-brown towards the tip. There is a continuous fine median ridge on the dorsal surface (discus) of the pronotum, usually continued forward as a yellowish line over the head.
A minority of individuals of both sexes in most populations are of the long-winged (macropterous) form (f. diluta). Although the wing tips in this form soon become tattered, it is usually possible to see that the wings are very long – sometimes extending beyond the tip of the abdomen by as much as one third to a half of the total wing length.
The ground colour is usually grey-brown with darker markings. There is often a fish-bone-shaped darker pattern on the outer surface of the rear femora, and the side flaps (paranota) of the pronotum are usually black, with a continuous cream-yellow border. The sides of the visible thoracic segments to the rear of the pronotum also have yellow patches. The underside of the abdomen is yellow.
A variable proportion of most populations have pale green sides and hind femora. In this form, the paranota are often greenish, with pale green borders.
Similar species
The pale yellowish (or greenish) borders to the paranota are distinctive. In the bog bush-cricket (M. brachyptera), the pale borders are limited to the rear edges only, and usually buff-cream in colour. The bog bush-cricket also lacks the yellow patches on the sides of the thoracic segments, and the ovipositor of the female bog bush-cricket is relatively longer, and less strongly up-curved.
For distinctions between Roesel’s and the dark and grey bush-crickets (P. griseoaptera and P. albopunctata), see the accounts of those species.
Life cycle
The female lays her eggs singly, in stems of coarse grasses or rushes. She bites a hole in the outer layers of the plant with her mandibles and raises her ovipositor under her abdomen to insert the tip into the cavity. The rest of the ovipositor is then pressed into the stem and she remains quiescent for some minutes while a single egg is placed in the plant tissue, parallel to the sides of the stem (see DVD). The egg is elongated, cylindrical and approximately 4 mm by 0.5 mm. Eggs laid early in the season may undergo sufficient embryonic development to hatch the following spring, while late-laid eggs pass two winters before hatching. The newly hatched nymphs appear in late May or early June, and pass through six nymphal instars before becoming adult from late June (Widgery, 2001b) or early July onwards. Adults and late instar nymphs can be found together through most of July. The adults persist until the end of September, and in declining numbers thereafter, until the end of October in most years.

FIGS F–G. Early instar nymph; and final instar green nymph, female.
The nymphs are usually pale brown, with a broad black band along each side of the abdomen, continued onto the paranota, with the pale border of the latter often more extensive than in the adults. A median black line runs along the whole dorsal surface of the insect, bordering the fine median ridge of the pronotum. The antennae are black. As in the adults, there is a form of the nymph with green sides, shading to brown dorsally. Up-turned wing stubs can be seen in the final two instars (see also Chapter 3, Fig 66).
Habitat
This is a species of open grassland with tall grasses. Although common on large expanses of grassland, such as south-facing downland and coastal grazing marshes, it is quite capable of surviving in small neglected field corners or roadside verges. It is common on sea walls and inner ‘foldings’, and is one of the few species to thrive in narrow conservation strips of coarse grasses at the edges of arable fields. However, it also occurs in overgrown and unmanaged sites, where there is succession to scrub, as well as along south-facing hedgerows, woodland edges and open woodland rides.
Behaviour
Roesel’s bush-cricket is a sun-loving species, the females, especially, spending much of their time basking. They are omnivorous but feed mainly on vegetable matter, particularly grasses.
On sunny days the males sing in prolonged continuous bursts. Although the song is high-pitched, like that of most bush-crickets, it can be heard by most people as a distinctive ‘buzz’. In most extensive grassland habitats the males are evenly spaced out, and sing from perches. These are usually exposed to the sun, but not conspicuous as they are surrounded by rank vegetation. However, there is some competition between males for song perches. Where wind or rain has broken clusters of grass stems to form a rough ‘platform’, three or more males may compete to occupy the perch, often emitting short bursts of song as they move about, and briefly skirmishing on contact. Clusters of both males and females may sometimes be seen on leaves of low scrub such as oak saplings or bramble.
At close quarters, courting males approach females, ‘fencing’ with their antennae, and continuing to emit bursts of stridulation (see DVD).
Long-winged forms appear to behave in similar ways to the typical one, but they are noticeably less mobile among the long grasses. The stridulation of the male seems to be unaffected by wing length. However, the long-winged form, even where it is relatively common, soon declines as a proportion of the population. This may be simply because, as the dispersive form, they emigrate to colonise other suitable habitat. One remarkable description of this refers to many macropterous Roesel’s bush-crickets taking to the wing in one field, the flight characterised as ‘ponderous and straight, with no apparent means of steering to left or right – the legs protruding behind their bodies like twin tails’ (Smith, 2007).

FIGS H–I. Singing male with raised fore wings; and courtship. Note the open wings of the singing male.
Clearly the macropterous form of roeselii is not a skillful flier, and as well as its disadvantage of lower mobility in tall vegetation, is also rendered vulnerable to predation when on the wing. There is believed to be a trade-off between the dispersal ability of the long-winged form and its lower fecundity, compared with the more usual short-winged form (see Chapter 3).
Enemies
The simultaneous flight of numerous long-winged individuals described above was sufficient to attract the interest of avian predators – in the shape of two pairs of hobbys and another pair of kestrels. The kestrels, feeding lower down, could be seen plucking the crickets out of the air, partially dismembering them and swallowing the remainder. After feasting for about an hour, the raptors were joined by approximately sixty black-headed gulls and one common gull, which also could be seen catching flying bush-crickets.
History
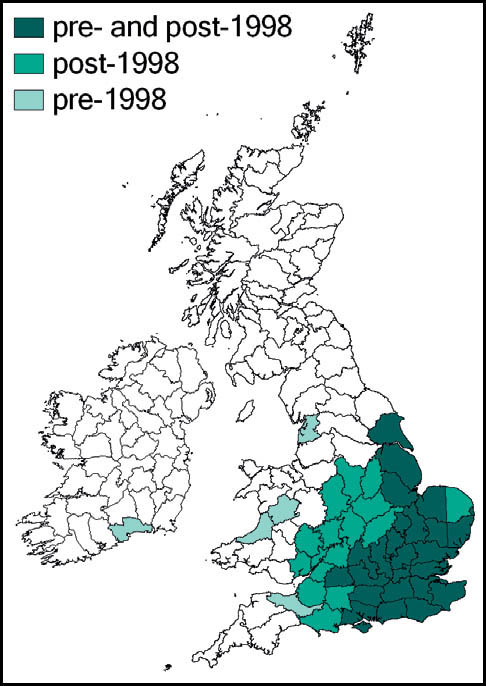
Stephens recorded it from Hampstead in 1835, but until the beginning of the 20th century it had been recorded only from east coastal areas from Kent northwards to the Humber estuary (Marshall, 1974). Burr (1936) considered it to be restricted to the east coast – from Herne Bay, Kent in the south to the Humber estuary in the north. He had records from Kent, Essex, Lincoln and Yorkshire, with a ‘doubtful suggestion’ from Cambridgeshire. Pickard (1954) adds Hampshire, Surrey, and coastal areas of Suffolk. According to Ragge (1965) its ‘liking for flat, estuarine localities’ limited it to the areas around the Solent, Thames and Humber, with other records from south Suffolk, Cambridgeshire and Surrey. The reports from Kent and Surrey included downland localities, indicating that earlier assumptions associating it solely with low-lying damp grassland were perhaps too restrictive. It was discovered in West Wales in 1970 (Ragge, 1973), and subsequently in south-east Ireland.
Marshall & Haes (1988) argue that the species is probably a late arrival in Britain from Dogger Land in the North Sea, before that area’s submergence, with the Thames estuary as the centre of its distribution in this country. They note its recent rapid range expansion, aided by the macropterous form – reported in exceptional numbers in London during the warm summers of 1983 and 1984. There is evidence that hot weather between April and July leads to accelerated nymphal development and earlier sightings of macropterous forms (Gardiner, 2009b; see also Chapter 2).
The expansion of range detected during the 1980s has continued apace. Widgery (2001b) reported many new records for 1998–2000 from Norfolk, Suffolk, Hampshire, Wiltshire (K. Rylands), Sussex (R. Becker) and Isle of Wight (B. Pinchen). In 2001 there were new reports from Hertfordshire, Oxfordshire and Gloucestershire, with evidence that at least some of these represented new colonisation. Subsequent new county records include:
Distribution
This species is widespread in Europe, except for the south. In Britain and southern Scandinavia it is close to the northern limit of its range. However, it continues to extend its range in Britain from the south and eastern coastal areas and is now common and widespread to the south and east of a line from the Bristol Channel to the Wash, and a more scattered distribution west to north Somerset, and north to Lancashire and Yorkshire. It is present in a restricted area of west Wales, and also in the south of Ireland.
Status and conservation
It seems likely that the recent range extension of Roesel’s bush-cricket will be continued, and aided in the longer term by climate change. Its long-grass habitats are likely to be a persistent, if marginal, feature of the farmed landscape, as well as of neglected and relatively lightly managed land such as roadside verges, flood defences and railway embankments. Agricultural set-aside and subsequently grant-aided stewardship schemes have no doubt favoured its range extension, and continue to provide both breeding habitat and, probably, connectivity between subpopulations (Simmons & Thomas, 2004; Gardiner, 2009b).
References: Gardiner, 2009b; Marshall & Haes, 1988; Simmons & Thomas, 2004; Smith, 2007; Sutton, 2005b, 2007b, 2008a, 2010a; Widgery, 2001b.
THE BOG BUSH-CRICKET
Metrioptera brachyptera (Linnaeus, 1761)
MEASUREMENTS: Male: 11–18 mm in length; female: 13–21 mm in length.

FIGS A–D. Green male; green female; brown female; male, dorsal view.

FIG E. The rare macropterous form, f. marginata (© P. Sutton).
Description
This medium-sized bush-cricket is quite similar in appearance to its relative, Roesel’s bush-cricket. In the typical form the hind wings are almost absent, while the fore wings are reduced to short stubs, reaching back only as far as the third or fourth abdominal segment. In the male the fore wings (tegmina) are modified for stridulation. There is a fine median longitudinal ridge along the pronotum. The male cerci have a black inward-pointing spine about halfway along. The ovipositor ofthe female is relatively long, and gently up-curved.
There are two basic colour forms, both of which occur quite frequently in British populations. One form has a brown ground colour, with dark brown-black markings. These are generally very fine speckles as well as larger patches of blackish coloration on the sides of the head, pronotum and the abdominal segments. There is often a fine pale line through the black around the eye, leading back to the front edge of the pronotum, and there are usually black stripes along the sides of the hind femora. The ovipositor is blackish for most of its length, but paler towards the base. The underside of the abdomen, and, often, the ventral surface of the hind femora, are green.
The other colour form is similar, except that the dorsal surface of the head and pronotum, and both leading and hind edges of the tegmina, are bright green.
In all forms, the paranota (side flaps of the pronotum) have a pale buff or cream-coloured hind margin.
There is a rare macropterous form (f. marginata (Thunberg)) in which both pairs of wings are fully developed (Ragge, 1973).
Similar species
Roesel’s bush-cricket has the pale border to the paranota running round the lower and fore margins, but in the bog bush-cricket it is confined to the rear margin. The ovipositor of the bog bush-cricket is longer relative to its body and more gently up-curved than that of Roesel’s bush-cricket. The subgenital plate of the female is deeply notched in the latter species, but only very shallowly so in the female bog bush-cricket (see the Key, Figs K8a and b). These species do occur together, so it is important for recorders to have clear identifying characters.

FIG F. Final instar male nymph.
The dark bush-cricket is superficially very similar, especially to the brown form of the bog bush-cricket. However, the wings of the dark bush-cricket are much more drastically reduced than those of the bog bush-cricket – being virtually absent in females and reduced to the stridulatory apparatus in the males.
The grey bush-cricket is fully winged and so quite different in appearance to the typical form of the bog bush-cricket.
Life cycle
The eggs are probably laid in the stems of plants during the summer, and are thought to overwinter twice before hatching in May or early June. The resulting nymphs pass through six instars before completing their development during July or early August. The adults can be found through August and September, becoming more scarce through October, and sometimes surviving into November.
The up-turned wing stubs are just visible in the fifth instar nymphs, and more clearly so in the final instar. The nymphs are always brown in ground colour, and darker-brown to blackish on the paranota and sides of the abdomen. As in the adults, there is a pale rear border to the paranota.
Habitat
The bog bush-cricket, as its name implies, is an inhabitant of the wetter parts of heaths and moors, usually at low altitudes. It is associated in these habitats with tall, dense tufts of vegetation such as cross-leaved heath (Erica tetralix) and other heathers, bog myrtle (Myrica gale) and grasses such as purple moor-grass (Molinia caerulea). In the wet heaths of Dorset and the New Forest it is often found together with the large marsh grasshopper (Stethophyma grossum).
Behaviour
The adults feed on seed heads of heathers and grasses, and probably also on other invertebrates. They make good use of the shelter provided by the grass tufts and low shrubs they inhabit, the males usually singing from cover. When they bask on outer branches or leaves they are easily alarmed by a careless approach and disappear down into the depths of the vegetation. A patient (and often waterlogged!) wait will sometimes be rewarded by their reappearance, and the males do sometimes sing while moving around over more exposed patches of scrub (see DVD).
The males sing through the day, and will often sing in overcast conditions. Their song is a short chirp, repeated at a variable rate of 2 to 6 chirps per second. It is rather quiet, and for many people a bat detector is the best way of locating the males.
Distribution
The bog bush-cricket occurs in suitable habitat through central and northern Europe, but is absent from the south and from northern Scandinavia. It also occurs in temperate Asia. In Britain it has a scattered distribution because of its rather specialised habitat requirements. It is present in heaths of Dorset, Hampshire, Surrey, Sussex, Kent and the south-west peninsula, then more locally northwards to the mosses of Lancashire and Cumbria (e.g. Winmarleigh Moss, Lancashire, and Meathop Moss, Cumbria (Widgery, 2001a)). Its most northerly known location is Aucheninnes (Cloak) Moss, Kirkudbright, Scotland. Formerly under threat from habitat destruction, the habitat has been saved following an intervention by the charity ‘Buglife’ (Sutton, 2010a). Its most northerly English population is isolated at Wedholme Flow NNR (Sutton, 2008a), and it has a scattered distribution in Wales. In East Anglia it is apparently absent from the Suffolk heaths, but relatively widespread in Norfolk (rediscovered in west Norfolk after gap of more than 50 years (Richmond, via Sutton, 2007b)).
Status and conservation
The bog bush-cricket was presumably much more widely distributed in the past and must have suffered considerably from the loss of wet heath to ‘development’ and agricultural intensification. However, it is abundant in its remaining strongholds, such as the heaths of Dorset, Surrey and the New Forest. In the longer term, the effects of climate change on these more southerly habitats may pose a serious threat to this, as to other species of lowland damp heaths and bogs.
References: Ragge, 1973; Sutton, 2007d, 2008a, 2010a; Widgery, 2001a.
THE LONG-WINGED CONEHEAD
Conocephalus discolor (Thunberg, 1815)
MEASUREMENTS: Male: 15–22 mm in length; female: 16–22 mm in length.
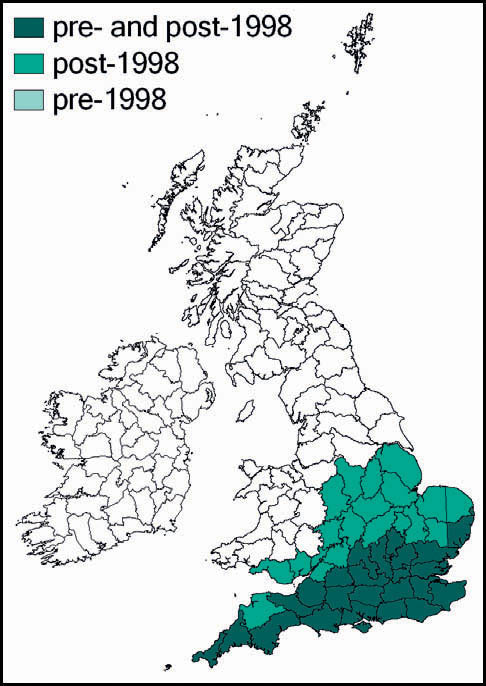

FIGS A–B. Male; female.
Description
The long-winged conehead is a rather delicately built species, with a distinctively triangular head (viewed from the side) that explains its vernacular name. Both pairs of wings are fully formed and extend back beyond the tip of the abdomen in both sexes (the hind wings usually projecting a little further back). The pronotum is saddle-shaped and without a median ridge on the dorsal surface. The basal area of the fore wings in males is modified for stridulation. The ovipositor is long, relatively narrow and almost straight, with minute serrations on the dorsal edge. The male cerci are almost straight at the tip.
There are two colour forms. The more common form is green with a dark brown dorsal band on the head and pronotum (and also usually on the abdomen, although this is largely obscured by the wings). This is bordered, at the sides, by a paler, yellowish band that shades into the green of the sides of the head and paranota. The wings are usually pale brown, with a transparent anterior section. The eyes are fawn, sometimes with a pinkish or violet tint, and the legs have tiny grey-black spots.
A minority of specimens have pale brown replacing the green coloration of the body, with darker brown on the dorsal surface of head and pronotum. The incidence of this form varies considerably from population to population (e.g up to 10 per cent of the population in the Plymouth area (Evans & Edmondson, 2007)), and also from year to year. In the south-east, 2009 was exceptionally dry, and this coincided with an apparent increase in numbers of the brown form.
There is also an extra-long-winged form of both sexes of this species, in which the wings reach almost to the tip of the ovipositor in females, and extend well beyond the tip of the abdomen in males. Ragge (1973) reports the discovery of this form by B. C. Pickard and S. House at Chapman’s Pool, Dorset in 1969. This is presumably the first British record of the form, and it coincided with an ‘enormous increase’ in the size of the population. Simmons & Thomas (2004) found this form to be eight times more frequent in populations at the margin of their (expanding) range than in the core (see Chapter 3 for more detail).

FIGS C–D. Extra-macropterous form of male and female.
Similar species
The long-winged conehead is much smaller than the great green bush-cricket, and is unlikely to be confused with any other established British species except its close relative, the short-winged conehead.
The short-winged conehead, as its name implies, is brachypterous in its typical form, and so confusion is unlikely. However, there is an uncommon long-winged form of the short-winged conehead which is very similar to C. discolor. In females, the shape of the ovipositor - almost straight in C. discolor, strongly up-curved in C. dorsalis - is the best guide to identification. Males of C. discolor are much more difficult to distinguish from the long-winged form of C. dorsalis. The shapes of the rear dorsal edge of the final abdominal segment and of the male cerci are different in the two species. In C. dorsalis there is a more prominent notched process on the final abdominal plate, and the male cerci narrow abruptly and are slightly up-turned at the tip. The male cerci in C. discolor narrow gradually towards the tip and are straight (see photographs under C. dorsalis, and the Key, Fig K4).
The songs of the two species are different (see DVD, and account of the following species).
The sickle-bearing bush-cricket (Phaneroptera falcata) has so far only a very tentative foothold in Britain, but could be confused with the long-winged conehead. However, P. falcata is much larger, is covered in tiny black spots, and both male cerci (strongly in-curved in P. falcata) and ovipositor (short, wide and strongly up-curved in P. falcata) are quite distinctive.
The large conehead (Ruspolia nitidula) is currently only doubtfully resident in mainland Britain, but may well become established in future years. It is much larger than the long-winged conehead, has a much more strongly conical head (i.e. the ‘forehead’ is sharply angled), and is bright green (without the dark brown on head and pronotum).
Life cycle
The cylindrical, buff-coloured eggs measure 6 mm by 1 mm, and are laid singly in plant stems during the summer and autumn. They hatch rather late the following year – in late April or May and into June. They develop through five nymphal instars to emerge as adults from late July onwards. Both late instar nymphs and adults can be found together at the end of August, and adults persist through October into November in most years.
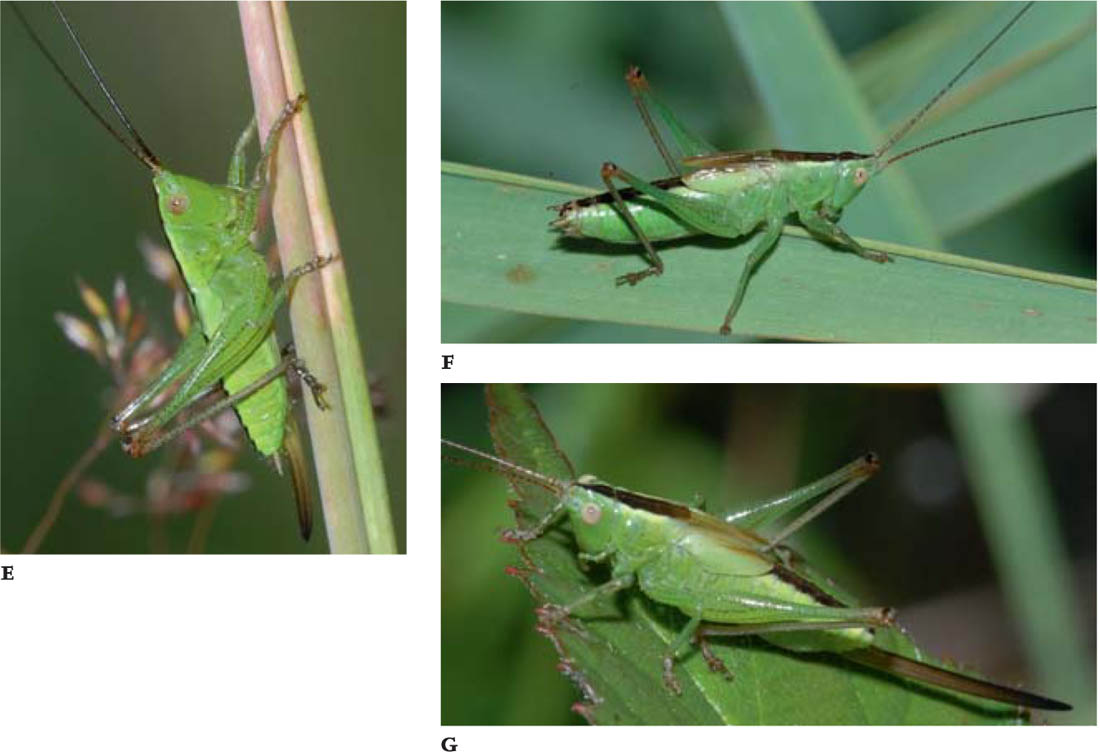
FIGS E–G. 4th instar female nymph; 5th instar male nymph; 5th instar female nymph.
The nymphs are bright green, with a dark brown to blackish band over the dorsal surface of the head, pronotum and abdominal segments. The up-turned wing stubs are clearly visible in the final two nymphal stages.
Habitat
This species inhabits a wide range of habitats – such as downland, coastal flood defences, conservation strips in agricultural land, roadside verges and unmanaged grassland – with long, coarse grasses, rushes or reeds, often with low scrub. In moist habitats it often coexists with the short-winged conehead, but it is also common in dry grassland, often in association with Roesel’s bush-cricket within the geographical range of both species.
Behaviour
Both males and females are active in rank vegetation through the day. They feed on grasses, seed heads and pollen, as well as small invertebrates, including aphids and caterpillars. They can fly but are more likely to descend into the vegetation when disturbed.
The males sing in long, continuous bursts. The frequency is at the limit of human hearing, and is rather quiet, so for most people a bat detector is the best way of locating singing males. Heard in this way, the song resembles a persistent ‘chugging’ motor. Singing males are usually evenly spread out in their habitat, although silent (‘satellite’) males can often be seen close to songsters. The females are attracted to the male song, and courtship begins with prolonged ‘fencing’ with the antennae, usually with the pair facing one another along the stem of a grass or rush. This is followed by a mutual approach, with male and female on opposite sides of the stem. As they slowly pass one another, the male curves round the tip of his abdomen in an attempt to ‘lock’ his genitalia onto their counterpart at the tip of the female’s abdomen. However, even though she clearly co-operates in this, the pair usually break apart after a few seconds, and the exercise is repeated – with sometime two or more females being involved (see DVD). Numerous ‘trial’ or ‘failed’ mating attempts can be observed without full copulation occurring.
Vahed & Gilbert (1996) give the spermatophore in this species as 9.6 per cent of the male body weight. After mating and consumption of the spermatophylax, the female lays her eggs in the stems of a wide range of plants – including reeds, rushes, sedges, coarse grasses, and even bramble. Her usual practice is to hold onto a plant stem while raising the rear part of her abdomen and bending her ovipositor down so that it is roughly at right-angles to her body, with the point close to the surface of the plant stem. She then probes the plant stem with the tip of her ovipositor, often twirling round the stem as she does so, until she finds a weak point that will permit insertion of the ovipositor (see DVD). The eggs are then laid one at a time, although often with several within any stem, lying along the line of the stem. Females spend much of their time in the ‘ovipositing mode’ testing stems, often paying attention to leaf sheaths, and sometimes resorting to chewing a hole in a tough stem. This again involves prolonged trial and error, with the female often having some difficulty probing with her ovipositor to locate the hole she has chewed.
Distribution
The long-winged conehead is widely distributed in Europe (except the far north), North Africa and temperate Asia. In Britain it was first discovered in 1931, but only recognised as this species (under the name C. fuscus) some time later (Blair, 1936). Here it is at the northern limit of its range, and until the mid-1980s was known only from a few southern coastal counties in England. Ragge (1965) reported it from Dorset, Hampshire, Sussex, Kent and the Isle of Wight, and noted that it was ‘seldom found far from tidal water’.
However, it began to expand its range beyond these limits during the 1980s – an extension that has accelerated since. It was first recorded from Hertfordshire in 1994, and from neighbouring Essex the following year (Wake, 1997). Widgery (2001b) reported new 10 km-square records from Hampshire and Kent, together with the first reports from Gloucestershire and Oxfordshire in 2001. The first Welsh record was from Cardiff in 1999, followed by Monmouth in 2004, with a report of it as widespread in Monmouthshire by 2007 (Sutton, 2008a). Its northward spread continued in the period from 2000, with new county records from Leicester (2001), Worcestershire and Norfolk (2003) (Sutton, 2004b), and Lincolnshire in 2006 (Sutton, 2007e).
Although the typical form is capable of flight (10–20 metres) (Sutton, 2003a), the extra-long-winged form is believed to have played a key role in its range expansion. (See Chapters 3 and 9 for more detail.)
Status and conservation
As it has spread from its former coastal habitats, this species seems to have become less specialised in its habitat requirements. Where it has become established it is found in almost all suitable habitat. Although many of its habitats are ephemeral, its proven dispersal ability indicates that it is unlikely to be at risk in Britain. It, like Roesel’s bush-cricket, has probably been aided in its expansion by a combination of agricultural set-aside and stewardship schemes and a succession of warm summers. Its further spread is likely to be favoured by climate change.
References: Blair, 1936; Marshall, 1974; Ragge, 1965; Ragge, 1973; Simmons & Thomas, 2004; Sutton, 2003a, 2004b, 2007e, 2008a; Wake 1997; Widgery 2001b.
THE SHORT-WINGED CONEHEAD
Conocephalus dorsalis (Latreille, 1804)
MEASUREMENTS: Male: 11–15 mm in length; female: 12–18 mm in length.
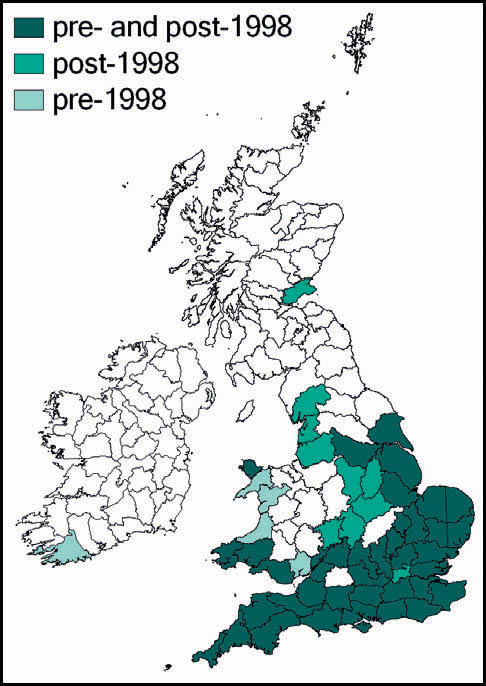

FIGS A–B. Male; female.

FIGS C–D. Ovipositor of female; female f. burri.
Description
This small bush-cricket closely resembles its long-winged relative. In the typical form the wings are short, but of variable length, reaching back over the first three to five abdominal segments. The basal part of the fore wings is modified for stridulation in the male. In both sexes the leading section of the fore wing is transparent, sometimes with a greenish tint. The ovipositor is long, up-curved and minutely serrated on the dorsal edge. The male cerci narrow abruptly close to the tip, and the tip is slightly up-turned. The rear edge of the final dorsal plate of the abdomen has a pronounced median notched projection.
The sides of the head, pronotum, abdomen and legs are green, and there is a dark brown band running from the top of the head back over the dorsal surface of the pronotum and abdomen. Often this band shades medially into paler reddish or violet tints on the dorsal surface of the abdomen, and there is usually a narrow and indistinct yellowish border between the brown band and the green of the sides of the body. The ovipositor is pale brown shading to dark brown around the tip. The eyes are fawn, often with pinkish or lilac tints, and there are tiny grey-black points on the legs.
There is a rare form in which the green coloration is replaced by brown. This form was reportedly quite common in the females on the south side of Poole Harbour in 1969 and 1970, although only one male was found (Payne, 1973; Ragge, 1973).
A small minority of specimens are of the long-winged form f. burri Ebner. In this form, the wings extend well beyond the tip of the abdomen – and, in females, beyond the tip of the ovipositor. The Colchester naturalist W. H. Harwood seems to have been the first to recognise this form, on ‘one hot day’ near Clacton-on-Sea, Essex, in August 1899 (Lucas, 1920; Marshall, 1974). There are also intermediate forms, in which the wings reach back approximately to the tip of the abdomen.
Similar species
In its typical, brachypterous, form this species is unlikely to be confused with any other. However, the long-winged form f. burri could easily be confused with the typical form of the long-winged conehead. The shape of the ovipositor is a useful way of distinguishing the females, and the shape of the cerci and final abdominal plate can be used to distinguish the males. The songs of the males of the two species also differ.
The speckled bush-cricket (Leptophyes punctatissima) is also green and brachypterous. However, the body shape is quite different, and the wing stubs much more severely reduced. The shapes of the male cerci and of the ovipositor, too, are quite distinctive. The two species are unlikely to be found in the same habitat.

FIGS E–G. Male cerci: C. discolor; C. dorsalis, lateral; C. dorsalis, dorsal view.
Life cycle
The eggs are pale whitish-buff, elongated flask-shaped, and approximately 5 mm by 0.75 mm. They are laid in the sturdy stems of plants such as marram grass (A. arenaria) and sea club-rush (Bolboschoenus maritimus). This is where they spend the winter, emerging in late spring or early summer the following year. The resulting nymphs pass through five instars before emerging as adults in late July or August. However, nymphs continue to be seen well into September (and even into October) (Ragge, 1965; B. Pinchen, pers. comm.). August and September are the peak months for the adults, and numbers tail off through October.
The nymphs are green, or yellow-green, with a black or very dark brown band along the dorsal surface of the head, pronotum and abdomen. The up-turned wing stubs are just visible in the final two instars (see Chapter 3, Fig 52).
Habitat
This species is particularly abundant among the rushes, club-rushes, reeds and coarse grasses fringing coastal and estuarine ditches. It also occurs among mature tussocks of marram grass (Ammophila arenaria) on exposed coastal sand dune systems. However, it can also be found inland in marshy areas in river flood plains and around fresh water ponds and lakes.

FIG H. Early instar nymph.
Behaviour
The short-winged conehead is omnivorous, feeding on small insects and also on vegetable matter, such as the seeds of rushes and reeds. According to Lucas (1920), it may also be cannibalistic.
When disturbed, the insects swivel round to the opposite side of a plant stem from the intruder, and adopt a posture that flattens the body against the plant stem – with hind legs stretched out behind, fore and mid legs aligned with the stem, and antennae pointing forwards. When slowly circled, they simply move around the stem to maintain their position relative to the observer, a game they seem able to play for quite some time!
They are active during the day. Females spend much of their time basking on the higher leaves of plants, or egg-laying, while the males are engaged in more active movement among the vegetation, or singing. The males stridulate for most of the day, with a song that superficially resembles that of the long-winged conehead. The sound is produced in prolonged, continuous bursts, but the more high-pitched ‘chugging’ (described as a ‘hiss’ by those who can hear it without the aid of a bat detector) alternates at irregular intervals with a lower-pitched ‘clicking’ sound (see DVD). This may be produced by alternately speeding up and slowing down the rate of vibration of the tegmina.
Although they sometimes move around as they stridulate, the males have favoured song perches. They can be seen to exude fluid from the tip of the abdomen and dab it along the surface of a leaf, in what appears to be a scent-marking activity. Singing males can often be seen competing physically for occupation of such a perch, sometimes by an intruder lunging at a defender, or sometimes by an approach that resembles a mating attempt – in which an intruder curves round his abdomen in the direction of his competitor. These interactions usually end very quickly with the retreat of one or other of them, but when two males are competing for the attentions of a female, the conflict can be much more prolonged, with repeated kicking, as well as the ‘abdomen-curving’ posture (see DVD). This seems to be counterproductive for both combatants, as the female invariably departs.
The females typically switch from basking in late afternoon and become more active, presumably searching for the singing males, although lunges by males at approaching females frequently result in the females’ jumping away.
After mating, the females lay their eggs, one at a time, in holes in the stems of water-side plants. They spend a great deal of time chewing the holes, with repeated trials, followed by more chewing until, finally, the hole is large enough for the ovipositor to be inserted fully into it. This performance is slightly different from that of the long-winged conehead, because of the differently shaped ovipositor. In C. dorsalis, the ovipositor is lowered and bent round under the abdomen of the female, with the point sticking out. The point is then inserted into the hole in the plant stem, and the ovipositor stretched out from the female’s body as she presses the shaft into the plant tissue and moves forwards (see DVD).
Enemies
Their excellent camouflage, and the hide-and-seek response to intruders, suggest adaptation to visual predators – possibly wetland birds such as reed and sedge warblers.
Distribution
Although somewhat localised because of its habitat requirements, this species is widespread through most of Europe, but absent from the south and far north.
The British distribution of the short-winged conehead seems to have remained stable until a significant extension of range began in the 1990s. Ragge’s (1965) distribution maps show a mainly southern and south-eastern pattern, with records from the southern coastal counties, the south-west peninsula, including the Somerset Levels, and East Anglia. At that time, the range extended to west and north Wales, and, in the east, as far north as south Yorkshire, with a majority of records within a few kilometres of the coast. This pattern is retained in the maps prepared by the UK Biological Records Centre (BRC), using records collected up to mid-1988, and published in Marshall & Haes (1988). A subsequent atlas (Haes & Harding, 1997) showed a large increase in inland records, especially from central southern England. The authors note that this partly reflects past under-recording, but state that the species is ‘certainly spreading in the vicinity of the Thames Valley and Hertfordshire’. This expansion into new areas in the south was confirmed by reports to Widgery, (2000b, 2001a), with a further extension to mainland west Cornwall (Haes, via Widgery, 2000c). This has been followed by a significant northward extension into Lancashire (2002 and 2005) (Sutton, 2005a; Smith & Newton, 2007), Cumbria (2006) (Sutton, 2007d), Leicestershire, Derbyshire and North Fife, in Scotland by 2009 (Sutton, 2010b). It has also been recorded from southwest Ireland.
Interestingly, the recently noted extension of range has not been accompanied by reports of increased numbers of the long-winged form, f. burri.
Status and conservation
Many of the habitats of this species are quite ephemeral, but partly as a result of human activity in maintaining flood defences and managing drainage ditches and ponds, suitable conditions are regularly reproduced. As many of its sites are quite isolated, it presumably has good powers of local dispersal, although it is unclear how this is achieved.
References: Haes & Harding, 1997; Lucas, 1920; Marshall, 1974; Marshall & Haes, 1988; Payne, 1973; Ragge, 1965, 1973; Smith & Newton, 2007; Sutton, 2005a, 2007d, 2010b; Widgery, 2000b, 2000c, 2001a.
THE SICKLE-BEARING BUSH-CRICKET
Phaneroptera falcata (Poda, 1761)
MEASUREMENTS: Male and female: 12–18 mm in length.
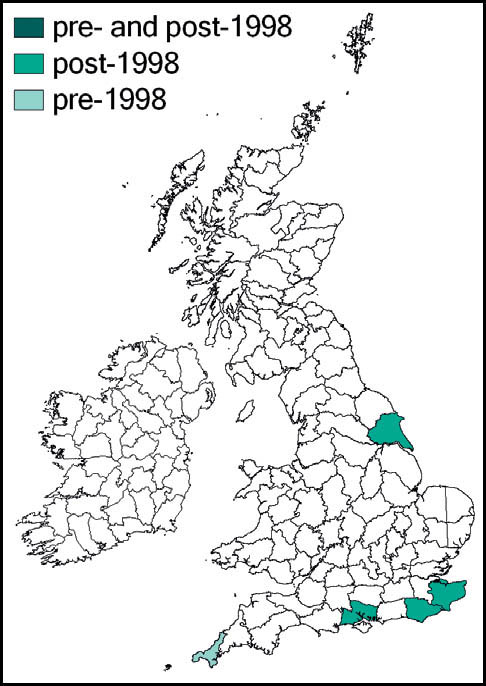

FIGS A–C. Male; male dorsal view; female.
Description
This relatively large bush-cricket is rather delicately built, with long thin legs, and very long wings. The fore wings reach back well beyond the tip of the abdomen, and the hind wings are significantly longer. The male cerci are long and upcurved, while the ovipositor is short, broad and strongly up-curved. The ground colour of the body and legs is pale yellowish green, with fine black speckles. The green shades to white on the ventral surface of the abdomen, and the face, too, is greenish white. The upper part of the eyes is red-brown, and the rear margin of the pronotum and rear edge of the wings are often also of this tint. There are two darker patches on the stridulatory apparatus on the male fore wing, and the dorsal surface of the abdomen (normally covered by the wings when at rest) shades from green to purple. The hearing organ at the base of the fore tibiae is very noticeable (see Chapter 1, Fig 9).
Similar species
The great green bush-cricket (Tettigonia viridissima) is also mainly green, fully winged and relatively long-bodied. However, that species is much more robust in appearance, lacks black spots on the body, and has a very differently shaped ovipositor (long and straight in viridissima) and male cerci (gently incurved in viridissima). The marked difference in length between fore wings (tegmina) and hind wings in P. falcata is also quite distinctive.
The long-winged conehead (Conocephalus discolor) is much smaller, and it, too, has very differently shaped ovipositor and male cerci.
The southern sickle-bearing bush-cricket (Phaneroptera nana) is very similar. The hind legs are thinner than those of P. falcata, and the side plates of the pronotum (paranota) are longer than deep (deeper than long in P. nana). However, P. nana has no known breeding colonies in Britain, and is an occasional accidental import only (e.g. a plant nursery in Nottinghamshire in 2007 (Sutton, 2008a)).
Life cycle
The eggs are laid between the surfaces of leaves. Adults emerge in July and last until October. In Britain August to September seems to be the peak period for finding the adults.
Habitat
P. falcata is regarded as an inhabitant of dry tall grassland and scrub on the mainland of Europe. The only currently known British population is confined to a substantial stand of rose-bay willowherb (Chamerion angustifolium) with a small tree in its midst, and some low bramble nearby and on an adjacent south-facing slope. The habitat is shared with Conocephalus discolor, Pholidoptera griseoaptera and Chorthippus parallelus.
Behaviour
The adults bask for brief periods on the higher leaves of the willowherb in sunshine, but retreat to the lower vegetation in cool weather. They rely greatly on their camouflage for protection, and move rather gingerly and deliberately through the vegetation. When seriously disturbed they can fly some distance and are then quite difficult to re-locate. The long back legs are stretched out and trail behind them when in flight.
The males sing from mid-afternoon into the night, the song being variously described as ‘a faint tss tss tss’ (Burr, 1936) and ‘tzpp tzpp tzpp’ (Evans & Edmondson, 2007). According to Ragge & Reynolds (1998) the calling song consists of single syllables repeated at irregular intervals, or, sometimes, more regularly at a rate of 1 to 4 per second. There is also a longer song consisting of 8 to 20 syllables. Phaneroptera seems to be unique in that the song is produced only during the opening strokes of the fore wings (Ragge & Reynolds, 1998).
History
This species is widespread in Europe north of the Mediterranean area, and its range extends eastwards through Turkey and central Asia to China and Japan. Despite occasional reports of single specimens, most commentators have not considered it to be a breeding species in Britain. However, Lucas (1920) reported two specimens that had been collected in Cornwall (in 1881 and 1884), and considered there was ‘a chance’ of it being included as a British species ‘if some enterprising entomologist will search the Land’s End district at the end of Summer’ (Lucas, 1920: 199). Burr (1936) and Ragge (1965) both mention the Cornish records, and Ragge also mentions a ‘dubious’ sighting from Dorset. One ‘enterprising entomologist’, R. M. Payne, did follow Lucas’s suggestion, and searched for the species in Cornwall without success (Payne, 1969).

FIG D. Habitat: the only known locality in Britain.
However, the species has been extending its range in mainland Europe for several decades, and prior to its discovery in England had made ‘spectacular’ gains of territory in the Netherlands (Sutton, 2006b; Kleukers, 2002; Kleukers & Krekels, 2004).
Single individuals were reported in 2004 (from Yorkshire) and in 2006 (from Hampshire) but are presumed to be migrants or accidental introductions.
The first confirmed breeding colony in Britain was discovered by a group including P. Hodge, M. Edwards, G. Collins and A. Phillips at Hastings Country Park, in East Sussex on 11 August 2006 (Collins et al., 2007; Sutton, 2006b). As many as 20 nymphs and adults were found in a patch of rose-bay willowherb at the corner of a rough, sloping meadow overlooking the sea. The presence of nymphs indicates that breeding had taken place at the site, implying that the species must have been present at least since the previous year. Visits to the site in subsequent years (2007, 2008) confirmed the survival of this breeding colony, but it is unclear whether it still survives.
However, there have been several reports of single specimens – either presumed migrants, or, just possibly, new colonists: one male, B. Pinchen, Hampshire, 2006 (Sutton, 2006b); one female (possibly of the closely related P. nana), Dungeness, 2009 (Sutton, 2010a); and one male, Humber estuary, east Yorkshire, 2010 (Sutton, 2010b). Also three nymphs were found in imported plants in Derbyshire (Sutton, 2007b).
Status and conservation
Repeated sightings of individuals of this species, together with its apparently successful breeding here for several years at one site, suggest that it is likely to establish itself in this country in the near future. In mainland Europe it appears not to be a habitat specialist and it flies readily, so there is no obvious reason why it should not spread to other localities in Britain.
References: Burr, 1936; Collins et al., 2007; Evans & Edmondson, 2007; Harz, 1969; Kleukers, 2002; Kleukers & Krekels, 2004; Lucas, 1920; Marshall & Haes, 1988; Payne, 1969; Ragge, 1965; Ragge & Reynolds, 1998; Sutton, 2006b, 2007b, 2008a, 2010a, 2010b.
THE SPECKLED BUSH-CRICKET
Leptophyes punctatissima (Bosc, 1792)
MEASUREMENTS: Male: 9–16 mm in length; female: 11–18 mm in length.
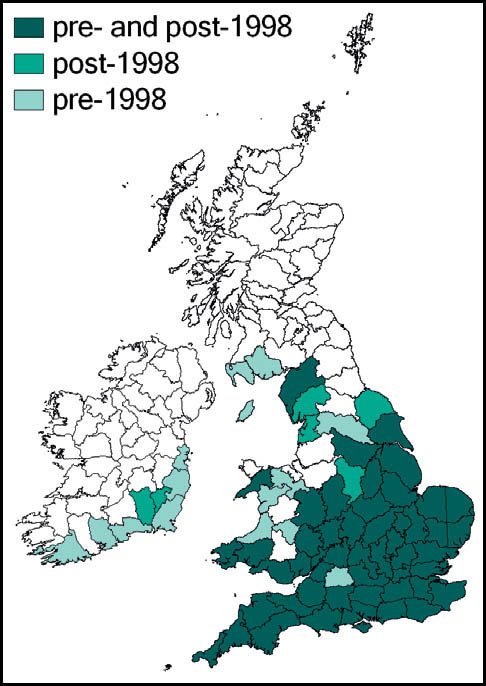
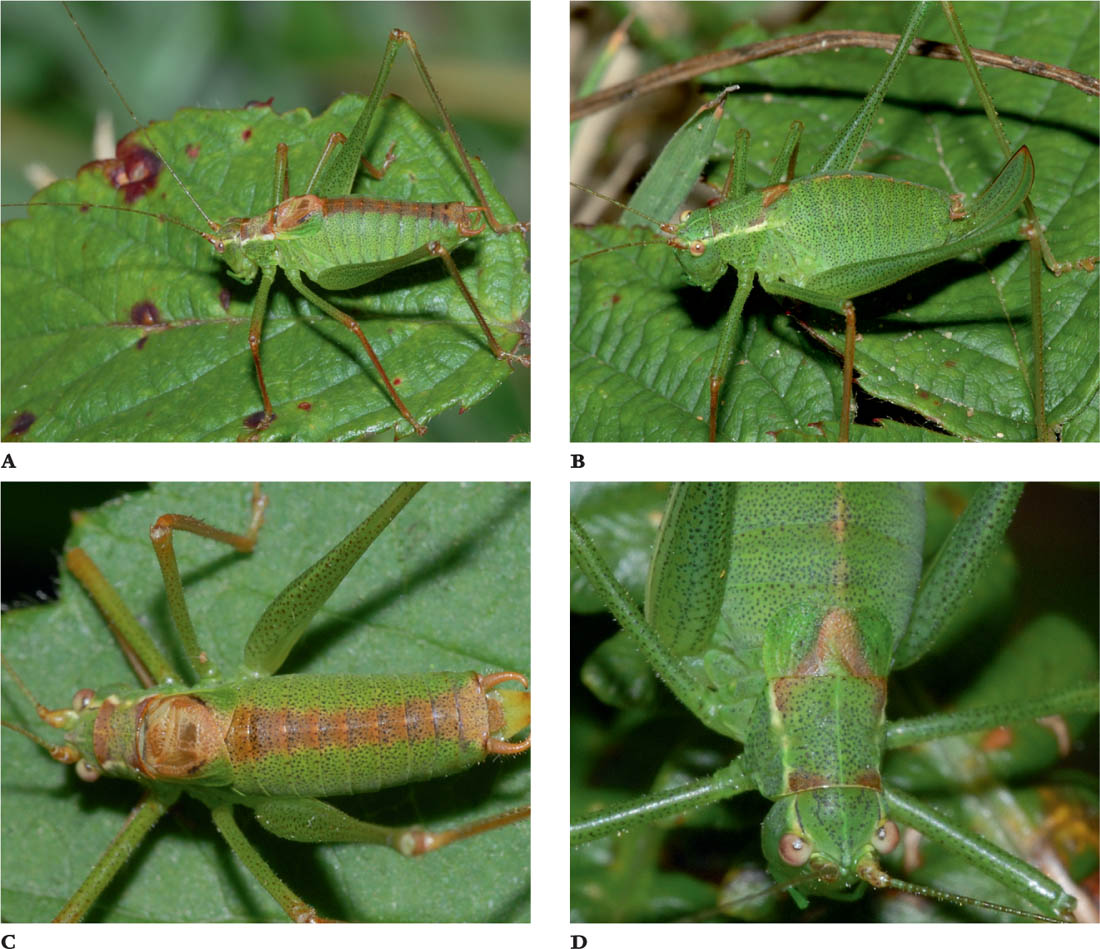
FIG A–D. Male; female; dorsal male; dorsal female showing wing-stubs.
Description
This small to medium-sized bush-cricket has a smoothly saddle-shaped pronotum, and long, spindly legs. The wings are greatly reduced to small flaps in both sexes. In the male, the wing-stubs overlap and are clearly modified for stridulation. In the female they are smaller, and project, side by side, behind the pronotum. The ovipositor is broad and scimitar-shaped, with minute serrations on both edges towards the tip. The male cerci are long and in-curved. The ground colour is pale green, densely flecked with tiny black spots (hence its vernacular name). The green shades paler towards the yellowish ventral surface. The male wing stubs are yellow-brown, as are the cerci, and there are similarly coloured patches on the pronotum. The tibiae and the eyes also tend to yellow-brown, and sometimes have a deeper, reddish tint. There is a dark band along the dorsal surface of the abdomen that varies from brown to violet-purple.
The female is similarly coloured except that the broad dorsal band on the abdomen is replaced in her case by a fine median yellow-brown (or violet) line. Her wing stubs are green with pale brown rear margins. The ovipositor is green with dark red-brown edging towards the tip. Both sexes have a yellow line along each edge of the dorsal surface of the pronotum (sometimes broken in the middle).
Similar species
Only two other British bush-crickets are green and brachypterous. One is the southern oak bush-cricket (Meconema meridionale). However, that species lacks the minute black spots, and has a yellow median line along its dorsal surface. The shape of the ovipositor also clearly distinguishes the females (long, narrow and gently up-curved in M. meridionale, short, broad and more strongly up-curved in L. punctatissima).
The other is the short-winged conehead (Conocephalus dorsalis). However, this species also lacks the black speckles of L. punctatissima, and, although usually brachypterous, has much less drastically reduced wings than L. punctatissima. The shapes of the male cerci and of the ovipositor are quite distinctive. C. dorsalis is unlikely to be found in the same habitat as L. punctatissima.
Nymphs of L. punctatissima could be confused with those – especially in the early instars – of the great green bush-cricket (Tettigonia viridissima), which often have black speckles on legs, abdomen and pronotum. However, the body shape of T. viridissima nymphs is more elongate, and the antennae are more or less uniformly brown (with strongly contrasting narrow bands of black and pale yellow-green in L. punctatissima nymphs).
Life cycle
The eggs are flat and ovoid in shape, approximately 1.5 mm by 3 mm. They are laid in the bark of trees or shrubs, or, where the bark is tough, in crevices. The nymphs emerge in May or early June and pass through six instars, before reaching adulthood from late July onwards. The adults persist into October, and even November in some years. In cooler parts of its range, the eggs undergo a double diapause, and the full life cycle takes two years (Deura & Hartley, 1982).
The nymphs are pale green, with quite prominent black spots on the body and legs. The antennae have distinctive alternating bands of black and pale yellow-green. The wing pads are visible in the final nymphal instar. The nymphs can often be seen sunning themselves on leaves of bramble or other low vegetation. They feed mainly on herbaceous plant species such as wood sage, dog’s mercury, nettles and golden rod in the early instars, initially grazing on leaf cuticle, but later biting small holes in the leaf lamina and so leaving a distinctive feeding pattern. With maturity they migrate to higher vegetation and their diet shifts to tougher foliage, especially the leaves of bramble (Hall, Ash, Robinson, pers. comm.).
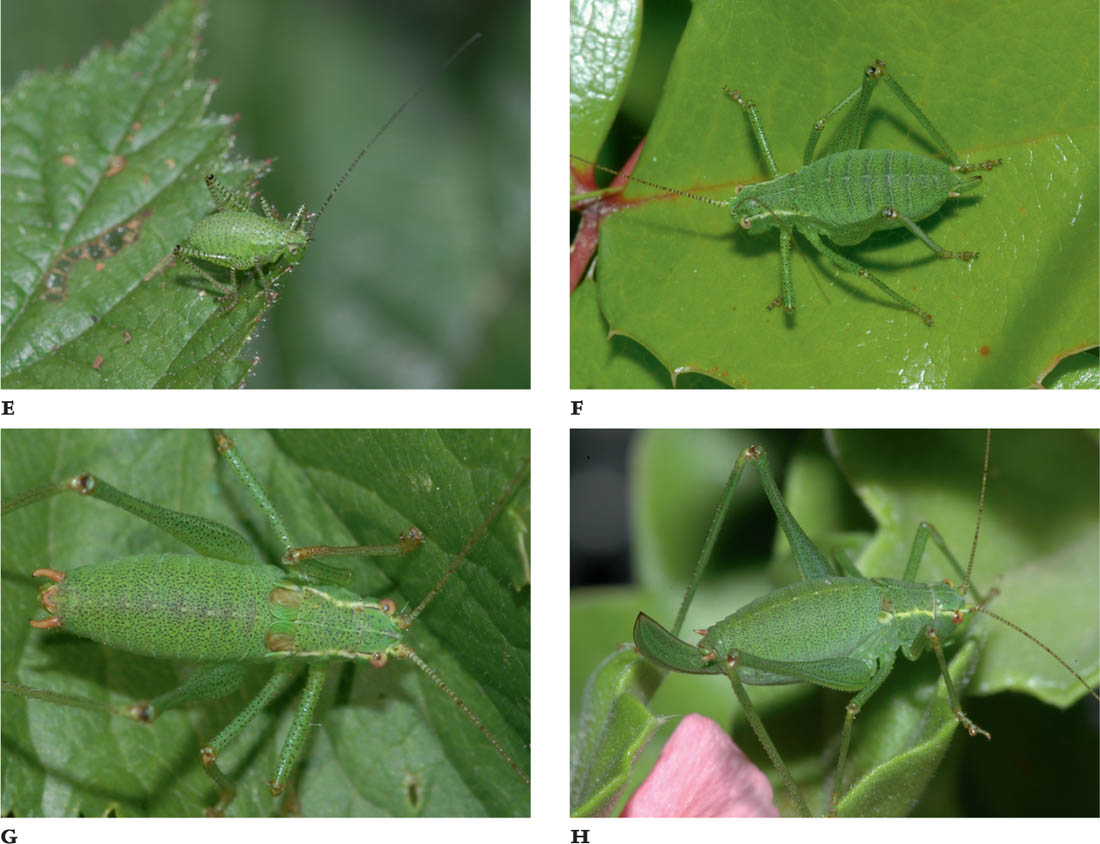
FIGS E–H. 1st instar nymph; 5th instar female nymph; 6th instar male nymph; 6th instar female nymph.
Habitat
This species inhabits trees and shrubs at various heights. Hedgerows, bramble patches, woodland edges and gardens may all be occupied. Although it may be found on a very wide range of woody plants, there is a strong association with bramble. A longstanding research project conducted by P. Ash, M. Hall and D. Robinson focused on this species has provided much new information on both its ecology and behaviour.
Behaviour
The adults are mainly vegetarian, feeding on the leaves of various plants. On sunny days they may be seen basking on open vegetation, especially on the upper surface of bramble leaves. The song of the male is a very quiet and high-pitched short chirp, which is repeated at variable intervals. Even at close quarters this is barely audible, and use of a bat detector enables detection of many more singing individuals. Modified by this device, the sound is a very brief ‘tic’. They sing from vegetation at a wide range of heights above the ground – from 1.5 metres up to 14 metres in trees (Robinson et al., 2009). Most frequently they sing from deep within foliage, but they will often walk around on exposed branches and leaves while singing. Singing males can be detected in this way at almost any time during the day, but they become more active from mid-afternoon onwards into the night.
The subfamily Phaneropterinae, to which this species belongs, is unusual in that receptive females respond to the male calling song with a song of their own. In L. punctatissima, this resembles that of the male, but without amplification it is even less audible to humans. The female response must occur within a very narrow ‘time window’ of 20 to 50 milliseconds. Allowing for the time the male song takes to reach the ‘ear’ of the female, her physiological processing of her response and the return of her stridulation to the male, the maximum distance across which effective communication can take place is approximately three metres (Heller & von Helversen, 1986; Robinson, 1980; Robinson et al., 1990). On hearing an appropriately timed response from a female, the male increases his chirp rate, and by call and response he orientates himself to her location and moves towards her (Kostarakos et al., 2007, Ofner et al., 2007, Rheinlaender et al., 2007, Zimmerman et al., 1989. See Chapters 2 and 5 for more detail).

FIG I. Male, showing tibial ‘ears’.
Mating generally takes place in the canopy, with the female mounting the male following antennal contact. The spermatophore is relatively small (approximately 4 per cent of the male body weight (Vahed & Gilbert, 1996). Following mating, the female consumes the spermatophore, taking some 30 minutes to do so, then descends to lower branches for oviposition (see Chapter 6, Fig 113). The eggs are laid at night, and are stuck to the substrate with a gum secreted by the female. Egg-laying sites at heights between 90 and 120 cm above the ground are usually chosen. Multiply-mated females lay more eggs than those with just one mating. This is not a function of how many spermatophores are consumed, and may be a consequence either of the female acquiring insufficient sperm at a single mating to fertilise all her eggs, or of stimulants transferred in the male ejaculate (Vahed, 2003b).
Enemies
There are four species of bats in Britain that forage by picking insects from vegetation at night. A legacy of predation by bats and other acoustically orienting predators may explain the extremely brief and relatively infrequent chirps emitted by this species as it moves through the vegetation (Robinson, 1990).
Distribution
This species is widespread through Europe, including southern Scandinavia, extending eastwards to the former Soviet Union.
In Britain it is common and widespread south of a line from the Wash to the Bristol Channel, including the south-west peninsula. Further north it has a more scattered distribution in England, but there are reports from south-western Scotland, the Isle of Man, western coastal districts of Wales, and in southern and eastern coastal areas of Ireland.
Status and Conservation
This is a common species and in many areas of southern Britain it is ubiquitous in gardens and hedgerows.
References: Deura & Hartley, 1982; Hartley & Robinson, 1976; Harz, 1969; Heller & von Helversen, 1986; Kostarakos et al., 2007; Offner et al., 2007; Rheinlaender et al., 2007; Robinson, 1980, 1990; Robinson, et al., 1986, 2009; Vahed, 2003b; Zimmerman et al., 1989.
FAMILY GRYLLIDAE: THE CRICKETS
Field crickets (© Tim Bernhard)
THE HOUSE CRICKET
Acheta domesticus (Linnaeus, 1758)
MEASUREMENTS: Male: 14–20 mm in length; female: 14–18 mm in length.
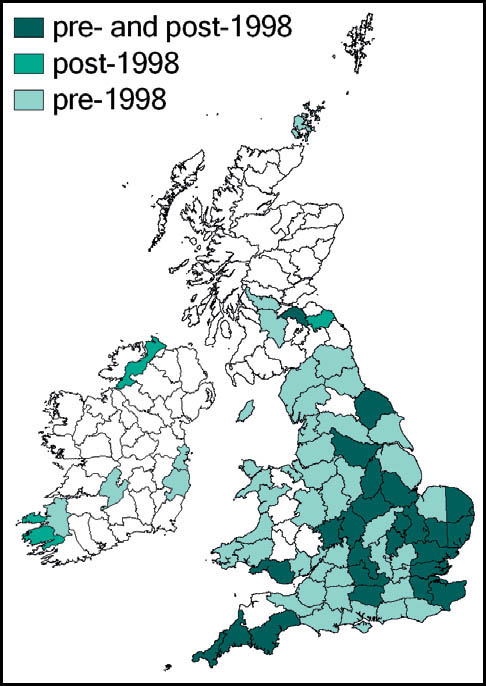

FIG A–D. Male lateral; male dorsal; female lateral; female dorsal.
Description
Both sexes are fully winged, with the tegmina reaching back approximately to the tip of the abdomen in the female, and somewhat shorter in the male. The male tegmina are modified for stridulation. In both sexes the longer hind wings, when folded, project back well beyond the tip of the abdomen (and beyond the tip of the ovipositor in the female). Both sexes have long, straight tapering cerci that point back beyond the tip of the abdomen. The ovipositor is also long, straight and narrow, pointing directly back from the tip of the female abdomen.
The ground colour is pale fawn-brown, with dark brown patches and markings especially on the head and pronotum. The dark brown areas form lateral bands on the head. The dorsal areas of the tegmina are also tinted darker brown than the ground colour.
Similar species
There are two species that are commonly kept as food for reptiles or other exotic pets:
Life cycle
Given appropriate conditions, they reproduce continuously, so that all stages in the life cycle may be seen at any time of year. The eggs are white and cylindrical, about 2.5 mm by 0.5 mm. They are laid singly or in clusters loosely in the soil, and each female may lay several hundred eggs.
The eggs usually hatch in two or three weeks, but take longer at low temperatures. The resulting nymphs may develop to adulthood in as few as seven instars, but may pass through twelve or more. Development takes from three to eight months, depending on temperature and availability of food. The nymphs are similar to the adults in colour.
Habitat
In Britain house crickets are found almost exclusively in association with human settlements. Formerly they could be found in a range of habitats such as private homes, rubbish dumps, bakeries, hotel and restaurant kitchens, farm out-houses and factories – anywhere that offered a combination of winter warmth and scraps of food. They need artificial warmth during the winter, but in summer can live in habitats such as hedgerows, gardens and allotments. Precautions against rats, mice and cockroaches have eliminated them from many former sites.
Behaviour
The males sing mainly in the evening or at night, with a high-pitched, repeated chirp.
There is also a distinct courtship stridulation, which is softer and interspersed with ‘clicks’ like those of the courting male field cricket. The female mounts the male to mate, and he transfers a spermatophore to her.
They are omnivorous scavengers, consuming decaying vegetable matter and scraps of food waste. As Gilbert White noted: ‘These crickets are not only very thirsty, but very voracious; for they will eat the scummings of pots, and yeast, salt, and crumbs of bread; and in any kitchen offal or sweepings.’ (White, 1971 [1789]: 246)
They can be active during the day, and are able to fly. Again, on White’s account: ‘In the summer we have observed them to fly, when it became dusk, out of the windows, and over the neighbouring roofs. This feat of activity accounts for the sudden manner in which they often leave their haunts, as it does for the method by which they come to houses where they were not known before.’
Although unable to survive British winters, there are numerous reports of house crickets singing outdoors, with possible temporary colonisation of crevices in walls or pavements and domestic gardens (Gardiner, 2005, 2006a, 2007a)
Enemies
The main enemy of the house cricket seems to be our contemporary concern with hygiene, but it is possible that competition with other commensals of human habitation, such as the house mouse and brown rat, have contributed to its demise.
White describes other threats: ‘Cats catch hearth-crickets, and, playing with them as they do with mice, devour them. Crickets may be destroyed, like wasps, by phials half-filled with beer or any liquid, and set in their haunts; for, being always eager to drink, they will crowd in till the bottles are full.’ (White, 1971 [1789]: 247)
History
Although the species is now cosmopolitan in distribution, its origin was probably in dry areas of north Africa and south-west Asia (Ragge, 1965). One view is that it may have been brought back to Britain by Crusaders in the 13th century (Kevan, 1955; Marshall, 1974), but it was certainly here by the 16th century (Moffet, 1634). Forster (1770) included it in his list of British species, and, as we saw, it figures in Gilbert White’s Natural History of Selborne (1971 [1789]). Given its close association with human habitation, it is not surprising that the house cricket (or ‘cricket on the hearth’) figured in poetry and folk lore. White quotes Milton’s Il Penseroso: ‘Far from all resort of mirth, save the cricket on the hearth.’ He also refers to it as ‘the housewife’s barometer’, foretelling rain, but also good or ill luck.
In Dickens’s The Cricket on the Hearth, the house cricket symbolises and mediates the relations between the humans in the story: ‘And it’s sure to bring us good fortune, John! It always has done so. To have a cricket on the hearth is the luckiest thing in all the world!’ (2004 [1845]). However, as some of the less savoury characters in Dickens’ story indicate, and White’s recipes for destroying them also suggest, the house cricket was not universally loved.
Lucas (1920) notes that not many British records of this species are ‘to hand’, and speculates that this may be owing to most entomologists’ thinking that so common an insect does not need to be recorded. However, Lucas’s view was that the species was undoubtedly declining at that time, possibly being displaced by the cockroach.
Burr (1936) refers to an inquest that came before the East Cheshire coroner in 1934, when ‘… it was officially stated that the sanitary inspector to Hale U.D.C. had been driven by crickets to desperation and suicide’ (p. 121).
Pickard (1954) thought it ‘not so common now as formerly’. Marshall & Haes (1988) comment on the loss of habitat owing to redevelopment of rubbish tips, the loss of many bakeries, and the use of insecticides such as DDT as responsible for a further decline in this species.
Distribution
There are historic records from almost all the English counties, as well as parts of Scotland, Wales and Ireland. The current distribution map shows it to be still widespread, although undoubtedly much less common than it once was.
Recent reports include its presence in manure heaps, a former landfill site, and along old railway track in Cambridgeshire (reported in Sutton, 2006b), a waste recycling plant in Pitsea, Essex (2009), manure heaps at West Stowe, Suffolk in 2002, and a pet-food factory in Suffolk in 2010 (A. Kettle, D. Underwood, pers. corr.)
Status and conservation
The house cricket is almost certainly an introduced species, once common and widespread, but now quite rarely found except for populations bred as food for reptiles. It seems likely that many of the frequent reports of new colonies of this species result from accidental or deliberate release of such captive populations.
References: Baldock, 1999; Burr, 1936; Dickens, 2004 [1845]; Forster, 1770; Gardiner, 2005, 2006a, 2007a; Kevan, 1955; Marshall, 1974; Marshall & Haes, 1988; Moffet, 1634; Pickard, 1954; Ragge, 1965; Sutton, 2006b; White, 1971 [1789].
THE FIELD CRICKET
Gryllus campestris (Linnaeus, 1758)
MEASUREMENTS: Male and female: 18–23 mm in length.
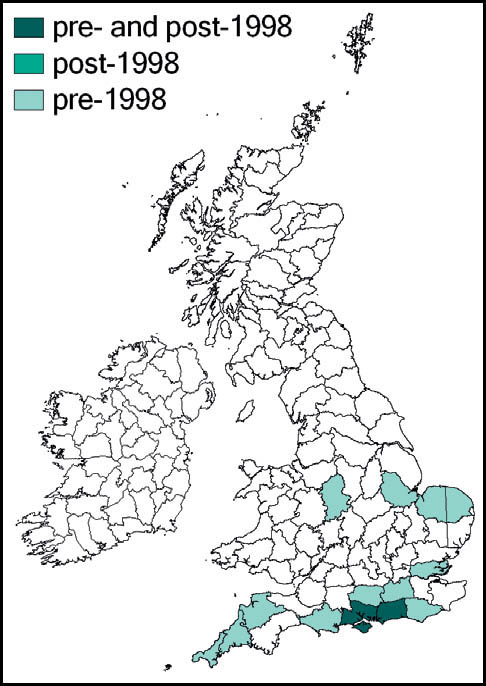

FIGS A–B. Male; female.
Description
Both sexes have large, shiny rounded heads. The eyes and long antennae are dark brown to black. The fore and rear edges of the smoothly saddle-shaped pronotum are approximately parallel, giving a rectangular appearance when viewed from above. The fore wings reach back towards the tip of the abdomen, leaving 3-4 abdominal segments exposed, and fold down at the sides. Both fore wings (tegmina) of the male are modified for stridulation, with a clear area (the harp and mirror) on each, which augments the sound. There are two rows of spines on each hind tibia. Both sexes have long tapering cerci, and the female has a long, needle-shaped ovipositor.
The colour of head, pronotum and abdomen is black. The legs, too, are mainly black, although the hind tibiae may be dark red-brown. The tegmina are also brown, but with a yellow band across the base. This is most clearly marked in the male. Parts of the body, especially the sides of the abdomen, often have minute hairs that shine golden.
Similar species
No other native species is remotely similar. However, a close relative, the southern field cricket (Gryllus bimaculatus), occurs naturally further south in Europe and is occasionally introduced into Britain. Although any resulting populations appear to be short-lived, they may still cause confusion. In the southern field cricket, the hind wings are fully developed, and when at rest they are folded and reach back to a point well beyond the tip of the abdomen in both sexes. In addition, the tegmina of the male G. bimaculatus are relatively longer than those of G. campestris, reaching to the tip of the abdomen, or almost so. The wings of G. bimaculatus are also darker in colour, especially in the female.
Life cycle
The eggs are laid during June in soil, either on bare ground or among tufts of short grasses. They hatch a few weeks later, by mid-July, and the nymphs congregate on bare patches of ground through their first three instars – presumably because warmth is important for their rapid development. In their early stages they are believed to feed on fungi and small plant fragments, graduating to tougher plant material as they develop. In the sixth instar, reached by mid-August, the nymphs begin to establish their own burrows. This is done by digging with the large mandibles, and passing the sand or soil back by kicks of their legs. According to Ragge (1965) the burrows are as much as 20 mm in length, and usually have a sharp bend near the entrance. Development continues through the rest of the summer, and winter is usually spent in the tenth (penultimate) nymphal instar. At this stage (see illustration) up-turned wing stubs are present, and, in females, the ovipositor is also just visible. Parts of the body, especially the dorsal surface of the abdomen, have a fine golden pubescence. Field crickets apparently may be seen foraging on mild winter days, but become fully active again in March or April. The ovipositor of the female becomes visible following the first spring moult, and the final moult, from which the fully adult insect emerges, takes place in mid-May.
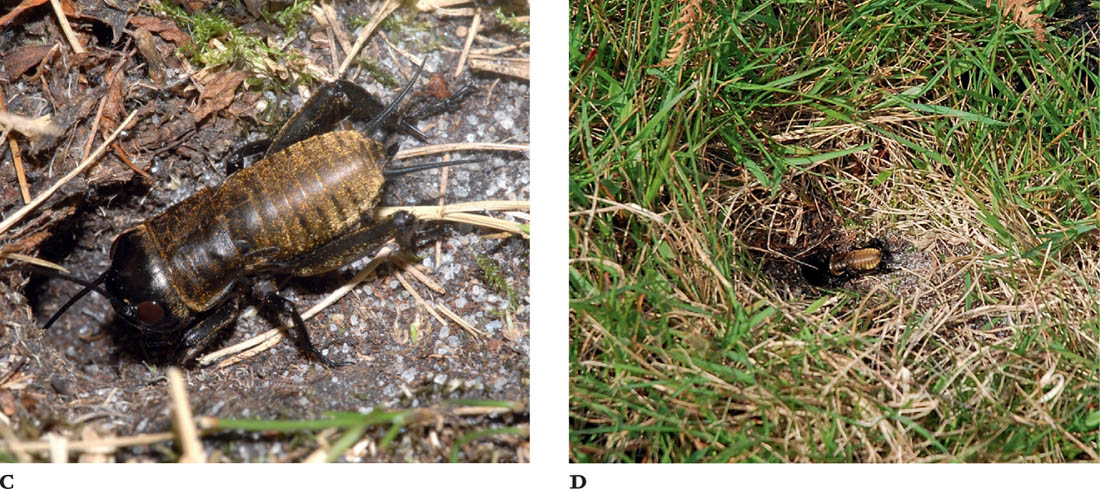
FIGS C–D. 10th instar nymph; nymph entering its burrow.
Habitat
The field cricket requires warm, sunny conditions if it is to complete its annual life cycle in southern Britain, and its known sites have accordingly been open, south-facing but sheltered slopes, with areas of bare ground and short vegetation. Both over-wintering nymphs and adults dig burrows, and so require a loose, sandy or chalk soil substrate. They are vulnerable to shading out of their habitat by succession to scrub, but, in a few sites, colonies have survived in woodland that has been regularly clear-felled. Grazing by rabbits, or by sheep or cattle at appropriate stocking levels, seems to be essential to maintain sufficiently short turf.
Behaviour
Last instar nymphs chew hollow, boat-shaped ‘platforms’ at the entrance to their burrows and also establish ‘runs’ through adjacent grass tufts. The presence of droppings is an indication that a platform and its burrow are occupied. The males generally sing from the platform outside a burrow, but sometimes will sing from the cover of a nearby run. The song, as with other crickets, is produced by rubbing the right tegmen over the left, and is a pure-toned repetitive chirp. Although each chirp is brief, it is repeated several times per second (usually 3–4), and when numerous individuals are singing simultaneously (‘chorusing’), the effect is of an almost continuous sound, resembling the stridulation of cicadas. The song is produced during both day and night, but sometimes ceases in the middle of very hot days. The females, too, live in burrows, and emerge to wander about, feeding on grasses and seed heads of various kinds, as well as to approach singing males and to search for suitable sites for egg-laying.

FIG E. Male producing ‘courtship song’ in contact with a female.
The calling song of the male attracts females, which enter the platform area. Sometimes this results in a very speedy change on the part of the male from the calling song to a more subdued, repeated metallic ‘click’. This is produced while he faces away from the female who, while probing him with her antennae, climbs onto his back. He meanwhile wriggles from side to side excitedly, and mating takes place. During this, a small white spermatophore is transferred to the female. This can be seen just below the base of her ovipositor (see DVD). However, on other occasions, male and female seem to spend extended periods of time together, sometimes motionless near the burrow entrance, sometimes moving in and out of the entrance. These periods are interspersed with persistent courtship activity on the part of the male, repeatedly edging, rear end first, towards the female, while making either the calling or courtship notes. In this mode, the females often retreat into a nearby ‘run’ among the grass stems, the male following on closely. The impression given is that male and female may cohabit in a burrow for substantial periods of time, with repeated matings or mating attempts on the part of the male. Edwards et al. (1996) reported instances of males using coercion to keep females in their burrows, and getting mauled by the females in return. However females often enter the burrows of males without any sign of coercion (pers. obs.). Formerly it was supposed that males were territorial, and would defend their burrow against other males. However, Edwards et al. demonstrated that individual crickets move frequently between burrows, and also dig new ones during the breeding season. Although they are not territorial, males can show aggression when they encounter one another. They may produce a more extended version of the calling chirp, and threaten one another with their large mandibles. Actual physical conflict may ensue, but usually neither male sustains injury. The adults emerge about the middle of May, and continue until the end of July or even early August. However, mating and egg-laying are mainly performed in the latter part of May and through June.
Enemies
During their research, Edwards et al. found two nymphs that had been attacked but then left by predators. In one of these examples there were bird bill feathers, suggesting that the insects were distasteful.
History
The field cricket was described by Thomas Moffet (1658, cited in Marshall, 1974), who noted the mechanism of its singing, and its habit of living in burrows. He also described a method of drawing it from its burrow by putting a tethered ant down the hole, but preferred the simpler technique, described later by White, and still used, of putting a twig or grass stem into the burrow and gently withdrawing it. Lucas (1920) included reports of this species from Devon and Cornwall, Norfolk and north Staffordshire, but expressed doubts about two Scottish reports. However, even at that time, it seems that the distribution of the field cricket was largely limited to the counties of Hampshire, Surrey and Sussex. Even here, he regarded it as ‘very rare and local’. Gilbert White (1971 [1789]) regarded the insect as ‘frequent in these parts’, but also noted that it was not common in other counties (Letter XLVI). White’s vivid descriptions of the habitat and behaviour of the field cricket have served to make it a well-known British insect – albeit one that is seldom encountered directly. Pickard (1954) added south Lincolnshire to the list of counties from which the field cricket had been reported, but Ragge (1965) commented that it was ‘rapidly becoming one of our rarest Orthoptera’, with flourishing colonies known only from south Hampshire, West Sussex and Surrey. Marshall (1974) reported several failed attempts to find the insect at sites where it had formerly been abundant, concluding that only two known breeding populations survived: one, near Arundel (discovered in 1956) and another, in West Sussex, discovered in 1971. The species was listed in Schedule 5 of the Wildlife and Countryside Act, 1981. Haes (1987) noted that there may have been a colony near Salisbury, in Wiltshire, up to the end of the 1960s, but confirmed the loss of all but two known colonies. One of these, on the chalk (presumably near Arundel) was reported as small and unmonitored. The other, on greensand, might produce over 100 singing males in warm years, but fewer than 30 in a cold one such as 1977.
In 1991, English Nature launched a ‘species recovery programme’, shortlisting a small number of species for urgent conservation action on the basis of their impending extinction, their potential to recover given conservation measures, and their attractiveness to humans. Initially field crickets were not included, but survey work in 1991 demonstrated that they had not been seen for some years on one of their presumed sites, and were present in much smaller numbers than had been thought at the other. This led to the subsequent inclusion of the species in the ‘target’ list for urgent action (Edwards, et al., 1996). The introduction of an appropriate management regime at the remaining site, together with a captive breeding programme undertaken by the Invertebrate Conservation Centre, London Zoo, and reintroduction at a small number of sites within its historic range has so far yielded positive results, with three populations now well established (Edwards, 2008).
Distribution
The species is widespread in mainland Europe, south to North Africa, and east to the Caucasus and west Asia. However, it is much more localised than formerly in Europe, especially towards its northern limits (Kleukers & Krekels, 2004). As recounted above this long term trend of decline is also true of the British population, possibly as a result of a combination of climatic conditions and habitat loss. It has persisted in only one site, in Sussex, but there are now at least three surviving colonies at nearby re-introduction sites. There is also a plan to increase the area of its last native site (Edwards, pers. comm).
Status and conservation
It was assigned the highest conservation priority (in British Red Data Books: 2) by Haes (in Shirt, 1987). Thanks to the timely conservation work begun in 1991, and the co-operation of landowners, it now seems that this species is no longer in immediate danger of extinction. However, its future does depend on continued appropriate management of its small number of habitats. Edwards et al. (1996) note that for such a large insect to complete its life cycle in one year in the British climate requires favourable weather in spring and summer. Haes’s report on annual fluctuations in numbers at one of its remaining sites through the 1970s supports the view of Edwards et al. that its remaining populations may undergo ‘boom-and-bust’ cycles in response to variations in weather from year to year. Local extinctions of isolated populations may have resulted from inclement weather through a succession of seasons, with no nearby populations to re-colonise the habitat.
References: Edwards, 2008; Edwards et al., 1996; Harz, 1969; Kleukers & Krekels, 2004; Lucas, 1920; Marshall, 1974; Ragge, 1965; Shirt, 1987; White, 1971 [1789].
THE SOUTHERN FIELD CRICKET
Gryllus bimaculatus (De Geer, 1773)
MEASUREMENTS: Male 19–23 mm in length; female 17–22 mm in length.
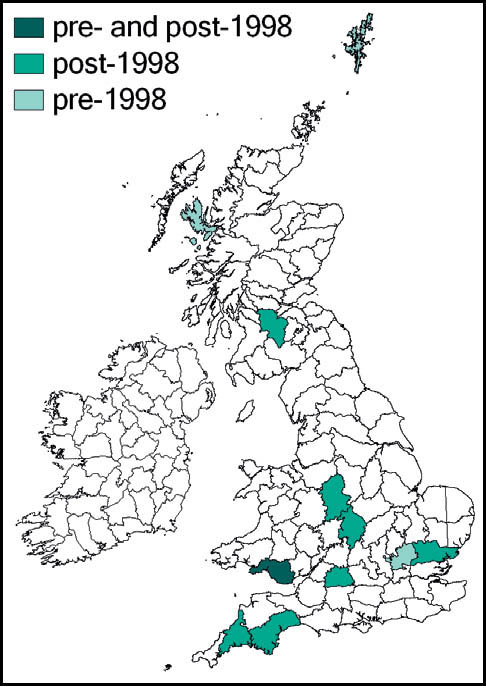

FIG A–D. Male dorsal; male lateral; female dorsal; female lateral.
Description
The head, pronotum and abdomen are black, as are the mouthparts, eyes and antennae. The legs are black, with variable amounts of reddish coloration. There are two rows of spines on the hind tibiae. Head and pronotum are approximately equal in width, and the pronotum is parallel sided, with a slightly wavy hind margin, especially in the female. The hind wings are long in both sexes – significantly projecting beyond the tip of the abdomen when furled. The tegmina are folded down over the sides of the abdomen. Both sexes have long cerci that curve outwards slightly. The ovipositor is straight and markedly longer than the cerci. The tegmina are black in the female, and dark brown in the male, with yellow towards the base – often reduced to a yellow patch on each tegmen.
Similar species
The field cricket (G. campestris) is very similar, but has shorter wings, not reaching the tip of the abdomen. The tegmina are paler in females of G. campestris.
Life cycle
In captivity it is continuously brooded, but in its natural habitat the adults emerge much later than those of the field cricket (G. campestris), and can be seen from July to September. The nymphs are pale fawn-brown, with dark brown to black markings, especially on head and pronotum. The dorsal surface of the abdomen is suffused blackish. In the final two nymphal instars the wing stubs are inverted, and in female nymphs the ovipositor can then be seen (see Chapter 3, Fig 55).
Habitat
Fabre (1917) says it does not burrow but lives in piles of rotting grass. According to Bellmann & Luquet (2009) it lives on the ground, taking shelter under crevices, stones and fragments of wood.
Distribution
It is present in all the French departments along the coast of the Mediterranean, west through the Iberian peninsula, and east into Greece. It is local, but sometimes abundant in dune habitats in France (Bellmann & Luquet, 2009).
In Britain there are occasional reports of short-lived colonies, presumably derived from crickets kept as food for reptiles. A colony was discovered in allotments near Epping, in Essex, by Mark Hanson (Sutton, 2006b). Another was reported from Chelmsford in 2008 (Sutton, 2008a).
Status and conservation
The current European distribution of this species remains close to the Mediterranean coast and it seems likely that climatic conditions will preclude its establishing a permanent population here.
References: Bellmann & Luquet, 2009; Fabre, 1917; Sutton, 2006b, 2008a.
THE WOOD CRICKET
Nemobius sylvestris (Bosc, 1792)
MEASUREMENTS: Male: 6.7–8.0 mm in length; female: 7.7–9.0 mm in length.
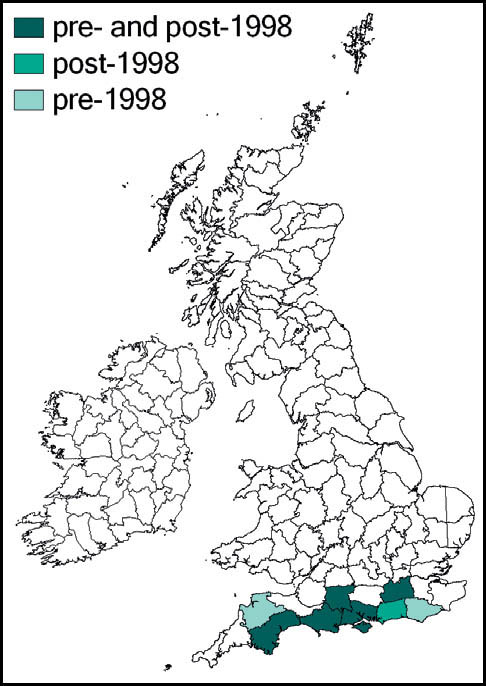

FIGS A–C. Male; male dorsal view; female.
Description
Both sexes are very small. The head is rounded and approximately as wide as the smoothly saddle-shaped pronotum (which looks broadly rectangular when viewed from above). The body is rather flattened dorso-ventrally, and both sexes have long, tapering cerci. The hind wings are absent, and the fore wings (tegmina) are short, but especially so in the female. The tegmina of the male reach back to cover roughly half of the abdomen, and are folded down at the sides. They are modified for stridulation, with the right tegmen overlapping the left when at rest. The female’s tegmina are reduced to very short stubs projecting back from the rear edge of the pronotum. The ovipositor is long, straight and needle-shaped, as is typical in crickets. There is a double row of spines on the hind tibiae, and there are fine, long black hairs sparsely distributed on the body, especially on the hind margins of the abdominal segments and around the edges of the pronotum.
The colour is a somewhat variable mixture of pale fawn and dark brown. The pronotum is often predominantly fawn with darker brown patches, while the abdominal segments tend to be predominantly dark brown in the males, and patterned dark and pale brown in the females. The wing stubs in the female have the veins outlined in dark brown. In both sexes the head is dark brown and the antennae are black. The head has a distinctive diamond-shaped cream marking. The legs are mottled dark and pale brown, often with reddish tints on the hind femora.
Similar species
The small size, distinctive habitat and the presence of reduced wings distinguish the wood cricket from all other British species. The only species comparable in size and general colouration is the scaly cricket, but this is wingless, and is exceedingly unlikely to be found in the woodland haunts of the wood cricket.
Life cycle
In Britain this species takes two years to complete its life cycle. Eggs laid in summer or in autumn enter a resting stage through the winter. They hatch in spring or early summer the following year, and the resulting nymphs pass through their first five or six instars by autumn, when they enter another period of arrested development through the winter (although they do not hibernate, as they are reported to be active on mild winter days (B. Pinchen, pers. comm.)). They become fully active in April and go on to complete their development in June or, more usually, July. In early August both adults and some final instar nymphs can be found together, along with younger nymphs that have hatched from eggs laid the previous summer or autumn. The adults continue until November, or, in a few cases, last through yet another winter into early spring.
In all, the nymphs pass through eight instars. The wing rudiments are visible for the final two nymphal instars, as is the ovipositor in females.
Habitat
As its name implies, the wood cricket is a woodland species, living among deep leaf-litter in clearings, along the edges of rides, and among bracken around woodland edges. It is found primarily in broad-leaved woodland, especially large, ancient oak forests, but it also occurs in mixed woodland. Ragge (1965) adds: ‘The wood cricket does occasionally occur in more open situations, such as on roadside banks or under a growth of bracken, but it is seldom found very far from trees’. Marshall & Haes (1988) also mention old stone walls, earthbanks and, on the Isle of Wight, crumbling sea cliffs, but it is always found close to trees or dense scrub.
Research on the Isle of Wight found it most abundant in older broad-leaved and mixed woodland, and associated with leaf litter, bare ground and low vegetation where the canopy cover was limited (in glades, rides and woodland edges.). The species was found to have good powers of dispersal in the absence of habitat barriers such as arable land (Brouwers & Newton, 2009a, 2009b; Brouwers, et al., 2011).
Behaviour
Wood crickets typically live among deep leaf-litter, and when disturbed disappear rapidly into it. They can run quite rapidly, and also jump when disappearing into the leaf litter is not an option. They feed on vegetable matter, supplemented by dead small invertebrates. According to Ragge (1965), in captivity they will eat a wide range of leaves, fruit, bread and dead insects. Although they will feed on the dead bodies of their own kind, there is little evidence of aggression even in crowded conditions.
The song is a low, churring sound, uttered in prolonged bursts. The crickets live in quite dense aggregations, and many males sing simultaneously (‘chorusing’), giving a continuous sound. This is produced throughout the day in warm conditions, and sometimes into the night.
Ragge (ibid.) gives a detailed account of the extraordinary courtship and mating behaviour of this species, and this is the main source for the following. In the presence of a female, the male produces a courtship song that is more subdued than the calling song, and he jerks back and forth. Eventually a small spermatophore is produced at the tip of his extended abdomen. His jerking movements become more violent until the female climbs onto his back. He remains still while the spermatophore is transferred to the tip of the female’s abdomen, below the base of the ovipositor. The pair then separate and the male resumes his jerky courtship movements until the female mounts him for a second time. This time they remain together for a longer period, and the female ‘licks’ an area on the right wing of the male that is covered in small hairs. The pair separate again, and a few minutes later the male produces a second, much larger, spermatophore. The male carries this with him for some time before resuming the jerky courtship movements, resulting in the female re-mounting him, mating for the second time, and taking the second spermatophore. The licking behaviour may be repeated. Sometimes the first spermatophore is removed and eaten by the female between the two matings, and sometimes it is retained, so that she carries both for some time. Eventually, the second spermatophore is rubbed off and eaten. Ragge notes that the female shows no inclination to remove and eat the spermatophore until at least a half hour has elapsed since she received it.
Current theoretical approaches suggest that edible spermatophores may function as nuptial gifts that convey nutrients to the female, as devices to give time for the male’s sperm to fully enter the female’s spermatheca, or as means of transferring stimulants to egg production or reduced receptivity to other males (see Chapter 6). The ‘licking’ behaviour, too, might perform any of these functions. However, this curious pattern of double mating with unequally sized spermatophores remains very puzzling.
Distribution
The wood cricket is widely distributed in Europe, including the Iberian peninsula, the Azores and Canary Islands, north Africa, and eastwards to the former Soviet Union. In the Alps it can be found up to an altitude of 1500 metres. In Britain, the Netherlands (where it is common in wooded areas) and Belgium it is close to the northern limit of its range.
In Britain it has a southerly distribution, south Devon, Hampshire and the Isle of Wight being its main strongholds. It has one population in Surrey, and there are previous records from Dorset and Sussex. Ragge (1965) mentions as ‘doubtful’ reports of the species from Cornwall, Worcestershire and Derbyshire.
Status and conservation
Widespread conversion of deciduous woodland to commercial conifer plantations must have caused many local extinctions of this species. Over-zealous woodland management, too, might lead to the elimination of the areas of warm, deep litter that they require. However, in their currently protected habitats, they remain very abundant and do not appear to be in immediate danger.
References: Baldock, 1999; Brouwers & Newton, 2009, 2010; Brouwers, Newton & Bailey, 2011; Harz, 1969; Marshall & Haes, 1988; Ragge, 1965.
FAMILY MOGOPLISTIDAE: SCALY CRICKETS
THE SCALY CRICKET
Pseudomogoplistes vicentae (Gorochov, 1996)
(Previously known as Mogoplistes squamiger, and Pseudomogoplistes squamiger.)
MEASUREMENTS: Male: 8–11 mm in length; female: 10–13 mm in length.
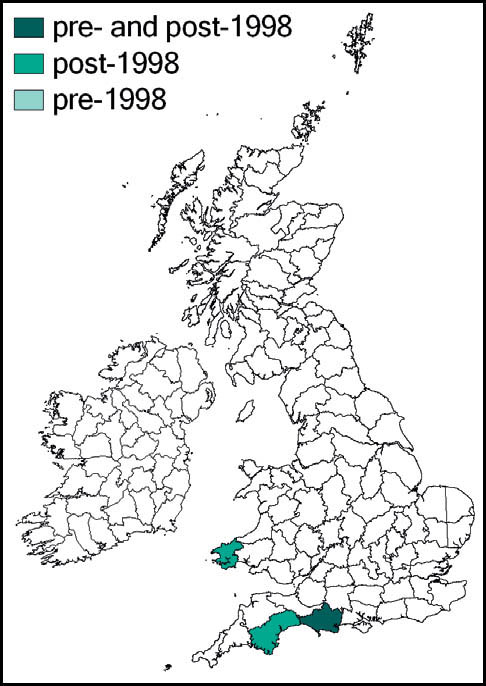

FIG A–D. Male; male lateral; female; female lateral.
Description
The scaly cricket has a slightly roughened appearance, especially on the abdomen, as a result of its covering of very fine scales. The head is as broad as the pronotum, narrowing at the front to a blunt ‘face’. The pronotum is roughly rectangular when viewed from above, and smoothly curved down at the sides. The antennae are as long as, or longer than, the body. Wings are completely absent in both sexes. The cerci are long, tapering and slightly outwardly curved in both sexes. The ovipositor of the female is relatively broader than that of our other crickets and very slightly up-curved. The sexes differ considerably in body shape, the females having rounder, fatter abdomens, the males having straight-sided abdomens that gradually widen to the broad, blunt rear end. In most specimens the main coloration of the head, pronotum and abdomen is uniformly dark brown. However, some individuals, especially females, have lighter brown on the pronotum and rear portion of the head. In both sexes the antennae, mouthparts and legs are lighter brown, often with some darker patterning on the outer surface of the hind femora. There are variable amounts of very fine, short greyish hairs on the body, especially on the dorsal surface of the abdomen, and a row of short, erect ginger hairs between the pronotum and the head. There are often fine ginger hairs on the tip of the abdomen and a few on the legs.
Similar species
The only similarly small and brown cricket to be found in Britain is the wood cricket (N. sylvestris). However, although brachypterous, this does have obvious wing rudiments, and is extremely unlikely to be found in the habitat occupied by the scaly cricket.
Life cycle
As adults and variously developed nymphs are frequently found together it is now supposed that they have a two or three-year life cycle. One suggested pattern that explains the co-existence of various different developmental stages at various times of year has been provided by P. Sutton. On this proposal, eggs may be laid before or after a first winter, hatching to give early nymphal stages during the first spring and summer, and nymphs overwintering at various developmental stages. Nymphal development continues through the second spring and summer, some individuals reaching adulthood by the onset of winter, overwintering as adults and laying eggs by the following autumn (Sutton, 1999). Other patterns have been proposed, however (Timmins, 1994; Kirby, 1995), and it may be that the seasonal cycle of development is variable. The number of nymphal instars is so far unknown.

FIGS E–G. Eggs; first instar nymph; final instar male nymph.
The nymphs are often paler in colour than the adults, and frequently have regular patterns of pale and dark brown markings on the dorsal surface of the abdomen. The different body shapes of the sexes enable them to be distinguished even prior to the development of the ovipositor in the female.
Habitat
This species lives among the pebbles, or under rocks, concrete installations and larger stones on shingle beaches. Although they are sometimes found inland of the shingle ridge at their best known locality, Chesil Beach, Dorset, by far the greatest numbers (both here and at other more recently discovered sites) live just above the high-tide mark on the exposed seaward aspect of the beach. This is referred to as the ‘seaweed strand line’, but at Chesil Beach there is little or no vegetation of any kind. Instead, there is a scattering of sea-borne detritus and litter. Despite earlier views that it required sheltered conditions, this species is now known to favour very bleak, exposed aspects, with the shingle as its only protection.
Behaviour
Scaly crickets are nocturnal, but when disturbed and exposed to light during the day they can move with astonishing speed. They escape by flattening their bodies and wriggling between the pebbles or into rock crevices. They are virtually impossible to recapture once they have disappeared by this means. Their relatively short but powerful hind legs enable them to jump effectively.
Numbers caught in pit fall traps or trapped by accident in plastic containers among the beach litter suggest not only very large populations where they occur, but also that they may be gregarious. In captivity they roost close together during the day, and often remain in contact by stroking one another with their antennae when active at night. Courtship involves protracted ‘fencing’ with the antennae, followed by a receptive female raising her abdomen while the male runs under and out again. This may be repeated on numerous occasions without appearing to culminate in mating. The female oviposits by raising her abdomen and lowering her ovipositor, so that it is roughly at right-angles to a substrate, and pressing it into the sand. Approximately 60 eggs were laid by one captive female, some up to two millimetres under the surface of the sand, others simply laid in loose clusters on the surface. The eggs are pale cream to yellow, 3 mm by 0.5 mm, and elongated with a narrowing towards one end (uppermost in those laid under the surface).
History
P. Sutton (1999) has provided a thorough chronology of the scaly cricket in Britain, from which much of the following is drawn.
The scaly cricket was discovered in 1949 at the eastern end of Chesil Beach, west of Weymouth, Dorset. Initially it was considered that this may have been a casual introduction. However, in 1954 another female was discovered, suggesting that the species was established at this site – although probably not native to Britain. Five more individuals (all females) were found in 1955. Ragge (1965), assuming the identification of it as M. squamiger to be correct, noted that the species was an inhabitant of the Mediterranean area, and raised the possibility that it might have been introduced to Chesil Beach from ships calling at Portland harbour. However, he also speculated that, given its extraordinary habitat, it might yet be found to have a more extensive distribution.
Small numbers of females were found on various occasions between 1965 and 1976, and it was speculated that the species might be parthenogenetic. However, on several dates in October 1977, R. Hansford collected seven males from Chesil Beach and nearby Smallmouth Sands, Portland.
The species continued to be reported from only one 10 km square in Britain, and was still considered a rarity. In 1984, a Russian entomologist, A. V. Gorochov, published a revised classification of the crickets, as a result of which it was proposed that the scaly cricket, formerly in the genus ‘Mogoplistes’, should be assigned to a new genus ‘Pseudomogoplistes’.
In 1985 extensive works were conducted to provide better pedestrian access to the reserve at the eastern end of Chesil Beach, and large numbers of the crickets (e.g. 100 reported by D. J. Moxom) were found under stones that were being laid to form a footpath. Further occasional reports of small numbers of specimens were received until 1992, when C. J. Timmins found 67 adult females, 22 adult males and a nymph, all dead in a margarine tub. This was the second indication that the Chesil Beach population could be much larger than previously thought. In 1994 a survey of the beach by P. Kirby confirmed this with the capture of 699 specimens using pitfall traps, and this was followed in 1995 by the capture of 207 crickets by Timmins using the same method. The co-existence of variously developed nymphs and adults led both observers to propose life-history patterns lasting more than one year. Kirby’s study revealed the presence of the cricket further north and west along the shingle ridge at Chesil Beach, so that its British distribution now included two 10 km squares (SY67 and SY68).
Searches on other shingle beaches on the south coast of England were carried out by Timmins in 1996 and proved negative, leading Haes & Harding (1997) to conclude that it was: ‘Probably introduced and successfully naturalised, occurring only at Chesil Beach, Dorset.’ However, this view was soon to be overturned, as a colony of the scaly cricket was discovered on 5 June 1998 on the Channel Island of Sark by P. and E. Brown. At almost the same time, C. Ash, an Exeter student carrying out a survey on the stretch of shingle between Branscombe and Beer Head in Devon, found a single scaly cricket nymph. Subsequent searches and pitfall trapping on this site later in the same year by P. Sutton, D. Cooper and C. J. Timmins confirmed the presence of a substantial colony of the species along approximately 1 km of the beach. These finds support the view that the scaly cricket is, after all, a native British species. In the same year, Peter Stallegger, the Orthoptera recorder for Normandy, informed the then British Orthoptera Recorder, John Widgery, that the scaly cricket had been discovered at Glanville, on the Cherbourg Peninsula in France (Widgery, 1998).
These discoveries were followed by reports from two localities in Guernsey and from another French locality in 1999 (Widgery, 2000a). Then, in 1999, a 13-year old, Beth Knight, found a scaly cricket in south-west Wales at Marloes, Pembrokeshire. Her parents collected more specimens, and these were subsequently confirmed as belonging to this species (Widgery, 2000b, 2000c). The same year, there were reports of a new colony from Guernsey, and two more on the coast of Brittany. Most recently, another population has been located at Dale Bay, to the south-east of its known population at Marloes, Pembrokeshire. In addition, the continuing presence of a large population at Chesil Beach was confirmed by O. Leyshon, who captured 68 in May 2010 (Sutton, 2010b).
Meanwhile, further examination of the Normandy population led to the recognition that the scaly cricket had been wrongly identified. It is now recognised as Pseudomogoplistes vicentae (itself only distinguished by Gorochov in 1996). On the basis of small differences in the genitalia, Morére & Livory assigned these specimens to subspecies septentrionalis. Specimens from Sark and Chesil Beach were confirmed as also belonging to P. vicentae (Gorochov & Marshall, 2001).
Distribution
The species was first distinguished on the basis of specimens from Morocco and Portugal (Gorochov 1996). So far they have also been reported from coastal sites in northern France and the Channel Islands. In Britain its known distribution remains confined to a small number of shingle beaches in Dorset, Devon and south-west Wales.
Status and conservation
The scaly cricket was designated ‘endangered’ in the Nature Conservancy Council’s Red Data Book 2: Insects (Haes, in Shirt 1987), and English Nature commissioned a survey carried out by C. J. Timmins in 1996. Since that time other populations have been discovered, and the large size of at least some of these has been demonstrated by trapping.
Despite its small number of localities, there has been concern that the large number of visitors to the first-discovered site might constitute a threat. The insect is potentially vulnerable to being crushed by people walking on the shingle, and there are reports of considerable numbers being killed by becoming accidentally trapped in litter. However, there is no evidence of a decline in the population at Chesil Beach, despite considerable visitor pressure. It has even been argued that there might be incidental benefits in the form of potential food items among litter (Gardiner, 2009c, and see also Chapter 9).
A more serious threat was illustrated by the off-shore grounding of the container ship NSC Napoli in January 2001. This resulted in oil pollution and large quantities of debris, some of it toxic, on Branscombe Beach. Both this and the subsequent clean-up operations, involving heavy vehicles and also physical cleaning of the shingle were a significant threat to the rich invertebrate fauna, and to the scaly cricket in particular (Sutton, 2007d). However, subsequent surveys revealed that the cricket had survived these operations (Sutton, 2008c), only to be challenged by winter storms in January 2010 that washed away large quantities of the shingle. Again, a visit by Beckman and Sutton in August that year confirmed the continued presence of the species there (Sutton, 2010b). Sutton (2008a) reported the possibility of another threat to the population at Marloes from disturbance due to excavation for cables for a tidal energy installation.
Although such potential threats may well recur, the remarkable persistence of the species in its few favoured haunts gives grounds for optimism about its future. Although a number of shingle beaches on the south coast have been searched without success, it remains quite possible that the species will be discovered in further localities.
References: Gardiner, 2009c; Gorochov, 1996; Gorochov & Marshall, 2001; Haes & Harding, 1997; Morère & Livory, 1999; Shirt, 1987; Sutton, 1999, 2002, 2007d, 2008a, 2008c, 2010b; Timmins, 1994; Widgery, 1998, 2000a, 2000b, 2000c.
FAMILY GRYLLOTALPIDAE: THE MOLE-CRICKETS
THE MOLE-CRICKET
Gryllotalpa gryllotalpa (Linnaeus, 1758)
MEASUREMENTS: Male: 35–45 mm in length; female: 40–45 mm in length.
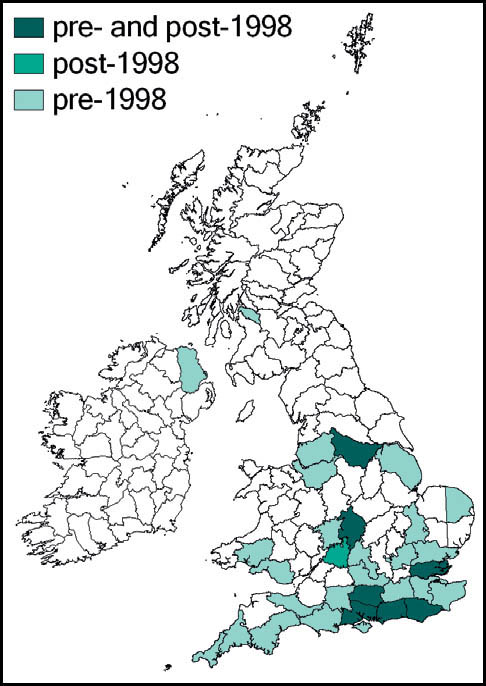

FIGS A–C. Male dorsal view (© B. Pinchen); male lateral view (© A. Kettle); head and pronotum, showing enlarged fore legs (© B. Pinchen).
Description
This species has a large, rather elongated body, with a tapering head, and an enlarged, rather funnel-shaped pronotum. The antennae are shorter than those of the crickets (approximately equalling the length of head plus pronotum). The fore wings are reduced, reaching back to about halfway along the abdomen in the male, and modified for stridulation in that sex. The hind wings are usually fully developed, and, furled up, they reach back beyond the tip of the abdomen. Both sexes have a pair of long, tapering cerci. The females do not have an external ovipositor, so that the simplest way of distinguishing males from females is by noting the different pattern of veins on the tegmina. Most of the body is covered by a fine pubescence. The colour is dark brown, paler below.
There is a rare form (f. cophta Haan) in which the hind wings are not fully developed, but this is apparently absent from Britain.
Similar species
There are no similar native species, but closely related mole-crickets are sometimes introduced accidentally in imported plant material. (e.g. an African mole-cricket Gryllotalpa africana found in York in 1999 (Widgery, 2000b), and Gryllotalpa quindecim found in Oxfordshire in 2005 (Pinchen, 2006)). Identification of species using morphological characters is extremely difficult.
Life cycle
Because of the difficulty in locating breeding colonies in Britain, most of the account below is drawn from mainland European studies of populations at similar latitudes. The information was collected together in Ragge (1965), which, together with Pinchen (2005), has been the main source used here.
Like the wood cricket, the mole-cricket has a two-year life cycle. The females begin to lay eggs from late April to early May. The eggs are laid over a one-to two-week period, in a heap within an underground chamber. Each batch may number from 30 to 50 eggs. However, a female may lay several egg batches during a summer, totalling 100 to 300, although as many as 640 have been recorded. Gilbert White described the accidentally exposed nest and eggs of a mole-cricket.
There were many caverns and winding passages leading to a kind of chamber, neatly smoothed and rounded, about the size of a moderate snuff-box. Within this secret nursery were deposited near an hundred eggs of a dirty yellow colour, and enveloped in a tough skin, but too lately excluded to contain any rudiments of young, being full of a viscous substance. The eggs lay but shallow, and within the influence of the sun, just under a little heap of fresh-moved mould... (G. White, 1971 [1789] letter XLVIII).
The eggs take from two to four weeks to hatch, depending on the temperature, and, uniquely among British Orthoptera, the mother engages in maternal care. She both guards the nest, and repeatedly cleans the eggs with her mouthparts – behaviour that is believed to protect them against mould.
After hatching, the small nymphs stay in the nest for a few weeks, feeding on humus and rootlets. When they emerge from the nest they continue for a while to be protected by the female, becoming independent by the fourth instar.
These nymphs continue to develop through the summer but do not reach the final instar before the onset of winter hibernation. As egg-laying continues into the summer, the offspring from later batches arrive at hibernation at earlier stages of development.
The following spring and summer the nymphs continue their development. The more advanced ones may reach adulthood by their second winter, but probably do not become sexually mature until the following spring. The nymphs that passed through their first winter in earlier developmental stages do not reach adulthood and sexual maturity until the summer after their second winter.
The number of nymphal instars appears to be variable, but Ragge gives ten as probably the most usual. Wing pads are just visible at the eighth instar, and clearly visible in the ninth and tenth.
Habitat
The mole-cricket makes its burrows in soft, moist soils, usually near rivers, streams canals or ponds. The edges of water meadows or river flood plains are its most common habitats, but it can be found in gardens and allotments. It prefers free-draining, loamy soils, with a fluctuating water table and with areas of short turf and occasionally disturbed soil, in open, sunny locations (Pinchen, 2005). An occasional source of records has been gardeners’ complaints that its burrowing and feeding on roots is a cause of damage. When burrowing close to the surface of the soil, as it does in wet ground, its burrows cause ridges to appear on the surface, similar to those made by moles.
Behaviour
The mole-cricket is seldom seen, even when present in an area, as it spends most of its time underground. The burrows may be close to the soil surface, if the ground is wet, or to a depth of a metre or more when conditions are drier, and for hibernation.
The males sing below ground in a resonating chamber formed within the burrow system. The sound is a low, continuous ‘churr’ that has been likened to the call of the nightjar (Caprimulgus europaeus), and is emitted during warm evenings and nights in spring and early summer.
When a female arrives, the male continues to sing, while drooping its wings on either side of its body and swaying from side to side. Mating is reputedly accomplished by the female climbing onto the back of the male, while the male transfers a spermatophore to the female. However, video footage of an above-ground mating attempt shows the male attempting to reverse over the female (B. Pinchen, pers. comm.).
Both adults and nymphs are able to swim, and they are also able to run quickly backwards. Although they do not fly during the day, they do fly on some warm evenings. In Gilbert White’s words, they ‘move “curso undoso”, rising and falling in curves’ (G. White 1971 [1789]).
The song and habitat of the mole-cricket have given rise to an exceptional range of vernacular names for it: fen-cricket, churr-worm, eve-churr, jar-worm, Cambridge nightingale, and, in south Wales, rhing y les (Baldock, 1999; Marshall & Haes, 1988). Other local names include croaker, land crab, bog mole and jarr-bob. Eve-churr and jarr-bob are also local names for the nightjar (B. Pinchen, pers. comm.)
Enemies
Baldock (1999) cites an early comment by the eminent 19th-century Godalming naturalist, Edward Newman, to the effect that the mole-cricket was ‘occasionally found in the craw of these birds [nightjars] when shot’. Newman’s speculation was that the similar call of the nightjar served as a ‘decoy’ for the male mole-cricket (Baldock, 1999: 71). In the New Forest, B. Pinchen reports that fox and tawny owl came close to investigate the sound source when males were singing, while fox, magpie, nightjar and tawny owl were attracted to sound tapes of the mole-cricket. Song-tapes used in Guernsey attracted hobbys, and one householder spoke of mole-crickets being caught by her cat (B. Pinchen, pers. comm.).
Distribution
The mole-cricket is still widespread in much of Europe, except for Norway and Finland, and also occurs in north Africa and western Asia. However, in Britain it has undergone a long drawn-out decline, with what appears to be only one probably native population known on the mainland at the time of writing.
Lucas (1920) listed reports from Berkshire, Cambridgeshire, Cornwall, Derbyshire, Devon, Hampshire, Kent, Lancashire, Lincolnshire, Norfolk, Oxfordshire, Staffordshire, Surrey, Sussex and Wiltshire, although many of these were old records, and others referred to single specimens that may have been accidental introductions. The same may be true of the records from Scotland (Renfrewshire) and Ireland (Derry). He considered it likely that it was less common than formerly. According to Ragge, it had become ‘very much rarer’ by 1965, and he gave reports from the Avon and Test valleys in South Hampshire, and the River Wey and its tributaries in Surrey.
Marshall & Haes (1988) note the destruction of sites in Surrey, Dorset and Hampshire during the 1940s and 1950s, citing recent reports of colonies from the northern edge of the New Forest, on allotments close to the river Itchen near Southampton, and in East Sussex around Bucksted and Uckfield only.
By the 1960s and early 1970s only two small colonies were known, one in a Southampton garden, the other in a meadow on the Hampshire/Wiltshire border, but both are thought to have become extinct. There seem to have been no reports between that time and 1988, when a single male was dug up from a field near Wareham, Dorset (Pinchen, 2005). There were unconfirmed reports from central Hertfordshire up to the 1990s (Widgery, 2000a). These were followed by reports of single specimens from Macclesfield (1996), the Vauxhall car factory in Luton (1999), Chelmsford (2000), and a garden in South Woodham Ferrers, Essex (also in 2000) (Pinchen, 2003). Widgery (2001b) reported that mole-cricket stridulation was heard at Old Marsden, Oxfordshire, and burrows were found at a locality in south Wales. Credible reports of mole-cricket song include one from an Oxford allotment in 2001 (Sutton, 2004b and Pinchen, 2005). Subsequent investigation of these localities turned up no further evidence of the crickets. However, song was heard from a small area of the New Forest in 2003, and a survey of the site a month later revealed surface burrows. Mole-cricket song heard in 2008 gave evidence of the continuing presence of the species at this site (Pinchen, 2009).
There are other occasional reports, usually with evidence that the specimens concerned have been incidentally imported in plant material: Southend-on-Sea, Essex, in imported root vegetables at a greengrocers (Pinchen, 2004); Ashington, Sussex, in plant material imported from Italy, August 2003 (Sutton, 2003c); Gloucestershire, one male in plants imported from mainland Europe, September 2004 (Pinchen, 2006); Oxfordshire, nymphs and adults in a compost heap, almost certainly imports from mainland Europe; Hampshire, in the root stock of an imported palm; south Wales, one adult in a garden; Hampshire, a nymph in a garden centre (all 2005, Pinchen, 2006); and Winchelsea beach, East Sussex, one adult in 2006 (Pinchen, 2009).
Status and conservation
The mole-cricket is very elusive, spending most of its life underground, and males singing at the surface only on rare occasions. The native population may now be close to extinction, but there remains the possibility that other surviving colonies await discovery. The mole-cricket was included in Schedule 5 of the Wildlife and Countryside Act, 1981, and listed as ‘endangered’ in the Nature Conservancy Council’s Red Data Book 2: Insects (Haes, in Shirt 1987). The species was included in English Nature’s Species Recovery Programme in 1994, and data was collected by M. Edwards. Searches for surviving colonies focused on the valley of the river Wey, where apparently suitable habitat still exists, but to no avail. It is also listed as a UK Biodiversity Action Plan priority species. Since 1999, the co-ordinator of the Species Recovery Project has been B. Pinchen, from whom much of the above information has been derived.
References: Baldock, 1999; Harz, 1969; Lucas, 1920; Pinchen, 2003, 2005, 2006, 2009; Ragge, 1965; Marshall & Haes, 1988; Shirt, 1987; Sutton, 2003c, 2004b; White, 1971 [1789]; Widgery, 2000a, 2001b.